User login
Bisphosphonates improve BMD in pediatric rheumatic disease
Prophylactic treatment with bisphosphonates could significantly improve bone mineral density (BMD) in children and adolescents receiving steroids for chronic rheumatic disease, a study has found.
A paper published in EClinicalMedicine reported the outcomes of a multicenter, double-dummy, double-blind, placebo-controlled trial involving 217 patients who were receiving steroid therapy for juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or juvenile vasculitis. The patients were randomized to risedronate, alfacalcidol, or placebo, and all of the participants received 500 mg calcium and 400 IU vitamin D daily.
Lumbar spine and total body (less head) BMD increased in all groups, but the greatest increase was seen in patients treated with risedronate.
After 1 year, lumbar spine and total body (less head) BMD had increased in all groups, compared with baseline, but the greatest increase was seen in patients who had been treated with risedronate.
The lumbar spine areal BMD z score remained the same in the placebo group (−1.15 to −1.13), decreased from −0.96 to −1.00 in the alfacalcidol group, and increased from −0.99 to −0.75 in the risedronate group.
The change in z scores was significantly different between placebo and risedronate groups, and between risedronate and alfacalcidol groups, but not between placebo and alfacalcidol.
“The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal,” wrote Madeleine Rooney, MBBCH, from the Queens University of Belfast, Northern Ireland, and associates.
There were no significant differences between the three groups in fracture rates. However, researchers were also able to compare Genant scores for vertebral fractures in 187 patients with pre- and posttreatment lateral spinal x-rays. That showed that the 54 patients in the placebo arm and 52 patients in the alfacalcidol arm had no change in their baseline Genant score of 0 (normal). However, although all 53 patients in the risedronate group had a Genant score of 0 at baseline, at 1-year follow-up, 2 patients had a Genant score of 1 (mild fracture), and 1 patient had a score of 3 (severe fracture).
In biochemical parameters, researchers saw a drop in parathyroid hormone in the placebo and alfacalcidol groups, but a rise in the risedronate group. However, the authors were not able to see any changes in bone markers that might have indicated which patients responded better to treatment.
Around 90% of participants in each group were also being treated with disease-modifying antirheumatic drugs. The rates of biologic use were 10.5% in the placebo group, 23.9% in the alfacalcidol group, and 10.1% in the risedronate group.
The researchers also noted a 7% higher rate of serious adverse events in the risedronate group, but emphasized that there were no differences in events related to the treatment.
In an accompanying editorial, Ian R. Reid, MBBCH, of the department of medicine, University of Auckland (New Zealand) noted that the study was an important step toward finding interventions for the prevention of steroid-induced bone loss in children. “The present study indicates that risedronate, and probably other potent bisphosphonates, can provide bone preservation in children and young people receiving therapeutic doses of glucocorticoid drugs, whereas alfacalcidol is without benefit. The targeted use of bisphosphonates in children and young people judged to be at significant fracture risk is appropriate. However, whether preventing loss of bone density will reduce fracture incidence remains to be established.”
The study was funded by Arthritis Research UK. No conflicts of interest were declared.
SOURCE: Rooney M et al. EClinicalMedicine. 2019 Jul 3. doi: 10.1016/j.eclinm.2019.06.004.
Prophylactic treatment with bisphosphonates could significantly improve bone mineral density (BMD) in children and adolescents receiving steroids for chronic rheumatic disease, a study has found.
A paper published in EClinicalMedicine reported the outcomes of a multicenter, double-dummy, double-blind, placebo-controlled trial involving 217 patients who were receiving steroid therapy for juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or juvenile vasculitis. The patients were randomized to risedronate, alfacalcidol, or placebo, and all of the participants received 500 mg calcium and 400 IU vitamin D daily.
Lumbar spine and total body (less head) BMD increased in all groups, but the greatest increase was seen in patients treated with risedronate.
After 1 year, lumbar spine and total body (less head) BMD had increased in all groups, compared with baseline, but the greatest increase was seen in patients who had been treated with risedronate.
The lumbar spine areal BMD z score remained the same in the placebo group (−1.15 to −1.13), decreased from −0.96 to −1.00 in the alfacalcidol group, and increased from −0.99 to −0.75 in the risedronate group.
The change in z scores was significantly different between placebo and risedronate groups, and between risedronate and alfacalcidol groups, but not between placebo and alfacalcidol.
“The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal,” wrote Madeleine Rooney, MBBCH, from the Queens University of Belfast, Northern Ireland, and associates.
There were no significant differences between the three groups in fracture rates. However, researchers were also able to compare Genant scores for vertebral fractures in 187 patients with pre- and posttreatment lateral spinal x-rays. That showed that the 54 patients in the placebo arm and 52 patients in the alfacalcidol arm had no change in their baseline Genant score of 0 (normal). However, although all 53 patients in the risedronate group had a Genant score of 0 at baseline, at 1-year follow-up, 2 patients had a Genant score of 1 (mild fracture), and 1 patient had a score of 3 (severe fracture).
In biochemical parameters, researchers saw a drop in parathyroid hormone in the placebo and alfacalcidol groups, but a rise in the risedronate group. However, the authors were not able to see any changes in bone markers that might have indicated which patients responded better to treatment.
Around 90% of participants in each group were also being treated with disease-modifying antirheumatic drugs. The rates of biologic use were 10.5% in the placebo group, 23.9% in the alfacalcidol group, and 10.1% in the risedronate group.
The researchers also noted a 7% higher rate of serious adverse events in the risedronate group, but emphasized that there were no differences in events related to the treatment.
In an accompanying editorial, Ian R. Reid, MBBCH, of the department of medicine, University of Auckland (New Zealand) noted that the study was an important step toward finding interventions for the prevention of steroid-induced bone loss in children. “The present study indicates that risedronate, and probably other potent bisphosphonates, can provide bone preservation in children and young people receiving therapeutic doses of glucocorticoid drugs, whereas alfacalcidol is without benefit. The targeted use of bisphosphonates in children and young people judged to be at significant fracture risk is appropriate. However, whether preventing loss of bone density will reduce fracture incidence remains to be established.”
The study was funded by Arthritis Research UK. No conflicts of interest were declared.
SOURCE: Rooney M et al. EClinicalMedicine. 2019 Jul 3. doi: 10.1016/j.eclinm.2019.06.004.
Prophylactic treatment with bisphosphonates could significantly improve bone mineral density (BMD) in children and adolescents receiving steroids for chronic rheumatic disease, a study has found.
A paper published in EClinicalMedicine reported the outcomes of a multicenter, double-dummy, double-blind, placebo-controlled trial involving 217 patients who were receiving steroid therapy for juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or juvenile vasculitis. The patients were randomized to risedronate, alfacalcidol, or placebo, and all of the participants received 500 mg calcium and 400 IU vitamin D daily.
Lumbar spine and total body (less head) BMD increased in all groups, but the greatest increase was seen in patients treated with risedronate.
After 1 year, lumbar spine and total body (less head) BMD had increased in all groups, compared with baseline, but the greatest increase was seen in patients who had been treated with risedronate.
The lumbar spine areal BMD z score remained the same in the placebo group (−1.15 to −1.13), decreased from −0.96 to −1.00 in the alfacalcidol group, and increased from −0.99 to −0.75 in the risedronate group.
The change in z scores was significantly different between placebo and risedronate groups, and between risedronate and alfacalcidol groups, but not between placebo and alfacalcidol.
“The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal,” wrote Madeleine Rooney, MBBCH, from the Queens University of Belfast, Northern Ireland, and associates.
There were no significant differences between the three groups in fracture rates. However, researchers were also able to compare Genant scores for vertebral fractures in 187 patients with pre- and posttreatment lateral spinal x-rays. That showed that the 54 patients in the placebo arm and 52 patients in the alfacalcidol arm had no change in their baseline Genant score of 0 (normal). However, although all 53 patients in the risedronate group had a Genant score of 0 at baseline, at 1-year follow-up, 2 patients had a Genant score of 1 (mild fracture), and 1 patient had a score of 3 (severe fracture).
In biochemical parameters, researchers saw a drop in parathyroid hormone in the placebo and alfacalcidol groups, but a rise in the risedronate group. However, the authors were not able to see any changes in bone markers that might have indicated which patients responded better to treatment.
Around 90% of participants in each group were also being treated with disease-modifying antirheumatic drugs. The rates of biologic use were 10.5% in the placebo group, 23.9% in the alfacalcidol group, and 10.1% in the risedronate group.
The researchers also noted a 7% higher rate of serious adverse events in the risedronate group, but emphasized that there were no differences in events related to the treatment.
In an accompanying editorial, Ian R. Reid, MBBCH, of the department of medicine, University of Auckland (New Zealand) noted that the study was an important step toward finding interventions for the prevention of steroid-induced bone loss in children. “The present study indicates that risedronate, and probably other potent bisphosphonates, can provide bone preservation in children and young people receiving therapeutic doses of glucocorticoid drugs, whereas alfacalcidol is without benefit. The targeted use of bisphosphonates in children and young people judged to be at significant fracture risk is appropriate. However, whether preventing loss of bone density will reduce fracture incidence remains to be established.”
The study was funded by Arthritis Research UK. No conflicts of interest were declared.
SOURCE: Rooney M et al. EClinicalMedicine. 2019 Jul 3. doi: 10.1016/j.eclinm.2019.06.004.
FROM ECLINICALMEDICINE
Heart of the Matter
During the last year, the field of psoriasis has continued to expand, with new therapies, new guidelines, and further understanding of the impact of treatment on associated comorbidities.
One of the most exciting prospects of the treatment of psoriasis with biologics is the potential for the reduction in major adverse cardiovascular events (MACEs). It has been well established that psoriasis is associated with increased cardiovascular risk.1,2 Ahlehoff et al1 conducted a cohort study of the entire Danish population 18 years and older followed from 1997 to 2006 by individual-level linkage of nationwide registers. They concluded that psoriasis is associated with increased risk for adverse cardiovascular events and all-cause mortality. Young age, severe skin affection, and/or psoriatic arthritis (PsA) carry the most risk. They suggested that patients with psoriasis may be candidates for early cardiovascular risk factor modification.1
Ogdie et al2 endeavored to quantify the risk for MACE among patients with PsA, rheumatoid arthritis (RA), and psoriasis without known PsA compared to the general population after adjusting for traditional cardiovascular risk factors. Patients with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573) were identified. After adjustment for traditional risk factors, the risk for MACE was higher in patients with PsA not prescribed a disease-modifying antirheumatic drug (DMARD)(hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).2 These data are one aspect of our increasing insight into the management of psoriasis.
To expand on the new guidelines and new therapies presented in 2019, this issue includes review articles looking at these facets. Wu and Weinberg3 review the impact of diet on psoriasis. Pithadia et al4 explain the American Academy of Dermatology and National Psoriasis Foundation guidelines of care for the management of psoriasis with biologics for the prescribing dermatologist, with an emphasis on the most clinically significant considerations during each step of treatment with biologic therapies (ie, choosing a biologic, initiating therapy, assessing treatment response, switching biologics). Havnaer et al5 provide an update on the newly approved and pipeline systemic agents for psoriasis.
We hope that you find this issue enjoyable and informative.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Wu AG, Weinberg JM. The impact of diet on psoriasis. Cutis. 2019;104(suppl 2):7-10.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF guidelines of care for the management of psoriasis with biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16, 20.
- Havnaer A, Weinberg JM, Han G. Systemic therapies in psoriasis: an update on newly approved and pipeline biologics and oral treatments. Cutis. 2019;104(suppl 2):17-20.
During the last year, the field of psoriasis has continued to expand, with new therapies, new guidelines, and further understanding of the impact of treatment on associated comorbidities.
One of the most exciting prospects of the treatment of psoriasis with biologics is the potential for the reduction in major adverse cardiovascular events (MACEs). It has been well established that psoriasis is associated with increased cardiovascular risk.1,2 Ahlehoff et al1 conducted a cohort study of the entire Danish population 18 years and older followed from 1997 to 2006 by individual-level linkage of nationwide registers. They concluded that psoriasis is associated with increased risk for adverse cardiovascular events and all-cause mortality. Young age, severe skin affection, and/or psoriatic arthritis (PsA) carry the most risk. They suggested that patients with psoriasis may be candidates for early cardiovascular risk factor modification.1
Ogdie et al2 endeavored to quantify the risk for MACE among patients with PsA, rheumatoid arthritis (RA), and psoriasis without known PsA compared to the general population after adjusting for traditional cardiovascular risk factors. Patients with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573) were identified. After adjustment for traditional risk factors, the risk for MACE was higher in patients with PsA not prescribed a disease-modifying antirheumatic drug (DMARD)(hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).2 These data are one aspect of our increasing insight into the management of psoriasis.
To expand on the new guidelines and new therapies presented in 2019, this issue includes review articles looking at these facets. Wu and Weinberg3 review the impact of diet on psoriasis. Pithadia et al4 explain the American Academy of Dermatology and National Psoriasis Foundation guidelines of care for the management of psoriasis with biologics for the prescribing dermatologist, with an emphasis on the most clinically significant considerations during each step of treatment with biologic therapies (ie, choosing a biologic, initiating therapy, assessing treatment response, switching biologics). Havnaer et al5 provide an update on the newly approved and pipeline systemic agents for psoriasis.
We hope that you find this issue enjoyable and informative.
During the last year, the field of psoriasis has continued to expand, with new therapies, new guidelines, and further understanding of the impact of treatment on associated comorbidities.
One of the most exciting prospects of the treatment of psoriasis with biologics is the potential for the reduction in major adverse cardiovascular events (MACEs). It has been well established that psoriasis is associated with increased cardiovascular risk.1,2 Ahlehoff et al1 conducted a cohort study of the entire Danish population 18 years and older followed from 1997 to 2006 by individual-level linkage of nationwide registers. They concluded that psoriasis is associated with increased risk for adverse cardiovascular events and all-cause mortality. Young age, severe skin affection, and/or psoriatic arthritis (PsA) carry the most risk. They suggested that patients with psoriasis may be candidates for early cardiovascular risk factor modification.1
Ogdie et al2 endeavored to quantify the risk for MACE among patients with PsA, rheumatoid arthritis (RA), and psoriasis without known PsA compared to the general population after adjusting for traditional cardiovascular risk factors. Patients with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573) were identified. After adjustment for traditional risk factors, the risk for MACE was higher in patients with PsA not prescribed a disease-modifying antirheumatic drug (DMARD)(hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).2 These data are one aspect of our increasing insight into the management of psoriasis.
To expand on the new guidelines and new therapies presented in 2019, this issue includes review articles looking at these facets. Wu and Weinberg3 review the impact of diet on psoriasis. Pithadia et al4 explain the American Academy of Dermatology and National Psoriasis Foundation guidelines of care for the management of psoriasis with biologics for the prescribing dermatologist, with an emphasis on the most clinically significant considerations during each step of treatment with biologic therapies (ie, choosing a biologic, initiating therapy, assessing treatment response, switching biologics). Havnaer et al5 provide an update on the newly approved and pipeline systemic agents for psoriasis.
We hope that you find this issue enjoyable and informative.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Wu AG, Weinberg JM. The impact of diet on psoriasis. Cutis. 2019;104(suppl 2):7-10.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF guidelines of care for the management of psoriasis with biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16, 20.
- Havnaer A, Weinberg JM, Han G. Systemic therapies in psoriasis: an update on newly approved and pipeline biologics and oral treatments. Cutis. 2019;104(suppl 2):17-20.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Wu AG, Weinberg JM. The impact of diet on psoriasis. Cutis. 2019;104(suppl 2):7-10.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF guidelines of care for the management of psoriasis with biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16, 20.
- Havnaer A, Weinberg JM, Han G. Systemic therapies in psoriasis: an update on newly approved and pipeline biologics and oral treatments. Cutis. 2019;104(suppl 2):17-20.
Survey: PCI use has declined in ES-SCLC
A nationwide survey of radiation oncologists showed a decline in the use of prophylactic cranial irradiation (PCI) for extensive-stage small cell lung cancer (ES-SCLC) after the release of phase 3 study results by Takahashi et al.
The recent study by Toshiaki Takahashi, MD, and colleagues (Lancet Oncol. 2017;18[5]:663-71) demonstrated no overall survival benefit when comparing active magnetic resonance imaging surveillance with PCI in patients with ES-SCLC. The results challenged the formerly accepted belief of the benefit of PCI for patients with ES-SCLC.
“We conducted a nationwide survey study of radiation oncologists to assess changes in the use of PCI for patients with ES-SCLC following publication of the trial by Takahashi et al.,” wrote Olsi Gjyshi, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in a research letter published in JAMA Network Open.
Dr. Gjyshi and colleagues distributed the anonymous survey to 3,851 United States–based radiation oncologists registered with the American Society for Radiation Oncology (ASTRO). In total, 487 members (12.6%) completed the survey.
After analysis, the researchers found that 72% of respondents regularly offered PCI to patients before the publication of the study by Takahashi et al., while only 44% do so currently (difference, 28%; 95% confidence interval, 25%-31%; P less than .001).
With respect to radiation oncologists in private versus academic settings, no significant difference in practice patterns was observed (P = .71).
“Regression analysis showed no difference in likelihood of offering PCI based on practice setting, location, or size; volume of patients treated for lung cancer; or years in practice,” the researchers wrote.
In respondents unaware of the Takahashi et al. study, 85% continued to propose PCI, which was greater than those aware of the trial (odds ratio, 0.11; 95% CI, 0.04-0.32; P less than .001).
The researchers acknowledged a key limitation of the study was that radiation oncologists familiar with the study by Dr. Takahashi and colleagues may have been more apt to respond to the survey. As a result, participation bias could have influenced the results.
“These results highlight the continued lack of consensus for PCI in SCLC and support ongoing investigations,” they concluded.
No funding sources were reported. One co-author reported financial affiliations with Novocure, Inc. The other authors reported no conflicts of interest.
SOURCE: Gjyshi O et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9135.
A nationwide survey of radiation oncologists showed a decline in the use of prophylactic cranial irradiation (PCI) for extensive-stage small cell lung cancer (ES-SCLC) after the release of phase 3 study results by Takahashi et al.
The recent study by Toshiaki Takahashi, MD, and colleagues (Lancet Oncol. 2017;18[5]:663-71) demonstrated no overall survival benefit when comparing active magnetic resonance imaging surveillance with PCI in patients with ES-SCLC. The results challenged the formerly accepted belief of the benefit of PCI for patients with ES-SCLC.
“We conducted a nationwide survey study of radiation oncologists to assess changes in the use of PCI for patients with ES-SCLC following publication of the trial by Takahashi et al.,” wrote Olsi Gjyshi, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in a research letter published in JAMA Network Open.
Dr. Gjyshi and colleagues distributed the anonymous survey to 3,851 United States–based radiation oncologists registered with the American Society for Radiation Oncology (ASTRO). In total, 487 members (12.6%) completed the survey.
After analysis, the researchers found that 72% of respondents regularly offered PCI to patients before the publication of the study by Takahashi et al., while only 44% do so currently (difference, 28%; 95% confidence interval, 25%-31%; P less than .001).
With respect to radiation oncologists in private versus academic settings, no significant difference in practice patterns was observed (P = .71).
“Regression analysis showed no difference in likelihood of offering PCI based on practice setting, location, or size; volume of patients treated for lung cancer; or years in practice,” the researchers wrote.
In respondents unaware of the Takahashi et al. study, 85% continued to propose PCI, which was greater than those aware of the trial (odds ratio, 0.11; 95% CI, 0.04-0.32; P less than .001).
The researchers acknowledged a key limitation of the study was that radiation oncologists familiar with the study by Dr. Takahashi and colleagues may have been more apt to respond to the survey. As a result, participation bias could have influenced the results.
“These results highlight the continued lack of consensus for PCI in SCLC and support ongoing investigations,” they concluded.
No funding sources were reported. One co-author reported financial affiliations with Novocure, Inc. The other authors reported no conflicts of interest.
SOURCE: Gjyshi O et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9135.
A nationwide survey of radiation oncologists showed a decline in the use of prophylactic cranial irradiation (PCI) for extensive-stage small cell lung cancer (ES-SCLC) after the release of phase 3 study results by Takahashi et al.
The recent study by Toshiaki Takahashi, MD, and colleagues (Lancet Oncol. 2017;18[5]:663-71) demonstrated no overall survival benefit when comparing active magnetic resonance imaging surveillance with PCI in patients with ES-SCLC. The results challenged the formerly accepted belief of the benefit of PCI for patients with ES-SCLC.
“We conducted a nationwide survey study of radiation oncologists to assess changes in the use of PCI for patients with ES-SCLC following publication of the trial by Takahashi et al.,” wrote Olsi Gjyshi, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in a research letter published in JAMA Network Open.
Dr. Gjyshi and colleagues distributed the anonymous survey to 3,851 United States–based radiation oncologists registered with the American Society for Radiation Oncology (ASTRO). In total, 487 members (12.6%) completed the survey.
After analysis, the researchers found that 72% of respondents regularly offered PCI to patients before the publication of the study by Takahashi et al., while only 44% do so currently (difference, 28%; 95% confidence interval, 25%-31%; P less than .001).
With respect to radiation oncologists in private versus academic settings, no significant difference in practice patterns was observed (P = .71).
“Regression analysis showed no difference in likelihood of offering PCI based on practice setting, location, or size; volume of patients treated for lung cancer; or years in practice,” the researchers wrote.
In respondents unaware of the Takahashi et al. study, 85% continued to propose PCI, which was greater than those aware of the trial (odds ratio, 0.11; 95% CI, 0.04-0.32; P less than .001).
The researchers acknowledged a key limitation of the study was that radiation oncologists familiar with the study by Dr. Takahashi and colleagues may have been more apt to respond to the survey. As a result, participation bias could have influenced the results.
“These results highlight the continued lack of consensus for PCI in SCLC and support ongoing investigations,” they concluded.
No funding sources were reported. One co-author reported financial affiliations with Novocure, Inc. The other authors reported no conflicts of interest.
SOURCE: Gjyshi O et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9135.
FROM JAMA NETWORK OPEN
Vaccination Not Associated With Increased Risk of MS
Key clinical point: Data do not support an association between vaccination and increased risk of MS.
Major finding: The odds of MS were lower in participants who received vaccination, compared with participants without autoimmune disease (odds ratio, 0.870).
Study details: A systematic retrospective analysis of claims data for 12,262 patients with MS and 210,773 controls.
Disclosures: A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no relevant conflicts.
Citation: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
Key clinical point: Data do not support an association between vaccination and increased risk of MS.
Major finding: The odds of MS were lower in participants who received vaccination, compared with participants without autoimmune disease (odds ratio, 0.870).
Study details: A systematic retrospective analysis of claims data for 12,262 patients with MS and 210,773 controls.
Disclosures: A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no relevant conflicts.
Citation: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
Key clinical point: Data do not support an association between vaccination and increased risk of MS.
Major finding: The odds of MS were lower in participants who received vaccination, compared with participants without autoimmune disease (odds ratio, 0.870).
Study details: A systematic retrospective analysis of claims data for 12,262 patients with MS and 210,773 controls.
Disclosures: A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no relevant conflicts.
Citation: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
Black Holes Associated With Impaired Cognition in MS
Key clinical point: Evaluating black holes as part of routine clinical practice could be a quick method for screening people with MS for referral to a comprehensive cognitive assessment.
Major finding: Mean Symbol Digit Modalities Test score was 49.0 in patients without a black hole and 42.9 in patients with at least one black hole.
Study details: A prospective study of 226 patients with MS.
Disclosures: The investigators had no disclosures and conducted their study without financial support.
Citation: Özakbas S et al. CMSC 2019, Abstract IMG02.
Key clinical point: Evaluating black holes as part of routine clinical practice could be a quick method for screening people with MS for referral to a comprehensive cognitive assessment.
Major finding: Mean Symbol Digit Modalities Test score was 49.0 in patients without a black hole and 42.9 in patients with at least one black hole.
Study details: A prospective study of 226 patients with MS.
Disclosures: The investigators had no disclosures and conducted their study without financial support.
Citation: Özakbas S et al. CMSC 2019, Abstract IMG02.
Key clinical point: Evaluating black holes as part of routine clinical practice could be a quick method for screening people with MS for referral to a comprehensive cognitive assessment.
Major finding: Mean Symbol Digit Modalities Test score was 49.0 in patients without a black hole and 42.9 in patients with at least one black hole.
Study details: A prospective study of 226 patients with MS.
Disclosures: The investigators had no disclosures and conducted their study without financial support.
Citation: Özakbas S et al. CMSC 2019, Abstract IMG02.
Neutrophils May Decline in Patients on Fingolimod
Key clinical point: Neutrophil levels may decline in patients with relapsing multiple sclerosis (MS) who have been on fingolimod for 2 or more years.
Major finding: In a cohort of patients continuously treated with fingolimod for at least 2 years, neutrophils declined over 6 months by about 9%, from an average of 3,698.56 cells per microliter to 3,336.13 cells per microliter.
Study details: Analysis of interim, 6-month data from the ongoing, open-label, phase 4 FLUENT study, which is a 12-month, prospective, multicenter, nonrandomized study to assess changes in the immune cell profiles of patients with relapsing MS who receive fingolimod. The interim results include data from 216 treatment-experienced patients and 166 treatment-naive patients.
Disclosures: Novartis funded the study, and four of the authors are Novartis employees. Dr. Cree disclosed consulting fees from Novartis and other pharmaceutical companies. His coauthors disclosed consulting fees, speaking fees, research support, and serving on advisory boards for pharmaceutical companies, including Novartis.
Citation: Mao-Draayer Y et al. CMSC 2019, Abstract DXM03.
Key clinical point: Neutrophil levels may decline in patients with relapsing multiple sclerosis (MS) who have been on fingolimod for 2 or more years.
Major finding: In a cohort of patients continuously treated with fingolimod for at least 2 years, neutrophils declined over 6 months by about 9%, from an average of 3,698.56 cells per microliter to 3,336.13 cells per microliter.
Study details: Analysis of interim, 6-month data from the ongoing, open-label, phase 4 FLUENT study, which is a 12-month, prospective, multicenter, nonrandomized study to assess changes in the immune cell profiles of patients with relapsing MS who receive fingolimod. The interim results include data from 216 treatment-experienced patients and 166 treatment-naive patients.
Disclosures: Novartis funded the study, and four of the authors are Novartis employees. Dr. Cree disclosed consulting fees from Novartis and other pharmaceutical companies. His coauthors disclosed consulting fees, speaking fees, research support, and serving on advisory boards for pharmaceutical companies, including Novartis.
Citation: Mao-Draayer Y et al. CMSC 2019, Abstract DXM03.
Key clinical point: Neutrophil levels may decline in patients with relapsing multiple sclerosis (MS) who have been on fingolimod for 2 or more years.
Major finding: In a cohort of patients continuously treated with fingolimod for at least 2 years, neutrophils declined over 6 months by about 9%, from an average of 3,698.56 cells per microliter to 3,336.13 cells per microliter.
Study details: Analysis of interim, 6-month data from the ongoing, open-label, phase 4 FLUENT study, which is a 12-month, prospective, multicenter, nonrandomized study to assess changes in the immune cell profiles of patients with relapsing MS who receive fingolimod. The interim results include data from 216 treatment-experienced patients and 166 treatment-naive patients.
Disclosures: Novartis funded the study, and four of the authors are Novartis employees. Dr. Cree disclosed consulting fees from Novartis and other pharmaceutical companies. His coauthors disclosed consulting fees, speaking fees, research support, and serving on advisory boards for pharmaceutical companies, including Novartis.
Citation: Mao-Draayer Y et al. CMSC 2019, Abstract DXM03.
Timing of adjuvant treatment impacts pancreatic cancer survival
The timing for adjuvant treatment following surgery for pancreatic cancer appears to have a sweet spot associated with the best survival outcomes, according to a study published in JAMA Network Open.
Researchers analyzed data from the National Cancer Database for 7,548 patients with stage I-II resected pancreatic cancer, 5,453 of whom had received adjuvant therapy and 2,095 who did not.
“While the benefit of adjuvant therapy to patients with resected pancreatic cancer is accepted, its optimal timing after surgery remains under investigation,” wrote Sung Jun Ma, MD, from the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and coauthors.
After a median overall follow-up of 38.6 months, they found the lowest mortality risk was in the reference cohort of patients who started adjuvant therapy 28-59 days after surgery. In comparison, patients who received early adjuvant therapy – within 28 days of surgery – had a 17% higher mortality (P = .03), and those who received adjuvant therapy late – 59 days or more after surgery – had a 9% higher mortality (P = .008)
The overall survival rate at 2 years was 45.2% for the early adjuvant therapy cohort and 52.5% for the reference cohort.
Despite the higher mortality among the early adjuvant therapy cohort, patients treated with adjuvant therapy more than 12 weeks after surgery still showed improved survival, compared with patient treated with surgery alone, particular those with node-positive disease.
“To our knowledge, it is the first study to suggest that patients who commence adjuvant therapy within 28-59 days after primary surgical resection of pancreatic adenocarcinoma have improved survival outcomes compared with those who waited for more than 59 days,” the authors wrote. “However, patients who recover slowly from surgery may still benefit from delayed adjuvant therapy initiated more than 12 weeks after surgery.”
No treatment interactions were seen for other variables such as age, comorbidity score, tumor size, pathologic T stages, surgical margin, duration of postoperative inpatient admission, unplanned readmission within 30 days after surgery, and time from diagnosis to surgery.
The analysis also revealed that patients with a primary tumor at the pancreatic body and tail and those receiving multiagent chemotherapy or radiation therapy were less likely to receive delayed adjuvant therapy, However, older or black patients, those with lower income, with postoperative inpatient admission longer than 1 week or with unplanned readmission within 30 days after surgery were more likely to have delayed initiation of adjuvant therapy.
No conflicts of interest were reported.
SOURCE: Ma SJ et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126.
The timing for adjuvant treatment following surgery for pancreatic cancer appears to have a sweet spot associated with the best survival outcomes, according to a study published in JAMA Network Open.
Researchers analyzed data from the National Cancer Database for 7,548 patients with stage I-II resected pancreatic cancer, 5,453 of whom had received adjuvant therapy and 2,095 who did not.
“While the benefit of adjuvant therapy to patients with resected pancreatic cancer is accepted, its optimal timing after surgery remains under investigation,” wrote Sung Jun Ma, MD, from the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and coauthors.
After a median overall follow-up of 38.6 months, they found the lowest mortality risk was in the reference cohort of patients who started adjuvant therapy 28-59 days after surgery. In comparison, patients who received early adjuvant therapy – within 28 days of surgery – had a 17% higher mortality (P = .03), and those who received adjuvant therapy late – 59 days or more after surgery – had a 9% higher mortality (P = .008)
The overall survival rate at 2 years was 45.2% for the early adjuvant therapy cohort and 52.5% for the reference cohort.
Despite the higher mortality among the early adjuvant therapy cohort, patients treated with adjuvant therapy more than 12 weeks after surgery still showed improved survival, compared with patient treated with surgery alone, particular those with node-positive disease.
“To our knowledge, it is the first study to suggest that patients who commence adjuvant therapy within 28-59 days after primary surgical resection of pancreatic adenocarcinoma have improved survival outcomes compared with those who waited for more than 59 days,” the authors wrote. “However, patients who recover slowly from surgery may still benefit from delayed adjuvant therapy initiated more than 12 weeks after surgery.”
No treatment interactions were seen for other variables such as age, comorbidity score, tumor size, pathologic T stages, surgical margin, duration of postoperative inpatient admission, unplanned readmission within 30 days after surgery, and time from diagnosis to surgery.
The analysis also revealed that patients with a primary tumor at the pancreatic body and tail and those receiving multiagent chemotherapy or radiation therapy were less likely to receive delayed adjuvant therapy, However, older or black patients, those with lower income, with postoperative inpatient admission longer than 1 week or with unplanned readmission within 30 days after surgery were more likely to have delayed initiation of adjuvant therapy.
No conflicts of interest were reported.
SOURCE: Ma SJ et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126.
The timing for adjuvant treatment following surgery for pancreatic cancer appears to have a sweet spot associated with the best survival outcomes, according to a study published in JAMA Network Open.
Researchers analyzed data from the National Cancer Database for 7,548 patients with stage I-II resected pancreatic cancer, 5,453 of whom had received adjuvant therapy and 2,095 who did not.
“While the benefit of adjuvant therapy to patients with resected pancreatic cancer is accepted, its optimal timing after surgery remains under investigation,” wrote Sung Jun Ma, MD, from the Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., and coauthors.
After a median overall follow-up of 38.6 months, they found the lowest mortality risk was in the reference cohort of patients who started adjuvant therapy 28-59 days after surgery. In comparison, patients who received early adjuvant therapy – within 28 days of surgery – had a 17% higher mortality (P = .03), and those who received adjuvant therapy late – 59 days or more after surgery – had a 9% higher mortality (P = .008)
The overall survival rate at 2 years was 45.2% for the early adjuvant therapy cohort and 52.5% for the reference cohort.
Despite the higher mortality among the early adjuvant therapy cohort, patients treated with adjuvant therapy more than 12 weeks after surgery still showed improved survival, compared with patient treated with surgery alone, particular those with node-positive disease.
“To our knowledge, it is the first study to suggest that patients who commence adjuvant therapy within 28-59 days after primary surgical resection of pancreatic adenocarcinoma have improved survival outcomes compared with those who waited for more than 59 days,” the authors wrote. “However, patients who recover slowly from surgery may still benefit from delayed adjuvant therapy initiated more than 12 weeks after surgery.”
No treatment interactions were seen for other variables such as age, comorbidity score, tumor size, pathologic T stages, surgical margin, duration of postoperative inpatient admission, unplanned readmission within 30 days after surgery, and time from diagnosis to surgery.
The analysis also revealed that patients with a primary tumor at the pancreatic body and tail and those receiving multiagent chemotherapy or radiation therapy were less likely to receive delayed adjuvant therapy, However, older or black patients, those with lower income, with postoperative inpatient admission longer than 1 week or with unplanned readmission within 30 days after surgery were more likely to have delayed initiation of adjuvant therapy.
No conflicts of interest were reported.
SOURCE: Ma SJ et al. JAMA Netw Open. 2019 Aug 14. doi: 10.1001/jamanetworkopen.2019.9126.
FROM JAMA NETWORK OPEN
Tamoxifen benefit in lower-risk breast cancer varies by intrinsic subtype
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
, finds a secondary analysis of the Stockholm Tamoxifen (STO-3) trial.
“Patients with estrogen receptor (ER)–positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood,” noted the investigators, who conducted the research under senior investigator Linda Lindström, MSc, PhD, department of biosciences and nutrition, Karolinska Institutet, Stockholm.
The STO-3 trial spanned 1976 to 1990 and randomized postmenopausal patients with lymph node–negative breast cancer to receive at least 2 years of adjuvant tamoxifen or no endocrine therapy.
Dr. Lindström and coinvestigators used immunohistochemistry and Agilent microarrays to define tumor molecular subtype. Analyses were based on 462 patients with ER-positive disease: 336 with luminal A subtype tumors and 126 with luminal B subtype tumors.
Results reported in JAMA Oncology showed that the distant recurrence–free interval (DRFI) was significantly better with tamoxifen than with no endocrine therapy in both the luminal A group (P less than .001) and the luminal B group (P = .04).
Among patients given tamoxifen, the 25-year DRFI rate was 87% (95% confidence interval, 82%-93%) for those with luminal A tumors vs. 67% (95% CI, 56%-82%) for those with luminal B tumors. Among patients not given any endocrine therapy, it was 70% (95% CI, 62%-79%) vs. 54% (95% CI, 42%-70%), respectively.
Tamoxifen had a significant DRFI benefit for 15 years after diagnosis in the luminal A group (hazard ratio, 0.57; 95% CI, 0.35-0.94). In contrast, the benefit was significant for only 5 years in the luminal B group (HR, 0.38; 95% CI, 0.24-0.59).
“We conclude that tamoxifen appears to confer a long-term benefit for patients with lymph node–negative, ER-positive, luminal A subtype tumors, and a short-term benefit for patients with luminal B subtype tumors. Given that the risk of distant metastatic disease is low for patients with the luminal A subtype but persists in the long term, whereas the risk for patients with luminal B subtype is higher initially but decreases after 5 years, tamoxifen treatment is beneficial for patients with luminal A or luminal B subtype tumors,” Dr. Lindström and coinvestigators maintained.
“In patients with luminal B subtype, up-front chemotherapy should be discussed and endocrine therapy potentially extended for up to 10 years, particularly in those in the higher risk strata according to other tumor characteristics,” they recommended.
Dr. Lindström disclosed no conflicts of interest. The study was supported by the Swedish Research Council, FORTE, The Gösta Milton Donation Fund, the California Breast Cancer Research Program, The Iris, Stig och Gerry Castenbäcks Stiftelse för Cancerforskning, and Konung Gustaf V:s Jubileumsfond from Radiumhemmets Forskningsfonder.
SOURCE: Yu NY et al. JAMA Oncol. 2019 Aug 8. doi: 10.1001/jamaoncol.2019.1856.
FROM JAMA ONCOLOGY
Safety of ondansetron for nausea and vomiting of pregnancy
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
Nausea and vomiting of pregnancy (NVP) affects up to 80% of pregnant women, most commonly between 5 and 18 weeks of gestation. In addition, its extreme form, hyperemesis gravidarum, affects less than 3% of pregnancies.1 Certainly with hyperemesis gravidarum, and oftentimes with less severe NVP, pharmacologic treatment is desired or required. One of the choices for such treatment has been ondansetron, a 5-HT3 receptor antagonist, which has been used off label for NVP and is now available in generic form. However, there have been concerns raised regarding the fetal safety of this medication, last reviewed in Ob.Gyn. News by Gideon Koren, MD, in a commentary published in 2013.
Since then, the escalating use of ondansetron in the United States has been described using a large dataset covering 2.3 million, predominantly commercially insured, pregnancies that resulted in live births from 2001 to 2015.1 Over that period of time, any outpatient pharmacy dispensing of an antiemetic in pregnancy increased from 17.0% in 2001 to 27.2% in 2014. That increase was entirely accounted for by a dramatic rise in oral ondansetron use beginning in 2006. By 2014, 22.4% of pregnancies in the database had received a prescription for ondansetron.
There have been two studies that have suggested an increased risk in specific major birth defects with first-trimester ondansetron use. The first, published in 2012, used data from the National Birth Defects Prevention case control study from 1997 to 2004 to examine risks with NVP and its treatments for the most common noncardiac defects in the dataset. These included cleft lip with or without cleft palate, cleft palate alone, neural tube defects, and hypospadias. NVP itself was not associated with any increased risks for the selected defects. In contrast, ondansetron was associated with an increased risk for cleft palate alone based on seven exposed cases (adjusted odds ratio, 2.37; 95% confidence interval, 1.18-4.76).2
A second study published in 2014 used data from the Swedish Medical Birth Register from 1998 to 2012 to identify 1,349 infants whose mothers reported taking ondansetron in early pregnancy. While no overall increased risk of major birth defects was found with early pregnancy ondansetron use, compared with no such use, there was a significant increased risk noted for cardiovascular defects, particularly cardiac septum defects (any cardiac defect OR, 1.62; 95% CI, 1.04-2.14; cardiac septum defects risk ratio, 2.05; 95% CI, 1.19-3.28).3 No cases of cleft palate were reported among exposed cases in that study.
In contrast, in another study, Danish National Birth Cohort data on 608,385 pregnancies from 2004 to 2011 were used to compare major birth defect outcomes among 1,233 women exposed to ondansetron in the first trimester with those of 4,392 unexposed women.4 The birth prevalence of any major birth defect was identical (2.9%) in both exposed and unexposed groups (adjusted prevalence OR, 1.12; 95% CI, 0.69-1.82). No cases of cleft palate were reported among exposed cases and the crude OR for any cardiac defect approximated the null (1.04; 95% CI, 0.52-1.95). Two other smaller or less well-designed studies did not support an increased risk for major birth defects overall (Fejzo et al. 2016 Jul;62:87-91; Einarson et al. 2004Aug 23. doi: 10.1111/j.1471-0528.2004.00236.x).
To date, although the data are conflicting, they are consistent with either a small increased risk for selected cardiac defects and perhaps cleft palate, or no increased risk at all. However, with recent data indicating that nearly one-quarter of insured pregnant women in the United States have been prescribed ondansetron in early pregnancy, there is an urgency to conduct additional rigorous studies of sufficient sample size to determine on balance if there is a small individual increased risk associated with this treatment that translates to a larger public health problem.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no conflicts of interest to disclose related to this column.
References:
1. Taylor LG et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017 May;26(5):592-6.
2. Anderka M et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012 Jan;94(1):22-30.
3. Danielsson B et al. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014 Dec;50:134-7.
4. Pasternak B et al. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013 Feb 28;368(9):814-23.
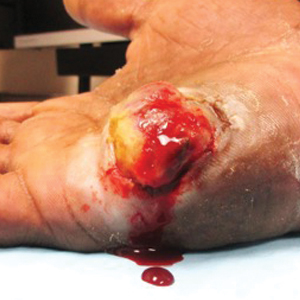
Well-Circumscribed Tumor on the Hand
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.

The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.

The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.

The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.

A 52-year-old man presented to the dermatology clinic with a 2×3-cm, fungating, dome-shaped, ulcerative, moist, well-circumscribed tumor with peripheral maceration on the volar aspect of the right hand of 3 months’ duration. The tumor was malodorous, painful, and hemorrhaged easily with minimal trauma. The patient’s medical history was notable for human immunodeficiency virus and latent syphilis, with elevated rapid plasma reagin titers and a positive Treponema palladium antibody on chemiluminescent immunoassay, that was refractory to 3 treatments with penicillin. The patient was not on antiretroviral therapy. He had a CD4+ lymphocyte count of 980 cells/µL (reference range, 359–1519 cells/µL) and a viral load of 8560 copies/mL (reference range, <200 copies/mL). No other skin or systemic concerns were noted, and the patient denied any recent travel, exposure to animals, or constitutional symptoms. A deep shave biopsy of the lesion was performed.