User login
Launching a surgical comanagement project
Improving quality, patient satisfaction
When hospital medicine and surgical departments (usually orthopedics or neurosurgery) have joined in comanagement programs, improvements in quality metrics and patient satisfaction have often resulted.
At the Level 1 regional trauma center in which he works, Charles L. Kast, MD, and his colleagues wanted to try a comanagement agreement between hospital medicine and trauma surgery.
“The surgical team identified a need within their own department, which was to improve patient mortality and satisfaction in the inpatient setting,” said Dr. Kast, who is based at North Shore University Hospital, Manhasset, N.Y. “Their leadership sought out our hospital medicine leadership team, who then worked together to synthesize their metrics. We were able to identify other quality indicators, such as readmission rates and hospital-acquired conditions, which we felt could also benefit from our services in order to help them improve.”
Five hospitalists became members of the comanagement team. A single hospitalist rotated for 2 weeks at a time, during which they were relieved of routine hospital medicine rounding responsibilities. The hospitalist attended daily interdisciplinary rounds with the trauma surgery team, during which he/she identified patients that could benefit from hospital medicine comanagement: Patients who were over age 65 years, had multiple chronic medical conditions, or were on high-risk medications were preferentially selected. Approximately 10 patients were seen daily.
The comanagement program was well received by trauma surgeons, who talked about improved patient communication and a fostered sense of collegiality. Preliminary quality and patient satisfaction metrics were also positive.
A top takeaway is that the benefits of surgical comanagement can be demonstrated in “atypical” collaborations, depending on the needs of the department and the hospital’s vision.
“The gains in improved patient quality metrics are only half of the story,” Dr. Kast said. “Collaborating in surgical comanagement improves the satisfaction of the hospitalists and surgeons involved and can lead to future quality improvement projects or original research, both of which we are currently pursuing.”
Reference
Kast C et al. The successful development of a hospital medicine-trauma surgery co-management program [abstract]. J Hosp Med. 2017;12(suppl 2). Accessed Feb. 2, 2018.
Improving quality, patient satisfaction
Improving quality, patient satisfaction
When hospital medicine and surgical departments (usually orthopedics or neurosurgery) have joined in comanagement programs, improvements in quality metrics and patient satisfaction have often resulted.
At the Level 1 regional trauma center in which he works, Charles L. Kast, MD, and his colleagues wanted to try a comanagement agreement between hospital medicine and trauma surgery.
“The surgical team identified a need within their own department, which was to improve patient mortality and satisfaction in the inpatient setting,” said Dr. Kast, who is based at North Shore University Hospital, Manhasset, N.Y. “Their leadership sought out our hospital medicine leadership team, who then worked together to synthesize their metrics. We were able to identify other quality indicators, such as readmission rates and hospital-acquired conditions, which we felt could also benefit from our services in order to help them improve.”
Five hospitalists became members of the comanagement team. A single hospitalist rotated for 2 weeks at a time, during which they were relieved of routine hospital medicine rounding responsibilities. The hospitalist attended daily interdisciplinary rounds with the trauma surgery team, during which he/she identified patients that could benefit from hospital medicine comanagement: Patients who were over age 65 years, had multiple chronic medical conditions, or were on high-risk medications were preferentially selected. Approximately 10 patients were seen daily.
The comanagement program was well received by trauma surgeons, who talked about improved patient communication and a fostered sense of collegiality. Preliminary quality and patient satisfaction metrics were also positive.
A top takeaway is that the benefits of surgical comanagement can be demonstrated in “atypical” collaborations, depending on the needs of the department and the hospital’s vision.
“The gains in improved patient quality metrics are only half of the story,” Dr. Kast said. “Collaborating in surgical comanagement improves the satisfaction of the hospitalists and surgeons involved and can lead to future quality improvement projects or original research, both of which we are currently pursuing.”
Reference
Kast C et al. The successful development of a hospital medicine-trauma surgery co-management program [abstract]. J Hosp Med. 2017;12(suppl 2). Accessed Feb. 2, 2018.
When hospital medicine and surgical departments (usually orthopedics or neurosurgery) have joined in comanagement programs, improvements in quality metrics and patient satisfaction have often resulted.
At the Level 1 regional trauma center in which he works, Charles L. Kast, MD, and his colleagues wanted to try a comanagement agreement between hospital medicine and trauma surgery.
“The surgical team identified a need within their own department, which was to improve patient mortality and satisfaction in the inpatient setting,” said Dr. Kast, who is based at North Shore University Hospital, Manhasset, N.Y. “Their leadership sought out our hospital medicine leadership team, who then worked together to synthesize their metrics. We were able to identify other quality indicators, such as readmission rates and hospital-acquired conditions, which we felt could also benefit from our services in order to help them improve.”
Five hospitalists became members of the comanagement team. A single hospitalist rotated for 2 weeks at a time, during which they were relieved of routine hospital medicine rounding responsibilities. The hospitalist attended daily interdisciplinary rounds with the trauma surgery team, during which he/she identified patients that could benefit from hospital medicine comanagement: Patients who were over age 65 years, had multiple chronic medical conditions, or were on high-risk medications were preferentially selected. Approximately 10 patients were seen daily.
The comanagement program was well received by trauma surgeons, who talked about improved patient communication and a fostered sense of collegiality. Preliminary quality and patient satisfaction metrics were also positive.
A top takeaway is that the benefits of surgical comanagement can be demonstrated in “atypical” collaborations, depending on the needs of the department and the hospital’s vision.
“The gains in improved patient quality metrics are only half of the story,” Dr. Kast said. “Collaborating in surgical comanagement improves the satisfaction of the hospitalists and surgeons involved and can lead to future quality improvement projects or original research, both of which we are currently pursuing.”
Reference
Kast C et al. The successful development of a hospital medicine-trauma surgery co-management program [abstract]. J Hosp Med. 2017;12(suppl 2). Accessed Feb. 2, 2018.
Risk score validated for major NSAID adverse events
CHICAGO – Researchers have derived and validated a 10-item formula to estimate a patient’s risk for developing a major adverse event while on NSAID treatment.
The calculator could “help guide use of NSAIDs in clinical practice,” said Daniel H. Solomon, MD, at the annual meeting of the American College of Rheumatology. Although he called for further validation of the risk-score formula using other databases, he noted that it uses readily available data and could easily be calculated with standard inputs in an electronic medical record. The formula predicts the risk for a major adverse effect during 1 year of daily NSAID use.
Dr. Solomon and his associates devised the risk-score calculator with data collected in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial, a safety study designed to test whether treatment with celecoxib was noninferior to treatment with naproxen or ibuprofen for producing cardiovascular adverse events, a hypothesis proven by the study’s results (N Engl J Med. 2016 Dec 29;375[26]:2519-29). They had full data available for 23,950 of the more than 24,000 enrolled patients. The patients averaged 63 years old, just over a third were men, their average body mass index was 31 kg/m2, and 90% had osteoarthritis and 10% had rheumatoid arthritis. The study enrolled patients with an elevated risk for a cardiovascular event, so 63% had hypertension and 36% had diabetes.
The adverse events included as possible outcomes estimated by the formula were all-cause death, major adverse cardiovascular events, clinically significant GI events, or renal insufficiency or failure. The investigators used data from more than 15,000 patients enrolled during the first 4 years of the study to derive the risk-score formula, and data from the nearly 9,000 patients enrolled during the next 5 years to validate it.
The analysis identified and validated 10 baseline items that, when plugged into the formula, calculated a predicted rate for the subsequent development of a major averse event during 1 year of NSAID treatment. The 10 parameters are: age, sex, known cardiovascular disease, hypertension, diabetes, current cigarette use, on treatment with a statin, baseline serum creatinine level, rheumatoid arthritis, and hematocrit.
As examples of the accuracy of the prediction score, Dr. Solomon reported that, among the patients with a predicted risk for a major adverse event of less than 1%, the observed rate was 0.4%; among people with a predicted rate of 1%-4%, the observed rate was 1.7%; and among those with a predicted risk of more than 4% the observed rate was 5.6%. Major cardiovascular events were the most common type of adverse events observed among the nearly 24,000 patients enrolled in PRECISION. A total of 5% of the patients fell into the lowest risk category, with a risk of less than 1%; 70% were in the intermediate risk category, with a predicted risk of 1%-4%; and 25% had a predicted risk of more than 4%, reported Dr. Solomon, a professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston.
Age is a major driver of risk, he noted. A patient who is at least 65 years old would have a greater than 1% risk for an adverse event regardless of the other nine risk factors in the scoring formula.
PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
SOURCE: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
CHICAGO – Researchers have derived and validated a 10-item formula to estimate a patient’s risk for developing a major adverse event while on NSAID treatment.
The calculator could “help guide use of NSAIDs in clinical practice,” said Daniel H. Solomon, MD, at the annual meeting of the American College of Rheumatology. Although he called for further validation of the risk-score formula using other databases, he noted that it uses readily available data and could easily be calculated with standard inputs in an electronic medical record. The formula predicts the risk for a major adverse effect during 1 year of daily NSAID use.
Dr. Solomon and his associates devised the risk-score calculator with data collected in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial, a safety study designed to test whether treatment with celecoxib was noninferior to treatment with naproxen or ibuprofen for producing cardiovascular adverse events, a hypothesis proven by the study’s results (N Engl J Med. 2016 Dec 29;375[26]:2519-29). They had full data available for 23,950 of the more than 24,000 enrolled patients. The patients averaged 63 years old, just over a third were men, their average body mass index was 31 kg/m2, and 90% had osteoarthritis and 10% had rheumatoid arthritis. The study enrolled patients with an elevated risk for a cardiovascular event, so 63% had hypertension and 36% had diabetes.
The adverse events included as possible outcomes estimated by the formula were all-cause death, major adverse cardiovascular events, clinically significant GI events, or renal insufficiency or failure. The investigators used data from more than 15,000 patients enrolled during the first 4 years of the study to derive the risk-score formula, and data from the nearly 9,000 patients enrolled during the next 5 years to validate it.
The analysis identified and validated 10 baseline items that, when plugged into the formula, calculated a predicted rate for the subsequent development of a major averse event during 1 year of NSAID treatment. The 10 parameters are: age, sex, known cardiovascular disease, hypertension, diabetes, current cigarette use, on treatment with a statin, baseline serum creatinine level, rheumatoid arthritis, and hematocrit.
As examples of the accuracy of the prediction score, Dr. Solomon reported that, among the patients with a predicted risk for a major adverse event of less than 1%, the observed rate was 0.4%; among people with a predicted rate of 1%-4%, the observed rate was 1.7%; and among those with a predicted risk of more than 4% the observed rate was 5.6%. Major cardiovascular events were the most common type of adverse events observed among the nearly 24,000 patients enrolled in PRECISION. A total of 5% of the patients fell into the lowest risk category, with a risk of less than 1%; 70% were in the intermediate risk category, with a predicted risk of 1%-4%; and 25% had a predicted risk of more than 4%, reported Dr. Solomon, a professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston.
Age is a major driver of risk, he noted. A patient who is at least 65 years old would have a greater than 1% risk for an adverse event regardless of the other nine risk factors in the scoring formula.
PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
SOURCE: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
CHICAGO – Researchers have derived and validated a 10-item formula to estimate a patient’s risk for developing a major adverse event while on NSAID treatment.
The calculator could “help guide use of NSAIDs in clinical practice,” said Daniel H. Solomon, MD, at the annual meeting of the American College of Rheumatology. Although he called for further validation of the risk-score formula using other databases, he noted that it uses readily available data and could easily be calculated with standard inputs in an electronic medical record. The formula predicts the risk for a major adverse effect during 1 year of daily NSAID use.
Dr. Solomon and his associates devised the risk-score calculator with data collected in the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen) trial, a safety study designed to test whether treatment with celecoxib was noninferior to treatment with naproxen or ibuprofen for producing cardiovascular adverse events, a hypothesis proven by the study’s results (N Engl J Med. 2016 Dec 29;375[26]:2519-29). They had full data available for 23,950 of the more than 24,000 enrolled patients. The patients averaged 63 years old, just over a third were men, their average body mass index was 31 kg/m2, and 90% had osteoarthritis and 10% had rheumatoid arthritis. The study enrolled patients with an elevated risk for a cardiovascular event, so 63% had hypertension and 36% had diabetes.
The adverse events included as possible outcomes estimated by the formula were all-cause death, major adverse cardiovascular events, clinically significant GI events, or renal insufficiency or failure. The investigators used data from more than 15,000 patients enrolled during the first 4 years of the study to derive the risk-score formula, and data from the nearly 9,000 patients enrolled during the next 5 years to validate it.
The analysis identified and validated 10 baseline items that, when plugged into the formula, calculated a predicted rate for the subsequent development of a major averse event during 1 year of NSAID treatment. The 10 parameters are: age, sex, known cardiovascular disease, hypertension, diabetes, current cigarette use, on treatment with a statin, baseline serum creatinine level, rheumatoid arthritis, and hematocrit.
As examples of the accuracy of the prediction score, Dr. Solomon reported that, among the patients with a predicted risk for a major adverse event of less than 1%, the observed rate was 0.4%; among people with a predicted rate of 1%-4%, the observed rate was 1.7%; and among those with a predicted risk of more than 4% the observed rate was 5.6%. Major cardiovascular events were the most common type of adverse events observed among the nearly 24,000 patients enrolled in PRECISION. A total of 5% of the patients fell into the lowest risk category, with a risk of less than 1%; 70% were in the intermediate risk category, with a predicted risk of 1%-4%; and 25% had a predicted risk of more than 4%, reported Dr. Solomon, a professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital in Boston.
Age is a major driver of risk, he noted. A patient who is at least 65 years old would have a greater than 1% risk for an adverse event regardless of the other nine risk factors in the scoring formula.
PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
SOURCE: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Five percent of patients had a predicted risk below 1%; 70% had 1%-4% risk; 25% had greater than 4% risk.
Study details: Derivation and validation of the risk score used data from 23,950 patients in the PRECISION trial.
Disclosures: PRECISION was funded by Pfizer. Dr. Solomon has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, and Pfizer.
Source: Solomon D et al. ACR Annual Meeting, Abstract 2952. Arthritis Rheumatol. 2018;70(Suppl 10).
Higher BMI associated with greater loss of gray matter volume in MS
ATLANTA – Among patients with relapsing-remitting multiple sclerosis, higher body mass index, but not vitamin D status, appears to be related to greater loss of gray matter brain volume over time, results from a 5-year analysis showed.
“We had previously known that obesity is a risk factor for developing MS, and among those who already have the disease, obesity-related comorbidities are associated with increased morbidity and mortality,” lead study author Ellen M. Mowry, MD, said in an interview at the annual meeting of the American Neurological Association. “Loss of brain tissue, especially as measured by reduced volume of gray matter noted on brain MRI, is predictive of long-term disability in MS. While we await the results of confirmatory studies and randomized trials, this study adds to the growing body of evidence suggesting there may be a role for modification of lifestyle factors in mitigating longer-term MS-related disability risk.”
In an effort to determine if body mass index (BMI) or vitamin D status is associated with longer-term MRI measures of neurodegeneration, Dr. Mowry and her colleagues drew from 469 patients participating in a longitudinal MS cohort study at the University of California, San Francisco, known as EPIC. Participants had clinical evaluations, brain MRI, and blood draws annually and were followed for 5 years. The main outcomes of interest were BMI and serum 25-hydroxyvitamin D levels measured over the time period, and their relationship to brain volume.
At baseline, the mean age of patients was 42 years, 70% were female, their mean BMI was 25 kg/m2, and their mean serum vitamin D level was 27.8 ng/mL. Dr. Mowry, a neurologist at Johns Hopkins University, Baltimore, and her colleagues found that over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001). In addition, each 1-kg/m2 higher BMI over time was independently associated with greater declines in normalized brain parenchymal brain volume (–1.1 mL; P = .039). Elevated vitamin D levels, however, did not appear to be meaningfully associated with brain volumes.
Dr. Mowry acknowledged certain limitations of the study, including its nonrandomized design. “Such a trial may be warranted but I believe will be challenging to conduct,” she said. “Also, this cohort was designed to assess the association of genes with brain MRI outcomes, and so the people included were racially homogeneous – only Caucasians were included. Since MS risk is especially high among African Americans in recent years, and African Americans appear overall to have a higher risk of long-term disability, it is important to evaluate these and other prognostic factors amongst a more representative group of people with MS.”
The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
SOURCE: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
ATLANTA – Among patients with relapsing-remitting multiple sclerosis, higher body mass index, but not vitamin D status, appears to be related to greater loss of gray matter brain volume over time, results from a 5-year analysis showed.
“We had previously known that obesity is a risk factor for developing MS, and among those who already have the disease, obesity-related comorbidities are associated with increased morbidity and mortality,” lead study author Ellen M. Mowry, MD, said in an interview at the annual meeting of the American Neurological Association. “Loss of brain tissue, especially as measured by reduced volume of gray matter noted on brain MRI, is predictive of long-term disability in MS. While we await the results of confirmatory studies and randomized trials, this study adds to the growing body of evidence suggesting there may be a role for modification of lifestyle factors in mitigating longer-term MS-related disability risk.”
In an effort to determine if body mass index (BMI) or vitamin D status is associated with longer-term MRI measures of neurodegeneration, Dr. Mowry and her colleagues drew from 469 patients participating in a longitudinal MS cohort study at the University of California, San Francisco, known as EPIC. Participants had clinical evaluations, brain MRI, and blood draws annually and were followed for 5 years. The main outcomes of interest were BMI and serum 25-hydroxyvitamin D levels measured over the time period, and their relationship to brain volume.
At baseline, the mean age of patients was 42 years, 70% were female, their mean BMI was 25 kg/m2, and their mean serum vitamin D level was 27.8 ng/mL. Dr. Mowry, a neurologist at Johns Hopkins University, Baltimore, and her colleagues found that over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001). In addition, each 1-kg/m2 higher BMI over time was independently associated with greater declines in normalized brain parenchymal brain volume (–1.1 mL; P = .039). Elevated vitamin D levels, however, did not appear to be meaningfully associated with brain volumes.
Dr. Mowry acknowledged certain limitations of the study, including its nonrandomized design. “Such a trial may be warranted but I believe will be challenging to conduct,” she said. “Also, this cohort was designed to assess the association of genes with brain MRI outcomes, and so the people included were racially homogeneous – only Caucasians were included. Since MS risk is especially high among African Americans in recent years, and African Americans appear overall to have a higher risk of long-term disability, it is important to evaluate these and other prognostic factors amongst a more representative group of people with MS.”
The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
SOURCE: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
ATLANTA – Among patients with relapsing-remitting multiple sclerosis, higher body mass index, but not vitamin D status, appears to be related to greater loss of gray matter brain volume over time, results from a 5-year analysis showed.
“We had previously known that obesity is a risk factor for developing MS, and among those who already have the disease, obesity-related comorbidities are associated with increased morbidity and mortality,” lead study author Ellen M. Mowry, MD, said in an interview at the annual meeting of the American Neurological Association. “Loss of brain tissue, especially as measured by reduced volume of gray matter noted on brain MRI, is predictive of long-term disability in MS. While we await the results of confirmatory studies and randomized trials, this study adds to the growing body of evidence suggesting there may be a role for modification of lifestyle factors in mitigating longer-term MS-related disability risk.”
In an effort to determine if body mass index (BMI) or vitamin D status is associated with longer-term MRI measures of neurodegeneration, Dr. Mowry and her colleagues drew from 469 patients participating in a longitudinal MS cohort study at the University of California, San Francisco, known as EPIC. Participants had clinical evaluations, brain MRI, and blood draws annually and were followed for 5 years. The main outcomes of interest were BMI and serum 25-hydroxyvitamin D levels measured over the time period, and their relationship to brain volume.
At baseline, the mean age of patients was 42 years, 70% were female, their mean BMI was 25 kg/m2, and their mean serum vitamin D level was 27.8 ng/mL. Dr. Mowry, a neurologist at Johns Hopkins University, Baltimore, and her colleagues found that over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001). In addition, each 1-kg/m2 higher BMI over time was independently associated with greater declines in normalized brain parenchymal brain volume (–1.1 mL; P = .039). Elevated vitamin D levels, however, did not appear to be meaningfully associated with brain volumes.
Dr. Mowry acknowledged certain limitations of the study, including its nonrandomized design. “Such a trial may be warranted but I believe will be challenging to conduct,” she said. “Also, this cohort was designed to assess the association of genes with brain MRI outcomes, and so the people included were racially homogeneous – only Caucasians were included. Since MS risk is especially high among African Americans in recent years, and African Americans appear overall to have a higher risk of long-term disability, it is important to evaluate these and other prognostic factors amongst a more representative group of people with MS.”
The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
SOURCE: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
AT ANA 2018
Key clinical point: Higher body mass in MS patients appears to be related to greater brain atrophy over time.
Major finding: Over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001).
Study details: An analysis of 469 patients participating in a longitudinal MS cohort study.
Disclosures: The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
Source: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
Shorter interpregnancy intervals may increase risk of adverse outcomes
Short interpregnancy intervals carry an increased risk of adverse pregnancy outcomes for women of all ages and increased adverse fetal and infant outcome risks for women between 20 and 34 years old, according to research published in JAMA Internal Medicine.
“This finding may be reassuring particularly for older women who must weigh the competing risks of increasing maternal age with longer interpregnancy intervals (including infertility and chromosomal anomalies) against the risks of short interpregnancy intervals,” wrote Laura Schummers, SD, of the department of epidemiology at Harvard T. H. Chan School of Public Health, Boston, and her colleagues.
The researchers examined 148,544 pregnancies of women in British Columbia who were younger than 20 years old at the index (5%), 20-34 years at the index birth (83%), and 35 years or older (12%). The women had two or more consecutive singleton pregnancies that resulted in a live birth between 2004 and 2014 and were recorded in the British Columbia Perinatal Data Registry. There was a lower number of short interpregnancy intervals, defined as less than 6 months between the index and second pregnancy, among women in the 35-years-or-older group, compared with the 20- to 34-year-old group (4.4% vs. 5.5%); the 35-years-or-older group instead had a higher number of interpregnancy intervals between 6 and 11 months and between 12 and 17 months, compared with the 20- to 34-year-old group (17.7% vs. 16.6%, and 25.2% vs. 22.5%, respectively).
The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk [aRR], 2.39). There was no significant increase in those aged between 20 and 34 years at 6 months, compared with 18 months (0.23% vs. 0.25%; aRR, 0.92). However, the 20- to 34-year-old group did have an increased risk of fetal and infant adverse outcomes at 6 months, compared with 18 months (2.0% vs. 1.4%; aRR, 1.42) and compared with women in the 35-years-or-older group at 6 months and 18 months (2.1% vs. 1.8%; aRR, 1.15).
There was a 5.3% increased risk at 6 months and a 3.2% increased risk at 18 months of spontaneous preterm delivery in the 20- to 34-year-old group (aRR, 1.65), compared with a 5.0% risk at 6 months and 3.6% at 18 months in the 35-years-or-older group (aRR, 1.40). The researchers noted “modest increases” in newborns who were born small for their gestational age and indicated preterm delivery at short intervals that did not differ by age group.
The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by various other awards.
SOURCE: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
While the findings of Schummers et al. appear to encourage pregnancy spacing among women of all ages, women who are 35 or older should be counseled differently than women aged 20-34 years, Stephanie B. Teal, MD, MPH, and Jeanelle Sheeder, MSPH, PhD, wrote in a related editorial.
“Clinicians should understand that women delivering at age 35 years or later may desire more children and may wish to conceive sooner than recommended,” the authors wrote.
Women who are 35 years old or older may not have 6-12 months to delay pregnancy, the authors explained, and thus should be counseled differently than younger patients. Delaying pregnancy in older women may increase the risk of miscarriage and chromosomal abnormalities, and may cause families to miss out on their desired family size. In addition, spacing out births up to 24 months apart does not significantly diminish the risk of fetal or infant risk among women 35 years and older as it does for younger women, which may make short interpregnancy intervals in this group a “rational choice.”
“Simply telling older women to delay conception is not likely to improve health outcomes, as women are aware of their ‘biological clocks’ and many will value their desire for another child over their physician’s warnings,” Dr. Teal and Dr. Sheeder noted. “Clinicians should use patient-centered counseling and shared decision-making strategies that respect women’s desires for pregnancy, possibly at short intervals in women 35 years or older, and adequately discuss fetal, infant, and maternal risks in this context.”
Dr. Teal and Dr. Sheeder are in the division of family planning in the department of obstetrics and gynecology at the University of Colorado in Aurora. Their their comments were made in an editorial in JAMA Internal Medicine (2018 Oct 29. doi: 10.1001/jamainternmed.2018.4734 ). They reported no conflicts of interest.
While the findings of Schummers et al. appear to encourage pregnancy spacing among women of all ages, women who are 35 or older should be counseled differently than women aged 20-34 years, Stephanie B. Teal, MD, MPH, and Jeanelle Sheeder, MSPH, PhD, wrote in a related editorial.
“Clinicians should understand that women delivering at age 35 years or later may desire more children and may wish to conceive sooner than recommended,” the authors wrote.
Women who are 35 years old or older may not have 6-12 months to delay pregnancy, the authors explained, and thus should be counseled differently than younger patients. Delaying pregnancy in older women may increase the risk of miscarriage and chromosomal abnormalities, and may cause families to miss out on their desired family size. In addition, spacing out births up to 24 months apart does not significantly diminish the risk of fetal or infant risk among women 35 years and older as it does for younger women, which may make short interpregnancy intervals in this group a “rational choice.”
“Simply telling older women to delay conception is not likely to improve health outcomes, as women are aware of their ‘biological clocks’ and many will value their desire for another child over their physician’s warnings,” Dr. Teal and Dr. Sheeder noted. “Clinicians should use patient-centered counseling and shared decision-making strategies that respect women’s desires for pregnancy, possibly at short intervals in women 35 years or older, and adequately discuss fetal, infant, and maternal risks in this context.”
Dr. Teal and Dr. Sheeder are in the division of family planning in the department of obstetrics and gynecology at the University of Colorado in Aurora. Their their comments were made in an editorial in JAMA Internal Medicine (2018 Oct 29. doi: 10.1001/jamainternmed.2018.4734 ). They reported no conflicts of interest.
While the findings of Schummers et al. appear to encourage pregnancy spacing among women of all ages, women who are 35 or older should be counseled differently than women aged 20-34 years, Stephanie B. Teal, MD, MPH, and Jeanelle Sheeder, MSPH, PhD, wrote in a related editorial.
“Clinicians should understand that women delivering at age 35 years or later may desire more children and may wish to conceive sooner than recommended,” the authors wrote.
Women who are 35 years old or older may not have 6-12 months to delay pregnancy, the authors explained, and thus should be counseled differently than younger patients. Delaying pregnancy in older women may increase the risk of miscarriage and chromosomal abnormalities, and may cause families to miss out on their desired family size. In addition, spacing out births up to 24 months apart does not significantly diminish the risk of fetal or infant risk among women 35 years and older as it does for younger women, which may make short interpregnancy intervals in this group a “rational choice.”
“Simply telling older women to delay conception is not likely to improve health outcomes, as women are aware of their ‘biological clocks’ and many will value their desire for another child over their physician’s warnings,” Dr. Teal and Dr. Sheeder noted. “Clinicians should use patient-centered counseling and shared decision-making strategies that respect women’s desires for pregnancy, possibly at short intervals in women 35 years or older, and adequately discuss fetal, infant, and maternal risks in this context.”
Dr. Teal and Dr. Sheeder are in the division of family planning in the department of obstetrics and gynecology at the University of Colorado in Aurora. Their their comments were made in an editorial in JAMA Internal Medicine (2018 Oct 29. doi: 10.1001/jamainternmed.2018.4734 ). They reported no conflicts of interest.
Short interpregnancy intervals carry an increased risk of adverse pregnancy outcomes for women of all ages and increased adverse fetal and infant outcome risks for women between 20 and 34 years old, according to research published in JAMA Internal Medicine.
“This finding may be reassuring particularly for older women who must weigh the competing risks of increasing maternal age with longer interpregnancy intervals (including infertility and chromosomal anomalies) against the risks of short interpregnancy intervals,” wrote Laura Schummers, SD, of the department of epidemiology at Harvard T. H. Chan School of Public Health, Boston, and her colleagues.
The researchers examined 148,544 pregnancies of women in British Columbia who were younger than 20 years old at the index (5%), 20-34 years at the index birth (83%), and 35 years or older (12%). The women had two or more consecutive singleton pregnancies that resulted in a live birth between 2004 and 2014 and were recorded in the British Columbia Perinatal Data Registry. There was a lower number of short interpregnancy intervals, defined as less than 6 months between the index and second pregnancy, among women in the 35-years-or-older group, compared with the 20- to 34-year-old group (4.4% vs. 5.5%); the 35-years-or-older group instead had a higher number of interpregnancy intervals between 6 and 11 months and between 12 and 17 months, compared with the 20- to 34-year-old group (17.7% vs. 16.6%, and 25.2% vs. 22.5%, respectively).
The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk [aRR], 2.39). There was no significant increase in those aged between 20 and 34 years at 6 months, compared with 18 months (0.23% vs. 0.25%; aRR, 0.92). However, the 20- to 34-year-old group did have an increased risk of fetal and infant adverse outcomes at 6 months, compared with 18 months (2.0% vs. 1.4%; aRR, 1.42) and compared with women in the 35-years-or-older group at 6 months and 18 months (2.1% vs. 1.8%; aRR, 1.15).
There was a 5.3% increased risk at 6 months and a 3.2% increased risk at 18 months of spontaneous preterm delivery in the 20- to 34-year-old group (aRR, 1.65), compared with a 5.0% risk at 6 months and 3.6% at 18 months in the 35-years-or-older group (aRR, 1.40). The researchers noted “modest increases” in newborns who were born small for their gestational age and indicated preterm delivery at short intervals that did not differ by age group.
The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by various other awards.
SOURCE: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
Short interpregnancy intervals carry an increased risk of adverse pregnancy outcomes for women of all ages and increased adverse fetal and infant outcome risks for women between 20 and 34 years old, according to research published in JAMA Internal Medicine.
“This finding may be reassuring particularly for older women who must weigh the competing risks of increasing maternal age with longer interpregnancy intervals (including infertility and chromosomal anomalies) against the risks of short interpregnancy intervals,” wrote Laura Schummers, SD, of the department of epidemiology at Harvard T. H. Chan School of Public Health, Boston, and her colleagues.
The researchers examined 148,544 pregnancies of women in British Columbia who were younger than 20 years old at the index (5%), 20-34 years at the index birth (83%), and 35 years or older (12%). The women had two or more consecutive singleton pregnancies that resulted in a live birth between 2004 and 2014 and were recorded in the British Columbia Perinatal Data Registry. There was a lower number of short interpregnancy intervals, defined as less than 6 months between the index and second pregnancy, among women in the 35-years-or-older group, compared with the 20- to 34-year-old group (4.4% vs. 5.5%); the 35-years-or-older group instead had a higher number of interpregnancy intervals between 6 and 11 months and between 12 and 17 months, compared with the 20- to 34-year-old group (17.7% vs. 16.6%, and 25.2% vs. 22.5%, respectively).
The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk [aRR], 2.39). There was no significant increase in those aged between 20 and 34 years at 6 months, compared with 18 months (0.23% vs. 0.25%; aRR, 0.92). However, the 20- to 34-year-old group did have an increased risk of fetal and infant adverse outcomes at 6 months, compared with 18 months (2.0% vs. 1.4%; aRR, 1.42) and compared with women in the 35-years-or-older group at 6 months and 18 months (2.1% vs. 1.8%; aRR, 1.15).
There was a 5.3% increased risk at 6 months and a 3.2% increased risk at 18 months of spontaneous preterm delivery in the 20- to 34-year-old group (aRR, 1.65), compared with a 5.0% risk at 6 months and 3.6% at 18 months in the 35-years-or-older group (aRR, 1.40). The researchers noted “modest increases” in newborns who were born small for their gestational age and indicated preterm delivery at short intervals that did not differ by age group.
The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by various other awards.
SOURCE: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: The risk for maternal mortality or severe morbidity was higher in women who were a minimum 35 years old with 6 months between pregnancies (0.62%), compared with women who had 18 months (0.26%) between pregnancies (adjusted relative risk, 2.39).
Study details: A cohort study of 148,544 pregnancies in Canada between 2004 and 2014.
Disclosures: The authors reported no conflicts of interest. Dr Schummers was supported a National Research Service Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and received a grant from the Canadian Institutes for Health Research and the Public Health Agency of Canada Family Planning Public Health Chair Seed Grant. Two of her coauthors were supported by other awards.
Source: Schummers L et al. JAMA Intern Med. 2018 Oct 29. doi: 10.1001/jamainternmed.2018.4696.
Heart cell transplant rejections
The concept that cell transplantation is the answer to the treatment of heart failure may have suffered a major setback as a result of a research scam perpetrated at Harvard University’s Brigham and Women’s Hospital in Boston.
The fraudulent cardiac research at that institution was from the lab of Piero Anversa, MD, which falsified or fabricated data. Dr. Anversa was one of the leaders in pursuing the concept that adult cardiomyocytes can not only regenerate by cell division but can also be transplanted from one animal to another, in his case a mouse.
The original reports over a decade ago caused a major stir in heart failure research. They also were met with considerable skepticism in the research field and have not been reproduced in other laboratories. Numerous small trials in humans have been unsuccessful in demonstrating the survival of autologous cells transplanted in both animal and humans. Dr. Anversa’s research has been under scrutiny by Harvard and Brigham and Women’s since 2013, ultimately resulting in the call to retract 31 published studies in mid-October. In the meantime, admission of fraud in regard to the original Anversa papers resulted in a fine of $10 million paid by the institutions to the National Institutes of Health in a 2017 settlement. Dr. Anversa left Harvard in 2015.
Nevertheless Dr. Anversa’s concepts led to the initiation of an NIH-supported multicenter trial in humans of the implantation of autologous bone marrow cells using endocardial devices to transplant cells in 144 patients with heart failure. The Combination of Autologous Bone Marrow Derived Mesenchymal and C-Kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) was started in 2015 and was still recruiting until Oct. 29, when the National Heart, Lung, and Blood Institute announced that a pause in recruitment was called in order to review the fraudulent data that led to the trial’s initiation. Patients were to be followed over a 1-year period using delayed-enhanced magnetic resonance imaging (DEMRI) scans to assess scar size and left ventricular function and structure at baseline and at 6 and 12 months post study product ad-ministration. For the purpose of the endpoint analysis and safety evaluations, the investigators planned to use an intention-to-treat study population evaluating a number of clinical parameters.
Anatomists and physiologists have long been of the opinion that adult human and mammalian cardiomyocytes are terminally differentiated and do not undergo cell division. However, over time, cardiomyocytes can undergo hypertrophy as a result of increased workload. Cells can die as a result of ischemia, infarction, or stress mediated through unchecked inflammatory processes. It also has been shown that cells can die as a result of apoptosis, particularly in areas in proximity to myocardial scars. It is generally believed that these three methods of cell depletion contribute to the progression of heart failure. Dr. Anversa also proposed that there is some degree of replication of cardiomyocytes but not to a degree that can have a meaningful replacement value.
The concept of cell transplantation in medicine certainly has been in the forefront of clinical research in medicine for the last decade based in part on research by Dr. Anversa and others in cardiology and numerous investigators in other medical disciplines. With few exceptions, there has been little support for the clinical benefit of these clinical studies. We are unfortunately now faced with the release of the tainted fraudulent research that led to CONCERT-HF. Whether there is anything of scientific value to come of the trial is highly unlikely.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This article was updated Oct. 30, 2018.
The concept that cell transplantation is the answer to the treatment of heart failure may have suffered a major setback as a result of a research scam perpetrated at Harvard University’s Brigham and Women’s Hospital in Boston.
The fraudulent cardiac research at that institution was from the lab of Piero Anversa, MD, which falsified or fabricated data. Dr. Anversa was one of the leaders in pursuing the concept that adult cardiomyocytes can not only regenerate by cell division but can also be transplanted from one animal to another, in his case a mouse.
The original reports over a decade ago caused a major stir in heart failure research. They also were met with considerable skepticism in the research field and have not been reproduced in other laboratories. Numerous small trials in humans have been unsuccessful in demonstrating the survival of autologous cells transplanted in both animal and humans. Dr. Anversa’s research has been under scrutiny by Harvard and Brigham and Women’s since 2013, ultimately resulting in the call to retract 31 published studies in mid-October. In the meantime, admission of fraud in regard to the original Anversa papers resulted in a fine of $10 million paid by the institutions to the National Institutes of Health in a 2017 settlement. Dr. Anversa left Harvard in 2015.
Nevertheless Dr. Anversa’s concepts led to the initiation of an NIH-supported multicenter trial in humans of the implantation of autologous bone marrow cells using endocardial devices to transplant cells in 144 patients with heart failure. The Combination of Autologous Bone Marrow Derived Mesenchymal and C-Kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) was started in 2015 and was still recruiting until Oct. 29, when the National Heart, Lung, and Blood Institute announced that a pause in recruitment was called in order to review the fraudulent data that led to the trial’s initiation. Patients were to be followed over a 1-year period using delayed-enhanced magnetic resonance imaging (DEMRI) scans to assess scar size and left ventricular function and structure at baseline and at 6 and 12 months post study product ad-ministration. For the purpose of the endpoint analysis and safety evaluations, the investigators planned to use an intention-to-treat study population evaluating a number of clinical parameters.
Anatomists and physiologists have long been of the opinion that adult human and mammalian cardiomyocytes are terminally differentiated and do not undergo cell division. However, over time, cardiomyocytes can undergo hypertrophy as a result of increased workload. Cells can die as a result of ischemia, infarction, or stress mediated through unchecked inflammatory processes. It also has been shown that cells can die as a result of apoptosis, particularly in areas in proximity to myocardial scars. It is generally believed that these three methods of cell depletion contribute to the progression of heart failure. Dr. Anversa also proposed that there is some degree of replication of cardiomyocytes but not to a degree that can have a meaningful replacement value.
The concept of cell transplantation in medicine certainly has been in the forefront of clinical research in medicine for the last decade based in part on research by Dr. Anversa and others in cardiology and numerous investigators in other medical disciplines. With few exceptions, there has been little support for the clinical benefit of these clinical studies. We are unfortunately now faced with the release of the tainted fraudulent research that led to CONCERT-HF. Whether there is anything of scientific value to come of the trial is highly unlikely.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This article was updated Oct. 30, 2018.
The concept that cell transplantation is the answer to the treatment of heart failure may have suffered a major setback as a result of a research scam perpetrated at Harvard University’s Brigham and Women’s Hospital in Boston.
The fraudulent cardiac research at that institution was from the lab of Piero Anversa, MD, which falsified or fabricated data. Dr. Anversa was one of the leaders in pursuing the concept that adult cardiomyocytes can not only regenerate by cell division but can also be transplanted from one animal to another, in his case a mouse.
The original reports over a decade ago caused a major stir in heart failure research. They also were met with considerable skepticism in the research field and have not been reproduced in other laboratories. Numerous small trials in humans have been unsuccessful in demonstrating the survival of autologous cells transplanted in both animal and humans. Dr. Anversa’s research has been under scrutiny by Harvard and Brigham and Women’s since 2013, ultimately resulting in the call to retract 31 published studies in mid-October. In the meantime, admission of fraud in regard to the original Anversa papers resulted in a fine of $10 million paid by the institutions to the National Institutes of Health in a 2017 settlement. Dr. Anversa left Harvard in 2015.
Nevertheless Dr. Anversa’s concepts led to the initiation of an NIH-supported multicenter trial in humans of the implantation of autologous bone marrow cells using endocardial devices to transplant cells in 144 patients with heart failure. The Combination of Autologous Bone Marrow Derived Mesenchymal and C-Kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) was started in 2015 and was still recruiting until Oct. 29, when the National Heart, Lung, and Blood Institute announced that a pause in recruitment was called in order to review the fraudulent data that led to the trial’s initiation. Patients were to be followed over a 1-year period using delayed-enhanced magnetic resonance imaging (DEMRI) scans to assess scar size and left ventricular function and structure at baseline and at 6 and 12 months post study product ad-ministration. For the purpose of the endpoint analysis and safety evaluations, the investigators planned to use an intention-to-treat study population evaluating a number of clinical parameters.
Anatomists and physiologists have long been of the opinion that adult human and mammalian cardiomyocytes are terminally differentiated and do not undergo cell division. However, over time, cardiomyocytes can undergo hypertrophy as a result of increased workload. Cells can die as a result of ischemia, infarction, or stress mediated through unchecked inflammatory processes. It also has been shown that cells can die as a result of apoptosis, particularly in areas in proximity to myocardial scars. It is generally believed that these three methods of cell depletion contribute to the progression of heart failure. Dr. Anversa also proposed that there is some degree of replication of cardiomyocytes but not to a degree that can have a meaningful replacement value.
The concept of cell transplantation in medicine certainly has been in the forefront of clinical research in medicine for the last decade based in part on research by Dr. Anversa and others in cardiology and numerous investigators in other medical disciplines. With few exceptions, there has been little support for the clinical benefit of these clinical studies. We are unfortunately now faced with the release of the tainted fraudulent research that led to CONCERT-HF. Whether there is anything of scientific value to come of the trial is highly unlikely.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This article was updated Oct. 30, 2018.
SVS announces honor recognizing surgeons in community practice
The Society for Vascular Surgery Community Practice Committee announces the Excellence in Community Service Award, honoring a member who has made contributions not only to the profession but to the community as well. Applications are due Feb. 1, 2019. The recipient will be announced and recognized at the 2019 Vascular Annual Meeting in June. Nominees must have practiced vascular surgery for at least 20 years and been an SVS member for at least five. They also must present evidence of impact on vascular care or community health.
The Society for Vascular Surgery Community Practice Committee announces the Excellence in Community Service Award, honoring a member who has made contributions not only to the profession but to the community as well. Applications are due Feb. 1, 2019. The recipient will be announced and recognized at the 2019 Vascular Annual Meeting in June. Nominees must have practiced vascular surgery for at least 20 years and been an SVS member for at least five. They also must present evidence of impact on vascular care or community health.
The Society for Vascular Surgery Community Practice Committee announces the Excellence in Community Service Award, honoring a member who has made contributions not only to the profession but to the community as well. Applications are due Feb. 1, 2019. The recipient will be announced and recognized at the 2019 Vascular Annual Meeting in June. Nominees must have practiced vascular surgery for at least 20 years and been an SVS member for at least five. They also must present evidence of impact on vascular care or community health.
Read the new 2018 Foundation Annual Report
Download Below
The SVS Foundation’s 2018 Annual Report has just been published online. The report highlights the Foundation’s work, the money raised, and money spent. It tells why people give and the profound difference SVS members make, not only in their research labs but also in their communities. The Foundation offers a number of ways to give and a number of funds – the general fund, disaster relief, research and more – to which donations may be directed. This is the season for the SVS annual Giving Campaign. Please read the report and give today.
Download Below
The SVS Foundation’s 2018 Annual Report has just been published online. The report highlights the Foundation’s work, the money raised, and money spent. It tells why people give and the profound difference SVS members make, not only in their research labs but also in their communities. The Foundation offers a number of ways to give and a number of funds – the general fund, disaster relief, research and more – to which donations may be directed. This is the season for the SVS annual Giving Campaign. Please read the report and give today.
Download Below
The SVS Foundation’s 2018 Annual Report has just been published online. The report highlights the Foundation’s work, the money raised, and money spent. It tells why people give and the profound difference SVS members make, not only in their research labs but also in their communities. The Foundation offers a number of ways to give and a number of funds – the general fund, disaster relief, research and more – to which donations may be directed. This is the season for the SVS annual Giving Campaign. Please read the report and give today.
AKI linked to later dementia
ACIP votes unanimously in favor of immunization schedule updated and redesign, could daptomycin/fosfomycin be a new standard for MRSA bacteremia? Plus, expert analysis on Justice Kavanaugh’s lasting healthcare impact.
Amazon Alexa
Apple Podcasts
Spotify
ACIP votes unanimously in favor of immunization schedule updated and redesign, could daptomycin/fosfomycin be a new standard for MRSA bacteremia? Plus, expert analysis on Justice Kavanaugh’s lasting healthcare impact.
Amazon Alexa
Apple Podcasts
Spotify
ACIP votes unanimously in favor of immunization schedule updated and redesign, could daptomycin/fosfomycin be a new standard for MRSA bacteremia? Plus, expert analysis on Justice Kavanaugh’s lasting healthcare impact.
Amazon Alexa
Apple Podcasts
Spotify
An Imposter Twice Over: A Case of IgG4-Related Disease
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
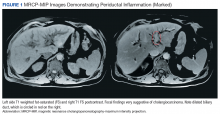
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
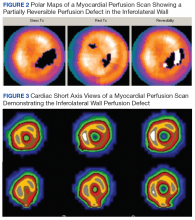
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Does Coffee Help or Harm Patients With HBV?
Coffee drinking has been linked to the reduced risk of fibrosis progression, liver cirrhosis, and hepatocellular carcinoma in some patients, including those with hepatitis C virus (HCV) infection, but the results of studies in patients with hepatitis B virus (HBV) infection have been inconsistent. Given the “global impact of HBV infection and the wide consumption of coffee,” researchers from Tzu Chi University in Taiwan, wanted to find out more.
They analyzed data from 328 patients with chronic HBV infection who were enrolled in a population-based gastroesophageal reflux disease study. Of those, 155 patients also entered into a 5-year follow-up study.
Among the patients with chronic HBV, 137 did not drink coffee. Of the 191 who did, 61 drank it on < 4 days a week, and 130 drank it ≥ 4 days.
Initially, the researchers observed an inverse association between coffee drinking and serum aspartate aminotransferase (AST) levels, as well as predicting indices of liver fibrosis in patients with HBV infection. Patients who drank ≥ 4 cups of coffee per day had a 70% decrease of serum AST, a 70% decrease of the AST to platelet ratio index, and a 70% decrease of fibrosis-4 index values.
Those findings indicated that coffee might have a “generally beneficial” effect on liver inflammation and fibrosis progression in patients with chronic liver disease, the researchers say. However, at the end of the 5-year follow-up, the incidences of liver cirrhosis complications and changes of serum predicting indices of liver fibrosis were comparable between HBV coffee drinkers and nondrinkers. That indicated, the researchers believe, that the beneficial effect “seems to be outweighed” in patients with chronic HBV infection.
The researchers suggest that the protective effects of coffee consumption on liver inflammation and insulin resistance may not be able to surpass the direct carcinogenic effect of HBV, and even the HBV virus replication.
Coffee drinking has been linked to the reduced risk of fibrosis progression, liver cirrhosis, and hepatocellular carcinoma in some patients, including those with hepatitis C virus (HCV) infection, but the results of studies in patients with hepatitis B virus (HBV) infection have been inconsistent. Given the “global impact of HBV infection and the wide consumption of coffee,” researchers from Tzu Chi University in Taiwan, wanted to find out more.
They analyzed data from 328 patients with chronic HBV infection who were enrolled in a population-based gastroesophageal reflux disease study. Of those, 155 patients also entered into a 5-year follow-up study.
Among the patients with chronic HBV, 137 did not drink coffee. Of the 191 who did, 61 drank it on < 4 days a week, and 130 drank it ≥ 4 days.
Initially, the researchers observed an inverse association between coffee drinking and serum aspartate aminotransferase (AST) levels, as well as predicting indices of liver fibrosis in patients with HBV infection. Patients who drank ≥ 4 cups of coffee per day had a 70% decrease of serum AST, a 70% decrease of the AST to platelet ratio index, and a 70% decrease of fibrosis-4 index values.
Those findings indicated that coffee might have a “generally beneficial” effect on liver inflammation and fibrosis progression in patients with chronic liver disease, the researchers say. However, at the end of the 5-year follow-up, the incidences of liver cirrhosis complications and changes of serum predicting indices of liver fibrosis were comparable between HBV coffee drinkers and nondrinkers. That indicated, the researchers believe, that the beneficial effect “seems to be outweighed” in patients with chronic HBV infection.
The researchers suggest that the protective effects of coffee consumption on liver inflammation and insulin resistance may not be able to surpass the direct carcinogenic effect of HBV, and even the HBV virus replication.
Coffee drinking has been linked to the reduced risk of fibrosis progression, liver cirrhosis, and hepatocellular carcinoma in some patients, including those with hepatitis C virus (HCV) infection, but the results of studies in patients with hepatitis B virus (HBV) infection have been inconsistent. Given the “global impact of HBV infection and the wide consumption of coffee,” researchers from Tzu Chi University in Taiwan, wanted to find out more.
They analyzed data from 328 patients with chronic HBV infection who were enrolled in a population-based gastroesophageal reflux disease study. Of those, 155 patients also entered into a 5-year follow-up study.
Among the patients with chronic HBV, 137 did not drink coffee. Of the 191 who did, 61 drank it on < 4 days a week, and 130 drank it ≥ 4 days.
Initially, the researchers observed an inverse association between coffee drinking and serum aspartate aminotransferase (AST) levels, as well as predicting indices of liver fibrosis in patients with HBV infection. Patients who drank ≥ 4 cups of coffee per day had a 70% decrease of serum AST, a 70% decrease of the AST to platelet ratio index, and a 70% decrease of fibrosis-4 index values.
Those findings indicated that coffee might have a “generally beneficial” effect on liver inflammation and fibrosis progression in patients with chronic liver disease, the researchers say. However, at the end of the 5-year follow-up, the incidences of liver cirrhosis complications and changes of serum predicting indices of liver fibrosis were comparable between HBV coffee drinkers and nondrinkers. That indicated, the researchers believe, that the beneficial effect “seems to be outweighed” in patients with chronic HBV infection.
The researchers suggest that the protective effects of coffee consumption on liver inflammation and insulin resistance may not be able to surpass the direct carcinogenic effect of HBV, and even the HBV virus replication.