User login
Risk factors identified for urinary retention after lap hernia repair
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
was more likely in patients aged more than 60 years, patients with benign prostatic hyperplasia, and patients with a decreased body mass index, according to findings published in the Journal of Surgical Research.
The researchers note that, while laparoscopic TEP is growing in popularity for inguinal hernia repair, postop urinary retention (POUR) is estimated at 2%-30%, but for open procedures, it is estimated at 0.4%-3%. POUR is linked to the development of urinary tract infections and also hospital readmissions. Since TEP may eventually become the norm, the study authors suggest that identifying patients at higher risk for POUR would contribute to the safety and quality of care for this operation.
In a retrospective chart review of 578 patients who had the procedure between 2009 and 2016, patients over age 60 years, patients with benign prostatic hyperplasia, and those with a body mass index (BMI) of less than or equal to 25.8 kg/m2 were more likely to develop postoperative urinary retention (POUR). Patients with these risk factors were also more likely to develop a urinary tract infection within 30 days, reported Daniel Roadman, a medical student in the department of surgery at Medical College of Wisconsin in Milwaukee, and coauthors.
Investigators conducted a retrospective chart review of patients 18 years of age or older with a direct, indirect, and/or femoral inguinal hernia. POUR was defined as “inability to void spontaneously prior to hospital discharge, requiring straight or indwelling catheter placement,” the authors wrote.
Patients were required to void before being discharged. For patients unable to void, an indwelling catheter was placed and removed the following morning. Patients still unable to void at this point were discharged with an indwelling catheter and scheduled for follow-up within 1 week.
POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI. Patients with POUR had increased incidence of UTI (6.3%), compared with patients without POUR (0.6%; P less than .0001).
Among patients who developed POUR, 54 (84.3%) were admitted for overnight observation with a stay of approximately 1.5 days. Three of these patients (5.6%) had a straight catheterization, and 51 (94.4%) had an indwelling urinary catheter placed. Two patients developed a UTI.
Of the 10 patients discharged home, six (60%) returned to the emergency department for catheterization; two (33.3%) patients had straight catheterization, and four (66.7%) were discharged home with an indwelling catheter. Two of these patients developed a UTI. In both groups, all patients who developed a UTI had an indwelling catheter placed, Mr. Roadman and colleagues reported.
During the study period, institutional protocol changed from routine intraoperative urinary catheterization to catheterization per surgeon discretion, though this did not affect POUR incidence.
“This is the first study to show a significant increase in UTI within 30 days after POUR,” the authors wrote. “Urinary stasis within the bladder due to the inability to void could lead to increased bacterial load and risk of infection.”
“It is important for providers, especially surgeons, to understand the risk of POUR and therefore increased risk of UTI after laparoscopic TEP inguinal hernia repair,” they added. “Identifying patients at higher risk … can help with patient education and expectations.”
No disclosures were reported by the study authors.
SOURCE: Roadman D et al. J Surg Res. 2018;11(231):309-15.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Postoperative urinary retention was more likely to occur in patients more than 60 years of age and patients with benign prostatic hyperplasia.
Major finding: POUR occurred in 64 of the 578 patients (11.1%), and was significantly associated with benign prostatic hyperplasia, age of 60 years or older, development of urinary tract infection (UTI) within 30 days, and decreased BMI.
Study details: A retrospective chart review of 578 patients who had laparoscopic total extraperitoneal inguinal hernia repair between 2009 and 2016.
Disclosures: No disclosures were reported.
Source: Roadman D et al. J Surg Res. 2018;11(231):309-15.
Topical treatment with retinoid/benzoyl peroxide combination reduced acne scars
PARIS – Treatment with the fixed combination in a multicenter, randomized trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“To my knowledge, this is the first time that we have seen a topical therapy showing a reduction in atrophic acne scars,” said Dr. Dreno, professor and chair of the department of dermatology at Nantes (France) University Hospital.
She reported on 67 adolescents and adults with mainly moderate facial acne randomized to treat half their face with adapalene 0.3%/benzoyl peroxide 2.5% gel (Epiduo Forte) and the other half with the product’s vehicle daily for 6 months. Investigators were blinded as to which side was which. At baseline, patients averaged 40 acne lesions and 12 scars per half face.
The primary efficacy endpoint was the atrophic acne scar count per half face at week 24. At that point, the mean total was 9.5 scars on the active treatment side, compared with 13.3 on the control side. This translated to a statistically significant and clinically meaningful 15.5% decrease in scars with active treatment versus a 14.4% increase with vehicle. The between-side difference achieved statistical significance at week 1 and remained so at all follow-up visits through week 24.
By Scar Global Assessment at week 24, 32.9% of half faces treated with the combination product were rated clear or almost clear, compared with 16.4% with vehicle.
At 24 weeks, 24.1% of participants reported having moderately or very visible holes or indents on the active treatment side of their face, compared with 51.8% on the control side. The number of inflammatory acne lesions fell by 86.7% with the active treatment and 57.9% with vehicle over the course of 24 weeks. Again, the difference became statistically significant starting at week 1. By the Investigator’s Global Assessment at week 24, 64.2% of adapalene/benzoyl peroxide gel–treated faces were rated clear or almost clear, as were 19.4% with vehicle. In addition, 32% of patients reported a marked improvement in skin texture on their active treatment side at 24 weeks, as did 14% on the control side.
The salutary effect on acne scars documented with a topical therapy in this study represents a real advance in clinical care.
“Facial acne scars are a very important and difficult problem for our patients and also for dermatologists,” Dr. Dreno observed, adding that the evidence base for procedural interventions for acne scars, such as dermabrasion and laser resurfacing, is not top quality.
Not surprisingly with a topical retinoid, skin irritation was the most common treatment-emergent adverse event, reported by 14.9% of patients on their active treatment side and 6% with vehicle. This side effect was typically mild and resolved within the first 2-3 weeks.
The improvement in preexisting acne scars documented in this trial was probably caused by drug-induced remodeling of the dermal matrix, according to Dr. Dreno.
The study was funded by Galderma. Dr. Dreno reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
PARIS – Treatment with the fixed combination in a multicenter, randomized trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“To my knowledge, this is the first time that we have seen a topical therapy showing a reduction in atrophic acne scars,” said Dr. Dreno, professor and chair of the department of dermatology at Nantes (France) University Hospital.
She reported on 67 adolescents and adults with mainly moderate facial acne randomized to treat half their face with adapalene 0.3%/benzoyl peroxide 2.5% gel (Epiduo Forte) and the other half with the product’s vehicle daily for 6 months. Investigators were blinded as to which side was which. At baseline, patients averaged 40 acne lesions and 12 scars per half face.
The primary efficacy endpoint was the atrophic acne scar count per half face at week 24. At that point, the mean total was 9.5 scars on the active treatment side, compared with 13.3 on the control side. This translated to a statistically significant and clinically meaningful 15.5% decrease in scars with active treatment versus a 14.4% increase with vehicle. The between-side difference achieved statistical significance at week 1 and remained so at all follow-up visits through week 24.
By Scar Global Assessment at week 24, 32.9% of half faces treated with the combination product were rated clear or almost clear, compared with 16.4% with vehicle.
At 24 weeks, 24.1% of participants reported having moderately or very visible holes or indents on the active treatment side of their face, compared with 51.8% on the control side. The number of inflammatory acne lesions fell by 86.7% with the active treatment and 57.9% with vehicle over the course of 24 weeks. Again, the difference became statistically significant starting at week 1. By the Investigator’s Global Assessment at week 24, 64.2% of adapalene/benzoyl peroxide gel–treated faces were rated clear or almost clear, as were 19.4% with vehicle. In addition, 32% of patients reported a marked improvement in skin texture on their active treatment side at 24 weeks, as did 14% on the control side.
The salutary effect on acne scars documented with a topical therapy in this study represents a real advance in clinical care.
“Facial acne scars are a very important and difficult problem for our patients and also for dermatologists,” Dr. Dreno observed, adding that the evidence base for procedural interventions for acne scars, such as dermabrasion and laser resurfacing, is not top quality.
Not surprisingly with a topical retinoid, skin irritation was the most common treatment-emergent adverse event, reported by 14.9% of patients on their active treatment side and 6% with vehicle. This side effect was typically mild and resolved within the first 2-3 weeks.
The improvement in preexisting acne scars documented in this trial was probably caused by drug-induced remodeling of the dermal matrix, according to Dr. Dreno.
The study was funded by Galderma. Dr. Dreno reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
PARIS – Treatment with the fixed combination in a multicenter, randomized trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“To my knowledge, this is the first time that we have seen a topical therapy showing a reduction in atrophic acne scars,” said Dr. Dreno, professor and chair of the department of dermatology at Nantes (France) University Hospital.
She reported on 67 adolescents and adults with mainly moderate facial acne randomized to treat half their face with adapalene 0.3%/benzoyl peroxide 2.5% gel (Epiduo Forte) and the other half with the product’s vehicle daily for 6 months. Investigators were blinded as to which side was which. At baseline, patients averaged 40 acne lesions and 12 scars per half face.
The primary efficacy endpoint was the atrophic acne scar count per half face at week 24. At that point, the mean total was 9.5 scars on the active treatment side, compared with 13.3 on the control side. This translated to a statistically significant and clinically meaningful 15.5% decrease in scars with active treatment versus a 14.4% increase with vehicle. The between-side difference achieved statistical significance at week 1 and remained so at all follow-up visits through week 24.
By Scar Global Assessment at week 24, 32.9% of half faces treated with the combination product were rated clear or almost clear, compared with 16.4% with vehicle.
At 24 weeks, 24.1% of participants reported having moderately or very visible holes or indents on the active treatment side of their face, compared with 51.8% on the control side. The number of inflammatory acne lesions fell by 86.7% with the active treatment and 57.9% with vehicle over the course of 24 weeks. Again, the difference became statistically significant starting at week 1. By the Investigator’s Global Assessment at week 24, 64.2% of adapalene/benzoyl peroxide gel–treated faces were rated clear or almost clear, as were 19.4% with vehicle. In addition, 32% of patients reported a marked improvement in skin texture on their active treatment side at 24 weeks, as did 14% on the control side.
The salutary effect on acne scars documented with a topical therapy in this study represents a real advance in clinical care.
“Facial acne scars are a very important and difficult problem for our patients and also for dermatologists,” Dr. Dreno observed, adding that the evidence base for procedural interventions for acne scars, such as dermabrasion and laser resurfacing, is not top quality.
Not surprisingly with a topical retinoid, skin irritation was the most common treatment-emergent adverse event, reported by 14.9% of patients on their active treatment side and 6% with vehicle. This side effect was typically mild and resolved within the first 2-3 weeks.
The improvement in preexisting acne scars documented in this trial was probably caused by drug-induced remodeling of the dermal matrix, according to Dr. Dreno.
The study was funded by Galderma. Dr. Dreno reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A fixed combination adapalene 0.3%/benzoyl peroxide 2.5% gel reduced the number of preexisting facial atrophic acne scars and prevented new scar formation.
Major finding: Facial atrophic acne scar count dropped by 15.5% with 6 months of treatment with adapalene 0.3%/benzoyl peroxide 2.5% gel while increasing by 14.4% with vehicle.
Study details: This was a 6-month, prospective, multicenter, randomized, vehicle-controlled, split-face study involving 67 acne patients.
Disclosures: The study was funded by Galderma. The presenter reported receiving research grants from and/or serving as a consultant to Galderma, Bioderma, Pierre Fabre, and La Roche–Posay.
Aripiprazole lauroxil nanocrystal suspension

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL
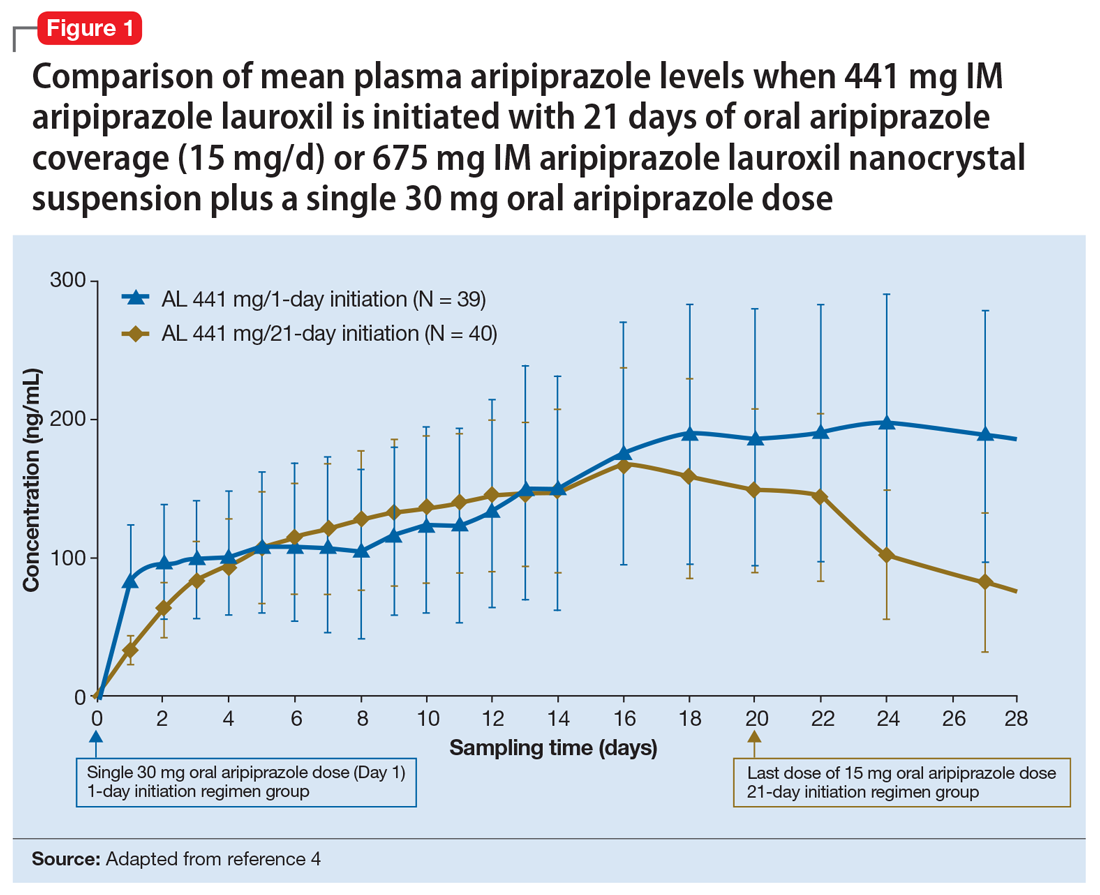
Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL
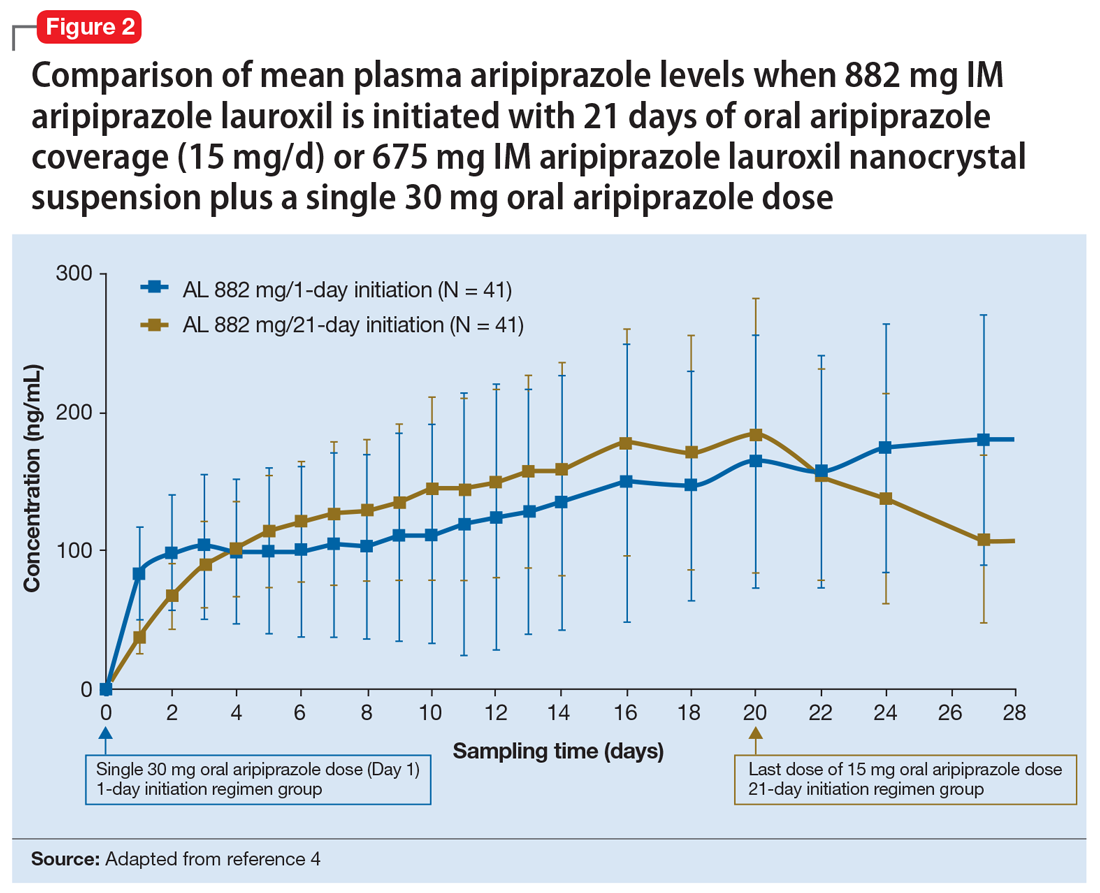
Continue to: Clinical considerations
Clinical considerations
AL
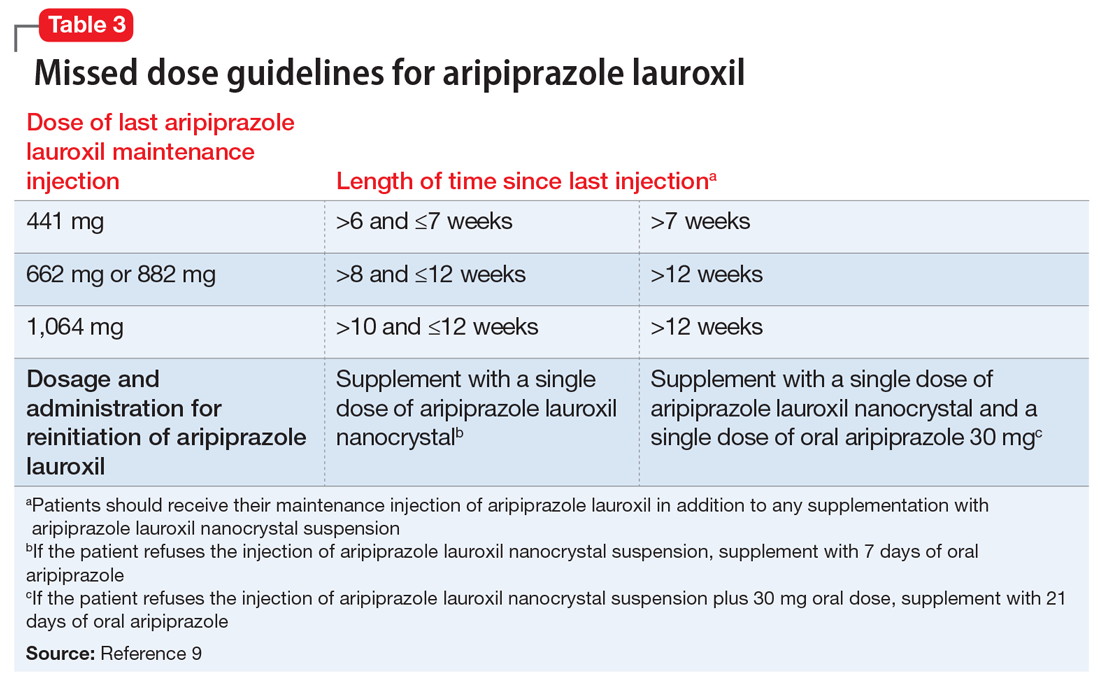
Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL

Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL

Continue to: Clinical considerations
Clinical considerations
AL

Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL

Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL

Continue to: Clinical considerations
Clinical considerations
AL

Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.
Challenging Dogma: The banana bag
Necessary, or just another pretty fluid?
The dogma
Patients with alcohol use disorders (AUD) are at risk for nutritional and vitamin deficiencies and may suffer from linked disease states, including Wernicke’s encephalopathy. These conditions may be underrecognized; for instance, an autopsy study suggests that Wernicke’s encephalopathy may have a prevalence rate of 12.5% among alcoholics.1
When patients with AUD are hospitalized, they have often already received a standard IV solution (100 mg of thiamine, 1 mg of folate, 1-2 g of magnesium, and a multivitamin dissolved in saline or dextrose). The practice is common enough that the solution is informally referred to as a “banana bag,” due to the yellow hue imparted by thiamine and multivitamin. These fluids might then be readministered daily during the inpatient stay. But what is the evidence supporting this widespread practice?
The evidence
While the banana bag (or “rally pack”, as it’s also colloquially known) hanging at the patient’s side may look cool, it may not be helping her. Let’s break down the ingredients:
- Folate: Patients with alcohol use disorder are at higher risk for folate deficiency (attributable to poor intake and decreased absorption), but overall rates of folate deficiency are still quite low.2 In addition, most oral and parenteral multivitamins already contain at least 400 mcg folate – the benefit of adding further intravenous folate is not clear.
- Magnesium. Patients with AUD are also at higher risk for magnesium deficiency attributable to increased excretion. While decreased magnesium levels could theoretically increase the risk of alcohol withdrawal symptoms, a Cochrane review found no evidence to support routine supplementation.3
- Multivitamin. Despite theoretical advantages in these (often) malnourished patients, there are no published studies on the benefit or harm of administering a “pan-vitamin” injection. The standard IV formulation is slightly different than an oral vitamin (the IV contains vitamin K, for instance, and lacks calcium), but the bioavailability should be roughly the same, except in rare patients with intestinal malabsorption.4
- IV fluids. Pharmacies typically mix these ingredients in a liter of normal saline or 5% dextrose. Once again, though, individual patients will have different needs. A dehydrated patient would benefit more from normal saline, a patient with alcoholic ketoacidosis would benefit more from dextrose, and a patient with alcohol-related cardiomyopathy likely shouldn’t be getting large volume IV fluids at all.
- Thiamine. Thiamine deficiency is likely the most common and most concerning vitamin deficiency in this patient population. The typical banana bag contains 100 mg of thiamine, which has been the traditional recommended daily amount for Wernicke’s treatment. This dosage, however, was apparently chosen arbitrarily in the 1950s (based on what the authors considered to be a high dose) and current recommendations suggest higher doses given more frequently because of the relatively short half-life of parenteral thiamine.5
Takeaway
The banana bag is a “one-size-fits-all” approach that offers too much of some of its ingredients and not enough of others. It’s better to individualize treatment based on a patient’s needs and consider high-dose thiamine (500 mg one to three times daily) for those at risk for, or showing signs of, Wernicke’s encephalopathy.
Dr. Sehgal and Dr. Hanson are clinical associate professors of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. Dr. Sehgal (@rtsehgal) is a member of the editorial advisory board for The Hospitalist.
References
1. Torvik A et al. Brain lesions in alcoholics: a neuropathological study with clinical correlation. J Neurol Sci. 1982 Nov;56(2-3):233-48.
2. Schwab RA et al. Prevalence in folate deficiency in emergency department patients with alcohol-related illness or injury. Am J Emerg Med. 1992 May;10(3):203-7.
3. Sarai M et al. Magnesium for alcohol withdrawal. Cochrane Database Syst Rev. 2013 Jun 5;(6):CD008358.
4. Krishel S et al. Intravenous vitamins for alcoholics in the emergency department: a review. J Emerg Med. 1998 May-Jun;16(3):419-24.
5. Donnino MW et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6): 715-21.
Necessary, or just another pretty fluid?
Necessary, or just another pretty fluid?
The dogma
Patients with alcohol use disorders (AUD) are at risk for nutritional and vitamin deficiencies and may suffer from linked disease states, including Wernicke’s encephalopathy. These conditions may be underrecognized; for instance, an autopsy study suggests that Wernicke’s encephalopathy may have a prevalence rate of 12.5% among alcoholics.1
When patients with AUD are hospitalized, they have often already received a standard IV solution (100 mg of thiamine, 1 mg of folate, 1-2 g of magnesium, and a multivitamin dissolved in saline or dextrose). The practice is common enough that the solution is informally referred to as a “banana bag,” due to the yellow hue imparted by thiamine and multivitamin. These fluids might then be readministered daily during the inpatient stay. But what is the evidence supporting this widespread practice?
The evidence
While the banana bag (or “rally pack”, as it’s also colloquially known) hanging at the patient’s side may look cool, it may not be helping her. Let’s break down the ingredients:
- Folate: Patients with alcohol use disorder are at higher risk for folate deficiency (attributable to poor intake and decreased absorption), but overall rates of folate deficiency are still quite low.2 In addition, most oral and parenteral multivitamins already contain at least 400 mcg folate – the benefit of adding further intravenous folate is not clear.
- Magnesium. Patients with AUD are also at higher risk for magnesium deficiency attributable to increased excretion. While decreased magnesium levels could theoretically increase the risk of alcohol withdrawal symptoms, a Cochrane review found no evidence to support routine supplementation.3
- Multivitamin. Despite theoretical advantages in these (often) malnourished patients, there are no published studies on the benefit or harm of administering a “pan-vitamin” injection. The standard IV formulation is slightly different than an oral vitamin (the IV contains vitamin K, for instance, and lacks calcium), but the bioavailability should be roughly the same, except in rare patients with intestinal malabsorption.4
- IV fluids. Pharmacies typically mix these ingredients in a liter of normal saline or 5% dextrose. Once again, though, individual patients will have different needs. A dehydrated patient would benefit more from normal saline, a patient with alcoholic ketoacidosis would benefit more from dextrose, and a patient with alcohol-related cardiomyopathy likely shouldn’t be getting large volume IV fluids at all.
- Thiamine. Thiamine deficiency is likely the most common and most concerning vitamin deficiency in this patient population. The typical banana bag contains 100 mg of thiamine, which has been the traditional recommended daily amount for Wernicke’s treatment. This dosage, however, was apparently chosen arbitrarily in the 1950s (based on what the authors considered to be a high dose) and current recommendations suggest higher doses given more frequently because of the relatively short half-life of parenteral thiamine.5
Takeaway
The banana bag is a “one-size-fits-all” approach that offers too much of some of its ingredients and not enough of others. It’s better to individualize treatment based on a patient’s needs and consider high-dose thiamine (500 mg one to three times daily) for those at risk for, or showing signs of, Wernicke’s encephalopathy.
Dr. Sehgal and Dr. Hanson are clinical associate professors of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. Dr. Sehgal (@rtsehgal) is a member of the editorial advisory board for The Hospitalist.
References
1. Torvik A et al. Brain lesions in alcoholics: a neuropathological study with clinical correlation. J Neurol Sci. 1982 Nov;56(2-3):233-48.
2. Schwab RA et al. Prevalence in folate deficiency in emergency department patients with alcohol-related illness or injury. Am J Emerg Med. 1992 May;10(3):203-7.
3. Sarai M et al. Magnesium for alcohol withdrawal. Cochrane Database Syst Rev. 2013 Jun 5;(6):CD008358.
4. Krishel S et al. Intravenous vitamins for alcoholics in the emergency department: a review. J Emerg Med. 1998 May-Jun;16(3):419-24.
5. Donnino MW et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6): 715-21.
The dogma
Patients with alcohol use disorders (AUD) are at risk for nutritional and vitamin deficiencies and may suffer from linked disease states, including Wernicke’s encephalopathy. These conditions may be underrecognized; for instance, an autopsy study suggests that Wernicke’s encephalopathy may have a prevalence rate of 12.5% among alcoholics.1
When patients with AUD are hospitalized, they have often already received a standard IV solution (100 mg of thiamine, 1 mg of folate, 1-2 g of magnesium, and a multivitamin dissolved in saline or dextrose). The practice is common enough that the solution is informally referred to as a “banana bag,” due to the yellow hue imparted by thiamine and multivitamin. These fluids might then be readministered daily during the inpatient stay. But what is the evidence supporting this widespread practice?
The evidence
While the banana bag (or “rally pack”, as it’s also colloquially known) hanging at the patient’s side may look cool, it may not be helping her. Let’s break down the ingredients:
- Folate: Patients with alcohol use disorder are at higher risk for folate deficiency (attributable to poor intake and decreased absorption), but overall rates of folate deficiency are still quite low.2 In addition, most oral and parenteral multivitamins already contain at least 400 mcg folate – the benefit of adding further intravenous folate is not clear.
- Magnesium. Patients with AUD are also at higher risk for magnesium deficiency attributable to increased excretion. While decreased magnesium levels could theoretically increase the risk of alcohol withdrawal symptoms, a Cochrane review found no evidence to support routine supplementation.3
- Multivitamin. Despite theoretical advantages in these (often) malnourished patients, there are no published studies on the benefit or harm of administering a “pan-vitamin” injection. The standard IV formulation is slightly different than an oral vitamin (the IV contains vitamin K, for instance, and lacks calcium), but the bioavailability should be roughly the same, except in rare patients with intestinal malabsorption.4
- IV fluids. Pharmacies typically mix these ingredients in a liter of normal saline or 5% dextrose. Once again, though, individual patients will have different needs. A dehydrated patient would benefit more from normal saline, a patient with alcoholic ketoacidosis would benefit more from dextrose, and a patient with alcohol-related cardiomyopathy likely shouldn’t be getting large volume IV fluids at all.
- Thiamine. Thiamine deficiency is likely the most common and most concerning vitamin deficiency in this patient population. The typical banana bag contains 100 mg of thiamine, which has been the traditional recommended daily amount for Wernicke’s treatment. This dosage, however, was apparently chosen arbitrarily in the 1950s (based on what the authors considered to be a high dose) and current recommendations suggest higher doses given more frequently because of the relatively short half-life of parenteral thiamine.5
Takeaway
The banana bag is a “one-size-fits-all” approach that offers too much of some of its ingredients and not enough of others. It’s better to individualize treatment based on a patient’s needs and consider high-dose thiamine (500 mg one to three times daily) for those at risk for, or showing signs of, Wernicke’s encephalopathy.
Dr. Sehgal and Dr. Hanson are clinical associate professors of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. Dr. Sehgal (@rtsehgal) is a member of the editorial advisory board for The Hospitalist.
References
1. Torvik A et al. Brain lesions in alcoholics: a neuropathological study with clinical correlation. J Neurol Sci. 1982 Nov;56(2-3):233-48.
2. Schwab RA et al. Prevalence in folate deficiency in emergency department patients with alcohol-related illness or injury. Am J Emerg Med. 1992 May;10(3):203-7.
3. Sarai M et al. Magnesium for alcohol withdrawal. Cochrane Database Syst Rev. 2013 Jun 5;(6):CD008358.
4. Krishel S et al. Intravenous vitamins for alcoholics in the emergency department: a review. J Emerg Med. 1998 May-Jun;16(3):419-24.
5. Donnino MW et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50(6): 715-21.
Antipsychotic drugs failed to shorten ICU delirium
The antipsychotic medications in patients in intensive care, new research has found.
In a paper published in the New England Journal of Medicine, researchers reported the results of a randomized, double-blind, placebo-controlled trial in 566 patients with acute respiratory failure or shock and hypoactive or hyperactive delirium. Participants were randomized either to a maximum of 20 mg IV haloperidol daily, maximum 40 mg ziprasidone daily, or placebo.
At the end of the 14-day intervention period, the placebo group had a median of 8.5 days alive without delirium or coma, the haloperidol group had a median of 7.9 days, and the ziprasidone group had a median of 8.7 days. The difference between groups was not statistically significant.
There were also no significant differences between the three groups in the secondary end point of duration of delirium and coma, 30-day and 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Timothy D. Girard, MD, from the department of critical care at the University of Pittsburgh, and his coauthors wrote that their findings echoed those of two previous placebo-controlled trials in smaller numbers of ICU patients.
“One possible reason that we found no evidence that the use of haloperidol or ziprasidone resulted in a fewer days with delirium or coma than placebo is that the mechanism of brain dysfunction that is considered to be targeted by antipsychotic medications – increased dopamine signaling – may not play a major role in the pathogenesis of delirium during critical illness,” they wrote.
“In the current trial, approximately 90% of the patients received one or more doses of sedatives or analgesics, and the doses of sedatives and offtrial antipsychotic medications and the durations of exposures to those agents were similar in all trial groups,” the authors added.
Most of the patients in the trial had hypotensive delirium, which made it difficult to assess the effects of antipsychotics on hypertensive delirium.
The authors also commented that the patients enrolled were a mixed group, so their findings did not rule out the possibility that certain subgroups of patients – such as nonintubated patients with hyperactive delirium, those with alcohol withdrawal, or with other delirium phenotypes – may still benefit from antipsychotics.
Patients treated with ziprasidone were more likely to experience prolongation of the corrected QT interval. Two patients in the haloperidol group developed torsades de pointes but neither had received haloperidol in the 4 days preceding the onset of the arrhythmia.
One patient in each group – including the placebo group – experienced extrapyramidal symptoms and had treatment withheld. One patient in the haloperidol group also had the trial drug withheld because of suspected neuroleptic malignant syndrome, but this was later ruled out, and one patient had haloperidol withheld because of dystonia.
The dose of haloperidol used in the study was considered high, the authors said, but they left open the possibility that even higher doses might help. However, they also noted that doses of 25 mg and above were known to have adverse effects on cognition, which is why they chose the 20-mg dosage.
The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors declared support from the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
SOURCE: Girard TD et al. N Engl J Med.2018 Oct 22. doi: 10.1056/NEJMoa1808217.
In a comment published with this study, Thomas P. Bleck, MD, of the department of neurologic sciences at Rush Medical College, Chicago, wrote, “A change in mental status in a patient in intensive care can be one of the most vexing problems. In the past 2 decades, the idea has arisen that antipsychotic drugs – and particularly dopamine antagonists, which ameliorate thought disorders in psychotic patients – could help patients with disordered thinking in other contexts, such as the intensive care unit. However, yet another trial has now called this idea into question.”
He noted that, in the study group, a bolus of placebo was just as effective as a bolus of active medication, which may be because of the majority of patients having hypoactive delirium, which the active drugs may not impact.
“I would still consider using dopamine agonists in patients at imminent risk of injurious behaviors but have less confidence in their benefits than I once had,” Dr. Bleck wrote.
Dr. Bleck did not report any conflicts of interest.
In a comment published with this study, Thomas P. Bleck, MD, of the department of neurologic sciences at Rush Medical College, Chicago, wrote, “A change in mental status in a patient in intensive care can be one of the most vexing problems. In the past 2 decades, the idea has arisen that antipsychotic drugs – and particularly dopamine antagonists, which ameliorate thought disorders in psychotic patients – could help patients with disordered thinking in other contexts, such as the intensive care unit. However, yet another trial has now called this idea into question.”
He noted that, in the study group, a bolus of placebo was just as effective as a bolus of active medication, which may be because of the majority of patients having hypoactive delirium, which the active drugs may not impact.
“I would still consider using dopamine agonists in patients at imminent risk of injurious behaviors but have less confidence in their benefits than I once had,” Dr. Bleck wrote.
Dr. Bleck did not report any conflicts of interest.
In a comment published with this study, Thomas P. Bleck, MD, of the department of neurologic sciences at Rush Medical College, Chicago, wrote, “A change in mental status in a patient in intensive care can be one of the most vexing problems. In the past 2 decades, the idea has arisen that antipsychotic drugs – and particularly dopamine antagonists, which ameliorate thought disorders in psychotic patients – could help patients with disordered thinking in other contexts, such as the intensive care unit. However, yet another trial has now called this idea into question.”
He noted that, in the study group, a bolus of placebo was just as effective as a bolus of active medication, which may be because of the majority of patients having hypoactive delirium, which the active drugs may not impact.
“I would still consider using dopamine agonists in patients at imminent risk of injurious behaviors but have less confidence in their benefits than I once had,” Dr. Bleck wrote.
Dr. Bleck did not report any conflicts of interest.
The antipsychotic medications in patients in intensive care, new research has found.
In a paper published in the New England Journal of Medicine, researchers reported the results of a randomized, double-blind, placebo-controlled trial in 566 patients with acute respiratory failure or shock and hypoactive or hyperactive delirium. Participants were randomized either to a maximum of 20 mg IV haloperidol daily, maximum 40 mg ziprasidone daily, or placebo.
At the end of the 14-day intervention period, the placebo group had a median of 8.5 days alive without delirium or coma, the haloperidol group had a median of 7.9 days, and the ziprasidone group had a median of 8.7 days. The difference between groups was not statistically significant.
There were also no significant differences between the three groups in the secondary end point of duration of delirium and coma, 30-day and 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Timothy D. Girard, MD, from the department of critical care at the University of Pittsburgh, and his coauthors wrote that their findings echoed those of two previous placebo-controlled trials in smaller numbers of ICU patients.
“One possible reason that we found no evidence that the use of haloperidol or ziprasidone resulted in a fewer days with delirium or coma than placebo is that the mechanism of brain dysfunction that is considered to be targeted by antipsychotic medications – increased dopamine signaling – may not play a major role in the pathogenesis of delirium during critical illness,” they wrote.
“In the current trial, approximately 90% of the patients received one or more doses of sedatives or analgesics, and the doses of sedatives and offtrial antipsychotic medications and the durations of exposures to those agents were similar in all trial groups,” the authors added.
Most of the patients in the trial had hypotensive delirium, which made it difficult to assess the effects of antipsychotics on hypertensive delirium.
The authors also commented that the patients enrolled were a mixed group, so their findings did not rule out the possibility that certain subgroups of patients – such as nonintubated patients with hyperactive delirium, those with alcohol withdrawal, or with other delirium phenotypes – may still benefit from antipsychotics.
Patients treated with ziprasidone were more likely to experience prolongation of the corrected QT interval. Two patients in the haloperidol group developed torsades de pointes but neither had received haloperidol in the 4 days preceding the onset of the arrhythmia.
One patient in each group – including the placebo group – experienced extrapyramidal symptoms and had treatment withheld. One patient in the haloperidol group also had the trial drug withheld because of suspected neuroleptic malignant syndrome, but this was later ruled out, and one patient had haloperidol withheld because of dystonia.
The dose of haloperidol used in the study was considered high, the authors said, but they left open the possibility that even higher doses might help. However, they also noted that doses of 25 mg and above were known to have adverse effects on cognition, which is why they chose the 20-mg dosage.
The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors declared support from the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
SOURCE: Girard TD et al. N Engl J Med.2018 Oct 22. doi: 10.1056/NEJMoa1808217.
The antipsychotic medications in patients in intensive care, new research has found.
In a paper published in the New England Journal of Medicine, researchers reported the results of a randomized, double-blind, placebo-controlled trial in 566 patients with acute respiratory failure or shock and hypoactive or hyperactive delirium. Participants were randomized either to a maximum of 20 mg IV haloperidol daily, maximum 40 mg ziprasidone daily, or placebo.
At the end of the 14-day intervention period, the placebo group had a median of 8.5 days alive without delirium or coma, the haloperidol group had a median of 7.9 days, and the ziprasidone group had a median of 8.7 days. The difference between groups was not statistically significant.
There were also no significant differences between the three groups in the secondary end point of duration of delirium and coma, 30-day and 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Timothy D. Girard, MD, from the department of critical care at the University of Pittsburgh, and his coauthors wrote that their findings echoed those of two previous placebo-controlled trials in smaller numbers of ICU patients.
“One possible reason that we found no evidence that the use of haloperidol or ziprasidone resulted in a fewer days with delirium or coma than placebo is that the mechanism of brain dysfunction that is considered to be targeted by antipsychotic medications – increased dopamine signaling – may not play a major role in the pathogenesis of delirium during critical illness,” they wrote.
“In the current trial, approximately 90% of the patients received one or more doses of sedatives or analgesics, and the doses of sedatives and offtrial antipsychotic medications and the durations of exposures to those agents were similar in all trial groups,” the authors added.
Most of the patients in the trial had hypotensive delirium, which made it difficult to assess the effects of antipsychotics on hypertensive delirium.
The authors also commented that the patients enrolled were a mixed group, so their findings did not rule out the possibility that certain subgroups of patients – such as nonintubated patients with hyperactive delirium, those with alcohol withdrawal, or with other delirium phenotypes – may still benefit from antipsychotics.
Patients treated with ziprasidone were more likely to experience prolongation of the corrected QT interval. Two patients in the haloperidol group developed torsades de pointes but neither had received haloperidol in the 4 days preceding the onset of the arrhythmia.
One patient in each group – including the placebo group – experienced extrapyramidal symptoms and had treatment withheld. One patient in the haloperidol group also had the trial drug withheld because of suspected neuroleptic malignant syndrome, but this was later ruled out, and one patient had haloperidol withheld because of dystonia.
The dose of haloperidol used in the study was considered high, the authors said, but they left open the possibility that even higher doses might help. However, they also noted that doses of 25 mg and above were known to have adverse effects on cognition, which is why they chose the 20-mg dosage.
The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors declared support from the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
SOURCE: Girard TD et al. N Engl J Med.2018 Oct 22. doi: 10.1056/NEJMoa1808217.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Antipsychotics do not reduce the duration or incidence of delirium in intensive care.
Major finding: Patients treated with antipsychotics showed similar median days without delirium or coma, compared with those treated with placebo.
Study details: A randomized, double-blind, placebo-controlled trial in 566 intensive care patients.
Disclosures: The study was supported by the National Institutes of Health and the Department of Veterans Affairs Geriatric Research Education and Clinical Center. Most authors were supported by the NIH or VA during the course of the study. Four authors also reported fees and grants from private industry outside the context of the study.
Source: Girard TD et al. N Engl J Med. 2018 Oct 22. doi: 10.1056/NEJMoa1808217.
Technology offers new tools, challenges for rare-disease patients
WASHINGTON – Genetic developments may create a new medical model for patients with rare diseases and the doctors who treat them, according to Marshall Summar, MD, chief of genetics and metabolism at Children’s National Medical Center in Washington, D.C.
In an interview at the NORD Rare Summit, held by the National Organization for Rare Disorders, Dr. Summar and Peter L. Saltonstall, president and CEO of NORD, discussed hot topics in the rare disease field. Those include new knowledge of the natural history of rare diseases, made possible by the creation of patient databases and the expansion of genetic technology. In addition, some DNA therapies “are finally crossing the finish line,” said Dr. Summar. That means clinicians will be looking at some rare diseases as acute conditions rather than chronic.
However, patients with rare diseases continue to face challenges in terms of the need for prior authorization and for drug access. One of NORD’s missions is to help patients access treatment. “We are seeing these prior authorizations take weeks or even longer,” Mr. Saltonstall said – and meanwhile, patients aren’t receiving therapy.
Visit rarediseases.org for more information about NORD’s ongoing research and advocacy efforts.
Dr. Summar and Mr. Saltonstall had no financial conflicts to disclose.
WASHINGTON – Genetic developments may create a new medical model for patients with rare diseases and the doctors who treat them, according to Marshall Summar, MD, chief of genetics and metabolism at Children’s National Medical Center in Washington, D.C.
In an interview at the NORD Rare Summit, held by the National Organization for Rare Disorders, Dr. Summar and Peter L. Saltonstall, president and CEO of NORD, discussed hot topics in the rare disease field. Those include new knowledge of the natural history of rare diseases, made possible by the creation of patient databases and the expansion of genetic technology. In addition, some DNA therapies “are finally crossing the finish line,” said Dr. Summar. That means clinicians will be looking at some rare diseases as acute conditions rather than chronic.
However, patients with rare diseases continue to face challenges in terms of the need for prior authorization and for drug access. One of NORD’s missions is to help patients access treatment. “We are seeing these prior authorizations take weeks or even longer,” Mr. Saltonstall said – and meanwhile, patients aren’t receiving therapy.
Visit rarediseases.org for more information about NORD’s ongoing research and advocacy efforts.
Dr. Summar and Mr. Saltonstall had no financial conflicts to disclose.
WASHINGTON – Genetic developments may create a new medical model for patients with rare diseases and the doctors who treat them, according to Marshall Summar, MD, chief of genetics and metabolism at Children’s National Medical Center in Washington, D.C.
In an interview at the NORD Rare Summit, held by the National Organization for Rare Disorders, Dr. Summar and Peter L. Saltonstall, president and CEO of NORD, discussed hot topics in the rare disease field. Those include new knowledge of the natural history of rare diseases, made possible by the creation of patient databases and the expansion of genetic technology. In addition, some DNA therapies “are finally crossing the finish line,” said Dr. Summar. That means clinicians will be looking at some rare diseases as acute conditions rather than chronic.
However, patients with rare diseases continue to face challenges in terms of the need for prior authorization and for drug access. One of NORD’s missions is to help patients access treatment. “We are seeing these prior authorizations take weeks or even longer,” Mr. Saltonstall said – and meanwhile, patients aren’t receiving therapy.
Visit rarediseases.org for more information about NORD’s ongoing research and advocacy efforts.
Dr. Summar and Mr. Saltonstall had no financial conflicts to disclose.
REPORTING FROM NORD SUMMIT 2018
Education and support enhance care for rare-disease patients
WASHINGTON – Physicians in primary and specialty care can provide guidance and support to patients with rare diseases by educating themselves about the resources available, according to Tim Boyd, director of state policy for the National Organization for Rare Disorders (NORD).
In an interview at the NORD Rare Summit, held by the National Organization for Rare Disorders, Mr. Boyd and Melinda Burnworth, PharmD, a pharmacist and NORD state volunteer from Arizona, discussed challenges faced by patients with rare diseases, including securing a correct diagnosis, accessing medication, and managing treatment going forward.
Physicians who understand some of the barriers to medication access can help advocate for their patients, explained Mr. Boyd, and those who know about resources for rare disorders can help make a diagnosis.
“All health care providers have an opportunity to enhance care for patients with rare disorders,” said Dr. Burnworth, author of the Rare Disease eResource Guide, available through the American Society of Health-System Pharmacists. Visit rarediseases.org for more information about NORD’s ongoing research and advocacy efforts.
Mr. Boyd and Dr. Burnworth had no financial conflicts to disclose.
WASHINGTON – Physicians in primary and specialty care can provide guidance and support to patients with rare diseases by educating themselves about the resources available, according to Tim Boyd, director of state policy for the National Organization for Rare Disorders (NORD).
In an interview at the NORD Rare Summit, held by the National Organization for Rare Disorders, Mr. Boyd and Melinda Burnworth, PharmD, a pharmacist and NORD state volunteer from Arizona, discussed challenges faced by patients with rare diseases, including securing a correct diagnosis, accessing medication, and managing treatment going forward.
Physicians who understand some of the barriers to medication access can help advocate for their patients, explained Mr. Boyd, and those who know about resources for rare disorders can help make a diagnosis.
“All health care providers have an opportunity to enhance care for patients with rare disorders,” said Dr. Burnworth, author of the Rare Disease eResource Guide, available through the American Society of Health-System Pharmacists. Visit rarediseases.org for more information about NORD’s ongoing research and advocacy efforts.
Mr. Boyd and Dr. Burnworth had no financial conflicts to disclose.
WASHINGTON – Physicians in primary and specialty care can provide guidance and support to patients with rare diseases by educating themselves about the resources available, according to Tim Boyd, director of state policy for the National Organization for Rare Disorders (NORD).
In an interview at the NORD Rare Summit, held by the National Organization for Rare Disorders, Mr. Boyd and Melinda Burnworth, PharmD, a pharmacist and NORD state volunteer from Arizona, discussed challenges faced by patients with rare diseases, including securing a correct diagnosis, accessing medication, and managing treatment going forward.
Physicians who understand some of the barriers to medication access can help advocate for their patients, explained Mr. Boyd, and those who know about resources for rare disorders can help make a diagnosis.
“All health care providers have an opportunity to enhance care for patients with rare disorders,” said Dr. Burnworth, author of the Rare Disease eResource Guide, available through the American Society of Health-System Pharmacists. Visit rarediseases.org for more information about NORD’s ongoing research and advocacy efforts.
Mr. Boyd and Dr. Burnworth had no financial conflicts to disclose.
REPORTING FROM NORD SUMMIT 2018
VTE risk after gynecologic surgery lower with laparoscopic procedures
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
The aim of this study was to determine the 3-month postoperative incidence of venous thromboembolism among patients undergoing gynecologic surgery. The study also addressed the mode of surgery to allow a comparison between laparotomy and minimally invasive approaches.
Postoperative VTE was defined as deep venous thrombosis of the lower extremities, pulmonary embolism, or both that occurred within 90 days of surgery. A key component of the study was that clinically recognized VTEs that required treatment with anticoagulation, vena caval filter, or both were included.
The study evaluated 43,751 gynecological cases among 37,485 patients. As expected, 59% of the cases were classified as vaginal surgery, 24% were laparoscopic cases, and 17% of the cases were laparotomies.
Of the 8,273 hysterectomies, 57% were via an abdominal approach, 34% were laparoscopic, and 9 were vaginal cases.
Overall, 0.2% of patients were diagnosed with a VTE. As expected, the greatest incidence of VTE was in patients with cancer who underwent a laparotomy. Those with a VTE were significantly more likely to have had an inpatient stay (longer than 24 hours), a cancer diagnosis, a longer surgical time, and an American Society of Anesthesiologists score of 3 or more. They also were older (mean age 56 years vs. 44 years). Of note, 20% of the VTE group identified as black.
Among patients who had a hysterectomy, there were VTEs in 0.7%: 1% in the laparotomy group, 0.3% in the laparoscopic group, and only 0.1% in the vaginal hysterectomy group.
It is interesting to note that 91% of the patients diagnosed with a VTE did received preoperative VTE prophylaxis. The authors noted that the high rate of prophylaxis may have reflected the surgeon’s ability to identify patients who are at high risk.
The authors recognized that the current guidelines do not stratify VTE risk based on the mode of surgery. Further, they noted that low-risk patients undergoing low-risk surgery may be receiving pharmacologic VTE prophylaxis, thus placing these patients at risk for complications related to such therapy.
This paper by Jorgensen et al. should remind us that VTE prophylaxis should be individualized. Patients may not fit nicely into boxes on our EMR; each clinical decision should be made for each patient and for each clinical scenario. The surgeon’s responsibility is to adopt the evidence-based guidelines that serve each individual patient’s unique risk/benefit profile.
David M. Jaspan, DO, is director of minimally invasive and pelvic surgery and chairman of the department of obstetrics and gynecology at the Einstein Medical Center in Philadelphia. Dr. Jaspan, who was asked to comment on the Jorgenson et al. article, said he had no relevant financial disclosures.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
according to a study published in Obstetrics & Gynecology.

The retrospective cohort study looked at data from 37,485 patients who underwent 43,751 gynecologic surgical procedures, including hysterectomy and myomectomy, at two tertiary care academic hospitals.
Overall, 96 patients (0.2%) were diagnosed with postoperative venous thromboembolism. However patients who underwent laparoscopic or vaginal surgery had a significant 78% and 93% lower risk of venous thromboembolism, respectively, than those who underwent laparotomy, even after adjusting for potential confounders such as age, cancer, race, pharmacologic thromboprophylaxis, and surgical time.
The incidence of postoperative thromboembolism was significantly higher among patients undergoing gynecologic surgery for cancer (1.1%). The incidence among those undergoing surgery for benign indications was only 0.2%, and the highest incidence was among patients with cancer who underwent laparotomy (2.2%).
“This study adds to data demonstrating that venous thromboembolism is rare in gynecologic surgery, particularly when a patient undergoes a minimally invasive procedure for benign indications,” wrote Dr. Elisa M. Jorgensen of Beth Israel Deaconess Medical Center, and her coauthors.
Among the 8,273 patients who underwent a hysterectomy, there were 55 cases of venous thromboembolism – representing an 0.7% incidence. However patients who underwent laparotomy had a 1% incidence of postoperative venous thromboembolism, while those who underwent laparoscopic hysterectomy had an 0.3% incidence and those who underwent vaginal hysterectomy had an 0.1% incidence.
Laparotomy was the most common mode of surgery for hysterectomy – accounting for 57% of operations – while 34% were laparoscopic and 9% were vaginal.
However, the authors noted that the use of laparoscopy increased and laparotomy declined over the 9 years of the study. In 2006, 12% of hysterectomies were laparoscopic, compared with 55% in 2015, while over that same period the percentage of laparotomies dropped from 74% to 41%, and the percentage of vaginal procedures declined from 14% to 4%.
“Because current practice guidelines do not account for mode of surgery, we find them to be insufficient for the modern gynecologic surgeon to counsel patients on their individual venous thromboembolism risk or to make ideal decisions regarding selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Only 5 patients of the 2,851 who underwent myomectomy developed postoperative VTE – an overall incidence of 0.2% – and the authors said numbers were too small to analyze. Vaginal or hysteroscopic myomectomy was the most common surgical method, accounting for 62% of procedures, compared with 23% for laparotomies and 15% for laparoscopies.
More than 90% of patients who experienced postoperative thromboembolism had received some form of thromboprophylaxis before surgery, either mechanical, pharmacologic, or both. In comparison, only 55% of the group who didn’t experience thromboembolism had received thromboprophylaxis.
“The high rate of prophylaxis among patients who developed postoperative venous thromboembolism may reflect surgeons’ abilities to preoperatively identify patients at increased risk, guiding appropriate selection of thromboprophylaxis,” Dr. Jorgenson and her associates wrote.
Addressing the study’s limitations, the authors noted that they were not able to capture data on patients’ body mass index and also were unable to account for patients who might have been diagnosed and treated for postoperative VTE at other hospitals.
No conflicts of interest were declared.
SOURCE: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Laparoscopic gynecologic surgery is associated with a lower risk of postoperative VTE than laparotomy.
Major finding: Laparoscopic hysterectomy was associated with a 78% lower incidence of postoperative VTE than laparotomy.
Study details: Retrospective cohort study of 37,485 patients who underwent 43,751 gynecologic surgical procedures
Disclosures: No conflicts of interest were declared.
Source: Jorgensen EM et al. Obstet Gynecol. 2018 Nov;132:1275-84.
Most profiles of mass shooters do not include mental illness
AUSTIN, TEX. – Mass shootings make up only a tiny percentage of annual gun violence deaths in the United States, but they capture the attention of the nation – and of media that do not always accurately represent their context.
Experts tend to identify the first modern U.S. mass shooting event as the University of Texas Tower shooting in Austin by Charles Whitman in 1966, Corina Freitas, MD, said at the annual meeting of the American Academy of Psychiatry and the Law.
But two previous incidents preceded Whitman’s: Howard Unruh’s 12-minute killing spree in his neighborhood in Camden, N.J., in 1949, and Andrew Kehoe’s 1927 series of bombings that killed 43 people in the Bath School disaster in Michigan. Studies of these events and the hundreds since have led to a better understanding of what motivates mass shooters (or bombers in Kehoe’s case) and how to potentially identify them and prevent such events, said Dr. Freitas, of the department of psychiatry and behavioral sciences at George Washington University in Washington.
Dr. Freitas provided an overview of mass shooting history in the United States before Karen B. Rosenbaum, MD, clinical assistant professor at New York University and clinical instructor at New York Presbyterian–Weill Cornell Medical Center, spoke about the social, political, and legal implications of the intersection between mental illness and mass shootings.
She began by explaining how the FBI’s definition of mass shootings has changed from “four or more people at one location within one event” in 2005 to its redefinition in 2012-2013 to “three or more killings in a single incident and in a place of public use.”
Mass shootings usually are not impulse kills, Dr. Freitas said, noting that 77% of shooters plan their shooting for at least a week, and 46% of people spend about a week preparing. The perpetrators are potentially recognizable, typically displaying four to five concerning behaviors up to 1 year before the shooting, such as talking about their plans or purchasing supplies. But only a minority of people who observe these behaviors ever speak up about them or take any actions, she said.
, but they also display numerous other psychosocial characteristics, such as self-esteem issues, paranoia, narcissism, depression, and suicidality.
“Almost half of them are suicidal, and they actually proclaim it up to 1 year ahead of the shooting,” Dr. Freitas said. “We could catch them if we paid more attention to that.”
Mass killers tend to fall into three categories, as classified by psychiatrist Park Dietz, MD, in 1986:
- Family annihilators, such as George Banks, are typically depressed, paranoid, suicidal older males who might be intoxicated at the time of their attack. Banks shot 13 people, including 5 of his own children and 2 other children and their mothers, in Pennsylvania in 1982.
- Pseudocommandos, such as Charles Whitman, are usually preoccupied with firearms and plan heavily. “They usually end up killing themselves by cop,” Dr. Freitas said.
- Set-and-run killers, the rarest type, include perpetrators like Kehoe; their method of killing gives them an escape (though Kehoe blew himself up as well).
But mental illness is not a major feature of mass killers: Only about a quarter of mass shooters have a diagnosed mental illness, and the illness might not necessarily be related to their crime. Of that quarter, about 75% of mass shooters had a mood disorder, 25% had an anxiety disorder,19% had psychosis, and 1% had the developmental condition, such as autism spectrum disorder.
Yet, as mass shootings have dramatically increased, mental illness has become inextricably associated with these events in the media and popular opinion, Dr. Rosenbaum said. There have been 74 school shootings since the Newtown, Conn., tragedy, and mental illness is repeatedly brought up as a contributor, she said.
A 2014 study that analyzed 25% of a random sample of news stories from 1997 to 2012 on serious mental illness and gun violence (before Newtown) found that most of the coverage occurred after mass shootings and “ ‘dangerous people’ with serious mental illness were more likely to be mentioned than ‘dangerous weapons’ as a cause of gun violence” (Am J Public Health. 2014 Mar;104[3]:406-13).
Yet this association does not reflect reality, Dr. Rosenbaum said. One meta-analysis found that prevention of one stranger homicide by someone with psychosis would require detaining 35,000 people with schizophrenia who had been judged as being at high risk for violence (Schizophr Bull. 2011 May;37[3]:572-9).
Further, the relationship between violence and mental illness is not simple. Complex historical factors are usually involved, including past violence, juvenile detection, physical abuse, substance abuse, age, parental arrest record, and life circumstances – such as a recent divorce, unemployment, or victimization.
The greater danger of a person with mental illness is the harm they will do to themselves, research shows. A study of 255 recently discharged psychiatric patients and 490 matched community residents found that the patients were no more likely to perpetuate violence than were the community members, but they were significantly more likely to report being suicidal (Int J Law Psychiatry. 2018 Jan-Feb;56:44-9).
Rather than mental illness, what is associated with violence is substance use and access to weapons, Dr. Rosenbaum said.
“The United States is one of only three countries in the world with a constitutionally protected right to own firearms,” Dr. Rosenbaum said, citing a 2017 study by John S. Rozel, MD, and Edward P. Mulvey, PhD, (Annu Rev Clin Psychol. 2017 May 8;13:445-9). And the United States has few restrictions on that right. With more than 350 million privately owned firearms – approximately 30% of all privately owned firearms in the world – the U.S. population exceeds all other countries in both per capita and absolute gun ownership.
And research shows that guns don’t make a country safer: Guns per capita are significantly correlated with firearm-related deaths; mental illness is only of borderline significance (Am J Med. 2013 Oct;126[10]:873-6).
Substance use – including use of cocaine, hallucinogens, methamphetamine, ecstasy, and prescription medications – has a stronger correlation with gun-carrying and gun-related behaviors (Inj Prev. 2017 Dec; 23[6]:383-7 and Epidemiol Rev. 2016;38[1]:46-61). Both acute and chronic alcohol misuse also are linked to firearm ownership and violence toward others and one’s self (Prev Med. 2015 Oct;79:15-21).
Yet public misperceptions of mental illness as a contributor to violence persists, research shows (Aust N Z J Psychiatry. 2014 Aug;48[8]:764-71), further stigmatizing people with psychiatric conditions and potentially reducing the likelihood of their seeking treatment. Politicians contribute to these misperceptions; an example is House Speaker Paul Ryan’s comment after the Parkland, Fla., school shooting: “Mental health is often a big problem underlying these tragedies.”
“The media sensationalizes violent crimes committed by people with mental illness, especially after mass shooting, and this societal bias contributes to the stigma that leads to decreased treatment seeking and discrimination,” Dr. Rosenbaum said, citing research from Mohit Varshney, MD, and his associates (J Epidemiol Community Health. 2016 Mar;70[3]:223-5). “It is important to dissociate the concept of mental illness from dangerousness.”
AUSTIN, TEX. – Mass shootings make up only a tiny percentage of annual gun violence deaths in the United States, but they capture the attention of the nation – and of media that do not always accurately represent their context.
Experts tend to identify the first modern U.S. mass shooting event as the University of Texas Tower shooting in Austin by Charles Whitman in 1966, Corina Freitas, MD, said at the annual meeting of the American Academy of Psychiatry and the Law.
But two previous incidents preceded Whitman’s: Howard Unruh’s 12-minute killing spree in his neighborhood in Camden, N.J., in 1949, and Andrew Kehoe’s 1927 series of bombings that killed 43 people in the Bath School disaster in Michigan. Studies of these events and the hundreds since have led to a better understanding of what motivates mass shooters (or bombers in Kehoe’s case) and how to potentially identify them and prevent such events, said Dr. Freitas, of the department of psychiatry and behavioral sciences at George Washington University in Washington.
Dr. Freitas provided an overview of mass shooting history in the United States before Karen B. Rosenbaum, MD, clinical assistant professor at New York University and clinical instructor at New York Presbyterian–Weill Cornell Medical Center, spoke about the social, political, and legal implications of the intersection between mental illness and mass shootings.
She began by explaining how the FBI’s definition of mass shootings has changed from “four or more people at one location within one event” in 2005 to its redefinition in 2012-2013 to “three or more killings in a single incident and in a place of public use.”
Mass shootings usually are not impulse kills, Dr. Freitas said, noting that 77% of shooters plan their shooting for at least a week, and 46% of people spend about a week preparing. The perpetrators are potentially recognizable, typically displaying four to five concerning behaviors up to 1 year before the shooting, such as talking about their plans or purchasing supplies. But only a minority of people who observe these behaviors ever speak up about them or take any actions, she said.
, but they also display numerous other psychosocial characteristics, such as self-esteem issues, paranoia, narcissism, depression, and suicidality.
“Almost half of them are suicidal, and they actually proclaim it up to 1 year ahead of the shooting,” Dr. Freitas said. “We could catch them if we paid more attention to that.”
Mass killers tend to fall into three categories, as classified by psychiatrist Park Dietz, MD, in 1986:
- Family annihilators, such as George Banks, are typically depressed, paranoid, suicidal older males who might be intoxicated at the time of their attack. Banks shot 13 people, including 5 of his own children and 2 other children and their mothers, in Pennsylvania in 1982.
- Pseudocommandos, such as Charles Whitman, are usually preoccupied with firearms and plan heavily. “They usually end up killing themselves by cop,” Dr. Freitas said.
- Set-and-run killers, the rarest type, include perpetrators like Kehoe; their method of killing gives them an escape (though Kehoe blew himself up as well).
But mental illness is not a major feature of mass killers: Only about a quarter of mass shooters have a diagnosed mental illness, and the illness might not necessarily be related to their crime. Of that quarter, about 75% of mass shooters had a mood disorder, 25% had an anxiety disorder,19% had psychosis, and 1% had the developmental condition, such as autism spectrum disorder.
Yet, as mass shootings have dramatically increased, mental illness has become inextricably associated with these events in the media and popular opinion, Dr. Rosenbaum said. There have been 74 school shootings since the Newtown, Conn., tragedy, and mental illness is repeatedly brought up as a contributor, she said.
A 2014 study that analyzed 25% of a random sample of news stories from 1997 to 2012 on serious mental illness and gun violence (before Newtown) found that most of the coverage occurred after mass shootings and “ ‘dangerous people’ with serious mental illness were more likely to be mentioned than ‘dangerous weapons’ as a cause of gun violence” (Am J Public Health. 2014 Mar;104[3]:406-13).
Yet this association does not reflect reality, Dr. Rosenbaum said. One meta-analysis found that prevention of one stranger homicide by someone with psychosis would require detaining 35,000 people with schizophrenia who had been judged as being at high risk for violence (Schizophr Bull. 2011 May;37[3]:572-9).
Further, the relationship between violence and mental illness is not simple. Complex historical factors are usually involved, including past violence, juvenile detection, physical abuse, substance abuse, age, parental arrest record, and life circumstances – such as a recent divorce, unemployment, or victimization.
The greater danger of a person with mental illness is the harm they will do to themselves, research shows. A study of 255 recently discharged psychiatric patients and 490 matched community residents found that the patients were no more likely to perpetuate violence than were the community members, but they were significantly more likely to report being suicidal (Int J Law Psychiatry. 2018 Jan-Feb;56:44-9).
Rather than mental illness, what is associated with violence is substance use and access to weapons, Dr. Rosenbaum said.
“The United States is one of only three countries in the world with a constitutionally protected right to own firearms,” Dr. Rosenbaum said, citing a 2017 study by John S. Rozel, MD, and Edward P. Mulvey, PhD, (Annu Rev Clin Psychol. 2017 May 8;13:445-9). And the United States has few restrictions on that right. With more than 350 million privately owned firearms – approximately 30% of all privately owned firearms in the world – the U.S. population exceeds all other countries in both per capita and absolute gun ownership.
And research shows that guns don’t make a country safer: Guns per capita are significantly correlated with firearm-related deaths; mental illness is only of borderline significance (Am J Med. 2013 Oct;126[10]:873-6).
Substance use – including use of cocaine, hallucinogens, methamphetamine, ecstasy, and prescription medications – has a stronger correlation with gun-carrying and gun-related behaviors (Inj Prev. 2017 Dec; 23[6]:383-7 and Epidemiol Rev. 2016;38[1]:46-61). Both acute and chronic alcohol misuse also are linked to firearm ownership and violence toward others and one’s self (Prev Med. 2015 Oct;79:15-21).
Yet public misperceptions of mental illness as a contributor to violence persists, research shows (Aust N Z J Psychiatry. 2014 Aug;48[8]:764-71), further stigmatizing people with psychiatric conditions and potentially reducing the likelihood of their seeking treatment. Politicians contribute to these misperceptions; an example is House Speaker Paul Ryan’s comment after the Parkland, Fla., school shooting: “Mental health is often a big problem underlying these tragedies.”
“The media sensationalizes violent crimes committed by people with mental illness, especially after mass shooting, and this societal bias contributes to the stigma that leads to decreased treatment seeking and discrimination,” Dr. Rosenbaum said, citing research from Mohit Varshney, MD, and his associates (J Epidemiol Community Health. 2016 Mar;70[3]:223-5). “It is important to dissociate the concept of mental illness from dangerousness.”
AUSTIN, TEX. – Mass shootings make up only a tiny percentage of annual gun violence deaths in the United States, but they capture the attention of the nation – and of media that do not always accurately represent their context.
Experts tend to identify the first modern U.S. mass shooting event as the University of Texas Tower shooting in Austin by Charles Whitman in 1966, Corina Freitas, MD, said at the annual meeting of the American Academy of Psychiatry and the Law.
But two previous incidents preceded Whitman’s: Howard Unruh’s 12-minute killing spree in his neighborhood in Camden, N.J., in 1949, and Andrew Kehoe’s 1927 series of bombings that killed 43 people in the Bath School disaster in Michigan. Studies of these events and the hundreds since have led to a better understanding of what motivates mass shooters (or bombers in Kehoe’s case) and how to potentially identify them and prevent such events, said Dr. Freitas, of the department of psychiatry and behavioral sciences at George Washington University in Washington.
Dr. Freitas provided an overview of mass shooting history in the United States before Karen B. Rosenbaum, MD, clinical assistant professor at New York University and clinical instructor at New York Presbyterian–Weill Cornell Medical Center, spoke about the social, political, and legal implications of the intersection between mental illness and mass shootings.
She began by explaining how the FBI’s definition of mass shootings has changed from “four or more people at one location within one event” in 2005 to its redefinition in 2012-2013 to “three or more killings in a single incident and in a place of public use.”
Mass shootings usually are not impulse kills, Dr. Freitas said, noting that 77% of shooters plan their shooting for at least a week, and 46% of people spend about a week preparing. The perpetrators are potentially recognizable, typically displaying four to five concerning behaviors up to 1 year before the shooting, such as talking about their plans or purchasing supplies. But only a minority of people who observe these behaviors ever speak up about them or take any actions, she said.
, but they also display numerous other psychosocial characteristics, such as self-esteem issues, paranoia, narcissism, depression, and suicidality.
“Almost half of them are suicidal, and they actually proclaim it up to 1 year ahead of the shooting,” Dr. Freitas said. “We could catch them if we paid more attention to that.”
Mass killers tend to fall into three categories, as classified by psychiatrist Park Dietz, MD, in 1986:
- Family annihilators, such as George Banks, are typically depressed, paranoid, suicidal older males who might be intoxicated at the time of their attack. Banks shot 13 people, including 5 of his own children and 2 other children and their mothers, in Pennsylvania in 1982.
- Pseudocommandos, such as Charles Whitman, are usually preoccupied with firearms and plan heavily. “They usually end up killing themselves by cop,” Dr. Freitas said.
- Set-and-run killers, the rarest type, include perpetrators like Kehoe; their method of killing gives them an escape (though Kehoe blew himself up as well).
But mental illness is not a major feature of mass killers: Only about a quarter of mass shooters have a diagnosed mental illness, and the illness might not necessarily be related to their crime. Of that quarter, about 75% of mass shooters had a mood disorder, 25% had an anxiety disorder,19% had psychosis, and 1% had the developmental condition, such as autism spectrum disorder.
Yet, as mass shootings have dramatically increased, mental illness has become inextricably associated with these events in the media and popular opinion, Dr. Rosenbaum said. There have been 74 school shootings since the Newtown, Conn., tragedy, and mental illness is repeatedly brought up as a contributor, she said.
A 2014 study that analyzed 25% of a random sample of news stories from 1997 to 2012 on serious mental illness and gun violence (before Newtown) found that most of the coverage occurred after mass shootings and “ ‘dangerous people’ with serious mental illness were more likely to be mentioned than ‘dangerous weapons’ as a cause of gun violence” (Am J Public Health. 2014 Mar;104[3]:406-13).
Yet this association does not reflect reality, Dr. Rosenbaum said. One meta-analysis found that prevention of one stranger homicide by someone with psychosis would require detaining 35,000 people with schizophrenia who had been judged as being at high risk for violence (Schizophr Bull. 2011 May;37[3]:572-9).
Further, the relationship between violence and mental illness is not simple. Complex historical factors are usually involved, including past violence, juvenile detection, physical abuse, substance abuse, age, parental arrest record, and life circumstances – such as a recent divorce, unemployment, or victimization.
The greater danger of a person with mental illness is the harm they will do to themselves, research shows. A study of 255 recently discharged psychiatric patients and 490 matched community residents found that the patients were no more likely to perpetuate violence than were the community members, but they were significantly more likely to report being suicidal (Int J Law Psychiatry. 2018 Jan-Feb;56:44-9).
Rather than mental illness, what is associated with violence is substance use and access to weapons, Dr. Rosenbaum said.
“The United States is one of only three countries in the world with a constitutionally protected right to own firearms,” Dr. Rosenbaum said, citing a 2017 study by John S. Rozel, MD, and Edward P. Mulvey, PhD, (Annu Rev Clin Psychol. 2017 May 8;13:445-9). And the United States has few restrictions on that right. With more than 350 million privately owned firearms – approximately 30% of all privately owned firearms in the world – the U.S. population exceeds all other countries in both per capita and absolute gun ownership.
And research shows that guns don’t make a country safer: Guns per capita are significantly correlated with firearm-related deaths; mental illness is only of borderline significance (Am J Med. 2013 Oct;126[10]:873-6).
Substance use – including use of cocaine, hallucinogens, methamphetamine, ecstasy, and prescription medications – has a stronger correlation with gun-carrying and gun-related behaviors (Inj Prev. 2017 Dec; 23[6]:383-7 and Epidemiol Rev. 2016;38[1]:46-61). Both acute and chronic alcohol misuse also are linked to firearm ownership and violence toward others and one’s self (Prev Med. 2015 Oct;79:15-21).
Yet public misperceptions of mental illness as a contributor to violence persists, research shows (Aust N Z J Psychiatry. 2014 Aug;48[8]:764-71), further stigmatizing people with psychiatric conditions and potentially reducing the likelihood of their seeking treatment. Politicians contribute to these misperceptions; an example is House Speaker Paul Ryan’s comment after the Parkland, Fla., school shooting: “Mental health is often a big problem underlying these tragedies.”
“The media sensationalizes violent crimes committed by people with mental illness, especially after mass shooting, and this societal bias contributes to the stigma that leads to decreased treatment seeking and discrimination,” Dr. Rosenbaum said, citing research from Mohit Varshney, MD, and his associates (J Epidemiol Community Health. 2016 Mar;70[3]:223-5). “It is important to dissociate the concept of mental illness from dangerousness.”
REPORTING FROM THE AAPL ANNUAL MEETING
Vascular emergencies on the rise, but more patients surviving
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Rates of endovascular repair for nontraumatic vascular emergencies rose sharply.
Major finding: Endovascular repair rates for nontraumatic vascular emergencies climbed from 24% to 36% of cases from 2005 to 2014 (P for trend, less than .0001).
Study details: A 10-year sample of hospitalizations for nontraumatic vascular emergencies from the U.S. National Inpatient Sample.
Disclosures: Dr. Vogel reported no outside sources of funding and no conflicts of interest.