User login
Dual immunotherapy goes the distance in MSI-H colorectal cancer
SAN FRANCISCO – First-line dual immune checkpoint inhibitor therapy for microsatellite instability–high/DNA mismatch repair–deficient (MSI-H/dMMR) metastatic colorectal cancer has impressive durability, an update of the multicohort CheckMate 142 trial shows.
“We all know that MSI-H colorectal cancer patients have a poor prognosis,” said lead investigator Heinz-Josef Lenz, MD, codirector of the Colorectal Center and section head of the GI oncology program at the University of Southern California Norris Comprehensive Cancer Center, Los Angeles. First-line chemotherapy in this population yields median overall survival on the order of 20-22 months.
The cohort of 45 patients treated on the phase 2 trial received the programmed death–1 inhibitor nivolumab (Opdivo) every 2 weeks, plus a low dose of the CTLA4 inhibitor ipilimumab (Yervoy) every 6 weeks as first-line therapy for MSI-H metastatic colorectal cancer. (Nivolumab, with or without ipilimumab, has received Food and Drug Administration accelerated approval as second-line therapy based on data from other cohorts in the trial.)
Previously reported initial results, at a median follow-up of 13.8 months, showed that the investigator-assessed objective response rate was 60% (ESMO 2018, Abstract LBA18_PR). As of the update, now at a median follow-up of 19.9 months, that rate was 64%, according to data reported at the 2020 GI Cancers Symposium.
“Nivolumab and ipilimumab demonstrates clinically meaningful durable benefits and may present an option for first-line treatment for MSI-H metastatic colorectal cancer patients,” Dr. Lenz summarized.
“The incredible complete response rates and overall response rates seen are never seen with chemotherapy, and it’s very well tolerated, so if I have a choice, I would start with nivolumab-ipilimumab in first-line MSI-H,” he added. “The [National Comprehensive Cancer Network] guidelines recommend that you can start in first line with this combination if patients are not candidates for chemotherapy. So they leave this door open, that you have an opportunity to use immunotherapy in this patient population.”
Choosing single or dual immunotherapy
Given the so-called financial toxicity of dual nivolumab and ipilimumab therapy and lack of a randomized comparison against single-agent nivolumab, a session attendee said, can clinicians simply use the latter?
“Very importantly, in this clinical trial, ipilimumab was given every 6 weeks. As you know, the 2-week regimens are significantly more toxic,” Dr. Lenz noted. The combination using this low dose has safety similar to that seen with nivolumab alone. And in cross-trial comparisons, at least, “the combination seems to be a little bit more active, so I always choose both,” he said, adding that the financial aspects are beyond his purview.
However, session cochair Joseph J.Y. Sung, MD, PhD, MBBS, the Mok Hing Yiu Professor of Medicine in the department of medicine and therapeutics at the Chinese University of Hong Kong, had a different view.
“I think there is still room for a randomized study to prove the combination’s superiority against single immunotherapy,” he said in an interview. “If I’m treating an MSI patient, I would stick to single immunotherapy until I see more evidence that this combination has a substantial improvement in the outcome that is worth the money.”
Study details
The patients studied were unselected for baseline tumor expression of programmed death–ligand 1, with 27% having expression of 1% or greater and 58% having expression below that threshold.
As of the data cutoff for the update, 47% of patients were still on treatment, Dr. Lenz reported at the symposium, sponsored by the American Gastroenterological Association, American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The most common reason for stopping treatment was disease progression.
The investigator-assessed objective response rate, the primary endpoint, consisted of complete responses in 9% of patients and partial responses in 56%. (The rate as assessed by blinded independent central reviewers was 58%, consisting of complete responses in 18% and partial responses in 40%.)
“The high response rate was consistent among all evaluated subgroups,” Dr. Lenz noted, with no differences detected by presence or absence of Lynch syndrome and/or specific microsatellite-related mutations.
Median time to response was 2.6 months, and median duration of response was not reached. Median progression-free and overall survival were also not reached, but 15-month rates were 75% and 84%, respectively.
The rate of grade 3-4 treatment-related adverse events was 20%, and the rate of grade 3-4 serious treatment-related adverse events was 11%. Only 4% of patients discontinued treatment because of such events.
Ongoing trials may take immunotherapy even further in this patient population, according to Dr. Lenz. “We are all waiting for the treatments with the immunotherapy in combination with chemo and bevacizumab in first line. Will that change the efficacy and toxicity?” he elaborated. “You can imagine, in the MSI-H patients with the immunotherapy alone, we haven’t reached median progression-free and overall survival, so it may take a long time to get this data.”
Dr. Lenz reported receiving honoraria from Bayer, Boehringer Ingelheim, Merck Serono, and Roche; consulting or advising with Bayer, Merck Serono, Pfizer, and Roche; receiving travel expenses from Bayer, Merck Serono, and Roche. The trial was funded by Bristol-Myers Squibb. Dr. Sung reported that he had no relevant conflicts of interest.
SOURCE: Lenz H-J et al. 2020 GI Cancers Symposium, Abstract 11.
SAN FRANCISCO – First-line dual immune checkpoint inhibitor therapy for microsatellite instability–high/DNA mismatch repair–deficient (MSI-H/dMMR) metastatic colorectal cancer has impressive durability, an update of the multicohort CheckMate 142 trial shows.
“We all know that MSI-H colorectal cancer patients have a poor prognosis,” said lead investigator Heinz-Josef Lenz, MD, codirector of the Colorectal Center and section head of the GI oncology program at the University of Southern California Norris Comprehensive Cancer Center, Los Angeles. First-line chemotherapy in this population yields median overall survival on the order of 20-22 months.
The cohort of 45 patients treated on the phase 2 trial received the programmed death–1 inhibitor nivolumab (Opdivo) every 2 weeks, plus a low dose of the CTLA4 inhibitor ipilimumab (Yervoy) every 6 weeks as first-line therapy for MSI-H metastatic colorectal cancer. (Nivolumab, with or without ipilimumab, has received Food and Drug Administration accelerated approval as second-line therapy based on data from other cohorts in the trial.)
Previously reported initial results, at a median follow-up of 13.8 months, showed that the investigator-assessed objective response rate was 60% (ESMO 2018, Abstract LBA18_PR). As of the update, now at a median follow-up of 19.9 months, that rate was 64%, according to data reported at the 2020 GI Cancers Symposium.
“Nivolumab and ipilimumab demonstrates clinically meaningful durable benefits and may present an option for first-line treatment for MSI-H metastatic colorectal cancer patients,” Dr. Lenz summarized.
“The incredible complete response rates and overall response rates seen are never seen with chemotherapy, and it’s very well tolerated, so if I have a choice, I would start with nivolumab-ipilimumab in first-line MSI-H,” he added. “The [National Comprehensive Cancer Network] guidelines recommend that you can start in first line with this combination if patients are not candidates for chemotherapy. So they leave this door open, that you have an opportunity to use immunotherapy in this patient population.”
Choosing single or dual immunotherapy
Given the so-called financial toxicity of dual nivolumab and ipilimumab therapy and lack of a randomized comparison against single-agent nivolumab, a session attendee said, can clinicians simply use the latter?
“Very importantly, in this clinical trial, ipilimumab was given every 6 weeks. As you know, the 2-week regimens are significantly more toxic,” Dr. Lenz noted. The combination using this low dose has safety similar to that seen with nivolumab alone. And in cross-trial comparisons, at least, “the combination seems to be a little bit more active, so I always choose both,” he said, adding that the financial aspects are beyond his purview.
However, session cochair Joseph J.Y. Sung, MD, PhD, MBBS, the Mok Hing Yiu Professor of Medicine in the department of medicine and therapeutics at the Chinese University of Hong Kong, had a different view.
“I think there is still room for a randomized study to prove the combination’s superiority against single immunotherapy,” he said in an interview. “If I’m treating an MSI patient, I would stick to single immunotherapy until I see more evidence that this combination has a substantial improvement in the outcome that is worth the money.”
Study details
The patients studied were unselected for baseline tumor expression of programmed death–ligand 1, with 27% having expression of 1% or greater and 58% having expression below that threshold.
As of the data cutoff for the update, 47% of patients were still on treatment, Dr. Lenz reported at the symposium, sponsored by the American Gastroenterological Association, American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The most common reason for stopping treatment was disease progression.
The investigator-assessed objective response rate, the primary endpoint, consisted of complete responses in 9% of patients and partial responses in 56%. (The rate as assessed by blinded independent central reviewers was 58%, consisting of complete responses in 18% and partial responses in 40%.)
“The high response rate was consistent among all evaluated subgroups,” Dr. Lenz noted, with no differences detected by presence or absence of Lynch syndrome and/or specific microsatellite-related mutations.
Median time to response was 2.6 months, and median duration of response was not reached. Median progression-free and overall survival were also not reached, but 15-month rates were 75% and 84%, respectively.
The rate of grade 3-4 treatment-related adverse events was 20%, and the rate of grade 3-4 serious treatment-related adverse events was 11%. Only 4% of patients discontinued treatment because of such events.
Ongoing trials may take immunotherapy even further in this patient population, according to Dr. Lenz. “We are all waiting for the treatments with the immunotherapy in combination with chemo and bevacizumab in first line. Will that change the efficacy and toxicity?” he elaborated. “You can imagine, in the MSI-H patients with the immunotherapy alone, we haven’t reached median progression-free and overall survival, so it may take a long time to get this data.”
Dr. Lenz reported receiving honoraria from Bayer, Boehringer Ingelheim, Merck Serono, and Roche; consulting or advising with Bayer, Merck Serono, Pfizer, and Roche; receiving travel expenses from Bayer, Merck Serono, and Roche. The trial was funded by Bristol-Myers Squibb. Dr. Sung reported that he had no relevant conflicts of interest.
SOURCE: Lenz H-J et al. 2020 GI Cancers Symposium, Abstract 11.
SAN FRANCISCO – First-line dual immune checkpoint inhibitor therapy for microsatellite instability–high/DNA mismatch repair–deficient (MSI-H/dMMR) metastatic colorectal cancer has impressive durability, an update of the multicohort CheckMate 142 trial shows.
“We all know that MSI-H colorectal cancer patients have a poor prognosis,” said lead investigator Heinz-Josef Lenz, MD, codirector of the Colorectal Center and section head of the GI oncology program at the University of Southern California Norris Comprehensive Cancer Center, Los Angeles. First-line chemotherapy in this population yields median overall survival on the order of 20-22 months.
The cohort of 45 patients treated on the phase 2 trial received the programmed death–1 inhibitor nivolumab (Opdivo) every 2 weeks, plus a low dose of the CTLA4 inhibitor ipilimumab (Yervoy) every 6 weeks as first-line therapy for MSI-H metastatic colorectal cancer. (Nivolumab, with or without ipilimumab, has received Food and Drug Administration accelerated approval as second-line therapy based on data from other cohorts in the trial.)
Previously reported initial results, at a median follow-up of 13.8 months, showed that the investigator-assessed objective response rate was 60% (ESMO 2018, Abstract LBA18_PR). As of the update, now at a median follow-up of 19.9 months, that rate was 64%, according to data reported at the 2020 GI Cancers Symposium.
“Nivolumab and ipilimumab demonstrates clinically meaningful durable benefits and may present an option for first-line treatment for MSI-H metastatic colorectal cancer patients,” Dr. Lenz summarized.
“The incredible complete response rates and overall response rates seen are never seen with chemotherapy, and it’s very well tolerated, so if I have a choice, I would start with nivolumab-ipilimumab in first-line MSI-H,” he added. “The [National Comprehensive Cancer Network] guidelines recommend that you can start in first line with this combination if patients are not candidates for chemotherapy. So they leave this door open, that you have an opportunity to use immunotherapy in this patient population.”
Choosing single or dual immunotherapy
Given the so-called financial toxicity of dual nivolumab and ipilimumab therapy and lack of a randomized comparison against single-agent nivolumab, a session attendee said, can clinicians simply use the latter?
“Very importantly, in this clinical trial, ipilimumab was given every 6 weeks. As you know, the 2-week regimens are significantly more toxic,” Dr. Lenz noted. The combination using this low dose has safety similar to that seen with nivolumab alone. And in cross-trial comparisons, at least, “the combination seems to be a little bit more active, so I always choose both,” he said, adding that the financial aspects are beyond his purview.
However, session cochair Joseph J.Y. Sung, MD, PhD, MBBS, the Mok Hing Yiu Professor of Medicine in the department of medicine and therapeutics at the Chinese University of Hong Kong, had a different view.
“I think there is still room for a randomized study to prove the combination’s superiority against single immunotherapy,” he said in an interview. “If I’m treating an MSI patient, I would stick to single immunotherapy until I see more evidence that this combination has a substantial improvement in the outcome that is worth the money.”
Study details
The patients studied were unselected for baseline tumor expression of programmed death–ligand 1, with 27% having expression of 1% or greater and 58% having expression below that threshold.
As of the data cutoff for the update, 47% of patients were still on treatment, Dr. Lenz reported at the symposium, sponsored by the American Gastroenterological Association, American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The most common reason for stopping treatment was disease progression.
The investigator-assessed objective response rate, the primary endpoint, consisted of complete responses in 9% of patients and partial responses in 56%. (The rate as assessed by blinded independent central reviewers was 58%, consisting of complete responses in 18% and partial responses in 40%.)
“The high response rate was consistent among all evaluated subgroups,” Dr. Lenz noted, with no differences detected by presence or absence of Lynch syndrome and/or specific microsatellite-related mutations.
Median time to response was 2.6 months, and median duration of response was not reached. Median progression-free and overall survival were also not reached, but 15-month rates were 75% and 84%, respectively.
The rate of grade 3-4 treatment-related adverse events was 20%, and the rate of grade 3-4 serious treatment-related adverse events was 11%. Only 4% of patients discontinued treatment because of such events.
Ongoing trials may take immunotherapy even further in this patient population, according to Dr. Lenz. “We are all waiting for the treatments with the immunotherapy in combination with chemo and bevacizumab in first line. Will that change the efficacy and toxicity?” he elaborated. “You can imagine, in the MSI-H patients with the immunotherapy alone, we haven’t reached median progression-free and overall survival, so it may take a long time to get this data.”
Dr. Lenz reported receiving honoraria from Bayer, Boehringer Ingelheim, Merck Serono, and Roche; consulting or advising with Bayer, Merck Serono, Pfizer, and Roche; receiving travel expenses from Bayer, Merck Serono, and Roche. The trial was funded by Bristol-Myers Squibb. Dr. Sung reported that he had no relevant conflicts of interest.
SOURCE: Lenz H-J et al. 2020 GI Cancers Symposium, Abstract 11.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
REPORTING FROM ACR 2019
Modafinil use in pregnancy tied to congenital malformations
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
FROM JAMA
The Clinical Conundrum in Managing Preterm Birth: Balancing Historical Trial Results, Society Guidelines, and Clinical Experience with a Contradictory Trial Outcome
This CME supplement details results of the PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial evaluating 17-OHPC in patients with a history of a prior spontaneous singleton preterm delivery.
To receive CME credit, please
read the article and go to www.omniaeducation.com/ptb
to access the posttest and evaluation.
This CME supplement details results of the PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial evaluating 17-OHPC in patients with a history of a prior spontaneous singleton preterm delivery.
To receive CME credit, please
read the article and go to www.omniaeducation.com/ptb
to access the posttest and evaluation.
This CME supplement details results of the PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial evaluating 17-OHPC in patients with a history of a prior spontaneous singleton preterm delivery.
To receive CME credit, please
read the article and go to www.omniaeducation.com/ptb
to access the posttest and evaluation.
Rural treatment of opioid use disorder increasingly driven by nonphysician workforce
Nurse practitioners and physician assistants, rather than physicians, are the clinicians who have boosted capacity for buprenorphine prescribing in rural America, according to a study in a rural health–focused issue of the journal Health Affairs.
In the face of an ongoing crisis of opioid use disorder, and associated overdoses and deaths that have spared no sector of the U.S. population, the federal government expanded its waiver program for buprenorphine prescribing in 2017. The waiver expansion allows nurse practitioners (NPs) and physician assistants (PAs) – along with clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives – to use the drug for medication-assisted treatment (MAT) for opioid use disorder after completing 24 hours of mandated training; physicians are required to complete 8 hours of training to receive their waiver.
From 2016 to 2019, capacity for MAT in rural areas increased, with the number of clinicians with buprenorphine waivers more than doubling. Of the newly waivered prescribers accounting for this 111% increase, more than half were NPs and PAs.
In many areas, NPs and PAs led the way forward, wrote the study’s lead author Michael L. Barnett, MD, and coauthors, noting in the abstract accompanying the paper that “NPs and PAs accounted for more than half of this increase and were the first waivered clinicians in 285 rural counties with 5.7 million residents.” Overall, the proportion of people living in a county without a waivered clinician has decreased by 36% since NPs and PAs were permitted to obtain waivers.
SAMHSA data identifies trends
In an in-depth interview, Dr. Barnett, an internal medicine physician and health services researcher at the Harvard School of Public Health, Boston, said the issue today is “not so much continuing to dissect the risks and benefits of opioids as a treatment for pain, but more trying to address the current overdose crisis, and the fact that our patient treatment infrastructure is woefully inadequate for the magnitude of the problem that we face.”
Dr. Barnett’s chief intention for this study, he said, was to generate information that will drive policy to implement effective opioid treatment. He’d always been interested in models of care delivery that move beyond seeing just the physician-patient dyad.
“There are a whole range of nonphysician providers that are probably better at providing many different types of care – things that physicians aren’t necessarily that well trained to do,” he said.
Expansion of buprenorphine waivers to NPs and PAs, said Dr. Barnett, presented “a very interesting opportunity to see: How does a nonphysician workforce respond to a new practice opportunity, to really be engaged in areas that many physicians really were neglecting?”
The researchers used information drawn from what Dr. Barnett characterized as a “gold-standard” dataset maintained by the federal Substance Abuse and Mental Health Services Administration. They found that, by March 2019, 52% of U.S. rural residents lived in counties with at least one NP or PA holding a buprenorphine waiver, though there was wide geographic variation: Every county in Maine and New Hampshire had waivered NPs or PAs, but in Tennessee, just 3 of 95 counties had an NP or PA with a waiver.
Scope-of-practice regulations matter
The scope of practice permitted NPs and PAs varies by state, and Dr. Barnett and coauthors also looked to see whether broader scope of practice meant that more advanced practice clinicians were getting buprenorphine waivers. This did appear to be the case: In an analysis that dichotomized scope of practice into “broad” and “restricted,” states with broader practice scope saw twice as many waivered NPs per 100,000 rural residents as those with restrictive practice scope. This association was not seen for PAs, but Dr. Barnett pointed out that PAs are less likely overall to work in primary care.
This, he added, is where scope of practice starts to matter. “A lot of states are still bickering about scope of practice. We show in our paper the clear relationship between scope of practice and the degree to which providers are able to take up these waivers. We can’t prove causality, but I think it’s not a big stretch to think that these policies are playing a big role. I hope we’re working to try to advance that conversation.”
Helping address the unmet need for evidence-based treatment of opioid use disorder, he said, “is one of the more important examples, because doctors have been leaving rural areas in droves. We are lucky that there is a workforce of NPs that still seem to recognize the market opportunity; rural areas still need providers, and they have been willing to fill the gap.”
Waivered NPs or PAs can apply for an expanded waiver, permitting expansion of the buprenorphine panel from 30 to 100 patients after 1 year of holding their initial waiver. Physicians may apply for a waiver to treat up to 275 patients.
Effect on quality of care
The evidence doesn’t support big worries about quality of care, he said. “We don’t have any data on this in the clinical context of addiction, but all of the data that are out there in terms of evaluating the quality of care and level of care being offered by NPs and PAs versus primary care doctors – the types of things that we think of as within the scope of NP and PA practice typically – have shown that they are the same.” Dr. Barnett acknowledged that “there are a little bit of mixed results here and there in one direction or another, but largely, the care being delivered is much more the same than different.”
In addressing the opioid crisis as in the rest of medicine, it’s a mistake not to include this sector of the health care workforce when policies are being crafted, said Dr. Barnett. “People who are making policy and aren’t familiar with the workforce in rural areas could miss the boat. ... NPs aren’t just an asterisk to the workforce – they are an essential part of delivering medicine, just as much as physicians are.”
Dr. Barnett said that, in his estimation, “a lot of protectionist myths get physicians worked up around increased scope of practice for NPs.” However, “The truth is that there’s enough health care spending to go around for everybody and there’s plenty of work to go around.”
Dr. Barnett acknowledged that the current study captured only prescribing capacity, and not actual prescription volume. But, based on some preliminary data, “my sense is that NPs and PAs who acquire waivers are more likely to be prescribing to a larger number of patients proportionately than MDs.” He wasn’t surprised to see this, since the many more hours of training required for NPs and PAs to acquire a waiver means they’re likely to be committed to using the waiver in practice.
Stepping back to look at the bigger picture, Dr. Barnett remarked that, “taking a look at the waiver requirement, a part of me feels that it’s a bit of an anachronistic regulation, anyway – it’s really hard to justify clinically or ethically versus other things that we do.” The waiver program he said, is “a regulation barrier whose time should be limited. ... I’m hoping that the waiver disappears soon.”
Prescribing issues will linger beyond any future abolition of the waiver program, since many clinicians will still not be comfortable prescribing medication for MAT of opioid use disorder, said Dr. Barnett. “It’ll be a lot of the same stigma and structural barriers that were in place prior to the waiver.”
Dr. Barnett reported that he has been retained as an expert witness for plaintiffs in lawsuits against opioid manufacturers. The study was partly funded by the National Institutes of Health.
SOURCE: Barnett ML et al. Health Aff. 2019 Jan;38(12):2048-56.
Nurse practitioners and physician assistants, rather than physicians, are the clinicians who have boosted capacity for buprenorphine prescribing in rural America, according to a study in a rural health–focused issue of the journal Health Affairs.
In the face of an ongoing crisis of opioid use disorder, and associated overdoses and deaths that have spared no sector of the U.S. population, the federal government expanded its waiver program for buprenorphine prescribing in 2017. The waiver expansion allows nurse practitioners (NPs) and physician assistants (PAs) – along with clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives – to use the drug for medication-assisted treatment (MAT) for opioid use disorder after completing 24 hours of mandated training; physicians are required to complete 8 hours of training to receive their waiver.
From 2016 to 2019, capacity for MAT in rural areas increased, with the number of clinicians with buprenorphine waivers more than doubling. Of the newly waivered prescribers accounting for this 111% increase, more than half were NPs and PAs.
In many areas, NPs and PAs led the way forward, wrote the study’s lead author Michael L. Barnett, MD, and coauthors, noting in the abstract accompanying the paper that “NPs and PAs accounted for more than half of this increase and were the first waivered clinicians in 285 rural counties with 5.7 million residents.” Overall, the proportion of people living in a county without a waivered clinician has decreased by 36% since NPs and PAs were permitted to obtain waivers.
SAMHSA data identifies trends
In an in-depth interview, Dr. Barnett, an internal medicine physician and health services researcher at the Harvard School of Public Health, Boston, said the issue today is “not so much continuing to dissect the risks and benefits of opioids as a treatment for pain, but more trying to address the current overdose crisis, and the fact that our patient treatment infrastructure is woefully inadequate for the magnitude of the problem that we face.”
Dr. Barnett’s chief intention for this study, he said, was to generate information that will drive policy to implement effective opioid treatment. He’d always been interested in models of care delivery that move beyond seeing just the physician-patient dyad.
“There are a whole range of nonphysician providers that are probably better at providing many different types of care – things that physicians aren’t necessarily that well trained to do,” he said.
Expansion of buprenorphine waivers to NPs and PAs, said Dr. Barnett, presented “a very interesting opportunity to see: How does a nonphysician workforce respond to a new practice opportunity, to really be engaged in areas that many physicians really were neglecting?”
The researchers used information drawn from what Dr. Barnett characterized as a “gold-standard” dataset maintained by the federal Substance Abuse and Mental Health Services Administration. They found that, by March 2019, 52% of U.S. rural residents lived in counties with at least one NP or PA holding a buprenorphine waiver, though there was wide geographic variation: Every county in Maine and New Hampshire had waivered NPs or PAs, but in Tennessee, just 3 of 95 counties had an NP or PA with a waiver.
Scope-of-practice regulations matter
The scope of practice permitted NPs and PAs varies by state, and Dr. Barnett and coauthors also looked to see whether broader scope of practice meant that more advanced practice clinicians were getting buprenorphine waivers. This did appear to be the case: In an analysis that dichotomized scope of practice into “broad” and “restricted,” states with broader practice scope saw twice as many waivered NPs per 100,000 rural residents as those with restrictive practice scope. This association was not seen for PAs, but Dr. Barnett pointed out that PAs are less likely overall to work in primary care.
This, he added, is where scope of practice starts to matter. “A lot of states are still bickering about scope of practice. We show in our paper the clear relationship between scope of practice and the degree to which providers are able to take up these waivers. We can’t prove causality, but I think it’s not a big stretch to think that these policies are playing a big role. I hope we’re working to try to advance that conversation.”
Helping address the unmet need for evidence-based treatment of opioid use disorder, he said, “is one of the more important examples, because doctors have been leaving rural areas in droves. We are lucky that there is a workforce of NPs that still seem to recognize the market opportunity; rural areas still need providers, and they have been willing to fill the gap.”
Waivered NPs or PAs can apply for an expanded waiver, permitting expansion of the buprenorphine panel from 30 to 100 patients after 1 year of holding their initial waiver. Physicians may apply for a waiver to treat up to 275 patients.
Effect on quality of care
The evidence doesn’t support big worries about quality of care, he said. “We don’t have any data on this in the clinical context of addiction, but all of the data that are out there in terms of evaluating the quality of care and level of care being offered by NPs and PAs versus primary care doctors – the types of things that we think of as within the scope of NP and PA practice typically – have shown that they are the same.” Dr. Barnett acknowledged that “there are a little bit of mixed results here and there in one direction or another, but largely, the care being delivered is much more the same than different.”
In addressing the opioid crisis as in the rest of medicine, it’s a mistake not to include this sector of the health care workforce when policies are being crafted, said Dr. Barnett. “People who are making policy and aren’t familiar with the workforce in rural areas could miss the boat. ... NPs aren’t just an asterisk to the workforce – they are an essential part of delivering medicine, just as much as physicians are.”
Dr. Barnett said that, in his estimation, “a lot of protectionist myths get physicians worked up around increased scope of practice for NPs.” However, “The truth is that there’s enough health care spending to go around for everybody and there’s plenty of work to go around.”
Dr. Barnett acknowledged that the current study captured only prescribing capacity, and not actual prescription volume. But, based on some preliminary data, “my sense is that NPs and PAs who acquire waivers are more likely to be prescribing to a larger number of patients proportionately than MDs.” He wasn’t surprised to see this, since the many more hours of training required for NPs and PAs to acquire a waiver means they’re likely to be committed to using the waiver in practice.
Stepping back to look at the bigger picture, Dr. Barnett remarked that, “taking a look at the waiver requirement, a part of me feels that it’s a bit of an anachronistic regulation, anyway – it’s really hard to justify clinically or ethically versus other things that we do.” The waiver program he said, is “a regulation barrier whose time should be limited. ... I’m hoping that the waiver disappears soon.”
Prescribing issues will linger beyond any future abolition of the waiver program, since many clinicians will still not be comfortable prescribing medication for MAT of opioid use disorder, said Dr. Barnett. “It’ll be a lot of the same stigma and structural barriers that were in place prior to the waiver.”
Dr. Barnett reported that he has been retained as an expert witness for plaintiffs in lawsuits against opioid manufacturers. The study was partly funded by the National Institutes of Health.
SOURCE: Barnett ML et al. Health Aff. 2019 Jan;38(12):2048-56.
Nurse practitioners and physician assistants, rather than physicians, are the clinicians who have boosted capacity for buprenorphine prescribing in rural America, according to a study in a rural health–focused issue of the journal Health Affairs.
In the face of an ongoing crisis of opioid use disorder, and associated overdoses and deaths that have spared no sector of the U.S. population, the federal government expanded its waiver program for buprenorphine prescribing in 2017. The waiver expansion allows nurse practitioners (NPs) and physician assistants (PAs) – along with clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives – to use the drug for medication-assisted treatment (MAT) for opioid use disorder after completing 24 hours of mandated training; physicians are required to complete 8 hours of training to receive their waiver.
From 2016 to 2019, capacity for MAT in rural areas increased, with the number of clinicians with buprenorphine waivers more than doubling. Of the newly waivered prescribers accounting for this 111% increase, more than half were NPs and PAs.
In many areas, NPs and PAs led the way forward, wrote the study’s lead author Michael L. Barnett, MD, and coauthors, noting in the abstract accompanying the paper that “NPs and PAs accounted for more than half of this increase and were the first waivered clinicians in 285 rural counties with 5.7 million residents.” Overall, the proportion of people living in a county without a waivered clinician has decreased by 36% since NPs and PAs were permitted to obtain waivers.
SAMHSA data identifies trends
In an in-depth interview, Dr. Barnett, an internal medicine physician and health services researcher at the Harvard School of Public Health, Boston, said the issue today is “not so much continuing to dissect the risks and benefits of opioids as a treatment for pain, but more trying to address the current overdose crisis, and the fact that our patient treatment infrastructure is woefully inadequate for the magnitude of the problem that we face.”
Dr. Barnett’s chief intention for this study, he said, was to generate information that will drive policy to implement effective opioid treatment. He’d always been interested in models of care delivery that move beyond seeing just the physician-patient dyad.
“There are a whole range of nonphysician providers that are probably better at providing many different types of care – things that physicians aren’t necessarily that well trained to do,” he said.
Expansion of buprenorphine waivers to NPs and PAs, said Dr. Barnett, presented “a very interesting opportunity to see: How does a nonphysician workforce respond to a new practice opportunity, to really be engaged in areas that many physicians really were neglecting?”
The researchers used information drawn from what Dr. Barnett characterized as a “gold-standard” dataset maintained by the federal Substance Abuse and Mental Health Services Administration. They found that, by March 2019, 52% of U.S. rural residents lived in counties with at least one NP or PA holding a buprenorphine waiver, though there was wide geographic variation: Every county in Maine and New Hampshire had waivered NPs or PAs, but in Tennessee, just 3 of 95 counties had an NP or PA with a waiver.
Scope-of-practice regulations matter
The scope of practice permitted NPs and PAs varies by state, and Dr. Barnett and coauthors also looked to see whether broader scope of practice meant that more advanced practice clinicians were getting buprenorphine waivers. This did appear to be the case: In an analysis that dichotomized scope of practice into “broad” and “restricted,” states with broader practice scope saw twice as many waivered NPs per 100,000 rural residents as those with restrictive practice scope. This association was not seen for PAs, but Dr. Barnett pointed out that PAs are less likely overall to work in primary care.
This, he added, is where scope of practice starts to matter. “A lot of states are still bickering about scope of practice. We show in our paper the clear relationship between scope of practice and the degree to which providers are able to take up these waivers. We can’t prove causality, but I think it’s not a big stretch to think that these policies are playing a big role. I hope we’re working to try to advance that conversation.”
Helping address the unmet need for evidence-based treatment of opioid use disorder, he said, “is one of the more important examples, because doctors have been leaving rural areas in droves. We are lucky that there is a workforce of NPs that still seem to recognize the market opportunity; rural areas still need providers, and they have been willing to fill the gap.”
Waivered NPs or PAs can apply for an expanded waiver, permitting expansion of the buprenorphine panel from 30 to 100 patients after 1 year of holding their initial waiver. Physicians may apply for a waiver to treat up to 275 patients.
Effect on quality of care
The evidence doesn’t support big worries about quality of care, he said. “We don’t have any data on this in the clinical context of addiction, but all of the data that are out there in terms of evaluating the quality of care and level of care being offered by NPs and PAs versus primary care doctors – the types of things that we think of as within the scope of NP and PA practice typically – have shown that they are the same.” Dr. Barnett acknowledged that “there are a little bit of mixed results here and there in one direction or another, but largely, the care being delivered is much more the same than different.”
In addressing the opioid crisis as in the rest of medicine, it’s a mistake not to include this sector of the health care workforce when policies are being crafted, said Dr. Barnett. “People who are making policy and aren’t familiar with the workforce in rural areas could miss the boat. ... NPs aren’t just an asterisk to the workforce – they are an essential part of delivering medicine, just as much as physicians are.”
Dr. Barnett said that, in his estimation, “a lot of protectionist myths get physicians worked up around increased scope of practice for NPs.” However, “The truth is that there’s enough health care spending to go around for everybody and there’s plenty of work to go around.”
Dr. Barnett acknowledged that the current study captured only prescribing capacity, and not actual prescription volume. But, based on some preliminary data, “my sense is that NPs and PAs who acquire waivers are more likely to be prescribing to a larger number of patients proportionately than MDs.” He wasn’t surprised to see this, since the many more hours of training required for NPs and PAs to acquire a waiver means they’re likely to be committed to using the waiver in practice.
Stepping back to look at the bigger picture, Dr. Barnett remarked that, “taking a look at the waiver requirement, a part of me feels that it’s a bit of an anachronistic regulation, anyway – it’s really hard to justify clinically or ethically versus other things that we do.” The waiver program he said, is “a regulation barrier whose time should be limited. ... I’m hoping that the waiver disappears soon.”
Prescribing issues will linger beyond any future abolition of the waiver program, since many clinicians will still not be comfortable prescribing medication for MAT of opioid use disorder, said Dr. Barnett. “It’ll be a lot of the same stigma and structural barriers that were in place prior to the waiver.”
Dr. Barnett reported that he has been retained as an expert witness for plaintiffs in lawsuits against opioid manufacturers. The study was partly funded by the National Institutes of Health.
SOURCE: Barnett ML et al. Health Aff. 2019 Jan;38(12):2048-56.
FROM HEALTH AFFAIRS
What 2019’s top five CAD trials tell us
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
Large percentage of psychiatrists sued for malpractice at least once
Forty-one percent of U.S. psychiatrists have been sued for malpractice at least once, findings from the newly released Medscape Psychiatrist Malpractice Report 2019 show.
The top reason for the legal action was wrongful death (31%), followed by poor outcome/disease progression (23%), failure to treat/delayed treatment (11%), errors in medication administration (10%), and complications from treatment/surgery (8%).
Only 7% of psychiatrists said failure to treat/delayed treatment was the reason for the lawsuit, whereas this was the top reason for physicians overall (33%) in the report.
Medscape surveyed 4,360 physician members in more than 25 specialties about whether they have been sued for malpractice, reasons for a lawsuit, what happened, and how the experience affected the way they practice medicine and interact with patients.
Among psychiatrists named in a lawsuit, 44% said they were very surprised to be the subject of litigation. A similar percentage reported they were somewhat surprised (41%), while 15% were not at all surprised.
The vast majority of psychiatrists (87%) believed the lawsuit was not warranted, while 11% were unsure. Only a small percentage (2%) believed that legal action was justified, the lowest percentage of all physicians (6%).
Among psychiatrists who were sued, 42% were able to identify the incident that sparked the lawsuit. A slightly higher percentage (47%) said there was no specific incident that spurred legal action; 11% couldn’t recall.
Psychological factors
“There’s a whole host of what you could call psychological factors that can contribute to the filing of a claim,” David S. Szabo, a malpractice defense attorney with Locke Lord LLP, Boston, said in an interview.
“These can occur when a patient perceives a breakdown in the doctor-patient relationship or is pretty certain that there’s been a mistake and they feel like they’ve been shut out of productive conversation with their health care provider or providers,” said Mr. Szabo.
Legal action eats up time. A total of 43% of psychiatrists reported spending more than 40 hours on their defense, which involved gathering records, meeting with attorneys, and preparing for depositions.
Forty-six percent reported that the entire process took 1-2 years to resolve, but nearly a quarter (23%) said the process dragged on for 3-5 years.
One-third of psychiatrists who were named in a malpractice lawsuit said the case was settled out of court. Of the cases that went to trial, 12% of psychiatrists reported that the verdict was in their favor; 3% reported that the outcome of the case was in the plaintiff’s favor.
Asked why they think most malpractice lawsuits occurred, 61% of psychiatrists said that patients don’t understand medical risks and blame the doctor for bad outcomes even if the doctor does everything right.
A similar percentage of psychiatrists recognized that if a true medical error has occurred, patients wanted to seek restitution and/or assign blame. Only 29% of psychiatrists felt that constant advertising by lawyers to get new clients was the reason for most malpractice cases.
The overwhelming majority of psychiatrists (93%) who responded to the survey carry malpractice insurance, about the same as physicians overall (94%).
Among those with malpractice coverage who either settled or went to trial, about half were either encouraged by their insurer to settle the case or were required by their insurer to do so.
“Generally, if a physician senses that he or she is heading toward a difference of opinion with the insurer about settlement, they probably ought to invest a little time in having personal counsel look at the case,” Mr. Szabo said.
Practice changing?
Facing a lawsuit can be devastating for any physician, but nearly half (48%) of psychiatrists surveyed said they made no changes after the case was resolved.
Just over a quarter (27%) of psychiatrists said the legal action prompted a change in their approach to patients. In addition, 8% said they left their practice setting, and 3% said they bought more malpractice insurance.
Among psychiatrist cases that resulted in a settlement or a verdict in the plaintiff’s favor, nearly half (48%) of monetary awards maxed out at $100,000, while 31% maxed out at $500,000, and 8% at $1 million.
More than half of psychiatrists (55%) named in a lawsuit believed the outcome of the case was fair; 45% felt it was unfair.
Psychiatrists reported that, in retrospect, they would have done several things differently. These included maintaining better documentation of their patient’s chart (20%) and not taking on the patient in the first place (15%), followed by spending more time with the patient and his/her family (11%), getting a second opinion from a colleague (9%), and reviewing the history/chart more carefully (7%).
About three-quarters of psychiatrists felt that saying sorry or offering an apology to the patient would not have prevented the lawsuit. This is a lower percentage than was indicated by all physicians (82%) who have been sued.
Psychiatrists believe the best ways to discourage lawsuits is through better patient communication and rapport (59%) and having a medical panel screen cases for merit (50%).
About half of psychiatrists (51%) and more than half (56%) of all physicians believe that medical organizations or state societies are not doing enough to discourage malpractice cases.
This article first appeared on Medscape.com.
Forty-one percent of U.S. psychiatrists have been sued for malpractice at least once, findings from the newly released Medscape Psychiatrist Malpractice Report 2019 show.
The top reason for the legal action was wrongful death (31%), followed by poor outcome/disease progression (23%), failure to treat/delayed treatment (11%), errors in medication administration (10%), and complications from treatment/surgery (8%).
Only 7% of psychiatrists said failure to treat/delayed treatment was the reason for the lawsuit, whereas this was the top reason for physicians overall (33%) in the report.
Medscape surveyed 4,360 physician members in more than 25 specialties about whether they have been sued for malpractice, reasons for a lawsuit, what happened, and how the experience affected the way they practice medicine and interact with patients.
Among psychiatrists named in a lawsuit, 44% said they were very surprised to be the subject of litigation. A similar percentage reported they were somewhat surprised (41%), while 15% were not at all surprised.
The vast majority of psychiatrists (87%) believed the lawsuit was not warranted, while 11% were unsure. Only a small percentage (2%) believed that legal action was justified, the lowest percentage of all physicians (6%).
Among psychiatrists who were sued, 42% were able to identify the incident that sparked the lawsuit. A slightly higher percentage (47%) said there was no specific incident that spurred legal action; 11% couldn’t recall.
Psychological factors
“There’s a whole host of what you could call psychological factors that can contribute to the filing of a claim,” David S. Szabo, a malpractice defense attorney with Locke Lord LLP, Boston, said in an interview.
“These can occur when a patient perceives a breakdown in the doctor-patient relationship or is pretty certain that there’s been a mistake and they feel like they’ve been shut out of productive conversation with their health care provider or providers,” said Mr. Szabo.
Legal action eats up time. A total of 43% of psychiatrists reported spending more than 40 hours on their defense, which involved gathering records, meeting with attorneys, and preparing for depositions.
Forty-six percent reported that the entire process took 1-2 years to resolve, but nearly a quarter (23%) said the process dragged on for 3-5 years.
One-third of psychiatrists who were named in a malpractice lawsuit said the case was settled out of court. Of the cases that went to trial, 12% of psychiatrists reported that the verdict was in their favor; 3% reported that the outcome of the case was in the plaintiff’s favor.
Asked why they think most malpractice lawsuits occurred, 61% of psychiatrists said that patients don’t understand medical risks and blame the doctor for bad outcomes even if the doctor does everything right.
A similar percentage of psychiatrists recognized that if a true medical error has occurred, patients wanted to seek restitution and/or assign blame. Only 29% of psychiatrists felt that constant advertising by lawyers to get new clients was the reason for most malpractice cases.
The overwhelming majority of psychiatrists (93%) who responded to the survey carry malpractice insurance, about the same as physicians overall (94%).
Among those with malpractice coverage who either settled or went to trial, about half were either encouraged by their insurer to settle the case or were required by their insurer to do so.
“Generally, if a physician senses that he or she is heading toward a difference of opinion with the insurer about settlement, they probably ought to invest a little time in having personal counsel look at the case,” Mr. Szabo said.
Practice changing?
Facing a lawsuit can be devastating for any physician, but nearly half (48%) of psychiatrists surveyed said they made no changes after the case was resolved.
Just over a quarter (27%) of psychiatrists said the legal action prompted a change in their approach to patients. In addition, 8% said they left their practice setting, and 3% said they bought more malpractice insurance.
Among psychiatrist cases that resulted in a settlement or a verdict in the plaintiff’s favor, nearly half (48%) of monetary awards maxed out at $100,000, while 31% maxed out at $500,000, and 8% at $1 million.
More than half of psychiatrists (55%) named in a lawsuit believed the outcome of the case was fair; 45% felt it was unfair.
Psychiatrists reported that, in retrospect, they would have done several things differently. These included maintaining better documentation of their patient’s chart (20%) and not taking on the patient in the first place (15%), followed by spending more time with the patient and his/her family (11%), getting a second opinion from a colleague (9%), and reviewing the history/chart more carefully (7%).
About three-quarters of psychiatrists felt that saying sorry or offering an apology to the patient would not have prevented the lawsuit. This is a lower percentage than was indicated by all physicians (82%) who have been sued.
Psychiatrists believe the best ways to discourage lawsuits is through better patient communication and rapport (59%) and having a medical panel screen cases for merit (50%).
About half of psychiatrists (51%) and more than half (56%) of all physicians believe that medical organizations or state societies are not doing enough to discourage malpractice cases.
This article first appeared on Medscape.com.
Forty-one percent of U.S. psychiatrists have been sued for malpractice at least once, findings from the newly released Medscape Psychiatrist Malpractice Report 2019 show.
The top reason for the legal action was wrongful death (31%), followed by poor outcome/disease progression (23%), failure to treat/delayed treatment (11%), errors in medication administration (10%), and complications from treatment/surgery (8%).
Only 7% of psychiatrists said failure to treat/delayed treatment was the reason for the lawsuit, whereas this was the top reason for physicians overall (33%) in the report.
Medscape surveyed 4,360 physician members in more than 25 specialties about whether they have been sued for malpractice, reasons for a lawsuit, what happened, and how the experience affected the way they practice medicine and interact with patients.
Among psychiatrists named in a lawsuit, 44% said they were very surprised to be the subject of litigation. A similar percentage reported they were somewhat surprised (41%), while 15% were not at all surprised.
The vast majority of psychiatrists (87%) believed the lawsuit was not warranted, while 11% were unsure. Only a small percentage (2%) believed that legal action was justified, the lowest percentage of all physicians (6%).
Among psychiatrists who were sued, 42% were able to identify the incident that sparked the lawsuit. A slightly higher percentage (47%) said there was no specific incident that spurred legal action; 11% couldn’t recall.
Psychological factors
“There’s a whole host of what you could call psychological factors that can contribute to the filing of a claim,” David S. Szabo, a malpractice defense attorney with Locke Lord LLP, Boston, said in an interview.
“These can occur when a patient perceives a breakdown in the doctor-patient relationship or is pretty certain that there’s been a mistake and they feel like they’ve been shut out of productive conversation with their health care provider or providers,” said Mr. Szabo.
Legal action eats up time. A total of 43% of psychiatrists reported spending more than 40 hours on their defense, which involved gathering records, meeting with attorneys, and preparing for depositions.
Forty-six percent reported that the entire process took 1-2 years to resolve, but nearly a quarter (23%) said the process dragged on for 3-5 years.
One-third of psychiatrists who were named in a malpractice lawsuit said the case was settled out of court. Of the cases that went to trial, 12% of psychiatrists reported that the verdict was in their favor; 3% reported that the outcome of the case was in the plaintiff’s favor.
Asked why they think most malpractice lawsuits occurred, 61% of psychiatrists said that patients don’t understand medical risks and blame the doctor for bad outcomes even if the doctor does everything right.
A similar percentage of psychiatrists recognized that if a true medical error has occurred, patients wanted to seek restitution and/or assign blame. Only 29% of psychiatrists felt that constant advertising by lawyers to get new clients was the reason for most malpractice cases.
The overwhelming majority of psychiatrists (93%) who responded to the survey carry malpractice insurance, about the same as physicians overall (94%).
Among those with malpractice coverage who either settled or went to trial, about half were either encouraged by their insurer to settle the case or were required by their insurer to do so.
“Generally, if a physician senses that he or she is heading toward a difference of opinion with the insurer about settlement, they probably ought to invest a little time in having personal counsel look at the case,” Mr. Szabo said.
Practice changing?
Facing a lawsuit can be devastating for any physician, but nearly half (48%) of psychiatrists surveyed said they made no changes after the case was resolved.
Just over a quarter (27%) of psychiatrists said the legal action prompted a change in their approach to patients. In addition, 8% said they left their practice setting, and 3% said they bought more malpractice insurance.
Among psychiatrist cases that resulted in a settlement or a verdict in the plaintiff’s favor, nearly half (48%) of monetary awards maxed out at $100,000, while 31% maxed out at $500,000, and 8% at $1 million.
More than half of psychiatrists (55%) named in a lawsuit believed the outcome of the case was fair; 45% felt it was unfair.
Psychiatrists reported that, in retrospect, they would have done several things differently. These included maintaining better documentation of their patient’s chart (20%) and not taking on the patient in the first place (15%), followed by spending more time with the patient and his/her family (11%), getting a second opinion from a colleague (9%), and reviewing the history/chart more carefully (7%).
About three-quarters of psychiatrists felt that saying sorry or offering an apology to the patient would not have prevented the lawsuit. This is a lower percentage than was indicated by all physicians (82%) who have been sued.
Psychiatrists believe the best ways to discourage lawsuits is through better patient communication and rapport (59%) and having a medical panel screen cases for merit (50%).
About half of psychiatrists (51%) and more than half (56%) of all physicians believe that medical organizations or state societies are not doing enough to discourage malpractice cases.
This article first appeared on Medscape.com.
HHS: Coronavirus risk low in U.S., vaccine development underway
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
How to help patients become successful diabetes self-managers
Through the years, I have had the privilege of educating clinicians about scientific advances in diabetes care. Prior to displaying the first slide of my presentation, I ask the audience, “How many of you have seen a ‘noncompliant’ patient with diabetes within the past month?” Without fail, 99% of the attendees will raise their hand and start laughing, as if to say, “Well, of course they’re noncompliant. They just don’t get it! How incompetent can these people be?”
Blaming patients for failing to achieve metabolic control is inappropriate and misguided. How many physicians would be able or willing to monitor their blood glucose levels 4 times a day? Based on the premeal glucose level, how many physicians know how much insulin to inject in order to keep the postprandial excursions below 180 mg/dL? How many would remember to take 8 other medications daily without missing a dose? And how many physicians exercise daily; have actually looked at their feet in the past month; and have gained no weight in the past year?
Diabetes self-management is time-consuming and difficult for many patients, especially those with health illiteracy, financial restraints, or social barriers. Any patient who presents to the doctor is, in fact, “compliant.” These individuals expect to receive the safest and most effective treatments for their diabetes while learning as much as possible about lifestyle and behavioral interventions.
I challenge each of you to ask your patients: “What concerns you the most about having diabetes?” Initially, patients will express guilt and remorse about having diabetes. They will hang their heads in shame, admit to not going to the gym as often as they could, and promise to eat smaller portions. They will then look you in the eye and say, “I am worried about losing my eye, my leg, and my kidney to this life-threatening disease. I’m scared I won’t be able to see my daughter walk down the aisle at her wedding or my son graduate from college.”
These patients are terrified because they are unfamiliar with the advances in clinical science that aid our ability to improve the lives of all patients with diabetes. After hearing patients’ concerns about dying prematurely or losing an extremity to diabetes, I assure them that, “Nothing is going to happen to you on my watch. You are safe with me, and I will always have your back.” This level of trust is vitally important to the patient as well as to the treating physician. We all want our patients to achieve treatment success, just as any teacher would want their students to excel and graduate to the next grade level. Diabetes cannot be cured, yet we, as physicians, are able to heal with reassurance and expert guidance.
So, how do we help our patients achieve better adherence to their chronic disease state interventions? Here are 9 techniques that I have learned in my years helping patients manage their diabetes (all of which are more broadly applicable to any chronic disease state):
- Explain the disease state you are comanaging with the patient to the best of your ability. The more the patient understands, the easier your job as the “drug police” becomes.
- Remind the patient that he/she is the captain of the disease management team. You, the physician, serve as the personal coach. You can help the patient win the game, but he/she is ultimately responsible for achieving successful metabolic targets.
- Explain the risks, benefits, and any expected adverse effects that are likely to occur. Do this prior to initiating any medication.
- Discuss when metabolic change might be expected after initiating a given medication. Patients who observe rapid improvement in their glucose levels will be encouraged to adhere to their prescribed treatment regimen.
- Make certain that any and all screening tests are performed prior to initiating a medication. For example, renal function should be assessed prior to beginning most diabetes medications.
- Use shared decision-making to negotiate acceptable metabolic targets with each patient. Discuss the urgency and importance of achieving these goals.
- Assess the A1C reduction from baseline at 4 weeks after therapy initiation, rather than at 3 months. About 50% of the total A1C is reflective of the preceding 4 weeks of treatment.1 Thus, if the baseline A1C drops from 8.2% to 7.8%, the patient is moving in the right direction. However, if the A1C increases from 8.2% to 8.5%, the patient is not taking the prescribed medications.
- Recommend that the patient use a continuous glucose monitor; this newer technology is now readily available for patients who are covered by private insurance or Medicare. These devices allow patients to observe their glucose levels every 5 minutes of every day without fingersticks and actually cost less than self-monitoring one’s blood glucose.
- Find a reason to praise the patient at each visit. Patients who receive a powerful compliment, such as “I am so very proud of your efforts at improving your diabetes control,” will do their absolute best to remain adherent to their prescribed medication regimen. The response might be different if a patient is told, “Once again, your blood sugars are too high. At this rate, you are probably going to die, just like your father did 20 years ago. Now come back in 6 months and show me what you’re really made of!”
With more than 30 million Americans living with diabetes and another 84 million with prediabetes, the burden of preventive and intensive care lies squarely with family physicians.2 Rather than complain about our patients’ lack of metabolic control, we should provide them with the knowledge, skills, tools, and encouragement that they need to become successful diabetes self-managers.
1. Berard LD, Siemens R, Woo V; Diabetes Canada Clinical Practice Guidelines Expert Committee. Monitoring glycemic control. Can J Diabetes. 2018;42(suppl 1):S47-S325.
2. Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes. July 18, 2017. www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed January 15, 2020.
Through the years, I have had the privilege of educating clinicians about scientific advances in diabetes care. Prior to displaying the first slide of my presentation, I ask the audience, “How many of you have seen a ‘noncompliant’ patient with diabetes within the past month?” Without fail, 99% of the attendees will raise their hand and start laughing, as if to say, “Well, of course they’re noncompliant. They just don’t get it! How incompetent can these people be?”
Blaming patients for failing to achieve metabolic control is inappropriate and misguided. How many physicians would be able or willing to monitor their blood glucose levels 4 times a day? Based on the premeal glucose level, how many physicians know how much insulin to inject in order to keep the postprandial excursions below 180 mg/dL? How many would remember to take 8 other medications daily without missing a dose? And how many physicians exercise daily; have actually looked at their feet in the past month; and have gained no weight in the past year?
Diabetes self-management is time-consuming and difficult for many patients, especially those with health illiteracy, financial restraints, or social barriers. Any patient who presents to the doctor is, in fact, “compliant.” These individuals expect to receive the safest and most effective treatments for their diabetes while learning as much as possible about lifestyle and behavioral interventions.
I challenge each of you to ask your patients: “What concerns you the most about having diabetes?” Initially, patients will express guilt and remorse about having diabetes. They will hang their heads in shame, admit to not going to the gym as often as they could, and promise to eat smaller portions. They will then look you in the eye and say, “I am worried about losing my eye, my leg, and my kidney to this life-threatening disease. I’m scared I won’t be able to see my daughter walk down the aisle at her wedding or my son graduate from college.”
These patients are terrified because they are unfamiliar with the advances in clinical science that aid our ability to improve the lives of all patients with diabetes. After hearing patients’ concerns about dying prematurely or losing an extremity to diabetes, I assure them that, “Nothing is going to happen to you on my watch. You are safe with me, and I will always have your back.” This level of trust is vitally important to the patient as well as to the treating physician. We all want our patients to achieve treatment success, just as any teacher would want their students to excel and graduate to the next grade level. Diabetes cannot be cured, yet we, as physicians, are able to heal with reassurance and expert guidance.
So, how do we help our patients achieve better adherence to their chronic disease state interventions? Here are 9 techniques that I have learned in my years helping patients manage their diabetes (all of which are more broadly applicable to any chronic disease state):
- Explain the disease state you are comanaging with the patient to the best of your ability. The more the patient understands, the easier your job as the “drug police” becomes.
- Remind the patient that he/she is the captain of the disease management team. You, the physician, serve as the personal coach. You can help the patient win the game, but he/she is ultimately responsible for achieving successful metabolic targets.
- Explain the risks, benefits, and any expected adverse effects that are likely to occur. Do this prior to initiating any medication.
- Discuss when metabolic change might be expected after initiating a given medication. Patients who observe rapid improvement in their glucose levels will be encouraged to adhere to their prescribed treatment regimen.
- Make certain that any and all screening tests are performed prior to initiating a medication. For example, renal function should be assessed prior to beginning most diabetes medications.
- Use shared decision-making to negotiate acceptable metabolic targets with each patient. Discuss the urgency and importance of achieving these goals.
- Assess the A1C reduction from baseline at 4 weeks after therapy initiation, rather than at 3 months. About 50% of the total A1C is reflective of the preceding 4 weeks of treatment.1 Thus, if the baseline A1C drops from 8.2% to 7.8%, the patient is moving in the right direction. However, if the A1C increases from 8.2% to 8.5%, the patient is not taking the prescribed medications.
- Recommend that the patient use a continuous glucose monitor; this newer technology is now readily available for patients who are covered by private insurance or Medicare. These devices allow patients to observe their glucose levels every 5 minutes of every day without fingersticks and actually cost less than self-monitoring one’s blood glucose.
- Find a reason to praise the patient at each visit. Patients who receive a powerful compliment, such as “I am so very proud of your efforts at improving your diabetes control,” will do their absolute best to remain adherent to their prescribed medication regimen. The response might be different if a patient is told, “Once again, your blood sugars are too high. At this rate, you are probably going to die, just like your father did 20 years ago. Now come back in 6 months and show me what you’re really made of!”
With more than 30 million Americans living with diabetes and another 84 million with prediabetes, the burden of preventive and intensive care lies squarely with family physicians.2 Rather than complain about our patients’ lack of metabolic control, we should provide them with the knowledge, skills, tools, and encouragement that they need to become successful diabetes self-managers.
Through the years, I have had the privilege of educating clinicians about scientific advances in diabetes care. Prior to displaying the first slide of my presentation, I ask the audience, “How many of you have seen a ‘noncompliant’ patient with diabetes within the past month?” Without fail, 99% of the attendees will raise their hand and start laughing, as if to say, “Well, of course they’re noncompliant. They just don’t get it! How incompetent can these people be?”
Blaming patients for failing to achieve metabolic control is inappropriate and misguided. How many physicians would be able or willing to monitor their blood glucose levels 4 times a day? Based on the premeal glucose level, how many physicians know how much insulin to inject in order to keep the postprandial excursions below 180 mg/dL? How many would remember to take 8 other medications daily without missing a dose? And how many physicians exercise daily; have actually looked at their feet in the past month; and have gained no weight in the past year?
Diabetes self-management is time-consuming and difficult for many patients, especially those with health illiteracy, financial restraints, or social barriers. Any patient who presents to the doctor is, in fact, “compliant.” These individuals expect to receive the safest and most effective treatments for their diabetes while learning as much as possible about lifestyle and behavioral interventions.
I challenge each of you to ask your patients: “What concerns you the most about having diabetes?” Initially, patients will express guilt and remorse about having diabetes. They will hang their heads in shame, admit to not going to the gym as often as they could, and promise to eat smaller portions. They will then look you in the eye and say, “I am worried about losing my eye, my leg, and my kidney to this life-threatening disease. I’m scared I won’t be able to see my daughter walk down the aisle at her wedding or my son graduate from college.”
These patients are terrified because they are unfamiliar with the advances in clinical science that aid our ability to improve the lives of all patients with diabetes. After hearing patients’ concerns about dying prematurely or losing an extremity to diabetes, I assure them that, “Nothing is going to happen to you on my watch. You are safe with me, and I will always have your back.” This level of trust is vitally important to the patient as well as to the treating physician. We all want our patients to achieve treatment success, just as any teacher would want their students to excel and graduate to the next grade level. Diabetes cannot be cured, yet we, as physicians, are able to heal with reassurance and expert guidance.
So, how do we help our patients achieve better adherence to their chronic disease state interventions? Here are 9 techniques that I have learned in my years helping patients manage their diabetes (all of which are more broadly applicable to any chronic disease state):
- Explain the disease state you are comanaging with the patient to the best of your ability. The more the patient understands, the easier your job as the “drug police” becomes.
- Remind the patient that he/she is the captain of the disease management team. You, the physician, serve as the personal coach. You can help the patient win the game, but he/she is ultimately responsible for achieving successful metabolic targets.
- Explain the risks, benefits, and any expected adverse effects that are likely to occur. Do this prior to initiating any medication.
- Discuss when metabolic change might be expected after initiating a given medication. Patients who observe rapid improvement in their glucose levels will be encouraged to adhere to their prescribed treatment regimen.
- Make certain that any and all screening tests are performed prior to initiating a medication. For example, renal function should be assessed prior to beginning most diabetes medications.
- Use shared decision-making to negotiate acceptable metabolic targets with each patient. Discuss the urgency and importance of achieving these goals.
- Assess the A1C reduction from baseline at 4 weeks after therapy initiation, rather than at 3 months. About 50% of the total A1C is reflective of the preceding 4 weeks of treatment.1 Thus, if the baseline A1C drops from 8.2% to 7.8%, the patient is moving in the right direction. However, if the A1C increases from 8.2% to 8.5%, the patient is not taking the prescribed medications.
- Recommend that the patient use a continuous glucose monitor; this newer technology is now readily available for patients who are covered by private insurance or Medicare. These devices allow patients to observe their glucose levels every 5 minutes of every day without fingersticks and actually cost less than self-monitoring one’s blood glucose.
- Find a reason to praise the patient at each visit. Patients who receive a powerful compliment, such as “I am so very proud of your efforts at improving your diabetes control,” will do their absolute best to remain adherent to their prescribed medication regimen. The response might be different if a patient is told, “Once again, your blood sugars are too high. At this rate, you are probably going to die, just like your father did 20 years ago. Now come back in 6 months and show me what you’re really made of!”
With more than 30 million Americans living with diabetes and another 84 million with prediabetes, the burden of preventive and intensive care lies squarely with family physicians.2 Rather than complain about our patients’ lack of metabolic control, we should provide them with the knowledge, skills, tools, and encouragement that they need to become successful diabetes self-managers.
1. Berard LD, Siemens R, Woo V; Diabetes Canada Clinical Practice Guidelines Expert Committee. Monitoring glycemic control. Can J Diabetes. 2018;42(suppl 1):S47-S325.
2. Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes. July 18, 2017. www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed January 15, 2020.
1. Berard LD, Siemens R, Woo V; Diabetes Canada Clinical Practice Guidelines Expert Committee. Monitoring glycemic control. Can J Diabetes. 2018;42(suppl 1):S47-S325.
2. Centers for Disease Control and Prevention. New CDC report: More than 100 million Americans have diabetes or prediabetes. July 18, 2017. www.cdc.gov/media/releases/2017/p0718-diabetes-report.html. Accessed January 15, 2020.
Evidence-based tools for premenstrual disorders
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
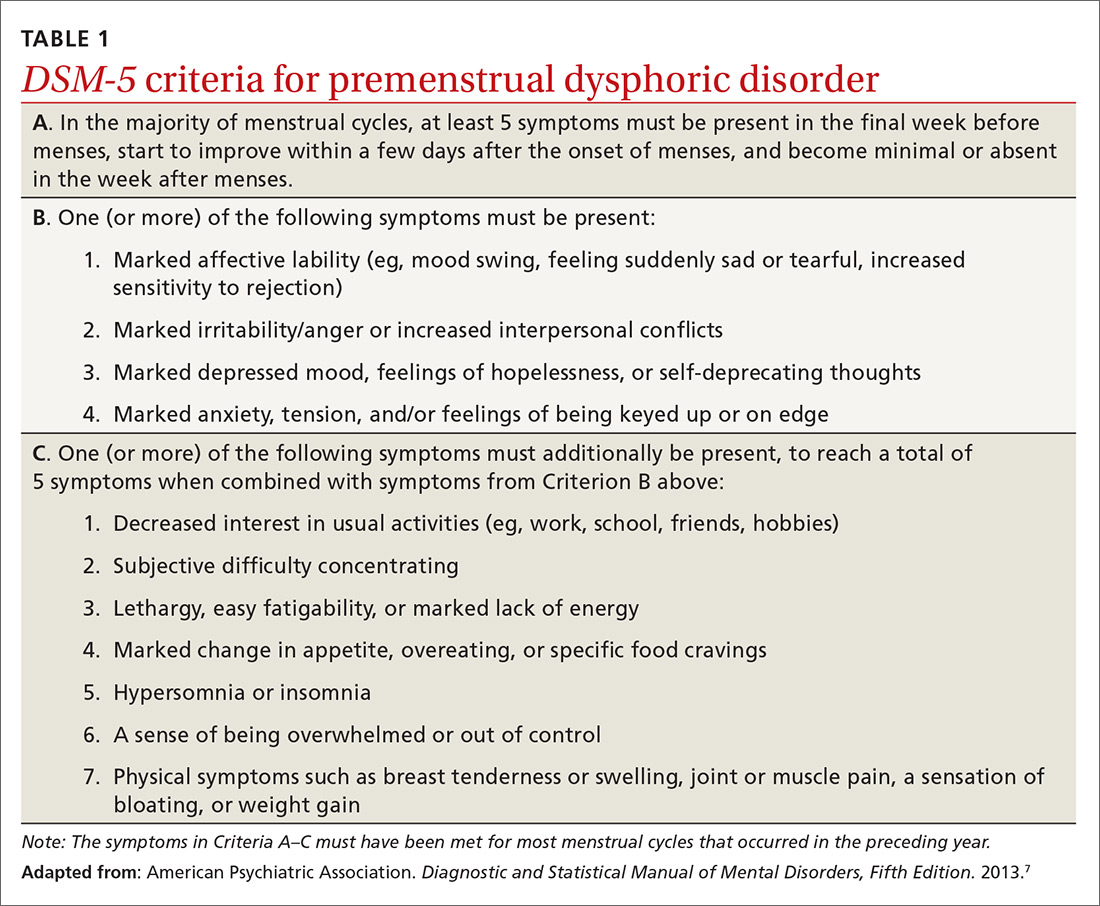
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
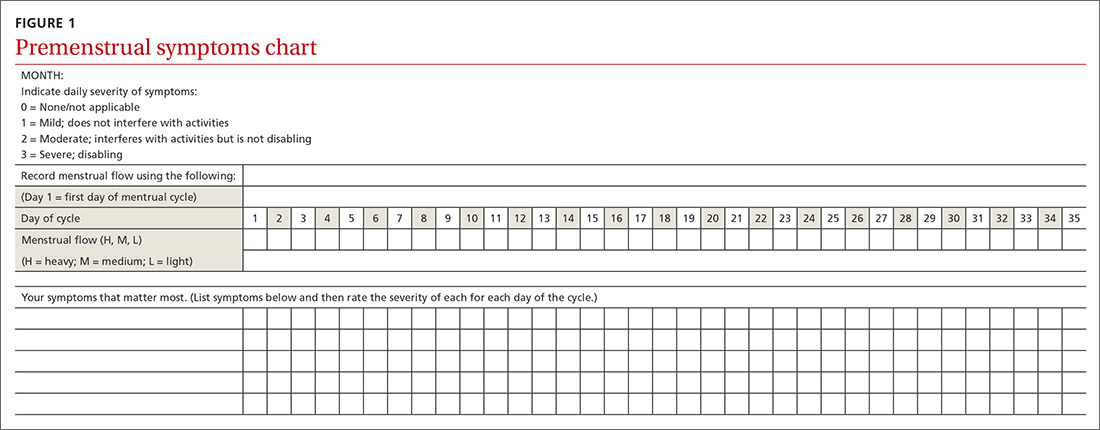
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
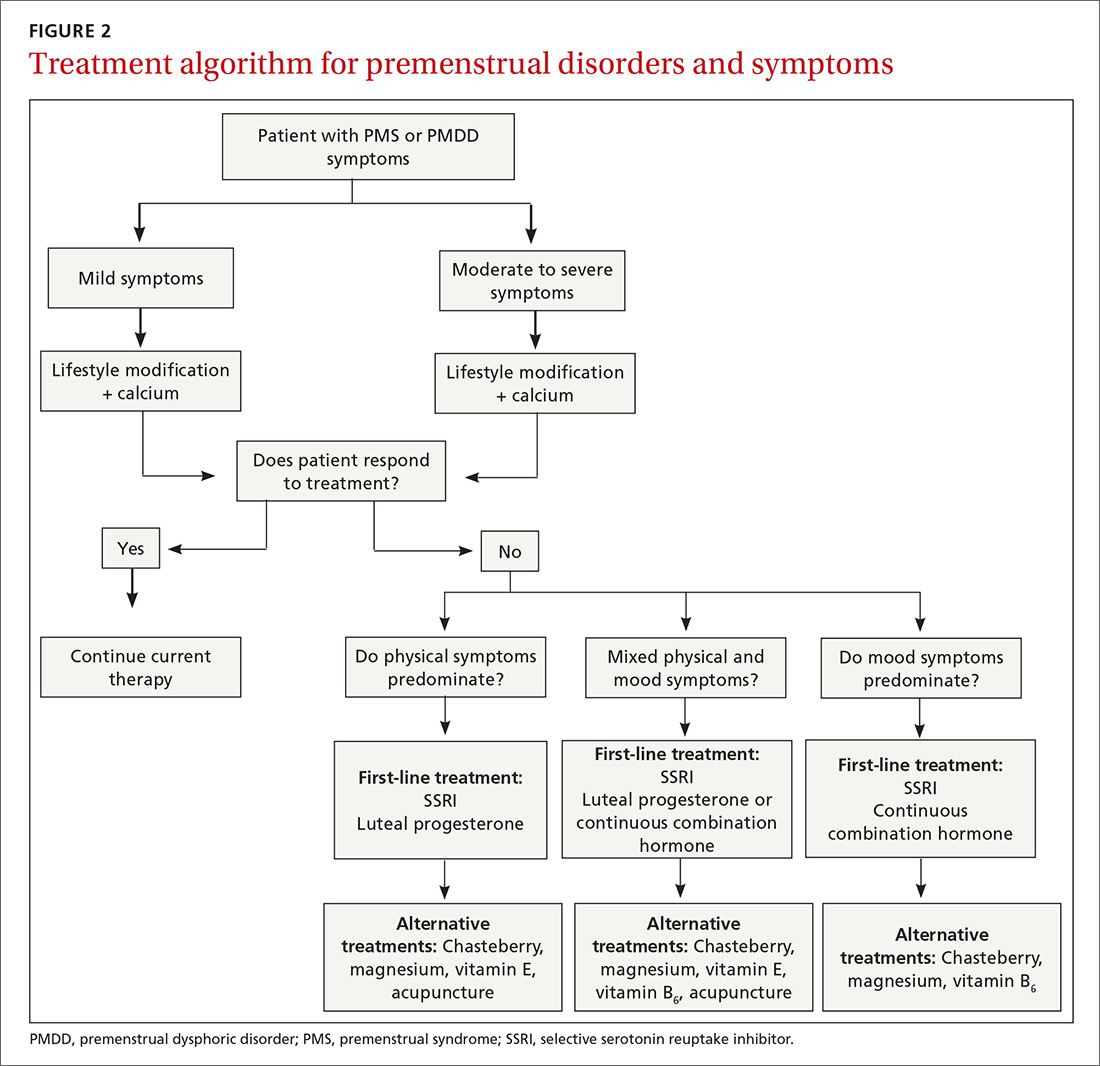
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
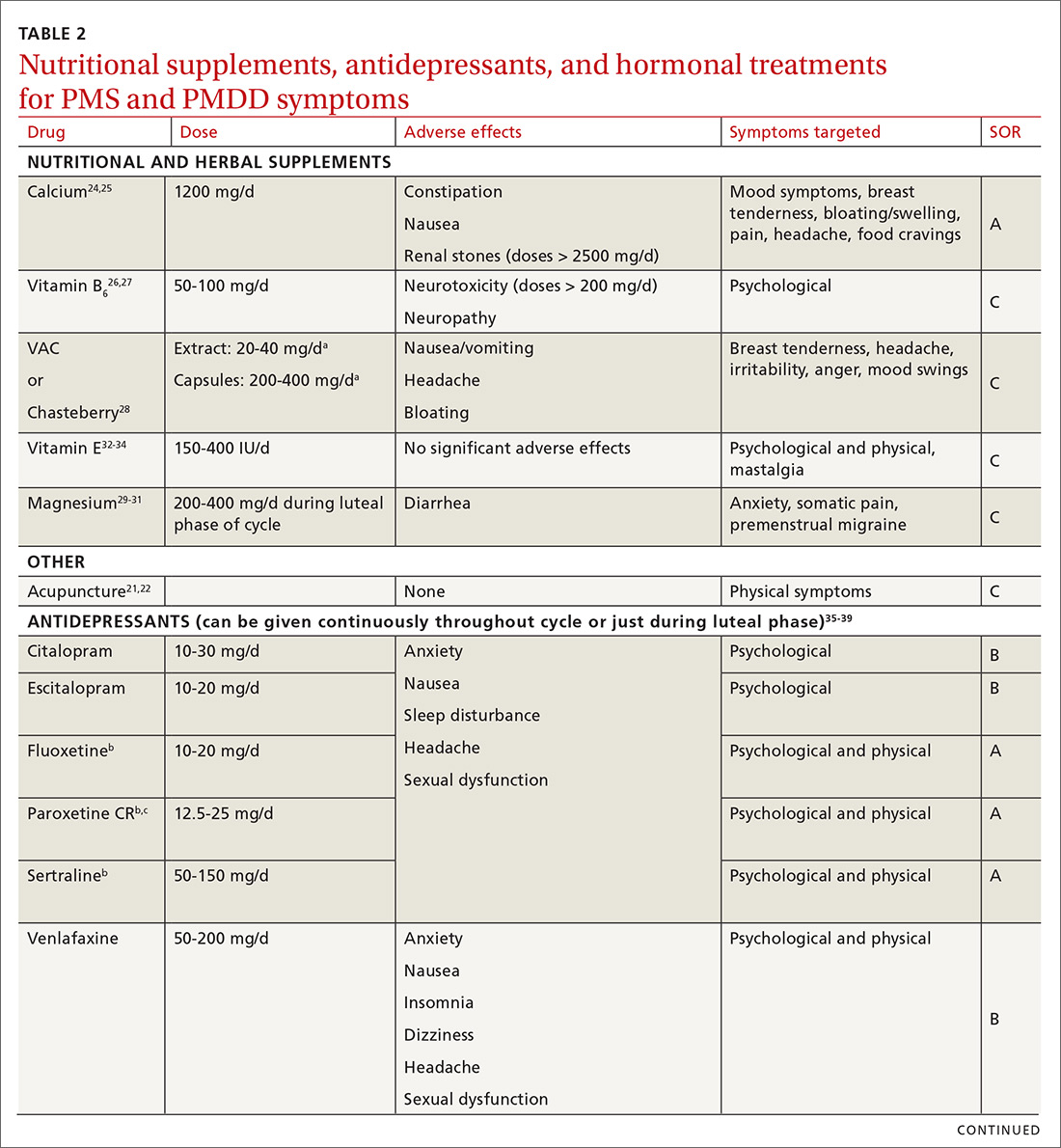
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
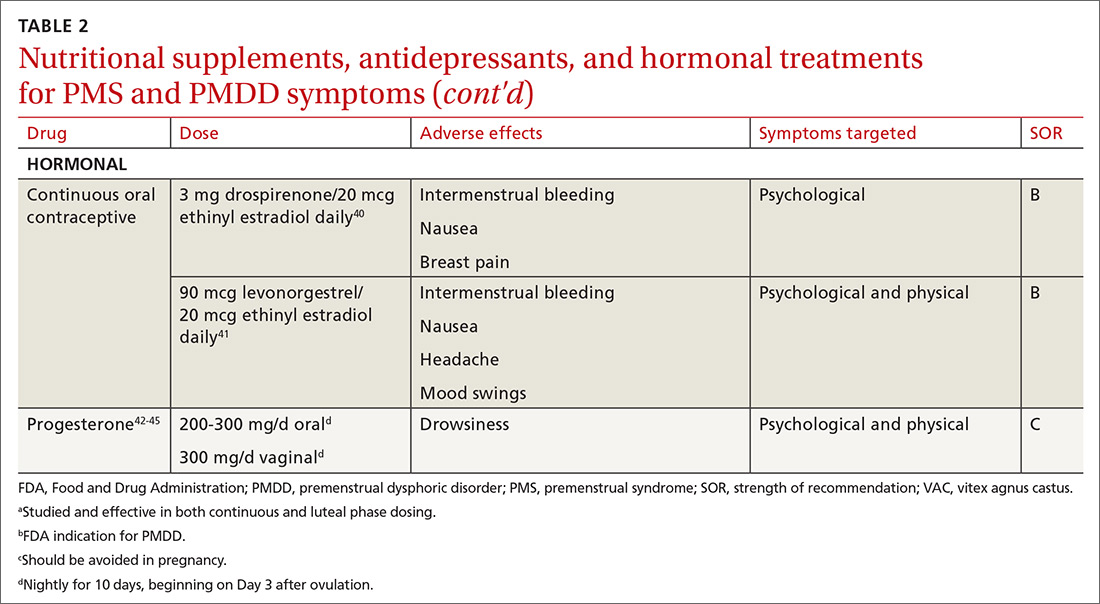
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7

Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.

Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness

Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25

Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27

Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7

Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.

Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness

Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25

Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27

Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
PRACTICE RECOMMENDATIONS
› Start calcium supplementation in all patients who report significant premenstrual symptoms. A
› Add a selective serotonin reuptake inhibitor (SSRI) to calcium supplementationfor patients who have more severe premenstrual psychological symptoms. A
› Consider hormonal treatment options for patients who require treatment beyond calcium and an SSRI. B
› Provide nutrition and exercise information to all patients who report significant premenstrual symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series











