User login
Experts propose new definition and recommendations for Alzheimer’s-like disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
FROM BRAIN
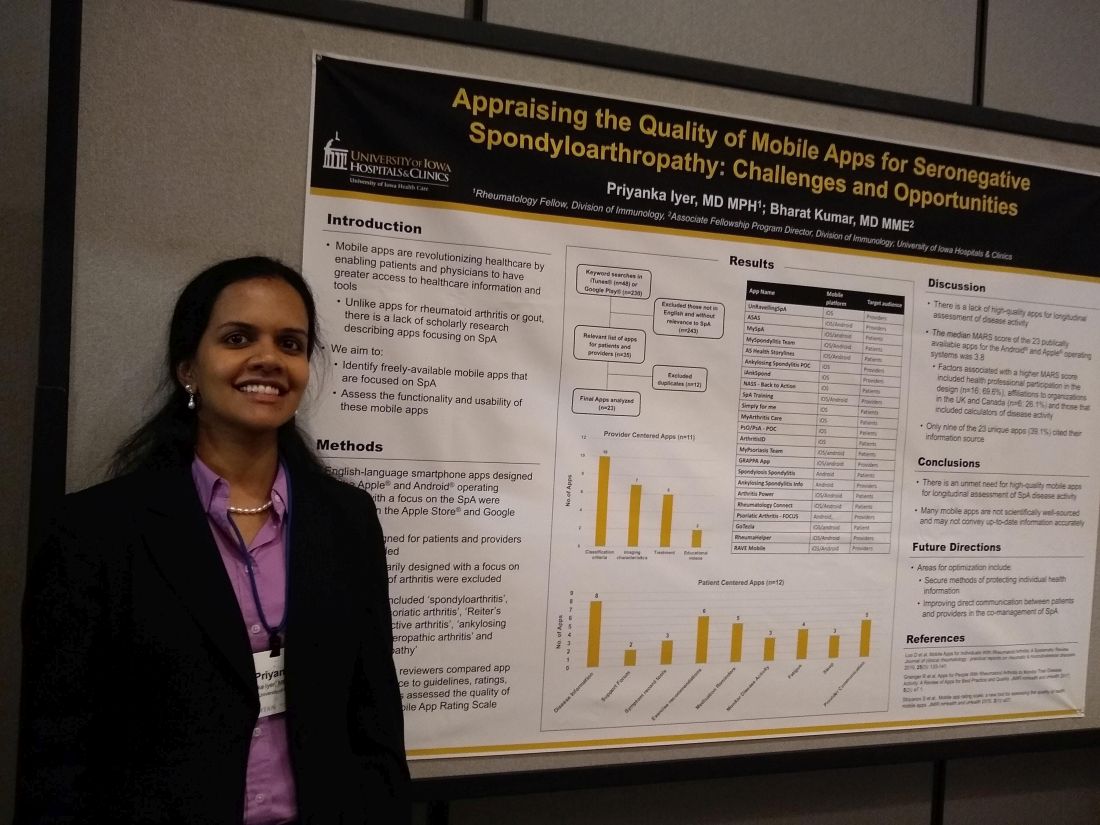
Mobile SpA apps abound, but there’s room for quality improvement
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
MADISON, WISC. – according to a recent review.
In assessing the 23 publicly available apps aimed at patients or providers, the median score on a common assessment of smartphone apps was just 3.8 on a 5-point scale, said Priyanka Iyer, MBBS, MPH.
Speaking in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN), Dr. Iyer pointed out several ways that apps could be optimized. Foremost, she said, is providing secure ways to store and transmit protected health information. Also, apps still haven’t realized their potential to support true comanagement of spondyloarthritis (SpA) via secure, direct patient-provider communication.
“This is an area that we researched previously in rheumatoid arthritis and gout,” explained Dr. Iyer, a rheumatology fellow at the University of Iowa, Iowa City. “We found 23 apps that are available between the Android and iOS platforms; most of them are actually centered towards patients.” In their review, Dr. Iyer and coauthor, Bharat Kumar, MD, had excluded apps that primarily focused on other types of arthritis, using search terms that focused on SpA.
In looking at the 11 provider-centered apps and the 12 that were patient focused, Dr. Iyer and coauthor independently reviewed features of each app. Factors they considered included adherence to guidelines, amount of correct medical information provided, and specific features including capacity to store imaging and test results, and ability to host patient-provider communication.
Of the provider-centered apps, 10 contained appropriate classification criteria, and 7 also contained medical imaging characteristics of the target conditions. Six apps guided providers through treatment options, and two had educational videos.
Of the 12 patient-centered apps, 8 provided disease information, and 6 gave exercise recommendations. Five of the apps had prompts that reminded patients to take medication, and three had tools to help patients record and track symptoms. Similarly, three apps had features to help patients monitor disease activity. Two of the apps were primarily access points for a patient support forum.
Additionally, each app was evaluated by each reviewer using the Mobile App Rating Scale (MARS), said Dr. Iyer. “The overall rating was pretty low, at 3.8 [of a possible 5.0]. Factors that increased the MARS scores included affiliations to organizations in the United Kingdom and Canada; for patients who use these apps, their information is automatically transmitted to their providers, and they are able to also access imaging and most of their other health care information on the app.”
Another factor associated with a higher MARS score was design that included health professional participation, which was the case for 16 apps (69.6%). Apps that included calculators of disease activity were also more likely to achieve a higher MARS score, Dr. Iyer and coauthor wrote.
Notably, just 9 of 23 apps (39.1%) included citations referencing their source for medical information.
“I think future areas for improvement and for development of apps include securing individual health information to allow direct communication between patients and providers,” Dr. Iyer said. “I hope that some patients use these apps to learn, and to help their self-management improve.”
“There is an unmet need for high-quality mobile apps for longitudinal assessment of SpA disease activity,” Dr. Iyer and colleagues wrote in the poster accompanying the presentation. “Many mobile apps are not scientifically well sourced and may not convey up-to-date information accurately.”
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Iyer P et al. SPARTAN 2019.
REPORTING FROM SPARTAN 2019
NHIA Conference Wrap-Up
This supplement to Neurology Reviews compiles news briefs from the 2019 National Home Infusion Association annual meeting, which was held in March in Orlando, Florida.
Click here to read the supplement.
This supplement to Neurology Reviews compiles news briefs from the 2019 National Home Infusion Association annual meeting, which was held in March in Orlando, Florida.
Click here to read the supplement.
This supplement to Neurology Reviews compiles news briefs from the 2019 National Home Infusion Association annual meeting, which was held in March in Orlando, Florida.
Click here to read the supplement.
Combo proves most effective in HMA-naive, higher-risk MDS
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Energy-based therapies in female genital cosmetic surgery: Hype, hope, and a way forward
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
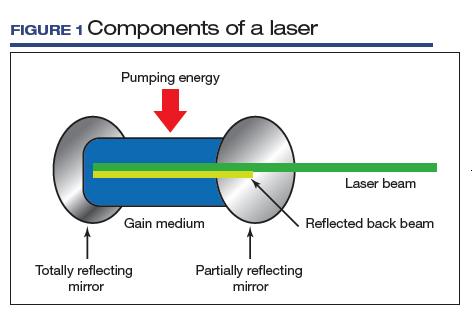
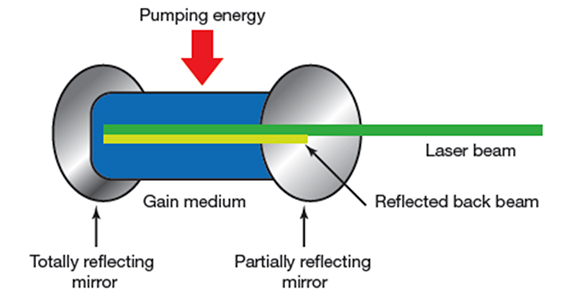
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
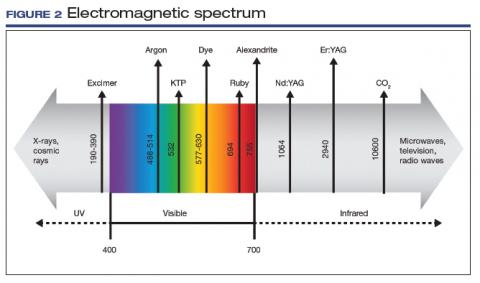
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
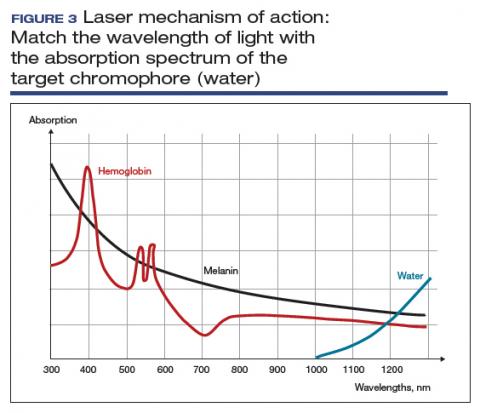
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
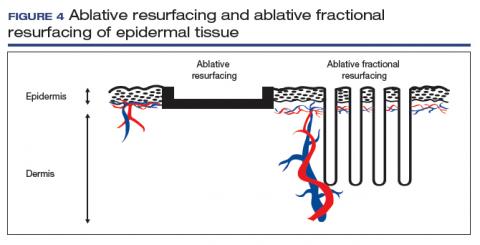
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
Assessing and treating sexual function after vaginal surgery
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
Top 10 tips community hospitalists need to know for implementing a QI project
Consider low-cost, high-impact projects
Quality improvement (QI) is essential to the advancement of medicine. QI differs from research as it focuses on already proven knowledge and aims to make quick, sustainable change in local health care systems. Community hospitals may not have organized quality improvement initiatives and often rely on individual hospitalists to be their champions.
Although there are resources for quality improvement projects, initiating a project can seem daunting to a hospitalist. Our aim is to equip the community hospitalist with basic skills to initiate their own successful project. We present our “Top 10” tips to review.
1. Start small: Many quality improvement ideas include grandiose changes that require a large buy-in or worse, more money. When starting a QI project, you need to consider low-cost, high-impact projects. Even the smallest projects can make considerable change. Focus on ideas that require only one or two improvement cycles to implement. Understand your hospital culture, flow, and processes, and then pick a project that is reasonable.
Projects can be as simple as decreasing the number of daily labs ordered by your hospitalist group. Projects that are small could still improve patient satisfaction and decrease costs. Listen to your colleagues, if they are discussing an issue, turn this into an idea! As you learn the culture of your hospital you will be able to tackle larger projects. Plus, it gets your name out there!
2. Establish buy-in: Surround yourself with champions of your cause. Properly identifying and engaging key players is paramount to a successful QI project. First, start with your hospital administration, and garner their support by aligning your project with the goals and objectives that the administration leaders have identified to be important for your institution. Next, select a motivated multidisciplinary team. When choosing your team, be sure to include a representative from the various stakeholders, that is, the individuals who have a variety of hospital roles likely to be affected by the outcome of the project. Stakeholders ensure the success of the project because they have a fundamental understanding of how the project will influence workflow, can predict issues before they arise, and often become empowered to make changes that directly influence their work.
Lastly, include at least one well-respected and highly influential member on your team. Change is always hard, and this person’s support and endorsement of the project, can often move mountains when challenges arise.
3. Know the data collector: It is important to understand what data can be collected because, without data, you cannot measure your success. Arrange a meeting and develop a partnership with the data collector. Obtain a general understanding of how and what specific data is collected. Be sure the data collector has a clear understanding of the project design and the specific details of the project. Include the overall project mission, specific aims of the project, the time frame in which data should be collected, and specific inclusion and exclusion criteria.
Often, data collectors prefer to collect extra data points upfront, even if you end up not using some of them, rather than having to find missing data after the fact. Communication is key, so be available for questions and open to the suggestions of the data collector.
4. Don’t reinvent the wheel: Prior to starting any QI projects, evaluate available resources for project ideas and implementation. The Society of Hospital Medicine and the American College of Physicians outline multiple projects on their websites. Reach out to colleagues at other institutions and obtain their input as they are likely struggling with similar issues and/or have worked on similar project ideas. Use these resources as scaffolding and edit them to fit your institution’s processes and culture, and use their metrics as your measures of success.
5. Remove waste: When determining QI projects, consider focusing on health care waste. Many of the current processes at our institutions have redundancies that add unhealthy time, effort, and inefficiency to our days that can not only impede patient care but also can lead to burnout. When outlining a project idea, consider mapping the process in your interested area to identify those redundancies and inefficiencies. Consider focusing on these instead of building an entirely new process. Improving inefficiencies also can help with provider buy-in with process changes, especially if this helps in improving their everyday frustrations.
6. Express your values: Create a sense of urgency around the problem you are trying to solve. Educate your colleagues to understand the depth of the QI initiative and its impact on their ability to care for patients and patient safety. Express genuine interest in improving your colleagues’ ability to care for patients and improve their days.
Sharing your passion about your project allows people to understand your vested interest in improving the system. This will inspire team members to lead the way to change and encourage colleagues to adopt the recommended changes.
7. Recognize and reward your team: Involve “champions” in every process change. Identify people who are part of your team and ensure they feel valued. Recognition and acknowledgment will allow people to feel more involved and to gain their buy-in. When it comes to results or progress, consider your group’s dynamics. If they are competitive, consider posting progress results on a publicly displayed run chart. If your group is less likely to be motivated by competition, hold individual meetings to help show progress. This is a crucial dynamic to understand, because creating a competitive environment may alienate some members of your group. Remember, the final result is not to blame those lagging behind but to encourage everyone to find the best pathway to success.
8. Be okay with failure: Celebrate your failures because failure is a chance to learn. Every failure is an educational opportunity to understand what not to do, or a chance to gain insight into a process that did not work.
Be a divergent thinker. Start considering problems as part of the path to solution, rather than a barrier in the way. Be open to change and learn from your mistakes. Don’t just be okay with your failures, own them. This will lead to trust with your team members and show your commitment.
9. Finish: This is key. You must finish your project. Even if you anticipate that the project will fail, you should see the project through to its completion. This proves both you and the process of QI are valid and worthwhile; you have to see results and share them with others.
Completing your project also shows your colleagues that you are resilient, committed, and dedicated. Completing a QI project, even with disappointing results, is a success in and of itself. In the end, it is most important to remember to show progress, not perfection.
10. Create sustainability: When your QI project is finished, you need to decide if the changes are sustainable. Some projects show small change and do not need permanent implementation, rather reminders over time. Other projects may be sustainable with EHR or organizational changes. Once you have successful results, your goal should be to find a way to ensure that the process stays in place over time. This is where all your hard work establishing buy-in comes in handy. Your team is more likely to create sustainable change with the hard work you forged through following these key tips.
These Top 10 tips are a hospitalist’s starting point to begin making changes at their own community hospital. Your motivation and effort in making quality change will not go unnoticed. Small ideas will open doors for larger, more sustainable QI projects. Remember, a failure just means a new idea for the next cycle! Enjoy the process of working collaboratively with your hospital on improving quality. Good luck!
Dr. Astik is a hospitalist and instructor of medicine at Northwestern Memorial Hospital, Chicago. Dr. Corbett is a hospitalist and assistant professor at the University of Oklahoma, Tulsa. Dr. Patel is a hospitalist and assistant professor at the University of Colorado, Denver. Dr. Ronan is a hospitalist and associate professor at Christus St. Vincent Regional Medical Center, Santa Fe, NM.
Consider low-cost, high-impact projects
Consider low-cost, high-impact projects
Quality improvement (QI) is essential to the advancement of medicine. QI differs from research as it focuses on already proven knowledge and aims to make quick, sustainable change in local health care systems. Community hospitals may not have organized quality improvement initiatives and often rely on individual hospitalists to be their champions.
Although there are resources for quality improvement projects, initiating a project can seem daunting to a hospitalist. Our aim is to equip the community hospitalist with basic skills to initiate their own successful project. We present our “Top 10” tips to review.
1. Start small: Many quality improvement ideas include grandiose changes that require a large buy-in or worse, more money. When starting a QI project, you need to consider low-cost, high-impact projects. Even the smallest projects can make considerable change. Focus on ideas that require only one or two improvement cycles to implement. Understand your hospital culture, flow, and processes, and then pick a project that is reasonable.
Projects can be as simple as decreasing the number of daily labs ordered by your hospitalist group. Projects that are small could still improve patient satisfaction and decrease costs. Listen to your colleagues, if they are discussing an issue, turn this into an idea! As you learn the culture of your hospital you will be able to tackle larger projects. Plus, it gets your name out there!
2. Establish buy-in: Surround yourself with champions of your cause. Properly identifying and engaging key players is paramount to a successful QI project. First, start with your hospital administration, and garner their support by aligning your project with the goals and objectives that the administration leaders have identified to be important for your institution. Next, select a motivated multidisciplinary team. When choosing your team, be sure to include a representative from the various stakeholders, that is, the individuals who have a variety of hospital roles likely to be affected by the outcome of the project. Stakeholders ensure the success of the project because they have a fundamental understanding of how the project will influence workflow, can predict issues before they arise, and often become empowered to make changes that directly influence their work.
Lastly, include at least one well-respected and highly influential member on your team. Change is always hard, and this person’s support and endorsement of the project, can often move mountains when challenges arise.
3. Know the data collector: It is important to understand what data can be collected because, without data, you cannot measure your success. Arrange a meeting and develop a partnership with the data collector. Obtain a general understanding of how and what specific data is collected. Be sure the data collector has a clear understanding of the project design and the specific details of the project. Include the overall project mission, specific aims of the project, the time frame in which data should be collected, and specific inclusion and exclusion criteria.
Often, data collectors prefer to collect extra data points upfront, even if you end up not using some of them, rather than having to find missing data after the fact. Communication is key, so be available for questions and open to the suggestions of the data collector.
4. Don’t reinvent the wheel: Prior to starting any QI projects, evaluate available resources for project ideas and implementation. The Society of Hospital Medicine and the American College of Physicians outline multiple projects on their websites. Reach out to colleagues at other institutions and obtain their input as they are likely struggling with similar issues and/or have worked on similar project ideas. Use these resources as scaffolding and edit them to fit your institution’s processes and culture, and use their metrics as your measures of success.
5. Remove waste: When determining QI projects, consider focusing on health care waste. Many of the current processes at our institutions have redundancies that add unhealthy time, effort, and inefficiency to our days that can not only impede patient care but also can lead to burnout. When outlining a project idea, consider mapping the process in your interested area to identify those redundancies and inefficiencies. Consider focusing on these instead of building an entirely new process. Improving inefficiencies also can help with provider buy-in with process changes, especially if this helps in improving their everyday frustrations.
6. Express your values: Create a sense of urgency around the problem you are trying to solve. Educate your colleagues to understand the depth of the QI initiative and its impact on their ability to care for patients and patient safety. Express genuine interest in improving your colleagues’ ability to care for patients and improve their days.
Sharing your passion about your project allows people to understand your vested interest in improving the system. This will inspire team members to lead the way to change and encourage colleagues to adopt the recommended changes.
7. Recognize and reward your team: Involve “champions” in every process change. Identify people who are part of your team and ensure they feel valued. Recognition and acknowledgment will allow people to feel more involved and to gain their buy-in. When it comes to results or progress, consider your group’s dynamics. If they are competitive, consider posting progress results on a publicly displayed run chart. If your group is less likely to be motivated by competition, hold individual meetings to help show progress. This is a crucial dynamic to understand, because creating a competitive environment may alienate some members of your group. Remember, the final result is not to blame those lagging behind but to encourage everyone to find the best pathway to success.
8. Be okay with failure: Celebrate your failures because failure is a chance to learn. Every failure is an educational opportunity to understand what not to do, or a chance to gain insight into a process that did not work.
Be a divergent thinker. Start considering problems as part of the path to solution, rather than a barrier in the way. Be open to change and learn from your mistakes. Don’t just be okay with your failures, own them. This will lead to trust with your team members and show your commitment.
9. Finish: This is key. You must finish your project. Even if you anticipate that the project will fail, you should see the project through to its completion. This proves both you and the process of QI are valid and worthwhile; you have to see results and share them with others.
Completing your project also shows your colleagues that you are resilient, committed, and dedicated. Completing a QI project, even with disappointing results, is a success in and of itself. In the end, it is most important to remember to show progress, not perfection.
10. Create sustainability: When your QI project is finished, you need to decide if the changes are sustainable. Some projects show small change and do not need permanent implementation, rather reminders over time. Other projects may be sustainable with EHR or organizational changes. Once you have successful results, your goal should be to find a way to ensure that the process stays in place over time. This is where all your hard work establishing buy-in comes in handy. Your team is more likely to create sustainable change with the hard work you forged through following these key tips.
These Top 10 tips are a hospitalist’s starting point to begin making changes at their own community hospital. Your motivation and effort in making quality change will not go unnoticed. Small ideas will open doors for larger, more sustainable QI projects. Remember, a failure just means a new idea for the next cycle! Enjoy the process of working collaboratively with your hospital on improving quality. Good luck!
Dr. Astik is a hospitalist and instructor of medicine at Northwestern Memorial Hospital, Chicago. Dr. Corbett is a hospitalist and assistant professor at the University of Oklahoma, Tulsa. Dr. Patel is a hospitalist and assistant professor at the University of Colorado, Denver. Dr. Ronan is a hospitalist and associate professor at Christus St. Vincent Regional Medical Center, Santa Fe, NM.
Quality improvement (QI) is essential to the advancement of medicine. QI differs from research as it focuses on already proven knowledge and aims to make quick, sustainable change in local health care systems. Community hospitals may not have organized quality improvement initiatives and often rely on individual hospitalists to be their champions.
Although there are resources for quality improvement projects, initiating a project can seem daunting to a hospitalist. Our aim is to equip the community hospitalist with basic skills to initiate their own successful project. We present our “Top 10” tips to review.
1. Start small: Many quality improvement ideas include grandiose changes that require a large buy-in or worse, more money. When starting a QI project, you need to consider low-cost, high-impact projects. Even the smallest projects can make considerable change. Focus on ideas that require only one or two improvement cycles to implement. Understand your hospital culture, flow, and processes, and then pick a project that is reasonable.
Projects can be as simple as decreasing the number of daily labs ordered by your hospitalist group. Projects that are small could still improve patient satisfaction and decrease costs. Listen to your colleagues, if they are discussing an issue, turn this into an idea! As you learn the culture of your hospital you will be able to tackle larger projects. Plus, it gets your name out there!
2. Establish buy-in: Surround yourself with champions of your cause. Properly identifying and engaging key players is paramount to a successful QI project. First, start with your hospital administration, and garner their support by aligning your project with the goals and objectives that the administration leaders have identified to be important for your institution. Next, select a motivated multidisciplinary team. When choosing your team, be sure to include a representative from the various stakeholders, that is, the individuals who have a variety of hospital roles likely to be affected by the outcome of the project. Stakeholders ensure the success of the project because they have a fundamental understanding of how the project will influence workflow, can predict issues before they arise, and often become empowered to make changes that directly influence their work.
Lastly, include at least one well-respected and highly influential member on your team. Change is always hard, and this person’s support and endorsement of the project, can often move mountains when challenges arise.
3. Know the data collector: It is important to understand what data can be collected because, without data, you cannot measure your success. Arrange a meeting and develop a partnership with the data collector. Obtain a general understanding of how and what specific data is collected. Be sure the data collector has a clear understanding of the project design and the specific details of the project. Include the overall project mission, specific aims of the project, the time frame in which data should be collected, and specific inclusion and exclusion criteria.
Often, data collectors prefer to collect extra data points upfront, even if you end up not using some of them, rather than having to find missing data after the fact. Communication is key, so be available for questions and open to the suggestions of the data collector.
4. Don’t reinvent the wheel: Prior to starting any QI projects, evaluate available resources for project ideas and implementation. The Society of Hospital Medicine and the American College of Physicians outline multiple projects on their websites. Reach out to colleagues at other institutions and obtain their input as they are likely struggling with similar issues and/or have worked on similar project ideas. Use these resources as scaffolding and edit them to fit your institution’s processes and culture, and use their metrics as your measures of success.
5. Remove waste: When determining QI projects, consider focusing on health care waste. Many of the current processes at our institutions have redundancies that add unhealthy time, effort, and inefficiency to our days that can not only impede patient care but also can lead to burnout. When outlining a project idea, consider mapping the process in your interested area to identify those redundancies and inefficiencies. Consider focusing on these instead of building an entirely new process. Improving inefficiencies also can help with provider buy-in with process changes, especially if this helps in improving their everyday frustrations.
6. Express your values: Create a sense of urgency around the problem you are trying to solve. Educate your colleagues to understand the depth of the QI initiative and its impact on their ability to care for patients and patient safety. Express genuine interest in improving your colleagues’ ability to care for patients and improve their days.
Sharing your passion about your project allows people to understand your vested interest in improving the system. This will inspire team members to lead the way to change and encourage colleagues to adopt the recommended changes.
7. Recognize and reward your team: Involve “champions” in every process change. Identify people who are part of your team and ensure they feel valued. Recognition and acknowledgment will allow people to feel more involved and to gain their buy-in. When it comes to results or progress, consider your group’s dynamics. If they are competitive, consider posting progress results on a publicly displayed run chart. If your group is less likely to be motivated by competition, hold individual meetings to help show progress. This is a crucial dynamic to understand, because creating a competitive environment may alienate some members of your group. Remember, the final result is not to blame those lagging behind but to encourage everyone to find the best pathway to success.
8. Be okay with failure: Celebrate your failures because failure is a chance to learn. Every failure is an educational opportunity to understand what not to do, or a chance to gain insight into a process that did not work.
Be a divergent thinker. Start considering problems as part of the path to solution, rather than a barrier in the way. Be open to change and learn from your mistakes. Don’t just be okay with your failures, own them. This will lead to trust with your team members and show your commitment.
9. Finish: This is key. You must finish your project. Even if you anticipate that the project will fail, you should see the project through to its completion. This proves both you and the process of QI are valid and worthwhile; you have to see results and share them with others.
Completing your project also shows your colleagues that you are resilient, committed, and dedicated. Completing a QI project, even with disappointing results, is a success in and of itself. In the end, it is most important to remember to show progress, not perfection.
10. Create sustainability: When your QI project is finished, you need to decide if the changes are sustainable. Some projects show small change and do not need permanent implementation, rather reminders over time. Other projects may be sustainable with EHR or organizational changes. Once you have successful results, your goal should be to find a way to ensure that the process stays in place over time. This is where all your hard work establishing buy-in comes in handy. Your team is more likely to create sustainable change with the hard work you forged through following these key tips.
These Top 10 tips are a hospitalist’s starting point to begin making changes at their own community hospital. Your motivation and effort in making quality change will not go unnoticed. Small ideas will open doors for larger, more sustainable QI projects. Remember, a failure just means a new idea for the next cycle! Enjoy the process of working collaboratively with your hospital on improving quality. Good luck!
Dr. Astik is a hospitalist and instructor of medicine at Northwestern Memorial Hospital, Chicago. Dr. Corbett is a hospitalist and assistant professor at the University of Oklahoma, Tulsa. Dr. Patel is a hospitalist and assistant professor at the University of Colorado, Denver. Dr. Ronan is a hospitalist and associate professor at Christus St. Vincent Regional Medical Center, Santa Fe, NM.
FDA approves first vaccine for prevention of dengue disease
The vaccine was approved for children aged 9-16 years who live in endemic areas and have previously had laboratory-confirmed dengue disease.
Dengue is endemic in the U.S. territories of American Samoa, Guam, Puerto Rico, and the U.S. Virgin Islands, according to an FDA statement announcing the approval.
While the first infection with dengue virus typically results in either no symptoms or a mild illness that can be mistaken for the flu, a second infection can lead to a more severe form of the disease, including dengue hemorrhagic fever, which can be fatal. About 95% of hospitalized patients with dengue disease have a second dengue virus infection.
FDA approval of Dengvaxia is based on results from three randomized, placebo-controlled studies of 35,000 individuals in dengue-endemic areas. The vaccine was about 76% effective in preventing symptomatic, laboratory-confirmed dengue disease in people aged 9-16 years with a previous dengue diagnosis. The most common adverse events were headache, muscle pain, joint pain, fatigue, injection site pain, and low-grade fever; the frequency of adverse events decreased after each subsequent dose.
“Infection by one type of dengue virus usually provides immunity against that specific serotype, but a subsequent infection by any of the other three serotypes of the virus increases the risk of developing severe dengue disease. ... The FDA’s approval of this vaccine will help protect people previously infected with dengue virus from subsequent development of dengue disease,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement.
The vaccine was approved for children aged 9-16 years who live in endemic areas and have previously had laboratory-confirmed dengue disease.
Dengue is endemic in the U.S. territories of American Samoa, Guam, Puerto Rico, and the U.S. Virgin Islands, according to an FDA statement announcing the approval.
While the first infection with dengue virus typically results in either no symptoms or a mild illness that can be mistaken for the flu, a second infection can lead to a more severe form of the disease, including dengue hemorrhagic fever, which can be fatal. About 95% of hospitalized patients with dengue disease have a second dengue virus infection.
FDA approval of Dengvaxia is based on results from three randomized, placebo-controlled studies of 35,000 individuals in dengue-endemic areas. The vaccine was about 76% effective in preventing symptomatic, laboratory-confirmed dengue disease in people aged 9-16 years with a previous dengue diagnosis. The most common adverse events were headache, muscle pain, joint pain, fatigue, injection site pain, and low-grade fever; the frequency of adverse events decreased after each subsequent dose.
“Infection by one type of dengue virus usually provides immunity against that specific serotype, but a subsequent infection by any of the other three serotypes of the virus increases the risk of developing severe dengue disease. ... The FDA’s approval of this vaccine will help protect people previously infected with dengue virus from subsequent development of dengue disease,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement.
The vaccine was approved for children aged 9-16 years who live in endemic areas and have previously had laboratory-confirmed dengue disease.
Dengue is endemic in the U.S. territories of American Samoa, Guam, Puerto Rico, and the U.S. Virgin Islands, according to an FDA statement announcing the approval.
While the first infection with dengue virus typically results in either no symptoms or a mild illness that can be mistaken for the flu, a second infection can lead to a more severe form of the disease, including dengue hemorrhagic fever, which can be fatal. About 95% of hospitalized patients with dengue disease have a second dengue virus infection.
FDA approval of Dengvaxia is based on results from three randomized, placebo-controlled studies of 35,000 individuals in dengue-endemic areas. The vaccine was about 76% effective in preventing symptomatic, laboratory-confirmed dengue disease in people aged 9-16 years with a previous dengue diagnosis. The most common adverse events were headache, muscle pain, joint pain, fatigue, injection site pain, and low-grade fever; the frequency of adverse events decreased after each subsequent dose.
“Infection by one type of dengue virus usually provides immunity against that specific serotype, but a subsequent infection by any of the other three serotypes of the virus increases the risk of developing severe dengue disease. ... The FDA’s approval of this vaccine will help protect people previously infected with dengue virus from subsequent development of dengue disease,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the FDA statement.
Liraglutide seems safe, effective in children already on metformin
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
FROM PAS 2019
Arthritis joint pain, inactivity vary greatly across U.S.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
FROM MMWR