User login
DOACs for treatment of cancer-associated venous thromboembolism
Bleeding risk may determine best option
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3
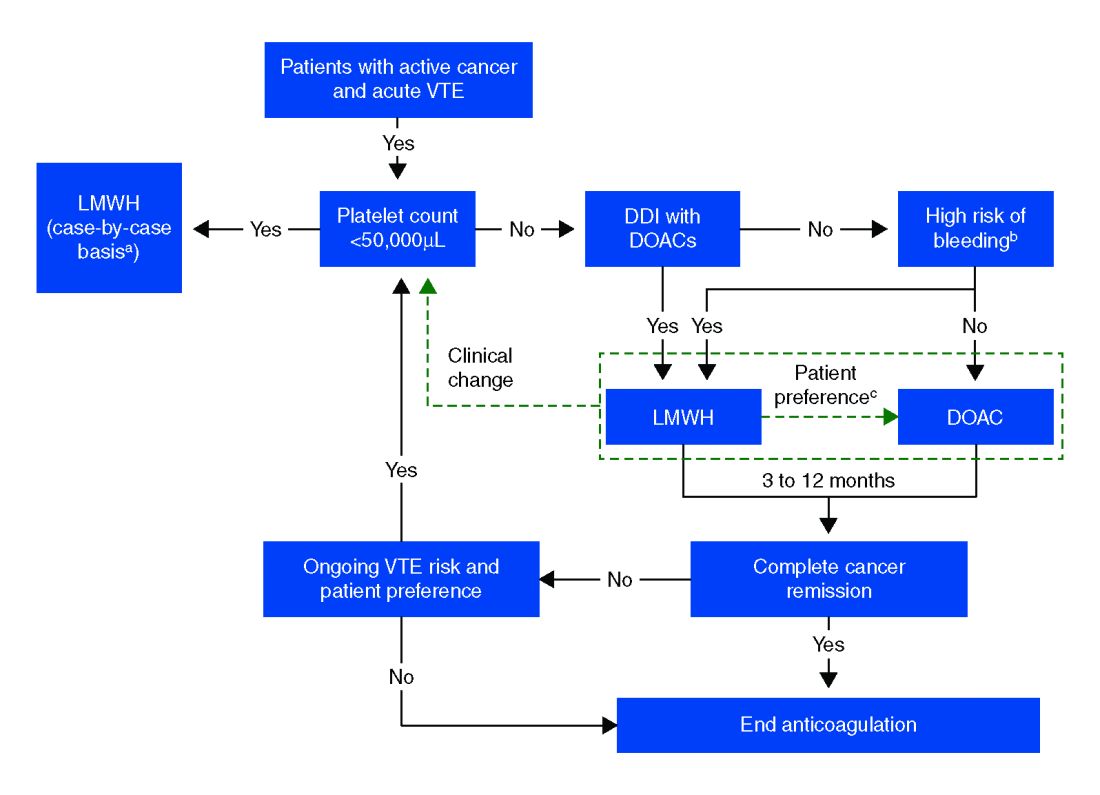
In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4
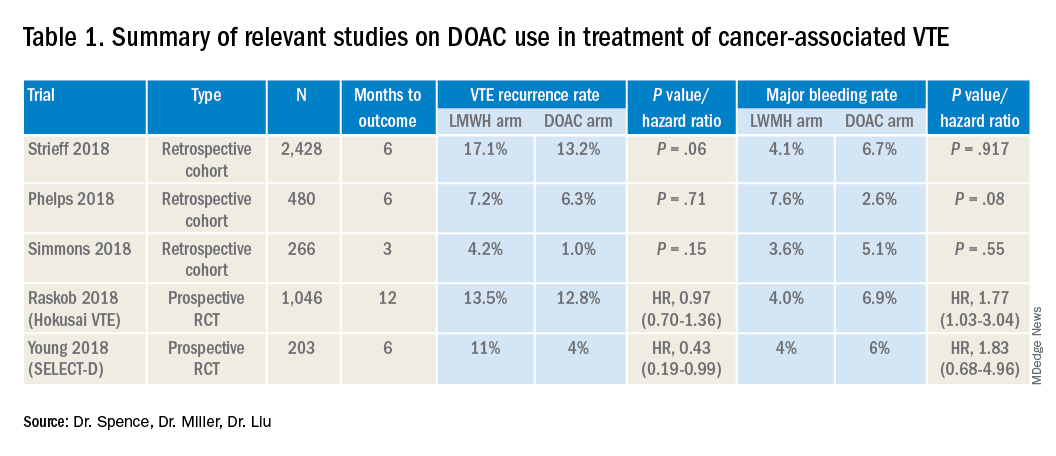
Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Bleeding risk may determine best option
Bleeding risk may determine best option
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3

In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4

Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3

In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4

Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Challenges, Evidence, and Treatment Options for Anticoagulation of Obese and Morbidly Obese Patients with Nonvalvular Atrial Fibrillation (NVAF)
- Understanding the obesity risk in patients with NVAF
- Challenges of anticoagulation with warfarin in patients with obesity
- NVAF patients with obesity
Click here to access the supplement.
January 2020 cp-127604v1
- Understanding the obesity risk in patients with NVAF
- Challenges of anticoagulation with warfarin in patients with obesity
- NVAF patients with obesity
Click here to access the supplement.
January 2020 cp-127604v1
- Understanding the obesity risk in patients with NVAF
- Challenges of anticoagulation with warfarin in patients with obesity
- NVAF patients with obesity
Click here to access the supplement.
January 2020 cp-127604v1
Challenges, Evidence, and Treatment Options for Anticoagulation of Obese and Morbidly Obese Patients with Nonvalvular Atrial Fibrillation (NVAF)
- Understanding the obesity risk in patients with NVAF
- Challenges of anticoagulation with warfarin in patients with obesity
- NVAF patients with obesity
Click here to access the supplement.
January 2020 cp-127604v1
- Understanding the obesity risk in patients with NVAF
- Challenges of anticoagulation with warfarin in patients with obesity
- NVAF patients with obesity
Click here to access the supplement.
January 2020 cp-127604v1
- Understanding the obesity risk in patients with NVAF
- Challenges of anticoagulation with warfarin in patients with obesity
- NVAF patients with obesity
Click here to access the supplement.
January 2020 cp-127604v1
Actor Alan Alda discusses using empathy as an antidote to burnout
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
Exogenous boosting against shingles not as robust as thought
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
FROM BMJ
The age of maximum misery, and why Marcus Welby was gray
A year to forget
47.2. Just another number, right? Nothing too special about it. But this innocent number is hiding a deep, dark secret. It is the number of misery.
More specifically, 47.2 is the age when human misery hits its peak, according to a study distributed by the National Bureau of Economic Research.
The data, collected from 132 countries, show that human happiness is actually U-shaped. We all start out pretty happy, you know, being infants and all. Sadly, life takes a pretty sharp downhill turn when we’re born, and that slide doesn’t abate until the magic age of 47.2. That’s the point in our lives when we’re at our most unhappy.
We do have some good news if you happen to have been born in early November 1972 and you’re having a rough time of things lately. That U-shaped curve will be your friend from now on, as your happiness will, according to the data at least, grow constantly from this point forward. Once you get past 70, at least in the United States, you’ll be as happy as you’ve ever been in your adult life.
Of course, that’s not much comfort for those of us who’ve yet to hit that magic number. So if you thought the daily existential crises were bad now, just wait: Apparently, they’ll only get worse. Won’t that be fun?
Why Marcus Welby was gray
Stress is a key ingredient in the Bureau of LOTME’s recipe for success. The deadlines. The office coffee. The serial commas. And what do we get for all that stress? Other than fan mail (thanks, Mom) and cease-and-desist orders?
Gray hair.
Is the correlation coefficient between stress and our silvering LOTME coifs truly zero? We think not. And now science agrees: Stress may indeed be gray hair’s follicular fertilizer.
Harvard University scientists say they’ve mapped the path from after-hours EHR data entry to premature silver fox status. Specifically, like a pharma rep with a new drug to detail, stress wears on nerves, which help spew norepinephrine and deplete the stem cells that regenerate your hair follicles’ pigment cells. Presto! You’ve got gray hair and a med closet bursting with more drug samples.
More accurately, the Harvard researchers found that stress damages the color-restorative function in the hair of mice. Which means 92-year-old Mickey Mouse is clearly hiding a dye job. (Ed. note: C’mon, people – another Disney cease-and-desist letter?)
We know no one knows stress as intimately as physicians. That’s why we’re planning a complete line of hair coloring products we call “Just for Docs,” featuring colors like “Pre-Auth Platinum Blonde,” “MOC Magenta,” and “EHR Red.” And, of course, “Burnout Brunette.”
Mr. Bedbug goes to Washington
You’ve heard it a million times: The old good news/bad news delivery.
Well, make that a million and one, because it’s time to play “Good news is bad news!”
Good news: Baltimore is no longer the bedbug capital of the United States. Bad news: It only dropped from first to second place on Orkin’s Top 50 Bed Bug Cities list. More bad news: Washington, D.C., the capital of the United States, is now the bedbug capital as well. [Insert joke about Congress here.]
Good news: Kids in England are getting less sugar and salt in their packed school lunches than they did a decade ago. Bad news: They are also getting less vitamin A, vitamin C, and fruit, according to a study in BMJ Open.
Good news: Drinking skim or 1% milk instead of 2% can add more than 4 years to your life, and the reduction in lifespan is even greater for whole milk. Bad news: “Children who drink whole milk are actually 40% less likely to be obese or overweight than kids drinking reduced-fat milk,” Study Finds reported.
Wait a second. That’s not exactly bad news, is it? Maybe for those who are drinking low-fat milk to add a few years to their lives. They will live longer, but they’ll be overweight while they’re doing it.
Thank you for watching “Good news is bad news.” Remember, if you’re not confused, you haven’t been paying attention.
A year to forget
47.2. Just another number, right? Nothing too special about it. But this innocent number is hiding a deep, dark secret. It is the number of misery.
More specifically, 47.2 is the age when human misery hits its peak, according to a study distributed by the National Bureau of Economic Research.
The data, collected from 132 countries, show that human happiness is actually U-shaped. We all start out pretty happy, you know, being infants and all. Sadly, life takes a pretty sharp downhill turn when we’re born, and that slide doesn’t abate until the magic age of 47.2. That’s the point in our lives when we’re at our most unhappy.
We do have some good news if you happen to have been born in early November 1972 and you’re having a rough time of things lately. That U-shaped curve will be your friend from now on, as your happiness will, according to the data at least, grow constantly from this point forward. Once you get past 70, at least in the United States, you’ll be as happy as you’ve ever been in your adult life.
Of course, that’s not much comfort for those of us who’ve yet to hit that magic number. So if you thought the daily existential crises were bad now, just wait: Apparently, they’ll only get worse. Won’t that be fun?
Why Marcus Welby was gray
Stress is a key ingredient in the Bureau of LOTME’s recipe for success. The deadlines. The office coffee. The serial commas. And what do we get for all that stress? Other than fan mail (thanks, Mom) and cease-and-desist orders?
Gray hair.
Is the correlation coefficient between stress and our silvering LOTME coifs truly zero? We think not. And now science agrees: Stress may indeed be gray hair’s follicular fertilizer.
Harvard University scientists say they’ve mapped the path from after-hours EHR data entry to premature silver fox status. Specifically, like a pharma rep with a new drug to detail, stress wears on nerves, which help spew norepinephrine and deplete the stem cells that regenerate your hair follicles’ pigment cells. Presto! You’ve got gray hair and a med closet bursting with more drug samples.
More accurately, the Harvard researchers found that stress damages the color-restorative function in the hair of mice. Which means 92-year-old Mickey Mouse is clearly hiding a dye job. (Ed. note: C’mon, people – another Disney cease-and-desist letter?)
We know no one knows stress as intimately as physicians. That’s why we’re planning a complete line of hair coloring products we call “Just for Docs,” featuring colors like “Pre-Auth Platinum Blonde,” “MOC Magenta,” and “EHR Red.” And, of course, “Burnout Brunette.”
Mr. Bedbug goes to Washington
You’ve heard it a million times: The old good news/bad news delivery.
Well, make that a million and one, because it’s time to play “Good news is bad news!”
Good news: Baltimore is no longer the bedbug capital of the United States. Bad news: It only dropped from first to second place on Orkin’s Top 50 Bed Bug Cities list. More bad news: Washington, D.C., the capital of the United States, is now the bedbug capital as well. [Insert joke about Congress here.]
Good news: Kids in England are getting less sugar and salt in their packed school lunches than they did a decade ago. Bad news: They are also getting less vitamin A, vitamin C, and fruit, according to a study in BMJ Open.
Good news: Drinking skim or 1% milk instead of 2% can add more than 4 years to your life, and the reduction in lifespan is even greater for whole milk. Bad news: “Children who drink whole milk are actually 40% less likely to be obese or overweight than kids drinking reduced-fat milk,” Study Finds reported.
Wait a second. That’s not exactly bad news, is it? Maybe for those who are drinking low-fat milk to add a few years to their lives. They will live longer, but they’ll be overweight while they’re doing it.
Thank you for watching “Good news is bad news.” Remember, if you’re not confused, you haven’t been paying attention.
A year to forget
47.2. Just another number, right? Nothing too special about it. But this innocent number is hiding a deep, dark secret. It is the number of misery.
More specifically, 47.2 is the age when human misery hits its peak, according to a study distributed by the National Bureau of Economic Research.
The data, collected from 132 countries, show that human happiness is actually U-shaped. We all start out pretty happy, you know, being infants and all. Sadly, life takes a pretty sharp downhill turn when we’re born, and that slide doesn’t abate until the magic age of 47.2. That’s the point in our lives when we’re at our most unhappy.
We do have some good news if you happen to have been born in early November 1972 and you’re having a rough time of things lately. That U-shaped curve will be your friend from now on, as your happiness will, according to the data at least, grow constantly from this point forward. Once you get past 70, at least in the United States, you’ll be as happy as you’ve ever been in your adult life.
Of course, that’s not much comfort for those of us who’ve yet to hit that magic number. So if you thought the daily existential crises were bad now, just wait: Apparently, they’ll only get worse. Won’t that be fun?
Why Marcus Welby was gray
Stress is a key ingredient in the Bureau of LOTME’s recipe for success. The deadlines. The office coffee. The serial commas. And what do we get for all that stress? Other than fan mail (thanks, Mom) and cease-and-desist orders?
Gray hair.
Is the correlation coefficient between stress and our silvering LOTME coifs truly zero? We think not. And now science agrees: Stress may indeed be gray hair’s follicular fertilizer.
Harvard University scientists say they’ve mapped the path from after-hours EHR data entry to premature silver fox status. Specifically, like a pharma rep with a new drug to detail, stress wears on nerves, which help spew norepinephrine and deplete the stem cells that regenerate your hair follicles’ pigment cells. Presto! You’ve got gray hair and a med closet bursting with more drug samples.
More accurately, the Harvard researchers found that stress damages the color-restorative function in the hair of mice. Which means 92-year-old Mickey Mouse is clearly hiding a dye job. (Ed. note: C’mon, people – another Disney cease-and-desist letter?)
We know no one knows stress as intimately as physicians. That’s why we’re planning a complete line of hair coloring products we call “Just for Docs,” featuring colors like “Pre-Auth Platinum Blonde,” “MOC Magenta,” and “EHR Red.” And, of course, “Burnout Brunette.”
Mr. Bedbug goes to Washington
You’ve heard it a million times: The old good news/bad news delivery.
Well, make that a million and one, because it’s time to play “Good news is bad news!”
Good news: Baltimore is no longer the bedbug capital of the United States. Bad news: It only dropped from first to second place on Orkin’s Top 50 Bed Bug Cities list. More bad news: Washington, D.C., the capital of the United States, is now the bedbug capital as well. [Insert joke about Congress here.]
Good news: Kids in England are getting less sugar and salt in their packed school lunches than they did a decade ago. Bad news: They are also getting less vitamin A, vitamin C, and fruit, according to a study in BMJ Open.
Good news: Drinking skim or 1% milk instead of 2% can add more than 4 years to your life, and the reduction in lifespan is even greater for whole milk. Bad news: “Children who drink whole milk are actually 40% less likely to be obese or overweight than kids drinking reduced-fat milk,” Study Finds reported.
Wait a second. That’s not exactly bad news, is it? Maybe for those who are drinking low-fat milk to add a few years to their lives. They will live longer, but they’ll be overweight while they’re doing it.
Thank you for watching “Good news is bad news.” Remember, if you’re not confused, you haven’t been paying attention.
Prenatal exposure to pollutants consumed through diet found to be associated with decreased fetal growth
Ideally, chemicals that are toxic to human health are identified and removed from use. Many such chemicals, known as persistent organic pollutants (POPs), have been studied individually for their ill effects in humans. DDT, for instance, once a widely used insecticide, is now classified as a probable human carcinogen and has not been used in the United States since the early 1970s.1 Other insecticides have similarly been banned but have long half-lives and can persist in the environment for decades. Humans are exposed to POPs mainly through diet, say Ouidir and colleagues, with exposure “nearly ubiquitous.”2
The association between POP exposure during pregnancy and birth weight has not been established, as results from previous studies have been inconsistent, according to researchers.2 In addition, birth weight does not distinguish growth-restricted fetuses from those that are constitutionally small. Therefore, Ouidir and colleagues analyzed maternal plasma levels of POPs and measures of fetal growth. Their research was published in JAMA Pediatrics.2
The investigators examined chemical class mixtures of organochlorine pesticides (OCPs), dioxin-like polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), among other groups of POPs, as well as individual chemicals, in 2,284 nonobese low-risk pregnant women before 14 weeks of gestation. They measured 14 fetal growth biometrics using ultrasonography throughout the women’s pregnancies, with researchers focusing their main findings on head and abdominal circumference and femur length measurements.
The researchers found that the OCP mixture was negatively associated with most fetal growth measures, with a reduction of 4.7 mm (95% confidence interval [CI], -6.7 to -2.8 mm) in head circumference, 3.5 mm (95% CI, -4.7 to -2.2) in abdominal circumference, and 0.6 mm (95% CI, -1.1 to -0.2 mm) in femur length. In addition, exposure to the PCB and PBDE mixtures were associated with reduced abdominal circumference.
OCPs have been associated with adverse effects on the endocrine system, lipid metabolism, and embryonic development. They also can result in hematologic and hepatic alterations.3
The JAMA Pediatrics study authors point out that their findings may not take into account the risk pregnant women with occupational exposure to POPs, or other higher exposure situations, may have, as the POP concentrations in their sample were low compared with levels in a nationally representative sample of pregnant women. They say that their findings provide insight into the “implications of POPs for fetal growth when exposures are low and suggest that, even if exposures could be successfully minimized, these associations may persist.”2
- United States Environmental Protection Agency. DDT: A brief history and status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status. Accessed January 16, 2020.
- Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. December 30, 2019. doi:10.1001/jamapediatrics.2019.5104.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
Ideally, chemicals that are toxic to human health are identified and removed from use. Many such chemicals, known as persistent organic pollutants (POPs), have been studied individually for their ill effects in humans. DDT, for instance, once a widely used insecticide, is now classified as a probable human carcinogen and has not been used in the United States since the early 1970s.1 Other insecticides have similarly been banned but have long half-lives and can persist in the environment for decades. Humans are exposed to POPs mainly through diet, say Ouidir and colleagues, with exposure “nearly ubiquitous.”2
The association between POP exposure during pregnancy and birth weight has not been established, as results from previous studies have been inconsistent, according to researchers.2 In addition, birth weight does not distinguish growth-restricted fetuses from those that are constitutionally small. Therefore, Ouidir and colleagues analyzed maternal plasma levels of POPs and measures of fetal growth. Their research was published in JAMA Pediatrics.2
The investigators examined chemical class mixtures of organochlorine pesticides (OCPs), dioxin-like polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), among other groups of POPs, as well as individual chemicals, in 2,284 nonobese low-risk pregnant women before 14 weeks of gestation. They measured 14 fetal growth biometrics using ultrasonography throughout the women’s pregnancies, with researchers focusing their main findings on head and abdominal circumference and femur length measurements.
The researchers found that the OCP mixture was negatively associated with most fetal growth measures, with a reduction of 4.7 mm (95% confidence interval [CI], -6.7 to -2.8 mm) in head circumference, 3.5 mm (95% CI, -4.7 to -2.2) in abdominal circumference, and 0.6 mm (95% CI, -1.1 to -0.2 mm) in femur length. In addition, exposure to the PCB and PBDE mixtures were associated with reduced abdominal circumference.
OCPs have been associated with adverse effects on the endocrine system, lipid metabolism, and embryonic development. They also can result in hematologic and hepatic alterations.3
The JAMA Pediatrics study authors point out that their findings may not take into account the risk pregnant women with occupational exposure to POPs, or other higher exposure situations, may have, as the POP concentrations in their sample were low compared with levels in a nationally representative sample of pregnant women. They say that their findings provide insight into the “implications of POPs for fetal growth when exposures are low and suggest that, even if exposures could be successfully minimized, these associations may persist.”2
Ideally, chemicals that are toxic to human health are identified and removed from use. Many such chemicals, known as persistent organic pollutants (POPs), have been studied individually for their ill effects in humans. DDT, for instance, once a widely used insecticide, is now classified as a probable human carcinogen and has not been used in the United States since the early 1970s.1 Other insecticides have similarly been banned but have long half-lives and can persist in the environment for decades. Humans are exposed to POPs mainly through diet, say Ouidir and colleagues, with exposure “nearly ubiquitous.”2
The association between POP exposure during pregnancy and birth weight has not been established, as results from previous studies have been inconsistent, according to researchers.2 In addition, birth weight does not distinguish growth-restricted fetuses from those that are constitutionally small. Therefore, Ouidir and colleagues analyzed maternal plasma levels of POPs and measures of fetal growth. Their research was published in JAMA Pediatrics.2
The investigators examined chemical class mixtures of organochlorine pesticides (OCPs), dioxin-like polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), among other groups of POPs, as well as individual chemicals, in 2,284 nonobese low-risk pregnant women before 14 weeks of gestation. They measured 14 fetal growth biometrics using ultrasonography throughout the women’s pregnancies, with researchers focusing their main findings on head and abdominal circumference and femur length measurements.
The researchers found that the OCP mixture was negatively associated with most fetal growth measures, with a reduction of 4.7 mm (95% confidence interval [CI], -6.7 to -2.8 mm) in head circumference, 3.5 mm (95% CI, -4.7 to -2.2) in abdominal circumference, and 0.6 mm (95% CI, -1.1 to -0.2 mm) in femur length. In addition, exposure to the PCB and PBDE mixtures were associated with reduced abdominal circumference.
OCPs have been associated with adverse effects on the endocrine system, lipid metabolism, and embryonic development. They also can result in hematologic and hepatic alterations.3
The JAMA Pediatrics study authors point out that their findings may not take into account the risk pregnant women with occupational exposure to POPs, or other higher exposure situations, may have, as the POP concentrations in their sample were low compared with levels in a nationally representative sample of pregnant women. They say that their findings provide insight into the “implications of POPs for fetal growth when exposures are low and suggest that, even if exposures could be successfully minimized, these associations may persist.”2
- United States Environmental Protection Agency. DDT: A brief history and status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status. Accessed January 16, 2020.
- Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. December 30, 2019. doi:10.1001/jamapediatrics.2019.5104.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
- United States Environmental Protection Agency. DDT: A brief history and status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status. Accessed January 16, 2020.
- Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. December 30, 2019. doi:10.1001/jamapediatrics.2019.5104.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
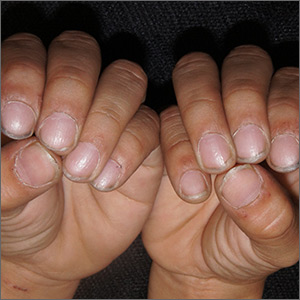
Pitting of fingernails

Given the constellation of non-scarring alopecia on the patient’s posterior scalp (with no scale, papules, or plaques), the physician diagnosed alopecia areata (AA) with associated nail pitting in this patient. Although a scalp biopsy could have confirmed the diagnosis, it was not needed because the clinical picture was sufficient.
Nail pitting is commonly associated with psoriasis (although it is often less dense in presentation), but it can occur with alopecia areata. It may occur concurrently or separately from active alopecia. Pitting of the nails may occur in one or multiple fingernails and occurs in up to a third of patients with AA.
The patient’s scalp was treated with intralesional triamcinolone diluted with normal saline to a concentration of 5 mg/mL (0.5%) and injected in dermal blebs over every square centimeter of involvement. Not every 10-year-old can tolerate this modality, and many families prefer observation or topical steroids. Other topical treatments for AA of the scalp include anthralin, minoxidil, and immunotherapy with squaric acid dibutyl ester or diphencyprone. None of these therapies are approved by the Food and Drug Administration for the treatment of nail disease. There are case reports of systemic tofacitinib clearing significant AA associated nail pitting in adults.
The physician counseled the family to observe the nails and not pursue any antifungal therapies for the nails.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Ferreira SB, Scheinberg M2, Steiner D, et al. Remarkable improvement of nail changes in alopecia areata universalis with 10 Months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8:262-266.

Given the constellation of non-scarring alopecia on the patient’s posterior scalp (with no scale, papules, or plaques), the physician diagnosed alopecia areata (AA) with associated nail pitting in this patient. Although a scalp biopsy could have confirmed the diagnosis, it was not needed because the clinical picture was sufficient.
Nail pitting is commonly associated with psoriasis (although it is often less dense in presentation), but it can occur with alopecia areata. It may occur concurrently or separately from active alopecia. Pitting of the nails may occur in one or multiple fingernails and occurs in up to a third of patients with AA.
The patient’s scalp was treated with intralesional triamcinolone diluted with normal saline to a concentration of 5 mg/mL (0.5%) and injected in dermal blebs over every square centimeter of involvement. Not every 10-year-old can tolerate this modality, and many families prefer observation or topical steroids. Other topical treatments for AA of the scalp include anthralin, minoxidil, and immunotherapy with squaric acid dibutyl ester or diphencyprone. None of these therapies are approved by the Food and Drug Administration for the treatment of nail disease. There are case reports of systemic tofacitinib clearing significant AA associated nail pitting in adults.
The physician counseled the family to observe the nails and not pursue any antifungal therapies for the nails.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Given the constellation of non-scarring alopecia on the patient’s posterior scalp (with no scale, papules, or plaques), the physician diagnosed alopecia areata (AA) with associated nail pitting in this patient. Although a scalp biopsy could have confirmed the diagnosis, it was not needed because the clinical picture was sufficient.
Nail pitting is commonly associated with psoriasis (although it is often less dense in presentation), but it can occur with alopecia areata. It may occur concurrently or separately from active alopecia. Pitting of the nails may occur in one or multiple fingernails and occurs in up to a third of patients with AA.
The patient’s scalp was treated with intralesional triamcinolone diluted with normal saline to a concentration of 5 mg/mL (0.5%) and injected in dermal blebs over every square centimeter of involvement. Not every 10-year-old can tolerate this modality, and many families prefer observation or topical steroids. Other topical treatments for AA of the scalp include anthralin, minoxidil, and immunotherapy with squaric acid dibutyl ester or diphencyprone. None of these therapies are approved by the Food and Drug Administration for the treatment of nail disease. There are case reports of systemic tofacitinib clearing significant AA associated nail pitting in adults.
The physician counseled the family to observe the nails and not pursue any antifungal therapies for the nails.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Ferreira SB, Scheinberg M2, Steiner D, et al. Remarkable improvement of nail changes in alopecia areata universalis with 10 Months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8:262-266.
Ferreira SB, Scheinberg M2, Steiner D, et al. Remarkable improvement of nail changes in alopecia areata universalis with 10 Months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8:262-266.
Phase 2 study shows regimen benefit with dasatinib in Ph+ALL therapy
ORLANDO – A dasatinib-based two-step treatment regimen before allogeneic hematopoietic cell transplantation (alloHCT) for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) reduces relapse and toxicity and improves survival versus an imatinib-based approach, according to findings from the phase 2 Ph+ALL213 study.
Of 78 evaluable patients aged 15-64 years with newly diagnosed BCR/ABL1-positive ALL in the single-arm, multicenter study conducted by the Japanese Adult Leukemia Study Group (JALSG), all but one experienced complete remission (CR or CRi) after dasatinib induction (step 1), and 56% achieved molecular complete response (MCR) after intensive consolidation (IC; step 2), Isamu Sugiura, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The MCR rate increased to 66.2% after the first cycle of consolidation, which included high-dose methotrexate/cytarabine followed by 21 days of 100-mg dasatinib (C1), said Dr. Sugiura of the division of hematology and oncology, Toyohashi Municipal Hospital, Japan.
After all cycles of treatment, the MCR rates before and at 30 and 100 days after transplant were 75.9%, 92.7%, and 93.6%, respectively, he added.
The current standard of care of Ph+ALL is tyrosine kinase inhibitor (TKI)-based chemotherapy followed by alloHCT in the first CR, he said noting that deeper MCR at the time of transplant is associated with the best prognosis.
However, early therapy-related mortality, relapse, and non-relapse mortality remain problematic, he said.
JALSG previously reported results from the Ph+ALL202 and Ph+ALL208 studies, which successfully introduced the TKI imatinib into IC followed by alloHCT for newly diagnosed PH+ALL, establishing the standard of care in Japan, Dr. Sugiura said.
“As the next step, Ph+ALL213 was started to evaluate the introduction of dasatinib and two-step chemotherapy,” he said, explaining that 30%-40% of patients in the prior studies were unable to undergo alloHCT at the first CR because of older age, early relapse, or therapy-related death; benefits in Ph+ALL202, for example, were largely seen in patients younger than age 55 years.
Ph+ALL213 was designed to assess to ability of dasatinib to improve efficacy and reduce toxicity in those settings.
Patients with Eastern Cooperative Oncology Group performance status scores of 0-3 and sufficient organ function were enrolled and underwent step 1 (induction), which targeted hematologic complete response (HCR) by day 28 of dasatinib at a dose of 140 mg daily and day 14 of 60 mg/m2 of prednisone, followed by step 2 (IC), which targeted MCR by day 28 of 100-mg dasatinib in combination with CALGB BFM-like intensive chemotherapy, Dr. Sugiura said.
Consolidation included four cycles alternating between the C1 methotrexate/cytarabine/dasatinib regimen and a CHOP-like regimen using vincristine/cyclophosphamide/daunorubicin followed by 21 days of 100-mg dasatinib (C2). Maintenance therapy included 12 cycles of 24 days of 100 mg DA with vincristine/prednisone.
Patients who achieved HCR and had an appropriate donor proceeded to alloHCT after the first cycle of C1 (C1-1), and in those who were minimal residual disease (MRD)–positive just before transplantation, 100 mg dasatinib was given for 10 cycles after alloHCT, whereas MRD-negative patients underwent observation.
Toxicities associated with dasatinib included liver dysfunction in 11 patients (14.1%), and pneumonitis with severe allergic reaction in 1 patient, Dr. Sugiura said, adding that no therapy-related mortality was reported.
Overall, 74.4% of patients underwent transplant, which was significantly greater than the 59.6% who did so in the JALSG Ph+ALL202 trial. Other significant differences between the Ph+ALL213 and 202 trials included the rates of related donor transplants (29.3% vs. 50.8%) and use of reduced-intensity conditioning (31.0% vs. 10.2%), respectively, he said.
At a median follow-up of 48.1 months, 3-year event-free survival in the current trial was 66.2%, and overall survival (OS) was 80.5%, and in the 58 patients who underwent transplant at the first CR, the rates, respectively, were 74.1% and 84.5%. In those with MCR they were 79.5% and 90.9%.
Of note, the presence of additional cytogenetic abnormalities at presentation was associated with worse OS (P = .0346), and the effect was greatest when derivative 22 syndrome was present (P = .00174), Dr. Sugiura said.
MRD state at the time of transplant in first CR also was associated with outcomes; 3-year event-free survival was 79.5% in 44 MRD-negative patients, compared with 57.1% in 14 MRD-positive patients, and 3-year overall survival was 90.9% vs. 64.3%, respectively.
“Survival curves for MRD-positive patients were inferior to those for MRD-negative patients not because of hematological relapse, but because of transplant-related mortality caused by therapy-related complications and gastrointestinal acute [graft-versus-host] disease,” he said.
The findings demonstrate that dasatinib-based two-step induction was highly effective and safe as pretransplant therapy, he said, noting that transplant was “maximally used,” and although 16% of patients relapsed, both relapse- and non-relapse-related mortality were minimized, with rates of 8.6% and 10.3%, respectively, after transplant.
Longer observation and a larger study are required to confirm these findings, Dr. Sugiura said, noting that the phase 2 JALSG Ph+ALL219 study will look at the potential for further improving outcomes with the addition of the multitargeted TKI ponatinib in patients who are MRD-positive after IC.
This study was funded by the Ministry of Health, Labor and Welfare of Japan. Dr. Sugiura reported having no disclosures.
SOURCE: Sugiura I et al. ASH 2019. Abstract 743.
ORLANDO – A dasatinib-based two-step treatment regimen before allogeneic hematopoietic cell transplantation (alloHCT) for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) reduces relapse and toxicity and improves survival versus an imatinib-based approach, according to findings from the phase 2 Ph+ALL213 study.
Of 78 evaluable patients aged 15-64 years with newly diagnosed BCR/ABL1-positive ALL in the single-arm, multicenter study conducted by the Japanese Adult Leukemia Study Group (JALSG), all but one experienced complete remission (CR or CRi) after dasatinib induction (step 1), and 56% achieved molecular complete response (MCR) after intensive consolidation (IC; step 2), Isamu Sugiura, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The MCR rate increased to 66.2% after the first cycle of consolidation, which included high-dose methotrexate/cytarabine followed by 21 days of 100-mg dasatinib (C1), said Dr. Sugiura of the division of hematology and oncology, Toyohashi Municipal Hospital, Japan.
After all cycles of treatment, the MCR rates before and at 30 and 100 days after transplant were 75.9%, 92.7%, and 93.6%, respectively, he added.
The current standard of care of Ph+ALL is tyrosine kinase inhibitor (TKI)-based chemotherapy followed by alloHCT in the first CR, he said noting that deeper MCR at the time of transplant is associated with the best prognosis.
However, early therapy-related mortality, relapse, and non-relapse mortality remain problematic, he said.
JALSG previously reported results from the Ph+ALL202 and Ph+ALL208 studies, which successfully introduced the TKI imatinib into IC followed by alloHCT for newly diagnosed PH+ALL, establishing the standard of care in Japan, Dr. Sugiura said.
“As the next step, Ph+ALL213 was started to evaluate the introduction of dasatinib and two-step chemotherapy,” he said, explaining that 30%-40% of patients in the prior studies were unable to undergo alloHCT at the first CR because of older age, early relapse, or therapy-related death; benefits in Ph+ALL202, for example, were largely seen in patients younger than age 55 years.
Ph+ALL213 was designed to assess to ability of dasatinib to improve efficacy and reduce toxicity in those settings.
Patients with Eastern Cooperative Oncology Group performance status scores of 0-3 and sufficient organ function were enrolled and underwent step 1 (induction), which targeted hematologic complete response (HCR) by day 28 of dasatinib at a dose of 140 mg daily and day 14 of 60 mg/m2 of prednisone, followed by step 2 (IC), which targeted MCR by day 28 of 100-mg dasatinib in combination with CALGB BFM-like intensive chemotherapy, Dr. Sugiura said.
Consolidation included four cycles alternating between the C1 methotrexate/cytarabine/dasatinib regimen and a CHOP-like regimen using vincristine/cyclophosphamide/daunorubicin followed by 21 days of 100-mg dasatinib (C2). Maintenance therapy included 12 cycles of 24 days of 100 mg DA with vincristine/prednisone.
Patients who achieved HCR and had an appropriate donor proceeded to alloHCT after the first cycle of C1 (C1-1), and in those who were minimal residual disease (MRD)–positive just before transplantation, 100 mg dasatinib was given for 10 cycles after alloHCT, whereas MRD-negative patients underwent observation.
Toxicities associated with dasatinib included liver dysfunction in 11 patients (14.1%), and pneumonitis with severe allergic reaction in 1 patient, Dr. Sugiura said, adding that no therapy-related mortality was reported.
Overall, 74.4% of patients underwent transplant, which was significantly greater than the 59.6% who did so in the JALSG Ph+ALL202 trial. Other significant differences between the Ph+ALL213 and 202 trials included the rates of related donor transplants (29.3% vs. 50.8%) and use of reduced-intensity conditioning (31.0% vs. 10.2%), respectively, he said.
At a median follow-up of 48.1 months, 3-year event-free survival in the current trial was 66.2%, and overall survival (OS) was 80.5%, and in the 58 patients who underwent transplant at the first CR, the rates, respectively, were 74.1% and 84.5%. In those with MCR they were 79.5% and 90.9%.
Of note, the presence of additional cytogenetic abnormalities at presentation was associated with worse OS (P = .0346), and the effect was greatest when derivative 22 syndrome was present (P = .00174), Dr. Sugiura said.
MRD state at the time of transplant in first CR also was associated with outcomes; 3-year event-free survival was 79.5% in 44 MRD-negative patients, compared with 57.1% in 14 MRD-positive patients, and 3-year overall survival was 90.9% vs. 64.3%, respectively.
“Survival curves for MRD-positive patients were inferior to those for MRD-negative patients not because of hematological relapse, but because of transplant-related mortality caused by therapy-related complications and gastrointestinal acute [graft-versus-host] disease,” he said.
The findings demonstrate that dasatinib-based two-step induction was highly effective and safe as pretransplant therapy, he said, noting that transplant was “maximally used,” and although 16% of patients relapsed, both relapse- and non-relapse-related mortality were minimized, with rates of 8.6% and 10.3%, respectively, after transplant.
Longer observation and a larger study are required to confirm these findings, Dr. Sugiura said, noting that the phase 2 JALSG Ph+ALL219 study will look at the potential for further improving outcomes with the addition of the multitargeted TKI ponatinib in patients who are MRD-positive after IC.
This study was funded by the Ministry of Health, Labor and Welfare of Japan. Dr. Sugiura reported having no disclosures.
SOURCE: Sugiura I et al. ASH 2019. Abstract 743.
ORLANDO – A dasatinib-based two-step treatment regimen before allogeneic hematopoietic cell transplantation (alloHCT) for Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) reduces relapse and toxicity and improves survival versus an imatinib-based approach, according to findings from the phase 2 Ph+ALL213 study.
Of 78 evaluable patients aged 15-64 years with newly diagnosed BCR/ABL1-positive ALL in the single-arm, multicenter study conducted by the Japanese Adult Leukemia Study Group (JALSG), all but one experienced complete remission (CR or CRi) after dasatinib induction (step 1), and 56% achieved molecular complete response (MCR) after intensive consolidation (IC; step 2), Isamu Sugiura, MD, PhD, reported at the annual meeting of the American Society of Hematology.
The MCR rate increased to 66.2% after the first cycle of consolidation, which included high-dose methotrexate/cytarabine followed by 21 days of 100-mg dasatinib (C1), said Dr. Sugiura of the division of hematology and oncology, Toyohashi Municipal Hospital, Japan.
After all cycles of treatment, the MCR rates before and at 30 and 100 days after transplant were 75.9%, 92.7%, and 93.6%, respectively, he added.
The current standard of care of Ph+ALL is tyrosine kinase inhibitor (TKI)-based chemotherapy followed by alloHCT in the first CR, he said noting that deeper MCR at the time of transplant is associated with the best prognosis.
However, early therapy-related mortality, relapse, and non-relapse mortality remain problematic, he said.
JALSG previously reported results from the Ph+ALL202 and Ph+ALL208 studies, which successfully introduced the TKI imatinib into IC followed by alloHCT for newly diagnosed PH+ALL, establishing the standard of care in Japan, Dr. Sugiura said.
“As the next step, Ph+ALL213 was started to evaluate the introduction of dasatinib and two-step chemotherapy,” he said, explaining that 30%-40% of patients in the prior studies were unable to undergo alloHCT at the first CR because of older age, early relapse, or therapy-related death; benefits in Ph+ALL202, for example, were largely seen in patients younger than age 55 years.
Ph+ALL213 was designed to assess to ability of dasatinib to improve efficacy and reduce toxicity in those settings.
Patients with Eastern Cooperative Oncology Group performance status scores of 0-3 and sufficient organ function were enrolled and underwent step 1 (induction), which targeted hematologic complete response (HCR) by day 28 of dasatinib at a dose of 140 mg daily and day 14 of 60 mg/m2 of prednisone, followed by step 2 (IC), which targeted MCR by day 28 of 100-mg dasatinib in combination with CALGB BFM-like intensive chemotherapy, Dr. Sugiura said.
Consolidation included four cycles alternating between the C1 methotrexate/cytarabine/dasatinib regimen and a CHOP-like regimen using vincristine/cyclophosphamide/daunorubicin followed by 21 days of 100-mg dasatinib (C2). Maintenance therapy included 12 cycles of 24 days of 100 mg DA with vincristine/prednisone.
Patients who achieved HCR and had an appropriate donor proceeded to alloHCT after the first cycle of C1 (C1-1), and in those who were minimal residual disease (MRD)–positive just before transplantation, 100 mg dasatinib was given for 10 cycles after alloHCT, whereas MRD-negative patients underwent observation.
Toxicities associated with dasatinib included liver dysfunction in 11 patients (14.1%), and pneumonitis with severe allergic reaction in 1 patient, Dr. Sugiura said, adding that no therapy-related mortality was reported.
Overall, 74.4% of patients underwent transplant, which was significantly greater than the 59.6% who did so in the JALSG Ph+ALL202 trial. Other significant differences between the Ph+ALL213 and 202 trials included the rates of related donor transplants (29.3% vs. 50.8%) and use of reduced-intensity conditioning (31.0% vs. 10.2%), respectively, he said.
At a median follow-up of 48.1 months, 3-year event-free survival in the current trial was 66.2%, and overall survival (OS) was 80.5%, and in the 58 patients who underwent transplant at the first CR, the rates, respectively, were 74.1% and 84.5%. In those with MCR they were 79.5% and 90.9%.
Of note, the presence of additional cytogenetic abnormalities at presentation was associated with worse OS (P = .0346), and the effect was greatest when derivative 22 syndrome was present (P = .00174), Dr. Sugiura said.
MRD state at the time of transplant in first CR also was associated with outcomes; 3-year event-free survival was 79.5% in 44 MRD-negative patients, compared with 57.1% in 14 MRD-positive patients, and 3-year overall survival was 90.9% vs. 64.3%, respectively.
“Survival curves for MRD-positive patients were inferior to those for MRD-negative patients not because of hematological relapse, but because of transplant-related mortality caused by therapy-related complications and gastrointestinal acute [graft-versus-host] disease,” he said.
The findings demonstrate that dasatinib-based two-step induction was highly effective and safe as pretransplant therapy, he said, noting that transplant was “maximally used,” and although 16% of patients relapsed, both relapse- and non-relapse-related mortality were minimized, with rates of 8.6% and 10.3%, respectively, after transplant.
Longer observation and a larger study are required to confirm these findings, Dr. Sugiura said, noting that the phase 2 JALSG Ph+ALL219 study will look at the potential for further improving outcomes with the addition of the multitargeted TKI ponatinib in patients who are MRD-positive after IC.
This study was funded by the Ministry of Health, Labor and Welfare of Japan. Dr. Sugiura reported having no disclosures.
SOURCE: Sugiura I et al. ASH 2019. Abstract 743.
REPORTING FROM ASH 2019
Apremilast more likely to succeed with moderate psoriatic arthritis activity
new research suggests.
A paper published in Arthritis Care & Research presents a pooled analysis of the PALACE 1-3 studies that included a total of 1,493 patients with active psoriatic arthritis whose disease had resisted treatment with tumor necrosis factor inhibitors or conventional disease-modifying antirheumatic drugs. Participants were randomized either to the oral phosphodiesterase 4 inhibitor apremilast (Otezla) 30 mg twice daily, 20 mg twice daily, or placebo for 24 weeks, after which all patients on placebo were rerandomized to one of the two apremilast doses until 52 weeks.
The analysis focused on 494 patients who were randomized to apremilast 30 mg twice daily at baseline.
At week 16, 40% patients with low disease activity at baseline had achieved remission on their clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) score, compared with 7% of patients with moderate disease activity and 2.1% of patients with high disease activity. The cDAPSA score is calculated as a composite score including swollen and tender joint counts, patient’s assessment of pain, and patient’s global assessment of disease activity, with possible scores from 0 to 154. Based on patients’ cDAPSA score, the researchers defined remission as a score of 4 or less, low disease activity as more than 4 and up to 13, moderate disease activity as more than 13 and up to 27, and high disease activity as greater than 27.
Among patients with moderate disease activity, 29.8% achieved low disease activity by week 16; among patients with high disease activity at baseline, 11.5% achieved low disease activity, and 38.1% achieved moderate disease activity.
The study found that patients who had moderate disease activity at baseline and achieved either low disease activity or remission by week 16 had a 58.9%-88.5% probability of remaining at those treatment targets by week 52. Patients with high disease activity at baseline who achieved low disease activity or remission by week 16 had a 64.3%-77.4% probability of achieving treatment targets by week 52.
Overall, nearly twice as many patients who had moderate disease activity at baseline achieved their treatment targets when compared with those who began with high disease activity (46.9% vs. 24.9%).
Any patient who achieved at least a 30% improvement in cDAPSA score by week 16 had a 63% probability of achieving treatment targets by week 52.
First author Philip J. Mease, MD, from the Swedish Medical Center and the University of Washington, Seattle, and coauthors noted that the absence of treatment response by week 16 should point to the need for a treatment adjustment. “Taken together, these findings provide a framework of reference for the selection and monitoring of patients with the highest likelihood of achieving optimal treatment responses with apremilast in clinical practice,” they wrote.
The authors also commented that their study provided support for the use of clinical Disease Activity Index for Psoriatic Arthritis score to monitor patients treated with apremilast.
The study was sponsored by Celgene. Three authors were employees of Celgene at the time of the study, and nine authors declared a range of consultancies, grants, research, and other support from the pharmaceutical sector, including from Celgene.
SOURCE: Mease P et al. Arthritis Care Res. 2019 Jan 7. doi: 10.1002/acr.24134
new research suggests.
A paper published in Arthritis Care & Research presents a pooled analysis of the PALACE 1-3 studies that included a total of 1,493 patients with active psoriatic arthritis whose disease had resisted treatment with tumor necrosis factor inhibitors or conventional disease-modifying antirheumatic drugs. Participants were randomized either to the oral phosphodiesterase 4 inhibitor apremilast (Otezla) 30 mg twice daily, 20 mg twice daily, or placebo for 24 weeks, after which all patients on placebo were rerandomized to one of the two apremilast doses until 52 weeks.
The analysis focused on 494 patients who were randomized to apremilast 30 mg twice daily at baseline.
At week 16, 40% patients with low disease activity at baseline had achieved remission on their clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) score, compared with 7% of patients with moderate disease activity and 2.1% of patients with high disease activity. The cDAPSA score is calculated as a composite score including swollen and tender joint counts, patient’s assessment of pain, and patient’s global assessment of disease activity, with possible scores from 0 to 154. Based on patients’ cDAPSA score, the researchers defined remission as a score of 4 or less, low disease activity as more than 4 and up to 13, moderate disease activity as more than 13 and up to 27, and high disease activity as greater than 27.
Among patients with moderate disease activity, 29.8% achieved low disease activity by week 16; among patients with high disease activity at baseline, 11.5% achieved low disease activity, and 38.1% achieved moderate disease activity.
The study found that patients who had moderate disease activity at baseline and achieved either low disease activity or remission by week 16 had a 58.9%-88.5% probability of remaining at those treatment targets by week 52. Patients with high disease activity at baseline who achieved low disease activity or remission by week 16 had a 64.3%-77.4% probability of achieving treatment targets by week 52.
Overall, nearly twice as many patients who had moderate disease activity at baseline achieved their treatment targets when compared with those who began with high disease activity (46.9% vs. 24.9%).
Any patient who achieved at least a 30% improvement in cDAPSA score by week 16 had a 63% probability of achieving treatment targets by week 52.
First author Philip J. Mease, MD, from the Swedish Medical Center and the University of Washington, Seattle, and coauthors noted that the absence of treatment response by week 16 should point to the need for a treatment adjustment. “Taken together, these findings provide a framework of reference for the selection and monitoring of patients with the highest likelihood of achieving optimal treatment responses with apremilast in clinical practice,” they wrote.
The authors also commented that their study provided support for the use of clinical Disease Activity Index for Psoriatic Arthritis score to monitor patients treated with apremilast.
The study was sponsored by Celgene. Three authors were employees of Celgene at the time of the study, and nine authors declared a range of consultancies, grants, research, and other support from the pharmaceutical sector, including from Celgene.
SOURCE: Mease P et al. Arthritis Care Res. 2019 Jan 7. doi: 10.1002/acr.24134
new research suggests.
A paper published in Arthritis Care & Research presents a pooled analysis of the PALACE 1-3 studies that included a total of 1,493 patients with active psoriatic arthritis whose disease had resisted treatment with tumor necrosis factor inhibitors or conventional disease-modifying antirheumatic drugs. Participants were randomized either to the oral phosphodiesterase 4 inhibitor apremilast (Otezla) 30 mg twice daily, 20 mg twice daily, or placebo for 24 weeks, after which all patients on placebo were rerandomized to one of the two apremilast doses until 52 weeks.
The analysis focused on 494 patients who were randomized to apremilast 30 mg twice daily at baseline.
At week 16, 40% patients with low disease activity at baseline had achieved remission on their clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) score, compared with 7% of patients with moderate disease activity and 2.1% of patients with high disease activity. The cDAPSA score is calculated as a composite score including swollen and tender joint counts, patient’s assessment of pain, and patient’s global assessment of disease activity, with possible scores from 0 to 154. Based on patients’ cDAPSA score, the researchers defined remission as a score of 4 or less, low disease activity as more than 4 and up to 13, moderate disease activity as more than 13 and up to 27, and high disease activity as greater than 27.
Among patients with moderate disease activity, 29.8% achieved low disease activity by week 16; among patients with high disease activity at baseline, 11.5% achieved low disease activity, and 38.1% achieved moderate disease activity.
The study found that patients who had moderate disease activity at baseline and achieved either low disease activity or remission by week 16 had a 58.9%-88.5% probability of remaining at those treatment targets by week 52. Patients with high disease activity at baseline who achieved low disease activity or remission by week 16 had a 64.3%-77.4% probability of achieving treatment targets by week 52.
Overall, nearly twice as many patients who had moderate disease activity at baseline achieved their treatment targets when compared with those who began with high disease activity (46.9% vs. 24.9%).
Any patient who achieved at least a 30% improvement in cDAPSA score by week 16 had a 63% probability of achieving treatment targets by week 52.
First author Philip J. Mease, MD, from the Swedish Medical Center and the University of Washington, Seattle, and coauthors noted that the absence of treatment response by week 16 should point to the need for a treatment adjustment. “Taken together, these findings provide a framework of reference for the selection and monitoring of patients with the highest likelihood of achieving optimal treatment responses with apremilast in clinical practice,” they wrote.
The authors also commented that their study provided support for the use of clinical Disease Activity Index for Psoriatic Arthritis score to monitor patients treated with apremilast.
The study was sponsored by Celgene. Three authors were employees of Celgene at the time of the study, and nine authors declared a range of consultancies, grants, research, and other support from the pharmaceutical sector, including from Celgene.
SOURCE: Mease P et al. Arthritis Care Res. 2019 Jan 7. doi: 10.1002/acr.24134
FROM ARTHRITIS CARE & RESEARCH