User login
Nonpharmacologic strategies for acute pain
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Pain is a very common cause for emergency room visits. Nonpharmacologic pain management is not well studied for adult patients visiting the ED.
Study design: Systematic review and meta-analysis.
Setting: Duke University, Durham, N.C.
Synopsis: Among the studies considered, 56 met inclusion criteria for summary analysis. Multiple interventions were used, mostly acupuncture and physical therapy. Of the 56 studies, 23 reported significantly reduced pain, compared with control; 24 showed no difference, compared with control; and 9 had no control group. Meta-analysis included 22 qualifying randomized, controlled trials and had a global standardized mean difference of –0.46 (95% confidence interval, –0.66 to –0.27) in favor of nonpharmacologic interventions for reducing pain.
Bottom line: Nonpharmacologic pain control approaches may be helpful for adult patients visiting the emergency room. Larger studies are needed to confirm efficacy.
Citation: JT Sakamoto et al. Are nonpharmacologic pain interventions effective at reducing pain in adult patients visiting the emergency department? A systematic review and meta‐analysis. Acad Emerg Med. 2018 Mar 15. doi: 10.1111/acem.13411.
Dr. Hasanein is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Obesity Extends Viral Shedding of Flu
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Obesity not only makes flu more severe, but also lengthens the period of viral shedding for influenza A, according to a study by University of Michigan researchers, partly funded by the National Institute of Allergy and Infectious Diseases.
Over 3 flu seasons, the researchers monitored 1,783 people from 320 households in Managua, Nicaragua. During that time, 87 people became ill with influenza A and 58 with influenza B.
More than 40% of the adults aged > 18 years were obese, as defined by body mass. Obese adults with ≥ 2 symptoms of influenza A (n = 62) shed the virus 42% longer than did nonobese adults, or 5.2 days compared with 3.7 days, respectively. Obese adults with 1 or no symptoms of influenza A (n = 25) shed the virus 104% longer than nonobese adults—3.2 days compared with 1.6 days, respectively.
Obesity was not a risk factor for increased viral shedding duration in children aged 5 to 17 years or for adults with influenza B.
The researchers suggest that chronic inflammation caused by obesity may be responsible for the increased viral shedding. Reducing obesity rates could be an important target to limit the spread of viral infectious diseases, they suggest. The findings may have particular significance in the US, where in 2014 35% of adults were obese compared with 17.4% of adults in Nicaragua.
Woman, 18, With Sore Throat, Fever, and Painful Rash
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
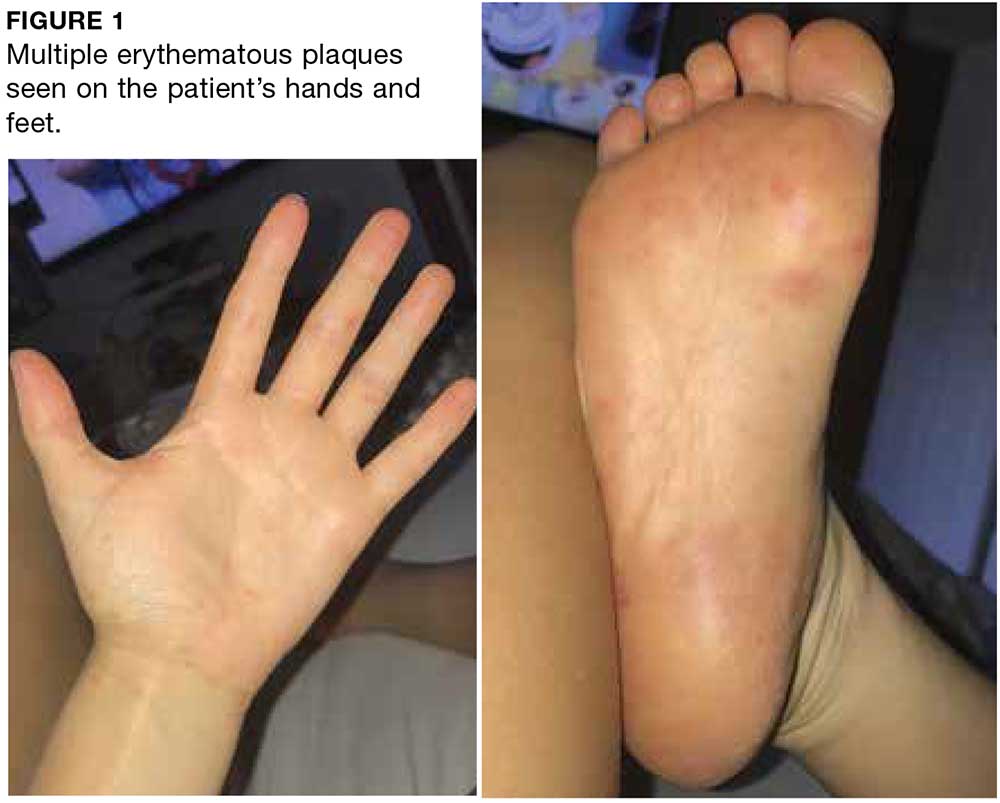
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).
DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.
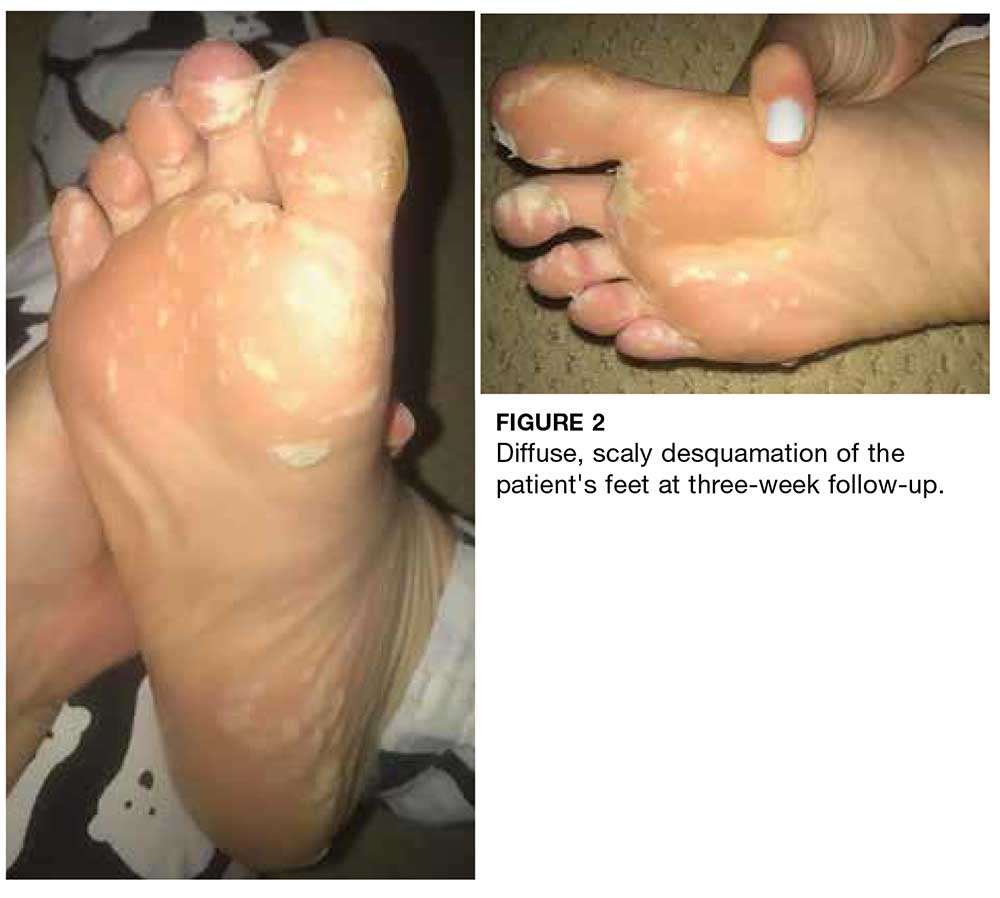
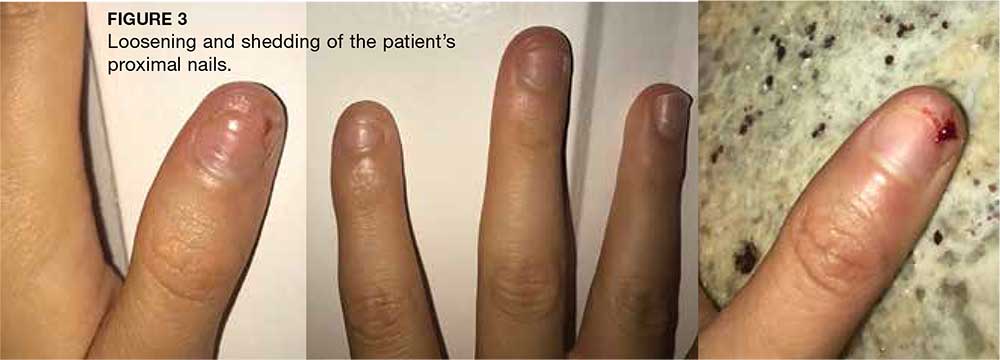
About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.
Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).

DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.

About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.

Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
IN THIS ARTICLE
- Diagnosis
- Management
- Outcome for the case patient
An 18-year-old woman presents to urgent care with a one-day history of sudden-onset sore throat, chills, malaise, and fever (102.5°F). On physical examination, the tonsils and pharynx are erythematous, and anterior cervical lymph nodes are tender on palpation. A rapid strep test is negative. The patient is instructed to use throat lozenges and take ibuprofen (400 mg every 8 h) as needed for pain.
On day 2, the patient’s fever resolves, but a painful rash develops on her palms and soles. Clinical examination reveals multiple erythematous plaques on the hands and feet (see Figure 1). The patient is diagnosed with hand-foot-and-mouth disease (HFMD).

DISCUSSION
HFMD is caused by enteroviruses, most commonly coxsackievirus A16 (CV-A16) or enterovirus 71 (EV71).1 However, a newly recognized strain, CV-A6, has caused worldwide outbreaks of HFMD in both children and adults. Although less common than CV-A16 or EV71, CV-A6 is associated with a more severe disease course.1 The CV-A6 strain was first identified in Finland in 2008 during a major outbreak of HFMD; it reached the United States in 2011.2,3 Accurate statistics on the prevalence of CV-A6 in the US are difficult to obtain because HFMD is not a reportable condition.
Clinical presentation
HFMD typically manifests with painful oral lesions, with or without a macular, maculopapular, or vesicular exanthema. If a fever is present, it is usually below 101°F. The oral lesions are typically benign and manifest as erythematous macules that progress to vesicles with an erythematous halo. These lesions tend to be painful and may interfere with eating or drinking. Signs and symptoms associated with the more virulent form, CV-A6, may include
- Higher fever
- Wider distribution of lesions
- More extensive skin involvement
- Longer duration
- Palmar and plantar desquamation
- Nail dystrophy.4,5
Laboratory diagnosis
Most symptomatic enterovirus infections are diagnosed based on clinical findings alone, reducing the need for laboratory testing. Laboratory confirmation may be warranted for more severe infections and during outbreaks. Molecular methods, such as reverse transcriptase polymerase chain reaction, are typically used for identifying enteroviruses, as they are rapid, sensitive, and widely available in hospital and commercial laboratories.6 Viral culture methods are labor-intensive, expensive, and reserved for typing the isolate. Serology is not useful in the diagnosis of acute infection.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis of HFMD includes conditions with oral lesions and maculopapular, vesicular lesions involving the palms and/or soles, as well as erythroderma.
Oral lesions. Aphthous ulcers are shallow, painful oral lesions not accompanied by skin rashes. Herpes gingivostomatitis, caused by herpes simplex virus (HSV), is often preceded by a prodrome of fever. The associated lesions manifest as vesicular clusters on a red base that evolve into large, painful ulcers. HSV mouth lesions can populate the gingivae, pharynx, hard palate, lips, and perioral skin. Skin lesions may occur unilaterally.
Rashes involving palms and soles. A number of conditions manifest with skin lesions similar to those of HFMD. An autoeczematization reaction consisting of a pruritic, papulovesicular eruption secondary to dermatophyte infection (eg, tinea pedis, tinea manuum, tinea cruris, tinea corporis, tinea capitis) should be ruled out. This type of reaction is thought to be a delayed hypersensitivity response to fungal antigens. Pruritus and the absence of mouth sores distinguishes this reaction from HFMD.7
Secondary syphilis can manifest with a short-lived macular rash involving the palm and soles, as well as oral mucous patches and generalized lymphadenopathy. Syphilis testing, including rapid plasma reagin or Venereal Disease Research Laboratory test with fluorescent treponemal antibody absorption, can rule out this diagnosis.
Erythema multiforme, which is more common in young adults, is characterized by target lesions on the palms and soles and erosions and/or bullae in the mouth and mucous membranes. It is usually preceded by a trigger, such as HSV infection.
Continue to: Erythroderma
Erythroderma. In addition to a rash, erythema multiforme can cause desquamation later in the disease course. Toxic shock syndrome (TSS), a life-threatening condition caused by Streptococcus or Staphylococcus, has an abrupt onset that is associated with high fever and hypotension. TSS causes a sunburn-like rash on the palms and soles that desquamates weeks after onset. Scarlet fever, caused by group A Streptococcus, can cause an erythematous rash with desquamation in children and adolescents. Scalded skin syndrome is a desquamative condition caused by Staphylococcus that occurs primarily in infants and young children.
Management
There is no specific antiviral treatment for HFMD, and thus management is mainly supportive. Fever and pain can be managed with ibuprofen or acetaminophen. Children who are unable to maintain oral hydration may require hospitalization for IV fluids.
Prevention of HFMD requires strict hand hygiene—washing with soap and water—as well as thoroughly cleaning and disinfecting surfaces that come in contact with infected oral secretions or feces.8
OUTCOME FOR THE CASE PATIENT
The patient was discharged and instructed to take ibuprofen as needed.

About three weeks later, the patient’s palms and soles began to peel. Clinical examination at follow-up revealed painful, diffuse, scaly desquamation of the hands and feet (see Figure 2). The patient also experienced loosening and shedding of the proximal nails (see Figure 3). She was diagnosed with postviral shedding.

Continue to: About eight weeks after the desquamatory rash manifested...
About eight weeks after the desquamatory rash manifested, complete resolution was seen. The patient experienced continued onychom
CONCLUSION
Clinicians should be mindful of the increasing incidence of HFMD in the adult population, since it may mimic other disease states. The extent and chronicity of this patient’s clinical manifestations were unusual and may have been caused by CV-A6.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
1. Ben-Chetrit E, Wiener-Well Y, Shulman LM, et al. Coxsackievirus A6-related hand foot and mouth disease: skin manifestations in a cluster of adult patients. J Clin Virol. 2014;59(3):201-203.
2. Blomqvist S, Klemola P, Kajalainen S, et al. Co-circulation of coxsackie viruses A6 and A10 in hand, foot, mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49-54.
3. Downing C, Ramirez-Fort MK, Doan HQ, et al. Coxsackievirus A6 associated hand, foot and mouth disease in adults: clinical presentation and review of the literature. J Clin Virol. 2014;60(4):381-386.
4. Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69(5):736-741.
5. Feder HM Jr, Bennett N, Modlin JF. Atypical hand, foot, and mouth disease: a vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14(1):83-86.
6. Pozo F, Casas I, Tenorio A, et al. Evaluation of a commercially available reverse transcription-PCR assay for diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J Clin Microbiol. 1998;36(6):1741-1745.
7. Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128(2):e453-e457.
8. Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4):e898- e904.
A postgraduate tour through the biliary tree, pancreas, and liver
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
For the pancreatobiliary session, Michelle Ann Anderson, MD, of the University of Michigan, Ann Arbor, reminded us about appropriate patient selection given the risk of pancreatitis after endoscopic retrograde cholangiopancreatography pancreatitis, also known as post-ERCP pancreatitis. Strategies to prevent post-ERCP pancreatitis include using pancreatic duct stents and using wire rather than contrast for cannulation. She recommended rectal indomethacin for all patients. Because of encouraging data, she recommended 2-3 L of lactated Ringer’s solution during the procedure and recovery.
Katie Morgan, MD, from the Medical University of South Carolina, Charleston, reviewed her group’s experience with 195 total pancreatectomies with islet autotransplants for chronic pancreatitis. Quality of life improved with major reductions in narcotic use, and 25% of patients were insulin free.
Bret Petersen, MD, of Mayo Clinic, Rochester, Minn., discussed multidrug resistant infection in ERCP endoscopes. He reminded us of the risk of lapses in endoscope reprocessing steps and the need for monitoring. He commented on recent Food and Drug Administration’s culture guidance and new technologies in development.
James Scheiman, MD, from the University of Virginia, Charlottesville, discussed pancreatic cysts. He reviewed the controversy between the more conservative American Gastroenterological Association guidelines and the more aggressive International Consensus guidelines. He advised considering patient preferences with a multidisciplinary approach.
For the liver session, Guadalupe García-Tsao, MD, of Yale University, New Haven, Conn., discussed the controversy regarding nonselective beta-blockers. She advised caution if refractory ascites are present because of risk for renal dysfunction, but she also highlighted the benefits including reduced first and recurrent variceal hemorrhage.
Rohit Loomba, MD, from the University of California at San Diego addressed fibrosis assessments in fatty liver. In his algorithm, patients with low Nonalcoholic Fatty Liver Disease Fibrosis Score or Fibrosis-4 scores would have continued observation, while patients with medium or high scores would undergo transient elastography or magnetic resonance elastography.
Patrick Northup, MD, from the University of Virginia discussed anticoagulation for portal vein thrombosis. He also discussed consideration of transjugular intrahepatic portosystemic shunt if there are high-risk varices. Duration of anticoagulation is controversial, but this strategy may prevent decompensation and affect transplant outcomes.
Daryl Lau, MD, MSc, MPH, from Harvard Medical School, Boston, reviewed the hepatitis B virus therapy controversy for e-antigen–negative patients with prolonged viral suppression. She recommended caution in general and emphasized that stage 3-4 fibrosis patients should not discontinue therapy.
The final talk was my review of hepatitis C virus treatment. I emphasized that pretreatment fibrosis assessments are critical given continued risk of hepatocellular carcinoma after cure. Challenges include identifying the remaining patients and supporting them through treatment. HCV therapies demonstrate what is possible when breakthroughs are translated to clinical care, and I was honored to participate in this course that highlighted many advances in our field.
Dr. Muir is a professor of medicine, director of gastroenterology & hepatology research at Duke Clinical Research Institute, and chief of the division of gastroenterology in the department of medicine at Duke University, all in Durham, N.C. He has received research grants from and served on the advisory boards for AbbVie, Gilead Sciences, Merck, and several other pharmaceutical companies. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
Treatment of inflammatory bowel disease
The inflammatory bowel disease (IBD) session covered optimal ways to initiate treatment for ulcerative colitis and Crohn’s disease, when and how to discontinue some of those treatments, and the use of biosimilars as substitutes for biologic therapies. Finally, suggestions were provided regarding the often-challenging identification of irritable bowel complaints in patients with IBD.
Uma Mahadevan, MD, opened by reviewing important patient factors to consider, such as disease type and severity, comorbidities, and previous therapies, before the initiation of anti-inflammatory, immunomodulatory, and/or biologic therapies in patients with IBD. She underlined the importance of risk stratification in selecting optimal therapies as well as the role of steroid-sparing agents, and she discussed the preferability of a “treat to target” strategy that emphasizes clinical, laboratory, and endoscopic response. Finally, Dr. Mahadevan, of the University of California, San Francisco, provided an overview of the role of tofacitinib (Xeljanz), which was recently approved for the treatment of moderate to severe ulcerative colitis.
Millie D. Long, MD, MPH, discussed the clinical challenges associated with stopping biologic and/or immunomodulator therapy in selected patients with IBD. Long-term use of immunomodulators and biologics can be associated with increased risk of malignancy. Dr. Long, of the University of North Carolina, Chapel Hill, reviewed data suggesting that approximately 50% of patients will relapse within 2 years of withdrawing from either class of agents. For patients on dual therapy, similar rates are seen on withdrawal of biologics alone, although most patients can be brought back under control with drug reinitiation. In general, withdrawal is predicted to be most effective in patients who have had a durable response for at least 6-12 months with clinical, laboratory, and endoscopic evidence of remission.
Around the world, use of biosimilar anti–tumor necrosis factor agents is growing. Peter Lakatos, MD, PhD, AGAF, director of IBD Centre at McGill University, Montreal, reviewed the current literature, which demonstrates very similar clinical performance between the first generation of biosimilars and more traditional agents from this class. Food and Drug Administration approval in the United States has been slower than elsewhere. However, it is expected that multiple agents already used or under development outside this country will be available in the United States in the next several years. Biosimilars have already captured 50% of the European market and, because of cost considerations, their use is mandatory in some non-U.S. settings.
In some patients, differentiating an IBD flare from superimposed irritable bowel syndrome symptoms can be difficult. Charles Bernstein, MD, of the University of Manitoba (Canada), emphasized the importance of exploring perceived stress in the life of patients with IBD. Stress can be the dominant factor driving clinical complaints in the absence of identifiable increases in inflammatory activity, he explained.
Dr. Weinberg is chairman of the department of medicine, Fox Chase Cancer Center, Philadelphia. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
The inflammatory bowel disease (IBD) session covered optimal ways to initiate treatment for ulcerative colitis and Crohn’s disease, when and how to discontinue some of those treatments, and the use of biosimilars as substitutes for biologic therapies. Finally, suggestions were provided regarding the often-challenging identification of irritable bowel complaints in patients with IBD.
Uma Mahadevan, MD, opened by reviewing important patient factors to consider, such as disease type and severity, comorbidities, and previous therapies, before the initiation of anti-inflammatory, immunomodulatory, and/or biologic therapies in patients with IBD. She underlined the importance of risk stratification in selecting optimal therapies as well as the role of steroid-sparing agents, and she discussed the preferability of a “treat to target” strategy that emphasizes clinical, laboratory, and endoscopic response. Finally, Dr. Mahadevan, of the University of California, San Francisco, provided an overview of the role of tofacitinib (Xeljanz), which was recently approved for the treatment of moderate to severe ulcerative colitis.
Millie D. Long, MD, MPH, discussed the clinical challenges associated with stopping biologic and/or immunomodulator therapy in selected patients with IBD. Long-term use of immunomodulators and biologics can be associated with increased risk of malignancy. Dr. Long, of the University of North Carolina, Chapel Hill, reviewed data suggesting that approximately 50% of patients will relapse within 2 years of withdrawing from either class of agents. For patients on dual therapy, similar rates are seen on withdrawal of biologics alone, although most patients can be brought back under control with drug reinitiation. In general, withdrawal is predicted to be most effective in patients who have had a durable response for at least 6-12 months with clinical, laboratory, and endoscopic evidence of remission.
Around the world, use of biosimilar anti–tumor necrosis factor agents is growing. Peter Lakatos, MD, PhD, AGAF, director of IBD Centre at McGill University, Montreal, reviewed the current literature, which demonstrates very similar clinical performance between the first generation of biosimilars and more traditional agents from this class. Food and Drug Administration approval in the United States has been slower than elsewhere. However, it is expected that multiple agents already used or under development outside this country will be available in the United States in the next several years. Biosimilars have already captured 50% of the European market and, because of cost considerations, their use is mandatory in some non-U.S. settings.
In some patients, differentiating an IBD flare from superimposed irritable bowel syndrome symptoms can be difficult. Charles Bernstein, MD, of the University of Manitoba (Canada), emphasized the importance of exploring perceived stress in the life of patients with IBD. Stress can be the dominant factor driving clinical complaints in the absence of identifiable increases in inflammatory activity, he explained.
Dr. Weinberg is chairman of the department of medicine, Fox Chase Cancer Center, Philadelphia. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
The inflammatory bowel disease (IBD) session covered optimal ways to initiate treatment for ulcerative colitis and Crohn’s disease, when and how to discontinue some of those treatments, and the use of biosimilars as substitutes for biologic therapies. Finally, suggestions were provided regarding the often-challenging identification of irritable bowel complaints in patients with IBD.
Uma Mahadevan, MD, opened by reviewing important patient factors to consider, such as disease type and severity, comorbidities, and previous therapies, before the initiation of anti-inflammatory, immunomodulatory, and/or biologic therapies in patients with IBD. She underlined the importance of risk stratification in selecting optimal therapies as well as the role of steroid-sparing agents, and she discussed the preferability of a “treat to target” strategy that emphasizes clinical, laboratory, and endoscopic response. Finally, Dr. Mahadevan, of the University of California, San Francisco, provided an overview of the role of tofacitinib (Xeljanz), which was recently approved for the treatment of moderate to severe ulcerative colitis.
Millie D. Long, MD, MPH, discussed the clinical challenges associated with stopping biologic and/or immunomodulator therapy in selected patients with IBD. Long-term use of immunomodulators and biologics can be associated with increased risk of malignancy. Dr. Long, of the University of North Carolina, Chapel Hill, reviewed data suggesting that approximately 50% of patients will relapse within 2 years of withdrawing from either class of agents. For patients on dual therapy, similar rates are seen on withdrawal of biologics alone, although most patients can be brought back under control with drug reinitiation. In general, withdrawal is predicted to be most effective in patients who have had a durable response for at least 6-12 months with clinical, laboratory, and endoscopic evidence of remission.
Around the world, use of biosimilar anti–tumor necrosis factor agents is growing. Peter Lakatos, MD, PhD, AGAF, director of IBD Centre at McGill University, Montreal, reviewed the current literature, which demonstrates very similar clinical performance between the first generation of biosimilars and more traditional agents from this class. Food and Drug Administration approval in the United States has been slower than elsewhere. However, it is expected that multiple agents already used or under development outside this country will be available in the United States in the next several years. Biosimilars have already captured 50% of the European market and, because of cost considerations, their use is mandatory in some non-U.S. settings.
In some patients, differentiating an IBD flare from superimposed irritable bowel syndrome symptoms can be difficult. Charles Bernstein, MD, of the University of Manitoba (Canada), emphasized the importance of exploring perceived stress in the life of patients with IBD. Stress can be the dominant factor driving clinical complaints in the absence of identifiable increases in inflammatory activity, he explained.
Dr. Weinberg is chairman of the department of medicine, Fox Chase Cancer Center, Philadelphia. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2018.
Eosinophilic esophagitis: Faces and facets of a new disease
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Patient-reported outcomes in esophageal diseases
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).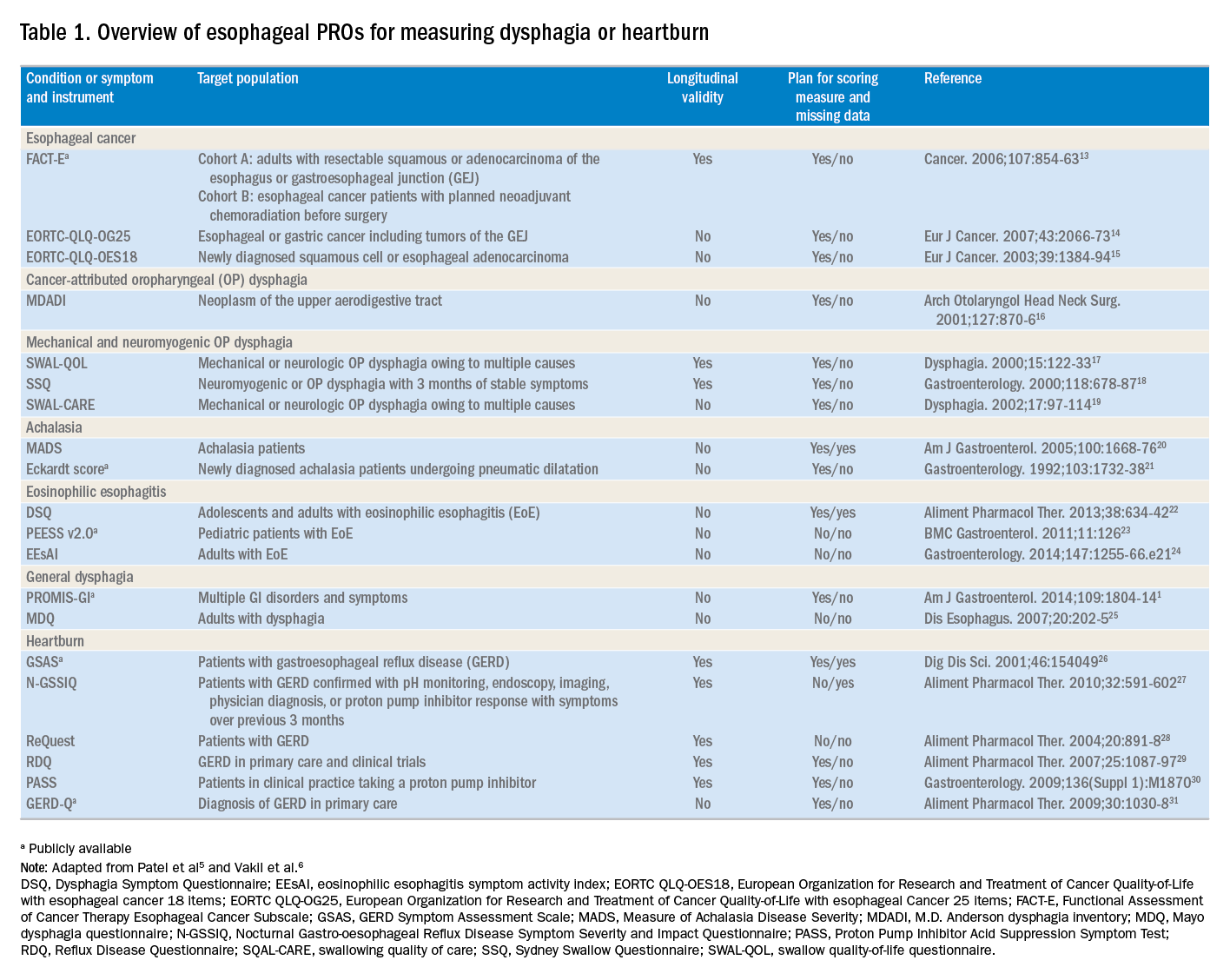
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
In my introductory comments to the practice management section last year, I wrote about cultivating competencies for value-based care. One of the key competencies was patient centeredness. Patient-reported outcomes (PROs) and patient experience measures specifically were highlighted as examples of meaningful tools for achieving patient centeredness. Starting with this month’s contribution by Drs Reed and Dellon on PROs in esophageal disease, we begin a series of articles focused on this important construct. We will follow this article with reports focused on PRO for patients with irritable bowel syndrome, inflammatory bowel disease, and chronic liver disease. These reports will not only review the importance of PROs, but also highlight the most practical approaches to measuring disease-specific PROs in clinical practice all with the goal of improving the care of our patients.
Ziad Gellad, MD, MPH, AGAF, Special Section Editor
Patients seek medical care for symptoms affecting their quality of life,1 and this is particularly true of digestive diseases, in which many common conditions are symptom predominant. However, clinician and patient perception of symptoms often conflict,2 and formalized measurement tools may have a role for optimizing symptom assessment. Patient-reported outcomes (PROs) directly capture patients’ health status from their own perspectives and can bridge the divide between patient and provider interpretation. The US Food and Drug Administration (FDA) defines PROs as “any report of the status of a patient’s health condition that comes directly from the patient without interpretation of the patient’s response by a clinician or anyone else.”3
For the clinical assessment of esophageal diseases, existing physiologic and structural testing modalities cannot ascertain patient disease perception or measure the impact of symptoms on health care–associated quality of life. In contrast, by capturing patient-centric data, PROs can provide insight into the psychosocial aspects of patient disease perceptions; capture health-related quality of life (HRQL); improve provider understanding; highlight discordance between physiologic, symptom, and HRQL measures; and formalize follow-up evaluation of treatment response.1,4 Following up symptoms such as dysphagia or heartburn over time in a structured way allows clinically obtained data to be used in pragmatic or comparative effectiveness studies. PROs are now an integral part of the FDA’s drug approval process.
In this article, we review the available PROs capturing esophageal symptoms with a focus on dysphagia and heartburn measures that were developed with rigorous methodology; it is beyond the scope of this article to perform a thorough review of all upper gastrointestinal (GI) PROs or quality-of-life PROs. We then discuss how esophageal PROs may be incorporated into clinical practice now, as well as opportunities for PRO use in the future.
Esophageal symptom-specific patient-reported outcomes
The literature pertinent to upper GI and esophageal-specific PROs is heterogeneous, and the development of PROs has been variable in rigor. Two recent systematic reviews identified PROs pertinent to dysphagia and heartburn (Table 1) and both emphasized rigorous measures developed in accordance with FDA guidance.3
Patel et al5 identified 34 dysphagia-specific PRO measures, of which 10 were rigorously developed (Table 1). These measures encompassed multiple conditions including esophageal cancer (Functional Assessment of Cancer Therapy Esophageal Cancer Subscale, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal Cancer 25 items, European Organization for Research and Treatment of Cancer Quality-of-Life with esophageal cancer 18 items, upper aerodigestive neoplasm-attributable oropharyngeal dysphagia [M.D. Anderson dysphagia inventory], mechanical and neuromyogenic oropharyngeal dysphagia [swallow quality-of-life questionnaire], Sydney Swallow Questionnaire, [swallowing quality of care], achalasia [Measure of Achalasia Disease Severity], eosinophilic esophagitis [Dysphagia Symptom Questionnaire], and general dysphagia symptoms and gastroesophageal reflux [Patient-Reported Outcomes Measurement Information System Gastrointestinal Symptom Scales (PROMIS-GI)]. PROMIS-GI, produced as part of the National Institutes of Health PROMIS program, includes rigorous measures for general dysphagia symptoms and gastroesophageal reflux in addition to lower gastrointestinal symptom measures.
The systematic review by Vakil et al6 found 15 PRO measures for gastroesophageal reflux disease (GERD) symptoms that underwent psychometric evaluation (Table 1). Of these, 5 measures were devised according to the developmental steps stipulated by the US FDA and the European Medicines Agency, and each measure has been used as an end point for a clinical trial. The 5 measures include the GERD Symptom Assessment Scale, the Nocturnal Gastro-oesophageal Reflux Disease Symptom Severity and Impact Questionnaire, the Reflux Questionnaire, the Reflux Disease Questionnaire, and the Proton Pump Inhibitor Acid Suppression Symptom Test (Table 1). Additional PROs capturing esophageal symptoms include the eosinophilic esophagitis symptom activity index, Eckardt score (used for achalasia), Mayo dysphagia questionnaire, and GERD-Q (Table 1).
Utilization of esophageal patient-reported outcomes in practice
Before incorporating a PRO into clinical practice, providers must appreciate the construct(s), intent, developmental measurement properties, validation strategies, and responsiveness characteristics associated with the measure.4 PROs can be symptom- and/or condition-specific. For example, this could include dysphagia associated with achalasia or eosinophilic esophagitis, postoperative dysphagia from spine surgery, or general dysphagia symptoms regardless of the etiology (Table 1). Intent refers to the context in which a PRO should be used and generally is stratified into 3 areas: population surveillance, individual patient-clinician interactions, and research studies.4 A thorough analysis of PRO developmental properties exceeds the scope of this article. However, several key considerations are worth discussing. Each measure should clearly delineate the construct, or outcome, in addition to the population used to create the measure (eg, patients with achalasia). PROs should be assessed for reliability, construct validity, and content validity. Reliability pertains to the degree in which scores are free from measurement error, the extent to which items (ie, questions) correlate, and test–retest reliability. Construct validity includes dimensionality (evidence of whether a single or multiple subscales exist in the measure), responsiveness to change (longitudinal validity), and convergent validity (correlation with additional construct-specific measures). Central to the PRO development process is the involvement of patients and content experts (content validity). PRO measures should be readily interpretable, and the handling of missing items should be stipulated. The burden, or time required for administering and scoring the instrument, and the reading level of the PRO need to be considered.8 In short, a PRO should measure something important to patients, in a way that patients can understand, and in a way that accurately reflects the underlying symptom and disease.
Although PROs traditionally represent a method for gathering data for research, they also should be viewed as a means of improving clinical care. The monitoring of change in a particular construct represents a common application of PROs in clinical practice. This helps quantify the efficacy of an intervention and can provide insight into the comparative effectiveness of alternative therapies. For example, in a patient with an esophageal stricture, a dysphagia-specific measure could be used at baseline before an endoscopy and dilation, in follow-up evaluation after dilation, and then as a monitoring tool to determine when repeat dilation may be needed. Similarly, the Eckardt score has been used commonly to monitor response to achalasia treatments. Clinicians also may use PROs in real time to optimize patient management. The data gathered from PROs may help triage patients into treatment pathways, trigger follow-up appointments, supply patient education prompts, and produce patient and provider alerts.8 For providers engaging in clinical research, PROs administered at the point of patient intake, whether electronically through a patient portal or in the clinic, provide a means of gathering baseline data.9 A key question, however, is whether it is practical to use a PRO routinely in the clinic, esophageal function laboratory, or endoscopy suite.
These practical issues include cultivating a conducive environment for PRO utilization, considering the burden of the measure on the patient, and utilization of the results in an expedient manner.9 To promote seamless use of a PRO in clinical work-flows, a multimodal means of collecting PRO data should be arranged. Electronic PROs available through a patient portal, designed with a user-friendly and intuitive interface, facilitate patient completion of PROs at their convenience, and ideally before a clinical or procedure visit. For patients without access to the internet, tablets and/or computer terminals within the office are convenient options. Nurses or clinic staff also could help patients complete a PRO during check-in for clinic, esophageal testing, or endoscopy. The burden a PRO imposes on patients also limits the utility of a measure. For instance, PROs with a small number of questions are more likely to be completed, while scales consisting of 30 of more items are infrequently finished. Clinicians also should consider how they plan to use the results of a PRO before implementing one; if the data will not be used, then the effort to implement and collect it will be wasted. Moreover, patients will anticipate that the time required to complete a PRO will translate to an impact on their management plan and will more readily complete additional PROs if previous measures expediently affected their care.9
Barriers to patient-reported outcome implementation and future directions
Given the potential benefits to PRO use, why are they not implemented routinely? In practice, there are multiple barriers that thwart the adoption of PROs into both health care systems and individual practices. The integration of PROs into large health care systems languishes partly because of technological and operational barriers.9 For instance, the manual distribution, collection, and transcription of handwritten information requires substantial investitures of time, which is magnified by the number of patients whose care is provided within a large health system. One approach to the technological barrier includes the creation of an electronic platform integrating with patient portals. Such a platform would obviate the need to manually collect and transcribe documents, and could import data directly into provider documentation and flowsheets. However, the programming time and costs are substantial upfront, and without clear data that this could lead to improved outcomes or decreased costs downstream there may be reluctance to devote resources to this. In clinical practice, the already significant demands on providers’ time mitigates enthusiasm to add additional tasks. Providers also could face annual licensing agreements, fees on a per-study basis, or royalties associated with particular PROs, and at the individual practice level, there may not be appropriate expertise to select and implement routine PRO monitoring. To address this, efforts are being made to simplify the process of incorporating PROs. For example, given the relatively large number of heterogeneous PROs, the PROMIS project1 endeavors to clarify which PROs constitute the best measure for each construct and condition.9 The PROMIS measures also are provided publicly and are available without license or fee.
Areas particularly well situated for growth in the use of PRO measures include comparative effectiveness studies and pragmatic clinical trials. PRO-derived data may promote a shift from explanatory randomized controlled trials to pragmatic randomized controlled trials because these data emphasize patient-centered care and are more broadly generalizable to clinical settings. Furthermore, the derivation of data directly from the health care delivery system through PROs, such as two-way text messages, increases the relevance and cost effectiveness of clinical trials. Given the current medical climate, pressures continue to mount to identify cost-efficient and efficacious medical therapies.10 In this capacity, PROs facilitate the understanding of changes in HRQL domains subject to treatment choices. PROs further consider the comparative symptom burden and side effects associated with competing treatment strategies.11 Finally, PROs also have enabled the procurement of data from patient-powered research networks. Although this concept has not yet been applied to esophageal diseases, one example of this in the GI field is the Crohn’s and Colitis Foundation of America Partners project, which has built an internet cohort consisting of approximately 14,200 inflammatory bowel disease patients who are monitored with a series of PROs.12 An endeavor such as this should be a model for esophageal conditions in the future.
Conclusions
PROs, as a structured means of directly assessing symptoms, help facilitate a provider’s understanding from a patient’s perspectives. Multiple PROs have been developed to characterize constructs pertinent to esophageal diseases and symptoms. These vary in methodologic rigor, but multiple well-constructed PROs exist for symptom domains such as dysphagia and heartburn, and can be used to monitor symptoms over time and assess treatment efficacy. Implementation of esophageal PROs, both in large health systems and in routine clinical practice, is not yet standard and faces a number of barriers. However, the potential benefits are substantial and include increased patient-centeredness, more accurate and timely disease monitoring, and applicability to comparative effectiveness studies, pragmatic clinical trials, and patient-powered research networks.
References
1. Spiegel B., Hays R., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
2. Chassany O., Shaheen N.J., Karlsson M., et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-21.
3. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. Available from:http://www.ncbi.nlm.nih.gov/pubmed/17034633%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1629006
Accessed May 23, 2017
4. Lipscomb J. Cancer outcomes research and the arenas of application. J Natl Cancer Inst Monogr. 2004;2004:1-7.
5. Patel D.A., Sharda R., Hovis K.L., et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1-23.
6. Vakil N.B., Halling K., Becher A., et al. Systematic review of patient-reported outcome instruments for gastroesophageal reflux disease symptoms. Eur J Gastroenterol Hepatol. 2013;25:2-14.
7. Bedell A., Taft T.H., Keefer L. Development of the Northwestern Esophageal Quality of Life Scale: a hybrid measure for use across esophageal conditions. Am J Gastroenterol. 2016;111:493-9.
8. Farnik M., Pierzchala W. Instrument development and evaluation for patient-related outcomes assessments. Patient Relat Outcome Meas. 2012;3:1-7.
9. Wagle N.W.. Implementing patient-reported outcome measures (PROMs). N Engl J Med Catal. 2016; :1-2. Available from:
http://catalyst.nejm.org/implementing-proms-patient-reported-outcome-measures/. Accessed July 14, 2017
10. Richesson R.L., Hammond W.E., Nahm M., et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Informatics Assoc. 2013;20: e226-e231.
11. Coon C.D., McLeod L.D. Patient-reported outcomes: current perspectives and future directions. Clin Ther. 2013;35:399-401.
12. Chung A.E., Sandler R.S., Long M.D., et al. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn’s and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners)
J Am Med Informatics Assoc. 2016;23:485-90.
13. Darling G., Eton D.T., Sulman J., et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854-63.
14. Lagergren P., Fayers P., Conroy T., et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer. 2007;43:2066-73.
15. Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
16. Chen A.Y., Frankowski R., Bishop-Leone J., et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870-6.
17. McHorney C.A., Bricker D.E., Robbins J., et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. item reduction and preliminary scaling. Dysphagia. 2000;15:122-33.
18. Wallace K.L., Middleton S., Cook I.J. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678-87.
19. McHorney C.A., Robbins J.A., Lomax K., et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97-114.
20. Urbach D.R., Tomlinson G.A., Harnish J.L., et al. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-76.
21. Eckardt V., Aignherr C., Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-8.
22. Dellon E.S., Irani A.M., Hill M.R., et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634-42.
23. Franciosi J.P., Hommel K., DeBrosse C.W., et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. 2011;11:126.
24. Schoepfer A.M., Straumann A., Panczak R., et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1-24.
25. Grudell A.B., Alexander J.A., Enders F.B., et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus. 2007;20:202-5.
26. Rothman M., Farup C., Steward W., et al. Symptoms associated with gastroesophageal reflux disease: Development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540-9.
27. Spiegel B.M., Roberts L., Mody R., et al. The development and validation of a nocturnal gastro-oesophageal reflux disease symptom severity and impact questionnaire for adults. Aliment Pharmacol Ther. 2010;32:591-602.
28. Bardhan K.D., Stanghellini V., Armstrong D., et al. International validation of ReQuest in patients with endoscopy-negative gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20:891-8.
29. Van Zanten S.V., Armstrong D., Barkun A., et al. Symptom overlap in patients with upper gastrointestinal complaints in the Canadian confirmatory acid suppression test (CAST) study: Further psychometric validation of the reflux disease questionnaire. Aliment Pharmacol Ther. 2007;25:1087-97.
30. Armstrong D., Moayyedi P., Hunt R., et al. M1870 resolution of persistent GERD symptoms after a change in therapy: EncomPASS - a cluster-randomized study in primary care. Gastroenterology. 2009;136(Suppl 1):A-435.
31. Jones R., Junghard O., Dent J., et al. Developement of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-8.
Dr. Reed is a senior fellow and Dr. Dillon is an associate professor of medicine and epidemiology, Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; he has been a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, and Shire; and has received an educational grant from Banner and Holoclara.
Delayed diagnosis of breast cancer: $15M award
Delayed diagnosis of breast cancer: $15M award
A woman in her mid-50s had been seen by a breast surgeon for 16 years for regular mammograms and sonograms. In May 2009, the breast surgeon misinterpreted a mammogram as negative, as did a radiologist who re-read the mammogram weeks later. In December 2010, the patient returned to the breast surgeon with nipple discharge. No further testing was conducted. In October 2011, the patient was found to have Stage IIIA breast cancer involving 4 lymph nodes. She underwent left radical mastectomy, chemotherapy, radiation therapy, and breast reconstruction. At time of trial, the cancer had invaded her vertebrae, was Stage IV, and most likely incurable.
PATIENT'S CLAIM: Although the surgeon admittedly did not possess the qualifications required under the Mammography Quality Standards Act, he interpreted about 5,000 mammograms per year in his office. In this case, he failed to detect a small breast tumor in May 2009. He also failed to perform testing when the patient reported nipple discharge. A more timely diagnosis of breast cancer at Stage I would have provided a 90% chance of long-term survival.
DEFENDANTS' DEFENSE: The defense held the radiologist fully liable because the surgeon was not a qualified interpreter of mammography, therefore relying on the radiologist’s interpretation. The radiologist was legally responsible for the missed diagnosis.
VERDICT: A $15M New York verdict was reached, finding the breast surgeon 75% at fault and the radiologist 25%. The radiologist settled before the trial (the jury was not informed of this). The breast surgeon was responsible for $11.25M. The defense indicated intent to appeal.
Alleged failure to evacuate uterus after cesarean delivery
A 37-year-old woman underwent cesarean delivery (CD) performed by 2 ObGyns. After delivery, she began to hemorrhage and the uterus became atonic. Hysterectomy was performed but the bleeding did not stop. The ObGyns called in 3 other ObGyns. During exploratory laparotomy, the bleeding was halted.
PATIENT'S CLAIM: She and her husband had hoped to have more children but the hysterectomy precluded that. She sued all 5 ObGyns, alleging that the delivering ObGyns failed to properly perform the CD and that each physician failed to properly perform the laparotomy, causing a large scar. The claim was discontinued against the 3 surgical ObGyns; trial addressed the 2 delivering ObGyns.
The patient’s expert ObGyn remarked that the hemorrhage was caused by a small placental remnant that remained in the uterus as a result of inadequate evacuation following delivery. The presence of the remnant was indicated by the uterine atony and should have prompted immediate investigation. The physicians’ notes did not document exploration of the uterus prior to closure.
PHYSICIAN'S DEFENSE: The defense’s expert contended that atony would not be a result of a small remnant of placenta. The patient’s uterus was properly evacuated, the hemorrhage was an unforeseeable complication, and the ObGyns properly addressed the hemorrhage.
VERDICT: A New York defense verdict was returned.
Alleged bowel injury during hysterectomy
Two days after a woman underwent a hysterectomy performed by her ObGyn, she went to the emergency department with increasing pain. Her ObGyn admitted her to the hospital. A general surgeon performed an exploratory laparotomy the next day that revealed an abscess; a 1-cm perforation of the patient’s bowel was surgically repaired. The patient had a difficult recovery. She developed pneumonia and respiratory failure. She underwent multiple repair surgeries for recurrent abscesses and fistulas because the wound was slow to heal.
PATIENT'S CLAIM: The ObGyn’s surgical technique was negligent. He injured the bowel when inserting a trocar and did not identify the injury in a timely manner. The expert witness commented that such an injury can sometimes be a surgical complication, but not in this case: the ObGyn rushed the procedure because he had another patient waiting for CD at another hospital.
PHYSICIAN'S DEFENSE: The ObGyn denied negligence and contended that the trocar used in surgery was too blunt to have caused a perforation. It would have been obvious to the ObGyn during surgery if a perforation had occurred. The perforation developed days after surgery within an abscess.
VERDICT: A Mississippi defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Delayed diagnosis of breast cancer: $15M award
A woman in her mid-50s had been seen by a breast surgeon for 16 years for regular mammograms and sonograms. In May 2009, the breast surgeon misinterpreted a mammogram as negative, as did a radiologist who re-read the mammogram weeks later. In December 2010, the patient returned to the breast surgeon with nipple discharge. No further testing was conducted. In October 2011, the patient was found to have Stage IIIA breast cancer involving 4 lymph nodes. She underwent left radical mastectomy, chemotherapy, radiation therapy, and breast reconstruction. At time of trial, the cancer had invaded her vertebrae, was Stage IV, and most likely incurable.
PATIENT'S CLAIM: Although the surgeon admittedly did not possess the qualifications required under the Mammography Quality Standards Act, he interpreted about 5,000 mammograms per year in his office. In this case, he failed to detect a small breast tumor in May 2009. He also failed to perform testing when the patient reported nipple discharge. A more timely diagnosis of breast cancer at Stage I would have provided a 90% chance of long-term survival.
DEFENDANTS' DEFENSE: The defense held the radiologist fully liable because the surgeon was not a qualified interpreter of mammography, therefore relying on the radiologist’s interpretation. The radiologist was legally responsible for the missed diagnosis.
VERDICT: A $15M New York verdict was reached, finding the breast surgeon 75% at fault and the radiologist 25%. The radiologist settled before the trial (the jury was not informed of this). The breast surgeon was responsible for $11.25M. The defense indicated intent to appeal.
Alleged failure to evacuate uterus after cesarean delivery
A 37-year-old woman underwent cesarean delivery (CD) performed by 2 ObGyns. After delivery, she began to hemorrhage and the uterus became atonic. Hysterectomy was performed but the bleeding did not stop. The ObGyns called in 3 other ObGyns. During exploratory laparotomy, the bleeding was halted.
PATIENT'S CLAIM: She and her husband had hoped to have more children but the hysterectomy precluded that. She sued all 5 ObGyns, alleging that the delivering ObGyns failed to properly perform the CD and that each physician failed to properly perform the laparotomy, causing a large scar. The claim was discontinued against the 3 surgical ObGyns; trial addressed the 2 delivering ObGyns.
The patient’s expert ObGyn remarked that the hemorrhage was caused by a small placental remnant that remained in the uterus as a result of inadequate evacuation following delivery. The presence of the remnant was indicated by the uterine atony and should have prompted immediate investigation. The physicians’ notes did not document exploration of the uterus prior to closure.
PHYSICIAN'S DEFENSE: The defense’s expert contended that atony would not be a result of a small remnant of placenta. The patient’s uterus was properly evacuated, the hemorrhage was an unforeseeable complication, and the ObGyns properly addressed the hemorrhage.
VERDICT: A New York defense verdict was returned.
Alleged bowel injury during hysterectomy
Two days after a woman underwent a hysterectomy performed by her ObGyn, she went to the emergency department with increasing pain. Her ObGyn admitted her to the hospital. A general surgeon performed an exploratory laparotomy the next day that revealed an abscess; a 1-cm perforation of the patient’s bowel was surgically repaired. The patient had a difficult recovery. She developed pneumonia and respiratory failure. She underwent multiple repair surgeries for recurrent abscesses and fistulas because the wound was slow to heal.
PATIENT'S CLAIM: The ObGyn’s surgical technique was negligent. He injured the bowel when inserting a trocar and did not identify the injury in a timely manner. The expert witness commented that such an injury can sometimes be a surgical complication, but not in this case: the ObGyn rushed the procedure because he had another patient waiting for CD at another hospital.
PHYSICIAN'S DEFENSE: The ObGyn denied negligence and contended that the trocar used in surgery was too blunt to have caused a perforation. It would have been obvious to the ObGyn during surgery if a perforation had occurred. The perforation developed days after surgery within an abscess.
VERDICT: A Mississippi defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Delayed diagnosis of breast cancer: $15M award
A woman in her mid-50s had been seen by a breast surgeon for 16 years for regular mammograms and sonograms. In May 2009, the breast surgeon misinterpreted a mammogram as negative, as did a radiologist who re-read the mammogram weeks later. In December 2010, the patient returned to the breast surgeon with nipple discharge. No further testing was conducted. In October 2011, the patient was found to have Stage IIIA breast cancer involving 4 lymph nodes. She underwent left radical mastectomy, chemotherapy, radiation therapy, and breast reconstruction. At time of trial, the cancer had invaded her vertebrae, was Stage IV, and most likely incurable.
PATIENT'S CLAIM: Although the surgeon admittedly did not possess the qualifications required under the Mammography Quality Standards Act, he interpreted about 5,000 mammograms per year in his office. In this case, he failed to detect a small breast tumor in May 2009. He also failed to perform testing when the patient reported nipple discharge. A more timely diagnosis of breast cancer at Stage I would have provided a 90% chance of long-term survival.
DEFENDANTS' DEFENSE: The defense held the radiologist fully liable because the surgeon was not a qualified interpreter of mammography, therefore relying on the radiologist’s interpretation. The radiologist was legally responsible for the missed diagnosis.
VERDICT: A $15M New York verdict was reached, finding the breast surgeon 75% at fault and the radiologist 25%. The radiologist settled before the trial (the jury was not informed of this). The breast surgeon was responsible for $11.25M. The defense indicated intent to appeal.
Alleged failure to evacuate uterus after cesarean delivery
A 37-year-old woman underwent cesarean delivery (CD) performed by 2 ObGyns. After delivery, she began to hemorrhage and the uterus became atonic. Hysterectomy was performed but the bleeding did not stop. The ObGyns called in 3 other ObGyns. During exploratory laparotomy, the bleeding was halted.
PATIENT'S CLAIM: She and her husband had hoped to have more children but the hysterectomy precluded that. She sued all 5 ObGyns, alleging that the delivering ObGyns failed to properly perform the CD and that each physician failed to properly perform the laparotomy, causing a large scar. The claim was discontinued against the 3 surgical ObGyns; trial addressed the 2 delivering ObGyns.
The patient’s expert ObGyn remarked that the hemorrhage was caused by a small placental remnant that remained in the uterus as a result of inadequate evacuation following delivery. The presence of the remnant was indicated by the uterine atony and should have prompted immediate investigation. The physicians’ notes did not document exploration of the uterus prior to closure.
PHYSICIAN'S DEFENSE: The defense’s expert contended that atony would not be a result of a small remnant of placenta. The patient’s uterus was properly evacuated, the hemorrhage was an unforeseeable complication, and the ObGyns properly addressed the hemorrhage.
VERDICT: A New York defense verdict was returned.
Alleged bowel injury during hysterectomy
Two days after a woman underwent a hysterectomy performed by her ObGyn, she went to the emergency department with increasing pain. Her ObGyn admitted her to the hospital. A general surgeon performed an exploratory laparotomy the next day that revealed an abscess; a 1-cm perforation of the patient’s bowel was surgically repaired. The patient had a difficult recovery. She developed pneumonia and respiratory failure. She underwent multiple repair surgeries for recurrent abscesses and fistulas because the wound was slow to heal.
PATIENT'S CLAIM: The ObGyn’s surgical technique was negligent. He injured the bowel when inserting a trocar and did not identify the injury in a timely manner. The expert witness commented that such an injury can sometimes be a surgical complication, but not in this case: the ObGyn rushed the procedure because he had another patient waiting for CD at another hospital.
PHYSICIAN'S DEFENSE: The ObGyn denied negligence and contended that the trocar used in surgery was too blunt to have caused a perforation. It would have been obvious to the ObGyn during surgery if a perforation had occurred. The perforation developed days after surgery within an abscess.
VERDICT: A Mississippi defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When is the right time to stop treatment?
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.