User login
How to bridge the gap for rural cancer patients
New research suggests that better access to quality care may reduce disparities in survival between cancer patients living in rural areas of the United States and those living in urban areas.
The study showed that urban and rural cancer patients had similar survival outcomes when they were enrolled in clinical trials.
These results, published in JAMA Network Open, cast new light on decades of research showing that cancer patients living in rural areas don’t live as long as do urban cancer patients.
“These findings were a surprise, since we thought we might find the same disparities others had found,” study author Joseph Unger, PhD, of Fred Hutchinson Cancer Research Center, Seattle, said in a statement.
“But clinical trials are a key difference here. In trials, patients are uniformly assessed, treated, and followed under a strict, guideline-driven protocol. This suggests that giving people with cancer access to uniform treatment strategies could help resolve the disparities in outcomes that we see between rural and urban patients.”
Dr. Unger and his colleagues studied data on 36,995 patients who were enrolled in 44 phase 3 or phase 2/3 SWOG trials from 1986 through 2012. All 50 states were represented.
Patients had 17 different cancer types, including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
A minority of patients (19.4%, n = 7,184) were from rural locations. They were significantly more likely than were urban patients to be 65 years or older (P less than .001) and significantly less likely to be black (vs. all other races; P less than .001).
However, there was no significant between-group differences in sex, and all major U.S. geographic regions (West, Midwest, South, and Northeast) were represented.
The researchers limited their analysis of survival to the first 5 years after trial enrollment to emphasize outcomes related to cancer and its treatment. They looked at overall survival (OS) as well as cancer-specific survival.
The team found no meaningful difference in OS or cancer-specific survival between rural and urban patients for 16 of the 17 cancer types.
The exception was estrogen receptor-–negative, progesterone receptor–negative breast cancer. Rural patients with this cancer didn’t live as long as did their urban counterparts. The hazard ratio (HR) was 1.27 (95% confidence interval, 1.06-1.51; P = .008) for OS and 1.26 (95% CI, 1.04-1.52; P = .02) for cancer-specific survival.
The researchers said this finding could be attributed to a few factors, including timely access to follow-up chemotherapy after patients’ first round of cancer treatment.
Although there were no significant survival differences for patients with hematologic malignancies, rural patients had slightly better OS if they had advanced indolent NHL or AML, but slightly worse OS if they had MM or advanced aggressive NHL.
Rural patients had slightly better cancer-specific survival if they had advanced indolent NHL but slightly worse cancer-specific survival if they had AML, MM, or advanced aggressive NHL.
The researchers said these findings suggest it is access to care, and not other characteristics, that drives the survival disparities typically observed between urban and rural cancer patients.
“If people diagnosed with cancer, regardless of where they live, receive similar care and have similar outcomes, then a reasonable inference is that the best way to improve outcomes for rural patients is to improve their access to quality care,” Dr. Unger said.
The National Cancer Institute and the HOPE Foundation supported the study. The researchers reported financial relationships with various pharmaceutical companies.
SOURCE: Unger JM et al. JAMA Network Open. 2018;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235.
New research suggests that better access to quality care may reduce disparities in survival between cancer patients living in rural areas of the United States and those living in urban areas.
The study showed that urban and rural cancer patients had similar survival outcomes when they were enrolled in clinical trials.
These results, published in JAMA Network Open, cast new light on decades of research showing that cancer patients living in rural areas don’t live as long as do urban cancer patients.
“These findings were a surprise, since we thought we might find the same disparities others had found,” study author Joseph Unger, PhD, of Fred Hutchinson Cancer Research Center, Seattle, said in a statement.
“But clinical trials are a key difference here. In trials, patients are uniformly assessed, treated, and followed under a strict, guideline-driven protocol. This suggests that giving people with cancer access to uniform treatment strategies could help resolve the disparities in outcomes that we see between rural and urban patients.”
Dr. Unger and his colleagues studied data on 36,995 patients who were enrolled in 44 phase 3 or phase 2/3 SWOG trials from 1986 through 2012. All 50 states were represented.
Patients had 17 different cancer types, including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
A minority of patients (19.4%, n = 7,184) were from rural locations. They were significantly more likely than were urban patients to be 65 years or older (P less than .001) and significantly less likely to be black (vs. all other races; P less than .001).
However, there was no significant between-group differences in sex, and all major U.S. geographic regions (West, Midwest, South, and Northeast) were represented.
The researchers limited their analysis of survival to the first 5 years after trial enrollment to emphasize outcomes related to cancer and its treatment. They looked at overall survival (OS) as well as cancer-specific survival.
The team found no meaningful difference in OS or cancer-specific survival between rural and urban patients for 16 of the 17 cancer types.
The exception was estrogen receptor-–negative, progesterone receptor–negative breast cancer. Rural patients with this cancer didn’t live as long as did their urban counterparts. The hazard ratio (HR) was 1.27 (95% confidence interval, 1.06-1.51; P = .008) for OS and 1.26 (95% CI, 1.04-1.52; P = .02) for cancer-specific survival.
The researchers said this finding could be attributed to a few factors, including timely access to follow-up chemotherapy after patients’ first round of cancer treatment.
Although there were no significant survival differences for patients with hematologic malignancies, rural patients had slightly better OS if they had advanced indolent NHL or AML, but slightly worse OS if they had MM or advanced aggressive NHL.
Rural patients had slightly better cancer-specific survival if they had advanced indolent NHL but slightly worse cancer-specific survival if they had AML, MM, or advanced aggressive NHL.
The researchers said these findings suggest it is access to care, and not other characteristics, that drives the survival disparities typically observed between urban and rural cancer patients.
“If people diagnosed with cancer, regardless of where they live, receive similar care and have similar outcomes, then a reasonable inference is that the best way to improve outcomes for rural patients is to improve their access to quality care,” Dr. Unger said.
The National Cancer Institute and the HOPE Foundation supported the study. The researchers reported financial relationships with various pharmaceutical companies.
SOURCE: Unger JM et al. JAMA Network Open. 2018;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235.
New research suggests that better access to quality care may reduce disparities in survival between cancer patients living in rural areas of the United States and those living in urban areas.
The study showed that urban and rural cancer patients had similar survival outcomes when they were enrolled in clinical trials.
These results, published in JAMA Network Open, cast new light on decades of research showing that cancer patients living in rural areas don’t live as long as do urban cancer patients.
“These findings were a surprise, since we thought we might find the same disparities others had found,” study author Joseph Unger, PhD, of Fred Hutchinson Cancer Research Center, Seattle, said in a statement.
“But clinical trials are a key difference here. In trials, patients are uniformly assessed, treated, and followed under a strict, guideline-driven protocol. This suggests that giving people with cancer access to uniform treatment strategies could help resolve the disparities in outcomes that we see between rural and urban patients.”
Dr. Unger and his colleagues studied data on 36,995 patients who were enrolled in 44 phase 3 or phase 2/3 SWOG trials from 1986 through 2012. All 50 states were represented.
Patients had 17 different cancer types, including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
A minority of patients (19.4%, n = 7,184) were from rural locations. They were significantly more likely than were urban patients to be 65 years or older (P less than .001) and significantly less likely to be black (vs. all other races; P less than .001).
However, there was no significant between-group differences in sex, and all major U.S. geographic regions (West, Midwest, South, and Northeast) were represented.
The researchers limited their analysis of survival to the first 5 years after trial enrollment to emphasize outcomes related to cancer and its treatment. They looked at overall survival (OS) as well as cancer-specific survival.
The team found no meaningful difference in OS or cancer-specific survival between rural and urban patients for 16 of the 17 cancer types.
The exception was estrogen receptor-–negative, progesterone receptor–negative breast cancer. Rural patients with this cancer didn’t live as long as did their urban counterparts. The hazard ratio (HR) was 1.27 (95% confidence interval, 1.06-1.51; P = .008) for OS and 1.26 (95% CI, 1.04-1.52; P = .02) for cancer-specific survival.
The researchers said this finding could be attributed to a few factors, including timely access to follow-up chemotherapy after patients’ first round of cancer treatment.
Although there were no significant survival differences for patients with hematologic malignancies, rural patients had slightly better OS if they had advanced indolent NHL or AML, but slightly worse OS if they had MM or advanced aggressive NHL.
Rural patients had slightly better cancer-specific survival if they had advanced indolent NHL but slightly worse cancer-specific survival if they had AML, MM, or advanced aggressive NHL.
The researchers said these findings suggest it is access to care, and not other characteristics, that drives the survival disparities typically observed between urban and rural cancer patients.
“If people diagnosed with cancer, regardless of where they live, receive similar care and have similar outcomes, then a reasonable inference is that the best way to improve outcomes for rural patients is to improve their access to quality care,” Dr. Unger said.
The National Cancer Institute and the HOPE Foundation supported the study. The researchers reported financial relationships with various pharmaceutical companies.
SOURCE: Unger JM et al. JAMA Network Open. 2018;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235.
FROM JAMA NETWORK OPEN
Key clinical point:
Major finding: Only rural patients with adjuvant-stage estrogen receptor–negative and progesterone receptor–negative breast cancer had worse overall survival (hazard ratio, 1.27) when patients had the same access to care.
Study details: A comparative effectiveness retrospective cohort analysis of 36,995 patients from all 50 states enrolled in 44 cancer trials from 1986 through 2012.
Disclosures: The National Cancer Institute and the HOPE Foundation supported the research. The researchers reported financial relationships with various pharmaceutical companies.
Source: Unger JM et al. JAMA Network Open. 2018;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235.
Eat/sleep/console approach almost eliminates morphine for NAS
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
REPORTING FROM PHM 2018
Key clinical point: When it comes to neonatal abstinence syndrome, treat the infant, not the Finnegan score.
Major finding: The University of North Carolina Children’s Hospital dropped the length of stay for neonatal abstinence syndrome from about 11 to 5 days by moving from scheduled to PRN morphine and abandoning Finnegan scoring. Morphine use fell more than 80%.
Study details: Review of a 7-month quality improvement project
Disclosures: There was no industry funding for the work. The lead investigator didn’t have any disclosures.
Blinatumomab gains European approval in children
The a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for children aged 1 year or older who have relapsed/refractory, Philadelphia chromosome–negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
The European Commission’s (EC) new approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
In 2015, the EC approved blinatumomab to treat adults with Philadelphia chromosome–negative, relapsed/refractory B-cell precursor ALL.The EC’s approval of blinatumomab in pediatric patients is based on results from a phase 1/2 study published in the Journal of Clinical Oncology in 2016. The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion – 49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were four dose-limiting toxicities during the phase 1 portion of the trial, two of which were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure. Based on the dose-limiting toxicities, the maximum tolerated dose of blinatumomab was 15 mcg/m2 per day, but a stepwise dosage was recommended to reduce the risk of CRS.
The recommended dose was 5 mcg/m2 per day on days 1-7 and 15 mcg/m2 per day on days 8-28 for cycle 1, and 15 mcg/m2 per day on days 1-28 for subsequent cycles, according to the study results.Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response within the first two cycles. A total of 14 of these patients (52%) achieved minimal residual disease negativity.
The a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for children aged 1 year or older who have relapsed/refractory, Philadelphia chromosome–negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
The European Commission’s (EC) new approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
In 2015, the EC approved blinatumomab to treat adults with Philadelphia chromosome–negative, relapsed/refractory B-cell precursor ALL.The EC’s approval of blinatumomab in pediatric patients is based on results from a phase 1/2 study published in the Journal of Clinical Oncology in 2016. The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion – 49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were four dose-limiting toxicities during the phase 1 portion of the trial, two of which were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure. Based on the dose-limiting toxicities, the maximum tolerated dose of blinatumomab was 15 mcg/m2 per day, but a stepwise dosage was recommended to reduce the risk of CRS.
The recommended dose was 5 mcg/m2 per day on days 1-7 and 15 mcg/m2 per day on days 8-28 for cycle 1, and 15 mcg/m2 per day on days 1-28 for subsequent cycles, according to the study results.Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response within the first two cycles. A total of 14 of these patients (52%) achieved minimal residual disease negativity.
The a bispecific, CD19-directed, CD3 T-cell engager immunotherapy.
Blinatumomab is now approved as monotherapy for children aged 1 year or older who have relapsed/refractory, Philadelphia chromosome–negative, CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). The patients must have received at least two prior therapies, or they must have relapsed after allogeneic hematopoietic stem cell transplant.
The European Commission’s (EC) new approval of blinatumomab extends to all countries in the European Union, as well as Norway, Iceland, and Liechtenstein.
In 2015, the EC approved blinatumomab to treat adults with Philadelphia chromosome–negative, relapsed/refractory B-cell precursor ALL.The EC’s approval of blinatumomab in pediatric patients is based on results from a phase 1/2 study published in the Journal of Clinical Oncology in 2016. The study included 93 pediatric patients with relapsed/refractory B-cell precursor ALL. Patients received blinatumomab as a continuous intravenous infusion – 49 patients in the phase 1 portion of the trial and 44 in phase 2. The patients were followed for 2 years.
There were four dose-limiting toxicities during the phase 1 portion of the trial, two of which were fatal. Three patients had grade 4 cytokine release syndrome (CRS), one had grade 5 cardiac failure (as well as grade 4 CRS), and one had grade 5 respiratory failure. Based on the dose-limiting toxicities, the maximum tolerated dose of blinatumomab was 15 mcg/m2 per day, but a stepwise dosage was recommended to reduce the risk of CRS.
The recommended dose was 5 mcg/m2 per day on days 1-7 and 15 mcg/m2 per day on days 8-28 for cycle 1, and 15 mcg/m2 per day on days 1-28 for subsequent cycles, according to the study results.Among the 70 patients who received the recommended dose of blinatumomab, 27 (39%) achieved a complete response within the first two cycles. A total of 14 of these patients (52%) achieved minimal residual disease negativity.
The techno vagina: The laser and radiofrequency device boom in gynecology
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
2018 Update on contraception
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.
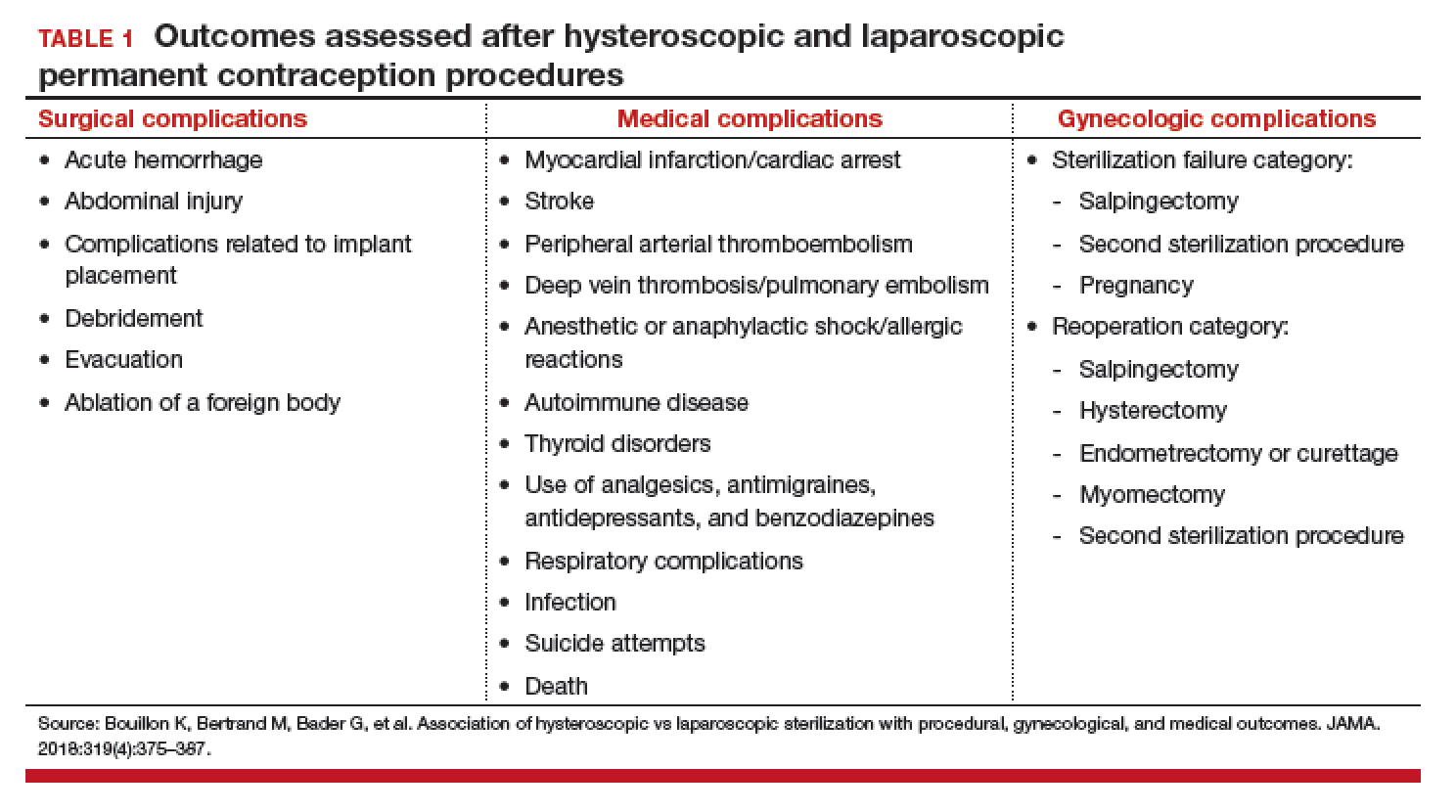
Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).
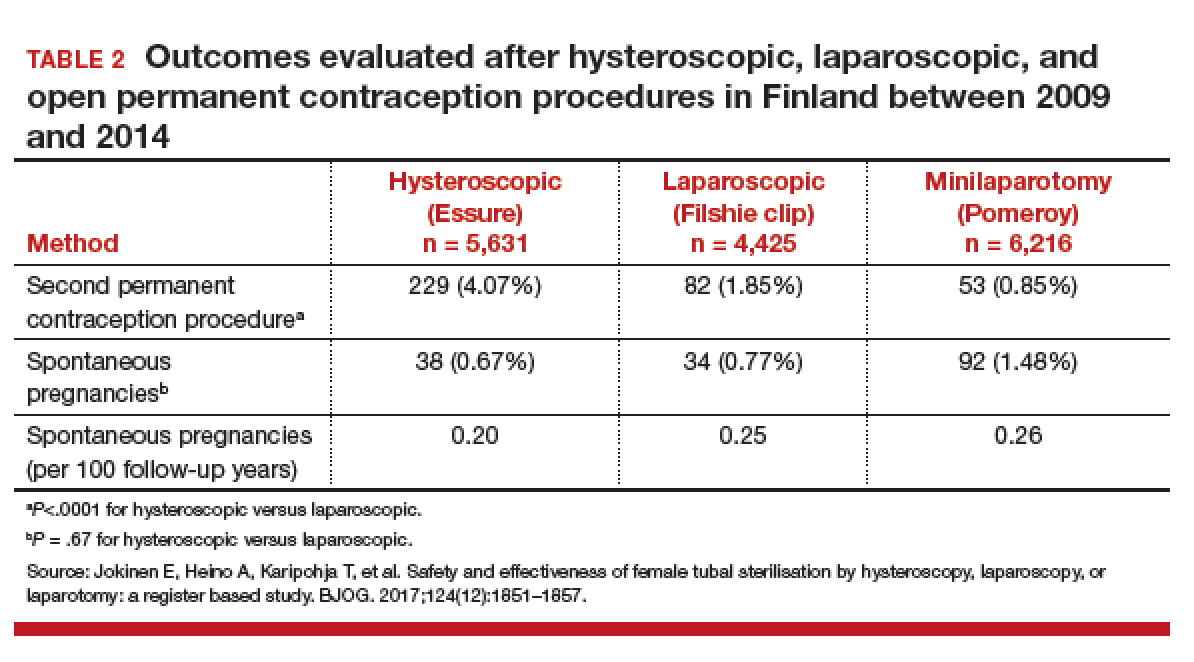
At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.
Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.

Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).

At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.

Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).

At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Importance of providing standardized management of hypertension in pregnancy
CASE Onset of nausea and headache, and elevated BP, at full term
A 24-year-old woman (G1P0) at 39 2/7 weeks of gestation without significant medical history and with uncomplicated prenatal care presents to labor and delivery reporting uterine contractions. She reports nausea and vomiting, and reports having a severe headache this morning. Blood pressure (BP) is 154/98 mm Hg. Urine dipstick analysis demonstrates absence of protein.
How should this patient be managed?
Although we have gained a greater understanding of hypertensive disorders in pregnancy—most notably, preeclampsia—during the past 15 years, management of these patients can, as evidenced in the case above, be complicated. Providers must respect this disease and be cognizant of the significant maternal, fetal, and neonatal complications that can be associated with hypertension during pregnancy—a leading cause of preterm birth and maternal mortality in the United States.1-3 Initiation of early and aggressive antihypertensive medical therapy, when indicated, plays a key role in preventing catastrophic complications of this disease.
Terminology and classification
Hypertension of pregnancy is classified as:
- chronic hypertension: BP≥140/90 mm Hg prior to pregnancy or prior to 20 weeks of gestation. Patients who have persistently elevated BP 12 weeks after delivery are also in this category.
- preeclampsia–eclampsia: hypertension along with multisystem involvement that occurs after 20 weeks of gestation.
- gestational hypertension: hypertension alone after 20 weeks of gestation; in approximately 15% to 25% of these patients, a diagnosis of preeclampsia will be made as pregnancy progresses.
- chronic hypertension with superimposed preeclampsia: hypertension complicated by development of multisystem involvement during the course of the pregnancy—often a challenging diagnosis, associated with greater perinatal morbidity than either chronic hypertension or preeclampsia alone.
Evaluation of the hypertensive gravida
Although most pregnant patients (approximately 90%) who have a diagnosis of chronic hypertension have primary or essential hypertension, a secondary cause—including thyroid disease, systemic lupus erythematosus (SLE), and underlying renal disease—might be present and should be sought out. It is important, therefore, to obtain a comprehensive history along with a directed physical examination and appropriate laboratory tests.
Ideally, a patient with chronic hypertension should be evaluated prior to pregnancy, but this rarely occurs. At the initial encounter, the patient should be informed of risks associated with chronic hypertension, as well as receive education on the signs and symptoms of preeclampsia. Obtain a thorough history—not only to evaluate for secondary causes of hypertension or end-organ involvement (eg, kidney disease), but to identify comorbidities (such as pregestational diabetes mellitus). The patient should be instructed to immediately discontinue any teratogenic medication (such as an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker).
Routine laboratory evaluation
Testing should comprise a chemistry panel to evaluate serum creatinine, electrolytes, and liver enzymes. A 24-hour urine collection for protein excretion and creatinine clearance or a urine protein–creatinine ratio should be obtained to record baseline kidney function.4 (Such testing is important, given that new-onset or worsening proteinuria is a manifestation of superimposed preeclampsia.) All pregnant patients with chronic hypertension also should have a complete blood count, including a platelet count, and an early screen for gestational diabetes.
Depending on what information is obtained from the history and physical examination, renal ultrasonography and any of several laboratory tests can be ordered, including thyroid function, an SLE panel, and vanillylmandelic acid/metanephrines. If the patient has a history of severe hypertension for greater than 5 years, is older than 40 years, or has cardiac symptoms, baseline electrocardio-graphy or echocardiography, or both, are recommended.
Clinical manifestations of chronic hypertension during pregnancy include5:
- in the mother: accelerated hypertension, with resulting target-organ damage involving heart, brain, and kidneys
- in the fetus: placental abruption, preterm birth, fetal growth restriction, and fetal death.
What should treatment seek to accomplish?
The goal of antihypertensive medication during pregnancy is to reduce maternal risk of stroke, congestive heart failure, renal failure, and severe hypertension. No convincing evidence exists that antihypertensive medications decrease the incidence of superimposed preeclampsia, preterm birth, placental abruption, or perinatal death.
According to the American College of Obstetricians and Gynecologists (ACOG), antihypertensive medication is not indicated in patients with uncomplicated chronic hypertension unless systolic BP is ≥ 160 mm Hg or diastolic BP is ≥ 105 mm Hg.3 The goal is to maintain systolic BP at 120–160 mm Hg and diastolic BP at 80–105 mm Hg. The National Institute for Health and Care Excellence recommends treatment of hypertension when systolic BP is ≥ 150 mm Hg or diastolic BP is ≥ 100 mm Hg.6 In patients with end-organ disease (chronic renal or cardiac disease) ACOG recommends treatment with an antihypertensive when systolic BP is >140 mm Hg or diastolic BP is >90 mm Hg.
First-line antihypertensives consideredsafe during pregnancy are methyldopa, labetalol, and nifedipine. Thiazide diuretics, although considered second-line agents, may be used during pregnancy—especially if BP is adequately controlled prior to pregnancy. Again, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are contraindicated during pregnancy (TABLE 1).3
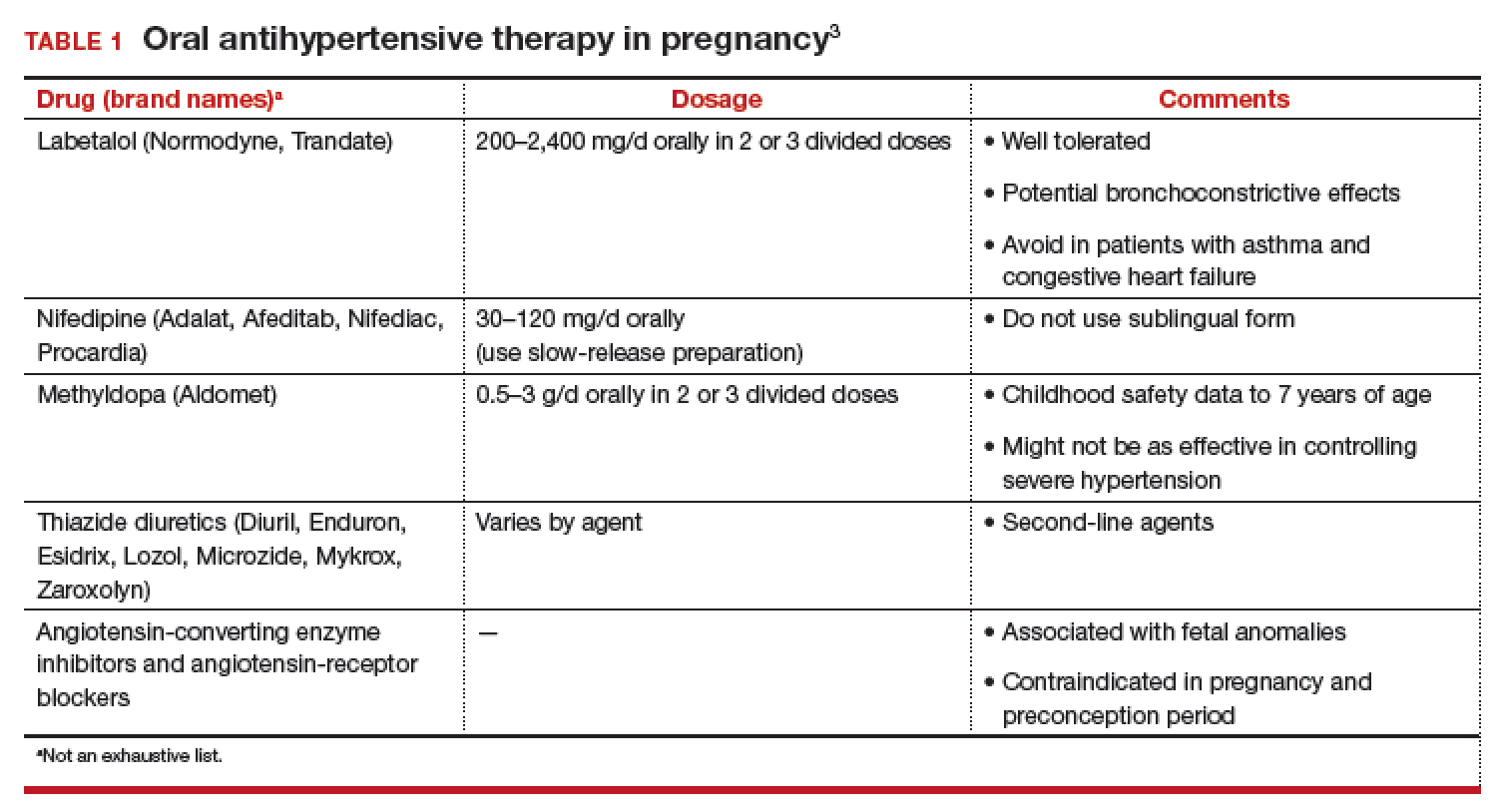
Continuing care in chronic hypertension
Given the maternal and fetal consequences of chronic hypertension, it is recommended that a hypertensive patient be followed closely as an outpatient; in fact, it is advisablethat she check her BP at least twice daily. Beginning at 24 weeks of gestation, serial ultrasonography should be performed every 4 to 6 weeks to evaluate interval fetal growth. Twice-weekly antepartum testing should begin at 32 to 34 weeks of gestation.
During the course of the pregnancy, the chronically hypertensive patient should be observed closely for development of superimposed preeclampsia. If she does not develop preeclampsia or fetal growth restriction, and has no other pregnancy complications that necessitate early delivery, 3 recommendations regarding timing of delivery apply7:
- If the patient is not taking antihypertensive medication, delivery should occur at 38 to 39 6/7 weeks of gestation
- If hypertension is controlled with medication, delivery is recommended at 37 to 39 6/7 weeks of gestation.
- If the patient has severe hypertension that is difficult to control, delivery might be advisable as early as 36 weeks of gestation.
Be vigilant for maternal complications (including cardiac compromise, congestive heart failure, cerebrovascular accident, hypertensive encephalopathy, and worsening renal disease) and fetal complications (such as placental abruption, fetal growth restriction, and fetal death). If any of these occur, management must be tailored and individualized accordingly. Study results have demonstrated that superimposed preeclampsia occurs in 20% to 30% of patients who have underlying mild chronic hypertension. This increases to 50% in women with underlying severe hypertension.8
Antihypertensive medication is the mainstay of treatment for severely elevated blood pressure (BP). To avoid fetal heart rate decelerations and possible emergent cesarean delivery, however, do not decrease BP too quickly or lower to values that might compromise perfusion to the fetus. The BP goal should be 140-155 mm Hg (systolic) and 90-105 mm Hg (diastolic). A
Be prepared for eclampsia, which is unpredictable and can occur in patients without symptoms or severely elevated BP and even postpartum in patients in whom the diagnosis of preeclampsia was never made prior to delivery. The response to eclamptic seizure includes administering magnesium sulfate, which is the approved initial therapy for an eclamptic seizure. A
Make algorithms for acute treatment of severe hypertension and eclampsia readily available or posted in labor and delivery units and in the emergency department. C
Counsel high-risk patients about the potential benefit of low-dosage aspirin to prevent preeclampsia. A
Strength of recommendation:
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The complex challenge of managing preeclampsia
Chronic hypertension is not the only risk factor for preeclampsia; others include nulliparity, history of preeclampsia, multifetal gestation, underlying renal disease, SLE, antiphospolipid syndrome, thyroid disease, and pregestational diabetes. Furthermore, preeclampsia has a bimodal age distribution, occurring more often in adolescent pregnancies and women of advanced maternal age. Risk is also increased in the presence of abnormal levels of various serum analytes or biochemical markers, such as a low level of pregnancy-associated plasma protein A or estriol or an elevated level of maternal serum α-fetoprotein, human chorionic gonadotropin, or inhibin—findings that might reflect abnormal placentation.9
In fact, the findings of most studies that have looked at the pathophysiology of preeclampsia appear to show that several noteworthy pathophysiologic changes are evident in early pregnancy10,11:
- incomplete trophoblastic invasion of spiral arteries
- retention of thick-walled, muscular arteries
- decreased placental perfusion
- early placental hypoxia
- placental release of factors that lead to endothelial dysfunction and endothelial damage.
Ultimately, vasoconstriction becomes evident, which leads to clinical manifestations of the disorder. In addition, there is an increase in the level of thromboxane (a vasoconstrictor and platelet aggregator), compared to the level of prostacyclin (a vasodilator).
ACOG revises nomenclature, provides recommendations
The considerable expansion of knowledge about preeclampsia over the past 10 to 15 years has not translated to better outcomes. In 2012, ACOG, in response to troubling observations about the condition (see “ACOG finds compelling motivation to boost understanding, management of preeclampsia,”), created a Task Force to investigate hypertension in pregnancy.
Findings and recommendations of the Task Force were published in November 2013,3 and have been endorsed and supported by professional organizations, including the American Academy of Neurology, American Society of Hypertension, Preeclampsia Foundation, and the Society for Maternal-Fetal Medicine. A major premise of the Task Force that has had a direct impact on recommendations for management of preeclampsia is that the condition is a progressive and dynamic process that involves multiple organ systems and is not specifically confined to the antepartum period.
The nomenclature of mild preeclampsia and severe preeclampsia was changed in the Task Force report to preeclampsia without severe features and preeclampsia with severe features. Preeclampsia without severe features is diagnosed when a patient has:
- systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg (measured twice at least 4 hours apart)
- proteinuria, defined as a 24-hour urine collection of ≥ 300 mg of protein or a urine protein–creatinine ratio of 0.3.
If a patient has elevated BP by those criteria, plus any of several laboratory indicators of multisystem involvement (platelet count, <100 × 103/μL; serum creatinine level, >1.1 mg/dL; doubling in the serum creatinine concentration; liver transaminase concentrations twice normal) or other findings (pulmonary edema, visual disturbance, headaches), she has preeclampsia with severe features. A diagnosis of preeclampsia without severe features is upgraded to preeclampsia with severe features if systolic BP increases to >160 mm Hgor diastolic BP increases to >110 mm Hg (determined by 2 measurements 4 hours apart) or if “severe”-range BP occurs with such rapidity that acute antihypertensive medication is required.
- Incidence of preeclampsia in the United States has increased by 25% over the past 2 decades
- Etiology remains unclear
- Leading cause of maternal and perinatal morbidity and mortality
- Risk factor for future cardiovascular disease and metabolic disease in women
- Hypertensive disorders of pregnancy are major contributors to prematurity
- New best-practice recommendations are urgently needed to guide clinicians in the care of women with all forms of preeclampsia and hypertension during pregnancy
- Improved patient education and counseling strategies are needed to convey, more effectively, the dangers of preeclampsia and hypertension during pregnancy
Reference
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
Pharmacotherapy for hypertensive emergency
Acute BP control with intravenous (IV) labetalol or hydralazine or oral nifedipine is recommended when a patient has a hypertensive emergency, defined as acute-onset severe hypertension that persists for ≥ 15 minutes (TABLE 2).12 The goal of management is not to completely normalize BP but to lower BP to the range of 140 to 155 mm Hg (systolic) and 90 to 105 mm Hg (diastolic). Of all proposed interventions, these agents are likely the most effective in preventing a maternal cerebrovascular or cardiovascular event. (Note: Labetalol is contraindicated in patients with severe asthma and in the setting of acute cocaine or methamphetamine intoxication. Hydralazine can cause tachycardia.)13,14
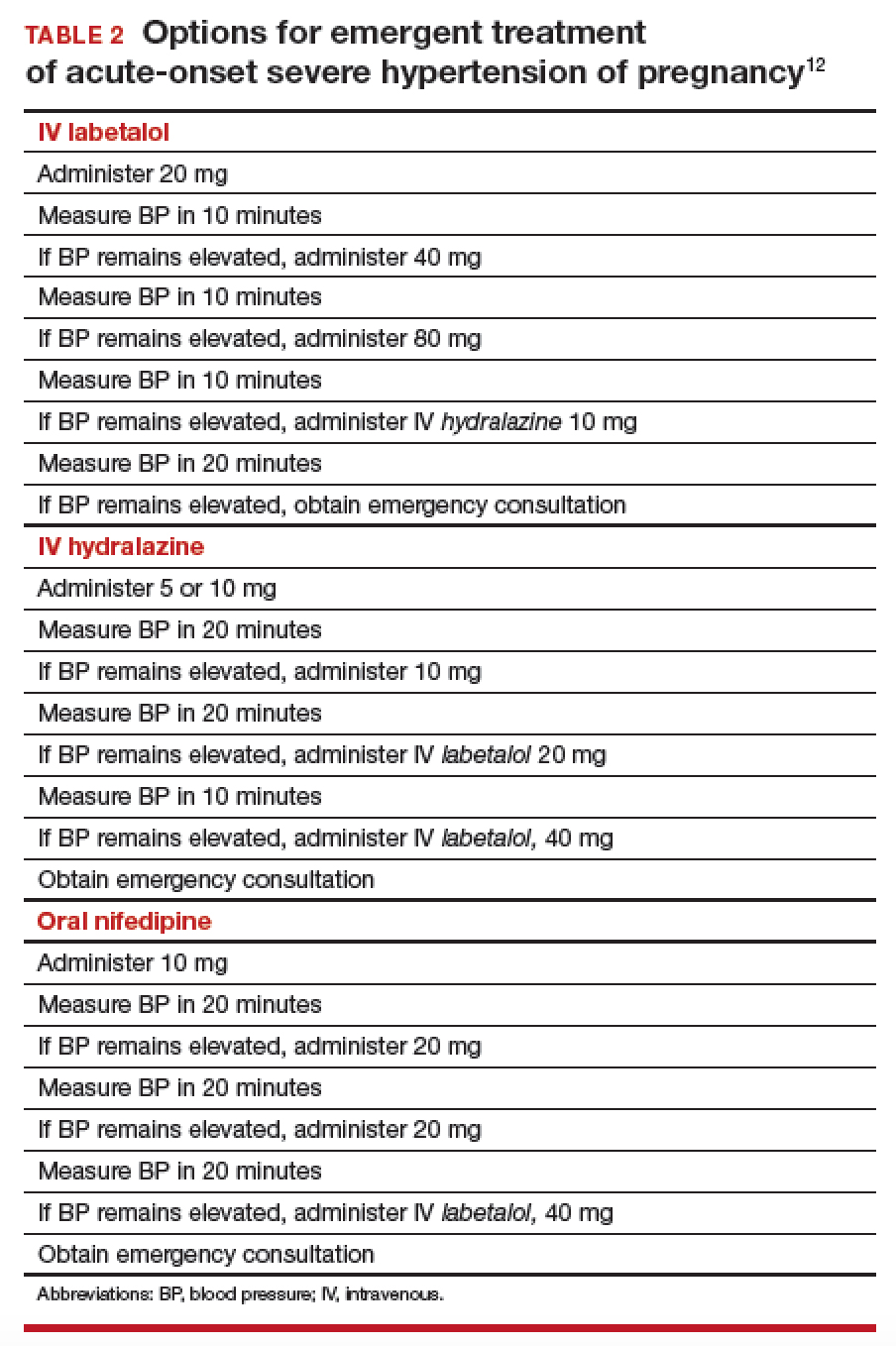
Once a diagnosis of preeclampsia with severe features or superimposed preeclampsia with severe features is made, the patient should remain hospitalized until delivery. If either of these diagnoses is made at ≥ 34 weeks of gestation, there is no reason to prolong pregnancy. Rather, the patient should be given prophylactic magnesium sulfate to prevent seizures and delivery should be accomplished.15,16 Earlier than 36 6/7 weeks of gestation, consider a late preterm course of corticosteroids; however, do not delay delivery in this situation.17
Planning for delivery
Route of delivery depends on customary obstetric indications. Before 34 weeks of gestation, corticosteroids, magnesium sulfate, and prolonging the pregnancy until 34 weeks of gestation are recommended. If, at any time, maternal or fetal condition deteriorates, delivery should be accomplished regardless of gestational age. If the patient is unwilling to accept the risks of expectant management of preeclampsia with severe features remote from term, delivery is indicated.18,19 If delivery is not likely to occur, magnesium sulfate can be discontinued after the patient has received a second dose of corticosteroids, with the plan to resume magnesium sulfate if she develops signs of worsening preeclampsia or eclampsia, or once the plan for delivery is made.
In patients who have either gestational hypertension or preeclampsia without severe features, the recommendation is to accomplish delivery no later than 37 weeks of gestation. While the patient is being expectantly managed, close maternal and fetal surveillance are necessary, comprising serial assessment of maternal symptoms and fetal movement; serial BP measurement (twice weekly); and weekly measurement of the platelet count, serum creatinine, and liver enzymes. At 34 weeks of gestation, conventional antepartum testing should begin. Again, if there is deterioration of the maternal or fetal condition, the patient should be hospitalized and delivery should be accomplished according to the recommendations above.3
Seizure management
If a patient has a tonic–clonic seizure consistent with eclampsia, management should be as follows:
- Preserve the airway and immediately tilt the head forward to prevent aspiration.
- If the patient is not receiving magnesium sulfate, immediately administer a loading dose of 4-6 g IV or 10 mg intramuscularly if IV access has not been established.20
- If the patient is already receiving magnesium sulfate, administer a loading dose of 2 g IV over 5 minutes.
- If the patient continues to have seizure activity, administer anticonvulsant medication(lorazepam, diazepam, midazolam, or phenytoin).
Eclamptic seizures are usually self-limited, lasting no longer than 1 or 2 minutes. Regrettably, these seizures are unpredictable and contribute significantly to maternal morbidity and mortality.21,22 A maternal seizure causes a significant interruption in the oxygen pathway to the fetus, with resultant late decelerations, prolonged decelerations, or bradycardia.
Resist the temptation to perform emergent cesarean delivery when eclamptic seizure occurs; rather, allow time for fetal recovery and then proceed with delivery in a controlled fashion. In many circumstances, the patient can undergo vaginal delivery after an eclamptic seizure. Keep in mind that the differential diagnosis of new-onset seizure in pregnancy includes cerebral pathology, such as a bleeding arteriovenous malformation or ruptured aneurysm. Therefore, brain-imaging studies might be indicated, especially in patients who have focal neurologic deficits, or who have seizures either while receiving magnesium sulfate or 48 to 72 hours after delivery.
Preeclampsia postpartum
More recent studies have demonstrated that preeclampsia can be exacerbated after delivery or might even present initially postpartum.23,24 In all women in whom gestational hypertension, preeclampsia, or superimposed preeclampsia is diagnosed, therefore, recommendations are that BP be monitored in the hospital or on an outpatient basis for at least 72 hours postpartum and again 7 to 10 days after delivery. For all women postpartum, the recommendation is that discharge instructions 1) include information about signs and symptoms of preeclampsia and 2) emphasize the importance of promptly reporting such developments to providers.25 Remember: Sequelae of preeclampsia have been reported as late as 4 to 6 weeks postpartum.
Magnesium sulfate is recommended when a patient presents postpartum with new-onset hypertension associated with headache or blurred vision, or with preeclampsia with severe hypertension. Because nonsteroidal anti-inflammatory drugs can be associated with elevated BP, these medications should be replaced by other analgesics in women with hypertension that persists for more than 1 day postpartum.
Prevention of preeclampsia
Given the significant maternal, fetal, and neonatal complications associated with preeclampsia, a number of studies have sought to determine ways in which this condition can be prevented. Currently, although no interventions appear to prevent preeclampsia in all patients, significant strides have been made in prevention for high-risk patients. Specifically, beginning low-dosage aspirin (most commonly, 81 mg/d, beginning at less than 16 weeks of gestation) has been shown to mitigate—although not eliminate—risk in patients with a history of preeclampsia and those who have chronic hypertension, multifetal gestation, pregestational diabetes, renal disease, SLE, or antiphospholipid syndrome.26,27Aspirin appears to act by preferentially blocking production of thromboxane, thus reducing the vasoconstrictive properties of this hormone.
Summing up
Hypertensive disorders during pregnancy are associated with significant morbidity and mortality for mother, fetus, and newborn. Preeclampsia, specifically, is recognized as a dynamic and progressive disease that has the potential to involve multiple organ systems, might present for the first time after delivery, and might be associated with long-term risk of hypertension, heart disease, stroke, and venous thromboembolism.28,29
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008; 199:133.e1-e8.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-1306.
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465.e1-e4.
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LL. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. CG107, August 2010. https://www.nice.org.uk/guidance/cg107. Accessed August 27, 2018. Last updated January 2011.
- Spong CY, Mercer BM, D'Alton M, et al. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers or aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052-1061.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193-201.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970-975.
- The American College of Obstetricians and Gynecologists Committee on Obstetric Practice; El-Sayed YY, Borders AE. Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period; April 2017. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co692.pdf?dmc=1. Accessed August 8, 2018.
- Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
- Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
- Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191-198.
- Norwitz E, Funai E. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008;199:209-212.
- Gordon R, Magee LA, Payne B, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J Obstet Gynaecol Can. 2014;36(2):154-163.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402-410.
- Liu S, Joseph KS, Liston, RM, et al; Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987-994.
- Yancey LM, Withers E, Bakes K, Abbot J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470-475.
- You WB, Wolf MS, Bailey SC, Grobman WA. Improving patient understanding of preeclampsia: a randomized controlled trial. Am J Obstet Gynecol. 2012;206:431.e1-e5.
- Henderson JT, Whitlock EP, O'Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-986.
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918-930.
CASE Onset of nausea and headache, and elevated BP, at full term
A 24-year-old woman (G1P0) at 39 2/7 weeks of gestation without significant medical history and with uncomplicated prenatal care presents to labor and delivery reporting uterine contractions. She reports nausea and vomiting, and reports having a severe headache this morning. Blood pressure (BP) is 154/98 mm Hg. Urine dipstick analysis demonstrates absence of protein.
How should this patient be managed?
Although we have gained a greater understanding of hypertensive disorders in pregnancy—most notably, preeclampsia—during the past 15 years, management of these patients can, as evidenced in the case above, be complicated. Providers must respect this disease and be cognizant of the significant maternal, fetal, and neonatal complications that can be associated with hypertension during pregnancy—a leading cause of preterm birth and maternal mortality in the United States.1-3 Initiation of early and aggressive antihypertensive medical therapy, when indicated, plays a key role in preventing catastrophic complications of this disease.
Terminology and classification
Hypertension of pregnancy is classified as:
- chronic hypertension: BP≥140/90 mm Hg prior to pregnancy or prior to 20 weeks of gestation. Patients who have persistently elevated BP 12 weeks after delivery are also in this category.
- preeclampsia–eclampsia: hypertension along with multisystem involvement that occurs after 20 weeks of gestation.
- gestational hypertension: hypertension alone after 20 weeks of gestation; in approximately 15% to 25% of these patients, a diagnosis of preeclampsia will be made as pregnancy progresses.
- chronic hypertension with superimposed preeclampsia: hypertension complicated by development of multisystem involvement during the course of the pregnancy—often a challenging diagnosis, associated with greater perinatal morbidity than either chronic hypertension or preeclampsia alone.
Evaluation of the hypertensive gravida
Although most pregnant patients (approximately 90%) who have a diagnosis of chronic hypertension have primary or essential hypertension, a secondary cause—including thyroid disease, systemic lupus erythematosus (SLE), and underlying renal disease—might be present and should be sought out. It is important, therefore, to obtain a comprehensive history along with a directed physical examination and appropriate laboratory tests.
Ideally, a patient with chronic hypertension should be evaluated prior to pregnancy, but this rarely occurs. At the initial encounter, the patient should be informed of risks associated with chronic hypertension, as well as receive education on the signs and symptoms of preeclampsia. Obtain a thorough history—not only to evaluate for secondary causes of hypertension or end-organ involvement (eg, kidney disease), but to identify comorbidities (such as pregestational diabetes mellitus). The patient should be instructed to immediately discontinue any teratogenic medication (such as an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker).
Routine laboratory evaluation
Testing should comprise a chemistry panel to evaluate serum creatinine, electrolytes, and liver enzymes. A 24-hour urine collection for protein excretion and creatinine clearance or a urine protein–creatinine ratio should be obtained to record baseline kidney function.4 (Such testing is important, given that new-onset or worsening proteinuria is a manifestation of superimposed preeclampsia.) All pregnant patients with chronic hypertension also should have a complete blood count, including a platelet count, and an early screen for gestational diabetes.
Depending on what information is obtained from the history and physical examination, renal ultrasonography and any of several laboratory tests can be ordered, including thyroid function, an SLE panel, and vanillylmandelic acid/metanephrines. If the patient has a history of severe hypertension for greater than 5 years, is older than 40 years, or has cardiac symptoms, baseline electrocardio-graphy or echocardiography, or both, are recommended.
Clinical manifestations of chronic hypertension during pregnancy include5:
- in the mother: accelerated hypertension, with resulting target-organ damage involving heart, brain, and kidneys
- in the fetus: placental abruption, preterm birth, fetal growth restriction, and fetal death.
What should treatment seek to accomplish?
The goal of antihypertensive medication during pregnancy is to reduce maternal risk of stroke, congestive heart failure, renal failure, and severe hypertension. No convincing evidence exists that antihypertensive medications decrease the incidence of superimposed preeclampsia, preterm birth, placental abruption, or perinatal death.
According to the American College of Obstetricians and Gynecologists (ACOG), antihypertensive medication is not indicated in patients with uncomplicated chronic hypertension unless systolic BP is ≥ 160 mm Hg or diastolic BP is ≥ 105 mm Hg.3 The goal is to maintain systolic BP at 120–160 mm Hg and diastolic BP at 80–105 mm Hg. The National Institute for Health and Care Excellence recommends treatment of hypertension when systolic BP is ≥ 150 mm Hg or diastolic BP is ≥ 100 mm Hg.6 In patients with end-organ disease (chronic renal or cardiac disease) ACOG recommends treatment with an antihypertensive when systolic BP is >140 mm Hg or diastolic BP is >90 mm Hg.
First-line antihypertensives consideredsafe during pregnancy are methyldopa, labetalol, and nifedipine. Thiazide diuretics, although considered second-line agents, may be used during pregnancy—especially if BP is adequately controlled prior to pregnancy. Again, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are contraindicated during pregnancy (TABLE 1).3

Continuing care in chronic hypertension
Given the maternal and fetal consequences of chronic hypertension, it is recommended that a hypertensive patient be followed closely as an outpatient; in fact, it is advisablethat she check her BP at least twice daily. Beginning at 24 weeks of gestation, serial ultrasonography should be performed every 4 to 6 weeks to evaluate interval fetal growth. Twice-weekly antepartum testing should begin at 32 to 34 weeks of gestation.
During the course of the pregnancy, the chronically hypertensive patient should be observed closely for development of superimposed preeclampsia. If she does not develop preeclampsia or fetal growth restriction, and has no other pregnancy complications that necessitate early delivery, 3 recommendations regarding timing of delivery apply7:
- If the patient is not taking antihypertensive medication, delivery should occur at 38 to 39 6/7 weeks of gestation
- If hypertension is controlled with medication, delivery is recommended at 37 to 39 6/7 weeks of gestation.
- If the patient has severe hypertension that is difficult to control, delivery might be advisable as early as 36 weeks of gestation.
Be vigilant for maternal complications (including cardiac compromise, congestive heart failure, cerebrovascular accident, hypertensive encephalopathy, and worsening renal disease) and fetal complications (such as placental abruption, fetal growth restriction, and fetal death). If any of these occur, management must be tailored and individualized accordingly. Study results have demonstrated that superimposed preeclampsia occurs in 20% to 30% of patients who have underlying mild chronic hypertension. This increases to 50% in women with underlying severe hypertension.8
Antihypertensive medication is the mainstay of treatment for severely elevated blood pressure (BP). To avoid fetal heart rate decelerations and possible emergent cesarean delivery, however, do not decrease BP too quickly or lower to values that might compromise perfusion to the fetus. The BP goal should be 140-155 mm Hg (systolic) and 90-105 mm Hg (diastolic). A
Be prepared for eclampsia, which is unpredictable and can occur in patients without symptoms or severely elevated BP and even postpartum in patients in whom the diagnosis of preeclampsia was never made prior to delivery. The response to eclamptic seizure includes administering magnesium sulfate, which is the approved initial therapy for an eclamptic seizure. A
Make algorithms for acute treatment of severe hypertension and eclampsia readily available or posted in labor and delivery units and in the emergency department. C
Counsel high-risk patients about the potential benefit of low-dosage aspirin to prevent preeclampsia. A
Strength of recommendation:
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The complex challenge of managing preeclampsia
Chronic hypertension is not the only risk factor for preeclampsia; others include nulliparity, history of preeclampsia, multifetal gestation, underlying renal disease, SLE, antiphospolipid syndrome, thyroid disease, and pregestational diabetes. Furthermore, preeclampsia has a bimodal age distribution, occurring more often in adolescent pregnancies and women of advanced maternal age. Risk is also increased in the presence of abnormal levels of various serum analytes or biochemical markers, such as a low level of pregnancy-associated plasma protein A or estriol or an elevated level of maternal serum α-fetoprotein, human chorionic gonadotropin, or inhibin—findings that might reflect abnormal placentation.9
In fact, the findings of most studies that have looked at the pathophysiology of preeclampsia appear to show that several noteworthy pathophysiologic changes are evident in early pregnancy10,11:
- incomplete trophoblastic invasion of spiral arteries
- retention of thick-walled, muscular arteries
- decreased placental perfusion
- early placental hypoxia
- placental release of factors that lead to endothelial dysfunction and endothelial damage.
Ultimately, vasoconstriction becomes evident, which leads to clinical manifestations of the disorder. In addition, there is an increase in the level of thromboxane (a vasoconstrictor and platelet aggregator), compared to the level of prostacyclin (a vasodilator).
ACOG revises nomenclature, provides recommendations
The considerable expansion of knowledge about preeclampsia over the past 10 to 15 years has not translated to better outcomes. In 2012, ACOG, in response to troubling observations about the condition (see “ACOG finds compelling motivation to boost understanding, management of preeclampsia,”), created a Task Force to investigate hypertension in pregnancy.
Findings and recommendations of the Task Force were published in November 2013,3 and have been endorsed and supported by professional organizations, including the American Academy of Neurology, American Society of Hypertension, Preeclampsia Foundation, and the Society for Maternal-Fetal Medicine. A major premise of the Task Force that has had a direct impact on recommendations for management of preeclampsia is that the condition is a progressive and dynamic process that involves multiple organ systems and is not specifically confined to the antepartum period.
The nomenclature of mild preeclampsia and severe preeclampsia was changed in the Task Force report to preeclampsia without severe features and preeclampsia with severe features. Preeclampsia without severe features is diagnosed when a patient has:
- systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg (measured twice at least 4 hours apart)
- proteinuria, defined as a 24-hour urine collection of ≥ 300 mg of protein or a urine protein–creatinine ratio of 0.3.
If a patient has elevated BP by those criteria, plus any of several laboratory indicators of multisystem involvement (platelet count, <100 × 103/μL; serum creatinine level, >1.1 mg/dL; doubling in the serum creatinine concentration; liver transaminase concentrations twice normal) or other findings (pulmonary edema, visual disturbance, headaches), she has preeclampsia with severe features. A diagnosis of preeclampsia without severe features is upgraded to preeclampsia with severe features if systolic BP increases to >160 mm Hgor diastolic BP increases to >110 mm Hg (determined by 2 measurements 4 hours apart) or if “severe”-range BP occurs with such rapidity that acute antihypertensive medication is required.
- Incidence of preeclampsia in the United States has increased by 25% over the past 2 decades
- Etiology remains unclear
- Leading cause of maternal and perinatal morbidity and mortality
- Risk factor for future cardiovascular disease and metabolic disease in women
- Hypertensive disorders of pregnancy are major contributors to prematurity
- New best-practice recommendations are urgently needed to guide clinicians in the care of women with all forms of preeclampsia and hypertension during pregnancy
- Improved patient education and counseling strategies are needed to convey, more effectively, the dangers of preeclampsia and hypertension during pregnancy
Reference
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
Pharmacotherapy for hypertensive emergency
Acute BP control with intravenous (IV) labetalol or hydralazine or oral nifedipine is recommended when a patient has a hypertensive emergency, defined as acute-onset severe hypertension that persists for ≥ 15 minutes (TABLE 2).12 The goal of management is not to completely normalize BP but to lower BP to the range of 140 to 155 mm Hg (systolic) and 90 to 105 mm Hg (diastolic). Of all proposed interventions, these agents are likely the most effective in preventing a maternal cerebrovascular or cardiovascular event. (Note: Labetalol is contraindicated in patients with severe asthma and in the setting of acute cocaine or methamphetamine intoxication. Hydralazine can cause tachycardia.)13,14

Once a diagnosis of preeclampsia with severe features or superimposed preeclampsia with severe features is made, the patient should remain hospitalized until delivery. If either of these diagnoses is made at ≥ 34 weeks of gestation, there is no reason to prolong pregnancy. Rather, the patient should be given prophylactic magnesium sulfate to prevent seizures and delivery should be accomplished.15,16 Earlier than 36 6/7 weeks of gestation, consider a late preterm course of corticosteroids; however, do not delay delivery in this situation.17
Planning for delivery
Route of delivery depends on customary obstetric indications. Before 34 weeks of gestation, corticosteroids, magnesium sulfate, and prolonging the pregnancy until 34 weeks of gestation are recommended. If, at any time, maternal or fetal condition deteriorates, delivery should be accomplished regardless of gestational age. If the patient is unwilling to accept the risks of expectant management of preeclampsia with severe features remote from term, delivery is indicated.18,19 If delivery is not likely to occur, magnesium sulfate can be discontinued after the patient has received a second dose of corticosteroids, with the plan to resume magnesium sulfate if she develops signs of worsening preeclampsia or eclampsia, or once the plan for delivery is made.
In patients who have either gestational hypertension or preeclampsia without severe features, the recommendation is to accomplish delivery no later than 37 weeks of gestation. While the patient is being expectantly managed, close maternal and fetal surveillance are necessary, comprising serial assessment of maternal symptoms and fetal movement; serial BP measurement (twice weekly); and weekly measurement of the platelet count, serum creatinine, and liver enzymes. At 34 weeks of gestation, conventional antepartum testing should begin. Again, if there is deterioration of the maternal or fetal condition, the patient should be hospitalized and delivery should be accomplished according to the recommendations above.3
Seizure management
If a patient has a tonic–clonic seizure consistent with eclampsia, management should be as follows:
- Preserve the airway and immediately tilt the head forward to prevent aspiration.
- If the patient is not receiving magnesium sulfate, immediately administer a loading dose of 4-6 g IV or 10 mg intramuscularly if IV access has not been established.20
- If the patient is already receiving magnesium sulfate, administer a loading dose of 2 g IV over 5 minutes.
- If the patient continues to have seizure activity, administer anticonvulsant medication(lorazepam, diazepam, midazolam, or phenytoin).
Eclamptic seizures are usually self-limited, lasting no longer than 1 or 2 minutes. Regrettably, these seizures are unpredictable and contribute significantly to maternal morbidity and mortality.21,22 A maternal seizure causes a significant interruption in the oxygen pathway to the fetus, with resultant late decelerations, prolonged decelerations, or bradycardia.
Resist the temptation to perform emergent cesarean delivery when eclamptic seizure occurs; rather, allow time for fetal recovery and then proceed with delivery in a controlled fashion. In many circumstances, the patient can undergo vaginal delivery after an eclamptic seizure. Keep in mind that the differential diagnosis of new-onset seizure in pregnancy includes cerebral pathology, such as a bleeding arteriovenous malformation or ruptured aneurysm. Therefore, brain-imaging studies might be indicated, especially in patients who have focal neurologic deficits, or who have seizures either while receiving magnesium sulfate or 48 to 72 hours after delivery.
Preeclampsia postpartum
More recent studies have demonstrated that preeclampsia can be exacerbated after delivery or might even present initially postpartum.23,24 In all women in whom gestational hypertension, preeclampsia, or superimposed preeclampsia is diagnosed, therefore, recommendations are that BP be monitored in the hospital or on an outpatient basis for at least 72 hours postpartum and again 7 to 10 days after delivery. For all women postpartum, the recommendation is that discharge instructions 1) include information about signs and symptoms of preeclampsia and 2) emphasize the importance of promptly reporting such developments to providers.25 Remember: Sequelae of preeclampsia have been reported as late as 4 to 6 weeks postpartum.
Magnesium sulfate is recommended when a patient presents postpartum with new-onset hypertension associated with headache or blurred vision, or with preeclampsia with severe hypertension. Because nonsteroidal anti-inflammatory drugs can be associated with elevated BP, these medications should be replaced by other analgesics in women with hypertension that persists for more than 1 day postpartum.
Prevention of preeclampsia
Given the significant maternal, fetal, and neonatal complications associated with preeclampsia, a number of studies have sought to determine ways in which this condition can be prevented. Currently, although no interventions appear to prevent preeclampsia in all patients, significant strides have been made in prevention for high-risk patients. Specifically, beginning low-dosage aspirin (most commonly, 81 mg/d, beginning at less than 16 weeks of gestation) has been shown to mitigate—although not eliminate—risk in patients with a history of preeclampsia and those who have chronic hypertension, multifetal gestation, pregestational diabetes, renal disease, SLE, or antiphospholipid syndrome.26,27Aspirin appears to act by preferentially blocking production of thromboxane, thus reducing the vasoconstrictive properties of this hormone.
Summing up
Hypertensive disorders during pregnancy are associated with significant morbidity and mortality for mother, fetus, and newborn. Preeclampsia, specifically, is recognized as a dynamic and progressive disease that has the potential to involve multiple organ systems, might present for the first time after delivery, and might be associated with long-term risk of hypertension, heart disease, stroke, and venous thromboembolism.28,29
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Onset of nausea and headache, and elevated BP, at full term
A 24-year-old woman (G1P0) at 39 2/7 weeks of gestation without significant medical history and with uncomplicated prenatal care presents to labor and delivery reporting uterine contractions. She reports nausea and vomiting, and reports having a severe headache this morning. Blood pressure (BP) is 154/98 mm Hg. Urine dipstick analysis demonstrates absence of protein.
How should this patient be managed?
Although we have gained a greater understanding of hypertensive disorders in pregnancy—most notably, preeclampsia—during the past 15 years, management of these patients can, as evidenced in the case above, be complicated. Providers must respect this disease and be cognizant of the significant maternal, fetal, and neonatal complications that can be associated with hypertension during pregnancy—a leading cause of preterm birth and maternal mortality in the United States.1-3 Initiation of early and aggressive antihypertensive medical therapy, when indicated, plays a key role in preventing catastrophic complications of this disease.
Terminology and classification
Hypertension of pregnancy is classified as:
- chronic hypertension: BP≥140/90 mm Hg prior to pregnancy or prior to 20 weeks of gestation. Patients who have persistently elevated BP 12 weeks after delivery are also in this category.
- preeclampsia–eclampsia: hypertension along with multisystem involvement that occurs after 20 weeks of gestation.
- gestational hypertension: hypertension alone after 20 weeks of gestation; in approximately 15% to 25% of these patients, a diagnosis of preeclampsia will be made as pregnancy progresses.
- chronic hypertension with superimposed preeclampsia: hypertension complicated by development of multisystem involvement during the course of the pregnancy—often a challenging diagnosis, associated with greater perinatal morbidity than either chronic hypertension or preeclampsia alone.
Evaluation of the hypertensive gravida
Although most pregnant patients (approximately 90%) who have a diagnosis of chronic hypertension have primary or essential hypertension, a secondary cause—including thyroid disease, systemic lupus erythematosus (SLE), and underlying renal disease—might be present and should be sought out. It is important, therefore, to obtain a comprehensive history along with a directed physical examination and appropriate laboratory tests.
Ideally, a patient with chronic hypertension should be evaluated prior to pregnancy, but this rarely occurs. At the initial encounter, the patient should be informed of risks associated with chronic hypertension, as well as receive education on the signs and symptoms of preeclampsia. Obtain a thorough history—not only to evaluate for secondary causes of hypertension or end-organ involvement (eg, kidney disease), but to identify comorbidities (such as pregestational diabetes mellitus). The patient should be instructed to immediately discontinue any teratogenic medication (such as an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker).
Routine laboratory evaluation
Testing should comprise a chemistry panel to evaluate serum creatinine, electrolytes, and liver enzymes. A 24-hour urine collection for protein excretion and creatinine clearance or a urine protein–creatinine ratio should be obtained to record baseline kidney function.4 (Such testing is important, given that new-onset or worsening proteinuria is a manifestation of superimposed preeclampsia.) All pregnant patients with chronic hypertension also should have a complete blood count, including a platelet count, and an early screen for gestational diabetes.
Depending on what information is obtained from the history and physical examination, renal ultrasonography and any of several laboratory tests can be ordered, including thyroid function, an SLE panel, and vanillylmandelic acid/metanephrines. If the patient has a history of severe hypertension for greater than 5 years, is older than 40 years, or has cardiac symptoms, baseline electrocardio-graphy or echocardiography, or both, are recommended.
Clinical manifestations of chronic hypertension during pregnancy include5:
- in the mother: accelerated hypertension, with resulting target-organ damage involving heart, brain, and kidneys
- in the fetus: placental abruption, preterm birth, fetal growth restriction, and fetal death.
What should treatment seek to accomplish?
The goal of antihypertensive medication during pregnancy is to reduce maternal risk of stroke, congestive heart failure, renal failure, and severe hypertension. No convincing evidence exists that antihypertensive medications decrease the incidence of superimposed preeclampsia, preterm birth, placental abruption, or perinatal death.
According to the American College of Obstetricians and Gynecologists (ACOG), antihypertensive medication is not indicated in patients with uncomplicated chronic hypertension unless systolic BP is ≥ 160 mm Hg or diastolic BP is ≥ 105 mm Hg.3 The goal is to maintain systolic BP at 120–160 mm Hg and diastolic BP at 80–105 mm Hg. The National Institute for Health and Care Excellence recommends treatment of hypertension when systolic BP is ≥ 150 mm Hg or diastolic BP is ≥ 100 mm Hg.6 In patients with end-organ disease (chronic renal or cardiac disease) ACOG recommends treatment with an antihypertensive when systolic BP is >140 mm Hg or diastolic BP is >90 mm Hg.
First-line antihypertensives consideredsafe during pregnancy are methyldopa, labetalol, and nifedipine. Thiazide diuretics, although considered second-line agents, may be used during pregnancy—especially if BP is adequately controlled prior to pregnancy. Again, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are contraindicated during pregnancy (TABLE 1).3

Continuing care in chronic hypertension
Given the maternal and fetal consequences of chronic hypertension, it is recommended that a hypertensive patient be followed closely as an outpatient; in fact, it is advisablethat she check her BP at least twice daily. Beginning at 24 weeks of gestation, serial ultrasonography should be performed every 4 to 6 weeks to evaluate interval fetal growth. Twice-weekly antepartum testing should begin at 32 to 34 weeks of gestation.
During the course of the pregnancy, the chronically hypertensive patient should be observed closely for development of superimposed preeclampsia. If she does not develop preeclampsia or fetal growth restriction, and has no other pregnancy complications that necessitate early delivery, 3 recommendations regarding timing of delivery apply7:
- If the patient is not taking antihypertensive medication, delivery should occur at 38 to 39 6/7 weeks of gestation
- If hypertension is controlled with medication, delivery is recommended at 37 to 39 6/7 weeks of gestation.
- If the patient has severe hypertension that is difficult to control, delivery might be advisable as early as 36 weeks of gestation.
Be vigilant for maternal complications (including cardiac compromise, congestive heart failure, cerebrovascular accident, hypertensive encephalopathy, and worsening renal disease) and fetal complications (such as placental abruption, fetal growth restriction, and fetal death). If any of these occur, management must be tailored and individualized accordingly. Study results have demonstrated that superimposed preeclampsia occurs in 20% to 30% of patients who have underlying mild chronic hypertension. This increases to 50% in women with underlying severe hypertension.8
Antihypertensive medication is the mainstay of treatment for severely elevated blood pressure (BP). To avoid fetal heart rate decelerations and possible emergent cesarean delivery, however, do not decrease BP too quickly or lower to values that might compromise perfusion to the fetus. The BP goal should be 140-155 mm Hg (systolic) and 90-105 mm Hg (diastolic). A
Be prepared for eclampsia, which is unpredictable and can occur in patients without symptoms or severely elevated BP and even postpartum in patients in whom the diagnosis of preeclampsia was never made prior to delivery. The response to eclamptic seizure includes administering magnesium sulfate, which is the approved initial therapy for an eclamptic seizure. A
Make algorithms for acute treatment of severe hypertension and eclampsia readily available or posted in labor and delivery units and in the emergency department. C
Counsel high-risk patients about the potential benefit of low-dosage aspirin to prevent preeclampsia. A
Strength of recommendation:
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The complex challenge of managing preeclampsia
Chronic hypertension is not the only risk factor for preeclampsia; others include nulliparity, history of preeclampsia, multifetal gestation, underlying renal disease, SLE, antiphospolipid syndrome, thyroid disease, and pregestational diabetes. Furthermore, preeclampsia has a bimodal age distribution, occurring more often in adolescent pregnancies and women of advanced maternal age. Risk is also increased in the presence of abnormal levels of various serum analytes or biochemical markers, such as a low level of pregnancy-associated plasma protein A or estriol or an elevated level of maternal serum α-fetoprotein, human chorionic gonadotropin, or inhibin—findings that might reflect abnormal placentation.9
In fact, the findings of most studies that have looked at the pathophysiology of preeclampsia appear to show that several noteworthy pathophysiologic changes are evident in early pregnancy10,11:
- incomplete trophoblastic invasion of spiral arteries
- retention of thick-walled, muscular arteries
- decreased placental perfusion
- early placental hypoxia
- placental release of factors that lead to endothelial dysfunction and endothelial damage.
Ultimately, vasoconstriction becomes evident, which leads to clinical manifestations of the disorder. In addition, there is an increase in the level of thromboxane (a vasoconstrictor and platelet aggregator), compared to the level of prostacyclin (a vasodilator).
ACOG revises nomenclature, provides recommendations
The considerable expansion of knowledge about preeclampsia over the past 10 to 15 years has not translated to better outcomes. In 2012, ACOG, in response to troubling observations about the condition (see “ACOG finds compelling motivation to boost understanding, management of preeclampsia,”), created a Task Force to investigate hypertension in pregnancy.
Findings and recommendations of the Task Force were published in November 2013,3 and have been endorsed and supported by professional organizations, including the American Academy of Neurology, American Society of Hypertension, Preeclampsia Foundation, and the Society for Maternal-Fetal Medicine. A major premise of the Task Force that has had a direct impact on recommendations for management of preeclampsia is that the condition is a progressive and dynamic process that involves multiple organ systems and is not specifically confined to the antepartum period.
The nomenclature of mild preeclampsia and severe preeclampsia was changed in the Task Force report to preeclampsia without severe features and preeclampsia with severe features. Preeclampsia without severe features is diagnosed when a patient has:
- systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg (measured twice at least 4 hours apart)
- proteinuria, defined as a 24-hour urine collection of ≥ 300 mg of protein or a urine protein–creatinine ratio of 0.3.
If a patient has elevated BP by those criteria, plus any of several laboratory indicators of multisystem involvement (platelet count, <100 × 103/μL; serum creatinine level, >1.1 mg/dL; doubling in the serum creatinine concentration; liver transaminase concentrations twice normal) or other findings (pulmonary edema, visual disturbance, headaches), she has preeclampsia with severe features. A diagnosis of preeclampsia without severe features is upgraded to preeclampsia with severe features if systolic BP increases to >160 mm Hgor diastolic BP increases to >110 mm Hg (determined by 2 measurements 4 hours apart) or if “severe”-range BP occurs with such rapidity that acute antihypertensive medication is required.
- Incidence of preeclampsia in the United States has increased by 25% over the past 2 decades
- Etiology remains unclear
- Leading cause of maternal and perinatal morbidity and mortality
- Risk factor for future cardiovascular disease and metabolic disease in women
- Hypertensive disorders of pregnancy are major contributors to prematurity
- New best-practice recommendations are urgently needed to guide clinicians in the care of women with all forms of preeclampsia and hypertension during pregnancy
- Improved patient education and counseling strategies are needed to convey, more effectively, the dangers of preeclampsia and hypertension during pregnancy
Reference
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
Pharmacotherapy for hypertensive emergency
Acute BP control with intravenous (IV) labetalol or hydralazine or oral nifedipine is recommended when a patient has a hypertensive emergency, defined as acute-onset severe hypertension that persists for ≥ 15 minutes (TABLE 2).12 The goal of management is not to completely normalize BP but to lower BP to the range of 140 to 155 mm Hg (systolic) and 90 to 105 mm Hg (diastolic). Of all proposed interventions, these agents are likely the most effective in preventing a maternal cerebrovascular or cardiovascular event. (Note: Labetalol is contraindicated in patients with severe asthma and in the setting of acute cocaine or methamphetamine intoxication. Hydralazine can cause tachycardia.)13,14

Once a diagnosis of preeclampsia with severe features or superimposed preeclampsia with severe features is made, the patient should remain hospitalized until delivery. If either of these diagnoses is made at ≥ 34 weeks of gestation, there is no reason to prolong pregnancy. Rather, the patient should be given prophylactic magnesium sulfate to prevent seizures and delivery should be accomplished.15,16 Earlier than 36 6/7 weeks of gestation, consider a late preterm course of corticosteroids; however, do not delay delivery in this situation.17
Planning for delivery
Route of delivery depends on customary obstetric indications. Before 34 weeks of gestation, corticosteroids, magnesium sulfate, and prolonging the pregnancy until 34 weeks of gestation are recommended. If, at any time, maternal or fetal condition deteriorates, delivery should be accomplished regardless of gestational age. If the patient is unwilling to accept the risks of expectant management of preeclampsia with severe features remote from term, delivery is indicated.18,19 If delivery is not likely to occur, magnesium sulfate can be discontinued after the patient has received a second dose of corticosteroids, with the plan to resume magnesium sulfate if she develops signs of worsening preeclampsia or eclampsia, or once the plan for delivery is made.
In patients who have either gestational hypertension or preeclampsia without severe features, the recommendation is to accomplish delivery no later than 37 weeks of gestation. While the patient is being expectantly managed, close maternal and fetal surveillance are necessary, comprising serial assessment of maternal symptoms and fetal movement; serial BP measurement (twice weekly); and weekly measurement of the platelet count, serum creatinine, and liver enzymes. At 34 weeks of gestation, conventional antepartum testing should begin. Again, if there is deterioration of the maternal or fetal condition, the patient should be hospitalized and delivery should be accomplished according to the recommendations above.3
Seizure management
If a patient has a tonic–clonic seizure consistent with eclampsia, management should be as follows:
- Preserve the airway and immediately tilt the head forward to prevent aspiration.
- If the patient is not receiving magnesium sulfate, immediately administer a loading dose of 4-6 g IV or 10 mg intramuscularly if IV access has not been established.20
- If the patient is already receiving magnesium sulfate, administer a loading dose of 2 g IV over 5 minutes.
- If the patient continues to have seizure activity, administer anticonvulsant medication(lorazepam, diazepam, midazolam, or phenytoin).
Eclamptic seizures are usually self-limited, lasting no longer than 1 or 2 minutes. Regrettably, these seizures are unpredictable and contribute significantly to maternal morbidity and mortality.21,22 A maternal seizure causes a significant interruption in the oxygen pathway to the fetus, with resultant late decelerations, prolonged decelerations, or bradycardia.
Resist the temptation to perform emergent cesarean delivery when eclamptic seizure occurs; rather, allow time for fetal recovery and then proceed with delivery in a controlled fashion. In many circumstances, the patient can undergo vaginal delivery after an eclamptic seizure. Keep in mind that the differential diagnosis of new-onset seizure in pregnancy includes cerebral pathology, such as a bleeding arteriovenous malformation or ruptured aneurysm. Therefore, brain-imaging studies might be indicated, especially in patients who have focal neurologic deficits, or who have seizures either while receiving magnesium sulfate or 48 to 72 hours after delivery.
Preeclampsia postpartum
More recent studies have demonstrated that preeclampsia can be exacerbated after delivery or might even present initially postpartum.23,24 In all women in whom gestational hypertension, preeclampsia, or superimposed preeclampsia is diagnosed, therefore, recommendations are that BP be monitored in the hospital or on an outpatient basis for at least 72 hours postpartum and again 7 to 10 days after delivery. For all women postpartum, the recommendation is that discharge instructions 1) include information about signs and symptoms of preeclampsia and 2) emphasize the importance of promptly reporting such developments to providers.25 Remember: Sequelae of preeclampsia have been reported as late as 4 to 6 weeks postpartum.
Magnesium sulfate is recommended when a patient presents postpartum with new-onset hypertension associated with headache or blurred vision, or with preeclampsia with severe hypertension. Because nonsteroidal anti-inflammatory drugs can be associated with elevated BP, these medications should be replaced by other analgesics in women with hypertension that persists for more than 1 day postpartum.
Prevention of preeclampsia
Given the significant maternal, fetal, and neonatal complications associated with preeclampsia, a number of studies have sought to determine ways in which this condition can be prevented. Currently, although no interventions appear to prevent preeclampsia in all patients, significant strides have been made in prevention for high-risk patients. Specifically, beginning low-dosage aspirin (most commonly, 81 mg/d, beginning at less than 16 weeks of gestation) has been shown to mitigate—although not eliminate—risk in patients with a history of preeclampsia and those who have chronic hypertension, multifetal gestation, pregestational diabetes, renal disease, SLE, or antiphospholipid syndrome.26,27Aspirin appears to act by preferentially blocking production of thromboxane, thus reducing the vasoconstrictive properties of this hormone.
Summing up
Hypertensive disorders during pregnancy are associated with significant morbidity and mortality for mother, fetus, and newborn. Preeclampsia, specifically, is recognized as a dynamic and progressive disease that has the potential to involve multiple organ systems, might present for the first time after delivery, and might be associated with long-term risk of hypertension, heart disease, stroke, and venous thromboembolism.28,29
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008; 199:133.e1-e8.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-1306.
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465.e1-e4.
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LL. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. CG107, August 2010. https://www.nice.org.uk/guidance/cg107. Accessed August 27, 2018. Last updated January 2011.
- Spong CY, Mercer BM, D'Alton M, et al. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers or aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052-1061.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193-201.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970-975.
- The American College of Obstetricians and Gynecologists Committee on Obstetric Practice; El-Sayed YY, Borders AE. Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period; April 2017. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co692.pdf?dmc=1. Accessed August 8, 2018.
- Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
- Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
- Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191-198.
- Norwitz E, Funai E. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008;199:209-212.
- Gordon R, Magee LA, Payne B, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J Obstet Gynaecol Can. 2014;36(2):154-163.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402-410.
- Liu S, Joseph KS, Liston, RM, et al; Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987-994.
- Yancey LM, Withers E, Bakes K, Abbot J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470-475.
- You WB, Wolf MS, Bailey SC, Grobman WA. Improving patient understanding of preeclampsia: a randomized controlled trial. Am J Obstet Gynecol. 2012;206:431.e1-e5.
- Henderson JT, Whitlock EP, O'Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-986.
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918-930.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008; 199:133.e1-e8.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-1306.
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465.e1-e4.
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LL. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. CG107, August 2010. https://www.nice.org.uk/guidance/cg107. Accessed August 27, 2018. Last updated January 2011.
- Spong CY, Mercer BM, D'Alton M, et al. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers or aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052-1061.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193-201.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970-975.
- The American College of Obstetricians and Gynecologists Committee on Obstetric Practice; El-Sayed YY, Borders AE. Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period; April 2017. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co692.pdf?dmc=1. Accessed August 8, 2018.
- Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
- Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
- Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191-198.
- Norwitz E, Funai E. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008;199:209-212.
- Gordon R, Magee LA, Payne B, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J Obstet Gynaecol Can. 2014;36(2):154-163.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402-410.
- Liu S, Joseph KS, Liston, RM, et al; Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987-994.
- Yancey LM, Withers E, Bakes K, Abbot J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470-475.
- You WB, Wolf MS, Bailey SC, Grobman WA. Improving patient understanding of preeclampsia: a randomized controlled trial. Am J Obstet Gynecol. 2012;206:431.e1-e5.
- Henderson JT, Whitlock EP, O'Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-986.
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918-930.
Physician groups call for CMS to drop E/M proposal
More than 170 physician groups are calling on the Centers for Medicare & Medicaid Services to withdraw a provision in the proposed 2019 physician fee schedule that would flatten evaluation and management payments.
The controversial proposal would set the payment rate for a level 1 evaluation and management (E/M) office visit for a new patient at $44, down from the $45 using the current methodology. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current payment of $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
In an Aug. 28 letter to the CMS, led by the American College of Rheumatology, physician groups applauded CMS recognition of the problems with the current E/M documentation guidelines and codes, but urged them to reconsider plans to “cut and consolidate evaluation and management services.” Doing so would “severely reduce Medicare patients’ access to care by cutting payments for complex office visits, adversely effecting the care and treatment of patients with complex conditions, and potentially exacerbate physician workforce shortages.”
A separate letter, led by the American Medical Association, made similar assertions that the current proposal has the potential to “hurt physicians and other health care professionals in specialties that treat the sickest patients, as well as those who provide comprehensive primary care, ultimately jeopardizing patients’ access to care.”
Daniel P. McQuillen, MD, of Lahey Hospital and Medical Center, Burlington, Mass. and member of the Infectious Diseases Society of America, said that IDSA is “worried that the implementation is being pushed ahead too fast without really considering whether it’s the right way of doing it and whether there might be some alternative ways of doing it that make more sense.”
Another concern related to the implementation of this proposal is the financial impact on physicians.
Implementation of the CMS proposal, as currently written, “would be amazingly expensive for private practice [doctors] and really for anyone else because we would have to change our EMRs,” Barbara Levy, MD, cochair of the CPT/RUC Work Group at the AMA.
“We would have to reprogram our billing software. All of that comes with a significant cost,” said Dr. Levy, who also serves as vice president of health policy at the American College of Obstetricians and Gynecologists.
Part of the selling point of the CMS proposal is the reduction in documentation that accompanies the E/M payment changes. The goal, according to the CMS, is to reduce time spent on paperwork and free up physicians to devote more time to patient care. But some physicians are skeptical it would work out that way.
“I don’t think it is going to change my documentation burden very much,” Jeffery Ward, MD, chair of the American Society of Clinical Oncology Government Relations Committee, said in an interview. “Their assumption is that I write my notes in order to meet a billing requirement and if I don’t have that billing requirement, I won’t have to write nearly as detailed notes. My notes are written so that if someone else needs to take care of my patient when I am not there, they can step in, read my notes, know what’s going on, and follow the patient.”
Dr. Ward suggested that the real purpose of the proposal is to shift more money to primary care, and raised the concern that the CMS is using this proposal “as a vehicle to take money away from specialists who take care of very complicated patients in order to give it to other people, particularly to primary care.”
ASCO and the Community Oncology Alliance both anticipate a 7%-8% reduction in payments to cancer specialists if this provision takes effect, Dr. Ward said.
Another element of the proposal that is raising concerns among physician groups is a proposed payment reduction when a visit involves more than one service. For example, when a single office visit includes both an E/M code and a procedure code, the proposal calls for the E/M code to be cut in half.
“From the patients’ perspective, the potential threat is that doctors could be incentivized to spend less time with patients or potentially bring patients back for subsequent visits to handle multiple problems,” Angus Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said in an interview.
Comments on the proposed update to the 2019 Medicare physician fee schedule are due Sept. 10.
More than 170 physician groups are calling on the Centers for Medicare & Medicaid Services to withdraw a provision in the proposed 2019 physician fee schedule that would flatten evaluation and management payments.
The controversial proposal would set the payment rate for a level 1 evaluation and management (E/M) office visit for a new patient at $44, down from the $45 using the current methodology. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current payment of $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
In an Aug. 28 letter to the CMS, led by the American College of Rheumatology, physician groups applauded CMS recognition of the problems with the current E/M documentation guidelines and codes, but urged them to reconsider plans to “cut and consolidate evaluation and management services.” Doing so would “severely reduce Medicare patients’ access to care by cutting payments for complex office visits, adversely effecting the care and treatment of patients with complex conditions, and potentially exacerbate physician workforce shortages.”
A separate letter, led by the American Medical Association, made similar assertions that the current proposal has the potential to “hurt physicians and other health care professionals in specialties that treat the sickest patients, as well as those who provide comprehensive primary care, ultimately jeopardizing patients’ access to care.”
Daniel P. McQuillen, MD, of Lahey Hospital and Medical Center, Burlington, Mass. and member of the Infectious Diseases Society of America, said that IDSA is “worried that the implementation is being pushed ahead too fast without really considering whether it’s the right way of doing it and whether there might be some alternative ways of doing it that make more sense.”
Another concern related to the implementation of this proposal is the financial impact on physicians.
Implementation of the CMS proposal, as currently written, “would be amazingly expensive for private practice [doctors] and really for anyone else because we would have to change our EMRs,” Barbara Levy, MD, cochair of the CPT/RUC Work Group at the AMA.
“We would have to reprogram our billing software. All of that comes with a significant cost,” said Dr. Levy, who also serves as vice president of health policy at the American College of Obstetricians and Gynecologists.
Part of the selling point of the CMS proposal is the reduction in documentation that accompanies the E/M payment changes. The goal, according to the CMS, is to reduce time spent on paperwork and free up physicians to devote more time to patient care. But some physicians are skeptical it would work out that way.
“I don’t think it is going to change my documentation burden very much,” Jeffery Ward, MD, chair of the American Society of Clinical Oncology Government Relations Committee, said in an interview. “Their assumption is that I write my notes in order to meet a billing requirement and if I don’t have that billing requirement, I won’t have to write nearly as detailed notes. My notes are written so that if someone else needs to take care of my patient when I am not there, they can step in, read my notes, know what’s going on, and follow the patient.”
Dr. Ward suggested that the real purpose of the proposal is to shift more money to primary care, and raised the concern that the CMS is using this proposal “as a vehicle to take money away from specialists who take care of very complicated patients in order to give it to other people, particularly to primary care.”
ASCO and the Community Oncology Alliance both anticipate a 7%-8% reduction in payments to cancer specialists if this provision takes effect, Dr. Ward said.
Another element of the proposal that is raising concerns among physician groups is a proposed payment reduction when a visit involves more than one service. For example, when a single office visit includes both an E/M code and a procedure code, the proposal calls for the E/M code to be cut in half.
“From the patients’ perspective, the potential threat is that doctors could be incentivized to spend less time with patients or potentially bring patients back for subsequent visits to handle multiple problems,” Angus Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said in an interview.
Comments on the proposed update to the 2019 Medicare physician fee schedule are due Sept. 10.
More than 170 physician groups are calling on the Centers for Medicare & Medicaid Services to withdraw a provision in the proposed 2019 physician fee schedule that would flatten evaluation and management payments.
The controversial proposal would set the payment rate for a level 1 evaluation and management (E/M) office visit for a new patient at $44, down from the $45 using the current methodology. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current payment of $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
In an Aug. 28 letter to the CMS, led by the American College of Rheumatology, physician groups applauded CMS recognition of the problems with the current E/M documentation guidelines and codes, but urged them to reconsider plans to “cut and consolidate evaluation and management services.” Doing so would “severely reduce Medicare patients’ access to care by cutting payments for complex office visits, adversely effecting the care and treatment of patients with complex conditions, and potentially exacerbate physician workforce shortages.”
A separate letter, led by the American Medical Association, made similar assertions that the current proposal has the potential to “hurt physicians and other health care professionals in specialties that treat the sickest patients, as well as those who provide comprehensive primary care, ultimately jeopardizing patients’ access to care.”
Daniel P. McQuillen, MD, of Lahey Hospital and Medical Center, Burlington, Mass. and member of the Infectious Diseases Society of America, said that IDSA is “worried that the implementation is being pushed ahead too fast without really considering whether it’s the right way of doing it and whether there might be some alternative ways of doing it that make more sense.”
Another concern related to the implementation of this proposal is the financial impact on physicians.
Implementation of the CMS proposal, as currently written, “would be amazingly expensive for private practice [doctors] and really for anyone else because we would have to change our EMRs,” Barbara Levy, MD, cochair of the CPT/RUC Work Group at the AMA.
“We would have to reprogram our billing software. All of that comes with a significant cost,” said Dr. Levy, who also serves as vice president of health policy at the American College of Obstetricians and Gynecologists.
Part of the selling point of the CMS proposal is the reduction in documentation that accompanies the E/M payment changes. The goal, according to the CMS, is to reduce time spent on paperwork and free up physicians to devote more time to patient care. But some physicians are skeptical it would work out that way.
“I don’t think it is going to change my documentation burden very much,” Jeffery Ward, MD, chair of the American Society of Clinical Oncology Government Relations Committee, said in an interview. “Their assumption is that I write my notes in order to meet a billing requirement and if I don’t have that billing requirement, I won’t have to write nearly as detailed notes. My notes are written so that if someone else needs to take care of my patient when I am not there, they can step in, read my notes, know what’s going on, and follow the patient.”
Dr. Ward suggested that the real purpose of the proposal is to shift more money to primary care, and raised the concern that the CMS is using this proposal “as a vehicle to take money away from specialists who take care of very complicated patients in order to give it to other people, particularly to primary care.”
ASCO and the Community Oncology Alliance both anticipate a 7%-8% reduction in payments to cancer specialists if this provision takes effect, Dr. Ward said.
Another element of the proposal that is raising concerns among physician groups is a proposed payment reduction when a visit involves more than one service. For example, when a single office visit includes both an E/M code and a procedure code, the proposal calls for the E/M code to be cut in half.
“From the patients’ perspective, the potential threat is that doctors could be incentivized to spend less time with patients or potentially bring patients back for subsequent visits to handle multiple problems,” Angus Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said in an interview.
Comments on the proposed update to the 2019 Medicare physician fee schedule are due Sept. 10.
Syphilis surge drives USPSTF reaffirmation of early screening for all pregnant women
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
FROM JAMA
An oath to save lives against a backdrop of growing disparities
Practicing in the field of obstetrics and gynecology affords us a special privilege: we are part of the most important and unforgettable events in our patients’ lives, both in sickness and in health. Along with the great joys we share comes profound responsibility and the recognition that we are only as effective as the team with whom we work. Although we live in a country that is home to some of the best health care systems in the world, the maternal mortality rates and disease burden among women in underserved communities belie this fact. A University of Washington study demonstrated a more than 20-year gap in life expectancy between wealthy and poor communities in the United States from 1980 to 2014.1 Not surprisingly, access to medical care was a contributing factor.
Poverty only partly explains this disparity. Racial differences are at play as well. In 1992, a seminal study by Schoendorf and colleagues2 demonstrated that the death rates of babies born to educated African American parents were higher due to lower birth weights. Concern recently has been amplified, and many lay publications have publicly raised the alarm.3 Several states have started investigating the causes, and the American College of Obstetrics and Gynecology, as well as other organizations, are studying possible solutions.
With nearly 50% of US births financed by Medicaid,5 there was great hope that the Patient Protection and Affordable Care Act and expansion of Medicaid would result in improved access and quality of health care for underserved patients; however, it has become apparent that coverage did not confer improved access to quality care, especially for medical specialties.
Urban and rural poor populations generally seek medical services from safety net clinics staffed by midlevel and physician primary care providers whose tight schedules, documentation demands, and low reimbursement rates are coupled with complex medical and socioeconomic patient populations. While these providers may be skilled in basic primary care, their patients often present with conditions outside their scope of practice. Our country’s growing physician shortage, along with patient location and personal logistics, adds to the challenges for patients and providers alike. And who among us is not asked several times a week, even by our well-insured patients, for a primary care or specialist physician recommendation? The barriers for seeking medical care in rural populations are even greater, as local hospitals and clinics are closing at an alarming rate.
Alumni at work
Communities of physicians across the country recognize both the access problem and the potential to create solutions. Organizations such as Project ECHO, launched in 2003 through the University of New Mexico, connect rural providers with university physicians to aid in treatment of hepatitis C and other illnesses.
As the date for implementation of the Patient Protection and Affordable Care Act approached, a group of medical school alumni leaders recognized that we could come together and offer our services to address growing health care disparities. Galvanized by the challenge, the Medical Alumni Volunteer Expert Network, or MAVEN Project, was, in our parlance, “born.”
While the concept of the MAVEN Project was germinating, we interviewed numerous colleagues for advice and input and were struck by their desire—especially among the newly retired—to continue to give back. Medicine is a calling, not just a job, and for many of us the joy of helping—the exhilaration of that first birth that sold us on our specialty—gives us meaning and purpose. Many physicians who had left full-time clinical medicine missed the collegiality of the “doctors’ lounge.” Throughout our careers, we are part of a cohort: our medical school class, our residency partners, our hospital staff—we all crave community. With 36% of US physicians older than age 55 and 240,000 retired doctors in the country, we realized a motivated, previously untapped workforce could be marshaled to form a community to serve the most vulnerable among us.5
At the same time, telemedicine had come into its own. Simple technology could enable us to see each other on smartphones and computers and even perform portions of a physical examination from afar.
We realized we could marry opportunity (the workforce), need (underserved populations across the country), and technology. The Harvard Medical School Center Primary Care supported a feasibility study, and the MAVEN Project began “seeing” patients in 2016.
What happens when a safety net clinic receives a donation of life-altering oral diabetes medications but their providers lack the expertise to use them appropriately? A closet full of drugs. That is what the MAVEN Project discovered at one of our partner clinics. Enter our volunteer endocrinologist. She consulted with the medical team, reviewed how each medication should be prescribed and monitored, and gave instructions on which patients with diabetes would benefit the most from them.
The closet is emptying, the clinic providers are confidently prescribing the newest therapies, and patients are enjoying improved blood sugars and quality of life!
A model of hope
The MAVEN Project matches physician volunteers with safety net clinics serving patients in need and provides malpractice insurance and a Health Information Portability and Accountability Act–compliant technology platform to facilitate remote communication. Our volunteers mentor and educate primary care providers in the field and offer both immediate and asynchronous advisory consults. Clinic providers can group cases for discussion, ask urgent questions, or receive advice and support for the day-to-day challenges facing clinicians today. Clinics choose educational topics, focusing on tools needed for patient care rather than esoteric mechanisms of disease. Patients receive best-in-class care conveniently and locally, and by making volunteering easy, we build partnerships that augment patient and provider satisfaction, support long-term capacity building, and improve service delivery.
Our volunteer physicians now represent more than 30 medical specialties and 25 medical schools, and we have completed more than 2,000 consultations to date. Our clinics are located in 6 states (California, Florida, Massachusetts, New York, South Dakota, and Washington), and thanks to our model, physician state of licensure is not an impediment to volunteering. Several colleagues in our specialty are providing advice in women’s health.
Driving innovative solutions
Elizabeth Kopin, MD, an ObGyn who practiced for 28 years in Worcester, Massachusetts, and volunteers for the MAVEN Project, eloquently described in correspondence with Project coordinators the spirit that embodies the pursuit of medicine and the organization’s mission. As Dr. Kopin stated, “The driving force behind my entering medicine was to help people in an essential and meaningful way. I was especially driven to participate in the care of women. I wanted to gain knowledge and skills to help women with health care throughout their lives.”
Dr. Kopin’s capacity to care for patients in the clinic and hospital was progressively reduced as her multiple sclerosis advanced. As a result, she retired from clinical practice, but her desire to participate and contribute to medicine with the passion with which she entered it remained.
Her father was an internist who started a charitable clinic in Georgia. Like her father, Dr. Kopin began her medical career in academic medicine. Her father felt that his last 15 years in medicine were the most meaningful of his career because of his work with underserved populations. Dr. Kopin is following in his footsteps. For her, “Looking for a telehealth vehicle helping communities in need gives me the opportunity to use my abilities in the best way possible.” Dr. Kopin also stated, “Helping the underserved was something I wanted to devote my time to and The MAVEN Project has given me that possibility.”
We like to think of ourselves as Match. com meets the Peace Corps, with the goal to reach underserved patients in all 50 states in both rural and urban communities. We ask for as little as 4 hours of your time per month, and all you need is a computer or smartphone and a medical license. We welcome volunteers in active or part-time practice, academics, and industry: your years of wisdom are invaluable.
The vast complexities of the US health care system are by no measure easy to address, but standing by and allowing a fractured system to rupture is not an option. Each of us has an expertise and an opportunity to make incremental steps to ensure that those who need health care do not slip through the cracks. Dr. Kopin and I are fortunate to have a skill to help others and, in the MAVEN Project, a robust, dedicated network of individuals who share our vision.
There are many who have and continue to inspire a guiding conscience to serve beyond oneself. George H.W. Bush said it best when explaining why he founded the Points of Light organization nearly 3 decades ago6:
I have pursued life itself over many years now and with varying degrees of happiness. Some of my happiness still comes from trying to be in my own small way a true “point of light.” I believe I was right when I said, as President, there can be no definition of a successful life that does not include service to others. So I do that now, and I gain happiness. I do not seek a Pulitzer Prize. I do not want press attention…. I have found happiness. I no longer pursue it, for it is mine.
Please join us on our mission!
How to join
We are actively seeking specialty and primary care physicians to provide advisory consultations, mentorship, and education via telehealth technology. We welcome physician volunteers who:
- are newly retired, semi-retired, in industry, or in clinical practice
- have a minimum of 2 years of clinical practice experience
- have been active in the medical community in the past 3 years
- have an active or volunteer US medical license (any state)
- are able to provide 3 professional references
- are willing to commit a minimum of 4 hours per month for 6 months.
Visit us online to complete our physician volunteer inquiry form (https://www.mavenproject.org/work-with-us/#wwu-volunteer-as-a-physician-lightblue).

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003-1011.
- Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326:1522-1526.
- Villarosa L. Why America's black mothers and babies are in a life-or-death crisis. New York Times. April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed August 14, 2018.
- Smith VK, Gifford K, Ellis E, et al; The Henry J. Kaiser Family Foundation; The National Association of Medical Directors. Implementing coverage and payment initiatives: results from a 50-state Medicaid budget survey for state fiscal years 2016 and 2017. http://files.kff.org/attachment/Report-Implementing-Coverage-and-Payment-Initiatives. Published October 2006. Accessed August 14, 2018.
- Association of American Medical Colleges. 2016 Physician Specialty Data Report: Executive Summary. https://www.aamc.org/download/471786/data/2016physicianspecialtydatareportexecutivesummary.pdf. Accessed August 23, 2018.
- Miller RW. Jenna Bush Hager shares George H.W. Bush 'point of light' letter after Trump jab. USA TODAY. July 7, 2018. https://www.usatoday.com/story/news/politics/onpolitics/2018/07/07/jenna-bush-hager-shares-george-h-w-bush-point-light-letter-donald-trump/765248002/. Accessed August 14, 2018.
Practicing in the field of obstetrics and gynecology affords us a special privilege: we are part of the most important and unforgettable events in our patients’ lives, both in sickness and in health. Along with the great joys we share comes profound responsibility and the recognition that we are only as effective as the team with whom we work. Although we live in a country that is home to some of the best health care systems in the world, the maternal mortality rates and disease burden among women in underserved communities belie this fact. A University of Washington study demonstrated a more than 20-year gap in life expectancy between wealthy and poor communities in the United States from 1980 to 2014.1 Not surprisingly, access to medical care was a contributing factor.
Poverty only partly explains this disparity. Racial differences are at play as well. In 1992, a seminal study by Schoendorf and colleagues2 demonstrated that the death rates of babies born to educated African American parents were higher due to lower birth weights. Concern recently has been amplified, and many lay publications have publicly raised the alarm.3 Several states have started investigating the causes, and the American College of Obstetrics and Gynecology, as well as other organizations, are studying possible solutions.
With nearly 50% of US births financed by Medicaid,5 there was great hope that the Patient Protection and Affordable Care Act and expansion of Medicaid would result in improved access and quality of health care for underserved patients; however, it has become apparent that coverage did not confer improved access to quality care, especially for medical specialties.
Urban and rural poor populations generally seek medical services from safety net clinics staffed by midlevel and physician primary care providers whose tight schedules, documentation demands, and low reimbursement rates are coupled with complex medical and socioeconomic patient populations. While these providers may be skilled in basic primary care, their patients often present with conditions outside their scope of practice. Our country’s growing physician shortage, along with patient location and personal logistics, adds to the challenges for patients and providers alike. And who among us is not asked several times a week, even by our well-insured patients, for a primary care or specialist physician recommendation? The barriers for seeking medical care in rural populations are even greater, as local hospitals and clinics are closing at an alarming rate.
Alumni at work
Communities of physicians across the country recognize both the access problem and the potential to create solutions. Organizations such as Project ECHO, launched in 2003 through the University of New Mexico, connect rural providers with university physicians to aid in treatment of hepatitis C and other illnesses.
As the date for implementation of the Patient Protection and Affordable Care Act approached, a group of medical school alumni leaders recognized that we could come together and offer our services to address growing health care disparities. Galvanized by the challenge, the Medical Alumni Volunteer Expert Network, or MAVEN Project, was, in our parlance, “born.”
While the concept of the MAVEN Project was germinating, we interviewed numerous colleagues for advice and input and were struck by their desire—especially among the newly retired—to continue to give back. Medicine is a calling, not just a job, and for many of us the joy of helping—the exhilaration of that first birth that sold us on our specialty—gives us meaning and purpose. Many physicians who had left full-time clinical medicine missed the collegiality of the “doctors’ lounge.” Throughout our careers, we are part of a cohort: our medical school class, our residency partners, our hospital staff—we all crave community. With 36% of US physicians older than age 55 and 240,000 retired doctors in the country, we realized a motivated, previously untapped workforce could be marshaled to form a community to serve the most vulnerable among us.5
At the same time, telemedicine had come into its own. Simple technology could enable us to see each other on smartphones and computers and even perform portions of a physical examination from afar.
We realized we could marry opportunity (the workforce), need (underserved populations across the country), and technology. The Harvard Medical School Center Primary Care supported a feasibility study, and the MAVEN Project began “seeing” patients in 2016.
What happens when a safety net clinic receives a donation of life-altering oral diabetes medications but their providers lack the expertise to use them appropriately? A closet full of drugs. That is what the MAVEN Project discovered at one of our partner clinics. Enter our volunteer endocrinologist. She consulted with the medical team, reviewed how each medication should be prescribed and monitored, and gave instructions on which patients with diabetes would benefit the most from them.
The closet is emptying, the clinic providers are confidently prescribing the newest therapies, and patients are enjoying improved blood sugars and quality of life!
A model of hope
The MAVEN Project matches physician volunteers with safety net clinics serving patients in need and provides malpractice insurance and a Health Information Portability and Accountability Act–compliant technology platform to facilitate remote communication. Our volunteers mentor and educate primary care providers in the field and offer both immediate and asynchronous advisory consults. Clinic providers can group cases for discussion, ask urgent questions, or receive advice and support for the day-to-day challenges facing clinicians today. Clinics choose educational topics, focusing on tools needed for patient care rather than esoteric mechanisms of disease. Patients receive best-in-class care conveniently and locally, and by making volunteering easy, we build partnerships that augment patient and provider satisfaction, support long-term capacity building, and improve service delivery.
Our volunteer physicians now represent more than 30 medical specialties and 25 medical schools, and we have completed more than 2,000 consultations to date. Our clinics are located in 6 states (California, Florida, Massachusetts, New York, South Dakota, and Washington), and thanks to our model, physician state of licensure is not an impediment to volunteering. Several colleagues in our specialty are providing advice in women’s health.
Driving innovative solutions
Elizabeth Kopin, MD, an ObGyn who practiced for 28 years in Worcester, Massachusetts, and volunteers for the MAVEN Project, eloquently described in correspondence with Project coordinators the spirit that embodies the pursuit of medicine and the organization’s mission. As Dr. Kopin stated, “The driving force behind my entering medicine was to help people in an essential and meaningful way. I was especially driven to participate in the care of women. I wanted to gain knowledge and skills to help women with health care throughout their lives.”
Dr. Kopin’s capacity to care for patients in the clinic and hospital was progressively reduced as her multiple sclerosis advanced. As a result, she retired from clinical practice, but her desire to participate and contribute to medicine with the passion with which she entered it remained.
Her father was an internist who started a charitable clinic in Georgia. Like her father, Dr. Kopin began her medical career in academic medicine. Her father felt that his last 15 years in medicine were the most meaningful of his career because of his work with underserved populations. Dr. Kopin is following in his footsteps. For her, “Looking for a telehealth vehicle helping communities in need gives me the opportunity to use my abilities in the best way possible.” Dr. Kopin also stated, “Helping the underserved was something I wanted to devote my time to and The MAVEN Project has given me that possibility.”
We like to think of ourselves as Match. com meets the Peace Corps, with the goal to reach underserved patients in all 50 states in both rural and urban communities. We ask for as little as 4 hours of your time per month, and all you need is a computer or smartphone and a medical license. We welcome volunteers in active or part-time practice, academics, and industry: your years of wisdom are invaluable.
The vast complexities of the US health care system are by no measure easy to address, but standing by and allowing a fractured system to rupture is not an option. Each of us has an expertise and an opportunity to make incremental steps to ensure that those who need health care do not slip through the cracks. Dr. Kopin and I are fortunate to have a skill to help others and, in the MAVEN Project, a robust, dedicated network of individuals who share our vision.
There are many who have and continue to inspire a guiding conscience to serve beyond oneself. George H.W. Bush said it best when explaining why he founded the Points of Light organization nearly 3 decades ago6:
I have pursued life itself over many years now and with varying degrees of happiness. Some of my happiness still comes from trying to be in my own small way a true “point of light.” I believe I was right when I said, as President, there can be no definition of a successful life that does not include service to others. So I do that now, and I gain happiness. I do not seek a Pulitzer Prize. I do not want press attention…. I have found happiness. I no longer pursue it, for it is mine.
Please join us on our mission!
How to join
We are actively seeking specialty and primary care physicians to provide advisory consultations, mentorship, and education via telehealth technology. We welcome physician volunteers who:
- are newly retired, semi-retired, in industry, or in clinical practice
- have a minimum of 2 years of clinical practice experience
- have been active in the medical community in the past 3 years
- have an active or volunteer US medical license (any state)
- are able to provide 3 professional references
- are willing to commit a minimum of 4 hours per month for 6 months.
Visit us online to complete our physician volunteer inquiry form (https://www.mavenproject.org/work-with-us/#wwu-volunteer-as-a-physician-lightblue).

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Practicing in the field of obstetrics and gynecology affords us a special privilege: we are part of the most important and unforgettable events in our patients’ lives, both in sickness and in health. Along with the great joys we share comes profound responsibility and the recognition that we are only as effective as the team with whom we work. Although we live in a country that is home to some of the best health care systems in the world, the maternal mortality rates and disease burden among women in underserved communities belie this fact. A University of Washington study demonstrated a more than 20-year gap in life expectancy between wealthy and poor communities in the United States from 1980 to 2014.1 Not surprisingly, access to medical care was a contributing factor.
Poverty only partly explains this disparity. Racial differences are at play as well. In 1992, a seminal study by Schoendorf and colleagues2 demonstrated that the death rates of babies born to educated African American parents were higher due to lower birth weights. Concern recently has been amplified, and many lay publications have publicly raised the alarm.3 Several states have started investigating the causes, and the American College of Obstetrics and Gynecology, as well as other organizations, are studying possible solutions.
With nearly 50% of US births financed by Medicaid,5 there was great hope that the Patient Protection and Affordable Care Act and expansion of Medicaid would result in improved access and quality of health care for underserved patients; however, it has become apparent that coverage did not confer improved access to quality care, especially for medical specialties.
Urban and rural poor populations generally seek medical services from safety net clinics staffed by midlevel and physician primary care providers whose tight schedules, documentation demands, and low reimbursement rates are coupled with complex medical and socioeconomic patient populations. While these providers may be skilled in basic primary care, their patients often present with conditions outside their scope of practice. Our country’s growing physician shortage, along with patient location and personal logistics, adds to the challenges for patients and providers alike. And who among us is not asked several times a week, even by our well-insured patients, for a primary care or specialist physician recommendation? The barriers for seeking medical care in rural populations are even greater, as local hospitals and clinics are closing at an alarming rate.
Alumni at work
Communities of physicians across the country recognize both the access problem and the potential to create solutions. Organizations such as Project ECHO, launched in 2003 through the University of New Mexico, connect rural providers with university physicians to aid in treatment of hepatitis C and other illnesses.
As the date for implementation of the Patient Protection and Affordable Care Act approached, a group of medical school alumni leaders recognized that we could come together and offer our services to address growing health care disparities. Galvanized by the challenge, the Medical Alumni Volunteer Expert Network, or MAVEN Project, was, in our parlance, “born.”
While the concept of the MAVEN Project was germinating, we interviewed numerous colleagues for advice and input and were struck by their desire—especially among the newly retired—to continue to give back. Medicine is a calling, not just a job, and for many of us the joy of helping—the exhilaration of that first birth that sold us on our specialty—gives us meaning and purpose. Many physicians who had left full-time clinical medicine missed the collegiality of the “doctors’ lounge.” Throughout our careers, we are part of a cohort: our medical school class, our residency partners, our hospital staff—we all crave community. With 36% of US physicians older than age 55 and 240,000 retired doctors in the country, we realized a motivated, previously untapped workforce could be marshaled to form a community to serve the most vulnerable among us.5
At the same time, telemedicine had come into its own. Simple technology could enable us to see each other on smartphones and computers and even perform portions of a physical examination from afar.
We realized we could marry opportunity (the workforce), need (underserved populations across the country), and technology. The Harvard Medical School Center Primary Care supported a feasibility study, and the MAVEN Project began “seeing” patients in 2016.
What happens when a safety net clinic receives a donation of life-altering oral diabetes medications but their providers lack the expertise to use them appropriately? A closet full of drugs. That is what the MAVEN Project discovered at one of our partner clinics. Enter our volunteer endocrinologist. She consulted with the medical team, reviewed how each medication should be prescribed and monitored, and gave instructions on which patients with diabetes would benefit the most from them.
The closet is emptying, the clinic providers are confidently prescribing the newest therapies, and patients are enjoying improved blood sugars and quality of life!
A model of hope
The MAVEN Project matches physician volunteers with safety net clinics serving patients in need and provides malpractice insurance and a Health Information Portability and Accountability Act–compliant technology platform to facilitate remote communication. Our volunteers mentor and educate primary care providers in the field and offer both immediate and asynchronous advisory consults. Clinic providers can group cases for discussion, ask urgent questions, or receive advice and support for the day-to-day challenges facing clinicians today. Clinics choose educational topics, focusing on tools needed for patient care rather than esoteric mechanisms of disease. Patients receive best-in-class care conveniently and locally, and by making volunteering easy, we build partnerships that augment patient and provider satisfaction, support long-term capacity building, and improve service delivery.
Our volunteer physicians now represent more than 30 medical specialties and 25 medical schools, and we have completed more than 2,000 consultations to date. Our clinics are located in 6 states (California, Florida, Massachusetts, New York, South Dakota, and Washington), and thanks to our model, physician state of licensure is not an impediment to volunteering. Several colleagues in our specialty are providing advice in women’s health.
Driving innovative solutions
Elizabeth Kopin, MD, an ObGyn who practiced for 28 years in Worcester, Massachusetts, and volunteers for the MAVEN Project, eloquently described in correspondence with Project coordinators the spirit that embodies the pursuit of medicine and the organization’s mission. As Dr. Kopin stated, “The driving force behind my entering medicine was to help people in an essential and meaningful way. I was especially driven to participate in the care of women. I wanted to gain knowledge and skills to help women with health care throughout their lives.”
Dr. Kopin’s capacity to care for patients in the clinic and hospital was progressively reduced as her multiple sclerosis advanced. As a result, she retired from clinical practice, but her desire to participate and contribute to medicine with the passion with which she entered it remained.
Her father was an internist who started a charitable clinic in Georgia. Like her father, Dr. Kopin began her medical career in academic medicine. Her father felt that his last 15 years in medicine were the most meaningful of his career because of his work with underserved populations. Dr. Kopin is following in his footsteps. For her, “Looking for a telehealth vehicle helping communities in need gives me the opportunity to use my abilities in the best way possible.” Dr. Kopin also stated, “Helping the underserved was something I wanted to devote my time to and The MAVEN Project has given me that possibility.”
We like to think of ourselves as Match. com meets the Peace Corps, with the goal to reach underserved patients in all 50 states in both rural and urban communities. We ask for as little as 4 hours of your time per month, and all you need is a computer or smartphone and a medical license. We welcome volunteers in active or part-time practice, academics, and industry: your years of wisdom are invaluable.
The vast complexities of the US health care system are by no measure easy to address, but standing by and allowing a fractured system to rupture is not an option. Each of us has an expertise and an opportunity to make incremental steps to ensure that those who need health care do not slip through the cracks. Dr. Kopin and I are fortunate to have a skill to help others and, in the MAVEN Project, a robust, dedicated network of individuals who share our vision.
There are many who have and continue to inspire a guiding conscience to serve beyond oneself. George H.W. Bush said it best when explaining why he founded the Points of Light organization nearly 3 decades ago6:
I have pursued life itself over many years now and with varying degrees of happiness. Some of my happiness still comes from trying to be in my own small way a true “point of light.” I believe I was right when I said, as President, there can be no definition of a successful life that does not include service to others. So I do that now, and I gain happiness. I do not seek a Pulitzer Prize. I do not want press attention…. I have found happiness. I no longer pursue it, for it is mine.
Please join us on our mission!
How to join
We are actively seeking specialty and primary care physicians to provide advisory consultations, mentorship, and education via telehealth technology. We welcome physician volunteers who:
- are newly retired, semi-retired, in industry, or in clinical practice
- have a minimum of 2 years of clinical practice experience
- have been active in the medical community in the past 3 years
- have an active or volunteer US medical license (any state)
- are able to provide 3 professional references
- are willing to commit a minimum of 4 hours per month for 6 months.
Visit us online to complete our physician volunteer inquiry form (https://www.mavenproject.org/work-with-us/#wwu-volunteer-as-a-physician-lightblue).

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003-1011.
- Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326:1522-1526.
- Villarosa L. Why America's black mothers and babies are in a life-or-death crisis. New York Times. April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed August 14, 2018.
- Smith VK, Gifford K, Ellis E, et al; The Henry J. Kaiser Family Foundation; The National Association of Medical Directors. Implementing coverage and payment initiatives: results from a 50-state Medicaid budget survey for state fiscal years 2016 and 2017. http://files.kff.org/attachment/Report-Implementing-Coverage-and-Payment-Initiatives. Published October 2006. Accessed August 14, 2018.
- Association of American Medical Colleges. 2016 Physician Specialty Data Report: Executive Summary. https://www.aamc.org/download/471786/data/2016physicianspecialtydatareportexecutivesummary.pdf. Accessed August 23, 2018.
- Miller RW. Jenna Bush Hager shares George H.W. Bush 'point of light' letter after Trump jab. USA TODAY. July 7, 2018. https://www.usatoday.com/story/news/politics/onpolitics/2018/07/07/jenna-bush-hager-shares-george-h-w-bush-point-light-letter-donald-trump/765248002/. Accessed August 14, 2018.
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003-1011.
- Schoendorf KC, Hogue CJ, Kleinman JC, et al. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326:1522-1526.
- Villarosa L. Why America's black mothers and babies are in a life-or-death crisis. New York Times. April 11, 2018. https://www.nytimes.com/2018/04/11/magazine/black-mothers-babies-death-maternal-mortality.html. Accessed August 14, 2018.
- Smith VK, Gifford K, Ellis E, et al; The Henry J. Kaiser Family Foundation; The National Association of Medical Directors. Implementing coverage and payment initiatives: results from a 50-state Medicaid budget survey for state fiscal years 2016 and 2017. http://files.kff.org/attachment/Report-Implementing-Coverage-and-Payment-Initiatives. Published October 2006. Accessed August 14, 2018.
- Association of American Medical Colleges. 2016 Physician Specialty Data Report: Executive Summary. https://www.aamc.org/download/471786/data/2016physicianspecialtydatareportexecutivesummary.pdf. Accessed August 23, 2018.
- Miller RW. Jenna Bush Hager shares George H.W. Bush 'point of light' letter after Trump jab. USA TODAY. July 7, 2018. https://www.usatoday.com/story/news/politics/onpolitics/2018/07/07/jenna-bush-hager-shares-george-h-w-bush-point-light-letter-donald-trump/765248002/. Accessed August 14, 2018.
Daratumumab approved in Europe for new myeloma indication
The drug is now authorized for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant, according to a press release published on the Genmab website.
Daratumumab was previously approved by the European Commission (EC) for use in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with MM who have received at least one prior therapy.
In addition, daratumumab is approved by the EC as monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who had disease progression on their last therapy.
The EC’s latest approval for daratumumab is based on results from the phase 3 ALCYONE trial. Results from this study were presented at the 2017 annual meeting of the American Society of Hematology and simultaneously published in the New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P less than.0001), and rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively, and the 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and in none of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm – 23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation caused by adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The drug is now authorized for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant, according to a press release published on the Genmab website.
Daratumumab was previously approved by the European Commission (EC) for use in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with MM who have received at least one prior therapy.
In addition, daratumumab is approved by the EC as monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who had disease progression on their last therapy.
The EC’s latest approval for daratumumab is based on results from the phase 3 ALCYONE trial. Results from this study were presented at the 2017 annual meeting of the American Society of Hematology and simultaneously published in the New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P less than.0001), and rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively, and the 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and in none of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm – 23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation caused by adverse events was 5% in the D-VMP arm and 9% in the VMP arm.
The drug is now authorized for use in combination with bortezomib, melphalan, and prednisone (VMP) to treat adults with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant, according to a press release published on the Genmab website.
Daratumumab was previously approved by the European Commission (EC) for use in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone to treat adults with MM who have received at least one prior therapy.
In addition, daratumumab is approved by the EC as monotherapy for adults with relapsed and refractory MM whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who had disease progression on their last therapy.
The EC’s latest approval for daratumumab is based on results from the phase 3 ALCYONE trial. Results from this study were presented at the 2017 annual meeting of the American Society of Hematology and simultaneously published in the New England Journal of Medicine.
ALCYONE enrolled 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant. Patients were randomized to receive VMP or daratumumab plus VMP (D-VMP).
The overall response rates were 91% in the D-VMP arm and 74% in the VMP arm (P less than.0001), and rates of complete response were 43% and 24%, respectively. Rates of minimal residual disease negativity were 22% and 6%, respectively.
The median progression-free survival (PFS) was not reached in the D-VMP arm and was 18.1 months in the VMP arm. The 12-month PFS was 87% and 76%, respectively, and the 18-month PFS was 72% and 50%, respectively.
The most common treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (50% and 53%), thrombocytopenia (49% and 54%), anemia (28% and 38%), peripheral sensory neuropathy (28% and 34%), upper respiratory tract infection (26% and 14%), diarrhea (24% and 25%), pyrexia (23% and 21%), and nausea (21% and 22%).
Infusion-related reactions occurred in 28% of patients in the D-VMP arm and in none of those in the VMP arm.
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm – 23% and 15%, respectively. In both arms, most infections resolved.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of discontinuation caused by adverse events was 5% in the D-VMP arm and 9% in the VMP arm.