User login
Zanubrutinib may be poised to challenge ibrutinib for CLL
The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib appears safe and effective for patients with B-cell malignancies, according to results from a phase 1 trial.
Among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), the overall response rate was 96.2%, reported Constantine Si Lun Tam, MD, of Peter MacCallum Cancer Centre in Melbourne and colleagues.
“Zanubrutinib (BGB-3111) is a highly specific next-generation BTK inhibitor with favorable oral bioavailability, as shown in preclinical studies,” the investigators wrote in Blood. “Compared with ibrutinib, zanubrutinib has shown greater selectivity for BTK and fewer off-target effects in multiple in vitro enzymatic and cell-based assays.”
The current, open-label trial involved 144 patients with B-cell malignancies. To determine optimal dosing, the investigators recruited 17 patients with relapsed/refractory B-cell malignancies who had received at least one prior therapy. The dose expansion part of the study assessed responses in multiple cohorts, including patients with CLL/SLL, mantle cell lymphoma, and Waldenström macroglobulinemia. The primary endpoints were safety and tolerability, including maximum tolerated dose. Efficacy findings were also reported.
During dose escalation, no dose-limiting toxicities were observed, so the highest dose – 320 mg once daily or 160 mg twice daily – was selected for further testing.
The investigators highlighted efficacy and safety findings from 94 patients with CLL/SLL who were involved in dose expansion. Although nearly one-quarter (23.4%) were treatment-naive, the median number of prior therapies was two, and some patients had high-risk features, such as adverse cytogenetics, including 19.1% with a TP53 mutation and 23.3% with a 17p deletion. After a median follow-up of 13.7 months, 94.7% of these patients were still undergoing treatment.
Out of the initial 94 patients with CLL/SLL, 78 were evaluable for efficacy. The overall response rate was 96.2%, including two (2.6%) complete responses, 63 (80.8%) partial responses, and 10 (12.8%) partial responses with lymphocytosis. The median progression-free survival had not been reached, and the 12-month estimated progression-free survival was 100%.
In regard to safety, the most common adverse events were contusion (35.1%), upper respiratory tract infection (33.0%), cough (25.5%), diarrhea (21.3%), fatigue (19.1%), back pain (14.9%), hematuria (14.9%), headache (13.8%), nausea (13.8%), rash (12.8%), arthralgia (11.7%), muscle spasms (11.7%), and urinary tract infection (10.6%).
A number of other adverse events were reported, although these occurred in less than 10% of patients.
More than one-third of patients (36.2%) experienced grade 3 or higher adverse events, with neutropenia being most common (6.4%), followed by pneumonia , hypertension, and anemia, which each occurred in 2.1% of patients, and less commonly, back pain, nausea, urinary tract infection, purpura, cellulitis, and squamous cell carcinoma of the skin, which each occurred in 1.1% of patients.
“In this first-in-human study, zanubrutinib demonstrated encouraging activity in patients with relapsed/refractory and treatment-naive CLL/SLL, with good tolerability,” the investigators concluded. “Two ongoing randomized studies of zanubrutinib versus ibrutinib (NCT03053440 and NCT03734016) aim to determine whether consistent, continuous BTK blockade with a selective inhibitor results in fewer off-target effects and translates into improvements in disease control.”
The study was funded by BeiGene USA, which is developing the drug. The investigators reported relationships with the study sponsor, as well as Janssen, Pharmacyclics, AbbVie, and others.
SOURCE: Tam CSL et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019001160.
The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib appears safe and effective for patients with B-cell malignancies, according to results from a phase 1 trial.
Among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), the overall response rate was 96.2%, reported Constantine Si Lun Tam, MD, of Peter MacCallum Cancer Centre in Melbourne and colleagues.
“Zanubrutinib (BGB-3111) is a highly specific next-generation BTK inhibitor with favorable oral bioavailability, as shown in preclinical studies,” the investigators wrote in Blood. “Compared with ibrutinib, zanubrutinib has shown greater selectivity for BTK and fewer off-target effects in multiple in vitro enzymatic and cell-based assays.”
The current, open-label trial involved 144 patients with B-cell malignancies. To determine optimal dosing, the investigators recruited 17 patients with relapsed/refractory B-cell malignancies who had received at least one prior therapy. The dose expansion part of the study assessed responses in multiple cohorts, including patients with CLL/SLL, mantle cell lymphoma, and Waldenström macroglobulinemia. The primary endpoints were safety and tolerability, including maximum tolerated dose. Efficacy findings were also reported.
During dose escalation, no dose-limiting toxicities were observed, so the highest dose – 320 mg once daily or 160 mg twice daily – was selected for further testing.
The investigators highlighted efficacy and safety findings from 94 patients with CLL/SLL who were involved in dose expansion. Although nearly one-quarter (23.4%) were treatment-naive, the median number of prior therapies was two, and some patients had high-risk features, such as adverse cytogenetics, including 19.1% with a TP53 mutation and 23.3% with a 17p deletion. After a median follow-up of 13.7 months, 94.7% of these patients were still undergoing treatment.
Out of the initial 94 patients with CLL/SLL, 78 were evaluable for efficacy. The overall response rate was 96.2%, including two (2.6%) complete responses, 63 (80.8%) partial responses, and 10 (12.8%) partial responses with lymphocytosis. The median progression-free survival had not been reached, and the 12-month estimated progression-free survival was 100%.
In regard to safety, the most common adverse events were contusion (35.1%), upper respiratory tract infection (33.0%), cough (25.5%), diarrhea (21.3%), fatigue (19.1%), back pain (14.9%), hematuria (14.9%), headache (13.8%), nausea (13.8%), rash (12.8%), arthralgia (11.7%), muscle spasms (11.7%), and urinary tract infection (10.6%).
A number of other adverse events were reported, although these occurred in less than 10% of patients.
More than one-third of patients (36.2%) experienced grade 3 or higher adverse events, with neutropenia being most common (6.4%), followed by pneumonia , hypertension, and anemia, which each occurred in 2.1% of patients, and less commonly, back pain, nausea, urinary tract infection, purpura, cellulitis, and squamous cell carcinoma of the skin, which each occurred in 1.1% of patients.
“In this first-in-human study, zanubrutinib demonstrated encouraging activity in patients with relapsed/refractory and treatment-naive CLL/SLL, with good tolerability,” the investigators concluded. “Two ongoing randomized studies of zanubrutinib versus ibrutinib (NCT03053440 and NCT03734016) aim to determine whether consistent, continuous BTK blockade with a selective inhibitor results in fewer off-target effects and translates into improvements in disease control.”
The study was funded by BeiGene USA, which is developing the drug. The investigators reported relationships with the study sponsor, as well as Janssen, Pharmacyclics, AbbVie, and others.
SOURCE: Tam CSL et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019001160.
The Bruton tyrosine kinase (BTK) inhibitor zanubrutinib appears safe and effective for patients with B-cell malignancies, according to results from a phase 1 trial.
Among patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), the overall response rate was 96.2%, reported Constantine Si Lun Tam, MD, of Peter MacCallum Cancer Centre in Melbourne and colleagues.
“Zanubrutinib (BGB-3111) is a highly specific next-generation BTK inhibitor with favorable oral bioavailability, as shown in preclinical studies,” the investigators wrote in Blood. “Compared with ibrutinib, zanubrutinib has shown greater selectivity for BTK and fewer off-target effects in multiple in vitro enzymatic and cell-based assays.”
The current, open-label trial involved 144 patients with B-cell malignancies. To determine optimal dosing, the investigators recruited 17 patients with relapsed/refractory B-cell malignancies who had received at least one prior therapy. The dose expansion part of the study assessed responses in multiple cohorts, including patients with CLL/SLL, mantle cell lymphoma, and Waldenström macroglobulinemia. The primary endpoints were safety and tolerability, including maximum tolerated dose. Efficacy findings were also reported.
During dose escalation, no dose-limiting toxicities were observed, so the highest dose – 320 mg once daily or 160 mg twice daily – was selected for further testing.
The investigators highlighted efficacy and safety findings from 94 patients with CLL/SLL who were involved in dose expansion. Although nearly one-quarter (23.4%) were treatment-naive, the median number of prior therapies was two, and some patients had high-risk features, such as adverse cytogenetics, including 19.1% with a TP53 mutation and 23.3% with a 17p deletion. After a median follow-up of 13.7 months, 94.7% of these patients were still undergoing treatment.
Out of the initial 94 patients with CLL/SLL, 78 were evaluable for efficacy. The overall response rate was 96.2%, including two (2.6%) complete responses, 63 (80.8%) partial responses, and 10 (12.8%) partial responses with lymphocytosis. The median progression-free survival had not been reached, and the 12-month estimated progression-free survival was 100%.
In regard to safety, the most common adverse events were contusion (35.1%), upper respiratory tract infection (33.0%), cough (25.5%), diarrhea (21.3%), fatigue (19.1%), back pain (14.9%), hematuria (14.9%), headache (13.8%), nausea (13.8%), rash (12.8%), arthralgia (11.7%), muscle spasms (11.7%), and urinary tract infection (10.6%).
A number of other adverse events were reported, although these occurred in less than 10% of patients.
More than one-third of patients (36.2%) experienced grade 3 or higher adverse events, with neutropenia being most common (6.4%), followed by pneumonia , hypertension, and anemia, which each occurred in 2.1% of patients, and less commonly, back pain, nausea, urinary tract infection, purpura, cellulitis, and squamous cell carcinoma of the skin, which each occurred in 1.1% of patients.
“In this first-in-human study, zanubrutinib demonstrated encouraging activity in patients with relapsed/refractory and treatment-naive CLL/SLL, with good tolerability,” the investigators concluded. “Two ongoing randomized studies of zanubrutinib versus ibrutinib (NCT03053440 and NCT03734016) aim to determine whether consistent, continuous BTK blockade with a selective inhibitor results in fewer off-target effects and translates into improvements in disease control.”
The study was funded by BeiGene USA, which is developing the drug. The investigators reported relationships with the study sponsor, as well as Janssen, Pharmacyclics, AbbVie, and others.
SOURCE: Tam CSL et al. Blood. 2019 Jul 24. doi: 10.1182/blood.2019001160.
FROM BLOOD
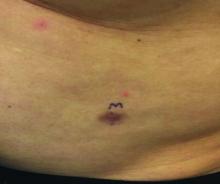
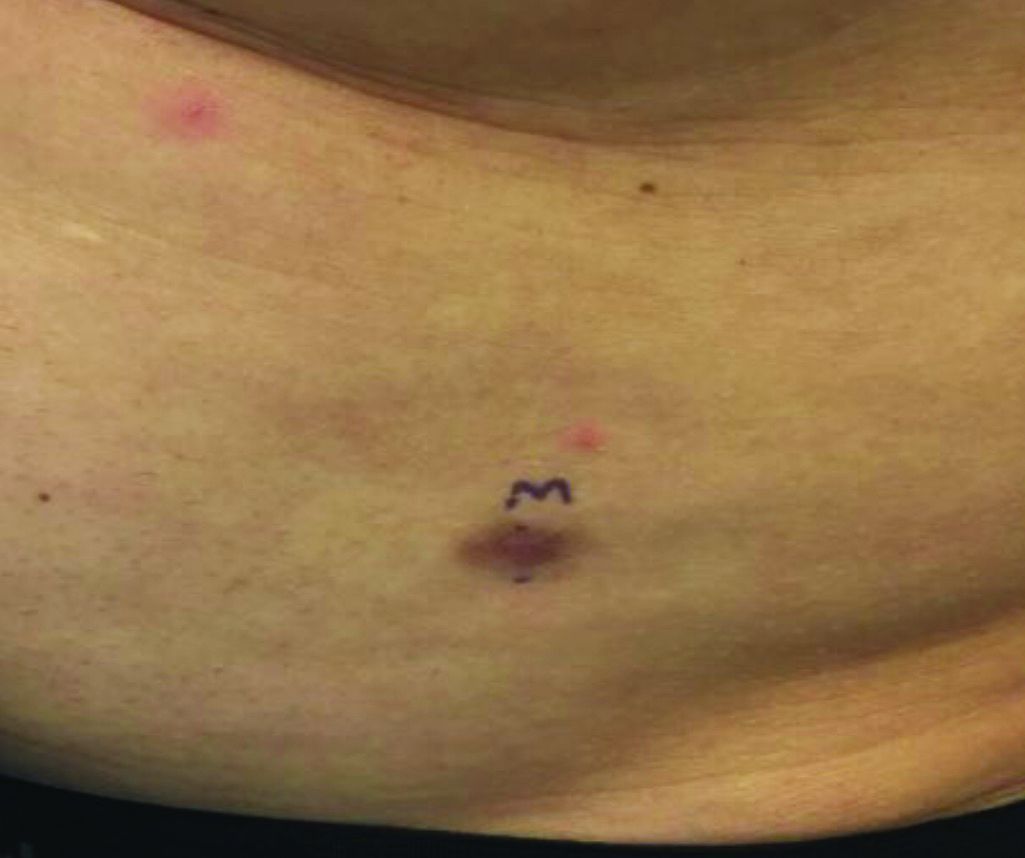
An asymptomatic reddish-brown plaque in a healthy adult man
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Chromosomal translocation abnormalities in the tumor cells involving chromosomes 17 and 22 resulting in the fusion gene COL1A1-PDGFB have been reported in DFSP. This translocation causes an overproduction of the protein platelet-derived growth factor, resulting in tumor growth.
Lesions are most common in the trunk and proximal extremities. Less commonly, the head and neck may be involved. Lesions present as painless slow-growing red-brown nodules that may become painful as they enlarge. The differential diagnosis for early DFSP includes large dermatofibroma, keloid, dermatomyofibroma, and morphea. DFSP in childhood tends to appear more atrophic. It may be difficult to diagnose DFSP if the initial biopsy is superficial. If clinical suspicion is high, rebiopsy, ideally into the fat, is recommended.
Histologically, there is a cellular proliferation of thin spindled fibroblasts and collagen in the dermis that extend into the fat, often in a multilayered pattern. Adnexal structures can be obliterated. Fibroblasts may form a cartwheel or storiform pattern. There is mild cytologic atypia. Fibrosarcomatous change may signal increased risk of metastasis. CD34 is often positive and factor XIIIa is negative, unlike in dermatofibroma, which is opposite. Forms of DFSP that can be seen histologically include atrophic DFSP (flat rather than nodular), myxoid DFSP, and pigmented DFSP (also known as Bednar tumor).
DFSP can have irregular shapes with extensions into the fat. Subsequently, DFSP has a high recurrence rate with traditional surgical removal. Mohs surgery is now the treatment of choice. Recurrent tumors should be resected. As metastasis is rare, further work-up is not routinely indicated unless history and physical examination warrant it. Imatinib mesylate (Gleevec), which targets the platelet-derived growth factor receptor, has been tried with patients with inoperable or metastatic DFSP with some success. Radiation may also be used as an adjuvant after surgery. Regular follow-up exams with examination of the surgical site for possible recurrence should be performed every 6-12 months.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Lower BMD found in patients with severe hemophilia A
Men with severe hemophilia A showed reduced levels of bone mineral density, compared with controls representative of the general population, according to findings from a case-control study.
In addition, the decrease in bone mineral density (BMD) was correlated with reduced functional ability and body mass index (BMI), and vitamin D insufficiency or deficiency.
“We aimed to investigate the presence of low BMD in adult patients diagnosed with severe hemophilia A and to evaluate the potential risk factors associated with low BMD and musculoskeletal function levels,” wrote Omer Ekinci, MD, of Firat University in Elazig, Turkey, and colleagues in Haemophilia.
The study included 41 men with severe hemophilia A and 40 men without hemophilia who were matched for age. All patients with hemophilia A received regular prophylactic therapy, and one patient had a high titre (greater than 5 Bethesda units) inhibitor against FVIII.
The researchers performed several laboratory tests: BMD was measured using dual-energy x-ray absorptiometry; BMI was recorded; and laboratory tests were performed to ascertain levels of vitamin D, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and hepatitis C and HIV antibodies. The Functional Independence Score in Hemophilia (FISH) was used to measure functional-ability status only in the study group.
After analysis, the researchers found a significant difference between patients in the case and control groups for femoral neck and total hip BMD (P = .017 and P less than .001, respectively), but not for lumbar spine BMD (P = .071).
In patients with hemophilia aged younger than 50 years, 27.8% were found to have “low normal” BMD levels, and 19.4% showed “lower than expected” BMD levels with respect to age.
“Vitamin D insufficiency and deficiency were present in 63.4% of the patients with hemophilia, significantly higher than the control group [37.5%; P less than .001],” the researchers wrote.
There were also statistically significant positive correlations between FISH score and femoral neck BMD (P = .001, r = .530), femoral neck z score (P = .001, r = .514), femoral neck T score (P = .002, r = .524), and lumbar spine BMD (P = .033, r = .334). No correlation was found between dual-energy x-ray absorptiometry measurements and the other variables (age, calcium, phosphorus, and alkaline phosphatase levels), and no results were reported for hepatitis C or HIV because none of the participants tested positive for those measures.
The most frequently reported causes of reduced BMD levels was vitamin D deficiency, low BMI, and low functional movement ability, although none of these was a strong independent risk factor in multivariate analysis, the authors reported.
They acknowledged that the results may not be generalizable to all patients because the study was conducted at a single center in Turkey.
“The results of our study emphasize the importance of early detection of comorbid conditions that decrease bone mass in severe hemophilia A patients,” they concluded.
The study was funded by the Yüzüncü Yıl University Scientific Research Project Committee. The authors reported no conflicts of interest.
SOURCE: Ekinci O et al. Haemophilia. 2019 Aug 8. doi: 10.1111/hae.13836.
Men with severe hemophilia A showed reduced levels of bone mineral density, compared with controls representative of the general population, according to findings from a case-control study.
In addition, the decrease in bone mineral density (BMD) was correlated with reduced functional ability and body mass index (BMI), and vitamin D insufficiency or deficiency.
“We aimed to investigate the presence of low BMD in adult patients diagnosed with severe hemophilia A and to evaluate the potential risk factors associated with low BMD and musculoskeletal function levels,” wrote Omer Ekinci, MD, of Firat University in Elazig, Turkey, and colleagues in Haemophilia.
The study included 41 men with severe hemophilia A and 40 men without hemophilia who were matched for age. All patients with hemophilia A received regular prophylactic therapy, and one patient had a high titre (greater than 5 Bethesda units) inhibitor against FVIII.
The researchers performed several laboratory tests: BMD was measured using dual-energy x-ray absorptiometry; BMI was recorded; and laboratory tests were performed to ascertain levels of vitamin D, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and hepatitis C and HIV antibodies. The Functional Independence Score in Hemophilia (FISH) was used to measure functional-ability status only in the study group.
After analysis, the researchers found a significant difference between patients in the case and control groups for femoral neck and total hip BMD (P = .017 and P less than .001, respectively), but not for lumbar spine BMD (P = .071).
In patients with hemophilia aged younger than 50 years, 27.8% were found to have “low normal” BMD levels, and 19.4% showed “lower than expected” BMD levels with respect to age.
“Vitamin D insufficiency and deficiency were present in 63.4% of the patients with hemophilia, significantly higher than the control group [37.5%; P less than .001],” the researchers wrote.
There were also statistically significant positive correlations between FISH score and femoral neck BMD (P = .001, r = .530), femoral neck z score (P = .001, r = .514), femoral neck T score (P = .002, r = .524), and lumbar spine BMD (P = .033, r = .334). No correlation was found between dual-energy x-ray absorptiometry measurements and the other variables (age, calcium, phosphorus, and alkaline phosphatase levels), and no results were reported for hepatitis C or HIV because none of the participants tested positive for those measures.
The most frequently reported causes of reduced BMD levels was vitamin D deficiency, low BMI, and low functional movement ability, although none of these was a strong independent risk factor in multivariate analysis, the authors reported.
They acknowledged that the results may not be generalizable to all patients because the study was conducted at a single center in Turkey.
“The results of our study emphasize the importance of early detection of comorbid conditions that decrease bone mass in severe hemophilia A patients,” they concluded.
The study was funded by the Yüzüncü Yıl University Scientific Research Project Committee. The authors reported no conflicts of interest.
SOURCE: Ekinci O et al. Haemophilia. 2019 Aug 8. doi: 10.1111/hae.13836.
Men with severe hemophilia A showed reduced levels of bone mineral density, compared with controls representative of the general population, according to findings from a case-control study.
In addition, the decrease in bone mineral density (BMD) was correlated with reduced functional ability and body mass index (BMI), and vitamin D insufficiency or deficiency.
“We aimed to investigate the presence of low BMD in adult patients diagnosed with severe hemophilia A and to evaluate the potential risk factors associated with low BMD and musculoskeletal function levels,” wrote Omer Ekinci, MD, of Firat University in Elazig, Turkey, and colleagues in Haemophilia.
The study included 41 men with severe hemophilia A and 40 men without hemophilia who were matched for age. All patients with hemophilia A received regular prophylactic therapy, and one patient had a high titre (greater than 5 Bethesda units) inhibitor against FVIII.
The researchers performed several laboratory tests: BMD was measured using dual-energy x-ray absorptiometry; BMI was recorded; and laboratory tests were performed to ascertain levels of vitamin D, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, and hepatitis C and HIV antibodies. The Functional Independence Score in Hemophilia (FISH) was used to measure functional-ability status only in the study group.
After analysis, the researchers found a significant difference between patients in the case and control groups for femoral neck and total hip BMD (P = .017 and P less than .001, respectively), but not for lumbar spine BMD (P = .071).
In patients with hemophilia aged younger than 50 years, 27.8% were found to have “low normal” BMD levels, and 19.4% showed “lower than expected” BMD levels with respect to age.
“Vitamin D insufficiency and deficiency were present in 63.4% of the patients with hemophilia, significantly higher than the control group [37.5%; P less than .001],” the researchers wrote.
There were also statistically significant positive correlations between FISH score and femoral neck BMD (P = .001, r = .530), femoral neck z score (P = .001, r = .514), femoral neck T score (P = .002, r = .524), and lumbar spine BMD (P = .033, r = .334). No correlation was found between dual-energy x-ray absorptiometry measurements and the other variables (age, calcium, phosphorus, and alkaline phosphatase levels), and no results were reported for hepatitis C or HIV because none of the participants tested positive for those measures.
The most frequently reported causes of reduced BMD levels was vitamin D deficiency, low BMI, and low functional movement ability, although none of these was a strong independent risk factor in multivariate analysis, the authors reported.
They acknowledged that the results may not be generalizable to all patients because the study was conducted at a single center in Turkey.
“The results of our study emphasize the importance of early detection of comorbid conditions that decrease bone mass in severe hemophilia A patients,” they concluded.
The study was funded by the Yüzüncü Yıl University Scientific Research Project Committee. The authors reported no conflicts of interest.
SOURCE: Ekinci O et al. Haemophilia. 2019 Aug 8. doi: 10.1111/hae.13836.
FROM HAEMOPHILIA
Diagnosing and managing diabetes and depression
SAN DIEGO – Nearly 350 years ago, British physician Thomas Willis wrote that diabetes seemed often to occur in patients who were experiencing “significant life stress, sadness, or long sorrow.” That, according to Ellen D. Mandel, DMH, MPA, MS, PA-C, RDN, CDE, a clinical professor at Pace University in New York City, was an important insight into the link between mind and body in patients with diabetes.
“As clinicians, we should be worried about mental illness in our patients with diabetes,” Dr. Mandel, a physician assistant educator, said during a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education.
In particular, she said, – and vice versa.
Dr. Mandel pointed to findings suggesting that 11% of patients with diabetes show signs of clinical depression, which is higher than in the general population, with many more believed to have subclinical depression (Diabetes Care. 2015;38[4]:551-60).
Anxiety can be a key factor in trying to understand how diabetes might contribute to depression. “Diabetes is a very stressful condition ... [and patients] may be fatigued and exhausted.” On top of that, they have to make nutrition changes, or at least pay attention to their diet and overall care, all of which can have a cumulatively negative impact on patient well-being.
Conversely, depression can contribute to diabetes. “They kind of go hand in hand,” she said, pointing to depression’s ability to disrupt appetite, diminish energy, and boost levels of cortisol.
Among the findings that provide evidence of a link between diabetes and depression are those from a study in which investigators estimated that for every 1-point increase in depression symptoms, the risk of diabetes will go up by as much as 5% (Clin Diabetes Endocrinol. 2018 Jan 4. doi: 10.1186/s40842-017-0052-1). Moreover, a 2013 review linked the combination of diabetes and depression to an adjusted 1.5-fold increase in risk of all-cause death (PLoS One. 2013 Mar 5. doi: 10.1371/journal.pone.0057058).
Dr. Mandel offered these tips about diagnosing depression in patients with diabetes and helping them feel comfortable:
- Put yourself in the patient’s shoes. “One of the biggest barriers to referring patients to diabetic education is that they don’t want to have to admit to a group that they have diabetes. They keep it to themselves, to their own detriment. In addition, there’s a lot of worry about insurance.” Patients with diabetes often have self-esteem issues and financial or insurance challenges, all of which need to be factored in when working with them, Dr. Mandel said.
- Ask questions and use screening tools. Two simple questions are helpful in starting a conversation and gathering useful information: Over the past 2 weeks, have you often been bothered by [having] little interest or pleasure in doing things? What about being bothered by feeling down, depressed, or hopeless? If the patient answers “yes” to either of these questions, it will be a positive screen, and two “no” answers will be a negative screen. With the “yes” responses, one should follow-up with a screening tool – typically, the one approved by your institution. Dr. Mandel also highlighted the Patient Health Questionnaire depression scale (PHQ-9), which is available online, or the brief, two-item Diabetes Distress Scale (DDS2) questionnaire.
- Keep your own language in mind. “The way you communicate with your patients can elevate their feeling about themselves or destroy how they feel about themselves,” Dr. Mandel said. “We’re trying to stop calling people with diabetes ‘diabetics.’ People don’t want to be labeled like that. Don’t blame yourself if you use this language, but work to make the changes,” Dr. Mandel suggested.
- Watch out for other forms of bias. Beware of unconsciously stereotyping your patients. “It affects how people relate to you, how they adhere to your suggestions, and how much they’ll trust [and confide in] you, which can have clinical implications,” Dr. Mandel said.
Global Academy and this news organization are owned by the same parent company. Dr. Mandel has no disclosures.
SAN DIEGO – Nearly 350 years ago, British physician Thomas Willis wrote that diabetes seemed often to occur in patients who were experiencing “significant life stress, sadness, or long sorrow.” That, according to Ellen D. Mandel, DMH, MPA, MS, PA-C, RDN, CDE, a clinical professor at Pace University in New York City, was an important insight into the link between mind and body in patients with diabetes.
“As clinicians, we should be worried about mental illness in our patients with diabetes,” Dr. Mandel, a physician assistant educator, said during a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education.
In particular, she said, – and vice versa.
Dr. Mandel pointed to findings suggesting that 11% of patients with diabetes show signs of clinical depression, which is higher than in the general population, with many more believed to have subclinical depression (Diabetes Care. 2015;38[4]:551-60).
Anxiety can be a key factor in trying to understand how diabetes might contribute to depression. “Diabetes is a very stressful condition ... [and patients] may be fatigued and exhausted.” On top of that, they have to make nutrition changes, or at least pay attention to their diet and overall care, all of which can have a cumulatively negative impact on patient well-being.
Conversely, depression can contribute to diabetes. “They kind of go hand in hand,” she said, pointing to depression’s ability to disrupt appetite, diminish energy, and boost levels of cortisol.
Among the findings that provide evidence of a link between diabetes and depression are those from a study in which investigators estimated that for every 1-point increase in depression symptoms, the risk of diabetes will go up by as much as 5% (Clin Diabetes Endocrinol. 2018 Jan 4. doi: 10.1186/s40842-017-0052-1). Moreover, a 2013 review linked the combination of diabetes and depression to an adjusted 1.5-fold increase in risk of all-cause death (PLoS One. 2013 Mar 5. doi: 10.1371/journal.pone.0057058).
Dr. Mandel offered these tips about diagnosing depression in patients with diabetes and helping them feel comfortable:
- Put yourself in the patient’s shoes. “One of the biggest barriers to referring patients to diabetic education is that they don’t want to have to admit to a group that they have diabetes. They keep it to themselves, to their own detriment. In addition, there’s a lot of worry about insurance.” Patients with diabetes often have self-esteem issues and financial or insurance challenges, all of which need to be factored in when working with them, Dr. Mandel said.
- Ask questions and use screening tools. Two simple questions are helpful in starting a conversation and gathering useful information: Over the past 2 weeks, have you often been bothered by [having] little interest or pleasure in doing things? What about being bothered by feeling down, depressed, or hopeless? If the patient answers “yes” to either of these questions, it will be a positive screen, and two “no” answers will be a negative screen. With the “yes” responses, one should follow-up with a screening tool – typically, the one approved by your institution. Dr. Mandel also highlighted the Patient Health Questionnaire depression scale (PHQ-9), which is available online, or the brief, two-item Diabetes Distress Scale (DDS2) questionnaire.
- Keep your own language in mind. “The way you communicate with your patients can elevate their feeling about themselves or destroy how they feel about themselves,” Dr. Mandel said. “We’re trying to stop calling people with diabetes ‘diabetics.’ People don’t want to be labeled like that. Don’t blame yourself if you use this language, but work to make the changes,” Dr. Mandel suggested.
- Watch out for other forms of bias. Beware of unconsciously stereotyping your patients. “It affects how people relate to you, how they adhere to your suggestions, and how much they’ll trust [and confide in] you, which can have clinical implications,” Dr. Mandel said.
Global Academy and this news organization are owned by the same parent company. Dr. Mandel has no disclosures.
SAN DIEGO – Nearly 350 years ago, British physician Thomas Willis wrote that diabetes seemed often to occur in patients who were experiencing “significant life stress, sadness, or long sorrow.” That, according to Ellen D. Mandel, DMH, MPA, MS, PA-C, RDN, CDE, a clinical professor at Pace University in New York City, was an important insight into the link between mind and body in patients with diabetes.
“As clinicians, we should be worried about mental illness in our patients with diabetes,” Dr. Mandel, a physician assistant educator, said during a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education.
In particular, she said, – and vice versa.
Dr. Mandel pointed to findings suggesting that 11% of patients with diabetes show signs of clinical depression, which is higher than in the general population, with many more believed to have subclinical depression (Diabetes Care. 2015;38[4]:551-60).
Anxiety can be a key factor in trying to understand how diabetes might contribute to depression. “Diabetes is a very stressful condition ... [and patients] may be fatigued and exhausted.” On top of that, they have to make nutrition changes, or at least pay attention to their diet and overall care, all of which can have a cumulatively negative impact on patient well-being.
Conversely, depression can contribute to diabetes. “They kind of go hand in hand,” she said, pointing to depression’s ability to disrupt appetite, diminish energy, and boost levels of cortisol.
Among the findings that provide evidence of a link between diabetes and depression are those from a study in which investigators estimated that for every 1-point increase in depression symptoms, the risk of diabetes will go up by as much as 5% (Clin Diabetes Endocrinol. 2018 Jan 4. doi: 10.1186/s40842-017-0052-1). Moreover, a 2013 review linked the combination of diabetes and depression to an adjusted 1.5-fold increase in risk of all-cause death (PLoS One. 2013 Mar 5. doi: 10.1371/journal.pone.0057058).
Dr. Mandel offered these tips about diagnosing depression in patients with diabetes and helping them feel comfortable:
- Put yourself in the patient’s shoes. “One of the biggest barriers to referring patients to diabetic education is that they don’t want to have to admit to a group that they have diabetes. They keep it to themselves, to their own detriment. In addition, there’s a lot of worry about insurance.” Patients with diabetes often have self-esteem issues and financial or insurance challenges, all of which need to be factored in when working with them, Dr. Mandel said.
- Ask questions and use screening tools. Two simple questions are helpful in starting a conversation and gathering useful information: Over the past 2 weeks, have you often been bothered by [having] little interest or pleasure in doing things? What about being bothered by feeling down, depressed, or hopeless? If the patient answers “yes” to either of these questions, it will be a positive screen, and two “no” answers will be a negative screen. With the “yes” responses, one should follow-up with a screening tool – typically, the one approved by your institution. Dr. Mandel also highlighted the Patient Health Questionnaire depression scale (PHQ-9), which is available online, or the brief, two-item Diabetes Distress Scale (DDS2) questionnaire.
- Keep your own language in mind. “The way you communicate with your patients can elevate their feeling about themselves or destroy how they feel about themselves,” Dr. Mandel said. “We’re trying to stop calling people with diabetes ‘diabetics.’ People don’t want to be labeled like that. Don’t blame yourself if you use this language, but work to make the changes,” Dr. Mandel suggested.
- Watch out for other forms of bias. Beware of unconsciously stereotyping your patients. “It affects how people relate to you, how they adhere to your suggestions, and how much they’ll trust [and confide in] you, which can have clinical implications,” Dr. Mandel said.
Global Academy and this news organization are owned by the same parent company. Dr. Mandel has no disclosures.
EXPERT ANALYSIS FROM MEDS 2019
Evinacumab shows promise for HoFH in top line results
(HoFH), according to a release from the company developing the drug.
LDL cholesterol levels were 255 mg/dL on average for patients at the outset of the trial despite treatment with other lipid-lowering therapies; however, combining this drug with lipid-lowering therapies including maximally-tolerated statins, PCSK9 inhibitors, and LDL apheresis, reduced LDL cholesterol by an average of 49% by week 24 relative to treatment with lipid-lowering therapies alone (P less than .0001). Furthermore, 47% of patients taking evinacumab achieved LDL cholesterol levels under 100 mg/dL by that time point versus 23% of those taking lipid-lowering therapies only.
Treatment with evinacumab showed lowering effects as early as the first assessment at 2 weeks, and these effects were maintained.
HoFH is an inherited, rare, but serious condition estimated to affect 1,300 people in the United States; it can lead to early atherosclerotic disease, and even teenagers with this genetic disorder can suffer cardiac events. Further details and data from the trial, called ELIPSE HoFH, will be reported at a future medical meeting, and will be submitted to the Food and Drug Administration for consideration.
Evinacumab is an investigational, fully-human, monoclonal antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3), which acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Evinacumab was granted breakthrough therapy designation for treatment of HoFH by the FDA in 2017, which entails an expedited review and development process for this drug because preliminary results have suggested it could have a substantial effect on a life-threatening or serious condition.
The company’s full release can be read on its website.
(HoFH), according to a release from the company developing the drug.
LDL cholesterol levels were 255 mg/dL on average for patients at the outset of the trial despite treatment with other lipid-lowering therapies; however, combining this drug with lipid-lowering therapies including maximally-tolerated statins, PCSK9 inhibitors, and LDL apheresis, reduced LDL cholesterol by an average of 49% by week 24 relative to treatment with lipid-lowering therapies alone (P less than .0001). Furthermore, 47% of patients taking evinacumab achieved LDL cholesterol levels under 100 mg/dL by that time point versus 23% of those taking lipid-lowering therapies only.
Treatment with evinacumab showed lowering effects as early as the first assessment at 2 weeks, and these effects were maintained.
HoFH is an inherited, rare, but serious condition estimated to affect 1,300 people in the United States; it can lead to early atherosclerotic disease, and even teenagers with this genetic disorder can suffer cardiac events. Further details and data from the trial, called ELIPSE HoFH, will be reported at a future medical meeting, and will be submitted to the Food and Drug Administration for consideration.
Evinacumab is an investigational, fully-human, monoclonal antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3), which acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Evinacumab was granted breakthrough therapy designation for treatment of HoFH by the FDA in 2017, which entails an expedited review and development process for this drug because preliminary results have suggested it could have a substantial effect on a life-threatening or serious condition.
The company’s full release can be read on its website.
(HoFH), according to a release from the company developing the drug.
LDL cholesterol levels were 255 mg/dL on average for patients at the outset of the trial despite treatment with other lipid-lowering therapies; however, combining this drug with lipid-lowering therapies including maximally-tolerated statins, PCSK9 inhibitors, and LDL apheresis, reduced LDL cholesterol by an average of 49% by week 24 relative to treatment with lipid-lowering therapies alone (P less than .0001). Furthermore, 47% of patients taking evinacumab achieved LDL cholesterol levels under 100 mg/dL by that time point versus 23% of those taking lipid-lowering therapies only.
Treatment with evinacumab showed lowering effects as early as the first assessment at 2 weeks, and these effects were maintained.
HoFH is an inherited, rare, but serious condition estimated to affect 1,300 people in the United States; it can lead to early atherosclerotic disease, and even teenagers with this genetic disorder can suffer cardiac events. Further details and data from the trial, called ELIPSE HoFH, will be reported at a future medical meeting, and will be submitted to the Food and Drug Administration for consideration.
Evinacumab is an investigational, fully-human, monoclonal antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3), which acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Evinacumab was granted breakthrough therapy designation for treatment of HoFH by the FDA in 2017, which entails an expedited review and development process for this drug because preliminary results have suggested it could have a substantial effect on a life-threatening or serious condition.
The company’s full release can be read on its website.
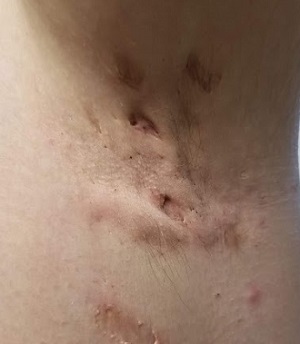
Boiling Points
This 37-year-old woman began developing “boils” under both arms at age 12. Over the years, the lesions have become more numerous and bothersome. They are often painful and large and are capable of bursting on their own, releasing purulent material. Occasionally, similar lesions appear under her breasts and in the groin. The problem seems to wax and wane with her menstrual cycle. Family history reveals that both her mother and one of her sisters have had the same problem, again starting around the time of menarche.
Whenever the patient seeks medical care, usually at the emergency department, the diagnosis is always the same: boils. Normally, the prescribed treatment includes incision, drainage, and packing of the largest lesions, followed by 2 weeks of oral antibiotics. While the problem generally improves after treatment, it invariably returns.
Her health is decent overall. However, she has been overweight for years and has been smoking since she was 14.
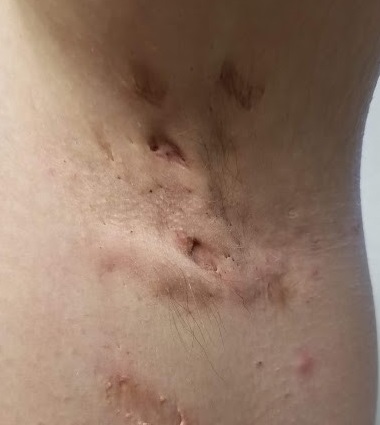
EXAMINATION
The patient’s left axilla shows ropy, hypertrophic scars, many comedones, and several fluctuant cystic subcutaneous masses. There is no frank erythema, although the patient indicates there often is.
No such changes are seen on examination of her right axilla. Instead, there is a slender 12-cm linear scar running across the axillary fold. Upon questioning, the patient reports that several years ago, a surgeon removed three-fourths of the skin and subcutaneous tissue from this area. This procedure cured the “boils” on her right arm, but it also left her with chronic lymphedema in that extremity.
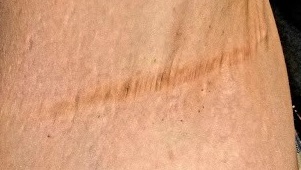
Other intertriginous areas are free of significant changes.
What’s the diagnosis?
DISCUSSION
In the US, hidradenitis suppurativa (HS) affects 1% to 4% of the population and about 4 times as many females as males. But as this case demonstrates, it is consistently misidentified as “boils” or “staph infection” by providers unfamiliar with the correct diagnosis.
HS involves hair follicles in intertriginous areas of the body that are rich with apocrine glands (eg, armpit, groin). The condition, initially known as acne inversa, was first described in 1833 by Dr. Alfred Velpeau, a French surgeon. Despite some minor similarities, HS is not actually a form of acne, nor is it an infection. About one-third of HS patients inherit the condition, and generally, onset occurs post puberty, suggesting a hormonal component.
With HS, the hair follicle and associated apocrine gland fail to function normally. As sweat accumulates in subcutaneous tissue, it creates a chronic inflammatory reaction manifesting with large comedones, cysts, and abscesses. Eventually, it can result in ropy, hypertrophic scars on the surface and deep tracts connecting multiple lesions. HS is classified as mild (stage 1), moderate (stage 2), or severe (stage 3) using the Hurley staging system.
HS is notoriously difficult to cure, but the anti-inflammatory effects of some antibiotics (eg, minocycline, doxycycline) can offer some relief, as can anti-androgens (eg, spironolactone). The use of isotretinoin has yielded disappointing results. For small lesions, intralesional injection of glucocorticoids can be useful for short-term relief of pain and swelling.
The most encouraging recent development in HS treatment is the approval for the use of adalimumab (Humira) in severe cases that have failed to respond to other modalities. Even with use of this biologic, decent control is probably the best outcome—and that’s at an annual cost of $50,000, plus the patient’s exposure to potentially serious adverse effects due to immunosuppression.
Another approach is surgical, with all its attendant risks, as this patient experienced in her right axilla. Simple incision and drainage offer little beyond temporary relief of pain.
Environmental factors should not be overlooked; obesity and smoking have both been linked to HS in multiple studies.
TAKE-HOME LEARNING POINTS
- Hidradenitis suppurativa, also known as acne inversa, results from malfunction of the hair follicle and associated apocrine glands in intertriginous areas.
- HS can range from mild (with minor pustules and sparse comedones) to and severe (diffuse disease, affecting multiple areas with heavy ropy scarring, large painful abscesses, and connecting tracts).
- HS affects approximately 4 times as many females as males, almost all with post-pubertal onset—strongly suggestive of a hormonal component.
- Treatment is problematic, although the recent approval of adalimumab for use in HS is proving to be helpful, if not curative. Some oral antibiotics and anti-androgens have shown mixed results.
This 37-year-old woman began developing “boils” under both arms at age 12. Over the years, the lesions have become more numerous and bothersome. They are often painful and large and are capable of bursting on their own, releasing purulent material. Occasionally, similar lesions appear under her breasts and in the groin. The problem seems to wax and wane with her menstrual cycle. Family history reveals that both her mother and one of her sisters have had the same problem, again starting around the time of menarche.
Whenever the patient seeks medical care, usually at the emergency department, the diagnosis is always the same: boils. Normally, the prescribed treatment includes incision, drainage, and packing of the largest lesions, followed by 2 weeks of oral antibiotics. While the problem generally improves after treatment, it invariably returns.
Her health is decent overall. However, she has been overweight for years and has been smoking since she was 14.

EXAMINATION
The patient’s left axilla shows ropy, hypertrophic scars, many comedones, and several fluctuant cystic subcutaneous masses. There is no frank erythema, although the patient indicates there often is.
No such changes are seen on examination of her right axilla. Instead, there is a slender 12-cm linear scar running across the axillary fold. Upon questioning, the patient reports that several years ago, a surgeon removed three-fourths of the skin and subcutaneous tissue from this area. This procedure cured the “boils” on her right arm, but it also left her with chronic lymphedema in that extremity.

Other intertriginous areas are free of significant changes.
What’s the diagnosis?
DISCUSSION
In the US, hidradenitis suppurativa (HS) affects 1% to 4% of the population and about 4 times as many females as males. But as this case demonstrates, it is consistently misidentified as “boils” or “staph infection” by providers unfamiliar with the correct diagnosis.
HS involves hair follicles in intertriginous areas of the body that are rich with apocrine glands (eg, armpit, groin). The condition, initially known as acne inversa, was first described in 1833 by Dr. Alfred Velpeau, a French surgeon. Despite some minor similarities, HS is not actually a form of acne, nor is it an infection. About one-third of HS patients inherit the condition, and generally, onset occurs post puberty, suggesting a hormonal component.
With HS, the hair follicle and associated apocrine gland fail to function normally. As sweat accumulates in subcutaneous tissue, it creates a chronic inflammatory reaction manifesting with large comedones, cysts, and abscesses. Eventually, it can result in ropy, hypertrophic scars on the surface and deep tracts connecting multiple lesions. HS is classified as mild (stage 1), moderate (stage 2), or severe (stage 3) using the Hurley staging system.
HS is notoriously difficult to cure, but the anti-inflammatory effects of some antibiotics (eg, minocycline, doxycycline) can offer some relief, as can anti-androgens (eg, spironolactone). The use of isotretinoin has yielded disappointing results. For small lesions, intralesional injection of glucocorticoids can be useful for short-term relief of pain and swelling.
The most encouraging recent development in HS treatment is the approval for the use of adalimumab (Humira) in severe cases that have failed to respond to other modalities. Even with use of this biologic, decent control is probably the best outcome—and that’s at an annual cost of $50,000, plus the patient’s exposure to potentially serious adverse effects due to immunosuppression.
Another approach is surgical, with all its attendant risks, as this patient experienced in her right axilla. Simple incision and drainage offer little beyond temporary relief of pain.
Environmental factors should not be overlooked; obesity and smoking have both been linked to HS in multiple studies.
TAKE-HOME LEARNING POINTS
- Hidradenitis suppurativa, also known as acne inversa, results from malfunction of the hair follicle and associated apocrine glands in intertriginous areas.
- HS can range from mild (with minor pustules and sparse comedones) to and severe (diffuse disease, affecting multiple areas with heavy ropy scarring, large painful abscesses, and connecting tracts).
- HS affects approximately 4 times as many females as males, almost all with post-pubertal onset—strongly suggestive of a hormonal component.
- Treatment is problematic, although the recent approval of adalimumab for use in HS is proving to be helpful, if not curative. Some oral antibiotics and anti-androgens have shown mixed results.
This 37-year-old woman began developing “boils” under both arms at age 12. Over the years, the lesions have become more numerous and bothersome. They are often painful and large and are capable of bursting on their own, releasing purulent material. Occasionally, similar lesions appear under her breasts and in the groin. The problem seems to wax and wane with her menstrual cycle. Family history reveals that both her mother and one of her sisters have had the same problem, again starting around the time of menarche.
Whenever the patient seeks medical care, usually at the emergency department, the diagnosis is always the same: boils. Normally, the prescribed treatment includes incision, drainage, and packing of the largest lesions, followed by 2 weeks of oral antibiotics. While the problem generally improves after treatment, it invariably returns.
Her health is decent overall. However, she has been overweight for years and has been smoking since she was 14.

EXAMINATION
The patient’s left axilla shows ropy, hypertrophic scars, many comedones, and several fluctuant cystic subcutaneous masses. There is no frank erythema, although the patient indicates there often is.
No such changes are seen on examination of her right axilla. Instead, there is a slender 12-cm linear scar running across the axillary fold. Upon questioning, the patient reports that several years ago, a surgeon removed three-fourths of the skin and subcutaneous tissue from this area. This procedure cured the “boils” on her right arm, but it also left her with chronic lymphedema in that extremity.

Other intertriginous areas are free of significant changes.
What’s the diagnosis?
DISCUSSION
In the US, hidradenitis suppurativa (HS) affects 1% to 4% of the population and about 4 times as many females as males. But as this case demonstrates, it is consistently misidentified as “boils” or “staph infection” by providers unfamiliar with the correct diagnosis.
HS involves hair follicles in intertriginous areas of the body that are rich with apocrine glands (eg, armpit, groin). The condition, initially known as acne inversa, was first described in 1833 by Dr. Alfred Velpeau, a French surgeon. Despite some minor similarities, HS is not actually a form of acne, nor is it an infection. About one-third of HS patients inherit the condition, and generally, onset occurs post puberty, suggesting a hormonal component.
With HS, the hair follicle and associated apocrine gland fail to function normally. As sweat accumulates in subcutaneous tissue, it creates a chronic inflammatory reaction manifesting with large comedones, cysts, and abscesses. Eventually, it can result in ropy, hypertrophic scars on the surface and deep tracts connecting multiple lesions. HS is classified as mild (stage 1), moderate (stage 2), or severe (stage 3) using the Hurley staging system.
HS is notoriously difficult to cure, but the anti-inflammatory effects of some antibiotics (eg, minocycline, doxycycline) can offer some relief, as can anti-androgens (eg, spironolactone). The use of isotretinoin has yielded disappointing results. For small lesions, intralesional injection of glucocorticoids can be useful for short-term relief of pain and swelling.
The most encouraging recent development in HS treatment is the approval for the use of adalimumab (Humira) in severe cases that have failed to respond to other modalities. Even with use of this biologic, decent control is probably the best outcome—and that’s at an annual cost of $50,000, plus the patient’s exposure to potentially serious adverse effects due to immunosuppression.
Another approach is surgical, with all its attendant risks, as this patient experienced in her right axilla. Simple incision and drainage offer little beyond temporary relief of pain.
Environmental factors should not be overlooked; obesity and smoking have both been linked to HS in multiple studies.
TAKE-HOME LEARNING POINTS
- Hidradenitis suppurativa, also known as acne inversa, results from malfunction of the hair follicle and associated apocrine glands in intertriginous areas.
- HS can range from mild (with minor pustules and sparse comedones) to and severe (diffuse disease, affecting multiple areas with heavy ropy scarring, large painful abscesses, and connecting tracts).
- HS affects approximately 4 times as many females as males, almost all with post-pubertal onset—strongly suggestive of a hormonal component.
- Treatment is problematic, although the recent approval of adalimumab for use in HS is proving to be helpful, if not curative. Some oral antibiotics and anti-androgens have shown mixed results.
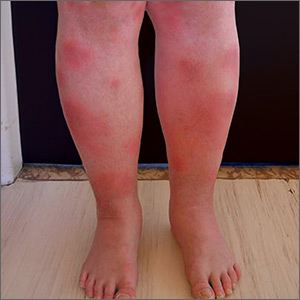
Tender swellings on legs
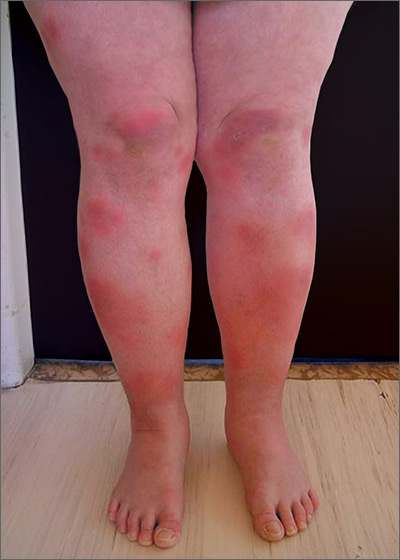
Based on the physical exam findings, the FP diagnosed erythema nodosum (EN) in this patient. He considered doing a punch biopsy down to the fat to prove that this was a panniculus, but realized that this was a classic presentation of EN. The lesions of EN are deep-seated nodules that may be more easily palpated than visualized. These lesions are initially firm, round or oval, and poorly demarcated. As seen in this case, the lesions may be bright red, warm, and painful.
The FP sought to consider the cause, and questioned the patient further about medications and other symptoms; however, he was unable to uncover any likely “suspects.” He then drew labs for a complete blood count, comprehensive metabolic panel, and uric acid and QuantiFERON TB gold tests. He started the patient on ibuprofen 400 mg tid with meals for the pain and inflammation.
On a follow-up visit 2 weeks later, all of the lab results were normal and the patient was about 50% improved. At this time, the FP obtained a chest x-ray to look for any evidence of sarcoidosis. The x-ray was also normal. (About half of all cases of EN are idiopathic, so the normal results were not surprising.) By the third visit the patient was 90% better and was happy to keep taking the ibuprofen to see if this would resolve completely.
After 6 weeks of treatment, there were no more tender erythematous nodules. All that remained was some postinflammatory hyperpigmentation. The patient was happy with these results and understood that she should return if the EN came back.
Photo courtesy of Hanuš Rozsypal, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the physical exam findings, the FP diagnosed erythema nodosum (EN) in this patient. He considered doing a punch biopsy down to the fat to prove that this was a panniculus, but realized that this was a classic presentation of EN. The lesions of EN are deep-seated nodules that may be more easily palpated than visualized. These lesions are initially firm, round or oval, and poorly demarcated. As seen in this case, the lesions may be bright red, warm, and painful.
The FP sought to consider the cause, and questioned the patient further about medications and other symptoms; however, he was unable to uncover any likely “suspects.” He then drew labs for a complete blood count, comprehensive metabolic panel, and uric acid and QuantiFERON TB gold tests. He started the patient on ibuprofen 400 mg tid with meals for the pain and inflammation.
On a follow-up visit 2 weeks later, all of the lab results were normal and the patient was about 50% improved. At this time, the FP obtained a chest x-ray to look for any evidence of sarcoidosis. The x-ray was also normal. (About half of all cases of EN are idiopathic, so the normal results were not surprising.) By the third visit the patient was 90% better and was happy to keep taking the ibuprofen to see if this would resolve completely.
After 6 weeks of treatment, there were no more tender erythematous nodules. All that remained was some postinflammatory hyperpigmentation. The patient was happy with these results and understood that she should return if the EN came back.
Photo courtesy of Hanuš Rozsypal, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

Based on the physical exam findings, the FP diagnosed erythema nodosum (EN) in this patient. He considered doing a punch biopsy down to the fat to prove that this was a panniculus, but realized that this was a classic presentation of EN. The lesions of EN are deep-seated nodules that may be more easily palpated than visualized. These lesions are initially firm, round or oval, and poorly demarcated. As seen in this case, the lesions may be bright red, warm, and painful.
The FP sought to consider the cause, and questioned the patient further about medications and other symptoms; however, he was unable to uncover any likely “suspects.” He then drew labs for a complete blood count, comprehensive metabolic panel, and uric acid and QuantiFERON TB gold tests. He started the patient on ibuprofen 400 mg tid with meals for the pain and inflammation.
On a follow-up visit 2 weeks later, all of the lab results were normal and the patient was about 50% improved. At this time, the FP obtained a chest x-ray to look for any evidence of sarcoidosis. The x-ray was also normal. (About half of all cases of EN are idiopathic, so the normal results were not surprising.) By the third visit the patient was 90% better and was happy to keep taking the ibuprofen to see if this would resolve completely.
After 6 weeks of treatment, there were no more tender erythematous nodules. All that remained was some postinflammatory hyperpigmentation. The patient was happy with these results and understood that she should return if the EN came back.
Photo courtesy of Hanuš Rozsypal, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Diaz L, Paulis R. Erythema nodosum. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Maternal factors impact childhood skin microbiota
Bacteria on children’s skin was similar to their mothers’ and affected by factors that included method of delivery and breastfeeding in a study of 154 children aged 10 years and younger.
Understanding the wrote Ting Zhu of Fudan University, Shanghai, China, and colleagues.
In a study published in the Journal of Investigative Dermatology, the researchers compared the skin microbiota of the 158 children aged 1-10 years and 50 mothers using 16S rRNA gene amplicon sequencing after collecting study samples from three skin areas: face, calf, and ventral forearm. The samples were pooled into 36 groups based on age, gender, and skin site.
“We observed significant differences in alpha diversity and the most prevalent taxa and identified factors that contributed to variation at each site,” the authors reported.
Overall, the “alpha diversity” – a measure of microbial diversity used in microbiome studies – of the skin microbiome increased with age, with the highest alpha diversity seen in the 10-year-olds (n = 28), notably on the face, but differences in alpha diversity between skin sites were seen only in the 1-year-olds (n = 26). Overall, the most commonly identified bacterial phyla at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%). In the three sites, the genera with high relative abundance (over 3%) included Streptococcus (13%), Enhydrobacter (6%), and Propionibacterium (5%). Of these, Streptococcus and Granulicatella showed negative linear correlations with age.
The researchers found significant differences between the bacterial communities of 10-year-olds delivered by Cesarean section and those delivered vaginally, particularly in the facial samples; however the difference wasn’t observed among face samples taken from 1-year-olds, according to the authors. They found significant variation in bacteria in calf samples based on whether the children were fed breast milk, formula, or a combination.
When the researchers examined the correlations between mother/child pairs, they found that the relative abundance of most bacteria in the children were more similar to their mothers than to unrelated adults, and they found the strongest correlations for the genera Deinococcus, Microbacterium, Chryseobacterium, Klebsiella, and Enhydrobacter. The relationships between the bacterial communities of mothers and children may be influenced by the shared living environment, topical products, and daily diet, they noted.
The study findings were limited by not controlling for certain variables, including daily diet, choice of topical products, bathing habits, and daily variation in environmental factors, the researchers wrote. However, the results show “that the skin microbiome is strongly affected by the surrounding microenvironment and that the alpha diversity of the skin microbiome increases during childhood,” they concluded.
The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
SOURCE: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
Bacteria on children’s skin was similar to their mothers’ and affected by factors that included method of delivery and breastfeeding in a study of 154 children aged 10 years and younger.
Understanding the wrote Ting Zhu of Fudan University, Shanghai, China, and colleagues.
In a study published in the Journal of Investigative Dermatology, the researchers compared the skin microbiota of the 158 children aged 1-10 years and 50 mothers using 16S rRNA gene amplicon sequencing after collecting study samples from three skin areas: face, calf, and ventral forearm. The samples were pooled into 36 groups based on age, gender, and skin site.
“We observed significant differences in alpha diversity and the most prevalent taxa and identified factors that contributed to variation at each site,” the authors reported.
Overall, the “alpha diversity” – a measure of microbial diversity used in microbiome studies – of the skin microbiome increased with age, with the highest alpha diversity seen in the 10-year-olds (n = 28), notably on the face, but differences in alpha diversity between skin sites were seen only in the 1-year-olds (n = 26). Overall, the most commonly identified bacterial phyla at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%). In the three sites, the genera with high relative abundance (over 3%) included Streptococcus (13%), Enhydrobacter (6%), and Propionibacterium (5%). Of these, Streptococcus and Granulicatella showed negative linear correlations with age.
The researchers found significant differences between the bacterial communities of 10-year-olds delivered by Cesarean section and those delivered vaginally, particularly in the facial samples; however the difference wasn’t observed among face samples taken from 1-year-olds, according to the authors. They found significant variation in bacteria in calf samples based on whether the children were fed breast milk, formula, or a combination.
When the researchers examined the correlations between mother/child pairs, they found that the relative abundance of most bacteria in the children were more similar to their mothers than to unrelated adults, and they found the strongest correlations for the genera Deinococcus, Microbacterium, Chryseobacterium, Klebsiella, and Enhydrobacter. The relationships between the bacterial communities of mothers and children may be influenced by the shared living environment, topical products, and daily diet, they noted.
The study findings were limited by not controlling for certain variables, including daily diet, choice of topical products, bathing habits, and daily variation in environmental factors, the researchers wrote. However, the results show “that the skin microbiome is strongly affected by the surrounding microenvironment and that the alpha diversity of the skin microbiome increases during childhood,” they concluded.
The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
SOURCE: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
Bacteria on children’s skin was similar to their mothers’ and affected by factors that included method of delivery and breastfeeding in a study of 154 children aged 10 years and younger.
Understanding the wrote Ting Zhu of Fudan University, Shanghai, China, and colleagues.
In a study published in the Journal of Investigative Dermatology, the researchers compared the skin microbiota of the 158 children aged 1-10 years and 50 mothers using 16S rRNA gene amplicon sequencing after collecting study samples from three skin areas: face, calf, and ventral forearm. The samples were pooled into 36 groups based on age, gender, and skin site.
“We observed significant differences in alpha diversity and the most prevalent taxa and identified factors that contributed to variation at each site,” the authors reported.
Overall, the “alpha diversity” – a measure of microbial diversity used in microbiome studies – of the skin microbiome increased with age, with the highest alpha diversity seen in the 10-year-olds (n = 28), notably on the face, but differences in alpha diversity between skin sites were seen only in the 1-year-olds (n = 26). Overall, the most commonly identified bacterial phyla at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%). In the three sites, the genera with high relative abundance (over 3%) included Streptococcus (13%), Enhydrobacter (6%), and Propionibacterium (5%). Of these, Streptococcus and Granulicatella showed negative linear correlations with age.
The researchers found significant differences between the bacterial communities of 10-year-olds delivered by Cesarean section and those delivered vaginally, particularly in the facial samples; however the difference wasn’t observed among face samples taken from 1-year-olds, according to the authors. They found significant variation in bacteria in calf samples based on whether the children were fed breast milk, formula, or a combination.
When the researchers examined the correlations between mother/child pairs, they found that the relative abundance of most bacteria in the children were more similar to their mothers than to unrelated adults, and they found the strongest correlations for the genera Deinococcus, Microbacterium, Chryseobacterium, Klebsiella, and Enhydrobacter. The relationships between the bacterial communities of mothers and children may be influenced by the shared living environment, topical products, and daily diet, they noted.
The study findings were limited by not controlling for certain variables, including daily diet, choice of topical products, bathing habits, and daily variation in environmental factors, the researchers wrote. However, the results show “that the skin microbiome is strongly affected by the surrounding microenvironment and that the alpha diversity of the skin microbiome increases during childhood,” they concluded.
The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
SOURCE: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Age, skin site, and maternal factors including delivery method and breastfeeding impact the bacterial makeup of children’s skin.
Major finding: The most common bacteria at all skin sites in children were Proteobacteria (42%), Firmicutes (25%), Actinobacteria (13%), and Bacteroidetes (11%).
Study details: The data come from 158 children aged 10 years and younger and included 474 skin samples.
Disclosures: The study was fully funded by Johnson & Johnson International, and several coauthors are employees of that company. Lead author Ms. Zhu had no financial conflicts to disclose.
Source: Zhu T et al. J Invest Dermatol. 2019 August 13. doi: 10.1016/j.jid.2019.05.018.
Hematuria is highly prevalent in pediatric hemophilia A and B
The presence of hematuria on screening urinalysis was found to be highly prevalent in children with hemophilia, according to results from a retrospective chart review.
The findings emphasize the need to measure the population‐wide prevalence of hematuria in pediatric patients with hemophilia, in addition to better understanding its influence on renal function.
“Little is known about the prevalence of haematuria in [patients with hemophilia] or its long‐term impact on the renal system,” wrote Kyle A. Davis, MD, of the Nationwide Children’s Hospital in Columbus, and his coauthors. The results were published in Haemophilia.
The researchers retrospectively reviewed data from 93 patients with hemophilia A and B who were treated at a pediatric hemophilia treatment center from August 2011 to September 2015. The children in the study were all male and aged 2 years and older.
The team detected the frequency of hematuria, defined as greater than or equal to three red blood cells (RBCs) for each high power field, on screening urinalysis.
Other clinical data, including demographic, treatment, and laboratory information were also collected and analyzed.
After analysis, the researchers found that hematuria was detected in 45% of patients with hemophilia (mean RBCs, 332; median RBCs, 7), 76% of whom were identified using urinalysis, rather than clinical symptoms.
In total, recurrent hematuria was seen in 52% of patients. Hemophilia A and older age were associated with a higher likelihood of hematuria. However, there was no correlation between the severity of hemophilia or the treatment regimen and hematuria.
“We suspect patients with haemophilia may be having unrecognized, recurrent microscopic haematuria,” the researchers wrote.
The authors noted a key limitation of the study was the retrospective design.
“We suggest paediatric haematologists should consider routine evaluation for hypertension and renal disease in their patients,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bioverativ, CSL Behring, Medscape, Shire, the American Society of Hematology, and others.
SOURCE: Davis KA et al. Haemophilia. 2019 Jul 10. doi: 10.1111/hae.13815.
The presence of hematuria on screening urinalysis was found to be highly prevalent in children with hemophilia, according to results from a retrospective chart review.
The findings emphasize the need to measure the population‐wide prevalence of hematuria in pediatric patients with hemophilia, in addition to better understanding its influence on renal function.
“Little is known about the prevalence of haematuria in [patients with hemophilia] or its long‐term impact on the renal system,” wrote Kyle A. Davis, MD, of the Nationwide Children’s Hospital in Columbus, and his coauthors. The results were published in Haemophilia.
The researchers retrospectively reviewed data from 93 patients with hemophilia A and B who were treated at a pediatric hemophilia treatment center from August 2011 to September 2015. The children in the study were all male and aged 2 years and older.
The team detected the frequency of hematuria, defined as greater than or equal to three red blood cells (RBCs) for each high power field, on screening urinalysis.
Other clinical data, including demographic, treatment, and laboratory information were also collected and analyzed.
After analysis, the researchers found that hematuria was detected in 45% of patients with hemophilia (mean RBCs, 332; median RBCs, 7), 76% of whom were identified using urinalysis, rather than clinical symptoms.
In total, recurrent hematuria was seen in 52% of patients. Hemophilia A and older age were associated with a higher likelihood of hematuria. However, there was no correlation between the severity of hemophilia or the treatment regimen and hematuria.
“We suspect patients with haemophilia may be having unrecognized, recurrent microscopic haematuria,” the researchers wrote.
The authors noted a key limitation of the study was the retrospective design.
“We suggest paediatric haematologists should consider routine evaluation for hypertension and renal disease in their patients,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bioverativ, CSL Behring, Medscape, Shire, the American Society of Hematology, and others.
SOURCE: Davis KA et al. Haemophilia. 2019 Jul 10. doi: 10.1111/hae.13815.
The presence of hematuria on screening urinalysis was found to be highly prevalent in children with hemophilia, according to results from a retrospective chart review.
The findings emphasize the need to measure the population‐wide prevalence of hematuria in pediatric patients with hemophilia, in addition to better understanding its influence on renal function.
“Little is known about the prevalence of haematuria in [patients with hemophilia] or its long‐term impact on the renal system,” wrote Kyle A. Davis, MD, of the Nationwide Children’s Hospital in Columbus, and his coauthors. The results were published in Haemophilia.
The researchers retrospectively reviewed data from 93 patients with hemophilia A and B who were treated at a pediatric hemophilia treatment center from August 2011 to September 2015. The children in the study were all male and aged 2 years and older.
The team detected the frequency of hematuria, defined as greater than or equal to three red blood cells (RBCs) for each high power field, on screening urinalysis.
Other clinical data, including demographic, treatment, and laboratory information were also collected and analyzed.
After analysis, the researchers found that hematuria was detected in 45% of patients with hemophilia (mean RBCs, 332; median RBCs, 7), 76% of whom were identified using urinalysis, rather than clinical symptoms.
In total, recurrent hematuria was seen in 52% of patients. Hemophilia A and older age were associated with a higher likelihood of hematuria. However, there was no correlation between the severity of hemophilia or the treatment regimen and hematuria.
“We suspect patients with haemophilia may be having unrecognized, recurrent microscopic haematuria,” the researchers wrote.
The authors noted a key limitation of the study was the retrospective design.
“We suggest paediatric haematologists should consider routine evaluation for hypertension and renal disease in their patients,” the researchers wrote.
No funding sources were reported. The authors reported financial affiliations with Bioverativ, CSL Behring, Medscape, Shire, the American Society of Hematology, and others.
SOURCE: Davis KA et al. Haemophilia. 2019 Jul 10. doi: 10.1111/hae.13815.
FROM HAEMOPHILIA
Two genetic variants modify risk of Alzheimer’s disease
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
according to research published online August 14 in Science Translational Medicine. The variants affect cerebrospinal fluid (CSF) concentrations of a soluble form of the TREM2 protein (sTREM2), which may be involved in Alzheimer’s disease pathology. “Increasing TREM2 or activating the TREM2 signaling pathway could offer a new therapeutic approach for treating Alzheimer’s disease,” wrote the researchers.
Yuetiva Deming, PhD, of the University of Wisconsin–Madison and colleagues conducted a genome-wide association study to identify genetic modifiers of CSF sTREM2. They analyzed CSF sTREM2 levels in 813 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Of this population, 172 participants had Alzheimer’s disease, 169 were cognitively normal, 183 had early mild cognitive impairment (MCI), 221 had late MCI, and 68 had significant memory concerns.
The rs1582763 single-nucleotide polymorphism (SNP) on chromosome 11 within the MS4A gene region was significantly associated with increased CSF levels of sTREM2. Conditional analyses of the MS4A locus indicated that rs6591561, a missense variant within MS4A4A, was associated with reduced CSF sTREM2. Analyzing 580 additional CSF sTREM2 samples, along with associated genetic data, from six other studies replicated these findings in an independent dataset.
Furthermore, Dr. Deming and colleagues found that rs1582763 was associated with reduced risk for Alzheimer’s disease and older age at Alzheimer’s disease onset. In addition, rs6591561 was associated with increased risk of Alzheimer’s disease and earlier onset of Alzheimer’s disease.
Subsequent analyses showed that rs1582763 modified the expression of the MS4A4A and MS4A6A genes in various tissues. This finding suggests that one or both of these genes are important for influencing the production of sTREM2, wrote Dr. Deming and colleagues. Using human macrophages as a proxy for microglia, the investigators observed that the MS4A4A and TREM2 proteins colocalized on lipid rafts at the plasma membrane. In addition, sTREM2 concentrations increased with MS4A4A overexpression, and silencing of MS4A4A reduced sTREM2 production.
These findings “provide a putative biological connection between the MS4A family, TREM2, and Alzheimer’s disease risk,” wrote the researchers. The data also suggest that MS4A4A is a potential therapeutic target in Alzheimer’s disease. Understanding the role of sTREM2 in Alzheimer’s disease will require additional research, but it may be involved in pathogenesis, wrote Dr. Deming and colleagues.
One of the study’s limitations is that the investigators included only common variants and thus could not determine the effect of genes that only harbor low-frequency or rare functional variants. Another limitation is that the data cannot support conclusions about whether other genes in the MS4A locus also modulate sTREM2, wrote Dr. Deming and colleagues.
Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
SOURCE: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Two variants of MS4A are associated with the risk of Alzheimer’s disease.
Major finding: The rs1582763 SNP is associated with reduced risk for Alzheimer’s disease, and rs6591561 is associated with increased risk of Alzheimer’s disease.
Study details: A genome-wide association study of 813 participants in the Alzheimer’s Disease Neuroimaging Initiative.
Disclosures: Grants from the National Institutes of Health supported this study. The investigators disclosed consulting and other relationships with various pharmaceutical companies.
Source: Deming Y et al. Sci Transl Med. 2019 Aug 14. doi: 10.1126/scitranslmed.aau2291.