User login
FDA approves fidaxomicin for treatment of C. difficile–associated diarrhea
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
The Food and Drug Administration has approved fidaxomicin (Dificid) for the treatment of Clostridioides difficile–associated diarrhea in children aged 6 months and older.
Approval was based on results from SUNSHINE, a phase 3, multicenter, investigator-blind, randomized, parallel-group study in 142 pediatric patients aged between 6 months and 18 years with confirmed C. difficile infection who received either fidaxomicin or vancomycin for 10 days. Clinical response 2 days after the conclusion of treatment was similar in both groups (77.6% for fidaxomicin vs. 70.5% for vancomycin), and fidaxomicin had a superior sustained response 30 days after the conclusion of treatment (68.4% vs. 50.0%).
The safety of fidaxomicin was assessed in a pair of clinical trials involving 136 patients; the most common adverse events were pyrexia, abdominal pain, vomiting, diarrhea, constipation, increased aminotransferases, and rash. Four patients discontinued fidaxomicin treatment because of adverse events, and four patients died during the trials, though all deaths were in patients aged younger than 2 years and seemed to be related to other comorbidities.
“C. difficile is an important cause of health care– and community-associated diarrheal illness in children, and sustained cure is difficult to achieve in some patients. The fidaxomicin pediatric trial was the first randomized, controlled trial of C. difficile infection treatment in children,” Larry K. Kociolek, MD, associate medical director of infection prevention and control at Ann & Robert H. Lurie Children’s Hospital of Chicago, said in the press release from Merck, manufacturer of fidaxomicin.
*This story was updated on 1/27/2020.
Zika virus: Birth defects rose fourfold in U.S. hardest-hit areas
according to the Centers for Disease Control and Prevention.
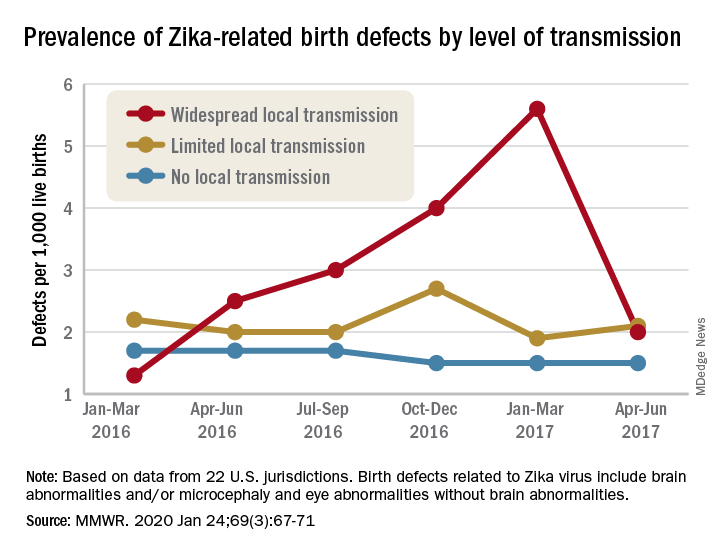
That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
FROM MMWR
Doctor wins $4.75 million award in defamation suit against hospital
Jurors awarded Carmel, Ind.–based ob.gyn. Rebecca Denman, MD, $4.75 million in damages against St. Vincent Carmel Hospital and St. Vincent Carmel Medical Group on Jan. 16, 2020, after a 4-day trial in Indiana Commercial Court. Dr. Denman sued after the hospital and medical group took a series of actions in response to a nurse practitioner’s claim that Dr. Denman smelled of alcohol while on duty. The doctor’s lawsuit alleged the NP’s claim was unproven; that administrators failed to conduct a proper peer-review investigation; and that repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
Indianapolis attorney Kathleen DeLaney, who represented Dr. Denman in the case, said that her client was pleased with the verdict.
“Dr. Denman feels vindicated that a group of jurors spent 4 days listening to all the evidence and gave her a resounding victory,” she said in an interview.
Dr. Denman declined to comment for this story through her attorney.
In a statement, a spokesman for Ascension, the hospital’s parent company, said the hospital was disappointed by the verdict and that it was “exploring all options available to us, including appeal.” The spokesman declined to answer further questions about the case or its peer-review process.
The case stems from an NP’s claim that Dr. Denman’s breath smelled of alcohol during an evening shift on Dec. 11, 2017. Dr. Denman was not informed of the allegation on Dec. 11 and was not tested for alcohol at the time, according to Dr. Denman’s lawsuit. Under hospital policy, if a physician is suspected of being under the influence of alcohol at work, the employer must immediately assess the doctor, relieve the doctor of duty, and request the physician submit to immediate blood testing at an external facility.
The NP reported the allegation to her supervisor through an email on Dec. 12, 2017. The supervisor relayed the information to the hospital’s chief medical officer who met with other administrators and physicians to discuss the claim. During the discussions, a previous concern about Dr. Denman’s drinking was raised, according to deposition information included in court documents. In 2015, two physicians had suggested Dr. Denman consider an assistance program after expressing concerns that she was arriving late to work and missing partner meetings. At the time, Dr. Denman did not enter an assistance program, but she changed her drinking habits, began seeing a therapist, and started arriving on-time to work and to partner meetings, according to court documents. No other criticism or complaints regarding her drinking or workplace behavior had been reported since, according to court documents.
When confronted with the NP’s claim on Dec. 13, 2017, Dr. Denman denied consuming alcohol on Dec. 11, 2017, and questioned why the hospital’s substance abuse protocol was not followed.
St. Vincent Carmel Hospital conducted a preliminary review of the allegation through its peer-review process and turned the matter over to St. Vincent Medical Group for further review, according to court documents. St. Vincent Medical Group later informed Dr. Denman they had reviewed the allegation through its peer-review process and that she was suspended with partial pay until she underwent an evaluation for alcohol abuse through the Indiana State Medical Association, according to the lawsuit.
“They falsely misrepresented to her that peer review had been done,” Ms. DeLaney said in an interview. “In spite of that statement, they never offered her a hearing before a peer-review committee, they never shared with her the substance of any evidence they had against her, they never gave her an opportunity to respond to the allegations. In fact, she wasn’t interviewed at all until the deposition in the lawsuit.”
According to the Indiana Peer Review law, a health care provider under investigation is permitted to see any records accumulated by a peer-review committee pertaining to the provider’s personal practice, and the provider shall be offered the opportunity to appear before the peer-review committee with adequate representation to hear all charges and findings concerning the provider and to offer rebuttal information. The rebuttal shall be part of the record before any disclosure of the charges and before any findings can be made, according to the statute.
Dr. Denman was referred by the medical association to an addiction treatment center that evaluated Dr. Denman and diagnosed her with alcohol use disorder, according to the lawsuit. As a result of the report and as a condition of retaining her medical license, the medical association and St. Vincent Medical Group required Dr. Denman to enter a treatment program at the same addiction treatment center. Dr. Denman was also required to sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit.
“The actions had life-changing consequences,” Ms. DeLaney said. “As a result, she was required to sign a contract that mandates she do a breathalyzer test four times a day for the first year and then three times a day for 4 more years. She has to go for random drug screenings. For the first year, she had to go to four [Alcoholics Anonymous] meetings a week. Now that number has been reduced, but she’s on a 5-year monitoring contract because of all of this.”
Dr. Denman sued the hospital, the medical group, and the NP in July 2018 alleging fraud, defamation, tortuous interference with an employment relationship, and negligent misrepresentation. The NP was dismissed from the case shortly before trial.
In its response to the lawsuit, attorneys for St. Vincent wrote that Dr. Denman’s action was frivolous, vexatious, and executed in bad faith. The defendants requested that a judge dismiss the lawsuit, noting that they were entitled to immunity pursuant to Indiana state and federal laws, including protection by Indiana’s Peer Review statute. In October 2019, a judge denied the hospital’s request to dismiss the lawsuit and allowed the case to proceed.
In their verdict, jurors awarded Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
Dr. Denman remains employed by the medical group and must continue the conditions of her 5-year monitoring contract, Ms. DeLaney said. She hopes Dr. Denman’s case raises awareness about physicians’ due process rights.
“We hope that Dr. Denman’s case emboldens physicians to stand up for themselves in the face of false accusations and rushes to judgment,” she said. “We hope the verdict leads to fair, prompt, and unbiased investigations by hospital and medical practice administrators, which include due process for accused physicians.”
Jurors awarded Carmel, Ind.–based ob.gyn. Rebecca Denman, MD, $4.75 million in damages against St. Vincent Carmel Hospital and St. Vincent Carmel Medical Group on Jan. 16, 2020, after a 4-day trial in Indiana Commercial Court. Dr. Denman sued after the hospital and medical group took a series of actions in response to a nurse practitioner’s claim that Dr. Denman smelled of alcohol while on duty. The doctor’s lawsuit alleged the NP’s claim was unproven; that administrators failed to conduct a proper peer-review investigation; and that repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
Indianapolis attorney Kathleen DeLaney, who represented Dr. Denman in the case, said that her client was pleased with the verdict.
“Dr. Denman feels vindicated that a group of jurors spent 4 days listening to all the evidence and gave her a resounding victory,” she said in an interview.
Dr. Denman declined to comment for this story through her attorney.
In a statement, a spokesman for Ascension, the hospital’s parent company, said the hospital was disappointed by the verdict and that it was “exploring all options available to us, including appeal.” The spokesman declined to answer further questions about the case or its peer-review process.
The case stems from an NP’s claim that Dr. Denman’s breath smelled of alcohol during an evening shift on Dec. 11, 2017. Dr. Denman was not informed of the allegation on Dec. 11 and was not tested for alcohol at the time, according to Dr. Denman’s lawsuit. Under hospital policy, if a physician is suspected of being under the influence of alcohol at work, the employer must immediately assess the doctor, relieve the doctor of duty, and request the physician submit to immediate blood testing at an external facility.
The NP reported the allegation to her supervisor through an email on Dec. 12, 2017. The supervisor relayed the information to the hospital’s chief medical officer who met with other administrators and physicians to discuss the claim. During the discussions, a previous concern about Dr. Denman’s drinking was raised, according to deposition information included in court documents. In 2015, two physicians had suggested Dr. Denman consider an assistance program after expressing concerns that she was arriving late to work and missing partner meetings. At the time, Dr. Denman did not enter an assistance program, but she changed her drinking habits, began seeing a therapist, and started arriving on-time to work and to partner meetings, according to court documents. No other criticism or complaints regarding her drinking or workplace behavior had been reported since, according to court documents.
When confronted with the NP’s claim on Dec. 13, 2017, Dr. Denman denied consuming alcohol on Dec. 11, 2017, and questioned why the hospital’s substance abuse protocol was not followed.
St. Vincent Carmel Hospital conducted a preliminary review of the allegation through its peer-review process and turned the matter over to St. Vincent Medical Group for further review, according to court documents. St. Vincent Medical Group later informed Dr. Denman they had reviewed the allegation through its peer-review process and that she was suspended with partial pay until she underwent an evaluation for alcohol abuse through the Indiana State Medical Association, according to the lawsuit.
“They falsely misrepresented to her that peer review had been done,” Ms. DeLaney said in an interview. “In spite of that statement, they never offered her a hearing before a peer-review committee, they never shared with her the substance of any evidence they had against her, they never gave her an opportunity to respond to the allegations. In fact, she wasn’t interviewed at all until the deposition in the lawsuit.”
According to the Indiana Peer Review law, a health care provider under investigation is permitted to see any records accumulated by a peer-review committee pertaining to the provider’s personal practice, and the provider shall be offered the opportunity to appear before the peer-review committee with adequate representation to hear all charges and findings concerning the provider and to offer rebuttal information. The rebuttal shall be part of the record before any disclosure of the charges and before any findings can be made, according to the statute.
Dr. Denman was referred by the medical association to an addiction treatment center that evaluated Dr. Denman and diagnosed her with alcohol use disorder, according to the lawsuit. As a result of the report and as a condition of retaining her medical license, the medical association and St. Vincent Medical Group required Dr. Denman to enter a treatment program at the same addiction treatment center. Dr. Denman was also required to sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit.
“The actions had life-changing consequences,” Ms. DeLaney said. “As a result, she was required to sign a contract that mandates she do a breathalyzer test four times a day for the first year and then three times a day for 4 more years. She has to go for random drug screenings. For the first year, she had to go to four [Alcoholics Anonymous] meetings a week. Now that number has been reduced, but she’s on a 5-year monitoring contract because of all of this.”
Dr. Denman sued the hospital, the medical group, and the NP in July 2018 alleging fraud, defamation, tortuous interference with an employment relationship, and negligent misrepresentation. The NP was dismissed from the case shortly before trial.
In its response to the lawsuit, attorneys for St. Vincent wrote that Dr. Denman’s action was frivolous, vexatious, and executed in bad faith. The defendants requested that a judge dismiss the lawsuit, noting that they were entitled to immunity pursuant to Indiana state and federal laws, including protection by Indiana’s Peer Review statute. In October 2019, a judge denied the hospital’s request to dismiss the lawsuit and allowed the case to proceed.
In their verdict, jurors awarded Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
Dr. Denman remains employed by the medical group and must continue the conditions of her 5-year monitoring contract, Ms. DeLaney said. She hopes Dr. Denman’s case raises awareness about physicians’ due process rights.
“We hope that Dr. Denman’s case emboldens physicians to stand up for themselves in the face of false accusations and rushes to judgment,” she said. “We hope the verdict leads to fair, prompt, and unbiased investigations by hospital and medical practice administrators, which include due process for accused physicians.”
Jurors awarded Carmel, Ind.–based ob.gyn. Rebecca Denman, MD, $4.75 million in damages against St. Vincent Carmel Hospital and St. Vincent Carmel Medical Group on Jan. 16, 2020, after a 4-day trial in Indiana Commercial Court. Dr. Denman sued after the hospital and medical group took a series of actions in response to a nurse practitioner’s claim that Dr. Denman smelled of alcohol while on duty. The doctor’s lawsuit alleged the NP’s claim was unproven; that administrators failed to conduct a proper peer-review investigation; and that repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
Indianapolis attorney Kathleen DeLaney, who represented Dr. Denman in the case, said that her client was pleased with the verdict.
“Dr. Denman feels vindicated that a group of jurors spent 4 days listening to all the evidence and gave her a resounding victory,” she said in an interview.
Dr. Denman declined to comment for this story through her attorney.
In a statement, a spokesman for Ascension, the hospital’s parent company, said the hospital was disappointed by the verdict and that it was “exploring all options available to us, including appeal.” The spokesman declined to answer further questions about the case or its peer-review process.
The case stems from an NP’s claim that Dr. Denman’s breath smelled of alcohol during an evening shift on Dec. 11, 2017. Dr. Denman was not informed of the allegation on Dec. 11 and was not tested for alcohol at the time, according to Dr. Denman’s lawsuit. Under hospital policy, if a physician is suspected of being under the influence of alcohol at work, the employer must immediately assess the doctor, relieve the doctor of duty, and request the physician submit to immediate blood testing at an external facility.
The NP reported the allegation to her supervisor through an email on Dec. 12, 2017. The supervisor relayed the information to the hospital’s chief medical officer who met with other administrators and physicians to discuss the claim. During the discussions, a previous concern about Dr. Denman’s drinking was raised, according to deposition information included in court documents. In 2015, two physicians had suggested Dr. Denman consider an assistance program after expressing concerns that she was arriving late to work and missing partner meetings. At the time, Dr. Denman did not enter an assistance program, but she changed her drinking habits, began seeing a therapist, and started arriving on-time to work and to partner meetings, according to court documents. No other criticism or complaints regarding her drinking or workplace behavior had been reported since, according to court documents.
When confronted with the NP’s claim on Dec. 13, 2017, Dr. Denman denied consuming alcohol on Dec. 11, 2017, and questioned why the hospital’s substance abuse protocol was not followed.
St. Vincent Carmel Hospital conducted a preliminary review of the allegation through its peer-review process and turned the matter over to St. Vincent Medical Group for further review, according to court documents. St. Vincent Medical Group later informed Dr. Denman they had reviewed the allegation through its peer-review process and that she was suspended with partial pay until she underwent an evaluation for alcohol abuse through the Indiana State Medical Association, according to the lawsuit.
“They falsely misrepresented to her that peer review had been done,” Ms. DeLaney said in an interview. “In spite of that statement, they never offered her a hearing before a peer-review committee, they never shared with her the substance of any evidence they had against her, they never gave her an opportunity to respond to the allegations. In fact, she wasn’t interviewed at all until the deposition in the lawsuit.”
According to the Indiana Peer Review law, a health care provider under investigation is permitted to see any records accumulated by a peer-review committee pertaining to the provider’s personal practice, and the provider shall be offered the opportunity to appear before the peer-review committee with adequate representation to hear all charges and findings concerning the provider and to offer rebuttal information. The rebuttal shall be part of the record before any disclosure of the charges and before any findings can be made, according to the statute.
Dr. Denman was referred by the medical association to an addiction treatment center that evaluated Dr. Denman and diagnosed her with alcohol use disorder, according to the lawsuit. As a result of the report and as a condition of retaining her medical license, the medical association and St. Vincent Medical Group required Dr. Denman to enter a treatment program at the same addiction treatment center. Dr. Denman was also required to sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit.
“The actions had life-changing consequences,” Ms. DeLaney said. “As a result, she was required to sign a contract that mandates she do a breathalyzer test four times a day for the first year and then three times a day for 4 more years. She has to go for random drug screenings. For the first year, she had to go to four [Alcoholics Anonymous] meetings a week. Now that number has been reduced, but she’s on a 5-year monitoring contract because of all of this.”
Dr. Denman sued the hospital, the medical group, and the NP in July 2018 alleging fraud, defamation, tortuous interference with an employment relationship, and negligent misrepresentation. The NP was dismissed from the case shortly before trial.
In its response to the lawsuit, attorneys for St. Vincent wrote that Dr. Denman’s action was frivolous, vexatious, and executed in bad faith. The defendants requested that a judge dismiss the lawsuit, noting that they were entitled to immunity pursuant to Indiana state and federal laws, including protection by Indiana’s Peer Review statute. In October 2019, a judge denied the hospital’s request to dismiss the lawsuit and allowed the case to proceed.
In their verdict, jurors awarded Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
Dr. Denman remains employed by the medical group and must continue the conditions of her 5-year monitoring contract, Ms. DeLaney said. She hopes Dr. Denman’s case raises awareness about physicians’ due process rights.
“We hope that Dr. Denman’s case emboldens physicians to stand up for themselves in the face of false accusations and rushes to judgment,” she said. “We hope the verdict leads to fair, prompt, and unbiased investigations by hospital and medical practice administrators, which include due process for accused physicians.”
Cisplatin-gemcitabine is highly active in BRCA/PALB2+ pancreatic cancer
SAN FRANCISCO – The combination of cisplatin and gemcitabine is highly efficacious as first-line therapy for advanced pancreatic cancer in patients with a germline BRCA1/2 or PALB2 mutation, with no additional benefit seen from concurrent veliparib, a phase 2 randomized controlled trial finds.
Up to 9% of pancreatic ductal adenocarcinomas occur in individuals with one of these germline mutations, which render cells more sensitive to platinum agents and PARP inhibitors because of ineffective DNA repair.
In the trial, nearly two-thirds of patients had a response to cisplatin-gemcitabine alone, according to results reported at the 2020 GI Cancers Symposium and simultaneously published (J Clin Oncol. 2020 Jan 24. doi: 10.1200/JCO.19.02931). Although almost three-quarters had a response when the investigational PARP 1/2 inhibitor veliparib was added, the difference was not statistically significant and toxicity was greater.
“Both arms were very active and substantially exceeded the prespecified thresholds,” said lead investigator Eileen Mary O’Reilly, MD, section head of Hepatopancreaticobilary & Neuroendocrine Cancers at Memorial Sloan Kettering Cancer Center in New York.
“The doublet is our recommendation for moving forward, and we believe these data define a reference regimen for germline BRCA-mutated or PALB2-mutated pancreas cancer,” she stated, noting that the PARP inhibitor olaparib (Lynparza) conferred benefit when used as maintenance therapy in the POLO trial. “I think the strategy that at the current time is best supported by the totality of the data is platinum-based therapy upfront and then sequential use of the PARP inhibitor as a maintenance approach.”
The trial was designed in 2012, before efficacy of the FOLFIRINOX regimen was well established, so an obvious question is which chemotherapy to use, Dr. O’Reilly acknowledged.
“I would say these are the only prospective randomized data we have with platinum-based therapy in germline-mutated pancreas cancer. So it comes with a high level of evidence,” she maintained. “Nonetheless, I think FOLFIRINOX is also an option. The options are good, and it probably will depend on the patient in front of you because there certainly are some lack of toxicity advantages, for the most part, to cisplatin and gemcitabine. This was a well-tolerated regimen.”
Implications for practice
“I wouldn’t say this is a negative study. It’s negative with respect to the veliparib question, but otherwise, it’s a very exciting study,” Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, said in an interview. “Seeing response rates of 60% and 70% in patients with metastatic pancreatic cancer is kind of unprecedented, and that’s quite remarkable.”
The reason for the lack of additional benefit from veliparib is unclear, he said. But it may be related to the specific PARP inhibitor or to the smaller sample size or very high response rate seen with chemotherapy alone, which possibly precluded detection of a significant difference.
When choosing between chemotherapy regimens, evidence favors the doublet, according to Dr. Schilsky. “FOLFIRINOX doesn’t have a 70% response rate; it may be 35%. If it was my family member, I would say, get gemcitabine-platinum if you have a BRCA mutation.”
“I think the real take-home message, quite honestly, for most oncologists is that patients with pancreatic cancer should be tested for germline BRCA mutations,” he recommended. “It can not only help inform the therapy choice, but of course, some of these cancers are going to run in families because these are germline mutations and they are going to reveal families that have a high risk of not only pancreatic cancer, but breast, ovarian, and all the other BRCA-associated cancers.”
Study details
The trial was conducted among 52 patients with untreated locally advanced or metastatic pancreatic ductal adenocarcinoma. Fully 94% had a BRCA mutation, while the rest had a PALB2 mutation, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The response rate – the trial’s primary endpoint – was 65.2% with cisplatin-gemcitabine alone and 74.1% with cisplatin-gemcitabine plus veliparib (P = .55), with both arms far exceeding not only the 10% prespecified as “acceptable,” but also the 30% prespecified as “promising.” The respective disease control rates were 78.3% and 100% (P = .02).
Median progression-free survival was 9.7 months with chemotherapy alone and 10.1 months with chemotherapy plus the PARP inhibitor (P = .73). “If you reference an unselected population, that would be about 5 to 7 months,” Dr. O’Reilly noted. Median overall survival was 16.4 months and 15.5 months, respectively (P = .6). For the entire trial cohort, the 2-year overall survival rate was 30.6% and the 3-year overall survival rate was 17.8%.
“The combination came at the expense of hematologic toxicity,” Dr. O’Reilly said, with patients given veliparib having higher rates of grade 3 or 4 neutropenia (48% vs. 30%), thrombocytopenia (55% vs. 9%), and anemia (52% vs. 35%).
In an exploratory analysis among all patients receiving 4 or more months of chemotherapy and then continuing on or starting a PARP inhibitor as their next therapy, without disease progression, median overall survival was 23.4 months, very similar to what was seen in the POLO trial, she noted.
Dr. O’Reilly disclosed that she has ties with Vesselon, Polaris, and CytomX, and that her institution receives grant/research support from Celgene, Sanofi, Genentech-Roche, AstraZeneca, BMS, Silenseed, MabVax, Halozyme, and Acta Biologica. The trial was funded by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Cancer Institute’s Cancer Therapy Evaluation Program, and NCI grants. Dr. Schilsky disclosed that he has no conflicts of interest.
SOURCE: O’Reilly EM et al. 2020 GI Cancers Symposium. Abstract 639.
SAN FRANCISCO – The combination of cisplatin and gemcitabine is highly efficacious as first-line therapy for advanced pancreatic cancer in patients with a germline BRCA1/2 or PALB2 mutation, with no additional benefit seen from concurrent veliparib, a phase 2 randomized controlled trial finds.
Up to 9% of pancreatic ductal adenocarcinomas occur in individuals with one of these germline mutations, which render cells more sensitive to platinum agents and PARP inhibitors because of ineffective DNA repair.
In the trial, nearly two-thirds of patients had a response to cisplatin-gemcitabine alone, according to results reported at the 2020 GI Cancers Symposium and simultaneously published (J Clin Oncol. 2020 Jan 24. doi: 10.1200/JCO.19.02931). Although almost three-quarters had a response when the investigational PARP 1/2 inhibitor veliparib was added, the difference was not statistically significant and toxicity was greater.
“Both arms were very active and substantially exceeded the prespecified thresholds,” said lead investigator Eileen Mary O’Reilly, MD, section head of Hepatopancreaticobilary & Neuroendocrine Cancers at Memorial Sloan Kettering Cancer Center in New York.
“The doublet is our recommendation for moving forward, and we believe these data define a reference regimen for germline BRCA-mutated or PALB2-mutated pancreas cancer,” she stated, noting that the PARP inhibitor olaparib (Lynparza) conferred benefit when used as maintenance therapy in the POLO trial. “I think the strategy that at the current time is best supported by the totality of the data is platinum-based therapy upfront and then sequential use of the PARP inhibitor as a maintenance approach.”
The trial was designed in 2012, before efficacy of the FOLFIRINOX regimen was well established, so an obvious question is which chemotherapy to use, Dr. O’Reilly acknowledged.
“I would say these are the only prospective randomized data we have with platinum-based therapy in germline-mutated pancreas cancer. So it comes with a high level of evidence,” she maintained. “Nonetheless, I think FOLFIRINOX is also an option. The options are good, and it probably will depend on the patient in front of you because there certainly are some lack of toxicity advantages, for the most part, to cisplatin and gemcitabine. This was a well-tolerated regimen.”
Implications for practice
“I wouldn’t say this is a negative study. It’s negative with respect to the veliparib question, but otherwise, it’s a very exciting study,” Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, said in an interview. “Seeing response rates of 60% and 70% in patients with metastatic pancreatic cancer is kind of unprecedented, and that’s quite remarkable.”
The reason for the lack of additional benefit from veliparib is unclear, he said. But it may be related to the specific PARP inhibitor or to the smaller sample size or very high response rate seen with chemotherapy alone, which possibly precluded detection of a significant difference.
When choosing between chemotherapy regimens, evidence favors the doublet, according to Dr. Schilsky. “FOLFIRINOX doesn’t have a 70% response rate; it may be 35%. If it was my family member, I would say, get gemcitabine-platinum if you have a BRCA mutation.”
“I think the real take-home message, quite honestly, for most oncologists is that patients with pancreatic cancer should be tested for germline BRCA mutations,” he recommended. “It can not only help inform the therapy choice, but of course, some of these cancers are going to run in families because these are germline mutations and they are going to reveal families that have a high risk of not only pancreatic cancer, but breast, ovarian, and all the other BRCA-associated cancers.”
Study details
The trial was conducted among 52 patients with untreated locally advanced or metastatic pancreatic ductal adenocarcinoma. Fully 94% had a BRCA mutation, while the rest had a PALB2 mutation, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The response rate – the trial’s primary endpoint – was 65.2% with cisplatin-gemcitabine alone and 74.1% with cisplatin-gemcitabine plus veliparib (P = .55), with both arms far exceeding not only the 10% prespecified as “acceptable,” but also the 30% prespecified as “promising.” The respective disease control rates were 78.3% and 100% (P = .02).
Median progression-free survival was 9.7 months with chemotherapy alone and 10.1 months with chemotherapy plus the PARP inhibitor (P = .73). “If you reference an unselected population, that would be about 5 to 7 months,” Dr. O’Reilly noted. Median overall survival was 16.4 months and 15.5 months, respectively (P = .6). For the entire trial cohort, the 2-year overall survival rate was 30.6% and the 3-year overall survival rate was 17.8%.
“The combination came at the expense of hematologic toxicity,” Dr. O’Reilly said, with patients given veliparib having higher rates of grade 3 or 4 neutropenia (48% vs. 30%), thrombocytopenia (55% vs. 9%), and anemia (52% vs. 35%).
In an exploratory analysis among all patients receiving 4 or more months of chemotherapy and then continuing on or starting a PARP inhibitor as their next therapy, without disease progression, median overall survival was 23.4 months, very similar to what was seen in the POLO trial, she noted.
Dr. O’Reilly disclosed that she has ties with Vesselon, Polaris, and CytomX, and that her institution receives grant/research support from Celgene, Sanofi, Genentech-Roche, AstraZeneca, BMS, Silenseed, MabVax, Halozyme, and Acta Biologica. The trial was funded by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Cancer Institute’s Cancer Therapy Evaluation Program, and NCI grants. Dr. Schilsky disclosed that he has no conflicts of interest.
SOURCE: O’Reilly EM et al. 2020 GI Cancers Symposium. Abstract 639.
SAN FRANCISCO – The combination of cisplatin and gemcitabine is highly efficacious as first-line therapy for advanced pancreatic cancer in patients with a germline BRCA1/2 or PALB2 mutation, with no additional benefit seen from concurrent veliparib, a phase 2 randomized controlled trial finds.
Up to 9% of pancreatic ductal adenocarcinomas occur in individuals with one of these germline mutations, which render cells more sensitive to platinum agents and PARP inhibitors because of ineffective DNA repair.
In the trial, nearly two-thirds of patients had a response to cisplatin-gemcitabine alone, according to results reported at the 2020 GI Cancers Symposium and simultaneously published (J Clin Oncol. 2020 Jan 24. doi: 10.1200/JCO.19.02931). Although almost three-quarters had a response when the investigational PARP 1/2 inhibitor veliparib was added, the difference was not statistically significant and toxicity was greater.
“Both arms were very active and substantially exceeded the prespecified thresholds,” said lead investigator Eileen Mary O’Reilly, MD, section head of Hepatopancreaticobilary & Neuroendocrine Cancers at Memorial Sloan Kettering Cancer Center in New York.
“The doublet is our recommendation for moving forward, and we believe these data define a reference regimen for germline BRCA-mutated or PALB2-mutated pancreas cancer,” she stated, noting that the PARP inhibitor olaparib (Lynparza) conferred benefit when used as maintenance therapy in the POLO trial. “I think the strategy that at the current time is best supported by the totality of the data is platinum-based therapy upfront and then sequential use of the PARP inhibitor as a maintenance approach.”
The trial was designed in 2012, before efficacy of the FOLFIRINOX regimen was well established, so an obvious question is which chemotherapy to use, Dr. O’Reilly acknowledged.
“I would say these are the only prospective randomized data we have with platinum-based therapy in germline-mutated pancreas cancer. So it comes with a high level of evidence,” she maintained. “Nonetheless, I think FOLFIRINOX is also an option. The options are good, and it probably will depend on the patient in front of you because there certainly are some lack of toxicity advantages, for the most part, to cisplatin and gemcitabine. This was a well-tolerated regimen.”
Implications for practice
“I wouldn’t say this is a negative study. It’s negative with respect to the veliparib question, but otherwise, it’s a very exciting study,” Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, said in an interview. “Seeing response rates of 60% and 70% in patients with metastatic pancreatic cancer is kind of unprecedented, and that’s quite remarkable.”
The reason for the lack of additional benefit from veliparib is unclear, he said. But it may be related to the specific PARP inhibitor or to the smaller sample size or very high response rate seen with chemotherapy alone, which possibly precluded detection of a significant difference.
When choosing between chemotherapy regimens, evidence favors the doublet, according to Dr. Schilsky. “FOLFIRINOX doesn’t have a 70% response rate; it may be 35%. If it was my family member, I would say, get gemcitabine-platinum if you have a BRCA mutation.”
“I think the real take-home message, quite honestly, for most oncologists is that patients with pancreatic cancer should be tested for germline BRCA mutations,” he recommended. “It can not only help inform the therapy choice, but of course, some of these cancers are going to run in families because these are germline mutations and they are going to reveal families that have a high risk of not only pancreatic cancer, but breast, ovarian, and all the other BRCA-associated cancers.”
Study details
The trial was conducted among 52 patients with untreated locally advanced or metastatic pancreatic ductal adenocarcinoma. Fully 94% had a BRCA mutation, while the rest had a PALB2 mutation, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
The response rate – the trial’s primary endpoint – was 65.2% with cisplatin-gemcitabine alone and 74.1% with cisplatin-gemcitabine plus veliparib (P = .55), with both arms far exceeding not only the 10% prespecified as “acceptable,” but also the 30% prespecified as “promising.” The respective disease control rates were 78.3% and 100% (P = .02).
Median progression-free survival was 9.7 months with chemotherapy alone and 10.1 months with chemotherapy plus the PARP inhibitor (P = .73). “If you reference an unselected population, that would be about 5 to 7 months,” Dr. O’Reilly noted. Median overall survival was 16.4 months and 15.5 months, respectively (P = .6). For the entire trial cohort, the 2-year overall survival rate was 30.6% and the 3-year overall survival rate was 17.8%.
“The combination came at the expense of hematologic toxicity,” Dr. O’Reilly said, with patients given veliparib having higher rates of grade 3 or 4 neutropenia (48% vs. 30%), thrombocytopenia (55% vs. 9%), and anemia (52% vs. 35%).
In an exploratory analysis among all patients receiving 4 or more months of chemotherapy and then continuing on or starting a PARP inhibitor as their next therapy, without disease progression, median overall survival was 23.4 months, very similar to what was seen in the POLO trial, she noted.
Dr. O’Reilly disclosed that she has ties with Vesselon, Polaris, and CytomX, and that her institution receives grant/research support from Celgene, Sanofi, Genentech-Roche, AstraZeneca, BMS, Silenseed, MabVax, Halozyme, and Acta Biologica. The trial was funded by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Cancer Institute’s Cancer Therapy Evaluation Program, and NCI grants. Dr. Schilsky disclosed that he has no conflicts of interest.
SOURCE: O’Reilly EM et al. 2020 GI Cancers Symposium. Abstract 639.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Barbers have role in encouraging diabetes screening in black men
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Barbershops could offer a way to encourage diabetes screening among black men.
Major finding: HbA1c testing in barbershops identified a significant number of individuals with undiagnosed diabetes.
Study details: Study involving 895 black men attending eight barbershops in Brooklyn.
Disclosures: The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
Source: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Sociodemographic disadvantage confers poorer survival in young adults with CRC
SAN FRANCISCO – Young adults with colorectal cancer who live in neighborhoods with higher levels of disadvantage differ on health measures, present with more advanced disease, and have poorer survival. These were among key findings of a retrospective cohort study reported at the 2020 GI Cancers Symposium.
The incidence of colorectal cancer has risen sharply – 51% – since 1994 among individuals aged younger than age 50 years, with the greatest uptick seen among those aged 20-29 years (J Natl Cancer Inst. 2017;109[8]. doi: 10.1093/jnci/djw322).
“Sociodemographic disparities have been linked to inferior survival. However, their impact and association with outcome in young adults is not well described,” said lead investigator Ashley Matusz-Fisher, MD, of the Levine Cancer Institute in Charlotte, N.C.
The investigators analyzed data from the National Cancer Database for the years 2004-2016, identifying 26,768 patients who received a colorectal cancer diagnosis when aged 18-40 years.
Results showed that those living in areas with low income (less than $38,000 annually) and low educational attainment (high school graduation rate less than 79%), and those living in urban or rural areas (versus metropolitan areas) had 24% and 10% higher risks of death, respectively.
Patients in the low-income, low-education group were more than six times as likely to be black and to lack private health insurance, had greater comorbidity, had larger tumors and more nodal involvement at diagnosis, and were less likely to undergo surgery.
Several factors may be at play for the low-income, low-education group, Dr. Matusz-Fisher speculated: limited access to care, lack of awareness of important symptoms, and inability to afford treatment when it is needed. “That could very well be contributing to them presenting at later stages and then maybe not getting the treatment that other people who have insurance would be getting.
“To try to eliminate these disparities, the first step is recognition, which is what we are doing – recognizing there are disparities – and then making people aware of these disparities,” she commented. “More efforts are needed to increase access and remove barriers to care, with the hope of eliminating disparities and achieving health equity.”
Mitigating disparities
Several studies have looked at mitigating sociodemographic-related disparities in colorectal cancer outcomes, according to session cochair John M. Carethers, MD, AGAF, professor and chair of the department of internal medicine at the University of Michigan, Ann Arbor.
A large Delaware initiative tackled the problem via screening (J Clin Oncol. 2013;31:1928-30). “Now this was over 50 – we don’t typically screen under 50 – but over 50, you can essentially eliminate this disparity with navigation services and screening. How do you do that under 50? I’m not quite sure,” he said in an interview, adding that some organizations are recommending lowering the screening age to 45 or even 40 years in light of rising incidence among young adults.
However, accumulating evidence suggests that there may be inherent biological differences that are harder to overcome. “There is a lot of data … showing that polyps happen earlier and they are bigger in certain racial groups, particularly African Americans and American Indians,” Dr. Carethers elaborated. What is driving the biology is unknown, but the microbiome has come under scrutiny.
“So you are a victim of your circumstances,” he summarized. “You are living in a low-income area, you are eating more proinflammatory-type foods, you are getting your polyps earlier, and then you are getting your cancers earlier.”
Study details
Rural, urban, or metropolitan status was ascertained for 25,861 patients in the study, and area income and education were ascertained for 7,743 patients, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Compared with counterparts living in areas with both high annual income (greater than $68,000) and education (greater than 93% high school graduation rate), patients living in areas with both low annual income (less than $38,000) and education ( less than 79% high school graduation rate) were significantly more likely to be black (odds ratio, 6.4), not have private insurance (odds ratio, 6.3), have pathologic T3/T4 stage (OR, 1.4), have positive nodes (OR, 1.2), and have a Charlson-Deyo comorbidity score of 1 or greater (OR, 1.6). They also were less likely to undergo surgery (OR, 0.63) and more likely to be rehospitalized within 30 days (OR, 1.3).
After adjusting for race, insurance status, T/N stage, and comorbidity score, relative to counterparts in the high-income, high-education group, patients in the low-income, low-education group had an increased risk of death (hazard ratio, 1.24; P = .004). And relative to counterparts living in metropolitan areas, patients living in urban or rural areas had an increased risk of death (HR, 1.10; P = .02).
Among patients with stage IV disease, median overall survival was 26.1 months for those from high-income, high-education areas, but 20.7 months for those from low-income, low-education areas (P less than .001).
Dr. Matusz-Fisher did not report any conflicts of interest. The study did not receive any funding.
SOURCE: Matusz-Fisher A et al. 2020 GI Cancers Symposium, Abstract 13.
SAN FRANCISCO – Young adults with colorectal cancer who live in neighborhoods with higher levels of disadvantage differ on health measures, present with more advanced disease, and have poorer survival. These were among key findings of a retrospective cohort study reported at the 2020 GI Cancers Symposium.
The incidence of colorectal cancer has risen sharply – 51% – since 1994 among individuals aged younger than age 50 years, with the greatest uptick seen among those aged 20-29 years (J Natl Cancer Inst. 2017;109[8]. doi: 10.1093/jnci/djw322).
“Sociodemographic disparities have been linked to inferior survival. However, their impact and association with outcome in young adults is not well described,” said lead investigator Ashley Matusz-Fisher, MD, of the Levine Cancer Institute in Charlotte, N.C.
The investigators analyzed data from the National Cancer Database for the years 2004-2016, identifying 26,768 patients who received a colorectal cancer diagnosis when aged 18-40 years.
Results showed that those living in areas with low income (less than $38,000 annually) and low educational attainment (high school graduation rate less than 79%), and those living in urban or rural areas (versus metropolitan areas) had 24% and 10% higher risks of death, respectively.
Patients in the low-income, low-education group were more than six times as likely to be black and to lack private health insurance, had greater comorbidity, had larger tumors and more nodal involvement at diagnosis, and were less likely to undergo surgery.
Several factors may be at play for the low-income, low-education group, Dr. Matusz-Fisher speculated: limited access to care, lack of awareness of important symptoms, and inability to afford treatment when it is needed. “That could very well be contributing to them presenting at later stages and then maybe not getting the treatment that other people who have insurance would be getting.
“To try to eliminate these disparities, the first step is recognition, which is what we are doing – recognizing there are disparities – and then making people aware of these disparities,” she commented. “More efforts are needed to increase access and remove barriers to care, with the hope of eliminating disparities and achieving health equity.”
Mitigating disparities
Several studies have looked at mitigating sociodemographic-related disparities in colorectal cancer outcomes, according to session cochair John M. Carethers, MD, AGAF, professor and chair of the department of internal medicine at the University of Michigan, Ann Arbor.
A large Delaware initiative tackled the problem via screening (J Clin Oncol. 2013;31:1928-30). “Now this was over 50 – we don’t typically screen under 50 – but over 50, you can essentially eliminate this disparity with navigation services and screening. How do you do that under 50? I’m not quite sure,” he said in an interview, adding that some organizations are recommending lowering the screening age to 45 or even 40 years in light of rising incidence among young adults.
However, accumulating evidence suggests that there may be inherent biological differences that are harder to overcome. “There is a lot of data … showing that polyps happen earlier and they are bigger in certain racial groups, particularly African Americans and American Indians,” Dr. Carethers elaborated. What is driving the biology is unknown, but the microbiome has come under scrutiny.
“So you are a victim of your circumstances,” he summarized. “You are living in a low-income area, you are eating more proinflammatory-type foods, you are getting your polyps earlier, and then you are getting your cancers earlier.”
Study details
Rural, urban, or metropolitan status was ascertained for 25,861 patients in the study, and area income and education were ascertained for 7,743 patients, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Compared with counterparts living in areas with both high annual income (greater than $68,000) and education (greater than 93% high school graduation rate), patients living in areas with both low annual income (less than $38,000) and education ( less than 79% high school graduation rate) were significantly more likely to be black (odds ratio, 6.4), not have private insurance (odds ratio, 6.3), have pathologic T3/T4 stage (OR, 1.4), have positive nodes (OR, 1.2), and have a Charlson-Deyo comorbidity score of 1 or greater (OR, 1.6). They also were less likely to undergo surgery (OR, 0.63) and more likely to be rehospitalized within 30 days (OR, 1.3).
After adjusting for race, insurance status, T/N stage, and comorbidity score, relative to counterparts in the high-income, high-education group, patients in the low-income, low-education group had an increased risk of death (hazard ratio, 1.24; P = .004). And relative to counterparts living in metropolitan areas, patients living in urban or rural areas had an increased risk of death (HR, 1.10; P = .02).
Among patients with stage IV disease, median overall survival was 26.1 months for those from high-income, high-education areas, but 20.7 months for those from low-income, low-education areas (P less than .001).
Dr. Matusz-Fisher did not report any conflicts of interest. The study did not receive any funding.
SOURCE: Matusz-Fisher A et al. 2020 GI Cancers Symposium, Abstract 13.
SAN FRANCISCO – Young adults with colorectal cancer who live in neighborhoods with higher levels of disadvantage differ on health measures, present with more advanced disease, and have poorer survival. These were among key findings of a retrospective cohort study reported at the 2020 GI Cancers Symposium.
The incidence of colorectal cancer has risen sharply – 51% – since 1994 among individuals aged younger than age 50 years, with the greatest uptick seen among those aged 20-29 years (J Natl Cancer Inst. 2017;109[8]. doi: 10.1093/jnci/djw322).
“Sociodemographic disparities have been linked to inferior survival. However, their impact and association with outcome in young adults is not well described,” said lead investigator Ashley Matusz-Fisher, MD, of the Levine Cancer Institute in Charlotte, N.C.
The investigators analyzed data from the National Cancer Database for the years 2004-2016, identifying 26,768 patients who received a colorectal cancer diagnosis when aged 18-40 years.
Results showed that those living in areas with low income (less than $38,000 annually) and low educational attainment (high school graduation rate less than 79%), and those living in urban or rural areas (versus metropolitan areas) had 24% and 10% higher risks of death, respectively.
Patients in the low-income, low-education group were more than six times as likely to be black and to lack private health insurance, had greater comorbidity, had larger tumors and more nodal involvement at diagnosis, and were less likely to undergo surgery.
Several factors may be at play for the low-income, low-education group, Dr. Matusz-Fisher speculated: limited access to care, lack of awareness of important symptoms, and inability to afford treatment when it is needed. “That could very well be contributing to them presenting at later stages and then maybe not getting the treatment that other people who have insurance would be getting.
“To try to eliminate these disparities, the first step is recognition, which is what we are doing – recognizing there are disparities – and then making people aware of these disparities,” she commented. “More efforts are needed to increase access and remove barriers to care, with the hope of eliminating disparities and achieving health equity.”
Mitigating disparities
Several studies have looked at mitigating sociodemographic-related disparities in colorectal cancer outcomes, according to session cochair John M. Carethers, MD, AGAF, professor and chair of the department of internal medicine at the University of Michigan, Ann Arbor.
A large Delaware initiative tackled the problem via screening (J Clin Oncol. 2013;31:1928-30). “Now this was over 50 – we don’t typically screen under 50 – but over 50, you can essentially eliminate this disparity with navigation services and screening. How do you do that under 50? I’m not quite sure,” he said in an interview, adding that some organizations are recommending lowering the screening age to 45 or even 40 years in light of rising incidence among young adults.
However, accumulating evidence suggests that there may be inherent biological differences that are harder to overcome. “There is a lot of data … showing that polyps happen earlier and they are bigger in certain racial groups, particularly African Americans and American Indians,” Dr. Carethers elaborated. What is driving the biology is unknown, but the microbiome has come under scrutiny.
“So you are a victim of your circumstances,” he summarized. “You are living in a low-income area, you are eating more proinflammatory-type foods, you are getting your polyps earlier, and then you are getting your cancers earlier.”
Study details
Rural, urban, or metropolitan status was ascertained for 25,861 patients in the study, and area income and education were ascertained for 7,743 patients, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Compared with counterparts living in areas with both high annual income (greater than $68,000) and education (greater than 93% high school graduation rate), patients living in areas with both low annual income (less than $38,000) and education ( less than 79% high school graduation rate) were significantly more likely to be black (odds ratio, 6.4), not have private insurance (odds ratio, 6.3), have pathologic T3/T4 stage (OR, 1.4), have positive nodes (OR, 1.2), and have a Charlson-Deyo comorbidity score of 1 or greater (OR, 1.6). They also were less likely to undergo surgery (OR, 0.63) and more likely to be rehospitalized within 30 days (OR, 1.3).
After adjusting for race, insurance status, T/N stage, and comorbidity score, relative to counterparts in the high-income, high-education group, patients in the low-income, low-education group had an increased risk of death (hazard ratio, 1.24; P = .004). And relative to counterparts living in metropolitan areas, patients living in urban or rural areas had an increased risk of death (HR, 1.10; P = .02).
Among patients with stage IV disease, median overall survival was 26.1 months for those from high-income, high-education areas, but 20.7 months for those from low-income, low-education areas (P less than .001).
Dr. Matusz-Fisher did not report any conflicts of interest. The study did not receive any funding.
SOURCE: Matusz-Fisher A et al. 2020 GI Cancers Symposium, Abstract 13.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Subungual Hemorrhage From an Epidermal Growth Factor Receptor Inhibitor
To the Editor:
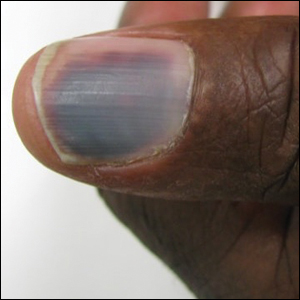
The epidermal growth factor receptor (EGFR) signaling pathway plays a role in the differentiation, proliferation, and survival of several cell types.1 Erlotinib is an EGFR inhibitor that targets aberrant cells that overexpress this receptor and has been used in the treatment of various solid malignant tumors.2,3 Common dermatologic side effects associated with EGFR inhibitors include papulopustular rash, xeroderma, and paronychia.2,3 We present a unique finding of subungual hemorrhage of the thumbnails in a patient taking erlotinib.
A 50-year-old man presented with acute-onset tenderness and discoloration of the thumbnails of 1 week’s duration. There was no preceding trauma or history of similar symptoms. His medical history was notable for recurrent lung adenocarcinoma with EGFR L858R mutation. Erlotinib therapy was initiated 5 weeks prior to symptom onset. He developed notable xeroderma of the palms and soles that preceded nail changes by a few days. He completed treatment with carboplatin and pemetrexed 16 months prior to relapse after paclitaxel failed due to a severe allergic reaction. There were no nail symptoms during that time. The patient did not have a documented coagulation disorder and was not on any known medications that would predispose him to bleeding. Physical examination demonstrated subungual hemorrhage of the thumbnails with tenderness on palpation (Figure). There was no evidence of periungual changes or nail plate abnormality. All other nails appeared normal. Laboratory test results showed normal platelets. Supportive therapeutic measures were recommended, and the patient was advised to avoid trauma to the nails.
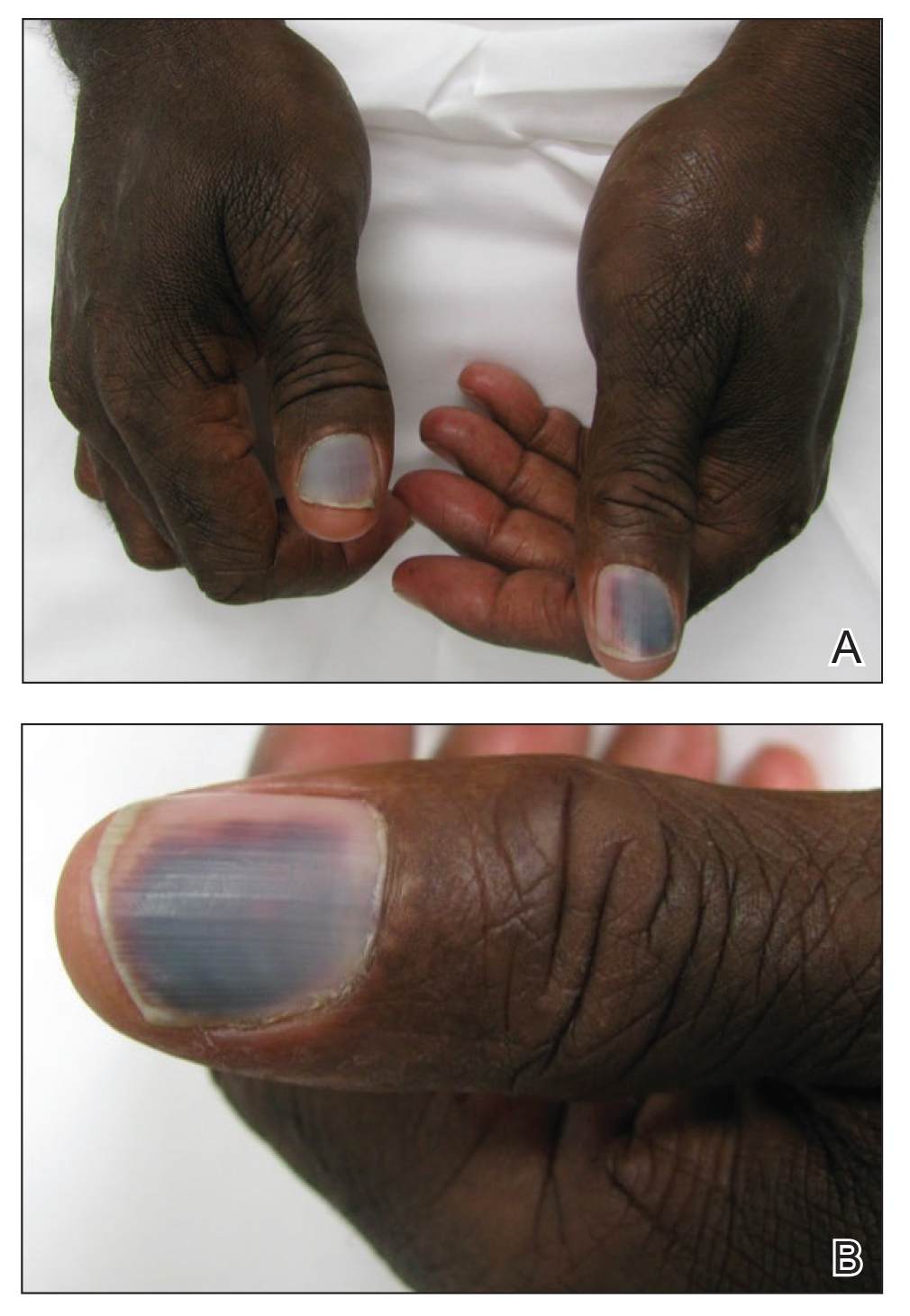
Nail toxicities reported with EGFR inhibitors include paronychia, periungual pyogenic granulomas, and ingrown nails.1-3 Inflammation of the nail bed also can lead to secondary nail changes, such as onychodystrophy or onycholysis.2 Subungual hemorrhage has been reported as a side effect of taxanes, anticoagulants, anthracyclines, anti-inflammatory agents, and retinoids.4,5
The pathogenesis of nail toxicity secondary to EGFR inhibitors is not entirely clear. Symptoms commonly occur several weeks to months after therapy initiation.6 Epidermal growth factor receptor inhibitors disrupt proliferation and promote apoptosis of keratinocytes that is thought to enhance fragility of the periungual skin and nail plate.1,3 Under the influence of EGFR inhibition, a proinflammatory microenvironment in the skin is created through a type I interferon response leading to tissue damage.7 These changes may predispose patients to develop subungual hemorrhage in response to repeated nail microtrauma. Subungual asymptomatic splinter hemorrhage is a nail finding described in patients treated with the multikinase inhibitors sorafenib and sunitinib. Splinter hemorrhages of the nails are thought to be secondary to capillary microinjuries of the digits that cannot be repaired due to inhibition of vascular EGFRs.4
The time course of erlotinib administration and the simultaneous onset of xeroderma, a known side effect of the drug, in our patient are consistent with other cases.6 Subungual hemorrhage, which the patient reported observing only days after the onset of xeroderma, provides increased support that the anti-EGFR medication was likely responsible for both side effects concurrently. Bilateral involvement of the thumbs makes trauma as an inciting event unlikely.
Incidence of nail changes secondary to anti-EGFR drugs are likely underestimated and underreported.3 Subungual hemorrhage should be considered as an additional, less common nail side effect of EGFR inhibitors that clinicians and patients may encounter. Improved awareness and understanding of nail toxicities associated with EGFR inhibitors may offer better insight into the pathogenesis of these side effects and management options.
- Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-626.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463-472.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;333-337.
- Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-408.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-465.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340-342.
- Lulli D, Carbone ML, Pastore S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget. 2016;7:47777-47793.
To the Editor:
The epidermal growth factor receptor (EGFR) signaling pathway plays a role in the differentiation, proliferation, and survival of several cell types.1 Erlotinib is an EGFR inhibitor that targets aberrant cells that overexpress this receptor and has been used in the treatment of various solid malignant tumors.2,3 Common dermatologic side effects associated with EGFR inhibitors include papulopustular rash, xeroderma, and paronychia.2,3 We present a unique finding of subungual hemorrhage of the thumbnails in a patient taking erlotinib.
A 50-year-old man presented with acute-onset tenderness and discoloration of the thumbnails of 1 week’s duration. There was no preceding trauma or history of similar symptoms. His medical history was notable for recurrent lung adenocarcinoma with EGFR L858R mutation. Erlotinib therapy was initiated 5 weeks prior to symptom onset. He developed notable xeroderma of the palms and soles that preceded nail changes by a few days. He completed treatment with carboplatin and pemetrexed 16 months prior to relapse after paclitaxel failed due to a severe allergic reaction. There were no nail symptoms during that time. The patient did not have a documented coagulation disorder and was not on any known medications that would predispose him to bleeding. Physical examination demonstrated subungual hemorrhage of the thumbnails with tenderness on palpation (Figure). There was no evidence of periungual changes or nail plate abnormality. All other nails appeared normal. Laboratory test results showed normal platelets. Supportive therapeutic measures were recommended, and the patient was advised to avoid trauma to the nails.

Nail toxicities reported with EGFR inhibitors include paronychia, periungual pyogenic granulomas, and ingrown nails.1-3 Inflammation of the nail bed also can lead to secondary nail changes, such as onychodystrophy or onycholysis.2 Subungual hemorrhage has been reported as a side effect of taxanes, anticoagulants, anthracyclines, anti-inflammatory agents, and retinoids.4,5
The pathogenesis of nail toxicity secondary to EGFR inhibitors is not entirely clear. Symptoms commonly occur several weeks to months after therapy initiation.6 Epidermal growth factor receptor inhibitors disrupt proliferation and promote apoptosis of keratinocytes that is thought to enhance fragility of the periungual skin and nail plate.1,3 Under the influence of EGFR inhibition, a proinflammatory microenvironment in the skin is created through a type I interferon response leading to tissue damage.7 These changes may predispose patients to develop subungual hemorrhage in response to repeated nail microtrauma. Subungual asymptomatic splinter hemorrhage is a nail finding described in patients treated with the multikinase inhibitors sorafenib and sunitinib. Splinter hemorrhages of the nails are thought to be secondary to capillary microinjuries of the digits that cannot be repaired due to inhibition of vascular EGFRs.4
The time course of erlotinib administration and the simultaneous onset of xeroderma, a known side effect of the drug, in our patient are consistent with other cases.6 Subungual hemorrhage, which the patient reported observing only days after the onset of xeroderma, provides increased support that the anti-EGFR medication was likely responsible for both side effects concurrently. Bilateral involvement of the thumbs makes trauma as an inciting event unlikely.
Incidence of nail changes secondary to anti-EGFR drugs are likely underestimated and underreported.3 Subungual hemorrhage should be considered as an additional, less common nail side effect of EGFR inhibitors that clinicians and patients may encounter. Improved awareness and understanding of nail toxicities associated with EGFR inhibitors may offer better insight into the pathogenesis of these side effects and management options.
To the Editor:
The epidermal growth factor receptor (EGFR) signaling pathway plays a role in the differentiation, proliferation, and survival of several cell types.1 Erlotinib is an EGFR inhibitor that targets aberrant cells that overexpress this receptor and has been used in the treatment of various solid malignant tumors.2,3 Common dermatologic side effects associated with EGFR inhibitors include papulopustular rash, xeroderma, and paronychia.2,3 We present a unique finding of subungual hemorrhage of the thumbnails in a patient taking erlotinib.
A 50-year-old man presented with acute-onset tenderness and discoloration of the thumbnails of 1 week’s duration. There was no preceding trauma or history of similar symptoms. His medical history was notable for recurrent lung adenocarcinoma with EGFR L858R mutation. Erlotinib therapy was initiated 5 weeks prior to symptom onset. He developed notable xeroderma of the palms and soles that preceded nail changes by a few days. He completed treatment with carboplatin and pemetrexed 16 months prior to relapse after paclitaxel failed due to a severe allergic reaction. There were no nail symptoms during that time. The patient did not have a documented coagulation disorder and was not on any known medications that would predispose him to bleeding. Physical examination demonstrated subungual hemorrhage of the thumbnails with tenderness on palpation (Figure). There was no evidence of periungual changes or nail plate abnormality. All other nails appeared normal. Laboratory test results showed normal platelets. Supportive therapeutic measures were recommended, and the patient was advised to avoid trauma to the nails.

Nail toxicities reported with EGFR inhibitors include paronychia, periungual pyogenic granulomas, and ingrown nails.1-3 Inflammation of the nail bed also can lead to secondary nail changes, such as onychodystrophy or onycholysis.2 Subungual hemorrhage has been reported as a side effect of taxanes, anticoagulants, anthracyclines, anti-inflammatory agents, and retinoids.4,5
The pathogenesis of nail toxicity secondary to EGFR inhibitors is not entirely clear. Symptoms commonly occur several weeks to months after therapy initiation.6 Epidermal growth factor receptor inhibitors disrupt proliferation and promote apoptosis of keratinocytes that is thought to enhance fragility of the periungual skin and nail plate.1,3 Under the influence of EGFR inhibition, a proinflammatory microenvironment in the skin is created through a type I interferon response leading to tissue damage.7 These changes may predispose patients to develop subungual hemorrhage in response to repeated nail microtrauma. Subungual asymptomatic splinter hemorrhage is a nail finding described in patients treated with the multikinase inhibitors sorafenib and sunitinib. Splinter hemorrhages of the nails are thought to be secondary to capillary microinjuries of the digits that cannot be repaired due to inhibition of vascular EGFRs.4
The time course of erlotinib administration and the simultaneous onset of xeroderma, a known side effect of the drug, in our patient are consistent with other cases.6 Subungual hemorrhage, which the patient reported observing only days after the onset of xeroderma, provides increased support that the anti-EGFR medication was likely responsible for both side effects concurrently. Bilateral involvement of the thumbs makes trauma as an inciting event unlikely.
Incidence of nail changes secondary to anti-EGFR drugs are likely underestimated and underreported.3 Subungual hemorrhage should be considered as an additional, less common nail side effect of EGFR inhibitors that clinicians and patients may encounter. Improved awareness and understanding of nail toxicities associated with EGFR inhibitors may offer better insight into the pathogenesis of these side effects and management options.
- Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-626.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463-472.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;333-337.
- Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-408.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-465.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340-342.
- Lulli D, Carbone ML, Pastore S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget. 2016;7:47777-47793.
- Piraccini BM, Alessandrini A. Drug-related nail disease. Clin Dermatol. 2013;31:618-626.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol. 2013;69:463-472.
- Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;333-337.
- Garden BC, Wu S, Lacouture ME. The risk of nail changes with epidermal growth factor receptor inhibitors: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2012;67:400-408.
- Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-465.
- Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with gefitinib, erlotinib or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340-342.
- Lulli D, Carbone ML, Pastore S. Epidermal growth factor receptor inhibitors trigger a type I interferon response in human skin. Oncotarget. 2016;7:47777-47793.
Practice Points
- Subungual hemorrhage is a potential adverse side effect of epidermal growth factor receptor inhibitors.
- Epidermal growth factor receptor inhibition may lead to enhanced fragility of the periungual skin and nail plate as well as a proinflammatory microenvironment in the skin, predisposing patients to nail toxicity.
Clinical Psychiatry News welcomes new board member
Clinical Psychiatry News is pleased to welcome Alice W. Lee, MD, to its editorial advisory board.
Dr. Lee, who works with children, adolescents and adults, specializes in integrative and holistic psychiatry. In her private practice in Gaithersburg, Md., she integrates functional/orthomolecular medicine and mind–body/energy medicine in her work with patients.
In addition, Dr. Lee is a Reiki master who integrates the biochemistry of chemistry with the quantum physics of healing. One of her specialties is helping patients withdraw from their psychiatric medications safely.
Dr. Lee is a member of the Academy of Integrative Health & Medicine, the Association for Comprehensive Energy Psychology, the Integrative Healthcare Symposium, and the Physicians Committee for Responsible Medicine.
Clinical Psychiatry News is pleased to welcome Alice W. Lee, MD, to its editorial advisory board.
Dr. Lee, who works with children, adolescents and adults, specializes in integrative and holistic psychiatry. In her private practice in Gaithersburg, Md., she integrates functional/orthomolecular medicine and mind–body/energy medicine in her work with patients.
In addition, Dr. Lee is a Reiki master who integrates the biochemistry of chemistry with the quantum physics of healing. One of her specialties is helping patients withdraw from their psychiatric medications safely.
Dr. Lee is a member of the Academy of Integrative Health & Medicine, the Association for Comprehensive Energy Psychology, the Integrative Healthcare Symposium, and the Physicians Committee for Responsible Medicine.
Clinical Psychiatry News is pleased to welcome Alice W. Lee, MD, to its editorial advisory board.
Dr. Lee, who works with children, adolescents and adults, specializes in integrative and holistic psychiatry. In her private practice in Gaithersburg, Md., she integrates functional/orthomolecular medicine and mind–body/energy medicine in her work with patients.
In addition, Dr. Lee is a Reiki master who integrates the biochemistry of chemistry with the quantum physics of healing. One of her specialties is helping patients withdraw from their psychiatric medications safely.
Dr. Lee is a member of the Academy of Integrative Health & Medicine, the Association for Comprehensive Energy Psychology, the Integrative Healthcare Symposium, and the Physicians Committee for Responsible Medicine.
Nonuremic Calciphylaxis Triggered by Rapid Weight Loss and Hypotension
Calciphylaxis, otherwise known as calcific uremic arteriolopathy, is characterized by calcification of the tunica media of the small- to medium-sized blood vessels of the dermis and subcutis, leading to ischemia and necrosis.1 It is a deadly disease with a 1-year mortality rate of more than 50%.2 End-stage renal disease (ESRD) is the most common risk factor for calciphylaxis, with a prevalence of 1% to 4% of hemodialysis patients with calciphylaxis in the United States.2-5 However, nonuremic calciphylaxis (NUC) has been increasingly reported in the literature and has risk factors other than ESRD, including but not limited to obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, and underlying malignancy.3,6-9 Triggers for calciphylaxis in at-risk patients include use of corticosteroids or warfarin, iron or albumin infusions, and rapid weight loss.3,6,9-11 We report an unusual case of NUC that most likely was triggered by rapid weight loss and hypotension in a patient with multiple risk factors for calciphylaxis.
Case Report
A 75-year-old white woman with history of morbid obesity (body mass index, 40 kg/m2), unexplained weight loss of 70 lb over the last year, and polymyalgia rheumatica requiring chronic prednisone therapy presented with painful lesions on the thighs, buttocks, and right shoulder of 4 months’ duration. She had multiple hospital admissions preceding the onset of lesions for severe infections resulting in sepsis with hypotension, including Enterococcus faecalis endocarditis, extended-spectrum beta-lactamase bacteremia, and Pseudomonas aeruginosa pneumonia. Physical examination revealed large well-demarcated ulcers and necrotic eschars with surrounding violaceous induration and stellate erythema on the anterior, medial, and posterior thighs and buttocks that were exquisitely tender (Figures 1 and 2).
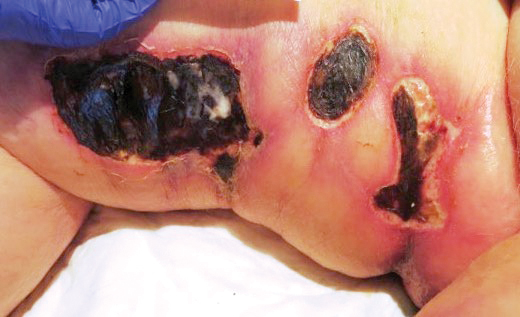

Notable laboratory results included hypoalbuminemia (1.3 g/dL [reference range, 3.5–5.0 g/dL]) with normal renal function, a corrected calcium level of 9.7 mg/dL (reference range, 8.2–10.2 mg/dL), a serum phosphorus level of 3.5 mg/dL (reference range, 2.3–4.7 mg/dL), a calcium-phosphate product of 27.3 mg2/dL2 (reference range, <55 mg2/dL2), and a parathyroid hormone level of 49.3 pg/mL (reference range, 10–65 pg/mL). Antinuclear antibodies were negative. A hypercoagulability evaluation showed normal protein C and S levels, negative lupus anticoagulant, and negative anticardiolipin antibodies.
Telescoping punch biopsies of the indurated borders of the eschars showed prominent calcification of the small- and medium-sized vessels in the mid and deep dermis, intravascular thrombi, and necrosis of the epidermis and subcutaneous fat consistent with calciphylaxis (Figure 3).
After the diagnosis of calciphylaxis was made, the patient was treated with intravenous sodium thiosulfate 25 mg 3 times weekly and alendronate 70 mg weekly. Daily arterial blood gas studies did not detect metabolic acidosis during the patient’s sodium thiosulfate therapy. The wounds were debrided, and we attempted to slowly taper the patient off the oral prednisone. Unfortunately, her condition slowly deteriorated secondary to sepsis, resulting in septic shock. The patient died 3 weeks after the diagnosis of calciphylaxis was made. At the time of diagnosis, the patient had a poor prognosis and notable risk for sepsis due to the large eschars on the thighs and abdomen as well as her relative immunosuppression due to chronic prednisone use.
Comment
Background on Calciphylaxis
Calciphylaxis is a rare but deadly disease that affects both ESRD patients receiving dialysis and patients without ESRD who have known risk factors for calciphylaxis, including female gender, white race, obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, underlying malignancy, protein C or S deficiency, corticosteroid use, warfarin use, diabetes, iron or albumin infusions, and rapid weight loss.3,6-9,11 Although the molecular pathogenesis of calciphylaxis is not completely understood, it is believed to be caused by local deposition of calcium in the tunica media of small- to medium-sized arterioles and venules in the skin.12 This deposition leads to intimal proliferation and progressive narrowing of the vessels with resultant thrombosis, ischemia, and necrosis. The cutaneous manifestations and histopathology of calciphylaxis classically follow its pathogenesis. Calciphylaxis typically presents with livedo reticularis as vessels narrow and then progresses to purpura, bullae, necrosis, and eschar formation with the onset of acute thrombosis and ischemia. Histopathology is characterized by small- and medium-sized vessel calcification and thrombus, dermal necrosis, and septal panniculitis, though the histology can be highly variable.12 Unfortunately, the already poor prognosis for calciphylaxis worsens when lesions become either ulcerative or present on the proximal extremities and trunk.4,13 Sepsis is the leading cause of death in calciphylaxis patients, affecting more than 50% of patients.2,3,14 The differential diagnoses for calciphylactic-appearing lesions include warfarin-induced skin necrosis, disseminated intravascular coagulation, pyoderma gangrenosum, cholesterol emboli, and various vasculitides and coagulopathies.
Risk Factors
Our case demonstrates the importance of risk factor minimization, trigger avoidance, and early intervention due to the high mortality rate of calciphylaxis. Selye et al15 coined the term calciphylaxis in 1961 based on experiments that induced calciphylaxis in rat models. Their research concluded that there were certain sensitizers (ie, risk factors) that predisposed patients to medial calcium deposition in blood vessels and other challengers (ie, triggers) that acted as inciting events to calcium deposition. Our patient presented with multiple known risk factors for calciphylaxis, including obesity (body mass index, 40 kg/m2), female gender, white race, hypoalbuminemia, and chronic corticosteroid use.16 In the presence of a milieu of risk factors, the patient’s rapid weight loss and episodes of hypotension likely were triggers for calciphylaxis.
Other case reports in the literature have suggested weight loss as a trigger for NUC. One morbidly obese patient with inactive rheumatoid arthritis had onset of calciphylaxis lesions after unintentional weight loss of approximately 50% body weight in 1 year17; however, the weight loss does not have to be drastic to trigger calciphylaxis. Another study of 16 patients with uremic calciphylaxis found that 7 of 16 (44%) patients lost 10 to 50 kg in the 6 months prior to calciphylaxis onset.14 One proposed mechanism by Munavalli et al10 is that elevated levels of matrix metalloproteinases during catabolic weight loss states enhance the deposition of calcium into elastic fibers of small vessels. The authors found elevated serum levels of matrix metalloproteinases in their patients with NUC induced by rapid weight loss.10
A meta-analysis by Nigwekar et al3 found a history of prior corticosteroid use in 61% (22/36) of NUC cases reviewed. However, it is unclear whether it is the use of corticosteroids or chronic inflammation that is implicated in NUC pathogenesis. Chronic inflammation causes downregulation of anticalcification signaling pathways.18-20 The role of 2 vascular calcification inhibitors has been evaluated in the pathogenesis of calciphylaxis: fetuin-A and matrix gla protein (MGP).21 The activity of these proteins is decreased not only in calciphylaxis but also in other inflammatory states and chronic renal failure.18-20 One study found lower fetuin-A levels in 312 hemodialysis patients compared to healthy controls and an association between low fetuin-A levels and increased C-reactive protein levels.22 Reduced fetuin-A and MGP levels may be the result of several calciphylaxis risk factors. Warfarin is believed to trigger calciphylaxis via inhibition of gamma-carboxylation of MGP, which is necessary for its anticalcification activity.23 Hypoalbuminemia and alcoholic liver disease also are risk factors that may be explained by the fact that fetuin-A is synthesized in the liver.24 Therefore, liver disease results in decreased production of fetuin-A that is permissive to vascular calcification in calciphylaxis patients.
There have been other reports of calciphylaxis patients who were originally hospitalized due to hypotension, which may serve as a trigger for calciphylaxis onset.25 Because calciphylaxis lesions are more likely to occur in the fatty areas of the abdomen and proximal thighs where blood flow is slower, hypotension likely accentuates the slowing of blood flow and subsequent blood vessel calcification. This theory is supported by studies showing that established calciphylactic lesions worsen more quickly in the presence of systemic hypotension.26 One patient with ESRD and calciphylaxis of the breasts had consistent systolic blood pressure readings in the high 60s to low 70s between dialysis sessions.27 Due to this association, we recommend that patients with calciphylaxis have close blood pressure monitoring to aid in preventing disease progression.28
Management
Calciphylaxis treatment has not yet been standardized, as it is an uncommon disease whose pathogenesis is not fully understood. Current management strategies aim to normalize metabolic abnormalities such as hypercalcemia if they are present and remove inciting agents such as warfarin and corticosteroids.29 Other medical treatments that have been successfully used include sodium thiosulfate, oral steroids, and adjunctive bisphosphonates.29-31 Sodium thiosulfate is known to cause metabolic acidosis by generating thiosulfuric acid in vivo in patients with or without renal disease; therefore, patients on sodium thiosulfate therapy should be monitored for development of metabolic acidosis and treated with oral sodium bicarbonate or dialysis as needed.30,32 Wound care also is an important element of calciphylaxis treatment; however, the debridement of wounds is controversial. Some argue that dry intact eschars serve to protect against sepsis, which is the leading cause of death in calciphylaxis.2,14,33 In contrast, a retrospective study of 63 calciphylaxis patients found a 1-year survival rate of 61.6% in 17 patients receiving wound debridement vs 27.4% in 46 patients who did not.2 The current consensus is that debridement should be considered on a case-by-case basis, factoring in the presence of wound infection, size of wounds, stability of eschars, and treatment goals of the patient.34 Future studies should be aimed at this issue, with special focus on how these factors and the decision to debride or not impact patient outcomes.
Conclusion
Calciphylaxis is a potentially fatal disease that impacts both patients with ESRD and those with nonuremic risk factors. The term calcific uremic arteriolopathy should be disregarded, as nonuremic causes are being reported with increased frequency in the literature. In such cases, patients often have multiple risk factors, including obesity, primary hyperparathyroidism, alcoholic liver disease, and underlying malignancy, among others. Certain triggers for onset of calciphylaxis should be avoided in at-risk patients, including the use of corticosteroids or warfarin; iron and albumin infusions; hypotension; and rapid weight loss. Our fatal case of NUC is a reminder to dermatologists treating at-risk patients to avoid these triggers and to keep calciphylaxis in the differential diagnosis when encountering early lesions such as livedo reticularis, as progression of these lesions has a 1-year mortality rate of more than 50% with the therapies being utilized at this time.
- Au S, Crawford RI. Three-dimensional analysis of a calciphylaxis plaque: clues to pathogenesis. J Am Acad Dermatol. 2007;47:53-57.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Angelis M, Wong LL, Myers SA, et al. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083-1090.
- Chavel SM, Taraszka KS, Schaffer JV, et al. Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis. J Am Acad Dermatol. 2004;50:125-128.
- Bosler DS, Amin MB, Gulli F, et al. Unusual case of calciphylaxis associated with metastatic breast carcinoma. Am J Dermatopathol. 2007;29:400-403.
- Buxtorf K, Cerottini JP, Panizzon RG. Lower limb skin ulcerations, intravascular calcifications and sensorimotor polyneuropathy: calciphylaxis as part of a hyperparathyroidism? Dermatology. 1999;198:423-425.
- Brouns K, Verbeken E, Degreef H, et al. Fatal calciphylaxis in two patients with giant cell arteritis. Clin Rheumatol. 2007;26:836-840.
- Munavalli G, Reisenauer A, Moses M, et al. Weight loss-induced calciphylaxis: potential role of matrix metalloproteinases. J Dermatol. 2003;30:915-919.
- Bae GH, Nambudiri VE, Bach DQ, et al. Rapidly progressive nonuremic calciphylaxis in setting of warfarin. Am J Med. 2015;128:E19-E21.
- Essary LR, Wick MR. Cutaneous calciphylaxis. an underrecognized clinicopathologic entity. Am J Clin Pathol. 2000;113:280-287.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Coates T, Kirkland GS, Dymock RB, et al. Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis. 1998;32:384-391.
- Selye H, Gentile G, Prioreschi P. Cutaneous molt induced by calciphylaxis in the rat. Science. 1961;134:1876-1877.
- Kalajian AH, Malhotra PS, Callen JP, et al. Calciphylaxis with normal renal and parathyroid function: not as rare as previously believed. Arch Dermatol. 2009;145:451-458.
- Malabu U, Roberts L, Sangla K. Calciphylaxis in a morbidly obese woman with rheumatoid arthritis presenting with severe weight loss and vitamin D deficiency. Endocr Pract. 2011;17:104-108.
- Schäfer C, Heiss A, Schwarz A, et al. The serum protein alpha 2–Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Cozzolino M, Galassi A, Biondi ML, et al. Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol. 2006;26:423-429.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. J Am Acad Dermatol. 2008;58:458-471.
- Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827-833.
- Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells a cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764-1767.
- Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3:109-121.
- Allegretti AS, Nazarian RM, Goverman J, et al. Calciphylaxis: a rare but fatal delayed complication of Roux-en-Y gastric bypass surgery. Am J Kidney Dis. 2014;64:274-277.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial. 2002;15:172-186.
- Gupta D, Tadros R, Mazumdar A, et al. Breast lesions with intractable pain in end-stage renal disease: calciphylaxis with chronic hypotensive dermatopathy related watershed breast lesions. J Palliat Med. 2013;16:551-554.
- Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588-597.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Bourgeois P, De Haes P. Sodium thiosulfate as a treatment for calciphylaxis: a case series. J Dermatolog Treat. 2016;27:520-524.
- Biswas A, Walsh NM, Tremaine R. A case of nonuremic calciphylaxis treated effectively with systemic corticosteroids. J Cutan Med Surg. 2016;20:275-278.
- Selk N, Rodby, RA. Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial. 2011;24:85-88.
- Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage. 2004:50:64-66, 68-70.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
Calciphylaxis, otherwise known as calcific uremic arteriolopathy, is characterized by calcification of the tunica media of the small- to medium-sized blood vessels of the dermis and subcutis, leading to ischemia and necrosis.1 It is a deadly disease with a 1-year mortality rate of more than 50%.2 End-stage renal disease (ESRD) is the most common risk factor for calciphylaxis, with a prevalence of 1% to 4% of hemodialysis patients with calciphylaxis in the United States.2-5 However, nonuremic calciphylaxis (NUC) has been increasingly reported in the literature and has risk factors other than ESRD, including but not limited to obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, and underlying malignancy.3,6-9 Triggers for calciphylaxis in at-risk patients include use of corticosteroids or warfarin, iron or albumin infusions, and rapid weight loss.3,6,9-11 We report an unusual case of NUC that most likely was triggered by rapid weight loss and hypotension in a patient with multiple risk factors for calciphylaxis.
Case Report
A 75-year-old white woman with history of morbid obesity (body mass index, 40 kg/m2), unexplained weight loss of 70 lb over the last year, and polymyalgia rheumatica requiring chronic prednisone therapy presented with painful lesions on the thighs, buttocks, and right shoulder of 4 months’ duration. She had multiple hospital admissions preceding the onset of lesions for severe infections resulting in sepsis with hypotension, including Enterococcus faecalis endocarditis, extended-spectrum beta-lactamase bacteremia, and Pseudomonas aeruginosa pneumonia. Physical examination revealed large well-demarcated ulcers and necrotic eschars with surrounding violaceous induration and stellate erythema on the anterior, medial, and posterior thighs and buttocks that were exquisitely tender (Figures 1 and 2).


Notable laboratory results included hypoalbuminemia (1.3 g/dL [reference range, 3.5–5.0 g/dL]) with normal renal function, a corrected calcium level of 9.7 mg/dL (reference range, 8.2–10.2 mg/dL), a serum phosphorus level of 3.5 mg/dL (reference range, 2.3–4.7 mg/dL), a calcium-phosphate product of 27.3 mg2/dL2 (reference range, <55 mg2/dL2), and a parathyroid hormone level of 49.3 pg/mL (reference range, 10–65 pg/mL). Antinuclear antibodies were negative. A hypercoagulability evaluation showed normal protein C and S levels, negative lupus anticoagulant, and negative anticardiolipin antibodies.
Telescoping punch biopsies of the indurated borders of the eschars showed prominent calcification of the small- and medium-sized vessels in the mid and deep dermis, intravascular thrombi, and necrosis of the epidermis and subcutaneous fat consistent with calciphylaxis (Figure 3).

After the diagnosis of calciphylaxis was made, the patient was treated with intravenous sodium thiosulfate 25 mg 3 times weekly and alendronate 70 mg weekly. Daily arterial blood gas studies did not detect metabolic acidosis during the patient’s sodium thiosulfate therapy. The wounds were debrided, and we attempted to slowly taper the patient off the oral prednisone. Unfortunately, her condition slowly deteriorated secondary to sepsis, resulting in septic shock. The patient died 3 weeks after the diagnosis of calciphylaxis was made. At the time of diagnosis, the patient had a poor prognosis and notable risk for sepsis due to the large eschars on the thighs and abdomen as well as her relative immunosuppression due to chronic prednisone use.
Comment
Background on Calciphylaxis
Calciphylaxis is a rare but deadly disease that affects both ESRD patients receiving dialysis and patients without ESRD who have known risk factors for calciphylaxis, including female gender, white race, obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, underlying malignancy, protein C or S deficiency, corticosteroid use, warfarin use, diabetes, iron or albumin infusions, and rapid weight loss.3,6-9,11 Although the molecular pathogenesis of calciphylaxis is not completely understood, it is believed to be caused by local deposition of calcium in the tunica media of small- to medium-sized arterioles and venules in the skin.12 This deposition leads to intimal proliferation and progressive narrowing of the vessels with resultant thrombosis, ischemia, and necrosis. The cutaneous manifestations and histopathology of calciphylaxis classically follow its pathogenesis. Calciphylaxis typically presents with livedo reticularis as vessels narrow and then progresses to purpura, bullae, necrosis, and eschar formation with the onset of acute thrombosis and ischemia. Histopathology is characterized by small- and medium-sized vessel calcification and thrombus, dermal necrosis, and septal panniculitis, though the histology can be highly variable.12 Unfortunately, the already poor prognosis for calciphylaxis worsens when lesions become either ulcerative or present on the proximal extremities and trunk.4,13 Sepsis is the leading cause of death in calciphylaxis patients, affecting more than 50% of patients.2,3,14 The differential diagnoses for calciphylactic-appearing lesions include warfarin-induced skin necrosis, disseminated intravascular coagulation, pyoderma gangrenosum, cholesterol emboli, and various vasculitides and coagulopathies.
Risk Factors
Our case demonstrates the importance of risk factor minimization, trigger avoidance, and early intervention due to the high mortality rate of calciphylaxis. Selye et al15 coined the term calciphylaxis in 1961 based on experiments that induced calciphylaxis in rat models. Their research concluded that there were certain sensitizers (ie, risk factors) that predisposed patients to medial calcium deposition in blood vessels and other challengers (ie, triggers) that acted as inciting events to calcium deposition. Our patient presented with multiple known risk factors for calciphylaxis, including obesity (body mass index, 40 kg/m2), female gender, white race, hypoalbuminemia, and chronic corticosteroid use.16 In the presence of a milieu of risk factors, the patient’s rapid weight loss and episodes of hypotension likely were triggers for calciphylaxis.
Other case reports in the literature have suggested weight loss as a trigger for NUC. One morbidly obese patient with inactive rheumatoid arthritis had onset of calciphylaxis lesions after unintentional weight loss of approximately 50% body weight in 1 year17; however, the weight loss does not have to be drastic to trigger calciphylaxis. Another study of 16 patients with uremic calciphylaxis found that 7 of 16 (44%) patients lost 10 to 50 kg in the 6 months prior to calciphylaxis onset.14 One proposed mechanism by Munavalli et al10 is that elevated levels of matrix metalloproteinases during catabolic weight loss states enhance the deposition of calcium into elastic fibers of small vessels. The authors found elevated serum levels of matrix metalloproteinases in their patients with NUC induced by rapid weight loss.10
A meta-analysis by Nigwekar et al3 found a history of prior corticosteroid use in 61% (22/36) of NUC cases reviewed. However, it is unclear whether it is the use of corticosteroids or chronic inflammation that is implicated in NUC pathogenesis. Chronic inflammation causes downregulation of anticalcification signaling pathways.18-20 The role of 2 vascular calcification inhibitors has been evaluated in the pathogenesis of calciphylaxis: fetuin-A and matrix gla protein (MGP).21 The activity of these proteins is decreased not only in calciphylaxis but also in other inflammatory states and chronic renal failure.18-20 One study found lower fetuin-A levels in 312 hemodialysis patients compared to healthy controls and an association between low fetuin-A levels and increased C-reactive protein levels.22 Reduced fetuin-A and MGP levels may be the result of several calciphylaxis risk factors. Warfarin is believed to trigger calciphylaxis via inhibition of gamma-carboxylation of MGP, which is necessary for its anticalcification activity.23 Hypoalbuminemia and alcoholic liver disease also are risk factors that may be explained by the fact that fetuin-A is synthesized in the liver.24 Therefore, liver disease results in decreased production of fetuin-A that is permissive to vascular calcification in calciphylaxis patients.
There have been other reports of calciphylaxis patients who were originally hospitalized due to hypotension, which may serve as a trigger for calciphylaxis onset.25 Because calciphylaxis lesions are more likely to occur in the fatty areas of the abdomen and proximal thighs where blood flow is slower, hypotension likely accentuates the slowing of blood flow and subsequent blood vessel calcification. This theory is supported by studies showing that established calciphylactic lesions worsen more quickly in the presence of systemic hypotension.26 One patient with ESRD and calciphylaxis of the breasts had consistent systolic blood pressure readings in the high 60s to low 70s between dialysis sessions.27 Due to this association, we recommend that patients with calciphylaxis have close blood pressure monitoring to aid in preventing disease progression.28
Management
Calciphylaxis treatment has not yet been standardized, as it is an uncommon disease whose pathogenesis is not fully understood. Current management strategies aim to normalize metabolic abnormalities such as hypercalcemia if they are present and remove inciting agents such as warfarin and corticosteroids.29 Other medical treatments that have been successfully used include sodium thiosulfate, oral steroids, and adjunctive bisphosphonates.29-31 Sodium thiosulfate is known to cause metabolic acidosis by generating thiosulfuric acid in vivo in patients with or without renal disease; therefore, patients on sodium thiosulfate therapy should be monitored for development of metabolic acidosis and treated with oral sodium bicarbonate or dialysis as needed.30,32 Wound care also is an important element of calciphylaxis treatment; however, the debridement of wounds is controversial. Some argue that dry intact eschars serve to protect against sepsis, which is the leading cause of death in calciphylaxis.2,14,33 In contrast, a retrospective study of 63 calciphylaxis patients found a 1-year survival rate of 61.6% in 17 patients receiving wound debridement vs 27.4% in 46 patients who did not.2 The current consensus is that debridement should be considered on a case-by-case basis, factoring in the presence of wound infection, size of wounds, stability of eschars, and treatment goals of the patient.34 Future studies should be aimed at this issue, with special focus on how these factors and the decision to debride or not impact patient outcomes.
Conclusion
Calciphylaxis is a potentially fatal disease that impacts both patients with ESRD and those with nonuremic risk factors. The term calcific uremic arteriolopathy should be disregarded, as nonuremic causes are being reported with increased frequency in the literature. In such cases, patients often have multiple risk factors, including obesity, primary hyperparathyroidism, alcoholic liver disease, and underlying malignancy, among others. Certain triggers for onset of calciphylaxis should be avoided in at-risk patients, including the use of corticosteroids or warfarin; iron and albumin infusions; hypotension; and rapid weight loss. Our fatal case of NUC is a reminder to dermatologists treating at-risk patients to avoid these triggers and to keep calciphylaxis in the differential diagnosis when encountering early lesions such as livedo reticularis, as progression of these lesions has a 1-year mortality rate of more than 50% with the therapies being utilized at this time.
Calciphylaxis, otherwise known as calcific uremic arteriolopathy, is characterized by calcification of the tunica media of the small- to medium-sized blood vessels of the dermis and subcutis, leading to ischemia and necrosis.1 It is a deadly disease with a 1-year mortality rate of more than 50%.2 End-stage renal disease (ESRD) is the most common risk factor for calciphylaxis, with a prevalence of 1% to 4% of hemodialysis patients with calciphylaxis in the United States.2-5 However, nonuremic calciphylaxis (NUC) has been increasingly reported in the literature and has risk factors other than ESRD, including but not limited to obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, and underlying malignancy.3,6-9 Triggers for calciphylaxis in at-risk patients include use of corticosteroids or warfarin, iron or albumin infusions, and rapid weight loss.3,6,9-11 We report an unusual case of NUC that most likely was triggered by rapid weight loss and hypotension in a patient with multiple risk factors for calciphylaxis.
Case Report
A 75-year-old white woman with history of morbid obesity (body mass index, 40 kg/m2), unexplained weight loss of 70 lb over the last year, and polymyalgia rheumatica requiring chronic prednisone therapy presented with painful lesions on the thighs, buttocks, and right shoulder of 4 months’ duration. She had multiple hospital admissions preceding the onset of lesions for severe infections resulting in sepsis with hypotension, including Enterococcus faecalis endocarditis, extended-spectrum beta-lactamase bacteremia, and Pseudomonas aeruginosa pneumonia. Physical examination revealed large well-demarcated ulcers and necrotic eschars with surrounding violaceous induration and stellate erythema on the anterior, medial, and posterior thighs and buttocks that were exquisitely tender (Figures 1 and 2).


Notable laboratory results included hypoalbuminemia (1.3 g/dL [reference range, 3.5–5.0 g/dL]) with normal renal function, a corrected calcium level of 9.7 mg/dL (reference range, 8.2–10.2 mg/dL), a serum phosphorus level of 3.5 mg/dL (reference range, 2.3–4.7 mg/dL), a calcium-phosphate product of 27.3 mg2/dL2 (reference range, <55 mg2/dL2), and a parathyroid hormone level of 49.3 pg/mL (reference range, 10–65 pg/mL). Antinuclear antibodies were negative. A hypercoagulability evaluation showed normal protein C and S levels, negative lupus anticoagulant, and negative anticardiolipin antibodies.
Telescoping punch biopsies of the indurated borders of the eschars showed prominent calcification of the small- and medium-sized vessels in the mid and deep dermis, intravascular thrombi, and necrosis of the epidermis and subcutaneous fat consistent with calciphylaxis (Figure 3).

After the diagnosis of calciphylaxis was made, the patient was treated with intravenous sodium thiosulfate 25 mg 3 times weekly and alendronate 70 mg weekly. Daily arterial blood gas studies did not detect metabolic acidosis during the patient’s sodium thiosulfate therapy. The wounds were debrided, and we attempted to slowly taper the patient off the oral prednisone. Unfortunately, her condition slowly deteriorated secondary to sepsis, resulting in septic shock. The patient died 3 weeks after the diagnosis of calciphylaxis was made. At the time of diagnosis, the patient had a poor prognosis and notable risk for sepsis due to the large eschars on the thighs and abdomen as well as her relative immunosuppression due to chronic prednisone use.
Comment
Background on Calciphylaxis
Calciphylaxis is a rare but deadly disease that affects both ESRD patients receiving dialysis and patients without ESRD who have known risk factors for calciphylaxis, including female gender, white race, obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, underlying malignancy, protein C or S deficiency, corticosteroid use, warfarin use, diabetes, iron or albumin infusions, and rapid weight loss.3,6-9,11 Although the molecular pathogenesis of calciphylaxis is not completely understood, it is believed to be caused by local deposition of calcium in the tunica media of small- to medium-sized arterioles and venules in the skin.12 This deposition leads to intimal proliferation and progressive narrowing of the vessels with resultant thrombosis, ischemia, and necrosis. The cutaneous manifestations and histopathology of calciphylaxis classically follow its pathogenesis. Calciphylaxis typically presents with livedo reticularis as vessels narrow and then progresses to purpura, bullae, necrosis, and eschar formation with the onset of acute thrombosis and ischemia. Histopathology is characterized by small- and medium-sized vessel calcification and thrombus, dermal necrosis, and septal panniculitis, though the histology can be highly variable.12 Unfortunately, the already poor prognosis for calciphylaxis worsens when lesions become either ulcerative or present on the proximal extremities and trunk.4,13 Sepsis is the leading cause of death in calciphylaxis patients, affecting more than 50% of patients.2,3,14 The differential diagnoses for calciphylactic-appearing lesions include warfarin-induced skin necrosis, disseminated intravascular coagulation, pyoderma gangrenosum, cholesterol emboli, and various vasculitides and coagulopathies.
Risk Factors
Our case demonstrates the importance of risk factor minimization, trigger avoidance, and early intervention due to the high mortality rate of calciphylaxis. Selye et al15 coined the term calciphylaxis in 1961 based on experiments that induced calciphylaxis in rat models. Their research concluded that there were certain sensitizers (ie, risk factors) that predisposed patients to medial calcium deposition in blood vessels and other challengers (ie, triggers) that acted as inciting events to calcium deposition. Our patient presented with multiple known risk factors for calciphylaxis, including obesity (body mass index, 40 kg/m2), female gender, white race, hypoalbuminemia, and chronic corticosteroid use.16 In the presence of a milieu of risk factors, the patient’s rapid weight loss and episodes of hypotension likely were triggers for calciphylaxis.
Other case reports in the literature have suggested weight loss as a trigger for NUC. One morbidly obese patient with inactive rheumatoid arthritis had onset of calciphylaxis lesions after unintentional weight loss of approximately 50% body weight in 1 year17; however, the weight loss does not have to be drastic to trigger calciphylaxis. Another study of 16 patients with uremic calciphylaxis found that 7 of 16 (44%) patients lost 10 to 50 kg in the 6 months prior to calciphylaxis onset.14 One proposed mechanism by Munavalli et al10 is that elevated levels of matrix metalloproteinases during catabolic weight loss states enhance the deposition of calcium into elastic fibers of small vessels. The authors found elevated serum levels of matrix metalloproteinases in their patients with NUC induced by rapid weight loss.10
A meta-analysis by Nigwekar et al3 found a history of prior corticosteroid use in 61% (22/36) of NUC cases reviewed. However, it is unclear whether it is the use of corticosteroids or chronic inflammation that is implicated in NUC pathogenesis. Chronic inflammation causes downregulation of anticalcification signaling pathways.18-20 The role of 2 vascular calcification inhibitors has been evaluated in the pathogenesis of calciphylaxis: fetuin-A and matrix gla protein (MGP).21 The activity of these proteins is decreased not only in calciphylaxis but also in other inflammatory states and chronic renal failure.18-20 One study found lower fetuin-A levels in 312 hemodialysis patients compared to healthy controls and an association between low fetuin-A levels and increased C-reactive protein levels.22 Reduced fetuin-A and MGP levels may be the result of several calciphylaxis risk factors. Warfarin is believed to trigger calciphylaxis via inhibition of gamma-carboxylation of MGP, which is necessary for its anticalcification activity.23 Hypoalbuminemia and alcoholic liver disease also are risk factors that may be explained by the fact that fetuin-A is synthesized in the liver.24 Therefore, liver disease results in decreased production of fetuin-A that is permissive to vascular calcification in calciphylaxis patients.
There have been other reports of calciphylaxis patients who were originally hospitalized due to hypotension, which may serve as a trigger for calciphylaxis onset.25 Because calciphylaxis lesions are more likely to occur in the fatty areas of the abdomen and proximal thighs where blood flow is slower, hypotension likely accentuates the slowing of blood flow and subsequent blood vessel calcification. This theory is supported by studies showing that established calciphylactic lesions worsen more quickly in the presence of systemic hypotension.26 One patient with ESRD and calciphylaxis of the breasts had consistent systolic blood pressure readings in the high 60s to low 70s between dialysis sessions.27 Due to this association, we recommend that patients with calciphylaxis have close blood pressure monitoring to aid in preventing disease progression.28
Management
Calciphylaxis treatment has not yet been standardized, as it is an uncommon disease whose pathogenesis is not fully understood. Current management strategies aim to normalize metabolic abnormalities such as hypercalcemia if they are present and remove inciting agents such as warfarin and corticosteroids.29 Other medical treatments that have been successfully used include sodium thiosulfate, oral steroids, and adjunctive bisphosphonates.29-31 Sodium thiosulfate is known to cause metabolic acidosis by generating thiosulfuric acid in vivo in patients with or without renal disease; therefore, patients on sodium thiosulfate therapy should be monitored for development of metabolic acidosis and treated with oral sodium bicarbonate or dialysis as needed.30,32 Wound care also is an important element of calciphylaxis treatment; however, the debridement of wounds is controversial. Some argue that dry intact eschars serve to protect against sepsis, which is the leading cause of death in calciphylaxis.2,14,33 In contrast, a retrospective study of 63 calciphylaxis patients found a 1-year survival rate of 61.6% in 17 patients receiving wound debridement vs 27.4% in 46 patients who did not.2 The current consensus is that debridement should be considered on a case-by-case basis, factoring in the presence of wound infection, size of wounds, stability of eschars, and treatment goals of the patient.34 Future studies should be aimed at this issue, with special focus on how these factors and the decision to debride or not impact patient outcomes.
Conclusion
Calciphylaxis is a potentially fatal disease that impacts both patients with ESRD and those with nonuremic risk factors. The term calcific uremic arteriolopathy should be disregarded, as nonuremic causes are being reported with increased frequency in the literature. In such cases, patients often have multiple risk factors, including obesity, primary hyperparathyroidism, alcoholic liver disease, and underlying malignancy, among others. Certain triggers for onset of calciphylaxis should be avoided in at-risk patients, including the use of corticosteroids or warfarin; iron and albumin infusions; hypotension; and rapid weight loss. Our fatal case of NUC is a reminder to dermatologists treating at-risk patients to avoid these triggers and to keep calciphylaxis in the differential diagnosis when encountering early lesions such as livedo reticularis, as progression of these lesions has a 1-year mortality rate of more than 50% with the therapies being utilized at this time.
- Au S, Crawford RI. Three-dimensional analysis of a calciphylaxis plaque: clues to pathogenesis. J Am Acad Dermatol. 2007;47:53-57.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Angelis M, Wong LL, Myers SA, et al. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083-1090.
- Chavel SM, Taraszka KS, Schaffer JV, et al. Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis. J Am Acad Dermatol. 2004;50:125-128.
- Bosler DS, Amin MB, Gulli F, et al. Unusual case of calciphylaxis associated with metastatic breast carcinoma. Am J Dermatopathol. 2007;29:400-403.
- Buxtorf K, Cerottini JP, Panizzon RG. Lower limb skin ulcerations, intravascular calcifications and sensorimotor polyneuropathy: calciphylaxis as part of a hyperparathyroidism? Dermatology. 1999;198:423-425.
- Brouns K, Verbeken E, Degreef H, et al. Fatal calciphylaxis in two patients with giant cell arteritis. Clin Rheumatol. 2007;26:836-840.
- Munavalli G, Reisenauer A, Moses M, et al. Weight loss-induced calciphylaxis: potential role of matrix metalloproteinases. J Dermatol. 2003;30:915-919.
- Bae GH, Nambudiri VE, Bach DQ, et al. Rapidly progressive nonuremic calciphylaxis in setting of warfarin. Am J Med. 2015;128:E19-E21.
- Essary LR, Wick MR. Cutaneous calciphylaxis. an underrecognized clinicopathologic entity. Am J Clin Pathol. 2000;113:280-287.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Coates T, Kirkland GS, Dymock RB, et al. Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis. 1998;32:384-391.
- Selye H, Gentile G, Prioreschi P. Cutaneous molt induced by calciphylaxis in the rat. Science. 1961;134:1876-1877.
- Kalajian AH, Malhotra PS, Callen JP, et al. Calciphylaxis with normal renal and parathyroid function: not as rare as previously believed. Arch Dermatol. 2009;145:451-458.
- Malabu U, Roberts L, Sangla K. Calciphylaxis in a morbidly obese woman with rheumatoid arthritis presenting with severe weight loss and vitamin D deficiency. Endocr Pract. 2011;17:104-108.
- Schäfer C, Heiss A, Schwarz A, et al. The serum protein alpha 2–Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Cozzolino M, Galassi A, Biondi ML, et al. Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol. 2006;26:423-429.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. J Am Acad Dermatol. 2008;58:458-471.
- Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827-833.
- Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells a cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764-1767.
- Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3:109-121.
- Allegretti AS, Nazarian RM, Goverman J, et al. Calciphylaxis: a rare but fatal delayed complication of Roux-en-Y gastric bypass surgery. Am J Kidney Dis. 2014;64:274-277.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial. 2002;15:172-186.
- Gupta D, Tadros R, Mazumdar A, et al. Breast lesions with intractable pain in end-stage renal disease: calciphylaxis with chronic hypotensive dermatopathy related watershed breast lesions. J Palliat Med. 2013;16:551-554.
- Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588-597.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Bourgeois P, De Haes P. Sodium thiosulfate as a treatment for calciphylaxis: a case series. J Dermatolog Treat. 2016;27:520-524.
- Biswas A, Walsh NM, Tremaine R. A case of nonuremic calciphylaxis treated effectively with systemic corticosteroids. J Cutan Med Surg. 2016;20:275-278.
- Selk N, Rodby, RA. Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial. 2011;24:85-88.
- Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage. 2004:50:64-66, 68-70.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Au S, Crawford RI. Three-dimensional analysis of a calciphylaxis plaque: clues to pathogenesis. J Am Acad Dermatol. 2007;47:53-57.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Angelis M, Wong LL, Myers SA, et al. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083-1090.
- Chavel SM, Taraszka KS, Schaffer JV, et al. Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis. J Am Acad Dermatol. 2004;50:125-128.
- Bosler DS, Amin MB, Gulli F, et al. Unusual case of calciphylaxis associated with metastatic breast carcinoma. Am J Dermatopathol. 2007;29:400-403.
- Buxtorf K, Cerottini JP, Panizzon RG. Lower limb skin ulcerations, intravascular calcifications and sensorimotor polyneuropathy: calciphylaxis as part of a hyperparathyroidism? Dermatology. 1999;198:423-425.
- Brouns K, Verbeken E, Degreef H, et al. Fatal calciphylaxis in two patients with giant cell arteritis. Clin Rheumatol. 2007;26:836-840.
- Munavalli G, Reisenauer A, Moses M, et al. Weight loss-induced calciphylaxis: potential role of matrix metalloproteinases. J Dermatol. 2003;30:915-919.
- Bae GH, Nambudiri VE, Bach DQ, et al. Rapidly progressive nonuremic calciphylaxis in setting of warfarin. Am J Med. 2015;128:E19-E21.
- Essary LR, Wick MR. Cutaneous calciphylaxis. an underrecognized clinicopathologic entity. Am J Clin Pathol. 2000;113:280-287.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Coates T, Kirkland GS, Dymock RB, et al. Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis. 1998;32:384-391.
- Selye H, Gentile G, Prioreschi P. Cutaneous molt induced by calciphylaxis in the rat. Science. 1961;134:1876-1877.
- Kalajian AH, Malhotra PS, Callen JP, et al. Calciphylaxis with normal renal and parathyroid function: not as rare as previously believed. Arch Dermatol. 2009;145:451-458.
- Malabu U, Roberts L, Sangla K. Calciphylaxis in a morbidly obese woman with rheumatoid arthritis presenting with severe weight loss and vitamin D deficiency. Endocr Pract. 2011;17:104-108.
- Schäfer C, Heiss A, Schwarz A, et al. The serum protein alpha 2–Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Cozzolino M, Galassi A, Biondi ML, et al. Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol. 2006;26:423-429.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. J Am Acad Dermatol. 2008;58:458-471.
- Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827-833.
- Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells a cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764-1767.
- Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3:109-121.
- Allegretti AS, Nazarian RM, Goverman J, et al. Calciphylaxis: a rare but fatal delayed complication of Roux-en-Y gastric bypass surgery. Am J Kidney Dis. 2014;64:274-277.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial. 2002;15:172-186.
- Gupta D, Tadros R, Mazumdar A, et al. Breast lesions with intractable pain in end-stage renal disease: calciphylaxis with chronic hypotensive dermatopathy related watershed breast lesions. J Palliat Med. 2013;16:551-554.
- Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588-597.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Bourgeois P, De Haes P. Sodium thiosulfate as a treatment for calciphylaxis: a case series. J Dermatolog Treat. 2016;27:520-524.
- Biswas A, Walsh NM, Tremaine R. A case of nonuremic calciphylaxis treated effectively with systemic corticosteroids. J Cutan Med Surg. 2016;20:275-278.
- Selk N, Rodby, RA. Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial. 2011;24:85-88.
- Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage. 2004:50:64-66, 68-70.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
Practice Points
- Calciphylaxis is a potentially fatal disease caused by metastatic calcification of cutaneous small- and medium-sized blood vessels leading to ischemia and necrosis.
- Calciphylaxis most commonly is seen in patients with renal disease requiring dialysis, but it also may be triggered by nonuremic causes in patients with known risk factors for calciphylaxis.
- Risk factors for calciphylaxis include female gender, white race, obesity, alcoholic liver disease, primary hyperparathyroidism, connective tissue disease, underlying malignancy, protein C or S deficiency, corticosteroid use, warfarin use, diabetes, iron or albumin infusions, and rapid weight loss.
- The term calcific uremic arteriolopathy should be disregarded, as nonuremic causes are being reported with increased frequency in the literature.
Antimalarial adherence is important for diabetes prevention in lupus
Adhering to antimalarial treatment offers some protection to patients with systemic lupus erythematosus (SLE) from developing type 2 diabetes mellitus (T2DM), according to new research.
Patients who took at least 90% of their prescribed antimalarial doses were 39% less likely to develop T2DM than patients who discontinued antimalarial therapy. Patients who took less than 90% of their prescribed doses but didn’t discontinue treatment were 22% less likely to develop T2DM.
“[O]ur study provides further support for the importance of adherence to antimalarials in SLE by demonstrating protective impacts on T2DM,” Shahrzad Salmasi, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in Arthritis Care & Research.
Dr. Salmasi and colleagues conducted this retrospective study using administrative health data on patients in British Columbia. The researchers analyzed 1,498 patients with SLE. Their mean age was about 44 years, and 91% were women.
The researchers used data on prescription dates and days’ supply to establish antimalarial drug courses and gaps in treatment. A new treatment course occurred when a 90-day gap was exceeded between refills. The researchers calculated the proportion of days covered (PDC) – the total number of days with antimalarials divided by the length of the course – and separated patients into three categories:
- Adherent to treatment – PDC of 0.90 or greater
- Nonadherent – PDC greater than 0 but less than 0.90
- Discontinuer – PDC of 0
The patients had a mean of about 23 antimalarial prescriptions and a mean of about two courses. The mean course duration was 554 days.
At a median follow-up of 4.6 years, there were 140 incident cases of T2DM. The researchers calculated the risk of T2DM among adherent and nonadherent patients, comparing these groups with the discontinuers and adjusting for age, sex, comorbidities, and concomitant medications.
The adjusted hazard ratio for developing T2DM was 0.61 among adherent patients and 0.78 among nonadherent patients.
“This population-based study highlighted that taking less than 90% of the prescribed antimalarials compromises their effect in preventing T2DM in SLE patients,” Dr. Salmasi and colleagues wrote. “Our findings should be used to emphasize the importance of medication adherence in not only treating SLE but also preventing its complications.”
The researchers reported having no conflicts of interest.
SOURCE: Salmasi S et al. Arthritis Care Res. 2020 Jan 21. doi: 10.1002/acr.24147.
Adhering to antimalarial treatment offers some protection to patients with systemic lupus erythematosus (SLE) from developing type 2 diabetes mellitus (T2DM), according to new research.
Patients who took at least 90% of their prescribed antimalarial doses were 39% less likely to develop T2DM than patients who discontinued antimalarial therapy. Patients who took less than 90% of their prescribed doses but didn’t discontinue treatment were 22% less likely to develop T2DM.
“[O]ur study provides further support for the importance of adherence to antimalarials in SLE by demonstrating protective impacts on T2DM,” Shahrzad Salmasi, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in Arthritis Care & Research.
Dr. Salmasi and colleagues conducted this retrospective study using administrative health data on patients in British Columbia. The researchers analyzed 1,498 patients with SLE. Their mean age was about 44 years, and 91% were women.
The researchers used data on prescription dates and days’ supply to establish antimalarial drug courses and gaps in treatment. A new treatment course occurred when a 90-day gap was exceeded between refills. The researchers calculated the proportion of days covered (PDC) – the total number of days with antimalarials divided by the length of the course – and separated patients into three categories:
- Adherent to treatment – PDC of 0.90 or greater
- Nonadherent – PDC greater than 0 but less than 0.90
- Discontinuer – PDC of 0
The patients had a mean of about 23 antimalarial prescriptions and a mean of about two courses. The mean course duration was 554 days.
At a median follow-up of 4.6 years, there were 140 incident cases of T2DM. The researchers calculated the risk of T2DM among adherent and nonadherent patients, comparing these groups with the discontinuers and adjusting for age, sex, comorbidities, and concomitant medications.
The adjusted hazard ratio for developing T2DM was 0.61 among adherent patients and 0.78 among nonadherent patients.
“This population-based study highlighted that taking less than 90% of the prescribed antimalarials compromises their effect in preventing T2DM in SLE patients,” Dr. Salmasi and colleagues wrote. “Our findings should be used to emphasize the importance of medication adherence in not only treating SLE but also preventing its complications.”
The researchers reported having no conflicts of interest.
SOURCE: Salmasi S et al. Arthritis Care Res. 2020 Jan 21. doi: 10.1002/acr.24147.
Adhering to antimalarial treatment offers some protection to patients with systemic lupus erythematosus (SLE) from developing type 2 diabetes mellitus (T2DM), according to new research.
Patients who took at least 90% of their prescribed antimalarial doses were 39% less likely to develop T2DM than patients who discontinued antimalarial therapy. Patients who took less than 90% of their prescribed doses but didn’t discontinue treatment were 22% less likely to develop T2DM.
“[O]ur study provides further support for the importance of adherence to antimalarials in SLE by demonstrating protective impacts on T2DM,” Shahrzad Salmasi, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in Arthritis Care & Research.
Dr. Salmasi and colleagues conducted this retrospective study using administrative health data on patients in British Columbia. The researchers analyzed 1,498 patients with SLE. Their mean age was about 44 years, and 91% were women.
The researchers used data on prescription dates and days’ supply to establish antimalarial drug courses and gaps in treatment. A new treatment course occurred when a 90-day gap was exceeded between refills. The researchers calculated the proportion of days covered (PDC) – the total number of days with antimalarials divided by the length of the course – and separated patients into three categories:
- Adherent to treatment – PDC of 0.90 or greater
- Nonadherent – PDC greater than 0 but less than 0.90
- Discontinuer – PDC of 0
The patients had a mean of about 23 antimalarial prescriptions and a mean of about two courses. The mean course duration was 554 days.
At a median follow-up of 4.6 years, there were 140 incident cases of T2DM. The researchers calculated the risk of T2DM among adherent and nonadherent patients, comparing these groups with the discontinuers and adjusting for age, sex, comorbidities, and concomitant medications.
The adjusted hazard ratio for developing T2DM was 0.61 among adherent patients and 0.78 among nonadherent patients.
“This population-based study highlighted that taking less than 90% of the prescribed antimalarials compromises their effect in preventing T2DM in SLE patients,” Dr. Salmasi and colleagues wrote. “Our findings should be used to emphasize the importance of medication adherence in not only treating SLE but also preventing its complications.”
The researchers reported having no conflicts of interest.
SOURCE: Salmasi S et al. Arthritis Care Res. 2020 Jan 21. doi: 10.1002/acr.24147.
FROM ARTHRITIS CARE & RESEARCH