User login
United Kingdom experience provides important lessons for controlling C. auris outbreaks
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at [email protected].”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Ten additional cases occurred in the 7 months after the axillary probes were removed from the environment.
Study details: A review of the epidemiology and control of a C. auris outbreak affecting 76 patients.
Disclosures: Dr. Eyre reported having no disclosures.
Source: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
Plan now for outpatient diabetes tech in the hospital
ORLANDO –
A third or more of patients with type 1 diabetes mellitus and growing numbers of patients with insulin-dependent type 2 diabetes mellitus patients are using pumps and sensor technology. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, and there’s general consensus that continuous glucose monitors (CGM) can be used in the hospital.
All in all, it’s a good thing, according to Guillermo E. Umpierrez, MD, professor of endocrinology at Emory University and chief of endocrinology at Grady Memorial Hospital, both in Atlanta.
In a talk at the annual scientific sessions of the American Diabetes Association, Dr. Umpierrez reviewed a number of studies showing that glycemic control with the new technology is no worse in the hospital – and sometimes even better – than with traditional point-of-care glucose testing and insulin administration. There is a lack of randomized, controlled trials to prove the point definitively, but what evidence does exist is promising.
“This technology is rapidly advancing, and I am very optimistic that we are going to see more and more of these devices in the hospital. If patients can manage themselves, allow them to use CGM, allow them to use their pumps,” he said.
As for closed loop systems – automated glucose sensing and insulin administration – emerging evidence suggests they “allow you to have very good glucose control and less glycemic variability,” both inside and outside of the ICU, he said. “I am very hopeful before I retire that there will be management of a significant number of patients with closed loop systems.”
To keep up, training for hospital providers on the new technology is now “mandatory at all levels,” Dr. Umpierrez said, and if they haven’t done so already, hospitals need to put policies and procedures in place for when, and when not, to allow patients to use their diabetes equipment, and how to integrate it into care.
Among many things to consider, patients must be well enough to use their pumps and monitors, be able to demonstrate their functions, and also want to participate in their own care.
Contraindications to inpatient pump use include impaired consciousness, critical illness, and hyperglycemic crises because insulin requirements change too rapidly and dramatically for pumps. Lack of trained providers and supplies is another hurdle. Pumps also need to come off for MRIs.
CGM, meanwhile, has been shown to improve glycemic control, detecting both hyperglycemia and hypoglycemia more readily than point-of-care testing. It’s good at picking up trends in glucose levels, and Dr. Umpierrez anticipates a time when readings will be transmitted to nurses’ stations automatically to track blood glucose trends. “I think that’s the future,” he said.
But, as with insulin pumps, there are caveats. Among them, it’s unclear how well CGM works during hypoxia, hypothermia, and hypotension. Thrombus formation and infections have been reported with intravascular monitors, and a number of agents can throw off some CGM devices, including acetaminophen, heparin, and dopamine.
Dr. Umpierrez disclosed relationships with AstraZeneca, Merck, Novo Nordisk, Sanofi, and other companies.
ORLANDO –
A third or more of patients with type 1 diabetes mellitus and growing numbers of patients with insulin-dependent type 2 diabetes mellitus patients are using pumps and sensor technology. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, and there’s general consensus that continuous glucose monitors (CGM) can be used in the hospital.
All in all, it’s a good thing, according to Guillermo E. Umpierrez, MD, professor of endocrinology at Emory University and chief of endocrinology at Grady Memorial Hospital, both in Atlanta.
In a talk at the annual scientific sessions of the American Diabetes Association, Dr. Umpierrez reviewed a number of studies showing that glycemic control with the new technology is no worse in the hospital – and sometimes even better – than with traditional point-of-care glucose testing and insulin administration. There is a lack of randomized, controlled trials to prove the point definitively, but what evidence does exist is promising.
“This technology is rapidly advancing, and I am very optimistic that we are going to see more and more of these devices in the hospital. If patients can manage themselves, allow them to use CGM, allow them to use their pumps,” he said.
As for closed loop systems – automated glucose sensing and insulin administration – emerging evidence suggests they “allow you to have very good glucose control and less glycemic variability,” both inside and outside of the ICU, he said. “I am very hopeful before I retire that there will be management of a significant number of patients with closed loop systems.”
To keep up, training for hospital providers on the new technology is now “mandatory at all levels,” Dr. Umpierrez said, and if they haven’t done so already, hospitals need to put policies and procedures in place for when, and when not, to allow patients to use their diabetes equipment, and how to integrate it into care.
Among many things to consider, patients must be well enough to use their pumps and monitors, be able to demonstrate their functions, and also want to participate in their own care.
Contraindications to inpatient pump use include impaired consciousness, critical illness, and hyperglycemic crises because insulin requirements change too rapidly and dramatically for pumps. Lack of trained providers and supplies is another hurdle. Pumps also need to come off for MRIs.
CGM, meanwhile, has been shown to improve glycemic control, detecting both hyperglycemia and hypoglycemia more readily than point-of-care testing. It’s good at picking up trends in glucose levels, and Dr. Umpierrez anticipates a time when readings will be transmitted to nurses’ stations automatically to track blood glucose trends. “I think that’s the future,” he said.
But, as with insulin pumps, there are caveats. Among them, it’s unclear how well CGM works during hypoxia, hypothermia, and hypotension. Thrombus formation and infections have been reported with intravascular monitors, and a number of agents can throw off some CGM devices, including acetaminophen, heparin, and dopamine.
Dr. Umpierrez disclosed relationships with AstraZeneca, Merck, Novo Nordisk, Sanofi, and other companies.
ORLANDO –
A third or more of patients with type 1 diabetes mellitus and growing numbers of patients with insulin-dependent type 2 diabetes mellitus patients are using pumps and sensor technology. The American Diabetes Association advocates allowing patients who are physically and mentally able to continue to use their pumps when hospitalized, and there’s general consensus that continuous glucose monitors (CGM) can be used in the hospital.
All in all, it’s a good thing, according to Guillermo E. Umpierrez, MD, professor of endocrinology at Emory University and chief of endocrinology at Grady Memorial Hospital, both in Atlanta.
In a talk at the annual scientific sessions of the American Diabetes Association, Dr. Umpierrez reviewed a number of studies showing that glycemic control with the new technology is no worse in the hospital – and sometimes even better – than with traditional point-of-care glucose testing and insulin administration. There is a lack of randomized, controlled trials to prove the point definitively, but what evidence does exist is promising.
“This technology is rapidly advancing, and I am very optimistic that we are going to see more and more of these devices in the hospital. If patients can manage themselves, allow them to use CGM, allow them to use their pumps,” he said.
As for closed loop systems – automated glucose sensing and insulin administration – emerging evidence suggests they “allow you to have very good glucose control and less glycemic variability,” both inside and outside of the ICU, he said. “I am very hopeful before I retire that there will be management of a significant number of patients with closed loop systems.”
To keep up, training for hospital providers on the new technology is now “mandatory at all levels,” Dr. Umpierrez said, and if they haven’t done so already, hospitals need to put policies and procedures in place for when, and when not, to allow patients to use their diabetes equipment, and how to integrate it into care.
Among many things to consider, patients must be well enough to use their pumps and monitors, be able to demonstrate their functions, and also want to participate in their own care.
Contraindications to inpatient pump use include impaired consciousness, critical illness, and hyperglycemic crises because insulin requirements change too rapidly and dramatically for pumps. Lack of trained providers and supplies is another hurdle. Pumps also need to come off for MRIs.
CGM, meanwhile, has been shown to improve glycemic control, detecting both hyperglycemia and hypoglycemia more readily than point-of-care testing. It’s good at picking up trends in glucose levels, and Dr. Umpierrez anticipates a time when readings will be transmitted to nurses’ stations automatically to track blood glucose trends. “I think that’s the future,” he said.
But, as with insulin pumps, there are caveats. Among them, it’s unclear how well CGM works during hypoxia, hypothermia, and hypotension. Thrombus formation and infections have been reported with intravascular monitors, and a number of agents can throw off some CGM devices, including acetaminophen, heparin, and dopamine.
Dr. Umpierrez disclosed relationships with AstraZeneca, Merck, Novo Nordisk, Sanofi, and other companies.
EXPERT ANALYSIS FROM ADA 2018
5 Points on Meniscal Allograft Transplantation
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.
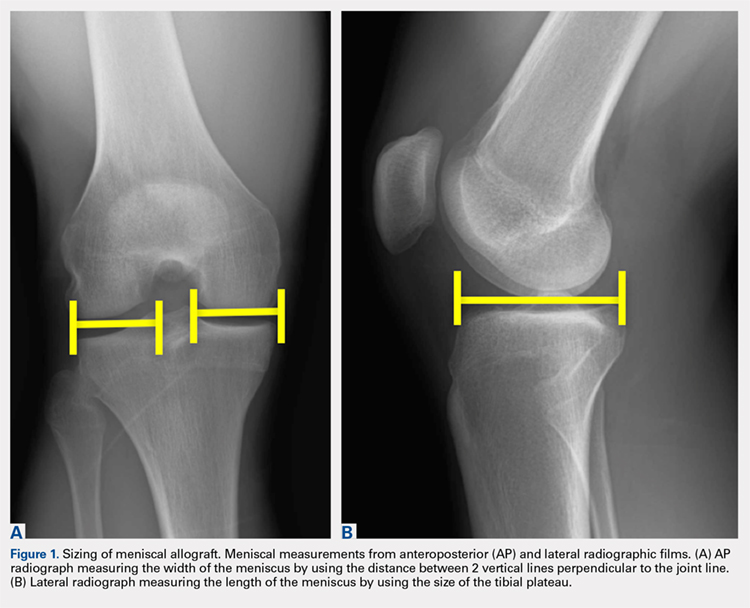
Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
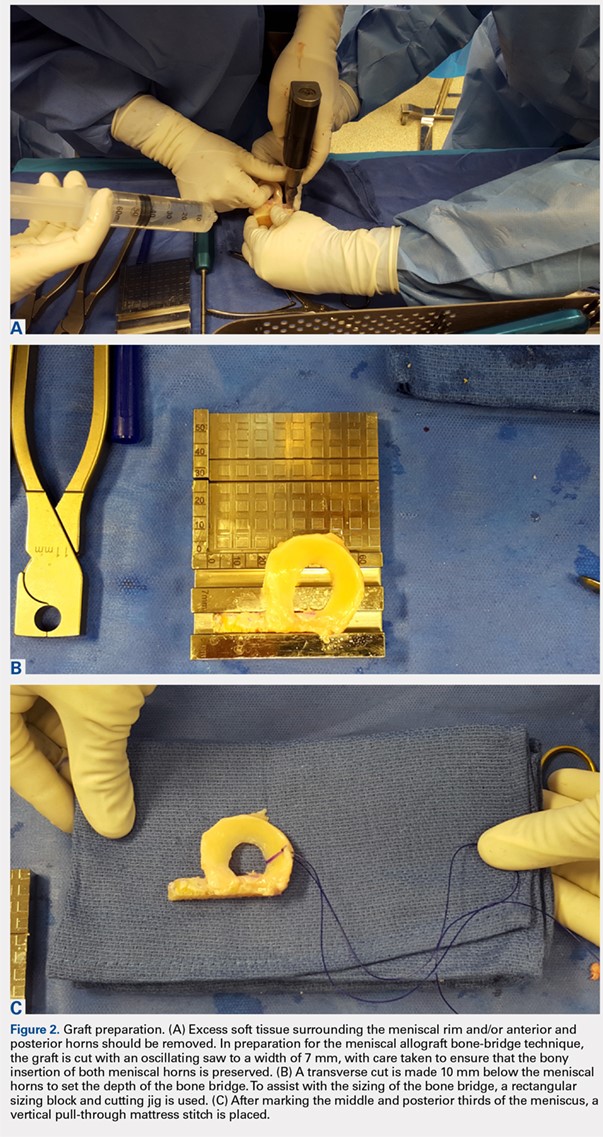
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.
Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
TAKE-HOME POINTS
- Patient selection is critical for obtaining long-term functional outcome improvements and reduced pain, with the ideal MAT candidate being a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency.
- Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure.
- Accurate graft width and length measurements are vital, and the most common technique used today includes measuring the meniscus on anteroposterior and lateral radiographic images.
- When preparing the graft for the bone-bridge technique, the bone is fashioned to create a bone bridge 10 mm in depth by approximately 7 mm in width, incorporating the anterior and posterior horns of the meniscus.
- Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Policy responses to opioid epidemic may have benefits, harms
Some policy responses to the opioid epidemic have immediate, beneficial effects, while others lead to short-term harms that might be offset by long-term health benefits, according to researchers who have mathematically modeled the impact of 11 interventions.
Policies that expand addiction treatment or curb harmful effects of addiction such as overdose and infection were immediately beneficial in the model, with no negative effects on life years (LYs), quality-adjusted life years (QALYs), or addiction deaths, the researchers reported.
In contrast, policies that constrain prescription opioid supply resulted in some benefits, but also short-term harms because of inadequate pain control and users switching to heroin.
However, the modeling study also suggests those harms might be mitigated over the long term as new addictions are averted, according to Allison L. Pitt, a PhD candidate in the department of management science and engineering at Stanford (Calif.) University, and her coauthors.
Combining different interventions had additive benefits in the model, prompting Ms. Pitt and her coauthors to recommend a multifaceted policy approach to curb opioid abuse and reduce addiction deaths.
“,” they wrote in the American Journal of Public Health. “Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects.”
In their study, Ms. Pitt and her colleagues projected the impact of 11 policies aimed at curbing opioid addiction and reducing addiction deaths. They used dynamic compartmental modeling, a technique commonly used for evaluating the spread of contagious disease.
This technique is appropriate for studying the opioid epidemic, because it allows for dynamic modeling of addiction incidence that reflects a changing number of prescription holders, the authors said in their report, which focused on projected outcomes of various interventions at 5 and 10 years.
None of the policies substantially reduced opioid-related deaths in 5-year outcomes projections, they found. Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over that time period.
However, interventions focused on providing services for people with addictions did generally provide uniform benefits over the 5-year horizon: “Naloxone availability, needle exchange, medication-assisted treatment, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group,” Ms. Pitt and her coauthors said.
Interventions that reduced opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, increased LYs and QALYs while decreasing total addiction deaths over 5 years. However, the investigators said, those benefits were partly offset by increases in heroin-related deaths.
Drug rescheduling was associated with a 45.6% increase in heroin-related deaths over 5 years in the model, the highest percentage increase of any intervention in the published data.
Over the 10-year horizon, addiction deaths continued to decrease proportionally for naloxone availability and needle-exchange policies, authors said. By comparison, policies focused on opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, averted substantially more deaths over 10 years than would be expected, compared with the 5-year results, investigators said.
Acute pain prescribing, which increased opioid-related deaths over 5 years in the model, was associated with a decrease in opioid-related deaths over 10 years, they added.
The report coauthors were Keith Humphreys, PhD, of Stanford’s department of psychiatry and behavioral sciences, and Margaret L. Brandeau, PhD, of the university’s department of management science and engineering.
The coauthors cited several limitations. One is that the opioid epidemic is changing in unpredictable ways. Therefore, numerous assumptions about the epidemic were made based on the opinions of clinicians and scientists.
The study was supported by a grant from the National Institute on Drug Abuse. Dr. Humphreys reported support through a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
SOURCE: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
Some policy responses to the opioid epidemic have immediate, beneficial effects, while others lead to short-term harms that might be offset by long-term health benefits, according to researchers who have mathematically modeled the impact of 11 interventions.
Policies that expand addiction treatment or curb harmful effects of addiction such as overdose and infection were immediately beneficial in the model, with no negative effects on life years (LYs), quality-adjusted life years (QALYs), or addiction deaths, the researchers reported.
In contrast, policies that constrain prescription opioid supply resulted in some benefits, but also short-term harms because of inadequate pain control and users switching to heroin.
However, the modeling study also suggests those harms might be mitigated over the long term as new addictions are averted, according to Allison L. Pitt, a PhD candidate in the department of management science and engineering at Stanford (Calif.) University, and her coauthors.
Combining different interventions had additive benefits in the model, prompting Ms. Pitt and her coauthors to recommend a multifaceted policy approach to curb opioid abuse and reduce addiction deaths.
“,” they wrote in the American Journal of Public Health. “Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects.”
In their study, Ms. Pitt and her colleagues projected the impact of 11 policies aimed at curbing opioid addiction and reducing addiction deaths. They used dynamic compartmental modeling, a technique commonly used for evaluating the spread of contagious disease.
This technique is appropriate for studying the opioid epidemic, because it allows for dynamic modeling of addiction incidence that reflects a changing number of prescription holders, the authors said in their report, which focused on projected outcomes of various interventions at 5 and 10 years.
None of the policies substantially reduced opioid-related deaths in 5-year outcomes projections, they found. Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over that time period.
However, interventions focused on providing services for people with addictions did generally provide uniform benefits over the 5-year horizon: “Naloxone availability, needle exchange, medication-assisted treatment, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group,” Ms. Pitt and her coauthors said.
Interventions that reduced opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, increased LYs and QALYs while decreasing total addiction deaths over 5 years. However, the investigators said, those benefits were partly offset by increases in heroin-related deaths.
Drug rescheduling was associated with a 45.6% increase in heroin-related deaths over 5 years in the model, the highest percentage increase of any intervention in the published data.
Over the 10-year horizon, addiction deaths continued to decrease proportionally for naloxone availability and needle-exchange policies, authors said. By comparison, policies focused on opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, averted substantially more deaths over 10 years than would be expected, compared with the 5-year results, investigators said.
Acute pain prescribing, which increased opioid-related deaths over 5 years in the model, was associated with a decrease in opioid-related deaths over 10 years, they added.
The report coauthors were Keith Humphreys, PhD, of Stanford’s department of psychiatry and behavioral sciences, and Margaret L. Brandeau, PhD, of the university’s department of management science and engineering.
The coauthors cited several limitations. One is that the opioid epidemic is changing in unpredictable ways. Therefore, numerous assumptions about the epidemic were made based on the opinions of clinicians and scientists.
The study was supported by a grant from the National Institute on Drug Abuse. Dr. Humphreys reported support through a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
SOURCE: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
Some policy responses to the opioid epidemic have immediate, beneficial effects, while others lead to short-term harms that might be offset by long-term health benefits, according to researchers who have mathematically modeled the impact of 11 interventions.
Policies that expand addiction treatment or curb harmful effects of addiction such as overdose and infection were immediately beneficial in the model, with no negative effects on life years (LYs), quality-adjusted life years (QALYs), or addiction deaths, the researchers reported.
In contrast, policies that constrain prescription opioid supply resulted in some benefits, but also short-term harms because of inadequate pain control and users switching to heroin.
However, the modeling study also suggests those harms might be mitigated over the long term as new addictions are averted, according to Allison L. Pitt, a PhD candidate in the department of management science and engineering at Stanford (Calif.) University, and her coauthors.
Combining different interventions had additive benefits in the model, prompting Ms. Pitt and her coauthors to recommend a multifaceted policy approach to curb opioid abuse and reduce addiction deaths.
“,” they wrote in the American Journal of Public Health. “Instead, portfolios of policies will likely be required, including those that prevent addiction, treat addiction, and mitigate its effects.”
In their study, Ms. Pitt and her colleagues projected the impact of 11 policies aimed at curbing opioid addiction and reducing addiction deaths. They used dynamic compartmental modeling, a technique commonly used for evaluating the spread of contagious disease.
This technique is appropriate for studying the opioid epidemic, because it allows for dynamic modeling of addiction incidence that reflects a changing number of prescription holders, the authors said in their report, which focused on projected outcomes of various interventions at 5 and 10 years.
None of the policies substantially reduced opioid-related deaths in 5-year outcomes projections, they found. Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over that time period.
However, interventions focused on providing services for people with addictions did generally provide uniform benefits over the 5-year horizon: “Naloxone availability, needle exchange, medication-assisted treatment, and psychosocial treatment policies generate gains in LYs and QALYs and reduce deaths, without harming any group,” Ms. Pitt and her coauthors said.
Interventions that reduced opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, increased LYs and QALYs while decreasing total addiction deaths over 5 years. However, the investigators said, those benefits were partly offset by increases in heroin-related deaths.
Drug rescheduling was associated with a 45.6% increase in heroin-related deaths over 5 years in the model, the highest percentage increase of any intervention in the published data.
Over the 10-year horizon, addiction deaths continued to decrease proportionally for naloxone availability and needle-exchange policies, authors said. By comparison, policies focused on opioid supply, such as excess opioid disposal and reduced prescribing for transitioning pain, averted substantially more deaths over 10 years than would be expected, compared with the 5-year results, investigators said.
Acute pain prescribing, which increased opioid-related deaths over 5 years in the model, was associated with a decrease in opioid-related deaths over 10 years, they added.
The report coauthors were Keith Humphreys, PhD, of Stanford’s department of psychiatry and behavioral sciences, and Margaret L. Brandeau, PhD, of the university’s department of management science and engineering.
The coauthors cited several limitations. One is that the opioid epidemic is changing in unpredictable ways. Therefore, numerous assumptions about the epidemic were made based on the opinions of clinicians and scientists.
The study was supported by a grant from the National Institute on Drug Abuse. Dr. Humphreys reported support through a Senior Career Research Scientist award from the VA Health Services Research and Development Service.
SOURCE: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
Key clinical point: Interventions focused on providing services for people with addictions generally provided uniform benefits over the 5-year horizon.
Major finding: Increasing availability of naloxone averted 4% of addiction deaths, the highest reduction of any intervention over a 5-year time period modeled in the study.
Study details: Mathematical modeling of 11 policy interventions and their effects on life years, quality-adjusted life years, and deaths over 5- and 10-year time horizons.
Disclosures: The study was supported by grant from the National Institute on Drug Abuse. One study author reported support from the VA Health Services Research and Development Service.
Source: Pitt AL et al. Am J Public Health. 2018 Aug 23. doi: 10.2105/AJPH.2018.304590.
Survivors of sexual abuse cope with stigma
The Roman Catholic Church continues to be rocked by the burgeoning reality of priests as sexual predators. Some Catholics, including the Pope, have stepped up and acknowledged the blame for the decades of abuse, but others have been less inclined to do so. The latter attitude was and still is crushing to some the victims.
“Being raised Catholic, I remember – you don’t speak out against your own church,” Jim VanSickle says in an interview with National Catholic Reporter. “Nobody’s going to listen to you.”
Many of the survivors, Mr. VanSickle included, belonged to very conservative parishes. Parishioners often showed no compassion. After being sexually assaulted at age 16, he suffered in silence for almost 4 decades before speaking out during the recent release of Grand Jury report on the church abuses by the Pennsylvania attorney general.
“I’ve known others [who] came forward. They were ridiculed and ostracized – even by their own family members,” Mr. VanSickle says.
“We lived in a neighborhood where most of the people in the subdivision were Catholic. Everything in our lives revolved around the church,” said another victim, Utah resident Judy Larson. To be in that kind of environment and try to say something horrible happened to you, by a person everybody thinks is a god on earth, you’re all alone.”
As reported by Zita Ballinger Fletcher of the Catholic News Service, “survivors also faced a stigma caused by sexual assault. The victims were molested at an age when they did not know about sex. Confused, they realized what happened when they grew up. Feeling disgust, anger, and shame, they feared hostile reactions from their traditional communities.”
with the Catholic Church.
“People say, ‘You’re a bad person,’ ” Mr. VanSickle says. “It’s amazing that they attack their own people. They attack their own faithful.”
Click here to read about the challenges faced by these survivors.
The Roman Catholic Church continues to be rocked by the burgeoning reality of priests as sexual predators. Some Catholics, including the Pope, have stepped up and acknowledged the blame for the decades of abuse, but others have been less inclined to do so. The latter attitude was and still is crushing to some the victims.
“Being raised Catholic, I remember – you don’t speak out against your own church,” Jim VanSickle says in an interview with National Catholic Reporter. “Nobody’s going to listen to you.”
Many of the survivors, Mr. VanSickle included, belonged to very conservative parishes. Parishioners often showed no compassion. After being sexually assaulted at age 16, he suffered in silence for almost 4 decades before speaking out during the recent release of Grand Jury report on the church abuses by the Pennsylvania attorney general.
“I’ve known others [who] came forward. They were ridiculed and ostracized – even by their own family members,” Mr. VanSickle says.
“We lived in a neighborhood where most of the people in the subdivision were Catholic. Everything in our lives revolved around the church,” said another victim, Utah resident Judy Larson. To be in that kind of environment and try to say something horrible happened to you, by a person everybody thinks is a god on earth, you’re all alone.”
As reported by Zita Ballinger Fletcher of the Catholic News Service, “survivors also faced a stigma caused by sexual assault. The victims were molested at an age when they did not know about sex. Confused, they realized what happened when they grew up. Feeling disgust, anger, and shame, they feared hostile reactions from their traditional communities.”
with the Catholic Church.
“People say, ‘You’re a bad person,’ ” Mr. VanSickle says. “It’s amazing that they attack their own people. They attack their own faithful.”
Click here to read about the challenges faced by these survivors.
The Roman Catholic Church continues to be rocked by the burgeoning reality of priests as sexual predators. Some Catholics, including the Pope, have stepped up and acknowledged the blame for the decades of abuse, but others have been less inclined to do so. The latter attitude was and still is crushing to some the victims.
“Being raised Catholic, I remember – you don’t speak out against your own church,” Jim VanSickle says in an interview with National Catholic Reporter. “Nobody’s going to listen to you.”
Many of the survivors, Mr. VanSickle included, belonged to very conservative parishes. Parishioners often showed no compassion. After being sexually assaulted at age 16, he suffered in silence for almost 4 decades before speaking out during the recent release of Grand Jury report on the church abuses by the Pennsylvania attorney general.
“I’ve known others [who] came forward. They were ridiculed and ostracized – even by their own family members,” Mr. VanSickle says.
“We lived in a neighborhood where most of the people in the subdivision were Catholic. Everything in our lives revolved around the church,” said another victim, Utah resident Judy Larson. To be in that kind of environment and try to say something horrible happened to you, by a person everybody thinks is a god on earth, you’re all alone.”
As reported by Zita Ballinger Fletcher of the Catholic News Service, “survivors also faced a stigma caused by sexual assault. The victims were molested at an age when they did not know about sex. Confused, they realized what happened when they grew up. Feeling disgust, anger, and shame, they feared hostile reactions from their traditional communities.”
with the Catholic Church.
“People say, ‘You’re a bad person,’ ” Mr. VanSickle says. “It’s amazing that they attack their own people. They attack their own faithful.”
Click here to read about the challenges faced by these survivors.
ATTR-ACT shows treatment breakthrough in amyloid cardiomyopathy
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
MUNICH – The big news in the field of heart failure at the annual congress of the European Society of Cardiology concerned an obscure form of the disease traditionally considered rare: transthyretin amyloid cardiomyopathy (TAC).
It turns out that TAC is far more common than previously recognized; it can now be diagnosed and staged noninvasively; and – most important of all – there is for the first time an effective disease-modifying treatment in the form of a novel oral drug called tafamidis, as demonstrated in the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) presented at the meeting.
“This is the first phase 3 trial that can offer a chance for people with a terrible, severe disease. And within the last year, while the trial was being conducted, it became clear that this disease is much more underdiagnosed than rare,” said Claudio Rapezzi, MD, ATTR-ACT principal investigator and director of the school of cardiovascular diseases at the University of Bologna, Italy.
ATTR-ACT participants randomized to tafamidis showed significant reductions in all-cause mortality and cardiovascular hospitalizations, compared with placebo-treated controls at 30 months follow-up. They also experienced significantly lesser declines in both quality of life as reflected in Kansas City Cardiomyopathy Questionnaire scores and in physical function as captured in 6-minute walk distance.
Designated discussant Jacob George, MD, was over the moon regarding the results.
“This is a pioneering, game-changing trial that is likely to transform the way we diagnose and treat patients with cardiac amyloidosis,” said Dr. George of Kaplan Medical Center in Rehovot, Israel.
“We’re now in an era that, to my opinion, any patient with nonischemic unexplained heart failure should be screened for the presence of amyloidosis because, first, we now know how to prognosticate these patients, and second, we can offer them a real disease-modifying agent,” he added.
An underdiagnosed disease
Transthyretin amyloid cardiomyopathy occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium. This results in progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild type protein.
Think of TAC as a sort of dementia of the heart. As Dr. George noted, the cardiac disease bears “remarkable similarities” to Alzheimer’s disease, with both conditions entailing extracellular deposition of amyloid.
In the heritable form of TAC, patients typically present with heart failure symptoms at about age 50-55, while the wild type form becomes symptomatic much later at a mean age of about 75. Average survival from time of diagnosis is only about 3 years.
Recent studies from multiple centers have reported that the prevalence of TAC was 16% in patients undergoing transcatheter aortic valve replacement for severe aortic stenosis, 13% among patients with heart failure with preserved ejection fraction, and 5% in patients who had been presumed to have hypertrophic cardiomyopathy: So, not a rare condition.
“In our clinic, vast and surprising numbers of patients with unexplained nonischemic heart failure are scan positive [for TAC],” according to Dr. George.
Breakthroughs in diagnosis and staging
The echocardiographic red flag for TAC in a patient with heart failure symptoms is symmetric hypertrophy with a normal end-diastolic volume and thickened ventricles. The end-diastolic interventricular septal wall thickness is typically about 15 mm. The left ventricular ejection fraction is typically in the normal range, “but the clue is not the preservation of the ejection fraction, it’s the [normal] quality of the volume,” Dr. Rapezzi said.
A clinical clue suggestive of TAC upon physical examination, even in the absence of heart failure symptoms, is development of bilateral carpal tunnel syndrome in an older man. That’s because the same disease process that results in TAC can involve deposition of amyloid fibrils in peripheral nerves. Indeed, tafamidis is already approved in Europe and Japan under the trade name Vyndaqel as a treatment for familial amyloid polyneuropathy. For TAC, however, tafamidis remains investigational with fast-track status provided by both the Food and Drug Administration and the European Medicines Agency.
When TAC is suspected, it’s no longer necessary to subject patients to an onerous myocardial biopsy. Total body scintigraphy with bone tracers has been shown to be nearly as sensitive and specific as biopsy for the diagnosis.
Staging can now be done noninvasively as well. Investigators at the U.K. National Amyloidosis Centre recently reported that patients with TAC can be accurately staged using two biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP) and estimated glomerular filtration rate (eGFR). In their series of 869 patients with TAC, median survival for those with stage I disease as defined by their protocol was 69 months, compared with 47 months for stage II disease and 24 months for those with stage III disease. This simple U.K. staging system was then validated in a separate French cohort of TAC patients (Eur Heart J. 2018 Aug 7;39[30]:2799-806).
The ATTR-ACT trial
Dr. Rapezzi reported on 441 patients with TAC who were randomized to oral tafamidis at either 20 mg or 80 mg per day or placebo and followed prospectively for 30 months in the 13-country, double-blind, phase 3 trial. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a 30% relative risk reduction. The rate of cardiovascular hospitalizations was 0.48 per year with tafamidis, compared with 0.70 per year with placebo, for a 38% relative risk reduction. The mortality benefit didn’t achieve significance until 15-18 months into the trial, as to be expected given tafamidis’ mechanism of action, which involves binding to transthyretin, gradually stabilizing it, and curbing amyloid fibril deposition.
Of note, the benefit was similar regardless of the dose used and whether patients had hereditary or wild type TAC.
Tafamidis proved safe and well tolerated, with a side-effect profile similar to placebo. While diarrhea and urinary tract infections have been an issue in tafamidis-treated patients with familial amyloid polyneuropathy, these adverse events were actually less common in TAC patients who received tafamidis than with placebo, according to Dr. Rapezzi.
A key point, the cardiologist emphasized, is that the benefits of active treatment were greatest in patients with earlier-stage disease. Therefore it’s vital that the diagnosis of TAC be made early, with prompt initiation of treatment to follow, in order to catch the disease at a more reversible stage. That could mean there will be a whole lot more bone scintigraphy being done in patients with unexplained nonischemic heart failure.
Dr. Rapezzi reported receiving research grants, speaker honoraria, and consulting fees from Pfizer, which sponsored the ATTR-ACT trial. Simultaneous with his presentation in Munich, the study results were published online at NEJM.org (doi: 10.1056/NEJMoa1805689). Dr. George reported no financial conflicts.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Tafamidis is the first-ever proven disease-modifying therapy for patients with a rapidly progressive form of cardiomyopathy.
Major finding: .
Study details: This 13-country, randomized, phase 3, double-blind trial included 441 patients with transthyretin amyloid cardiomyopathy.
Disclosures: The presenter reported receiving research grants, speaker honoraria, and consultant fees from Pfizer, which sponsored the ATTR-ACT trial.
The aftermath of a mother’s suicide attempt
A suicide attempt can be devastating for family members. For a son or daughter, the fallout can include feelings of guilt at missing the warning signs and the knowledge that they may have contributed to the despair that might have driven their loved one to trying to end their own lives.
There also can be the feeling that the attempt to deliberately exit this life is an indication that those left behind are not valued.
As related in a StoryCorps episode, which described the attempted suicide of Linda Kwong and the toll on her daughter Emily, Linda’s longstanding suicidal depression was separate from her love for her family.
The news of the suicide attempt rocked Emily. “I described our family as a table, and you were the most important leg. So you disappearing just knocked the whole thing over,” she says.
Yet, the past had seen Emily distancing herself from Linda, with the reality of her mother’s ongoing darkness. “I thought if I spent too much time with you, I would become like you,” Emily says to Linda during their StoryCorps interview.
In the aftermath of Linda’s suicide attempt, the mother-daughter bond could have been shattered. Instead, in the intervening 5 years, it has been stripped down and rebuilt, with both individuals coming to a better understanding of one another and the pain in their lives.
“I mean,
“I can’t believe that you can use the word ‘proud,’ but it makes me feel like that bond between us will always be there,” Linda says. “And that means the world to me.”
Click here to listen to their StoryCorps episode, broadcast on NPR.
A suicide attempt can be devastating for family members. For a son or daughter, the fallout can include feelings of guilt at missing the warning signs and the knowledge that they may have contributed to the despair that might have driven their loved one to trying to end their own lives.
There also can be the feeling that the attempt to deliberately exit this life is an indication that those left behind are not valued.
As related in a StoryCorps episode, which described the attempted suicide of Linda Kwong and the toll on her daughter Emily, Linda’s longstanding suicidal depression was separate from her love for her family.
The news of the suicide attempt rocked Emily. “I described our family as a table, and you were the most important leg. So you disappearing just knocked the whole thing over,” she says.
Yet, the past had seen Emily distancing herself from Linda, with the reality of her mother’s ongoing darkness. “I thought if I spent too much time with you, I would become like you,” Emily says to Linda during their StoryCorps interview.
In the aftermath of Linda’s suicide attempt, the mother-daughter bond could have been shattered. Instead, in the intervening 5 years, it has been stripped down and rebuilt, with both individuals coming to a better understanding of one another and the pain in their lives.
“I mean,
“I can’t believe that you can use the word ‘proud,’ but it makes me feel like that bond between us will always be there,” Linda says. “And that means the world to me.”
Click here to listen to their StoryCorps episode, broadcast on NPR.
A suicide attempt can be devastating for family members. For a son or daughter, the fallout can include feelings of guilt at missing the warning signs and the knowledge that they may have contributed to the despair that might have driven their loved one to trying to end their own lives.
There also can be the feeling that the attempt to deliberately exit this life is an indication that those left behind are not valued.
As related in a StoryCorps episode, which described the attempted suicide of Linda Kwong and the toll on her daughter Emily, Linda’s longstanding suicidal depression was separate from her love for her family.
The news of the suicide attempt rocked Emily. “I described our family as a table, and you were the most important leg. So you disappearing just knocked the whole thing over,” she says.
Yet, the past had seen Emily distancing herself from Linda, with the reality of her mother’s ongoing darkness. “I thought if I spent too much time with you, I would become like you,” Emily says to Linda during their StoryCorps interview.
In the aftermath of Linda’s suicide attempt, the mother-daughter bond could have been shattered. Instead, in the intervening 5 years, it has been stripped down and rebuilt, with both individuals coming to a better understanding of one another and the pain in their lives.
“I mean,
“I can’t believe that you can use the word ‘proud,’ but it makes me feel like that bond between us will always be there,” Linda says. “And that means the world to me.”
Click here to listen to their StoryCorps episode, broadcast on NPR.
Product Update: PICO NPWT; Encision; TimerCap; AMA
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
SURGICAL SITE WOUND THERAPY
PICO NPWT is a negative-pressure wound therapy device to treat surgical site infection (SSI). According to Smith & Nephew, a new meta-analysis demonstrates that the prophylactic application of PICO with AIRLOCK™ Technology significantly reduces surgical site complications by 58%, the rate of dehiscence by 26%, and length of stay by one-half day when compared with standard care.
The PICO System is canister-free and disposable. Patients can be discharged safely with PICO in place. Seven days of therapy are provided in each kit, with 1 pump, 2 dressings, and fixation strips to allow for a dressing change.
PICO uses a 4-layer multifunction dressing design in which the layers work together to ensure that negative pressure is delivered to the wound bed and exudate is removed through absorption and evaporation. Approximately 20% of fluid still remains in the dressing. The top film layer has a high-moisture vapor transmission rate to transpire as much as 80% of the exudate, says Smith & Nephew.
FOR MORE INFORMATION, VISIT: http://www.smith-nephew.com/
SHIELDED LAPAROSCOPIC INSTRUMENTS PREVENT BURNS
Encision’s patented Active Electrode Monitoring (AEM®) Shielded Laparoscopic Instruments eliminate patient burns and the associated complications.
Every 90 minutes in the United States, a patient is severely injured from a stray energy burn during laparoscopic surgery, according to Encision. The AEM® Shielded Instruments are designed to eliminate burns caused by monopolar energy insulation failure and capacitive coupling, reducing complications and re-admissions.
In addition to helping health care professionals improve patient safety in line with a recent FDA safety communication, Active Electrode Monitoring is a recommended practice of AORN and AAGL.
Encision offers a complete line of premium laparoscopic monopolar surgical instruments with integrated AEM® technology as well as complimentary products to improve clinical effectiveness and patient safety, including bipolar and cold instrumentation.
FOR MORE INFORMATION, VISIT: https://www.encision.com/
iSORT: 7-DAY BLUETOOTH PILLBOX
TimerCap has a new Bluetooth-enabled 7-day pill box called the iSort that sends reminders to take medication to a patient’s phone using a free TimerCap App found at the AppStore and Android Market.
The iSort automatically records and stores the times when each door/slot is opened and closed. It knows which door has been used and seamlessly updates the TimerCap App. The app will notify the patient and, if designated, a caregiver, whenever a dose is due or missed using pictures to show what and how many meds are scheduled. More than one iSort box can be used with the app.
iSort provides reminders that help improve adherence to medication dosing instructions and eliminates annoying false alarms, double entries, and unnecessary reminders when pills already have been taken. The portable iSort uses 2 AA batteries that need to be changed about once per year.
FOR MORE INFORMATION, VISIT: https://www.timercap.com/isort
PLATFORM TO COORDINATE HEALTH AND TECHNOLOGY
The American Medical Association (AMA) recently has established a new initiative that introduces a solution to improve, organize, and share health care information. The Integrated Health Model Initiative (IHMI) is a platform that coordinates the health and technology sectors around a common data model. IHMI fills the national imperative to pioneer a shared framework for organizing health data, emphasizing patient-centric information, and refining data elements to those most predictive of better outcomes. The AMA says that evolving available health data to depict a complete picture of a patient’s journey from wellness to illness to treatment and beyond allows health care delivery to fully focus on patient outcomes, goals, and wellness. Participation in IHMI is open to all health care and technology stakeholders.
FOR MORE INFORMATION, VISIT: www.ama-assn.org/ihmi
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Over past 20 years, the percentage of children with ADHD nearly doubles
The number of children diagnosed with ADHD has reached more than 10%, a significant increase during the past 20 years, according to a recent study in JAMA.
The rise was most pronounced in minority groups, suggesting that better access to health insurance and mental health treatment through the Affordable Care Act might have played some role in the increase. The rate of diagnosis during that time period doubled in girls, although it was still much lower than in boys.
But the researchers say they found no evidence confirming frequent complaints that the condition is overdiagnosed or misdiagnosed.
The United States has significantly more instances of ADHD than do other developed countries, which researchers said has led some to think Americans are overdiagnosing children. Wei Bao, MD, PhD, lead author of the study, said in an interview that a review of studies around the world does not support that.
”I don’t think overdiagnosis is the main issue,” he said.
Nonetheless, those doubts persist. Stephen Hinshaw, MD, who coauthored a 2014 book called “The ADHD Explosion: Myths, Medication, Money, and Today’s Push for Performance,” compared ADHD with depression. He said in an interview that neither condition has unequivocal biological markers, which makes it hard to determine if a patient truly has the condition without lengthy psychological evaluations. Symptoms of ADHD can include inattention, fidgety behavior, and impulsivity.
“It’s probably not a true epidemic of ADHD,” said Dr. Hinshaw, a professor of psychology at the University of California, Berkeley, and a professor of psychiatry at University of California, San Francisco. “It might be an epidemic of diagnosing it.”
In interpreting their results, however, the study’s authors tied the higher numbers to better understanding of the condition by doctors and the public, new standards for diagnosis and an increase in access to health insurance through the ACA.
Because of the ACA, “some low-income families have improved access to services and referrals,” said Dr. Bao, an epidemiologist at the University of Iowa College of Public Health in Iowa City.
The study, published in JAMA Network Open, used data from the National Health Interview Survey, an annual federal survey of about 35,000 households. It found a steady increase in diagnoses in children from about 6% during 1997-1998 to more than 10% during 2015-2016.
Advances in medical technology also may have contributed to the increase, according to the research. Twenty years ago, preterm or low-birth-weight babies had a harder time surviving. Those factors increase the risk of being diagnosed with ADHD.
The study also suggests that fewer stigmas about mental health care in minority communities also may lead to more people receiving an ADHD diagnosis.
In the late 1990s, 7.2% of non-Hispanic white children, 4.7% of non-Hispanic black children, and 3.6% of Hispanic children were diagnosed with ADHD, according to the study. By 2016, it was 12% of white kids, 12.8% of blacks, and 6.1% of Hispanics.
Over the past several decades, Dr. Hinshaw said, there’s been an expanded view of who can develop ADHD. It’s no longer viewed as a disease that affects only white middle-class boys, as eating disorders are no longer seen as afflicting only white middle-class girls.
Still, he cautioned against overdiagnosing ADHD in communities in which behavioral issues could be the result of social or environmental factors such as overcrowded classrooms.
The study found rates of ADHD among girls rose from 3% to more than 6% over the study period. It said that was partly a result of a change in how the condition is classified. For years, ADHD pertained to children who were hyperactive. But in recent years, the American Psychiatric Association added to its guide of mental health conditions that diagnosis should also include some children who are inattentive, Dr. Bao said. That raised the number of girls, he explained, because it seems they are more likely to be in that second subtype.
“If we compare these two, you can easily imagine people will easily recognize hyperactivity,” he said.
That rang true for Ruth Hay, a 25-year-old student and cook from New York who now lives in Jerusalem. She was diagnosed with what was then called ADD the summer between second and third grade.
Ms. Hay said her hyperactive tendencies aren’t as “loud” as some people’s. She’s less likely to bounce around a room than she is to bounce in her chair, she said.
Yet, despite her early diagnosis, Ms. Hay said, no one ever told her about other symptoms. For example, she said, she suffers from executive dysfunction, which leaves her feeling unable to accomplish tasks, no matter how much she wanted to or tried.
“I grew up being called lazy in periods of time when I wasn’t,” Ms. Hay said. “If you look at a list of all the various ADHD symptoms, I have all of them to one degree or another, but the only ones ever discussed with me was you might be less focused and more fidgety.”
“I don’t know how my brain would be if I didn’t have it,” she added. “I don’t know if I’d still be me, but all it has been for me is a disability.”
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The number of children diagnosed with ADHD has reached more than 10%, a significant increase during the past 20 years, according to a recent study in JAMA.
The rise was most pronounced in minority groups, suggesting that better access to health insurance and mental health treatment through the Affordable Care Act might have played some role in the increase. The rate of diagnosis during that time period doubled in girls, although it was still much lower than in boys.
But the researchers say they found no evidence confirming frequent complaints that the condition is overdiagnosed or misdiagnosed.
The United States has significantly more instances of ADHD than do other developed countries, which researchers said has led some to think Americans are overdiagnosing children. Wei Bao, MD, PhD, lead author of the study, said in an interview that a review of studies around the world does not support that.
”I don’t think overdiagnosis is the main issue,” he said.
Nonetheless, those doubts persist. Stephen Hinshaw, MD, who coauthored a 2014 book called “The ADHD Explosion: Myths, Medication, Money, and Today’s Push for Performance,” compared ADHD with depression. He said in an interview that neither condition has unequivocal biological markers, which makes it hard to determine if a patient truly has the condition without lengthy psychological evaluations. Symptoms of ADHD can include inattention, fidgety behavior, and impulsivity.
“It’s probably not a true epidemic of ADHD,” said Dr. Hinshaw, a professor of psychology at the University of California, Berkeley, and a professor of psychiatry at University of California, San Francisco. “It might be an epidemic of diagnosing it.”
In interpreting their results, however, the study’s authors tied the higher numbers to better understanding of the condition by doctors and the public, new standards for diagnosis and an increase in access to health insurance through the ACA.
Because of the ACA, “some low-income families have improved access to services and referrals,” said Dr. Bao, an epidemiologist at the University of Iowa College of Public Health in Iowa City.
The study, published in JAMA Network Open, used data from the National Health Interview Survey, an annual federal survey of about 35,000 households. It found a steady increase in diagnoses in children from about 6% during 1997-1998 to more than 10% during 2015-2016.
Advances in medical technology also may have contributed to the increase, according to the research. Twenty years ago, preterm or low-birth-weight babies had a harder time surviving. Those factors increase the risk of being diagnosed with ADHD.
The study also suggests that fewer stigmas about mental health care in minority communities also may lead to more people receiving an ADHD diagnosis.
In the late 1990s, 7.2% of non-Hispanic white children, 4.7% of non-Hispanic black children, and 3.6% of Hispanic children were diagnosed with ADHD, according to the study. By 2016, it was 12% of white kids, 12.8% of blacks, and 6.1% of Hispanics.
Over the past several decades, Dr. Hinshaw said, there’s been an expanded view of who can develop ADHD. It’s no longer viewed as a disease that affects only white middle-class boys, as eating disorders are no longer seen as afflicting only white middle-class girls.
Still, he cautioned against overdiagnosing ADHD in communities in which behavioral issues could be the result of social or environmental factors such as overcrowded classrooms.
The study found rates of ADHD among girls rose from 3% to more than 6% over the study period. It said that was partly a result of a change in how the condition is classified. For years, ADHD pertained to children who were hyperactive. But in recent years, the American Psychiatric Association added to its guide of mental health conditions that diagnosis should also include some children who are inattentive, Dr. Bao said. That raised the number of girls, he explained, because it seems they are more likely to be in that second subtype.
“If we compare these two, you can easily imagine people will easily recognize hyperactivity,” he said.
That rang true for Ruth Hay, a 25-year-old student and cook from New York who now lives in Jerusalem. She was diagnosed with what was then called ADD the summer between second and third grade.
Ms. Hay said her hyperactive tendencies aren’t as “loud” as some people’s. She’s less likely to bounce around a room than she is to bounce in her chair, she said.
Yet, despite her early diagnosis, Ms. Hay said, no one ever told her about other symptoms. For example, she said, she suffers from executive dysfunction, which leaves her feeling unable to accomplish tasks, no matter how much she wanted to or tried.
“I grew up being called lazy in periods of time when I wasn’t,” Ms. Hay said. “If you look at a list of all the various ADHD symptoms, I have all of them to one degree or another, but the only ones ever discussed with me was you might be less focused and more fidgety.”
“I don’t know how my brain would be if I didn’t have it,” she added. “I don’t know if I’d still be me, but all it has been for me is a disability.”
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The number of children diagnosed with ADHD has reached more than 10%, a significant increase during the past 20 years, according to a recent study in JAMA.
The rise was most pronounced in minority groups, suggesting that better access to health insurance and mental health treatment through the Affordable Care Act might have played some role in the increase. The rate of diagnosis during that time period doubled in girls, although it was still much lower than in boys.
But the researchers say they found no evidence confirming frequent complaints that the condition is overdiagnosed or misdiagnosed.
The United States has significantly more instances of ADHD than do other developed countries, which researchers said has led some to think Americans are overdiagnosing children. Wei Bao, MD, PhD, lead author of the study, said in an interview that a review of studies around the world does not support that.
”I don’t think overdiagnosis is the main issue,” he said.
Nonetheless, those doubts persist. Stephen Hinshaw, MD, who coauthored a 2014 book called “The ADHD Explosion: Myths, Medication, Money, and Today’s Push for Performance,” compared ADHD with depression. He said in an interview that neither condition has unequivocal biological markers, which makes it hard to determine if a patient truly has the condition without lengthy psychological evaluations. Symptoms of ADHD can include inattention, fidgety behavior, and impulsivity.
“It’s probably not a true epidemic of ADHD,” said Dr. Hinshaw, a professor of psychology at the University of California, Berkeley, and a professor of psychiatry at University of California, San Francisco. “It might be an epidemic of diagnosing it.”
In interpreting their results, however, the study’s authors tied the higher numbers to better understanding of the condition by doctors and the public, new standards for diagnosis and an increase in access to health insurance through the ACA.
Because of the ACA, “some low-income families have improved access to services and referrals,” said Dr. Bao, an epidemiologist at the University of Iowa College of Public Health in Iowa City.
The study, published in JAMA Network Open, used data from the National Health Interview Survey, an annual federal survey of about 35,000 households. It found a steady increase in diagnoses in children from about 6% during 1997-1998 to more than 10% during 2015-2016.
Advances in medical technology also may have contributed to the increase, according to the research. Twenty years ago, preterm or low-birth-weight babies had a harder time surviving. Those factors increase the risk of being diagnosed with ADHD.
The study also suggests that fewer stigmas about mental health care in minority communities also may lead to more people receiving an ADHD diagnosis.
In the late 1990s, 7.2% of non-Hispanic white children, 4.7% of non-Hispanic black children, and 3.6% of Hispanic children were diagnosed with ADHD, according to the study. By 2016, it was 12% of white kids, 12.8% of blacks, and 6.1% of Hispanics.
Over the past several decades, Dr. Hinshaw said, there’s been an expanded view of who can develop ADHD. It’s no longer viewed as a disease that affects only white middle-class boys, as eating disorders are no longer seen as afflicting only white middle-class girls.
Still, he cautioned against overdiagnosing ADHD in communities in which behavioral issues could be the result of social or environmental factors such as overcrowded classrooms.
The study found rates of ADHD among girls rose from 3% to more than 6% over the study period. It said that was partly a result of a change in how the condition is classified. For years, ADHD pertained to children who were hyperactive. But in recent years, the American Psychiatric Association added to its guide of mental health conditions that diagnosis should also include some children who are inattentive, Dr. Bao said. That raised the number of girls, he explained, because it seems they are more likely to be in that second subtype.
“If we compare these two, you can easily imagine people will easily recognize hyperactivity,” he said.
That rang true for Ruth Hay, a 25-year-old student and cook from New York who now lives in Jerusalem. She was diagnosed with what was then called ADD the summer between second and third grade.
Ms. Hay said her hyperactive tendencies aren’t as “loud” as some people’s. She’s less likely to bounce around a room than she is to bounce in her chair, she said.
Yet, despite her early diagnosis, Ms. Hay said, no one ever told her about other symptoms. For example, she said, she suffers from executive dysfunction, which leaves her feeling unable to accomplish tasks, no matter how much she wanted to or tried.
“I grew up being called lazy in periods of time when I wasn’t,” Ms. Hay said. “If you look at a list of all the various ADHD symptoms, I have all of them to one degree or another, but the only ones ever discussed with me was you might be less focused and more fidgety.”
“I don’t know how my brain would be if I didn’t have it,” she added. “I don’t know if I’d still be me, but all it has been for me is a disability.”
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
E-cigarette use highest among adults aged under 35 years
Almost 11 million adults use e-cigarettes in the United States, and the majority are under the age of 35 years, according to the American Heart Association.
As of 2016, an estimated 4.5% of adults – more than 10.8 million individuals – used e-cigarettes every day or some days, which defined current use for the 466,842 people who responded to the Behavioral Risk Factor Surveillance System survey and were included in the study conducted by the AHA’s Tobacco Regulation and Addiction Center and published in the Annals of Internal Medicine.
Based on that survey data, an estimated 51% of current users were under the age of 35 years in 2016. Daily use was highest among those aged 18-24 years, and of those respondents, 44% said that they had never been regular cigarette users. “It’s particularly disturbing to see these younger people who have never been regular cigarette smokers taking up the use of e-cigarettes, perhaps with the assumption that this alternative nicotine delivery system has been proven to be safe,” said Rose Marie Robertson, MD, who is the AHA’s chief science and medical officer.
The analysis also showed that about 60% of e-cigarette users were men and that use was higher among LGBT people. The first-ever estimates of current use by state put the prevalence highest in Oklahoma at 7.0% and lowest in South Dakota (3.1%) and the District of Columbia (2.3%), the AHA said.
The study was funded through a grant from the National Institutes of Health and the Food and Drug Administration’s Center for Tobacco Products.
[email protected]
SOURCE: Mirbolouk M et al. Ann Intern Med. 2018 Aug 28. doi: 10.7326/M17-3440.
Almost 11 million adults use e-cigarettes in the United States, and the majority are under the age of 35 years, according to the American Heart Association.

As of 2016, an estimated 4.5% of adults – more than 10.8 million individuals – used e-cigarettes every day or some days, which defined current use for the 466,842 people who responded to the Behavioral Risk Factor Surveillance System survey and were included in the study conducted by the AHA’s Tobacco Regulation and Addiction Center and published in the Annals of Internal Medicine.
Based on that survey data, an estimated 51% of current users were under the age of 35 years in 2016. Daily use was highest among those aged 18-24 years, and of those respondents, 44% said that they had never been regular cigarette users. “It’s particularly disturbing to see these younger people who have never been regular cigarette smokers taking up the use of e-cigarettes, perhaps with the assumption that this alternative nicotine delivery system has been proven to be safe,” said Rose Marie Robertson, MD, who is the AHA’s chief science and medical officer.
The analysis also showed that about 60% of e-cigarette users were men and that use was higher among LGBT people. The first-ever estimates of current use by state put the prevalence highest in Oklahoma at 7.0% and lowest in South Dakota (3.1%) and the District of Columbia (2.3%), the AHA said.
The study was funded through a grant from the National Institutes of Health and the Food and Drug Administration’s Center for Tobacco Products.
[email protected]
SOURCE: Mirbolouk M et al. Ann Intern Med. 2018 Aug 28. doi: 10.7326/M17-3440.
Almost 11 million adults use e-cigarettes in the United States, and the majority are under the age of 35 years, according to the American Heart Association.

As of 2016, an estimated 4.5% of adults – more than 10.8 million individuals – used e-cigarettes every day or some days, which defined current use for the 466,842 people who responded to the Behavioral Risk Factor Surveillance System survey and were included in the study conducted by the AHA’s Tobacco Regulation and Addiction Center and published in the Annals of Internal Medicine.
Based on that survey data, an estimated 51% of current users were under the age of 35 years in 2016. Daily use was highest among those aged 18-24 years, and of those respondents, 44% said that they had never been regular cigarette users. “It’s particularly disturbing to see these younger people who have never been regular cigarette smokers taking up the use of e-cigarettes, perhaps with the assumption that this alternative nicotine delivery system has been proven to be safe,” said Rose Marie Robertson, MD, who is the AHA’s chief science and medical officer.
The analysis also showed that about 60% of e-cigarette users were men and that use was higher among LGBT people. The first-ever estimates of current use by state put the prevalence highest in Oklahoma at 7.0% and lowest in South Dakota (3.1%) and the District of Columbia (2.3%), the AHA said.
The study was funded through a grant from the National Institutes of Health and the Food and Drug Administration’s Center for Tobacco Products.
[email protected]
SOURCE: Mirbolouk M et al. Ann Intern Med. 2018 Aug 28. doi: 10.7326/M17-3440.
FROM ANNALS OF INTERNAL MEDICINE











