User login
Silent ischemia isn’t what it used to be
SNOWMASS, COLO. – The concept that silent myocardial ischemia is clinically detrimental has fallen by the wayside, and routine screening for this phenomenon can no longer be recommended, Patrick T. O’Gara, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
What a difference a decade or 2 can make.
“Think about where we were 25 years ago, when we worried about people who had transient ST-segment depression without angina on Holter monitoring. We would wig out, chase them down the street, try to tackle them and load them up with medications and think about balloon [percutaneous transluminal coronary angioplasty]. And now we’re at the point where it doesn’t seem to help with respect to quality of life, let alone death or myocardial infarction,” observed Dr. O’Gara, director of clinical cardiology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
The end of the line for the now-discredited notion that silent ischemia carries clinical significance approaching that of ischemia plus angina pectoris was the landmark ISCHEMIA trial, reported in November 2019 at the annual scientific sessions of the American Heart Association. This randomized trial asked the question: Is there any high-risk subgroup of patients with stable ischemic heart disease not involving the left main coronary artery for whom a strategy of routine revascularization improves hard outcomes in the current era of highly effective, guideline-directed medical therapy?
The answer turned out to be no. At 5 years of follow-up of 5,179 randomized patients with baseline stable coronary artery disease (CAD) and rigorously determined baseline moderate or severe ischemia affecting more than 10% of the myocardium, there was no difference between patients randomized to routine revascularization plus optimal medical therapy versus those on optimal medical therapy alone in the primary combined outcome of cardiovascular death, MI, heart failure, cardiac arrest, or hospitalization for unstable angina.
Of note, 35% of participants in the ISCHEMIA trial had moderate or severe silent ischemia. Like those who had angina, they achieved no additional benefit from a strategy of routine revascularization in terms of the primary outcome. ISCHEMIA participants with angina did show significant and durable improvements in quality of life and angina control with routine revascularization; however, those with silent ischemia showed little or no such improvement with an invasive strategy.
That being said, Dr. O’Gara added that he supports the ISCHEMIA investigators’ efforts to obtain funding from the National Institutes of Health for another 5 years or so of follow-up in order to determine whether revascularization actually does lead to improvement in the hard outcomes.
“Remember, in the STICH trial it took 10 years to show superiority of CABG [coronary artery bypass surgery] versus medical therapy to treat ischemic cardiomyopathy [N Engl J Med 2016; 374:1511-20]. My own view is that it’s too premature to throw the baby out with the bathwater. I think shared decision making is still very important, and I think, for many of our patients, relief of angina and improved quality of life are legitimate reasons in a low-risk situation with a good interventionalist to proceed,” he said.
Dr. O’Gara traced the history of medical thinking about silent ischemia. The notion that silent ischemia carried a clinical significance comparable with ischemia with angina gained wide credence more than 30 years ago, when investigators from the National Institutes of Health–sponsored Coronary Artery Surgery Study registry reported: “Patients with either silent or symptomatic ischemia during exercise testing have a similar risk of developing an acute myocardial infarction or sudden death – except in the three-vessel CAD subgroup, where the risk is greater in silent ischemia” (Am J Cardiol. 1988 Dec 1;62[17]:1155-8).
“This was a very important observation and led to many, many recommendations about screening and making sure that you took the expression of ST-segment depression on exercise treadmill testing pretty seriously, even if your patient did not have angina,” Dr. O’Gara recalled.
The prevailing wisdom that silent ischemia was detrimental took a hit in the Detection of Ischemia in Asymptomatic Diabetics (DIAC) trial. DIAC was conducted at a time when it had become clear that type 2 diabetes was a condition associated with increased cardiovascular risk, and that various methods of imaging were more accurate than treadmill exercise testing for the detection of underlying CAD. But when 1,123 DIAC participants with type 2 diabetes were randomized to screening with adenosine-stress radionuclide myocardial perfusion imaging or not and prospectively followed for roughly 5 years, it turned out there was no between-group difference in cardiac death or MI (JAMA. 2009 Apr 15;301[15]:1547-55).
“This pretty much put the lid on going out of one’s way to do routine screening of this nature in persons with diabetes who were considered to be at higher than average risk for the development of coronary disease,” the cardiologist commented.
Another fissure in the idea that silent ischemia was worth searching for and treating came from CLARIFY, an observational international registry of more than 20,000 individuals with stable CAD, roughly 12% of whom had silent ischemia, a figure in line with the prevalence reported in other studies. The 2-year rate of cardiovascular death or MI in the group with silent ischemia didn’t differ from the rate in patients with neither angina nor provocable ischemia. In contrast, rates of cardiovascular death or MI were significantly higher in the groups with angina but no ischemia or angina with ischemia (JAMA Intern Med. 2014 Oct;174[10]:1651-9).
“There’s something about the expression of angina that’s a very key clinical marker,” Dr. O’Gara observed.
He noted that just a few months before the ISCHEMIA trial results were released, a report from the far-smaller, randomized second Medicine, Angioplasty, or Surgery Study “threw cold water” on the notion that stress-induced ischemia in patients with multivessel CAD is a bad thing. Over 10 years of follow-up, the risk of major adverse cardiovascular events or deterioration in left ventricular function was identical in patients with or without baseline ischemia on stress testing performed after percutaneous coronary intervention, CABG surgery, or initiation of medical therapy (JAMA Intern Med. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2227).
What the guidelines say
The 6-year-old U.S. guidelines on the diagnosis and management of patients with stable ischemic heart disease are clearly out of date on the topic of silent ischemia (Circulation. 2014 Nov 4;130[19]:1749-67). The recommendations are based on expert opinion formed prior to the massive amount of new evidence that has since become available. For example, the current guidelines state as a class IIa, level of evidence C recommendation that exercise or pharmacologic stress can be useful for follow-up assessment at 2-year or greater intervals in patients with stable ischemic heart disease with prior evidence of silent ischemia.
“This is a very weak recommendation. The class of recommendation says it would be reasonable, but in the absence of an evidence base and in light of newer information, I’m not sure that it approaches even a class IIa level of recommendation,” according to Dr. O’Gara.
The 2019 European Society of Cardiology guidelines on chronic coronary syndromes are similarly weak on silent ischemia. The European guidelines state that patients with diabetes or chronic kidney disease may have a higher burden of silent ischemia, might be at higher risk for atherosclerotic cardiovascular disease events, and that periodic ECGs and functional testing every 3-5 years might be considered.
“Obviously there’s a lot of leeway there in how you wish to interpret that,” Dr. O’Gara said. “And this did not rise to the level where they’d put it in the table of recommendations, but it’s simply included as part of the explanatory text.”
What’s coming next in stable ischemic heart disease
“Nowadays all the rage has to do with coronary microvascular dysfunction,” according to Dr. O’Gara. “I think all of the research interest currently is focused on the coronary microcirculation as perhaps the next frontier in our understanding of why it is that ischemia can occur in the absence of epicardial coronary disease.”
He highly recommended a review article entitled: “Reappraisal of Ischemic Heart Disease,” in which an international trio of prominent cardiologists asserted that coronary microvascular dysfunction not only plays a pivotal pathogenic role in angina pectoris, but also in a phenomenon known as microvascular angina – that is, angina in the absence of obstructive CAD. Microvascular angina may explain the roughly one-third of patients who experience acute coronary syndrome without epicardial coronary artery stenosis or thrombosis. The authors delved into the structural and functional mechanisms underlying coronary microvascular dysfunction, while noting that effective treatment of this common phenomenon remains a major unmet need (Circulation. 2018 Oct 2;138[14]:1463-80).
Dr. O’Gara reported receiving funding from the National Heart, Lung, and Blood Institute; from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial; from Edwards Lifesciences for the ongoing EARLY TAVR trial; and from Medtrace Pharma, a Danish company developing an innovative form of PET diagnostic imaging.
SNOWMASS, COLO. – The concept that silent myocardial ischemia is clinically detrimental has fallen by the wayside, and routine screening for this phenomenon can no longer be recommended, Patrick T. O’Gara, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
What a difference a decade or 2 can make.
“Think about where we were 25 years ago, when we worried about people who had transient ST-segment depression without angina on Holter monitoring. We would wig out, chase them down the street, try to tackle them and load them up with medications and think about balloon [percutaneous transluminal coronary angioplasty]. And now we’re at the point where it doesn’t seem to help with respect to quality of life, let alone death or myocardial infarction,” observed Dr. O’Gara, director of clinical cardiology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
The end of the line for the now-discredited notion that silent ischemia carries clinical significance approaching that of ischemia plus angina pectoris was the landmark ISCHEMIA trial, reported in November 2019 at the annual scientific sessions of the American Heart Association. This randomized trial asked the question: Is there any high-risk subgroup of patients with stable ischemic heart disease not involving the left main coronary artery for whom a strategy of routine revascularization improves hard outcomes in the current era of highly effective, guideline-directed medical therapy?
The answer turned out to be no. At 5 years of follow-up of 5,179 randomized patients with baseline stable coronary artery disease (CAD) and rigorously determined baseline moderate or severe ischemia affecting more than 10% of the myocardium, there was no difference between patients randomized to routine revascularization plus optimal medical therapy versus those on optimal medical therapy alone in the primary combined outcome of cardiovascular death, MI, heart failure, cardiac arrest, or hospitalization for unstable angina.
Of note, 35% of participants in the ISCHEMIA trial had moderate or severe silent ischemia. Like those who had angina, they achieved no additional benefit from a strategy of routine revascularization in terms of the primary outcome. ISCHEMIA participants with angina did show significant and durable improvements in quality of life and angina control with routine revascularization; however, those with silent ischemia showed little or no such improvement with an invasive strategy.
That being said, Dr. O’Gara added that he supports the ISCHEMIA investigators’ efforts to obtain funding from the National Institutes of Health for another 5 years or so of follow-up in order to determine whether revascularization actually does lead to improvement in the hard outcomes.
“Remember, in the STICH trial it took 10 years to show superiority of CABG [coronary artery bypass surgery] versus medical therapy to treat ischemic cardiomyopathy [N Engl J Med 2016; 374:1511-20]. My own view is that it’s too premature to throw the baby out with the bathwater. I think shared decision making is still very important, and I think, for many of our patients, relief of angina and improved quality of life are legitimate reasons in a low-risk situation with a good interventionalist to proceed,” he said.
Dr. O’Gara traced the history of medical thinking about silent ischemia. The notion that silent ischemia carried a clinical significance comparable with ischemia with angina gained wide credence more than 30 years ago, when investigators from the National Institutes of Health–sponsored Coronary Artery Surgery Study registry reported: “Patients with either silent or symptomatic ischemia during exercise testing have a similar risk of developing an acute myocardial infarction or sudden death – except in the three-vessel CAD subgroup, where the risk is greater in silent ischemia” (Am J Cardiol. 1988 Dec 1;62[17]:1155-8).
“This was a very important observation and led to many, many recommendations about screening and making sure that you took the expression of ST-segment depression on exercise treadmill testing pretty seriously, even if your patient did not have angina,” Dr. O’Gara recalled.
The prevailing wisdom that silent ischemia was detrimental took a hit in the Detection of Ischemia in Asymptomatic Diabetics (DIAC) trial. DIAC was conducted at a time when it had become clear that type 2 diabetes was a condition associated with increased cardiovascular risk, and that various methods of imaging were more accurate than treadmill exercise testing for the detection of underlying CAD. But when 1,123 DIAC participants with type 2 diabetes were randomized to screening with adenosine-stress radionuclide myocardial perfusion imaging or not and prospectively followed for roughly 5 years, it turned out there was no between-group difference in cardiac death or MI (JAMA. 2009 Apr 15;301[15]:1547-55).
“This pretty much put the lid on going out of one’s way to do routine screening of this nature in persons with diabetes who were considered to be at higher than average risk for the development of coronary disease,” the cardiologist commented.
Another fissure in the idea that silent ischemia was worth searching for and treating came from CLARIFY, an observational international registry of more than 20,000 individuals with stable CAD, roughly 12% of whom had silent ischemia, a figure in line with the prevalence reported in other studies. The 2-year rate of cardiovascular death or MI in the group with silent ischemia didn’t differ from the rate in patients with neither angina nor provocable ischemia. In contrast, rates of cardiovascular death or MI were significantly higher in the groups with angina but no ischemia or angina with ischemia (JAMA Intern Med. 2014 Oct;174[10]:1651-9).
“There’s something about the expression of angina that’s a very key clinical marker,” Dr. O’Gara observed.
He noted that just a few months before the ISCHEMIA trial results were released, a report from the far-smaller, randomized second Medicine, Angioplasty, or Surgery Study “threw cold water” on the notion that stress-induced ischemia in patients with multivessel CAD is a bad thing. Over 10 years of follow-up, the risk of major adverse cardiovascular events or deterioration in left ventricular function was identical in patients with or without baseline ischemia on stress testing performed after percutaneous coronary intervention, CABG surgery, or initiation of medical therapy (JAMA Intern Med. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2227).
What the guidelines say
The 6-year-old U.S. guidelines on the diagnosis and management of patients with stable ischemic heart disease are clearly out of date on the topic of silent ischemia (Circulation. 2014 Nov 4;130[19]:1749-67). The recommendations are based on expert opinion formed prior to the massive amount of new evidence that has since become available. For example, the current guidelines state as a class IIa, level of evidence C recommendation that exercise or pharmacologic stress can be useful for follow-up assessment at 2-year or greater intervals in patients with stable ischemic heart disease with prior evidence of silent ischemia.
“This is a very weak recommendation. The class of recommendation says it would be reasonable, but in the absence of an evidence base and in light of newer information, I’m not sure that it approaches even a class IIa level of recommendation,” according to Dr. O’Gara.
The 2019 European Society of Cardiology guidelines on chronic coronary syndromes are similarly weak on silent ischemia. The European guidelines state that patients with diabetes or chronic kidney disease may have a higher burden of silent ischemia, might be at higher risk for atherosclerotic cardiovascular disease events, and that periodic ECGs and functional testing every 3-5 years might be considered.
“Obviously there’s a lot of leeway there in how you wish to interpret that,” Dr. O’Gara said. “And this did not rise to the level where they’d put it in the table of recommendations, but it’s simply included as part of the explanatory text.”
What’s coming next in stable ischemic heart disease
“Nowadays all the rage has to do with coronary microvascular dysfunction,” according to Dr. O’Gara. “I think all of the research interest currently is focused on the coronary microcirculation as perhaps the next frontier in our understanding of why it is that ischemia can occur in the absence of epicardial coronary disease.”
He highly recommended a review article entitled: “Reappraisal of Ischemic Heart Disease,” in which an international trio of prominent cardiologists asserted that coronary microvascular dysfunction not only plays a pivotal pathogenic role in angina pectoris, but also in a phenomenon known as microvascular angina – that is, angina in the absence of obstructive CAD. Microvascular angina may explain the roughly one-third of patients who experience acute coronary syndrome without epicardial coronary artery stenosis or thrombosis. The authors delved into the structural and functional mechanisms underlying coronary microvascular dysfunction, while noting that effective treatment of this common phenomenon remains a major unmet need (Circulation. 2018 Oct 2;138[14]:1463-80).
Dr. O’Gara reported receiving funding from the National Heart, Lung, and Blood Institute; from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial; from Edwards Lifesciences for the ongoing EARLY TAVR trial; and from Medtrace Pharma, a Danish company developing an innovative form of PET diagnostic imaging.
SNOWMASS, COLO. – The concept that silent myocardial ischemia is clinically detrimental has fallen by the wayside, and routine screening for this phenomenon can no longer be recommended, Patrick T. O’Gara, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
What a difference a decade or 2 can make.
“Think about where we were 25 years ago, when we worried about people who had transient ST-segment depression without angina on Holter monitoring. We would wig out, chase them down the street, try to tackle them and load them up with medications and think about balloon [percutaneous transluminal coronary angioplasty]. And now we’re at the point where it doesn’t seem to help with respect to quality of life, let alone death or myocardial infarction,” observed Dr. O’Gara, director of clinical cardiology at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
The end of the line for the now-discredited notion that silent ischemia carries clinical significance approaching that of ischemia plus angina pectoris was the landmark ISCHEMIA trial, reported in November 2019 at the annual scientific sessions of the American Heart Association. This randomized trial asked the question: Is there any high-risk subgroup of patients with stable ischemic heart disease not involving the left main coronary artery for whom a strategy of routine revascularization improves hard outcomes in the current era of highly effective, guideline-directed medical therapy?
The answer turned out to be no. At 5 years of follow-up of 5,179 randomized patients with baseline stable coronary artery disease (CAD) and rigorously determined baseline moderate or severe ischemia affecting more than 10% of the myocardium, there was no difference between patients randomized to routine revascularization plus optimal medical therapy versus those on optimal medical therapy alone in the primary combined outcome of cardiovascular death, MI, heart failure, cardiac arrest, or hospitalization for unstable angina.
Of note, 35% of participants in the ISCHEMIA trial had moderate or severe silent ischemia. Like those who had angina, they achieved no additional benefit from a strategy of routine revascularization in terms of the primary outcome. ISCHEMIA participants with angina did show significant and durable improvements in quality of life and angina control with routine revascularization; however, those with silent ischemia showed little or no such improvement with an invasive strategy.
That being said, Dr. O’Gara added that he supports the ISCHEMIA investigators’ efforts to obtain funding from the National Institutes of Health for another 5 years or so of follow-up in order to determine whether revascularization actually does lead to improvement in the hard outcomes.
“Remember, in the STICH trial it took 10 years to show superiority of CABG [coronary artery bypass surgery] versus medical therapy to treat ischemic cardiomyopathy [N Engl J Med 2016; 374:1511-20]. My own view is that it’s too premature to throw the baby out with the bathwater. I think shared decision making is still very important, and I think, for many of our patients, relief of angina and improved quality of life are legitimate reasons in a low-risk situation with a good interventionalist to proceed,” he said.
Dr. O’Gara traced the history of medical thinking about silent ischemia. The notion that silent ischemia carried a clinical significance comparable with ischemia with angina gained wide credence more than 30 years ago, when investigators from the National Institutes of Health–sponsored Coronary Artery Surgery Study registry reported: “Patients with either silent or symptomatic ischemia during exercise testing have a similar risk of developing an acute myocardial infarction or sudden death – except in the three-vessel CAD subgroup, where the risk is greater in silent ischemia” (Am J Cardiol. 1988 Dec 1;62[17]:1155-8).
“This was a very important observation and led to many, many recommendations about screening and making sure that you took the expression of ST-segment depression on exercise treadmill testing pretty seriously, even if your patient did not have angina,” Dr. O’Gara recalled.
The prevailing wisdom that silent ischemia was detrimental took a hit in the Detection of Ischemia in Asymptomatic Diabetics (DIAC) trial. DIAC was conducted at a time when it had become clear that type 2 diabetes was a condition associated with increased cardiovascular risk, and that various methods of imaging were more accurate than treadmill exercise testing for the detection of underlying CAD. But when 1,123 DIAC participants with type 2 diabetes were randomized to screening with adenosine-stress radionuclide myocardial perfusion imaging or not and prospectively followed for roughly 5 years, it turned out there was no between-group difference in cardiac death or MI (JAMA. 2009 Apr 15;301[15]:1547-55).
“This pretty much put the lid on going out of one’s way to do routine screening of this nature in persons with diabetes who were considered to be at higher than average risk for the development of coronary disease,” the cardiologist commented.
Another fissure in the idea that silent ischemia was worth searching for and treating came from CLARIFY, an observational international registry of more than 20,000 individuals with stable CAD, roughly 12% of whom had silent ischemia, a figure in line with the prevalence reported in other studies. The 2-year rate of cardiovascular death or MI in the group with silent ischemia didn’t differ from the rate in patients with neither angina nor provocable ischemia. In contrast, rates of cardiovascular death or MI were significantly higher in the groups with angina but no ischemia or angina with ischemia (JAMA Intern Med. 2014 Oct;174[10]:1651-9).
“There’s something about the expression of angina that’s a very key clinical marker,” Dr. O’Gara observed.
He noted that just a few months before the ISCHEMIA trial results were released, a report from the far-smaller, randomized second Medicine, Angioplasty, or Surgery Study “threw cold water” on the notion that stress-induced ischemia in patients with multivessel CAD is a bad thing. Over 10 years of follow-up, the risk of major adverse cardiovascular events or deterioration in left ventricular function was identical in patients with or without baseline ischemia on stress testing performed after percutaneous coronary intervention, CABG surgery, or initiation of medical therapy (JAMA Intern Med. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2227).
What the guidelines say
The 6-year-old U.S. guidelines on the diagnosis and management of patients with stable ischemic heart disease are clearly out of date on the topic of silent ischemia (Circulation. 2014 Nov 4;130[19]:1749-67). The recommendations are based on expert opinion formed prior to the massive amount of new evidence that has since become available. For example, the current guidelines state as a class IIa, level of evidence C recommendation that exercise or pharmacologic stress can be useful for follow-up assessment at 2-year or greater intervals in patients with stable ischemic heart disease with prior evidence of silent ischemia.
“This is a very weak recommendation. The class of recommendation says it would be reasonable, but in the absence of an evidence base and in light of newer information, I’m not sure that it approaches even a class IIa level of recommendation,” according to Dr. O’Gara.
The 2019 European Society of Cardiology guidelines on chronic coronary syndromes are similarly weak on silent ischemia. The European guidelines state that patients with diabetes or chronic kidney disease may have a higher burden of silent ischemia, might be at higher risk for atherosclerotic cardiovascular disease events, and that periodic ECGs and functional testing every 3-5 years might be considered.
“Obviously there’s a lot of leeway there in how you wish to interpret that,” Dr. O’Gara said. “And this did not rise to the level where they’d put it in the table of recommendations, but it’s simply included as part of the explanatory text.”
What’s coming next in stable ischemic heart disease
“Nowadays all the rage has to do with coronary microvascular dysfunction,” according to Dr. O’Gara. “I think all of the research interest currently is focused on the coronary microcirculation as perhaps the next frontier in our understanding of why it is that ischemia can occur in the absence of epicardial coronary disease.”
He highly recommended a review article entitled: “Reappraisal of Ischemic Heart Disease,” in which an international trio of prominent cardiologists asserted that coronary microvascular dysfunction not only plays a pivotal pathogenic role in angina pectoris, but also in a phenomenon known as microvascular angina – that is, angina in the absence of obstructive CAD. Microvascular angina may explain the roughly one-third of patients who experience acute coronary syndrome without epicardial coronary artery stenosis or thrombosis. The authors delved into the structural and functional mechanisms underlying coronary microvascular dysfunction, while noting that effective treatment of this common phenomenon remains a major unmet need (Circulation. 2018 Oct 2;138[14]:1463-80).
Dr. O’Gara reported receiving funding from the National Heart, Lung, and Blood Institute; from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial; from Edwards Lifesciences for the ongoing EARLY TAVR trial; and from Medtrace Pharma, a Danish company developing an innovative form of PET diagnostic imaging.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Docs weigh pulling out of MIPS over paltry payments
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Risk of Suicide in the Year After an ED Visit
What happens to patients at risk for suicide after they leave the emergency department (ED)? According to a new study funded by the National Institute of Mental Health and published in JAMA Network Open, people who presented to California EDs with deliberate self-harm or suicidal ideation were not just at risk for a future suicide—they were at extreme risk.
The researchers divided patients into 3 groups: 83,507 who had deliberately self-harmed with or without co-occurring suicidal ideation; 67,379 presenting with suicidal ideation but without deliberate self-harm; and 497,760 without either self-harm or suicidal ideation.
In the first year after the ED visit, the patients who had presented with deliberate self-harm had a suicide rate almost 57 times higher than that of demographically similar Californians. Among those with suicidal ideation, the suicide rate was about 31 times higher. The suicide rate for the reference group, while the lowest of the 3 groups, was still twice the rate among Californians overall.
The researchers found certain clinical and demographic characteristics predicted subsequent suicide. Men and patients aged > 65 years had higher rates of suicide when compared with women or people aged 10 to 24 years. In all groups, suicide rates were higher for non-Hispanic, white patients.
Comorbid diagnoses were associated with suicide risk but with “striking differences” among the groups, the researchers say. Among patients presenting with deliberate self-harm, those with a comorbid diagnosis of bipolar disorder, anxiety disorder, or a psychotic disorder were more likely to die of suicide. Among reference patients, those with bipolar disorder, depression, or alcohol use disorder had a higher risk.
Of note, the researchers say, patients in the deliberate self-harm group who presented with a firearm-related injury had a subsequent suicide rate of 4.4% in the following year, a far higher rate than that in any other patient group. The researchers urge ED physicians to be aware that patients who present with self-harm who use high-lethality methods at a nonfatal event remain at highly increased risk for future suicide.
To the researchers’ knowledge, this is the first US population-based study to examine 12-month suicide rates after an index ED visit. These findings reinforce the importance of universal screening for suicide risk in EDs and the need for follow-up care, the researchers add, “an approach that has been found to increase the number of ED patients identified as warranting treatment for suicide risk by approximately 2-fold, but which is also not yet widespread.”
What happens to patients at risk for suicide after they leave the emergency department (ED)? According to a new study funded by the National Institute of Mental Health and published in JAMA Network Open, people who presented to California EDs with deliberate self-harm or suicidal ideation were not just at risk for a future suicide—they were at extreme risk.
The researchers divided patients into 3 groups: 83,507 who had deliberately self-harmed with or without co-occurring suicidal ideation; 67,379 presenting with suicidal ideation but without deliberate self-harm; and 497,760 without either self-harm or suicidal ideation.
In the first year after the ED visit, the patients who had presented with deliberate self-harm had a suicide rate almost 57 times higher than that of demographically similar Californians. Among those with suicidal ideation, the suicide rate was about 31 times higher. The suicide rate for the reference group, while the lowest of the 3 groups, was still twice the rate among Californians overall.
The researchers found certain clinical and demographic characteristics predicted subsequent suicide. Men and patients aged > 65 years had higher rates of suicide when compared with women or people aged 10 to 24 years. In all groups, suicide rates were higher for non-Hispanic, white patients.
Comorbid diagnoses were associated with suicide risk but with “striking differences” among the groups, the researchers say. Among patients presenting with deliberate self-harm, those with a comorbid diagnosis of bipolar disorder, anxiety disorder, or a psychotic disorder were more likely to die of suicide. Among reference patients, those with bipolar disorder, depression, or alcohol use disorder had a higher risk.
Of note, the researchers say, patients in the deliberate self-harm group who presented with a firearm-related injury had a subsequent suicide rate of 4.4% in the following year, a far higher rate than that in any other patient group. The researchers urge ED physicians to be aware that patients who present with self-harm who use high-lethality methods at a nonfatal event remain at highly increased risk for future suicide.
To the researchers’ knowledge, this is the first US population-based study to examine 12-month suicide rates after an index ED visit. These findings reinforce the importance of universal screening for suicide risk in EDs and the need for follow-up care, the researchers add, “an approach that has been found to increase the number of ED patients identified as warranting treatment for suicide risk by approximately 2-fold, but which is also not yet widespread.”
What happens to patients at risk for suicide after they leave the emergency department (ED)? According to a new study funded by the National Institute of Mental Health and published in JAMA Network Open, people who presented to California EDs with deliberate self-harm or suicidal ideation were not just at risk for a future suicide—they were at extreme risk.
The researchers divided patients into 3 groups: 83,507 who had deliberately self-harmed with or without co-occurring suicidal ideation; 67,379 presenting with suicidal ideation but without deliberate self-harm; and 497,760 without either self-harm or suicidal ideation.
In the first year after the ED visit, the patients who had presented with deliberate self-harm had a suicide rate almost 57 times higher than that of demographically similar Californians. Among those with suicidal ideation, the suicide rate was about 31 times higher. The suicide rate for the reference group, while the lowest of the 3 groups, was still twice the rate among Californians overall.
The researchers found certain clinical and demographic characteristics predicted subsequent suicide. Men and patients aged > 65 years had higher rates of suicide when compared with women or people aged 10 to 24 years. In all groups, suicide rates were higher for non-Hispanic, white patients.
Comorbid diagnoses were associated with suicide risk but with “striking differences” among the groups, the researchers say. Among patients presenting with deliberate self-harm, those with a comorbid diagnosis of bipolar disorder, anxiety disorder, or a psychotic disorder were more likely to die of suicide. Among reference patients, those with bipolar disorder, depression, or alcohol use disorder had a higher risk.
Of note, the researchers say, patients in the deliberate self-harm group who presented with a firearm-related injury had a subsequent suicide rate of 4.4% in the following year, a far higher rate than that in any other patient group. The researchers urge ED physicians to be aware that patients who present with self-harm who use high-lethality methods at a nonfatal event remain at highly increased risk for future suicide.
To the researchers’ knowledge, this is the first US population-based study to examine 12-month suicide rates after an index ED visit. These findings reinforce the importance of universal screening for suicide risk in EDs and the need for follow-up care, the researchers add, “an approach that has been found to increase the number of ED patients identified as warranting treatment for suicide risk by approximately 2-fold, but which is also not yet widespread.”
CDC: Risk in U.S. from 2019-nCoV remains low
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
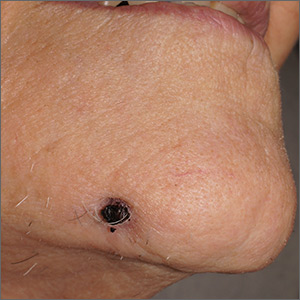
Draining papule on chin
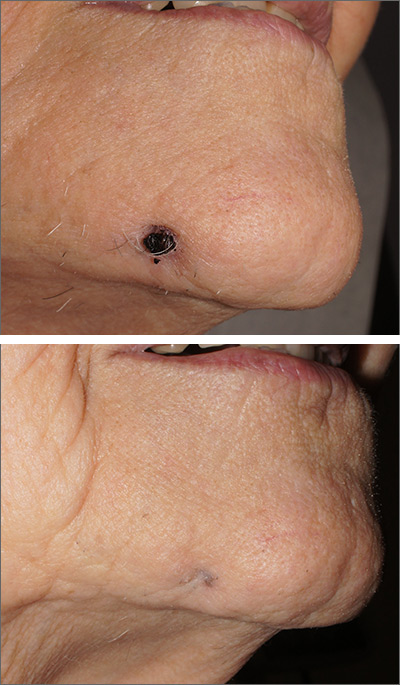
A biopsy of the skin was consistent with folliculitis, but a dental panoramic x-ray revealed an abscess of tooth number 30, which is on the right side. This combination of findings was consistent with a dental sinus.
This unusual diagnosis is most common in adults and arises from dental disease in the mid mandibular pre-molars. It will appear as an abscess or crusted papule and can occur in the lower jaw, neck, or occasionally, the mid cheek. Children are rarely affected. Because the sinus opening relieves pressure from the dental abscess, there is often little to no pain.
It can be challenging to convince a patient that his or her skin lesion derives from dental disease. Often, though, patients are glad it is not cancer. Patients should be referred to an endodontist for a work-up and a root canal, which is the preferred treatment. If a root canal is not feasible, dental extraction will lead to cure.
In this case, the patient promptly underwent a root canal and was cured in 2 weeks. The scar from the papule can have a depressed appearance (FIGURE 2), which can be addressed with excisional scar revision once the underlying abscess is cured.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Janev E, Redzep E. Managing the cutaneous sinus tract of dental origine. Open Access Maced J Med Sci. 2016;4:489-492.

A biopsy of the skin was consistent with folliculitis, but a dental panoramic x-ray revealed an abscess of tooth number 30, which is on the right side. This combination of findings was consistent with a dental sinus.
This unusual diagnosis is most common in adults and arises from dental disease in the mid mandibular pre-molars. It will appear as an abscess or crusted papule and can occur in the lower jaw, neck, or occasionally, the mid cheek. Children are rarely affected. Because the sinus opening relieves pressure from the dental abscess, there is often little to no pain.
It can be challenging to convince a patient that his or her skin lesion derives from dental disease. Often, though, patients are glad it is not cancer. Patients should be referred to an endodontist for a work-up and a root canal, which is the preferred treatment. If a root canal is not feasible, dental extraction will lead to cure.
In this case, the patient promptly underwent a root canal and was cured in 2 weeks. The scar from the papule can have a depressed appearance (FIGURE 2), which can be addressed with excisional scar revision once the underlying abscess is cured.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A biopsy of the skin was consistent with folliculitis, but a dental panoramic x-ray revealed an abscess of tooth number 30, which is on the right side. This combination of findings was consistent with a dental sinus.
This unusual diagnosis is most common in adults and arises from dental disease in the mid mandibular pre-molars. It will appear as an abscess or crusted papule and can occur in the lower jaw, neck, or occasionally, the mid cheek. Children are rarely affected. Because the sinus opening relieves pressure from the dental abscess, there is often little to no pain.
It can be challenging to convince a patient that his or her skin lesion derives from dental disease. Often, though, patients are glad it is not cancer. Patients should be referred to an endodontist for a work-up and a root canal, which is the preferred treatment. If a root canal is not feasible, dental extraction will lead to cure.
In this case, the patient promptly underwent a root canal and was cured in 2 weeks. The scar from the papule can have a depressed appearance (FIGURE 2), which can be addressed with excisional scar revision once the underlying abscess is cured.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Janev E, Redzep E. Managing the cutaneous sinus tract of dental origine. Open Access Maced J Med Sci. 2016;4:489-492.
Janev E, Redzep E. Managing the cutaneous sinus tract of dental origine. Open Access Maced J Med Sci. 2016;4:489-492.
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.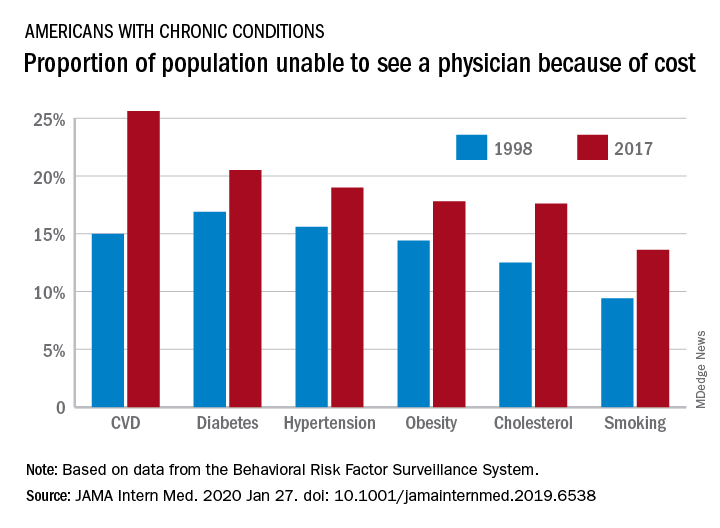
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE
RCT confirms CT scan screening catches lung cancer early
CT scan screening of older people with heavy smoking histories – using lesion volume, not diameter, as a trigger for further work-up – reduced lung cancer deaths by about 30% in a randomized trial from the Netherlands and Belgium with almost 16,000 current and former smokers, investigators reported in the New England Journal of Medicine.
The Dutch-Belgian lung-cancer screening trial (Nederlands-Leuvens Longkanker Screenings Onderzoek [NELSON]) is “arguably the only adequately powered trial other than the” National Lung Screening Trial (NLST) in the United States to assess the role of CT scan screening among smokers, wrote University of London cancer epidemiologist Stephen Duffy, MSc, and University of Liverpool molecular oncology professor John Field, PhD, in an accompanying editorial.
NLST, which used lesion diameter, found an approximately 20% lower lung cancer mortality than screening with chest x-rays among 53,454 heavy smokers after a median follow-up of 6.5 years. The trial ultimately led the U.S. Preventive Services Task Force to recommend annual screening for people aged 55-80 years with a history of at least 30 pack-years.
European countries have considered similar programs but have hesitated “partly due to doubts fostered by the early publication of inconclusive results of a number of smaller trials in Europe. These doubts should be laid to rest,” Mr. Duffy and Dr. Field wrote.
“With the NELSON results, the efficacy of low-dose CT screening for lung cancer is confirmed. Our job is no longer to assess whether low-dose CT screening for lung cancer works; it does. Our job is to identify the target population in which it will be acceptable and cost effective,” they added.
The 15,789 NELSON participants (84% men, with a median age of 58 years and 38 pack-year history) were randomized about 1:1 to either low-dose CT scan screening at baseline and 1, 3, and 5.5 years, or to no screening.
At 10 years follow-up, there were 5.58 lung cancer cases and 2.5 deaths per 1,000 person-years in the screened group versus 4.91 cases and 3.3 deaths per 1,000 person-years among controls. Lung-cancer mortality was 24% lower among screened subjects overall, and 33% lower among screened women. The team estimated that screening prevented about 60 lung cancer deaths.
Using volume instead of diameter “resulted in low[er] referral rates” – 2.1% with a positive predictive value of 43.5% versus 24% with a positive predictive value of 3.8% in NLST – for additional work-up, explained investigators led by H.J. de Koning, MD, PhD, of the department of public health at Erasmus University Medical Center in Rotterdam, the Netherlands.
The upper limit of overdiagnosis risk – a major concern with any screening program – was 18.5% with NLST versus 8.9% with NELSON, they wrote.
In short: “Volume CT screening enabled a significant reduction of harms (e.g., false positive tests and unnecessary work-up procedures) without jeopardizing favorable outcomes,” the investigators wrote. Indeed, an ad hoc analysis suggested “more-favorable effects on lung-cancer mortality than in the NLST, despite lower referral rates for suspicious lesions” and the fact that NLST used annual screening.
“Recently,” Mr. Duffy and Dr. Field explained in their editorial, “the NELSON investigators evaluated both diameter and volume measurement to estimate lung-nodule size as an imaging biomarker for nodule management; this provided evidence that using mean or maximum axial diameter to assess nodule volume led to a substantial overestimation of nodule volume.” Direct measurement of volume “resulted in a substantial number of early-stage cancers identified at the time of diagnosis and avoided false positives from the overestimation incurred by management based on diameter.”
“The lung-nodule management system used in the NELSON trial has been advocated in the European position statement on lung-cancer screening. This will improve the acceptability of the intervention, because the rate of further investigation has been a major concern in lung cancer screening,” they wrote.
Baseline characteristics did not differ significantly between the screened and unscreened in NELSON, except for a slightly longer duration of smoking in the screened group.
The work was funded by the Netherlands Organization of Health Research and Development, among others. Mr. Duffy and Dr. de Koning didn’t report any disclosures. Dr. Field is an advisor for AstraZeneca, Epigenomics, and Nucleix, and has a research grant to his university from Janssen.
SOURCE: de Honing HJ et al. N Engl J Med. 2020 Jan 29. doi: 10.1056/NEJMoa1911793.
CT scan screening of older people with heavy smoking histories – using lesion volume, not diameter, as a trigger for further work-up – reduced lung cancer deaths by about 30% in a randomized trial from the Netherlands and Belgium with almost 16,000 current and former smokers, investigators reported in the New England Journal of Medicine.
The Dutch-Belgian lung-cancer screening trial (Nederlands-Leuvens Longkanker Screenings Onderzoek [NELSON]) is “arguably the only adequately powered trial other than the” National Lung Screening Trial (NLST) in the United States to assess the role of CT scan screening among smokers, wrote University of London cancer epidemiologist Stephen Duffy, MSc, and University of Liverpool molecular oncology professor John Field, PhD, in an accompanying editorial.
NLST, which used lesion diameter, found an approximately 20% lower lung cancer mortality than screening with chest x-rays among 53,454 heavy smokers after a median follow-up of 6.5 years. The trial ultimately led the U.S. Preventive Services Task Force to recommend annual screening for people aged 55-80 years with a history of at least 30 pack-years.
European countries have considered similar programs but have hesitated “partly due to doubts fostered by the early publication of inconclusive results of a number of smaller trials in Europe. These doubts should be laid to rest,” Mr. Duffy and Dr. Field wrote.
“With the NELSON results, the efficacy of low-dose CT screening for lung cancer is confirmed. Our job is no longer to assess whether low-dose CT screening for lung cancer works; it does. Our job is to identify the target population in which it will be acceptable and cost effective,” they added.
The 15,789 NELSON participants (84% men, with a median age of 58 years and 38 pack-year history) were randomized about 1:1 to either low-dose CT scan screening at baseline and 1, 3, and 5.5 years, or to no screening.
At 10 years follow-up, there were 5.58 lung cancer cases and 2.5 deaths per 1,000 person-years in the screened group versus 4.91 cases and 3.3 deaths per 1,000 person-years among controls. Lung-cancer mortality was 24% lower among screened subjects overall, and 33% lower among screened women. The team estimated that screening prevented about 60 lung cancer deaths.
Using volume instead of diameter “resulted in low[er] referral rates” – 2.1% with a positive predictive value of 43.5% versus 24% with a positive predictive value of 3.8% in NLST – for additional work-up, explained investigators led by H.J. de Koning, MD, PhD, of the department of public health at Erasmus University Medical Center in Rotterdam, the Netherlands.
The upper limit of overdiagnosis risk – a major concern with any screening program – was 18.5% with NLST versus 8.9% with NELSON, they wrote.
In short: “Volume CT screening enabled a significant reduction of harms (e.g., false positive tests and unnecessary work-up procedures) without jeopardizing favorable outcomes,” the investigators wrote. Indeed, an ad hoc analysis suggested “more-favorable effects on lung-cancer mortality than in the NLST, despite lower referral rates for suspicious lesions” and the fact that NLST used annual screening.
“Recently,” Mr. Duffy and Dr. Field explained in their editorial, “the NELSON investigators evaluated both diameter and volume measurement to estimate lung-nodule size as an imaging biomarker for nodule management; this provided evidence that using mean or maximum axial diameter to assess nodule volume led to a substantial overestimation of nodule volume.” Direct measurement of volume “resulted in a substantial number of early-stage cancers identified at the time of diagnosis and avoided false positives from the overestimation incurred by management based on diameter.”
“The lung-nodule management system used in the NELSON trial has been advocated in the European position statement on lung-cancer screening. This will improve the acceptability of the intervention, because the rate of further investigation has been a major concern in lung cancer screening,” they wrote.
Baseline characteristics did not differ significantly between the screened and unscreened in NELSON, except for a slightly longer duration of smoking in the screened group.
The work was funded by the Netherlands Organization of Health Research and Development, among others. Mr. Duffy and Dr. de Koning didn’t report any disclosures. Dr. Field is an advisor for AstraZeneca, Epigenomics, and Nucleix, and has a research grant to his university from Janssen.
SOURCE: de Honing HJ et al. N Engl J Med. 2020 Jan 29. doi: 10.1056/NEJMoa1911793.
CT scan screening of older people with heavy smoking histories – using lesion volume, not diameter, as a trigger for further work-up – reduced lung cancer deaths by about 30% in a randomized trial from the Netherlands and Belgium with almost 16,000 current and former smokers, investigators reported in the New England Journal of Medicine.
The Dutch-Belgian lung-cancer screening trial (Nederlands-Leuvens Longkanker Screenings Onderzoek [NELSON]) is “arguably the only adequately powered trial other than the” National Lung Screening Trial (NLST) in the United States to assess the role of CT scan screening among smokers, wrote University of London cancer epidemiologist Stephen Duffy, MSc, and University of Liverpool molecular oncology professor John Field, PhD, in an accompanying editorial.
NLST, which used lesion diameter, found an approximately 20% lower lung cancer mortality than screening with chest x-rays among 53,454 heavy smokers after a median follow-up of 6.5 years. The trial ultimately led the U.S. Preventive Services Task Force to recommend annual screening for people aged 55-80 years with a history of at least 30 pack-years.
European countries have considered similar programs but have hesitated “partly due to doubts fostered by the early publication of inconclusive results of a number of smaller trials in Europe. These doubts should be laid to rest,” Mr. Duffy and Dr. Field wrote.
“With the NELSON results, the efficacy of low-dose CT screening for lung cancer is confirmed. Our job is no longer to assess whether low-dose CT screening for lung cancer works; it does. Our job is to identify the target population in which it will be acceptable and cost effective,” they added.
The 15,789 NELSON participants (84% men, with a median age of 58 years and 38 pack-year history) were randomized about 1:1 to either low-dose CT scan screening at baseline and 1, 3, and 5.5 years, or to no screening.
At 10 years follow-up, there were 5.58 lung cancer cases and 2.5 deaths per 1,000 person-years in the screened group versus 4.91 cases and 3.3 deaths per 1,000 person-years among controls. Lung-cancer mortality was 24% lower among screened subjects overall, and 33% lower among screened women. The team estimated that screening prevented about 60 lung cancer deaths.
Using volume instead of diameter “resulted in low[er] referral rates” – 2.1% with a positive predictive value of 43.5% versus 24% with a positive predictive value of 3.8% in NLST – for additional work-up, explained investigators led by H.J. de Koning, MD, PhD, of the department of public health at Erasmus University Medical Center in Rotterdam, the Netherlands.
The upper limit of overdiagnosis risk – a major concern with any screening program – was 18.5% with NLST versus 8.9% with NELSON, they wrote.
In short: “Volume CT screening enabled a significant reduction of harms (e.g., false positive tests and unnecessary work-up procedures) without jeopardizing favorable outcomes,” the investigators wrote. Indeed, an ad hoc analysis suggested “more-favorable effects on lung-cancer mortality than in the NLST, despite lower referral rates for suspicious lesions” and the fact that NLST used annual screening.
“Recently,” Mr. Duffy and Dr. Field explained in their editorial, “the NELSON investigators evaluated both diameter and volume measurement to estimate lung-nodule size as an imaging biomarker for nodule management; this provided evidence that using mean or maximum axial diameter to assess nodule volume led to a substantial overestimation of nodule volume.” Direct measurement of volume “resulted in a substantial number of early-stage cancers identified at the time of diagnosis and avoided false positives from the overestimation incurred by management based on diameter.”
“The lung-nodule management system used in the NELSON trial has been advocated in the European position statement on lung-cancer screening. This will improve the acceptability of the intervention, because the rate of further investigation has been a major concern in lung cancer screening,” they wrote.
Baseline characteristics did not differ significantly between the screened and unscreened in NELSON, except for a slightly longer duration of smoking in the screened group.
The work was funded by the Netherlands Organization of Health Research and Development, among others. Mr. Duffy and Dr. de Koning didn’t report any disclosures. Dr. Field is an advisor for AstraZeneca, Epigenomics, and Nucleix, and has a research grant to his university from Janssen.
SOURCE: de Honing HJ et al. N Engl J Med. 2020 Jan 29. doi: 10.1056/NEJMoa1911793.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
The scents-less life and the speaking mummy
If I only had a nose
Deaf and blind people get all the attention. Special schools, Braille, sign language, even a pinball-focused rock opera. And it is easy to see why: Those senses are kind of important when it comes to navigating the world. But what if you have to live without one of the less-cool senses? What if the nose doesn’t know?
According to research published in Clinical Otolaryngology, up to 5% of the world’s population has some sort of smell disorder, preventing them from either smelling correctly or smelling anything at all. And the effects of this on everyday life are drastic.
In a survey of 71 people with smell disorders, the researchers found that patients experience a smorgasbord of negative effects – ranging from poor hazard perception and poor sense of personal hygiene, to an inability to enjoy food and an inability to link smell to happy memories. The whiff of gingerbread on Christmas morning, the smoke of a bonfire on a summer evening – the smell-deprived miss out on them all. The negative emotions those people experience read like a recipe for your very own homemade Sith lord: sadness, regret, isolation, anxiety, anger, frustration. A path to the dark side, losing your scent is.
Speaking of fictional bad guys, this nasal-based research really could have benefited one Lord Volde ... fine, You-Know-Who. Just look at that face. That’s a man who can’t smell. You can’t tell us he wouldn’t have turned out better if only Dorothy had picked him up on the yellow brick road instead of some dumb scarecrow.
The sound of hieroglyphics
The Rosetta Stone revealed the meaning of Egyptian hieroglyphics and unlocked the ancient language of the Pharaohs for modern humans. But that mute stele said nothing about what those who uttered that ancient tongue sounded like.
Researchers at London’s Royal Holloway College may now know the answer. At least, a monosyllabic one.
The answer comes (indirectly) from Egyptian priest Nesyamun, a former resident of Thebes who worked at the temple of Karnak, but who now calls the Leeds City Museum home. Or, to be precise, Nesyamun’s 3,000-year-old mummified remains live on in Leeds. Nesyamun’s religious duties during his Karnak career likely required a smooth singing style and an accomplished speaking voice.
In a paper published in Scientific Reports, the British scientists say they’ve now heard the sound of the Egyptian priest’s long-silenced liturgical voice.
Working from CT scans of Nesyamun’s relatively well-preserved vocal-tract soft tissue, the scientists used a 3D-printed vocal tract and an electronic larynx to synthesize the actual sound of his voice.
And the result? Did the crooning priest of Karnak utter a Boris Karloffian curse upon those who had disturbed his millennia-long slumber? Did he deliver a rousing hymn of praise to Egypt’s ruler during the turbulent 1070s bce, Ramses XI?
In fact, what emerged from Nesyamun’s synthesized throat was ... “eh.” Maybe “a,” as in “bad.”
Given the state of the priest’s tongue (shrunken) and his soft palate (missing), the researchers say those monosyllabic sounds are the best Nesyamun can muster in his present state. Other experts say actual words from the ancients are likely impossible.
Perhaps one day, science will indeed be able to synthesize whole words or sentences from other well-preserved residents of the distant past. May we all live to hear an unyielding Ramses II himself chew the scenery like his Hollywood doppelganger, Yul Brynner: “So let it be written! So let it be done!”
To beard or not to beard
People are funny, and men, who happen to be people, are no exception.
Men, you see, have these things called beards, and there are definitely more men running around with facial hair these days. A lot of women go through a lot of trouble to get rid of a lot of their hair. But men, well, we grow extra hair. Why?
That’s what Honest Amish, a maker of beard-care products, wanted to know. They commissioned OnePoll to conduct a survey of 2,000 Americans, both men and women, to learn all kinds of things about beards.
So what did they find? Facial hair confidence, that’s what. Three-quarters of men said that a beard made them feel more confident than did a bare face, and 73% said that facial hair makes a man more attractive. That number was a bit lower among women, 63% of whom said that facial hair made a man more attractive.
That doesn’t seem very funny, does it? We’re getting there.
Male respondents also were asked what they would do to get the perfect beard: 40% would be willing to spend a night in jail or give up coffee for a year, and 38% would stand in line at the DMV for an entire day. Somewhat less popular responses included giving up sex for a year (22%) – seems like a waste of all that new-found confidence – and shaving their heads (18%).
And that, we don’t mind saying, is a hair-raising conclusion.
If I only had a nose
Deaf and blind people get all the attention. Special schools, Braille, sign language, even a pinball-focused rock opera. And it is easy to see why: Those senses are kind of important when it comes to navigating the world. But what if you have to live without one of the less-cool senses? What if the nose doesn’t know?
According to research published in Clinical Otolaryngology, up to 5% of the world’s population has some sort of smell disorder, preventing them from either smelling correctly or smelling anything at all. And the effects of this on everyday life are drastic.
In a survey of 71 people with smell disorders, the researchers found that patients experience a smorgasbord of negative effects – ranging from poor hazard perception and poor sense of personal hygiene, to an inability to enjoy food and an inability to link smell to happy memories. The whiff of gingerbread on Christmas morning, the smoke of a bonfire on a summer evening – the smell-deprived miss out on them all. The negative emotions those people experience read like a recipe for your very own homemade Sith lord: sadness, regret, isolation, anxiety, anger, frustration. A path to the dark side, losing your scent is.
Speaking of fictional bad guys, this nasal-based research really could have benefited one Lord Volde ... fine, You-Know-Who. Just look at that face. That’s a man who can’t smell. You can’t tell us he wouldn’t have turned out better if only Dorothy had picked him up on the yellow brick road instead of some dumb scarecrow.
The sound of hieroglyphics
The Rosetta Stone revealed the meaning of Egyptian hieroglyphics and unlocked the ancient language of the Pharaohs for modern humans. But that mute stele said nothing about what those who uttered that ancient tongue sounded like.
Researchers at London’s Royal Holloway College may now know the answer. At least, a monosyllabic one.
The answer comes (indirectly) from Egyptian priest Nesyamun, a former resident of Thebes who worked at the temple of Karnak, but who now calls the Leeds City Museum home. Or, to be precise, Nesyamun’s 3,000-year-old mummified remains live on in Leeds. Nesyamun’s religious duties during his Karnak career likely required a smooth singing style and an accomplished speaking voice.
In a paper published in Scientific Reports, the British scientists say they’ve now heard the sound of the Egyptian priest’s long-silenced liturgical voice.
Working from CT scans of Nesyamun’s relatively well-preserved vocal-tract soft tissue, the scientists used a 3D-printed vocal tract and an electronic larynx to synthesize the actual sound of his voice.
And the result? Did the crooning priest of Karnak utter a Boris Karloffian curse upon those who had disturbed his millennia-long slumber? Did he deliver a rousing hymn of praise to Egypt’s ruler during the turbulent 1070s bce, Ramses XI?
In fact, what emerged from Nesyamun’s synthesized throat was ... “eh.” Maybe “a,” as in “bad.”
Given the state of the priest’s tongue (shrunken) and his soft palate (missing), the researchers say those monosyllabic sounds are the best Nesyamun can muster in his present state. Other experts say actual words from the ancients are likely impossible.
Perhaps one day, science will indeed be able to synthesize whole words or sentences from other well-preserved residents of the distant past. May we all live to hear an unyielding Ramses II himself chew the scenery like his Hollywood doppelganger, Yul Brynner: “So let it be written! So let it be done!”
To beard or not to beard
People are funny, and men, who happen to be people, are no exception.
Men, you see, have these things called beards, and there are definitely more men running around with facial hair these days. A lot of women go through a lot of trouble to get rid of a lot of their hair. But men, well, we grow extra hair. Why?
That’s what Honest Amish, a maker of beard-care products, wanted to know. They commissioned OnePoll to conduct a survey of 2,000 Americans, both men and women, to learn all kinds of things about beards.
So what did they find? Facial hair confidence, that’s what. Three-quarters of men said that a beard made them feel more confident than did a bare face, and 73% said that facial hair makes a man more attractive. That number was a bit lower among women, 63% of whom said that facial hair made a man more attractive.
That doesn’t seem very funny, does it? We’re getting there.
Male respondents also were asked what they would do to get the perfect beard: 40% would be willing to spend a night in jail or give up coffee for a year, and 38% would stand in line at the DMV for an entire day. Somewhat less popular responses included giving up sex for a year (22%) – seems like a waste of all that new-found confidence – and shaving their heads (18%).
And that, we don’t mind saying, is a hair-raising conclusion.
If I only had a nose
Deaf and blind people get all the attention. Special schools, Braille, sign language, even a pinball-focused rock opera. And it is easy to see why: Those senses are kind of important when it comes to navigating the world. But what if you have to live without one of the less-cool senses? What if the nose doesn’t know?
According to research published in Clinical Otolaryngology, up to 5% of the world’s population has some sort of smell disorder, preventing them from either smelling correctly or smelling anything at all. And the effects of this on everyday life are drastic.
In a survey of 71 people with smell disorders, the researchers found that patients experience a smorgasbord of negative effects – ranging from poor hazard perception and poor sense of personal hygiene, to an inability to enjoy food and an inability to link smell to happy memories. The whiff of gingerbread on Christmas morning, the smoke of a bonfire on a summer evening – the smell-deprived miss out on them all. The negative emotions those people experience read like a recipe for your very own homemade Sith lord: sadness, regret, isolation, anxiety, anger, frustration. A path to the dark side, losing your scent is.
Speaking of fictional bad guys, this nasal-based research really could have benefited one Lord Volde ... fine, You-Know-Who. Just look at that face. That’s a man who can’t smell. You can’t tell us he wouldn’t have turned out better if only Dorothy had picked him up on the yellow brick road instead of some dumb scarecrow.
The sound of hieroglyphics
The Rosetta Stone revealed the meaning of Egyptian hieroglyphics and unlocked the ancient language of the Pharaohs for modern humans. But that mute stele said nothing about what those who uttered that ancient tongue sounded like.
Researchers at London’s Royal Holloway College may now know the answer. At least, a monosyllabic one.
The answer comes (indirectly) from Egyptian priest Nesyamun, a former resident of Thebes who worked at the temple of Karnak, but who now calls the Leeds City Museum home. Or, to be precise, Nesyamun’s 3,000-year-old mummified remains live on in Leeds. Nesyamun’s religious duties during his Karnak career likely required a smooth singing style and an accomplished speaking voice.
In a paper published in Scientific Reports, the British scientists say they’ve now heard the sound of the Egyptian priest’s long-silenced liturgical voice.
Working from CT scans of Nesyamun’s relatively well-preserved vocal-tract soft tissue, the scientists used a 3D-printed vocal tract and an electronic larynx to synthesize the actual sound of his voice.
And the result? Did the crooning priest of Karnak utter a Boris Karloffian curse upon those who had disturbed his millennia-long slumber? Did he deliver a rousing hymn of praise to Egypt’s ruler during the turbulent 1070s bce, Ramses XI?
In fact, what emerged from Nesyamun’s synthesized throat was ... “eh.” Maybe “a,” as in “bad.”
Given the state of the priest’s tongue (shrunken) and his soft palate (missing), the researchers say those monosyllabic sounds are the best Nesyamun can muster in his present state. Other experts say actual words from the ancients are likely impossible.
Perhaps one day, science will indeed be able to synthesize whole words or sentences from other well-preserved residents of the distant past. May we all live to hear an unyielding Ramses II himself chew the scenery like his Hollywood doppelganger, Yul Brynner: “So let it be written! So let it be done!”
To beard or not to beard
People are funny, and men, who happen to be people, are no exception.
Men, you see, have these things called beards, and there are definitely more men running around with facial hair these days. A lot of women go through a lot of trouble to get rid of a lot of their hair. But men, well, we grow extra hair. Why?
That’s what Honest Amish, a maker of beard-care products, wanted to know. They commissioned OnePoll to conduct a survey of 2,000 Americans, both men and women, to learn all kinds of things about beards.
So what did they find? Facial hair confidence, that’s what. Three-quarters of men said that a beard made them feel more confident than did a bare face, and 73% said that facial hair makes a man more attractive. That number was a bit lower among women, 63% of whom said that facial hair made a man more attractive.
That doesn’t seem very funny, does it? We’re getting there.
Male respondents also were asked what they would do to get the perfect beard: 40% would be willing to spend a night in jail or give up coffee for a year, and 38% would stand in line at the DMV for an entire day. Somewhat less popular responses included giving up sex for a year (22%) – seems like a waste of all that new-found confidence – and shaving their heads (18%).
And that, we don’t mind saying, is a hair-raising conclusion.
Psoriasis: A look back over the past 50 years, and forward to next steps
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Are unmatched residency graduates a solution for ‘shrinking shrinks’?
‘Physician associates’ could be used to expand the reach of psychiatry
For many years now, we have been lamenting the shortage of psychiatrists practicing in the United States. At this point, we must identify possible solutions.1,2 Currently, the shortage of practicing psychiatrists in the United States could be as high as 45,000.3 The major problem is that the number of psychiatry residency positions will not increase in the foreseeable future, thus generating more psychiatrists is not an option.
Medicare pays about $150,000 per residency slot per year. To solve the mental health access problem, $27 billion (45,000 x $150,000 x 4 years)* would be required from Medicare, which is not feasible.4 The national average starting salary for psychiatrists from 2018-2019 was about $273,000 (much lower in academic institutions), according to Merritt Hawkins, the physician recruiting firm. That salary is modest, compared with those offered in other medical specialties. For this reason, many graduates choose other lucrative specialties. And we know that increasing the salaries of psychiatrists alone would not lead more people to choose psychiatry. On paper, it may say they work a 40-hour week, but they end up working 60 hours a week.
To make matters worse, family medicine and internal medicine doctors generally would rather not deal with people with mental illness and do “cherry-picking and lemon-dropping.” While many patients present to primary care with mental health issues, lack of time and education in psychiatric disorders and treatment hinder these physicians. In short, the mental health field cannot count on primary care physicians.
Meanwhile, there are thousands of unmatched residency graduates. In light of those realities, perhaps psychiatry residency programs could provide these unmatched graduates with 6 months of training and use them to supplement the workforce. These medical doctors, or “physician associates,” could be paired with a few psychiatrists to do clinical and administrative work. With one in four individuals having mental health issues, and more and more people seeking help because of increasing awareness and the benefits that accompanied the Affordable Care Act (ACA), physician associates might ease the workload of psychiatrists so that they can deliver better care to more people. We must take advantage of these two trends: The surge in unmatched graduates and “shrinking shrinks,” or the decline in the psychiatric workforce pool. (The Royal College of Physicians has established a category of clinicians called physician associates,5 but they are comparable to physician assistants in the United States. As you will see, the construct I am proposing is different.)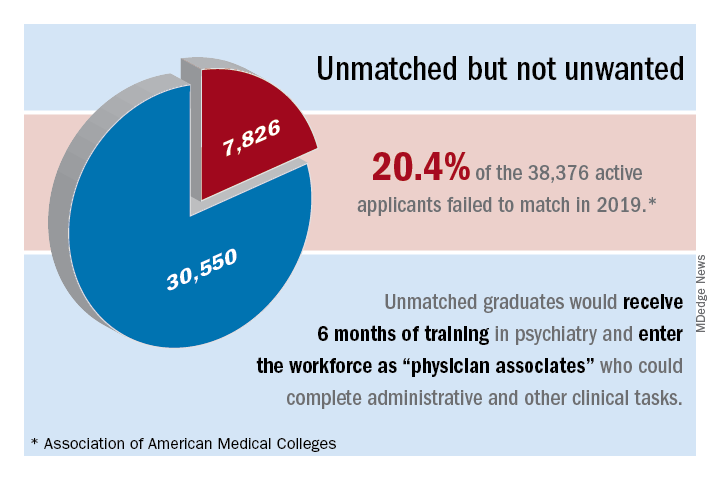
The current landscape
Currently, psychiatrists are under a lot of pressure to see a certain number of patients. Patients consistently complain that psychiatrists spend a maximum of 15 minutes with them, that the visits are interrupted by phone calls, and that they are not being heard and helped. Burnout, a silent epidemic among physicians, is relatively prevalent in psychiatry.6 Hence, some psychiatrists are reducing their hours and retiring early. Psychiatry has the third-oldest workforce, with 59% of current psychiatrists aged 55 years or older.7 A better pay/work ratio and work/life balance would enable psychiatrists to enjoy more fulfilling careers.
Many psychiatrists are spending a lot of their time in research, administration, and the classroom. In addition to those issues, the United States currently has a broken mental health care system.8 Finally, the medical practice landscape has changed dramatically in recent years, and those changes undermine both the effectiveness and well-being of clinicians.
The historical landscape
Some people proudly refer to the deinstitutionalization of mental asylums and state mental hospitals in the United States. But where have these patients gone? According to a U.S. Justice Department report, 2,220,300 adults were incarcerated in U.S. federal and state prisons and county jails in 2013.9 In addition, 4,751,400 adults in 2013 were on probation or parole. The percentages of inmates in state and federal prisons and local jails with a psychiatric diagnosis were 56%, 45%, and 64%, respectively.
I work at the Maryland correctional institutions, part of the Maryland Department of Public Safety and Correctional Services. One thing that I consistently hear from several correctional officers is “had these inmates received timely help and care, they wouldn’t have ended up behind bars.” Because of the criminalization of mental illness, in 44 states, the number of people with mental illness is higher in a jail or prison than in the largest state psychiatric hospital, according to the Treatment Advocacy Center. We have to be responsible for many of the inmates currently in correctional facilities for committing crimes related to mental health problems. In Maryland, a small state, there are 30,000 inmates in jails, and state and federal prison. The average cost of a meal is $1.36, thus $1.36 x 3 meals x 30,000 inmates = $122,400.00 for food alone for 1 day – this average does not take other expenses into account. By using money and manpower wisely and taking care of individuals’ mental health problems before they commit crimes, better outcomes could be achieved.
I used to work for MedOptions Inc. doing psychiatry consults at nursing homes and assisted-living facilities. Because of the shortage of psychiatrists and nurse practitioners, especially in the suburbs and rural areas, those patients could not be seen in a timely manner even for their 3-month routine follow-ups. As my colleagues and I have written previously, many elderly individuals with major neurocognitive disorders are not on the Food and Drug Administration–approved cognitive enhancers, such as donepezil, galantamine, and memantine.10 Instead, those patients are on benzodiazepines, which are associated with cognitive impairments, and increased risk of pneumonia and falls. Benzodiazepines also can cause and/or worsen disinhibited behavior. Also, in those settings, crisis situations often are addressed days to weeks later because of the doctor shortage. This situation is going to get worse, because this patient population is growing.
Child and geriatric psychiatry shortages
Child and geriatric psychiatrist shortages are even higher than those in general psychiatry.11 Many years of training and low salaries are a few of the reasons some choose not to do a fellowship. These residency graduates would rather join a practice at an attending salary than at a fellow’s salary, which requires an additional 1 to 2 years of training. Student loans of $100,000–$500,000 after residency also discourage some from pursuing fellowship opportunities. We need to consider models such as 2 years of residency with 2 years of a child psychiatry fellowship or 3 years of residency with 1 year of geriatric psychiatry fellowship. Working as an adult attending physician (50% of the time) and concurrently doing a fellowship (50% of the time) while receiving an attending salary might motivate more people to complete a fellowship.
In specialties such as radiology, international medical graduates (IMGs) who have completed residency training in radiology in other countries can complete a radiology fellowship in a particular area for several years and can practice in the United States as board-eligible certified MDs. Likewise, in line with the model proposed here, we could provide unmatched graduates who have no residency training with 3 to 4 years of child psychiatry and geriatric psychiatry training in addition to some adult psychiatry training.
Implementation of such a model might take care of the shortage of child and geriatric psychiatrists. In 2015, there were 56 geriatric psychiatry fellowship programs; 54 positions were filled, and 51 fellows completed training.12 “It appears that a reasonable percentage of IMGs who obtain a fellowship in geriatric psychiatry do not have an intent of pursuing a career in the field,” Marc H. Zisselman, MD, former geriatric psychiatry fellowship director and currently with the Einstein Medical Center in Philadelphia, told me in 2016. These numbers are not at all sufficient to take care of the nation’s unmet need. Hence, implementing alternate strategies is imperative.
Administrative tasks and care
What consumes a psychiatrist’s time and leads to burnout? The answer has to do with administrative tasks at work. Administrative tasks are not an effective use of time for an MD who has spent more than a decade in medical school, residency, and fellowship training. Although electronic medical record (EMR) systems are considered a major advancement, engaging in the process throughout the day is associated with exhaustion.
Many physicians feel that EMRs have slowed them down, and some are not well-equipped to use them in quick and efficient ways. EMRs also have led to physicians making minimal eye contact in interviews with patients. Patients often complain: “I am talking, and the doctor is looking at the computer and typing.” Patients consider this behavior to be unprofessional and rude. In a survey of 57 U.S. physicians in family medicine, internal medicine, cardiology, and orthopedics, results showed that during the work day, 27% of their time was spent on direct clinical face time with patients and 49.2% was spent on EMR and desk work. While in the examination room with patients, physicians spent 52.9% of their time on direct clinical face time and 37.0% on EMR and desk work. Outside office hours, physicians spend up to 2 hours of personal time each night doing additional computer and other clerical work.13
Several EMR software systems, such as CareLogic, Cerner, Epic,NextGen, PointClickCare, and Sunrise, are used in the United States. The U.S. Veterans Affairs Medical Centers (VAMCs) use the computerized patient record system (CPRS) across the country. VA clinicians find CPRS extremely useful when they move from one VAMC to another. Likewise, hospitals and universities may use one software system such as the CPRS and thus, when clinicians change jobs, they find it hard to adapt to the new system.
Because psychiatrists are wasting a lot of time doing administrative tasks, they might be unable to do a good job with regard to making the right diagnoses and prescribing the best treatments.When I ask patients what are they diagnosed with, they tell me: “It depends on who you ask,” or “I’ve been diagnosed with everything.” This shows that we are not doing a good job or something is not right.
Currently, psychiatrists do not have the time and/or interest to make the right diagnoses and provide adequate psychoeducation for their patients. This also could be attributable to a variety of factors, including, but not limited to, time constraints, cynicism, and apathy. Time constraints also lead to the gross underutilization14 of relapse prevention strategies such as long-acting injectables and medications that can prevent suicide, such as lithium and clozapine.15
Other factors that undermine good care include not participating in continuing medical education (CME) and not staying up to date with the literature. For example, haloperidol continues to be one of the most frequently prescribed (probably, the most common) antipsychotic, although it is clearly neurotoxic16,17 and other safer options are available.18 Board certification and maintenance of certification (MOC) are not synonymous with good clinical practice. Many physicians are finding it hard to complete daily documentation, let alone time for MOC. For a variety of reasons, many are not maintaining certification, and this number is likely to increase. Think about how much time is devoted to the one-to-one interview with the patient and direct patient care during the 15-minute medical check appointment and the hour-long new evaluation. In some clinics, psychiatrists are asked to see more than 25 patients in 4 hours. Some U.S.-based psychiatrists see 65 inpatients and initiate 10 new evaluations in a single day. Under those kinds of time constraints, how can we provide quality care?
A model that would address the shortage
Overall, 7,826 PGY-1 applicants were unmatched in 2019, according to data from the 2019 Main Residency Match.19 Psychiatry residency programs could give these unmatched graduates 6 months of training (arbitrary duration) in psychiatry, which is not at all difficult with the program modules that are available.20 We could use them as physician associates as a major contributor to our workforce to complete administrative and other clinical tasks.
Administrative tasks are not necessarily negative, as all psychiatrists have done administrative tasks as medical students, residents, and fellows. However, at this point, administrative tasks are not an effective use of a psychiatrist’s time. Those physician associates could be paired with two to three psychiatrists to do administrative tasks (for making daytime and overnight phone calls; handling prescriptions, prior authorizations, and medication orders, especially over-the-counter and comfort medications in the inpatient units; doing chart reviews; ordering and checking laboratory tests; collecting collateral information from previous clinicians and records; printing medication education pamphlets; faxing; corresponding with insurance companies/utilization review; performing documentation; billing; and taking care of other clinical and administrative paperwork).
In addition, physician associates could collect information using rating scales such as the 9-item Patient Health Questionnaire for measurement-based care21 and Geriatric Depression Scale, both of which are currently not used in psychiatric practice because of time constraints and lack of manpower. Keep in mind that these individuals are medical doctors and could do a good job with these kinds of tasks. Most of them already have clinical experience in the United States and know the health care system. These MDs could conduct an initial interview (what medical students, residents, and fellows do) and present to the attending psychiatrist. Psychiatrists could then focus on the follow-up interview; diagnoses and treatment; major medical decision making, including shared decision making (patients feel that they are not part of the treatment plan); and seeing more patients, which is a more effective use of their time. This training would give these physician associates a chance to work as doctors and make a living. These MDs have completed medical school training after passing Medical College Admission Test – equivalent exams in their countries. They have passed all steps of the U.S. Medical Licensing Examination and have received Educational Commission for Foreign Medical Graduates certification. Some have even completed residency programs in their home countries.
Some U.S. states already have implemented these kinds of programs. In Arkansas, Kansas, and Missouri,22,23 legislators have passed laws allowing unmatched graduates who have not completed a residency program to work in medically underserved areas with a collaborating physician. These physicians must directly supervise the new doctors for at least a month before they can see patients on their own. Another proposal that has been suggested to address the psychiatrist shortage is employing physician assistants to provide care.24-26
The model proposed here is comparable to postdoctoral fellow-principal investigator and resident-attending collaborative work. At hospitals, a certified nurse assistant helps patients with health care needs under the supervision of a nurse. Similarly, a physician associate could help a psychiatrist under his or her supervision. In the Sheppard Pratt Health System in Baltimore, where I worked previously, for example, nurses dictate and prepare discharge summaries for the attending physician with whom they work. These are the kinds of tasks that physician associates could do as well.
The wait time to get a new evaluation with a psychiatrist is enormous. The policy is that a new patient admitted to an inpatient unit must be seen within 24 hours. With this model, the physician associates could see patients within a few hours, take care of their most immediate needs, take a history and conduct a physical, and write an admission note for the attending psychiatrist to sign. Currently, the outpatient practice is so busy that psychiatrists do not have the time to read the discharge summaries of patients referred to them after a recent hospitalization, which often leads to poor aftercare. The physician associates could read the discharge summaries and provide pertinent information to the attending psychiatrists.
In the inpatient units and emergency departments, nurses and social workers see patients before the attending physician, present patient information to the attending psychiatrist, and document their findings. It is redundant for the physician to write the same narrative again. Rather, the physician could add an addendum to the nurse’s or social worker’s notes and sign off. This would save a lot of time.
Numerous well-designed studies support the adoption of collaborative care models as one means of providing quality psychiatric care to larger populations.27,28 The American Psychiatric Association (APA) is currently training 3,500 psychiatrists in collaborative care through the Centers for Medicare and Medicaid Services’ Transforming Clinical Practice Initiative.29,30 Despite this training and the services provided by the nurse practitioners and physician assistants, the shortage of psychiatrists has not been adequately addressed. Hence, we need to think outside the box to find other potential pragmatic solutions.
Simply increasing the hours of work or the number of nurse practitioners or physician assistants already in practice is not going to solve the problem completely. The model proposed here and previously31 is likely to improve the quality of care that we now provide. This model should not be seen as exploiting these unmatched medical graduates and setting up a two-tiered health care system. The salary for these physicians would be a small percentage (5%-10%; these are arbitrary percentages) from the reimbursement of the attending psychiatrist. This model would not affect the salary of the attending psychiatrists; with this model, they would be able to see 25%-50% more patients (again, arbitrary percentages) with the help and support from these physician associates.
Potential barriers to implementation
There could be inherent barriers and complications to implementation of this model that are difficult to foresee at this point. Nurse practitioners (222,000 plus) and physician assistants (83,000 plus) have a fixed and structured curriculum, have national examining boards and national organizations with recertification requirements, and are licensed as independent practitioners, at least as far as CME is concerned.
Physician associates would need a standardized curriculum and examinations to validate what they have studied and learned. This process might be an important part of the credentialing of these individuals, as well as evaluation of cultural competency. If this model is to successfully lead to formation of a specific clinical group, it might need its own specific identity, national organization, national standards of competency, national certification and recertification processes, and national conference and CME or at least a subsection in a national behavioral and medical health organization, such as the APA or the American Academy of Child and Adolescent Psychiatry.
It would be desirable to “field test” the physician associate concept to clarify implementation difficulties, including the ones described above, that could arise. The cost of implementation of this program should not be of much concern; the 6-month training could be on a volunteer basis, or a small stipend might be paid by graduate medical education funding. This model could prove to be rewarding long term, save trillions of health care dollars, and allow us to provide exceptional and timely care.
Conclusion
The 2020 Mental Health America annual State of Mental Health in America report found that more than 70% of youth with severe major depressive disorder were in need of treatment in 2017. The percentage of adults with any mental illness who did not receive treatment stood at about 57.2%.32 Meanwhile, from 1999 through 2014, the age-adjusted suicide rate in the United States increased 24%.33 More individuals are seeking help because of increased awareness.34,35 In light of the access to services afforded by the ACA, physician associates might ease the workload of psychiatrists and enable them to deliver better care to more people. We would not necessarily have to use the term “physician associate” and could generate better terminologies later. In short, let’s tap into the pools of unmatched graduates and shrinking shrinks! If this model is successful, it could be used in other specialties and countries. The stakes for our patients have never been higher.
References
1. Bishop TF et al. Health Aff. 2016;35(7):1271-7.
2. National Council Medical Director Institute. The psychiatric shortage: Causes and solutions. 2017. Washington: National Council for Behavioral Health.
3. Satiani A et al. Psychiatric Serv. 2018;69:710-3.
4. Carlat D. Psychiatric Times. 2010 Aug 3;27(8).
5. McCartney M. BMJ. 2017;359:j5022.
6. Maslach C and Leiter MP. World Psychiatry. 2016 Jun 5;15:103-11.
7. Merritt Hawkins. “The silent shortage: A white paper examining supply, demand and recruitment trends in psychiatry.” 2018.
8. Sederer LI and Sharfstein SS. JAMA. 2014 Sep 24;312:1195-6.
9. James DJ and Glaze LE. Mental health problems of prison and jail inmates. 2006 Sep. U.S. Justice Department, Bureau of Justice Statistics Special Report.
10. Koola MM et al. J Geriatr Care Res. 2018;5(2):57-67.
11. Buckley PF and Nasrallah HA. Curr Psychiatr. 2016;15:23-4.
12. American Medical Association Database. Open Residency and Fellowship Positions.
13. Sinsky C et al. Ann Intern Med. 2016;165:753-60.
14. Koola MM. Curr Psychiatr. 2017 Mar. 16(3):19-20,47,e1.
15. Koola MM and Sebastian J. HSOA J Psychiatry Depress Anxiety. 2016;(2):1-11.
16. Nasrallah HA and Chen AT. Ann Clin Psychiatry. 2017 Aug;29(3):195-202.
17. Nasrallah HA. Curr Psychiatr. 2013 Jul;7-8.
18. Chen AT and Nasrallah HA. Schizophr Res. 2019 Jun;208:1-7.
19. National Resident Matching Program, Results and Data: 2019 Main Residency Match. National Resident Matching Program, Washington, 2019.
20. Masters KJ. J Physician Assist Educ. 2015 Sep;26(3):136-43.
21. Koola MM et al. J Nerv Ment Dis. 2011;199(12):989-90.
22. “New Missouri licensing offers ‘Band-Aid’ for physician shortages.” Kansas City Business Journal. Updated 2017 May 16.
23. “After earning an MD, she’s headed back to school – to become a nurse.” STAT. 2016 Nov 8.
24. Keizer TB and Trangle MA. Acad Psychiatry. 2015 Dec;39(6):691-4.
25. Miller JG and Peterson DJ. Acad Psychiatry. 2015 Dec;39(6):685-6.
26. Smith MS. Curr Psychiatr. 2019 Sep;18(9):17-24.
27. Osofsky HJ et al. Acad Psychiatry. 2016 Oct;40(5):747-54.
28. Dreier-Wolfgramm A et al. Z Gerontol Geriatr. 2017 May;50(Suppl 2):68-77.
29. Huang H and Barkil-Oteo A. Psychosomatics. 2015 Nov-Dec;56(6):658-61.
30. Raney L et al. Fam Syst Health. 2014 Jun;32(2):147-8.
31. Koola MM. Curr Psychiatr. 2016 Dec. 15(12):33-4.
32. Mental Health America. State of Mental Health in America 2020.
33. Curtin SC et al. NCHS Data Brief. 2016 Apr;(241):1-8.
34. Kelly DL et al. Ann Intern Med. 2020;172(2):167-8.
35. Miller JP and Nasrallah HA. Curr Psychiatr. 2015;14(12):45-6.
Dr. Koola is an associate professor in the department of psychiatry and behavioral health at Stony Brook (N.Y.) University. His main area of interest is novel therapeutic discovery in the treatment of schizophrenia. He has a particular interest in improving the health care delivery system for people with psychiatric illness. Dr. Koola declared no conflicts of interest. He can be reached at [email protected].
*This commentary was updated 2/2/2020.
‘Physician associates’ could be used to expand the reach of psychiatry
‘Physician associates’ could be used to expand the reach of psychiatry
For many years now, we have been lamenting the shortage of psychiatrists practicing in the United States. At this point, we must identify possible solutions.1,2 Currently, the shortage of practicing psychiatrists in the United States could be as high as 45,000.3 The major problem is that the number of psychiatry residency positions will not increase in the foreseeable future, thus generating more psychiatrists is not an option.
Medicare pays about $150,000 per residency slot per year. To solve the mental health access problem, $27 billion (45,000 x $150,000 x 4 years)* would be required from Medicare, which is not feasible.4 The national average starting salary for psychiatrists from 2018-2019 was about $273,000 (much lower in academic institutions), according to Merritt Hawkins, the physician recruiting firm. That salary is modest, compared with those offered in other medical specialties. For this reason, many graduates choose other lucrative specialties. And we know that increasing the salaries of psychiatrists alone would not lead more people to choose psychiatry. On paper, it may say they work a 40-hour week, but they end up working 60 hours a week.
To make matters worse, family medicine and internal medicine doctors generally would rather not deal with people with mental illness and do “cherry-picking and lemon-dropping.” While many patients present to primary care with mental health issues, lack of time and education in psychiatric disorders and treatment hinder these physicians. In short, the mental health field cannot count on primary care physicians.
Meanwhile, there are thousands of unmatched residency graduates. In light of those realities, perhaps psychiatry residency programs could provide these unmatched graduates with 6 months of training and use them to supplement the workforce. These medical doctors, or “physician associates,” could be paired with a few psychiatrists to do clinical and administrative work. With one in four individuals having mental health issues, and more and more people seeking help because of increasing awareness and the benefits that accompanied the Affordable Care Act (ACA), physician associates might ease the workload of psychiatrists so that they can deliver better care to more people. We must take advantage of these two trends: The surge in unmatched graduates and “shrinking shrinks,” or the decline in the psychiatric workforce pool. (The Royal College of Physicians has established a category of clinicians called physician associates,5 but they are comparable to physician assistants in the United States. As you will see, the construct I am proposing is different.)
The current landscape
Currently, psychiatrists are under a lot of pressure to see a certain number of patients. Patients consistently complain that psychiatrists spend a maximum of 15 minutes with them, that the visits are interrupted by phone calls, and that they are not being heard and helped. Burnout, a silent epidemic among physicians, is relatively prevalent in psychiatry.6 Hence, some psychiatrists are reducing their hours and retiring early. Psychiatry has the third-oldest workforce, with 59% of current psychiatrists aged 55 years or older.7 A better pay/work ratio and work/life balance would enable psychiatrists to enjoy more fulfilling careers.
Many psychiatrists are spending a lot of their time in research, administration, and the classroom. In addition to those issues, the United States currently has a broken mental health care system.8 Finally, the medical practice landscape has changed dramatically in recent years, and those changes undermine both the effectiveness and well-being of clinicians.
The historical landscape
Some people proudly refer to the deinstitutionalization of mental asylums and state mental hospitals in the United States. But where have these patients gone? According to a U.S. Justice Department report, 2,220,300 adults were incarcerated in U.S. federal and state prisons and county jails in 2013.9 In addition, 4,751,400 adults in 2013 were on probation or parole. The percentages of inmates in state and federal prisons and local jails with a psychiatric diagnosis were 56%, 45%, and 64%, respectively.
I work at the Maryland correctional institutions, part of the Maryland Department of Public Safety and Correctional Services. One thing that I consistently hear from several correctional officers is “had these inmates received timely help and care, they wouldn’t have ended up behind bars.” Because of the criminalization of mental illness, in 44 states, the number of people with mental illness is higher in a jail or prison than in the largest state psychiatric hospital, according to the Treatment Advocacy Center. We have to be responsible for many of the inmates currently in correctional facilities for committing crimes related to mental health problems. In Maryland, a small state, there are 30,000 inmates in jails, and state and federal prison. The average cost of a meal is $1.36, thus $1.36 x 3 meals x 30,000 inmates = $122,400.00 for food alone for 1 day – this average does not take other expenses into account. By using money and manpower wisely and taking care of individuals’ mental health problems before they commit crimes, better outcomes could be achieved.
I used to work for MedOptions Inc. doing psychiatry consults at nursing homes and assisted-living facilities. Because of the shortage of psychiatrists and nurse practitioners, especially in the suburbs and rural areas, those patients could not be seen in a timely manner even for their 3-month routine follow-ups. As my colleagues and I have written previously, many elderly individuals with major neurocognitive disorders are not on the Food and Drug Administration–approved cognitive enhancers, such as donepezil, galantamine, and memantine.10 Instead, those patients are on benzodiazepines, which are associated with cognitive impairments, and increased risk of pneumonia and falls. Benzodiazepines also can cause and/or worsen disinhibited behavior. Also, in those settings, crisis situations often are addressed days to weeks later because of the doctor shortage. This situation is going to get worse, because this patient population is growing.
Child and geriatric psychiatry shortages
Child and geriatric psychiatrist shortages are even higher than those in general psychiatry.11 Many years of training and low salaries are a few of the reasons some choose not to do a fellowship. These residency graduates would rather join a practice at an attending salary than at a fellow’s salary, which requires an additional 1 to 2 years of training. Student loans of $100,000–$500,000 after residency also discourage some from pursuing fellowship opportunities. We need to consider models such as 2 years of residency with 2 years of a child psychiatry fellowship or 3 years of residency with 1 year of geriatric psychiatry fellowship. Working as an adult attending physician (50% of the time) and concurrently doing a fellowship (50% of the time) while receiving an attending salary might motivate more people to complete a fellowship.
In specialties such as radiology, international medical graduates (IMGs) who have completed residency training in radiology in other countries can complete a radiology fellowship in a particular area for several years and can practice in the United States as board-eligible certified MDs. Likewise, in line with the model proposed here, we could provide unmatched graduates who have no residency training with 3 to 4 years of child psychiatry and geriatric psychiatry training in addition to some adult psychiatry training.
Implementation of such a model might take care of the shortage of child and geriatric psychiatrists. In 2015, there were 56 geriatric psychiatry fellowship programs; 54 positions were filled, and 51 fellows completed training.12 “It appears that a reasonable percentage of IMGs who obtain a fellowship in geriatric psychiatry do not have an intent of pursuing a career in the field,” Marc H. Zisselman, MD, former geriatric psychiatry fellowship director and currently with the Einstein Medical Center in Philadelphia, told me in 2016. These numbers are not at all sufficient to take care of the nation’s unmet need. Hence, implementing alternate strategies is imperative.
Administrative tasks and care
What consumes a psychiatrist’s time and leads to burnout? The answer has to do with administrative tasks at work. Administrative tasks are not an effective use of time for an MD who has spent more than a decade in medical school, residency, and fellowship training. Although electronic medical record (EMR) systems are considered a major advancement, engaging in the process throughout the day is associated with exhaustion.
Many physicians feel that EMRs have slowed them down, and some are not well-equipped to use them in quick and efficient ways. EMRs also have led to physicians making minimal eye contact in interviews with patients. Patients often complain: “I am talking, and the doctor is looking at the computer and typing.” Patients consider this behavior to be unprofessional and rude. In a survey of 57 U.S. physicians in family medicine, internal medicine, cardiology, and orthopedics, results showed that during the work day, 27% of their time was spent on direct clinical face time with patients and 49.2% was spent on EMR and desk work. While in the examination room with patients, physicians spent 52.9% of their time on direct clinical face time and 37.0% on EMR and desk work. Outside office hours, physicians spend up to 2 hours of personal time each night doing additional computer and other clerical work.13
Several EMR software systems, such as CareLogic, Cerner, Epic,NextGen, PointClickCare, and Sunrise, are used in the United States. The U.S. Veterans Affairs Medical Centers (VAMCs) use the computerized patient record system (CPRS) across the country. VA clinicians find CPRS extremely useful when they move from one VAMC to another. Likewise, hospitals and universities may use one software system such as the CPRS and thus, when clinicians change jobs, they find it hard to adapt to the new system.
Because psychiatrists are wasting a lot of time doing administrative tasks, they might be unable to do a good job with regard to making the right diagnoses and prescribing the best treatments.When I ask patients what are they diagnosed with, they tell me: “It depends on who you ask,” or “I’ve been diagnosed with everything.” This shows that we are not doing a good job or something is not right.
Currently, psychiatrists do not have the time and/or interest to make the right diagnoses and provide adequate psychoeducation for their patients. This also could be attributable to a variety of factors, including, but not limited to, time constraints, cynicism, and apathy. Time constraints also lead to the gross underutilization14 of relapse prevention strategies such as long-acting injectables and medications that can prevent suicide, such as lithium and clozapine.15
Other factors that undermine good care include not participating in continuing medical education (CME) and not staying up to date with the literature. For example, haloperidol continues to be one of the most frequently prescribed (probably, the most common) antipsychotic, although it is clearly neurotoxic16,17 and other safer options are available.18 Board certification and maintenance of certification (MOC) are not synonymous with good clinical practice. Many physicians are finding it hard to complete daily documentation, let alone time for MOC. For a variety of reasons, many are not maintaining certification, and this number is likely to increase. Think about how much time is devoted to the one-to-one interview with the patient and direct patient care during the 15-minute medical check appointment and the hour-long new evaluation. In some clinics, psychiatrists are asked to see more than 25 patients in 4 hours. Some U.S.-based psychiatrists see 65 inpatients and initiate 10 new evaluations in a single day. Under those kinds of time constraints, how can we provide quality care?
A model that would address the shortage
Overall, 7,826 PGY-1 applicants were unmatched in 2019, according to data from the 2019 Main Residency Match.19 Psychiatry residency programs could give these unmatched graduates 6 months of training (arbitrary duration) in psychiatry, which is not at all difficult with the program modules that are available.20 We could use them as physician associates as a major contributor to our workforce to complete administrative and other clinical tasks.
Administrative tasks are not necessarily negative, as all psychiatrists have done administrative tasks as medical students, residents, and fellows. However, at this point, administrative tasks are not an effective use of a psychiatrist’s time. Those physician associates could be paired with two to three psychiatrists to do administrative tasks (for making daytime and overnight phone calls; handling prescriptions, prior authorizations, and medication orders, especially over-the-counter and comfort medications in the inpatient units; doing chart reviews; ordering and checking laboratory tests; collecting collateral information from previous clinicians and records; printing medication education pamphlets; faxing; corresponding with insurance companies/utilization review; performing documentation; billing; and taking care of other clinical and administrative paperwork).
In addition, physician associates could collect information using rating scales such as the 9-item Patient Health Questionnaire for measurement-based care21 and Geriatric Depression Scale, both of which are currently not used in psychiatric practice because of time constraints and lack of manpower. Keep in mind that these individuals are medical doctors and could do a good job with these kinds of tasks. Most of them already have clinical experience in the United States and know the health care system. These MDs could conduct an initial interview (what medical students, residents, and fellows do) and present to the attending psychiatrist. Psychiatrists could then focus on the follow-up interview; diagnoses and treatment; major medical decision making, including shared decision making (patients feel that they are not part of the treatment plan); and seeing more patients, which is a more effective use of their time. This training would give these physician associates a chance to work as doctors and make a living. These MDs have completed medical school training after passing Medical College Admission Test – equivalent exams in their countries. They have passed all steps of the U.S. Medical Licensing Examination and have received Educational Commission for Foreign Medical Graduates certification. Some have even completed residency programs in their home countries.
Some U.S. states already have implemented these kinds of programs. In Arkansas, Kansas, and Missouri,22,23 legislators have passed laws allowing unmatched graduates who have not completed a residency program to work in medically underserved areas with a collaborating physician. These physicians must directly supervise the new doctors for at least a month before they can see patients on their own. Another proposal that has been suggested to address the psychiatrist shortage is employing physician assistants to provide care.24-26
The model proposed here is comparable to postdoctoral fellow-principal investigator and resident-attending collaborative work. At hospitals, a certified nurse assistant helps patients with health care needs under the supervision of a nurse. Similarly, a physician associate could help a psychiatrist under his or her supervision. In the Sheppard Pratt Health System in Baltimore, where I worked previously, for example, nurses dictate and prepare discharge summaries for the attending physician with whom they work. These are the kinds of tasks that physician associates could do as well.
The wait time to get a new evaluation with a psychiatrist is enormous. The policy is that a new patient admitted to an inpatient unit must be seen within 24 hours. With this model, the physician associates could see patients within a few hours, take care of their most immediate needs, take a history and conduct a physical, and write an admission note for the attending psychiatrist to sign. Currently, the outpatient practice is so busy that psychiatrists do not have the time to read the discharge summaries of patients referred to them after a recent hospitalization, which often leads to poor aftercare. The physician associates could read the discharge summaries and provide pertinent information to the attending psychiatrists.
In the inpatient units and emergency departments, nurses and social workers see patients before the attending physician, present patient information to the attending psychiatrist, and document their findings. It is redundant for the physician to write the same narrative again. Rather, the physician could add an addendum to the nurse’s or social worker’s notes and sign off. This would save a lot of time.
Numerous well-designed studies support the adoption of collaborative care models as one means of providing quality psychiatric care to larger populations.27,28 The American Psychiatric Association (APA) is currently training 3,500 psychiatrists in collaborative care through the Centers for Medicare and Medicaid Services’ Transforming Clinical Practice Initiative.29,30 Despite this training and the services provided by the nurse practitioners and physician assistants, the shortage of psychiatrists has not been adequately addressed. Hence, we need to think outside the box to find other potential pragmatic solutions.
Simply increasing the hours of work or the number of nurse practitioners or physician assistants already in practice is not going to solve the problem completely. The model proposed here and previously31 is likely to improve the quality of care that we now provide. This model should not be seen as exploiting these unmatched medical graduates and setting up a two-tiered health care system. The salary for these physicians would be a small percentage (5%-10%; these are arbitrary percentages) from the reimbursement of the attending psychiatrist. This model would not affect the salary of the attending psychiatrists; with this model, they would be able to see 25%-50% more patients (again, arbitrary percentages) with the help and support from these physician associates.
Potential barriers to implementation
There could be inherent barriers and complications to implementation of this model that are difficult to foresee at this point. Nurse practitioners (222,000 plus) and physician assistants (83,000 plus) have a fixed and structured curriculum, have national examining boards and national organizations with recertification requirements, and are licensed as independent practitioners, at least as far as CME is concerned.
Physician associates would need a standardized curriculum and examinations to validate what they have studied and learned. This process might be an important part of the credentialing of these individuals, as well as evaluation of cultural competency. If this model is to successfully lead to formation of a specific clinical group, it might need its own specific identity, national organization, national standards of competency, national certification and recertification processes, and national conference and CME or at least a subsection in a national behavioral and medical health organization, such as the APA or the American Academy of Child and Adolescent Psychiatry.
It would be desirable to “field test” the physician associate concept to clarify implementation difficulties, including the ones described above, that could arise. The cost of implementation of this program should not be of much concern; the 6-month training could be on a volunteer basis, or a small stipend might be paid by graduate medical education funding. This model could prove to be rewarding long term, save trillions of health care dollars, and allow us to provide exceptional and timely care.
Conclusion
The 2020 Mental Health America annual State of Mental Health in America report found that more than 70% of youth with severe major depressive disorder were in need of treatment in 2017. The percentage of adults with any mental illness who did not receive treatment stood at about 57.2%.32 Meanwhile, from 1999 through 2014, the age-adjusted suicide rate in the United States increased 24%.33 More individuals are seeking help because of increased awareness.34,35 In light of the access to services afforded by the ACA, physician associates might ease the workload of psychiatrists and enable them to deliver better care to more people. We would not necessarily have to use the term “physician associate” and could generate better terminologies later. In short, let’s tap into the pools of unmatched graduates and shrinking shrinks! If this model is successful, it could be used in other specialties and countries. The stakes for our patients have never been higher.
References
1. Bishop TF et al. Health Aff. 2016;35(7):1271-7.
2. National Council Medical Director Institute. The psychiatric shortage: Causes and solutions. 2017. Washington: National Council for Behavioral Health.
3. Satiani A et al. Psychiatric Serv. 2018;69:710-3.
4. Carlat D. Psychiatric Times. 2010 Aug 3;27(8).
5. McCartney M. BMJ. 2017;359:j5022.
6. Maslach C and Leiter MP. World Psychiatry. 2016 Jun 5;15:103-11.
7. Merritt Hawkins. “The silent shortage: A white paper examining supply, demand and recruitment trends in psychiatry.” 2018.
8. Sederer LI and Sharfstein SS. JAMA. 2014 Sep 24;312:1195-6.
9. James DJ and Glaze LE. Mental health problems of prison and jail inmates. 2006 Sep. U.S. Justice Department, Bureau of Justice Statistics Special Report.
10. Koola MM et al. J Geriatr Care Res. 2018;5(2):57-67.
11. Buckley PF and Nasrallah HA. Curr Psychiatr. 2016;15:23-4.
12. American Medical Association Database. Open Residency and Fellowship Positions.
13. Sinsky C et al. Ann Intern Med. 2016;165:753-60.
14. Koola MM. Curr Psychiatr. 2017 Mar. 16(3):19-20,47,e1.
15. Koola MM and Sebastian J. HSOA J Psychiatry Depress Anxiety. 2016;(2):1-11.
16. Nasrallah HA and Chen AT. Ann Clin Psychiatry. 2017 Aug;29(3):195-202.
17. Nasrallah HA. Curr Psychiatr. 2013 Jul;7-8.
18. Chen AT and Nasrallah HA. Schizophr Res. 2019 Jun;208:1-7.
19. National Resident Matching Program, Results and Data: 2019 Main Residency Match. National Resident Matching Program, Washington, 2019.
20. Masters KJ. J Physician Assist Educ. 2015 Sep;26(3):136-43.
21. Koola MM et al. J Nerv Ment Dis. 2011;199(12):989-90.
22. “New Missouri licensing offers ‘Band-Aid’ for physician shortages.” Kansas City Business Journal. Updated 2017 May 16.
23. “After earning an MD, she’s headed back to school – to become a nurse.” STAT. 2016 Nov 8.
24. Keizer TB and Trangle MA. Acad Psychiatry. 2015 Dec;39(6):691-4.
25. Miller JG and Peterson DJ. Acad Psychiatry. 2015 Dec;39(6):685-6.
26. Smith MS. Curr Psychiatr. 2019 Sep;18(9):17-24.
27. Osofsky HJ et al. Acad Psychiatry. 2016 Oct;40(5):747-54.
28. Dreier-Wolfgramm A et al. Z Gerontol Geriatr. 2017 May;50(Suppl 2):68-77.
29. Huang H and Barkil-Oteo A. Psychosomatics. 2015 Nov-Dec;56(6):658-61.
30. Raney L et al. Fam Syst Health. 2014 Jun;32(2):147-8.
31. Koola MM. Curr Psychiatr. 2016 Dec. 15(12):33-4.
32. Mental Health America. State of Mental Health in America 2020.
33. Curtin SC et al. NCHS Data Brief. 2016 Apr;(241):1-8.
34. Kelly DL et al. Ann Intern Med. 2020;172(2):167-8.
35. Miller JP and Nasrallah HA. Curr Psychiatr. 2015;14(12):45-6.
Dr. Koola is an associate professor in the department of psychiatry and behavioral health at Stony Brook (N.Y.) University. His main area of interest is novel therapeutic discovery in the treatment of schizophrenia. He has a particular interest in improving the health care delivery system for people with psychiatric illness. Dr. Koola declared no conflicts of interest. He can be reached at [email protected].
*This commentary was updated 2/2/2020.
For many years now, we have been lamenting the shortage of psychiatrists practicing in the United States. At this point, we must identify possible solutions.1,2 Currently, the shortage of practicing psychiatrists in the United States could be as high as 45,000.3 The major problem is that the number of psychiatry residency positions will not increase in the foreseeable future, thus generating more psychiatrists is not an option.
Medicare pays about $150,000 per residency slot per year. To solve the mental health access problem, $27 billion (45,000 x $150,000 x 4 years)* would be required from Medicare, which is not feasible.4 The national average starting salary for psychiatrists from 2018-2019 was about $273,000 (much lower in academic institutions), according to Merritt Hawkins, the physician recruiting firm. That salary is modest, compared with those offered in other medical specialties. For this reason, many graduates choose other lucrative specialties. And we know that increasing the salaries of psychiatrists alone would not lead more people to choose psychiatry. On paper, it may say they work a 40-hour week, but they end up working 60 hours a week.
To make matters worse, family medicine and internal medicine doctors generally would rather not deal with people with mental illness and do “cherry-picking and lemon-dropping.” While many patients present to primary care with mental health issues, lack of time and education in psychiatric disorders and treatment hinder these physicians. In short, the mental health field cannot count on primary care physicians.
Meanwhile, there are thousands of unmatched residency graduates. In light of those realities, perhaps psychiatry residency programs could provide these unmatched graduates with 6 months of training and use them to supplement the workforce. These medical doctors, or “physician associates,” could be paired with a few psychiatrists to do clinical and administrative work. With one in four individuals having mental health issues, and more and more people seeking help because of increasing awareness and the benefits that accompanied the Affordable Care Act (ACA), physician associates might ease the workload of psychiatrists so that they can deliver better care to more people. We must take advantage of these two trends: The surge in unmatched graduates and “shrinking shrinks,” or the decline in the psychiatric workforce pool. (The Royal College of Physicians has established a category of clinicians called physician associates,5 but they are comparable to physician assistants in the United States. As you will see, the construct I am proposing is different.)
The current landscape
Currently, psychiatrists are under a lot of pressure to see a certain number of patients. Patients consistently complain that psychiatrists spend a maximum of 15 minutes with them, that the visits are interrupted by phone calls, and that they are not being heard and helped. Burnout, a silent epidemic among physicians, is relatively prevalent in psychiatry.6 Hence, some psychiatrists are reducing their hours and retiring early. Psychiatry has the third-oldest workforce, with 59% of current psychiatrists aged 55 years or older.7 A better pay/work ratio and work/life balance would enable psychiatrists to enjoy more fulfilling careers.
Many psychiatrists are spending a lot of their time in research, administration, and the classroom. In addition to those issues, the United States currently has a broken mental health care system.8 Finally, the medical practice landscape has changed dramatically in recent years, and those changes undermine both the effectiveness and well-being of clinicians.
The historical landscape
Some people proudly refer to the deinstitutionalization of mental asylums and state mental hospitals in the United States. But where have these patients gone? According to a U.S. Justice Department report, 2,220,300 adults were incarcerated in U.S. federal and state prisons and county jails in 2013.9 In addition, 4,751,400 adults in 2013 were on probation or parole. The percentages of inmates in state and federal prisons and local jails with a psychiatric diagnosis were 56%, 45%, and 64%, respectively.
I work at the Maryland correctional institutions, part of the Maryland Department of Public Safety and Correctional Services. One thing that I consistently hear from several correctional officers is “had these inmates received timely help and care, they wouldn’t have ended up behind bars.” Because of the criminalization of mental illness, in 44 states, the number of people with mental illness is higher in a jail or prison than in the largest state psychiatric hospital, according to the Treatment Advocacy Center. We have to be responsible for many of the inmates currently in correctional facilities for committing crimes related to mental health problems. In Maryland, a small state, there are 30,000 inmates in jails, and state and federal prison. The average cost of a meal is $1.36, thus $1.36 x 3 meals x 30,000 inmates = $122,400.00 for food alone for 1 day – this average does not take other expenses into account. By using money and manpower wisely and taking care of individuals’ mental health problems before they commit crimes, better outcomes could be achieved.
I used to work for MedOptions Inc. doing psychiatry consults at nursing homes and assisted-living facilities. Because of the shortage of psychiatrists and nurse practitioners, especially in the suburbs and rural areas, those patients could not be seen in a timely manner even for their 3-month routine follow-ups. As my colleagues and I have written previously, many elderly individuals with major neurocognitive disorders are not on the Food and Drug Administration–approved cognitive enhancers, such as donepezil, galantamine, and memantine.10 Instead, those patients are on benzodiazepines, which are associated with cognitive impairments, and increased risk of pneumonia and falls. Benzodiazepines also can cause and/or worsen disinhibited behavior. Also, in those settings, crisis situations often are addressed days to weeks later because of the doctor shortage. This situation is going to get worse, because this patient population is growing.
Child and geriatric psychiatry shortages
Child and geriatric psychiatrist shortages are even higher than those in general psychiatry.11 Many years of training and low salaries are a few of the reasons some choose not to do a fellowship. These residency graduates would rather join a practice at an attending salary than at a fellow’s salary, which requires an additional 1 to 2 years of training. Student loans of $100,000–$500,000 after residency also discourage some from pursuing fellowship opportunities. We need to consider models such as 2 years of residency with 2 years of a child psychiatry fellowship or 3 years of residency with 1 year of geriatric psychiatry fellowship. Working as an adult attending physician (50% of the time) and concurrently doing a fellowship (50% of the time) while receiving an attending salary might motivate more people to complete a fellowship.
In specialties such as radiology, international medical graduates (IMGs) who have completed residency training in radiology in other countries can complete a radiology fellowship in a particular area for several years and can practice in the United States as board-eligible certified MDs. Likewise, in line with the model proposed here, we could provide unmatched graduates who have no residency training with 3 to 4 years of child psychiatry and geriatric psychiatry training in addition to some adult psychiatry training.
Implementation of such a model might take care of the shortage of child and geriatric psychiatrists. In 2015, there were 56 geriatric psychiatry fellowship programs; 54 positions were filled, and 51 fellows completed training.12 “It appears that a reasonable percentage of IMGs who obtain a fellowship in geriatric psychiatry do not have an intent of pursuing a career in the field,” Marc H. Zisselman, MD, former geriatric psychiatry fellowship director and currently with the Einstein Medical Center in Philadelphia, told me in 2016. These numbers are not at all sufficient to take care of the nation’s unmet need. Hence, implementing alternate strategies is imperative.
Administrative tasks and care
What consumes a psychiatrist’s time and leads to burnout? The answer has to do with administrative tasks at work. Administrative tasks are not an effective use of time for an MD who has spent more than a decade in medical school, residency, and fellowship training. Although electronic medical record (EMR) systems are considered a major advancement, engaging in the process throughout the day is associated with exhaustion.
Many physicians feel that EMRs have slowed them down, and some are not well-equipped to use them in quick and efficient ways. EMRs also have led to physicians making minimal eye contact in interviews with patients. Patients often complain: “I am talking, and the doctor is looking at the computer and typing.” Patients consider this behavior to be unprofessional and rude. In a survey of 57 U.S. physicians in family medicine, internal medicine, cardiology, and orthopedics, results showed that during the work day, 27% of their time was spent on direct clinical face time with patients and 49.2% was spent on EMR and desk work. While in the examination room with patients, physicians spent 52.9% of their time on direct clinical face time and 37.0% on EMR and desk work. Outside office hours, physicians spend up to 2 hours of personal time each night doing additional computer and other clerical work.13
Several EMR software systems, such as CareLogic, Cerner, Epic,NextGen, PointClickCare, and Sunrise, are used in the United States. The U.S. Veterans Affairs Medical Centers (VAMCs) use the computerized patient record system (CPRS) across the country. VA clinicians find CPRS extremely useful when they move from one VAMC to another. Likewise, hospitals and universities may use one software system such as the CPRS and thus, when clinicians change jobs, they find it hard to adapt to the new system.
Because psychiatrists are wasting a lot of time doing administrative tasks, they might be unable to do a good job with regard to making the right diagnoses and prescribing the best treatments.When I ask patients what are they diagnosed with, they tell me: “It depends on who you ask,” or “I’ve been diagnosed with everything.” This shows that we are not doing a good job or something is not right.
Currently, psychiatrists do not have the time and/or interest to make the right diagnoses and provide adequate psychoeducation for their patients. This also could be attributable to a variety of factors, including, but not limited to, time constraints, cynicism, and apathy. Time constraints also lead to the gross underutilization14 of relapse prevention strategies such as long-acting injectables and medications that can prevent suicide, such as lithium and clozapine.15
Other factors that undermine good care include not participating in continuing medical education (CME) and not staying up to date with the literature. For example, haloperidol continues to be one of the most frequently prescribed (probably, the most common) antipsychotic, although it is clearly neurotoxic16,17 and other safer options are available.18 Board certification and maintenance of certification (MOC) are not synonymous with good clinical practice. Many physicians are finding it hard to complete daily documentation, let alone time for MOC. For a variety of reasons, many are not maintaining certification, and this number is likely to increase. Think about how much time is devoted to the one-to-one interview with the patient and direct patient care during the 15-minute medical check appointment and the hour-long new evaluation. In some clinics, psychiatrists are asked to see more than 25 patients in 4 hours. Some U.S.-based psychiatrists see 65 inpatients and initiate 10 new evaluations in a single day. Under those kinds of time constraints, how can we provide quality care?
A model that would address the shortage
Overall, 7,826 PGY-1 applicants were unmatched in 2019, according to data from the 2019 Main Residency Match.19 Psychiatry residency programs could give these unmatched graduates 6 months of training (arbitrary duration) in psychiatry, which is not at all difficult with the program modules that are available.20 We could use them as physician associates as a major contributor to our workforce to complete administrative and other clinical tasks.
Administrative tasks are not necessarily negative, as all psychiatrists have done administrative tasks as medical students, residents, and fellows. However, at this point, administrative tasks are not an effective use of a psychiatrist’s time. Those physician associates could be paired with two to three psychiatrists to do administrative tasks (for making daytime and overnight phone calls; handling prescriptions, prior authorizations, and medication orders, especially over-the-counter and comfort medications in the inpatient units; doing chart reviews; ordering and checking laboratory tests; collecting collateral information from previous clinicians and records; printing medication education pamphlets; faxing; corresponding with insurance companies/utilization review; performing documentation; billing; and taking care of other clinical and administrative paperwork).
In addition, physician associates could collect information using rating scales such as the 9-item Patient Health Questionnaire for measurement-based care21 and Geriatric Depression Scale, both of which are currently not used in psychiatric practice because of time constraints and lack of manpower. Keep in mind that these individuals are medical doctors and could do a good job with these kinds of tasks. Most of them already have clinical experience in the United States and know the health care system. These MDs could conduct an initial interview (what medical students, residents, and fellows do) and present to the attending psychiatrist. Psychiatrists could then focus on the follow-up interview; diagnoses and treatment; major medical decision making, including shared decision making (patients feel that they are not part of the treatment plan); and seeing more patients, which is a more effective use of their time. This training would give these physician associates a chance to work as doctors and make a living. These MDs have completed medical school training after passing Medical College Admission Test – equivalent exams in their countries. They have passed all steps of the U.S. Medical Licensing Examination and have received Educational Commission for Foreign Medical Graduates certification. Some have even completed residency programs in their home countries.
Some U.S. states already have implemented these kinds of programs. In Arkansas, Kansas, and Missouri,22,23 legislators have passed laws allowing unmatched graduates who have not completed a residency program to work in medically underserved areas with a collaborating physician. These physicians must directly supervise the new doctors for at least a month before they can see patients on their own. Another proposal that has been suggested to address the psychiatrist shortage is employing physician assistants to provide care.24-26
The model proposed here is comparable to postdoctoral fellow-principal investigator and resident-attending collaborative work. At hospitals, a certified nurse assistant helps patients with health care needs under the supervision of a nurse. Similarly, a physician associate could help a psychiatrist under his or her supervision. In the Sheppard Pratt Health System in Baltimore, where I worked previously, for example, nurses dictate and prepare discharge summaries for the attending physician with whom they work. These are the kinds of tasks that physician associates could do as well.
The wait time to get a new evaluation with a psychiatrist is enormous. The policy is that a new patient admitted to an inpatient unit must be seen within 24 hours. With this model, the physician associates could see patients within a few hours, take care of their most immediate needs, take a history and conduct a physical, and write an admission note for the attending psychiatrist to sign. Currently, the outpatient practice is so busy that psychiatrists do not have the time to read the discharge summaries of patients referred to them after a recent hospitalization, which often leads to poor aftercare. The physician associates could read the discharge summaries and provide pertinent information to the attending psychiatrists.
In the inpatient units and emergency departments, nurses and social workers see patients before the attending physician, present patient information to the attending psychiatrist, and document their findings. It is redundant for the physician to write the same narrative again. Rather, the physician could add an addendum to the nurse’s or social worker’s notes and sign off. This would save a lot of time.
Numerous well-designed studies support the adoption of collaborative care models as one means of providing quality psychiatric care to larger populations.27,28 The American Psychiatric Association (APA) is currently training 3,500 psychiatrists in collaborative care through the Centers for Medicare and Medicaid Services’ Transforming Clinical Practice Initiative.29,30 Despite this training and the services provided by the nurse practitioners and physician assistants, the shortage of psychiatrists has not been adequately addressed. Hence, we need to think outside the box to find other potential pragmatic solutions.
Simply increasing the hours of work or the number of nurse practitioners or physician assistants already in practice is not going to solve the problem completely. The model proposed here and previously31 is likely to improve the quality of care that we now provide. This model should not be seen as exploiting these unmatched medical graduates and setting up a two-tiered health care system. The salary for these physicians would be a small percentage (5%-10%; these are arbitrary percentages) from the reimbursement of the attending psychiatrist. This model would not affect the salary of the attending psychiatrists; with this model, they would be able to see 25%-50% more patients (again, arbitrary percentages) with the help and support from these physician associates.
Potential barriers to implementation
There could be inherent barriers and complications to implementation of this model that are difficult to foresee at this point. Nurse practitioners (222,000 plus) and physician assistants (83,000 plus) have a fixed and structured curriculum, have national examining boards and national organizations with recertification requirements, and are licensed as independent practitioners, at least as far as CME is concerned.
Physician associates would need a standardized curriculum and examinations to validate what they have studied and learned. This process might be an important part of the credentialing of these individuals, as well as evaluation of cultural competency. If this model is to successfully lead to formation of a specific clinical group, it might need its own specific identity, national organization, national standards of competency, national certification and recertification processes, and national conference and CME or at least a subsection in a national behavioral and medical health organization, such as the APA or the American Academy of Child and Adolescent Psychiatry.
It would be desirable to “field test” the physician associate concept to clarify implementation difficulties, including the ones described above, that could arise. The cost of implementation of this program should not be of much concern; the 6-month training could be on a volunteer basis, or a small stipend might be paid by graduate medical education funding. This model could prove to be rewarding long term, save trillions of health care dollars, and allow us to provide exceptional and timely care.
Conclusion
The 2020 Mental Health America annual State of Mental Health in America report found that more than 70% of youth with severe major depressive disorder were in need of treatment in 2017. The percentage of adults with any mental illness who did not receive treatment stood at about 57.2%.32 Meanwhile, from 1999 through 2014, the age-adjusted suicide rate in the United States increased 24%.33 More individuals are seeking help because of increased awareness.34,35 In light of the access to services afforded by the ACA, physician associates might ease the workload of psychiatrists and enable them to deliver better care to more people. We would not necessarily have to use the term “physician associate” and could generate better terminologies later. In short, let’s tap into the pools of unmatched graduates and shrinking shrinks! If this model is successful, it could be used in other specialties and countries. The stakes for our patients have never been higher.
References
1. Bishop TF et al. Health Aff. 2016;35(7):1271-7.
2. National Council Medical Director Institute. The psychiatric shortage: Causes and solutions. 2017. Washington: National Council for Behavioral Health.
3. Satiani A et al. Psychiatric Serv. 2018;69:710-3.
4. Carlat D. Psychiatric Times. 2010 Aug 3;27(8).
5. McCartney M. BMJ. 2017;359:j5022.
6. Maslach C and Leiter MP. World Psychiatry. 2016 Jun 5;15:103-11.
7. Merritt Hawkins. “The silent shortage: A white paper examining supply, demand and recruitment trends in psychiatry.” 2018.
8. Sederer LI and Sharfstein SS. JAMA. 2014 Sep 24;312:1195-6.
9. James DJ and Glaze LE. Mental health problems of prison and jail inmates. 2006 Sep. U.S. Justice Department, Bureau of Justice Statistics Special Report.
10. Koola MM et al. J Geriatr Care Res. 2018;5(2):57-67.
11. Buckley PF and Nasrallah HA. Curr Psychiatr. 2016;15:23-4.
12. American Medical Association Database. Open Residency and Fellowship Positions.
13. Sinsky C et al. Ann Intern Med. 2016;165:753-60.
14. Koola MM. Curr Psychiatr. 2017 Mar. 16(3):19-20,47,e1.
15. Koola MM and Sebastian J. HSOA J Psychiatry Depress Anxiety. 2016;(2):1-11.
16. Nasrallah HA and Chen AT. Ann Clin Psychiatry. 2017 Aug;29(3):195-202.
17. Nasrallah HA. Curr Psychiatr. 2013 Jul;7-8.
18. Chen AT and Nasrallah HA. Schizophr Res. 2019 Jun;208:1-7.
19. National Resident Matching Program, Results and Data: 2019 Main Residency Match. National Resident Matching Program, Washington, 2019.
20. Masters KJ. J Physician Assist Educ. 2015 Sep;26(3):136-43.
21. Koola MM et al. J Nerv Ment Dis. 2011;199(12):989-90.
22. “New Missouri licensing offers ‘Band-Aid’ for physician shortages.” Kansas City Business Journal. Updated 2017 May 16.
23. “After earning an MD, she’s headed back to school – to become a nurse.” STAT. 2016 Nov 8.
24. Keizer TB and Trangle MA. Acad Psychiatry. 2015 Dec;39(6):691-4.
25. Miller JG and Peterson DJ. Acad Psychiatry. 2015 Dec;39(6):685-6.
26. Smith MS. Curr Psychiatr. 2019 Sep;18(9):17-24.
27. Osofsky HJ et al. Acad Psychiatry. 2016 Oct;40(5):747-54.
28. Dreier-Wolfgramm A et al. Z Gerontol Geriatr. 2017 May;50(Suppl 2):68-77.
29. Huang H and Barkil-Oteo A. Psychosomatics. 2015 Nov-Dec;56(6):658-61.
30. Raney L et al. Fam Syst Health. 2014 Jun;32(2):147-8.
31. Koola MM. Curr Psychiatr. 2016 Dec. 15(12):33-4.
32. Mental Health America. State of Mental Health in America 2020.
33. Curtin SC et al. NCHS Data Brief. 2016 Apr;(241):1-8.
34. Kelly DL et al. Ann Intern Med. 2020;172(2):167-8.
35. Miller JP and Nasrallah HA. Curr Psychiatr. 2015;14(12):45-6.
Dr. Koola is an associate professor in the department of psychiatry and behavioral health at Stony Brook (N.Y.) University. His main area of interest is novel therapeutic discovery in the treatment of schizophrenia. He has a particular interest in improving the health care delivery system for people with psychiatric illness. Dr. Koola declared no conflicts of interest. He can be reached at [email protected].
*This commentary was updated 2/2/2020.