User login
Endocrine Society raises concerns about FDA’s “safe” classification of bisphenol A in food containers
An initial report from the Endocrine Society has raised concerns about bisphenol A use in products such as food and drink containers, toys, and medical devices, citing recent data that show the synthetic compound is linked to reproductive, behavioral, and metabolic disorders.
Although the Food and Drug Administration classifies bisphenol A (BPA) as safe to use in food containers, there have been hundreds of studies tying BPA to health problems such as “neurological outcomes, abnormal metabolism, reproductive effects as well as growth and development effects,” according to Laura N. Vandenberg, PhD, an Endocrine Society spokesperson, who spoke at a press briefing held on Oct. 23. Dr. Vandenberg explained the FDA’s 2014 position on BPA safety comes from a small subset of publicly available data, but these are not all the data on BPA, as some academic data are still under review.
The Endocrine Society recently held the news conference because they are concerned the FDA has “jumped the gun” before all the research has been published. “Even considering the fact that the data that have been presented by FDA have been interpreted by FDA as suggesting that BPA is safe, scientists still disagree,” Dr. Vandenberg said.
However, the Endocrine Society noted there is an issue with the current literature, which can be used to interpret and report different results. Heather Patisaul, PhD, cited a joint report from the Food and Agriculture Organization of the United Nations and World Health Organization, as well as a report from the National Toxicology Program (NTP), to illustrate this problem. Both reports expressed concern about BPA safety but took different approaches and a wider viewpoint, and came to different conclusions, she said.
“These two documents both concluded that there was some concern about bisphenol A and behavior, but they identified there was a big problem with trying to pool all this literature together because the experimental protocols were different, the animals were different, the dosing was different,” she said. “It was not a very harmonious literature.”
To combat this issue, the National Institute of Environmental Health Sciences and the FDA have funded the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) study. Dr. Patisaul said CLARITY-BPA is “the most ambitious project that’s ever been done” to study the health effects of a chemical, bringing together scientists from academic institutions, the NTP, and the FDA to help create data for risk assessment.
“The goal was to create this culture of partnership and communication between the agencies that have to make these decisions about safety and the scientists who are producing the data that’s trying to inform those assessments,” Dr. Patisaul said.
Dr. Vandenberg and Dr. Patisaul presented results from the CLARITY-BPA Core Study, which studied the effects of continuous doses of BPA in rats starting from 6 days of pregnancy; after birth, the rat offspring were fed doses of BPA for 1 year and 2 years. A second group of rats in a stop-dose group were fed BPA from early development, where the mothers were fed BPA at day 6 of pregnancy and the offspring fed BPA from birth until puberty (21 days) and followed for 1 year or 2 years. The researchers also examined 2.5 mcg/kg, 25 mcg/kg, 250 mcg/kg, 2,500 mcg/kg, and 25,000 mcg/kg doses of BPA exposure as well as continuous ethinyl estradiol exposure as a positive control.
In the FDA Core Study, there was a significantly increased incidence of mammary adenocarcinoma in the stop dose group and inflammation of the dorsal and lateral lobes of the prostate in the continuous dose group at a dose of 2.5 mcg/kg. In addition, kidney nephropathy and increased body weight in female rats in the continuous group were also seen at the 2.5 mcg/kg dose, Dr. Vandenberg noted.
“I think one of the reasons why FDA is dismissing those low-dose effects is that there’s an expectation with increasing dose, there should be an increase in an effect,” Dr. Vandenberg said.
In the low-dose range, BPA could be acting as a hormone such as estrogen, but also could be acting through other hormone receptors or as a toxicant at the high-dose range, she explained.
Dr. Patisaul also presented results of BPA-related effects on the brain and behavior in the existing literature from the TEDX Low-dose Bisphenol A project, which is a comparison of 391 in vivo and in vitro studies of BPA prior to 2009. The results showed brain and behavior was “heavily impacted” by BPA, as were organ systems such as the heart, which supports the results from the CLARITY-BPA study, Dr. Patisaul noted.
“When you think about reproducibility in the broadest sense, and you look at the effects that the FDA found at low dose, you look at the effects the CLARITY investigators found at low dose, and you go back and look at the existing literature, you see a very clear picture of BPA-produced effects on brain and behavior, female reproductive systems, and the cardiovascular system,” she said.
Dr. Patisaul is a study investigator for CLARITY-BPA.
An initial report from the Endocrine Society has raised concerns about bisphenol A use in products such as food and drink containers, toys, and medical devices, citing recent data that show the synthetic compound is linked to reproductive, behavioral, and metabolic disorders.
Although the Food and Drug Administration classifies bisphenol A (BPA) as safe to use in food containers, there have been hundreds of studies tying BPA to health problems such as “neurological outcomes, abnormal metabolism, reproductive effects as well as growth and development effects,” according to Laura N. Vandenberg, PhD, an Endocrine Society spokesperson, who spoke at a press briefing held on Oct. 23. Dr. Vandenberg explained the FDA’s 2014 position on BPA safety comes from a small subset of publicly available data, but these are not all the data on BPA, as some academic data are still under review.
The Endocrine Society recently held the news conference because they are concerned the FDA has “jumped the gun” before all the research has been published. “Even considering the fact that the data that have been presented by FDA have been interpreted by FDA as suggesting that BPA is safe, scientists still disagree,” Dr. Vandenberg said.
However, the Endocrine Society noted there is an issue with the current literature, which can be used to interpret and report different results. Heather Patisaul, PhD, cited a joint report from the Food and Agriculture Organization of the United Nations and World Health Organization, as well as a report from the National Toxicology Program (NTP), to illustrate this problem. Both reports expressed concern about BPA safety but took different approaches and a wider viewpoint, and came to different conclusions, she said.
“These two documents both concluded that there was some concern about bisphenol A and behavior, but they identified there was a big problem with trying to pool all this literature together because the experimental protocols were different, the animals were different, the dosing was different,” she said. “It was not a very harmonious literature.”
To combat this issue, the National Institute of Environmental Health Sciences and the FDA have funded the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) study. Dr. Patisaul said CLARITY-BPA is “the most ambitious project that’s ever been done” to study the health effects of a chemical, bringing together scientists from academic institutions, the NTP, and the FDA to help create data for risk assessment.
“The goal was to create this culture of partnership and communication between the agencies that have to make these decisions about safety and the scientists who are producing the data that’s trying to inform those assessments,” Dr. Patisaul said.
Dr. Vandenberg and Dr. Patisaul presented results from the CLARITY-BPA Core Study, which studied the effects of continuous doses of BPA in rats starting from 6 days of pregnancy; after birth, the rat offspring were fed doses of BPA for 1 year and 2 years. A second group of rats in a stop-dose group were fed BPA from early development, where the mothers were fed BPA at day 6 of pregnancy and the offspring fed BPA from birth until puberty (21 days) and followed for 1 year or 2 years. The researchers also examined 2.5 mcg/kg, 25 mcg/kg, 250 mcg/kg, 2,500 mcg/kg, and 25,000 mcg/kg doses of BPA exposure as well as continuous ethinyl estradiol exposure as a positive control.
In the FDA Core Study, there was a significantly increased incidence of mammary adenocarcinoma in the stop dose group and inflammation of the dorsal and lateral lobes of the prostate in the continuous dose group at a dose of 2.5 mcg/kg. In addition, kidney nephropathy and increased body weight in female rats in the continuous group were also seen at the 2.5 mcg/kg dose, Dr. Vandenberg noted.
“I think one of the reasons why FDA is dismissing those low-dose effects is that there’s an expectation with increasing dose, there should be an increase in an effect,” Dr. Vandenberg said.
In the low-dose range, BPA could be acting as a hormone such as estrogen, but also could be acting through other hormone receptors or as a toxicant at the high-dose range, she explained.
Dr. Patisaul also presented results of BPA-related effects on the brain and behavior in the existing literature from the TEDX Low-dose Bisphenol A project, which is a comparison of 391 in vivo and in vitro studies of BPA prior to 2009. The results showed brain and behavior was “heavily impacted” by BPA, as were organ systems such as the heart, which supports the results from the CLARITY-BPA study, Dr. Patisaul noted.
“When you think about reproducibility in the broadest sense, and you look at the effects that the FDA found at low dose, you look at the effects the CLARITY investigators found at low dose, and you go back and look at the existing literature, you see a very clear picture of BPA-produced effects on brain and behavior, female reproductive systems, and the cardiovascular system,” she said.
Dr. Patisaul is a study investigator for CLARITY-BPA.
An initial report from the Endocrine Society has raised concerns about bisphenol A use in products such as food and drink containers, toys, and medical devices, citing recent data that show the synthetic compound is linked to reproductive, behavioral, and metabolic disorders.
Although the Food and Drug Administration classifies bisphenol A (BPA) as safe to use in food containers, there have been hundreds of studies tying BPA to health problems such as “neurological outcomes, abnormal metabolism, reproductive effects as well as growth and development effects,” according to Laura N. Vandenberg, PhD, an Endocrine Society spokesperson, who spoke at a press briefing held on Oct. 23. Dr. Vandenberg explained the FDA’s 2014 position on BPA safety comes from a small subset of publicly available data, but these are not all the data on BPA, as some academic data are still under review.
The Endocrine Society recently held the news conference because they are concerned the FDA has “jumped the gun” before all the research has been published. “Even considering the fact that the data that have been presented by FDA have been interpreted by FDA as suggesting that BPA is safe, scientists still disagree,” Dr. Vandenberg said.
However, the Endocrine Society noted there is an issue with the current literature, which can be used to interpret and report different results. Heather Patisaul, PhD, cited a joint report from the Food and Agriculture Organization of the United Nations and World Health Organization, as well as a report from the National Toxicology Program (NTP), to illustrate this problem. Both reports expressed concern about BPA safety but took different approaches and a wider viewpoint, and came to different conclusions, she said.
“These two documents both concluded that there was some concern about bisphenol A and behavior, but they identified there was a big problem with trying to pool all this literature together because the experimental protocols were different, the animals were different, the dosing was different,” she said. “It was not a very harmonious literature.”
To combat this issue, the National Institute of Environmental Health Sciences and the FDA have funded the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) study. Dr. Patisaul said CLARITY-BPA is “the most ambitious project that’s ever been done” to study the health effects of a chemical, bringing together scientists from academic institutions, the NTP, and the FDA to help create data for risk assessment.
“The goal was to create this culture of partnership and communication between the agencies that have to make these decisions about safety and the scientists who are producing the data that’s trying to inform those assessments,” Dr. Patisaul said.
Dr. Vandenberg and Dr. Patisaul presented results from the CLARITY-BPA Core Study, which studied the effects of continuous doses of BPA in rats starting from 6 days of pregnancy; after birth, the rat offspring were fed doses of BPA for 1 year and 2 years. A second group of rats in a stop-dose group were fed BPA from early development, where the mothers were fed BPA at day 6 of pregnancy and the offspring fed BPA from birth until puberty (21 days) and followed for 1 year or 2 years. The researchers also examined 2.5 mcg/kg, 25 mcg/kg, 250 mcg/kg, 2,500 mcg/kg, and 25,000 mcg/kg doses of BPA exposure as well as continuous ethinyl estradiol exposure as a positive control.
In the FDA Core Study, there was a significantly increased incidence of mammary adenocarcinoma in the stop dose group and inflammation of the dorsal and lateral lobes of the prostate in the continuous dose group at a dose of 2.5 mcg/kg. In addition, kidney nephropathy and increased body weight in female rats in the continuous group were also seen at the 2.5 mcg/kg dose, Dr. Vandenberg noted.
“I think one of the reasons why FDA is dismissing those low-dose effects is that there’s an expectation with increasing dose, there should be an increase in an effect,” Dr. Vandenberg said.
In the low-dose range, BPA could be acting as a hormone such as estrogen, but also could be acting through other hormone receptors or as a toxicant at the high-dose range, she explained.
Dr. Patisaul also presented results of BPA-related effects on the brain and behavior in the existing literature from the TEDX Low-dose Bisphenol A project, which is a comparison of 391 in vivo and in vitro studies of BPA prior to 2009. The results showed brain and behavior was “heavily impacted” by BPA, as were organ systems such as the heart, which supports the results from the CLARITY-BPA study, Dr. Patisaul noted.
“When you think about reproducibility in the broadest sense, and you look at the effects that the FDA found at low dose, you look at the effects the CLARITY investigators found at low dose, and you go back and look at the existing literature, you see a very clear picture of BPA-produced effects on brain and behavior, female reproductive systems, and the cardiovascular system,” she said.
Dr. Patisaul is a study investigator for CLARITY-BPA.
Key clinical point: Despite claims from the Food and Drug Administration, results from the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) Core Study show serious effects in humans of bisphenol A at low doses.
Major finding: Research from CLARITY-BPA has shown brain and behavior, female reproduction, and organ systems such as the heart can be adversely affected by bisphenol A even at low doses.
Study details: An initial report from the CLARITY-BPA Core Study.
Disclosures: Dr. Patisaul is a study investigator for CLARITY-BPA.
Booming economy helps flatten Medicaid enrollment and limit costs, states report
Medicaid enrollment fell by 0.6% in 2018 – its first drop since 2007 – because of the strong economy and increased efforts in some states to verify eligibility, a new report finds.
But costs continue to go up. Total Medicaid spending rose 4.2% in 2018, same as a year ago, as a result of rising costs for drugs, long-term care, and mental health services, according to the study released Oct. 25 by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
States expect total Medicaid spending growth to accelerate modestly to 5.3% in 2019 as enrollment increases by about 1%, according to the annual survey of state Medicaid directors.
About 73 million people were enrolled in Medicaid in August, according to a federal report released Wednesday.
Medicaid, the state-federal health insurance program for low-income Americans, has seen its rolls soar in the past decade – initially as a result of massive job losses during the Great Recession and in recent years when dozens of states expanded eligibility using federal financing provided by the Affordable Care Act. Thirty-three states expanded their programs to cover people with incomes under 138% of the federal poverty level, or an income of about $16,750 for an individual in 2018.
Medicaid spending and enrollment typically rise during economic downturns as more people lose jobs and health benefits. When the economy is humming, Medicaid enrollment flattens as more people get back to work and can get coverage at work or can afford to buy it on their own. The national unemployment rate was 3.7% in September, the lowest since 1969.
The falling unemployment rate is the main reason for the drop in Medicaid enrollment, but some states have reduced their rolls by requiring adults and families to verify their eligibility. Arkansas, for example, has cut thousands of people after instituting new steps to confirm eligibility.
The brightening economic outlook for states has led many to increase benefits to enrollees and payment rates for health providers.
“A total of 19 states expanded or enhanced covered benefits in fiscal 2018 and 24 states plan to add or enhance benefits for the current fiscal year, which for most states started in July,” the Kaiser report said. “The most common benefit enhancements reported were for mental health and substance abuse services. A handful of states reported expansions related to dental services, telehealth, physical or occupational therapies and home visiting services for pregnant women.”
A dozen states increased pay to dentists and 18 states added to primary care doctors’ reimbursements for fiscal year 2019.
Medicaid covers about 20% of U.S. residents and accounts for nearly one-sixth of health care expenditures. Nearly half of enrollees are children.
Overall, the federal government pays about 62% of Medicaid costs with state’s picking up the rest. Poorer states get a higher federal match rate.
Seventeen Republican-controlled states have not expanded Medicaid. For individuals accepted into the program as part of the ACA expansion, the federal government paid the full cost of coverage from 2014 through 2016. It will pay no less than 90% thereafter.
In 2018, the states’ share of spending rose 4.9%. This was the first full year that states were responsible for part of the cost of the expansion. States expect their spending will grow about 3.5% in 2019.
Robin Rudowitz, one of the authors of the study and associate director of the Kaiser Commission on Medicaid and the Uninsured, said the survey found many states were using Medicaid to address the opioid crisis by expanding benefits for substance disorders and also by implementing tougher restrictions on prescriptions.
“Almost every governor wants to do something, and Medicaid is generally a large part of it,” she said.
While the Trump administration’s approval of work requirements for some adults on Medicaid has generated controversy over the past year, the report shows that states are making many other changes to the program, such as increasing benefits and changing how it pays providers to get better value.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Medicaid enrollment fell by 0.6% in 2018 – its first drop since 2007 – because of the strong economy and increased efforts in some states to verify eligibility, a new report finds.
But costs continue to go up. Total Medicaid spending rose 4.2% in 2018, same as a year ago, as a result of rising costs for drugs, long-term care, and mental health services, according to the study released Oct. 25 by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
States expect total Medicaid spending growth to accelerate modestly to 5.3% in 2019 as enrollment increases by about 1%, according to the annual survey of state Medicaid directors.
About 73 million people were enrolled in Medicaid in August, according to a federal report released Wednesday.
Medicaid, the state-federal health insurance program for low-income Americans, has seen its rolls soar in the past decade – initially as a result of massive job losses during the Great Recession and in recent years when dozens of states expanded eligibility using federal financing provided by the Affordable Care Act. Thirty-three states expanded their programs to cover people with incomes under 138% of the federal poverty level, or an income of about $16,750 for an individual in 2018.
Medicaid spending and enrollment typically rise during economic downturns as more people lose jobs and health benefits. When the economy is humming, Medicaid enrollment flattens as more people get back to work and can get coverage at work or can afford to buy it on their own. The national unemployment rate was 3.7% in September, the lowest since 1969.
The falling unemployment rate is the main reason for the drop in Medicaid enrollment, but some states have reduced their rolls by requiring adults and families to verify their eligibility. Arkansas, for example, has cut thousands of people after instituting new steps to confirm eligibility.
The brightening economic outlook for states has led many to increase benefits to enrollees and payment rates for health providers.
“A total of 19 states expanded or enhanced covered benefits in fiscal 2018 and 24 states plan to add or enhance benefits for the current fiscal year, which for most states started in July,” the Kaiser report said. “The most common benefit enhancements reported were for mental health and substance abuse services. A handful of states reported expansions related to dental services, telehealth, physical or occupational therapies and home visiting services for pregnant women.”
A dozen states increased pay to dentists and 18 states added to primary care doctors’ reimbursements for fiscal year 2019.
Medicaid covers about 20% of U.S. residents and accounts for nearly one-sixth of health care expenditures. Nearly half of enrollees are children.
Overall, the federal government pays about 62% of Medicaid costs with state’s picking up the rest. Poorer states get a higher federal match rate.
Seventeen Republican-controlled states have not expanded Medicaid. For individuals accepted into the program as part of the ACA expansion, the federal government paid the full cost of coverage from 2014 through 2016. It will pay no less than 90% thereafter.
In 2018, the states’ share of spending rose 4.9%. This was the first full year that states were responsible for part of the cost of the expansion. States expect their spending will grow about 3.5% in 2019.
Robin Rudowitz, one of the authors of the study and associate director of the Kaiser Commission on Medicaid and the Uninsured, said the survey found many states were using Medicaid to address the opioid crisis by expanding benefits for substance disorders and also by implementing tougher restrictions on prescriptions.
“Almost every governor wants to do something, and Medicaid is generally a large part of it,” she said.
While the Trump administration’s approval of work requirements for some adults on Medicaid has generated controversy over the past year, the report shows that states are making many other changes to the program, such as increasing benefits and changing how it pays providers to get better value.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Medicaid enrollment fell by 0.6% in 2018 – its first drop since 2007 – because of the strong economy and increased efforts in some states to verify eligibility, a new report finds.
But costs continue to go up. Total Medicaid spending rose 4.2% in 2018, same as a year ago, as a result of rising costs for drugs, long-term care, and mental health services, according to the study released Oct. 25 by the Kaiser Family Foundation. (Kaiser Health News is an editorially independent program of the foundation.)
States expect total Medicaid spending growth to accelerate modestly to 5.3% in 2019 as enrollment increases by about 1%, according to the annual survey of state Medicaid directors.
About 73 million people were enrolled in Medicaid in August, according to a federal report released Wednesday.
Medicaid, the state-federal health insurance program for low-income Americans, has seen its rolls soar in the past decade – initially as a result of massive job losses during the Great Recession and in recent years when dozens of states expanded eligibility using federal financing provided by the Affordable Care Act. Thirty-three states expanded their programs to cover people with incomes under 138% of the federal poverty level, or an income of about $16,750 for an individual in 2018.
Medicaid spending and enrollment typically rise during economic downturns as more people lose jobs and health benefits. When the economy is humming, Medicaid enrollment flattens as more people get back to work and can get coverage at work or can afford to buy it on their own. The national unemployment rate was 3.7% in September, the lowest since 1969.
The falling unemployment rate is the main reason for the drop in Medicaid enrollment, but some states have reduced their rolls by requiring adults and families to verify their eligibility. Arkansas, for example, has cut thousands of people after instituting new steps to confirm eligibility.
The brightening economic outlook for states has led many to increase benefits to enrollees and payment rates for health providers.
“A total of 19 states expanded or enhanced covered benefits in fiscal 2018 and 24 states plan to add or enhance benefits for the current fiscal year, which for most states started in July,” the Kaiser report said. “The most common benefit enhancements reported were for mental health and substance abuse services. A handful of states reported expansions related to dental services, telehealth, physical or occupational therapies and home visiting services for pregnant women.”
A dozen states increased pay to dentists and 18 states added to primary care doctors’ reimbursements for fiscal year 2019.
Medicaid covers about 20% of U.S. residents and accounts for nearly one-sixth of health care expenditures. Nearly half of enrollees are children.
Overall, the federal government pays about 62% of Medicaid costs with state’s picking up the rest. Poorer states get a higher federal match rate.
Seventeen Republican-controlled states have not expanded Medicaid. For individuals accepted into the program as part of the ACA expansion, the federal government paid the full cost of coverage from 2014 through 2016. It will pay no less than 90% thereafter.
In 2018, the states’ share of spending rose 4.9%. This was the first full year that states were responsible for part of the cost of the expansion. States expect their spending will grow about 3.5% in 2019.
Robin Rudowitz, one of the authors of the study and associate director of the Kaiser Commission on Medicaid and the Uninsured, said the survey found many states were using Medicaid to address the opioid crisis by expanding benefits for substance disorders and also by implementing tougher restrictions on prescriptions.
“Almost every governor wants to do something, and Medicaid is generally a large part of it,” she said.
While the Trump administration’s approval of work requirements for some adults on Medicaid has generated controversy over the past year, the report shows that states are making many other changes to the program, such as increasing benefits and changing how it pays providers to get better value.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
What infectious disease should parents be most worried about?
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
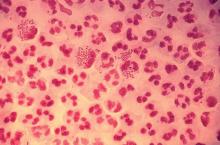
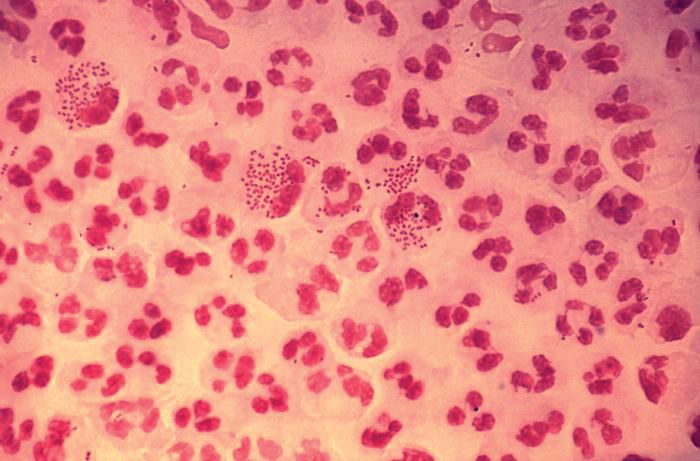
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
FDA clears Abbott’s Influenza A & B 2, Strep A 2 assays
The Food and Drug Administration has cleared Abbott Laboratories’ next-generation Influenza A & B 2 and Strep A 2 molecular assays for point-of-care testing.
The Influenza A & B 2 assay can detect and differentiate influenza A and B in 13 minutes, with a call-out of positive results at 5 minutes. It can be stored at room temperature, simplifying storage and ordering. The Strep A 2 assay detects group A streptococcus bacterial nucleic acid in 6 minutes, with a call-out of positive results at 2 minutes. Both will be the fastest tests currently on the market in their respective fields, according to a corporate press release.
The assays will be available in a variety of inpatient and outpatient settings, particularly in locations where patients commonly access health care services, such as EDs, physician offices, walk-in clinics, and urgent care centers. This will allow health care providers to make a fast, informed diagnosis and provide appropriate treatment within the span of a single patient visit.
“The ability to obtain early call outs for positive test results with molecular accuracy in as little as 5 minutes for influenza and 2 minutes for strep A is a game-changing development that allows prompt treatment decisions at the point of care. Rapid testing may also help reduce improper antibiotic usage, which can occur when treatment is based exclusively on a patient’s symptoms, and contributes to antibiotic resistance,” Gregory J. Berry, PhD, director of molecular diagnostics at Northwell Health Laboratories in Lake Success, N.Y., said in the press release.
Find the full press release on the Abbott Laboratories website.
The Food and Drug Administration has cleared Abbott Laboratories’ next-generation Influenza A & B 2 and Strep A 2 molecular assays for point-of-care testing.
The Influenza A & B 2 assay can detect and differentiate influenza A and B in 13 minutes, with a call-out of positive results at 5 minutes. It can be stored at room temperature, simplifying storage and ordering. The Strep A 2 assay detects group A streptococcus bacterial nucleic acid in 6 minutes, with a call-out of positive results at 2 minutes. Both will be the fastest tests currently on the market in their respective fields, according to a corporate press release.
The assays will be available in a variety of inpatient and outpatient settings, particularly in locations where patients commonly access health care services, such as EDs, physician offices, walk-in clinics, and urgent care centers. This will allow health care providers to make a fast, informed diagnosis and provide appropriate treatment within the span of a single patient visit.
“The ability to obtain early call outs for positive test results with molecular accuracy in as little as 5 minutes for influenza and 2 minutes for strep A is a game-changing development that allows prompt treatment decisions at the point of care. Rapid testing may also help reduce improper antibiotic usage, which can occur when treatment is based exclusively on a patient’s symptoms, and contributes to antibiotic resistance,” Gregory J. Berry, PhD, director of molecular diagnostics at Northwell Health Laboratories in Lake Success, N.Y., said in the press release.
Find the full press release on the Abbott Laboratories website.
The Food and Drug Administration has cleared Abbott Laboratories’ next-generation Influenza A & B 2 and Strep A 2 molecular assays for point-of-care testing.
The Influenza A & B 2 assay can detect and differentiate influenza A and B in 13 minutes, with a call-out of positive results at 5 minutes. It can be stored at room temperature, simplifying storage and ordering. The Strep A 2 assay detects group A streptococcus bacterial nucleic acid in 6 minutes, with a call-out of positive results at 2 minutes. Both will be the fastest tests currently on the market in their respective fields, according to a corporate press release.
The assays will be available in a variety of inpatient and outpatient settings, particularly in locations where patients commonly access health care services, such as EDs, physician offices, walk-in clinics, and urgent care centers. This will allow health care providers to make a fast, informed diagnosis and provide appropriate treatment within the span of a single patient visit.
“The ability to obtain early call outs for positive test results with molecular accuracy in as little as 5 minutes for influenza and 2 minutes for strep A is a game-changing development that allows prompt treatment decisions at the point of care. Rapid testing may also help reduce improper antibiotic usage, which can occur when treatment is based exclusively on a patient’s symptoms, and contributes to antibiotic resistance,” Gregory J. Berry, PhD, director of molecular diagnostics at Northwell Health Laboratories in Lake Success, N.Y., said in the press release.
Find the full press release on the Abbott Laboratories website.
ACIP votes unanimously in favor of immunization schedule update and redesign
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
AT AN ACIP MEETING
Please stay involved as leaders
By the time you receive this issue, we will know election results. The effects on medical care, medical coverage, Medicare, and Medicaid will be profound. American medicine is integrally linked to Congress and the Supreme Court because on July 30, 1965, Lyndon Johnson signed Title 18 of the Social Security Act and created Medicare – a move that took medical care out of personal law and into public law.
In November, CMS will publish its “final rule” about documentation and reimbursement changes, site of service reimbursement, and several other impactful policy changes. We have an extended article from the AGA Partners in Value conference about these potential changes.
This month we highlight the medical home concept for IBD – an idea that is gaining traction. More intense colon cancer screening may be needed for families with nonhereditary colon cancer. An interesting article from JAMA suggests that obesity may play a role in colon cancer rates in young women.
Antibiotic resistance in H. pylori infections is reaching alarming levels and this information may alter our practice. We feature an “In Focus” section on endoscopic treatment for obese patients. We also continue highlighting some popular and interesting discussion chains emanating from the AGA Community.
Please stay involved as leaders in health care economics, delivery, and politics. We need thoughtful discussions and we need to bring patient stories to our politicians. It often seems that our advocacy does little to alter the national debate but who better to speak for the people that entrust us with their care?
John I. Allen, MD, MBA, AGAF
Editor in Chief
By the time you receive this issue, we will know election results. The effects on medical care, medical coverage, Medicare, and Medicaid will be profound. American medicine is integrally linked to Congress and the Supreme Court because on July 30, 1965, Lyndon Johnson signed Title 18 of the Social Security Act and created Medicare – a move that took medical care out of personal law and into public law.
In November, CMS will publish its “final rule” about documentation and reimbursement changes, site of service reimbursement, and several other impactful policy changes. We have an extended article from the AGA Partners in Value conference about these potential changes.
This month we highlight the medical home concept for IBD – an idea that is gaining traction. More intense colon cancer screening may be needed for families with nonhereditary colon cancer. An interesting article from JAMA suggests that obesity may play a role in colon cancer rates in young women.
Antibiotic resistance in H. pylori infections is reaching alarming levels and this information may alter our practice. We feature an “In Focus” section on endoscopic treatment for obese patients. We also continue highlighting some popular and interesting discussion chains emanating from the AGA Community.
Please stay involved as leaders in health care economics, delivery, and politics. We need thoughtful discussions and we need to bring patient stories to our politicians. It often seems that our advocacy does little to alter the national debate but who better to speak for the people that entrust us with their care?
John I. Allen, MD, MBA, AGAF
Editor in Chief
By the time you receive this issue, we will know election results. The effects on medical care, medical coverage, Medicare, and Medicaid will be profound. American medicine is integrally linked to Congress and the Supreme Court because on July 30, 1965, Lyndon Johnson signed Title 18 of the Social Security Act and created Medicare – a move that took medical care out of personal law and into public law.
In November, CMS will publish its “final rule” about documentation and reimbursement changes, site of service reimbursement, and several other impactful policy changes. We have an extended article from the AGA Partners in Value conference about these potential changes.
This month we highlight the medical home concept for IBD – an idea that is gaining traction. More intense colon cancer screening may be needed for families with nonhereditary colon cancer. An interesting article from JAMA suggests that obesity may play a role in colon cancer rates in young women.
Antibiotic resistance in H. pylori infections is reaching alarming levels and this information may alter our practice. We feature an “In Focus” section on endoscopic treatment for obese patients. We also continue highlighting some popular and interesting discussion chains emanating from the AGA Community.
Please stay involved as leaders in health care economics, delivery, and politics. We need thoughtful discussions and we need to bring patient stories to our politicians. It often seems that our advocacy does little to alter the national debate but who better to speak for the people that entrust us with their care?
John I. Allen, MD, MBA, AGAF
Editor in Chief
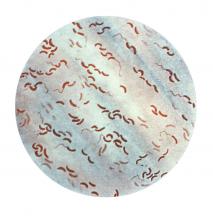
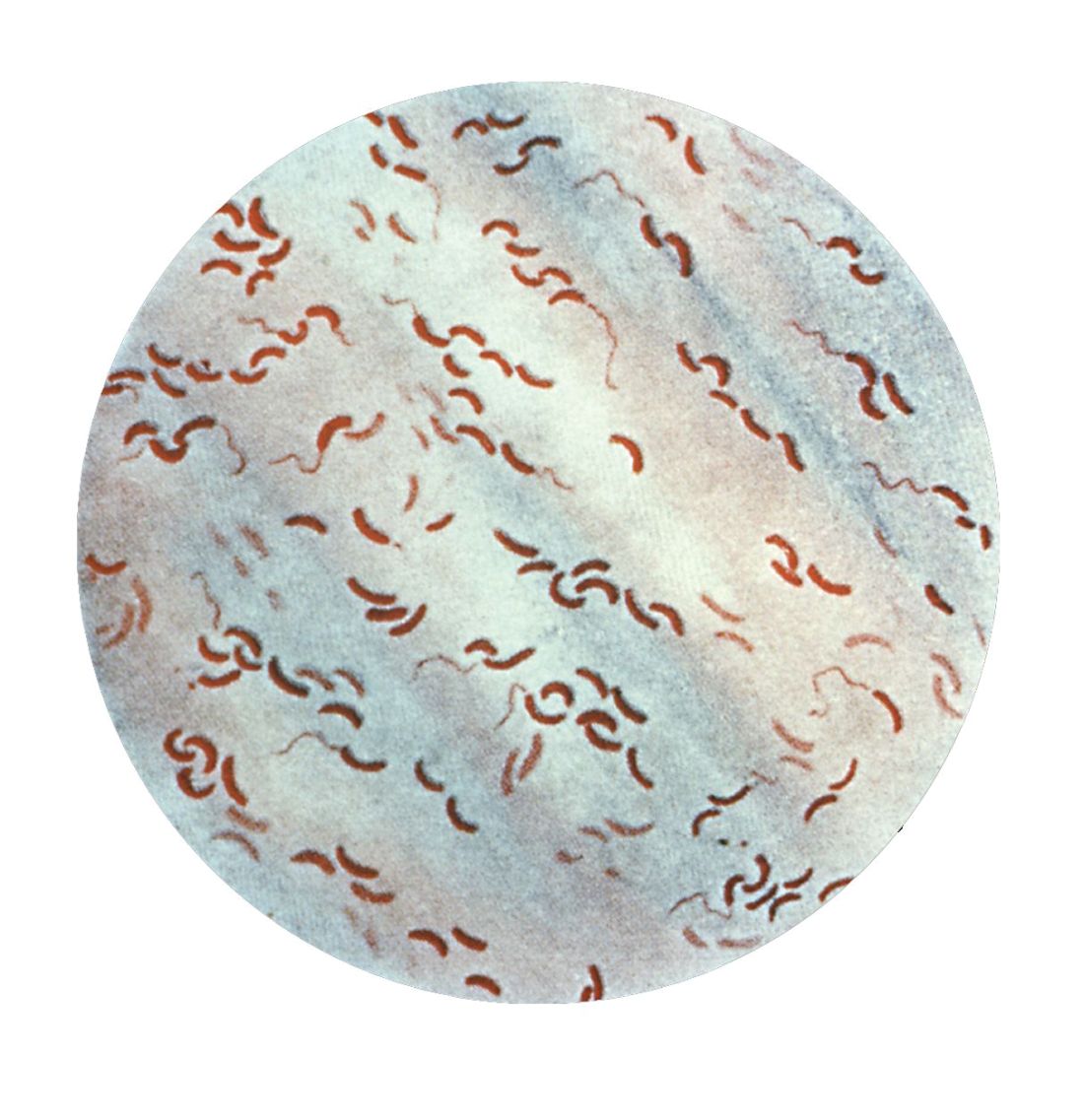
Cholera, bacteriophage in an epic evolutionary struggle
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
SAN FRANCISCO – A new analysis of cholera strains suggests that bacteriophages – viruses that prey on bacteria – are engaged in an evolutionary arms race with the Vibrio cholerae bacteria, and the dynamic between the two organisms may be an important factor in determining which strain of cholera goes on to cause a pandemic.
The work, presented by Kim Seed, PhD, at IDWeek, an annual scientific meeting on infectious diseases, examined a defense mechanism in V. cholerae, called phage inducible chromosomal island like element (PLE), as well as a unique mechanism in the bacteriophage to counter it. The work adds insight into the cholera strains that could emerge to produce future epidemics, and could even inform the use of bacteriophages as prophylactic agents to counter V. cholerae infection
In her talk, Dr. Seed described the dynamics of the current cholera pandemic, which is the seventh in recorded history and began in the 1960s. Over the past 100 years, six previous strains arose and then vanished, yielding each time to a new strain that became the predominant cholera-causing agent.
“This pattern of evolution, this so-called disappearing act, drives my research – I’m trying to understand what factors promote the evolution of novel genetic variants, and what factors contribute to why those variants disappear,” said Dr. Seed.
That quest brought her to the Bay of Bengal and Bangladesh. Genetic studies have shown this region to be the epicenter of cholera strains. It appears that cholera strains evolve there and then invade other regions of the world as a result of human travel and activity. Go to places in Africa or Asia where there is a cholera outbreak, and you can find cholera bacteria in the water that has the potential to cause human disease – but it won’t be the strain that is causing disease nearby. “(The culprit) is these introduced strains that come from Southeast Asia,” said Dr. Seed.
So her team went to Bangladesh, and studied cholera bacteria isolated from patients at the International Centre for Diarrhoeal Disease Research. The current strain is antibiotic resistant, as has been well documented. But Dr. Seed was interested in bacteriophages – viruses that prey on bacteria – because they live in the water supply and can also be isolated from the stool of cholera-infected patients, and it seemed likely that they could be an important selective force.
Indeed, her team found only a few bacteriophages that prey on V. cholerae in the samples from this hospital, and one type predominated in samples collected between 2001 and 2017; a bacteriophage known as ICP1. “This set up a very nice dynamic to be able to study the molecular mechanisms by which co-evolution was occurring in this one specific phage and Vibrio cholerae,” said Dr. Seed.
Genetic analysis revealed a mobile genetic element in V. cholerae – PLE –that conferred specific resistance against ICP1. After an infection, one of the bacteriophage’s proteins leads to excision and transcription of PLE. That produces a predicted 25 proteins, which in turn interfere with ICP1 through an as yet undetermined mechanism. But it’s effective, completely shutting down bacteriophage replication.
That couldn’t be the end of the story, Dr. Seed reasoned. Otherwise the bacteriophage would die out entirely for lack of a vulnerable host. More searching revealed the biggest surprise of all – ICP1, even though it is a virus, contains a complete suite of CRISPR (clustered regularly interspaced short palindromic repeats) apparatus that directly targets the PLE sequence. CRISPR is currently all the rage as a potential tool for genetic modification. It was discovered in bacteria, as a sort of immune response against bacteriophages. The CRISPR DNA contains a guide sequence that is complementary to and binds viral DNA, and then recruits other proteins to destroy the viral blueprint.
But here, for the first and only time, Dr. Seed’s team found that a bacteriophage had turned the tables, somehow capturing a CRISPR system of its own and turning it against its host’s defense system. Soon after infection, PLE switches on in response to its bacteriophage trigger, but the ICP1 counters by activating its CRISPR system, which is effective enough to allow the bacteriophage to reproduce.
The researchers then examined historical samples, and found another surprise: The appearance of CRISPR in ICP1 predated the appearance of the PLE variant that it targeted in V. cholerae. A little more digging revealed older variants of PLE, now gone from the V. cholerae population. “This explains why ICP1 had to have CRISPR, so it could overcome these previously prevalent genetic variants,” said Dr. Seed.
All told, the researchers found five unique PLE variants dating back to 1931, and the co-evolution of V. cholerae and ICP1 no doubt stretch much farther into the dim past. More recently, they found that previous strains of V. cholerae that went extinct also had different variations of PLE, suggesting that it may have been a temporary evolutionary victory by ICP1 over a PLE variant that caused the demise of an existing V. cholerae strain. But each time, it seems the bacteria responded with a new PLE variant, prolonging the arms race.
The work has the potential to affect other bacterial diseases, since most bacteria have phages that prey on them. “I have no doubt that they are a strong presence and selective force on all pathogens. People haven’t done so much work on that yet, but I think it’s coming,” said Dr. Seed.
SOURCE: Seed K. et al. ID Week 2018. Abstract 954.
REPORTING FROM IDWEEK 2018
Key clinical point: Bacteriophages place selective pressure on bacteria that may influence the emergence of new pathogenic strains.
Major finding: Bacteriophages turn on their own CRISPR system to target cholera defensive genes.
Study details: Genetic analysis of an antibiotic resistant strain and attacking bacteriophage strains.
Disclosures: The National Institutes of Health and the Chan Zuckerberg Biohub provided research funding.
Source: Seed K et al. ID Week 2018. Abstract 954.
Obesity, weight gain linked to CRC risk in younger women
Obesity and weight gain are linked to increased risk of colorectal cancer in younger women, according to an analysis of a large, prospective U.S. cohort study.
, authors of the study reported in JAMA Oncology.
The findings suggest body weight could be used to “personalize and complement” early cancer screening strategies among adults younger than 50 years, said investigator Po-Hong Liu, MD, of Washington University, St. Louis, and coauthors.
“Given that most of these younger cases are diagnosed symptomatically with more advanced tumors and with a significant influence on years of life lost, our findings reinforce the benefits of maintaining a healthy weight throughout life,” Dr. Liu and coinvestigators said in their report.
Their analysis was based on the ongoing Nurses Health Study II, which began in 1989 and enrolled a total of 116,430 women between the ages of 25 and 42 years in 14 U.S. states. Women completed questionnaires on demographics, medical and health information, and lifestyle factors every 2 years after enrollment.
Dr. Liu and colleagues were able to document 114 cases of colorectal cancer over a median of 13.9 years of follow-up in 85,256 women who had no cancer or inflammatory bowel disease when they were enrolled in the study. The median age at diagnosis for these cancers was 45 years.
Obesity was independently associated with increased risk of these early-onset colorectal cancers, the investigators found in multivariable analysis.
Women with a body mass index of 30 kg/m2 or higher had a relative risk of 1.93 (95% confidence interval, 1.15-3.25) versus women with normal BMIs in the 18.5 to 22.9-kg/m2 range, according to results of the analysis, they reported.
There was an apparent linear trend between increasing weight and increasing colorectal cancer risk, they added in their report.
They also found links between BMI in early adulthood and risk of early-onset colorectal cancer, including a relative risk of 1.63 for women who reported a BMI of 23 kg/m2 or higher at 18 years of age, versus women with a BMI of 18.5-20.9 kg/m2 at that age.
Similarly, increase in weight since early adulthood was associated with increased cancer risk, they reported.
While the link between excess weight and colorectal cancer incidence and mortality is well established in previous studies, this study is one of few reports looking at the association in younger individuals, according to Dr. Liu and colleagues.
This is thought to be the first prospective study looking at the link between obesity and risk of colo-rectal cancer diagnosed before the age of 50, they added.
The study was funded by grants from the National Institutes of Health. Dr. Liu had no conflict of interest disclosures related to the study. One coauthor reported consulting fees from Bayer Pharma AG, Janssen, and Pfizer.
SOURCE: Liu P-H et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4280.
AGA Resource
Visit the AGA GI Patient Center for education to share with your patients about obesity, colorectal cancer and other GI disorders at patient.gastro.org.
Obesity and weight gain are linked to increased risk of colorectal cancer in younger women, according to an analysis of a large, prospective U.S. cohort study.
, authors of the study reported in JAMA Oncology.
The findings suggest body weight could be used to “personalize and complement” early cancer screening strategies among adults younger than 50 years, said investigator Po-Hong Liu, MD, of Washington University, St. Louis, and coauthors.
“Given that most of these younger cases are diagnosed symptomatically with more advanced tumors and with a significant influence on years of life lost, our findings reinforce the benefits of maintaining a healthy weight throughout life,” Dr. Liu and coinvestigators said in their report.
Their analysis was based on the ongoing Nurses Health Study II, which began in 1989 and enrolled a total of 116,430 women between the ages of 25 and 42 years in 14 U.S. states. Women completed questionnaires on demographics, medical and health information, and lifestyle factors every 2 years after enrollment.
Dr. Liu and colleagues were able to document 114 cases of colorectal cancer over a median of 13.9 years of follow-up in 85,256 women who had no cancer or inflammatory bowel disease when they were enrolled in the study. The median age at diagnosis for these cancers was 45 years.
Obesity was independently associated with increased risk of these early-onset colorectal cancers, the investigators found in multivariable analysis.
Women with a body mass index of 30 kg/m2 or higher had a relative risk of 1.93 (95% confidence interval, 1.15-3.25) versus women with normal BMIs in the 18.5 to 22.9-kg/m2 range, according to results of the analysis, they reported.
There was an apparent linear trend between increasing weight and increasing colorectal cancer risk, they added in their report.
They also found links between BMI in early adulthood and risk of early-onset colorectal cancer, including a relative risk of 1.63 for women who reported a BMI of 23 kg/m2 or higher at 18 years of age, versus women with a BMI of 18.5-20.9 kg/m2 at that age.
Similarly, increase in weight since early adulthood was associated with increased cancer risk, they reported.
While the link between excess weight and colorectal cancer incidence and mortality is well established in previous studies, this study is one of few reports looking at the association in younger individuals, according to Dr. Liu and colleagues.
This is thought to be the first prospective study looking at the link between obesity and risk of colo-rectal cancer diagnosed before the age of 50, they added.
The study was funded by grants from the National Institutes of Health. Dr. Liu had no conflict of interest disclosures related to the study. One coauthor reported consulting fees from Bayer Pharma AG, Janssen, and Pfizer.
SOURCE: Liu P-H et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4280.
AGA Resource
Visit the AGA GI Patient Center for education to share with your patients about obesity, colorectal cancer and other GI disorders at patient.gastro.org.
Obesity and weight gain are linked to increased risk of colorectal cancer in younger women, according to an analysis of a large, prospective U.S. cohort study.
, authors of the study reported in JAMA Oncology.
The findings suggest body weight could be used to “personalize and complement” early cancer screening strategies among adults younger than 50 years, said investigator Po-Hong Liu, MD, of Washington University, St. Louis, and coauthors.
“Given that most of these younger cases are diagnosed symptomatically with more advanced tumors and with a significant influence on years of life lost, our findings reinforce the benefits of maintaining a healthy weight throughout life,” Dr. Liu and coinvestigators said in their report.
Their analysis was based on the ongoing Nurses Health Study II, which began in 1989 and enrolled a total of 116,430 women between the ages of 25 and 42 years in 14 U.S. states. Women completed questionnaires on demographics, medical and health information, and lifestyle factors every 2 years after enrollment.
Dr. Liu and colleagues were able to document 114 cases of colorectal cancer over a median of 13.9 years of follow-up in 85,256 women who had no cancer or inflammatory bowel disease when they were enrolled in the study. The median age at diagnosis for these cancers was 45 years.
Obesity was independently associated with increased risk of these early-onset colorectal cancers, the investigators found in multivariable analysis.
Women with a body mass index of 30 kg/m2 or higher had a relative risk of 1.93 (95% confidence interval, 1.15-3.25) versus women with normal BMIs in the 18.5 to 22.9-kg/m2 range, according to results of the analysis, they reported.
There was an apparent linear trend between increasing weight and increasing colorectal cancer risk, they added in their report.
They also found links between BMI in early adulthood and risk of early-onset colorectal cancer, including a relative risk of 1.63 for women who reported a BMI of 23 kg/m2 or higher at 18 years of age, versus women with a BMI of 18.5-20.9 kg/m2 at that age.
Similarly, increase in weight since early adulthood was associated with increased cancer risk, they reported.
While the link between excess weight and colorectal cancer incidence and mortality is well established in previous studies, this study is one of few reports looking at the association in younger individuals, according to Dr. Liu and colleagues.
This is thought to be the first prospective study looking at the link between obesity and risk of colo-rectal cancer diagnosed before the age of 50, they added.
The study was funded by grants from the National Institutes of Health. Dr. Liu had no conflict of interest disclosures related to the study. One coauthor reported consulting fees from Bayer Pharma AG, Janssen, and Pfizer.
SOURCE: Liu P-H et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4280.
AGA Resource
Visit the AGA GI Patient Center for education to share with your patients about obesity, colorectal cancer and other GI disorders at patient.gastro.org.
First-line bortezomib prolongs survival in MCL
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
FROM THE LANCET ONCOLOGY
Key clinical point:
Major finding: Median overall survival was 90.7 months in the intervention arm (bortezomib in addition to rituximab plus chemotherapy) versus 55.7 months in the control arm (hazard ratio, 0.66; 95% confidence interval, 0.51-0.85; P = .001).
Study details: LYM-3002 was a phase 3, randomized, open-label study of 487 transplant-ineligible patients with untreated mantle cell lymphoma.
Disclosures: The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties with Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
Source: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
Checkpoint inhibitor doubles 3-year survival rate of BRAF wild-type advanced melanoma
For previously untreated patients with BRAF wild-type advanced melanoma, the antiprogrammed death-ligand 1 agent nivolumab dramatically improved overall survival (OS), compared with standard first-line chemotherapy, according to new data from the CheckMate 066 trial.
At the 3-year follow-up, more than twice as many nivolumab-treated patients were alive, compared with those who received dacarbazine, reported lead author Paolo A. Ascierto, MD, director of the unit of melanoma, cancer immunotherapy, and innovative therapy at the Istituto Nazionale Tumori Fondazione Pascale in Naples, Italy, and his colleagues. Longer median progression-free survival (PFS) and higher complete response rates were also reported.
“The results of this 3-year follow-up analysis provided evidence for a durable survival benefit with nivolumab monotherapy in patients with previously untreated BRAF wild-type advanced melanoma,” the investigators wrote in JAMA Oncology.
The double-blind, phase 3 trial involved 418 patients with unresectable, previously untreated stage III or IV melanoma not exhibiting a BRAF mutation. Patients were randomized to receive either nivolumab (n = 210; 3 mg/kg every 2 weeks plus placebo every 3 weeks) or dacarbazine (n = 208; 1,000 mg/m2 every 3 weeks plus placebo every 2 weeks). Treatment continued until unacceptable toxicity or disease progression occurred.
The results showed dramatic benefits, measured by OS and PFS, when patients were treated with nivolumab. The 3-year OS rate was 51.2% in the nivolumab group, compared with 21.6% in the dacarbazine group, an approximate 130% difference. Median OS and median PFS were also multiplied by the checkpoint inhibitor, showing 230% and 130% improvements, respectively (OS, 37.5 months vs. 11.2 months; PFS, 5.1 months vs. 2.2 months; P less than .001). Grade 3 or higher treatment-related adverse events (AEs) were comparable between treatment arms (nivolumab, 15.0% vs. dacarbazine, 17.6%).
“Responses to nivolumab were long lasting in many patients who discontinued treatment, with most patients who stopped treatment still alive and without disease progression at the time of the last assessment,” the investigators wrote.
“Collectively, our results showed durable responses and long-term survival with nivolumab monotherapy, with no new AEs developing at late time points,” they concluded.
The study was funded by Bristol-Myers Squibb. The authors reported financial relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, Amgen, and others.
SOURCE: Ascierto PA et al. JAMA Oncol. 2018 Oct 25. doi: 10.1001/jamaoncol.2018.4514.
For previously untreated patients with BRAF wild-type advanced melanoma, the antiprogrammed death-ligand 1 agent nivolumab dramatically improved overall survival (OS), compared with standard first-line chemotherapy, according to new data from the CheckMate 066 trial.
At the 3-year follow-up, more than twice as many nivolumab-treated patients were alive, compared with those who received dacarbazine, reported lead author Paolo A. Ascierto, MD, director of the unit of melanoma, cancer immunotherapy, and innovative therapy at the Istituto Nazionale Tumori Fondazione Pascale in Naples, Italy, and his colleagues. Longer median progression-free survival (PFS) and higher complete response rates were also reported.
“The results of this 3-year follow-up analysis provided evidence for a durable survival benefit with nivolumab monotherapy in patients with previously untreated BRAF wild-type advanced melanoma,” the investigators wrote in JAMA Oncology.
The double-blind, phase 3 trial involved 418 patients with unresectable, previously untreated stage III or IV melanoma not exhibiting a BRAF mutation. Patients were randomized to receive either nivolumab (n = 210; 3 mg/kg every 2 weeks plus placebo every 3 weeks) or dacarbazine (n = 208; 1,000 mg/m2 every 3 weeks plus placebo every 2 weeks). Treatment continued until unacceptable toxicity or disease progression occurred.
The results showed dramatic benefits, measured by OS and PFS, when patients were treated with nivolumab. The 3-year OS rate was 51.2% in the nivolumab group, compared with 21.6% in the dacarbazine group, an approximate 130% difference. Median OS and median PFS were also multiplied by the checkpoint inhibitor, showing 230% and 130% improvements, respectively (OS, 37.5 months vs. 11.2 months; PFS, 5.1 months vs. 2.2 months; P less than .001). Grade 3 or higher treatment-related adverse events (AEs) were comparable between treatment arms (nivolumab, 15.0% vs. dacarbazine, 17.6%).
“Responses to nivolumab were long lasting in many patients who discontinued treatment, with most patients who stopped treatment still alive and without disease progression at the time of the last assessment,” the investigators wrote.
“Collectively, our results showed durable responses and long-term survival with nivolumab monotherapy, with no new AEs developing at late time points,” they concluded.
The study was funded by Bristol-Myers Squibb. The authors reported financial relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, Amgen, and others.
SOURCE: Ascierto PA et al. JAMA Oncol. 2018 Oct 25. doi: 10.1001/jamaoncol.2018.4514.
For previously untreated patients with BRAF wild-type advanced melanoma, the antiprogrammed death-ligand 1 agent nivolumab dramatically improved overall survival (OS), compared with standard first-line chemotherapy, according to new data from the CheckMate 066 trial.
At the 3-year follow-up, more than twice as many nivolumab-treated patients were alive, compared with those who received dacarbazine, reported lead author Paolo A. Ascierto, MD, director of the unit of melanoma, cancer immunotherapy, and innovative therapy at the Istituto Nazionale Tumori Fondazione Pascale in Naples, Italy, and his colleagues. Longer median progression-free survival (PFS) and higher complete response rates were also reported.
“The results of this 3-year follow-up analysis provided evidence for a durable survival benefit with nivolumab monotherapy in patients with previously untreated BRAF wild-type advanced melanoma,” the investigators wrote in JAMA Oncology.
The double-blind, phase 3 trial involved 418 patients with unresectable, previously untreated stage III or IV melanoma not exhibiting a BRAF mutation. Patients were randomized to receive either nivolumab (n = 210; 3 mg/kg every 2 weeks plus placebo every 3 weeks) or dacarbazine (n = 208; 1,000 mg/m2 every 3 weeks plus placebo every 2 weeks). Treatment continued until unacceptable toxicity or disease progression occurred.
The results showed dramatic benefits, measured by OS and PFS, when patients were treated with nivolumab. The 3-year OS rate was 51.2% in the nivolumab group, compared with 21.6% in the dacarbazine group, an approximate 130% difference. Median OS and median PFS were also multiplied by the checkpoint inhibitor, showing 230% and 130% improvements, respectively (OS, 37.5 months vs. 11.2 months; PFS, 5.1 months vs. 2.2 months; P less than .001). Grade 3 or higher treatment-related adverse events (AEs) were comparable between treatment arms (nivolumab, 15.0% vs. dacarbazine, 17.6%).
“Responses to nivolumab were long lasting in many patients who discontinued treatment, with most patients who stopped treatment still alive and without disease progression at the time of the last assessment,” the investigators wrote.
“Collectively, our results showed durable responses and long-term survival with nivolumab monotherapy, with no new AEs developing at late time points,” they concluded.
The study was funded by Bristol-Myers Squibb. The authors reported financial relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, Amgen, and others.
SOURCE: Ascierto PA et al. JAMA Oncol. 2018 Oct 25. doi: 10.1001/jamaoncol.2018.4514.
FROM JAMA ONCOLOGY
Key clinical point: For previously untreated patients with BRAF wild-type advanced melanoma, the checkpoint inhibitor nivolumab dramatically improved overall survival, compared with standard first-line chemotherapy.
Major finding: The 3-year overall survival rate for patients treated with nivolumab was 51.2%, compared with 21.6% for those who received dacarbazine.
Study details: CheckMate 066 is an ongoing, phase 3, double-blind trial involving 418 patients with BRAF wild-type advanced melanoma.
Disclosures: The study was funded by Bristol-Myers Squibb. The authors reported financial relationships with Bristol-Myers Squibb, Roche/Genentech, Novartis, Amgen, and others.
Source: Ascierto PA et al. JAMA Oncol. 2018 Oct 25. doi: 10.1001/jamaoncol.2018.4514.