User login
PEXIVAS trial results will likely change ANCA-associated vasculitis therapy
CHICAGO – Results of the landmark PEXIVAS study – far and away the largest randomized trial ever done in ANCA-associated vasculitis – will likely change treatment in a couple of major ways.
“I think this trial will have an impact on care. Based on these findings, physicians should strongly reconsider the utility of plasma exchange as a treatment for AAV [antineutrophil cytoplasmic antibody–associated vasculitis] patients and should now consider using lower cumulative doses of glucocorticoids for the treatment of severe AAV,” PEXIVAS coprincipal investigator Peter A. Merkel, MD, said at the annual meeting of the American College of Rheumatology.
That’s because the trial demonstrated that plasma exchange neither saved lives nor avoided end-stage renal disease, while utilization of oral glucocorticoids in doses substantially lower than the high-dose current standard significantly reduced the serious infection rate without causing less effective disease control, according to Dr. Merkel, chief of rheumatology and professor of medicine and epidemiology at the University of Pennsylvania in Philadelphia.
PEXIVAS comprised 704 patients with severe granulomatosis with polyangiitis or microscopic polyangiitis, making it more than twice as large as any other trial in AAV. This was a multicenter, international, open-label, randomized trial with a 2-by-2 factorial design. To qualify as having severe AAV, participants had to have an estimated glomerular filtration rate below 50 mL/min per 1.73 m2 and/or lung hemorrhage.
“This was in essence two trials embedded within one protocol in the factorial design,” he explained.
The impetus for this major clinical trial was a recognition that mortality due to AAV remains high, especially in the first year, with a clear unmet need for better, less toxic therapies. Indeed, it’s estimated that only 29% of deaths in the first year after diagnosis are due to the vasculitis disease itself, while over 50% of the mortality is caused by infection, much of it collateral damage from immunosuppressive therapies.
Dr. Merkel, who heads the National Institutes of Health–supported Vasculitis Clinical Research Consortium, said the time was right for a clinical trial aimed at improving patient management: “Clinical equipoise exists for the efficacy of both plasma exchange and reduced-dose glucocorticoids in ANCA-associated vasculitis.”
The patients underwent induction therapy with cyclophosphamide or rituximab (Rituxan) plus IV methylprednisolone. Then they were randomized to seven plasma exchange sessions in 14 days or no plasma exchange, and further randomized to conventional weight-based, high-dose oral glucocorticoids or a lower-dose regimen. Those on the reduced-dose regimen received 54% of the cumulative amount of glucocorticoids used in the standard-dose group through the first 3 months, and 61% over the course of 6 months. By week 4, those on the reduced-dose regimen were on an average of 25 mg/day, while those on standard therapy were on 50 mg/day. Adherence to assigned study arms exceeded 90%. Patients were followed prospectively for 1-7 years.
The primary endpoint, a composite of all-cause mortality or development of end-stage renal disease, occurred in 28% of patients on plasma exchange and 31% of those who did not undergo plasma exchange, a nonsignificant difference indicative of a lack of benefit for the intervention. No differential effect was seen in prespecified subgroups based on age, creatinine clearance, ANCA type, form of immunosuppression, or presence or absence of lung hemorrhage.
Further, the primary endpoint occurred in 28% of patients on reduced-dose glucocorticoids, compared with 26% on full-dose therapy; again, a nonsignificant difference, meaning lower-dose therapy didn’t result in less effective disease control. But it did result in a significant reduction in the prespecified endpoint of serious infections in the first year: 27% versus 33% with full-dose therapy, representing a 30% relative risk reduction.
Audience members wanted to know if there are any circumstances at all in which Dr. Merkel would now consider resorting to plasma exchange, such as maybe in AAV patients at the most extreme end of the severity spectrum.
“I’m not sure I should be the one dictating that; I think the world needs to see the data,” he replied.
That being said, he added, “I think these data are incredibly helpful to physicians and patients as they face this decision. I think plasma exchange is an expensive therapy and somewhat invasive. I think our results indicate that the benefit that we may have thought was there is not there.”
The study was sponsored by the National Institutes of Health, the Food and Drug Administration, the U.K. Medical Research Council and the National Institute for Health Research, the Canadian Institutes of Health Research, and the governments of France, Australia, and New Zealand. The presenter reported receiving research funding from the ACR, EULAR, FDA, NIH, Patient-Centered Outcomes Research Institute, and the Vasculitis Foundation. He also receives research funding from and/or serves as a consultant to more than a dozen pharmaceutical companies.
SOURCE: Merkel PA et al. Arthritis Rheumatol. 2018;70(Suppl 10):Abstract 2788.
CHICAGO – Results of the landmark PEXIVAS study – far and away the largest randomized trial ever done in ANCA-associated vasculitis – will likely change treatment in a couple of major ways.
“I think this trial will have an impact on care. Based on these findings, physicians should strongly reconsider the utility of plasma exchange as a treatment for AAV [antineutrophil cytoplasmic antibody–associated vasculitis] patients and should now consider using lower cumulative doses of glucocorticoids for the treatment of severe AAV,” PEXIVAS coprincipal investigator Peter A. Merkel, MD, said at the annual meeting of the American College of Rheumatology.
That’s because the trial demonstrated that plasma exchange neither saved lives nor avoided end-stage renal disease, while utilization of oral glucocorticoids in doses substantially lower than the high-dose current standard significantly reduced the serious infection rate without causing less effective disease control, according to Dr. Merkel, chief of rheumatology and professor of medicine and epidemiology at the University of Pennsylvania in Philadelphia.
PEXIVAS comprised 704 patients with severe granulomatosis with polyangiitis or microscopic polyangiitis, making it more than twice as large as any other trial in AAV. This was a multicenter, international, open-label, randomized trial with a 2-by-2 factorial design. To qualify as having severe AAV, participants had to have an estimated glomerular filtration rate below 50 mL/min per 1.73 m2 and/or lung hemorrhage.
“This was in essence two trials embedded within one protocol in the factorial design,” he explained.
The impetus for this major clinical trial was a recognition that mortality due to AAV remains high, especially in the first year, with a clear unmet need for better, less toxic therapies. Indeed, it’s estimated that only 29% of deaths in the first year after diagnosis are due to the vasculitis disease itself, while over 50% of the mortality is caused by infection, much of it collateral damage from immunosuppressive therapies.
Dr. Merkel, who heads the National Institutes of Health–supported Vasculitis Clinical Research Consortium, said the time was right for a clinical trial aimed at improving patient management: “Clinical equipoise exists for the efficacy of both plasma exchange and reduced-dose glucocorticoids in ANCA-associated vasculitis.”
The patients underwent induction therapy with cyclophosphamide or rituximab (Rituxan) plus IV methylprednisolone. Then they were randomized to seven plasma exchange sessions in 14 days or no plasma exchange, and further randomized to conventional weight-based, high-dose oral glucocorticoids or a lower-dose regimen. Those on the reduced-dose regimen received 54% of the cumulative amount of glucocorticoids used in the standard-dose group through the first 3 months, and 61% over the course of 6 months. By week 4, those on the reduced-dose regimen were on an average of 25 mg/day, while those on standard therapy were on 50 mg/day. Adherence to assigned study arms exceeded 90%. Patients were followed prospectively for 1-7 years.
The primary endpoint, a composite of all-cause mortality or development of end-stage renal disease, occurred in 28% of patients on plasma exchange and 31% of those who did not undergo plasma exchange, a nonsignificant difference indicative of a lack of benefit for the intervention. No differential effect was seen in prespecified subgroups based on age, creatinine clearance, ANCA type, form of immunosuppression, or presence or absence of lung hemorrhage.
Further, the primary endpoint occurred in 28% of patients on reduced-dose glucocorticoids, compared with 26% on full-dose therapy; again, a nonsignificant difference, meaning lower-dose therapy didn’t result in less effective disease control. But it did result in a significant reduction in the prespecified endpoint of serious infections in the first year: 27% versus 33% with full-dose therapy, representing a 30% relative risk reduction.
Audience members wanted to know if there are any circumstances at all in which Dr. Merkel would now consider resorting to plasma exchange, such as maybe in AAV patients at the most extreme end of the severity spectrum.
“I’m not sure I should be the one dictating that; I think the world needs to see the data,” he replied.
That being said, he added, “I think these data are incredibly helpful to physicians and patients as they face this decision. I think plasma exchange is an expensive therapy and somewhat invasive. I think our results indicate that the benefit that we may have thought was there is not there.”
The study was sponsored by the National Institutes of Health, the Food and Drug Administration, the U.K. Medical Research Council and the National Institute for Health Research, the Canadian Institutes of Health Research, and the governments of France, Australia, and New Zealand. The presenter reported receiving research funding from the ACR, EULAR, FDA, NIH, Patient-Centered Outcomes Research Institute, and the Vasculitis Foundation. He also receives research funding from and/or serves as a consultant to more than a dozen pharmaceutical companies.
SOURCE: Merkel PA et al. Arthritis Rheumatol. 2018;70(Suppl 10):Abstract 2788.
CHICAGO – Results of the landmark PEXIVAS study – far and away the largest randomized trial ever done in ANCA-associated vasculitis – will likely change treatment in a couple of major ways.
“I think this trial will have an impact on care. Based on these findings, physicians should strongly reconsider the utility of plasma exchange as a treatment for AAV [antineutrophil cytoplasmic antibody–associated vasculitis] patients and should now consider using lower cumulative doses of glucocorticoids for the treatment of severe AAV,” PEXIVAS coprincipal investigator Peter A. Merkel, MD, said at the annual meeting of the American College of Rheumatology.
That’s because the trial demonstrated that plasma exchange neither saved lives nor avoided end-stage renal disease, while utilization of oral glucocorticoids in doses substantially lower than the high-dose current standard significantly reduced the serious infection rate without causing less effective disease control, according to Dr. Merkel, chief of rheumatology and professor of medicine and epidemiology at the University of Pennsylvania in Philadelphia.
PEXIVAS comprised 704 patients with severe granulomatosis with polyangiitis or microscopic polyangiitis, making it more than twice as large as any other trial in AAV. This was a multicenter, international, open-label, randomized trial with a 2-by-2 factorial design. To qualify as having severe AAV, participants had to have an estimated glomerular filtration rate below 50 mL/min per 1.73 m2 and/or lung hemorrhage.
“This was in essence two trials embedded within one protocol in the factorial design,” he explained.
The impetus for this major clinical trial was a recognition that mortality due to AAV remains high, especially in the first year, with a clear unmet need for better, less toxic therapies. Indeed, it’s estimated that only 29% of deaths in the first year after diagnosis are due to the vasculitis disease itself, while over 50% of the mortality is caused by infection, much of it collateral damage from immunosuppressive therapies.
Dr. Merkel, who heads the National Institutes of Health–supported Vasculitis Clinical Research Consortium, said the time was right for a clinical trial aimed at improving patient management: “Clinical equipoise exists for the efficacy of both plasma exchange and reduced-dose glucocorticoids in ANCA-associated vasculitis.”
The patients underwent induction therapy with cyclophosphamide or rituximab (Rituxan) plus IV methylprednisolone. Then they were randomized to seven plasma exchange sessions in 14 days or no plasma exchange, and further randomized to conventional weight-based, high-dose oral glucocorticoids or a lower-dose regimen. Those on the reduced-dose regimen received 54% of the cumulative amount of glucocorticoids used in the standard-dose group through the first 3 months, and 61% over the course of 6 months. By week 4, those on the reduced-dose regimen were on an average of 25 mg/day, while those on standard therapy were on 50 mg/day. Adherence to assigned study arms exceeded 90%. Patients were followed prospectively for 1-7 years.
The primary endpoint, a composite of all-cause mortality or development of end-stage renal disease, occurred in 28% of patients on plasma exchange and 31% of those who did not undergo plasma exchange, a nonsignificant difference indicative of a lack of benefit for the intervention. No differential effect was seen in prespecified subgroups based on age, creatinine clearance, ANCA type, form of immunosuppression, or presence or absence of lung hemorrhage.
Further, the primary endpoint occurred in 28% of patients on reduced-dose glucocorticoids, compared with 26% on full-dose therapy; again, a nonsignificant difference, meaning lower-dose therapy didn’t result in less effective disease control. But it did result in a significant reduction in the prespecified endpoint of serious infections in the first year: 27% versus 33% with full-dose therapy, representing a 30% relative risk reduction.
Audience members wanted to know if there are any circumstances at all in which Dr. Merkel would now consider resorting to plasma exchange, such as maybe in AAV patients at the most extreme end of the severity spectrum.
“I’m not sure I should be the one dictating that; I think the world needs to see the data,” he replied.
That being said, he added, “I think these data are incredibly helpful to physicians and patients as they face this decision. I think plasma exchange is an expensive therapy and somewhat invasive. I think our results indicate that the benefit that we may have thought was there is not there.”
The study was sponsored by the National Institutes of Health, the Food and Drug Administration, the U.K. Medical Research Council and the National Institute for Health Research, the Canadian Institutes of Health Research, and the governments of France, Australia, and New Zealand. The presenter reported receiving research funding from the ACR, EULAR, FDA, NIH, Patient-Centered Outcomes Research Institute, and the Vasculitis Foundation. He also receives research funding from and/or serves as a consultant to more than a dozen pharmaceutical companies.
SOURCE: Merkel PA et al. Arthritis Rheumatol. 2018;70(Suppl 10):Abstract 2788.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Plasma exchange was without benefit and reduced-dose oral glucocorticoids safely decreased serious infections in ANCA-associated vasculitis.
Major finding: The rate of serious infections in the first year was 27% in patients on reduced-dose oral glucocorticoids and 33% with standard high-dose therapy, for a significant 30% relative risk reduction.
Study details: PEXIVAS was a multicenter, international, open-label, randomized trial with a 2-by-2 factorial design comprising 704 patients with severe ANCA-associated vasculitis.
Disclosures: The study was sponsored by the National Institutes of Health, the Food and Drug Administration, the U.K. Medical Research Council and the National Institute of Health Research, the Canadian Institutes of Health Research, and the governments of France, Australia, and New Zealand. The presenter reported receiving research funding from the ACR, EULAR, FDA, NIH, Patient-Centered Outcomes Research Institute, and the Vasculitis Foundation. He also receives research funding from and/or serves as a consultant to more than a dozen pharmaceutical companies.
Source: Merkel PA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2788.
AGA report from Capitol Hill
On Sept. 14, AGA held Advocacy Day. This was a day in which several AGA members met with staff of Congressional representatives on Capitol Hill to advocate for important issues within the field of gastroenterology. The three primary issues involved:
- Support of increased NIH funding.
- Requesting increased transparency in insurance-driven step-therapy protocols.
- Removal of the coinsurance or copayment for screening colonoscopies that become therapeutic, once polyps are identified and removed.
These issues support growth and autonomy of our field, while supporting the interests of our patients.
Advocacy is not difficult. Many of my fellow GIs are unnecessarily intimidated by this word; however, each individual has the ability and, arguably, the responsibility to shape the environment in which we practice. Opportunities to engage your representatives may be as simple as clicking a link, leaving a voicemail, or signing a petition, to testifying at hearings or hosting a representative at your own institution. AGA staff made participating in Advocacy Day very easy. Staff at AGA coordinate meetings between each advocate, and the offices of his or her local Congress members. AGA also provides brief training prior to these meetings; thus, no prior experience is required. I felt well prepared for the meetings with my local Congress staff members.
I chose to participate in Advocacy Day because I want to bring the experiences of my colleagues and patients to the doorsteps of those who make decisions about how we practice. I feel that it is important to stand up for our field and our patients, lest others make decisions for us. We do not have to feel powerless in a changing field. Let your voice be heard.
Dr. Anjou is a gastroenterologist at the University of Connecticut Health Center, Farmington, and member of the AGA Trainee and Early Career Committee and Quality Measures Committee.
On Sept. 14, AGA held Advocacy Day. This was a day in which several AGA members met with staff of Congressional representatives on Capitol Hill to advocate for important issues within the field of gastroenterology. The three primary issues involved:
- Support of increased NIH funding.
- Requesting increased transparency in insurance-driven step-therapy protocols.
- Removal of the coinsurance or copayment for screening colonoscopies that become therapeutic, once polyps are identified and removed.
These issues support growth and autonomy of our field, while supporting the interests of our patients.
Advocacy is not difficult. Many of my fellow GIs are unnecessarily intimidated by this word; however, each individual has the ability and, arguably, the responsibility to shape the environment in which we practice. Opportunities to engage your representatives may be as simple as clicking a link, leaving a voicemail, or signing a petition, to testifying at hearings or hosting a representative at your own institution. AGA staff made participating in Advocacy Day very easy. Staff at AGA coordinate meetings between each advocate, and the offices of his or her local Congress members. AGA also provides brief training prior to these meetings; thus, no prior experience is required. I felt well prepared for the meetings with my local Congress staff members.
I chose to participate in Advocacy Day because I want to bring the experiences of my colleagues and patients to the doorsteps of those who make decisions about how we practice. I feel that it is important to stand up for our field and our patients, lest others make decisions for us. We do not have to feel powerless in a changing field. Let your voice be heard.
Dr. Anjou is a gastroenterologist at the University of Connecticut Health Center, Farmington, and member of the AGA Trainee and Early Career Committee and Quality Measures Committee.
On Sept. 14, AGA held Advocacy Day. This was a day in which several AGA members met with staff of Congressional representatives on Capitol Hill to advocate for important issues within the field of gastroenterology. The three primary issues involved:
- Support of increased NIH funding.
- Requesting increased transparency in insurance-driven step-therapy protocols.
- Removal of the coinsurance or copayment for screening colonoscopies that become therapeutic, once polyps are identified and removed.
These issues support growth and autonomy of our field, while supporting the interests of our patients.
Advocacy is not difficult. Many of my fellow GIs are unnecessarily intimidated by this word; however, each individual has the ability and, arguably, the responsibility to shape the environment in which we practice. Opportunities to engage your representatives may be as simple as clicking a link, leaving a voicemail, or signing a petition, to testifying at hearings or hosting a representative at your own institution. AGA staff made participating in Advocacy Day very easy. Staff at AGA coordinate meetings between each advocate, and the offices of his or her local Congress members. AGA also provides brief training prior to these meetings; thus, no prior experience is required. I felt well prepared for the meetings with my local Congress staff members.
I chose to participate in Advocacy Day because I want to bring the experiences of my colleagues and patients to the doorsteps of those who make decisions about how we practice. I feel that it is important to stand up for our field and our patients, lest others make decisions for us. We do not have to feel powerless in a changing field. Let your voice be heard.
Dr. Anjou is a gastroenterologist at the University of Connecticut Health Center, Farmington, and member of the AGA Trainee and Early Career Committee and Quality Measures Committee.
Drafts of new classification criteria presented for GCA, Takayasu’s arteritis
CHICAGO – Drafts of new classification criteria for giant cell arteritis and Takayasu’s arteritis developed by the American College of Rheumatology and the European League Against Rheumatism (EULAR) reflect the increasingly important role of advanced vascular imaging in the diagnosis and management of large-vessel vasculitis, according to Peter A. Merkel, MD.
The drafts, which are the result of a multiyear collaboration between the ACR and EULAR, were presented at the annual meeting of the ACR and will be submitted to the ACR/EULAR committee overseeing the work for comprehensive review and possible revisions. Once endorsed, the new criteria will replace the “extremely important,” but outdated, existing classification criteria, which were published in 1990.
“What we’ve done is, rather than purely revise the 1990 [criteria], we’ve started again from scratch ... with a great number of cases from a wide variety of centers throughout the world. This was a very large international effort ... really a great community effort in the field of rheumatology,” Dr. Merkel, professor and chief of the division of rheumatology at the University of Pennsylvania, Philadelphia, and one of the chief investigators for the project, said in a video interview.
The new criteria will allow for better classification of patients with giant cell arteritis versus Takayasu’s arteritis versus another form of vasculitis, he said, noting that advances in imaging that allow for “more enriched data with which to make decisions” play a large role.
However, the new criteria are not meant to be used for diagnosis, but to “sort out among the different types of vasculitis,” he said.
“It provides awareness and it provides a tool, especially for research investigation, but that seeps out into the broader community,” he added.
Dr. Merkel reported having no disclosures.
SOURCE: Merkel PA et al. ACR Annual Meeting, Presentation 5T116.
CHICAGO – Drafts of new classification criteria for giant cell arteritis and Takayasu’s arteritis developed by the American College of Rheumatology and the European League Against Rheumatism (EULAR) reflect the increasingly important role of advanced vascular imaging in the diagnosis and management of large-vessel vasculitis, according to Peter A. Merkel, MD.
The drafts, which are the result of a multiyear collaboration between the ACR and EULAR, were presented at the annual meeting of the ACR and will be submitted to the ACR/EULAR committee overseeing the work for comprehensive review and possible revisions. Once endorsed, the new criteria will replace the “extremely important,” but outdated, existing classification criteria, which were published in 1990.
“What we’ve done is, rather than purely revise the 1990 [criteria], we’ve started again from scratch ... with a great number of cases from a wide variety of centers throughout the world. This was a very large international effort ... really a great community effort in the field of rheumatology,” Dr. Merkel, professor and chief of the division of rheumatology at the University of Pennsylvania, Philadelphia, and one of the chief investigators for the project, said in a video interview.
The new criteria will allow for better classification of patients with giant cell arteritis versus Takayasu’s arteritis versus another form of vasculitis, he said, noting that advances in imaging that allow for “more enriched data with which to make decisions” play a large role.
However, the new criteria are not meant to be used for diagnosis, but to “sort out among the different types of vasculitis,” he said.
“It provides awareness and it provides a tool, especially for research investigation, but that seeps out into the broader community,” he added.
Dr. Merkel reported having no disclosures.
SOURCE: Merkel PA et al. ACR Annual Meeting, Presentation 5T116.
CHICAGO – Drafts of new classification criteria for giant cell arteritis and Takayasu’s arteritis developed by the American College of Rheumatology and the European League Against Rheumatism (EULAR) reflect the increasingly important role of advanced vascular imaging in the diagnosis and management of large-vessel vasculitis, according to Peter A. Merkel, MD.
The drafts, which are the result of a multiyear collaboration between the ACR and EULAR, were presented at the annual meeting of the ACR and will be submitted to the ACR/EULAR committee overseeing the work for comprehensive review and possible revisions. Once endorsed, the new criteria will replace the “extremely important,” but outdated, existing classification criteria, which were published in 1990.
“What we’ve done is, rather than purely revise the 1990 [criteria], we’ve started again from scratch ... with a great number of cases from a wide variety of centers throughout the world. This was a very large international effort ... really a great community effort in the field of rheumatology,” Dr. Merkel, professor and chief of the division of rheumatology at the University of Pennsylvania, Philadelphia, and one of the chief investigators for the project, said in a video interview.
The new criteria will allow for better classification of patients with giant cell arteritis versus Takayasu’s arteritis versus another form of vasculitis, he said, noting that advances in imaging that allow for “more enriched data with which to make decisions” play a large role.
However, the new criteria are not meant to be used for diagnosis, but to “sort out among the different types of vasculitis,” he said.
“It provides awareness and it provides a tool, especially for research investigation, but that seeps out into the broader community,” he added.
Dr. Merkel reported having no disclosures.
SOURCE: Merkel PA et al. ACR Annual Meeting, Presentation 5T116.
REPORTING FROM THE ACR ANNUAL MEETING
Length of stay, complications predict readmission for cirrhosis patients
PHILADELPHIA – Patients with cirrhosis have a higher risk of hospital readmission if their length of stay is less than 4 days, if they have cirrhosis-related complications, and if they are discharged to an extended-care facility or to home health care, according to a recent presentation at the annual meeting of the American College of Gastroenterology.
“The presence of cirrhosis-related complications is very strongly associated with readmissions,” Chandraprakash Umapathy, MD, MS, from the University of California, San Francisco, Fresno, said during his presentation. “Quality improvement efforts should focus on optimizing the management of complications of cirrhosis in the outpatient setting to reduce readmissions.”
In a retrospective cohort study, Dr. Umapathy and colleagues identified 230,036 patients from the Healthcare Cost and Utilization Project National Readmission Database for 2014 who had been discharged with a diagnosis of cirrhosis; of these patients, there were 185,737 index cases after excluding readmissions. Included patients had a mean age of 60.2 years and mean length of stay of 6.4 days, with 46% of patients having a length of stay longer than 4 days and mean total charges of $56,519. With regard to cirrhosis, 55% of patients displayed cirrhosis complications and 6.7% had more than three cirrhosis-related complications; the most common complication was ascites, in 32% of patients.
Overall, 11.09% of patients were readmitted at 30 days and 18.74% of patients were readmitted at 90 days, Dr. Umapathy said. Patients were more likely to be readmitted at 30 days if they were originally admitted on a weekend (adjusted prevalence ratio, 1.06; P = .001); into a medium (1.09; P = .009) or large (1.11; P less than .001) hospital; were admitted at a metropolitan teaching hospital (1.07; P less than .001); were insured by Medicaid (1.07; P less than .001); or were transferred to an extended care (1.51; P less than .001) facility or discharged to home health care (1.43; P less than .001).
Compared with patients who were not readmitted at 30 days, patients with 30-day readmission had a higher rate of alcoholic liver disease (43% vs. 46%; P less than .001), hepatitis C (28% vs. 32%; P less than .001), ascites (31% vs. 43%; P less than .001), hepatic encephalopathy (15% vs. 22%; P less than .001), hepatorenal syndrome (2.3% vs. 4.9%; P less than .001), hepatocellular cancer (5.1% vs. 5.7%; P = .001), presence of any cirrhosis complications (54% vs. 65%; P less than .001), and presence of more than three cirrhosis-related complications (6.3% vs. 10%; P less than .001). When adjusted in a multivariate analysis, association with readmission at 30 days for patients with cirrhosis-related complications such as ascites (1.42; P less than .001), hepatic encephalopathy (1.44; P less than .001), and hepatorenal syndrome (1.34; P less than .001) remained, Dr. Umapathy noted.
Length of stay longer than 4 days (0.84; P less than .001) and variceal hemorrhage (0.74; P = .002) were associated with reduced risk of readmissions at 30 days. “Focus on length of stay may result in patients being discharged prematurely, leading to higher early readmission,” Dr. Umapathy said.
Dr. Umapathy reports no relevant conflicts of interest.
SOURCE: Umapathy C et al. ACG 2018, Presentation 60
PHILADELPHIA – Patients with cirrhosis have a higher risk of hospital readmission if their length of stay is less than 4 days, if they have cirrhosis-related complications, and if they are discharged to an extended-care facility or to home health care, according to a recent presentation at the annual meeting of the American College of Gastroenterology.
“The presence of cirrhosis-related complications is very strongly associated with readmissions,” Chandraprakash Umapathy, MD, MS, from the University of California, San Francisco, Fresno, said during his presentation. “Quality improvement efforts should focus on optimizing the management of complications of cirrhosis in the outpatient setting to reduce readmissions.”
In a retrospective cohort study, Dr. Umapathy and colleagues identified 230,036 patients from the Healthcare Cost and Utilization Project National Readmission Database for 2014 who had been discharged with a diagnosis of cirrhosis; of these patients, there were 185,737 index cases after excluding readmissions. Included patients had a mean age of 60.2 years and mean length of stay of 6.4 days, with 46% of patients having a length of stay longer than 4 days and mean total charges of $56,519. With regard to cirrhosis, 55% of patients displayed cirrhosis complications and 6.7% had more than three cirrhosis-related complications; the most common complication was ascites, in 32% of patients.
Overall, 11.09% of patients were readmitted at 30 days and 18.74% of patients were readmitted at 90 days, Dr. Umapathy said. Patients were more likely to be readmitted at 30 days if they were originally admitted on a weekend (adjusted prevalence ratio, 1.06; P = .001); into a medium (1.09; P = .009) or large (1.11; P less than .001) hospital; were admitted at a metropolitan teaching hospital (1.07; P less than .001); were insured by Medicaid (1.07; P less than .001); or were transferred to an extended care (1.51; P less than .001) facility or discharged to home health care (1.43; P less than .001).
Compared with patients who were not readmitted at 30 days, patients with 30-day readmission had a higher rate of alcoholic liver disease (43% vs. 46%; P less than .001), hepatitis C (28% vs. 32%; P less than .001), ascites (31% vs. 43%; P less than .001), hepatic encephalopathy (15% vs. 22%; P less than .001), hepatorenal syndrome (2.3% vs. 4.9%; P less than .001), hepatocellular cancer (5.1% vs. 5.7%; P = .001), presence of any cirrhosis complications (54% vs. 65%; P less than .001), and presence of more than three cirrhosis-related complications (6.3% vs. 10%; P less than .001). When adjusted in a multivariate analysis, association with readmission at 30 days for patients with cirrhosis-related complications such as ascites (1.42; P less than .001), hepatic encephalopathy (1.44; P less than .001), and hepatorenal syndrome (1.34; P less than .001) remained, Dr. Umapathy noted.
Length of stay longer than 4 days (0.84; P less than .001) and variceal hemorrhage (0.74; P = .002) were associated with reduced risk of readmissions at 30 days. “Focus on length of stay may result in patients being discharged prematurely, leading to higher early readmission,” Dr. Umapathy said.
Dr. Umapathy reports no relevant conflicts of interest.
SOURCE: Umapathy C et al. ACG 2018, Presentation 60
PHILADELPHIA – Patients with cirrhosis have a higher risk of hospital readmission if their length of stay is less than 4 days, if they have cirrhosis-related complications, and if they are discharged to an extended-care facility or to home health care, according to a recent presentation at the annual meeting of the American College of Gastroenterology.
“The presence of cirrhosis-related complications is very strongly associated with readmissions,” Chandraprakash Umapathy, MD, MS, from the University of California, San Francisco, Fresno, said during his presentation. “Quality improvement efforts should focus on optimizing the management of complications of cirrhosis in the outpatient setting to reduce readmissions.”
In a retrospective cohort study, Dr. Umapathy and colleagues identified 230,036 patients from the Healthcare Cost and Utilization Project National Readmission Database for 2014 who had been discharged with a diagnosis of cirrhosis; of these patients, there were 185,737 index cases after excluding readmissions. Included patients had a mean age of 60.2 years and mean length of stay of 6.4 days, with 46% of patients having a length of stay longer than 4 days and mean total charges of $56,519. With regard to cirrhosis, 55% of patients displayed cirrhosis complications and 6.7% had more than three cirrhosis-related complications; the most common complication was ascites, in 32% of patients.
Overall, 11.09% of patients were readmitted at 30 days and 18.74% of patients were readmitted at 90 days, Dr. Umapathy said. Patients were more likely to be readmitted at 30 days if they were originally admitted on a weekend (adjusted prevalence ratio, 1.06; P = .001); into a medium (1.09; P = .009) or large (1.11; P less than .001) hospital; were admitted at a metropolitan teaching hospital (1.07; P less than .001); were insured by Medicaid (1.07; P less than .001); or were transferred to an extended care (1.51; P less than .001) facility or discharged to home health care (1.43; P less than .001).
Compared with patients who were not readmitted at 30 days, patients with 30-day readmission had a higher rate of alcoholic liver disease (43% vs. 46%; P less than .001), hepatitis C (28% vs. 32%; P less than .001), ascites (31% vs. 43%; P less than .001), hepatic encephalopathy (15% vs. 22%; P less than .001), hepatorenal syndrome (2.3% vs. 4.9%; P less than .001), hepatocellular cancer (5.1% vs. 5.7%; P = .001), presence of any cirrhosis complications (54% vs. 65%; P less than .001), and presence of more than three cirrhosis-related complications (6.3% vs. 10%; P less than .001). When adjusted in a multivariate analysis, association with readmission at 30 days for patients with cirrhosis-related complications such as ascites (1.42; P less than .001), hepatic encephalopathy (1.44; P less than .001), and hepatorenal syndrome (1.34; P less than .001) remained, Dr. Umapathy noted.
Length of stay longer than 4 days (0.84; P less than .001) and variceal hemorrhage (0.74; P = .002) were associated with reduced risk of readmissions at 30 days. “Focus on length of stay may result in patients being discharged prematurely, leading to higher early readmission,” Dr. Umapathy said.
Dr. Umapathy reports no relevant conflicts of interest.
SOURCE: Umapathy C et al. ACG 2018, Presentation 60
REPORTING FROM ACG 2018
Key clinical point: Cirrhosis-related complications and shorter length of stay were associated with higher rates of readmissions for patients with cirrhosis.
Major finding: 11.09% of patients were readmitted at 30 days and 18.74% of patients at 90 days, with the most common reasons for readmission including presence of cirrhosis complications and length of stay less than 4 days.
Study details: A retrospective cohort study of 185,737 index cases in the Healthcare Cost and Utilization Project National Readmission Database.
Disclosures: Dr. Umapathy reports no relevant conflicts of interest.
Source: Umapathy C et al. ACG 2018, Presentation 60.
“It Gets Better With Age”
Since birth, this now–13-year-old boy has had redness on his face— the intensity of which has slowly increased with time. Various providers have offered a plethora of diagnoses, but no treatment attempts thus far have helped. The condition is asymptomatic but nonetheless distressing to the patient.
More history-taking reveals that, when he was about 6, crops of tiny papules developed on both triceps, his buttocks, and his upper back. These, too, have resisted treatment with OTC creams.
Neither of the boy’s two siblings have had any similar lesions, and no one in the family has any related health problems (eg, atopic diatheses).
EXAMINATION
The posterior 2/3 of both sides of the patient’s face are strikingly red. His nasolabial folds are spared, but the redness extends posteriorly to the immediate preauricular areas and vertically from the zygoma to the jawline. The erythema is highly blanchable with digital pressure and has a uniformly rough, papular feel. There is no tenderness or increased warmth on palpation.
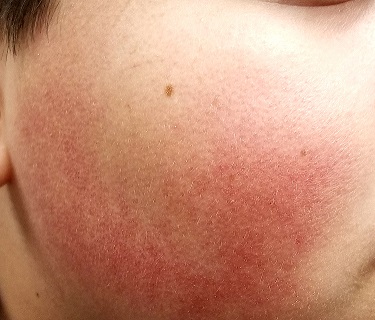
The papules on the triceps, anterior thighs, and upper back are uniform in size (pinpoint, measuring ≤ 1 mm) and distribution, obviously originating from follicles. Unlike the face, these areas are not erythematous.
What’s the diagnosis?
DISCUSSION
There are several types of keratosis pilaris (KP), including rubra faceii, the form affecting this patient (distinguished in part by involvement of the facial skin). KP is utterly common, affecting 30% to 50% of the white population worldwide, with no gender preference. This autosomal dominant disorder involves follicular keratinization—normal keratin (produced in the hair follicle) builds up and creates a “plug” that manifests as a firm, dry papule. Obstruction of the follicular orifice may be significant enough to prevent hair from exiting, in which case, the hair continues to grow but simply curls in on itself and accentuates the appearance of the papule.
Although KP is a condition and not a disease, it is often considered part of the atopic diatheses, which include the major diagnostic criteria of eczema, urticaria, and seasonal allergies. KP is often mistaken for acne, especially when it affects the face, but its lack of comedones and pustules is a distinguishing characteristic.
Keratosis follicularis (Darier disease) also features follicular papules, but the distribution and morphology differ significantly. Darier is a more serious problem in terms of extent and symptomatology.
Treatment of KP is unsatisfactory at best, but emollients can make it less bumpy. Salicylic acid and lactic acid–containing preparations can also help, but only temporarily. Gentle exfoliation followed by the application of heavy oils is considered the most effective treatment method. The most encouraging thing we can tell our patients: The problem tends to lessen with age.
TAKE-HOME LEARNING POINTS
- Keratosis pilaris (KP) is a common inherited defect of follicular keratinization that affects 30% to 50% of the white population worldwide.
- KP results in a distribution of follicular rough papules across the face, triceps, thighs, buttocks, and upper back, beginning in early childhood.
- A significant percentage of affected patients exhibit the variant termed rubra faceii, which involves the posterior 2/3 of the bilateral face.
- Treatment is problematic, but the application of emollients after gentle exfoliation can help; most cases improve as the patient ages.
Since birth, this now–13-year-old boy has had redness on his face— the intensity of which has slowly increased with time. Various providers have offered a plethora of diagnoses, but no treatment attempts thus far have helped. The condition is asymptomatic but nonetheless distressing to the patient.
More history-taking reveals that, when he was about 6, crops of tiny papules developed on both triceps, his buttocks, and his upper back. These, too, have resisted treatment with OTC creams.
Neither of the boy’s two siblings have had any similar lesions, and no one in the family has any related health problems (eg, atopic diatheses).
EXAMINATION
The posterior 2/3 of both sides of the patient’s face are strikingly red. His nasolabial folds are spared, but the redness extends posteriorly to the immediate preauricular areas and vertically from the zygoma to the jawline. The erythema is highly blanchable with digital pressure and has a uniformly rough, papular feel. There is no tenderness or increased warmth on palpation.

The papules on the triceps, anterior thighs, and upper back are uniform in size (pinpoint, measuring ≤ 1 mm) and distribution, obviously originating from follicles. Unlike the face, these areas are not erythematous.
What’s the diagnosis?
DISCUSSION
There are several types of keratosis pilaris (KP), including rubra faceii, the form affecting this patient (distinguished in part by involvement of the facial skin). KP is utterly common, affecting 30% to 50% of the white population worldwide, with no gender preference. This autosomal dominant disorder involves follicular keratinization—normal keratin (produced in the hair follicle) builds up and creates a “plug” that manifests as a firm, dry papule. Obstruction of the follicular orifice may be significant enough to prevent hair from exiting, in which case, the hair continues to grow but simply curls in on itself and accentuates the appearance of the papule.
Although KP is a condition and not a disease, it is often considered part of the atopic diatheses, which include the major diagnostic criteria of eczema, urticaria, and seasonal allergies. KP is often mistaken for acne, especially when it affects the face, but its lack of comedones and pustules is a distinguishing characteristic.
Keratosis follicularis (Darier disease) also features follicular papules, but the distribution and morphology differ significantly. Darier is a more serious problem in terms of extent and symptomatology.
Treatment of KP is unsatisfactory at best, but emollients can make it less bumpy. Salicylic acid and lactic acid–containing preparations can also help, but only temporarily. Gentle exfoliation followed by the application of heavy oils is considered the most effective treatment method. The most encouraging thing we can tell our patients: The problem tends to lessen with age.
TAKE-HOME LEARNING POINTS
- Keratosis pilaris (KP) is a common inherited defect of follicular keratinization that affects 30% to 50% of the white population worldwide.
- KP results in a distribution of follicular rough papules across the face, triceps, thighs, buttocks, and upper back, beginning in early childhood.
- A significant percentage of affected patients exhibit the variant termed rubra faceii, which involves the posterior 2/3 of the bilateral face.
- Treatment is problematic, but the application of emollients after gentle exfoliation can help; most cases improve as the patient ages.
Since birth, this now–13-year-old boy has had redness on his face— the intensity of which has slowly increased with time. Various providers have offered a plethora of diagnoses, but no treatment attempts thus far have helped. The condition is asymptomatic but nonetheless distressing to the patient.
More history-taking reveals that, when he was about 6, crops of tiny papules developed on both triceps, his buttocks, and his upper back. These, too, have resisted treatment with OTC creams.
Neither of the boy’s two siblings have had any similar lesions, and no one in the family has any related health problems (eg, atopic diatheses).
EXAMINATION
The posterior 2/3 of both sides of the patient’s face are strikingly red. His nasolabial folds are spared, but the redness extends posteriorly to the immediate preauricular areas and vertically from the zygoma to the jawline. The erythema is highly blanchable with digital pressure and has a uniformly rough, papular feel. There is no tenderness or increased warmth on palpation.

The papules on the triceps, anterior thighs, and upper back are uniform in size (pinpoint, measuring ≤ 1 mm) and distribution, obviously originating from follicles. Unlike the face, these areas are not erythematous.
What’s the diagnosis?
DISCUSSION
There are several types of keratosis pilaris (KP), including rubra faceii, the form affecting this patient (distinguished in part by involvement of the facial skin). KP is utterly common, affecting 30% to 50% of the white population worldwide, with no gender preference. This autosomal dominant disorder involves follicular keratinization—normal keratin (produced in the hair follicle) builds up and creates a “plug” that manifests as a firm, dry papule. Obstruction of the follicular orifice may be significant enough to prevent hair from exiting, in which case, the hair continues to grow but simply curls in on itself and accentuates the appearance of the papule.
Although KP is a condition and not a disease, it is often considered part of the atopic diatheses, which include the major diagnostic criteria of eczema, urticaria, and seasonal allergies. KP is often mistaken for acne, especially when it affects the face, but its lack of comedones and pustules is a distinguishing characteristic.
Keratosis follicularis (Darier disease) also features follicular papules, but the distribution and morphology differ significantly. Darier is a more serious problem in terms of extent and symptomatology.
Treatment of KP is unsatisfactory at best, but emollients can make it less bumpy. Salicylic acid and lactic acid–containing preparations can also help, but only temporarily. Gentle exfoliation followed by the application of heavy oils is considered the most effective treatment method. The most encouraging thing we can tell our patients: The problem tends to lessen with age.
TAKE-HOME LEARNING POINTS
- Keratosis pilaris (KP) is a common inherited defect of follicular keratinization that affects 30% to 50% of the white population worldwide.
- KP results in a distribution of follicular rough papules across the face, triceps, thighs, buttocks, and upper back, beginning in early childhood.
- A significant percentage of affected patients exhibit the variant termed rubra faceii, which involves the posterior 2/3 of the bilateral face.
- Treatment is problematic, but the application of emollients after gentle exfoliation can help; most cases improve as the patient ages.
Innovations in Dermatology: Antibiotic Stewardship in Acne Therapy



Child-Pugh class does not affect HE recurrence in patients taking rifaximin
PHILADELPHIA – according to a recent presentation at the annual meeting of the American College of Gastroenterology.
Steven L. Flamm, MD, from Northwestern University, Chicago, and his colleagues examined results from a previous randomized, double-blinded trial of 140 patients receiving twice-daily rifaximin at 550 mg for 6 months in which the results showed rifaximin successfully maintained remission of hepatic encephalopathy (HE), compared with 159 patients receiving placebo.
“This pivotal study was published in March of 2010, but one of the post hoc assessments that was not performed was looking at various different phases of hepatic impairment as dictated by [Child-Pugh] class and each of those responses to this product,” Dr. Flamm said in his presentation.
Patients in the study were included if they had a Model For End-Stage Liver Disease score of 25 or less and two or more overt HE within 6 months (Conn score 1 or less) but were currently in remission. The researchers allowed the use of concomitant lactulose during the study period, which was used in 94.1% of rifaximin and 91.2% of placebo patients.
In the post hoc analysis, Dr. Flamm and his colleagues divided rifaximin and placebo patients into Child-Pugh class A (46 patients vs. 56 patients), class B (65 patients vs. 72 patients), and class C (17 patients vs. 14 patients) groups. For rifaximin and placebo patients, the mean age was 57.3 years and 57.2 years in the class A group, 54.4 years and 57.0 years in the class B group, and 56.1 years and 57.6 years in the class C group, respectively.
Overall, 8 of 46 rifaximin patients (17.4%) in the Child-Pugh class A and 15 of 65 rifaximin patients (23.1%) in the class B groups experienced an overt HE event during the 6-month study period, compared with 26 of 56 patients in the class A (46.4%) and 32 of 72 patients (44.4%) in the class B placebo groups; 5 of 17 rifaximin patients (29.4%) in the Child-Pugh class C group experienced their first overt HE event, compared with 9 of 14 (64.3%) patients in the placebo group.
With regard to first HE-related hospitalization, 5 of 46 patients (10.9%) in the rifaximin Child-Pugh class A group, 8 of 65 rifaximin patients (12.3%) in the class B group, and 4 of 17 rifaximin patients (23.5%) in the class C group experienced hospitalization because of HE, compared with 15 of 56 patients (23.2%) in the Child-Pugh class A group, 15 of 72 patients (20.8%) in the class B group, and 5 of 14 patients (35.7%) in the class C placebo group. The researchers noted lactulose use in the majority of patient cases in the study “provided further benefit” in reducing overt HE events.
“Although numeric differences were observed favoring rifaximin for the incidence of HE-related hospitalizations, a risk reduction versus placebo could not be firmly established, and presumably this was largely due to a lack of adequate power because of small sample size,” Dr. Flamm said.
This study and its analysis were supported by Salix Pharmaceuticals. Dr. Flamm and other coauthors report advisory committee membership, board membership, employment, or consultancy with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Intercept Pharmaceuticals, Merck and Salix Pharmaceuticals. One coauthor reported no relevant conflicts of interest.
SOURCE: Flamm SL et al ACG 2018, Presentation 58.
PHILADELPHIA – according to a recent presentation at the annual meeting of the American College of Gastroenterology.
Steven L. Flamm, MD, from Northwestern University, Chicago, and his colleagues examined results from a previous randomized, double-blinded trial of 140 patients receiving twice-daily rifaximin at 550 mg for 6 months in which the results showed rifaximin successfully maintained remission of hepatic encephalopathy (HE), compared with 159 patients receiving placebo.
“This pivotal study was published in March of 2010, but one of the post hoc assessments that was not performed was looking at various different phases of hepatic impairment as dictated by [Child-Pugh] class and each of those responses to this product,” Dr. Flamm said in his presentation.
Patients in the study were included if they had a Model For End-Stage Liver Disease score of 25 or less and two or more overt HE within 6 months (Conn score 1 or less) but were currently in remission. The researchers allowed the use of concomitant lactulose during the study period, which was used in 94.1% of rifaximin and 91.2% of placebo patients.
In the post hoc analysis, Dr. Flamm and his colleagues divided rifaximin and placebo patients into Child-Pugh class A (46 patients vs. 56 patients), class B (65 patients vs. 72 patients), and class C (17 patients vs. 14 patients) groups. For rifaximin and placebo patients, the mean age was 57.3 years and 57.2 years in the class A group, 54.4 years and 57.0 years in the class B group, and 56.1 years and 57.6 years in the class C group, respectively.
Overall, 8 of 46 rifaximin patients (17.4%) in the Child-Pugh class A and 15 of 65 rifaximin patients (23.1%) in the class B groups experienced an overt HE event during the 6-month study period, compared with 26 of 56 patients in the class A (46.4%) and 32 of 72 patients (44.4%) in the class B placebo groups; 5 of 17 rifaximin patients (29.4%) in the Child-Pugh class C group experienced their first overt HE event, compared with 9 of 14 (64.3%) patients in the placebo group.
With regard to first HE-related hospitalization, 5 of 46 patients (10.9%) in the rifaximin Child-Pugh class A group, 8 of 65 rifaximin patients (12.3%) in the class B group, and 4 of 17 rifaximin patients (23.5%) in the class C group experienced hospitalization because of HE, compared with 15 of 56 patients (23.2%) in the Child-Pugh class A group, 15 of 72 patients (20.8%) in the class B group, and 5 of 14 patients (35.7%) in the class C placebo group. The researchers noted lactulose use in the majority of patient cases in the study “provided further benefit” in reducing overt HE events.
“Although numeric differences were observed favoring rifaximin for the incidence of HE-related hospitalizations, a risk reduction versus placebo could not be firmly established, and presumably this was largely due to a lack of adequate power because of small sample size,” Dr. Flamm said.
This study and its analysis were supported by Salix Pharmaceuticals. Dr. Flamm and other coauthors report advisory committee membership, board membership, employment, or consultancy with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Intercept Pharmaceuticals, Merck and Salix Pharmaceuticals. One coauthor reported no relevant conflicts of interest.
SOURCE: Flamm SL et al ACG 2018, Presentation 58.
PHILADELPHIA – according to a recent presentation at the annual meeting of the American College of Gastroenterology.
Steven L. Flamm, MD, from Northwestern University, Chicago, and his colleagues examined results from a previous randomized, double-blinded trial of 140 patients receiving twice-daily rifaximin at 550 mg for 6 months in which the results showed rifaximin successfully maintained remission of hepatic encephalopathy (HE), compared with 159 patients receiving placebo.
“This pivotal study was published in March of 2010, but one of the post hoc assessments that was not performed was looking at various different phases of hepatic impairment as dictated by [Child-Pugh] class and each of those responses to this product,” Dr. Flamm said in his presentation.
Patients in the study were included if they had a Model For End-Stage Liver Disease score of 25 or less and two or more overt HE within 6 months (Conn score 1 or less) but were currently in remission. The researchers allowed the use of concomitant lactulose during the study period, which was used in 94.1% of rifaximin and 91.2% of placebo patients.
In the post hoc analysis, Dr. Flamm and his colleagues divided rifaximin and placebo patients into Child-Pugh class A (46 patients vs. 56 patients), class B (65 patients vs. 72 patients), and class C (17 patients vs. 14 patients) groups. For rifaximin and placebo patients, the mean age was 57.3 years and 57.2 years in the class A group, 54.4 years and 57.0 years in the class B group, and 56.1 years and 57.6 years in the class C group, respectively.
Overall, 8 of 46 rifaximin patients (17.4%) in the Child-Pugh class A and 15 of 65 rifaximin patients (23.1%) in the class B groups experienced an overt HE event during the 6-month study period, compared with 26 of 56 patients in the class A (46.4%) and 32 of 72 patients (44.4%) in the class B placebo groups; 5 of 17 rifaximin patients (29.4%) in the Child-Pugh class C group experienced their first overt HE event, compared with 9 of 14 (64.3%) patients in the placebo group.
With regard to first HE-related hospitalization, 5 of 46 patients (10.9%) in the rifaximin Child-Pugh class A group, 8 of 65 rifaximin patients (12.3%) in the class B group, and 4 of 17 rifaximin patients (23.5%) in the class C group experienced hospitalization because of HE, compared with 15 of 56 patients (23.2%) in the Child-Pugh class A group, 15 of 72 patients (20.8%) in the class B group, and 5 of 14 patients (35.7%) in the class C placebo group. The researchers noted lactulose use in the majority of patient cases in the study “provided further benefit” in reducing overt HE events.
“Although numeric differences were observed favoring rifaximin for the incidence of HE-related hospitalizations, a risk reduction versus placebo could not be firmly established, and presumably this was largely due to a lack of adequate power because of small sample size,” Dr. Flamm said.
This study and its analysis were supported by Salix Pharmaceuticals. Dr. Flamm and other coauthors report advisory committee membership, board membership, employment, or consultancy with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Intercept Pharmaceuticals, Merck and Salix Pharmaceuticals. One coauthor reported no relevant conflicts of interest.
SOURCE: Flamm SL et al ACG 2018, Presentation 58.
REPORTING FROM ACG 2018
Key clinical point: Child-Pugh class does not significantly affect the overt hepatic encephalopathy recurrence rate in patients taking rifaximin, compared with placebo.
Major finding: A total of 17.4% of Child-Pugh class A, 23.1% of class B, and 29.4% class C patients taking rifaximin experienced overt hepatic encephalopathy, compared with 46.4% of Child-Pugh class A, 44.4% of class B, and 64.3% of class C patients receiving placebo.
Study details: A post hoc analysis of 299 patients receiving twice-daily rifaximin or placebo for 6 months.
Disclosures: This study and its analysis were supported by Salix Pharmaceuticals. Dr. Flamm and other coauthors reported advisory committee membership, board memberships, employment, or consultancy with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Intercept Pharmaceuticals, Merck, and Salix Pharmaceuticals. One coauthor reported no relevant conflicts of interest.
Source: Flamm SL et al. ACG 2018, Presentation 58.
Screening for intimate partner violence
Also today, drug overdose deaths are down since late 2017, the risk for stroke in the elderly after an acute MI extends to 12 weeks, and high-dose flu vaccine for patients with rheumatoid arthritis beats the standard dose.
Amazon Alexa
Apple Podcasts
Spotify
Also today, drug overdose deaths are down since late 2017, the risk for stroke in the elderly after an acute MI extends to 12 weeks, and high-dose flu vaccine for patients with rheumatoid arthritis beats the standard dose.
Amazon Alexa
Apple Podcasts
Spotify
Also today, drug overdose deaths are down since late 2017, the risk for stroke in the elderly after an acute MI extends to 12 weeks, and high-dose flu vaccine for patients with rheumatoid arthritis beats the standard dose.
Amazon Alexa
Apple Podcasts
Spotify
“Unique” Challenges for Screening Native American Women
American Indian/Alaska Native (AI/AN) women face the same barriers as all low-income minority women face in accessing preventive care, but according to researchers from Rutgers University in New Jersey and University of Arizona, they also face “unique challenges and circumstances.” The researchers reviewed 18 studies to find out more about facilitators of, and barriers to, breast cancer screening.
Low-income women are more likely to be diagnosed at a later stage and to die of breast cancer, one study found. The factors are well known: cost, lack of a usual source of care, lack of insurance, distance from a facility, and lack of transportation.
However, the researchers of the meta-analysis say, “compounding these barriers,” AI/AN women expressed the belief that preventive care is not a priority, especially when it is their own preventive care. Moreover, some barriers that might be unique to the AI/AN women included concern with “manifest destiny”: the assumption that thinking or talking about breast cancer can cause it, for instance. One study examined “traditionality” and found women who could be seen as more traditional, defining themselves as living an “Indian way of life,” were less likely to be current with screening. Other women expressed mistrust in the technology of screening or spoke about perception of discrimination in the health care system.
Although this population has access to screening through IHS facilities, women who also have insurance (typically Medicaid) are more likely to get screened. Women in rural areas who lived near an IHS facility were more likely than were urban women to get mammograms. The researchers suggest this could be because rural women are more likely to be isolated from other mammogram facilities. Too, the IHS is “chronically underfunded,” the researchers note, likely a cause of the health disparities and limiting scope of services.
Their review made clear that efforts to intervene with AI/AN women to increase breast cancer screening have been limited, the researchers say. The intervention studies they reviewed “were not successful in improving screening rates or adherence.” The qualitative studies, on the other hand, suggest that women may be more responsive to locally supportive, targeted, and culturally appropriate interventions that respect traditionality yet encourage trust in the medical system.
American Indian/Alaska Native (AI/AN) women face the same barriers as all low-income minority women face in accessing preventive care, but according to researchers from Rutgers University in New Jersey and University of Arizona, they also face “unique challenges and circumstances.” The researchers reviewed 18 studies to find out more about facilitators of, and barriers to, breast cancer screening.
Low-income women are more likely to be diagnosed at a later stage and to die of breast cancer, one study found. The factors are well known: cost, lack of a usual source of care, lack of insurance, distance from a facility, and lack of transportation.
However, the researchers of the meta-analysis say, “compounding these barriers,” AI/AN women expressed the belief that preventive care is not a priority, especially when it is their own preventive care. Moreover, some barriers that might be unique to the AI/AN women included concern with “manifest destiny”: the assumption that thinking or talking about breast cancer can cause it, for instance. One study examined “traditionality” and found women who could be seen as more traditional, defining themselves as living an “Indian way of life,” were less likely to be current with screening. Other women expressed mistrust in the technology of screening or spoke about perception of discrimination in the health care system.
Although this population has access to screening through IHS facilities, women who also have insurance (typically Medicaid) are more likely to get screened. Women in rural areas who lived near an IHS facility were more likely than were urban women to get mammograms. The researchers suggest this could be because rural women are more likely to be isolated from other mammogram facilities. Too, the IHS is “chronically underfunded,” the researchers note, likely a cause of the health disparities and limiting scope of services.
Their review made clear that efforts to intervene with AI/AN women to increase breast cancer screening have been limited, the researchers say. The intervention studies they reviewed “were not successful in improving screening rates or adherence.” The qualitative studies, on the other hand, suggest that women may be more responsive to locally supportive, targeted, and culturally appropriate interventions that respect traditionality yet encourage trust in the medical system.
American Indian/Alaska Native (AI/AN) women face the same barriers as all low-income minority women face in accessing preventive care, but according to researchers from Rutgers University in New Jersey and University of Arizona, they also face “unique challenges and circumstances.” The researchers reviewed 18 studies to find out more about facilitators of, and barriers to, breast cancer screening.
Low-income women are more likely to be diagnosed at a later stage and to die of breast cancer, one study found. The factors are well known: cost, lack of a usual source of care, lack of insurance, distance from a facility, and lack of transportation.
However, the researchers of the meta-analysis say, “compounding these barriers,” AI/AN women expressed the belief that preventive care is not a priority, especially when it is their own preventive care. Moreover, some barriers that might be unique to the AI/AN women included concern with “manifest destiny”: the assumption that thinking or talking about breast cancer can cause it, for instance. One study examined “traditionality” and found women who could be seen as more traditional, defining themselves as living an “Indian way of life,” were less likely to be current with screening. Other women expressed mistrust in the technology of screening or spoke about perception of discrimination in the health care system.
Although this population has access to screening through IHS facilities, women who also have insurance (typically Medicaid) are more likely to get screened. Women in rural areas who lived near an IHS facility were more likely than were urban women to get mammograms. The researchers suggest this could be because rural women are more likely to be isolated from other mammogram facilities. Too, the IHS is “chronically underfunded,” the researchers note, likely a cause of the health disparities and limiting scope of services.
Their review made clear that efforts to intervene with AI/AN women to increase breast cancer screening have been limited, the researchers say. The intervention studies they reviewed “were not successful in improving screening rates or adherence.” The qualitative studies, on the other hand, suggest that women may be more responsive to locally supportive, targeted, and culturally appropriate interventions that respect traditionality yet encourage trust in the medical system.
Canada approves Jivi for hemophilia A
Health Canada has approved Jivi® (antihemophilic factor [recombinant, B-domain deleted, PEGylated]) for use in patients with hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate developed by Bayer.
Health Canada has approved Jivi for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in previously treated hemophilia A patients age 12 and older.
Jivi is also approved for the control and prevention of episodic bleeding and for perioperative management of bleeding.
The recommended initial dosing for Jivi as prophylaxis is twice weekly, with the ability to dose every 5 days and further adjust dosing based on bleeding episodes.
Health Canada’s approval of Jivi is based on the PROTECT VIII trial. Results from this trial are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A. In part B, Jivi was used for perioperative management of bleeding.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
Health Canada has approved Jivi® (antihemophilic factor [recombinant, B-domain deleted, PEGylated]) for use in patients with hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate developed by Bayer.
Health Canada has approved Jivi for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in previously treated hemophilia A patients age 12 and older.
Jivi is also approved for the control and prevention of episodic bleeding and for perioperative management of bleeding.
The recommended initial dosing for Jivi as prophylaxis is twice weekly, with the ability to dose every 5 days and further adjust dosing based on bleeding episodes.
Health Canada’s approval of Jivi is based on the PROTECT VIII trial. Results from this trial are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A. In part B, Jivi was used for perioperative management of bleeding.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
Health Canada has approved Jivi® (antihemophilic factor [recombinant, B-domain deleted, PEGylated]) for use in patients with hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate developed by Bayer.
Health Canada has approved Jivi for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in previously treated hemophilia A patients age 12 and older.
Jivi is also approved for the control and prevention of episodic bleeding and for perioperative management of bleeding.
The recommended initial dosing for Jivi as prophylaxis is twice weekly, with the ability to dose every 5 days and further adjust dosing based on bleeding episodes.
Health Canada’s approval of Jivi is based on the PROTECT VIII trial. Results from this trial are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A. In part B, Jivi was used for perioperative management of bleeding.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.