User login
The search for a life-changing innovation
It might be the ultimate medical innovation – an artificial heart – and generations of physicians have pursued it, a story told in “Ticker: The Quest to Create an Artificial Heart.”
Author Mimi Swartz feared this history was being forgotten. “The larger-than-life personalities – Dr. Michael DeBakey and Dr. Denton Cooley – were such dominant figures for more than 50 years; I couldn’t stand for that history to be lost,” she said. “Also, so many innovations happened in Houston, including the implantation of the first artificial heart and the development of the Left Ventricular Assist Device – I couldn’t stand for that information to be lost too.”
Writing this book taught her a lot about innovation in medicine, the trade-offs involved in medical progress, even who benefits most.
“One of the most important things to think about is how many of these high-tech devices we need, and who will get them – who will be able to afford them,” she said.
“Medical innovation over the last 50 years is a global, billion dollar business, fraught with pitfalls: legal, governmental, ethical, financial, and, finally, personal,” Ms. Swartz said. “A great invention that could save millions of lives can end up on the junk heap because a hedge fund lost interest, while another great invention moves forward, but was stolen from the lab of another researcher. The persistence required to bring a medical device to market is daunting. One inventor told me, ‘If I’d known what was going to happen, I never would have even started.’ ”
Reference
Swartz M. Ticker: The Quest to Create an Artificial Heart. New York: Penguin Random House, 2018.
It might be the ultimate medical innovation – an artificial heart – and generations of physicians have pursued it, a story told in “Ticker: The Quest to Create an Artificial Heart.”
Author Mimi Swartz feared this history was being forgotten. “The larger-than-life personalities – Dr. Michael DeBakey and Dr. Denton Cooley – were such dominant figures for more than 50 years; I couldn’t stand for that history to be lost,” she said. “Also, so many innovations happened in Houston, including the implantation of the first artificial heart and the development of the Left Ventricular Assist Device – I couldn’t stand for that information to be lost too.”
Writing this book taught her a lot about innovation in medicine, the trade-offs involved in medical progress, even who benefits most.
“One of the most important things to think about is how many of these high-tech devices we need, and who will get them – who will be able to afford them,” she said.
“Medical innovation over the last 50 years is a global, billion dollar business, fraught with pitfalls: legal, governmental, ethical, financial, and, finally, personal,” Ms. Swartz said. “A great invention that could save millions of lives can end up on the junk heap because a hedge fund lost interest, while another great invention moves forward, but was stolen from the lab of another researcher. The persistence required to bring a medical device to market is daunting. One inventor told me, ‘If I’d known what was going to happen, I never would have even started.’ ”
Reference
Swartz M. Ticker: The Quest to Create an Artificial Heart. New York: Penguin Random House, 2018.
It might be the ultimate medical innovation – an artificial heart – and generations of physicians have pursued it, a story told in “Ticker: The Quest to Create an Artificial Heart.”
Author Mimi Swartz feared this history was being forgotten. “The larger-than-life personalities – Dr. Michael DeBakey and Dr. Denton Cooley – were such dominant figures for more than 50 years; I couldn’t stand for that history to be lost,” she said. “Also, so many innovations happened in Houston, including the implantation of the first artificial heart and the development of the Left Ventricular Assist Device – I couldn’t stand for that information to be lost too.”
Writing this book taught her a lot about innovation in medicine, the trade-offs involved in medical progress, even who benefits most.
“One of the most important things to think about is how many of these high-tech devices we need, and who will get them – who will be able to afford them,” she said.
“Medical innovation over the last 50 years is a global, billion dollar business, fraught with pitfalls: legal, governmental, ethical, financial, and, finally, personal,” Ms. Swartz said. “A great invention that could save millions of lives can end up on the junk heap because a hedge fund lost interest, while another great invention moves forward, but was stolen from the lab of another researcher. The persistence required to bring a medical device to market is daunting. One inventor told me, ‘If I’d known what was going to happen, I never would have even started.’ ”
Reference
Swartz M. Ticker: The Quest to Create an Artificial Heart. New York: Penguin Random House, 2018.
Laquinimod may not improve motor function in Huntington’s disease
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
PHILADELPHIA – Laquinimod appears not to improve motor function or clinical outcomes in patients with Huntington’s disease, according to a study presented at the annual meeting of the American Academy of Neurology. However, the drug reduced brain volume loss in the caudate and other regions.
Laquinimod is an investigational immunomodulatory drug that prevents inflammation and neurodegeneration in the CNS. The treatment was studied as a therapy for multiple sclerosis (MS), but development for this indication has been stopped. Researchers have observed that laquinimod modulates inflammatory pathways that are involved in Huntington’s disease pathology.
Ralf Reilmann, MD, PhD, founder of the George Huntington Institute and chair of the Huntington unit at the University of Münster (Germany), and colleagues conducted the phase 2 LEGATO-HD study at 48 sites in 10 countries to examine the efficacy and safety of laquinimod in patients with early Huntington’s disease. Participants were randomized in double-blind fashion to daily placebo or 0.5-mg, 1.0-mg, or 1.5-mg doses of laquinimod for 52 weeks. After the initiation of this trial, studies of the drug in MS indicated that the 1.5-mg dose was associated with cardiovascular risks, and Dr. Reilmann and his colleagues discontinued the 1.5-mg arm of their trial as a precaution.
The primary endpoint of LEGATO-HD was the change from baseline in the Unified Huntington’s Disease Rating Scale (UHDRS)–Total Motor Score (TMS). The secondary endpoint was the percent change in caudate volume at week 52 for the 1.0-mg dose group, compared with controls. The investigators also examined exploratory endpoints such as changes in MRI volume measures and Quantitative Motor, Clinician Interview-Based Impression of Change plus caregiver input, UHDRS–Total Functional Capacity and UHDRS–Functional Assessment scores. Adverse event reporting and clinical and laboratory examinations constituted the safety measures.
Dr. Reilmann and colleagues found no difference between the treated patients and controls in UHDRS-TMS. However, they did observe less caudate volume loss in the laquinimod group, compared with controls. All MRI exploratory measures also favored laquinimod. The researchers found no treatment effects of laquinimod in rater-dependent clinical outcome measures. Laquinimod was well tolerated, and the study yielded no new safety findings.
Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018 sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
SOURCE: Reilmann R et al. AAN 2019, Abstract S16.007.
REPORTING FROM AAN 2019
Key clinical point: Investigators found no difference between laquinimod and placebo on motor function in Huntington’s disease.
Major finding: The study examined 0.5-mg, 1.0-mg, and 1.5-mg doses of laquinimod.
Study details: The phase 2 LEGATO-HD trial included 352 patients with Huntington’s disease who underwent a 52-week treatment period.
Disclosures: Dr. Reilmann has received research support from Teva, which supported the LEGATO-HD trial and in 2018, sold development and commercial rights for laquinimod to Active Biotech. He has received research support from a variety of other pharmaceutical companies and organizations, including the CHDI Foundation and the European Huntington Disease Network.
Source: Reilmann R et al. AAN 2019, Abstract S16.007.
Eculizumab cuts relapse risk in NMO spectrum disorder
PHILADELPHIA – Treatment with the monoclonal antibody eculizumab substantially reduced the risk of relapse versus placebo in patients with aquaporin-4 positive neuromyelitis optica (NMO) spectrum disorder, according to the results of a phase 3 trial reported at the annual meeting of the American Academy of Neurology. Nearly 98% of patients with this autoimmune inflammatory CNS disorder were relapse-free at 48 weeks in the PREVENT trial, according to principal investigator Sean J. Pittock, MD, director of the Mayo Clinic’s Center for Multiple Sclerosis and Autoimmune Neurology in Rochester, Minn.
“This was a dramatic result, I think, really showing a significant amount of hope for people with this disease,” Dr. Pittock said in a press conference.
Most cases of NMO are associated with aquaporin-4 antibodies and complement-mediated CNS damage, and eculizumab (Soliris) is an inhibitor of complement protein C5 shown to reduce relapse frequency in a previous, small open-label study, according to Dr. Pittock.
In the current global phase 3 trial, conducted at 70 centers in 18 countries, 143 adult patients with aquaporin-4 positive NMO spectrum disorder were randomized to eculizumab every 2 weeks or placebo. The trial allowed for supportive immunosuppressive therapy and excluded patients who had received rituximab in the past 3 months. A total of 124 patients completed the study, which was stopped after 23 adjudicated relapses had occurred.
Time to first adjudicated relapse on trial, the primary endpoint of the study, showed a significant effect (P less than .0001) in favor of monoclonal antibody treatment over placebo, with a 94.2% reduction in risk of relapse, according to Dr. Pittock and coinvestigators.
At 48 weeks, 97.9% of patients were relapse free in the eculizumab group, versus 63.2% in the placebo group, they added in their report, which was published May 3 online ahead of print in the New England Journal of Medicine (doi: 10.1056/NEJMoa1900866).
Longer-term follow-up showed that, at 144 weeks, 96% of eculizumab-treated patients remained relapse free, while 45% of the placebo group were relapse free, Dr. Pittock said in the press conference.
Most adverse events seen on treatment were mild to moderate, and no meningococcal infections were observed.
One death occurred in the study from pulmonary empyema in an eculizumab-treated patient, but the associated cultures yielded microorganisms not associated with complement deficiency, investigators said in their published report.
“The concept that you can discover a target, understand the immunopathology of a disease, identify a novel mechanism, then identify a precision drug that targets that mechanism, and essentially turn off, or switch off, the disease is very, very exciting,” Dr. Pittock said in the press conference.
The study was supported by Alexion Pharmaceuticals. Dr. Pittock provided disclosures related to Alexion Pharmaceuticals, MedImmune, and Grifols, along with patents related to administration of eculizumab and cancer markers in neuromyelitis optica.
SOURCE: Pittock SJ et al. AAN 2019, Emerging Science Abstract 009.
PHILADELPHIA – Treatment with the monoclonal antibody eculizumab substantially reduced the risk of relapse versus placebo in patients with aquaporin-4 positive neuromyelitis optica (NMO) spectrum disorder, according to the results of a phase 3 trial reported at the annual meeting of the American Academy of Neurology. Nearly 98% of patients with this autoimmune inflammatory CNS disorder were relapse-free at 48 weeks in the PREVENT trial, according to principal investigator Sean J. Pittock, MD, director of the Mayo Clinic’s Center for Multiple Sclerosis and Autoimmune Neurology in Rochester, Minn.
“This was a dramatic result, I think, really showing a significant amount of hope for people with this disease,” Dr. Pittock said in a press conference.
Most cases of NMO are associated with aquaporin-4 antibodies and complement-mediated CNS damage, and eculizumab (Soliris) is an inhibitor of complement protein C5 shown to reduce relapse frequency in a previous, small open-label study, according to Dr. Pittock.
In the current global phase 3 trial, conducted at 70 centers in 18 countries, 143 adult patients with aquaporin-4 positive NMO spectrum disorder were randomized to eculizumab every 2 weeks or placebo. The trial allowed for supportive immunosuppressive therapy and excluded patients who had received rituximab in the past 3 months. A total of 124 patients completed the study, which was stopped after 23 adjudicated relapses had occurred.
Time to first adjudicated relapse on trial, the primary endpoint of the study, showed a significant effect (P less than .0001) in favor of monoclonal antibody treatment over placebo, with a 94.2% reduction in risk of relapse, according to Dr. Pittock and coinvestigators.
At 48 weeks, 97.9% of patients were relapse free in the eculizumab group, versus 63.2% in the placebo group, they added in their report, which was published May 3 online ahead of print in the New England Journal of Medicine (doi: 10.1056/NEJMoa1900866).
Longer-term follow-up showed that, at 144 weeks, 96% of eculizumab-treated patients remained relapse free, while 45% of the placebo group were relapse free, Dr. Pittock said in the press conference.
Most adverse events seen on treatment were mild to moderate, and no meningococcal infections were observed.
One death occurred in the study from pulmonary empyema in an eculizumab-treated patient, but the associated cultures yielded microorganisms not associated with complement deficiency, investigators said in their published report.
“The concept that you can discover a target, understand the immunopathology of a disease, identify a novel mechanism, then identify a precision drug that targets that mechanism, and essentially turn off, or switch off, the disease is very, very exciting,” Dr. Pittock said in the press conference.
The study was supported by Alexion Pharmaceuticals. Dr. Pittock provided disclosures related to Alexion Pharmaceuticals, MedImmune, and Grifols, along with patents related to administration of eculizumab and cancer markers in neuromyelitis optica.
SOURCE: Pittock SJ et al. AAN 2019, Emerging Science Abstract 009.
PHILADELPHIA – Treatment with the monoclonal antibody eculizumab substantially reduced the risk of relapse versus placebo in patients with aquaporin-4 positive neuromyelitis optica (NMO) spectrum disorder, according to the results of a phase 3 trial reported at the annual meeting of the American Academy of Neurology. Nearly 98% of patients with this autoimmune inflammatory CNS disorder were relapse-free at 48 weeks in the PREVENT trial, according to principal investigator Sean J. Pittock, MD, director of the Mayo Clinic’s Center for Multiple Sclerosis and Autoimmune Neurology in Rochester, Minn.
“This was a dramatic result, I think, really showing a significant amount of hope for people with this disease,” Dr. Pittock said in a press conference.
Most cases of NMO are associated with aquaporin-4 antibodies and complement-mediated CNS damage, and eculizumab (Soliris) is an inhibitor of complement protein C5 shown to reduce relapse frequency in a previous, small open-label study, according to Dr. Pittock.
In the current global phase 3 trial, conducted at 70 centers in 18 countries, 143 adult patients with aquaporin-4 positive NMO spectrum disorder were randomized to eculizumab every 2 weeks or placebo. The trial allowed for supportive immunosuppressive therapy and excluded patients who had received rituximab in the past 3 months. A total of 124 patients completed the study, which was stopped after 23 adjudicated relapses had occurred.
Time to first adjudicated relapse on trial, the primary endpoint of the study, showed a significant effect (P less than .0001) in favor of monoclonal antibody treatment over placebo, with a 94.2% reduction in risk of relapse, according to Dr. Pittock and coinvestigators.
At 48 weeks, 97.9% of patients were relapse free in the eculizumab group, versus 63.2% in the placebo group, they added in their report, which was published May 3 online ahead of print in the New England Journal of Medicine (doi: 10.1056/NEJMoa1900866).
Longer-term follow-up showed that, at 144 weeks, 96% of eculizumab-treated patients remained relapse free, while 45% of the placebo group were relapse free, Dr. Pittock said in the press conference.
Most adverse events seen on treatment were mild to moderate, and no meningococcal infections were observed.
One death occurred in the study from pulmonary empyema in an eculizumab-treated patient, but the associated cultures yielded microorganisms not associated with complement deficiency, investigators said in their published report.
“The concept that you can discover a target, understand the immunopathology of a disease, identify a novel mechanism, then identify a precision drug that targets that mechanism, and essentially turn off, or switch off, the disease is very, very exciting,” Dr. Pittock said in the press conference.
The study was supported by Alexion Pharmaceuticals. Dr. Pittock provided disclosures related to Alexion Pharmaceuticals, MedImmune, and Grifols, along with patents related to administration of eculizumab and cancer markers in neuromyelitis optica.
SOURCE: Pittock SJ et al. AAN 2019, Emerging Science Abstract 009.
FROM AAN 2019
Key clinical point: Treatment with eculizumab substantially reduced risk of relapse versus placebo in patients with aquaporin-4 positive neuromyelitis optica spectrum disorder.
Major finding: Time to first adjudicated relapse on trial, the primary endpoint of the study, showed a significant (P less than .0001) effect in favor of monoclonal antibody treatment over placebo, with a 94.2% reduction in risk of relapse.
Study details: A phase 3, randomized, double-blind, placebo-controlled, multicenter trial (PREVENT) including 143 adult patients.
Disclosures: The study was supported by Alexion Pharmaceuticals. Dr. Pittock provided disclosures related to Alexion Pharmaceuticals, MedImmune, and Grifols, along with patents related to administration of eculizumab and cancer markers in neuromyelitis optica.
Source: Pittock SJ et al. AAN 2019, Emerging Science Abstract 009.
Fournier gangrene cases surge in patients using SGLT2 inhibitors
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: The number of Fournier gangrene cases reported in patients receiving sodium-glucose cotransporter-2 (SGLT2) inhibitors has increased in the time since an FDA warning was issued about this rare but potentially serious infection.
Major finding: The previous FDA warning noted 12 reported cases from March 1, 2013 through March 1, 2018. This latest report included a total of 55 cases reported through January 31, 2019.
Study details: A review of spontaneous postmarketing cases of Fournier gangrene reported to the FDA or in the medical literature.
Disclosures: Authors disclosed no conflicts of interest related to the study.
Source: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6.
Employed physicians now outnumber independent doctors
For the first time, employed physicians outnumber independent physicians, according to a survey from the American Medical Association.
The AMA’s annual Physician Practice Benchmark Survey, which queried 3,500 doctors, showed that 47% of all physicians in 2018 were employed, compared with 46% of doctors who were self-employed that year. The number of employed physicians has risen 6 percentage points since 2012, while the number of self-employed doctors has fallen by 7 percentage points over the same period, according to the study published May 6 on the AMA website.
Younger physicians and women doctors were more likely to be employed than their counterparts. Nearly 70% of physicians under age 40 years were employees in 2018, compared with 38% of physicians 55 years and older, the study found. About 35% of physicians worked either directly for a hospital or in a practice at least partly owned by a hospital in 2018, up from 29% in 2012.
More than half of physicians surveyed (54%) worked in physician-owned practices in 2018 either as an owner, employee, or contractor, a decrease from 60% in 2012. Male physicians were more likely to be practice owners than female physicians. Among female doctors, 58% were employees, compared with 34% who were practice owners, while 52% of men physicians were practice owners, compared with 42% who were employees.
Surgical subspecialists had the highest share of owners (65%) followed by obstetrician-gynecologists (54%) and internal medicine subspecialists (52%). Emergency physicians had the lowest share of owners (26%) and the highest share of independent contractors (27%). Family physicians, meanwhile, had the highest share of employed physicians (57%).
A majority of doctors still work in small practices, the analysis found. In 2018, 57% of physicians worked in practices with 10 or fewer physicians versus 61% in 2012. However, fewer physicians work in solo practice. Between 2012 and 2018 the percentage of physicians in solo practice fell from 18% in 2012 to 15% in 2018.
“Transformational change continues in the delivery of health care and physicians are responding by reevaluating their practice arrangements,” AMA President Barbara L. McAneny, MD, said in a statement. “Physicians must assess many factors and carefully determine for themselves what settings they find professionally rewarding when considering independence or employment.”
The AMA’s Physician Practice Benchmark Survey is a nationally representative survey of post-residency physicians who provide at least 20 hours of patient care per week, are not employed by the federal government, and practice in one of the 50 states or the District of Columbia. The 2018 survey was conducted in September 2018, and the final data included 3,500 physicians.
For the first time, employed physicians outnumber independent physicians, according to a survey from the American Medical Association.
The AMA’s annual Physician Practice Benchmark Survey, which queried 3,500 doctors, showed that 47% of all physicians in 2018 were employed, compared with 46% of doctors who were self-employed that year. The number of employed physicians has risen 6 percentage points since 2012, while the number of self-employed doctors has fallen by 7 percentage points over the same period, according to the study published May 6 on the AMA website.
Younger physicians and women doctors were more likely to be employed than their counterparts. Nearly 70% of physicians under age 40 years were employees in 2018, compared with 38% of physicians 55 years and older, the study found. About 35% of physicians worked either directly for a hospital or in a practice at least partly owned by a hospital in 2018, up from 29% in 2012.
More than half of physicians surveyed (54%) worked in physician-owned practices in 2018 either as an owner, employee, or contractor, a decrease from 60% in 2012. Male physicians were more likely to be practice owners than female physicians. Among female doctors, 58% were employees, compared with 34% who were practice owners, while 52% of men physicians were practice owners, compared with 42% who were employees.
Surgical subspecialists had the highest share of owners (65%) followed by obstetrician-gynecologists (54%) and internal medicine subspecialists (52%). Emergency physicians had the lowest share of owners (26%) and the highest share of independent contractors (27%). Family physicians, meanwhile, had the highest share of employed physicians (57%).
A majority of doctors still work in small practices, the analysis found. In 2018, 57% of physicians worked in practices with 10 or fewer physicians versus 61% in 2012. However, fewer physicians work in solo practice. Between 2012 and 2018 the percentage of physicians in solo practice fell from 18% in 2012 to 15% in 2018.
“Transformational change continues in the delivery of health care and physicians are responding by reevaluating their practice arrangements,” AMA President Barbara L. McAneny, MD, said in a statement. “Physicians must assess many factors and carefully determine for themselves what settings they find professionally rewarding when considering independence or employment.”
The AMA’s Physician Practice Benchmark Survey is a nationally representative survey of post-residency physicians who provide at least 20 hours of patient care per week, are not employed by the federal government, and practice in one of the 50 states or the District of Columbia. The 2018 survey was conducted in September 2018, and the final data included 3,500 physicians.
For the first time, employed physicians outnumber independent physicians, according to a survey from the American Medical Association.
The AMA’s annual Physician Practice Benchmark Survey, which queried 3,500 doctors, showed that 47% of all physicians in 2018 were employed, compared with 46% of doctors who were self-employed that year. The number of employed physicians has risen 6 percentage points since 2012, while the number of self-employed doctors has fallen by 7 percentage points over the same period, according to the study published May 6 on the AMA website.
Younger physicians and women doctors were more likely to be employed than their counterparts. Nearly 70% of physicians under age 40 years were employees in 2018, compared with 38% of physicians 55 years and older, the study found. About 35% of physicians worked either directly for a hospital or in a practice at least partly owned by a hospital in 2018, up from 29% in 2012.
More than half of physicians surveyed (54%) worked in physician-owned practices in 2018 either as an owner, employee, or contractor, a decrease from 60% in 2012. Male physicians were more likely to be practice owners than female physicians. Among female doctors, 58% were employees, compared with 34% who were practice owners, while 52% of men physicians were practice owners, compared with 42% who were employees.
Surgical subspecialists had the highest share of owners (65%) followed by obstetrician-gynecologists (54%) and internal medicine subspecialists (52%). Emergency physicians had the lowest share of owners (26%) and the highest share of independent contractors (27%). Family physicians, meanwhile, had the highest share of employed physicians (57%).
A majority of doctors still work in small practices, the analysis found. In 2018, 57% of physicians worked in practices with 10 or fewer physicians versus 61% in 2012. However, fewer physicians work in solo practice. Between 2012 and 2018 the percentage of physicians in solo practice fell from 18% in 2012 to 15% in 2018.
“Transformational change continues in the delivery of health care and physicians are responding by reevaluating their practice arrangements,” AMA President Barbara L. McAneny, MD, said in a statement. “Physicians must assess many factors and carefully determine for themselves what settings they find professionally rewarding when considering independence or employment.”
The AMA’s Physician Practice Benchmark Survey is a nationally representative survey of post-residency physicians who provide at least 20 hours of patient care per week, are not employed by the federal government, and practice in one of the 50 states or the District of Columbia. The 2018 survey was conducted in September 2018, and the final data included 3,500 physicians.
Key clinical point: More doctors are employees vs. business owners for the first time.
Major finding: Of surveyed physicians, 47% are employees versus 46% who are self-employed.
Study details: Annual survey of 3,500 physicians.
Disclosures: The survey was conducted by the American Medical Association.
Source: Kane C. Updated Data on Physician Practice Arrangements. May 6, 2019.
Simple initiative boosted proportion of residents who screen for obesity
LOS ANGELES – After a simple educational initiative was implemented, the proportion of internal medicine residents who discussed the topic of overweight and obesity with patients improved from 17% to 69%, results from a single center have demonstrated.
“Everybody knows about the obesity epidemic, but nobody’s talking about it,” Hassan Mehmood, MD, said in an interview at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “They’re not doing enough to treat obesity. The United States Preventive Services Task Force [USPSTF] recommends screening all adults over the age of 18 years for obesity.”
In an effort to determine how often internal medicine residents are discussing obesity with patients, Dr. Mehmood and colleagues retrospectively reviewed the medical charts of 301 adults with a body mass index of 30 kg/m2 or greater who were seen at Temple University/Conemaugh Memorial Medical Center in Johnstown, Pa., between May and June of 2018. They recorded the total number of problems addressed, type of visit, whether obesity was discussed, and the resources used by the residents and attendings for management.
Between July and December of 2018, residents received education through lectures and conferences on obesity screening and management tools. The educational initiative included placement of posters in the clinic about obesity, and all physicians were encouraged to schedule separate visits to discuss the topic with patients. To evaluate the effects of the initiative, the researchers collected data on 255 adults with a BMI of 30 or greater who were seen in the clinic between May and June of 2018.
The mean age of patients in the study sample was between 40 and 50 years, 61% were women, and 91% of the office visits were for follow-up. The patients’ average BMI was 38, they had an average of two comorbidities, and residents most often addressed five diagnoses. From preintervention to postintervention, the researchers observed a statistically significant improvement in the frequency with which residents addressed general health maintenance with patients (from 62% to 83%; P less than .0005) and obesity (from 17% to 69%; P less than .0005). The discussions around obesity included talking about lifestyle modification, medication management, or bariatric surgical intervention.
Before the intervention, many residents reported not being aware of the USPSTF recommendations to screen all patients aged 18 years or older for obesity. They also felt pinched for time during office visits.
“They have only 30 minutes for treatment of chronic problems, so they didn’t find time to talk about obesity,” said Dr. Mehmood, who is a third-year resident at the medical center. “If residents don’t find time for talking about obesity, they can ask patients to return for a separate visit to talk about obesity and give management options to patients. Patients should know what their BMI is so that they can discuss it with their physician.”
He acknowledged that few patients are comfortable talking about their weight, and he suggested starting the conversation by asking “How do you feel about your weight?” during office visits. “That is the best question you can ask,” he said. “[It] helps open the conversation.”
Dr Mehmood reported having no financial disclosures.
LOS ANGELES – After a simple educational initiative was implemented, the proportion of internal medicine residents who discussed the topic of overweight and obesity with patients improved from 17% to 69%, results from a single center have demonstrated.
“Everybody knows about the obesity epidemic, but nobody’s talking about it,” Hassan Mehmood, MD, said in an interview at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “They’re not doing enough to treat obesity. The United States Preventive Services Task Force [USPSTF] recommends screening all adults over the age of 18 years for obesity.”
In an effort to determine how often internal medicine residents are discussing obesity with patients, Dr. Mehmood and colleagues retrospectively reviewed the medical charts of 301 adults with a body mass index of 30 kg/m2 or greater who were seen at Temple University/Conemaugh Memorial Medical Center in Johnstown, Pa., between May and June of 2018. They recorded the total number of problems addressed, type of visit, whether obesity was discussed, and the resources used by the residents and attendings for management.
Between July and December of 2018, residents received education through lectures and conferences on obesity screening and management tools. The educational initiative included placement of posters in the clinic about obesity, and all physicians were encouraged to schedule separate visits to discuss the topic with patients. To evaluate the effects of the initiative, the researchers collected data on 255 adults with a BMI of 30 or greater who were seen in the clinic between May and June of 2018.
The mean age of patients in the study sample was between 40 and 50 years, 61% were women, and 91% of the office visits were for follow-up. The patients’ average BMI was 38, they had an average of two comorbidities, and residents most often addressed five diagnoses. From preintervention to postintervention, the researchers observed a statistically significant improvement in the frequency with which residents addressed general health maintenance with patients (from 62% to 83%; P less than .0005) and obesity (from 17% to 69%; P less than .0005). The discussions around obesity included talking about lifestyle modification, medication management, or bariatric surgical intervention.
Before the intervention, many residents reported not being aware of the USPSTF recommendations to screen all patients aged 18 years or older for obesity. They also felt pinched for time during office visits.
“They have only 30 minutes for treatment of chronic problems, so they didn’t find time to talk about obesity,” said Dr. Mehmood, who is a third-year resident at the medical center. “If residents don’t find time for talking about obesity, they can ask patients to return for a separate visit to talk about obesity and give management options to patients. Patients should know what their BMI is so that they can discuss it with their physician.”
He acknowledged that few patients are comfortable talking about their weight, and he suggested starting the conversation by asking “How do you feel about your weight?” during office visits. “That is the best question you can ask,” he said. “[It] helps open the conversation.”
Dr Mehmood reported having no financial disclosures.
LOS ANGELES – After a simple educational initiative was implemented, the proportion of internal medicine residents who discussed the topic of overweight and obesity with patients improved from 17% to 69%, results from a single center have demonstrated.
“Everybody knows about the obesity epidemic, but nobody’s talking about it,” Hassan Mehmood, MD, said in an interview at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “They’re not doing enough to treat obesity. The United States Preventive Services Task Force [USPSTF] recommends screening all adults over the age of 18 years for obesity.”
In an effort to determine how often internal medicine residents are discussing obesity with patients, Dr. Mehmood and colleagues retrospectively reviewed the medical charts of 301 adults with a body mass index of 30 kg/m2 or greater who were seen at Temple University/Conemaugh Memorial Medical Center in Johnstown, Pa., between May and June of 2018. They recorded the total number of problems addressed, type of visit, whether obesity was discussed, and the resources used by the residents and attendings for management.
Between July and December of 2018, residents received education through lectures and conferences on obesity screening and management tools. The educational initiative included placement of posters in the clinic about obesity, and all physicians were encouraged to schedule separate visits to discuss the topic with patients. To evaluate the effects of the initiative, the researchers collected data on 255 adults with a BMI of 30 or greater who were seen in the clinic between May and June of 2018.
The mean age of patients in the study sample was between 40 and 50 years, 61% were women, and 91% of the office visits were for follow-up. The patients’ average BMI was 38, they had an average of two comorbidities, and residents most often addressed five diagnoses. From preintervention to postintervention, the researchers observed a statistically significant improvement in the frequency with which residents addressed general health maintenance with patients (from 62% to 83%; P less than .0005) and obesity (from 17% to 69%; P less than .0005). The discussions around obesity included talking about lifestyle modification, medication management, or bariatric surgical intervention.
Before the intervention, many residents reported not being aware of the USPSTF recommendations to screen all patients aged 18 years or older for obesity. They also felt pinched for time during office visits.
“They have only 30 minutes for treatment of chronic problems, so they didn’t find time to talk about obesity,” said Dr. Mehmood, who is a third-year resident at the medical center. “If residents don’t find time for talking about obesity, they can ask patients to return for a separate visit to talk about obesity and give management options to patients. Patients should know what their BMI is so that they can discuss it with their physician.”
He acknowledged that few patients are comfortable talking about their weight, and he suggested starting the conversation by asking “How do you feel about your weight?” during office visits. “That is the best question you can ask,” he said. “[It] helps open the conversation.”
Dr Mehmood reported having no financial disclosures.
REPORTING FROM AACE 2019
Key clinical point:
Major finding: From preintervention to postintervention, the researchers observed a statistically significant improvement in the frequency with which residents addressed obesity with patients (from 17% to 69%; P less than .0005).
Study details: A retrospective analysis of 301 patient visits before and 255 after implementation of an educational initiative.
Disclosures: Dr. Mehmood reported having no financial disclosures.
Weight-loss drug options expand, but beware cardiac risk
LOS ANGELES – Newer medications are much more powerful, but they come with cautions – insurer coverage can be a hurdle, and there are significant gaps in knowledge about their risks for patients with heart disease, Ken Fujioka, MD, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Fujioka, of Scripps Clinic in San Diego, shared some tips with his peers about using medications to reduce weight.
Diabetes drugs help, but may need a boost
Metformin can reduce weight by as much as 3%, Dr. Fujioka said. And there may be another benefit related to long-term weight loss maintenance, he said, citing a 15-year study of overweight or obese patients at high risk for diabetes who either received metformin, underwent an intensive lifestyle intervention, or took a placebo. Of the participants with weight loss of at least 5% after the first year, those originally assigned to receive metformin had the greatest weight loss during years 6-15. Older age, the amount of weight initially lost, and continued used of metformin were predictors of long-term weight loss maintenance, according to the researchers (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M18-1605).
There are other options among diabetes drugs. Sodium-glucose cotransporter 2 (SGLT2) inhibitors – a class of drugs that includes canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have a striking effect on weight loss, Dr. Fujioka said. They can cause 300 calories to be flushed out in the urine each day. But that typically doesn’t translate into weight loss of more than 20 pounds, he said, because the body doesn’t fully adjust to fewer calories.
“The patients begin to eat more,” he said. “They have to take in more calories to make up for [the loss]. They’re not consciously trying to do this. It’s a metabolic adaptation, so 2%-3% [weight loss] is about all you’ll get. You won’t get 10% or 20%.”
To drive up weight loss, Dr. Fujioka recommended adding the glucagonlike peptide–1 [GLP1] receptor diabetes drug exenatide (Byetta; Bydureon) or the appetite suppressant phentermine (Adipex-p; Lomaira) to an SGLT2 inhibitor. Recent studies have shown that the drug combinations have a greater impact on weight loss than when taken separately (Lancet Diabetes Endocrinol. 2016 Dec;4[12]:1004-16; Diabetes Care. 2017 May;40[5]:632-9).
In regard to phentermine, which acts similarly to amphetamine, Dr. Fujioka advised colleagues to be aware that “15 mg or less is really safe, but you drive pulse and heart rate beyond that.”
Consider insurance coverage and other factors
Often, insurers will pay for GLP1-receptor and SGLT2-inhibitor medications in patients with diabetes, even if their hemoglobin A1c is in the healthy range, Dr. Fujioka said, but they’ll balk at paying for specific weight-loss medications, although that can vary by the region of the country. He added that cash discount cards are available for several weight-loss drugs.
Newer weight-loss drugs ...
Dr. Fujioka highlighted a quartet of weight-loss drugs that have been approved in recent years.
- Lorcaserin (Belviq), a selective serotonin 2C receptor agonist, has shown unique benefits in patients with diabetes. A large, multinational, randomized controlled trial found that the drug reduced the risk for incident diabetes, induced remission of hyperglycemia, and reduced the risk of microvascular complications in obese and overweight patients (Lancet. 2018 Nov 24;392[10161]:2269-79).
- Phentermine/topiramate (Qsymia), a combination of an antiseizure medication (topiramate) and an appetite suppressant (phentermine). A 2014 study found that the drug, together with lifestyle modification, effectively promoted weight loss and improved glycemic control in obese or overweight patients with type 2 diabetes (Diabetes Care. 2014 Dec;37[12]:3309-16).
- Naltrexone/bupropion (Contrave), a combination of an addiction drug (naltrexone) and an antidepressant (bupropion). Findings from a 2013 study reported that the drug “in overweight/obese patients with type 2 diabetes induced weight loss... was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.” (Diabetes Care. 2013 Dec;36[12]:4022-9).
- Liraglutide, an injectable GLP1 agonist that has been approved for diabetes (Victoza) and weight loss (Saxenda). Dr. Fujioka was coauthor for a study in which the findings suggested that the drug could prevent prediabetes from turning into diabetes. (Lancet. 2017 Apr 8;389[10077]:1399-409).
... but watch out for safety in patients with heart disease
Two of the newer weight-loss drugs are OK to prescribe for diabetic patients with heart disease, Dr. Fujioka said, but two are not, because no cardiac safety trials have been completed for them.
Liraglutide (at a dose of 3.0 mg) is considered safe based on previous data (Diabetes Obes Metab. 2018 Mar;20[3]:734-9), Dr. Fujioka said. Likewise, findings from a trial with lorcaserin in which 12,000 overweight or obese patients with atherosclerotic cardiovascular disease or multiple cardiovascular risk factors received either lorcaserin (10 mg twice daily) or placebo, suggested that lorcaserin helped sustain weight loss without a higher rate of major cardiovascular events compared with placebo (N Engl J Med. 2018 Sep 20;379[12]:1107-17).However, no such cardiac safety trials have been completed for naltrexone/bupropion or phentermine/topiramate, said Dr. Fujioka. As a result, he said he could not recommend either of them for patients with high-risk cardiovascular disease.
Dr. Fujioka disclosed relationships of various types with Novo Nordisk, Eisai, Gelesis, KVK Tech, Amgen, Sunovion, Boehringer Ingelheim, and Janssen Global Services.
LOS ANGELES – Newer medications are much more powerful, but they come with cautions – insurer coverage can be a hurdle, and there are significant gaps in knowledge about their risks for patients with heart disease, Ken Fujioka, MD, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Fujioka, of Scripps Clinic in San Diego, shared some tips with his peers about using medications to reduce weight.
Diabetes drugs help, but may need a boost
Metformin can reduce weight by as much as 3%, Dr. Fujioka said. And there may be another benefit related to long-term weight loss maintenance, he said, citing a 15-year study of overweight or obese patients at high risk for diabetes who either received metformin, underwent an intensive lifestyle intervention, or took a placebo. Of the participants with weight loss of at least 5% after the first year, those originally assigned to receive metformin had the greatest weight loss during years 6-15. Older age, the amount of weight initially lost, and continued used of metformin were predictors of long-term weight loss maintenance, according to the researchers (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M18-1605).
There are other options among diabetes drugs. Sodium-glucose cotransporter 2 (SGLT2) inhibitors – a class of drugs that includes canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have a striking effect on weight loss, Dr. Fujioka said. They can cause 300 calories to be flushed out in the urine each day. But that typically doesn’t translate into weight loss of more than 20 pounds, he said, because the body doesn’t fully adjust to fewer calories.
“The patients begin to eat more,” he said. “They have to take in more calories to make up for [the loss]. They’re not consciously trying to do this. It’s a metabolic adaptation, so 2%-3% [weight loss] is about all you’ll get. You won’t get 10% or 20%.”
To drive up weight loss, Dr. Fujioka recommended adding the glucagonlike peptide–1 [GLP1] receptor diabetes drug exenatide (Byetta; Bydureon) or the appetite suppressant phentermine (Adipex-p; Lomaira) to an SGLT2 inhibitor. Recent studies have shown that the drug combinations have a greater impact on weight loss than when taken separately (Lancet Diabetes Endocrinol. 2016 Dec;4[12]:1004-16; Diabetes Care. 2017 May;40[5]:632-9).
In regard to phentermine, which acts similarly to amphetamine, Dr. Fujioka advised colleagues to be aware that “15 mg or less is really safe, but you drive pulse and heart rate beyond that.”
Consider insurance coverage and other factors
Often, insurers will pay for GLP1-receptor and SGLT2-inhibitor medications in patients with diabetes, even if their hemoglobin A1c is in the healthy range, Dr. Fujioka said, but they’ll balk at paying for specific weight-loss medications, although that can vary by the region of the country. He added that cash discount cards are available for several weight-loss drugs.
Newer weight-loss drugs ...
Dr. Fujioka highlighted a quartet of weight-loss drugs that have been approved in recent years.
- Lorcaserin (Belviq), a selective serotonin 2C receptor agonist, has shown unique benefits in patients with diabetes. A large, multinational, randomized controlled trial found that the drug reduced the risk for incident diabetes, induced remission of hyperglycemia, and reduced the risk of microvascular complications in obese and overweight patients (Lancet. 2018 Nov 24;392[10161]:2269-79).
- Phentermine/topiramate (Qsymia), a combination of an antiseizure medication (topiramate) and an appetite suppressant (phentermine). A 2014 study found that the drug, together with lifestyle modification, effectively promoted weight loss and improved glycemic control in obese or overweight patients with type 2 diabetes (Diabetes Care. 2014 Dec;37[12]:3309-16).
- Naltrexone/bupropion (Contrave), a combination of an addiction drug (naltrexone) and an antidepressant (bupropion). Findings from a 2013 study reported that the drug “in overweight/obese patients with type 2 diabetes induced weight loss... was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.” (Diabetes Care. 2013 Dec;36[12]:4022-9).
- Liraglutide, an injectable GLP1 agonist that has been approved for diabetes (Victoza) and weight loss (Saxenda). Dr. Fujioka was coauthor for a study in which the findings suggested that the drug could prevent prediabetes from turning into diabetes. (Lancet. 2017 Apr 8;389[10077]:1399-409).
... but watch out for safety in patients with heart disease
Two of the newer weight-loss drugs are OK to prescribe for diabetic patients with heart disease, Dr. Fujioka said, but two are not, because no cardiac safety trials have been completed for them.
Liraglutide (at a dose of 3.0 mg) is considered safe based on previous data (Diabetes Obes Metab. 2018 Mar;20[3]:734-9), Dr. Fujioka said. Likewise, findings from a trial with lorcaserin in which 12,000 overweight or obese patients with atherosclerotic cardiovascular disease or multiple cardiovascular risk factors received either lorcaserin (10 mg twice daily) or placebo, suggested that lorcaserin helped sustain weight loss without a higher rate of major cardiovascular events compared with placebo (N Engl J Med. 2018 Sep 20;379[12]:1107-17).However, no such cardiac safety trials have been completed for naltrexone/bupropion or phentermine/topiramate, said Dr. Fujioka. As a result, he said he could not recommend either of them for patients with high-risk cardiovascular disease.
Dr. Fujioka disclosed relationships of various types with Novo Nordisk, Eisai, Gelesis, KVK Tech, Amgen, Sunovion, Boehringer Ingelheim, and Janssen Global Services.
LOS ANGELES – Newer medications are much more powerful, but they come with cautions – insurer coverage can be a hurdle, and there are significant gaps in knowledge about their risks for patients with heart disease, Ken Fujioka, MD, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Fujioka, of Scripps Clinic in San Diego, shared some tips with his peers about using medications to reduce weight.
Diabetes drugs help, but may need a boost
Metformin can reduce weight by as much as 3%, Dr. Fujioka said. And there may be another benefit related to long-term weight loss maintenance, he said, citing a 15-year study of overweight or obese patients at high risk for diabetes who either received metformin, underwent an intensive lifestyle intervention, or took a placebo. Of the participants with weight loss of at least 5% after the first year, those originally assigned to receive metformin had the greatest weight loss during years 6-15. Older age, the amount of weight initially lost, and continued used of metformin were predictors of long-term weight loss maintenance, according to the researchers (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M18-1605).
There are other options among diabetes drugs. Sodium-glucose cotransporter 2 (SGLT2) inhibitors – a class of drugs that includes canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have a striking effect on weight loss, Dr. Fujioka said. They can cause 300 calories to be flushed out in the urine each day. But that typically doesn’t translate into weight loss of more than 20 pounds, he said, because the body doesn’t fully adjust to fewer calories.
“The patients begin to eat more,” he said. “They have to take in more calories to make up for [the loss]. They’re not consciously trying to do this. It’s a metabolic adaptation, so 2%-3% [weight loss] is about all you’ll get. You won’t get 10% or 20%.”
To drive up weight loss, Dr. Fujioka recommended adding the glucagonlike peptide–1 [GLP1] receptor diabetes drug exenatide (Byetta; Bydureon) or the appetite suppressant phentermine (Adipex-p; Lomaira) to an SGLT2 inhibitor. Recent studies have shown that the drug combinations have a greater impact on weight loss than when taken separately (Lancet Diabetes Endocrinol. 2016 Dec;4[12]:1004-16; Diabetes Care. 2017 May;40[5]:632-9).
In regard to phentermine, which acts similarly to amphetamine, Dr. Fujioka advised colleagues to be aware that “15 mg or less is really safe, but you drive pulse and heart rate beyond that.”
Consider insurance coverage and other factors
Often, insurers will pay for GLP1-receptor and SGLT2-inhibitor medications in patients with diabetes, even if their hemoglobin A1c is in the healthy range, Dr. Fujioka said, but they’ll balk at paying for specific weight-loss medications, although that can vary by the region of the country. He added that cash discount cards are available for several weight-loss drugs.
Newer weight-loss drugs ...
Dr. Fujioka highlighted a quartet of weight-loss drugs that have been approved in recent years.
- Lorcaserin (Belviq), a selective serotonin 2C receptor agonist, has shown unique benefits in patients with diabetes. A large, multinational, randomized controlled trial found that the drug reduced the risk for incident diabetes, induced remission of hyperglycemia, and reduced the risk of microvascular complications in obese and overweight patients (Lancet. 2018 Nov 24;392[10161]:2269-79).
- Phentermine/topiramate (Qsymia), a combination of an antiseizure medication (topiramate) and an appetite suppressant (phentermine). A 2014 study found that the drug, together with lifestyle modification, effectively promoted weight loss and improved glycemic control in obese or overweight patients with type 2 diabetes (Diabetes Care. 2014 Dec;37[12]:3309-16).
- Naltrexone/bupropion (Contrave), a combination of an addiction drug (naltrexone) and an antidepressant (bupropion). Findings from a 2013 study reported that the drug “in overweight/obese patients with type 2 diabetes induced weight loss... was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.” (Diabetes Care. 2013 Dec;36[12]:4022-9).
- Liraglutide, an injectable GLP1 agonist that has been approved for diabetes (Victoza) and weight loss (Saxenda). Dr. Fujioka was coauthor for a study in which the findings suggested that the drug could prevent prediabetes from turning into diabetes. (Lancet. 2017 Apr 8;389[10077]:1399-409).
... but watch out for safety in patients with heart disease
Two of the newer weight-loss drugs are OK to prescribe for diabetic patients with heart disease, Dr. Fujioka said, but two are not, because no cardiac safety trials have been completed for them.
Liraglutide (at a dose of 3.0 mg) is considered safe based on previous data (Diabetes Obes Metab. 2018 Mar;20[3]:734-9), Dr. Fujioka said. Likewise, findings from a trial with lorcaserin in which 12,000 overweight or obese patients with atherosclerotic cardiovascular disease or multiple cardiovascular risk factors received either lorcaserin (10 mg twice daily) or placebo, suggested that lorcaserin helped sustain weight loss without a higher rate of major cardiovascular events compared with placebo (N Engl J Med. 2018 Sep 20;379[12]:1107-17).However, no such cardiac safety trials have been completed for naltrexone/bupropion or phentermine/topiramate, said Dr. Fujioka. As a result, he said he could not recommend either of them for patients with high-risk cardiovascular disease.
Dr. Fujioka disclosed relationships of various types with Novo Nordisk, Eisai, Gelesis, KVK Tech, Amgen, Sunovion, Boehringer Ingelheim, and Janssen Global Services.
EXPERT ANALYSIS FROM AACE 2019
Gabapentin falls short in treating sickle cell pain
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
REPORTING FROM THE 2019 ASPHO CONFERENCE
Key clinical point:
Major finding: The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23).
Study details: A phase 2 trial of 86 evaluable patients.
Disclosures: The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. The speaker did not provide disclosure information at the meeting.
Source: Puri L et al. 2019 ASPHO Conference, Abstract 2011.
Painful, slow-growing recurrent nodules
A 67-year-old woman presented with multiple painful nodules that had developed on her scalp, face, and neck over the course of 1 year. She also had a few nodules on her trunk and hip. There was no associated bleeding, ulceration, or drainage from the lesions. She had no systemic symptoms. The patient reported that she’d had a similar lesion on her frontal scalp about 15 years earlier, and it was excised completely. (She was not aware of the diagnosis.) She indicated that her mother and son had similar lesions in the past.
Her medical history was remarkable for diabetes and hypertension, which were well controlled on metformin and lisinopril, respectively. The patient had cancer of the left breast that was treated with mastectomy and chemotherapy 3 years prior.
On physical examination, the patient had multiple firm, rubbery, tender nodules with tan or pink hue, measuring 1 to 1.5 cm. The nodules were located on the left side of her chin and right preauricular area (FIGURE 1), as well as the right sides of her neck and hip. Most of the nodules were solitary; the preauricular area had 2 clustered pink lesions. The largest nodule was located on the patient’s chin and had overlying telangiectasias.
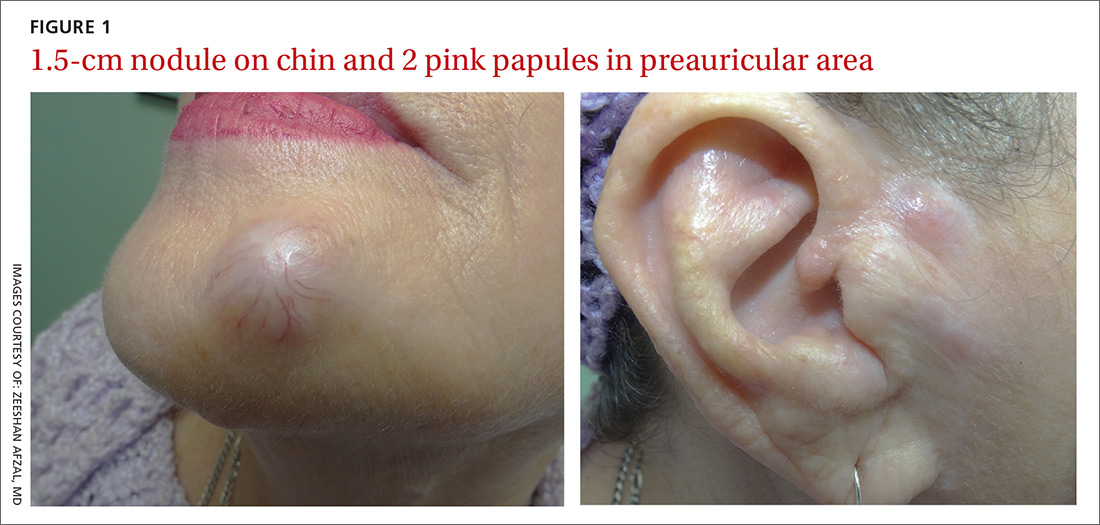
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cylindroma
Definitive diagnosis was made by shave biopsy of the left hip lesion. Histopathology demonstrated various-sized discrete aggregates of basaloid cell nests in a jigsaw puzzle–like configuration (FIGURE 2), surrounded by rims of homogenous eosinophilic material. Histologic findings were consistent with cylindroma.1
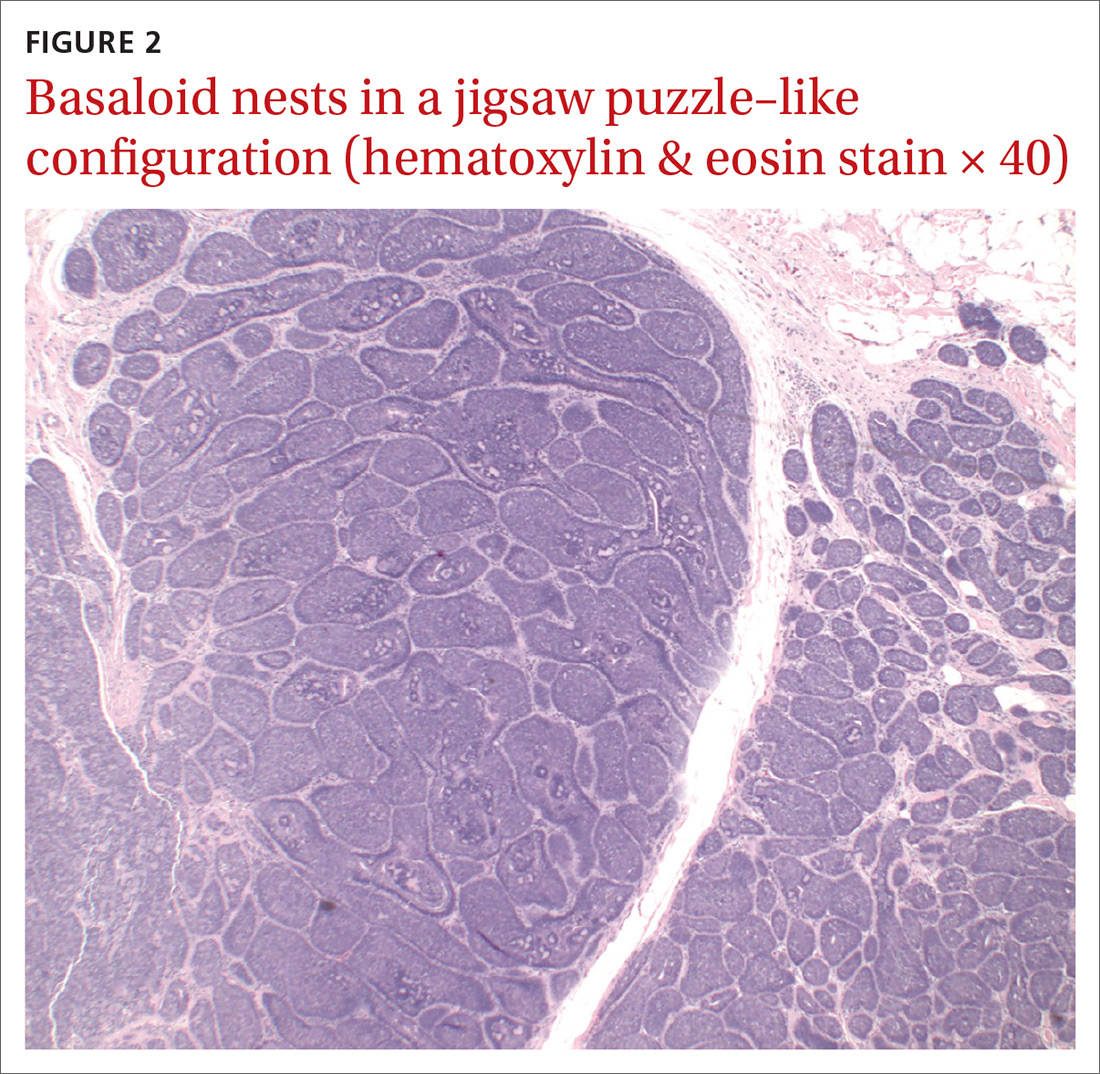
Rare with a female predominance. Solitary cylindromas occur sporadically and usually affect middle-aged and elderly patients. Incidence is rare, but there is a female predominance of 6 to 9:1.2 Clinical appearance shows a slow-growing, firm mass that can range from a few millimeters to a few centimeters in diameter. The masses can have a pink or blue hue and usually are nontender unless there is nerve impingement.2
If multiple tumors are present or the patient has a family history of similar lesions, the disorder is likely inherited in an autosomal dominant pattern (with variable expression), which can be associated with Brooke-Spiegler syndrome. This syndrome is related to a mutation of the cylindromatosis gene on chromosome 16. This is a tumor suppressor gene, inactivation of which can lead to uninhibited action of NF-
Rarely, cylindromas can undergo malignant transformation; signs include ulceration, bleeding, rapid growth, or color change.2 In these cases, appropriate imaging such as computed tomography, magnetic resonance imaging, or positron emission tomography should be sought as there have been case reports of cylindroma extension to bone, as well as metastases to sites including the lymph nodes, thyroid, liver, lungs, bones, and meninges.4
Lipomas and pilar cysts comprise the differential
Lipomas are soft, painless, flesh-colored masses that typically appear on the trunk and arms but are uncommon on the face. Telangiectasias are not seen.
Continue to: Pilar cysts
Pilar cysts can mimic cylindromas in clinical presentation. Derived from the root sheath of hair follicles, they typically appear on the scalp but rarely on the face. Pilar cysts are slow growing, firm, and whitish in color5; several cysts can appear at a time.
Pilomatricomas are firm skin masses—usually < 3 cm in size—that can vary in color. There may be an extrusion of calcified material within the nodules, which does not occur in cylindromas.
Sebaceous adenomas are yellowish papules, usually < 1 cm in size, that appear on the head and neck area.6
Making the diagnosis. The clinical appearance of cylindromas and their location will point to the diagnosis, as will a family history of similar lesions. Ultimately, the diagnosis is confirmed by biopsy.
Treatment mainstay includes excision
Complete excision of all lesions is key due to the possibility of malignant transformation and metastasis. Options for removal include electrodesiccation, curettage, cryosurgery,3 high-dose radiation, and the use of a CO2 laser.2 For multiple large tumors, or ones in cosmetically sensitive areas, consider referral to Dermatology or Plastic Surgery. Further imaging should be sought to rule out extension to bone or metastasis. Patients with multiple cylindromas and Brooke-Spiegler syndrome need lifelong follow-up to monitor for recurrence and malignant transformation.3,7,8
Continue to: Our patient...
Our patient underwent complete excision of the left hip lesion. Other trunk lesions were excised serially. The patient was referred to a surgeon specializing in Mohs micrographic surgery for excision of the facial lesions. The patient and her family members were referred for genetic testing.
CORRESPONDENCE
Zeeshan Afzal, MD, McAllen Family Medicine Residency Program, University of Texas Rio Grande Valley, 205 E Toronto Ave, McAllen, TX 78503; [email protected]
1. Elder D, Elenitsas R, Jaworsky CH, et al. Tumors of the epidermal appendages. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:775-777.
2. Singh DD, Naujoks C, Depprich R, et al. Cylindroma of head and neck: Review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516-521.
3. Sicinska J, Rakowska A, Czuwara-Ladykowska J, et al. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1:4-9.
4. Jordão C, de Magalhães TC, Cuzzi T, et al. Cylindroma: an update. Int J Dermatol. 2015;54:275-278.
5. Stone M. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1920.
6. McCalmont T, Pincus L. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1938-1942.
7. Gerretsen AL, Van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
8. Manicketh I, Singh R, Ghosh PK. Eccrine cylindroma of the face and scalp. Indian Dermatol Online J. 2016;7:203-205.
A 67-year-old woman presented with multiple painful nodules that had developed on her scalp, face, and neck over the course of 1 year. She also had a few nodules on her trunk and hip. There was no associated bleeding, ulceration, or drainage from the lesions. She had no systemic symptoms. The patient reported that she’d had a similar lesion on her frontal scalp about 15 years earlier, and it was excised completely. (She was not aware of the diagnosis.) She indicated that her mother and son had similar lesions in the past.
Her medical history was remarkable for diabetes and hypertension, which were well controlled on metformin and lisinopril, respectively. The patient had cancer of the left breast that was treated with mastectomy and chemotherapy 3 years prior.
On physical examination, the patient had multiple firm, rubbery, tender nodules with tan or pink hue, measuring 1 to 1.5 cm. The nodules were located on the left side of her chin and right preauricular area (FIGURE 1), as well as the right sides of her neck and hip. Most of the nodules were solitary; the preauricular area had 2 clustered pink lesions. The largest nodule was located on the patient’s chin and had overlying telangiectasias.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cylindroma
Definitive diagnosis was made by shave biopsy of the left hip lesion. Histopathology demonstrated various-sized discrete aggregates of basaloid cell nests in a jigsaw puzzle–like configuration (FIGURE 2), surrounded by rims of homogenous eosinophilic material. Histologic findings were consistent with cylindroma.1

Rare with a female predominance. Solitary cylindromas occur sporadically and usually affect middle-aged and elderly patients. Incidence is rare, but there is a female predominance of 6 to 9:1.2 Clinical appearance shows a slow-growing, firm mass that can range from a few millimeters to a few centimeters in diameter. The masses can have a pink or blue hue and usually are nontender unless there is nerve impingement.2
If multiple tumors are present or the patient has a family history of similar lesions, the disorder is likely inherited in an autosomal dominant pattern (with variable expression), which can be associated with Brooke-Spiegler syndrome. This syndrome is related to a mutation of the cylindromatosis gene on chromosome 16. This is a tumor suppressor gene, inactivation of which can lead to uninhibited action of NF-
Rarely, cylindromas can undergo malignant transformation; signs include ulceration, bleeding, rapid growth, or color change.2 In these cases, appropriate imaging such as computed tomography, magnetic resonance imaging, or positron emission tomography should be sought as there have been case reports of cylindroma extension to bone, as well as metastases to sites including the lymph nodes, thyroid, liver, lungs, bones, and meninges.4
Lipomas and pilar cysts comprise the differential
Lipomas are soft, painless, flesh-colored masses that typically appear on the trunk and arms but are uncommon on the face. Telangiectasias are not seen.
Continue to: Pilar cysts
Pilar cysts can mimic cylindromas in clinical presentation. Derived from the root sheath of hair follicles, they typically appear on the scalp but rarely on the face. Pilar cysts are slow growing, firm, and whitish in color5; several cysts can appear at a time.
Pilomatricomas are firm skin masses—usually < 3 cm in size—that can vary in color. There may be an extrusion of calcified material within the nodules, which does not occur in cylindromas.
Sebaceous adenomas are yellowish papules, usually < 1 cm in size, that appear on the head and neck area.6
Making the diagnosis. The clinical appearance of cylindromas and their location will point to the diagnosis, as will a family history of similar lesions. Ultimately, the diagnosis is confirmed by biopsy.
Treatment mainstay includes excision
Complete excision of all lesions is key due to the possibility of malignant transformation and metastasis. Options for removal include electrodesiccation, curettage, cryosurgery,3 high-dose radiation, and the use of a CO2 laser.2 For multiple large tumors, or ones in cosmetically sensitive areas, consider referral to Dermatology or Plastic Surgery. Further imaging should be sought to rule out extension to bone or metastasis. Patients with multiple cylindromas and Brooke-Spiegler syndrome need lifelong follow-up to monitor for recurrence and malignant transformation.3,7,8
Continue to: Our patient...
Our patient underwent complete excision of the left hip lesion. Other trunk lesions were excised serially. The patient was referred to a surgeon specializing in Mohs micrographic surgery for excision of the facial lesions. The patient and her family members were referred for genetic testing.
CORRESPONDENCE
Zeeshan Afzal, MD, McAllen Family Medicine Residency Program, University of Texas Rio Grande Valley, 205 E Toronto Ave, McAllen, TX 78503; [email protected]
A 67-year-old woman presented with multiple painful nodules that had developed on her scalp, face, and neck over the course of 1 year. She also had a few nodules on her trunk and hip. There was no associated bleeding, ulceration, or drainage from the lesions. She had no systemic symptoms. The patient reported that she’d had a similar lesion on her frontal scalp about 15 years earlier, and it was excised completely. (She was not aware of the diagnosis.) She indicated that her mother and son had similar lesions in the past.
Her medical history was remarkable for diabetes and hypertension, which were well controlled on metformin and lisinopril, respectively. The patient had cancer of the left breast that was treated with mastectomy and chemotherapy 3 years prior.
On physical examination, the patient had multiple firm, rubbery, tender nodules with tan or pink hue, measuring 1 to 1.5 cm. The nodules were located on the left side of her chin and right preauricular area (FIGURE 1), as well as the right sides of her neck and hip. Most of the nodules were solitary; the preauricular area had 2 clustered pink lesions. The largest nodule was located on the patient’s chin and had overlying telangiectasias.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cylindroma
Definitive diagnosis was made by shave biopsy of the left hip lesion. Histopathology demonstrated various-sized discrete aggregates of basaloid cell nests in a jigsaw puzzle–like configuration (FIGURE 2), surrounded by rims of homogenous eosinophilic material. Histologic findings were consistent with cylindroma.1

Rare with a female predominance. Solitary cylindromas occur sporadically and usually affect middle-aged and elderly patients. Incidence is rare, but there is a female predominance of 6 to 9:1.2 Clinical appearance shows a slow-growing, firm mass that can range from a few millimeters to a few centimeters in diameter. The masses can have a pink or blue hue and usually are nontender unless there is nerve impingement.2
If multiple tumors are present or the patient has a family history of similar lesions, the disorder is likely inherited in an autosomal dominant pattern (with variable expression), which can be associated with Brooke-Spiegler syndrome. This syndrome is related to a mutation of the cylindromatosis gene on chromosome 16. This is a tumor suppressor gene, inactivation of which can lead to uninhibited action of NF-
Rarely, cylindromas can undergo malignant transformation; signs include ulceration, bleeding, rapid growth, or color change.2 In these cases, appropriate imaging such as computed tomography, magnetic resonance imaging, or positron emission tomography should be sought as there have been case reports of cylindroma extension to bone, as well as metastases to sites including the lymph nodes, thyroid, liver, lungs, bones, and meninges.4
Lipomas and pilar cysts comprise the differential
Lipomas are soft, painless, flesh-colored masses that typically appear on the trunk and arms but are uncommon on the face. Telangiectasias are not seen.
Continue to: Pilar cysts
Pilar cysts can mimic cylindromas in clinical presentation. Derived from the root sheath of hair follicles, they typically appear on the scalp but rarely on the face. Pilar cysts are slow growing, firm, and whitish in color5; several cysts can appear at a time.
Pilomatricomas are firm skin masses—usually < 3 cm in size—that can vary in color. There may be an extrusion of calcified material within the nodules, which does not occur in cylindromas.
Sebaceous adenomas are yellowish papules, usually < 1 cm in size, that appear on the head and neck area.6
Making the diagnosis. The clinical appearance of cylindromas and their location will point to the diagnosis, as will a family history of similar lesions. Ultimately, the diagnosis is confirmed by biopsy.
Treatment mainstay includes excision
Complete excision of all lesions is key due to the possibility of malignant transformation and metastasis. Options for removal include electrodesiccation, curettage, cryosurgery,3 high-dose radiation, and the use of a CO2 laser.2 For multiple large tumors, or ones in cosmetically sensitive areas, consider referral to Dermatology or Plastic Surgery. Further imaging should be sought to rule out extension to bone or metastasis. Patients with multiple cylindromas and Brooke-Spiegler syndrome need lifelong follow-up to monitor for recurrence and malignant transformation.3,7,8
Continue to: Our patient...
Our patient underwent complete excision of the left hip lesion. Other trunk lesions were excised serially. The patient was referred to a surgeon specializing in Mohs micrographic surgery for excision of the facial lesions. The patient and her family members were referred for genetic testing.
CORRESPONDENCE
Zeeshan Afzal, MD, McAllen Family Medicine Residency Program, University of Texas Rio Grande Valley, 205 E Toronto Ave, McAllen, TX 78503; [email protected]
1. Elder D, Elenitsas R, Jaworsky CH, et al. Tumors of the epidermal appendages. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:775-777.
2. Singh DD, Naujoks C, Depprich R, et al. Cylindroma of head and neck: Review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516-521.
3. Sicinska J, Rakowska A, Czuwara-Ladykowska J, et al. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1:4-9.
4. Jordão C, de Magalhães TC, Cuzzi T, et al. Cylindroma: an update. Int J Dermatol. 2015;54:275-278.
5. Stone M. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1920.
6. McCalmont T, Pincus L. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1938-1942.
7. Gerretsen AL, Van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
8. Manicketh I, Singh R, Ghosh PK. Eccrine cylindroma of the face and scalp. Indian Dermatol Online J. 2016;7:203-205.
1. Elder D, Elenitsas R, Jaworsky CH, et al. Tumors of the epidermal appendages. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:775-777.
2. Singh DD, Naujoks C, Depprich R, et al. Cylindroma of head and neck: Review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516-521.
3. Sicinska J, Rakowska A, Czuwara-Ladykowska J, et al. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1:4-9.
4. Jordão C, de Magalhães TC, Cuzzi T, et al. Cylindroma: an update. Int J Dermatol. 2015;54:275-278.
5. Stone M. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1920.
6. McCalmont T, Pincus L. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1938-1942.
7. Gerretsen AL, Van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
8. Manicketh I, Singh R, Ghosh PK. Eccrine cylindroma of the face and scalp. Indian Dermatol Online J. 2016;7:203-205.
Mantle Cell Lymphoma Roundtable Discussion
Mantle cell lymphoma (MCL) is a rare, often aggressive form of non-Hodgkin lymphoma that develops when the body makes abnormal B-cells, and it is typically diagnosed at a later stage of disease. In this video series, Dr. Andre Goy sits down with Drs. Matthew Matasar and Peter Martin to discuss diagnosis, treatment, and unmet needs in MCL.

This video roundtable was produced by the Custom Programs division. The faculty received modest honoraria from Custom Programs for participating in this roundtable.
The faculty was solely responsible for the content presented.
Disclosures
Dr. Goy is on the speaker’s bureau and reports grant/research support from Acerta, Genentech, Kite/Gilead, Janssen, Pharmacyclics, and Takeda, and stocks/shares with COTA.
Dr. Matasar reports stock and other ownership interests with Merck; receiving honoraria from Bayer, Genentech, GlaxoSmithKline, Janssen, Pharmacyclics, Roche, and Seattle Genetics; consulting or advisory roles with Bayer, Genentech, Daiichi Sankyo, Juno Therapeutics, Merck, Roche, Rocket Medical, Seattle Genetics, and Teva; and research funding from Bayer, Genentech, GlaxoSmithKline, Janssen, Pharmacyclics, Roche, Rocket Medical, and Seattle Genetics.
Dr. Martin reports consulting for AstraZeneca, Bayer, Celgene, and Janssen.
Mantle cell lymphoma (MCL) is a rare, often aggressive form of non-Hodgkin lymphoma that develops when the body makes abnormal B-cells, and it is typically diagnosed at a later stage of disease. In this video series, Dr. Andre Goy sits down with Drs. Matthew Matasar and Peter Martin to discuss diagnosis, treatment, and unmet needs in MCL.

This video roundtable was produced by the Custom Programs division. The faculty received modest honoraria from Custom Programs for participating in this roundtable.
The faculty was solely responsible for the content presented.
Disclosures
Dr. Goy is on the speaker’s bureau and reports grant/research support from Acerta, Genentech, Kite/Gilead, Janssen, Pharmacyclics, and Takeda, and stocks/shares with COTA.
Dr. Matasar reports stock and other ownership interests with Merck; receiving honoraria from Bayer, Genentech, GlaxoSmithKline, Janssen, Pharmacyclics, Roche, and Seattle Genetics; consulting or advisory roles with Bayer, Genentech, Daiichi Sankyo, Juno Therapeutics, Merck, Roche, Rocket Medical, Seattle Genetics, and Teva; and research funding from Bayer, Genentech, GlaxoSmithKline, Janssen, Pharmacyclics, Roche, Rocket Medical, and Seattle Genetics.
Dr. Martin reports consulting for AstraZeneca, Bayer, Celgene, and Janssen.
Mantle cell lymphoma (MCL) is a rare, often aggressive form of non-Hodgkin lymphoma that develops when the body makes abnormal B-cells, and it is typically diagnosed at a later stage of disease. In this video series, Dr. Andre Goy sits down with Drs. Matthew Matasar and Peter Martin to discuss diagnosis, treatment, and unmet needs in MCL.

This video roundtable was produced by the Custom Programs division. The faculty received modest honoraria from Custom Programs for participating in this roundtable.
The faculty was solely responsible for the content presented.
Disclosures
Dr. Goy is on the speaker’s bureau and reports grant/research support from Acerta, Genentech, Kite/Gilead, Janssen, Pharmacyclics, and Takeda, and stocks/shares with COTA.
Dr. Matasar reports stock and other ownership interests with Merck; receiving honoraria from Bayer, Genentech, GlaxoSmithKline, Janssen, Pharmacyclics, Roche, and Seattle Genetics; consulting or advisory roles with Bayer, Genentech, Daiichi Sankyo, Juno Therapeutics, Merck, Roche, Rocket Medical, Seattle Genetics, and Teva; and research funding from Bayer, Genentech, GlaxoSmithKline, Janssen, Pharmacyclics, Roche, Rocket Medical, and Seattle Genetics.
Dr. Martin reports consulting for AstraZeneca, Bayer, Celgene, and Janssen.