User login
Conspiracy theory or delusion? 3 questions to tell them apart
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
Believing in conspiracy theories is not delusional
When many people across the country, not to mention in public office, believe that the world is run by a group of Satanic pedophiles that includes top Democrats and Hollywood elites, and that former President Trump is leading a secret mission to bring these evildoers to justice, one can’t help but ask if they’re at least to some degree mentally impaired.
Conspiracy theories are often received with psychiatric connotations; associated with paranoid plan-hatchers, and nonbelieving outsiders. But whereas theories such as QAnon strain credibility for many people, we would argue that they are likely not the product of psychosis or mental illness; nor do conspiracy theories in general represent delusions.
For one thing, surveys have consistently revealed that about 50% of the population believes in at least one conspiracy theory. Furthermore, there are several substantive differences between conspiracy theory beliefs and delusions.
Some researchers consider conspiracy theories to be “a subset of false beliefs,” but most scholars, ourselves included, do not prejudge their validity or veracity. Real-life conspiracies, such as the CIA’s MK-Ultra program, have clearly occurred throughout history.
Our central contention is that belief in conspiracy theories is distinct from psychosis, and more closely resembles extreme but subculturally sanctioned religious or political beliefs. However, the line between believing in conspiracies and being delusional becomes blurred when the believer becomes part of the conspiracy theory and feels compelled to act on the belief as part of a personal mission.
Take Edgar Maddison Welch, a 28-year-old man who firmly believed the so-called “Pizzagate” conspiracy theory – the baseless claim that Hillary Clinton and Democratic elites were running a child sex-trafficking ring out of a Washington, DC, pizzeria. Seeing himself a potential savior of children, Mr. Welch drove 350 miles to the pizza shop from his home in North Carolina in December 2016 and fired three shots from an AR-15 style rifle into a locked closet door, ultimately surrendering to police. However, on questioning he quickly conceded, “The intel on this wasn’t 100%.”
Who believes in conspiracy theories?
Given that half the population believes in at least one conspiracy theory, it should come as little surprise that there is no reliable “profile” for believers. Although some studies have suggested associations with low education, right-wing political orientation, and certain personality traits like subclinical paranoia and schizotypy, such findings have been inconsistent and may vary across specific conspiracy theory. Associations between conspiracy belief and paranoia suggest overlap within a “conspiratorial mindset,” with recent evidence that “distrust of officialdom” is a key mediator between believing in conspiracies and political ideology.
Other quantitative “cognitive quirks” reported in those who believe in conspiracies are a need for certainty and control, a need for uniqueness, illusory pattern perception, and lack of analytical thinking. It’s unclear which of these factors may represent universal cognitive explanations for conspiratorial beliefs, vs. those that might be related to specific beliefs, such as the need for certainty during times of crisis and societal upheaval, when conspiracy theories tend to flourish.
Much of the research on conspiracy theory belief is based on the questionable premise that it’s best understood at the level of the individual’s psychopathology, or the “deficit model,” as it’s called. One of us (JMP) has instead proposed a two-component model that includes social and informational contexts. The first component – epistemic mistrust – involves mistrusting conventional, “authoritative” knowledge. The second involves biased information processing and exposure to misinformation, often transmitted by word of mouth, or through social networks. With this model, believing in conspiracy theories could be conceived as involving “delusion-like beliefs,” but not frank psychosis or full-blown delusions, as one might see, for example, in schizophrenia.
Indeed, many of the cognitive characteristics associated with conspiracy theory belief are universal, continuously distributed traits, varying in quantity, rather than all-or-none variables or distinct symptoms of mental illness.
Essentially, delusions are fixed, false, usually unshared beliefs, often based on subjective “inner” experience. (One rare exception is the so-called folie à deux, in which two people appear to “share” the same delusion; however, psychiatrists have long debated whether both individuals should be considered truly delusional). The delusion’s content is often “self-referential”; i.e., focused primarily on the believer.
In contrast, conspiracy theories are usually, but not necessarily, false. They are typically shared beliefs that don’t explicitly or directly involve the believer, and are based on evidence that one finds “out there,” such as on the Internet. This speaks to the highly communal nature of so many conspiracy theories – networks of like-minded individuals reinforcing one another’s beliefs in a particular socio-cultural context.
Conspiracy theory belief, COVID-19, and medical intervention
As for medical conspiracy theories, none have flourished recently more so than those involving the COVID-19 pandemic. As a recent editorial by Stein and colleagues noted, “Some conspiratorial claims include assertions that COVID‐19 is a hoax; arguments that the virus was created artificially and spread on purpose as a bioweapon; or allegations that governments are using the emergency situation to pursue their antidemocratic goals. … Other conspiracies argued that people in power are taking advantage of the pandemic as a plan to inject microchip quantum-dot spy software and monitor people.”
Stein and colleagues make the important point that a “key difference between COVID‐19 and the 1918 flu pandemic ... is that [now] a highly interconnected world, to a great extent on social media, is setting the stage for distributing information and misinformation about COVID‐19.”
Consider the following composite vignette: Mr. A is a 70-year-old retiree with a history of COPD who has been advised by his PCP to get vaccinated against COVID-19. He is extremely reluctant to do so, fearing that “the vaccine is going to change my DNA” and “might even give me COVID.” He has heard from friends on social media that vaccine developers “faked the results” and are “in cahoots with the federal government.” Mr. A has heard “experts” declare the vaccines safe, but does not trust them. Mr. A has no psychiatric or substance abuse history, and there are no cognitive, perceptual, or other abnormalities in Mr. A’s mental status exam.
Mr. A’s beliefs qualify as a “conspiracy theory,” but probably represent widely held misconceptions about COVID-19 vaccines, as well as widespread mistrust of pharmaceutical companies and the federal government. Based on the information provided, there is no basis for concluding that Mr. A is psychotic or delusional. His beliefs appear to be the result of “epistemic mistrust” of authoritative informational accounts, biased information processing, and exposure to misinformation.
How should the physician manage and care for patients like Mr. A? Absent frank delusions, there is no role for antipsychotic medication, though . In addition to providing accurate medical information to the patient, the physician should avoid arguing, or trying to “talk the patient out of” his or her belief. Instead, the focus should be on sustaining and strengthening the physician-patient alliance, establishing an atmosphere of respect and safety, clarifying differences in trusted sources of medical information, and allowing the patient time to process the physician’s recommendations.
One-to-one engagement with health care providers has proved effective in reducing vaccine hesitancy and correcting misinformation. For patients with less fixed conspiracy theory beliefs, it may sometimes be helpful to gently offer alternative hypotheses to the patient’s conspiracy theory, using elements of cognitive-behavioral therapy (CBT). For example, a physician might ask, “Is it possible that the online source you read was mistaken about the vaccine changing your DNA?” while reminding patients that – contrary to popular belief – mRNA vaccines have been in development against cancer for several decades.
Challenging beliefs collaboratively and acknowledging areas of uncertainty, rather than confronting or arguing about false beliefs, can foster trust between physician and patient and, at the very least, open a dialogue regarding potential exposure to medical misinformation. “Inoculation” strategies that present and then dispel misinformation before patients become aware of it are among the best supported strategies for mitigating conspiracy theory belief. Ideally, physicians and health care systems should maintain an ongoing “inventory” of medical misinformation circulating online and “beat it to the punch” with reliable information.
Finally, because believing in conspiracy theories is often associated with a sense of uncertainty, and feeling that one’s life is “out of control,” medical interventions can be framed as ways of regaining control and appealing to patients’ values; for example, saying, “By getting the vaccine, you’ll be more likely to stay in good health, protect your family, and do all the things you want to do.”
Dr. Pies is professor of psychiatry and a lecturer on bioethics and humanities at State University of New York, Syracuse. Dr. Pierre is a health sciences clinical professor in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles. A version of this article first appeared on Medscape.com.
When many people across the country, not to mention in public office, believe that the world is run by a group of Satanic pedophiles that includes top Democrats and Hollywood elites, and that former President Trump is leading a secret mission to bring these evildoers to justice, one can’t help but ask if they’re at least to some degree mentally impaired.
Conspiracy theories are often received with psychiatric connotations; associated with paranoid plan-hatchers, and nonbelieving outsiders. But whereas theories such as QAnon strain credibility for many people, we would argue that they are likely not the product of psychosis or mental illness; nor do conspiracy theories in general represent delusions.
For one thing, surveys have consistently revealed that about 50% of the population believes in at least one conspiracy theory. Furthermore, there are several substantive differences between conspiracy theory beliefs and delusions.
Some researchers consider conspiracy theories to be “a subset of false beliefs,” but most scholars, ourselves included, do not prejudge their validity or veracity. Real-life conspiracies, such as the CIA’s MK-Ultra program, have clearly occurred throughout history.
Our central contention is that belief in conspiracy theories is distinct from psychosis, and more closely resembles extreme but subculturally sanctioned religious or political beliefs. However, the line between believing in conspiracies and being delusional becomes blurred when the believer becomes part of the conspiracy theory and feels compelled to act on the belief as part of a personal mission.
Take Edgar Maddison Welch, a 28-year-old man who firmly believed the so-called “Pizzagate” conspiracy theory – the baseless claim that Hillary Clinton and Democratic elites were running a child sex-trafficking ring out of a Washington, DC, pizzeria. Seeing himself a potential savior of children, Mr. Welch drove 350 miles to the pizza shop from his home in North Carolina in December 2016 and fired three shots from an AR-15 style rifle into a locked closet door, ultimately surrendering to police. However, on questioning he quickly conceded, “The intel on this wasn’t 100%.”
Who believes in conspiracy theories?
Given that half the population believes in at least one conspiracy theory, it should come as little surprise that there is no reliable “profile” for believers. Although some studies have suggested associations with low education, right-wing political orientation, and certain personality traits like subclinical paranoia and schizotypy, such findings have been inconsistent and may vary across specific conspiracy theory. Associations between conspiracy belief and paranoia suggest overlap within a “conspiratorial mindset,” with recent evidence that “distrust of officialdom” is a key mediator between believing in conspiracies and political ideology.
Other quantitative “cognitive quirks” reported in those who believe in conspiracies are a need for certainty and control, a need for uniqueness, illusory pattern perception, and lack of analytical thinking. It’s unclear which of these factors may represent universal cognitive explanations for conspiratorial beliefs, vs. those that might be related to specific beliefs, such as the need for certainty during times of crisis and societal upheaval, when conspiracy theories tend to flourish.
Much of the research on conspiracy theory belief is based on the questionable premise that it’s best understood at the level of the individual’s psychopathology, or the “deficit model,” as it’s called. One of us (JMP) has instead proposed a two-component model that includes social and informational contexts. The first component – epistemic mistrust – involves mistrusting conventional, “authoritative” knowledge. The second involves biased information processing and exposure to misinformation, often transmitted by word of mouth, or through social networks. With this model, believing in conspiracy theories could be conceived as involving “delusion-like beliefs,” but not frank psychosis or full-blown delusions, as one might see, for example, in schizophrenia.
Indeed, many of the cognitive characteristics associated with conspiracy theory belief are universal, continuously distributed traits, varying in quantity, rather than all-or-none variables or distinct symptoms of mental illness.
Essentially, delusions are fixed, false, usually unshared beliefs, often based on subjective “inner” experience. (One rare exception is the so-called folie à deux, in which two people appear to “share” the same delusion; however, psychiatrists have long debated whether both individuals should be considered truly delusional). The delusion’s content is often “self-referential”; i.e., focused primarily on the believer.
In contrast, conspiracy theories are usually, but not necessarily, false. They are typically shared beliefs that don’t explicitly or directly involve the believer, and are based on evidence that one finds “out there,” such as on the Internet. This speaks to the highly communal nature of so many conspiracy theories – networks of like-minded individuals reinforcing one another’s beliefs in a particular socio-cultural context.
Conspiracy theory belief, COVID-19, and medical intervention
As for medical conspiracy theories, none have flourished recently more so than those involving the COVID-19 pandemic. As a recent editorial by Stein and colleagues noted, “Some conspiratorial claims include assertions that COVID‐19 is a hoax; arguments that the virus was created artificially and spread on purpose as a bioweapon; or allegations that governments are using the emergency situation to pursue their antidemocratic goals. … Other conspiracies argued that people in power are taking advantage of the pandemic as a plan to inject microchip quantum-dot spy software and monitor people.”
Stein and colleagues make the important point that a “key difference between COVID‐19 and the 1918 flu pandemic ... is that [now] a highly interconnected world, to a great extent on social media, is setting the stage for distributing information and misinformation about COVID‐19.”
Consider the following composite vignette: Mr. A is a 70-year-old retiree with a history of COPD who has been advised by his PCP to get vaccinated against COVID-19. He is extremely reluctant to do so, fearing that “the vaccine is going to change my DNA” and “might even give me COVID.” He has heard from friends on social media that vaccine developers “faked the results” and are “in cahoots with the federal government.” Mr. A has heard “experts” declare the vaccines safe, but does not trust them. Mr. A has no psychiatric or substance abuse history, and there are no cognitive, perceptual, or other abnormalities in Mr. A’s mental status exam.
Mr. A’s beliefs qualify as a “conspiracy theory,” but probably represent widely held misconceptions about COVID-19 vaccines, as well as widespread mistrust of pharmaceutical companies and the federal government. Based on the information provided, there is no basis for concluding that Mr. A is psychotic or delusional. His beliefs appear to be the result of “epistemic mistrust” of authoritative informational accounts, biased information processing, and exposure to misinformation.
How should the physician manage and care for patients like Mr. A? Absent frank delusions, there is no role for antipsychotic medication, though . In addition to providing accurate medical information to the patient, the physician should avoid arguing, or trying to “talk the patient out of” his or her belief. Instead, the focus should be on sustaining and strengthening the physician-patient alliance, establishing an atmosphere of respect and safety, clarifying differences in trusted sources of medical information, and allowing the patient time to process the physician’s recommendations.
One-to-one engagement with health care providers has proved effective in reducing vaccine hesitancy and correcting misinformation. For patients with less fixed conspiracy theory beliefs, it may sometimes be helpful to gently offer alternative hypotheses to the patient’s conspiracy theory, using elements of cognitive-behavioral therapy (CBT). For example, a physician might ask, “Is it possible that the online source you read was mistaken about the vaccine changing your DNA?” while reminding patients that – contrary to popular belief – mRNA vaccines have been in development against cancer for several decades.
Challenging beliefs collaboratively and acknowledging areas of uncertainty, rather than confronting or arguing about false beliefs, can foster trust between physician and patient and, at the very least, open a dialogue regarding potential exposure to medical misinformation. “Inoculation” strategies that present and then dispel misinformation before patients become aware of it are among the best supported strategies for mitigating conspiracy theory belief. Ideally, physicians and health care systems should maintain an ongoing “inventory” of medical misinformation circulating online and “beat it to the punch” with reliable information.
Finally, because believing in conspiracy theories is often associated with a sense of uncertainty, and feeling that one’s life is “out of control,” medical interventions can be framed as ways of regaining control and appealing to patients’ values; for example, saying, “By getting the vaccine, you’ll be more likely to stay in good health, protect your family, and do all the things you want to do.”
Dr. Pies is professor of psychiatry and a lecturer on bioethics and humanities at State University of New York, Syracuse. Dr. Pierre is a health sciences clinical professor in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles. A version of this article first appeared on Medscape.com.
When many people across the country, not to mention in public office, believe that the world is run by a group of Satanic pedophiles that includes top Democrats and Hollywood elites, and that former President Trump is leading a secret mission to bring these evildoers to justice, one can’t help but ask if they’re at least to some degree mentally impaired.
Conspiracy theories are often received with psychiatric connotations; associated with paranoid plan-hatchers, and nonbelieving outsiders. But whereas theories such as QAnon strain credibility for many people, we would argue that they are likely not the product of psychosis or mental illness; nor do conspiracy theories in general represent delusions.
For one thing, surveys have consistently revealed that about 50% of the population believes in at least one conspiracy theory. Furthermore, there are several substantive differences between conspiracy theory beliefs and delusions.
Some researchers consider conspiracy theories to be “a subset of false beliefs,” but most scholars, ourselves included, do not prejudge their validity or veracity. Real-life conspiracies, such as the CIA’s MK-Ultra program, have clearly occurred throughout history.
Our central contention is that belief in conspiracy theories is distinct from psychosis, and more closely resembles extreme but subculturally sanctioned religious or political beliefs. However, the line between believing in conspiracies and being delusional becomes blurred when the believer becomes part of the conspiracy theory and feels compelled to act on the belief as part of a personal mission.
Take Edgar Maddison Welch, a 28-year-old man who firmly believed the so-called “Pizzagate” conspiracy theory – the baseless claim that Hillary Clinton and Democratic elites were running a child sex-trafficking ring out of a Washington, DC, pizzeria. Seeing himself a potential savior of children, Mr. Welch drove 350 miles to the pizza shop from his home in North Carolina in December 2016 and fired three shots from an AR-15 style rifle into a locked closet door, ultimately surrendering to police. However, on questioning he quickly conceded, “The intel on this wasn’t 100%.”
Who believes in conspiracy theories?
Given that half the population believes in at least one conspiracy theory, it should come as little surprise that there is no reliable “profile” for believers. Although some studies have suggested associations with low education, right-wing political orientation, and certain personality traits like subclinical paranoia and schizotypy, such findings have been inconsistent and may vary across specific conspiracy theory. Associations between conspiracy belief and paranoia suggest overlap within a “conspiratorial mindset,” with recent evidence that “distrust of officialdom” is a key mediator between believing in conspiracies and political ideology.
Other quantitative “cognitive quirks” reported in those who believe in conspiracies are a need for certainty and control, a need for uniqueness, illusory pattern perception, and lack of analytical thinking. It’s unclear which of these factors may represent universal cognitive explanations for conspiratorial beliefs, vs. those that might be related to specific beliefs, such as the need for certainty during times of crisis and societal upheaval, when conspiracy theories tend to flourish.
Much of the research on conspiracy theory belief is based on the questionable premise that it’s best understood at the level of the individual’s psychopathology, or the “deficit model,” as it’s called. One of us (JMP) has instead proposed a two-component model that includes social and informational contexts. The first component – epistemic mistrust – involves mistrusting conventional, “authoritative” knowledge. The second involves biased information processing and exposure to misinformation, often transmitted by word of mouth, or through social networks. With this model, believing in conspiracy theories could be conceived as involving “delusion-like beliefs,” but not frank psychosis or full-blown delusions, as one might see, for example, in schizophrenia.
Indeed, many of the cognitive characteristics associated with conspiracy theory belief are universal, continuously distributed traits, varying in quantity, rather than all-or-none variables or distinct symptoms of mental illness.
Essentially, delusions are fixed, false, usually unshared beliefs, often based on subjective “inner” experience. (One rare exception is the so-called folie à deux, in which two people appear to “share” the same delusion; however, psychiatrists have long debated whether both individuals should be considered truly delusional). The delusion’s content is often “self-referential”; i.e., focused primarily on the believer.
In contrast, conspiracy theories are usually, but not necessarily, false. They are typically shared beliefs that don’t explicitly or directly involve the believer, and are based on evidence that one finds “out there,” such as on the Internet. This speaks to the highly communal nature of so many conspiracy theories – networks of like-minded individuals reinforcing one another’s beliefs in a particular socio-cultural context.
Conspiracy theory belief, COVID-19, and medical intervention
As for medical conspiracy theories, none have flourished recently more so than those involving the COVID-19 pandemic. As a recent editorial by Stein and colleagues noted, “Some conspiratorial claims include assertions that COVID‐19 is a hoax; arguments that the virus was created artificially and spread on purpose as a bioweapon; or allegations that governments are using the emergency situation to pursue their antidemocratic goals. … Other conspiracies argued that people in power are taking advantage of the pandemic as a plan to inject microchip quantum-dot spy software and monitor people.”
Stein and colleagues make the important point that a “key difference between COVID‐19 and the 1918 flu pandemic ... is that [now] a highly interconnected world, to a great extent on social media, is setting the stage for distributing information and misinformation about COVID‐19.”
Consider the following composite vignette: Mr. A is a 70-year-old retiree with a history of COPD who has been advised by his PCP to get vaccinated against COVID-19. He is extremely reluctant to do so, fearing that “the vaccine is going to change my DNA” and “might even give me COVID.” He has heard from friends on social media that vaccine developers “faked the results” and are “in cahoots with the federal government.” Mr. A has heard “experts” declare the vaccines safe, but does not trust them. Mr. A has no psychiatric or substance abuse history, and there are no cognitive, perceptual, or other abnormalities in Mr. A’s mental status exam.
Mr. A’s beliefs qualify as a “conspiracy theory,” but probably represent widely held misconceptions about COVID-19 vaccines, as well as widespread mistrust of pharmaceutical companies and the federal government. Based on the information provided, there is no basis for concluding that Mr. A is psychotic or delusional. His beliefs appear to be the result of “epistemic mistrust” of authoritative informational accounts, biased information processing, and exposure to misinformation.
How should the physician manage and care for patients like Mr. A? Absent frank delusions, there is no role for antipsychotic medication, though . In addition to providing accurate medical information to the patient, the physician should avoid arguing, or trying to “talk the patient out of” his or her belief. Instead, the focus should be on sustaining and strengthening the physician-patient alliance, establishing an atmosphere of respect and safety, clarifying differences in trusted sources of medical information, and allowing the patient time to process the physician’s recommendations.
One-to-one engagement with health care providers has proved effective in reducing vaccine hesitancy and correcting misinformation. For patients with less fixed conspiracy theory beliefs, it may sometimes be helpful to gently offer alternative hypotheses to the patient’s conspiracy theory, using elements of cognitive-behavioral therapy (CBT). For example, a physician might ask, “Is it possible that the online source you read was mistaken about the vaccine changing your DNA?” while reminding patients that – contrary to popular belief – mRNA vaccines have been in development against cancer for several decades.
Challenging beliefs collaboratively and acknowledging areas of uncertainty, rather than confronting or arguing about false beliefs, can foster trust between physician and patient and, at the very least, open a dialogue regarding potential exposure to medical misinformation. “Inoculation” strategies that present and then dispel misinformation before patients become aware of it are among the best supported strategies for mitigating conspiracy theory belief. Ideally, physicians and health care systems should maintain an ongoing “inventory” of medical misinformation circulating online and “beat it to the punch” with reliable information.
Finally, because believing in conspiracy theories is often associated with a sense of uncertainty, and feeling that one’s life is “out of control,” medical interventions can be framed as ways of regaining control and appealing to patients’ values; for example, saying, “By getting the vaccine, you’ll be more likely to stay in good health, protect your family, and do all the things you want to do.”
Dr. Pies is professor of psychiatry and a lecturer on bioethics and humanities at State University of New York, Syracuse. Dr. Pierre is a health sciences clinical professor in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles. A version of this article first appeared on Medscape.com.
Time to retire haloperidol?
For more than half a century, haloperidol has been used as a first-line medication for psychiatric agitation constituting a “behavioral emergency” when a patient cannot or will not take oral medication. Today, haloperidol is most commonly administered as an IM injection along with an anticholinergic medication to minimize extrapyramidal symptoms (EPS) and a benzodiazepine for additional sedation. The multiple-medication “cocktail” is often referred to by double-entendre nicknames, such as “B-52” or “5250” (ie, haloperidol, 5 mg; lorazepam, 2 mg; and
Earlier evidence of haloperidol’s efficacy
The initial “discovery” of antipsychotic medications was made in 1951 based on the inadvertent observation that chlorpromazine had the potential to calm surgical patients with autonomic activation. This calming effect, described as “désintéressment” (meaning a kind of “indifference to the world”),1 resulted in a new class of medications replacing barbiturates and bromides as go-to options to achieve “rapid tranquilization” of psychiatric agitation.2 Although the ability of antipsychotic medications to gradually reduce positive symptoms, such as delusions and hallucinations, has been attributed to dopamine (D2) antagonism, their more immediate sedating and anti-agitation effects are the result of broader effects as histamine (H1) and alpha-1 adrenergic antagonists.
In the 1970s, haloperidol emerged as a first-line option to manage agitation due to its IM and IV availability, as well as its relative lack of sedation and orthostasis compared with low-potency D2 antagonists such as chlorpromazine. However, haloperidol was observed to have a significant risk of acute EPS, including dystonic reactions.2 From the 1970s to the 1990s, numerous prospective clinical trials of haloperidol for the treatment of acute psychotic agitation, including several randomized controlled trials (RCTs) comparing haloperidol to lorazepam, were conducted.3 The design and outcomes of the haloperidol vs lorazepam RCTs were fairly consistent4-7:
- adult participants with acute agitation and a variety of psychiatric diagnoses, for whom informed consent often was waived due to agitation severity
- randomization to either IM haloperidol, 5 mg, or IM lorazepam, 2 mg, administered every 30 minutes until agitation resolved
- behavioral outcomes measured over several hours using various rating scales, without consistent assessment of EPS
- equivalent efficacy of haloperidol and lorazepam, with symptom resolution usually achieved after 1 to 2 doses (in 30 to 60 minutes), but sometimes longer
- anticholinergic “rescue” allowed for EPS, but not administered prophylactically
- EPS, including dystonia and akathisia, were significantly more frequent with haloperidol compared with lorazepam.8
In recognition of the greater risk of EPS with haloperidol compared with lorazepam, and the fact that most study participants were already taking standing doses of antipsychotic medications, some researchers have recommended using benzodiazepines alone as the optimal treatment for agitation.4,9 A 2012 Cochrane review concluded that the involuntary use of haloperidol alone “could be considered unethical.”10,11 However, other studies that examined the combination of haloperidol and lorazepam compared with either medication alone found that the combination of the 2 medications was associated with a more rapid resolution of symptoms, which suggests a superior synergistic effect.6,7,12 By the late 1990s, combined haloperidol and lorazepam, often mixed within a single injection, became the most common strategy to achieve rapid tranquilization in the psychiatric emergency setting.13 However, while the combination has been justified as a way to reduce the antipsychotic medication dose and EPS risk,2 few studies have compared combinations containing <5 mg of haloperidol. As a result, the apparent superiority of combined haloperidol and lorazepam compared with either medication alone may be a simple cumulative dose effect rather than true synergism. It is also important to note that adding lorazepam to haloperidol does not mitigate the risk of EPS such as dystonia in the absence of anticholinergic medication.8 To date, however, there have been no clinical trials investigating the efficacy of IM haloperidol, lorazepam, and
Newer RCTs tell a different story
With the availability of second-generation antipsychotics (SGAs) in IM formulations, clinical trials over the past 2 decades have focused on comparing SGAs with haloperidol alone as the “gold standard” control for acute agitation. Compared with previous trials of
- Study participants who signed informed consent (and were likely less agitated)
- IM haloperidol doses typically >5 mg (eg, 6.5 to 10 mg).
As with studies comparing lorazepam with haloperidol, the results of these RCTs revealed that IM
An updated 2017 Cochrane review of haloperidol for psychosis-induced aggression or agitation concluded that9:
- haloperidol is an effective intervention, although the evidence is “weak”
- significant treatment effects may take as long as 1 to 2 hours following multiple IM injections
- in contrast to SGAs, treatment with haloperidol carries a significant risk of EPS
- adding a benzodiazepine “does not have strong evidence of benefit and carries risk of additional harm.”
Continue to: Haloperidol's well-known toxicity
Haloperidol’s well-known toxicity
Haloperidol has been associated with numerous adverse effects:
Akathisia and other acute EPS. Treatment with even a single dose of IM haloperidol can result in acute EPS, including dystonia and akathisia. At best, such adverse effects are subjectively troubling and unpleasant; at worst, akathisia can exacerbate and be mistaken for agitation, leading to administration of more medication23 and the possible development of suicidal or violent behavior.24-25 In the studies reviewed above, the overall rate of EPS was as high as 21% after treatment with haloperidol,16 with parkinsonism occurring in up to 17% of patients,19 dystonia in up to 11%,7 and akathisia in up to 10%.15 However, because specific EPS were assessed inconsistently, and sometimes not at all, the rate of akathisia—arguably the most relevant and counter-therapeutic adverse effect related to agitation—remains unclear.
In another study that specifically assessed for akathisia in patients treated with haloperidol, up to 40% experienced akathisia 6 hours after a single oral dose of 5 mg.26 Even a single dose of IV
Although anticholinergic medications or benzodiazepinesare often administered as part of a haloperidol “cocktail,” these medications often do not adequately resolve emergent akathisia.26,28 No clinical trials of IM haloperidol combined with benztropine or diphenhydramine have been published, but several studies suggest that combining haloperidol with
Cardiotoxicity. Although low-potency antipsychotic medications such as
Continue to: Although there is no direct evidence...
Although there is no direct evidence that the cardiac risks associated with IV haloperidol apply to IM administration, epidemiologic studies indicate that oral haloperidol carries an elevated risk of ventricular arrhythmia and sudden cardiac death,35,36 with 1 study reporting greater risk compared with other SGAs.37 Haloperidol, whether administered orally or IM, may therefore be an especially poor choice for patients with agitation who are at risk for arrhythmia, including those with relevant medical comorbidities or delirium.34
Neuronal cell death. Several lines of research evidence have demonstrated that haloperidol can cause cellular injury or death in neuronal tissue in a dose-dependent fashion through a variety of mechanisms.38 By contrast, SGAs have been shown to have neuroprotective effects.39 While these findings have mostly come from studies conducted in animals or in vitro human tumor cell lines, some researchers have nonetheless called for haloperidol to be banned, noting that if its neurotoxic effects were more widely known, “we would realize what a travesty it is to use [such] a brain-unfriendly drug.”40
Several reasonable alternatives
Echoing the earlier Cochrane review of haloperidol for psychosis-induced aggression or agitation,10 a 2017 update concluded, “If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical.”9
What then are reasonable alternatives to replace IM haloperidol for agitation? Clinicians should consider the following nonpharmacologic and pharmacologic interventions:
Nonpharmacologic interventions. Several behavioral interventions have been demonstrated to be effective for managing acute agitation, including verbal de-escalation, enhanced “programming” on the inpatient units, and the judicious use of seclusion.41-43 While such interventions may demand additional staff or resources, they have the potential to lower long-term costs, reduce injuries to patients and staff, and improve the quality of care.43 The use of IM haloperidol as a form of “chemical restraint” does not represent standard-of-care treatment,3 and from an ethical perspective, should never be implemented punitively or to compensate for substandard care in the form of inadequate staffing or staff training.
Continue to: Benzodiazepines
Benzodiazepines. Lorazepam offers an attractive alternative to haloperidol without the risk of EPS.2,4,8 However, lorazepam alone may be perceived as less efficacious than a haloperidol “cocktail” because it represents less overall medication. Some evidence has suggested that lorazepam, 4 mg, might be the most appropriate dose, although it has only rarely been studied in clinical trials of acute agitation.3
Respiratory depression is frequently cited as an argument against using lorazepam for agitation, as if the therapeutic window is extremely narrow with ineffectiveness at 2 mg, but potential lethality beyond that dose. In fact, serious respiratory depression with lorazepam is unlikely in the absence of chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, or concomitant alcohol or other sedative use.46 Case reports have documented therapeutic lorazepam dosing of 2 to 4 mg every 2 hours up to 20 to 30 mg/d in patients with manic agitation.47 Even in patients with COPD, significant respiratory depression tends not to occur at doses <8 mg.48 A more evidence-based concern about lorazepam dosing is that 2 mg might be ineffective in patients with established tolerance. For example, 1 report described a patient in acute alcohol withdrawal who required dosing lorazepam to 1,600 mg within 24 hours.49 Collectively, these reports suggest that lorazepam has a much wider therapeutic window than is typically perceived, and that dosing with 3 to 4 mg IM is a reasonable option for agitation when 2 mg is likely to be inadequate.
Paradoxical disinhibition is another concern that might prevent benzodiazepines from being used alone as a first-line intervention for emergency treatment of agitation. However, similar to respiratory depression, this adverse event is relatively rare and tends to occur in children and geriatric patients, individuals intoxicated with alcohol or other sedatives, and patients with brain injury, developmental delay, or dementia.23,46 Although exacerbation of aggression has not been demonstrated in the RCTs examining benzodiazepines for agitation reviewed above, based on other research, some clinicians have expressed concerns about the potential for benzodiazepines to exacerbate aggression in patients with impulse control disorders and a history of violent behavior.50
The 2005 Expert Consensus Panel for Behavioral Emergencies51 recommended the use of lorazepam alone over haloperidol for agitation for patients for whom the diagnosis is unknown or includes the following:
- stimulant intoxication
- personality disorder
- comorbid obesity
- comorbid cardiac arrhythmia
- a history of akathisia and other EPS
- a history of amenorrhea/galactorrhea
- a history of seizures.
In surveys, patients have ranked lorazepam as the preferred medication for emergency agitation, whereas haloperidol was ranked as one of the least-preferred options.51,52
Continue to: Second-generation antipsychotics
Second-generation antipsychotics. The SGAs available in IM formulations, such as aripiprazole, olanzapine, and ziprasidone, have been shown to be at least as effective as haloperidol for the treatment of acute agitation (in 2015, the short-acting injectable formulation of aripiprazole was discontinued in the United States independent of safety or efficacy issues53). A review of RCTs examining IM SGAs for the treatment of agitation concluded that the number needed to treat for response compared with placebo was 5 for aripiprazole, 3 for olanzapine, and 3 for ziprasidone.54 In terms of safety, a meta-analysis of studies examining IM medications for agitation confirmed that the risk of acute EPS, including dystonia, akathisia, and parkinsonism, is significantly lower with SGAs compared with haloperidol.55 An RCT comparing IM ziprasidone with haloperidol found equivalently modest effects on QTc prolongation.56 Therefore, SGAs are an obvious and evidence-based option for replacing haloperidol as a treatment for acute agitation.
Unfortunately, for clinicians hoping to replace haloperidol within a multiple-medication IM “cocktail,” there have been no published controlled trials of SGAs combined with benzodiazepines. Although a short report indicated that aripiprazole and lorazepam are chemically compatible to be combined within a single injection,57 the package insert for aripiprazole warns that “If parenteral benzodiazepine therapy is deemed necessary in addition to ABILIFY injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension.”58 The package insert for olanzapine likewise lists the combination of lorazepam and olanzapine as a drug interaction that can potentiate sedation, and the manufacturer issued specific warnings about parenteral combination.59,60 A single published case of significant hypotension with combined IM olanzapine and lorazepam,60 together with the fact that IM olanzapine can cause hypotension by itself,61 has discouraged the coadministration of these medications. Nonetheless, the combination is used in some emergency settings, with several retrospective studies failing to provide evidence of hypotension or respiratory depression as adverse effects.62-64
Droperidol.
Over the past decade, however, droperidol has returned to the US market68 and its IV and IM usage has been revitalized for managing patients with agitation within or en route to the ED. Studies have demonstrated droperidol efficacy comparable to midazolam, ziprasidone, or olanzapine, as well as effectiveness as an IV adjunct to midazolam.69-71 In contrast to the FDA black-box warning, retrospective studies and RCTs of both IV and IM droperidol suggest that QTc prolongation and torsades de pointes are rare events that do not occur any more frequently than they do with haloperidol, even at doses >10 mg.72,73 However, in studies involving patients with drug intoxication and treatment with multiple medications, oversedation to the point of needing rescue intervention was reported. In an emergency setting where these issues are relatively easily managed, such risks may be better tolerated than in psychiatric settings.
With earlier studies examining the use of droperidol in an acute psychiatric setting that reported a more rapid onset of action than haloperidol,65-67 a 2016 Cochrane review concluded that there was high-quality evidence to support droperidol’s use for psychosis-induced agitation.74 However, a 2015 RCT comparing IM droperidol, 10 mg, to haloperidol, 10 mg, found equivalent efficacy and response times (with maximal response occurring within 2 hours) and concluded that droperidol had no advantage over haloperidol.75 Because none of the clinical trials that evaluated droperidol have included assessments for EPS, its risk of akathisia remains uncertain.
Continue to: Ketamine
Ketamine. In recent years, ketamine has been used to treat acute agitation within or en route to the ED. Preliminary observational studies support ketamine’s efficacy when administered via IV or IM routes,76 with more rapid symptomatic improvement compared with haloperidol, lorazepam, or midazolam alone.77 Reported adverse effects of ketamine include dissociation, psychotic exacerbation, and respiratory depression,76 although 1 small naturalistic study found no evidence of exacerbation of psychotic or other psychiatric symptoms.78 An ongoing RCT is comparing IM ketamine, 5 mg/kg, to combined IM haloperidol, 5 mg, and midazolam, 5 mg.79 Although various ketamine formulations are increasingly being used in psychiatry, active psychosis is generally regarded as a contraindication. It is premature to recommend parenteral ketamine administration for agitation within most psychiatric settings until more research on safety has been completed.
Haloperidol, or something else? Practical considerations
Consider the following factors when deciding whether to use haloperidol or one of its alternatives:
Limitations of the evidence. Modern clinical trials requiring informed consent often do not include the kind of severe agitation that clinicians encounter in acute psychiatric, emergency, or forensic settings. In addition, standard interventions, such as 3-medication haloperidol “cocktails,” have not been evaluated in clinical trials. Clinicians are therefore often in the dark about optimal evidence-based practices.
Treatment goals. Psychiatric agitation has many causes, with a range of severity that warrants a commensurate range of responses. Protocols for managing acute agitation should include graded interventions that begin with nonpharmacologic interventions and voluntary oral medications, and move to involuntary IM medications when necessary.
While treatment guidelines clearly recommend against IM medications as “chemical restraint” with a goal of sedating a patient until he/she is unconscious,3,51 such outcomes are nonetheless often sought by staff who are concerned about the risk of injuries during a behavioral emergency. In such instances, the risks of violence towards patients and staff may outweigh concerns about adverse effects in a risk-benefit analysis. Consequently, clinicians may be prone to “skip over” graded interventions because they assume they “won’t work” in favor of administering involuntary multiple-medication haloperidol “cocktails” despite risks of excess sedation, EPS, and cardiotoxicity. Treatment settings should critically evaluate such biased preferences, with a goal of developing tailored, evidence-based strategies that maximize benefits while minimizing excess sedation and other untoward adverse effects, with an eye towards promoting better overall patient care and reducing length of stay.42,43,80
Continue to: Limitations of available medications
Limitations of available medications. There is no perfect medication for the management of acute agitation. Evidence indicates that pharmacologic options take 15 minutes to several hours to resolve acute agitation, even potentially more rapid-acting medications such as midazolam and droperidol. This is well beyond most clinicians’ desired window for response time in a behavioral emergency. Multiple-medication “cocktails” may be used with the hope of hastening response time, but may not achieve this goal at the expense of increasing the risk of adverse effects and the likelihood that a patient will remain sedated for a prolonged time. In the real world, this often means that by the time a psychiatrist comes to evaluate a patient who has been given emergency medications, the patient cannot be aroused for an interview. Ideally, medications would calm an agitated patient rapidly, without excess or prolonged sedation.80 Less-sedating SGAs, such as ziprasidone, might have this potential, but can sometimes be perceived as ineffective.
Avoiding akathisia. Akathisia’s potential to worsen and be mistaken for agitation makes it an especially concerning, if underappreciated, adverse effect of haloperidol that is often not adequately assessed in clinical trials or practice. In light of evidence that akathisia can occur in nearly half of patients receiving a single 5 mg-dose of haloperidol, it is difficult to justify the use of this medication for agitation when equally effective options exist with a lower risk of EPS.
While haloperidol-induced akathisia could in theory be mitigated by adding anticholinergic medications or benzodiazepines, previous studies have found that such strategies have limited effectiveness compared to “gold standard” treatment with propranolol.28,81,82 Furthermore, the half-lives of anticholinergic medications, such as benztropine or diphenhydramine, are significantly shorter than that of a single dose of haloperidol, which can be as long as 37 hours.83 Therefore, akathisia and other EPS could emerge or worsen several hours or even days after receiving an IM haloperidol “cocktail” as the shorter-acting medications wear off. Akathisia is best minimized by avoiding FGAs, such as haloperidol, when treating acute agitation.
Promoting adherence. Although haloperidol is often recommended for acute agitation in patients with schizophrenia or bipolar disorder on the basis that it would treat the underlying condition, many patients who receive IM medications for acute agitation are already prescribed standing doses of oral medication, which increases the risk of cumulative toxicity. In addition, receiving a medication likely to cause acute EPS that is ranked near the bottom of patient preferences may erode the potential for a therapeutic alliance and hamper longer-term antipsychotic medication adherence.

Time for a change
For nearly half a century, haloperidol has been a “gold standard” intervention for IM control in patients with agitation. However, given its potential to produce adverse effects, including a significant risk of akathisia that can worsen agitation, along with the availability of newer pharmacologic options that are at least as effective (Table 1, and Table 2), haloperidol should be retired as a first-line medication for the treatment of agitation. Clinicians would benefit from RCTs investigating the safety and efficacy of novel interventions including frequently-used, but untested medication combinations, as well as nonpharmacologic interventions.

Continue to: Bottom Line
Bottom Line
Although there is no perfect IM medication to treat acute agitation, haloperidol’s higher risk of adverse effects relative to newer alternatives suggest that it should no longer be considered a first-line intervention.
Related Resources
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 1: onset of efficacy. J Emerg Med. 2018;54(3):364-374.
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 2: safety. J Emerg Med. 2018;54(4): 522-532.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Chlorpromazine • Thorazine
Diphenhydramine • Benadryl
Droperidol • Inapsine
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Midazolam • Versed
Olanzapine • Zyprexa
Prochlorperazine • Compazine
Promethazine • Phenergan
Propranolol • Inderal, Pronol
Ziprasidone • Geodon
1. Shorter E. A history of psychiatry. New York, NY: John Wiley & Sons, Inc.; 1997:249.
2. Salzman C, Green AI, Rodriguez-Villa F, et al. Benzodiazepines combined with neuroleptics for management of severe disruptive behavior. Psychosomatics. 1986;27(suppl 1):17-22.
3. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatr. 2000;61(suppl 14):11-20.
4. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatr. 1991:52(4):177-180.
5. Allen MH, Currier GW, Hughes DH, et al. The expert consensus guideline series: treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1-88; quiz 89-90.
6. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12(3):175-179.
7. Garza-Trevino WS, Hollister LE, Overall JE, et al. Efficacy of combinations of intramuscular antipsychotics and sedative-hypnotics for control of psychotic agitation. Am J Psychiatr. 1989:146(12):1598-1601.
8. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective double-blind, emergency study. Am J Emerg Med 1997;15(4):335-340.
9. Ostinelli EG, Brooke-Powney MJ, Li X, et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2017; 7:CD009377. doi: 10.1002/14651858.CD009377.pub3.
10. Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2.
11. Citrome L. Review: limited evidence on effects of haloperidol alone for rapid tranquillisation in psychosis-induced aggression. Evid Based Ment Health. 2013;16(2):47.
12. Bienek SA, Ownby R, Penalver A, et al. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacother. 1998;18(1):57-62.
13. Binder RL, McNiel DE. Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Serv. 1999;50(2):1553-1554.
14. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl). 2006;188(3):281-292.
15. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatr. 2007;68(1):111-119.
16. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatr. 2000;61(12):933-941.
17. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
18. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatr. 2001;158(7):1149-1151.
19. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psych. 2002;59(5):441-448.
20. Hsu W, Huang S, Lee B, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30(3):230-234.
21. Chan H, Ree S, Su L, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34(3):355-358.
22. Baldaçara L, Sanches M, Cordeiro DC, et al. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30-39.
23. Hillard JR. Defusing patient violence. Current Psychiatry. 2002;1(4):22-29.
24. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
25. Eikelenboom-Schieveld SJM, Lucire Y, Fogleman JC. The relevance of cytochrome P450 polymorphism in forensic medicine and akathisia-related violence and suicide. J Forens Leg Med. 2016;41:65-71.
26. Van Putten T, May PRA, Marder SR. Akathisia with haloperidol and thiothixene. Arch Gen Psych. 1984;41:1036-1039.
27. Drotts DL, Vinson DR. Prochlorperazine induced akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
28. Salem H, Negpal C, Pigott T. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
29. Huf G, Coutinho ESF, Adams CE. Rapid tranquilization in psychiatric emergency settings in Brazil: pragmatic randomized controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869.
30. Mantovani C, Labate CM, Sponholz A, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306-312.
31. Darwish H, Grant R, Haslam R, et al. Promethazine-induced acute dystonic reactions. Am J Dis Child. 1980;134(10):990-991.
32. Jyothi CH, Rudraiah HGM, Vidya HK, et al. Promethazine induced acute dystonia: a case report. Manipal J Med Sci. 2016;1(2):63-64.
33. Ames D, Carr-Lopez SM, Gutierrez MA, et al. Detecting and managing adverse effects of antipsychotic medications: current state of play. Psychiatr Clin North Am. 2016;39(2):275-311.
34. Meyer-Massetti C, Cheng CM, Sharpe MA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5(4):E8-E16. doi: 10.1002/jhm.691.
35. Wu C, Tsai Y, Tsai H. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Dis. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568.
36. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointe, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(1):105-122.
37. Leonard CE, Freeman CP, Newcomb CW, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;suppl 10(6):1-9.
38. Nasrallah H, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatr. 2017;29(3):195-202.
39. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
40. Nasrallah HA. Haloperidol clearly is neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
41. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Comm Psychiatr. 1993;44(2):125-133.
42. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172.
43. Vieta E, Garriga M, Cardete L, et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatr. 2017;17:328.
44. Nobay F, Simon BC, Levitt A, et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
45. Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385.
46. Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatr. 1998;59(suppl 1):57-60.
47. Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985;5(2):109-110.
48. Denaut M, Yernault JC, De Coster A. Double-blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Curr Med Res Opin. 1975;2(10):611-615.
49. Kahn DR, Barnhorst AV, Bourgeois JA. A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours. CNS Spectr. 2009;14(7):385-389.
50. Jones KA. Benzodiazepines: their role in aggression and why GPs should prescribe with caution. Austral Fam Physician. 2011;40(11):862-865.
51. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-108.
52. Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58.
53. Han DH. Some Abilify formulations to discontinue in 2015. MPR. https://www.empr.com/home/news/some-abilify-formulations-to-discontinue-in-2015/. Published January 13, 2015. Accessed April 17, 2020.
54. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
55. Satterthwaite TD, Wolf DH, Rosenheck RA, et al. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment for agitation. J Clin Psychiatr. 2008;69(12):1869-1879.
56. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491.
57. Kovalick LJ, Pikalov AA, Ni N, et al. Short-term physical compatibility of intramuscular aripiprazole with intramuscular lorazepam. Am J Health-Syst Pharm. 2008;65(21):2007-2008.
58. Abilify [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
59. Zyprexa [package insert]. Indianapolis, IN: Lilly Research Laboratories; 2005.
60. Zacher JL, Roche-Desilets J. Hypotension secondary to the combination of intramuscular olanzapine and intramuscular lorazepam. J Clin Psychiatr. 2005;66(12):1614-1615.
61. Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatr 2010;71(4):433-441.
62. Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-797.
63. Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-896.
64. Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin [Internet]. 2018;8(5):208-213.
65. Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298-299.
66. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407-413.
67. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567-573.
68. Boyer EW. Droperidol is back (and here’s what you need to know). ACEP Now. https://www.acepnow.com/article/droperidol-is-back-and-heres-what-you-need-to-know/. Published September 16, 2019. Accessed April 17, 2020.
69. Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167-1172.
70. Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72-81.
71. Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392-401.
72. Macht M, Mull AC, McVaney KE, et al. Comparison of droperidol and haloperidol for use by paramedics assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18(3):375-380.
73. Calver L, Page CB, Downes MA, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med. 2015;66(3):230-238.
74. Kohokar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12:CD002830.
75. Calver L, Drinkwater V, Gupta R, et al. Droperidol v. haloperidol for sedation of aggressive behavior in acute mental health: randomized controlled trial. Brit J Psychiatr. 2015;206(3):223-228.
76. Hopper AB, Vilke GM, Castillo EM, et al. Ketamine use for acute agitation in the emergency department. J Emerg Med. 2015;48(6):712-719.
77. Riddell J, Tran A, Bengiamin R, et al. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35:1000-1004.
78. Lebin JA, Akhavan AR, Hippe DS, et al. Psychiatric outcomes of patients with severe agitation following administration of prehospital ketamine. Acad Emerg Med. 2019;26(8):889-896.
79. Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department (RACKED): a randomized controlled trial protocol. Trials. 2018;19(1):651.
80. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatr. 2016;17(2):86-128.
81. Adler L, Angrist B, Peselow E, et al. Efficacy of propranolol in neuroleptic-induced akathesia. J Clin Psychopharmacol. 1985;5(3):164-166.
82. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
83. de Leon J, Diaz FJ, Wedlund P, et al. Haloperidol half-life after chronic dosing. J Clin Psychopharmacol. 2004;24(6):656-660.
For more than half a century, haloperidol has been used as a first-line medication for psychiatric agitation constituting a “behavioral emergency” when a patient cannot or will not take oral medication. Today, haloperidol is most commonly administered as an IM injection along with an anticholinergic medication to minimize extrapyramidal symptoms (EPS) and a benzodiazepine for additional sedation. The multiple-medication “cocktail” is often referred to by double-entendre nicknames, such as “B-52” or “5250” (ie, haloperidol, 5 mg; lorazepam, 2 mg; and
Earlier evidence of haloperidol’s efficacy
The initial “discovery” of antipsychotic medications was made in 1951 based on the inadvertent observation that chlorpromazine had the potential to calm surgical patients with autonomic activation. This calming effect, described as “désintéressment” (meaning a kind of “indifference to the world”),1 resulted in a new class of medications replacing barbiturates and bromides as go-to options to achieve “rapid tranquilization” of psychiatric agitation.2 Although the ability of antipsychotic medications to gradually reduce positive symptoms, such as delusions and hallucinations, has been attributed to dopamine (D2) antagonism, their more immediate sedating and anti-agitation effects are the result of broader effects as histamine (H1) and alpha-1 adrenergic antagonists.
In the 1970s, haloperidol emerged as a first-line option to manage agitation due to its IM and IV availability, as well as its relative lack of sedation and orthostasis compared with low-potency D2 antagonists such as chlorpromazine. However, haloperidol was observed to have a significant risk of acute EPS, including dystonic reactions.2 From the 1970s to the 1990s, numerous prospective clinical trials of haloperidol for the treatment of acute psychotic agitation, including several randomized controlled trials (RCTs) comparing haloperidol to lorazepam, were conducted.3 The design and outcomes of the haloperidol vs lorazepam RCTs were fairly consistent4-7:
- adult participants with acute agitation and a variety of psychiatric diagnoses, for whom informed consent often was waived due to agitation severity
- randomization to either IM haloperidol, 5 mg, or IM lorazepam, 2 mg, administered every 30 minutes until agitation resolved
- behavioral outcomes measured over several hours using various rating scales, without consistent assessment of EPS
- equivalent efficacy of haloperidol and lorazepam, with symptom resolution usually achieved after 1 to 2 doses (in 30 to 60 minutes), but sometimes longer
- anticholinergic “rescue” allowed for EPS, but not administered prophylactically
- EPS, including dystonia and akathisia, were significantly more frequent with haloperidol compared with lorazepam.8
In recognition of the greater risk of EPS with haloperidol compared with lorazepam, and the fact that most study participants were already taking standing doses of antipsychotic medications, some researchers have recommended using benzodiazepines alone as the optimal treatment for agitation.4,9 A 2012 Cochrane review concluded that the involuntary use of haloperidol alone “could be considered unethical.”10,11 However, other studies that examined the combination of haloperidol and lorazepam compared with either medication alone found that the combination of the 2 medications was associated with a more rapid resolution of symptoms, which suggests a superior synergistic effect.6,7,12 By the late 1990s, combined haloperidol and lorazepam, often mixed within a single injection, became the most common strategy to achieve rapid tranquilization in the psychiatric emergency setting.13 However, while the combination has been justified as a way to reduce the antipsychotic medication dose and EPS risk,2 few studies have compared combinations containing <5 mg of haloperidol. As a result, the apparent superiority of combined haloperidol and lorazepam compared with either medication alone may be a simple cumulative dose effect rather than true synergism. It is also important to note that adding lorazepam to haloperidol does not mitigate the risk of EPS such as dystonia in the absence of anticholinergic medication.8 To date, however, there have been no clinical trials investigating the efficacy of IM haloperidol, lorazepam, and
Newer RCTs tell a different story
With the availability of second-generation antipsychotics (SGAs) in IM formulations, clinical trials over the past 2 decades have focused on comparing SGAs with haloperidol alone as the “gold standard” control for acute agitation. Compared with previous trials of
- Study participants who signed informed consent (and were likely less agitated)
- IM haloperidol doses typically >5 mg (eg, 6.5 to 10 mg).
As with studies comparing lorazepam with haloperidol, the results of these RCTs revealed that IM
An updated 2017 Cochrane review of haloperidol for psychosis-induced aggression or agitation concluded that9:
- haloperidol is an effective intervention, although the evidence is “weak”
- significant treatment effects may take as long as 1 to 2 hours following multiple IM injections
- in contrast to SGAs, treatment with haloperidol carries a significant risk of EPS
- adding a benzodiazepine “does not have strong evidence of benefit and carries risk of additional harm.”
Continue to: Haloperidol's well-known toxicity
Haloperidol’s well-known toxicity
Haloperidol has been associated with numerous adverse effects:
Akathisia and other acute EPS. Treatment with even a single dose of IM haloperidol can result in acute EPS, including dystonia and akathisia. At best, such adverse effects are subjectively troubling and unpleasant; at worst, akathisia can exacerbate and be mistaken for agitation, leading to administration of more medication23 and the possible development of suicidal or violent behavior.24-25 In the studies reviewed above, the overall rate of EPS was as high as 21% after treatment with haloperidol,16 with parkinsonism occurring in up to 17% of patients,19 dystonia in up to 11%,7 and akathisia in up to 10%.15 However, because specific EPS were assessed inconsistently, and sometimes not at all, the rate of akathisia—arguably the most relevant and counter-therapeutic adverse effect related to agitation—remains unclear.
In another study that specifically assessed for akathisia in patients treated with haloperidol, up to 40% experienced akathisia 6 hours after a single oral dose of 5 mg.26 Even a single dose of IV
Although anticholinergic medications or benzodiazepinesare often administered as part of a haloperidol “cocktail,” these medications often do not adequately resolve emergent akathisia.26,28 No clinical trials of IM haloperidol combined with benztropine or diphenhydramine have been published, but several studies suggest that combining haloperidol with
Cardiotoxicity. Although low-potency antipsychotic medications such as
Continue to: Although there is no direct evidence...
Although there is no direct evidence that the cardiac risks associated with IV haloperidol apply to IM administration, epidemiologic studies indicate that oral haloperidol carries an elevated risk of ventricular arrhythmia and sudden cardiac death,35,36 with 1 study reporting greater risk compared with other SGAs.37 Haloperidol, whether administered orally or IM, may therefore be an especially poor choice for patients with agitation who are at risk for arrhythmia, including those with relevant medical comorbidities or delirium.34
Neuronal cell death. Several lines of research evidence have demonstrated that haloperidol can cause cellular injury or death in neuronal tissue in a dose-dependent fashion through a variety of mechanisms.38 By contrast, SGAs have been shown to have neuroprotective effects.39 While these findings have mostly come from studies conducted in animals or in vitro human tumor cell lines, some researchers have nonetheless called for haloperidol to be banned, noting that if its neurotoxic effects were more widely known, “we would realize what a travesty it is to use [such] a brain-unfriendly drug.”40
Several reasonable alternatives
Echoing the earlier Cochrane review of haloperidol for psychosis-induced aggression or agitation,10 a 2017 update concluded, “If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical.”9
What then are reasonable alternatives to replace IM haloperidol for agitation? Clinicians should consider the following nonpharmacologic and pharmacologic interventions:
Nonpharmacologic interventions. Several behavioral interventions have been demonstrated to be effective for managing acute agitation, including verbal de-escalation, enhanced “programming” on the inpatient units, and the judicious use of seclusion.41-43 While such interventions may demand additional staff or resources, they have the potential to lower long-term costs, reduce injuries to patients and staff, and improve the quality of care.43 The use of IM haloperidol as a form of “chemical restraint” does not represent standard-of-care treatment,3 and from an ethical perspective, should never be implemented punitively or to compensate for substandard care in the form of inadequate staffing or staff training.
Continue to: Benzodiazepines
Benzodiazepines. Lorazepam offers an attractive alternative to haloperidol without the risk of EPS.2,4,8 However, lorazepam alone may be perceived as less efficacious than a haloperidol “cocktail” because it represents less overall medication. Some evidence has suggested that lorazepam, 4 mg, might be the most appropriate dose, although it has only rarely been studied in clinical trials of acute agitation.3
Respiratory depression is frequently cited as an argument against using lorazepam for agitation, as if the therapeutic window is extremely narrow with ineffectiveness at 2 mg, but potential lethality beyond that dose. In fact, serious respiratory depression with lorazepam is unlikely in the absence of chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, or concomitant alcohol or other sedative use.46 Case reports have documented therapeutic lorazepam dosing of 2 to 4 mg every 2 hours up to 20 to 30 mg/d in patients with manic agitation.47 Even in patients with COPD, significant respiratory depression tends not to occur at doses <8 mg.48 A more evidence-based concern about lorazepam dosing is that 2 mg might be ineffective in patients with established tolerance. For example, 1 report described a patient in acute alcohol withdrawal who required dosing lorazepam to 1,600 mg within 24 hours.49 Collectively, these reports suggest that lorazepam has a much wider therapeutic window than is typically perceived, and that dosing with 3 to 4 mg IM is a reasonable option for agitation when 2 mg is likely to be inadequate.
Paradoxical disinhibition is another concern that might prevent benzodiazepines from being used alone as a first-line intervention for emergency treatment of agitation. However, similar to respiratory depression, this adverse event is relatively rare and tends to occur in children and geriatric patients, individuals intoxicated with alcohol or other sedatives, and patients with brain injury, developmental delay, or dementia.23,46 Although exacerbation of aggression has not been demonstrated in the RCTs examining benzodiazepines for agitation reviewed above, based on other research, some clinicians have expressed concerns about the potential for benzodiazepines to exacerbate aggression in patients with impulse control disorders and a history of violent behavior.50
The 2005 Expert Consensus Panel for Behavioral Emergencies51 recommended the use of lorazepam alone over haloperidol for agitation for patients for whom the diagnosis is unknown or includes the following:
- stimulant intoxication
- personality disorder
- comorbid obesity
- comorbid cardiac arrhythmia
- a history of akathisia and other EPS
- a history of amenorrhea/galactorrhea
- a history of seizures.
In surveys, patients have ranked lorazepam as the preferred medication for emergency agitation, whereas haloperidol was ranked as one of the least-preferred options.51,52
Continue to: Second-generation antipsychotics
Second-generation antipsychotics. The SGAs available in IM formulations, such as aripiprazole, olanzapine, and ziprasidone, have been shown to be at least as effective as haloperidol for the treatment of acute agitation (in 2015, the short-acting injectable formulation of aripiprazole was discontinued in the United States independent of safety or efficacy issues53). A review of RCTs examining IM SGAs for the treatment of agitation concluded that the number needed to treat for response compared with placebo was 5 for aripiprazole, 3 for olanzapine, and 3 for ziprasidone.54 In terms of safety, a meta-analysis of studies examining IM medications for agitation confirmed that the risk of acute EPS, including dystonia, akathisia, and parkinsonism, is significantly lower with SGAs compared with haloperidol.55 An RCT comparing IM ziprasidone with haloperidol found equivalently modest effects on QTc prolongation.56 Therefore, SGAs are an obvious and evidence-based option for replacing haloperidol as a treatment for acute agitation.
Unfortunately, for clinicians hoping to replace haloperidol within a multiple-medication IM “cocktail,” there have been no published controlled trials of SGAs combined with benzodiazepines. Although a short report indicated that aripiprazole and lorazepam are chemically compatible to be combined within a single injection,57 the package insert for aripiprazole warns that “If parenteral benzodiazepine therapy is deemed necessary in addition to ABILIFY injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension.”58 The package insert for olanzapine likewise lists the combination of lorazepam and olanzapine as a drug interaction that can potentiate sedation, and the manufacturer issued specific warnings about parenteral combination.59,60 A single published case of significant hypotension with combined IM olanzapine and lorazepam,60 together with the fact that IM olanzapine can cause hypotension by itself,61 has discouraged the coadministration of these medications. Nonetheless, the combination is used in some emergency settings, with several retrospective studies failing to provide evidence of hypotension or respiratory depression as adverse effects.62-64
Droperidol.
Over the past decade, however, droperidol has returned to the US market68 and its IV and IM usage has been revitalized for managing patients with agitation within or en route to the ED. Studies have demonstrated droperidol efficacy comparable to midazolam, ziprasidone, or olanzapine, as well as effectiveness as an IV adjunct to midazolam.69-71 In contrast to the FDA black-box warning, retrospective studies and RCTs of both IV and IM droperidol suggest that QTc prolongation and torsades de pointes are rare events that do not occur any more frequently than they do with haloperidol, even at doses >10 mg.72,73 However, in studies involving patients with drug intoxication and treatment with multiple medications, oversedation to the point of needing rescue intervention was reported. In an emergency setting where these issues are relatively easily managed, such risks may be better tolerated than in psychiatric settings.
With earlier studies examining the use of droperidol in an acute psychiatric setting that reported a more rapid onset of action than haloperidol,65-67 a 2016 Cochrane review concluded that there was high-quality evidence to support droperidol’s use for psychosis-induced agitation.74 However, a 2015 RCT comparing IM droperidol, 10 mg, to haloperidol, 10 mg, found equivalent efficacy and response times (with maximal response occurring within 2 hours) and concluded that droperidol had no advantage over haloperidol.75 Because none of the clinical trials that evaluated droperidol have included assessments for EPS, its risk of akathisia remains uncertain.
Continue to: Ketamine
Ketamine. In recent years, ketamine has been used to treat acute agitation within or en route to the ED. Preliminary observational studies support ketamine’s efficacy when administered via IV or IM routes,76 with more rapid symptomatic improvement compared with haloperidol, lorazepam, or midazolam alone.77 Reported adverse effects of ketamine include dissociation, psychotic exacerbation, and respiratory depression,76 although 1 small naturalistic study found no evidence of exacerbation of psychotic or other psychiatric symptoms.78 An ongoing RCT is comparing IM ketamine, 5 mg/kg, to combined IM haloperidol, 5 mg, and midazolam, 5 mg.79 Although various ketamine formulations are increasingly being used in psychiatry, active psychosis is generally regarded as a contraindication. It is premature to recommend parenteral ketamine administration for agitation within most psychiatric settings until more research on safety has been completed.
Haloperidol, or something else? Practical considerations
Consider the following factors when deciding whether to use haloperidol or one of its alternatives:
Limitations of the evidence. Modern clinical trials requiring informed consent often do not include the kind of severe agitation that clinicians encounter in acute psychiatric, emergency, or forensic settings. In addition, standard interventions, such as 3-medication haloperidol “cocktails,” have not been evaluated in clinical trials. Clinicians are therefore often in the dark about optimal evidence-based practices.
Treatment goals. Psychiatric agitation has many causes, with a range of severity that warrants a commensurate range of responses. Protocols for managing acute agitation should include graded interventions that begin with nonpharmacologic interventions and voluntary oral medications, and move to involuntary IM medications when necessary.
While treatment guidelines clearly recommend against IM medications as “chemical restraint” with a goal of sedating a patient until he/she is unconscious,3,51 such outcomes are nonetheless often sought by staff who are concerned about the risk of injuries during a behavioral emergency. In such instances, the risks of violence towards patients and staff may outweigh concerns about adverse effects in a risk-benefit analysis. Consequently, clinicians may be prone to “skip over” graded interventions because they assume they “won’t work” in favor of administering involuntary multiple-medication haloperidol “cocktails” despite risks of excess sedation, EPS, and cardiotoxicity. Treatment settings should critically evaluate such biased preferences, with a goal of developing tailored, evidence-based strategies that maximize benefits while minimizing excess sedation and other untoward adverse effects, with an eye towards promoting better overall patient care and reducing length of stay.42,43,80
Continue to: Limitations of available medications
Limitations of available medications. There is no perfect medication for the management of acute agitation. Evidence indicates that pharmacologic options take 15 minutes to several hours to resolve acute agitation, even potentially more rapid-acting medications such as midazolam and droperidol. This is well beyond most clinicians’ desired window for response time in a behavioral emergency. Multiple-medication “cocktails” may be used with the hope of hastening response time, but may not achieve this goal at the expense of increasing the risk of adverse effects and the likelihood that a patient will remain sedated for a prolonged time. In the real world, this often means that by the time a psychiatrist comes to evaluate a patient who has been given emergency medications, the patient cannot be aroused for an interview. Ideally, medications would calm an agitated patient rapidly, without excess or prolonged sedation.80 Less-sedating SGAs, such as ziprasidone, might have this potential, but can sometimes be perceived as ineffective.
Avoiding akathisia. Akathisia’s potential to worsen and be mistaken for agitation makes it an especially concerning, if underappreciated, adverse effect of haloperidol that is often not adequately assessed in clinical trials or practice. In light of evidence that akathisia can occur in nearly half of patients receiving a single 5 mg-dose of haloperidol, it is difficult to justify the use of this medication for agitation when equally effective options exist with a lower risk of EPS.
While haloperidol-induced akathisia could in theory be mitigated by adding anticholinergic medications or benzodiazepines, previous studies have found that such strategies have limited effectiveness compared to “gold standard” treatment with propranolol.28,81,82 Furthermore, the half-lives of anticholinergic medications, such as benztropine or diphenhydramine, are significantly shorter than that of a single dose of haloperidol, which can be as long as 37 hours.83 Therefore, akathisia and other EPS could emerge or worsen several hours or even days after receiving an IM haloperidol “cocktail” as the shorter-acting medications wear off. Akathisia is best minimized by avoiding FGAs, such as haloperidol, when treating acute agitation.
Promoting adherence. Although haloperidol is often recommended for acute agitation in patients with schizophrenia or bipolar disorder on the basis that it would treat the underlying condition, many patients who receive IM medications for acute agitation are already prescribed standing doses of oral medication, which increases the risk of cumulative toxicity. In addition, receiving a medication likely to cause acute EPS that is ranked near the bottom of patient preferences may erode the potential for a therapeutic alliance and hamper longer-term antipsychotic medication adherence.

Time for a change
For nearly half a century, haloperidol has been a “gold standard” intervention for IM control in patients with agitation. However, given its potential to produce adverse effects, including a significant risk of akathisia that can worsen agitation, along with the availability of newer pharmacologic options that are at least as effective (Table 1, and Table 2), haloperidol should be retired as a first-line medication for the treatment of agitation. Clinicians would benefit from RCTs investigating the safety and efficacy of novel interventions including frequently-used, but untested medication combinations, as well as nonpharmacologic interventions.

Continue to: Bottom Line
Bottom Line
Although there is no perfect IM medication to treat acute agitation, haloperidol’s higher risk of adverse effects relative to newer alternatives suggest that it should no longer be considered a first-line intervention.
Related Resources
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 1: onset of efficacy. J Emerg Med. 2018;54(3):364-374.
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 2: safety. J Emerg Med. 2018;54(4): 522-532.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Chlorpromazine • Thorazine
Diphenhydramine • Benadryl
Droperidol • Inapsine
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Midazolam • Versed
Olanzapine • Zyprexa
Prochlorperazine • Compazine
Promethazine • Phenergan
Propranolol • Inderal, Pronol
Ziprasidone • Geodon
For more than half a century, haloperidol has been used as a first-line medication for psychiatric agitation constituting a “behavioral emergency” when a patient cannot or will not take oral medication. Today, haloperidol is most commonly administered as an IM injection along with an anticholinergic medication to minimize extrapyramidal symptoms (EPS) and a benzodiazepine for additional sedation. The multiple-medication “cocktail” is often referred to by double-entendre nicknames, such as “B-52” or “5250” (ie, haloperidol, 5 mg; lorazepam, 2 mg; and
Earlier evidence of haloperidol’s efficacy
The initial “discovery” of antipsychotic medications was made in 1951 based on the inadvertent observation that chlorpromazine had the potential to calm surgical patients with autonomic activation. This calming effect, described as “désintéressment” (meaning a kind of “indifference to the world”),1 resulted in a new class of medications replacing barbiturates and bromides as go-to options to achieve “rapid tranquilization” of psychiatric agitation.2 Although the ability of antipsychotic medications to gradually reduce positive symptoms, such as delusions and hallucinations, has been attributed to dopamine (D2) antagonism, their more immediate sedating and anti-agitation effects are the result of broader effects as histamine (H1) and alpha-1 adrenergic antagonists.
In the 1970s, haloperidol emerged as a first-line option to manage agitation due to its IM and IV availability, as well as its relative lack of sedation and orthostasis compared with low-potency D2 antagonists such as chlorpromazine. However, haloperidol was observed to have a significant risk of acute EPS, including dystonic reactions.2 From the 1970s to the 1990s, numerous prospective clinical trials of haloperidol for the treatment of acute psychotic agitation, including several randomized controlled trials (RCTs) comparing haloperidol to lorazepam, were conducted.3 The design and outcomes of the haloperidol vs lorazepam RCTs were fairly consistent4-7:
- adult participants with acute agitation and a variety of psychiatric diagnoses, for whom informed consent often was waived due to agitation severity
- randomization to either IM haloperidol, 5 mg, or IM lorazepam, 2 mg, administered every 30 minutes until agitation resolved
- behavioral outcomes measured over several hours using various rating scales, without consistent assessment of EPS
- equivalent efficacy of haloperidol and lorazepam, with symptom resolution usually achieved after 1 to 2 doses (in 30 to 60 minutes), but sometimes longer
- anticholinergic “rescue” allowed for EPS, but not administered prophylactically
- EPS, including dystonia and akathisia, were significantly more frequent with haloperidol compared with lorazepam.8
In recognition of the greater risk of EPS with haloperidol compared with lorazepam, and the fact that most study participants were already taking standing doses of antipsychotic medications, some researchers have recommended using benzodiazepines alone as the optimal treatment for agitation.4,9 A 2012 Cochrane review concluded that the involuntary use of haloperidol alone “could be considered unethical.”10,11 However, other studies that examined the combination of haloperidol and lorazepam compared with either medication alone found that the combination of the 2 medications was associated with a more rapid resolution of symptoms, which suggests a superior synergistic effect.6,7,12 By the late 1990s, combined haloperidol and lorazepam, often mixed within a single injection, became the most common strategy to achieve rapid tranquilization in the psychiatric emergency setting.13 However, while the combination has been justified as a way to reduce the antipsychotic medication dose and EPS risk,2 few studies have compared combinations containing <5 mg of haloperidol. As a result, the apparent superiority of combined haloperidol and lorazepam compared with either medication alone may be a simple cumulative dose effect rather than true synergism. It is also important to note that adding lorazepam to haloperidol does not mitigate the risk of EPS such as dystonia in the absence of anticholinergic medication.8 To date, however, there have been no clinical trials investigating the efficacy of IM haloperidol, lorazepam, and
Newer RCTs tell a different story
With the availability of second-generation antipsychotics (SGAs) in IM formulations, clinical trials over the past 2 decades have focused on comparing SGAs with haloperidol alone as the “gold standard” control for acute agitation. Compared with previous trials of
- Study participants who signed informed consent (and were likely less agitated)
- IM haloperidol doses typically >5 mg (eg, 6.5 to 10 mg).
As with studies comparing lorazepam with haloperidol, the results of these RCTs revealed that IM
An updated 2017 Cochrane review of haloperidol for psychosis-induced aggression or agitation concluded that9:
- haloperidol is an effective intervention, although the evidence is “weak”
- significant treatment effects may take as long as 1 to 2 hours following multiple IM injections
- in contrast to SGAs, treatment with haloperidol carries a significant risk of EPS
- adding a benzodiazepine “does not have strong evidence of benefit and carries risk of additional harm.”
Continue to: Haloperidol's well-known toxicity
Haloperidol’s well-known toxicity
Haloperidol has been associated with numerous adverse effects:
Akathisia and other acute EPS. Treatment with even a single dose of IM haloperidol can result in acute EPS, including dystonia and akathisia. At best, such adverse effects are subjectively troubling and unpleasant; at worst, akathisia can exacerbate and be mistaken for agitation, leading to administration of more medication23 and the possible development of suicidal or violent behavior.24-25 In the studies reviewed above, the overall rate of EPS was as high as 21% after treatment with haloperidol,16 with parkinsonism occurring in up to 17% of patients,19 dystonia in up to 11%,7 and akathisia in up to 10%.15 However, because specific EPS were assessed inconsistently, and sometimes not at all, the rate of akathisia—arguably the most relevant and counter-therapeutic adverse effect related to agitation—remains unclear.
In another study that specifically assessed for akathisia in patients treated with haloperidol, up to 40% experienced akathisia 6 hours after a single oral dose of 5 mg.26 Even a single dose of IV
Although anticholinergic medications or benzodiazepinesare often administered as part of a haloperidol “cocktail,” these medications often do not adequately resolve emergent akathisia.26,28 No clinical trials of IM haloperidol combined with benztropine or diphenhydramine have been published, but several studies suggest that combining haloperidol with
Cardiotoxicity. Although low-potency antipsychotic medications such as
Continue to: Although there is no direct evidence...
Although there is no direct evidence that the cardiac risks associated with IV haloperidol apply to IM administration, epidemiologic studies indicate that oral haloperidol carries an elevated risk of ventricular arrhythmia and sudden cardiac death,35,36 with 1 study reporting greater risk compared with other SGAs.37 Haloperidol, whether administered orally or IM, may therefore be an especially poor choice for patients with agitation who are at risk for arrhythmia, including those with relevant medical comorbidities or delirium.34
Neuronal cell death. Several lines of research evidence have demonstrated that haloperidol can cause cellular injury or death in neuronal tissue in a dose-dependent fashion through a variety of mechanisms.38 By contrast, SGAs have been shown to have neuroprotective effects.39 While these findings have mostly come from studies conducted in animals or in vitro human tumor cell lines, some researchers have nonetheless called for haloperidol to be banned, noting that if its neurotoxic effects were more widely known, “we would realize what a travesty it is to use [such] a brain-unfriendly drug.”40
Several reasonable alternatives
Echoing the earlier Cochrane review of haloperidol for psychosis-induced aggression or agitation,10 a 2017 update concluded, “If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical.”9
What then are reasonable alternatives to replace IM haloperidol for agitation? Clinicians should consider the following nonpharmacologic and pharmacologic interventions:
Nonpharmacologic interventions. Several behavioral interventions have been demonstrated to be effective for managing acute agitation, including verbal de-escalation, enhanced “programming” on the inpatient units, and the judicious use of seclusion.41-43 While such interventions may demand additional staff or resources, they have the potential to lower long-term costs, reduce injuries to patients and staff, and improve the quality of care.43 The use of IM haloperidol as a form of “chemical restraint” does not represent standard-of-care treatment,3 and from an ethical perspective, should never be implemented punitively or to compensate for substandard care in the form of inadequate staffing or staff training.
Continue to: Benzodiazepines
Benzodiazepines. Lorazepam offers an attractive alternative to haloperidol without the risk of EPS.2,4,8 However, lorazepam alone may be perceived as less efficacious than a haloperidol “cocktail” because it represents less overall medication. Some evidence has suggested that lorazepam, 4 mg, might be the most appropriate dose, although it has only rarely been studied in clinical trials of acute agitation.3
Respiratory depression is frequently cited as an argument against using lorazepam for agitation, as if the therapeutic window is extremely narrow with ineffectiveness at 2 mg, but potential lethality beyond that dose. In fact, serious respiratory depression with lorazepam is unlikely in the absence of chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, or concomitant alcohol or other sedative use.46 Case reports have documented therapeutic lorazepam dosing of 2 to 4 mg every 2 hours up to 20 to 30 mg/d in patients with manic agitation.47 Even in patients with COPD, significant respiratory depression tends not to occur at doses <8 mg.48 A more evidence-based concern about lorazepam dosing is that 2 mg might be ineffective in patients with established tolerance. For example, 1 report described a patient in acute alcohol withdrawal who required dosing lorazepam to 1,600 mg within 24 hours.49 Collectively, these reports suggest that lorazepam has a much wider therapeutic window than is typically perceived, and that dosing with 3 to 4 mg IM is a reasonable option for agitation when 2 mg is likely to be inadequate.
Paradoxical disinhibition is another concern that might prevent benzodiazepines from being used alone as a first-line intervention for emergency treatment of agitation. However, similar to respiratory depression, this adverse event is relatively rare and tends to occur in children and geriatric patients, individuals intoxicated with alcohol or other sedatives, and patients with brain injury, developmental delay, or dementia.23,46 Although exacerbation of aggression has not been demonstrated in the RCTs examining benzodiazepines for agitation reviewed above, based on other research, some clinicians have expressed concerns about the potential for benzodiazepines to exacerbate aggression in patients with impulse control disorders and a history of violent behavior.50
The 2005 Expert Consensus Panel for Behavioral Emergencies51 recommended the use of lorazepam alone over haloperidol for agitation for patients for whom the diagnosis is unknown or includes the following:
- stimulant intoxication
- personality disorder
- comorbid obesity
- comorbid cardiac arrhythmia
- a history of akathisia and other EPS
- a history of amenorrhea/galactorrhea
- a history of seizures.
In surveys, patients have ranked lorazepam as the preferred medication for emergency agitation, whereas haloperidol was ranked as one of the least-preferred options.51,52
Continue to: Second-generation antipsychotics
Second-generation antipsychotics. The SGAs available in IM formulations, such as aripiprazole, olanzapine, and ziprasidone, have been shown to be at least as effective as haloperidol for the treatment of acute agitation (in 2015, the short-acting injectable formulation of aripiprazole was discontinued in the United States independent of safety or efficacy issues53). A review of RCTs examining IM SGAs for the treatment of agitation concluded that the number needed to treat for response compared with placebo was 5 for aripiprazole, 3 for olanzapine, and 3 for ziprasidone.54 In terms of safety, a meta-analysis of studies examining IM medications for agitation confirmed that the risk of acute EPS, including dystonia, akathisia, and parkinsonism, is significantly lower with SGAs compared with haloperidol.55 An RCT comparing IM ziprasidone with haloperidol found equivalently modest effects on QTc prolongation.56 Therefore, SGAs are an obvious and evidence-based option for replacing haloperidol as a treatment for acute agitation.
Unfortunately, for clinicians hoping to replace haloperidol within a multiple-medication IM “cocktail,” there have been no published controlled trials of SGAs combined with benzodiazepines. Although a short report indicated that aripiprazole and lorazepam are chemically compatible to be combined within a single injection,57 the package insert for aripiprazole warns that “If parenteral benzodiazepine therapy is deemed necessary in addition to ABILIFY injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension.”58 The package insert for olanzapine likewise lists the combination of lorazepam and olanzapine as a drug interaction that can potentiate sedation, and the manufacturer issued specific warnings about parenteral combination.59,60 A single published case of significant hypotension with combined IM olanzapine and lorazepam,60 together with the fact that IM olanzapine can cause hypotension by itself,61 has discouraged the coadministration of these medications. Nonetheless, the combination is used in some emergency settings, with several retrospective studies failing to provide evidence of hypotension or respiratory depression as adverse effects.62-64
Droperidol.
Over the past decade, however, droperidol has returned to the US market68 and its IV and IM usage has been revitalized for managing patients with agitation within or en route to the ED. Studies have demonstrated droperidol efficacy comparable to midazolam, ziprasidone, or olanzapine, as well as effectiveness as an IV adjunct to midazolam.69-71 In contrast to the FDA black-box warning, retrospective studies and RCTs of both IV and IM droperidol suggest that QTc prolongation and torsades de pointes are rare events that do not occur any more frequently than they do with haloperidol, even at doses >10 mg.72,73 However, in studies involving patients with drug intoxication and treatment with multiple medications, oversedation to the point of needing rescue intervention was reported. In an emergency setting where these issues are relatively easily managed, such risks may be better tolerated than in psychiatric settings.
With earlier studies examining the use of droperidol in an acute psychiatric setting that reported a more rapid onset of action than haloperidol,65-67 a 2016 Cochrane review concluded that there was high-quality evidence to support droperidol’s use for psychosis-induced agitation.74 However, a 2015 RCT comparing IM droperidol, 10 mg, to haloperidol, 10 mg, found equivalent efficacy and response times (with maximal response occurring within 2 hours) and concluded that droperidol had no advantage over haloperidol.75 Because none of the clinical trials that evaluated droperidol have included assessments for EPS, its risk of akathisia remains uncertain.
Continue to: Ketamine
Ketamine. In recent years, ketamine has been used to treat acute agitation within or en route to the ED. Preliminary observational studies support ketamine’s efficacy when administered via IV or IM routes,76 with more rapid symptomatic improvement compared with haloperidol, lorazepam, or midazolam alone.77 Reported adverse effects of ketamine include dissociation, psychotic exacerbation, and respiratory depression,76 although 1 small naturalistic study found no evidence of exacerbation of psychotic or other psychiatric symptoms.78 An ongoing RCT is comparing IM ketamine, 5 mg/kg, to combined IM haloperidol, 5 mg, and midazolam, 5 mg.79 Although various ketamine formulations are increasingly being used in psychiatry, active psychosis is generally regarded as a contraindication. It is premature to recommend parenteral ketamine administration for agitation within most psychiatric settings until more research on safety has been completed.
Haloperidol, or something else? Practical considerations
Consider the following factors when deciding whether to use haloperidol or one of its alternatives:
Limitations of the evidence. Modern clinical trials requiring informed consent often do not include the kind of severe agitation that clinicians encounter in acute psychiatric, emergency, or forensic settings. In addition, standard interventions, such as 3-medication haloperidol “cocktails,” have not been evaluated in clinical trials. Clinicians are therefore often in the dark about optimal evidence-based practices.
Treatment goals. Psychiatric agitation has many causes, with a range of severity that warrants a commensurate range of responses. Protocols for managing acute agitation should include graded interventions that begin with nonpharmacologic interventions and voluntary oral medications, and move to involuntary IM medications when necessary.
While treatment guidelines clearly recommend against IM medications as “chemical restraint” with a goal of sedating a patient until he/she is unconscious,3,51 such outcomes are nonetheless often sought by staff who are concerned about the risk of injuries during a behavioral emergency. In such instances, the risks of violence towards patients and staff may outweigh concerns about adverse effects in a risk-benefit analysis. Consequently, clinicians may be prone to “skip over” graded interventions because they assume they “won’t work” in favor of administering involuntary multiple-medication haloperidol “cocktails” despite risks of excess sedation, EPS, and cardiotoxicity. Treatment settings should critically evaluate such biased preferences, with a goal of developing tailored, evidence-based strategies that maximize benefits while minimizing excess sedation and other untoward adverse effects, with an eye towards promoting better overall patient care and reducing length of stay.42,43,80
Continue to: Limitations of available medications
Limitations of available medications. There is no perfect medication for the management of acute agitation. Evidence indicates that pharmacologic options take 15 minutes to several hours to resolve acute agitation, even potentially more rapid-acting medications such as midazolam and droperidol. This is well beyond most clinicians’ desired window for response time in a behavioral emergency. Multiple-medication “cocktails” may be used with the hope of hastening response time, but may not achieve this goal at the expense of increasing the risk of adverse effects and the likelihood that a patient will remain sedated for a prolonged time. In the real world, this often means that by the time a psychiatrist comes to evaluate a patient who has been given emergency medications, the patient cannot be aroused for an interview. Ideally, medications would calm an agitated patient rapidly, without excess or prolonged sedation.80 Less-sedating SGAs, such as ziprasidone, might have this potential, but can sometimes be perceived as ineffective.
Avoiding akathisia. Akathisia’s potential to worsen and be mistaken for agitation makes it an especially concerning, if underappreciated, adverse effect of haloperidol that is often not adequately assessed in clinical trials or practice. In light of evidence that akathisia can occur in nearly half of patients receiving a single 5 mg-dose of haloperidol, it is difficult to justify the use of this medication for agitation when equally effective options exist with a lower risk of EPS.
While haloperidol-induced akathisia could in theory be mitigated by adding anticholinergic medications or benzodiazepines, previous studies have found that such strategies have limited effectiveness compared to “gold standard” treatment with propranolol.28,81,82 Furthermore, the half-lives of anticholinergic medications, such as benztropine or diphenhydramine, are significantly shorter than that of a single dose of haloperidol, which can be as long as 37 hours.83 Therefore, akathisia and other EPS could emerge or worsen several hours or even days after receiving an IM haloperidol “cocktail” as the shorter-acting medications wear off. Akathisia is best minimized by avoiding FGAs, such as haloperidol, when treating acute agitation.
Promoting adherence. Although haloperidol is often recommended for acute agitation in patients with schizophrenia or bipolar disorder on the basis that it would treat the underlying condition, many patients who receive IM medications for acute agitation are already prescribed standing doses of oral medication, which increases the risk of cumulative toxicity. In addition, receiving a medication likely to cause acute EPS that is ranked near the bottom of patient preferences may erode the potential for a therapeutic alliance and hamper longer-term antipsychotic medication adherence.

Time for a change
For nearly half a century, haloperidol has been a “gold standard” intervention for IM control in patients with agitation. However, given its potential to produce adverse effects, including a significant risk of akathisia that can worsen agitation, along with the availability of newer pharmacologic options that are at least as effective (Table 1, and Table 2), haloperidol should be retired as a first-line medication for the treatment of agitation. Clinicians would benefit from RCTs investigating the safety and efficacy of novel interventions including frequently-used, but untested medication combinations, as well as nonpharmacologic interventions.

Continue to: Bottom Line
Bottom Line
Although there is no perfect IM medication to treat acute agitation, haloperidol’s higher risk of adverse effects relative to newer alternatives suggest that it should no longer be considered a first-line intervention.
Related Resources
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 1: onset of efficacy. J Emerg Med. 2018;54(3):364-374.
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 2: safety. J Emerg Med. 2018;54(4): 522-532.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Chlorpromazine • Thorazine
Diphenhydramine • Benadryl
Droperidol • Inapsine
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Midazolam • Versed
Olanzapine • Zyprexa
Prochlorperazine • Compazine
Promethazine • Phenergan
Propranolol • Inderal, Pronol
Ziprasidone • Geodon
1. Shorter E. A history of psychiatry. New York, NY: John Wiley & Sons, Inc.; 1997:249.
2. Salzman C, Green AI, Rodriguez-Villa F, et al. Benzodiazepines combined with neuroleptics for management of severe disruptive behavior. Psychosomatics. 1986;27(suppl 1):17-22.
3. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatr. 2000;61(suppl 14):11-20.
4. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatr. 1991:52(4):177-180.
5. Allen MH, Currier GW, Hughes DH, et al. The expert consensus guideline series: treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1-88; quiz 89-90.
6. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12(3):175-179.
7. Garza-Trevino WS, Hollister LE, Overall JE, et al. Efficacy of combinations of intramuscular antipsychotics and sedative-hypnotics for control of psychotic agitation. Am J Psychiatr. 1989:146(12):1598-1601.
8. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective double-blind, emergency study. Am J Emerg Med 1997;15(4):335-340.
9. Ostinelli EG, Brooke-Powney MJ, Li X, et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2017; 7:CD009377. doi: 10.1002/14651858.CD009377.pub3.
10. Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2.
11. Citrome L. Review: limited evidence on effects of haloperidol alone for rapid tranquillisation in psychosis-induced aggression. Evid Based Ment Health. 2013;16(2):47.
12. Bienek SA, Ownby R, Penalver A, et al. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacother. 1998;18(1):57-62.
13. Binder RL, McNiel DE. Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Serv. 1999;50(2):1553-1554.
14. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl). 2006;188(3):281-292.
15. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatr. 2007;68(1):111-119.
16. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatr. 2000;61(12):933-941.
17. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
18. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatr. 2001;158(7):1149-1151.
19. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psych. 2002;59(5):441-448.
20. Hsu W, Huang S, Lee B, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30(3):230-234.
21. Chan H, Ree S, Su L, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34(3):355-358.
22. Baldaçara L, Sanches M, Cordeiro DC, et al. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30-39.
23. Hillard JR. Defusing patient violence. Current Psychiatry. 2002;1(4):22-29.
24. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
25. Eikelenboom-Schieveld SJM, Lucire Y, Fogleman JC. The relevance of cytochrome P450 polymorphism in forensic medicine and akathisia-related violence and suicide. J Forens Leg Med. 2016;41:65-71.
26. Van Putten T, May PRA, Marder SR. Akathisia with haloperidol and thiothixene. Arch Gen Psych. 1984;41:1036-1039.
27. Drotts DL, Vinson DR. Prochlorperazine induced akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
28. Salem H, Negpal C, Pigott T. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
29. Huf G, Coutinho ESF, Adams CE. Rapid tranquilization in psychiatric emergency settings in Brazil: pragmatic randomized controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869.
30. Mantovani C, Labate CM, Sponholz A, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306-312.
31. Darwish H, Grant R, Haslam R, et al. Promethazine-induced acute dystonic reactions. Am J Dis Child. 1980;134(10):990-991.
32. Jyothi CH, Rudraiah HGM, Vidya HK, et al. Promethazine induced acute dystonia: a case report. Manipal J Med Sci. 2016;1(2):63-64.
33. Ames D, Carr-Lopez SM, Gutierrez MA, et al. Detecting and managing adverse effects of antipsychotic medications: current state of play. Psychiatr Clin North Am. 2016;39(2):275-311.
34. Meyer-Massetti C, Cheng CM, Sharpe MA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5(4):E8-E16. doi: 10.1002/jhm.691.
35. Wu C, Tsai Y, Tsai H. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Dis. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568.
36. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointe, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(1):105-122.
37. Leonard CE, Freeman CP, Newcomb CW, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;suppl 10(6):1-9.
38. Nasrallah H, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatr. 2017;29(3):195-202.
39. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
40. Nasrallah HA. Haloperidol clearly is neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
41. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Comm Psychiatr. 1993;44(2):125-133.
42. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172.
43. Vieta E, Garriga M, Cardete L, et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatr. 2017;17:328.
44. Nobay F, Simon BC, Levitt A, et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
45. Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385.
46. Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatr. 1998;59(suppl 1):57-60.
47. Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985;5(2):109-110.
48. Denaut M, Yernault JC, De Coster A. Double-blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Curr Med Res Opin. 1975;2(10):611-615.
49. Kahn DR, Barnhorst AV, Bourgeois JA. A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours. CNS Spectr. 2009;14(7):385-389.
50. Jones KA. Benzodiazepines: their role in aggression and why GPs should prescribe with caution. Austral Fam Physician. 2011;40(11):862-865.
51. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-108.
52. Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58.
53. Han DH. Some Abilify formulations to discontinue in 2015. MPR. https://www.empr.com/home/news/some-abilify-formulations-to-discontinue-in-2015/. Published January 13, 2015. Accessed April 17, 2020.
54. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
55. Satterthwaite TD, Wolf DH, Rosenheck RA, et al. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment for agitation. J Clin Psychiatr. 2008;69(12):1869-1879.
56. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491.
57. Kovalick LJ, Pikalov AA, Ni N, et al. Short-term physical compatibility of intramuscular aripiprazole with intramuscular lorazepam. Am J Health-Syst Pharm. 2008;65(21):2007-2008.
58. Abilify [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
59. Zyprexa [package insert]. Indianapolis, IN: Lilly Research Laboratories; 2005.
60. Zacher JL, Roche-Desilets J. Hypotension secondary to the combination of intramuscular olanzapine and intramuscular lorazepam. J Clin Psychiatr. 2005;66(12):1614-1615.
61. Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatr 2010;71(4):433-441.
62. Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-797.
63. Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-896.
64. Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin [Internet]. 2018;8(5):208-213.
65. Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298-299.
66. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407-413.
67. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567-573.
68. Boyer EW. Droperidol is back (and here’s what you need to know). ACEP Now. https://www.acepnow.com/article/droperidol-is-back-and-heres-what-you-need-to-know/. Published September 16, 2019. Accessed April 17, 2020.
69. Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167-1172.
70. Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72-81.
71. Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392-401.
72. Macht M, Mull AC, McVaney KE, et al. Comparison of droperidol and haloperidol for use by paramedics assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18(3):375-380.
73. Calver L, Page CB, Downes MA, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med. 2015;66(3):230-238.
74. Kohokar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12:CD002830.
75. Calver L, Drinkwater V, Gupta R, et al. Droperidol v. haloperidol for sedation of aggressive behavior in acute mental health: randomized controlled trial. Brit J Psychiatr. 2015;206(3):223-228.
76. Hopper AB, Vilke GM, Castillo EM, et al. Ketamine use for acute agitation in the emergency department. J Emerg Med. 2015;48(6):712-719.
77. Riddell J, Tran A, Bengiamin R, et al. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35:1000-1004.
78. Lebin JA, Akhavan AR, Hippe DS, et al. Psychiatric outcomes of patients with severe agitation following administration of prehospital ketamine. Acad Emerg Med. 2019;26(8):889-896.
79. Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department (RACKED): a randomized controlled trial protocol. Trials. 2018;19(1):651.
80. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatr. 2016;17(2):86-128.
81. Adler L, Angrist B, Peselow E, et al. Efficacy of propranolol in neuroleptic-induced akathesia. J Clin Psychopharmacol. 1985;5(3):164-166.
82. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
83. de Leon J, Diaz FJ, Wedlund P, et al. Haloperidol half-life after chronic dosing. J Clin Psychopharmacol. 2004;24(6):656-660.
1. Shorter E. A history of psychiatry. New York, NY: John Wiley & Sons, Inc.; 1997:249.
2. Salzman C, Green AI, Rodriguez-Villa F, et al. Benzodiazepines combined with neuroleptics for management of severe disruptive behavior. Psychosomatics. 1986;27(suppl 1):17-22.
3. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatr. 2000;61(suppl 14):11-20.
4. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatr. 1991:52(4):177-180.
5. Allen MH, Currier GW, Hughes DH, et al. The expert consensus guideline series: treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1-88; quiz 89-90.
6. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12(3):175-179.
7. Garza-Trevino WS, Hollister LE, Overall JE, et al. Efficacy of combinations of intramuscular antipsychotics and sedative-hypnotics for control of psychotic agitation. Am J Psychiatr. 1989:146(12):1598-1601.
8. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective double-blind, emergency study. Am J Emerg Med 1997;15(4):335-340.
9. Ostinelli EG, Brooke-Powney MJ, Li X, et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2017; 7:CD009377. doi: 10.1002/14651858.CD009377.pub3.
10. Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2.
11. Citrome L. Review: limited evidence on effects of haloperidol alone for rapid tranquillisation in psychosis-induced aggression. Evid Based Ment Health. 2013;16(2):47.
12. Bienek SA, Ownby R, Penalver A, et al. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacother. 1998;18(1):57-62.
13. Binder RL, McNiel DE. Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Serv. 1999;50(2):1553-1554.
14. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl). 2006;188(3):281-292.
15. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatr. 2007;68(1):111-119.
16. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatr. 2000;61(12):933-941.
17. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
18. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatr. 2001;158(7):1149-1151.
19. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psych. 2002;59(5):441-448.
20. Hsu W, Huang S, Lee B, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30(3):230-234.
21. Chan H, Ree S, Su L, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34(3):355-358.
22. Baldaçara L, Sanches M, Cordeiro DC, et al. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30-39.
23. Hillard JR. Defusing patient violence. Current Psychiatry. 2002;1(4):22-29.
24. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
25. Eikelenboom-Schieveld SJM, Lucire Y, Fogleman JC. The relevance of cytochrome P450 polymorphism in forensic medicine and akathisia-related violence and suicide. J Forens Leg Med. 2016;41:65-71.
26. Van Putten T, May PRA, Marder SR. Akathisia with haloperidol and thiothixene. Arch Gen Psych. 1984;41:1036-1039.
27. Drotts DL, Vinson DR. Prochlorperazine induced akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
28. Salem H, Negpal C, Pigott T. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
29. Huf G, Coutinho ESF, Adams CE. Rapid tranquilization in psychiatric emergency settings in Brazil: pragmatic randomized controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869.
30. Mantovani C, Labate CM, Sponholz A, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306-312.
31. Darwish H, Grant R, Haslam R, et al. Promethazine-induced acute dystonic reactions. Am J Dis Child. 1980;134(10):990-991.
32. Jyothi CH, Rudraiah HGM, Vidya HK, et al. Promethazine induced acute dystonia: a case report. Manipal J Med Sci. 2016;1(2):63-64.
33. Ames D, Carr-Lopez SM, Gutierrez MA, et al. Detecting and managing adverse effects of antipsychotic medications: current state of play. Psychiatr Clin North Am. 2016;39(2):275-311.
34. Meyer-Massetti C, Cheng CM, Sharpe MA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5(4):E8-E16. doi: 10.1002/jhm.691.
35. Wu C, Tsai Y, Tsai H. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Dis. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568.
36. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointe, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(1):105-122.
37. Leonard CE, Freeman CP, Newcomb CW, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;suppl 10(6):1-9.
38. Nasrallah H, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatr. 2017;29(3):195-202.
39. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
40. Nasrallah HA. Haloperidol clearly is neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
41. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Comm Psychiatr. 1993;44(2):125-133.
42. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172.
43. Vieta E, Garriga M, Cardete L, et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatr. 2017;17:328.
44. Nobay F, Simon BC, Levitt A, et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
45. Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385.
46. Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatr. 1998;59(suppl 1):57-60.
47. Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985;5(2):109-110.
48. Denaut M, Yernault JC, De Coster A. Double-blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Curr Med Res Opin. 1975;2(10):611-615.
49. Kahn DR, Barnhorst AV, Bourgeois JA. A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours. CNS Spectr. 2009;14(7):385-389.
50. Jones KA. Benzodiazepines: their role in aggression and why GPs should prescribe with caution. Austral Fam Physician. 2011;40(11):862-865.
51. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-108.
52. Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58.
53. Han DH. Some Abilify formulations to discontinue in 2015. MPR. https://www.empr.com/home/news/some-abilify-formulations-to-discontinue-in-2015/. Published January 13, 2015. Accessed April 17, 2020.
54. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
55. Satterthwaite TD, Wolf DH, Rosenheck RA, et al. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment for agitation. J Clin Psychiatr. 2008;69(12):1869-1879.
56. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491.
57. Kovalick LJ, Pikalov AA, Ni N, et al. Short-term physical compatibility of intramuscular aripiprazole with intramuscular lorazepam. Am J Health-Syst Pharm. 2008;65(21):2007-2008.
58. Abilify [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
59. Zyprexa [package insert]. Indianapolis, IN: Lilly Research Laboratories; 2005.
60. Zacher JL, Roche-Desilets J. Hypotension secondary to the combination of intramuscular olanzapine and intramuscular lorazepam. J Clin Psychiatr. 2005;66(12):1614-1615.
61. Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatr 2010;71(4):433-441.
62. Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-797.
63. Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-896.
64. Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin [Internet]. 2018;8(5):208-213.
65. Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298-299.
66. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407-413.
67. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567-573.
68. Boyer EW. Droperidol is back (and here’s what you need to know). ACEP Now. https://www.acepnow.com/article/droperidol-is-back-and-heres-what-you-need-to-know/. Published September 16, 2019. Accessed April 17, 2020.
69. Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167-1172.
70. Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72-81.
71. Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392-401.
72. Macht M, Mull AC, McVaney KE, et al. Comparison of droperidol and haloperidol for use by paramedics assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18(3):375-380.
73. Calver L, Page CB, Downes MA, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med. 2015;66(3):230-238.
74. Kohokar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12:CD002830.
75. Calver L, Drinkwater V, Gupta R, et al. Droperidol v. haloperidol for sedation of aggressive behavior in acute mental health: randomized controlled trial. Brit J Psychiatr. 2015;206(3):223-228.
76. Hopper AB, Vilke GM, Castillo EM, et al. Ketamine use for acute agitation in the emergency department. J Emerg Med. 2015;48(6):712-719.
77. Riddell J, Tran A, Bengiamin R, et al. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35:1000-1004.
78. Lebin JA, Akhavan AR, Hippe DS, et al. Psychiatric outcomes of patients with severe agitation following administration of prehospital ketamine. Acad Emerg Med. 2019;26(8):889-896.
79. Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department (RACKED): a randomized controlled trial protocol. Trials. 2018;19(1):651.
80. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatr. 2016;17(2):86-128.
81. Adler L, Angrist B, Peselow E, et al. Efficacy of propranolol in neuroleptic-induced akathesia. J Clin Psychopharmacol. 1985;5(3):164-166.
82. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
83. de Leon J, Diaz FJ, Wedlund P, et al. Haloperidol half-life after chronic dosing. J Clin Psychopharmacol. 2004;24(6):656-660.
Cannabidiol (CBD) for schizophrenia: Promise or pipe dream?
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
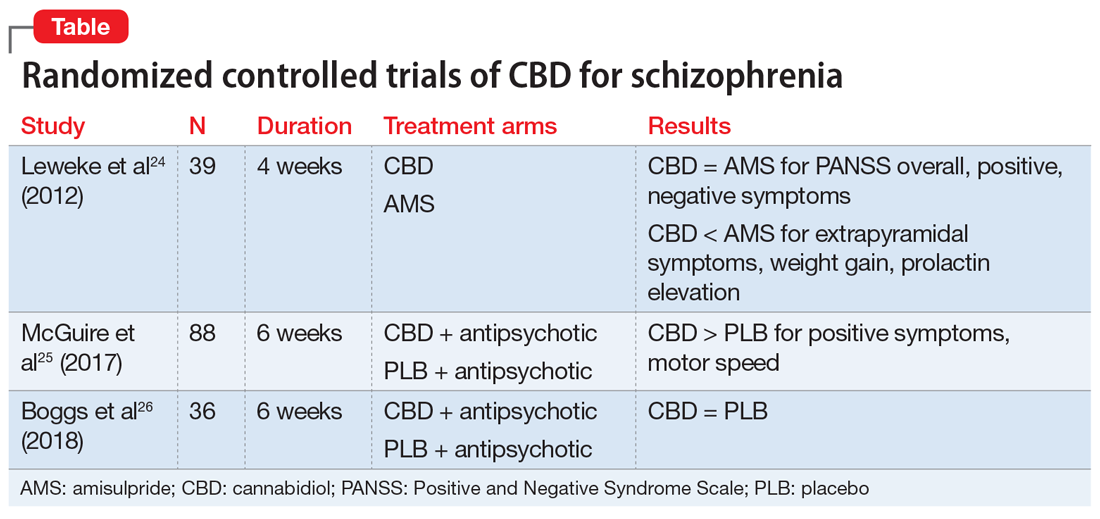
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.

Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.

Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Abuse of psychiatric medications: Not just stimulants and benzodiazepines
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
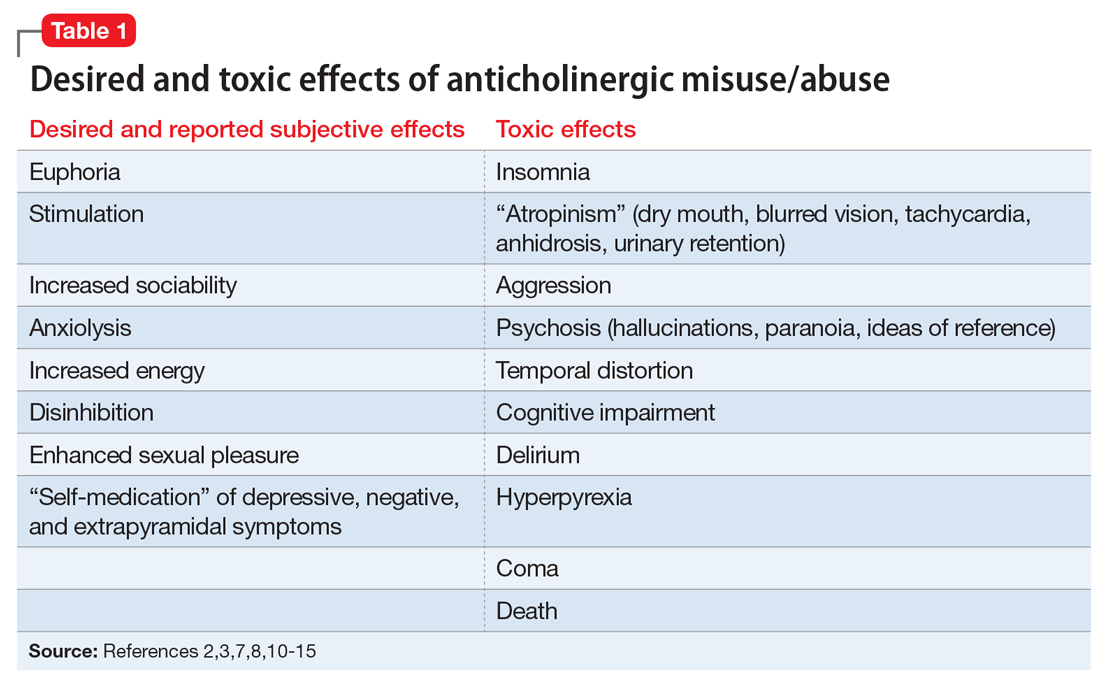
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15

Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15

Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
Real-world challenges in managing ‘dual diagnosis’ patients
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
Risks of increasingly potent Cannabis: The joint effects of potency and frequency
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.
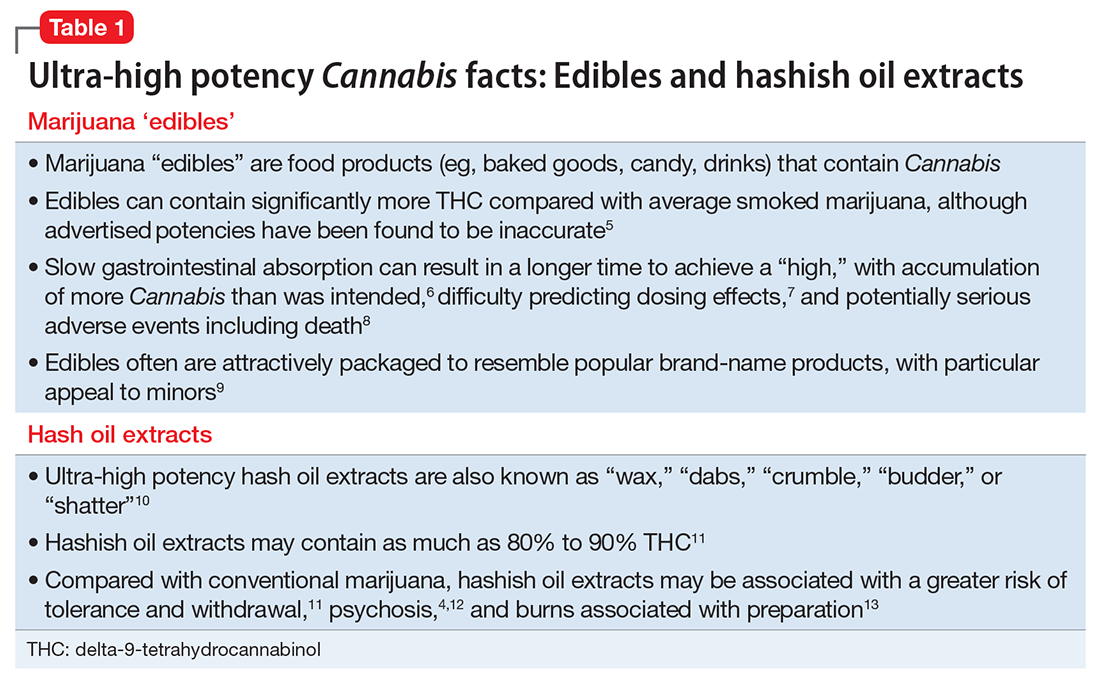
The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2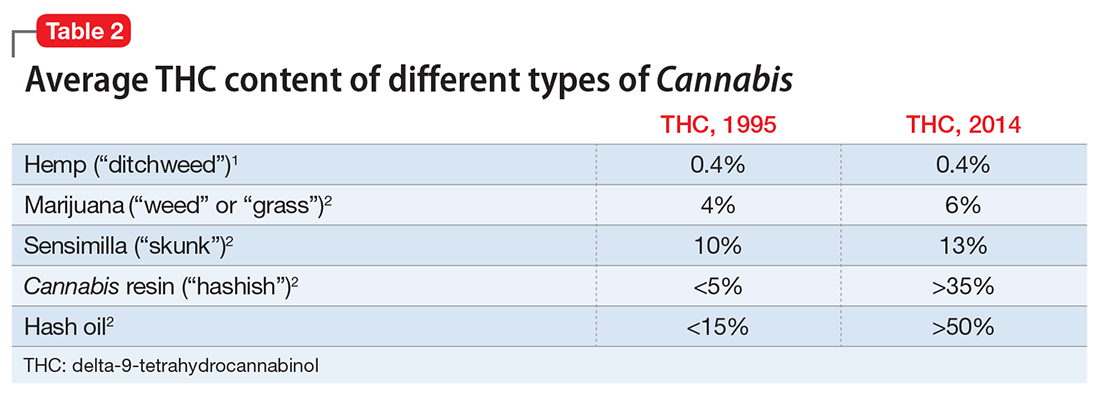
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.

The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.

The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
Cannabis, synthetic cannabinoids, and psychosis risk: What the evidence says
Discuss this article at www.facebook.com/CurrentPsychiatry
Over the past 50 years, anecdotal reports linking cannabis sativa (marijuana) and psychosis have been steadily accumulating, giving rise to the notion of “cannabis psychosis.” Despite this historic connection, marijuana often is regarded as a “soft drug” with few harmful effects. However, this benign view is now being revised, along with mounting research demonstrating a clear association between cannabis and psychosis.
In this article, I review evidence on marijuana’s impact on the risk of developing psychotic disorders, as well as the potential contributions of “medical” marijuana and other legally available products containing synthetic cannabinoids to psychosis risk.
Cannabis use and psychosis
Cannabis use has a largely deleterious effect on patients with psychotic disorders, and typically is associated with relapse, poor treatment adherence, and worsening psychotic symptoms.1,2 There is, however, evidence that some patients with schizophrenia might benefit from treatment with cannabidiol,3-5 another constituent of marijuana, as well as delta-9-tetrahydrocannabinol (Δ-9-THC), the principle psychoactive constituent of cannabis.6,7
The acute psychotic potential of cannabis has been demonstrated by studies that documented psychotic symptoms (eg, hallucinations, paranoid delusions, derealization) in a dose-dependent manner among healthy volunteers administered Δ-9-THC under experimental conditions.8-10 Various cross-sectional epidemiologic studies also have revealed an association between cannabis use and acute or chronic psychosis.11,12
In the absence of definitive evidence from randomized, long-term, placebo-controlled trials, the strongest evidence of a connection between cannabis use and development of a psychotic disorder comes from prospective, longitudinal cohort studies. In the past 15 years, new evidence has emerged from 7 such studies that cumulatively provide strong support for an association between cannabis use as an adolescent or young adult and a greater risk for developing a psychotic disorder such as schizophrenia.13-19 These longitudinal studies surveyed for self-reported cannabis use before psychosis onset and controlled for a variety of potential confounding factors (eg, other drug use and demographic, social, and psychological variables). Three meta-analyses of these and other studies concluded an increased risk of psychosis is associated with cannabis use, with an odds ratio of 1.4 to 2.9 (meaning the risk of developing psychosis with any history of cannabis use is up to 3-fold higher compared with those who did not use cannabis).11,20,21 In addition, this association appears to be dose-related, with increasing amounts of cannabis use linked to greater risk—1 study found an odds ratio of 7 for psychosis among daily cannabis users.16
There are several ways to explain the link between cannabis use and psychosis, and a causal relationship has not yet been firmly established (Table 1).1-7,11-19,21-25 Current evidence supports that cannabis is a “component cause” of chronic psychosis, meaning although neither necessary nor sufficient, cannabis use at a young age increases the likelihood of developing schizophrenia or other psychotic disorders.26 This risk may be greatest for young persons with some psychosis vulnerability (eg, those with attenuated psychotic symptoms).16,18
The overall magnitude of risk appears to be modest, and cannabis use is only 1 of myriad factors that increase the risk of psychosis.27 Furthermore, most cannabis users do not develop psychosis. However, the risk associated with cannabis occurs during a vulnerable time of development and is modifiable. Based on conservative estimates, 8% of emergent schizophrenia cases and 14% of more broadly defined emergent psychosis cases could be prevented if it were possible to eliminate cannabis use among young people.11,26 Therefore, reducing cannabis use among young people vulnerable to psychosis should be a clinical and public health priority.
Table 1
Hypotheses linking cannabis and psychosis
| Hypothesis | Strength of evidence | Evidence for | Evidence against |
|---|---|---|---|
| Cannabis does not cause chronic psychosis | Weak |
| |
| Cannabis can cause schizophrenia | Equivocal | Cannabis use precedes the onset of schizophrenia in longitudinal studies13-19 | The incidence of schizophrenia has not been clearly increasing as expected with increasing cannabis use11,21 |
| Cannabis worsens existing psychotic disorders | Strong | Cannabidiol and Δ-9-THC improve symptoms in some patients with schizophrenia3-7 | |
| Cannabis increases the risk of chronic psychosis among vulnerable individuals | Strong | Cannabis use is not always a risk factor for conversion to psychosis in studies of prodromal schizophrenia25 | |
| Δ-9-THC: delta-9-tetrahydrocannabinol | |||
Medical marijuana
Although cannabis extracts were marketed by major pharmaceutical companies and widely used by consumers for various ailments during the late 1800s, medicinal cannabis use in the United States declined significantly during the early 20th century. In 1937, the Marihuana Tax Act was passed, effectively putting a stop to physicians prescribing cannabis for medical purposes. The FDA currently classifies cannabis as a Schedule I drug (eg, high abuse potential, no currently accepted medical use, lack of safety data) and the use of cannabis and its prescription by physicians are prohibited under federal law.
However, in recognition of the potential medical benefits of cannabis, 16 states have legalized medicinal use (“medical marijuana”) over the past several years. Laws and regulations governing medical marijuana vary from state to state. For example, in California, adults who obtain a recommendation from a physician and register for a Medical Marijuana Identification Card can legally purchase cannabis from a state-recognized dispensary and use it in a non-public setting. The physician’s “recommendation” (not a prescription) is based upon the determination that “the person’s health would benefit from the use of marijuana in the treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief”28 (emphasis added). Although no state has yet legalized cannabis use for recreational purposes, with such regulations, an increasing number of jurisdictions have provided a way for consumers to easily obtain marijuana for loosely defined medical purposes.
Medical marijuana dispensaries offer a variety of cannabis strains, each with a different advertised “high” based upon variable proportions of Δ-9-THC and other constituents. The Δ-9-THC content of medical marijuana is about twice that of “street” marijuana, even with the latter’s Δ-9-THC content rising to >10% over the past 15 years.29,30 Therefore, medical marijuana is not only legal, but generally offers a more potent Δ-9-THC dose than typical street marijuana.
A single case of psychosis emerging in the context of medical marijuana has been reported in the literature.31 A 24-year-old man with mild, transient psychotic symptoms switched from street cannabis to medical marijuana for its superior potency and to conform with the law. He obtained a physician’s recommendation based on diagnoses of “posttraumatic stress disorder” and “pain.” After several months of increasingly frequent medical marijuana use, he developed florid and persistent psychotic symptoms necessitating antipsychotic medication, and was diagnosed with schizophrenia.
Although causality cannot be established based on this report, taken together with evidence that higher-potency cannabis is associated with a greater risk of psychotic emergence,32 this case raises concerns about the iatrogenic and psychotoxic liability of medical marijuana use among those vulnerable to psychosis. Policy decisions about medical marijuana and its use among patients with psychiatric illness must be informed by evidence of its psychotic potential.
Synthetic cannabinoids
Synthetic cannabinoids were developed in the 1960s for research purposes and potential clinical applications, but have not been FDA-approved for therapeutic use.33 Over the past 5 years, however, a variety of “herbal incense” products bearing names such as “Spice,” “K2,” and “Aroma” have emerged in Europe and the United States that contain botanicals laced with synthetic cannabinoids (Table 2).
Although herbal incense products are labeled “not for human consumption,” they are sold by “head shops” and on the Internet without age restrictions and typically are purchased for the sole purpose of ingesting them, usually by smoking. Their desired effects resemble cannabis intoxication, including sedation, relaxation, altered consciousness, and euphoria. The products initially had the added appeal of being legal and undetectable in routine drug screening. Although not listed among the product’s ingredients, chemical analyses confirmed these products typically contained 1 of 3 families of synthetic cannabinoid1 and cannabinoid2 (CB1/CB2) receptor agonists, designated by the prefixes JWH-, CP-, and HU-.34 The compounds most commonly found in these analyses (JWH-018; CP-47,497; HU-210) have significantly greater potency (ie, CB1 receptor affinity) compared with Δ-9-THC.33,34
The growing popularity of herbal incense products has prompted health concerns based on reports of emergency presentations for adverse effects, including tachycardia, agitation, excess sedation, and loss of consciousness.33,35,36 In addition, 8 anecdotal reports of psychosis associated with herbal incense (with a total of 33 patients) have emerged since 2010 (Table 3). Among them, a variety of psychotic symptoms are described in patients ranging in age from adolescence to adulthood, both with and without histories of psychosis. For those without a pre-existing psychotic disorder, symptoms were typically self-limited.
In the most recently presented case series of patients without pre-existing psychosis (N = 10), symptoms resolved in 70% of patients within 8 days, but 30% had psychosis that persisted beyond 5-month follow-up.37 Collectively, these reports suggest that synthetic cannabinoid intoxication is associated with acute psychosis as well as exacerbations of previously stable psychotic disorders, and also may have a propensity to trigger a chronic psychotic disorder among vulnerable individuals.
Because of health concerns and the abuse potential of herbal incense products, many have been banned in several European countries, 18 U.S. states, and the U.S. military.33,38 In March 2011, the FDA placed 5 synthetic cannabinoids (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) on Schedule I, making them illegal to possess or sell in the United States.38 However, there are hundreds of synthetic cannabinoid homologues, and herbal incense manufacturers have rapidly adapted by substituting other synthetic cannabinoids not yet banned by existing legislation.34 The effects of these newly arising compounds in humans, including their psychotic potential, are largely unknown.
Table 2
Herbal incense products and synthetic cannabinoids
| Herbal incense brand names | Cannabinoids they may contain |
|---|---|
| Spice, K2, Mojo, Aroma, Dream, Chill, Chaos, Sence, Smoke, Skunk, Space Diamond, Silent Black, Genie, Algerian Blend, Yucatan Fire, Tai Fun, Sensation, SpicyXXX, Spike 99, Bonsai-18, Banana Cream Nuke, Wicked X, Natures Organic, Zen |
|
Table 3
Case reports of psychosis associated with synthetic cannabinoids
| Study | N (age) | Herbal product or suspected cannabinoid | Previous psychotic disorder | Symptoms |
|---|---|---|---|---|
| Müller et al, 2010a | 1 (25) | JWH-018 “Spice” | Yes | Anxiety, exacerbation of paranoid delusions, delusions of control, auditory hallucinations |
| Vearrier et al, 2010b | 1 (17) | JWH-018 | No | Tachycardia, hypokalemia, agitation, visual hallucinations |
| Every-Palmer, 2010c | 5 | JWH-018 CP-47,497 | Yes | Agitation, disorganization, paranoid and grandiose delusions |
| Rodgman et al, 2011d | 3 | JWH-018 (“Mojo”) | – | “Mojo psychosis” |
| Benford et al, 2011e | 1 (20) | JWH-018 (“Spice”) | – | Tachycardia, anxiety, paranoia, auditory and visual hallucinations |
| Van Der Veer et al, 2011f | 3 (20 to 30) | “Spice” “Spike 99” | No | Anxiety, disorganization, paranoia, Capgras delusion |
| Every-Palmer, 2011g | 9 (20s to 40s) | JWH-018 (“Aroma”) | Yes | Anxiety, agitation, paranoia |
| Hurst et al, 2011h | 10 (21 to 25) | “Spice” | No | Anxiety, agitation, confusion, disorganization, paranoia, ideas of reference, hallucinations |
| Source: References a. Müller H, Sperling W, Köhrmann M, et al. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. 2010;118(1-3):309-310. b. Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care. 2010;26(6):462-465. c. Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105(10):1859-1860. d. Rodgman C, Kinzie E, Leimbach E. Bad Mojo: use of the new marijuana substitute leads to more and more ED visits for acute psychosis. Am J Emerg Med. 2011;29(2):232. e. Benford DM, Caplan JP. Psychiatric sequelae of spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics. 2011;52(3):295. f. Van Der Veer N, Friday J. Persistent psychosis following the use of Spice. Schizophr Res. 2011;130(1-3):285-286. g. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011. [Epub ahead of print]. h. Hurst D, Loeffler G, McLay R. Synthetic cannabinoid agonist induced psychosis: a case series. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, HI | ||||
Related Resources
- Murray RM, Morrison PD, Henquet C, et al. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8(11):885-895.
- European Monitoring Centre for Drugs and Drug Addiction: Synthetic cannabinoids and “spice.” www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids.
- U.S. Department of Justice, Drug Enforcement Agency, Office of Diversion Control: Schedules of controlled substances: temporary placement of five synthetic cannabinoids into Schedule I. www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm.
1. Degenhardt L, Tennant C, Gilmour S, et al. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med. 2007;37(7):927-934.
2. Zammit S, Moore TH, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193(5):357-363.
3. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39(4):421-429.
4. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
5. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
6. Schwarcz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
7. Schwarcz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
8. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558-1572.
9. Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39(10):1607-1616.
10. Favrat B, Ménétrey A, Augsburger M, et al. Two cases of “cannabis acute psychosis” following the administration of oral cannabis. BMC Psychiatry. 2005;5:17.-
11. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328
12. Minozzi S, Davoli M, Bargagli AM, et al. An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug Alcohol Rev. 2010;29(3):304-317.
13. Andréasson S, Allebeck P, Engström A, et al. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483-1486
14. Zammit S, Allebeck P, Andreasson S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325(7374):1199.-
15. Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212-1213
16. van Os J, Bak M, Hanssen M, et al. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156(4):319-327
17. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21
18. Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330(7481):11.-
19. Kuepper R, van Os J, Lieb R, et al. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738.-
20. Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608-612
21. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194
22. D’Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594-608
23. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561
24. Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151(1-2):151-154.
25. Phillips LJ, Curry C, Yung AR, et al. Cannabis use is not associated with the development of psychosis in an “ultra” high-risk group. Aust N Z J Psychiatry. 2002;36(6):800-806
26. Arseneault L, Cannon M, Witton J, et al. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110-117.
27. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102(1-3):1-18.
28. California Secretary of State. California Proposition 215: Text of proposed law. Available at: http://vote96.sos.ca.gov/Vote96/html/BP/215text.htm. Accessed July 27, 2011.
29. Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117(1):59-61
30. Gieringer D. Medical cannabis potency testing project. Bulletin of the Multidisciplinary Association for Psychedelic Studies. 1999;9(3):20 22. Available at: http://www.maps.org/news-letters/v09n3/09320gie.html. Accessed July 27, 2011.
31. Pierre JM. Psychosis associated with medical marijuana: risk vs. benefits of medicinal cannabis use. Am J Psychiatry. 2010;167(5):598-599
32. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195(6):488-491.
33. Vardakou I, Pistos C, Spiliopoulou CH. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157-162
34. Dresen S, Ferreirós N, Pütz M, et al. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45(10):1186-1194.
35. Simmons JR, Skinner CG, Williams J, et al. Intoxication from smoking “spice.” Ann Emerg Med. 2011;57(2):187-188
36. Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299
37. Hurst D, Loeffler G, McLay R. Synthetic cannabinoid agonist induced psychosis: a case series. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, HI.
38. U.S. Department of Justice Drug Enforcement Agency. Temporary placement of five synthetic cannabinoids into schedule I. Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm. Accessed July 27, 2011.
Discuss this article at www.facebook.com/CurrentPsychiatry
Over the past 50 years, anecdotal reports linking cannabis sativa (marijuana) and psychosis have been steadily accumulating, giving rise to the notion of “cannabis psychosis.” Despite this historic connection, marijuana often is regarded as a “soft drug” with few harmful effects. However, this benign view is now being revised, along with mounting research demonstrating a clear association between cannabis and psychosis.
In this article, I review evidence on marijuana’s impact on the risk of developing psychotic disorders, as well as the potential contributions of “medical” marijuana and other legally available products containing synthetic cannabinoids to psychosis risk.
Cannabis use and psychosis
Cannabis use has a largely deleterious effect on patients with psychotic disorders, and typically is associated with relapse, poor treatment adherence, and worsening psychotic symptoms.1,2 There is, however, evidence that some patients with schizophrenia might benefit from treatment with cannabidiol,3-5 another constituent of marijuana, as well as delta-9-tetrahydrocannabinol (Δ-9-THC), the principle psychoactive constituent of cannabis.6,7
The acute psychotic potential of cannabis has been demonstrated by studies that documented psychotic symptoms (eg, hallucinations, paranoid delusions, derealization) in a dose-dependent manner among healthy volunteers administered Δ-9-THC under experimental conditions.8-10 Various cross-sectional epidemiologic studies also have revealed an association between cannabis use and acute or chronic psychosis.11,12
In the absence of definitive evidence from randomized, long-term, placebo-controlled trials, the strongest evidence of a connection between cannabis use and development of a psychotic disorder comes from prospective, longitudinal cohort studies. In the past 15 years, new evidence has emerged from 7 such studies that cumulatively provide strong support for an association between cannabis use as an adolescent or young adult and a greater risk for developing a psychotic disorder such as schizophrenia.13-19 These longitudinal studies surveyed for self-reported cannabis use before psychosis onset and controlled for a variety of potential confounding factors (eg, other drug use and demographic, social, and psychological variables). Three meta-analyses of these and other studies concluded an increased risk of psychosis is associated with cannabis use, with an odds ratio of 1.4 to 2.9 (meaning the risk of developing psychosis with any history of cannabis use is up to 3-fold higher compared with those who did not use cannabis).11,20,21 In addition, this association appears to be dose-related, with increasing amounts of cannabis use linked to greater risk—1 study found an odds ratio of 7 for psychosis among daily cannabis users.16
There are several ways to explain the link between cannabis use and psychosis, and a causal relationship has not yet been firmly established (Table 1).1-7,11-19,21-25 Current evidence supports that cannabis is a “component cause” of chronic psychosis, meaning although neither necessary nor sufficient, cannabis use at a young age increases the likelihood of developing schizophrenia or other psychotic disorders.26 This risk may be greatest for young persons with some psychosis vulnerability (eg, those with attenuated psychotic symptoms).16,18
The overall magnitude of risk appears to be modest, and cannabis use is only 1 of myriad factors that increase the risk of psychosis.27 Furthermore, most cannabis users do not develop psychosis. However, the risk associated with cannabis occurs during a vulnerable time of development and is modifiable. Based on conservative estimates, 8% of emergent schizophrenia cases and 14% of more broadly defined emergent psychosis cases could be prevented if it were possible to eliminate cannabis use among young people.11,26 Therefore, reducing cannabis use among young people vulnerable to psychosis should be a clinical and public health priority.
Table 1
Hypotheses linking cannabis and psychosis
| Hypothesis | Strength of evidence | Evidence for | Evidence against |
|---|---|---|---|
| Cannabis does not cause chronic psychosis | Weak |
| |
| Cannabis can cause schizophrenia | Equivocal | Cannabis use precedes the onset of schizophrenia in longitudinal studies13-19 | The incidence of schizophrenia has not been clearly increasing as expected with increasing cannabis use11,21 |
| Cannabis worsens existing psychotic disorders | Strong | Cannabidiol and Δ-9-THC improve symptoms in some patients with schizophrenia3-7 | |
| Cannabis increases the risk of chronic psychosis among vulnerable individuals | Strong | Cannabis use is not always a risk factor for conversion to psychosis in studies of prodromal schizophrenia25 | |
| Δ-9-THC: delta-9-tetrahydrocannabinol | |||
Medical marijuana
Although cannabis extracts were marketed by major pharmaceutical companies and widely used by consumers for various ailments during the late 1800s, medicinal cannabis use in the United States declined significantly during the early 20th century. In 1937, the Marihuana Tax Act was passed, effectively putting a stop to physicians prescribing cannabis for medical purposes. The FDA currently classifies cannabis as a Schedule I drug (eg, high abuse potential, no currently accepted medical use, lack of safety data) and the use of cannabis and its prescription by physicians are prohibited under federal law.
However, in recognition of the potential medical benefits of cannabis, 16 states have legalized medicinal use (“medical marijuana”) over the past several years. Laws and regulations governing medical marijuana vary from state to state. For example, in California, adults who obtain a recommendation from a physician and register for a Medical Marijuana Identification Card can legally purchase cannabis from a state-recognized dispensary and use it in a non-public setting. The physician’s “recommendation” (not a prescription) is based upon the determination that “the person’s health would benefit from the use of marijuana in the treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief”28 (emphasis added). Although no state has yet legalized cannabis use for recreational purposes, with such regulations, an increasing number of jurisdictions have provided a way for consumers to easily obtain marijuana for loosely defined medical purposes.
Medical marijuana dispensaries offer a variety of cannabis strains, each with a different advertised “high” based upon variable proportions of Δ-9-THC and other constituents. The Δ-9-THC content of medical marijuana is about twice that of “street” marijuana, even with the latter’s Δ-9-THC content rising to >10% over the past 15 years.29,30 Therefore, medical marijuana is not only legal, but generally offers a more potent Δ-9-THC dose than typical street marijuana.
A single case of psychosis emerging in the context of medical marijuana has been reported in the literature.31 A 24-year-old man with mild, transient psychotic symptoms switched from street cannabis to medical marijuana for its superior potency and to conform with the law. He obtained a physician’s recommendation based on diagnoses of “posttraumatic stress disorder” and “pain.” After several months of increasingly frequent medical marijuana use, he developed florid and persistent psychotic symptoms necessitating antipsychotic medication, and was diagnosed with schizophrenia.
Although causality cannot be established based on this report, taken together with evidence that higher-potency cannabis is associated with a greater risk of psychotic emergence,32 this case raises concerns about the iatrogenic and psychotoxic liability of medical marijuana use among those vulnerable to psychosis. Policy decisions about medical marijuana and its use among patients with psychiatric illness must be informed by evidence of its psychotic potential.
Synthetic cannabinoids
Synthetic cannabinoids were developed in the 1960s for research purposes and potential clinical applications, but have not been FDA-approved for therapeutic use.33 Over the past 5 years, however, a variety of “herbal incense” products bearing names such as “Spice,” “K2,” and “Aroma” have emerged in Europe and the United States that contain botanicals laced with synthetic cannabinoids (Table 2).
Although herbal incense products are labeled “not for human consumption,” they are sold by “head shops” and on the Internet without age restrictions and typically are purchased for the sole purpose of ingesting them, usually by smoking. Their desired effects resemble cannabis intoxication, including sedation, relaxation, altered consciousness, and euphoria. The products initially had the added appeal of being legal and undetectable in routine drug screening. Although not listed among the product’s ingredients, chemical analyses confirmed these products typically contained 1 of 3 families of synthetic cannabinoid1 and cannabinoid2 (CB1/CB2) receptor agonists, designated by the prefixes JWH-, CP-, and HU-.34 The compounds most commonly found in these analyses (JWH-018; CP-47,497; HU-210) have significantly greater potency (ie, CB1 receptor affinity) compared with Δ-9-THC.33,34
The growing popularity of herbal incense products has prompted health concerns based on reports of emergency presentations for adverse effects, including tachycardia, agitation, excess sedation, and loss of consciousness.33,35,36 In addition, 8 anecdotal reports of psychosis associated with herbal incense (with a total of 33 patients) have emerged since 2010 (Table 3). Among them, a variety of psychotic symptoms are described in patients ranging in age from adolescence to adulthood, both with and without histories of psychosis. For those without a pre-existing psychotic disorder, symptoms were typically self-limited.
In the most recently presented case series of patients without pre-existing psychosis (N = 10), symptoms resolved in 70% of patients within 8 days, but 30% had psychosis that persisted beyond 5-month follow-up.37 Collectively, these reports suggest that synthetic cannabinoid intoxication is associated with acute psychosis as well as exacerbations of previously stable psychotic disorders, and also may have a propensity to trigger a chronic psychotic disorder among vulnerable individuals.
Because of health concerns and the abuse potential of herbal incense products, many have been banned in several European countries, 18 U.S. states, and the U.S. military.33,38 In March 2011, the FDA placed 5 synthetic cannabinoids (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) on Schedule I, making them illegal to possess or sell in the United States.38 However, there are hundreds of synthetic cannabinoid homologues, and herbal incense manufacturers have rapidly adapted by substituting other synthetic cannabinoids not yet banned by existing legislation.34 The effects of these newly arising compounds in humans, including their psychotic potential, are largely unknown.
Table 2
Herbal incense products and synthetic cannabinoids
| Herbal incense brand names | Cannabinoids they may contain |
|---|---|
| Spice, K2, Mojo, Aroma, Dream, Chill, Chaos, Sence, Smoke, Skunk, Space Diamond, Silent Black, Genie, Algerian Blend, Yucatan Fire, Tai Fun, Sensation, SpicyXXX, Spike 99, Bonsai-18, Banana Cream Nuke, Wicked X, Natures Organic, Zen |
|
Table 3
Case reports of psychosis associated with synthetic cannabinoids
| Study | N (age) | Herbal product or suspected cannabinoid | Previous psychotic disorder | Symptoms |
|---|---|---|---|---|
| Müller et al, 2010a | 1 (25) | JWH-018 “Spice” | Yes | Anxiety, exacerbation of paranoid delusions, delusions of control, auditory hallucinations |
| Vearrier et al, 2010b | 1 (17) | JWH-018 | No | Tachycardia, hypokalemia, agitation, visual hallucinations |
| Every-Palmer, 2010c | 5 | JWH-018 CP-47,497 | Yes | Agitation, disorganization, paranoid and grandiose delusions |
| Rodgman et al, 2011d | 3 | JWH-018 (“Mojo”) | – | “Mojo psychosis” |
| Benford et al, 2011e | 1 (20) | JWH-018 (“Spice”) | – | Tachycardia, anxiety, paranoia, auditory and visual hallucinations |
| Van Der Veer et al, 2011f | 3 (20 to 30) | “Spice” “Spike 99” | No | Anxiety, disorganization, paranoia, Capgras delusion |
| Every-Palmer, 2011g | 9 (20s to 40s) | JWH-018 (“Aroma”) | Yes | Anxiety, agitation, paranoia |
| Hurst et al, 2011h | 10 (21 to 25) | “Spice” | No | Anxiety, agitation, confusion, disorganization, paranoia, ideas of reference, hallucinations |
| Source: References a. Müller H, Sperling W, Köhrmann M, et al. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. 2010;118(1-3):309-310. b. Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care. 2010;26(6):462-465. c. Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105(10):1859-1860. d. Rodgman C, Kinzie E, Leimbach E. Bad Mojo: use of the new marijuana substitute leads to more and more ED visits for acute psychosis. Am J Emerg Med. 2011;29(2):232. e. Benford DM, Caplan JP. Psychiatric sequelae of spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics. 2011;52(3):295. f. Van Der Veer N, Friday J. Persistent psychosis following the use of Spice. Schizophr Res. 2011;130(1-3):285-286. g. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011. [Epub ahead of print]. h. Hurst D, Loeffler G, McLay R. Synthetic cannabinoid agonist induced psychosis: a case series. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, HI | ||||
Related Resources
- Murray RM, Morrison PD, Henquet C, et al. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8(11):885-895.
- European Monitoring Centre for Drugs and Drug Addiction: Synthetic cannabinoids and “spice.” www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids.
- U.S. Department of Justice, Drug Enforcement Agency, Office of Diversion Control: Schedules of controlled substances: temporary placement of five synthetic cannabinoids into Schedule I. www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm.
Discuss this article at www.facebook.com/CurrentPsychiatry
Over the past 50 years, anecdotal reports linking cannabis sativa (marijuana) and psychosis have been steadily accumulating, giving rise to the notion of “cannabis psychosis.” Despite this historic connection, marijuana often is regarded as a “soft drug” with few harmful effects. However, this benign view is now being revised, along with mounting research demonstrating a clear association between cannabis and psychosis.
In this article, I review evidence on marijuana’s impact on the risk of developing psychotic disorders, as well as the potential contributions of “medical” marijuana and other legally available products containing synthetic cannabinoids to psychosis risk.
Cannabis use and psychosis
Cannabis use has a largely deleterious effect on patients with psychotic disorders, and typically is associated with relapse, poor treatment adherence, and worsening psychotic symptoms.1,2 There is, however, evidence that some patients with schizophrenia might benefit from treatment with cannabidiol,3-5 another constituent of marijuana, as well as delta-9-tetrahydrocannabinol (Δ-9-THC), the principle psychoactive constituent of cannabis.6,7
The acute psychotic potential of cannabis has been demonstrated by studies that documented psychotic symptoms (eg, hallucinations, paranoid delusions, derealization) in a dose-dependent manner among healthy volunteers administered Δ-9-THC under experimental conditions.8-10 Various cross-sectional epidemiologic studies also have revealed an association between cannabis use and acute or chronic psychosis.11,12
In the absence of definitive evidence from randomized, long-term, placebo-controlled trials, the strongest evidence of a connection between cannabis use and development of a psychotic disorder comes from prospective, longitudinal cohort studies. In the past 15 years, new evidence has emerged from 7 such studies that cumulatively provide strong support for an association between cannabis use as an adolescent or young adult and a greater risk for developing a psychotic disorder such as schizophrenia.13-19 These longitudinal studies surveyed for self-reported cannabis use before psychosis onset and controlled for a variety of potential confounding factors (eg, other drug use and demographic, social, and psychological variables). Three meta-analyses of these and other studies concluded an increased risk of psychosis is associated with cannabis use, with an odds ratio of 1.4 to 2.9 (meaning the risk of developing psychosis with any history of cannabis use is up to 3-fold higher compared with those who did not use cannabis).11,20,21 In addition, this association appears to be dose-related, with increasing amounts of cannabis use linked to greater risk—1 study found an odds ratio of 7 for psychosis among daily cannabis users.16
There are several ways to explain the link between cannabis use and psychosis, and a causal relationship has not yet been firmly established (Table 1).1-7,11-19,21-25 Current evidence supports that cannabis is a “component cause” of chronic psychosis, meaning although neither necessary nor sufficient, cannabis use at a young age increases the likelihood of developing schizophrenia or other psychotic disorders.26 This risk may be greatest for young persons with some psychosis vulnerability (eg, those with attenuated psychotic symptoms).16,18
The overall magnitude of risk appears to be modest, and cannabis use is only 1 of myriad factors that increase the risk of psychosis.27 Furthermore, most cannabis users do not develop psychosis. However, the risk associated with cannabis occurs during a vulnerable time of development and is modifiable. Based on conservative estimates, 8% of emergent schizophrenia cases and 14% of more broadly defined emergent psychosis cases could be prevented if it were possible to eliminate cannabis use among young people.11,26 Therefore, reducing cannabis use among young people vulnerable to psychosis should be a clinical and public health priority.
Table 1
Hypotheses linking cannabis and psychosis
| Hypothesis | Strength of evidence | Evidence for | Evidence against |
|---|---|---|---|
| Cannabis does not cause chronic psychosis | Weak |
| |
| Cannabis can cause schizophrenia | Equivocal | Cannabis use precedes the onset of schizophrenia in longitudinal studies13-19 | The incidence of schizophrenia has not been clearly increasing as expected with increasing cannabis use11,21 |
| Cannabis worsens existing psychotic disorders | Strong | Cannabidiol and Δ-9-THC improve symptoms in some patients with schizophrenia3-7 | |
| Cannabis increases the risk of chronic psychosis among vulnerable individuals | Strong | Cannabis use is not always a risk factor for conversion to psychosis in studies of prodromal schizophrenia25 | |
| Δ-9-THC: delta-9-tetrahydrocannabinol | |||
Medical marijuana
Although cannabis extracts were marketed by major pharmaceutical companies and widely used by consumers for various ailments during the late 1800s, medicinal cannabis use in the United States declined significantly during the early 20th century. In 1937, the Marihuana Tax Act was passed, effectively putting a stop to physicians prescribing cannabis for medical purposes. The FDA currently classifies cannabis as a Schedule I drug (eg, high abuse potential, no currently accepted medical use, lack of safety data) and the use of cannabis and its prescription by physicians are prohibited under federal law.
However, in recognition of the potential medical benefits of cannabis, 16 states have legalized medicinal use (“medical marijuana”) over the past several years. Laws and regulations governing medical marijuana vary from state to state. For example, in California, adults who obtain a recommendation from a physician and register for a Medical Marijuana Identification Card can legally purchase cannabis from a state-recognized dispensary and use it in a non-public setting. The physician’s “recommendation” (not a prescription) is based upon the determination that “the person’s health would benefit from the use of marijuana in the treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief”28 (emphasis added). Although no state has yet legalized cannabis use for recreational purposes, with such regulations, an increasing number of jurisdictions have provided a way for consumers to easily obtain marijuana for loosely defined medical purposes.
Medical marijuana dispensaries offer a variety of cannabis strains, each with a different advertised “high” based upon variable proportions of Δ-9-THC and other constituents. The Δ-9-THC content of medical marijuana is about twice that of “street” marijuana, even with the latter’s Δ-9-THC content rising to >10% over the past 15 years.29,30 Therefore, medical marijuana is not only legal, but generally offers a more potent Δ-9-THC dose than typical street marijuana.
A single case of psychosis emerging in the context of medical marijuana has been reported in the literature.31 A 24-year-old man with mild, transient psychotic symptoms switched from street cannabis to medical marijuana for its superior potency and to conform with the law. He obtained a physician’s recommendation based on diagnoses of “posttraumatic stress disorder” and “pain.” After several months of increasingly frequent medical marijuana use, he developed florid and persistent psychotic symptoms necessitating antipsychotic medication, and was diagnosed with schizophrenia.
Although causality cannot be established based on this report, taken together with evidence that higher-potency cannabis is associated with a greater risk of psychotic emergence,32 this case raises concerns about the iatrogenic and psychotoxic liability of medical marijuana use among those vulnerable to psychosis. Policy decisions about medical marijuana and its use among patients with psychiatric illness must be informed by evidence of its psychotic potential.
Synthetic cannabinoids
Synthetic cannabinoids were developed in the 1960s for research purposes and potential clinical applications, but have not been FDA-approved for therapeutic use.33 Over the past 5 years, however, a variety of “herbal incense” products bearing names such as “Spice,” “K2,” and “Aroma” have emerged in Europe and the United States that contain botanicals laced with synthetic cannabinoids (Table 2).
Although herbal incense products are labeled “not for human consumption,” they are sold by “head shops” and on the Internet without age restrictions and typically are purchased for the sole purpose of ingesting them, usually by smoking. Their desired effects resemble cannabis intoxication, including sedation, relaxation, altered consciousness, and euphoria. The products initially had the added appeal of being legal and undetectable in routine drug screening. Although not listed among the product’s ingredients, chemical analyses confirmed these products typically contained 1 of 3 families of synthetic cannabinoid1 and cannabinoid2 (CB1/CB2) receptor agonists, designated by the prefixes JWH-, CP-, and HU-.34 The compounds most commonly found in these analyses (JWH-018; CP-47,497; HU-210) have significantly greater potency (ie, CB1 receptor affinity) compared with Δ-9-THC.33,34
The growing popularity of herbal incense products has prompted health concerns based on reports of emergency presentations for adverse effects, including tachycardia, agitation, excess sedation, and loss of consciousness.33,35,36 In addition, 8 anecdotal reports of psychosis associated with herbal incense (with a total of 33 patients) have emerged since 2010 (Table 3). Among them, a variety of psychotic symptoms are described in patients ranging in age from adolescence to adulthood, both with and without histories of psychosis. For those without a pre-existing psychotic disorder, symptoms were typically self-limited.
In the most recently presented case series of patients without pre-existing psychosis (N = 10), symptoms resolved in 70% of patients within 8 days, but 30% had psychosis that persisted beyond 5-month follow-up.37 Collectively, these reports suggest that synthetic cannabinoid intoxication is associated with acute psychosis as well as exacerbations of previously stable psychotic disorders, and also may have a propensity to trigger a chronic psychotic disorder among vulnerable individuals.
Because of health concerns and the abuse potential of herbal incense products, many have been banned in several European countries, 18 U.S. states, and the U.S. military.33,38 In March 2011, the FDA placed 5 synthetic cannabinoids (JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol) on Schedule I, making them illegal to possess or sell in the United States.38 However, there are hundreds of synthetic cannabinoid homologues, and herbal incense manufacturers have rapidly adapted by substituting other synthetic cannabinoids not yet banned by existing legislation.34 The effects of these newly arising compounds in humans, including their psychotic potential, are largely unknown.
Table 2
Herbal incense products and synthetic cannabinoids
| Herbal incense brand names | Cannabinoids they may contain |
|---|---|
| Spice, K2, Mojo, Aroma, Dream, Chill, Chaos, Sence, Smoke, Skunk, Space Diamond, Silent Black, Genie, Algerian Blend, Yucatan Fire, Tai Fun, Sensation, SpicyXXX, Spike 99, Bonsai-18, Banana Cream Nuke, Wicked X, Natures Organic, Zen |
|
Table 3
Case reports of psychosis associated with synthetic cannabinoids
| Study | N (age) | Herbal product or suspected cannabinoid | Previous psychotic disorder | Symptoms |
|---|---|---|---|---|
| Müller et al, 2010a | 1 (25) | JWH-018 “Spice” | Yes | Anxiety, exacerbation of paranoid delusions, delusions of control, auditory hallucinations |
| Vearrier et al, 2010b | 1 (17) | JWH-018 | No | Tachycardia, hypokalemia, agitation, visual hallucinations |
| Every-Palmer, 2010c | 5 | JWH-018 CP-47,497 | Yes | Agitation, disorganization, paranoid and grandiose delusions |
| Rodgman et al, 2011d | 3 | JWH-018 (“Mojo”) | – | “Mojo psychosis” |
| Benford et al, 2011e | 1 (20) | JWH-018 (“Spice”) | – | Tachycardia, anxiety, paranoia, auditory and visual hallucinations |
| Van Der Veer et al, 2011f | 3 (20 to 30) | “Spice” “Spike 99” | No | Anxiety, disorganization, paranoia, Capgras delusion |
| Every-Palmer, 2011g | 9 (20s to 40s) | JWH-018 (“Aroma”) | Yes | Anxiety, agitation, paranoia |
| Hurst et al, 2011h | 10 (21 to 25) | “Spice” | No | Anxiety, agitation, confusion, disorganization, paranoia, ideas of reference, hallucinations |
| Source: References a. Müller H, Sperling W, Köhrmann M, et al. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. 2010;118(1-3):309-310. b. Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care. 2010;26(6):462-465. c. Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105(10):1859-1860. d. Rodgman C, Kinzie E, Leimbach E. Bad Mojo: use of the new marijuana substitute leads to more and more ED visits for acute psychosis. Am J Emerg Med. 2011;29(2):232. e. Benford DM, Caplan JP. Psychiatric sequelae of spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics. 2011;52(3):295. f. Van Der Veer N, Friday J. Persistent psychosis following the use of Spice. Schizophr Res. 2011;130(1-3):285-286. g. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011. [Epub ahead of print]. h. Hurst D, Loeffler G, McLay R. Synthetic cannabinoid agonist induced psychosis: a case series. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, HI | ||||
Related Resources
- Murray RM, Morrison PD, Henquet C, et al. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8(11):885-895.
- European Monitoring Centre for Drugs and Drug Addiction: Synthetic cannabinoids and “spice.” www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids.
- U.S. Department of Justice, Drug Enforcement Agency, Office of Diversion Control: Schedules of controlled substances: temporary placement of five synthetic cannabinoids into Schedule I. www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm.
1. Degenhardt L, Tennant C, Gilmour S, et al. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med. 2007;37(7):927-934.
2. Zammit S, Moore TH, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193(5):357-363.
3. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39(4):421-429.
4. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
5. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
6. Schwarcz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
7. Schwarcz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
8. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558-1572.
9. Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39(10):1607-1616.
10. Favrat B, Ménétrey A, Augsburger M, et al. Two cases of “cannabis acute psychosis” following the administration of oral cannabis. BMC Psychiatry. 2005;5:17.-
11. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328
12. Minozzi S, Davoli M, Bargagli AM, et al. An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug Alcohol Rev. 2010;29(3):304-317.
13. Andréasson S, Allebeck P, Engström A, et al. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483-1486
14. Zammit S, Allebeck P, Andreasson S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325(7374):1199.-
15. Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212-1213
16. van Os J, Bak M, Hanssen M, et al. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156(4):319-327
17. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21
18. Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330(7481):11.-
19. Kuepper R, van Os J, Lieb R, et al. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738.-
20. Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608-612
21. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194
22. D’Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594-608
23. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561
24. Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151(1-2):151-154.
25. Phillips LJ, Curry C, Yung AR, et al. Cannabis use is not associated with the development of psychosis in an “ultra” high-risk group. Aust N Z J Psychiatry. 2002;36(6):800-806
26. Arseneault L, Cannon M, Witton J, et al. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110-117.
27. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102(1-3):1-18.
28. California Secretary of State. California Proposition 215: Text of proposed law. Available at: http://vote96.sos.ca.gov/Vote96/html/BP/215text.htm. Accessed July 27, 2011.
29. Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117(1):59-61
30. Gieringer D. Medical cannabis potency testing project. Bulletin of the Multidisciplinary Association for Psychedelic Studies. 1999;9(3):20 22. Available at: http://www.maps.org/news-letters/v09n3/09320gie.html. Accessed July 27, 2011.
31. Pierre JM. Psychosis associated with medical marijuana: risk vs. benefits of medicinal cannabis use. Am J Psychiatry. 2010;167(5):598-599
32. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195(6):488-491.
33. Vardakou I, Pistos C, Spiliopoulou CH. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157-162
34. Dresen S, Ferreirós N, Pütz M, et al. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45(10):1186-1194.
35. Simmons JR, Skinner CG, Williams J, et al. Intoxication from smoking “spice.” Ann Emerg Med. 2011;57(2):187-188
36. Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299
37. Hurst D, Loeffler G, McLay R. Synthetic cannabinoid agonist induced psychosis: a case series. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, HI.
38. U.S. Department of Justice Drug Enforcement Agency. Temporary placement of five synthetic cannabinoids into schedule I. Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm. Accessed July 27, 2011.
1. Degenhardt L, Tennant C, Gilmour S, et al. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med. 2007;37(7):927-934.
2. Zammit S, Moore TH, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193(5):357-363.
3. Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39(4):421-429.
4. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
5. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
6. Schwarcz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
7. Schwarcz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
8. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558-1572.
9. Morrison PD, Zois V, McKeown DA, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39(10):1607-1616.
10. Favrat B, Ménétrey A, Augsburger M, et al. Two cases of “cannabis acute psychosis” following the administration of oral cannabis. BMC Psychiatry. 2005;5:17.-
11. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328
12. Minozzi S, Davoli M, Bargagli AM, et al. An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug Alcohol Rev. 2010;29(3):304-317.
13. Andréasson S, Allebeck P, Engström A, et al. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483-1486
14. Zammit S, Allebeck P, Andreasson S, et al. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325(7374):1199.-
15. Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212-1213
16. van Os J, Bak M, Hanssen M, et al. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156(4):319-327
17. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21
18. Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330(7481):11.-
19. Kuepper R, van Os J, Lieb R, et al. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738.-
20. Henquet C, Murray R, Linszen D, et al. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608-612
21. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194
22. D’Souza DC, Abi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594-608
23. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561
24. Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151(1-2):151-154.
25. Phillips LJ, Curry C, Yung AR, et al. Cannabis use is not associated with the development of psychosis in an “ultra” high-risk group. Aust N Z J Psychiatry. 2002;36(6):800-806
26. Arseneault L, Cannon M, Witton J, et al. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110-117.
27. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102(1-3):1-18.
28. California Secretary of State. California Proposition 215: Text of proposed law. Available at: http://vote96.sos.ca.gov/Vote96/html/BP/215text.htm. Accessed July 27, 2011.
29. Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117(1):59-61
30. Gieringer D. Medical cannabis potency testing project. Bulletin of the Multidisciplinary Association for Psychedelic Studies. 1999;9(3):20 22. Available at: http://www.maps.org/news-letters/v09n3/09320gie.html. Accessed July 27, 2011.
31. Pierre JM. Psychosis associated with medical marijuana: risk vs. benefits of medicinal cannabis use. Am J Psychiatry. 2010;167(5):598-599
32. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195(6):488-491.
33. Vardakou I, Pistos C, Spiliopoulou CH. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157-162
34. Dresen S, Ferreirós N, Pütz M, et al. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. 2010;45(10):1186-1194.
35. Simmons JR, Skinner CG, Williams J, et al. Intoxication from smoking “spice.” Ann Emerg Med. 2011;57(2):187-188
36. Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299
37. Hurst D, Loeffler G, McLay R. Synthetic cannabinoid agonist induced psychosis: a case series. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, HI.
38. U.S. Department of Justice Drug Enforcement Agency. Temporary placement of five synthetic cannabinoids into schedule I. Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm. Accessed July 27, 2011.
High-dose antipsychotics: Desperation or data-driven?
When nothing else works, desperate clinicians are resorting to progressively more-tenuous and unpredictable treatments, trying to improve the lives of patients with refractory schizophrenia. High-dose antipsychotics is a common strategy.
Does boosting antipsychotic doses beyond the recommended range—but short of the neuroleptic threshold—enhance efficacy? This article attempts to answer that question by presenting the evidence on higher-than-recommended doses of atypical antipsychotics.
Lessons from neuroleptics
Up to 30% of patients with schizophrenia do not respond to antipsychotics and are considered “treatment refractory.”1 Even among those who do respond, improving symptoms by 20%—as research defines “treatment response”—does not necessarily yield clinical or functional improvement. Clozapine is the only atypical antipsychotic with well-established efficacy in these chronically ill patients,2 but its daunting side effects greatly curtail its use.
Before atypical antipsychotics, patients who did not respond to usual dosages of the typical neuroleptics were treated with higher dosages or switched to another drug class. Although many clinicians embraced high-dose neuroleptics, subsequent research discredited “rapid neuroleptization” in any clinical circumstance and showed that exceeding an antipsychotic’s neuroleptic threshold—the dose at which extrapyramidal side effects (EPS) occur—reduces its efficacy (Figure 1).3-5 In some instances, reducing neuroleptic dosages improves treatment-resistant patients’ symptoms and reduces druginduced side effects.6
Figure 1 Typical antipsychotics’ dose-response curve
Narrow therapeutic window between antipsychotic effect and neuroleptic threshold. Dotted line indicates declining efficacy.
Figure 2 Atypical antipsychotics’ dose-response curve
Wider therapeutic window with atypicals, compared with typical antipsychotics, as neuroleptic threshold (dotted line) moves right.Atypical antipsychotics are defined by their relative lack of EPS at recommended dosages (Figure 2). Because these agents can cause EPS if dosed too high, however, our historical habit of testing this dose limit risks losing “atypicality” and encountering other untoward events (Figure 3).
What is the safest, most effective dosage? Consider the evidence for each atypical antipsychotic.
Risperidone
Recommended dosage too high? When using atypicals at recommended doses, you are most likely to encounter the neuroleptic threshold with risperidone, with EPS risk increasing substantially at >6 mg/d.7 Post-approval studies set the most effective and safest dosage at approximately 4 mg/d, though this dosage was not studied in North American pre-approval trials. Dosages of 2 to 4 mg/d have been associated with more-favorable outcomes, suggesting that the initial recommendation to titrate to 6 mg/d within the first 3 days was ill-advised.8
In our study of patients with treatment-refractory schizophrenia,9 those treated with risperidone, 6 mg/d, improved significantly more after 4 weeks than did those receiving haloperidol, 15 mg/d, based on Brief Psychiatric Rating Scale (BPRS) scores. No additional benefit was seen after risperidone was increased to >6 mg/d at 8 weeks. Akathisia and tardive dyskinesia occurred significantly more often in the haloperidol group.
Conclusion. Some patients respond to higher-dose risperidone, but emerging EPS suggest the need to reduce the dosage rather than add an antiparkinsonian agent.
Figure 3 Unknown effects of high-dose atypical antipsychotic therapy
Dotted line indicates potential for greater antipsychotic effect with increasing dose.
Olanzapine
Mixed results. Case reports suggest that some patients who did not respond to previous antipsychotic trials or olanzapine, 20 mg/d, improved sig-nificantly—without substanial side effects—when olanzapine was increased up to 60 mg/d.10-14 Other case studies, however, report EPS, increased heart rate, increased transaminases, hyperprolactinemia, and prolonged QTc interval with high-dose olanzapine.14-16
In an open-label trial,17 43 patients with schizophrenia received olanzapine, up to 40 mg/d, after inadequate response to neuroleptics and risperidone or clozapine. Olanzapine was titrated to 20 mg/d by week 4 and increased 5 mg every 2 weeks if symptoms did not improve. After 14 weeks, improvement was modest and only 17% of patients met response criteria. However, >20 mg/d reduced symptoms more than did <20 mg/d, suggesting that high-dose olanzapine was more effective.
In a randomized trial,18 patients who did not respond to at least one atypical antipsychotic then received 8 weeks of fixed, standard-dose treatment with (mean dosages):
- haloperidol, 18.9 mg/d
- risperidone, 7.9 mg/d
- olanzapine, 19.6 mg/d
- clozapine, 401.6 mg/d.
Flexible dosing was then allowed for 6 weeks, and mean dosages were:
- haloperidol, 25.7 mg/d
- risperidone, 11.6 mg/d
- olanzapine, 30.4 mg/d
- clozapine, 526.6 mg/d.
Symptoms improved modestly at best for all medications, although patients taking olanzapine or clozapine improved significantly more than those treated with haloperidol as shown by mean changes in total Positive and Negative Syndrome Scale (PANSS) scores.
PANSS scores for olanzapine-treated patients showed additional improvement at week 14—when higher dosages were used—compared with week 8. This was not the case for the other medications, for which response plateaued. These findings suggest that high-dose risperidone and haloperidol are incrementally ineffective, but high-dose olanzapine could help some patients with refractory symptoms.
Results were different in a randomized, double-blind, 16-week, crossover study,19 when 13 patients with inadequate response to neuroleptics, risperidone, or conventional-dose olanzapine then received olanzapine, 50 mg/d, or clozapine, 450 mg/d. No olanzapine-treated patients and 20% of clozapine-treated patients met criteria for treatment response (20% improvement in BPRS score and final BPRS score <35 or 1-point improvement on Clinical Global Impressions-Severity of Illness scale).
Negative results don’t make headlines. Published clinical trials and case reports are subject to selective reporting of positive outcomes. Cases in which high-dose therapy proved ineffectivemay outnumber positive results but are less likely to be published.
Numbers don’t lie. Using high doses will almost always increase side effect risk and drug therapy costs, contributing to a poor risk-benefit ratio when efficacy remains unchanged. Resorting to an “if-it’s-not-working, double-it” strategy may seem reasonable, but two times zero is still zero.
Desperation warps perception. Clinicians tend to rely on observational experience. The desperation inherent in treating refractory patients, however, often creates a strong desire for improvement and therefore a potentially biased perception of outcome.
Likewise, patients may inaccurately portray themselves as improved to avoid disappointing their doctors. Controlled trials reduce these biases to better assess efficacy.
Antipsychotics work in 6 to 8 weeks. Improvements seen when pushing medications beyond recommended dosing may not be an effect of dose but of additional time on the medication. Antipsychotics usually take 6 to 8 weeks to produce maximal response, so high-dose therapy should not be started during this initial phase. This pace may not satisfy pressures for expedient stabilization and hospital discharge, but it is unrealistic to expect antipsychotics to work more quickly than they do.
Oversedation does not equal improvement. Patients who become excessively sedated from high-dose therapy or adjunctive medications may appear less psychotic but may not be so. The family or hospital staff may desire such sedation, but it can adversely affect the patient’s quality of life or medication adherence.
Polypharmacy clouds the issue. Many patients treated with high-dose antipsychotics are taking multiple agents, making it difficult to attribute improvement (or side effects) to any single one. A well-designed study of high-dose therapy would therefore:
- control for time
- examine concomitant medications’ effects
- determine whether “improvements” are related to sedation or reduced psychosis.
Medication may not need to change. When a patient decompensates, many forces pressure clinicians to change or add medications or increase dosages. Change may not be necessary, however, as nonadherence or substance abuse often trigger psychotic exacerbations. For example, Steingard et al27 added fluphenazine or placebo to antipsychotic regimens of newly hospitalized patients and found that increasing antipsychotic dosage did not improve outcome.
Subjects switching from clozapine to olanzapine tended to worsen, whereas those switching from olanzapine to clozapine tended to improve. Olanzapine-treated patients experienced more anticholinergic side effects and more weight gain than did clozapine-treated subjects.20
Conclusion. These mixed findings on high-dose olanzapine suggest questionable efficacy in patients with treatment-resistant schizophrenia and an uncertain risk of increased toxicity.
Quetiapine
Early placebo-controlled studies of quetiapine in schizophrenia concluded that statistically significant improvement begins at 150 mg/d and falls off after 600 mg/d.21 Although few high-dose quetiapine cases have been presented, clinical opinion holds that:
- most patients with chronic schizophrenia require 400 to 800 mg/d
- some treatment-refractory patients might benefit from >800 mg/d.
One patient responded to quetiapine, 1,600 mg/d, after not responding to olanzapine, 40 mg/d, and quetiapine, 800 mg/d. Constipation was the only reported side effect.22
Our group23 reported a series of 7 patients who responded (by clinician report) to quetiapine, 1,200 to 2,400 mg/d, after not responding to quetiapine, 800 mg/d, or to neuroleptics, risperidone, or olanzapine. Six responded to high-dose quetiapine and 1 to high-dose quetiapine plus risperidone, 2 mg/d; 4 received adjunctive dival-proex sodium, 1,500 to 3,000 mg/d. Psychopathology, violence, and behavioral disturbances were reduced throughout 5 to 14 months of monitoring. Side effects included sedation, orthostasis, and dysphagia.
When Nelson et al24 treated 13 subjects for 14 weeks with quetiapine, 1,000 to 1,400 mg/d, mean weight, glucose, total cholesterol, prolactin, and QTc interval duration did not change significantly. Heart rate increased significantly (though not to tachycardia), and headache, constipation, and lethargy were the most frequent side effects.
Summary. Although encouraging, these reports are preliminary, unpublished, and lack peer review. Controlled trials of high-dose quetiapine’s efficacy and safety are needed.
Ziprasidone and aripiprazole
No studies of high-dose ziprasidone or aripiprazole have been published. In premarketing trials:
- ziprasidone was studied at 200 mg/d and released with a maximum recommended dosage of 160 mg/d
- aripiprazole, 30 mg/d, was not more effective than 15 mg/d.25
Deutschman et al26 reviewed the charts of 31 patients who received ziprasidone, 240 to 320 mg/d, after an “incomplete” response to 160 mg/d. At the higher dosing:
- psychosis, affective symptoms, or anxiety improved in nearly one-half of patients
- 15% reported sedation, but most reported no side effects
- none developed QTc intervals >500 msec.
Caveats and precautions
These uncontrolled case reports and open-label studies do not “prove” efficacy or safety but reflect clinical practice. More than anything, they show that we need controlled trials to gauge high-dose antipsychotic therapy’s efficacy and safety and to curb our collective habit of relying on anecdotal experience and idiosyncratic beliefs.
Despite its side-effect profile, clozapine remains the treatment of choice for refractory schizophrenia. Given high-dose antipsychotic therapy’s uncertain efficacy and unknown risks, the evidence supports a clozapine trial before higher-than-recommended dosing is attempted.
Because educated guesswork plays a role in premarketing dosing studies, a medication’s optimal dose may be:
- overestimated (as with risperidone)
- underestimated (as perhaps with olanzapine and quetiapine).
Keep in mind some important caveats when you consider giving a patient high-dose antipsychotic therapy (Box).27 Of course, nonadherence is often the cause of apparent medication nonresponse. Increasing the dosage of a medication a patient is not taking rarely improves adherence. Interventions to enhance adherence—careful assessment, psychoeducation, and using longacting intramuscular medication—may be useful.
Related resources
- Marder SR, Essock SM, Miller AL, et al. The Mount Sinai Conference on the pharmacotherapy of schizophrenia. Schizophrenia Bull 2002;28:5-16.
- Practice guideline for the treatment of patients with schizophrenia (2nd ed). Am J Psychiatry 2004;161(suppl):1-56.
- Texas Medication Algorithm Project antipsychotic algorithm. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/timascz1algo.pdf
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Pierre receives research support from Cephalon Inc., and is a consultant to and/or speaker for Pfizer Inc., Bristol-Myers Squibb Co., AstraZeneca Pharmaceuticals, and Janssen Pharmaceutica.
Dr. Donna Wirshing receives research support from, is a consultant to, and/or is a speaker for Bristol-Myers Squibb Co., Pfizer Inc., Eli Lilly & Co., Janssen Pharmaceutica, AstraZeneca Pharmaceuticals, and Abbott Laboratories.
Dr. William Wirshing receives research support from, is a consultant to, and/or is a speaker for Bristol-Myers Squibb Co., Pfizer Inc., Eli Lilly & Co., Janssen Pharmaceutica, and AstraZeneca Pharmaceuticals.
1. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997;23:663-74.
2. Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: A review and meta-analysis of randomized trials. Am J Psychiatry 2001;158:518-26.
3. Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psych 1988;45:79-91.
4. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: A controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991;48:739-45.
5. Van Putten T, Marder SR, Mintz J, Poland R. Haloperidol plasma levels and clinical response: A therapeutic window relationship. Am J Psychiatry 1992;149:500-5.
6. Van Putten T, Marshall BD, Liberman R, et al. Systematic dosage reduction in treatment-resistant schizophrenic patients. Psychopharmacol Bull 1993;29:315-20.
7. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994;151:825-36.
8. Love RC, Conley RR, Kelly DL, Bartko JJ. A dose-outcome analysis of risperidone. J Clin Psychiatry 1999;60:771-5.
9. Wirshing DA, Marshall BD, Jr, Green MF, et al. Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 1999;156:1374-9.
10. Fanous A, Lindenmayer JP. Schizophrenia and schizoaffective disorder treated with high doses of olanzapine. J Clin Psychopharmacol 1999;19:275-6.
11. Reich J. Use of high-dose olanzapine in refractory psychosis. Am J Psychiatry 1999;156:661.-
12. Dursun SM, Gardner DM, Bird DC, Flinn J. Olanzapine for patients with treatment-resistant schizophrenia: A naturalistic case-series outcome study. Can J Psychiatry 1999;44:701-4.
13. Lerner V. High-dose olanzapine for treatment-refractory schizophrenia. Clin Neuropharmacol 2003;26:58-61.
14. Sheitman BB, Lindgren JC, Early JE, Sved M. High-dose olanzapine for treatment-refractory schizophrenia. Am J Psychiatry 1997;154:1626.
15. Bronson BD, Lindenmayer JP. Adverse effects of high-dose olanzapine in treatment-refractory schizophrenia. J Clin Psychopharmacol 2000;20:383-4.
16. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics 2003;44:174-5.
17. Lindenmayer JP, Volavka J, Lieberman J, et al. Olanzapine for schizophrenia refractory to typical and atypical antipsychotics: An open-label, prospective trial. J Clin Psychopharmacol. 2001;21:448-53.
18. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002;159:255-62.
19. Conley RR, Kelly DL, Richardson CM, et al. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: A double-blind cross-over study. J Clin Psychopharmacol 2003;23:668-71.
20. Kelly DL, Conley RR, Richardson CM, et al. Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Ann Clin Psychiatry 2003;15:181-6.
21. Arvanitis LA, Miller BG. and the Seroquel Trial 13 Study Group. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: A comparison with haloperidol and placebo. Biol Psychiatry 1997;42:233-46.
22. Bobes J, Garcia-Portilla MP, Saiz PA, et al. High degree of tolerability for monotherapy with high doses of quetiapine: A case report. J Clin Psychiatry 2002;63:1048-9.
23. Pierre JM, Wirshing DA, Cannell J, et al. High-dose quetiapine in treatment refractory schizophrenia (poster). Colorado Springs, CO: International Congress of Schizophrenia Research, 2003; abstracted in Schizophrenia Res 2003;60(supp):299.-
24. Nelson MW, Reynolds R, Kelly DL, et al. Safety and tolerability of high-dose quetiapine in treatment-refractory schizophrenia: Preliminary results from an open-label trial (poster). Colorado Springs, CO: International Congress of Schizophrenia Research, 2003; abstracted in Schizophrenia Res 2003;60(supp):363.-
25. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2003;60:681-90.
26. Deutschman DA, Deutschman DH. High-dose ziprasidone: effectiveness and tolerability in clinical practice (poster). Boston, MA: American Psychiatric Association Institute on Psychiatric Services annual meeting, 2003.
27. Steingard S, Allen M, Schooler MR. A study of pharmacologic treatment on medication-compliant schizophrenics who relapse. J Clin Psychiatry 1994;55:470-2.
When nothing else works, desperate clinicians are resorting to progressively more-tenuous and unpredictable treatments, trying to improve the lives of patients with refractory schizophrenia. High-dose antipsychotics is a common strategy.
Does boosting antipsychotic doses beyond the recommended range—but short of the neuroleptic threshold—enhance efficacy? This article attempts to answer that question by presenting the evidence on higher-than-recommended doses of atypical antipsychotics.
Lessons from neuroleptics
Up to 30% of patients with schizophrenia do not respond to antipsychotics and are considered “treatment refractory.”1 Even among those who do respond, improving symptoms by 20%—as research defines “treatment response”—does not necessarily yield clinical or functional improvement. Clozapine is the only atypical antipsychotic with well-established efficacy in these chronically ill patients,2 but its daunting side effects greatly curtail its use.
Before atypical antipsychotics, patients who did not respond to usual dosages of the typical neuroleptics were treated with higher dosages or switched to another drug class. Although many clinicians embraced high-dose neuroleptics, subsequent research discredited “rapid neuroleptization” in any clinical circumstance and showed that exceeding an antipsychotic’s neuroleptic threshold—the dose at which extrapyramidal side effects (EPS) occur—reduces its efficacy (Figure 1).3-5 In some instances, reducing neuroleptic dosages improves treatment-resistant patients’ symptoms and reduces druginduced side effects.6
Figure 1 Typical antipsychotics’ dose-response curve
Narrow therapeutic window between antipsychotic effect and neuroleptic threshold. Dotted line indicates declining efficacy.
Figure 2 Atypical antipsychotics’ dose-response curve
Wider therapeutic window with atypicals, compared with typical antipsychotics, as neuroleptic threshold (dotted line) moves right.Atypical antipsychotics are defined by their relative lack of EPS at recommended dosages (Figure 2). Because these agents can cause EPS if dosed too high, however, our historical habit of testing this dose limit risks losing “atypicality” and encountering other untoward events (Figure 3).
What is the safest, most effective dosage? Consider the evidence for each atypical antipsychotic.
Risperidone
Recommended dosage too high? When using atypicals at recommended doses, you are most likely to encounter the neuroleptic threshold with risperidone, with EPS risk increasing substantially at >6 mg/d.7 Post-approval studies set the most effective and safest dosage at approximately 4 mg/d, though this dosage was not studied in North American pre-approval trials. Dosages of 2 to 4 mg/d have been associated with more-favorable outcomes, suggesting that the initial recommendation to titrate to 6 mg/d within the first 3 days was ill-advised.8
In our study of patients with treatment-refractory schizophrenia,9 those treated with risperidone, 6 mg/d, improved significantly more after 4 weeks than did those receiving haloperidol, 15 mg/d, based on Brief Psychiatric Rating Scale (BPRS) scores. No additional benefit was seen after risperidone was increased to >6 mg/d at 8 weeks. Akathisia and tardive dyskinesia occurred significantly more often in the haloperidol group.
Conclusion. Some patients respond to higher-dose risperidone, but emerging EPS suggest the need to reduce the dosage rather than add an antiparkinsonian agent.
Figure 3 Unknown effects of high-dose atypical antipsychotic therapy
Dotted line indicates potential for greater antipsychotic effect with increasing dose.
Olanzapine
Mixed results. Case reports suggest that some patients who did not respond to previous antipsychotic trials or olanzapine, 20 mg/d, improved sig-nificantly—without substanial side effects—when olanzapine was increased up to 60 mg/d.10-14 Other case studies, however, report EPS, increased heart rate, increased transaminases, hyperprolactinemia, and prolonged QTc interval with high-dose olanzapine.14-16
In an open-label trial,17 43 patients with schizophrenia received olanzapine, up to 40 mg/d, after inadequate response to neuroleptics and risperidone or clozapine. Olanzapine was titrated to 20 mg/d by week 4 and increased 5 mg every 2 weeks if symptoms did not improve. After 14 weeks, improvement was modest and only 17% of patients met response criteria. However, >20 mg/d reduced symptoms more than did <20 mg/d, suggesting that high-dose olanzapine was more effective.
In a randomized trial,18 patients who did not respond to at least one atypical antipsychotic then received 8 weeks of fixed, standard-dose treatment with (mean dosages):
- haloperidol, 18.9 mg/d
- risperidone, 7.9 mg/d
- olanzapine, 19.6 mg/d
- clozapine, 401.6 mg/d.
Flexible dosing was then allowed for 6 weeks, and mean dosages were:
- haloperidol, 25.7 mg/d
- risperidone, 11.6 mg/d
- olanzapine, 30.4 mg/d
- clozapine, 526.6 mg/d.
Symptoms improved modestly at best for all medications, although patients taking olanzapine or clozapine improved significantly more than those treated with haloperidol as shown by mean changes in total Positive and Negative Syndrome Scale (PANSS) scores.
PANSS scores for olanzapine-treated patients showed additional improvement at week 14—when higher dosages were used—compared with week 8. This was not the case for the other medications, for which response plateaued. These findings suggest that high-dose risperidone and haloperidol are incrementally ineffective, but high-dose olanzapine could help some patients with refractory symptoms.
Results were different in a randomized, double-blind, 16-week, crossover study,19 when 13 patients with inadequate response to neuroleptics, risperidone, or conventional-dose olanzapine then received olanzapine, 50 mg/d, or clozapine, 450 mg/d. No olanzapine-treated patients and 20% of clozapine-treated patients met criteria for treatment response (20% improvement in BPRS score and final BPRS score <35 or 1-point improvement on Clinical Global Impressions-Severity of Illness scale).
Negative results don’t make headlines. Published clinical trials and case reports are subject to selective reporting of positive outcomes. Cases in which high-dose therapy proved ineffectivemay outnumber positive results but are less likely to be published.
Numbers don’t lie. Using high doses will almost always increase side effect risk and drug therapy costs, contributing to a poor risk-benefit ratio when efficacy remains unchanged. Resorting to an “if-it’s-not-working, double-it” strategy may seem reasonable, but two times zero is still zero.
Desperation warps perception. Clinicians tend to rely on observational experience. The desperation inherent in treating refractory patients, however, often creates a strong desire for improvement and therefore a potentially biased perception of outcome.
Likewise, patients may inaccurately portray themselves as improved to avoid disappointing their doctors. Controlled trials reduce these biases to better assess efficacy.
Antipsychotics work in 6 to 8 weeks. Improvements seen when pushing medications beyond recommended dosing may not be an effect of dose but of additional time on the medication. Antipsychotics usually take 6 to 8 weeks to produce maximal response, so high-dose therapy should not be started during this initial phase. This pace may not satisfy pressures for expedient stabilization and hospital discharge, but it is unrealistic to expect antipsychotics to work more quickly than they do.
Oversedation does not equal improvement. Patients who become excessively sedated from high-dose therapy or adjunctive medications may appear less psychotic but may not be so. The family or hospital staff may desire such sedation, but it can adversely affect the patient’s quality of life or medication adherence.
Polypharmacy clouds the issue. Many patients treated with high-dose antipsychotics are taking multiple agents, making it difficult to attribute improvement (or side effects) to any single one. A well-designed study of high-dose therapy would therefore:
- control for time
- examine concomitant medications’ effects
- determine whether “improvements” are related to sedation or reduced psychosis.
Medication may not need to change. When a patient decompensates, many forces pressure clinicians to change or add medications or increase dosages. Change may not be necessary, however, as nonadherence or substance abuse often trigger psychotic exacerbations. For example, Steingard et al27 added fluphenazine or placebo to antipsychotic regimens of newly hospitalized patients and found that increasing antipsychotic dosage did not improve outcome.
Subjects switching from clozapine to olanzapine tended to worsen, whereas those switching from olanzapine to clozapine tended to improve. Olanzapine-treated patients experienced more anticholinergic side effects and more weight gain than did clozapine-treated subjects.20
Conclusion. These mixed findings on high-dose olanzapine suggest questionable efficacy in patients with treatment-resistant schizophrenia and an uncertain risk of increased toxicity.
Quetiapine
Early placebo-controlled studies of quetiapine in schizophrenia concluded that statistically significant improvement begins at 150 mg/d and falls off after 600 mg/d.21 Although few high-dose quetiapine cases have been presented, clinical opinion holds that:
- most patients with chronic schizophrenia require 400 to 800 mg/d
- some treatment-refractory patients might benefit from >800 mg/d.
One patient responded to quetiapine, 1,600 mg/d, after not responding to olanzapine, 40 mg/d, and quetiapine, 800 mg/d. Constipation was the only reported side effect.22
Our group23 reported a series of 7 patients who responded (by clinician report) to quetiapine, 1,200 to 2,400 mg/d, after not responding to quetiapine, 800 mg/d, or to neuroleptics, risperidone, or olanzapine. Six responded to high-dose quetiapine and 1 to high-dose quetiapine plus risperidone, 2 mg/d; 4 received adjunctive dival-proex sodium, 1,500 to 3,000 mg/d. Psychopathology, violence, and behavioral disturbances were reduced throughout 5 to 14 months of monitoring. Side effects included sedation, orthostasis, and dysphagia.
When Nelson et al24 treated 13 subjects for 14 weeks with quetiapine, 1,000 to 1,400 mg/d, mean weight, glucose, total cholesterol, prolactin, and QTc interval duration did not change significantly. Heart rate increased significantly (though not to tachycardia), and headache, constipation, and lethargy were the most frequent side effects.
Summary. Although encouraging, these reports are preliminary, unpublished, and lack peer review. Controlled trials of high-dose quetiapine’s efficacy and safety are needed.
Ziprasidone and aripiprazole
No studies of high-dose ziprasidone or aripiprazole have been published. In premarketing trials:
- ziprasidone was studied at 200 mg/d and released with a maximum recommended dosage of 160 mg/d
- aripiprazole, 30 mg/d, was not more effective than 15 mg/d.25
Deutschman et al26 reviewed the charts of 31 patients who received ziprasidone, 240 to 320 mg/d, after an “incomplete” response to 160 mg/d. At the higher dosing:
- psychosis, affective symptoms, or anxiety improved in nearly one-half of patients
- 15% reported sedation, but most reported no side effects
- none developed QTc intervals >500 msec.
Caveats and precautions
These uncontrolled case reports and open-label studies do not “prove” efficacy or safety but reflect clinical practice. More than anything, they show that we need controlled trials to gauge high-dose antipsychotic therapy’s efficacy and safety and to curb our collective habit of relying on anecdotal experience and idiosyncratic beliefs.
Despite its side-effect profile, clozapine remains the treatment of choice for refractory schizophrenia. Given high-dose antipsychotic therapy’s uncertain efficacy and unknown risks, the evidence supports a clozapine trial before higher-than-recommended dosing is attempted.
Because educated guesswork plays a role in premarketing dosing studies, a medication’s optimal dose may be:
- overestimated (as with risperidone)
- underestimated (as perhaps with olanzapine and quetiapine).
Keep in mind some important caveats when you consider giving a patient high-dose antipsychotic therapy (Box).27 Of course, nonadherence is often the cause of apparent medication nonresponse. Increasing the dosage of a medication a patient is not taking rarely improves adherence. Interventions to enhance adherence—careful assessment, psychoeducation, and using longacting intramuscular medication—may be useful.
Related resources
- Marder SR, Essock SM, Miller AL, et al. The Mount Sinai Conference on the pharmacotherapy of schizophrenia. Schizophrenia Bull 2002;28:5-16.
- Practice guideline for the treatment of patients with schizophrenia (2nd ed). Am J Psychiatry 2004;161(suppl):1-56.
- Texas Medication Algorithm Project antipsychotic algorithm. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/timascz1algo.pdf
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Pierre receives research support from Cephalon Inc., and is a consultant to and/or speaker for Pfizer Inc., Bristol-Myers Squibb Co., AstraZeneca Pharmaceuticals, and Janssen Pharmaceutica.
Dr. Donna Wirshing receives research support from, is a consultant to, and/or is a speaker for Bristol-Myers Squibb Co., Pfizer Inc., Eli Lilly & Co., Janssen Pharmaceutica, AstraZeneca Pharmaceuticals, and Abbott Laboratories.
Dr. William Wirshing receives research support from, is a consultant to, and/or is a speaker for Bristol-Myers Squibb Co., Pfizer Inc., Eli Lilly & Co., Janssen Pharmaceutica, and AstraZeneca Pharmaceuticals.
When nothing else works, desperate clinicians are resorting to progressively more-tenuous and unpredictable treatments, trying to improve the lives of patients with refractory schizophrenia. High-dose antipsychotics is a common strategy.
Does boosting antipsychotic doses beyond the recommended range—but short of the neuroleptic threshold—enhance efficacy? This article attempts to answer that question by presenting the evidence on higher-than-recommended doses of atypical antipsychotics.
Lessons from neuroleptics
Up to 30% of patients with schizophrenia do not respond to antipsychotics and are considered “treatment refractory.”1 Even among those who do respond, improving symptoms by 20%—as research defines “treatment response”—does not necessarily yield clinical or functional improvement. Clozapine is the only atypical antipsychotic with well-established efficacy in these chronically ill patients,2 but its daunting side effects greatly curtail its use.
Before atypical antipsychotics, patients who did not respond to usual dosages of the typical neuroleptics were treated with higher dosages or switched to another drug class. Although many clinicians embraced high-dose neuroleptics, subsequent research discredited “rapid neuroleptization” in any clinical circumstance and showed that exceeding an antipsychotic’s neuroleptic threshold—the dose at which extrapyramidal side effects (EPS) occur—reduces its efficacy (Figure 1).3-5 In some instances, reducing neuroleptic dosages improves treatment-resistant patients’ symptoms and reduces druginduced side effects.6
Figure 1 Typical antipsychotics’ dose-response curve
Narrow therapeutic window between antipsychotic effect and neuroleptic threshold. Dotted line indicates declining efficacy.
Figure 2 Atypical antipsychotics’ dose-response curve
Wider therapeutic window with atypicals, compared with typical antipsychotics, as neuroleptic threshold (dotted line) moves right.Atypical antipsychotics are defined by their relative lack of EPS at recommended dosages (Figure 2). Because these agents can cause EPS if dosed too high, however, our historical habit of testing this dose limit risks losing “atypicality” and encountering other untoward events (Figure 3).
What is the safest, most effective dosage? Consider the evidence for each atypical antipsychotic.
Risperidone
Recommended dosage too high? When using atypicals at recommended doses, you are most likely to encounter the neuroleptic threshold with risperidone, with EPS risk increasing substantially at >6 mg/d.7 Post-approval studies set the most effective and safest dosage at approximately 4 mg/d, though this dosage was not studied in North American pre-approval trials. Dosages of 2 to 4 mg/d have been associated with more-favorable outcomes, suggesting that the initial recommendation to titrate to 6 mg/d within the first 3 days was ill-advised.8
In our study of patients with treatment-refractory schizophrenia,9 those treated with risperidone, 6 mg/d, improved significantly more after 4 weeks than did those receiving haloperidol, 15 mg/d, based on Brief Psychiatric Rating Scale (BPRS) scores. No additional benefit was seen after risperidone was increased to >6 mg/d at 8 weeks. Akathisia and tardive dyskinesia occurred significantly more often in the haloperidol group.
Conclusion. Some patients respond to higher-dose risperidone, but emerging EPS suggest the need to reduce the dosage rather than add an antiparkinsonian agent.
Figure 3 Unknown effects of high-dose atypical antipsychotic therapy
Dotted line indicates potential for greater antipsychotic effect with increasing dose.
Olanzapine
Mixed results. Case reports suggest that some patients who did not respond to previous antipsychotic trials or olanzapine, 20 mg/d, improved sig-nificantly—without substanial side effects—when olanzapine was increased up to 60 mg/d.10-14 Other case studies, however, report EPS, increased heart rate, increased transaminases, hyperprolactinemia, and prolonged QTc interval with high-dose olanzapine.14-16
In an open-label trial,17 43 patients with schizophrenia received olanzapine, up to 40 mg/d, after inadequate response to neuroleptics and risperidone or clozapine. Olanzapine was titrated to 20 mg/d by week 4 and increased 5 mg every 2 weeks if symptoms did not improve. After 14 weeks, improvement was modest and only 17% of patients met response criteria. However, >20 mg/d reduced symptoms more than did <20 mg/d, suggesting that high-dose olanzapine was more effective.
In a randomized trial,18 patients who did not respond to at least one atypical antipsychotic then received 8 weeks of fixed, standard-dose treatment with (mean dosages):
- haloperidol, 18.9 mg/d
- risperidone, 7.9 mg/d
- olanzapine, 19.6 mg/d
- clozapine, 401.6 mg/d.
Flexible dosing was then allowed for 6 weeks, and mean dosages were:
- haloperidol, 25.7 mg/d
- risperidone, 11.6 mg/d
- olanzapine, 30.4 mg/d
- clozapine, 526.6 mg/d.
Symptoms improved modestly at best for all medications, although patients taking olanzapine or clozapine improved significantly more than those treated with haloperidol as shown by mean changes in total Positive and Negative Syndrome Scale (PANSS) scores.
PANSS scores for olanzapine-treated patients showed additional improvement at week 14—when higher dosages were used—compared with week 8. This was not the case for the other medications, for which response plateaued. These findings suggest that high-dose risperidone and haloperidol are incrementally ineffective, but high-dose olanzapine could help some patients with refractory symptoms.
Results were different in a randomized, double-blind, 16-week, crossover study,19 when 13 patients with inadequate response to neuroleptics, risperidone, or conventional-dose olanzapine then received olanzapine, 50 mg/d, or clozapine, 450 mg/d. No olanzapine-treated patients and 20% of clozapine-treated patients met criteria for treatment response (20% improvement in BPRS score and final BPRS score <35 or 1-point improvement on Clinical Global Impressions-Severity of Illness scale).
Negative results don’t make headlines. Published clinical trials and case reports are subject to selective reporting of positive outcomes. Cases in which high-dose therapy proved ineffectivemay outnumber positive results but are less likely to be published.
Numbers don’t lie. Using high doses will almost always increase side effect risk and drug therapy costs, contributing to a poor risk-benefit ratio when efficacy remains unchanged. Resorting to an “if-it’s-not-working, double-it” strategy may seem reasonable, but two times zero is still zero.
Desperation warps perception. Clinicians tend to rely on observational experience. The desperation inherent in treating refractory patients, however, often creates a strong desire for improvement and therefore a potentially biased perception of outcome.
Likewise, patients may inaccurately portray themselves as improved to avoid disappointing their doctors. Controlled trials reduce these biases to better assess efficacy.
Antipsychotics work in 6 to 8 weeks. Improvements seen when pushing medications beyond recommended dosing may not be an effect of dose but of additional time on the medication. Antipsychotics usually take 6 to 8 weeks to produce maximal response, so high-dose therapy should not be started during this initial phase. This pace may not satisfy pressures for expedient stabilization and hospital discharge, but it is unrealistic to expect antipsychotics to work more quickly than they do.
Oversedation does not equal improvement. Patients who become excessively sedated from high-dose therapy or adjunctive medications may appear less psychotic but may not be so. The family or hospital staff may desire such sedation, but it can adversely affect the patient’s quality of life or medication adherence.
Polypharmacy clouds the issue. Many patients treated with high-dose antipsychotics are taking multiple agents, making it difficult to attribute improvement (or side effects) to any single one. A well-designed study of high-dose therapy would therefore:
- control for time
- examine concomitant medications’ effects
- determine whether “improvements” are related to sedation or reduced psychosis.
Medication may not need to change. When a patient decompensates, many forces pressure clinicians to change or add medications or increase dosages. Change may not be necessary, however, as nonadherence or substance abuse often trigger psychotic exacerbations. For example, Steingard et al27 added fluphenazine or placebo to antipsychotic regimens of newly hospitalized patients and found that increasing antipsychotic dosage did not improve outcome.
Subjects switching from clozapine to olanzapine tended to worsen, whereas those switching from olanzapine to clozapine tended to improve. Olanzapine-treated patients experienced more anticholinergic side effects and more weight gain than did clozapine-treated subjects.20
Conclusion. These mixed findings on high-dose olanzapine suggest questionable efficacy in patients with treatment-resistant schizophrenia and an uncertain risk of increased toxicity.
Quetiapine
Early placebo-controlled studies of quetiapine in schizophrenia concluded that statistically significant improvement begins at 150 mg/d and falls off after 600 mg/d.21 Although few high-dose quetiapine cases have been presented, clinical opinion holds that:
- most patients with chronic schizophrenia require 400 to 800 mg/d
- some treatment-refractory patients might benefit from >800 mg/d.
One patient responded to quetiapine, 1,600 mg/d, after not responding to olanzapine, 40 mg/d, and quetiapine, 800 mg/d. Constipation was the only reported side effect.22
Our group23 reported a series of 7 patients who responded (by clinician report) to quetiapine, 1,200 to 2,400 mg/d, after not responding to quetiapine, 800 mg/d, or to neuroleptics, risperidone, or olanzapine. Six responded to high-dose quetiapine and 1 to high-dose quetiapine plus risperidone, 2 mg/d; 4 received adjunctive dival-proex sodium, 1,500 to 3,000 mg/d. Psychopathology, violence, and behavioral disturbances were reduced throughout 5 to 14 months of monitoring. Side effects included sedation, orthostasis, and dysphagia.
When Nelson et al24 treated 13 subjects for 14 weeks with quetiapine, 1,000 to 1,400 mg/d, mean weight, glucose, total cholesterol, prolactin, and QTc interval duration did not change significantly. Heart rate increased significantly (though not to tachycardia), and headache, constipation, and lethargy were the most frequent side effects.
Summary. Although encouraging, these reports are preliminary, unpublished, and lack peer review. Controlled trials of high-dose quetiapine’s efficacy and safety are needed.
Ziprasidone and aripiprazole
No studies of high-dose ziprasidone or aripiprazole have been published. In premarketing trials:
- ziprasidone was studied at 200 mg/d and released with a maximum recommended dosage of 160 mg/d
- aripiprazole, 30 mg/d, was not more effective than 15 mg/d.25
Deutschman et al26 reviewed the charts of 31 patients who received ziprasidone, 240 to 320 mg/d, after an “incomplete” response to 160 mg/d. At the higher dosing:
- psychosis, affective symptoms, or anxiety improved in nearly one-half of patients
- 15% reported sedation, but most reported no side effects
- none developed QTc intervals >500 msec.
Caveats and precautions
These uncontrolled case reports and open-label studies do not “prove” efficacy or safety but reflect clinical practice. More than anything, they show that we need controlled trials to gauge high-dose antipsychotic therapy’s efficacy and safety and to curb our collective habit of relying on anecdotal experience and idiosyncratic beliefs.
Despite its side-effect profile, clozapine remains the treatment of choice for refractory schizophrenia. Given high-dose antipsychotic therapy’s uncertain efficacy and unknown risks, the evidence supports a clozapine trial before higher-than-recommended dosing is attempted.
Because educated guesswork plays a role in premarketing dosing studies, a medication’s optimal dose may be:
- overestimated (as with risperidone)
- underestimated (as perhaps with olanzapine and quetiapine).
Keep in mind some important caveats when you consider giving a patient high-dose antipsychotic therapy (Box).27 Of course, nonadherence is often the cause of apparent medication nonresponse. Increasing the dosage of a medication a patient is not taking rarely improves adherence. Interventions to enhance adherence—careful assessment, psychoeducation, and using longacting intramuscular medication—may be useful.
Related resources
- Marder SR, Essock SM, Miller AL, et al. The Mount Sinai Conference on the pharmacotherapy of schizophrenia. Schizophrenia Bull 2002;28:5-16.
- Practice guideline for the treatment of patients with schizophrenia (2nd ed). Am J Psychiatry 2004;161(suppl):1-56.
- Texas Medication Algorithm Project antipsychotic algorithm. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/timascz1algo.pdf
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Pierre receives research support from Cephalon Inc., and is a consultant to and/or speaker for Pfizer Inc., Bristol-Myers Squibb Co., AstraZeneca Pharmaceuticals, and Janssen Pharmaceutica.
Dr. Donna Wirshing receives research support from, is a consultant to, and/or is a speaker for Bristol-Myers Squibb Co., Pfizer Inc., Eli Lilly & Co., Janssen Pharmaceutica, AstraZeneca Pharmaceuticals, and Abbott Laboratories.
Dr. William Wirshing receives research support from, is a consultant to, and/or is a speaker for Bristol-Myers Squibb Co., Pfizer Inc., Eli Lilly & Co., Janssen Pharmaceutica, and AstraZeneca Pharmaceuticals.
1. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997;23:663-74.
2. Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: A review and meta-analysis of randomized trials. Am J Psychiatry 2001;158:518-26.
3. Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psych 1988;45:79-91.
4. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: A controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991;48:739-45.
5. Van Putten T, Marder SR, Mintz J, Poland R. Haloperidol plasma levels and clinical response: A therapeutic window relationship. Am J Psychiatry 1992;149:500-5.
6. Van Putten T, Marshall BD, Liberman R, et al. Systematic dosage reduction in treatment-resistant schizophrenic patients. Psychopharmacol Bull 1993;29:315-20.
7. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994;151:825-36.
8. Love RC, Conley RR, Kelly DL, Bartko JJ. A dose-outcome analysis of risperidone. J Clin Psychiatry 1999;60:771-5.
9. Wirshing DA, Marshall BD, Jr, Green MF, et al. Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 1999;156:1374-9.
10. Fanous A, Lindenmayer JP. Schizophrenia and schizoaffective disorder treated with high doses of olanzapine. J Clin Psychopharmacol 1999;19:275-6.
11. Reich J. Use of high-dose olanzapine in refractory psychosis. Am J Psychiatry 1999;156:661.-
12. Dursun SM, Gardner DM, Bird DC, Flinn J. Olanzapine for patients with treatment-resistant schizophrenia: A naturalistic case-series outcome study. Can J Psychiatry 1999;44:701-4.
13. Lerner V. High-dose olanzapine for treatment-refractory schizophrenia. Clin Neuropharmacol 2003;26:58-61.
14. Sheitman BB, Lindgren JC, Early JE, Sved M. High-dose olanzapine for treatment-refractory schizophrenia. Am J Psychiatry 1997;154:1626.
15. Bronson BD, Lindenmayer JP. Adverse effects of high-dose olanzapine in treatment-refractory schizophrenia. J Clin Psychopharmacol 2000;20:383-4.
16. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics 2003;44:174-5.
17. Lindenmayer JP, Volavka J, Lieberman J, et al. Olanzapine for schizophrenia refractory to typical and atypical antipsychotics: An open-label, prospective trial. J Clin Psychopharmacol. 2001;21:448-53.
18. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002;159:255-62.
19. Conley RR, Kelly DL, Richardson CM, et al. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: A double-blind cross-over study. J Clin Psychopharmacol 2003;23:668-71.
20. Kelly DL, Conley RR, Richardson CM, et al. Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Ann Clin Psychiatry 2003;15:181-6.
21. Arvanitis LA, Miller BG. and the Seroquel Trial 13 Study Group. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: A comparison with haloperidol and placebo. Biol Psychiatry 1997;42:233-46.
22. Bobes J, Garcia-Portilla MP, Saiz PA, et al. High degree of tolerability for monotherapy with high doses of quetiapine: A case report. J Clin Psychiatry 2002;63:1048-9.
23. Pierre JM, Wirshing DA, Cannell J, et al. High-dose quetiapine in treatment refractory schizophrenia (poster). Colorado Springs, CO: International Congress of Schizophrenia Research, 2003; abstracted in Schizophrenia Res 2003;60(supp):299.-
24. Nelson MW, Reynolds R, Kelly DL, et al. Safety and tolerability of high-dose quetiapine in treatment-refractory schizophrenia: Preliminary results from an open-label trial (poster). Colorado Springs, CO: International Congress of Schizophrenia Research, 2003; abstracted in Schizophrenia Res 2003;60(supp):363.-
25. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2003;60:681-90.
26. Deutschman DA, Deutschman DH. High-dose ziprasidone: effectiveness and tolerability in clinical practice (poster). Boston, MA: American Psychiatric Association Institute on Psychiatric Services annual meeting, 2003.
27. Steingard S, Allen M, Schooler MR. A study of pharmacologic treatment on medication-compliant schizophrenics who relapse. J Clin Psychiatry 1994;55:470-2.
1. Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull 1997;23:663-74.
2. Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: A review and meta-analysis of randomized trials. Am J Psychiatry 2001;158:518-26.
3. Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psych 1988;45:79-91.
4. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: A controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991;48:739-45.
5. Van Putten T, Marder SR, Mintz J, Poland R. Haloperidol plasma levels and clinical response: A therapeutic window relationship. Am J Psychiatry 1992;149:500-5.
6. Van Putten T, Marshall BD, Liberman R, et al. Systematic dosage reduction in treatment-resistant schizophrenic patients. Psychopharmacol Bull 1993;29:315-20.
7. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994;151:825-36.
8. Love RC, Conley RR, Kelly DL, Bartko JJ. A dose-outcome analysis of risperidone. J Clin Psychiatry 1999;60:771-5.
9. Wirshing DA, Marshall BD, Jr, Green MF, et al. Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 1999;156:1374-9.
10. Fanous A, Lindenmayer JP. Schizophrenia and schizoaffective disorder treated with high doses of olanzapine. J Clin Psychopharmacol 1999;19:275-6.
11. Reich J. Use of high-dose olanzapine in refractory psychosis. Am J Psychiatry 1999;156:661.-
12. Dursun SM, Gardner DM, Bird DC, Flinn J. Olanzapine for patients with treatment-resistant schizophrenia: A naturalistic case-series outcome study. Can J Psychiatry 1999;44:701-4.
13. Lerner V. High-dose olanzapine for treatment-refractory schizophrenia. Clin Neuropharmacol 2003;26:58-61.
14. Sheitman BB, Lindgren JC, Early JE, Sved M. High-dose olanzapine for treatment-refractory schizophrenia. Am J Psychiatry 1997;154:1626.
15. Bronson BD, Lindenmayer JP. Adverse effects of high-dose olanzapine in treatment-refractory schizophrenia. J Clin Psychopharmacol 2000;20:383-4.
16. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics 2003;44:174-5.
17. Lindenmayer JP, Volavka J, Lieberman J, et al. Olanzapine for schizophrenia refractory to typical and atypical antipsychotics: An open-label, prospective trial. J Clin Psychopharmacol. 2001;21:448-53.
18. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002;159:255-62.
19. Conley RR, Kelly DL, Richardson CM, et al. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: A double-blind cross-over study. J Clin Psychopharmacol 2003;23:668-71.
20. Kelly DL, Conley RR, Richardson CM, et al. Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Ann Clin Psychiatry 2003;15:181-6.
21. Arvanitis LA, Miller BG. and the Seroquel Trial 13 Study Group. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: A comparison with haloperidol and placebo. Biol Psychiatry 1997;42:233-46.
22. Bobes J, Garcia-Portilla MP, Saiz PA, et al. High degree of tolerability for monotherapy with high doses of quetiapine: A case report. J Clin Psychiatry 2002;63:1048-9.
23. Pierre JM, Wirshing DA, Cannell J, et al. High-dose quetiapine in treatment refractory schizophrenia (poster). Colorado Springs, CO: International Congress of Schizophrenia Research, 2003; abstracted in Schizophrenia Res 2003;60(supp):299.-
24. Nelson MW, Reynolds R, Kelly DL, et al. Safety and tolerability of high-dose quetiapine in treatment-refractory schizophrenia: Preliminary results from an open-label trial (poster). Colorado Springs, CO: International Congress of Schizophrenia Research, 2003; abstracted in Schizophrenia Res 2003;60(supp):363.-
25. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2003;60:681-90.
26. Deutschman DA, Deutschman DH. High-dose ziprasidone: effectiveness and tolerability in clinical practice (poster). Boston, MA: American Psychiatric Association Institute on Psychiatric Services annual meeting, 2003.
27. Steingard S, Allen M, Schooler MR. A study of pharmacologic treatment on medication-compliant schizophrenics who relapse. J Clin Psychiatry 1994;55:470-2.