User login
New medical ethics series debuts
Dear colleagues,
The first issue of The New Gastroenterologist in 2020 consists of a particularly interesting array of articles and the introduction of a new medical ethics series!
This month’s “In Focus” article, brought to you by Jennifer Maratt (Indiana University) and Elena Stoffel (University of Michigan), provides a high yield overview of hereditary colorectal cancer and polyposis syndromes, with guidance on when a referral to a high risk cancer specialist and geneticist is warranted.
Daniel Mills (Cunningham, Meyer & Vedrine P.C.) gives us a valuable legal perspective of the role of electronic patient portals in the dissemination of information and medical advice to patients – such an important topic for everyone to be aware of as the nature of patient communication now strongly relies on electronic messaging.
R. Thomas Finn III (Palo Alto Medical Foundation) and David Leiman (Duke) nicely broach the issue of patient satisfaction. This is a timely topic as many institutions are not only publishing patient reviews online so that they are readily available to the public, but are also making financial incentives contingent on high patient ratings. The article discusses the evolution of the emphasis placed on patient satisfaction throughout the years with tips on how to navigate some of the distinct challenges within gastroenterology.
As part of our DHPA Private Practice Perspectives series, David Stokesberry (Digestive Disease Specialists Inc, Oklahoma City) discusses the nuts and bolts of ambulatory endoscopy centers and some of the challenges and benefits that accompany ownership of such centers.
An often overlooked aspect of gastroenterology training is nutrition. In our postfellowship pathways section, Dejan Micic (University of Chicago) outlines his decision to pursue a career in nutrition support, small bowel disorders, and the practice of deep enteroscopy.
Finally, this quarter’s newsletter features the start of a new section, which I am very excited to introduce – a case based series which will address issues in clinical medical ethics specific to gastroenterology. Lauren Feld (University of Washington) writes the inaugural piece for the section, providing a systematic approach to the patient with an existing do-not-resuscitate (DNR) order that is about to undergo endoscopy.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dear colleagues,
The first issue of The New Gastroenterologist in 2020 consists of a particularly interesting array of articles and the introduction of a new medical ethics series!
This month’s “In Focus” article, brought to you by Jennifer Maratt (Indiana University) and Elena Stoffel (University of Michigan), provides a high yield overview of hereditary colorectal cancer and polyposis syndromes, with guidance on when a referral to a high risk cancer specialist and geneticist is warranted.
Daniel Mills (Cunningham, Meyer & Vedrine P.C.) gives us a valuable legal perspective of the role of electronic patient portals in the dissemination of information and medical advice to patients – such an important topic for everyone to be aware of as the nature of patient communication now strongly relies on electronic messaging.
R. Thomas Finn III (Palo Alto Medical Foundation) and David Leiman (Duke) nicely broach the issue of patient satisfaction. This is a timely topic as many institutions are not only publishing patient reviews online so that they are readily available to the public, but are also making financial incentives contingent on high patient ratings. The article discusses the evolution of the emphasis placed on patient satisfaction throughout the years with tips on how to navigate some of the distinct challenges within gastroenterology.
As part of our DHPA Private Practice Perspectives series, David Stokesberry (Digestive Disease Specialists Inc, Oklahoma City) discusses the nuts and bolts of ambulatory endoscopy centers and some of the challenges and benefits that accompany ownership of such centers.
An often overlooked aspect of gastroenterology training is nutrition. In our postfellowship pathways section, Dejan Micic (University of Chicago) outlines his decision to pursue a career in nutrition support, small bowel disorders, and the practice of deep enteroscopy.
Finally, this quarter’s newsletter features the start of a new section, which I am very excited to introduce – a case based series which will address issues in clinical medical ethics specific to gastroenterology. Lauren Feld (University of Washington) writes the inaugural piece for the section, providing a systematic approach to the patient with an existing do-not-resuscitate (DNR) order that is about to undergo endoscopy.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dear colleagues,
The first issue of The New Gastroenterologist in 2020 consists of a particularly interesting array of articles and the introduction of a new medical ethics series!
This month’s “In Focus” article, brought to you by Jennifer Maratt (Indiana University) and Elena Stoffel (University of Michigan), provides a high yield overview of hereditary colorectal cancer and polyposis syndromes, with guidance on when a referral to a high risk cancer specialist and geneticist is warranted.
Daniel Mills (Cunningham, Meyer & Vedrine P.C.) gives us a valuable legal perspective of the role of electronic patient portals in the dissemination of information and medical advice to patients – such an important topic for everyone to be aware of as the nature of patient communication now strongly relies on electronic messaging.
R. Thomas Finn III (Palo Alto Medical Foundation) and David Leiman (Duke) nicely broach the issue of patient satisfaction. This is a timely topic as many institutions are not only publishing patient reviews online so that they are readily available to the public, but are also making financial incentives contingent on high patient ratings. The article discusses the evolution of the emphasis placed on patient satisfaction throughout the years with tips on how to navigate some of the distinct challenges within gastroenterology.
As part of our DHPA Private Practice Perspectives series, David Stokesberry (Digestive Disease Specialists Inc, Oklahoma City) discusses the nuts and bolts of ambulatory endoscopy centers and some of the challenges and benefits that accompany ownership of such centers.
An often overlooked aspect of gastroenterology training is nutrition. In our postfellowship pathways section, Dejan Micic (University of Chicago) outlines his decision to pursue a career in nutrition support, small bowel disorders, and the practice of deep enteroscopy.
Finally, this quarter’s newsletter features the start of a new section, which I am very excited to introduce – a case based series which will address issues in clinical medical ethics specific to gastroenterology. Lauren Feld (University of Washington) writes the inaugural piece for the section, providing a systematic approach to the patient with an existing do-not-resuscitate (DNR) order that is about to undergo endoscopy.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Colorectal polyps and cancer – when to refer to genetics
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
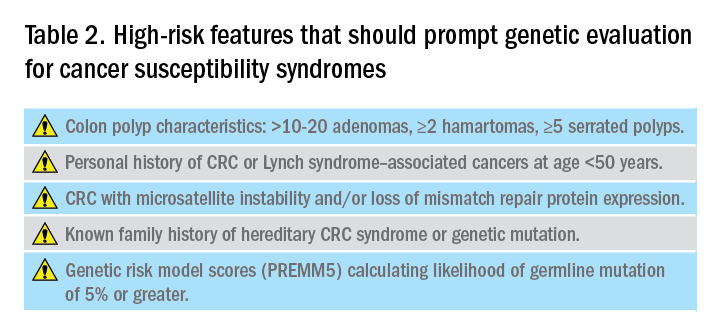
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.
As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Hope springs eternal
As practicing clinicians, we all want to do what is best for patients. We hope our treatments will improve actual health outcomes (and not intermediate process metrics), so we make decisions based on “evidence” that lies on a continuum from “I hope” on one end to “I’m sure” on the other. This month, our three lead articles represent differing points along that continuum.
First, we consider H. pylori and gastric cancer. We know H. pylori eradication reduces ulcer risk and that H. pylori is a risk for gastric cancer. We did not know whether eradication reduces cancer risk. In a large retrospective study from the VA, Kumar et al demonstrated that eradication (not just treatment) substantially reduced subsequent gastric cancers. These data are not definitive, but they nudge us towards the “I’m sure” end of the continuum.
A second group of studies (both retrospective and prospective) suggests that successful weight loss after bariatric surgery was associated with a substantial reduction of risk for 13 cancer types related to obesity. Moderate evidence but again nudging us away from “I hope.”
A third article highlights the recent Clinical Practice Update on Barrett’s esophagus published by the AGA Clinical Practice Update Committee in Gastroenterology’s February 2020 issue. This practice update helps us understand the impact we will make on cancer reduction with surveillance and treatment of Barrett’s. Despite this publication, Barrett’s management remains closer to “hope” than “sure.”
The difficulty we face, as clinician or patient, is what to do when outcomes are really serious but evidence remains close to the “I hope” end. Take a reasonably healthy 68-year-old man with asymptomatic coronary disease, but a very high (and increasing) coronary artery calcium score, despite maximum statins and appropriate lifestyle practices. Should he initiate a PCSK9 inhibitor ($14,000 per year) absent evidence that it would alter cardiac risk? Recently, a retrospective study nudged us along the continuum (Peng et al. JACC Cardiovascular Imaging. 2020 Jan;13[1 Pt 1]:83-93). A serious outcome, suggestive but not definitive evidence, and no time for an RCT. Will such aggressive therapy help? I sure hope so.
John I. Allen, MD, MBA, AGAF
Editor in Chief
As practicing clinicians, we all want to do what is best for patients. We hope our treatments will improve actual health outcomes (and not intermediate process metrics), so we make decisions based on “evidence” that lies on a continuum from “I hope” on one end to “I’m sure” on the other. This month, our three lead articles represent differing points along that continuum.
First, we consider H. pylori and gastric cancer. We know H. pylori eradication reduces ulcer risk and that H. pylori is a risk for gastric cancer. We did not know whether eradication reduces cancer risk. In a large retrospective study from the VA, Kumar et al demonstrated that eradication (not just treatment) substantially reduced subsequent gastric cancers. These data are not definitive, but they nudge us towards the “I’m sure” end of the continuum.
A second group of studies (both retrospective and prospective) suggests that successful weight loss after bariatric surgery was associated with a substantial reduction of risk for 13 cancer types related to obesity. Moderate evidence but again nudging us away from “I hope.”
A third article highlights the recent Clinical Practice Update on Barrett’s esophagus published by the AGA Clinical Practice Update Committee in Gastroenterology’s February 2020 issue. This practice update helps us understand the impact we will make on cancer reduction with surveillance and treatment of Barrett’s. Despite this publication, Barrett’s management remains closer to “hope” than “sure.”
The difficulty we face, as clinician or patient, is what to do when outcomes are really serious but evidence remains close to the “I hope” end. Take a reasonably healthy 68-year-old man with asymptomatic coronary disease, but a very high (and increasing) coronary artery calcium score, despite maximum statins and appropriate lifestyle practices. Should he initiate a PCSK9 inhibitor ($14,000 per year) absent evidence that it would alter cardiac risk? Recently, a retrospective study nudged us along the continuum (Peng et al. JACC Cardiovascular Imaging. 2020 Jan;13[1 Pt 1]:83-93). A serious outcome, suggestive but not definitive evidence, and no time for an RCT. Will such aggressive therapy help? I sure hope so.
John I. Allen, MD, MBA, AGAF
Editor in Chief
As practicing clinicians, we all want to do what is best for patients. We hope our treatments will improve actual health outcomes (and not intermediate process metrics), so we make decisions based on “evidence” that lies on a continuum from “I hope” on one end to “I’m sure” on the other. This month, our three lead articles represent differing points along that continuum.
First, we consider H. pylori and gastric cancer. We know H. pylori eradication reduces ulcer risk and that H. pylori is a risk for gastric cancer. We did not know whether eradication reduces cancer risk. In a large retrospective study from the VA, Kumar et al demonstrated that eradication (not just treatment) substantially reduced subsequent gastric cancers. These data are not definitive, but they nudge us towards the “I’m sure” end of the continuum.
A second group of studies (both retrospective and prospective) suggests that successful weight loss after bariatric surgery was associated with a substantial reduction of risk for 13 cancer types related to obesity. Moderate evidence but again nudging us away from “I hope.”
A third article highlights the recent Clinical Practice Update on Barrett’s esophagus published by the AGA Clinical Practice Update Committee in Gastroenterology’s February 2020 issue. This practice update helps us understand the impact we will make on cancer reduction with surveillance and treatment of Barrett’s. Despite this publication, Barrett’s management remains closer to “hope” than “sure.”
The difficulty we face, as clinician or patient, is what to do when outcomes are really serious but evidence remains close to the “I hope” end. Take a reasonably healthy 68-year-old man with asymptomatic coronary disease, but a very high (and increasing) coronary artery calcium score, despite maximum statins and appropriate lifestyle practices. Should he initiate a PCSK9 inhibitor ($14,000 per year) absent evidence that it would alter cardiac risk? Recently, a retrospective study nudged us along the continuum (Peng et al. JACC Cardiovascular Imaging. 2020 Jan;13[1 Pt 1]:83-93). A serious outcome, suggestive but not definitive evidence, and no time for an RCT. Will such aggressive therapy help? I sure hope so.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Dependent trait in chronic migraine may predict nonresponse to onabotulinumtoxin A
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
according to research published in the January issue of Headache. The research may be the first to show that personality traits predict response to onabotulinumtoxin A in this population.
“These findings point out that conducting an evaluation of personality traits in patients with chronic migraine might be helpful in the prediction of the course and election of the treatment, as well as identifying patients who might benefit from a multidisciplinary approach,” wrote Alicia Gonzalez-Martinez, MD, of the Hospital Universitario de La Princesa and Instituto de Investigación Sanitaria de La Princesa in Madrid and colleagues. “Categorical questionnaires such as the Salamanca screening test seem to be useful for this purpose.”
Researchers used ICD-10 personality criteria
Personality patterns in patients with migraine and other primary headaches have been the subject of decades of research. Munoz et al. found that certain personality traits are associated with migraine and chronic migraine, and this association may influence clinical management and treatment. The effect of personality traits on response to treatment, however, had not been studied previously.
Dr. Gonzalez-Martinez and colleagues hypothesized that cluster C traits (e.g., obsessive-compulsive, dependent, and anxious), as defined by ICD-10, are associated with nonresponse to onabotulinumtoxin A. To test this hypothesis, they conducted a case-control observational study in a cohort of patients with chronic migraine. Eligible patients presented to one of two headache units of a tertiary hospital between January and May 2018. The investigators obtained a complete headache history and demographic information from each patient. Patients had at least two treatment cycles of onabotulinumtoxin A. Dr. Gonzalez-Martinez and colleagues defined treatment response as a reduction in the number of monthly migraine days of at least 50% after at least two treatment cycles.
The investigators assessed participants’ personality traits by administering the Salamanca test, a brief categorical inventory that examines 11 personality traits using 22 questions. Patients completed the test at the beginning of the study period and before they were classified as responders or nonresponders.
Medication overuse was a potential confounder
The study population included 112 patients with chronic migraine. One hundred patients (89%) were women. Participants’ mean age at initiation of onabotulinumtoxin A treatment was 43 years. The population’s mean duration of chronic migraine was 29 months. Eighty-three patients (74.1%) had medication overuse, and 96 (85.7%) responded to onabotulinumtoxin A.
Cluster A traits in the population included paranoid (prevalence, 10.7%), schizoid (38.4%), and schizotypal (7.1%). Cluster B traits included histrionic (50%), antisocial (1.8%), narcissistic (9.8%), emotional instability subtype impulsive (27.7%), and emotional instability subtype limit (EISL, 24.1%). Cluster C traits were anxious (58.9%) anancastic (i.e., obsessive-compulsive, 54.5%), and dependent (32.1%).
The investigators found no differences in demographics between responders and nonresponders. In a univariate analysis, dependent traits (e.g., passivity and emotional overdependence on others) and EISL traits (e.g., impulsivity and disturbed self-image) were significantly more common among nonresponders. In a multivariate analysis, dependent traits remained significantly associated with nonresponse to onabotulinumtoxin A.
Medication overuse was a potential confounder in the study, according to Dr. Gonzalez-Martinez and colleagues. One of the study’s limitations was its absence of a healthy control group. Another was the fact that the psychometrics of the Salamanca screening test have not been published in a peer-reviewed journal and may need further examination.
Dependent personality “may also be part of the proposed chronic pain sufferer personality,” wrote the investigators. “Early detection of personality traits could improve management and outcome of chronic migraine patients. Additionally, the possibility to predict the effectiveness of onabotulinumtoxin A therapy may reduce costs and latency time of effect in patients with improbable effectiveness.”
The study had no outside funding, and the authors reported no conflicts of interest.
SOURCE: Gonzalez-Martinez A et al. Headache. 2020;60(1):153-61.
FROM HEADACHE
HHS declares coronavirus emergency, orders quarantine
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”

The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
Is anxiety about the coronavirus out of proportion?
A number of years ago, a patient I was treating mentioned that she was not eating tomatoes. There had been stories in the news about people contracting bacterial infections from tomatoes, but I paused for a moment, then asked her: “Have there been any contaminated tomatoes here in Maryland?” There had not been and I was still happily eating salsa, but my patient thought about this differently: If disease-causing tomatoes were to come to our state, someone would be the first person to become ill. She did not want to take any risks. My patient, however, was a heavy smoker and already grappling with health issues that were caused by smoking, so I found her choice of what she should worry about and how it influenced her behavior to be perplexing. I realize it’s not the same; nicotine is an addiction, while tomatoes remain a choice for most of us, and it’s common for people to worry about very unlikely events even when we are surrounded by very real and statistically more probable threats to our well-being.
Today’s news reports are filled with stories about 2019 Novel Coronavirus (2019-nCoV), an illness that started in Wuhan, China; as of Jan. 31, 2020, there were 9,776 confirmed cases and 213 deaths. There have been an additional 118 cases reported outside of mainland China, including 6 in the United States, and no one outside of China has died.
The response to the virus has been remarkable: Wuhan, a city of more than 11 million inhabitants, is on lockdown, as are 15 other cities in China; 46 million people have been affected, the largest quarantine in human history. Travel is restricted in parts of China, airports all over the world are screening those who fly in from Wuhan, foreign governments are bringing their citizens home from Wuhan, and even Starbucks has temporarily closed half its stores in China. The economics of containing this virus are astounding.
In the meantime, the Centers for Disease Control and Prevention reports that, as of the week of Jan. 25, there have been 19 million cases of the flu in the United States. Of those stricken, 180,000 people have been hospitalized and 10,000 have died, including 68 pediatric patients. No cities are on lockdown, public transportation runs as usual, airports don’t screen passengers for flu symptoms, and Starbucks continues to serve vanilla lattes to any willing customer. Anxiety about illness is not new; we’ve seen it with SARS, Ebola, measles, and even around Chipotle’s food poisoning cases – to name just a few recent scares. We have also seen a lot of media on vaping-related deaths, and as of early January 2020, vaping-related illnesses affected 2,602 people with 59 deaths. It has been a topic of discussion among legislators, with an emphasis on either outlawing the flavoring that might appeal to younger people or simply outlawing e-cigarettes. No one, however, is talking about outlawing regular cigarettes, despite the fact that many people have switched from cigarettes to vaping products as a way to quit smoking. So, while vaping has caused 59 deaths since 2018, cigarettes are responsible for 480,000 fatalities a year in the United States and smokers live, on average, 10 years less than nonsmokers.
So what fuels anxiety about the latest health scare, and why aren’t we more anxious about the more common causes of premature mortality? Certainly, the newness and the unknown are factors in the coronavirus scare. It’s not certain how this illness was introduced into the human population, although one theory is that it started with the consumption of bats who carry the virus. It’s spreading fast, and in some people, it has been lethal. The incubation period is not known, or whether it is contagious before symptoms appear. Coronavirus is getting a lot of public health attention and the World Health Organization just announced that the virus is a public health emergency of international concern. On the televised news on Jan. 29, 2020, coronavirus was the top story in the United States, even though an impeachment trial is in progress for our country’s president.
The public health response of locking down cities may help contain the outbreak and prevent a global epidemic, although millions of people had already left Wuhan, so the heavy-handed attempt to prevent spread of the virus may well be too late. In the case of the Ebola virus – a much more lethal disease that was also thought to be introduced by bats – public health measures certainly curtailed global spread, and the epidemic of 2014-2016 was limited to 28,600 cases and 11,325 deaths, nearly all of them in West Africa.
Most of the things that cause people to die are not new and are not topics the media chooses to sensationalize. Dissemination of news has changed over the decades, with so much more of it, instant reports on social media, and competition for viewers that leads journalists to pull at our emotions. And while we may, or may not, get flu shots and avoid those who have the flu, how and where we position both our anxiety and our resources does not always make sense. Certainly some people are predisposed to worry about both common and uncommon dangers, while others seem never to worry and engage in acts that many of us would consider dangerous. If we are looking for logic, it may be hard to find – there are those who would happily go bungee jumping but wouldn’t dream of leaving the house out without hand sanitizer.
The repercussions from this massive response to the Wuhan coronavirus are significant. For the millions of people on lockdown in China, each day gets emotionally harder; some may begin to have issues procuring food, and the financial losses for the economy will be significant. It’s not really possible to know yet if this response is warranted; we do know that infectious diseases can kill millions. The AIDS pandemic has taken the lives of 36 million people since 1981, and the influenza pandemic of 1918 resulted in an estimated 20 million to 50 million deaths after infecting 500 million people. Still, one might wonder if other, more mundane causes of morbidity and mortality – the ones that no longer garner our dread or make it to the front pages – might also be worthy of more hype and resources.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
A number of years ago, a patient I was treating mentioned that she was not eating tomatoes. There had been stories in the news about people contracting bacterial infections from tomatoes, but I paused for a moment, then asked her: “Have there been any contaminated tomatoes here in Maryland?” There had not been and I was still happily eating salsa, but my patient thought about this differently: If disease-causing tomatoes were to come to our state, someone would be the first person to become ill. She did not want to take any risks. My patient, however, was a heavy smoker and already grappling with health issues that were caused by smoking, so I found her choice of what she should worry about and how it influenced her behavior to be perplexing. I realize it’s not the same; nicotine is an addiction, while tomatoes remain a choice for most of us, and it’s common for people to worry about very unlikely events even when we are surrounded by very real and statistically more probable threats to our well-being.
Today’s news reports are filled with stories about 2019 Novel Coronavirus (2019-nCoV), an illness that started in Wuhan, China; as of Jan. 31, 2020, there were 9,776 confirmed cases and 213 deaths. There have been an additional 118 cases reported outside of mainland China, including 6 in the United States, and no one outside of China has died.
The response to the virus has been remarkable: Wuhan, a city of more than 11 million inhabitants, is on lockdown, as are 15 other cities in China; 46 million people have been affected, the largest quarantine in human history. Travel is restricted in parts of China, airports all over the world are screening those who fly in from Wuhan, foreign governments are bringing their citizens home from Wuhan, and even Starbucks has temporarily closed half its stores in China. The economics of containing this virus are astounding.
In the meantime, the Centers for Disease Control and Prevention reports that, as of the week of Jan. 25, there have been 19 million cases of the flu in the United States. Of those stricken, 180,000 people have been hospitalized and 10,000 have died, including 68 pediatric patients. No cities are on lockdown, public transportation runs as usual, airports don’t screen passengers for flu symptoms, and Starbucks continues to serve vanilla lattes to any willing customer. Anxiety about illness is not new; we’ve seen it with SARS, Ebola, measles, and even around Chipotle’s food poisoning cases – to name just a few recent scares. We have also seen a lot of media on vaping-related deaths, and as of early January 2020, vaping-related illnesses affected 2,602 people with 59 deaths. It has been a topic of discussion among legislators, with an emphasis on either outlawing the flavoring that might appeal to younger people or simply outlawing e-cigarettes. No one, however, is talking about outlawing regular cigarettes, despite the fact that many people have switched from cigarettes to vaping products as a way to quit smoking. So, while vaping has caused 59 deaths since 2018, cigarettes are responsible for 480,000 fatalities a year in the United States and smokers live, on average, 10 years less than nonsmokers.
So what fuels anxiety about the latest health scare, and why aren’t we more anxious about the more common causes of premature mortality? Certainly, the newness and the unknown are factors in the coronavirus scare. It’s not certain how this illness was introduced into the human population, although one theory is that it started with the consumption of bats who carry the virus. It’s spreading fast, and in some people, it has been lethal. The incubation period is not known, or whether it is contagious before symptoms appear. Coronavirus is getting a lot of public health attention and the World Health Organization just announced that the virus is a public health emergency of international concern. On the televised news on Jan. 29, 2020, coronavirus was the top story in the United States, even though an impeachment trial is in progress for our country’s president.
The public health response of locking down cities may help contain the outbreak and prevent a global epidemic, although millions of people had already left Wuhan, so the heavy-handed attempt to prevent spread of the virus may well be too late. In the case of the Ebola virus – a much more lethal disease that was also thought to be introduced by bats – public health measures certainly curtailed global spread, and the epidemic of 2014-2016 was limited to 28,600 cases and 11,325 deaths, nearly all of them in West Africa.
Most of the things that cause people to die are not new and are not topics the media chooses to sensationalize. Dissemination of news has changed over the decades, with so much more of it, instant reports on social media, and competition for viewers that leads journalists to pull at our emotions. And while we may, or may not, get flu shots and avoid those who have the flu, how and where we position both our anxiety and our resources does not always make sense. Certainly some people are predisposed to worry about both common and uncommon dangers, while others seem never to worry and engage in acts that many of us would consider dangerous. If we are looking for logic, it may be hard to find – there are those who would happily go bungee jumping but wouldn’t dream of leaving the house out without hand sanitizer.
The repercussions from this massive response to the Wuhan coronavirus are significant. For the millions of people on lockdown in China, each day gets emotionally harder; some may begin to have issues procuring food, and the financial losses for the economy will be significant. It’s not really possible to know yet if this response is warranted; we do know that infectious diseases can kill millions. The AIDS pandemic has taken the lives of 36 million people since 1981, and the influenza pandemic of 1918 resulted in an estimated 20 million to 50 million deaths after infecting 500 million people. Still, one might wonder if other, more mundane causes of morbidity and mortality – the ones that no longer garner our dread or make it to the front pages – might also be worthy of more hype and resources.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
A number of years ago, a patient I was treating mentioned that she was not eating tomatoes. There had been stories in the news about people contracting bacterial infections from tomatoes, but I paused for a moment, then asked her: “Have there been any contaminated tomatoes here in Maryland?” There had not been and I was still happily eating salsa, but my patient thought about this differently: If disease-causing tomatoes were to come to our state, someone would be the first person to become ill. She did not want to take any risks. My patient, however, was a heavy smoker and already grappling with health issues that were caused by smoking, so I found her choice of what she should worry about and how it influenced her behavior to be perplexing. I realize it’s not the same; nicotine is an addiction, while tomatoes remain a choice for most of us, and it’s common for people to worry about very unlikely events even when we are surrounded by very real and statistically more probable threats to our well-being.
Today’s news reports are filled with stories about 2019 Novel Coronavirus (2019-nCoV), an illness that started in Wuhan, China; as of Jan. 31, 2020, there were 9,776 confirmed cases and 213 deaths. There have been an additional 118 cases reported outside of mainland China, including 6 in the United States, and no one outside of China has died.
The response to the virus has been remarkable: Wuhan, a city of more than 11 million inhabitants, is on lockdown, as are 15 other cities in China; 46 million people have been affected, the largest quarantine in human history. Travel is restricted in parts of China, airports all over the world are screening those who fly in from Wuhan, foreign governments are bringing their citizens home from Wuhan, and even Starbucks has temporarily closed half its stores in China. The economics of containing this virus are astounding.
In the meantime, the Centers for Disease Control and Prevention reports that, as of the week of Jan. 25, there have been 19 million cases of the flu in the United States. Of those stricken, 180,000 people have been hospitalized and 10,000 have died, including 68 pediatric patients. No cities are on lockdown, public transportation runs as usual, airports don’t screen passengers for flu symptoms, and Starbucks continues to serve vanilla lattes to any willing customer. Anxiety about illness is not new; we’ve seen it with SARS, Ebola, measles, and even around Chipotle’s food poisoning cases – to name just a few recent scares. We have also seen a lot of media on vaping-related deaths, and as of early January 2020, vaping-related illnesses affected 2,602 people with 59 deaths. It has been a topic of discussion among legislators, with an emphasis on either outlawing the flavoring that might appeal to younger people or simply outlawing e-cigarettes. No one, however, is talking about outlawing regular cigarettes, despite the fact that many people have switched from cigarettes to vaping products as a way to quit smoking. So, while vaping has caused 59 deaths since 2018, cigarettes are responsible for 480,000 fatalities a year in the United States and smokers live, on average, 10 years less than nonsmokers.
So what fuels anxiety about the latest health scare, and why aren’t we more anxious about the more common causes of premature mortality? Certainly, the newness and the unknown are factors in the coronavirus scare. It’s not certain how this illness was introduced into the human population, although one theory is that it started with the consumption of bats who carry the virus. It’s spreading fast, and in some people, it has been lethal. The incubation period is not known, or whether it is contagious before symptoms appear. Coronavirus is getting a lot of public health attention and the World Health Organization just announced that the virus is a public health emergency of international concern. On the televised news on Jan. 29, 2020, coronavirus was the top story in the United States, even though an impeachment trial is in progress for our country’s president.
The public health response of locking down cities may help contain the outbreak and prevent a global epidemic, although millions of people had already left Wuhan, so the heavy-handed attempt to prevent spread of the virus may well be too late. In the case of the Ebola virus – a much more lethal disease that was also thought to be introduced by bats – public health measures certainly curtailed global spread, and the epidemic of 2014-2016 was limited to 28,600 cases and 11,325 deaths, nearly all of them in West Africa.
Most of the things that cause people to die are not new and are not topics the media chooses to sensationalize. Dissemination of news has changed over the decades, with so much more of it, instant reports on social media, and competition for viewers that leads journalists to pull at our emotions. And while we may, or may not, get flu shots and avoid those who have the flu, how and where we position both our anxiety and our resources does not always make sense. Certainly some people are predisposed to worry about both common and uncommon dangers, while others seem never to worry and engage in acts that many of us would consider dangerous. If we are looking for logic, it may be hard to find – there are those who would happily go bungee jumping but wouldn’t dream of leaving the house out without hand sanitizer.
The repercussions from this massive response to the Wuhan coronavirus are significant. For the millions of people on lockdown in China, each day gets emotionally harder; some may begin to have issues procuring food, and the financial losses for the economy will be significant. It’s not really possible to know yet if this response is warranted; we do know that infectious diseases can kill millions. The AIDS pandemic has taken the lives of 36 million people since 1981, and the influenza pandemic of 1918 resulted in an estimated 20 million to 50 million deaths after infecting 500 million people. Still, one might wonder if other, more mundane causes of morbidity and mortality – the ones that no longer garner our dread or make it to the front pages – might also be worthy of more hype and resources.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Developing guidance for patient movement requests
Clear guidelines in policy needed
In hospital medicine, inpatients often request more freedom to move within the hospital complex for a wide range of both benign and potentially concerning reasons, says Sara Stream, MD.
“Hospitalists are often confronted with a dilemma when considering these patient requests: how to promote patient-centered care and autonomy while balancing patient safety, concerns for hospital liability, and the delivery of timely, efficient medical care,” said Dr. Stream, a hospitalist at the VA New York Harbor Healthcare System. Guidance from medical literature and institutional policies on inpatient movement are lacking, so Dr. Stream coauthored an article seeking to develop a framework with which hospitalists can approach patient requests for liberalized movement.
The authors concluded that for a small subset of patients, liberalized movement within the hospital may be clinically feasible: those who are medically, physically, and psychiatrically stable enough to move off their assigned floors without inordinate risk. “For the rest of inpatients, movement outside their monitored inpatient settings may interfere with appropriate medical care and undermine the indications for acute hospitalization,” Dr. Stream said.
Creating institutional policy that identifies relevant clinical, legal and ethical considerations, while incorporating the varied perspectives of physicians, patients, nurses, and hospital administration/risk management will allow requests for increased movement to be evaluated systematically and transparently.
“When patients request liberalized movement, hospitalists should consider the requests systematically: first to identify the intent behind requests, and then to follow a framework to determine whether increased movement would be safe and allow appropriate medical care without creating additional risks,” Dr. Stream said.
Hospitalists should assess and compile individual patient requests for liberalized movement and work with other physicians, nurses, hospital administration, and risk management to devise pertinent policy on this issue that is specific to their institutions. “By eventually creating clear guidelines in policy, health care providers will spend less time managing each individual request to leave the floor because they have a systematic strategy for making consistent decisions about patient movement,” the authors concluded.
Reference
1. Stream S, Alfandre D. “Just Getting a Cup of Coffee” – Considering Best Practices for Patients’ Movement off the Hospital Floor. J Hosp Med. 2019 Nov. doi: 10.12788/jhm.3227.
Clear guidelines in policy needed
Clear guidelines in policy needed
In hospital medicine, inpatients often request more freedom to move within the hospital complex for a wide range of both benign and potentially concerning reasons, says Sara Stream, MD.
“Hospitalists are often confronted with a dilemma when considering these patient requests: how to promote patient-centered care and autonomy while balancing patient safety, concerns for hospital liability, and the delivery of timely, efficient medical care,” said Dr. Stream, a hospitalist at the VA New York Harbor Healthcare System. Guidance from medical literature and institutional policies on inpatient movement are lacking, so Dr. Stream coauthored an article seeking to develop a framework with which hospitalists can approach patient requests for liberalized movement.
The authors concluded that for a small subset of patients, liberalized movement within the hospital may be clinically feasible: those who are medically, physically, and psychiatrically stable enough to move off their assigned floors without inordinate risk. “For the rest of inpatients, movement outside their monitored inpatient settings may interfere with appropriate medical care and undermine the indications for acute hospitalization,” Dr. Stream said.
Creating institutional policy that identifies relevant clinical, legal and ethical considerations, while incorporating the varied perspectives of physicians, patients, nurses, and hospital administration/risk management will allow requests for increased movement to be evaluated systematically and transparently.
“When patients request liberalized movement, hospitalists should consider the requests systematically: first to identify the intent behind requests, and then to follow a framework to determine whether increased movement would be safe and allow appropriate medical care without creating additional risks,” Dr. Stream said.
Hospitalists should assess and compile individual patient requests for liberalized movement and work with other physicians, nurses, hospital administration, and risk management to devise pertinent policy on this issue that is specific to their institutions. “By eventually creating clear guidelines in policy, health care providers will spend less time managing each individual request to leave the floor because they have a systematic strategy for making consistent decisions about patient movement,” the authors concluded.
Reference
1. Stream S, Alfandre D. “Just Getting a Cup of Coffee” – Considering Best Practices for Patients’ Movement off the Hospital Floor. J Hosp Med. 2019 Nov. doi: 10.12788/jhm.3227.
In hospital medicine, inpatients often request more freedom to move within the hospital complex for a wide range of both benign and potentially concerning reasons, says Sara Stream, MD.
“Hospitalists are often confronted with a dilemma when considering these patient requests: how to promote patient-centered care and autonomy while balancing patient safety, concerns for hospital liability, and the delivery of timely, efficient medical care,” said Dr. Stream, a hospitalist at the VA New York Harbor Healthcare System. Guidance from medical literature and institutional policies on inpatient movement are lacking, so Dr. Stream coauthored an article seeking to develop a framework with which hospitalists can approach patient requests for liberalized movement.
The authors concluded that for a small subset of patients, liberalized movement within the hospital may be clinically feasible: those who are medically, physically, and psychiatrically stable enough to move off their assigned floors without inordinate risk. “For the rest of inpatients, movement outside their monitored inpatient settings may interfere with appropriate medical care and undermine the indications for acute hospitalization,” Dr. Stream said.
Creating institutional policy that identifies relevant clinical, legal and ethical considerations, while incorporating the varied perspectives of physicians, patients, nurses, and hospital administration/risk management will allow requests for increased movement to be evaluated systematically and transparently.
“When patients request liberalized movement, hospitalists should consider the requests systematically: first to identify the intent behind requests, and then to follow a framework to determine whether increased movement would be safe and allow appropriate medical care without creating additional risks,” Dr. Stream said.
Hospitalists should assess and compile individual patient requests for liberalized movement and work with other physicians, nurses, hospital administration, and risk management to devise pertinent policy on this issue that is specific to their institutions. “By eventually creating clear guidelines in policy, health care providers will spend less time managing each individual request to leave the floor because they have a systematic strategy for making consistent decisions about patient movement,” the authors concluded.
Reference
1. Stream S, Alfandre D. “Just Getting a Cup of Coffee” – Considering Best Practices for Patients’ Movement off the Hospital Floor. J Hosp Med. 2019 Nov. doi: 10.12788/jhm.3227.
CDC: Opioid prescribing and use rates down since 2010
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
FROM MMWR SURVEILLANCE SUMMARIES
Systemic therapy options for pediatric skin diseases are improving
ORLANDO – Because Food and Drug Administration–approved treatment options for medications. However, this scenario is changing, A. Yasmine Kirkorian, MD, said at the ODAC Dermatology, Aesthetic & Surgical Conference.
“I really would like to emphasize that children with severe disease need to be treated,” added Dr. Kirkorian, a pediatric dermatologist at George Washington University, Washington, and Children’s National Health System, where she is interim chief of the division of dermatology.
Current on-label systemic therapies for pediatric skin disease include etanercept for psoriasis (4 years and older), ustekinumab for psoriasis (12 years and older), adalimumab for hidradenitis suppurativa (12 years and older), and omalizumab for chronic idiopathic urticaria (12 years and older). A new addition to the list is dupilumab, which was approved for children and adolescents with atopic dermatitis (AD) aged 12 years and older in 2019, she noted.
Dupilumab is currently being studied in children aged 6 months to 12 years, and other clinical trials are evaluating more options for pediatric patients with AD, alopecia areata, and psoriasis. They include a clinical trial of the oral Janus kinase 3 (JAK3) inhibitor PF-06651600 in patients aged 12 years and older with alopecia areata. Six biologic therapies are being evaluated for psoriasis in patients beginning at 6 years: ixekizumab, secukinumab, ustekinumab, guselkumab, brodalumab, and apremilast.
Some systemic therapies are off-label “but used all the time” for dermatologic diseases in pediatrics, Dr. Kirkorian noted. One example is methotrexate, which is approved by the FDA for acute lymphoblastic leukemia, meningeal leukemia, and juvenile idiopathic arthritis down to infancy. Having existing efficacy and safety data for a medication in a pediatric population, even for a different disease, can be helpful when counseling parents of children with severe dermatologic disease. “If you have something, even in an older population of children, it can be reassuring, or you can use evidence from other diseases,” she said.
While methotrexate is a cheap option and approved by the FDA for other pediatric indications down to infancy, the cons of using it to treat AD in pediatric patients are numerous. Treatment requires a number of blood draws for lab testing, which can be discouraging for younger patients, and the reported adverse effect profile may be concerning to some parents, while “in practice doesn’t really occur,” she said. Methotrexate is a teratogen so is not appropriate for teenagers who are sexually active and not using contraception.
The “biggest problem,” though, is the issue of whether methotrexate is effective, since it doesn’t always work for AD, Dr. Kirkorian said. “Even at the highest doses, I often feel that we fail the atopic children,” as opposed to using it to treat psoriasis, “where you know I’m going to get you on something that works.”
In contrast, cyclosporine is FDA approved down to infancy, and works quickly as a bridge to other therapy, and is not expensive, Dr. Kirkorian said. Cons include the need for blood draws, blood pressure checks, drug interactions, and adverse effects, she noted, adding that she tries to use cyclosporine as a bridge to on-label and off-label dupilumab.
Even with FDA approval for dupilumab down to age 12 years, she said it can be difficult to get insurance approval for the on-label treatment for patients in this age group with AD, before they first fail other therapies (even with off-label systemic drugs). For patients under age 12 years, getting approval is even more challenging and requires rigorous documentation of what therapies the child has failed, and how it has affected their quality of life, she said.
“If you send in a letter to the insurance company without an IGA [Investigator Global Assessment] or SCORAD, you’re going to get rejected,” Dr. Kirkorian said. In addition to those two measures, she provides “everything else,” including the impact of the disease on quality of life of patients, and school, she said, adding, “Did they miss school, did they get hospitalized for infections? And do they have comorbid diseases that might help you get approval?”
In pediatric patients with psoriasis, common issues are more likely to be about how insurance dictates step therapy. She has often found that young children may stop responding to etanercept after a few years, which can justify a switch to ustekinumab or a new treatment in a clinical trial, she said. Adolescents with psoriasis can receive ustekinumab, which is approved for psoriasis in patients aged 12-17 years, she said, noting that the infrequent ustekinumab dosing schedule is often beneficial in this population.
When all other approved options fail for young patients with psoriasis, justifying off-label use isn’t always easy. “You just have to make a justification based on the literature, even though it’s off label,” citing available safety information for other diseases, and “demonstrate over and over the impact on quality of life,” which works “most of the time,” Dr. Kirkorian said.
She reported having no conflicts of interest.
ORLANDO – Because Food and Drug Administration–approved treatment options for medications. However, this scenario is changing, A. Yasmine Kirkorian, MD, said at the ODAC Dermatology, Aesthetic & Surgical Conference.
“I really would like to emphasize that children with severe disease need to be treated,” added Dr. Kirkorian, a pediatric dermatologist at George Washington University, Washington, and Children’s National Health System, where she is interim chief of the division of dermatology.
Current on-label systemic therapies for pediatric skin disease include etanercept for psoriasis (4 years and older), ustekinumab for psoriasis (12 years and older), adalimumab for hidradenitis suppurativa (12 years and older), and omalizumab for chronic idiopathic urticaria (12 years and older). A new addition to the list is dupilumab, which was approved for children and adolescents with atopic dermatitis (AD) aged 12 years and older in 2019, she noted.
Dupilumab is currently being studied in children aged 6 months to 12 years, and other clinical trials are evaluating more options for pediatric patients with AD, alopecia areata, and psoriasis. They include a clinical trial of the oral Janus kinase 3 (JAK3) inhibitor PF-06651600 in patients aged 12 years and older with alopecia areata. Six biologic therapies are being evaluated for psoriasis in patients beginning at 6 years: ixekizumab, secukinumab, ustekinumab, guselkumab, brodalumab, and apremilast.
Some systemic therapies are off-label “but used all the time” for dermatologic diseases in pediatrics, Dr. Kirkorian noted. One example is methotrexate, which is approved by the FDA for acute lymphoblastic leukemia, meningeal leukemia, and juvenile idiopathic arthritis down to infancy. Having existing efficacy and safety data for a medication in a pediatric population, even for a different disease, can be helpful when counseling parents of children with severe dermatologic disease. “If you have something, even in an older population of children, it can be reassuring, or you can use evidence from other diseases,” she said.
While methotrexate is a cheap option and approved by the FDA for other pediatric indications down to infancy, the cons of using it to treat AD in pediatric patients are numerous. Treatment requires a number of blood draws for lab testing, which can be discouraging for younger patients, and the reported adverse effect profile may be concerning to some parents, while “in practice doesn’t really occur,” she said. Methotrexate is a teratogen so is not appropriate for teenagers who are sexually active and not using contraception.
The “biggest problem,” though, is the issue of whether methotrexate is effective, since it doesn’t always work for AD, Dr. Kirkorian said. “Even at the highest doses, I often feel that we fail the atopic children,” as opposed to using it to treat psoriasis, “where you know I’m going to get you on something that works.”
In contrast, cyclosporine is FDA approved down to infancy, and works quickly as a bridge to other therapy, and is not expensive, Dr. Kirkorian said. Cons include the need for blood draws, blood pressure checks, drug interactions, and adverse effects, she noted, adding that she tries to use cyclosporine as a bridge to on-label and off-label dupilumab.
Even with FDA approval for dupilumab down to age 12 years, she said it can be difficult to get insurance approval for the on-label treatment for patients in this age group with AD, before they first fail other therapies (even with off-label systemic drugs). For patients under age 12 years, getting approval is even more challenging and requires rigorous documentation of what therapies the child has failed, and how it has affected their quality of life, she said.
“If you send in a letter to the insurance company without an IGA [Investigator Global Assessment] or SCORAD, you’re going to get rejected,” Dr. Kirkorian said. In addition to those two measures, she provides “everything else,” including the impact of the disease on quality of life of patients, and school, she said, adding, “Did they miss school, did they get hospitalized for infections? And do they have comorbid diseases that might help you get approval?”
In pediatric patients with psoriasis, common issues are more likely to be about how insurance dictates step therapy. She has often found that young children may stop responding to etanercept after a few years, which can justify a switch to ustekinumab or a new treatment in a clinical trial, she said. Adolescents with psoriasis can receive ustekinumab, which is approved for psoriasis in patients aged 12-17 years, she said, noting that the infrequent ustekinumab dosing schedule is often beneficial in this population.
When all other approved options fail for young patients with psoriasis, justifying off-label use isn’t always easy. “You just have to make a justification based on the literature, even though it’s off label,” citing available safety information for other diseases, and “demonstrate over and over the impact on quality of life,” which works “most of the time,” Dr. Kirkorian said.
She reported having no conflicts of interest.
ORLANDO – Because Food and Drug Administration–approved treatment options for medications. However, this scenario is changing, A. Yasmine Kirkorian, MD, said at the ODAC Dermatology, Aesthetic & Surgical Conference.
“I really would like to emphasize that children with severe disease need to be treated,” added Dr. Kirkorian, a pediatric dermatologist at George Washington University, Washington, and Children’s National Health System, where she is interim chief of the division of dermatology.
Current on-label systemic therapies for pediatric skin disease include etanercept for psoriasis (4 years and older), ustekinumab for psoriasis (12 years and older), adalimumab for hidradenitis suppurativa (12 years and older), and omalizumab for chronic idiopathic urticaria (12 years and older). A new addition to the list is dupilumab, which was approved for children and adolescents with atopic dermatitis (AD) aged 12 years and older in 2019, she noted.
Dupilumab is currently being studied in children aged 6 months to 12 years, and other clinical trials are evaluating more options for pediatric patients with AD, alopecia areata, and psoriasis. They include a clinical trial of the oral Janus kinase 3 (JAK3) inhibitor PF-06651600 in patients aged 12 years and older with alopecia areata. Six biologic therapies are being evaluated for psoriasis in patients beginning at 6 years: ixekizumab, secukinumab, ustekinumab, guselkumab, brodalumab, and apremilast.
Some systemic therapies are off-label “but used all the time” for dermatologic diseases in pediatrics, Dr. Kirkorian noted. One example is methotrexate, which is approved by the FDA for acute lymphoblastic leukemia, meningeal leukemia, and juvenile idiopathic arthritis down to infancy. Having existing efficacy and safety data for a medication in a pediatric population, even for a different disease, can be helpful when counseling parents of children with severe dermatologic disease. “If you have something, even in an older population of children, it can be reassuring, or you can use evidence from other diseases,” she said.
While methotrexate is a cheap option and approved by the FDA for other pediatric indications down to infancy, the cons of using it to treat AD in pediatric patients are numerous. Treatment requires a number of blood draws for lab testing, which can be discouraging for younger patients, and the reported adverse effect profile may be concerning to some parents, while “in practice doesn’t really occur,” she said. Methotrexate is a teratogen so is not appropriate for teenagers who are sexually active and not using contraception.
The “biggest problem,” though, is the issue of whether methotrexate is effective, since it doesn’t always work for AD, Dr. Kirkorian said. “Even at the highest doses, I often feel that we fail the atopic children,” as opposed to using it to treat psoriasis, “where you know I’m going to get you on something that works.”
In contrast, cyclosporine is FDA approved down to infancy, and works quickly as a bridge to other therapy, and is not expensive, Dr. Kirkorian said. Cons include the need for blood draws, blood pressure checks, drug interactions, and adverse effects, she noted, adding that she tries to use cyclosporine as a bridge to on-label and off-label dupilumab.
Even with FDA approval for dupilumab down to age 12 years, she said it can be difficult to get insurance approval for the on-label treatment for patients in this age group with AD, before they first fail other therapies (even with off-label systemic drugs). For patients under age 12 years, getting approval is even more challenging and requires rigorous documentation of what therapies the child has failed, and how it has affected their quality of life, she said.
“If you send in a letter to the insurance company without an IGA [Investigator Global Assessment] or SCORAD, you’re going to get rejected,” Dr. Kirkorian said. In addition to those two measures, she provides “everything else,” including the impact of the disease on quality of life of patients, and school, she said, adding, “Did they miss school, did they get hospitalized for infections? And do they have comorbid diseases that might help you get approval?”
In pediatric patients with psoriasis, common issues are more likely to be about how insurance dictates step therapy. She has often found that young children may stop responding to etanercept after a few years, which can justify a switch to ustekinumab or a new treatment in a clinical trial, she said. Adolescents with psoriasis can receive ustekinumab, which is approved for psoriasis in patients aged 12-17 years, she said, noting that the infrequent ustekinumab dosing schedule is often beneficial in this population.
When all other approved options fail for young patients with psoriasis, justifying off-label use isn’t always easy. “You just have to make a justification based on the literature, even though it’s off label,” citing available safety information for other diseases, and “demonstrate over and over the impact on quality of life,” which works “most of the time,” Dr. Kirkorian said.
She reported having no conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2020
Streamlining the transition from pediatric to adult care
Diabetes is a complex disease with a range of nuanced therapy options and a plethora of risk factors that could significantly affect patient quality of life and long-term outcomes. From the outset, after diagnosis, a selected regimen has to be meticulously tailored to a patient’s clinical needs and monitored over time, and many other nonclinical variables, such as patient preference, social history, access to care, and support systems, as well as the cost of the drugs and its impact on the patient, must also be considered.
The increase in the incidence of youth-onset diabetes means that more young adults are making the transition from pediatric to adult care, and careful care coordination is paramount at the handover point to ensure that a full and complete account of the history gets transferred to the adult-care provider.
So how do you distill the information from all those records (on paper and online) that you’ve accumulated during the time you’ve been treating a young adult who is now transitioning to adult care?
Transition summary
One resource that can facilitate this handover is the transition summary. It effectively consolidates and packages the aforementioned aspects of care and patient history so that the adult-care provider does not have to collect the patient’s history from the start. The transition summary should not be confused with the discharge or medical summary, which focuses only on the preceding clinical care.
It is important to stress at this stage that collaboration between the pediatric- and adult-care providers is crucial to the success of such a summary, from its creation, to its implementation, and through the subsequent and inevitable revisions and updates.
Benefits all around
After we introduced the transition summary at my institution, we found that the average initial patient visit with the new adult-care provider decreased by 12 minutes (with a range of 6-19 min). The adult-care providers welcomed receiving such detailed, important patient information packaged in a concise and readily accessible format. It helped them identify the preceding care team members, which facilitated continuity of care, and it also helped them forge a better therapeutic relationship with the patient earlier on in their engagement.
We also learned that patients were more comfortable with the transition, and the referring providers were relieved and reassured that their patients would continue to receive personalized care with the new adult-care provider.
At a personal level, I found I was less stressed as I could spend better-quality clinical time with patients. And I got to eliminate those unwieldy stacks of medical records since getting buy-in from divisional and IT leadership enabled us to automate the entire process of information transfer.
It is important to note that the patient has to consent to release of medical records to other institutions.
Setting up the summary
At our clinic, I started out by adapting the transition summary from guidelines provided by the Endocrine Society to make a template. Then, in collaboration with my pediatric colleagues, I removed and added information so that the revised document would contain information that is vitally important and not readily available in the chart and would be feasible to fill out. For example, we included details such as the patient’s psychosocial history, an estimation of the patient barriers to diabetes management, family relationship issues, and the patient’s reasons for not adopting advanced diabetes technology (see accompanying example of a transition summary) .
I kept the summary brief, at two pages, and piloted it with referring providers who were interested in using the summary and with related supporting services. I also sought buy-in from my institution. This meant that I needed pediatric and adult divisional leadership support, which offered me information technology, resources, and expertise to automate the summary within the electronic health record. Once I had feedback from would-be users, we revised and updated the summary. We set up training for staff, including pediatric providers, nurse practitioners, social workers, and nurses who could fill out the summary, and ultimately succeeded in making it mandatory that the adult-care provider receive a summary before scheduling or seeing the transfer patient.
I started out with a paper version, and once we’d refined the questions, we incorporated it into the electronic medical record.
The information we use in our summary is grouped under the following headings:
- Reason for transition.
- Diabetes type.
- Degree of diabetes control.
- Type of insulin therapy and supplies.
- Current and former insulin regimen: reasons for discontinuation of any therapies or reluctance to start any therapies.
- Diabetes health maintenance.
- Social history and support, including living situation, main social support network, child protective services involvement.
- Other pertinent medical surgical history, including psychiatric disease.
Tips and takeaways
Top of the list of takeaways is that you should make the final document work for you, your colleagues, and ultimately, your patients – customize it as you see fit, but be sure to keep it short and easy to fill out. Make a note as you start using it in practice of what you think might be missing from the chart and whether updates are needed. If you can, it’s a great idea to fold the transfer summary into the electronic medical record, though it’s not imperative. Care coordination is key to successful transfer of patients, whether from pediatric to adult care or hospital to home. A small change to work flow can result in a huge change in patient and provider satisfaction, as well as a reduction in visit times.
Dr. Agarwal is director of the Supporting Emerging Adults With Diabetes (SEAD) program at Montefiore Medical Center and assistant professor of medicine at Albert Einstein College of Medicine, New York. She reports no disclosures or financial conflicts of interest. Write to her at [email protected].
Diabetes is a complex disease with a range of nuanced therapy options and a plethora of risk factors that could significantly affect patient quality of life and long-term outcomes. From the outset, after diagnosis, a selected regimen has to be meticulously tailored to a patient’s clinical needs and monitored over time, and many other nonclinical variables, such as patient preference, social history, access to care, and support systems, as well as the cost of the drugs and its impact on the patient, must also be considered.
The increase in the incidence of youth-onset diabetes means that more young adults are making the transition from pediatric to adult care, and careful care coordination is paramount at the handover point to ensure that a full and complete account of the history gets transferred to the adult-care provider.
So how do you distill the information from all those records (on paper and online) that you’ve accumulated during the time you’ve been treating a young adult who is now transitioning to adult care?
Transition summary
One resource that can facilitate this handover is the transition summary. It effectively consolidates and packages the aforementioned aspects of care and patient history so that the adult-care provider does not have to collect the patient’s history from the start. The transition summary should not be confused with the discharge or medical summary, which focuses only on the preceding clinical care.
It is important to stress at this stage that collaboration between the pediatric- and adult-care providers is crucial to the success of such a summary, from its creation, to its implementation, and through the subsequent and inevitable revisions and updates.
Benefits all around
After we introduced the transition summary at my institution, we found that the average initial patient visit with the new adult-care provider decreased by 12 minutes (with a range of 6-19 min). The adult-care providers welcomed receiving such detailed, important patient information packaged in a concise and readily accessible format. It helped them identify the preceding care team members, which facilitated continuity of care, and it also helped them forge a better therapeutic relationship with the patient earlier on in their engagement.
We also learned that patients were more comfortable with the transition, and the referring providers were relieved and reassured that their patients would continue to receive personalized care with the new adult-care provider.
At a personal level, I found I was less stressed as I could spend better-quality clinical time with patients. And I got to eliminate those unwieldy stacks of medical records since getting buy-in from divisional and IT leadership enabled us to automate the entire process of information transfer.
It is important to note that the patient has to consent to release of medical records to other institutions.
Setting up the summary
At our clinic, I started out by adapting the transition summary from guidelines provided by the Endocrine Society to make a template. Then, in collaboration with my pediatric colleagues, I removed and added information so that the revised document would contain information that is vitally important and not readily available in the chart and would be feasible to fill out. For example, we included details such as the patient’s psychosocial history, an estimation of the patient barriers to diabetes management, family relationship issues, and the patient’s reasons for not adopting advanced diabetes technology (see accompanying example of a transition summary) .
I kept the summary brief, at two pages, and piloted it with referring providers who were interested in using the summary and with related supporting services. I also sought buy-in from my institution. This meant that I needed pediatric and adult divisional leadership support, which offered me information technology, resources, and expertise to automate the summary within the electronic health record. Once I had feedback from would-be users, we revised and updated the summary. We set up training for staff, including pediatric providers, nurse practitioners, social workers, and nurses who could fill out the summary, and ultimately succeeded in making it mandatory that the adult-care provider receive a summary before scheduling or seeing the transfer patient.
I started out with a paper version, and once we’d refined the questions, we incorporated it into the electronic medical record.
The information we use in our summary is grouped under the following headings:
- Reason for transition.
- Diabetes type.
- Degree of diabetes control.
- Type of insulin therapy and supplies.
- Current and former insulin regimen: reasons for discontinuation of any therapies or reluctance to start any therapies.
- Diabetes health maintenance.
- Social history and support, including living situation, main social support network, child protective services involvement.
- Other pertinent medical surgical history, including psychiatric disease.
Tips and takeaways
Top of the list of takeaways is that you should make the final document work for you, your colleagues, and ultimately, your patients – customize it as you see fit, but be sure to keep it short and easy to fill out. Make a note as you start using it in practice of what you think might be missing from the chart and whether updates are needed. If you can, it’s a great idea to fold the transfer summary into the electronic medical record, though it’s not imperative. Care coordination is key to successful transfer of patients, whether from pediatric to adult care or hospital to home. A small change to work flow can result in a huge change in patient and provider satisfaction, as well as a reduction in visit times.
Dr. Agarwal is director of the Supporting Emerging Adults With Diabetes (SEAD) program at Montefiore Medical Center and assistant professor of medicine at Albert Einstein College of Medicine, New York. She reports no disclosures or financial conflicts of interest. Write to her at [email protected].
Diabetes is a complex disease with a range of nuanced therapy options and a plethora of risk factors that could significantly affect patient quality of life and long-term outcomes. From the outset, after diagnosis, a selected regimen has to be meticulously tailored to a patient’s clinical needs and monitored over time, and many other nonclinical variables, such as patient preference, social history, access to care, and support systems, as well as the cost of the drugs and its impact on the patient, must also be considered.
The increase in the incidence of youth-onset diabetes means that more young adults are making the transition from pediatric to adult care, and careful care coordination is paramount at the handover point to ensure that a full and complete account of the history gets transferred to the adult-care provider.
So how do you distill the information from all those records (on paper and online) that you’ve accumulated during the time you’ve been treating a young adult who is now transitioning to adult care?
Transition summary
One resource that can facilitate this handover is the transition summary. It effectively consolidates and packages the aforementioned aspects of care and patient history so that the adult-care provider does not have to collect the patient’s history from the start. The transition summary should not be confused with the discharge or medical summary, which focuses only on the preceding clinical care.
It is important to stress at this stage that collaboration between the pediatric- and adult-care providers is crucial to the success of such a summary, from its creation, to its implementation, and through the subsequent and inevitable revisions and updates.
Benefits all around
After we introduced the transition summary at my institution, we found that the average initial patient visit with the new adult-care provider decreased by 12 minutes (with a range of 6-19 min). The adult-care providers welcomed receiving such detailed, important patient information packaged in a concise and readily accessible format. It helped them identify the preceding care team members, which facilitated continuity of care, and it also helped them forge a better therapeutic relationship with the patient earlier on in their engagement.
We also learned that patients were more comfortable with the transition, and the referring providers were relieved and reassured that their patients would continue to receive personalized care with the new adult-care provider.
At a personal level, I found I was less stressed as I could spend better-quality clinical time with patients. And I got to eliminate those unwieldy stacks of medical records since getting buy-in from divisional and IT leadership enabled us to automate the entire process of information transfer.
It is important to note that the patient has to consent to release of medical records to other institutions.
Setting up the summary
At our clinic, I started out by adapting the transition summary from guidelines provided by the Endocrine Society to make a template. Then, in collaboration with my pediatric colleagues, I removed and added information so that the revised document would contain information that is vitally important and not readily available in the chart and would be feasible to fill out. For example, we included details such as the patient’s psychosocial history, an estimation of the patient barriers to diabetes management, family relationship issues, and the patient’s reasons for not adopting advanced diabetes technology (see accompanying example of a transition summary) .
I kept the summary brief, at two pages, and piloted it with referring providers who were interested in using the summary and with related supporting services. I also sought buy-in from my institution. This meant that I needed pediatric and adult divisional leadership support, which offered me information technology, resources, and expertise to automate the summary within the electronic health record. Once I had feedback from would-be users, we revised and updated the summary. We set up training for staff, including pediatric providers, nurse practitioners, social workers, and nurses who could fill out the summary, and ultimately succeeded in making it mandatory that the adult-care provider receive a summary before scheduling or seeing the transfer patient.
I started out with a paper version, and once we’d refined the questions, we incorporated it into the electronic medical record.
The information we use in our summary is grouped under the following headings:
- Reason for transition.
- Diabetes type.
- Degree of diabetes control.
- Type of insulin therapy and supplies.
- Current and former insulin regimen: reasons for discontinuation of any therapies or reluctance to start any therapies.
- Diabetes health maintenance.
- Social history and support, including living situation, main social support network, child protective services involvement.
- Other pertinent medical surgical history, including psychiatric disease.
Tips and takeaways
Top of the list of takeaways is that you should make the final document work for you, your colleagues, and ultimately, your patients – customize it as you see fit, but be sure to keep it short and easy to fill out. Make a note as you start using it in practice of what you think might be missing from the chart and whether updates are needed. If you can, it’s a great idea to fold the transfer summary into the electronic medical record, though it’s not imperative. Care coordination is key to successful transfer of patients, whether from pediatric to adult care or hospital to home. A small change to work flow can result in a huge change in patient and provider satisfaction, as well as a reduction in visit times.
Dr. Agarwal is director of the Supporting Emerging Adults With Diabetes (SEAD) program at Montefiore Medical Center and assistant professor of medicine at Albert Einstein College of Medicine, New York. She reports no disclosures or financial conflicts of interest. Write to her at [email protected].