User login
Nabilone May Reduce Agitation in People With Alzheimer’s Disease
The treatment also improves behavioral symptoms such as delusions, hallucinations, anxiety, and apathy.
CHICAGO—Nabilone, a synthetic cannabinoid, may effectively treat agitation in people with Alzheimer’s disease, according to a randomized, double-blind clinical trial presented at AAIC 2018.
“Agitation, including verbal or physical outbursts, general emotional distress, restlessness, and pacing, is one of the most common behavioral changes associated with Alzheimer’s disease as it progresses and can be a significant cause of caregiver stress,” said Krista L. Lanctôt, PhD, Senior Scientist at Sunnybrook Health Sciences Centre and Professor of Pharmacology and Psychiatry at the University of Toronto.
Dr. Lanctôt and colleagues investigated the potential benefits of nabilone for adults with moderate-to-severe Alzheimer’s dementia and clinically significant agitation. During the 14-week trial, 39 participants (77% male; average age, 87) received nabilone in capsule form (mean therapeutic dose, 1.6 mg) for six weeks, followed by one week without treatment and six weeks of placebo. In addition to measuring agitation, the researchers assessed overall behavioral symptoms, memory, physical changes, and safety.
Dr. Lanctôt’s group found that agitation improved significantly when participants were taking nabilone, compared with when they were receiving placebo, as measured by the Cohen-Mansfield Agitation Inventory. Nabilone also significantly improved overall behavioral symptoms, compared with placebo, as measured by the Neuropsychiatric Inventory.
In addition, the researchers observed small benefits in cognition and nutrition when participants received nabilone during the study. More people in the study experienced sedation when taking nabilone (45%) than when taking placebo (16%).
“Currently prescribed treatments for agitation in Alzheimer’s disease do not work in everybody. And when they do work, the effect is small, and they increase the risk of harmful side effects, including increased risk of death. As a result, there is an urgent need for safer medication options,” said Dr. Lanctôt. “These findings suggest that nabilone may be an effective treatment for agitation; however, the risk of sedation must be carefully monitored. A larger clinical trial would allow us to confirm our findings regarding how effective and safe nabilone is in the treatment of agitation for Alzheimer’s disease.”The FDA has not approved marijuana for the treatment or management of Alzheimer’s disease or other dementias. The use of marijuana as medical treatment is increasingly common, but much about the drug’s use in people with Alzheimer’s disease or other dementias is unknown. No robust, consistent clinical trial data support marijuana for the treatment of Alzheimer’s disease dementia or for related issues.
The treatment also improves behavioral symptoms such as delusions, hallucinations, anxiety, and apathy.
The treatment also improves behavioral symptoms such as delusions, hallucinations, anxiety, and apathy.
CHICAGO—Nabilone, a synthetic cannabinoid, may effectively treat agitation in people with Alzheimer’s disease, according to a randomized, double-blind clinical trial presented at AAIC 2018.
“Agitation, including verbal or physical outbursts, general emotional distress, restlessness, and pacing, is one of the most common behavioral changes associated with Alzheimer’s disease as it progresses and can be a significant cause of caregiver stress,” said Krista L. Lanctôt, PhD, Senior Scientist at Sunnybrook Health Sciences Centre and Professor of Pharmacology and Psychiatry at the University of Toronto.
Dr. Lanctôt and colleagues investigated the potential benefits of nabilone for adults with moderate-to-severe Alzheimer’s dementia and clinically significant agitation. During the 14-week trial, 39 participants (77% male; average age, 87) received nabilone in capsule form (mean therapeutic dose, 1.6 mg) for six weeks, followed by one week without treatment and six weeks of placebo. In addition to measuring agitation, the researchers assessed overall behavioral symptoms, memory, physical changes, and safety.
Dr. Lanctôt’s group found that agitation improved significantly when participants were taking nabilone, compared with when they were receiving placebo, as measured by the Cohen-Mansfield Agitation Inventory. Nabilone also significantly improved overall behavioral symptoms, compared with placebo, as measured by the Neuropsychiatric Inventory.
In addition, the researchers observed small benefits in cognition and nutrition when participants received nabilone during the study. More people in the study experienced sedation when taking nabilone (45%) than when taking placebo (16%).
“Currently prescribed treatments for agitation in Alzheimer’s disease do not work in everybody. And when they do work, the effect is small, and they increase the risk of harmful side effects, including increased risk of death. As a result, there is an urgent need for safer medication options,” said Dr. Lanctôt. “These findings suggest that nabilone may be an effective treatment for agitation; however, the risk of sedation must be carefully monitored. A larger clinical trial would allow us to confirm our findings regarding how effective and safe nabilone is in the treatment of agitation for Alzheimer’s disease.”The FDA has not approved marijuana for the treatment or management of Alzheimer’s disease or other dementias. The use of marijuana as medical treatment is increasingly common, but much about the drug’s use in people with Alzheimer’s disease or other dementias is unknown. No robust, consistent clinical trial data support marijuana for the treatment of Alzheimer’s disease dementia or for related issues.
CHICAGO—Nabilone, a synthetic cannabinoid, may effectively treat agitation in people with Alzheimer’s disease, according to a randomized, double-blind clinical trial presented at AAIC 2018.
“Agitation, including verbal or physical outbursts, general emotional distress, restlessness, and pacing, is one of the most common behavioral changes associated with Alzheimer’s disease as it progresses and can be a significant cause of caregiver stress,” said Krista L. Lanctôt, PhD, Senior Scientist at Sunnybrook Health Sciences Centre and Professor of Pharmacology and Psychiatry at the University of Toronto.
Dr. Lanctôt and colleagues investigated the potential benefits of nabilone for adults with moderate-to-severe Alzheimer’s dementia and clinically significant agitation. During the 14-week trial, 39 participants (77% male; average age, 87) received nabilone in capsule form (mean therapeutic dose, 1.6 mg) for six weeks, followed by one week without treatment and six weeks of placebo. In addition to measuring agitation, the researchers assessed overall behavioral symptoms, memory, physical changes, and safety.
Dr. Lanctôt’s group found that agitation improved significantly when participants were taking nabilone, compared with when they were receiving placebo, as measured by the Cohen-Mansfield Agitation Inventory. Nabilone also significantly improved overall behavioral symptoms, compared with placebo, as measured by the Neuropsychiatric Inventory.
In addition, the researchers observed small benefits in cognition and nutrition when participants received nabilone during the study. More people in the study experienced sedation when taking nabilone (45%) than when taking placebo (16%).
“Currently prescribed treatments for agitation in Alzheimer’s disease do not work in everybody. And when they do work, the effect is small, and they increase the risk of harmful side effects, including increased risk of death. As a result, there is an urgent need for safer medication options,” said Dr. Lanctôt. “These findings suggest that nabilone may be an effective treatment for agitation; however, the risk of sedation must be carefully monitored. A larger clinical trial would allow us to confirm our findings regarding how effective and safe nabilone is in the treatment of agitation for Alzheimer’s disease.”The FDA has not approved marijuana for the treatment or management of Alzheimer’s disease or other dementias. The use of marijuana as medical treatment is increasingly common, but much about the drug’s use in people with Alzheimer’s disease or other dementias is unknown. No robust, consistent clinical trial data support marijuana for the treatment of Alzheimer’s disease dementia or for related issues.
Pregnancy and Years of Reproductive Capability Are Associated With Dementia Risk
Miscarriages and age at menarche and menopause may influence the likelihood of dementia.
CHICAGO—More pregnancies and a longer span of reproductive years appear to protect women against dementia, according to a study presented at AAIC 2018. The results suggest that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study by Paola Gilsanz, ScD, staff scientist at Kaiser Permanente in Oakland, California, and colleagues included more than 14,500 women and 50 years of follow-up data. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Reproductive Years
The researchers analyzed data from a cohort of 14,595 women in the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup between 1964 and 1973 when they were ages 40 to 55. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the women in the group (68%) were white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996 to 2017, when the women were ages 62 to 86.
A multivariate regression model controlled for age, race, education, midlife health issues (eg, hypertension, smoking, and BMI), hysterectomy, and late-life health issues (eg, stroke, heart failure, and diabetes).
Half of the cohort had at least three children, and 75% had at least one miscarriage. The average age at menarche was 13, and the average age at last natural menstrual period was 47. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child. The association remained significant even after researchers controlled for age, race, education, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who did not report a miscarriage were 20% less likely to develop dementia than were those who had experienced at least one miscarriage. The benefit of no miscarriage was greater among women with at least three children, conferring a 28% reduced risk.
A shorter reproductive period increased the risk of dementia. Those who experienced menarche at age 16 or older had a 31% increased risk of dementia, and those who experienced their last period at age 45 or younger had a 28% greater risk. Each additional year of reproductive capability was associated with a 2% decreased risk.
Women with between 21 and 30 reproductive years were 33% more likely to develop dementia than were those with longer reproductive periods.
Renewed Interest
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period, may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieus that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
Researchers are exploring the link between hormones and cognition with renewed interest, said Suzanne Craft, PhD, of Wake Forest University in Winston-Salem, North Carolina, who moderated a press briefing on the topic. The Women’s Heath Initiative study had a chilling effect on funding for this area of research, Dr. Craft said. “But now I think the pendulum is slowly moving back” toward supporting investigations of hormones and cognition. “It’s clear that something is going on, that there is a link. I am glad we are starting to explore this again.”
—Michele G. Sullivan
Miscarriages and age at menarche and menopause may influence the likelihood of dementia.
Miscarriages and age at menarche and menopause may influence the likelihood of dementia.
CHICAGO—More pregnancies and a longer span of reproductive years appear to protect women against dementia, according to a study presented at AAIC 2018. The results suggest that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study by Paola Gilsanz, ScD, staff scientist at Kaiser Permanente in Oakland, California, and colleagues included more than 14,500 women and 50 years of follow-up data. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Reproductive Years
The researchers analyzed data from a cohort of 14,595 women in the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup between 1964 and 1973 when they were ages 40 to 55. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the women in the group (68%) were white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996 to 2017, when the women were ages 62 to 86.
A multivariate regression model controlled for age, race, education, midlife health issues (eg, hypertension, smoking, and BMI), hysterectomy, and late-life health issues (eg, stroke, heart failure, and diabetes).
Half of the cohort had at least three children, and 75% had at least one miscarriage. The average age at menarche was 13, and the average age at last natural menstrual period was 47. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child. The association remained significant even after researchers controlled for age, race, education, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who did not report a miscarriage were 20% less likely to develop dementia than were those who had experienced at least one miscarriage. The benefit of no miscarriage was greater among women with at least three children, conferring a 28% reduced risk.
A shorter reproductive period increased the risk of dementia. Those who experienced menarche at age 16 or older had a 31% increased risk of dementia, and those who experienced their last period at age 45 or younger had a 28% greater risk. Each additional year of reproductive capability was associated with a 2% decreased risk.
Women with between 21 and 30 reproductive years were 33% more likely to develop dementia than were those with longer reproductive periods.
Renewed Interest
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period, may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieus that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
Researchers are exploring the link between hormones and cognition with renewed interest, said Suzanne Craft, PhD, of Wake Forest University in Winston-Salem, North Carolina, who moderated a press briefing on the topic. The Women’s Heath Initiative study had a chilling effect on funding for this area of research, Dr. Craft said. “But now I think the pendulum is slowly moving back” toward supporting investigations of hormones and cognition. “It’s clear that something is going on, that there is a link. I am glad we are starting to explore this again.”
—Michele G. Sullivan
CHICAGO—More pregnancies and a longer span of reproductive years appear to protect women against dementia, according to a study presented at AAIC 2018. The results suggest that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study by Paola Gilsanz, ScD, staff scientist at Kaiser Permanente in Oakland, California, and colleagues included more than 14,500 women and 50 years of follow-up data. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Reproductive Years
The researchers analyzed data from a cohort of 14,595 women in the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup between 1964 and 1973 when they were ages 40 to 55. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the women in the group (68%) were white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996 to 2017, when the women were ages 62 to 86.
A multivariate regression model controlled for age, race, education, midlife health issues (eg, hypertension, smoking, and BMI), hysterectomy, and late-life health issues (eg, stroke, heart failure, and diabetes).
Half of the cohort had at least three children, and 75% had at least one miscarriage. The average age at menarche was 13, and the average age at last natural menstrual period was 47. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child. The association remained significant even after researchers controlled for age, race, education, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who did not report a miscarriage were 20% less likely to develop dementia than were those who had experienced at least one miscarriage. The benefit of no miscarriage was greater among women with at least three children, conferring a 28% reduced risk.
A shorter reproductive period increased the risk of dementia. Those who experienced menarche at age 16 or older had a 31% increased risk of dementia, and those who experienced their last period at age 45 or younger had a 28% greater risk. Each additional year of reproductive capability was associated with a 2% decreased risk.
Women with between 21 and 30 reproductive years were 33% more likely to develop dementia than were those with longer reproductive periods.
Renewed Interest
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period, may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieus that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
Researchers are exploring the link between hormones and cognition with renewed interest, said Suzanne Craft, PhD, of Wake Forest University in Winston-Salem, North Carolina, who moderated a press briefing on the topic. The Women’s Heath Initiative study had a chilling effect on funding for this area of research, Dr. Craft said. “But now I think the pendulum is slowly moving back” toward supporting investigations of hormones and cognition. “It’s clear that something is going on, that there is a link. I am glad we are starting to explore this again.”
—Michele G. Sullivan
Quality and safety of hospital care from the patient perspective
Background: Delivery of high-quality, safe care is key to earning the trust and confidence of patients. Patients can be a valuable asset in determining and evaluating the quality and safety of the care they receive. Collectively analyzing patient perceptions remains a challenge.
Study design: Multicenter, wait-list design, cluster-randomized controlled trial.
Setting: Five National Health Service Trusts Hospital sites in the north of England.
Synopsis: Data were collected via validated survey of inpatients; 1,155 patient incident reports were gathered from 579 patients. Patient volunteers were trained to group these reports into 14 categories. Next, clinical researchers and physicians independently reviewed all reports for presence of a patient safety incident (PSI) using a previously determined consensus definition.
One in 10 patients identified a PSI. There was variability in classifying incidents as PSIs in some categories. Of the concerns expressed by patients, 65% were not classified as PSI. Limitations included a focus on patient’s concerns rather than safety, PSI estimates based on patient’s feedback without clinical information, and lack of inter-rater reliability estimates.
Bottom line: Effective translation of patient experience can provide valuable insights about safety and quality of care in hospital.
Citation: O’Hara JK et al. What can patients tell us about the quality and safety of hospital care? Findings from a UK multicentre survey study. BMJ Qual Saf. 2018 Mar 15. doi: 10.1136/bmjqs-2017-006974.
Dr. Chikkanna is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Delivery of high-quality, safe care is key to earning the trust and confidence of patients. Patients can be a valuable asset in determining and evaluating the quality and safety of the care they receive. Collectively analyzing patient perceptions remains a challenge.
Study design: Multicenter, wait-list design, cluster-randomized controlled trial.
Setting: Five National Health Service Trusts Hospital sites in the north of England.
Synopsis: Data were collected via validated survey of inpatients; 1,155 patient incident reports were gathered from 579 patients. Patient volunteers were trained to group these reports into 14 categories. Next, clinical researchers and physicians independently reviewed all reports for presence of a patient safety incident (PSI) using a previously determined consensus definition.
One in 10 patients identified a PSI. There was variability in classifying incidents as PSIs in some categories. Of the concerns expressed by patients, 65% were not classified as PSI. Limitations included a focus on patient’s concerns rather than safety, PSI estimates based on patient’s feedback without clinical information, and lack of inter-rater reliability estimates.
Bottom line: Effective translation of patient experience can provide valuable insights about safety and quality of care in hospital.
Citation: O’Hara JK et al. What can patients tell us about the quality and safety of hospital care? Findings from a UK multicentre survey study. BMJ Qual Saf. 2018 Mar 15. doi: 10.1136/bmjqs-2017-006974.
Dr. Chikkanna is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Background: Delivery of high-quality, safe care is key to earning the trust and confidence of patients. Patients can be a valuable asset in determining and evaluating the quality and safety of the care they receive. Collectively analyzing patient perceptions remains a challenge.
Study design: Multicenter, wait-list design, cluster-randomized controlled trial.
Setting: Five National Health Service Trusts Hospital sites in the north of England.
Synopsis: Data were collected via validated survey of inpatients; 1,155 patient incident reports were gathered from 579 patients. Patient volunteers were trained to group these reports into 14 categories. Next, clinical researchers and physicians independently reviewed all reports for presence of a patient safety incident (PSI) using a previously determined consensus definition.
One in 10 patients identified a PSI. There was variability in classifying incidents as PSIs in some categories. Of the concerns expressed by patients, 65% were not classified as PSI. Limitations included a focus on patient’s concerns rather than safety, PSI estimates based on patient’s feedback without clinical information, and lack of inter-rater reliability estimates.
Bottom line: Effective translation of patient experience can provide valuable insights about safety and quality of care in hospital.
Citation: O’Hara JK et al. What can patients tell us about the quality and safety of hospital care? Findings from a UK multicentre survey study. BMJ Qual Saf. 2018 Mar 15. doi: 10.1136/bmjqs-2017-006974.
Dr. Chikkanna is an assistant professor in the division of hospital medicine at the University of Kentucky, Lexington.
Does BAN2401 Benefit Patients With Alzheimer’s Disease?
The study arms did not contain comparable numbers of patients who carried APOE4.
CHICAGO—BAN2401, a monoclonal antibody that targets soluble amyloid-beta oligomers, slows cognitive decline by as much as 47% and clears brain amyloid in 81% of patients with mild cognitive impairment (MCI) and very mild Alzheimer’s disease, according to the results of a phase II study presented at AAIC 2018.
Imbalanced Treatment Groups
The treatment groups were not well balanced in at least one respect, however, which could influence the interpretation of the data. APOE4-positive patients were unequally distributed through the six treatment groups, which included BAN2401 (2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly) and placebo. This imbalance could have biased cognitive results in the antibody’s favor. APOE4 carriers represented between 70% and 80% of every unsuccessful treatment arm in the trial and approximately 29% of the arm that enjoyed significant cognitive benefits.
“This is a big confound,” said Keith Fargo, PhD, Director of Scientific Programs and Outreach at the Alzheimer’s Association. “According to the trial sponsors, … a regulatory body requested that people with the APOE4 Alzheimer’s risk gene not be included in the highest dose group for safety reasons. Because of this [request], the people in the highest-dose group are different from people in the other groups on an important dimension: they were much less likely to have the APOE4 gene, which is known to be a major risk factor for cognitive decline. So, it is plausible that the people on the highest dose declined differently due to genetic differences, rather than due to being on the highest dose. A planned subgroup analysis will shed more light on this [question] and [on] whether it reduces confidence in the overall findings.”
Regulators Influenced the Trial
Eisai, which is headquartered in Tokyo, and Biogen, which is based in Cambridge, Massachusetts, are developing the antibody. The decision to restructure the randomization was not Eisai’s, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“European regulators did not allow randomization of [some] APOE4 carriers to the highest dose,” he said in an interview. “I ultimately don’t know what it would do to the results except make them even more difficult to justify as sufficient for registration. In general, in symptomatic Alzheimer’s dementia patients, APOE4 carriage has no substantial impact on rate of decline, but whether [APOE4] status interacted with the treatment is of course completely unknown. Bottom line: just another feature that makes this a phase II study that needs to be followed by a phase III study with a simple design using the high dose.”
“Regardless of who made the decision and why, the data is what it is, and the question remains,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas in Lawrence. “Given the numbers of E4-positive [patients] versus E4-negative [patients] for each treatment group, the interpretation of the results is now seriously thrown into question. The one group that showed a clear slowing of cognitive decline versus placebo—10 mg/kg biweekly—also has far fewer E4-positive [patients] versus E4-negative [patients]. This difference in proportion of E4-positive [patients] could be a major factor in the apparent reduced rate of cognitive decline and confounds the ability to tell if the 10-mg/kg biweekly dose is effective. In contrast, the 10-mg/kg monthly dose group has an E4-positive to E4-negative ratio more comparable to that of placebo, and the effect of the drug on cognitive decline under this dosing regimen is not clearly distinguishable from that seen with placebo.”
Treatment Reduced Amyloid Levels
In addition, BAN2401 failed to meet its 12-month prespecified primary cognitive end points. The investigators reached this conclusion through Bayesian analysis that strove to predict an 80% probability of achieving at least a 25% cognitive benefit. In December 2017, Eisai announced that the treatment had reached 64% probability. Because the company was optimistic about the treatment’s success, it continued with the additional six months of treatment, as the study design allowed, and reanalyzed results with a simpler and more straightforward method. This analysis concluded that the 10-mg/kg biweekly dose slowed decline on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, by 30%. It also found that treatment slowed decline on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) by 47%. Treatment cleared brain amyloid in 81% of subjects and had positive effects on CSF biomarkers. CSF amyloid levels increased, and CSF total tau levels decreased, which indicated decreased neuronal injury.
—Michele G. Sullivan
The study arms did not contain comparable numbers of patients who carried APOE4.
The study arms did not contain comparable numbers of patients who carried APOE4.
CHICAGO—BAN2401, a monoclonal antibody that targets soluble amyloid-beta oligomers, slows cognitive decline by as much as 47% and clears brain amyloid in 81% of patients with mild cognitive impairment (MCI) and very mild Alzheimer’s disease, according to the results of a phase II study presented at AAIC 2018.
Imbalanced Treatment Groups
The treatment groups were not well balanced in at least one respect, however, which could influence the interpretation of the data. APOE4-positive patients were unequally distributed through the six treatment groups, which included BAN2401 (2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly) and placebo. This imbalance could have biased cognitive results in the antibody’s favor. APOE4 carriers represented between 70% and 80% of every unsuccessful treatment arm in the trial and approximately 29% of the arm that enjoyed significant cognitive benefits.
“This is a big confound,” said Keith Fargo, PhD, Director of Scientific Programs and Outreach at the Alzheimer’s Association. “According to the trial sponsors, … a regulatory body requested that people with the APOE4 Alzheimer’s risk gene not be included in the highest dose group for safety reasons. Because of this [request], the people in the highest-dose group are different from people in the other groups on an important dimension: they were much less likely to have the APOE4 gene, which is known to be a major risk factor for cognitive decline. So, it is plausible that the people on the highest dose declined differently due to genetic differences, rather than due to being on the highest dose. A planned subgroup analysis will shed more light on this [question] and [on] whether it reduces confidence in the overall findings.”
Regulators Influenced the Trial
Eisai, which is headquartered in Tokyo, and Biogen, which is based in Cambridge, Massachusetts, are developing the antibody. The decision to restructure the randomization was not Eisai’s, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“European regulators did not allow randomization of [some] APOE4 carriers to the highest dose,” he said in an interview. “I ultimately don’t know what it would do to the results except make them even more difficult to justify as sufficient for registration. In general, in symptomatic Alzheimer’s dementia patients, APOE4 carriage has no substantial impact on rate of decline, but whether [APOE4] status interacted with the treatment is of course completely unknown. Bottom line: just another feature that makes this a phase II study that needs to be followed by a phase III study with a simple design using the high dose.”
“Regardless of who made the decision and why, the data is what it is, and the question remains,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas in Lawrence. “Given the numbers of E4-positive [patients] versus E4-negative [patients] for each treatment group, the interpretation of the results is now seriously thrown into question. The one group that showed a clear slowing of cognitive decline versus placebo—10 mg/kg biweekly—also has far fewer E4-positive [patients] versus E4-negative [patients]. This difference in proportion of E4-positive [patients] could be a major factor in the apparent reduced rate of cognitive decline and confounds the ability to tell if the 10-mg/kg biweekly dose is effective. In contrast, the 10-mg/kg monthly dose group has an E4-positive to E4-negative ratio more comparable to that of placebo, and the effect of the drug on cognitive decline under this dosing regimen is not clearly distinguishable from that seen with placebo.”
Treatment Reduced Amyloid Levels
In addition, BAN2401 failed to meet its 12-month prespecified primary cognitive end points. The investigators reached this conclusion through Bayesian analysis that strove to predict an 80% probability of achieving at least a 25% cognitive benefit. In December 2017, Eisai announced that the treatment had reached 64% probability. Because the company was optimistic about the treatment’s success, it continued with the additional six months of treatment, as the study design allowed, and reanalyzed results with a simpler and more straightforward method. This analysis concluded that the 10-mg/kg biweekly dose slowed decline on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, by 30%. It also found that treatment slowed decline on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) by 47%. Treatment cleared brain amyloid in 81% of subjects and had positive effects on CSF biomarkers. CSF amyloid levels increased, and CSF total tau levels decreased, which indicated decreased neuronal injury.
—Michele G. Sullivan
CHICAGO—BAN2401, a monoclonal antibody that targets soluble amyloid-beta oligomers, slows cognitive decline by as much as 47% and clears brain amyloid in 81% of patients with mild cognitive impairment (MCI) and very mild Alzheimer’s disease, according to the results of a phase II study presented at AAIC 2018.
Imbalanced Treatment Groups
The treatment groups were not well balanced in at least one respect, however, which could influence the interpretation of the data. APOE4-positive patients were unequally distributed through the six treatment groups, which included BAN2401 (2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly) and placebo. This imbalance could have biased cognitive results in the antibody’s favor. APOE4 carriers represented between 70% and 80% of every unsuccessful treatment arm in the trial and approximately 29% of the arm that enjoyed significant cognitive benefits.
“This is a big confound,” said Keith Fargo, PhD, Director of Scientific Programs and Outreach at the Alzheimer’s Association. “According to the trial sponsors, … a regulatory body requested that people with the APOE4 Alzheimer’s risk gene not be included in the highest dose group for safety reasons. Because of this [request], the people in the highest-dose group are different from people in the other groups on an important dimension: they were much less likely to have the APOE4 gene, which is known to be a major risk factor for cognitive decline. So, it is plausible that the people on the highest dose declined differently due to genetic differences, rather than due to being on the highest dose. A planned subgroup analysis will shed more light on this [question] and [on] whether it reduces confidence in the overall findings.”
Regulators Influenced the Trial
Eisai, which is headquartered in Tokyo, and Biogen, which is based in Cambridge, Massachusetts, are developing the antibody. The decision to restructure the randomization was not Eisai’s, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“European regulators did not allow randomization of [some] APOE4 carriers to the highest dose,” he said in an interview. “I ultimately don’t know what it would do to the results except make them even more difficult to justify as sufficient for registration. In general, in symptomatic Alzheimer’s dementia patients, APOE4 carriage has no substantial impact on rate of decline, but whether [APOE4] status interacted with the treatment is of course completely unknown. Bottom line: just another feature that makes this a phase II study that needs to be followed by a phase III study with a simple design using the high dose.”
“Regardless of who made the decision and why, the data is what it is, and the question remains,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas in Lawrence. “Given the numbers of E4-positive [patients] versus E4-negative [patients] for each treatment group, the interpretation of the results is now seriously thrown into question. The one group that showed a clear slowing of cognitive decline versus placebo—10 mg/kg biweekly—also has far fewer E4-positive [patients] versus E4-negative [patients]. This difference in proportion of E4-positive [patients] could be a major factor in the apparent reduced rate of cognitive decline and confounds the ability to tell if the 10-mg/kg biweekly dose is effective. In contrast, the 10-mg/kg monthly dose group has an E4-positive to E4-negative ratio more comparable to that of placebo, and the effect of the drug on cognitive decline under this dosing regimen is not clearly distinguishable from that seen with placebo.”
Treatment Reduced Amyloid Levels
In addition, BAN2401 failed to meet its 12-month prespecified primary cognitive end points. The investigators reached this conclusion through Bayesian analysis that strove to predict an 80% probability of achieving at least a 25% cognitive benefit. In December 2017, Eisai announced that the treatment had reached 64% probability. Because the company was optimistic about the treatment’s success, it continued with the additional six months of treatment, as the study design allowed, and reanalyzed results with a simpler and more straightforward method. This analysis concluded that the 10-mg/kg biweekly dose slowed decline on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, by 30%. It also found that treatment slowed decline on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) by 47%. Treatment cleared brain amyloid in 81% of subjects and had positive effects on CSF biomarkers. CSF amyloid levels increased, and CSF total tau levels decreased, which indicated decreased neuronal injury.
—Michele G. Sullivan
Are Nonbenzodiazepines Appropriate for Treating Sleep Disturbance in Dementia?
A review of research and hospital data indicates that this drug class increases the risk of fractures.
CHICAGO—Nonbenzodiazepine hypnotic “Z-drugs” (eg, zolpidem, zopiclone, and zaleplon) increase the risk of fractures in a dose-dependent manner in people with dementia, according to research presented at AAIC 2018. Patients with dementia who are receiving these drugs should be monitored, according to the researchers.
Approximately 60% of people with dementia have sleep disturbance. Z-drugs are often prescribed to help treat insomnia in older adults, but observers have raised concerns that these treatments may cause problems such as falls and fractures and increase confusion. Researchers have not fully investigated the safety and efficacy of Z-drugs in this patient population, however.
Chris Fox, MD, Professor of Psychiatry at Norwich Medical School at the University of East Anglia in the United Kingdom, and colleagues analyzed cohort studies using primary care data from the UK Clinical Practice Research Datalink that was linked to hospital admissions data. They also examined data from three clinical studies of people with dementia. To evaluate the benefits and harms of these medicines, the researchers compared data for 2,952 people with dementia who were newly prescribed Z-drugs with data for 1,651 people who were not prescribed sedatives or hypnotics.
Dr. Fox and colleagues defined the index date as the first date of a diagnosis of sleep disturbance or of a Z-drug prescription after dementia diagnosis. They excluded patients who had been prescribed a sedative during the previous year. Patients were followed for as long as two years or until 90 days after their last prescription. Dr. Fox’s group compared the two arms’ outcomes using Cox regression. They adjusted the data for sociodemographic variables, BMI, systolic blood pressure, diagnosed health conditions, and comedications.
The use of Z-drugs was associated with a 47% increased risk of any type of fracture. The risk increased among patients on higher doses. Z-drug use was also associated with greater risks of hip fracture and mortality. The study did not identify a higher risk of other events
“Fractures in people with dementia can have a devastating impact, including loss of mobility, increased dependency, and worsening dementia,” said Dr. Fox. “We desperately need better alternatives to the drugs currently being prescribed for sleep problems and other noncognitive symptoms of dementia. Wherever possible, suitable nonpharmacologic alternatives should be considered, and where Z-drugs are prescribed, patients should receive care that reduces or prevents the occurrence of falls.”
A review of research and hospital data indicates that this drug class increases the risk of fractures.
A review of research and hospital data indicates that this drug class increases the risk of fractures.
CHICAGO—Nonbenzodiazepine hypnotic “Z-drugs” (eg, zolpidem, zopiclone, and zaleplon) increase the risk of fractures in a dose-dependent manner in people with dementia, according to research presented at AAIC 2018. Patients with dementia who are receiving these drugs should be monitored, according to the researchers.
Approximately 60% of people with dementia have sleep disturbance. Z-drugs are often prescribed to help treat insomnia in older adults, but observers have raised concerns that these treatments may cause problems such as falls and fractures and increase confusion. Researchers have not fully investigated the safety and efficacy of Z-drugs in this patient population, however.
Chris Fox, MD, Professor of Psychiatry at Norwich Medical School at the University of East Anglia in the United Kingdom, and colleagues analyzed cohort studies using primary care data from the UK Clinical Practice Research Datalink that was linked to hospital admissions data. They also examined data from three clinical studies of people with dementia. To evaluate the benefits and harms of these medicines, the researchers compared data for 2,952 people with dementia who were newly prescribed Z-drugs with data for 1,651 people who were not prescribed sedatives or hypnotics.
Dr. Fox and colleagues defined the index date as the first date of a diagnosis of sleep disturbance or of a Z-drug prescription after dementia diagnosis. They excluded patients who had been prescribed a sedative during the previous year. Patients were followed for as long as two years or until 90 days after their last prescription. Dr. Fox’s group compared the two arms’ outcomes using Cox regression. They adjusted the data for sociodemographic variables, BMI, systolic blood pressure, diagnosed health conditions, and comedications.
The use of Z-drugs was associated with a 47% increased risk of any type of fracture. The risk increased among patients on higher doses. Z-drug use was also associated with greater risks of hip fracture and mortality. The study did not identify a higher risk of other events
“Fractures in people with dementia can have a devastating impact, including loss of mobility, increased dependency, and worsening dementia,” said Dr. Fox. “We desperately need better alternatives to the drugs currently being prescribed for sleep problems and other noncognitive symptoms of dementia. Wherever possible, suitable nonpharmacologic alternatives should be considered, and where Z-drugs are prescribed, patients should receive care that reduces or prevents the occurrence of falls.”
CHICAGO—Nonbenzodiazepine hypnotic “Z-drugs” (eg, zolpidem, zopiclone, and zaleplon) increase the risk of fractures in a dose-dependent manner in people with dementia, according to research presented at AAIC 2018. Patients with dementia who are receiving these drugs should be monitored, according to the researchers.
Approximately 60% of people with dementia have sleep disturbance. Z-drugs are often prescribed to help treat insomnia in older adults, but observers have raised concerns that these treatments may cause problems such as falls and fractures and increase confusion. Researchers have not fully investigated the safety and efficacy of Z-drugs in this patient population, however.
Chris Fox, MD, Professor of Psychiatry at Norwich Medical School at the University of East Anglia in the United Kingdom, and colleagues analyzed cohort studies using primary care data from the UK Clinical Practice Research Datalink that was linked to hospital admissions data. They also examined data from three clinical studies of people with dementia. To evaluate the benefits and harms of these medicines, the researchers compared data for 2,952 people with dementia who were newly prescribed Z-drugs with data for 1,651 people who were not prescribed sedatives or hypnotics.
Dr. Fox and colleagues defined the index date as the first date of a diagnosis of sleep disturbance or of a Z-drug prescription after dementia diagnosis. They excluded patients who had been prescribed a sedative during the previous year. Patients were followed for as long as two years or until 90 days after their last prescription. Dr. Fox’s group compared the two arms’ outcomes using Cox regression. They adjusted the data for sociodemographic variables, BMI, systolic blood pressure, diagnosed health conditions, and comedications.
The use of Z-drugs was associated with a 47% increased risk of any type of fracture. The risk increased among patients on higher doses. Z-drug use was also associated with greater risks of hip fracture and mortality. The study did not identify a higher risk of other events
“Fractures in people with dementia can have a devastating impact, including loss of mobility, increased dependency, and worsening dementia,” said Dr. Fox. “We desperately need better alternatives to the drugs currently being prescribed for sleep problems and other noncognitive symptoms of dementia. Wherever possible, suitable nonpharmacologic alternatives should be considered, and where Z-drugs are prescribed, patients should receive care that reduces or prevents the occurrence of falls.”
Laser tattoo removal techniques continue to be refined
SAN DIEGO –
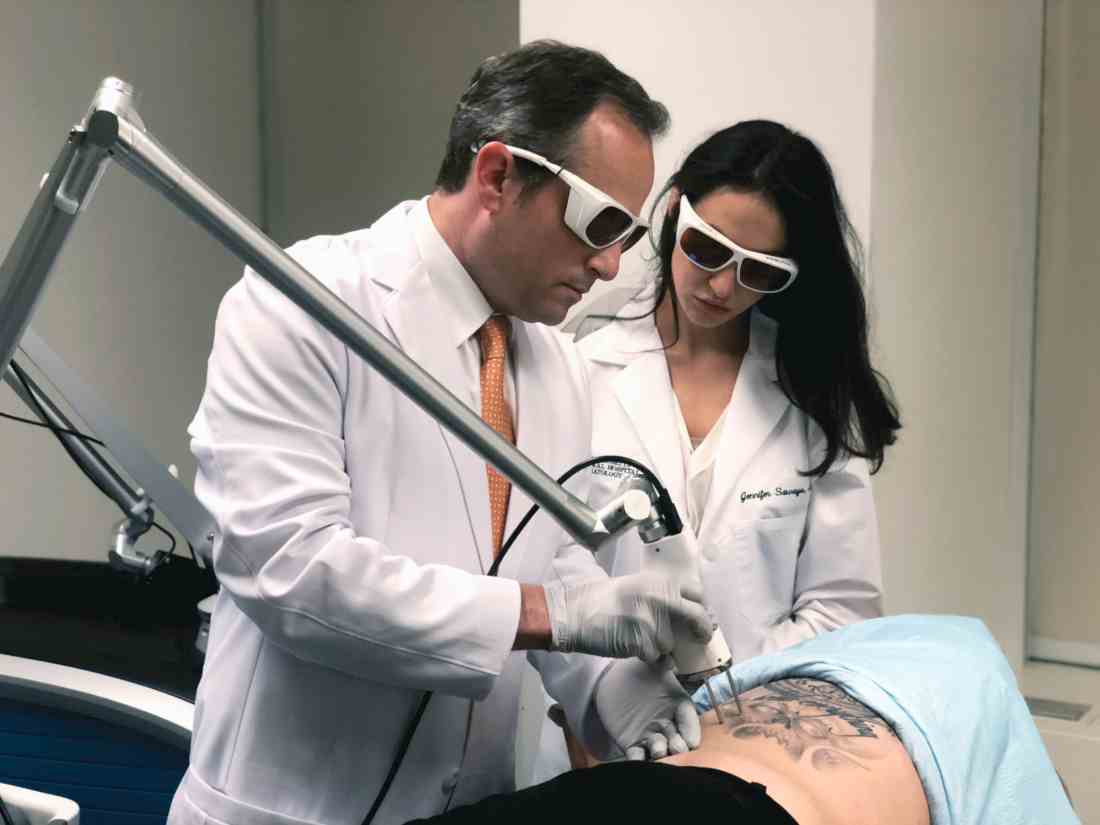
“A picosecond is to a second as 1 second is to 37,000 years,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “That’s equivalent to the total energy of the city of San Diego for 300-750 trillionths of a second.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, picosecond lasers produce extreme cavitation and cell rupture, with a desired clinical endpoint of immediate dermal whitening of tattooed skin. The process causes transdermal elimination of the tattoo ink. Some of the ink flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Commercially available picosecond lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 picoseconds. Nd:YAG lasers work best for red and black ink, while alexandrite lasers work best for green and blue ink. In Dr. Avram’s experience, picosecond lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There is some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos, but the devices are more expensive,” he said.
Dr. Avram, who is also the faculty director for laser and cosmetic dermatology training at Harvard Medical School, also in Boston, advises against promising a certain number of laser treatments during initial patient consultations. “You will regret it,” he said. “Tattoos are notoriously unpredictable in how they respond. I often hear people say they get rid of these in three to five treatments. That isn’t my experience with these lasers. Often, all you’re going to be able to do is get significant clearing rather than tattoo removal. Professional tattoos are the most difficult to treat because they are the deepest and they have the most amount of ink.”
On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos require far fewer treatments. “The color is important,” he said. “Multicolored tattoos, regardless of the colors, are always going to be more difficult to clear than a single-color tattoo.” Black and dark-blue tattoos respond best to laser light; light-blue and green also respond well. Red responds well, while purple can be challenging. Yellow and orange do not respond very well, but they do respond partially.
According to a trial that analyzed variables influencing the outcome of tattoos treated by Q-switched lasers, 47% were cleared after 10 sessions, while 75% were cleared after 15 sessions (Arch Dermatol 2012;148[12]:1364-9). “It’s very important to message to your patients how many treatments this might take, because there is going to be an annuity of patients who are unhappy because they have to keep coming back,” said Dr. Avram, who is the immediate past president of the American Society for Laser Medicine and Surgery. Skin type and pigmentation also affect treatment outcomes. “For darker skin types or tanned individuals, hyper- or hypopigmentation is a greater concern than in patients with lighter skin types,” he said. “A test spot may be beneficial. The 1,064-nm Q-switched Nd:YAG laser is least likely to affect skin pigment; it’s safest for skin types IV-VI . This is great if it’s a black tattoo. But if it’s a green, blue, or red tattoo, you have a problem because you’re not going to target it very effectively.”
Some degree of posttreatment hypopigmentation is likely to occur, regardless of skin type. “Let patients know this is going to happen, but over time, this usually resolves, because you’re not destroying the melanocytes, unless you’re going too strong,” Dr. Avram said. “It may take a few months. It may take a year or 2, but the pigment should recur.”
He emphasized that the key variable during laser treatment of tattoos is the clinical endpoint, not the energy setting of the device. “What you want to see is immediate whitening of the treated area,” he said. “With the 1,064-nm Nd:YAG, you may get a little pinpoint bleeding in addition to whitening. Do not memorize treatment settings. Many Q-switched lasers are not externally calibrated. Thus, energy levels may change day to day or before and after servicing [of the device]. Trust your eyes; trust your clinical skills.” If you see epidermal disruption and bleeding during treatment, you’re probably being too aggressive. If that happens, “decrease your fluence,” he recommended. “You also want to decrease fluences when treating tattoos that are placed over other tattoos.”
Another rule of thumb is to use larger spot sizes during treatment sessions. “The larger the spot size, the more efficient the energy is going to get more deeply, and less is going to be at the dermal-epidermal junction,” Dr. Avram said. “So you’re going to get less hypopigmentation and less hyperpigmentation. Follow your endpoints and you are less likely to get pigmentation changes.”
Posttreatment care typically includes the application of topical petroleum jelly and a Telfa dressing. “Wait about a week to heal, counsel patients to keep out of the sun, and avoid friction to the treated area during healing,” he said. Patients can be rescheduled for retreatment 6-8 weeks later.
Common adverse events during laser treatment of tattoos include erythema, blistering, hyperpigmentation, hypopigmentation, and scarring, which occurs in about 5% of cases. Less common adverse events include allergic reaction, darkening of cosmetic tattoos, immune reaction, and chrysiasis, which is a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold salt ingestion. “Any history of gold salt ingestion will produce this characteristic finding, even if they took it when they were 5 years old and they come to you when they’re 85,” Dr. Avram said. “All of our intake forms include a question about this, and before I treat patients I always ask if they have a history of gold ingestion, because it’s very difficult to treat.”
Surgical excision may be an alternative for smaller tattoos. “Another option is ablative fractional resurfacing as a solo treatment or in combination with the Q-switched or picosecond laser, which has better efficacy,” he said. “The ablative fractional laser also may help with fibrosis after multiple treatments in a recalcitrant tattoo.” He noted that cosmetic tattoos such as lip liner and blush tattoos might darken because of oxidation of ferric oxide or titanium oxide pigment. The best approach to such cases is to perform an inconspicuous test spot prior to treatment.
Clinicians continue to explore the optimal interval between treatments. For example, the “R20” method consists of four consecutive treatment passes separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol 2012;66[2]:271-7). A follow-up study by Dr. Avram and his colleagues contradicted these findings, while another follow-up study was supportive.
Another technology playing a role in such repeat treatments is a perfluorodecalin-infused silicone patch, which is placed over the treatment area. According to Dr. Avram, the FDA-cleared patch helps reduce scatter during treatment and likely improves efficacy. It also allows for performing consecutive repeat laser treatments at the same visit. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side treated without the patch (Laser Surg Med 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
SAN DIEGO –

“A picosecond is to a second as 1 second is to 37,000 years,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “That’s equivalent to the total energy of the city of San Diego for 300-750 trillionths of a second.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, picosecond lasers produce extreme cavitation and cell rupture, with a desired clinical endpoint of immediate dermal whitening of tattooed skin. The process causes transdermal elimination of the tattoo ink. Some of the ink flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Commercially available picosecond lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 picoseconds. Nd:YAG lasers work best for red and black ink, while alexandrite lasers work best for green and blue ink. In Dr. Avram’s experience, picosecond lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There is some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos, but the devices are more expensive,” he said.
Dr. Avram, who is also the faculty director for laser and cosmetic dermatology training at Harvard Medical School, also in Boston, advises against promising a certain number of laser treatments during initial patient consultations. “You will regret it,” he said. “Tattoos are notoriously unpredictable in how they respond. I often hear people say they get rid of these in three to five treatments. That isn’t my experience with these lasers. Often, all you’re going to be able to do is get significant clearing rather than tattoo removal. Professional tattoos are the most difficult to treat because they are the deepest and they have the most amount of ink.”
On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos require far fewer treatments. “The color is important,” he said. “Multicolored tattoos, regardless of the colors, are always going to be more difficult to clear than a single-color tattoo.” Black and dark-blue tattoos respond best to laser light; light-blue and green also respond well. Red responds well, while purple can be challenging. Yellow and orange do not respond very well, but they do respond partially.
According to a trial that analyzed variables influencing the outcome of tattoos treated by Q-switched lasers, 47% were cleared after 10 sessions, while 75% were cleared after 15 sessions (Arch Dermatol 2012;148[12]:1364-9). “It’s very important to message to your patients how many treatments this might take, because there is going to be an annuity of patients who are unhappy because they have to keep coming back,” said Dr. Avram, who is the immediate past president of the American Society for Laser Medicine and Surgery. Skin type and pigmentation also affect treatment outcomes. “For darker skin types or tanned individuals, hyper- or hypopigmentation is a greater concern than in patients with lighter skin types,” he said. “A test spot may be beneficial. The 1,064-nm Q-switched Nd:YAG laser is least likely to affect skin pigment; it’s safest for skin types IV-VI . This is great if it’s a black tattoo. But if it’s a green, blue, or red tattoo, you have a problem because you’re not going to target it very effectively.”
Some degree of posttreatment hypopigmentation is likely to occur, regardless of skin type. “Let patients know this is going to happen, but over time, this usually resolves, because you’re not destroying the melanocytes, unless you’re going too strong,” Dr. Avram said. “It may take a few months. It may take a year or 2, but the pigment should recur.”
He emphasized that the key variable during laser treatment of tattoos is the clinical endpoint, not the energy setting of the device. “What you want to see is immediate whitening of the treated area,” he said. “With the 1,064-nm Nd:YAG, you may get a little pinpoint bleeding in addition to whitening. Do not memorize treatment settings. Many Q-switched lasers are not externally calibrated. Thus, energy levels may change day to day or before and after servicing [of the device]. Trust your eyes; trust your clinical skills.” If you see epidermal disruption and bleeding during treatment, you’re probably being too aggressive. If that happens, “decrease your fluence,” he recommended. “You also want to decrease fluences when treating tattoos that are placed over other tattoos.”
Another rule of thumb is to use larger spot sizes during treatment sessions. “The larger the spot size, the more efficient the energy is going to get more deeply, and less is going to be at the dermal-epidermal junction,” Dr. Avram said. “So you’re going to get less hypopigmentation and less hyperpigmentation. Follow your endpoints and you are less likely to get pigmentation changes.”
Posttreatment care typically includes the application of topical petroleum jelly and a Telfa dressing. “Wait about a week to heal, counsel patients to keep out of the sun, and avoid friction to the treated area during healing,” he said. Patients can be rescheduled for retreatment 6-8 weeks later.
Common adverse events during laser treatment of tattoos include erythema, blistering, hyperpigmentation, hypopigmentation, and scarring, which occurs in about 5% of cases. Less common adverse events include allergic reaction, darkening of cosmetic tattoos, immune reaction, and chrysiasis, which is a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold salt ingestion. “Any history of gold salt ingestion will produce this characteristic finding, even if they took it when they were 5 years old and they come to you when they’re 85,” Dr. Avram said. “All of our intake forms include a question about this, and before I treat patients I always ask if they have a history of gold ingestion, because it’s very difficult to treat.”
Surgical excision may be an alternative for smaller tattoos. “Another option is ablative fractional resurfacing as a solo treatment or in combination with the Q-switched or picosecond laser, which has better efficacy,” he said. “The ablative fractional laser also may help with fibrosis after multiple treatments in a recalcitrant tattoo.” He noted that cosmetic tattoos such as lip liner and blush tattoos might darken because of oxidation of ferric oxide or titanium oxide pigment. The best approach to such cases is to perform an inconspicuous test spot prior to treatment.
Clinicians continue to explore the optimal interval between treatments. For example, the “R20” method consists of four consecutive treatment passes separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol 2012;66[2]:271-7). A follow-up study by Dr. Avram and his colleagues contradicted these findings, while another follow-up study was supportive.
Another technology playing a role in such repeat treatments is a perfluorodecalin-infused silicone patch, which is placed over the treatment area. According to Dr. Avram, the FDA-cleared patch helps reduce scatter during treatment and likely improves efficacy. It also allows for performing consecutive repeat laser treatments at the same visit. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side treated without the patch (Laser Surg Med 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
SAN DIEGO –

“A picosecond is to a second as 1 second is to 37,000 years,” Mathew M. Avram, MD, JD, said at the annual Masters of Aesthetics Symposium. “That’s equivalent to the total energy of the city of San Diego for 300-750 trillionths of a second.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, picosecond lasers produce extreme cavitation and cell rupture, with a desired clinical endpoint of immediate dermal whitening of tattooed skin. The process causes transdermal elimination of the tattoo ink. Some of the ink flows into the lymphatic system, while the rest undergoes rephagocytosis by dermal scavenger cells.
Commercially available picosecond lasers include devices with wavelengths of 532 nm, 755 nm, and 1,064 nm that deliver energy in a range of 300-750 picoseconds. Nd:YAG lasers work best for red and black ink, while alexandrite lasers work best for green and blue ink. In Dr. Avram’s experience, picosecond lasers are generally more effective for tattoo removal, compared with nanosecond lasers. “There is some nonselective targeting of other pigments, and they’re particularly effective for faded tattoos, but the devices are more expensive,” he said.
Dr. Avram, who is also the faculty director for laser and cosmetic dermatology training at Harvard Medical School, also in Boston, advises against promising a certain number of laser treatments during initial patient consultations. “You will regret it,” he said. “Tattoos are notoriously unpredictable in how they respond. I often hear people say they get rid of these in three to five treatments. That isn’t my experience with these lasers. Often, all you’re going to be able to do is get significant clearing rather than tattoo removal. Professional tattoos are the most difficult to treat because they are the deepest and they have the most amount of ink.”
On the other hand, amateur tattoos, traumatic tattoos, and radiation tattoos require far fewer treatments. “The color is important,” he said. “Multicolored tattoos, regardless of the colors, are always going to be more difficult to clear than a single-color tattoo.” Black and dark-blue tattoos respond best to laser light; light-blue and green also respond well. Red responds well, while purple can be challenging. Yellow and orange do not respond very well, but they do respond partially.
According to a trial that analyzed variables influencing the outcome of tattoos treated by Q-switched lasers, 47% were cleared after 10 sessions, while 75% were cleared after 15 sessions (Arch Dermatol 2012;148[12]:1364-9). “It’s very important to message to your patients how many treatments this might take, because there is going to be an annuity of patients who are unhappy because they have to keep coming back,” said Dr. Avram, who is the immediate past president of the American Society for Laser Medicine and Surgery. Skin type and pigmentation also affect treatment outcomes. “For darker skin types or tanned individuals, hyper- or hypopigmentation is a greater concern than in patients with lighter skin types,” he said. “A test spot may be beneficial. The 1,064-nm Q-switched Nd:YAG laser is least likely to affect skin pigment; it’s safest for skin types IV-VI . This is great if it’s a black tattoo. But if it’s a green, blue, or red tattoo, you have a problem because you’re not going to target it very effectively.”
Some degree of posttreatment hypopigmentation is likely to occur, regardless of skin type. “Let patients know this is going to happen, but over time, this usually resolves, because you’re not destroying the melanocytes, unless you’re going too strong,” Dr. Avram said. “It may take a few months. It may take a year or 2, but the pigment should recur.”
He emphasized that the key variable during laser treatment of tattoos is the clinical endpoint, not the energy setting of the device. “What you want to see is immediate whitening of the treated area,” he said. “With the 1,064-nm Nd:YAG, you may get a little pinpoint bleeding in addition to whitening. Do not memorize treatment settings. Many Q-switched lasers are not externally calibrated. Thus, energy levels may change day to day or before and after servicing [of the device]. Trust your eyes; trust your clinical skills.” If you see epidermal disruption and bleeding during treatment, you’re probably being too aggressive. If that happens, “decrease your fluence,” he recommended. “You also want to decrease fluences when treating tattoos that are placed over other tattoos.”
Another rule of thumb is to use larger spot sizes during treatment sessions. “The larger the spot size, the more efficient the energy is going to get more deeply, and less is going to be at the dermal-epidermal junction,” Dr. Avram said. “So you’re going to get less hypopigmentation and less hyperpigmentation. Follow your endpoints and you are less likely to get pigmentation changes.”
Posttreatment care typically includes the application of topical petroleum jelly and a Telfa dressing. “Wait about a week to heal, counsel patients to keep out of the sun, and avoid friction to the treated area during healing,” he said. Patients can be rescheduled for retreatment 6-8 weeks later.
Common adverse events during laser treatment of tattoos include erythema, blistering, hyperpigmentation, hypopigmentation, and scarring, which occurs in about 5% of cases. Less common adverse events include allergic reaction, darkening of cosmetic tattoos, immune reaction, and chrysiasis, which is a dark-blue pigmentation caused by Q-switched laser treatment in patients with a history of gold salt ingestion. “Any history of gold salt ingestion will produce this characteristic finding, even if they took it when they were 5 years old and they come to you when they’re 85,” Dr. Avram said. “All of our intake forms include a question about this, and before I treat patients I always ask if they have a history of gold ingestion, because it’s very difficult to treat.”
Surgical excision may be an alternative for smaller tattoos. “Another option is ablative fractional resurfacing as a solo treatment or in combination with the Q-switched or picosecond laser, which has better efficacy,” he said. “The ablative fractional laser also may help with fibrosis after multiple treatments in a recalcitrant tattoo.” He noted that cosmetic tattoos such as lip liner and blush tattoos might darken because of oxidation of ferric oxide or titanium oxide pigment. The best approach to such cases is to perform an inconspicuous test spot prior to treatment.
Clinicians continue to explore the optimal interval between treatments. For example, the “R20” method consists of four consecutive treatment passes separated by 20 minutes. The initial study found that this approach led to better outcomes, compared with conventional, single-pass laser treatment (J Am Acad Dermatol 2012;66[2]:271-7). A follow-up study by Dr. Avram and his colleagues contradicted these findings, while another follow-up study was supportive.
Another technology playing a role in such repeat treatments is a perfluorodecalin-infused silicone patch, which is placed over the treatment area. According to Dr. Avram, the FDA-cleared patch helps reduce scatter during treatment and likely improves efficacy. It also allows for performing consecutive repeat laser treatments at the same visit. In one study, 11 of 17 patients had more rapid clearance on the side treated with the perfluorodecalin patch, compared with the side treated without the patch (Laser Surg Med 2015;47[8]:613-8).
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea.
[email protected]
AT MOAS 2018
Recent Thrombectomy Trials Do Not Reduce Pressure to Treat Acute Stroke Urgently
While findings from the DAWN and DEFUSE3 trials support late thrombectomy, rapid intervention remains the preferred goal.
HILTON HEAD, SC—Although two recent studies demonstrated that endovascular thrombectomy is effective up to 24 hours after acute stroke onset in patients with large vessel occlusions, the findings do not diminish the urgency of rapid intervention. According to one expert who spoke at the 41st Annual Contemporary Clinical Neurology Symposium, findings from studies of late thrombectomy are important to the management of only a small group of acute stroke patients and do nothing to alter the premise that time is brain. For better outcomes, “we need to get more patients into therapy more quickly. If we optimize our systems of care, we can achieve that,” said Michael Froehler, MD, PhD, Director of the Cerebrovascular Program at Vanderbilt University Medical Center in Nashville.
Two Trials of Late Thrombectomy
In an analysis of the significance of these two studies as well as of other advances in stroke management, Dr. Froehler explained that rapid intervention is always the goal. The data from these multicenter trials, DAWN and DEFUSE3, were published earlier this year. Both randomized studies compared
The primary end points of the two trials differed, but the advantage of endovascular thrombectomy was comparable at 90 days when examining a modified Rankin score (mRS). A good outcome, defined as an mRS of 2 or less, was achieved with late endovascular thrombectomy in 49% and 45% of patients in DAWN and DEFUSE3, respectively, versus 13% and 17% of those treated with standard care. According to Dr. Froehler, these results were a surprise, because the effect size was greater in these two late treatment trials when compared with that of early endovascular thrombectomy (46% vs 27%) in a five-trial meta-analysis by Goyal et al published in 2016.
Entry criteria of these late endovascular thrombectomy trials are critical for understanding the results and their clinical significance, according to Dr. Froehler. He explained that both DAWN and DEFUSE3 were designed to enroll patients with salvageable tissue. Selection criteria such as a small infarct volume on imaging assessed with RAPID software ensured a “good collateral” patient population, Dr. Froehler said. Unlike the majority of patients with rapidly advancing infarcts, “good collateral patients hang on to salvageable brain for much longer,” Dr. Froehler explained.
The results of DAWN and DEFUSE3 thus are relevant to a small subpopulation of stroke patients. According to Dr. Froehler, only about 3% of acute stroke patients would meet entry criteria for DAWN or DEFUSE3, and only about 1.1% would meet the criteria for both.
“Unfortunately, the vast majority of patients we are seeing in real life are not going to be eligible for thrombectomy in the six- to 24-hour window,” Dr. Froehler emphasized. As a result, the data from DAWN and DEFUSE3, “do not change the importance of time” as the key factor in achieving good outcomes in patients with acute stroke.
Time Is Still Brain
The standard of care for management of acute stroke is IV t-PA within 4.5 hours, whether or not endovascular thrombectomy is offered, according to Dr. Froehler, but he cited data from the SWIFT PRIME trial, which employed endovascular thrombectomy after t-PA, to emphasize that the earlier the treatment, the better the outcome. In SWIFT PRIME, which was stopped early because of efficacy, the greater overall rate of good outcome (mRS ≤ 2) in the endovascular thrombectomy/t-PA versus t-PA alone groups were impressive (60% vs 35%), but time mattered. “Of those treated within 2.5 hours, 91% went home essentially normal,” according to Dr. Froehler.
Returning to his message that early reperfusion is the critical predictor of a good outcome, Dr. Froehler noted that an estimated 1.9 million neurons die for every minute of ischemia. In one analysis he cited, good outcomes dropped by 10% between 2.5 and 3.5 hours and then 20% for every hour thereafter.
One approach to accelerating time to appropriate therapy is optimizing triage strategies, particularly when patients who will benefit from endovascular thrombectomy will require transfer to a center that offers this intervention. Of triage strategies, Dr. Froehler singled out the 10-point ASPECTS scoring system, which is based on a CT scan. If the score is low, endovascular thrombectomy is not an option. Higher scores, particularly 6 or greater, can be a reason to consider and accelerate the time to transfer, which may mean the difference for a full recovery.
Reevaluating t-PA
“When you look at what t-PA has done for patients with large vessel occlusion, it is noteworthy, but it is not that great,” cautioned Dr. Froehler in making a case for endovascular thrombectomy in eligible patients. He called recanalization rates with IV t-PA in those with the largest clots “pretty low,” showing that the majority of patients achieve either partial or no recanalization with this treatment alone.
In fact, the therapeutic margin is “rather narrow” for t-PA overall, according to Dr. Froehler, citing data from 12 trials with alteplase. He noted that a review of the original publications reveals that only two of the investigating teams characterized their results as positive. Although almost all the others discussed risk-to-benefit ratios without labeling the findings positive or negative, he believes clinician should be aware of the limitations of these data.
For the newer thrombolytic tenecteplase, which was included as an alternative to alteplase in the most recent American Heart Association/American Stroke Association guidelines, Dr. Froehler said the evidence is even more limited, particularly regarding the optimal dose. In the recently published guidelines, the recommended dose was 0.4 mg/kg , even though this dose has been associated with intracranial hemorrhage in at least one clinical study. Lower doses such as 0.25 mg/kg may be a safer alternative, but Dr. Froehler recommended caution. “I do not think there is evidence that we should be transitioning to tenecteplase now,” he said, concluding that more data regarding the most appropriate dose are needed.
Patients with acute stroke can anticipate a favorable outcome with current therapies, but the urgency of reperfusion remains unchanged despite advances. Dr. Froehler concluded, “We must now work toward optimizing stroke systems of care for endovascular thrombectomy” to increase the proportion of patients who benefit.
Dr. Froehler disclosed financial relationships with Balt USA, Control Medical, EndoPhys, Genentech, Medtronic, Microvention, NeurVana, Penumbra, Stryker, and Viz.ai.
Suggested Reading
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21.
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295.
While findings from the DAWN and DEFUSE3 trials support late thrombectomy, rapid intervention remains the preferred goal.
While findings from the DAWN and DEFUSE3 trials support late thrombectomy, rapid intervention remains the preferred goal.
HILTON HEAD, SC—Although two recent studies demonstrated that endovascular thrombectomy is effective up to 24 hours after acute stroke onset in patients with large vessel occlusions, the findings do not diminish the urgency of rapid intervention. According to one expert who spoke at the 41st Annual Contemporary Clinical Neurology Symposium, findings from studies of late thrombectomy are important to the management of only a small group of acute stroke patients and do nothing to alter the premise that time is brain. For better outcomes, “we need to get more patients into therapy more quickly. If we optimize our systems of care, we can achieve that,” said Michael Froehler, MD, PhD, Director of the Cerebrovascular Program at Vanderbilt University Medical Center in Nashville.
Two Trials of Late Thrombectomy
In an analysis of the significance of these two studies as well as of other advances in stroke management, Dr. Froehler explained that rapid intervention is always the goal. The data from these multicenter trials, DAWN and DEFUSE3, were published earlier this year. Both randomized studies compared
The primary end points of the two trials differed, but the advantage of endovascular thrombectomy was comparable at 90 days when examining a modified Rankin score (mRS). A good outcome, defined as an mRS of 2 or less, was achieved with late endovascular thrombectomy in 49% and 45% of patients in DAWN and DEFUSE3, respectively, versus 13% and 17% of those treated with standard care. According to Dr. Froehler, these results were a surprise, because the effect size was greater in these two late treatment trials when compared with that of early endovascular thrombectomy (46% vs 27%) in a five-trial meta-analysis by Goyal et al published in 2016.
Entry criteria of these late endovascular thrombectomy trials are critical for understanding the results and their clinical significance, according to Dr. Froehler. He explained that both DAWN and DEFUSE3 were designed to enroll patients with salvageable tissue. Selection criteria such as a small infarct volume on imaging assessed with RAPID software ensured a “good collateral” patient population, Dr. Froehler said. Unlike the majority of patients with rapidly advancing infarcts, “good collateral patients hang on to salvageable brain for much longer,” Dr. Froehler explained.
The results of DAWN and DEFUSE3 thus are relevant to a small subpopulation of stroke patients. According to Dr. Froehler, only about 3% of acute stroke patients would meet entry criteria for DAWN or DEFUSE3, and only about 1.1% would meet the criteria for both.
“Unfortunately, the vast majority of patients we are seeing in real life are not going to be eligible for thrombectomy in the six- to 24-hour window,” Dr. Froehler emphasized. As a result, the data from DAWN and DEFUSE3, “do not change the importance of time” as the key factor in achieving good outcomes in patients with acute stroke.
Time Is Still Brain
The standard of care for management of acute stroke is IV t-PA within 4.5 hours, whether or not endovascular thrombectomy is offered, according to Dr. Froehler, but he cited data from the SWIFT PRIME trial, which employed endovascular thrombectomy after t-PA, to emphasize that the earlier the treatment, the better the outcome. In SWIFT PRIME, which was stopped early because of efficacy, the greater overall rate of good outcome (mRS ≤ 2) in the endovascular thrombectomy/t-PA versus t-PA alone groups were impressive (60% vs 35%), but time mattered. “Of those treated within 2.5 hours, 91% went home essentially normal,” according to Dr. Froehler.
Returning to his message that early reperfusion is the critical predictor of a good outcome, Dr. Froehler noted that an estimated 1.9 million neurons die for every minute of ischemia. In one analysis he cited, good outcomes dropped by 10% between 2.5 and 3.5 hours and then 20% for every hour thereafter.
One approach to accelerating time to appropriate therapy is optimizing triage strategies, particularly when patients who will benefit from endovascular thrombectomy will require transfer to a center that offers this intervention. Of triage strategies, Dr. Froehler singled out the 10-point ASPECTS scoring system, which is based on a CT scan. If the score is low, endovascular thrombectomy is not an option. Higher scores, particularly 6 or greater, can be a reason to consider and accelerate the time to transfer, which may mean the difference for a full recovery.
Reevaluating t-PA
“When you look at what t-PA has done for patients with large vessel occlusion, it is noteworthy, but it is not that great,” cautioned Dr. Froehler in making a case for endovascular thrombectomy in eligible patients. He called recanalization rates with IV t-PA in those with the largest clots “pretty low,” showing that the majority of patients achieve either partial or no recanalization with this treatment alone.
In fact, the therapeutic margin is “rather narrow” for t-PA overall, according to Dr. Froehler, citing data from 12 trials with alteplase. He noted that a review of the original publications reveals that only two of the investigating teams characterized their results as positive. Although almost all the others discussed risk-to-benefit ratios without labeling the findings positive or negative, he believes clinician should be aware of the limitations of these data.
For the newer thrombolytic tenecteplase, which was included as an alternative to alteplase in the most recent American Heart Association/American Stroke Association guidelines, Dr. Froehler said the evidence is even more limited, particularly regarding the optimal dose. In the recently published guidelines, the recommended dose was 0.4 mg/kg , even though this dose has been associated with intracranial hemorrhage in at least one clinical study. Lower doses such as 0.25 mg/kg may be a safer alternative, but Dr. Froehler recommended caution. “I do not think there is evidence that we should be transitioning to tenecteplase now,” he said, concluding that more data regarding the most appropriate dose are needed.
Patients with acute stroke can anticipate a favorable outcome with current therapies, but the urgency of reperfusion remains unchanged despite advances. Dr. Froehler concluded, “We must now work toward optimizing stroke systems of care for endovascular thrombectomy” to increase the proportion of patients who benefit.
Dr. Froehler disclosed financial relationships with Balt USA, Control Medical, EndoPhys, Genentech, Medtronic, Microvention, NeurVana, Penumbra, Stryker, and Viz.ai.
Suggested Reading
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21.
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295.
HILTON HEAD, SC—Although two recent studies demonstrated that endovascular thrombectomy is effective up to 24 hours after acute stroke onset in patients with large vessel occlusions, the findings do not diminish the urgency of rapid intervention. According to one expert who spoke at the 41st Annual Contemporary Clinical Neurology Symposium, findings from studies of late thrombectomy are important to the management of only a small group of acute stroke patients and do nothing to alter the premise that time is brain. For better outcomes, “we need to get more patients into therapy more quickly. If we optimize our systems of care, we can achieve that,” said Michael Froehler, MD, PhD, Director of the Cerebrovascular Program at Vanderbilt University Medical Center in Nashville.
Two Trials of Late Thrombectomy
In an analysis of the significance of these two studies as well as of other advances in stroke management, Dr. Froehler explained that rapid intervention is always the goal. The data from these multicenter trials, DAWN and DEFUSE3, were published earlier this year. Both randomized studies compared
The primary end points of the two trials differed, but the advantage of endovascular thrombectomy was comparable at 90 days when examining a modified Rankin score (mRS). A good outcome, defined as an mRS of 2 or less, was achieved with late endovascular thrombectomy in 49% and 45% of patients in DAWN and DEFUSE3, respectively, versus 13% and 17% of those treated with standard care. According to Dr. Froehler, these results were a surprise, because the effect size was greater in these two late treatment trials when compared with that of early endovascular thrombectomy (46% vs 27%) in a five-trial meta-analysis by Goyal et al published in 2016.
Entry criteria of these late endovascular thrombectomy trials are critical for understanding the results and their clinical significance, according to Dr. Froehler. He explained that both DAWN and DEFUSE3 were designed to enroll patients with salvageable tissue. Selection criteria such as a small infarct volume on imaging assessed with RAPID software ensured a “good collateral” patient population, Dr. Froehler said. Unlike the majority of patients with rapidly advancing infarcts, “good collateral patients hang on to salvageable brain for much longer,” Dr. Froehler explained.
The results of DAWN and DEFUSE3 thus are relevant to a small subpopulation of stroke patients. According to Dr. Froehler, only about 3% of acute stroke patients would meet entry criteria for DAWN or DEFUSE3, and only about 1.1% would meet the criteria for both.
“Unfortunately, the vast majority of patients we are seeing in real life are not going to be eligible for thrombectomy in the six- to 24-hour window,” Dr. Froehler emphasized. As a result, the data from DAWN and DEFUSE3, “do not change the importance of time” as the key factor in achieving good outcomes in patients with acute stroke.
Time Is Still Brain
The standard of care for management of acute stroke is IV t-PA within 4.5 hours, whether or not endovascular thrombectomy is offered, according to Dr. Froehler, but he cited data from the SWIFT PRIME trial, which employed endovascular thrombectomy after t-PA, to emphasize that the earlier the treatment, the better the outcome. In SWIFT PRIME, which was stopped early because of efficacy, the greater overall rate of good outcome (mRS ≤ 2) in the endovascular thrombectomy/t-PA versus t-PA alone groups were impressive (60% vs 35%), but time mattered. “Of those treated within 2.5 hours, 91% went home essentially normal,” according to Dr. Froehler.
Returning to his message that early reperfusion is the critical predictor of a good outcome, Dr. Froehler noted that an estimated 1.9 million neurons die for every minute of ischemia. In one analysis he cited, good outcomes dropped by 10% between 2.5 and 3.5 hours and then 20% for every hour thereafter.
One approach to accelerating time to appropriate therapy is optimizing triage strategies, particularly when patients who will benefit from endovascular thrombectomy will require transfer to a center that offers this intervention. Of triage strategies, Dr. Froehler singled out the 10-point ASPECTS scoring system, which is based on a CT scan. If the score is low, endovascular thrombectomy is not an option. Higher scores, particularly 6 or greater, can be a reason to consider and accelerate the time to transfer, which may mean the difference for a full recovery.
Reevaluating t-PA
“When you look at what t-PA has done for patients with large vessel occlusion, it is noteworthy, but it is not that great,” cautioned Dr. Froehler in making a case for endovascular thrombectomy in eligible patients. He called recanalization rates with IV t-PA in those with the largest clots “pretty low,” showing that the majority of patients achieve either partial or no recanalization with this treatment alone.
In fact, the therapeutic margin is “rather narrow” for t-PA overall, according to Dr. Froehler, citing data from 12 trials with alteplase. He noted that a review of the original publications reveals that only two of the investigating teams characterized their results as positive. Although almost all the others discussed risk-to-benefit ratios without labeling the findings positive or negative, he believes clinician should be aware of the limitations of these data.
For the newer thrombolytic tenecteplase, which was included as an alternative to alteplase in the most recent American Heart Association/American Stroke Association guidelines, Dr. Froehler said the evidence is even more limited, particularly regarding the optimal dose. In the recently published guidelines, the recommended dose was 0.4 mg/kg , even though this dose has been associated with intracranial hemorrhage in at least one clinical study. Lower doses such as 0.25 mg/kg may be a safer alternative, but Dr. Froehler recommended caution. “I do not think there is evidence that we should be transitioning to tenecteplase now,” he said, concluding that more data regarding the most appropriate dose are needed.
Patients with acute stroke can anticipate a favorable outcome with current therapies, but the urgency of reperfusion remains unchanged despite advances. Dr. Froehler concluded, “We must now work toward optimizing stroke systems of care for endovascular thrombectomy” to increase the proportion of patients who benefit.
Dr. Froehler disclosed financial relationships with Balt USA, Control Medical, EndoPhys, Genentech, Medtronic, Microvention, NeurVana, Penumbra, Stryker, and Viz.ai.
Suggested Reading
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21.
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295.
Artificial Intelligence for Clinical Decision Support
There is abundant research being conducted on the use of artificial intelligence (AI) to improve diagnosis in dermatology. Recently, convolutional neural networks trained using large image libraries have achieved parity with dermatologists in discriminating between benign and malignant lesions.1 There are expectations that these systems, as they improve and are implemented in mobile electronic devices, will revolutionize diagnosis. Substantially less attention has been given to the use of AI to guide management options following a diagnosis. There are several reasons this area lends itself to the application of AI.
In 2015, the National Library of Medicine indexed more than 800,000 articles.2 Medical literature is growing at an overwhelming pace that makes it challenging for health care professionals to read, retain, and appropriately implement the latest research into their care. One survey found that physicians spend no more than 4 hours per week reading medical journals, and for the majority of articles, only the abstracts are read.3 Conversely, AI networks today are able to interpret millions of pages of data within seconds. It is worth investigating how AI can be used to improve treatment and management decisions made by physicians.
Cognitive computing is a modern approach to AI that incorporates natural language processing, machine learning, and other techniques to answer questions. One cognitive computing system developed by IBM research in 2007, Watson, can interpret a user’s query using natural language processing and then generate hypotheses. It searches data sources extensively to find and score evidence for each candidate hypothesis.4 This information is synthesized to provide a simple output: ranked answers with associated confidence scores. Machine learning is used to improve the answers with feedback, training, and repetition.4,5
Watson Oncology, an ongoing collaboration between IBM and Memorial Sloan Kettering Cancer Center, is an application of cognitive computing to medicine. At Memorial Sloan Kettering, Watson has been trained by expert clinicians to provide an individualized, evidence-based list of therapeutic options for oncologists and patients to discuss. Furthermore, Watson is capable of taking patient preferences into consideration.4
In the near future, there also may be a role that cognitive computing could play in aiding dermatologists. Dermatologists manage a multitude of conditions requiring systemic therapies such as chemotherapeutics, biologics, and immunosuppressant medications. Frequently, the patient population has a complicated medical history with multiple comorbidities. Although current electronic health record (EHR) systems are able to assist physicians with structured numerical data such as vitals and laboratory results, cognitive computing systems could interpret the natural language of journal articles, textbooks, and published guidelines, as well as the narrative components of EHR notes. Outcomes from similar patients also could be used as inputs. With enough data, cognitive computing systems may be able to identify associations and epidemiologic trends that would not otherwise be noticed. As described by Miotto et al,6 one system, “deep patient,” was able to accurately predict the development of schizophrenia, diabetes mellitus, and various cancers based on EHR data. Patient genetic information also could one day be used to generate new insights into pharmacogenomics.
The benefit of a cognitive computing decision support system is that ineffective treatments and adverse reactions could be minimized, which may improve outcomes and reduce costs. Artificial intelligence also could help to decrease work burden so that physicians can spend more time with their patients, resulting in improved patient satisfaction and overall increased access to the specialty.
As with other clinical decision support systems, a number of challenges exist with the integration of cognitive computing into real care. One obstacle unique to machine learning algorithms is the black box problem. For instance, the skin lesion–identifying neural network cannot be questioned to determine which factors it used to arrive at its diagnosis. This shortcoming can lead to dangerous situations, such as the one reported by Caruana et al.7 A predictive model classified patients with pneumonia and a history of asthma as having a lower mortality risk than those with pneumonia alone because the model was unable to recognize the confounder that asthmatic patients were preemptively admitted to the intensive care unit and treated more aggressively, which is another reason that AI recommendations must always be evaluated by a physician.7 Physician and patient input also will be integral to incorporate contextual and qualitative information that may not be accessible to computers.8
As cognitive computing decision support systems are primarily used in oncology, they will need to be adjusted to optimize them for dermatologic conditions. It also will be up to health care providers to benchmark the performance of these systems.
Current clinical decision support systems that do not use AI have struggled to improve major patient outcomes such as mortality. These systems have been hobbled by poor usability and human-computer integration. Clinicians find their alerts and warnings to be a nuisance. The adoption of cognitive computing systems has the potential to give clinicians an intelligent partner. Their natural language processing, ability to comprehend questions, and easily understandable output give them an inherent ease of use that simplifies interactions with clinicians. Rather than replacing physicians, these systems will free clinicians to spend more of their time on the components of care that only a human can provide.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The National Library of Medicine fact sheet. U.S. National Library of Medicine website https://www.nlm.nih.gov/pubs/factsheets/nlm.html. Updated October 20, 2016. Accessed June 18, 2018.
- Saint S, Christakis DA, Saha S, et al. Journal reading habits of internists. J Gen Intern Med. 2000;15:881-884.
- Kelly JE III, Hamm S. Smart Machines: IBM’s Watson and the Era of Cognitive Computing. New York, NY: Columbia University Press; 2013.
- Ferrucci D, Levas A, Bagchi S, et al. Watson: beyond Jeopardy! Artificial Intelligence. 2013;199:93-105.
- Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094.
- Caruana R, Lou Y, Gehrke J, et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. Paper presented at: 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2015; August 10-13, 2015; Sydney, Australia.
- Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319:19-20.
There is abundant research being conducted on the use of artificial intelligence (AI) to improve diagnosis in dermatology. Recently, convolutional neural networks trained using large image libraries have achieved parity with dermatologists in discriminating between benign and malignant lesions.1 There are expectations that these systems, as they improve and are implemented in mobile electronic devices, will revolutionize diagnosis. Substantially less attention has been given to the use of AI to guide management options following a diagnosis. There are several reasons this area lends itself to the application of AI.
In 2015, the National Library of Medicine indexed more than 800,000 articles.2 Medical literature is growing at an overwhelming pace that makes it challenging for health care professionals to read, retain, and appropriately implement the latest research into their care. One survey found that physicians spend no more than 4 hours per week reading medical journals, and for the majority of articles, only the abstracts are read.3 Conversely, AI networks today are able to interpret millions of pages of data within seconds. It is worth investigating how AI can be used to improve treatment and management decisions made by physicians.
Cognitive computing is a modern approach to AI that incorporates natural language processing, machine learning, and other techniques to answer questions. One cognitive computing system developed by IBM research in 2007, Watson, can interpret a user’s query using natural language processing and then generate hypotheses. It searches data sources extensively to find and score evidence for each candidate hypothesis.4 This information is synthesized to provide a simple output: ranked answers with associated confidence scores. Machine learning is used to improve the answers with feedback, training, and repetition.4,5
Watson Oncology, an ongoing collaboration between IBM and Memorial Sloan Kettering Cancer Center, is an application of cognitive computing to medicine. At Memorial Sloan Kettering, Watson has been trained by expert clinicians to provide an individualized, evidence-based list of therapeutic options for oncologists and patients to discuss. Furthermore, Watson is capable of taking patient preferences into consideration.4
In the near future, there also may be a role that cognitive computing could play in aiding dermatologists. Dermatologists manage a multitude of conditions requiring systemic therapies such as chemotherapeutics, biologics, and immunosuppressant medications. Frequently, the patient population has a complicated medical history with multiple comorbidities. Although current electronic health record (EHR) systems are able to assist physicians with structured numerical data such as vitals and laboratory results, cognitive computing systems could interpret the natural language of journal articles, textbooks, and published guidelines, as well as the narrative components of EHR notes. Outcomes from similar patients also could be used as inputs. With enough data, cognitive computing systems may be able to identify associations and epidemiologic trends that would not otherwise be noticed. As described by Miotto et al,6 one system, “deep patient,” was able to accurately predict the development of schizophrenia, diabetes mellitus, and various cancers based on EHR data. Patient genetic information also could one day be used to generate new insights into pharmacogenomics.
The benefit of a cognitive computing decision support system is that ineffective treatments and adverse reactions could be minimized, which may improve outcomes and reduce costs. Artificial intelligence also could help to decrease work burden so that physicians can spend more time with their patients, resulting in improved patient satisfaction and overall increased access to the specialty.
As with other clinical decision support systems, a number of challenges exist with the integration of cognitive computing into real care. One obstacle unique to machine learning algorithms is the black box problem. For instance, the skin lesion–identifying neural network cannot be questioned to determine which factors it used to arrive at its diagnosis. This shortcoming can lead to dangerous situations, such as the one reported by Caruana et al.7 A predictive model classified patients with pneumonia and a history of asthma as having a lower mortality risk than those with pneumonia alone because the model was unable to recognize the confounder that asthmatic patients were preemptively admitted to the intensive care unit and treated more aggressively, which is another reason that AI recommendations must always be evaluated by a physician.7 Physician and patient input also will be integral to incorporate contextual and qualitative information that may not be accessible to computers.8
As cognitive computing decision support systems are primarily used in oncology, they will need to be adjusted to optimize them for dermatologic conditions. It also will be up to health care providers to benchmark the performance of these systems.
Current clinical decision support systems that do not use AI have struggled to improve major patient outcomes such as mortality. These systems have been hobbled by poor usability and human-computer integration. Clinicians find their alerts and warnings to be a nuisance. The adoption of cognitive computing systems has the potential to give clinicians an intelligent partner. Their natural language processing, ability to comprehend questions, and easily understandable output give them an inherent ease of use that simplifies interactions with clinicians. Rather than replacing physicians, these systems will free clinicians to spend more of their time on the components of care that only a human can provide.
There is abundant research being conducted on the use of artificial intelligence (AI) to improve diagnosis in dermatology. Recently, convolutional neural networks trained using large image libraries have achieved parity with dermatologists in discriminating between benign and malignant lesions.1 There are expectations that these systems, as they improve and are implemented in mobile electronic devices, will revolutionize diagnosis. Substantially less attention has been given to the use of AI to guide management options following a diagnosis. There are several reasons this area lends itself to the application of AI.
In 2015, the National Library of Medicine indexed more than 800,000 articles.2 Medical literature is growing at an overwhelming pace that makes it challenging for health care professionals to read, retain, and appropriately implement the latest research into their care. One survey found that physicians spend no more than 4 hours per week reading medical journals, and for the majority of articles, only the abstracts are read.3 Conversely, AI networks today are able to interpret millions of pages of data within seconds. It is worth investigating how AI can be used to improve treatment and management decisions made by physicians.
Cognitive computing is a modern approach to AI that incorporates natural language processing, machine learning, and other techniques to answer questions. One cognitive computing system developed by IBM research in 2007, Watson, can interpret a user’s query using natural language processing and then generate hypotheses. It searches data sources extensively to find and score evidence for each candidate hypothesis.4 This information is synthesized to provide a simple output: ranked answers with associated confidence scores. Machine learning is used to improve the answers with feedback, training, and repetition.4,5
Watson Oncology, an ongoing collaboration between IBM and Memorial Sloan Kettering Cancer Center, is an application of cognitive computing to medicine. At Memorial Sloan Kettering, Watson has been trained by expert clinicians to provide an individualized, evidence-based list of therapeutic options for oncologists and patients to discuss. Furthermore, Watson is capable of taking patient preferences into consideration.4
In the near future, there also may be a role that cognitive computing could play in aiding dermatologists. Dermatologists manage a multitude of conditions requiring systemic therapies such as chemotherapeutics, biologics, and immunosuppressant medications. Frequently, the patient population has a complicated medical history with multiple comorbidities. Although current electronic health record (EHR) systems are able to assist physicians with structured numerical data such as vitals and laboratory results, cognitive computing systems could interpret the natural language of journal articles, textbooks, and published guidelines, as well as the narrative components of EHR notes. Outcomes from similar patients also could be used as inputs. With enough data, cognitive computing systems may be able to identify associations and epidemiologic trends that would not otherwise be noticed. As described by Miotto et al,6 one system, “deep patient,” was able to accurately predict the development of schizophrenia, diabetes mellitus, and various cancers based on EHR data. Patient genetic information also could one day be used to generate new insights into pharmacogenomics.
The benefit of a cognitive computing decision support system is that ineffective treatments and adverse reactions could be minimized, which may improve outcomes and reduce costs. Artificial intelligence also could help to decrease work burden so that physicians can spend more time with their patients, resulting in improved patient satisfaction and overall increased access to the specialty.
As with other clinical decision support systems, a number of challenges exist with the integration of cognitive computing into real care. One obstacle unique to machine learning algorithms is the black box problem. For instance, the skin lesion–identifying neural network cannot be questioned to determine which factors it used to arrive at its diagnosis. This shortcoming can lead to dangerous situations, such as the one reported by Caruana et al.7 A predictive model classified patients with pneumonia and a history of asthma as having a lower mortality risk than those with pneumonia alone because the model was unable to recognize the confounder that asthmatic patients were preemptively admitted to the intensive care unit and treated more aggressively, which is another reason that AI recommendations must always be evaluated by a physician.7 Physician and patient input also will be integral to incorporate contextual and qualitative information that may not be accessible to computers.8
As cognitive computing decision support systems are primarily used in oncology, they will need to be adjusted to optimize them for dermatologic conditions. It also will be up to health care providers to benchmark the performance of these systems.
Current clinical decision support systems that do not use AI have struggled to improve major patient outcomes such as mortality. These systems have been hobbled by poor usability and human-computer integration. Clinicians find their alerts and warnings to be a nuisance. The adoption of cognitive computing systems has the potential to give clinicians an intelligent partner. Their natural language processing, ability to comprehend questions, and easily understandable output give them an inherent ease of use that simplifies interactions with clinicians. Rather than replacing physicians, these systems will free clinicians to spend more of their time on the components of care that only a human can provide.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The National Library of Medicine fact sheet. U.S. National Library of Medicine website https://www.nlm.nih.gov/pubs/factsheets/nlm.html. Updated October 20, 2016. Accessed June 18, 2018.
- Saint S, Christakis DA, Saha S, et al. Journal reading habits of internists. J Gen Intern Med. 2000;15:881-884.
- Kelly JE III, Hamm S. Smart Machines: IBM’s Watson and the Era of Cognitive Computing. New York, NY: Columbia University Press; 2013.
- Ferrucci D, Levas A, Bagchi S, et al. Watson: beyond Jeopardy! Artificial Intelligence. 2013;199:93-105.
- Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094.
- Caruana R, Lou Y, Gehrke J, et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. Paper presented at: 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2015; August 10-13, 2015; Sydney, Australia.
- Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319:19-20.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- The National Library of Medicine fact sheet. U.S. National Library of Medicine website https://www.nlm.nih.gov/pubs/factsheets/nlm.html. Updated October 20, 2016. Accessed June 18, 2018.
- Saint S, Christakis DA, Saha S, et al. Journal reading habits of internists. J Gen Intern Med. 2000;15:881-884.
- Kelly JE III, Hamm S. Smart Machines: IBM’s Watson and the Era of Cognitive Computing. New York, NY: Columbia University Press; 2013.
- Ferrucci D, Levas A, Bagchi S, et al. Watson: beyond Jeopardy! Artificial Intelligence. 2013;199:93-105.
- Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094.
- Caruana R, Lou Y, Gehrke J, et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. Paper presented at: 21st ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 2015; August 10-13, 2015; Sydney, Australia.
- Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319:19-20.
Topical Corticosteroids for Treatment-Resistant Atopic Dermatitis
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
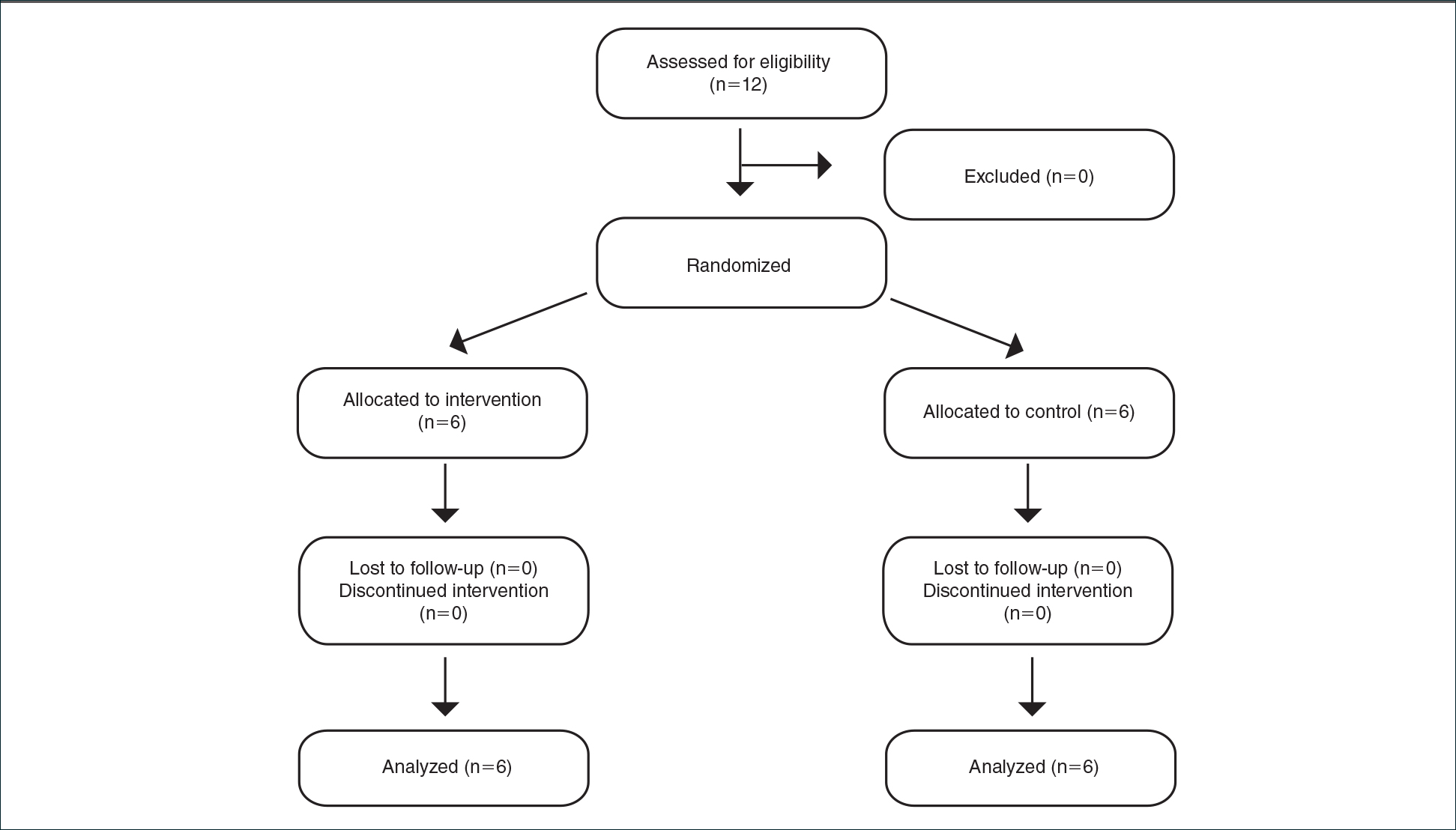
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.
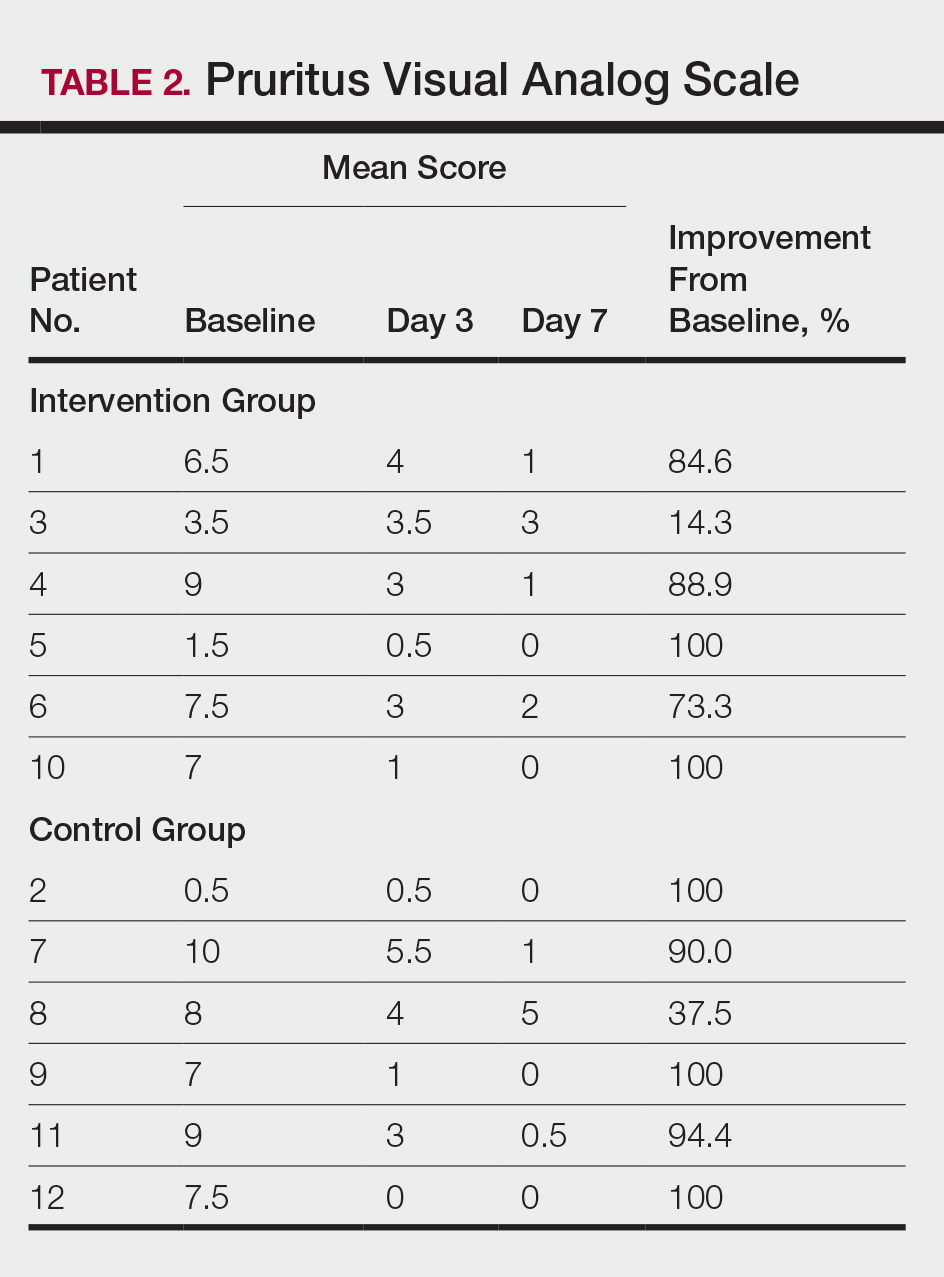
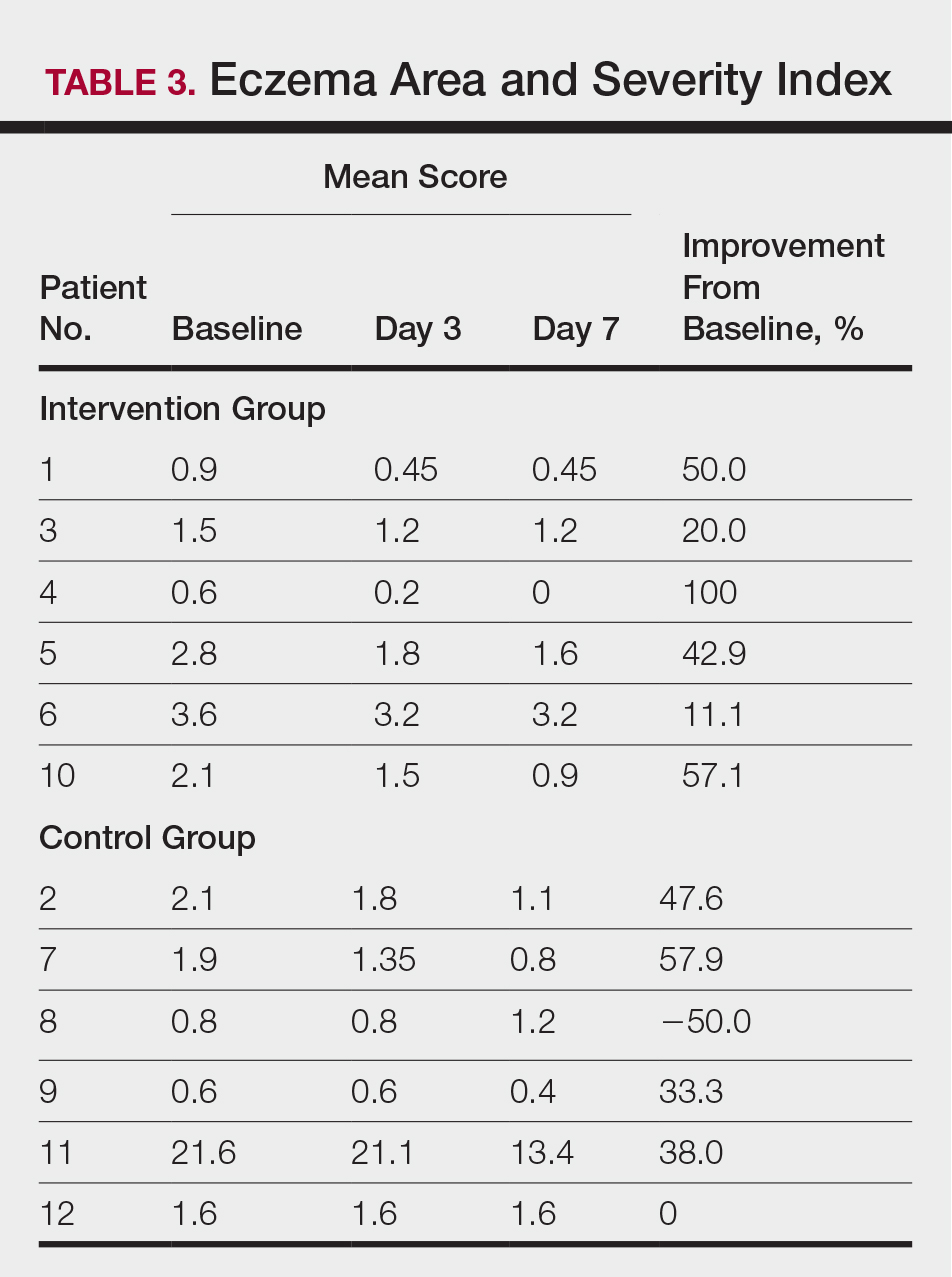
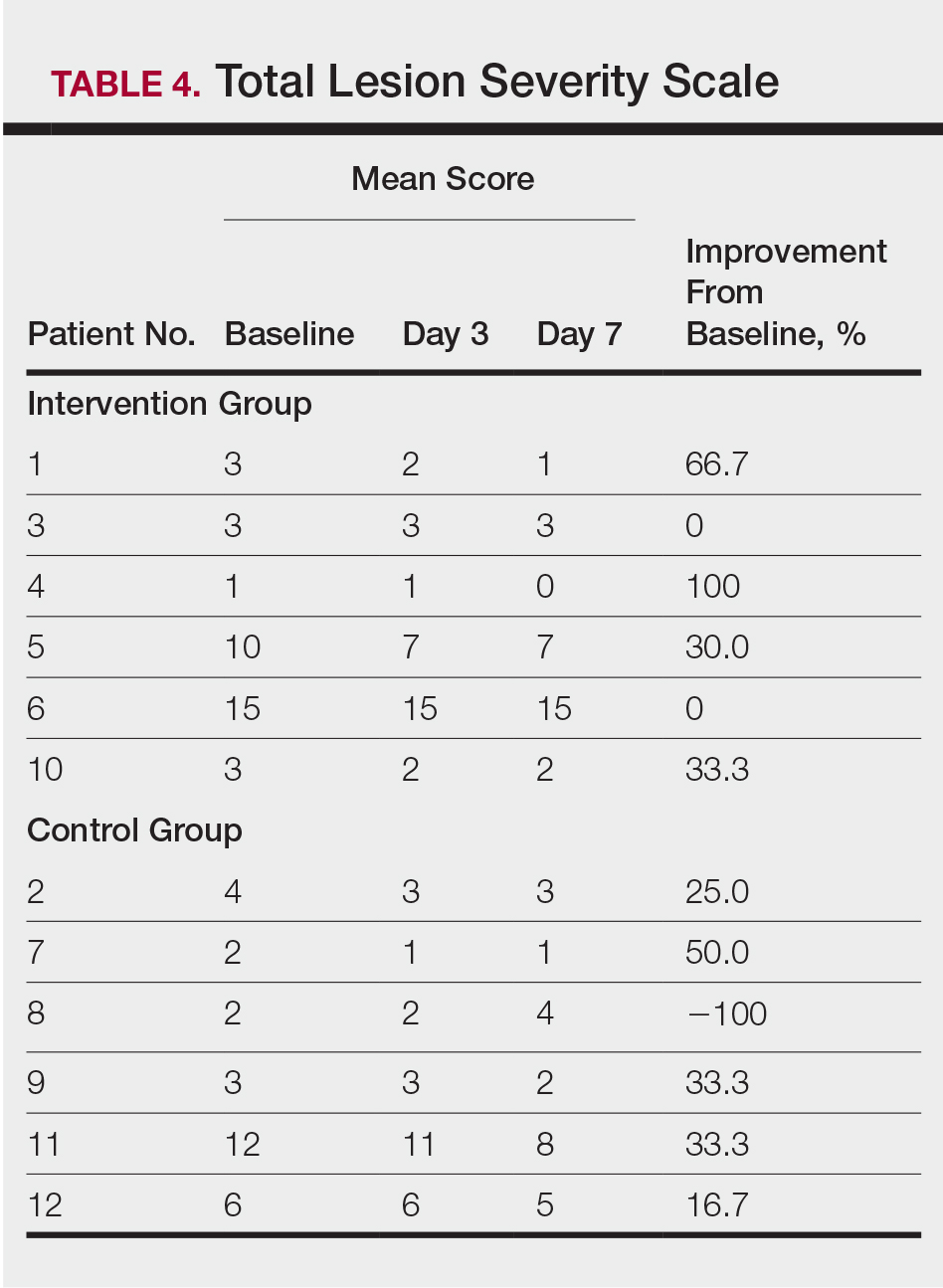
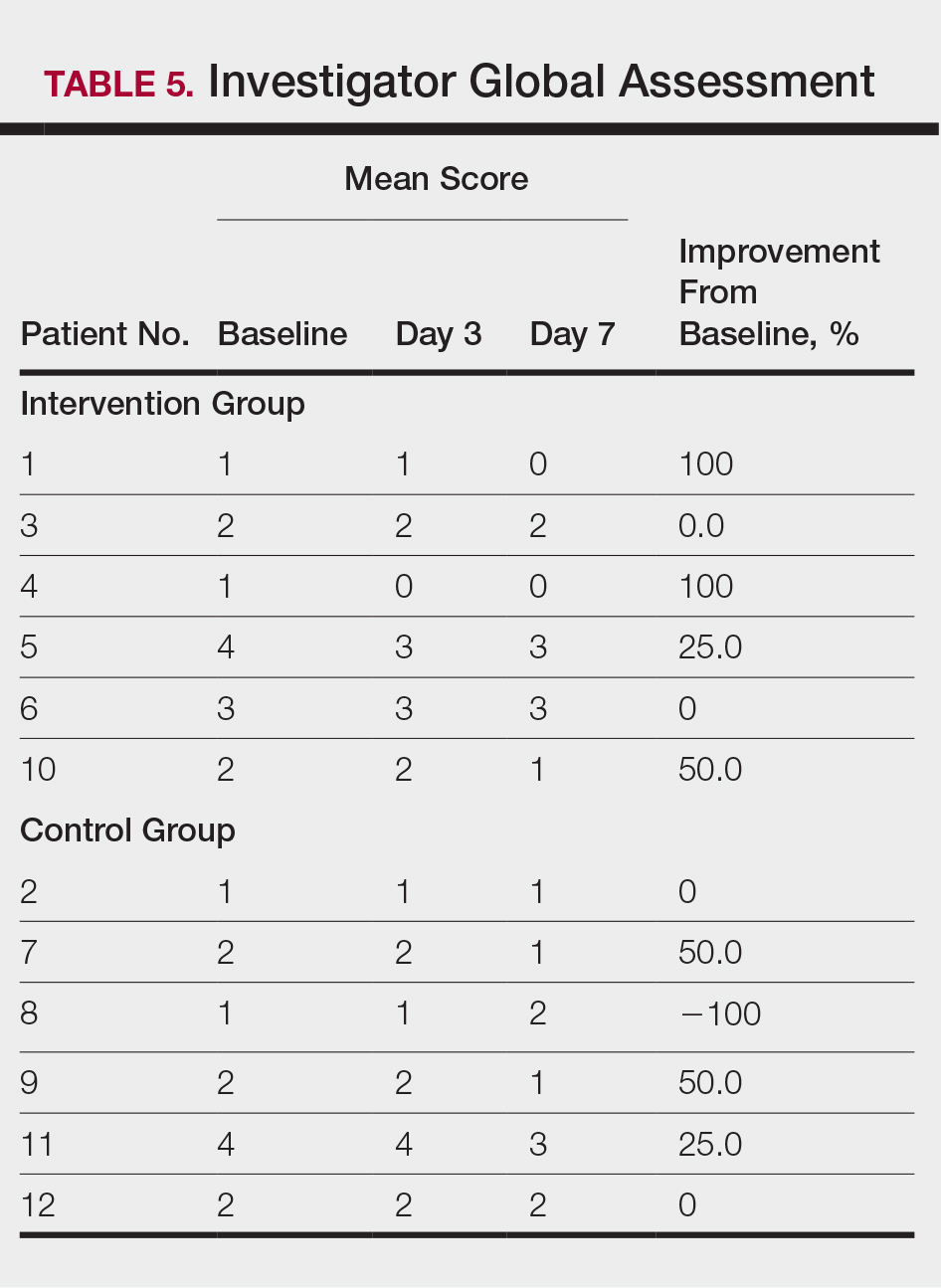
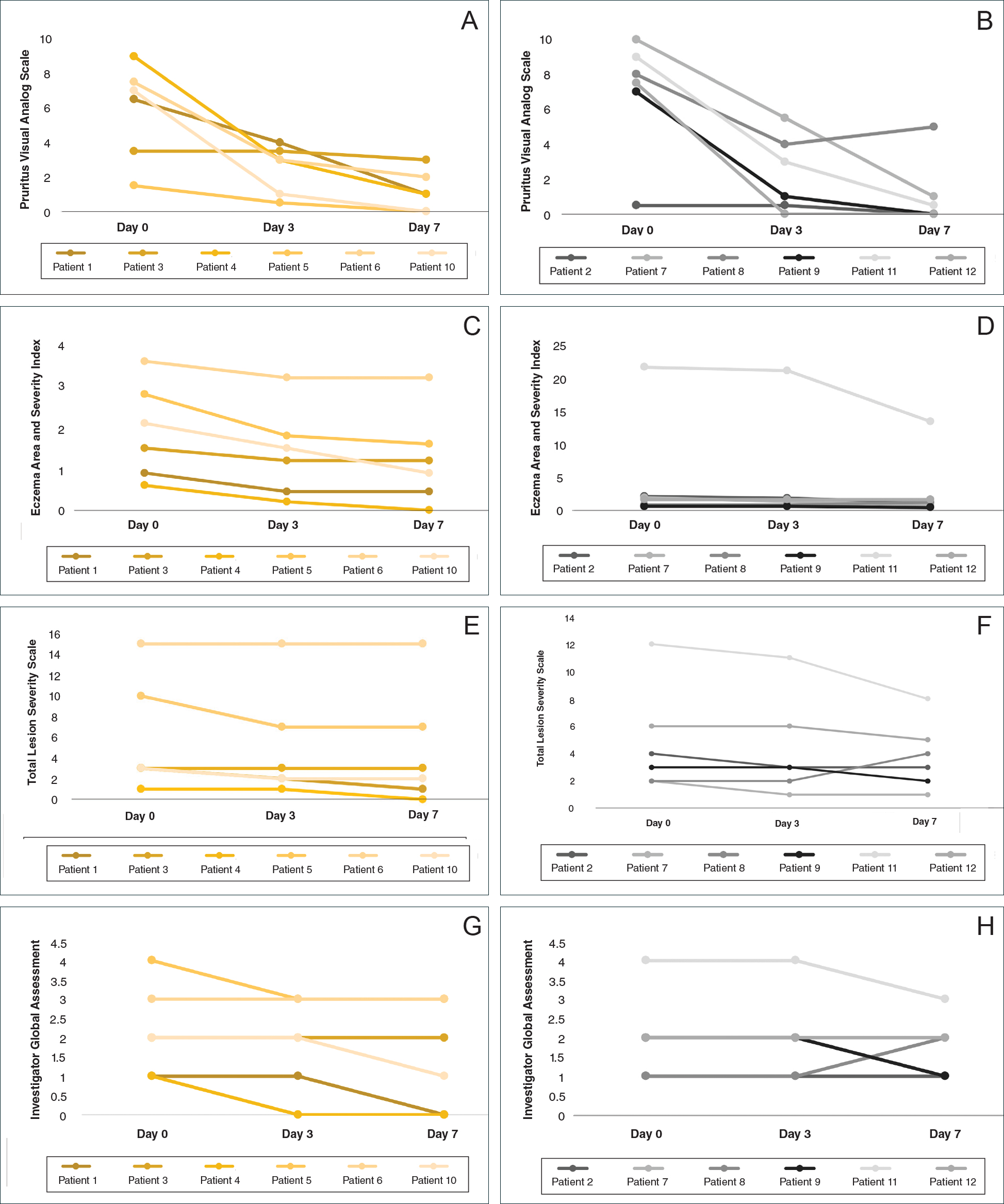
Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.

Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.

All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.





Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.

Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.

All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.





Comment
Although topical corticosteroids are the mainstay for treatment of AD, many patients report treatment resistance after a period of a few doses or longer.6-9 There is strong evidence demonstrating rapid corticosteroid receptor downregulation in tissues after corticosteroid therapy, which is the accepted mechanism for tachyphylaxis, but the timing of this effect does not match up with clinical experiences. The physiologic significance of corticosteroid agonist-induced receptor downregulation is unknown and may not have any considerable effect on corticosteroid efficacy.3 A systematic review by Taheri et al3 on the development of resistance to topical corticosteroids proposed 2 theories for the underlying pathogenesis of tachyphylaxis: (1) long-term patient nonadherence, and (2) the initial maximal response during the first few weeks of therapy eventually plateaus. Because corticosteroids may plateau after a certain number of doses, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis.10 The treatment “resistance” reported by the patients in our study may have been due to this plateau effect or to poor adherence.
Our finding that nearly all patients had rapid improvement of AD with the topical corticosteroid is not definitive proof but supports the notion that tachyphylaxis is largely mediated by poor adherence to treatment. Patients rapidly improved over the short study period. The short duration of treatment and multiple visits over the study period were designed to help ensure patient adherence. Rapid improvement in AD when topical corticosteroids are used should be expected, as AD patients have rapid improvement with application of topical corticosteroids in inpatient settings.11,12
Poor adherence to topical medication is common. In a Danish study, 99 of 322 patients (31%) did not redeem their AD prescriptions.13 In a single-center, 5-day, prospective study evaluating the use of fluocinonide cream 0.1% for treatment of children and adults with AD, the median percentage of prescribed doses taken was 40%, according to objective electronic monitors, even though patients reported 100% adherence in their medication diaries.Better adherence was seen on day 1 of treatment in which 66.6% (6/9) of patients adhered to their treatment strategy versus day 5 in which only 11.1% (1/9) of patients used their medication.1
Topical corticosteroids are safe and efficacious if used appropriately; however, patients commonly express fear and anxiety about using them. Topical corticosteroid phobia may stem from a misconception that these products carry the same adverse effects as their oral and systemic counterparts, which may be perpetuated by the media.1 Of 200 dermatology patients surveyed, 72.5% expressed concern about using topical corticosteroids on themselves or their children’s skin, and 24% of these patients stated they were noncompliant with their medication because of these worries. Almost 50% of patients requested prescriptions for corticosteroid-sparing medications such as tacrolimus.1 Patient education is important to help ensure treatment adherence. Other factors that can affect treatment adherence include forgetfulness; the chronic nature of AD; the need for ongoing application of topical treatments; prohibitive costs of some topical agents; and complexities in coordinating school, work, and family plans with the treatment regimen.2
We attempted to ensure good treatment adherence in our study by calling the patients in the intervention group twice daily. The mean improvement in EASI, TLSS, and IGA scores was higher in the intervention group versus the control group, which suggests that patient reminders have at least some benefit. Because AD treatment resistance appears more closely tied to nonadherence rather than loss of medication efficacy, it seems prudent to focus on interventions that would improve treatment adherence; however, such interventions generally are not well tested. Recommended interventions have included educating patients about the side effects of topical corticosteroids, avoiding use of medical jargon, and taking patient vehicle preference into account when prescribing treatments.8 Patients should be scheduled for a return visit within 1 to 2 weeks, as early return visits can augment treatment adherence.14 At the return visit, there can be a more detailed discussion of long-term management and side effects.8
Limitations of our study included a small sample size and brief treatment duration. Even though the patients had previously reported treatment failure with topical corticosteroids, all demonstrated improvement in only 1 week with a potent topical corticosteroid. The treatment resistance that initially was reported likely was due to poor adherence, but it is possible for AD patients to be resistant to treatment with topical corticosteroids due to allergic contact dermatitis. Patients could theoretically be allergic to components of the vehicle used in topical corticosteroids, which could aggravate their dermatitis; however, this effect seems unlikely in our patient population, as all the patients in our study showed improvement following treatment. Another study limitation was that adherence was not measured. The frequent follow-up visits were designed to encourage treatment adherence, but adherence was not specifically assessed. Although patients were encouraged to only use the desoximetasone spray during the study, it is not known whether patients used other products.
Conclusion
Some AD patients exhibit apparent decreased efficacy of topical corticosteroids over time, but this tachyphylaxis phenomenon is more likely due to poor treatment adherence than to loss of corticosteroid responsiveness. In our study, AD patients who reported treatment failure with topical corticosteroids improved rapidly with topical corticosteroids under conditions designed to promote good adherence to treatment. The majority of patients improved in all 4 parameters used for evaluating disease severity, with 100% of patients reporting improvement in pruritus. Intervention to improve treatment adherence may lead to better health outcomes. When AD appears resistant to topical corticosteroids, addressing adherence issues may be critical.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18:323-332.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J. 2013;19:18954.
- Miller JJ, Roling D, Margolis D, et al. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol. 1999;41:546-549.
- Smith SD, Harris V, Lee A, et al. General practitioners knowledge about use of topical corticosteroids in paediatric atopic dermatitis in Australia. Aust Fam Physician. 2017;46:335-340.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656-661.
- Reitamo S, Remitz A. Topical agents for atopic dermatitis. In: Bieber T, ed. Advances in the Management of Atopic Dermatitis. London, United Kingdom: Future Medicine Ltd; 2013:62-72.
- Krejci-Manwaring J, Tusa MG, Carroll C, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211-216.
- Fukaya M. Cortisol homeostasis in the epidermis is influenced by topical corticosteroids in patients with atopic dermatitis. Indian J Dermatol. 2017;62:440.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- van der Schaft J, Keijzer WW, Sanders KJ, et al. Is there an additional value of inpatient treatment for patients with atopic dermatitis? Acta Derm Venereol. 2016;96:797-801.
- Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2011;67:100-106.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Sagransky MJ, Yentzer BA, Williams LL, et al. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol. 2010;146:1428-1430.
Practice Points
- Mid-potency corticosteroids are the first-line treatment of atopic dermatitis (AD).
- Atopic dermatitis may fail to respond to topical corticosteroids initially or lose response over time, a phenomenon known as tachyphylaxis.
- Nonadherence to medication is the most likely cause of treatment resistance in patients with AD.
Dupilumab for Off-Label Treatment of Moderate to Severe Childhood Atopic Dermatitis
Case Report
A 7-year-old boy with a history of shellfish anaphylaxis, pollen allergy, asthma, rhinoconjunctivitis, frequent headaches and ear infections, sinusitis, bronchitis, vitiligo, warts, and cold sores presented to our dermatology clinic for evaluation of a widespread crusting, cracking, red rash that had been present since 6 months of age. The patient’s mother reported that he had many sleepless nights from uncontrolled itching. His medications included albuterol solution for nebulization, loratadine, and montelukast. Prior to the current presentation he had been treated with triamcinolone and betamethasone creams by the pediatrician. Despite compliance with topical therapy, his mother stated the itching persisted and lesions lingered with minimal improvement. He also was treated with oral corticosteroids for episodic sinusitis and bronchitis, which was beneficial to the skin lesions for only a short duration. The patient was adopted and therefore his family history was unavailable.
During physical examination, the patient was in the fetal position on the examination table and appeared uncomfortable, scratching himself. The patient admitted to severe widespread itching and burning. On skin examination, multiple thick, lichenified, highly pruritic plaques coalesced on the knees, ankles, arms, and wrists, and very discreet scaly patches were present on the scalp. Annular patches covered 50% of the patient’s body, with highly inflamed lesions concentrated in skin folds (Figure 1), leading to diagnosis of atopic dermatitis (AD).
Over the course of several months, a number of topical therapies were prescribed. The calcineurin inhibitor pimecrolimus cream 1% proffered minimal relief, and the patient experienced burning with crisaborole despite attempts to combine it with emollients and topical corticosteroids. The patient and his mother favored intermittent use of topical corticosteroids alone; however, he experienced frequent disease flares. Stabilized hypochlorous acid spray and mupirocin 2% antibiotic ointment were included in the treatment regimen as adjunctive topical therapies. Additionally, the patient underwent bleach and vinegar bath therapy without success.
Although UVA and UVB phototherapy has shown to be safe and effective in children, our patient had limited treatment options due to insurance restrictions. The patient had been taking oral corticosteroids on and off for years prior to presentation to our dermatology clinic.
Our patient weighed approximately 40 lb and was prescribed methotrexate 5 mg once weekly for 2 weeks along with oral folic acid 1 mg once daily, except when taking the methotrexate. Laboratory workup was ordered at 2- and then 4-week intervals. After 2 weeks of treatment, methotrexate was increased to 10 mg once weekly. His asthma was carefully monitored by the allergist, and his mother was instructed to stop the medication if he had worsening shortness of breath or exacerbation of asthma symptoms. He tolerated methotrexate at 10 mg once weekly well without clinical side effects for 6 months. His mother observed less frequent ear and sinus infections during methotrexate therapy; however, he developed anemia over time and the methotrexate was discontinued. Understanding the nature of off-label use in administering dupilumab, the patient’s mother consented to a scheduled dosage of 300 mg subcutaneous (SQ) injection every month in the absence of a loading dose with the assumption of future modifications pending his response to therapy.
Five days after treatment with a 300-mg SQ dupilumab injection, the patient returned to clinic for evaluation of a vesicular rash with subsequent peeling confined to the shoulders (Figure 2). He and his mother denied any UV exposure, citing he had been completely out of the sun. He denied constitutional symptoms including fever, malaise, swelling, joint pain, headache, muscle pain, nausea, vomiting, diarrhea, enlarged lymph glands, difficulty urinating, breathing, or neurological disturbance. Upon physical examination, the rash was not considered to be a drug eruption. Had a mild drug reaction been suspected, a careful rechallenge, weighing the risks and benefits, would have been considered and was discussed with the mother and patient. New-onset or worsening eye symptoms should be reported; therefore, a referral to ophthalmology was prompted due to our patient’s history of rhinoconjunctivitis and persistent conjunctival injection observed early after initiating dupilumab therapy. Nothing remarkable was found.
The patient was eager to continue dupilumab therapy due to considerable reduction of itching and elimination of lesions. His mother reported that the greatest benefit 1 month after starting dupilumab was almost no itching (Figure 3A). Additionally, he denied headache or nasopharyngitis at his 1-month office visit. After 2 months of dupilumab therapy, the patient reported persistent lesions on the feet and ankles despite concomitant treatment with topical corticosteroids. The decision to increase the dupilumab dose to 300-mg SQ injection once every 3 weeks for a total of 3 doses was made, which resulted in resolution of all lesions (Figure 3B).
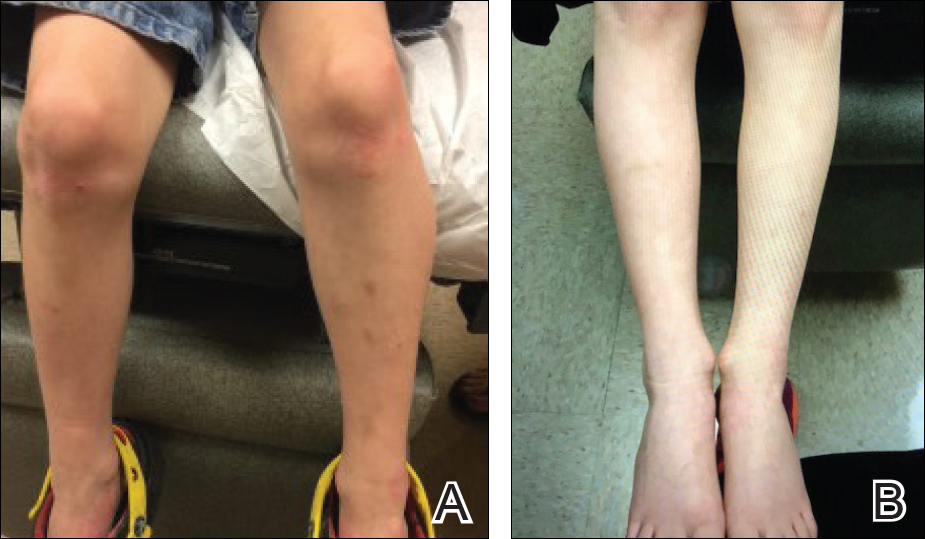
Comment
Prevalence and Pathogenesis
Atopic dermatitis affects 31.6 million individuals in the United States, with 17.8 million experiencing moderate to severe lesions.1 The current prevalence of AD in the pediatric population ranges from 10% to 30% compared to 2% to 10% in adults. Fortunately, up to 70% of young children enter remission or improve by 12 years of age. Atopic diatheses may simultaneously occur, which includes asthma and rhinoconjunctivitis.2
Complications from AD include bacterial and viral infections and ocular disease. Furthermore, impaired growth in stature has been correlated with individuals who have extensive disease.2 Of interest, our 7-year-old patient gained 7 lb and grew almost 3 in within 6 months of being on immunosuppressant therapy. Children with AD have poorer sleep efficiency in contrast to children without AD.3 Eczema is associated with more frequent headaches in childhood, especially in those with sleep disturbances,4 as our patient had experienced prior to systemic therapy.
The pathogenesis of AD is complex, and one must take into consideration the multiple cellular activities including inflammatory mechanics in the absence of IgE-mediated sensitization, epidermal barrier changes, epicutaneous sensitization, dendritic cell roles, T-cell responses and cytokine orchestrations, actions of microbial colonization, and involvement of autoimmunity.5 Select patients with AD have IgE antibodies focused against self-proteins. Disease severity correlates with ubiquity of these antibodies. Moreover, certain autoallergens induce helper T cell (TH1) responses.5 Circulating TH2 cytokines and chemokines IL-4, IL4ra, and IL-13 also have been linked to AD pathogenesis. Additionally, nonlesional skin abnormalities have been observed.6 Most recently, researchers have identified a caspase recruitment domain family member 11 (CARD11) gene mutation possibly leading to AD.7 Clinically, our patient responded favorably to dupilumab, which inhibits TH2 cytokines IL-4 and IL13. He experienced a considerable decrease in itching and inflammation and reduced lesion count after 1 month of treatment with dupilumab. No skin lesions were identified on visual examination at week 17 and inevitably the patient discontinued messy topicals.
Treatment Options
Because AD is characterized by episodes of remission and relapse, management generally is comprised of trigger avoidance, including known allergens and irritants; a skin care regimen that promotes healthy epidermal barrier function; anti-inflammatory therapies to control both flares and subclinical inflammation; and adjunctive therapy for additional symptomatic control (eg, phototherapy, stabilized hypochlorous acid, topical antibiotic treatment) when needed. Avoidance of excessive washing or irritants, food provocation, and emotional stress, as well as toleration of body temperature fluctuations and humidity, is recommended to amend exacerbations.5
Current topical therapies include emollients; corticosteroids; calcineurin inhibitors; and crisaborole, a newer phosphodiesterase 4 inhibitor. There are a number of emollients and moisturizers available, and one over-the-counter preparation showed tolerability and improved skin hydration in AD patients and demonstrated less transepidermal water loss than the control group.8 Ointments such as petrolatum usually do not include ingredients such as preservatives, gelling agents, or humectants that can promote stinging or burning.9 Topical corticosteroids, which ameliorate inflammation by subduing proinflammatory cytokine expression, have been the mainstay of treatment for more than 60 years; however, caution should be used due to the potential for side effects, mainly but not limited to systemic absorption in children, development of striae, and skin atrophy. Calcineurin inhibitors prohibit T-cell activity, modify mast cell response, and decrease dendritic cells in the epidermis. Since 2000, calcineurin inhibitors have been utilized as steroid-sparing agents10; however, prior authorization is still necessary with some insurance providers. Crisaborole ointment 2%, the newest topical agent for AD treatment in the market, has shown improvement of erythema, exudation, and pruritus. Approved for patients aged 2 years and older, twice-daily application of topical crisaborole as a steroid-sparing agent has rendered AD symptom relief.11 It has been reported that 4% of patients encounter stinging or burning with topical crisaborole application, whereas up to 50% of calcineurin inhibitors induce these adverse effects.12 Stabilized hypochlorous acid spray or gel acts as an antipruritic and antimicrobial agent, relieving pain associated with skin irritations. Topical antimicrobial preparations such as mupirocin 2% antibiotic ointment can reduce Staphylococcus colonization when applied in the nasal passage as well as to affected skin lesions.2
In children, UVA and UVB phototherapy has proven safe and effective and can be utilized in AD when suitable.13 When patients inadequately respond to topical therapies and phototherapy, systemic immunomodulatory agents have been recommended as treatment options.A child’s developing immune system indeed may be sensitive to systemic therapies as the innate immune system fully matures in adolescence and his/her adaptive immune system is undergoing vigorous definition.14 Systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate have been used off label for years and pose certain challenges in being identified as durable therapies due to potential side effects. Cyclosporine is effective for the treatment of AD; however, long-term administration should be dosed up to a 12-month period and then stopped to decrease cumulative exposure to the drug. Therefore, further treatment options must be considered. For children, cyclosporine should be administered in a dose of 3 to 6 mg/kg daily. Fluctuations in blood pressure and renal function should be monitored. The recommended pediatric dose for azathioprine is 1 to 4 mg/kg daily with laboratory monitoring, particularly of liver enzymes and complete blood cell count. Obtaining the patient’s thiopurine methyltransferase level may aid in dosing. Gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea are common. Phototherapy is not advised in conjunction with azathioprine due to an increased risk of photocarcinogenicity.13 The literature supporting mycophenolate mofetil in children with AD is limited.
Biologic therapies targeting IgE, B-lymphocyte antigen CD20, IL-5, thymic stromal lymphopoietin, TH17 cells, IL-12, IL-23, interferon gamma, IL-6 receptors, tumor necrosis factor, phosphodiesterase 4, Janus kinase, chymase, and nuclear receptors expressed on adipocytes and immune cells have undergone investigation for treatment of AD.17 Additionally, biologic agents targeting IL-31, IL-13, and IL-22 also have been evaluated.1 Currently, there are no US Food and Drug Administration–approved biologic agents for moderate to severe childhood AD.
Dupilumab, an IL-4Rα and IL-13Rα antagonist, recently has been approved for treatment of moderate to severe AD in adults but not yet for children. Potential side effects include nasopharyngitis, headache, hypersensitivity reactions, and ocular symptoms,11 namely keratitis and conjunctivitis.18 Less than 1% of patients experienced keratitis in clinical trials, while conjunctivitis was reported in 4% of patients taking dupilumab with topical corticosteroids at 52 weeks.18 However, possible ocular findings on slit-lamp examination in AD patients include atopic keratoconjunctivitis, blepharitis, palpebral conjunctival scarring, papillary conjunctival reaction, Horner-Trantas dots, keratoconus, and atopic cataracts. Spontaneous retinal detachment is seen more commonly in individuals with AD than in the general population.19
In clinical trials, hypersensitivity reactions included urticaria and serum sickness or serum sickness–like reactions in less than 1% of patients taking dupilumab.18
Conclusion
Childhood AD can be debilitating, and affected individuals often lead a poorer quality of life if left untreated. Embarrassment and isolation are commonly experienced. Increased responsibility and work in tending for a child with eczema may result in parental exhaustion.21 As with psoriasis, AD can impair activity and productivity.22 Currently, dupilumab has proven to positively impact health-related quality of life for adults.23 Pending the outcome of ongoing pediatric clinical trials, dupilumab may become a benchmark therapy for children younger than 18 years.
- Samalonis L. What’s new in eczema and atopic dermatitis research. The Dermatologist. November 19, 2015. http://www.the-dermatologist.com/content/whats-new-eczema-and-atopic-dermatitis-research. Accessed July 19, 2018.
- Habif T. Atopic dermatitis. In: Bonnet C, Pinczewski A, Cook L, eds. Clinical Dermatology. 5th ed. Edinburgh, Scotland: Mosby Elsevier; 2010:160-180.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case control study [published online October 28, 2017]. J Am Acad Dermatol. 2018;78:336-341.
- Silverberg J. Association between childhood eczema and headaches: an analysis of 19 US population-based studies [published online August 29, 2015]. J Allergy Clin Immunol. 2016;137:492-499.e5.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Saunders Elsevier; 2012:203-216.
- Suarez-Farinas M, Tintle S, Shemer A, et al. Non-lesional atopic dermatitis (AD) skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954-964.
- Hilton L. AD gene mutation identified: discovery may lead to new therapeutic option for patients. Dermatol Times. 2017;38:30.
- Zeichner JA, Dryer L. Effect of CeraVe Healing Ointment on skin hydration and barrier function on normal and barrier-impaired skin. Poster presented at: Orlando Dermatology Aesthetic & Clinical Conference; January 15-16, 2016; Orlando, FL.
- Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22:969-987.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Koutnik-Fotopoulous E. Update on the latest eczema treatments. The Dermatologist. February 17, 2016. http://www.the-dermatologist.com/content/update-latest-eczema-treatments. Accessed August 16, 2018.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Sidbury R, Davis D, Cohen D, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents . J Am Acad Dermatol. 2014;71:327-349.
- van der Merwe R, Gianella-Borradori A. Industry perspective on the clinical development of systemic products for the treatment of atopic dermatitis in pediatric patients with inadequate response to topical prescription therapy. Presented at: FDA Dermatologic and Ophthalmic Drugs Advisory Committee Meeting; March 9, 2015; Silver Spring, MD.
- Heller M, Shin HT, Orlow SJ, et al. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127-132.
- Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Saunders Elsevier; 2013:169-181.
- Guttman-Yassky E, Dhingra N, Leung DY. New era of biological therapeutics in atopic dermatitis [published online January 16, 2013]. Expert Opin Biol Ther. 2013;13:549-561.
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Lowery RS. Ophthalmologic manifestations of atopic dermatitis clinical presentation. Medscape website. emedicine.medscape.com/article/1197636-clinical#b4. Updated September 7, 2016. Accessed July 19, 2018.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the Unites States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol, 2017;77:274-279.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial [published online January 11, 2018]. Br J Dermatol. 2018;178:406-414.
Case Report
A 7-year-old boy with a history of shellfish anaphylaxis, pollen allergy, asthma, rhinoconjunctivitis, frequent headaches and ear infections, sinusitis, bronchitis, vitiligo, warts, and cold sores presented to our dermatology clinic for evaluation of a widespread crusting, cracking, red rash that had been present since 6 months of age. The patient’s mother reported that he had many sleepless nights from uncontrolled itching. His medications included albuterol solution for nebulization, loratadine, and montelukast. Prior to the current presentation he had been treated with triamcinolone and betamethasone creams by the pediatrician. Despite compliance with topical therapy, his mother stated the itching persisted and lesions lingered with minimal improvement. He also was treated with oral corticosteroids for episodic sinusitis and bronchitis, which was beneficial to the skin lesions for only a short duration. The patient was adopted and therefore his family history was unavailable.
During physical examination, the patient was in the fetal position on the examination table and appeared uncomfortable, scratching himself. The patient admitted to severe widespread itching and burning. On skin examination, multiple thick, lichenified, highly pruritic plaques coalesced on the knees, ankles, arms, and wrists, and very discreet scaly patches were present on the scalp. Annular patches covered 50% of the patient’s body, with highly inflamed lesions concentrated in skin folds (Figure 1), leading to diagnosis of atopic dermatitis (AD).

Over the course of several months, a number of topical therapies were prescribed. The calcineurin inhibitor pimecrolimus cream 1% proffered minimal relief, and the patient experienced burning with crisaborole despite attempts to combine it with emollients and topical corticosteroids. The patient and his mother favored intermittent use of topical corticosteroids alone; however, he experienced frequent disease flares. Stabilized hypochlorous acid spray and mupirocin 2% antibiotic ointment were included in the treatment regimen as adjunctive topical therapies. Additionally, the patient underwent bleach and vinegar bath therapy without success.
Although UVA and UVB phototherapy has shown to be safe and effective in children, our patient had limited treatment options due to insurance restrictions. The patient had been taking oral corticosteroids on and off for years prior to presentation to our dermatology clinic.
Our patient weighed approximately 40 lb and was prescribed methotrexate 5 mg once weekly for 2 weeks along with oral folic acid 1 mg once daily, except when taking the methotrexate. Laboratory workup was ordered at 2- and then 4-week intervals. After 2 weeks of treatment, methotrexate was increased to 10 mg once weekly. His asthma was carefully monitored by the allergist, and his mother was instructed to stop the medication if he had worsening shortness of breath or exacerbation of asthma symptoms. He tolerated methotrexate at 10 mg once weekly well without clinical side effects for 6 months. His mother observed less frequent ear and sinus infections during methotrexate therapy; however, he developed anemia over time and the methotrexate was discontinued. Understanding the nature of off-label use in administering dupilumab, the patient’s mother consented to a scheduled dosage of 300 mg subcutaneous (SQ) injection every month in the absence of a loading dose with the assumption of future modifications pending his response to therapy.
Five days after treatment with a 300-mg SQ dupilumab injection, the patient returned to clinic for evaluation of a vesicular rash with subsequent peeling confined to the shoulders (Figure 2). He and his mother denied any UV exposure, citing he had been completely out of the sun. He denied constitutional symptoms including fever, malaise, swelling, joint pain, headache, muscle pain, nausea, vomiting, diarrhea, enlarged lymph glands, difficulty urinating, breathing, or neurological disturbance. Upon physical examination, the rash was not considered to be a drug eruption. Had a mild drug reaction been suspected, a careful rechallenge, weighing the risks and benefits, would have been considered and was discussed with the mother and patient. New-onset or worsening eye symptoms should be reported; therefore, a referral to ophthalmology was prompted due to our patient’s history of rhinoconjunctivitis and persistent conjunctival injection observed early after initiating dupilumab therapy. Nothing remarkable was found.

The patient was eager to continue dupilumab therapy due to considerable reduction of itching and elimination of lesions. His mother reported that the greatest benefit 1 month after starting dupilumab was almost no itching (Figure 3A). Additionally, he denied headache or nasopharyngitis at his 1-month office visit. After 2 months of dupilumab therapy, the patient reported persistent lesions on the feet and ankles despite concomitant treatment with topical corticosteroids. The decision to increase the dupilumab dose to 300-mg SQ injection once every 3 weeks for a total of 3 doses was made, which resulted in resolution of all lesions (Figure 3B).

Comment
Prevalence and Pathogenesis
Atopic dermatitis affects 31.6 million individuals in the United States, with 17.8 million experiencing moderate to severe lesions.1 The current prevalence of AD in the pediatric population ranges from 10% to 30% compared to 2% to 10% in adults. Fortunately, up to 70% of young children enter remission or improve by 12 years of age. Atopic diatheses may simultaneously occur, which includes asthma and rhinoconjunctivitis.2
Complications from AD include bacterial and viral infections and ocular disease. Furthermore, impaired growth in stature has been correlated with individuals who have extensive disease.2 Of interest, our 7-year-old patient gained 7 lb and grew almost 3 in within 6 months of being on immunosuppressant therapy. Children with AD have poorer sleep efficiency in contrast to children without AD.3 Eczema is associated with more frequent headaches in childhood, especially in those with sleep disturbances,4 as our patient had experienced prior to systemic therapy.
The pathogenesis of AD is complex, and one must take into consideration the multiple cellular activities including inflammatory mechanics in the absence of IgE-mediated sensitization, epidermal barrier changes, epicutaneous sensitization, dendritic cell roles, T-cell responses and cytokine orchestrations, actions of microbial colonization, and involvement of autoimmunity.5 Select patients with AD have IgE antibodies focused against self-proteins. Disease severity correlates with ubiquity of these antibodies. Moreover, certain autoallergens induce helper T cell (TH1) responses.5 Circulating TH2 cytokines and chemokines IL-4, IL4ra, and IL-13 also have been linked to AD pathogenesis. Additionally, nonlesional skin abnormalities have been observed.6 Most recently, researchers have identified a caspase recruitment domain family member 11 (CARD11) gene mutation possibly leading to AD.7 Clinically, our patient responded favorably to dupilumab, which inhibits TH2 cytokines IL-4 and IL13. He experienced a considerable decrease in itching and inflammation and reduced lesion count after 1 month of treatment with dupilumab. No skin lesions were identified on visual examination at week 17 and inevitably the patient discontinued messy topicals.
Treatment Options
Because AD is characterized by episodes of remission and relapse, management generally is comprised of trigger avoidance, including known allergens and irritants; a skin care regimen that promotes healthy epidermal barrier function; anti-inflammatory therapies to control both flares and subclinical inflammation; and adjunctive therapy for additional symptomatic control (eg, phototherapy, stabilized hypochlorous acid, topical antibiotic treatment) when needed. Avoidance of excessive washing or irritants, food provocation, and emotional stress, as well as toleration of body temperature fluctuations and humidity, is recommended to amend exacerbations.5
Current topical therapies include emollients; corticosteroids; calcineurin inhibitors; and crisaborole, a newer phosphodiesterase 4 inhibitor. There are a number of emollients and moisturizers available, and one over-the-counter preparation showed tolerability and improved skin hydration in AD patients and demonstrated less transepidermal water loss than the control group.8 Ointments such as petrolatum usually do not include ingredients such as preservatives, gelling agents, or humectants that can promote stinging or burning.9 Topical corticosteroids, which ameliorate inflammation by subduing proinflammatory cytokine expression, have been the mainstay of treatment for more than 60 years; however, caution should be used due to the potential for side effects, mainly but not limited to systemic absorption in children, development of striae, and skin atrophy. Calcineurin inhibitors prohibit T-cell activity, modify mast cell response, and decrease dendritic cells in the epidermis. Since 2000, calcineurin inhibitors have been utilized as steroid-sparing agents10; however, prior authorization is still necessary with some insurance providers. Crisaborole ointment 2%, the newest topical agent for AD treatment in the market, has shown improvement of erythema, exudation, and pruritus. Approved for patients aged 2 years and older, twice-daily application of topical crisaborole as a steroid-sparing agent has rendered AD symptom relief.11 It has been reported that 4% of patients encounter stinging or burning with topical crisaborole application, whereas up to 50% of calcineurin inhibitors induce these adverse effects.12 Stabilized hypochlorous acid spray or gel acts as an antipruritic and antimicrobial agent, relieving pain associated with skin irritations. Topical antimicrobial preparations such as mupirocin 2% antibiotic ointment can reduce Staphylococcus colonization when applied in the nasal passage as well as to affected skin lesions.2
In children, UVA and UVB phototherapy has proven safe and effective and can be utilized in AD when suitable.13 When patients inadequately respond to topical therapies and phototherapy, systemic immunomodulatory agents have been recommended as treatment options.A child’s developing immune system indeed may be sensitive to systemic therapies as the innate immune system fully matures in adolescence and his/her adaptive immune system is undergoing vigorous definition.14 Systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate have been used off label for years and pose certain challenges in being identified as durable therapies due to potential side effects. Cyclosporine is effective for the treatment of AD; however, long-term administration should be dosed up to a 12-month period and then stopped to decrease cumulative exposure to the drug. Therefore, further treatment options must be considered. For children, cyclosporine should be administered in a dose of 3 to 6 mg/kg daily. Fluctuations in blood pressure and renal function should be monitored. The recommended pediatric dose for azathioprine is 1 to 4 mg/kg daily with laboratory monitoring, particularly of liver enzymes and complete blood cell count. Obtaining the patient’s thiopurine methyltransferase level may aid in dosing. Gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea are common. Phototherapy is not advised in conjunction with azathioprine due to an increased risk of photocarcinogenicity.13 The literature supporting mycophenolate mofetil in children with AD is limited.
Biologic therapies targeting IgE, B-lymphocyte antigen CD20, IL-5, thymic stromal lymphopoietin, TH17 cells, IL-12, IL-23, interferon gamma, IL-6 receptors, tumor necrosis factor, phosphodiesterase 4, Janus kinase, chymase, and nuclear receptors expressed on adipocytes and immune cells have undergone investigation for treatment of AD.17 Additionally, biologic agents targeting IL-31, IL-13, and IL-22 also have been evaluated.1 Currently, there are no US Food and Drug Administration–approved biologic agents for moderate to severe childhood AD.
Dupilumab, an IL-4Rα and IL-13Rα antagonist, recently has been approved for treatment of moderate to severe AD in adults but not yet for children. Potential side effects include nasopharyngitis, headache, hypersensitivity reactions, and ocular symptoms,11 namely keratitis and conjunctivitis.18 Less than 1% of patients experienced keratitis in clinical trials, while conjunctivitis was reported in 4% of patients taking dupilumab with topical corticosteroids at 52 weeks.18 However, possible ocular findings on slit-lamp examination in AD patients include atopic keratoconjunctivitis, blepharitis, palpebral conjunctival scarring, papillary conjunctival reaction, Horner-Trantas dots, keratoconus, and atopic cataracts. Spontaneous retinal detachment is seen more commonly in individuals with AD than in the general population.19
In clinical trials, hypersensitivity reactions included urticaria and serum sickness or serum sickness–like reactions in less than 1% of patients taking dupilumab.18
Conclusion
Childhood AD can be debilitating, and affected individuals often lead a poorer quality of life if left untreated. Embarrassment and isolation are commonly experienced. Increased responsibility and work in tending for a child with eczema may result in parental exhaustion.21 As with psoriasis, AD can impair activity and productivity.22 Currently, dupilumab has proven to positively impact health-related quality of life for adults.23 Pending the outcome of ongoing pediatric clinical trials, dupilumab may become a benchmark therapy for children younger than 18 years.
Case Report
A 7-year-old boy with a history of shellfish anaphylaxis, pollen allergy, asthma, rhinoconjunctivitis, frequent headaches and ear infections, sinusitis, bronchitis, vitiligo, warts, and cold sores presented to our dermatology clinic for evaluation of a widespread crusting, cracking, red rash that had been present since 6 months of age. The patient’s mother reported that he had many sleepless nights from uncontrolled itching. His medications included albuterol solution for nebulization, loratadine, and montelukast. Prior to the current presentation he had been treated with triamcinolone and betamethasone creams by the pediatrician. Despite compliance with topical therapy, his mother stated the itching persisted and lesions lingered with minimal improvement. He also was treated with oral corticosteroids for episodic sinusitis and bronchitis, which was beneficial to the skin lesions for only a short duration. The patient was adopted and therefore his family history was unavailable.
During physical examination, the patient was in the fetal position on the examination table and appeared uncomfortable, scratching himself. The patient admitted to severe widespread itching and burning. On skin examination, multiple thick, lichenified, highly pruritic plaques coalesced on the knees, ankles, arms, and wrists, and very discreet scaly patches were present on the scalp. Annular patches covered 50% of the patient’s body, with highly inflamed lesions concentrated in skin folds (Figure 1), leading to diagnosis of atopic dermatitis (AD).

Over the course of several months, a number of topical therapies were prescribed. The calcineurin inhibitor pimecrolimus cream 1% proffered minimal relief, and the patient experienced burning with crisaborole despite attempts to combine it with emollients and topical corticosteroids. The patient and his mother favored intermittent use of topical corticosteroids alone; however, he experienced frequent disease flares. Stabilized hypochlorous acid spray and mupirocin 2% antibiotic ointment were included in the treatment regimen as adjunctive topical therapies. Additionally, the patient underwent bleach and vinegar bath therapy without success.
Although UVA and UVB phototherapy has shown to be safe and effective in children, our patient had limited treatment options due to insurance restrictions. The patient had been taking oral corticosteroids on and off for years prior to presentation to our dermatology clinic.
Our patient weighed approximately 40 lb and was prescribed methotrexate 5 mg once weekly for 2 weeks along with oral folic acid 1 mg once daily, except when taking the methotrexate. Laboratory workup was ordered at 2- and then 4-week intervals. After 2 weeks of treatment, methotrexate was increased to 10 mg once weekly. His asthma was carefully monitored by the allergist, and his mother was instructed to stop the medication if he had worsening shortness of breath or exacerbation of asthma symptoms. He tolerated methotrexate at 10 mg once weekly well without clinical side effects for 6 months. His mother observed less frequent ear and sinus infections during methotrexate therapy; however, he developed anemia over time and the methotrexate was discontinued. Understanding the nature of off-label use in administering dupilumab, the patient’s mother consented to a scheduled dosage of 300 mg subcutaneous (SQ) injection every month in the absence of a loading dose with the assumption of future modifications pending his response to therapy.
Five days after treatment with a 300-mg SQ dupilumab injection, the patient returned to clinic for evaluation of a vesicular rash with subsequent peeling confined to the shoulders (Figure 2). He and his mother denied any UV exposure, citing he had been completely out of the sun. He denied constitutional symptoms including fever, malaise, swelling, joint pain, headache, muscle pain, nausea, vomiting, diarrhea, enlarged lymph glands, difficulty urinating, breathing, or neurological disturbance. Upon physical examination, the rash was not considered to be a drug eruption. Had a mild drug reaction been suspected, a careful rechallenge, weighing the risks and benefits, would have been considered and was discussed with the mother and patient. New-onset or worsening eye symptoms should be reported; therefore, a referral to ophthalmology was prompted due to our patient’s history of rhinoconjunctivitis and persistent conjunctival injection observed early after initiating dupilumab therapy. Nothing remarkable was found.

The patient was eager to continue dupilumab therapy due to considerable reduction of itching and elimination of lesions. His mother reported that the greatest benefit 1 month after starting dupilumab was almost no itching (Figure 3A). Additionally, he denied headache or nasopharyngitis at his 1-month office visit. After 2 months of dupilumab therapy, the patient reported persistent lesions on the feet and ankles despite concomitant treatment with topical corticosteroids. The decision to increase the dupilumab dose to 300-mg SQ injection once every 3 weeks for a total of 3 doses was made, which resulted in resolution of all lesions (Figure 3B).

Comment
Prevalence and Pathogenesis
Atopic dermatitis affects 31.6 million individuals in the United States, with 17.8 million experiencing moderate to severe lesions.1 The current prevalence of AD in the pediatric population ranges from 10% to 30% compared to 2% to 10% in adults. Fortunately, up to 70% of young children enter remission or improve by 12 years of age. Atopic diatheses may simultaneously occur, which includes asthma and rhinoconjunctivitis.2
Complications from AD include bacterial and viral infections and ocular disease. Furthermore, impaired growth in stature has been correlated with individuals who have extensive disease.2 Of interest, our 7-year-old patient gained 7 lb and grew almost 3 in within 6 months of being on immunosuppressant therapy. Children with AD have poorer sleep efficiency in contrast to children without AD.3 Eczema is associated with more frequent headaches in childhood, especially in those with sleep disturbances,4 as our patient had experienced prior to systemic therapy.
The pathogenesis of AD is complex, and one must take into consideration the multiple cellular activities including inflammatory mechanics in the absence of IgE-mediated sensitization, epidermal barrier changes, epicutaneous sensitization, dendritic cell roles, T-cell responses and cytokine orchestrations, actions of microbial colonization, and involvement of autoimmunity.5 Select patients with AD have IgE antibodies focused against self-proteins. Disease severity correlates with ubiquity of these antibodies. Moreover, certain autoallergens induce helper T cell (TH1) responses.5 Circulating TH2 cytokines and chemokines IL-4, IL4ra, and IL-13 also have been linked to AD pathogenesis. Additionally, nonlesional skin abnormalities have been observed.6 Most recently, researchers have identified a caspase recruitment domain family member 11 (CARD11) gene mutation possibly leading to AD.7 Clinically, our patient responded favorably to dupilumab, which inhibits TH2 cytokines IL-4 and IL13. He experienced a considerable decrease in itching and inflammation and reduced lesion count after 1 month of treatment with dupilumab. No skin lesions were identified on visual examination at week 17 and inevitably the patient discontinued messy topicals.
Treatment Options
Because AD is characterized by episodes of remission and relapse, management generally is comprised of trigger avoidance, including known allergens and irritants; a skin care regimen that promotes healthy epidermal barrier function; anti-inflammatory therapies to control both flares and subclinical inflammation; and adjunctive therapy for additional symptomatic control (eg, phototherapy, stabilized hypochlorous acid, topical antibiotic treatment) when needed. Avoidance of excessive washing or irritants, food provocation, and emotional stress, as well as toleration of body temperature fluctuations and humidity, is recommended to amend exacerbations.5
Current topical therapies include emollients; corticosteroids; calcineurin inhibitors; and crisaborole, a newer phosphodiesterase 4 inhibitor. There are a number of emollients and moisturizers available, and one over-the-counter preparation showed tolerability and improved skin hydration in AD patients and demonstrated less transepidermal water loss than the control group.8 Ointments such as petrolatum usually do not include ingredients such as preservatives, gelling agents, or humectants that can promote stinging or burning.9 Topical corticosteroids, which ameliorate inflammation by subduing proinflammatory cytokine expression, have been the mainstay of treatment for more than 60 years; however, caution should be used due to the potential for side effects, mainly but not limited to systemic absorption in children, development of striae, and skin atrophy. Calcineurin inhibitors prohibit T-cell activity, modify mast cell response, and decrease dendritic cells in the epidermis. Since 2000, calcineurin inhibitors have been utilized as steroid-sparing agents10; however, prior authorization is still necessary with some insurance providers. Crisaborole ointment 2%, the newest topical agent for AD treatment in the market, has shown improvement of erythema, exudation, and pruritus. Approved for patients aged 2 years and older, twice-daily application of topical crisaborole as a steroid-sparing agent has rendered AD symptom relief.11 It has been reported that 4% of patients encounter stinging or burning with topical crisaborole application, whereas up to 50% of calcineurin inhibitors induce these adverse effects.12 Stabilized hypochlorous acid spray or gel acts as an antipruritic and antimicrobial agent, relieving pain associated with skin irritations. Topical antimicrobial preparations such as mupirocin 2% antibiotic ointment can reduce Staphylococcus colonization when applied in the nasal passage as well as to affected skin lesions.2
In children, UVA and UVB phototherapy has proven safe and effective and can be utilized in AD when suitable.13 When patients inadequately respond to topical therapies and phototherapy, systemic immunomodulatory agents have been recommended as treatment options.A child’s developing immune system indeed may be sensitive to systemic therapies as the innate immune system fully matures in adolescence and his/her adaptive immune system is undergoing vigorous definition.14 Systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate have been used off label for years and pose certain challenges in being identified as durable therapies due to potential side effects. Cyclosporine is effective for the treatment of AD; however, long-term administration should be dosed up to a 12-month period and then stopped to decrease cumulative exposure to the drug. Therefore, further treatment options must be considered. For children, cyclosporine should be administered in a dose of 3 to 6 mg/kg daily. Fluctuations in blood pressure and renal function should be monitored. The recommended pediatric dose for azathioprine is 1 to 4 mg/kg daily with laboratory monitoring, particularly of liver enzymes and complete blood cell count. Obtaining the patient’s thiopurine methyltransferase level may aid in dosing. Gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea are common. Phototherapy is not advised in conjunction with azathioprine due to an increased risk of photocarcinogenicity.13 The literature supporting mycophenolate mofetil in children with AD is limited.
Biologic therapies targeting IgE, B-lymphocyte antigen CD20, IL-5, thymic stromal lymphopoietin, TH17 cells, IL-12, IL-23, interferon gamma, IL-6 receptors, tumor necrosis factor, phosphodiesterase 4, Janus kinase, chymase, and nuclear receptors expressed on adipocytes and immune cells have undergone investigation for treatment of AD.17 Additionally, biologic agents targeting IL-31, IL-13, and IL-22 also have been evaluated.1 Currently, there are no US Food and Drug Administration–approved biologic agents for moderate to severe childhood AD.
Dupilumab, an IL-4Rα and IL-13Rα antagonist, recently has been approved for treatment of moderate to severe AD in adults but not yet for children. Potential side effects include nasopharyngitis, headache, hypersensitivity reactions, and ocular symptoms,11 namely keratitis and conjunctivitis.18 Less than 1% of patients experienced keratitis in clinical trials, while conjunctivitis was reported in 4% of patients taking dupilumab with topical corticosteroids at 52 weeks.18 However, possible ocular findings on slit-lamp examination in AD patients include atopic keratoconjunctivitis, blepharitis, palpebral conjunctival scarring, papillary conjunctival reaction, Horner-Trantas dots, keratoconus, and atopic cataracts. Spontaneous retinal detachment is seen more commonly in individuals with AD than in the general population.19
In clinical trials, hypersensitivity reactions included urticaria and serum sickness or serum sickness–like reactions in less than 1% of patients taking dupilumab.18
Conclusion
Childhood AD can be debilitating, and affected individuals often lead a poorer quality of life if left untreated. Embarrassment and isolation are commonly experienced. Increased responsibility and work in tending for a child with eczema may result in parental exhaustion.21 As with psoriasis, AD can impair activity and productivity.22 Currently, dupilumab has proven to positively impact health-related quality of life for adults.23 Pending the outcome of ongoing pediatric clinical trials, dupilumab may become a benchmark therapy for children younger than 18 years.
- Samalonis L. What’s new in eczema and atopic dermatitis research. The Dermatologist. November 19, 2015. http://www.the-dermatologist.com/content/whats-new-eczema-and-atopic-dermatitis-research. Accessed July 19, 2018.
- Habif T. Atopic dermatitis. In: Bonnet C, Pinczewski A, Cook L, eds. Clinical Dermatology. 5th ed. Edinburgh, Scotland: Mosby Elsevier; 2010:160-180.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case control study [published online October 28, 2017]. J Am Acad Dermatol. 2018;78:336-341.
- Silverberg J. Association between childhood eczema and headaches: an analysis of 19 US population-based studies [published online August 29, 2015]. J Allergy Clin Immunol. 2016;137:492-499.e5.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Saunders Elsevier; 2012:203-216.
- Suarez-Farinas M, Tintle S, Shemer A, et al. Non-lesional atopic dermatitis (AD) skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954-964.
- Hilton L. AD gene mutation identified: discovery may lead to new therapeutic option for patients. Dermatol Times. 2017;38:30.
- Zeichner JA, Dryer L. Effect of CeraVe Healing Ointment on skin hydration and barrier function on normal and barrier-impaired skin. Poster presented at: Orlando Dermatology Aesthetic & Clinical Conference; January 15-16, 2016; Orlando, FL.
- Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22:969-987.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Koutnik-Fotopoulous E. Update on the latest eczema treatments. The Dermatologist. February 17, 2016. http://www.the-dermatologist.com/content/update-latest-eczema-treatments. Accessed August 16, 2018.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Sidbury R, Davis D, Cohen D, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents . J Am Acad Dermatol. 2014;71:327-349.
- van der Merwe R, Gianella-Borradori A. Industry perspective on the clinical development of systemic products for the treatment of atopic dermatitis in pediatric patients with inadequate response to topical prescription therapy. Presented at: FDA Dermatologic and Ophthalmic Drugs Advisory Committee Meeting; March 9, 2015; Silver Spring, MD.
- Heller M, Shin HT, Orlow SJ, et al. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127-132.
- Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Saunders Elsevier; 2013:169-181.
- Guttman-Yassky E, Dhingra N, Leung DY. New era of biological therapeutics in atopic dermatitis [published online January 16, 2013]. Expert Opin Biol Ther. 2013;13:549-561.
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Lowery RS. Ophthalmologic manifestations of atopic dermatitis clinical presentation. Medscape website. emedicine.medscape.com/article/1197636-clinical#b4. Updated September 7, 2016. Accessed July 19, 2018.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the Unites States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol, 2017;77:274-279.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial [published online January 11, 2018]. Br J Dermatol. 2018;178:406-414.
- Samalonis L. What’s new in eczema and atopic dermatitis research. The Dermatologist. November 19, 2015. http://www.the-dermatologist.com/content/whats-new-eczema-and-atopic-dermatitis-research. Accessed July 19, 2018.
- Habif T. Atopic dermatitis. In: Bonnet C, Pinczewski A, Cook L, eds. Clinical Dermatology. 5th ed. Edinburgh, Scotland: Mosby Elsevier; 2010:160-180.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case control study [published online October 28, 2017]. J Am Acad Dermatol. 2018;78:336-341.
- Silverberg J. Association between childhood eczema and headaches: an analysis of 19 US population-based studies [published online August 29, 2015]. J Allergy Clin Immunol. 2016;137:492-499.e5.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Saunders Elsevier; 2012:203-216.
- Suarez-Farinas M, Tintle S, Shemer A, et al. Non-lesional atopic dermatitis (AD) skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954-964.
- Hilton L. AD gene mutation identified: discovery may lead to new therapeutic option for patients. Dermatol Times. 2017;38:30.
- Zeichner JA, Dryer L. Effect of CeraVe Healing Ointment on skin hydration and barrier function on normal and barrier-impaired skin. Poster presented at: Orlando Dermatology Aesthetic & Clinical Conference; January 15-16, 2016; Orlando, FL.
- Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22:969-987.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Koutnik-Fotopoulous E. Update on the latest eczema treatments. The Dermatologist. February 17, 2016. http://www.the-dermatologist.com/content/update-latest-eczema-treatments. Accessed August 16, 2018.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel phosphodiesterase 4 inhibitor for the topical treatment of AD in children and adults [published online July 11, 2016]. J Am Acad Dermatol. 2016;75:494-503.
- Sidbury R, Davis D, Cohen D, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents . J Am Acad Dermatol. 2014;71:327-349.
- van der Merwe R, Gianella-Borradori A. Industry perspective on the clinical development of systemic products for the treatment of atopic dermatitis in pediatric patients with inadequate response to topical prescription therapy. Presented at: FDA Dermatologic and Ophthalmic Drugs Advisory Committee Meeting; March 9, 2015; Silver Spring, MD.
- Heller M, Shin HT, Orlow SJ, et al. Mycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patients. Br J Dermatol. 2007;157:127-132.
- Callen JP, Kulp-Shorten CL. Methotrexate. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Saunders Elsevier; 2013:169-181.
- Guttman-Yassky E, Dhingra N, Leung DY. New era of biological therapeutics in atopic dermatitis [published online January 16, 2013]. Expert Opin Biol Ther. 2013;13:549-561.
- Dupixent [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2017.
- Lowery RS. Ophthalmologic manifestations of atopic dermatitis clinical presentation. Medscape website. emedicine.medscape.com/article/1197636-clinical#b4. Updated September 7, 2016. Accessed July 19, 2018.
- Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601-609.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984-992.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the Unites States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol, 2017;77:274-279.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial [published online January 11, 2018]. Br J Dermatol. 2018;178:406-414.
Practice Points
- Childhood atopic dermatitis can be debilitating, and affected individuals often experience poorer quality of life if left untreated.
- Dupilumab may become a benchmark therapy for children younger than 18 years.