User login
Coalescing Papules on Bilateral Mastectomy Scars
The Diagnosis: Scar Sarcoidosis
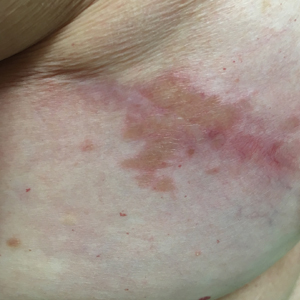
Although scars on both breasts were involved, the decision was made to biopsy the right breast because the patient reported more pain on the left breast. Biopsy showed noncaseating granulomas consistent with scar sarcoidosis (Figure). Additional screening tests were performed to evaluate for any systemic involvement of sarcoidosis, including a complete blood cell count, comprehensive metabolic panel, angiotensin-converting enzyme level, tuberculosis serology screening, electrocardiogram, chest radiograph, and pulmonary function tests. She also was referred to rheumatology and ophthalmology for consultation. The results of all screenings were within reference range, and no sign of systemic sarcoidosis was found. She was treated with hydrocortisone ointment 2.5% for several weeks without notable improvement. She elected not to pursue any additional treatment and to monitor the symptoms with close follow-up only. One year after the initial visit, the skin lesions spontaneously and notably improved.
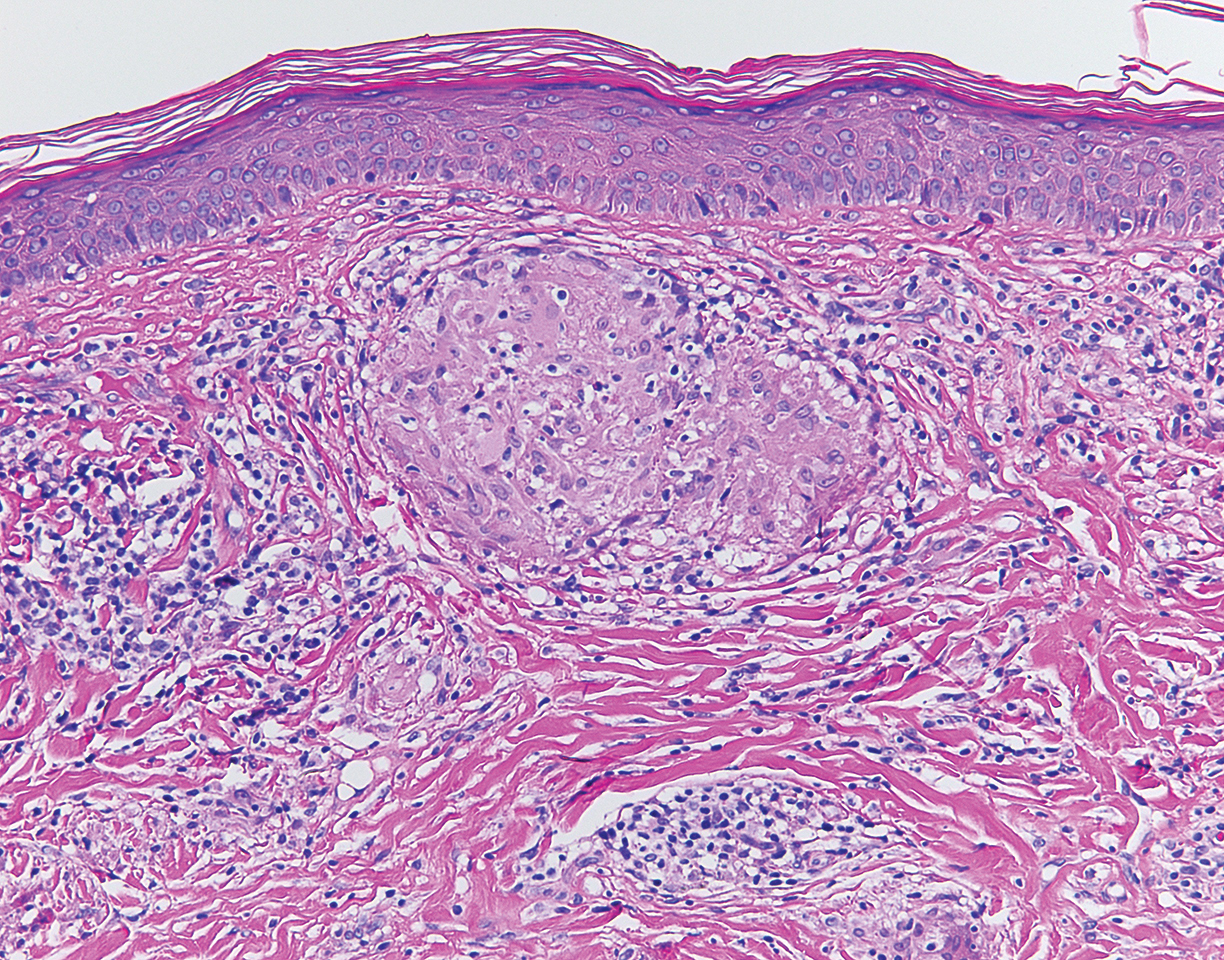
Sarcoidosis is a systemic granulomatous disorder of unknown etiology that most commonly affects the lungs. It also can involve the lymph nodes, liver, spleen, bones, gastrointestinal tract, eyes, and skin. Cutaneous sarcoidosis has been documented in the literature since the late 1800s and occurs in up to one-third of sarcoid patients.1 Cutaneous lesions developing within a preexisting scar is a well-known variant, occurring in 29% of patients with cutaneous sarcoidosis in one clinical study (N=818).2 There have been many reports describing scar sarcoidosis, with its development at prior sites of surgery, trauma, acne, or venipuncture.3 Other case reports have described variants of scar sarcoidosis developing at sites of hyaluronic acid injection, laser surgery, ritual scarification, tattoos, and desensitization injections, as well as prior herpes zoster infections.4-9
Cutaneous sarcoidosis has a wide range of clinical presentations. Lesions can be described as specific or nonspecific. Specific lesions demonstrate the typical sarcoid granuloma on histology and more often are seen in chronic disease, while nonspecific lesions more often are seen in acute disease.3,10 Scar sarcoidosis is an example of a specific lesion in which old scars become infiltrated with noncaseating granulomas. The granulomas typically are in the superficial dermis but may involve the full thickness of the dermis, extending into the subcutaneous tissue.11 The cause of granulomas developing in scars is unknown. Prior contamination of the scar with foreign material, possibly at the time of the trauma, is a possible underlying cause.12
Typical scar sarcoidosis presents as swollen, erythematous, indurated lesions with a purple-red hue that may become brown.3,12 Tenderness or pruritus also may be present.13 Interestingly, our patient's scar sarcoidosis presented with a yellow hue at both mastectomy sites.
Diagnosing scar sarcoidosis can be challenging. Patients are diagnosed with sarcoidosis when a compatible clinical or radiologic picture is present along with histologic evidence of a noncaseating granuloma and other potential causes are excluded.11 The differential includes an infectious etiology, other types of granulomatous dermatitis, hypertrophic scar, keloid, or foreign body granuloma.
Scar sarcoidosis can be isolated in occurrence. It also can precede or occur concomitantly or during a relapse of systemic sarcoidosis.10 Most commonly, patients with scar sarcoidosis also have systemic manifestations of sarcoidosis, and changing scars may be an indicator of disease exacerbation or relapse.10 For patients who only demonstrate specific skin lesions of cutaneous sarcoidosis, approximately 30% develop systemic involvement later in life.3 For this reason, close monitoring and regular follow-up are necessary.
Treatment of scar sarcoidosis is dependent on the extent of the disease and presence of systemic sarcoidosis. Topical and systemic corticosteroids, hydroxychloroquine, chloroquine phosphate, and methotrexate all have been shown to be helpful in treating cutaneous sarcoidosis.3 For scar sarcoidosis that is limited to only the scar site, as seen in our case, monitoring and close follow-up is acceptable. Topical steroids can be prescribed for symptomatic relief. Scar sarcoidosis can resolve slowly and spontaneously over time.10 Our patient notably improved 1 year after the initial presentation without treatment.
Scar sarcoidosis is a well-documented variant of cutaneous sarcoidosis that can have important implications for diagnosing systemic sarcoidosis. Although there are typical lesions that represent scar sarcoidosis, it is important to have a high degree of suspicion with any changing scar. Once diagnosed through biopsy, a thorough investigation for systemic signs of sarcoidosis needs to be performed to guide treatment.
- Bolognia JL, Jorizzo JL, Shaffer JV, eds. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis: relationship to systemic disease. Arch Dermatol. 1997;133:882-888.
- Dal Sacco D, Cozzani E, Parodi A, et al. Scar sarcoidosis after hyaluronic acid injection. Int J Dermatol. 2005;44:411-412.
- Kormeili T, Neel V, Moy RL. Cutaneous sarcoidosis at sites of previous laser surgery. Cutis. 2004;73:53-55.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- James WD, Elston DM, Berger TG, et al. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
- Healsmith MF, Hutchinson PE. The development of scar sarcoidosis at the site of desensitization injections. Clin Exp Dermatol. 1992;17:369-370.
- Singal A, Vij A, Pandhi D. Post herpes-zoster scar sarcoidosis with pulmonary involvement. Indian Dermatol Online J. 2014;5:77-79.
- Chudomirova K, Velichkova L, Anavi B, et al. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venereol. 2003;17:360-361.
- Selim A, Ehrsam E, Atassi MB, et al. Scar sarcoidosis: a case report and brief review. Cutis. 2006;78:418-422.
- Singal A, Thami GP, Goraya JS. Scar sarcoidosis in childhood: case report and review of the literature. Clin Exp Dermatol. 2005;30:244-246.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
The Diagnosis: Scar Sarcoidosis
Although scars on both breasts were involved, the decision was made to biopsy the right breast because the patient reported more pain on the left breast. Biopsy showed noncaseating granulomas consistent with scar sarcoidosis (Figure). Additional screening tests were performed to evaluate for any systemic involvement of sarcoidosis, including a complete blood cell count, comprehensive metabolic panel, angiotensin-converting enzyme level, tuberculosis serology screening, electrocardiogram, chest radiograph, and pulmonary function tests. She also was referred to rheumatology and ophthalmology for consultation. The results of all screenings were within reference range, and no sign of systemic sarcoidosis was found. She was treated with hydrocortisone ointment 2.5% for several weeks without notable improvement. She elected not to pursue any additional treatment and to monitor the symptoms with close follow-up only. One year after the initial visit, the skin lesions spontaneously and notably improved.

Sarcoidosis is a systemic granulomatous disorder of unknown etiology that most commonly affects the lungs. It also can involve the lymph nodes, liver, spleen, bones, gastrointestinal tract, eyes, and skin. Cutaneous sarcoidosis has been documented in the literature since the late 1800s and occurs in up to one-third of sarcoid patients.1 Cutaneous lesions developing within a preexisting scar is a well-known variant, occurring in 29% of patients with cutaneous sarcoidosis in one clinical study (N=818).2 There have been many reports describing scar sarcoidosis, with its development at prior sites of surgery, trauma, acne, or venipuncture.3 Other case reports have described variants of scar sarcoidosis developing at sites of hyaluronic acid injection, laser surgery, ritual scarification, tattoos, and desensitization injections, as well as prior herpes zoster infections.4-9
Cutaneous sarcoidosis has a wide range of clinical presentations. Lesions can be described as specific or nonspecific. Specific lesions demonstrate the typical sarcoid granuloma on histology and more often are seen in chronic disease, while nonspecific lesions more often are seen in acute disease.3,10 Scar sarcoidosis is an example of a specific lesion in which old scars become infiltrated with noncaseating granulomas. The granulomas typically are in the superficial dermis but may involve the full thickness of the dermis, extending into the subcutaneous tissue.11 The cause of granulomas developing in scars is unknown. Prior contamination of the scar with foreign material, possibly at the time of the trauma, is a possible underlying cause.12
Typical scar sarcoidosis presents as swollen, erythematous, indurated lesions with a purple-red hue that may become brown.3,12 Tenderness or pruritus also may be present.13 Interestingly, our patient's scar sarcoidosis presented with a yellow hue at both mastectomy sites.
Diagnosing scar sarcoidosis can be challenging. Patients are diagnosed with sarcoidosis when a compatible clinical or radiologic picture is present along with histologic evidence of a noncaseating granuloma and other potential causes are excluded.11 The differential includes an infectious etiology, other types of granulomatous dermatitis, hypertrophic scar, keloid, or foreign body granuloma.
Scar sarcoidosis can be isolated in occurrence. It also can precede or occur concomitantly or during a relapse of systemic sarcoidosis.10 Most commonly, patients with scar sarcoidosis also have systemic manifestations of sarcoidosis, and changing scars may be an indicator of disease exacerbation or relapse.10 For patients who only demonstrate specific skin lesions of cutaneous sarcoidosis, approximately 30% develop systemic involvement later in life.3 For this reason, close monitoring and regular follow-up are necessary.
Treatment of scar sarcoidosis is dependent on the extent of the disease and presence of systemic sarcoidosis. Topical and systemic corticosteroids, hydroxychloroquine, chloroquine phosphate, and methotrexate all have been shown to be helpful in treating cutaneous sarcoidosis.3 For scar sarcoidosis that is limited to only the scar site, as seen in our case, monitoring and close follow-up is acceptable. Topical steroids can be prescribed for symptomatic relief. Scar sarcoidosis can resolve slowly and spontaneously over time.10 Our patient notably improved 1 year after the initial presentation without treatment.
Scar sarcoidosis is a well-documented variant of cutaneous sarcoidosis that can have important implications for diagnosing systemic sarcoidosis. Although there are typical lesions that represent scar sarcoidosis, it is important to have a high degree of suspicion with any changing scar. Once diagnosed through biopsy, a thorough investigation for systemic signs of sarcoidosis needs to be performed to guide treatment.
The Diagnosis: Scar Sarcoidosis
Although scars on both breasts were involved, the decision was made to biopsy the right breast because the patient reported more pain on the left breast. Biopsy showed noncaseating granulomas consistent with scar sarcoidosis (Figure). Additional screening tests were performed to evaluate for any systemic involvement of sarcoidosis, including a complete blood cell count, comprehensive metabolic panel, angiotensin-converting enzyme level, tuberculosis serology screening, electrocardiogram, chest radiograph, and pulmonary function tests. She also was referred to rheumatology and ophthalmology for consultation. The results of all screenings were within reference range, and no sign of systemic sarcoidosis was found. She was treated with hydrocortisone ointment 2.5% for several weeks without notable improvement. She elected not to pursue any additional treatment and to monitor the symptoms with close follow-up only. One year after the initial visit, the skin lesions spontaneously and notably improved.

Sarcoidosis is a systemic granulomatous disorder of unknown etiology that most commonly affects the lungs. It also can involve the lymph nodes, liver, spleen, bones, gastrointestinal tract, eyes, and skin. Cutaneous sarcoidosis has been documented in the literature since the late 1800s and occurs in up to one-third of sarcoid patients.1 Cutaneous lesions developing within a preexisting scar is a well-known variant, occurring in 29% of patients with cutaneous sarcoidosis in one clinical study (N=818).2 There have been many reports describing scar sarcoidosis, with its development at prior sites of surgery, trauma, acne, or venipuncture.3 Other case reports have described variants of scar sarcoidosis developing at sites of hyaluronic acid injection, laser surgery, ritual scarification, tattoos, and desensitization injections, as well as prior herpes zoster infections.4-9
Cutaneous sarcoidosis has a wide range of clinical presentations. Lesions can be described as specific or nonspecific. Specific lesions demonstrate the typical sarcoid granuloma on histology and more often are seen in chronic disease, while nonspecific lesions more often are seen in acute disease.3,10 Scar sarcoidosis is an example of a specific lesion in which old scars become infiltrated with noncaseating granulomas. The granulomas typically are in the superficial dermis but may involve the full thickness of the dermis, extending into the subcutaneous tissue.11 The cause of granulomas developing in scars is unknown. Prior contamination of the scar with foreign material, possibly at the time of the trauma, is a possible underlying cause.12
Typical scar sarcoidosis presents as swollen, erythematous, indurated lesions with a purple-red hue that may become brown.3,12 Tenderness or pruritus also may be present.13 Interestingly, our patient's scar sarcoidosis presented with a yellow hue at both mastectomy sites.
Diagnosing scar sarcoidosis can be challenging. Patients are diagnosed with sarcoidosis when a compatible clinical or radiologic picture is present along with histologic evidence of a noncaseating granuloma and other potential causes are excluded.11 The differential includes an infectious etiology, other types of granulomatous dermatitis, hypertrophic scar, keloid, or foreign body granuloma.
Scar sarcoidosis can be isolated in occurrence. It also can precede or occur concomitantly or during a relapse of systemic sarcoidosis.10 Most commonly, patients with scar sarcoidosis also have systemic manifestations of sarcoidosis, and changing scars may be an indicator of disease exacerbation or relapse.10 For patients who only demonstrate specific skin lesions of cutaneous sarcoidosis, approximately 30% develop systemic involvement later in life.3 For this reason, close monitoring and regular follow-up are necessary.
Treatment of scar sarcoidosis is dependent on the extent of the disease and presence of systemic sarcoidosis. Topical and systemic corticosteroids, hydroxychloroquine, chloroquine phosphate, and methotrexate all have been shown to be helpful in treating cutaneous sarcoidosis.3 For scar sarcoidosis that is limited to only the scar site, as seen in our case, monitoring and close follow-up is acceptable. Topical steroids can be prescribed for symptomatic relief. Scar sarcoidosis can resolve slowly and spontaneously over time.10 Our patient notably improved 1 year after the initial presentation without treatment.
Scar sarcoidosis is a well-documented variant of cutaneous sarcoidosis that can have important implications for diagnosing systemic sarcoidosis. Although there are typical lesions that represent scar sarcoidosis, it is important to have a high degree of suspicion with any changing scar. Once diagnosed through biopsy, a thorough investigation for systemic signs of sarcoidosis needs to be performed to guide treatment.
- Bolognia JL, Jorizzo JL, Shaffer JV, eds. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis: relationship to systemic disease. Arch Dermatol. 1997;133:882-888.
- Dal Sacco D, Cozzani E, Parodi A, et al. Scar sarcoidosis after hyaluronic acid injection. Int J Dermatol. 2005;44:411-412.
- Kormeili T, Neel V, Moy RL. Cutaneous sarcoidosis at sites of previous laser surgery. Cutis. 2004;73:53-55.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- James WD, Elston DM, Berger TG, et al. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
- Healsmith MF, Hutchinson PE. The development of scar sarcoidosis at the site of desensitization injections. Clin Exp Dermatol. 1992;17:369-370.
- Singal A, Vij A, Pandhi D. Post herpes-zoster scar sarcoidosis with pulmonary involvement. Indian Dermatol Online J. 2014;5:77-79.
- Chudomirova K, Velichkova L, Anavi B, et al. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venereol. 2003;17:360-361.
- Selim A, Ehrsam E, Atassi MB, et al. Scar sarcoidosis: a case report and brief review. Cutis. 2006;78:418-422.
- Singal A, Thami GP, Goraya JS. Scar sarcoidosis in childhood: case report and review of the literature. Clin Exp Dermatol. 2005;30:244-246.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Bolognia JL, Jorizzo JL, Shaffer JV, eds. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
- Mañá J, Marcoval J, Graells J, et al. Cutaneous involvement in sarcoidosis: relationship to systemic disease. Arch Dermatol. 1997;133:882-888.
- Dal Sacco D, Cozzani E, Parodi A, et al. Scar sarcoidosis after hyaluronic acid injection. Int J Dermatol. 2005;44:411-412.
- Kormeili T, Neel V, Moy RL. Cutaneous sarcoidosis at sites of previous laser surgery. Cutis. 2004;73:53-55.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- James WD, Elston DM, Berger TG, et al. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
- Healsmith MF, Hutchinson PE. The development of scar sarcoidosis at the site of desensitization injections. Clin Exp Dermatol. 1992;17:369-370.
- Singal A, Vij A, Pandhi D. Post herpes-zoster scar sarcoidosis with pulmonary involvement. Indian Dermatol Online J. 2014;5:77-79.
- Chudomirova K, Velichkova L, Anavi B, et al. Recurrent sarcoidosis in skin scars accompanying systemic sarcoidosis. J Eur Acad Dermatol Venereol. 2003;17:360-361.
- Selim A, Ehrsam E, Atassi MB, et al. Scar sarcoidosis: a case report and brief review. Cutis. 2006;78:418-422.
- Singal A, Thami GP, Goraya JS. Scar sarcoidosis in childhood: case report and review of the literature. Clin Exp Dermatol. 2005;30:244-246.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
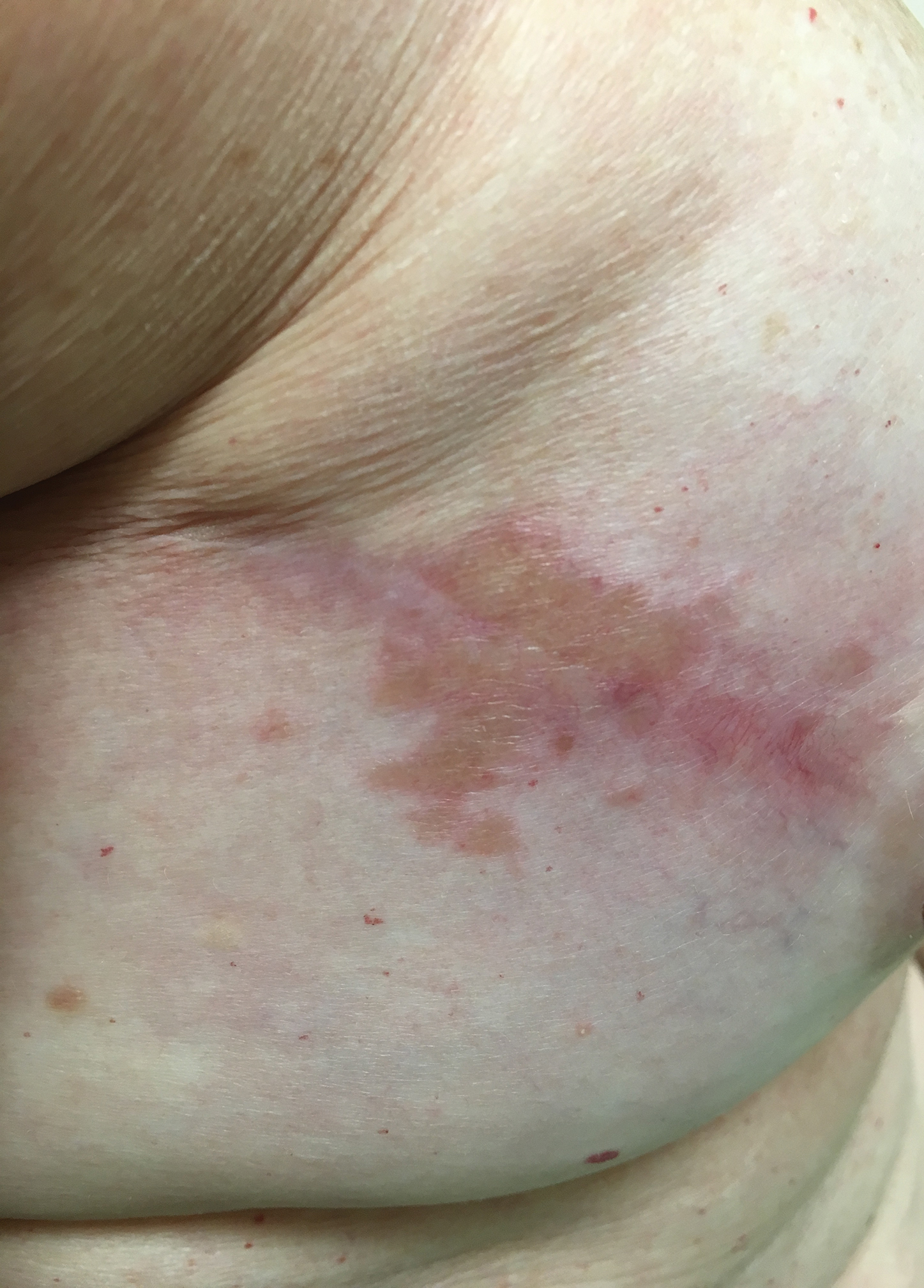
A 57-year-old woman with triple-negative ductal breast cancer presented with a mildly pruritic rash on bilateral mastectomy scars of 3 to 4 months' duration. More than a year prior to presentation, she was diagnosed with breast cancer and treated with a bilateral mastectomy and chemotherapy. On physical examination, faintly yellow, slightly indurated, coalescing papules with red rims were present on the bilateral mastectomy scars, with the scar on the left side appearing worse than the right. She previously had not sought treatment.
Patients describe significant impact of epilepsy on their lives
PHILADELPHIA – said Jacqueline French, MD, a professor at the Comprehensive Epilepsy Center at New York University.
“This underscores the need to consider these experiences, and potentially the stage of disease, when developing patient-reported outcome measures,” she said at the annual meeting of the American Academy of Neurology.
To describe the patient’s experience of living with epilepsy, including the occurrence of disease-related signs and symptoms and impact on daily life at different disease stages, Dr. French conducted qualitative, semistructured interviews with adults with focal epilepsy at the following stages: early (1 year or less since diagnosis), middle (1-5 years since diagnosis), and late (more than 5 years since diagnosis). The patients had varying seizure frequency and treatment experiences. They were asked to describe the symptoms and functional impact they had experienced related to epilepsy, and then to rate the degree to which each symptom and impact “bothered” them, using a disturbance rating scale from 0 (not at all) to 10 (extremely).
A total of 62 patients who were aged 18-60 years (mean age, 37 years; 73% female) were interviewed. In all, 19 of the patients had early-stage disease, 17 had middle-stage, and 26 had late-stage disease. Symptoms reported with the highest frequency and highest average disturbance (AD) ratings across all cohorts included twitching/tremors (80% of patients; AD, 5.3), confusion (78%; AD, 7.8), difficulty talking (75%; AD, 8.1), impaired/loss of consciousness (70%; AD, 6.8), stiffening (65%; AD, 5.4), déjà vu (62%; AD, 5.1), difficulty remembering (60%; AD, 8.5), and dizziness/light-headedness (58%; AD, 6.4).
The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (74%; AD, 7.1), limited ability to work and/or go to school (61%; AD, 6.7), limitations on leisure and social activities (58%; AD, 6.3), and memory loss (47%; AD, 8.4).
Dr French noted that, although disease experiences were similar among the cohorts, some heterogeneity across patient subgroups was observed.
Eisai sponsored the study.
PHILADELPHIA – said Jacqueline French, MD, a professor at the Comprehensive Epilepsy Center at New York University.
“This underscores the need to consider these experiences, and potentially the stage of disease, when developing patient-reported outcome measures,” she said at the annual meeting of the American Academy of Neurology.
To describe the patient’s experience of living with epilepsy, including the occurrence of disease-related signs and symptoms and impact on daily life at different disease stages, Dr. French conducted qualitative, semistructured interviews with adults with focal epilepsy at the following stages: early (1 year or less since diagnosis), middle (1-5 years since diagnosis), and late (more than 5 years since diagnosis). The patients had varying seizure frequency and treatment experiences. They were asked to describe the symptoms and functional impact they had experienced related to epilepsy, and then to rate the degree to which each symptom and impact “bothered” them, using a disturbance rating scale from 0 (not at all) to 10 (extremely).
A total of 62 patients who were aged 18-60 years (mean age, 37 years; 73% female) were interviewed. In all, 19 of the patients had early-stage disease, 17 had middle-stage, and 26 had late-stage disease. Symptoms reported with the highest frequency and highest average disturbance (AD) ratings across all cohorts included twitching/tremors (80% of patients; AD, 5.3), confusion (78%; AD, 7.8), difficulty talking (75%; AD, 8.1), impaired/loss of consciousness (70%; AD, 6.8), stiffening (65%; AD, 5.4), déjà vu (62%; AD, 5.1), difficulty remembering (60%; AD, 8.5), and dizziness/light-headedness (58%; AD, 6.4).
The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (74%; AD, 7.1), limited ability to work and/or go to school (61%; AD, 6.7), limitations on leisure and social activities (58%; AD, 6.3), and memory loss (47%; AD, 8.4).
Dr French noted that, although disease experiences were similar among the cohorts, some heterogeneity across patient subgroups was observed.
Eisai sponsored the study.
PHILADELPHIA – said Jacqueline French, MD, a professor at the Comprehensive Epilepsy Center at New York University.
“This underscores the need to consider these experiences, and potentially the stage of disease, when developing patient-reported outcome measures,” she said at the annual meeting of the American Academy of Neurology.
To describe the patient’s experience of living with epilepsy, including the occurrence of disease-related signs and symptoms and impact on daily life at different disease stages, Dr. French conducted qualitative, semistructured interviews with adults with focal epilepsy at the following stages: early (1 year or less since diagnosis), middle (1-5 years since diagnosis), and late (more than 5 years since diagnosis). The patients had varying seizure frequency and treatment experiences. They were asked to describe the symptoms and functional impact they had experienced related to epilepsy, and then to rate the degree to which each symptom and impact “bothered” them, using a disturbance rating scale from 0 (not at all) to 10 (extremely).
A total of 62 patients who were aged 18-60 years (mean age, 37 years; 73% female) were interviewed. In all, 19 of the patients had early-stage disease, 17 had middle-stage, and 26 had late-stage disease. Symptoms reported with the highest frequency and highest average disturbance (AD) ratings across all cohorts included twitching/tremors (80% of patients; AD, 5.3), confusion (78%; AD, 7.8), difficulty talking (75%; AD, 8.1), impaired/loss of consciousness (70%; AD, 6.8), stiffening (65%; AD, 5.4), déjà vu (62%; AD, 5.1), difficulty remembering (60%; AD, 8.5), and dizziness/light-headedness (58%; AD, 6.4).
The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (74%; AD, 7.1), limited ability to work and/or go to school (61%; AD, 6.7), limitations on leisure and social activities (58%; AD, 6.3), and memory loss (47%; AD, 8.4).
Dr French noted that, although disease experiences were similar among the cohorts, some heterogeneity across patient subgroups was observed.
Eisai sponsored the study.
REPORTING FROM AAN 2019
Key clinical point: Adults with focal epilepsy report a range of high-disturbance symptoms and impacts on daily life.
Major finding: The high-frequency/high-disturbance daily impact of epilepsy included the inability to drive (reported by 74% of respondents), limited ability to work and/or go to school (61%), limitations on leisure and social activities (58%), and memory loss (47%).
Study details: Qualitative, semistructured interviews with 62 adults with focal epilepsy at different stages of illness: early, middle, and late.
Disclosures: Eisai sponsored the study.
Physical activity slows cognitive decline in patients with Parkinson’s disease
PHILADELPHIA – according to Sneha Mantri, MD, of Duke University in Durham, N.C., and colleagues, who presented the results of their study at the annual meeting of the American Academy of Neurology.
Physical activity is an important component of the management of Parkinson’s disease and is shown to mitigate cognitive decline among patients with moderate disease, said Dr. Mantri and colleagues. “Exercise levels in de novo and early disease may influence risk of future cognitive decline; early disease also presents an opportunity for early intervention and possible disease modification,” Dr. Mantri said.
Physical activity levels in early disease are known to be low, but the effects of activity on cognition are currently unclear. To assess the relationship between physical activity and cognition, Dr. Mantri and colleagues examined patients with Parkinson’s disease who were enrolled in the prospective Parkinson’s Progression Markers Initiative (PPMI) cohort. At annual study visits, participants completed the Physical Activity Scale for the Elderly (PASE), a validated self-reported questionnaire assessing household, leisure, and work activities over the previous 7 days. The researchers used a linear mixed-effects model to compare rates of change in the Montreal Cognitive Assessment (MoCA) according to PASE scores; covariates included age, sex, Unified Parkinson’s Disease Rating Scale (UPDRS) part III score, and baseline MoCA.
A total of 379 patients completed at least one PASE questionnaire over the course of the study. PASE scores in this cohort have been previously described (Mantri S et al. J Park Dis. 2018;8[1]:107-11). Although overall rates of cognitive decline are known to be modest in this early cohort, PASE over time has a significant effect on MoCA during follow-up (P = 0.02) which suggest that higher levels of activity throughout disease are associated with better cognitive performance.
Dr. Mantri had nothing to disclose. Among her coauthors, Dr. Tropea received personal compensation from Genzyme and Medtronics and research support from Sanofi. Dr. Morley had nothing to disclose.
SOURCE: Mantri S et al. AAN 2019, Abstract P2.8-021.
PHILADELPHIA – according to Sneha Mantri, MD, of Duke University in Durham, N.C., and colleagues, who presented the results of their study at the annual meeting of the American Academy of Neurology.
Physical activity is an important component of the management of Parkinson’s disease and is shown to mitigate cognitive decline among patients with moderate disease, said Dr. Mantri and colleagues. “Exercise levels in de novo and early disease may influence risk of future cognitive decline; early disease also presents an opportunity for early intervention and possible disease modification,” Dr. Mantri said.
Physical activity levels in early disease are known to be low, but the effects of activity on cognition are currently unclear. To assess the relationship between physical activity and cognition, Dr. Mantri and colleagues examined patients with Parkinson’s disease who were enrolled in the prospective Parkinson’s Progression Markers Initiative (PPMI) cohort. At annual study visits, participants completed the Physical Activity Scale for the Elderly (PASE), a validated self-reported questionnaire assessing household, leisure, and work activities over the previous 7 days. The researchers used a linear mixed-effects model to compare rates of change in the Montreal Cognitive Assessment (MoCA) according to PASE scores; covariates included age, sex, Unified Parkinson’s Disease Rating Scale (UPDRS) part III score, and baseline MoCA.
A total of 379 patients completed at least one PASE questionnaire over the course of the study. PASE scores in this cohort have been previously described (Mantri S et al. J Park Dis. 2018;8[1]:107-11). Although overall rates of cognitive decline are known to be modest in this early cohort, PASE over time has a significant effect on MoCA during follow-up (P = 0.02) which suggest that higher levels of activity throughout disease are associated with better cognitive performance.
Dr. Mantri had nothing to disclose. Among her coauthors, Dr. Tropea received personal compensation from Genzyme and Medtronics and research support from Sanofi. Dr. Morley had nothing to disclose.
SOURCE: Mantri S et al. AAN 2019, Abstract P2.8-021.
PHILADELPHIA – according to Sneha Mantri, MD, of Duke University in Durham, N.C., and colleagues, who presented the results of their study at the annual meeting of the American Academy of Neurology.
Physical activity is an important component of the management of Parkinson’s disease and is shown to mitigate cognitive decline among patients with moderate disease, said Dr. Mantri and colleagues. “Exercise levels in de novo and early disease may influence risk of future cognitive decline; early disease also presents an opportunity for early intervention and possible disease modification,” Dr. Mantri said.
Physical activity levels in early disease are known to be low, but the effects of activity on cognition are currently unclear. To assess the relationship between physical activity and cognition, Dr. Mantri and colleagues examined patients with Parkinson’s disease who were enrolled in the prospective Parkinson’s Progression Markers Initiative (PPMI) cohort. At annual study visits, participants completed the Physical Activity Scale for the Elderly (PASE), a validated self-reported questionnaire assessing household, leisure, and work activities over the previous 7 days. The researchers used a linear mixed-effects model to compare rates of change in the Montreal Cognitive Assessment (MoCA) according to PASE scores; covariates included age, sex, Unified Parkinson’s Disease Rating Scale (UPDRS) part III score, and baseline MoCA.
A total of 379 patients completed at least one PASE questionnaire over the course of the study. PASE scores in this cohort have been previously described (Mantri S et al. J Park Dis. 2018;8[1]:107-11). Although overall rates of cognitive decline are known to be modest in this early cohort, PASE over time has a significant effect on MoCA during follow-up (P = 0.02) which suggest that higher levels of activity throughout disease are associated with better cognitive performance.
Dr. Mantri had nothing to disclose. Among her coauthors, Dr. Tropea received personal compensation from Genzyme and Medtronics and research support from Sanofi. Dr. Morley had nothing to disclose.
SOURCE: Mantri S et al. AAN 2019, Abstract P2.8-021.
REPORTING FROM AAN 2019
Key clinical point: Physical activity is associated with slower cognitive decline in patients with de novo Parkinson’s disease.
Major finding: Higher scores on the Physical Activity Scale for the Elderly over time had a significant effect on cognitive function.
Study details: A prospective study of 379 patients enrolled in the Parkinson’s Progression Markers Initiative.
Disclosures: Dr. Mantri had no relevant financial disclosures. Among her coauthors, Dr. Tropea received personal compensation from Genzyme and Medtronics and research support from Sanofi. Dr. Morley had nothing to disclose.
Source: Mantri S et al. AAN 2019, Abstract P2.8-021.
Methylenetetrahydrofolate Reductase Screening in Treatment-Resistant Depression
Therapeutic response to antidepressant drugs is often partial. Multiple trials of medications may be prescribed before a patient achieves remission of symptoms. Further, no universally accepted definition for treatment-resistant depression (TRD) has been established. The most commonly proposed definition (and the definition used in this article) is the failure to achieve remission with 2 or more adequate antidepressant treatments.1
About 20% to 30% of patients with depression are treatment resistant. The overall Canada-wide prevalence of TRD in primary care was 21.7%.2 In the US, about 15.7 million adults have had at least 1 major depressive episode in the past year, and 10% to 15% of major depressive disorder (MDD) cases can be classified as treatment resistant.3,4 In a retrospective, longitudinal cohort analysis in a Medicaid population, 25.9% of pharmacologically treated adults with MDD met criteria for TRD.5 Similarly, TRD in this review was defined as starting a third treatment regimen after 2 adequate regimens of antidepressants.
Why is this important? Treatment resistance is often associated with high rates of disability and comorbidity. Given the significant prevalence and impact of TRD, research into better understanding and treating these patients is paramount. Pharmacogenetics has been proposed for tailoring therapy and theoretically circumventing treatment resistance to achieve better outcomes.
Methylenetetrahydrofolate reductase (MTHFR) is a gene that encodes an enzyme similarly called MTHFR. The enzyme converts 5,10-MTHF to 5-MTHF. 5-MTHF then donates a methyl group in the conversion of homocysteine to methionine. Decreased or absent expression of MTHFR leads to decreased levels of 5-MTHF, which then leads to high levels of homocysteine. This results in suboptimal production of monoamines, including serotonin, dopamine, and norepinephrine as well as subsequent abnormalities in neural and vascular pathways.6
Screening for MTHFR polymorphisms has been proposed in past years due to weak associations with conditions such as cardiac disease, poor pregnancy outcomes, and colorectal cancer.7 Recently, an increasing number of studies suggest screening for MTHFR polymorphisms in patients with depression. This proposal is based on demonstrated links between abnormal folate metabolism and high levels of homocysteine and an increased risk for MDD and reduced antidepressant effectiveness.
In a meta-analysis by Wu and colleagues of 26 published studies, including 4,992 depression cases and 17,082 controls, MTHFR C677T polymorphism was associated with an increased risk of depression especially in Asian populations. This relationship was not observed in the elderly.8 A more recent article reviewing 6 small studies from 2005 to 2016 suggested that the MTHFR A1298C polymorphism (via abnormal homocysteine metabolism and folate cycles) may play a role in identifying those at risk of developing MDD particularly women in white populations.9
As the proposed mechanism of treatment resistance associated with the MTHFR polymorphisms seems to be related to folate metabolism, L-methylfolate supplementation has been recommended. In a 60-day randomized trial of a selective serotonin reuptake inhibitor (SSRI) and L-methylfolate vs SSRI and placebo, patients prescribed an SSRI with L-methylfolate had a greater response rate (reduction of baseline symptoms by at least 50%) that was statistically significant (P = .04) vs patients taking the placebo.10
In primary care and specialty settings, screening patients with TRD for MTHFR polymorphisms has been proposed. LabCorp (Burlington, NC) and Quest Diagnostics (Secaucus, NJ) have a DNA assay that detects C677T and A1298C mutations in the MTHFR gene, using whole blood samples; however, the cost is high. In the DC/Maryland/Virginia region, test cost varies from $390 if the patient requests it from the lab to $325 if requested through an institution that has an account with LabCorp. Although there are little data regarding false positive and false negative rates, 1 source suggested an analytic sensitivity and specificity of 99% for the tests.11
Once obtained, positive screening results may assist in directing next steps in terms of adjunctive or next-line therapies. Given the high price of the test and positive responses with L-methylfolate supplementation thus far, the question remains: Why not supplement patients with TRD with folate and forego screening? For these 2 reasons: The treatment dosage in the studies referenced is 15 mg of L-methylfolate. This dosage is often unavailable over-the-counter and can cost as much as $75 for 90 capsules. Additionally, the high dosage of methylfolate may increase the risk of colon cancer in certain subpopulations, such as those with precancerous lesions.12Although the current data seem promising, further research is needed to explore the benefits of folate supplementation in larger study samples and perhaps other targeted treatment options for patients with TRD with MTHFR gene polymorphisms.
1. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7.
2. Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349-357.
3. Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739-744.
4. Little A. Treatment-resistant depression. Am Fam Physician. 2009;80(2):167-172.
5. Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of Medicaid beneficiaries with treatment resistant depression. J Manag Care Spec Pharm. 2018;24(3):226-236.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
7. Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237-240.
8. Wu YL, Ding XX, Sun YH, et al. Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:78-85.
9. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrogolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9(10):e1734.
10. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
11. Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15(2):153-156.
12. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
Therapeutic response to antidepressant drugs is often partial. Multiple trials of medications may be prescribed before a patient achieves remission of symptoms. Further, no universally accepted definition for treatment-resistant depression (TRD) has been established. The most commonly proposed definition (and the definition used in this article) is the failure to achieve remission with 2 or more adequate antidepressant treatments.1
About 20% to 30% of patients with depression are treatment resistant. The overall Canada-wide prevalence of TRD in primary care was 21.7%.2 In the US, about 15.7 million adults have had at least 1 major depressive episode in the past year, and 10% to 15% of major depressive disorder (MDD) cases can be classified as treatment resistant.3,4 In a retrospective, longitudinal cohort analysis in a Medicaid population, 25.9% of pharmacologically treated adults with MDD met criteria for TRD.5 Similarly, TRD in this review was defined as starting a third treatment regimen after 2 adequate regimens of antidepressants.
Why is this important? Treatment resistance is often associated with high rates of disability and comorbidity. Given the significant prevalence and impact of TRD, research into better understanding and treating these patients is paramount. Pharmacogenetics has been proposed for tailoring therapy and theoretically circumventing treatment resistance to achieve better outcomes.
Methylenetetrahydrofolate reductase (MTHFR) is a gene that encodes an enzyme similarly called MTHFR. The enzyme converts 5,10-MTHF to 5-MTHF. 5-MTHF then donates a methyl group in the conversion of homocysteine to methionine. Decreased or absent expression of MTHFR leads to decreased levels of 5-MTHF, which then leads to high levels of homocysteine. This results in suboptimal production of monoamines, including serotonin, dopamine, and norepinephrine as well as subsequent abnormalities in neural and vascular pathways.6
Screening for MTHFR polymorphisms has been proposed in past years due to weak associations with conditions such as cardiac disease, poor pregnancy outcomes, and colorectal cancer.7 Recently, an increasing number of studies suggest screening for MTHFR polymorphisms in patients with depression. This proposal is based on demonstrated links between abnormal folate metabolism and high levels of homocysteine and an increased risk for MDD and reduced antidepressant effectiveness.
In a meta-analysis by Wu and colleagues of 26 published studies, including 4,992 depression cases and 17,082 controls, MTHFR C677T polymorphism was associated with an increased risk of depression especially in Asian populations. This relationship was not observed in the elderly.8 A more recent article reviewing 6 small studies from 2005 to 2016 suggested that the MTHFR A1298C polymorphism (via abnormal homocysteine metabolism and folate cycles) may play a role in identifying those at risk of developing MDD particularly women in white populations.9
As the proposed mechanism of treatment resistance associated with the MTHFR polymorphisms seems to be related to folate metabolism, L-methylfolate supplementation has been recommended. In a 60-day randomized trial of a selective serotonin reuptake inhibitor (SSRI) and L-methylfolate vs SSRI and placebo, patients prescribed an SSRI with L-methylfolate had a greater response rate (reduction of baseline symptoms by at least 50%) that was statistically significant (P = .04) vs patients taking the placebo.10
In primary care and specialty settings, screening patients with TRD for MTHFR polymorphisms has been proposed. LabCorp (Burlington, NC) and Quest Diagnostics (Secaucus, NJ) have a DNA assay that detects C677T and A1298C mutations in the MTHFR gene, using whole blood samples; however, the cost is high. In the DC/Maryland/Virginia region, test cost varies from $390 if the patient requests it from the lab to $325 if requested through an institution that has an account with LabCorp. Although there are little data regarding false positive and false negative rates, 1 source suggested an analytic sensitivity and specificity of 99% for the tests.11
Once obtained, positive screening results may assist in directing next steps in terms of adjunctive or next-line therapies. Given the high price of the test and positive responses with L-methylfolate supplementation thus far, the question remains: Why not supplement patients with TRD with folate and forego screening? For these 2 reasons: The treatment dosage in the studies referenced is 15 mg of L-methylfolate. This dosage is often unavailable over-the-counter and can cost as much as $75 for 90 capsules. Additionally, the high dosage of methylfolate may increase the risk of colon cancer in certain subpopulations, such as those with precancerous lesions.12Although the current data seem promising, further research is needed to explore the benefits of folate supplementation in larger study samples and perhaps other targeted treatment options for patients with TRD with MTHFR gene polymorphisms.
Therapeutic response to antidepressant drugs is often partial. Multiple trials of medications may be prescribed before a patient achieves remission of symptoms. Further, no universally accepted definition for treatment-resistant depression (TRD) has been established. The most commonly proposed definition (and the definition used in this article) is the failure to achieve remission with 2 or more adequate antidepressant treatments.1
About 20% to 30% of patients with depression are treatment resistant. The overall Canada-wide prevalence of TRD in primary care was 21.7%.2 In the US, about 15.7 million adults have had at least 1 major depressive episode in the past year, and 10% to 15% of major depressive disorder (MDD) cases can be classified as treatment resistant.3,4 In a retrospective, longitudinal cohort analysis in a Medicaid population, 25.9% of pharmacologically treated adults with MDD met criteria for TRD.5 Similarly, TRD in this review was defined as starting a third treatment regimen after 2 adequate regimens of antidepressants.
Why is this important? Treatment resistance is often associated with high rates of disability and comorbidity. Given the significant prevalence and impact of TRD, research into better understanding and treating these patients is paramount. Pharmacogenetics has been proposed for tailoring therapy and theoretically circumventing treatment resistance to achieve better outcomes.
Methylenetetrahydrofolate reductase (MTHFR) is a gene that encodes an enzyme similarly called MTHFR. The enzyme converts 5,10-MTHF to 5-MTHF. 5-MTHF then donates a methyl group in the conversion of homocysteine to methionine. Decreased or absent expression of MTHFR leads to decreased levels of 5-MTHF, which then leads to high levels of homocysteine. This results in suboptimal production of monoamines, including serotonin, dopamine, and norepinephrine as well as subsequent abnormalities in neural and vascular pathways.6
Screening for MTHFR polymorphisms has been proposed in past years due to weak associations with conditions such as cardiac disease, poor pregnancy outcomes, and colorectal cancer.7 Recently, an increasing number of studies suggest screening for MTHFR polymorphisms in patients with depression. This proposal is based on demonstrated links between abnormal folate metabolism and high levels of homocysteine and an increased risk for MDD and reduced antidepressant effectiveness.
In a meta-analysis by Wu and colleagues of 26 published studies, including 4,992 depression cases and 17,082 controls, MTHFR C677T polymorphism was associated with an increased risk of depression especially in Asian populations. This relationship was not observed in the elderly.8 A more recent article reviewing 6 small studies from 2005 to 2016 suggested that the MTHFR A1298C polymorphism (via abnormal homocysteine metabolism and folate cycles) may play a role in identifying those at risk of developing MDD particularly women in white populations.9
As the proposed mechanism of treatment resistance associated with the MTHFR polymorphisms seems to be related to folate metabolism, L-methylfolate supplementation has been recommended. In a 60-day randomized trial of a selective serotonin reuptake inhibitor (SSRI) and L-methylfolate vs SSRI and placebo, patients prescribed an SSRI with L-methylfolate had a greater response rate (reduction of baseline symptoms by at least 50%) that was statistically significant (P = .04) vs patients taking the placebo.10
In primary care and specialty settings, screening patients with TRD for MTHFR polymorphisms has been proposed. LabCorp (Burlington, NC) and Quest Diagnostics (Secaucus, NJ) have a DNA assay that detects C677T and A1298C mutations in the MTHFR gene, using whole blood samples; however, the cost is high. In the DC/Maryland/Virginia region, test cost varies from $390 if the patient requests it from the lab to $325 if requested through an institution that has an account with LabCorp. Although there are little data regarding false positive and false negative rates, 1 source suggested an analytic sensitivity and specificity of 99% for the tests.11
Once obtained, positive screening results may assist in directing next steps in terms of adjunctive or next-line therapies. Given the high price of the test and positive responses with L-methylfolate supplementation thus far, the question remains: Why not supplement patients with TRD with folate and forego screening? For these 2 reasons: The treatment dosage in the studies referenced is 15 mg of L-methylfolate. This dosage is often unavailable over-the-counter and can cost as much as $75 for 90 capsules. Additionally, the high dosage of methylfolate may increase the risk of colon cancer in certain subpopulations, such as those with precancerous lesions.12Although the current data seem promising, further research is needed to explore the benefits of folate supplementation in larger study samples and perhaps other targeted treatment options for patients with TRD with MTHFR gene polymorphisms.
1. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7.
2. Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349-357.
3. Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739-744.
4. Little A. Treatment-resistant depression. Am Fam Physician. 2009;80(2):167-172.
5. Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of Medicaid beneficiaries with treatment resistant depression. J Manag Care Spec Pharm. 2018;24(3):226-236.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
7. Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237-240.
8. Wu YL, Ding XX, Sun YH, et al. Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:78-85.
9. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrogolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9(10):e1734.
10. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
11. Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15(2):153-156.
12. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
1. McIntyre RS, Filteau MJ, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1-7.
2. Rizvi SJ, Grima E, Tan M, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349-357.
3. Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739-744.
4. Little A. Treatment-resistant depression. Am Fam Physician. 2009;80(2):167-172.
5. Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of Medicaid beneficiaries with treatment resistant depression. J Manag Care Spec Pharm. 2018;24(3):226-236.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228-232.
7. Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237-240.
8. Wu YL, Ding XX, Sun YH, et al. Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:78-85.
9. Cho K, Amin ZM, An J, Rambaran KA, Johnson TB, Alzghari SK. Methylenetetrahydrogolate reductase A1298C polymorphism and major depressive disorder. Cureus. 2017;9(10):e1734.
10. Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
11. Hickey SE, Curry CJ, Toriello HV. ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15(2):153-156.
12. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
Can Medicine Bring Good Out of War?
The title of this essay is more often posed as “Is War Good for Medicine?”2 The career VA physician in me, and the daughter and granddaughter of combat veterans, finds this question historically accurate, but ethically problematic. So I have rewritten the question to one that enables us to examine the historic relationship of medical advances and war from a more ethically justifiable posture. I am by no means ascribing to authors of other publications with this title anything but the highest motives of education and edification.
Yet the more I read and thought about the question(s), I realized that the moral assumptions underlying and supporting each concept are significantly different. What led me to that realization was a story my father told me when I was young which in my youthful ignorance I either dismissed or ignored. I now see that the narrative captured a profound truth about how war is not good especially for those who must wage it, but good may come from it for those who now live in peace.
My father was one of the founders of military pediatrics. Surprisingly, pediatricians were valuable members of the military medical forces because of their knowledge of infectious diseases.3 My father had gone in to the then new specialty of pediatrics because in the 1930s, infectious diseases were the primary cause of death in children. Before antibiotics, children would often die of common infections. Service as a combat medical officer in World War II stationed in the European Theater, my father had experience with and access to penicillin. After returning from the war to work in an Army hospital, he and his staff went into the acute pediatric ward and gave the drug to several very sick children, many of whom were likely to die. The next morning on rounds, they noted that many of the children were feeling much better, some even bouncing on their beds.
Perhaps either his telling or my remembering of these events is partly apocryphal, but the reality is that those lethal microbes had no idea what had hit them. Before human physicians overused the new drugs and nature struck back with antibiotic resistance, penicillin seemed miraculous.
Most likely, in 1945 those children would never have been prescribed penicillin, much less survived, if not for the unprecedented and war-driven consortium of industry and government that mass-produced penicillin to treat the troops with infections. Without a doubt then, from the sacrifice and devastation of World War II came the benefits and boons of the antibiotic era—one of the greatest discoveries in medical science.4
Penicillin is but one of legions of scientific discoveries that emerged during wartime. Many of these dramatic improvements, especially those in surgical techniques and emergency medicine, quickly entered the civilian sector. The French surgeon Amboise Paré, for example, revived an old Roman Army practice of using ligatures or tourniquets to stop excessive blood loss, now a staple of emergency responders in disasters. The ambulance services that transported wounded troops to the hospital began on the battlefields of the Civil War.5
These impressive contributions are the direct result of military medicine intended to preserve fighting strength. There are also indirect, although just as revolutionary, efforts of DoD and VA scientists and health care professionals to minimize disability and prevent progression especially of service-connected injuries and illnesses. Among the most groundbreaking is the VA’s 3D-printed artificial lung. I have to admit at first I thought that it was futuristic, but quickly I learned that it was a realistic possibility for the coming decades.6 VA researchers hope the lung will offer a treatment option for patients with chronic obstructive pulmonary disease (COPD), a lung condition more prevalent in veterans than in the civilian population.7 One contributing factor to the increased risk of COPD among former military is the higher rate of smoking among both active duty and veterans than that in the civilian population.8 And the last chain in the link of causation is that smoking is more common in those service members who have posttraumatic stress disorder.9
However, there also is a very dark side to the link between wartime research and medicine—most infamously the Nazi hypothermia experiments conducted at concentration camps. The proposed publication aroused a decades long ethical controversy regarding whether the data should be published, much less used, in research and practice even if it could save the lives of present or future warriors. In 1990, Marcia Angel, MD, then editor-in-chief of the prestigious New England Journal of Medicine, published the information with an accompanying ethical justification. “Finally, refusal to publish the unethical work serves notice to society at large that even scientists do not consider science the primary measure of a civilization. Knowledge, although important, may be less important to a decent society than the way it is obtained.”10 Ethicist Stephen Post writing on behalf of Holocaust victims strenuously disagreed with the decision to publish the research, “Because the Nazi experiments on human beings were so appallingly unethical, it follows, prima facie, that the use of the records is unethical.”11
This debate is key to the distinction between the 2 questions posed at the beginning of this column. Few who have been on a battlefield or who have cared for those who were can suggest or defend that wars should be fought as a catalyst for scientific research or an impetus to medical advancement. Such an instrumentalist view justifies the end of healing with the means of death, which is an intrinsic contradiction that would eventually corrode the integrity of the medical and scientific professions. Conversely, the second question challenges all of us in federal practice to assume a mantle of obligation to take the interventions that enabled combat medicine to save soldiers and apply them to improve the health and save the lives of veterans and civilians alike. It summons scientists laboring in the hundreds of DoD and VA laboratories to use the unparalleled funding and infrastructure of the institutions to develop promising therapeutics to treat the psychological toll and physical cost of war. And finally it charges the citizens whose family and friends have and will serve in uniform to enlist in a political process that enables military medicine and science to achieve the greatest good-health in peace.
1. Remarque EM. All Quiet on the Western Front. New York, NY: Fawcett Books; 1929:228.
2. Connell C. Is war good for medicine: war’s medical legacy? http://sm.stanford.edu/archive/stanmed/2007summer/main.html. Published 2007. Accessed April 18, 2019.
3. Burnett MW, Callahan CW. American pediatricians at war; a legacy of service. Pediatrics. 2012;129(suppl 1):S33-S49.
4. Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52-57.
5. Samuel L. 6 medical innovations that moved from the battlefield to mainstream medicine. https://www.scientificamercan.com/article/6-medical-innovations-that-moved-from-the-battlefield-to-mainstream-medicine. Published November 11, 2017. Accessed April 18, 2019.
6. Richman M. Breathing easier. https://www.research.va.gov/currents/0818-Researchers-strive-to-make-3D-printed-artificial-lung-to-help-Vets-with-respiratory-disease.cfm. Published August 1, 2018. Accessed April 18, 2019.
7. Murphy DE, Chaudry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban Midwest. Mill Med. 2011;176(5):552-560.
8. Thompson WH, St-Hilaire C. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555-560.
9. Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189-1195.
10. Angell M. The Nazi hypothermia experiments and unethical research today. N Eng J Med 1990;322(20):1462-1464.
11. Post SG. The echo of Nuremberg: Nazi data and ethics. J Med Ethics. 1991;17(1):42-44.
The title of this essay is more often posed as “Is War Good for Medicine?”2 The career VA physician in me, and the daughter and granddaughter of combat veterans, finds this question historically accurate, but ethically problematic. So I have rewritten the question to one that enables us to examine the historic relationship of medical advances and war from a more ethically justifiable posture. I am by no means ascribing to authors of other publications with this title anything but the highest motives of education and edification.
Yet the more I read and thought about the question(s), I realized that the moral assumptions underlying and supporting each concept are significantly different. What led me to that realization was a story my father told me when I was young which in my youthful ignorance I either dismissed or ignored. I now see that the narrative captured a profound truth about how war is not good especially for those who must wage it, but good may come from it for those who now live in peace.
My father was one of the founders of military pediatrics. Surprisingly, pediatricians were valuable members of the military medical forces because of their knowledge of infectious diseases.3 My father had gone in to the then new specialty of pediatrics because in the 1930s, infectious diseases were the primary cause of death in children. Before antibiotics, children would often die of common infections. Service as a combat medical officer in World War II stationed in the European Theater, my father had experience with and access to penicillin. After returning from the war to work in an Army hospital, he and his staff went into the acute pediatric ward and gave the drug to several very sick children, many of whom were likely to die. The next morning on rounds, they noted that many of the children were feeling much better, some even bouncing on their beds.
Perhaps either his telling or my remembering of these events is partly apocryphal, but the reality is that those lethal microbes had no idea what had hit them. Before human physicians overused the new drugs and nature struck back with antibiotic resistance, penicillin seemed miraculous.
Most likely, in 1945 those children would never have been prescribed penicillin, much less survived, if not for the unprecedented and war-driven consortium of industry and government that mass-produced penicillin to treat the troops with infections. Without a doubt then, from the sacrifice and devastation of World War II came the benefits and boons of the antibiotic era—one of the greatest discoveries in medical science.4
Penicillin is but one of legions of scientific discoveries that emerged during wartime. Many of these dramatic improvements, especially those in surgical techniques and emergency medicine, quickly entered the civilian sector. The French surgeon Amboise Paré, for example, revived an old Roman Army practice of using ligatures or tourniquets to stop excessive blood loss, now a staple of emergency responders in disasters. The ambulance services that transported wounded troops to the hospital began on the battlefields of the Civil War.5
These impressive contributions are the direct result of military medicine intended to preserve fighting strength. There are also indirect, although just as revolutionary, efforts of DoD and VA scientists and health care professionals to minimize disability and prevent progression especially of service-connected injuries and illnesses. Among the most groundbreaking is the VA’s 3D-printed artificial lung. I have to admit at first I thought that it was futuristic, but quickly I learned that it was a realistic possibility for the coming decades.6 VA researchers hope the lung will offer a treatment option for patients with chronic obstructive pulmonary disease (COPD), a lung condition more prevalent in veterans than in the civilian population.7 One contributing factor to the increased risk of COPD among former military is the higher rate of smoking among both active duty and veterans than that in the civilian population.8 And the last chain in the link of causation is that smoking is more common in those service members who have posttraumatic stress disorder.9
However, there also is a very dark side to the link between wartime research and medicine—most infamously the Nazi hypothermia experiments conducted at concentration camps. The proposed publication aroused a decades long ethical controversy regarding whether the data should be published, much less used, in research and practice even if it could save the lives of present or future warriors. In 1990, Marcia Angel, MD, then editor-in-chief of the prestigious New England Journal of Medicine, published the information with an accompanying ethical justification. “Finally, refusal to publish the unethical work serves notice to society at large that even scientists do not consider science the primary measure of a civilization. Knowledge, although important, may be less important to a decent society than the way it is obtained.”10 Ethicist Stephen Post writing on behalf of Holocaust victims strenuously disagreed with the decision to publish the research, “Because the Nazi experiments on human beings were so appallingly unethical, it follows, prima facie, that the use of the records is unethical.”11
This debate is key to the distinction between the 2 questions posed at the beginning of this column. Few who have been on a battlefield or who have cared for those who were can suggest or defend that wars should be fought as a catalyst for scientific research or an impetus to medical advancement. Such an instrumentalist view justifies the end of healing with the means of death, which is an intrinsic contradiction that would eventually corrode the integrity of the medical and scientific professions. Conversely, the second question challenges all of us in federal practice to assume a mantle of obligation to take the interventions that enabled combat medicine to save soldiers and apply them to improve the health and save the lives of veterans and civilians alike. It summons scientists laboring in the hundreds of DoD and VA laboratories to use the unparalleled funding and infrastructure of the institutions to develop promising therapeutics to treat the psychological toll and physical cost of war. And finally it charges the citizens whose family and friends have and will serve in uniform to enlist in a political process that enables military medicine and science to achieve the greatest good-health in peace.
The title of this essay is more often posed as “Is War Good for Medicine?”2 The career VA physician in me, and the daughter and granddaughter of combat veterans, finds this question historically accurate, but ethically problematic. So I have rewritten the question to one that enables us to examine the historic relationship of medical advances and war from a more ethically justifiable posture. I am by no means ascribing to authors of other publications with this title anything but the highest motives of education and edification.
Yet the more I read and thought about the question(s), I realized that the moral assumptions underlying and supporting each concept are significantly different. What led me to that realization was a story my father told me when I was young which in my youthful ignorance I either dismissed or ignored. I now see that the narrative captured a profound truth about how war is not good especially for those who must wage it, but good may come from it for those who now live in peace.
My father was one of the founders of military pediatrics. Surprisingly, pediatricians were valuable members of the military medical forces because of their knowledge of infectious diseases.3 My father had gone in to the then new specialty of pediatrics because in the 1930s, infectious diseases were the primary cause of death in children. Before antibiotics, children would often die of common infections. Service as a combat medical officer in World War II stationed in the European Theater, my father had experience with and access to penicillin. After returning from the war to work in an Army hospital, he and his staff went into the acute pediatric ward and gave the drug to several very sick children, many of whom were likely to die. The next morning on rounds, they noted that many of the children were feeling much better, some even bouncing on their beds.
Perhaps either his telling or my remembering of these events is partly apocryphal, but the reality is that those lethal microbes had no idea what had hit them. Before human physicians overused the new drugs and nature struck back with antibiotic resistance, penicillin seemed miraculous.
Most likely, in 1945 those children would never have been prescribed penicillin, much less survived, if not for the unprecedented and war-driven consortium of industry and government that mass-produced penicillin to treat the troops with infections. Without a doubt then, from the sacrifice and devastation of World War II came the benefits and boons of the antibiotic era—one of the greatest discoveries in medical science.4
Penicillin is but one of legions of scientific discoveries that emerged during wartime. Many of these dramatic improvements, especially those in surgical techniques and emergency medicine, quickly entered the civilian sector. The French surgeon Amboise Paré, for example, revived an old Roman Army practice of using ligatures or tourniquets to stop excessive blood loss, now a staple of emergency responders in disasters. The ambulance services that transported wounded troops to the hospital began on the battlefields of the Civil War.5
These impressive contributions are the direct result of military medicine intended to preserve fighting strength. There are also indirect, although just as revolutionary, efforts of DoD and VA scientists and health care professionals to minimize disability and prevent progression especially of service-connected injuries and illnesses. Among the most groundbreaking is the VA’s 3D-printed artificial lung. I have to admit at first I thought that it was futuristic, but quickly I learned that it was a realistic possibility for the coming decades.6 VA researchers hope the lung will offer a treatment option for patients with chronic obstructive pulmonary disease (COPD), a lung condition more prevalent in veterans than in the civilian population.7 One contributing factor to the increased risk of COPD among former military is the higher rate of smoking among both active duty and veterans than that in the civilian population.8 And the last chain in the link of causation is that smoking is more common in those service members who have posttraumatic stress disorder.9
However, there also is a very dark side to the link between wartime research and medicine—most infamously the Nazi hypothermia experiments conducted at concentration camps. The proposed publication aroused a decades long ethical controversy regarding whether the data should be published, much less used, in research and practice even if it could save the lives of present or future warriors. In 1990, Marcia Angel, MD, then editor-in-chief of the prestigious New England Journal of Medicine, published the information with an accompanying ethical justification. “Finally, refusal to publish the unethical work serves notice to society at large that even scientists do not consider science the primary measure of a civilization. Knowledge, although important, may be less important to a decent society than the way it is obtained.”10 Ethicist Stephen Post writing on behalf of Holocaust victims strenuously disagreed with the decision to publish the research, “Because the Nazi experiments on human beings were so appallingly unethical, it follows, prima facie, that the use of the records is unethical.”11
This debate is key to the distinction between the 2 questions posed at the beginning of this column. Few who have been on a battlefield or who have cared for those who were can suggest or defend that wars should be fought as a catalyst for scientific research or an impetus to medical advancement. Such an instrumentalist view justifies the end of healing with the means of death, which is an intrinsic contradiction that would eventually corrode the integrity of the medical and scientific professions. Conversely, the second question challenges all of us in federal practice to assume a mantle of obligation to take the interventions that enabled combat medicine to save soldiers and apply them to improve the health and save the lives of veterans and civilians alike. It summons scientists laboring in the hundreds of DoD and VA laboratories to use the unparalleled funding and infrastructure of the institutions to develop promising therapeutics to treat the psychological toll and physical cost of war. And finally it charges the citizens whose family and friends have and will serve in uniform to enlist in a political process that enables military medicine and science to achieve the greatest good-health in peace.
1. Remarque EM. All Quiet on the Western Front. New York, NY: Fawcett Books; 1929:228.
2. Connell C. Is war good for medicine: war’s medical legacy? http://sm.stanford.edu/archive/stanmed/2007summer/main.html. Published 2007. Accessed April 18, 2019.
3. Burnett MW, Callahan CW. American pediatricians at war; a legacy of service. Pediatrics. 2012;129(suppl 1):S33-S49.
4. Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52-57.
5. Samuel L. 6 medical innovations that moved from the battlefield to mainstream medicine. https://www.scientificamercan.com/article/6-medical-innovations-that-moved-from-the-battlefield-to-mainstream-medicine. Published November 11, 2017. Accessed April 18, 2019.
6. Richman M. Breathing easier. https://www.research.va.gov/currents/0818-Researchers-strive-to-make-3D-printed-artificial-lung-to-help-Vets-with-respiratory-disease.cfm. Published August 1, 2018. Accessed April 18, 2019.
7. Murphy DE, Chaudry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban Midwest. Mill Med. 2011;176(5):552-560.
8. Thompson WH, St-Hilaire C. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555-560.
9. Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189-1195.
10. Angell M. The Nazi hypothermia experiments and unethical research today. N Eng J Med 1990;322(20):1462-1464.
11. Post SG. The echo of Nuremberg: Nazi data and ethics. J Med Ethics. 1991;17(1):42-44.
1. Remarque EM. All Quiet on the Western Front. New York, NY: Fawcett Books; 1929:228.
2. Connell C. Is war good for medicine: war’s medical legacy? http://sm.stanford.edu/archive/stanmed/2007summer/main.html. Published 2007. Accessed April 18, 2019.
3. Burnett MW, Callahan CW. American pediatricians at war; a legacy of service. Pediatrics. 2012;129(suppl 1):S33-S49.
4. Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52-57.
5. Samuel L. 6 medical innovations that moved from the battlefield to mainstream medicine. https://www.scientificamercan.com/article/6-medical-innovations-that-moved-from-the-battlefield-to-mainstream-medicine. Published November 11, 2017. Accessed April 18, 2019.
6. Richman M. Breathing easier. https://www.research.va.gov/currents/0818-Researchers-strive-to-make-3D-printed-artificial-lung-to-help-Vets-with-respiratory-disease.cfm. Published August 1, 2018. Accessed April 18, 2019.
7. Murphy DE, Chaudry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban Midwest. Mill Med. 2011;176(5):552-560.
8. Thompson WH, St-Hilaire C. Prevalence of chronic obstructive pulmonary disease and tobacco use in veterans at Boise Veterans Affairs Medical Center. Respir Care. 2010;55(5):555-560.
9. Cook J, Jakupcak M, Rosenheck R, Fontana A, McFall M. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189-1195.
10. Angell M. The Nazi hypothermia experiments and unethical research today. N Eng J Med 1990;322(20):1462-1464.
11. Post SG. The echo of Nuremberg: Nazi data and ethics. J Med Ethics. 1991;17(1):42-44.
Universal adolescent anxiety screening is feasible in primary care
BALTIMORE – according to a new study.
The findings suggest that implementing a universal anxiety screening for teen patients is feasible and improves detection of patients with anxiety.
“Our providers were able to act on these positive screens and are able to catch a really serious entry-level condition that may have otherwise been missed,” presenter Sarah Malik, MD, a resident at Penn State Children’s Hospital, told attendees at the Pediatric Academic Societies annual meeting. “Hopefully, this will make a really meaningful difference in these kids’ lives, which is, of course, what we all want.”
An estimated 32% of U.S. teens have anxiety, according to the National Institute of Mental Health, and “8.3% of adolescents with anxiety have severe impairment defined by DSM4 criteria,” according to the study’s background information. Yet neither the American Academy of Pediatrics nor the U.S. Preventive Services Task Force has issued recommendations regarding screening for anxiety in teens.
“For this reason, we developed a study in which we implemented and measured the effect of a universal anxiety screening program in the pediatric primary care setting,” Dr Malik said.
The screening intervention took place in a single Penn State Health Children’s Hospital primary care practice in Hershey, Pa., that typically received 37,000 visits a year from 12,500 patients. The practice has 19 attending physicians, 4 nurse practitioners, and 21 residents.
Providers asked patients aged 11-18 years to fill out a nine-question Generalized Anxiety Disorder subscale of the Screen for Child Anxiety Related Disorders (SCARED) during their well-child visits from April 2017 to March 2018. Two-thirds of the patients had private insurance, 80% were white and 8% were black; 10% were Hispanic.
Providers had access to the screening results after nurses transcribed them into electronic medical records. The researchers used EMRs to determine how many patients completed a SCARED at their well-child visit and how many screened positive for anxiety, defined as a score of at least 9/18.
Then the providers compared the prevalence of anxiety 1 year after implementing the routine screening with the prevalence of teens with an ICD-10 anxiety diagnosis within the 36 months before the screening was implemented. The practice’s prevalence of adolescent anxiety was 13.3% 1 year after implementing universal anxiety screening, compared with 9.6% in the previous 3 years (P less than .0001).
Among 2,276 well-child visits for adolescents during the study period, 80% completed a SCARED. Of those who completed the screening, 17% screened positive. The physicians identified 70% of those patients with positive screens (214/306) as having anxiety, and 82% of those patients (n = 176) were diagnosed with anxiety.
About half of those diagnosed with anxiety (n = 93) received one or more interventions: 77 received referrals for counseling, 15 received psychiatric referrals, and 20 were prescribed new anxiety medication.
“We did find that a universal screening program for anxiety is very useful to implement in the primary care setting, and it’s also really effective at identifying adolescents with anxiety symptoms,” Dr. Malik said.
The study’s generalizability is limited by its implementation at a single academic center with integrated behavioral health, and the use of the SCARED, a portion of the GAD scale, is not considered a standard of care.
The researchers used no external funding, and they had no disclosures.
BALTIMORE – according to a new study.
The findings suggest that implementing a universal anxiety screening for teen patients is feasible and improves detection of patients with anxiety.
“Our providers were able to act on these positive screens and are able to catch a really serious entry-level condition that may have otherwise been missed,” presenter Sarah Malik, MD, a resident at Penn State Children’s Hospital, told attendees at the Pediatric Academic Societies annual meeting. “Hopefully, this will make a really meaningful difference in these kids’ lives, which is, of course, what we all want.”
An estimated 32% of U.S. teens have anxiety, according to the National Institute of Mental Health, and “8.3% of adolescents with anxiety have severe impairment defined by DSM4 criteria,” according to the study’s background information. Yet neither the American Academy of Pediatrics nor the U.S. Preventive Services Task Force has issued recommendations regarding screening for anxiety in teens.
“For this reason, we developed a study in which we implemented and measured the effect of a universal anxiety screening program in the pediatric primary care setting,” Dr Malik said.
The screening intervention took place in a single Penn State Health Children’s Hospital primary care practice in Hershey, Pa., that typically received 37,000 visits a year from 12,500 patients. The practice has 19 attending physicians, 4 nurse practitioners, and 21 residents.
Providers asked patients aged 11-18 years to fill out a nine-question Generalized Anxiety Disorder subscale of the Screen for Child Anxiety Related Disorders (SCARED) during their well-child visits from April 2017 to March 2018. Two-thirds of the patients had private insurance, 80% were white and 8% were black; 10% were Hispanic.
Providers had access to the screening results after nurses transcribed them into electronic medical records. The researchers used EMRs to determine how many patients completed a SCARED at their well-child visit and how many screened positive for anxiety, defined as a score of at least 9/18.
Then the providers compared the prevalence of anxiety 1 year after implementing the routine screening with the prevalence of teens with an ICD-10 anxiety diagnosis within the 36 months before the screening was implemented. The practice’s prevalence of adolescent anxiety was 13.3% 1 year after implementing universal anxiety screening, compared with 9.6% in the previous 3 years (P less than .0001).
Among 2,276 well-child visits for adolescents during the study period, 80% completed a SCARED. Of those who completed the screening, 17% screened positive. The physicians identified 70% of those patients with positive screens (214/306) as having anxiety, and 82% of those patients (n = 176) were diagnosed with anxiety.
About half of those diagnosed with anxiety (n = 93) received one or more interventions: 77 received referrals for counseling, 15 received psychiatric referrals, and 20 were prescribed new anxiety medication.
“We did find that a universal screening program for anxiety is very useful to implement in the primary care setting, and it’s also really effective at identifying adolescents with anxiety symptoms,” Dr. Malik said.
The study’s generalizability is limited by its implementation at a single academic center with integrated behavioral health, and the use of the SCARED, a portion of the GAD scale, is not considered a standard of care.
The researchers used no external funding, and they had no disclosures.
BALTIMORE – according to a new study.
The findings suggest that implementing a universal anxiety screening for teen patients is feasible and improves detection of patients with anxiety.
“Our providers were able to act on these positive screens and are able to catch a really serious entry-level condition that may have otherwise been missed,” presenter Sarah Malik, MD, a resident at Penn State Children’s Hospital, told attendees at the Pediatric Academic Societies annual meeting. “Hopefully, this will make a really meaningful difference in these kids’ lives, which is, of course, what we all want.”
An estimated 32% of U.S. teens have anxiety, according to the National Institute of Mental Health, and “8.3% of adolescents with anxiety have severe impairment defined by DSM4 criteria,” according to the study’s background information. Yet neither the American Academy of Pediatrics nor the U.S. Preventive Services Task Force has issued recommendations regarding screening for anxiety in teens.
“For this reason, we developed a study in which we implemented and measured the effect of a universal anxiety screening program in the pediatric primary care setting,” Dr Malik said.
The screening intervention took place in a single Penn State Health Children’s Hospital primary care practice in Hershey, Pa., that typically received 37,000 visits a year from 12,500 patients. The practice has 19 attending physicians, 4 nurse practitioners, and 21 residents.
Providers asked patients aged 11-18 years to fill out a nine-question Generalized Anxiety Disorder subscale of the Screen for Child Anxiety Related Disorders (SCARED) during their well-child visits from April 2017 to March 2018. Two-thirds of the patients had private insurance, 80% were white and 8% were black; 10% were Hispanic.
Providers had access to the screening results after nurses transcribed them into electronic medical records. The researchers used EMRs to determine how many patients completed a SCARED at their well-child visit and how many screened positive for anxiety, defined as a score of at least 9/18.
Then the providers compared the prevalence of anxiety 1 year after implementing the routine screening with the prevalence of teens with an ICD-10 anxiety diagnosis within the 36 months before the screening was implemented. The practice’s prevalence of adolescent anxiety was 13.3% 1 year after implementing universal anxiety screening, compared with 9.6% in the previous 3 years (P less than .0001).
Among 2,276 well-child visits for adolescents during the study period, 80% completed a SCARED. Of those who completed the screening, 17% screened positive. The physicians identified 70% of those patients with positive screens (214/306) as having anxiety, and 82% of those patients (n = 176) were diagnosed with anxiety.
About half of those diagnosed with anxiety (n = 93) received one or more interventions: 77 received referrals for counseling, 15 received psychiatric referrals, and 20 were prescribed new anxiety medication.
“We did find that a universal screening program for anxiety is very useful to implement in the primary care setting, and it’s also really effective at identifying adolescents with anxiety symptoms,” Dr. Malik said.
The study’s generalizability is limited by its implementation at a single academic center with integrated behavioral health, and the use of the SCARED, a portion of the GAD scale, is not considered a standard of care.
The researchers used no external funding, and they had no disclosures.
REPORTING FROM PAS 2019
Key clinical point: Universal anxiety screening for adolescents is feasible and effective in pediatric primary care.
Major finding: Adolescent anxiety diagnoses increased from 9.6% to 13.3% 1 year after university screening (P less than .0001).
Study details: The findings are based on assessment of a universal anxiety screening program implemented at a single academic pediatric primary care practice, involving 2,276 well visits between April 2017 and March 2018 for patients aged 11-18 years.
Disclosures: The researchers used no external funding, and they had no disclosures.
Cannabidiol reduces seizures in Dravet syndrome
Philadelphia – according to research presented at the annual meeting of the American Academy of Neurology. The two dosages in the study appear to have comparable efficacy.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said Ian Miller, MD, director of the epilepsy and neurophysiology program at Nicklaus Children’s Hospital in Miami, in a press release. “The children in this study had already tried an average of four epilepsy drugs with no success and at the time were taking an average of three additional drugs, so to have this measure of success with CBD is a major victory.”
Dravet syndrome is a rare developmental and epileptic encephalopathy. Onset occurs during infancy, and the syndrome is associated with drug-resistant seizures. Dr. Miller and colleagues designed a trial to evaluate the efficacy of two dosages of CBD as adjunctive anticonvulsant therapy in patients with Dravet syndrome and drug-resistant seizures.
The study population included 199 patients whose seizures were recorded for 4 weeks at baseline. The investigators randomized participants in approximately equal groups to receive placebo or highly purified CBD (the formulation approved under the name Epidiolex) at 20 mg/kg per day or 10 mg/kg per day. The study’s primary outcome was the change from baseline in frequency of convulsive seizures over 14 weeks of treatment.
Participants’ mean age was 9 years. Patients were taking a median of three concomitant antiepileptic drugs and had discontinued a median of four such drugs previously.
The reduction in the frequency of convulsive seizures was 46% for the high dose of CBD, 49% for the low dose of CBD, and 27% for placebo. The proportion of participants with a 50% or greater reduction in seizures was 49% in the high-dose group, 44% in the low-dose group, and 26% among controls. In addition, the reduction in the rate of total seizures was 47% for the high-dose group, 56% for the low-dose group, and 30% among controls.
The rate of adverse events was similar in all groups (90% for the high-dose group, 88% for the low-dose group, and 89% for controls). The five most common adverse events were diarrhea, somnolence, pyrexia, fatigue, and decreased appetite. The rate of serious adverse events was 25% for the high-dose group, 20% for the low-dose group, and 15% for controls. Discontinuations because of adverse events were limited to the high-dose group (7%). The rate of transaminases that exceeded three times the upper limit of normal was 19% in the high-dose group and 5% in the low-dose group. All of these elevations resolved. No patients died.
“Based on these results, dose increases above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” said Dr. Miller.
GW Research, the developer of cannabidiol, supported the study. GW operates through its affiliate Greenwich Biosciences in the United States. Dr. Miller has received compensation and research support from several companies, including GW Pharma.
SOURCE: Miller I et al. AAN 2019, Abstract P3.6-0.76.
Philadelphia – according to research presented at the annual meeting of the American Academy of Neurology. The two dosages in the study appear to have comparable efficacy.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said Ian Miller, MD, director of the epilepsy and neurophysiology program at Nicklaus Children’s Hospital in Miami, in a press release. “The children in this study had already tried an average of four epilepsy drugs with no success and at the time were taking an average of three additional drugs, so to have this measure of success with CBD is a major victory.”
Dravet syndrome is a rare developmental and epileptic encephalopathy. Onset occurs during infancy, and the syndrome is associated with drug-resistant seizures. Dr. Miller and colleagues designed a trial to evaluate the efficacy of two dosages of CBD as adjunctive anticonvulsant therapy in patients with Dravet syndrome and drug-resistant seizures.
The study population included 199 patients whose seizures were recorded for 4 weeks at baseline. The investigators randomized participants in approximately equal groups to receive placebo or highly purified CBD (the formulation approved under the name Epidiolex) at 20 mg/kg per day or 10 mg/kg per day. The study’s primary outcome was the change from baseline in frequency of convulsive seizures over 14 weeks of treatment.
Participants’ mean age was 9 years. Patients were taking a median of three concomitant antiepileptic drugs and had discontinued a median of four such drugs previously.
The reduction in the frequency of convulsive seizures was 46% for the high dose of CBD, 49% for the low dose of CBD, and 27% for placebo. The proportion of participants with a 50% or greater reduction in seizures was 49% in the high-dose group, 44% in the low-dose group, and 26% among controls. In addition, the reduction in the rate of total seizures was 47% for the high-dose group, 56% for the low-dose group, and 30% among controls.
The rate of adverse events was similar in all groups (90% for the high-dose group, 88% for the low-dose group, and 89% for controls). The five most common adverse events were diarrhea, somnolence, pyrexia, fatigue, and decreased appetite. The rate of serious adverse events was 25% for the high-dose group, 20% for the low-dose group, and 15% for controls. Discontinuations because of adverse events were limited to the high-dose group (7%). The rate of transaminases that exceeded three times the upper limit of normal was 19% in the high-dose group and 5% in the low-dose group. All of these elevations resolved. No patients died.
“Based on these results, dose increases above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” said Dr. Miller.
GW Research, the developer of cannabidiol, supported the study. GW operates through its affiliate Greenwich Biosciences in the United States. Dr. Miller has received compensation and research support from several companies, including GW Pharma.
SOURCE: Miller I et al. AAN 2019, Abstract P3.6-0.76.
Philadelphia – according to research presented at the annual meeting of the American Academy of Neurology. The two dosages in the study appear to have comparable efficacy.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said Ian Miller, MD, director of the epilepsy and neurophysiology program at Nicklaus Children’s Hospital in Miami, in a press release. “The children in this study had already tried an average of four epilepsy drugs with no success and at the time were taking an average of three additional drugs, so to have this measure of success with CBD is a major victory.”
Dravet syndrome is a rare developmental and epileptic encephalopathy. Onset occurs during infancy, and the syndrome is associated with drug-resistant seizures. Dr. Miller and colleagues designed a trial to evaluate the efficacy of two dosages of CBD as adjunctive anticonvulsant therapy in patients with Dravet syndrome and drug-resistant seizures.
The study population included 199 patients whose seizures were recorded for 4 weeks at baseline. The investigators randomized participants in approximately equal groups to receive placebo or highly purified CBD (the formulation approved under the name Epidiolex) at 20 mg/kg per day or 10 mg/kg per day. The study’s primary outcome was the change from baseline in frequency of convulsive seizures over 14 weeks of treatment.
Participants’ mean age was 9 years. Patients were taking a median of three concomitant antiepileptic drugs and had discontinued a median of four such drugs previously.
The reduction in the frequency of convulsive seizures was 46% for the high dose of CBD, 49% for the low dose of CBD, and 27% for placebo. The proportion of participants with a 50% or greater reduction in seizures was 49% in the high-dose group, 44% in the low-dose group, and 26% among controls. In addition, the reduction in the rate of total seizures was 47% for the high-dose group, 56% for the low-dose group, and 30% among controls.
The rate of adverse events was similar in all groups (90% for the high-dose group, 88% for the low-dose group, and 89% for controls). The five most common adverse events were diarrhea, somnolence, pyrexia, fatigue, and decreased appetite. The rate of serious adverse events was 25% for the high-dose group, 20% for the low-dose group, and 15% for controls. Discontinuations because of adverse events were limited to the high-dose group (7%). The rate of transaminases that exceeded three times the upper limit of normal was 19% in the high-dose group and 5% in the low-dose group. All of these elevations resolved. No patients died.
“Based on these results, dose increases above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” said Dr. Miller.
GW Research, the developer of cannabidiol, supported the study. GW operates through its affiliate Greenwich Biosciences in the United States. Dr. Miller has received compensation and research support from several companies, including GW Pharma.
SOURCE: Miller I et al. AAN 2019, Abstract P3.6-0.76.
REPORTING FROM AAN 2019
PsA Fast Facts: Prevalence and incidence



Management of Rodenticide Poisoning Associated with Synthetic Cannabinoids
Between March 7, 2018, and May 9, 2018, at least 164 people in Illinois were sickened by synthetic cannabinoids laced with rodenticides. The Illinois Department of Public Health has reported 4 deaths connected with the use of synthetic cannabinoids (sold under names such as Spice, K2, Legal Weed, etc).1 Synthetic cannabinoids are mind-altering chemicals that are sprayed on dried plant material and often sold at convenience stores. Some users have reported smoking these substances because they are generally not detected by standard urine toxicology tests.
Recreational use of synthetic cannabinoids can lead to serious and, at times, deadly complications. Chemicals found in rat poison have contaminated batches of synthetic cannabinoids, leading to coagulopathy and severe bleeding. Affected patients have reported hemoptysis, hematuria, severe epistaxis, bleeding gums, conjunctival hemorrhages, and gastrointestinal bleeding. The following case is of a patient who presented to an emergency department (ED) with severe coagulopathy and cardiotoxicity after using an adulterated synthetic cannabinoid product.
Case Presentation
A 65-year-old man presented to the ED reporting hematochezia, hematuria, and hemoptysis. He reported that these symptoms began about 1 day after he had smoked a synthetic cannabinoid called K2. The patient stated that some of his friends who used the same product were experiencing similar symptoms. He reported mild generalized abdominal pain but reported no chest pain, dyspnea, headache, fevers, chills, or dysuria.
The patient’s past medical history included hypertension, dyslipidemia, chronic lower back pain, and vitamin D deficiency. His past surgical history was notable for an exploratory laparotomy after a stab wound to the abdomen. The patient reported taking the following medications: morphine SA 30 mg bid, meloxicam 15 mg daily, amitriptyline 100 mg qhs, amlodipine 5 mg daily, hydrocodone/acetaminophen 5/325 mg q12h prn, atorvastatin 20 mg qhs, omeprazole 20 mg qam, senna 187 mg daily prn, psyllium 1 packet dissolved in water daily prn, and cholecalciferol 1,000 IU daily.
The patient’s temperature was 98o F, blood pressure, 144/80 mm Hg; pulse, 131 beats per minute; respiratory rate, 18 breaths per minute; and O2 saturation, 98% (ambient air). A physical examination revealed no acute distress; he was coughing up blood; clear lungs; heart sounds were tachycardic and irregularly irregular; soft, nondistended, mild generalized tenderness in the abdomen with no guarding and no rebound. The pertinent laboratory tests were international normalized ratio (INR), > 20; prothrombin time, > 150 seconds; prothrombin thromboplastin time, 157 seconds; hemoglobin, 13.3 g/dL; platelet count, 195 k/uL; white blood count, 11.3 k/uL; creatinine, 0.57mg/dL; potassium, 3.8 mmol/L, D-dimertest, 0.87 ug/mL fibrinogen equivalent units; fibrinogen level, 624 mg/dL; troponin, < 0.04 ng/mL; lactic acid, 1.3 mmol/L; total bilirubin, 0.8 mg/dL; alanine aminotransferase, 22 U/L, aspartate aminotransferase, 22 U/L; alkaline phosphatase, 89 U/L; urinalysis with > 50 red blood cells/high power field; large blood, negative leukocyte esterase, negative nitrite. The patient’s urine toxicology was negative for cannabinoids, methadone, amphetamines, cocaine, and benzodiazepines; but was positive for opiates. An anticoagulant poisoning panel also was ordered.
An electrocardiogram (ECG) and imaging studies were ordered. The ECG showed atrial fibrillation (AF) with rapid ventricular response (Figure 1). A chest X-ray indicated bibasilar consolidations that were worse on the right side. A noncontrast computed tomography (CT) of the head did not show intracranial bleeding. An abdomen/pelvis CT showed bilateral diffuse patchy peribronchovascular ground-glass opacities in the lung bases that could represent pulmonary hemorrhage, but no peritoneal or retroperitoneal bleeding.
Treatment
In the ED, the case was discussed with the Illinois Poison Control Center. The patient was diagnosed with coagulopathy likely due to anticoagulant poisoning. He was immediately treated with 10 mg of IV vitamin K, a fixed dose of 2,000 units of 4-factor prothrombin complex concentrate, and 4 units of fresh frozen plasma. His INR improved to 1.42 within several hours. He received 5 mg of IV metoprolol for uncontrolled AF and was admitted to the intensive care unit (ICU) for further care.
In the ICU the patient was started on oral vitamin K 50 mg tid for ongoing treatment of coagulopathy due to concern for possible rodenticide poisoning associated with very long half-life. This dose was then decreased to 50 mg bid. He was given IV fluid resuscitation with normal saline and started on rate control for AF with oral metoprolol. His heart rate improved. An echocardiogram showed new cardiomyopathy with an ejection fraction of 25% to 30%. Given basilar infiltrates and 1 episode of low-grade fever, he was started on ceftriaxone for possible community-acquired pneumonia. The patient was started on cholestyramine to help with washout of the possible rodenticide. No endoscopic interventions were performed.
The patient was transferred to an inpatient telemetry floor 24 hours after admission to the ICU once his tachycardia and bleeding improved. He did not require transfusion of packed red blood cells. In the ICU his INR had ranged between 1.62 and 2.46 (down from > 20 in the ED). His hemoglobin dropped from 13.3 g/dL on admission to 12 g/dL on transfer from the ICU, before stabilizing around 11 g/dL on the floor. The patient’s heart rate required better control, so metoprolol was increased to a total daily dose of 200 mg on the telemetry floor. Oral digoxin was then added after a digoxin load for additional rate control, as the patient remained tachycardic. Twice a day the patient continued to take 50 mg vitamin K. Cholestyramine and ceftriaxone were initially continued, but when the INR started increasing again, the cholestyramine was stopped to allow for an increase to more frequent 3-times daily vitamin 50 mg K administration (cholestyramine can interfere with vitamin K absorption). According to the toxicology service, there was only weak evidence to support use of cholestyramine in this setting.
Given his ongoing mild hemoptysis, the patient received first 1 unit, and then another 4 units of FFP when the INR increased to 3.96 despite oral vitamin K. After FFP, the INR decreased to 1.93 and subsequently to 1.52. A CT of the chest showed patchy ground-glass densities throughout the lungs, predominantly at the lung bases and to a lesser extent in the upper lobes. The findings were felt to represent pulmonary hemorrhage given the patient’s history of hemoptysis (Figure 2).
The patient’s heart rate control improved, and he remained hemodynamically stable. A thyroid function test was within normal limits. Lisinopril was added to metoprolol and digoxin given his newly diagnosed cardiomyopathy. The patient was observed for a total of 4 days on the inpatient floor and discharged after his INR stabilized around 1.5 on twice daily 50 mg vitamin K. The patient’s hematuria and hematochezia completely resolved, and hemoptysis was much improved at the time of discharge. His hemoglobin remained stable. The anticoagulant poisoning panel came back positive for
The patient has remained in AF at all follow-up visits. The INR normalized by 6 weeks after hospital discharge, and the dose of vitamin K slowly was tapered with close monitoring of the INR. Vitamin K was tapered for about 6 months after his initial presentation, and the patient was started on a direct oral anticoagulant (DOAC) for anticoagulation when the INR remained stable off vitamin K. He subsequently underwent a transesophageal echocardiogram followed by an attempt at direct current (DC) cardioversion; however, he did not remain in sinus rhythm, and is being continued on anticoagulation and rate control for his AF.
Discussion
Users generally smoke synthetic cannabinoids, which produce cannabis-like effects. However, atypical intoxication effects with worse complications often occur.2 These products typically contain dried shredded plant material that is soaked in or sprayed with several synthetic cannabinoids, varying in dosage and combination.3 Synthetic cannabinoids have been associated with serious adverse effects (AEs), including drowsiness, light-headedness, and fast or irregular heartbeat.4 More severe clinical features such as psychosis, delirium, cardiotoxicity, seizures, rhabdomyolysis, acute kidney injury, hyperthermia, myocardial ischemia, ischemic strokes, and death have also been noted.4
It is not known how some batches of synthetic cannabinoids came to be contaminated with rat poison or how commonly such an adulteration is found across the country. Several different guidelines provide pathways for the treatment of acute bleeding in the setting of coagulopathy due to vitamin K antagonists.5,6 Each guideline divides the indications for reversal into either severity of bleeding or the criticality of the bleeding based on location.5,6 All guidelines recommend the use of vitamin K (either oral or IV) followed by FFP or 4-factor prothrombin complex concentrate (PCC) for more severe bleeding.5,6 However, recommendations regarding the use of PCC vary in dosing for vitamin K antagonists (in contrast to treatment of coagulopathy due to DOACs). Recent studies and guidelines suggest that fixed-dose (rather than weight-based dose) PCC is effective for the reversal of coagulopathy due to vitamin K antagonists.6,7 Using fixed rather than weight-based dosing decreases cost and may decrease the possibility of thrombotic AEs.7 In this patient, a fixed-dose of 2,000 units of PCC was given based on data that were extrapolated from warfarin reversal using PCC.7
The vitamin K antagonists that adulterated this patient’s synthetic cannabinoid were difenacoum and brodifacoum, which are 4-hydroxycoumarin derivatives. These are second-generation long-acting anticoagulant rodenticides (LAARs) that are about 100 times more potent than warfarin.8 As the name implies, LAARs have a longer duration of action in the body of any organism that ingests the poison, which is due to the highly lipophilic groups that have been added to the warfarin molecule to combat resistance in rodents.9
As a result of the deposition in the tissues, there have been reports of the duration of action of brodifacoum ranging from 51 days to 9 months after ingestion, with the latter caused by an intentional overdose in a human.9-12 Reports suggest that coagulopathy is not likely to occur when the serum brodifacoum concentration is < 10 ng/mL.13,14 Animal models show difenacoum has a tissue half-life of about 62 days.15 Reports of difenacoum poisoning in humans have shown variable lengths of treatment, ranging from 30 to 47 days.16-18 The length of treatment for either brodifacoum or difenacoum will depend on the amount of poison exposure.
The long duration of action and treatment duration may lead to problems with drug procurement, especially in the early phase of treatment in which IV vitamin K is used. The supply of IV vitamin K recently has been limited for at least some manufacturers. According to the American Society of Health System Pharmacists Current Drug Shortage List, the increased demand is thought to be due to increased use of synthetic inhaled cannabinoids laced with anticoagulant.19 IV vitamin K products are available from suppliers such as Amphastar (Rancho Cucamonga, CA) and Hospira (Lake Forest, IL).
The American College of Chest Physicians recommends IV vitamin K administration in patients with major bleeding secondary to vitamin K antagonists.20 The oral route is thought to be more effective than a subcutaneous route in the treatment of nonbleeding patients with rodenticide-associated coagulopathy. Due to erratic and unpredictable absorption, the subcutaneous route of administration has fallen out of favor. Oral vitamin K products were not affected by the recent shortage. However, large doses of oral vitamin K can be costly. Due to the long half-life of LAAR, many patients are discharged with a prescription for oral vitamin K. Although vitamin K is found in most over-the-counter (OTC) multivitamins, the strength is insufficient. Most OTC formulations are ≤ 100 μg, whereas the prescription strength is 5 mg, but patients being treated for rodenticide poisoning require much larger doses.
Commercial insurance carriers and Medicare Part D usually do not cover vitamins and minerals unless it is for a medically accepted indication or is an indication supported by citation in either the American Hospital Formulary System, United States Pharmacopeia drug information book, or an electronic information resource that is supported by evidence such as Micromedex.21 For a patient without insurance coverage being treated with high-dose vitamin K therapy for rodenticide poisoning outside of a federal health care system, the cost could be as high as $500 to $1,000 per day, depending on the dose of vitamin K needed to maintain an acceptable INR.
Conclusion
In addition to bleeding as a result of coagulopathy, this patient presented with new onset of AF with rapid ventricular response and a newly diagnosed cardiomyopathy. Although the patient had other cardiovascular risk factors, such as hypertension, dyslipidemia, and a remote history of cocaine use, it is likely that the use of the synthetic cannabinoids contributed to the development and/or worsening of this arrhythmia and cardiomyopathy. The patient remained in AF 6 weeks after hospital discharge with a controlled ventricular rate on metoprolol and digoxin. An interval echocardiogram 6 weeks after hospital discharge showed a recovered ejection fraction. In cases of tachycardia-induced cardiomyopathy, the ejection fraction often recovers with control of the tachycardia. The patient was weaned off vitamin K about 6 months after his initial presentation and started on a DOAC for anticoagulation. He subsequently underwent a transesophageal echocardiogram followed by an attempt at DC cardioversion; however, he did not remain in sinus rhythm and is being continued on anticoagulation and rate control for his AF.
Although unclear how synthetic cannabinoids became adulterated with a potent vitamin K antagonist, health care practitioners should consider this if a patient presents with unexplained coagulopathy and widespread bleeding. Fixed-dose PCC should be considered as an alternative to weight-based dosing in these cases. Physicians and pharmacy personnel should anticipate a need for long-term high doses of vitamin K in order to begin work early to obtain sufficient supplies to treat presenting patients.
1. Illinois Department of Public Health. Synthetic cannabinoids. http://dph.illinois.gov/topics-services/prevention-wellness/medical-cannabis/synthetic-cannabinoids. Updated May 30, 2018. Accessed April 8, 2019.
2. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: update 2015. Subst Abus. 2017;38(3):344-366.
3. United Nations Office on Drugs and Crime. Global SMART update. https://www.unodc.org/documents/scientific/Global_SMART_Update_13_web.pdf. Published March 2015. Accessed April 8, 2019.
4. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235-242.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
6. Cushman M, Lim W, Zakai NA. 2011 Clinical Practice guide on anticoagulant dosing and management of anticoagulant-associated bleeding complications in adults. http://www.hematology.org/Clinicians/Guidelines-Quality/Quick-Ref/525.aspx. Published 2011. Accessed April 8, 2019.
7. Klein L, Peters J, Miner J, Gorlin J. Evaluation of fixed dose 4-factor prothrombin complex concentrate for emergent warfarin reversal. Am J Emerg Med. 2015;33(9):1213-1218.
8. Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281-288.
9. Lipton RA, Klass EM. Human ingestion of ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004-3005.
10. Chong LL, Chau WK, Ho CH. A case of ‘superwarfarin’ poisoning. Scand J Haematol. 1986;36(3):314-331.
11. Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
12. Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15(1):126-130.
13. Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med. 1993;153(16):1925-1928.
14. Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36(3):262-267.
15. Vandenbrouke V, Bousquet-Meloua A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31(5):437-445.
16. Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed). 1982;285(6341):541.
17. Katona B, Wason S. Superwarfarin poisoning. J Emerg Med. 1989;7(6):627-631.
18. Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol. 1992;11(6):553-554.
19. American Society of Health System Pharmacists. Current drug shortages. Vitamin K (phytonadione) injection. https://www.ashp.org/drug-shortages/current-shortages/Drug-Shortage-Detail.aspx?id=100. Updated July 5, 2018. Accessed April 8, 2019.
20. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
21. Centers for Medicare and Medicaid Services. Part D Excluded Drugs. https://www.medicareadvocacy.org/old-site/News/Archives/PartD_ExcludedDrugsByState.htm. Accessed on August 23, 2018.
Between March 7, 2018, and May 9, 2018, at least 164 people in Illinois were sickened by synthetic cannabinoids laced with rodenticides. The Illinois Department of Public Health has reported 4 deaths connected with the use of synthetic cannabinoids (sold under names such as Spice, K2, Legal Weed, etc).1 Synthetic cannabinoids are mind-altering chemicals that are sprayed on dried plant material and often sold at convenience stores. Some users have reported smoking these substances because they are generally not detected by standard urine toxicology tests.
Recreational use of synthetic cannabinoids can lead to serious and, at times, deadly complications. Chemicals found in rat poison have contaminated batches of synthetic cannabinoids, leading to coagulopathy and severe bleeding. Affected patients have reported hemoptysis, hematuria, severe epistaxis, bleeding gums, conjunctival hemorrhages, and gastrointestinal bleeding. The following case is of a patient who presented to an emergency department (ED) with severe coagulopathy and cardiotoxicity after using an adulterated synthetic cannabinoid product.
Case Presentation
A 65-year-old man presented to the ED reporting hematochezia, hematuria, and hemoptysis. He reported that these symptoms began about 1 day after he had smoked a synthetic cannabinoid called K2. The patient stated that some of his friends who used the same product were experiencing similar symptoms. He reported mild generalized abdominal pain but reported no chest pain, dyspnea, headache, fevers, chills, or dysuria.
The patient’s past medical history included hypertension, dyslipidemia, chronic lower back pain, and vitamin D deficiency. His past surgical history was notable for an exploratory laparotomy after a stab wound to the abdomen. The patient reported taking the following medications: morphine SA 30 mg bid, meloxicam 15 mg daily, amitriptyline 100 mg qhs, amlodipine 5 mg daily, hydrocodone/acetaminophen 5/325 mg q12h prn, atorvastatin 20 mg qhs, omeprazole 20 mg qam, senna 187 mg daily prn, psyllium 1 packet dissolved in water daily prn, and cholecalciferol 1,000 IU daily.
The patient’s temperature was 98o F, blood pressure, 144/80 mm Hg; pulse, 131 beats per minute; respiratory rate, 18 breaths per minute; and O2 saturation, 98% (ambient air). A physical examination revealed no acute distress; he was coughing up blood; clear lungs; heart sounds were tachycardic and irregularly irregular; soft, nondistended, mild generalized tenderness in the abdomen with no guarding and no rebound. The pertinent laboratory tests were international normalized ratio (INR), > 20; prothrombin time, > 150 seconds; prothrombin thromboplastin time, 157 seconds; hemoglobin, 13.3 g/dL; platelet count, 195 k/uL; white blood count, 11.3 k/uL; creatinine, 0.57mg/dL; potassium, 3.8 mmol/L, D-dimertest, 0.87 ug/mL fibrinogen equivalent units; fibrinogen level, 624 mg/dL; troponin, < 0.04 ng/mL; lactic acid, 1.3 mmol/L; total bilirubin, 0.8 mg/dL; alanine aminotransferase, 22 U/L, aspartate aminotransferase, 22 U/L; alkaline phosphatase, 89 U/L; urinalysis with > 50 red blood cells/high power field; large blood, negative leukocyte esterase, negative nitrite. The patient’s urine toxicology was negative for cannabinoids, methadone, amphetamines, cocaine, and benzodiazepines; but was positive for opiates. An anticoagulant poisoning panel also was ordered.
An electrocardiogram (ECG) and imaging studies were ordered. The ECG showed atrial fibrillation (AF) with rapid ventricular response (Figure 1). A chest X-ray indicated bibasilar consolidations that were worse on the right side. A noncontrast computed tomography (CT) of the head did not show intracranial bleeding. An abdomen/pelvis CT showed bilateral diffuse patchy peribronchovascular ground-glass opacities in the lung bases that could represent pulmonary hemorrhage, but no peritoneal or retroperitoneal bleeding.
Treatment
In the ED, the case was discussed with the Illinois Poison Control Center. The patient was diagnosed with coagulopathy likely due to anticoagulant poisoning. He was immediately treated with 10 mg of IV vitamin K, a fixed dose of 2,000 units of 4-factor prothrombin complex concentrate, and 4 units of fresh frozen plasma. His INR improved to 1.42 within several hours. He received 5 mg of IV metoprolol for uncontrolled AF and was admitted to the intensive care unit (ICU) for further care.
In the ICU the patient was started on oral vitamin K 50 mg tid for ongoing treatment of coagulopathy due to concern for possible rodenticide poisoning associated with very long half-life. This dose was then decreased to 50 mg bid. He was given IV fluid resuscitation with normal saline and started on rate control for AF with oral metoprolol. His heart rate improved. An echocardiogram showed new cardiomyopathy with an ejection fraction of 25% to 30%. Given basilar infiltrates and 1 episode of low-grade fever, he was started on ceftriaxone for possible community-acquired pneumonia. The patient was started on cholestyramine to help with washout of the possible rodenticide. No endoscopic interventions were performed.
The patient was transferred to an inpatient telemetry floor 24 hours after admission to the ICU once his tachycardia and bleeding improved. He did not require transfusion of packed red blood cells. In the ICU his INR had ranged between 1.62 and 2.46 (down from > 20 in the ED). His hemoglobin dropped from 13.3 g/dL on admission to 12 g/dL on transfer from the ICU, before stabilizing around 11 g/dL on the floor. The patient’s heart rate required better control, so metoprolol was increased to a total daily dose of 200 mg on the telemetry floor. Oral digoxin was then added after a digoxin load for additional rate control, as the patient remained tachycardic. Twice a day the patient continued to take 50 mg vitamin K. Cholestyramine and ceftriaxone were initially continued, but when the INR started increasing again, the cholestyramine was stopped to allow for an increase to more frequent 3-times daily vitamin 50 mg K administration (cholestyramine can interfere with vitamin K absorption). According to the toxicology service, there was only weak evidence to support use of cholestyramine in this setting.
Given his ongoing mild hemoptysis, the patient received first 1 unit, and then another 4 units of FFP when the INR increased to 3.96 despite oral vitamin K. After FFP, the INR decreased to 1.93 and subsequently to 1.52. A CT of the chest showed patchy ground-glass densities throughout the lungs, predominantly at the lung bases and to a lesser extent in the upper lobes. The findings were felt to represent pulmonary hemorrhage given the patient’s history of hemoptysis (Figure 2).
The patient’s heart rate control improved, and he remained hemodynamically stable. A thyroid function test was within normal limits. Lisinopril was added to metoprolol and digoxin given his newly diagnosed cardiomyopathy. The patient was observed for a total of 4 days on the inpatient floor and discharged after his INR stabilized around 1.5 on twice daily 50 mg vitamin K. The patient’s hematuria and hematochezia completely resolved, and hemoptysis was much improved at the time of discharge. His hemoglobin remained stable. The anticoagulant poisoning panel came back positive for
The patient has remained in AF at all follow-up visits. The INR normalized by 6 weeks after hospital discharge, and the dose of vitamin K slowly was tapered with close monitoring of the INR. Vitamin K was tapered for about 6 months after his initial presentation, and the patient was started on a direct oral anticoagulant (DOAC) for anticoagulation when the INR remained stable off vitamin K. He subsequently underwent a transesophageal echocardiogram followed by an attempt at direct current (DC) cardioversion; however, he did not remain in sinus rhythm, and is being continued on anticoagulation and rate control for his AF.
Discussion
Users generally smoke synthetic cannabinoids, which produce cannabis-like effects. However, atypical intoxication effects with worse complications often occur.2 These products typically contain dried shredded plant material that is soaked in or sprayed with several synthetic cannabinoids, varying in dosage and combination.3 Synthetic cannabinoids have been associated with serious adverse effects (AEs), including drowsiness, light-headedness, and fast or irregular heartbeat.4 More severe clinical features such as psychosis, delirium, cardiotoxicity, seizures, rhabdomyolysis, acute kidney injury, hyperthermia, myocardial ischemia, ischemic strokes, and death have also been noted.4
It is not known how some batches of synthetic cannabinoids came to be contaminated with rat poison or how commonly such an adulteration is found across the country. Several different guidelines provide pathways for the treatment of acute bleeding in the setting of coagulopathy due to vitamin K antagonists.5,6 Each guideline divides the indications for reversal into either severity of bleeding or the criticality of the bleeding based on location.5,6 All guidelines recommend the use of vitamin K (either oral or IV) followed by FFP or 4-factor prothrombin complex concentrate (PCC) for more severe bleeding.5,6 However, recommendations regarding the use of PCC vary in dosing for vitamin K antagonists (in contrast to treatment of coagulopathy due to DOACs). Recent studies and guidelines suggest that fixed-dose (rather than weight-based dose) PCC is effective for the reversal of coagulopathy due to vitamin K antagonists.6,7 Using fixed rather than weight-based dosing decreases cost and may decrease the possibility of thrombotic AEs.7 In this patient, a fixed-dose of 2,000 units of PCC was given based on data that were extrapolated from warfarin reversal using PCC.7
The vitamin K antagonists that adulterated this patient’s synthetic cannabinoid were difenacoum and brodifacoum, which are 4-hydroxycoumarin derivatives. These are second-generation long-acting anticoagulant rodenticides (LAARs) that are about 100 times more potent than warfarin.8 As the name implies, LAARs have a longer duration of action in the body of any organism that ingests the poison, which is due to the highly lipophilic groups that have been added to the warfarin molecule to combat resistance in rodents.9
As a result of the deposition in the tissues, there have been reports of the duration of action of brodifacoum ranging from 51 days to 9 months after ingestion, with the latter caused by an intentional overdose in a human.9-12 Reports suggest that coagulopathy is not likely to occur when the serum brodifacoum concentration is < 10 ng/mL.13,14 Animal models show difenacoum has a tissue half-life of about 62 days.15 Reports of difenacoum poisoning in humans have shown variable lengths of treatment, ranging from 30 to 47 days.16-18 The length of treatment for either brodifacoum or difenacoum will depend on the amount of poison exposure.
The long duration of action and treatment duration may lead to problems with drug procurement, especially in the early phase of treatment in which IV vitamin K is used. The supply of IV vitamin K recently has been limited for at least some manufacturers. According to the American Society of Health System Pharmacists Current Drug Shortage List, the increased demand is thought to be due to increased use of synthetic inhaled cannabinoids laced with anticoagulant.19 IV vitamin K products are available from suppliers such as Amphastar (Rancho Cucamonga, CA) and Hospira (Lake Forest, IL).
The American College of Chest Physicians recommends IV vitamin K administration in patients with major bleeding secondary to vitamin K antagonists.20 The oral route is thought to be more effective than a subcutaneous route in the treatment of nonbleeding patients with rodenticide-associated coagulopathy. Due to erratic and unpredictable absorption, the subcutaneous route of administration has fallen out of favor. Oral vitamin K products were not affected by the recent shortage. However, large doses of oral vitamin K can be costly. Due to the long half-life of LAAR, many patients are discharged with a prescription for oral vitamin K. Although vitamin K is found in most over-the-counter (OTC) multivitamins, the strength is insufficient. Most OTC formulations are ≤ 100 μg, whereas the prescription strength is 5 mg, but patients being treated for rodenticide poisoning require much larger doses.
Commercial insurance carriers and Medicare Part D usually do not cover vitamins and minerals unless it is for a medically accepted indication or is an indication supported by citation in either the American Hospital Formulary System, United States Pharmacopeia drug information book, or an electronic information resource that is supported by evidence such as Micromedex.21 For a patient without insurance coverage being treated with high-dose vitamin K therapy for rodenticide poisoning outside of a federal health care system, the cost could be as high as $500 to $1,000 per day, depending on the dose of vitamin K needed to maintain an acceptable INR.
Conclusion
In addition to bleeding as a result of coagulopathy, this patient presented with new onset of AF with rapid ventricular response and a newly diagnosed cardiomyopathy. Although the patient had other cardiovascular risk factors, such as hypertension, dyslipidemia, and a remote history of cocaine use, it is likely that the use of the synthetic cannabinoids contributed to the development and/or worsening of this arrhythmia and cardiomyopathy. The patient remained in AF 6 weeks after hospital discharge with a controlled ventricular rate on metoprolol and digoxin. An interval echocardiogram 6 weeks after hospital discharge showed a recovered ejection fraction. In cases of tachycardia-induced cardiomyopathy, the ejection fraction often recovers with control of the tachycardia. The patient was weaned off vitamin K about 6 months after his initial presentation and started on a DOAC for anticoagulation. He subsequently underwent a transesophageal echocardiogram followed by an attempt at DC cardioversion; however, he did not remain in sinus rhythm and is being continued on anticoagulation and rate control for his AF.
Although unclear how synthetic cannabinoids became adulterated with a potent vitamin K antagonist, health care practitioners should consider this if a patient presents with unexplained coagulopathy and widespread bleeding. Fixed-dose PCC should be considered as an alternative to weight-based dosing in these cases. Physicians and pharmacy personnel should anticipate a need for long-term high doses of vitamin K in order to begin work early to obtain sufficient supplies to treat presenting patients.
Between March 7, 2018, and May 9, 2018, at least 164 people in Illinois were sickened by synthetic cannabinoids laced with rodenticides. The Illinois Department of Public Health has reported 4 deaths connected with the use of synthetic cannabinoids (sold under names such as Spice, K2, Legal Weed, etc).1 Synthetic cannabinoids are mind-altering chemicals that are sprayed on dried plant material and often sold at convenience stores. Some users have reported smoking these substances because they are generally not detected by standard urine toxicology tests.
Recreational use of synthetic cannabinoids can lead to serious and, at times, deadly complications. Chemicals found in rat poison have contaminated batches of synthetic cannabinoids, leading to coagulopathy and severe bleeding. Affected patients have reported hemoptysis, hematuria, severe epistaxis, bleeding gums, conjunctival hemorrhages, and gastrointestinal bleeding. The following case is of a patient who presented to an emergency department (ED) with severe coagulopathy and cardiotoxicity after using an adulterated synthetic cannabinoid product.
Case Presentation
A 65-year-old man presented to the ED reporting hematochezia, hematuria, and hemoptysis. He reported that these symptoms began about 1 day after he had smoked a synthetic cannabinoid called K2. The patient stated that some of his friends who used the same product were experiencing similar symptoms. He reported mild generalized abdominal pain but reported no chest pain, dyspnea, headache, fevers, chills, or dysuria.
The patient’s past medical history included hypertension, dyslipidemia, chronic lower back pain, and vitamin D deficiency. His past surgical history was notable for an exploratory laparotomy after a stab wound to the abdomen. The patient reported taking the following medications: morphine SA 30 mg bid, meloxicam 15 mg daily, amitriptyline 100 mg qhs, amlodipine 5 mg daily, hydrocodone/acetaminophen 5/325 mg q12h prn, atorvastatin 20 mg qhs, omeprazole 20 mg qam, senna 187 mg daily prn, psyllium 1 packet dissolved in water daily prn, and cholecalciferol 1,000 IU daily.
The patient’s temperature was 98o F, blood pressure, 144/80 mm Hg; pulse, 131 beats per minute; respiratory rate, 18 breaths per minute; and O2 saturation, 98% (ambient air). A physical examination revealed no acute distress; he was coughing up blood; clear lungs; heart sounds were tachycardic and irregularly irregular; soft, nondistended, mild generalized tenderness in the abdomen with no guarding and no rebound. The pertinent laboratory tests were international normalized ratio (INR), > 20; prothrombin time, > 150 seconds; prothrombin thromboplastin time, 157 seconds; hemoglobin, 13.3 g/dL; platelet count, 195 k/uL; white blood count, 11.3 k/uL; creatinine, 0.57mg/dL; potassium, 3.8 mmol/L, D-dimertest, 0.87 ug/mL fibrinogen equivalent units; fibrinogen level, 624 mg/dL; troponin, < 0.04 ng/mL; lactic acid, 1.3 mmol/L; total bilirubin, 0.8 mg/dL; alanine aminotransferase, 22 U/L, aspartate aminotransferase, 22 U/L; alkaline phosphatase, 89 U/L; urinalysis with > 50 red blood cells/high power field; large blood, negative leukocyte esterase, negative nitrite. The patient’s urine toxicology was negative for cannabinoids, methadone, amphetamines, cocaine, and benzodiazepines; but was positive for opiates. An anticoagulant poisoning panel also was ordered.
An electrocardiogram (ECG) and imaging studies were ordered. The ECG showed atrial fibrillation (AF) with rapid ventricular response (Figure 1). A chest X-ray indicated bibasilar consolidations that were worse on the right side. A noncontrast computed tomography (CT) of the head did not show intracranial bleeding. An abdomen/pelvis CT showed bilateral diffuse patchy peribronchovascular ground-glass opacities in the lung bases that could represent pulmonary hemorrhage, but no peritoneal or retroperitoneal bleeding.
Treatment
In the ED, the case was discussed with the Illinois Poison Control Center. The patient was diagnosed with coagulopathy likely due to anticoagulant poisoning. He was immediately treated with 10 mg of IV vitamin K, a fixed dose of 2,000 units of 4-factor prothrombin complex concentrate, and 4 units of fresh frozen plasma. His INR improved to 1.42 within several hours. He received 5 mg of IV metoprolol for uncontrolled AF and was admitted to the intensive care unit (ICU) for further care.
In the ICU the patient was started on oral vitamin K 50 mg tid for ongoing treatment of coagulopathy due to concern for possible rodenticide poisoning associated with very long half-life. This dose was then decreased to 50 mg bid. He was given IV fluid resuscitation with normal saline and started on rate control for AF with oral metoprolol. His heart rate improved. An echocardiogram showed new cardiomyopathy with an ejection fraction of 25% to 30%. Given basilar infiltrates and 1 episode of low-grade fever, he was started on ceftriaxone for possible community-acquired pneumonia. The patient was started on cholestyramine to help with washout of the possible rodenticide. No endoscopic interventions were performed.
The patient was transferred to an inpatient telemetry floor 24 hours after admission to the ICU once his tachycardia and bleeding improved. He did not require transfusion of packed red blood cells. In the ICU his INR had ranged between 1.62 and 2.46 (down from > 20 in the ED). His hemoglobin dropped from 13.3 g/dL on admission to 12 g/dL on transfer from the ICU, before stabilizing around 11 g/dL on the floor. The patient’s heart rate required better control, so metoprolol was increased to a total daily dose of 200 mg on the telemetry floor. Oral digoxin was then added after a digoxin load for additional rate control, as the patient remained tachycardic. Twice a day the patient continued to take 50 mg vitamin K. Cholestyramine and ceftriaxone were initially continued, but when the INR started increasing again, the cholestyramine was stopped to allow for an increase to more frequent 3-times daily vitamin 50 mg K administration (cholestyramine can interfere with vitamin K absorption). According to the toxicology service, there was only weak evidence to support use of cholestyramine in this setting.
Given his ongoing mild hemoptysis, the patient received first 1 unit, and then another 4 units of FFP when the INR increased to 3.96 despite oral vitamin K. After FFP, the INR decreased to 1.93 and subsequently to 1.52. A CT of the chest showed patchy ground-glass densities throughout the lungs, predominantly at the lung bases and to a lesser extent in the upper lobes. The findings were felt to represent pulmonary hemorrhage given the patient’s history of hemoptysis (Figure 2).
The patient’s heart rate control improved, and he remained hemodynamically stable. A thyroid function test was within normal limits. Lisinopril was added to metoprolol and digoxin given his newly diagnosed cardiomyopathy. The patient was observed for a total of 4 days on the inpatient floor and discharged after his INR stabilized around 1.5 on twice daily 50 mg vitamin K. The patient’s hematuria and hematochezia completely resolved, and hemoptysis was much improved at the time of discharge. His hemoglobin remained stable. The anticoagulant poisoning panel came back positive for
The patient has remained in AF at all follow-up visits. The INR normalized by 6 weeks after hospital discharge, and the dose of vitamin K slowly was tapered with close monitoring of the INR. Vitamin K was tapered for about 6 months after his initial presentation, and the patient was started on a direct oral anticoagulant (DOAC) for anticoagulation when the INR remained stable off vitamin K. He subsequently underwent a transesophageal echocardiogram followed by an attempt at direct current (DC) cardioversion; however, he did not remain in sinus rhythm, and is being continued on anticoagulation and rate control for his AF.
Discussion
Users generally smoke synthetic cannabinoids, which produce cannabis-like effects. However, atypical intoxication effects with worse complications often occur.2 These products typically contain dried shredded plant material that is soaked in or sprayed with several synthetic cannabinoids, varying in dosage and combination.3 Synthetic cannabinoids have been associated with serious adverse effects (AEs), including drowsiness, light-headedness, and fast or irregular heartbeat.4 More severe clinical features such as psychosis, delirium, cardiotoxicity, seizures, rhabdomyolysis, acute kidney injury, hyperthermia, myocardial ischemia, ischemic strokes, and death have also been noted.4
It is not known how some batches of synthetic cannabinoids came to be contaminated with rat poison or how commonly such an adulteration is found across the country. Several different guidelines provide pathways for the treatment of acute bleeding in the setting of coagulopathy due to vitamin K antagonists.5,6 Each guideline divides the indications for reversal into either severity of bleeding or the criticality of the bleeding based on location.5,6 All guidelines recommend the use of vitamin K (either oral or IV) followed by FFP or 4-factor prothrombin complex concentrate (PCC) for more severe bleeding.5,6 However, recommendations regarding the use of PCC vary in dosing for vitamin K antagonists (in contrast to treatment of coagulopathy due to DOACs). Recent studies and guidelines suggest that fixed-dose (rather than weight-based dose) PCC is effective for the reversal of coagulopathy due to vitamin K antagonists.6,7 Using fixed rather than weight-based dosing decreases cost and may decrease the possibility of thrombotic AEs.7 In this patient, a fixed-dose of 2,000 units of PCC was given based on data that were extrapolated from warfarin reversal using PCC.7
The vitamin K antagonists that adulterated this patient’s synthetic cannabinoid were difenacoum and brodifacoum, which are 4-hydroxycoumarin derivatives. These are second-generation long-acting anticoagulant rodenticides (LAARs) that are about 100 times more potent than warfarin.8 As the name implies, LAARs have a longer duration of action in the body of any organism that ingests the poison, which is due to the highly lipophilic groups that have been added to the warfarin molecule to combat resistance in rodents.9
As a result of the deposition in the tissues, there have been reports of the duration of action of brodifacoum ranging from 51 days to 9 months after ingestion, with the latter caused by an intentional overdose in a human.9-12 Reports suggest that coagulopathy is not likely to occur when the serum brodifacoum concentration is < 10 ng/mL.13,14 Animal models show difenacoum has a tissue half-life of about 62 days.15 Reports of difenacoum poisoning in humans have shown variable lengths of treatment, ranging from 30 to 47 days.16-18 The length of treatment for either brodifacoum or difenacoum will depend on the amount of poison exposure.
The long duration of action and treatment duration may lead to problems with drug procurement, especially in the early phase of treatment in which IV vitamin K is used. The supply of IV vitamin K recently has been limited for at least some manufacturers. According to the American Society of Health System Pharmacists Current Drug Shortage List, the increased demand is thought to be due to increased use of synthetic inhaled cannabinoids laced with anticoagulant.19 IV vitamin K products are available from suppliers such as Amphastar (Rancho Cucamonga, CA) and Hospira (Lake Forest, IL).
The American College of Chest Physicians recommends IV vitamin K administration in patients with major bleeding secondary to vitamin K antagonists.20 The oral route is thought to be more effective than a subcutaneous route in the treatment of nonbleeding patients with rodenticide-associated coagulopathy. Due to erratic and unpredictable absorption, the subcutaneous route of administration has fallen out of favor. Oral vitamin K products were not affected by the recent shortage. However, large doses of oral vitamin K can be costly. Due to the long half-life of LAAR, many patients are discharged with a prescription for oral vitamin K. Although vitamin K is found in most over-the-counter (OTC) multivitamins, the strength is insufficient. Most OTC formulations are ≤ 100 μg, whereas the prescription strength is 5 mg, but patients being treated for rodenticide poisoning require much larger doses.
Commercial insurance carriers and Medicare Part D usually do not cover vitamins and minerals unless it is for a medically accepted indication or is an indication supported by citation in either the American Hospital Formulary System, United States Pharmacopeia drug information book, or an electronic information resource that is supported by evidence such as Micromedex.21 For a patient without insurance coverage being treated with high-dose vitamin K therapy for rodenticide poisoning outside of a federal health care system, the cost could be as high as $500 to $1,000 per day, depending on the dose of vitamin K needed to maintain an acceptable INR.
Conclusion
In addition to bleeding as a result of coagulopathy, this patient presented with new onset of AF with rapid ventricular response and a newly diagnosed cardiomyopathy. Although the patient had other cardiovascular risk factors, such as hypertension, dyslipidemia, and a remote history of cocaine use, it is likely that the use of the synthetic cannabinoids contributed to the development and/or worsening of this arrhythmia and cardiomyopathy. The patient remained in AF 6 weeks after hospital discharge with a controlled ventricular rate on metoprolol and digoxin. An interval echocardiogram 6 weeks after hospital discharge showed a recovered ejection fraction. In cases of tachycardia-induced cardiomyopathy, the ejection fraction often recovers with control of the tachycardia. The patient was weaned off vitamin K about 6 months after his initial presentation and started on a DOAC for anticoagulation. He subsequently underwent a transesophageal echocardiogram followed by an attempt at DC cardioversion; however, he did not remain in sinus rhythm and is being continued on anticoagulation and rate control for his AF.
Although unclear how synthetic cannabinoids became adulterated with a potent vitamin K antagonist, health care practitioners should consider this if a patient presents with unexplained coagulopathy and widespread bleeding. Fixed-dose PCC should be considered as an alternative to weight-based dosing in these cases. Physicians and pharmacy personnel should anticipate a need for long-term high doses of vitamin K in order to begin work early to obtain sufficient supplies to treat presenting patients.
1. Illinois Department of Public Health. Synthetic cannabinoids. http://dph.illinois.gov/topics-services/prevention-wellness/medical-cannabis/synthetic-cannabinoids. Updated May 30, 2018. Accessed April 8, 2019.
2. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: update 2015. Subst Abus. 2017;38(3):344-366.
3. United Nations Office on Drugs and Crime. Global SMART update. https://www.unodc.org/documents/scientific/Global_SMART_Update_13_web.pdf. Published March 2015. Accessed April 8, 2019.
4. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235-242.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
6. Cushman M, Lim W, Zakai NA. 2011 Clinical Practice guide on anticoagulant dosing and management of anticoagulant-associated bleeding complications in adults. http://www.hematology.org/Clinicians/Guidelines-Quality/Quick-Ref/525.aspx. Published 2011. Accessed April 8, 2019.
7. Klein L, Peters J, Miner J, Gorlin J. Evaluation of fixed dose 4-factor prothrombin complex concentrate for emergent warfarin reversal. Am J Emerg Med. 2015;33(9):1213-1218.
8. Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281-288.
9. Lipton RA, Klass EM. Human ingestion of ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004-3005.
10. Chong LL, Chau WK, Ho CH. A case of ‘superwarfarin’ poisoning. Scand J Haematol. 1986;36(3):314-331.
11. Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
12. Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15(1):126-130.
13. Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med. 1993;153(16):1925-1928.
14. Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36(3):262-267.
15. Vandenbrouke V, Bousquet-Meloua A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31(5):437-445.
16. Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed). 1982;285(6341):541.
17. Katona B, Wason S. Superwarfarin poisoning. J Emerg Med. 1989;7(6):627-631.
18. Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol. 1992;11(6):553-554.
19. American Society of Health System Pharmacists. Current drug shortages. Vitamin K (phytonadione) injection. https://www.ashp.org/drug-shortages/current-shortages/Drug-Shortage-Detail.aspx?id=100. Updated July 5, 2018. Accessed April 8, 2019.
20. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
21. Centers for Medicare and Medicaid Services. Part D Excluded Drugs. https://www.medicareadvocacy.org/old-site/News/Archives/PartD_ExcludedDrugsByState.htm. Accessed on August 23, 2018.
1. Illinois Department of Public Health. Synthetic cannabinoids. http://dph.illinois.gov/topics-services/prevention-wellness/medical-cannabis/synthetic-cannabinoids. Updated May 30, 2018. Accessed April 8, 2019.
2. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: update 2015. Subst Abus. 2017;38(3):344-366.
3. United Nations Office on Drugs and Crime. Global SMART update. https://www.unodc.org/documents/scientific/Global_SMART_Update_13_web.pdf. Published March 2015. Accessed April 8, 2019.
4. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235-242.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
6. Cushman M, Lim W, Zakai NA. 2011 Clinical Practice guide on anticoagulant dosing and management of anticoagulant-associated bleeding complications in adults. http://www.hematology.org/Clinicians/Guidelines-Quality/Quick-Ref/525.aspx. Published 2011. Accessed April 8, 2019.
7. Klein L, Peters J, Miner J, Gorlin J. Evaluation of fixed dose 4-factor prothrombin complex concentrate for emergent warfarin reversal. Am J Emerg Med. 2015;33(9):1213-1218.
8. Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281-288.
9. Lipton RA, Klass EM. Human ingestion of ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004-3005.
10. Chong LL, Chau WK, Ho CH. A case of ‘superwarfarin’ poisoning. Scand J Haematol. 1986;36(3):314-331.
11. Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
12. Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15(1):126-130.
13. Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med. 1993;153(16):1925-1928.
14. Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36(3):262-267.
15. Vandenbrouke V, Bousquet-Meloua A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31(5):437-445.
16. Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed). 1982;285(6341):541.
17. Katona B, Wason S. Superwarfarin poisoning. J Emerg Med. 1989;7(6):627-631.
18. Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol. 1992;11(6):553-554.
19. American Society of Health System Pharmacists. Current drug shortages. Vitamin K (phytonadione) injection. https://www.ashp.org/drug-shortages/current-shortages/Drug-Shortage-Detail.aspx?id=100. Updated July 5, 2018. Accessed April 8, 2019.
20. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
21. Centers for Medicare and Medicaid Services. Part D Excluded Drugs. https://www.medicareadvocacy.org/old-site/News/Archives/PartD_ExcludedDrugsByState.htm. Accessed on August 23, 2018.
Higher infection risk in RA seen with high blood biologic levels
BIRMINGHAM, ENGLAND – Higher blood biologic drug levels in the first year of treatment for rheumatoid arthritis independently increased the risk of any infection by about 50% when compared against low or normal levels in a new observational cohort study, providing support for monitoring biologic drug levels to help to predict infection risk.
Data from the British Society for Rheumatology Biologics Register – Rheumatoid Arthritis (BSRBR-RA) that were presented at the British Society for Rheumatology annual conference showed that the adjusted hazard ratio for any infection occurring within the first year among patients with high drug levels was 1.51, with a 95% confidence interval (CI) of 1.14 to 2.01. The adjustments took into account patients’ age, gender, disease activity score, and use of methotrexate.
There are more than 10 biologics now available for use in rheumatoid arthritis but deciding which to use in a particular patient remains very much “a trial and error approach,” first author Meghna Jani, MBChB, said at the conference.
“From a patient perspective, one of the most important concerns continues to be the risk of serious infections and adverse events,” added Dr. Jani, a National Institute for Health Research Academic Clinical Lecturer in Rheumatology at the University of Manchester (England).
The link between biologic agents and infections, including those that could result in hospitalization or other serious consequences, has been well studied in biologics registries. It is known, for example, that the risk of infections with tumor necrosis factor inhibitor treatment seems to be highest during the first 6-12 months of treatment.
According to Dr. Jani, conventional means of determining risk – such as patient age and the presence of comorbid factors – have limited benefit in terms of deciding which patients could be at heightened risk of infections. “Ideally, we need biomarkers in rheumatology that can be implemented in clinical practice and help us predict efficacy and safety, as well as help us use these medications much more cost-effectively,” she said.
Four years ago, a meta-analysis (Lancet. 2015;386:258-65) suggested that the risk of infection may be linked to using higher doses of anti–tumor necrosis factor drugs, which led the BSRBR-RA team to see if elevated levels of these drugs in the serum could be predictive of the infection risk and thus used as a possible biomarker. There was also prior evidence that serum drug concentrations of biologics were associated with long-term treatment response and that a certain level was needed to determine the likely treatment response.
In the current study, Dr. Jani and colleagues used data on 703 patients with rheumatoid arthritis starting biologic therapy who were simultaneously recruited into the BSRBR-RA, which has been running since 2001, and the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate (BRAGGSS). The BSRBR-RA did not collect biological samples, but in BRAGGSS serological samples were collected at 3-, 6-, and 12-month intervals after the start of a biologic treatment, along with other assessments. This is the first time two national, U.K.-based, rheumatoid arthritis cohorts have been linked in this way, Dr. Jani said.
Serum samples taken from the patients were assessed via enzyme-linked immunoassay to determine levels of the biologic agent used, with high drug levels defined as more than 4 mcg/mL for etanercept (n = 286), tocilizumab (n = 104), and infliximab (n = 14); more than 8 mcg/mL for adalimumab (n = 179), and 25 mcg/mL or more for certolizumab pegol (n = 120).
In the study, about three-quarters of the patients were women. The mean age was 58 years, and disease duration was just under 6 years. Most patients were starting their first biologic.
The crude rate of all infections at 1 year, including recurrent infections, was 464 per 1,000 patient-years in the high biologic drug level group versus 314 per 1,000 patient-years in the low biologic drug level group. When only the first infections were considered, the crude rate of all infections within the first year were a respective 300 and 229 per 1,000 patient-years, with an adjusted hazard ratio of 1.27, Dr. Jani reported.
As expected, lower respiratory tract infections were the most common type of infection, occurring in 34% of patients with high drug levels versus around 10% in the low drug level group. Upper respiratory tract, urinary tract, and skin infections including shingles were seen in a respective 16%, 15%, and 8% in the high drug level group, with rates less than 5% in the low drug level group.
Of note, there were certain types of infections present in the high but not low drug level groups: bacterial peritonitis, neutropenic sepsis, and herpes zoster.
Crude rates for serious infections at 1 year were 76 and 54 per 1,000 patient-years, respectively, for the high and low drug level groups. The crude rates for the first serious infection within the first year were 44 and 29 per 1,000 patient-years. The adjusted hazard ratio for the risk of serious infection at 1 year was 1.26. Serious infections were rare events, Dr. Jani emphasized, so the power was reduced, but “there was a slightly increased risk.”
Aside from the low statistical power to assess the rarer serious infections, another limitation was that drug levels were not measured at the time of the adverse event.
Concluding, Dr. Jani suggested that perhaps monitoring drug levels could be useful in predicting the risk of infection in patients being treated with biologics for rheumatoid arthritis.
Furthermore, “in patients with remission, dose-tapering guided by therapeutic drug monitoring may help lower infection risk and help us balance safety and efficacy.”
When asked to comment, Tore K. Kvien, MD, PhD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, supported this conclusion. “Therapeutic drug monitoring [TDM] is widely used among gastroenterologists when treating inflammatory bowel diseases with TNF inhibitors. In recent years, data from several research groups in rheumatology have indicated that TDM may help to optimize drug efficacy. The results from Dr. Jani and her colleagues also support that TDM may be important for safety. The importance of TDM as a ‘new’ hot topic in rheumatology is also supported by the recent establishment of a EULAR [European League Against Rheumatism] task force to further explore the value of TDM when treating patients with inflammatory joint diseases.”
The BSRBR-RA is funded through the BSR, which receives restricted income from several U.K. pharmaceutical companies. These currently include AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche, and in the past, Swedish Orphan Biovitrum and Merck. The pharmaceutical company funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Jani has no personal conflicts of interest to disclose.
SOURCE: Jani M et al. Rheumatology, 2019 April;58(Suppl 3):kez105.018.
In this study, the authors use the major British Society for Rheumatology Biologics Register – Rheumatoid Arthritis and examine infections and serious infections across biologics. They define “low/normal” blood levels versus “high” blood levels based on concentration-effect curves. Examining data censored at 1 year versus incidence during 1 year, the results are somewhat inconsistent. With larger numbers available for data censored at 1 year, there is some increased risk using hazard ratios for both all infections and serious infections. With smaller numbers for incident infections during the first year, this hazard ratio does not show an effect.
Daniel E. Furst, MD, is professor of medicine (emeritus) at the University of California, Los Angeles, an adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy). He is also practices part-time in Los Angeles and Seattle.
In this study, the authors use the major British Society for Rheumatology Biologics Register – Rheumatoid Arthritis and examine infections and serious infections across biologics. They define “low/normal” blood levels versus “high” blood levels based on concentration-effect curves. Examining data censored at 1 year versus incidence during 1 year, the results are somewhat inconsistent. With larger numbers available for data censored at 1 year, there is some increased risk using hazard ratios for both all infections and serious infections. With smaller numbers for incident infections during the first year, this hazard ratio does not show an effect.
Daniel E. Furst, MD, is professor of medicine (emeritus) at the University of California, Los Angeles, an adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy). He is also practices part-time in Los Angeles and Seattle.
In this study, the authors use the major British Society for Rheumatology Biologics Register – Rheumatoid Arthritis and examine infections and serious infections across biologics. They define “low/normal” blood levels versus “high” blood levels based on concentration-effect curves. Examining data censored at 1 year versus incidence during 1 year, the results are somewhat inconsistent. With larger numbers available for data censored at 1 year, there is some increased risk using hazard ratios for both all infections and serious infections. With smaller numbers for incident infections during the first year, this hazard ratio does not show an effect.
Daniel E. Furst, MD, is professor of medicine (emeritus) at the University of California, Los Angeles, an adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy). He is also practices part-time in Los Angeles and Seattle.
BIRMINGHAM, ENGLAND – Higher blood biologic drug levels in the first year of treatment for rheumatoid arthritis independently increased the risk of any infection by about 50% when compared against low or normal levels in a new observational cohort study, providing support for monitoring biologic drug levels to help to predict infection risk.
Data from the British Society for Rheumatology Biologics Register – Rheumatoid Arthritis (BSRBR-RA) that were presented at the British Society for Rheumatology annual conference showed that the adjusted hazard ratio for any infection occurring within the first year among patients with high drug levels was 1.51, with a 95% confidence interval (CI) of 1.14 to 2.01. The adjustments took into account patients’ age, gender, disease activity score, and use of methotrexate.
There are more than 10 biologics now available for use in rheumatoid arthritis but deciding which to use in a particular patient remains very much “a trial and error approach,” first author Meghna Jani, MBChB, said at the conference.
“From a patient perspective, one of the most important concerns continues to be the risk of serious infections and adverse events,” added Dr. Jani, a National Institute for Health Research Academic Clinical Lecturer in Rheumatology at the University of Manchester (England).
The link between biologic agents and infections, including those that could result in hospitalization or other serious consequences, has been well studied in biologics registries. It is known, for example, that the risk of infections with tumor necrosis factor inhibitor treatment seems to be highest during the first 6-12 months of treatment.
According to Dr. Jani, conventional means of determining risk – such as patient age and the presence of comorbid factors – have limited benefit in terms of deciding which patients could be at heightened risk of infections. “Ideally, we need biomarkers in rheumatology that can be implemented in clinical practice and help us predict efficacy and safety, as well as help us use these medications much more cost-effectively,” she said.
Four years ago, a meta-analysis (Lancet. 2015;386:258-65) suggested that the risk of infection may be linked to using higher doses of anti–tumor necrosis factor drugs, which led the BSRBR-RA team to see if elevated levels of these drugs in the serum could be predictive of the infection risk and thus used as a possible biomarker. There was also prior evidence that serum drug concentrations of biologics were associated with long-term treatment response and that a certain level was needed to determine the likely treatment response.
In the current study, Dr. Jani and colleagues used data on 703 patients with rheumatoid arthritis starting biologic therapy who were simultaneously recruited into the BSRBR-RA, which has been running since 2001, and the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate (BRAGGSS). The BSRBR-RA did not collect biological samples, but in BRAGGSS serological samples were collected at 3-, 6-, and 12-month intervals after the start of a biologic treatment, along with other assessments. This is the first time two national, U.K.-based, rheumatoid arthritis cohorts have been linked in this way, Dr. Jani said.
Serum samples taken from the patients were assessed via enzyme-linked immunoassay to determine levels of the biologic agent used, with high drug levels defined as more than 4 mcg/mL for etanercept (n = 286), tocilizumab (n = 104), and infliximab (n = 14); more than 8 mcg/mL for adalimumab (n = 179), and 25 mcg/mL or more for certolizumab pegol (n = 120).
In the study, about three-quarters of the patients were women. The mean age was 58 years, and disease duration was just under 6 years. Most patients were starting their first biologic.
The crude rate of all infections at 1 year, including recurrent infections, was 464 per 1,000 patient-years in the high biologic drug level group versus 314 per 1,000 patient-years in the low biologic drug level group. When only the first infections were considered, the crude rate of all infections within the first year were a respective 300 and 229 per 1,000 patient-years, with an adjusted hazard ratio of 1.27, Dr. Jani reported.
As expected, lower respiratory tract infections were the most common type of infection, occurring in 34% of patients with high drug levels versus around 10% in the low drug level group. Upper respiratory tract, urinary tract, and skin infections including shingles were seen in a respective 16%, 15%, and 8% in the high drug level group, with rates less than 5% in the low drug level group.
Of note, there were certain types of infections present in the high but not low drug level groups: bacterial peritonitis, neutropenic sepsis, and herpes zoster.
Crude rates for serious infections at 1 year were 76 and 54 per 1,000 patient-years, respectively, for the high and low drug level groups. The crude rates for the first serious infection within the first year were 44 and 29 per 1,000 patient-years. The adjusted hazard ratio for the risk of serious infection at 1 year was 1.26. Serious infections were rare events, Dr. Jani emphasized, so the power was reduced, but “there was a slightly increased risk.”
Aside from the low statistical power to assess the rarer serious infections, another limitation was that drug levels were not measured at the time of the adverse event.
Concluding, Dr. Jani suggested that perhaps monitoring drug levels could be useful in predicting the risk of infection in patients being treated with biologics for rheumatoid arthritis.
Furthermore, “in patients with remission, dose-tapering guided by therapeutic drug monitoring may help lower infection risk and help us balance safety and efficacy.”
When asked to comment, Tore K. Kvien, MD, PhD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, supported this conclusion. “Therapeutic drug monitoring [TDM] is widely used among gastroenterologists when treating inflammatory bowel diseases with TNF inhibitors. In recent years, data from several research groups in rheumatology have indicated that TDM may help to optimize drug efficacy. The results from Dr. Jani and her colleagues also support that TDM may be important for safety. The importance of TDM as a ‘new’ hot topic in rheumatology is also supported by the recent establishment of a EULAR [European League Against Rheumatism] task force to further explore the value of TDM when treating patients with inflammatory joint diseases.”
The BSRBR-RA is funded through the BSR, which receives restricted income from several U.K. pharmaceutical companies. These currently include AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche, and in the past, Swedish Orphan Biovitrum and Merck. The pharmaceutical company funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Jani has no personal conflicts of interest to disclose.
SOURCE: Jani M et al. Rheumatology, 2019 April;58(Suppl 3):kez105.018.
BIRMINGHAM, ENGLAND – Higher blood biologic drug levels in the first year of treatment for rheumatoid arthritis independently increased the risk of any infection by about 50% when compared against low or normal levels in a new observational cohort study, providing support for monitoring biologic drug levels to help to predict infection risk.
Data from the British Society for Rheumatology Biologics Register – Rheumatoid Arthritis (BSRBR-RA) that were presented at the British Society for Rheumatology annual conference showed that the adjusted hazard ratio for any infection occurring within the first year among patients with high drug levels was 1.51, with a 95% confidence interval (CI) of 1.14 to 2.01. The adjustments took into account patients’ age, gender, disease activity score, and use of methotrexate.
There are more than 10 biologics now available for use in rheumatoid arthritis but deciding which to use in a particular patient remains very much “a trial and error approach,” first author Meghna Jani, MBChB, said at the conference.
“From a patient perspective, one of the most important concerns continues to be the risk of serious infections and adverse events,” added Dr. Jani, a National Institute for Health Research Academic Clinical Lecturer in Rheumatology at the University of Manchester (England).
The link between biologic agents and infections, including those that could result in hospitalization or other serious consequences, has been well studied in biologics registries. It is known, for example, that the risk of infections with tumor necrosis factor inhibitor treatment seems to be highest during the first 6-12 months of treatment.
According to Dr. Jani, conventional means of determining risk – such as patient age and the presence of comorbid factors – have limited benefit in terms of deciding which patients could be at heightened risk of infections. “Ideally, we need biomarkers in rheumatology that can be implemented in clinical practice and help us predict efficacy and safety, as well as help us use these medications much more cost-effectively,” she said.
Four years ago, a meta-analysis (Lancet. 2015;386:258-65) suggested that the risk of infection may be linked to using higher doses of anti–tumor necrosis factor drugs, which led the BSRBR-RA team to see if elevated levels of these drugs in the serum could be predictive of the infection risk and thus used as a possible biomarker. There was also prior evidence that serum drug concentrations of biologics were associated with long-term treatment response and that a certain level was needed to determine the likely treatment response.
In the current study, Dr. Jani and colleagues used data on 703 patients with rheumatoid arthritis starting biologic therapy who were simultaneously recruited into the BSRBR-RA, which has been running since 2001, and the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate (BRAGGSS). The BSRBR-RA did not collect biological samples, but in BRAGGSS serological samples were collected at 3-, 6-, and 12-month intervals after the start of a biologic treatment, along with other assessments. This is the first time two national, U.K.-based, rheumatoid arthritis cohorts have been linked in this way, Dr. Jani said.
Serum samples taken from the patients were assessed via enzyme-linked immunoassay to determine levels of the biologic agent used, with high drug levels defined as more than 4 mcg/mL for etanercept (n = 286), tocilizumab (n = 104), and infliximab (n = 14); more than 8 mcg/mL for adalimumab (n = 179), and 25 mcg/mL or more for certolizumab pegol (n = 120).
In the study, about three-quarters of the patients were women. The mean age was 58 years, and disease duration was just under 6 years. Most patients were starting their first biologic.
The crude rate of all infections at 1 year, including recurrent infections, was 464 per 1,000 patient-years in the high biologic drug level group versus 314 per 1,000 patient-years in the low biologic drug level group. When only the first infections were considered, the crude rate of all infections within the first year were a respective 300 and 229 per 1,000 patient-years, with an adjusted hazard ratio of 1.27, Dr. Jani reported.
As expected, lower respiratory tract infections were the most common type of infection, occurring in 34% of patients with high drug levels versus around 10% in the low drug level group. Upper respiratory tract, urinary tract, and skin infections including shingles were seen in a respective 16%, 15%, and 8% in the high drug level group, with rates less than 5% in the low drug level group.
Of note, there were certain types of infections present in the high but not low drug level groups: bacterial peritonitis, neutropenic sepsis, and herpes zoster.
Crude rates for serious infections at 1 year were 76 and 54 per 1,000 patient-years, respectively, for the high and low drug level groups. The crude rates for the first serious infection within the first year were 44 and 29 per 1,000 patient-years. The adjusted hazard ratio for the risk of serious infection at 1 year was 1.26. Serious infections were rare events, Dr. Jani emphasized, so the power was reduced, but “there was a slightly increased risk.”
Aside from the low statistical power to assess the rarer serious infections, another limitation was that drug levels were not measured at the time of the adverse event.
Concluding, Dr. Jani suggested that perhaps monitoring drug levels could be useful in predicting the risk of infection in patients being treated with biologics for rheumatoid arthritis.
Furthermore, “in patients with remission, dose-tapering guided by therapeutic drug monitoring may help lower infection risk and help us balance safety and efficacy.”
When asked to comment, Tore K. Kvien, MD, PhD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, supported this conclusion. “Therapeutic drug monitoring [TDM] is widely used among gastroenterologists when treating inflammatory bowel diseases with TNF inhibitors. In recent years, data from several research groups in rheumatology have indicated that TDM may help to optimize drug efficacy. The results from Dr. Jani and her colleagues also support that TDM may be important for safety. The importance of TDM as a ‘new’ hot topic in rheumatology is also supported by the recent establishment of a EULAR [European League Against Rheumatism] task force to further explore the value of TDM when treating patients with inflammatory joint diseases.”
The BSRBR-RA is funded through the BSR, which receives restricted income from several U.K. pharmaceutical companies. These currently include AbbVie, Celltrion, Hospira, Pfizer, UCB, and Roche, and in the past, Swedish Orphan Biovitrum and Merck. The pharmaceutical company funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Jani has no personal conflicts of interest to disclose.
SOURCE: Jani M et al. Rheumatology, 2019 April;58(Suppl 3):kez105.018.
REPORTING FROM BSR 2019