User login
GI symptoms in Parkinson’s disease correlate with less microbial diversity
according to research presented online as part of the 2020 American Academy of Neurology Science Highlights.
Jade E. Kenna, a PhD candidate and research assistant at the Perron Institute of Neurological and Translational Science in Perth, Australia, described findings from a multicenter assessment of 167 patients with Parkinson’s disease and 100 controls from movement disorders clinics in Australia. Participants completed the self-report Gastrointestinal Symptom Rating Scale (GSRS), which rates the frequency and severity of 15 GI symptoms. In addition, stool samples were analyzed using targeted sequencing to characterize gut microbiome composition.
Although Parkinson’s disease is recognized primarily as a motor disorder, GI dysfunction may be one of the first symptoms. “This is hypothesized to result from a change in microbiota towards an inflammatory, dysbiotic composition,” Ms. Kenna said. A limited number of studies have reported an association between altered microbiota composition, GI symptoms, and Parkinson’s disease, but not in Australian cohorts.
Total GSRS score was significantly higher in patients with Parkinson’s disease, compared with controls. Eight of the symptoms – heartburn, acid reflux, nausea or vomiting, borborygmus, increased flatus, decreased passage of stools, feeling of incomplete evacuation, and passing hard stools – were significantly increased in patients with Parkinson’s disease. GSRS symptoms can be categorized as upper, lower, general, hypoactive, or hyperactive, and patients with Parkinson’s disease had significantly increased ratings in the upper, lower, and hypoactive GI symptom domains.
“This is quite a novel finding as not only has this not been assessed in an Australian cohort of individuals before, but the majority of existing literature focuses on the presence of constipation only,” Ms. Kenna said. “The treatment and understanding of nonmotor symptoms of Parkinson’s disease, in particular GI symptoms, remain as one of the top unmet needs reported by patients with Parkinson’s disease themselves. Therefore, a better, more thorough understanding of these symptoms is clearly needed, and research into this area has such value in terms of improving current therapeutic approaches, management strategies, and patient education.”
Microbial analyses found that Firmicutes and Proteobacteria were significantly increased and Verrucomicrobia trended toward an increase in patients with Parkinson’s disease. Fusobacteria was increased in controls. “Proteobacteria and Verrucomicrobia are known to promote inflammation, which can lead to GI symptoms. Furthermore, Faecalibacterium and Ruminococcus, which are reduced in [Parkinson’s disease], can metabolize various substrates to produce [short-chain fatty acids] like butyrate, which are known to aid against intestinal barrier dysfunction and inflammation,” she said.
Individuals with Parkinson’s disease had significantly less microbial diversity. As Parkinson’s disease severity and GI symptom severity increased, microbiome diversity decreased, Ms. Kenna said. “As reduced diversity is associated with increased intestinal inflammation, this indicates that the altered microbiome we saw in [individuals with Parkinson’s disease] may be instigating the increase in incidence and severity of GI symptoms.”
Ms. Kenna reported that she had no disclosures.
SOURCE: Kenna JE. AAN 2020, Abstract S17.006.
according to research presented online as part of the 2020 American Academy of Neurology Science Highlights.
Jade E. Kenna, a PhD candidate and research assistant at the Perron Institute of Neurological and Translational Science in Perth, Australia, described findings from a multicenter assessment of 167 patients with Parkinson’s disease and 100 controls from movement disorders clinics in Australia. Participants completed the self-report Gastrointestinal Symptom Rating Scale (GSRS), which rates the frequency and severity of 15 GI symptoms. In addition, stool samples were analyzed using targeted sequencing to characterize gut microbiome composition.
Although Parkinson’s disease is recognized primarily as a motor disorder, GI dysfunction may be one of the first symptoms. “This is hypothesized to result from a change in microbiota towards an inflammatory, dysbiotic composition,” Ms. Kenna said. A limited number of studies have reported an association between altered microbiota composition, GI symptoms, and Parkinson’s disease, but not in Australian cohorts.
Total GSRS score was significantly higher in patients with Parkinson’s disease, compared with controls. Eight of the symptoms – heartburn, acid reflux, nausea or vomiting, borborygmus, increased flatus, decreased passage of stools, feeling of incomplete evacuation, and passing hard stools – were significantly increased in patients with Parkinson’s disease. GSRS symptoms can be categorized as upper, lower, general, hypoactive, or hyperactive, and patients with Parkinson’s disease had significantly increased ratings in the upper, lower, and hypoactive GI symptom domains.
“This is quite a novel finding as not only has this not been assessed in an Australian cohort of individuals before, but the majority of existing literature focuses on the presence of constipation only,” Ms. Kenna said. “The treatment and understanding of nonmotor symptoms of Parkinson’s disease, in particular GI symptoms, remain as one of the top unmet needs reported by patients with Parkinson’s disease themselves. Therefore, a better, more thorough understanding of these symptoms is clearly needed, and research into this area has such value in terms of improving current therapeutic approaches, management strategies, and patient education.”
Microbial analyses found that Firmicutes and Proteobacteria were significantly increased and Verrucomicrobia trended toward an increase in patients with Parkinson’s disease. Fusobacteria was increased in controls. “Proteobacteria and Verrucomicrobia are known to promote inflammation, which can lead to GI symptoms. Furthermore, Faecalibacterium and Ruminococcus, which are reduced in [Parkinson’s disease], can metabolize various substrates to produce [short-chain fatty acids] like butyrate, which are known to aid against intestinal barrier dysfunction and inflammation,” she said.
Individuals with Parkinson’s disease had significantly less microbial diversity. As Parkinson’s disease severity and GI symptom severity increased, microbiome diversity decreased, Ms. Kenna said. “As reduced diversity is associated with increased intestinal inflammation, this indicates that the altered microbiome we saw in [individuals with Parkinson’s disease] may be instigating the increase in incidence and severity of GI symptoms.”
Ms. Kenna reported that she had no disclosures.
SOURCE: Kenna JE. AAN 2020, Abstract S17.006.
according to research presented online as part of the 2020 American Academy of Neurology Science Highlights.
Jade E. Kenna, a PhD candidate and research assistant at the Perron Institute of Neurological and Translational Science in Perth, Australia, described findings from a multicenter assessment of 167 patients with Parkinson’s disease and 100 controls from movement disorders clinics in Australia. Participants completed the self-report Gastrointestinal Symptom Rating Scale (GSRS), which rates the frequency and severity of 15 GI symptoms. In addition, stool samples were analyzed using targeted sequencing to characterize gut microbiome composition.
Although Parkinson’s disease is recognized primarily as a motor disorder, GI dysfunction may be one of the first symptoms. “This is hypothesized to result from a change in microbiota towards an inflammatory, dysbiotic composition,” Ms. Kenna said. A limited number of studies have reported an association between altered microbiota composition, GI symptoms, and Parkinson’s disease, but not in Australian cohorts.
Total GSRS score was significantly higher in patients with Parkinson’s disease, compared with controls. Eight of the symptoms – heartburn, acid reflux, nausea or vomiting, borborygmus, increased flatus, decreased passage of stools, feeling of incomplete evacuation, and passing hard stools – were significantly increased in patients with Parkinson’s disease. GSRS symptoms can be categorized as upper, lower, general, hypoactive, or hyperactive, and patients with Parkinson’s disease had significantly increased ratings in the upper, lower, and hypoactive GI symptom domains.
“This is quite a novel finding as not only has this not been assessed in an Australian cohort of individuals before, but the majority of existing literature focuses on the presence of constipation only,” Ms. Kenna said. “The treatment and understanding of nonmotor symptoms of Parkinson’s disease, in particular GI symptoms, remain as one of the top unmet needs reported by patients with Parkinson’s disease themselves. Therefore, a better, more thorough understanding of these symptoms is clearly needed, and research into this area has such value in terms of improving current therapeutic approaches, management strategies, and patient education.”
Microbial analyses found that Firmicutes and Proteobacteria were significantly increased and Verrucomicrobia trended toward an increase in patients with Parkinson’s disease. Fusobacteria was increased in controls. “Proteobacteria and Verrucomicrobia are known to promote inflammation, which can lead to GI symptoms. Furthermore, Faecalibacterium and Ruminococcus, which are reduced in [Parkinson’s disease], can metabolize various substrates to produce [short-chain fatty acids] like butyrate, which are known to aid against intestinal barrier dysfunction and inflammation,” she said.
Individuals with Parkinson’s disease had significantly less microbial diversity. As Parkinson’s disease severity and GI symptom severity increased, microbiome diversity decreased, Ms. Kenna said. “As reduced diversity is associated with increased intestinal inflammation, this indicates that the altered microbiome we saw in [individuals with Parkinson’s disease] may be instigating the increase in incidence and severity of GI symptoms.”
Ms. Kenna reported that she had no disclosures.
SOURCE: Kenna JE. AAN 2020, Abstract S17.006.
FROM AAN 2020
AHA emphasizes the need for cardio-obstetrics teams
according to a statement from the American Heart Association.
Cardiovascular disease (CVD) remains the leading cause of pregnancy-related mortality in the United States, and accounted for approximately 17 deaths per 100,000 live births in 2015, wrote Laxmi S. Mehta, MD, of The Ohio State University, Columbus, and colleagues.
Ideally, a woman with CVD at the time of pregnancy should be managed by a multidisciplinary cardio-obstetrics team that can assess cardiovascular risk, obstetric risk, and fetal risk throughout pregnancy, delivery, and up to a year post partum. The team should develop a shared strategy to promote best outcomes, according to the statement. The cardio-obstetrics team may include obstetricians, cardiologists, anesthesiologists, maternal-fetal medicine specialists, geneticists, neurologists, nurses, and pharmacists, according to the statement.
Women with preexisting CVD should receive counseling about maternal and fetal risks before conception, if possible, to involve the women in shared decision-making and to develop strategies for each stage of pregnancy and delivery, Dr. Mehta and associates said. Such counseling should include a review of all medications and assessment of risk factors.
However, some women present already in the early stages of pregnancy even with severe conditions such as pulmonary arterial hypertension, severe ventricular dysfunction, severe left-sided heart obstruction, and significant aortic dilatation with underlying connective tissue disease. Women with these conditions often are counseled to avoid pregnancy, but if they already are pregnant, a high-risk cardio-obstetrics team will need to work together to discover the best strategies going forward to mitigate risk, Dr. Mehta and associates said.
Common CVD conditions that affect pregnancy include hypertensive disorders, notably preeclampsia, defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg in women after 20 weeks of gestation whose blood pressure was normal prior to pregnancy. A management strategy to reduce the risk of pregnancy-related complications from hypertension includes healthy lifestyle behaviors such as exercise, nutrition, and smoking cessation, according to the statement. However, patients with severe hypertension may require intravenous labetalol or hydralazine. The statement gives more information about handling preeclampsia with pulmonary edema, and prevention of eclampsia and treatment of seizures.
It is important to recognize that severe hypertension or superimposed preeclampsia may occur for the first time post partum. Early ambulatory visits in the first 1-2 weeks are sensible. Medications may be needed to keep a systolic blood pressure not higher than 150 mm Hg and a diastolic blood pressure not higher than 100 mm Hg, Dr. Mehta and associates said.
According to the statement, severe hypertriglyceridemia and familial hypercholesterolemia are the two most common conditions in which lipids should be addressed during pregnancy, with consideration of the fetal risks associated with certain medications.
“Statins are contraindicated during pregnancy, and all women who are on any lipid-lowering agents should review with their physician the safety of treatment during pregnancy and whether to discontinue treatment before pregnancy,” according to the statement. A heart-healthy lifestyle can help improve lipid profiles in all pregnant patients, Dr. Mehta and associates said. Patients with extremely high triglycerides above 500 mg/dL are at risk of pancreatitis and “may benefit from pharmacological agents (omega-3 fatty acids with or without fenofibrate or gemfibrozil) during the second trimester,” they noted. Pregnant women with familial hypercholesterolemia might take bile acid sequestrants, or as a last resort, low-density lipoprotein apheresis.
Other conditions calling for a multidisciplinary cardio-obstetric approach include preexisting coronary artery disease, cardiomyopathies, arrhythmias, valvular heart disease, cerebrovascular disease, and deep venous thrombosis, according to the statement, which provides information about the risks, diagnosis, and management.
When it is time for delivery, spontaneous labor and vaginal birth are preferable for most women with heart disease, as cesarean delivery is associated with increased risk of infection, thrombotic complications, and blood loss, according to the statement.
Women with CVD and associated complications will require “specialized long-term cardiovascular follow-up,” Dr. Mehta and associates said. “In women with a high-risk pregnancy, a cardio-obstetrics team is essential to prevent maternal morbidity and mortality during the length of the pregnancy and post partum.”
“The release of this document demonstrates the AHA’s recognition of the importance of CVD in pregnancy-related death and their commitment to education and ensuring best practices in this field,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and chief medical officer at Texas Children’s Health Plan, Bellaire.
“I think one of the most important outcomes from the release of this scientific statement from AHA will be increased implementation of cardio-obstetrics teams,” she said in an interview.
“In the United States, cardiovascular disease and cardiomyopathy together are now the leading cause of death in pregnancy and the postpartum period, and constitute 26.5% of pregnancy-related deaths, with higher rates of mortality among women of color and women with lower incomes,” she said. “The rising trend in cardiovascular-related maternal deaths appears to be due to acquired, not congenital, heart disease.”
During her tenure as president of ACOG, Dr. Hollier convened a task force on cardiovascular disease in pregnancy that developed guidance that outlines screening, diagnosis, and management of CVD for women from prepregnancy through post partum.
Dr. Hollier noted that COVID-19 emphasizes racial disparities for maternal mortality.
“Pregnant patients with comorbidities, like heart conditions, may be at increased risk for severe illness from COVID-19 – consistent with the general population with similar comorbidities,” she said. “And as we know, black women’s risk of dying from CVD-related pregnancy complications is 3.4 times higher than that of white women. During the COVID-19 pandemic, we are seeing these racial health disparities exacerbated.”
However, any pregnant patients should not hesitate to communicate with their health care providers despite the pandemic situation, Dr. Hollier emphasized. “Communication between a patient and her ob.gyn., cardiologist, or other clinician is even more critical now during the COVID-19 pandemic. We’re hearing reports that patients who are experiencing symptoms or those with known cardiac conditions are avoiding the hospital and delaying or not seeking necessary treatment. This has the very real possibility of worsening the devastating maternal mortality crisis that we’re already experiencing in this country.”
To help overcome barriers to treatment, “collaboration between ob.gyns. and cardiologists, such as the cardio-obstetrics team or pregnancy heart team, is critical,” said Dr. Hollier. “These collaborative teams with a multidisciplinary approach can prospectively reduce the communication gaps across specialties when patients are seen separately. They can also improve the communication during care transitions such as between outpatient and inpatient care.
“In reviews of maternal deaths, we have found that there are often delays in diagnosis of heart conditions during and after pregnancy,” Dr. Hollier added. “Most maternal deaths from CVD are due to either undiagnosed cardiovascular disease or new-onset cardiomyopathy. ACOG recommends that all women be assessed for cardiovascular disease in the antepartum and postpartum periods using a recently developed algorithm,” she said. “Women who have known CVD and women who have concerning symptoms should have a consultation with this team. With increased awareness and screening, women can receive the additional care that they need.
“Because management of cardiac conditions in pregnancy is so complex, it is important to ensure that women receive care with teams and in facilities that have appropriate resources,” explained Dr. Hollier. “Women with known heart disease should see a cardiologist prior to pregnancy and receive prepregnancy counseling,” as noted in the AHA statement. “Patients determined to have moderate and high-risk CVD should be managed during pregnancy, delivery, and post partum in a medical center that is able to provide a higher level of care, including a cardio-obstetrics team.”
Early recognition of cardiovascular conditions is essential to help manage care and reduce risks to mother and baby, said Dr. Hollier. “Identification before a woman becomes pregnant means the patient’s care can be properly managed throughout the pregnancy and a detailed delivery plan can be developed through shared decision making between the patient and provider. We must think of heart disease as a possibility in every pregnant or postpartum patient we see to detect and treat at-risk mothers,” she said.
Additional research should focus on identifying risk factors prior to pregnancy, said Dr. Hollier. “There are often delays in recognizing symptoms during pregnancy and post partum, particularly for black women. We need data to understand which protocols are best to identify heart disease,”
Dr. Hollier had no financial conflicts to disclose. The authors of the AHA statement had no financial conflicts to disclose. The scientific statement was produced on behalf of the American Heart Association Council on Clinical Cardiology; Council on Atherosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
SOURCE: Mehta LS et al. Circulation. 2020 May 4. doi: 10.1161/CIR.0000000000000772.
according to a statement from the American Heart Association.
Cardiovascular disease (CVD) remains the leading cause of pregnancy-related mortality in the United States, and accounted for approximately 17 deaths per 100,000 live births in 2015, wrote Laxmi S. Mehta, MD, of The Ohio State University, Columbus, and colleagues.
Ideally, a woman with CVD at the time of pregnancy should be managed by a multidisciplinary cardio-obstetrics team that can assess cardiovascular risk, obstetric risk, and fetal risk throughout pregnancy, delivery, and up to a year post partum. The team should develop a shared strategy to promote best outcomes, according to the statement. The cardio-obstetrics team may include obstetricians, cardiologists, anesthesiologists, maternal-fetal medicine specialists, geneticists, neurologists, nurses, and pharmacists, according to the statement.
Women with preexisting CVD should receive counseling about maternal and fetal risks before conception, if possible, to involve the women in shared decision-making and to develop strategies for each stage of pregnancy and delivery, Dr. Mehta and associates said. Such counseling should include a review of all medications and assessment of risk factors.
However, some women present already in the early stages of pregnancy even with severe conditions such as pulmonary arterial hypertension, severe ventricular dysfunction, severe left-sided heart obstruction, and significant aortic dilatation with underlying connective tissue disease. Women with these conditions often are counseled to avoid pregnancy, but if they already are pregnant, a high-risk cardio-obstetrics team will need to work together to discover the best strategies going forward to mitigate risk, Dr. Mehta and associates said.
Common CVD conditions that affect pregnancy include hypertensive disorders, notably preeclampsia, defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg in women after 20 weeks of gestation whose blood pressure was normal prior to pregnancy. A management strategy to reduce the risk of pregnancy-related complications from hypertension includes healthy lifestyle behaviors such as exercise, nutrition, and smoking cessation, according to the statement. However, patients with severe hypertension may require intravenous labetalol or hydralazine. The statement gives more information about handling preeclampsia with pulmonary edema, and prevention of eclampsia and treatment of seizures.
It is important to recognize that severe hypertension or superimposed preeclampsia may occur for the first time post partum. Early ambulatory visits in the first 1-2 weeks are sensible. Medications may be needed to keep a systolic blood pressure not higher than 150 mm Hg and a diastolic blood pressure not higher than 100 mm Hg, Dr. Mehta and associates said.
According to the statement, severe hypertriglyceridemia and familial hypercholesterolemia are the two most common conditions in which lipids should be addressed during pregnancy, with consideration of the fetal risks associated with certain medications.
“Statins are contraindicated during pregnancy, and all women who are on any lipid-lowering agents should review with their physician the safety of treatment during pregnancy and whether to discontinue treatment before pregnancy,” according to the statement. A heart-healthy lifestyle can help improve lipid profiles in all pregnant patients, Dr. Mehta and associates said. Patients with extremely high triglycerides above 500 mg/dL are at risk of pancreatitis and “may benefit from pharmacological agents (omega-3 fatty acids with or without fenofibrate or gemfibrozil) during the second trimester,” they noted. Pregnant women with familial hypercholesterolemia might take bile acid sequestrants, or as a last resort, low-density lipoprotein apheresis.
Other conditions calling for a multidisciplinary cardio-obstetric approach include preexisting coronary artery disease, cardiomyopathies, arrhythmias, valvular heart disease, cerebrovascular disease, and deep venous thrombosis, according to the statement, which provides information about the risks, diagnosis, and management.
When it is time for delivery, spontaneous labor and vaginal birth are preferable for most women with heart disease, as cesarean delivery is associated with increased risk of infection, thrombotic complications, and blood loss, according to the statement.
Women with CVD and associated complications will require “specialized long-term cardiovascular follow-up,” Dr. Mehta and associates said. “In women with a high-risk pregnancy, a cardio-obstetrics team is essential to prevent maternal morbidity and mortality during the length of the pregnancy and post partum.”
“The release of this document demonstrates the AHA’s recognition of the importance of CVD in pregnancy-related death and their commitment to education and ensuring best practices in this field,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and chief medical officer at Texas Children’s Health Plan, Bellaire.
“I think one of the most important outcomes from the release of this scientific statement from AHA will be increased implementation of cardio-obstetrics teams,” she said in an interview.
“In the United States, cardiovascular disease and cardiomyopathy together are now the leading cause of death in pregnancy and the postpartum period, and constitute 26.5% of pregnancy-related deaths, with higher rates of mortality among women of color and women with lower incomes,” she said. “The rising trend in cardiovascular-related maternal deaths appears to be due to acquired, not congenital, heart disease.”
During her tenure as president of ACOG, Dr. Hollier convened a task force on cardiovascular disease in pregnancy that developed guidance that outlines screening, diagnosis, and management of CVD for women from prepregnancy through post partum.
Dr. Hollier noted that COVID-19 emphasizes racial disparities for maternal mortality.
“Pregnant patients with comorbidities, like heart conditions, may be at increased risk for severe illness from COVID-19 – consistent with the general population with similar comorbidities,” she said. “And as we know, black women’s risk of dying from CVD-related pregnancy complications is 3.4 times higher than that of white women. During the COVID-19 pandemic, we are seeing these racial health disparities exacerbated.”
However, any pregnant patients should not hesitate to communicate with their health care providers despite the pandemic situation, Dr. Hollier emphasized. “Communication between a patient and her ob.gyn., cardiologist, or other clinician is even more critical now during the COVID-19 pandemic. We’re hearing reports that patients who are experiencing symptoms or those with known cardiac conditions are avoiding the hospital and delaying or not seeking necessary treatment. This has the very real possibility of worsening the devastating maternal mortality crisis that we’re already experiencing in this country.”
To help overcome barriers to treatment, “collaboration between ob.gyns. and cardiologists, such as the cardio-obstetrics team or pregnancy heart team, is critical,” said Dr. Hollier. “These collaborative teams with a multidisciplinary approach can prospectively reduce the communication gaps across specialties when patients are seen separately. They can also improve the communication during care transitions such as between outpatient and inpatient care.
“In reviews of maternal deaths, we have found that there are often delays in diagnosis of heart conditions during and after pregnancy,” Dr. Hollier added. “Most maternal deaths from CVD are due to either undiagnosed cardiovascular disease or new-onset cardiomyopathy. ACOG recommends that all women be assessed for cardiovascular disease in the antepartum and postpartum periods using a recently developed algorithm,” she said. “Women who have known CVD and women who have concerning symptoms should have a consultation with this team. With increased awareness and screening, women can receive the additional care that they need.
“Because management of cardiac conditions in pregnancy is so complex, it is important to ensure that women receive care with teams and in facilities that have appropriate resources,” explained Dr. Hollier. “Women with known heart disease should see a cardiologist prior to pregnancy and receive prepregnancy counseling,” as noted in the AHA statement. “Patients determined to have moderate and high-risk CVD should be managed during pregnancy, delivery, and post partum in a medical center that is able to provide a higher level of care, including a cardio-obstetrics team.”
Early recognition of cardiovascular conditions is essential to help manage care and reduce risks to mother and baby, said Dr. Hollier. “Identification before a woman becomes pregnant means the patient’s care can be properly managed throughout the pregnancy and a detailed delivery plan can be developed through shared decision making between the patient and provider. We must think of heart disease as a possibility in every pregnant or postpartum patient we see to detect and treat at-risk mothers,” she said.
Additional research should focus on identifying risk factors prior to pregnancy, said Dr. Hollier. “There are often delays in recognizing symptoms during pregnancy and post partum, particularly for black women. We need data to understand which protocols are best to identify heart disease,”
Dr. Hollier had no financial conflicts to disclose. The authors of the AHA statement had no financial conflicts to disclose. The scientific statement was produced on behalf of the American Heart Association Council on Clinical Cardiology; Council on Atherosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
SOURCE: Mehta LS et al. Circulation. 2020 May 4. doi: 10.1161/CIR.0000000000000772.
according to a statement from the American Heart Association.
Cardiovascular disease (CVD) remains the leading cause of pregnancy-related mortality in the United States, and accounted for approximately 17 deaths per 100,000 live births in 2015, wrote Laxmi S. Mehta, MD, of The Ohio State University, Columbus, and colleagues.
Ideally, a woman with CVD at the time of pregnancy should be managed by a multidisciplinary cardio-obstetrics team that can assess cardiovascular risk, obstetric risk, and fetal risk throughout pregnancy, delivery, and up to a year post partum. The team should develop a shared strategy to promote best outcomes, according to the statement. The cardio-obstetrics team may include obstetricians, cardiologists, anesthesiologists, maternal-fetal medicine specialists, geneticists, neurologists, nurses, and pharmacists, according to the statement.
Women with preexisting CVD should receive counseling about maternal and fetal risks before conception, if possible, to involve the women in shared decision-making and to develop strategies for each stage of pregnancy and delivery, Dr. Mehta and associates said. Such counseling should include a review of all medications and assessment of risk factors.
However, some women present already in the early stages of pregnancy even with severe conditions such as pulmonary arterial hypertension, severe ventricular dysfunction, severe left-sided heart obstruction, and significant aortic dilatation with underlying connective tissue disease. Women with these conditions often are counseled to avoid pregnancy, but if they already are pregnant, a high-risk cardio-obstetrics team will need to work together to discover the best strategies going forward to mitigate risk, Dr. Mehta and associates said.
Common CVD conditions that affect pregnancy include hypertensive disorders, notably preeclampsia, defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg in women after 20 weeks of gestation whose blood pressure was normal prior to pregnancy. A management strategy to reduce the risk of pregnancy-related complications from hypertension includes healthy lifestyle behaviors such as exercise, nutrition, and smoking cessation, according to the statement. However, patients with severe hypertension may require intravenous labetalol or hydralazine. The statement gives more information about handling preeclampsia with pulmonary edema, and prevention of eclampsia and treatment of seizures.
It is important to recognize that severe hypertension or superimposed preeclampsia may occur for the first time post partum. Early ambulatory visits in the first 1-2 weeks are sensible. Medications may be needed to keep a systolic blood pressure not higher than 150 mm Hg and a diastolic blood pressure not higher than 100 mm Hg, Dr. Mehta and associates said.
According to the statement, severe hypertriglyceridemia and familial hypercholesterolemia are the two most common conditions in which lipids should be addressed during pregnancy, with consideration of the fetal risks associated with certain medications.
“Statins are contraindicated during pregnancy, and all women who are on any lipid-lowering agents should review with their physician the safety of treatment during pregnancy and whether to discontinue treatment before pregnancy,” according to the statement. A heart-healthy lifestyle can help improve lipid profiles in all pregnant patients, Dr. Mehta and associates said. Patients with extremely high triglycerides above 500 mg/dL are at risk of pancreatitis and “may benefit from pharmacological agents (omega-3 fatty acids with or without fenofibrate or gemfibrozil) during the second trimester,” they noted. Pregnant women with familial hypercholesterolemia might take bile acid sequestrants, or as a last resort, low-density lipoprotein apheresis.
Other conditions calling for a multidisciplinary cardio-obstetric approach include preexisting coronary artery disease, cardiomyopathies, arrhythmias, valvular heart disease, cerebrovascular disease, and deep venous thrombosis, according to the statement, which provides information about the risks, diagnosis, and management.
When it is time for delivery, spontaneous labor and vaginal birth are preferable for most women with heart disease, as cesarean delivery is associated with increased risk of infection, thrombotic complications, and blood loss, according to the statement.
Women with CVD and associated complications will require “specialized long-term cardiovascular follow-up,” Dr. Mehta and associates said. “In women with a high-risk pregnancy, a cardio-obstetrics team is essential to prevent maternal morbidity and mortality during the length of the pregnancy and post partum.”
“The release of this document demonstrates the AHA’s recognition of the importance of CVD in pregnancy-related death and their commitment to education and ensuring best practices in this field,” said Lisa M. Hollier, MD, past president of the American College of Obstetricians and Gynecologists and chief medical officer at Texas Children’s Health Plan, Bellaire.
“I think one of the most important outcomes from the release of this scientific statement from AHA will be increased implementation of cardio-obstetrics teams,” she said in an interview.
“In the United States, cardiovascular disease and cardiomyopathy together are now the leading cause of death in pregnancy and the postpartum period, and constitute 26.5% of pregnancy-related deaths, with higher rates of mortality among women of color and women with lower incomes,” she said. “The rising trend in cardiovascular-related maternal deaths appears to be due to acquired, not congenital, heart disease.”
During her tenure as president of ACOG, Dr. Hollier convened a task force on cardiovascular disease in pregnancy that developed guidance that outlines screening, diagnosis, and management of CVD for women from prepregnancy through post partum.
Dr. Hollier noted that COVID-19 emphasizes racial disparities for maternal mortality.
“Pregnant patients with comorbidities, like heart conditions, may be at increased risk for severe illness from COVID-19 – consistent with the general population with similar comorbidities,” she said. “And as we know, black women’s risk of dying from CVD-related pregnancy complications is 3.4 times higher than that of white women. During the COVID-19 pandemic, we are seeing these racial health disparities exacerbated.”
However, any pregnant patients should not hesitate to communicate with their health care providers despite the pandemic situation, Dr. Hollier emphasized. “Communication between a patient and her ob.gyn., cardiologist, or other clinician is even more critical now during the COVID-19 pandemic. We’re hearing reports that patients who are experiencing symptoms or those with known cardiac conditions are avoiding the hospital and delaying or not seeking necessary treatment. This has the very real possibility of worsening the devastating maternal mortality crisis that we’re already experiencing in this country.”
To help overcome barriers to treatment, “collaboration between ob.gyns. and cardiologists, such as the cardio-obstetrics team or pregnancy heart team, is critical,” said Dr. Hollier. “These collaborative teams with a multidisciplinary approach can prospectively reduce the communication gaps across specialties when patients are seen separately. They can also improve the communication during care transitions such as between outpatient and inpatient care.
“In reviews of maternal deaths, we have found that there are often delays in diagnosis of heart conditions during and after pregnancy,” Dr. Hollier added. “Most maternal deaths from CVD are due to either undiagnosed cardiovascular disease or new-onset cardiomyopathy. ACOG recommends that all women be assessed for cardiovascular disease in the antepartum and postpartum periods using a recently developed algorithm,” she said. “Women who have known CVD and women who have concerning symptoms should have a consultation with this team. With increased awareness and screening, women can receive the additional care that they need.
“Because management of cardiac conditions in pregnancy is so complex, it is important to ensure that women receive care with teams and in facilities that have appropriate resources,” explained Dr. Hollier. “Women with known heart disease should see a cardiologist prior to pregnancy and receive prepregnancy counseling,” as noted in the AHA statement. “Patients determined to have moderate and high-risk CVD should be managed during pregnancy, delivery, and post partum in a medical center that is able to provide a higher level of care, including a cardio-obstetrics team.”
Early recognition of cardiovascular conditions is essential to help manage care and reduce risks to mother and baby, said Dr. Hollier. “Identification before a woman becomes pregnant means the patient’s care can be properly managed throughout the pregnancy and a detailed delivery plan can be developed through shared decision making between the patient and provider. We must think of heart disease as a possibility in every pregnant or postpartum patient we see to detect and treat at-risk mothers,” she said.
Additional research should focus on identifying risk factors prior to pregnancy, said Dr. Hollier. “There are often delays in recognizing symptoms during pregnancy and post partum, particularly for black women. We need data to understand which protocols are best to identify heart disease,”
Dr. Hollier had no financial conflicts to disclose. The authors of the AHA statement had no financial conflicts to disclose. The scientific statement was produced on behalf of the American Heart Association Council on Clinical Cardiology; Council on Atherosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
SOURCE: Mehta LS et al. Circulation. 2020 May 4. doi: 10.1161/CIR.0000000000000772.
FROM CIRCULATION
Hand Hygiene in Preventing COVID-19 Transmission
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8
Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8

Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8

Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
Practice Points
- Alcohol-based sanitizers are as or even more effective as handwashing with soap and water for preventing disease transmission of enveloped viruses such as severe acute respiratory syndrome coronavirus.
- Although perceived as more irritating, alcohol-based sanitizers are less likely to cause irritant contact dermatitis of the hands than handwashing with soap and water.
- Use of humectants, moisturizers, and/or emollients in combination with alcohol-based sanitizers allows for effective hand hygiene without irritating the skin.
Analysis of Education on Nail Conditions at the American Academy of Dermatology Annual Meetings
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
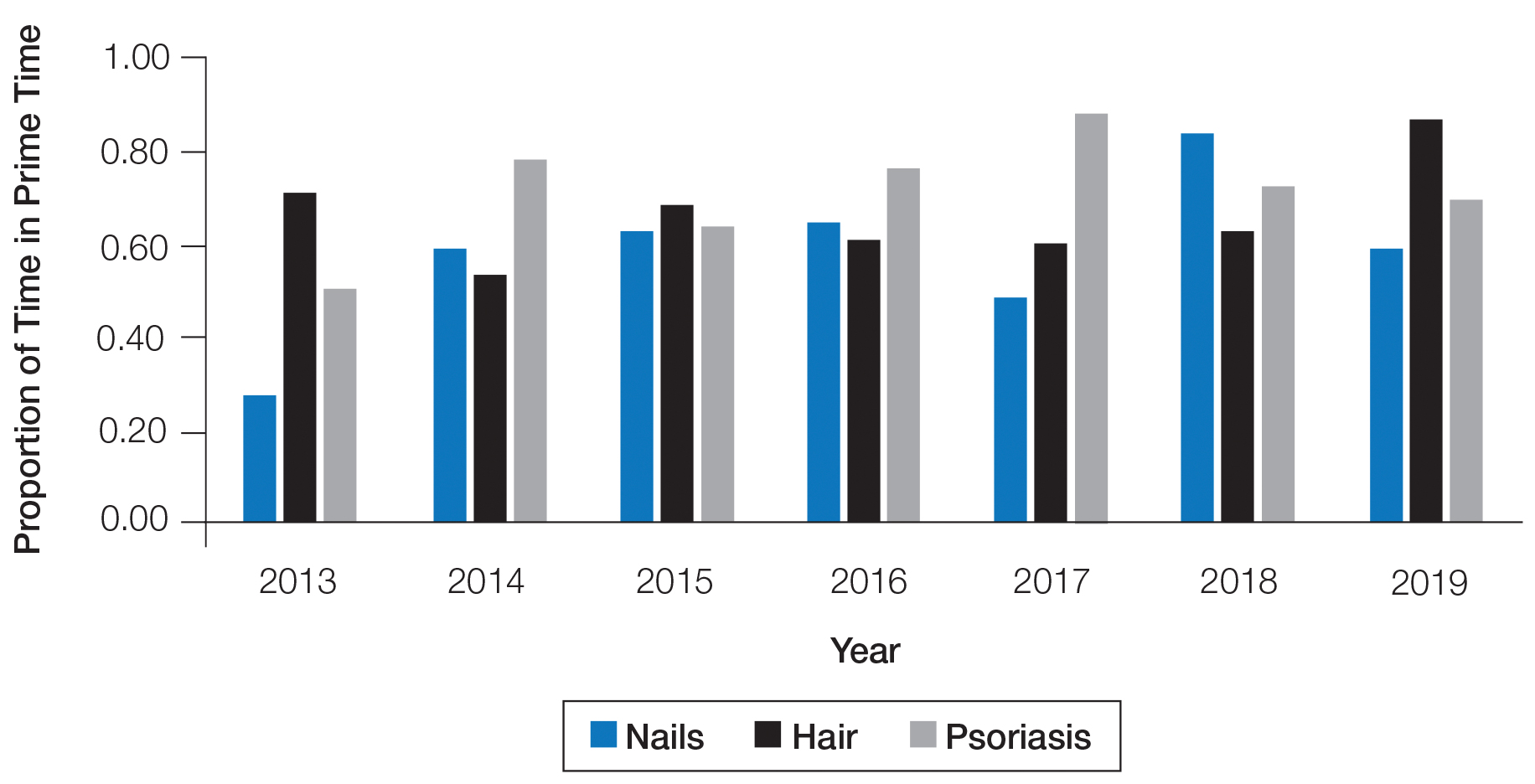
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.

Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
Practice Points
- Diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.
- We recommend increasing the number of hours and overall content of didactic nail sessions at the American Academy of Dermatology annual meeting to further the knowledge and procedural skills of dermatologists caring for patients with nail disorders.
Facial Malignancies in Patients Referred for Mohs Micrographic Surgery: A Retrospective Review of the Impact of Hair Growth on Tumor and Defect Size
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).

We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).

Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).

We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).

Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).

We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).

Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
Practice Points
- In our study, men with cutaneous tumors who had facial hair exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men who do not have any facial hair growth.
- Both patients and dermatologists should have a high index of suspicion for any concerning lesion contained within skin underlying facial hair to ensure prompt diagnosis and treatment of cutaneous tumors.
More on How to Decrease Dermatology Interview Costs
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
Interleukin-27 increased cytotoxic effects of bone marrow NK cells in CLL
Chronic lymphocytic leukemia is characterized by significant immune perturbation, including significant impairment of natural killer (NK) cells, which leads to disease complications and reduced effectiveness of treatment.
However, the use of , according to an in vitro study conducted by Maral Hemati, a student researcher at the Semnan (Iran) University of Medical Sciences, and colleagues.
Ms. Hemati and her colleagues obtained bone marrow aspirates (BM) and peripheral blood samples (PB) were from 12 untreated CLL patients (9 men and 3 women) with a median age of 61 years. The cells were cultured in vitro, according to their report in International Immunopharmacology.
The researchers found that the use of recombinant human interleukin-27 (IL-27) stimulated NK cells in the cultured BM and PB cells of CLL patients, based upon assessment using cell surface flow cytometry and a cytotoxicity assay.
Treatment with IL-27 also increased CD69 (a marker for NK cell activity) on NK cells both in BM and PB. In addition, , whereas it did not improve NK cell activity of PB, according to the researchers.
The research was supported by Semnan (Iran) University of Medical Sciences. The authors reported that they had no conflicts of interest.
SOURCE: Hemati M et al. Int Immunopharmacol. 2020;82:doi.org/10.1016/j.intimp.2020.106350.
Chronic lymphocytic leukemia is characterized by significant immune perturbation, including significant impairment of natural killer (NK) cells, which leads to disease complications and reduced effectiveness of treatment.
However, the use of , according to an in vitro study conducted by Maral Hemati, a student researcher at the Semnan (Iran) University of Medical Sciences, and colleagues.
Ms. Hemati and her colleagues obtained bone marrow aspirates (BM) and peripheral blood samples (PB) were from 12 untreated CLL patients (9 men and 3 women) with a median age of 61 years. The cells were cultured in vitro, according to their report in International Immunopharmacology.
The researchers found that the use of recombinant human interleukin-27 (IL-27) stimulated NK cells in the cultured BM and PB cells of CLL patients, based upon assessment using cell surface flow cytometry and a cytotoxicity assay.
Treatment with IL-27 also increased CD69 (a marker for NK cell activity) on NK cells both in BM and PB. In addition, , whereas it did not improve NK cell activity of PB, according to the researchers.
The research was supported by Semnan (Iran) University of Medical Sciences. The authors reported that they had no conflicts of interest.
SOURCE: Hemati M et al. Int Immunopharmacol. 2020;82:doi.org/10.1016/j.intimp.2020.106350.
Chronic lymphocytic leukemia is characterized by significant immune perturbation, including significant impairment of natural killer (NK) cells, which leads to disease complications and reduced effectiveness of treatment.
However, the use of , according to an in vitro study conducted by Maral Hemati, a student researcher at the Semnan (Iran) University of Medical Sciences, and colleagues.
Ms. Hemati and her colleagues obtained bone marrow aspirates (BM) and peripheral blood samples (PB) were from 12 untreated CLL patients (9 men and 3 women) with a median age of 61 years. The cells were cultured in vitro, according to their report in International Immunopharmacology.
The researchers found that the use of recombinant human interleukin-27 (IL-27) stimulated NK cells in the cultured BM and PB cells of CLL patients, based upon assessment using cell surface flow cytometry and a cytotoxicity assay.
Treatment with IL-27 also increased CD69 (a marker for NK cell activity) on NK cells both in BM and PB. In addition, , whereas it did not improve NK cell activity of PB, according to the researchers.
The research was supported by Semnan (Iran) University of Medical Sciences. The authors reported that they had no conflicts of interest.
SOURCE: Hemati M et al. Int Immunopharmacol. 2020;82:doi.org/10.1016/j.intimp.2020.106350.
FROM INTERNATIONAL IMMUNOPHARMACOLOGY
Patient Questionnaire to Reduce Anxiety Prior to Full-Body Skin Examination
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
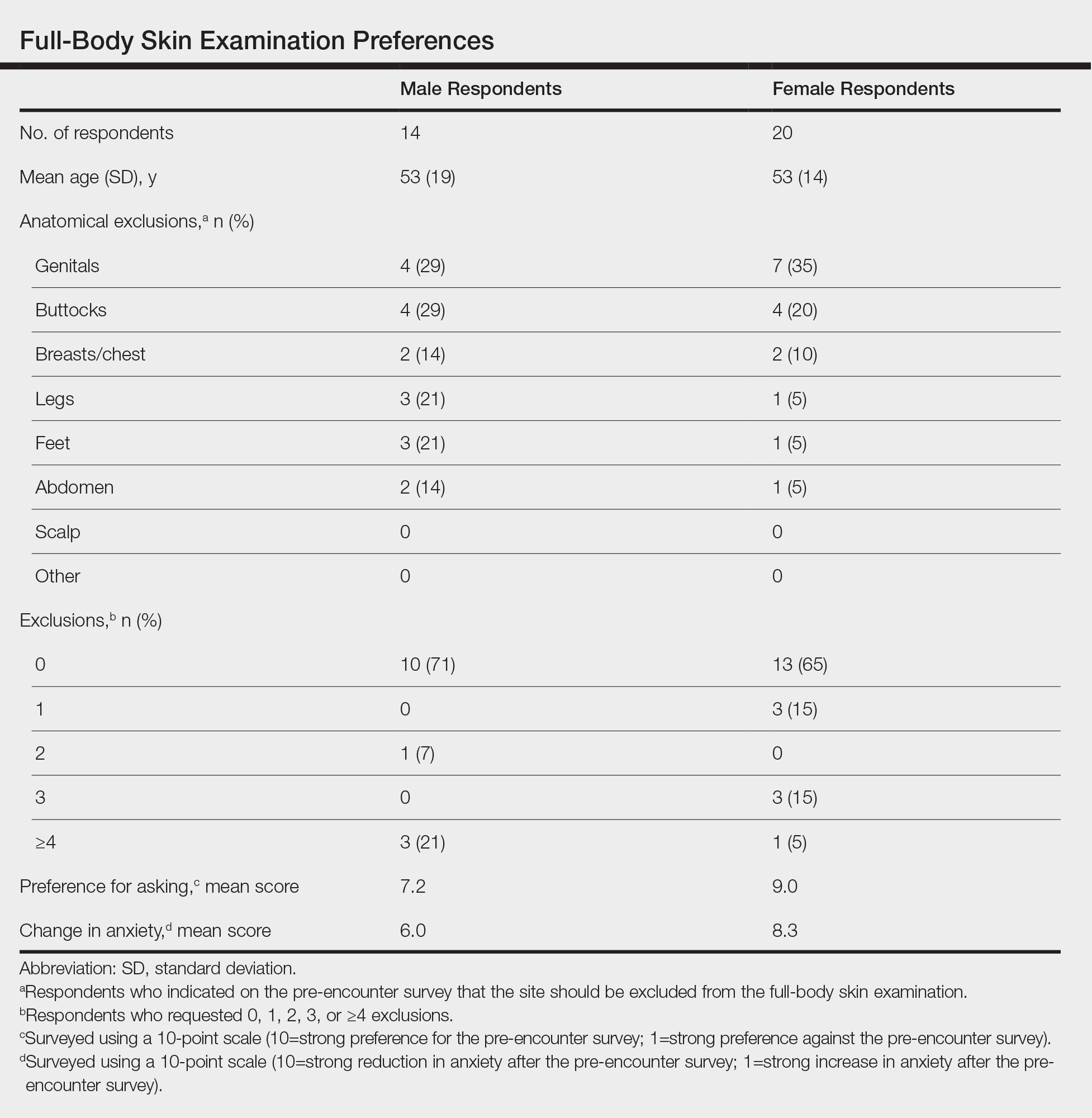
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.

After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.

After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Practice Points
- Full-body skin examination (FBSE) is an assessment that requires examination of sensitive body areas, any of which can be seen as intrusive by certain patients.
- A pre-encounter survey on the FBSE can offer an efficient means by which to determine patient preference and reduce visit-associated anxiety.
CO2 Laser Ablative Fractional Resurfacing Photodynamic Therapy for Actinic Keratosis and Nonmelanoma Skin Cancer: A Randomized Split-Side Study
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
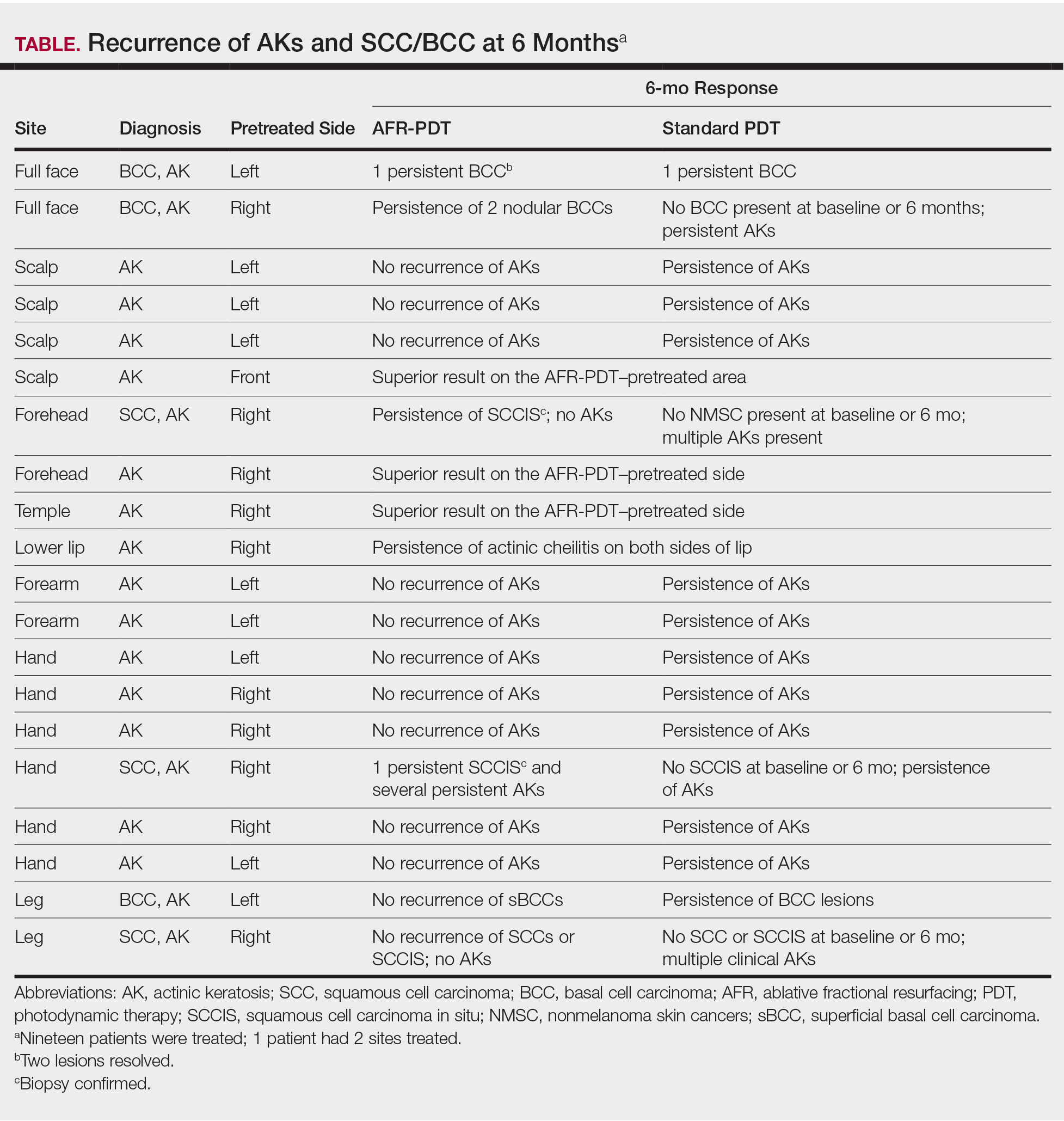
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
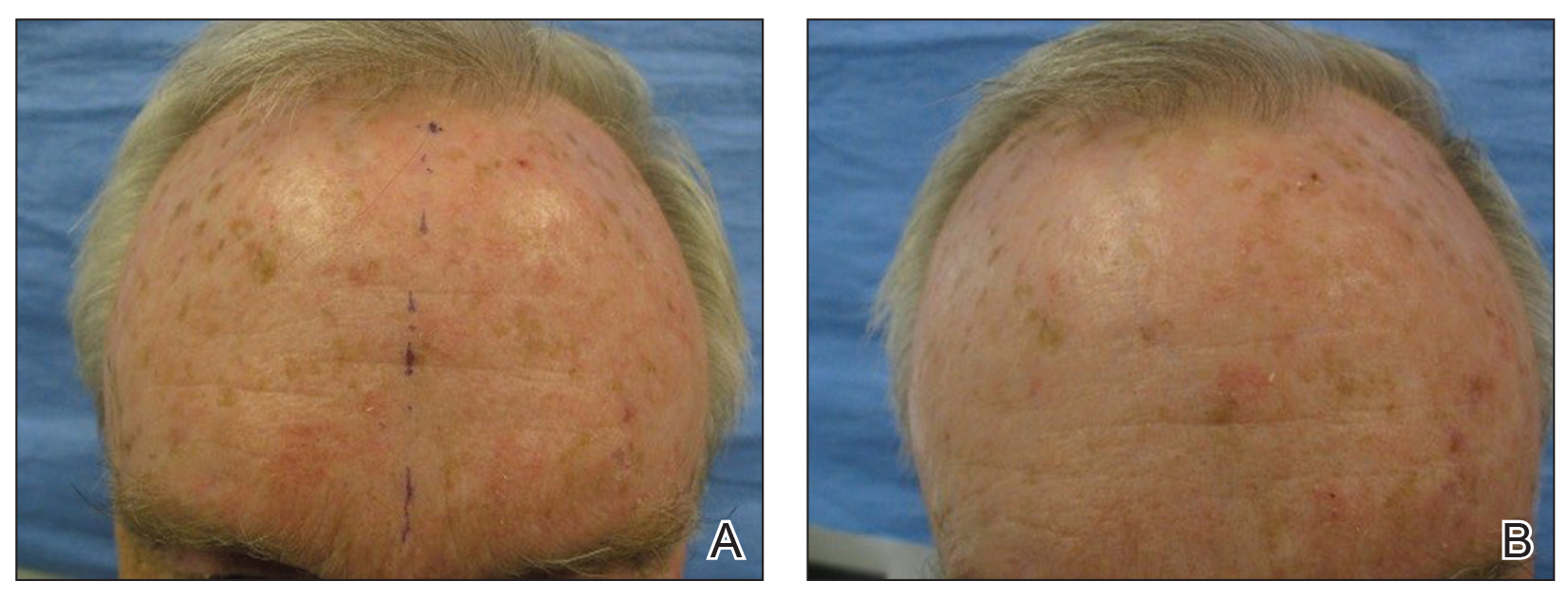
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.

Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.

Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.

Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.

Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Practice Points
- Pretreatment with CO2 laser ablative fractional resurfacing (AFR) before photodynamic therapy (PDT) provided efficient clearance of actinic keratosis (AK).
- Superior clearance of lesions was seen at 6 months for AK and thin nonmelanoma skin cancers (NMSCs) on pretreated sites compared to PDT alone, with no novel adverse events reported.
- A reduced incubation period for aminolevulinic acid (ALA) absorption before PDT was used, leading to a shorter overall treatment time.
Multisociety roadmap eyes restarting elective cardiac cases
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.