User login
Teprotumumab gets FDA go-ahead for thyroid eye disease
according to a press release.
Thyroid eye disease is a rare, progressive, autoimmune condition that causes the eyes to bulge (proptosis) and can lead to blindness. Until now, treatment has focused on managing its symptoms – which can include eye pain, double vision, or sensitivity to light – with steroids, and in some cases, multiple invasive surgeries.
The human monoclonal antibody and a targeted inhibitor of the insulinlike growth factor-1 receptor is administered to patients once every 3 weeks, for a total of eight infusions, according to a statement from Horizon Therapeutics, which manufactures the drug.
The approval was based on the findings from two similarly designed, parallel-group studies (Studies 1 and 2) involving 170 patients with thyroid eye disease who were randomized to receive either teprotumumab or placebo. Of those receiving the study drug, 71% in Study 1 and 83% in Study 2 had a reduction of more than 2 mm in eye protrusion, compared with 20% and 10%, respectively, among the placebo participants.
The most common adverse reactions in patients receiving teprotumumab were muscle spasm, nausea, alopecia, diarrhea, fatigue, and hyperglycemia. The treatment is contraindicated for pregnancy.
according to a press release.
Thyroid eye disease is a rare, progressive, autoimmune condition that causes the eyes to bulge (proptosis) and can lead to blindness. Until now, treatment has focused on managing its symptoms – which can include eye pain, double vision, or sensitivity to light – with steroids, and in some cases, multiple invasive surgeries.
The human monoclonal antibody and a targeted inhibitor of the insulinlike growth factor-1 receptor is administered to patients once every 3 weeks, for a total of eight infusions, according to a statement from Horizon Therapeutics, which manufactures the drug.
The approval was based on the findings from two similarly designed, parallel-group studies (Studies 1 and 2) involving 170 patients with thyroid eye disease who were randomized to receive either teprotumumab or placebo. Of those receiving the study drug, 71% in Study 1 and 83% in Study 2 had a reduction of more than 2 mm in eye protrusion, compared with 20% and 10%, respectively, among the placebo participants.
The most common adverse reactions in patients receiving teprotumumab were muscle spasm, nausea, alopecia, diarrhea, fatigue, and hyperglycemia. The treatment is contraindicated for pregnancy.
according to a press release.
Thyroid eye disease is a rare, progressive, autoimmune condition that causes the eyes to bulge (proptosis) and can lead to blindness. Until now, treatment has focused on managing its symptoms – which can include eye pain, double vision, or sensitivity to light – with steroids, and in some cases, multiple invasive surgeries.
The human monoclonal antibody and a targeted inhibitor of the insulinlike growth factor-1 receptor is administered to patients once every 3 weeks, for a total of eight infusions, according to a statement from Horizon Therapeutics, which manufactures the drug.
The approval was based on the findings from two similarly designed, parallel-group studies (Studies 1 and 2) involving 170 patients with thyroid eye disease who were randomized to receive either teprotumumab or placebo. Of those receiving the study drug, 71% in Study 1 and 83% in Study 2 had a reduction of more than 2 mm in eye protrusion, compared with 20% and 10%, respectively, among the placebo participants.
The most common adverse reactions in patients receiving teprotumumab were muscle spasm, nausea, alopecia, diarrhea, fatigue, and hyperglycemia. The treatment is contraindicated for pregnancy.
FROM THE FDA
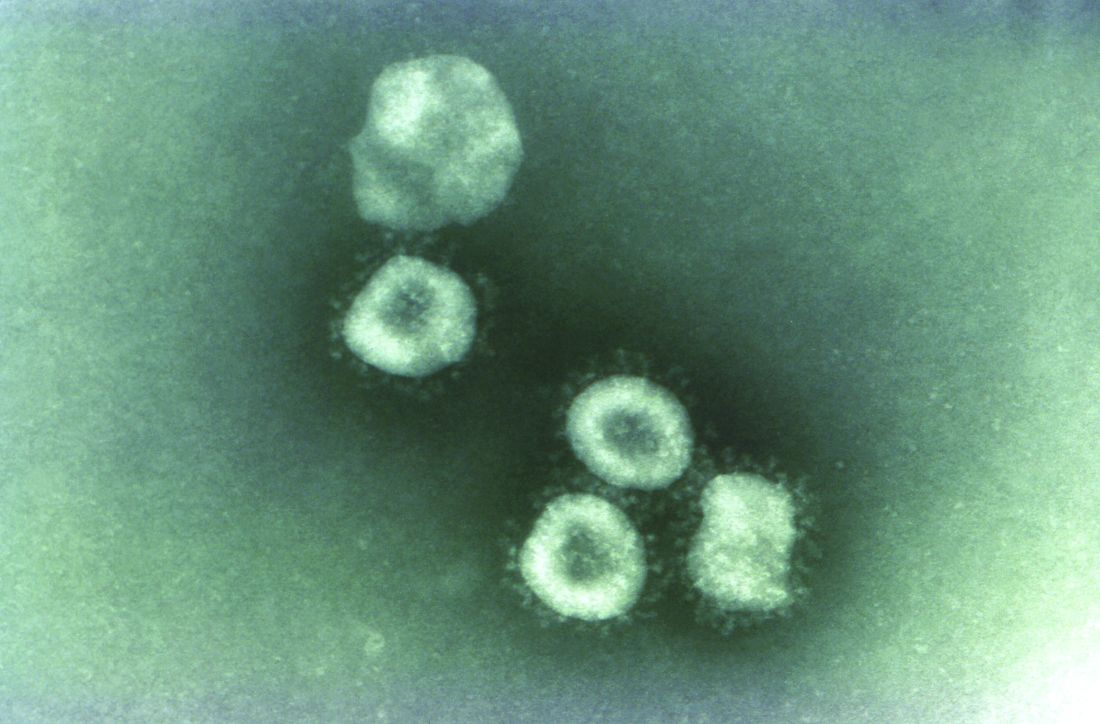
Washington state patient is first U.S. case of novel coronavirus
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
REPORTING FROM CDC
Dermatology News welcomes new advisory board member Dr. Jonathan I. Silverberg
Dermatology News welcomes Jonathan I. Silverberg, MD, PhD, MPH, to its editorial advisory board. Dr. Silverberg is an associate professor of dermatology at George Washington University in Washington, where he is also director of clinical research and contact dermatitis.
Dr. Silverberg’s clinical subspecialty is inflammatory skin disease, particularly atopic dermatitis and contact dermatitis. His research interests include comorbidities and burden of itch and inflammatory skin disease, evidence-based dermatology, patient-reported outcomes, and dermatoepidemiology, as well as health services research, drug development, clinical trial design, and biomarkers. He has published more than 600 peer-reviewed articles, abstracts, and book chapters, and has appeared frequently on the pages of Dermatology News.
‑Elizabeth Mechcatie, editor
Dermatology News welcomes Jonathan I. Silverberg, MD, PhD, MPH, to its editorial advisory board. Dr. Silverberg is an associate professor of dermatology at George Washington University in Washington, where he is also director of clinical research and contact dermatitis.
Dr. Silverberg’s clinical subspecialty is inflammatory skin disease, particularly atopic dermatitis and contact dermatitis. His research interests include comorbidities and burden of itch and inflammatory skin disease, evidence-based dermatology, patient-reported outcomes, and dermatoepidemiology, as well as health services research, drug development, clinical trial design, and biomarkers. He has published more than 600 peer-reviewed articles, abstracts, and book chapters, and has appeared frequently on the pages of Dermatology News.
‑Elizabeth Mechcatie, editor
Dermatology News welcomes Jonathan I. Silverberg, MD, PhD, MPH, to its editorial advisory board. Dr. Silverberg is an associate professor of dermatology at George Washington University in Washington, where he is also director of clinical research and contact dermatitis.
Dr. Silverberg’s clinical subspecialty is inflammatory skin disease, particularly atopic dermatitis and contact dermatitis. His research interests include comorbidities and burden of itch and inflammatory skin disease, evidence-based dermatology, patient-reported outcomes, and dermatoepidemiology, as well as health services research, drug development, clinical trial design, and biomarkers. He has published more than 600 peer-reviewed articles, abstracts, and book chapters, and has appeared frequently on the pages of Dermatology News.
‑Elizabeth Mechcatie, editor
FDA advisers set high bar for new opioids
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
During an opioid-addiction epidemic, can any new opioid pain drug meet prevailing safety demands to gain regulatory approval?
On Jan. 14 and 15, a Food and Drug Administration advisory committee voted virtually unanimously against two new opioid formulations and evenly split for and against a third; the 2 days of data and discussion showed how high a bar new opioids face these days for getting onto the U.S. market.
The bar’s height is very understandable given how many Americans have become addicted to opioids over the past decade, more often than not by accident while using pain medications as they believed they had been directed, said experts during the sessions held on the FDA’s campus in White Oak, Md.
Among the many upshots of the opioid crisis, the meetings held to discuss these three contender opioids highlighted the bitter irony confronting attempts to bring new, safer opioids to the U.S. market: While less abusable pain-relief medications that still harness the potent analgesic power of mu opioid receptor agonists are desperately desired, new agents in this space now receive withering scrutiny over their safeguards against misuse and abuse, and over whether they add anything meaningfully new to what’s already available. While these demands seem reasonable, perhaps even essential, it’s unclear whether any new opioid-based pain drugs will ever fully meet the safety that researchers, clinicians, and the public now seek.
A special FDA advisory committee that combined the Anesthetic and Analgesic Drug Products Advisory Committee with members of the Drug Safety and Risk Management Advisory Committee considered the application for three different opioid drugs from three separate companies. None received a clear endorsement. Oxycodegol, a new type of orally delivered opioid molecule engineered to slow brain entry and thereby delay an abuser’s high, got voted down without any votes in favor and 27 votes against agency approval. Aximris XR, an extended-release oxycodone formulation that successfully deterred intravenous abuse but had no deterrence efficacy for intranasal or oral abuse failed by a 2-24 vote against. The third agent, CTC, a novel formulation of the schedule IV opioid tramadol with the NSAID celecoxib designed to be analgesic but with limited opioid-abuse appeal, came the closest to meaningful support with a tied 13-13 vote from advisory committee members for and against agency approval. FDA staff takes advisory committee opinions and votes into account when making their final decisions about drug marketing approvals.
In each case, the committee members, mostly the same roster assembled for each of the three agents, identified specific concerns with the data purported to show each drug’s safety and efficacy. But the gathered experts and consumer representatives also consistently cited holistic challenges to approving new opioids and the stiffer criteria these agents face amid a continuing wave of opioid misuse and abuse.
“In the context of the public health issues, we don’t want to be perceived in any way of taking shortcuts,” said Linda S. Tyler, PharmD,, an advisory committee member and professor of pharmacy and chief pharmacy officer at the University of Utah in Salt Lake City. “There is no question that for a new product to come to market in this space it needs to add to what’s on the market, meet a high bar, and provide advantages compared with what’s already on the market,” she said.
Tramadol plus celecoxib gains some support
The proposed combined formulation of tramadol and celecoxib came closest to meeting that bar, as far as the advisory committee was concerned, coming away with 13 votes favoring approval to match 13 votes against. The premise behind this agent, know as CTC (cocrystal of tramadol and celecoxib), was that it combined a modest dose (44 mg) of the schedule IV opioid tramadol with a 56-mg dose of celecoxib in a twice-daily pill. Eugene R. Viscusi, MD, professor of anesthesiology and director of acute pain management at Thomas Jefferson University in Philadelphia and a speaker at the session on behalf of the applicant company, spelled out the rationale behind CTC: “We are caught in a dilemma. We need to reduce opioid use, but we also need to treat pain. We have an urgent need to have pain treatment options that are effective but have low potential for abuse and dependence. We are looking at multimodal analgesia, that uses combination of agents, recognizing that postoperative pain is a mixed pain syndrome. Multimodal pain treatments are now considered standard care. We want to minimize opioids to the lowest dose possible to produce safe analgesia. Tramadol is the least-preferred opioid for abuse,” and is rated as schedule IV, the U.S. designation for drugs considered to have a low level of potential for causing abuse or dependence. “Opioids used as stand-alone agents have contributed to the current opioid crisis,” Dr. Viscusi told the committee.
In contrast to tramadol’s schedule IV status, the mainstays of recent opioid pain therapy have been hydrocodone and oxycodone, schedule II opioids rated as having a “high potential for abuse.”
Several advisory committee members agreed that CTC minimized patient exposure to an opioid. “This drug isn’t even tramadol; it’s tramadol light. It has about as low a dose [of an opioid] as you can have and still have a drug,” said member Lee A. Hoffer, PhD, a medical anthropologist at Case Western Reserve University, Cleveland, who studies substance use disorders. “All opioids are dangerous, even at a low dose, but there is a linear relationship based on potency, so if we want to have an opioid for acute pain, I’d like it to have the lowest morphine milligram equivalent possible. The ideal is no opioids, but that is not what happens,” he said. The CTC formulation delivers 17.6 morphine milligram equivalents (MME) per pill, the manufacturer’s representatives said. The Centers for Disease Control and Prevention defines a “relatively low” daily opioid dose as 20-50 MME.
Some committee members hailed the CTC formulation as a meaningful step toward cutting opioid consumption.
“We may be very nervous about abuse of scheduled opioids, but a schedule IV opioid in an opioid-sparing formulation is as good as it gets in 2020,” said committee member Kevin L. Zacharoff, MD, a pain medicine specialist at the State University of New York at Stony Brook. “Any opioid has potential for abuse, but this is a safer alternative to the schedule II drugs. There is less public health risk with this,” said committee member Sherif Zaafran, MD, a Houston anesthesiologist. “This represents an incremental but important approach to addressing the opioid crisis, especially if used to replace schedule II opioids,” said Brandon D.L. Marshall, PhD, an epidemiologist and substance abuse researcher at Brown University in Providence, R.I.
But despite agreement that CTC represented a new low in the MME of an opioid given to patients, several committee members still saw the formulation as problematic by introducing any opioid, no matter how small the dose.
“The landscape of tramadol use and prescribing is evolving. There’s been an exponential upturn in tramadol prescribing. It’s perceived [as] safer, but it’s not completely safe. Will this change tramadol abuse and open the door to abuse of other opioids? This is what got us into trouble with opioids in the first place. Patients start with a prescription opioid that they perceive is safe. Patients don’t start with oxycodone or heroin. They start with drugs that are believed to be safe. I feel this combination has less risk for abuse, but I’m worried that it would produce a false sense of security for tolerability and safety,” said committee member Maryann E. Amirshahi, MD, a medical toxicologist at Georgetown University and MedStar Health in Washington.
Several other committee members returned to this point throughout the 2 days of discussions: The majority of Americans who have become hooked on opioids reached that point by taking an opioid pain medication for a legitimate medical reason and using the drug the way they had understood they should.
“I’m most concerned about unintentional misuse leading to addiction and abuse. Most people with an opioid addiction got it inadvertently, misusing it by mistake,” said committee member Suzanne B. Robotti, a consumer representative and executive director of DES Action USA. “I’m concerned about approving an opioid, even an opioid with a low abuse history, without a clearer picture of the human abuse potential data and what would happen if this drug were abused,” she added, referring to the proposed CTC formulation.
“All the patients I work with started [their opioid addiction] as pain patients,” Dr. Hoffer said.
“The most common use and abuse of opioids is orally. We need to avoid having patients who use the drug as prescribed and still end up addicted,” said committee member Friedhelm Sandbrink, MD, a neurologist and director of pain management at the Veterans Affairs (VA) Medical Center in Washington.
What this means, said several panelists, is functionally clamping down a class-wide lid on new opioids. “The way to reduce deaths from abuse is to reduce addiction, and to have an impact you need to reduce opioid exposure.” said committee member Sonia Hernandez-Diaz, MD, professor of epidemiology at the Harvard School of Public Health in Boston.
“In this opioid crisis, we ask for data that we wouldn’t ordinarily ask for. I feel there are unanswered questions about the abuse potential [of CTC]. We have seen a recent reduction in oxycodone use, which is great, but also an increase in tramadol use. We should not be fooled. Tramadol is an opioid, even if it’s schedule IV,” Dr. Tyler said.
Two other opioids faced greater opposition
The other two agents that the committee considered received much less support and sharper skepticism. The application for Aximris XR, an extended release form of oxycodone with a purported abuse-deterrent formulation (ADF) that relies on being difficult to extract for intravenous use as well as possibly having effective deterrence mechanisms for other forms of abuse. But FDA staffers reported that the only effective deterrence they could document was against manipulation for intravenous use, making Aximris XR the first opioid seeking ADF labeling based on deterrence to a single delivery route. This led several committee members, as well as the FDA, to comment on the clinical meaningfulness of ADF for one route. So far, the FDA approved ADF labeling for seven opioids, most notably OxyContin, an extended-release oxycodone with the biggest share of the U.S. market for opioids with ADF labeling.
“For ADF, we label based on what we expect from the premarket data. We don’t really know how that translates into what happens once the drug is on the market. Every company with an ADF in their label is required to do postmarketing studies on the abuse routes that are supposed to be deterred. We see shifts to other routes. Assessment of ADF is incredibly challenging, both scientifically and logistically, because there has not been a lot of uptake of these products, for a variety of reasons,” said Judy Staffa, PhD, associate director for Public Health Initiatives in the Office of Surveillance & Epidemiology in the FDA’s Center for Drug Evaluation and Research. The company that markets OxyContin has been the first to submit to the FDA all of its required postmarketing data on ADF efficacy, and the agency is now reviewing this filing, Dr. Staffa said.
The data presented for Aximris XR appeared to generally fail to convince committee members that it provided a meaningful addition to the range of opioids with ADF designations already available, which meant that their decision mostly came down to whether they felt it made sense to bring a me-too opioid to the U.S. market. Their answer was mostly no.
“In the end, it’s another opioid, and I’m not sure we need another opioid,” said committee member Lonnie K. Zeltzer, MD, professor of pediatrics, anesthesiology, psychiatry, and biobehavioral sciences and director of pediatric pain at the University of California, Los Angeles “There are so many options for patients and for people who abuse these drug. I don’t see this formulation as having a profound impact, but I’m very concerned about adding more prescription opioids,” said Martin Garcia-Bunuel, MD, deputy chief of staff for the VA Maryland Health Care System in Baltimore. Another concern of some committee members was that ADF remains a designation with an uncertain meaning, pending the FDA’s analysis of the OxyContin data.
“At the end of the day, we don’t know whether any of the [ADF] stuff makes a difference,” noted Steve B. Meisel, PharmD, system director of medication safety for M Health Fairview in Minneapolis and a committee member,
The third agent, oxycodegol, a molecule designed to pass more slowly across the blood-brain barrier because of an attached polyethylene glycol chain that’s supposed to prevent a rapid high after ingestion and hence cut abuse potential. It received unanimous committee rejection, primarily because its safety and efficacy evidence had so many holes, but the shadow of opioid abuse permeated the committee’s discussion.
“One dogma in the abuse world is that slowing entry into the brain reduces abuse potential, but the opioid crisis showed that this is not the only factor. Some people have become addicted to slow-acting drugs. The abuse potential of this drug, oxycodegol, needs to be considered given where we’ve been with the opioid crisis,” said Jane B. Acri, PhD, chief of the Medications Discovery and Toxicology Branch of the National Institute on Drug Abuse.
“During the opioid epidemic, do we want to approve more opioids? If the [pain] efficacy is about the same as oxycodone, is better safety or abuse potential a reason to approve it? We need guidance [from the FDA] about what is ‘better enough.’ No opioid will ever be perfect; there will always be abuse and misuse. But what is good enough to justify bringing another opioid onto the market? What is a good enough improvement? I don’t have an answer,” Dr. Hernandez-Diaz said.
Adviser comments showed that the continued threat of widespread opioid addiction has cooled prospects for new opioid approvals by making FDA advisers skittish over how to properly score the incremental value of a new opioid.
“Do we need to go back to the drawing board on how we make decisions on exposing the American public to these kinds of agents?” Dr. Garcia-Bunuel asked. “I don’t think we have the tools to make these decisions.”
Renal denervation rebounds
PHILADELPHIA – Enthusiasm for catheter-based renal denervation as a potential nondrug treatment for hypertension is once again on the rise, Michael Bohm, MD, observed at the American Heart Association scientific sessions.
The field experienced “a big depression” in 2014 with the publication of the unexpectedly negative results of the Symplicity HTN-3 trial (N Engl J Med. 2014;370:1393-401), he said. But post hoc analysis of the trial revealed significant shortcomings in design and execution.
“All of the flaws of this trial have been eliminated and now there is a very tightly controlled program to show whether renal denervation will work or not,” according to Dr. Bohm, director of the department of internal medicine and professor of cardiology at Saarland University in Homburg, Germany.
Indeed, three randomized, double-blind, sham-controlled, proof-of-concept clinical trials – all with strongly positive results – were published in Lancet in 2017 and 2018: SPYRAL HTN-OFF (2017 Nov 11;390:2160-70), RADIANCE SOLO (2018 Jun 9;391:2335-45), and SPYRAL HTN-ON (2018 May 23;391:2346-55). Based on the encouraging findings, four large pivotal trials of renal denervation (RDN) for hypertension are ongoing: RADIANCE HTN, REQUIRE, RADIANCE II, and SPYRAL HTN-ON MED. In addition, the SPYRAL HTN-OFF MED pivotal trial has been completed and will be presented soon, Dr. Bohm said.
Defining who’s most likely to benefit
Treatment response has been quite variable within the various RDN trials. A reliable predictor of response would be an important advance because it would enable physicians to select the best candidates for treatment while sparing others from an invasive procedure – albeit a relatively safe one – that they may not benefit from. On this front, Dr. Bohm and colleagues have recently reported that a baseline 24-hour heart rate above the median value of 73.5 bpm in the SPYRAL HTN-OFF MED trial – a marker for sympathetic overdrive – was associated with a 10.7/7.5 mm Hg greater reduction in average ambulatory blood pressures post-RDN than with a sham procedure. In contrast, blood pressure changes in RDN recipients with a below-median baseline 24-hour heart rate weren’t significant (Eur Heart J. 2019 Mar 1;40:743-51).
“Although this is a little bit rough, there is no other really true and reliable marker,” the cardiologist observed.
A pressing need exists for a reliable intraprocedural indicator of success. Dr. Bohm noted that Australian investigators are pursuing a promising approach in animal studies: intraprocedural transvascular high-frequency pacing of the aorticorenal ganglia. Abolition of the pacing-induced increase in blood pressure may be an indicator of complete RDN (JACC Cardiovasc Interv. 2019 Jun 24;12:1109-20).
Applications other than hypertension
Renal denervation is under early-stage investigation for a range of other cardiovascular diseases in which sympathetic overdrive figures prominently.
“The truly interesting things in renal denervation are what happens beyond hypertension. There are a lot of potential applications,” according to Dr. Bohm.
For example, when RDN was performed alongside pulmonary vein isolation for treatment of paroxysmal atrial fibrillation in hypertensive patients, the arrhythmia recurrence rate was significantly reduced during 1 year of follow-up, compared with AF ablation alone, in the randomized, multicenter, 302-patient ERADICATE-AF trial, presented at the most recent meeting of the Heart Rhythm Society.
Also, a small, uncontrolled registry study of RDN in patients with cardiomyopathy and electrical storm suggests the procedure may have an immediate anti–ventricular arrhythmia effect.
Meanwhile, Dr. Bohm is pressing the German government to sponsor an independent randomized controlled trial of RDN for heart failure. He and others have shown in small pilot studies a promising signal that the treatment may improve myocardial function and the signs and symptoms of heart failure in both patients with reduced and preserved left ventricular ejection fraction – and without reducing their blood pressure, which is often already low.
Dr. Bohm and others have also been exploring the impact of RDN in patients with metabolic syndrome. The treatment has a sound pathophysiologic rationale because insulin resistance is dependent upon sympathetic nervous system activation. Preliminary reports show improved insulin sensitivity in response to RDN. Patients also report better quality of life, presumably because of the reduction in sympathetic overactivity.
A couple of small Chinese studies suggest denervating the pulmonary vein in patients with pulmonary hypertension leads to a salutary reduction in pulmonary blood pressures.
“We haven’t done that yet. There is no properly designed catheter. They’ve used a Spyra unipolar catheter. It could work, but it hasn’t been rigorously investigated,” the cardiologist said.
Dr. Bohm reported serving as a scientific adviser to Abbott, AstraZeneca, BMS, Boehringer Ingelheim, and Servier.
PHILADELPHIA – Enthusiasm for catheter-based renal denervation as a potential nondrug treatment for hypertension is once again on the rise, Michael Bohm, MD, observed at the American Heart Association scientific sessions.
The field experienced “a big depression” in 2014 with the publication of the unexpectedly negative results of the Symplicity HTN-3 trial (N Engl J Med. 2014;370:1393-401), he said. But post hoc analysis of the trial revealed significant shortcomings in design and execution.
“All of the flaws of this trial have been eliminated and now there is a very tightly controlled program to show whether renal denervation will work or not,” according to Dr. Bohm, director of the department of internal medicine and professor of cardiology at Saarland University in Homburg, Germany.
Indeed, three randomized, double-blind, sham-controlled, proof-of-concept clinical trials – all with strongly positive results – were published in Lancet in 2017 and 2018: SPYRAL HTN-OFF (2017 Nov 11;390:2160-70), RADIANCE SOLO (2018 Jun 9;391:2335-45), and SPYRAL HTN-ON (2018 May 23;391:2346-55). Based on the encouraging findings, four large pivotal trials of renal denervation (RDN) for hypertension are ongoing: RADIANCE HTN, REQUIRE, RADIANCE II, and SPYRAL HTN-ON MED. In addition, the SPYRAL HTN-OFF MED pivotal trial has been completed and will be presented soon, Dr. Bohm said.
Defining who’s most likely to benefit
Treatment response has been quite variable within the various RDN trials. A reliable predictor of response would be an important advance because it would enable physicians to select the best candidates for treatment while sparing others from an invasive procedure – albeit a relatively safe one – that they may not benefit from. On this front, Dr. Bohm and colleagues have recently reported that a baseline 24-hour heart rate above the median value of 73.5 bpm in the SPYRAL HTN-OFF MED trial – a marker for sympathetic overdrive – was associated with a 10.7/7.5 mm Hg greater reduction in average ambulatory blood pressures post-RDN than with a sham procedure. In contrast, blood pressure changes in RDN recipients with a below-median baseline 24-hour heart rate weren’t significant (Eur Heart J. 2019 Mar 1;40:743-51).
“Although this is a little bit rough, there is no other really true and reliable marker,” the cardiologist observed.
A pressing need exists for a reliable intraprocedural indicator of success. Dr. Bohm noted that Australian investigators are pursuing a promising approach in animal studies: intraprocedural transvascular high-frequency pacing of the aorticorenal ganglia. Abolition of the pacing-induced increase in blood pressure may be an indicator of complete RDN (JACC Cardiovasc Interv. 2019 Jun 24;12:1109-20).
Applications other than hypertension
Renal denervation is under early-stage investigation for a range of other cardiovascular diseases in which sympathetic overdrive figures prominently.
“The truly interesting things in renal denervation are what happens beyond hypertension. There are a lot of potential applications,” according to Dr. Bohm.
For example, when RDN was performed alongside pulmonary vein isolation for treatment of paroxysmal atrial fibrillation in hypertensive patients, the arrhythmia recurrence rate was significantly reduced during 1 year of follow-up, compared with AF ablation alone, in the randomized, multicenter, 302-patient ERADICATE-AF trial, presented at the most recent meeting of the Heart Rhythm Society.
Also, a small, uncontrolled registry study of RDN in patients with cardiomyopathy and electrical storm suggests the procedure may have an immediate anti–ventricular arrhythmia effect.
Meanwhile, Dr. Bohm is pressing the German government to sponsor an independent randomized controlled trial of RDN for heart failure. He and others have shown in small pilot studies a promising signal that the treatment may improve myocardial function and the signs and symptoms of heart failure in both patients with reduced and preserved left ventricular ejection fraction – and without reducing their blood pressure, which is often already low.
Dr. Bohm and others have also been exploring the impact of RDN in patients with metabolic syndrome. The treatment has a sound pathophysiologic rationale because insulin resistance is dependent upon sympathetic nervous system activation. Preliminary reports show improved insulin sensitivity in response to RDN. Patients also report better quality of life, presumably because of the reduction in sympathetic overactivity.
A couple of small Chinese studies suggest denervating the pulmonary vein in patients with pulmonary hypertension leads to a salutary reduction in pulmonary blood pressures.
“We haven’t done that yet. There is no properly designed catheter. They’ve used a Spyra unipolar catheter. It could work, but it hasn’t been rigorously investigated,” the cardiologist said.
Dr. Bohm reported serving as a scientific adviser to Abbott, AstraZeneca, BMS, Boehringer Ingelheim, and Servier.
PHILADELPHIA – Enthusiasm for catheter-based renal denervation as a potential nondrug treatment for hypertension is once again on the rise, Michael Bohm, MD, observed at the American Heart Association scientific sessions.
The field experienced “a big depression” in 2014 with the publication of the unexpectedly negative results of the Symplicity HTN-3 trial (N Engl J Med. 2014;370:1393-401), he said. But post hoc analysis of the trial revealed significant shortcomings in design and execution.
“All of the flaws of this trial have been eliminated and now there is a very tightly controlled program to show whether renal denervation will work or not,” according to Dr. Bohm, director of the department of internal medicine and professor of cardiology at Saarland University in Homburg, Germany.
Indeed, three randomized, double-blind, sham-controlled, proof-of-concept clinical trials – all with strongly positive results – were published in Lancet in 2017 and 2018: SPYRAL HTN-OFF (2017 Nov 11;390:2160-70), RADIANCE SOLO (2018 Jun 9;391:2335-45), and SPYRAL HTN-ON (2018 May 23;391:2346-55). Based on the encouraging findings, four large pivotal trials of renal denervation (RDN) for hypertension are ongoing: RADIANCE HTN, REQUIRE, RADIANCE II, and SPYRAL HTN-ON MED. In addition, the SPYRAL HTN-OFF MED pivotal trial has been completed and will be presented soon, Dr. Bohm said.
Defining who’s most likely to benefit
Treatment response has been quite variable within the various RDN trials. A reliable predictor of response would be an important advance because it would enable physicians to select the best candidates for treatment while sparing others from an invasive procedure – albeit a relatively safe one – that they may not benefit from. On this front, Dr. Bohm and colleagues have recently reported that a baseline 24-hour heart rate above the median value of 73.5 bpm in the SPYRAL HTN-OFF MED trial – a marker for sympathetic overdrive – was associated with a 10.7/7.5 mm Hg greater reduction in average ambulatory blood pressures post-RDN than with a sham procedure. In contrast, blood pressure changes in RDN recipients with a below-median baseline 24-hour heart rate weren’t significant (Eur Heart J. 2019 Mar 1;40:743-51).
“Although this is a little bit rough, there is no other really true and reliable marker,” the cardiologist observed.
A pressing need exists for a reliable intraprocedural indicator of success. Dr. Bohm noted that Australian investigators are pursuing a promising approach in animal studies: intraprocedural transvascular high-frequency pacing of the aorticorenal ganglia. Abolition of the pacing-induced increase in blood pressure may be an indicator of complete RDN (JACC Cardiovasc Interv. 2019 Jun 24;12:1109-20).
Applications other than hypertension
Renal denervation is under early-stage investigation for a range of other cardiovascular diseases in which sympathetic overdrive figures prominently.
“The truly interesting things in renal denervation are what happens beyond hypertension. There are a lot of potential applications,” according to Dr. Bohm.
For example, when RDN was performed alongside pulmonary vein isolation for treatment of paroxysmal atrial fibrillation in hypertensive patients, the arrhythmia recurrence rate was significantly reduced during 1 year of follow-up, compared with AF ablation alone, in the randomized, multicenter, 302-patient ERADICATE-AF trial, presented at the most recent meeting of the Heart Rhythm Society.
Also, a small, uncontrolled registry study of RDN in patients with cardiomyopathy and electrical storm suggests the procedure may have an immediate anti–ventricular arrhythmia effect.
Meanwhile, Dr. Bohm is pressing the German government to sponsor an independent randomized controlled trial of RDN for heart failure. He and others have shown in small pilot studies a promising signal that the treatment may improve myocardial function and the signs and symptoms of heart failure in both patients with reduced and preserved left ventricular ejection fraction – and without reducing their blood pressure, which is often already low.
Dr. Bohm and others have also been exploring the impact of RDN in patients with metabolic syndrome. The treatment has a sound pathophysiologic rationale because insulin resistance is dependent upon sympathetic nervous system activation. Preliminary reports show improved insulin sensitivity in response to RDN. Patients also report better quality of life, presumably because of the reduction in sympathetic overactivity.
A couple of small Chinese studies suggest denervating the pulmonary vein in patients with pulmonary hypertension leads to a salutary reduction in pulmonary blood pressures.
“We haven’t done that yet. There is no properly designed catheter. They’ve used a Spyra unipolar catheter. It could work, but it hasn’t been rigorously investigated,” the cardiologist said.
Dr. Bohm reported serving as a scientific adviser to Abbott, AstraZeneca, BMS, Boehringer Ingelheim, and Servier.
EXPERT ANALYSIS FROM AHA 2019
Start of myeloma therapy may be delayed for women, minorities
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
FROM JCO ONCOLOGY PRACTICE
Key clinical point:
Major finding: Patients with delays in treatment were more likely to be women (odds ratio, 1.15) and more likely to be non-Hispanic blacks (OR, 1.21).
Study details: Retrospective analysis of 74,722 patients in the National Cancer Database diagnosed with multiple myeloma between 2004 and 2015.
Disclosures: Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
Source: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
The rise of U.S. dermatology: A brief history from the 1800s to 1970
As Dermatology News (formerly Skin and Allergy News) reaches its 50th-year milestone, a reflection on the history of the discipline, especially in the United States, up to the time of the launch of this publication is in order. Such an overview must, of course, be cursory in this context. Yet, for those who want to learn more, a large body of historical references and research has been created to fill in the gaps, as modern dermatology has always been cognizant of the importance of its history, with many individuals and groups drawn to the subject.
Two excellent sources for the history of the field can be found in work by William Allen Pusey, MD (1865-1940), and Herbert Rattner, MD (1900-1962), “The History of Dermatology” published in 1933 and research by members of the History of Dermatology Society, founded in 1973 in New York.
Modern dermatology
The development of the field of modern dermatology can be traced back to the early to mid-19th century. During the first half of the 19th century, England and France dominated the study of dermatology, but by the middle of the century, the German revolution in microparasitology shifted that focus “with remarkable German discoveries,” according to Bernard S. Potter, MD, in his review of bibliographic landmarks of the history of dermatology (J Am Acad Dermatol 2003;48:919-32). For example, Johann Lucas Schoenlein (1793-1864) in 1839 discovered the fungal origin of favus, and in 1841 Jacob Henle (1809-1885) discovered Demodex folliculorum. Karl Ferdinand Eichstedt (1816-1892) in 1846 followed with the discovery of the causative agent of pityriasis versicolor, and Friedrich Wilhelm Felix von Barensprung (1822-1864) in 1862 coined the term erythrasma and named the organism responsible for this condition Microsporum minutissimum.
Dr. Potter described how American dermatology originated in New York City in 1836 when Henry Daggett Bulkley, MD, (1803-1872) opened the first dispensary for skin diseases, the Broome Street Infirmary for Diseases of the Skin, thus creating the first institution in the United States for the treatment of cutaneous disease. As the first American dermatologist, he was also the first in the United States to lecture on and to exclusively practice dermatology.
The rise of interest in the importance of dermatology led to the organization of the early American Dermatological Association in 1886.
However, the state of dermatology as a science in the 19th century was not always looked upon favorably, even by its practitioners, especially in the United States. In 1871, in a “Review on Modern Dermatology,” given as a series of lectures on skin disease at Harvard University, James C. White, MD (1833-1916) of Massachusetts General Hospital, stated that: “Were the literature of skin diseases previous to that of the last half-century absolutely annihilated, and with it, the influence it has exercised upon that of the present day, it would be an immense gain to dermatology, although much of real value would perish.” He lamented that America had contributed little so far to the study of dermatology, and that the discipline was only taught in some of its largest schools, and he urged that this be changed. He also lamented that The American Journal of Syphilography and Dermatology, established the year before, had so far proved itself heavy on syphilis, but light on dermatology, a situation he also hoped would change dramatically.
By the late-19th century, the conviction that diseases of the skin needed to be connected to the overall metabolism and physiology of the patient as a whole was becoming more mainstream.
“It has been, and still is, too much the custom to study diseases of the skin in the light of pathological pictures, to name the local manifestation and to so label it as disease. It is much easier to give the disease name and to label it than it is to comprehend the process at work. The former is comparatively unimportant for the patient, the latter point upon which recovery may depend. The nature and meaning of the process in connection with the cutaneous symptoms has not received enough attention, and I believe this to be one reason why the treatment of many of these diseases in the past has been so notoriously unsatisfactory,” Louis A. Duhring, MD (1845-1913) chided his colleagues in the Section of Dermatology and Syphilography, at the Forty-fourth Annual Meeting of the American Medical Association in 1894. (collections.nlm.nih.gov/ext/dw/101489447/PDF/101489447.pdf)
In the early-20th century, German dermatology influenced American dermatology more than any other, according to Karl Holubar, MD, of the Institute for the History of Medicine, University of Vienna, in his lecture on the history of European dermatopathology.
He stated that, with regard to dermatopathology, it was Oscar Gans, MD (1888-1983) who brought the latest knowledge into the United States by delivering a series of lectures at Mayo Clinic in the late 1920s upon the invitation of Paul A. O’Leary, MD, (1891-1955) who then headed the Mayo section of dermatology.
By the 1930s, a flurry of organizational activity overtook American dermatology. In 1932, the American Board of Dermatology was established, with its first exams given in 1933 (20 students passed, 7 failed). The Society for Investigative Dermatology was founded in 1937, and the American Academy of Dermatology and Syphilology (now the American Academy of Dermatology), founded in 1938.
The 1930s also saw a major influx of German and other European Jews fleeing Nazi oppression who would forever change the face of American dermatology. “Between 1933 and 1938, a series of repressive measures eliminated them from the practice of medicine in Germany and other countries. Although some died in concentration camps and others committed suicide, many were able to emigrate from Europe. Dermatology in the United States particularly benefited from the influx of several stellar Jewish dermatologists who were major contributors to the subsequent flowering of academic dermatology in the United States” (JAMA Derm. 2013;149[9]:1090-4).
“The overtures of the holocaust and the rising power of Hitler in Europe finally brought over to the United States the flower of dermatologists and investigators of the German School, e.g., Alexander and Walter Lever, Felix and Hermann Pinkus, the Epsteins, Erich Auerbach, Stephen Rothman, to name just a few. With this exodus and transfer of brain power, Europe lost its leading role to never again regain it,” according to Dr. Holubar. Walter F. Lever, MD (1909-1992) was especially well-known for his landmark textbook on dermatology, “Histopathology of the Skin,” published in the United States in 1949.
The therapeutic era
Throughout the 19th century, a variety of soaps and patent medicines were touted as cure-alls for a host of skin diseases. Other than their benefits to surface cleanliness and their antiseptic properties, however, they were of little effect.
It wasn’t until the 20th century that truly effective therapeutics entered the dermatologic pharmacopoeia. In their 1989 review, Diane Quintal, MD, and Robert Jackson, MD, discussed the origins of the most important of these drugs and pointed out that, “Until this century, the essence of dermatology resided in the realm of morphology. Early contributors largely confined their activities to the classification of skin diseases and to the elaboration of clinical dermatologic entities based on morphologic features. ... but “in the last 50 years, there have been significant scientific discoveries in the field of therapeutics that have revolutionized the practice of dermatology.“ (Clin Dermatol. 1989;7[3]38-47).
These key drugs comprised:
- Quinacrine was introduced in 1932 by Walter Kikuth, MD, as an antimalarial drug. But it was not until 1940, that A.J. Prokoptchouksi, MD, reported on its effectiveness 35 patients with lupus erythematosus.
- Para-aminobenzoic acid (PABA) came into prominence in 1942, when Stephen Rothman, MD, and Jack Rubin, MD, at the University of Chicago, published the results of their experiment, showing that when PABA was incorporated in an ointment base and applied to the skin, it could protect against sunburn.
- Dapsone. The effectiveness of sulfapyridine was demonstrated in 1947 by M.J. Costello, MD, who reported its usefulness in a patient with dermatitis herpetiformis, which he believed to be caused by a bacterial allergy. Sulfapyridine controlled the disease, but gastrointestinal intolerance and sulfonamide sensitivity were side effects. Ultimately, in 1951, Theodore Cornbleet, MD, introduced the use of sulfoxones in his article entitled “Sulfoxone (diasones) sodium for dermatitis herpetiformis,” considered more effective than sulfapyridine. Dapsone is the active principal ingredient.
- Hydrocortisone. In August 1952, Marion Sulzberger, MD, and Victor H. Witten, MD (both members of the first Skin & Allergy News editorial advisory board), described use of Compound F (17-hydroxycorticosterone-21-acetate, hydrocortisone) in seven cases of atopic dermatitis and one case of discoid or subacute lupus erythematosus, reporting improvement in all of these cases.
- Benzoyl peroxide. Canadian dermatologist William E. Pace, MD, reported on the beneficial effects of benzoyl peroxide on acne in 1953. The product had originally been used for chronic Staphylococcus aureus folliculitis of the beard.
- Griseofulvin, a metabolic byproduct of a number of species of Penicillium, was first isolated in 1939. But in 1958, Harvey Blank, MD, at the University of Miami (also on the first Skin & Allergy News editorial advisory board), and Stanley I. Cullen, MD, administered the drug to a patient with Trichophyton rubrum granuloma, in the first human trial. In 1959, they reported the drug’s benefits on 31 patients with various fungal infections.
- Methotrexate. In 1951, R. Gubner, MD, and colleagues noticed the rapid clearing of skin lesions in a patient with rheumatoid arthritis who had been treated with the folic acid antagonist, aminopterin. And in 1958, W.F. Edmundson, MD, and W.B. Guy, MD, reported on the oral use of the folic acid antagonist, methotrexate. This was followed by multiple reports on the successful use of methotrexate in psoriasis.
- 5-fluorouracil (5-FU). In 1957, 5-FU, an antimetabolite of uracil, was first synthesized. In 1962, G. Falkson, MD, and E.J. Schulz, MD, reported on skin changes observed in 85 patients being treated with systemic 5-FU for advanced carcinomatosis. They found that 31 of the 85 patients developed sensitivity to sunlight and subsequent disappearance of actinic keratoses in these same patients.
Technology in skin care also was developing in the era just before the launch of Skin & Allergy News. For example, Leon Goldman, MD, then chairman of the department of dermatology at the University of Cincinnati, was the first physician to use a laser for tattoo removal. His publication in 1965 helped to solidify its use, leading him to be “regarded by many in the dermatologic community as the ‘godfather of lasers in medicine and surgery’ ” (Clin Dermatol. 2007;25:434-42).
So, by 1970, dermatology as a field had established itself fully with a strong societal infrastructure, a vibrant base of journals and books, and an evolving set of scientific and technical tools. The launch of Skin & Allergy News (now Dermatology News) that year would chronicle dermatology’s commitment to the development of new therapeutics and technologies in service of patient needs – the stories of which would grace the newspaper’s pages for 5 decades and counting.
As Dermatology News (formerly Skin and Allergy News) reaches its 50th-year milestone, a reflection on the history of the discipline, especially in the United States, up to the time of the launch of this publication is in order. Such an overview must, of course, be cursory in this context. Yet, for those who want to learn more, a large body of historical references and research has been created to fill in the gaps, as modern dermatology has always been cognizant of the importance of its history, with many individuals and groups drawn to the subject.
Two excellent sources for the history of the field can be found in work by William Allen Pusey, MD (1865-1940), and Herbert Rattner, MD (1900-1962), “The History of Dermatology” published in 1933 and research by members of the History of Dermatology Society, founded in 1973 in New York.
Modern dermatology
The development of the field of modern dermatology can be traced back to the early to mid-19th century. During the first half of the 19th century, England and France dominated the study of dermatology, but by the middle of the century, the German revolution in microparasitology shifted that focus “with remarkable German discoveries,” according to Bernard S. Potter, MD, in his review of bibliographic landmarks of the history of dermatology (J Am Acad Dermatol 2003;48:919-32). For example, Johann Lucas Schoenlein (1793-1864) in 1839 discovered the fungal origin of favus, and in 1841 Jacob Henle (1809-1885) discovered Demodex folliculorum. Karl Ferdinand Eichstedt (1816-1892) in 1846 followed with the discovery of the causative agent of pityriasis versicolor, and Friedrich Wilhelm Felix von Barensprung (1822-1864) in 1862 coined the term erythrasma and named the organism responsible for this condition Microsporum minutissimum.
Dr. Potter described how American dermatology originated in New York City in 1836 when Henry Daggett Bulkley, MD, (1803-1872) opened the first dispensary for skin diseases, the Broome Street Infirmary for Diseases of the Skin, thus creating the first institution in the United States for the treatment of cutaneous disease. As the first American dermatologist, he was also the first in the United States to lecture on and to exclusively practice dermatology.
The rise of interest in the importance of dermatology led to the organization of the early American Dermatological Association in 1886.
However, the state of dermatology as a science in the 19th century was not always looked upon favorably, even by its practitioners, especially in the United States. In 1871, in a “Review on Modern Dermatology,” given as a series of lectures on skin disease at Harvard University, James C. White, MD (1833-1916) of Massachusetts General Hospital, stated that: “Were the literature of skin diseases previous to that of the last half-century absolutely annihilated, and with it, the influence it has exercised upon that of the present day, it would be an immense gain to dermatology, although much of real value would perish.” He lamented that America had contributed little so far to the study of dermatology, and that the discipline was only taught in some of its largest schools, and he urged that this be changed. He also lamented that The American Journal of Syphilography and Dermatology, established the year before, had so far proved itself heavy on syphilis, but light on dermatology, a situation he also hoped would change dramatically.
By the late-19th century, the conviction that diseases of the skin needed to be connected to the overall metabolism and physiology of the patient as a whole was becoming more mainstream.
“It has been, and still is, too much the custom to study diseases of the skin in the light of pathological pictures, to name the local manifestation and to so label it as disease. It is much easier to give the disease name and to label it than it is to comprehend the process at work. The former is comparatively unimportant for the patient, the latter point upon which recovery may depend. The nature and meaning of the process in connection with the cutaneous symptoms has not received enough attention, and I believe this to be one reason why the treatment of many of these diseases in the past has been so notoriously unsatisfactory,” Louis A. Duhring, MD (1845-1913) chided his colleagues in the Section of Dermatology and Syphilography, at the Forty-fourth Annual Meeting of the American Medical Association in 1894. (collections.nlm.nih.gov/ext/dw/101489447/PDF/101489447.pdf)
In the early-20th century, German dermatology influenced American dermatology more than any other, according to Karl Holubar, MD, of the Institute for the History of Medicine, University of Vienna, in his lecture on the history of European dermatopathology.
He stated that, with regard to dermatopathology, it was Oscar Gans, MD (1888-1983) who brought the latest knowledge into the United States by delivering a series of lectures at Mayo Clinic in the late 1920s upon the invitation of Paul A. O’Leary, MD, (1891-1955) who then headed the Mayo section of dermatology.
By the 1930s, a flurry of organizational activity overtook American dermatology. In 1932, the American Board of Dermatology was established, with its first exams given in 1933 (20 students passed, 7 failed). The Society for Investigative Dermatology was founded in 1937, and the American Academy of Dermatology and Syphilology (now the American Academy of Dermatology), founded in 1938.
The 1930s also saw a major influx of German and other European Jews fleeing Nazi oppression who would forever change the face of American dermatology. “Between 1933 and 1938, a series of repressive measures eliminated them from the practice of medicine in Germany and other countries. Although some died in concentration camps and others committed suicide, many were able to emigrate from Europe. Dermatology in the United States particularly benefited from the influx of several stellar Jewish dermatologists who were major contributors to the subsequent flowering of academic dermatology in the United States” (JAMA Derm. 2013;149[9]:1090-4).
“The overtures of the holocaust and the rising power of Hitler in Europe finally brought over to the United States the flower of dermatologists and investigators of the German School, e.g., Alexander and Walter Lever, Felix and Hermann Pinkus, the Epsteins, Erich Auerbach, Stephen Rothman, to name just a few. With this exodus and transfer of brain power, Europe lost its leading role to never again regain it,” according to Dr. Holubar. Walter F. Lever, MD (1909-1992) was especially well-known for his landmark textbook on dermatology, “Histopathology of the Skin,” published in the United States in 1949.
The therapeutic era
Throughout the 19th century, a variety of soaps and patent medicines were touted as cure-alls for a host of skin diseases. Other than their benefits to surface cleanliness and their antiseptic properties, however, they were of little effect.
It wasn’t until the 20th century that truly effective therapeutics entered the dermatologic pharmacopoeia. In their 1989 review, Diane Quintal, MD, and Robert Jackson, MD, discussed the origins of the most important of these drugs and pointed out that, “Until this century, the essence of dermatology resided in the realm of morphology. Early contributors largely confined their activities to the classification of skin diseases and to the elaboration of clinical dermatologic entities based on morphologic features. ... but “in the last 50 years, there have been significant scientific discoveries in the field of therapeutics that have revolutionized the practice of dermatology.“ (Clin Dermatol. 1989;7[3]38-47).
These key drugs comprised:
- Quinacrine was introduced in 1932 by Walter Kikuth, MD, as an antimalarial drug. But it was not until 1940, that A.J. Prokoptchouksi, MD, reported on its effectiveness 35 patients with lupus erythematosus.
- Para-aminobenzoic acid (PABA) came into prominence in 1942, when Stephen Rothman, MD, and Jack Rubin, MD, at the University of Chicago, published the results of their experiment, showing that when PABA was incorporated in an ointment base and applied to the skin, it could protect against sunburn.
- Dapsone. The effectiveness of sulfapyridine was demonstrated in 1947 by M.J. Costello, MD, who reported its usefulness in a patient with dermatitis herpetiformis, which he believed to be caused by a bacterial allergy. Sulfapyridine controlled the disease, but gastrointestinal intolerance and sulfonamide sensitivity were side effects. Ultimately, in 1951, Theodore Cornbleet, MD, introduced the use of sulfoxones in his article entitled “Sulfoxone (diasones) sodium for dermatitis herpetiformis,” considered more effective than sulfapyridine. Dapsone is the active principal ingredient.
- Hydrocortisone. In August 1952, Marion Sulzberger, MD, and Victor H. Witten, MD (both members of the first Skin & Allergy News editorial advisory board), described use of Compound F (17-hydroxycorticosterone-21-acetate, hydrocortisone) in seven cases of atopic dermatitis and one case of discoid or subacute lupus erythematosus, reporting improvement in all of these cases.
- Benzoyl peroxide. Canadian dermatologist William E. Pace, MD, reported on the beneficial effects of benzoyl peroxide on acne in 1953. The product had originally been used for chronic Staphylococcus aureus folliculitis of the beard.
- Griseofulvin, a metabolic byproduct of a number of species of Penicillium, was first isolated in 1939. But in 1958, Harvey Blank, MD, at the University of Miami (also on the first Skin & Allergy News editorial advisory board), and Stanley I. Cullen, MD, administered the drug to a patient with Trichophyton rubrum granuloma, in the first human trial. In 1959, they reported the drug’s benefits on 31 patients with various fungal infections.
- Methotrexate. In 1951, R. Gubner, MD, and colleagues noticed the rapid clearing of skin lesions in a patient with rheumatoid arthritis who had been treated with the folic acid antagonist, aminopterin. And in 1958, W.F. Edmundson, MD, and W.B. Guy, MD, reported on the oral use of the folic acid antagonist, methotrexate. This was followed by multiple reports on the successful use of methotrexate in psoriasis.
- 5-fluorouracil (5-FU). In 1957, 5-FU, an antimetabolite of uracil, was first synthesized. In 1962, G. Falkson, MD, and E.J. Schulz, MD, reported on skin changes observed in 85 patients being treated with systemic 5-FU for advanced carcinomatosis. They found that 31 of the 85 patients developed sensitivity to sunlight and subsequent disappearance of actinic keratoses in these same patients.
Technology in skin care also was developing in the era just before the launch of Skin & Allergy News. For example, Leon Goldman, MD, then chairman of the department of dermatology at the University of Cincinnati, was the first physician to use a laser for tattoo removal. His publication in 1965 helped to solidify its use, leading him to be “regarded by many in the dermatologic community as the ‘godfather of lasers in medicine and surgery’ ” (Clin Dermatol. 2007;25:434-42).
So, by 1970, dermatology as a field had established itself fully with a strong societal infrastructure, a vibrant base of journals and books, and an evolving set of scientific and technical tools. The launch of Skin & Allergy News (now Dermatology News) that year would chronicle dermatology’s commitment to the development of new therapeutics and technologies in service of patient needs – the stories of which would grace the newspaper’s pages for 5 decades and counting.
As Dermatology News (formerly Skin and Allergy News) reaches its 50th-year milestone, a reflection on the history of the discipline, especially in the United States, up to the time of the launch of this publication is in order. Such an overview must, of course, be cursory in this context. Yet, for those who want to learn more, a large body of historical references and research has been created to fill in the gaps, as modern dermatology has always been cognizant of the importance of its history, with many individuals and groups drawn to the subject.
Two excellent sources for the history of the field can be found in work by William Allen Pusey, MD (1865-1940), and Herbert Rattner, MD (1900-1962), “The History of Dermatology” published in 1933 and research by members of the History of Dermatology Society, founded in 1973 in New York.
Modern dermatology
The development of the field of modern dermatology can be traced back to the early to mid-19th century. During the first half of the 19th century, England and France dominated the study of dermatology, but by the middle of the century, the German revolution in microparasitology shifted that focus “with remarkable German discoveries,” according to Bernard S. Potter, MD, in his review of bibliographic landmarks of the history of dermatology (J Am Acad Dermatol 2003;48:919-32). For example, Johann Lucas Schoenlein (1793-1864) in 1839 discovered the fungal origin of favus, and in 1841 Jacob Henle (1809-1885) discovered Demodex folliculorum. Karl Ferdinand Eichstedt (1816-1892) in 1846 followed with the discovery of the causative agent of pityriasis versicolor, and Friedrich Wilhelm Felix von Barensprung (1822-1864) in 1862 coined the term erythrasma and named the organism responsible for this condition Microsporum minutissimum.
Dr. Potter described how American dermatology originated in New York City in 1836 when Henry Daggett Bulkley, MD, (1803-1872) opened the first dispensary for skin diseases, the Broome Street Infirmary for Diseases of the Skin, thus creating the first institution in the United States for the treatment of cutaneous disease. As the first American dermatologist, he was also the first in the United States to lecture on and to exclusively practice dermatology.
The rise of interest in the importance of dermatology led to the organization of the early American Dermatological Association in 1886.
However, the state of dermatology as a science in the 19th century was not always looked upon favorably, even by its practitioners, especially in the United States. In 1871, in a “Review on Modern Dermatology,” given as a series of lectures on skin disease at Harvard University, James C. White, MD (1833-1916) of Massachusetts General Hospital, stated that: “Were the literature of skin diseases previous to that of the last half-century absolutely annihilated, and with it, the influence it has exercised upon that of the present day, it would be an immense gain to dermatology, although much of real value would perish.” He lamented that America had contributed little so far to the study of dermatology, and that the discipline was only taught in some of its largest schools, and he urged that this be changed. He also lamented that The American Journal of Syphilography and Dermatology, established the year before, had so far proved itself heavy on syphilis, but light on dermatology, a situation he also hoped would change dramatically.
By the late-19th century, the conviction that diseases of the skin needed to be connected to the overall metabolism and physiology of the patient as a whole was becoming more mainstream.
“It has been, and still is, too much the custom to study diseases of the skin in the light of pathological pictures, to name the local manifestation and to so label it as disease. It is much easier to give the disease name and to label it than it is to comprehend the process at work. The former is comparatively unimportant for the patient, the latter point upon which recovery may depend. The nature and meaning of the process in connection with the cutaneous symptoms has not received enough attention, and I believe this to be one reason why the treatment of many of these diseases in the past has been so notoriously unsatisfactory,” Louis A. Duhring, MD (1845-1913) chided his colleagues in the Section of Dermatology and Syphilography, at the Forty-fourth Annual Meeting of the American Medical Association in 1894. (collections.nlm.nih.gov/ext/dw/101489447/PDF/101489447.pdf)
In the early-20th century, German dermatology influenced American dermatology more than any other, according to Karl Holubar, MD, of the Institute for the History of Medicine, University of Vienna, in his lecture on the history of European dermatopathology.
He stated that, with regard to dermatopathology, it was Oscar Gans, MD (1888-1983) who brought the latest knowledge into the United States by delivering a series of lectures at Mayo Clinic in the late 1920s upon the invitation of Paul A. O’Leary, MD, (1891-1955) who then headed the Mayo section of dermatology.
By the 1930s, a flurry of organizational activity overtook American dermatology. In 1932, the American Board of Dermatology was established, with its first exams given in 1933 (20 students passed, 7 failed). The Society for Investigative Dermatology was founded in 1937, and the American Academy of Dermatology and Syphilology (now the American Academy of Dermatology), founded in 1938.
The 1930s also saw a major influx of German and other European Jews fleeing Nazi oppression who would forever change the face of American dermatology. “Between 1933 and 1938, a series of repressive measures eliminated them from the practice of medicine in Germany and other countries. Although some died in concentration camps and others committed suicide, many were able to emigrate from Europe. Dermatology in the United States particularly benefited from the influx of several stellar Jewish dermatologists who were major contributors to the subsequent flowering of academic dermatology in the United States” (JAMA Derm. 2013;149[9]:1090-4).
“The overtures of the holocaust and the rising power of Hitler in Europe finally brought over to the United States the flower of dermatologists and investigators of the German School, e.g., Alexander and Walter Lever, Felix and Hermann Pinkus, the Epsteins, Erich Auerbach, Stephen Rothman, to name just a few. With this exodus and transfer of brain power, Europe lost its leading role to never again regain it,” according to Dr. Holubar. Walter F. Lever, MD (1909-1992) was especially well-known for his landmark textbook on dermatology, “Histopathology of the Skin,” published in the United States in 1949.
The therapeutic era
Throughout the 19th century, a variety of soaps and patent medicines were touted as cure-alls for a host of skin diseases. Other than their benefits to surface cleanliness and their antiseptic properties, however, they were of little effect.
It wasn’t until the 20th century that truly effective therapeutics entered the dermatologic pharmacopoeia. In their 1989 review, Diane Quintal, MD, and Robert Jackson, MD, discussed the origins of the most important of these drugs and pointed out that, “Until this century, the essence of dermatology resided in the realm of morphology. Early contributors largely confined their activities to the classification of skin diseases and to the elaboration of clinical dermatologic entities based on morphologic features. ... but “in the last 50 years, there have been significant scientific discoveries in the field of therapeutics that have revolutionized the practice of dermatology.“ (Clin Dermatol. 1989;7[3]38-47).
These key drugs comprised:
- Quinacrine was introduced in 1932 by Walter Kikuth, MD, as an antimalarial drug. But it was not until 1940, that A.J. Prokoptchouksi, MD, reported on its effectiveness 35 patients with lupus erythematosus.
- Para-aminobenzoic acid (PABA) came into prominence in 1942, when Stephen Rothman, MD, and Jack Rubin, MD, at the University of Chicago, published the results of their experiment, showing that when PABA was incorporated in an ointment base and applied to the skin, it could protect against sunburn.
- Dapsone. The effectiveness of sulfapyridine was demonstrated in 1947 by M.J. Costello, MD, who reported its usefulness in a patient with dermatitis herpetiformis, which he believed to be caused by a bacterial allergy. Sulfapyridine controlled the disease, but gastrointestinal intolerance and sulfonamide sensitivity were side effects. Ultimately, in 1951, Theodore Cornbleet, MD, introduced the use of sulfoxones in his article entitled “Sulfoxone (diasones) sodium for dermatitis herpetiformis,” considered more effective than sulfapyridine. Dapsone is the active principal ingredient.
- Hydrocortisone. In August 1952, Marion Sulzberger, MD, and Victor H. Witten, MD (both members of the first Skin & Allergy News editorial advisory board), described use of Compound F (17-hydroxycorticosterone-21-acetate, hydrocortisone) in seven cases of atopic dermatitis and one case of discoid or subacute lupus erythematosus, reporting improvement in all of these cases.
- Benzoyl peroxide. Canadian dermatologist William E. Pace, MD, reported on the beneficial effects of benzoyl peroxide on acne in 1953. The product had originally been used for chronic Staphylococcus aureus folliculitis of the beard.
- Griseofulvin, a metabolic byproduct of a number of species of Penicillium, was first isolated in 1939. But in 1958, Harvey Blank, MD, at the University of Miami (also on the first Skin & Allergy News editorial advisory board), and Stanley I. Cullen, MD, administered the drug to a patient with Trichophyton rubrum granuloma, in the first human trial. In 1959, they reported the drug’s benefits on 31 patients with various fungal infections.
- Methotrexate. In 1951, R. Gubner, MD, and colleagues noticed the rapid clearing of skin lesions in a patient with rheumatoid arthritis who had been treated with the folic acid antagonist, aminopterin. And in 1958, W.F. Edmundson, MD, and W.B. Guy, MD, reported on the oral use of the folic acid antagonist, methotrexate. This was followed by multiple reports on the successful use of methotrexate in psoriasis.
- 5-fluorouracil (5-FU). In 1957, 5-FU, an antimetabolite of uracil, was first synthesized. In 1962, G. Falkson, MD, and E.J. Schulz, MD, reported on skin changes observed in 85 patients being treated with systemic 5-FU for advanced carcinomatosis. They found that 31 of the 85 patients developed sensitivity to sunlight and subsequent disappearance of actinic keratoses in these same patients.
Technology in skin care also was developing in the era just before the launch of Skin & Allergy News. For example, Leon Goldman, MD, then chairman of the department of dermatology at the University of Cincinnati, was the first physician to use a laser for tattoo removal. His publication in 1965 helped to solidify its use, leading him to be “regarded by many in the dermatologic community as the ‘godfather of lasers in medicine and surgery’ ” (Clin Dermatol. 2007;25:434-42).
So, by 1970, dermatology as a field had established itself fully with a strong societal infrastructure, a vibrant base of journals and books, and an evolving set of scientific and technical tools. The launch of Skin & Allergy News (now Dermatology News) that year would chronicle dermatology’s commitment to the development of new therapeutics and technologies in service of patient needs – the stories of which would grace the newspaper’s pages for 5 decades and counting.
IHS and AAP Issue Recommendations on Prenatal Exposure to Opioids
The opioid crisis has hit the American Indian and Alaska Native (AI/AN) communities particularly hard, and “[i]nfants born withdrawing from opioids represent one of the most heartbreaking aspects,” says US Department of Health and Human Services Secretary Alex Azar.
Intrauterine exposure to opioids can induce symptoms that may result in spontaneous abortion, placental injury, and reduced nutrients for the fetus. Moreover, as many as 55% to 94% of infants prenatally exposed to opioids develop neonatal opioid withdrawal syndrome (NOWS), which can vary in severity from mild to life-threatening.
AI/AN women face significant barriers to obtaining appropriate care for substance use disorders, which may delay early interventions for the newborn’s health, said Shaquita Bell, MD, FAAP, chair of the American Academy of Pediatrics (AAP) Committee on Native American Child Health. The Indian Health Service (IHS) and the AAP have recently released clinical recommendations on NOWS for IHS, tribal, and urban Indian organization health care facilities.
The recommendations describe supportive, culturally appropriate standards of care for screening, diagnosing, and treating pregnant mothers and infants affected by prenatal opioid exposure. Management of NOWS begins with identifying women at risk, says the multidisciplinary panel responsible for the recommendations. Among other things, the experts advise screening a pregnant woman at the initial presentation for risk of substance use disorder, as well as for prescription opioid use for treatment of pain, and other risk factors for NOWS.
The panel notes that early application of nonpharmacologic treatment and support can reduce the need for pharmacologic treatment and transfer. Patient education should be a “key component of every prenatal care visit,” the panel says, provided in a nonjudgmental, culturally competent way to increase engagement, involving the partner and other family members if possible. Discussion topics may include the physical effects of continued substance use on both the woman and her infant, but also may include social and legal consequences of continued use.
The recommendations are also a companion guide to clinical recommendations for improving care of AI/AN pregnant women and women of childbearing age with opioid use disorder, which were announced by IHS and the American College of Obstetricians and Gynecologists in March 2019.
The opioid crisis has hit the American Indian and Alaska Native (AI/AN) communities particularly hard, and “[i]nfants born withdrawing from opioids represent one of the most heartbreaking aspects,” says US Department of Health and Human Services Secretary Alex Azar.
Intrauterine exposure to opioids can induce symptoms that may result in spontaneous abortion, placental injury, and reduced nutrients for the fetus. Moreover, as many as 55% to 94% of infants prenatally exposed to opioids develop neonatal opioid withdrawal syndrome (NOWS), which can vary in severity from mild to life-threatening.
AI/AN women face significant barriers to obtaining appropriate care for substance use disorders, which may delay early interventions for the newborn’s health, said Shaquita Bell, MD, FAAP, chair of the American Academy of Pediatrics (AAP) Committee on Native American Child Health. The Indian Health Service (IHS) and the AAP have recently released clinical recommendations on NOWS for IHS, tribal, and urban Indian organization health care facilities.
The recommendations describe supportive, culturally appropriate standards of care for screening, diagnosing, and treating pregnant mothers and infants affected by prenatal opioid exposure. Management of NOWS begins with identifying women at risk, says the multidisciplinary panel responsible for the recommendations. Among other things, the experts advise screening a pregnant woman at the initial presentation for risk of substance use disorder, as well as for prescription opioid use for treatment of pain, and other risk factors for NOWS.
The panel notes that early application of nonpharmacologic treatment and support can reduce the need for pharmacologic treatment and transfer. Patient education should be a “key component of every prenatal care visit,” the panel says, provided in a nonjudgmental, culturally competent way to increase engagement, involving the partner and other family members if possible. Discussion topics may include the physical effects of continued substance use on both the woman and her infant, but also may include social and legal consequences of continued use.
The recommendations are also a companion guide to clinical recommendations for improving care of AI/AN pregnant women and women of childbearing age with opioid use disorder, which were announced by IHS and the American College of Obstetricians and Gynecologists in March 2019.
The opioid crisis has hit the American Indian and Alaska Native (AI/AN) communities particularly hard, and “[i]nfants born withdrawing from opioids represent one of the most heartbreaking aspects,” says US Department of Health and Human Services Secretary Alex Azar.
Intrauterine exposure to opioids can induce symptoms that may result in spontaneous abortion, placental injury, and reduced nutrients for the fetus. Moreover, as many as 55% to 94% of infants prenatally exposed to opioids develop neonatal opioid withdrawal syndrome (NOWS), which can vary in severity from mild to life-threatening.
AI/AN women face significant barriers to obtaining appropriate care for substance use disorders, which may delay early interventions for the newborn’s health, said Shaquita Bell, MD, FAAP, chair of the American Academy of Pediatrics (AAP) Committee on Native American Child Health. The Indian Health Service (IHS) and the AAP have recently released clinical recommendations on NOWS for IHS, tribal, and urban Indian organization health care facilities.
The recommendations describe supportive, culturally appropriate standards of care for screening, diagnosing, and treating pregnant mothers and infants affected by prenatal opioid exposure. Management of NOWS begins with identifying women at risk, says the multidisciplinary panel responsible for the recommendations. Among other things, the experts advise screening a pregnant woman at the initial presentation for risk of substance use disorder, as well as for prescription opioid use for treatment of pain, and other risk factors for NOWS.
The panel notes that early application of nonpharmacologic treatment and support can reduce the need for pharmacologic treatment and transfer. Patient education should be a “key component of every prenatal care visit,” the panel says, provided in a nonjudgmental, culturally competent way to increase engagement, involving the partner and other family members if possible. Discussion topics may include the physical effects of continued substance use on both the woman and her infant, but also may include social and legal consequences of continued use.
The recommendations are also a companion guide to clinical recommendations for improving care of AI/AN pregnant women and women of childbearing age with opioid use disorder, which were announced by IHS and the American College of Obstetricians and Gynecologists in March 2019.
Sevuparin failed for acute VOC in sickle cell, but may have preventive potential
ORLANDO – Sevuparin, a novel nonanticoagulant heparinoid drug, showed no efficacy for acute vaso-occlusive crisis (VOC) in patients with sickle cell disease (SCD) in the randomized, controlled, phase 2 TVOC01 trial, but its promising safety and broad mechanism of action warrant further exploration in the prodromal VOC setting, according to investigators.
Time to VOC resolution – the primary study endpoint – was similar at about 168 hours in 71 hospitalized patients randomized to receive sevuparin and in 76 who received placebo (intention-to-treat hazard ratio, 0.89), Bart J. Biemond, MD, explained during a presentation of the findings at the annual meeting of the American Society of Hematology.
A per-protocol analysis based on the 69 and 75 patients dosed in the treatment and placebo arms, respectively, showed a similar result (HR, 0.81), said Dr. Biemond of the department of clinical hematology, Amsterdam UMC, Academic Medical Center, the Netherlands.
Secondary endpoints, including mean change in pain intensity from baseline on a visual analogue scale (VAS), duration of severest pain measured as time to achieve a 30% reduction in VAS score from baseline, and cumulative use of parenteral opioids, also did not differ between the treatment and placebo arms, he added.
Patients in the global, double-blind, multicenter trial were aged 12-50 years (median, 22 years) with any type of SCD. They were enrolled from 16 sites in 7 countries to receive a loading dose of 3 mg/kg of sevuparin and continuous 18 mg/kg per day infusions or placebo. Patients in both arms also received standard-of-care and parenteral opioid therapy.
The groups were generally balanced with respect to demographic and baseline characteristics, Dr. Biemond said, noting that the treatment was safe: No serious adverse events occurred, and any mild-to-moderate adverse events were transient.
The findings were disappointing given the lack of approved treatment options other than pain management for acute VOC in hospitalized patients with SCD, and they were somewhat surprising given that preclinical and clinical data in recent years have demonstrated that “you can actually prevent those crises by antiadhesive strategies,” he said.
“So we hypothesized that, if you perform such an antiadhesive strategy in a patient already having a crisis and admitted in the hospital, you may shorten their period of admission and perhaps also shorten the severity of their pain,” he said.
In fact, a single-center, randomized, controlled trial conducted by Qari et al. in 2007 (Thromb Haemost. 2007 Aug;98[2]:392-6) showed that full-dose tinzaparin reduced pain severity and duration of admission among sickle cell patients with acute VOC – perhaps because of the antiadhesive properties of heparin – but that study was never repeated, Dr. Biemond said, noting that those antiadhesive properties have been well documented in animal studies.
“Heparin is able to inhibit P-selectin, L-selectin, thrombospondin, fibronectin, and von Willebrand activity, which are all involved in vaso-occlusion in patients with sickle cell disease, and very likely also involved during a vaso-occlusive crisis,” he explained, adding that sevuparin, a low-molecular-weight heparin without functional antithrombin binding domain, seemed to be a good candidate for testing that hypothesis.
“It has no anticoagulant effects on factor Xa and IIa,” he said. “It retains, however, its antiadhesive and antiaggregation properties.”
Since it has no anticoagulation activity, it can be dosed at up to 20-fold the therapeutic dose of low-molecular-weight heparin to optimize the antiadhesive effects, he noted.
However, the data indicate that “antiadhesive therapies are clearly not effective in patients with vaso-occlusive crisis,” he said, noting that this was also affirmed by a similar 2019 study of the investigational panselectin inhibitor rivipansel, as reported in a Pfizer press release.
Intriguingly, the difference between the current study and the 2007 study by Qari et al. raises questions about whether anticoagulation, rather than antiadhesion, helped resolve VOC in that study, he said, noting that future studies should focus on whether that is the case.
As for the role of antiadhesive therapy, the mode of action of sevuparin and the current findings taken together suggest that future studies should also assess whether it can be used to prevent VOC.
“Perhaps sevuparin could be administered to patients in a prodromal phase – just before a real vaso-occlusive crisis appears – to prevent such a crisis from happening,” he said. “It would be interesting to use the drug that way.”
Dr. Biemond reported research funding from Sanquin and honoraria from Novartis and GBT.
SOURCE: Biemond B et al. ASH 2019, Abstract 614.
ORLANDO – Sevuparin, a novel nonanticoagulant heparinoid drug, showed no efficacy for acute vaso-occlusive crisis (VOC) in patients with sickle cell disease (SCD) in the randomized, controlled, phase 2 TVOC01 trial, but its promising safety and broad mechanism of action warrant further exploration in the prodromal VOC setting, according to investigators.
Time to VOC resolution – the primary study endpoint – was similar at about 168 hours in 71 hospitalized patients randomized to receive sevuparin and in 76 who received placebo (intention-to-treat hazard ratio, 0.89), Bart J. Biemond, MD, explained during a presentation of the findings at the annual meeting of the American Society of Hematology.
A per-protocol analysis based on the 69 and 75 patients dosed in the treatment and placebo arms, respectively, showed a similar result (HR, 0.81), said Dr. Biemond of the department of clinical hematology, Amsterdam UMC, Academic Medical Center, the Netherlands.
Secondary endpoints, including mean change in pain intensity from baseline on a visual analogue scale (VAS), duration of severest pain measured as time to achieve a 30% reduction in VAS score from baseline, and cumulative use of parenteral opioids, also did not differ between the treatment and placebo arms, he added.
Patients in the global, double-blind, multicenter trial were aged 12-50 years (median, 22 years) with any type of SCD. They were enrolled from 16 sites in 7 countries to receive a loading dose of 3 mg/kg of sevuparin and continuous 18 mg/kg per day infusions or placebo. Patients in both arms also received standard-of-care and parenteral opioid therapy.
The groups were generally balanced with respect to demographic and baseline characteristics, Dr. Biemond said, noting that the treatment was safe: No serious adverse events occurred, and any mild-to-moderate adverse events were transient.
The findings were disappointing given the lack of approved treatment options other than pain management for acute VOC in hospitalized patients with SCD, and they were somewhat surprising given that preclinical and clinical data in recent years have demonstrated that “you can actually prevent those crises by antiadhesive strategies,” he said.
“So we hypothesized that, if you perform such an antiadhesive strategy in a patient already having a crisis and admitted in the hospital, you may shorten their period of admission and perhaps also shorten the severity of their pain,” he said.
In fact, a single-center, randomized, controlled trial conducted by Qari et al. in 2007 (Thromb Haemost. 2007 Aug;98[2]:392-6) showed that full-dose tinzaparin reduced pain severity and duration of admission among sickle cell patients with acute VOC – perhaps because of the antiadhesive properties of heparin – but that study was never repeated, Dr. Biemond said, noting that those antiadhesive properties have been well documented in animal studies.
“Heparin is able to inhibit P-selectin, L-selectin, thrombospondin, fibronectin, and von Willebrand activity, which are all involved in vaso-occlusion in patients with sickle cell disease, and very likely also involved during a vaso-occlusive crisis,” he explained, adding that sevuparin, a low-molecular-weight heparin without functional antithrombin binding domain, seemed to be a good candidate for testing that hypothesis.
“It has no anticoagulant effects on factor Xa and IIa,” he said. “It retains, however, its antiadhesive and antiaggregation properties.”
Since it has no anticoagulation activity, it can be dosed at up to 20-fold the therapeutic dose of low-molecular-weight heparin to optimize the antiadhesive effects, he noted.
However, the data indicate that “antiadhesive therapies are clearly not effective in patients with vaso-occlusive crisis,” he said, noting that this was also affirmed by a similar 2019 study of the investigational panselectin inhibitor rivipansel, as reported in a Pfizer press release.
Intriguingly, the difference between the current study and the 2007 study by Qari et al. raises questions about whether anticoagulation, rather than antiadhesion, helped resolve VOC in that study, he said, noting that future studies should focus on whether that is the case.
As for the role of antiadhesive therapy, the mode of action of sevuparin and the current findings taken together suggest that future studies should also assess whether it can be used to prevent VOC.
“Perhaps sevuparin could be administered to patients in a prodromal phase – just before a real vaso-occlusive crisis appears – to prevent such a crisis from happening,” he said. “It would be interesting to use the drug that way.”
Dr. Biemond reported research funding from Sanquin and honoraria from Novartis and GBT.
SOURCE: Biemond B et al. ASH 2019, Abstract 614.
ORLANDO – Sevuparin, a novel nonanticoagulant heparinoid drug, showed no efficacy for acute vaso-occlusive crisis (VOC) in patients with sickle cell disease (SCD) in the randomized, controlled, phase 2 TVOC01 trial, but its promising safety and broad mechanism of action warrant further exploration in the prodromal VOC setting, according to investigators.
Time to VOC resolution – the primary study endpoint – was similar at about 168 hours in 71 hospitalized patients randomized to receive sevuparin and in 76 who received placebo (intention-to-treat hazard ratio, 0.89), Bart J. Biemond, MD, explained during a presentation of the findings at the annual meeting of the American Society of Hematology.
A per-protocol analysis based on the 69 and 75 patients dosed in the treatment and placebo arms, respectively, showed a similar result (HR, 0.81), said Dr. Biemond of the department of clinical hematology, Amsterdam UMC, Academic Medical Center, the Netherlands.
Secondary endpoints, including mean change in pain intensity from baseline on a visual analogue scale (VAS), duration of severest pain measured as time to achieve a 30% reduction in VAS score from baseline, and cumulative use of parenteral opioids, also did not differ between the treatment and placebo arms, he added.
Patients in the global, double-blind, multicenter trial were aged 12-50 years (median, 22 years) with any type of SCD. They were enrolled from 16 sites in 7 countries to receive a loading dose of 3 mg/kg of sevuparin and continuous 18 mg/kg per day infusions or placebo. Patients in both arms also received standard-of-care and parenteral opioid therapy.
The groups were generally balanced with respect to demographic and baseline characteristics, Dr. Biemond said, noting that the treatment was safe: No serious adverse events occurred, and any mild-to-moderate adverse events were transient.
The findings were disappointing given the lack of approved treatment options other than pain management for acute VOC in hospitalized patients with SCD, and they were somewhat surprising given that preclinical and clinical data in recent years have demonstrated that “you can actually prevent those crises by antiadhesive strategies,” he said.
“So we hypothesized that, if you perform such an antiadhesive strategy in a patient already having a crisis and admitted in the hospital, you may shorten their period of admission and perhaps also shorten the severity of their pain,” he said.
In fact, a single-center, randomized, controlled trial conducted by Qari et al. in 2007 (Thromb Haemost. 2007 Aug;98[2]:392-6) showed that full-dose tinzaparin reduced pain severity and duration of admission among sickle cell patients with acute VOC – perhaps because of the antiadhesive properties of heparin – but that study was never repeated, Dr. Biemond said, noting that those antiadhesive properties have been well documented in animal studies.
“Heparin is able to inhibit P-selectin, L-selectin, thrombospondin, fibronectin, and von Willebrand activity, which are all involved in vaso-occlusion in patients with sickle cell disease, and very likely also involved during a vaso-occlusive crisis,” he explained, adding that sevuparin, a low-molecular-weight heparin without functional antithrombin binding domain, seemed to be a good candidate for testing that hypothesis.
“It has no anticoagulant effects on factor Xa and IIa,” he said. “It retains, however, its antiadhesive and antiaggregation properties.”
Since it has no anticoagulation activity, it can be dosed at up to 20-fold the therapeutic dose of low-molecular-weight heparin to optimize the antiadhesive effects, he noted.
However, the data indicate that “antiadhesive therapies are clearly not effective in patients with vaso-occlusive crisis,” he said, noting that this was also affirmed by a similar 2019 study of the investigational panselectin inhibitor rivipansel, as reported in a Pfizer press release.
Intriguingly, the difference between the current study and the 2007 study by Qari et al. raises questions about whether anticoagulation, rather than antiadhesion, helped resolve VOC in that study, he said, noting that future studies should focus on whether that is the case.
As for the role of antiadhesive therapy, the mode of action of sevuparin and the current findings taken together suggest that future studies should also assess whether it can be used to prevent VOC.
“Perhaps sevuparin could be administered to patients in a prodromal phase – just before a real vaso-occlusive crisis appears – to prevent such a crisis from happening,” he said. “It would be interesting to use the drug that way.”
Dr. Biemond reported research funding from Sanquin and honoraria from Novartis and GBT.
SOURCE: Biemond B et al. ASH 2019, Abstract 614.
REPORTING FROM ASH 2019
Common drug with lots of surprising side effects
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.