User login
Team tracks changes in height, weight in pediatric ALL
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
New research suggests several factors may be associated with the risk of short stature and excess weight gain in children with acute lymphoblastic leukemia (ALL).
Researchers found that patients who were younger at ALL diagnosis had an increased risk of becoming overweight or obese both during and after therapy.
Patients had an increased risk of short stature after therapy if they were older at diagnosis or had standard or high-risk disease, higher white blood cell counts at diagnosis, and central nervous system disease.
The researchers reported these findings in Cancer.
The team looked at 372 children with ALL, reviewing changes in their body mass index (BMI), weight, and height from diagnosis to 5 years after treatment ended.
The patients were treated with the Total XV protocol between 2000 and 2007 (NCT00137111). They received 6 weeks of induction therapy, 8 weeks of consolidation, and continuation for 120 weeks in females and 146 weeks in males.
BMI changes
Roughly a quarter of patients were overweight or obese at diagnosis, but that increased to roughly half of patients by the time they had been off therapy for 5 years.
“Over the whole population that was studied, we found statistically significant weight gain even during remission-induction therapy,” said study author Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Patients’ median BMI z scores increased significantly during induction (P<0.001) and reinduction (P=0.001) with glucocorticoid therapy as well as in the first year after therapy ended (P=0.006).
At various points during treatment, there were significant differences in BMI z scores according to sex, race, and disease risk group. However, these differences were not present after therapy.
On the other hand, there were significant differences in BMI z scores according to age both during and after therapy.
Between week 21 of treatment and 3 years after therapy ended, patients who were ages 2 to 9 at diagnosis had median BMI z scores that were significantly higher than scores of patients who were age 10 or older at diagnosis (P≤0.033 for all time points).
The researchers also found that patients who were of a healthy weight or underweight at the time of diagnosis had a significantly higher risk of becoming overweight or obese during or after therapy if they were ages 2 to 9 at diagnosis, compared to the older patients (P=0.001).
Height changes
The researchers found that height z scores declined during treatment and improved after it ended, although z scores “never improved to the levels noted at the time of diagnosis.”
Median height z scores at the end of induction and in continuation weeks 1 to 21 were significantly higher in patients age 10 or older at diagnosis than in patients ages to 2 to 9 at diagnosis (P≤0.038 for all time points).
However, the median height z scores at 5 years off therapy were significantly higher for the younger patients than for the older patients (P=0.011).
The median height z scores were higher for patients with low-risk disease than for standard- or high-risk patients in weeks 17, 21, 48, and 146 of treatment and at 1 to 3 years after therapy ended (P≤0.024 for all time points).
At 3 years to 5 years after treatment ended, the median height z scores were significantly higher among patients with white blood cell counts below 50 × 109/L at diagnosis (P≤0.018 for all time points).
Patients without central nervous system disease had significantly higher median height z scores at 3 years after treatment ended (P=0.029).
Males had significantly higher median height z scores than females in weeks 96 and 120 of therapy (P≤0.009 for both time points).
And white patients had higher median height z scores than black patients at 2 to 4 years after treatment ended (P≤0.027 for all time points).
Implications
To address the issue of excess weight gain in ALL patients, the researchers suggested early interventions, such as education about proper diet and exercise.
“When you look at the literature of childhood obesity prevention for the general population, there are interventions that could also help ALL patients,” said study author Emily Browne, of St. Jude.
“But we need to adapt those recommendations to take the cancer therapy into account.”
For the issue of height, the researchers recommended evaluating certain patients for growth hormone deficiency.
The team also noted that further study is needed to determine whether emerging therapeutic approaches can reduce toxicities without compromising antileukemic effects.
“We are hoping new therapeutic options can decrease intensity of chemotherapy and keep normal tissues intact,” Dr. Inaba said. “But until then, we’re collaborating with multiple clinical departments to help ensure a good, quality cure and a good quality of life in survivorship.”
This research was supported by grants from the National Institutes of Health and ALSAC, the fundraising and awareness organization of St. Jude.
Primary renal synovial sarcoma – a diagnostic dilemma
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
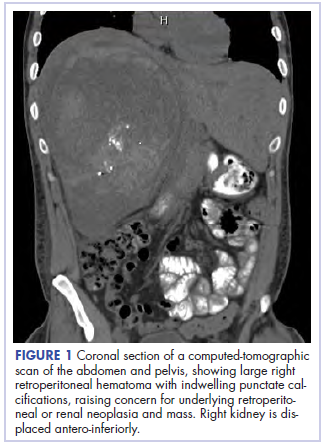
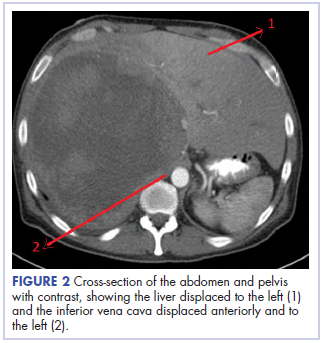
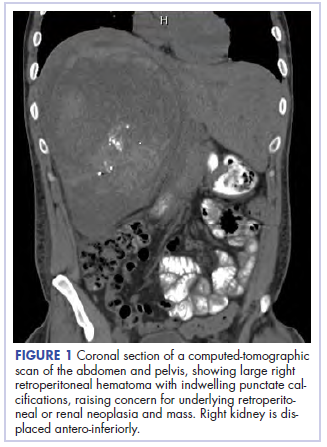
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
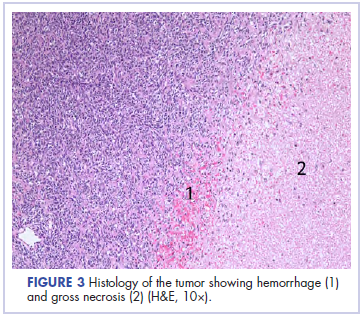
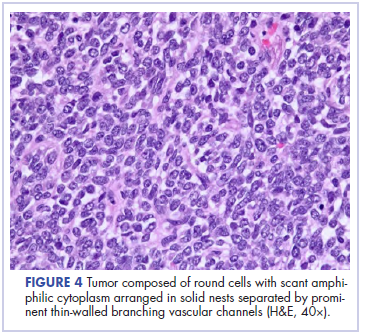
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
Marriage predicts for survival in patients with stage III non–small-cell lung cancer
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
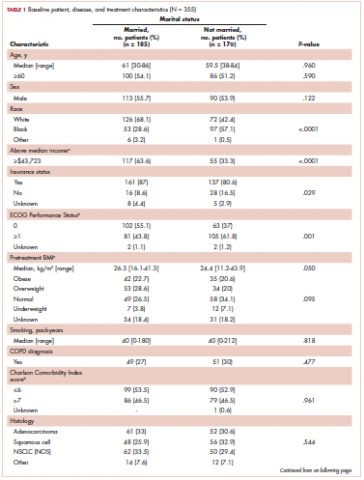
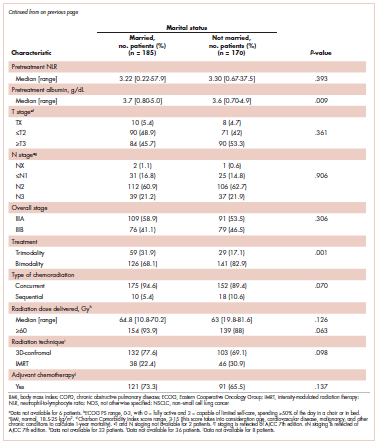
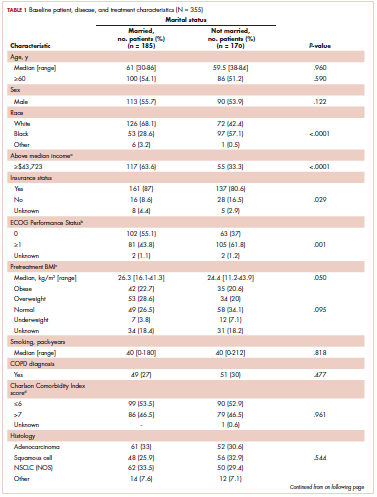
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
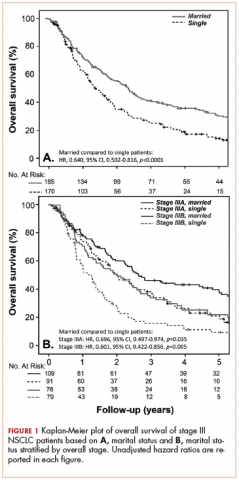
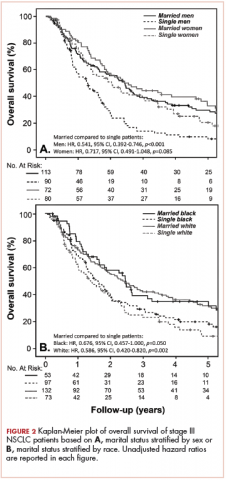
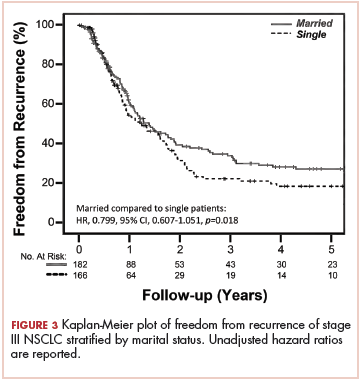
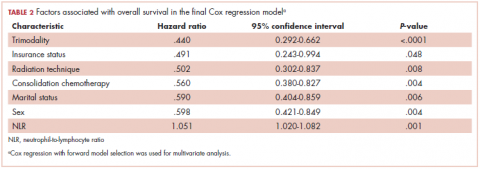
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
Effect of time of admission to treatment initiation on outcomes of patients with acute myeloid leukemia: a tertiary care referral center experience
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
Venetoclax approved to treat CLL patients regardless of genotype
The approval of Bcl-2 inhibitor venetoclax was expanded by the US Food and Drug Administration in June 2018 to include the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL), regardless of their genotype, who have received at least 1 prior therapy.1 It was previously approved in 2016 for the treatment of patients who had a chromosome 17p deletion, which leads to loss of the tumor-suppressor gene TP53.
Approval was based on the positive results of the phase 3, randomized, multicenter, open-label MURANO trial in which 389 patients were randomized 1:1 to receive a combination of venetoclax and the CD20-targeting monoclonal antibody rituximab (venetoclax–rituximab) or bendamustine in combination with rituximab (bendamustine–rituximab).
Eligible patients were 18 years of age or older, had been diagnosed with relapsed/refractory CLL that required treatment, had received 1-3 prior therapies (including at least 1 chemotherapy regimen), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (on a 5-point scale, with 5 indicating the greatest level of disability), and had adequate bone marrow, renal, and hepatic function.
Patients who had received prior bendamustine treatment were eligible for the trial provided they had experienced a duration of response of 24 months or longer. However, patients with transformed CLL, central nervous system involvement, prior treatment with allogeneic or autologous stem cell transplant, major organ dysfunction, other active malignancy, or who were pregnant or breastfeeding, were excluded from the study.
Patients in the venetoclax arm received a 5-week ramp-up schedule, followed by a dose of 400 mg once daily for 24 months. Rituximab treatment started at the end of the venetoclax ramp-up period and was administered at a dose of 375 mg/m2 intravenously on cycle 1 day 1 and 500 mg/m2 on day 1 of cycles 2-6. In the control arm, patients received 6 cycles with the same rituxima
The primary endpoint was progression-free survival (PFS), as assessed by an independent review committee over a median follow-up of 23 months. Median PFS was significantly improved in the venetoclax arm (not yet reached versus 18.1 months in the bendamustine arm [HR, 0.19; P < .001]). In addition, objective response rate (ORR) and event-free survival (EFS) also favored the venetoclax arm; ORR was 92% compared with 72%, respectively, and 2-year EFS was 84.9% compared with 34.8%. There was also a trend toward improved 24-month overall survival (OS) rate (91.9% vs 86.6%), however this did not achieve statistical significance, nor did median OS.
The most common adverse events (AEs) in patients treated with venetoclax were neutropenia, diarrhea, upper-respiratory tract infection, fatigue, cough, and nausea. Grade 3/4 neutropenia occurred in 64% of patients, and serious AEs in 46% of patients. Serious infections occurred in 21% of patients, most commonly pneumonia. Ten deaths in the venetoclax arm were attributed to treatment, compared with 11 deaths in the bendamustine arm.2
The prescribing information details warnings and precautions relating to the risk of tumor lysis syndrome, which is increased in patients with higher tumor burden, reduced renal function, or in receipt of strong or moderate CYP3A inhibitors or P-gp inhibitors during the ramp-up stage. Patients should receive appropriate preventive strategies, including hydration and antihyperuricemics, blood chemistry should be monitored and abnormalities managed promptly, and dosing should be interrupted or adjusted as necessary.
Other warnings relate to neutropenia (complete blood counts should be monitored throughout treatment and venetoclax treatment interrupted or dose reduced for severe neutropenia, alongside possible use of supportive measures), immunization (live vaccines should not be administered before or during treatment or after treatment until B-cell recovery, and patients should be advised of the potentially reduced efficacy of vaccines), and embryofetal toxicity (patients should be advised of the risks and the need for effective contraception during and after treatment). Venetoclax is marketed as Venclexta by Genentech.3
1. US Food and Drug Administration website. FDA approves venetoclax for CLL or SLL, with or without 17p deletion, after one prior therapy. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610308.htm. Last updated June 8, 2018. Accessed July 29, 2018.
2. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107-1120.
3. Venclexta (venetoclax tablets) for oral use. Prescribing information. Genentech USA, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Last updated June 2018. Accessed July 29, 2018.
The approval of Bcl-2 inhibitor venetoclax was expanded by the US Food and Drug Administration in June 2018 to include the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL), regardless of their genotype, who have received at least 1 prior therapy.1 It was previously approved in 2016 for the treatment of patients who had a chromosome 17p deletion, which leads to loss of the tumor-suppressor gene TP53.
Approval was based on the positive results of the phase 3, randomized, multicenter, open-label MURANO trial in which 389 patients were randomized 1:1 to receive a combination of venetoclax and the CD20-targeting monoclonal antibody rituximab (venetoclax–rituximab) or bendamustine in combination with rituximab (bendamustine–rituximab).
Eligible patients were 18 years of age or older, had been diagnosed with relapsed/refractory CLL that required treatment, had received 1-3 prior therapies (including at least 1 chemotherapy regimen), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (on a 5-point scale, with 5 indicating the greatest level of disability), and had adequate bone marrow, renal, and hepatic function.
Patients who had received prior bendamustine treatment were eligible for the trial provided they had experienced a duration of response of 24 months or longer. However, patients with transformed CLL, central nervous system involvement, prior treatment with allogeneic or autologous stem cell transplant, major organ dysfunction, other active malignancy, or who were pregnant or breastfeeding, were excluded from the study.
Patients in the venetoclax arm received a 5-week ramp-up schedule, followed by a dose of 400 mg once daily for 24 months. Rituximab treatment started at the end of the venetoclax ramp-up period and was administered at a dose of 375 mg/m2 intravenously on cycle 1 day 1 and 500 mg/m2 on day 1 of cycles 2-6. In the control arm, patients received 6 cycles with the same rituxima
The primary endpoint was progression-free survival (PFS), as assessed by an independent review committee over a median follow-up of 23 months. Median PFS was significantly improved in the venetoclax arm (not yet reached versus 18.1 months in the bendamustine arm [HR, 0.19; P < .001]). In addition, objective response rate (ORR) and event-free survival (EFS) also favored the venetoclax arm; ORR was 92% compared with 72%, respectively, and 2-year EFS was 84.9% compared with 34.8%. There was also a trend toward improved 24-month overall survival (OS) rate (91.9% vs 86.6%), however this did not achieve statistical significance, nor did median OS.
The most common adverse events (AEs) in patients treated with venetoclax were neutropenia, diarrhea, upper-respiratory tract infection, fatigue, cough, and nausea. Grade 3/4 neutropenia occurred in 64% of patients, and serious AEs in 46% of patients. Serious infections occurred in 21% of patients, most commonly pneumonia. Ten deaths in the venetoclax arm were attributed to treatment, compared with 11 deaths in the bendamustine arm.2
The prescribing information details warnings and precautions relating to the risk of tumor lysis syndrome, which is increased in patients with higher tumor burden, reduced renal function, or in receipt of strong or moderate CYP3A inhibitors or P-gp inhibitors during the ramp-up stage. Patients should receive appropriate preventive strategies, including hydration and antihyperuricemics, blood chemistry should be monitored and abnormalities managed promptly, and dosing should be interrupted or adjusted as necessary.
Other warnings relate to neutropenia (complete blood counts should be monitored throughout treatment and venetoclax treatment interrupted or dose reduced for severe neutropenia, alongside possible use of supportive measures), immunization (live vaccines should not be administered before or during treatment or after treatment until B-cell recovery, and patients should be advised of the potentially reduced efficacy of vaccines), and embryofetal toxicity (patients should be advised of the risks and the need for effective contraception during and after treatment). Venetoclax is marketed as Venclexta by Genentech.3
The approval of Bcl-2 inhibitor venetoclax was expanded by the US Food and Drug Administration in June 2018 to include the treatment of patients with chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL), regardless of their genotype, who have received at least 1 prior therapy.1 It was previously approved in 2016 for the treatment of patients who had a chromosome 17p deletion, which leads to loss of the tumor-suppressor gene TP53.
Approval was based on the positive results of the phase 3, randomized, multicenter, open-label MURANO trial in which 389 patients were randomized 1:1 to receive a combination of venetoclax and the CD20-targeting monoclonal antibody rituximab (venetoclax–rituximab) or bendamustine in combination with rituximab (bendamustine–rituximab).
Eligible patients were 18 years of age or older, had been diagnosed with relapsed/refractory CLL that required treatment, had received 1-3 prior therapies (including at least 1 chemotherapy regimen), had an Eastern Cooperative Oncology Group performance status of 0 or 1 (on a 5-point scale, with 5 indicating the greatest level of disability), and had adequate bone marrow, renal, and hepatic function.
Patients who had received prior bendamustine treatment were eligible for the trial provided they had experienced a duration of response of 24 months or longer. However, patients with transformed CLL, central nervous system involvement, prior treatment with allogeneic or autologous stem cell transplant, major organ dysfunction, other active malignancy, or who were pregnant or breastfeeding, were excluded from the study.
Patients in the venetoclax arm received a 5-week ramp-up schedule, followed by a dose of 400 mg once daily for 24 months. Rituximab treatment started at the end of the venetoclax ramp-up period and was administered at a dose of 375 mg/m2 intravenously on cycle 1 day 1 and 500 mg/m2 on day 1 of cycles 2-6. In the control arm, patients received 6 cycles with the same rituxima
The primary endpoint was progression-free survival (PFS), as assessed by an independent review committee over a median follow-up of 23 months. Median PFS was significantly improved in the venetoclax arm (not yet reached versus 18.1 months in the bendamustine arm [HR, 0.19; P < .001]). In addition, objective response rate (ORR) and event-free survival (EFS) also favored the venetoclax arm; ORR was 92% compared with 72%, respectively, and 2-year EFS was 84.9% compared with 34.8%. There was also a trend toward improved 24-month overall survival (OS) rate (91.9% vs 86.6%), however this did not achieve statistical significance, nor did median OS.
The most common adverse events (AEs) in patients treated with venetoclax were neutropenia, diarrhea, upper-respiratory tract infection, fatigue, cough, and nausea. Grade 3/4 neutropenia occurred in 64% of patients, and serious AEs in 46% of patients. Serious infections occurred in 21% of patients, most commonly pneumonia. Ten deaths in the venetoclax arm were attributed to treatment, compared with 11 deaths in the bendamustine arm.2
The prescribing information details warnings and precautions relating to the risk of tumor lysis syndrome, which is increased in patients with higher tumor burden, reduced renal function, or in receipt of strong or moderate CYP3A inhibitors or P-gp inhibitors during the ramp-up stage. Patients should receive appropriate preventive strategies, including hydration and antihyperuricemics, blood chemistry should be monitored and abnormalities managed promptly, and dosing should be interrupted or adjusted as necessary.
Other warnings relate to neutropenia (complete blood counts should be monitored throughout treatment and venetoclax treatment interrupted or dose reduced for severe neutropenia, alongside possible use of supportive measures), immunization (live vaccines should not be administered before or during treatment or after treatment until B-cell recovery, and patients should be advised of the potentially reduced efficacy of vaccines), and embryofetal toxicity (patients should be advised of the risks and the need for effective contraception during and after treatment). Venetoclax is marketed as Venclexta by Genentech.3
1. US Food and Drug Administration website. FDA approves venetoclax for CLL or SLL, with or without 17p deletion, after one prior therapy. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610308.htm. Last updated June 8, 2018. Accessed July 29, 2018.
2. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107-1120.
3. Venclexta (venetoclax tablets) for oral use. Prescribing information. Genentech USA, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Last updated June 2018. Accessed July 29, 2018.
1. US Food and Drug Administration website. FDA approves venetoclax for CLL or SLL, with or without 17p deletion, after one prior therapy. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610308.htm. Last updated June 8, 2018. Accessed July 29, 2018.
2. Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107-1120.
3. Venclexta (venetoclax tablets) for oral use. Prescribing information. Genentech USA, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Last updated June 2018. Accessed July 29, 2018.
Nivolumab and ipilimumab combination promises new standard of care for advanced RCC
In April 2018, the US Food and Drug Administration expanded the approval of the combination of nivolumab and ipilimumab into a new indication, following a previous approval in patients with metastatic melanoma. The double immune checkpoint inhibitor combination was approved on the basis of the phase 3 CheckMate-214 study for the treatment of patients with intermediate- or poor-risk, previously untreated advanced renal cell carcinoma (RCC).1
Nivolumab monotherapy is already approved in the second-line setting for the treatment of advanced RCC, and the demonstration of significantly improved overall survival (OS) in this study suggests that the combination should supplant sunitinib in the front-line setting in the treatment of this type of cancer.
A total of 1,096 patients at 175 sites in 28 countries were randomized 1:1 to receive nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) intravenously every 3 weeks for 4 doses in an induction phase, followed by nivolumab monotherapy (3 mg/kg) every 2 weeks in a maintenance phase or sunitinib (50 mg) orally daily for 4 weeks of each 6-week cycle.
Eligible patients were 18 years or older, had previously untreated advanced RCC with a clear-cell component, had measurable disease according to Response Evaluation Criteria in Solid Tumors (version 1.1), and had a Karnofsky performance status of at least 70 (on a scale from 0 to 100, with lower scores indicating greater disability). Patients with central nervous system metastases or autoimmune disease who were being treated with glucocorticoids and immunosuppressants were excluded from the study.
Around three-quarters of patients with advanced RCC have intermediate- or poor-risk disease and experience worse outcomes than patients with favorable-risk disease. Patients in CheckMate-214 were stratified according to International Metastatic Renal Cell Carcinoma Database Consortium risk score as favorable (score of 0), intermediate (score of 1 or 2) or poor risk (score of 3-6), according to the number of risk factors present.
Risk factors included a Karnofsky performance score of 70, time from initial diagnosis to randomization of <1 year, a hemoglobin level below the lower limit of normal, a corrected serum calcium concentration of >10 mg/dL, or an absolute neutrophil count or platelet count above the upper limit of normal. Patients were also stratified according to geographic region (United States versus Canada and Europe versus the rest of the world).
The coprimary endpoints were objective response rate (ORR), progression-free survival (PFS), and OS in a subset of 847 intermediate- and poor-risk patients. Over a median follow-up of 25.2 months, there was a statistically significant improvement in OS and ORR in patients treated with nivolumab and ipilimumab (mPFS not reached; ORR, 41.6%), compared with sunitinib (OS, 25.9 months; ORR, 26.5%), with P <.001 for both. The immunotherapy combination was favored across subgroups.
The most common adverse events (AEs) in patients treated with nivolumab and ipilimumab included fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, and decreased appetite. The combination was associated with fewer grade 3/4 AEs (63% vs 46% for sunitinib), but a higher rate of treatment discontinuations because of AEs (31% vs 21%, respectively). There were 8 deaths in the combination arm, and 4 in the sunitinib arm that were reported to be treatment related.2
The warnings and precautions related to nivolumab–ipilimumab combination therapy outlined in the prescribing information include mostly immune-mediated AEs, such as immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, nephritis and renal dysfunction, skin adverse reactions, and encephalitis. There are also warnings relating to the risk of infusion reactions and the potential for embryofetal toxicity.
Patients should be monitored for hyperglycemia and for changes in liver, thyroid, renal, and neurologic function. Treatment with nivolumab and ipilimumab should be withheld for moderate and permanently discontinued for severe or life-threatening immune-mediated pneumonitis, colitis, and hepatitis, as well as transaminase or total bilirubin elevation. It should also be withheld for moderate or severe hypophysitis and serum creatinine elevation, moderate adrenal insufficiency and severe hyperglycemia, and permanently discontinued for life-threatening hypophysitis and serum creatinine elevation, severe or life-threatening adrenal insufficiency, and life-threatening hyperglycemia.
New-onset moderate to severe neurologic signs or symptoms warrant treatment being withheld, and immune-mediated encephalitis should lead to treatment discontinuation. For mild or moderate infusion reactions, the infusion rate can be slowed or interrupted, and infusions should be discontinued in the event of severe or life-threatening infusion reactions. Patients should be advised of the potential for fetal harm and the need for effective contraception during and after treatment. Ipilimumab and nivolumab are marketed as Yervoy and Opdivo, respectively, by Bristol-Myers Squibb.3,4
1. US Food and Drug Administration website. FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm604685.htm. Last updated April 16, 2018. Accessed July 25, 2018.
2. Motzer RJ, Tannir NM, McDermott O, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277-1290.
3. Opdivo (nivolumab) injection, for intravenous use. Prescribing information. Bristol-Myers Squibb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf. Revised April 2018.
4. Yervoy (ipilimumab) injection, for intravenous use. Prescribing information. Bristol-Myers Squibb. July 2018. https://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed September, 2018.
In April 2018, the US Food and Drug Administration expanded the approval of the combination of nivolumab and ipilimumab into a new indication, following a previous approval in patients with metastatic melanoma. The double immune checkpoint inhibitor combination was approved on the basis of the phase 3 CheckMate-214 study for the treatment of patients with intermediate- or poor-risk, previously untreated advanced renal cell carcinoma (RCC).1
Nivolumab monotherapy is already approved in the second-line setting for the treatment of advanced RCC, and the demonstration of significantly improved overall survival (OS) in this study suggests that the combination should supplant sunitinib in the front-line setting in the treatment of this type of cancer.
A total of 1,096 patients at 175 sites in 28 countries were randomized 1:1 to receive nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) intravenously every 3 weeks for 4 doses in an induction phase, followed by nivolumab monotherapy (3 mg/kg) every 2 weeks in a maintenance phase or sunitinib (50 mg) orally daily for 4 weeks of each 6-week cycle.
Eligible patients were 18 years or older, had previously untreated advanced RCC with a clear-cell component, had measurable disease according to Response Evaluation Criteria in Solid Tumors (version 1.1), and had a Karnofsky performance status of at least 70 (on a scale from 0 to 100, with lower scores indicating greater disability). Patients with central nervous system metastases or autoimmune disease who were being treated with glucocorticoids and immunosuppressants were excluded from the study.
Around three-quarters of patients with advanced RCC have intermediate- or poor-risk disease and experience worse outcomes than patients with favorable-risk disease. Patients in CheckMate-214 were stratified according to International Metastatic Renal Cell Carcinoma Database Consortium risk score as favorable (score of 0), intermediate (score of 1 or 2) or poor risk (score of 3-6), according to the number of risk factors present.
Risk factors included a Karnofsky performance score of 70, time from initial diagnosis to randomization of <1 year, a hemoglobin level below the lower limit of normal, a corrected serum calcium concentration of >10 mg/dL, or an absolute neutrophil count or platelet count above the upper limit of normal. Patients were also stratified according to geographic region (United States versus Canada and Europe versus the rest of the world).
The coprimary endpoints were objective response rate (ORR), progression-free survival (PFS), and OS in a subset of 847 intermediate- and poor-risk patients. Over a median follow-up of 25.2 months, there was a statistically significant improvement in OS and ORR in patients treated with nivolumab and ipilimumab (mPFS not reached; ORR, 41.6%), compared with sunitinib (OS, 25.9 months; ORR, 26.5%), with P <.001 for both. The immunotherapy combination was favored across subgroups.
The most common adverse events (AEs) in patients treated with nivolumab and ipilimumab included fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, and decreased appetite. The combination was associated with fewer grade 3/4 AEs (63% vs 46% for sunitinib), but a higher rate of treatment discontinuations because of AEs (31% vs 21%, respectively). There were 8 deaths in the combination arm, and 4 in the sunitinib arm that were reported to be treatment related.2
The warnings and precautions related to nivolumab–ipilimumab combination therapy outlined in the prescribing information include mostly immune-mediated AEs, such as immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, nephritis and renal dysfunction, skin adverse reactions, and encephalitis. There are also warnings relating to the risk of infusion reactions and the potential for embryofetal toxicity.
Patients should be monitored for hyperglycemia and for changes in liver, thyroid, renal, and neurologic function. Treatment with nivolumab and ipilimumab should be withheld for moderate and permanently discontinued for severe or life-threatening immune-mediated pneumonitis, colitis, and hepatitis, as well as transaminase or total bilirubin elevation. It should also be withheld for moderate or severe hypophysitis and serum creatinine elevation, moderate adrenal insufficiency and severe hyperglycemia, and permanently discontinued for life-threatening hypophysitis and serum creatinine elevation, severe or life-threatening adrenal insufficiency, and life-threatening hyperglycemia.
New-onset moderate to severe neurologic signs or symptoms warrant treatment being withheld, and immune-mediated encephalitis should lead to treatment discontinuation. For mild or moderate infusion reactions, the infusion rate can be slowed or interrupted, and infusions should be discontinued in the event of severe or life-threatening infusion reactions. Patients should be advised of the potential for fetal harm and the need for effective contraception during and after treatment. Ipilimumab and nivolumab are marketed as Yervoy and Opdivo, respectively, by Bristol-Myers Squibb.3,4
In April 2018, the US Food and Drug Administration expanded the approval of the combination of nivolumab and ipilimumab into a new indication, following a previous approval in patients with metastatic melanoma. The double immune checkpoint inhibitor combination was approved on the basis of the phase 3 CheckMate-214 study for the treatment of patients with intermediate- or poor-risk, previously untreated advanced renal cell carcinoma (RCC).1
Nivolumab monotherapy is already approved in the second-line setting for the treatment of advanced RCC, and the demonstration of significantly improved overall survival (OS) in this study suggests that the combination should supplant sunitinib in the front-line setting in the treatment of this type of cancer.
A total of 1,096 patients at 175 sites in 28 countries were randomized 1:1 to receive nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) intravenously every 3 weeks for 4 doses in an induction phase, followed by nivolumab monotherapy (3 mg/kg) every 2 weeks in a maintenance phase or sunitinib (50 mg) orally daily for 4 weeks of each 6-week cycle.
Eligible patients were 18 years or older, had previously untreated advanced RCC with a clear-cell component, had measurable disease according to Response Evaluation Criteria in Solid Tumors (version 1.1), and had a Karnofsky performance status of at least 70 (on a scale from 0 to 100, with lower scores indicating greater disability). Patients with central nervous system metastases or autoimmune disease who were being treated with glucocorticoids and immunosuppressants were excluded from the study.
Around three-quarters of patients with advanced RCC have intermediate- or poor-risk disease and experience worse outcomes than patients with favorable-risk disease. Patients in CheckMate-214 were stratified according to International Metastatic Renal Cell Carcinoma Database Consortium risk score as favorable (score of 0), intermediate (score of 1 or 2) or poor risk (score of 3-6), according to the number of risk factors present.
Risk factors included a Karnofsky performance score of 70, time from initial diagnosis to randomization of <1 year, a hemoglobin level below the lower limit of normal, a corrected serum calcium concentration of >10 mg/dL, or an absolute neutrophil count or platelet count above the upper limit of normal. Patients were also stratified according to geographic region (United States versus Canada and Europe versus the rest of the world).
The coprimary endpoints were objective response rate (ORR), progression-free survival (PFS), and OS in a subset of 847 intermediate- and poor-risk patients. Over a median follow-up of 25.2 months, there was a statistically significant improvement in OS and ORR in patients treated with nivolumab and ipilimumab (mPFS not reached; ORR, 41.6%), compared with sunitinib (OS, 25.9 months; ORR, 26.5%), with P <.001 for both. The immunotherapy combination was favored across subgroups.
The most common adverse events (AEs) in patients treated with nivolumab and ipilimumab included fatigue, rash, diarrhea, musculoskeletal pain, pruritus, nausea, cough, pyrexia, arthralgia, and decreased appetite. The combination was associated with fewer grade 3/4 AEs (63% vs 46% for sunitinib), but a higher rate of treatment discontinuations because of AEs (31% vs 21%, respectively). There were 8 deaths in the combination arm, and 4 in the sunitinib arm that were reported to be treatment related.2
The warnings and precautions related to nivolumab–ipilimumab combination therapy outlined in the prescribing information include mostly immune-mediated AEs, such as immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, nephritis and renal dysfunction, skin adverse reactions, and encephalitis. There are also warnings relating to the risk of infusion reactions and the potential for embryofetal toxicity.
Patients should be monitored for hyperglycemia and for changes in liver, thyroid, renal, and neurologic function. Treatment with nivolumab and ipilimumab should be withheld for moderate and permanently discontinued for severe or life-threatening immune-mediated pneumonitis, colitis, and hepatitis, as well as transaminase or total bilirubin elevation. It should also be withheld for moderate or severe hypophysitis and serum creatinine elevation, moderate adrenal insufficiency and severe hyperglycemia, and permanently discontinued for life-threatening hypophysitis and serum creatinine elevation, severe or life-threatening adrenal insufficiency, and life-threatening hyperglycemia.
New-onset moderate to severe neurologic signs or symptoms warrant treatment being withheld, and immune-mediated encephalitis should lead to treatment discontinuation. For mild or moderate infusion reactions, the infusion rate can be slowed or interrupted, and infusions should be discontinued in the event of severe or life-threatening infusion reactions. Patients should be advised of the potential for fetal harm and the need for effective contraception during and after treatment. Ipilimumab and nivolumab are marketed as Yervoy and Opdivo, respectively, by Bristol-Myers Squibb.3,4
1. US Food and Drug Administration website. FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm604685.htm. Last updated April 16, 2018. Accessed July 25, 2018.
2. Motzer RJ, Tannir NM, McDermott O, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277-1290.
3. Opdivo (nivolumab) injection, for intravenous use. Prescribing information. Bristol-Myers Squibb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf. Revised April 2018.
4. Yervoy (ipilimumab) injection, for intravenous use. Prescribing information. Bristol-Myers Squibb. July 2018. https://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed September, 2018.
1. US Food and Drug Administration website. FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm604685.htm. Last updated April 16, 2018. Accessed July 25, 2018.
2. Motzer RJ, Tannir NM, McDermott O, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277-1290.
3. Opdivo (nivolumab) injection, for intravenous use. Prescribing information. Bristol-Myers Squibb. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf. Revised April 2018.
4. Yervoy (ipilimumab) injection, for intravenous use. Prescribing information. Bristol-Myers Squibb. July 2018. https://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed September, 2018.
Hemithyroidectomy rates rose after guideline update
BOSTON – that position the procedure as equivalent to total thyroidectomy, an analysis of U.S. hospital data shows.
Patients undergoing hemithyroidectomy had fewer complications and shorter length of stay versus patients who received a bilateral procedure, with no corresponding increase in completion thyroidectomy, according to results of the retrospective analysis reported here at the annual clinical congress of the American College of Surgeons.
“We think this suggests that surgeons might be changing their practice at these hospitals, at least in part in response to the guidelines,” investigator Timothy M. Ullmann, MD, endocrine oncology research fellow in the department of surgery at New York Presbyterian Hospital–Weill Cornell Medical Center, New York.
Dr. Ullmann and colleagues queried the American College of Surgeons National Surgical Quality Improvement Program database for the 2014-2016 period to illustrate operative trends before and after release of the 2015 guidelines from the American Thyroid Association guidelines. They looked at a total of 26,562 procedures done before the guidelines were release, and 7,422 done after.
The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P less than .001), according to Dr. Ullmann. By contrast, the rates of completion thyroidectomy were 7.8% for the pre-guidelines period and 7.4% post-guidelines (P = .19).
The increase was gradual throughout the 2014-2016 period, though it was especially steep after the guideline introduction, according to co-investigator Toni Beninato, MD, of Weill Cornell Medicine.
“While we can’t say that the guidelines directly caused an increase, it’s a pretty good association,” Dr. Beninato said in a press conference. “I think going forward, we would expect this to continue to increase, because the vast majority of patients with thyroid cancer probably fit criteria to have a hemithyroidectomy rather than a total thyroidectomy.”
Patients treated by otolaryngologists were more likely to undergo hemithyroidectomies versus those treated by general surgeons, multivariate analysis of this data set suggested (odds ratio, 1.13, P less than .001). On the other hand, Hispanic patients and those with a higher operative risk classification were less likely to undergo a unilateral procedure.
Complications were less likely in the hemithyroidectomy patients, according to investigators. There were significantly fewer superficial surgical site infections, at 0.2% versus 0.4% for total thyroidectomy, and operative time was 91.6 minutes versus 141.1 minutes.
Hemithyroidectomy patients were less likely to be reintubated after surgery, had a shorter length of stay, and were more likely to be managed on an outpatient basis, they added at the press conference.
Prior ATA guidelines, in place since 2009, called for near-total or total thyroidectomy for cancers that were at least 1 cm in size in patients with no contraindications to the procedure. The 2015 update says the initial surgical procedure could also be a unilateral procedure, or lobectomy, in cancers greater than 1 cm, or smaller than 4 cm with no extrathyroidal extension.
Dr. Ullmann and Dr. Beninato had no relevant financial relationships with commercial interests pertaining to the content of their presentation.
SOURCE: Ullmann TM et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
BOSTON – that position the procedure as equivalent to total thyroidectomy, an analysis of U.S. hospital data shows.
Patients undergoing hemithyroidectomy had fewer complications and shorter length of stay versus patients who received a bilateral procedure, with no corresponding increase in completion thyroidectomy, according to results of the retrospective analysis reported here at the annual clinical congress of the American College of Surgeons.
“We think this suggests that surgeons might be changing their practice at these hospitals, at least in part in response to the guidelines,” investigator Timothy M. Ullmann, MD, endocrine oncology research fellow in the department of surgery at New York Presbyterian Hospital–Weill Cornell Medical Center, New York.
Dr. Ullmann and colleagues queried the American College of Surgeons National Surgical Quality Improvement Program database for the 2014-2016 period to illustrate operative trends before and after release of the 2015 guidelines from the American Thyroid Association guidelines. They looked at a total of 26,562 procedures done before the guidelines were release, and 7,422 done after.
The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P less than .001), according to Dr. Ullmann. By contrast, the rates of completion thyroidectomy were 7.8% for the pre-guidelines period and 7.4% post-guidelines (P = .19).
The increase was gradual throughout the 2014-2016 period, though it was especially steep after the guideline introduction, according to co-investigator Toni Beninato, MD, of Weill Cornell Medicine.
“While we can’t say that the guidelines directly caused an increase, it’s a pretty good association,” Dr. Beninato said in a press conference. “I think going forward, we would expect this to continue to increase, because the vast majority of patients with thyroid cancer probably fit criteria to have a hemithyroidectomy rather than a total thyroidectomy.”
Patients treated by otolaryngologists were more likely to undergo hemithyroidectomies versus those treated by general surgeons, multivariate analysis of this data set suggested (odds ratio, 1.13, P less than .001). On the other hand, Hispanic patients and those with a higher operative risk classification were less likely to undergo a unilateral procedure.
Complications were less likely in the hemithyroidectomy patients, according to investigators. There were significantly fewer superficial surgical site infections, at 0.2% versus 0.4% for total thyroidectomy, and operative time was 91.6 minutes versus 141.1 minutes.
Hemithyroidectomy patients were less likely to be reintubated after surgery, had a shorter length of stay, and were more likely to be managed on an outpatient basis, they added at the press conference.
Prior ATA guidelines, in place since 2009, called for near-total or total thyroidectomy for cancers that were at least 1 cm in size in patients with no contraindications to the procedure. The 2015 update says the initial surgical procedure could also be a unilateral procedure, or lobectomy, in cancers greater than 1 cm, or smaller than 4 cm with no extrathyroidal extension.
Dr. Ullmann and Dr. Beninato had no relevant financial relationships with commercial interests pertaining to the content of their presentation.
SOURCE: Ullmann TM et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
BOSTON – that position the procedure as equivalent to total thyroidectomy, an analysis of U.S. hospital data shows.
Patients undergoing hemithyroidectomy had fewer complications and shorter length of stay versus patients who received a bilateral procedure, with no corresponding increase in completion thyroidectomy, according to results of the retrospective analysis reported here at the annual clinical congress of the American College of Surgeons.
“We think this suggests that surgeons might be changing their practice at these hospitals, at least in part in response to the guidelines,” investigator Timothy M. Ullmann, MD, endocrine oncology research fellow in the department of surgery at New York Presbyterian Hospital–Weill Cornell Medical Center, New York.
Dr. Ullmann and colleagues queried the American College of Surgeons National Surgical Quality Improvement Program database for the 2014-2016 period to illustrate operative trends before and after release of the 2015 guidelines from the American Thyroid Association guidelines. They looked at a total of 26,562 procedures done before the guidelines were release, and 7,422 done after.
The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P less than .001), according to Dr. Ullmann. By contrast, the rates of completion thyroidectomy were 7.8% for the pre-guidelines period and 7.4% post-guidelines (P = .19).
The increase was gradual throughout the 2014-2016 period, though it was especially steep after the guideline introduction, according to co-investigator Toni Beninato, MD, of Weill Cornell Medicine.
“While we can’t say that the guidelines directly caused an increase, it’s a pretty good association,” Dr. Beninato said in a press conference. “I think going forward, we would expect this to continue to increase, because the vast majority of patients with thyroid cancer probably fit criteria to have a hemithyroidectomy rather than a total thyroidectomy.”
Patients treated by otolaryngologists were more likely to undergo hemithyroidectomies versus those treated by general surgeons, multivariate analysis of this data set suggested (odds ratio, 1.13, P less than .001). On the other hand, Hispanic patients and those with a higher operative risk classification were less likely to undergo a unilateral procedure.
Complications were less likely in the hemithyroidectomy patients, according to investigators. There were significantly fewer superficial surgical site infections, at 0.2% versus 0.4% for total thyroidectomy, and operative time was 91.6 minutes versus 141.1 minutes.
Hemithyroidectomy patients were less likely to be reintubated after surgery, had a shorter length of stay, and were more likely to be managed on an outpatient basis, they added at the press conference.
Prior ATA guidelines, in place since 2009, called for near-total or total thyroidectomy for cancers that were at least 1 cm in size in patients with no contraindications to the procedure. The 2015 update says the initial surgical procedure could also be a unilateral procedure, or lobectomy, in cancers greater than 1 cm, or smaller than 4 cm with no extrathyroidal extension.
Dr. Ullmann and Dr. Beninato had no relevant financial relationships with commercial interests pertaining to the content of their presentation.
SOURCE: Ullmann TM et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Hemithyroidectomy rates had a robust uptick following release of 2015 clinical practice guidelines, suggesting surgeons may be changing their practice in response.
Major finding: The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P < 0.001).
Study details: Analysis of the American College of Surgeons-NSQIP database from 2014 to 2016 including nearly 34,000 procedures.
Disclosures: Study authors reported no disclosures.
Source: Ullmann TM et al. Abstract SF125 presented at American College of Surgeons Clinical Congress
Survey: Humanitarian surgical groups need best-practices guidelines
BOSTON – A survey of U.S. and suggests that a best-practices guide would be beneficial in helping them adhere more closely to standard protocols.
While most NGOs follow guideline-based practices, some deviations from standard of care do occur. Deviations occurred most often in the areas of preoperative workup, operative technique, and pain management, according to survey results presented at the annual clinical congress of the American College of Surgeons.
Consensus guidelines specific to the NGO sector would be used by the great majority of NGOs surveyed, reported Peter F. Johnston, MD, a general surgery resident and the Ben Rush Global Surgery Fellow at Rutgers New Jersey Medical School.
“There is a lot of heterogeneity in the sector, based on the different organizations doing general surgery and organizations doing plastics,” Dr. Johnston said in an interview. “What we think we can do in terms of low-hanging fruit is come up with guidelines for things like perioperative antibiotics that are pretty much common to all types of surgeries.”
The survey conducted by Dr. Johnston and colleagues is one of the first to characterize the clinical practices of U.S. humanitarian organizations that provide general or subspecialty care through short-term surgical missions. It was completed by representatives of 30 of 83 organizations (36%) that were contacted.
Of respondents, 20% said their organizations deviated from standard U.S. practice often or very often, Dr. Johnston said in his presentation. The respondents mentioned deviation from standard practice in pain management (18%), preoperative workup (16%), and operative technique (16%).
Only about one-third of respondents said they believed those deviations impacted patient outcomes, the results show.
In all, 67% of respondents adhered to at least four protocol-driven practices. Those NGOs that adhered most closely to standard protocol tended to be older, more established organizations, compared with those less protocolized organizations, according to Dr. Johnston (age of organization 22 vs. 14 years, P < .05).
“It makes sense... from my own experience of going back to the same countries,” said Dr. Johnston, who has participated in missions in countries including Ghana, Sierra Leone, and Peru. “As an organization, and even within different countries, the process gets smoother as you keep working at it.”
A total of 85% of respondents expressed interest in best-practice guidelines to guide short-term surgical missions, according to the survey data.
Dr. Johnston reported no conflicts of interest related to his presentation.
SOURCE: Johnston P, et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
BOSTON – A survey of U.S. and suggests that a best-practices guide would be beneficial in helping them adhere more closely to standard protocols.
While most NGOs follow guideline-based practices, some deviations from standard of care do occur. Deviations occurred most often in the areas of preoperative workup, operative technique, and pain management, according to survey results presented at the annual clinical congress of the American College of Surgeons.
Consensus guidelines specific to the NGO sector would be used by the great majority of NGOs surveyed, reported Peter F. Johnston, MD, a general surgery resident and the Ben Rush Global Surgery Fellow at Rutgers New Jersey Medical School.
“There is a lot of heterogeneity in the sector, based on the different organizations doing general surgery and organizations doing plastics,” Dr. Johnston said in an interview. “What we think we can do in terms of low-hanging fruit is come up with guidelines for things like perioperative antibiotics that are pretty much common to all types of surgeries.”
The survey conducted by Dr. Johnston and colleagues is one of the first to characterize the clinical practices of U.S. humanitarian organizations that provide general or subspecialty care through short-term surgical missions. It was completed by representatives of 30 of 83 organizations (36%) that were contacted.
Of respondents, 20% said their organizations deviated from standard U.S. practice often or very often, Dr. Johnston said in his presentation. The respondents mentioned deviation from standard practice in pain management (18%), preoperative workup (16%), and operative technique (16%).
Only about one-third of respondents said they believed those deviations impacted patient outcomes, the results show.
In all, 67% of respondents adhered to at least four protocol-driven practices. Those NGOs that adhered most closely to standard protocol tended to be older, more established organizations, compared with those less protocolized organizations, according to Dr. Johnston (age of organization 22 vs. 14 years, P < .05).
“It makes sense... from my own experience of going back to the same countries,” said Dr. Johnston, who has participated in missions in countries including Ghana, Sierra Leone, and Peru. “As an organization, and even within different countries, the process gets smoother as you keep working at it.”
A total of 85% of respondents expressed interest in best-practice guidelines to guide short-term surgical missions, according to the survey data.
Dr. Johnston reported no conflicts of interest related to his presentation.
SOURCE: Johnston P, et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
BOSTON – A survey of U.S. and suggests that a best-practices guide would be beneficial in helping them adhere more closely to standard protocols.
While most NGOs follow guideline-based practices, some deviations from standard of care do occur. Deviations occurred most often in the areas of preoperative workup, operative technique, and pain management, according to survey results presented at the annual clinical congress of the American College of Surgeons.
Consensus guidelines specific to the NGO sector would be used by the great majority of NGOs surveyed, reported Peter F. Johnston, MD, a general surgery resident and the Ben Rush Global Surgery Fellow at Rutgers New Jersey Medical School.
“There is a lot of heterogeneity in the sector, based on the different organizations doing general surgery and organizations doing plastics,” Dr. Johnston said in an interview. “What we think we can do in terms of low-hanging fruit is come up with guidelines for things like perioperative antibiotics that are pretty much common to all types of surgeries.”
The survey conducted by Dr. Johnston and colleagues is one of the first to characterize the clinical practices of U.S. humanitarian organizations that provide general or subspecialty care through short-term surgical missions. It was completed by representatives of 30 of 83 organizations (36%) that were contacted.
Of respondents, 20% said their organizations deviated from standard U.S. practice often or very often, Dr. Johnston said in his presentation. The respondents mentioned deviation from standard practice in pain management (18%), preoperative workup (16%), and operative technique (16%).
Only about one-third of respondents said they believed those deviations impacted patient outcomes, the results show.
In all, 67% of respondents adhered to at least four protocol-driven practices. Those NGOs that adhered most closely to standard protocol tended to be older, more established organizations, compared with those less protocolized organizations, according to Dr. Johnston (age of organization 22 vs. 14 years, P < .05).
“It makes sense... from my own experience of going back to the same countries,” said Dr. Johnston, who has participated in missions in countries including Ghana, Sierra Leone, and Peru. “As an organization, and even within different countries, the process gets smoother as you keep working at it.”
A total of 85% of respondents expressed interest in best-practice guidelines to guide short-term surgical missions, according to the survey data.
Dr. Johnston reported no conflicts of interest related to his presentation.
SOURCE: Johnston P, et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
AT THE ACS CLINICAL CONGRESS
Key clinical point: The clinical practice of some humanitarian surgical organizations deviated from standard practice, suggesting a need for NGO-specific guidelines.
Major finding: The most common deviations from standard practice were in pain management (18%), preoperative workup (16%), and operative technique (16%).
Study details: 30 Responses from a survey of 83 organizations that provide general or subspecialty care through short-term surgical missions.
Disclosures: Study authors reported no conflicts.
Source: Johnston PF et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
Statins cut all-cause mortality in spinal cord injury
ATLANTA – Statin use among a cohort of veterans with traumatic spinal cord injury reduced all-cause mortality, results from a novel observational study showed.
“This is the first clinical study to show that administration of statins irrespective of the lipid levels reduces all-cause mortality, not just cardiovascular mortality,” lead study author Meheroz H. Rabadi, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “This clinical study confirms the impression of several prior studies in animal models with spinal cord injury, which have shown the anti-inflammatory and neuro-protective effects of statins.”
To determine whether statin use in a cohort of patients with traumatic spinal cord injuries (SCI) reduced overall and cause-specific mortality, Dr. Rabadi and his colleagues retrospectively reviewed the medical charts and death records of 163 individuals with SCI who were treated at the Oklahoma City Veterans Administration Medical Center Spinal Cord Injury & Disease, Multiple Sclerosis, and ALS Program, an outpatient clinic, from 2000 to 2014. They collected data on statin use, duration of statin use, and intensity of statin therapy, as well as cause-specific mortality.
Of the 163 subjects studied, 75 (46%) had taken statins for an average of 5.7 years, and had greater cardiovascular risk burdens than those who had not taken statins. The mortality rate for patients on statins, however, was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins. Kaplan-Meier survival curves showed a significant difference between the two groups (P less than .0052). Within the statin group, neither duration nor average intensity of statin therapy affected mortality.
“We were surprised to note statins reduced pneumonia-related mortality in patients with SCI,” Dr. Rabadi said. “Since our publication there have been several publications, including a meta-analysis of statins reducing community-acquired pneumonia-related mortality and reducing the need for mechanical ventilation or ICU admission (see CHEST 2015;148:523-32, Clin Med (Lond) 2017;17(5):403-7, and Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):30-40). Another surprise was neither the intensity, duration, or types of statin affected the result.”
He acknowledged certain limitations of the analysis, including its retrospective design, its relatively small sample size, and the fact that most of the subjects were non-Hispanic white men. “Routine prescription of statins in any dose in patients with SCI – even if the lipid profile is normal – is more beneficial than detrimental over the long haul,” concluded Dr. Rabadi, who also directs the Oklahoma VAMC Stroke Program. “Nearly all our patients with SCI continue to be on varying doses of statins.”
Dr. Rabadi reported having no financial disclosures.
[email protected]
SOURCE: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
ATLANTA – Statin use among a cohort of veterans with traumatic spinal cord injury reduced all-cause mortality, results from a novel observational study showed.
“This is the first clinical study to show that administration of statins irrespective of the lipid levels reduces all-cause mortality, not just cardiovascular mortality,” lead study author Meheroz H. Rabadi, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “This clinical study confirms the impression of several prior studies in animal models with spinal cord injury, which have shown the anti-inflammatory and neuro-protective effects of statins.”
To determine whether statin use in a cohort of patients with traumatic spinal cord injuries (SCI) reduced overall and cause-specific mortality, Dr. Rabadi and his colleagues retrospectively reviewed the medical charts and death records of 163 individuals with SCI who were treated at the Oklahoma City Veterans Administration Medical Center Spinal Cord Injury & Disease, Multiple Sclerosis, and ALS Program, an outpatient clinic, from 2000 to 2014. They collected data on statin use, duration of statin use, and intensity of statin therapy, as well as cause-specific mortality.
Of the 163 subjects studied, 75 (46%) had taken statins for an average of 5.7 years, and had greater cardiovascular risk burdens than those who had not taken statins. The mortality rate for patients on statins, however, was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins. Kaplan-Meier survival curves showed a significant difference between the two groups (P less than .0052). Within the statin group, neither duration nor average intensity of statin therapy affected mortality.
“We were surprised to note statins reduced pneumonia-related mortality in patients with SCI,” Dr. Rabadi said. “Since our publication there have been several publications, including a meta-analysis of statins reducing community-acquired pneumonia-related mortality and reducing the need for mechanical ventilation or ICU admission (see CHEST 2015;148:523-32, Clin Med (Lond) 2017;17(5):403-7, and Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):30-40). Another surprise was neither the intensity, duration, or types of statin affected the result.”
He acknowledged certain limitations of the analysis, including its retrospective design, its relatively small sample size, and the fact that most of the subjects were non-Hispanic white men. “Routine prescription of statins in any dose in patients with SCI – even if the lipid profile is normal – is more beneficial than detrimental over the long haul,” concluded Dr. Rabadi, who also directs the Oklahoma VAMC Stroke Program. “Nearly all our patients with SCI continue to be on varying doses of statins.”
Dr. Rabadi reported having no financial disclosures.
[email protected]
SOURCE: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
ATLANTA – Statin use among a cohort of veterans with traumatic spinal cord injury reduced all-cause mortality, results from a novel observational study showed.
“This is the first clinical study to show that administration of statins irrespective of the lipid levels reduces all-cause mortality, not just cardiovascular mortality,” lead study author Meheroz H. Rabadi, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “This clinical study confirms the impression of several prior studies in animal models with spinal cord injury, which have shown the anti-inflammatory and neuro-protective effects of statins.”
To determine whether statin use in a cohort of patients with traumatic spinal cord injuries (SCI) reduced overall and cause-specific mortality, Dr. Rabadi and his colleagues retrospectively reviewed the medical charts and death records of 163 individuals with SCI who were treated at the Oklahoma City Veterans Administration Medical Center Spinal Cord Injury & Disease, Multiple Sclerosis, and ALS Program, an outpatient clinic, from 2000 to 2014. They collected data on statin use, duration of statin use, and intensity of statin therapy, as well as cause-specific mortality.
Of the 163 subjects studied, 75 (46%) had taken statins for an average of 5.7 years, and had greater cardiovascular risk burdens than those who had not taken statins. The mortality rate for patients on statins, however, was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins. Kaplan-Meier survival curves showed a significant difference between the two groups (P less than .0052). Within the statin group, neither duration nor average intensity of statin therapy affected mortality.
“We were surprised to note statins reduced pneumonia-related mortality in patients with SCI,” Dr. Rabadi said. “Since our publication there have been several publications, including a meta-analysis of statins reducing community-acquired pneumonia-related mortality and reducing the need for mechanical ventilation or ICU admission (see CHEST 2015;148:523-32, Clin Med (Lond) 2017;17(5):403-7, and Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):30-40). Another surprise was neither the intensity, duration, or types of statin affected the result.”
He acknowledged certain limitations of the analysis, including its retrospective design, its relatively small sample size, and the fact that most of the subjects were non-Hispanic white men. “Routine prescription of statins in any dose in patients with SCI – even if the lipid profile is normal – is more beneficial than detrimental over the long haul,” concluded Dr. Rabadi, who also directs the Oklahoma VAMC Stroke Program. “Nearly all our patients with SCI continue to be on varying doses of statins.”
Dr. Rabadi reported having no financial disclosures.
[email protected]
SOURCE: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
AT ANA 2018
Key clinical point:
Major finding: The mortality rate for patients on statins was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins (P less than .0052).
Study details: A retrospective review of 163 individuals with traumatic spinal cord injuries.
Disclosures: Dr. Rabadi reported having no financial disclosures.
Source: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
Septic shock: Innovative treatment options in the wings
BOSTON– treatment that require more investigation, but nevertheless appear promising, Rishi Rattan, MD, said at the annual clinical congress of the American College of Surgeons.
Trials evaluating vitamin C in this setting have demonstrated a large mortality impact with an absence of side effects, according to Dr. Rattan, a trauma and critical care surgeon with the Ryder Trauma Center at the University of Miami.
“It’s something that I have decided to start early adopting, and many of my colleagues at University of Miami do as well,” Dr. Rattan said in a panel session on updates in septic shock. “We’re anecdotally so far at least seeing good results and are going to be excited to see what these ongoing trials show.”
As an antioxidant, vitamin C has anti-inflammatory properties that may possibly attenuate the overly exuberant inflammatory response seen in septic shock, Dr. Rattan said in his presentation.
The limited clinical data for vitamin C in refractory shock include three studies, of which two are randomized controlled trials, comprising a total of 146 patients, he added.
“I will admit an N of 146 is hardly practice-changing for most people,” Dr. Rattan said. “There’s still a significant and sustained large mortality effect for the use of vitamin C, with nearly no adverse effects.”
Pooled analysis of all three studies revealed a marked reduction in mortality with the use of vitamin C (odds ratio, 0.17, 95% confidence interval 0.07–0.40; P less than .001), according to a meta-analysis recently just published in Critical Care that Dr. Rattan referenced in his presentation (Critical Care 2018;22:258, DOI:10.1186/s13054-018-2191-x).
When taken in recommended dosages, vitamin C given with corticosteroids and thiamine is without known side effects, researcher Paul E. Marik wrote earlier this year in an editorial in Pharmacology & Therapeutics (2018;189[9]:63-70, DOI:10.1016/j.pharmthera.2018.04.007) noted Dr. Rattan, who said he uses the intravenous vitamin C, thiamine, and hydrocortisone protocol previously reported by Dr. Marik and colleagues.
There are 13 ongoing trials, including some prospective blinded, randomized trials, looking at the role of vitamin C in refractory shock, he added.
Angiotensin-II is another intervention that may be promising in refractory septic shock, Dr. Rattan told attendees, pointing to the 2017 publication of the ATHOS-3 trial in the New England Journal of Medicine (2017; 377:419-430,DOI: 10.1056/NEJMoa1704154) showing that treatment increased blood pressure in patients with vasodilatory shock not responding to conventional vasopressors at high doses.
Likewise, methylene blue has shown promise in septic shock, at least in some limited clinical investigations and anecdotally in patients not improving despite standard interventions. “I’ve been able to have a couple patients walk out of the hospital with the use of methylene blue,” Dr. Rattan said. “Again, the plural of ‘anecdote’ is not ‘data,’ but it’s something to consider for the early adopters.”
Dr. Rattan had no disclosures related to his presentation.
BOSTON– treatment that require more investigation, but nevertheless appear promising, Rishi Rattan, MD, said at the annual clinical congress of the American College of Surgeons.
Trials evaluating vitamin C in this setting have demonstrated a large mortality impact with an absence of side effects, according to Dr. Rattan, a trauma and critical care surgeon with the Ryder Trauma Center at the University of Miami.
“It’s something that I have decided to start early adopting, and many of my colleagues at University of Miami do as well,” Dr. Rattan said in a panel session on updates in septic shock. “We’re anecdotally so far at least seeing good results and are going to be excited to see what these ongoing trials show.”
As an antioxidant, vitamin C has anti-inflammatory properties that may possibly attenuate the overly exuberant inflammatory response seen in septic shock, Dr. Rattan said in his presentation.
The limited clinical data for vitamin C in refractory shock include three studies, of which two are randomized controlled trials, comprising a total of 146 patients, he added.
“I will admit an N of 146 is hardly practice-changing for most people,” Dr. Rattan said. “There’s still a significant and sustained large mortality effect for the use of vitamin C, with nearly no adverse effects.”
Pooled analysis of all three studies revealed a marked reduction in mortality with the use of vitamin C (odds ratio, 0.17, 95% confidence interval 0.07–0.40; P less than .001), according to a meta-analysis recently just published in Critical Care that Dr. Rattan referenced in his presentation (Critical Care 2018;22:258, DOI:10.1186/s13054-018-2191-x).
When taken in recommended dosages, vitamin C given with corticosteroids and thiamine is without known side effects, researcher Paul E. Marik wrote earlier this year in an editorial in Pharmacology & Therapeutics (2018;189[9]:63-70, DOI:10.1016/j.pharmthera.2018.04.007) noted Dr. Rattan, who said he uses the intravenous vitamin C, thiamine, and hydrocortisone protocol previously reported by Dr. Marik and colleagues.
There are 13 ongoing trials, including some prospective blinded, randomized trials, looking at the role of vitamin C in refractory shock, he added.
Angiotensin-II is another intervention that may be promising in refractory septic shock, Dr. Rattan told attendees, pointing to the 2017 publication of the ATHOS-3 trial in the New England Journal of Medicine (2017; 377:419-430,DOI: 10.1056/NEJMoa1704154) showing that treatment increased blood pressure in patients with vasodilatory shock not responding to conventional vasopressors at high doses.
Likewise, methylene blue has shown promise in septic shock, at least in some limited clinical investigations and anecdotally in patients not improving despite standard interventions. “I’ve been able to have a couple patients walk out of the hospital with the use of methylene blue,” Dr. Rattan said. “Again, the plural of ‘anecdote’ is not ‘data,’ but it’s something to consider for the early adopters.”
Dr. Rattan had no disclosures related to his presentation.
BOSTON– treatment that require more investigation, but nevertheless appear promising, Rishi Rattan, MD, said at the annual clinical congress of the American College of Surgeons.
Trials evaluating vitamin C in this setting have demonstrated a large mortality impact with an absence of side effects, according to Dr. Rattan, a trauma and critical care surgeon with the Ryder Trauma Center at the University of Miami.
“It’s something that I have decided to start early adopting, and many of my colleagues at University of Miami do as well,” Dr. Rattan said in a panel session on updates in septic shock. “We’re anecdotally so far at least seeing good results and are going to be excited to see what these ongoing trials show.”
As an antioxidant, vitamin C has anti-inflammatory properties that may possibly attenuate the overly exuberant inflammatory response seen in septic shock, Dr. Rattan said in his presentation.
The limited clinical data for vitamin C in refractory shock include three studies, of which two are randomized controlled trials, comprising a total of 146 patients, he added.
“I will admit an N of 146 is hardly practice-changing for most people,” Dr. Rattan said. “There’s still a significant and sustained large mortality effect for the use of vitamin C, with nearly no adverse effects.”
Pooled analysis of all three studies revealed a marked reduction in mortality with the use of vitamin C (odds ratio, 0.17, 95% confidence interval 0.07–0.40; P less than .001), according to a meta-analysis recently just published in Critical Care that Dr. Rattan referenced in his presentation (Critical Care 2018;22:258, DOI:10.1186/s13054-018-2191-x).
When taken in recommended dosages, vitamin C given with corticosteroids and thiamine is without known side effects, researcher Paul E. Marik wrote earlier this year in an editorial in Pharmacology & Therapeutics (2018;189[9]:63-70, DOI:10.1016/j.pharmthera.2018.04.007) noted Dr. Rattan, who said he uses the intravenous vitamin C, thiamine, and hydrocortisone protocol previously reported by Dr. Marik and colleagues.
There are 13 ongoing trials, including some prospective blinded, randomized trials, looking at the role of vitamin C in refractory shock, he added.
Angiotensin-II is another intervention that may be promising in refractory septic shock, Dr. Rattan told attendees, pointing to the 2017 publication of the ATHOS-3 trial in the New England Journal of Medicine (2017; 377:419-430,DOI: 10.1056/NEJMoa1704154) showing that treatment increased blood pressure in patients with vasodilatory shock not responding to conventional vasopressors at high doses.
Likewise, methylene blue has shown promise in septic shock, at least in some limited clinical investigations and anecdotally in patients not improving despite standard interventions. “I’ve been able to have a couple patients walk out of the hospital with the use of methylene blue,” Dr. Rattan said. “Again, the plural of ‘anecdote’ is not ‘data,’ but it’s something to consider for the early adopters.”
Dr. Rattan had no disclosures related to his presentation.
AT THE ACS CLINICAL CONGRESS