User login
Should a normal-appearing hippocampus be resected in a patient with temporal lobe epilepsy?
BALTIMORE – according to an analysis presented at the annual meeting of the American Epilepsy Society. Long-term seizure outcomes, however, are similar between resected and nonresected patients. In addition, sparing a normal-appearing hippocampus is correlated with a lower risk of verbal deficits, but long-term outcomes are unclear.
Neurologists have not arrived at a consensus about the best surgical management of patients with temporal lobe epilepsy and a hippocampus that appears normal on MRI. Few studies have examined seizure and neuropsychologic outcomes in this population, and this scarcity of data makes counseling patients difficult.
A review of data for surgical patients
To investigate this question, Marcia E. Morita-Sherman, MD, from the Cleveland Clinic, and colleagues retrospectively reviewed data for 152 patients who underwent surgery for temporal lobe epilepsy at the Cleveland Clinic during 2010-2018. Eligible participants were older than 16 years, and the researchers excluded patients with MRI or pathologic signs of hippocampal sclerosis and those with prior surgeries from the analysis.
To examine neuropsychological outcomes, Dr. Morita-Sherman and colleagues compared measures of verbal memory, visual memory, and confrontation naming that had been obtained before surgery and at 6 months after surgery. They measured hippocampal volume using Neuroquant. They categorized resections as dominant or nondominant according to patients’ handedness or language lateralization. The investigators classified 74 patients as having a spared hippocampus and 78 patients as having a resected hippocampus. They classified neuropsychological outcomes as showing decline or no decline using epilepsy-specific reliable change indexes.
Type of surgery affected memory and naming
Approximately 40% of patients had seizure recurrence within 1 year after surgery, and 63% had seizure recurrence within 6 years after surgery. The rate of invasive EEG was similar between patients with a spared hippocampus (50%) and those with a resected hippocampus (47%). In a univariate analysis, male sex, longer epilepsy duration, normal MRI, history of invasive evaluation, and acute postoperative seizures were associated with a higher risk of seizure recurrence. Patients with a spared hippocampus had a higher risk of early seizure recurrence, compared with patients with a resected hippocampus, but the difference was not statistically significant. Long-term seizure outcomes were similar between the two groups.
Neuropsychological outcomes were available for 86 patients. Among 56 patients who underwent surgery on the dominant side, those with spared-hippocampal surgery, compared with those with resected-hippocampal surgery, had lower rates of clinically meaningful declines in verbal memory (39.7% vs. 70.4%) and naming (40.7% vs. 79.2%). The investigators found no significant difference in the 30 patients with nondominant surgeries.
“Sparing the hippocampus in a tailored temporal lobe resection doesn’t necessarily prevent any memory decline. Close to 40% of our patients where the hippocampus was spared had a clinically significant memory loss,” said Lara E. Jehi, MD, an epileptologist at the Cleveland Clinic and one of the investigators. “Including the hippocampus in the resection seems to correlate with better odds of seizure freedom, at least in the short term. We need more research to study the long-term memory and naming implications of hippocampal sparing versus resection.”
The study was funded by a grant from the National Institutes of Health. The investigators reported no conflicts of interest. [email protected]
SOURCE: Morita-Sherman ME et al. AES 2019, Abstract 1.336.
BALTIMORE – according to an analysis presented at the annual meeting of the American Epilepsy Society. Long-term seizure outcomes, however, are similar between resected and nonresected patients. In addition, sparing a normal-appearing hippocampus is correlated with a lower risk of verbal deficits, but long-term outcomes are unclear.
Neurologists have not arrived at a consensus about the best surgical management of patients with temporal lobe epilepsy and a hippocampus that appears normal on MRI. Few studies have examined seizure and neuropsychologic outcomes in this population, and this scarcity of data makes counseling patients difficult.
A review of data for surgical patients
To investigate this question, Marcia E. Morita-Sherman, MD, from the Cleveland Clinic, and colleagues retrospectively reviewed data for 152 patients who underwent surgery for temporal lobe epilepsy at the Cleveland Clinic during 2010-2018. Eligible participants were older than 16 years, and the researchers excluded patients with MRI or pathologic signs of hippocampal sclerosis and those with prior surgeries from the analysis.
To examine neuropsychological outcomes, Dr. Morita-Sherman and colleagues compared measures of verbal memory, visual memory, and confrontation naming that had been obtained before surgery and at 6 months after surgery. They measured hippocampal volume using Neuroquant. They categorized resections as dominant or nondominant according to patients’ handedness or language lateralization. The investigators classified 74 patients as having a spared hippocampus and 78 patients as having a resected hippocampus. They classified neuropsychological outcomes as showing decline or no decline using epilepsy-specific reliable change indexes.
Type of surgery affected memory and naming
Approximately 40% of patients had seizure recurrence within 1 year after surgery, and 63% had seizure recurrence within 6 years after surgery. The rate of invasive EEG was similar between patients with a spared hippocampus (50%) and those with a resected hippocampus (47%). In a univariate analysis, male sex, longer epilepsy duration, normal MRI, history of invasive evaluation, and acute postoperative seizures were associated with a higher risk of seizure recurrence. Patients with a spared hippocampus had a higher risk of early seizure recurrence, compared with patients with a resected hippocampus, but the difference was not statistically significant. Long-term seizure outcomes were similar between the two groups.
Neuropsychological outcomes were available for 86 patients. Among 56 patients who underwent surgery on the dominant side, those with spared-hippocampal surgery, compared with those with resected-hippocampal surgery, had lower rates of clinically meaningful declines in verbal memory (39.7% vs. 70.4%) and naming (40.7% vs. 79.2%). The investigators found no significant difference in the 30 patients with nondominant surgeries.
“Sparing the hippocampus in a tailored temporal lobe resection doesn’t necessarily prevent any memory decline. Close to 40% of our patients where the hippocampus was spared had a clinically significant memory loss,” said Lara E. Jehi, MD, an epileptologist at the Cleveland Clinic and one of the investigators. “Including the hippocampus in the resection seems to correlate with better odds of seizure freedom, at least in the short term. We need more research to study the long-term memory and naming implications of hippocampal sparing versus resection.”
The study was funded by a grant from the National Institutes of Health. The investigators reported no conflicts of interest. [email protected]
SOURCE: Morita-Sherman ME et al. AES 2019, Abstract 1.336.
BALTIMORE – according to an analysis presented at the annual meeting of the American Epilepsy Society. Long-term seizure outcomes, however, are similar between resected and nonresected patients. In addition, sparing a normal-appearing hippocampus is correlated with a lower risk of verbal deficits, but long-term outcomes are unclear.
Neurologists have not arrived at a consensus about the best surgical management of patients with temporal lobe epilepsy and a hippocampus that appears normal on MRI. Few studies have examined seizure and neuropsychologic outcomes in this population, and this scarcity of data makes counseling patients difficult.
A review of data for surgical patients
To investigate this question, Marcia E. Morita-Sherman, MD, from the Cleveland Clinic, and colleagues retrospectively reviewed data for 152 patients who underwent surgery for temporal lobe epilepsy at the Cleveland Clinic during 2010-2018. Eligible participants were older than 16 years, and the researchers excluded patients with MRI or pathologic signs of hippocampal sclerosis and those with prior surgeries from the analysis.
To examine neuropsychological outcomes, Dr. Morita-Sherman and colleagues compared measures of verbal memory, visual memory, and confrontation naming that had been obtained before surgery and at 6 months after surgery. They measured hippocampal volume using Neuroquant. They categorized resections as dominant or nondominant according to patients’ handedness or language lateralization. The investigators classified 74 patients as having a spared hippocampus and 78 patients as having a resected hippocampus. They classified neuropsychological outcomes as showing decline or no decline using epilepsy-specific reliable change indexes.
Type of surgery affected memory and naming
Approximately 40% of patients had seizure recurrence within 1 year after surgery, and 63% had seizure recurrence within 6 years after surgery. The rate of invasive EEG was similar between patients with a spared hippocampus (50%) and those with a resected hippocampus (47%). In a univariate analysis, male sex, longer epilepsy duration, normal MRI, history of invasive evaluation, and acute postoperative seizures were associated with a higher risk of seizure recurrence. Patients with a spared hippocampus had a higher risk of early seizure recurrence, compared with patients with a resected hippocampus, but the difference was not statistically significant. Long-term seizure outcomes were similar between the two groups.
Neuropsychological outcomes were available for 86 patients. Among 56 patients who underwent surgery on the dominant side, those with spared-hippocampal surgery, compared with those with resected-hippocampal surgery, had lower rates of clinically meaningful declines in verbal memory (39.7% vs. 70.4%) and naming (40.7% vs. 79.2%). The investigators found no significant difference in the 30 patients with nondominant surgeries.
“Sparing the hippocampus in a tailored temporal lobe resection doesn’t necessarily prevent any memory decline. Close to 40% of our patients where the hippocampus was spared had a clinically significant memory loss,” said Lara E. Jehi, MD, an epileptologist at the Cleveland Clinic and one of the investigators. “Including the hippocampus in the resection seems to correlate with better odds of seizure freedom, at least in the short term. We need more research to study the long-term memory and naming implications of hippocampal sparing versus resection.”
The study was funded by a grant from the National Institutes of Health. The investigators reported no conflicts of interest. [email protected]
SOURCE: Morita-Sherman ME et al. AES 2019, Abstract 1.336.
REPORTING FROM AES 2019
Anxiety may be a part of healthy development, sometimes
Anxiety is probably the most common behavioral health complaint that presents in the pediatrician’s office. The prevalence of anxiety is going up every year, and we do not have a good understanding why. Is it the pressure to perform at earlier and earlier ages? Is it the press of information or rapid communication of every disaster on Earth? Or are children not developing appropriate coping skills for the expectable challenges and stresses they will face through development? We do not know.
Anxiety disorders are most likely to present in the early school years – latency – between the ages of 6 and 12 years. Teenagers may present with new anxiety disorders or may disclose symptoms that they have been quietly managing since they were younger, when they were thought to be “shy.” These disorders include separation anxiety disorder, social phobia, selective mutism, specific phobia, and generalized anxiety disorder. This age period also is marked by high levels of normal anxiety because children’s cognitive development has advanced beyond their emotional development. They are capable of logic, can understand cause and effect, and can appreciate the passage of time and serious matters such as the permanence of death. Gone is the magical thinking of the preschool years! When an elementary student learns about global warming or a refugee crisis, they can fully appreciate the serious implications of the subject. What they lack is experience with tolerating uncertainty and worry and proceeding with life, focusing on what they might address or even bearing the fact that life is sometimes unfair. This mismatch of relative cognitive maturity with emotional immaturity can lead to anxiety and distress. This is particularly true as they face these challenges while they have new independence, spending longer days at school and less time with parents. Bearing this distress with caring adults, learning to focus on what they can do, and discovering that they and the world can go on even when something very unfair has happened is central to how they develop emotional maturity.
How a child learns to manage anxiety is very much determined by how their parents manage anxiety and how well their parents can tolerate their children’s distress. A parent who becomes overwhelmed when their child is upset about missing a goal in soccer will have a difficult time helping their child learn how to manage distress. And children who are facing chronic severe stress, such as poverty, domestic violence, or chronic illness in a parent, are facing the double challenge of managing persistent anxiety that may be impairing their parents’ ability to support them. When the child and their family are connected to a community that has not been able to effectively respond to larger problems, such as creating safe schools or neighborhoods, anxiety can become entrenched in despair.
It often is tempting to jump in with reassurance when your patient or their parents present with anxiety. But when you calmly show curiosity, you model tolerance of their distress. Are they fearful about very specific situations, such as being called on in class? Or do they become dysregulated when facing a separation from their parents, such as at bedtime or before school, seeking contact with their parents with endless questions? Find out how the parents are managing separations and whether they may be inadvertently rewarding by staying with them to negotiate or answer endless questions. Find out if parents may be accommodating anxiety by allowing their children to avoid normal situations that are stirring anxiety. Do they give in anytime their child shows resistance or have they learned to pick their battles and help their children face more-modest stress while avoiding only the most intensely anxious situations? Are the parents able to speak calmly and with good humor about these challenges or do they become very stressed and defensive? Is there a family history of anxiety? Managing a child’s anxiety every day can be exhausting, and parents might need a referral in addition to a discussion about how anxiety is developmentally normal.
For those parents that can manage this discussion, suggest that, like you, if they can remain calm during these times with their child (even if they don’t feel calm), it will help their child get better at managing anxiety, even if their child has an anxiety disorder. They also should be curious about their child’s worries, learning about the details and scenarios their children may be anticipating. They should express compassion about how uncomfortable anxiety is, coupled with their confident belief that the child will be able to tolerate and manage the situation even though it’s uncomfortable. This acknowledgment should not be a dismissal of the anxiety, instead it should be confidence that the child will learn to bear it.
When your patient is a teenager describing anxiety, unpack. Are they anxious about their performance on their five Advanced Placement exams? If their anxiety sounds more like appropriate stress, be compassionate and then curious about how they are learning to relax. Are they using drugs and alcohol? Or have they found healthy ways to unwind and recharge? Focusing on ways in which they are learning to care for themselves, making time for sleep and exercise, live time with friends, and senseless fun is therapeutic. Find out if their parents are supportive of their self-care. You might even give them a prescription!
Anxiety is often a private experience, and parents might not know about it until it presents with an explosion of distress or obstinacy when an anxious child is pushed into scary territory. Asking questions about specific worries (something happening to parents, germs, weather events) can illuminate the extent of anxiety. It also is worth exploring if there are rituals that help them manage their worries, whether they are common (finding a parent, hugging a pet, prayer) or more compulsive (repetitive undoing, hair pulling). Find out if there has recently been any serious stress or change for the family, such as the loss of a job or illness in a grandparent, that may be contributing to a child’s anxiety.
Anytime you see anxieties that are broad or extreme, disrupt their ability to function (go to school, participate in activities, build friendships), or if their parents are clearly struggling with managing their child’s distress, it is worthwhile to find a referral to a psychiatrist or psychologist for evaluation and further treatment. School avoidance constitutes an urgent need for evaluation, as every day of school missed makes it harder for the child to return to school. For all of your anxious patients, even when you make a referral to a psychiatrist for evaluation, teach your patients and parents about how critical adequate sleep and regular exercise are to managing anxiety. Remind them that an appropriate level of anxiety is normal and promotes performance and grit, despite the discomfort, and that learning how to manage anxiety is essential to growing up and building mental health.
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
Anxiety is probably the most common behavioral health complaint that presents in the pediatrician’s office. The prevalence of anxiety is going up every year, and we do not have a good understanding why. Is it the pressure to perform at earlier and earlier ages? Is it the press of information or rapid communication of every disaster on Earth? Or are children not developing appropriate coping skills for the expectable challenges and stresses they will face through development? We do not know.
Anxiety disorders are most likely to present in the early school years – latency – between the ages of 6 and 12 years. Teenagers may present with new anxiety disorders or may disclose symptoms that they have been quietly managing since they were younger, when they were thought to be “shy.” These disorders include separation anxiety disorder, social phobia, selective mutism, specific phobia, and generalized anxiety disorder. This age period also is marked by high levels of normal anxiety because children’s cognitive development has advanced beyond their emotional development. They are capable of logic, can understand cause and effect, and can appreciate the passage of time and serious matters such as the permanence of death. Gone is the magical thinking of the preschool years! When an elementary student learns about global warming or a refugee crisis, they can fully appreciate the serious implications of the subject. What they lack is experience with tolerating uncertainty and worry and proceeding with life, focusing on what they might address or even bearing the fact that life is sometimes unfair. This mismatch of relative cognitive maturity with emotional immaturity can lead to anxiety and distress. This is particularly true as they face these challenges while they have new independence, spending longer days at school and less time with parents. Bearing this distress with caring adults, learning to focus on what they can do, and discovering that they and the world can go on even when something very unfair has happened is central to how they develop emotional maturity.
How a child learns to manage anxiety is very much determined by how their parents manage anxiety and how well their parents can tolerate their children’s distress. A parent who becomes overwhelmed when their child is upset about missing a goal in soccer will have a difficult time helping their child learn how to manage distress. And children who are facing chronic severe stress, such as poverty, domestic violence, or chronic illness in a parent, are facing the double challenge of managing persistent anxiety that may be impairing their parents’ ability to support them. When the child and their family are connected to a community that has not been able to effectively respond to larger problems, such as creating safe schools or neighborhoods, anxiety can become entrenched in despair.
It often is tempting to jump in with reassurance when your patient or their parents present with anxiety. But when you calmly show curiosity, you model tolerance of their distress. Are they fearful about very specific situations, such as being called on in class? Or do they become dysregulated when facing a separation from their parents, such as at bedtime or before school, seeking contact with their parents with endless questions? Find out how the parents are managing separations and whether they may be inadvertently rewarding by staying with them to negotiate or answer endless questions. Find out if parents may be accommodating anxiety by allowing their children to avoid normal situations that are stirring anxiety. Do they give in anytime their child shows resistance or have they learned to pick their battles and help their children face more-modest stress while avoiding only the most intensely anxious situations? Are the parents able to speak calmly and with good humor about these challenges or do they become very stressed and defensive? Is there a family history of anxiety? Managing a child’s anxiety every day can be exhausting, and parents might need a referral in addition to a discussion about how anxiety is developmentally normal.
For those parents that can manage this discussion, suggest that, like you, if they can remain calm during these times with their child (even if they don’t feel calm), it will help their child get better at managing anxiety, even if their child has an anxiety disorder. They also should be curious about their child’s worries, learning about the details and scenarios their children may be anticipating. They should express compassion about how uncomfortable anxiety is, coupled with their confident belief that the child will be able to tolerate and manage the situation even though it’s uncomfortable. This acknowledgment should not be a dismissal of the anxiety, instead it should be confidence that the child will learn to bear it.
When your patient is a teenager describing anxiety, unpack. Are they anxious about their performance on their five Advanced Placement exams? If their anxiety sounds more like appropriate stress, be compassionate and then curious about how they are learning to relax. Are they using drugs and alcohol? Or have they found healthy ways to unwind and recharge? Focusing on ways in which they are learning to care for themselves, making time for sleep and exercise, live time with friends, and senseless fun is therapeutic. Find out if their parents are supportive of their self-care. You might even give them a prescription!
Anxiety is often a private experience, and parents might not know about it until it presents with an explosion of distress or obstinacy when an anxious child is pushed into scary territory. Asking questions about specific worries (something happening to parents, germs, weather events) can illuminate the extent of anxiety. It also is worth exploring if there are rituals that help them manage their worries, whether they are common (finding a parent, hugging a pet, prayer) or more compulsive (repetitive undoing, hair pulling). Find out if there has recently been any serious stress or change for the family, such as the loss of a job or illness in a grandparent, that may be contributing to a child’s anxiety.
Anytime you see anxieties that are broad or extreme, disrupt their ability to function (go to school, participate in activities, build friendships), or if their parents are clearly struggling with managing their child’s distress, it is worthwhile to find a referral to a psychiatrist or psychologist for evaluation and further treatment. School avoidance constitutes an urgent need for evaluation, as every day of school missed makes it harder for the child to return to school. For all of your anxious patients, even when you make a referral to a psychiatrist for evaluation, teach your patients and parents about how critical adequate sleep and regular exercise are to managing anxiety. Remind them that an appropriate level of anxiety is normal and promotes performance and grit, despite the discomfort, and that learning how to manage anxiety is essential to growing up and building mental health.
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
Anxiety is probably the most common behavioral health complaint that presents in the pediatrician’s office. The prevalence of anxiety is going up every year, and we do not have a good understanding why. Is it the pressure to perform at earlier and earlier ages? Is it the press of information or rapid communication of every disaster on Earth? Or are children not developing appropriate coping skills for the expectable challenges and stresses they will face through development? We do not know.
Anxiety disorders are most likely to present in the early school years – latency – between the ages of 6 and 12 years. Teenagers may present with new anxiety disorders or may disclose symptoms that they have been quietly managing since they were younger, when they were thought to be “shy.” These disorders include separation anxiety disorder, social phobia, selective mutism, specific phobia, and generalized anxiety disorder. This age period also is marked by high levels of normal anxiety because children’s cognitive development has advanced beyond their emotional development. They are capable of logic, can understand cause and effect, and can appreciate the passage of time and serious matters such as the permanence of death. Gone is the magical thinking of the preschool years! When an elementary student learns about global warming or a refugee crisis, they can fully appreciate the serious implications of the subject. What they lack is experience with tolerating uncertainty and worry and proceeding with life, focusing on what they might address or even bearing the fact that life is sometimes unfair. This mismatch of relative cognitive maturity with emotional immaturity can lead to anxiety and distress. This is particularly true as they face these challenges while they have new independence, spending longer days at school and less time with parents. Bearing this distress with caring adults, learning to focus on what they can do, and discovering that they and the world can go on even when something very unfair has happened is central to how they develop emotional maturity.
How a child learns to manage anxiety is very much determined by how their parents manage anxiety and how well their parents can tolerate their children’s distress. A parent who becomes overwhelmed when their child is upset about missing a goal in soccer will have a difficult time helping their child learn how to manage distress. And children who are facing chronic severe stress, such as poverty, domestic violence, or chronic illness in a parent, are facing the double challenge of managing persistent anxiety that may be impairing their parents’ ability to support them. When the child and their family are connected to a community that has not been able to effectively respond to larger problems, such as creating safe schools or neighborhoods, anxiety can become entrenched in despair.
It often is tempting to jump in with reassurance when your patient or their parents present with anxiety. But when you calmly show curiosity, you model tolerance of their distress. Are they fearful about very specific situations, such as being called on in class? Or do they become dysregulated when facing a separation from their parents, such as at bedtime or before school, seeking contact with their parents with endless questions? Find out how the parents are managing separations and whether they may be inadvertently rewarding by staying with them to negotiate or answer endless questions. Find out if parents may be accommodating anxiety by allowing their children to avoid normal situations that are stirring anxiety. Do they give in anytime their child shows resistance or have they learned to pick their battles and help their children face more-modest stress while avoiding only the most intensely anxious situations? Are the parents able to speak calmly and with good humor about these challenges or do they become very stressed and defensive? Is there a family history of anxiety? Managing a child’s anxiety every day can be exhausting, and parents might need a referral in addition to a discussion about how anxiety is developmentally normal.
For those parents that can manage this discussion, suggest that, like you, if they can remain calm during these times with their child (even if they don’t feel calm), it will help their child get better at managing anxiety, even if their child has an anxiety disorder. They also should be curious about their child’s worries, learning about the details and scenarios their children may be anticipating. They should express compassion about how uncomfortable anxiety is, coupled with their confident belief that the child will be able to tolerate and manage the situation even though it’s uncomfortable. This acknowledgment should not be a dismissal of the anxiety, instead it should be confidence that the child will learn to bear it.
When your patient is a teenager describing anxiety, unpack. Are they anxious about their performance on their five Advanced Placement exams? If their anxiety sounds more like appropriate stress, be compassionate and then curious about how they are learning to relax. Are they using drugs and alcohol? Or have they found healthy ways to unwind and recharge? Focusing on ways in which they are learning to care for themselves, making time for sleep and exercise, live time with friends, and senseless fun is therapeutic. Find out if their parents are supportive of their self-care. You might even give them a prescription!
Anxiety is often a private experience, and parents might not know about it until it presents with an explosion of distress or obstinacy when an anxious child is pushed into scary territory. Asking questions about specific worries (something happening to parents, germs, weather events) can illuminate the extent of anxiety. It also is worth exploring if there are rituals that help them manage their worries, whether they are common (finding a parent, hugging a pet, prayer) or more compulsive (repetitive undoing, hair pulling). Find out if there has recently been any serious stress or change for the family, such as the loss of a job or illness in a grandparent, that may be contributing to a child’s anxiety.
Anytime you see anxieties that are broad or extreme, disrupt their ability to function (go to school, participate in activities, build friendships), or if their parents are clearly struggling with managing their child’s distress, it is worthwhile to find a referral to a psychiatrist or psychologist for evaluation and further treatment. School avoidance constitutes an urgent need for evaluation, as every day of school missed makes it harder for the child to return to school. For all of your anxious patients, even when you make a referral to a psychiatrist for evaluation, teach your patients and parents about how critical adequate sleep and regular exercise are to managing anxiety. Remind them that an appropriate level of anxiety is normal and promotes performance and grit, despite the discomfort, and that learning how to manage anxiety is essential to growing up and building mental health.
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at [email protected].
Runaway youth: Knowing the risk factors and care needs
As many as 1 in 20 youth run away from home each year, and you can play a critical role in identifying adolescents at high risk through confidential social histories and discussions, according to a clinical report from the American Academy of Pediatrics.
The academy’s data-rich report, “Runaway Youth: Caring for the Nation’s Largest Segment of Missing Children,” details how unaccompanied youth who run away – either on their own or who are asked to leave home – have high rates of trauma and neglect, mental illness, substance abuse, family dysfunction, and disengagement from school.
Children who identify as lesbian, gay, bisexual, transgender, and questioning or queer (LGBTQ) and youth in protective custody also are at high risk of running away and of becoming homeless – and once away from home, they and other runaways are at high risk for additional trauma, victimization, and violence, including sexual exploitation, according to the report published in Pediatrics.
“There clearly are certain populations at higher risk, and we really need to be aware of and in tune with these risks, and ask about the home and the household in order to try to decrease the risk of these kids getting into dangerous situations,” Thresia B. Gambon, MD, said in an interview. She is coauthor of the report and a pediatrician with the Citrus Health Network in Miami.
Among the AAP’s recommendations for practice is the guidance to conduct a thorough and confidential psychosocial assessment such as the HEEADSSS assessment (home environment, education and employment, eating peer-related activities, drugs, sexuality, suicide/depression, and safety) and to use a validated depression screening tool for adolescents, such as the Patient Health Questionnaire for Adolescents (PHQ-A) and the primary care version of the Beck Depression Inventory (BDI).
Broadly speaking, which involves being aware of trauma and adverse childhood experiences that can affect health,” according to the report. The AAP Trauma Toolbox for Primary Care is mentioned as a resource.
Most surprising to Dr. Gambon in the research and report-writing process were data showing that disengagement from school is a significant risk factor. “This stood out to me,” she said. “If there are school problems [of various types], kids might run away to avoid attending school.”
Tasked with updating the AAP’s 2004 clinical report, “The Pediatrician’s Role in the Prevention of Missing Children,” Dr. Gambon and coauthor, Janna R. Gewirtz O’Brien, MD, decided to look more closely at runaway youth after studying the numbers – some studies estimate that between 5% and 8% of adolescents run away every year. They saw that, “in general, the number of kids who just go missing has actually decreased [with the help of] cell phones,” Dr. Gambon said in an interview.
“The numbers of kids who are actually running away are high,” she said, “and probably we’re underidentifying these in our primary care clinics.”
Because a significant number of runaway youth become homeless, data on the homeless offers a valuable window not only into the health risks of homelessness for teens (substance abuse, pregnancy, STDs,) but also into risk factors for leaving home in the first place, she noted. Research shows, for instance, that about 20%-40% of teenagers who are homeless identify as LGBTQ, compared with 4%-10% of their nonhomeless peers.
When an adolescent at high risk for running away is identified, you should use practice- and community-based resources to address key issues, support psychological and behavioral health needs of the child and family, and ensure safety.
For youth who have run away, you can share information on local resources, as well as the national Runaway Safeline (1-800-RUNAWAY), which provides 24-hour referrals to community resources, including shelter, food banks, social services, and counseling. You also can ask adolescents whether they have sources of support and shelter (safe, supportive adults who might help in a crisis), and discuss safety plans for leaving home that include health care to mitigate risk, such as reliable contraception and access to mental health care.
“The goal with talking about a safety plan isn’t, of course, to encourage a child to run away, but if they feel as if they need to find somewhere else to live or stay, to discuss what resources are available to them to try to keep them as safe as possible when they’re out of their home,” Dr. Gambon said.
Dr. Gambon speaks partly from experience. She works routinely with youth who have run away from foster care homes, youth who have been trafficked, and other runaways. “I always try to talk with them about safety. I try not to put them down for their decisions but to work with them to make better decisions,” she said. “I work closely with a psychologist because a big part of this is getting them to have self-worth. They often feel as if no one cares, and some just want to be heard and to be able to talk about their situations.”
The AAP report notes that, of more than 70,000 contacts made to Runaway Safeline in 2017, 31% were about youth who were contemplating running away, 16% were about youth who had run away, 5% were about youth asked to leave home or prevented from returning, and 9% concerned youth experiencing homelessness. About three-quarters of the calls came from the youth themselves.
Dr. Gambon and Dr. Gewirtz O’Brien, of the department of pediatrics at the University of Minnesota in Minneapolis, worked with the AAP Committee on Psychosocial Aspects of Child and Family Health and the AAP Council on Community Pediatrics in producing the report. There was no external funding for this report and the authors said they had no conflicts of interest.
SOURCE: Gambon TB et al. Pediatrics. 2020 Jan 21. doi: 10.1542/peds.2019-3752.
As many as 1 in 20 youth run away from home each year, and you can play a critical role in identifying adolescents at high risk through confidential social histories and discussions, according to a clinical report from the American Academy of Pediatrics.
The academy’s data-rich report, “Runaway Youth: Caring for the Nation’s Largest Segment of Missing Children,” details how unaccompanied youth who run away – either on their own or who are asked to leave home – have high rates of trauma and neglect, mental illness, substance abuse, family dysfunction, and disengagement from school.
Children who identify as lesbian, gay, bisexual, transgender, and questioning or queer (LGBTQ) and youth in protective custody also are at high risk of running away and of becoming homeless – and once away from home, they and other runaways are at high risk for additional trauma, victimization, and violence, including sexual exploitation, according to the report published in Pediatrics.
“There clearly are certain populations at higher risk, and we really need to be aware of and in tune with these risks, and ask about the home and the household in order to try to decrease the risk of these kids getting into dangerous situations,” Thresia B. Gambon, MD, said in an interview. She is coauthor of the report and a pediatrician with the Citrus Health Network in Miami.
Among the AAP’s recommendations for practice is the guidance to conduct a thorough and confidential psychosocial assessment such as the HEEADSSS assessment (home environment, education and employment, eating peer-related activities, drugs, sexuality, suicide/depression, and safety) and to use a validated depression screening tool for adolescents, such as the Patient Health Questionnaire for Adolescents (PHQ-A) and the primary care version of the Beck Depression Inventory (BDI).
Broadly speaking, which involves being aware of trauma and adverse childhood experiences that can affect health,” according to the report. The AAP Trauma Toolbox for Primary Care is mentioned as a resource.
Most surprising to Dr. Gambon in the research and report-writing process were data showing that disengagement from school is a significant risk factor. “This stood out to me,” she said. “If there are school problems [of various types], kids might run away to avoid attending school.”
Tasked with updating the AAP’s 2004 clinical report, “The Pediatrician’s Role in the Prevention of Missing Children,” Dr. Gambon and coauthor, Janna R. Gewirtz O’Brien, MD, decided to look more closely at runaway youth after studying the numbers – some studies estimate that between 5% and 8% of adolescents run away every year. They saw that, “in general, the number of kids who just go missing has actually decreased [with the help of] cell phones,” Dr. Gambon said in an interview.
“The numbers of kids who are actually running away are high,” she said, “and probably we’re underidentifying these in our primary care clinics.”
Because a significant number of runaway youth become homeless, data on the homeless offers a valuable window not only into the health risks of homelessness for teens (substance abuse, pregnancy, STDs,) but also into risk factors for leaving home in the first place, she noted. Research shows, for instance, that about 20%-40% of teenagers who are homeless identify as LGBTQ, compared with 4%-10% of their nonhomeless peers.
When an adolescent at high risk for running away is identified, you should use practice- and community-based resources to address key issues, support psychological and behavioral health needs of the child and family, and ensure safety.
For youth who have run away, you can share information on local resources, as well as the national Runaway Safeline (1-800-RUNAWAY), which provides 24-hour referrals to community resources, including shelter, food banks, social services, and counseling. You also can ask adolescents whether they have sources of support and shelter (safe, supportive adults who might help in a crisis), and discuss safety plans for leaving home that include health care to mitigate risk, such as reliable contraception and access to mental health care.
“The goal with talking about a safety plan isn’t, of course, to encourage a child to run away, but if they feel as if they need to find somewhere else to live or stay, to discuss what resources are available to them to try to keep them as safe as possible when they’re out of their home,” Dr. Gambon said.
Dr. Gambon speaks partly from experience. She works routinely with youth who have run away from foster care homes, youth who have been trafficked, and other runaways. “I always try to talk with them about safety. I try not to put them down for their decisions but to work with them to make better decisions,” she said. “I work closely with a psychologist because a big part of this is getting them to have self-worth. They often feel as if no one cares, and some just want to be heard and to be able to talk about their situations.”
The AAP report notes that, of more than 70,000 contacts made to Runaway Safeline in 2017, 31% were about youth who were contemplating running away, 16% were about youth who had run away, 5% were about youth asked to leave home or prevented from returning, and 9% concerned youth experiencing homelessness. About three-quarters of the calls came from the youth themselves.
Dr. Gambon and Dr. Gewirtz O’Brien, of the department of pediatrics at the University of Minnesota in Minneapolis, worked with the AAP Committee on Psychosocial Aspects of Child and Family Health and the AAP Council on Community Pediatrics in producing the report. There was no external funding for this report and the authors said they had no conflicts of interest.
SOURCE: Gambon TB et al. Pediatrics. 2020 Jan 21. doi: 10.1542/peds.2019-3752.
As many as 1 in 20 youth run away from home each year, and you can play a critical role in identifying adolescents at high risk through confidential social histories and discussions, according to a clinical report from the American Academy of Pediatrics.
The academy’s data-rich report, “Runaway Youth: Caring for the Nation’s Largest Segment of Missing Children,” details how unaccompanied youth who run away – either on their own or who are asked to leave home – have high rates of trauma and neglect, mental illness, substance abuse, family dysfunction, and disengagement from school.
Children who identify as lesbian, gay, bisexual, transgender, and questioning or queer (LGBTQ) and youth in protective custody also are at high risk of running away and of becoming homeless – and once away from home, they and other runaways are at high risk for additional trauma, victimization, and violence, including sexual exploitation, according to the report published in Pediatrics.
“There clearly are certain populations at higher risk, and we really need to be aware of and in tune with these risks, and ask about the home and the household in order to try to decrease the risk of these kids getting into dangerous situations,” Thresia B. Gambon, MD, said in an interview. She is coauthor of the report and a pediatrician with the Citrus Health Network in Miami.
Among the AAP’s recommendations for practice is the guidance to conduct a thorough and confidential psychosocial assessment such as the HEEADSSS assessment (home environment, education and employment, eating peer-related activities, drugs, sexuality, suicide/depression, and safety) and to use a validated depression screening tool for adolescents, such as the Patient Health Questionnaire for Adolescents (PHQ-A) and the primary care version of the Beck Depression Inventory (BDI).
Broadly speaking, which involves being aware of trauma and adverse childhood experiences that can affect health,” according to the report. The AAP Trauma Toolbox for Primary Care is mentioned as a resource.
Most surprising to Dr. Gambon in the research and report-writing process were data showing that disengagement from school is a significant risk factor. “This stood out to me,” she said. “If there are school problems [of various types], kids might run away to avoid attending school.”
Tasked with updating the AAP’s 2004 clinical report, “The Pediatrician’s Role in the Prevention of Missing Children,” Dr. Gambon and coauthor, Janna R. Gewirtz O’Brien, MD, decided to look more closely at runaway youth after studying the numbers – some studies estimate that between 5% and 8% of adolescents run away every year. They saw that, “in general, the number of kids who just go missing has actually decreased [with the help of] cell phones,” Dr. Gambon said in an interview.
“The numbers of kids who are actually running away are high,” she said, “and probably we’re underidentifying these in our primary care clinics.”
Because a significant number of runaway youth become homeless, data on the homeless offers a valuable window not only into the health risks of homelessness for teens (substance abuse, pregnancy, STDs,) but also into risk factors for leaving home in the first place, she noted. Research shows, for instance, that about 20%-40% of teenagers who are homeless identify as LGBTQ, compared with 4%-10% of their nonhomeless peers.
When an adolescent at high risk for running away is identified, you should use practice- and community-based resources to address key issues, support psychological and behavioral health needs of the child and family, and ensure safety.
For youth who have run away, you can share information on local resources, as well as the national Runaway Safeline (1-800-RUNAWAY), which provides 24-hour referrals to community resources, including shelter, food banks, social services, and counseling. You also can ask adolescents whether they have sources of support and shelter (safe, supportive adults who might help in a crisis), and discuss safety plans for leaving home that include health care to mitigate risk, such as reliable contraception and access to mental health care.
“The goal with talking about a safety plan isn’t, of course, to encourage a child to run away, but if they feel as if they need to find somewhere else to live or stay, to discuss what resources are available to them to try to keep them as safe as possible when they’re out of their home,” Dr. Gambon said.
Dr. Gambon speaks partly from experience. She works routinely with youth who have run away from foster care homes, youth who have been trafficked, and other runaways. “I always try to talk with them about safety. I try not to put them down for their decisions but to work with them to make better decisions,” she said. “I work closely with a psychologist because a big part of this is getting them to have self-worth. They often feel as if no one cares, and some just want to be heard and to be able to talk about their situations.”
The AAP report notes that, of more than 70,000 contacts made to Runaway Safeline in 2017, 31% were about youth who were contemplating running away, 16% were about youth who had run away, 5% were about youth asked to leave home or prevented from returning, and 9% concerned youth experiencing homelessness. About three-quarters of the calls came from the youth themselves.
Dr. Gambon and Dr. Gewirtz O’Brien, of the department of pediatrics at the University of Minnesota in Minneapolis, worked with the AAP Committee on Psychosocial Aspects of Child and Family Health and the AAP Council on Community Pediatrics in producing the report. There was no external funding for this report and the authors said they had no conflicts of interest.
SOURCE: Gambon TB et al. Pediatrics. 2020 Jan 21. doi: 10.1542/peds.2019-3752.
FROM PEDIATRICS
ACP maps two potential paths to universal health care
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
FROM ANNALS OF INTERNAL MEDICINE
CD1a and cosmetic-related contact dermatitis
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
A quick guide to PrEP: Steps to take & insurance coverage changes to watch for
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. Accessed January 17, 2020.
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. CDC Web Site. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed January 17, 2020.
- US Preventive Services Task Force. Final recommendation statement: prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Published June 2019. Accessed January 17, 2020.
- Campos-Outcalt D. A look at new guidelines for HIV treatment and prevention. J Fam Pract. 2018;67:768-772.
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. Accessed January 17, 2020.
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. CDC Web Site. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed January 17, 2020.
- US Preventive Services Task Force. Final recommendation statement: prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Published June 2019. Accessed January 17, 2020.
- Campos-Outcalt D. A look at new guidelines for HIV treatment and prevention. J Fam Pract. 2018;67:768-772.
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. Accessed January 17, 2020.
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. CDC Web Site. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed January 17, 2020.
- US Preventive Services Task Force. Final recommendation statement: prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Published June 2019. Accessed January 17, 2020.
- Campos-Outcalt D. A look at new guidelines for HIV treatment and prevention. J Fam Pract. 2018;67:768-772.
The evolving landscape of complement inhibition therapy
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
CAR T-cell therapy may worsen mental health in some patients
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Medscape survey points to generational differences in physician burnout
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”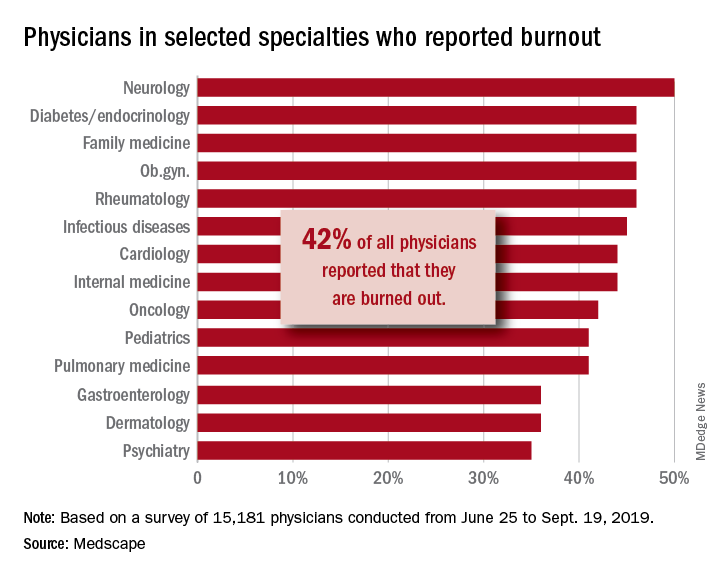
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Adult survivors of childhood cancer are experiencing fewer major cardiac events
Adult survivors of pediatric cancers appear to be experiencing fewer major cardiac events in adulthood partly because of reduced radiotherapy exposure, especially among survivors of Hodgkin lymphoma, recent research published in BMJ has shown.
“Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects,” Daniel A. Mulrooney, MD, of the Division of Cancer Survivorship, Department of Oncology, at St. Jude Children’s Research Hospital, Arlington, Va., and colleagues wrote. “Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late-onset heart failure became apparent.”
Although research has been published on improved survival rates of children who underwent cancer treatment in the 1990s, compared with those who received treatment in the 1980s and 1970s, Dr. Mulrooney and colleagues set out to determine whether cardiac outcomes were reduced as well. They conducted a retrospective study of 23,462 5-year survivors of pediatric cancer, which consisted of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas, and bone sarcomas diagnosed between January 1970 and December 1999. Researchers compared the cardiac outcomes of the survivors, including heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias, with a comparison group of their siblings (n = 5,057) separated by decade. The adult survivors tended to be women (46% vs. 40%) with a median age of 6.1 years at diagnosis and 27.7 years at final follow-up.
Of the 6,193 participants treated for cancer in the 1970s, the 20-year cumulative incidence of heart failure was 0.69%, while the 9,363 participants treated in the 1980s had an incidence of 0.74%, and 7,906 participants in the 1990s had a cumulative incidence of 0.54% over 20 years. The 20-year cumulative incidence for coronary artery disease (CAD) was 0.38% for participants in the 1970s, 0.24% for participants in the 1980s, and 0.19% for participants in the 1990s (P less than .01). Researchers noted the 20-year cumulative incidence of valvular disease, pericardial disease, and arrhythmias did not decrease between the 1970s and the 1990s.
When comparing the rate of major cardiac events of participants in the 1980s and 1990s with those of the 1970s, CAD diagnoses significantly decreased in the 1980s (hazard ratio, 0.65; 95% confidence interval, 0.45-0.92) and 1990s (HR, 0.53; 95% CI, 0.36-0.77), while there was no significant decrease in heart failure or valvular heart disease risk over time. After adjusting for cardiac radiation, overall risk for CAD was attenuated (HR, 0.90; 0.78-1.05), and Hodgkin lymphoma survivors saw the greatest change between unadjusted (HR, 0.77; 95% CI, 0.66-0.89) and adjusted risk (HR, 0.87; 95% CI, 0.69-1.10) when accounting for cardiac radiation.
“While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects,” the researchers concluded.
In a related editorial, Mike Hawkins, DPhil, of the Centre for Childhood Cancer Survivor Studies, Institute of Applied Health Research at the University of Birmingham (England), and colleagues said that, while measuring cardiotoxicity is important for this patient population, traditional risk factors with independent associations to cardiac outcomes should also be studied. Guidelines on follow-up for these patients are also needed to inform clinical practice, such as those produced by the International Late Effects of Childhood Cancer Guideline Harmonization Group, they added.
“Survivorship issues are extremely important to patients, their families, and their doctors,” they said. “In two research priority setting initiatives in the United Kingdom, detailed consultation with patients with cancer, survivors, families, friends, and healthcare professionals identified further research into the consequences of cancer as a top priority.”
This study was funded by grants from the National Cancer Institute, Cancer Center Support (CORE) to St. Jude Children’s Research Hospital and American Lebanese Syrian Associated Charities. The authors of the study and the editorial reported no relevant conflicts of interest.
SOURCE: Mulrooney A et al. BMJ. 2020. doi: 10.1136/bmj.l6794.
Adult survivors of pediatric cancers appear to be experiencing fewer major cardiac events in adulthood partly because of reduced radiotherapy exposure, especially among survivors of Hodgkin lymphoma, recent research published in BMJ has shown.
“Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects,” Daniel A. Mulrooney, MD, of the Division of Cancer Survivorship, Department of Oncology, at St. Jude Children’s Research Hospital, Arlington, Va., and colleagues wrote. “Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late-onset heart failure became apparent.”
Although research has been published on improved survival rates of children who underwent cancer treatment in the 1990s, compared with those who received treatment in the 1980s and 1970s, Dr. Mulrooney and colleagues set out to determine whether cardiac outcomes were reduced as well. They conducted a retrospective study of 23,462 5-year survivors of pediatric cancer, which consisted of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas, and bone sarcomas diagnosed between January 1970 and December 1999. Researchers compared the cardiac outcomes of the survivors, including heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias, with a comparison group of their siblings (n = 5,057) separated by decade. The adult survivors tended to be women (46% vs. 40%) with a median age of 6.1 years at diagnosis and 27.7 years at final follow-up.
Of the 6,193 participants treated for cancer in the 1970s, the 20-year cumulative incidence of heart failure was 0.69%, while the 9,363 participants treated in the 1980s had an incidence of 0.74%, and 7,906 participants in the 1990s had a cumulative incidence of 0.54% over 20 years. The 20-year cumulative incidence for coronary artery disease (CAD) was 0.38% for participants in the 1970s, 0.24% for participants in the 1980s, and 0.19% for participants in the 1990s (P less than .01). Researchers noted the 20-year cumulative incidence of valvular disease, pericardial disease, and arrhythmias did not decrease between the 1970s and the 1990s.
When comparing the rate of major cardiac events of participants in the 1980s and 1990s with those of the 1970s, CAD diagnoses significantly decreased in the 1980s (hazard ratio, 0.65; 95% confidence interval, 0.45-0.92) and 1990s (HR, 0.53; 95% CI, 0.36-0.77), while there was no significant decrease in heart failure or valvular heart disease risk over time. After adjusting for cardiac radiation, overall risk for CAD was attenuated (HR, 0.90; 0.78-1.05), and Hodgkin lymphoma survivors saw the greatest change between unadjusted (HR, 0.77; 95% CI, 0.66-0.89) and adjusted risk (HR, 0.87; 95% CI, 0.69-1.10) when accounting for cardiac radiation.
“While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects,” the researchers concluded.
In a related editorial, Mike Hawkins, DPhil, of the Centre for Childhood Cancer Survivor Studies, Institute of Applied Health Research at the University of Birmingham (England), and colleagues said that, while measuring cardiotoxicity is important for this patient population, traditional risk factors with independent associations to cardiac outcomes should also be studied. Guidelines on follow-up for these patients are also needed to inform clinical practice, such as those produced by the International Late Effects of Childhood Cancer Guideline Harmonization Group, they added.
“Survivorship issues are extremely important to patients, their families, and their doctors,” they said. “In two research priority setting initiatives in the United Kingdom, detailed consultation with patients with cancer, survivors, families, friends, and healthcare professionals identified further research into the consequences of cancer as a top priority.”
This study was funded by grants from the National Cancer Institute, Cancer Center Support (CORE) to St. Jude Children’s Research Hospital and American Lebanese Syrian Associated Charities. The authors of the study and the editorial reported no relevant conflicts of interest.
SOURCE: Mulrooney A et al. BMJ. 2020. doi: 10.1136/bmj.l6794.
Adult survivors of pediatric cancers appear to be experiencing fewer major cardiac events in adulthood partly because of reduced radiotherapy exposure, especially among survivors of Hodgkin lymphoma, recent research published in BMJ has shown.
“Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects,” Daniel A. Mulrooney, MD, of the Division of Cancer Survivorship, Department of Oncology, at St. Jude Children’s Research Hospital, Arlington, Va., and colleagues wrote. “Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late-onset heart failure became apparent.”
Although research has been published on improved survival rates of children who underwent cancer treatment in the 1990s, compared with those who received treatment in the 1980s and 1970s, Dr. Mulrooney and colleagues set out to determine whether cardiac outcomes were reduced as well. They conducted a retrospective study of 23,462 5-year survivors of pediatric cancer, which consisted of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft-tissue sarcomas, and bone sarcomas diagnosed between January 1970 and December 1999. Researchers compared the cardiac outcomes of the survivors, including heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias, with a comparison group of their siblings (n = 5,057) separated by decade. The adult survivors tended to be women (46% vs. 40%) with a median age of 6.1 years at diagnosis and 27.7 years at final follow-up.
Of the 6,193 participants treated for cancer in the 1970s, the 20-year cumulative incidence of heart failure was 0.69%, while the 9,363 participants treated in the 1980s had an incidence of 0.74%, and 7,906 participants in the 1990s had a cumulative incidence of 0.54% over 20 years. The 20-year cumulative incidence for coronary artery disease (CAD) was 0.38% for participants in the 1970s, 0.24% for participants in the 1980s, and 0.19% for participants in the 1990s (P less than .01). Researchers noted the 20-year cumulative incidence of valvular disease, pericardial disease, and arrhythmias did not decrease between the 1970s and the 1990s.
When comparing the rate of major cardiac events of participants in the 1980s and 1990s with those of the 1970s, CAD diagnoses significantly decreased in the 1980s (hazard ratio, 0.65; 95% confidence interval, 0.45-0.92) and 1990s (HR, 0.53; 95% CI, 0.36-0.77), while there was no significant decrease in heart failure or valvular heart disease risk over time. After adjusting for cardiac radiation, overall risk for CAD was attenuated (HR, 0.90; 0.78-1.05), and Hodgkin lymphoma survivors saw the greatest change between unadjusted (HR, 0.77; 95% CI, 0.66-0.89) and adjusted risk (HR, 0.87; 95% CI, 0.69-1.10) when accounting for cardiac radiation.
“While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects,” the researchers concluded.
In a related editorial, Mike Hawkins, DPhil, of the Centre for Childhood Cancer Survivor Studies, Institute of Applied Health Research at the University of Birmingham (England), and colleagues said that, while measuring cardiotoxicity is important for this patient population, traditional risk factors with independent associations to cardiac outcomes should also be studied. Guidelines on follow-up for these patients are also needed to inform clinical practice, such as those produced by the International Late Effects of Childhood Cancer Guideline Harmonization Group, they added.
“Survivorship issues are extremely important to patients, their families, and their doctors,” they said. “In two research priority setting initiatives in the United Kingdom, detailed consultation with patients with cancer, survivors, families, friends, and healthcare professionals identified further research into the consequences of cancer as a top priority.”
This study was funded by grants from the National Cancer Institute, Cancer Center Support (CORE) to St. Jude Children’s Research Hospital and American Lebanese Syrian Associated Charities. The authors of the study and the editorial reported no relevant conflicts of interest.
SOURCE: Mulrooney A et al. BMJ. 2020. doi: 10.1136/bmj.l6794.
FROM BMJ















