User login
Legal duty to nonpatients: Driving accidents
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
‘Substantial burden’ of enterovirus meningitis in young infants
LJUBLJANA, SLOVENIA – A prospective international surveillance study has provided new insights into the surprisingly substantial clinical burden of viral meningitis caused by enteroviruses and human parechoviruses in young infants, Seilesh Kadambari, MBBS, PhD, said in his ESPID Young Investigator Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
This comprehensive study captured all cases of laboratory-confirmed enterovirus (EV) and human parechovirus (HPeV) meningitis in infants less than 90 days old seen by pediatricians in the United Kingdom and Ireland during a 13-month period starting in July 2014, a time free of outbreaks. Dr. Kadambari, a pediatrician at the University of Oxford (England), was first author of the study. It was for this project, as well as his earlier studies shedding light on congenital viral infections, that he received the Young Investigator honor.
Among the key findings of the U.K./Ireland surveillance study: The incidence of EV/HPeV meningitis was more than twice that of bacterial meningitis in the same age group and more than fivefold higher than that of group B streptococcal meningitis, the No. 1 cause of bacterial meningitis in early infancy. Moreover, more than one-half of infants with EV/HPeV meningitis had low levels of inflammatory markers and no cerebrospinal fluid pleocytosis, which underscores the importance of routinely testing the cerebrospinal fluid for viral causes of meningitis in such patients using modern molecular tools such as multiplex polymerase chain reaction, according to Dr. Kadambari.
“Also, not a single one of the patients with EV/HPeV meningitis had a secondary bacterial infection – and that has important implications for management of our antibiotic stewardship programs,” he observed.
The study (Arch Dis Child. 2019 Jun;104(6):552-7) identified 668 cases of EV meningitis and 35 of HPeV meningitis, for an incidence of 0.79 and 0.04 per 1,000 live births, respectively. The most common clinical presentations were those generally seen in meningitis: fever, irritability, and reduced feeding. Circulatory shock was present in 43% of the infants with HPeV and 27% of the infants with EV infections.
Of infants with EV meningitis, 11% required admission to an intensive care unit, as did 23% of those with HPeV meningitis. Two babies with EV meningitis died and four others had continued neurologic complications at 12 months of follow-up. In contrast, all infants with HPeV survived without long-term sequelae.
Reassuringly, none of the 189 infants who underwent formal hearing testing had sensorineural hearing loss.
The surveillance study data have played an influential role in evidence-based guidelines for EV diagnosis and characterization published by the European Society of Clinical Virology (J Clin Virol. 2018 Apr;101:11-7).
An earlier study led by Dr. Kadambari documented a hefty sevenfold increase in the rate of laboratory-confirmed viral meningo-encephalitis in England and Wales during 2004-2013 across all age groups (J Infect. 2014 Oct;69[4]:326-32).
He attributed this increase to improved diagnosis of viral forms of meningitis through greater use of polymerase chain reaction. The study, based upon National Health Service hospital records, showed that more than 90% of all cases of viral meningo-encephalitis in infants less than 90 days old were caused by EV, a finding that prompted the subsequent prospective U.K./Ireland surveillance study.
Dr. Kadambari closed by noting the past decade had seen a greatly improved ability to diagnose congenital viral infections, but those improvements are not good enough.
“In the decade ahead, we hope to improve the management of this poorly understood group of infections,” the pediatrician promised.
Planned efforts include a cost-effectiveness analysis of a cytomegalovirus vaccine, an ESPID-funded research project aimed at identifying which EV/HPeV strains are most responsible for outbreaks and isolated severe disease, and gaining insight into the host-immunity factors associated with a proclivity to develop EV/HPeV meningitis in early infancy.
Dr. Kadambari reported having no financial conflicts regarding his studies, which was funded largely by Public Health England and university grants.
SOURCE: Kadambari S et al. Arch Dis Child. 2019;104:552-7.
LJUBLJANA, SLOVENIA – A prospective international surveillance study has provided new insights into the surprisingly substantial clinical burden of viral meningitis caused by enteroviruses and human parechoviruses in young infants, Seilesh Kadambari, MBBS, PhD, said in his ESPID Young Investigator Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
This comprehensive study captured all cases of laboratory-confirmed enterovirus (EV) and human parechovirus (HPeV) meningitis in infants less than 90 days old seen by pediatricians in the United Kingdom and Ireland during a 13-month period starting in July 2014, a time free of outbreaks. Dr. Kadambari, a pediatrician at the University of Oxford (England), was first author of the study. It was for this project, as well as his earlier studies shedding light on congenital viral infections, that he received the Young Investigator honor.
Among the key findings of the U.K./Ireland surveillance study: The incidence of EV/HPeV meningitis was more than twice that of bacterial meningitis in the same age group and more than fivefold higher than that of group B streptococcal meningitis, the No. 1 cause of bacterial meningitis in early infancy. Moreover, more than one-half of infants with EV/HPeV meningitis had low levels of inflammatory markers and no cerebrospinal fluid pleocytosis, which underscores the importance of routinely testing the cerebrospinal fluid for viral causes of meningitis in such patients using modern molecular tools such as multiplex polymerase chain reaction, according to Dr. Kadambari.
“Also, not a single one of the patients with EV/HPeV meningitis had a secondary bacterial infection – and that has important implications for management of our antibiotic stewardship programs,” he observed.
The study (Arch Dis Child. 2019 Jun;104(6):552-7) identified 668 cases of EV meningitis and 35 of HPeV meningitis, for an incidence of 0.79 and 0.04 per 1,000 live births, respectively. The most common clinical presentations were those generally seen in meningitis: fever, irritability, and reduced feeding. Circulatory shock was present in 43% of the infants with HPeV and 27% of the infants with EV infections.
Of infants with EV meningitis, 11% required admission to an intensive care unit, as did 23% of those with HPeV meningitis. Two babies with EV meningitis died and four others had continued neurologic complications at 12 months of follow-up. In contrast, all infants with HPeV survived without long-term sequelae.
Reassuringly, none of the 189 infants who underwent formal hearing testing had sensorineural hearing loss.
The surveillance study data have played an influential role in evidence-based guidelines for EV diagnosis and characterization published by the European Society of Clinical Virology (J Clin Virol. 2018 Apr;101:11-7).
An earlier study led by Dr. Kadambari documented a hefty sevenfold increase in the rate of laboratory-confirmed viral meningo-encephalitis in England and Wales during 2004-2013 across all age groups (J Infect. 2014 Oct;69[4]:326-32).
He attributed this increase to improved diagnosis of viral forms of meningitis through greater use of polymerase chain reaction. The study, based upon National Health Service hospital records, showed that more than 90% of all cases of viral meningo-encephalitis in infants less than 90 days old were caused by EV, a finding that prompted the subsequent prospective U.K./Ireland surveillance study.
Dr. Kadambari closed by noting the past decade had seen a greatly improved ability to diagnose congenital viral infections, but those improvements are not good enough.
“In the decade ahead, we hope to improve the management of this poorly understood group of infections,” the pediatrician promised.
Planned efforts include a cost-effectiveness analysis of a cytomegalovirus vaccine, an ESPID-funded research project aimed at identifying which EV/HPeV strains are most responsible for outbreaks and isolated severe disease, and gaining insight into the host-immunity factors associated with a proclivity to develop EV/HPeV meningitis in early infancy.
Dr. Kadambari reported having no financial conflicts regarding his studies, which was funded largely by Public Health England and university grants.
SOURCE: Kadambari S et al. Arch Dis Child. 2019;104:552-7.
LJUBLJANA, SLOVENIA – A prospective international surveillance study has provided new insights into the surprisingly substantial clinical burden of viral meningitis caused by enteroviruses and human parechoviruses in young infants, Seilesh Kadambari, MBBS, PhD, said in his ESPID Young Investigator Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
This comprehensive study captured all cases of laboratory-confirmed enterovirus (EV) and human parechovirus (HPeV) meningitis in infants less than 90 days old seen by pediatricians in the United Kingdom and Ireland during a 13-month period starting in July 2014, a time free of outbreaks. Dr. Kadambari, a pediatrician at the University of Oxford (England), was first author of the study. It was for this project, as well as his earlier studies shedding light on congenital viral infections, that he received the Young Investigator honor.
Among the key findings of the U.K./Ireland surveillance study: The incidence of EV/HPeV meningitis was more than twice that of bacterial meningitis in the same age group and more than fivefold higher than that of group B streptococcal meningitis, the No. 1 cause of bacterial meningitis in early infancy. Moreover, more than one-half of infants with EV/HPeV meningitis had low levels of inflammatory markers and no cerebrospinal fluid pleocytosis, which underscores the importance of routinely testing the cerebrospinal fluid for viral causes of meningitis in such patients using modern molecular tools such as multiplex polymerase chain reaction, according to Dr. Kadambari.
“Also, not a single one of the patients with EV/HPeV meningitis had a secondary bacterial infection – and that has important implications for management of our antibiotic stewardship programs,” he observed.
The study (Arch Dis Child. 2019 Jun;104(6):552-7) identified 668 cases of EV meningitis and 35 of HPeV meningitis, for an incidence of 0.79 and 0.04 per 1,000 live births, respectively. The most common clinical presentations were those generally seen in meningitis: fever, irritability, and reduced feeding. Circulatory shock was present in 43% of the infants with HPeV and 27% of the infants with EV infections.
Of infants with EV meningitis, 11% required admission to an intensive care unit, as did 23% of those with HPeV meningitis. Two babies with EV meningitis died and four others had continued neurologic complications at 12 months of follow-up. In contrast, all infants with HPeV survived without long-term sequelae.
Reassuringly, none of the 189 infants who underwent formal hearing testing had sensorineural hearing loss.
The surveillance study data have played an influential role in evidence-based guidelines for EV diagnosis and characterization published by the European Society of Clinical Virology (J Clin Virol. 2018 Apr;101:11-7).
An earlier study led by Dr. Kadambari documented a hefty sevenfold increase in the rate of laboratory-confirmed viral meningo-encephalitis in England and Wales during 2004-2013 across all age groups (J Infect. 2014 Oct;69[4]:326-32).
He attributed this increase to improved diagnosis of viral forms of meningitis through greater use of polymerase chain reaction. The study, based upon National Health Service hospital records, showed that more than 90% of all cases of viral meningo-encephalitis in infants less than 90 days old were caused by EV, a finding that prompted the subsequent prospective U.K./Ireland surveillance study.
Dr. Kadambari closed by noting the past decade had seen a greatly improved ability to diagnose congenital viral infections, but those improvements are not good enough.
“In the decade ahead, we hope to improve the management of this poorly understood group of infections,” the pediatrician promised.
Planned efforts include a cost-effectiveness analysis of a cytomegalovirus vaccine, an ESPID-funded research project aimed at identifying which EV/HPeV strains are most responsible for outbreaks and isolated severe disease, and gaining insight into the host-immunity factors associated with a proclivity to develop EV/HPeV meningitis in early infancy.
Dr. Kadambari reported having no financial conflicts regarding his studies, which was funded largely by Public Health England and university grants.
SOURCE: Kadambari S et al. Arch Dis Child. 2019;104:552-7.
REPORTING FROM ESPID 2019
FDA approves fedratinib for myelofibrosis
The Food and Drug Administration has approved fedratinib (Inrebic), an oral JAK2/FLT3 inhibitor, to treat myelofibrosis.
Fedratinib is approved to treat adults with intermediate-2 or high-risk primary or secondary (post–polycythemia vera or post–essential thrombocythemia) myelofibrosis.
The prescribing information for fedratinib includes a boxed warning detailing the risk of serious and fatal encephalopathy, including Wernicke’s.
The encephalopathy risk prompted Sanofi to stop developing fedratinib in 2013. The FDA placed a clinical hold on all trials of fedratinib after potential cases of Wernicke’s encephalopathy were observed in eight patients.
The FDA lifted the clinical hold in 2017, and Celgene Corporation decided to develop fedratinib when the company acquired Impact Biomedicines in 2018.
In the phase 3 JAKARTA trial, fedratinib significantly reduced splenomegaly and symptom burden in patients with primary or secondary myelofibrosis (JAMA Oncol. 2015 Aug;1[5]:643-51). In the phase 2 JAKARTA2 trial, fedratinib produced responses in myelofibrosis patients previously treated with ruxolitinib (Lancet Haematol. 2017 Jul;4[7]:e317-e324).
Fedratinib received orphan drug designation from the FDA, and the application for fedratinib received priority review.
The FDA granted approval of fedratinib to Impact Biomedicines, a wholly owned subsidiary of Celgene.
The Food and Drug Administration has approved fedratinib (Inrebic), an oral JAK2/FLT3 inhibitor, to treat myelofibrosis.
Fedratinib is approved to treat adults with intermediate-2 or high-risk primary or secondary (post–polycythemia vera or post–essential thrombocythemia) myelofibrosis.
The prescribing information for fedratinib includes a boxed warning detailing the risk of serious and fatal encephalopathy, including Wernicke’s.
The encephalopathy risk prompted Sanofi to stop developing fedratinib in 2013. The FDA placed a clinical hold on all trials of fedratinib after potential cases of Wernicke’s encephalopathy were observed in eight patients.
The FDA lifted the clinical hold in 2017, and Celgene Corporation decided to develop fedratinib when the company acquired Impact Biomedicines in 2018.
In the phase 3 JAKARTA trial, fedratinib significantly reduced splenomegaly and symptom burden in patients with primary or secondary myelofibrosis (JAMA Oncol. 2015 Aug;1[5]:643-51). In the phase 2 JAKARTA2 trial, fedratinib produced responses in myelofibrosis patients previously treated with ruxolitinib (Lancet Haematol. 2017 Jul;4[7]:e317-e324).
Fedratinib received orphan drug designation from the FDA, and the application for fedratinib received priority review.
The FDA granted approval of fedratinib to Impact Biomedicines, a wholly owned subsidiary of Celgene.
The Food and Drug Administration has approved fedratinib (Inrebic), an oral JAK2/FLT3 inhibitor, to treat myelofibrosis.
Fedratinib is approved to treat adults with intermediate-2 or high-risk primary or secondary (post–polycythemia vera or post–essential thrombocythemia) myelofibrosis.
The prescribing information for fedratinib includes a boxed warning detailing the risk of serious and fatal encephalopathy, including Wernicke’s.
The encephalopathy risk prompted Sanofi to stop developing fedratinib in 2013. The FDA placed a clinical hold on all trials of fedratinib after potential cases of Wernicke’s encephalopathy were observed in eight patients.
The FDA lifted the clinical hold in 2017, and Celgene Corporation decided to develop fedratinib when the company acquired Impact Biomedicines in 2018.
In the phase 3 JAKARTA trial, fedratinib significantly reduced splenomegaly and symptom burden in patients with primary or secondary myelofibrosis (JAMA Oncol. 2015 Aug;1[5]:643-51). In the phase 2 JAKARTA2 trial, fedratinib produced responses in myelofibrosis patients previously treated with ruxolitinib (Lancet Haematol. 2017 Jul;4[7]:e317-e324).
Fedratinib received orphan drug designation from the FDA, and the application for fedratinib received priority review.
The FDA granted approval of fedratinib to Impact Biomedicines, a wholly owned subsidiary of Celgene.
Screening for pancreatic and lung cancers
In this edition of “How I will treat my next patient,” I examine the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement regarding screening for pancreatic cancer in normal-risk populations. I also review newly published information regarding low-dose CT screening (LDCT) for lung cancer in a commonly screened population – individuals with chronic obstructive pulmonary disease (COPD). Both publications highlight the complexity of implementing shared decision making in clinicians’ efforts to find these highly lethal cancers in their earliest, most curable stages.
Pancreatic cancer screening
In their recommendation, the USPSTF considered data relevant to the benefits and harms (exclusive of costs) of screening for pancreatic cancer in the 85%-90% of individuals who are at normal risk because they lack a known familial or genetic syndrome and do not have at least two affected relatives or one first-degree affected relative (JAMA. 2019;322[5]:438-44).
After reviewing 13 cohort studies employing image-based technologies (CT, MRI, endoscopic ultrasound) and biomarkers for screening, the USPSTF reaffirmed its 2004 recommendation against pancreatic cancer screening. They found no new evidence of sufficient strength and quality to alter their previous “D grade” for screening (i.e., “Don’t do it.”). There were at least moderate harms of screening and subsequent treatment in normal-risk populations. These recommendations apply to asymptomatic individuals with new-onset diabetes mellitus, smokers, older adults, obese patients, and patients with a history of chronic pancreatitis.
What this means in practice
In the nicely written and comprehensive recommendation statement and in the two accompanying editorials (JAMA Surg. 2019 Aug 6. doi: 10.1001/jamasurg.2019.2832; JAMA. 2019;322[5]:407-8), the authors were explicit that the “D grade” for pancreatic cancer screening did not apply to individuals from familial pancreatic cancer kindreds and those with germline mutations and Lynch syndrome mismatch repair genes. For them, the relative risk of pancreatic cancer (greater than 5%) may justify the morbidity of available surveillance technologies, especially since U.S. and International screening studies in these high-risk individuals have generated data suggesting a benefit for treatment of screen-detected cancers.
The USPSTF and the editorial authors were strongly supportive of, and enthusiastic about, ongoing research efforts. Recently, a joint effort at the National Institutes of Health began recruiting centers for a study to assess the sensitivity of novel biomarkers in detecting pancreatic cancer among adults with new-onset diabetes (A211701).
To me, it is clear that the pathway to identifying effective screening for pancreatic cancer – which is forecast to become the second leading cause of cancer death in the United States by 2020 – will focus on high-risk populations first, enabling accurate determination of sensitivity and specificity before being applied to the general population. This is as it should be.
Lung cancer screening
Currently, guidelines from the National Comprehensive Cancer Network recommend LDCT screening annually for high-risk smokers, former smokers, and individuals with additional risk factors aged 55-77 years. The National Lung Screening Trial indicated that LDCT screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in a similar population. NCCN guidelines stress the importance of shared decision making and include a table of risks and benefits that should be considered.
Jonathan M. Iaccarino, MD, and colleagues quantified the risks of screening among COPD patients in a secondary analysis of the more than 75,000 LDCT scans that were performed among the more than 26,000 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016). In comparison with participants who did not self-report a diagnosis of COPD, the 4,632 participants with self-reported COPD were significantly more likely to require further diagnostic studies, have an invasive procedure, have a complication of any type from the invasive procedure, and suffer a serious complication. The establishment of a lung cancer diagnosis from the invasive procedure, however, occurred in just 6.1% of COPD patients versus 3.6% of patients without COPD.
What this means in practice
At a consensus conference convened by the National Quality Forum, shared decision making was defined as a process of communication in which clinicians and patients work together to make decisions that align with what matters most to patients. Ideally, shared decision making requires clear, accurate, unbiased medical evidence about reasonable alternatives; tailored evidence for individual patients; and the incorporation of patient values, goals, informed preferences and concerns, including a discussion of treatment burdens. All of us wrestle with the challenge of conducting these conversations in a comprehensive and unbiased manner. I am not sure that I have ever achieved an ideal shared decision-making conversation in my practice.
Despite the limitations acknowledged by the authors – self-reported diagnosis of COPD, outcomes that were not the primary focus of the trial, failure to incorporate other important comorbid conditions – the study by Dr. Iaccarino and colleagues helps to quantify risks and benefits for a commonly screened population, specifically COPD patients. Most importantly, it focuses our attention on the key goal of all cancer-screening efforts – applying our personal and technological resources to patients who benefit the most and will suffer the least harm from our efforts.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I examine the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement regarding screening for pancreatic cancer in normal-risk populations. I also review newly published information regarding low-dose CT screening (LDCT) for lung cancer in a commonly screened population – individuals with chronic obstructive pulmonary disease (COPD). Both publications highlight the complexity of implementing shared decision making in clinicians’ efforts to find these highly lethal cancers in their earliest, most curable stages.
Pancreatic cancer screening
In their recommendation, the USPSTF considered data relevant to the benefits and harms (exclusive of costs) of screening for pancreatic cancer in the 85%-90% of individuals who are at normal risk because they lack a known familial or genetic syndrome and do not have at least two affected relatives or one first-degree affected relative (JAMA. 2019;322[5]:438-44).
After reviewing 13 cohort studies employing image-based technologies (CT, MRI, endoscopic ultrasound) and biomarkers for screening, the USPSTF reaffirmed its 2004 recommendation against pancreatic cancer screening. They found no new evidence of sufficient strength and quality to alter their previous “D grade” for screening (i.e., “Don’t do it.”). There were at least moderate harms of screening and subsequent treatment in normal-risk populations. These recommendations apply to asymptomatic individuals with new-onset diabetes mellitus, smokers, older adults, obese patients, and patients with a history of chronic pancreatitis.
What this means in practice
In the nicely written and comprehensive recommendation statement and in the two accompanying editorials (JAMA Surg. 2019 Aug 6. doi: 10.1001/jamasurg.2019.2832; JAMA. 2019;322[5]:407-8), the authors were explicit that the “D grade” for pancreatic cancer screening did not apply to individuals from familial pancreatic cancer kindreds and those with germline mutations and Lynch syndrome mismatch repair genes. For them, the relative risk of pancreatic cancer (greater than 5%) may justify the morbidity of available surveillance technologies, especially since U.S. and International screening studies in these high-risk individuals have generated data suggesting a benefit for treatment of screen-detected cancers.
The USPSTF and the editorial authors were strongly supportive of, and enthusiastic about, ongoing research efforts. Recently, a joint effort at the National Institutes of Health began recruiting centers for a study to assess the sensitivity of novel biomarkers in detecting pancreatic cancer among adults with new-onset diabetes (A211701).
To me, it is clear that the pathway to identifying effective screening for pancreatic cancer – which is forecast to become the second leading cause of cancer death in the United States by 2020 – will focus on high-risk populations first, enabling accurate determination of sensitivity and specificity before being applied to the general population. This is as it should be.
Lung cancer screening
Currently, guidelines from the National Comprehensive Cancer Network recommend LDCT screening annually for high-risk smokers, former smokers, and individuals with additional risk factors aged 55-77 years. The National Lung Screening Trial indicated that LDCT screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in a similar population. NCCN guidelines stress the importance of shared decision making and include a table of risks and benefits that should be considered.
Jonathan M. Iaccarino, MD, and colleagues quantified the risks of screening among COPD patients in a secondary analysis of the more than 75,000 LDCT scans that were performed among the more than 26,000 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016). In comparison with participants who did not self-report a diagnosis of COPD, the 4,632 participants with self-reported COPD were significantly more likely to require further diagnostic studies, have an invasive procedure, have a complication of any type from the invasive procedure, and suffer a serious complication. The establishment of a lung cancer diagnosis from the invasive procedure, however, occurred in just 6.1% of COPD patients versus 3.6% of patients without COPD.
What this means in practice
At a consensus conference convened by the National Quality Forum, shared decision making was defined as a process of communication in which clinicians and patients work together to make decisions that align with what matters most to patients. Ideally, shared decision making requires clear, accurate, unbiased medical evidence about reasonable alternatives; tailored evidence for individual patients; and the incorporation of patient values, goals, informed preferences and concerns, including a discussion of treatment burdens. All of us wrestle with the challenge of conducting these conversations in a comprehensive and unbiased manner. I am not sure that I have ever achieved an ideal shared decision-making conversation in my practice.
Despite the limitations acknowledged by the authors – self-reported diagnosis of COPD, outcomes that were not the primary focus of the trial, failure to incorporate other important comorbid conditions – the study by Dr. Iaccarino and colleagues helps to quantify risks and benefits for a commonly screened population, specifically COPD patients. Most importantly, it focuses our attention on the key goal of all cancer-screening efforts – applying our personal and technological resources to patients who benefit the most and will suffer the least harm from our efforts.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I examine the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement regarding screening for pancreatic cancer in normal-risk populations. I also review newly published information regarding low-dose CT screening (LDCT) for lung cancer in a commonly screened population – individuals with chronic obstructive pulmonary disease (COPD). Both publications highlight the complexity of implementing shared decision making in clinicians’ efforts to find these highly lethal cancers in their earliest, most curable stages.
Pancreatic cancer screening
In their recommendation, the USPSTF considered data relevant to the benefits and harms (exclusive of costs) of screening for pancreatic cancer in the 85%-90% of individuals who are at normal risk because they lack a known familial or genetic syndrome and do not have at least two affected relatives or one first-degree affected relative (JAMA. 2019;322[5]:438-44).
After reviewing 13 cohort studies employing image-based technologies (CT, MRI, endoscopic ultrasound) and biomarkers for screening, the USPSTF reaffirmed its 2004 recommendation against pancreatic cancer screening. They found no new evidence of sufficient strength and quality to alter their previous “D grade” for screening (i.e., “Don’t do it.”). There were at least moderate harms of screening and subsequent treatment in normal-risk populations. These recommendations apply to asymptomatic individuals with new-onset diabetes mellitus, smokers, older adults, obese patients, and patients with a history of chronic pancreatitis.
What this means in practice
In the nicely written and comprehensive recommendation statement and in the two accompanying editorials (JAMA Surg. 2019 Aug 6. doi: 10.1001/jamasurg.2019.2832; JAMA. 2019;322[5]:407-8), the authors were explicit that the “D grade” for pancreatic cancer screening did not apply to individuals from familial pancreatic cancer kindreds and those with germline mutations and Lynch syndrome mismatch repair genes. For them, the relative risk of pancreatic cancer (greater than 5%) may justify the morbidity of available surveillance technologies, especially since U.S. and International screening studies in these high-risk individuals have generated data suggesting a benefit for treatment of screen-detected cancers.
The USPSTF and the editorial authors were strongly supportive of, and enthusiastic about, ongoing research efforts. Recently, a joint effort at the National Institutes of Health began recruiting centers for a study to assess the sensitivity of novel biomarkers in detecting pancreatic cancer among adults with new-onset diabetes (A211701).
To me, it is clear that the pathway to identifying effective screening for pancreatic cancer – which is forecast to become the second leading cause of cancer death in the United States by 2020 – will focus on high-risk populations first, enabling accurate determination of sensitivity and specificity before being applied to the general population. This is as it should be.
Lung cancer screening
Currently, guidelines from the National Comprehensive Cancer Network recommend LDCT screening annually for high-risk smokers, former smokers, and individuals with additional risk factors aged 55-77 years. The National Lung Screening Trial indicated that LDCT screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in a similar population. NCCN guidelines stress the importance of shared decision making and include a table of risks and benefits that should be considered.
Jonathan M. Iaccarino, MD, and colleagues quantified the risks of screening among COPD patients in a secondary analysis of the more than 75,000 LDCT scans that were performed among the more than 26,000 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016). In comparison with participants who did not self-report a diagnosis of COPD, the 4,632 participants with self-reported COPD were significantly more likely to require further diagnostic studies, have an invasive procedure, have a complication of any type from the invasive procedure, and suffer a serious complication. The establishment of a lung cancer diagnosis from the invasive procedure, however, occurred in just 6.1% of COPD patients versus 3.6% of patients without COPD.
What this means in practice
At a consensus conference convened by the National Quality Forum, shared decision making was defined as a process of communication in which clinicians and patients work together to make decisions that align with what matters most to patients. Ideally, shared decision making requires clear, accurate, unbiased medical evidence about reasonable alternatives; tailored evidence for individual patients; and the incorporation of patient values, goals, informed preferences and concerns, including a discussion of treatment burdens. All of us wrestle with the challenge of conducting these conversations in a comprehensive and unbiased manner. I am not sure that I have ever achieved an ideal shared decision-making conversation in my practice.
Despite the limitations acknowledged by the authors – self-reported diagnosis of COPD, outcomes that were not the primary focus of the trial, failure to incorporate other important comorbid conditions – the study by Dr. Iaccarino and colleagues helps to quantify risks and benefits for a commonly screened population, specifically COPD patients. Most importantly, it focuses our attention on the key goal of all cancer-screening efforts – applying our personal and technological resources to patients who benefit the most and will suffer the least harm from our efforts.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
FDA takes another swing at updating cigarette pack warnings
illustrating the harms of smoking, but this could be subjected to legal challenge.
Several years ago, tobacco companies filed a lawsuit, which ultimately shut down a similar proposal.
The warnings focus on lesser-known complications – including diabetes, cataracts, gangrene, stroke, bladder cancer, erectile dysfunction, and obstructive pulmonary disease – and would take up the top half of the front and back of cigarette packs, and at least the top 20% of print advertisements. Each pack and ad would be required to carry 1 of the 13 proposed warnings, according to the announcement.
The approach would be similar to, but not as aggressive as Canada’s. For years, cigarettes packs sold in Canada have included disturbing photographs of diseased lungs, rotted teeth, and dying patients. The lasting impact of such imagery has been demonstrated in the literature (for example, Am J Prev Med. 2007 Mar;32[3]:202-9).
The new proposal is the FDA’s second attempt to enact something comparable in the United States, after being directed to do so by the Tobacco Control Act of 2009.
The first effort to add strong, illustrated warnings to cigarette packs was widely backed by medical groups, but challenged in the courts by R.J. Reynolds and other tobacco companies, and blocked on appeal in 2012 as an abridgment of commercial free speech. The federal government dropped the case in 2013.
The American Lung Association and other public health groups subsequently sued the FDA in 2016 to enact the Tobacco Act mandate. Subsequently, a federal judge ordered the agency to publish a new rule by August 2019, and issue a final rule in March 2020.
This time around, the FDA “took the necessary time to get these new proposed warnings right ... based on – and within the limits of – both science and the law,” the agency said. The new images, though graphic, are less disturbing than those used in Canada and the agency’s previous proposals, which included an apparent corpse with a sternotomy. The 1-800-Quit-Now cessation hotline number, which was a sticking point in the 2012 ruling, has also been dropped.
When asked about the new efforts, R.J. Reynolds spokesperson Kaelan Hollon said, “We are carefully reviewing FDA’s latest proposal for graphic warnings on cigarettes. We firmly support public awareness of the harms of smoking cigarettes, but the manner in which those messages are delivered to the public cannot run afoul of the First Amendment protections that apply to all speakers, including cigarette manufacturers.”
Warnings on U.S. cigarettes haven’t changed since 1984, when the risks of lung cancer, heart disease, emphysema, and pregnancy complications were added to the side of cigarette packs. With time, the FDA said the surgeon general’s warnings have become “virtually invisible” to consumers.
The American Lung Association, American Academy of Pediatrics, and other plaintiffs in the 2016 suit called the new proposal a “dramatic improvement” over the current situation and “long overdue” in a joint statement on Aug. 15.
Although rates have declined substantially in recent decades, about 34.3 million U.S. adults and almost 1.4 million teenagers still smoke. The habit kills about a half million Americans every year, at a health cost of more than $300 billion, the FDA said.
Comments on the proposed rule are being accepted through Oct. 15. The agency is open to suggestions for alternative text and images.
illustrating the harms of smoking, but this could be subjected to legal challenge.
Several years ago, tobacco companies filed a lawsuit, which ultimately shut down a similar proposal.
The warnings focus on lesser-known complications – including diabetes, cataracts, gangrene, stroke, bladder cancer, erectile dysfunction, and obstructive pulmonary disease – and would take up the top half of the front and back of cigarette packs, and at least the top 20% of print advertisements. Each pack and ad would be required to carry 1 of the 13 proposed warnings, according to the announcement.
The approach would be similar to, but not as aggressive as Canada’s. For years, cigarettes packs sold in Canada have included disturbing photographs of diseased lungs, rotted teeth, and dying patients. The lasting impact of such imagery has been demonstrated in the literature (for example, Am J Prev Med. 2007 Mar;32[3]:202-9).
The new proposal is the FDA’s second attempt to enact something comparable in the United States, after being directed to do so by the Tobacco Control Act of 2009.
The first effort to add strong, illustrated warnings to cigarette packs was widely backed by medical groups, but challenged in the courts by R.J. Reynolds and other tobacco companies, and blocked on appeal in 2012 as an abridgment of commercial free speech. The federal government dropped the case in 2013.
The American Lung Association and other public health groups subsequently sued the FDA in 2016 to enact the Tobacco Act mandate. Subsequently, a federal judge ordered the agency to publish a new rule by August 2019, and issue a final rule in March 2020.
This time around, the FDA “took the necessary time to get these new proposed warnings right ... based on – and within the limits of – both science and the law,” the agency said. The new images, though graphic, are less disturbing than those used in Canada and the agency’s previous proposals, which included an apparent corpse with a sternotomy. The 1-800-Quit-Now cessation hotline number, which was a sticking point in the 2012 ruling, has also been dropped.
When asked about the new efforts, R.J. Reynolds spokesperson Kaelan Hollon said, “We are carefully reviewing FDA’s latest proposal for graphic warnings on cigarettes. We firmly support public awareness of the harms of smoking cigarettes, but the manner in which those messages are delivered to the public cannot run afoul of the First Amendment protections that apply to all speakers, including cigarette manufacturers.”
Warnings on U.S. cigarettes haven’t changed since 1984, when the risks of lung cancer, heart disease, emphysema, and pregnancy complications were added to the side of cigarette packs. With time, the FDA said the surgeon general’s warnings have become “virtually invisible” to consumers.
The American Lung Association, American Academy of Pediatrics, and other plaintiffs in the 2016 suit called the new proposal a “dramatic improvement” over the current situation and “long overdue” in a joint statement on Aug. 15.
Although rates have declined substantially in recent decades, about 34.3 million U.S. adults and almost 1.4 million teenagers still smoke. The habit kills about a half million Americans every year, at a health cost of more than $300 billion, the FDA said.
Comments on the proposed rule are being accepted through Oct. 15. The agency is open to suggestions for alternative text and images.
illustrating the harms of smoking, but this could be subjected to legal challenge.
Several years ago, tobacco companies filed a lawsuit, which ultimately shut down a similar proposal.
The warnings focus on lesser-known complications – including diabetes, cataracts, gangrene, stroke, bladder cancer, erectile dysfunction, and obstructive pulmonary disease – and would take up the top half of the front and back of cigarette packs, and at least the top 20% of print advertisements. Each pack and ad would be required to carry 1 of the 13 proposed warnings, according to the announcement.
The approach would be similar to, but not as aggressive as Canada’s. For years, cigarettes packs sold in Canada have included disturbing photographs of diseased lungs, rotted teeth, and dying patients. The lasting impact of such imagery has been demonstrated in the literature (for example, Am J Prev Med. 2007 Mar;32[3]:202-9).
The new proposal is the FDA’s second attempt to enact something comparable in the United States, after being directed to do so by the Tobacco Control Act of 2009.
The first effort to add strong, illustrated warnings to cigarette packs was widely backed by medical groups, but challenged in the courts by R.J. Reynolds and other tobacco companies, and blocked on appeal in 2012 as an abridgment of commercial free speech. The federal government dropped the case in 2013.
The American Lung Association and other public health groups subsequently sued the FDA in 2016 to enact the Tobacco Act mandate. Subsequently, a federal judge ordered the agency to publish a new rule by August 2019, and issue a final rule in March 2020.
This time around, the FDA “took the necessary time to get these new proposed warnings right ... based on – and within the limits of – both science and the law,” the agency said. The new images, though graphic, are less disturbing than those used in Canada and the agency’s previous proposals, which included an apparent corpse with a sternotomy. The 1-800-Quit-Now cessation hotline number, which was a sticking point in the 2012 ruling, has also been dropped.
When asked about the new efforts, R.J. Reynolds spokesperson Kaelan Hollon said, “We are carefully reviewing FDA’s latest proposal for graphic warnings on cigarettes. We firmly support public awareness of the harms of smoking cigarettes, but the manner in which those messages are delivered to the public cannot run afoul of the First Amendment protections that apply to all speakers, including cigarette manufacturers.”
Warnings on U.S. cigarettes haven’t changed since 1984, when the risks of lung cancer, heart disease, emphysema, and pregnancy complications were added to the side of cigarette packs. With time, the FDA said the surgeon general’s warnings have become “virtually invisible” to consumers.
The American Lung Association, American Academy of Pediatrics, and other plaintiffs in the 2016 suit called the new proposal a “dramatic improvement” over the current situation and “long overdue” in a joint statement on Aug. 15.
Although rates have declined substantially in recent decades, about 34.3 million U.S. adults and almost 1.4 million teenagers still smoke. The habit kills about a half million Americans every year, at a health cost of more than $300 billion, the FDA said.
Comments on the proposed rule are being accepted through Oct. 15. The agency is open to suggestions for alternative text and images.
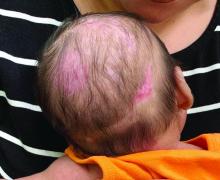
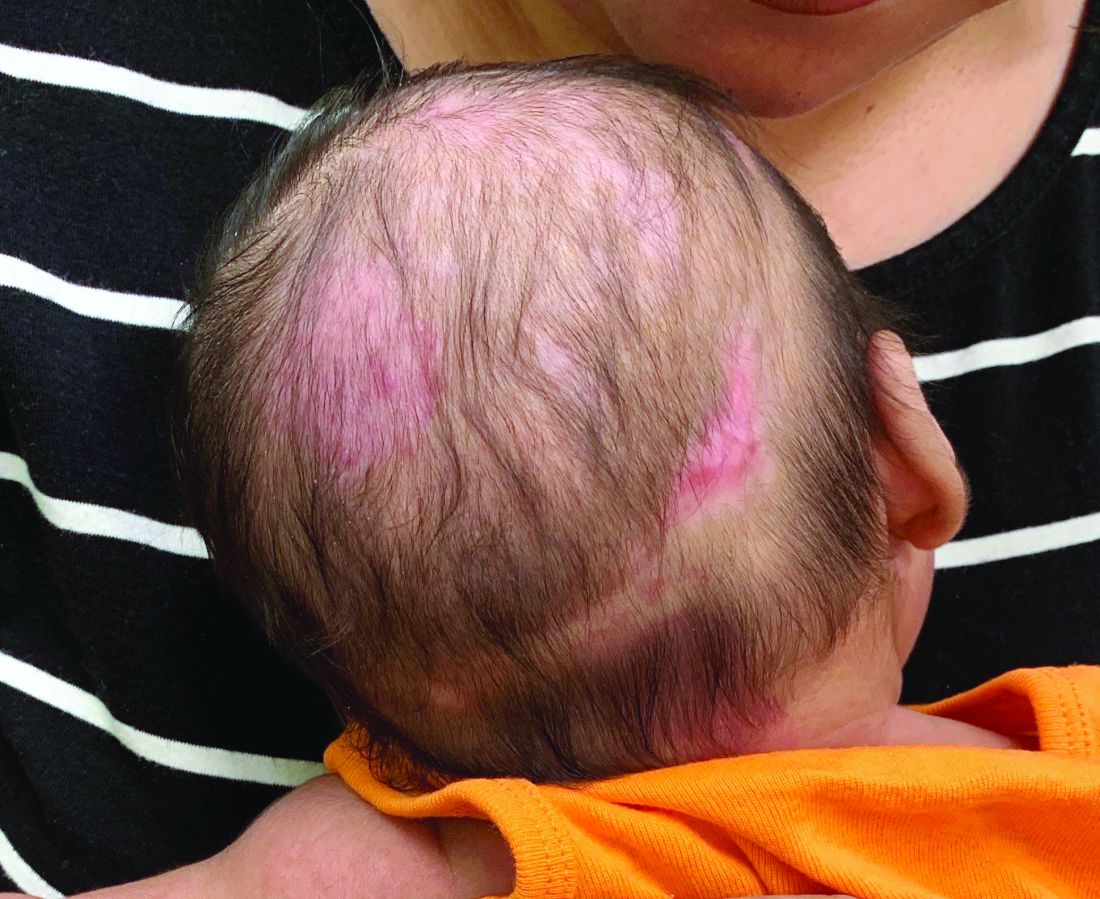
A 2-month-old infant with a scalp rash that appeared after birth
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
A 2-month-old male is referred to our pediatric dermatology clinic for evaluation of persistent seborrheic dermatitis. The mother reports that he presented with a rash on his scalp a few days after birth. She has been treating the crusted areas with clotrimazole and hydrocortisone and has noted improvement on the crusting, but now is worried that there is some scarring. The affected areas are not bleeding or tender. There are no other rashes elsewhere in the body.
He was born at 36 weeks from a 35-year-old gravida 1 para 0 woman with adequate prenatal care. The mother was diagnosed with preeclampsia and was induced. She had a prolonged labor and had premature rupture of membranes. The baby was delivered via cesarean section because of failure to progress and fetal distress; forceps, vacuum, and a scalp probe were not used during delivery. He was admitted to the neonatal unit for 5 days for sepsis work-up and respiratory distress. No intubation was needed.
Besides the preeclampsia, the mother denied any other medical conditions and was not taking any medications. He has met all developmental milestones for his age. He has no history of seizures.
On physical exam, there are semicircular patches of alopecia on the scalp. Some areas have pink, rubbery plaques with loss of hair follicles. On the frontal scalp, there are waxy plaques.
There is a blanchable violaceous patch on the occiput and there are some erythematous papules on the cheeks.
Diabetes targets remain elusive for patients
Some things never change: In 2005, most adults with diabetes missed their treatment targets. In 2016, most adults with diabetes missed their treatment targets. And during that time, from 2005 to 2016, around 96% of men and 94% of women were linked to care.
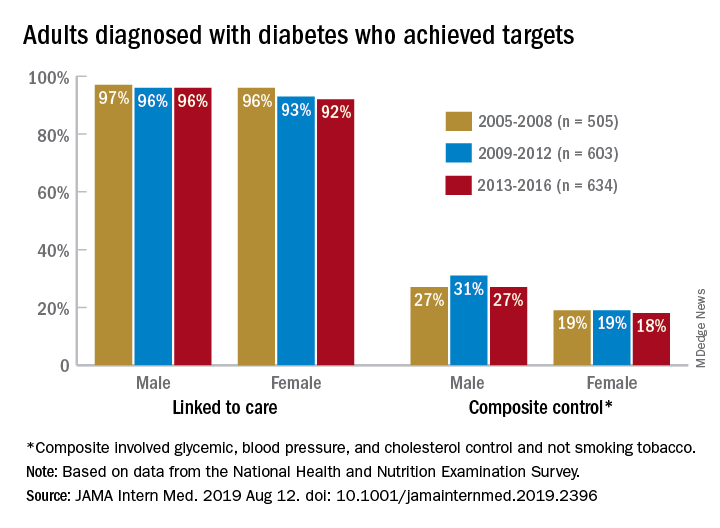
“Fewer than one in four American adults with diagnosed diabetes achieve a controlled level of blood sugar, blood pressure, and cholesterol, and do not smoke tobacco. Our results suggest that, despite major advances in diabetes drug discovery and movement to develop innovative care delivery models over the past two decades, achievement of diabetes care targets has not improved in the United States since 2005,” Pooyan Kazemian, PhD, of Massachusetts General Hospital, Boston, said in a written statement.
During 2013-2016, only 23% of adults with diabetes met a combined composite target of glycemic (HbA1c below a liberal personalized level), blood pressure (less than 140/90 mm Hg), and cholesterol (LDL cholesterol level less than 100 mg/dL) control, as well as not smoking tobacco, Dr. Kazemian and associates reported in JAMA Internal Medicine. The corresponding figures were 25% (2009-2012) and 23% (2005-2008) for the two earlier time periods covered in the study,
The investigators used data for 1,742 nonpregnant adults from the National Health and Nutrition Examination Survey to evaluate the diabetes care cascade, which they defined as “diagnosis, linkage to care, achievement of individual treatment targets, and a composite of all individual targets.”
In 2013-2016, 94% of those diagnosed were linked to care, 64% met their HbA1c target, 70% achieved blood pressure control, 57% met the cholesterol target, and 85% were nonsmokers. When targets were combined, 41% achieved blood pressure and cholesterol control, and 25% met the glycemic, blood pressure, and cholesterol targets, they said.
“We found that none of the U.S. diabetes care variables improved from 2005 to 2016,” Dr. Kazemian and associates noted. Women were less likely than men to meet their treatment goals (see graph) over the course of the study, as were adults aged 18-44 years and black and Hispanic individuals.
“Recent advances in [treatments for diabetes] have not effectively reached the populations at risk and may indicate an immediate need for better approaches to the delivery of diabetes care, including a continued focus on reaching underserved populations with persistent disparities in care,” they wrote.
The study was supported by the Boston Area Diabetes Endocrinology Research Center and Massachusetts General Hospital. One investigator reported that her husband has equity in Apolo1bio. No other disclosures were reported.
SOURCE: Kazemian P et al. JAMA Intern Med. 2019 Aug 12. doi: 10.1001/jamainternmed.2019.2396.
Some things never change: In 2005, most adults with diabetes missed their treatment targets. In 2016, most adults with diabetes missed their treatment targets. And during that time, from 2005 to 2016, around 96% of men and 94% of women were linked to care.

“Fewer than one in four American adults with diagnosed diabetes achieve a controlled level of blood sugar, blood pressure, and cholesterol, and do not smoke tobacco. Our results suggest that, despite major advances in diabetes drug discovery and movement to develop innovative care delivery models over the past two decades, achievement of diabetes care targets has not improved in the United States since 2005,” Pooyan Kazemian, PhD, of Massachusetts General Hospital, Boston, said in a written statement.
During 2013-2016, only 23% of adults with diabetes met a combined composite target of glycemic (HbA1c below a liberal personalized level), blood pressure (less than 140/90 mm Hg), and cholesterol (LDL cholesterol level less than 100 mg/dL) control, as well as not smoking tobacco, Dr. Kazemian and associates reported in JAMA Internal Medicine. The corresponding figures were 25% (2009-2012) and 23% (2005-2008) for the two earlier time periods covered in the study,
The investigators used data for 1,742 nonpregnant adults from the National Health and Nutrition Examination Survey to evaluate the diabetes care cascade, which they defined as “diagnosis, linkage to care, achievement of individual treatment targets, and a composite of all individual targets.”
In 2013-2016, 94% of those diagnosed were linked to care, 64% met their HbA1c target, 70% achieved blood pressure control, 57% met the cholesterol target, and 85% were nonsmokers. When targets were combined, 41% achieved blood pressure and cholesterol control, and 25% met the glycemic, blood pressure, and cholesterol targets, they said.
“We found that none of the U.S. diabetes care variables improved from 2005 to 2016,” Dr. Kazemian and associates noted. Women were less likely than men to meet their treatment goals (see graph) over the course of the study, as were adults aged 18-44 years and black and Hispanic individuals.
“Recent advances in [treatments for diabetes] have not effectively reached the populations at risk and may indicate an immediate need for better approaches to the delivery of diabetes care, including a continued focus on reaching underserved populations with persistent disparities in care,” they wrote.
The study was supported by the Boston Area Diabetes Endocrinology Research Center and Massachusetts General Hospital. One investigator reported that her husband has equity in Apolo1bio. No other disclosures were reported.
SOURCE: Kazemian P et al. JAMA Intern Med. 2019 Aug 12. doi: 10.1001/jamainternmed.2019.2396.
Some things never change: In 2005, most adults with diabetes missed their treatment targets. In 2016, most adults with diabetes missed their treatment targets. And during that time, from 2005 to 2016, around 96% of men and 94% of women were linked to care.

“Fewer than one in four American adults with diagnosed diabetes achieve a controlled level of blood sugar, blood pressure, and cholesterol, and do not smoke tobacco. Our results suggest that, despite major advances in diabetes drug discovery and movement to develop innovative care delivery models over the past two decades, achievement of diabetes care targets has not improved in the United States since 2005,” Pooyan Kazemian, PhD, of Massachusetts General Hospital, Boston, said in a written statement.
During 2013-2016, only 23% of adults with diabetes met a combined composite target of glycemic (HbA1c below a liberal personalized level), blood pressure (less than 140/90 mm Hg), and cholesterol (LDL cholesterol level less than 100 mg/dL) control, as well as not smoking tobacco, Dr. Kazemian and associates reported in JAMA Internal Medicine. The corresponding figures were 25% (2009-2012) and 23% (2005-2008) for the two earlier time periods covered in the study,
The investigators used data for 1,742 nonpregnant adults from the National Health and Nutrition Examination Survey to evaluate the diabetes care cascade, which they defined as “diagnosis, linkage to care, achievement of individual treatment targets, and a composite of all individual targets.”
In 2013-2016, 94% of those diagnosed were linked to care, 64% met their HbA1c target, 70% achieved blood pressure control, 57% met the cholesterol target, and 85% were nonsmokers. When targets were combined, 41% achieved blood pressure and cholesterol control, and 25% met the glycemic, blood pressure, and cholesterol targets, they said.
“We found that none of the U.S. diabetes care variables improved from 2005 to 2016,” Dr. Kazemian and associates noted. Women were less likely than men to meet their treatment goals (see graph) over the course of the study, as were adults aged 18-44 years and black and Hispanic individuals.
“Recent advances in [treatments for diabetes] have not effectively reached the populations at risk and may indicate an immediate need for better approaches to the delivery of diabetes care, including a continued focus on reaching underserved populations with persistent disparities in care,” they wrote.
The study was supported by the Boston Area Diabetes Endocrinology Research Center and Massachusetts General Hospital. One investigator reported that her husband has equity in Apolo1bio. No other disclosures were reported.
SOURCE: Kazemian P et al. JAMA Intern Med. 2019 Aug 12. doi: 10.1001/jamainternmed.2019.2396.
FROM JAMA INTERNAL MEDICINE
USPSTF draft guidance calls for drug use screening
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
FROM THE USPSTF
Lamotrigine-Induced Cutaneous Pseudolymphoma
To the Editor:
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.
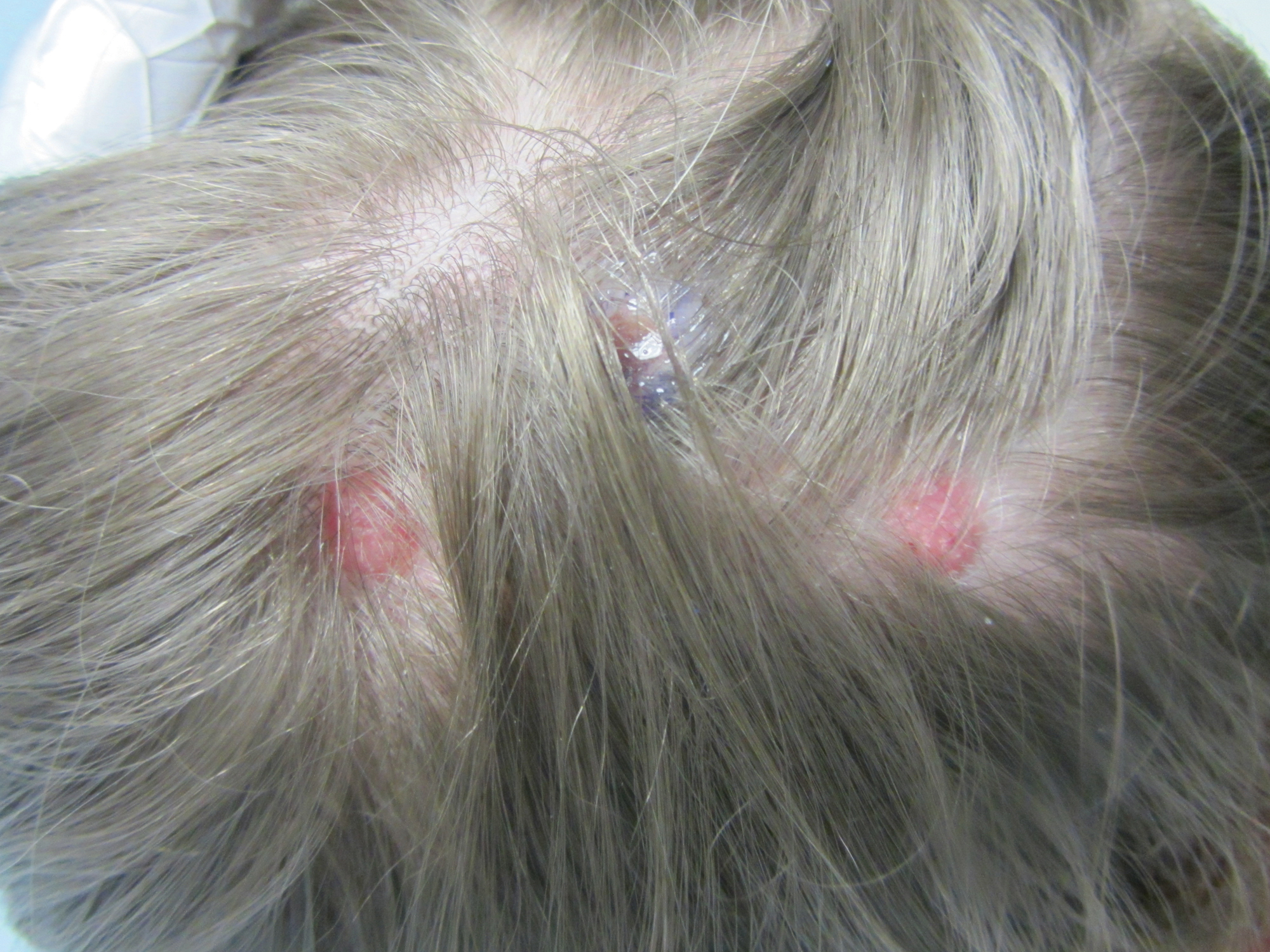
Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).
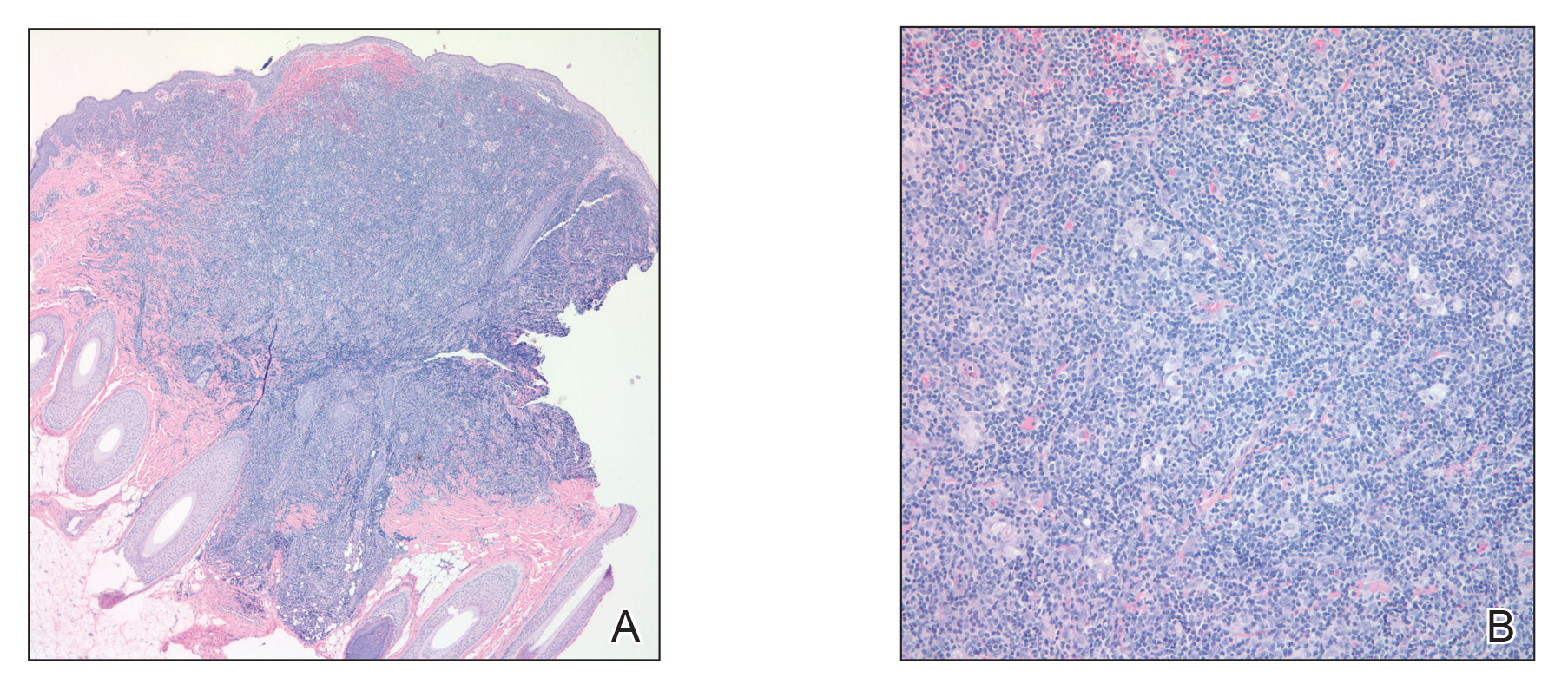
Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2
The clinical appearance of cutaneous pseudolymphoma is variable, ranging from discrete nodules or papules to even confluent erythroderma in certain cases.2 The high clinical variability further complicates diagnosis. Although our patient presented with 9 individual nodular lesions, this finding alone is not sufficient to have high suspicion for cutaneous pseudolymphoma without including a much broader differential diagnosis. In our case, the differential diagnosis also included cutaneous lymphoma, arthropod bite reaction, lymphomatoid papulosis, tumid lupus, follicular mucinosis, lymphocytic infiltrate of Jessner, and leukemia cutis.
The primary concern regarding diagnosis of cutaneous pseudolymphoma is the clinician’s ability to effectively differentiate this entity from a true malignant lymphoma. Immunostaining has some value by identification of heterogeneous cell–type populations with a mixed T-cell and B-cell infiltrate that is more characteristic of a benign reactive process. Subsequent polymerase chain reaction analysis can detect the presence or absence of monoclonal T-cell receptor gene rearrangement or immunoglobulin heavy chain rearrangement.3 If these monoclonal rearrangements are absent, a benign diagnosis is favored; however, these rearrangements also have been shown to exist in a case of cutaneous pseudolymphoma that earned the final diagnosis when removal of the offending agent led to spontaneous lesion regression, similar to our case.4
Many different entities have been described as causative factors for the development of cutaneous pseudolymphoma. Of those that have been considered causative, simple categories have emerged, including endogenous, exogenous, and iatrogenic causes. One potential endogenous etiology of cutaneous pseudolymphoma is IgG4-related disease.5 A multitude of exogenous causes have been reported, including several cases of cutaneous pseudolymphoma developing in a prior tattoo site.6 Viruses, specifically molluscum contagiosum, also have been implicated as exogenous causes, and a report of cutaneous pseudolymphoma development at a prior site of herpes zoster lesions has been described.7 Development of cutaneous pseudolymphoma in vaccination sites also has been reported,8 as well as more obscure inciting events such as Leishmania donovani infection and medicinal leech therapy.9
A considerable number of reported cases of cutaneous pseudolymphoma have been attributed to drugs, including monoclonal antibodies,10 herbal supplements,11 and a multitude of other medications.1 As a class, anticonvulsants are considered more likely to cause lymph node pseudolymphomas than strictly cutaneous pseudolymphomas12; however, many drugs in this class of medications have been described in the development of cutaneous pseudolymphoma.3 A review of the literature by Ploysangam et al1 revealed reports of the development of cutaneous pseudolymphomas after administration of phenytoin, carbamazepine, mephenytoin, trimethadione, phenobarbital, primidone, butabarbital, methsuximide, phensuximide, and valproic acid.
Our patient represents a rare case of strictly cutaneous pseudolymphoma caused by administration of lamotrigine. Our case demonstrated a clear temporal relation between the cessation of lamotrigine and rapid and spontaneous disappearance of cutaneous lesions. We found another case of pseudolymphoma in which lamotrigine was deemed causative, but only lymph node involvement was observed.12
Proper diagnosis of cutaneous pseudolymphoma is important not only with regard to the initial differentiation from true malignant lymphoma but in allowing for appropriate follow-up and vigilant surveillance. Cases of progression from cutaneous pseudolymphoma to true lymphoma have been reported.1,2 It is recommended that watchful follow-up for these patients be carried out until at least 5 years after the diagnosis of cutaneous pseudolymphoma is made to rule out the possibility of malignant transformation, particularly in idiopathic cases.13
- Ploysangam T, Breneman D, Mutasim D. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38:877-898.
- Bergman R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28:568-574.
- Braddock S, Harrington D, Vose J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. J Am Acad Dermatol. 1992;27:337-340.
- Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235-1238.
- Cheuk W, Lee K, Chong L, et al. IgG4-related sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009;33:1713-1719.
- Marchesi A, Parodi P, Brioschi M, et al. Tattoo ink-related cutaneous pseudolymphomas: a rare but significant complication. case report and review of the literature. Aesthetic Plast Surg. 2014;38:471-478.
- Gonzalez J, Sanz A, Martin T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47:502-504.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Altamura D, Calonje E, Liau J, et al. Diffuse cutaneous pseudolymphoma due to therapy with medicinal leeches. JAMA Dermatol. 2014;150:783-784.
- Imafuku S, Ito K, Nakayama J. Cutaneous pseudolymphoma induced by adalimumab and reproduced by infliximab in a patient with arthopathic psoriasis. Br J Dermatol. 2011;166:675-678.
- Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214:94-96.
- Pathak P, McLachlan R. Drug-induced pseudolymphoma secondary to lamotrigine. Neurology. 1998;50:1509-1510.
- Prabu V, Shivani A, Pawar V. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5:224-226.
To the Editor:
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.

Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).

Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2
The clinical appearance of cutaneous pseudolymphoma is variable, ranging from discrete nodules or papules to even confluent erythroderma in certain cases.2 The high clinical variability further complicates diagnosis. Although our patient presented with 9 individual nodular lesions, this finding alone is not sufficient to have high suspicion for cutaneous pseudolymphoma without including a much broader differential diagnosis. In our case, the differential diagnosis also included cutaneous lymphoma, arthropod bite reaction, lymphomatoid papulosis, tumid lupus, follicular mucinosis, lymphocytic infiltrate of Jessner, and leukemia cutis.
The primary concern regarding diagnosis of cutaneous pseudolymphoma is the clinician’s ability to effectively differentiate this entity from a true malignant lymphoma. Immunostaining has some value by identification of heterogeneous cell–type populations with a mixed T-cell and B-cell infiltrate that is more characteristic of a benign reactive process. Subsequent polymerase chain reaction analysis can detect the presence or absence of monoclonal T-cell receptor gene rearrangement or immunoglobulin heavy chain rearrangement.3 If these monoclonal rearrangements are absent, a benign diagnosis is favored; however, these rearrangements also have been shown to exist in a case of cutaneous pseudolymphoma that earned the final diagnosis when removal of the offending agent led to spontaneous lesion regression, similar to our case.4
Many different entities have been described as causative factors for the development of cutaneous pseudolymphoma. Of those that have been considered causative, simple categories have emerged, including endogenous, exogenous, and iatrogenic causes. One potential endogenous etiology of cutaneous pseudolymphoma is IgG4-related disease.5 A multitude of exogenous causes have been reported, including several cases of cutaneous pseudolymphoma developing in a prior tattoo site.6 Viruses, specifically molluscum contagiosum, also have been implicated as exogenous causes, and a report of cutaneous pseudolymphoma development at a prior site of herpes zoster lesions has been described.7 Development of cutaneous pseudolymphoma in vaccination sites also has been reported,8 as well as more obscure inciting events such as Leishmania donovani infection and medicinal leech therapy.9
A considerable number of reported cases of cutaneous pseudolymphoma have been attributed to drugs, including monoclonal antibodies,10 herbal supplements,11 and a multitude of other medications.1 As a class, anticonvulsants are considered more likely to cause lymph node pseudolymphomas than strictly cutaneous pseudolymphomas12; however, many drugs in this class of medications have been described in the development of cutaneous pseudolymphoma.3 A review of the literature by Ploysangam et al1 revealed reports of the development of cutaneous pseudolymphomas after administration of phenytoin, carbamazepine, mephenytoin, trimethadione, phenobarbital, primidone, butabarbital, methsuximide, phensuximide, and valproic acid.
Our patient represents a rare case of strictly cutaneous pseudolymphoma caused by administration of lamotrigine. Our case demonstrated a clear temporal relation between the cessation of lamotrigine and rapid and spontaneous disappearance of cutaneous lesions. We found another case of pseudolymphoma in which lamotrigine was deemed causative, but only lymph node involvement was observed.12
Proper diagnosis of cutaneous pseudolymphoma is important not only with regard to the initial differentiation from true malignant lymphoma but in allowing for appropriate follow-up and vigilant surveillance. Cases of progression from cutaneous pseudolymphoma to true lymphoma have been reported.1,2 It is recommended that watchful follow-up for these patients be carried out until at least 5 years after the diagnosis of cutaneous pseudolymphoma is made to rule out the possibility of malignant transformation, particularly in idiopathic cases.13
To the Editor:
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.

Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).

Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2
The clinical appearance of cutaneous pseudolymphoma is variable, ranging from discrete nodules or papules to even confluent erythroderma in certain cases.2 The high clinical variability further complicates diagnosis. Although our patient presented with 9 individual nodular lesions, this finding alone is not sufficient to have high suspicion for cutaneous pseudolymphoma without including a much broader differential diagnosis. In our case, the differential diagnosis also included cutaneous lymphoma, arthropod bite reaction, lymphomatoid papulosis, tumid lupus, follicular mucinosis, lymphocytic infiltrate of Jessner, and leukemia cutis.
The primary concern regarding diagnosis of cutaneous pseudolymphoma is the clinician’s ability to effectively differentiate this entity from a true malignant lymphoma. Immunostaining has some value by identification of heterogeneous cell–type populations with a mixed T-cell and B-cell infiltrate that is more characteristic of a benign reactive process. Subsequent polymerase chain reaction analysis can detect the presence or absence of monoclonal T-cell receptor gene rearrangement or immunoglobulin heavy chain rearrangement.3 If these monoclonal rearrangements are absent, a benign diagnosis is favored; however, these rearrangements also have been shown to exist in a case of cutaneous pseudolymphoma that earned the final diagnosis when removal of the offending agent led to spontaneous lesion regression, similar to our case.4
Many different entities have been described as causative factors for the development of cutaneous pseudolymphoma. Of those that have been considered causative, simple categories have emerged, including endogenous, exogenous, and iatrogenic causes. One potential endogenous etiology of cutaneous pseudolymphoma is IgG4-related disease.5 A multitude of exogenous causes have been reported, including several cases of cutaneous pseudolymphoma developing in a prior tattoo site.6 Viruses, specifically molluscum contagiosum, also have been implicated as exogenous causes, and a report of cutaneous pseudolymphoma development at a prior site of herpes zoster lesions has been described.7 Development of cutaneous pseudolymphoma in vaccination sites also has been reported,8 as well as more obscure inciting events such as Leishmania donovani infection and medicinal leech therapy.9
A considerable number of reported cases of cutaneous pseudolymphoma have been attributed to drugs, including monoclonal antibodies,10 herbal supplements,11 and a multitude of other medications.1 As a class, anticonvulsants are considered more likely to cause lymph node pseudolymphomas than strictly cutaneous pseudolymphomas12; however, many drugs in this class of medications have been described in the development of cutaneous pseudolymphoma.3 A review of the literature by Ploysangam et al1 revealed reports of the development of cutaneous pseudolymphomas after administration of phenytoin, carbamazepine, mephenytoin, trimethadione, phenobarbital, primidone, butabarbital, methsuximide, phensuximide, and valproic acid.
Our patient represents a rare case of strictly cutaneous pseudolymphoma caused by administration of lamotrigine. Our case demonstrated a clear temporal relation between the cessation of lamotrigine and rapid and spontaneous disappearance of cutaneous lesions. We found another case of pseudolymphoma in which lamotrigine was deemed causative, but only lymph node involvement was observed.12
Proper diagnosis of cutaneous pseudolymphoma is important not only with regard to the initial differentiation from true malignant lymphoma but in allowing for appropriate follow-up and vigilant surveillance. Cases of progression from cutaneous pseudolymphoma to true lymphoma have been reported.1,2 It is recommended that watchful follow-up for these patients be carried out until at least 5 years after the diagnosis of cutaneous pseudolymphoma is made to rule out the possibility of malignant transformation, particularly in idiopathic cases.13
- Ploysangam T, Breneman D, Mutasim D. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38:877-898.
- Bergman R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28:568-574.
- Braddock S, Harrington D, Vose J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. J Am Acad Dermatol. 1992;27:337-340.
- Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235-1238.
- Cheuk W, Lee K, Chong L, et al. IgG4-related sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009;33:1713-1719.
- Marchesi A, Parodi P, Brioschi M, et al. Tattoo ink-related cutaneous pseudolymphomas: a rare but significant complication. case report and review of the literature. Aesthetic Plast Surg. 2014;38:471-478.
- Gonzalez J, Sanz A, Martin T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47:502-504.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Altamura D, Calonje E, Liau J, et al. Diffuse cutaneous pseudolymphoma due to therapy with medicinal leeches. JAMA Dermatol. 2014;150:783-784.
- Imafuku S, Ito K, Nakayama J. Cutaneous pseudolymphoma induced by adalimumab and reproduced by infliximab in a patient with arthopathic psoriasis. Br J Dermatol. 2011;166:675-678.
- Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214:94-96.
- Pathak P, McLachlan R. Drug-induced pseudolymphoma secondary to lamotrigine. Neurology. 1998;50:1509-1510.
- Prabu V, Shivani A, Pawar V. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5:224-226.
- Ploysangam T, Breneman D, Mutasim D. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38:877-898.
- Bergman R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28:568-574.
- Braddock S, Harrington D, Vose J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. J Am Acad Dermatol. 1992;27:337-340.
- Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235-1238.
- Cheuk W, Lee K, Chong L, et al. IgG4-related sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009;33:1713-1719.
- Marchesi A, Parodi P, Brioschi M, et al. Tattoo ink-related cutaneous pseudolymphomas: a rare but significant complication. case report and review of the literature. Aesthetic Plast Surg. 2014;38:471-478.
- Gonzalez J, Sanz A, Martin T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47:502-504.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Altamura D, Calonje E, Liau J, et al. Diffuse cutaneous pseudolymphoma due to therapy with medicinal leeches. JAMA Dermatol. 2014;150:783-784.
- Imafuku S, Ito K, Nakayama J. Cutaneous pseudolymphoma induced by adalimumab and reproduced by infliximab in a patient with arthopathic psoriasis. Br J Dermatol. 2011;166:675-678.
- Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214:94-96.
- Pathak P, McLachlan R. Drug-induced pseudolymphoma secondary to lamotrigine. Neurology. 1998;50:1509-1510.
- Prabu V, Shivani A, Pawar V. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5:224-226.
Practice Points
- Cutaneous pseudolymphomas are a heterogenous group of benign T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.
- Cutaneous pseudolymphomas have many causative factors, including medications, infections, tattoo ink, vaccinations, and insect bites.
- Lamotrigine is a potential inciting factor of cutaneous pseudolymphoma.
Social determinants of health gaining prominence
Fragmented, essentializing, simplistic. That’s how students at Perelman School of Medicine at the University of Pennsylvania, Philadelphia, described their required course on cultural competence. Lectures and discussions about cultural groups and communication issues weren’t providing them with the skills they needed to navigate doctor-patient relationships.
Their criticism was a wake-up call that Horace Delisser, MD, associate dean for diversity and inclusion at the school, took to heart. He enlisted medical students to help reinvent the curriculum. The result, Introduction to Medicine and Society, launched in 2013 and described in an article published in 2017 (Acad Med. 2017;92[3]:335-43), emphasizes self-awareness and reflection about one’s own biases and the adoption of a less hierarchical and more respectful “other-oriented” approach to the patient relationship.
The course examines social determinants of health (SDHs) – the influences of society, government, culture, and health systems. Students analyze how health and health outcomes are affected by a patient’s income, education, and living and working conditions, as well as access to healthy food, safe water, and transportation.
A host of policy makers, advisory groups, and organized medicine groups have called in recent years for educational efforts to boost all physicians’ working knowledge of health inequities and SDHs.
Dr. Delisser, associate professor of medicine who also practices as a pulmonologist at the Harron Lung Center in the Perelman Center for Advanced Medicine, said SDHs play into daily care.
Consider the patient who is chronically late for appointments. “It may not be an issue of the patient being disinterested in their health care, but maybe the public transportation system is unreliable, or maybe the patient has to take two buses and a subway to get there. I need [this knowledge] to inform my care and to engage my patient. I need to know, ‘what does it take for you to get here?’ That factors into how I [make the care plan],” said Dr. Delisser.
Malika Fair, MD, MPH, who teaches a longitudinal professional development class at George Washington University, Washington, and is senior director of health equity partnerships and programs at the American Association of Medical Colleges, provided the example of how her medical students intervened during their rotation in the emergency department on behalf of a newly-diagnosed patient with diabetes who had been unable to fill a prescribed medication. After determining where the patient lived, the students ensured that she had transportation and was able to get the needed medication at a local grocery store. They asked about her barriers to healthy eating, researched local grocery stores, and made practical recommendations that the patient was amenable to implementing. They identified a clinic closer to the patient’s home, and worked with her on making an appointment at a time when she could take off from work.
“Because of their training, these students were able to identify and address social risks in their first month on the ward,” said Dr. Fair, who also practices emergency medicine. They had learned about how to ask about food access and how safe it was for the patient to walk and exercise in her neighborhood.
At Perelman, most students work in student-led community clinics, and some fourth-year students participate in an elective rotation as apprentices to community health workers, learning to address SDHs and develop the cultural humility that they learned about in the classroom. The rotation was similarly created in 2013 and is described in a 2018 article (J Health Care Poor Underserved. 2018;29[2]:581-90).“Being a good physician involves being technically competent as well as what I call relationally competent,” Dr. Delisser said. “And [this involves] being aware that my relationship with a patient doesn’t exist in a vacuum ... that there’s a bigger, broader social and structural context that I need to know and understand. I [then need] to use that to inform how I mediate and empower that relationship.”
Aletha Maybank, MD, who became the American Medical Association’s first chief health equity officer earlier this year, explained that “the medical profession had a very strong social context at one point in time,” but this was dampened by the Flexner Report of 1910.*
The report revolutionized medical education by increasing its rigor, but “it was really focused on clinical and basic science and took out the social context, the context of what medicine is about,” said Dr. Maybank, a pediatrician with a board certification in preventive medicine/public health. “[Now] we’re asking, how do we revolutionize medical education again at this point in time, recognizing the confluence of information and data that we now have available to us about inequities and disparities ... and the sense of urgency from students.”
Students driving practice change
Students nationally are “the most important” drivers of the increasing focus on SDHs in medical education, according to Dr. Fair. “They are demanding experiences to learn about the entire patient. We know that only 20% of a patient’s health is dependent on their health care. Our students are demanding education about the other 80%.”
More and more, communities are identifying needs and “students will then come up with initiatives to meet those needs,” Dr. Fair said.
Others interviewed for this story predicted this trend will only intensify, since not-for-profit hospitals are required under the Affordable Care Act regulations to assess community health needs every few years and to intervene accordingly.
Education on health care systems is also advancing. Penn State University, for instance, utilized a million-dollar grant from the AMA’s Accelerating Change in Medical Education initiative to design and implement a 4-year curriculum on the health system sciences that started in 2014. The curriculum includes an immersive experience in patient navigation.
“Students were taught to be patient navigators, and they were assigned within the clinical context to work on issues like, why are [patients] having trouble getting their medications?” said Susan E. Skochelak, MD, MPH, who leads the 6-year-old Accelerating Change initiative as vice president for medical education at the AMA.
From the start, she noted, students at Penn State are encouraged to question inequities, social and structural barriers to health, and faults in the health care system. “The message given at their white coat ceremony is ‘Welcome to medicine. Now that you’re here, you’re a member of the health care team, and we want you to speak up if you think there are things that need to be addressed. We want you to tell us when the system is working and not working,’ ” said Dr Skochelak, who previously served as the senior associate dean for academic affairs at the University of Wisconsin School of Medicine and Public Health, where she had been a tenured professor of family medicine.
Tomorrow’s physician partners
Approximately 80% of medical school graduates who participated in the AAMC’s 2018 survey of graduates said they had received significant training on health disparities—up from 71% in 2014.
“There’s a huge amount [of innovation] happening, but on the flip side, there’s not really a set of accepted tools and practices, and certainly no robust evaluation [of the training],” said Philip M. Alberti, PhD, senior director for health equity research and policy at the American Association of Medical Colleges. A recently published review (J Gen Intern Med. 2019;34[5]:720-30) shows growing interest in the teaching of SDHs in undergraduate medical education but variable content, strategies, and instructional practices.
Health care systems and practicing physicians are still very much feeling their way with SDHs. Screening tools are being developed and tested, and academic medical centers are trying to determine their roles in addressing issues such as transportation and housing – and what funding and structural levers can be pulled to fulfill these roles. “As we learn more about [these issues], it will become clearer what the right baseline set of competencies might be for all physicians,” Dr. Alberti noted.
In the meantime, some basic expectations for medical education are taking root officially. The National Board of Medical Examiners, with whom the AMA has partnered in its Accelerating Change initiative, has included questions in the United States Medical Licensing Examination on population health and SDHs, and plans to add more exam content on these topics and on health systems science, said Dr. Skochelak.
And through its site visit program (the Clinical Learning Environment Review program), the Accreditation Council for Graduate Medical Education has “made it pretty clear that there’s an expectation that residents and fellows are learning about the health system’s approach to identifying and addressing health care disparities – and that they’re given opportunities to develop quality improvement initiatives that target those disparities,” Dr. Alberti said.
In hopes of achieving consistency across medical specialties and in national accreditation and board certifications exams, the American Association of Medical Colleges is developing its first set of competencies in quality improvement and patient safety, with health equity being one of these competencies’ domains .
The competencies are tiered for medical school graduates, residency graduates, and faculty physicians who are 3-5 years post residency. At this point in time, said Dr. Alberti, the consensus among medical educators has been that physicians “need to be able to understand and consider [social, economic, and structural] contexts when they’re seeing patients, when they’re developing care plans, when they’re talking with caregivers, and when they’re looking at their own quality data.”
Elisabeth Poorman, MD, MPH, an internist at UW Medicine in Kent, Washington, said she worries that the passion of medical students for SDHs will too often be crushed, especially during residency and with immersion in the productivity-focused health care system. Studies show a drop in mental wellness and empathy and a rise in cynicism as training advances, said Dr. Poorman, who also writes about health care and issues of equity and serves on the editorial advisory board of Internal Medicine News.
With similar concerns, the AMA has recently launched a “Reimagining Residency” initiative that aims to improve transitions from medical school to residency and the wellness of residents and faculty, and expand educational content relating to SDHs.
Dr. Fair is optimistic that new physicians’ knowledge of SDHs will permeate medical practices.
“Physicians who are out practicing are going to be working with our graduates, and they’re going to be asking in [job] interviews, do you have flexible hours for patients? What community partnerships do you have? Are there other professionals on staff to help us address social determinants of health? What data [relating to SDHs] are you collecting?” she said.
Correction, 8/26/2019: An earlier version of this story misstated the title of Aletha Maybank, MD. Dr. Maybank's correct title is the first chief health equity officer of the American Medical Association.
Fragmented, essentializing, simplistic. That’s how students at Perelman School of Medicine at the University of Pennsylvania, Philadelphia, described their required course on cultural competence. Lectures and discussions about cultural groups and communication issues weren’t providing them with the skills they needed to navigate doctor-patient relationships.
Their criticism was a wake-up call that Horace Delisser, MD, associate dean for diversity and inclusion at the school, took to heart. He enlisted medical students to help reinvent the curriculum. The result, Introduction to Medicine and Society, launched in 2013 and described in an article published in 2017 (Acad Med. 2017;92[3]:335-43), emphasizes self-awareness and reflection about one’s own biases and the adoption of a less hierarchical and more respectful “other-oriented” approach to the patient relationship.
The course examines social determinants of health (SDHs) – the influences of society, government, culture, and health systems. Students analyze how health and health outcomes are affected by a patient’s income, education, and living and working conditions, as well as access to healthy food, safe water, and transportation.
A host of policy makers, advisory groups, and organized medicine groups have called in recent years for educational efforts to boost all physicians’ working knowledge of health inequities and SDHs.
Dr. Delisser, associate professor of medicine who also practices as a pulmonologist at the Harron Lung Center in the Perelman Center for Advanced Medicine, said SDHs play into daily care.
Consider the patient who is chronically late for appointments. “It may not be an issue of the patient being disinterested in their health care, but maybe the public transportation system is unreliable, or maybe the patient has to take two buses and a subway to get there. I need [this knowledge] to inform my care and to engage my patient. I need to know, ‘what does it take for you to get here?’ That factors into how I [make the care plan],” said Dr. Delisser.
Malika Fair, MD, MPH, who teaches a longitudinal professional development class at George Washington University, Washington, and is senior director of health equity partnerships and programs at the American Association of Medical Colleges, provided the example of how her medical students intervened during their rotation in the emergency department on behalf of a newly-diagnosed patient with diabetes who had been unable to fill a prescribed medication. After determining where the patient lived, the students ensured that she had transportation and was able to get the needed medication at a local grocery store. They asked about her barriers to healthy eating, researched local grocery stores, and made practical recommendations that the patient was amenable to implementing. They identified a clinic closer to the patient’s home, and worked with her on making an appointment at a time when she could take off from work.
“Because of their training, these students were able to identify and address social risks in their first month on the ward,” said Dr. Fair, who also practices emergency medicine. They had learned about how to ask about food access and how safe it was for the patient to walk and exercise in her neighborhood.
At Perelman, most students work in student-led community clinics, and some fourth-year students participate in an elective rotation as apprentices to community health workers, learning to address SDHs and develop the cultural humility that they learned about in the classroom. The rotation was similarly created in 2013 and is described in a 2018 article (J Health Care Poor Underserved. 2018;29[2]:581-90).“Being a good physician involves being technically competent as well as what I call relationally competent,” Dr. Delisser said. “And [this involves] being aware that my relationship with a patient doesn’t exist in a vacuum ... that there’s a bigger, broader social and structural context that I need to know and understand. I [then need] to use that to inform how I mediate and empower that relationship.”
Aletha Maybank, MD, who became the American Medical Association’s first chief health equity officer earlier this year, explained that “the medical profession had a very strong social context at one point in time,” but this was dampened by the Flexner Report of 1910.*
The report revolutionized medical education by increasing its rigor, but “it was really focused on clinical and basic science and took out the social context, the context of what medicine is about,” said Dr. Maybank, a pediatrician with a board certification in preventive medicine/public health. “[Now] we’re asking, how do we revolutionize medical education again at this point in time, recognizing the confluence of information and data that we now have available to us about inequities and disparities ... and the sense of urgency from students.”
Students driving practice change
Students nationally are “the most important” drivers of the increasing focus on SDHs in medical education, according to Dr. Fair. “They are demanding experiences to learn about the entire patient. We know that only 20% of a patient’s health is dependent on their health care. Our students are demanding education about the other 80%.”
More and more, communities are identifying needs and “students will then come up with initiatives to meet those needs,” Dr. Fair said.
Others interviewed for this story predicted this trend will only intensify, since not-for-profit hospitals are required under the Affordable Care Act regulations to assess community health needs every few years and to intervene accordingly.
Education on health care systems is also advancing. Penn State University, for instance, utilized a million-dollar grant from the AMA’s Accelerating Change in Medical Education initiative to design and implement a 4-year curriculum on the health system sciences that started in 2014. The curriculum includes an immersive experience in patient navigation.
“Students were taught to be patient navigators, and they were assigned within the clinical context to work on issues like, why are [patients] having trouble getting their medications?” said Susan E. Skochelak, MD, MPH, who leads the 6-year-old Accelerating Change initiative as vice president for medical education at the AMA.
From the start, she noted, students at Penn State are encouraged to question inequities, social and structural barriers to health, and faults in the health care system. “The message given at their white coat ceremony is ‘Welcome to medicine. Now that you’re here, you’re a member of the health care team, and we want you to speak up if you think there are things that need to be addressed. We want you to tell us when the system is working and not working,’ ” said Dr Skochelak, who previously served as the senior associate dean for academic affairs at the University of Wisconsin School of Medicine and Public Health, where she had been a tenured professor of family medicine.
Tomorrow’s physician partners
Approximately 80% of medical school graduates who participated in the AAMC’s 2018 survey of graduates said they had received significant training on health disparities—up from 71% in 2014.
“There’s a huge amount [of innovation] happening, but on the flip side, there’s not really a set of accepted tools and practices, and certainly no robust evaluation [of the training],” said Philip M. Alberti, PhD, senior director for health equity research and policy at the American Association of Medical Colleges. A recently published review (J Gen Intern Med. 2019;34[5]:720-30) shows growing interest in the teaching of SDHs in undergraduate medical education but variable content, strategies, and instructional practices.
Health care systems and practicing physicians are still very much feeling their way with SDHs. Screening tools are being developed and tested, and academic medical centers are trying to determine their roles in addressing issues such as transportation and housing – and what funding and structural levers can be pulled to fulfill these roles. “As we learn more about [these issues], it will become clearer what the right baseline set of competencies might be for all physicians,” Dr. Alberti noted.
In the meantime, some basic expectations for medical education are taking root officially. The National Board of Medical Examiners, with whom the AMA has partnered in its Accelerating Change initiative, has included questions in the United States Medical Licensing Examination on population health and SDHs, and plans to add more exam content on these topics and on health systems science, said Dr. Skochelak.
And through its site visit program (the Clinical Learning Environment Review program), the Accreditation Council for Graduate Medical Education has “made it pretty clear that there’s an expectation that residents and fellows are learning about the health system’s approach to identifying and addressing health care disparities – and that they’re given opportunities to develop quality improvement initiatives that target those disparities,” Dr. Alberti said.
In hopes of achieving consistency across medical specialties and in national accreditation and board certifications exams, the American Association of Medical Colleges is developing its first set of competencies in quality improvement and patient safety, with health equity being one of these competencies’ domains .
The competencies are tiered for medical school graduates, residency graduates, and faculty physicians who are 3-5 years post residency. At this point in time, said Dr. Alberti, the consensus among medical educators has been that physicians “need to be able to understand and consider [social, economic, and structural] contexts when they’re seeing patients, when they’re developing care plans, when they’re talking with caregivers, and when they’re looking at their own quality data.”
Elisabeth Poorman, MD, MPH, an internist at UW Medicine in Kent, Washington, said she worries that the passion of medical students for SDHs will too often be crushed, especially during residency and with immersion in the productivity-focused health care system. Studies show a drop in mental wellness and empathy and a rise in cynicism as training advances, said Dr. Poorman, who also writes about health care and issues of equity and serves on the editorial advisory board of Internal Medicine News.
With similar concerns, the AMA has recently launched a “Reimagining Residency” initiative that aims to improve transitions from medical school to residency and the wellness of residents and faculty, and expand educational content relating to SDHs.
Dr. Fair is optimistic that new physicians’ knowledge of SDHs will permeate medical practices.
“Physicians who are out practicing are going to be working with our graduates, and they’re going to be asking in [job] interviews, do you have flexible hours for patients? What community partnerships do you have? Are there other professionals on staff to help us address social determinants of health? What data [relating to SDHs] are you collecting?” she said.
Correction, 8/26/2019: An earlier version of this story misstated the title of Aletha Maybank, MD. Dr. Maybank's correct title is the first chief health equity officer of the American Medical Association.
Fragmented, essentializing, simplistic. That’s how students at Perelman School of Medicine at the University of Pennsylvania, Philadelphia, described their required course on cultural competence. Lectures and discussions about cultural groups and communication issues weren’t providing them with the skills they needed to navigate doctor-patient relationships.
Their criticism was a wake-up call that Horace Delisser, MD, associate dean for diversity and inclusion at the school, took to heart. He enlisted medical students to help reinvent the curriculum. The result, Introduction to Medicine and Society, launched in 2013 and described in an article published in 2017 (Acad Med. 2017;92[3]:335-43), emphasizes self-awareness and reflection about one’s own biases and the adoption of a less hierarchical and more respectful “other-oriented” approach to the patient relationship.
The course examines social determinants of health (SDHs) – the influences of society, government, culture, and health systems. Students analyze how health and health outcomes are affected by a patient’s income, education, and living and working conditions, as well as access to healthy food, safe water, and transportation.
A host of policy makers, advisory groups, and organized medicine groups have called in recent years for educational efforts to boost all physicians’ working knowledge of health inequities and SDHs.
Dr. Delisser, associate professor of medicine who also practices as a pulmonologist at the Harron Lung Center in the Perelman Center for Advanced Medicine, said SDHs play into daily care.
Consider the patient who is chronically late for appointments. “It may not be an issue of the patient being disinterested in their health care, but maybe the public transportation system is unreliable, or maybe the patient has to take two buses and a subway to get there. I need [this knowledge] to inform my care and to engage my patient. I need to know, ‘what does it take for you to get here?’ That factors into how I [make the care plan],” said Dr. Delisser.
Malika Fair, MD, MPH, who teaches a longitudinal professional development class at George Washington University, Washington, and is senior director of health equity partnerships and programs at the American Association of Medical Colleges, provided the example of how her medical students intervened during their rotation in the emergency department on behalf of a newly-diagnosed patient with diabetes who had been unable to fill a prescribed medication. After determining where the patient lived, the students ensured that she had transportation and was able to get the needed medication at a local grocery store. They asked about her barriers to healthy eating, researched local grocery stores, and made practical recommendations that the patient was amenable to implementing. They identified a clinic closer to the patient’s home, and worked with her on making an appointment at a time when she could take off from work.
“Because of their training, these students were able to identify and address social risks in their first month on the ward,” said Dr. Fair, who also practices emergency medicine. They had learned about how to ask about food access and how safe it was for the patient to walk and exercise in her neighborhood.
At Perelman, most students work in student-led community clinics, and some fourth-year students participate in an elective rotation as apprentices to community health workers, learning to address SDHs and develop the cultural humility that they learned about in the classroom. The rotation was similarly created in 2013 and is described in a 2018 article (J Health Care Poor Underserved. 2018;29[2]:581-90).“Being a good physician involves being technically competent as well as what I call relationally competent,” Dr. Delisser said. “And [this involves] being aware that my relationship with a patient doesn’t exist in a vacuum ... that there’s a bigger, broader social and structural context that I need to know and understand. I [then need] to use that to inform how I mediate and empower that relationship.”
Aletha Maybank, MD, who became the American Medical Association’s first chief health equity officer earlier this year, explained that “the medical profession had a very strong social context at one point in time,” but this was dampened by the Flexner Report of 1910.*
The report revolutionized medical education by increasing its rigor, but “it was really focused on clinical and basic science and took out the social context, the context of what medicine is about,” said Dr. Maybank, a pediatrician with a board certification in preventive medicine/public health. “[Now] we’re asking, how do we revolutionize medical education again at this point in time, recognizing the confluence of information and data that we now have available to us about inequities and disparities ... and the sense of urgency from students.”
Students driving practice change
Students nationally are “the most important” drivers of the increasing focus on SDHs in medical education, according to Dr. Fair. “They are demanding experiences to learn about the entire patient. We know that only 20% of a patient’s health is dependent on their health care. Our students are demanding education about the other 80%.”
More and more, communities are identifying needs and “students will then come up with initiatives to meet those needs,” Dr. Fair said.
Others interviewed for this story predicted this trend will only intensify, since not-for-profit hospitals are required under the Affordable Care Act regulations to assess community health needs every few years and to intervene accordingly.
Education on health care systems is also advancing. Penn State University, for instance, utilized a million-dollar grant from the AMA’s Accelerating Change in Medical Education initiative to design and implement a 4-year curriculum on the health system sciences that started in 2014. The curriculum includes an immersive experience in patient navigation.
“Students were taught to be patient navigators, and they were assigned within the clinical context to work on issues like, why are [patients] having trouble getting their medications?” said Susan E. Skochelak, MD, MPH, who leads the 6-year-old Accelerating Change initiative as vice president for medical education at the AMA.
From the start, she noted, students at Penn State are encouraged to question inequities, social and structural barriers to health, and faults in the health care system. “The message given at their white coat ceremony is ‘Welcome to medicine. Now that you’re here, you’re a member of the health care team, and we want you to speak up if you think there are things that need to be addressed. We want you to tell us when the system is working and not working,’ ” said Dr Skochelak, who previously served as the senior associate dean for academic affairs at the University of Wisconsin School of Medicine and Public Health, where she had been a tenured professor of family medicine.
Tomorrow’s physician partners
Approximately 80% of medical school graduates who participated in the AAMC’s 2018 survey of graduates said they had received significant training on health disparities—up from 71% in 2014.
“There’s a huge amount [of innovation] happening, but on the flip side, there’s not really a set of accepted tools and practices, and certainly no robust evaluation [of the training],” said Philip M. Alberti, PhD, senior director for health equity research and policy at the American Association of Medical Colleges. A recently published review (J Gen Intern Med. 2019;34[5]:720-30) shows growing interest in the teaching of SDHs in undergraduate medical education but variable content, strategies, and instructional practices.
Health care systems and practicing physicians are still very much feeling their way with SDHs. Screening tools are being developed and tested, and academic medical centers are trying to determine their roles in addressing issues such as transportation and housing – and what funding and structural levers can be pulled to fulfill these roles. “As we learn more about [these issues], it will become clearer what the right baseline set of competencies might be for all physicians,” Dr. Alberti noted.
In the meantime, some basic expectations for medical education are taking root officially. The National Board of Medical Examiners, with whom the AMA has partnered in its Accelerating Change initiative, has included questions in the United States Medical Licensing Examination on population health and SDHs, and plans to add more exam content on these topics and on health systems science, said Dr. Skochelak.
And through its site visit program (the Clinical Learning Environment Review program), the Accreditation Council for Graduate Medical Education has “made it pretty clear that there’s an expectation that residents and fellows are learning about the health system’s approach to identifying and addressing health care disparities – and that they’re given opportunities to develop quality improvement initiatives that target those disparities,” Dr. Alberti said.
In hopes of achieving consistency across medical specialties and in national accreditation and board certifications exams, the American Association of Medical Colleges is developing its first set of competencies in quality improvement and patient safety, with health equity being one of these competencies’ domains .
The competencies are tiered for medical school graduates, residency graduates, and faculty physicians who are 3-5 years post residency. At this point in time, said Dr. Alberti, the consensus among medical educators has been that physicians “need to be able to understand and consider [social, economic, and structural] contexts when they’re seeing patients, when they’re developing care plans, when they’re talking with caregivers, and when they’re looking at their own quality data.”
Elisabeth Poorman, MD, MPH, an internist at UW Medicine in Kent, Washington, said she worries that the passion of medical students for SDHs will too often be crushed, especially during residency and with immersion in the productivity-focused health care system. Studies show a drop in mental wellness and empathy and a rise in cynicism as training advances, said Dr. Poorman, who also writes about health care and issues of equity and serves on the editorial advisory board of Internal Medicine News.
With similar concerns, the AMA has recently launched a “Reimagining Residency” initiative that aims to improve transitions from medical school to residency and the wellness of residents and faculty, and expand educational content relating to SDHs.
Dr. Fair is optimistic that new physicians’ knowledge of SDHs will permeate medical practices.
“Physicians who are out practicing are going to be working with our graduates, and they’re going to be asking in [job] interviews, do you have flexible hours for patients? What community partnerships do you have? Are there other professionals on staff to help us address social determinants of health? What data [relating to SDHs] are you collecting?” she said.
Correction, 8/26/2019: An earlier version of this story misstated the title of Aletha Maybank, MD. Dr. Maybank's correct title is the first chief health equity officer of the American Medical Association.