User login
More abnormal cells linked to poorer ASCT outcomes in MDS
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
What do patients want in a migraine preventive?
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
FROM HEADACHE
FDA approves ivosidenib frontline for certain AML patients
The Food and Drug Administration has approved ivosidenib (Tibsovo) for newly diagnosed acute myeloid leukemia (AML) with a susceptible IDH1 mutation in patients who are at least 75 years old or have comorbidities preventing the use of intensive induction chemotherapy.
In July 2018, the FDA approved ivosidenib for adults with relapsed or refractory AML with a susceptible IDH1 mutation.
The latest approval was based on results from an open-label, single-arm, multicenter trial of patients with newly diagnosed AML with an IDH1 mutation. Patients were treated with 500 mg ivosidenib daily until disease progression, development of unacceptable toxicity, or hematopoietic stem cell transplantation; the median age of the 28 patients treated with ivosidenib was 77 years.
Of the 28 patients treated, 12 achieved complete remission or complete remission with partial hematologic recovery; 7 of the 17 transfusion-dependent patients achieved transfusion independence for at least 8 weeks.
The most common adverse events were diarrhea, fatigue, edema, decreased appetite, leukocytosis, nausea, arthralgia, abdominal pain, dyspnea, differentiation syndrome, and myalgia. The drug’s prescribing information includes a boxed warning on the risk of differentiation syndrome.
“The recommended ivosidenib dose is 500 mg orally once daily with or without food until disease progression or unacceptable toxicity. For patients without disease progression or unacceptable toxicity, treatment is recommended for a minimum of 6 months to allow time for clinical response,” the FDA noted.
Find the full press release on the FDA website.
The Food and Drug Administration has approved ivosidenib (Tibsovo) for newly diagnosed acute myeloid leukemia (AML) with a susceptible IDH1 mutation in patients who are at least 75 years old or have comorbidities preventing the use of intensive induction chemotherapy.
In July 2018, the FDA approved ivosidenib for adults with relapsed or refractory AML with a susceptible IDH1 mutation.
The latest approval was based on results from an open-label, single-arm, multicenter trial of patients with newly diagnosed AML with an IDH1 mutation. Patients were treated with 500 mg ivosidenib daily until disease progression, development of unacceptable toxicity, or hematopoietic stem cell transplantation; the median age of the 28 patients treated with ivosidenib was 77 years.
Of the 28 patients treated, 12 achieved complete remission or complete remission with partial hematologic recovery; 7 of the 17 transfusion-dependent patients achieved transfusion independence for at least 8 weeks.
The most common adverse events were diarrhea, fatigue, edema, decreased appetite, leukocytosis, nausea, arthralgia, abdominal pain, dyspnea, differentiation syndrome, and myalgia. The drug’s prescribing information includes a boxed warning on the risk of differentiation syndrome.
“The recommended ivosidenib dose is 500 mg orally once daily with or without food until disease progression or unacceptable toxicity. For patients without disease progression or unacceptable toxicity, treatment is recommended for a minimum of 6 months to allow time for clinical response,” the FDA noted.
Find the full press release on the FDA website.
The Food and Drug Administration has approved ivosidenib (Tibsovo) for newly diagnosed acute myeloid leukemia (AML) with a susceptible IDH1 mutation in patients who are at least 75 years old or have comorbidities preventing the use of intensive induction chemotherapy.
In July 2018, the FDA approved ivosidenib for adults with relapsed or refractory AML with a susceptible IDH1 mutation.
The latest approval was based on results from an open-label, single-arm, multicenter trial of patients with newly diagnosed AML with an IDH1 mutation. Patients were treated with 500 mg ivosidenib daily until disease progression, development of unacceptable toxicity, or hematopoietic stem cell transplantation; the median age of the 28 patients treated with ivosidenib was 77 years.
Of the 28 patients treated, 12 achieved complete remission or complete remission with partial hematologic recovery; 7 of the 17 transfusion-dependent patients achieved transfusion independence for at least 8 weeks.
The most common adverse events were diarrhea, fatigue, edema, decreased appetite, leukocytosis, nausea, arthralgia, abdominal pain, dyspnea, differentiation syndrome, and myalgia. The drug’s prescribing information includes a boxed warning on the risk of differentiation syndrome.
“The recommended ivosidenib dose is 500 mg orally once daily with or without food until disease progression or unacceptable toxicity. For patients without disease progression or unacceptable toxicity, treatment is recommended for a minimum of 6 months to allow time for clinical response,” the FDA noted.
Find the full press release on the FDA website.
LentiGlobin reduces transfusion dependence in young thalassemia patients
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
REPORTING FROM 2019 ASPHO CONFERENCE
Topical Chemotherapy for Numerous Superficial Basal Cell Carcinomas Years After Isolated Limb Perfusion for Melanoma
Isolated limb perfusion (ILP) for the adjuvant treatment of melanoma involves isolating the blood flow of a limb from the rest of the body to allow for high concentrations of chemotherapeutic agents locally. Chemotherapy with nitrogen mustard is the preferred chemotherapeutic agent in ILP for the adjuvant treatment of locally advanced melanoma.1 Systemic exposure to nitrogen mustard has shown to be carcinogenic, and its topical application has been associated with the development of actinic keratosis, basal cell carcinoma (BCC), and squamous cell carcinoma.2,3 However, the long-term effects of ILP with nitrogen mustard are not well defined. In 1998, one of the authors (R.L.M.) described a patient with melanoma of the left leg that was treated with ILP with nitrogen mustard who subsequently developed numerous BCCs on the same leg.4 This same patient has since been successfully managed with only topical chemotherapeutic agents for the last 21 years.
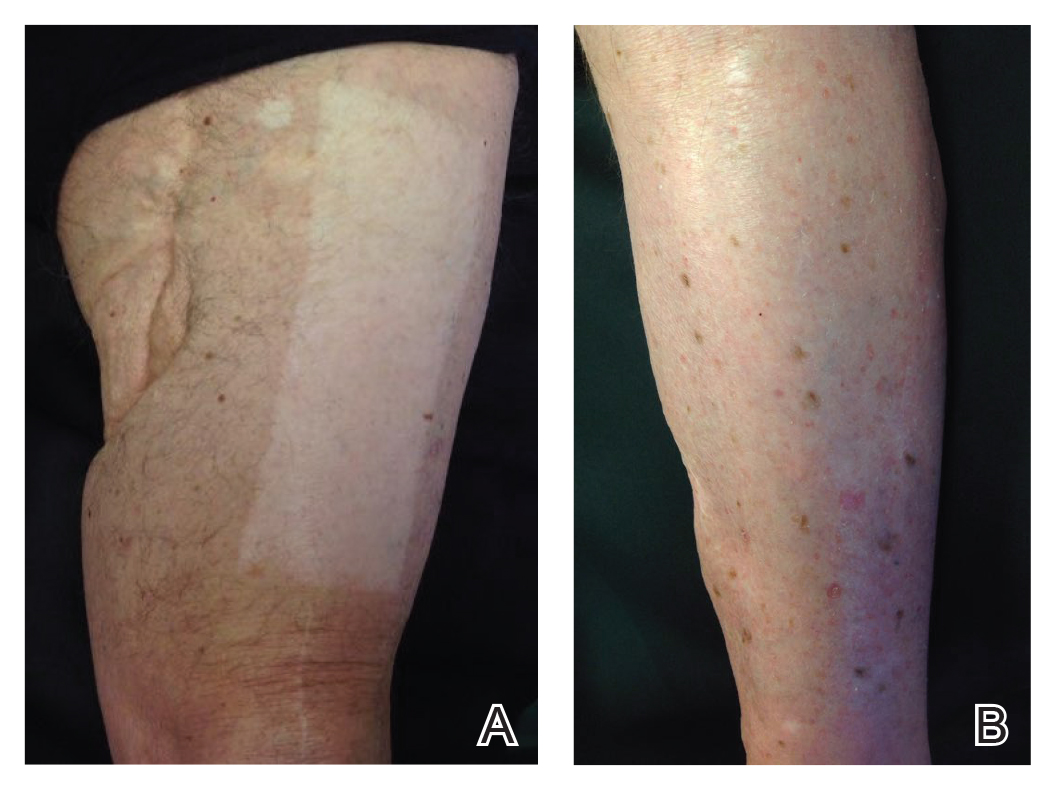
An 86-year-old man with a history of melanoma underwent wide resection, lymph node dissection, and adjuvant ILP with nitrogen mustard for the treatment of melanoma of the medial left thigh approximately 50 years ago. He denied any prior radiation treatment. He subsequently presented years later to our dermatology clinic with many biopsy-proven superficial and nodular BCCs of the left leg over the course of the last 30 years. On physical examination, the patient had several pink papules and macules on the left lower leg (Figure). The patient had previously undergone multiple invasive excisions with grafting for the treatment of BCCs by a plastic surgeon prior to presentation to our clinic but has since had many years of control under our care with only topical chemotherapeutic agents. His current medication regimen consists of 5-fluorouracil twice daily, which he tolerates without serious side effects. He also has used imiquimod in the past.
Isolated limb perfusion was first described by Creech et al5 in 1958. Chemotherapy in ILP is designed to maximize limb perfusion while minimizing systemic absorption.1 Meta
Topical use of nitrogen mustard has been linked to the development of nonmelanoma skin cancer (NMSC)2,3; however, a 30-year population-based study found no significant increase in secondary malignancies, including NMSC or melanoma, following use of topical nitrogen mustard.6 There also have been reported cases of secondary cancers following ILP reported in the literature, including pleomorphic sarcoma and Merkel cell carcinoma.7 We hypothesize that our patient’s exposure to nitrogen mustard during ILP led to the development of numerous BCCs, but further research is necessary to confirm this relationship.
Treatment modalities for NMSC include surgical excision with defined margins, Mohs micrographic surgery, radiotherapy, electrodesiccation and curettage, cryotherapy, photodynamic therapy, and topical therapy. Our patient experienced such a high volume of superficial BCCs that the decision was made to avoid frequent surgical procedures and to treat with topical chemotherapeutic agents. He had an excellent response to topical 5-fluorouracil, and the treatment has been well tolerated. This case is valuable for clinicians, as it demonstrates that topical chemotherapy can be a well-tolerated option for patients who present with frequent superficial BCCs to prevent numerous invasive surgical treatments.
- Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157-163.
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapy for mycosis fungoides. J Am Acad Dermatol. 1986;14:1029-1038.
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. 1982;7:590-598.
- Lamb PM, Menaker GM, Moy RL. Multiple basal cell carcinomas of the limb after adjuvant treatment of melanoma with isolated limb perfusion. J Am Acad Dermatol. 1998;38:767-768.
- Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporal circuit. Ann Surg. 1958;148:616-632.
- Lindahl L, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170:699-704.
- Lenormand C, Pelletier C, Goeldel AL, et al. Second malignant neoplasm occurring years after hyperthermic isolated limb perfusion for melanoma. Arch Dermatol. 2010;146:319-321.
Isolated limb perfusion (ILP) for the adjuvant treatment of melanoma involves isolating the blood flow of a limb from the rest of the body to allow for high concentrations of chemotherapeutic agents locally. Chemotherapy with nitrogen mustard is the preferred chemotherapeutic agent in ILP for the adjuvant treatment of locally advanced melanoma.1 Systemic exposure to nitrogen mustard has shown to be carcinogenic, and its topical application has been associated with the development of actinic keratosis, basal cell carcinoma (BCC), and squamous cell carcinoma.2,3 However, the long-term effects of ILP with nitrogen mustard are not well defined. In 1998, one of the authors (R.L.M.) described a patient with melanoma of the left leg that was treated with ILP with nitrogen mustard who subsequently developed numerous BCCs on the same leg.4 This same patient has since been successfully managed with only topical chemotherapeutic agents for the last 21 years.
An 86-year-old man with a history of melanoma underwent wide resection, lymph node dissection, and adjuvant ILP with nitrogen mustard for the treatment of melanoma of the medial left thigh approximately 50 years ago. He denied any prior radiation treatment. He subsequently presented years later to our dermatology clinic with many biopsy-proven superficial and nodular BCCs of the left leg over the course of the last 30 years. On physical examination, the patient had several pink papules and macules on the left lower leg (Figure). The patient had previously undergone multiple invasive excisions with grafting for the treatment of BCCs by a plastic surgeon prior to presentation to our clinic but has since had many years of control under our care with only topical chemotherapeutic agents. His current medication regimen consists of 5-fluorouracil twice daily, which he tolerates without serious side effects. He also has used imiquimod in the past.

Isolated limb perfusion was first described by Creech et al5 in 1958. Chemotherapy in ILP is designed to maximize limb perfusion while minimizing systemic absorption.1 Meta
Topical use of nitrogen mustard has been linked to the development of nonmelanoma skin cancer (NMSC)2,3; however, a 30-year population-based study found no significant increase in secondary malignancies, including NMSC or melanoma, following use of topical nitrogen mustard.6 There also have been reported cases of secondary cancers following ILP reported in the literature, including pleomorphic sarcoma and Merkel cell carcinoma.7 We hypothesize that our patient’s exposure to nitrogen mustard during ILP led to the development of numerous BCCs, but further research is necessary to confirm this relationship.
Treatment modalities for NMSC include surgical excision with defined margins, Mohs micrographic surgery, radiotherapy, electrodesiccation and curettage, cryotherapy, photodynamic therapy, and topical therapy. Our patient experienced such a high volume of superficial BCCs that the decision was made to avoid frequent surgical procedures and to treat with topical chemotherapeutic agents. He had an excellent response to topical 5-fluorouracil, and the treatment has been well tolerated. This case is valuable for clinicians, as it demonstrates that topical chemotherapy can be a well-tolerated option for patients who present with frequent superficial BCCs to prevent numerous invasive surgical treatments.
Isolated limb perfusion (ILP) for the adjuvant treatment of melanoma involves isolating the blood flow of a limb from the rest of the body to allow for high concentrations of chemotherapeutic agents locally. Chemotherapy with nitrogen mustard is the preferred chemotherapeutic agent in ILP for the adjuvant treatment of locally advanced melanoma.1 Systemic exposure to nitrogen mustard has shown to be carcinogenic, and its topical application has been associated with the development of actinic keratosis, basal cell carcinoma (BCC), and squamous cell carcinoma.2,3 However, the long-term effects of ILP with nitrogen mustard are not well defined. In 1998, one of the authors (R.L.M.) described a patient with melanoma of the left leg that was treated with ILP with nitrogen mustard who subsequently developed numerous BCCs on the same leg.4 This same patient has since been successfully managed with only topical chemotherapeutic agents for the last 21 years.
An 86-year-old man with a history of melanoma underwent wide resection, lymph node dissection, and adjuvant ILP with nitrogen mustard for the treatment of melanoma of the medial left thigh approximately 50 years ago. He denied any prior radiation treatment. He subsequently presented years later to our dermatology clinic with many biopsy-proven superficial and nodular BCCs of the left leg over the course of the last 30 years. On physical examination, the patient had several pink papules and macules on the left lower leg (Figure). The patient had previously undergone multiple invasive excisions with grafting for the treatment of BCCs by a plastic surgeon prior to presentation to our clinic but has since had many years of control under our care with only topical chemotherapeutic agents. His current medication regimen consists of 5-fluorouracil twice daily, which he tolerates without serious side effects. He also has used imiquimod in the past.

Isolated limb perfusion was first described by Creech et al5 in 1958. Chemotherapy in ILP is designed to maximize limb perfusion while minimizing systemic absorption.1 Meta
Topical use of nitrogen mustard has been linked to the development of nonmelanoma skin cancer (NMSC)2,3; however, a 30-year population-based study found no significant increase in secondary malignancies, including NMSC or melanoma, following use of topical nitrogen mustard.6 There also have been reported cases of secondary cancers following ILP reported in the literature, including pleomorphic sarcoma and Merkel cell carcinoma.7 We hypothesize that our patient’s exposure to nitrogen mustard during ILP led to the development of numerous BCCs, but further research is necessary to confirm this relationship.
Treatment modalities for NMSC include surgical excision with defined margins, Mohs micrographic surgery, radiotherapy, electrodesiccation and curettage, cryotherapy, photodynamic therapy, and topical therapy. Our patient experienced such a high volume of superficial BCCs that the decision was made to avoid frequent surgical procedures and to treat with topical chemotherapeutic agents. He had an excellent response to topical 5-fluorouracil, and the treatment has been well tolerated. This case is valuable for clinicians, as it demonstrates that topical chemotherapy can be a well-tolerated option for patients who present with frequent superficial BCCs to prevent numerous invasive surgical treatments.
- Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157-163.
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapy for mycosis fungoides. J Am Acad Dermatol. 1986;14:1029-1038.
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. 1982;7:590-598.
- Lamb PM, Menaker GM, Moy RL. Multiple basal cell carcinomas of the limb after adjuvant treatment of melanoma with isolated limb perfusion. J Am Acad Dermatol. 1998;38:767-768.
- Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporal circuit. Ann Surg. 1958;148:616-632.
- Lindahl L, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170:699-704.
- Lenormand C, Pelletier C, Goeldel AL, et al. Second malignant neoplasm occurring years after hyperthermic isolated limb perfusion for melanoma. Arch Dermatol. 2010;146:319-321.
- Benckhuijsen C, Kroon BB, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157-163.
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapy for mycosis fungoides. J Am Acad Dermatol. 1986;14:1029-1038.
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol. 1982;7:590-598.
- Lamb PM, Menaker GM, Moy RL. Multiple basal cell carcinomas of the limb after adjuvant treatment of melanoma with isolated limb perfusion. J Am Acad Dermatol. 1998;38:767-768.
- Creech O Jr, Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporal circuit. Ann Surg. 1958;148:616-632.
- Lindahl L, Fenger-Grøn M, Iversen L. Secondary cancers, comorbidities and mortality associated with nitrogen mustard therapy in patients with mycosis fungoides: a 30-year population-based cohort study. Br J Dermatol. 2014;170:699-704.
- Lenormand C, Pelletier C, Goeldel AL, et al. Second malignant neoplasm occurring years after hyperthermic isolated limb perfusion for melanoma. Arch Dermatol. 2010;146:319-321.
Only 1.5% of individuals at high risk of opioid overdose receive naloxone
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
FROM JAMA NETWORK OPEN
Optimal Cosmetic Outcomes for Basal Cell Carcinoma: A Retrospective Study of Nonablative Laser Management
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
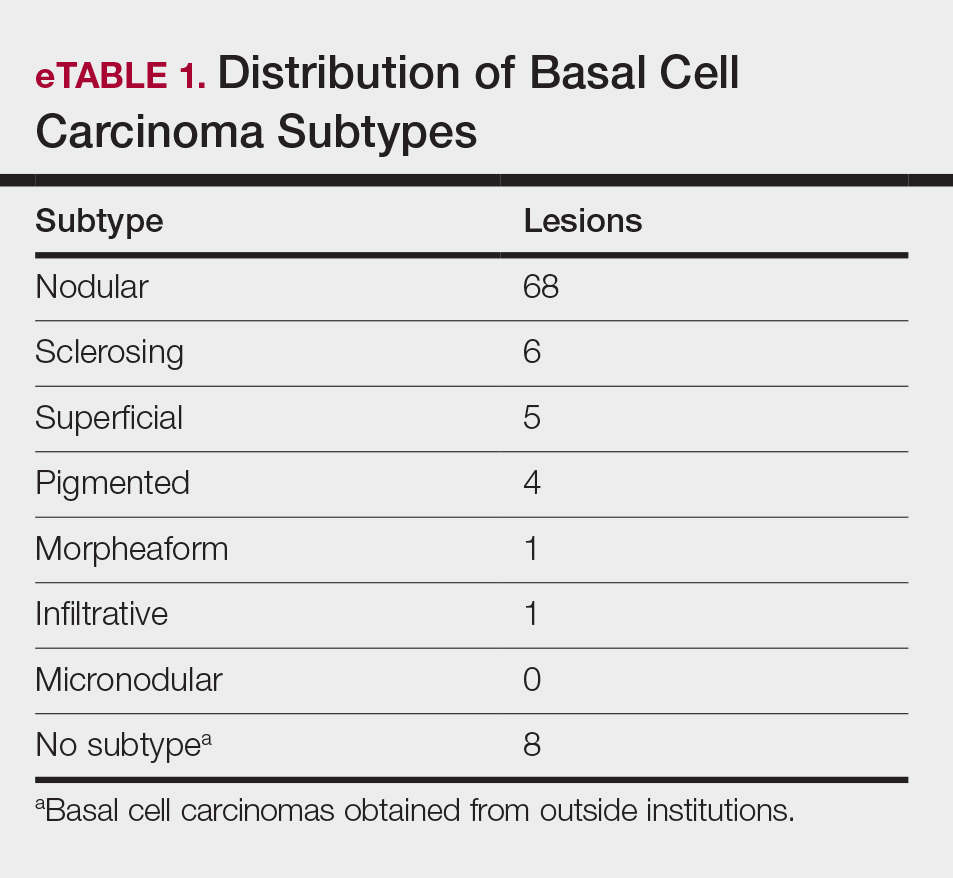
Patient and Lesion Characteristics
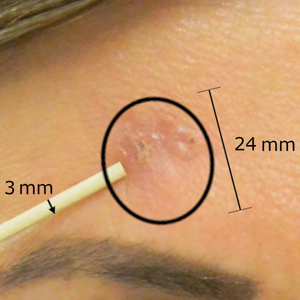
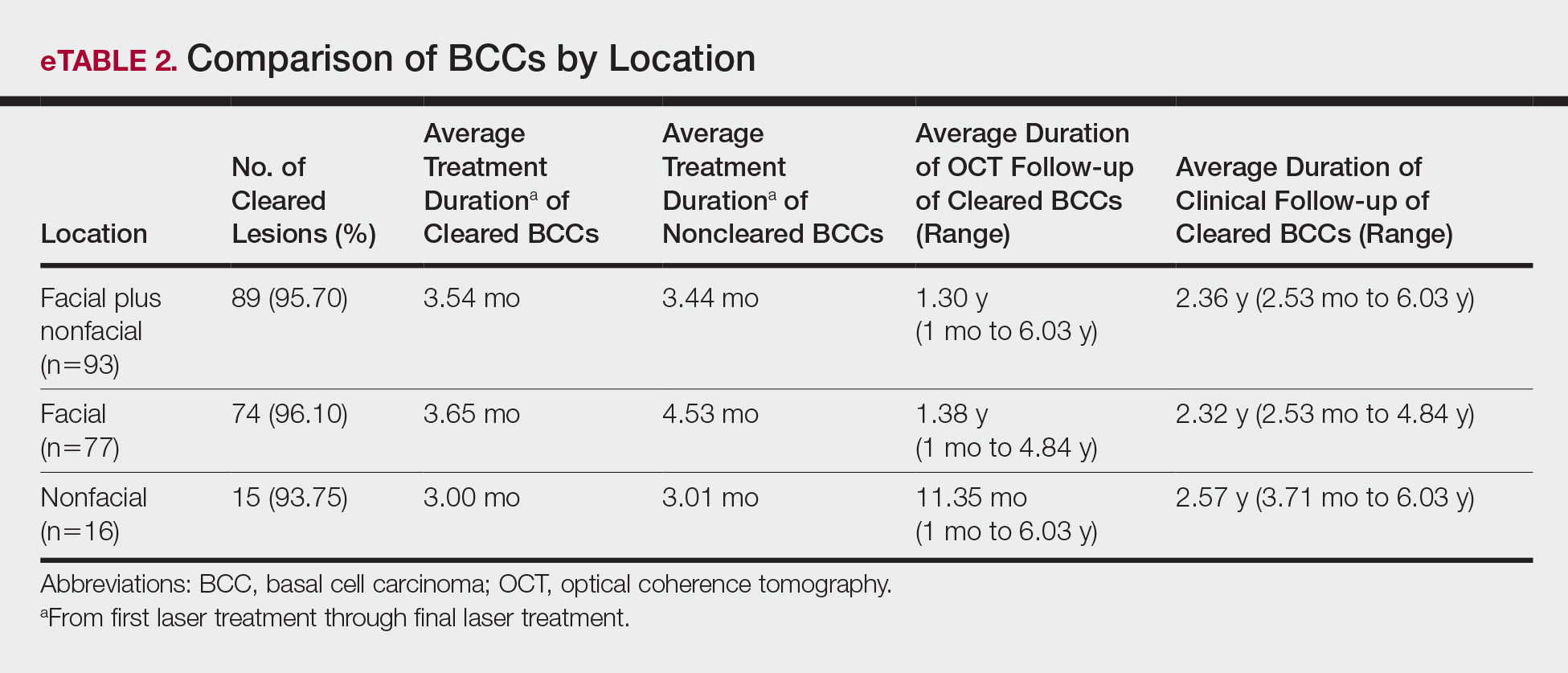
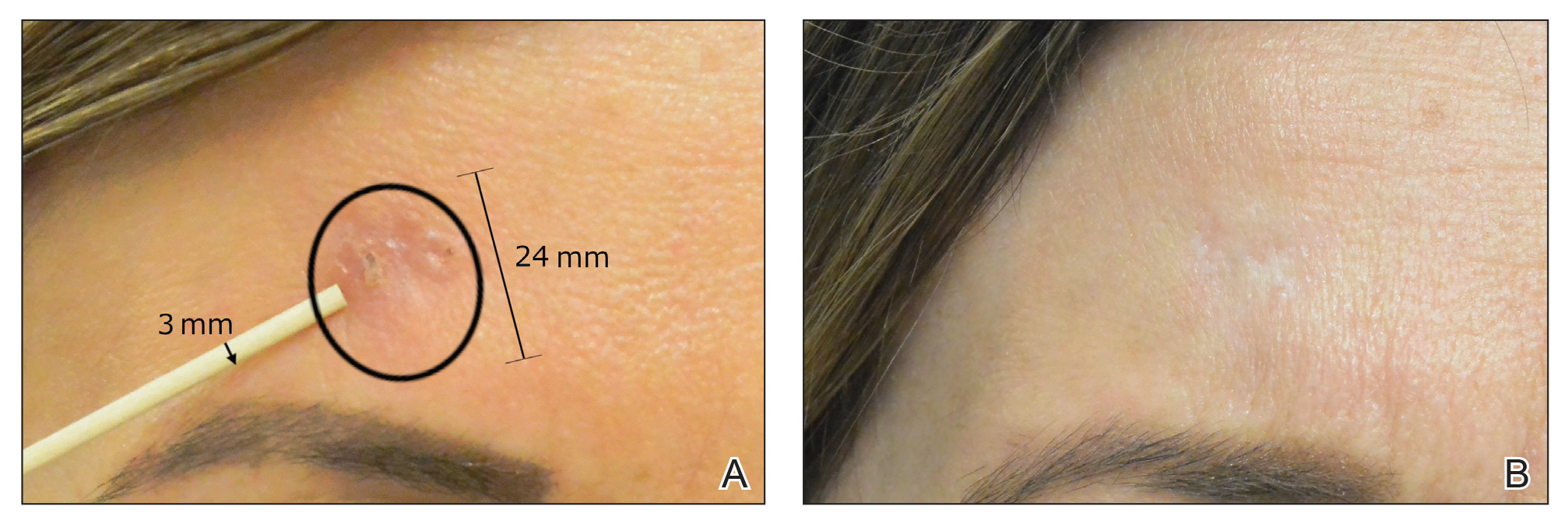
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
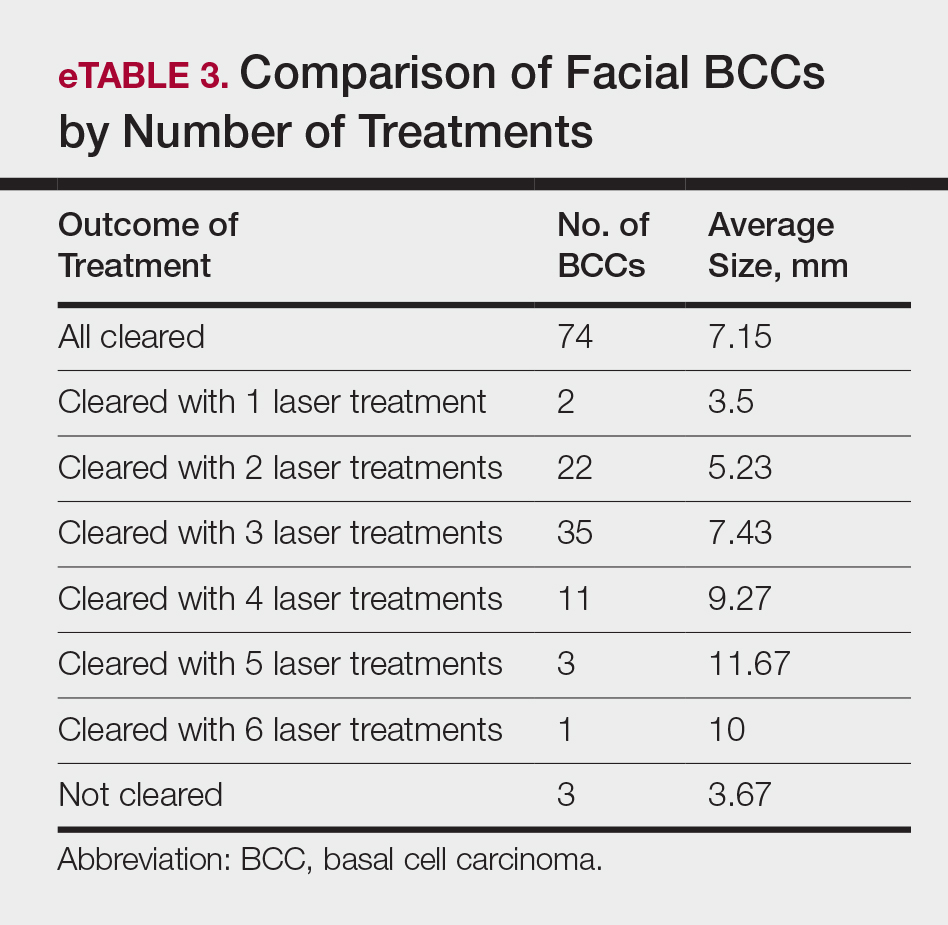
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
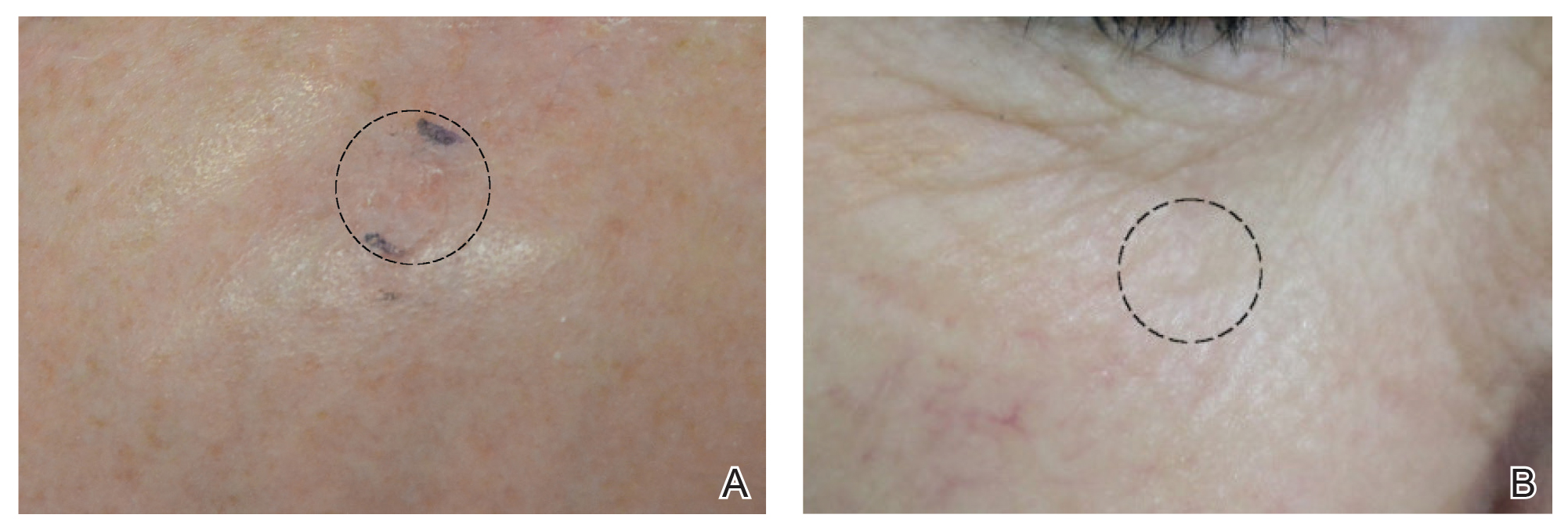
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.

Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
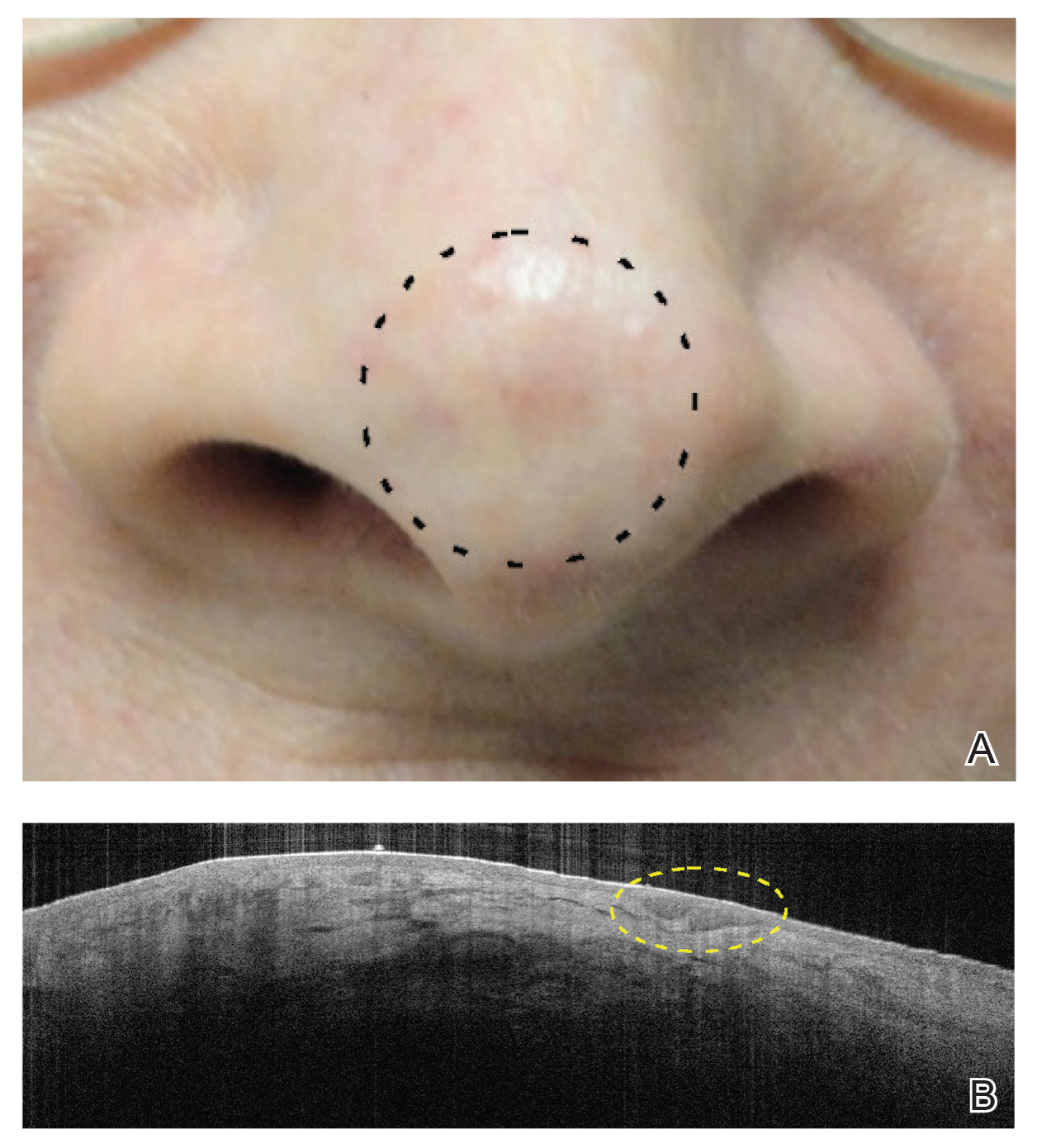
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.

Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.

Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).

Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.

Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.


Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29

After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.

there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.

Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.

Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).

Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.

Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.


Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29

After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.

there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Practice Points
- A major benefit of nonablative laser therapy over more invasive options in the management of basal cell carcinoma (BCC) is minimal scarring.
- When patients are managed with nonablative laser therapy, follow-up with clinical, dermoscopic, and/or noninvasive imaging is more efficient during treatment as well as when assessing for recurrences.
- Optical coherence tomography in combination with nonablative laser therapy allows for detection of residual skin cancers that would not be evident on clinical and/or dermoscopic examination.
- Utilizing early detection techniques, such as a color wheel dermoscopic approach, along with other noninvasive imaging modalities facilitates the use of less invasive treatment options for primary and/or recurrent BCCs.
Basal Cell Carcinoma Masquerading as a Dermoid Cyst and Bursitis of the Knee
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).
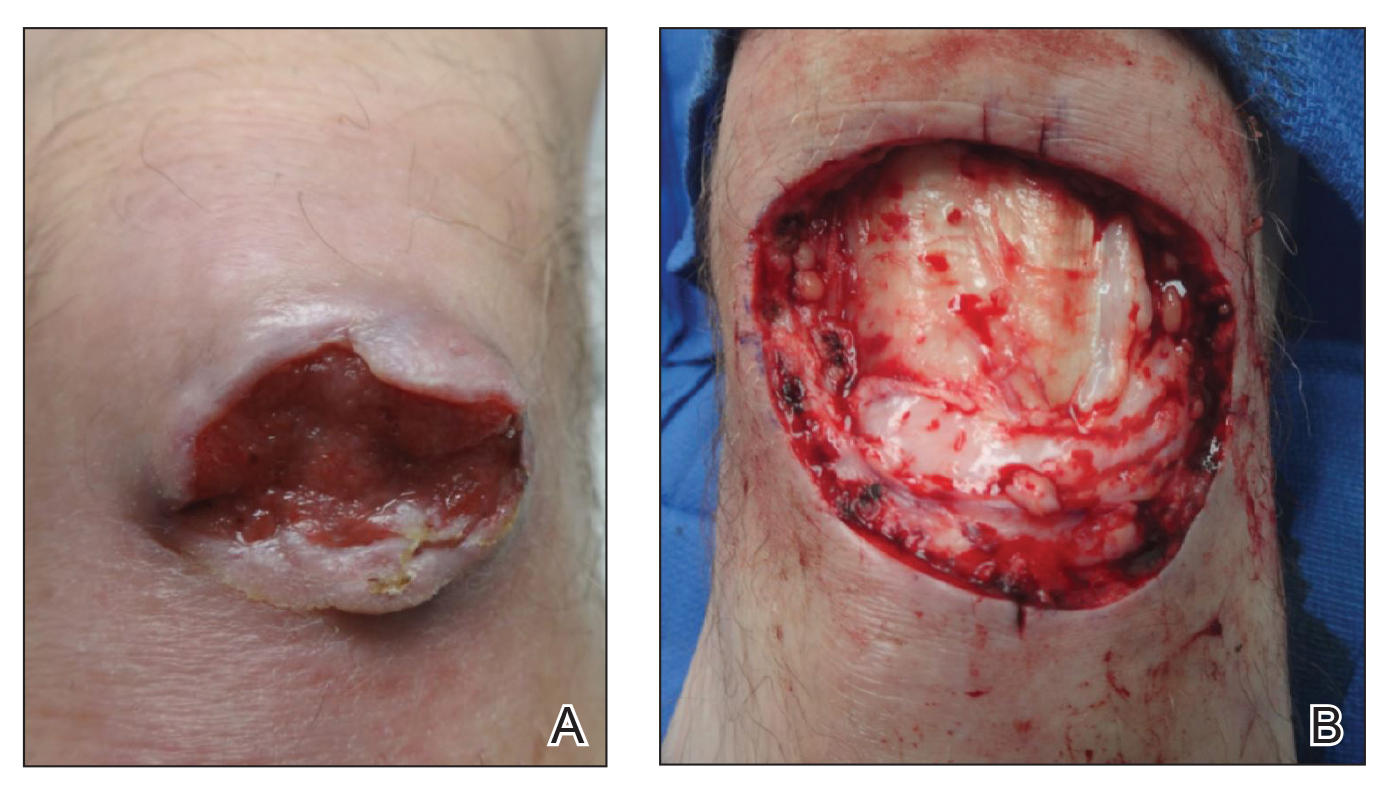
Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).


Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).


Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
Practice Points
- This case highlights an unusual presentation of basal cell carcinoma masquerading as bursitis.
- Clinicians should be aware of confirmation bias, especially when multiple physicians and specialists are involved in a case.
- When the initial clinical impression is not corroborated by objective data or the condition is not responding to conventional therapy, it is important for clinicians to revisit the possibility of an inaccurate diagnosis.
Quantity and Characteristics of Flap or Graft Repairs for Skin Cancer on the Nose or Ears: A Comparison Between Mohs Micrographic Surgery and Plastic Surgery
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.
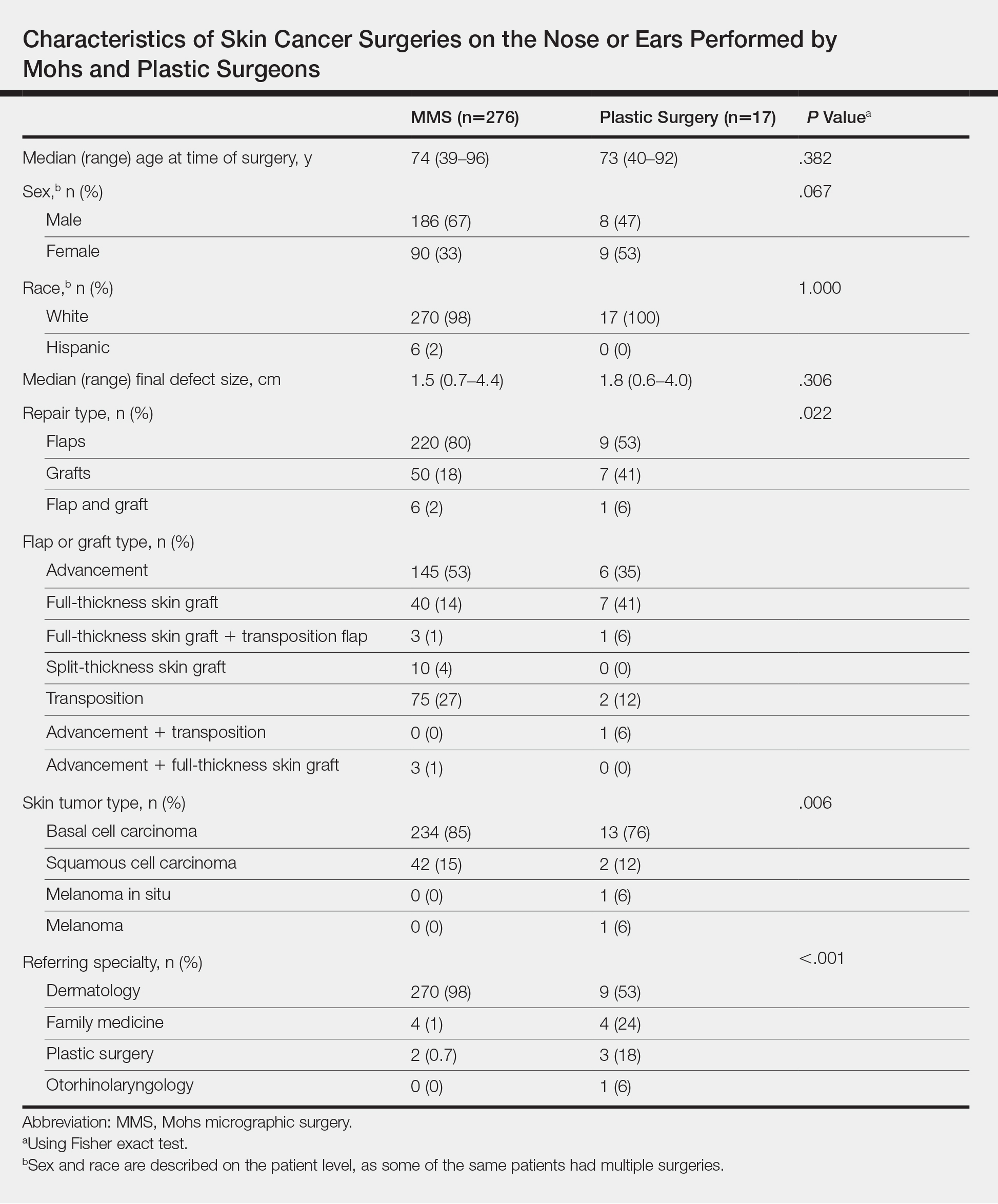
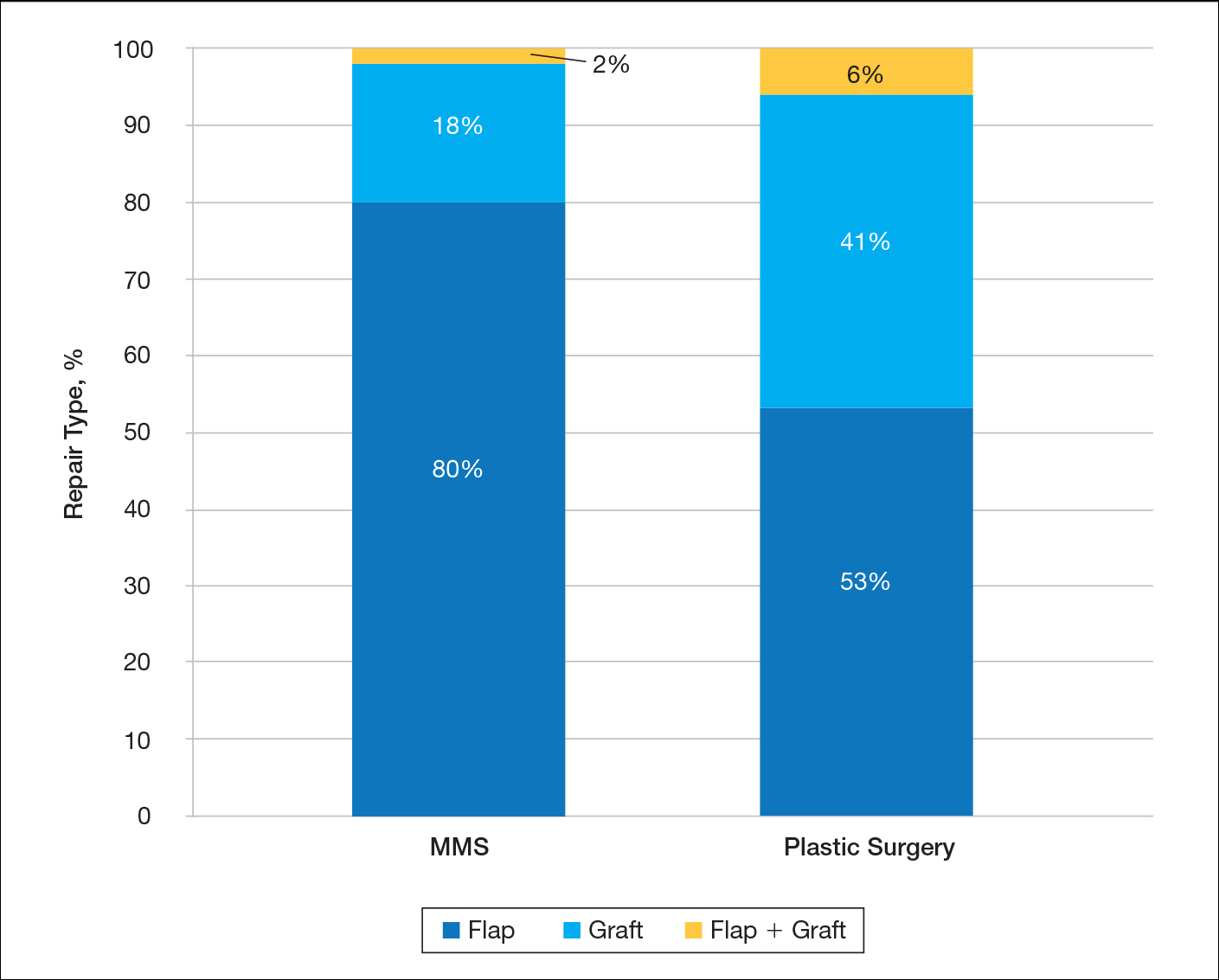
Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.


Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.


Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
Practice Points
- Patients and nondermatologist physicians may be unaware of how frequently Mohs surgeons perform complex surgical repairs compared to other specialists.
- Compared to plastic surgeons, Mohs surgeons performed a larger number of complex skin cancer repairs on the nose or ears with similar-sized defects.
- Primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer through awareness of this type of data.
Dermoscopic Patterns of Acral Melanocytic Lesions in Skin of Color
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
Practice Points
- Dermatologists should be familiar with common dermoscopic patterns seen at acral sites in patients with skin of color as well as the most up-to-date diagnostic algorithms.
- Acral lentiginous melanoma should be strongly suspected if dermoscopy reveals a parallel ridge pattern or if dermoscopy of volar skin reveals a lack of typical dermoscopic patterns in lesions with a diameter greater than 7 mm.