User login
Bedside Microscopy for the Beginner
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
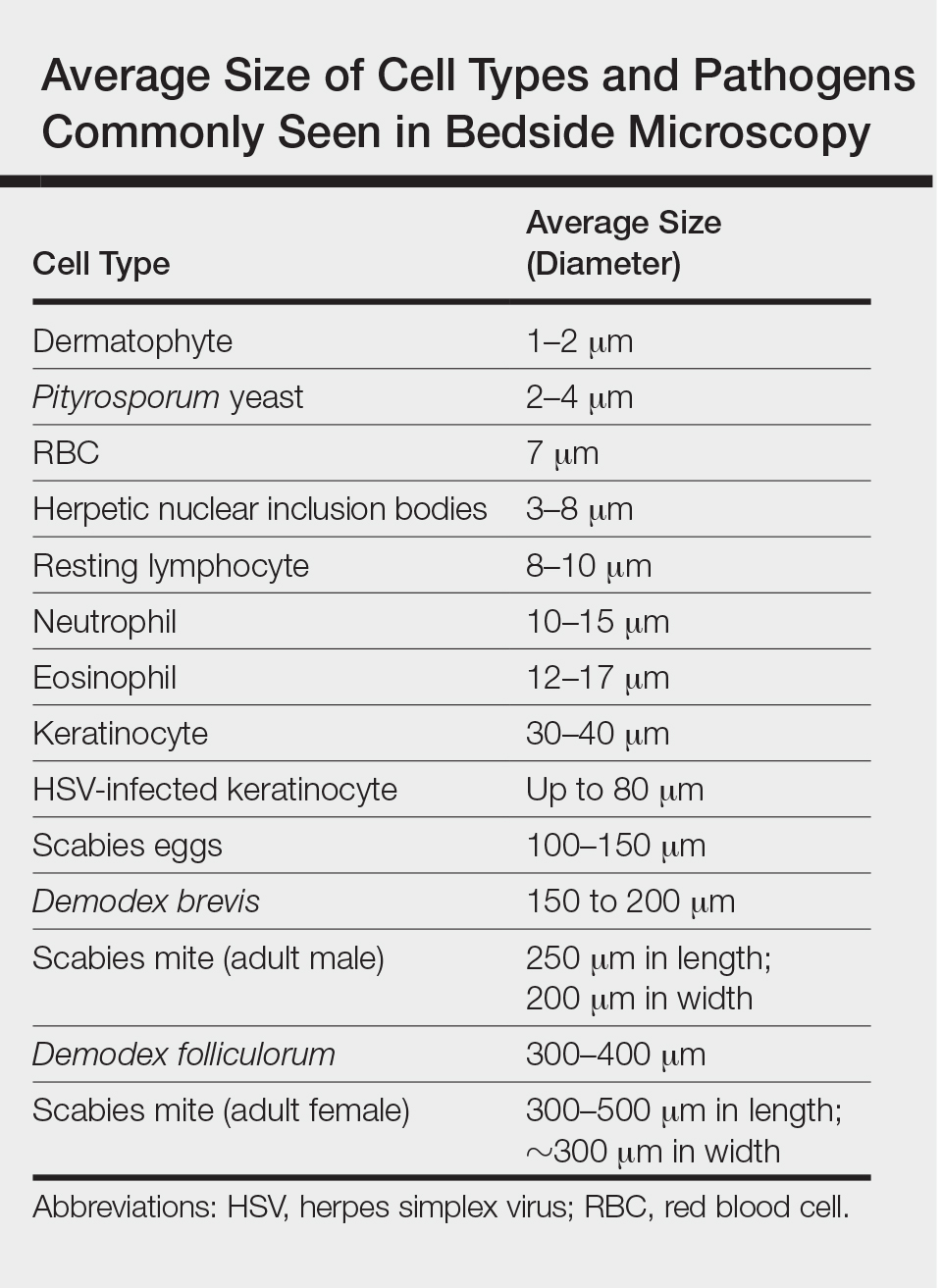
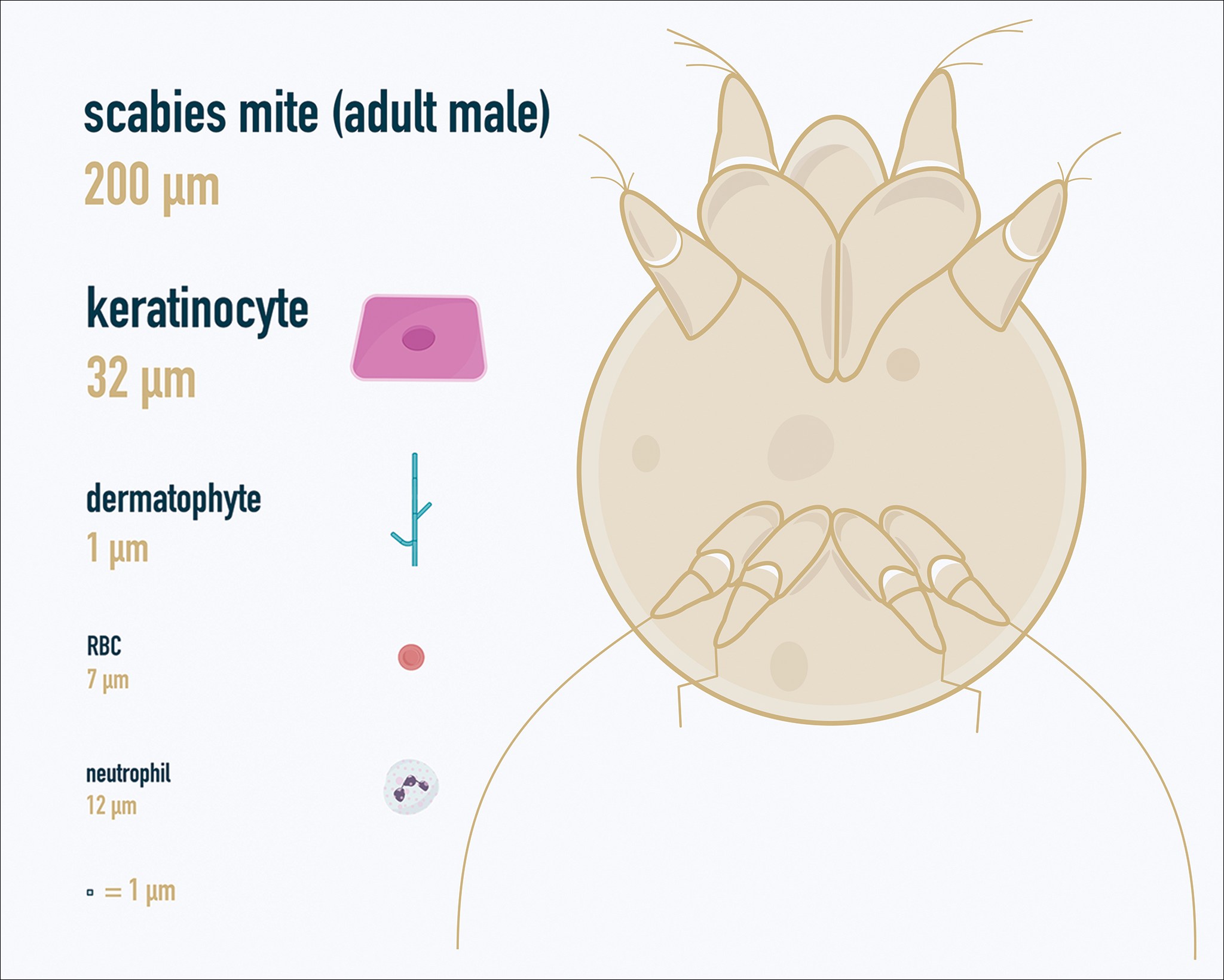
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.
Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
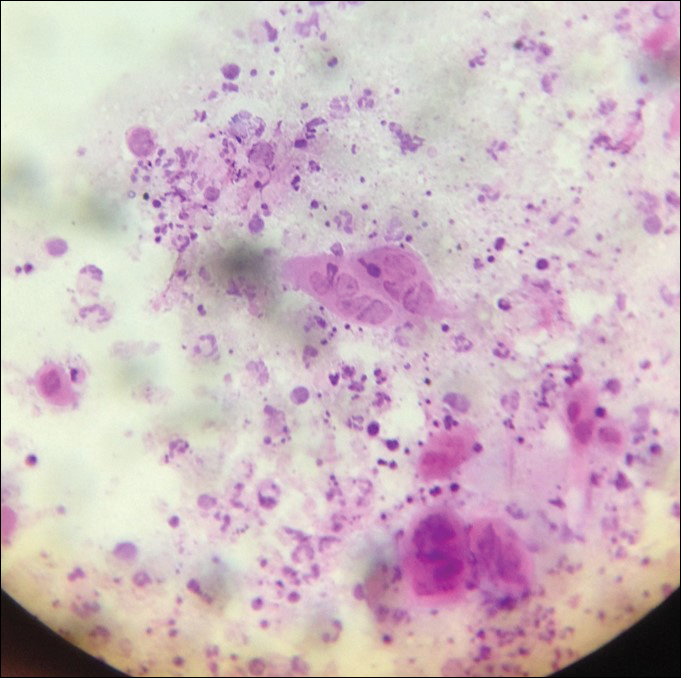
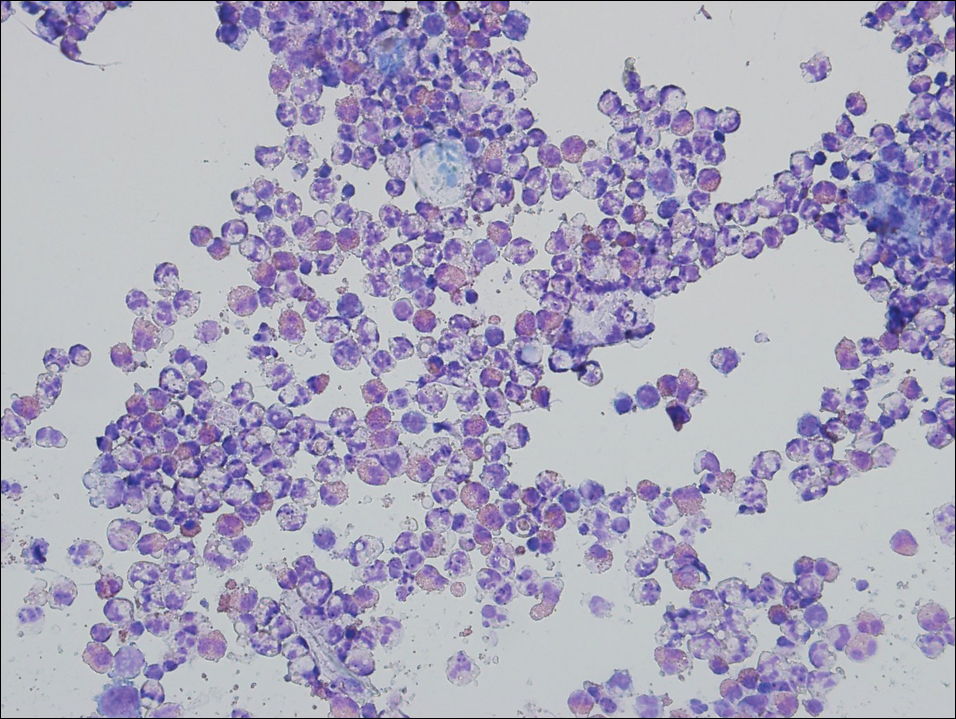
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2
Gout Preparation
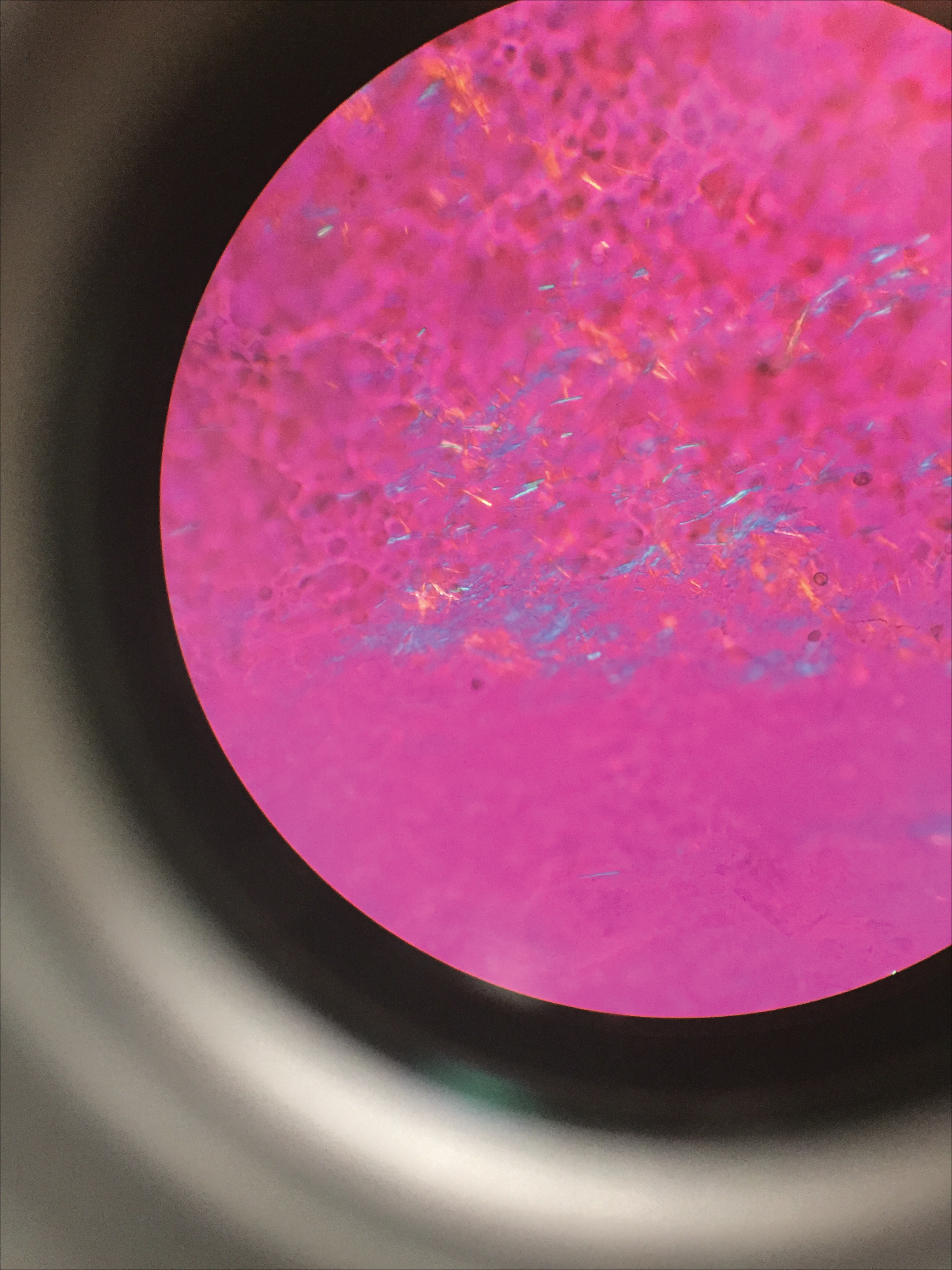
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12
Parasitic Infections
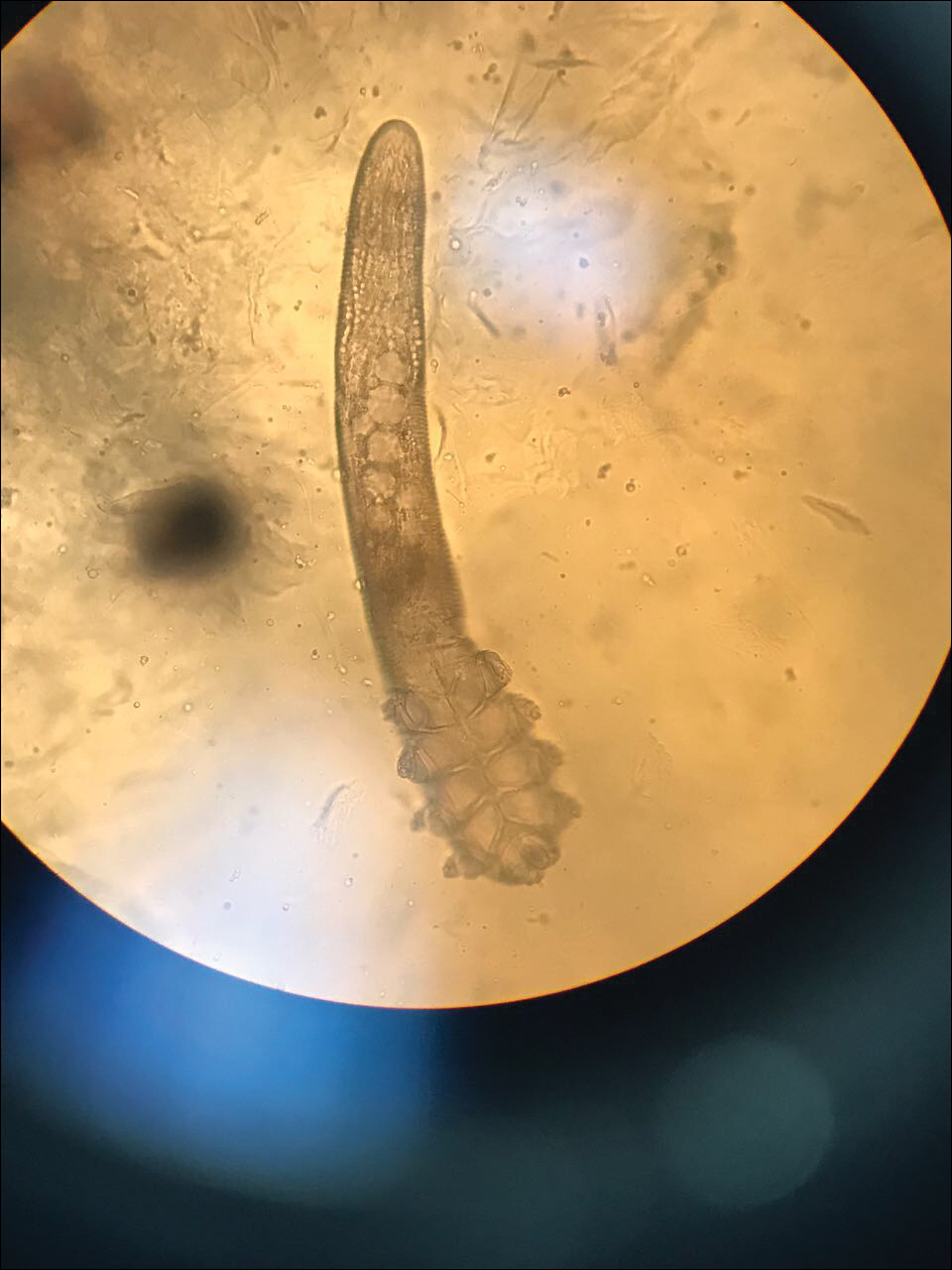
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.
Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.


Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.


Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Docs push back on step therapy in Medicare Advantage
A new policy that allows Medicare Advantage plans to use step therapy to control spending on prescription drug administered in the office is not going over well with doctors.
The Centers for Medicare & Medicaid Services announced the policy change Aug. 7, which will give Medicare Advantage plan sponsors the “choice of implementing step therapy to manage Part B drugs, beginning Jan. 1, 2019,” the agency said in a statement.
The action is part of the broader Trump administration initiative to lower the prices and out-of-pocket costs of prescription drugs as outlined in the American Patients First blueprint.
By “implementing step therapy along with care coordination and drug adherence programs in [Medicare Advantage], it will lower costs and improve the quality of care for Medicare beneficiaries,” CMS officials said in a statement. The move to allow step therapy will give Medicare Advantage plan sponsors the ability to negotiate the designation of a preferred drug, something the agency believes could result in lower prices for these drugs, which in turn will lower the copays for Medicare beneficiaries.
Plan sponsors will be required to pass savings onto beneficiaries through some sort of rewards program, according to a memo detailing the policy change, which also notes that plan rewards “cannot be offered in the form of cash or monetary rebate, but may be offered as gift cards or other items value to all eligible enrollees.”
The value of the rewards must be more than half of the savings generated from implementing the step therapy program, according to the memo.
CMS officials noted that there will be a process that beneficiaries can follow if they believe they need direct access to a drug that would otherwise be available only after failing on another drug.
The American Gastroenterological Association “is concerned that the proposal could limit access for current and future beneficiaries and could add to the growing regulatory burden that physicians already face,” according to a statement. AGA stated that “any change in policy must ensure that patients have access to the appropriate therapies to manage their diseases and not contribute to additional administrative burdens for physician practices.” In addition to responding to CMS, AGA continues to advocate to Congress for patient protections for those subject to step therapy protocols in employer-sponsored health plans; learn more at http:/ow.ly/kp8l30lnDmp.
The new policy applies to only new prescriptions or administrations of Part B drugs. Patients will not have current treatments disrupted if that drug is not the first drug on the step therapy ladder. Additionally, patients will have the opportunity to make a one-time change in plans during the first quarter annually if they are finding the plan is not working for them. Plan sponsors must disclose that Part B drugs may be subject to step therapy.
A new policy that allows Medicare Advantage plans to use step therapy to control spending on prescription drug administered in the office is not going over well with doctors.
The Centers for Medicare & Medicaid Services announced the policy change Aug. 7, which will give Medicare Advantage plan sponsors the “choice of implementing step therapy to manage Part B drugs, beginning Jan. 1, 2019,” the agency said in a statement.
The action is part of the broader Trump administration initiative to lower the prices and out-of-pocket costs of prescription drugs as outlined in the American Patients First blueprint.
By “implementing step therapy along with care coordination and drug adherence programs in [Medicare Advantage], it will lower costs and improve the quality of care for Medicare beneficiaries,” CMS officials said in a statement. The move to allow step therapy will give Medicare Advantage plan sponsors the ability to negotiate the designation of a preferred drug, something the agency believes could result in lower prices for these drugs, which in turn will lower the copays for Medicare beneficiaries.
Plan sponsors will be required to pass savings onto beneficiaries through some sort of rewards program, according to a memo detailing the policy change, which also notes that plan rewards “cannot be offered in the form of cash or monetary rebate, but may be offered as gift cards or other items value to all eligible enrollees.”
The value of the rewards must be more than half of the savings generated from implementing the step therapy program, according to the memo.
CMS officials noted that there will be a process that beneficiaries can follow if they believe they need direct access to a drug that would otherwise be available only after failing on another drug.
The American Gastroenterological Association “is concerned that the proposal could limit access for current and future beneficiaries and could add to the growing regulatory burden that physicians already face,” according to a statement. AGA stated that “any change in policy must ensure that patients have access to the appropriate therapies to manage their diseases and not contribute to additional administrative burdens for physician practices.” In addition to responding to CMS, AGA continues to advocate to Congress for patient protections for those subject to step therapy protocols in employer-sponsored health plans; learn more at http:/ow.ly/kp8l30lnDmp.
The new policy applies to only new prescriptions or administrations of Part B drugs. Patients will not have current treatments disrupted if that drug is not the first drug on the step therapy ladder. Additionally, patients will have the opportunity to make a one-time change in plans during the first quarter annually if they are finding the plan is not working for them. Plan sponsors must disclose that Part B drugs may be subject to step therapy.
A new policy that allows Medicare Advantage plans to use step therapy to control spending on prescription drug administered in the office is not going over well with doctors.
The Centers for Medicare & Medicaid Services announced the policy change Aug. 7, which will give Medicare Advantage plan sponsors the “choice of implementing step therapy to manage Part B drugs, beginning Jan. 1, 2019,” the agency said in a statement.
The action is part of the broader Trump administration initiative to lower the prices and out-of-pocket costs of prescription drugs as outlined in the American Patients First blueprint.
By “implementing step therapy along with care coordination and drug adherence programs in [Medicare Advantage], it will lower costs and improve the quality of care for Medicare beneficiaries,” CMS officials said in a statement. The move to allow step therapy will give Medicare Advantage plan sponsors the ability to negotiate the designation of a preferred drug, something the agency believes could result in lower prices for these drugs, which in turn will lower the copays for Medicare beneficiaries.
Plan sponsors will be required to pass savings onto beneficiaries through some sort of rewards program, according to a memo detailing the policy change, which also notes that plan rewards “cannot be offered in the form of cash or monetary rebate, but may be offered as gift cards or other items value to all eligible enrollees.”
The value of the rewards must be more than half of the savings generated from implementing the step therapy program, according to the memo.
CMS officials noted that there will be a process that beneficiaries can follow if they believe they need direct access to a drug that would otherwise be available only after failing on another drug.
The American Gastroenterological Association “is concerned that the proposal could limit access for current and future beneficiaries and could add to the growing regulatory burden that physicians already face,” according to a statement. AGA stated that “any change in policy must ensure that patients have access to the appropriate therapies to manage their diseases and not contribute to additional administrative burdens for physician practices.” In addition to responding to CMS, AGA continues to advocate to Congress for patient protections for those subject to step therapy protocols in employer-sponsored health plans; learn more at http:/ow.ly/kp8l30lnDmp.
The new policy applies to only new prescriptions or administrations of Part B drugs. Patients will not have current treatments disrupted if that drug is not the first drug on the step therapy ladder. Additionally, patients will have the opportunity to make a one-time change in plans during the first quarter annually if they are finding the plan is not working for them. Plan sponsors must disclose that Part B drugs may be subject to step therapy.
CMS proposes site-neutral payments for hospital outpatient settings
In the proposed update to the Outpatient Prospective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Payment System for 2019, CMS is proposing to apply a physician fee schedule–equivalent for the clinic visit service when provided at an off-campus, provider-based department that is paid under OPPS.
According to CMS, the average current clinical visit paid by CMS is $116 with $23 being the average copay by the patient. If the proposal is finalized, the payment would drop to about $46 with an average patient copay of $9.
“This is intended to address concerns about recent consolidations in the market that reduce competition,” CMS Administrator Seema Verma said during a July 25 press conference.
The American Hospital Association already is pushing back on this proposal.
“With today’s proposed rule, CMS has once again showed a lack of understanding about the reality in which hospitals and health systems operate daily to serve the needs of their communities,” AHA Executive Vice President Tom Nickels said in a statement. “In 2015, Congress clearly intended to provide current off-campus hospital clinics with the existing outpatient payment rate in recognition of the critical role they play in their communities. But CMS’s proposal runs counter to this and will instead impede access to care for the most vulnerable patients.”
The OPPS/ASC update also includes proposals to expand the list of covered surgical procedures that can be performed in an ASC, a move that Ms. Verma said would “provide patients with more choices and options for lower-priced care.”
“For CY 2019, CMS is proposing to allow certain CPT codes outside of the surgical code range that directly crosswalk or are clinically similar to procedures within the CPT surgical code range to be included on the [covered procedure list] and is proposing to add certain cardiovascular codes to the ASC [covered procedure list] as a result,” the CMS fact sheet notes.
Another change proposed by CMS relates to how ASC reimbursement rates are updated. They have been based on the consumer price index-urban, which has resulted in a decline in ASC payments relative to hospitals for the same service. For 2019-2023, CMS proposes to use the hospital market basket instead, which will help promote site neutrality between hospitals and ASCs. The AGA applauds this proposal, and has been working for it with the ACG and ASGE for nearly a decade.
In addition, the OPPS is seeking feedback on a number of topics.
One is related to price transparency. The agency is asking “whether providers and suppliers can and should be required to inform patients about charges and payment information for healthcare services and out-of-pocket costs, what data elements the public would find most useful, and what other charges are needed to empower patients,” according to the fact sheet.
Finally, the agency is seeking more information on solutions to better promote interoperability.
In the proposed update to the Outpatient Prospective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Payment System for 2019, CMS is proposing to apply a physician fee schedule–equivalent for the clinic visit service when provided at an off-campus, provider-based department that is paid under OPPS.
According to CMS, the average current clinical visit paid by CMS is $116 with $23 being the average copay by the patient. If the proposal is finalized, the payment would drop to about $46 with an average patient copay of $9.
“This is intended to address concerns about recent consolidations in the market that reduce competition,” CMS Administrator Seema Verma said during a July 25 press conference.
The American Hospital Association already is pushing back on this proposal.
“With today’s proposed rule, CMS has once again showed a lack of understanding about the reality in which hospitals and health systems operate daily to serve the needs of their communities,” AHA Executive Vice President Tom Nickels said in a statement. “In 2015, Congress clearly intended to provide current off-campus hospital clinics with the existing outpatient payment rate in recognition of the critical role they play in their communities. But CMS’s proposal runs counter to this and will instead impede access to care for the most vulnerable patients.”
The OPPS/ASC update also includes proposals to expand the list of covered surgical procedures that can be performed in an ASC, a move that Ms. Verma said would “provide patients with more choices and options for lower-priced care.”
“For CY 2019, CMS is proposing to allow certain CPT codes outside of the surgical code range that directly crosswalk or are clinically similar to procedures within the CPT surgical code range to be included on the [covered procedure list] and is proposing to add certain cardiovascular codes to the ASC [covered procedure list] as a result,” the CMS fact sheet notes.
Another change proposed by CMS relates to how ASC reimbursement rates are updated. They have been based on the consumer price index-urban, which has resulted in a decline in ASC payments relative to hospitals for the same service. For 2019-2023, CMS proposes to use the hospital market basket instead, which will help promote site neutrality between hospitals and ASCs. The AGA applauds this proposal, and has been working for it with the ACG and ASGE for nearly a decade.
In addition, the OPPS is seeking feedback on a number of topics.
One is related to price transparency. The agency is asking “whether providers and suppliers can and should be required to inform patients about charges and payment information for healthcare services and out-of-pocket costs, what data elements the public would find most useful, and what other charges are needed to empower patients,” according to the fact sheet.
Finally, the agency is seeking more information on solutions to better promote interoperability.
In the proposed update to the Outpatient Prospective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Payment System for 2019, CMS is proposing to apply a physician fee schedule–equivalent for the clinic visit service when provided at an off-campus, provider-based department that is paid under OPPS.
According to CMS, the average current clinical visit paid by CMS is $116 with $23 being the average copay by the patient. If the proposal is finalized, the payment would drop to about $46 with an average patient copay of $9.
“This is intended to address concerns about recent consolidations in the market that reduce competition,” CMS Administrator Seema Verma said during a July 25 press conference.
The American Hospital Association already is pushing back on this proposal.
“With today’s proposed rule, CMS has once again showed a lack of understanding about the reality in which hospitals and health systems operate daily to serve the needs of their communities,” AHA Executive Vice President Tom Nickels said in a statement. “In 2015, Congress clearly intended to provide current off-campus hospital clinics with the existing outpatient payment rate in recognition of the critical role they play in their communities. But CMS’s proposal runs counter to this and will instead impede access to care for the most vulnerable patients.”
The OPPS/ASC update also includes proposals to expand the list of covered surgical procedures that can be performed in an ASC, a move that Ms. Verma said would “provide patients with more choices and options for lower-priced care.”
“For CY 2019, CMS is proposing to allow certain CPT codes outside of the surgical code range that directly crosswalk or are clinically similar to procedures within the CPT surgical code range to be included on the [covered procedure list] and is proposing to add certain cardiovascular codes to the ASC [covered procedure list] as a result,” the CMS fact sheet notes.
Another change proposed by CMS relates to how ASC reimbursement rates are updated. They have been based on the consumer price index-urban, which has resulted in a decline in ASC payments relative to hospitals for the same service. For 2019-2023, CMS proposes to use the hospital market basket instead, which will help promote site neutrality between hospitals and ASCs. The AGA applauds this proposal, and has been working for it with the ACG and ASGE for nearly a decade.
In addition, the OPPS is seeking feedback on a number of topics.
One is related to price transparency. The agency is asking “whether providers and suppliers can and should be required to inform patients about charges and payment information for healthcare services and out-of-pocket costs, what data elements the public would find most useful, and what other charges are needed to empower patients,” according to the fact sheet.
Finally, the agency is seeking more information on solutions to better promote interoperability.
CMS proposal to level E/M payments raises concerns
Citing the need to reduce paperwork hassles, officials at the Centers for Medicare & Medicaid Services are proposing to flatten the payment for evaluation and management (E/M) visits coded at levels 2-5.
The CMS outlined how the proposal would affect payment using 2018 rates to model the change. The proposal would set the payment rate for level 1 E/M office visits for new patients at $44, down from the $45 using the current methodology. Levels 2-5 would receive $135. Currently, payments for level 2 visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For office visits with established patients, the proposed rate would be $24, up from the current payment of $22 for a level 1 visit. Levels 2-5 would receive $93. Under the current methodology, payments for level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
The change also comes with a reduced documentation burden, so the same documentation is needed regardless of which level between 2 and 5 the office visit is, a move that is expected to save time.
The CMS outlined its vision for changes to the E/M payment in the proposed update to the 2019 Medicare physician fee schedule. Comments on the proposal are due Sept. 10, 2018.
The agency estimated that for most specialties, there would be minimal effect on this proposed change. However, for 10 specialties, payment reductions could result from this change. The proposal is raising concerns, particularly from those who stand to see their pay reduced.
CMS officials estimate the proposal would save time. CMS Administrator Seema Verma said that the documentation change would result in an additional 51 hours for patient care per clinician per year.
“The agency has clearly heard from physicians about the need to reduce administrative burdens for physicians,” stated Lisa Gangarosa, MD, AGAF, chair, AGA Government Affairs Committee. “In that regard, CMS should be commended. Unfortunately, in their efforts to reduce burden, CMS has proposed changes that drastically undervalue the care gastroenterologists and hepatologists provide to patients with inflammatory bowel disease, motility disorders, chronic liver disease and other complex gastrointestinal diseases.”
Angus B. Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said he was doubtful that any increase in volume would offset the losses from the proposed flat payment across levels 2-5 E/M visits, especially if the pay decrease results in access issues.
SOURCE: CMS proposed rule, CMS-1693-P.
On July 12, 2018, CMS published a set of “proposed rules” that will have substantial impact on your practice. CMS released a 665-page document with 26 proposed changes in Medicare. A public comment period is open until Sept. 10, 2018. Final rules will be published in the fall with implementation expected in January 2019.
Medicare proposes to reduce the number of E/M coding levels to two (from five: these relate to current CPT codes 99201-99205 and 99211-99215), with documentation requirements reduced to those required for current level 2. If you tend to bill levels 4-5, your bottom line will be affected.
CMS wants to eliminate site-of-service differences in both clinic and ASC payments. This will modify the financial advantages gained by practices who sold their centers to hospital systems and for health systems that have HOPD endoscopy centers and clinics.
Endoscopy with biopsy and colonoscopy with polypectomy were again identified as being potentially overvalued and thus may trigger a re-analysis.
A policy change announced recently by CMS would allow Medicare Advantage plans to establish sequence requirements (step therapy) for medical therapies, including biologics.
While community practices clearly will be affected by these changes, the financial pressures on academic medical centers will be immense. AMC’s have high fixed costs and deteriorating clinical margins. Clinical revenue supports not only clinical enterprises (including faculty salaries) but also a large portion of research and education costs. Loss of 340b pharmacy income, the more government payers, CMS regulations and potential penalties, and narrowing clinical networks all have reduced revenue for many AMCs. Adding these proposed rule changes will send many AMCs further into negative margins: This will affect the training of our next-generation leaders and discoveries of new science.
John I. Allen, MD, MBA, AGAF, professor of medicine, division of gastroenterology, University of Michigan School of Medicine, Ann Arbor. He reported no conflicts.
On July 12, 2018, CMS published a set of “proposed rules” that will have substantial impact on your practice. CMS released a 665-page document with 26 proposed changes in Medicare. A public comment period is open until Sept. 10, 2018. Final rules will be published in the fall with implementation expected in January 2019.
Medicare proposes to reduce the number of E/M coding levels to two (from five: these relate to current CPT codes 99201-99205 and 99211-99215), with documentation requirements reduced to those required for current level 2. If you tend to bill levels 4-5, your bottom line will be affected.
CMS wants to eliminate site-of-service differences in both clinic and ASC payments. This will modify the financial advantages gained by practices who sold their centers to hospital systems and for health systems that have HOPD endoscopy centers and clinics.
Endoscopy with biopsy and colonoscopy with polypectomy were again identified as being potentially overvalued and thus may trigger a re-analysis.
A policy change announced recently by CMS would allow Medicare Advantage plans to establish sequence requirements (step therapy) for medical therapies, including biologics.
While community practices clearly will be affected by these changes, the financial pressures on academic medical centers will be immense. AMC’s have high fixed costs and deteriorating clinical margins. Clinical revenue supports not only clinical enterprises (including faculty salaries) but also a large portion of research and education costs. Loss of 340b pharmacy income, the more government payers, CMS regulations and potential penalties, and narrowing clinical networks all have reduced revenue for many AMCs. Adding these proposed rule changes will send many AMCs further into negative margins: This will affect the training of our next-generation leaders and discoveries of new science.
John I. Allen, MD, MBA, AGAF, professor of medicine, division of gastroenterology, University of Michigan School of Medicine, Ann Arbor. He reported no conflicts.
On July 12, 2018, CMS published a set of “proposed rules” that will have substantial impact on your practice. CMS released a 665-page document with 26 proposed changes in Medicare. A public comment period is open until Sept. 10, 2018. Final rules will be published in the fall with implementation expected in January 2019.
Medicare proposes to reduce the number of E/M coding levels to two (from five: these relate to current CPT codes 99201-99205 and 99211-99215), with documentation requirements reduced to those required for current level 2. If you tend to bill levels 4-5, your bottom line will be affected.
CMS wants to eliminate site-of-service differences in both clinic and ASC payments. This will modify the financial advantages gained by practices who sold their centers to hospital systems and for health systems that have HOPD endoscopy centers and clinics.
Endoscopy with biopsy and colonoscopy with polypectomy were again identified as being potentially overvalued and thus may trigger a re-analysis.
A policy change announced recently by CMS would allow Medicare Advantage plans to establish sequence requirements (step therapy) for medical therapies, including biologics.
While community practices clearly will be affected by these changes, the financial pressures on academic medical centers will be immense. AMC’s have high fixed costs and deteriorating clinical margins. Clinical revenue supports not only clinical enterprises (including faculty salaries) but also a large portion of research and education costs. Loss of 340b pharmacy income, the more government payers, CMS regulations and potential penalties, and narrowing clinical networks all have reduced revenue for many AMCs. Adding these proposed rule changes will send many AMCs further into negative margins: This will affect the training of our next-generation leaders and discoveries of new science.
John I. Allen, MD, MBA, AGAF, professor of medicine, division of gastroenterology, University of Michigan School of Medicine, Ann Arbor. He reported no conflicts.
Citing the need to reduce paperwork hassles, officials at the Centers for Medicare & Medicaid Services are proposing to flatten the payment for evaluation and management (E/M) visits coded at levels 2-5.
The CMS outlined how the proposal would affect payment using 2018 rates to model the change. The proposal would set the payment rate for level 1 E/M office visits for new patients at $44, down from the $45 using the current methodology. Levels 2-5 would receive $135. Currently, payments for level 2 visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For office visits with established patients, the proposed rate would be $24, up from the current payment of $22 for a level 1 visit. Levels 2-5 would receive $93. Under the current methodology, payments for level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
The change also comes with a reduced documentation burden, so the same documentation is needed regardless of which level between 2 and 5 the office visit is, a move that is expected to save time.
The CMS outlined its vision for changes to the E/M payment in the proposed update to the 2019 Medicare physician fee schedule. Comments on the proposal are due Sept. 10, 2018.
The agency estimated that for most specialties, there would be minimal effect on this proposed change. However, for 10 specialties, payment reductions could result from this change. The proposal is raising concerns, particularly from those who stand to see their pay reduced.
CMS officials estimate the proposal would save time. CMS Administrator Seema Verma said that the documentation change would result in an additional 51 hours for patient care per clinician per year.
“The agency has clearly heard from physicians about the need to reduce administrative burdens for physicians,” stated Lisa Gangarosa, MD, AGAF, chair, AGA Government Affairs Committee. “In that regard, CMS should be commended. Unfortunately, in their efforts to reduce burden, CMS has proposed changes that drastically undervalue the care gastroenterologists and hepatologists provide to patients with inflammatory bowel disease, motility disorders, chronic liver disease and other complex gastrointestinal diseases.”
Angus B. Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said he was doubtful that any increase in volume would offset the losses from the proposed flat payment across levels 2-5 E/M visits, especially if the pay decrease results in access issues.
SOURCE: CMS proposed rule, CMS-1693-P.
Citing the need to reduce paperwork hassles, officials at the Centers for Medicare & Medicaid Services are proposing to flatten the payment for evaluation and management (E/M) visits coded at levels 2-5.
The CMS outlined how the proposal would affect payment using 2018 rates to model the change. The proposal would set the payment rate for level 1 E/M office visits for new patients at $44, down from the $45 using the current methodology. Levels 2-5 would receive $135. Currently, payments for level 2 visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For office visits with established patients, the proposed rate would be $24, up from the current payment of $22 for a level 1 visit. Levels 2-5 would receive $93. Under the current methodology, payments for level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
The change also comes with a reduced documentation burden, so the same documentation is needed regardless of which level between 2 and 5 the office visit is, a move that is expected to save time.
The CMS outlined its vision for changes to the E/M payment in the proposed update to the 2019 Medicare physician fee schedule. Comments on the proposal are due Sept. 10, 2018.
The agency estimated that for most specialties, there would be minimal effect on this proposed change. However, for 10 specialties, payment reductions could result from this change. The proposal is raising concerns, particularly from those who stand to see their pay reduced.
CMS officials estimate the proposal would save time. CMS Administrator Seema Verma said that the documentation change would result in an additional 51 hours for patient care per clinician per year.
“The agency has clearly heard from physicians about the need to reduce administrative burdens for physicians,” stated Lisa Gangarosa, MD, AGAF, chair, AGA Government Affairs Committee. “In that regard, CMS should be commended. Unfortunately, in their efforts to reduce burden, CMS has proposed changes that drastically undervalue the care gastroenterologists and hepatologists provide to patients with inflammatory bowel disease, motility disorders, chronic liver disease and other complex gastrointestinal diseases.”
Angus B. Worthing, MD, chair of the American College of Rheumatology’s Committee on Government Affairs, said he was doubtful that any increase in volume would offset the losses from the proposed flat payment across levels 2-5 E/M visits, especially if the pay decrease results in access issues.
SOURCE: CMS proposed rule, CMS-1693-P.
Rapid-onset rash in child
A 7-year-old boy was brought to his family physician for evaluation of a mildly pruritic spreading rash. Ten days earlier, the skin eruption had appeared, and he was given a diagnosis of streptococcal pharyngitis, which was confirmed by a throat swab and a positive antistreptolysin O titer. The child had no personal or family history of skin disorders, including eczema or psoriasis. He hadn’t used any topical agents or new medications recently, nor had he been exposed to triggering plants, animals, or chemicals. There was no history of trauma, friction, or rubbing in the area.
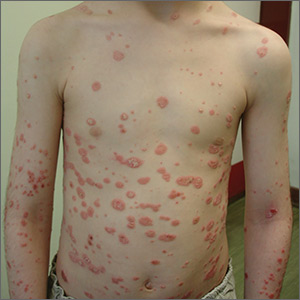
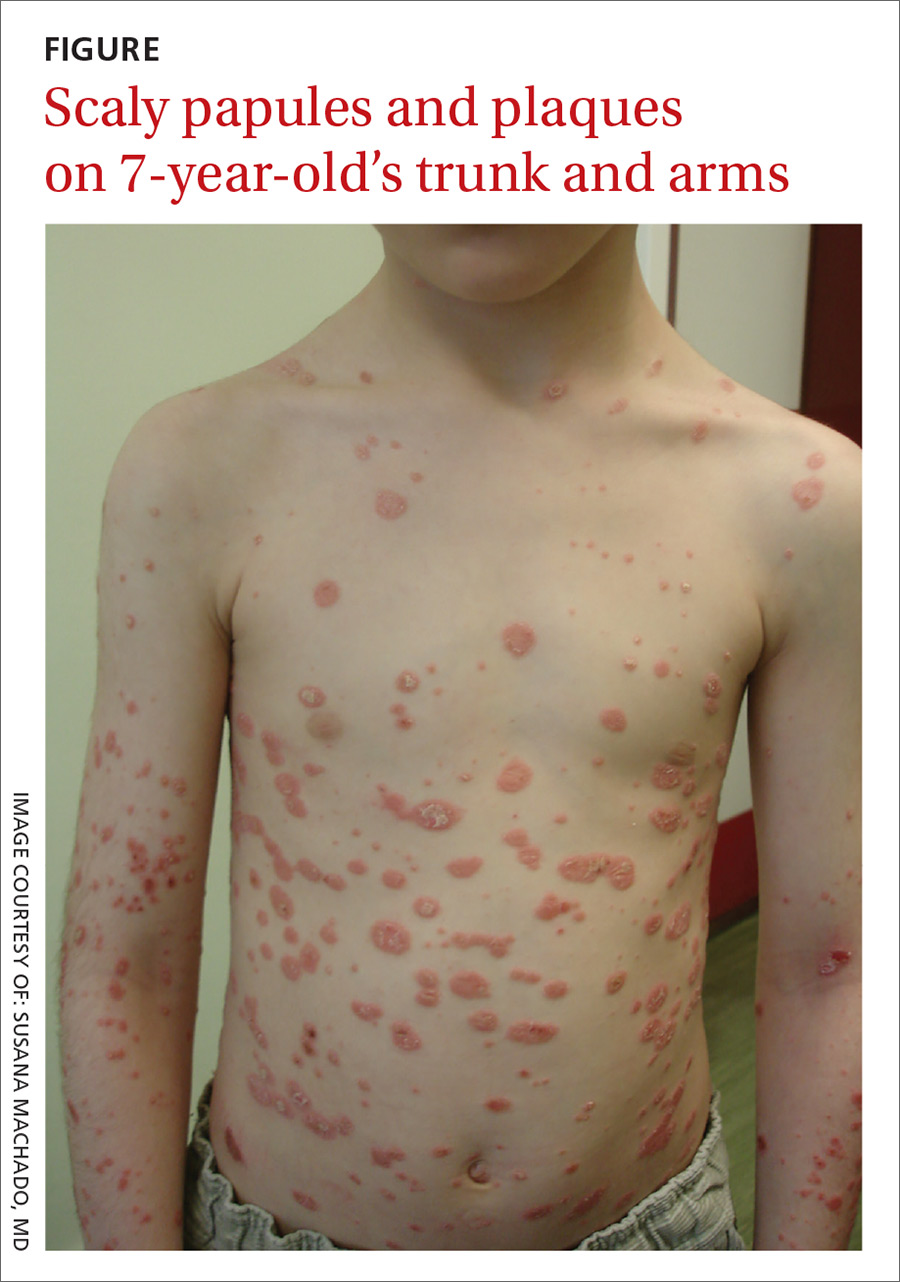
Physical examination revealed multiple erythematous, scaly papules and plaques of varying size on the patient’s trunk, arms, and legs (FIGURE). His palms and soles were spared.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
A diagnosis of guttate psoriasis was made based on the physical exam findings and the preceding group A beta-hemolytic streptococcal infection.
This condition affects approximately 2% of all patients with psoriasis; it is characterized by the acute onset of multiple erythematosquamous papules and small plaques that look like droplets (“gutta”).1 It tends to affect children and young adults and typically occurs following an acute infection (eg, streptococcal pharyngitis).2,3 In this case, a rapid strep test and throat culture positive for group A Streptococcus supported the diagnosis.
Although this particular phenotype of psoriasis is usually associated with streptococcal infection and mainly occurs in patients with the HLA-Cw6+ allele, the specific immunologic response that causes these skin lesions is poorly understood.4 Antigenic similarities between streptococcal proteins and keratinocyte antigens might explain why the condition is triggered by streptococcal infections.5
Pityriasis rosea and tinea corporis are part of the differential
The differential includes skin conditions such as pityriasis rosea, tinea corporis, varicella, and insect bites.
Pityriasis rosea can manifest as a papulosquamous eruption, but it has an inward-facing scale, called a collarette. The “Christmas tree” pattern on the back that is preceded by a solitary 2- to 10-cm oval, pink, scaly herald patch (in 17%-50% of cases) is key to the diagnosis.6 (For more information, see “Rash on trunk and upper arms.”)
Continue to: Tinea corporis...
Tinea corporis is a dermatophyte infection that causes flat, red, scaly lesions that progress into annular lesions with central clearing or brown discoloration. The plaques can range from a few centimeters to several inches in size, but are always characterized by the slowly advancing border.6
Varicella also affects the trunk and extremities, but a key clinical finding is crops of characteristic lesions, including papules, vesicles, pustules, and crusted lesions in different stages that manifest simultaneously.6
Insect bites usually appear as urticarial papules and plaques associated with outdoor exposure. The lesions are distributed where insects are likely to bite.6
Treat the infection, control the psoriasis
The first-line treatment for streptococcal infection is amoxicillin (50 mg/kg/d [maximum: 1000 mg/d] orally for 10 d) or penicillin G benzathine (for children < 60 lb, 6 × 105 units intramuscularly; children ≥ 60 lb, 1.2 × 106 units intramuscularly).7 For the psoriasis lesions, treatment options include topical glucocorticosteroids, vitamin D derivatives, or combinations of both.5 In most cases, guttate psoriasis completely resolves. However, one-third of children with guttate psoriasis go on to develop plaque psoriasis later in life.8
Our patient was treated with penicillin G benzathine (1.2 × 106 units intramuscularly) and a calcipotriol/betamethasone combination gel. The streptococcal infection and skin lesions completely resolved. No adverse events were reported, and no relapse was observed after 3 months.
CORRESPONDENCE
Rita Matos, MD, Rua Actor Mário Viegas SN Rio Tinto, Portugal; [email protected]
1. Maciejewska-Radomska A, Szczerkowska-Dobosz A, Rebała K, et al. Frequency of streptococcal upper respiratory tract infections and HLA-Cw*06 allele in 70 patients with guttate psoriasis from northern Poland. Postepy Dermatol Alergol. 2015;32:455-458.
2. Garritsen FM, Kraag DE, de Graaf M. Guttate psoriasis triggered by perianal streptococcal infection. Clin Exp Dermatol. 2017;42:536-538.
3. Pfingstler LF, Maroon M, Mowad C. Guttate psoriasis outcomes. Cutis. 2016;97:140-144.
4. Ruiz-Romeu E, Ferran M, Sagristà M, et al. Streptococcus pyogenes-induced cutaneous lymphocyte antigen-positive T cell-dependent epidermal cell activation triggers TH17 responses in patients with guttate psoriasis. J Allergy Clin Immunol. 2016;138:491-499.
5. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
6. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
7. Kalra MG, Higgins KE, Perez ED. Common questions about streptococcal pharyngitis. Am Fam Physician. 2016;94:24-31.
8. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132: 717-718.
A 7-year-old boy was brought to his family physician for evaluation of a mildly pruritic spreading rash. Ten days earlier, the skin eruption had appeared, and he was given a diagnosis of streptococcal pharyngitis, which was confirmed by a throat swab and a positive antistreptolysin O titer. The child had no personal or family history of skin disorders, including eczema or psoriasis. He hadn’t used any topical agents or new medications recently, nor had he been exposed to triggering plants, animals, or chemicals. There was no history of trauma, friction, or rubbing in the area.
Physical examination revealed multiple erythematous, scaly papules and plaques of varying size on the patient’s trunk, arms, and legs (FIGURE). His palms and soles were spared.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
A diagnosis of guttate psoriasis was made based on the physical exam findings and the preceding group A beta-hemolytic streptococcal infection.
This condition affects approximately 2% of all patients with psoriasis; it is characterized by the acute onset of multiple erythematosquamous papules and small plaques that look like droplets (“gutta”).1 It tends to affect children and young adults and typically occurs following an acute infection (eg, streptococcal pharyngitis).2,3 In this case, a rapid strep test and throat culture positive for group A Streptococcus supported the diagnosis.
Although this particular phenotype of psoriasis is usually associated with streptococcal infection and mainly occurs in patients with the HLA-Cw6+ allele, the specific immunologic response that causes these skin lesions is poorly understood.4 Antigenic similarities between streptococcal proteins and keratinocyte antigens might explain why the condition is triggered by streptococcal infections.5
Pityriasis rosea and tinea corporis are part of the differential
The differential includes skin conditions such as pityriasis rosea, tinea corporis, varicella, and insect bites.
Pityriasis rosea can manifest as a papulosquamous eruption, but it has an inward-facing scale, called a collarette. The “Christmas tree” pattern on the back that is preceded by a solitary 2- to 10-cm oval, pink, scaly herald patch (in 17%-50% of cases) is key to the diagnosis.6 (For more information, see “Rash on trunk and upper arms.”)
Continue to: Tinea corporis...
Tinea corporis is a dermatophyte infection that causes flat, red, scaly lesions that progress into annular lesions with central clearing or brown discoloration. The plaques can range from a few centimeters to several inches in size, but are always characterized by the slowly advancing border.6
Varicella also affects the trunk and extremities, but a key clinical finding is crops of characteristic lesions, including papules, vesicles, pustules, and crusted lesions in different stages that manifest simultaneously.6
Insect bites usually appear as urticarial papules and plaques associated with outdoor exposure. The lesions are distributed where insects are likely to bite.6
Treat the infection, control the psoriasis
The first-line treatment for streptococcal infection is amoxicillin (50 mg/kg/d [maximum: 1000 mg/d] orally for 10 d) or penicillin G benzathine (for children < 60 lb, 6 × 105 units intramuscularly; children ≥ 60 lb, 1.2 × 106 units intramuscularly).7 For the psoriasis lesions, treatment options include topical glucocorticosteroids, vitamin D derivatives, or combinations of both.5 In most cases, guttate psoriasis completely resolves. However, one-third of children with guttate psoriasis go on to develop plaque psoriasis later in life.8
Our patient was treated with penicillin G benzathine (1.2 × 106 units intramuscularly) and a calcipotriol/betamethasone combination gel. The streptococcal infection and skin lesions completely resolved. No adverse events were reported, and no relapse was observed after 3 months.
CORRESPONDENCE
Rita Matos, MD, Rua Actor Mário Viegas SN Rio Tinto, Portugal; [email protected]
A 7-year-old boy was brought to his family physician for evaluation of a mildly pruritic spreading rash. Ten days earlier, the skin eruption had appeared, and he was given a diagnosis of streptococcal pharyngitis, which was confirmed by a throat swab and a positive antistreptolysin O titer. The child had no personal or family history of skin disorders, including eczema or psoriasis. He hadn’t used any topical agents or new medications recently, nor had he been exposed to triggering plants, animals, or chemicals. There was no history of trauma, friction, or rubbing in the area.
Physical examination revealed multiple erythematous, scaly papules and plaques of varying size on the patient’s trunk, arms, and legs (FIGURE). His palms and soles were spared.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
A diagnosis of guttate psoriasis was made based on the physical exam findings and the preceding group A beta-hemolytic streptococcal infection.
This condition affects approximately 2% of all patients with psoriasis; it is characterized by the acute onset of multiple erythematosquamous papules and small plaques that look like droplets (“gutta”).1 It tends to affect children and young adults and typically occurs following an acute infection (eg, streptococcal pharyngitis).2,3 In this case, a rapid strep test and throat culture positive for group A Streptococcus supported the diagnosis.
Although this particular phenotype of psoriasis is usually associated with streptococcal infection and mainly occurs in patients with the HLA-Cw6+ allele, the specific immunologic response that causes these skin lesions is poorly understood.4 Antigenic similarities between streptococcal proteins and keratinocyte antigens might explain why the condition is triggered by streptococcal infections.5
Pityriasis rosea and tinea corporis are part of the differential
The differential includes skin conditions such as pityriasis rosea, tinea corporis, varicella, and insect bites.
Pityriasis rosea can manifest as a papulosquamous eruption, but it has an inward-facing scale, called a collarette. The “Christmas tree” pattern on the back that is preceded by a solitary 2- to 10-cm oval, pink, scaly herald patch (in 17%-50% of cases) is key to the diagnosis.6 (For more information, see “Rash on trunk and upper arms.”)
Continue to: Tinea corporis...
Tinea corporis is a dermatophyte infection that causes flat, red, scaly lesions that progress into annular lesions with central clearing or brown discoloration. The plaques can range from a few centimeters to several inches in size, but are always characterized by the slowly advancing border.6
Varicella also affects the trunk and extremities, but a key clinical finding is crops of characteristic lesions, including papules, vesicles, pustules, and crusted lesions in different stages that manifest simultaneously.6
Insect bites usually appear as urticarial papules and plaques associated with outdoor exposure. The lesions are distributed where insects are likely to bite.6
Treat the infection, control the psoriasis
The first-line treatment for streptococcal infection is amoxicillin (50 mg/kg/d [maximum: 1000 mg/d] orally for 10 d) or penicillin G benzathine (for children < 60 lb, 6 × 105 units intramuscularly; children ≥ 60 lb, 1.2 × 106 units intramuscularly).7 For the psoriasis lesions, treatment options include topical glucocorticosteroids, vitamin D derivatives, or combinations of both.5 In most cases, guttate psoriasis completely resolves. However, one-third of children with guttate psoriasis go on to develop plaque psoriasis later in life.8
Our patient was treated with penicillin G benzathine (1.2 × 106 units intramuscularly) and a calcipotriol/betamethasone combination gel. The streptococcal infection and skin lesions completely resolved. No adverse events were reported, and no relapse was observed after 3 months.
CORRESPONDENCE
Rita Matos, MD, Rua Actor Mário Viegas SN Rio Tinto, Portugal; [email protected]
1. Maciejewska-Radomska A, Szczerkowska-Dobosz A, Rebała K, et al. Frequency of streptococcal upper respiratory tract infections and HLA-Cw*06 allele in 70 patients with guttate psoriasis from northern Poland. Postepy Dermatol Alergol. 2015;32:455-458.
2. Garritsen FM, Kraag DE, de Graaf M. Guttate psoriasis triggered by perianal streptococcal infection. Clin Exp Dermatol. 2017;42:536-538.
3. Pfingstler LF, Maroon M, Mowad C. Guttate psoriasis outcomes. Cutis. 2016;97:140-144.
4. Ruiz-Romeu E, Ferran M, Sagristà M, et al. Streptococcus pyogenes-induced cutaneous lymphocyte antigen-positive T cell-dependent epidermal cell activation triggers TH17 responses in patients with guttate psoriasis. J Allergy Clin Immunol. 2016;138:491-499.
5. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
6. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
7. Kalra MG, Higgins KE, Perez ED. Common questions about streptococcal pharyngitis. Am Fam Physician. 2016;94:24-31.
8. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132: 717-718.
1. Maciejewska-Radomska A, Szczerkowska-Dobosz A, Rebała K, et al. Frequency of streptococcal upper respiratory tract infections and HLA-Cw*06 allele in 70 patients with guttate psoriasis from northern Poland. Postepy Dermatol Alergol. 2015;32:455-458.
2. Garritsen FM, Kraag DE, de Graaf M. Guttate psoriasis triggered by perianal streptococcal infection. Clin Exp Dermatol. 2017;42:536-538.
3. Pfingstler LF, Maroon M, Mowad C. Guttate psoriasis outcomes. Cutis. 2016;97:140-144.
4. Ruiz-Romeu E, Ferran M, Sagristà M, et al. Streptococcus pyogenes-induced cutaneous lymphocyte antigen-positive T cell-dependent epidermal cell activation triggers TH17 responses in patients with guttate psoriasis. J Allergy Clin Immunol. 2016;138:491-499.
5. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
6. Ely JW, Seabury Stone M. The generalized rash: part I. Differential diagnosis. Am Fam Physician. 2010;81:726-734.
7. Kalra MG, Higgins KE, Perez ED. Common questions about streptococcal pharyngitis. Am Fam Physician. 2016;94:24-31.
8. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132: 717-718.
Strides in digestive cancer research: Two research projects to note
The AGA Research Foundation Research Awards Program includes two grants dedicated to digestive cancer research: the AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer and the AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer.
Continue reading to learn about the novel research projects being conducted by our 2018 digestive cancer grant recipients.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Ravikanth Maddipati, MD
University of Pennsylvania Hospital System, Philadelphia
Dr. Maddipati’s research focuses on understanding and treating metastatic disease in pancreatic cancer. With this grant, Dr. Maddipati will use advanced lineage-traced mouse models and innovative bioengineering approaches to identify the molecular pathways involved in tumor cell cooperation and define the role of circulating tumor cell-clusters in pancreatic cancer progression. This work will lead to a noninvasive method for monitoring disease progression and response to treatment in pancreatic cancer patients.
AGA’s take: Pancreatic cancer, namely pancreatic ductal adenocarcinoma, is one of the deadliest cancers in the U.S. The AGA Research Foundation is pleased to fund Dr. Maddipati’s research. As a physician-scientist, he is in a unique position to translate findings from basic research, using preclinical models, to develop improved approaches to treating patients with pancreatic cancer.
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Jingwu Xie, PhD
Indiana University, Indianapolis
Dr. Xie’s research focuses on drug resistance in gastric cancer. His team recently had a monumental discovery — activated hedgehog signaling, via GLI1 and GLI2 gene up-regulation, is responsible for drug resistance in gastric cancer. Dr. Xie’s AGA-funded research will work to identify novel ways to sensitize gastric cancer cells to drug treatment by suppressing GLI1 and GLI2 activity.
AGA’s take: Gastric cancer is a very underfunded area of research in the U.S., and with limited treatment options for patients, there is a great need for novel research projects. The AGA Research Foundation is proud to fund Dr. Xie’s research, which we believe has the potential to translate into a new treatment that will improve outcomes for gastric cancer patients.
To see the full class of 2018 AGA Research Foundation awardees, visit the Meet Our Awardees section of our website, www.gastro.org/foundation-awardees.
The AGA Research Foundation Research Awards Program includes two grants dedicated to digestive cancer research: the AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer and the AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer.
Continue reading to learn about the novel research projects being conducted by our 2018 digestive cancer grant recipients.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Ravikanth Maddipati, MD
University of Pennsylvania Hospital System, Philadelphia
Dr. Maddipati’s research focuses on understanding and treating metastatic disease in pancreatic cancer. With this grant, Dr. Maddipati will use advanced lineage-traced mouse models and innovative bioengineering approaches to identify the molecular pathways involved in tumor cell cooperation and define the role of circulating tumor cell-clusters in pancreatic cancer progression. This work will lead to a noninvasive method for monitoring disease progression and response to treatment in pancreatic cancer patients.
AGA’s take: Pancreatic cancer, namely pancreatic ductal adenocarcinoma, is one of the deadliest cancers in the U.S. The AGA Research Foundation is pleased to fund Dr. Maddipati’s research. As a physician-scientist, he is in a unique position to translate findings from basic research, using preclinical models, to develop improved approaches to treating patients with pancreatic cancer.
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Jingwu Xie, PhD
Indiana University, Indianapolis
Dr. Xie’s research focuses on drug resistance in gastric cancer. His team recently had a monumental discovery — activated hedgehog signaling, via GLI1 and GLI2 gene up-regulation, is responsible for drug resistance in gastric cancer. Dr. Xie’s AGA-funded research will work to identify novel ways to sensitize gastric cancer cells to drug treatment by suppressing GLI1 and GLI2 activity.
AGA’s take: Gastric cancer is a very underfunded area of research in the U.S., and with limited treatment options for patients, there is a great need for novel research projects. The AGA Research Foundation is proud to fund Dr. Xie’s research, which we believe has the potential to translate into a new treatment that will improve outcomes for gastric cancer patients.
To see the full class of 2018 AGA Research Foundation awardees, visit the Meet Our Awardees section of our website, www.gastro.org/foundation-awardees.
The AGA Research Foundation Research Awards Program includes two grants dedicated to digestive cancer research: the AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer and the AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer.
Continue reading to learn about the novel research projects being conducted by our 2018 digestive cancer grant recipients.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Ravikanth Maddipati, MD
University of Pennsylvania Hospital System, Philadelphia
Dr. Maddipati’s research focuses on understanding and treating metastatic disease in pancreatic cancer. With this grant, Dr. Maddipati will use advanced lineage-traced mouse models and innovative bioengineering approaches to identify the molecular pathways involved in tumor cell cooperation and define the role of circulating tumor cell-clusters in pancreatic cancer progression. This work will lead to a noninvasive method for monitoring disease progression and response to treatment in pancreatic cancer patients.
AGA’s take: Pancreatic cancer, namely pancreatic ductal adenocarcinoma, is one of the deadliest cancers in the U.S. The AGA Research Foundation is pleased to fund Dr. Maddipati’s research. As a physician-scientist, he is in a unique position to translate findings from basic research, using preclinical models, to develop improved approaches to treating patients with pancreatic cancer.
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Jingwu Xie, PhD
Indiana University, Indianapolis
Dr. Xie’s research focuses on drug resistance in gastric cancer. His team recently had a monumental discovery — activated hedgehog signaling, via GLI1 and GLI2 gene up-regulation, is responsible for drug resistance in gastric cancer. Dr. Xie’s AGA-funded research will work to identify novel ways to sensitize gastric cancer cells to drug treatment by suppressing GLI1 and GLI2 activity.
AGA’s take: Gastric cancer is a very underfunded area of research in the U.S., and with limited treatment options for patients, there is a great need for novel research projects. The AGA Research Foundation is proud to fund Dr. Xie’s research, which we believe has the potential to translate into a new treatment that will improve outcomes for gastric cancer patients.
To see the full class of 2018 AGA Research Foundation awardees, visit the Meet Our Awardees section of our website, www.gastro.org/foundation-awardees.
Rivaroxaban superior to aspirin for extended VTE treatment
Both low-dose and full-dose rivaroxaban had superior benefit-risk profiles for extended venous thromboembolism (VTE) treatment compared with aspirin, according to results published in Thrombosis Research.
Incidences of the combined outcome of recurrent VTE and major bleeding were 2.8% and 3.4% lower for patients treated with rivaroxaban at 20 mg and 10 mg, respectively, than for those treated with aspirin, reported Paolo Prandoni, MD, of the department of cardiothoracic and vascular sciences at the University of Padua, Italy, and his coauthors.
Investigators analyzed data from the EINSTEIN-CHOICE trial, a double-blind, randomized study of 3,365 patients aged 18 years or older with deep vein thrombosis or pulmonary embolism who had previously received anticoagulant treatment for 6-12 months. Patients were given either once-daily rivaroxaban at a low dose (10 mg), once-daily rivaroxaban at full dose (20 mg), or once- daily aspirin at a dose of 100 mg.
Benefit and risk were calculated using “excess numbers of events,” or the difference in cumulative incidences in a hypothetical population of 10,000 VTE patients treated for 1 year. Excess numbers of events were defined as the number of patients in this hypothetical population who would experience a particular event when treated with rivaroxaban (at either dose), minus that in the same population treated with aspirin.
The cumulative incidences of recurrent VTE in the full-dose rivaroxaban, low-dose rivaroxaban, and aspirin groups were 1.9%, 1.6%, and 5.0%, respectively. The cumulative incidences of major bleeding in these groups were 0.7%, 0.4% and 0.5%, respectively.
In patients treated with 20 mg of rivaroxaban instead of aspirin, there would be 123 fewer episodes of pulmonary embolism (95% confidence interval, 21-226) and 198 fewer episodes of deep vein thrombosis (95% CI, 62-333).
In patients given 10 mg of rivaroxaban instead of aspirin, there would be 121 fewer episodes of pulmonary embolism (95% CI, 4-238) and 217 fewer episodes of deep vein thrombosis (95% CI, 92-342), Dr. Prandoni and his colleagues wrote.
Net clinical benefit was defined as the composite of symptomatic recurrent VTE and major bleeding events, and occurred in 23 patients in the full-dose rivaroxaban group, 17 patients in the low-dose rivaroxaban group, and 53 patients in the aspirin group.
For 10,000 patients treated for 1 year with rivaroxaban instead of aspirin, there would be 284 fewer net clinical benefit outcomes for the 20-mg dose (95% CI, 106-462) and 339 fewer (95% CI, 165-512) for the 10-mg dose. “Thus, compared with aspirin, one additional symptomatic recurrent VTE or major bleed would be avoided for every 36 or 30 patients treated with rivaroxaban 20 mg or 10 mg, respectively,” the investigators wrote.
The findings indicate that there is “no longer a place” for extended VTE treatment with aspirin, the investigators said.
“Extended anticoagulation with once daily rivaroxaban ... provides a clinically important benefit in terms of reduction in recurrent VTE,” they wrote. “Regardless of which dose is chosen ... rivaroxaban has a favourable benefit-risk profile relative to aspirin.”
Bayer AG funded the study. Dr. Prandoni reported financial relationships with Bayer, Sanofi, Daiichi Sankyo, and Pfizer.
SOURCE: Prandoni P et al. Thromb Res. 2018 Aug;168:121-9.
Both low-dose and full-dose rivaroxaban had superior benefit-risk profiles for extended venous thromboembolism (VTE) treatment compared with aspirin, according to results published in Thrombosis Research.
Incidences of the combined outcome of recurrent VTE and major bleeding were 2.8% and 3.4% lower for patients treated with rivaroxaban at 20 mg and 10 mg, respectively, than for those treated with aspirin, reported Paolo Prandoni, MD, of the department of cardiothoracic and vascular sciences at the University of Padua, Italy, and his coauthors.
Investigators analyzed data from the EINSTEIN-CHOICE trial, a double-blind, randomized study of 3,365 patients aged 18 years or older with deep vein thrombosis or pulmonary embolism who had previously received anticoagulant treatment for 6-12 months. Patients were given either once-daily rivaroxaban at a low dose (10 mg), once-daily rivaroxaban at full dose (20 mg), or once- daily aspirin at a dose of 100 mg.
Benefit and risk were calculated using “excess numbers of events,” or the difference in cumulative incidences in a hypothetical population of 10,000 VTE patients treated for 1 year. Excess numbers of events were defined as the number of patients in this hypothetical population who would experience a particular event when treated with rivaroxaban (at either dose), minus that in the same population treated with aspirin.
The cumulative incidences of recurrent VTE in the full-dose rivaroxaban, low-dose rivaroxaban, and aspirin groups were 1.9%, 1.6%, and 5.0%, respectively. The cumulative incidences of major bleeding in these groups were 0.7%, 0.4% and 0.5%, respectively.
In patients treated with 20 mg of rivaroxaban instead of aspirin, there would be 123 fewer episodes of pulmonary embolism (95% confidence interval, 21-226) and 198 fewer episodes of deep vein thrombosis (95% CI, 62-333).
In patients given 10 mg of rivaroxaban instead of aspirin, there would be 121 fewer episodes of pulmonary embolism (95% CI, 4-238) and 217 fewer episodes of deep vein thrombosis (95% CI, 92-342), Dr. Prandoni and his colleagues wrote.
Net clinical benefit was defined as the composite of symptomatic recurrent VTE and major bleeding events, and occurred in 23 patients in the full-dose rivaroxaban group, 17 patients in the low-dose rivaroxaban group, and 53 patients in the aspirin group.
For 10,000 patients treated for 1 year with rivaroxaban instead of aspirin, there would be 284 fewer net clinical benefit outcomes for the 20-mg dose (95% CI, 106-462) and 339 fewer (95% CI, 165-512) for the 10-mg dose. “Thus, compared with aspirin, one additional symptomatic recurrent VTE or major bleed would be avoided for every 36 or 30 patients treated with rivaroxaban 20 mg or 10 mg, respectively,” the investigators wrote.
The findings indicate that there is “no longer a place” for extended VTE treatment with aspirin, the investigators said.
“Extended anticoagulation with once daily rivaroxaban ... provides a clinically important benefit in terms of reduction in recurrent VTE,” they wrote. “Regardless of which dose is chosen ... rivaroxaban has a favourable benefit-risk profile relative to aspirin.”
Bayer AG funded the study. Dr. Prandoni reported financial relationships with Bayer, Sanofi, Daiichi Sankyo, and Pfizer.
SOURCE: Prandoni P et al. Thromb Res. 2018 Aug;168:121-9.
Both low-dose and full-dose rivaroxaban had superior benefit-risk profiles for extended venous thromboembolism (VTE) treatment compared with aspirin, according to results published in Thrombosis Research.
Incidences of the combined outcome of recurrent VTE and major bleeding were 2.8% and 3.4% lower for patients treated with rivaroxaban at 20 mg and 10 mg, respectively, than for those treated with aspirin, reported Paolo Prandoni, MD, of the department of cardiothoracic and vascular sciences at the University of Padua, Italy, and his coauthors.
Investigators analyzed data from the EINSTEIN-CHOICE trial, a double-blind, randomized study of 3,365 patients aged 18 years or older with deep vein thrombosis or pulmonary embolism who had previously received anticoagulant treatment for 6-12 months. Patients were given either once-daily rivaroxaban at a low dose (10 mg), once-daily rivaroxaban at full dose (20 mg), or once- daily aspirin at a dose of 100 mg.
Benefit and risk were calculated using “excess numbers of events,” or the difference in cumulative incidences in a hypothetical population of 10,000 VTE patients treated for 1 year. Excess numbers of events were defined as the number of patients in this hypothetical population who would experience a particular event when treated with rivaroxaban (at either dose), minus that in the same population treated with aspirin.
The cumulative incidences of recurrent VTE in the full-dose rivaroxaban, low-dose rivaroxaban, and aspirin groups were 1.9%, 1.6%, and 5.0%, respectively. The cumulative incidences of major bleeding in these groups were 0.7%, 0.4% and 0.5%, respectively.
In patients treated with 20 mg of rivaroxaban instead of aspirin, there would be 123 fewer episodes of pulmonary embolism (95% confidence interval, 21-226) and 198 fewer episodes of deep vein thrombosis (95% CI, 62-333).
In patients given 10 mg of rivaroxaban instead of aspirin, there would be 121 fewer episodes of pulmonary embolism (95% CI, 4-238) and 217 fewer episodes of deep vein thrombosis (95% CI, 92-342), Dr. Prandoni and his colleagues wrote.
Net clinical benefit was defined as the composite of symptomatic recurrent VTE and major bleeding events, and occurred in 23 patients in the full-dose rivaroxaban group, 17 patients in the low-dose rivaroxaban group, and 53 patients in the aspirin group.
For 10,000 patients treated for 1 year with rivaroxaban instead of aspirin, there would be 284 fewer net clinical benefit outcomes for the 20-mg dose (95% CI, 106-462) and 339 fewer (95% CI, 165-512) for the 10-mg dose. “Thus, compared with aspirin, one additional symptomatic recurrent VTE or major bleed would be avoided for every 36 or 30 patients treated with rivaroxaban 20 mg or 10 mg, respectively,” the investigators wrote.
The findings indicate that there is “no longer a place” for extended VTE treatment with aspirin, the investigators said.
“Extended anticoagulation with once daily rivaroxaban ... provides a clinically important benefit in terms of reduction in recurrent VTE,” they wrote. “Regardless of which dose is chosen ... rivaroxaban has a favourable benefit-risk profile relative to aspirin.”
Bayer AG funded the study. Dr. Prandoni reported financial relationships with Bayer, Sanofi, Daiichi Sankyo, and Pfizer.
SOURCE: Prandoni P et al. Thromb Res. 2018 Aug;168:121-9.
FROM THROMBOSIS RESEARCH
Key clinical point:
Major finding: Incidences of the combined outcome of recurrent VTE and major bleeding were 2.8% and 3.4% lower in the rivaroxaban 20-mg and 10-mg groups, respectively, than in the aspirin group.
Study details: Analysis of data from 3,365 patients in the EINSTEIN-CHOICE trial, a double-blind, randomized study comparing rivaroxaban with aspirin for extended treatment of VTE.
Disclosures: Bayer AG funded the study. Dr. Prandoni disclosed financial relationships with Bayer, Sanofi, Daiichi Sankyo, and Pfizer.
Source: Prandoni P et al. Thromb Res. 2018 Aug;168:121-9.
Headless Compression Screw Fixation of Vertical Medial Malleolus Fractures is Superior to Unicortical Screw Fixation
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.
Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.
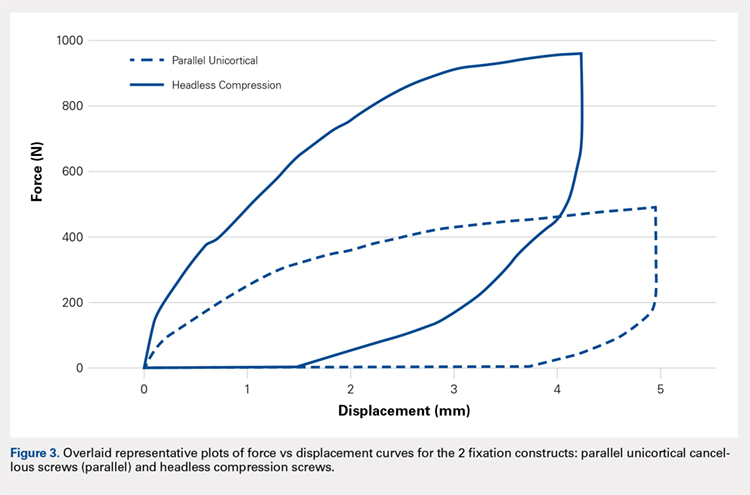
RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.
Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).
Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.

Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.

RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.

Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).

Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study is the first biomechanical research of headless compression screws for fixation of vertical shear fractures of the medial malleolus, a promising alternative that potentially offers several advantages for fixation.
Vertical shear fractures were simulated by osteotomies in 20 synthetic distal tibiae. Models were randomly assigned to fixation with either 2 parallel cancellous screws or 2 parallel Acutrak 2 headless compression screws (Acumed). Specimens were subjected to offset axial loading to simulate supination-adduction loading and tracked using high-resolution video.
The headless compression screw construct was significantly stiffer (P < .0001) (360 ± 131 N/mm) than the partially threaded cancellous screws (180 ± 48 N/mm) and demonstrated a significantly increased (P < .0001) mean load to clinical failure (719 ± 91 N vs 343 ± 83 N). When specimens were displaced to 6 mm and allowed to relax, the headless compression screw constructs demonstrated an elastic recoil and were reduced to the pretesting fragment alignment, whereas the parallel cancellous screw constructs remained displaced.
Along with the headless design that may decrease soft tissue irritation, the increased stiffness and elastic recoil of the headless compression screw construct offers improved fixation of medial malleolus vertical shear fractures over the traditional methods.
Continue to: Headless compressions screws...
Headless compressions screws are cannulated tapered titanium screws with variable thread pitch angle, allowing a fully threaded screw to apply compression along its entire length. These screws have been most commonly used for scaphoid fractures1 but have also been studied in fractures of small bones, such as capitellum, midfoot, and talar neck,2-4 and arthrodesis in the foot, ankle, and hand.5-7 Headless compression screws have been found to produce equivalent fragment compression to partially threaded cancellous screws while allowing less fragment displacement.8,9 The lack of a head may decrease soft tissue irritation compared with the partially threaded cancellous screws. Finally, headless compression screws are independent of cortical integrity, as the entire length of the screw features a wide thread diameter to capture cancellous bone in the proximal fragment, unlike partially threaded cancellous screws, which only possess a thread purchase in the distal fragment and depend on an intact cortex.
Vertical shear fractures of the medial malleolus occur through the supination-adduction of the talus exerted onto the articular surface of the medial malleolus.10 Optimal fixation of these fractures must be sufficient to maintain stable anatomic reduction of the ankle joint articular surface, allowing early range of motion, maintaining congruency of the ankle joint, and decreasing the risk of future post-traumatic arthritis to maximize functional outcome.11
A wide variety of techniques are available for fixation of these fractures, including various configurations of cortical screws, cancellous screws, tension bands, and antiglide plates. Clinically, 2 parallel 4.0-mm partially threaded cancellous screws are most often used. Limited evidence indicates that headless compression screws may be a viable option for fixation of medial malleolus fractures. One case reports the use of a headless compression screw for a horizontal medial malleolar fracture,12 and a small retrospective case series that used headless compression screws for all medial malleolar fractures showed satisfactory outcomes, a high union rate, and low patient-reported pain.13
We evaluate the stiffness, force to 2-mm displacement of the joint surface, and elastic properties of these 2 different constructs in vertical medial malleolar fractures in synthetic distal tibiae. We hypothesize that the parallel headless compression screw fixation will be stiffer and require more force to 2-mm displacement than parallel unicortical cancellous screw fixation.
MATERIALS AND METHODS
Identical vertical osteotomies (17.5 mm) were made from the medial border of the medial malleolus using a custom jig in 20 left 4th-generation composite synthetic distal tibiae (Sawbones, Pacific Research Labs; Model No. 3401) to simulate an Orthopaedic Trauma Association type 44-A2.3 fracture. The tibiae were then cut 18 cm from the tibial plafond and randomized to 2 fixation groups (n = 10 specimens for each group): parallel unicortical screw fixation or parallel unicortical headless compression screw fixation (Figures 1A-1D). Custom polymethylmethacrylate jigs were used to reproducibly drill identical holes with a 3.2-mm drill for the parallel unicortical screw construct and the drill bits provided by the Acutrak 2 Headless Compression Screw System (Acumed). The parallel unicortical screw construct consisted of 2 parallel 4.0-mm-diameter, 40-mm partially threaded cancellous screws (Depuy Synthes), and the headless compression fixation construct consisted of 2 parallel 4.7-mm-diameter, 45-mm titanium Acutrak 2 screws parallel to each other in the transverse plane. The Acutrak screws were placed per manufacturer instructions by first drilling with the Acutrak 2-4.7 Long Drill bit (Acumed), followed by the Acutrak 2-4.7 Profile Drill bit for the near cortex.

Continue to: Specimens...
Specimens were fixed to the base of a servohydraulic testing machine (Model 809, MTS Systems Corporation) with an axial-torsional load transducer (Model No. 662.20-01; Axial capacity of 250 kg, torsional capacity 2.88 kg-m; MTS Systems Corporation). The specimens were set in a vice tilted at 17° in the coronal plane to allow the MTS crosshead to apply an offset axial load simulating supination-adduction loading, which has been described previously (Figure 2).14,15 Load was applied to the inferolateral articular surface of the medial malleolus at 1 mm/s to a crosshead displacement of 6 mm and then cycled back to 0 mm. Load and axial displacement were measured at 60 Hz. The markers on the distal tibia and medial malleolus fracture fragment were tracked using high-resolution video (Fastcam PCI, Photron USA Inc). The motion of the video markers was determined using digitization and motion analysis software (Motus 9, Vicon).

Stiffness was calculated as the slope of the linear portion of the load-displacement curve over a range of 0.5 to 2.0 mm (Figure 3) and reported as mean (standard deviation). The force at 2 mm of fragment displacement was defined as a clinical failure.16,17 Student’s t test was used to determine the difference in construct stiffness and force for 2 mm displacement of the 2 groups. Significance was defined as P < .05. Institutional Review Board approval was not required for this study.

RESULTS
With offset axial testing to simulate supination-adduction force along with video motion analysis, the mean stiffness (± standard deviation) measured 180 ± 48 N/mm for the parallel unicortical screw fixation construct and 360 ± 131 N/mm for the headless compression screw fixation construct (Figure 4A). The headless compression screw fixation construct was over 2 times stiffer than the parallel unicortical construct during initial displacement of the fracture, indicating a statistically significant difference (P < .0001).
The mean force for 2 mm of fracture displacement, defined as clinical failure, reached 342 ± 83 N for the parallel unicortical screw fixation construct and 719 ± 91 N for the headless compression screw fixation construct (Figure 4B). The headless compression screw fixation construct resisted displacement significantly more (P = .0001) than the parallel unicortical screw construct, presenting a 100% increase.

Upon cycling of the servohydraulic testing machine back to 0-mm displacement, the parallel unicortical construct demonstrated no elastic recoil, remaining displaced at 4 mm, whereas the headless compression screw construct rebounded to almost 0-mm displacement, which is well below the clinical definition of fixation failure of 2 mm (Figure 5).

Continue to: Discussion...
DISCUSSION
When subjected to offset axial load, we observed that the headless compression screw construct exhibited significantly increased stiffness and load to 2 mm of displacement compared with a parallel unicortical screw construct. The headless compression screw also demonstrated elastic recoil to almost 0 mm of displacement, which is well below the 2-mm displacement.
We made reproducible fractures and fixation methods in synthetic distal tibiae, which feature less variability in size and quality than the cadaveric bone. Offset axial loading, rather than direct axial loading previously described by Amanatullah and colleagues,18 is the most physiologically relevant mode of force application to simulate the loading of the tauls onto the medial malleolus in the supination-adduction mechanism of injury.
The limitations of this study include the use of synthetic rather than cadaveric bone. Fourth-generation sawbones have been validated as possessing similar biomechanical properties as real bone.7,19 These results may also be inapplicable to osteoporotic bone, which would be significantly less dense than sawbones. This study is also an artificial situation designed to only test construct stiffness and load to clinical failure in a single mode of stress, offset axial loading and neglects other possible modes of force. This testing setup also disregards the structures surrounding the medial malleolus and tibia, including the talus, fibula, or soft tissue attachments, including the deltoid ligament and flexor retinaculum. These results are only relevant immediately after fixation and before bone healing occurs. We also tested the load to clinical failure rather than cyclic loading. Our testing more closely modeled a single traumatic force rather than the considerably smaller stresses that would be repeatedly exerted on the construct over several weeks after fixation in a clinical situation. This research is also not a clinical outcome study, rather, it suggests that headless compression screws are a viable, stronger, and possibly superior method for the initial fixation of vertical medial malleolar fractures.
As the load is offset axial, the larger thread purchase of the headless compression screws may lead to increased pullout strength, possibly increasing headless compression screw construct stiffness. Also, the variable diameter of headless compression screw, which reaches up to 4.7 mm, would increase the stiffness of the construct compared with the diameter of the cancellous screws. The elasticity of the headless compression construct may be because screws are made of titanium rather than stainless steel. Such property and given that the screws are cannulated rather than solid may also play a role, although several studies have shown variable results for cannulated vs solid screws of the same diameter.20,21 The elastic section modulus of both screws would have to be calculated to determine their exact effect on fixation.
CONCLUSION
The headless compression screw construct was found to be stiffer and features a higher load to clinical failure than a parallel unicortical cancellous screw construct for fixation of vertical medial malleolus fractures. Although significantly increased cost occurs with this construct, the headless design may decrease soft tissue irritation, and the elastic recoil of the construct after displacement may decrease clinical failure rates of this fixation method. This condition would eliminate the need for revision surgeries and thus be a cost effective alternative overall.
This paper will be judged for the Resident Writer’s Award.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
- Fowler JR, Ilyas AM. Headless compression screw fixation of scaphoid fractures. Hand Clin. 2010;26(3):351-361, vi. doi:10.1016/j.hcl.2010.04.005.
- Karakasli A, Hapa O, Erduran M, Dincer C, Cecen B, Havitcioglu H. Mechanical comparison of headless screw fixation and locking plate fixation for talar neck fractures. J Foot Ankle Surg. 2015;54(5):905-909. doi:10.1053/j.jfas.2015.04.002.
- Elkowitz SJ, Polatsch DB, Egol KA, Kummer FJ, Koval KJ. Capitellum fractures: a biomechanical evaluation of three fixation methods. J Orthop Trauma. 2002;16(7):503-506. doi:10.1097/00005131-200208000-00009.
- Zhang H, Min L, Wang GL, et al. Primary open reduction and internal fixation with headless compression screws in the treatment of Chinese patients with acute Lisfranc joint injuries. J Trauma Acute Care Surg. 2012;72(5):1380-1385. doi:10.1097/TA.0b013e318246eabc.
- Lucas KJ, Morris RP, Buford WL Jr, Panchbhavi VK. Biomechanical comparison of first metatarsophalangeal joint arthrodeses using triple-threaded headless screws versus partially threaded lag screws. Foot Ankle Surg. 2014;20(2):144-148. doi:10.1016/j.fas.2014.02.009.
- Iwamoto T, Matsumura N, Sato K, Momohara S, Toyama Y, Nakamura T. An obliquely placed headless compression screw for distal interphalangeal joint arthrodesis. J Hand Surg. 2013;38(12):2360-2364. doi:10.1016/j.jhsa.2013.09.026.
- Odutola AA, Sheridan BD, Kelly AJ. Headless compression screw fixation prevents symptomatic metalwork in arthroscopic ankle arthrodesis. Foot Ankle Surg. 2012;18(2):111-113. doi:10.1016/j.fas.2011.03.013.
- Capelle JH, Couch CG, Wells KM, et al. Fixation strength of anteriorly inserted headless screws for talar neck fractures. Foot Ankle Int. 2013;34(7):1012-1016. doi:10.1177/1071100713479586.
- Wheeler DL, McLoughlin SW. Biomechanical assessment of compression screws. Clin Orthop Relat Res. 1998;350(350):237-245. doi:10.1097/00003086-199805000-00032.
- Rockwood CA, Green DP, Bucholz RW. Rockwood and Green's Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
- Simanski CJ, Maegele MG, Lefering R, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20(2):108-114. doi:10.1097/01.bot.0000197701.96954.8c.
- Reimer H, Kreibich M, Oettinger W. Extended uses for the Herbert/Whipple screw: six case reports out of 35 illustrating technique. J Orthop Trauma. 1996;10(1):7-14. doi:10.1097/00005131-199601000-00002.
- Barnes H, Cannada LK, Watson JT. A clinical evaluation of alternative fixation techniques for medial malleolus fractures. Injury. 2014;45(9):1365-1367. doi:10.1016/j.injury.2014.05.031.
- Dumigan RM, Bronson DG, Early JS. Analysis of fixation methods for vertical shear fractures of the medial malleolus. J Orthop Trauma. 2006;20(10):687-691. doi:10.1097/01.bot.0000247075.17548.3a.
- Toolan BC, Koval KJ, Kummer FJ, Sanders R, Zuckerman JD. Vertical shear fractures of the medial malleolus: a biomechanical study of five internal fixation techniques. Foot Ankle Int. 1994;15(9):483-489. doi:10.1177/107110079401500905.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58(3):356-357. doi:10.2106/00004623-197658030-00010.
- Thordarson DB, Motamed S, Hedman T, Ebramzadeh E, Bakshian S. The effect of fibular malreduction on contact pressures in an ankle fracture malunion model. J Bone Joint Surg Am. 1997;79(12):1809-1815. doi:10.2106/00004623-199712000-00006.
- Amanatullah DF, Khan SN, Curtiss S, Wolinsky PR. Effect of divergent screw fixation in vertical medial malleolus fractures. J Trauma Acute Care Surg. 2012;72(3):751-754. doi:10.1097/TA.0b013e31823b8b9f.
- Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284. doi:10.1016/j.jbiomech.2008.08.013.
- Brown GA, McCarthy T, Bourgeault CA, Callahan DJ. Mechanical performance of standard and cannulated 4.0-mm cancellous bone screws. J Orthop Res. 2000;18(2):307-312. doi:10.1002/jor.1100180220.
- Merk BR, Stern SH, Cordes S, Lautenschlager EP. A fatigue life analysis of small fragment screws. J Orthop Trauma. 2001;15(7):494-499. doi:10.1097/00005131-200109000-00006.
TAKE-HOME POINTS
- Optimal fixation of vertical sheer ankle fractures is unknown.
- Headless compression screws are stiffer than cancellous screws in offset axial load.
- Headless compression screws have a higher load to failure than cancellous screws.
- Headless compression screws may offer a soft tissue friendly fixation of method for vertical sheer ankle fractures.
- These findings may not apply when subject to cyclic loads or in osteoporotic bone.
Patient transfers between hospitals contribute substantially to CDI burden
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more at http://ow.ly/WdQE30lBuSu
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more at http://ow.ly/WdQE30lBuSu
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more at http://ow.ly/WdQE30lBuSu
REPORTING FROM ICEID 2018
Key clinical point: Patient sharing among hospitals contributes substantially to Clostridium difficile infection (CDI) rates.
Major finding: Patient transfers account for 7.6% of the overall CDI burden.
Study details: A statistical analysis to estimate interhospital CDI transmissions.
Disclosures: Dr. Sewell reported that he had no disclosures.
Bone biopsy in suspected osteomyelitis: Culture and histology matter
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
EXPERT ANALYSIS FROM ADA 2018