User login
Pubertal suppression reduces risk of later suicidal ideation in transgender people
Transgender adults who, as adolescents, desired and received pubertal suppression had reduced odds of suicidal ideation, compared with those who wanted but didn’t receive pubertal suppression during their teen years.
Raw frequency of lifetime suicidal ideation was 90% in transgender adults who wanted, but did not receive, pubertal suppression in adolescence, compared with 75% in those who did receive pubertal suppression in adolescence, according to a new analysis of a nationwide survey of transgender people reported in Pediatrics. After controlling for demographic variables, the lifetime adjusted odds ratio for suicidal ideation was 0.3 for those receiving pubertal suppression, compared with those who wanted but didn’t receive pubertal suppression.
The study was the first to examine this association, and findings were drawn from the 2015 U.S. Transgender Survey, the largest known dataset of transgender adults, wrote the study’s lead author Jack Turban, MD, and coinvestigators.
“Suicidality is of particular concern for this population because the estimated lifetime prevalence of suicide attempts among transgender people is as high as 40%,” noted Dr. Turban, a psychiatry resident at Harvard Medical School and Massachusetts General Hospital, Boston, and McLean Hospital, Belmont, Mass., and coauthors. Anxiety, depression, and suicidality all are more common among transgender youth, who make up almost 2% of the nation’s adolescent population, they said.
Among transgender youth, the researchers explained, a spectrum exists: “Some have minimal body dysphoria and do not desire pubertal suppression, whereas others report significant dysphoria around the physical changes related to puberty.” Accordingly, they said, “We examined only those youth who desired pubertal suppression,” because this is the population of youth about whom clinicians need to make treatment decisions.
For individuals who might experience distress from the irreversible bodily changes of endogenous puberty, suppression via gonadotropin releasing hormone analogues (GnRHas) “allows these adolescents more time to decide if they wish to either induce exogenous gender-congruent puberty or allow endogenous puberty to progress,” wrote Dr. Turban and his collaborators.
The U.S. Transgender Survey dataset includes response from over 27,000 transgender adults with nationwide representation. However, this study included only participants who were younger than 17 years in 1998, when GnRHas for pubertal suppression became available. Filtering this group further to just those respondents between the ages of 18 and 36 years whose survey responses indicated they had ever wanted pubertal suppression yielded 3,494 individuals. Of these individuals, just 2.5% (89 participants) had ever received pubertal suppression.
“Results from this study suggest that the majority of transgender adults in the United States who have wanted pubertal suppression did not receive it,” noted the authors. Even among the youngest respondents – who received care during puberty most recently – just 5% of the 18-year-olds in 2015 desiring pubertal suppression actually received the treatment.
Among other associations, individuals who were younger, those with feminine gender identity, those with male sex assigned at birth, and those reporting heterosexual sexual orientation were more likely to have received pubertal suppression.
Receiving GnRHas also was more likely for individuals with higher household income and more family support of their gender identity. Without insurance, studies have indicated that the annual cost of GnRHA treatment can be $4,000-$25,000. Another study noted that at the Boston Children’s Hospital Gender Management Service before 2012, fewer than 20% of patients were able to get insurance coverage for pubertal suppression, according to Dr. Turban and colleagues.
The study looked at suicidality over the past year and lifetime suicidality, as well as severe psychological distress and binge drinking over the past month. Investigators also asked about lifetime history of illicit drug use, hypothesizing that those who received pubertal suppression would have “superior mental health outcomes” when compared to those who desired – but didn’t receive – pubertal suppression, wrote Dr. Turban and coauthors.
Suicidality within the past 12 months and severe psychological distress were both significantly more common among those who did not receive pubertal suppression, but these associations lost significance after multivariable analysis. There was no difference in odds of suicide attempts, although the study may have been underpowered to detect some of these associations, said the investigators.
After statistical analysis to control for demographic variables, pubertal suppression still was associated with decreased odds of having suicidal ideation over the lifespan.
Dr. Turban and colleagues acknowledged that reverse causation may have been in play, because adolescents with better mental health might have been considered better candidates for GnRHa therapy. But the study’s large sample size and wide geographic reach are strengths, they said, concluding that overall, the findings lend support to existing recommendations from the Endocrine Society and the World Professional Association for Transgender Health that pubertal suppression therapy be available to those adolescents who desire it.
Investigators were supported by the U.S. Health Resources and Services Administration, the Patient-Centered Outcomes Research Institute, and the American Academy of Child & Adolescent Psychiatry. The authors reported that they had no financial conflicts of interest.
SOURCE: Turban JL et al. Pediatrics. 2020;145(2):e20191725.
Access to good medical care for transgender adolescents remains very limited. When it is available, puberty blockers are an excellent, conservative option for trans adolescents entering puberty so that they have time to consider longer-term treatment options with their providers and families. As demonstrated by the data in this study, good attention to transgender children can substantially improve their mental health.
The biggest barrier to health care for trans adolescents is access to knowledgeable providers – even more than affordability, which is improving with better coverage by payers. As noted in the study by Turban et al., the lack of access to care remains a huge problem.
It is not surprising that, when we neglect health care, we get bad outcomes. In that sense, the study by Turban et al. is quite intuitive. The few adolescents with access to the appropriate health care had better immediate outcomes. Still, as a scientist I take nothing for granted, and a study confirming what seems logical is important confirmation.
Joshua D. Safer, MD, who is the executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System, and professor of medicine at Icahn School of Medicine at Mount Sinai, both in New York, was asked to comment on the article by Turban et al. He said he had no relevant financial disclosures.
Access to good medical care for transgender adolescents remains very limited. When it is available, puberty blockers are an excellent, conservative option for trans adolescents entering puberty so that they have time to consider longer-term treatment options with their providers and families. As demonstrated by the data in this study, good attention to transgender children can substantially improve their mental health.
The biggest barrier to health care for trans adolescents is access to knowledgeable providers – even more than affordability, which is improving with better coverage by payers. As noted in the study by Turban et al., the lack of access to care remains a huge problem.
It is not surprising that, when we neglect health care, we get bad outcomes. In that sense, the study by Turban et al. is quite intuitive. The few adolescents with access to the appropriate health care had better immediate outcomes. Still, as a scientist I take nothing for granted, and a study confirming what seems logical is important confirmation.
Joshua D. Safer, MD, who is the executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System, and professor of medicine at Icahn School of Medicine at Mount Sinai, both in New York, was asked to comment on the article by Turban et al. He said he had no relevant financial disclosures.
Access to good medical care for transgender adolescents remains very limited. When it is available, puberty blockers are an excellent, conservative option for trans adolescents entering puberty so that they have time to consider longer-term treatment options with their providers and families. As demonstrated by the data in this study, good attention to transgender children can substantially improve their mental health.
The biggest barrier to health care for trans adolescents is access to knowledgeable providers – even more than affordability, which is improving with better coverage by payers. As noted in the study by Turban et al., the lack of access to care remains a huge problem.
It is not surprising that, when we neglect health care, we get bad outcomes. In that sense, the study by Turban et al. is quite intuitive. The few adolescents with access to the appropriate health care had better immediate outcomes. Still, as a scientist I take nothing for granted, and a study confirming what seems logical is important confirmation.
Joshua D. Safer, MD, who is the executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System, and professor of medicine at Icahn School of Medicine at Mount Sinai, both in New York, was asked to comment on the article by Turban et al. He said he had no relevant financial disclosures.
Transgender adults who, as adolescents, desired and received pubertal suppression had reduced odds of suicidal ideation, compared with those who wanted but didn’t receive pubertal suppression during their teen years.
Raw frequency of lifetime suicidal ideation was 90% in transgender adults who wanted, but did not receive, pubertal suppression in adolescence, compared with 75% in those who did receive pubertal suppression in adolescence, according to a new analysis of a nationwide survey of transgender people reported in Pediatrics. After controlling for demographic variables, the lifetime adjusted odds ratio for suicidal ideation was 0.3 for those receiving pubertal suppression, compared with those who wanted but didn’t receive pubertal suppression.
The study was the first to examine this association, and findings were drawn from the 2015 U.S. Transgender Survey, the largest known dataset of transgender adults, wrote the study’s lead author Jack Turban, MD, and coinvestigators.
“Suicidality is of particular concern for this population because the estimated lifetime prevalence of suicide attempts among transgender people is as high as 40%,” noted Dr. Turban, a psychiatry resident at Harvard Medical School and Massachusetts General Hospital, Boston, and McLean Hospital, Belmont, Mass., and coauthors. Anxiety, depression, and suicidality all are more common among transgender youth, who make up almost 2% of the nation’s adolescent population, they said.
Among transgender youth, the researchers explained, a spectrum exists: “Some have minimal body dysphoria and do not desire pubertal suppression, whereas others report significant dysphoria around the physical changes related to puberty.” Accordingly, they said, “We examined only those youth who desired pubertal suppression,” because this is the population of youth about whom clinicians need to make treatment decisions.
For individuals who might experience distress from the irreversible bodily changes of endogenous puberty, suppression via gonadotropin releasing hormone analogues (GnRHas) “allows these adolescents more time to decide if they wish to either induce exogenous gender-congruent puberty or allow endogenous puberty to progress,” wrote Dr. Turban and his collaborators.
The U.S. Transgender Survey dataset includes response from over 27,000 transgender adults with nationwide representation. However, this study included only participants who were younger than 17 years in 1998, when GnRHas for pubertal suppression became available. Filtering this group further to just those respondents between the ages of 18 and 36 years whose survey responses indicated they had ever wanted pubertal suppression yielded 3,494 individuals. Of these individuals, just 2.5% (89 participants) had ever received pubertal suppression.
“Results from this study suggest that the majority of transgender adults in the United States who have wanted pubertal suppression did not receive it,” noted the authors. Even among the youngest respondents – who received care during puberty most recently – just 5% of the 18-year-olds in 2015 desiring pubertal suppression actually received the treatment.
Among other associations, individuals who were younger, those with feminine gender identity, those with male sex assigned at birth, and those reporting heterosexual sexual orientation were more likely to have received pubertal suppression.
Receiving GnRHas also was more likely for individuals with higher household income and more family support of their gender identity. Without insurance, studies have indicated that the annual cost of GnRHA treatment can be $4,000-$25,000. Another study noted that at the Boston Children’s Hospital Gender Management Service before 2012, fewer than 20% of patients were able to get insurance coverage for pubertal suppression, according to Dr. Turban and colleagues.
The study looked at suicidality over the past year and lifetime suicidality, as well as severe psychological distress and binge drinking over the past month. Investigators also asked about lifetime history of illicit drug use, hypothesizing that those who received pubertal suppression would have “superior mental health outcomes” when compared to those who desired – but didn’t receive – pubertal suppression, wrote Dr. Turban and coauthors.
Suicidality within the past 12 months and severe psychological distress were both significantly more common among those who did not receive pubertal suppression, but these associations lost significance after multivariable analysis. There was no difference in odds of suicide attempts, although the study may have been underpowered to detect some of these associations, said the investigators.
After statistical analysis to control for demographic variables, pubertal suppression still was associated with decreased odds of having suicidal ideation over the lifespan.
Dr. Turban and colleagues acknowledged that reverse causation may have been in play, because adolescents with better mental health might have been considered better candidates for GnRHa therapy. But the study’s large sample size and wide geographic reach are strengths, they said, concluding that overall, the findings lend support to existing recommendations from the Endocrine Society and the World Professional Association for Transgender Health that pubertal suppression therapy be available to those adolescents who desire it.
Investigators were supported by the U.S. Health Resources and Services Administration, the Patient-Centered Outcomes Research Institute, and the American Academy of Child & Adolescent Psychiatry. The authors reported that they had no financial conflicts of interest.
SOURCE: Turban JL et al. Pediatrics. 2020;145(2):e20191725.
Transgender adults who, as adolescents, desired and received pubertal suppression had reduced odds of suicidal ideation, compared with those who wanted but didn’t receive pubertal suppression during their teen years.
Raw frequency of lifetime suicidal ideation was 90% in transgender adults who wanted, but did not receive, pubertal suppression in adolescence, compared with 75% in those who did receive pubertal suppression in adolescence, according to a new analysis of a nationwide survey of transgender people reported in Pediatrics. After controlling for demographic variables, the lifetime adjusted odds ratio for suicidal ideation was 0.3 for those receiving pubertal suppression, compared with those who wanted but didn’t receive pubertal suppression.
The study was the first to examine this association, and findings were drawn from the 2015 U.S. Transgender Survey, the largest known dataset of transgender adults, wrote the study’s lead author Jack Turban, MD, and coinvestigators.
“Suicidality is of particular concern for this population because the estimated lifetime prevalence of suicide attempts among transgender people is as high as 40%,” noted Dr. Turban, a psychiatry resident at Harvard Medical School and Massachusetts General Hospital, Boston, and McLean Hospital, Belmont, Mass., and coauthors. Anxiety, depression, and suicidality all are more common among transgender youth, who make up almost 2% of the nation’s adolescent population, they said.
Among transgender youth, the researchers explained, a spectrum exists: “Some have minimal body dysphoria and do not desire pubertal suppression, whereas others report significant dysphoria around the physical changes related to puberty.” Accordingly, they said, “We examined only those youth who desired pubertal suppression,” because this is the population of youth about whom clinicians need to make treatment decisions.
For individuals who might experience distress from the irreversible bodily changes of endogenous puberty, suppression via gonadotropin releasing hormone analogues (GnRHas) “allows these adolescents more time to decide if they wish to either induce exogenous gender-congruent puberty or allow endogenous puberty to progress,” wrote Dr. Turban and his collaborators.
The U.S. Transgender Survey dataset includes response from over 27,000 transgender adults with nationwide representation. However, this study included only participants who were younger than 17 years in 1998, when GnRHas for pubertal suppression became available. Filtering this group further to just those respondents between the ages of 18 and 36 years whose survey responses indicated they had ever wanted pubertal suppression yielded 3,494 individuals. Of these individuals, just 2.5% (89 participants) had ever received pubertal suppression.
“Results from this study suggest that the majority of transgender adults in the United States who have wanted pubertal suppression did not receive it,” noted the authors. Even among the youngest respondents – who received care during puberty most recently – just 5% of the 18-year-olds in 2015 desiring pubertal suppression actually received the treatment.
Among other associations, individuals who were younger, those with feminine gender identity, those with male sex assigned at birth, and those reporting heterosexual sexual orientation were more likely to have received pubertal suppression.
Receiving GnRHas also was more likely for individuals with higher household income and more family support of their gender identity. Without insurance, studies have indicated that the annual cost of GnRHA treatment can be $4,000-$25,000. Another study noted that at the Boston Children’s Hospital Gender Management Service before 2012, fewer than 20% of patients were able to get insurance coverage for pubertal suppression, according to Dr. Turban and colleagues.
The study looked at suicidality over the past year and lifetime suicidality, as well as severe psychological distress and binge drinking over the past month. Investigators also asked about lifetime history of illicit drug use, hypothesizing that those who received pubertal suppression would have “superior mental health outcomes” when compared to those who desired – but didn’t receive – pubertal suppression, wrote Dr. Turban and coauthors.
Suicidality within the past 12 months and severe psychological distress were both significantly more common among those who did not receive pubertal suppression, but these associations lost significance after multivariable analysis. There was no difference in odds of suicide attempts, although the study may have been underpowered to detect some of these associations, said the investigators.
After statistical analysis to control for demographic variables, pubertal suppression still was associated with decreased odds of having suicidal ideation over the lifespan.
Dr. Turban and colleagues acknowledged that reverse causation may have been in play, because adolescents with better mental health might have been considered better candidates for GnRHa therapy. But the study’s large sample size and wide geographic reach are strengths, they said, concluding that overall, the findings lend support to existing recommendations from the Endocrine Society and the World Professional Association for Transgender Health that pubertal suppression therapy be available to those adolescents who desire it.
Investigators were supported by the U.S. Health Resources and Services Administration, the Patient-Centered Outcomes Research Institute, and the American Academy of Child & Adolescent Psychiatry. The authors reported that they had no financial conflicts of interest.
SOURCE: Turban JL et al. Pediatrics. 2020;145(2):e20191725.
FROM PEDIATRICS
The Lowdown on Low-Dose Naltrexone
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
Resident Pearl
- Low-dose naltrexone is an alternative antiinflammatory treatment to consider in patients with inflammatory skin diseases, with a minimal side-effect profile.
Adding lymphopenia component ‘improves’ FLIPI
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
Incorporating lymphopenia into the Follicular Lymphoma International Prognostic Index (FLIPI) can improve prognostication, according to researchers.
The team added lymphopenia as a point in a revised FLIPI scoring system, called FLIPI-L, and found the new system could better predict overall survival (OS), progression-free survival, and histologic transformation in patients with follicular lymphoma.
George Yang, MD, of Moffitt Cancer Center in Tampa, Fla., and his colleagues described results with the FLIPI-L in a letter published in Blood Cancer Journal.
“Prior studies have demonstrated that lymphopenia was associated with worsened OS in [follicular lymphoma],” Dr. Yang and his colleagues wrote. “Therefore, we hypothesized that lymphopenia may be integrated with existing FLIPI to better stratify long-term survival outcomes and predict for transformation.”
The researchers tested this theory in 736 follicular lymphoma patients who were followed for a median of 72 months (range, 2-211 months). The 5-year OS in this cohort was 81.3%, the 10-year OS was 67.3%, and 18% of patients experienced transformation to high-grade lymphoma.
The researchers defined absolute lymphopenia as less than 1.0 × 109 lymphocytes per liter. In multivariate analyses, lymphopenia was an independent predictor of OS (hazard ratio, 1.74; P less than .01) and transformation (odds ratio, 2.1; P less than .01).
To incorporate lymphopenia into the FLIPI, the researchers created a model in which 1 point was given for each of the standard FLIPI components (age, Ann Arbor stage, number of nodal areas, lactate dehydrogenase, and hemoglobin level), and one point was given for the presence of lymphopenia. Patients in the low-risk FLIPI-L category had 0-1 points, those in the intermediate-risk category had 2-3 points, and patients in the high-risk FLIPI-L category had 4-6 points.
Using the original FLIPI, the 5-year OS was 91% in the low-risk group (0-1), 82.7% in the intermediate-risk group (2), and 66% in the high-risk group (3-5). The 10-year OS was 80.4%, 66%, and 45.8%, respectively.
Using the FLIPI-L, the 5-year OS was 94.5% in the low-risk group (0-1), 89% in the intermediate-risk group (2-3), and 61% in the high-risk group (4-6). The 10-year OS was 83.9%, 68.5%, and 34.5%, respectively.
In a univariate Cox regression analysis of OS, each point increase in FLIPI-L score was associated with a significant increase in hazard ratio. For example, the hazard ratio was 3.4 for patients with a FLIPI-L score of 1 and 30.9 for those with a FLIPI-L score of 6 (P less than .02 for all FLIPI-L scores). Conversely, increases in hazard ratio were not significant with the original FLIPI (P greater than .05 for all FLIPI scores).
The FLIPI-L was prognostic for OS in different treatment groups. In patients who received rituximab alone, radiation alone, or rituximab plus chemotherapy, the scoring system differentiated low-, intermediate-, and high-risk groups (P less than .04). In patients under observation, the FLIPI-L distinguished low/intermediate-risk and high-risk groups (P less than .01).
For patients who progressed within 24 months, the FLIPI-L was more predictive of progression-free survival (P = .05) than was the original FLIPI (P = .11).
Increasing FLIPI-L was an independent predictor of transformation, both when assessed as a continuous variable (P less than .01) and stepwise for FLIPI-L 3-5 (P = .004-.01). The original FLIPI, on the other hand, was not an independent predictor of transformation.
“Our analysis of a lymphopenia cutoff as an addition to the original FLIPI is simple yet improves risk stratification to differentiate between prognostic groups and, importantly, to predict transformation,” Dr. Yang and his colleagues wrote.
The authors reported having no conflicts of interest.
SOURCE: Yang G et al. Blood Cancer J. 2020 Jan 2;9(12):104. doi: 10.1038/s41408-019-0269-6.
FROM BLOOD CANCER JOURNAL
Infant deaths from birth defects decline, but some disparities widen
according to the Centers for Disease Control and Prevention.
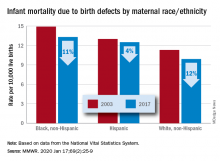
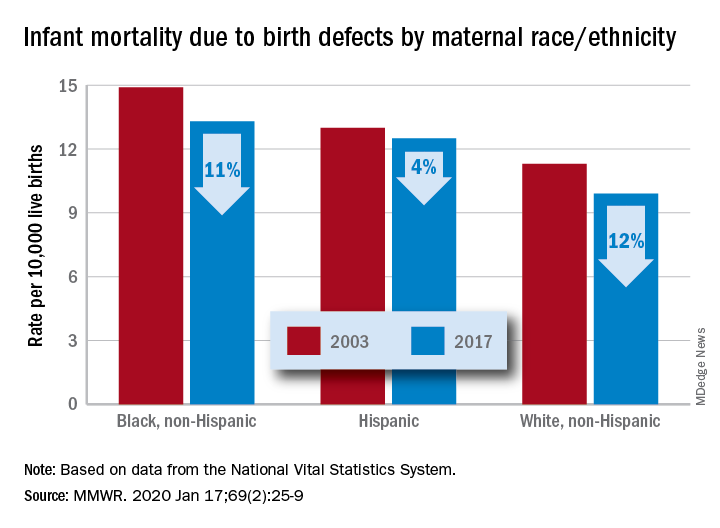
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
according to the Centers for Disease Control and Prevention.
The total rate of IMBD dropped from 12.2 cases per 10,000 live births in 2003 to 11 cases per 10,000 in 2017, with decreases occurring “across the categories of maternal race/ethnicity, infant sex, and infant age at death,” Lynn M. Almli, PhD, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates wrote in the Morbidity and Mortality Weekly Report.
Rates were down for infants of white non-Hispanic, black non-Hispanic, and Hispanic mothers, but disparities among races/ethnicities persisted or even increased. The IMBD rate for infants born to Hispanic mothers, which was 15% higher than that of infants born to white mothers in 2003, was 26% higher by 2017. The difference between infants born to black mothers and those born to whites rose from 32% in 2003 to 34% in 2017, the investigators reported.
The disparities were even greater among subgroups of infants categorized by gestational age. From 2003 to 2017, IMBD rates dropped by 20% for infants in the youngest group (20-27 weeks), 25% for infants in the oldest group (41-44 weeks), and 29% among those born at 39-40 weeks, they said.
For moderate- and late-preterm infants, however, IMBD rates went up: Infants born at 32-33 weeks and 34-36 weeks each had an increase of 17% over the study period, Dr. Almli and associates noted, based on data from the National Vital Statistics System.
“The observed differences in IMBD rates by race/ethnicity might be influenced by access to and utilization of health care before and during pregnancy, prenatal screening, losses of pregnancies with fetal anomalies, and insurance type,” they wrote, and trends by gestational age “could be influenced by the quantity and quality of care for infants born before 30 weeks’ gestation, compared with that of those born closer to term.”
Birth defects occur in approximately 3% of all births in the United States but accounted for 20% of infant deaths during 2003-2017, the investigators wrote, suggesting that “the results from this analysis can inform future research into areas where efforts to reduce IMBD rates are needed.”
SOURCE: Almli LM et al. MMWR. 2020 Jan 17;69(2):25-9.
FROM MMWR
AFib link with titin mutations warrants selected genetic testing
NATIONAL HARBOR, MD. – Testing to identify mutations in the gene that codes for the muscle protein titin is now a reasonable step in routine clinical practice for selected people with either early-onset atrial fibrillation (AFib) or a family history of atrial fibrillation, or other cardiac disorders that have been strongly linked with titin-gene mutations, Patrick T. Ellinor, MD, said at the annual International AF Symposium.
About one out of every 250 people carries a loss of function (LOF) mutation in one of their TTN genes that codes for the titin protein, making these mutations about as common as mutations for familial hypercholesterolemia, noted Dr. Ellinor, professor of medicine at Harvard Medical School in Boston and director of the Cardiovascular Disease Initiative at the Broad Institute in Cambridge, Mass. TTN LOF mutations are “bad and very frequent,” he said in an interview. “This is evolving quickly as we start to appreciate how frequent these mutations are.”
Several commercial genetic testing companies now offer testing of blood specimens for TTN LOF mutations, often as part of an “arrhythmia test” panel, with a turnaround time of about 4 weeks at a cost to the patient of about $100, noted Dr. Ellinor, who said that he has begun to discuss such testing with a small number of patients in his practice. “It’s reasonable for selected people; the jury is still out on which ones,” a subject that guideline writers will soon need to address, he said.
Patients already diagnosed with early-onset atrial fibrillation (AFib) could benefit from knowing if they had a TTN LOF mutation because that diagnosis would warrant a magnetic resonance scan to look for “subtle myopathies” not detectable with echocardiography, Dr. Ellinor explained. Identification of a TTN LOF would also be a reason to then test the patient’s children. “Perhaps we should offer testing to everyone 40 years old or younger with AFib,” Dr. Ellinor suggested. Many of these patients are now getting genetic testing for TTN on their own “whether or not their physician wants it done,” he noted.
The most recent, and perhaps most persuasive evidence for the link between TTN LOF mutations and AFib came from a recent report from Dr. Ellinor and associates that examined genome-wide associations in 1,546 people with AFib and 41,593 controls using information contained in the UK Biobank, which holds complete gene sequencing data for about half a million U.K. residents (Circ Res. 2020 Jan 17;126[2]: 200-9). The results showed that just under 0.5% of the entire population carried a TTN LOF mutation, and among patients with AFib the prevalence of a TTN LOF mutation was about 2%, but among people who carry this type of mutation 14% were diagnosed with AFib. This penetrance of 14% for AFib among people with a TTN LOF mutation makes AFib the most frequent clinical consequence identified so far for people with this type of mutation. Other cardiac disorders linked with TTN LOF mutations include heart failure and nonischemic cardiomyopathy. The Biobank study findings showed a penetrance for heart failure of about 7% among those with a TTN LOF mutation, and a penetrance of these mutations for nonischemic cardiomyopathy of about 3%.
Dr. Ellinor cited three other recently published studies with consistent results documenting a strong link between TTN LOF mutations and AFib: a study he worked on with lead author Seung H. Choi, Ph.D., and associates that ran an analysis on 2,781 AFib patients and 4,959 controls in a U.S. database of people who underwent whole-genome sequencing (JAMA. 2018 Dec 11; 320[22]:2354-64); a study of 24 Danish families with clusters of three or more affected members with AFib as well as 399 Danish residents with lone, early-onset AFib (Nat Commun. 2018 Oct 17;9[1]:4316); and a study of 25 patients with “very early onset” (less than 45 years old) AFib, which identified four of the 25 patients with a TTN LOF mutation (Circ Genom Precis Med. 2019 Nov 12[11]; 526-8).
Titin is the largest protein in humans and is critical for normal myocardial function. Titin acts as a molecular scaffold for sarcomere assembly and signaling, providing passive stiffness to the sarcomere. Mutations in TTN have been associated with tibial muscular dystrophy, hypertrophic cardiomyopathy, and dilated cardiomyopathy. The relationship now established between TTN mutations and AFib, cardiomyopathy, and heart failure may in the future help explain the tight clinical association of AFib and heart failure, Dr. Ellinor noted. The TTN gene is also notable as the largest gene in the human genome.
Dr. Ellinor has received research funding from Bayer, and he has served as an adviser or consultant to Bayer, Quest Diagnostics, and Novartis.
SOURCE: Choi SH et al. Circ Res. 2020 Jan 17;126[2]: 200-9.
NATIONAL HARBOR, MD. – Testing to identify mutations in the gene that codes for the muscle protein titin is now a reasonable step in routine clinical practice for selected people with either early-onset atrial fibrillation (AFib) or a family history of atrial fibrillation, or other cardiac disorders that have been strongly linked with titin-gene mutations, Patrick T. Ellinor, MD, said at the annual International AF Symposium.
About one out of every 250 people carries a loss of function (LOF) mutation in one of their TTN genes that codes for the titin protein, making these mutations about as common as mutations for familial hypercholesterolemia, noted Dr. Ellinor, professor of medicine at Harvard Medical School in Boston and director of the Cardiovascular Disease Initiative at the Broad Institute in Cambridge, Mass. TTN LOF mutations are “bad and very frequent,” he said in an interview. “This is evolving quickly as we start to appreciate how frequent these mutations are.”
Several commercial genetic testing companies now offer testing of blood specimens for TTN LOF mutations, often as part of an “arrhythmia test” panel, with a turnaround time of about 4 weeks at a cost to the patient of about $100, noted Dr. Ellinor, who said that he has begun to discuss such testing with a small number of patients in his practice. “It’s reasonable for selected people; the jury is still out on which ones,” a subject that guideline writers will soon need to address, he said.
Patients already diagnosed with early-onset atrial fibrillation (AFib) could benefit from knowing if they had a TTN LOF mutation because that diagnosis would warrant a magnetic resonance scan to look for “subtle myopathies” not detectable with echocardiography, Dr. Ellinor explained. Identification of a TTN LOF would also be a reason to then test the patient’s children. “Perhaps we should offer testing to everyone 40 years old or younger with AFib,” Dr. Ellinor suggested. Many of these patients are now getting genetic testing for TTN on their own “whether or not their physician wants it done,” he noted.
The most recent, and perhaps most persuasive evidence for the link between TTN LOF mutations and AFib came from a recent report from Dr. Ellinor and associates that examined genome-wide associations in 1,546 people with AFib and 41,593 controls using information contained in the UK Biobank, which holds complete gene sequencing data for about half a million U.K. residents (Circ Res. 2020 Jan 17;126[2]: 200-9). The results showed that just under 0.5% of the entire population carried a TTN LOF mutation, and among patients with AFib the prevalence of a TTN LOF mutation was about 2%, but among people who carry this type of mutation 14% were diagnosed with AFib. This penetrance of 14% for AFib among people with a TTN LOF mutation makes AFib the most frequent clinical consequence identified so far for people with this type of mutation. Other cardiac disorders linked with TTN LOF mutations include heart failure and nonischemic cardiomyopathy. The Biobank study findings showed a penetrance for heart failure of about 7% among those with a TTN LOF mutation, and a penetrance of these mutations for nonischemic cardiomyopathy of about 3%.
Dr. Ellinor cited three other recently published studies with consistent results documenting a strong link between TTN LOF mutations and AFib: a study he worked on with lead author Seung H. Choi, Ph.D., and associates that ran an analysis on 2,781 AFib patients and 4,959 controls in a U.S. database of people who underwent whole-genome sequencing (JAMA. 2018 Dec 11; 320[22]:2354-64); a study of 24 Danish families with clusters of three or more affected members with AFib as well as 399 Danish residents with lone, early-onset AFib (Nat Commun. 2018 Oct 17;9[1]:4316); and a study of 25 patients with “very early onset” (less than 45 years old) AFib, which identified four of the 25 patients with a TTN LOF mutation (Circ Genom Precis Med. 2019 Nov 12[11]; 526-8).
Titin is the largest protein in humans and is critical for normal myocardial function. Titin acts as a molecular scaffold for sarcomere assembly and signaling, providing passive stiffness to the sarcomere. Mutations in TTN have been associated with tibial muscular dystrophy, hypertrophic cardiomyopathy, and dilated cardiomyopathy. The relationship now established between TTN mutations and AFib, cardiomyopathy, and heart failure may in the future help explain the tight clinical association of AFib and heart failure, Dr. Ellinor noted. The TTN gene is also notable as the largest gene in the human genome.
Dr. Ellinor has received research funding from Bayer, and he has served as an adviser or consultant to Bayer, Quest Diagnostics, and Novartis.
SOURCE: Choi SH et al. Circ Res. 2020 Jan 17;126[2]: 200-9.
NATIONAL HARBOR, MD. – Testing to identify mutations in the gene that codes for the muscle protein titin is now a reasonable step in routine clinical practice for selected people with either early-onset atrial fibrillation (AFib) or a family history of atrial fibrillation, or other cardiac disorders that have been strongly linked with titin-gene mutations, Patrick T. Ellinor, MD, said at the annual International AF Symposium.
About one out of every 250 people carries a loss of function (LOF) mutation in one of their TTN genes that codes for the titin protein, making these mutations about as common as mutations for familial hypercholesterolemia, noted Dr. Ellinor, professor of medicine at Harvard Medical School in Boston and director of the Cardiovascular Disease Initiative at the Broad Institute in Cambridge, Mass. TTN LOF mutations are “bad and very frequent,” he said in an interview. “This is evolving quickly as we start to appreciate how frequent these mutations are.”
Several commercial genetic testing companies now offer testing of blood specimens for TTN LOF mutations, often as part of an “arrhythmia test” panel, with a turnaround time of about 4 weeks at a cost to the patient of about $100, noted Dr. Ellinor, who said that he has begun to discuss such testing with a small number of patients in his practice. “It’s reasonable for selected people; the jury is still out on which ones,” a subject that guideline writers will soon need to address, he said.
Patients already diagnosed with early-onset atrial fibrillation (AFib) could benefit from knowing if they had a TTN LOF mutation because that diagnosis would warrant a magnetic resonance scan to look for “subtle myopathies” not detectable with echocardiography, Dr. Ellinor explained. Identification of a TTN LOF would also be a reason to then test the patient’s children. “Perhaps we should offer testing to everyone 40 years old or younger with AFib,” Dr. Ellinor suggested. Many of these patients are now getting genetic testing for TTN on their own “whether or not their physician wants it done,” he noted.
The most recent, and perhaps most persuasive evidence for the link between TTN LOF mutations and AFib came from a recent report from Dr. Ellinor and associates that examined genome-wide associations in 1,546 people with AFib and 41,593 controls using information contained in the UK Biobank, which holds complete gene sequencing data for about half a million U.K. residents (Circ Res. 2020 Jan 17;126[2]: 200-9). The results showed that just under 0.5% of the entire population carried a TTN LOF mutation, and among patients with AFib the prevalence of a TTN LOF mutation was about 2%, but among people who carry this type of mutation 14% were diagnosed with AFib. This penetrance of 14% for AFib among people with a TTN LOF mutation makes AFib the most frequent clinical consequence identified so far for people with this type of mutation. Other cardiac disorders linked with TTN LOF mutations include heart failure and nonischemic cardiomyopathy. The Biobank study findings showed a penetrance for heart failure of about 7% among those with a TTN LOF mutation, and a penetrance of these mutations for nonischemic cardiomyopathy of about 3%.
Dr. Ellinor cited three other recently published studies with consistent results documenting a strong link between TTN LOF mutations and AFib: a study he worked on with lead author Seung H. Choi, Ph.D., and associates that ran an analysis on 2,781 AFib patients and 4,959 controls in a U.S. database of people who underwent whole-genome sequencing (JAMA. 2018 Dec 11; 320[22]:2354-64); a study of 24 Danish families with clusters of three or more affected members with AFib as well as 399 Danish residents with lone, early-onset AFib (Nat Commun. 2018 Oct 17;9[1]:4316); and a study of 25 patients with “very early onset” (less than 45 years old) AFib, which identified four of the 25 patients with a TTN LOF mutation (Circ Genom Precis Med. 2019 Nov 12[11]; 526-8).
Titin is the largest protein in humans and is critical for normal myocardial function. Titin acts as a molecular scaffold for sarcomere assembly and signaling, providing passive stiffness to the sarcomere. Mutations in TTN have been associated with tibial muscular dystrophy, hypertrophic cardiomyopathy, and dilated cardiomyopathy. The relationship now established between TTN mutations and AFib, cardiomyopathy, and heart failure may in the future help explain the tight clinical association of AFib and heart failure, Dr. Ellinor noted. The TTN gene is also notable as the largest gene in the human genome.
Dr. Ellinor has received research funding from Bayer, and he has served as an adviser or consultant to Bayer, Quest Diagnostics, and Novartis.
SOURCE: Choi SH et al. Circ Res. 2020 Jan 17;126[2]: 200-9.
REPORTING FROM THE AF SYMPOSIUM 2020
Hospitalist movers and shakers – January 2020
Hyung (Harry) Cho, MD, SFHM, and Christopher Moriates, MD, SFHM, have been honored by Modern Healthcare as two of 25 emerging young executives in health care management.
Dr. Cho is chief value officer for NYC Health and Hospitals, where his focus is on eliminating unnecessary testing and treatments within the New York City public health system, which includes 11 hospitals and five post-acute care facilities. Before landing with NYC Health and Hospitals, Dr. Cho was director of quality, safety and value at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Moriates is assistant dean for health care and value at the University of Texas at Austin’s Dell Medical School, where he has created the Discovering Value-Based Health Care online learning platform. In addition, Dr. Moriates has helped design a care model to enhance the treatment of patients who suffer from opioid use disorder. Prior to arriving at Dell, he helped create curriculum to educate students about costs and value at the University of California, San Francisco.
Trina Abla, DO, was appointed chief medical officer at Mercy Catholic Medical Center in Darby, Pa. A practicing hospitalist, Dr. Abla will be in charge of the hospital budget, the recruiting and training of physicians, and maintaining safety standards and quality care at the facility.
Prior to taking the position at Mercy Catholic, Dr. Abla was chief quality officer and associate CMO at Penn State Health St. Joseph in Reading, Pa.
Ghania El Akiki, MD, has been named to the board of advisors at Beth Israel Deaconess Hospital in Needham, Mass. Dr. Akiki is chief of hospitalist services at Beth Israel Deaconess, landing there after a fellowship in geriatrics at Beth Israel Deaconess Medical Center.
Dr. Akiki completed a physician leadership program at BID Medical Center in 2018, and serves as instructor of medicine at Harvard Medical School, Boston.
Michael Schandorf-Lartey, MD, has been named the chief medical officer at Doctors Hospital in Sarasota, Fla. Dr. Schandorf-Lartey has been a hospitalist at Doctors Hospital for the past 12 years.
In his time at Doctors, Dr. Schandorf-Lartey also has been chief of medicine, president-elect, and president of the medical staff. A native of Ghana, he has had experience working in rural and urban hospitals in Africa before coming to the United States.
Michael Roberts, MD, was named chief of staff at East Alabama Medical Center in Opeleika, Ala. He has been part of EAMC since 2008, when he became a hospitalist there through Internal Medicine Associates.
As chief of staff, Dr. Roberts will work with different components of the medical staff and serve as a liaison between the hospital board and its staff; assist in developing policies alongside the chief medical officer; and serve on many of the medical staff’s committees.
Brian Dawson, MD, has been named chief medical officer for Ballad Health, Southwest Region, based in Johnson City, Tenn. Dr. Dawson will lead Ballad Health locations in Washington County, which include Franklin Woods Community Hospital, Johnson City Medical Center, Niswonger Children Hospital, and Woodridge Hospital.
Dr. Dawson comes to Ballad Health after serving as vice president at VEP Healthcare, where he focused on contract management for the emergency medicine and hospitalist firm. Previously, he was chief of staff and Northeast regional director for emergency medicine at Johnston Memorial Hospital, Abington, Va.
Eagle Telemedicine (Atlanta, Ga.) recently agreed to begin a telehospitalist program at Jersey Community Hospital in Jerseyville, Ill. Eagle Telemedicine offers telehospitalist services to more than 150 hospitals nationwide.
A rural facility with fewer than 50 beds, JCH will use Eagle to make up for the lack of a full-time, onsite hospitalist program, taking strain off of physicians handling emergency calls. At JCH, telehospitalists work closely with onsite nurse practitioners to guide patients through their hospital stay.
Hyung (Harry) Cho, MD, SFHM, and Christopher Moriates, MD, SFHM, have been honored by Modern Healthcare as two of 25 emerging young executives in health care management.
Dr. Cho is chief value officer for NYC Health and Hospitals, where his focus is on eliminating unnecessary testing and treatments within the New York City public health system, which includes 11 hospitals and five post-acute care facilities. Before landing with NYC Health and Hospitals, Dr. Cho was director of quality, safety and value at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Moriates is assistant dean for health care and value at the University of Texas at Austin’s Dell Medical School, where he has created the Discovering Value-Based Health Care online learning platform. In addition, Dr. Moriates has helped design a care model to enhance the treatment of patients who suffer from opioid use disorder. Prior to arriving at Dell, he helped create curriculum to educate students about costs and value at the University of California, San Francisco.
Trina Abla, DO, was appointed chief medical officer at Mercy Catholic Medical Center in Darby, Pa. A practicing hospitalist, Dr. Abla will be in charge of the hospital budget, the recruiting and training of physicians, and maintaining safety standards and quality care at the facility.
Prior to taking the position at Mercy Catholic, Dr. Abla was chief quality officer and associate CMO at Penn State Health St. Joseph in Reading, Pa.
Ghania El Akiki, MD, has been named to the board of advisors at Beth Israel Deaconess Hospital in Needham, Mass. Dr. Akiki is chief of hospitalist services at Beth Israel Deaconess, landing there after a fellowship in geriatrics at Beth Israel Deaconess Medical Center.
Dr. Akiki completed a physician leadership program at BID Medical Center in 2018, and serves as instructor of medicine at Harvard Medical School, Boston.
Michael Schandorf-Lartey, MD, has been named the chief medical officer at Doctors Hospital in Sarasota, Fla. Dr. Schandorf-Lartey has been a hospitalist at Doctors Hospital for the past 12 years.
In his time at Doctors, Dr. Schandorf-Lartey also has been chief of medicine, president-elect, and president of the medical staff. A native of Ghana, he has had experience working in rural and urban hospitals in Africa before coming to the United States.
Michael Roberts, MD, was named chief of staff at East Alabama Medical Center in Opeleika, Ala. He has been part of EAMC since 2008, when he became a hospitalist there through Internal Medicine Associates.
As chief of staff, Dr. Roberts will work with different components of the medical staff and serve as a liaison between the hospital board and its staff; assist in developing policies alongside the chief medical officer; and serve on many of the medical staff’s committees.
Brian Dawson, MD, has been named chief medical officer for Ballad Health, Southwest Region, based in Johnson City, Tenn. Dr. Dawson will lead Ballad Health locations in Washington County, which include Franklin Woods Community Hospital, Johnson City Medical Center, Niswonger Children Hospital, and Woodridge Hospital.
Dr. Dawson comes to Ballad Health after serving as vice president at VEP Healthcare, where he focused on contract management for the emergency medicine and hospitalist firm. Previously, he was chief of staff and Northeast regional director for emergency medicine at Johnston Memorial Hospital, Abington, Va.
Eagle Telemedicine (Atlanta, Ga.) recently agreed to begin a telehospitalist program at Jersey Community Hospital in Jerseyville, Ill. Eagle Telemedicine offers telehospitalist services to more than 150 hospitals nationwide.
A rural facility with fewer than 50 beds, JCH will use Eagle to make up for the lack of a full-time, onsite hospitalist program, taking strain off of physicians handling emergency calls. At JCH, telehospitalists work closely with onsite nurse practitioners to guide patients through their hospital stay.
Hyung (Harry) Cho, MD, SFHM, and Christopher Moriates, MD, SFHM, have been honored by Modern Healthcare as two of 25 emerging young executives in health care management.
Dr. Cho is chief value officer for NYC Health and Hospitals, where his focus is on eliminating unnecessary testing and treatments within the New York City public health system, which includes 11 hospitals and five post-acute care facilities. Before landing with NYC Health and Hospitals, Dr. Cho was director of quality, safety and value at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Moriates is assistant dean for health care and value at the University of Texas at Austin’s Dell Medical School, where he has created the Discovering Value-Based Health Care online learning platform. In addition, Dr. Moriates has helped design a care model to enhance the treatment of patients who suffer from opioid use disorder. Prior to arriving at Dell, he helped create curriculum to educate students about costs and value at the University of California, San Francisco.
Trina Abla, DO, was appointed chief medical officer at Mercy Catholic Medical Center in Darby, Pa. A practicing hospitalist, Dr. Abla will be in charge of the hospital budget, the recruiting and training of physicians, and maintaining safety standards and quality care at the facility.
Prior to taking the position at Mercy Catholic, Dr. Abla was chief quality officer and associate CMO at Penn State Health St. Joseph in Reading, Pa.
Ghania El Akiki, MD, has been named to the board of advisors at Beth Israel Deaconess Hospital in Needham, Mass. Dr. Akiki is chief of hospitalist services at Beth Israel Deaconess, landing there after a fellowship in geriatrics at Beth Israel Deaconess Medical Center.
Dr. Akiki completed a physician leadership program at BID Medical Center in 2018, and serves as instructor of medicine at Harvard Medical School, Boston.
Michael Schandorf-Lartey, MD, has been named the chief medical officer at Doctors Hospital in Sarasota, Fla. Dr. Schandorf-Lartey has been a hospitalist at Doctors Hospital for the past 12 years.
In his time at Doctors, Dr. Schandorf-Lartey also has been chief of medicine, president-elect, and president of the medical staff. A native of Ghana, he has had experience working in rural and urban hospitals in Africa before coming to the United States.
Michael Roberts, MD, was named chief of staff at East Alabama Medical Center in Opeleika, Ala. He has been part of EAMC since 2008, when he became a hospitalist there through Internal Medicine Associates.
As chief of staff, Dr. Roberts will work with different components of the medical staff and serve as a liaison between the hospital board and its staff; assist in developing policies alongside the chief medical officer; and serve on many of the medical staff’s committees.
Brian Dawson, MD, has been named chief medical officer for Ballad Health, Southwest Region, based in Johnson City, Tenn. Dr. Dawson will lead Ballad Health locations in Washington County, which include Franklin Woods Community Hospital, Johnson City Medical Center, Niswonger Children Hospital, and Woodridge Hospital.
Dr. Dawson comes to Ballad Health after serving as vice president at VEP Healthcare, where he focused on contract management for the emergency medicine and hospitalist firm. Previously, he was chief of staff and Northeast regional director for emergency medicine at Johnston Memorial Hospital, Abington, Va.
Eagle Telemedicine (Atlanta, Ga.) recently agreed to begin a telehospitalist program at Jersey Community Hospital in Jerseyville, Ill. Eagle Telemedicine offers telehospitalist services to more than 150 hospitals nationwide.
A rural facility with fewer than 50 beds, JCH will use Eagle to make up for the lack of a full-time, onsite hospitalist program, taking strain off of physicians handling emergency calls. At JCH, telehospitalists work closely with onsite nurse practitioners to guide patients through their hospital stay.
GAO Finds DoD Can Do More to Recruit and Retain Physicians and Dentists
Is the US Department of Defense (DoD) doing enough—or the right things—to attract and keep physicians and dentists? According to a new report by the Government Accountability Office (GAO), although the DoD is hitting the mark in some areas, there’s room for improvement in others.
It’s a crucial question. The GAO reported in 2018 that DoD officials cited “a number of challenges” that made it difficult to attract and retain physicians and dentists, such as national shortages and competition with the private sector. Indeed, military health system physicians and dentists make less than do their counterparts in the private sector, the GAO says. For 21 of 27 specialties studied in the new report, the maximum cash compensation was less than the civilian median within 4 officer pay grades (O-3 to O-6). Moreover, cash compensation even for the most senior military physicians and dentists was less than that of the civilian median at “key retention points,” such as after physicians and dentists fulfill their initial active-duty service.
The DoD provides “substantial” deferred and noncash benefits, the GAO notes, such as retirement pensions and tuition-free education, but adds that the value to service members is “difficult to determine.” The DoD also recruits with a package of incentives, including multi-year retention bonuses.
In general, the GAO found, the DoD applies several “effective human capital management” principles. For instance, it relies on clearly defined criteria on when to use incentives (such as rules-based pay plans). It also identifies and evaluates unique staffing situations. For example, to attract physicians and dentists in “critically short wartime specialties,” it offers a Critical Wartime Skills Accession Bonus.
However, the report says, the DoD does not consistently collect information that could help inform its recruitment/retention decisions. At the time of the study, the DoD had not identified replacement costs for physicians or dentists as it does, for instance, with nuclear propulsion personnel. Nor did it gather current and historical retention information. Specifically, the GAO report says, Navy and Air Force officials said they don’t have readily available information to determine the percentage of those who accepted a retention bonus. Conversely, Army officials don’t have a framework in place that uses retention information to determine the effectiveness of retention bonuses (as do the Navy, Marine Corps, and Air Force).
Extending Service Obligations
The DoD is considering extending service obligations for students receiving DoD-funded assistance for physician or dentist education. Students in the DoD scholarship program have a 2-year minimum service obligation, with 6months of active-duty service obligations for each 6 months of benefits received. Medical students attending the Uniformed Services University of the Health Sciences (USUHS), have a 7-year active-duty service obligation.
The GAO held 8 focus groups with students and found 68% of USUHS students and 46% of scholarship students would be willing to accept 1 more year of obligation (although only 34% and 16%, respectively, would agree to 2). The participants expressed concern that longer service obligations would delay their eligibility for retention bonuses—resulting in a reduction of cash compensation over the course of a career. However, 80% and 63%, respectively, would accept an additional year of service obligation if accompanied by additional cash incentives.
Further, the GAO notes, longer obligations could have “unintended consequences.” For example, students might decide to separate and train in a civilian program after 1 or more tours as general medical officers to complete their active duty service obligation, decline further medical training and specialization via a military fellowship program, or separate from the military sooner than planned.
Potential Reductions in Health Care Force
The DoD, according to the report, also is considering reducing the overall number of active-duty physicians, including “targeted reductions” to certain specialties, raising concerns among participants in all 8 focus groups.
Given that the DoD spends millions of dollars annually to train medical and dental students and that almost half of the special pay budget is dedicated to retaining them once they’re fully trained, consistently collecting information to help inform investment decisions is “critical to ensuring the efficiency of these significant resources,” the GAO says. Collecting such information, the GAO says, and using it, would help inform its decision making. For instance, such information would help officials decide whether it would be more cost effective to focus on retaining medical personnel rather than training new staff.
Retaining “top talent,” the DoD says, is “essential to sustaining mission readiness that is adaptable and responsive.” The GAO report cites a 2012 study that found compensation for military physicians had “a large impact on the decision to remain in the military in the first unobligated year of service and just a small impact on retention in the years afterward.”
DoD officials told the GAO that budget considerations and statutory limitations hinder their ability to change the rate of special and incentive pays. The GAO calls these “valid considerations” but suggests that collecting information on replacement costs, retention, and civilian wages would allow DoD departments to “provide greater stewardship of available funding by ensuring its efficient application.” It may be, the GAO says, that retaining fully trained physicians within the DoD is “highly economical.”. Most important, using such data to inform investment decisions will allow the DoD to “efficiently and effectively meet its mission of providing health care during times of war and peace.”
In response to the GAO findings, DoD officials have a group working on a plan to recruit and retain critical specialties, which will be released by June 2020. They also concurred with other GAO recommendations, saying changes will be made within 2 years.
Is the US Department of Defense (DoD) doing enough—or the right things—to attract and keep physicians and dentists? According to a new report by the Government Accountability Office (GAO), although the DoD is hitting the mark in some areas, there’s room for improvement in others.
It’s a crucial question. The GAO reported in 2018 that DoD officials cited “a number of challenges” that made it difficult to attract and retain physicians and dentists, such as national shortages and competition with the private sector. Indeed, military health system physicians and dentists make less than do their counterparts in the private sector, the GAO says. For 21 of 27 specialties studied in the new report, the maximum cash compensation was less than the civilian median within 4 officer pay grades (O-3 to O-6). Moreover, cash compensation even for the most senior military physicians and dentists was less than that of the civilian median at “key retention points,” such as after physicians and dentists fulfill their initial active-duty service.
The DoD provides “substantial” deferred and noncash benefits, the GAO notes, such as retirement pensions and tuition-free education, but adds that the value to service members is “difficult to determine.” The DoD also recruits with a package of incentives, including multi-year retention bonuses.
In general, the GAO found, the DoD applies several “effective human capital management” principles. For instance, it relies on clearly defined criteria on when to use incentives (such as rules-based pay plans). It also identifies and evaluates unique staffing situations. For example, to attract physicians and dentists in “critically short wartime specialties,” it offers a Critical Wartime Skills Accession Bonus.
However, the report says, the DoD does not consistently collect information that could help inform its recruitment/retention decisions. At the time of the study, the DoD had not identified replacement costs for physicians or dentists as it does, for instance, with nuclear propulsion personnel. Nor did it gather current and historical retention information. Specifically, the GAO report says, Navy and Air Force officials said they don’t have readily available information to determine the percentage of those who accepted a retention bonus. Conversely, Army officials don’t have a framework in place that uses retention information to determine the effectiveness of retention bonuses (as do the Navy, Marine Corps, and Air Force).
Extending Service Obligations
The DoD is considering extending service obligations for students receiving DoD-funded assistance for physician or dentist education. Students in the DoD scholarship program have a 2-year minimum service obligation, with 6months of active-duty service obligations for each 6 months of benefits received. Medical students attending the Uniformed Services University of the Health Sciences (USUHS), have a 7-year active-duty service obligation.
The GAO held 8 focus groups with students and found 68% of USUHS students and 46% of scholarship students would be willing to accept 1 more year of obligation (although only 34% and 16%, respectively, would agree to 2). The participants expressed concern that longer service obligations would delay their eligibility for retention bonuses—resulting in a reduction of cash compensation over the course of a career. However, 80% and 63%, respectively, would accept an additional year of service obligation if accompanied by additional cash incentives.
Further, the GAO notes, longer obligations could have “unintended consequences.” For example, students might decide to separate and train in a civilian program after 1 or more tours as general medical officers to complete their active duty service obligation, decline further medical training and specialization via a military fellowship program, or separate from the military sooner than planned.
Potential Reductions in Health Care Force
The DoD, according to the report, also is considering reducing the overall number of active-duty physicians, including “targeted reductions” to certain specialties, raising concerns among participants in all 8 focus groups.
Given that the DoD spends millions of dollars annually to train medical and dental students and that almost half of the special pay budget is dedicated to retaining them once they’re fully trained, consistently collecting information to help inform investment decisions is “critical to ensuring the efficiency of these significant resources,” the GAO says. Collecting such information, the GAO says, and using it, would help inform its decision making. For instance, such information would help officials decide whether it would be more cost effective to focus on retaining medical personnel rather than training new staff.
Retaining “top talent,” the DoD says, is “essential to sustaining mission readiness that is adaptable and responsive.” The GAO report cites a 2012 study that found compensation for military physicians had “a large impact on the decision to remain in the military in the first unobligated year of service and just a small impact on retention in the years afterward.”
DoD officials told the GAO that budget considerations and statutory limitations hinder their ability to change the rate of special and incentive pays. The GAO calls these “valid considerations” but suggests that collecting information on replacement costs, retention, and civilian wages would allow DoD departments to “provide greater stewardship of available funding by ensuring its efficient application.” It may be, the GAO says, that retaining fully trained physicians within the DoD is “highly economical.”. Most important, using such data to inform investment decisions will allow the DoD to “efficiently and effectively meet its mission of providing health care during times of war and peace.”
In response to the GAO findings, DoD officials have a group working on a plan to recruit and retain critical specialties, which will be released by June 2020. They also concurred with other GAO recommendations, saying changes will be made within 2 years.
Is the US Department of Defense (DoD) doing enough—or the right things—to attract and keep physicians and dentists? According to a new report by the Government Accountability Office (GAO), although the DoD is hitting the mark in some areas, there’s room for improvement in others.
It’s a crucial question. The GAO reported in 2018 that DoD officials cited “a number of challenges” that made it difficult to attract and retain physicians and dentists, such as national shortages and competition with the private sector. Indeed, military health system physicians and dentists make less than do their counterparts in the private sector, the GAO says. For 21 of 27 specialties studied in the new report, the maximum cash compensation was less than the civilian median within 4 officer pay grades (O-3 to O-6). Moreover, cash compensation even for the most senior military physicians and dentists was less than that of the civilian median at “key retention points,” such as after physicians and dentists fulfill their initial active-duty service.
The DoD provides “substantial” deferred and noncash benefits, the GAO notes, such as retirement pensions and tuition-free education, but adds that the value to service members is “difficult to determine.” The DoD also recruits with a package of incentives, including multi-year retention bonuses.
In general, the GAO found, the DoD applies several “effective human capital management” principles. For instance, it relies on clearly defined criteria on when to use incentives (such as rules-based pay plans). It also identifies and evaluates unique staffing situations. For example, to attract physicians and dentists in “critically short wartime specialties,” it offers a Critical Wartime Skills Accession Bonus.
However, the report says, the DoD does not consistently collect information that could help inform its recruitment/retention decisions. At the time of the study, the DoD had not identified replacement costs for physicians or dentists as it does, for instance, with nuclear propulsion personnel. Nor did it gather current and historical retention information. Specifically, the GAO report says, Navy and Air Force officials said they don’t have readily available information to determine the percentage of those who accepted a retention bonus. Conversely, Army officials don’t have a framework in place that uses retention information to determine the effectiveness of retention bonuses (as do the Navy, Marine Corps, and Air Force).
Extending Service Obligations
The DoD is considering extending service obligations for students receiving DoD-funded assistance for physician or dentist education. Students in the DoD scholarship program have a 2-year minimum service obligation, with 6months of active-duty service obligations for each 6 months of benefits received. Medical students attending the Uniformed Services University of the Health Sciences (USUHS), have a 7-year active-duty service obligation.
The GAO held 8 focus groups with students and found 68% of USUHS students and 46% of scholarship students would be willing to accept 1 more year of obligation (although only 34% and 16%, respectively, would agree to 2). The participants expressed concern that longer service obligations would delay their eligibility for retention bonuses—resulting in a reduction of cash compensation over the course of a career. However, 80% and 63%, respectively, would accept an additional year of service obligation if accompanied by additional cash incentives.
Further, the GAO notes, longer obligations could have “unintended consequences.” For example, students might decide to separate and train in a civilian program after 1 or more tours as general medical officers to complete their active duty service obligation, decline further medical training and specialization via a military fellowship program, or separate from the military sooner than planned.
Potential Reductions in Health Care Force
The DoD, according to the report, also is considering reducing the overall number of active-duty physicians, including “targeted reductions” to certain specialties, raising concerns among participants in all 8 focus groups.
Given that the DoD spends millions of dollars annually to train medical and dental students and that almost half of the special pay budget is dedicated to retaining them once they’re fully trained, consistently collecting information to help inform investment decisions is “critical to ensuring the efficiency of these significant resources,” the GAO says. Collecting such information, the GAO says, and using it, would help inform its decision making. For instance, such information would help officials decide whether it would be more cost effective to focus on retaining medical personnel rather than training new staff.
Retaining “top talent,” the DoD says, is “essential to sustaining mission readiness that is adaptable and responsive.” The GAO report cites a 2012 study that found compensation for military physicians had “a large impact on the decision to remain in the military in the first unobligated year of service and just a small impact on retention in the years afterward.”
DoD officials told the GAO that budget considerations and statutory limitations hinder their ability to change the rate of special and incentive pays. The GAO calls these “valid considerations” but suggests that collecting information on replacement costs, retention, and civilian wages would allow DoD departments to “provide greater stewardship of available funding by ensuring its efficient application.” It may be, the GAO says, that retaining fully trained physicians within the DoD is “highly economical.”. Most important, using such data to inform investment decisions will allow the DoD to “efficiently and effectively meet its mission of providing health care during times of war and peace.”
In response to the GAO findings, DoD officials have a group working on a plan to recruit and retain critical specialties, which will be released by June 2020. They also concurred with other GAO recommendations, saying changes will be made within 2 years.
Second U.S. coronavirus patient confirmed
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
FDA: Cybersecurity vulnerabilities identified in GE Healthcare monitoring devices
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
Neurologic disease doesn’t discriminate against anyone
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.