User login
Single-center study outlines stroke risk, DOAC type in nonvalvular AFib patients
PHILADELPHIA – A disproportionate number of breakthrough strokes were observed among patients receiving rivaroxaban for nonvalvular atrial fibrillation in a stroke unit, according to a small, single-center, retrospective study presented at the annual meeting of the American Academy of Neurology.
The researchers reviewed all patients presenting to a tertiary care stroke unit in Australia from January 2015 to June 2018.
A total of 56 patients (median age was 74 years; 61% were male) had received direct oral anticoagulant (DOAC) therapy and then had an ischemic stroke. Of those patients, 37 (66%) had strokes while receiving the treatment; 14 patients (25%) had a stroke after recently stopping a DOAC, often prior to a medical procedure; and 5 patients (9%) were not adherent to their DOAC regimen.
Of the 37 patients who had strokes during DOAC treatment, 48% were on rivaroxaban, 9% were on dabigatran, and 9% were on apixaban, Fiona Chan, MD, of The Princess Alexandra Hospital, Brisbane, Australia, and coinvestigators reported in a poster presentation.
While these findings need to be replicated in a larger study, they do “raise concern for inadequate stroke prevention within this cohort,” they said.
Moreover, the findings illustrate the importance of bridging anticoagulation prior to procedures, when appropriate, to minimize stroke risk, they added, as 25% of the strokes had occurred in patients who recently stopped the DOACs due to procedures.
To determine which DOAC was most often associated with breakthrough ischemic strokes in patients with nonvalvular atrial fibrillation, the investigators compared the proportion of DOACs prescribed in Australia to the proportion of observed strokes in their cohort.
Despite accounting for about 51% of Australian DOAC prescriptions, rivaroxaban represented nearly 73% of breakthrough strokes among the patients who had strokes while receiving the treatment (P = .001), the investigators reported.
Conversely, apixaban accounted for about 35% of prescriptions but 14% of the breakthrough strokes (P = .0007), while dabigatran accounted for 14% of prescriptions and 14% of the strokes (P = 0.99), the investigators said in their poster.
One limitation of this retrospective study is that the patient cohort came from a single specialized center, and may not reflect the true incidence of nonvalvular atrial fibrillation across Australia, the researchers noted.
Dr. Chan and coinvestigators reported that they had no relevant financial disclosures.
SOURCE: Chan F et al. AAN 2019. P1.3-001.
PHILADELPHIA – A disproportionate number of breakthrough strokes were observed among patients receiving rivaroxaban for nonvalvular atrial fibrillation in a stroke unit, according to a small, single-center, retrospective study presented at the annual meeting of the American Academy of Neurology.
The researchers reviewed all patients presenting to a tertiary care stroke unit in Australia from January 2015 to June 2018.
A total of 56 patients (median age was 74 years; 61% were male) had received direct oral anticoagulant (DOAC) therapy and then had an ischemic stroke. Of those patients, 37 (66%) had strokes while receiving the treatment; 14 patients (25%) had a stroke after recently stopping a DOAC, often prior to a medical procedure; and 5 patients (9%) were not adherent to their DOAC regimen.
Of the 37 patients who had strokes during DOAC treatment, 48% were on rivaroxaban, 9% were on dabigatran, and 9% were on apixaban, Fiona Chan, MD, of The Princess Alexandra Hospital, Brisbane, Australia, and coinvestigators reported in a poster presentation.
While these findings need to be replicated in a larger study, they do “raise concern for inadequate stroke prevention within this cohort,” they said.
Moreover, the findings illustrate the importance of bridging anticoagulation prior to procedures, when appropriate, to minimize stroke risk, they added, as 25% of the strokes had occurred in patients who recently stopped the DOACs due to procedures.
To determine which DOAC was most often associated with breakthrough ischemic strokes in patients with nonvalvular atrial fibrillation, the investigators compared the proportion of DOACs prescribed in Australia to the proportion of observed strokes in their cohort.
Despite accounting for about 51% of Australian DOAC prescriptions, rivaroxaban represented nearly 73% of breakthrough strokes among the patients who had strokes while receiving the treatment (P = .001), the investigators reported.
Conversely, apixaban accounted for about 35% of prescriptions but 14% of the breakthrough strokes (P = .0007), while dabigatran accounted for 14% of prescriptions and 14% of the strokes (P = 0.99), the investigators said in their poster.
One limitation of this retrospective study is that the patient cohort came from a single specialized center, and may not reflect the true incidence of nonvalvular atrial fibrillation across Australia, the researchers noted.
Dr. Chan and coinvestigators reported that they had no relevant financial disclosures.
SOURCE: Chan F et al. AAN 2019. P1.3-001.
PHILADELPHIA – A disproportionate number of breakthrough strokes were observed among patients receiving rivaroxaban for nonvalvular atrial fibrillation in a stroke unit, according to a small, single-center, retrospective study presented at the annual meeting of the American Academy of Neurology.
The researchers reviewed all patients presenting to a tertiary care stroke unit in Australia from January 2015 to June 2018.
A total of 56 patients (median age was 74 years; 61% were male) had received direct oral anticoagulant (DOAC) therapy and then had an ischemic stroke. Of those patients, 37 (66%) had strokes while receiving the treatment; 14 patients (25%) had a stroke after recently stopping a DOAC, often prior to a medical procedure; and 5 patients (9%) were not adherent to their DOAC regimen.
Of the 37 patients who had strokes during DOAC treatment, 48% were on rivaroxaban, 9% were on dabigatran, and 9% were on apixaban, Fiona Chan, MD, of The Princess Alexandra Hospital, Brisbane, Australia, and coinvestigators reported in a poster presentation.
While these findings need to be replicated in a larger study, they do “raise concern for inadequate stroke prevention within this cohort,” they said.
Moreover, the findings illustrate the importance of bridging anticoagulation prior to procedures, when appropriate, to minimize stroke risk, they added, as 25% of the strokes had occurred in patients who recently stopped the DOACs due to procedures.
To determine which DOAC was most often associated with breakthrough ischemic strokes in patients with nonvalvular atrial fibrillation, the investigators compared the proportion of DOACs prescribed in Australia to the proportion of observed strokes in their cohort.
Despite accounting for about 51% of Australian DOAC prescriptions, rivaroxaban represented nearly 73% of breakthrough strokes among the patients who had strokes while receiving the treatment (P = .001), the investigators reported.
Conversely, apixaban accounted for about 35% of prescriptions but 14% of the breakthrough strokes (P = .0007), while dabigatran accounted for 14% of prescriptions and 14% of the strokes (P = 0.99), the investigators said in their poster.
One limitation of this retrospective study is that the patient cohort came from a single specialized center, and may not reflect the true incidence of nonvalvular atrial fibrillation across Australia, the researchers noted.
Dr. Chan and coinvestigators reported that they had no relevant financial disclosures.
SOURCE: Chan F et al. AAN 2019. P1.3-001.
REPORTING FROM AAN 2019
Key clinical point: Rivaroxaban was associated with a disproportionate number of breakthrough strokes among patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants at one stroke unit in Australia.
Major finding: Despite accounting for about 51% of Australian DOAC prescriptions, rivaroxaban represented nearly 73% of breakthrough strokes among the patients who had strokes while receiving treatment (P = .001).
Study details: Retrospective study of 56 patients with nonvalvular atrial fibrillation reporting to a tertiary care stroke unit in Australia.
Disclosures: The authors reported no financial disclosures.
Source: Chan F et al. AAN 019. Poster P1.3-001.
Immunotherapy induces improvements in PML
Philadelphia – Adoptive transfer of donor-derived T cells represents a potentially life-saving treatment of severely immunocompromised patients with progressive multifocal leukoencephalopathy (PML).
“We think this strategy has real potential to be one of the first effective treatments for PML,” Erin Beck, MD, PhD, said during a press conference at the annual meeting of the American Academy of Neurology.
In a pilot study including 12 patients with PML, the 7 who survived had substantial neurological improvement after treatment with this immunotherapeutic approach, said Dr. Beck of the National Institute of Neurological Disorders and Stroke.
“This was very encouraging to us because these patients were worsening at the time of their treatment and we did not expect them to do well,” she said.
There were no serious adverse events related to treatment, which suggests the approach is safe for patients with PML, according to Dr. Beck.
“This is a phenomenal breakthrough,” Natalia S. Rost, MD, MPH, chair of the meeting’s science committee, said at the press conference. “Giving a diagnosis of PML to a patient is basically giving them a death sentence, and it’s one of the most dreaded scenarios in our clinical practice.”
PML, an opportunistic infection of the central nervous system caused by JC polyomavirus, is usually fatal unless adaptive immunity to JC polyomavirus can be restored, Dr. Beck said.
Cases of PML surged in the 1980s and 1990s as a result of the HIV/AIDS epidemic, she said, and an increase in cases has been observed more recently related to new immunosuppressive treatments for cancer and autoimmune diseases.
The pilot study described by Dr. Beck included 12 patients with refractory PML who underwent adoptive transfer of polyomavirus-specific T cells (PyVSTs) that were generated from partially matched first-degree relatives of the patients. Up to three infusions were given at least 28 days apart.
Although five patients died of PML, the remaining seven patients stabilized and in some cases experienced significant neurological improvement, according to the investigators. Two of the seven died of PML a year after their final infusion.
No overt immune reconstitution inflammatory syndrome (IRIS) was observed in treated patients.
It’s not clear to date which PML patients may be most likely to benefit from this treatment approach because there were no differences in age, sex, baseline leukocyte count numbers, or time since their initial diagnosis between responders and nonresponders, Dr. Beck said.
However, patients who died tended to have much higher JC virus copy numbers in their spinal fluid, as did some patients who were enrolled but died before they could receive any treatment, she added.
“That high range, I think, is a very bad prognostic sign,” Dr. Beck said. “A number in the lower range doesn’t mean a good prognosis, but it means potentially that you could respond to a treatment such as this one.”
The study was funded by National Institute of Neurological Disorders and Stroke. The investigators disclosed no conflicts related to the study.
SOURCE: Cortese I et al. AAN 2019, Abstract Plen01.002.
Philadelphia – Adoptive transfer of donor-derived T cells represents a potentially life-saving treatment of severely immunocompromised patients with progressive multifocal leukoencephalopathy (PML).
“We think this strategy has real potential to be one of the first effective treatments for PML,” Erin Beck, MD, PhD, said during a press conference at the annual meeting of the American Academy of Neurology.
In a pilot study including 12 patients with PML, the 7 who survived had substantial neurological improvement after treatment with this immunotherapeutic approach, said Dr. Beck of the National Institute of Neurological Disorders and Stroke.
“This was very encouraging to us because these patients were worsening at the time of their treatment and we did not expect them to do well,” she said.
There were no serious adverse events related to treatment, which suggests the approach is safe for patients with PML, according to Dr. Beck.
“This is a phenomenal breakthrough,” Natalia S. Rost, MD, MPH, chair of the meeting’s science committee, said at the press conference. “Giving a diagnosis of PML to a patient is basically giving them a death sentence, and it’s one of the most dreaded scenarios in our clinical practice.”
PML, an opportunistic infection of the central nervous system caused by JC polyomavirus, is usually fatal unless adaptive immunity to JC polyomavirus can be restored, Dr. Beck said.
Cases of PML surged in the 1980s and 1990s as a result of the HIV/AIDS epidemic, she said, and an increase in cases has been observed more recently related to new immunosuppressive treatments for cancer and autoimmune diseases.
The pilot study described by Dr. Beck included 12 patients with refractory PML who underwent adoptive transfer of polyomavirus-specific T cells (PyVSTs) that were generated from partially matched first-degree relatives of the patients. Up to three infusions were given at least 28 days apart.
Although five patients died of PML, the remaining seven patients stabilized and in some cases experienced significant neurological improvement, according to the investigators. Two of the seven died of PML a year after their final infusion.
No overt immune reconstitution inflammatory syndrome (IRIS) was observed in treated patients.
It’s not clear to date which PML patients may be most likely to benefit from this treatment approach because there were no differences in age, sex, baseline leukocyte count numbers, or time since their initial diagnosis between responders and nonresponders, Dr. Beck said.
However, patients who died tended to have much higher JC virus copy numbers in their spinal fluid, as did some patients who were enrolled but died before they could receive any treatment, she added.
“That high range, I think, is a very bad prognostic sign,” Dr. Beck said. “A number in the lower range doesn’t mean a good prognosis, but it means potentially that you could respond to a treatment such as this one.”
The study was funded by National Institute of Neurological Disorders and Stroke. The investigators disclosed no conflicts related to the study.
SOURCE: Cortese I et al. AAN 2019, Abstract Plen01.002.
Philadelphia – Adoptive transfer of donor-derived T cells represents a potentially life-saving treatment of severely immunocompromised patients with progressive multifocal leukoencephalopathy (PML).
“We think this strategy has real potential to be one of the first effective treatments for PML,” Erin Beck, MD, PhD, said during a press conference at the annual meeting of the American Academy of Neurology.
In a pilot study including 12 patients with PML, the 7 who survived had substantial neurological improvement after treatment with this immunotherapeutic approach, said Dr. Beck of the National Institute of Neurological Disorders and Stroke.
“This was very encouraging to us because these patients were worsening at the time of their treatment and we did not expect them to do well,” she said.
There were no serious adverse events related to treatment, which suggests the approach is safe for patients with PML, according to Dr. Beck.
“This is a phenomenal breakthrough,” Natalia S. Rost, MD, MPH, chair of the meeting’s science committee, said at the press conference. “Giving a diagnosis of PML to a patient is basically giving them a death sentence, and it’s one of the most dreaded scenarios in our clinical practice.”
PML, an opportunistic infection of the central nervous system caused by JC polyomavirus, is usually fatal unless adaptive immunity to JC polyomavirus can be restored, Dr. Beck said.
Cases of PML surged in the 1980s and 1990s as a result of the HIV/AIDS epidemic, she said, and an increase in cases has been observed more recently related to new immunosuppressive treatments for cancer and autoimmune diseases.
The pilot study described by Dr. Beck included 12 patients with refractory PML who underwent adoptive transfer of polyomavirus-specific T cells (PyVSTs) that were generated from partially matched first-degree relatives of the patients. Up to three infusions were given at least 28 days apart.
Although five patients died of PML, the remaining seven patients stabilized and in some cases experienced significant neurological improvement, according to the investigators. Two of the seven died of PML a year after their final infusion.
No overt immune reconstitution inflammatory syndrome (IRIS) was observed in treated patients.
It’s not clear to date which PML patients may be most likely to benefit from this treatment approach because there were no differences in age, sex, baseline leukocyte count numbers, or time since their initial diagnosis between responders and nonresponders, Dr. Beck said.
However, patients who died tended to have much higher JC virus copy numbers in their spinal fluid, as did some patients who were enrolled but died before they could receive any treatment, she added.
“That high range, I think, is a very bad prognostic sign,” Dr. Beck said. “A number in the lower range doesn’t mean a good prognosis, but it means potentially that you could respond to a treatment such as this one.”
The study was funded by National Institute of Neurological Disorders and Stroke. The investigators disclosed no conflicts related to the study.
SOURCE: Cortese I et al. AAN 2019, Abstract Plen01.002.
FROM AAN 2019
Key clinical point: Adoptive transfer of donor-derived T cells represents a potentially life-saving strategy for patients with progressive multifocal leukoencephalopathy.
Major finding: Seven of 12 patients stabilized and in some cases experienced significant neurological improvement.
Study details: A pilot study including 12 patients with refractory PML.
Disclosures: The study was funded by NINDS. The investigators disclosed no conflicts related to the study.
Source: Cortese I et al. AAN 2019, Abstract Plen01.002.
United States up to 764 measles cases for the year
The number of U.S. measles cases in 2019 rose by 60 during the week ending May 3, which puts the new postelimination high at 764 cases for the year, according to the Centers for Disease Control and Prevention.
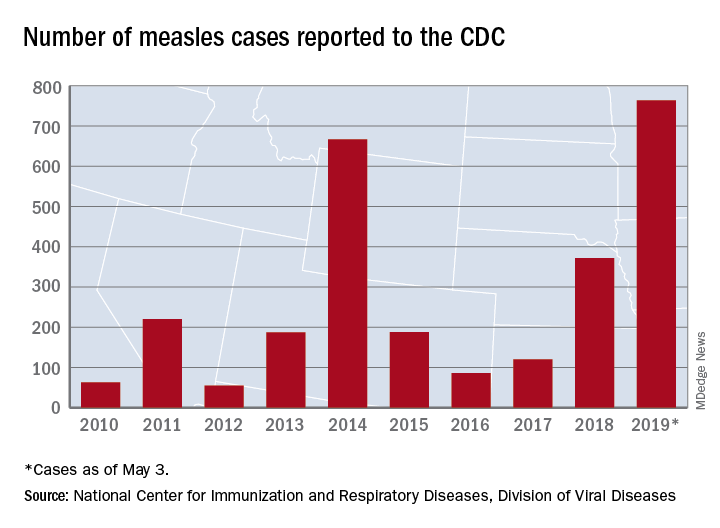
The CDC is currently tracking nine measles outbreaks in six states. The largest outbreak this year has been in New York City, mainly Brooklyn, which has almost half (367) of all cases in the country. An outbreak in New York’s Rockland County has resulted in another 109 cases so far this year. California is experiencing outbreaks in three counties – Butte, Los Angeles, and Sacramento – and the state was up to 40 confirmed cases as of May 1. The other states with outbreaks are Michigan, New Jersey, Georgia, and Maryland.
Twenty-three states now have reported measles cases in 2019, as officials in Pittsburgh reported Pennsylvania’s first case on April 30. Four additional cases in the area were reported on May 2 by the Allegheny County Health Department. A measles outbreak is “defined as three or more cases” by the CDC, but the situation in Pennsylvania is not yet being reported as such.
One Pennsylvania legislator in the state, Rep. Daryl Metcalfe (R) of Butler County, has authored a bill that would “bar health care practitioners and facilities and insurance companies from denying care if parents refuse or delay” recommended vaccinations, PennLive.com reported.
Pennsylvania Governor Tom Wolf (D) said that the “bill would put children, pregnant women, and vulnerable patients at risk of being exposed to horrific diseases – at the doctor’s office.”
The number of U.S. measles cases in 2019 rose by 60 during the week ending May 3, which puts the new postelimination high at 764 cases for the year, according to the Centers for Disease Control and Prevention.

The CDC is currently tracking nine measles outbreaks in six states. The largest outbreak this year has been in New York City, mainly Brooklyn, which has almost half (367) of all cases in the country. An outbreak in New York’s Rockland County has resulted in another 109 cases so far this year. California is experiencing outbreaks in three counties – Butte, Los Angeles, and Sacramento – and the state was up to 40 confirmed cases as of May 1. The other states with outbreaks are Michigan, New Jersey, Georgia, and Maryland.
Twenty-three states now have reported measles cases in 2019, as officials in Pittsburgh reported Pennsylvania’s first case on April 30. Four additional cases in the area were reported on May 2 by the Allegheny County Health Department. A measles outbreak is “defined as three or more cases” by the CDC, but the situation in Pennsylvania is not yet being reported as such.
One Pennsylvania legislator in the state, Rep. Daryl Metcalfe (R) of Butler County, has authored a bill that would “bar health care practitioners and facilities and insurance companies from denying care if parents refuse or delay” recommended vaccinations, PennLive.com reported.
Pennsylvania Governor Tom Wolf (D) said that the “bill would put children, pregnant women, and vulnerable patients at risk of being exposed to horrific diseases – at the doctor’s office.”
The number of U.S. measles cases in 2019 rose by 60 during the week ending May 3, which puts the new postelimination high at 764 cases for the year, according to the Centers for Disease Control and Prevention.

The CDC is currently tracking nine measles outbreaks in six states. The largest outbreak this year has been in New York City, mainly Brooklyn, which has almost half (367) of all cases in the country. An outbreak in New York’s Rockland County has resulted in another 109 cases so far this year. California is experiencing outbreaks in three counties – Butte, Los Angeles, and Sacramento – and the state was up to 40 confirmed cases as of May 1. The other states with outbreaks are Michigan, New Jersey, Georgia, and Maryland.
Twenty-three states now have reported measles cases in 2019, as officials in Pittsburgh reported Pennsylvania’s first case on April 30. Four additional cases in the area were reported on May 2 by the Allegheny County Health Department. A measles outbreak is “defined as three or more cases” by the CDC, but the situation in Pennsylvania is not yet being reported as such.
One Pennsylvania legislator in the state, Rep. Daryl Metcalfe (R) of Butler County, has authored a bill that would “bar health care practitioners and facilities and insurance companies from denying care if parents refuse or delay” recommended vaccinations, PennLive.com reported.
Pennsylvania Governor Tom Wolf (D) said that the “bill would put children, pregnant women, and vulnerable patients at risk of being exposed to horrific diseases – at the doctor’s office.”
ARCHES: Enzalutamide improves mHSPC survival regardless of baseline PSA
For men with metastatic hormone-sensitive prostate cancer (mHSPC), enzalutamide with androgen deprivation therapy (ADT) offers longer radiographic progression-free survival (rPFS) compared with placebo and ADT, regardless of baseline prostate-specific antigen level (PSA), based on results from the phase 3 ARCHES trial.
Although initial PSA level was not predictive of response, treatment with enzalutamide and ADT improved PSA-related efficacy measures, reported lead author Arnulf Stenzl, MD, of the University of Tübingen, Germany, and colleagues. The adverse effect profile of enzalutamide was similar to previous experiences with castrate-resistant patients, they noted in their abstract, which was presented at the annual meeting of the American Urological Association.
ARCHES was a double-blind, placebo-controlled trial involving 1,150 patients with mHSPC, divided approximately 1:1 to receive either enzalutamide with ADT (n = 574) or placebo with ADT (n = 576). Eligibility required that patients had not exhibited radiographic disease progression or rising PSA levels for up to 3 months of ADT, or up to 6 months with prior docetaxel. The primary endpoints were death within 24 weeks of stopping treatment and rPFS. Treatment was continued until unacceptable toxicity or disease progression. PSA levels were measured at baseline and at follow-up, which was an average of 14.4 months.
Median baseline PSA level across both cohorts was 5.21 ng/mL, with most patients having received prior ADT (91%). Baseline PSA was not predictive of response, “suggesting the limitation of baseline PSA as a predictive factor in this population in which most [patients] received prior ADT,” the investigators wrote. Although PSA levels were not predictive, enzalutamide did have a greater impact on PSA-related efficacy endpoints; compared with the placebo group, patients in the enzalutamide arm were significantly more likely to have PSA reductions from baseline of at least 50% (92.9% vs. 56.8%) and at least 90% (72.8% vs. 30.0%). In further support of the efficacy of enzalutamide, median rPFS in the enzalutamide group was significantly better than in the placebo group (not reached vs. 19.4 months). Adverse events were comparable between enzalutamide (85.1%) and placebo (85.9%) cohorts.
Astellas Pharma and Medivation funded the study. The investigators reported no conflicts of interest.
SOURCE: Stenzl et al. AUA 2019. Abstract LBA-10.
For men with metastatic hormone-sensitive prostate cancer (mHSPC), enzalutamide with androgen deprivation therapy (ADT) offers longer radiographic progression-free survival (rPFS) compared with placebo and ADT, regardless of baseline prostate-specific antigen level (PSA), based on results from the phase 3 ARCHES trial.
Although initial PSA level was not predictive of response, treatment with enzalutamide and ADT improved PSA-related efficacy measures, reported lead author Arnulf Stenzl, MD, of the University of Tübingen, Germany, and colleagues. The adverse effect profile of enzalutamide was similar to previous experiences with castrate-resistant patients, they noted in their abstract, which was presented at the annual meeting of the American Urological Association.
ARCHES was a double-blind, placebo-controlled trial involving 1,150 patients with mHSPC, divided approximately 1:1 to receive either enzalutamide with ADT (n = 574) or placebo with ADT (n = 576). Eligibility required that patients had not exhibited radiographic disease progression or rising PSA levels for up to 3 months of ADT, or up to 6 months with prior docetaxel. The primary endpoints were death within 24 weeks of stopping treatment and rPFS. Treatment was continued until unacceptable toxicity or disease progression. PSA levels were measured at baseline and at follow-up, which was an average of 14.4 months.
Median baseline PSA level across both cohorts was 5.21 ng/mL, with most patients having received prior ADT (91%). Baseline PSA was not predictive of response, “suggesting the limitation of baseline PSA as a predictive factor in this population in which most [patients] received prior ADT,” the investigators wrote. Although PSA levels were not predictive, enzalutamide did have a greater impact on PSA-related efficacy endpoints; compared with the placebo group, patients in the enzalutamide arm were significantly more likely to have PSA reductions from baseline of at least 50% (92.9% vs. 56.8%) and at least 90% (72.8% vs. 30.0%). In further support of the efficacy of enzalutamide, median rPFS in the enzalutamide group was significantly better than in the placebo group (not reached vs. 19.4 months). Adverse events were comparable between enzalutamide (85.1%) and placebo (85.9%) cohorts.
Astellas Pharma and Medivation funded the study. The investigators reported no conflicts of interest.
SOURCE: Stenzl et al. AUA 2019. Abstract LBA-10.
For men with metastatic hormone-sensitive prostate cancer (mHSPC), enzalutamide with androgen deprivation therapy (ADT) offers longer radiographic progression-free survival (rPFS) compared with placebo and ADT, regardless of baseline prostate-specific antigen level (PSA), based on results from the phase 3 ARCHES trial.
Although initial PSA level was not predictive of response, treatment with enzalutamide and ADT improved PSA-related efficacy measures, reported lead author Arnulf Stenzl, MD, of the University of Tübingen, Germany, and colleagues. The adverse effect profile of enzalutamide was similar to previous experiences with castrate-resistant patients, they noted in their abstract, which was presented at the annual meeting of the American Urological Association.
ARCHES was a double-blind, placebo-controlled trial involving 1,150 patients with mHSPC, divided approximately 1:1 to receive either enzalutamide with ADT (n = 574) or placebo with ADT (n = 576). Eligibility required that patients had not exhibited radiographic disease progression or rising PSA levels for up to 3 months of ADT, or up to 6 months with prior docetaxel. The primary endpoints were death within 24 weeks of stopping treatment and rPFS. Treatment was continued until unacceptable toxicity or disease progression. PSA levels were measured at baseline and at follow-up, which was an average of 14.4 months.
Median baseline PSA level across both cohorts was 5.21 ng/mL, with most patients having received prior ADT (91%). Baseline PSA was not predictive of response, “suggesting the limitation of baseline PSA as a predictive factor in this population in which most [patients] received prior ADT,” the investigators wrote. Although PSA levels were not predictive, enzalutamide did have a greater impact on PSA-related efficacy endpoints; compared with the placebo group, patients in the enzalutamide arm were significantly more likely to have PSA reductions from baseline of at least 50% (92.9% vs. 56.8%) and at least 90% (72.8% vs. 30.0%). In further support of the efficacy of enzalutamide, median rPFS in the enzalutamide group was significantly better than in the placebo group (not reached vs. 19.4 months). Adverse events were comparable between enzalutamide (85.1%) and placebo (85.9%) cohorts.
Astellas Pharma and Medivation funded the study. The investigators reported no conflicts of interest.
SOURCE: Stenzl et al. AUA 2019. Abstract LBA-10.
FROM AUA 2019
Key clinical point: For men with metastatic hormone-sensitive prostate cancer (mHSPC), enzalutamide with androgen deprivation therapy offers longer radiographic progression-free survival (rPFS) compared with placebo and androgen deprivation therapy, regardless of baseline prostate-specific antigen level (PSA).
Major finding: A greater percentage of patients treated with enzalutamide and androgen deprivation therapy had a prostate-specific antigen reduction from baseline of at least 90%, compared with those who received placebo and androgen deprivation therapy (72.8% vs. 30.0%).
Study details: ARCHES was a double-blind, placebo-controlled, phase 3 study involving 1,150 men with metastatic hormone-sensitive prostate cancer.
Disclosures: The study was funded by Astellas Pharma and Medivation. The investigators reported no conflicts of interest.
Source: Stenzl et al. AUA 2019. Abstract LBA-10.
Angelman syndrome treatment safe, well-tolerated, and effective in exploratory analyses
Philadelphia – according to results of a study presented at the annual meeting of the American Academy of Neurology.
Clinician-rated clinical global impressions of improvement (CGI-I) were improved versus placebo in the randomized study, as were other outcomes in post hoc analyses, including measures of sleep and motor function, said Alexander Kolevzon, MD, professor of psychiatry and pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
This study of OV101 was a genetics-driven trial for the rare genetic disorder, which is caused by mutations in UBE3A and characterized by seizures, speech impairments, profound intellectual disability, gait problems, and anxiety, Dr. Kolevzon said in a press conference.
“The only treatments that exist are really very symptomatically driven,” Dr. Kolevzon said. “Here, we are taking a genetics-first approach. Having identified the gene, there is some understanding of what the underlying biology is, and it seems to relate to deficits in tonic inhibition.”
OV101 is a delta-selective type A gamma-aminobutyric acid receptor agonist that may potentially normalize the tonic inhibition that is decreased in Angelman syndrome. “What we think this compound is doing is actually reversing the deficits of tonic inhibition, and sort of restoring that state to these patients,” Dr. Kolevzon said in the press conference.
A total of 78 patients completed the phase 2, randomized study, known as STARS, which had a primary endpoint of safety and tolerability over 12 weeks of treatment with OV101 once daily, twice daily, or placebo. The mean age of the 87 patients who enrolled and had at least one dose of study drug was 22.6 years.
Most adverse events were mild, and frequencies of specific adverse events were similar for OV101 and placebo treatment groups, according to Dr. Kolevzon and his coinvestigators.
Improvements in motor function, sleep, and behavior were seen in a series of exploratory analyses, including global improvement at week 12, as captured by CGI-I, which was significantly improved for daily OV101 versus placebo (P = .0006).
These phase 2 results have informed discussions of which specific endpoints might be incorporated into the design of a planned phase 3 trial in pediatric patients. The CGI-I may be especially useful to measure clinical improvement in Angelman syndrome, which is a very “heterogeneous” disorder, Dr. Kolevzon said in the press conference.
“Every child has a different composite of symptoms, so that is the big challenge,” Dr. Kolevzon said. “I do not think we are going to have one singular outcome. The idea is to have a global measure that really captures heterogeneity across each trial and allows for children to be compared to their baseline, and each as their own control, in essence, but with specific domains in mind.”
Funding for the study came from Ovid Therapeutics. Dr. Kolevzon reported disclosures related to Ovid Therapeutics, as well as Coronis Neurosciences, 5AM Ventures, SEMA4, LabCorp, and AMO Pharma.
Philadelphia – according to results of a study presented at the annual meeting of the American Academy of Neurology.
Clinician-rated clinical global impressions of improvement (CGI-I) were improved versus placebo in the randomized study, as were other outcomes in post hoc analyses, including measures of sleep and motor function, said Alexander Kolevzon, MD, professor of psychiatry and pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
This study of OV101 was a genetics-driven trial for the rare genetic disorder, which is caused by mutations in UBE3A and characterized by seizures, speech impairments, profound intellectual disability, gait problems, and anxiety, Dr. Kolevzon said in a press conference.
“The only treatments that exist are really very symptomatically driven,” Dr. Kolevzon said. “Here, we are taking a genetics-first approach. Having identified the gene, there is some understanding of what the underlying biology is, and it seems to relate to deficits in tonic inhibition.”
OV101 is a delta-selective type A gamma-aminobutyric acid receptor agonist that may potentially normalize the tonic inhibition that is decreased in Angelman syndrome. “What we think this compound is doing is actually reversing the deficits of tonic inhibition, and sort of restoring that state to these patients,” Dr. Kolevzon said in the press conference.
A total of 78 patients completed the phase 2, randomized study, known as STARS, which had a primary endpoint of safety and tolerability over 12 weeks of treatment with OV101 once daily, twice daily, or placebo. The mean age of the 87 patients who enrolled and had at least one dose of study drug was 22.6 years.
Most adverse events were mild, and frequencies of specific adverse events were similar for OV101 and placebo treatment groups, according to Dr. Kolevzon and his coinvestigators.
Improvements in motor function, sleep, and behavior were seen in a series of exploratory analyses, including global improvement at week 12, as captured by CGI-I, which was significantly improved for daily OV101 versus placebo (P = .0006).
These phase 2 results have informed discussions of which specific endpoints might be incorporated into the design of a planned phase 3 trial in pediatric patients. The CGI-I may be especially useful to measure clinical improvement in Angelman syndrome, which is a very “heterogeneous” disorder, Dr. Kolevzon said in the press conference.
“Every child has a different composite of symptoms, so that is the big challenge,” Dr. Kolevzon said. “I do not think we are going to have one singular outcome. The idea is to have a global measure that really captures heterogeneity across each trial and allows for children to be compared to their baseline, and each as their own control, in essence, but with specific domains in mind.”
Funding for the study came from Ovid Therapeutics. Dr. Kolevzon reported disclosures related to Ovid Therapeutics, as well as Coronis Neurosciences, 5AM Ventures, SEMA4, LabCorp, and AMO Pharma.
Philadelphia – according to results of a study presented at the annual meeting of the American Academy of Neurology.
Clinician-rated clinical global impressions of improvement (CGI-I) were improved versus placebo in the randomized study, as were other outcomes in post hoc analyses, including measures of sleep and motor function, said Alexander Kolevzon, MD, professor of psychiatry and pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
This study of OV101 was a genetics-driven trial for the rare genetic disorder, which is caused by mutations in UBE3A and characterized by seizures, speech impairments, profound intellectual disability, gait problems, and anxiety, Dr. Kolevzon said in a press conference.
“The only treatments that exist are really very symptomatically driven,” Dr. Kolevzon said. “Here, we are taking a genetics-first approach. Having identified the gene, there is some understanding of what the underlying biology is, and it seems to relate to deficits in tonic inhibition.”
OV101 is a delta-selective type A gamma-aminobutyric acid receptor agonist that may potentially normalize the tonic inhibition that is decreased in Angelman syndrome. “What we think this compound is doing is actually reversing the deficits of tonic inhibition, and sort of restoring that state to these patients,” Dr. Kolevzon said in the press conference.
A total of 78 patients completed the phase 2, randomized study, known as STARS, which had a primary endpoint of safety and tolerability over 12 weeks of treatment with OV101 once daily, twice daily, or placebo. The mean age of the 87 patients who enrolled and had at least one dose of study drug was 22.6 years.
Most adverse events were mild, and frequencies of specific adverse events were similar for OV101 and placebo treatment groups, according to Dr. Kolevzon and his coinvestigators.
Improvements in motor function, sleep, and behavior were seen in a series of exploratory analyses, including global improvement at week 12, as captured by CGI-I, which was significantly improved for daily OV101 versus placebo (P = .0006).
These phase 2 results have informed discussions of which specific endpoints might be incorporated into the design of a planned phase 3 trial in pediatric patients. The CGI-I may be especially useful to measure clinical improvement in Angelman syndrome, which is a very “heterogeneous” disorder, Dr. Kolevzon said in the press conference.
“Every child has a different composite of symptoms, so that is the big challenge,” Dr. Kolevzon said. “I do not think we are going to have one singular outcome. The idea is to have a global measure that really captures heterogeneity across each trial and allows for children to be compared to their baseline, and each as their own control, in essence, but with specific domains in mind.”
Funding for the study came from Ovid Therapeutics. Dr. Kolevzon reported disclosures related to Ovid Therapeutics, as well as Coronis Neurosciences, 5AM Ventures, SEMA4, LabCorp, and AMO Pharma.
REPORTING FROM AAN 2019
Biomarkers in tears may help identify patients with Parkinson’s disease
PHILADELPHIA – according to research presented at the annual meeting of the American Academy of Neurology. Chemokine (C-C motif) ligand 2 (CCL2), a cytokine, may be used for the same purpose, according to researchers.
Lacrimal glands have high numbers of cholinergic and other neurons. Parasympathetic and sympathetic neural pathways stimulate the tears that lacrimal grands secrete. It is possible that the production, packaging, and secretion of proteins into tears may alter when nerve function in the lacrimal glands and cornea changes. This idea may be tested by collecting reflex tears (i.e., stimulated tears provoked by an unanesthetized Schirmer’s test).
Mark Lew, MD, professor of clinical neurology at University of Southern California, Los Angeles, and colleagues previously studied patients using basal tears collected from an anesthetized Schirmer’s test. To examine whether the protein composition of reflex tears differs in patients with Parkinson’s disease, compared with healthy controls, they collected reflex tears from 85 patients with Parkinson’s disease and 80 age- and sex-matched healthy controls using an unanesthetized Schirmer’s test. The researchers pooled samples from both eyes to analyze alpha synuclein, CCL2, and total protein using enzyme-linked immunosorbent assays or multiplex ELISA.
Eligible participants were aged 30-85 years and had a Montreal Cognitive Assessment (MoCA) score of 21 or higher. Patients with Parkinson’s disease had a lower MoCA score than controls did, although it was still in the normal range. Tear flow was significantly decreased in patients with Parkinson’s disease, compared with controls.
Dr. Lew and colleagues found that the amount of oligomeric alpha synuclein was increased nearly 400% in the tears of patients with Parkinson’s disease, compared with those of healthy controls (4.21 ng/mg tear protein vs. 0.90 ng/mg tear protein). This difference was statistically significant. Similarly, CCL2 was significantly increased in the tears of patients with Parkinson’s disease, compared with those of healthy controls (165.8 pg/mg tear protein vs. 116.3 pg/mg tear protein). Among men, Parkinson’s disease was associated with greater rises in oligomeric alpha synuclein (4.95 ng/mg tear protein vs. 0.89 ng/mg tear protein in healthy controls) and CCL2 (201.5 pg/mg tear protein vs. 117.9 pg/mg tear protein in healthy controls) than in women. The origin of sex differences in these biomarker values requires further study, the investigators said.
Dr. Lew reported receiving research support from the Parkinson’s Study Group, the Michael J. Fox Foundation, Biotie Therapies, NeuroDerm, Enterin, Pharm2B Fellowship Grants, Allergan, and Medtronic.
SOURCE: Lew M et al. AAN 2019, Abstract S10.001
PHILADELPHIA – according to research presented at the annual meeting of the American Academy of Neurology. Chemokine (C-C motif) ligand 2 (CCL2), a cytokine, may be used for the same purpose, according to researchers.
Lacrimal glands have high numbers of cholinergic and other neurons. Parasympathetic and sympathetic neural pathways stimulate the tears that lacrimal grands secrete. It is possible that the production, packaging, and secretion of proteins into tears may alter when nerve function in the lacrimal glands and cornea changes. This idea may be tested by collecting reflex tears (i.e., stimulated tears provoked by an unanesthetized Schirmer’s test).
Mark Lew, MD, professor of clinical neurology at University of Southern California, Los Angeles, and colleagues previously studied patients using basal tears collected from an anesthetized Schirmer’s test. To examine whether the protein composition of reflex tears differs in patients with Parkinson’s disease, compared with healthy controls, they collected reflex tears from 85 patients with Parkinson’s disease and 80 age- and sex-matched healthy controls using an unanesthetized Schirmer’s test. The researchers pooled samples from both eyes to analyze alpha synuclein, CCL2, and total protein using enzyme-linked immunosorbent assays or multiplex ELISA.
Eligible participants were aged 30-85 years and had a Montreal Cognitive Assessment (MoCA) score of 21 or higher. Patients with Parkinson’s disease had a lower MoCA score than controls did, although it was still in the normal range. Tear flow was significantly decreased in patients with Parkinson’s disease, compared with controls.
Dr. Lew and colleagues found that the amount of oligomeric alpha synuclein was increased nearly 400% in the tears of patients with Parkinson’s disease, compared with those of healthy controls (4.21 ng/mg tear protein vs. 0.90 ng/mg tear protein). This difference was statistically significant. Similarly, CCL2 was significantly increased in the tears of patients with Parkinson’s disease, compared with those of healthy controls (165.8 pg/mg tear protein vs. 116.3 pg/mg tear protein). Among men, Parkinson’s disease was associated with greater rises in oligomeric alpha synuclein (4.95 ng/mg tear protein vs. 0.89 ng/mg tear protein in healthy controls) and CCL2 (201.5 pg/mg tear protein vs. 117.9 pg/mg tear protein in healthy controls) than in women. The origin of sex differences in these biomarker values requires further study, the investigators said.
Dr. Lew reported receiving research support from the Parkinson’s Study Group, the Michael J. Fox Foundation, Biotie Therapies, NeuroDerm, Enterin, Pharm2B Fellowship Grants, Allergan, and Medtronic.
SOURCE: Lew M et al. AAN 2019, Abstract S10.001
PHILADELPHIA – according to research presented at the annual meeting of the American Academy of Neurology. Chemokine (C-C motif) ligand 2 (CCL2), a cytokine, may be used for the same purpose, according to researchers.
Lacrimal glands have high numbers of cholinergic and other neurons. Parasympathetic and sympathetic neural pathways stimulate the tears that lacrimal grands secrete. It is possible that the production, packaging, and secretion of proteins into tears may alter when nerve function in the lacrimal glands and cornea changes. This idea may be tested by collecting reflex tears (i.e., stimulated tears provoked by an unanesthetized Schirmer’s test).
Mark Lew, MD, professor of clinical neurology at University of Southern California, Los Angeles, and colleagues previously studied patients using basal tears collected from an anesthetized Schirmer’s test. To examine whether the protein composition of reflex tears differs in patients with Parkinson’s disease, compared with healthy controls, they collected reflex tears from 85 patients with Parkinson’s disease and 80 age- and sex-matched healthy controls using an unanesthetized Schirmer’s test. The researchers pooled samples from both eyes to analyze alpha synuclein, CCL2, and total protein using enzyme-linked immunosorbent assays or multiplex ELISA.
Eligible participants were aged 30-85 years and had a Montreal Cognitive Assessment (MoCA) score of 21 or higher. Patients with Parkinson’s disease had a lower MoCA score than controls did, although it was still in the normal range. Tear flow was significantly decreased in patients with Parkinson’s disease, compared with controls.
Dr. Lew and colleagues found that the amount of oligomeric alpha synuclein was increased nearly 400% in the tears of patients with Parkinson’s disease, compared with those of healthy controls (4.21 ng/mg tear protein vs. 0.90 ng/mg tear protein). This difference was statistically significant. Similarly, CCL2 was significantly increased in the tears of patients with Parkinson’s disease, compared with those of healthy controls (165.8 pg/mg tear protein vs. 116.3 pg/mg tear protein). Among men, Parkinson’s disease was associated with greater rises in oligomeric alpha synuclein (4.95 ng/mg tear protein vs. 0.89 ng/mg tear protein in healthy controls) and CCL2 (201.5 pg/mg tear protein vs. 117.9 pg/mg tear protein in healthy controls) than in women. The origin of sex differences in these biomarker values requires further study, the investigators said.
Dr. Lew reported receiving research support from the Parkinson’s Study Group, the Michael J. Fox Foundation, Biotie Therapies, NeuroDerm, Enterin, Pharm2B Fellowship Grants, Allergan, and Medtronic.
SOURCE: Lew M et al. AAN 2019, Abstract S10.001
REPORTING FROM AAN 2019
Researchers win Stand Up To Cancer grants
Stand Up To Cancer has awarded 1- and 2-year grants to five teams conducting cancer research.
Alan D’Andrea, MD, of the Dana-Farber Cancer Institute in Boston and Juan Cubillos-Ruiz, PhD, of Weill Cornell Medicine in New York won a 2-year grant totaling $250,000.
Dr. Cubillos-Ruiz and Dr. D’Andrea will use this funding to evaluate gene signatures controlled by phospholipid messengers and endoplasmic reticulum stress in responding and nonresponding ovarian cancer patients, and the team will use tumor organoids to test whether pharmacological inhibition of these pathways can fight ovarian cancer.
Denada Dibra, PhD, of the University of Texas MD Anderson Cancer Center in Houston and Peter Lee, MD, of City of Hope in Duarte, Calif., won a 1-year grant of $250,000.
Dr. Lee and Dr. Dibra will use mouse models to study how TP53 may influence the tumor microenvironment in triple-negative breast cancer and to characterize tumor cells and immune cells in the tumor tissue.
Maximilian Diehn, MD, PhD, of Stanford (Calif.) University and Aaron Hata, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston won a 2-year grant totaling $225,000.
Dr. Hata and Dr. Diehn will work on developing a novel method for analyzing cell-free RNA associated with resistance to tyrosine kinase inhibitors in patients with non–small cell lung cancer. The team’s goal is to develop a noninvasive approach to better characterize tumors and detect phenotypic changes during treatment.
Sarah Tasian, MD, of Children’s Hospital of Philadelphia and Kimberly Stegmaier, MD, of the Dana-Farber Cancer Institute won a 2-year grant totaling $250,000, which was funded with support from the Emily Whitehead Foundation.
Dr. Stegmaier and Dr. Tasian will test chimeric antigen receptor T cells targeting two or more neoantigens in preclinical models of childhood Down syndrome–associated acute lymphoblastic leukemia and Ph-like acute lymphoblastic leukemia.
Robert Vonderheide, MD, of the University of Pennsylvania Abramson Cancer Center in Philadelphia and Vinod Balachandran, MD, of Memorial Sloan Kettering Cancer Center in New York won a 2-year grant totaling $225,000.
Dr. Balachandran and Dr. Vonderheide will analyze short- and long-term pancreatic cancer survivors, patients with primary resected pancreatic cancers and patients with mKRAS lung and colon cancers to evaluate how mKRAS immunogenicity may affect outcomes.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Stand Up To Cancer has awarded 1- and 2-year grants to five teams conducting cancer research.
Alan D’Andrea, MD, of the Dana-Farber Cancer Institute in Boston and Juan Cubillos-Ruiz, PhD, of Weill Cornell Medicine in New York won a 2-year grant totaling $250,000.
Dr. Cubillos-Ruiz and Dr. D’Andrea will use this funding to evaluate gene signatures controlled by phospholipid messengers and endoplasmic reticulum stress in responding and nonresponding ovarian cancer patients, and the team will use tumor organoids to test whether pharmacological inhibition of these pathways can fight ovarian cancer.
Denada Dibra, PhD, of the University of Texas MD Anderson Cancer Center in Houston and Peter Lee, MD, of City of Hope in Duarte, Calif., won a 1-year grant of $250,000.
Dr. Lee and Dr. Dibra will use mouse models to study how TP53 may influence the tumor microenvironment in triple-negative breast cancer and to characterize tumor cells and immune cells in the tumor tissue.
Maximilian Diehn, MD, PhD, of Stanford (Calif.) University and Aaron Hata, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston won a 2-year grant totaling $225,000.
Dr. Hata and Dr. Diehn will work on developing a novel method for analyzing cell-free RNA associated with resistance to tyrosine kinase inhibitors in patients with non–small cell lung cancer. The team’s goal is to develop a noninvasive approach to better characterize tumors and detect phenotypic changes during treatment.
Sarah Tasian, MD, of Children’s Hospital of Philadelphia and Kimberly Stegmaier, MD, of the Dana-Farber Cancer Institute won a 2-year grant totaling $250,000, which was funded with support from the Emily Whitehead Foundation.
Dr. Stegmaier and Dr. Tasian will test chimeric antigen receptor T cells targeting two or more neoantigens in preclinical models of childhood Down syndrome–associated acute lymphoblastic leukemia and Ph-like acute lymphoblastic leukemia.
Robert Vonderheide, MD, of the University of Pennsylvania Abramson Cancer Center in Philadelphia and Vinod Balachandran, MD, of Memorial Sloan Kettering Cancer Center in New York won a 2-year grant totaling $225,000.
Dr. Balachandran and Dr. Vonderheide will analyze short- and long-term pancreatic cancer survivors, patients with primary resected pancreatic cancers and patients with mKRAS lung and colon cancers to evaluate how mKRAS immunogenicity may affect outcomes.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Stand Up To Cancer has awarded 1- and 2-year grants to five teams conducting cancer research.
Alan D’Andrea, MD, of the Dana-Farber Cancer Institute in Boston and Juan Cubillos-Ruiz, PhD, of Weill Cornell Medicine in New York won a 2-year grant totaling $250,000.
Dr. Cubillos-Ruiz and Dr. D’Andrea will use this funding to evaluate gene signatures controlled by phospholipid messengers and endoplasmic reticulum stress in responding and nonresponding ovarian cancer patients, and the team will use tumor organoids to test whether pharmacological inhibition of these pathways can fight ovarian cancer.
Denada Dibra, PhD, of the University of Texas MD Anderson Cancer Center in Houston and Peter Lee, MD, of City of Hope in Duarte, Calif., won a 1-year grant of $250,000.
Dr. Lee and Dr. Dibra will use mouse models to study how TP53 may influence the tumor microenvironment in triple-negative breast cancer and to characterize tumor cells and immune cells in the tumor tissue.
Maximilian Diehn, MD, PhD, of Stanford (Calif.) University and Aaron Hata, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston won a 2-year grant totaling $225,000.
Dr. Hata and Dr. Diehn will work on developing a novel method for analyzing cell-free RNA associated with resistance to tyrosine kinase inhibitors in patients with non–small cell lung cancer. The team’s goal is to develop a noninvasive approach to better characterize tumors and detect phenotypic changes during treatment.
Sarah Tasian, MD, of Children’s Hospital of Philadelphia and Kimberly Stegmaier, MD, of the Dana-Farber Cancer Institute won a 2-year grant totaling $250,000, which was funded with support from the Emily Whitehead Foundation.
Dr. Stegmaier and Dr. Tasian will test chimeric antigen receptor T cells targeting two or more neoantigens in preclinical models of childhood Down syndrome–associated acute lymphoblastic leukemia and Ph-like acute lymphoblastic leukemia.
Robert Vonderheide, MD, of the University of Pennsylvania Abramson Cancer Center in Philadelphia and Vinod Balachandran, MD, of Memorial Sloan Kettering Cancer Center in New York won a 2-year grant totaling $225,000.
Dr. Balachandran and Dr. Vonderheide will analyze short- and long-term pancreatic cancer survivors, patients with primary resected pancreatic cancers and patients with mKRAS lung and colon cancers to evaluate how mKRAS immunogenicity may affect outcomes.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Interplay of TP53, ESR2 may expand treatment options for some TNBCs
, an interaction that may have therapeutic implications for triple-negative disease, a new study suggests.
Up to 80% of triple-negative breast cancers (TNBCs) express ESR2, but its role has been hard to pin down because of conflicting data, according to the investigators, who were led by senior author Gokul M. Das, PhD, of the department of pharmacology and therapeutics, Center for Genetics and Pharmacology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Expression has been found to have both antiproliferative activity and proproliferative activity, depending on the experimental conditions.
Dr. Das and colleagues performed a series of in vitro assays in breast cancer cell lines and a clinical study of patients with basal-like TNBC from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohort (308 patients with TP53 mutational status determined by sequencing) and a Roswell cohort (46 patients with TP53 mutational status determined by immunohistochemistry).
Results showed that in vitro, ESR2 interaction with wild-type TP53 had proproliferative effects, whereas interaction of ESR2 with mutant TP53 had antiproliferative effects. The report is in the Journal of the National Cancer Institute.
In luminal breast cancer cell lines with wild-type TP53, compared with control, depleting ESR2 increased expression of the TP53 target genes CDKN1A and BBC3 (P = .003 and P = .003, respectively); in contrast, in those with mutant TP53, depleting ESR2 decreased their expression (P = .02 and P = .008). Opposite results were seen when ESR2 was instead overexpressed.
In cells with mutant TP53, tamoxifen treatment increased interaction of ESR2 with the mutant protein, ultimately leading to reactivation of TP73 and apoptosis.
Finally, among women with basal-like TNBC in the METABRIC cohort, high ESR2 expression was associated with better overall survival for those with mutant TP53 tumors (hazard ratio for death, 0.26) but a trend toward poorer overall survival for those with wild-type TP53 tumors (P = .05). Results were similar in the Roswell cohort.
“These findings suggest that ESR2-TP53 combination can be used to stratify TNBC into therapeutically actionable subgroups,” Dr. Das and coinvestigators proposed.
“Our data that treatment with tamoxifen can lead to sequestration of mutant TP53 away from TP73 and thereby reactivate tumor suppressor activities of TP73, provide for the first time, a strong rationale for suggesting that tamoxifen therapy could be beneficial to basal-type/TNBC patients expressing mutant TP53,” they concluded. “On the other hand, based on our data that ESR2 is proproliferative in the WT [wild-type] TP53 context, we predict that in TNBCs expressing WT TP53, tamoxifen or other agents that increase ESR2-WTP53 interaction may not only be ineffective as antitumor agents, but also may contribute to adverse outcome.”
The authors disclosed that they had no conflicts of interest. The study was supported by the several sources including the National Cancer Institute and Susan G. Komen for the Cure.
SOURCE: Mukhopadhyay UK et al. J Natl Cancer Inst. 2019 Apr 16. doi: 10.1093/jnci/djz051.
“The ability to selectively administer endocrine therapy should, in principle, lead to greater response rates,” according to an editorial by Sunil Badve, MBBS, FRCPath, and Yesim Gokmen-Polar, PhD (J Natl Cancer Inst. 2019 Apr 16. doi: 10.1093/jnci/djz052).
The study illustrates that “company matters” when it comes to the impact of markers and mutations in cancers, they maintain. “The intracellular environment is a complex milieu wherein changes in one player can have a dramatic impact on DNA, RNA and protein interactions. The players in the neighborhood could further affect cellular phenotype.
“Acknowledging these processes also provides a reality check for those of us involved in precision medicine, wherein treatments are being prescribed based on the presence of single gene mutations ... ” Dr. Badve and Dr. Gokmen-Polar noted. “The cooperativity and interactions of cellular networks may, to a large extent, determine the prognostic and predictive utility of mutations in patients.”
The new study “is a good step in this direction and provides compelling reasons to understand the combinatorial impact to determine clinically actionable strategies and solutions,” they concluded.
Dr. Badve and Dr. Gokmen-Polar are in the department of pathology and laboratory medicine at Indiana University, Indianapolis.
“The ability to selectively administer endocrine therapy should, in principle, lead to greater response rates,” according to an editorial by Sunil Badve, MBBS, FRCPath, and Yesim Gokmen-Polar, PhD (J Natl Cancer Inst. 2019 Apr 16. doi: 10.1093/jnci/djz052).
The study illustrates that “company matters” when it comes to the impact of markers and mutations in cancers, they maintain. “The intracellular environment is a complex milieu wherein changes in one player can have a dramatic impact on DNA, RNA and protein interactions. The players in the neighborhood could further affect cellular phenotype.
“Acknowledging these processes also provides a reality check for those of us involved in precision medicine, wherein treatments are being prescribed based on the presence of single gene mutations ... ” Dr. Badve and Dr. Gokmen-Polar noted. “The cooperativity and interactions of cellular networks may, to a large extent, determine the prognostic and predictive utility of mutations in patients.”
The new study “is a good step in this direction and provides compelling reasons to understand the combinatorial impact to determine clinically actionable strategies and solutions,” they concluded.
Dr. Badve and Dr. Gokmen-Polar are in the department of pathology and laboratory medicine at Indiana University, Indianapolis.
“The ability to selectively administer endocrine therapy should, in principle, lead to greater response rates,” according to an editorial by Sunil Badve, MBBS, FRCPath, and Yesim Gokmen-Polar, PhD (J Natl Cancer Inst. 2019 Apr 16. doi: 10.1093/jnci/djz052).
The study illustrates that “company matters” when it comes to the impact of markers and mutations in cancers, they maintain. “The intracellular environment is a complex milieu wherein changes in one player can have a dramatic impact on DNA, RNA and protein interactions. The players in the neighborhood could further affect cellular phenotype.
“Acknowledging these processes also provides a reality check for those of us involved in precision medicine, wherein treatments are being prescribed based on the presence of single gene mutations ... ” Dr. Badve and Dr. Gokmen-Polar noted. “The cooperativity and interactions of cellular networks may, to a large extent, determine the prognostic and predictive utility of mutations in patients.”
The new study “is a good step in this direction and provides compelling reasons to understand the combinatorial impact to determine clinically actionable strategies and solutions,” they concluded.
Dr. Badve and Dr. Gokmen-Polar are in the department of pathology and laboratory medicine at Indiana University, Indianapolis.
, an interaction that may have therapeutic implications for triple-negative disease, a new study suggests.
Up to 80% of triple-negative breast cancers (TNBCs) express ESR2, but its role has been hard to pin down because of conflicting data, according to the investigators, who were led by senior author Gokul M. Das, PhD, of the department of pharmacology and therapeutics, Center for Genetics and Pharmacology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Expression has been found to have both antiproliferative activity and proproliferative activity, depending on the experimental conditions.
Dr. Das and colleagues performed a series of in vitro assays in breast cancer cell lines and a clinical study of patients with basal-like TNBC from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohort (308 patients with TP53 mutational status determined by sequencing) and a Roswell cohort (46 patients with TP53 mutational status determined by immunohistochemistry).
Results showed that in vitro, ESR2 interaction with wild-type TP53 had proproliferative effects, whereas interaction of ESR2 with mutant TP53 had antiproliferative effects. The report is in the Journal of the National Cancer Institute.
In luminal breast cancer cell lines with wild-type TP53, compared with control, depleting ESR2 increased expression of the TP53 target genes CDKN1A and BBC3 (P = .003 and P = .003, respectively); in contrast, in those with mutant TP53, depleting ESR2 decreased their expression (P = .02 and P = .008). Opposite results were seen when ESR2 was instead overexpressed.
In cells with mutant TP53, tamoxifen treatment increased interaction of ESR2 with the mutant protein, ultimately leading to reactivation of TP73 and apoptosis.
Finally, among women with basal-like TNBC in the METABRIC cohort, high ESR2 expression was associated with better overall survival for those with mutant TP53 tumors (hazard ratio for death, 0.26) but a trend toward poorer overall survival for those with wild-type TP53 tumors (P = .05). Results were similar in the Roswell cohort.
“These findings suggest that ESR2-TP53 combination can be used to stratify TNBC into therapeutically actionable subgroups,” Dr. Das and coinvestigators proposed.
“Our data that treatment with tamoxifen can lead to sequestration of mutant TP53 away from TP73 and thereby reactivate tumor suppressor activities of TP73, provide for the first time, a strong rationale for suggesting that tamoxifen therapy could be beneficial to basal-type/TNBC patients expressing mutant TP53,” they concluded. “On the other hand, based on our data that ESR2 is proproliferative in the WT [wild-type] TP53 context, we predict that in TNBCs expressing WT TP53, tamoxifen or other agents that increase ESR2-WTP53 interaction may not only be ineffective as antitumor agents, but also may contribute to adverse outcome.”
The authors disclosed that they had no conflicts of interest. The study was supported by the several sources including the National Cancer Institute and Susan G. Komen for the Cure.
SOURCE: Mukhopadhyay UK et al. J Natl Cancer Inst. 2019 Apr 16. doi: 10.1093/jnci/djz051.
, an interaction that may have therapeutic implications for triple-negative disease, a new study suggests.
Up to 80% of triple-negative breast cancers (TNBCs) express ESR2, but its role has been hard to pin down because of conflicting data, according to the investigators, who were led by senior author Gokul M. Das, PhD, of the department of pharmacology and therapeutics, Center for Genetics and Pharmacology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Expression has been found to have both antiproliferative activity and proproliferative activity, depending on the experimental conditions.
Dr. Das and colleagues performed a series of in vitro assays in breast cancer cell lines and a clinical study of patients with basal-like TNBC from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohort (308 patients with TP53 mutational status determined by sequencing) and a Roswell cohort (46 patients with TP53 mutational status determined by immunohistochemistry).
Results showed that in vitro, ESR2 interaction with wild-type TP53 had proproliferative effects, whereas interaction of ESR2 with mutant TP53 had antiproliferative effects. The report is in the Journal of the National Cancer Institute.
In luminal breast cancer cell lines with wild-type TP53, compared with control, depleting ESR2 increased expression of the TP53 target genes CDKN1A and BBC3 (P = .003 and P = .003, respectively); in contrast, in those with mutant TP53, depleting ESR2 decreased their expression (P = .02 and P = .008). Opposite results were seen when ESR2 was instead overexpressed.
In cells with mutant TP53, tamoxifen treatment increased interaction of ESR2 with the mutant protein, ultimately leading to reactivation of TP73 and apoptosis.
Finally, among women with basal-like TNBC in the METABRIC cohort, high ESR2 expression was associated with better overall survival for those with mutant TP53 tumors (hazard ratio for death, 0.26) but a trend toward poorer overall survival for those with wild-type TP53 tumors (P = .05). Results were similar in the Roswell cohort.
“These findings suggest that ESR2-TP53 combination can be used to stratify TNBC into therapeutically actionable subgroups,” Dr. Das and coinvestigators proposed.
“Our data that treatment with tamoxifen can lead to sequestration of mutant TP53 away from TP73 and thereby reactivate tumor suppressor activities of TP73, provide for the first time, a strong rationale for suggesting that tamoxifen therapy could be beneficial to basal-type/TNBC patients expressing mutant TP53,” they concluded. “On the other hand, based on our data that ESR2 is proproliferative in the WT [wild-type] TP53 context, we predict that in TNBCs expressing WT TP53, tamoxifen or other agents that increase ESR2-WTP53 interaction may not only be ineffective as antitumor agents, but also may contribute to adverse outcome.”
The authors disclosed that they had no conflicts of interest. The study was supported by the several sources including the National Cancer Institute and Susan G. Komen for the Cure.
SOURCE: Mukhopadhyay UK et al. J Natl Cancer Inst. 2019 Apr 16. doi: 10.1093/jnci/djz051.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: In triple-negative breast cancer, TP53 mutational status influences the prognostic impact of estrogen receptor-beta (ESR2) expression.
Major finding: High ESR2 expression was associated with better overall survival among women with mutant TP53 tumors (hazard ratio for death, 0.26) but a trend toward poorer overall survival among women with wild-type TP53 tumors (P = .05).
Study details: In vitro study and retrospective cohort study of 354 women with basal-like triple-negative breast cancer.
Disclosures: The authors disclosed that they had no conflicts of interest. The study was supported by the several sources including the National Cancer Institute and Susan G. Komen for the Cure.
Source: Mukhopadhyay UK et al. J Natl Cancer Inst 2019 Apr 16 doi: 10.1093/jnci/djz051.
Depressive symptoms are associated with stroke risk
PHILADELPHIA – according to preliminary data from a prospective cohort study presented at the annual meeting of the American Academy of Neurology.
Prior research has found that depression may increase the likelihood of hypertension, cardiac morbidity and mortality, and stroke mortality. How depression affects the risk of incident stroke, however, “is underexplored, especially among Latinos and African Americans,” said study author Marialaura Simonetto, MD, of the University of Miami and associates.
To assess the relationship between depressive symptoms and risk of incident ischemic stroke, the researchers analyzed data from more than 1,100 participants in the MRI substudy of the Northern Manhattan Study. The cohort includes older adults who were clinically stroke free at baseline.
Researchers assessed depressive symptoms at baseline using the Center for Epidemiological Studies–Depression Scale (CES-D). Scores range from 0-60, and they considered CES-D scores of 16 or greater to be elevated.
The investigators used Cox proportional hazards models to estimate hazard ratios of incident ischemic stroke after adjusting for age, sex, race, ethnicity, years of education, smoking, physical activity, alcohol consumption, diabetes, and hypertension.
In all, 1,104 participants were included in the analysis. Participants had an average age of 70 years, 61% were women, and 69% were Hispanic. At baseline, 198 participants (18%) had elevated depressive symptoms.
During 14 years of follow-up, 101 participants had incident strokes, 87 of which were ischemic strokes. In adjusted models, participants with elevated depressive symptoms had a significantly greater risk of ischemic stroke (HR, 1.75; 95% confidence interval, 1.06-2.88). “Every 5-point increase in CES-D score conferred a 12% greater risk of ischemic stroke,” the researchers reported.
“Depression is common and often goes untreated,” Dr. Simonetto said in a news release. “If people with depression are at elevated risk of stroke, early detection and treatment will be even more important.”
The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Simonetto reported no disclosures. Coauthors disclosed personal compensation and research support from pharmaceutical and medical device companies.
SOURCE: Simonetto M et al. AAN 2019, Abstract S1.003.
PHILADELPHIA – according to preliminary data from a prospective cohort study presented at the annual meeting of the American Academy of Neurology.
Prior research has found that depression may increase the likelihood of hypertension, cardiac morbidity and mortality, and stroke mortality. How depression affects the risk of incident stroke, however, “is underexplored, especially among Latinos and African Americans,” said study author Marialaura Simonetto, MD, of the University of Miami and associates.
To assess the relationship between depressive symptoms and risk of incident ischemic stroke, the researchers analyzed data from more than 1,100 participants in the MRI substudy of the Northern Manhattan Study. The cohort includes older adults who were clinically stroke free at baseline.
Researchers assessed depressive symptoms at baseline using the Center for Epidemiological Studies–Depression Scale (CES-D). Scores range from 0-60, and they considered CES-D scores of 16 or greater to be elevated.
The investigators used Cox proportional hazards models to estimate hazard ratios of incident ischemic stroke after adjusting for age, sex, race, ethnicity, years of education, smoking, physical activity, alcohol consumption, diabetes, and hypertension.
In all, 1,104 participants were included in the analysis. Participants had an average age of 70 years, 61% were women, and 69% were Hispanic. At baseline, 198 participants (18%) had elevated depressive symptoms.
During 14 years of follow-up, 101 participants had incident strokes, 87 of which were ischemic strokes. In adjusted models, participants with elevated depressive symptoms had a significantly greater risk of ischemic stroke (HR, 1.75; 95% confidence interval, 1.06-2.88). “Every 5-point increase in CES-D score conferred a 12% greater risk of ischemic stroke,” the researchers reported.
“Depression is common and often goes untreated,” Dr. Simonetto said in a news release. “If people with depression are at elevated risk of stroke, early detection and treatment will be even more important.”
The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Simonetto reported no disclosures. Coauthors disclosed personal compensation and research support from pharmaceutical and medical device companies.
SOURCE: Simonetto M et al. AAN 2019, Abstract S1.003.
PHILADELPHIA – according to preliminary data from a prospective cohort study presented at the annual meeting of the American Academy of Neurology.
Prior research has found that depression may increase the likelihood of hypertension, cardiac morbidity and mortality, and stroke mortality. How depression affects the risk of incident stroke, however, “is underexplored, especially among Latinos and African Americans,” said study author Marialaura Simonetto, MD, of the University of Miami and associates.
To assess the relationship between depressive symptoms and risk of incident ischemic stroke, the researchers analyzed data from more than 1,100 participants in the MRI substudy of the Northern Manhattan Study. The cohort includes older adults who were clinically stroke free at baseline.
Researchers assessed depressive symptoms at baseline using the Center for Epidemiological Studies–Depression Scale (CES-D). Scores range from 0-60, and they considered CES-D scores of 16 or greater to be elevated.
The investigators used Cox proportional hazards models to estimate hazard ratios of incident ischemic stroke after adjusting for age, sex, race, ethnicity, years of education, smoking, physical activity, alcohol consumption, diabetes, and hypertension.
In all, 1,104 participants were included in the analysis. Participants had an average age of 70 years, 61% were women, and 69% were Hispanic. At baseline, 198 participants (18%) had elevated depressive symptoms.
During 14 years of follow-up, 101 participants had incident strokes, 87 of which were ischemic strokes. In adjusted models, participants with elevated depressive symptoms had a significantly greater risk of ischemic stroke (HR, 1.75; 95% confidence interval, 1.06-2.88). “Every 5-point increase in CES-D score conferred a 12% greater risk of ischemic stroke,” the researchers reported.
“Depression is common and often goes untreated,” Dr. Simonetto said in a news release. “If people with depression are at elevated risk of stroke, early detection and treatment will be even more important.”
The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Simonetto reported no disclosures. Coauthors disclosed personal compensation and research support from pharmaceutical and medical device companies.
SOURCE: Simonetto M et al. AAN 2019, Abstract S1.003.
REPORTING FROM AAN 2019
Direct pharmacy dispensing of naloxone linked to drop in fatal overdoses
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
FROM JAMA Internal Medicine
Key clinical point: State laws granting pharmacists direct authority to dispense naloxone were linked to significant drops in opioid-related fatal overdoses.
Major finding: The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Study details: Analysis of naloxone distribution data and opioid-related mortality data from 2005 to 2016 for all 50 states and the District of Columbia.
Disclosures: Study authors reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
Source: Abouk R, et al. JAMA Intern Med. 2019 May 6.














