User login
2019 USPSTF update
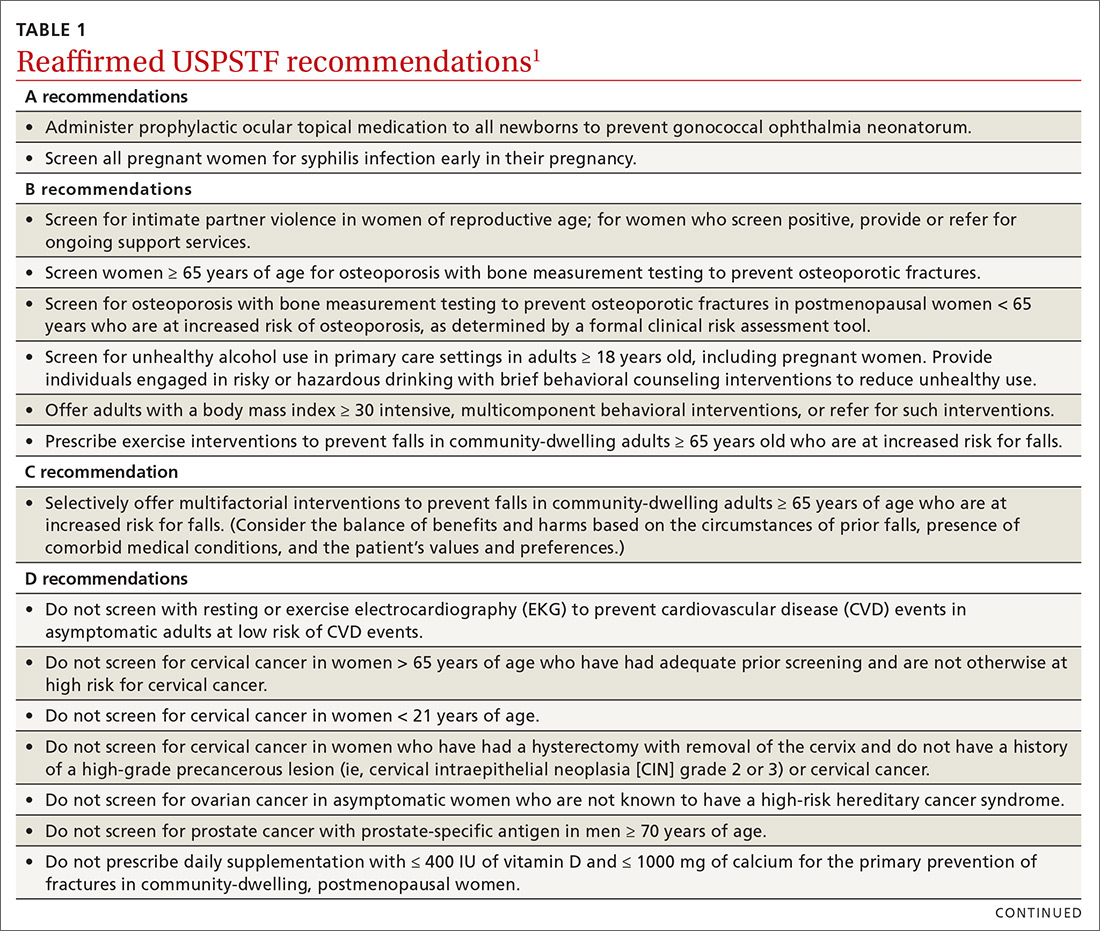
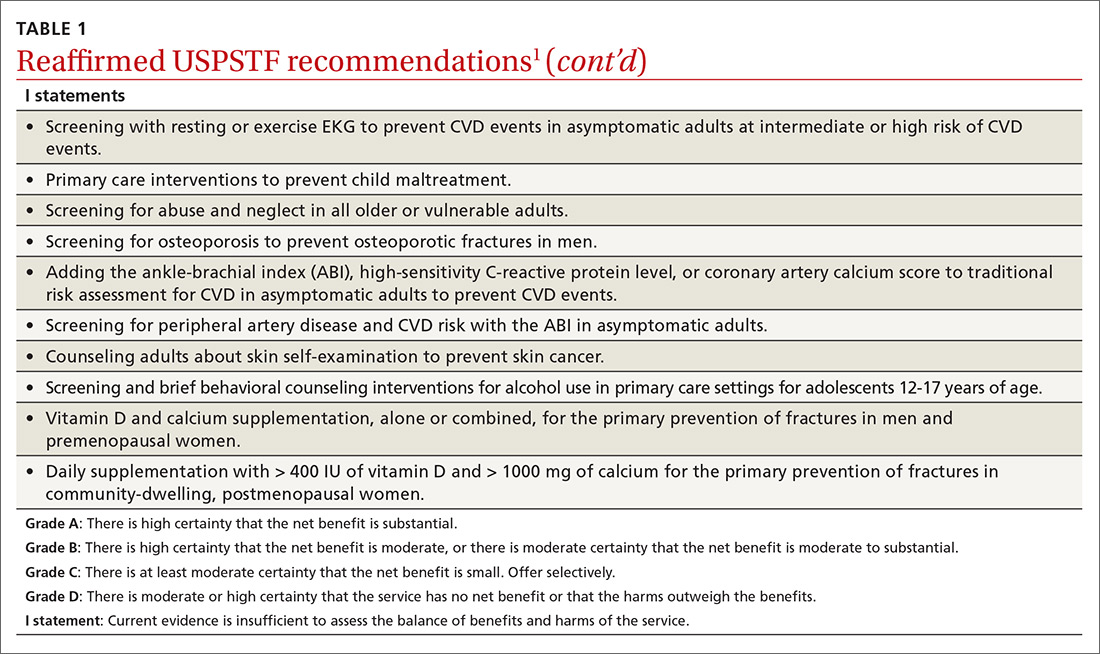
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
A practical guide to the care of ingrown toenails
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
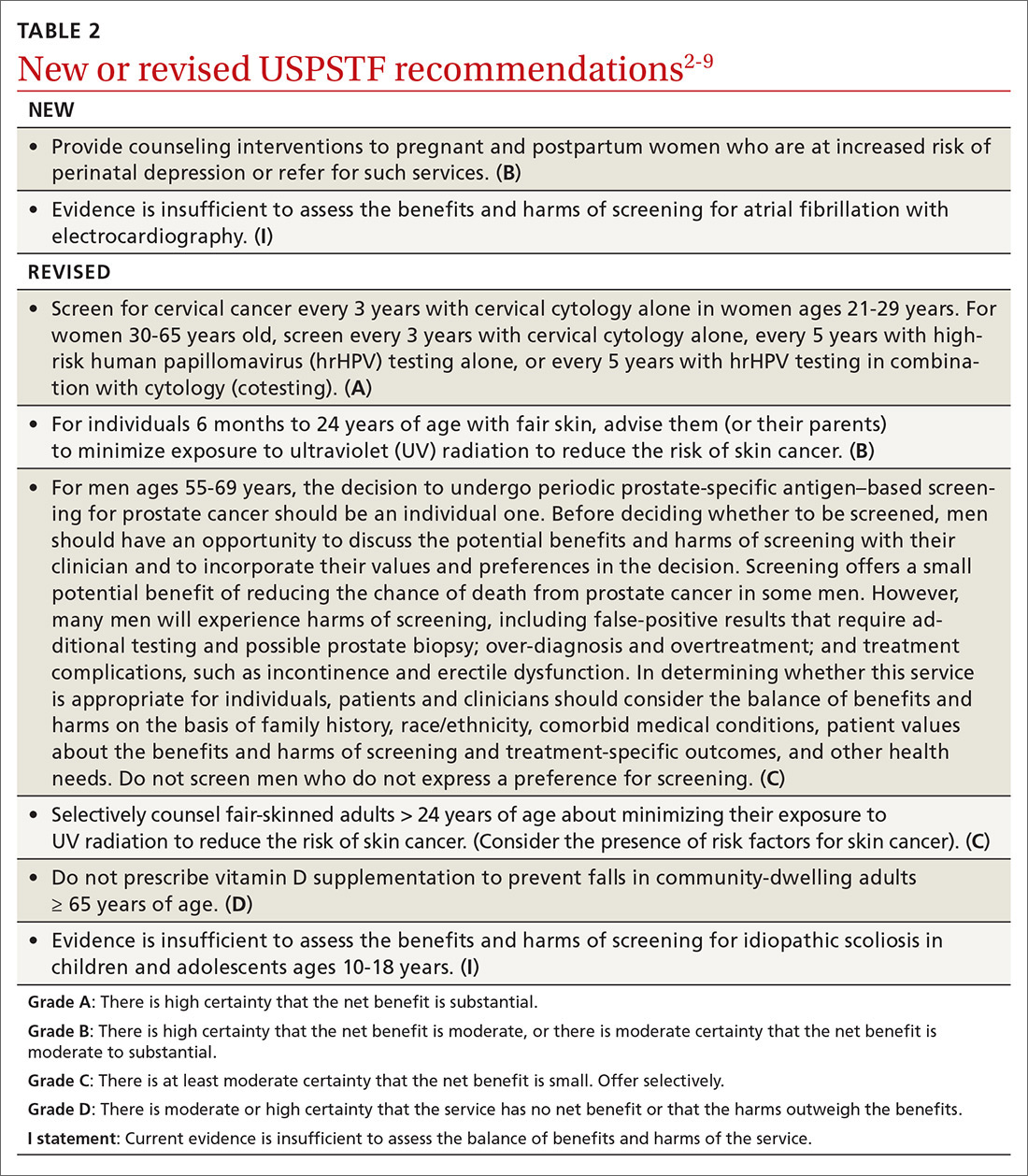
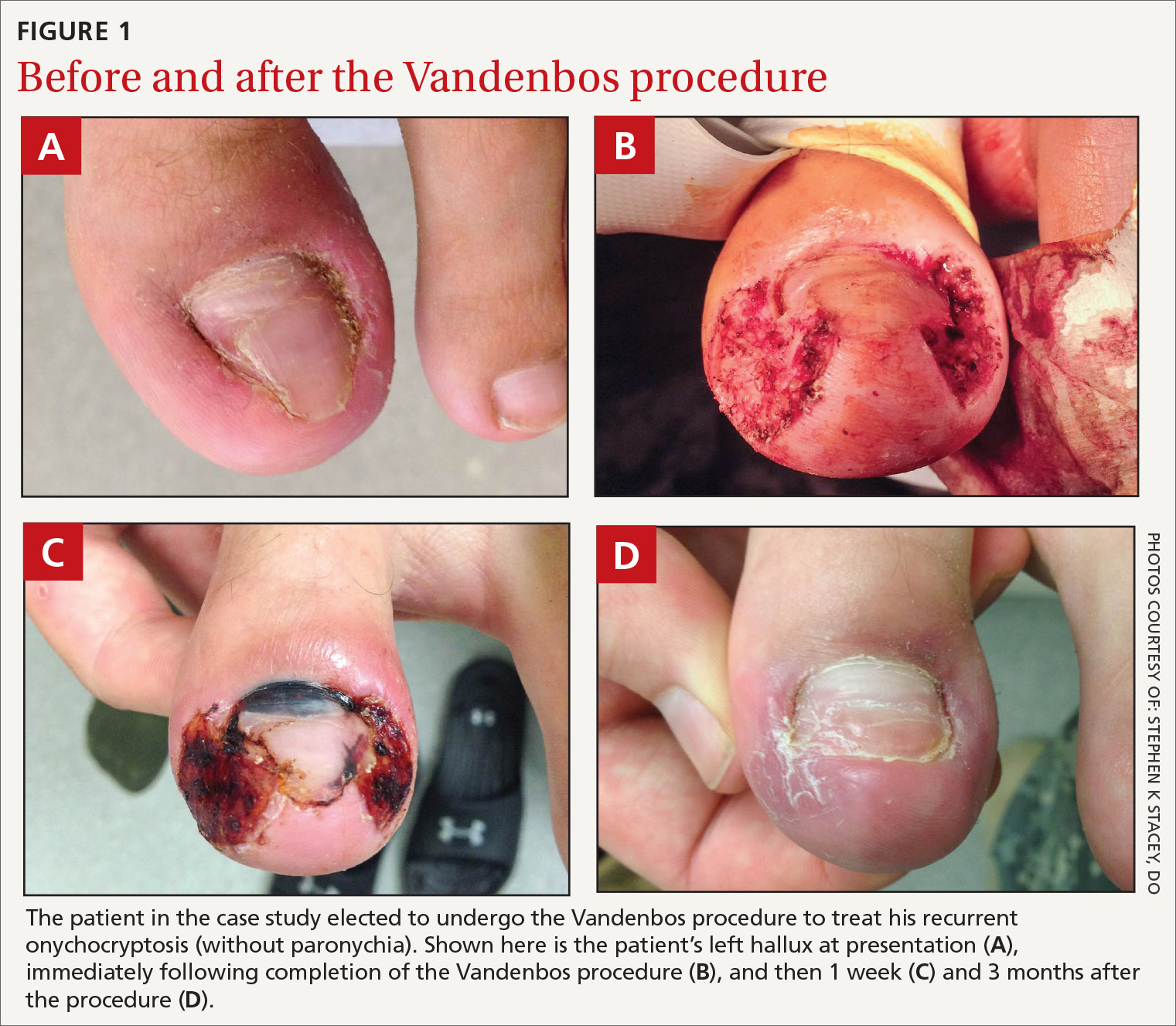
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.
How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.

How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.

How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
Translating AHA/ACC cholesterol guidelines into meaningful risk reduction
A new cholesterol guideline1 builds on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol guidelines,2 which were a major paradigm shift in the evaluation and management of blood cholesterol levels and risk for atherosclerotic cardiovascular disease (ASCVD). The work was presented (and simultaneously published) on November 10, 2018, at the annual AHA Scientific Sessions in Chicago. Full text,1 an executive summary,3 and accompanying systematic review of evidence4 are available online.
The 2018 AHA/ACC cholesterol guideline represents a step forward in ASCVD prevention—especially in primary prevention, where it provides guidance for risk refinement and personalization. In this article, we mine the details of what has changed and what is new in this guideline so that you can prepare to adopt the recommendations in your practice.

2013 and 2018 guidelines: Similarities, differences
As in earlier iterations, the 2018 guideline emphasizes healthy lifestyle across the life-course as the basis of ASCVD prevention—as elaborated in the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.5 In contrast to the 2013 guidelines,2 the 2018 guideline is more comprehensive and more personalized, focusing on risk assessment for individual patients, rather than simply providing population-based approaches. Moreover, the guideline isn’t limited to adults: It makes recommendations pertaining to children and adolescents.1
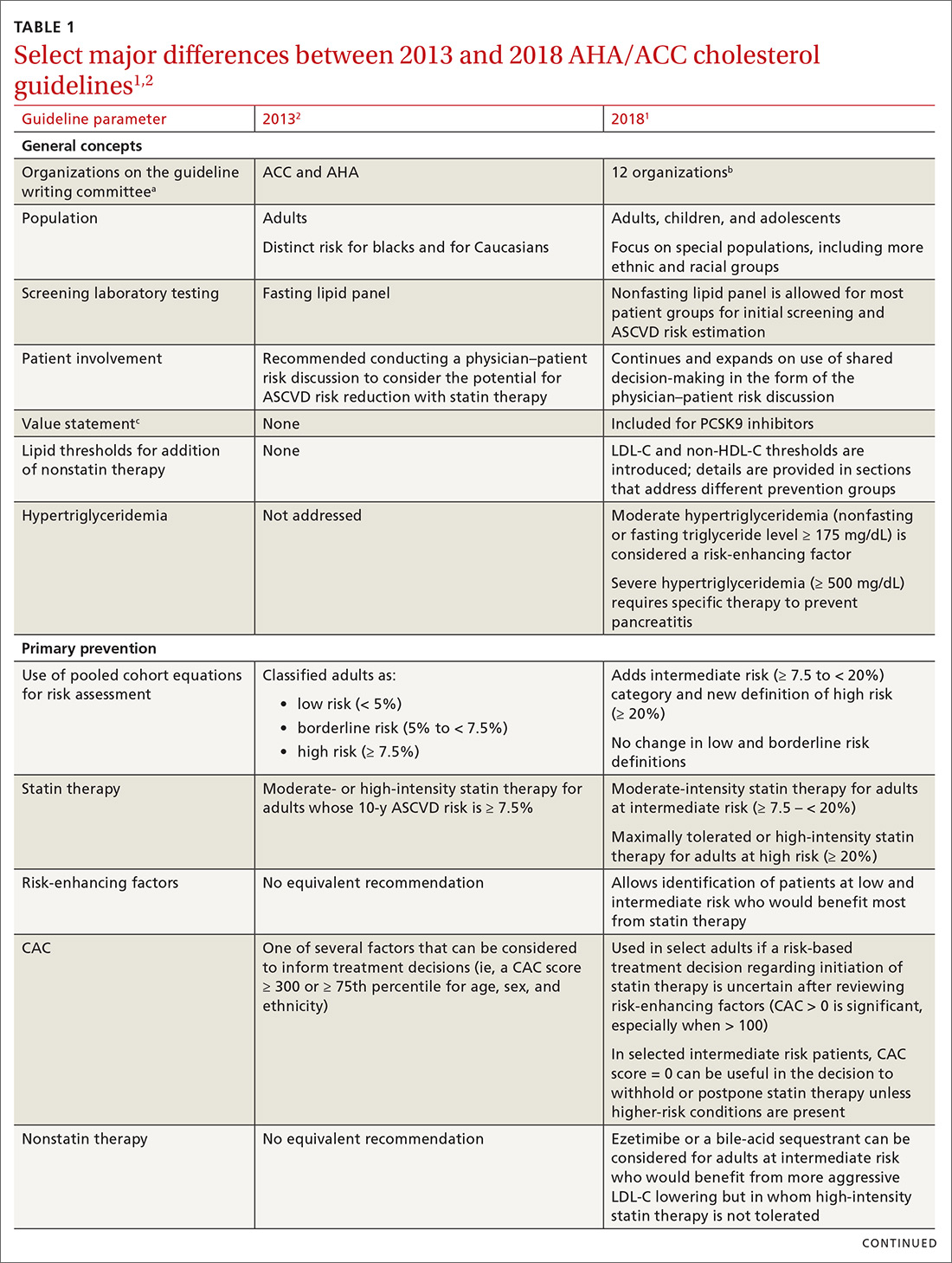
TABLE 11,2 compares the most important differences between the 2013 and 2018 guidelines.

The 2013 ACC/AHA guidelines eliminated low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C)a goals of therapy and replaced them with the concept of 4 “statin benefit groups”—that is, patient populations for which clear evidence supports the role of statin therapy.4 In the 2018 guideline, statin benefit groups have been maintained, although without explicit use of this term.1
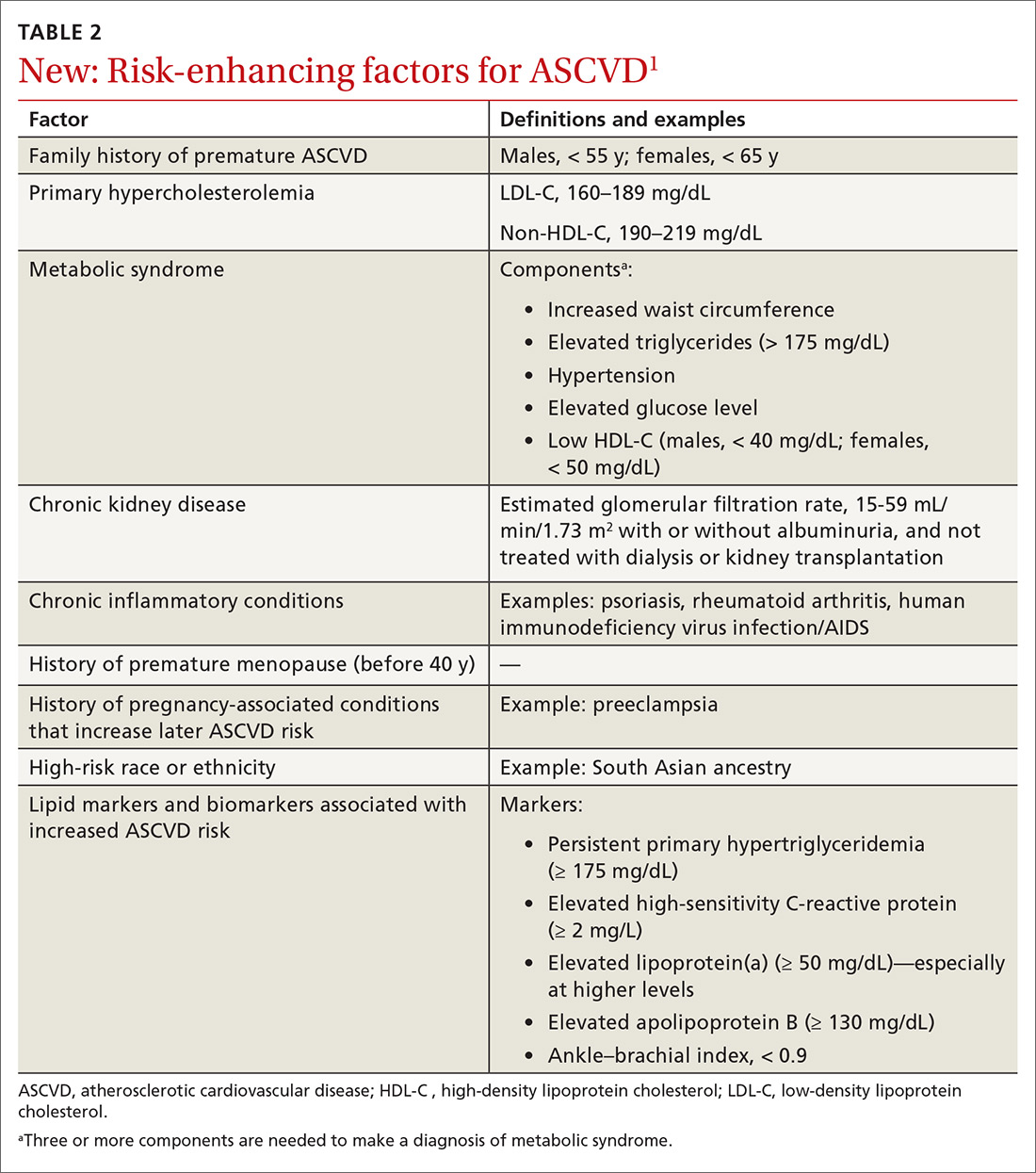
Primary prevention. Although no major changes in statin indications are made for patients with (1) established ASCVD (ie, for secondary prevention), (2) diabetes mellitus (DM) and who are 40 to 75 years of age, or (3) a primary LDL-C elevation ≥ 190 mg/dL, significant changes were made for primary prevention patients ages 40 to 75 years.1 ASCVD risk calculation using the 2013 pooled cohort equations (PCE) is still recommended4; however, risk estimation is refined by the use of specific so-called risk-enhancing factors (TABLE 21). In cases in which the risk decision remains uncertain, obtaining the coronary artery calcium (CAC) score (which we’ll describe shortly) using specialized computed tomography (CT) is advised to facilitate the shared physician–patient decision-making process.1
LDL-C and non-HDL-C thresholds. Although LDL-C and non-HDL-C goals are not overtly brought back from the 2002 National Cholesterol Education Program/Adult Treatment Panel guidelines,6 the new guideline does introduce LDL-C and non-HDL-C thresholds—levels at which adding nonstatin therapy can be considered, in contrast to previous goals to which therapy was titrated. Definitions of statin intensity remain the same: Moderate-intensity statin therapy is expected to reduce the LDL-C level by 30% to 50%; high-intensity statin therapy, by ≥ 50%.1 The intensity of statin therapy has been de-escalated in the intermediate-risk group, where previous guidelines advised high-intensity statin therapy,4 and replaced with moderate-intensity statin therapy (similar to 2016 US Preventive Services Task Force [USPSTF] recommendations7).
[polldaddy:10312157]
Continue to: Fasting vs nonfasting lipid profiles
Fasting vs nonfasting lipid profiles. In contrast to previous guidelines,2,8 which used fasting lipid profiles, nonfasting lipid profiles are now recommended for establishing a baseline LDL-C level and for ASCVD risk estimation for most patients—as long as the triglycerides (TG) level is < 400 mg/dL. When the calculated LDL-C level is < 70 mg/dL using the standard Friedewald formula, obtaining a direct LDL-C or a modified LDL-C estimate9 is deemed reasonable to improve accuracy. (The modified LDL-C can be estimated using The Johns Hopkins Hospital’s free “LDL Cholesterol Calculator” [www.hopkinsmedicine.org/apps/all-apps/ldl-cholesterol-calculator]).
A fasting lipid profile is still preferred for patients who have a family history of a lipid disorder. The definition of hypertriglyceridemia has been revised from a fasting TG level ≥ 150 mg/dL to a nonfasting or fasting TG level ≥ 175 mg/dL.1
Nonstatin add-on therapy. The new guideline supports the addition of nonstatin therapies to maximally tolerated statin therapy in patients who have established ASCVD or a primary LDL-C elevation ≥ 190 mg/dL when (1) the LDL-C level has not been reduced by the expected percentage (≥ 50% for high-intensity statin therapy) or (2) explicit LDL-C level thresholds have been met.1
The principal 2 groups of recommended nonstatins for which there is randomized, controlled trial evidence of cardiovascular benefit are (1) the cholesterol-absorbing agent ezetimibe10 and (2) the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab11 and alirocumab.12
AAFP’s guarded positions on the 2013 and 2018 guidelines
The American Academy of Family Physicians (AAFP) welcomed the patient-centered and outcome-oriented aspects of the 2013 ACC/AHA guidelines, endorsing them with 3 qualifications.13
- Many of the recommendations were based on expert opinion, not rigorous research results—in particular, not on the findings of randomized controlled trials (although key points are based on high-quality evidence).
- There were conflicts of interest disclosed for 15 members of the guidelines panel, including a vice chair.
- Validation of the PCE risk estimation tool was lacking.
Continue to: AAFP announced...
AAFP announced in March that it does not endorse the 2018 AHA/ACC guideline, asserting that (1) only a small portion of the recommendations, primarily focused on the addition of nonstatin therapy, were addressed by an independent systematic review and (2) many of the guideline recommendations are based on low-quality or insufficient evidence. AAFP nevertheless bestowed an “affirmation of value” designation on the guideline—meaning that it provides some benefit for family physicians’ practice without fulfilling all criteria for full endorsement.14
Detailed recommendations from the 2018 guideline
Lifestyle modification
When talking about ASCVD risk with patients, it is important to review current lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). Subsequent to that conversation, a healthy lifestyle should be endorsed and relevant advice provided. In addition, patient-directed materials (eg, ACC’s CardioSmart [www.cardiosmart.org]; AHA’s Life’s Simple 7 [www.heart.org/en/professional/workplace-health/lifes-simple-7]; and the National Lipid Association’s Patient Tear Sheets [www.lipid.org/practicetools/tools/tearsheets] and Clinicians’ Lifestyle Modification Toolbox [www.lipid.org/CLMT]) and referrals (eg, to cardiac rehabilitation, a dietitian, a smoking-cessation program) should be provided.1
Primary prevention of ASCVD
Risk assessment for primary prevention is now approached as a process, rather than the simple risk calculation used in the 2013 ACC/AHA guidelines.2 Assessment involves risk estimation followed by risk personalization, which, in some cases, is followed by risk reclassification using CAC scoring.1
Patients are classified into 1 of 4 risk groups, based on the PCE1:
- low (< 5%)
- borderline (5%-7.5%)
- intermediate (7.5%-19.9%)
- high (≥ 20%).
However, the PCE-based risk score is a population-based tool, which might not reflect the actual risk of individual patients. In some populations, PCE underestimates ASCVD risk; in others, it overestimates risk. A central tenet of the new guideline is personalization of risk, taking into account the unique circumstances of each patient. Moreover, the new guideline provides guidance on how to interpret the PCE risk score for several different ethnic and racial groups.1
Continue to: Medical therapy
Medical therapy. The decision to start lipid-lowering therapy should be made after a physician–patient discussion that considers costs of therapy as well as patient preferences and values in the context of shared decision-making. Discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, and the LDL-C level), the PCE risk score, the presence of risk-enhancing factors (TABLE 21), potential benefits of lifestyle changes and statin therapy, and the potential for adverse drug effects and drug–drug interactions.1
If the estimated ASCVD risk is 7.5%-19.9%, starting moderate-intensity statin therapy is recommended. Risk-enhancing factors favor initiation of statin therapy, even in patients at borderline risk (5%-7.5%). If risk is uncertain, the CAC score can be used to facilitate shared decision-making.1 The use of CAC is in agreement with the USPSTF statement that CAC can moderately improve discrimination and reclassification, but has an unclear effect on downstream health care utilization.15 Importantly, CAC should not be measured routinely in patients already taking a statin because its primary role is to facilitate shared decision-making regarding initiation of statin therapy.16
If the 10-year ASCVD risk is ≥ 20%, high-intensity statin therapy is advised, without need to obtain the CAC score. If high-intensity statin therapy is advisable but not acceptable to, or tolerated by, the patient, it might be reasonable to add a nonstatin drug (ezetimibe or a bile-acid sequestrant) to moderate-intensity statin therapy.1
Risk-enhancing factors (TABLE 21) apply to intermediate- and borderline-risk patients. Importantly, these factors include membership in specific ethnic groups, conditions specific to females, and male–female distinctions in risk. Risk-enhancing factors also incorporate biomarkers that are often measured by lipid specialists, such as lipoprotein(a) (Lp[a]) and apolipoprotein B (ApoB).1
Lp(a) is an atherogenic particle, akin to an LDL particle, that consists of a molecule of apolipoprotein (a) (a nonfunctional mimic of a portion of plasminogen) covalently bound to ApoB, like the one found on the LDL particle. Lp(a) is proportionally associated with an increased risk for ASCVD and aortic stenosis at a level > 50 mg/dL.17 A family history of premature ASCVD is a relative indication for measuring Lp(a).1
Continue to: When and why to measure CAC
When and why to measure CAC
If the decision to initiate statin therapy is still uncertain after risk estimation and personalization, or when a patient is undecided about committing to lifelong lipid-lowering therapy, the new guideline recommends obtaining a CAC score to inform the shared decision-making process.1,18 Measurement of CAC is obtained by noncontrast, electrocardiographic-gated CT that can be performed in 10 to 15 minutes, requiring approximately 1 millisievert of radiation (equivalent of the approximate dose absorbed during 2 mammograms). Although measurement of the CAC score is generally not covered by insurance, its cost ($50-$450) nationwide makes it accessible.19
CAC measures the presence (or absence) of subclinical atherosclerosis by detecting calcified plaque in coronary arteries. The absolute CAC score is expressed in Agatston units; an age–gender population percentile is also provided. Keep in mind that the presence of any CAC (ie, a score > 0) is abnormal and demonstrates the presence of subclinical coronary artery disease. The prevalence of CAC > 0 increases with age, but a significant percentage of older people have a CAC score = 0. When CAC > 0, additional information is provided by the distribution of plaque burden among the different coronary arteries.20
Among intermediate-risk patients, 50% have CAC = 0 and, therefore, a very low event rate over the ensuing 10 years, which allows statin therapy to be safely deferred unless certain risk factors are present (eg, family history, smoking, DM).1,18 It is reasonable to repeat CAC testing in 5 to 10 years to assess whether subclinical atherosclerosis has developed. The 2018 guideline emphasizes that, when the CAC score is > 0 but < 100 Agatston units, statin therapy is favored, especially in patients > 55 years of age; when the CAC score is ≥ 100 Agatston units or at the ≥ 75th percentile, statin therapy is indicated regardless of age.1
Patients who might benefit from knowing their CAC score include those who are:
- reluctant to initiate statin therapy but who want to understand their risk and potential for benefit more precisely
- concerned about the need to reinstitute statin therapy after discontinuing it because of statin-associated adverse effects
- older (men, 55-80 years; women, 60-80 years) who have a low burden of risk factors and who question whether they would benefit from statin therapy
- middle-aged (40-55 years) and who have a PCE-calculated risk of 5% to < 7.5% for ASCVD and factors that increase their risk for ASCVD, even though they are in a borderline-risk group.1
Primary prevention in special populations
Older patients. In adults ≥ 75 years who have an LDL-C level 70 to 189 mg/dL, initiating a moderate-intensity statin might be reasonable; however, it might also be reasonable to stop treatment in this population when physical or cognitive decline, multiple morbidities, frailty, or reduced life expectancy limits the potential benefit of statin therapy. It might be reasonable to use the CAC score in adults 76 to 80 years of age who have an LDL-C level of 70 to 189 mg/dL to reclassify those whose CAC score = 0, so that they can avoid statin therapy.1
Continue to: Children and adolescents
Children and adolescents. In alignment with current pediatric guidelines,21 but in contrast to USPSTF reccomendations,22 the 2018 ACC/AHA guideline endorses universal lipid screening for pediatric patients (see TABLE W11,21,22). It is reasonable to obtain a fasting lipid profile or nonfasting non-HDL-C in all children and adolescents who have neither cardiovascular risk factors nor a family history of early cardiovascular disease to detect moderate-to-severe lipid abnormalities. Screening should be done once at 9 to 11 years of age and again at 17 to 21 years.1
A screening test as early as 2 years of age to detect familial hypercholesterolemia (FH) is reasonable when a family history of either early CVD or significant hypercholesterolemia is present. The guideline endorses reverse cascade screening for detection of FH in family members of children and adolescents who have severe hypercholesterolemia.1
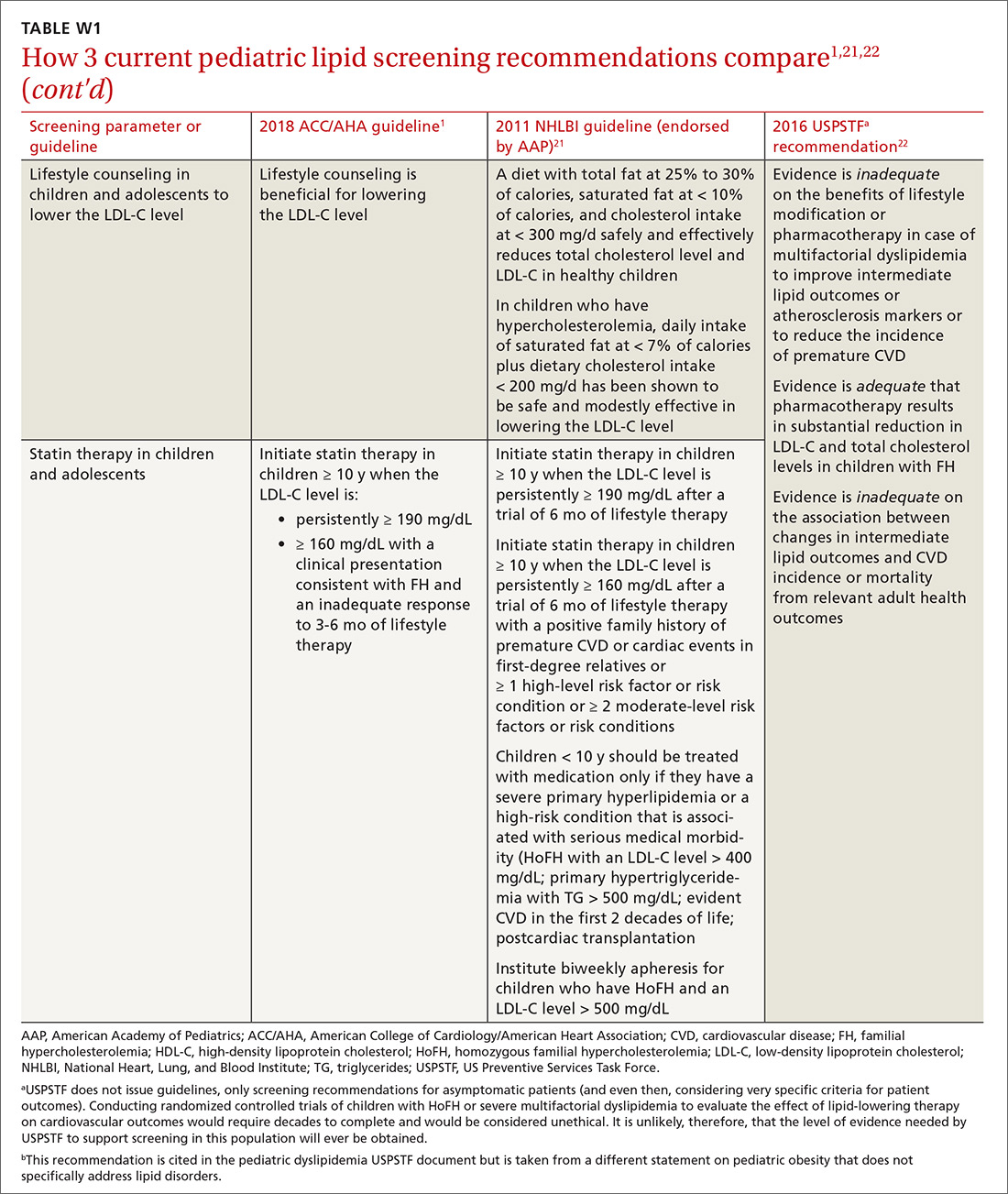
In children and adolescents with a lipid abnormality, especially when associated with the metabolic syndrome, lifestyle counseling is beneficial for lowering the LDL-C level. In children and adolescents ≥ 10 years of age with (1) an LDL-C level persistently ≥ 190 mg/dL or (2) an LDL level ≥ 160 mg/dL plus a clinical presentation consistent with FH, it is reasonable to initiate statin therapy if they do not respond adequately to 3 to 6 months of lifestyle therapy.1
Ethnicity as a risk-modifying factor. The PCE distinguishes between US adults of European ancestry and African ancestry, but no other ethnic groups are distinguished.4 The new guideline advocates for the use of PCE in other populations; however, it states that, for clinical decision-making purposes, it is reasonable, in adults of different races and ethnicities, for the physician to review racial and ethnic features that can influence ASCVD risk to allow adjustment of the choice of statin or intensity of treatment. Specifically, South Asian ancestry is now treated as a risk-enhancing factor, given the high prevalence of premature and extensive ASCVD in this patient population.1
Concerns specific to women. Considering conditions specific to women as potential risk-enhancing factors is advised when discussing lifestyle intervention and the potential for benefit from statin therapy—in particular, (1) in the setting of premature menopause (< 40 years) and (2) when there is a history of a pregnancy-associated disorder (eg, hypertension, preeclampsia, gestational DM, a small-for-gestational-age infant, and preterm delivery). If the decision is made to initiate statin therapy in women of childbearing age who are sexually active, there is a guideline mandate to counsel patients on using reliable contraception. When pregnancy is planned, statin therapy should be discontinued 1 to 2 months before pregnancy is attempted; when pregnancy occurs while a patient is taking a statin, therapy should be stopped as soon as the pregnancy is discovered.1
Continue to: Adults with chronic kidney disease
Adults with chronic kidney disease. Chronic kidney disease that is not treated with dialysis or kidney transplantation is considered a risk-enhancing factor; initiation of a moderate-intensity statin or a moderate-intensity statin plus ezetimibe can be useful in patients with chronic kidney disease who are 40 to 75 years of age and have an LDL-C level of 70 to 189 mg/dL and a PCE-calculated risk ≥ 7.5%. In adults with advanced kidney disease that requires dialysis who are already taking a statin, it may be reasonable to continue the statin; however, initiation of a statin in adults with advanced kidney disease who require dialysis is not recommended because of an apparent lack of benefit.1
Adults with a chronic inflammatory disorder or human immunodeficiency virus infection. Any of these conditions are treated as risk-enhancing factors; in a risk discussion with affected patients, therefore, moderate-intensity statin therapy or high-intensity statin therapy is favored for those 40 to 75 years of age who have an LDL-C level of 70 to 189 mg/dL and PCE-calculated risk ≥ 7.5%. A fasting lipid profile and assessment of ASCVD risk factors for these patients can be useful (1) as a guide to the potential benefit of statin therapy and (2) for monitoring or adjusting lipid-lowering drug therapy before, and 4 to 12 weeks after, starting inflammatory disease-modifying therapy or antiretroviral therapy.
In adults with rheumatoid arthritis who undergo ASCVD risk assessment with a lipid profile, it can be useful to recheck lipid values and other major ASCVD risk factors 2 to 4 months after the inflammatory disease has been controlled.1
Primary hypercholesterolemia
The diagnosis and management of heterozygous or homozygous familial hypercholesterolemia (HeFH or HoFH) is beyond the scope of the 2018 ACC/AHA cholesterol guidelines; instead, the 2015 AHA Scientific Statement, “The Agenda for Familial Hypercholesterolemia,” provides a contemporary review of these topics.23 However, the 2018 cholesterol guideline does acknowledge that an LDL-C level ≥ 190 mg/dL often corresponds to primary (ie, genetic) hypercholesterolemia.
In patients 20 to 75 years of age who have a primary elevation of LDL-C level ≥ 190 mg/dL, the guideline recommends initiation of high-intensity statin therapy without calculating ASCVD risk using the PCE. If a > 50% LDL-C reduction is not achieved, or if the LDL-C level on maximally tolerated statin therapy remains ≥ 100 mg/dL, adding ezetimibe is considered reasonable. If there is < 50% reduction in the LDL-C level while taking maximally tolerated statin and ezetimibe therapy, adding a bile-acid sequestrant can be considered, as long as the TG level is not > 300 mg/dL (ie, bile-acid sequestrants can elevate the TG level significantly).
Continue to: In patients 30 to 75 years of age...
In patients 30 to 75 years of age who have a diagnosis of HeFH and an LDL-C level ≥ 100 mg/dL while taking maximally tolerated statin and ezetimibe therapy, the addition of a PCSK9 inhibitor can be considered. Regardless of whether there is a diagnosis of HeFH, addition of a PCSK9 inhibitor can be considered in patients 40 to 75 years of age who have a baseline LDL-C level ≥ 220 mg/dL and who achieve an on-treatment LDL-C level ≥ 130 mg/dL while receiving maximally tolerated statin therapy and ezetimibe.1
Diabetes mellitus
In patients with DM who are 40 to 75 years of age, moderate-intensity statin therapy is recommended without calculating the 10-year ASCVD risk. When the LDL-C level is 70 to 189 mg/dL, however, it is reasonable to use the PCE to assess 10-year ASCVD risk to facilitate risk stratification.
In patients with DM who are at higher risk, especially those who have multiple risk factors or are 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥ 50 %. In adults > 75 years of age with DM who are already on statin therapy, it is reasonable to continue statin therapy; for those that age who are not on statin therapy, it might be reasonable to initiate statin therapy after a physician–patient discussion of potential benefits and risks.
In adults with DM and PCE-calculated risk ≥ 20%, it might be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce the LDL-C level by ≥ 50%. In adults 20 to 39 years of age with DM of long duration (≥ 10 years of type 2 DM, ≥ 20 years of type 1 DM), albuminuria (≥ 30 μg of albumin/mg creatinine), estimated glomerular filtration rate < 60 mL/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index < 0.9, it might be reasonable to initiate statin therapy.1
Secondary prevention
Presence of clinical ASCVD. In patients with clinical ASCVD who are ≤ 75 years of age, high-intensity statin therapy should be initiated or continued, with the aim of achieving ≥ 50% reduction in the LDL-C level. When high-intensity statin therapy is contraindicated or if a patient experiences statin-associated adverse effects, moderate-intensity statin therapy should be initiated or continued with the aim of achieving a 30% to 49% reduction in the LDL-C level.
Continue to: In patients...
In patients > 75 years of age with clinical ASCVD, it is reasonable to initiate or continue moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preference.1
Very high risk. In patients at very high risk (this includes a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions), maximally tolerated LDL-C-lowering therapy should include maximally tolerated statin therapy and ezetimibe before considering a PCSK9 inhibitor. An LDL-C level ≥ 70 mg/dL or a non-HDL-C level ≥ 100 mg/dL is considered a reasonable threshold for adding a PCSK9 inhibitor to background lipid-lowering therapy1 (TABLE 31).
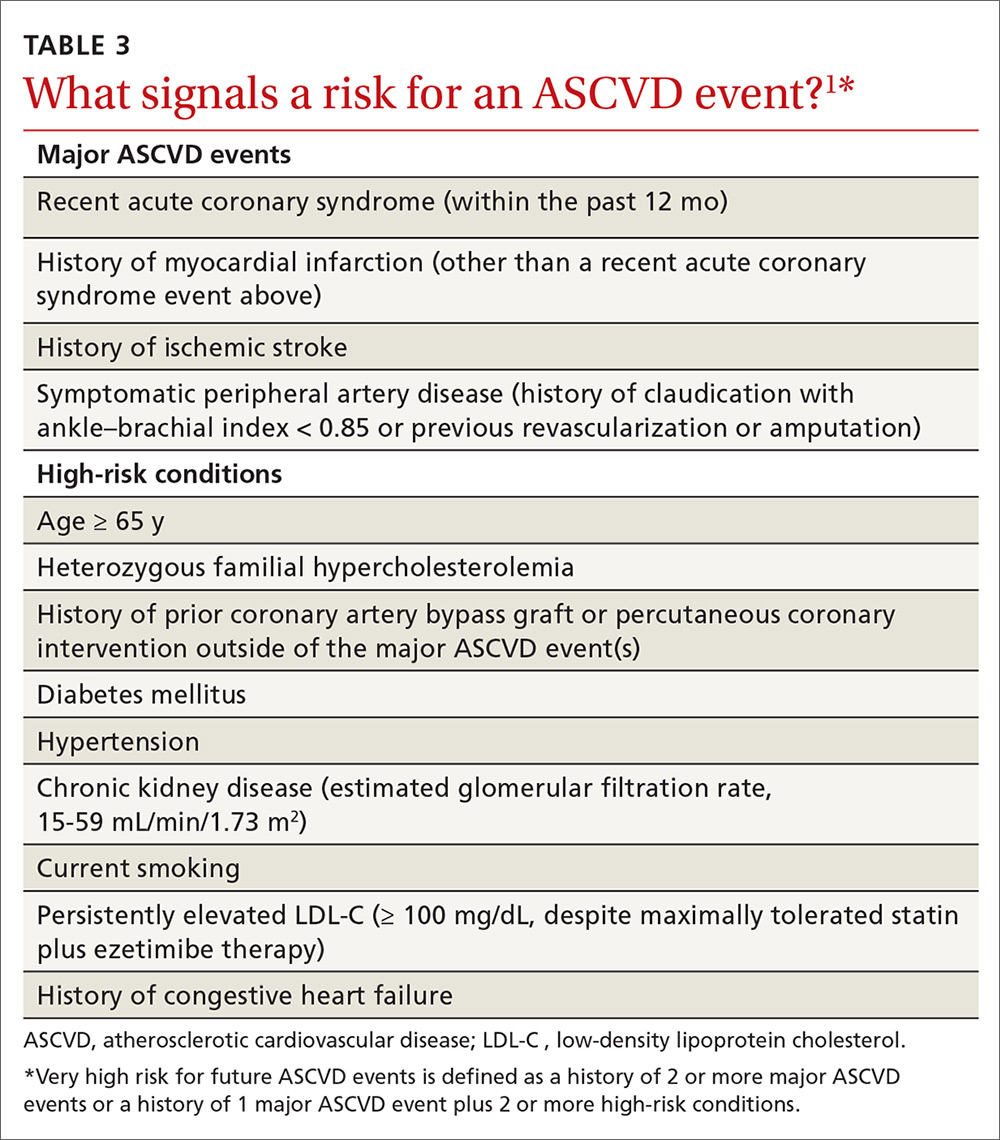
Heart failure. In patients with heart failure who have (1) a reduced ejection fraction attributable to ischemic heart disease, (2) a reasonable life expectancy (3-5 years), and (3) are not already on a statin because of ASCVD, consider initiating moderate-intensity statin therapy to reduce the risk for an ASCVD event.1
Reduction of elevated triglycerides
The guideline defines moderate hypertriglyceridemia as a nonfasting or fasting TG level of 175 to 499 mg/dL. Such a finding is considered a risk-enhancing factor and is 1 of 5 components of the metabolic syndrome. Three independent measurements are advised to diagnose primary moderate hypertriglyceridemia. Severe hypertriglyceridemia is diagnosed when the fasting TG level is ≥ 500 mg/dL.1
In moderate hypertriglyceridemia, most TGs are carried in very-low-density lipoprotein particles; in severe hypertriglyceridemia, on the other hand, chylomicrons predominate, raising the risk for pancreatitis. In adults with severe hypertriglyceridemia, therefore—especially when the fasting TG level is ≥ 1000 mg/dL—it is reasonable to identify and address other causes of hypertriglyceridemia. If TGs are persistently elevated or increasing, levels should be reduced to prevent acute pancreatitis with a very low-fat diet and by avoiding refined carbohydrates and alcohol; consuming omega-3 fatty acids; and, if necessary, taking a fibrate.1
Continue to: In adults...
In adults ≥ 20 years of age with moderate hypertriglyceridemia, lifestyle factors (eg, obesity, metabolic syndrome), secondary factors (eg, DM, chronic liver or kidney disease, nephrotic syndrome, hypothyroidism), and medications that increase the TG level need to be addressed first. In adults 40 to 75 years of age with moderate or severe hypertriglyceridemia and a PCE-calculated ASCVD risk ≥ 7.5%, it is reasonable to reevaluate risk after lifestyle and secondary factors are addressed and to consider a persistently elevated TG level as a factor favoring initiation or intensification of statin therapy. In adults 40 to 75 years of age with severe hypertriglyceridemia and ASCVD risk ≥ 7.5%, it is reasonable to address reversible causes of a high TG level and to initiate statin therapy.1
Other considerations in cholesterol management
Tools to assess adherence
The response to lifestyle and statin therapy should be evaluated by the percentage reduction in the LDL-C level compared with baseline, not by assessment of the absolute LDL-C level. When seeing a patient whose treatment is ongoing, a baseline level can be estimated using a desktop LDL-calculator app.
Adherence and percentage response to LDL-C–lowering medications and lifestyle changes should be evaluated with repeat lipid measurement 4 to 12 weeks after either a statin is initiated or the dosage is adjusted, and repeated every 3 to 12 months as needed. In patients with established ASCVD who are at very high risk, triggers for adding nonstatin therapy are defined by a threshold LDL-C level ≥ 70 mg/dL on maximal statin therapy.1
Interventions focused on improving adherence to prescribed therapy are recommended for management of adults with an elevated cholesterol level. These interventions include telephone reminders, calendar reminders, integrated multidisciplinary educational activities, and pharmacist-led interventions, such as simplification of the medication regimen to once-daily dosing.1
Statin safety and associated adverse effects
A physician–patient risk discussion is recommended before initiating statin therapy to review net clinical benefit, during which the 2 parties weigh the potential for ASCVD risk reduction against the potential for statin-associated adverse effects, statin–drug interactions, and safety, with the physician emphasizing that adverse effects can be addressed successfully.
Continue to: Statins are one of...
Statins are one of the safest classes of medication, with an excellent risk-benefit ratio. However, there are myriad confusing media reports regarding potential adverse effects and safety of the statin class—reports that often lead patients to discontinue or refuse statins.
Statin-associated adverse effects include the common statin-associated muscle symptoms (SAMS), new-onset DM, cognitive effects, and hepatic injury. The frequency of new-onset DM depends on the population exposed to statins, with a higher incidence of new-onset DM found in patients who are already predisposed, such as those with obesity, prediabetes, and metabolic syndrome. Cognitive effects are rare and difficult to interpret; they were not reported in the large statin mega-trials but have been described in case reports. Significant transaminase elevations > 3 times the upper limit of normal are infrequent; hepatic failure with statins is extremely rare and found at the same incidence in the general population.1
SAMS include (in order of decreasing prevalence)24:
- myalgias with a normal creatine kinase (CK) level
- conditions such as myositis or myopathy (elevated CK level)
- rhabdomyolysis (CK level > 10 times the upper limit of normal, plus renal injury)
- extremely rare statin-associated autoimmune myopathy, with detectable 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase antibodies.
In patients with SAMS, thorough assessment of symptoms is recommended, in addition to evaluation for nonstatin causes and predisposing factors. Identification of potential SAMS-predisposing factors is recommended before initiation of treatment, including demographics (eg, East-Asian ancestry), comorbid conditions (eg, hypothyroidism and vitamin D deficiency), and use of medications adversely affecting statin metabolism (eg, cyclosporine).
In patients with statin-associated adverse effects that are not severe, it is recommended to reassess and rechallenge to achieve a maximal lowering of the LDL-C level by a modified dosing regimen or an alternate statin or by combining a statin with nonstatin therapy. In patients with increased risk for DM or new-onset DM, it is recommended to continue statin therapy.
Continue to: Routine CK and liver function testing...
Routine CK and liver function testing is not useful in patients treated with statins; however, it is recommended that CK be measured in patients with severe SAMS or objective muscle weakness, or both, and to measure liver function if symptoms suggest hepatotoxicity. In patients at increased risk for ASCVD who have chronic, stable liver disease (including non-alcoholic fatty liver disease), it is reasonable, when appropriately indicated, to use statins after obtaining baseline measurements and determining a schedule of monitoring and safety checks.
In patients at increased risk for ASCVD who have severe or recurrent SAMS after appropriate statin rechallenge, it is reasonable to use nonstatin therapy that is likely to provide net clinical benefit. The guideline does not recommend routine use of coenzyme Q10 supplementation for the treatment or prevention of SAMS.1
Guideline criticism
Guideline development is challenging on multiple levels, including balancing perspectives from multiple stakeholders. Nevertheless, the 2018 AHA/ACC cholesterol guideline builds nicely on progress made since its 2013 predecessor was released.4 This document was developed with the participation of representatives from 10 professional societies in addition to the ACC and AHA—notably, the National Lipid Association and American Society for Preventive Cardiology.1
To refine risk estimation and facilitate shared decision-making, the new guideline introduced so-called risk-enhancing factors and use of the CAC.1 However, some potential risk-enhancing factors were left out: erectile dysfunction, for example, often a marker of increased cardiovascular risk in men < 50 years of age.25 In addition, although pretreatment ApoB was introduced as a risk-enhancing factor,1 no recommendation is given to measure ApoB after initiation of therapy for evaluation of residual cardiovascular risk, as endorsed in other guidelines.26,27
Moreover, the guideline does not include the “extreme risk” category in the guideline developed by the American Association of Clinical Endocrinologists (AACE).28 Although the 2018 AHA/ACC guideline introduces < 70 mg/dL and < 100 mg/dL LDL-C thresholds,1 the < 55 mg/dL LDL-C threshold used for patients in the AACE/American College of Endocrinology extreme-risk category is not mentioned.26 This omission might leave patients who are at extreme ASCVD risk without optimal lipid-lowering therapy. Similarly, the guideline does not elaborate on the diagnosis and treatment of HoFH and HeFH.1 The age cutoff of 30 years for the recommendation to consider PCSK9 inhibitors in patients with HeFH appears arbitrary and excludes younger FH patients who have an extreme LDL-C elevation from potentially important therapy.23
Continue to: Guidelines are dynamic instruments...
Guidelines are dynamic instruments that require constant updating, given the production of new evidence. In fact, the results of the Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) were presented at the same meeting at which this guideline was unveiled.29 REDUCE-IT demonstrated an astonishing highly significant 25% reduction in the composite primary major adverse cardiovascular event outcome in patients with an LDL-C level of 44 to 100 mg/dL on statin therapy, who had a TG level of 135 to 499 mg/dL and had been treated for a median of 4.9 years with 4 g of pure eicosapentaenoic acid.
In addition, the guideline’s value statements, which address the need to consider the cost of drugs in determining most appropriate treatment, are no longer accurate because the price of PCSK9 inhibitors has dropped by more than half since the guideline was issued.30
An upward climb to clinical payoff
Even after close study of the 2018 AHA/ACC cholesterol guideline, implementing it in practice might remain a challenge to clinicians who are inexperienced in ordering lipid markers such as Lp(a) and interpreting the CAC score. Moreover, initiating and monitoring nonstatin therapies will be a demanding task—especially with PCSK9 inhibitors, which present access difficulties because they are relatively expensive (even after the recent price cut). That’s why, when there is doubt in the mind of the physician or other provider, we will likely see more referrals to specialists in lipid management and ASCVD risk estimation to optimize preventive therapy.31
CORRESPONDENCE
Cezary Wójcik, MD, PhD, FNLA, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; [email protected]
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. pii: S0735-1097(18)39034-X. [Epub ahead of print]
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-S45.
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39033-8. [Epub ahead of print]
4. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39035-1. [Epub ahead of print]
5. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
6. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III): final report. Circulation. 2002;106:3143-3421.
7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997-2007.
8. National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel II). Circulation. 1994;89:1333-1445.
9. Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749-753.
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
12. Szarek M, White HD, Schwartz GG, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387-396.
13. Crawford C. AAFP endorses ACC/AHA cholesterol management guidelines with qualifications. Leawood, KS: American Academy of Family Physicians; 2014 June 18. www.aafp.org/news/health-of-the-public/20140618cholesterolgdlnendorse.html. Accessed March 20, 2019.
14. Crawford C. AAFP News. AAFP affirms value of new cholesterol management guideline. March 20, 2019. www.aafp.org/news/health-of-the-public/20190320acc-ahacholguidln.html?cmpid=em_AP_20190320. Accessed April 1, 2019.
15. Lin JS, Evans CV, Johnson E, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Evidence Synthesis, No. 166. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 Jul. Report No.: 17-05225-EF-1.
16. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273-1282.
17. Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553-1560.
18. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92:1831-1841.
19. MDsave. Cardiac CT calcium scoring. www.mdsave.com/procedures/cardiac-ct-calcium-scoring/d785f4cf. Accessed Aprl 1, 2019.
20. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407-1416.
21. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213-S256.
22. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633.
23. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167-2192.
24. Newman CB, Preiss D, Tobert JA, et al; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38-e81.
25. Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sex Med Rev. 2018 Jan 27. pii: S2050-0521(18)30009-X. [Epub ahead of print]
26. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
27. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282.
28. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
29. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
30. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC Managed Markets Network. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed April 12, 2019.
31. Kaufman TM, Duell PB, Purnell JQ, et al. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499-501.
A new cholesterol guideline1 builds on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol guidelines,2 which were a major paradigm shift in the evaluation and management of blood cholesterol levels and risk for atherosclerotic cardiovascular disease (ASCVD). The work was presented (and simultaneously published) on November 10, 2018, at the annual AHA Scientific Sessions in Chicago. Full text,1 an executive summary,3 and accompanying systematic review of evidence4 are available online.
The 2018 AHA/ACC cholesterol guideline represents a step forward in ASCVD prevention—especially in primary prevention, where it provides guidance for risk refinement and personalization. In this article, we mine the details of what has changed and what is new in this guideline so that you can prepare to adopt the recommendations in your practice.

2013 and 2018 guidelines: Similarities, differences
As in earlier iterations, the 2018 guideline emphasizes healthy lifestyle across the life-course as the basis of ASCVD prevention—as elaborated in the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.5 In contrast to the 2013 guidelines,2 the 2018 guideline is more comprehensive and more personalized, focusing on risk assessment for individual patients, rather than simply providing population-based approaches. Moreover, the guideline isn’t limited to adults: It makes recommendations pertaining to children and adolescents.1

TABLE 11,2 compares the most important differences between the 2013 and 2018 guidelines.

The 2013 ACC/AHA guidelines eliminated low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C)a goals of therapy and replaced them with the concept of 4 “statin benefit groups”—that is, patient populations for which clear evidence supports the role of statin therapy.4 In the 2018 guideline, statin benefit groups have been maintained, although without explicit use of this term.1
Primary prevention. Although no major changes in statin indications are made for patients with (1) established ASCVD (ie, for secondary prevention), (2) diabetes mellitus (DM) and who are 40 to 75 years of age, or (3) a primary LDL-C elevation ≥ 190 mg/dL, significant changes were made for primary prevention patients ages 40 to 75 years.1 ASCVD risk calculation using the 2013 pooled cohort equations (PCE) is still recommended4; however, risk estimation is refined by the use of specific so-called risk-enhancing factors (TABLE 21). In cases in which the risk decision remains uncertain, obtaining the coronary artery calcium (CAC) score (which we’ll describe shortly) using specialized computed tomography (CT) is advised to facilitate the shared physician–patient decision-making process.1

LDL-C and non-HDL-C thresholds. Although LDL-C and non-HDL-C goals are not overtly brought back from the 2002 National Cholesterol Education Program/Adult Treatment Panel guidelines,6 the new guideline does introduce LDL-C and non-HDL-C thresholds—levels at which adding nonstatin therapy can be considered, in contrast to previous goals to which therapy was titrated. Definitions of statin intensity remain the same: Moderate-intensity statin therapy is expected to reduce the LDL-C level by 30% to 50%; high-intensity statin therapy, by ≥ 50%.1 The intensity of statin therapy has been de-escalated in the intermediate-risk group, where previous guidelines advised high-intensity statin therapy,4 and replaced with moderate-intensity statin therapy (similar to 2016 US Preventive Services Task Force [USPSTF] recommendations7).
[polldaddy:10312157]
Continue to: Fasting vs nonfasting lipid profiles
Fasting vs nonfasting lipid profiles. In contrast to previous guidelines,2,8 which used fasting lipid profiles, nonfasting lipid profiles are now recommended for establishing a baseline LDL-C level and for ASCVD risk estimation for most patients—as long as the triglycerides (TG) level is < 400 mg/dL. When the calculated LDL-C level is < 70 mg/dL using the standard Friedewald formula, obtaining a direct LDL-C or a modified LDL-C estimate9 is deemed reasonable to improve accuracy. (The modified LDL-C can be estimated using The Johns Hopkins Hospital’s free “LDL Cholesterol Calculator” [www.hopkinsmedicine.org/apps/all-apps/ldl-cholesterol-calculator]).
A fasting lipid profile is still preferred for patients who have a family history of a lipid disorder. The definition of hypertriglyceridemia has been revised from a fasting TG level ≥ 150 mg/dL to a nonfasting or fasting TG level ≥ 175 mg/dL.1
Nonstatin add-on therapy. The new guideline supports the addition of nonstatin therapies to maximally tolerated statin therapy in patients who have established ASCVD or a primary LDL-C elevation ≥ 190 mg/dL when (1) the LDL-C level has not been reduced by the expected percentage (≥ 50% for high-intensity statin therapy) or (2) explicit LDL-C level thresholds have been met.1
The principal 2 groups of recommended nonstatins for which there is randomized, controlled trial evidence of cardiovascular benefit are (1) the cholesterol-absorbing agent ezetimibe10 and (2) the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab11 and alirocumab.12
AAFP’s guarded positions on the 2013 and 2018 guidelines
The American Academy of Family Physicians (AAFP) welcomed the patient-centered and outcome-oriented aspects of the 2013 ACC/AHA guidelines, endorsing them with 3 qualifications.13
- Many of the recommendations were based on expert opinion, not rigorous research results—in particular, not on the findings of randomized controlled trials (although key points are based on high-quality evidence).
- There were conflicts of interest disclosed for 15 members of the guidelines panel, including a vice chair.
- Validation of the PCE risk estimation tool was lacking.
Continue to: AAFP announced...
AAFP announced in March that it does not endorse the 2018 AHA/ACC guideline, asserting that (1) only a small portion of the recommendations, primarily focused on the addition of nonstatin therapy, were addressed by an independent systematic review and (2) many of the guideline recommendations are based on low-quality or insufficient evidence. AAFP nevertheless bestowed an “affirmation of value” designation on the guideline—meaning that it provides some benefit for family physicians’ practice without fulfilling all criteria for full endorsement.14
Detailed recommendations from the 2018 guideline
Lifestyle modification
When talking about ASCVD risk with patients, it is important to review current lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). Subsequent to that conversation, a healthy lifestyle should be endorsed and relevant advice provided. In addition, patient-directed materials (eg, ACC’s CardioSmart [www.cardiosmart.org]; AHA’s Life’s Simple 7 [www.heart.org/en/professional/workplace-health/lifes-simple-7]; and the National Lipid Association’s Patient Tear Sheets [www.lipid.org/practicetools/tools/tearsheets] and Clinicians’ Lifestyle Modification Toolbox [www.lipid.org/CLMT]) and referrals (eg, to cardiac rehabilitation, a dietitian, a smoking-cessation program) should be provided.1
Primary prevention of ASCVD
Risk assessment for primary prevention is now approached as a process, rather than the simple risk calculation used in the 2013 ACC/AHA guidelines.2 Assessment involves risk estimation followed by risk personalization, which, in some cases, is followed by risk reclassification using CAC scoring.1
Patients are classified into 1 of 4 risk groups, based on the PCE1:
- low (< 5%)
- borderline (5%-7.5%)
- intermediate (7.5%-19.9%)
- high (≥ 20%).
However, the PCE-based risk score is a population-based tool, which might not reflect the actual risk of individual patients. In some populations, PCE underestimates ASCVD risk; in others, it overestimates risk. A central tenet of the new guideline is personalization of risk, taking into account the unique circumstances of each patient. Moreover, the new guideline provides guidance on how to interpret the PCE risk score for several different ethnic and racial groups.1
Continue to: Medical therapy
Medical therapy. The decision to start lipid-lowering therapy should be made after a physician–patient discussion that considers costs of therapy as well as patient preferences and values in the context of shared decision-making. Discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, and the LDL-C level), the PCE risk score, the presence of risk-enhancing factors (TABLE 21), potential benefits of lifestyle changes and statin therapy, and the potential for adverse drug effects and drug–drug interactions.1
If the estimated ASCVD risk is 7.5%-19.9%, starting moderate-intensity statin therapy is recommended. Risk-enhancing factors favor initiation of statin therapy, even in patients at borderline risk (5%-7.5%). If risk is uncertain, the CAC score can be used to facilitate shared decision-making.1 The use of CAC is in agreement with the USPSTF statement that CAC can moderately improve discrimination and reclassification, but has an unclear effect on downstream health care utilization.15 Importantly, CAC should not be measured routinely in patients already taking a statin because its primary role is to facilitate shared decision-making regarding initiation of statin therapy.16
If the 10-year ASCVD risk is ≥ 20%, high-intensity statin therapy is advised, without need to obtain the CAC score. If high-intensity statin therapy is advisable but not acceptable to, or tolerated by, the patient, it might be reasonable to add a nonstatin drug (ezetimibe or a bile-acid sequestrant) to moderate-intensity statin therapy.1
Risk-enhancing factors (TABLE 21) apply to intermediate- and borderline-risk patients. Importantly, these factors include membership in specific ethnic groups, conditions specific to females, and male–female distinctions in risk. Risk-enhancing factors also incorporate biomarkers that are often measured by lipid specialists, such as lipoprotein(a) (Lp[a]) and apolipoprotein B (ApoB).1
Lp(a) is an atherogenic particle, akin to an LDL particle, that consists of a molecule of apolipoprotein (a) (a nonfunctional mimic of a portion of plasminogen) covalently bound to ApoB, like the one found on the LDL particle. Lp(a) is proportionally associated with an increased risk for ASCVD and aortic stenosis at a level > 50 mg/dL.17 A family history of premature ASCVD is a relative indication for measuring Lp(a).1
Continue to: When and why to measure CAC
When and why to measure CAC
If the decision to initiate statin therapy is still uncertain after risk estimation and personalization, or when a patient is undecided about committing to lifelong lipid-lowering therapy, the new guideline recommends obtaining a CAC score to inform the shared decision-making process.1,18 Measurement of CAC is obtained by noncontrast, electrocardiographic-gated CT that can be performed in 10 to 15 minutes, requiring approximately 1 millisievert of radiation (equivalent of the approximate dose absorbed during 2 mammograms). Although measurement of the CAC score is generally not covered by insurance, its cost ($50-$450) nationwide makes it accessible.19
CAC measures the presence (or absence) of subclinical atherosclerosis by detecting calcified plaque in coronary arteries. The absolute CAC score is expressed in Agatston units; an age–gender population percentile is also provided. Keep in mind that the presence of any CAC (ie, a score > 0) is abnormal and demonstrates the presence of subclinical coronary artery disease. The prevalence of CAC > 0 increases with age, but a significant percentage of older people have a CAC score = 0. When CAC > 0, additional information is provided by the distribution of plaque burden among the different coronary arteries.20
Among intermediate-risk patients, 50% have CAC = 0 and, therefore, a very low event rate over the ensuing 10 years, which allows statin therapy to be safely deferred unless certain risk factors are present (eg, family history, smoking, DM).1,18 It is reasonable to repeat CAC testing in 5 to 10 years to assess whether subclinical atherosclerosis has developed. The 2018 guideline emphasizes that, when the CAC score is > 0 but < 100 Agatston units, statin therapy is favored, especially in patients > 55 years of age; when the CAC score is ≥ 100 Agatston units or at the ≥ 75th percentile, statin therapy is indicated regardless of age.1
Patients who might benefit from knowing their CAC score include those who are:
- reluctant to initiate statin therapy but who want to understand their risk and potential for benefit more precisely
- concerned about the need to reinstitute statin therapy after discontinuing it because of statin-associated adverse effects
- older (men, 55-80 years; women, 60-80 years) who have a low burden of risk factors and who question whether they would benefit from statin therapy
- middle-aged (40-55 years) and who have a PCE-calculated risk of 5% to < 7.5% for ASCVD and factors that increase their risk for ASCVD, even though they are in a borderline-risk group.1
Primary prevention in special populations
Older patients. In adults ≥ 75 years who have an LDL-C level 70 to 189 mg/dL, initiating a moderate-intensity statin might be reasonable; however, it might also be reasonable to stop treatment in this population when physical or cognitive decline, multiple morbidities, frailty, or reduced life expectancy limits the potential benefit of statin therapy. It might be reasonable to use the CAC score in adults 76 to 80 years of age who have an LDL-C level of 70 to 189 mg/dL to reclassify those whose CAC score = 0, so that they can avoid statin therapy.1
Continue to: Children and adolescents
Children and adolescents. In alignment with current pediatric guidelines,21 but in contrast to USPSTF reccomendations,22 the 2018 ACC/AHA guideline endorses universal lipid screening for pediatric patients (see TABLE W11,21,22). It is reasonable to obtain a fasting lipid profile or nonfasting non-HDL-C in all children and adolescents who have neither cardiovascular risk factors nor a family history of early cardiovascular disease to detect moderate-to-severe lipid abnormalities. Screening should be done once at 9 to 11 years of age and again at 17 to 21 years.1

A screening test as early as 2 years of age to detect familial hypercholesterolemia (FH) is reasonable when a family history of either early CVD or significant hypercholesterolemia is present. The guideline endorses reverse cascade screening for detection of FH in family members of children and adolescents who have severe hypercholesterolemia.1

In children and adolescents with a lipid abnormality, especially when associated with the metabolic syndrome, lifestyle counseling is beneficial for lowering the LDL-C level. In children and adolescents ≥ 10 years of age with (1) an LDL-C level persistently ≥ 190 mg/dL or (2) an LDL level ≥ 160 mg/dL plus a clinical presentation consistent with FH, it is reasonable to initiate statin therapy if they do not respond adequately to 3 to 6 months of lifestyle therapy.1
Ethnicity as a risk-modifying factor. The PCE distinguishes between US adults of European ancestry and African ancestry, but no other ethnic groups are distinguished.4 The new guideline advocates for the use of PCE in other populations; however, it states that, for clinical decision-making purposes, it is reasonable, in adults of different races and ethnicities, for the physician to review racial and ethnic features that can influence ASCVD risk to allow adjustment of the choice of statin or intensity of treatment. Specifically, South Asian ancestry is now treated as a risk-enhancing factor, given the high prevalence of premature and extensive ASCVD in this patient population.1
Concerns specific to women. Considering conditions specific to women as potential risk-enhancing factors is advised when discussing lifestyle intervention and the potential for benefit from statin therapy—in particular, (1) in the setting of premature menopause (< 40 years) and (2) when there is a history of a pregnancy-associated disorder (eg, hypertension, preeclampsia, gestational DM, a small-for-gestational-age infant, and preterm delivery). If the decision is made to initiate statin therapy in women of childbearing age who are sexually active, there is a guideline mandate to counsel patients on using reliable contraception. When pregnancy is planned, statin therapy should be discontinued 1 to 2 months before pregnancy is attempted; when pregnancy occurs while a patient is taking a statin, therapy should be stopped as soon as the pregnancy is discovered.1
Continue to: Adults with chronic kidney disease
Adults with chronic kidney disease. Chronic kidney disease that is not treated with dialysis or kidney transplantation is considered a risk-enhancing factor; initiation of a moderate-intensity statin or a moderate-intensity statin plus ezetimibe can be useful in patients with chronic kidney disease who are 40 to 75 years of age and have an LDL-C level of 70 to 189 mg/dL and a PCE-calculated risk ≥ 7.5%. In adults with advanced kidney disease that requires dialysis who are already taking a statin, it may be reasonable to continue the statin; however, initiation of a statin in adults with advanced kidney disease who require dialysis is not recommended because of an apparent lack of benefit.1
Adults with a chronic inflammatory disorder or human immunodeficiency virus infection. Any of these conditions are treated as risk-enhancing factors; in a risk discussion with affected patients, therefore, moderate-intensity statin therapy or high-intensity statin therapy is favored for those 40 to 75 years of age who have an LDL-C level of 70 to 189 mg/dL and PCE-calculated risk ≥ 7.5%. A fasting lipid profile and assessment of ASCVD risk factors for these patients can be useful (1) as a guide to the potential benefit of statin therapy and (2) for monitoring or adjusting lipid-lowering drug therapy before, and 4 to 12 weeks after, starting inflammatory disease-modifying therapy or antiretroviral therapy.
In adults with rheumatoid arthritis who undergo ASCVD risk assessment with a lipid profile, it can be useful to recheck lipid values and other major ASCVD risk factors 2 to 4 months after the inflammatory disease has been controlled.1
Primary hypercholesterolemia
The diagnosis and management of heterozygous or homozygous familial hypercholesterolemia (HeFH or HoFH) is beyond the scope of the 2018 ACC/AHA cholesterol guidelines; instead, the 2015 AHA Scientific Statement, “The Agenda for Familial Hypercholesterolemia,” provides a contemporary review of these topics.23 However, the 2018 cholesterol guideline does acknowledge that an LDL-C level ≥ 190 mg/dL often corresponds to primary (ie, genetic) hypercholesterolemia.
In patients 20 to 75 years of age who have a primary elevation of LDL-C level ≥ 190 mg/dL, the guideline recommends initiation of high-intensity statin therapy without calculating ASCVD risk using the PCE. If a > 50% LDL-C reduction is not achieved, or if the LDL-C level on maximally tolerated statin therapy remains ≥ 100 mg/dL, adding ezetimibe is considered reasonable. If there is < 50% reduction in the LDL-C level while taking maximally tolerated statin and ezetimibe therapy, adding a bile-acid sequestrant can be considered, as long as the TG level is not > 300 mg/dL (ie, bile-acid sequestrants can elevate the TG level significantly).
Continue to: In patients 30 to 75 years of age...
In patients 30 to 75 years of age who have a diagnosis of HeFH and an LDL-C level ≥ 100 mg/dL while taking maximally tolerated statin and ezetimibe therapy, the addition of a PCSK9 inhibitor can be considered. Regardless of whether there is a diagnosis of HeFH, addition of a PCSK9 inhibitor can be considered in patients 40 to 75 years of age who have a baseline LDL-C level ≥ 220 mg/dL and who achieve an on-treatment LDL-C level ≥ 130 mg/dL while receiving maximally tolerated statin therapy and ezetimibe.1
Diabetes mellitus
In patients with DM who are 40 to 75 years of age, moderate-intensity statin therapy is recommended without calculating the 10-year ASCVD risk. When the LDL-C level is 70 to 189 mg/dL, however, it is reasonable to use the PCE to assess 10-year ASCVD risk to facilitate risk stratification.
In patients with DM who are at higher risk, especially those who have multiple risk factors or are 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥ 50 %. In adults > 75 years of age with DM who are already on statin therapy, it is reasonable to continue statin therapy; for those that age who are not on statin therapy, it might be reasonable to initiate statin therapy after a physician–patient discussion of potential benefits and risks.
In adults with DM and PCE-calculated risk ≥ 20%, it might be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce the LDL-C level by ≥ 50%. In adults 20 to 39 years of age with DM of long duration (≥ 10 years of type 2 DM, ≥ 20 years of type 1 DM), albuminuria (≥ 30 μg of albumin/mg creatinine), estimated glomerular filtration rate < 60 mL/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index < 0.9, it might be reasonable to initiate statin therapy.1
Secondary prevention
Presence of clinical ASCVD. In patients with clinical ASCVD who are ≤ 75 years of age, high-intensity statin therapy should be initiated or continued, with the aim of achieving ≥ 50% reduction in the LDL-C level. When high-intensity statin therapy is contraindicated or if a patient experiences statin-associated adverse effects, moderate-intensity statin therapy should be initiated or continued with the aim of achieving a 30% to 49% reduction in the LDL-C level.
Continue to: In patients...
In patients > 75 years of age with clinical ASCVD, it is reasonable to initiate or continue moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preference.1
Very high risk. In patients at very high risk (this includes a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions), maximally tolerated LDL-C-lowering therapy should include maximally tolerated statin therapy and ezetimibe before considering a PCSK9 inhibitor. An LDL-C level ≥ 70 mg/dL or a non-HDL-C level ≥ 100 mg/dL is considered a reasonable threshold for adding a PCSK9 inhibitor to background lipid-lowering therapy1 (TABLE 31).

Heart failure. In patients with heart failure who have (1) a reduced ejection fraction attributable to ischemic heart disease, (2) a reasonable life expectancy (3-5 years), and (3) are not already on a statin because of ASCVD, consider initiating moderate-intensity statin therapy to reduce the risk for an ASCVD event.1
Reduction of elevated triglycerides
The guideline defines moderate hypertriglyceridemia as a nonfasting or fasting TG level of 175 to 499 mg/dL. Such a finding is considered a risk-enhancing factor and is 1 of 5 components of the metabolic syndrome. Three independent measurements are advised to diagnose primary moderate hypertriglyceridemia. Severe hypertriglyceridemia is diagnosed when the fasting TG level is ≥ 500 mg/dL.1
In moderate hypertriglyceridemia, most TGs are carried in very-low-density lipoprotein particles; in severe hypertriglyceridemia, on the other hand, chylomicrons predominate, raising the risk for pancreatitis. In adults with severe hypertriglyceridemia, therefore—especially when the fasting TG level is ≥ 1000 mg/dL—it is reasonable to identify and address other causes of hypertriglyceridemia. If TGs are persistently elevated or increasing, levels should be reduced to prevent acute pancreatitis with a very low-fat diet and by avoiding refined carbohydrates and alcohol; consuming omega-3 fatty acids; and, if necessary, taking a fibrate.1
Continue to: In adults...
In adults ≥ 20 years of age with moderate hypertriglyceridemia, lifestyle factors (eg, obesity, metabolic syndrome), secondary factors (eg, DM, chronic liver or kidney disease, nephrotic syndrome, hypothyroidism), and medications that increase the TG level need to be addressed first. In adults 40 to 75 years of age with moderate or severe hypertriglyceridemia and a PCE-calculated ASCVD risk ≥ 7.5%, it is reasonable to reevaluate risk after lifestyle and secondary factors are addressed and to consider a persistently elevated TG level as a factor favoring initiation or intensification of statin therapy. In adults 40 to 75 years of age with severe hypertriglyceridemia and ASCVD risk ≥ 7.5%, it is reasonable to address reversible causes of a high TG level and to initiate statin therapy.1
Other considerations in cholesterol management
Tools to assess adherence
The response to lifestyle and statin therapy should be evaluated by the percentage reduction in the LDL-C level compared with baseline, not by assessment of the absolute LDL-C level. When seeing a patient whose treatment is ongoing, a baseline level can be estimated using a desktop LDL-calculator app.
Adherence and percentage response to LDL-C–lowering medications and lifestyle changes should be evaluated with repeat lipid measurement 4 to 12 weeks after either a statin is initiated or the dosage is adjusted, and repeated every 3 to 12 months as needed. In patients with established ASCVD who are at very high risk, triggers for adding nonstatin therapy are defined by a threshold LDL-C level ≥ 70 mg/dL on maximal statin therapy.1
Interventions focused on improving adherence to prescribed therapy are recommended for management of adults with an elevated cholesterol level. These interventions include telephone reminders, calendar reminders, integrated multidisciplinary educational activities, and pharmacist-led interventions, such as simplification of the medication regimen to once-daily dosing.1
Statin safety and associated adverse effects
A physician–patient risk discussion is recommended before initiating statin therapy to review net clinical benefit, during which the 2 parties weigh the potential for ASCVD risk reduction against the potential for statin-associated adverse effects, statin–drug interactions, and safety, with the physician emphasizing that adverse effects can be addressed successfully.
Continue to: Statins are one of...
Statins are one of the safest classes of medication, with an excellent risk-benefit ratio. However, there are myriad confusing media reports regarding potential adverse effects and safety of the statin class—reports that often lead patients to discontinue or refuse statins.
Statin-associated adverse effects include the common statin-associated muscle symptoms (SAMS), new-onset DM, cognitive effects, and hepatic injury. The frequency of new-onset DM depends on the population exposed to statins, with a higher incidence of new-onset DM found in patients who are already predisposed, such as those with obesity, prediabetes, and metabolic syndrome. Cognitive effects are rare and difficult to interpret; they were not reported in the large statin mega-trials but have been described in case reports. Significant transaminase elevations > 3 times the upper limit of normal are infrequent; hepatic failure with statins is extremely rare and found at the same incidence in the general population.1
SAMS include (in order of decreasing prevalence)24:
- myalgias with a normal creatine kinase (CK) level
- conditions such as myositis or myopathy (elevated CK level)
- rhabdomyolysis (CK level > 10 times the upper limit of normal, plus renal injury)
- extremely rare statin-associated autoimmune myopathy, with detectable 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase antibodies.
In patients with SAMS, thorough assessment of symptoms is recommended, in addition to evaluation for nonstatin causes and predisposing factors. Identification of potential SAMS-predisposing factors is recommended before initiation of treatment, including demographics (eg, East-Asian ancestry), comorbid conditions (eg, hypothyroidism and vitamin D deficiency), and use of medications adversely affecting statin metabolism (eg, cyclosporine).
In patients with statin-associated adverse effects that are not severe, it is recommended to reassess and rechallenge to achieve a maximal lowering of the LDL-C level by a modified dosing regimen or an alternate statin or by combining a statin with nonstatin therapy. In patients with increased risk for DM or new-onset DM, it is recommended to continue statin therapy.
Continue to: Routine CK and liver function testing...
Routine CK and liver function testing is not useful in patients treated with statins; however, it is recommended that CK be measured in patients with severe SAMS or objective muscle weakness, or both, and to measure liver function if symptoms suggest hepatotoxicity. In patients at increased risk for ASCVD who have chronic, stable liver disease (including non-alcoholic fatty liver disease), it is reasonable, when appropriately indicated, to use statins after obtaining baseline measurements and determining a schedule of monitoring and safety checks.
In patients at increased risk for ASCVD who have severe or recurrent SAMS after appropriate statin rechallenge, it is reasonable to use nonstatin therapy that is likely to provide net clinical benefit. The guideline does not recommend routine use of coenzyme Q10 supplementation for the treatment or prevention of SAMS.1
Guideline criticism
Guideline development is challenging on multiple levels, including balancing perspectives from multiple stakeholders. Nevertheless, the 2018 AHA/ACC cholesterol guideline builds nicely on progress made since its 2013 predecessor was released.4 This document was developed with the participation of representatives from 10 professional societies in addition to the ACC and AHA—notably, the National Lipid Association and American Society for Preventive Cardiology.1
To refine risk estimation and facilitate shared decision-making, the new guideline introduced so-called risk-enhancing factors and use of the CAC.1 However, some potential risk-enhancing factors were left out: erectile dysfunction, for example, often a marker of increased cardiovascular risk in men < 50 years of age.25 In addition, although pretreatment ApoB was introduced as a risk-enhancing factor,1 no recommendation is given to measure ApoB after initiation of therapy for evaluation of residual cardiovascular risk, as endorsed in other guidelines.26,27
Moreover, the guideline does not include the “extreme risk” category in the guideline developed by the American Association of Clinical Endocrinologists (AACE).28 Although the 2018 AHA/ACC guideline introduces < 70 mg/dL and < 100 mg/dL LDL-C thresholds,1 the < 55 mg/dL LDL-C threshold used for patients in the AACE/American College of Endocrinology extreme-risk category is not mentioned.26 This omission might leave patients who are at extreme ASCVD risk without optimal lipid-lowering therapy. Similarly, the guideline does not elaborate on the diagnosis and treatment of HoFH and HeFH.1 The age cutoff of 30 years for the recommendation to consider PCSK9 inhibitors in patients with HeFH appears arbitrary and excludes younger FH patients who have an extreme LDL-C elevation from potentially important therapy.23
Continue to: Guidelines are dynamic instruments...
Guidelines are dynamic instruments that require constant updating, given the production of new evidence. In fact, the results of the Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) were presented at the same meeting at which this guideline was unveiled.29 REDUCE-IT demonstrated an astonishing highly significant 25% reduction in the composite primary major adverse cardiovascular event outcome in patients with an LDL-C level of 44 to 100 mg/dL on statin therapy, who had a TG level of 135 to 499 mg/dL and had been treated for a median of 4.9 years with 4 g of pure eicosapentaenoic acid.
In addition, the guideline’s value statements, which address the need to consider the cost of drugs in determining most appropriate treatment, are no longer accurate because the price of PCSK9 inhibitors has dropped by more than half since the guideline was issued.30
An upward climb to clinical payoff
Even after close study of the 2018 AHA/ACC cholesterol guideline, implementing it in practice might remain a challenge to clinicians who are inexperienced in ordering lipid markers such as Lp(a) and interpreting the CAC score. Moreover, initiating and monitoring nonstatin therapies will be a demanding task—especially with PCSK9 inhibitors, which present access difficulties because they are relatively expensive (even after the recent price cut). That’s why, when there is doubt in the mind of the physician or other provider, we will likely see more referrals to specialists in lipid management and ASCVD risk estimation to optimize preventive therapy.31
CORRESPONDENCE
Cezary Wójcik, MD, PhD, FNLA, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; [email protected]
A new cholesterol guideline1 builds on the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol guidelines,2 which were a major paradigm shift in the evaluation and management of blood cholesterol levels and risk for atherosclerotic cardiovascular disease (ASCVD). The work was presented (and simultaneously published) on November 10, 2018, at the annual AHA Scientific Sessions in Chicago. Full text,1 an executive summary,3 and accompanying systematic review of evidence4 are available online.
The 2018 AHA/ACC cholesterol guideline represents a step forward in ASCVD prevention—especially in primary prevention, where it provides guidance for risk refinement and personalization. In this article, we mine the details of what has changed and what is new in this guideline so that you can prepare to adopt the recommendations in your practice.

2013 and 2018 guidelines: Similarities, differences
As in earlier iterations, the 2018 guideline emphasizes healthy lifestyle across the life-course as the basis of ASCVD prevention—as elaborated in the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.5 In contrast to the 2013 guidelines,2 the 2018 guideline is more comprehensive and more personalized, focusing on risk assessment for individual patients, rather than simply providing population-based approaches. Moreover, the guideline isn’t limited to adults: It makes recommendations pertaining to children and adolescents.1

TABLE 11,2 compares the most important differences between the 2013 and 2018 guidelines.

The 2013 ACC/AHA guidelines eliminated low-density lipoprotein cholesterol (LDL-C) and non-high-density lipoprotein cholesterol (non-HDL-C)a goals of therapy and replaced them with the concept of 4 “statin benefit groups”—that is, patient populations for which clear evidence supports the role of statin therapy.4 In the 2018 guideline, statin benefit groups have been maintained, although without explicit use of this term.1
Primary prevention. Although no major changes in statin indications are made for patients with (1) established ASCVD (ie, for secondary prevention), (2) diabetes mellitus (DM) and who are 40 to 75 years of age, or (3) a primary LDL-C elevation ≥ 190 mg/dL, significant changes were made for primary prevention patients ages 40 to 75 years.1 ASCVD risk calculation using the 2013 pooled cohort equations (PCE) is still recommended4; however, risk estimation is refined by the use of specific so-called risk-enhancing factors (TABLE 21). In cases in which the risk decision remains uncertain, obtaining the coronary artery calcium (CAC) score (which we’ll describe shortly) using specialized computed tomography (CT) is advised to facilitate the shared physician–patient decision-making process.1

LDL-C and non-HDL-C thresholds. Although LDL-C and non-HDL-C goals are not overtly brought back from the 2002 National Cholesterol Education Program/Adult Treatment Panel guidelines,6 the new guideline does introduce LDL-C and non-HDL-C thresholds—levels at which adding nonstatin therapy can be considered, in contrast to previous goals to which therapy was titrated. Definitions of statin intensity remain the same: Moderate-intensity statin therapy is expected to reduce the LDL-C level by 30% to 50%; high-intensity statin therapy, by ≥ 50%.1 The intensity of statin therapy has been de-escalated in the intermediate-risk group, where previous guidelines advised high-intensity statin therapy,4 and replaced with moderate-intensity statin therapy (similar to 2016 US Preventive Services Task Force [USPSTF] recommendations7).
[polldaddy:10312157]
Continue to: Fasting vs nonfasting lipid profiles
Fasting vs nonfasting lipid profiles. In contrast to previous guidelines,2,8 which used fasting lipid profiles, nonfasting lipid profiles are now recommended for establishing a baseline LDL-C level and for ASCVD risk estimation for most patients—as long as the triglycerides (TG) level is < 400 mg/dL. When the calculated LDL-C level is < 70 mg/dL using the standard Friedewald formula, obtaining a direct LDL-C or a modified LDL-C estimate9 is deemed reasonable to improve accuracy. (The modified LDL-C can be estimated using The Johns Hopkins Hospital’s free “LDL Cholesterol Calculator” [www.hopkinsmedicine.org/apps/all-apps/ldl-cholesterol-calculator]).
A fasting lipid profile is still preferred for patients who have a family history of a lipid disorder. The definition of hypertriglyceridemia has been revised from a fasting TG level ≥ 150 mg/dL to a nonfasting or fasting TG level ≥ 175 mg/dL.1
Nonstatin add-on therapy. The new guideline supports the addition of nonstatin therapies to maximally tolerated statin therapy in patients who have established ASCVD or a primary LDL-C elevation ≥ 190 mg/dL when (1) the LDL-C level has not been reduced by the expected percentage (≥ 50% for high-intensity statin therapy) or (2) explicit LDL-C level thresholds have been met.1
The principal 2 groups of recommended nonstatins for which there is randomized, controlled trial evidence of cardiovascular benefit are (1) the cholesterol-absorbing agent ezetimibe10 and (2) the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors evolocumab11 and alirocumab.12
AAFP’s guarded positions on the 2013 and 2018 guidelines
The American Academy of Family Physicians (AAFP) welcomed the patient-centered and outcome-oriented aspects of the 2013 ACC/AHA guidelines, endorsing them with 3 qualifications.13
- Many of the recommendations were based on expert opinion, not rigorous research results—in particular, not on the findings of randomized controlled trials (although key points are based on high-quality evidence).
- There were conflicts of interest disclosed for 15 members of the guidelines panel, including a vice chair.
- Validation of the PCE risk estimation tool was lacking.
Continue to: AAFP announced...
AAFP announced in March that it does not endorse the 2018 AHA/ACC guideline, asserting that (1) only a small portion of the recommendations, primarily focused on the addition of nonstatin therapy, were addressed by an independent systematic review and (2) many of the guideline recommendations are based on low-quality or insufficient evidence. AAFP nevertheless bestowed an “affirmation of value” designation on the guideline—meaning that it provides some benefit for family physicians’ practice without fulfilling all criteria for full endorsement.14
Detailed recommendations from the 2018 guideline
Lifestyle modification
When talking about ASCVD risk with patients, it is important to review current lifestyle habits (eg, diet, physical activity, weight or body mass index, and tobacco use). Subsequent to that conversation, a healthy lifestyle should be endorsed and relevant advice provided. In addition, patient-directed materials (eg, ACC’s CardioSmart [www.cardiosmart.org]; AHA’s Life’s Simple 7 [www.heart.org/en/professional/workplace-health/lifes-simple-7]; and the National Lipid Association’s Patient Tear Sheets [www.lipid.org/practicetools/tools/tearsheets] and Clinicians’ Lifestyle Modification Toolbox [www.lipid.org/CLMT]) and referrals (eg, to cardiac rehabilitation, a dietitian, a smoking-cessation program) should be provided.1
Primary prevention of ASCVD
Risk assessment for primary prevention is now approached as a process, rather than the simple risk calculation used in the 2013 ACC/AHA guidelines.2 Assessment involves risk estimation followed by risk personalization, which, in some cases, is followed by risk reclassification using CAC scoring.1
Patients are classified into 1 of 4 risk groups, based on the PCE1:
- low (< 5%)
- borderline (5%-7.5%)
- intermediate (7.5%-19.9%)
- high (≥ 20%).
However, the PCE-based risk score is a population-based tool, which might not reflect the actual risk of individual patients. In some populations, PCE underestimates ASCVD risk; in others, it overestimates risk. A central tenet of the new guideline is personalization of risk, taking into account the unique circumstances of each patient. Moreover, the new guideline provides guidance on how to interpret the PCE risk score for several different ethnic and racial groups.1
Continue to: Medical therapy
Medical therapy. The decision to start lipid-lowering therapy should be made after a physician–patient discussion that considers costs of therapy as well as patient preferences and values in the context of shared decision-making. Discussion should include a review of major risk factors (eg, cigarette smoking, elevated blood pressure, and the LDL-C level), the PCE risk score, the presence of risk-enhancing factors (TABLE 21), potential benefits of lifestyle changes and statin therapy, and the potential for adverse drug effects and drug–drug interactions.1
If the estimated ASCVD risk is 7.5%-19.9%, starting moderate-intensity statin therapy is recommended. Risk-enhancing factors favor initiation of statin therapy, even in patients at borderline risk (5%-7.5%). If risk is uncertain, the CAC score can be used to facilitate shared decision-making.1 The use of CAC is in agreement with the USPSTF statement that CAC can moderately improve discrimination and reclassification, but has an unclear effect on downstream health care utilization.15 Importantly, CAC should not be measured routinely in patients already taking a statin because its primary role is to facilitate shared decision-making regarding initiation of statin therapy.16
If the 10-year ASCVD risk is ≥ 20%, high-intensity statin therapy is advised, without need to obtain the CAC score. If high-intensity statin therapy is advisable but not acceptable to, or tolerated by, the patient, it might be reasonable to add a nonstatin drug (ezetimibe or a bile-acid sequestrant) to moderate-intensity statin therapy.1
Risk-enhancing factors (TABLE 21) apply to intermediate- and borderline-risk patients. Importantly, these factors include membership in specific ethnic groups, conditions specific to females, and male–female distinctions in risk. Risk-enhancing factors also incorporate biomarkers that are often measured by lipid specialists, such as lipoprotein(a) (Lp[a]) and apolipoprotein B (ApoB).1
Lp(a) is an atherogenic particle, akin to an LDL particle, that consists of a molecule of apolipoprotein (a) (a nonfunctional mimic of a portion of plasminogen) covalently bound to ApoB, like the one found on the LDL particle. Lp(a) is proportionally associated with an increased risk for ASCVD and aortic stenosis at a level > 50 mg/dL.17 A family history of premature ASCVD is a relative indication for measuring Lp(a).1
Continue to: When and why to measure CAC
When and why to measure CAC
If the decision to initiate statin therapy is still uncertain after risk estimation and personalization, or when a patient is undecided about committing to lifelong lipid-lowering therapy, the new guideline recommends obtaining a CAC score to inform the shared decision-making process.1,18 Measurement of CAC is obtained by noncontrast, electrocardiographic-gated CT that can be performed in 10 to 15 minutes, requiring approximately 1 millisievert of radiation (equivalent of the approximate dose absorbed during 2 mammograms). Although measurement of the CAC score is generally not covered by insurance, its cost ($50-$450) nationwide makes it accessible.19
CAC measures the presence (or absence) of subclinical atherosclerosis by detecting calcified plaque in coronary arteries. The absolute CAC score is expressed in Agatston units; an age–gender population percentile is also provided. Keep in mind that the presence of any CAC (ie, a score > 0) is abnormal and demonstrates the presence of subclinical coronary artery disease. The prevalence of CAC > 0 increases with age, but a significant percentage of older people have a CAC score = 0. When CAC > 0, additional information is provided by the distribution of plaque burden among the different coronary arteries.20
Among intermediate-risk patients, 50% have CAC = 0 and, therefore, a very low event rate over the ensuing 10 years, which allows statin therapy to be safely deferred unless certain risk factors are present (eg, family history, smoking, DM).1,18 It is reasonable to repeat CAC testing in 5 to 10 years to assess whether subclinical atherosclerosis has developed. The 2018 guideline emphasizes that, when the CAC score is > 0 but < 100 Agatston units, statin therapy is favored, especially in patients > 55 years of age; when the CAC score is ≥ 100 Agatston units or at the ≥ 75th percentile, statin therapy is indicated regardless of age.1
Patients who might benefit from knowing their CAC score include those who are:
- reluctant to initiate statin therapy but who want to understand their risk and potential for benefit more precisely
- concerned about the need to reinstitute statin therapy after discontinuing it because of statin-associated adverse effects
- older (men, 55-80 years; women, 60-80 years) who have a low burden of risk factors and who question whether they would benefit from statin therapy
- middle-aged (40-55 years) and who have a PCE-calculated risk of 5% to < 7.5% for ASCVD and factors that increase their risk for ASCVD, even though they are in a borderline-risk group.1
Primary prevention in special populations
Older patients. In adults ≥ 75 years who have an LDL-C level 70 to 189 mg/dL, initiating a moderate-intensity statin might be reasonable; however, it might also be reasonable to stop treatment in this population when physical or cognitive decline, multiple morbidities, frailty, or reduced life expectancy limits the potential benefit of statin therapy. It might be reasonable to use the CAC score in adults 76 to 80 years of age who have an LDL-C level of 70 to 189 mg/dL to reclassify those whose CAC score = 0, so that they can avoid statin therapy.1
Continue to: Children and adolescents
Children and adolescents. In alignment with current pediatric guidelines,21 but in contrast to USPSTF reccomendations,22 the 2018 ACC/AHA guideline endorses universal lipid screening for pediatric patients (see TABLE W11,21,22). It is reasonable to obtain a fasting lipid profile or nonfasting non-HDL-C in all children and adolescents who have neither cardiovascular risk factors nor a family history of early cardiovascular disease to detect moderate-to-severe lipid abnormalities. Screening should be done once at 9 to 11 years of age and again at 17 to 21 years.1

A screening test as early as 2 years of age to detect familial hypercholesterolemia (FH) is reasonable when a family history of either early CVD or significant hypercholesterolemia is present. The guideline endorses reverse cascade screening for detection of FH in family members of children and adolescents who have severe hypercholesterolemia.1

In children and adolescents with a lipid abnormality, especially when associated with the metabolic syndrome, lifestyle counseling is beneficial for lowering the LDL-C level. In children and adolescents ≥ 10 years of age with (1) an LDL-C level persistently ≥ 190 mg/dL or (2) an LDL level ≥ 160 mg/dL plus a clinical presentation consistent with FH, it is reasonable to initiate statin therapy if they do not respond adequately to 3 to 6 months of lifestyle therapy.1
Ethnicity as a risk-modifying factor. The PCE distinguishes between US adults of European ancestry and African ancestry, but no other ethnic groups are distinguished.4 The new guideline advocates for the use of PCE in other populations; however, it states that, for clinical decision-making purposes, it is reasonable, in adults of different races and ethnicities, for the physician to review racial and ethnic features that can influence ASCVD risk to allow adjustment of the choice of statin or intensity of treatment. Specifically, South Asian ancestry is now treated as a risk-enhancing factor, given the high prevalence of premature and extensive ASCVD in this patient population.1
Concerns specific to women. Considering conditions specific to women as potential risk-enhancing factors is advised when discussing lifestyle intervention and the potential for benefit from statin therapy—in particular, (1) in the setting of premature menopause (< 40 years) and (2) when there is a history of a pregnancy-associated disorder (eg, hypertension, preeclampsia, gestational DM, a small-for-gestational-age infant, and preterm delivery). If the decision is made to initiate statin therapy in women of childbearing age who are sexually active, there is a guideline mandate to counsel patients on using reliable contraception. When pregnancy is planned, statin therapy should be discontinued 1 to 2 months before pregnancy is attempted; when pregnancy occurs while a patient is taking a statin, therapy should be stopped as soon as the pregnancy is discovered.1
Continue to: Adults with chronic kidney disease
Adults with chronic kidney disease. Chronic kidney disease that is not treated with dialysis or kidney transplantation is considered a risk-enhancing factor; initiation of a moderate-intensity statin or a moderate-intensity statin plus ezetimibe can be useful in patients with chronic kidney disease who are 40 to 75 years of age and have an LDL-C level of 70 to 189 mg/dL and a PCE-calculated risk ≥ 7.5%. In adults with advanced kidney disease that requires dialysis who are already taking a statin, it may be reasonable to continue the statin; however, initiation of a statin in adults with advanced kidney disease who require dialysis is not recommended because of an apparent lack of benefit.1
Adults with a chronic inflammatory disorder or human immunodeficiency virus infection. Any of these conditions are treated as risk-enhancing factors; in a risk discussion with affected patients, therefore, moderate-intensity statin therapy or high-intensity statin therapy is favored for those 40 to 75 years of age who have an LDL-C level of 70 to 189 mg/dL and PCE-calculated risk ≥ 7.5%. A fasting lipid profile and assessment of ASCVD risk factors for these patients can be useful (1) as a guide to the potential benefit of statin therapy and (2) for monitoring or adjusting lipid-lowering drug therapy before, and 4 to 12 weeks after, starting inflammatory disease-modifying therapy or antiretroviral therapy.
In adults with rheumatoid arthritis who undergo ASCVD risk assessment with a lipid profile, it can be useful to recheck lipid values and other major ASCVD risk factors 2 to 4 months after the inflammatory disease has been controlled.1
Primary hypercholesterolemia
The diagnosis and management of heterozygous or homozygous familial hypercholesterolemia (HeFH or HoFH) is beyond the scope of the 2018 ACC/AHA cholesterol guidelines; instead, the 2015 AHA Scientific Statement, “The Agenda for Familial Hypercholesterolemia,” provides a contemporary review of these topics.23 However, the 2018 cholesterol guideline does acknowledge that an LDL-C level ≥ 190 mg/dL often corresponds to primary (ie, genetic) hypercholesterolemia.
In patients 20 to 75 years of age who have a primary elevation of LDL-C level ≥ 190 mg/dL, the guideline recommends initiation of high-intensity statin therapy without calculating ASCVD risk using the PCE. If a > 50% LDL-C reduction is not achieved, or if the LDL-C level on maximally tolerated statin therapy remains ≥ 100 mg/dL, adding ezetimibe is considered reasonable. If there is < 50% reduction in the LDL-C level while taking maximally tolerated statin and ezetimibe therapy, adding a bile-acid sequestrant can be considered, as long as the TG level is not > 300 mg/dL (ie, bile-acid sequestrants can elevate the TG level significantly).
Continue to: In patients 30 to 75 years of age...
In patients 30 to 75 years of age who have a diagnosis of HeFH and an LDL-C level ≥ 100 mg/dL while taking maximally tolerated statin and ezetimibe therapy, the addition of a PCSK9 inhibitor can be considered. Regardless of whether there is a diagnosis of HeFH, addition of a PCSK9 inhibitor can be considered in patients 40 to 75 years of age who have a baseline LDL-C level ≥ 220 mg/dL and who achieve an on-treatment LDL-C level ≥ 130 mg/dL while receiving maximally tolerated statin therapy and ezetimibe.1
Diabetes mellitus
In patients with DM who are 40 to 75 years of age, moderate-intensity statin therapy is recommended without calculating the 10-year ASCVD risk. When the LDL-C level is 70 to 189 mg/dL, however, it is reasonable to use the PCE to assess 10-year ASCVD risk to facilitate risk stratification.
In patients with DM who are at higher risk, especially those who have multiple risk factors or are 50 to 75 years of age, it is reasonable to use a high-intensity statin to reduce the LDL-C level by ≥ 50 %. In adults > 75 years of age with DM who are already on statin therapy, it is reasonable to continue statin therapy; for those that age who are not on statin therapy, it might be reasonable to initiate statin therapy after a physician–patient discussion of potential benefits and risks.
In adults with DM and PCE-calculated risk ≥ 20%, it might be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce the LDL-C level by ≥ 50%. In adults 20 to 39 years of age with DM of long duration (≥ 10 years of type 2 DM, ≥ 20 years of type 1 DM), albuminuria (≥ 30 μg of albumin/mg creatinine), estimated glomerular filtration rate < 60 mL/min/1.73 m2, retinopathy, neuropathy, or ankle-brachial index < 0.9, it might be reasonable to initiate statin therapy.1
Secondary prevention
Presence of clinical ASCVD. In patients with clinical ASCVD who are ≤ 75 years of age, high-intensity statin therapy should be initiated or continued, with the aim of achieving ≥ 50% reduction in the LDL-C level. When high-intensity statin therapy is contraindicated or if a patient experiences statin-associated adverse effects, moderate-intensity statin therapy should be initiated or continued with the aim of achieving a 30% to 49% reduction in the LDL-C level.
Continue to: In patients...
In patients > 75 years of age with clinical ASCVD, it is reasonable to initiate or continue moderate- or high-intensity statin therapy after evaluation of the potential for ASCVD risk reduction, adverse effects, and drug–drug interactions, as well as patient frailty and patient preference.1
Very high risk. In patients at very high risk (this includes a history of multiple major ASCVD events or 1 major ASCVD event plus multiple high-risk conditions), maximally tolerated LDL-C-lowering therapy should include maximally tolerated statin therapy and ezetimibe before considering a PCSK9 inhibitor. An LDL-C level ≥ 70 mg/dL or a non-HDL-C level ≥ 100 mg/dL is considered a reasonable threshold for adding a PCSK9 inhibitor to background lipid-lowering therapy1 (TABLE 31).

Heart failure. In patients with heart failure who have (1) a reduced ejection fraction attributable to ischemic heart disease, (2) a reasonable life expectancy (3-5 years), and (3) are not already on a statin because of ASCVD, consider initiating moderate-intensity statin therapy to reduce the risk for an ASCVD event.1
Reduction of elevated triglycerides
The guideline defines moderate hypertriglyceridemia as a nonfasting or fasting TG level of 175 to 499 mg/dL. Such a finding is considered a risk-enhancing factor and is 1 of 5 components of the metabolic syndrome. Three independent measurements are advised to diagnose primary moderate hypertriglyceridemia. Severe hypertriglyceridemia is diagnosed when the fasting TG level is ≥ 500 mg/dL.1
In moderate hypertriglyceridemia, most TGs are carried in very-low-density lipoprotein particles; in severe hypertriglyceridemia, on the other hand, chylomicrons predominate, raising the risk for pancreatitis. In adults with severe hypertriglyceridemia, therefore—especially when the fasting TG level is ≥ 1000 mg/dL—it is reasonable to identify and address other causes of hypertriglyceridemia. If TGs are persistently elevated or increasing, levels should be reduced to prevent acute pancreatitis with a very low-fat diet and by avoiding refined carbohydrates and alcohol; consuming omega-3 fatty acids; and, if necessary, taking a fibrate.1
Continue to: In adults...
In adults ≥ 20 years of age with moderate hypertriglyceridemia, lifestyle factors (eg, obesity, metabolic syndrome), secondary factors (eg, DM, chronic liver or kidney disease, nephrotic syndrome, hypothyroidism), and medications that increase the TG level need to be addressed first. In adults 40 to 75 years of age with moderate or severe hypertriglyceridemia and a PCE-calculated ASCVD risk ≥ 7.5%, it is reasonable to reevaluate risk after lifestyle and secondary factors are addressed and to consider a persistently elevated TG level as a factor favoring initiation or intensification of statin therapy. In adults 40 to 75 years of age with severe hypertriglyceridemia and ASCVD risk ≥ 7.5%, it is reasonable to address reversible causes of a high TG level and to initiate statin therapy.1
Other considerations in cholesterol management
Tools to assess adherence
The response to lifestyle and statin therapy should be evaluated by the percentage reduction in the LDL-C level compared with baseline, not by assessment of the absolute LDL-C level. When seeing a patient whose treatment is ongoing, a baseline level can be estimated using a desktop LDL-calculator app.
Adherence and percentage response to LDL-C–lowering medications and lifestyle changes should be evaluated with repeat lipid measurement 4 to 12 weeks after either a statin is initiated or the dosage is adjusted, and repeated every 3 to 12 months as needed. In patients with established ASCVD who are at very high risk, triggers for adding nonstatin therapy are defined by a threshold LDL-C level ≥ 70 mg/dL on maximal statin therapy.1
Interventions focused on improving adherence to prescribed therapy are recommended for management of adults with an elevated cholesterol level. These interventions include telephone reminders, calendar reminders, integrated multidisciplinary educational activities, and pharmacist-led interventions, such as simplification of the medication regimen to once-daily dosing.1
Statin safety and associated adverse effects
A physician–patient risk discussion is recommended before initiating statin therapy to review net clinical benefit, during which the 2 parties weigh the potential for ASCVD risk reduction against the potential for statin-associated adverse effects, statin–drug interactions, and safety, with the physician emphasizing that adverse effects can be addressed successfully.
Continue to: Statins are one of...
Statins are one of the safest classes of medication, with an excellent risk-benefit ratio. However, there are myriad confusing media reports regarding potential adverse effects and safety of the statin class—reports that often lead patients to discontinue or refuse statins.
Statin-associated adverse effects include the common statin-associated muscle symptoms (SAMS), new-onset DM, cognitive effects, and hepatic injury. The frequency of new-onset DM depends on the population exposed to statins, with a higher incidence of new-onset DM found in patients who are already predisposed, such as those with obesity, prediabetes, and metabolic syndrome. Cognitive effects are rare and difficult to interpret; they were not reported in the large statin mega-trials but have been described in case reports. Significant transaminase elevations > 3 times the upper limit of normal are infrequent; hepatic failure with statins is extremely rare and found at the same incidence in the general population.1
SAMS include (in order of decreasing prevalence)24:
- myalgias with a normal creatine kinase (CK) level
- conditions such as myositis or myopathy (elevated CK level)
- rhabdomyolysis (CK level > 10 times the upper limit of normal, plus renal injury)
- extremely rare statin-associated autoimmune myopathy, with detectable 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase antibodies.
In patients with SAMS, thorough assessment of symptoms is recommended, in addition to evaluation for nonstatin causes and predisposing factors. Identification of potential SAMS-predisposing factors is recommended before initiation of treatment, including demographics (eg, East-Asian ancestry), comorbid conditions (eg, hypothyroidism and vitamin D deficiency), and use of medications adversely affecting statin metabolism (eg, cyclosporine).
In patients with statin-associated adverse effects that are not severe, it is recommended to reassess and rechallenge to achieve a maximal lowering of the LDL-C level by a modified dosing regimen or an alternate statin or by combining a statin with nonstatin therapy. In patients with increased risk for DM or new-onset DM, it is recommended to continue statin therapy.
Continue to: Routine CK and liver function testing...
Routine CK and liver function testing is not useful in patients treated with statins; however, it is recommended that CK be measured in patients with severe SAMS or objective muscle weakness, or both, and to measure liver function if symptoms suggest hepatotoxicity. In patients at increased risk for ASCVD who have chronic, stable liver disease (including non-alcoholic fatty liver disease), it is reasonable, when appropriately indicated, to use statins after obtaining baseline measurements and determining a schedule of monitoring and safety checks.
In patients at increased risk for ASCVD who have severe or recurrent SAMS after appropriate statin rechallenge, it is reasonable to use nonstatin therapy that is likely to provide net clinical benefit. The guideline does not recommend routine use of coenzyme Q10 supplementation for the treatment or prevention of SAMS.1
Guideline criticism
Guideline development is challenging on multiple levels, including balancing perspectives from multiple stakeholders. Nevertheless, the 2018 AHA/ACC cholesterol guideline builds nicely on progress made since its 2013 predecessor was released.4 This document was developed with the participation of representatives from 10 professional societies in addition to the ACC and AHA—notably, the National Lipid Association and American Society for Preventive Cardiology.1
To refine risk estimation and facilitate shared decision-making, the new guideline introduced so-called risk-enhancing factors and use of the CAC.1 However, some potential risk-enhancing factors were left out: erectile dysfunction, for example, often a marker of increased cardiovascular risk in men < 50 years of age.25 In addition, although pretreatment ApoB was introduced as a risk-enhancing factor,1 no recommendation is given to measure ApoB after initiation of therapy for evaluation of residual cardiovascular risk, as endorsed in other guidelines.26,27
Moreover, the guideline does not include the “extreme risk” category in the guideline developed by the American Association of Clinical Endocrinologists (AACE).28 Although the 2018 AHA/ACC guideline introduces < 70 mg/dL and < 100 mg/dL LDL-C thresholds,1 the < 55 mg/dL LDL-C threshold used for patients in the AACE/American College of Endocrinology extreme-risk category is not mentioned.26 This omission might leave patients who are at extreme ASCVD risk without optimal lipid-lowering therapy. Similarly, the guideline does not elaborate on the diagnosis and treatment of HoFH and HeFH.1 The age cutoff of 30 years for the recommendation to consider PCSK9 inhibitors in patients with HeFH appears arbitrary and excludes younger FH patients who have an extreme LDL-C elevation from potentially important therapy.23
Continue to: Guidelines are dynamic instruments...
Guidelines are dynamic instruments that require constant updating, given the production of new evidence. In fact, the results of the Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial (REDUCE-IT) were presented at the same meeting at which this guideline was unveiled.29 REDUCE-IT demonstrated an astonishing highly significant 25% reduction in the composite primary major adverse cardiovascular event outcome in patients with an LDL-C level of 44 to 100 mg/dL on statin therapy, who had a TG level of 135 to 499 mg/dL and had been treated for a median of 4.9 years with 4 g of pure eicosapentaenoic acid.
In addition, the guideline’s value statements, which address the need to consider the cost of drugs in determining most appropriate treatment, are no longer accurate because the price of PCSK9 inhibitors has dropped by more than half since the guideline was issued.30
An upward climb to clinical payoff
Even after close study of the 2018 AHA/ACC cholesterol guideline, implementing it in practice might remain a challenge to clinicians who are inexperienced in ordering lipid markers such as Lp(a) and interpreting the CAC score. Moreover, initiating and monitoring nonstatin therapies will be a demanding task—especially with PCSK9 inhibitors, which present access difficulties because they are relatively expensive (even after the recent price cut). That’s why, when there is doubt in the mind of the physician or other provider, we will likely see more referrals to specialists in lipid management and ASCVD risk estimation to optimize preventive therapy.31
CORRESPONDENCE
Cezary Wójcik, MD, PhD, FNLA, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; [email protected]
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. pii: S0735-1097(18)39034-X. [Epub ahead of print]
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-S45.
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39033-8. [Epub ahead of print]
4. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39035-1. [Epub ahead of print]
5. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
6. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III): final report. Circulation. 2002;106:3143-3421.
7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997-2007.
8. National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel II). Circulation. 1994;89:1333-1445.
9. Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749-753.
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
12. Szarek M, White HD, Schwartz GG, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387-396.
13. Crawford C. AAFP endorses ACC/AHA cholesterol management guidelines with qualifications. Leawood, KS: American Academy of Family Physicians; 2014 June 18. www.aafp.org/news/health-of-the-public/20140618cholesterolgdlnendorse.html. Accessed March 20, 2019.
14. Crawford C. AAFP News. AAFP affirms value of new cholesterol management guideline. March 20, 2019. www.aafp.org/news/health-of-the-public/20190320acc-ahacholguidln.html?cmpid=em_AP_20190320. Accessed April 1, 2019.
15. Lin JS, Evans CV, Johnson E, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Evidence Synthesis, No. 166. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 Jul. Report No.: 17-05225-EF-1.
16. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273-1282.
17. Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553-1560.
18. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92:1831-1841.
19. MDsave. Cardiac CT calcium scoring. www.mdsave.com/procedures/cardiac-ct-calcium-scoring/d785f4cf. Accessed Aprl 1, 2019.
20. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407-1416.
21. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213-S256.
22. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633.
23. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167-2192.
24. Newman CB, Preiss D, Tobert JA, et al; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38-e81.
25. Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sex Med Rev. 2018 Jan 27. pii: S2050-0521(18)30009-X. [Epub ahead of print]
26. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
27. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282.
28. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
29. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
30. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC Managed Markets Network. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed April 12, 2019.
31. Kaufman TM, Duell PB, Purnell JQ, et al. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499-501.
1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 8. pii: S0735-1097(18)39034-X. [Epub ahead of print]
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1-S45.
3. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39033-8. [Epub ahead of print]
4. Wilson PWF, Polonsky TS, Miedema MD, et al. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 Nov 3. pii: S0735-1097(18)39035-1. [Epub ahead of print]
5. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984.
6. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III): final report. Circulation. 2002;106:3143-3421.
7. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997-2007.
8. National Cholesterol Education Program. Second report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel II). Circulation. 1994;89:1333-1445.
9. Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 2018;3:749-753.
10. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
11. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
12. Szarek M, White HD, Schwartz GG, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab reduces total nonfatal cardiovascular and fatal events in the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73:387-396.
13. Crawford C. AAFP endorses ACC/AHA cholesterol management guidelines with qualifications. Leawood, KS: American Academy of Family Physicians; 2014 June 18. www.aafp.org/news/health-of-the-public/20140618cholesterolgdlnendorse.html. Accessed March 20, 2019.
14. Crawford C. AAFP News. AAFP affirms value of new cholesterol management guideline. March 20, 2019. www.aafp.org/news/health-of-the-public/20190320acc-ahacholguidln.html?cmpid=em_AP_20190320. Accessed April 1, 2019.
15. Lin JS, Evans CV, Johnson E, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Evidence Synthesis, No. 166. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018 Jul. Report No.: 17-05225-EF-1.
16. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273-1282.
17. Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553-1560.
18. Michos ED, Blaha MJ, Blumenthal RS. Use of the coronary artery calcium score in discussion of initiation of statin therapy in primary prevention. Mayo Clin Proc. 2017;92:1831-1841.
19. MDsave. Cardiac CT calcium scoring. www.mdsave.com/procedures/cardiac-ct-calcium-scoring/d785f4cf. Accessed Aprl 1, 2019.
20. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:1407-1416.
21. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213-S256.
22. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633.
23. Gidding SS, Champagne MA, de Ferranti SD, et al; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167-2192.
24. Newman CB, Preiss D, Tobert JA, et al; American Heart Association Clinical Lipidology, Lipoprotein, Metabolism and Thrombosis Committee, a Joint Committee of the Council on Atherosclerosis, Thrombosis and Vascular Biology and Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38-e81.
25. Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sex Med Rev. 2018 Jan 27. pii: S2050-0521(18)30009-X. [Epub ahead of print]
26. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
27. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263-1282.
28. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
29. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
30. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC Managed Markets Network. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed April 12, 2019.
31. Kaufman TM, Duell PB, Purnell JQ, et al. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499-501.
PRACTICE RECOMMENDATIONS
› Reduce the low-density lipoprotein cholesterol (LDL-C) level in patients with clinical atherosclerotic cardiovascular disease (ASCVD) using high-intensity statin therapy or maximally tolerated statin therapy. A
› Use an LDL-C threshold of 70 mg/dL to prompt consideration of adding nonstatin therapy in patients who have very high-risk ASCVD. A
› Start high-intensity statin therapy in patients who have primary hypercholesterolemia (LDL-C level ≥ 190 mg/dL) without calculating the 10-year ASCVD risk. A
› Begin moderate-intensity statin therapy in patients 40 to 75 years of age who have diabetes mellitus and an LDL-C level ≥ 70 mg/dL without calculating 10-year ASCVD risk. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Females with acne stay on spironolactone longer than antibiotics in real-world usage study
according to a retrospective study of women with acne published in the Journal of the American Academy of Dermatology.
Among those treated for at least a year, patients continued spironolactone for about 90 days longer on average, than those on antibiotic therapy, reported John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and associates. “The extended drug usage survival of spironolactone suggests that, in routine clinical practice, spironolactone may have good long-term effectiveness and tolerability,” they wrote. “Since female patients often have persistent acne into adulthood and given concerns regarding antibiotic overuse among acne patients, it is possible that using spironolactone as a first-line agent before oral antibiotics could improve outcomes for female patients with acne,” they added.
In the study, they pointed out that spironolactone is emerging as a possible alternative to oral antibiotic therapy, but “little is known about long-term outcomes with spironolactone for those who have an initial positive response and how it compares to other alternatives.”
To examine the duration of acne treatment with spironolactone versus oral antibiotics, the researchers analyzed data during 2010-2016 in the Optum Clinformatics Data Mart. They included data on female patients aged 12-40 years, with at least two diagnosis codes for acne, who received spironolactone or oral antibiotics for at least 12 months. They used multivariate Cox proportional hazard models to assess differences in duration of therapy for spironolactone, compared with oral antibiotics.
The mean duration of a treatment course was significantly longer among the 4,321 patients treated with spironolactone than among the 7,517 patients treated with oral tetracycline-class antibiotics (697.8 days vs. 604.4 days; P less than .001). Compared with treatment with oral tetracyclines, the hazard ratio for discontinuing spironolactone treatment was 0.74, after researchers controlled for the age at diagnosis and treatment, history of polycystic ovarian syndrome, and history of combined oral contraceptive or topical retinoid treatment.
Patients who receive spironolactone and patients who receive oral antibiotics may represent different populations, the authors noted. In addition, guidelines advise limiting antibiotic treatment to 3-6 months, and antibiotic discontinuations may have been related to these recommendations. “It is not possible to determine whether medication discontinuation occurred due to lack of efficacy, cost, side effects, resolution of acne, or other factors,” they said, adding that prospective studies are needed “to identify the optimal treatment approaches for female patients with moderate to severe acne.”
The study was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri is supported by NIAMS and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania.
SOURCE: Barbieri JS et al. J Am Acad Dermatol. 2019 Mar 21. doi: 10.1016/j.jaad.2019.03.036.
according to a retrospective study of women with acne published in the Journal of the American Academy of Dermatology.
Among those treated for at least a year, patients continued spironolactone for about 90 days longer on average, than those on antibiotic therapy, reported John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and associates. “The extended drug usage survival of spironolactone suggests that, in routine clinical practice, spironolactone may have good long-term effectiveness and tolerability,” they wrote. “Since female patients often have persistent acne into adulthood and given concerns regarding antibiotic overuse among acne patients, it is possible that using spironolactone as a first-line agent before oral antibiotics could improve outcomes for female patients with acne,” they added.
In the study, they pointed out that spironolactone is emerging as a possible alternative to oral antibiotic therapy, but “little is known about long-term outcomes with spironolactone for those who have an initial positive response and how it compares to other alternatives.”
To examine the duration of acne treatment with spironolactone versus oral antibiotics, the researchers analyzed data during 2010-2016 in the Optum Clinformatics Data Mart. They included data on female patients aged 12-40 years, with at least two diagnosis codes for acne, who received spironolactone or oral antibiotics for at least 12 months. They used multivariate Cox proportional hazard models to assess differences in duration of therapy for spironolactone, compared with oral antibiotics.
The mean duration of a treatment course was significantly longer among the 4,321 patients treated with spironolactone than among the 7,517 patients treated with oral tetracycline-class antibiotics (697.8 days vs. 604.4 days; P less than .001). Compared with treatment with oral tetracyclines, the hazard ratio for discontinuing spironolactone treatment was 0.74, after researchers controlled for the age at diagnosis and treatment, history of polycystic ovarian syndrome, and history of combined oral contraceptive or topical retinoid treatment.
Patients who receive spironolactone and patients who receive oral antibiotics may represent different populations, the authors noted. In addition, guidelines advise limiting antibiotic treatment to 3-6 months, and antibiotic discontinuations may have been related to these recommendations. “It is not possible to determine whether medication discontinuation occurred due to lack of efficacy, cost, side effects, resolution of acne, or other factors,” they said, adding that prospective studies are needed “to identify the optimal treatment approaches for female patients with moderate to severe acne.”
The study was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri is supported by NIAMS and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania.
SOURCE: Barbieri JS et al. J Am Acad Dermatol. 2019 Mar 21. doi: 10.1016/j.jaad.2019.03.036.
according to a retrospective study of women with acne published in the Journal of the American Academy of Dermatology.
Among those treated for at least a year, patients continued spironolactone for about 90 days longer on average, than those on antibiotic therapy, reported John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and associates. “The extended drug usage survival of spironolactone suggests that, in routine clinical practice, spironolactone may have good long-term effectiveness and tolerability,” they wrote. “Since female patients often have persistent acne into adulthood and given concerns regarding antibiotic overuse among acne patients, it is possible that using spironolactone as a first-line agent before oral antibiotics could improve outcomes for female patients with acne,” they added.
In the study, they pointed out that spironolactone is emerging as a possible alternative to oral antibiotic therapy, but “little is known about long-term outcomes with spironolactone for those who have an initial positive response and how it compares to other alternatives.”
To examine the duration of acne treatment with spironolactone versus oral antibiotics, the researchers analyzed data during 2010-2016 in the Optum Clinformatics Data Mart. They included data on female patients aged 12-40 years, with at least two diagnosis codes for acne, who received spironolactone or oral antibiotics for at least 12 months. They used multivariate Cox proportional hazard models to assess differences in duration of therapy for spironolactone, compared with oral antibiotics.
The mean duration of a treatment course was significantly longer among the 4,321 patients treated with spironolactone than among the 7,517 patients treated with oral tetracycline-class antibiotics (697.8 days vs. 604.4 days; P less than .001). Compared with treatment with oral tetracyclines, the hazard ratio for discontinuing spironolactone treatment was 0.74, after researchers controlled for the age at diagnosis and treatment, history of polycystic ovarian syndrome, and history of combined oral contraceptive or topical retinoid treatment.
Patients who receive spironolactone and patients who receive oral antibiotics may represent different populations, the authors noted. In addition, guidelines advise limiting antibiotic treatment to 3-6 months, and antibiotic discontinuations may have been related to these recommendations. “It is not possible to determine whether medication discontinuation occurred due to lack of efficacy, cost, side effects, resolution of acne, or other factors,” they said, adding that prospective studies are needed “to identify the optimal treatment approaches for female patients with moderate to severe acne.”
The study was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri is supported by NIAMS and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania.
SOURCE: Barbieri JS et al. J Am Acad Dermatol. 2019 Mar 21. doi: 10.1016/j.jaad.2019.03.036.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
FDA approves Qternmet XR as adjunct therapy for glycemic improvement in type 2 diabetes
The Food and Drug Administration has approved Qternmet XR (dapagliflozin/saxagliptin/metformin) as an oral adjunct therapy to diet and exercise for the improvement of glycemic control in patients with type 2 diabetes, according to AstraZeneca.
FDA approval is based on results from a pair of phase 3 trials that tested different combinations of dapagliflozin and saxagliptin in patients with inadequately controlled type 2 diabetes who were also receiving metformin over a 24-week period. In both trials, treatment with dapagliflozin/saxagliptin/metformin decreased hemoglobin A1c by statistically significant amounts, and increased the number of patients with HbA1c levels below 7%
The safety results of Qternmet XR was consistent with each component medication’s known profile.
The Food and Drug Administration has approved Qternmet XR (dapagliflozin/saxagliptin/metformin) as an oral adjunct therapy to diet and exercise for the improvement of glycemic control in patients with type 2 diabetes, according to AstraZeneca.
FDA approval is based on results from a pair of phase 3 trials that tested different combinations of dapagliflozin and saxagliptin in patients with inadequately controlled type 2 diabetes who were also receiving metformin over a 24-week period. In both trials, treatment with dapagliflozin/saxagliptin/metformin decreased hemoglobin A1c by statistically significant amounts, and increased the number of patients with HbA1c levels below 7%
The safety results of Qternmet XR was consistent with each component medication’s known profile.
The Food and Drug Administration has approved Qternmet XR (dapagliflozin/saxagliptin/metformin) as an oral adjunct therapy to diet and exercise for the improvement of glycemic control in patients with type 2 diabetes, according to AstraZeneca.
FDA approval is based on results from a pair of phase 3 trials that tested different combinations of dapagliflozin and saxagliptin in patients with inadequately controlled type 2 diabetes who were also receiving metformin over a 24-week period. In both trials, treatment with dapagliflozin/saxagliptin/metformin decreased hemoglobin A1c by statistically significant amounts, and increased the number of patients with HbA1c levels below 7%
The safety results of Qternmet XR was consistent with each component medication’s known profile.
Bioimpedance spectroscopy may better identify lymphedema progression
Bioimpedance spectroscopy may better identify lymphedema progression in women at risk for breast cancer–related lymphedema over the traditionally used method of monitoring arm circumference with a tape measure, according to results from an interim analysis of the PREVENT trial presented in a recent webcast from the annual meeting of the American Society of Breast Surgeons.
“Despite advances in breast-conserving surgery, improved radiation protocol, the advent of sentinel [node] biopsies and recent improvement in chemotherapy regimens, breast cancer–related lymphedema ... remains a major source of morbidity and concern in this patient population,” Sheila Ridner, PhD, RN, FAAN, professor of nursing at the Vanderbilt University School of Nursing in Nashville, Tenn., said in her presentation. “Because it is thought that early identification of swelling in the limbs coupled with a compression intervention may reduce the risk of patients developing full-blown clinical lymphedema, clinicians are proposing to use a prospective surveillance model to follow breast cancer survivors post surgery in order to assess limbs in a routine fashion and perhaps instigate preventative mechanisms early.”
The larger randomized controlled Prevention of Lymphedema Following Locoregional Treatment for Breast Cancer (PREVENT) trial enrolled 1,201 patients, and 200 patients overall have completed the full protocol. The researchers plan to follow patients for 3 years after surgery. In this interim analysis, Dr. Ridner and colleagues analyzed data from 508 patients at eight sites in the United States and four sites in Sydney who had stage I through stage III or ductal carcinoma in situ (DCIS) breast cancer and underwent mastectomy, taxane-based chemotherapy, or an axillary treatment such as axillary radiation, axillary lymph node dissection, or sentinel lymph nose biopsy with more than 6 nodes.
The patients were randomized to be measured using either traditional tape measurement or bioimpedance spectroscopy (BIS). Patients were moved to a prevention intervention if there was a change in baseline volume of 5% or greater but less than 10% in the tape measure group and change from baseline L-Dex measurement of 6.5 or greater in the BIS group, which consisted of wearing an arm compression sleeve and chest gauntlet for 12 hours a day over 4 weeks. After surgery, the patients were followed up at 3 months, 6 months, 12 months, 18 months, and 24 months, with optional follow-up visits at 15 months and 21 months. Progression to full lymphedema was defined as a 10% or greater change in pretreatment baseline measurements in the tape measure group.
Of the 508 patients analyzed, 10 patients had already progressed to full lymphedema, leaving 498 patients available for the interim analysis. There were 68 patients in the tape measure group (28.5%) and 41 patients in the BIS group (15.8%) who received the prevention intervention, and 10 patients in the tape measure group (14.7%) and 2 patients in the BIS group (4.9%) eventually progressed to full lymphedema. In the BIS group, there was a 10% absolute reduction and 67% relative reduction in lymphedema progression, compared with the tape measure group.
“We believe that the 10% absolute reduction is clinically significant for this patient population,” said Dr. Ridner. “We also believe that our interim results may support the concept of posttreatment surveillance using BIS for early detection of subclinical lymphedema coupled with early intervention as our preliminary data suggest this does have clinical advantages to the patient.”
The study was funded by ImpediMed. Dr. Ridner reports being the principal investigator for ImpediMed and Tactile Medical through work agreements contracted between the companies and her institution.
Bioimpedance spectroscopy may better identify lymphedema progression in women at risk for breast cancer–related lymphedema over the traditionally used method of monitoring arm circumference with a tape measure, according to results from an interim analysis of the PREVENT trial presented in a recent webcast from the annual meeting of the American Society of Breast Surgeons.
“Despite advances in breast-conserving surgery, improved radiation protocol, the advent of sentinel [node] biopsies and recent improvement in chemotherapy regimens, breast cancer–related lymphedema ... remains a major source of morbidity and concern in this patient population,” Sheila Ridner, PhD, RN, FAAN, professor of nursing at the Vanderbilt University School of Nursing in Nashville, Tenn., said in her presentation. “Because it is thought that early identification of swelling in the limbs coupled with a compression intervention may reduce the risk of patients developing full-blown clinical lymphedema, clinicians are proposing to use a prospective surveillance model to follow breast cancer survivors post surgery in order to assess limbs in a routine fashion and perhaps instigate preventative mechanisms early.”
The larger randomized controlled Prevention of Lymphedema Following Locoregional Treatment for Breast Cancer (PREVENT) trial enrolled 1,201 patients, and 200 patients overall have completed the full protocol. The researchers plan to follow patients for 3 years after surgery. In this interim analysis, Dr. Ridner and colleagues analyzed data from 508 patients at eight sites in the United States and four sites in Sydney who had stage I through stage III or ductal carcinoma in situ (DCIS) breast cancer and underwent mastectomy, taxane-based chemotherapy, or an axillary treatment such as axillary radiation, axillary lymph node dissection, or sentinel lymph nose biopsy with more than 6 nodes.
The patients were randomized to be measured using either traditional tape measurement or bioimpedance spectroscopy (BIS). Patients were moved to a prevention intervention if there was a change in baseline volume of 5% or greater but less than 10% in the tape measure group and change from baseline L-Dex measurement of 6.5 or greater in the BIS group, which consisted of wearing an arm compression sleeve and chest gauntlet for 12 hours a day over 4 weeks. After surgery, the patients were followed up at 3 months, 6 months, 12 months, 18 months, and 24 months, with optional follow-up visits at 15 months and 21 months. Progression to full lymphedema was defined as a 10% or greater change in pretreatment baseline measurements in the tape measure group.
Of the 508 patients analyzed, 10 patients had already progressed to full lymphedema, leaving 498 patients available for the interim analysis. There were 68 patients in the tape measure group (28.5%) and 41 patients in the BIS group (15.8%) who received the prevention intervention, and 10 patients in the tape measure group (14.7%) and 2 patients in the BIS group (4.9%) eventually progressed to full lymphedema. In the BIS group, there was a 10% absolute reduction and 67% relative reduction in lymphedema progression, compared with the tape measure group.
“We believe that the 10% absolute reduction is clinically significant for this patient population,” said Dr. Ridner. “We also believe that our interim results may support the concept of posttreatment surveillance using BIS for early detection of subclinical lymphedema coupled with early intervention as our preliminary data suggest this does have clinical advantages to the patient.”
The study was funded by ImpediMed. Dr. Ridner reports being the principal investigator for ImpediMed and Tactile Medical through work agreements contracted between the companies and her institution.
Bioimpedance spectroscopy may better identify lymphedema progression in women at risk for breast cancer–related lymphedema over the traditionally used method of monitoring arm circumference with a tape measure, according to results from an interim analysis of the PREVENT trial presented in a recent webcast from the annual meeting of the American Society of Breast Surgeons.
“Despite advances in breast-conserving surgery, improved radiation protocol, the advent of sentinel [node] biopsies and recent improvement in chemotherapy regimens, breast cancer–related lymphedema ... remains a major source of morbidity and concern in this patient population,” Sheila Ridner, PhD, RN, FAAN, professor of nursing at the Vanderbilt University School of Nursing in Nashville, Tenn., said in her presentation. “Because it is thought that early identification of swelling in the limbs coupled with a compression intervention may reduce the risk of patients developing full-blown clinical lymphedema, clinicians are proposing to use a prospective surveillance model to follow breast cancer survivors post surgery in order to assess limbs in a routine fashion and perhaps instigate preventative mechanisms early.”
The larger randomized controlled Prevention of Lymphedema Following Locoregional Treatment for Breast Cancer (PREVENT) trial enrolled 1,201 patients, and 200 patients overall have completed the full protocol. The researchers plan to follow patients for 3 years after surgery. In this interim analysis, Dr. Ridner and colleagues analyzed data from 508 patients at eight sites in the United States and four sites in Sydney who had stage I through stage III or ductal carcinoma in situ (DCIS) breast cancer and underwent mastectomy, taxane-based chemotherapy, or an axillary treatment such as axillary radiation, axillary lymph node dissection, or sentinel lymph nose biopsy with more than 6 nodes.
The patients were randomized to be measured using either traditional tape measurement or bioimpedance spectroscopy (BIS). Patients were moved to a prevention intervention if there was a change in baseline volume of 5% or greater but less than 10% in the tape measure group and change from baseline L-Dex measurement of 6.5 or greater in the BIS group, which consisted of wearing an arm compression sleeve and chest gauntlet for 12 hours a day over 4 weeks. After surgery, the patients were followed up at 3 months, 6 months, 12 months, 18 months, and 24 months, with optional follow-up visits at 15 months and 21 months. Progression to full lymphedema was defined as a 10% or greater change in pretreatment baseline measurements in the tape measure group.
Of the 508 patients analyzed, 10 patients had already progressed to full lymphedema, leaving 498 patients available for the interim analysis. There were 68 patients in the tape measure group (28.5%) and 41 patients in the BIS group (15.8%) who received the prevention intervention, and 10 patients in the tape measure group (14.7%) and 2 patients in the BIS group (4.9%) eventually progressed to full lymphedema. In the BIS group, there was a 10% absolute reduction and 67% relative reduction in lymphedema progression, compared with the tape measure group.
“We believe that the 10% absolute reduction is clinically significant for this patient population,” said Dr. Ridner. “We also believe that our interim results may support the concept of posttreatment surveillance using BIS for early detection of subclinical lymphedema coupled with early intervention as our preliminary data suggest this does have clinical advantages to the patient.”
The study was funded by ImpediMed. Dr. Ridner reports being the principal investigator for ImpediMed and Tactile Medical through work agreements contracted between the companies and her institution.
REPORTING FROM ASBS 2019
Patch testing in atopic dermatitis: when and how
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
The art of selecting an MS therapy
DALLAS – , said Mark Freedman, MD, MSc, in a presentation at ACTRIMS Forum 2019, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “Right now, we are probably dealing with more of an imprecise medicine,” said Dr. Freedman.
Information such as a patient’s ability to recover from relapses may indicate MS severity or the likelihood of disease progression, but selecting a therapy remains “an art of medicine,” said Dr. Freedman, professor of neurology at the University of Ottawa, director of the multiple sclerosis research unit at Ottawa Hospital, and senior scientist at the Ottawa Hospital Research Institute.
When prescribing a DMT, neurologists tend to consider three key elements: the disease, the treatment, and patient expectations. “Focus on these three aspects,” Dr. Freedman said.
It is no longer sufficient for neurologists to diagnose MS, hand the patient a drug, and “expect that things are going to go the way you want them to go,” he said.
Immunomodulating, anti–cell trafficking, or cell-depleting therapy?
Genetics, sex, types of relapses, recovery from relapses, response to therapy, MRI burden, and other biomarkers such as oligoclonal bands and neurofilaments may indicate which patients have severe disease and should receive aggressive treatment.
Determining the phase of the disease is a crucial first step “that is going to drive your choice of therapy,” he said.
Dr. Freedman likened the development of progressive MS to approaching the edge of a cliff. If patients appear to be nearing the progressive phase, “then your choice of therapy has to be an aggressive one – one that will hopefully hold them back from falling,” he said. In the earlier phases of MS, on the other hand, “you are looking at a long-term treatment that should probably be safe and still able to contain the disease,” such as an immunomodulator. If a patient is “about to fall off, you may want to go for temporary use of an antitrafficker to control things, and then eventually deplete the cells that are going to be causing the patient to fall off the cliff.”
Prognostic factors
Disease activity over time, and whether the disease is progressing faster or slower than would be expected, may be important prognostic factors. A patient’s sex also may be a factor because women tend to have more attacks and to have their attacks at a younger age, Dr. Freedman said.
The types of relapses and a patient’s ability to recover from them may provide important information. “Some attacks are quite mild. Others tend to build up disease,” Dr. Freedman said. “Some people are better healers than others. We have all seen people who have been quadriplegic in an ICU on a ventilator walk out of the hospital without even a numb toe. And other people who have a little bit of weakness in one leg seem to never be able to recover from that. Exactly what drives repair is still not clear.” Most patients do recover, however, “and the inability to recover early on is a bad omen,” Dr. Freedman said.
When researchers examined the relationship between functional components of the Expanded Disability Status Scale (EDSS) and disability progression, “not surprisingly ... pyramidal and spinal cord and cerebellar [functioning] are more associated with earlier progression” (Neuroepidemiology. 2015;44[1]:16-23).
A study by Lublin et al. found that patients with MS whose attacks left them with residual deficits had more EDSS accumulation over time (Neurology. 2003 Dec 9;61[11]:1528-32.).
Response to immunomodulators
“The inability to control the disease with an immunomodulator is a bad sign,” Dr. Freedman said. He pointed to data from a trial of teriflunomide that included patients who had had suboptimal responses to first-line therapy as well as patients who were treatment naive (Mult Scler. 2018 Apr;24[4]:535-9.). Some of the patients who had received prior MS therapy were randomized to placebo, which “is not something that would happen today,” he said.
“If you just focus on the [patients who received placebo] and look at the rate of attack in patients who had no prior DMT, at least one prior DMT, or two or more prior DMTs, the attack rates are much higher in those individuals who tried and failed first-line therapies,” Dr. Freedman said. These patients also had more EDSS progression. “The majority of people do respond [to first-line treatment], but those who do not you need to worry about a little bit more than those who do respond.”
MRI lesions and brain reserve
MRI activity over time tends to predict disease progression, and lesion location is important. One cohort study found that the likelihood of developing secondary progressive MS was lower among patients who did not develop new spinal cord or brainstem lesions in the first three years of the disease, compared with those who did.
In addition, patients who presented with more lesions were more likely to reach an EDSS score 3 or 6 over 10 years (Brain. 2008 Mar;131[Pt 3]:808-17.).
Brain reserve also may be important. Among 52 treatment-naive Serbian adults with MS, Sumowski et al. found that maximal lifetime brain growth as estimated with intracranial volume was associated with risk of disability progression over 5 years (Neurology. 2016 May 24;86[21]:2006-9.). “Those who had a greater reserve had a much lower risk of disease progression,” Dr. Freedman said. The results suggest that patients with more brain reserve may be better able to sustain damage as the disease progresses and they age, he said.
Comorbidities
In the past, neurologists may have left it up to general practitioners “to sort out the rest of the patient’s health,” Dr. Freedman said. “But we now recognize that having certain comorbidities already puts a higher burden onto the disease. And those patients who have more comorbidities ... are going to do worse. But not only are they going to do worse ... it turns out that patients who have more comorbidities are going to have less of a response to your various therapies.” Vascular comorbidities, in particular, may affect treatment response (Neurology. 2017 Nov 28;89[22]:2222-9.).
If hypertension or diabetes clinics can help control those conditions in patients with MS, “it will help us a lot in getting what we are expecting from the [MS] medications,” Dr. Freedman said.
Adherence, expectations, and symptomatic treatment
Ultimately, selecting an MS therapy is a decision that doctors share with their patients. “You’re going to have a discussion with them,” he said. “You can see what fits their lifestyle.” For example, a world traveler might not be a good candidate for a drug that requires regular monitoring. A patient’s risk averseness also may influence treatment choice.
If you involve patients in the selection process, it may improve medication adherence. In addition, patients need to understand what you aim to accomplish with a DMT, said Dr. Freedman. “That may sound like a trivial thing. But how many times has the patient come in and said, ‘The drug is not working. ... My eye is not better’” when that was not the goal of treatment to begin with. Let patients know that symptomatic treatments may address problems apart from MS DMT. This personalized but imprecise approach to treatment is “probably the best we can do for now,” Dr. Freedman said.
Dr. Freedman has received a research grant from Genzyme and is on the company’s speakers bureau. He has received honoraria and consulting fees from various pharmaceutical companies and serves on companies’ advisory boards.
SOURCE: Freedman MS. ACTRIMS Forum 2019, Session 2.
DALLAS – , said Mark Freedman, MD, MSc, in a presentation at ACTRIMS Forum 2019, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “Right now, we are probably dealing with more of an imprecise medicine,” said Dr. Freedman.
Information such as a patient’s ability to recover from relapses may indicate MS severity or the likelihood of disease progression, but selecting a therapy remains “an art of medicine,” said Dr. Freedman, professor of neurology at the University of Ottawa, director of the multiple sclerosis research unit at Ottawa Hospital, and senior scientist at the Ottawa Hospital Research Institute.
When prescribing a DMT, neurologists tend to consider three key elements: the disease, the treatment, and patient expectations. “Focus on these three aspects,” Dr. Freedman said.
It is no longer sufficient for neurologists to diagnose MS, hand the patient a drug, and “expect that things are going to go the way you want them to go,” he said.
Immunomodulating, anti–cell trafficking, or cell-depleting therapy?
Genetics, sex, types of relapses, recovery from relapses, response to therapy, MRI burden, and other biomarkers such as oligoclonal bands and neurofilaments may indicate which patients have severe disease and should receive aggressive treatment.
Determining the phase of the disease is a crucial first step “that is going to drive your choice of therapy,” he said.
Dr. Freedman likened the development of progressive MS to approaching the edge of a cliff. If patients appear to be nearing the progressive phase, “then your choice of therapy has to be an aggressive one – one that will hopefully hold them back from falling,” he said. In the earlier phases of MS, on the other hand, “you are looking at a long-term treatment that should probably be safe and still able to contain the disease,” such as an immunomodulator. If a patient is “about to fall off, you may want to go for temporary use of an antitrafficker to control things, and then eventually deplete the cells that are going to be causing the patient to fall off the cliff.”
Prognostic factors
Disease activity over time, and whether the disease is progressing faster or slower than would be expected, may be important prognostic factors. A patient’s sex also may be a factor because women tend to have more attacks and to have their attacks at a younger age, Dr. Freedman said.
The types of relapses and a patient’s ability to recover from them may provide important information. “Some attacks are quite mild. Others tend to build up disease,” Dr. Freedman said. “Some people are better healers than others. We have all seen people who have been quadriplegic in an ICU on a ventilator walk out of the hospital without even a numb toe. And other people who have a little bit of weakness in one leg seem to never be able to recover from that. Exactly what drives repair is still not clear.” Most patients do recover, however, “and the inability to recover early on is a bad omen,” Dr. Freedman said.
When researchers examined the relationship between functional components of the Expanded Disability Status Scale (EDSS) and disability progression, “not surprisingly ... pyramidal and spinal cord and cerebellar [functioning] are more associated with earlier progression” (Neuroepidemiology. 2015;44[1]:16-23).
A study by Lublin et al. found that patients with MS whose attacks left them with residual deficits had more EDSS accumulation over time (Neurology. 2003 Dec 9;61[11]:1528-32.).
Response to immunomodulators
“The inability to control the disease with an immunomodulator is a bad sign,” Dr. Freedman said. He pointed to data from a trial of teriflunomide that included patients who had had suboptimal responses to first-line therapy as well as patients who were treatment naive (Mult Scler. 2018 Apr;24[4]:535-9.). Some of the patients who had received prior MS therapy were randomized to placebo, which “is not something that would happen today,” he said.
“If you just focus on the [patients who received placebo] and look at the rate of attack in patients who had no prior DMT, at least one prior DMT, or two or more prior DMTs, the attack rates are much higher in those individuals who tried and failed first-line therapies,” Dr. Freedman said. These patients also had more EDSS progression. “The majority of people do respond [to first-line treatment], but those who do not you need to worry about a little bit more than those who do respond.”
MRI lesions and brain reserve
MRI activity over time tends to predict disease progression, and lesion location is important. One cohort study found that the likelihood of developing secondary progressive MS was lower among patients who did not develop new spinal cord or brainstem lesions in the first three years of the disease, compared with those who did.
In addition, patients who presented with more lesions were more likely to reach an EDSS score 3 or 6 over 10 years (Brain. 2008 Mar;131[Pt 3]:808-17.).
Brain reserve also may be important. Among 52 treatment-naive Serbian adults with MS, Sumowski et al. found that maximal lifetime brain growth as estimated with intracranial volume was associated with risk of disability progression over 5 years (Neurology. 2016 May 24;86[21]:2006-9.). “Those who had a greater reserve had a much lower risk of disease progression,” Dr. Freedman said. The results suggest that patients with more brain reserve may be better able to sustain damage as the disease progresses and they age, he said.
Comorbidities
In the past, neurologists may have left it up to general practitioners “to sort out the rest of the patient’s health,” Dr. Freedman said. “But we now recognize that having certain comorbidities already puts a higher burden onto the disease. And those patients who have more comorbidities ... are going to do worse. But not only are they going to do worse ... it turns out that patients who have more comorbidities are going to have less of a response to your various therapies.” Vascular comorbidities, in particular, may affect treatment response (Neurology. 2017 Nov 28;89[22]:2222-9.).
If hypertension or diabetes clinics can help control those conditions in patients with MS, “it will help us a lot in getting what we are expecting from the [MS] medications,” Dr. Freedman said.
Adherence, expectations, and symptomatic treatment
Ultimately, selecting an MS therapy is a decision that doctors share with their patients. “You’re going to have a discussion with them,” he said. “You can see what fits their lifestyle.” For example, a world traveler might not be a good candidate for a drug that requires regular monitoring. A patient’s risk averseness also may influence treatment choice.
If you involve patients in the selection process, it may improve medication adherence. In addition, patients need to understand what you aim to accomplish with a DMT, said Dr. Freedman. “That may sound like a trivial thing. But how many times has the patient come in and said, ‘The drug is not working. ... My eye is not better’” when that was not the goal of treatment to begin with. Let patients know that symptomatic treatments may address problems apart from MS DMT. This personalized but imprecise approach to treatment is “probably the best we can do for now,” Dr. Freedman said.
Dr. Freedman has received a research grant from Genzyme and is on the company’s speakers bureau. He has received honoraria and consulting fees from various pharmaceutical companies and serves on companies’ advisory boards.
SOURCE: Freedman MS. ACTRIMS Forum 2019, Session 2.
DALLAS – , said Mark Freedman, MD, MSc, in a presentation at ACTRIMS Forum 2019, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “Right now, we are probably dealing with more of an imprecise medicine,” said Dr. Freedman.
Information such as a patient’s ability to recover from relapses may indicate MS severity or the likelihood of disease progression, but selecting a therapy remains “an art of medicine,” said Dr. Freedman, professor of neurology at the University of Ottawa, director of the multiple sclerosis research unit at Ottawa Hospital, and senior scientist at the Ottawa Hospital Research Institute.
When prescribing a DMT, neurologists tend to consider three key elements: the disease, the treatment, and patient expectations. “Focus on these three aspects,” Dr. Freedman said.
It is no longer sufficient for neurologists to diagnose MS, hand the patient a drug, and “expect that things are going to go the way you want them to go,” he said.
Immunomodulating, anti–cell trafficking, or cell-depleting therapy?
Genetics, sex, types of relapses, recovery from relapses, response to therapy, MRI burden, and other biomarkers such as oligoclonal bands and neurofilaments may indicate which patients have severe disease and should receive aggressive treatment.
Determining the phase of the disease is a crucial first step “that is going to drive your choice of therapy,” he said.
Dr. Freedman likened the development of progressive MS to approaching the edge of a cliff. If patients appear to be nearing the progressive phase, “then your choice of therapy has to be an aggressive one – one that will hopefully hold them back from falling,” he said. In the earlier phases of MS, on the other hand, “you are looking at a long-term treatment that should probably be safe and still able to contain the disease,” such as an immunomodulator. If a patient is “about to fall off, you may want to go for temporary use of an antitrafficker to control things, and then eventually deplete the cells that are going to be causing the patient to fall off the cliff.”
Prognostic factors
Disease activity over time, and whether the disease is progressing faster or slower than would be expected, may be important prognostic factors. A patient’s sex also may be a factor because women tend to have more attacks and to have their attacks at a younger age, Dr. Freedman said.
The types of relapses and a patient’s ability to recover from them may provide important information. “Some attacks are quite mild. Others tend to build up disease,” Dr. Freedman said. “Some people are better healers than others. We have all seen people who have been quadriplegic in an ICU on a ventilator walk out of the hospital without even a numb toe. And other people who have a little bit of weakness in one leg seem to never be able to recover from that. Exactly what drives repair is still not clear.” Most patients do recover, however, “and the inability to recover early on is a bad omen,” Dr. Freedman said.
When researchers examined the relationship between functional components of the Expanded Disability Status Scale (EDSS) and disability progression, “not surprisingly ... pyramidal and spinal cord and cerebellar [functioning] are more associated with earlier progression” (Neuroepidemiology. 2015;44[1]:16-23).
A study by Lublin et al. found that patients with MS whose attacks left them with residual deficits had more EDSS accumulation over time (Neurology. 2003 Dec 9;61[11]:1528-32.).
Response to immunomodulators
“The inability to control the disease with an immunomodulator is a bad sign,” Dr. Freedman said. He pointed to data from a trial of teriflunomide that included patients who had had suboptimal responses to first-line therapy as well as patients who were treatment naive (Mult Scler. 2018 Apr;24[4]:535-9.). Some of the patients who had received prior MS therapy were randomized to placebo, which “is not something that would happen today,” he said.
“If you just focus on the [patients who received placebo] and look at the rate of attack in patients who had no prior DMT, at least one prior DMT, or two or more prior DMTs, the attack rates are much higher in those individuals who tried and failed first-line therapies,” Dr. Freedman said. These patients also had more EDSS progression. “The majority of people do respond [to first-line treatment], but those who do not you need to worry about a little bit more than those who do respond.”
MRI lesions and brain reserve
MRI activity over time tends to predict disease progression, and lesion location is important. One cohort study found that the likelihood of developing secondary progressive MS was lower among patients who did not develop new spinal cord or brainstem lesions in the first three years of the disease, compared with those who did.
In addition, patients who presented with more lesions were more likely to reach an EDSS score 3 or 6 over 10 years (Brain. 2008 Mar;131[Pt 3]:808-17.).
Brain reserve also may be important. Among 52 treatment-naive Serbian adults with MS, Sumowski et al. found that maximal lifetime brain growth as estimated with intracranial volume was associated with risk of disability progression over 5 years (Neurology. 2016 May 24;86[21]:2006-9.). “Those who had a greater reserve had a much lower risk of disease progression,” Dr. Freedman said. The results suggest that patients with more brain reserve may be better able to sustain damage as the disease progresses and they age, he said.
Comorbidities
In the past, neurologists may have left it up to general practitioners “to sort out the rest of the patient’s health,” Dr. Freedman said. “But we now recognize that having certain comorbidities already puts a higher burden onto the disease. And those patients who have more comorbidities ... are going to do worse. But not only are they going to do worse ... it turns out that patients who have more comorbidities are going to have less of a response to your various therapies.” Vascular comorbidities, in particular, may affect treatment response (Neurology. 2017 Nov 28;89[22]:2222-9.).
If hypertension or diabetes clinics can help control those conditions in patients with MS, “it will help us a lot in getting what we are expecting from the [MS] medications,” Dr. Freedman said.
Adherence, expectations, and symptomatic treatment
Ultimately, selecting an MS therapy is a decision that doctors share with their patients. “You’re going to have a discussion with them,” he said. “You can see what fits their lifestyle.” For example, a world traveler might not be a good candidate for a drug that requires regular monitoring. A patient’s risk averseness also may influence treatment choice.
If you involve patients in the selection process, it may improve medication adherence. In addition, patients need to understand what you aim to accomplish with a DMT, said Dr. Freedman. “That may sound like a trivial thing. But how many times has the patient come in and said, ‘The drug is not working. ... My eye is not better’” when that was not the goal of treatment to begin with. Let patients know that symptomatic treatments may address problems apart from MS DMT. This personalized but imprecise approach to treatment is “probably the best we can do for now,” Dr. Freedman said.
Dr. Freedman has received a research grant from Genzyme and is on the company’s speakers bureau. He has received honoraria and consulting fees from various pharmaceutical companies and serves on companies’ advisory boards.
SOURCE: Freedman MS. ACTRIMS Forum 2019, Session 2.
EXPERT ANALYSIS FROM ACTRIMS
Most pregnancy-related deaths are preventable
according to the Centers for Disease Control and Prevention.
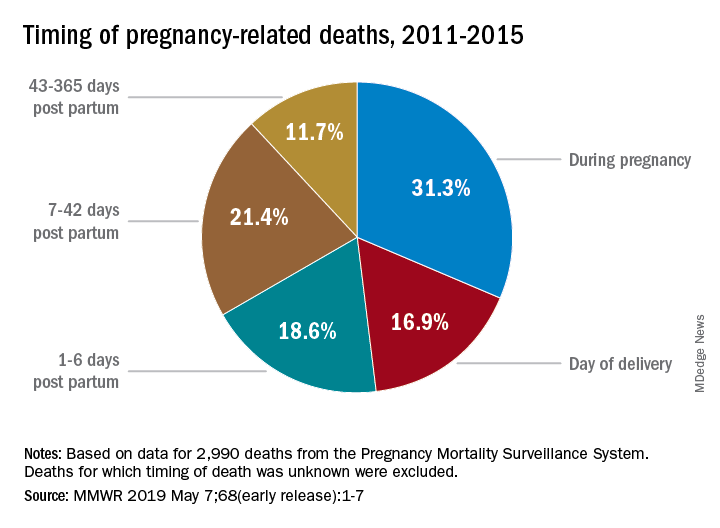
Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
according to the Centers for Disease Control and Prevention.

Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
according to the Centers for Disease Control and Prevention.

Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
PCV13 vaccine reduces frequency of otitis media visits
The mean number of office visits for otitis media in children younger than 5 years dropped significantly after the introduction of the 13-valent pneumococcal conjugate vaccine, according to findings published in the International Journal of Pediatric Otorhinolaryngology.
Previous studies have shown that more than half of children with otitis media (OM) have serotypes included in the PCV7 vaccine (4, 6B, 9V, 14, 18C, 19F, and 23F), wrote Xiaofeng Zhou, MD, of Pfizer, New York, and colleagues.
To assess the impact of PCV13, with the additional serotypes 1, 3, 5, 6A, 7F, and 19A, the researchers analyzed data from the U.S. National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey for three time periods: pre-PCV7 (1997-1999), after the introduction of PCV7 (2001-2009), and after the introduction of PCV13 (2011-2013).
Between the pre-PCV7 and PCV13 time periods, the researchers found significant reductions in the mean rates of OM visits of 48% and 41% among children younger than 2 years and younger than 5 years, respectively; reductions were 24% and 22%, respectively, when comparing PCV13 and PCV7. Ambulatory care visits for skin rash and trauma were not significantly different among the study periods.
Comparing the PCV7 and PCV13 time periods, the mean number of OM visits per 100 children declined from 84 to 64 per 100 children younger than 2 years, 41 to 34 per 100 children between ages 2 and 5 years, and from 59 to 46 per 100 children younger than 5 years.
The study findings were limited by several factors including the use of an ecologic study design, which was chosen to help reduce selection bias, but that did not show evidence of the field effectiveness of the PCV13 vaccine. Another limitation was the potential misclassification of patients with OM given clinician variability in diagnostic criteria, the researchers noted.
“Our results in this study, while not providing direct evidence of causality, nonetheless suggest a significant and positive impact of the PCV13 vaccination program on otitis media for children less than 5 years of age in the U.S., with further reductions in OM visits observed in PCV13 period following a decade of PCV7 use,” Dr. Zhou and associates said.
The investigators are employed by Pfizer, which funded the study.
SOURCE: Zhou X et al. Int J Pediatr Otorhinolaryngol. 2019 Apr. 119:96-102.
The mean number of office visits for otitis media in children younger than 5 years dropped significantly after the introduction of the 13-valent pneumococcal conjugate vaccine, according to findings published in the International Journal of Pediatric Otorhinolaryngology.
Previous studies have shown that more than half of children with otitis media (OM) have serotypes included in the PCV7 vaccine (4, 6B, 9V, 14, 18C, 19F, and 23F), wrote Xiaofeng Zhou, MD, of Pfizer, New York, and colleagues.
To assess the impact of PCV13, with the additional serotypes 1, 3, 5, 6A, 7F, and 19A, the researchers analyzed data from the U.S. National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey for three time periods: pre-PCV7 (1997-1999), after the introduction of PCV7 (2001-2009), and after the introduction of PCV13 (2011-2013).
Between the pre-PCV7 and PCV13 time periods, the researchers found significant reductions in the mean rates of OM visits of 48% and 41% among children younger than 2 years and younger than 5 years, respectively; reductions were 24% and 22%, respectively, when comparing PCV13 and PCV7. Ambulatory care visits for skin rash and trauma were not significantly different among the study periods.
Comparing the PCV7 and PCV13 time periods, the mean number of OM visits per 100 children declined from 84 to 64 per 100 children younger than 2 years, 41 to 34 per 100 children between ages 2 and 5 years, and from 59 to 46 per 100 children younger than 5 years.
The study findings were limited by several factors including the use of an ecologic study design, which was chosen to help reduce selection bias, but that did not show evidence of the field effectiveness of the PCV13 vaccine. Another limitation was the potential misclassification of patients with OM given clinician variability in diagnostic criteria, the researchers noted.
“Our results in this study, while not providing direct evidence of causality, nonetheless suggest a significant and positive impact of the PCV13 vaccination program on otitis media for children less than 5 years of age in the U.S., with further reductions in OM visits observed in PCV13 period following a decade of PCV7 use,” Dr. Zhou and associates said.
The investigators are employed by Pfizer, which funded the study.
SOURCE: Zhou X et al. Int J Pediatr Otorhinolaryngol. 2019 Apr. 119:96-102.
The mean number of office visits for otitis media in children younger than 5 years dropped significantly after the introduction of the 13-valent pneumococcal conjugate vaccine, according to findings published in the International Journal of Pediatric Otorhinolaryngology.
Previous studies have shown that more than half of children with otitis media (OM) have serotypes included in the PCV7 vaccine (4, 6B, 9V, 14, 18C, 19F, and 23F), wrote Xiaofeng Zhou, MD, of Pfizer, New York, and colleagues.
To assess the impact of PCV13, with the additional serotypes 1, 3, 5, 6A, 7F, and 19A, the researchers analyzed data from the U.S. National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey for three time periods: pre-PCV7 (1997-1999), after the introduction of PCV7 (2001-2009), and after the introduction of PCV13 (2011-2013).
Between the pre-PCV7 and PCV13 time periods, the researchers found significant reductions in the mean rates of OM visits of 48% and 41% among children younger than 2 years and younger than 5 years, respectively; reductions were 24% and 22%, respectively, when comparing PCV13 and PCV7. Ambulatory care visits for skin rash and trauma were not significantly different among the study periods.
Comparing the PCV7 and PCV13 time periods, the mean number of OM visits per 100 children declined from 84 to 64 per 100 children younger than 2 years, 41 to 34 per 100 children between ages 2 and 5 years, and from 59 to 46 per 100 children younger than 5 years.
The study findings were limited by several factors including the use of an ecologic study design, which was chosen to help reduce selection bias, but that did not show evidence of the field effectiveness of the PCV13 vaccine. Another limitation was the potential misclassification of patients with OM given clinician variability in diagnostic criteria, the researchers noted.
“Our results in this study, while not providing direct evidence of causality, nonetheless suggest a significant and positive impact of the PCV13 vaccination program on otitis media for children less than 5 years of age in the U.S., with further reductions in OM visits observed in PCV13 period following a decade of PCV7 use,” Dr. Zhou and associates said.
The investigators are employed by Pfizer, which funded the study.
SOURCE: Zhou X et al. Int J Pediatr Otorhinolaryngol. 2019 Apr. 119:96-102.
FROM THE INTERNATIONAL JOURNAL OF PEDIATRIC OTORHINOLARYNGOLOGY