User login
How Does Sleep Affect Migraine Risk?
Sleep efficiency, sleep duration, and wake after sleep onset may modify the risk of headache.
BALTIMORE—Multiple dimensions of sleep may temporally precede acute risk of migraine attack among patients with episodic migraine, according to a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies. While some aspects of sleep appear to protect against next-day headache, “these novel pilot data support the hypothesis that self-reported fragmented sleep is associated with higher risk of migraine two days later,” said Suzanne M. Bertisch, MD, MPH, and colleagues. Dr. Bertisch is an Assistant Professor of Medicine at Harvard Medical School and a Director of Behavioral Sleep Medicine at Brigham and Women’s Hospital in Boston.
Retrospective studies have indicated that nearly half of patients with migraine identify too little or too much sleep as a migraine trigger. One prospective study reported a higher incidence of headache, both migraine and tension type, after two consecutive nights of four or fewer hours of sleep, as measured by self-report. This study accounted for daily stress but not other potential triggers of migraine. “To date, there have been no prospective studies on the association between objectively assessed sleep parameters and migraine incidence while accounting for other potential triggers of migraine,” Dr. Bertisch said.
A Cohort Study of Migraine Triggers
She and her colleagues assessed the independent contribution of sleep characteristics as temporal precedents of migraine. “We were particularly interested … in sleep duration, fragmentation, and self-reported quality.” To examine these factors, they developed a cohort study of migraine triggers that they conducted from March 2016 to August 2017.
The researchers enrolled 101 adults with episodic migraine from the greater Boston area. Inclusion criteria included at least two migraines per month but fewer than 15 headache days per month, a history of migraine for at least three years, and fulfillment of ICHD-3 criteria for episodic migraine. Exclusion criteria included untreated obstructive sleep apnea, pregnancy, and current opioid use.
Data were collected for six weeks. Study participants were prompted to complete morning and evening diaries that recorded information on sleep, including the pattern, fragmentation, and sleep quality; physical activity and daily mood; medications; and headache characteristics. Patients also wore wrist actigraphs for the duration of the six-week study. Each patient had about 40 days of diary and actigraphy data.
Patients reported the onset and duration of headaches, associated symptoms, whether their pain was a headache or a migraine, maximum pain intensity, and any abortive medications used. Data on daily covariates such as alcohol and caffeine consumption, self-reported physical activity, menstrual cycle, stress, and mood prior to bedtime also were collected.
Dr. Bertisch and colleagues used self-matched case–crossover analyses. “Each person served as [his or her] own control. This approach accounts for time-invariant confounders, including sex, genetics, and usual migraine frequency. We used a conditional logistic regression model that was self-matched by day of the week, because there might be an influence of weekend versus weekday sleep patterns, as well as migraine, and we adjusted for time-dependent covariates, including daily alcohol and caffeine use.”
High WASO Protected Against Next-Day Headache
The 98 participants included in the analyses reflected the known migraine prevalence. “They were generally younger women with an average age of 35, and generally a healthy population,” Dr. Bertisch said. “About one-third reported a history of migraine with aura, and about one-quarter used daily medications to prevent migraines. About 60% reported that sleeping too little triggered their migraines.”
The researchers collected data by actigraphy and diary during approximately 4,500 nights and found that their cohort’s sleep was relatively healthy. “They slept over seven hours per night, they had few sleep problems, they had high sleep efficiency, and they had modest alcohol or caffeine consumption during the study,” Dr. Bertisch said.
In an analysis of diary data, there was no association between odds of a next-day headache and sleep duration, wake after sleep onset (WASO), sleep efficiency, or sleep quality. “However, when we looked at the actigraphy measures, we did find associations with lower sleep efficiency and higher WASO, each associated with lower risk of next-day headache,” Dr. Bertisch said.
“When we looked at odds of headache two days later, we did find that a low sleep efficiency based on self-report was associated with a higher risk of migraine. We also noted a similar trend with self-reported short sleep duration. For actigraphy data, we found a somewhat similar pattern. We also found that sleep duration greater than 8.5 hours was associated with lower
In summary, sleep efficiency and high WASO may be associated with lower odds of next-day headache. For sleep two nights before the headache, low sleep efficiency was associated with higher odds of headache. Long sleep duration, as assessed by actigraphy, was associated with lower odds of headache two days later.
—Glenn S. Williams
Sleep efficiency, sleep duration, and wake after sleep onset may modify the risk of headache.
Sleep efficiency, sleep duration, and wake after sleep onset may modify the risk of headache.
BALTIMORE—Multiple dimensions of sleep may temporally precede acute risk of migraine attack among patients with episodic migraine, according to a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies. While some aspects of sleep appear to protect against next-day headache, “these novel pilot data support the hypothesis that self-reported fragmented sleep is associated with higher risk of migraine two days later,” said Suzanne M. Bertisch, MD, MPH, and colleagues. Dr. Bertisch is an Assistant Professor of Medicine at Harvard Medical School and a Director of Behavioral Sleep Medicine at Brigham and Women’s Hospital in Boston.
Retrospective studies have indicated that nearly half of patients with migraine identify too little or too much sleep as a migraine trigger. One prospective study reported a higher incidence of headache, both migraine and tension type, after two consecutive nights of four or fewer hours of sleep, as measured by self-report. This study accounted for daily stress but not other potential triggers of migraine. “To date, there have been no prospective studies on the association between objectively assessed sleep parameters and migraine incidence while accounting for other potential triggers of migraine,” Dr. Bertisch said.
A Cohort Study of Migraine Triggers
She and her colleagues assessed the independent contribution of sleep characteristics as temporal precedents of migraine. “We were particularly interested … in sleep duration, fragmentation, and self-reported quality.” To examine these factors, they developed a cohort study of migraine triggers that they conducted from March 2016 to August 2017.
The researchers enrolled 101 adults with episodic migraine from the greater Boston area. Inclusion criteria included at least two migraines per month but fewer than 15 headache days per month, a history of migraine for at least three years, and fulfillment of ICHD-3 criteria for episodic migraine. Exclusion criteria included untreated obstructive sleep apnea, pregnancy, and current opioid use.
Data were collected for six weeks. Study participants were prompted to complete morning and evening diaries that recorded information on sleep, including the pattern, fragmentation, and sleep quality; physical activity and daily mood; medications; and headache characteristics. Patients also wore wrist actigraphs for the duration of the six-week study. Each patient had about 40 days of diary and actigraphy data.
Patients reported the onset and duration of headaches, associated symptoms, whether their pain was a headache or a migraine, maximum pain intensity, and any abortive medications used. Data on daily covariates such as alcohol and caffeine consumption, self-reported physical activity, menstrual cycle, stress, and mood prior to bedtime also were collected.
Dr. Bertisch and colleagues used self-matched case–crossover analyses. “Each person served as [his or her] own control. This approach accounts for time-invariant confounders, including sex, genetics, and usual migraine frequency. We used a conditional logistic regression model that was self-matched by day of the week, because there might be an influence of weekend versus weekday sleep patterns, as well as migraine, and we adjusted for time-dependent covariates, including daily alcohol and caffeine use.”
High WASO Protected Against Next-Day Headache
The 98 participants included in the analyses reflected the known migraine prevalence. “They were generally younger women with an average age of 35, and generally a healthy population,” Dr. Bertisch said. “About one-third reported a history of migraine with aura, and about one-quarter used daily medications to prevent migraines. About 60% reported that sleeping too little triggered their migraines.”
The researchers collected data by actigraphy and diary during approximately 4,500 nights and found that their cohort’s sleep was relatively healthy. “They slept over seven hours per night, they had few sleep problems, they had high sleep efficiency, and they had modest alcohol or caffeine consumption during the study,” Dr. Bertisch said.
In an analysis of diary data, there was no association between odds of a next-day headache and sleep duration, wake after sleep onset (WASO), sleep efficiency, or sleep quality. “However, when we looked at the actigraphy measures, we did find associations with lower sleep efficiency and higher WASO, each associated with lower risk of next-day headache,” Dr. Bertisch said.
“When we looked at odds of headache two days later, we did find that a low sleep efficiency based on self-report was associated with a higher risk of migraine. We also noted a similar trend with self-reported short sleep duration. For actigraphy data, we found a somewhat similar pattern. We also found that sleep duration greater than 8.5 hours was associated with lower
In summary, sleep efficiency and high WASO may be associated with lower odds of next-day headache. For sleep two nights before the headache, low sleep efficiency was associated with higher odds of headache. Long sleep duration, as assessed by actigraphy, was associated with lower odds of headache two days later.
—Glenn S. Williams
BALTIMORE—Multiple dimensions of sleep may temporally precede acute risk of migraine attack among patients with episodic migraine, according to a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies. While some aspects of sleep appear to protect against next-day headache, “these novel pilot data support the hypothesis that self-reported fragmented sleep is associated with higher risk of migraine two days later,” said Suzanne M. Bertisch, MD, MPH, and colleagues. Dr. Bertisch is an Assistant Professor of Medicine at Harvard Medical School and a Director of Behavioral Sleep Medicine at Brigham and Women’s Hospital in Boston.
Retrospective studies have indicated that nearly half of patients with migraine identify too little or too much sleep as a migraine trigger. One prospective study reported a higher incidence of headache, both migraine and tension type, after two consecutive nights of four or fewer hours of sleep, as measured by self-report. This study accounted for daily stress but not other potential triggers of migraine. “To date, there have been no prospective studies on the association between objectively assessed sleep parameters and migraine incidence while accounting for other potential triggers of migraine,” Dr. Bertisch said.
A Cohort Study of Migraine Triggers
She and her colleagues assessed the independent contribution of sleep characteristics as temporal precedents of migraine. “We were particularly interested … in sleep duration, fragmentation, and self-reported quality.” To examine these factors, they developed a cohort study of migraine triggers that they conducted from March 2016 to August 2017.
The researchers enrolled 101 adults with episodic migraine from the greater Boston area. Inclusion criteria included at least two migraines per month but fewer than 15 headache days per month, a history of migraine for at least three years, and fulfillment of ICHD-3 criteria for episodic migraine. Exclusion criteria included untreated obstructive sleep apnea, pregnancy, and current opioid use.
Data were collected for six weeks. Study participants were prompted to complete morning and evening diaries that recorded information on sleep, including the pattern, fragmentation, and sleep quality; physical activity and daily mood; medications; and headache characteristics. Patients also wore wrist actigraphs for the duration of the six-week study. Each patient had about 40 days of diary and actigraphy data.
Patients reported the onset and duration of headaches, associated symptoms, whether their pain was a headache or a migraine, maximum pain intensity, and any abortive medications used. Data on daily covariates such as alcohol and caffeine consumption, self-reported physical activity, menstrual cycle, stress, and mood prior to bedtime also were collected.
Dr. Bertisch and colleagues used self-matched case–crossover analyses. “Each person served as [his or her] own control. This approach accounts for time-invariant confounders, including sex, genetics, and usual migraine frequency. We used a conditional logistic regression model that was self-matched by day of the week, because there might be an influence of weekend versus weekday sleep patterns, as well as migraine, and we adjusted for time-dependent covariates, including daily alcohol and caffeine use.”
High WASO Protected Against Next-Day Headache
The 98 participants included in the analyses reflected the known migraine prevalence. “They were generally younger women with an average age of 35, and generally a healthy population,” Dr. Bertisch said. “About one-third reported a history of migraine with aura, and about one-quarter used daily medications to prevent migraines. About 60% reported that sleeping too little triggered their migraines.”
The researchers collected data by actigraphy and diary during approximately 4,500 nights and found that their cohort’s sleep was relatively healthy. “They slept over seven hours per night, they had few sleep problems, they had high sleep efficiency, and they had modest alcohol or caffeine consumption during the study,” Dr. Bertisch said.
In an analysis of diary data, there was no association between odds of a next-day headache and sleep duration, wake after sleep onset (WASO), sleep efficiency, or sleep quality. “However, when we looked at the actigraphy measures, we did find associations with lower sleep efficiency and higher WASO, each associated with lower risk of next-day headache,” Dr. Bertisch said.
“When we looked at odds of headache two days later, we did find that a low sleep efficiency based on self-report was associated with a higher risk of migraine. We also noted a similar trend with self-reported short sleep duration. For actigraphy data, we found a somewhat similar pattern. We also found that sleep duration greater than 8.5 hours was associated with lower
In summary, sleep efficiency and high WASO may be associated with lower odds of next-day headache. For sleep two nights before the headache, low sleep efficiency was associated with higher odds of headache. Long sleep duration, as assessed by actigraphy, was associated with lower odds of headache two days later.
—Glenn S. Williams
Product News: 09 2018
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Almirall Acquires 5 Products From Allergan
Almirall, SA, announces the acquisition of 5 products from Allergan: Aczone (dapsone), Tazorac (tazarotene), Azelex (azelaic acid), Cordran Tape (flurandrenolide), and Seysara (sarecycline). Seysara is a tetracycline-derived antibiotic with anti-inflammatory properties for the treatment of acne vulgaris in patients aged 9 years and older. Approval for Seysara is anticipated later in 2018. For more information, visit www.almirall.com.
Aspire Higher Scholarship Honorees Selected
Ortho Dermatologics announces the 2018 honorees of the Aspire Higher scholarship program. Nine students—3 pursuing undergraduate degrees, 3 pursuing graduate degrees, and 3 who are mothers pursuing either degree—will receive scholarships of $10,000 each. The 2018 honorees were selected from nearly 1200 applicants who shared their experience of living with a dermatologic condition, as well as the role that a dermatologist, physician assistant, or nurse practitioner has played in helping treat it. The applications were judged by an independent panel of dermatologists from across the country. Since 2013 the program has granted more than $450,000 in scholarships to students who have been affected by dermatologic conditions. For more information, visit www.aspirehigherscholarships.com.
Duobrii NDA Resubmitted to FDA
Ortho Dermatologics resubmits a New Drug Application to the US Food and Drug Administration (FDA) for Duobrii (halobetasol propionate and tazarotene) for plaque psoriasis with additional pharmacokinetic data as requested by the FDA in June 2018. The unique formulation will allow for a potentially expanded duration of use if approved. For more information, visit www.ortho-dermatologics.com.
JubliApp Now Available for Onychomycosis Patients
Ortho Dermatologics launches JubliApp, a mobile application (app) designed to encourage patient adherence to long-term treatment with Jublia (efinaconazole topical solution 10%) for onychomycosis. The app offers treatment and refill reminders, as well as a game (Mission Plu-Toe) to keep patients engaged while the daily application is drying, helping to make the 48-week long therapy less intimidating. The app also includes efficacy tracking with side-by-side photographs that can be shared with health care providers to monitor progress. It is available in the Apple App Store and the Google Play store. For more information, visit www.jubliarx.com.
Qbrexza Receives FDA Approval for Hyperhidrosis
Dermira, Inc, receives US Food and Drug Administration approval of Qbrexza (glycopyrronium cloth 2.4%) for the topical treatment of primary axillary hyperhidrosis in patients 9 years and older. Qbrexza works by blocking receptors responsible for sweat gland activation and can be used once daily. Qbrexza is expected to be available in October 2018. For more information, visit www.qbrexza.com/hcp.
Rituxan Announces FDA Approval for Pemphigus Vulgaris
Genentech USA, Inc, announces US Food and Drug Administration approval of Rituxan (rituximab) for the treatment of adults with moderate to severe pemphigus vulgaris, making it the first approved treatment for the condition in more than 60 years. Rituxan also is indicated for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and microscopic polyangiitis. Clinical trials indicated th
Verrica Is Developing a Topical Treatment for Molluscum Contagiosum
Verrica Pharmaceuticals, a clinical-stage medical dermatology company committed to identifying, developing, and commercializing pharmaceutical products for underserved patients, is working on a novel therapy for molluscum contagiosum and verruca vulgaris (common warts). Verrica’s lead product VP-102 is a proprietary drug-device combination of a topical solution of cantharidin administered through a single-use precision applicator. Top-line phase 3 trial results are expected in 2019. For more information, visit www.verrica.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Molluscum Contagiosum Virus Infection Can Trigger Atopic Dermatitis Disease Onset or Flare
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).
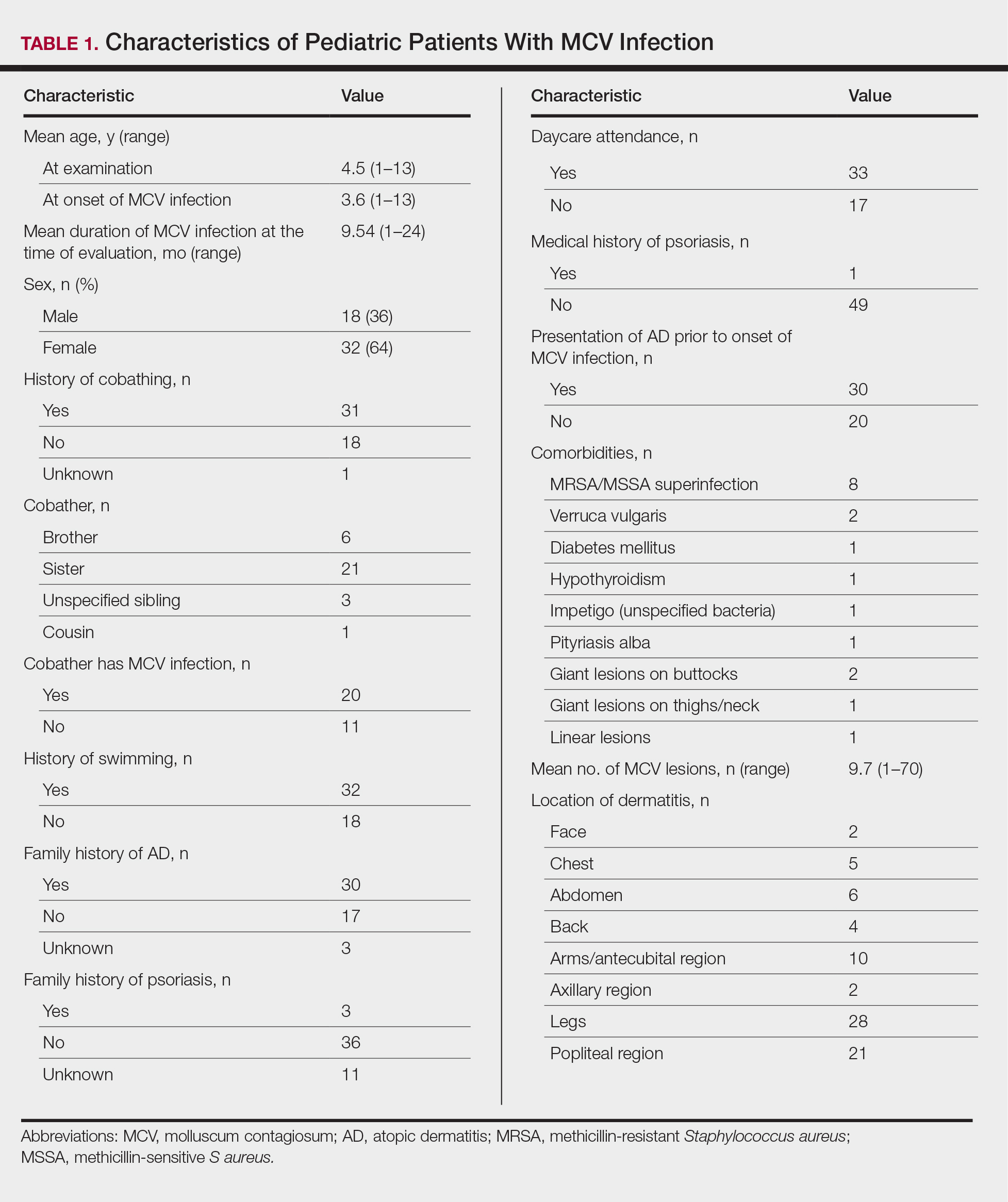
The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
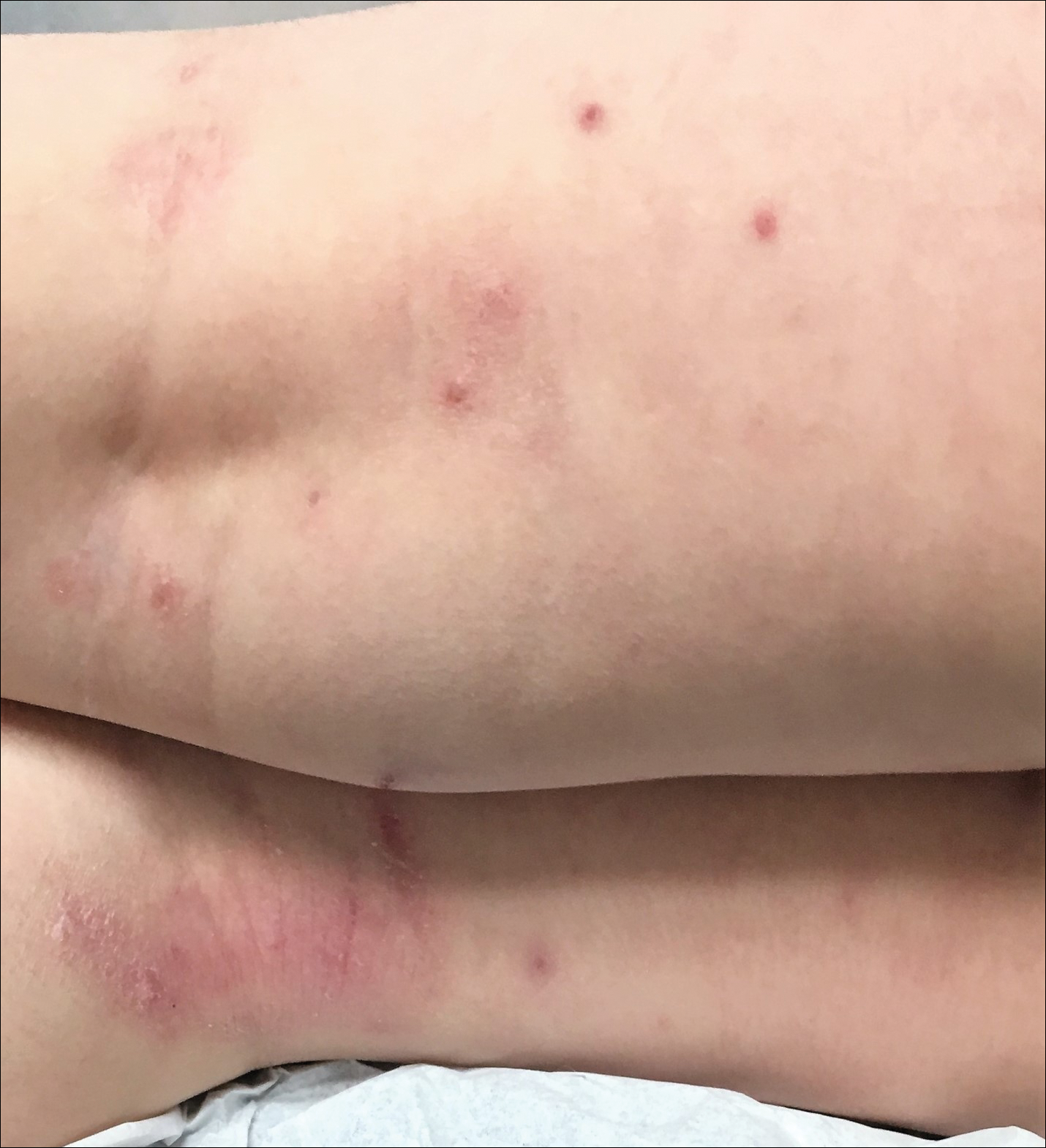
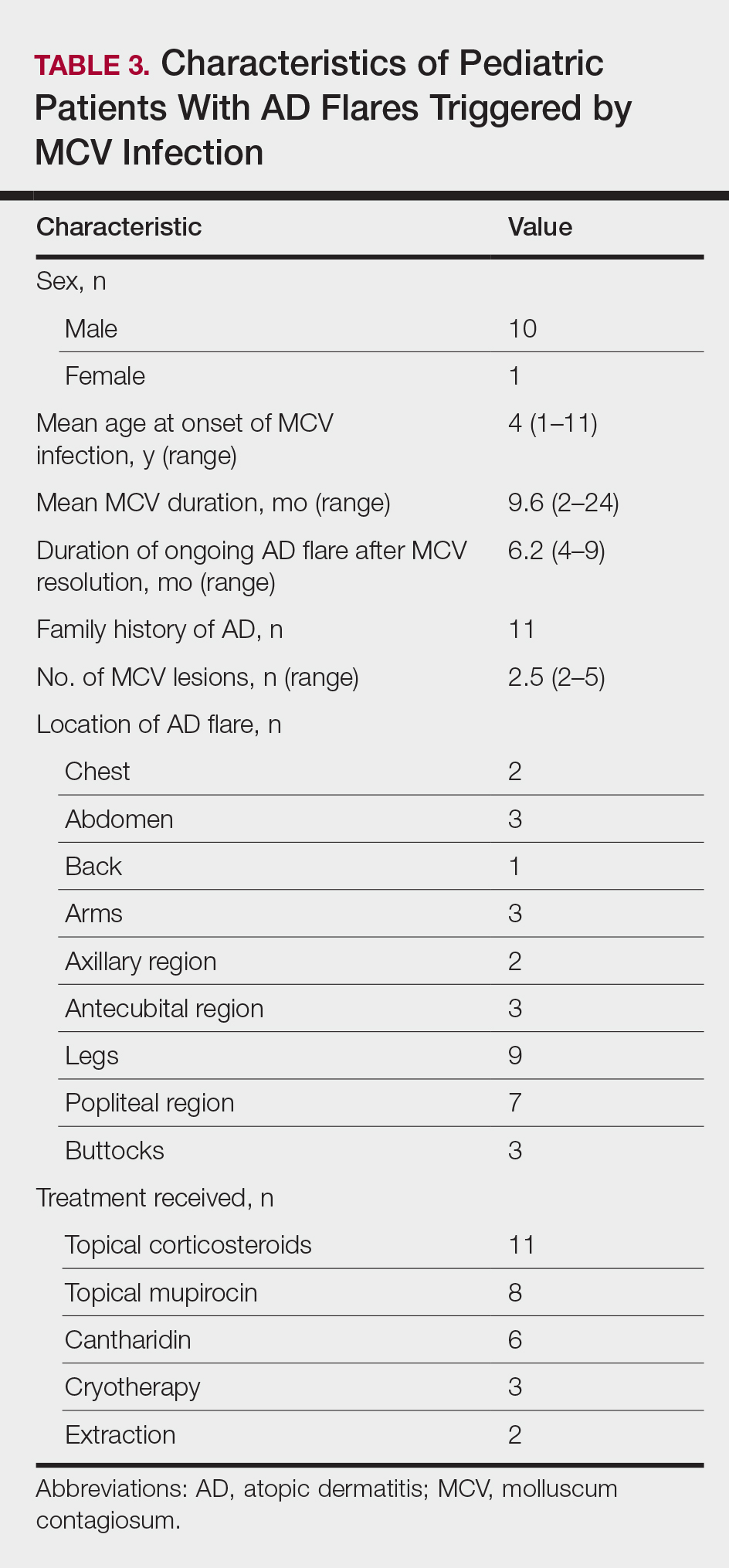
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.
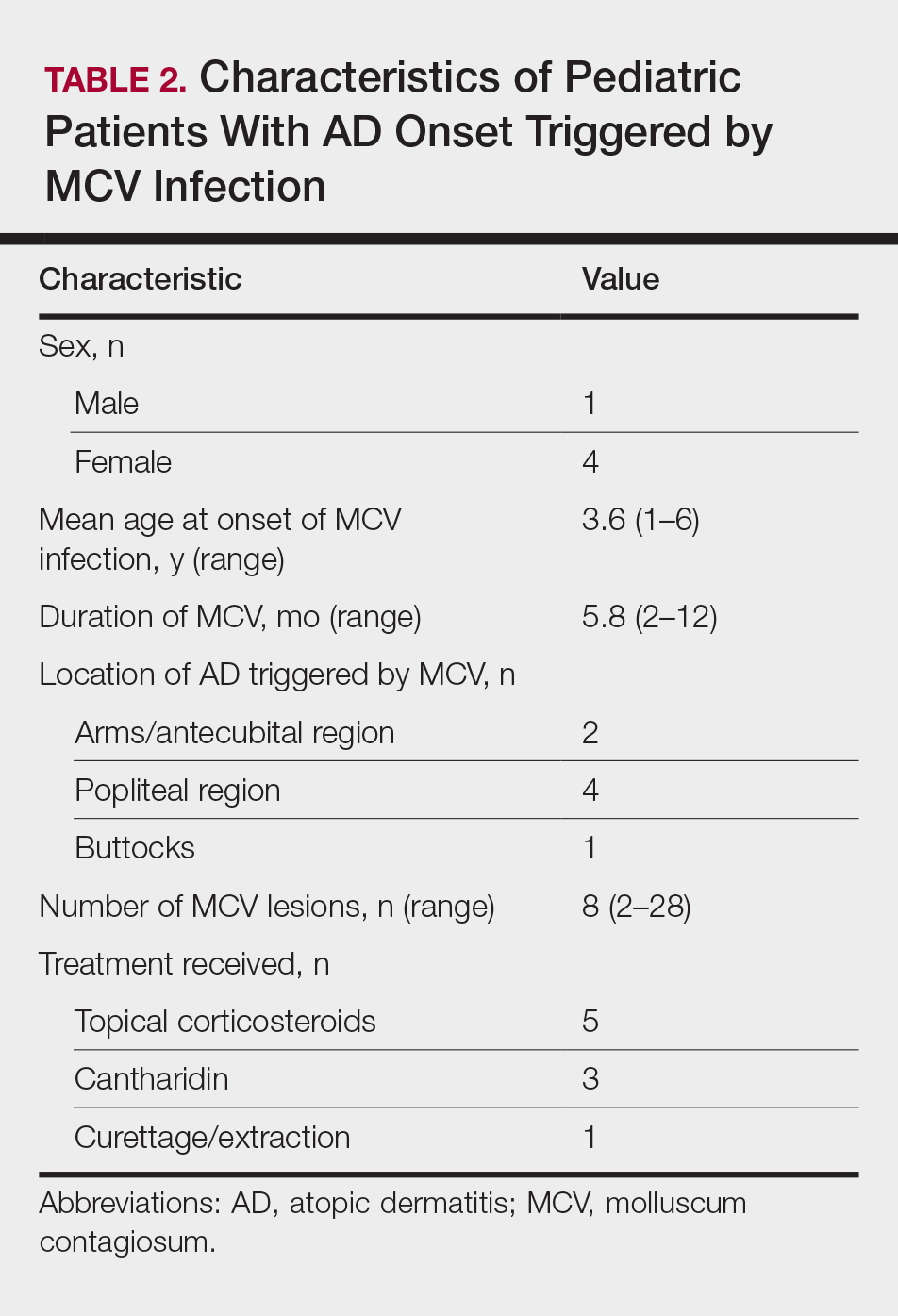
Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).

The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.



Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
Molluscum contagiosum virus (MCV) is a common pediatric viral infection of the skin and/or mucous membranes.1 It has been noted in increasingly younger patient populations, ranging from congenital cases resulting from perinatal/vertical transmission to transmission from cobathing and pool usage.2,3
An association between MCV infection and atopic dermatitis (AD) has been reported to be caused by a predisposition to prolonged and severe cutaneous viral infections.4 However, the exact nature of the relationship between MCV and AD is unknown.
The purpose of this study was to identify pediatric patients with AD onset or flare of AD triggered by MCV infection as well as to characterize the setting under which MCV may trigger AD onset or flares in children.
Methods
Medical records for 50 children with prior or current MCV infection who presented sequentially to an outpatient pediatric dermatology practice over a 1-month period were identified. Institutional review board approval was obtained.
Results
The age range of the 50 patients with MCV infection was 1 to 13 years, with an average age of 3.6 years at the onset of infection (reported by parents/guardians) and 4.5 years at presentation to the pediatric dermatology office (Table 1).

The role of cobathing is unknown; however, 62% (31/50) of patients previously or currently cobathed at home, suggesting it may be a risk factor for MCV infection. An association of MCV lesions in the popliteal region trended toward being more likely with cobathing, but the association was not statistically significant.
Children with AD onset triggered by MCV infection statistically were more likely to have flexural localization of MCV and AD lesions and were statistically more likely to have a family history of AD (P<.04)(Table 2). Children with AD flares triggered by MCV infection were more likely to have MCV and AD lesions of the popliteal region and legs (P<.05)(Figure) and family history of AD (P<.04)(Table 3). Location of MCV lesions on the upper and lower extremities, buttocks, and genitalia were more likely to be associated with presence of any dermatitis than facial and/or truncal lesions (P<.05). Treatment of the MCV infection did not appear to impact the course of AD when present, but prospective interventions would be needed to assess this issue.



Superinfection with methicillin-resistant and methicillin-sensitive Staphylococcus aureus as well as atypical giant lesions of the intertriginous neck, inner thighs, and buttocks also were noted, but AD was uncommon in these cases. Given the limited number of cases, statistical significance could not be assessed.
Comment
Cutaneous infections with Malassezia have been postulated to trigger AD in infancy,1 while systemic viral infections such as varicella-zoster virus may be protective against AD when acquired in younger children.7 It appears that MCV infection in young children (eg, 3 years or younger) with specific localization to the flexural areas has the potential to trigger AD in susceptible hosts. Larger studies are needed to chart the long-term disease course of AD in these children. Due to the small size of this study, it is unclear if the rise of MCV infections since the 1980s has contributed to increased AD.8 Susceptible children appear to have a family history of AD and localization of MCV lesions on the legs, buttocks, and antecubital region. Atopic dermatitis risk appears to be highest when MCV lesions are localized to intertriginous or flexural locations.
In addition to triggering the onset of AD, MCV infection also can trigger persistent flaring of AD, especially in the popliteal region and legs. Atopic dermatitis flares can occur at any age, but they appear to cluster in preschoolers and typically are not prevented by AD or MCV treatments; however, randomized trials are needed to identify if early intervention of MCV has a preventive benefit on AD onset or flares, and longer-term observation is needed to identify true disease course modification. Reduction of the number of MCV lesions previously has been demonstrated with institution of topical corticosteroid therapy.6 Therefore, institution of atopic skin care generally is advisable in the setting of MCV infection. Future studies should address the potential use of interventions to prevent the triggering of AD onset or flares in the setting of MCV infection in children.5
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99.
- Connell CO, Oranje A, Van Gysel D, et al. Congenital molluscum contagiosum: report of four cases and review of the literature. Pediatr Dermatol. 2008;25:553-556.
- Luke JD, Silverberg NB. Vertically transmitted molluscum contagiosum infection. Pediatrics. 2010;125:E423-E425.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357.
- Berger EM, Orlow SJ, Patel RR, et al. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148:1257-1264.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Oriel JD. The increase in molluscum contagiosum. Br Med J (Clin Res Ed). 1987;294:74.
Practice Points
- Molluscum contagiosum virus (MCV) infection appears to aggravate atopic dermatitis (AD) symptoms in a subset of pediatric patients.
- In susceptible children, the first onset of AD symptoms can occur during the course of MCV infection.
Concurrent Notalgia Paresthetica and Brachioradial Pruritus Associated With Cervical Degenerative Disc Disease
Case Report
A 74-year-old man presented with persistent episodes of severe pruritus with exacerbations on the bilateral forearms, arms, and left side of the mid back of 4 years’ duration. He had a refractory and debilitating disease that had failed extensive therapies including topical antipruritics, antihistamines, oral hydroxyzine, capsaicin, potent topical steroids (ie, clobetasol, fluocinonide, triamcinolone), phototherapy with narrowband UVB, and various dietary modifications including a gluten-free trial. The patient reported he had exhausted all medical evaluation through care with more than 7 physicians and multiple dermatologists, including a university-based dermatology department for repeated consultations; he was seen by our dermatology center for an eighth opinion.
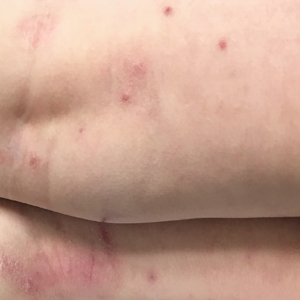
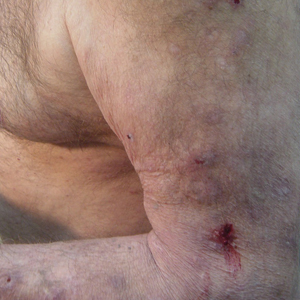
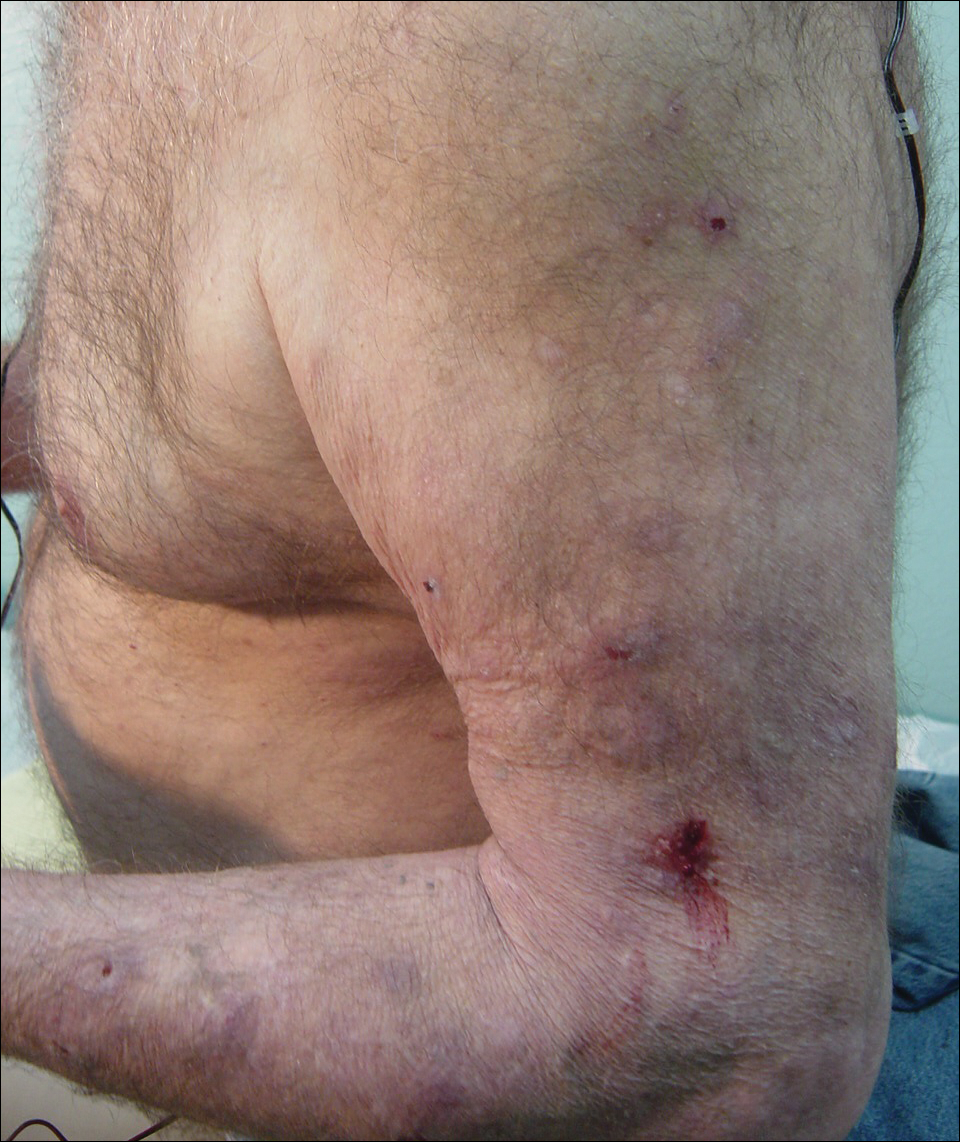
Initial dermatologic examination revealed multiple secondarily excoriated, hemorrhagic, hyperpigmented plaques and nodules on the right side of the mid upper back indicative of notalgia paresthetica (NP) with secondary chronic skin changes (Figure 1). Additional examination of the left arm and forearm revealed several open erosions, raised nodules, and lichenified skin plaques indicative of brachioradial pruritus (BP) with secondary skin changes (Figure 2). In addition, multiple lichenified plaques of the left side of the mid back were associated with decreased sensory alternations to light touch and pin prick. Of note, the localized pruritus pattern, particularly of the unilateral infrascapular back region, heralded the possibility of a neuropathic pruritus condition originating from the cervical spine. Examination confirmed decreased range of motion in the neck with associated marked palpable bilateral cervical muscle spasm and tenderness. Laboratory testing confirmed Staphylococcus aureus secondary skin infection that was treated empirically with chlorhexidine wash. General pruritus serology and imaging workup was ordered with contributory results. The patient’s medical history was notable for noninsulin-dependent diabetes mellitus, obesity, deep venous thrombosis, asthma, vein surgery, cardiovascular disease, atrial fibrillation, atopy, allergies, asthma, and keratosis pilaris, as well as drug intolerances of warfarin sodium, sitagliptin, and clopidogrel. His medications on presentation included glyburide, digoxin, prednisone, aspirin, cetirizine, cimetidine, and hydroxyzine. Based on the relatively classic localized pruritus symptoms and the anatomical distribution of skin findings, a clinical diagnosis of concurrent NP and BRP was made, and radiologic studies of the cervical spine were ordered.

Magnetic resonance imaging (MRI) of the cervical spine showed severe central canal stenosis at C3-C4 secondary to disc disease slight asymmetric toward the right side, severe central canal stenosis at C4-C5 slightly more prominent in the midline, severe central stenosis at C5-C6 more prominent in the midline, and mild changes at other levels as described. Laboratory workup revealed an abnormal complete blood cell count with mildly elevated white blood cell count (11,800/µL [reference range, 4000–10,500/µL]), elevated neutrophils (8600/µL [reference range, 1800–7800/µL]), elevated eosinophils (600/µL [reference range, 0–450/µL ]), and elevated IgE (160 IU/mL [reference range, 0–100 IU/mL]). Further testing revealed negative results for Helicobacter pylori IgG and IgM, human immunodeficiency virus, and hepatitis B and C screening panels; antinuclear antibody negative; normal thyroid-stimulating hormone; and normal thyroid peroxidase antibody. Chest radiograph and computed tomography of the chest, abdomen, and pelvis were negative.
We referred the patient for a neurosurgical consultation that uncovered newly diagnosed severe cervical stenosis with mild to moderate canal compromise at C3, C4, C5, and C6. His motor examination revealed full strength in the upper extremities (5/5). Sensory examination showed patchy sensory alteration on the mid back. He declined oral antibiotics as advised for the skin staphylococcal infection and neurosurgical treatment for the cervical disease.
During the 4 years prior to presentation at our center, the patient reported failure to improve with a dermatologically prescribed gluten-free diet as well as all topical and oral steroid treatments. He was presented at a university grand rounds where a suggestion for UVB light treatment was made; the patient reported possible worsening of symptoms with narrowband UVB phototherapy.
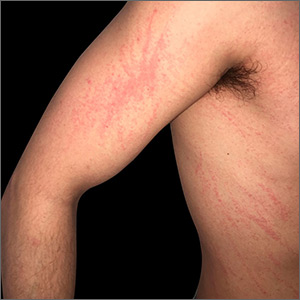
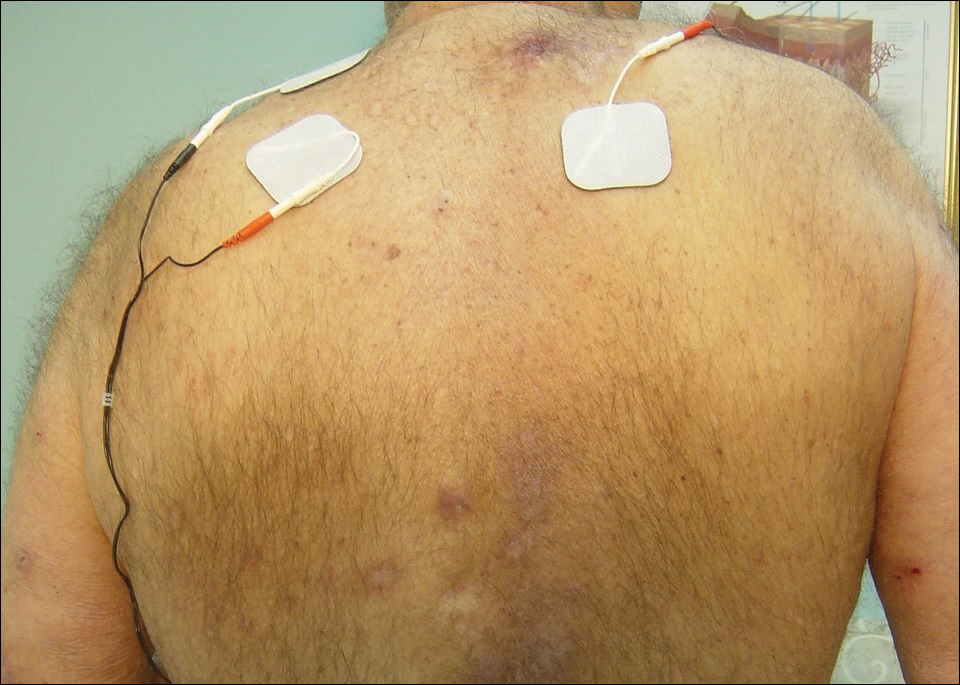
At the patient’s first visit at our center, for immediate symptom relief he underwent therapy with transcutaneous electronic nerve stimulation (TENS) with acupuncture of the cervicothoracic spine (Figure 3). He agreed to discontinue oral prednisone and begin chlorhexidine cleansing body wash, low-dose hydroxyzine 10-mg tablets up to 60 mg every 6 hours as required for pruritus, and mupirocin intranasal ointment. At 1-week follow-up, he reported at least 50% improvement in his symptoms with decreased pruritus, improved sleep, and enhanced quality of life. Within 2 weeks of initial assessment, there was a notable 70% clinical improvement of both the NP and BRP, with a notable decrease in cutaneous erosions and flattening of the pruritic skin nodules. He reported adequate control of symptoms with continued TENS for at-home use 3 times daily for 5- to 10-minute intervals.
Comment
NP Presentation
Notalgia paresthetica is a common, albeit heavily unrecognized and underdiagnosed, sensory neuropathic syndrome of the back, classically of the unilateral infrascapular region. Notalg
The dermatologic condition may consist of other symptoms that include but are not limited to localized burning, pain, tenderness, hyperalgesia, or dysesthesia. Notalgia paresthetica typically is associated with a poorly confined tan or hyperpigmented patch in the symptomatic area, though the skin may have no visible findings in many early cases. Notalgia paresthetica tends to be a chronic condition with periodic remissions and exacerbations. It is generally not associated with other comorbidities and is not life threatening; however, it does frequently decrease quality of life, causing much discomfort and annoyance to the affected patients.
Treatment
Topical therapies for NP have generally failed and are considered difficult because of the out-of-reach affected location. There is no uniformly effective treatment of NP.
Pathogenesis
The etiologies for NP and BRP are evolving and remain to be fully elucidated. Although the exact etiology remains uncertain, there are several possible mechanisms that have been proposed for NP: (1) neuropathy from degenerative cervicothoracic disc disease or direct nerve impingement,1 and (2) localized increased sensory innervations of the affected skin areas.2
Differential and Workup
The differential diagnosis in NP may include allergic or irritant contact dermatitis, fixed drug eruption, dermatophytosis, neoplasm, lichen amyloidosis, arthropod reaction, lichenified skin reactions including lichen simplex chronicus, neurodermatitis, infection, and other hypersensitivity reaction.
It is important during the initial assessment of patients with NP and/or BRP to obtain a thorough history of osteoarthritis, neck trauma, motor vehicle accident(s), vertebral fracture, cervical neoplasm or malignancy, family history of NP or BRP, or cervical disc disease. Radiographs or MRIs of the cervical spine may aid in diagnosis and treatment, and perhaps more so if there is an absence of contributory medical history. Radiographic imaging also may be indicated if there is a positive family history of osteoarthritis or vertebral disc disease.
A full laboratory workup including complete blood cell count, chemistry panel including renal and liver functions, and other laboratory tests (eg, IgE levels) may be warranted if pruritus is generalized and persistent to exclude other causes. Proper management of NP and BRP may involve a multispecialty effort of dermatology with radiology; orthopedic surgery; neurosurgery; neurology; and adjunctive fields including acupuncture, massage, chiropractic, and physical therapy.
NP and BRB Overlap
Because NP and BRP often originate from varying degrees of cervical disease, particularly at the C5-C6 level, traditional topical therapies aimed at treating the affected skin of the mid back and forearms may be ineffectual or partially effective as basic emollients. Due to NP and BRP’s periodic spontaneous remissions and exacerbations, it may be reasonably difficult to accurately measure direct response to various therapies. Some topical therapies aimed at the mid back skin or forearms may appear partially effective from a placebo-type perspective.
Currently, uniformly effective treatment of NP and BRP include the following: (1) therapies aimed at the cervical spine at C5-C6, including TENS, cervical massage, physical therapy, and acupuncture; and (2) therapies targeting the underlying lowered pruritus threshold such as oral antihistamines and narrowband UVB. Although uniformly effective treatments in this space had been previously lacking, traditional therapeutic options for NP and BRP included capsaicin cream, eutectic mixture of local anesthetic cream, topical steroids, pramoxine cream, topical cooling, oral steroids, menthol creams, various commercially available topical mixtures of menthol and methyl salicylate, cordran tape, intralesional corticosteroid injections, botulinum toxin injections,3 oral antihistamines, hydroxyzine, doxepin, topiramate, carbamazepine, antidepressants, gabapentin, oxcarbazepine, topiramate, thalidomide,4 paravertebral local anesthetic block, cervical epidural injection, surgical resection of the rib, and many others. Some of the tried systemic therapies exert their effect through the spinal nerves and central nervous system, thereby supporting the neuropathic etiology of NP.
Cervical Disc Disease
Alai et al1 reported a 37-year-old with documented NP on the right side of the back with MRI findings of disc disease at C5-C6 and mild nerve impingement that strongly suggest the association of cervical degenerative disc disease and NP.
Savk and Savk5 reported that 7 of 10 patients with NP demonstrated normal neurological examination and standard electrodiagnostic results. All had skin histopathology compatible with postinflammatory hyperpigmentation. There were no amyloid deposits or other described pathology on pathologic examination of the skin. Seven of 10 cases confirmed radiographic changes in the vertebra corresponding to the dermatome of the cutaneous lesion.5
An earlier study by Springall et al6 evaluating the mechanism of NP studied whether the cutaneous symptoms were caused by alternations on the cutaneous innervation of the involved infrascapular area. They p
Histologic studies have shown cutaneous changes in a few cases including lichen amyloidosis that may be secondary to the localized chronic scratching and rubbing.7 Clinical observations in orthopedics have established a clear relationship between the upper thoracic and interscapular region and the lower cervical spine. Frequently, cervical disc disease presents as referred pain in the upper thoracic and interscapular area. Similarly, some tumors of the cervical medulla also have presented as interscapular pain.8
Some have speculated direct involvement and actual entrapment of the posterior rami of T2-T6 spinal nerves.9 However, there are referred symptoms from the cervical area directly to the interscapular back. Degenerative vertebral and disc changes corresponding to the affected dermatome may be observed in some cases. Radiographic imaging of cervical and thoracic spine will help to exclude disc disease and possible nerve compromise.8
With advances in radiography and availability of MRI, earlier detection and intervention of cervical disc disease is possible. Early recognition may promote timely intervention and treatment to prevent cervical spine disease progression. In addition to degenerative cervical discs, osteoarthritis, and cervical spine strain and muscle spasm, there may be neoplasms or other pathology of the cervical spine contributing to NP and BRP.
There is some thought that there may be a relationship between NP and BRP. The described association of many cases of BRP and cervical spine disease6 and description of these diseases as likely neuropathic/neurogenic pruritic conditions also support a probable association of these two conditions. In contrast, NP has classically been described as unilateral in distribution, while BRP may involve unilateral or bilateral dorsolateral forearms. Most recently, as seen in our case, there are increasing incidences of nonclassic presentations of both of these diseases that may involve additional skin areas and be a basis for diagnostic challenges to the clinician.
First-line therapies for NP and BRP with associated cervical spinal disease are currently evolving and may include nondermatologic and noninvasive treatments such as spinal manipulation, physical therapy, acupuncture, cervical soft collars, massage, cervical traction, cervical muscle strengthening and increased range on motion, oral nonsteroidal anti-inflammatory medications (eg, ibuprofen, celecoxib, ketorolac), and oral muscle relaxants (eg, carisoprodol, cyclobenzaprine, methocarbamol, metaxalone). Curren
Conclusion
Notalgia paresthetica and BRP may not be solely skin diseases but rather cutaneous signs of an underlying cervical spine disease. The striking association of NP with BRP we present as well as the degenerative and/or traumatic cervicothoracic spine disease suggests that early spinal nerve impingement or cervical muscle spasm may contribute to the pathogenesis of these skin symptoms. Additional studies are needed to further assess the relationship of NP and BRP as well as the association of each disease entity independently with cervical spine disease, as it is unknown if these are causal or coincidental findings. Although topical therapies may seemingly help decrease the localized symptoms in NP and BRP in some cases, systemic or broader-scope cervical spinal evaluation may be warranted to fully evaluate refractory cases. Cervical spinal imaging and treatment, particularly at C5-C6 levels, may be appropriate as primary or first-line therapy in many cases of NP and BRP. The paradigm shift in thinking will more likely than not be to treat the cervical spine and the skin will follow.
- Alai NN, Skinner HB, Nabili S, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77-81.
- Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170.
- Goodless DR, Eaglstein WH. Brachioradial pruritus treatment with topical capsaicin. J Am Acad Dermatol. 1993;29(5, pt 1):783-784.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
- Springall DR, Karanth SS, Kirkham N, et al. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-561.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
- Misery L. What is notalgia paresthetica? Dermatology. 2002;204:86-87.
- Pleet AB, Massey EW. Notalgia paresthetica. Neurology. 1978;28:1310-1312.
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion. Bone Joint J. 2018;100-B:991-1001.
Case Report
A 74-year-old man presented with persistent episodes of severe pruritus with exacerbations on the bilateral forearms, arms, and left side of the mid back of 4 years’ duration. He had a refractory and debilitating disease that had failed extensive therapies including topical antipruritics, antihistamines, oral hydroxyzine, capsaicin, potent topical steroids (ie, clobetasol, fluocinonide, triamcinolone), phototherapy with narrowband UVB, and various dietary modifications including a gluten-free trial. The patient reported he had exhausted all medical evaluation through care with more than 7 physicians and multiple dermatologists, including a university-based dermatology department for repeated consultations; he was seen by our dermatology center for an eighth opinion.
Initial dermatologic examination revealed multiple secondarily excoriated, hemorrhagic, hyperpigmented plaques and nodules on the right side of the mid upper back indicative of notalgia paresthetica (NP) with secondary chronic skin changes (Figure 1). Additional examination of the left arm and forearm revealed several open erosions, raised nodules, and lichenified skin plaques indicative of brachioradial pruritus (BP) with secondary skin changes (Figure 2). In addition, multiple lichenified plaques of the left side of the mid back were associated with decreased sensory alternations to light touch and pin prick. Of note, the localized pruritus pattern, particularly of the unilateral infrascapular back region, heralded the possibility of a neuropathic pruritus condition originating from the cervical spine. Examination confirmed decreased range of motion in the neck with associated marked palpable bilateral cervical muscle spasm and tenderness. Laboratory testing confirmed Staphylococcus aureus secondary skin infection that was treated empirically with chlorhexidine wash. General pruritus serology and imaging workup was ordered with contributory results. The patient’s medical history was notable for noninsulin-dependent diabetes mellitus, obesity, deep venous thrombosis, asthma, vein surgery, cardiovascular disease, atrial fibrillation, atopy, allergies, asthma, and keratosis pilaris, as well as drug intolerances of warfarin sodium, sitagliptin, and clopidogrel. His medications on presentation included glyburide, digoxin, prednisone, aspirin, cetirizine, cimetidine, and hydroxyzine. Based on the relatively classic localized pruritus symptoms and the anatomical distribution of skin findings, a clinical diagnosis of concurrent NP and BRP was made, and radiologic studies of the cervical spine were ordered.


Magnetic resonance imaging (MRI) of the cervical spine showed severe central canal stenosis at C3-C4 secondary to disc disease slight asymmetric toward the right side, severe central canal stenosis at C4-C5 slightly more prominent in the midline, severe central stenosis at C5-C6 more prominent in the midline, and mild changes at other levels as described. Laboratory workup revealed an abnormal complete blood cell count with mildly elevated white blood cell count (11,800/µL [reference range, 4000–10,500/µL]), elevated neutrophils (8600/µL [reference range, 1800–7800/µL]), elevated eosinophils (600/µL [reference range, 0–450/µL ]), and elevated IgE (160 IU/mL [reference range, 0–100 IU/mL]). Further testing revealed negative results for Helicobacter pylori IgG and IgM, human immunodeficiency virus, and hepatitis B and C screening panels; antinuclear antibody negative; normal thyroid-stimulating hormone; and normal thyroid peroxidase antibody. Chest radiograph and computed tomography of the chest, abdomen, and pelvis were negative.
We referred the patient for a neurosurgical consultation that uncovered newly diagnosed severe cervical stenosis with mild to moderate canal compromise at C3, C4, C5, and C6. His motor examination revealed full strength in the upper extremities (5/5). Sensory examination showed patchy sensory alteration on the mid back. He declined oral antibiotics as advised for the skin staphylococcal infection and neurosurgical treatment for the cervical disease.
During the 4 years prior to presentation at our center, the patient reported failure to improve with a dermatologically prescribed gluten-free diet as well as all topical and oral steroid treatments. He was presented at a university grand rounds where a suggestion for UVB light treatment was made; the patient reported possible worsening of symptoms with narrowband UVB phototherapy.
At the patient’s first visit at our center, for immediate symptom relief he underwent therapy with transcutaneous electronic nerve stimulation (TENS) with acupuncture of the cervicothoracic spine (Figure 3). He agreed to discontinue oral prednisone and begin chlorhexidine cleansing body wash, low-dose hydroxyzine 10-mg tablets up to 60 mg every 6 hours as required for pruritus, and mupirocin intranasal ointment. At 1-week follow-up, he reported at least 50% improvement in his symptoms with decreased pruritus, improved sleep, and enhanced quality of life. Within 2 weeks of initial assessment, there was a notable 70% clinical improvement of both the NP and BRP, with a notable decrease in cutaneous erosions and flattening of the pruritic skin nodules. He reported adequate control of symptoms with continued TENS for at-home use 3 times daily for 5- to 10-minute intervals.

Comment
NP Presentation
Notalgia paresthetica is a common, albeit heavily unrecognized and underdiagnosed, sensory neuropathic syndrome of the back, classically of the unilateral infrascapular region. Notalg
The dermatologic condition may consist of other symptoms that include but are not limited to localized burning, pain, tenderness, hyperalgesia, or dysesthesia. Notalgia paresthetica typically is associated with a poorly confined tan or hyperpigmented patch in the symptomatic area, though the skin may have no visible findings in many early cases. Notalgia paresthetica tends to be a chronic condition with periodic remissions and exacerbations. It is generally not associated with other comorbidities and is not life threatening; however, it does frequently decrease quality of life, causing much discomfort and annoyance to the affected patients.
Treatment
Topical therapies for NP have generally failed and are considered difficult because of the out-of-reach affected location. There is no uniformly effective treatment of NP.
Pathogenesis
The etiologies for NP and BRP are evolving and remain to be fully elucidated. Although the exact etiology remains uncertain, there are several possible mechanisms that have been proposed for NP: (1) neuropathy from degenerative cervicothoracic disc disease or direct nerve impingement,1 and (2) localized increased sensory innervations of the affected skin areas.2
Differential and Workup
The differential diagnosis in NP may include allergic or irritant contact dermatitis, fixed drug eruption, dermatophytosis, neoplasm, lichen amyloidosis, arthropod reaction, lichenified skin reactions including lichen simplex chronicus, neurodermatitis, infection, and other hypersensitivity reaction.
It is important during the initial assessment of patients with NP and/or BRP to obtain a thorough history of osteoarthritis, neck trauma, motor vehicle accident(s), vertebral fracture, cervical neoplasm or malignancy, family history of NP or BRP, or cervical disc disease. Radiographs or MRIs of the cervical spine may aid in diagnosis and treatment, and perhaps more so if there is an absence of contributory medical history. Radiographic imaging also may be indicated if there is a positive family history of osteoarthritis or vertebral disc disease.
A full laboratory workup including complete blood cell count, chemistry panel including renal and liver functions, and other laboratory tests (eg, IgE levels) may be warranted if pruritus is generalized and persistent to exclude other causes. Proper management of NP and BRP may involve a multispecialty effort of dermatology with radiology; orthopedic surgery; neurosurgery; neurology; and adjunctive fields including acupuncture, massage, chiropractic, and physical therapy.
NP and BRB Overlap
Because NP and BRP often originate from varying degrees of cervical disease, particularly at the C5-C6 level, traditional topical therapies aimed at treating the affected skin of the mid back and forearms may be ineffectual or partially effective as basic emollients. Due to NP and BRP’s periodic spontaneous remissions and exacerbations, it may be reasonably difficult to accurately measure direct response to various therapies. Some topical therapies aimed at the mid back skin or forearms may appear partially effective from a placebo-type perspective.
Currently, uniformly effective treatment of NP and BRP include the following: (1) therapies aimed at the cervical spine at C5-C6, including TENS, cervical massage, physical therapy, and acupuncture; and (2) therapies targeting the underlying lowered pruritus threshold such as oral antihistamines and narrowband UVB. Although uniformly effective treatments in this space had been previously lacking, traditional therapeutic options for NP and BRP included capsaicin cream, eutectic mixture of local anesthetic cream, topical steroids, pramoxine cream, topical cooling, oral steroids, menthol creams, various commercially available topical mixtures of menthol and methyl salicylate, cordran tape, intralesional corticosteroid injections, botulinum toxin injections,3 oral antihistamines, hydroxyzine, doxepin, topiramate, carbamazepine, antidepressants, gabapentin, oxcarbazepine, topiramate, thalidomide,4 paravertebral local anesthetic block, cervical epidural injection, surgical resection of the rib, and many others. Some of the tried systemic therapies exert their effect through the spinal nerves and central nervous system, thereby supporting the neuropathic etiology of NP.
Cervical Disc Disease
Alai et al1 reported a 37-year-old with documented NP on the right side of the back with MRI findings of disc disease at C5-C6 and mild nerve impingement that strongly suggest the association of cervical degenerative disc disease and NP.
Savk and Savk5 reported that 7 of 10 patients with NP demonstrated normal neurological examination and standard electrodiagnostic results. All had skin histopathology compatible with postinflammatory hyperpigmentation. There were no amyloid deposits or other described pathology on pathologic examination of the skin. Seven of 10 cases confirmed radiographic changes in the vertebra corresponding to the dermatome of the cutaneous lesion.5
An earlier study by Springall et al6 evaluating the mechanism of NP studied whether the cutaneous symptoms were caused by alternations on the cutaneous innervation of the involved infrascapular area. They p
Histologic studies have shown cutaneous changes in a few cases including lichen amyloidosis that may be secondary to the localized chronic scratching and rubbing.7 Clinical observations in orthopedics have established a clear relationship between the upper thoracic and interscapular region and the lower cervical spine. Frequently, cervical disc disease presents as referred pain in the upper thoracic and interscapular area. Similarly, some tumors of the cervical medulla also have presented as interscapular pain.8
Some have speculated direct involvement and actual entrapment of the posterior rami of T2-T6 spinal nerves.9 However, there are referred symptoms from the cervical area directly to the interscapular back. Degenerative vertebral and disc changes corresponding to the affected dermatome may be observed in some cases. Radiographic imaging of cervical and thoracic spine will help to exclude disc disease and possible nerve compromise.8
With advances in radiography and availability of MRI, earlier detection and intervention of cervical disc disease is possible. Early recognition may promote timely intervention and treatment to prevent cervical spine disease progression. In addition to degenerative cervical discs, osteoarthritis, and cervical spine strain and muscle spasm, there may be neoplasms or other pathology of the cervical spine contributing to NP and BRP.
There is some thought that there may be a relationship between NP and BRP. The described association of many cases of BRP and cervical spine disease6 and description of these diseases as likely neuropathic/neurogenic pruritic conditions also support a probable association of these two conditions. In contrast, NP has classically been described as unilateral in distribution, while BRP may involve unilateral or bilateral dorsolateral forearms. Most recently, as seen in our case, there are increasing incidences of nonclassic presentations of both of these diseases that may involve additional skin areas and be a basis for diagnostic challenges to the clinician.
First-line therapies for NP and BRP with associated cervical spinal disease are currently evolving and may include nondermatologic and noninvasive treatments such as spinal manipulation, physical therapy, acupuncture, cervical soft collars, massage, cervical traction, cervical muscle strengthening and increased range on motion, oral nonsteroidal anti-inflammatory medications (eg, ibuprofen, celecoxib, ketorolac), and oral muscle relaxants (eg, carisoprodol, cyclobenzaprine, methocarbamol, metaxalone). Curren
Conclusion
Notalgia paresthetica and BRP may not be solely skin diseases but rather cutaneous signs of an underlying cervical spine disease. The striking association of NP with BRP we present as well as the degenerative and/or traumatic cervicothoracic spine disease suggests that early spinal nerve impingement or cervical muscle spasm may contribute to the pathogenesis of these skin symptoms. Additional studies are needed to further assess the relationship of NP and BRP as well as the association of each disease entity independently with cervical spine disease, as it is unknown if these are causal or coincidental findings. Although topical therapies may seemingly help decrease the localized symptoms in NP and BRP in some cases, systemic or broader-scope cervical spinal evaluation may be warranted to fully evaluate refractory cases. Cervical spinal imaging and treatment, particularly at C5-C6 levels, may be appropriate as primary or first-line therapy in many cases of NP and BRP. The paradigm shift in thinking will more likely than not be to treat the cervical spine and the skin will follow.
Case Report
A 74-year-old man presented with persistent episodes of severe pruritus with exacerbations on the bilateral forearms, arms, and left side of the mid back of 4 years’ duration. He had a refractory and debilitating disease that had failed extensive therapies including topical antipruritics, antihistamines, oral hydroxyzine, capsaicin, potent topical steroids (ie, clobetasol, fluocinonide, triamcinolone), phototherapy with narrowband UVB, and various dietary modifications including a gluten-free trial. The patient reported he had exhausted all medical evaluation through care with more than 7 physicians and multiple dermatologists, including a university-based dermatology department for repeated consultations; he was seen by our dermatology center for an eighth opinion.
Initial dermatologic examination revealed multiple secondarily excoriated, hemorrhagic, hyperpigmented plaques and nodules on the right side of the mid upper back indicative of notalgia paresthetica (NP) with secondary chronic skin changes (Figure 1). Additional examination of the left arm and forearm revealed several open erosions, raised nodules, and lichenified skin plaques indicative of brachioradial pruritus (BP) with secondary skin changes (Figure 2). In addition, multiple lichenified plaques of the left side of the mid back were associated with decreased sensory alternations to light touch and pin prick. Of note, the localized pruritus pattern, particularly of the unilateral infrascapular back region, heralded the possibility of a neuropathic pruritus condition originating from the cervical spine. Examination confirmed decreased range of motion in the neck with associated marked palpable bilateral cervical muscle spasm and tenderness. Laboratory testing confirmed Staphylococcus aureus secondary skin infection that was treated empirically with chlorhexidine wash. General pruritus serology and imaging workup was ordered with contributory results. The patient’s medical history was notable for noninsulin-dependent diabetes mellitus, obesity, deep venous thrombosis, asthma, vein surgery, cardiovascular disease, atrial fibrillation, atopy, allergies, asthma, and keratosis pilaris, as well as drug intolerances of warfarin sodium, sitagliptin, and clopidogrel. His medications on presentation included glyburide, digoxin, prednisone, aspirin, cetirizine, cimetidine, and hydroxyzine. Based on the relatively classic localized pruritus symptoms and the anatomical distribution of skin findings, a clinical diagnosis of concurrent NP and BRP was made, and radiologic studies of the cervical spine were ordered.


Magnetic resonance imaging (MRI) of the cervical spine showed severe central canal stenosis at C3-C4 secondary to disc disease slight asymmetric toward the right side, severe central canal stenosis at C4-C5 slightly more prominent in the midline, severe central stenosis at C5-C6 more prominent in the midline, and mild changes at other levels as described. Laboratory workup revealed an abnormal complete blood cell count with mildly elevated white blood cell count (11,800/µL [reference range, 4000–10,500/µL]), elevated neutrophils (8600/µL [reference range, 1800–7800/µL]), elevated eosinophils (600/µL [reference range, 0–450/µL ]), and elevated IgE (160 IU/mL [reference range, 0–100 IU/mL]). Further testing revealed negative results for Helicobacter pylori IgG and IgM, human immunodeficiency virus, and hepatitis B and C screening panels; antinuclear antibody negative; normal thyroid-stimulating hormone; and normal thyroid peroxidase antibody. Chest radiograph and computed tomography of the chest, abdomen, and pelvis were negative.
We referred the patient for a neurosurgical consultation that uncovered newly diagnosed severe cervical stenosis with mild to moderate canal compromise at C3, C4, C5, and C6. His motor examination revealed full strength in the upper extremities (5/5). Sensory examination showed patchy sensory alteration on the mid back. He declined oral antibiotics as advised for the skin staphylococcal infection and neurosurgical treatment for the cervical disease.
During the 4 years prior to presentation at our center, the patient reported failure to improve with a dermatologically prescribed gluten-free diet as well as all topical and oral steroid treatments. He was presented at a university grand rounds where a suggestion for UVB light treatment was made; the patient reported possible worsening of symptoms with narrowband UVB phototherapy.
At the patient’s first visit at our center, for immediate symptom relief he underwent therapy with transcutaneous electronic nerve stimulation (TENS) with acupuncture of the cervicothoracic spine (Figure 3). He agreed to discontinue oral prednisone and begin chlorhexidine cleansing body wash, low-dose hydroxyzine 10-mg tablets up to 60 mg every 6 hours as required for pruritus, and mupirocin intranasal ointment. At 1-week follow-up, he reported at least 50% improvement in his symptoms with decreased pruritus, improved sleep, and enhanced quality of life. Within 2 weeks of initial assessment, there was a notable 70% clinical improvement of both the NP and BRP, with a notable decrease in cutaneous erosions and flattening of the pruritic skin nodules. He reported adequate control of symptoms with continued TENS for at-home use 3 times daily for 5- to 10-minute intervals.

Comment
NP Presentation
Notalgia paresthetica is a common, albeit heavily unrecognized and underdiagnosed, sensory neuropathic syndrome of the back, classically of the unilateral infrascapular region. Notalg
The dermatologic condition may consist of other symptoms that include but are not limited to localized burning, pain, tenderness, hyperalgesia, or dysesthesia. Notalgia paresthetica typically is associated with a poorly confined tan or hyperpigmented patch in the symptomatic area, though the skin may have no visible findings in many early cases. Notalgia paresthetica tends to be a chronic condition with periodic remissions and exacerbations. It is generally not associated with other comorbidities and is not life threatening; however, it does frequently decrease quality of life, causing much discomfort and annoyance to the affected patients.
Treatment
Topical therapies for NP have generally failed and are considered difficult because of the out-of-reach affected location. There is no uniformly effective treatment of NP.
Pathogenesis
The etiologies for NP and BRP are evolving and remain to be fully elucidated. Although the exact etiology remains uncertain, there are several possible mechanisms that have been proposed for NP: (1) neuropathy from degenerative cervicothoracic disc disease or direct nerve impingement,1 and (2) localized increased sensory innervations of the affected skin areas.2
Differential and Workup
The differential diagnosis in NP may include allergic or irritant contact dermatitis, fixed drug eruption, dermatophytosis, neoplasm, lichen amyloidosis, arthropod reaction, lichenified skin reactions including lichen simplex chronicus, neurodermatitis, infection, and other hypersensitivity reaction.
It is important during the initial assessment of patients with NP and/or BRP to obtain a thorough history of osteoarthritis, neck trauma, motor vehicle accident(s), vertebral fracture, cervical neoplasm or malignancy, family history of NP or BRP, or cervical disc disease. Radiographs or MRIs of the cervical spine may aid in diagnosis and treatment, and perhaps more so if there is an absence of contributory medical history. Radiographic imaging also may be indicated if there is a positive family history of osteoarthritis or vertebral disc disease.
A full laboratory workup including complete blood cell count, chemistry panel including renal and liver functions, and other laboratory tests (eg, IgE levels) may be warranted if pruritus is generalized and persistent to exclude other causes. Proper management of NP and BRP may involve a multispecialty effort of dermatology with radiology; orthopedic surgery; neurosurgery; neurology; and adjunctive fields including acupuncture, massage, chiropractic, and physical therapy.
NP and BRB Overlap
Because NP and BRP often originate from varying degrees of cervical disease, particularly at the C5-C6 level, traditional topical therapies aimed at treating the affected skin of the mid back and forearms may be ineffectual or partially effective as basic emollients. Due to NP and BRP’s periodic spontaneous remissions and exacerbations, it may be reasonably difficult to accurately measure direct response to various therapies. Some topical therapies aimed at the mid back skin or forearms may appear partially effective from a placebo-type perspective.
Currently, uniformly effective treatment of NP and BRP include the following: (1) therapies aimed at the cervical spine at C5-C6, including TENS, cervical massage, physical therapy, and acupuncture; and (2) therapies targeting the underlying lowered pruritus threshold such as oral antihistamines and narrowband UVB. Although uniformly effective treatments in this space had been previously lacking, traditional therapeutic options for NP and BRP included capsaicin cream, eutectic mixture of local anesthetic cream, topical steroids, pramoxine cream, topical cooling, oral steroids, menthol creams, various commercially available topical mixtures of menthol and methyl salicylate, cordran tape, intralesional corticosteroid injections, botulinum toxin injections,3 oral antihistamines, hydroxyzine, doxepin, topiramate, carbamazepine, antidepressants, gabapentin, oxcarbazepine, topiramate, thalidomide,4 paravertebral local anesthetic block, cervical epidural injection, surgical resection of the rib, and many others. Some of the tried systemic therapies exert their effect through the spinal nerves and central nervous system, thereby supporting the neuropathic etiology of NP.
Cervical Disc Disease
Alai et al1 reported a 37-year-old with documented NP on the right side of the back with MRI findings of disc disease at C5-C6 and mild nerve impingement that strongly suggest the association of cervical degenerative disc disease and NP.
Savk and Savk5 reported that 7 of 10 patients with NP demonstrated normal neurological examination and standard electrodiagnostic results. All had skin histopathology compatible with postinflammatory hyperpigmentation. There were no amyloid deposits or other described pathology on pathologic examination of the skin. Seven of 10 cases confirmed radiographic changes in the vertebra corresponding to the dermatome of the cutaneous lesion.5
An earlier study by Springall et al6 evaluating the mechanism of NP studied whether the cutaneous symptoms were caused by alternations on the cutaneous innervation of the involved infrascapular area. They p
Histologic studies have shown cutaneous changes in a few cases including lichen amyloidosis that may be secondary to the localized chronic scratching and rubbing.7 Clinical observations in orthopedics have established a clear relationship between the upper thoracic and interscapular region and the lower cervical spine. Frequently, cervical disc disease presents as referred pain in the upper thoracic and interscapular area. Similarly, some tumors of the cervical medulla also have presented as interscapular pain.8
Some have speculated direct involvement and actual entrapment of the posterior rami of T2-T6 spinal nerves.9 However, there are referred symptoms from the cervical area directly to the interscapular back. Degenerative vertebral and disc changes corresponding to the affected dermatome may be observed in some cases. Radiographic imaging of cervical and thoracic spine will help to exclude disc disease and possible nerve compromise.8
With advances in radiography and availability of MRI, earlier detection and intervention of cervical disc disease is possible. Early recognition may promote timely intervention and treatment to prevent cervical spine disease progression. In addition to degenerative cervical discs, osteoarthritis, and cervical spine strain and muscle spasm, there may be neoplasms or other pathology of the cervical spine contributing to NP and BRP.
There is some thought that there may be a relationship between NP and BRP. The described association of many cases of BRP and cervical spine disease6 and description of these diseases as likely neuropathic/neurogenic pruritic conditions also support a probable association of these two conditions. In contrast, NP has classically been described as unilateral in distribution, while BRP may involve unilateral or bilateral dorsolateral forearms. Most recently, as seen in our case, there are increasing incidences of nonclassic presentations of both of these diseases that may involve additional skin areas and be a basis for diagnostic challenges to the clinician.
First-line therapies for NP and BRP with associated cervical spinal disease are currently evolving and may include nondermatologic and noninvasive treatments such as spinal manipulation, physical therapy, acupuncture, cervical soft collars, massage, cervical traction, cervical muscle strengthening and increased range on motion, oral nonsteroidal anti-inflammatory medications (eg, ibuprofen, celecoxib, ketorolac), and oral muscle relaxants (eg, carisoprodol, cyclobenzaprine, methocarbamol, metaxalone). Curren
Conclusion
Notalgia paresthetica and BRP may not be solely skin diseases but rather cutaneous signs of an underlying cervical spine disease. The striking association of NP with BRP we present as well as the degenerative and/or traumatic cervicothoracic spine disease suggests that early spinal nerve impingement or cervical muscle spasm may contribute to the pathogenesis of these skin symptoms. Additional studies are needed to further assess the relationship of NP and BRP as well as the association of each disease entity independently with cervical spine disease, as it is unknown if these are causal or coincidental findings. Although topical therapies may seemingly help decrease the localized symptoms in NP and BRP in some cases, systemic or broader-scope cervical spinal evaluation may be warranted to fully evaluate refractory cases. Cervical spinal imaging and treatment, particularly at C5-C6 levels, may be appropriate as primary or first-line therapy in many cases of NP and BRP. The paradigm shift in thinking will more likely than not be to treat the cervical spine and the skin will follow.
- Alai NN, Skinner HB, Nabili S, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77-81.
- Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170.
- Goodless DR, Eaglstein WH. Brachioradial pruritus treatment with topical capsaicin. J Am Acad Dermatol. 1993;29(5, pt 1):783-784.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
- Springall DR, Karanth SS, Kirkham N, et al. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-561.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
- Misery L. What is notalgia paresthetica? Dermatology. 2002;204:86-87.
- Pleet AB, Massey EW. Notalgia paresthetica. Neurology. 1978;28:1310-1312.
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion. Bone Joint J. 2018;100-B:991-1001.
- Alai NN, Skinner HB, Nabili S, et al. Notalgia paresthetica associated with cervical spinal stenosis and cervicothoracic disk disease at C4 through C7. Cutis. 2010;85:77-81.
- Savk E, Savk O, Bolukbasi O, et al. Notalgia paresthetica: a study on pathogenesis. Int J Dermatol. 2000;39:754-759.
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170.
- Goodless DR, Eaglstein WH. Brachioradial pruritus treatment with topical capsaicin. J Am Acad Dermatol. 1993;29(5, pt 1):783-784.
- Savk O, Savk E. Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-1087.
- Springall DR, Karanth SS, Kirkham N, et al. Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-561.
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch Dermatol. 2007;143:980-982.
- Misery L. What is notalgia paresthetica? Dermatology. 2002;204:86-87.
- Pleet AB, Massey EW. Notalgia paresthetica. Neurology. 1978;28:1310-1312.
- Findlay C, Ayis S, Demetriades AK. Total disc replacement versus anterior cervical discectomy and fusion. Bone Joint J. 2018;100-B:991-1001.
Practice Points
- Assess the spine in patients with notalgia paresthetica (NP) and brachioradial pruritus (BRP). Cervical spinal disease, especially at the C5-C6 level, may be the root cause of BRP and/or NP.
- Consider a multimodality approach to treatment, including physical therapy, acupuncture, massage, and transcutaneous electronic nerve stimulation.
- Treat the neck (underlying cause) and expect the skin (cutaneous symptoms) to follow.
Sustained minimal disease activity in PsA reduced CV risk factors
Achieving sustained minimal disease activity in psoriatic arthritis could slow or prevent the progression of carotid atherosclerosis and arterial stiffness, new research suggests.
A prospective cohort study, published online Aug. 25 in Arthritis & Rheumatology, involved 90 patients with psoriatic arthritis (PsA) who were followed for at least 24 months. High-resolution carotid ultrasound and arterial stiffness markers were assessed annually.
Overall, 57 patients (63%) achieved minimal disease activity (MDA) – defined as meeting five or more cutoffs for seven domains of disease activity – at 1 year of follow-up, 69% achieved it by 2 years, and 46% sustained MDA from the 1-year follow-up to the 2-year follow-up.
The 41 patients who sustained MDA over the follow-up points showed significantly lower odds of progression of atherosclerotic carotid plaques (odds ratio = 0.273; P = .024). They were also significantly less likely to show increases in total carotid plaque area, increased intima-media thickness, or increased measures of arterial stiffness. The researchers also noted a trend toward improvements in pulse wave velocity in this group.
Patients who achieved sustained MDA had lower disease activity at baseline, and a higher proportion of them were already in a state of MDA at baseline, compared with the group of patients who did not achieved sustained MDA.
Sustained MDA appeared to be key to reducing atherosclerosis, as researchers did not see any significant differences in measures of arterial stiffness between those who achieved MDA – sustained or otherwise – at 2 years and those who did not, after adjusting for baseline differences and use of disease-modifying antirheumatic drugs (DMARDs).
“The most important finding in this study is that long-term control of inflammation is important in preventing progression of subclinical atherosclerosis and arterial stiffness, independent of traditional CV risk factors,” wrote Isaac T. Cheng of Prince of Wales Hospital at The Chinese University of Hong Kong, and his coauthors. “These data suggest that low stable disease activity over a prolonged period of time may have a significant protective effect against CVD in PsA compared with those patients with intermittent flare-ups.”
The study also looked at treatment effect. More patients treated with biologic DMARDs achieved MDA, but the researchers saw no association between the use of conventional synthetic DMARDs and the likelihood of achieving MDA.
They noted that the protective effects of achieving sustained MDA seemed to be independent of biologic DMARDs, “suggesting that controlling disease activity using various combinations of conventional and biological DMARD may be useful in improving CV risk in these patients.”
However, the investigators noted that the study was conducted in Hong Kong, where biologics are not reimbursed by the government. Patients in the study had to pay for these medications themselves, and some could not afford them.
They raised the possibility that early initiation with biologic DMARDs might further improve cardiovascular outcomes, but said this needed to be addressed in future studies.
“These data support the EULAR recommendation that disease activity should be controlled optimally in order to lower CVD risk in patients with PsA,” the authors wrote. “Data from the current study confirm that using a treat-to-target strategy, MDA is indeed an achievable target even in a health care system with resource constrain.”
The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
SOURCE: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
Achieving sustained minimal disease activity in psoriatic arthritis could slow or prevent the progression of carotid atherosclerosis and arterial stiffness, new research suggests.
A prospective cohort study, published online Aug. 25 in Arthritis & Rheumatology, involved 90 patients with psoriatic arthritis (PsA) who were followed for at least 24 months. High-resolution carotid ultrasound and arterial stiffness markers were assessed annually.
Overall, 57 patients (63%) achieved minimal disease activity (MDA) – defined as meeting five or more cutoffs for seven domains of disease activity – at 1 year of follow-up, 69% achieved it by 2 years, and 46% sustained MDA from the 1-year follow-up to the 2-year follow-up.
The 41 patients who sustained MDA over the follow-up points showed significantly lower odds of progression of atherosclerotic carotid plaques (odds ratio = 0.273; P = .024). They were also significantly less likely to show increases in total carotid plaque area, increased intima-media thickness, or increased measures of arterial stiffness. The researchers also noted a trend toward improvements in pulse wave velocity in this group.
Patients who achieved sustained MDA had lower disease activity at baseline, and a higher proportion of them were already in a state of MDA at baseline, compared with the group of patients who did not achieved sustained MDA.
Sustained MDA appeared to be key to reducing atherosclerosis, as researchers did not see any significant differences in measures of arterial stiffness between those who achieved MDA – sustained or otherwise – at 2 years and those who did not, after adjusting for baseline differences and use of disease-modifying antirheumatic drugs (DMARDs).
“The most important finding in this study is that long-term control of inflammation is important in preventing progression of subclinical atherosclerosis and arterial stiffness, independent of traditional CV risk factors,” wrote Isaac T. Cheng of Prince of Wales Hospital at The Chinese University of Hong Kong, and his coauthors. “These data suggest that low stable disease activity over a prolonged period of time may have a significant protective effect against CVD in PsA compared with those patients with intermittent flare-ups.”
The study also looked at treatment effect. More patients treated with biologic DMARDs achieved MDA, but the researchers saw no association between the use of conventional synthetic DMARDs and the likelihood of achieving MDA.
They noted that the protective effects of achieving sustained MDA seemed to be independent of biologic DMARDs, “suggesting that controlling disease activity using various combinations of conventional and biological DMARD may be useful in improving CV risk in these patients.”
However, the investigators noted that the study was conducted in Hong Kong, where biologics are not reimbursed by the government. Patients in the study had to pay for these medications themselves, and some could not afford them.
They raised the possibility that early initiation with biologic DMARDs might further improve cardiovascular outcomes, but said this needed to be addressed in future studies.
“These data support the EULAR recommendation that disease activity should be controlled optimally in order to lower CVD risk in patients with PsA,” the authors wrote. “Data from the current study confirm that using a treat-to-target strategy, MDA is indeed an achievable target even in a health care system with resource constrain.”
The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
SOURCE: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
Achieving sustained minimal disease activity in psoriatic arthritis could slow or prevent the progression of carotid atherosclerosis and arterial stiffness, new research suggests.
A prospective cohort study, published online Aug. 25 in Arthritis & Rheumatology, involved 90 patients with psoriatic arthritis (PsA) who were followed for at least 24 months. High-resolution carotid ultrasound and arterial stiffness markers were assessed annually.
Overall, 57 patients (63%) achieved minimal disease activity (MDA) – defined as meeting five or more cutoffs for seven domains of disease activity – at 1 year of follow-up, 69% achieved it by 2 years, and 46% sustained MDA from the 1-year follow-up to the 2-year follow-up.
The 41 patients who sustained MDA over the follow-up points showed significantly lower odds of progression of atherosclerotic carotid plaques (odds ratio = 0.273; P = .024). They were also significantly less likely to show increases in total carotid plaque area, increased intima-media thickness, or increased measures of arterial stiffness. The researchers also noted a trend toward improvements in pulse wave velocity in this group.
Patients who achieved sustained MDA had lower disease activity at baseline, and a higher proportion of them were already in a state of MDA at baseline, compared with the group of patients who did not achieved sustained MDA.
Sustained MDA appeared to be key to reducing atherosclerosis, as researchers did not see any significant differences in measures of arterial stiffness between those who achieved MDA – sustained or otherwise – at 2 years and those who did not, after adjusting for baseline differences and use of disease-modifying antirheumatic drugs (DMARDs).
“The most important finding in this study is that long-term control of inflammation is important in preventing progression of subclinical atherosclerosis and arterial stiffness, independent of traditional CV risk factors,” wrote Isaac T. Cheng of Prince of Wales Hospital at The Chinese University of Hong Kong, and his coauthors. “These data suggest that low stable disease activity over a prolonged period of time may have a significant protective effect against CVD in PsA compared with those patients with intermittent flare-ups.”
The study also looked at treatment effect. More patients treated with biologic DMARDs achieved MDA, but the researchers saw no association between the use of conventional synthetic DMARDs and the likelihood of achieving MDA.
They noted that the protective effects of achieving sustained MDA seemed to be independent of biologic DMARDs, “suggesting that controlling disease activity using various combinations of conventional and biological DMARD may be useful in improving CV risk in these patients.”
However, the investigators noted that the study was conducted in Hong Kong, where biologics are not reimbursed by the government. Patients in the study had to pay for these medications themselves, and some could not afford them.
They raised the possibility that early initiation with biologic DMARDs might further improve cardiovascular outcomes, but said this needed to be addressed in future studies.
“These data support the EULAR recommendation that disease activity should be controlled optimally in order to lower CVD risk in patients with PsA,” the authors wrote. “Data from the current study confirm that using a treat-to-target strategy, MDA is indeed an achievable target even in a health care system with resource constrain.”
The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
SOURCE: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: Patients who achieved sustained minimal disease activity had lower odds of progression of atherosclerotic carotid plaques.
Study details: Prospective cohort study of 90 patients with psoriatic arthritis.
Disclosures: The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
Source: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
Angioimmunoblastic T-Cell Lymphoma Mimicking Diffuse Large B-Cell Lymphoma
Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma. It comprises 18% of peripheral T-cell lymphomas and 1% to 2% of all non-Hodgkin lymphomas.1 The incidence of AITL in the United States is estimated to be 0.05 cases per 100,000 person-years,2 and there is a slight male predominance.1,3,4 It typically presents in the seventh decade of life; however, cases have been reported in adults ranging from 20 to 91 years of age.3
Angioimmunoblastic T-cell lymphoma presents with lymphadenopathy, hepatosplenomegaly, and systemic B symptoms (eg, fever, night sweats, weight loss, generalized pruritus).4-6 There are cutaneous manifestations in up to 50% of cases4,5,7 and frequently signs of autoimmune disorder.4,5 The diagnosis often is made by excisional lymph node biopsy. Lymph node specimens characteristically have a mixed inflammatory infiltrate that includes numerous B cells often infected with Epstein-Barr virus (EBV) and a relatively small population of atypical T lymphocytes.8 Identification of this neoplastic population of CD4+CD8− T lymphocytes expressing normal follicular helper T-cell markers CD10, chemokine CXCL13, programmed cell death protein 1 (PD-1), and B-cell lymphoma 6 (BCL-6) confirms the diagnosis of AITL.9,10 These malignant cells can be identified in skin specimens in cases of cutaneous metastatic disease.11,12 We present a case originally misdiagnosed as diffuse large B-cell lymphoma that was later identified as AITL on skin biopsy.
Case Report
A 72-year-old woman presented with a pruritic erythematous eruption around the neck of 3 weeks’ duration (Figure 1). Her medical history was notable for diffuse large B-cell non-Hodgkin lymphoma diagnosed 3 months prior based on results from a right cervical lymph node biopsy. She was treated with bendamustine and rituximab. On physical examination there were erythematous edematous papules coalescing into indurated plaques around the neck. The differential diagnosis included drug hypersensitivity reaction, herpes zoster, urticaria, and cutaneous metastasis. Two punch biopsies were taken for hematoxylin and eosin and tissue culture.
Tissue cultures and viral polymerase chain reaction were negative. Histopathologic examination revealed a scant atypical lymphoid infiltrate focally involving the deep dermis. The cells were medium to large in size and contained hyperchromatic pleomorphic nuclei (Figure 2). They were positive for CD3 and CD4, which was concerning for T-cell lymphoma. The histologic report of the excisional lymph node biopsy done 3 months prior described an atypical lymphoid neoplasm with extensive necrosis and extranodal spread that stained positively for CD20 (Figure 3).
Further staining of this cervical lymph node specimen revealed large atypical lymphoid cells positive for CD3, CD10, B-cell lymphoma 2 (BCL-2), BCL-6, and PD-1. There were intermixed mature B lymphocytes positive for CD20 and BCL-2. Chromogenic in situ hybridization with probes for EBV showed numerous positive cells throughout the infiltrate. Polymerase chain reaction demonstrated a T-cell population with clonally rearranged T-cell receptor genes. Primers for immunoglobulin heavy and light chains showed no evidence of a clonal B-cell population.
Additional staining of the atypical cutaneous lymphocytes revealed positivity for CD3, CD10, and PD-1. The morphologic and immunophenotypic findings of both specimens supported the diagnosis of AITL.
The patient declined further treatment and chose hospice care.
Comment
Etiology
Angioimmunoblastic T-cell lymphoma was originally named angioimmunoblastic lymphadenopathy with dysproteinemia. It was initially thought to be a benign hyperreactive immune process driven by B cells, and patients often died of infectious complications not long after the diagnosis was made.13 As more cases were reported with clonal rearrangements and signs of progressive lymphoma, AITL was recognized as a malignancy.
Presentation
Patients with AITL often present with advanced stage III or IV disease with extranodal and bone marrow involvement.3-6 Cutaneous disease occurs in up to half of patients and portends a poor prognosis.7 The rash often is a nonspecific erythematous macular and papular eruption mimicking a morbilliform viral exanthem or drug reaction. Urticarial, nodular, petechial, purpuric, eczematous, erythrodermic, and vesiculobullous presentations have been described.4,11,12 In up to one-third of cases, the eruption occurs in association with a new medication, often leading to an initial misdiagnosis of drug hypersensitivity reaction.4,11 In a review conducted by Balaraman et al,14 84% of patients with AITL reported having pruritus.
There is an association of autoimmune phenomena in patients with AITL, which is likely a result of immune dysregulation associated with poorly functioning follicular helper T cells. Patients may present with arthralgia, hemolytic anemia, or thrombocytopenic purpura. Hypergammaglobulinemia has been reported in 30% to 50% of AITL patients.4,6 Other pertinent immunologic findings include positive Coombs test, cold agglutinins, cryoglobulinemia, hypocomplementemia, and positive antinuclear antibodies.4-7
Gene Analysis
Affected lymph nodes have a characteristically effaced architecture with proliferative arborizing venules; a hyperplastic population of follicular dendritic cells; and a mixed inflammatory infiltrate that is comprised of atypical lymphocytes and variable numbers of reactive lymphocytes, histiocytes, eosinophils, and plasma cells. The malignant T lymphocytes often account for only a small portion of the infiltrate.8 T-cell gene rearrangement studies identify clonal cells with β and γ rearrangements in the majority of cases.4 These cells are predominantly CD4+CD8− and express normal follicular helper T-cell markers CD10, CXCL13, BCL-6,5,9 and PD-1.10 Numerous B cells are seen intermixed with follicular dendritic cells. They are frequently infected with EBV and can have an atypical Reed-Sternberg cell–like appearance.4,5,15 In the evaluation of AITL, polymerase chain reaction studies with primers for immunoglobulin heavy and light chain should be performed to look for clonal B-cell populations and rule out a possible secondary B-cell lymphoma.
Histology
Five histologic patterns have been described with cutaneous AITL: (1) superficial perivascular infiltrate of eosinophils and lymphocytes that lack atypia, (2) sparse perivascular infiltrate with atypical lymphocytes, (3) dense dermal infiltrate of pleomorphic lymphocytes, (4) leukocytoclastic vasculitis without atypical lymphocytes,11 and (5) necrotizing vasculitis.12 The finding of vascular hyperplasia, perivascular infiltrate, or vasculitis has been reported in 91% of cases in the literature. Although these findings are nonspecific, an analysis of cutaneous cases reported in the literature found that 87% demonstrated T-cell receptor gene rearrangements.14 Lymphoid cells are positive for CD10 and PD-1, as was demonstrated in our case, and are CXCL13 positive in the majority of cases.12 Atypical and EBV-infected B cells also can be found in the skin.11,12
Differential Diagnosis
Angioimmunoblastic T-cell lymphoma can mimic infectious, autoimmune, or allergic etiologies, and misdiagnosis of another type of lymphoma is not uncommon, as occurred in our case. Patients who have a delay in the correct diagnosis have similar outcomes to those correctly diagnosed at first presentation.4
Treatment
There are no effective therapies for AITL. Poor prognostic factors include age (>60 years), stages III to IV disease, male gender, elevated serum lactate dehydrogenase level,3,5,10 and cutaneous involvement.7 Corticosteroids, anthracycline-based chemotherapy, and autologous stem cell transplant are currently the mainstays of therapy. Initial response to chemotherapy is promising, but duration of response is poor overall and there is no increased survival.5,15 A large population-based study of 1207 cases by Xu and Liu3 showed the overall survival rate at 2 and 10 years was 46.8% and 21.9%, respectively. Ten-year disease-specific survival was 35.9%, and there was no demonstrable improvement in survival over the last 2 decades.3 Case reports have demonstrated that thalidomide,16 lenalidomide,17 and cyclosporine plus dexamethasone18 have been successfully used to achieve remission for up to 3 years.
Conclusion
Angioimmunoblastic T-cell lymphoma is difficult to diagnose due to nonspecific clinical and histologic findings. Cutaneous manifestations are seen in AITL in up to half of cases that may occur early or in advanced disease. Similar to all cutaneous metastases, the appearance of the lesions can greatly vary. Our case demonstrates that dermatologists and dermatopathologists can make this diagnosis in the appropriate clinicopathologic context utilizing appropriate immunohistochemical staining and gene rearrangement studies.
- Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkins Lymphoma Classification Project. Ann Oncol. 2002;13:140-149.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-276.
- Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9:e92585.
- Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282-292.
- Mourad N, Mounier N, Briére J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470.
- Frederico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-cell Lymphoma Project. J Clin Oncol. 2013;31:240-246.
- Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lym-phadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659-664.
- Attygalle AD, Chuang SS, Diss TC, et al. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype, and molecular genetics. Histopathology. 2007;50:498-508.
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490-494.
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293-1296.
- Martel P, Laroche L, Courville P, et al. Cutaneous involvementin patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881-886.
- Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068-1076.
- Frizzera G, Moran E, Rappaport H. Angioimmunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070-1073.
- Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma [published online May 6, 2011]. J Am Acad Dermatol. 2011;65:855-862.
- Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837-2843.
- Dogan A, Ngu LSP, Ng SH, et al. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19:873-875.
- Fabbri A, Cencini E, Pietrini A, et al. Impressive activity of lenalidomide monotherapy in refractory angioimmunoblastic T-cell lymphoma: report of a case with long-term follow-up. Hematol Oncol. 2013;31:213-217.
- Kobayashi T, Kuroda J, Uchiyama H, et al. Successful treatment of chemotherapy-refractory angioimmunoblastic T cell lymphoma with cyclosporin A. Acta Haematol. 2012;127:10-15.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma. It comprises 18% of peripheral T-cell lymphomas and 1% to 2% of all non-Hodgkin lymphomas.1 The incidence of AITL in the United States is estimated to be 0.05 cases per 100,000 person-years,2 and there is a slight male predominance.1,3,4 It typically presents in the seventh decade of life; however, cases have been reported in adults ranging from 20 to 91 years of age.3
Angioimmunoblastic T-cell lymphoma presents with lymphadenopathy, hepatosplenomegaly, and systemic B symptoms (eg, fever, night sweats, weight loss, generalized pruritus).4-6 There are cutaneous manifestations in up to 50% of cases4,5,7 and frequently signs of autoimmune disorder.4,5 The diagnosis often is made by excisional lymph node biopsy. Lymph node specimens characteristically have a mixed inflammatory infiltrate that includes numerous B cells often infected with Epstein-Barr virus (EBV) and a relatively small population of atypical T lymphocytes.8 Identification of this neoplastic population of CD4+CD8− T lymphocytes expressing normal follicular helper T-cell markers CD10, chemokine CXCL13, programmed cell death protein 1 (PD-1), and B-cell lymphoma 6 (BCL-6) confirms the diagnosis of AITL.9,10 These malignant cells can be identified in skin specimens in cases of cutaneous metastatic disease.11,12 We present a case originally misdiagnosed as diffuse large B-cell lymphoma that was later identified as AITL on skin biopsy.
Case Report
A 72-year-old woman presented with a pruritic erythematous eruption around the neck of 3 weeks’ duration (Figure 1). Her medical history was notable for diffuse large B-cell non-Hodgkin lymphoma diagnosed 3 months prior based on results from a right cervical lymph node biopsy. She was treated with bendamustine and rituximab. On physical examination there were erythematous edematous papules coalescing into indurated plaques around the neck. The differential diagnosis included drug hypersensitivity reaction, herpes zoster, urticaria, and cutaneous metastasis. Two punch biopsies were taken for hematoxylin and eosin and tissue culture.

Tissue cultures and viral polymerase chain reaction were negative. Histopathologic examination revealed a scant atypical lymphoid infiltrate focally involving the deep dermis. The cells were medium to large in size and contained hyperchromatic pleomorphic nuclei (Figure 2). They were positive for CD3 and CD4, which was concerning for T-cell lymphoma. The histologic report of the excisional lymph node biopsy done 3 months prior described an atypical lymphoid neoplasm with extensive necrosis and extranodal spread that stained positively for CD20 (Figure 3).

Further staining of this cervical lymph node specimen revealed large atypical lymphoid cells positive for CD3, CD10, B-cell lymphoma 2 (BCL-2), BCL-6, and PD-1. There were intermixed mature B lymphocytes positive for CD20 and BCL-2. Chromogenic in situ hybridization with probes for EBV showed numerous positive cells throughout the infiltrate. Polymerase chain reaction demonstrated a T-cell population with clonally rearranged T-cell receptor genes. Primers for immunoglobulin heavy and light chains showed no evidence of a clonal B-cell population.
Additional staining of the atypical cutaneous lymphocytes revealed positivity for CD3, CD10, and PD-1. The morphologic and immunophenotypic findings of both specimens supported the diagnosis of AITL.
The patient declined further treatment and chose hospice care.
Comment
Etiology
Angioimmunoblastic T-cell lymphoma was originally named angioimmunoblastic lymphadenopathy with dysproteinemia. It was initially thought to be a benign hyperreactive immune process driven by B cells, and patients often died of infectious complications not long after the diagnosis was made.13 As more cases were reported with clonal rearrangements and signs of progressive lymphoma, AITL was recognized as a malignancy.
Presentation
Patients with AITL often present with advanced stage III or IV disease with extranodal and bone marrow involvement.3-6 Cutaneous disease occurs in up to half of patients and portends a poor prognosis.7 The rash often is a nonspecific erythematous macular and papular eruption mimicking a morbilliform viral exanthem or drug reaction. Urticarial, nodular, petechial, purpuric, eczematous, erythrodermic, and vesiculobullous presentations have been described.4,11,12 In up to one-third of cases, the eruption occurs in association with a new medication, often leading to an initial misdiagnosis of drug hypersensitivity reaction.4,11 In a review conducted by Balaraman et al,14 84% of patients with AITL reported having pruritus.
There is an association of autoimmune phenomena in patients with AITL, which is likely a result of immune dysregulation associated with poorly functioning follicular helper T cells. Patients may present with arthralgia, hemolytic anemia, or thrombocytopenic purpura. Hypergammaglobulinemia has been reported in 30% to 50% of AITL patients.4,6 Other pertinent immunologic findings include positive Coombs test, cold agglutinins, cryoglobulinemia, hypocomplementemia, and positive antinuclear antibodies.4-7
Gene Analysis
Affected lymph nodes have a characteristically effaced architecture with proliferative arborizing venules; a hyperplastic population of follicular dendritic cells; and a mixed inflammatory infiltrate that is comprised of atypical lymphocytes and variable numbers of reactive lymphocytes, histiocytes, eosinophils, and plasma cells. The malignant T lymphocytes often account for only a small portion of the infiltrate.8 T-cell gene rearrangement studies identify clonal cells with β and γ rearrangements in the majority of cases.4 These cells are predominantly CD4+CD8− and express normal follicular helper T-cell markers CD10, CXCL13, BCL-6,5,9 and PD-1.10 Numerous B cells are seen intermixed with follicular dendritic cells. They are frequently infected with EBV and can have an atypical Reed-Sternberg cell–like appearance.4,5,15 In the evaluation of AITL, polymerase chain reaction studies with primers for immunoglobulin heavy and light chain should be performed to look for clonal B-cell populations and rule out a possible secondary B-cell lymphoma.
Histology
Five histologic patterns have been described with cutaneous AITL: (1) superficial perivascular infiltrate of eosinophils and lymphocytes that lack atypia, (2) sparse perivascular infiltrate with atypical lymphocytes, (3) dense dermal infiltrate of pleomorphic lymphocytes, (4) leukocytoclastic vasculitis without atypical lymphocytes,11 and (5) necrotizing vasculitis.12 The finding of vascular hyperplasia, perivascular infiltrate, or vasculitis has been reported in 91% of cases in the literature. Although these findings are nonspecific, an analysis of cutaneous cases reported in the literature found that 87% demonstrated T-cell receptor gene rearrangements.14 Lymphoid cells are positive for CD10 and PD-1, as was demonstrated in our case, and are CXCL13 positive in the majority of cases.12 Atypical and EBV-infected B cells also can be found in the skin.11,12
Differential Diagnosis
Angioimmunoblastic T-cell lymphoma can mimic infectious, autoimmune, or allergic etiologies, and misdiagnosis of another type of lymphoma is not uncommon, as occurred in our case. Patients who have a delay in the correct diagnosis have similar outcomes to those correctly diagnosed at first presentation.4
Treatment
There are no effective therapies for AITL. Poor prognostic factors include age (>60 years), stages III to IV disease, male gender, elevated serum lactate dehydrogenase level,3,5,10 and cutaneous involvement.7 Corticosteroids, anthracycline-based chemotherapy, and autologous stem cell transplant are currently the mainstays of therapy. Initial response to chemotherapy is promising, but duration of response is poor overall and there is no increased survival.5,15 A large population-based study of 1207 cases by Xu and Liu3 showed the overall survival rate at 2 and 10 years was 46.8% and 21.9%, respectively. Ten-year disease-specific survival was 35.9%, and there was no demonstrable improvement in survival over the last 2 decades.3 Case reports have demonstrated that thalidomide,16 lenalidomide,17 and cyclosporine plus dexamethasone18 have been successfully used to achieve remission for up to 3 years.
Conclusion
Angioimmunoblastic T-cell lymphoma is difficult to diagnose due to nonspecific clinical and histologic findings. Cutaneous manifestations are seen in AITL in up to half of cases that may occur early or in advanced disease. Similar to all cutaneous metastases, the appearance of the lesions can greatly vary. Our case demonstrates that dermatologists and dermatopathologists can make this diagnosis in the appropriate clinicopathologic context utilizing appropriate immunohistochemical staining and gene rearrangement studies.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma. It comprises 18% of peripheral T-cell lymphomas and 1% to 2% of all non-Hodgkin lymphomas.1 The incidence of AITL in the United States is estimated to be 0.05 cases per 100,000 person-years,2 and there is a slight male predominance.1,3,4 It typically presents in the seventh decade of life; however, cases have been reported in adults ranging from 20 to 91 years of age.3
Angioimmunoblastic T-cell lymphoma presents with lymphadenopathy, hepatosplenomegaly, and systemic B symptoms (eg, fever, night sweats, weight loss, generalized pruritus).4-6 There are cutaneous manifestations in up to 50% of cases4,5,7 and frequently signs of autoimmune disorder.4,5 The diagnosis often is made by excisional lymph node biopsy. Lymph node specimens characteristically have a mixed inflammatory infiltrate that includes numerous B cells often infected with Epstein-Barr virus (EBV) and a relatively small population of atypical T lymphocytes.8 Identification of this neoplastic population of CD4+CD8− T lymphocytes expressing normal follicular helper T-cell markers CD10, chemokine CXCL13, programmed cell death protein 1 (PD-1), and B-cell lymphoma 6 (BCL-6) confirms the diagnosis of AITL.9,10 These malignant cells can be identified in skin specimens in cases of cutaneous metastatic disease.11,12 We present a case originally misdiagnosed as diffuse large B-cell lymphoma that was later identified as AITL on skin biopsy.
Case Report
A 72-year-old woman presented with a pruritic erythematous eruption around the neck of 3 weeks’ duration (Figure 1). Her medical history was notable for diffuse large B-cell non-Hodgkin lymphoma diagnosed 3 months prior based on results from a right cervical lymph node biopsy. She was treated with bendamustine and rituximab. On physical examination there were erythematous edematous papules coalescing into indurated plaques around the neck. The differential diagnosis included drug hypersensitivity reaction, herpes zoster, urticaria, and cutaneous metastasis. Two punch biopsies were taken for hematoxylin and eosin and tissue culture.

Tissue cultures and viral polymerase chain reaction were negative. Histopathologic examination revealed a scant atypical lymphoid infiltrate focally involving the deep dermis. The cells were medium to large in size and contained hyperchromatic pleomorphic nuclei (Figure 2). They were positive for CD3 and CD4, which was concerning for T-cell lymphoma. The histologic report of the excisional lymph node biopsy done 3 months prior described an atypical lymphoid neoplasm with extensive necrosis and extranodal spread that stained positively for CD20 (Figure 3).

Further staining of this cervical lymph node specimen revealed large atypical lymphoid cells positive for CD3, CD10, B-cell lymphoma 2 (BCL-2), BCL-6, and PD-1. There were intermixed mature B lymphocytes positive for CD20 and BCL-2. Chromogenic in situ hybridization with probes for EBV showed numerous positive cells throughout the infiltrate. Polymerase chain reaction demonstrated a T-cell population with clonally rearranged T-cell receptor genes. Primers for immunoglobulin heavy and light chains showed no evidence of a clonal B-cell population.
Additional staining of the atypical cutaneous lymphocytes revealed positivity for CD3, CD10, and PD-1. The morphologic and immunophenotypic findings of both specimens supported the diagnosis of AITL.
The patient declined further treatment and chose hospice care.
Comment
Etiology
Angioimmunoblastic T-cell lymphoma was originally named angioimmunoblastic lymphadenopathy with dysproteinemia. It was initially thought to be a benign hyperreactive immune process driven by B cells, and patients often died of infectious complications not long after the diagnosis was made.13 As more cases were reported with clonal rearrangements and signs of progressive lymphoma, AITL was recognized as a malignancy.
Presentation
Patients with AITL often present with advanced stage III or IV disease with extranodal and bone marrow involvement.3-6 Cutaneous disease occurs in up to half of patients and portends a poor prognosis.7 The rash often is a nonspecific erythematous macular and papular eruption mimicking a morbilliform viral exanthem or drug reaction. Urticarial, nodular, petechial, purpuric, eczematous, erythrodermic, and vesiculobullous presentations have been described.4,11,12 In up to one-third of cases, the eruption occurs in association with a new medication, often leading to an initial misdiagnosis of drug hypersensitivity reaction.4,11 In a review conducted by Balaraman et al,14 84% of patients with AITL reported having pruritus.
There is an association of autoimmune phenomena in patients with AITL, which is likely a result of immune dysregulation associated with poorly functioning follicular helper T cells. Patients may present with arthralgia, hemolytic anemia, or thrombocytopenic purpura. Hypergammaglobulinemia has been reported in 30% to 50% of AITL patients.4,6 Other pertinent immunologic findings include positive Coombs test, cold agglutinins, cryoglobulinemia, hypocomplementemia, and positive antinuclear antibodies.4-7
Gene Analysis
Affected lymph nodes have a characteristically effaced architecture with proliferative arborizing venules; a hyperplastic population of follicular dendritic cells; and a mixed inflammatory infiltrate that is comprised of atypical lymphocytes and variable numbers of reactive lymphocytes, histiocytes, eosinophils, and plasma cells. The malignant T lymphocytes often account for only a small portion of the infiltrate.8 T-cell gene rearrangement studies identify clonal cells with β and γ rearrangements in the majority of cases.4 These cells are predominantly CD4+CD8− and express normal follicular helper T-cell markers CD10, CXCL13, BCL-6,5,9 and PD-1.10 Numerous B cells are seen intermixed with follicular dendritic cells. They are frequently infected with EBV and can have an atypical Reed-Sternberg cell–like appearance.4,5,15 In the evaluation of AITL, polymerase chain reaction studies with primers for immunoglobulin heavy and light chain should be performed to look for clonal B-cell populations and rule out a possible secondary B-cell lymphoma.
Histology
Five histologic patterns have been described with cutaneous AITL: (1) superficial perivascular infiltrate of eosinophils and lymphocytes that lack atypia, (2) sparse perivascular infiltrate with atypical lymphocytes, (3) dense dermal infiltrate of pleomorphic lymphocytes, (4) leukocytoclastic vasculitis without atypical lymphocytes,11 and (5) necrotizing vasculitis.12 The finding of vascular hyperplasia, perivascular infiltrate, or vasculitis has been reported in 91% of cases in the literature. Although these findings are nonspecific, an analysis of cutaneous cases reported in the literature found that 87% demonstrated T-cell receptor gene rearrangements.14 Lymphoid cells are positive for CD10 and PD-1, as was demonstrated in our case, and are CXCL13 positive in the majority of cases.12 Atypical and EBV-infected B cells also can be found in the skin.11,12
Differential Diagnosis
Angioimmunoblastic T-cell lymphoma can mimic infectious, autoimmune, or allergic etiologies, and misdiagnosis of another type of lymphoma is not uncommon, as occurred in our case. Patients who have a delay in the correct diagnosis have similar outcomes to those correctly diagnosed at first presentation.4
Treatment
There are no effective therapies for AITL. Poor prognostic factors include age (>60 years), stages III to IV disease, male gender, elevated serum lactate dehydrogenase level,3,5,10 and cutaneous involvement.7 Corticosteroids, anthracycline-based chemotherapy, and autologous stem cell transplant are currently the mainstays of therapy. Initial response to chemotherapy is promising, but duration of response is poor overall and there is no increased survival.5,15 A large population-based study of 1207 cases by Xu and Liu3 showed the overall survival rate at 2 and 10 years was 46.8% and 21.9%, respectively. Ten-year disease-specific survival was 35.9%, and there was no demonstrable improvement in survival over the last 2 decades.3 Case reports have demonstrated that thalidomide,16 lenalidomide,17 and cyclosporine plus dexamethasone18 have been successfully used to achieve remission for up to 3 years.
Conclusion
Angioimmunoblastic T-cell lymphoma is difficult to diagnose due to nonspecific clinical and histologic findings. Cutaneous manifestations are seen in AITL in up to half of cases that may occur early or in advanced disease. Similar to all cutaneous metastases, the appearance of the lesions can greatly vary. Our case demonstrates that dermatologists and dermatopathologists can make this diagnosis in the appropriate clinicopathologic context utilizing appropriate immunohistochemical staining and gene rearrangement studies.
- Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkins Lymphoma Classification Project. Ann Oncol. 2002;13:140-149.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-276.
- Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9:e92585.
- Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282-292.
- Mourad N, Mounier N, Briére J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470.
- Frederico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-cell Lymphoma Project. J Clin Oncol. 2013;31:240-246.
- Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lym-phadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659-664.
- Attygalle AD, Chuang SS, Diss TC, et al. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype, and molecular genetics. Histopathology. 2007;50:498-508.
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490-494.
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293-1296.
- Martel P, Laroche L, Courville P, et al. Cutaneous involvementin patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881-886.
- Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068-1076.
- Frizzera G, Moran E, Rappaport H. Angioimmunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070-1073.
- Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma [published online May 6, 2011]. J Am Acad Dermatol. 2011;65:855-862.
- Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837-2843.
- Dogan A, Ngu LSP, Ng SH, et al. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19:873-875.
- Fabbri A, Cencini E, Pietrini A, et al. Impressive activity of lenalidomide monotherapy in refractory angioimmunoblastic T-cell lymphoma: report of a case with long-term follow-up. Hematol Oncol. 2013;31:213-217.
- Kobayashi T, Kuroda J, Uchiyama H, et al. Successful treatment of chemotherapy-refractory angioimmunoblastic T cell lymphoma with cyclosporin A. Acta Haematol. 2012;127:10-15.
- Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkins Lymphoma Classification Project. Ann Oncol. 2002;13:140-149.
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265-276.
- Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9:e92585.
- Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282-292.
- Mourad N, Mounier N, Briére J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470.
- Frederico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-cell Lymphoma Project. J Clin Oncol. 2013;31:240-246.
- Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lym-phadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659-664.
- Attygalle AD, Chuang SS, Diss TC, et al. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype, and molecular genetics. Histopathology. 2007;50:498-508.
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490-494.
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293-1296.
- Martel P, Laroche L, Courville P, et al. Cutaneous involvementin patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881-886.
- Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068-1076.
- Frizzera G, Moran E, Rappaport H. Angioimmunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070-1073.
- Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma [published online May 6, 2011]. J Am Acad Dermatol. 2011;65:855-862.
- Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119:2837-2843.
- Dogan A, Ngu LSP, Ng SH, et al. Pathology and clinical features of angioimmunoblastic T-cell lymphoma after successful treatment with thalidomide. Leukemia. 2005;19:873-875.
- Fabbri A, Cencini E, Pietrini A, et al. Impressive activity of lenalidomide monotherapy in refractory angioimmunoblastic T-cell lymphoma: report of a case with long-term follow-up. Hematol Oncol. 2013;31:213-217.
- Kobayashi T, Kuroda J, Uchiyama H, et al. Successful treatment of chemotherapy-refractory angioimmunoblastic T cell lymphoma with cyclosporin A. Acta Haematol. 2012;127:10-15.
Practice Points
- Angioimmunoblastic T-cell lymphoma (AITL) is a rare, often aggressive type of peripheral T-cell lymphoma.
- Cutaneous manifestations have been seen in up to 50% of cases.
- Immunohistochemical markers for normal follicular helper T cells—CD-10, chemokine CXCL-13, and programmed cell death protein 1 (PD-1)—can be used to differentiate AITL from other types of lymphoma.
- The prognosis of AITL is poor.
Linear streaks on trunk, extremities
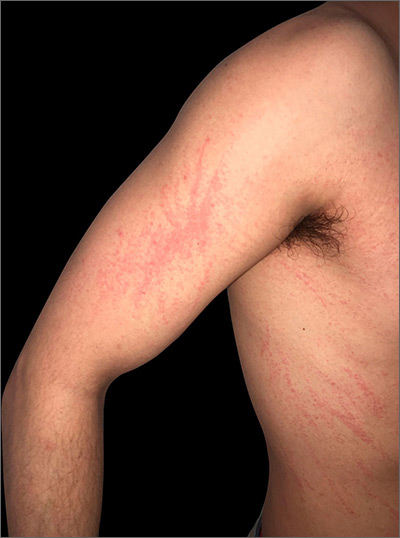
Based on the clinical findings, the patient was diagnosed with flagellate shiitake mushroom dermatitis. (Subsequent treponemal and nontreponemal tests were negative for syphilis.)
Shiitake dermatitis is a rare disease that appears in susceptible patients after the consumption of large amounts of raw or undercooked shiitake mushrooms. The eruption is believed to be attributable to either a toxic or hypersensitivity reaction to lentinan, a polysaccharide component found within the mushroom cell wall.1 Shiitake dermatitis is self-limiting and treatment focuses on symptomatic management.
Early recognition and proper counseling should ensure symptomatic relief and prevent future episodes. In addition, anyone preparing shiitake mushrooms should make sure that they are fully cooked before serving or eating them.2
In the case described here, the patient was advised to avoid eating undercooked shiitake mushrooms in the future and he was prescribed topical steroids (mometasone furoate 0.1% cream). The eruption resolved 2 weeks later.
References
1. Nguyen AH, Gonzaga MI, Lim VM, et al. Clinical features of shiitake dermatitis: a systematic review. Int J Dermatol. 2017;56:610-616.
2. Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.

Based on the clinical findings, the patient was diagnosed with flagellate shiitake mushroom dermatitis. (Subsequent treponemal and nontreponemal tests were negative for syphilis.)
Shiitake dermatitis is a rare disease that appears in susceptible patients after the consumption of large amounts of raw or undercooked shiitake mushrooms. The eruption is believed to be attributable to either a toxic or hypersensitivity reaction to lentinan, a polysaccharide component found within the mushroom cell wall.1 Shiitake dermatitis is self-limiting and treatment focuses on symptomatic management.
Early recognition and proper counseling should ensure symptomatic relief and prevent future episodes. In addition, anyone preparing shiitake mushrooms should make sure that they are fully cooked before serving or eating them.2
In the case described here, the patient was advised to avoid eating undercooked shiitake mushrooms in the future and he was prescribed topical steroids (mometasone furoate 0.1% cream). The eruption resolved 2 weeks later.
References
1. Nguyen AH, Gonzaga MI, Lim VM, et al. Clinical features of shiitake dermatitis: a systematic review. Int J Dermatol. 2017;56:610-616.
2. Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.

Based on the clinical findings, the patient was diagnosed with flagellate shiitake mushroom dermatitis. (Subsequent treponemal and nontreponemal tests were negative for syphilis.)
Shiitake dermatitis is a rare disease that appears in susceptible patients after the consumption of large amounts of raw or undercooked shiitake mushrooms. The eruption is believed to be attributable to either a toxic or hypersensitivity reaction to lentinan, a polysaccharide component found within the mushroom cell wall.1 Shiitake dermatitis is self-limiting and treatment focuses on symptomatic management.
Early recognition and proper counseling should ensure symptomatic relief and prevent future episodes. In addition, anyone preparing shiitake mushrooms should make sure that they are fully cooked before serving or eating them.2
In the case described here, the patient was advised to avoid eating undercooked shiitake mushrooms in the future and he was prescribed topical steroids (mometasone furoate 0.1% cream). The eruption resolved 2 weeks later.
References
1. Nguyen AH, Gonzaga MI, Lim VM, et al. Clinical features of shiitake dermatitis: a systematic review. Int J Dermatol. 2017;56:610-616.
2. Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
Levamisole-Induced Vasculopathy With Gastric Involvement in a Cocaine User
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.
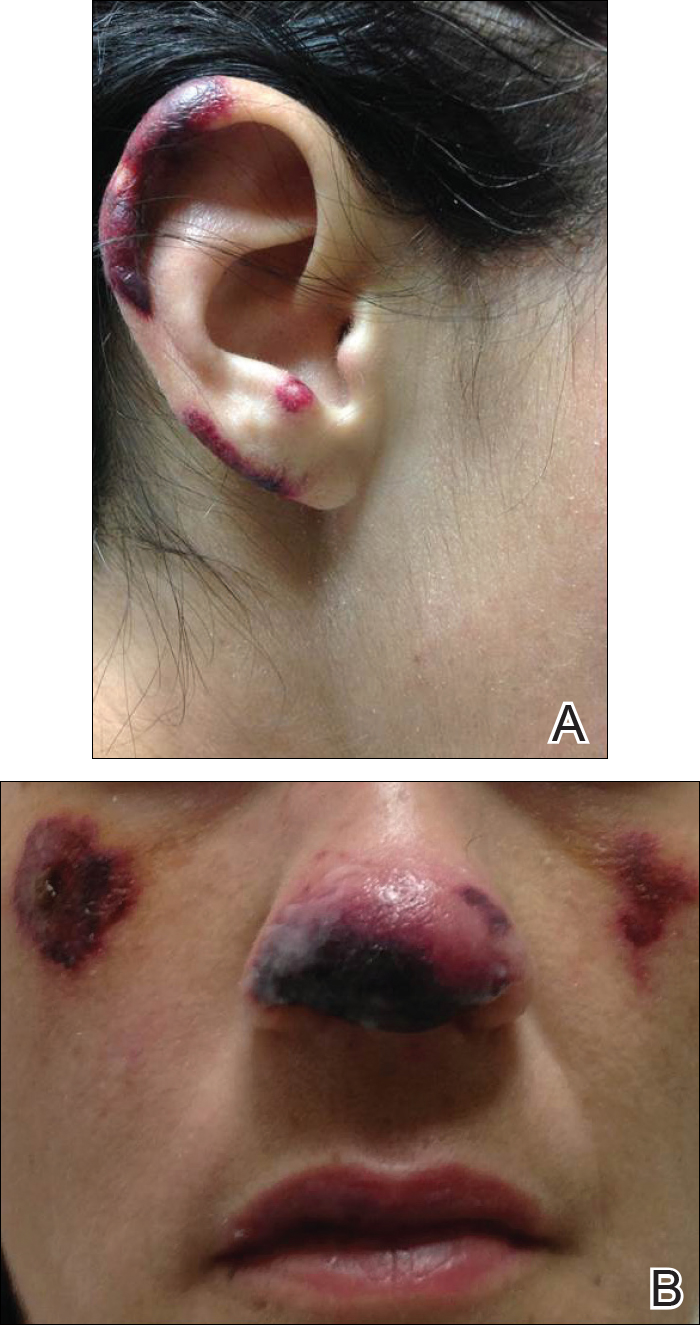
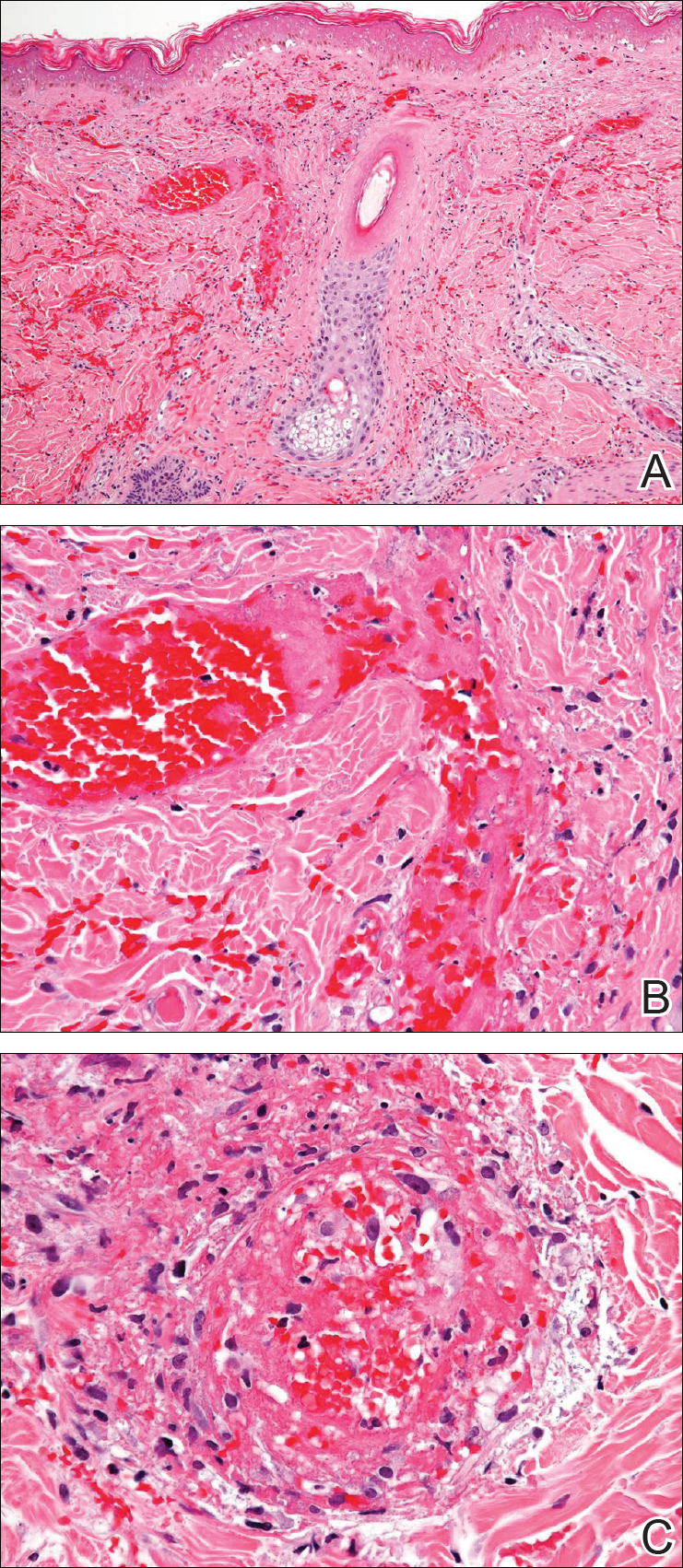
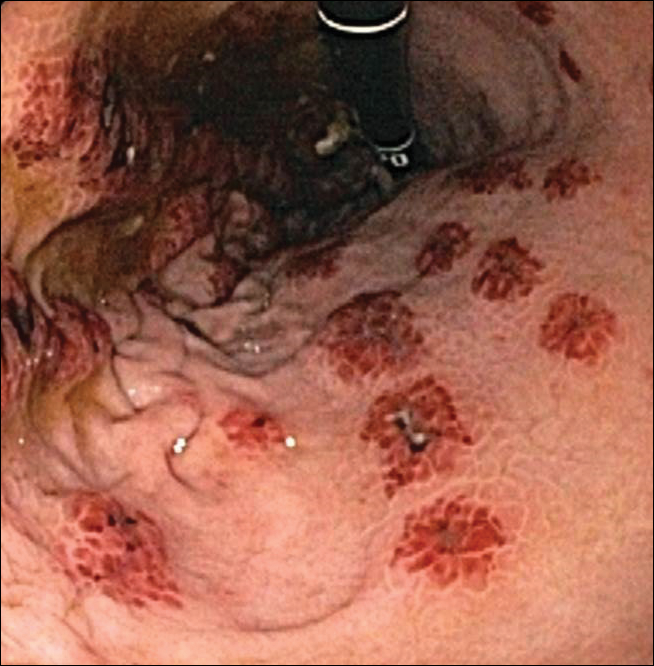
Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
Practice Points
- More than half of the cocaine illicitly consumed in the United States is contaminated with levamisole, a veterinary drug that can incite a vasculitic/vasculopathic response in the skin as well as in other organ systems.
- Because dermatologists often are the specialists to make the diagnosis of levamisole-induced vasculopathy, clinicians should be made aware that consumption of levamisole-contaminated cocaine may affect more than the skin alone.
Can Lighting Improve Sleep, Mood, and Behavior in Patients With Dementia?
Nonpharmacologic stimulation of the circadian system may benefit people in long-term care facilities.
BALTIMORE—Among patients with Alzheimer’s disease and related dementias, a lighting intervention may improve sleep, mood, and agitation, according to a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
The intervention “holds considerable promise as a novel, practically applied, nonpharmacologic intervention” for people with dementia who live in long-term care facilities, said Mariana G. Figueiro, PhD, Director of the Lighting Research Center and Professor of Architecture at Rensselaer Polytechnic Institute in Troy, New York.
People with dementia often have problems related to sleep and daytime irritability. A study in office workers found that exposure to light that stimulates the circadian system early in the day is associated with better sleep and improved behavior and mood. To assess whether a lighting intervention that delivers a circadian stimulus may improve sleep and behavior in patients with dementia in long-term care facilities, Dr. Figueiro and colleagues conducted a crossover, repeated-measures, within-subjects study. The study included 43 subjects (31 female) with Alzheimer’s disease or related dementias and Mini-Mental State Examination scores of less than 24.
The intervention consisted of an LED light table and individual room lights that were placed where patients spent most of their waking hours. The lights were turned on when participants woke up and remained on until 6 PM. Participants wore calibrated light meters that monitored their light exposure.
The trial included four weeks with an active circadian stimulus, four weeks with an inactive lighting intervention, and a four-week washout period in between.
In addition, researchers collected subjective measures of sleep disturbances (Pittsburgh Sleep Quality Index [PSQI]), mood (Cornell Scale for Depression in Dementia [CSDD]), and agitation (Cohen-Mansfield Agitation Index [CMAI]) at baseline and during the last week of the active and placebo intervention periods.
The active lighting intervention significantly decreased scores for sleep disturbances, depression, and agitation, compared with placebo. During the active intervention, mean PSQI scores decreased from 10.3 to 6.7, CSDD scores decreased from 10.5 to 7.3, and CMAI scores decreased from 42.9 to 37.4.
A six-month study of the lighting intervention is underway. “A logical next step would be to test the short- and long-term effects of the tailored lighting intervention among those living at home,” Dr. Figueiro and colleagues said.
—Jake Remaly
Suggested Reading
Figueiro MG, Steverson B, Heerwagen J, et al. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3(3):204-215.
Nonpharmacologic stimulation of the circadian system may benefit people in long-term care facilities.
Nonpharmacologic stimulation of the circadian system may benefit people in long-term care facilities.
BALTIMORE—Among patients with Alzheimer’s disease and related dementias, a lighting intervention may improve sleep, mood, and agitation, according to a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
The intervention “holds considerable promise as a novel, practically applied, nonpharmacologic intervention” for people with dementia who live in long-term care facilities, said Mariana G. Figueiro, PhD, Director of the Lighting Research Center and Professor of Architecture at Rensselaer Polytechnic Institute in Troy, New York.
People with dementia often have problems related to sleep and daytime irritability. A study in office workers found that exposure to light that stimulates the circadian system early in the day is associated with better sleep and improved behavior and mood. To assess whether a lighting intervention that delivers a circadian stimulus may improve sleep and behavior in patients with dementia in long-term care facilities, Dr. Figueiro and colleagues conducted a crossover, repeated-measures, within-subjects study. The study included 43 subjects (31 female) with Alzheimer’s disease or related dementias and Mini-Mental State Examination scores of less than 24.
The intervention consisted of an LED light table and individual room lights that were placed where patients spent most of their waking hours. The lights were turned on when participants woke up and remained on until 6 PM. Participants wore calibrated light meters that monitored their light exposure.
The trial included four weeks with an active circadian stimulus, four weeks with an inactive lighting intervention, and a four-week washout period in between.
In addition, researchers collected subjective measures of sleep disturbances (Pittsburgh Sleep Quality Index [PSQI]), mood (Cornell Scale for Depression in Dementia [CSDD]), and agitation (Cohen-Mansfield Agitation Index [CMAI]) at baseline and during the last week of the active and placebo intervention periods.
The active lighting intervention significantly decreased scores for sleep disturbances, depression, and agitation, compared with placebo. During the active intervention, mean PSQI scores decreased from 10.3 to 6.7, CSDD scores decreased from 10.5 to 7.3, and CMAI scores decreased from 42.9 to 37.4.
A six-month study of the lighting intervention is underway. “A logical next step would be to test the short- and long-term effects of the tailored lighting intervention among those living at home,” Dr. Figueiro and colleagues said.
—Jake Remaly
Suggested Reading
Figueiro MG, Steverson B, Heerwagen J, et al. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3(3):204-215.
BALTIMORE—Among patients with Alzheimer’s disease and related dementias, a lighting intervention may improve sleep, mood, and agitation, according to a study presented at the 32nd Annual Meeting of the Associated Professional Sleep Societies.
The intervention “holds considerable promise as a novel, practically applied, nonpharmacologic intervention” for people with dementia who live in long-term care facilities, said Mariana G. Figueiro, PhD, Director of the Lighting Research Center and Professor of Architecture at Rensselaer Polytechnic Institute in Troy, New York.
People with dementia often have problems related to sleep and daytime irritability. A study in office workers found that exposure to light that stimulates the circadian system early in the day is associated with better sleep and improved behavior and mood. To assess whether a lighting intervention that delivers a circadian stimulus may improve sleep and behavior in patients with dementia in long-term care facilities, Dr. Figueiro and colleagues conducted a crossover, repeated-measures, within-subjects study. The study included 43 subjects (31 female) with Alzheimer’s disease or related dementias and Mini-Mental State Examination scores of less than 24.
The intervention consisted of an LED light table and individual room lights that were placed where patients spent most of their waking hours. The lights were turned on when participants woke up and remained on until 6 PM. Participants wore calibrated light meters that monitored their light exposure.
The trial included four weeks with an active circadian stimulus, four weeks with an inactive lighting intervention, and a four-week washout period in between.
In addition, researchers collected subjective measures of sleep disturbances (Pittsburgh Sleep Quality Index [PSQI]), mood (Cornell Scale for Depression in Dementia [CSDD]), and agitation (Cohen-Mansfield Agitation Index [CMAI]) at baseline and during the last week of the active and placebo intervention periods.
The active lighting intervention significantly decreased scores for sleep disturbances, depression, and agitation, compared with placebo. During the active intervention, mean PSQI scores decreased from 10.3 to 6.7, CSDD scores decreased from 10.5 to 7.3, and CMAI scores decreased from 42.9 to 37.4.
A six-month study of the lighting intervention is underway. “A logical next step would be to test the short- and long-term effects of the tailored lighting intervention among those living at home,” Dr. Figueiro and colleagues said.
—Jake Remaly
Suggested Reading
Figueiro MG, Steverson B, Heerwagen J, et al. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3(3):204-215.
Huntington’s Disease Symptoms Vary by Age of Onset
Earlier age of onset may be associated with higher severity of behavioral symptoms.
MIAMI—The greater the age of Huntington’s disease onset, the lower the likelihood that the patient’s major symptom type at disease presentation will be behavioral or cognitive, according to research presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Increasing age at onset also is associated with a higher likelihood that motor symptoms will be the major symptom type at disease presentation.
Examining Enroll-HD Data
A patient may have subtle changes in movement, thinking, or behavior as early as 20 years before the onset of Huntington’s disease. Allison Daley, a clinical research specialist at Ohio State University in Columbus, and colleagues noted anecdotally that the severity of behavioral symptoms was decreased in older patients with newly diagnosed Huntington’s disease, compared with younger patients. The group set out to investigate the relationship between behavioral symptoms and age at onset of clinical symptoms in Huntington’s disease.
Ms. Daley and colleagues examined data for 8,714 participants registered in the Enroll-HD database as of 2016. They established three categories of patients based on age of onset. Early-onset Huntington’s disease was defined as onset at an age younger than 30. Earlier adult-onset Huntington’s disease was defined as onset between ages 30 and 59. Later adult-onset Huntington’s disease was defined as onset at an age above 59. Ms. Daley’s group examined the frequency and severity of behavioral symptoms at disease presentation in all three groups. They used the Clinical Characteristics form and the Problem Behaviors Assessment form to evaluate symptom presence and severity.
Motor Symptoms More Common in Late-Onset Disease
Of the entire sample, 4,469 participants had manifest Huntington’s disease. Motor symptoms were present in 42% of participants with early-onset Huntington’s disease, 50% of participants with earlier adult-onset Huntington’s disease, and 67% of participants with later adult-onset Huntington’s disease. Cognitive symptoms were recorded in 9% of the early-onset group, 10% of the earlier adult-onset group, and 4% of the later adult-onset group. Behavioral symptoms were observed in 26% of the early-onset group, 19% of the earlier adult-onset group, and 11% of the later adult-onset group.
A one-year increase in age at onset was associated with a 5.5% decrease in the odds of severe behavioral symptom of any type. In addition, a one-year increase in age at onset was associated with a 9.4% decrease in the odds of severe disorientation, an 8.8% decrease in the odds of severe delusions, a 6.8% decrease in the odds of severe obsessive–compulsive behavior, and a 5.8% decrease in the odds of severe apathy.
At baseline, 56% of participants had apathy, 55% had anxiety, 54% had irritability, 51% had depression, 44% had perseverative thinking, and 29% had anger or aggression.
“Specific behavioral symptoms, particularly disorientation, delusions, and obsessive–compulsive behaviors, may tend to manifest more severely in individuals with earlier age at onset. Earlier age at onset may be associated with higher severity of behavioral symptoms overall,” said Ms. Daley. “Further research exploring biologic mechanisms and environmental factors that interact with CAG repeat size to affect symptom presentation in Huntington’s disease at different ages at onset may help identify additional therapeutic strategies and individualized treatment approaches for Huntington’s disease.”
—Erik Greb
Earlier age of onset may be associated with higher severity of behavioral symptoms.
Earlier age of onset may be associated with higher severity of behavioral symptoms.
MIAMI—The greater the age of Huntington’s disease onset, the lower the likelihood that the patient’s major symptom type at disease presentation will be behavioral or cognitive, according to research presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Increasing age at onset also is associated with a higher likelihood that motor symptoms will be the major symptom type at disease presentation.
Examining Enroll-HD Data
A patient may have subtle changes in movement, thinking, or behavior as early as 20 years before the onset of Huntington’s disease. Allison Daley, a clinical research specialist at Ohio State University in Columbus, and colleagues noted anecdotally that the severity of behavioral symptoms was decreased in older patients with newly diagnosed Huntington’s disease, compared with younger patients. The group set out to investigate the relationship between behavioral symptoms and age at onset of clinical symptoms in Huntington’s disease.
Ms. Daley and colleagues examined data for 8,714 participants registered in the Enroll-HD database as of 2016. They established three categories of patients based on age of onset. Early-onset Huntington’s disease was defined as onset at an age younger than 30. Earlier adult-onset Huntington’s disease was defined as onset between ages 30 and 59. Later adult-onset Huntington’s disease was defined as onset at an age above 59. Ms. Daley’s group examined the frequency and severity of behavioral symptoms at disease presentation in all three groups. They used the Clinical Characteristics form and the Problem Behaviors Assessment form to evaluate symptom presence and severity.
Motor Symptoms More Common in Late-Onset Disease
Of the entire sample, 4,469 participants had manifest Huntington’s disease. Motor symptoms were present in 42% of participants with early-onset Huntington’s disease, 50% of participants with earlier adult-onset Huntington’s disease, and 67% of participants with later adult-onset Huntington’s disease. Cognitive symptoms were recorded in 9% of the early-onset group, 10% of the earlier adult-onset group, and 4% of the later adult-onset group. Behavioral symptoms were observed in 26% of the early-onset group, 19% of the earlier adult-onset group, and 11% of the later adult-onset group.
A one-year increase in age at onset was associated with a 5.5% decrease in the odds of severe behavioral symptom of any type. In addition, a one-year increase in age at onset was associated with a 9.4% decrease in the odds of severe disorientation, an 8.8% decrease in the odds of severe delusions, a 6.8% decrease in the odds of severe obsessive–compulsive behavior, and a 5.8% decrease in the odds of severe apathy.
At baseline, 56% of participants had apathy, 55% had anxiety, 54% had irritability, 51% had depression, 44% had perseverative thinking, and 29% had anger or aggression.
“Specific behavioral symptoms, particularly disorientation, delusions, and obsessive–compulsive behaviors, may tend to manifest more severely in individuals with earlier age at onset. Earlier age at onset may be associated with higher severity of behavioral symptoms overall,” said Ms. Daley. “Further research exploring biologic mechanisms and environmental factors that interact with CAG repeat size to affect symptom presentation in Huntington’s disease at different ages at onset may help identify additional therapeutic strategies and individualized treatment approaches for Huntington’s disease.”
—Erik Greb
MIAMI—The greater the age of Huntington’s disease onset, the lower the likelihood that the patient’s major symptom type at disease presentation will be behavioral or cognitive, according to research presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Increasing age at onset also is associated with a higher likelihood that motor symptoms will be the major symptom type at disease presentation.
Examining Enroll-HD Data
A patient may have subtle changes in movement, thinking, or behavior as early as 20 years before the onset of Huntington’s disease. Allison Daley, a clinical research specialist at Ohio State University in Columbus, and colleagues noted anecdotally that the severity of behavioral symptoms was decreased in older patients with newly diagnosed Huntington’s disease, compared with younger patients. The group set out to investigate the relationship between behavioral symptoms and age at onset of clinical symptoms in Huntington’s disease.
Ms. Daley and colleagues examined data for 8,714 participants registered in the Enroll-HD database as of 2016. They established three categories of patients based on age of onset. Early-onset Huntington’s disease was defined as onset at an age younger than 30. Earlier adult-onset Huntington’s disease was defined as onset between ages 30 and 59. Later adult-onset Huntington’s disease was defined as onset at an age above 59. Ms. Daley’s group examined the frequency and severity of behavioral symptoms at disease presentation in all three groups. They used the Clinical Characteristics form and the Problem Behaviors Assessment form to evaluate symptom presence and severity.
Motor Symptoms More Common in Late-Onset Disease
Of the entire sample, 4,469 participants had manifest Huntington’s disease. Motor symptoms were present in 42% of participants with early-onset Huntington’s disease, 50% of participants with earlier adult-onset Huntington’s disease, and 67% of participants with later adult-onset Huntington’s disease. Cognitive symptoms were recorded in 9% of the early-onset group, 10% of the earlier adult-onset group, and 4% of the later adult-onset group. Behavioral symptoms were observed in 26% of the early-onset group, 19% of the earlier adult-onset group, and 11% of the later adult-onset group.
A one-year increase in age at onset was associated with a 5.5% decrease in the odds of severe behavioral symptom of any type. In addition, a one-year increase in age at onset was associated with a 9.4% decrease in the odds of severe disorientation, an 8.8% decrease in the odds of severe delusions, a 6.8% decrease in the odds of severe obsessive–compulsive behavior, and a 5.8% decrease in the odds of severe apathy.
At baseline, 56% of participants had apathy, 55% had anxiety, 54% had irritability, 51% had depression, 44% had perseverative thinking, and 29% had anger or aggression.
“Specific behavioral symptoms, particularly disorientation, delusions, and obsessive–compulsive behaviors, may tend to manifest more severely in individuals with earlier age at onset. Earlier age at onset may be associated with higher severity of behavioral symptoms overall,” said Ms. Daley. “Further research exploring biologic mechanisms and environmental factors that interact with CAG repeat size to affect symptom presentation in Huntington’s disease at different ages at onset may help identify additional therapeutic strategies and individualized treatment approaches for Huntington’s disease.”
—Erik Greb