User login
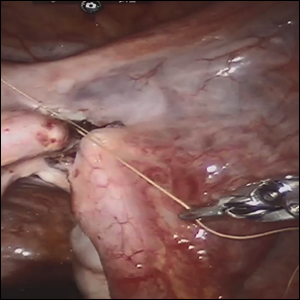
Robot-assisted laparoscopic tubal anastomosis following sterilization

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
Understanding hypertensive disorders in pregnancy
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Preeclampsia is one of the most significant medical complications in pregnancy because of the acute onset it can have in so many affected patients. This acute onset may then rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth.
It is an obstetrical syndrome with serious implications for the fetus, the infant at birth, and the mother, and it is one whose incidence has been increasing. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for the health and well-being of both the mother and fetus.
A new classification system introduced in 2013 by the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy has added further complexity to an already complicated disease. On one hand, attempting to precisely achieve a diagnosis with such an imprecise and insidious disease seems ill advised. On the other hand, it is important to achieve some level of clarity with respect to diagnosis and management. In doing so, we must lean toward overdiagnosis and maintain a low threshold for treatment and intervention in the interest of the mother and infant.
I have engaged Baha M. Sibai, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to introduce a practical approach for interpreting and utilizing the ACOG report. This installment is the first of a two-part series in which we hope to provide practical clinical strategies for this complex disease.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Clarifying the categories of hypertensive disorders in pregnancy
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Prenatal care always has been in part about identifying women with medical complications including preeclampsia. We have long measured blood pressure, checked the urine for high levels of protein, and monitored weight gain. We still do.
However, over the years, the diagnostic criteria for preeclampsia have evolved, first with the exclusion of edema and more recently with the exclusion of proteinuria as a necessary element of the diagnosis. The American College of Obstetricians and Gynecologists’ Task Force Report, Hypertension in Pregnancy, published in 2013, concluded that while preeclampsia may still be defined by the occurrence of hypertension with proteinuria, it also may be diagnosed when hypertension occurs in association with other multisystemic signs indicative of disease severity. The change came based on evidence that some women develop eclampsia, HELLP syndrome, and other serious complications in the absence of proteinuria.
The 2013 document also attempted to review and clarify various issues relating to the classifications, diagnosis, prediction and prevention, and management of hypertension during pregnancy, including the postpartum period. In many respects, it was successful in doing so. However, there is still much confusion regarding the diagnosis of certain categories of hypertensive disorders – particularly preeclampsia with severe features and superimposed preeclampsia with or without severe features.
While it is difficult to establish precise definitions given the often insidious nature of preeclampsia, it still is important to achieve a higher level of clarity with respect to these categories. Overdiagnosis may be preferable. However, improper classification also may influence management decisions that could prove detrimental to the fetus.
Severe gestational hypertension
ACOG’s 2013 Report on Hypertension in Pregnancy classifies hypertensive disorders of pregnancy into these categories: Gestational hypertension (GHTN), preeclampsia, preeclampsia with severe features (this includes HELLP), chronic hypertension (CHTN), superimposed preeclampsia with or without severe features, and eclampsia.
Some of the definitions and diagnostic criteria are clear. For instance, GHTN is defined as the new onset of hypertension after 20 weeks’ gestation in the absence of proteinuria or systemic findings such as thrombocytopenia or impaired liver function. CHTN is defined as hypertension that predates conception or is detected before 20 weeks’ gestation. In both cases there should be elevated blood pressure on two occasions at least 4 hours apart.
A major omission is the lack of a definition for severe GHTN. Removal of this previously well-understood classification category combined with unclear statements regarding preeclampsia with or without severe features has made it difficult for physicians to know in some cases of severe hypertension only what diagnosis a woman should receive and how she should be managed.
I recommend that we maintain the category of severe GHTN, and that it be defined as a systolic blood pressure (BP) greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions at least 4 hours apart when antihypertensive medications have not been initiated. There should be no proteinuria or severe features such as thrombocytopenia or impaired liver function.
The physician may elect in these cases to administer antihypertensive medication and observe the patient in the hospital. An individualized decision can then be made regarding how the patient should be managed, including whether she should be admitted and whether the pregnancy should continue beyond 34 weeks. Blood pressure, gestational age at diagnosis, the presence or absence of symptoms, and laboratory tests all should be taken into consideration.
Preeclampsia with or without severe features
We need to clarify and simplify how we think about GHTN and preeclampsia with or without severe features.
Most cases of preeclampsia will involve new-onset proteinuria, with proteinuria being defined as greater than or equal to 300 mg/day or a protein-creatinine ratio of greater than or equal to 0.3 mg/dL. In cases in which a dipstick test must be used, proteinuria is suggested by a urine protein reading of 1+. (It is important to note that dipstick readings should be taken on two separate occasions.) According to the report, preeclampsia also may be established by the presence of GHTN in association with any one of a list of features that are generally referred to as “severe features.”
Various boxes and textual descriptions in the report offer a sometimes confusing picture, however, of the terms preeclampsia and preeclampsia with severe features and their differences. For clarification, I recommend that we define preeclampsia with severe features as GHTN (mild or severe) in association with any one of the severe features.
Severe features of preeclampsia
- Platelet count less than 100,000/microliter.
- Elevated hepatic transaminases greater than two times the upper limit of normal for specific laboratory adult reference ranges.
- Severe persistent right upper quadrant abdominal pain or epigastric pain unresponsive to analgesics and unexplained by other etiology.
- Serum creatinine greater than 1.1 mg/dL.
- Pulmonary edema.
- Persistent cerebral disturbances such as severe persistent new-onset headaches unresponsive to nonnarcotic analgesics, altered mental status or other neurologic deficits.
- Visual disturbances such as blurred vision, scotomata, photophobia, or loss of vision.
I also suggest that we think of “mild” GHTN (systolic BP of 140-159 mm Hg or diastolic BP 90-109 mm Hg) and preeclampsia without severe features as one in the same, and that we manage them similarly. The presence or absence of proteinuria is currently the only difference diagnostically. The only difference with respect to management – aside from a weekly urine protein check in the case of GHTN – is the frequency of nonstress testing (NST) and amniotic fluid index (AFI) measurement (currently once a week for GHTN and twice a week for preeclampsia).
Given that unnecessary time and energy may be spent differentiating the two when management is essentially the same, I suggest that preeclampsia be diagnosed in any patient with GHTN with or without proteinuria. All patients can then be managed with blood pressure checks twice a week; symptoms and kick count daily; NST and AFI twice a week; estimated fetal weight by ultrasound every third week; lab tests (CBC, liver enzymes, and creatinine) once a week, and delivery at 37 weeks.
Superimposed preeclampsia with or without severe features
As the report states, the recognition of preeclampsia superimposed on chronic hypertension is “perhaps the greatest challenge” in the diagnosis and management of hypertensive disorders in pregnancy. Overdiagnosis “may be preferable,” the report says, given the high risk of adverse pregnancy outcomes with superimposed preeclampsia. On the other hand, it says, a “more stratified approach based on severity and predictors of adverse outcome may be useful” in avoiding unnecessary preterm births.
Ultimately, the task force proposed that we utilize the two categories of “superimposed preeclampsia” and “superimposed preeclampsia with severe features,” and in doing so, it noted that there “often is ambiguity in the diagnosis of superimposed preeclampsia and that the clinical spectrum of disease is broad.” Indeed, the diagnosis of superimposed preeclampsia as presented in the report remains vague and open to interpretation. In my institution, it has created significant confusion.
The report states that superimposed preeclampsia is likely when any of the following are present: 1) a sudden increase in blood pressure that was previously well controlled or escalation of antihypertensive medications to control blood pressure, or 2) new onset of proteinuria or a sudden increase in proteinuria in a woman with known proteinuria before or early in pregnancy.
It is not clear, however, what is considered a sudden increase in blood pressure, and it is concerning that any escalation of medication could potentially prompt this diagnosis. Is an increase in systolic blood pressure from 140 mm Hg to 150 mm Hg or an increase in diastolic blood pressure from 90 mm Hg to 100 mm Hg between two prenatal visits considered a “sudden increase”? Does an increase in methyldopa dosage from 250 mg daily to 500 mg daily to keep blood pressure within the range of mild hypertension mean that the patient should be diagnosed with superimposed preeclampsia? Hypertension is likely to increase and require an escalation of antihypertensive medications as patients with chronic hypertension progress through their pregnancies.
Similarly, a “sudden increase in proteinuria” – or “sudden, substantial, and sustained increases in protein excretion,” as written elsewhere in the report with respect to superimposed preeclampsia – also is undefined. What exactly does this mean? That we lack clinically meaningful parameters and clear descriptions of acceptable criteria/scenarios for observation rather than intervention is troubling, particularly because some of these women may have preexisting renal disease with expected increases and fluctuations in protein excretion during advanced gestation.
We must be cautious about making a diagnosis of superimposed preeclampsia based on changes in blood pressure or urinary protein alone, lest we have unnecessary hospitalizations and interventions. I recommend that the diagnosis of superimposed preeclampsia be made based on either the new onset of proteinuria in association with mild hypertension after 20 weeks or on elevation in blood pressure to severe ranges (systolic BP greater than or equal to160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of maximum doses of one antihypertensive drug.
Regarding superimposed preeclampsia with severe features, I recommend that in the case of blood pressure elevation, it be diagnosed only after maximal doses of two medications have been used. Specifically, I recommend that superimposed preeclampsia with severe features be defined as either CHTN or superimposed preeclampsia in association with either systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg on at least two occasions despite use of maximum doses of labetalol (2,400 mg/day) plus long-acting nifedipine (120 mg/day), or with any of the other severe features.
In a second installment of the Master Class, I will elaborate on the treatment of severe GHTN and address the management of preeclampsia with severe features as well as postpartum management of hypertension during pregnancy.
Suggested diagnostic definitions
- Preeclampsia with severe features: GHTN in association with severe features.
- Superimposed preeclampsia: CHTN with either the new onset of proteinuria in association with mild hypertension after 20 weeks, or an elevation in blood pressure to severe ranges (systolic BP greater than or equal to 160 mm Hg and/or diastolic BP greater than or equal to 110 mm Hg) despite the use of the maximal dose of one antihypertensive drug.
- Superimposed preeclampsia with severe features: CHTN or superimposed preeclampsia with severe features or with a rise in blood pressure to severe ranges despite the maximal doses of two antihypertensive drugs (e.g. 2,400 mg/day labetalol plus 120 mg/day long-acting nifedipine).
Note: These definitions reflect adaptations and clarifications of ACOG’s 2013 Task Force Report on Hypertension in Pregnancy.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Experts: Kavanaugh may have lasting impact on health care
As the Supreme Court sits for its second session on Oct. 29, legal analysts question what impact its newest Associate Justice, Brett M. Kavanaugh, may have on health care cases that might come before the court.
The addition of Justice Kavanaugh cements a conservative majority on the high court, said Eric J. Segall, a constitutional law professor at Georgia State University in Atlanta.
“Where Justice Kennedy was moderate or liberal, Justice Kavanaugh will move the court dramatically to the right in most areas of law,” Mr. Segall said in an interview.
One area drawing considerable attention is abortion, with abortion rights advocates raising concerns that Justice Kavanaugh’s appointment may mean the reversal of Roe v. Wade. Legal analysts, however, say fall of the momentous ruling is not probable.
Based on his decisions written while he sat on the Court of Appeals for the District of Columbia Circuit, it’s more likely that Justice Kavanaugh would attempt to narrow the instances in which state abortion restrictions are considered to impede a woman’s constitutional right to an abortion, according to Timothy S. Jost, a legal analyst and retired health law professor at Washington and Lee University in Lexington, Va.
In Garza v. Hargan for example, then-Judge Kavanaugh dissented from a majority decision which ultimately allowed a teenage immigrant in U.S. custody to have an abortion. Judge Kavanaugh argued that it was wrong for unlawful immigrant minors in U.S. detention to obtain “immediate abortion on demand.” However, his dissent was not as far-reaching as that of another judge who argued that a minor undocumented immigrant had no constitutional right to an abortion.
“[Justice] Kavanaugh certainly could vote to overturn Roe v. Wade, but based on his past opinions, I think it much more likely that he would simply expand Roe’s undue burden exception on a case by case basis until it was meaningless,” Mr. Jost said in an interview. “He doesn’t have to overturn Roe v. Wade to allow states that want to effectively bar abortion [to succeed].”
Mr. Segall agrees that Justice Kavanaugh will likely water down the undue burden test for abortion and through this softening, essentially dismantle Roe and return the issue of abortion to states.
As for the fate of the Affordable Care Act, legal experts foresee a restrained stance by Justice Kavanaugh, rather than a strong rejection of the entire law. In 2011, Judge Kavanaugh wrote a dissenting opinion in Seven-Sky v. Holder, a case that challenged the constitutionality of the health law’s individual mandate. While his fellow judges ruled that Congress had the authority to enact the mandate, Judge Kavanaugh argued the mandate was a tax and thus, it was too early for the court to hear the case since the tax had not yet been levied.
The dissent by Judge Kavanaugh in this case amounted to a “procedural cop-out,” according to Thomas P. Miller, a resident fellow at the conservative American Enterprise Institute and a health care policy scholar.
“That tells you he is a relatively cautious judge as opposed to going right to the metal because other circuit court of appeals judges were able to say this is an unconstitutional mandate,” Mr. Miller said in an interview. “That suggests a degree of tactical caution on these issues, very similar to [Supreme Court Chief Justice John] Roberts.”
Justice Kavanaugh may get his first chance to rule on the ACA’s future through Texas v. United States. The case centers on a challenge by 20 Republican state attorneys general over the constitutionality of the health law’s individual mandate. The Trump administration has reduced the penalty for failing to have health insurance to $0 starting in 2019. The plaintiffs allege that the mandate cannot be severed from the rest of the ACA and that if the mandate is eliminated, the rest of the law should fall. Because the Trump administration has opted not to protect the ACA, Democratic state attorneys general in 16 states have intervened to defend the health law. Arguments were heard before the federal district court in the Northern District of Texas in September and a decision is expected any day.
If appealed, the Fifth Circuit would next take on the case, but the challenge could eventually reach the Supreme Court, Mr. Jost said.
“Chief Justice Roberts could save [the ACA] one more time, but I could see [Justice] Kavanaugh voting to uphold the ACA as the question would likely be a question of severability of the individual mandate from the rest of the ACA, and I can see [Kavanaugh] holding it severable,” he said.
Justice Kavanaugh may also weigh in on a handful of Medicaid cases that could go before the Supreme Court. The first case involves how much deference the federal government should have in allowing states to impose work requirements on Medicaid patients. In June, a federal judge in Washington D.C. struck down the federal government’s approval of a Kentucky Medicaid waiver that would have imposed work requirements and other rules for eligibility. That judge ruled that the Centers for Medicare & Medicaid Services did not adequately evaluate whether Kentucky’s requirements were consistent with federal Medicaid law.
Mr. Miller said it is too early to tell which way Justice Kavanaugh would vote on a Medicaid work requirements case, and that the decision depends on the context of the case and the reading of the law involved.
“[Justice] Kavanaugh has a less predictable record in this more narrow area of administrative law,” Mr. Miller said. “I doubt he alone is going to drive the court anywhere on this issue it doesn’t otherwise want to go – or at least drift.”
Two other Medicaid cases pending before the Supreme Court revolve around the right of a private Medicaid patient to sue a state over the exclusion of Planned Parenthood in its Medicaid program. Gee v. Planned Parenthood of Gulf Coast Inc. and its sister case, Anderson v. Planned Parenthood of Kansas and Mid-Missouri, stem from separate efforts by Kansas and Louisiana to remove Planned Parenthood from their Medicaid programs. The question is whether the Medicaid patients have a right to challenge the exclusions.
If the Supreme Court accepts the cases, Mr. Jost said it’s likely that Justice Kavanaugh would side with the states and bar the patients from suing.
“I could see him holding that Medicaid recipients can’t sue if a state violates federal law, which would effectively end Medicaid as an entitlement,” Mr. Jost said. “This would have disastrous consequences for low-income Americans.”
Justice Kavanaugh also could make an impact on gun control, Mr. Segall noted. The Supreme Court has not taken a Second Amendment case in several years; in the past, there was uncertainty about how Justice Kennedy would vote on a gun control case. Not so with Justice Kavanaugh, Mr. Segall said. In Heller v. District of Columbia (known as Heller II), Judge Kavanaugh dissented from the majority, writing that the District’s ban on semiautomatic rifles and its requirement that handguns be registered were unconstitutional.
“[The dissent] gave the Second Amendment as broad a reading as any judge has ever given,” Mr. Segall said. “Gun control, gun reform, and gun limits ... this is where [Kavanaugh] is going to make the biggest difference.”
As the Supreme Court sits for its second session on Oct. 29, legal analysts question what impact its newest Associate Justice, Brett M. Kavanaugh, may have on health care cases that might come before the court.
The addition of Justice Kavanaugh cements a conservative majority on the high court, said Eric J. Segall, a constitutional law professor at Georgia State University in Atlanta.
“Where Justice Kennedy was moderate or liberal, Justice Kavanaugh will move the court dramatically to the right in most areas of law,” Mr. Segall said in an interview.
One area drawing considerable attention is abortion, with abortion rights advocates raising concerns that Justice Kavanaugh’s appointment may mean the reversal of Roe v. Wade. Legal analysts, however, say fall of the momentous ruling is not probable.
Based on his decisions written while he sat on the Court of Appeals for the District of Columbia Circuit, it’s more likely that Justice Kavanaugh would attempt to narrow the instances in which state abortion restrictions are considered to impede a woman’s constitutional right to an abortion, according to Timothy S. Jost, a legal analyst and retired health law professor at Washington and Lee University in Lexington, Va.
In Garza v. Hargan for example, then-Judge Kavanaugh dissented from a majority decision which ultimately allowed a teenage immigrant in U.S. custody to have an abortion. Judge Kavanaugh argued that it was wrong for unlawful immigrant minors in U.S. detention to obtain “immediate abortion on demand.” However, his dissent was not as far-reaching as that of another judge who argued that a minor undocumented immigrant had no constitutional right to an abortion.
“[Justice] Kavanaugh certainly could vote to overturn Roe v. Wade, but based on his past opinions, I think it much more likely that he would simply expand Roe’s undue burden exception on a case by case basis until it was meaningless,” Mr. Jost said in an interview. “He doesn’t have to overturn Roe v. Wade to allow states that want to effectively bar abortion [to succeed].”
Mr. Segall agrees that Justice Kavanaugh will likely water down the undue burden test for abortion and through this softening, essentially dismantle Roe and return the issue of abortion to states.
As for the fate of the Affordable Care Act, legal experts foresee a restrained stance by Justice Kavanaugh, rather than a strong rejection of the entire law. In 2011, Judge Kavanaugh wrote a dissenting opinion in Seven-Sky v. Holder, a case that challenged the constitutionality of the health law’s individual mandate. While his fellow judges ruled that Congress had the authority to enact the mandate, Judge Kavanaugh argued the mandate was a tax and thus, it was too early for the court to hear the case since the tax had not yet been levied.
The dissent by Judge Kavanaugh in this case amounted to a “procedural cop-out,” according to Thomas P. Miller, a resident fellow at the conservative American Enterprise Institute and a health care policy scholar.
“That tells you he is a relatively cautious judge as opposed to going right to the metal because other circuit court of appeals judges were able to say this is an unconstitutional mandate,” Mr. Miller said in an interview. “That suggests a degree of tactical caution on these issues, very similar to [Supreme Court Chief Justice John] Roberts.”
Justice Kavanaugh may get his first chance to rule on the ACA’s future through Texas v. United States. The case centers on a challenge by 20 Republican state attorneys general over the constitutionality of the health law’s individual mandate. The Trump administration has reduced the penalty for failing to have health insurance to $0 starting in 2019. The plaintiffs allege that the mandate cannot be severed from the rest of the ACA and that if the mandate is eliminated, the rest of the law should fall. Because the Trump administration has opted not to protect the ACA, Democratic state attorneys general in 16 states have intervened to defend the health law. Arguments were heard before the federal district court in the Northern District of Texas in September and a decision is expected any day.
If appealed, the Fifth Circuit would next take on the case, but the challenge could eventually reach the Supreme Court, Mr. Jost said.
“Chief Justice Roberts could save [the ACA] one more time, but I could see [Justice] Kavanaugh voting to uphold the ACA as the question would likely be a question of severability of the individual mandate from the rest of the ACA, and I can see [Kavanaugh] holding it severable,” he said.
Justice Kavanaugh may also weigh in on a handful of Medicaid cases that could go before the Supreme Court. The first case involves how much deference the federal government should have in allowing states to impose work requirements on Medicaid patients. In June, a federal judge in Washington D.C. struck down the federal government’s approval of a Kentucky Medicaid waiver that would have imposed work requirements and other rules for eligibility. That judge ruled that the Centers for Medicare & Medicaid Services did not adequately evaluate whether Kentucky’s requirements were consistent with federal Medicaid law.
Mr. Miller said it is too early to tell which way Justice Kavanaugh would vote on a Medicaid work requirements case, and that the decision depends on the context of the case and the reading of the law involved.
“[Justice] Kavanaugh has a less predictable record in this more narrow area of administrative law,” Mr. Miller said. “I doubt he alone is going to drive the court anywhere on this issue it doesn’t otherwise want to go – or at least drift.”
Two other Medicaid cases pending before the Supreme Court revolve around the right of a private Medicaid patient to sue a state over the exclusion of Planned Parenthood in its Medicaid program. Gee v. Planned Parenthood of Gulf Coast Inc. and its sister case, Anderson v. Planned Parenthood of Kansas and Mid-Missouri, stem from separate efforts by Kansas and Louisiana to remove Planned Parenthood from their Medicaid programs. The question is whether the Medicaid patients have a right to challenge the exclusions.
If the Supreme Court accepts the cases, Mr. Jost said it’s likely that Justice Kavanaugh would side with the states and bar the patients from suing.
“I could see him holding that Medicaid recipients can’t sue if a state violates federal law, which would effectively end Medicaid as an entitlement,” Mr. Jost said. “This would have disastrous consequences for low-income Americans.”
Justice Kavanaugh also could make an impact on gun control, Mr. Segall noted. The Supreme Court has not taken a Second Amendment case in several years; in the past, there was uncertainty about how Justice Kennedy would vote on a gun control case. Not so with Justice Kavanaugh, Mr. Segall said. In Heller v. District of Columbia (known as Heller II), Judge Kavanaugh dissented from the majority, writing that the District’s ban on semiautomatic rifles and its requirement that handguns be registered were unconstitutional.
“[The dissent] gave the Second Amendment as broad a reading as any judge has ever given,” Mr. Segall said. “Gun control, gun reform, and gun limits ... this is where [Kavanaugh] is going to make the biggest difference.”
As the Supreme Court sits for its second session on Oct. 29, legal analysts question what impact its newest Associate Justice, Brett M. Kavanaugh, may have on health care cases that might come before the court.
The addition of Justice Kavanaugh cements a conservative majority on the high court, said Eric J. Segall, a constitutional law professor at Georgia State University in Atlanta.
“Where Justice Kennedy was moderate or liberal, Justice Kavanaugh will move the court dramatically to the right in most areas of law,” Mr. Segall said in an interview.
One area drawing considerable attention is abortion, with abortion rights advocates raising concerns that Justice Kavanaugh’s appointment may mean the reversal of Roe v. Wade. Legal analysts, however, say fall of the momentous ruling is not probable.
Based on his decisions written while he sat on the Court of Appeals for the District of Columbia Circuit, it’s more likely that Justice Kavanaugh would attempt to narrow the instances in which state abortion restrictions are considered to impede a woman’s constitutional right to an abortion, according to Timothy S. Jost, a legal analyst and retired health law professor at Washington and Lee University in Lexington, Va.
In Garza v. Hargan for example, then-Judge Kavanaugh dissented from a majority decision which ultimately allowed a teenage immigrant in U.S. custody to have an abortion. Judge Kavanaugh argued that it was wrong for unlawful immigrant minors in U.S. detention to obtain “immediate abortion on demand.” However, his dissent was not as far-reaching as that of another judge who argued that a minor undocumented immigrant had no constitutional right to an abortion.
“[Justice] Kavanaugh certainly could vote to overturn Roe v. Wade, but based on his past opinions, I think it much more likely that he would simply expand Roe’s undue burden exception on a case by case basis until it was meaningless,” Mr. Jost said in an interview. “He doesn’t have to overturn Roe v. Wade to allow states that want to effectively bar abortion [to succeed].”
Mr. Segall agrees that Justice Kavanaugh will likely water down the undue burden test for abortion and through this softening, essentially dismantle Roe and return the issue of abortion to states.
As for the fate of the Affordable Care Act, legal experts foresee a restrained stance by Justice Kavanaugh, rather than a strong rejection of the entire law. In 2011, Judge Kavanaugh wrote a dissenting opinion in Seven-Sky v. Holder, a case that challenged the constitutionality of the health law’s individual mandate. While his fellow judges ruled that Congress had the authority to enact the mandate, Judge Kavanaugh argued the mandate was a tax and thus, it was too early for the court to hear the case since the tax had not yet been levied.
The dissent by Judge Kavanaugh in this case amounted to a “procedural cop-out,” according to Thomas P. Miller, a resident fellow at the conservative American Enterprise Institute and a health care policy scholar.
“That tells you he is a relatively cautious judge as opposed to going right to the metal because other circuit court of appeals judges were able to say this is an unconstitutional mandate,” Mr. Miller said in an interview. “That suggests a degree of tactical caution on these issues, very similar to [Supreme Court Chief Justice John] Roberts.”
Justice Kavanaugh may get his first chance to rule on the ACA’s future through Texas v. United States. The case centers on a challenge by 20 Republican state attorneys general over the constitutionality of the health law’s individual mandate. The Trump administration has reduced the penalty for failing to have health insurance to $0 starting in 2019. The plaintiffs allege that the mandate cannot be severed from the rest of the ACA and that if the mandate is eliminated, the rest of the law should fall. Because the Trump administration has opted not to protect the ACA, Democratic state attorneys general in 16 states have intervened to defend the health law. Arguments were heard before the federal district court in the Northern District of Texas in September and a decision is expected any day.
If appealed, the Fifth Circuit would next take on the case, but the challenge could eventually reach the Supreme Court, Mr. Jost said.
“Chief Justice Roberts could save [the ACA] one more time, but I could see [Justice] Kavanaugh voting to uphold the ACA as the question would likely be a question of severability of the individual mandate from the rest of the ACA, and I can see [Kavanaugh] holding it severable,” he said.
Justice Kavanaugh may also weigh in on a handful of Medicaid cases that could go before the Supreme Court. The first case involves how much deference the federal government should have in allowing states to impose work requirements on Medicaid patients. In June, a federal judge in Washington D.C. struck down the federal government’s approval of a Kentucky Medicaid waiver that would have imposed work requirements and other rules for eligibility. That judge ruled that the Centers for Medicare & Medicaid Services did not adequately evaluate whether Kentucky’s requirements were consistent with federal Medicaid law.
Mr. Miller said it is too early to tell which way Justice Kavanaugh would vote on a Medicaid work requirements case, and that the decision depends on the context of the case and the reading of the law involved.
“[Justice] Kavanaugh has a less predictable record in this more narrow area of administrative law,” Mr. Miller said. “I doubt he alone is going to drive the court anywhere on this issue it doesn’t otherwise want to go – or at least drift.”
Two other Medicaid cases pending before the Supreme Court revolve around the right of a private Medicaid patient to sue a state over the exclusion of Planned Parenthood in its Medicaid program. Gee v. Planned Parenthood of Gulf Coast Inc. and its sister case, Anderson v. Planned Parenthood of Kansas and Mid-Missouri, stem from separate efforts by Kansas and Louisiana to remove Planned Parenthood from their Medicaid programs. The question is whether the Medicaid patients have a right to challenge the exclusions.
If the Supreme Court accepts the cases, Mr. Jost said it’s likely that Justice Kavanaugh would side with the states and bar the patients from suing.
“I could see him holding that Medicaid recipients can’t sue if a state violates federal law, which would effectively end Medicaid as an entitlement,” Mr. Jost said. “This would have disastrous consequences for low-income Americans.”
Justice Kavanaugh also could make an impact on gun control, Mr. Segall noted. The Supreme Court has not taken a Second Amendment case in several years; in the past, there was uncertainty about how Justice Kennedy would vote on a gun control case. Not so with Justice Kavanaugh, Mr. Segall said. In Heller v. District of Columbia (known as Heller II), Judge Kavanaugh dissented from the majority, writing that the District’s ban on semiautomatic rifles and its requirement that handguns be registered were unconstitutional.
“[The dissent] gave the Second Amendment as broad a reading as any judge has ever given,” Mr. Segall said. “Gun control, gun reform, and gun limits ... this is where [Kavanaugh] is going to make the biggest difference.”
IDEAS study meets first aim of changing 3-month clinical management, health outcomes
BARCELONA – Amyloid PET brain imaging changed clinical management in 60% of patients with a diagnosis of mild cognitive impairment or dementia and confirmed a presumptive Alzheimer’s diagnosis in 95% of those with positive scans.
But the scans also benefited amyloid-negative patients, Gil Rabinovici, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Before the test, 71% carried an Alzheimer’s disease (AD) diagnosis; after the test, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
“These patients were saved from unnecessary treatment for Alzheimer’s,” said Dr. Rabinovici, the Edward Fein and Pearl Landrith Endowed Professor in Memory & Aging at the University of California, San Francisco. They received more suitable care plans because of the confirmation.
He presented final results of aim one of the IDEAS (Imaging Dementia–Evidence for Amyloid Scanning) study, which seeks to prove that amyloid imaging changes clinical management and improves health outcomes in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two aims are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and that this impacts major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
Ultimately, investigators hope the U.S.-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service.
So far, IDEAS has accrued data on 11,409 patients and is quickly closing in on the 18,000-patient target. The patients reported on at CTAD were aged a mean of 75 years and were largely white; only 4% were black and 4% Hispanic. The mean Mini-Mental Scale Exam score was 26. AD was the leading suspect pathology in 73% of the 6,905 with MCI and in 83% of those with dementia of uncertain etiology. Overall, 44% were taking AD medications at baseline.
Scans were positive in 55% of those with MCI and in 70% of those with dementia. Overall, the scans changed clinical management in 61% (7,018), including 60% of those with MCI and 63% of those with dementia.
“We also asked physicians how much the scan results contributed to these changes, and 86.7% replied that they ‘contributed significantly,’ ” Dr. Rabinovici said.
Most changes involved adjustments to medication. AD drugs were started in 44% of MCI patients and in 45% of dementia patients, and non-AD drugs started in 22% and 25%, respectively. About a fifth of the patients received counseling in wake of the scan results.
Medication adjustments also varied by scan result. Among amyloid-positive MCI patients, AD drug use increased from 40% before imaging to 81% after; among amyloid-negative MCI patients, drug use decreased slightly from 27% to 24%. Among amyloid-positive dementia patients, AD drug use increased from 63% to 91%, and among amyloid-negative patients, it dropped from 50% to 44%. All these changes were statistically significant.
The primary diagnosis changed from AD to non-AD in 25%, and from non-AD to AD in 10%. Among amyloid-positive patients, the diagnosis prevalence jumped from 80.0% to 95.5%; among amyloid-negative patients, it dropped from 71% to just 10%.
“IDEAS now provides the strongest data we have supporting the beneficial impact of amyloid PET on patient management,” said Dr. Rabinovici. “Aim two, which is the 12-month health outcomes, we expect to be completed by the end of next year.”
The IDEAS team is also looking at a furthering the investigation with a study called, aptly, “NEW IDEAS.” That would reach out to recruit the minorities that were so underrepresented in the main study and include patients with early-onset MCI or dementia. Building up a library of DNA and blood plasma samples might also fit into the new project.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
BARCELONA – Amyloid PET brain imaging changed clinical management in 60% of patients with a diagnosis of mild cognitive impairment or dementia and confirmed a presumptive Alzheimer’s diagnosis in 95% of those with positive scans.
But the scans also benefited amyloid-negative patients, Gil Rabinovici, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Before the test, 71% carried an Alzheimer’s disease (AD) diagnosis; after the test, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
“These patients were saved from unnecessary treatment for Alzheimer’s,” said Dr. Rabinovici, the Edward Fein and Pearl Landrith Endowed Professor in Memory & Aging at the University of California, San Francisco. They received more suitable care plans because of the confirmation.
He presented final results of aim one of the IDEAS (Imaging Dementia–Evidence for Amyloid Scanning) study, which seeks to prove that amyloid imaging changes clinical management and improves health outcomes in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two aims are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and that this impacts major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
Ultimately, investigators hope the U.S.-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service.
So far, IDEAS has accrued data on 11,409 patients and is quickly closing in on the 18,000-patient target. The patients reported on at CTAD were aged a mean of 75 years and were largely white; only 4% were black and 4% Hispanic. The mean Mini-Mental Scale Exam score was 26. AD was the leading suspect pathology in 73% of the 6,905 with MCI and in 83% of those with dementia of uncertain etiology. Overall, 44% were taking AD medications at baseline.
Scans were positive in 55% of those with MCI and in 70% of those with dementia. Overall, the scans changed clinical management in 61% (7,018), including 60% of those with MCI and 63% of those with dementia.
“We also asked physicians how much the scan results contributed to these changes, and 86.7% replied that they ‘contributed significantly,’ ” Dr. Rabinovici said.
Most changes involved adjustments to medication. AD drugs were started in 44% of MCI patients and in 45% of dementia patients, and non-AD drugs started in 22% and 25%, respectively. About a fifth of the patients received counseling in wake of the scan results.
Medication adjustments also varied by scan result. Among amyloid-positive MCI patients, AD drug use increased from 40% before imaging to 81% after; among amyloid-negative MCI patients, drug use decreased slightly from 27% to 24%. Among amyloid-positive dementia patients, AD drug use increased from 63% to 91%, and among amyloid-negative patients, it dropped from 50% to 44%. All these changes were statistically significant.
The primary diagnosis changed from AD to non-AD in 25%, and from non-AD to AD in 10%. Among amyloid-positive patients, the diagnosis prevalence jumped from 80.0% to 95.5%; among amyloid-negative patients, it dropped from 71% to just 10%.
“IDEAS now provides the strongest data we have supporting the beneficial impact of amyloid PET on patient management,” said Dr. Rabinovici. “Aim two, which is the 12-month health outcomes, we expect to be completed by the end of next year.”
The IDEAS team is also looking at a furthering the investigation with a study called, aptly, “NEW IDEAS.” That would reach out to recruit the minorities that were so underrepresented in the main study and include patients with early-onset MCI or dementia. Building up a library of DNA and blood plasma samples might also fit into the new project.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
BARCELONA – Amyloid PET brain imaging changed clinical management in 60% of patients with a diagnosis of mild cognitive impairment or dementia and confirmed a presumptive Alzheimer’s diagnosis in 95% of those with positive scans.
But the scans also benefited amyloid-negative patients, Gil Rabinovici, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Before the test, 71% carried an Alzheimer’s disease (AD) diagnosis; after the test, just 10% did, opening the way for an accurate diagnosis and more effective treatment.
“These patients were saved from unnecessary treatment for Alzheimer’s,” said Dr. Rabinovici, the Edward Fein and Pearl Landrith Endowed Professor in Memory & Aging at the University of California, San Francisco. They received more suitable care plans because of the confirmation.
He presented final results of aim one of the IDEAS (Imaging Dementia–Evidence for Amyloid Scanning) study, which seeks to prove that amyloid imaging changes clinical management and improves health outcomes in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two aims are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and that this impacts major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
Ultimately, investigators hope the U.S.-wide, open-label study will prove the clinical value of amyloid PET scanning and convince the Centers for Medicare & Medicaid Services to make the test a fully covered service.
So far, IDEAS has accrued data on 11,409 patients and is quickly closing in on the 18,000-patient target. The patients reported on at CTAD were aged a mean of 75 years and were largely white; only 4% were black and 4% Hispanic. The mean Mini-Mental Scale Exam score was 26. AD was the leading suspect pathology in 73% of the 6,905 with MCI and in 83% of those with dementia of uncertain etiology. Overall, 44% were taking AD medications at baseline.
Scans were positive in 55% of those with MCI and in 70% of those with dementia. Overall, the scans changed clinical management in 61% (7,018), including 60% of those with MCI and 63% of those with dementia.
“We also asked physicians how much the scan results contributed to these changes, and 86.7% replied that they ‘contributed significantly,’ ” Dr. Rabinovici said.
Most changes involved adjustments to medication. AD drugs were started in 44% of MCI patients and in 45% of dementia patients, and non-AD drugs started in 22% and 25%, respectively. About a fifth of the patients received counseling in wake of the scan results.
Medication adjustments also varied by scan result. Among amyloid-positive MCI patients, AD drug use increased from 40% before imaging to 81% after; among amyloid-negative MCI patients, drug use decreased slightly from 27% to 24%. Among amyloid-positive dementia patients, AD drug use increased from 63% to 91%, and among amyloid-negative patients, it dropped from 50% to 44%. All these changes were statistically significant.
The primary diagnosis changed from AD to non-AD in 25%, and from non-AD to AD in 10%. Among amyloid-positive patients, the diagnosis prevalence jumped from 80.0% to 95.5%; among amyloid-negative patients, it dropped from 71% to just 10%.
“IDEAS now provides the strongest data we have supporting the beneficial impact of amyloid PET on patient management,” said Dr. Rabinovici. “Aim two, which is the 12-month health outcomes, we expect to be completed by the end of next year.”
The IDEAS team is also looking at a furthering the investigation with a study called, aptly, “NEW IDEAS.” That would reach out to recruit the minorities that were so underrepresented in the main study and include patients with early-onset MCI or dementia. Building up a library of DNA and blood plasma samples might also fit into the new project.
IDEAS is a funding collaboration of the CMS, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
REPORTING FROM CTAD
Key clinical point: Amyloid PET imaging can refine equivocal dementia diagnoses.
Major finding:
Study details: The IDEAS study has thus far accrued data on 11,409 subjects.
Disclosures: IDEAS is a funding collaboration of the Centers for Medicare & Medicaid Services, the Alzheimer’s Association, Avid Radiopharmaceuticals/Eli Lilly, General Electric Healthcare, Piramal Imaging, and the American College of Radiology. Dr. Rabinovici had no financial disclosures.
Transition to high-sensitivity troponin T assay helped boost ED discharge rates
SAN DIEGO – After transitioning to using the high-sensitivity troponin T assay for patients over the age of 35 years who presented to the ED with chest pain or tightness, an increased number of patients were discharged, yet no impact was observed on the time to admission and/or observation, according to a study conducted at two Philadelphia-based hospitals.
“Biomarkers have been a cornerstone of evaluating patients who present with chest pain,” Frederick T. Randolph, MD, said at the annual meeting of the American College of Emergency Physicians. “Recently in the United States, fifth-generation or high-sensitivity troponin [assays] have been approved for use. Our institution made the plan to transition to utilization of high-sensitivity troponins. At that time, we were aware of European studies that they were able to discharge a higher percentage of patients from the ED. There have been no prior studies of the clinical impact of transition to high-sensitivity troponin [assays] in the U.S.”
Dr. Randolph, director of the Chest Pain Center at Thomas Jefferson University, Philadelphia, and his colleagues performed a before and after study assessing all patients over age 35 years who presented to two hospitals with chest pain or tightness and who received a troponin assay draw in the ED. They conducted two town halls, in-services with cardiology and emergency medicine, and distributed a slide set to all providers. “There was no guidance as to who to order the troponin on,” said Dr. Randolph, who is also vice chief of emergency medicine at the university. “Essentially, the recommendation was, ‘Keep using troponins the same way you’ve been using troponins for years.’ The education was centered on how frequently we were going to order repeat troponins and how to interpret those results.”
The researchers used a 0- and 2-hour sampling strategy (unless the first value was less than 6 ng/L) and used a cut-point of 19 ng/L to define “ruled out” and a cut-point of 53 ng/L to define “consistent with acute myocardial infarction.” Indeterminate values were repeated every 2 hours. Dr. Randolph and his associates collected data for 1 year prior to transition to high-sensitivity troponins in 4,295 patients, and 2 months post transition in 769 patients. The primary outcome was discharge rate from the ED. Secondary outcome was time from presentation to admission/observation decision.
There were no statistically significant differences between the pre- and posttransition groups in the percentage of those aged 36-64 years (74% vs. 76%, respectively) or in the percentage of those who were nonwhite (61% vs. 59%). The only statistically significant difference was a higher percentage of females in the preimplementation group (49% vs. 46%; P = .02).
In all, the researchers were able to discharge 45.2% of all patients during the pretransition period, compared with 58.7% of patients in the posttransition period, for an absolute increase of 13% (P less than .001). There was no difference between the two groups in time to decision to admit or to observe (a mean of 199.1 vs. 192.2 minutes, respectively; P = .96). “Areas of future study would be to evaluate those patients who were discharged and monitor them for any changes in outcomes, to see if there is any increase in morbidity or complications,” said Dr. Randolph. “An additional research curiosity would be the downstream consequences on cost or revenue to the institution given the increased number of admissions and the decreased use of diagnostic testing such as coronary computed tomography angiography and stress testing.”
Dr. Randolph disclosed that some of the study authors have participated in multiple biomarker studies over the years. He reported having no financial disclosures.
Source: Randolph FT et al. Ann Emerg Med. 2018 Oct;72;4:S2-3. doi. 10.1016/j.annemergmed.2018.08.009.
SAN DIEGO – After transitioning to using the high-sensitivity troponin T assay for patients over the age of 35 years who presented to the ED with chest pain or tightness, an increased number of patients were discharged, yet no impact was observed on the time to admission and/or observation, according to a study conducted at two Philadelphia-based hospitals.
“Biomarkers have been a cornerstone of evaluating patients who present with chest pain,” Frederick T. Randolph, MD, said at the annual meeting of the American College of Emergency Physicians. “Recently in the United States, fifth-generation or high-sensitivity troponin [assays] have been approved for use. Our institution made the plan to transition to utilization of high-sensitivity troponins. At that time, we were aware of European studies that they were able to discharge a higher percentage of patients from the ED. There have been no prior studies of the clinical impact of transition to high-sensitivity troponin [assays] in the U.S.”
Dr. Randolph, director of the Chest Pain Center at Thomas Jefferson University, Philadelphia, and his colleagues performed a before and after study assessing all patients over age 35 years who presented to two hospitals with chest pain or tightness and who received a troponin assay draw in the ED. They conducted two town halls, in-services with cardiology and emergency medicine, and distributed a slide set to all providers. “There was no guidance as to who to order the troponin on,” said Dr. Randolph, who is also vice chief of emergency medicine at the university. “Essentially, the recommendation was, ‘Keep using troponins the same way you’ve been using troponins for years.’ The education was centered on how frequently we were going to order repeat troponins and how to interpret those results.”
The researchers used a 0- and 2-hour sampling strategy (unless the first value was less than 6 ng/L) and used a cut-point of 19 ng/L to define “ruled out” and a cut-point of 53 ng/L to define “consistent with acute myocardial infarction.” Indeterminate values were repeated every 2 hours. Dr. Randolph and his associates collected data for 1 year prior to transition to high-sensitivity troponins in 4,295 patients, and 2 months post transition in 769 patients. The primary outcome was discharge rate from the ED. Secondary outcome was time from presentation to admission/observation decision.
There were no statistically significant differences between the pre- and posttransition groups in the percentage of those aged 36-64 years (74% vs. 76%, respectively) or in the percentage of those who were nonwhite (61% vs. 59%). The only statistically significant difference was a higher percentage of females in the preimplementation group (49% vs. 46%; P = .02).
In all, the researchers were able to discharge 45.2% of all patients during the pretransition period, compared with 58.7% of patients in the posttransition period, for an absolute increase of 13% (P less than .001). There was no difference between the two groups in time to decision to admit or to observe (a mean of 199.1 vs. 192.2 minutes, respectively; P = .96). “Areas of future study would be to evaluate those patients who were discharged and monitor them for any changes in outcomes, to see if there is any increase in morbidity or complications,” said Dr. Randolph. “An additional research curiosity would be the downstream consequences on cost or revenue to the institution given the increased number of admissions and the decreased use of diagnostic testing such as coronary computed tomography angiography and stress testing.”
Dr. Randolph disclosed that some of the study authors have participated in multiple biomarker studies over the years. He reported having no financial disclosures.
Source: Randolph FT et al. Ann Emerg Med. 2018 Oct;72;4:S2-3. doi. 10.1016/j.annemergmed.2018.08.009.
SAN DIEGO – After transitioning to using the high-sensitivity troponin T assay for patients over the age of 35 years who presented to the ED with chest pain or tightness, an increased number of patients were discharged, yet no impact was observed on the time to admission and/or observation, according to a study conducted at two Philadelphia-based hospitals.
“Biomarkers have been a cornerstone of evaluating patients who present with chest pain,” Frederick T. Randolph, MD, said at the annual meeting of the American College of Emergency Physicians. “Recently in the United States, fifth-generation or high-sensitivity troponin [assays] have been approved for use. Our institution made the plan to transition to utilization of high-sensitivity troponins. At that time, we were aware of European studies that they were able to discharge a higher percentage of patients from the ED. There have been no prior studies of the clinical impact of transition to high-sensitivity troponin [assays] in the U.S.”
Dr. Randolph, director of the Chest Pain Center at Thomas Jefferson University, Philadelphia, and his colleagues performed a before and after study assessing all patients over age 35 years who presented to two hospitals with chest pain or tightness and who received a troponin assay draw in the ED. They conducted two town halls, in-services with cardiology and emergency medicine, and distributed a slide set to all providers. “There was no guidance as to who to order the troponin on,” said Dr. Randolph, who is also vice chief of emergency medicine at the university. “Essentially, the recommendation was, ‘Keep using troponins the same way you’ve been using troponins for years.’ The education was centered on how frequently we were going to order repeat troponins and how to interpret those results.”
The researchers used a 0- and 2-hour sampling strategy (unless the first value was less than 6 ng/L) and used a cut-point of 19 ng/L to define “ruled out” and a cut-point of 53 ng/L to define “consistent with acute myocardial infarction.” Indeterminate values were repeated every 2 hours. Dr. Randolph and his associates collected data for 1 year prior to transition to high-sensitivity troponins in 4,295 patients, and 2 months post transition in 769 patients. The primary outcome was discharge rate from the ED. Secondary outcome was time from presentation to admission/observation decision.
There were no statistically significant differences between the pre- and posttransition groups in the percentage of those aged 36-64 years (74% vs. 76%, respectively) or in the percentage of those who were nonwhite (61% vs. 59%). The only statistically significant difference was a higher percentage of females in the preimplementation group (49% vs. 46%; P = .02).
In all, the researchers were able to discharge 45.2% of all patients during the pretransition period, compared with 58.7% of patients in the posttransition period, for an absolute increase of 13% (P less than .001). There was no difference between the two groups in time to decision to admit or to observe (a mean of 199.1 vs. 192.2 minutes, respectively; P = .96). “Areas of future study would be to evaluate those patients who were discharged and monitor them for any changes in outcomes, to see if there is any increase in morbidity or complications,” said Dr. Randolph. “An additional research curiosity would be the downstream consequences on cost or revenue to the institution given the increased number of admissions and the decreased use of diagnostic testing such as coronary computed tomography angiography and stress testing.”
Dr. Randolph disclosed that some of the study authors have participated in multiple biomarker studies over the years. He reported having no financial disclosures.
Source: Randolph FT et al. Ann Emerg Med. 2018 Oct;72;4:S2-3. doi. 10.1016/j.annemergmed.2018.08.009.
AT ACEP18
Key clinical point: .
Major finding: After conversion to using the high-sensitivity troponin T assay, the ED discharge rate increased to 58.7%, from 45.2% (P less than 0.001).
Study details: A before and after study of 5,064 patients aged 35 and older who presented to the ED with chest pain or tightness.
Disclosures: Dr. Randolph disclosed that some of the study authors have participated in multiple biomarker studies over the years. He reported having no financial disclosures.
Source: Randolph FT et al. Ann Emerg Med. 2018 Oct;72;4:S2-3. doi: 10.1016/j.annemergmed.2018.08.009.
Serlopitant quells prurigo nodularis in phase 2 study
PARIS – The in a 127-patient, randomized, placebo-controlled trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She used her speaker’s platform not only to present key outcomes of the phase 2 study, but also to explain some of the underappreciated aspects of prurigo nodularis and how the results shed new light on the pathogenesis of the disease.
“There is a knowledge gap regarding this disease,” observed Dr. Stander, professor of dermatology at the University of Munster (Germany).
Prurigo nodularis is a chronic skin condition characterized by numerous intensely pruritic, symmetrically distributed, erosive nodular lesions. As Dr. Stander and her fellow members of the EADV Task Force Pruritus recently reported, prurigo nodularis should be considered as one of the clinical manifestations of chronic prurigo, hallmarks of which include neuronal sensitization to itch and development of an itch-scratch cycle (J Eur Acad Dermatol Venereol. 2018 Jul;32[7]:1059-65). At present, there is no approved treatment for prurigo nodularis in the United States or Europe.
Despite its name, prurigo nodularis is not just about the itch. Additional symptoms were frequently reported by participants in the serlopitant study. Indeed, while 97% of participants reported experiencing devilish pruritus, 52% reported a burning sensation on involved skin, 41% said the lesions were painful, and 36% indicated they were plagued by a stinging sensation. All of these disturbing sensations were significantly reduced with 8 weeks of serlopitant in the trial.
The clinical implications of these baseline findings are clear: “We have to ask not only about itch, but also about pain and burning and stinging, which are really an issue with these patients,” the dermatologist said.
Dr. Stander noted that prurigo nodularis has a large negative impact on quality of life as reflected in study participants’ mean baseline Dermatology Life Quality Index score of 61. And it’s a disease that sticks around: At enrollment, 38% of subjects had a 1- to 5-year history of prurigo nodularis, 20% had had the disease for 5-10 years, and fully 29% had had prurigo nodularis for longer than 10 years.
“This is not a chronic relapsing disease. These patients had prurigo nodularis all the time,” Dr. Stander said.
Consistent with the findings of other studies, half of patients with prurigo nodularis had an atopic predisposition as defined by an Erlangen Atopy Questionnaire score of 10 or higher.
Patients in the phase 2 study were randomized to either a 15-mg loading dose of oral serlopitant followed by 5 mg/day for 8 weeks or to placebo. All four of the most common manifestations of prurigo nodularis – itching, burning, pain, and stinging – were reduced to a significantly greater extent in the serlopitant group than with placebo. For example, the proportion of serlopitant-treated patients who rated their itching as none or mild went from zero at baseline to 54% at week 8, as compared with 26% among placebo-treated controls. After 8 weeks, 46% of serlopitant-treated patients rated their itching as moderate, severe, or very severe, as did 71% of controls.
Similarly, the proportion of patients in the serlopitant group who reported no or only a mild burning sensation climbed from 41% at baseline to 73% after 8 weeks. In the control group, the proportion improved from 36% to 47% over the course of 8 weeks. At week 8, 27% of patients on serlopitant characterized themselves as experiencing a moderate, severe, or very severe burning sensation; the rate was twice as high in controls.
Although the stinging sensation associated with prurigo nodularis was significantly reduced by serlopitant, the effect was less robust than with the other common sensory expressions of the disease. The proportion of patients with no or mild stinging increased by an absolute 23% after 8 weeks on serlopitant and by 6% with placebo. At week 8, 22% of patients treated with the neurokinin-1 receptor antagonist complained of a moderate to very severe stinging sensation, as did 40% of controls.
As a neurokinin-1 receptor antagonist, serlopitant inhibits substance P. The drug’s efficacy suggests that substance P plays a role in inducing the itch and other sensory symptoms of prurigo nodularis, according to Dr. Stander.
Based upon the favorable phase 2 results, multiple phase 3 clinical trials of serlopitant for prurigo nodularis are underway in the United States and elsewhere, including a year-long safety study.
Serlopitant is being developed by Menlo Therapeutics. Dr. Stander reported receiving research funding from and serving as a consultant to the company.
PARIS – The in a 127-patient, randomized, placebo-controlled trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She used her speaker’s platform not only to present key outcomes of the phase 2 study, but also to explain some of the underappreciated aspects of prurigo nodularis and how the results shed new light on the pathogenesis of the disease.
“There is a knowledge gap regarding this disease,” observed Dr. Stander, professor of dermatology at the University of Munster (Germany).
Prurigo nodularis is a chronic skin condition characterized by numerous intensely pruritic, symmetrically distributed, erosive nodular lesions. As Dr. Stander and her fellow members of the EADV Task Force Pruritus recently reported, prurigo nodularis should be considered as one of the clinical manifestations of chronic prurigo, hallmarks of which include neuronal sensitization to itch and development of an itch-scratch cycle (J Eur Acad Dermatol Venereol. 2018 Jul;32[7]:1059-65). At present, there is no approved treatment for prurigo nodularis in the United States or Europe.
Despite its name, prurigo nodularis is not just about the itch. Additional symptoms were frequently reported by participants in the serlopitant study. Indeed, while 97% of participants reported experiencing devilish pruritus, 52% reported a burning sensation on involved skin, 41% said the lesions were painful, and 36% indicated they were plagued by a stinging sensation. All of these disturbing sensations were significantly reduced with 8 weeks of serlopitant in the trial.
The clinical implications of these baseline findings are clear: “We have to ask not only about itch, but also about pain and burning and stinging, which are really an issue with these patients,” the dermatologist said.
Dr. Stander noted that prurigo nodularis has a large negative impact on quality of life as reflected in study participants’ mean baseline Dermatology Life Quality Index score of 61. And it’s a disease that sticks around: At enrollment, 38% of subjects had a 1- to 5-year history of prurigo nodularis, 20% had had the disease for 5-10 years, and fully 29% had had prurigo nodularis for longer than 10 years.
“This is not a chronic relapsing disease. These patients had prurigo nodularis all the time,” Dr. Stander said.
Consistent with the findings of other studies, half of patients with prurigo nodularis had an atopic predisposition as defined by an Erlangen Atopy Questionnaire score of 10 or higher.
Patients in the phase 2 study were randomized to either a 15-mg loading dose of oral serlopitant followed by 5 mg/day for 8 weeks or to placebo. All four of the most common manifestations of prurigo nodularis – itching, burning, pain, and stinging – were reduced to a significantly greater extent in the serlopitant group than with placebo. For example, the proportion of serlopitant-treated patients who rated their itching as none or mild went from zero at baseline to 54% at week 8, as compared with 26% among placebo-treated controls. After 8 weeks, 46% of serlopitant-treated patients rated their itching as moderate, severe, or very severe, as did 71% of controls.
Similarly, the proportion of patients in the serlopitant group who reported no or only a mild burning sensation climbed from 41% at baseline to 73% after 8 weeks. In the control group, the proportion improved from 36% to 47% over the course of 8 weeks. At week 8, 27% of patients on serlopitant characterized themselves as experiencing a moderate, severe, or very severe burning sensation; the rate was twice as high in controls.
Although the stinging sensation associated with prurigo nodularis was significantly reduced by serlopitant, the effect was less robust than with the other common sensory expressions of the disease. The proportion of patients with no or mild stinging increased by an absolute 23% after 8 weeks on serlopitant and by 6% with placebo. At week 8, 22% of patients treated with the neurokinin-1 receptor antagonist complained of a moderate to very severe stinging sensation, as did 40% of controls.
As a neurokinin-1 receptor antagonist, serlopitant inhibits substance P. The drug’s efficacy suggests that substance P plays a role in inducing the itch and other sensory symptoms of prurigo nodularis, according to Dr. Stander.
Based upon the favorable phase 2 results, multiple phase 3 clinical trials of serlopitant for prurigo nodularis are underway in the United States and elsewhere, including a year-long safety study.
Serlopitant is being developed by Menlo Therapeutics. Dr. Stander reported receiving research funding from and serving as a consultant to the company.
PARIS – The in a 127-patient, randomized, placebo-controlled trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She used her speaker’s platform not only to present key outcomes of the phase 2 study, but also to explain some of the underappreciated aspects of prurigo nodularis and how the results shed new light on the pathogenesis of the disease.
“There is a knowledge gap regarding this disease,” observed Dr. Stander, professor of dermatology at the University of Munster (Germany).
Prurigo nodularis is a chronic skin condition characterized by numerous intensely pruritic, symmetrically distributed, erosive nodular lesions. As Dr. Stander and her fellow members of the EADV Task Force Pruritus recently reported, prurigo nodularis should be considered as one of the clinical manifestations of chronic prurigo, hallmarks of which include neuronal sensitization to itch and development of an itch-scratch cycle (J Eur Acad Dermatol Venereol. 2018 Jul;32[7]:1059-65). At present, there is no approved treatment for prurigo nodularis in the United States or Europe.
Despite its name, prurigo nodularis is not just about the itch. Additional symptoms were frequently reported by participants in the serlopitant study. Indeed, while 97% of participants reported experiencing devilish pruritus, 52% reported a burning sensation on involved skin, 41% said the lesions were painful, and 36% indicated they were plagued by a stinging sensation. All of these disturbing sensations were significantly reduced with 8 weeks of serlopitant in the trial.
The clinical implications of these baseline findings are clear: “We have to ask not only about itch, but also about pain and burning and stinging, which are really an issue with these patients,” the dermatologist said.
Dr. Stander noted that prurigo nodularis has a large negative impact on quality of life as reflected in study participants’ mean baseline Dermatology Life Quality Index score of 61. And it’s a disease that sticks around: At enrollment, 38% of subjects had a 1- to 5-year history of prurigo nodularis, 20% had had the disease for 5-10 years, and fully 29% had had prurigo nodularis for longer than 10 years.
“This is not a chronic relapsing disease. These patients had prurigo nodularis all the time,” Dr. Stander said.
Consistent with the findings of other studies, half of patients with prurigo nodularis had an atopic predisposition as defined by an Erlangen Atopy Questionnaire score of 10 or higher.
Patients in the phase 2 study were randomized to either a 15-mg loading dose of oral serlopitant followed by 5 mg/day for 8 weeks or to placebo. All four of the most common manifestations of prurigo nodularis – itching, burning, pain, and stinging – were reduced to a significantly greater extent in the serlopitant group than with placebo. For example, the proportion of serlopitant-treated patients who rated their itching as none or mild went from zero at baseline to 54% at week 8, as compared with 26% among placebo-treated controls. After 8 weeks, 46% of serlopitant-treated patients rated their itching as moderate, severe, or very severe, as did 71% of controls.
Similarly, the proportion of patients in the serlopitant group who reported no or only a mild burning sensation climbed from 41% at baseline to 73% after 8 weeks. In the control group, the proportion improved from 36% to 47% over the course of 8 weeks. At week 8, 27% of patients on serlopitant characterized themselves as experiencing a moderate, severe, or very severe burning sensation; the rate was twice as high in controls.
Although the stinging sensation associated with prurigo nodularis was significantly reduced by serlopitant, the effect was less robust than with the other common sensory expressions of the disease. The proportion of patients with no or mild stinging increased by an absolute 23% after 8 weeks on serlopitant and by 6% with placebo. At week 8, 22% of patients treated with the neurokinin-1 receptor antagonist complained of a moderate to very severe stinging sensation, as did 40% of controls.
As a neurokinin-1 receptor antagonist, serlopitant inhibits substance P. The drug’s efficacy suggests that substance P plays a role in inducing the itch and other sensory symptoms of prurigo nodularis, according to Dr. Stander.
Based upon the favorable phase 2 results, multiple phase 3 clinical trials of serlopitant for prurigo nodularis are underway in the United States and elsewhere, including a year-long safety study.
Serlopitant is being developed by Menlo Therapeutics. Dr. Stander reported receiving research funding from and serving as a consultant to the company.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Serlopitant decreases the disruptive itch, burning, pain, and stinging of prurigo nodularis.
Major finding: The proportion of prurigo nodularis patients who rated their itching as none or mild went from zero at baseline to 54% after 8 weeks on oral serlopitant.
Study details: This 8-week, randomized, double-blind phase 2 study included 127 patients with prurigo nodularis who were randomized to oral serlopitant or placebo.
Disclosures: Serlopitant is being developed by Menlo Therapeutics. The presenter reported receiving research funding from and serving as a consultant to the company.
Collaborative approach for suicidal youth helps providers, too
SEATTLE – When it comes to treating children and adolescents who have experienced trauma and present suicidality, it is not just the patients who need support. Clinicians also experience grave anxiety when dealing with a traumatized child exhibiting suicidal behavior. The Collaborative Assessment and Management of Suicide (CAMS) framework can help clinicians or health care workers manage the care of these challenging patients.
CAMS is well established in adults with suicidal behavior, but it is unproven in youth and adolescents. The key is to its success in younger patients will be whether the program is developmentally appropriate, according to Molly C. Adrian, PhD, assistant professor of psychiatry and behavioral medicine at the University of Washington, Seattle. Dr. Adrian discussed CAMS and its potential applications at the annual meeting of the American Academy of Child and Adolescent Psychiatry.
The CAMS approach emphasizes cooperation between the therapist and patient. “Adolescents are seeking autonomy and independence, and so it’s a good fit philosophically that you would partner alongside the teen as opposed to starting in a more adversarial sort of way – not that we as therapists ever do that. But it can be more tense when suicide is on the table and you feel unprepared,” Dr. Adrian said.
Seattle Children’s Hospital is persuaded enough to make CAMS part of its standard of care, Dr. Adrian said.
CAMS is a framework for patient management that has no prerequisites for the therapeutic approach. “It’s principle driven, not protocol driven. Clinicians can use whatever specific interventions they feel are appropriate for the drivers (of the suicidality). What we’re trying to change is the approach to the suicidal patient, so that it is not an anxiety-provoking, terrifying experience for the clinician – because we know that a suicidal patient is the most anxiety-provoking task for clinicians,” Dr. Adrian said. “We want clinicians to feel prepared and protected in providing the elements of care,” Dr. Adrian said in an interview.
The framework incorporates a suicide-status form (SSF), which assesses theory-driven and epidemiologically guided risk factors and helps to identify the drivers or the reasons why suicide is compelling to the patient. Those drivers then help inform crisis prevention efforts and the selection of interventions.
Three randomized, controlled trials have demonstrated the efficacy of CAMS over standard of care in adult patients, showing that the SSF is an effective assessment tool and that CAMS reduces suicidal ideation and leads to decreases in distress, depression, and hopelessness. There are no randomized, controlled trials showing its efficacy in children, but Seattle Children’s Hospital is conducting a pilot study in 12 youth and have found a 40% response rate at 8 weeks.
The SSF gauges the patient’s status and trajectory, while the CAMS therapeutic worksheet helps to distinguish direct and indirect drivers of suicidal behavior. In cases in which trauma symptoms or experiences are tied to suicidal behavior, they receive priority for treatment. The framework provides for discussion of options and treatment choice through collaboration between the clinician and the patient.
The patient and clinician fill out an SSF in the first session and use it to create a crisis convention plan, which includes gaining a commitment to treatment, removing or restricting access to lethal means, and incorporating parental monitoring.
Dr. Adrian is hopeful that the CAMS framework will help health care workers address suicidality in traumatized youth and adolescents. Currently, they may feel intimidated by a stricken child’s issues. “You don’t want to be responsible for a child dying, so you may overrespond with an intervention like an emergency department evaluation or an inpatient hospitalization that may be iatrogenic – there are data coming out from adults that hospitalization contributes to suicide risk above and beyond other risk factors,” Dr. Adrian said.
CAMS is simple and flexible, according to Dr. Adrian, and can be used by psychiatrists, social workers, substance use counselors, and others. At Seattle Children’s Hospital, clinicians have embraced it. “They feel it changes their practice. It gets to the heart of the matter quickly, and they feel more confident having that framework,” Dr. Adrian said.
Dr. Adrian has no conflicts of interest or disclosures.
SEATTLE – When it comes to treating children and adolescents who have experienced trauma and present suicidality, it is not just the patients who need support. Clinicians also experience grave anxiety when dealing with a traumatized child exhibiting suicidal behavior. The Collaborative Assessment and Management of Suicide (CAMS) framework can help clinicians or health care workers manage the care of these challenging patients.
CAMS is well established in adults with suicidal behavior, but it is unproven in youth and adolescents. The key is to its success in younger patients will be whether the program is developmentally appropriate, according to Molly C. Adrian, PhD, assistant professor of psychiatry and behavioral medicine at the University of Washington, Seattle. Dr. Adrian discussed CAMS and its potential applications at the annual meeting of the American Academy of Child and Adolescent Psychiatry.
The CAMS approach emphasizes cooperation between the therapist and patient. “Adolescents are seeking autonomy and independence, and so it’s a good fit philosophically that you would partner alongside the teen as opposed to starting in a more adversarial sort of way – not that we as therapists ever do that. But it can be more tense when suicide is on the table and you feel unprepared,” Dr. Adrian said.
Seattle Children’s Hospital is persuaded enough to make CAMS part of its standard of care, Dr. Adrian said.
CAMS is a framework for patient management that has no prerequisites for the therapeutic approach. “It’s principle driven, not protocol driven. Clinicians can use whatever specific interventions they feel are appropriate for the drivers (of the suicidality). What we’re trying to change is the approach to the suicidal patient, so that it is not an anxiety-provoking, terrifying experience for the clinician – because we know that a suicidal patient is the most anxiety-provoking task for clinicians,” Dr. Adrian said. “We want clinicians to feel prepared and protected in providing the elements of care,” Dr. Adrian said in an interview.
The framework incorporates a suicide-status form (SSF), which assesses theory-driven and epidemiologically guided risk factors and helps to identify the drivers or the reasons why suicide is compelling to the patient. Those drivers then help inform crisis prevention efforts and the selection of interventions.
Three randomized, controlled trials have demonstrated the efficacy of CAMS over standard of care in adult patients, showing that the SSF is an effective assessment tool and that CAMS reduces suicidal ideation and leads to decreases in distress, depression, and hopelessness. There are no randomized, controlled trials showing its efficacy in children, but Seattle Children’s Hospital is conducting a pilot study in 12 youth and have found a 40% response rate at 8 weeks.
The SSF gauges the patient’s status and trajectory, while the CAMS therapeutic worksheet helps to distinguish direct and indirect drivers of suicidal behavior. In cases in which trauma symptoms or experiences are tied to suicidal behavior, they receive priority for treatment. The framework provides for discussion of options and treatment choice through collaboration between the clinician and the patient.
The patient and clinician fill out an SSF in the first session and use it to create a crisis convention plan, which includes gaining a commitment to treatment, removing or restricting access to lethal means, and incorporating parental monitoring.
Dr. Adrian is hopeful that the CAMS framework will help health care workers address suicidality in traumatized youth and adolescents. Currently, they may feel intimidated by a stricken child’s issues. “You don’t want to be responsible for a child dying, so you may overrespond with an intervention like an emergency department evaluation or an inpatient hospitalization that may be iatrogenic – there are data coming out from adults that hospitalization contributes to suicide risk above and beyond other risk factors,” Dr. Adrian said.
CAMS is simple and flexible, according to Dr. Adrian, and can be used by psychiatrists, social workers, substance use counselors, and others. At Seattle Children’s Hospital, clinicians have embraced it. “They feel it changes their practice. It gets to the heart of the matter quickly, and they feel more confident having that framework,” Dr. Adrian said.
Dr. Adrian has no conflicts of interest or disclosures.
SEATTLE – When it comes to treating children and adolescents who have experienced trauma and present suicidality, it is not just the patients who need support. Clinicians also experience grave anxiety when dealing with a traumatized child exhibiting suicidal behavior. The Collaborative Assessment and Management of Suicide (CAMS) framework can help clinicians or health care workers manage the care of these challenging patients.
CAMS is well established in adults with suicidal behavior, but it is unproven in youth and adolescents. The key is to its success in younger patients will be whether the program is developmentally appropriate, according to Molly C. Adrian, PhD, assistant professor of psychiatry and behavioral medicine at the University of Washington, Seattle. Dr. Adrian discussed CAMS and its potential applications at the annual meeting of the American Academy of Child and Adolescent Psychiatry.
The CAMS approach emphasizes cooperation between the therapist and patient. “Adolescents are seeking autonomy and independence, and so it’s a good fit philosophically that you would partner alongside the teen as opposed to starting in a more adversarial sort of way – not that we as therapists ever do that. But it can be more tense when suicide is on the table and you feel unprepared,” Dr. Adrian said.
Seattle Children’s Hospital is persuaded enough to make CAMS part of its standard of care, Dr. Adrian said.
CAMS is a framework for patient management that has no prerequisites for the therapeutic approach. “It’s principle driven, not protocol driven. Clinicians can use whatever specific interventions they feel are appropriate for the drivers (of the suicidality). What we’re trying to change is the approach to the suicidal patient, so that it is not an anxiety-provoking, terrifying experience for the clinician – because we know that a suicidal patient is the most anxiety-provoking task for clinicians,” Dr. Adrian said. “We want clinicians to feel prepared and protected in providing the elements of care,” Dr. Adrian said in an interview.
The framework incorporates a suicide-status form (SSF), which assesses theory-driven and epidemiologically guided risk factors and helps to identify the drivers or the reasons why suicide is compelling to the patient. Those drivers then help inform crisis prevention efforts and the selection of interventions.
Three randomized, controlled trials have demonstrated the efficacy of CAMS over standard of care in adult patients, showing that the SSF is an effective assessment tool and that CAMS reduces suicidal ideation and leads to decreases in distress, depression, and hopelessness. There are no randomized, controlled trials showing its efficacy in children, but Seattle Children’s Hospital is conducting a pilot study in 12 youth and have found a 40% response rate at 8 weeks.
The SSF gauges the patient’s status and trajectory, while the CAMS therapeutic worksheet helps to distinguish direct and indirect drivers of suicidal behavior. In cases in which trauma symptoms or experiences are tied to suicidal behavior, they receive priority for treatment. The framework provides for discussion of options and treatment choice through collaboration between the clinician and the patient.
The patient and clinician fill out an SSF in the first session and use it to create a crisis convention plan, which includes gaining a commitment to treatment, removing or restricting access to lethal means, and incorporating parental monitoring.
Dr. Adrian is hopeful that the CAMS framework will help health care workers address suicidality in traumatized youth and adolescents. Currently, they may feel intimidated by a stricken child’s issues. “You don’t want to be responsible for a child dying, so you may overrespond with an intervention like an emergency department evaluation or an inpatient hospitalization that may be iatrogenic – there are data coming out from adults that hospitalization contributes to suicide risk above and beyond other risk factors,” Dr. Adrian said.
CAMS is simple and flexible, according to Dr. Adrian, and can be used by psychiatrists, social workers, substance use counselors, and others. At Seattle Children’s Hospital, clinicians have embraced it. “They feel it changes their practice. It gets to the heart of the matter quickly, and they feel more confident having that framework,” Dr. Adrian said.
Dr. Adrian has no conflicts of interest or disclosures.
FROM AACAP 2018
Heather Yeo: Surgical Residency Attrition
When Dr. Yeo was a resident, one of her co-residents left surgery. Dr. Yeo says that this resident was highly talented technically and on-point clinically. After this resident left, Dr. Yeo began investigating this and looking into what can be done to keep surgical trainees in surgery.
Subscribe here:
When Dr. Yeo was a resident, one of her co-residents left surgery. Dr. Yeo says that this resident was highly talented technically and on-point clinically. After this resident left, Dr. Yeo began investigating this and looking into what can be done to keep surgical trainees in surgery.
Subscribe here:
When Dr. Yeo was a resident, one of her co-residents left surgery. Dr. Yeo says that this resident was highly talented technically and on-point clinically. After this resident left, Dr. Yeo began investigating this and looking into what can be done to keep surgical trainees in surgery.
Subscribe here:
Validation for SLE low-disease definition
Click here to listen to the MDedge Postcall Podcast.
Also today, if you see a swollen knee in a kid, remember: above 9, treat for lyme, FDA approves Xofluza for treatment of influenza, and there are still problems with APMs.
Amazon Alexa
Apple Podcasts
Spotify
Click here to listen to the MDedge Postcall Podcast.
Also today, if you see a swollen knee in a kid, remember: above 9, treat for lyme, FDA approves Xofluza for treatment of influenza, and there are still problems with APMs.
Amazon Alexa
Apple Podcasts
Spotify
Click here to listen to the MDedge Postcall Podcast.
Also today, if you see a swollen knee in a kid, remember: above 9, treat for lyme, FDA approves Xofluza for treatment of influenza, and there are still problems with APMs.
Amazon Alexa
Apple Podcasts
Spotify