User login
FDA approves two once-daily HIV drugs
Two once-daily oral HIV-1 medicines have been approved by the Food and Drug Administration, according to Merck: Delstrigo, a fixed-dose combination tablet of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg), and Pifeltro (doravirine, 100 mg). Both drugs are indicated for treating HIV-1 infection in adult patients with no prior antiretroviral treatment.
Pifeltro is a nonnucleoside reverse transcriptase inhibitor to be used in combination with other antiretroviral medicines. Delstrigo contains a boxed warning regarding posttreatment acute exacerbation of hepatitis B infection.
Delstrigo and Pifeltro are not curative, according to the announcement by Merck, which manufactures both drugs.
The FDA approval of Delstrigo was based on findings from the DRIVE-AHEAD trial (NCT02403674), which randomized 728 participants with no history of antiretroviral treatment to receive once daily either Delstrigo or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV 600 mg/FTC 200 mg/TDF 300 mg). Delstrigo was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with EFV/FTC/TDF (84% vs. 81%, respectively), according to Merck.
Pifeltro was approved based on the results of the DRIVE-FORWARD trial (NCT02275780), which randomized 766 participants with no history of antiretroviral treatment to receive either Pifeltro once daily or darunavir 800 mg plus ritonavir 100 mg (DRV+r) once daily, each in combination with either emtricitabine (FTC)/TDF or abacavir (ABC)/lamivudine (3TC), as selected by the investigator. Pifeltro was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with DRV+r, each in combination with FTC/TDF or ABC/3TC (84% vs. 80%, respectively), according to the Merck announcement.
Two once-daily oral HIV-1 medicines have been approved by the Food and Drug Administration, according to Merck: Delstrigo, a fixed-dose combination tablet of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg), and Pifeltro (doravirine, 100 mg). Both drugs are indicated for treating HIV-1 infection in adult patients with no prior antiretroviral treatment.
Pifeltro is a nonnucleoside reverse transcriptase inhibitor to be used in combination with other antiretroviral medicines. Delstrigo contains a boxed warning regarding posttreatment acute exacerbation of hepatitis B infection.
Delstrigo and Pifeltro are not curative, according to the announcement by Merck, which manufactures both drugs.
The FDA approval of Delstrigo was based on findings from the DRIVE-AHEAD trial (NCT02403674), which randomized 728 participants with no history of antiretroviral treatment to receive once daily either Delstrigo or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV 600 mg/FTC 200 mg/TDF 300 mg). Delstrigo was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with EFV/FTC/TDF (84% vs. 81%, respectively), according to Merck.
Pifeltro was approved based on the results of the DRIVE-FORWARD trial (NCT02275780), which randomized 766 participants with no history of antiretroviral treatment to receive either Pifeltro once daily or darunavir 800 mg plus ritonavir 100 mg (DRV+r) once daily, each in combination with either emtricitabine (FTC)/TDF or abacavir (ABC)/lamivudine (3TC), as selected by the investigator. Pifeltro was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with DRV+r, each in combination with FTC/TDF or ABC/3TC (84% vs. 80%, respectively), according to the Merck announcement.
Two once-daily oral HIV-1 medicines have been approved by the Food and Drug Administration, according to Merck: Delstrigo, a fixed-dose combination tablet of doravirine (100 mg), lamivudine (300 mg), and tenofovir disoproxil fumarate (300 mg), and Pifeltro (doravirine, 100 mg). Both drugs are indicated for treating HIV-1 infection in adult patients with no prior antiretroviral treatment.
Pifeltro is a nonnucleoside reverse transcriptase inhibitor to be used in combination with other antiretroviral medicines. Delstrigo contains a boxed warning regarding posttreatment acute exacerbation of hepatitis B infection.
Delstrigo and Pifeltro are not curative, according to the announcement by Merck, which manufactures both drugs.
The FDA approval of Delstrigo was based on findings from the DRIVE-AHEAD trial (NCT02403674), which randomized 728 participants with no history of antiretroviral treatment to receive once daily either Delstrigo or efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV 600 mg/FTC 200 mg/TDF 300 mg). Delstrigo was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with EFV/FTC/TDF (84% vs. 81%, respectively), according to Merck.
Pifeltro was approved based on the results of the DRIVE-FORWARD trial (NCT02275780), which randomized 766 participants with no history of antiretroviral treatment to receive either Pifeltro once daily or darunavir 800 mg plus ritonavir 100 mg (DRV+r) once daily, each in combination with either emtricitabine (FTC)/TDF or abacavir (ABC)/lamivudine (3TC), as selected by the investigator. Pifeltro was associated with sustained viral suppression through 48 weeks, meeting its primary endpoint of noninferior efficacy when compared with DRV+r, each in combination with FTC/TDF or ABC/3TC (84% vs. 80%, respectively), according to the Merck announcement.
Dose-dense MVAC credited with better bladder cancer survival
In patients with muscle-invasive bladder cancer, a dose-dense neoadjuvant chemotherapy regimen followed by cystectomy was associated with a higher rate of complete responses, compared with standard gemcitabine-platinum neoadjuvant chemotherapy and cystectomy, results of a retrospective analysis indicate.
Among 1,113 patients who underwent neoadjuvant chemotherapy (NAC) and cystectomy, a regimen of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) was associated with a nearly threefold greater likelihood that patients would have a complete response, compared with gemcitabine and cisplatin, reported Scott M. Gilbert, MD, of the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and colleagues.
“We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival,” they wrote in JAMA Oncology.
The investigators noted that, despite clear evidence of a survival benefit associated with neoadjuvant chemotherapy followed by cystectomy, compared with cystectomy alone in patients with muscle-invasive bladder cancer, “the rates of adoption and routine use of NAC have been modest.”
Gemcitabine and cisplatin have become the de facto standard of care because of favorable toxicity profile and response rates comparable to those seen with ddMVAC. Yet there are few studies comparing disease control and survival outcomes for different neoadjuvant chemotherapy regimens, they noted, which prompted the current study.
The investigators conducted a cross-sectional analysis of data on 1,113 patients with bladder cancer treated with cystectomy at their center from January 2007 through May 2017. They compared rates of downstaging, complete responses, and overall survival with ddMVAC, compared with gemcitabine combined with either cisplatin or carboplatin, other neoadjuvant combinations (including etoposide- fluorouracil- and paclitaxel-based regimens) or no neoadjuvant chemotherapy.
Of the 1,113 patients, 824 had disease stage T2 or greater, and of this group, 332 had received neoadjuvant chemotherapy.
They found that ddMVAC was associated with a 52.2% downstaging rate, compared with 41.3% for gemcitabine-cisplatin, and 27% for gemcitabine-carboplatin. Respective pathologic complete response rates were 41.3%, 24.5%, and 9.4% (P less than .001).
Downstaging rates for patients treated with other regimens and patients who did not receive neoadjuvant chemotherapy were 42% and 25.7%, respectively. Complete response rates (downstaging to pT0N0) were 24% and 10.7%.
In a multivariable logistic regression model controlling for age, comorbidities, sex, clinical stage, and chemotherapy regimen, ddMVAC was associated with a significantly higher likelihood of pathologic complete response, with an odds ratio of 2.67 (P less than .001). Similarly, a propensity-score model weighted for clinical and demographic characteristics showed an OR for complete response with ddMVAC of 1.52 (P = .05).
The 2-year Kaplan-Meier survival probability estimate for ddMVAC was 73.3%, compared with 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Regardless of chemotherapy type, a complete pathologic response was a significant predictor for overall survival (P less than .001).
Although ddMVAC showed a trend toward better overall survival in both logistic regression and propensity score models, neither reached statistical significance.
The authors did not report survival results for patients who did not receive neoadjuvant chemotherapy.
The investigators acknowledged that the study is limited by its nonrandomized design and the relatively small sample of patients treated with ddMVAC (46 patients).
The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
SOURCE: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
In patients with muscle-invasive bladder cancer, a dose-dense neoadjuvant chemotherapy regimen followed by cystectomy was associated with a higher rate of complete responses, compared with standard gemcitabine-platinum neoadjuvant chemotherapy and cystectomy, results of a retrospective analysis indicate.
Among 1,113 patients who underwent neoadjuvant chemotherapy (NAC) and cystectomy, a regimen of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) was associated with a nearly threefold greater likelihood that patients would have a complete response, compared with gemcitabine and cisplatin, reported Scott M. Gilbert, MD, of the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and colleagues.
“We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival,” they wrote in JAMA Oncology.
The investigators noted that, despite clear evidence of a survival benefit associated with neoadjuvant chemotherapy followed by cystectomy, compared with cystectomy alone in patients with muscle-invasive bladder cancer, “the rates of adoption and routine use of NAC have been modest.”
Gemcitabine and cisplatin have become the de facto standard of care because of favorable toxicity profile and response rates comparable to those seen with ddMVAC. Yet there are few studies comparing disease control and survival outcomes for different neoadjuvant chemotherapy regimens, they noted, which prompted the current study.
The investigators conducted a cross-sectional analysis of data on 1,113 patients with bladder cancer treated with cystectomy at their center from January 2007 through May 2017. They compared rates of downstaging, complete responses, and overall survival with ddMVAC, compared with gemcitabine combined with either cisplatin or carboplatin, other neoadjuvant combinations (including etoposide- fluorouracil- and paclitaxel-based regimens) or no neoadjuvant chemotherapy.
Of the 1,113 patients, 824 had disease stage T2 or greater, and of this group, 332 had received neoadjuvant chemotherapy.
They found that ddMVAC was associated with a 52.2% downstaging rate, compared with 41.3% for gemcitabine-cisplatin, and 27% for gemcitabine-carboplatin. Respective pathologic complete response rates were 41.3%, 24.5%, and 9.4% (P less than .001).
Downstaging rates for patients treated with other regimens and patients who did not receive neoadjuvant chemotherapy were 42% and 25.7%, respectively. Complete response rates (downstaging to pT0N0) were 24% and 10.7%.
In a multivariable logistic regression model controlling for age, comorbidities, sex, clinical stage, and chemotherapy regimen, ddMVAC was associated with a significantly higher likelihood of pathologic complete response, with an odds ratio of 2.67 (P less than .001). Similarly, a propensity-score model weighted for clinical and demographic characteristics showed an OR for complete response with ddMVAC of 1.52 (P = .05).
The 2-year Kaplan-Meier survival probability estimate for ddMVAC was 73.3%, compared with 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Regardless of chemotherapy type, a complete pathologic response was a significant predictor for overall survival (P less than .001).
Although ddMVAC showed a trend toward better overall survival in both logistic regression and propensity score models, neither reached statistical significance.
The authors did not report survival results for patients who did not receive neoadjuvant chemotherapy.
The investigators acknowledged that the study is limited by its nonrandomized design and the relatively small sample of patients treated with ddMVAC (46 patients).
The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
SOURCE: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
In patients with muscle-invasive bladder cancer, a dose-dense neoadjuvant chemotherapy regimen followed by cystectomy was associated with a higher rate of complete responses, compared with standard gemcitabine-platinum neoadjuvant chemotherapy and cystectomy, results of a retrospective analysis indicate.
Among 1,113 patients who underwent neoadjuvant chemotherapy (NAC) and cystectomy, a regimen of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) was associated with a nearly threefold greater likelihood that patients would have a complete response, compared with gemcitabine and cisplatin, reported Scott M. Gilbert, MD, of the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and colleagues.
“We also found that ddMVAC was associated with longer survival intervals and a lower risk of death than the other treatments examined, although those findings did not reach statistical significance, indicating that larger comparative studies are needed to definitively answer questions regarding survival,” they wrote in JAMA Oncology.
The investigators noted that, despite clear evidence of a survival benefit associated with neoadjuvant chemotherapy followed by cystectomy, compared with cystectomy alone in patients with muscle-invasive bladder cancer, “the rates of adoption and routine use of NAC have been modest.”
Gemcitabine and cisplatin have become the de facto standard of care because of favorable toxicity profile and response rates comparable to those seen with ddMVAC. Yet there are few studies comparing disease control and survival outcomes for different neoadjuvant chemotherapy regimens, they noted, which prompted the current study.
The investigators conducted a cross-sectional analysis of data on 1,113 patients with bladder cancer treated with cystectomy at their center from January 2007 through May 2017. They compared rates of downstaging, complete responses, and overall survival with ddMVAC, compared with gemcitabine combined with either cisplatin or carboplatin, other neoadjuvant combinations (including etoposide- fluorouracil- and paclitaxel-based regimens) or no neoadjuvant chemotherapy.
Of the 1,113 patients, 824 had disease stage T2 or greater, and of this group, 332 had received neoadjuvant chemotherapy.
They found that ddMVAC was associated with a 52.2% downstaging rate, compared with 41.3% for gemcitabine-cisplatin, and 27% for gemcitabine-carboplatin. Respective pathologic complete response rates were 41.3%, 24.5%, and 9.4% (P less than .001).
Downstaging rates for patients treated with other regimens and patients who did not receive neoadjuvant chemotherapy were 42% and 25.7%, respectively. Complete response rates (downstaging to pT0N0) were 24% and 10.7%.
In a multivariable logistic regression model controlling for age, comorbidities, sex, clinical stage, and chemotherapy regimen, ddMVAC was associated with a significantly higher likelihood of pathologic complete response, with an odds ratio of 2.67 (P less than .001). Similarly, a propensity-score model weighted for clinical and demographic characteristics showed an OR for complete response with ddMVAC of 1.52 (P = .05).
The 2-year Kaplan-Meier survival probability estimate for ddMVAC was 73.3%, compared with 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Regardless of chemotherapy type, a complete pathologic response was a significant predictor for overall survival (P less than .001).
Although ddMVAC showed a trend toward better overall survival in both logistic regression and propensity score models, neither reached statistical significance.
The authors did not report survival results for patients who did not receive neoadjuvant chemotherapy.
The investigators acknowledged that the study is limited by its nonrandomized design and the relatively small sample of patients treated with ddMVAC (46 patients).
The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
SOURCE: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
FROM JAMA ONCOLOGY
Key clinical point: Neoadjuvant chemotherapy with dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) is associated with improved response and survival rates, compared with gemcitabine-based regimens.
Major finding: 2-year survival probability with ddMVAC was 73.3% vs. 62% for gemcitabine-cisplatin and 34.8% for gemcitabine-carboplatin (P = .002).
Study details: Retrospective cross-sectional analysis of data on 1,113 patients with muscle-invasive bladder cancer.
Disclosures: The study was supported in part by a National Cancer Institute grant to the H. Lee Moffitt Cancer Center. The authors reported having no conflicts of interest.
Source: Peyton CC et al. JAMA Oncology. 2018 Aug 30. doi: 10.1001/jamaoncol.2018.3542.
Sometimes talk is useless
“Alex, I understand that you are upset that you left your little bulldozer at home. Let’s try to think of something else you can play with here at the restaurant that is kind of like a bulldozer.”
Sounds like a reasonable strategy to calm an unruly preschooler, and it might have been had it not been the fifth attempt in a 45-minute dialogue between a mother and her overtired, misbehaving 3-year-old. There had been a lot of “I understand how you feel” and “use your words” woven into a gag-worthy and futile effort to forge a collaborative parent-child solution to the problem of an exhausted preschooler who is up past his bedtime in a public place.
My wife and I enjoy a night out with friends and prefer a quiet dining atmosphere. However, some evenings we eat earlier and choose a restaurant we know appeals to families with young children. At those meals, we anticipate being serenaded by a loud background buzz punctuated by the occasional shriek or short bout of crying. We expect a degree of childish behavior to come with the territory, and watching the dramas unfold brings back fond “been there, done that” memories. But, listening to those behaviors being horribly mismanaged can ruin even the most tolerant adult’s appetite in less time than it takes a parent to say, “I can see you’re unhappy, and we need to talk about why.”
In an op-ed piece, a psychotherapist asks the legitimate question, and provides the correct quick answer (“Which Is Better, Rewards or Punishments? Neither,” New York Times, Aug. 21, 2018). I couldn’t agree more. In my experience, rewards have a very short half-life and can become disastrously inflationary in the blink of an eye. On the other hand, punishments can be either too heavy-handed or so irrational that the child fails to make a logical connection between his misbehavior and his sentence.
Unfortunately, many child behavior advisers, including the op-ed author, offer alternatives to rewards and punishment that are unworkable in real-world circumstances, such as the restaurant scenario my wife and I endured.
While it sounds very democratic to ask a 3-year-old why he is misbehaving, more often than not it should be the parent who is asking what he or she could have done differently to avoid the situation. It is likely the child has been allowed to become overtired and/or the parent may be in denial about his/her child’s intolerance for stimulating environments.
Too often the parent takes too long to realize that the water is spilling over the dam and it is time to head for shore. Children who are overtired and having a tantrum can’t participate in a rational discussion about their feelings. If that dialogue needs to happen, and that is seldom, it should be the next day after the parent has time to consider his or her own mistakes.
When it comes to managing misbehaving children, I prefer well-tailored consequences and my favorite is a humanely crafted time-out. When presented and executed properly, a time-out can break the cycle of misbehavior and give both parent and child a chance to reconsider their positions.
But at 7 p.m. on a Friday evening in a busy restaurant, neither a time-out nor philosophizing with a 3-year-old is going to work. It’s time to ask for the check and head home to bed.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“Alex, I understand that you are upset that you left your little bulldozer at home. Let’s try to think of something else you can play with here at the restaurant that is kind of like a bulldozer.”
Sounds like a reasonable strategy to calm an unruly preschooler, and it might have been had it not been the fifth attempt in a 45-minute dialogue between a mother and her overtired, misbehaving 3-year-old. There had been a lot of “I understand how you feel” and “use your words” woven into a gag-worthy and futile effort to forge a collaborative parent-child solution to the problem of an exhausted preschooler who is up past his bedtime in a public place.
My wife and I enjoy a night out with friends and prefer a quiet dining atmosphere. However, some evenings we eat earlier and choose a restaurant we know appeals to families with young children. At those meals, we anticipate being serenaded by a loud background buzz punctuated by the occasional shriek or short bout of crying. We expect a degree of childish behavior to come with the territory, and watching the dramas unfold brings back fond “been there, done that” memories. But, listening to those behaviors being horribly mismanaged can ruin even the most tolerant adult’s appetite in less time than it takes a parent to say, “I can see you’re unhappy, and we need to talk about why.”
In an op-ed piece, a psychotherapist asks the legitimate question, and provides the correct quick answer (“Which Is Better, Rewards or Punishments? Neither,” New York Times, Aug. 21, 2018). I couldn’t agree more. In my experience, rewards have a very short half-life and can become disastrously inflationary in the blink of an eye. On the other hand, punishments can be either too heavy-handed or so irrational that the child fails to make a logical connection between his misbehavior and his sentence.
Unfortunately, many child behavior advisers, including the op-ed author, offer alternatives to rewards and punishment that are unworkable in real-world circumstances, such as the restaurant scenario my wife and I endured.
While it sounds very democratic to ask a 3-year-old why he is misbehaving, more often than not it should be the parent who is asking what he or she could have done differently to avoid the situation. It is likely the child has been allowed to become overtired and/or the parent may be in denial about his/her child’s intolerance for stimulating environments.
Too often the parent takes too long to realize that the water is spilling over the dam and it is time to head for shore. Children who are overtired and having a tantrum can’t participate in a rational discussion about their feelings. If that dialogue needs to happen, and that is seldom, it should be the next day after the parent has time to consider his or her own mistakes.
When it comes to managing misbehaving children, I prefer well-tailored consequences and my favorite is a humanely crafted time-out. When presented and executed properly, a time-out can break the cycle of misbehavior and give both parent and child a chance to reconsider their positions.
But at 7 p.m. on a Friday evening in a busy restaurant, neither a time-out nor philosophizing with a 3-year-old is going to work. It’s time to ask for the check and head home to bed.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“Alex, I understand that you are upset that you left your little bulldozer at home. Let’s try to think of something else you can play with here at the restaurant that is kind of like a bulldozer.”
Sounds like a reasonable strategy to calm an unruly preschooler, and it might have been had it not been the fifth attempt in a 45-minute dialogue between a mother and her overtired, misbehaving 3-year-old. There had been a lot of “I understand how you feel” and “use your words” woven into a gag-worthy and futile effort to forge a collaborative parent-child solution to the problem of an exhausted preschooler who is up past his bedtime in a public place.
My wife and I enjoy a night out with friends and prefer a quiet dining atmosphere. However, some evenings we eat earlier and choose a restaurant we know appeals to families with young children. At those meals, we anticipate being serenaded by a loud background buzz punctuated by the occasional shriek or short bout of crying. We expect a degree of childish behavior to come with the territory, and watching the dramas unfold brings back fond “been there, done that” memories. But, listening to those behaviors being horribly mismanaged can ruin even the most tolerant adult’s appetite in less time than it takes a parent to say, “I can see you’re unhappy, and we need to talk about why.”
In an op-ed piece, a psychotherapist asks the legitimate question, and provides the correct quick answer (“Which Is Better, Rewards or Punishments? Neither,” New York Times, Aug. 21, 2018). I couldn’t agree more. In my experience, rewards have a very short half-life and can become disastrously inflationary in the blink of an eye. On the other hand, punishments can be either too heavy-handed or so irrational that the child fails to make a logical connection between his misbehavior and his sentence.
Unfortunately, many child behavior advisers, including the op-ed author, offer alternatives to rewards and punishment that are unworkable in real-world circumstances, such as the restaurant scenario my wife and I endured.
While it sounds very democratic to ask a 3-year-old why he is misbehaving, more often than not it should be the parent who is asking what he or she could have done differently to avoid the situation. It is likely the child has been allowed to become overtired and/or the parent may be in denial about his/her child’s intolerance for stimulating environments.
Too often the parent takes too long to realize that the water is spilling over the dam and it is time to head for shore. Children who are overtired and having a tantrum can’t participate in a rational discussion about their feelings. If that dialogue needs to happen, and that is seldom, it should be the next day after the parent has time to consider his or her own mistakes.
When it comes to managing misbehaving children, I prefer well-tailored consequences and my favorite is a humanely crafted time-out. When presented and executed properly, a time-out can break the cycle of misbehavior and give both parent and child a chance to reconsider their positions.
But at 7 p.m. on a Friday evening in a busy restaurant, neither a time-out nor philosophizing with a 3-year-old is going to work. It’s time to ask for the check and head home to bed.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Case Series Evaluating the Operative and Nonoperative Treatment of Scapular Fractures
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
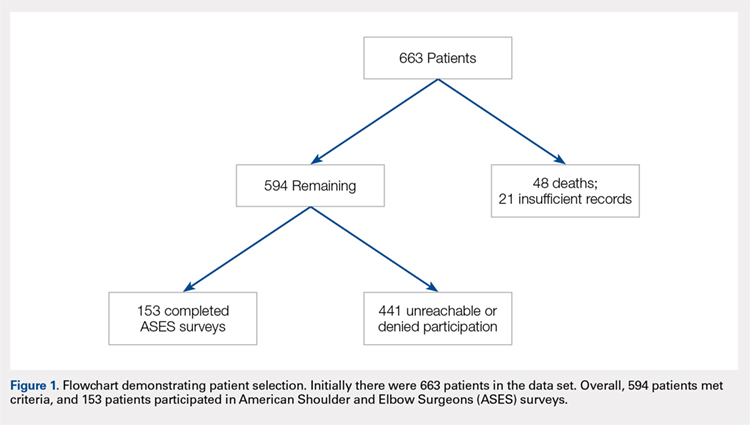
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.
Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
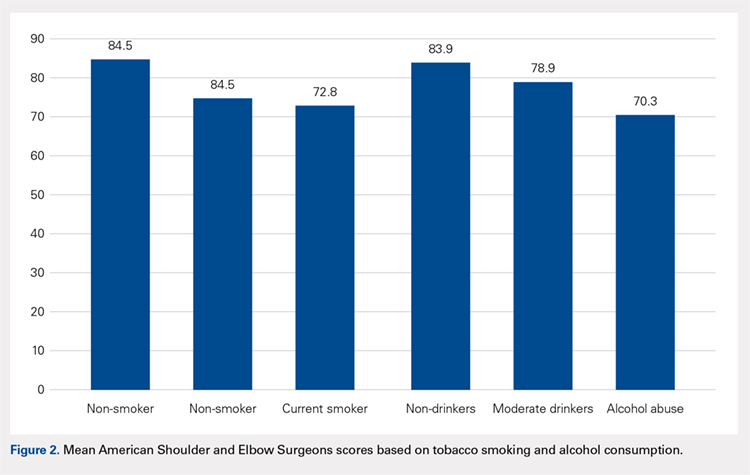
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.
Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.

Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.

Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
ABSTRACT
The injury parameters and patient characteristics that affect function after scapular fracture are poorly defined. We performed a retrospective review of 594 adult patients with a minimum 12-month follow-up after scapular fracture. Functional outcomes were prospectively assessed using the American Shoulder and Elbow Surgeons (ASES) survey in 153 patients after a mean of 62 months of follow-up. The population was 78% male, and 88% had injuries caused by a high-energy event. Only 4.6% had injuries isolated to the scapula. All fractures healed primarily and the mean ASES score was 79.3, indicating minimal functional impairment. However, 7 patients (4.6%) reported severe functional deficits. Fifteen patients (9.8%) underwent open reduction and internal fixation. These patients had a better mean ASES score than those who were treated nonoperatively (92.1 vs 77.9, P = .03). When fracture types were analyzed individually, there was an advantage to surgery in fractures involving the glenoid (96.0 vs 75.7, P < .05). Concomitant chest wall injury or the presence of adjacent fractures did not affect functional outcomes. Smokers had a worse mean score (73.3 vs 84.5, P = .01), as did patients with a history of alcohol abuse (70.3 vs 83.9, P < .05). In conclusion, mean ASES scores indicated good function overall. Patients with a history of tobacco use or alcohol abuse had worse outcome scores.
Continue to: Scapular fractures occur frequently due to high-energy trauma...
Scapular fractures occur frequently due to high-energy trauma, with concomitant injuries seen in approximately 90% of cases.1-4 As a result, treatment is often surrounded by other difficult medical decisions, and factors affecting outcomes can be multifaceted. The gaps in our understanding of long-term outcomes with current treatment modalities have recently come to light, especially when it comes to determining indications for surgery.
Specifically, there is very little literature on radiographic healing and long-term shoulder function in larger samples of scapular fractures; additionally, there is evidence that some patients do not experience full functional recovery.3,5-7 Studies assessing return of function in patients treated nonoperatively have shown decreased mobility and persistence of pain.7 Some of these findings could be due to variability in surgical indications.2,4 While the majority of fractures are treated nonoperatively, the decision to operate has recently been one of debate. Prior literature has suggested highly variable measurements of angulation and extra-articular displacement at which surgery is recommended.1 For example, indications for surgery measured by the medial displacement of extra-articular fractures range from >10 mm to >20 mm;8-11 similarly, the displacement of intra-articular fractures meriting surgery ranges from >2 mm to >5 mm, depending on the author.12-16
The current debate over surgical indications for less severe scapular fractures, as well as the potential for chronic pain and stiffness calls for a thorough examination of factors affecting functional outcomes. The purpose of this study is to determine which patient factors, fracture patterns, and treatment modalities were associated with differences in healing and return of shoulder function. We hypothesized that certain aspects of the patient’s social history (tobacco, alcohol) as well as concomitant chest wall injuries may be associated with poor outcome scores and lower levels of function. We further hypothesized that glenoid fractures would affect function more than body fractures, and we did not expect to see a significant difference in outcomes between operative and nonoperative treatment.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. A registry at our level 1 trauma center was queried to identify 663 skeletally mature patients with scapular fractures between 1999 and 2011. Forty-eight patients had died prior to the study, and 21 patients had insufficient radiography and/or clinical follow-up (Figure 1). To be included, patients were required to have at least 1 year of follow-up to assess healing. Data on patient demographics, fracture classification, etiology of injury, concomitant injuries (clavicle fractures, rib fractures, pulmonary injuries), comorbidities, alcohol use, and tobacco use were collected retrospectively for the remaining 594 patients. Patients were then prospectively contacted via telephone and mail, employing 3 Internet search engines as needed, in an attempt to obtain current contact information. Three patients declined to participate, and 438 were not reachable after multiple attempts. Outcome scores for the remaining 153 patients were determined with the Modified American Shoulder and Elbow Surgeons (ASES) Shoulder Form.17 Scores were measured out of 100, with 0 to 30 representing maximally impaired, 31 to 60 representing moderately impaired, and 61 to 100 representing minimally impaired shoulder function.18 Due to the retrospective identification of the patients, no pre-injury shoulder function scores were collected. Given that many patients were unreachable, or reachable but not living in close proximity to the hospital, patients did not routinely return for re-evaluation for this study.

Nonoperative management consisted of sling immobilization for comfort for up to 2 weeks, during which time Codman’s exercises and elbow, forearm, wrist, and hand motion were encouraged. Active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 8 to 10 weeks following the injury. Decision for surgery was at the surgeon’s discretion. Surgical indications included articular displacement and severely displaced glenoid neck fractures. Open reduction and internal fixation was performed by 1 of 4 fellowship-trained surgeons. Concomitant surgical procedures were not undertaken in the same setting. Postoperative activity consisted of sling immobilization for comfort for up to 6 weeks, during which time active and passive shoulder mobility without restriction were also recommended progressively as tolerated. Strengthening and unrestricted lifting activities were allowed after approximately 12 weeks following surgery. We considered fractures as healed if either X-rays showed healing progression to complete union or early X-rays showing signs of healing with subsequent follow-up visits indicating clinical healing (absence of pain, absence of shoulder dysfunction).
Continue to: STATISTICAL ANALYSIS...
STATISTICAL ANALYSIS
Statistical analysis was undertaken with GraphPad software. Associations were tested between positive predictive variables and functional outcomes. Variables included gender, mechanism, fracture classification, patient comorbidities, social factors, associated injuries, and type of treatment. A Mann-Whitney rank test was used to test for associations between nonparametric variables, including patient age. In all cases, P < .05 was considered significant.
RESULTS
Complete clinical and radiographic data were available for 594 patients. This included 462 men and 132 women, with a mean age of 42.8 years (range, 15-92 years). Twenty-four patients (4.0%) sustained bilateral fractures, and 31 fractures (5.0%) were open. All fractures healed primarily. A total of 153 patients completed the ASES questionnaire at a mean of 62 months after injury (Table 1). This group was similar to the entire population with respect to age, gender, and type of treatment. In all, 135 patients had been injured by a high-energy mechanism (88%), and the fracture pattern as per the Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association (AO/OTA) classification consisted of 14A (no glenoid involvement) (n = 139; 91%) and 14B/C (glenoid involvement) (n = 14; 9.2%).19 The mean ASES score for our entire sample was 79.3 (minimally functionally impaired). In all, 117 patients (76%) reported minimal functional deficit (ASES, 61-100), 29 (19%) reported moderate functional deficit (ASES, 31-60), and only 7 (4.6%) reported maximum functional deficit (ASES, 0-30). Gender and age were not associated with functional outcome scores.
Table 1. Patient Demographics and Etiology of Scapula Fractures.
| n |
Gender |
|
Men | 119 (77.8%) |
Women | 34 (22.2%) |
Mechanism |
|
Motorcycle crash | 48 (31.4%) |
Motor vehicle collision | 38 (24.8%) |
Fall from stand | 14 (9.2%) |
Fall from height | 13 (8.5%) |
Pedestrian vs vehicle | 11 (7.2%) |
Crush | 7 (4.5%) |
Gunshot | 5 (3.3%) |
Other | 17 (11.1%) |
Fracture Pattern |
|
14A | 139 (88.2%) |
14B/C | 14 (11.8%) |
Fifteen patients (9.8%) were treated surgically. They had a higher mean ASES score vs non-surgically treated patients (92.1 vs 77.9; P = .03) (Table 2). However, when patients were divided into 14A and 14B/C fracture patterns, there was only a significant advantage in outcome scores for operative vs nonoperative care in the 14B/C classification (96.0 vs 75.7; P < .05); meanwhile, surgery for scapular body fractures (14A) was not associated with better outcome scores (90.2 vs 78.3; P = .14). Unfortunately, assessment of these comparisons within classification groups resulted in underpowered analyses for these small groups.
Table 2. Number of ASES Surveys Completed and Mean ASES Score for Each Treatment Type and Fracture Classification
| n | Mean ASES | Standard Error |
Surgical (total) | 15 | 92.1a | 3.5 |
Surgical 14A | 10 | 90.2 | 4.9 |
Surgical 14B/C | 5 | 96.0a | 3.2 |
Non-surgical (total) | 138 | 77.9a | 2.1 |
Nonsurgical. 14A | 129 | 78.3 | 2.2 |
Nonsurgical 14B/C | 9 | 75.7a | 6.5 |
aP < 0.05.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Table 3 shows the ASES scores for patients with various types of associated chest and shoulder injuries. Only 7 patients (4.6%) had injuries isolated to the scapula. Thirty-three patients (22%) had associated clavicle fractures, and 102 patients (67%) sustained concomitant chest wall injuries, including rib fractures (n = 88) and pulmonary injuries (n = 71). Patients with associated chest wall injuries did not have worse mean ASES scores than those without chest wall injuries (80.9 vs 78.2; P = .49). Additionally, patients who had concomitant clavicle fractures did not report worse scores than those who did not (83.2 vs 78.6; P = .46).
Table 3. Concomitant Injuries and Mean American Shoulder and Elbow Surgeons (ASES) Scores
| n | Mean ASES | Standard Error |
Clavicle fracture | 33 (21.6%) | 83.2 | 3.6 |
No clavicle fracture | 120 (78.4%) | 78.6 | 2.2 |
Chest wall injury | 102 (66.7%) | 80.9 | 2.1 |
Rib fracture | 31 (20.3%) | 82.4 | 3.6 |
Lung Injury | 14 (9.2%) | 80.8 | 5.5 |
Rib Fracture + Lung Injury | 57 (37.3%) | 80.2 | 3.0 |
No chest wall injury | 51 (33.3%) | 78.2 | 3.8 |
Isolated scapula fracture | 7 (4.6%) | 92.4 | 6.5 |
The majority of patients were self-reported smokers (54%) and alcohol drinkers (64%) (Table 4). Aspects of social history were associated with differences in functional outcome scores. Non-smokers had a higher mean ASES score than both current smokers (84.5 vs 72.8; P = .02) and patients with any lifetime history of smoking (84.5 vs 73.3; P = .01) (Figure 2). There was no significant difference in shoulder function scores between patients identified as non-drinkers and those who reported consuming alcohol at moderate levels (83.9 vs 78.9; P = .26); however, patients who had a documented history of alcohol abuse had lower mean ASES scores than those who reported being non-drinkers (70.3 vs 83.9; P < .05).
Table 4. Substance Use and Functional Outcome Scores
| n | Mean ASES | Standard Error |
Non-smoker | 57 (46.3%) | 84.5a | 2.9 |
History of smoking | 66 (53.7%) | 73.3a | 3.0 |
Smoker | 45 (36.6%) | 72.8a | 3.8 |
Former | 21 (17.1%) | 74.6 | 5.1 |
No alcohol consumption | 46 (36.2%) | 83.9a | 3.1 |
Moderate alcohol use | 65 (51.2%) | 78.9 | 2.9 |
Alcohol abuse | 16 (12.6%) | 70.3a | 7.3 |
aP < 0.05.

Continue to: DISCUSSION...
DISCUSSION
Patients with scapular fractures often require a complex set of treatment decisions due to high rates of concomitant injuries.2,20-22 A lack of large studies on long-term scapular function, as well as evidence that some patients treated conservatively for scapular fractures experience functional deficit and pain, inspired us to investigate the recovery process after scapular fractures through radiographs and the ASES survey.7 Further, we attempted to identify any factors that may be associated with poor functional results. Our review of long-term outcomes after scapular fractures demonstrates that they not only heal well but also have a good functional outcome in most cases. Over 95% had acceptable ASES scores, with both 14A and 14B/C having similar return of function. While both operatively and nonoperatively treated patients had scores indicating minimal functional impairment, those treated surgically had better scores overall. Surprisingly, concomitant injuries, including chest wall injuries, did not portend a worse shoulder outcome in our patients. The factors that were associated with worse outcome were tobacco use and alcohol abuse.
Beyond these findings, we attempted to comment on surgical indications, which have been highly debated.2,3 For example, the medial displacement at which studies suggest extra-articular fractures merit surgery ranges from >10 mm to >20 mm;8-11 similarly, the indication for surgery based on displacement of intra-articular fractures ranges from >2 mm to >5 mm, depending on the author.12-16 Glenoid articular fractures or neck fractures are other potential indications for operative treatment. In fact, a review of 520 scapular fractures from multiple studies found that 80% of those with glenoid involvement were treated operatively, while only 52% of those with exclusive acromion and/or coracoid involvement, and 1% of those with exclusive scapular body involvement were treated operatively.5 Some reports indicate that 14B/C fractures, especially those that are displaced or complex, show good functional outcomes and low complication rates after fixation.5,23 In this study, articular fractures of the glenoid were treated operatively more often than extra-articular fractures. We attempted to determine the impact of surgical care on functional outcomes according to fracture type, but we were limited by the small number of surgical patients when reviewing the 14A and 14B/C groups. As a whole, surgical patients had better outcomes than non-surgical patients. We believe this difference is clinically relevant and suggests a potential group of patients who may benefit from fixation. Further investigation is needed to better characterize these injuries and to develop specific recommendations.
This study yielded interesting results related to substance abuse. It has previously been shown that tobacco smoking and alcohol abuse have both been associated with poor bone health.24 Studies have suggested that exposure to nicotine and other chemical components in cigarettes can lead to delayed healing, higher rates of nonunion, and decreased mechanical strength of bone.25-29 Additionally, alcohol abuse has been associated with decreased bone mass and poor bone formation.24,30,31 Although we did not measure bone density or quantitate time of healing, this study provides added insight in that the healed fractures of smokers and patients with a history of alcohol abuse showed lower levels of shoulder function, as measured by ASES scores after similar initial injuries and similar follow-up periods. These results suggest that chemical, social, or a combination of these factors affect muscular recovery, other aspects of post-fracture recovery, and/or levels of baseline physical or mental impairment beyond those detailed in previous studies of bone health and substance abuse. For example, return to work was a scored category in the ASES survey that we used to asses the return of shoulder function, and several studies have shown that factors such as education level, coping abilities, and baseline functioning (cognitive, social, and physical) all have a significant impact on rates of return to work, independently of injury type.6,32-35 It is possible, then, that other aspects of the ASES survey are affected by factors that may be more prevalent in populations engaging in substance abuse. From both perspectives, these data highlight the importance of addressing tobacco use and alcohol abuse as a part of caring for all trauma patients, including those with scapular fractures, regardless of their high rates of radiographic healing. They also provide insight for prognosticating and setting patient expectations after scapular fractures.
Continue to: This study addressed the relationship between...
This study addressed the relationship between concomitant chest wall injuries and recovery of shoulder function after scapular fracture. Previous studies have suggested that concomitant chest wall injuries, such as rib fractures, cause more pain and may adversely impact the return of function in patients who have sustained scapular body fractures.1 These results, however, occurred in the setting of a much shorter follow-up, in which Disability of Arm, Shoulder, and Hand (DASH) surveys were distributed 6 months post-injury, 12 months post-injury, and once at last follow-up (<3 years). At our significantly later average follow-up, chest wall injuries did not portend a worse return of shoulder function, in contrast to our hypothesis. Our lack of findings of a worse return of function in patients with chest wall injuries, in light of previous literature, suggests that this association could become less distinct as the initial injury becomes more remote and has had more time to heal. Farther out from injury, patients seem to function similarly, regardless of chest wall injury history.
This study was limited by several factors. First, the surgically treated group was considerably smaller than the nonoperative group, which made drawing statistically significant comparisons between them challenging. Although there were no apparent differences between the group who completed ASES surveys and those who did not, only collecting ASES data on 153 of the 663 patients introduces a possible selection bias in this analysis. Additionally, due to the retrospective nature of this study, we were not able to ascertain the specific surgical indications used by individual surgeons. Again, the nature of this study also made it implausible to separate fractures beyond the simple 14A vs 14B/C classification. For example, we did not routinely have access to computed tomography scans to provide exact measurements of displacement, angulation, or step-off; therefore, we were unable to compare our fracture parameters to those mentioned in studies with more specific surgical indications. We also did not have information regarding pre-existing shoulder dysfunction, which could negatively affect ASES scores. Finally, accurate measures of certain social history factors can be difficult to achieve; smoking, alcohol consumption, and alcohol abuse may be subject to underreporting.
CONCLUSION
We assessed parameters that may affect return of shoulder function after scapular fracture. Our results indicate that both 14A and 14B/C fractures have similarly high rates of healing and minimal functional impairment. Patients treated operatively typically had better shoulder functional outcomes. Current or past tobacco use or alcohol abuse was associated with worse functional outcome scores. This could suggest chemical, social, or a combination of these factors affecting muscular recovery and/or greater levels of baseline functional impairment. Finally, concomitant chest wall injuries may not negatively affect shoulder outcome, contrasting with data from previous studies on the more immediate post-injury period.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
1. Dimitroulias A, Molinero KG, Krenk DE, Muffly MT, Altman DT, Altman GT. Outcomes of nonoperatively treated displaced scapular body fractures. Clin Orthop Relat Res. 2011;469(5):1459-1465. doi:10.1007/s11999-010-1670-4.
2. Voleti PB, Namdari S, Mehta S. Fractures of the scapula. Adv Orthop. 2012;2012:903850. doi:10.1155/2012/903850.
3. Cole PA, Gauger EM, Schroder LK. Management of scapular fractures. J Am Acad Orthop Surg. 2012;20(3):130-141. doi:10.5435/JAAOS-20-03-130.
4. Salimi J, Khaji A, Karbakhsh M, Saadat S, Eftekhar B. Scapular fracture: lower severity and mortality. Sao Paulo Med J. 2008;126(3):186-189. doi:10.1590/S1516-31802008000300009.
5. Anavian J, Gauger EM, Schroder LK, Wijdicks CA, Cole PA. Surgical and functional outcomes After operative management of complex and displaced intra-articular glenoid fractures. J Bone Joint Surg Am. 2012;94(7):645-653. doi:10.2106/JBJS.J.00896.
6. Brenneman FD, Redelmeier DA, Boulanger BR, McLellan BA, Culhane JP. Long-term outcomes in blunt trauma: who goes back to work? J Trauma. 1997;42(5):778-781. doi:10.1097/00005373-199705000-00004.
7. Schofer MD, Sehrt AC, Timmesfeld N, Störmer S, Kortmann HR. Fractures of the scapula: long-term results after conservative treatment. Arch Orthop Trauma Surg. 2009;129(11):1511-1519. doi:10.1007/s00402-009-0855-3.
8. Ada JR, Miller ME. Scapular fractures - analysis of 113 cases. Clin Orthop Relat Res. 1991:174-180.
9. Herrera DA, Anavian J, Tarkin IS, Armitage BA, Schroder LK, Cole PA. Delayed operative management of fractures of the scapula. J Bone Joint Surg Br. 2009;91(5):619-626. doi:10.1302/0301-620X.91B5.22158.
10. Jones CB, Sietsema DL. Analysis of operative versus nonoperative treatment of displaced scapular fractures. Clin Orthop Relat Res. 2011;469(12):3379-3389. doi:10.1007/s11999-011-2016-6.
11. Khallaf F, Mikami A, Al-Akkad M. The use of surgery in displaced scapular neck fractures. Med Princ Pract. 2006;15(6):443-448. doi:10.1159/000095491.
12. Adam FF. Surgical treatment of displaced fractures of the glenoid cavity. Int Orthop. 2002;26(3):150-153. doi:10.1007/s00264-002-0342-8.
13. Kavanagh BF, Bradway JK, Cofield RH. Open reduction and internal fixation of displaced intraarticular fractures of the glenoid fossa. J Bone Joint Surg Am. 1993;75(4):479-484.
14. Leung KS, Lam TP, Poon KM. Operative treatment of displaced intra-articular glenoid fractures. Injury. 1993;24(5):324-328. doi:10.1016/0020-1383(93)90056-C.
15. Mayo KA, Benirschke SK, Mast JW. Displaced fractures of the glenoid fossa. Results of open reduction and internal fixation. Clin Orthop Relat Res. 1998:122-130. doi:10.1097/00003086-199802000-00015.
16. Schandelmaier P, Blauth M, Schneider C, Krettek C. Fractures of the glenoid treated by operation. A 5-to 23-year follow-up of 22 cases. J Bone Joint Surg Br. 2002;84(2):173-177. doi:10.1302/0301-620X.84B2.12357.
17. Beaton D, Richards RR. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7(6):565-572. doi:10.1016/S1058-2746(98)90002-7.
18. Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594. doi:10.1067/mse.2002.127096.
19. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium-2007 - Orthopedic Trauma Association classification. Orthop Trauma. 2007;21:S1-S133.
20. Armstrong CP, Van der Spuy J. The fractured scapula: importance and management based on a series of 62 patients. Injury. 1984;15(5):324-329. doi:10.1016/0020-1383(84)90056-1.
21. McGahan JP, Rab GT, Dublin A. Fractures of the scapula. J Trauma. 1980;20(10):880-883. doi:10.1097/00005373-198010000-00011.
22. Thompson DA, Flynn TC, Miller PW, Fischer RP. The significance of scapular fractures. J Trauma. 1985;25(10):974-977. doi:10.1097/00005373-198510000-00008.
23. Zlowodzki M, Bhandari M, Zelle BA, Kregor PJ, Cole PA. Treatment of scapula fractures: systematic review of 520 fractures in 22 case series. J Orthop Trauma. 2006;20(3):230-233. doi:10.1097/00005131-200603000-00013.
24. Fini M, Giavaresi G, Salamanna F, et al. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J Bone Miner Metab. 2011;29(6):633-644. doi:10.1007/s00774-011-0309-1.
25. Donigan JA, Fredericks DC, Nepola JV, Smucker JD. The effect of transdermal nicotine on fracture healing in a rabbit model. J Orthop Trauma. 2012;26(12):724-727. doi:10.1097/BOT.0b013e318270466f.
26. Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
27. Hernigou J, Schuind F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int Orthop. 2013;37(5):883-887. doi:10.1007/s00264-013-1809-5.
28. Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640-1644.
29. Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254-262.
30. Farley JR, Fitzsimmons R, Taylor AK, Jorch UM, Lau KH. Direct effects of ethanol on bone resorption and formation in vitro. Arch Biochem Biophys. 1985;238(1):305-314. doi:10.1016/0003-9861(85)90169-9.
31. Turner RT. Skeletal response to alcohol. Alcoholism Clin Exp Res. 2000;24(11):1693-1701. doi:10.1111/j.1530-0277.2000.tb01971.x.
32. MacKenzie EJ, Morris JA, Jurkovich GJ, et al. Return to work following injury: the role of economic, social, and job-related factors. Am J Public Health. 1998;88(11):1630-1637. doi:10.2105/AJPH.88.11.1630.
33. Schnyder U, Moergeli H, Klaghofer R, Sensky T, Buchi S. Does patient cognition predict time off from work after life-threatening accidents? Am J Psychiatry. 2003;160(11):2025-2031. doi:10.1176/appi.ajp.160.11.2025.
34. Soberg HL, Finset A, Bautz-Holter E, Sandvik L, Roise O. Return to work after severe multiple injuries: A multidimensional approach on status 1 and 2 years postinjury. J Trauma. 2007;62(2):471-481. doi:10.1097/TA.0b013e31802e95f4.
35. Soberg HL, Roise O, Bautz-Holter E, Finset A. Returning to work after severe multiple injuries: multidimensional functioning and the trajectory from injury to work at 5 years. J Trauma. 2011;71(2):425-434. doi:10.1097/TA.0b013e3181eff54f.
TAKE-HOME POINTS
- The majority of patients with scapula fractures are multiply-injured.
- Despite being multiply-injured, most heal with minimal functional shoulder impairment.
- While concomitant injuries do not appear to affect shoulder function scores, tobacco use and alcohol abuse are associated with worse outcomes after scapula fractures.
- Most scapula fractures can be treated successfully without surgery.
- Although patients had higher average function scores after open reduction and internal fixation, further research should be done to define indications for fixation.
September 2018 Question 2
Q2. Correct Answer: D
Rationale
This patient is on nadolol, a nonselective beta-blocker, for the primary prophylaxis of large esophageal varices. The dose of nonselective beta-blockers should be increased in a stepwise manner until the maximum tolerated dose or until a resting heart rate of 50-55/min is met. Since this patient is already at target heart rate, there is no indication to increase the dose. Repeat endoscopy is not indicated to assess change in size of varices once initiated on nonselective beta-blockers and at target heart rate. The choice between beta-blockers or endoscopic variceal ligation depends on local resources and expertise, patient preference and characteristics, side effects, and contraindications. Carvedilol, a nonselective beta-blocker with vasodilatory properties, is a promising alternative therapy that deserves further evaluation. However, given that nadolol has achieved target heart rate and patient is tolerating it, there is no indication to change management.
Reference
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., Carey W.. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. Tripathi D., Ferguson J.W., Kochar N., et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50(3):825-33.
[email protected]
Q2. Correct Answer: D
Rationale
This patient is on nadolol, a nonselective beta-blocker, for the primary prophylaxis of large esophageal varices. The dose of nonselective beta-blockers should be increased in a stepwise manner until the maximum tolerated dose or until a resting heart rate of 50-55/min is met. Since this patient is already at target heart rate, there is no indication to increase the dose. Repeat endoscopy is not indicated to assess change in size of varices once initiated on nonselective beta-blockers and at target heart rate. The choice between beta-blockers or endoscopic variceal ligation depends on local resources and expertise, patient preference and characteristics, side effects, and contraindications. Carvedilol, a nonselective beta-blocker with vasodilatory properties, is a promising alternative therapy that deserves further evaluation. However, given that nadolol has achieved target heart rate and patient is tolerating it, there is no indication to change management.
Reference
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., Carey W.. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. Tripathi D., Ferguson J.W., Kochar N., et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50(3):825-33.
[email protected]
Q2. Correct Answer: D
Rationale
This patient is on nadolol, a nonselective beta-blocker, for the primary prophylaxis of large esophageal varices. The dose of nonselective beta-blockers should be increased in a stepwise manner until the maximum tolerated dose or until a resting heart rate of 50-55/min is met. Since this patient is already at target heart rate, there is no indication to increase the dose. Repeat endoscopy is not indicated to assess change in size of varices once initiated on nonselective beta-blockers and at target heart rate. The choice between beta-blockers or endoscopic variceal ligation depends on local resources and expertise, patient preference and characteristics, side effects, and contraindications. Carvedilol, a nonselective beta-blocker with vasodilatory properties, is a promising alternative therapy that deserves further evaluation. However, given that nadolol has achieved target heart rate and patient is tolerating it, there is no indication to change management.
Reference
1. Garcia-Tsao G., Sanyal A.J., Grace N.D., Carey W.. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
2. Tripathi D., Ferguson J.W., Kochar N., et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50(3):825-33.
[email protected]
Q2. A 63-year-old man presents to your clinic for follow-up of his known cirrhosis. He had an upper endoscopy 1 month ago, where he was found to have large varices with no high-risk stigmata. The patient was placed on nadolol 20 mg daily, and is tolerating it without side effects. On physical exam, he has no clinical ascites. His vitals are as follows: temperature, 98.4º F; blood pressure, 114/75 mm Hg; heart rate 55 beats/minute.
What is the next most appropriate step to manage these varices?
September 2018 Question 1
Q1. Correct Answer: D
Rationale
There is a risk of perineal trauma with vaginal delivery, and therefore, patients with active perianal Crohn's disease should undergo cesarean delivery to avoid exacerbation of disease. Patients without a history of perianal disease or those with inactive perianal disease have a low rate of relapse, and cesarean delivery is not warranted. Aside from patients with active perineal disease, the mode of delivery should be left to the discretion of the obstetrician. None of the other choices above, including ileal pouch-anal anastomosis, justify the decision to perform cesarean section.
Q1. Correct Answer: D
Rationale
There is a risk of perineal trauma with vaginal delivery, and therefore, patients with active perianal Crohn's disease should undergo cesarean delivery to avoid exacerbation of disease. Patients without a history of perianal disease or those with inactive perianal disease have a low rate of relapse, and cesarean delivery is not warranted. Aside from patients with active perineal disease, the mode of delivery should be left to the discretion of the obstetrician. None of the other choices above, including ileal pouch-anal anastomosis, justify the decision to perform cesarean section.
Q1. Correct Answer: D
Rationale
There is a risk of perineal trauma with vaginal delivery, and therefore, patients with active perianal Crohn's disease should undergo cesarean delivery to avoid exacerbation of disease. Patients without a history of perianal disease or those with inactive perianal disease have a low rate of relapse, and cesarean delivery is not warranted. Aside from patients with active perineal disease, the mode of delivery should be left to the discretion of the obstetrician. None of the other choices above, including ileal pouch-anal anastomosis, justify the decision to perform cesarean section.
A 28-year-old woman with a history of Crohn's disease is 29 weeks pregnant. She has had an ileocolonic resection and continues to have a small enterocutaneous fistula. She is otherwise doing well, and is maintained on infliximab therapy. She is asking about the mode of delivery of her baby. She wants to know if she should have an elective cesarean delivery.
In which of the following clinical scenarios would a cesarean delivery be recommended?
What's your diagnosis? - September 2018
Endovascular walled-off pancreatic necrosis complicating a pancreatic duct-portal vein fistula
Pancreatic fistula occurs primarily as a result of abdominal trauma, pancreatic surgery, or disruption of the pancreatic duct. In the vast majority of the cases, the latter is encountered in the context of chronic pancreatitis, and results in chronic pancreatic or peripancreatic fluid or necrotic collections. Rarely, such ductal disruption leads to a direct communication between the ruptured duct and the portal vein lumen. Such pancreas-portal venous fistulas are extremely rare, with less than 20 cases reported in published literature.1 The location of the fistula is within the head of the pancreas in most cases, and it is associated with intrapancreatic necrotic collection in close proximity to the portal vein, as in the present case. The intravascular flow of pancreatic enzymes leads to local and progressively extensive portal vein thrombosis. Importantly, most portal vein thromboses in the context of acute or chronic pancreatitis do not result from pancreas-portal venous fistula, and are explained by local vascular compression by the inflammatory pancreatic head, and acquired coagulation abnormalities owing to the pancreatitis. In case of fistula, the resulting high blood level of the pancreatic enzymes may lead to a range of clinical presentations, from vague abdominal pain to disseminated fat necrosis. Painful erythematous lesions on the lower extremities and arthritis have also been described. The present case is an exceptional complication of pancreatic duct-portal vein fistula, with endovascular organization of walled-off pancreatic necrosis.2 The direct visualization of the fistula is difficult, endoscopic retrograde or, more frequently, magnetic resonance cholangiopancreatography being the most useful technique.3
Ultrasound imaging can be useful by showing the heterogeneous yet hypoechoic content of the portal venous system. Percutaneous transhepatic puncture has also been described, and is performed, as in our case, to obtain fluid sample and to perform evacuation of fluid/drainage if necessary. Percutaneous puncture may also provide precise extension of the portal venous invasion. The management of patients with pancreatic-portal vein fistula is poorly codified and relies on individual clinical and imaging analysis. Early surgical intervention has been described in patients with disseminated fat necrosis to limit morbidity and prevent mortality. Later in the evolution of the disease, surgery can be performed if the fistula remains active to alleviate the patient's symptoms and prevent future complications. Finally, conservative treatment can be proposed in selected patients with dried up fistula, as in the present report.
References
1. Brown A., Malden E., Kugelmas M., et al. Diagnosis of pancreatic duct-portal vein fistula; A case report and review of the literature. J Radiol Case Rep. 2014;8:31-8.
2. Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
3. Yoon S.E., Lee Y.H., Yoon K.H., et al. Spontaneous pancreatic pseudocyst-portal vein fistula presenting with pancreatic ascites: Strength of MR cholangiopancreatography. Br J Radiol. 2008;81:e13-6.
Endovascular walled-off pancreatic necrosis complicating a pancreatic duct-portal vein fistula
Pancreatic fistula occurs primarily as a result of abdominal trauma, pancreatic surgery, or disruption of the pancreatic duct. In the vast majority of the cases, the latter is encountered in the context of chronic pancreatitis, and results in chronic pancreatic or peripancreatic fluid or necrotic collections. Rarely, such ductal disruption leads to a direct communication between the ruptured duct and the portal vein lumen. Such pancreas-portal venous fistulas are extremely rare, with less than 20 cases reported in published literature.1 The location of the fistula is within the head of the pancreas in most cases, and it is associated with intrapancreatic necrotic collection in close proximity to the portal vein, as in the present case. The intravascular flow of pancreatic enzymes leads to local and progressively extensive portal vein thrombosis. Importantly, most portal vein thromboses in the context of acute or chronic pancreatitis do not result from pancreas-portal venous fistula, and are explained by local vascular compression by the inflammatory pancreatic head, and acquired coagulation abnormalities owing to the pancreatitis. In case of fistula, the resulting high blood level of the pancreatic enzymes may lead to a range of clinical presentations, from vague abdominal pain to disseminated fat necrosis. Painful erythematous lesions on the lower extremities and arthritis have also been described. The present case is an exceptional complication of pancreatic duct-portal vein fistula, with endovascular organization of walled-off pancreatic necrosis.2 The direct visualization of the fistula is difficult, endoscopic retrograde or, more frequently, magnetic resonance cholangiopancreatography being the most useful technique.3
Ultrasound imaging can be useful by showing the heterogeneous yet hypoechoic content of the portal venous system. Percutaneous transhepatic puncture has also been described, and is performed, as in our case, to obtain fluid sample and to perform evacuation of fluid/drainage if necessary. Percutaneous puncture may also provide precise extension of the portal venous invasion. The management of patients with pancreatic-portal vein fistula is poorly codified and relies on individual clinical and imaging analysis. Early surgical intervention has been described in patients with disseminated fat necrosis to limit morbidity and prevent mortality. Later in the evolution of the disease, surgery can be performed if the fistula remains active to alleviate the patient's symptoms and prevent future complications. Finally, conservative treatment can be proposed in selected patients with dried up fistula, as in the present report.
References
1. Brown A., Malden E., Kugelmas M., et al. Diagnosis of pancreatic duct-portal vein fistula; A case report and review of the literature. J Radiol Case Rep. 2014;8:31-8.
2. Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
3. Yoon S.E., Lee Y.H., Yoon K.H., et al. Spontaneous pancreatic pseudocyst-portal vein fistula presenting with pancreatic ascites: Strength of MR cholangiopancreatography. Br J Radiol. 2008;81:e13-6.
Endovascular walled-off pancreatic necrosis complicating a pancreatic duct-portal vein fistula
Pancreatic fistula occurs primarily as a result of abdominal trauma, pancreatic surgery, or disruption of the pancreatic duct. In the vast majority of the cases, the latter is encountered in the context of chronic pancreatitis, and results in chronic pancreatic or peripancreatic fluid or necrotic collections. Rarely, such ductal disruption leads to a direct communication between the ruptured duct and the portal vein lumen. Such pancreas-portal venous fistulas are extremely rare, with less than 20 cases reported in published literature.1 The location of the fistula is within the head of the pancreas in most cases, and it is associated with intrapancreatic necrotic collection in close proximity to the portal vein, as in the present case. The intravascular flow of pancreatic enzymes leads to local and progressively extensive portal vein thrombosis. Importantly, most portal vein thromboses in the context of acute or chronic pancreatitis do not result from pancreas-portal venous fistula, and are explained by local vascular compression by the inflammatory pancreatic head, and acquired coagulation abnormalities owing to the pancreatitis. In case of fistula, the resulting high blood level of the pancreatic enzymes may lead to a range of clinical presentations, from vague abdominal pain to disseminated fat necrosis. Painful erythematous lesions on the lower extremities and arthritis have also been described. The present case is an exceptional complication of pancreatic duct-portal vein fistula, with endovascular organization of walled-off pancreatic necrosis.2 The direct visualization of the fistula is difficult, endoscopic retrograde or, more frequently, magnetic resonance cholangiopancreatography being the most useful technique.3
Ultrasound imaging can be useful by showing the heterogeneous yet hypoechoic content of the portal venous system. Percutaneous transhepatic puncture has also been described, and is performed, as in our case, to obtain fluid sample and to perform evacuation of fluid/drainage if necessary. Percutaneous puncture may also provide precise extension of the portal venous invasion. The management of patients with pancreatic-portal vein fistula is poorly codified and relies on individual clinical and imaging analysis. Early surgical intervention has been described in patients with disseminated fat necrosis to limit morbidity and prevent mortality. Later in the evolution of the disease, surgery can be performed if the fistula remains active to alleviate the patient's symptoms and prevent future complications. Finally, conservative treatment can be proposed in selected patients with dried up fistula, as in the present report.
References
1. Brown A., Malden E., Kugelmas M., et al. Diagnosis of pancreatic duct-portal vein fistula; A case report and review of the literature. J Radiol Case Rep. 2014;8:31-8.
2. Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
3. Yoon S.E., Lee Y.H., Yoon K.H., et al. Spontaneous pancreatic pseudocyst-portal vein fistula presenting with pancreatic ascites: Strength of MR cholangiopancreatography. Br J Radiol. 2008;81:e13-6.
A 51-year-old man with a history of chronic pancreatitis presented with fatigue, weight loss, and right abdominal pain. He reported excessive alcohol consumption (> 200 g of alcohol per day during the past 35 years), active tobacco smoking (70 pack-years), and diabetes mellitus treated by insulin therapy. He had suffered from recurrent epigastric pain, left unexplored, for several weeks. Abdominal examination revealed no anomaly. Laboratory test results showed serum lipase 146 U/L (normal, < 78), alkaline phosphatase 477 U/L (normal, < 130), gamma-glutamyl transpeptidase 503 U/L (normal, < 55), albumin 23 g/L (normal, 40-49), and prealbumin 0.11 g/L (normal, 0.22-0.39).
Contrast-enhanced computed tomography scanning showed features of chronic pancreatitis including pancreatic atrophy, parenchymal calcifications, marked peripancreatic fat stranding, and cephalic hypoattenuating well-delineated collection (Figure A).
There was a chronic obstruction of the portal vein that was surrounded by numerous tortuous venous channels (Figure B). The entire intrahepatic portal branches were occluded, the right and left portal branches showed marked dilatation with a lumen filled with fluid-like material (mean density, 18 Hounsfield units), and no contrast uptake (Figure C).
Portal vein walls were thickened and showed contrast enhancement (Figure D). The superior mesenteric vein was also thrombosed, whereas the splenic vein remained patent. Bile ducts were unnoticeable.
Ultrasound-guided transhepatic puncture of the left portal branch was performed and allowed for the aspiration of a brown fluid. Analysis showed lipase 89,990 UI/dL and amylase 43,125 UI/dL. The patient was treated by parenteral nutrition, anticoagulation therapy, and somatostatin analogues. The patient is doing well at the 6-month follow-up.
New and Noteworthy Information—September 2018
Intrathecal Baclofen Reduces Pain in Poststroke Spasticity
Intrathecal baclofen (ITB) therapy improves pain and quality of life in patients with poststroke spasticity, according to a study published August 14 in Stroke. Patients with poststroke spasticity in two or more extremities and an Ashworth Scale score of 3 or higher in two or more affected lower extremity muscle groups were randomized to ITB or conventional medical management. At six months, ITB effectively reduced Numeric Pain Rating Scale scores for actual and least spasticity-related pain and improved quality of life, compared with medical management. In addition, 73% of patients given ITB therapy reported satisfaction with spasticity reduction at month six, versus 48% of patients given medical management. The researchers found no statistically significant differences between groups in reduction of worst pain.
Creamer M, Cloud G, Kossmehl P, et al. Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. Stroke. 2018 Aug 14 [Epub ahead of print].
FDA Approves Diacomit for Seizures Associated With Dravet Syndrome
The FDA has approved Diacomit (stiripentol) for the treatment of seizures associated with Dravet syndrome in patients age 2 and older who are taking clobazam. Diacomit will be available in 250-mg and 500-mg capsules and in fruit-flavored powder packets for oral suspension. In two studies of patients between ages 3 and 17 with Dravet syndrome, patients were randomized to Diacomit or placebo, along with their previous treatment with clobazam and valproate. In Study 1, 71% of patients in the Diacomit group were 50% responders versus 5% in the placebo group. In Study 2, 67% of patients treated with Diacomit were 50% responders versus 9.1% of controls. Diacomit is marketed by Biocodex, which is headquartered in Gentilly, France.
Retinal Thinning Is Associated With Dopaminergic Cell Loss
Retinal thinning is linked to the loss of brain cells in Parkinson’s disease, according to a study published online ahead of print August 15 in Neurology. Researchers examined 49 participants (average age, 69) who had been diagnosed with Parkinson’s disease an average of two years earlier, but who had not yet started medication. Participants were compared with 54 healthy controls who were matched for age. Participants underwent a complete eye exam and high-resolution eye scans. Patients with Parkinson’s disease had retinal layer thinning in the temporal and inferior 2.22-mm sectors. The thickness of these layers in the inferior 2.22-mm sector correlated negatively with Hoehn and Yahr stage. Retinal thinning was associated with dopaminergic loss in the left substantia nigra.
Ahn J, Lee JY, Kim TW, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018 Aug 15 [Epub ahead of print].
Contact Sports Linked to Vascular Risk Factors and Depression
Athletes with a history of playing professional contact sports have more vascular risk factors and higher depression scores, according to a study published online ahead of print August 3 in the Journal of Head Trauma Rehabilitation. This case–control study included 21 retired National Football League and National Hockey League players and 21 age-matched noncontact athlete controls. The investigators assessed participants for mild cognitive impairment (MCI) and measured depression using the Beck Depression Inventory-II (BDI). Eight contact sport athletes and three noncontact athletes met criteria for MCI. Contact sport athletes’ scores were significantly worse on Letter Fluency and List B Immediate Recall. Contact athletes were more obese, had more vascular risk factors, and had higher BDI scores.
Baker JG, Leddy JJ, Hinds AL, et al. An exploratory study of mild cognitive impairment of retired professional contact sport athletes. J Head Trauma Rehabil. 2018 Aug 3 [Epub ahead of print].
Managing Vegetative and Minimally Conscious States
The American Academy of Neurology; the American Congress of Rehabilitative Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research have published a practice guideline on the diagnosis and ongoing medical and rehabilitative care of patients in a vegetative or minimally conscious state caused by brain injury. The guideline was published online ahead of print August 8 in Neurology. The authors based their recommendations on a systematic review of the evidence using a modified Delphi consensus process. Clinicians should advise families that for adults, a minimally conscious state and traumatic etiology are associated with more favorable outcomes, according to the guideline. Structural MRI, SPECT, and the Coma Recovery Scale-Revised can assist prognostication in adults, but no tests improve prognostic accuracy in children, said the authors.
Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018 Aug 8 [Epub ahead of print].
FDA Approves Onpattro
The FDA has approved Onpattro (patisiran) lipid complex injection for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The approval of Onpattro was based on results from a randomized, double-blind, placebo-controlled phase III study. Of 225 patients, 148 were randomized to Onpattro infusion once every three weeks for 18 months. The other participants were randomized to placebo infusion at the same frequency. The patients who received Onpattro had better outcomes on measures of polyneuropathy, including muscle strength, sensation, reflexes, and autonomic symptoms, compared with participants receiving placebo infusions. Patients receiving Onpattro also scored better on assessments of walking, nutritional status, and the ability to perform activities of daily living. Alnylam Pharmaceuticals, which markets Onpattro, is headquartered in Cambridge, Massachusetts.
Ophthalmic Conditions May Indicate Increased Risk of Alzheimer’s Disease
People with recent diagnoses of glaucoma, established age-related macular degeneration, and recent and established diabetic retinopathy may have increased risk of Alzheimer’s disease, according to a study published online ahead of print August 2 in Alzheimer’s & Dementia. The investigators included 3,877 participants selected randomly from the Adult Changes in Thought study in their analysis. Participants were age 65 or older and did not have Alzheimer’s disease at the time of enrollment. During the five-year study, a committee of dementia experts diagnosed Alzheimer’s disease in 792 people. Patients with age-related macular degeneration, diabetic retinopathy, or glaucoma were at 40% to 50% greater risk of developing Alzheimer’s disease, compared with people without these eye conditions. Cataract diagnosis was not a risk factor for Alzheimer’s disease.
Lee CS, Larson EB, Gibbons LE, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2018 Aug 2 [Epub ahead of print].
Insulin Resistance in Nondiabetics With Parkinson’s Disease
Insulin resistance is prevalent in Parkinson’s disease and correlates with BMI, according to a study published in the August issue of the Journal of Parkinson’s Disease. The investigators included 154 nondiabetic patients with Parkinson’s disease in the study. Participants were tested for fasting insulin, fasting glucose, and hemoglobin A1c (HbA1c) and underwent a battery of clinical tests. Investigators recorded participants’ Parkinson’s disease medications, height, weight, and other demographic features. Ninety (58.4%) participants had abnormal insulin resistance. Insulin resistance was more prevalent in overweight and obese participants than in participants with a normal weight. BMI was the only significant predictor of insulin resistance. Insulin resistance did not correlate with cognition, functioning, or nonmotor symptoms.
Hogg E, Athreya K, Basile C, et al. High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson’s disease. J Parkinsons Dis. 2018;8(2):259-265.
FDA Approves Galafold for Fabry Disease in Adults
The FDA has approved Galafold (migalastat), the first oral medication for the treatment of adults with Fabry disease. The drug is available in a 123-mg capsule. The efficacy of Galafold was demonstrated in a six-month, placebo-controlled clinical trial in 45 adults with Fabry disease. Patients treated with Galafold over six months had a greater reduction in globotriaosylceramide in blood vessels of the kidneys, compared with patients taking placebo. Investigators studied the safety of Galafold in four clinical trials. The most common adverse drug reactions in patients taking Galafold in clinical trials were headache, nasal and throat irritation, urinary tract infection, nausea, and fever. Amicus Therapeutics, which markets the capsules, is headquartered in Cranbury, New Jersey.
Binge Drinking Increases Cardiovascular Risk in Men
Young adults who frequently binge drink are more likely to have higher blood pressure, higher cholesterol, and higher blood sugar at a younger age than nonbinge drinkers, according to a study published June 27 in the Journal of the American Heart Association. Researchers analyzed data from the US National Health and Nutrition Examination Survey for 4,710 adults from ages 18 to 45. After controlling for diet and physical activity, men who binge drank as many as 12 times per year, compared with nonbinge drinkers, had higher systolic blood pressure (121.8 mm Hg vs 117.5 mm Hg) and total cholesterol (215.5 mg/dL vs 207.8 mg/dL). Binge drinking did not affect systolic blood pressure or total cholesterol in women. The effects of binge drinking on glucose parameters in men and women varied.
Piano MR, Burke L, Kang M, Phillips SA. Effects of repeated binge drinking on blood pressure levels and other cardiovascular health metrics in young adults: National Health and Nutrition Examination Survey, 2011-2014. J Am Heart Assoc. 2018;7(13).
Brain SPECT Predicts Brain Aging and Psychiatric Disorders
Brain SPECT predicts chronologic age, and brain aging varies as a function of common psychiatric disorders, according to a study published online ahead of print August 3 in the Journal of Alzheimer’s Disease. A psychiatric cohort of 31,227 participants underwent brain SPECT at rest and during a concentration task for a total of 62,454 scans. Analysis of variance identified the mean age trends over the population’s age range (ie, nine months to 105 years). Researchers studied 128 brain regions to predict the chronologic age of each participant. Older age predicted from the scan, compared with actual chronologic age, was considered accelerated aging. Childhood, adolescence, and late life were associated with variations in perfusion. Alcohol use, cannabis use, anxiety, bipolar disorder, schizophrenia, and ADHD were associated with increased brain aging.
Amen DG, Egan S, Meysami S, et al. Patterns of regional cerebral blood flow as a function of age throughout the lifespan. J Alzheimers Dis. 2018 Aug 3 [Epub ahead of print].
Folic Acid May Prevent Language Delays Associated With AED Exposure
Folic acid use early in pregnancy may prevent language delay associated with in utero antiepileptic drug (AED) exposure, according to a study published online ahead of print August 1 in Neurology. The study included 335 AED-exposed children of mothers with epilepsy and 104,222 children of mothers without epilepsy. For children with no maternal periconceptional folic acid supplementation, the fully adjusted odds ratios for language delay in AED-exposed children, compared with controls, were 3.9 at 18 months and 4.7 at 36 months. When mothers took folic acid, the corresponding odds ratios for language delay were 1.7 and 1.7, respectively. The effect of folic acid supplementation on language delay in AED-exposed children was significant only when supplementation began four weeks before pregnancy and continued until the end of the first trimester.
Husebye ESN, Gilhus NE, Riedel B, et al. Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology. 2018 Aug 1 [Epub ahead of print].
—Kimberly Williams
Intrathecal Baclofen Reduces Pain in Poststroke Spasticity
Intrathecal baclofen (ITB) therapy improves pain and quality of life in patients with poststroke spasticity, according to a study published August 14 in Stroke. Patients with poststroke spasticity in two or more extremities and an Ashworth Scale score of 3 or higher in two or more affected lower extremity muscle groups were randomized to ITB or conventional medical management. At six months, ITB effectively reduced Numeric Pain Rating Scale scores for actual and least spasticity-related pain and improved quality of life, compared with medical management. In addition, 73% of patients given ITB therapy reported satisfaction with spasticity reduction at month six, versus 48% of patients given medical management. The researchers found no statistically significant differences between groups in reduction of worst pain.
Creamer M, Cloud G, Kossmehl P, et al. Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. Stroke. 2018 Aug 14 [Epub ahead of print].
FDA Approves Diacomit for Seizures Associated With Dravet Syndrome
The FDA has approved Diacomit (stiripentol) for the treatment of seizures associated with Dravet syndrome in patients age 2 and older who are taking clobazam. Diacomit will be available in 250-mg and 500-mg capsules and in fruit-flavored powder packets for oral suspension. In two studies of patients between ages 3 and 17 with Dravet syndrome, patients were randomized to Diacomit or placebo, along with their previous treatment with clobazam and valproate. In Study 1, 71% of patients in the Diacomit group were 50% responders versus 5% in the placebo group. In Study 2, 67% of patients treated with Diacomit were 50% responders versus 9.1% of controls. Diacomit is marketed by Biocodex, which is headquartered in Gentilly, France.
Retinal Thinning Is Associated With Dopaminergic Cell Loss
Retinal thinning is linked to the loss of brain cells in Parkinson’s disease, according to a study published online ahead of print August 15 in Neurology. Researchers examined 49 participants (average age, 69) who had been diagnosed with Parkinson’s disease an average of two years earlier, but who had not yet started medication. Participants were compared with 54 healthy controls who were matched for age. Participants underwent a complete eye exam and high-resolution eye scans. Patients with Parkinson’s disease had retinal layer thinning in the temporal and inferior 2.22-mm sectors. The thickness of these layers in the inferior 2.22-mm sector correlated negatively with Hoehn and Yahr stage. Retinal thinning was associated with dopaminergic loss in the left substantia nigra.
Ahn J, Lee JY, Kim TW, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018 Aug 15 [Epub ahead of print].
Contact Sports Linked to Vascular Risk Factors and Depression
Athletes with a history of playing professional contact sports have more vascular risk factors and higher depression scores, according to a study published online ahead of print August 3 in the Journal of Head Trauma Rehabilitation. This case–control study included 21 retired National Football League and National Hockey League players and 21 age-matched noncontact athlete controls. The investigators assessed participants for mild cognitive impairment (MCI) and measured depression using the Beck Depression Inventory-II (BDI). Eight contact sport athletes and three noncontact athletes met criteria for MCI. Contact sport athletes’ scores were significantly worse on Letter Fluency and List B Immediate Recall. Contact athletes were more obese, had more vascular risk factors, and had higher BDI scores.
Baker JG, Leddy JJ, Hinds AL, et al. An exploratory study of mild cognitive impairment of retired professional contact sport athletes. J Head Trauma Rehabil. 2018 Aug 3 [Epub ahead of print].
Managing Vegetative and Minimally Conscious States
The American Academy of Neurology; the American Congress of Rehabilitative Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research have published a practice guideline on the diagnosis and ongoing medical and rehabilitative care of patients in a vegetative or minimally conscious state caused by brain injury. The guideline was published online ahead of print August 8 in Neurology. The authors based their recommendations on a systematic review of the evidence using a modified Delphi consensus process. Clinicians should advise families that for adults, a minimally conscious state and traumatic etiology are associated with more favorable outcomes, according to the guideline. Structural MRI, SPECT, and the Coma Recovery Scale-Revised can assist prognostication in adults, but no tests improve prognostic accuracy in children, said the authors.
Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018 Aug 8 [Epub ahead of print].
FDA Approves Onpattro
The FDA has approved Onpattro (patisiran) lipid complex injection for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The approval of Onpattro was based on results from a randomized, double-blind, placebo-controlled phase III study. Of 225 patients, 148 were randomized to Onpattro infusion once every three weeks for 18 months. The other participants were randomized to placebo infusion at the same frequency. The patients who received Onpattro had better outcomes on measures of polyneuropathy, including muscle strength, sensation, reflexes, and autonomic symptoms, compared with participants receiving placebo infusions. Patients receiving Onpattro also scored better on assessments of walking, nutritional status, and the ability to perform activities of daily living. Alnylam Pharmaceuticals, which markets Onpattro, is headquartered in Cambridge, Massachusetts.
Ophthalmic Conditions May Indicate Increased Risk of Alzheimer’s Disease
People with recent diagnoses of glaucoma, established age-related macular degeneration, and recent and established diabetic retinopathy may have increased risk of Alzheimer’s disease, according to a study published online ahead of print August 2 in Alzheimer’s & Dementia. The investigators included 3,877 participants selected randomly from the Adult Changes in Thought study in their analysis. Participants were age 65 or older and did not have Alzheimer’s disease at the time of enrollment. During the five-year study, a committee of dementia experts diagnosed Alzheimer’s disease in 792 people. Patients with age-related macular degeneration, diabetic retinopathy, or glaucoma were at 40% to 50% greater risk of developing Alzheimer’s disease, compared with people without these eye conditions. Cataract diagnosis was not a risk factor for Alzheimer’s disease.
Lee CS, Larson EB, Gibbons LE, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2018 Aug 2 [Epub ahead of print].
Insulin Resistance in Nondiabetics With Parkinson’s Disease
Insulin resistance is prevalent in Parkinson’s disease and correlates with BMI, according to a study published in the August issue of the Journal of Parkinson’s Disease. The investigators included 154 nondiabetic patients with Parkinson’s disease in the study. Participants were tested for fasting insulin, fasting glucose, and hemoglobin A1c (HbA1c) and underwent a battery of clinical tests. Investigators recorded participants’ Parkinson’s disease medications, height, weight, and other demographic features. Ninety (58.4%) participants had abnormal insulin resistance. Insulin resistance was more prevalent in overweight and obese participants than in participants with a normal weight. BMI was the only significant predictor of insulin resistance. Insulin resistance did not correlate with cognition, functioning, or nonmotor symptoms.
Hogg E, Athreya K, Basile C, et al. High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson’s disease. J Parkinsons Dis. 2018;8(2):259-265.
FDA Approves Galafold for Fabry Disease in Adults
The FDA has approved Galafold (migalastat), the first oral medication for the treatment of adults with Fabry disease. The drug is available in a 123-mg capsule. The efficacy of Galafold was demonstrated in a six-month, placebo-controlled clinical trial in 45 adults with Fabry disease. Patients treated with Galafold over six months had a greater reduction in globotriaosylceramide in blood vessels of the kidneys, compared with patients taking placebo. Investigators studied the safety of Galafold in four clinical trials. The most common adverse drug reactions in patients taking Galafold in clinical trials were headache, nasal and throat irritation, urinary tract infection, nausea, and fever. Amicus Therapeutics, which markets the capsules, is headquartered in Cranbury, New Jersey.
Binge Drinking Increases Cardiovascular Risk in Men
Young adults who frequently binge drink are more likely to have higher blood pressure, higher cholesterol, and higher blood sugar at a younger age than nonbinge drinkers, according to a study published June 27 in the Journal of the American Heart Association. Researchers analyzed data from the US National Health and Nutrition Examination Survey for 4,710 adults from ages 18 to 45. After controlling for diet and physical activity, men who binge drank as many as 12 times per year, compared with nonbinge drinkers, had higher systolic blood pressure (121.8 mm Hg vs 117.5 mm Hg) and total cholesterol (215.5 mg/dL vs 207.8 mg/dL). Binge drinking did not affect systolic blood pressure or total cholesterol in women. The effects of binge drinking on glucose parameters in men and women varied.
Piano MR, Burke L, Kang M, Phillips SA. Effects of repeated binge drinking on blood pressure levels and other cardiovascular health metrics in young adults: National Health and Nutrition Examination Survey, 2011-2014. J Am Heart Assoc. 2018;7(13).
Brain SPECT Predicts Brain Aging and Psychiatric Disorders
Brain SPECT predicts chronologic age, and brain aging varies as a function of common psychiatric disorders, according to a study published online ahead of print August 3 in the Journal of Alzheimer’s Disease. A psychiatric cohort of 31,227 participants underwent brain SPECT at rest and during a concentration task for a total of 62,454 scans. Analysis of variance identified the mean age trends over the population’s age range (ie, nine months to 105 years). Researchers studied 128 brain regions to predict the chronologic age of each participant. Older age predicted from the scan, compared with actual chronologic age, was considered accelerated aging. Childhood, adolescence, and late life were associated with variations in perfusion. Alcohol use, cannabis use, anxiety, bipolar disorder, schizophrenia, and ADHD were associated with increased brain aging.
Amen DG, Egan S, Meysami S, et al. Patterns of regional cerebral blood flow as a function of age throughout the lifespan. J Alzheimers Dis. 2018 Aug 3 [Epub ahead of print].
Folic Acid May Prevent Language Delays Associated With AED Exposure
Folic acid use early in pregnancy may prevent language delay associated with in utero antiepileptic drug (AED) exposure, according to a study published online ahead of print August 1 in Neurology. The study included 335 AED-exposed children of mothers with epilepsy and 104,222 children of mothers without epilepsy. For children with no maternal periconceptional folic acid supplementation, the fully adjusted odds ratios for language delay in AED-exposed children, compared with controls, were 3.9 at 18 months and 4.7 at 36 months. When mothers took folic acid, the corresponding odds ratios for language delay were 1.7 and 1.7, respectively. The effect of folic acid supplementation on language delay in AED-exposed children was significant only when supplementation began four weeks before pregnancy and continued until the end of the first trimester.
Husebye ESN, Gilhus NE, Riedel B, et al. Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology. 2018 Aug 1 [Epub ahead of print].
—Kimberly Williams
Intrathecal Baclofen Reduces Pain in Poststroke Spasticity
Intrathecal baclofen (ITB) therapy improves pain and quality of life in patients with poststroke spasticity, according to a study published August 14 in Stroke. Patients with poststroke spasticity in two or more extremities and an Ashworth Scale score of 3 or higher in two or more affected lower extremity muscle groups were randomized to ITB or conventional medical management. At six months, ITB effectively reduced Numeric Pain Rating Scale scores for actual and least spasticity-related pain and improved quality of life, compared with medical management. In addition, 73% of patients given ITB therapy reported satisfaction with spasticity reduction at month six, versus 48% of patients given medical management. The researchers found no statistically significant differences between groups in reduction of worst pain.
Creamer M, Cloud G, Kossmehl P, et al. Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. Stroke. 2018 Aug 14 [Epub ahead of print].
FDA Approves Diacomit for Seizures Associated With Dravet Syndrome
The FDA has approved Diacomit (stiripentol) for the treatment of seizures associated with Dravet syndrome in patients age 2 and older who are taking clobazam. Diacomit will be available in 250-mg and 500-mg capsules and in fruit-flavored powder packets for oral suspension. In two studies of patients between ages 3 and 17 with Dravet syndrome, patients were randomized to Diacomit or placebo, along with their previous treatment with clobazam and valproate. In Study 1, 71% of patients in the Diacomit group were 50% responders versus 5% in the placebo group. In Study 2, 67% of patients treated with Diacomit were 50% responders versus 9.1% of controls. Diacomit is marketed by Biocodex, which is headquartered in Gentilly, France.
Retinal Thinning Is Associated With Dopaminergic Cell Loss
Retinal thinning is linked to the loss of brain cells in Parkinson’s disease, according to a study published online ahead of print August 15 in Neurology. Researchers examined 49 participants (average age, 69) who had been diagnosed with Parkinson’s disease an average of two years earlier, but who had not yet started medication. Participants were compared with 54 healthy controls who were matched for age. Participants underwent a complete eye exam and high-resolution eye scans. Patients with Parkinson’s disease had retinal layer thinning in the temporal and inferior 2.22-mm sectors. The thickness of these layers in the inferior 2.22-mm sector correlated negatively with Hoehn and Yahr stage. Retinal thinning was associated with dopaminergic loss in the left substantia nigra.
Ahn J, Lee JY, Kim TW, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018 Aug 15 [Epub ahead of print].
Contact Sports Linked to Vascular Risk Factors and Depression
Athletes with a history of playing professional contact sports have more vascular risk factors and higher depression scores, according to a study published online ahead of print August 3 in the Journal of Head Trauma Rehabilitation. This case–control study included 21 retired National Football League and National Hockey League players and 21 age-matched noncontact athlete controls. The investigators assessed participants for mild cognitive impairment (MCI) and measured depression using the Beck Depression Inventory-II (BDI). Eight contact sport athletes and three noncontact athletes met criteria for MCI. Contact sport athletes’ scores were significantly worse on Letter Fluency and List B Immediate Recall. Contact athletes were more obese, had more vascular risk factors, and had higher BDI scores.
Baker JG, Leddy JJ, Hinds AL, et al. An exploratory study of mild cognitive impairment of retired professional contact sport athletes. J Head Trauma Rehabil. 2018 Aug 3 [Epub ahead of print].
Managing Vegetative and Minimally Conscious States
The American Academy of Neurology; the American Congress of Rehabilitative Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research have published a practice guideline on the diagnosis and ongoing medical and rehabilitative care of patients in a vegetative or minimally conscious state caused by brain injury. The guideline was published online ahead of print August 8 in Neurology. The authors based their recommendations on a systematic review of the evidence using a modified Delphi consensus process. Clinicians should advise families that for adults, a minimally conscious state and traumatic etiology are associated with more favorable outcomes, according to the guideline. Structural MRI, SPECT, and the Coma Recovery Scale-Revised can assist prognostication in adults, but no tests improve prognostic accuracy in children, said the authors.
Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018 Aug 8 [Epub ahead of print].
FDA Approves Onpattro
The FDA has approved Onpattro (patisiran) lipid complex injection for the treatment of polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The approval of Onpattro was based on results from a randomized, double-blind, placebo-controlled phase III study. Of 225 patients, 148 were randomized to Onpattro infusion once every three weeks for 18 months. The other participants were randomized to placebo infusion at the same frequency. The patients who received Onpattro had better outcomes on measures of polyneuropathy, including muscle strength, sensation, reflexes, and autonomic symptoms, compared with participants receiving placebo infusions. Patients receiving Onpattro also scored better on assessments of walking, nutritional status, and the ability to perform activities of daily living. Alnylam Pharmaceuticals, which markets Onpattro, is headquartered in Cambridge, Massachusetts.
Ophthalmic Conditions May Indicate Increased Risk of Alzheimer’s Disease
People with recent diagnoses of glaucoma, established age-related macular degeneration, and recent and established diabetic retinopathy may have increased risk of Alzheimer’s disease, according to a study published online ahead of print August 2 in Alzheimer’s & Dementia. The investigators included 3,877 participants selected randomly from the Adult Changes in Thought study in their analysis. Participants were age 65 or older and did not have Alzheimer’s disease at the time of enrollment. During the five-year study, a committee of dementia experts diagnosed Alzheimer’s disease in 792 people. Patients with age-related macular degeneration, diabetic retinopathy, or glaucoma were at 40% to 50% greater risk of developing Alzheimer’s disease, compared with people without these eye conditions. Cataract diagnosis was not a risk factor for Alzheimer’s disease.
Lee CS, Larson EB, Gibbons LE, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2018 Aug 2 [Epub ahead of print].
Insulin Resistance in Nondiabetics With Parkinson’s Disease
Insulin resistance is prevalent in Parkinson’s disease and correlates with BMI, according to a study published in the August issue of the Journal of Parkinson’s Disease. The investigators included 154 nondiabetic patients with Parkinson’s disease in the study. Participants were tested for fasting insulin, fasting glucose, and hemoglobin A1c (HbA1c) and underwent a battery of clinical tests. Investigators recorded participants’ Parkinson’s disease medications, height, weight, and other demographic features. Ninety (58.4%) participants had abnormal insulin resistance. Insulin resistance was more prevalent in overweight and obese participants than in participants with a normal weight. BMI was the only significant predictor of insulin resistance. Insulin resistance did not correlate with cognition, functioning, or nonmotor symptoms.
Hogg E, Athreya K, Basile C, et al. High prevalence of undiagnosed insulin resistance in non-diabetic subjects with Parkinson’s disease. J Parkinsons Dis. 2018;8(2):259-265.
FDA Approves Galafold for Fabry Disease in Adults
The FDA has approved Galafold (migalastat), the first oral medication for the treatment of adults with Fabry disease. The drug is available in a 123-mg capsule. The efficacy of Galafold was demonstrated in a six-month, placebo-controlled clinical trial in 45 adults with Fabry disease. Patients treated with Galafold over six months had a greater reduction in globotriaosylceramide in blood vessels of the kidneys, compared with patients taking placebo. Investigators studied the safety of Galafold in four clinical trials. The most common adverse drug reactions in patients taking Galafold in clinical trials were headache, nasal and throat irritation, urinary tract infection, nausea, and fever. Amicus Therapeutics, which markets the capsules, is headquartered in Cranbury, New Jersey.
Binge Drinking Increases Cardiovascular Risk in Men
Young adults who frequently binge drink are more likely to have higher blood pressure, higher cholesterol, and higher blood sugar at a younger age than nonbinge drinkers, according to a study published June 27 in the Journal of the American Heart Association. Researchers analyzed data from the US National Health and Nutrition Examination Survey for 4,710 adults from ages 18 to 45. After controlling for diet and physical activity, men who binge drank as many as 12 times per year, compared with nonbinge drinkers, had higher systolic blood pressure (121.8 mm Hg vs 117.5 mm Hg) and total cholesterol (215.5 mg/dL vs 207.8 mg/dL). Binge drinking did not affect systolic blood pressure or total cholesterol in women. The effects of binge drinking on glucose parameters in men and women varied.
Piano MR, Burke L, Kang M, Phillips SA. Effects of repeated binge drinking on blood pressure levels and other cardiovascular health metrics in young adults: National Health and Nutrition Examination Survey, 2011-2014. J Am Heart Assoc. 2018;7(13).
Brain SPECT Predicts Brain Aging and Psychiatric Disorders
Brain SPECT predicts chronologic age, and brain aging varies as a function of common psychiatric disorders, according to a study published online ahead of print August 3 in the Journal of Alzheimer’s Disease. A psychiatric cohort of 31,227 participants underwent brain SPECT at rest and during a concentration task for a total of 62,454 scans. Analysis of variance identified the mean age trends over the population’s age range (ie, nine months to 105 years). Researchers studied 128 brain regions to predict the chronologic age of each participant. Older age predicted from the scan, compared with actual chronologic age, was considered accelerated aging. Childhood, adolescence, and late life were associated with variations in perfusion. Alcohol use, cannabis use, anxiety, bipolar disorder, schizophrenia, and ADHD were associated with increased brain aging.
Amen DG, Egan S, Meysami S, et al. Patterns of regional cerebral blood flow as a function of age throughout the lifespan. J Alzheimers Dis. 2018 Aug 3 [Epub ahead of print].
Folic Acid May Prevent Language Delays Associated With AED Exposure
Folic acid use early in pregnancy may prevent language delay associated with in utero antiepileptic drug (AED) exposure, according to a study published online ahead of print August 1 in Neurology. The study included 335 AED-exposed children of mothers with epilepsy and 104,222 children of mothers without epilepsy. For children with no maternal periconceptional folic acid supplementation, the fully adjusted odds ratios for language delay in AED-exposed children, compared with controls, were 3.9 at 18 months and 4.7 at 36 months. When mothers took folic acid, the corresponding odds ratios for language delay were 1.7 and 1.7, respectively. The effect of folic acid supplementation on language delay in AED-exposed children was significant only when supplementation began four weeks before pregnancy and continued until the end of the first trimester.
Husebye ESN, Gilhus NE, Riedel B, et al. Verbal abilities in children of mothers with epilepsy: association to maternal folate status. Neurology. 2018 Aug 1 [Epub ahead of print].
—Kimberly Williams
Epidemiology of Existing Extensor Mechanism Pathology in Primary Anterior Cruciate Ligament Ruptures in an Active-Duty Population
ABSTRACT
The purpose of this study is to determine the prevalence of potential graft-influencing pathologies of the extensor mechanism of the knee in patients presenting with a primary anterior cruciate ligament (ACL) rupture.
We performed a retrospective review of the plain radiographs and magnetic resonance imaging (MRI) of all active-duty patients presenting with a primary ACL rupture at our institution between July 2006 and February 2009. Imaging was reviewed to determine the presence of a multipartite patella, unresolved Osgood-Schlatter’s disease, and/or radiographic evidence suggestive of patella tendinopathy.
A total of 197 patients were reviewed, including 27 females and 170 males. One patient (0.5%) had a bipartite patella and 4 patients (2%) had free-floating ossicles about the tibial tuberosity consistent with unresolved Osgood-Schlatter’s disease. A total of 15 patients (7.6%) showed MRI evidence suggestive of patella tendinopathy.
This study revealed 20 patients out of 197 (10.1%) who presented with existing extensor mechanism pathologies in radiologic studies. While preoperative imaging is routinely used to confirm clinical suspicion of ACL rupture or identify associated injuries, this study shows that it can also identify existing extensor mechanism pathologies that could ultimately influence the use of an extensor mechanism graft.
Continue to: Anterior cruciate ligament (ACL) reconstruction...
Anterior cruciate ligament (ACL) reconstruction is an extremely common procedure; in fact, an estimated 60,000 to 175,000 ACL reconstructions are performed annually in the United States.1,2 One of the most widely debated aspects of ACL reconstruction is the choice of graft. Grafts are broadly categorized into allografts and autografts. The autograft selections for ACL reconstruction include patellar bone-tendon-bone (pBTB), combined semitendinosus and gracilis hamstrings (HS), free quadriceps tendon (QT)without accompanying bone block, and quadriceps tendon-bone (qTB). Allograft choices predominantly include pBTB and HS, as well as the tibialis anterior and Achilles tendons. The pBTB autograft is traditionally considered the reference standard for ACL reconstruction.3 Recent advances in allograft processing, along with improved fixation techniques and devices, have improved results following the use of soft-tissue autografts and both bony and soft tissue allografts.4 Thus, the optimal graft choice for ACL reconstruction has become controversial in light of several studies demonstrating no significant, long-term difference in clinical and/or functional outcomes based on graft selection.5-7
Given the lack of a clear gold standard in graft selection, multiple patient factors, such as age, activity demands, and patient preference, should be taken into account when considering the choice of graft. In addition, intrinsic factors that could potentially weaken an autograft should be considered. Several extensor mechanism pathological findings that are easily visualized on either plain radiographs or magnetic resonance imaging (MRI) could potentially affect graft selection. Findings such as a multipartite patella, free ossicles about the tibial tuberosity consistent with Osgood-Schlatter’s disease, and proximal patella tendon thickening suggestive of patellar tendinopathy are easily identifiable on preoperative imaging and could exert adverse effects on pBTB, QT, and qTB autografts. The purpose of this study is to identify the prevalence of these pre-existing conditions in active-duty military patients presenting with acute ACL tears.
METHODS
A retrospective review was conducted on all active-duty patients who underwent primary ACL reconstruction at our institution from July 2006 to February 2009. A systematic review of all plain radiographs and MRIs was performed on a calibrated picture archiving and communication system workstation. Imaging review was conducted by 2 of the authors. Pertinent findings included a multipartite patella, free ossicles within the patella tendon, and hypertrophy of the proximal aspect of the patella tendon. Assessment for multipartite patella and unresolved Osgood-Schlatter's disease was made using plain radiographs with MRI for confirmation. Measurements of the patella tendon were performed on the short tau inversion recovery and T2-weighted sagittal MRI images at the point of maximal tendon width. A width of ≥7 mm was considered suggestive of patella tendinopathy based on prior studies.8-10 The prevalence of each finding was then determined based on the total number of patients.
Continue to: RESULTS...
RESULTS
A total of 197 active-duty patients, including 27 females (13.7%) and 170 males (86.3%), underwent primary ACL reconstruction during the study time period. A total of 93 right knees and 104 left knees were evaluated. The average age at presentation was 29 years (range, 19-45 years).
Of the 197 patients, only 1 was found to have a multipartite patella (prevalence, 0.5%). This 37-year-old male patient showed a right bipartite patella located in the superior-lateral aspect (Figure 1).
Four patients had free ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease (prevalence, 2.0%) (Figure 2). All 4 patients were male, which is consistent with the higher incidence of Osgood-Schlatter’s disease in males than in females. The average age of these patients was 27.5 years (range, 22-33 years).
The most common extensor mechanism pathology present on preoperative imaging was proximal patella tendon thickening suggestive of patella tendinopathy. Thickening of the proximal portion of the patellar tendon was present in 15 of the 197 MRIs (prevalence, 7.6%) (Figure 3). The average width of this thickening was 8.49 mm (7.17-10.17 mm), and the average age of patients with radiographic evidence of patellar tendinopathy was 29.9 years (range, 20-43 years). Gender distribution was predominantly male (14 males, 1 female). Details of all extensor mechanism pathologies found are provided in the Table.
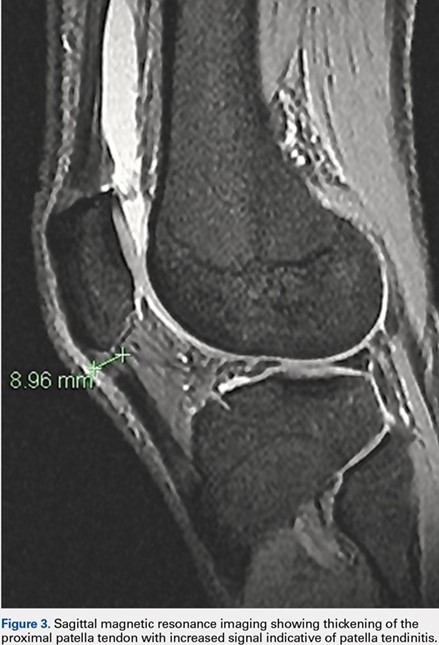
Table. Identified Extensor Mechanism Pathology
| Male | Female | Total |
Patients | 170 | 27 | 197 |
Multipartite Patella | 1 | 0 | 1 |
Osgood-Schlatter’s Disease | 4 | 0 | 4 |
Patella Tendinopathy | 14 | 1 | 15 |
|
| 20/97 (10.10%) |
|
DISCUSSION
When considering ACL reconstruction, determination of the graft type is one of the most important decisions to be made, perhaps second only to the decision to perform the surgery itself. Recent multiple, well-designed studies comparing differences among grafts have shown equivalent long-term results, leading to the lack of a universally accepted gold standard.5-7 Thus, both autograft and allograft ACL surgeries are routinely performed in the United States. Surgeons typically take into account factors such as patient age and physical demands, along with their own preferences and/or experience, when considering graft selection. A paucity of research concerning existing pathological conditions that could also influence preoperative decision-making has been observed; most reports consist only of expert opinion.11-13 Our goal is to determine the prevalence of several conditions that could potentially affect an autograft harvested from the extensor mechanism.
This study revealed an overall prevalence of 10.1% of existing extensor mechanism pathology in patients sustaining an acute ACL tear and presenting for ACL reconstruction. Only 1 (0.5%) showed evidence of a multipartite patella, which is below the reported prevalence of 0.2% to 6%.14 The presence of a multipartite patella could potentially have the most deleterious effect on a qTB autograft. Although not as commonly used as HS, QT, or pBTB autografts, some surgeons prefer a qTB autograft because of its increased surface area, bony fixation, and reported decreased donor site pain.15 A multipartite patella could complicate harvesting, disrupt the bone block, or lead to an unstable segment of the patella. These effects are of great concern since the most common location of a bipartite patella is superior-lateral and the quadriceps tendon has been shown to asymmetrically insert laterally.16 While these potential adverse effects have not been specifically studied, the availability of comparable options makes the use of a qTB autograft in the setting of a bipartite patella questionable.
Four patients (2%) revealed evidence of ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease. Osgood-Schlatter’s disease has been reported to occur in up to 21% of active adolescents and is historically considered a self-resolving process.17 Recent papers have reported persistent symptoms in up to 10% of patients, with a small percentage experiencing persistent free ossicles within their patella tendon on MRI.18,19 The presence of such ossicles raises concern about the integrity of the patellar tendon and questions its use as an autograft when present. This concern was published in a report with the surgeon opting to utilize an alternate graft due to the presence of unresolved Osgood-Schlatter’s disease.13
Fifteen patients (7.6%) demonstrated radiographic evidence suggestive of patella tendinopathy based on the thickness of the proximal patella tendon. Patella tendinopathy is the most common tendinopathy in skeletally mature athletes and one of the most common athletic injuries of the knee, with a reported career prevalence of 22%.20 It is described as an overuse injury due to the cumulative effect of micro trauma without an adequate healing interval. While it remains a clinical diagnosis, patellar tendinopathy often shows radiographic findings best assessed on sagittal MRIs. In general, the normal patella tendon appears as a homogenous low-intensity structure and is of uniform thickness. A tendon affected with tendinopathy typically demonstrates a focal increase in signal on T2-weighted sequences just distal to the tendon origin on the inferior pole of the patella. In addition, the patella tendon will usually demonstrate thickening, primarily in the proximal medial and posterior fibers. Patella marrow changes and indistinct tendon margins can also be present. The sensitivity and specificity of diagnosing patellar tendinopathy on MRI are 78% and 86%, respectively.20 We derived our criteria for MRI evidence suggestive of patella tendinopathy from studies by El-Khoury and colleagues,8 Johnson and colleagues,9 and Popp and colleagues.10 In a 1992 study, El-Khoury and colleagues8 compared MRI findings between a group of patients with a clinical diagnosis of patella tendonitis and a control group without knee complaints. The authors found that the average proximal patella tendon diameter in the control group was 3.7 mm while the average proximal patella tendon diameter in the patella tendinopathy group was 10.9 mm; no patella tendons in the control group were >7 mm.8 In a 1996 study, Johnson and colleagues9 determined that the most reliable MRI finding for patients with patellar tendonitis is significant thickening of the proximal patella tendon seen on the sagittal view. The average thickness in symptomatic patients was 8.5 mm (range, 5-15 mm). The average thickness in the control group was 5.5 mm. None of the control patients had a proximal tendon thickness >7 mm.9 Finally, Popp and colleagues10 reviewed the MRI of 11 knees of patients who underwent surgical débridement of chronic patellar tendonitis and reported an average proximal patella tendon thickness of 12 mm (range, 9-16 mm). We therefore used a proximal patella tendon thickness of >7 mm on the sagittal view as a radiographic finding suggestive of patella tendinopathy. No data regarding symptoms of anterior knee pain were available among our patients. Histological studies of patients with patella tendonitis have shown evidence of chronic inflammation, fibrinoid necrosis, mucoid degeneration, and synovial proliferation within the patella tendon insertion.21 Although no controlled data showing that patella tendons with a history of tendonitis are more prone to failure than those without such history when used as an autograft for ACL reconstruction, the idea of utilizing a diseased tendon for a graft is not ideal. Some surgeons question their patients regarding a history of anterior knee pain and will not use a pBTB autograft in a patient with a positive history.22
Continue to: The goal of this study is to obtain epidemiological evidence...
The goal of this study is to obtain epidemiological evidence of the prevalence of existing extensor mechanism pathologies in patients with acute ACL ruptures and determine how these pathologies may relate to the choice of graft. Out of 197 patients studied, over 10% presented with radiographic evidence of pathologies that could influence the choice of graft. This prevalence is certainly significant enough for surgeons to consider including a radiographic evaluation of the extensor mechanism in their standard ACL rupture work-up.
This study presents obvious limitations. While we report the prevalence of some extensor mechanism pathologies, no definitive evidence that recommends against the use of these autografts from these affected individuals has yet been published. In addition, our diagnosis of patella tendinopathy is based solely on MRI findings with no information regarding clinical symptoms. This limitation is a weakness as several additional studies have questioned the validity of a 7 mm proximal patella tendon thickness.23,24 Furthermore, no studies demonstrating the inferior strength of autografts with the co-existing findings described in our work have yet been performed.
CONCLUSION
We found that 10% of active-duty patients presenting for ACL reconstruction demonstrated radiographic evidence of an extensor mechanism pathology that could affect the harvesting of or integrity of select autografts. Given the recent trend of functionally equivocal results in ACL reconstructions utilizing a variety of grafts, this information could and should influence surgical recommendations for graft utilization to obtain optimal surgical results.
1. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321-2328. doi:10.2106/JBJS.H.00539.
2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135-2142. doi:10.1056/NEJMcp0804745.
3. Fu FH, Bennett CH, Lattermann CL, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27(6):821-830. doi:10.1177/03635465990270062501.
4. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft Versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(2):492-499. doi:10.1177/0363546513497566.
5. Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):214-220. doi:10.1177/03635465020300021201.
6. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: Allograft versus allograft. Arthroscopy. 2005;21(7):774-785. doi:10.1016/j.arthro.2005.04.112.
7. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):292-298. doi:10.1016/j.arthro.2007.08.029.
8. El-Khoury GY, Wira RL, Berbaum KS, Pope TL, Monu JUV. MR imaging of patellar tendinitis. Radiology. 1992;184(3):849-854. doi:10.1148/radiology.184.3.1509078.
9. Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Joint Surg Br. 1996;78(3):452-457. doi:10.1302/0301-620X.78B3.0780452.
10. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218-222. doi:10.1177/036354659702500214.
11. Provencher MT, Ryu JH, Gaston T, Dewing CB. Technique: bone-patellar tendon-bone autograft ACL reconstruction in the young, active patient. J Knee Surg. 2011;24(2):83-92. doi:10.1055/s-0031-1280875.
12. Fu F, Cohen S. Current Concepts in ACL Reconstruction. Thorofare: SLACK Incorporated; 2008.
13. Cosgarea AJ, Weng MS, Andrews M. Osgood Schlatter’s disease complicating anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(6):700-703. doi:10.1016/S0749-8063(05)80511-0.
14. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855. doi:10.1007/s11999-008-0367-4.
15. Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252-254. doi:10.1016/0749-8063(95)90078-0.
16. Scully WF, Wilson DJ, Arrington ED. “Central” quadriceps tendon harvest with patellar bone plug: surgical technique revisited. Arthrosc Tech. 2013;2(4):e427-e432.
17. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236-241. doi:10.1177/036354658501300404.
18. Pihlajamäki HK, Visuri TI. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 Pt 2):258-264. doi:10.2106/JBJS.J.00450.
19. Weiss JM, Jordan SS, Andersen JS, Lee BM, Kocher M. Surgical treatment of unresolved Osgood-Schlatter disease: ossicle resection with tibial tubercleplasty. J Pediatr Orthop. 2007;27(7):844-847. doi:10.1097/BPO.0b013e318155849b.
20. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee Among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561-567. doi:10.1177/0363546504270454.
21. O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725-739, vii. doi:10.1016/j.mric.2009.06.010.
22. Martens M, Wouters P, Burssens A, Mulier JC. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand. 1982;53(3):445-450. doi:10.3109/17453678208992239.
23. Shalaby M, Almekinders LC. Patellar tendinitis: the significance of magnetic resonance imaging findings. Am J Sports Med. 1999;27(3):345-349. doi:10.1177/03635465990270031301.
24. Reiff DB, Heenan SD, Heron CW. MRI appearances of the asymptomatic patellar tendon on gradient echo imaging. Skeletal Radiol. 1995;24(2):123-126. doi:10.1007/BF00198074.
ABSTRACT
The purpose of this study is to determine the prevalence of potential graft-influencing pathologies of the extensor mechanism of the knee in patients presenting with a primary anterior cruciate ligament (ACL) rupture.
We performed a retrospective review of the plain radiographs and magnetic resonance imaging (MRI) of all active-duty patients presenting with a primary ACL rupture at our institution between July 2006 and February 2009. Imaging was reviewed to determine the presence of a multipartite patella, unresolved Osgood-Schlatter’s disease, and/or radiographic evidence suggestive of patella tendinopathy.
A total of 197 patients were reviewed, including 27 females and 170 males. One patient (0.5%) had a bipartite patella and 4 patients (2%) had free-floating ossicles about the tibial tuberosity consistent with unresolved Osgood-Schlatter’s disease. A total of 15 patients (7.6%) showed MRI evidence suggestive of patella tendinopathy.
This study revealed 20 patients out of 197 (10.1%) who presented with existing extensor mechanism pathologies in radiologic studies. While preoperative imaging is routinely used to confirm clinical suspicion of ACL rupture or identify associated injuries, this study shows that it can also identify existing extensor mechanism pathologies that could ultimately influence the use of an extensor mechanism graft.
Continue to: Anterior cruciate ligament (ACL) reconstruction...
Anterior cruciate ligament (ACL) reconstruction is an extremely common procedure; in fact, an estimated 60,000 to 175,000 ACL reconstructions are performed annually in the United States.1,2 One of the most widely debated aspects of ACL reconstruction is the choice of graft. Grafts are broadly categorized into allografts and autografts. The autograft selections for ACL reconstruction include patellar bone-tendon-bone (pBTB), combined semitendinosus and gracilis hamstrings (HS), free quadriceps tendon (QT)without accompanying bone block, and quadriceps tendon-bone (qTB). Allograft choices predominantly include pBTB and HS, as well as the tibialis anterior and Achilles tendons. The pBTB autograft is traditionally considered the reference standard for ACL reconstruction.3 Recent advances in allograft processing, along with improved fixation techniques and devices, have improved results following the use of soft-tissue autografts and both bony and soft tissue allografts.4 Thus, the optimal graft choice for ACL reconstruction has become controversial in light of several studies demonstrating no significant, long-term difference in clinical and/or functional outcomes based on graft selection.5-7
Given the lack of a clear gold standard in graft selection, multiple patient factors, such as age, activity demands, and patient preference, should be taken into account when considering the choice of graft. In addition, intrinsic factors that could potentially weaken an autograft should be considered. Several extensor mechanism pathological findings that are easily visualized on either plain radiographs or magnetic resonance imaging (MRI) could potentially affect graft selection. Findings such as a multipartite patella, free ossicles about the tibial tuberosity consistent with Osgood-Schlatter’s disease, and proximal patella tendon thickening suggestive of patellar tendinopathy are easily identifiable on preoperative imaging and could exert adverse effects on pBTB, QT, and qTB autografts. The purpose of this study is to identify the prevalence of these pre-existing conditions in active-duty military patients presenting with acute ACL tears.
METHODS
A retrospective review was conducted on all active-duty patients who underwent primary ACL reconstruction at our institution from July 2006 to February 2009. A systematic review of all plain radiographs and MRIs was performed on a calibrated picture archiving and communication system workstation. Imaging review was conducted by 2 of the authors. Pertinent findings included a multipartite patella, free ossicles within the patella tendon, and hypertrophy of the proximal aspect of the patella tendon. Assessment for multipartite patella and unresolved Osgood-Schlatter's disease was made using plain radiographs with MRI for confirmation. Measurements of the patella tendon were performed on the short tau inversion recovery and T2-weighted sagittal MRI images at the point of maximal tendon width. A width of ≥7 mm was considered suggestive of patella tendinopathy based on prior studies.8-10 The prevalence of each finding was then determined based on the total number of patients.
Continue to: RESULTS...
RESULTS
A total of 197 active-duty patients, including 27 females (13.7%) and 170 males (86.3%), underwent primary ACL reconstruction during the study time period. A total of 93 right knees and 104 left knees were evaluated. The average age at presentation was 29 years (range, 19-45 years).
Of the 197 patients, only 1 was found to have a multipartite patella (prevalence, 0.5%). This 37-year-old male patient showed a right bipartite patella located in the superior-lateral aspect (Figure 1).

Four patients had free ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease (prevalence, 2.0%) (Figure 2). All 4 patients were male, which is consistent with the higher incidence of Osgood-Schlatter’s disease in males than in females. The average age of these patients was 27.5 years (range, 22-33 years).

The most common extensor mechanism pathology present on preoperative imaging was proximal patella tendon thickening suggestive of patella tendinopathy. Thickening of the proximal portion of the patellar tendon was present in 15 of the 197 MRIs (prevalence, 7.6%) (Figure 3). The average width of this thickening was 8.49 mm (7.17-10.17 mm), and the average age of patients with radiographic evidence of patellar tendinopathy was 29.9 years (range, 20-43 years). Gender distribution was predominantly male (14 males, 1 female). Details of all extensor mechanism pathologies found are provided in the Table.

Table. Identified Extensor Mechanism Pathology
| Male | Female | Total |
Patients | 170 | 27 | 197 |
Multipartite Patella | 1 | 0 | 1 |
Osgood-Schlatter’s Disease | 4 | 0 | 4 |
Patella Tendinopathy | 14 | 1 | 15 |
|
| 20/97 (10.10%) |
|
DISCUSSION
When considering ACL reconstruction, determination of the graft type is one of the most important decisions to be made, perhaps second only to the decision to perform the surgery itself. Recent multiple, well-designed studies comparing differences among grafts have shown equivalent long-term results, leading to the lack of a universally accepted gold standard.5-7 Thus, both autograft and allograft ACL surgeries are routinely performed in the United States. Surgeons typically take into account factors such as patient age and physical demands, along with their own preferences and/or experience, when considering graft selection. A paucity of research concerning existing pathological conditions that could also influence preoperative decision-making has been observed; most reports consist only of expert opinion.11-13 Our goal is to determine the prevalence of several conditions that could potentially affect an autograft harvested from the extensor mechanism.
This study revealed an overall prevalence of 10.1% of existing extensor mechanism pathology in patients sustaining an acute ACL tear and presenting for ACL reconstruction. Only 1 (0.5%) showed evidence of a multipartite patella, which is below the reported prevalence of 0.2% to 6%.14 The presence of a multipartite patella could potentially have the most deleterious effect on a qTB autograft. Although not as commonly used as HS, QT, or pBTB autografts, some surgeons prefer a qTB autograft because of its increased surface area, bony fixation, and reported decreased donor site pain.15 A multipartite patella could complicate harvesting, disrupt the bone block, or lead to an unstable segment of the patella. These effects are of great concern since the most common location of a bipartite patella is superior-lateral and the quadriceps tendon has been shown to asymmetrically insert laterally.16 While these potential adverse effects have not been specifically studied, the availability of comparable options makes the use of a qTB autograft in the setting of a bipartite patella questionable.
Four patients (2%) revealed evidence of ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease. Osgood-Schlatter’s disease has been reported to occur in up to 21% of active adolescents and is historically considered a self-resolving process.17 Recent papers have reported persistent symptoms in up to 10% of patients, with a small percentage experiencing persistent free ossicles within their patella tendon on MRI.18,19 The presence of such ossicles raises concern about the integrity of the patellar tendon and questions its use as an autograft when present. This concern was published in a report with the surgeon opting to utilize an alternate graft due to the presence of unresolved Osgood-Schlatter’s disease.13
Fifteen patients (7.6%) demonstrated radiographic evidence suggestive of patella tendinopathy based on the thickness of the proximal patella tendon. Patella tendinopathy is the most common tendinopathy in skeletally mature athletes and one of the most common athletic injuries of the knee, with a reported career prevalence of 22%.20 It is described as an overuse injury due to the cumulative effect of micro trauma without an adequate healing interval. While it remains a clinical diagnosis, patellar tendinopathy often shows radiographic findings best assessed on sagittal MRIs. In general, the normal patella tendon appears as a homogenous low-intensity structure and is of uniform thickness. A tendon affected with tendinopathy typically demonstrates a focal increase in signal on T2-weighted sequences just distal to the tendon origin on the inferior pole of the patella. In addition, the patella tendon will usually demonstrate thickening, primarily in the proximal medial and posterior fibers. Patella marrow changes and indistinct tendon margins can also be present. The sensitivity and specificity of diagnosing patellar tendinopathy on MRI are 78% and 86%, respectively.20 We derived our criteria for MRI evidence suggestive of patella tendinopathy from studies by El-Khoury and colleagues,8 Johnson and colleagues,9 and Popp and colleagues.10 In a 1992 study, El-Khoury and colleagues8 compared MRI findings between a group of patients with a clinical diagnosis of patella tendonitis and a control group without knee complaints. The authors found that the average proximal patella tendon diameter in the control group was 3.7 mm while the average proximal patella tendon diameter in the patella tendinopathy group was 10.9 mm; no patella tendons in the control group were >7 mm.8 In a 1996 study, Johnson and colleagues9 determined that the most reliable MRI finding for patients with patellar tendonitis is significant thickening of the proximal patella tendon seen on the sagittal view. The average thickness in symptomatic patients was 8.5 mm (range, 5-15 mm). The average thickness in the control group was 5.5 mm. None of the control patients had a proximal tendon thickness >7 mm.9 Finally, Popp and colleagues10 reviewed the MRI of 11 knees of patients who underwent surgical débridement of chronic patellar tendonitis and reported an average proximal patella tendon thickness of 12 mm (range, 9-16 mm). We therefore used a proximal patella tendon thickness of >7 mm on the sagittal view as a radiographic finding suggestive of patella tendinopathy. No data regarding symptoms of anterior knee pain were available among our patients. Histological studies of patients with patella tendonitis have shown evidence of chronic inflammation, fibrinoid necrosis, mucoid degeneration, and synovial proliferation within the patella tendon insertion.21 Although no controlled data showing that patella tendons with a history of tendonitis are more prone to failure than those without such history when used as an autograft for ACL reconstruction, the idea of utilizing a diseased tendon for a graft is not ideal. Some surgeons question their patients regarding a history of anterior knee pain and will not use a pBTB autograft in a patient with a positive history.22
Continue to: The goal of this study is to obtain epidemiological evidence...
The goal of this study is to obtain epidemiological evidence of the prevalence of existing extensor mechanism pathologies in patients with acute ACL ruptures and determine how these pathologies may relate to the choice of graft. Out of 197 patients studied, over 10% presented with radiographic evidence of pathologies that could influence the choice of graft. This prevalence is certainly significant enough for surgeons to consider including a radiographic evaluation of the extensor mechanism in their standard ACL rupture work-up.
This study presents obvious limitations. While we report the prevalence of some extensor mechanism pathologies, no definitive evidence that recommends against the use of these autografts from these affected individuals has yet been published. In addition, our diagnosis of patella tendinopathy is based solely on MRI findings with no information regarding clinical symptoms. This limitation is a weakness as several additional studies have questioned the validity of a 7 mm proximal patella tendon thickness.23,24 Furthermore, no studies demonstrating the inferior strength of autografts with the co-existing findings described in our work have yet been performed.
CONCLUSION
We found that 10% of active-duty patients presenting for ACL reconstruction demonstrated radiographic evidence of an extensor mechanism pathology that could affect the harvesting of or integrity of select autografts. Given the recent trend of functionally equivocal results in ACL reconstructions utilizing a variety of grafts, this information could and should influence surgical recommendations for graft utilization to obtain optimal surgical results.
ABSTRACT
The purpose of this study is to determine the prevalence of potential graft-influencing pathologies of the extensor mechanism of the knee in patients presenting with a primary anterior cruciate ligament (ACL) rupture.
We performed a retrospective review of the plain radiographs and magnetic resonance imaging (MRI) of all active-duty patients presenting with a primary ACL rupture at our institution between July 2006 and February 2009. Imaging was reviewed to determine the presence of a multipartite patella, unresolved Osgood-Schlatter’s disease, and/or radiographic evidence suggestive of patella tendinopathy.
A total of 197 patients were reviewed, including 27 females and 170 males. One patient (0.5%) had a bipartite patella and 4 patients (2%) had free-floating ossicles about the tibial tuberosity consistent with unresolved Osgood-Schlatter’s disease. A total of 15 patients (7.6%) showed MRI evidence suggestive of patella tendinopathy.
This study revealed 20 patients out of 197 (10.1%) who presented with existing extensor mechanism pathologies in radiologic studies. While preoperative imaging is routinely used to confirm clinical suspicion of ACL rupture or identify associated injuries, this study shows that it can also identify existing extensor mechanism pathologies that could ultimately influence the use of an extensor mechanism graft.
Continue to: Anterior cruciate ligament (ACL) reconstruction...
Anterior cruciate ligament (ACL) reconstruction is an extremely common procedure; in fact, an estimated 60,000 to 175,000 ACL reconstructions are performed annually in the United States.1,2 One of the most widely debated aspects of ACL reconstruction is the choice of graft. Grafts are broadly categorized into allografts and autografts. The autograft selections for ACL reconstruction include patellar bone-tendon-bone (pBTB), combined semitendinosus and gracilis hamstrings (HS), free quadriceps tendon (QT)without accompanying bone block, and quadriceps tendon-bone (qTB). Allograft choices predominantly include pBTB and HS, as well as the tibialis anterior and Achilles tendons. The pBTB autograft is traditionally considered the reference standard for ACL reconstruction.3 Recent advances in allograft processing, along with improved fixation techniques and devices, have improved results following the use of soft-tissue autografts and both bony and soft tissue allografts.4 Thus, the optimal graft choice for ACL reconstruction has become controversial in light of several studies demonstrating no significant, long-term difference in clinical and/or functional outcomes based on graft selection.5-7
Given the lack of a clear gold standard in graft selection, multiple patient factors, such as age, activity demands, and patient preference, should be taken into account when considering the choice of graft. In addition, intrinsic factors that could potentially weaken an autograft should be considered. Several extensor mechanism pathological findings that are easily visualized on either plain radiographs or magnetic resonance imaging (MRI) could potentially affect graft selection. Findings such as a multipartite patella, free ossicles about the tibial tuberosity consistent with Osgood-Schlatter’s disease, and proximal patella tendon thickening suggestive of patellar tendinopathy are easily identifiable on preoperative imaging and could exert adverse effects on pBTB, QT, and qTB autografts. The purpose of this study is to identify the prevalence of these pre-existing conditions in active-duty military patients presenting with acute ACL tears.
METHODS
A retrospective review was conducted on all active-duty patients who underwent primary ACL reconstruction at our institution from July 2006 to February 2009. A systematic review of all plain radiographs and MRIs was performed on a calibrated picture archiving and communication system workstation. Imaging review was conducted by 2 of the authors. Pertinent findings included a multipartite patella, free ossicles within the patella tendon, and hypertrophy of the proximal aspect of the patella tendon. Assessment for multipartite patella and unresolved Osgood-Schlatter's disease was made using plain radiographs with MRI for confirmation. Measurements of the patella tendon were performed on the short tau inversion recovery and T2-weighted sagittal MRI images at the point of maximal tendon width. A width of ≥7 mm was considered suggestive of patella tendinopathy based on prior studies.8-10 The prevalence of each finding was then determined based on the total number of patients.
Continue to: RESULTS...
RESULTS
A total of 197 active-duty patients, including 27 females (13.7%) and 170 males (86.3%), underwent primary ACL reconstruction during the study time period. A total of 93 right knees and 104 left knees were evaluated. The average age at presentation was 29 years (range, 19-45 years).
Of the 197 patients, only 1 was found to have a multipartite patella (prevalence, 0.5%). This 37-year-old male patient showed a right bipartite patella located in the superior-lateral aspect (Figure 1).

Four patients had free ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease (prevalence, 2.0%) (Figure 2). All 4 patients were male, which is consistent with the higher incidence of Osgood-Schlatter’s disease in males than in females. The average age of these patients was 27.5 years (range, 22-33 years).

The most common extensor mechanism pathology present on preoperative imaging was proximal patella tendon thickening suggestive of patella tendinopathy. Thickening of the proximal portion of the patellar tendon was present in 15 of the 197 MRIs (prevalence, 7.6%) (Figure 3). The average width of this thickening was 8.49 mm (7.17-10.17 mm), and the average age of patients with radiographic evidence of patellar tendinopathy was 29.9 years (range, 20-43 years). Gender distribution was predominantly male (14 males, 1 female). Details of all extensor mechanism pathologies found are provided in the Table.

Table. Identified Extensor Mechanism Pathology
| Male | Female | Total |
Patients | 170 | 27 | 197 |
Multipartite Patella | 1 | 0 | 1 |
Osgood-Schlatter’s Disease | 4 | 0 | 4 |
Patella Tendinopathy | 14 | 1 | 15 |
|
| 20/97 (10.10%) |
|
DISCUSSION
When considering ACL reconstruction, determination of the graft type is one of the most important decisions to be made, perhaps second only to the decision to perform the surgery itself. Recent multiple, well-designed studies comparing differences among grafts have shown equivalent long-term results, leading to the lack of a universally accepted gold standard.5-7 Thus, both autograft and allograft ACL surgeries are routinely performed in the United States. Surgeons typically take into account factors such as patient age and physical demands, along with their own preferences and/or experience, when considering graft selection. A paucity of research concerning existing pathological conditions that could also influence preoperative decision-making has been observed; most reports consist only of expert opinion.11-13 Our goal is to determine the prevalence of several conditions that could potentially affect an autograft harvested from the extensor mechanism.
This study revealed an overall prevalence of 10.1% of existing extensor mechanism pathology in patients sustaining an acute ACL tear and presenting for ACL reconstruction. Only 1 (0.5%) showed evidence of a multipartite patella, which is below the reported prevalence of 0.2% to 6%.14 The presence of a multipartite patella could potentially have the most deleterious effect on a qTB autograft. Although not as commonly used as HS, QT, or pBTB autografts, some surgeons prefer a qTB autograft because of its increased surface area, bony fixation, and reported decreased donor site pain.15 A multipartite patella could complicate harvesting, disrupt the bone block, or lead to an unstable segment of the patella. These effects are of great concern since the most common location of a bipartite patella is superior-lateral and the quadriceps tendon has been shown to asymmetrically insert laterally.16 While these potential adverse effects have not been specifically studied, the availability of comparable options makes the use of a qTB autograft in the setting of a bipartite patella questionable.
Four patients (2%) revealed evidence of ossicles within the inferior patellar tendon consistent with unresolved Osgood-Schlatter’s disease. Osgood-Schlatter’s disease has been reported to occur in up to 21% of active adolescents and is historically considered a self-resolving process.17 Recent papers have reported persistent symptoms in up to 10% of patients, with a small percentage experiencing persistent free ossicles within their patella tendon on MRI.18,19 The presence of such ossicles raises concern about the integrity of the patellar tendon and questions its use as an autograft when present. This concern was published in a report with the surgeon opting to utilize an alternate graft due to the presence of unresolved Osgood-Schlatter’s disease.13
Fifteen patients (7.6%) demonstrated radiographic evidence suggestive of patella tendinopathy based on the thickness of the proximal patella tendon. Patella tendinopathy is the most common tendinopathy in skeletally mature athletes and one of the most common athletic injuries of the knee, with a reported career prevalence of 22%.20 It is described as an overuse injury due to the cumulative effect of micro trauma without an adequate healing interval. While it remains a clinical diagnosis, patellar tendinopathy often shows radiographic findings best assessed on sagittal MRIs. In general, the normal patella tendon appears as a homogenous low-intensity structure and is of uniform thickness. A tendon affected with tendinopathy typically demonstrates a focal increase in signal on T2-weighted sequences just distal to the tendon origin on the inferior pole of the patella. In addition, the patella tendon will usually demonstrate thickening, primarily in the proximal medial and posterior fibers. Patella marrow changes and indistinct tendon margins can also be present. The sensitivity and specificity of diagnosing patellar tendinopathy on MRI are 78% and 86%, respectively.20 We derived our criteria for MRI evidence suggestive of patella tendinopathy from studies by El-Khoury and colleagues,8 Johnson and colleagues,9 and Popp and colleagues.10 In a 1992 study, El-Khoury and colleagues8 compared MRI findings between a group of patients with a clinical diagnosis of patella tendonitis and a control group without knee complaints. The authors found that the average proximal patella tendon diameter in the control group was 3.7 mm while the average proximal patella tendon diameter in the patella tendinopathy group was 10.9 mm; no patella tendons in the control group were >7 mm.8 In a 1996 study, Johnson and colleagues9 determined that the most reliable MRI finding for patients with patellar tendonitis is significant thickening of the proximal patella tendon seen on the sagittal view. The average thickness in symptomatic patients was 8.5 mm (range, 5-15 mm). The average thickness in the control group was 5.5 mm. None of the control patients had a proximal tendon thickness >7 mm.9 Finally, Popp and colleagues10 reviewed the MRI of 11 knees of patients who underwent surgical débridement of chronic patellar tendonitis and reported an average proximal patella tendon thickness of 12 mm (range, 9-16 mm). We therefore used a proximal patella tendon thickness of >7 mm on the sagittal view as a radiographic finding suggestive of patella tendinopathy. No data regarding symptoms of anterior knee pain were available among our patients. Histological studies of patients with patella tendonitis have shown evidence of chronic inflammation, fibrinoid necrosis, mucoid degeneration, and synovial proliferation within the patella tendon insertion.21 Although no controlled data showing that patella tendons with a history of tendonitis are more prone to failure than those without such history when used as an autograft for ACL reconstruction, the idea of utilizing a diseased tendon for a graft is not ideal. Some surgeons question their patients regarding a history of anterior knee pain and will not use a pBTB autograft in a patient with a positive history.22
Continue to: The goal of this study is to obtain epidemiological evidence...
The goal of this study is to obtain epidemiological evidence of the prevalence of existing extensor mechanism pathologies in patients with acute ACL ruptures and determine how these pathologies may relate to the choice of graft. Out of 197 patients studied, over 10% presented with radiographic evidence of pathologies that could influence the choice of graft. This prevalence is certainly significant enough for surgeons to consider including a radiographic evaluation of the extensor mechanism in their standard ACL rupture work-up.
This study presents obvious limitations. While we report the prevalence of some extensor mechanism pathologies, no definitive evidence that recommends against the use of these autografts from these affected individuals has yet been published. In addition, our diagnosis of patella tendinopathy is based solely on MRI findings with no information regarding clinical symptoms. This limitation is a weakness as several additional studies have questioned the validity of a 7 mm proximal patella tendon thickness.23,24 Furthermore, no studies demonstrating the inferior strength of autografts with the co-existing findings described in our work have yet been performed.
CONCLUSION
We found that 10% of active-duty patients presenting for ACL reconstruction demonstrated radiographic evidence of an extensor mechanism pathology that could affect the harvesting of or integrity of select autografts. Given the recent trend of functionally equivocal results in ACL reconstructions utilizing a variety of grafts, this information could and should influence surgical recommendations for graft utilization to obtain optimal surgical results.
1. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321-2328. doi:10.2106/JBJS.H.00539.
2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135-2142. doi:10.1056/NEJMcp0804745.
3. Fu FH, Bennett CH, Lattermann CL, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27(6):821-830. doi:10.1177/03635465990270062501.
4. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft Versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(2):492-499. doi:10.1177/0363546513497566.
5. Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):214-220. doi:10.1177/03635465020300021201.
6. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: Allograft versus allograft. Arthroscopy. 2005;21(7):774-785. doi:10.1016/j.arthro.2005.04.112.
7. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):292-298. doi:10.1016/j.arthro.2007.08.029.
8. El-Khoury GY, Wira RL, Berbaum KS, Pope TL, Monu JUV. MR imaging of patellar tendinitis. Radiology. 1992;184(3):849-854. doi:10.1148/radiology.184.3.1509078.
9. Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Joint Surg Br. 1996;78(3):452-457. doi:10.1302/0301-620X.78B3.0780452.
10. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218-222. doi:10.1177/036354659702500214.
11. Provencher MT, Ryu JH, Gaston T, Dewing CB. Technique: bone-patellar tendon-bone autograft ACL reconstruction in the young, active patient. J Knee Surg. 2011;24(2):83-92. doi:10.1055/s-0031-1280875.
12. Fu F, Cohen S. Current Concepts in ACL Reconstruction. Thorofare: SLACK Incorporated; 2008.
13. Cosgarea AJ, Weng MS, Andrews M. Osgood Schlatter’s disease complicating anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(6):700-703. doi:10.1016/S0749-8063(05)80511-0.
14. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855. doi:10.1007/s11999-008-0367-4.
15. Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252-254. doi:10.1016/0749-8063(95)90078-0.
16. Scully WF, Wilson DJ, Arrington ED. “Central” quadriceps tendon harvest with patellar bone plug: surgical technique revisited. Arthrosc Tech. 2013;2(4):e427-e432.
17. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236-241. doi:10.1177/036354658501300404.
18. Pihlajamäki HK, Visuri TI. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 Pt 2):258-264. doi:10.2106/JBJS.J.00450.
19. Weiss JM, Jordan SS, Andersen JS, Lee BM, Kocher M. Surgical treatment of unresolved Osgood-Schlatter disease: ossicle resection with tibial tubercleplasty. J Pediatr Orthop. 2007;27(7):844-847. doi:10.1097/BPO.0b013e318155849b.
20. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee Among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561-567. doi:10.1177/0363546504270454.
21. O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725-739, vii. doi:10.1016/j.mric.2009.06.010.
22. Martens M, Wouters P, Burssens A, Mulier JC. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand. 1982;53(3):445-450. doi:10.3109/17453678208992239.
23. Shalaby M, Almekinders LC. Patellar tendinitis: the significance of magnetic resonance imaging findings. Am J Sports Med. 1999;27(3):345-349. doi:10.1177/03635465990270031301.
24. Reiff DB, Heenan SD, Heron CW. MRI appearances of the asymptomatic patellar tendon on gradient echo imaging. Skeletal Radiol. 1995;24(2):123-126. doi:10.1007/BF00198074.
1. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321-2328. doi:10.2106/JBJS.H.00539.
2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359(20):2135-2142. doi:10.1056/NEJMcp0804745.
3. Fu FH, Bennett CH, Lattermann CL, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27(6):821-830. doi:10.1177/03635465990270062501.
4. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft Versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42(2):492-499. doi:10.1177/0363546513497566.
5. Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(2):214-220. doi:10.1177/03635465020300021201.
6. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: Allograft versus allograft. Arthroscopy. 2005;21(7):774-785. doi:10.1016/j.arthro.2005.04.112.
7. Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(3):292-298. doi:10.1016/j.arthro.2007.08.029.
8. El-Khoury GY, Wira RL, Berbaum KS, Pope TL, Monu JUV. MR imaging of patellar tendinitis. Radiology. 1992;184(3):849-854. doi:10.1148/radiology.184.3.1509078.
9. Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Joint Surg Br. 1996;78(3):452-457. doi:10.1302/0301-620X.78B3.0780452.
10. Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218-222. doi:10.1177/036354659702500214.
11. Provencher MT, Ryu JH, Gaston T, Dewing CB. Technique: bone-patellar tendon-bone autograft ACL reconstruction in the young, active patient. J Knee Surg. 2011;24(2):83-92. doi:10.1055/s-0031-1280875.
12. Fu F, Cohen S. Current Concepts in ACL Reconstruction. Thorofare: SLACK Incorporated; 2008.
13. Cosgarea AJ, Weng MS, Andrews M. Osgood Schlatter’s disease complicating anterior cruciate ligament reconstruction. Arthroscopy. 1993;9(6):700-703. doi:10.1016/S0749-8063(05)80511-0.
14. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855. doi:10.1007/s11999-008-0367-4.
15. Fulkerson JP, Langeland R. An alternative cruciate reconstruction graft: the central quadriceps tendon. Arthroscopy. 1995;11(2):252-254. doi:10.1016/0749-8063(95)90078-0.
16. Scully WF, Wilson DJ, Arrington ED. “Central” quadriceps tendon harvest with patellar bone plug: surgical technique revisited. Arthrosc Tech. 2013;2(4):e427-e432.
17. Kujala UM, Kvist M, Heinonen O. Osgood-Schlatter’s disease in adolescent athletes. Retrospective study of incidence and duration. Am J Sports Med. 1985;13(4):236-241. doi:10.1177/036354658501300404.
18. Pihlajamäki HK, Visuri TI. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 Pt 2):258-264. doi:10.2106/JBJS.J.00450.
19. Weiss JM, Jordan SS, Andersen JS, Lee BM, Kocher M. Surgical treatment of unresolved Osgood-Schlatter disease: ossicle resection with tibial tubercleplasty. J Pediatr Orthop. 2007;27(7):844-847. doi:10.1097/BPO.0b013e318155849b.
20. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee Among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33(4):561-567. doi:10.1177/0363546504270454.
21. O’Keeffe SA, Hogan BA, Eustace SJ, Kavanagh EC. Overuse injuries of the knee. Magn Reson Imaging Clin N Am. 2009;17(4):725-739, vii. doi:10.1016/j.mric.2009.06.010.
22. Martens M, Wouters P, Burssens A, Mulier JC. Patellar tendinitis: pathology and results of treatment. Acta Orthop Scand. 1982;53(3):445-450. doi:10.3109/17453678208992239.
23. Shalaby M, Almekinders LC. Patellar tendinitis: the significance of magnetic resonance imaging findings. Am J Sports Med. 1999;27(3):345-349. doi:10.1177/03635465990270031301.
24. Reiff DB, Heenan SD, Heron CW. MRI appearances of the asymptomatic patellar tendon on gradient echo imaging. Skeletal Radiol. 1995;24(2):123-126. doi:10.1007/BF00198074.
TAKE-HOME POINTS
- Extensor mechanism pathology is a common finding in patients with ACL injuries.
- Extensor mechanism pathology such as a multipartite patella, unresolved Osgood-Schlatter’s disease, and patella tendinopathy are easily identifiable on standard imaging.
- It is unknown what type of effect, if any, these pathologies may have on graft strength.
- The bone-patella tendon-bone and quadriceps autograft are the most likely to be affected.
- Surgeons should take into account existing extensor mechanism pathology when considering individual patient graft selection for ACL reconstruction.
How Teleneurologists Can Enhance Acute and Chronic Patient Care
HILTON HEAD, SC—When neurologists see patients remotely, the focus should be on the patient, not on the technology behind the virtual visit, according to one researcher.
“One of the big mistakes that is made with telehealth is focusing on the technology and not on the clinical care delivery,” said Kenneth Gaines, MD, Professor of Neurology at Vanderbilt University in Nashville. “It is the clinical care delivery that ought to drive the technology. Too often it happens in reverse…. That is a recipe for an ineffective program.”
Broadly speaking, telemedicine is medicine practiced at a distance. Ideally, it uses technology to facilitate a clinical care paradigm that improves efficiency, care coordination, and outcomes and lowers costs, Dr. Gaines said. The public increasingly expects this type of care to be available, he said.
“Why would telehealth be useful in neurology? In part because we deal with acute and chronic disease, which is what stroke is, for example, but also other diseases like epilepsy,” Dr. Gaines said at Vanderbilt’s 41st Annual Contemporary Clinical Neurology Symposium. In addition, neurologic diseases are complex, and generalists’ training in them may be limited. Telehealth could allow neurologists to assist general practitioners and provide neurologic care to areas with few neurologists.
Opportunity in Neurology
Systematic reviews in disease states like diabetes, hyperlipidemia, and hypertension have found that telemedicine may benefit patients. A 2015 Cochrane review of data from randomized controlled trials of interactive telemedicine found with moderate certainty that telemedicine decreased LDL and blood pressure, compared with usual care. It found with high certainty that among patients with diabetes, those who received telemedicine had lower glycated hemoglobin levels at nine months, compared with controls. Evidence to assess the effects of telemedicine in neurology, however, was inadequate.
Nevertheless, studies indicate telemedicine’s promise in neurology. Beck et al conducted the Connect.Parkinson trial, which included 195 patients with Parkinson’s disease who were randomized to usual care or usual care plus four virtual visits via video conferencing with a remote specialist. The researchers found that telemedicine was feasible and equivalent to usual care with regard to its effects on patients’ quality of life. The virtual house calls saved patients a median of 88 minutes and 38 miles per visit. Mammen et al analyzed survey data from patients with Parkinson’s disease and physicians and found that they generally were satisfied with the telemedicine approach, but technical problems affected individual experiences. Physicians’ greatest source of dissatisfaction was performing a detailed motor examination remotely.
Samii et al reported the experience of one Veterans Administration medical center that found it feasible to conduct follow-up visits with patients with Parkinson’s disease via telemedicine. Although the video quality initially was not sufficient to score the motor Unified Parkinson’s Disease Rating Scale, a videoconferencing unit upgrade allowed physicians to assess those measures, with the exception of elements that require physical contact, such as rigidity and retropulsion.
A study by Kane et al found that telemedicine assessments of patients with multiple sclerosis could reliably determine Expanded Disability Status Scale scores. Scores related to cerebellar and brainstem functions, however, were less consistent with the scores of a hands-on examiner than were scores related to optic, bowel, bladder, and cerebral functions.
In neurology, most studies of telemedicine have focused on stroke, perhaps because of the complex and time-sensitive nature of the disease and a maldistribution of health care providers, Dr. Gaines said.
The Telemedical Pilot Project for Integrative Stroke Care (TEMPiS) in Germany connected 12 community hospitals that had limited experience with stroke thrombolysis to two specialized stroke centers. In the first 22 months, patients treated at the community hospitals and at the stroke centers had equivalent rates of mortality and good functional outcomes that were similar to those in randomized trials, Schwab et al reported.
In the STRokE DOC trial, Meyer et al assessed whether telemedicine (ie, real-time, two-way audio and video communication and digital imaging interpretation) or telephone consultation was superior in acute stroke consultations. In all, 111 patients were randomized to telemedicine, and 111 were randomized to telephone consultation. Ninety-day functional outcomes and rates of intracerebral hemorrhage after treatment with thromblytics and mortality were equivalent between the groups. With telemedicine, neurologists were more likely to arrive at a correct treatment decision and less likely to violate trial protocols, Dr. Gaines said.
The Neglected First Year
Dr. Gaines helped create a comprehensive stroke care model at Ochsner Medical Center in New Orleans that incorporated telemedicine. The program targeted acute stroke treatment as well as what Dr. Gaines calls the neglected first year after hospitalization for stroke. “In the chronic phase of stroke, these folks have significant disability. It is a significant stressor for their families and their caregivers, and unfortunately we have relatively poor risk factor control and inadequate medication compliance in this segment,” he said. Patients have high death rates due to recurrent stroke, complications of stroke, and other cardiovascular diseases.
The medical center provided telemedicine consultations to 22 affiliated hospitals. In addition, a Stroke Mobile team that consisted of a nurse and a health educator visited patients’ homes monthly for one year to address stroke risks and complications. Stroke Mobile staff used HIPAA-compliant video communication to facilitate telemedicine consultations with a vascular neurologist or advanced practice clinician during the home visits.
This model decreased length of hospital stay by one day, decreased cost per case by 9%, and lowered readmission and stroke recurrence by about 17% each. Furthermore, between 85% and 90% of patients achieved control of risk factors such as blood pressure, diabetes, and cholesterol. “Projects like this can offer us a much better, more comprehensive approach,” said Dr. Gaines.
Performing Neurologic Exams Remotely
Researchers have found that the NIH Stroke Scale can be administered as reliably in a telehealth setting as in person, Dr. Gaines said. Shafqat et al found that four NIH Stroke Scale items had excellent agreement (ie, orientation, motor arm, motor leg, and neglect), and six items had good agreement (ie, language, dysarthria, sensation, visual fields, facial palsy, and gaze), whereas two items (ie, commands and ataxia) had poor agreement, when they compared remote and in-person assessments.
Certain examination elements require the assistance of a nurse or emergency department doctor who is with the patient, and a neurologist’s confidence in his or her ability to perform a neurologic examination via telehealth may depend on how much he or she is “willing to ask people to do,” Dr. Gaines said. “I will ask somebody to shine a light into the eyes so I can look at the pupils. I will have a person who is helping me in the ER setting do a Babinski sign and watch for the response. I have diagnosed a hereditary neuropathy with liability to pressure palsies via teleneurology.”
Emergency department doctors and nurses often know how to perform elements of neurologic examinations, even if they do not routinely use them. “We spend time training nurses at our sites to help us,” he said.
In addition, neurologists can assess gait, which typically is not tested in emergency rooms but can provide valuable information. A patient’s gait might help a neurologist identify cerebellar infarcts that otherwise would have been attributed to dizziness, for example.
Telemedicine presents “opportunities … to impact stroke care and hopefully other areas of neurology as well. It is not nearly as daunting as you might think to do these consults virtually,” Dr. Gaines said. “It is a great opportunity for us to put systems of care in place to deal with some of the inequities and problems with delivery that we have.”
—Jake Remaly
Suggested Reading
Beck CA, Beran DB, Biglan KM, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152-1161.
Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;(9):CD002098.
Kane RL, Bever CT, Ehrmantraut M, et al. Teleneurology in patients with multiple sclerosis: EDSS ratings derived remotely and from hands-on examination. J Telemed Telecare. 2008;14(4):190-194.
Mammen JR, Elson MJ, Java JJ, et al. Patient and physician perceptions of virtual visits for Parkinson’s disease: a qualitative study. Telemed J E Health. 2018;24(4):255-267.
Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795.
Samii A, Ryan-Dykes P, Tsukuda RA, et al. Telemedicine for delivery of health care in Parkinson’s disease. J Telemed Telecare. 2006;12(1):16-18.
Schwab S, Vatankhah B, Kukla C, et al. Long-term outcome after thrombolysis in telemedical stroke care. Neurology. 2007;69(9):898-903.
Shafqat S, Kvedar JC, Guanci MM, et al. Role for telemedicine in acute stroke. Feasibility and reliability of remote administration of the NIH stroke scale. Stroke. 1999;30(10):2141-2145.
HILTON HEAD, SC—When neurologists see patients remotely, the focus should be on the patient, not on the technology behind the virtual visit, according to one researcher.
“One of the big mistakes that is made with telehealth is focusing on the technology and not on the clinical care delivery,” said Kenneth Gaines, MD, Professor of Neurology at Vanderbilt University in Nashville. “It is the clinical care delivery that ought to drive the technology. Too often it happens in reverse…. That is a recipe for an ineffective program.”
Broadly speaking, telemedicine is medicine practiced at a distance. Ideally, it uses technology to facilitate a clinical care paradigm that improves efficiency, care coordination, and outcomes and lowers costs, Dr. Gaines said. The public increasingly expects this type of care to be available, he said.
“Why would telehealth be useful in neurology? In part because we deal with acute and chronic disease, which is what stroke is, for example, but also other diseases like epilepsy,” Dr. Gaines said at Vanderbilt’s 41st Annual Contemporary Clinical Neurology Symposium. In addition, neurologic diseases are complex, and generalists’ training in them may be limited. Telehealth could allow neurologists to assist general practitioners and provide neurologic care to areas with few neurologists.
Opportunity in Neurology
Systematic reviews in disease states like diabetes, hyperlipidemia, and hypertension have found that telemedicine may benefit patients. A 2015 Cochrane review of data from randomized controlled trials of interactive telemedicine found with moderate certainty that telemedicine decreased LDL and blood pressure, compared with usual care. It found with high certainty that among patients with diabetes, those who received telemedicine had lower glycated hemoglobin levels at nine months, compared with controls. Evidence to assess the effects of telemedicine in neurology, however, was inadequate.
Nevertheless, studies indicate telemedicine’s promise in neurology. Beck et al conducted the Connect.Parkinson trial, which included 195 patients with Parkinson’s disease who were randomized to usual care or usual care plus four virtual visits via video conferencing with a remote specialist. The researchers found that telemedicine was feasible and equivalent to usual care with regard to its effects on patients’ quality of life. The virtual house calls saved patients a median of 88 minutes and 38 miles per visit. Mammen et al analyzed survey data from patients with Parkinson’s disease and physicians and found that they generally were satisfied with the telemedicine approach, but technical problems affected individual experiences. Physicians’ greatest source of dissatisfaction was performing a detailed motor examination remotely.
Samii et al reported the experience of one Veterans Administration medical center that found it feasible to conduct follow-up visits with patients with Parkinson’s disease via telemedicine. Although the video quality initially was not sufficient to score the motor Unified Parkinson’s Disease Rating Scale, a videoconferencing unit upgrade allowed physicians to assess those measures, with the exception of elements that require physical contact, such as rigidity and retropulsion.
A study by Kane et al found that telemedicine assessments of patients with multiple sclerosis could reliably determine Expanded Disability Status Scale scores. Scores related to cerebellar and brainstem functions, however, were less consistent with the scores of a hands-on examiner than were scores related to optic, bowel, bladder, and cerebral functions.
In neurology, most studies of telemedicine have focused on stroke, perhaps because of the complex and time-sensitive nature of the disease and a maldistribution of health care providers, Dr. Gaines said.
The Telemedical Pilot Project for Integrative Stroke Care (TEMPiS) in Germany connected 12 community hospitals that had limited experience with stroke thrombolysis to two specialized stroke centers. In the first 22 months, patients treated at the community hospitals and at the stroke centers had equivalent rates of mortality and good functional outcomes that were similar to those in randomized trials, Schwab et al reported.
In the STRokE DOC trial, Meyer et al assessed whether telemedicine (ie, real-time, two-way audio and video communication and digital imaging interpretation) or telephone consultation was superior in acute stroke consultations. In all, 111 patients were randomized to telemedicine, and 111 were randomized to telephone consultation. Ninety-day functional outcomes and rates of intracerebral hemorrhage after treatment with thromblytics and mortality were equivalent between the groups. With telemedicine, neurologists were more likely to arrive at a correct treatment decision and less likely to violate trial protocols, Dr. Gaines said.
The Neglected First Year
Dr. Gaines helped create a comprehensive stroke care model at Ochsner Medical Center in New Orleans that incorporated telemedicine. The program targeted acute stroke treatment as well as what Dr. Gaines calls the neglected first year after hospitalization for stroke. “In the chronic phase of stroke, these folks have significant disability. It is a significant stressor for their families and their caregivers, and unfortunately we have relatively poor risk factor control and inadequate medication compliance in this segment,” he said. Patients have high death rates due to recurrent stroke, complications of stroke, and other cardiovascular diseases.
The medical center provided telemedicine consultations to 22 affiliated hospitals. In addition, a Stroke Mobile team that consisted of a nurse and a health educator visited patients’ homes monthly for one year to address stroke risks and complications. Stroke Mobile staff used HIPAA-compliant video communication to facilitate telemedicine consultations with a vascular neurologist or advanced practice clinician during the home visits.
This model decreased length of hospital stay by one day, decreased cost per case by 9%, and lowered readmission and stroke recurrence by about 17% each. Furthermore, between 85% and 90% of patients achieved control of risk factors such as blood pressure, diabetes, and cholesterol. “Projects like this can offer us a much better, more comprehensive approach,” said Dr. Gaines.
Performing Neurologic Exams Remotely
Researchers have found that the NIH Stroke Scale can be administered as reliably in a telehealth setting as in person, Dr. Gaines said. Shafqat et al found that four NIH Stroke Scale items had excellent agreement (ie, orientation, motor arm, motor leg, and neglect), and six items had good agreement (ie, language, dysarthria, sensation, visual fields, facial palsy, and gaze), whereas two items (ie, commands and ataxia) had poor agreement, when they compared remote and in-person assessments.
Certain examination elements require the assistance of a nurse or emergency department doctor who is with the patient, and a neurologist’s confidence in his or her ability to perform a neurologic examination via telehealth may depend on how much he or she is “willing to ask people to do,” Dr. Gaines said. “I will ask somebody to shine a light into the eyes so I can look at the pupils. I will have a person who is helping me in the ER setting do a Babinski sign and watch for the response. I have diagnosed a hereditary neuropathy with liability to pressure palsies via teleneurology.”
Emergency department doctors and nurses often know how to perform elements of neurologic examinations, even if they do not routinely use them. “We spend time training nurses at our sites to help us,” he said.
In addition, neurologists can assess gait, which typically is not tested in emergency rooms but can provide valuable information. A patient’s gait might help a neurologist identify cerebellar infarcts that otherwise would have been attributed to dizziness, for example.
Telemedicine presents “opportunities … to impact stroke care and hopefully other areas of neurology as well. It is not nearly as daunting as you might think to do these consults virtually,” Dr. Gaines said. “It is a great opportunity for us to put systems of care in place to deal with some of the inequities and problems with delivery that we have.”
—Jake Remaly
Suggested Reading
Beck CA, Beran DB, Biglan KM, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152-1161.
Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;(9):CD002098.
Kane RL, Bever CT, Ehrmantraut M, et al. Teleneurology in patients with multiple sclerosis: EDSS ratings derived remotely and from hands-on examination. J Telemed Telecare. 2008;14(4):190-194.
Mammen JR, Elson MJ, Java JJ, et al. Patient and physician perceptions of virtual visits for Parkinson’s disease: a qualitative study. Telemed J E Health. 2018;24(4):255-267.
Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795.
Samii A, Ryan-Dykes P, Tsukuda RA, et al. Telemedicine for delivery of health care in Parkinson’s disease. J Telemed Telecare. 2006;12(1):16-18.
Schwab S, Vatankhah B, Kukla C, et al. Long-term outcome after thrombolysis in telemedical stroke care. Neurology. 2007;69(9):898-903.
Shafqat S, Kvedar JC, Guanci MM, et al. Role for telemedicine in acute stroke. Feasibility and reliability of remote administration of the NIH stroke scale. Stroke. 1999;30(10):2141-2145.
HILTON HEAD, SC—When neurologists see patients remotely, the focus should be on the patient, not on the technology behind the virtual visit, according to one researcher.
“One of the big mistakes that is made with telehealth is focusing on the technology and not on the clinical care delivery,” said Kenneth Gaines, MD, Professor of Neurology at Vanderbilt University in Nashville. “It is the clinical care delivery that ought to drive the technology. Too often it happens in reverse…. That is a recipe for an ineffective program.”
Broadly speaking, telemedicine is medicine practiced at a distance. Ideally, it uses technology to facilitate a clinical care paradigm that improves efficiency, care coordination, and outcomes and lowers costs, Dr. Gaines said. The public increasingly expects this type of care to be available, he said.
“Why would telehealth be useful in neurology? In part because we deal with acute and chronic disease, which is what stroke is, for example, but also other diseases like epilepsy,” Dr. Gaines said at Vanderbilt’s 41st Annual Contemporary Clinical Neurology Symposium. In addition, neurologic diseases are complex, and generalists’ training in them may be limited. Telehealth could allow neurologists to assist general practitioners and provide neurologic care to areas with few neurologists.
Opportunity in Neurology
Systematic reviews in disease states like diabetes, hyperlipidemia, and hypertension have found that telemedicine may benefit patients. A 2015 Cochrane review of data from randomized controlled trials of interactive telemedicine found with moderate certainty that telemedicine decreased LDL and blood pressure, compared with usual care. It found with high certainty that among patients with diabetes, those who received telemedicine had lower glycated hemoglobin levels at nine months, compared with controls. Evidence to assess the effects of telemedicine in neurology, however, was inadequate.
Nevertheless, studies indicate telemedicine’s promise in neurology. Beck et al conducted the Connect.Parkinson trial, which included 195 patients with Parkinson’s disease who were randomized to usual care or usual care plus four virtual visits via video conferencing with a remote specialist. The researchers found that telemedicine was feasible and equivalent to usual care with regard to its effects on patients’ quality of life. The virtual house calls saved patients a median of 88 minutes and 38 miles per visit. Mammen et al analyzed survey data from patients with Parkinson’s disease and physicians and found that they generally were satisfied with the telemedicine approach, but technical problems affected individual experiences. Physicians’ greatest source of dissatisfaction was performing a detailed motor examination remotely.
Samii et al reported the experience of one Veterans Administration medical center that found it feasible to conduct follow-up visits with patients with Parkinson’s disease via telemedicine. Although the video quality initially was not sufficient to score the motor Unified Parkinson’s Disease Rating Scale, a videoconferencing unit upgrade allowed physicians to assess those measures, with the exception of elements that require physical contact, such as rigidity and retropulsion.
A study by Kane et al found that telemedicine assessments of patients with multiple sclerosis could reliably determine Expanded Disability Status Scale scores. Scores related to cerebellar and brainstem functions, however, were less consistent with the scores of a hands-on examiner than were scores related to optic, bowel, bladder, and cerebral functions.
In neurology, most studies of telemedicine have focused on stroke, perhaps because of the complex and time-sensitive nature of the disease and a maldistribution of health care providers, Dr. Gaines said.
The Telemedical Pilot Project for Integrative Stroke Care (TEMPiS) in Germany connected 12 community hospitals that had limited experience with stroke thrombolysis to two specialized stroke centers. In the first 22 months, patients treated at the community hospitals and at the stroke centers had equivalent rates of mortality and good functional outcomes that were similar to those in randomized trials, Schwab et al reported.
In the STRokE DOC trial, Meyer et al assessed whether telemedicine (ie, real-time, two-way audio and video communication and digital imaging interpretation) or telephone consultation was superior in acute stroke consultations. In all, 111 patients were randomized to telemedicine, and 111 were randomized to telephone consultation. Ninety-day functional outcomes and rates of intracerebral hemorrhage after treatment with thromblytics and mortality were equivalent between the groups. With telemedicine, neurologists were more likely to arrive at a correct treatment decision and less likely to violate trial protocols, Dr. Gaines said.
The Neglected First Year
Dr. Gaines helped create a comprehensive stroke care model at Ochsner Medical Center in New Orleans that incorporated telemedicine. The program targeted acute stroke treatment as well as what Dr. Gaines calls the neglected first year after hospitalization for stroke. “In the chronic phase of stroke, these folks have significant disability. It is a significant stressor for their families and their caregivers, and unfortunately we have relatively poor risk factor control and inadequate medication compliance in this segment,” he said. Patients have high death rates due to recurrent stroke, complications of stroke, and other cardiovascular diseases.
The medical center provided telemedicine consultations to 22 affiliated hospitals. In addition, a Stroke Mobile team that consisted of a nurse and a health educator visited patients’ homes monthly for one year to address stroke risks and complications. Stroke Mobile staff used HIPAA-compliant video communication to facilitate telemedicine consultations with a vascular neurologist or advanced practice clinician during the home visits.
This model decreased length of hospital stay by one day, decreased cost per case by 9%, and lowered readmission and stroke recurrence by about 17% each. Furthermore, between 85% and 90% of patients achieved control of risk factors such as blood pressure, diabetes, and cholesterol. “Projects like this can offer us a much better, more comprehensive approach,” said Dr. Gaines.
Performing Neurologic Exams Remotely
Researchers have found that the NIH Stroke Scale can be administered as reliably in a telehealth setting as in person, Dr. Gaines said. Shafqat et al found that four NIH Stroke Scale items had excellent agreement (ie, orientation, motor arm, motor leg, and neglect), and six items had good agreement (ie, language, dysarthria, sensation, visual fields, facial palsy, and gaze), whereas two items (ie, commands and ataxia) had poor agreement, when they compared remote and in-person assessments.
Certain examination elements require the assistance of a nurse or emergency department doctor who is with the patient, and a neurologist’s confidence in his or her ability to perform a neurologic examination via telehealth may depend on how much he or she is “willing to ask people to do,” Dr. Gaines said. “I will ask somebody to shine a light into the eyes so I can look at the pupils. I will have a person who is helping me in the ER setting do a Babinski sign and watch for the response. I have diagnosed a hereditary neuropathy with liability to pressure palsies via teleneurology.”
Emergency department doctors and nurses often know how to perform elements of neurologic examinations, even if they do not routinely use them. “We spend time training nurses at our sites to help us,” he said.
In addition, neurologists can assess gait, which typically is not tested in emergency rooms but can provide valuable information. A patient’s gait might help a neurologist identify cerebellar infarcts that otherwise would have been attributed to dizziness, for example.
Telemedicine presents “opportunities … to impact stroke care and hopefully other areas of neurology as well. It is not nearly as daunting as you might think to do these consults virtually,” Dr. Gaines said. “It is a great opportunity for us to put systems of care in place to deal with some of the inequities and problems with delivery that we have.”
—Jake Remaly
Suggested Reading
Beck CA, Beran DB, Biglan KM, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152-1161.
Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;(9):CD002098.
Kane RL, Bever CT, Ehrmantraut M, et al. Teleneurology in patients with multiple sclerosis: EDSS ratings derived remotely and from hands-on examination. J Telemed Telecare. 2008;14(4):190-194.
Mammen JR, Elson MJ, Java JJ, et al. Patient and physician perceptions of virtual visits for Parkinson’s disease: a qualitative study. Telemed J E Health. 2018;24(4):255-267.
Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795.
Samii A, Ryan-Dykes P, Tsukuda RA, et al. Telemedicine for delivery of health care in Parkinson’s disease. J Telemed Telecare. 2006;12(1):16-18.
Schwab S, Vatankhah B, Kukla C, et al. Long-term outcome after thrombolysis in telemedical stroke care. Neurology. 2007;69(9):898-903.
Shafqat S, Kvedar JC, Guanci MM, et al. Role for telemedicine in acute stroke. Feasibility and reliability of remote administration of the NIH stroke scale. Stroke. 1999;30(10):2141-2145.