User login
CHEST Foundation awards grants to scholars, young investigators, and community service volunteers
Each year, the CHEST Foundation offers grants to worthy research candidates, generous community service volunteers, and distinguished scholars. More than 1,000 recipients worldwide have received more than $10 million in support and recognition of outstanding contributions to chest medicine.
In 2018, the Foundation awarded than $500,000 to researchers who were honored during Sunday’s Opening Session.
Robert C. Hyzy, MD, FCCP, director of the Critical Care Medicine Unit at the University of Michigan, was awarded the 2018 Eli Lilly and Company Distinguished Scholar in Critical Care Medicine grant for his research titled “The Use of Electrical Impedance Tomography to Assess Mechanical Ventilation in Acute Respiratory Distress Syndrome.” The grant, sponsored by Eli Lilly, will further Dr. Hyzy’s research into vetting electrical impedance tomography (EIT).
“EIT is essentially a belt that’s worn around a patient’s chest that creates an image through a low, imperceptible electronic current,” Dr. Hyzy said, noting a CAT scanner can also be used to see how air gets into the lungs, but those are not a practical tool in the ICU. “EIT creates some images, and the images change with regard to how air gets into the lungs, particularly when the patient has ARDS. So the idea here, with this generous grant, would be to build a better mousetrap—to explore this technology as a means to see various ways to push air through the lungs. It’s using the images you get to guide the way mechanical ventilation is provided.”
The Foundation’s grants have made a difference in patients’ lives by aiding young investigators. Many of the supported projects have led to breakthroughs in the treatment of chest diseases and patient care. The Foundation encourages members to apply for grants so that chest medicine will continue to improve and evolve.
Congratulations to all of our 2018 CHEST Foundation grant winners!
Watch for the list of all CHEST 2018 winners in the December issue of CHEST Physician.
Each year, the CHEST Foundation offers grants to worthy research candidates, generous community service volunteers, and distinguished scholars. More than 1,000 recipients worldwide have received more than $10 million in support and recognition of outstanding contributions to chest medicine.
In 2018, the Foundation awarded than $500,000 to researchers who were honored during Sunday’s Opening Session.
Robert C. Hyzy, MD, FCCP, director of the Critical Care Medicine Unit at the University of Michigan, was awarded the 2018 Eli Lilly and Company Distinguished Scholar in Critical Care Medicine grant for his research titled “The Use of Electrical Impedance Tomography to Assess Mechanical Ventilation in Acute Respiratory Distress Syndrome.” The grant, sponsored by Eli Lilly, will further Dr. Hyzy’s research into vetting electrical impedance tomography (EIT).
“EIT is essentially a belt that’s worn around a patient’s chest that creates an image through a low, imperceptible electronic current,” Dr. Hyzy said, noting a CAT scanner can also be used to see how air gets into the lungs, but those are not a practical tool in the ICU. “EIT creates some images, and the images change with regard to how air gets into the lungs, particularly when the patient has ARDS. So the idea here, with this generous grant, would be to build a better mousetrap—to explore this technology as a means to see various ways to push air through the lungs. It’s using the images you get to guide the way mechanical ventilation is provided.”
The Foundation’s grants have made a difference in patients’ lives by aiding young investigators. Many of the supported projects have led to breakthroughs in the treatment of chest diseases and patient care. The Foundation encourages members to apply for grants so that chest medicine will continue to improve and evolve.
Congratulations to all of our 2018 CHEST Foundation grant winners!
Watch for the list of all CHEST 2018 winners in the December issue of CHEST Physician.
Each year, the CHEST Foundation offers grants to worthy research candidates, generous community service volunteers, and distinguished scholars. More than 1,000 recipients worldwide have received more than $10 million in support and recognition of outstanding contributions to chest medicine.
In 2018, the Foundation awarded than $500,000 to researchers who were honored during Sunday’s Opening Session.
Robert C. Hyzy, MD, FCCP, director of the Critical Care Medicine Unit at the University of Michigan, was awarded the 2018 Eli Lilly and Company Distinguished Scholar in Critical Care Medicine grant for his research titled “The Use of Electrical Impedance Tomography to Assess Mechanical Ventilation in Acute Respiratory Distress Syndrome.” The grant, sponsored by Eli Lilly, will further Dr. Hyzy’s research into vetting electrical impedance tomography (EIT).
“EIT is essentially a belt that’s worn around a patient’s chest that creates an image through a low, imperceptible electronic current,” Dr. Hyzy said, noting a CAT scanner can also be used to see how air gets into the lungs, but those are not a practical tool in the ICU. “EIT creates some images, and the images change with regard to how air gets into the lungs, particularly when the patient has ARDS. So the idea here, with this generous grant, would be to build a better mousetrap—to explore this technology as a means to see various ways to push air through the lungs. It’s using the images you get to guide the way mechanical ventilation is provided.”
The Foundation’s grants have made a difference in patients’ lives by aiding young investigators. Many of the supported projects have led to breakthroughs in the treatment of chest diseases and patient care. The Foundation encourages members to apply for grants so that chest medicine will continue to improve and evolve.
Congratulations to all of our 2018 CHEST Foundation grant winners!
Watch for the list of all CHEST 2018 winners in the December issue of CHEST Physician.
A year in review with CHEST President, John Studdard, MD, FCCP
Wow, what an incredible year this has been! Serving as CHEST President from the end of CHEST 2017 until the CHEST Annual Meeting in San Antonio—in early October 2018--means I’ve served one of the shortest presidencies in CHEST history. I must say, however, that it has been a phenomenal year for me personally, highlighted not only by the accomplishments outlined below, but by the opportunity to meet so many new people and to grow existing relationships both for myself and for CHEST. I am so proud and excited by the meaningful work being done by our volunteers, staff, and leadership. Thank you for the incredibly humbling opportunity to work with you and to serve CHEST this year.
Since joining CHEST in 1982, I’ve had the opportunity to observe and learn from so many great leaders, each with different strengths and styles of leadership. I also have learned so much from members of our staff at all levels, as well as members of our leadership who serve as committee chairs, NetWork leaders, faculty representatives, and more, all giving so unselfishly of their time and talent to this organization. In addition, I was blessed this year to work with a special Board of Regents—experienced, engaged, professional in their approach, supportive, strategic, representing diversity of thought and passionate about this organization.
Throughout the 2017-2018 fiscal year, CHEST’s Board of Regents worked tirelessly to refine CHEST’s mission and vision and to develop goals, strategies, and key performance indicators to develop a new, 5-year strategic plan. Our organizational goals going forward are focused on several broad areas of achievement. To achieve these goals, we need to continue investing in and expanding our efforts in key areas like Membership, Education, and Publishing. We need to focus our attention on key groups like clinician educators, young leaders and young members, and embracing diversity of thought and meaningful inclusion, paying attention to gaps, barriers, and opportunities.
As I look to our updated CHEST mission--“To champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research”—as I look at the areas of achievement over the past year, and as I look to the strategic plan and what lies ahead, in my opinion there is no finish line, and there will always be more work to do.
Thank you to the CHEST volunteers, staff, leadership, and partners for your unwavering support of CHEST and our mission. We could not be successful without you.
First released as a Thought Leaders Blog on chestnet.org, September 30, 2018.
Wow, what an incredible year this has been! Serving as CHEST President from the end of CHEST 2017 until the CHEST Annual Meeting in San Antonio—in early October 2018--means I’ve served one of the shortest presidencies in CHEST history. I must say, however, that it has been a phenomenal year for me personally, highlighted not only by the accomplishments outlined below, but by the opportunity to meet so many new people and to grow existing relationships both for myself and for CHEST. I am so proud and excited by the meaningful work being done by our volunteers, staff, and leadership. Thank you for the incredibly humbling opportunity to work with you and to serve CHEST this year.
Since joining CHEST in 1982, I’ve had the opportunity to observe and learn from so many great leaders, each with different strengths and styles of leadership. I also have learned so much from members of our staff at all levels, as well as members of our leadership who serve as committee chairs, NetWork leaders, faculty representatives, and more, all giving so unselfishly of their time and talent to this organization. In addition, I was blessed this year to work with a special Board of Regents—experienced, engaged, professional in their approach, supportive, strategic, representing diversity of thought and passionate about this organization.
Throughout the 2017-2018 fiscal year, CHEST’s Board of Regents worked tirelessly to refine CHEST’s mission and vision and to develop goals, strategies, and key performance indicators to develop a new, 5-year strategic plan. Our organizational goals going forward are focused on several broad areas of achievement. To achieve these goals, we need to continue investing in and expanding our efforts in key areas like Membership, Education, and Publishing. We need to focus our attention on key groups like clinician educators, young leaders and young members, and embracing diversity of thought and meaningful inclusion, paying attention to gaps, barriers, and opportunities.
As I look to our updated CHEST mission--“To champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research”—as I look at the areas of achievement over the past year, and as I look to the strategic plan and what lies ahead, in my opinion there is no finish line, and there will always be more work to do.
Thank you to the CHEST volunteers, staff, leadership, and partners for your unwavering support of CHEST and our mission. We could not be successful without you.
First released as a Thought Leaders Blog on chestnet.org, September 30, 2018.
Wow, what an incredible year this has been! Serving as CHEST President from the end of CHEST 2017 until the CHEST Annual Meeting in San Antonio—in early October 2018--means I’ve served one of the shortest presidencies in CHEST history. I must say, however, that it has been a phenomenal year for me personally, highlighted not only by the accomplishments outlined below, but by the opportunity to meet so many new people and to grow existing relationships both for myself and for CHEST. I am so proud and excited by the meaningful work being done by our volunteers, staff, and leadership. Thank you for the incredibly humbling opportunity to work with you and to serve CHEST this year.
Since joining CHEST in 1982, I’ve had the opportunity to observe and learn from so many great leaders, each with different strengths and styles of leadership. I also have learned so much from members of our staff at all levels, as well as members of our leadership who serve as committee chairs, NetWork leaders, faculty representatives, and more, all giving so unselfishly of their time and talent to this organization. In addition, I was blessed this year to work with a special Board of Regents—experienced, engaged, professional in their approach, supportive, strategic, representing diversity of thought and passionate about this organization.
Throughout the 2017-2018 fiscal year, CHEST’s Board of Regents worked tirelessly to refine CHEST’s mission and vision and to develop goals, strategies, and key performance indicators to develop a new, 5-year strategic plan. Our organizational goals going forward are focused on several broad areas of achievement. To achieve these goals, we need to continue investing in and expanding our efforts in key areas like Membership, Education, and Publishing. We need to focus our attention on key groups like clinician educators, young leaders and young members, and embracing diversity of thought and meaningful inclusion, paying attention to gaps, barriers, and opportunities.
As I look to our updated CHEST mission--“To champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research”—as I look at the areas of achievement over the past year, and as I look to the strategic plan and what lies ahead, in my opinion there is no finish line, and there will always be more work to do.
Thank you to the CHEST volunteers, staff, leadership, and partners for your unwavering support of CHEST and our mission. We could not be successful without you.
First released as a Thought Leaders Blog on chestnet.org, September 30, 2018.
The link between suicide and sleep
According to the Centers for Disease Control and Prevention, suicide is the 10th leading cause of mortality in the United States, with rates of suicide rising over the past 2 decades. In 2016, completed suicides accounted for approximately 45,000 deaths in the United States (Ivey-Stephenson AZ, et al. MMWR Surveill Summ. 2017;66[18]:1). While progress has been made to lower mortality rates of other leading causes of death, very little progress has been made on reducing the rates of suicide. The term “suicide,” as referred to in this article, encompasses suicidal ideation, suicidal behavior, and suicide death.
Researchers have been investigating potential risk factors and prevention strategies for suicide. The relationship between suicide and sleep disturbances, specifically insomnia and nightmares, has been well documented in the literature. Given that insomnia and nightmares are potentially modifiable risk factors, it continues to be an area of active exploration for suicide rate reduction. While there are many different types of sleep disorders, including excessive daytime sleepiness, parasomnias, obstructive sleep apnea, and restless legs syndrome, this article will focus on the relationship between insomnia and nightmares with suicide.
Insomnia
Insomnia disorder, according to the American Psychiatric Association’s DSM-5, is a dissatisfaction of sleep quantity or quality that occurs at least three nights per week for a minimum of 3 months despite adequate opportunity for sleep. This may present as difficulty with falling asleep, staying asleep, or early morning awakenings. The sleep disturbance results in functional impairment or significant distress in at least one area of life (American Psychiatric Association. Arlington, Virginia: APA; 2013). While insomnia is often a symptom of many psychiatric disorders, research has shown that insomnia is an independent risk factor for suicide, even when controlling for mental illness. Studies have shown that there is up to a 2.4 relative risk of suicide death with insomnia after adjusting for depression severity (McCall W, et al. J Clin Sleep Med. 2013;32[9]:135).
Nightmares
Nightmares, as defined by the American Psychiatric Association’s DSM-5, are “typically lengthy, elaborate, story-like sequences of dream imagery that seem real and incite anxiety, fear, or other dysphoric emotions” (American Psychiatric Association. Arlington, Virginia: APA; 2013). They are common symptoms in posttraumatic stress disorder (PTSD), with up to 90% of individuals with PTSD experiencing nightmares following a traumatic event (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393). Nightmares have also been shown to be an independent risk factor for suicide when controlling for mental illness. Studies have shown that nightmares are associated with an elevated risk factor of 1.5 to 3 times for suicidal ideation and 3 to 4 times for suicide attempts. The data suggest that nightmares may be a stronger risk factor for suicide than insomnia (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Proposed Mechanism
The mechanism linking insomnia and nightmares with suicide has been theorized and studied by researchers. A couple of the most noteworthy proposed psychological mechanisms involve dysfunctional beliefs and attitudes about sleep, as well as deficits in problem solving capability. Dysfunctional beliefs and attitudes about sleep (DBAS) are negative cognitions pertaining to sleep, and they have been shown to be related to the intensity of suicidal ideations. Many of the DBAS are pessimistic thoughts that contain a “hopelessness flavor” to them, which lead to the perpetuation of insomnia. Hopelessness has been found to be a strong risk factor for suicide. In addition to DBAS, insomnia has also shown to lead to impairments in complex problem solving. The lack of problem solving skills in these patients may lead to fewer quantity and quality of solutions during stressful situations and leave suicide as the perceived best or only option.
The biological theories focus on serotonin and hyperarousal mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Serotonin is a neurotransmitter that is involved in the induction and maintenance of sleep. Of interesting note, low levels of serotonin’s main metabolite, 5-hydroxyindoleacetic acid (5-HIAA) have been found in the cerebrospinal fluid of suicide victims. Evidence has also shown that sleep and the HPA axis are closely related. The HPA axis is activated by stress leading to a cascade of hormones that can cause susceptibility of hyperarousal, REM alterations, and suicide. Hyperarousal, shared in context with PTSD and insomnia, can lead to hyperactivation of the noradrenergic systems in the medial prefrontal cortex, which can lead to decrease in executive decision making (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Treatment Strategies
The benefit of treating insomnia and nightmares, in regards to reducing suicidality, continues to be an area of active research. Many of the previous studies have theorized that treating symptoms of insomnia and nightmares may indirectly reduce suicide. Pharmaceutical and nonpharmaceutical treatments are currently being used to help treat patients with insomnia and nightmares, but the benefit for reducing suicidality is still unknown.
One of the main treatment modalities for insomnia is hypnotic medication; however, these medications carry their own potential risk for suicide. Reports of suicide death in conjunction with hypnotic medication has led the FDA to add warnings about the increased risk of suicide with these medications. Some of these medications include zolpidem, zaleplon, eszopiclone, doxepin, ramelteon, and suvorexant. A review of research studies and case reports was completed in 2017 and showed that there was an odds ratio of 2 to 3 for hypnotic use in suicide deaths. However, most of the studies that were reviewed reported a potential confounding bias of the individual’s current mental health state. Furthermore, many of the suicide case reports that involved hypnotics also had additional substances detected, such as alcohol. Hypnotic medication has been shown to be an effective treatment for insomnia, but caution needs to be used when prescribing these medications. Strategies that may be beneficial when using hypnotic medication to reduce the risk of an adverse outcome include using the lowest effective dose and educating the patient of not combining the medication with alcohol or other sedative/hypnotics (McCall W, et al. Am J Psychiatry. 2017;174[1]:18).
For patients who have recurrent nightmares in the context of PTSD, the alpha-1 adrenergic receptor antagonist, prazosin, may provide some benefit; however, the literature is divided. There have been several randomized, placebo-controlled clinical trials with prazosin, which has shown a moderate to large effect for alleviating trauma-related nightmares and improving sleep quality. Some of the limitations of these studies were that the trials were small to moderate in size, and the length of the trials was 15 weeks or less. In 2018, Raskin and colleagues completed a follow-up randomized, placebo-controlled study for 26 weeks with 304 participants and did not find a significant difference between prazosin and placebo in regards to nightmares and sleep quality (Raskind MA, et al. N Engl J Med. 2018;378[6]:507).
Cognitive behavioral therapy for insomnia (CBT-I) and image rehearsal therapy (IRT) are two sleep-targeted therapy modalities that are evidence based. CBT-I targets dysfunctional beliefs and attitudes regarding sleep (McCall W, et al. J Clin Sleep Med. 2013;9[2]:135). IRT, on the other hand, specifically targets nightmares by having the patient write out a narrative of the nightmare, followed by re-scripting an alternative ending to something that is less distressing. The patient will rehearse the new dream narrative before going to sleep. There is still insufficient evidence to determine if these therapies have benefit in reducing suicide (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393).
While the jury is still out on how best to target and treat the risk factors of insomnia and nightmares in regards to suicide, there are still steps that health-care providers can take to help keep their patients safe. During the patient interview, new or worsening insomnia and nightmares should prompt further investigation of suicidal thoughts and behaviors. After a thorough interview, treatment options, with a discussion of risks and benefits, can be tailored to the individual’s needs. Managing insomnia and nightmares may be one avenue of suicide prevention.
Drs. Locrotondo and McCall are with the Department of Psychiatry and Health Behavior at the Medical College of Georgia, Augusta University, Augusta, Georgia.
According to the Centers for Disease Control and Prevention, suicide is the 10th leading cause of mortality in the United States, with rates of suicide rising over the past 2 decades. In 2016, completed suicides accounted for approximately 45,000 deaths in the United States (Ivey-Stephenson AZ, et al. MMWR Surveill Summ. 2017;66[18]:1). While progress has been made to lower mortality rates of other leading causes of death, very little progress has been made on reducing the rates of suicide. The term “suicide,” as referred to in this article, encompasses suicidal ideation, suicidal behavior, and suicide death.
Researchers have been investigating potential risk factors and prevention strategies for suicide. The relationship between suicide and sleep disturbances, specifically insomnia and nightmares, has been well documented in the literature. Given that insomnia and nightmares are potentially modifiable risk factors, it continues to be an area of active exploration for suicide rate reduction. While there are many different types of sleep disorders, including excessive daytime sleepiness, parasomnias, obstructive sleep apnea, and restless legs syndrome, this article will focus on the relationship between insomnia and nightmares with suicide.
Insomnia
Insomnia disorder, according to the American Psychiatric Association’s DSM-5, is a dissatisfaction of sleep quantity or quality that occurs at least three nights per week for a minimum of 3 months despite adequate opportunity for sleep. This may present as difficulty with falling asleep, staying asleep, or early morning awakenings. The sleep disturbance results in functional impairment or significant distress in at least one area of life (American Psychiatric Association. Arlington, Virginia: APA; 2013). While insomnia is often a symptom of many psychiatric disorders, research has shown that insomnia is an independent risk factor for suicide, even when controlling for mental illness. Studies have shown that there is up to a 2.4 relative risk of suicide death with insomnia after adjusting for depression severity (McCall W, et al. J Clin Sleep Med. 2013;32[9]:135).
Nightmares
Nightmares, as defined by the American Psychiatric Association’s DSM-5, are “typically lengthy, elaborate, story-like sequences of dream imagery that seem real and incite anxiety, fear, or other dysphoric emotions” (American Psychiatric Association. Arlington, Virginia: APA; 2013). They are common symptoms in posttraumatic stress disorder (PTSD), with up to 90% of individuals with PTSD experiencing nightmares following a traumatic event (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393). Nightmares have also been shown to be an independent risk factor for suicide when controlling for mental illness. Studies have shown that nightmares are associated with an elevated risk factor of 1.5 to 3 times for suicidal ideation and 3 to 4 times for suicide attempts. The data suggest that nightmares may be a stronger risk factor for suicide than insomnia (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Proposed Mechanism
The mechanism linking insomnia and nightmares with suicide has been theorized and studied by researchers. A couple of the most noteworthy proposed psychological mechanisms involve dysfunctional beliefs and attitudes about sleep, as well as deficits in problem solving capability. Dysfunctional beliefs and attitudes about sleep (DBAS) are negative cognitions pertaining to sleep, and they have been shown to be related to the intensity of suicidal ideations. Many of the DBAS are pessimistic thoughts that contain a “hopelessness flavor” to them, which lead to the perpetuation of insomnia. Hopelessness has been found to be a strong risk factor for suicide. In addition to DBAS, insomnia has also shown to lead to impairments in complex problem solving. The lack of problem solving skills in these patients may lead to fewer quantity and quality of solutions during stressful situations and leave suicide as the perceived best or only option.
The biological theories focus on serotonin and hyperarousal mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Serotonin is a neurotransmitter that is involved in the induction and maintenance of sleep. Of interesting note, low levels of serotonin’s main metabolite, 5-hydroxyindoleacetic acid (5-HIAA) have been found in the cerebrospinal fluid of suicide victims. Evidence has also shown that sleep and the HPA axis are closely related. The HPA axis is activated by stress leading to a cascade of hormones that can cause susceptibility of hyperarousal, REM alterations, and suicide. Hyperarousal, shared in context with PTSD and insomnia, can lead to hyperactivation of the noradrenergic systems in the medial prefrontal cortex, which can lead to decrease in executive decision making (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Treatment Strategies
The benefit of treating insomnia and nightmares, in regards to reducing suicidality, continues to be an area of active research. Many of the previous studies have theorized that treating symptoms of insomnia and nightmares may indirectly reduce suicide. Pharmaceutical and nonpharmaceutical treatments are currently being used to help treat patients with insomnia and nightmares, but the benefit for reducing suicidality is still unknown.
One of the main treatment modalities for insomnia is hypnotic medication; however, these medications carry their own potential risk for suicide. Reports of suicide death in conjunction with hypnotic medication has led the FDA to add warnings about the increased risk of suicide with these medications. Some of these medications include zolpidem, zaleplon, eszopiclone, doxepin, ramelteon, and suvorexant. A review of research studies and case reports was completed in 2017 and showed that there was an odds ratio of 2 to 3 for hypnotic use in suicide deaths. However, most of the studies that were reviewed reported a potential confounding bias of the individual’s current mental health state. Furthermore, many of the suicide case reports that involved hypnotics also had additional substances detected, such as alcohol. Hypnotic medication has been shown to be an effective treatment for insomnia, but caution needs to be used when prescribing these medications. Strategies that may be beneficial when using hypnotic medication to reduce the risk of an adverse outcome include using the lowest effective dose and educating the patient of not combining the medication with alcohol or other sedative/hypnotics (McCall W, et al. Am J Psychiatry. 2017;174[1]:18).
For patients who have recurrent nightmares in the context of PTSD, the alpha-1 adrenergic receptor antagonist, prazosin, may provide some benefit; however, the literature is divided. There have been several randomized, placebo-controlled clinical trials with prazosin, which has shown a moderate to large effect for alleviating trauma-related nightmares and improving sleep quality. Some of the limitations of these studies were that the trials were small to moderate in size, and the length of the trials was 15 weeks or less. In 2018, Raskin and colleagues completed a follow-up randomized, placebo-controlled study for 26 weeks with 304 participants and did not find a significant difference between prazosin and placebo in regards to nightmares and sleep quality (Raskind MA, et al. N Engl J Med. 2018;378[6]:507).
Cognitive behavioral therapy for insomnia (CBT-I) and image rehearsal therapy (IRT) are two sleep-targeted therapy modalities that are evidence based. CBT-I targets dysfunctional beliefs and attitudes regarding sleep (McCall W, et al. J Clin Sleep Med. 2013;9[2]:135). IRT, on the other hand, specifically targets nightmares by having the patient write out a narrative of the nightmare, followed by re-scripting an alternative ending to something that is less distressing. The patient will rehearse the new dream narrative before going to sleep. There is still insufficient evidence to determine if these therapies have benefit in reducing suicide (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393).
While the jury is still out on how best to target and treat the risk factors of insomnia and nightmares in regards to suicide, there are still steps that health-care providers can take to help keep their patients safe. During the patient interview, new or worsening insomnia and nightmares should prompt further investigation of suicidal thoughts and behaviors. After a thorough interview, treatment options, with a discussion of risks and benefits, can be tailored to the individual’s needs. Managing insomnia and nightmares may be one avenue of suicide prevention.
Drs. Locrotondo and McCall are with the Department of Psychiatry and Health Behavior at the Medical College of Georgia, Augusta University, Augusta, Georgia.
According to the Centers for Disease Control and Prevention, suicide is the 10th leading cause of mortality in the United States, with rates of suicide rising over the past 2 decades. In 2016, completed suicides accounted for approximately 45,000 deaths in the United States (Ivey-Stephenson AZ, et al. MMWR Surveill Summ. 2017;66[18]:1). While progress has been made to lower mortality rates of other leading causes of death, very little progress has been made on reducing the rates of suicide. The term “suicide,” as referred to in this article, encompasses suicidal ideation, suicidal behavior, and suicide death.
Researchers have been investigating potential risk factors and prevention strategies for suicide. The relationship between suicide and sleep disturbances, specifically insomnia and nightmares, has been well documented in the literature. Given that insomnia and nightmares are potentially modifiable risk factors, it continues to be an area of active exploration for suicide rate reduction. While there are many different types of sleep disorders, including excessive daytime sleepiness, parasomnias, obstructive sleep apnea, and restless legs syndrome, this article will focus on the relationship between insomnia and nightmares with suicide.
Insomnia
Insomnia disorder, according to the American Psychiatric Association’s DSM-5, is a dissatisfaction of sleep quantity or quality that occurs at least three nights per week for a minimum of 3 months despite adequate opportunity for sleep. This may present as difficulty with falling asleep, staying asleep, or early morning awakenings. The sleep disturbance results in functional impairment or significant distress in at least one area of life (American Psychiatric Association. Arlington, Virginia: APA; 2013). While insomnia is often a symptom of many psychiatric disorders, research has shown that insomnia is an independent risk factor for suicide, even when controlling for mental illness. Studies have shown that there is up to a 2.4 relative risk of suicide death with insomnia after adjusting for depression severity (McCall W, et al. J Clin Sleep Med. 2013;32[9]:135).
Nightmares
Nightmares, as defined by the American Psychiatric Association’s DSM-5, are “typically lengthy, elaborate, story-like sequences of dream imagery that seem real and incite anxiety, fear, or other dysphoric emotions” (American Psychiatric Association. Arlington, Virginia: APA; 2013). They are common symptoms in posttraumatic stress disorder (PTSD), with up to 90% of individuals with PTSD experiencing nightmares following a traumatic event (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393). Nightmares have also been shown to be an independent risk factor for suicide when controlling for mental illness. Studies have shown that nightmares are associated with an elevated risk factor of 1.5 to 3 times for suicidal ideation and 3 to 4 times for suicide attempts. The data suggest that nightmares may be a stronger risk factor for suicide than insomnia (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Proposed Mechanism
The mechanism linking insomnia and nightmares with suicide has been theorized and studied by researchers. A couple of the most noteworthy proposed psychological mechanisms involve dysfunctional beliefs and attitudes about sleep, as well as deficits in problem solving capability. Dysfunctional beliefs and attitudes about sleep (DBAS) are negative cognitions pertaining to sleep, and they have been shown to be related to the intensity of suicidal ideations. Many of the DBAS are pessimistic thoughts that contain a “hopelessness flavor” to them, which lead to the perpetuation of insomnia. Hopelessness has been found to be a strong risk factor for suicide. In addition to DBAS, insomnia has also shown to lead to impairments in complex problem solving. The lack of problem solving skills in these patients may lead to fewer quantity and quality of solutions during stressful situations and leave suicide as the perceived best or only option.
The biological theories focus on serotonin and hyperarousal mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Serotonin is a neurotransmitter that is involved in the induction and maintenance of sleep. Of interesting note, low levels of serotonin’s main metabolite, 5-hydroxyindoleacetic acid (5-HIAA) have been found in the cerebrospinal fluid of suicide victims. Evidence has also shown that sleep and the HPA axis are closely related. The HPA axis is activated by stress leading to a cascade of hormones that can cause susceptibility of hyperarousal, REM alterations, and suicide. Hyperarousal, shared in context with PTSD and insomnia, can lead to hyperactivation of the noradrenergic systems in the medial prefrontal cortex, which can lead to decrease in executive decision making (McCall W, et al. Curr Psychiatr Rep. 2013;15[9]:389).
Treatment Strategies
The benefit of treating insomnia and nightmares, in regards to reducing suicidality, continues to be an area of active research. Many of the previous studies have theorized that treating symptoms of insomnia and nightmares may indirectly reduce suicide. Pharmaceutical and nonpharmaceutical treatments are currently being used to help treat patients with insomnia and nightmares, but the benefit for reducing suicidality is still unknown.
One of the main treatment modalities for insomnia is hypnotic medication; however, these medications carry their own potential risk for suicide. Reports of suicide death in conjunction with hypnotic medication has led the FDA to add warnings about the increased risk of suicide with these medications. Some of these medications include zolpidem, zaleplon, eszopiclone, doxepin, ramelteon, and suvorexant. A review of research studies and case reports was completed in 2017 and showed that there was an odds ratio of 2 to 3 for hypnotic use in suicide deaths. However, most of the studies that were reviewed reported a potential confounding bias of the individual’s current mental health state. Furthermore, many of the suicide case reports that involved hypnotics also had additional substances detected, such as alcohol. Hypnotic medication has been shown to be an effective treatment for insomnia, but caution needs to be used when prescribing these medications. Strategies that may be beneficial when using hypnotic medication to reduce the risk of an adverse outcome include using the lowest effective dose and educating the patient of not combining the medication with alcohol or other sedative/hypnotics (McCall W, et al. Am J Psychiatry. 2017;174[1]:18).
For patients who have recurrent nightmares in the context of PTSD, the alpha-1 adrenergic receptor antagonist, prazosin, may provide some benefit; however, the literature is divided. There have been several randomized, placebo-controlled clinical trials with prazosin, which has shown a moderate to large effect for alleviating trauma-related nightmares and improving sleep quality. Some of the limitations of these studies were that the trials were small to moderate in size, and the length of the trials was 15 weeks or less. In 2018, Raskin and colleagues completed a follow-up randomized, placebo-controlled study for 26 weeks with 304 participants and did not find a significant difference between prazosin and placebo in regards to nightmares and sleep quality (Raskind MA, et al. N Engl J Med. 2018;378[6]:507).
Cognitive behavioral therapy for insomnia (CBT-I) and image rehearsal therapy (IRT) are two sleep-targeted therapy modalities that are evidence based. CBT-I targets dysfunctional beliefs and attitudes regarding sleep (McCall W, et al. J Clin Sleep Med. 2013;9[2]:135). IRT, on the other hand, specifically targets nightmares by having the patient write out a narrative of the nightmare, followed by re-scripting an alternative ending to something that is less distressing. The patient will rehearse the new dream narrative before going to sleep. There is still insufficient evidence to determine if these therapies have benefit in reducing suicide (Littlewood DL, et al. J Clin Sleep Med. 2016;12[3]:393).
While the jury is still out on how best to target and treat the risk factors of insomnia and nightmares in regards to suicide, there are still steps that health-care providers can take to help keep their patients safe. During the patient interview, new or worsening insomnia and nightmares should prompt further investigation of suicidal thoughts and behaviors. After a thorough interview, treatment options, with a discussion of risks and benefits, can be tailored to the individual’s needs. Managing insomnia and nightmares may be one avenue of suicide prevention.
Drs. Locrotondo and McCall are with the Department of Psychiatry and Health Behavior at the Medical College of Georgia, Augusta University, Augusta, Georgia.
Trump rule and ACA contraception
Also today, physical activity is tied to lower depression risk among older adults, high rates of prescription benzodiazepine observed in chronic liver disease, and rates of sexually transmitted infections are rising among U.S. teens who are sexually active.
Amazon Alexa
Apple Podcasts
Spotify
Also today, physical activity is tied to lower depression risk among older adults, high rates of prescription benzodiazepine observed in chronic liver disease, and rates of sexually transmitted infections are rising among U.S. teens who are sexually active.
Amazon Alexa
Apple Podcasts
Spotify
Also today, physical activity is tied to lower depression risk among older adults, high rates of prescription benzodiazepine observed in chronic liver disease, and rates of sexually transmitted infections are rising among U.S. teens who are sexually active.
Amazon Alexa
Apple Podcasts
Spotify
Can Probiotics Beat MRSA?
An unexpected finding in a study by National Institute of Health (NIH) researchers suggests that a “good” bacterium commonly found in probiotic digestive supplements works against Staphylococcus aureus (S aureus).
The researchers recruited 200 volunteers in Thailand for the study. They speculated that Thais would not be as affected by food sterilization or antibiotics as people in highly developed urban areas. The researchers analyzed fecal samples from each participant for bacteria correlated with the absence of S aureus. They found 101 samples positive for bacillus, primarily Bacillus subtilis (B subtilis), which is often mixed with other bacteria in probiotic products. The researchers then sampled for S aureus and found 25 positive gut samples and 26 positive nose samples. Strikingly, the researchers say, they found no S aureus in any of the samples that contained bacillus.
Using chromatography and mass spectrometry, the study team identified fengycins—a class of lipopeptides—as the specific bacillus substance that inhibited the S aureus sensing system. Other tests showed that fengycins had the same effect on several different strains of S aureus, including high-risk USA300 methicillin-resistant S aureus (MRSA).
To further validate their findings, the researchers colonized the gut of mice with S aureus and fed them B subtilis spores. Probiotic bacillus given every 2 days eliminated S aureus. The same test using bacillus where fengycin production had been removed had no effect: S aureus grew as expected.
An unexpected finding in a study by National Institute of Health (NIH) researchers suggests that a “good” bacterium commonly found in probiotic digestive supplements works against Staphylococcus aureus (S aureus).
The researchers recruited 200 volunteers in Thailand for the study. They speculated that Thais would not be as affected by food sterilization or antibiotics as people in highly developed urban areas. The researchers analyzed fecal samples from each participant for bacteria correlated with the absence of S aureus. They found 101 samples positive for bacillus, primarily Bacillus subtilis (B subtilis), which is often mixed with other bacteria in probiotic products. The researchers then sampled for S aureus and found 25 positive gut samples and 26 positive nose samples. Strikingly, the researchers say, they found no S aureus in any of the samples that contained bacillus.
Using chromatography and mass spectrometry, the study team identified fengycins—a class of lipopeptides—as the specific bacillus substance that inhibited the S aureus sensing system. Other tests showed that fengycins had the same effect on several different strains of S aureus, including high-risk USA300 methicillin-resistant S aureus (MRSA).
To further validate their findings, the researchers colonized the gut of mice with S aureus and fed them B subtilis spores. Probiotic bacillus given every 2 days eliminated S aureus. The same test using bacillus where fengycin production had been removed had no effect: S aureus grew as expected.
An unexpected finding in a study by National Institute of Health (NIH) researchers suggests that a “good” bacterium commonly found in probiotic digestive supplements works against Staphylococcus aureus (S aureus).
The researchers recruited 200 volunteers in Thailand for the study. They speculated that Thais would not be as affected by food sterilization or antibiotics as people in highly developed urban areas. The researchers analyzed fecal samples from each participant for bacteria correlated with the absence of S aureus. They found 101 samples positive for bacillus, primarily Bacillus subtilis (B subtilis), which is often mixed with other bacteria in probiotic products. The researchers then sampled for S aureus and found 25 positive gut samples and 26 positive nose samples. Strikingly, the researchers say, they found no S aureus in any of the samples that contained bacillus.
Using chromatography and mass spectrometry, the study team identified fengycins—a class of lipopeptides—as the specific bacillus substance that inhibited the S aureus sensing system. Other tests showed that fengycins had the same effect on several different strains of S aureus, including high-risk USA300 methicillin-resistant S aureus (MRSA).
To further validate their findings, the researchers colonized the gut of mice with S aureus and fed them B subtilis spores. Probiotic bacillus given every 2 days eliminated S aureus. The same test using bacillus where fengycin production had been removed had no effect: S aureus grew as expected.
This month in the journal CHEST®
Editor’s picks
ORIGINAL RESEARCH
Effectiveness of Reprocessing for Flexible Bronchoscopes and Endobronchial Ultrasound Bronchoscopes.
By Dr. C. L. Ofstead, et al.
Lower Glucose Target Is Associated With Improved 30-Day Mortality in Cardiac and Cardiothoracic Patients.
By Dr. A. M. Hersh, et al.
Sarcoidosis Diagnostic Score: A Systematic Evaluation to Enhance the Diagnosis of Sarcoidosis.
By Dr. A. N. Bickett, et al.
EVIDENCE-BASED MEDICINE
Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report.
By Dr. G. Y. H. Lip, et al.
Editor’s picks
ORIGINAL RESEARCH
Effectiveness of Reprocessing for Flexible Bronchoscopes and Endobronchial Ultrasound Bronchoscopes.
By Dr. C. L. Ofstead, et al.
Lower Glucose Target Is Associated With Improved 30-Day Mortality in Cardiac and Cardiothoracic Patients.
By Dr. A. M. Hersh, et al.
Sarcoidosis Diagnostic Score: A Systematic Evaluation to Enhance the Diagnosis of Sarcoidosis.
By Dr. A. N. Bickett, et al.
EVIDENCE-BASED MEDICINE
Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report.
By Dr. G. Y. H. Lip, et al.
Editor’s picks
ORIGINAL RESEARCH
Effectiveness of Reprocessing for Flexible Bronchoscopes and Endobronchial Ultrasound Bronchoscopes.
By Dr. C. L. Ofstead, et al.
Lower Glucose Target Is Associated With Improved 30-Day Mortality in Cardiac and Cardiothoracic Patients.
By Dr. A. M. Hersh, et al.
Sarcoidosis Diagnostic Score: A Systematic Evaluation to Enhance the Diagnosis of Sarcoidosis.
By Dr. A. N. Bickett, et al.
EVIDENCE-BASED MEDICINE
Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report.
By Dr. G. Y. H. Lip, et al.
Financial burden of blood cancers in the U.S.
An analysis of more than 2,000 U.S. patients with blood cancers revealed an average healthcare cost of almost $157,000 in the first year after diagnosis.
Costs were highest for acute leukemia patients—almost triple the average for all blood cancers.
Out-of-pocket (OOP) costs were initially highest for acute leukemia patients. However, over time, OOP costs became highest for patients with multiple myeloma.
These results are included in a report commissioned by the Leukemia & Lymphoma Society and prepared by the actuarial firm Milliman.
The report is based on data from the Truven Health MarketScan commercial claims databases.
The cost figures are drawn from data for 2,332 patients, ages 18 to 64, who were diagnosed with blood cancer in 2014 and followed through 2016. This includes the following:
- 1,468 patients with lymphoma
- 286 with chronic leukemia
- 282 with multiple myeloma
- 148 with acute leukemia
- 148 with bone marrow disorders (myelodysplastic syndromes).
The average allowed spending—the amount paid by the payer and patient combined—in the first 12 months after diagnosis was:
- $156,845 overall
- $463,414 for acute leukemia
- $213,879 for multiple myeloma
- $133,744 for bone marrow disorders
- $130,545 for lymphoma
- $88,913 for chronic leukemia.
Differences in OOP costs were smaller, although OOP spending was 32% higher for acute leukemia patients than the overall average.
Average OOP costs—which include coinsurance, copay, and deductible—in the first 12 months after diagnosis were:
- $3,877 overall
- $5,147 for acute leukemia
- $4,849 for multiple myeloma
- $3,695 for lymphoma
- $3,480 for chronic leukemia
- $3,336 for bone marrow disorders.
Although OOP costs were initially highest for acute leukemia patients, over time, costs for multiple myeloma patients became the highest.
The average OOP costs in the month of diagnosis were $1,637 for acute leukemia patients and $1,210 for multiple myeloma patients.
The total accumulated OOP costs 3 years after diagnosis were $8,797 for acute leukemia and $9,127 for multiple myeloma. For the other blood cancers, the average 3-year accumulated OOP costs were under $7,800.
The Leukemia & Lymphoma Society received support from Pfizer, Genentech, and Amgen for this work.
An analysis of more than 2,000 U.S. patients with blood cancers revealed an average healthcare cost of almost $157,000 in the first year after diagnosis.
Costs were highest for acute leukemia patients—almost triple the average for all blood cancers.
Out-of-pocket (OOP) costs were initially highest for acute leukemia patients. However, over time, OOP costs became highest for patients with multiple myeloma.
These results are included in a report commissioned by the Leukemia & Lymphoma Society and prepared by the actuarial firm Milliman.
The report is based on data from the Truven Health MarketScan commercial claims databases.
The cost figures are drawn from data for 2,332 patients, ages 18 to 64, who were diagnosed with blood cancer in 2014 and followed through 2016. This includes the following:
- 1,468 patients with lymphoma
- 286 with chronic leukemia
- 282 with multiple myeloma
- 148 with acute leukemia
- 148 with bone marrow disorders (myelodysplastic syndromes).
The average allowed spending—the amount paid by the payer and patient combined—in the first 12 months after diagnosis was:
- $156,845 overall
- $463,414 for acute leukemia
- $213,879 for multiple myeloma
- $133,744 for bone marrow disorders
- $130,545 for lymphoma
- $88,913 for chronic leukemia.
Differences in OOP costs were smaller, although OOP spending was 32% higher for acute leukemia patients than the overall average.
Average OOP costs—which include coinsurance, copay, and deductible—in the first 12 months after diagnosis were:
- $3,877 overall
- $5,147 for acute leukemia
- $4,849 for multiple myeloma
- $3,695 for lymphoma
- $3,480 for chronic leukemia
- $3,336 for bone marrow disorders.
Although OOP costs were initially highest for acute leukemia patients, over time, costs for multiple myeloma patients became the highest.
The average OOP costs in the month of diagnosis were $1,637 for acute leukemia patients and $1,210 for multiple myeloma patients.
The total accumulated OOP costs 3 years after diagnosis were $8,797 for acute leukemia and $9,127 for multiple myeloma. For the other blood cancers, the average 3-year accumulated OOP costs were under $7,800.
The Leukemia & Lymphoma Society received support from Pfizer, Genentech, and Amgen for this work.
An analysis of more than 2,000 U.S. patients with blood cancers revealed an average healthcare cost of almost $157,000 in the first year after diagnosis.
Costs were highest for acute leukemia patients—almost triple the average for all blood cancers.
Out-of-pocket (OOP) costs were initially highest for acute leukemia patients. However, over time, OOP costs became highest for patients with multiple myeloma.
These results are included in a report commissioned by the Leukemia & Lymphoma Society and prepared by the actuarial firm Milliman.
The report is based on data from the Truven Health MarketScan commercial claims databases.
The cost figures are drawn from data for 2,332 patients, ages 18 to 64, who were diagnosed with blood cancer in 2014 and followed through 2016. This includes the following:
- 1,468 patients with lymphoma
- 286 with chronic leukemia
- 282 with multiple myeloma
- 148 with acute leukemia
- 148 with bone marrow disorders (myelodysplastic syndromes).
The average allowed spending—the amount paid by the payer and patient combined—in the first 12 months after diagnosis was:
- $156,845 overall
- $463,414 for acute leukemia
- $213,879 for multiple myeloma
- $133,744 for bone marrow disorders
- $130,545 for lymphoma
- $88,913 for chronic leukemia.
Differences in OOP costs were smaller, although OOP spending was 32% higher for acute leukemia patients than the overall average.
Average OOP costs—which include coinsurance, copay, and deductible—in the first 12 months after diagnosis were:
- $3,877 overall
- $5,147 for acute leukemia
- $4,849 for multiple myeloma
- $3,695 for lymphoma
- $3,480 for chronic leukemia
- $3,336 for bone marrow disorders.
Although OOP costs were initially highest for acute leukemia patients, over time, costs for multiple myeloma patients became the highest.
The average OOP costs in the month of diagnosis were $1,637 for acute leukemia patients and $1,210 for multiple myeloma patients.
The total accumulated OOP costs 3 years after diagnosis were $8,797 for acute leukemia and $9,127 for multiple myeloma. For the other blood cancers, the average 3-year accumulated OOP costs were under $7,800.
The Leukemia & Lymphoma Society received support from Pfizer, Genentech, and Amgen for this work.
Nearly half of infants don’t sleep through the night at 1 year
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
FROM PEDIATRICS
Key clinical point: Sleeping through the night in infancy was not significantly associated with any variations in development or maternal mood.
Major finding: There were no associations between 6 or 8 hours of uninterrupted sleep and concurrent mental or psychomotor development or reported depressive symptoms.
Study details: An analysis of 388 mother-infant dyads in a longitudinal birth cohort study.
Disclosures: Authors said they had no financial relationships or potential conflicts of interest to disclose.
Source: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
The Unintended Off-road Experience
ANSWER
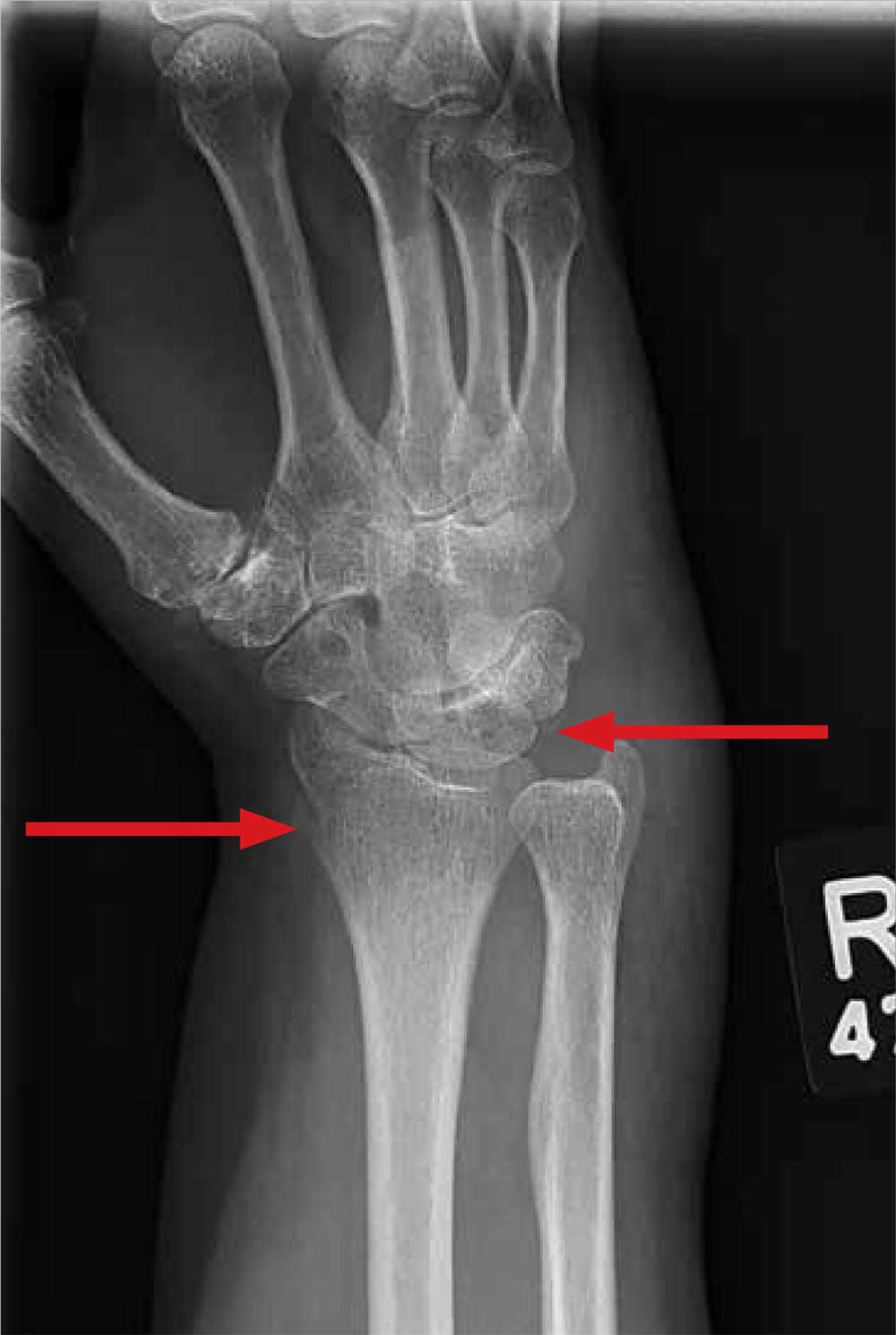
A moderate amount of soft-tissue swelling is evident. There is also a fracture of the distal radius and an avulsion fracture of the triquetrum. The latter is the second most commonly fractured carpal bone after the scaphoid. Triquetral fractures usually result from a hyperflexion injury.
The patient’s wrist was immobilized in a splint, and referral to a hand surgeon was coordinated.

ANSWER
A moderate amount of soft-tissue swelling is evident. There is also a fracture of the distal radius and an avulsion fracture of the triquetrum. The latter is the second most commonly fractured carpal bone after the scaphoid. Triquetral fractures usually result from a hyperflexion injury.
The patient’s wrist was immobilized in a splint, and referral to a hand surgeon was coordinated.

ANSWER
A moderate amount of soft-tissue swelling is evident. There is also a fracture of the distal radius and an avulsion fracture of the triquetrum. The latter is the second most commonly fractured carpal bone after the scaphoid. Triquetral fractures usually result from a hyperflexion injury.
The patient’s wrist was immobilized in a splint, and referral to a hand surgeon was coordinated.
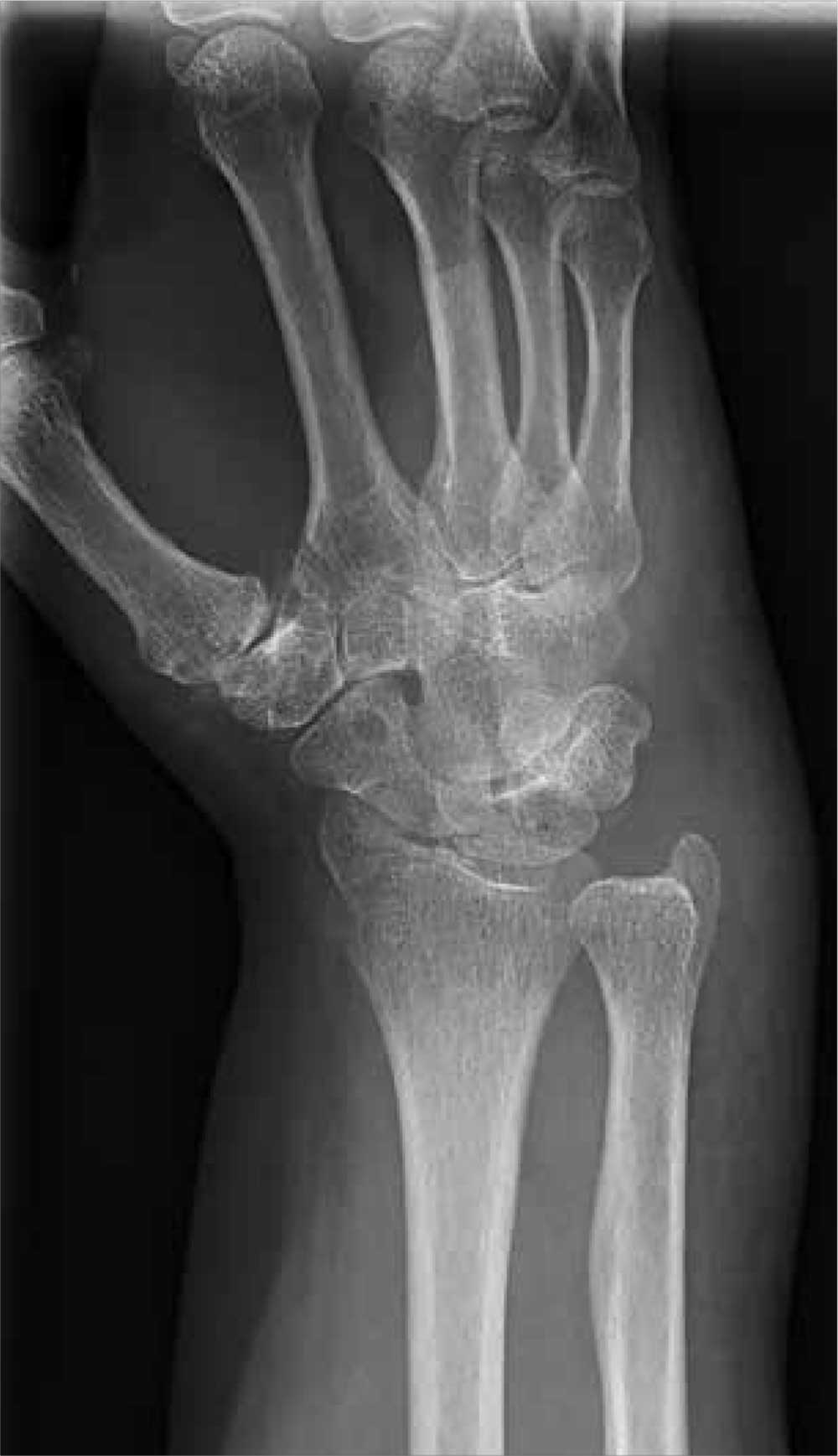
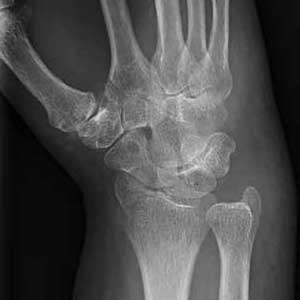
A 70-year-old woman is brought to the emergency department by EMS after being involved in a motor vehicle collision. She was a restrained driver in a vehicle that was hit by a tractor-trailer, causing her vehicle to go off the road and hit the guardrail. She does not recall if the airbag deployed, and she believes she momentarily lost consciousness. She is complaining of pain in the head and the right wrist.
Her medical history is significant for mild (controlled) hypertension. Primary survey reveals an elderly female who is awake, alert, and oriented and in mild distress. Her vital signs are stable.
The patient has a small laceration on her face. Her right wrist is moderately swollen, and she has decreased range of motion. There is also moderate tenderness to palpation. Pulses are good, as is capillary refill time in the fingernail bed.
Radiograph of the right wrist is obtained (oblique view shown). What is your impression?
PIONEER-HF secures place for sacubitril/valsartan in this heart failure doc’s practice
CHICAGO – Dr. Larry A. Allen will now have an easier time of treating hospitalized patients with acute decompensated heart failure because of the results of the PIONEER-HF trial.
That study examined whether in-hospital initiation of sacubitril/valsartan compared to enalapril is safe and effective in ADHF, a treatment that hasn’t been studied well or taken up in clinical practice.
, showed comparable safety, and reduced composite endpoint of death, rehospitalization for heart failure, implantation of a left-ventricular implant device, and need for a transplant by 46%.
Dr. Allen, of the University of Colorado, Denver, was the designated discussant for the PIONEER-HF presentation at the American Heart Association scientific sessions. In an interview, he explained how these results will change his practice as a heart failure specialist. “It simplifies things: I don’t have to start on an old therapy in the hospital, get the patients back in clinic, and switch the over to this newer therapy. I can just start from the beginning with the therapy that I think will be most effective.”
To find out why, watch the complete interview.
CHICAGO – Dr. Larry A. Allen will now have an easier time of treating hospitalized patients with acute decompensated heart failure because of the results of the PIONEER-HF trial.
That study examined whether in-hospital initiation of sacubitril/valsartan compared to enalapril is safe and effective in ADHF, a treatment that hasn’t been studied well or taken up in clinical practice.
, showed comparable safety, and reduced composite endpoint of death, rehospitalization for heart failure, implantation of a left-ventricular implant device, and need for a transplant by 46%.
Dr. Allen, of the University of Colorado, Denver, was the designated discussant for the PIONEER-HF presentation at the American Heart Association scientific sessions. In an interview, he explained how these results will change his practice as a heart failure specialist. “It simplifies things: I don’t have to start on an old therapy in the hospital, get the patients back in clinic, and switch the over to this newer therapy. I can just start from the beginning with the therapy that I think will be most effective.”
To find out why, watch the complete interview.
CHICAGO – Dr. Larry A. Allen will now have an easier time of treating hospitalized patients with acute decompensated heart failure because of the results of the PIONEER-HF trial.
That study examined whether in-hospital initiation of sacubitril/valsartan compared to enalapril is safe and effective in ADHF, a treatment that hasn’t been studied well or taken up in clinical practice.
, showed comparable safety, and reduced composite endpoint of death, rehospitalization for heart failure, implantation of a left-ventricular implant device, and need for a transplant by 46%.
Dr. Allen, of the University of Colorado, Denver, was the designated discussant for the PIONEER-HF presentation at the American Heart Association scientific sessions. In an interview, he explained how these results will change his practice as a heart failure specialist. “It simplifies things: I don’t have to start on an old therapy in the hospital, get the patients back in clinic, and switch the over to this newer therapy. I can just start from the beginning with the therapy that I think will be most effective.”
To find out why, watch the complete interview.
REPORTING FROM AHA 2018