User login
Group Clinic for Chemoprevention of Squamous Cell Carcinoma: A Pilot Study
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
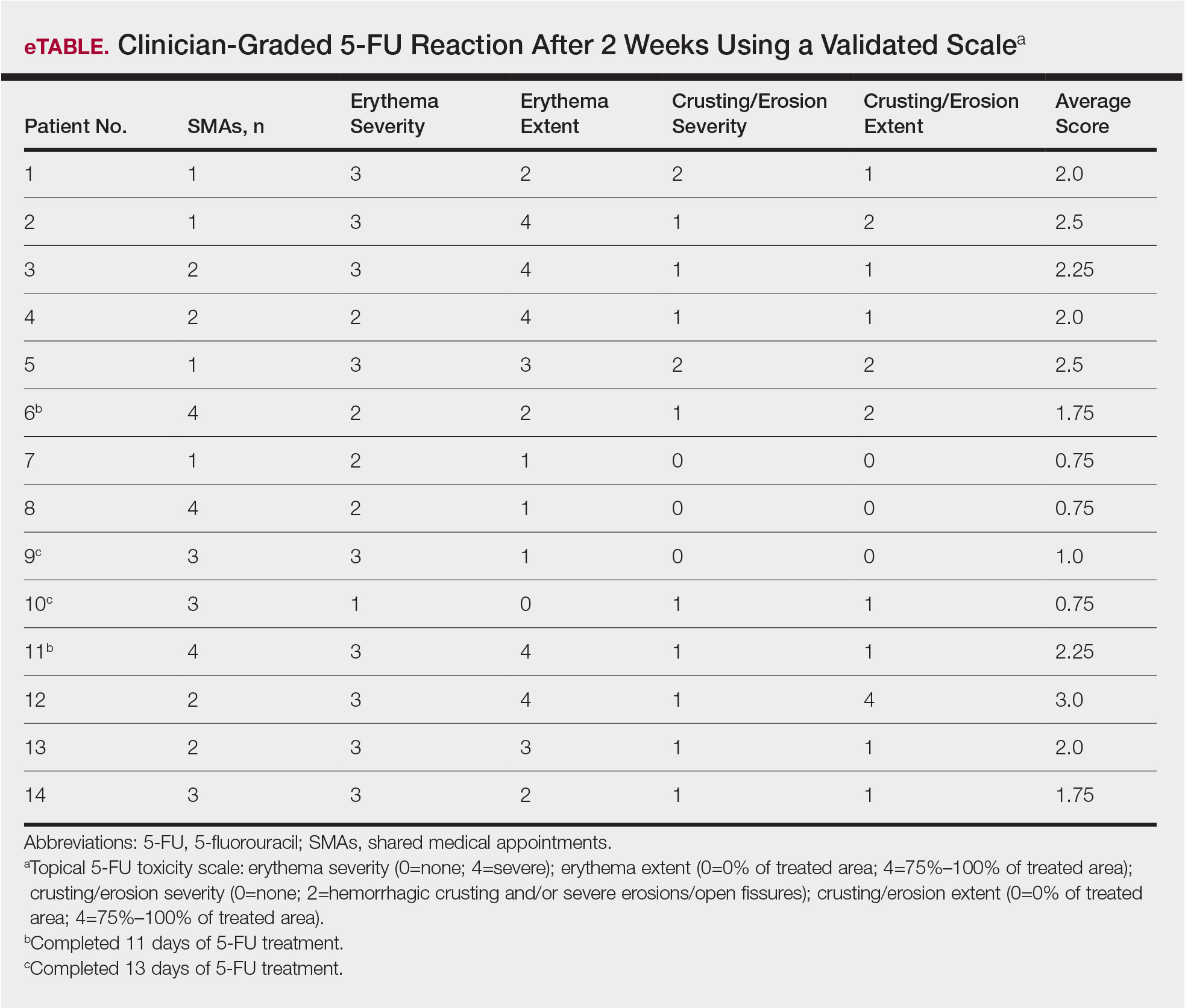
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.

None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.

None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Practice Points
- Shared medical appointments (SMAs) enhance patient experience with topical 5-fluorouracil (5-FU) treatment of actinic keratosis (AK).
- Dermatologists should consider utilizing the SMA model for their patients being treated with 5-FU, as patients demonstrated a positive emotional response to 5-FU therapy in the group clinic setting.
What’s Eating You? Bark Scorpions (Centruroides exilicauda and Centruroides sculpturatus)
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Practice Points
- Centruroides scorpions can inflict painful stings.
- Children are at greatest risk for systemic toxicity.
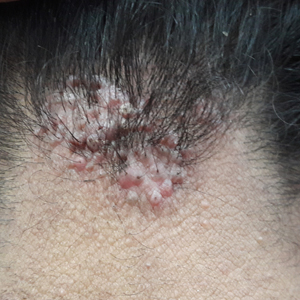
Acne Keloidalis Nuchae in the Armed Forces
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Practice Points
- Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder of the occipital scalp and posterior neck characterized by keloidlike papules, pustules, and plaques that develop following mechanical irritation.
- Military members are required to maintain short haircuts and may be disproportionately affected by AKN.
- In the military population, early identification and treatment, which includes topical steroids, oral antibiotics, UV light therapy, lasers, and surgical excision, can prevent further scarring, permanent hair loss, and disfigurement from AKN.
Triage, L&D, postpartum care during the COVID-19 pandemic
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
Obstetrics during the COVID-19 pandemic
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
You Need a Plan: A Stepwise Protocol for Operating Room Preparedness During an Infectious Pandemic
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
The worldwide spread of SARS-CoV-2, the coronavirus that causes the syndrome designated COVID-19 by the World Health Organization (WHO), presents a challenge for emergency operative care in a global pandemic setting that is novel for modern surgical practice. The virulence of this new pathogen has raised concern for how to protect operating room (OR) staff and their environs in the event that an infected patient requires urgent surgical care. Because coronaviridae spread mainly through contact with contaminated respiratory droplets or aerosolized virion-containing particles, personal protective equipment (PPE) is vital to personnel involved in these cases, and proper utilization of these scarce resources poses an additional challenge. Establishment of a clear protocol that adheres to rigorous infection control measures while providing a safe system for intrafacility transport and operative care is an essential component of a successful surgical pandemic response.
The first case of COVID-19 disease identified in the US was diagnosed in Everett, Washington, on January 21, 2020.1 In the succeeding months, the Seattle region became an early epicenter of the epidemic in the US, with Washington State becoming the first state to see in excess of 1,000 cases by mid-March 2020. As hospitalizations for COVID-19 increased, emergency surge preparations were enacted at medical centers across the region. Recommendations for how to manage infected patients evolved rapidly. Anticipating the need to provide surgical services during this pandemic, starting in early March 2020, the perioperative services staff at the US Department of Veterans Affairs (VA) Puget Sound Health Care System (PSHCS) convened to develop the protocol described here through a process of literature review, multidisciplinary discussion, and practical trial runs and drills. VAPSHCS is an urban academic medical center affiliated with the University of Washington, Seattle. The result of this collaboration is a detailed, step-by-step protocol that establishes the roles and responsibilities of the various personnel who intersect in the OR and recruits their teamwork to prevent environmental contamination and health care worker transmission of SARS-CoV-2.
The protocol is divided into discrete practice recommendations for the preoperative, intraoperative, and postoperative management of patients with confirmed or suspected COVID-19 infection, with a focus on maintaining Centers of Disease Control and Prevention-defined respiratory droplet and airborne precautions throughout the period of patient contact and mitigating infectious contamination of the operating suite.2 It is acknowledged that no written protocol can encompass all the possible considerations that attend the vast diversity of surgical scenarios which can transpire in the operative setting. Patient acuity must sometimes mandate modifications to even the most thoroughly laid plans; for instance, the exsanguinating patient requiring emergent surgery for hemorrhage control will undoubtedly require an urgent appraisal of the relative risks and benefits of certain elements of the practices here described. Nevertheless, we believe that this protocol provides a useful framework for mitigating the infection and contamination risks of operative care in an epidemic environment, and should be readily adaptable to any facility that may perform surgery in patients infected with a high-risk contagious pathogen.
Preoperative Management
In addition to introducing the risk of viral transmission, surgery in the patient with COVID-19 also imposes a large consumption of vital PPE, supplies and can become dangerously low in health care centers coping with an influx of infected patients. Early in the pandemic, to reduce exposure, conserve the medical workforce and lessen the resource strain on the overall health care infrastructure, the American College of Surgeons (ACS), American College of Gastroenterology, and other professional societies recommended cancellation of elective procedures, confining operations to urgent or emergent procedures for high-acuity diseases that would negatively impact morbidity or mortality if delayed.3,4 In each case, physicians from the surgical and anesthesia services should discuss the rationale for the operation and secure agreement to commit resources to the endeavor prior to reserving the OR. These considerations should be shared with the patient prior to obtaining informed consent.
Preoperatively, the surgical team, consisting of surgeon, anesthesiologist, OR nurse, surgical technician, and assistants to the surgeon, anesthesiologist and nurse, convene for a preoperative “team huddle.” While assistants will aid in patient transport and supplying equipment to the team during the procedure, they should not be in the OR during the case, to minimize personnel exposure and PPE consumption. All members of the surgical team remove their personal effects, including wallets, phones, badges, and jewelry; any pagers are handed to other members of the care team for the duration of the surgery. During this preoperative team huddle, proper setup and accounting of the surgical equipment is confirmed, as well as the availability of all necessary anesthesia equipment and medications.
A specific OR with versatile characteristics was chosen to be the designated OR for procedures in patients with confirmed or suspected COVID-19. The COVID OR is on standby when no such cases are active, and it is not used for surgeries in noninfected patients. This is in accord with published recommendations of anesthesiologists who, throughout the COVID-19 epidemic in China, maintained designated ORs and anesthesia machines for only infected patients.5 Strong consideration should be given to performing procedures for which endotracheal intubation is not required in the patient’s own respiratory isolation room, rather than the OR to avoid room contamination and excessive use of PPE.5,6
The availability of adequate PPE is confirmed during the preoperative team huddle. At a minimum, powered air purifying respirator devices (PAPRs) with hoods must be available for the anesthesia provider, surgeon and surgical technician, recognizing the Anesthesia Patient Safety Foundation (APSF) recommendation that these devices confer superior protection for those with the highest risk and most proximate exposure to the patient throughout the case.7,8 An N95 respirator, at minimum, must be available for the circulating OR nurse. Patient condition, need for critical care transport, anesthetic plan (monitored anesthesia care or general anesthesia), and availability of negative pressure isolation rooms in the ward vs in the operating suite should help decide patient transport strategies and help determine the most suitable location to secure the airway. In case of an inadvertent tube disconnection, transporting intubated patients carries the risk of disseminating virus laden aerosols into the environment. Risks of pre-OR intubation should be balanced with the potential benefit of securing the airway prior to transport and decreased gross OR contamination with intubation in the operating suite. Airway manipulation and intubation are among the highest risk procedures for nosocomial transmission and performance of these procedures should utilize precautions described in current APSF recommendations.3,9,10
For patients not requiring critical care transport, and when conditions favor intubation in the OR, patients should be transported in a gurney while wearing a surgical mask. Verification of the operative site, surgical plan, and other components of the WHO universal surgical safety checklist or time out are performed in the OR prior to induction of anesthesia, and a conscious patient can be an active participant.
If critical care transport is deemed necessary and/or a decision is made to intubate the patient outside the OR, preferably in a negative airflow respiratory isolation room, the perioperative team will confirm the availability of the following equipment needed for patient transport:
- Portable transport monitor;
- Video laryngoscope;
- Airway supplies and medications for induction of general anesthesia;
- Self-inflating bag-mask apparatus attached to an oxygen source;
- High-quality HMEF (heat and moisture exchanging filter) rated to remove at least 99.97% of airborne particles ≥ 0.3 microns to filter out viral particles attached to the expiratory outlet; and
- PPE including impermeable disposable gowns, gloves, and shoe covers.
While the surgical technician remains in the OR, the rest of the team will proceed to the patient’s location with these supplies, along with the necessary number of PAPRs and N95 respirators.
Outside the patient room, the team consisting of surgeon, anesthesia provider, OR nurse, and the assistant to each of these health care providers, gathers for the first time out, confirming the patient’s identification, intended procedure, surgical site, laterality, and informed consent. If the patient is verbal and has decision-making capacity, they confirm their identification, understanding of the planned procedure, and consent with the team over the phone from the confines of their room. If a patient lacks decision making capacity standard organization policies should be adhered to, most of which do not require direct patient contact and do not pose any unique infection control challenges. The anesthesia provider and surgeon don their PPE including PAPR devices with the aid of their assistants. Using a PPE checklist, the surgical team member dons with the assistance of a PPE partner, who is charged with reading the instructions on the checklist to the surgical team member step by step and inspecting the adequacy of the full PPE attire (Figure 1). A similar secondary check of appropriate PPE by an assistant during high risk encounters has also been advocated by other authors.6
Consideration should be given to intubating the patient prior to transport to the OR particularly if the patient originates in a respiratory isolation room with negative pressure airflow, being mindful that most operating suites are ventilated with positive airflow that could help disperse virus laden aerosols in the procedure area. It may also be beneficial to have a secure airway in a patient who is actively coughing, sneezing, and dispersing respiratory droplets to the surrounding environment prior to leaving respiratory isolation. When intubation prior to OR transport is chosen, the fully attired anesthesiologist enters the patient room first, with a video laryngoscope, medication, and other supplies needed to successfully induce general endotracheal tube anesthesia. The anesthesia and surgery assistants don droplet precaution PPE and remain outside the patient room. Whenever possible, a rapid sequence induction is performed with minimization of bag-mask ventilation. Video laryngoscopy is preferred over direct laryngoscopy in patients with COVID-19 as it enables a greater distance between the health care provider and the airway.5,6 The surgeon and OR nurse then enter the room, wearing PPE including PAPR, and assist with attaching the transport monitor and moving the patient bed out of the room. The OR nurse wipes the front face shield and PAPR hood of the anesthesia provider after intubation, to clean these presumably contaminated components prior to exiting the room. A second, clean disposable gown covers the one worn during intubation to minimize environmental contamination during transport.11,12
The patient is intubated, anesthetized, and, transported to the OR, with a self-inflating bag mask apparatus attached to an oxygen source and a second high-quality HMEF rated to remove at least 99.97% of airborne particles ≥ 0.3 microns is attached to the expiratory outlet, or a transport ventilator with HEPA filter attached to the expiratory limb. In the OR, the anesthesia provider, surgical technician, and OR nurse assist with moving the patient to the operating gurney and attaching the monitor. The surgeon remains outside the room in order to doff the gown and gloves worn during transport, disinfect their hands (preoperative scrubbing), and don sterile attire, all while continuing to wear the same PAPR and hood.
Intraoperative Management
Advance planning can help to ensure a safer intraoperative period when a COVID-19 patient is brought to the OR. Patient room airflow patterns and ventilation capacity should be considered when developing measures to prevent aerosol transmission of airborne infectious agents. Although negative pressure rooms are ideal for aerosol generating procedures such as intubation, most ORs are generally maintained at a positive pressure when compared with the surrounding areas. The feasibility of rapidly converting ORs into negative pressure rooms should be in facility planning for COVID-19; portable high-efficiency particulate air (HEPA) machines, for instance, can be set up to create negative pressure areas around the OR.13 We established a negative pressure anteroom outside our OR to be used for doffing and as an airlock, for use by staff who need to enter midcase or pass supplies or specimens into and out of the procedure room (Figure 2). By adding 2 portable HEPA filters and directing the HEPA-filtered exhaust into the OR ventilation return columns, we were able to establish negative pressure airflow in the OR (Figure 3).
The protocol was devised with the current pandemic-associated shortage of PPE taken into consideration. We decided to minimize staffing across disciplines by excluding all nonessential personal from entering the OR. This includes observers, researchers, and medical students. Residents and fellows may participate if their presence is deemed vital to the patient’s intraoperative care. To further prevent resource consumption, equipment in the designated COVID OR was reduced to essential elements such as the anesthesia machine, a minimized anesthesia drug cart and general supply cabinet, all of which were covered with disposable transparent covers (Figure 4).14 After transfer of the patient to the OR table, the patient stretcher is kept in the OR (space permitting) to minimize contamination of areas immediately outside the OR.
Prior to incision a second time out is performed to confirm the previously verified operative site and plan. During the case, the assistants to the OR nurse and anesthesia provider act as facilitators or “runners” for equipment retrieval and communication with the outside OR staff. These roles are assigned to personnel who are familiar with the layout and day-to-day functioning of the ORs, such as anesthesia technicians and OR circulating nurses. All staff agreed on a strategy of no breaks or alternations whenever possible to conserve PPEs.15 Near the conclusion of the surgical procedure, the receiving intensive care unit (ICU) is given a verbal report on patient status over the phone.
Postperative Management
Similar to intubation, extubation poses a risk of generating aerosolization of infectious airborne microbes.10 It is helpful for OR personnel to be aware of the airflow pattern in their ORs, whether it is positive, negative, or neutral. As the PSHCS ORs were originally engineered as positive pressure rooms, we elected to have to postoperative patients with COVID-19 transported intubated to a reverse airflow or negative pressure room in the ICU. Extubation is performed when the intensive care team has determined the patient meets extubation criteria and has passed a spontaneous breathing trial. When a negative pressure room in the ICU is not available for recovery, extubation may be performed in the OR.
In that circumstance, the patient remains in the OR for 30 minutes after extubation to allow for turnover of air in the room prior to the doors opening for patient transport to the ICU.16 A surgical mask is placed over the patient’s oxygenating face mask to reduce droplet spread during transport. Patients who are not intubated for the anesthetic may be first recovered in the operating room or transported under droplet precautions directly back to a negative pressure isolation room.
Prior to transport, the patient’s gurney is thoroughly cleaned with Environmental Protection Agency-approved disinfectant wipes, and a clean sheet is placed over the patient’s body below the head.17 The front face shield of the surgeon’s and anesthesiologist’s PAPR hood should be wiped down with an alcohol-based disinfectant. Both health care providers don a clean disposable gown as an outer layer to minimize contamination by their used attire during transport. Once the patient is transported out of the OR, all disposable items are discarded. Reusable medical equipment are cleaned and disinfected according to a thorough application of local environmental services standard operating procedures.18 The surgeon and anesthesia providers aid in transporting the patient to the ICU, along with their outside OR assistants. All personnel remaining in the OR exit and doff their PPE according to the doffing protocol, which is similar to the donning protocol, utilizes a PPE partner tasked with providing instructions to the surgical team member step by step (Figure 5).
After leaving the OR, terminal cleaning must be performed by environmental services (EVS) personnel, but they should delay entry into the room until a sufficient amount of time has elapsed after the last aerosol-generating procedure in the OR. Time determination will depend on the air change per hour (ACH) in the OR that will achieve 99.9% removal of airborne contaminates. For example, ventilation in our operating rooms operate at approximately 15 to 20 ACH, which should attain that level of air clearance in 21 to 28 minutes.16 Once the stipulated time has elapsed EVS personnel may enter the room but should wear a gown and gloves when performing terminal cleaning. A face mask and eye protection should be added if splashes or sprays during cleaning and disinfection activities are anticipated, or otherwise required based on the selected cleaning products. Anesthesia technicians can now also enter the room to disinfect the anesthesia machines and set up all disposable supplies for any potential following case.
Conclusions
The outbreak of COVID-19 has resulted in an unprecedented modern health care crisis across the globe. Perioperative management of patients with COVID-19 pose unique challenges to all personnel working in the OR, where the risk of nosocomial transmission of infection is ever present. It is essential that hospitals consider their local resources, infrastructure and capabilities when devising policies to respond to the COVID-19 emergency. In all perioperative situations, meticulous attention should be given to both donning and doffing of PPE, crucial for the safety of everyone involved in the care of patients with COVID-19.
Our experience also highlighted the importance of treating a new protocol as an evolving document, which can be modified and improved through the conduct of training and simulation exercises with providers across disciplines (Figure 6). In gathering nurses, anesthesia staff, and surgeons to perform drills and simulate their roles in an imaginary scenario, we gained new insights, and made corrections and additions that ultimately generated the presently described process. Modifications to any protocol may be necessary depending on the unique circumstances of individual health care systems and hospitals, the characteristics of the patient population they cater to, and the resources and expertise they have available. As the pandemic continues, we are bound to learn more about the epidemiology and modes of transmission of SARS-CoV-2, which may demand further changes to our practice. It is crucial to remember that while emergency policies must be rapidly developed, they should be collaboratively improved and incorporate new knowledge when it becomes available. This is essential to ensure the ultimate protocol is useful, up-to-date, easy to follow and tailored to the unique local environment of each health care setting.
After the initial apprehensions and struggles that attended our confrontation with the crisis, it is our hope that the experience we share will be helpful to surgical staff at other institutions grappling with the challenges of operative care in the pandemic environment. While this protocol focuses on the current COVID-19 pandemic, these recommendations serve as a template for surgical preparedness that can be readily adapted to the next infectious disease crisis that will inevitably emerge.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
1. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
2. Siegel S RE, Jackson M, Chiarello L. Healthcare Infection Control Practices Advisory Committee; Guideline for Isolation Precautions. Centers For Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html. Published 2007. Accessed March 28, 2020.
3. American College of Surgeons: COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/triage. Published March 17, 2020. Accessed April 19, 2020.
4. American College of Gastroenterology. Gastroenterology professional society Guidance on endoscopic procedures During the covid-19 pandemic. American College of Gastroenterology. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf. Published March 31, 2020. Accessed April 19, 2020.
5. Chen X, Liu Y, Gong Y, et al. Perioperative management of patients infected with the novel coronavirus: recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists [published online ahead of print, 2020 Mar 26]. Anesthesiology. 2020;10.1097/ALN.0000000000003301.
6. Zhang HF, Bo L, Lin Y, et al. Response of Chinese anesthesiologists to the COVID-19 outbreak [published online ahead of print, 2020 Mar 30]. Anesthesiology. 2020;10.1097/ALN.0000000000003300.
7. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. Br J Anaesth. 2003;90(6):715-718.
8. Zucco L LN, Ketchandji D, Aziz M, Ramachandran SK. Perioperative considerations for the 2019 novel coronavirus (COVID-19). https://www.apsf.org/news-updatesperioperative-considerations-for-the-2019-novel-coronavirus-covid-19/. Published Feb 12, 2020. Accessed March 30, 2020.
9. Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53(2):122-129.
10. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11(10):940.
11. Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497‐501.
12. Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance [published online ahead of print, 2020 Mar 6]. Can J Anaesth. 2020;1‐3.
13. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64(4):371-378.
14. Clark C, Taenzer A, Charette K, Whitty M. Decreasing contamination of the anesthesia environment. Am J Infect Control. 2014;42(11):1223-1225.
15. Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management [published online ahead of print, 2020 Mar 26]. Anesth Analg. 2020;10.1213/ANE.0000000000004829.
16. Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
17. US Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 16, 2020. Accessed April 19, 2020.
18. Munoz-Price LS, Bowdle A, Johnston BL, et al. Infection prevention in the operating room anesthesia work area [published correction appears in Infect Control Hosp Epidemiol. 2019 Apr;40(4):500]. Infect Control Hosp Epidemiol. 2018;1‐17.
Hydrogen peroxide reduces C. acnes cultures following shoulder surgery
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
FROM AAOS 2020
Expert discusses red flags for interstitial lung disease in pediatric rheumatology
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
REPORTING FROM RWCS 2020
FMT may improve outcomes without clearing multidrug-resistant organisms
For seriously ill patients with multidrug-resistant organisms (MDROs) in their gastrointestinal tract, performing a fecal microbiota transplant (FMT) may result in fewer and less severe infections, as well as shorter hospital stays, according to investigators.
Significant clinical improvements were observed across the group even though 59% of patients did not clear MDROs, which suggests that complete decolonization of resistant organisms may be unnecessary for patients to benefit from FMT, reported lead author Julian Marchesi, PhD, of Cardiff (Wales) University and Imperial College London (England).
“We see the quality of life for these patients is hugely improved even when we don’t get rid of the organism totally,” Dr. Marchesi said in a virtual press conference.
Although previous studies have suggested that FMT may be used to decolonize MDROs, little research has addressed other clinical outcomes, the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
The present study involved 20 patients with MDROs, including extended-spectrum beta-lactamase Enterobacteriaceae (ESBL), carbapenemase-producing Enterobacteriaceae (CPE), or vancomycin-resistant enterococci (VRE). Approximately half of the population (n = 11) had chronic hematological disease. The other half (n = 9) had recurrent urinary tract infections with ESBL, including patients who had undergone renal transplant or had recurrent Clostridioides difficile infection.
For each transplant, 200-300 mL of fecal slurry was delivered via nasogastric tube into the small intestine. Fecal donors underwent a strict screening process that included blood, fecal, and behavioral testing.
Multiple clinical outcomes were evaluated in the 6 months leading up to FMT, then compared with outcomes in the 6 months following fecal transplant. Out of 20 patients, 17 completed the 6-month follow-up. Although only 7 of these patients (41%) were decolonized of MDROs, multiple significant clinical improvements were observed across the group, including reductions in MDRO bloodstream infections (P = .047), all bloodstream infections (P = .03), length of stay in hospital (P = .0002), and duration of carbapenem use (P = .0005). Eight out of 11 patients with hematologic disease improved enough to undergo stem cell transplantation within 6 months of FMT, and in the subgroup of patients who had undergone renal transplant, the rate of urinary tract infections was significantly improved (P = .008).
No serious adverse events were encountered during the trial, which led the investigators to conclude that FMT was safe and well tolerated, even in patients with bloodstream infections and those who were highly immunosuppressed.
Beyond clinical implications, Dr. Marchesi suggested that the study findings should influence FMT trial methodology.
“We’ve got to start thinking a little bit differently in terms of how we measure the impact of FMT,” he said. “It’s not all about ... getting rid of these opportunistic pathogens. There are other quality-of-life factors that we need to measure, because they’re also important for the patient.”
Dr. Marchesi said that more research is needed to confirm findings and gain a mechanistic understanding of why patients may improve despite a lack of decolonization.
“We think we’re on a strong foundation here to take this into a clinical trial,” he said.
The research was funded by the National Institute for Health Research and the Medical Research Council. The investigators reported no conflicts of interest.
For seriously ill patients with multidrug-resistant organisms (MDROs) in their gastrointestinal tract, performing a fecal microbiota transplant (FMT) may result in fewer and less severe infections, as well as shorter hospital stays, according to investigators.
Significant clinical improvements were observed across the group even though 59% of patients did not clear MDROs, which suggests that complete decolonization of resistant organisms may be unnecessary for patients to benefit from FMT, reported lead author Julian Marchesi, PhD, of Cardiff (Wales) University and Imperial College London (England).
“We see the quality of life for these patients is hugely improved even when we don’t get rid of the organism totally,” Dr. Marchesi said in a virtual press conference.
Although previous studies have suggested that FMT may be used to decolonize MDROs, little research has addressed other clinical outcomes, the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
The present study involved 20 patients with MDROs, including extended-spectrum beta-lactamase Enterobacteriaceae (ESBL), carbapenemase-producing Enterobacteriaceae (CPE), or vancomycin-resistant enterococci (VRE). Approximately half of the population (n = 11) had chronic hematological disease. The other half (n = 9) had recurrent urinary tract infections with ESBL, including patients who had undergone renal transplant or had recurrent Clostridioides difficile infection.
For each transplant, 200-300 mL of fecal slurry was delivered via nasogastric tube into the small intestine. Fecal donors underwent a strict screening process that included blood, fecal, and behavioral testing.
Multiple clinical outcomes were evaluated in the 6 months leading up to FMT, then compared with outcomes in the 6 months following fecal transplant. Out of 20 patients, 17 completed the 6-month follow-up. Although only 7 of these patients (41%) were decolonized of MDROs, multiple significant clinical improvements were observed across the group, including reductions in MDRO bloodstream infections (P = .047), all bloodstream infections (P = .03), length of stay in hospital (P = .0002), and duration of carbapenem use (P = .0005). Eight out of 11 patients with hematologic disease improved enough to undergo stem cell transplantation within 6 months of FMT, and in the subgroup of patients who had undergone renal transplant, the rate of urinary tract infections was significantly improved (P = .008).
No serious adverse events were encountered during the trial, which led the investigators to conclude that FMT was safe and well tolerated, even in patients with bloodstream infections and those who were highly immunosuppressed.
Beyond clinical implications, Dr. Marchesi suggested that the study findings should influence FMT trial methodology.
“We’ve got to start thinking a little bit differently in terms of how we measure the impact of FMT,” he said. “It’s not all about ... getting rid of these opportunistic pathogens. There are other quality-of-life factors that we need to measure, because they’re also important for the patient.”
Dr. Marchesi said that more research is needed to confirm findings and gain a mechanistic understanding of why patients may improve despite a lack of decolonization.
“We think we’re on a strong foundation here to take this into a clinical trial,” he said.
The research was funded by the National Institute for Health Research and the Medical Research Council. The investigators reported no conflicts of interest.
For seriously ill patients with multidrug-resistant organisms (MDROs) in their gastrointestinal tract, performing a fecal microbiota transplant (FMT) may result in fewer and less severe infections, as well as shorter hospital stays, according to investigators.
Significant clinical improvements were observed across the group even though 59% of patients did not clear MDROs, which suggests that complete decolonization of resistant organisms may be unnecessary for patients to benefit from FMT, reported lead author Julian Marchesi, PhD, of Cardiff (Wales) University and Imperial College London (England).
“We see the quality of life for these patients is hugely improved even when we don’t get rid of the organism totally,” Dr. Marchesi said in a virtual press conference.
Although previous studies have suggested that FMT may be used to decolonize MDROs, little research has addressed other clinical outcomes, the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
The present study involved 20 patients with MDROs, including extended-spectrum beta-lactamase Enterobacteriaceae (ESBL), carbapenemase-producing Enterobacteriaceae (CPE), or vancomycin-resistant enterococci (VRE). Approximately half of the population (n = 11) had chronic hematological disease. The other half (n = 9) had recurrent urinary tract infections with ESBL, including patients who had undergone renal transplant or had recurrent Clostridioides difficile infection.
For each transplant, 200-300 mL of fecal slurry was delivered via nasogastric tube into the small intestine. Fecal donors underwent a strict screening process that included blood, fecal, and behavioral testing.
Multiple clinical outcomes were evaluated in the 6 months leading up to FMT, then compared with outcomes in the 6 months following fecal transplant. Out of 20 patients, 17 completed the 6-month follow-up. Although only 7 of these patients (41%) were decolonized of MDROs, multiple significant clinical improvements were observed across the group, including reductions in MDRO bloodstream infections (P = .047), all bloodstream infections (P = .03), length of stay in hospital (P = .0002), and duration of carbapenem use (P = .0005). Eight out of 11 patients with hematologic disease improved enough to undergo stem cell transplantation within 6 months of FMT, and in the subgroup of patients who had undergone renal transplant, the rate of urinary tract infections was significantly improved (P = .008).
No serious adverse events were encountered during the trial, which led the investigators to conclude that FMT was safe and well tolerated, even in patients with bloodstream infections and those who were highly immunosuppressed.
Beyond clinical implications, Dr. Marchesi suggested that the study findings should influence FMT trial methodology.
“We’ve got to start thinking a little bit differently in terms of how we measure the impact of FMT,” he said. “It’s not all about ... getting rid of these opportunistic pathogens. There are other quality-of-life factors that we need to measure, because they’re also important for the patient.”
Dr. Marchesi said that more research is needed to confirm findings and gain a mechanistic understanding of why patients may improve despite a lack of decolonization.
“We think we’re on a strong foundation here to take this into a clinical trial,” he said.
The research was funded by the National Institute for Health Research and the Medical Research Council. The investigators reported no conflicts of interest.
FROM DDW 2020
Researchers identify a cause of L-DOPA–induced dyskinesia in Parkinson’s disease
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
FROM Science Advances