User login
FDA approves dapagliflozin for low-EF heart failure
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has come through with the widely anticipated approval of dapagliflozin (Farxiga, AstraZeneca) for heart failure and reduced ejection fraction (HFrEF), adding to the rich array of medications lately available for this indication.
The approval follows the agency’s priority review of the sodium-glucose cotransporter 2 (SGLT2) inhibitor for reducing the risk of cardiovascular death and heart-failure hospitalization in adults with HFrEF following last year’s seminal results of the DAPA-HF trial.
In that study, treatment with dapagliflozin led to about a one-fourth reduction in risk of a primary endpoint consisting primarily of CV death or heart failure hospitalization in patients with chronic HFrEF, in both those with and without diabetes. The randomized, placebo-controlled trial had entered more than 4,700 patients.
Soon after, the FDA approved dapagliflozin for reducing the risk of heart failure hospitalization in adults with type 2 diabetes and other CV risk factors.
And of course, dapagliflozin – traditionally viewed only as an antidiabetic agent – has long been indicated for improvement of glycemic control in adults with type 2 diabetes.
The latest approval for patients with New York Heart Association functional class III-IV HFrEF makes dapagliflozin the only SGLT2 inhibitor to be indicated for heart failure in the absence of diabetes.
Soon after the DAPA-HF results had been unveiled at a major meeting, heart failure expert Christopher O’Connor, MD, expressed concern that dapagliflozin’s uptake for patients with HFrEF would be slow once it gained approval for patients without diabetes.
“We have to think of this as a drug that you would prescribe like an ACE inhibitor, or a beta-blocker, or a mineralocorticoid receptor antagonist, or sacubitril/valsartan [Entresto, Novartis],” Dr. O’Connor, of the Inova Heart and Vascular Institute, Falls Church, Va., said in an interview.
Dr. O’Connor was not associated with DAPA-HF and had previously disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Milestone’ study shows promise for pedophilic disorder
Testosterone-suppressing treatment with the gonadotropin-releasing hormone (GnRH) antagonist degarelix may reduce dynamic risk factors for sexual offense in men with pedophilic disorder, new research suggests.
In a first-of-its-kind randomized, controlled trial of 52 help-seeking men with the disorder, degarelix versus placebo significantly dampened two critical risk factors for committing abuse: high sexual desire and sexual attraction to children. In addition, effects were noticeable within 2 weeks.
“The medicine is quick-acting, not only on biological systems but also on thoughts and behavior,” coinvestigator and corresponding author Christoffer Rahm, MD, of the Centre for Psychiatry Research at Karolinska Institutet, Stockholm, said in an interview.
“The effect lasts and increases after 10 weeks, and especially so in the small group of high-risk individuals,” Dr. Rahm added.
The study findings were published in JAMA Psychiatry.
Opportunity for prevention
Although all men with pedophilic disorder do not commit a sexual offense, those who do generally report struggling with their sexual urges for 10 years before committing a sexual crime, the investigators noted.
This presents an opportunity for prevention by treating high-risk individuals without prior convictions. Effective treatment could prevent child sexual abuse and reduce psychosocial stress for the individual with pedophilic disorder, the researchers wrote.
GnRH antagonists are considered effective in reducing paraphilic symptoms, but their use has been limited to correctional settings. – and not just convicted men from prison and the probation system.
“It means the conclusions from the study are applicable to the patients you meet on sexual medicine and general psychiatry clinics too,” Dr. Rahm said.
The study included 52 men with a pedophilic disorder diagnosis and no contraindications to the intervention. All had contacted PrevenTell, the Swedish national telephone helpline for unwanted sexuality.
Half of the participants were randomly assigned to receive two subcutaneous 120-mg injections of degarelix acetate, while the other half received an equal volume of placebo.
The primary endpoint was efficacy at 2 weeks after injection in reducing a composite risk score of five domains for committing child sexual abuse; this risk score ranged from 0 to 15 points (each domain could be rated 0-3). Secondary endpoints included efficacy at 2 and 10 weeks in the composite score, each risk domain, quality of life, self-reported effects, and adverse events.
‘Positive effects’
At 2 weeks, the composite risk score decreased from 7.4 to 4.4 in the degarelix group and from 7.8 to 6.6 in the placebo group, which was a mean between-group difference of –1.8 (95% confidence interval, –3.2 to –0.5; P = .01).
Compared with placebo, the degarelix group also showed a decrease in the composite score at 10 weeks (−2.2; 95% CI, −3.6 to −0.7), in the domains of pedophilic disorder at 2 weeks (−0.7; 95% CI, −1.4 to 0.0) and 10 weeks (−1.1; 95% CI, −1.8 to −0.4), and in sexual preoccupation at 2 weeks (−0.7; 95% CI, −1.2 to −0.3) and 10 weeks (−0.8; 95% CI, −1.3 to −0.3).
There were no between-group differences in the other domains of self-rated risk, low empathy, and impaired self-regulation at 2 or 10 weeks, or in quality of life.
Injection-site reactions were more common with degarelix than placebo (88% vs. 4%, respectively), as were elevations in hepatobiliary enzyme levels (44% vs. 8%). Two patients in the degarelix group were hospitalized as a result of increased suicidal ideation, suggesting “vigilance for the risk of exacerbating suicidality in predisposed individuals is warranted,” the researchers wrote.
“Most patients tolerated it well, many experienced what they thought were positive effects on sexuality, and a majority wanted to continue with the medicine after the study was over and have another injection,” Dr. Rahm said.
Sexual science milestone
In an accompanying editorial, Peer Briken, MD, of the Institute for Sex Research, Sexual Medicine, and Forensic Psychiatry at University Medical Centre, Hamburg, Germany, wrote that the innovative potential of this study should “not be underestimated.”
It has previously been thought that randomized, controlled trials were not possible because it might be unethical to withhold therapy from high-risk participants and thus risk sexual assaults on children in a control group, Dr. Briken noted.
With the current study, “the situation has changed, which marks a milestone in clinical sexual science and the field of forensic psychiatry,” he wrote.
However, the “great benefit” of the study, which is the proof of feasibility of a randomized, controlled trial in this special group of patients and use of a new drug, comes with some “important limitations,” he added.
Only three participants in each treatment group were in the high-risk subgroup. In addition, the most important long-term outcome criterion – reduction in recidivism in high-risk individuals – could not be investigated, he said.
Dr. Briken agreed with the investigators that risk of suicidal tendencies during rapid testosterone withdrawal requires attention.
Despite its limitations, this study is “certainly the most important contribution to the field of pharmacotherapy of pedophilic disorders since Rösler and Witztum’s study on GnRH agonists in 1998. Also, a relevant number of the study participants (58%) were in favor of further application,” he concluded.
The study was funded by the Swedish Society of Medicine, the Söderström-Königska Foundation, the Fredrik and Ingrid Thuring Foundation, the Centre for Psychiatric Research at Karolinska Institutet, the Gothenburg Society of Medicine, Skaraborg Hospital research unit, Region Stockholm, and the Swedish Society for Medical Research. Dr. Rahm and Dr. Briken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Testosterone-suppressing treatment with the gonadotropin-releasing hormone (GnRH) antagonist degarelix may reduce dynamic risk factors for sexual offense in men with pedophilic disorder, new research suggests.
In a first-of-its-kind randomized, controlled trial of 52 help-seeking men with the disorder, degarelix versus placebo significantly dampened two critical risk factors for committing abuse: high sexual desire and sexual attraction to children. In addition, effects were noticeable within 2 weeks.
“The medicine is quick-acting, not only on biological systems but also on thoughts and behavior,” coinvestigator and corresponding author Christoffer Rahm, MD, of the Centre for Psychiatry Research at Karolinska Institutet, Stockholm, said in an interview.
“The effect lasts and increases after 10 weeks, and especially so in the small group of high-risk individuals,” Dr. Rahm added.
The study findings were published in JAMA Psychiatry.
Opportunity for prevention
Although all men with pedophilic disorder do not commit a sexual offense, those who do generally report struggling with their sexual urges for 10 years before committing a sexual crime, the investigators noted.
This presents an opportunity for prevention by treating high-risk individuals without prior convictions. Effective treatment could prevent child sexual abuse and reduce psychosocial stress for the individual with pedophilic disorder, the researchers wrote.
GnRH antagonists are considered effective in reducing paraphilic symptoms, but their use has been limited to correctional settings. – and not just convicted men from prison and the probation system.
“It means the conclusions from the study are applicable to the patients you meet on sexual medicine and general psychiatry clinics too,” Dr. Rahm said.
The study included 52 men with a pedophilic disorder diagnosis and no contraindications to the intervention. All had contacted PrevenTell, the Swedish national telephone helpline for unwanted sexuality.
Half of the participants were randomly assigned to receive two subcutaneous 120-mg injections of degarelix acetate, while the other half received an equal volume of placebo.
The primary endpoint was efficacy at 2 weeks after injection in reducing a composite risk score of five domains for committing child sexual abuse; this risk score ranged from 0 to 15 points (each domain could be rated 0-3). Secondary endpoints included efficacy at 2 and 10 weeks in the composite score, each risk domain, quality of life, self-reported effects, and adverse events.
‘Positive effects’
At 2 weeks, the composite risk score decreased from 7.4 to 4.4 in the degarelix group and from 7.8 to 6.6 in the placebo group, which was a mean between-group difference of –1.8 (95% confidence interval, –3.2 to –0.5; P = .01).
Compared with placebo, the degarelix group also showed a decrease in the composite score at 10 weeks (−2.2; 95% CI, −3.6 to −0.7), in the domains of pedophilic disorder at 2 weeks (−0.7; 95% CI, −1.4 to 0.0) and 10 weeks (−1.1; 95% CI, −1.8 to −0.4), and in sexual preoccupation at 2 weeks (−0.7; 95% CI, −1.2 to −0.3) and 10 weeks (−0.8; 95% CI, −1.3 to −0.3).
There were no between-group differences in the other domains of self-rated risk, low empathy, and impaired self-regulation at 2 or 10 weeks, or in quality of life.
Injection-site reactions were more common with degarelix than placebo (88% vs. 4%, respectively), as were elevations in hepatobiliary enzyme levels (44% vs. 8%). Two patients in the degarelix group were hospitalized as a result of increased suicidal ideation, suggesting “vigilance for the risk of exacerbating suicidality in predisposed individuals is warranted,” the researchers wrote.
“Most patients tolerated it well, many experienced what they thought were positive effects on sexuality, and a majority wanted to continue with the medicine after the study was over and have another injection,” Dr. Rahm said.
Sexual science milestone
In an accompanying editorial, Peer Briken, MD, of the Institute for Sex Research, Sexual Medicine, and Forensic Psychiatry at University Medical Centre, Hamburg, Germany, wrote that the innovative potential of this study should “not be underestimated.”
It has previously been thought that randomized, controlled trials were not possible because it might be unethical to withhold therapy from high-risk participants and thus risk sexual assaults on children in a control group, Dr. Briken noted.
With the current study, “the situation has changed, which marks a milestone in clinical sexual science and the field of forensic psychiatry,” he wrote.
However, the “great benefit” of the study, which is the proof of feasibility of a randomized, controlled trial in this special group of patients and use of a new drug, comes with some “important limitations,” he added.
Only three participants in each treatment group were in the high-risk subgroup. In addition, the most important long-term outcome criterion – reduction in recidivism in high-risk individuals – could not be investigated, he said.
Dr. Briken agreed with the investigators that risk of suicidal tendencies during rapid testosterone withdrawal requires attention.
Despite its limitations, this study is “certainly the most important contribution to the field of pharmacotherapy of pedophilic disorders since Rösler and Witztum’s study on GnRH agonists in 1998. Also, a relevant number of the study participants (58%) were in favor of further application,” he concluded.
The study was funded by the Swedish Society of Medicine, the Söderström-Königska Foundation, the Fredrik and Ingrid Thuring Foundation, the Centre for Psychiatric Research at Karolinska Institutet, the Gothenburg Society of Medicine, Skaraborg Hospital research unit, Region Stockholm, and the Swedish Society for Medical Research. Dr. Rahm and Dr. Briken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Testosterone-suppressing treatment with the gonadotropin-releasing hormone (GnRH) antagonist degarelix may reduce dynamic risk factors for sexual offense in men with pedophilic disorder, new research suggests.
In a first-of-its-kind randomized, controlled trial of 52 help-seeking men with the disorder, degarelix versus placebo significantly dampened two critical risk factors for committing abuse: high sexual desire and sexual attraction to children. In addition, effects were noticeable within 2 weeks.
“The medicine is quick-acting, not only on biological systems but also on thoughts and behavior,” coinvestigator and corresponding author Christoffer Rahm, MD, of the Centre for Psychiatry Research at Karolinska Institutet, Stockholm, said in an interview.
“The effect lasts and increases after 10 weeks, and especially so in the small group of high-risk individuals,” Dr. Rahm added.
The study findings were published in JAMA Psychiatry.
Opportunity for prevention
Although all men with pedophilic disorder do not commit a sexual offense, those who do generally report struggling with their sexual urges for 10 years before committing a sexual crime, the investigators noted.
This presents an opportunity for prevention by treating high-risk individuals without prior convictions. Effective treatment could prevent child sexual abuse and reduce psychosocial stress for the individual with pedophilic disorder, the researchers wrote.
GnRH antagonists are considered effective in reducing paraphilic symptoms, but their use has been limited to correctional settings. – and not just convicted men from prison and the probation system.
“It means the conclusions from the study are applicable to the patients you meet on sexual medicine and general psychiatry clinics too,” Dr. Rahm said.
The study included 52 men with a pedophilic disorder diagnosis and no contraindications to the intervention. All had contacted PrevenTell, the Swedish national telephone helpline for unwanted sexuality.
Half of the participants were randomly assigned to receive two subcutaneous 120-mg injections of degarelix acetate, while the other half received an equal volume of placebo.
The primary endpoint was efficacy at 2 weeks after injection in reducing a composite risk score of five domains for committing child sexual abuse; this risk score ranged from 0 to 15 points (each domain could be rated 0-3). Secondary endpoints included efficacy at 2 and 10 weeks in the composite score, each risk domain, quality of life, self-reported effects, and adverse events.
‘Positive effects’
At 2 weeks, the composite risk score decreased from 7.4 to 4.4 in the degarelix group and from 7.8 to 6.6 in the placebo group, which was a mean between-group difference of –1.8 (95% confidence interval, –3.2 to –0.5; P = .01).
Compared with placebo, the degarelix group also showed a decrease in the composite score at 10 weeks (−2.2; 95% CI, −3.6 to −0.7), in the domains of pedophilic disorder at 2 weeks (−0.7; 95% CI, −1.4 to 0.0) and 10 weeks (−1.1; 95% CI, −1.8 to −0.4), and in sexual preoccupation at 2 weeks (−0.7; 95% CI, −1.2 to −0.3) and 10 weeks (−0.8; 95% CI, −1.3 to −0.3).
There were no between-group differences in the other domains of self-rated risk, low empathy, and impaired self-regulation at 2 or 10 weeks, or in quality of life.
Injection-site reactions were more common with degarelix than placebo (88% vs. 4%, respectively), as were elevations in hepatobiliary enzyme levels (44% vs. 8%). Two patients in the degarelix group were hospitalized as a result of increased suicidal ideation, suggesting “vigilance for the risk of exacerbating suicidality in predisposed individuals is warranted,” the researchers wrote.
“Most patients tolerated it well, many experienced what they thought were positive effects on sexuality, and a majority wanted to continue with the medicine after the study was over and have another injection,” Dr. Rahm said.
Sexual science milestone
In an accompanying editorial, Peer Briken, MD, of the Institute for Sex Research, Sexual Medicine, and Forensic Psychiatry at University Medical Centre, Hamburg, Germany, wrote that the innovative potential of this study should “not be underestimated.”
It has previously been thought that randomized, controlled trials were not possible because it might be unethical to withhold therapy from high-risk participants and thus risk sexual assaults on children in a control group, Dr. Briken noted.
With the current study, “the situation has changed, which marks a milestone in clinical sexual science and the field of forensic psychiatry,” he wrote.
However, the “great benefit” of the study, which is the proof of feasibility of a randomized, controlled trial in this special group of patients and use of a new drug, comes with some “important limitations,” he added.
Only three participants in each treatment group were in the high-risk subgroup. In addition, the most important long-term outcome criterion – reduction in recidivism in high-risk individuals – could not be investigated, he said.
Dr. Briken agreed with the investigators that risk of suicidal tendencies during rapid testosterone withdrawal requires attention.
Despite its limitations, this study is “certainly the most important contribution to the field of pharmacotherapy of pedophilic disorders since Rösler and Witztum’s study on GnRH agonists in 1998. Also, a relevant number of the study participants (58%) were in favor of further application,” he concluded.
The study was funded by the Swedish Society of Medicine, the Söderström-Königska Foundation, the Fredrik and Ingrid Thuring Foundation, the Centre for Psychiatric Research at Karolinska Institutet, the Gothenburg Society of Medicine, Skaraborg Hospital research unit, Region Stockholm, and the Swedish Society for Medical Research. Dr. Rahm and Dr. Briken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Transplant beats bortezomib-based therapy for MM, but questions remain
While upfront autologous transplantation has bested bortezomib-based intensification therapy in a large, randomized multiple myeloma (MM) trial, investigators and observers say more research will be needed to determine the optimal treatment strategy in the era of novel agents.
Autologous hematopoietic stem cell transplantation (HSCT) extended progression-free survival by almost 15 months compared with bortezomib, melphalan, and prednisone (VMP) intensification therapy for the treatment of newly diagnosed multiple myeloma, according to results of the randomized, phase 3 trial of 1,503 patients enrolled at 172 centers in the European Myeloma Network.
That finding could provide more fodder for the ongoing debate over the role of upfront autologous HSCT as a gold-standard intensification treatment for patients who can tolerate myeloablative doses of chemotherapy in light of the proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies that are now available.
However, the study is not without limitations, including the use of bortezomib, cyclophosphamide, and dexamethasone (VCD) as induction therapy, according to the investigators, led by Michele Cavo, MD, with the Seràgnoli Institute of Hematology at Bologna (Italy) University.
The VCD regimen was one of the most frequently used induction regimens back when the trial was designed, but now it’s considered “less optimal” versus regimens such as bortezomib, lenalidomide, and dexamethasone (VRD), according to Dr. Cavo and coinvestigators.
Besides, the field is moving forward based on clinical trials of “highly active” daratumumab-based four-drug regimens, which appear to enhance rates of response and minimal residual disease (MRD) negativity when given as induction before autologous HSCT and as consolidation afterward, they said.
“Final results from these studies should be awaited before a shift from routine use of upfront autologous HSCT to delayed HSCT or alternative treatment strategies driven by MRD status can be offered to patients with newly diagnosed multiple myeloma who are fit for high-dose chemotherapy,” Dr. Cavo and colleagues noted in their report, available in The Lancet Hematology.
The multicenter, randomized, open-label, phase 3 study by Dr. Cavo and coinvestigators, known as EMN02/HO95, included patients up to 65 years of age with symptomatic multiple myeloma, measurable disease, and WHO performance of 0 to 2. Patients were treated with VCD induction therapy, followed by randomization to either VMP or autologous HSCT after high-dose melphalan. In a second randomization, patients were assigned to either VRD consolidation therapy or no consolidation. All patients then received lenalidomide maintenance therapy until progression.
Median progression-free survival was 56.7 months in patients initially randomized to HSCT, compared with 41.9 months for MP (hazard ratio, 0.73; 95% confidence interval, 0.62-0.85; P = .0001), according to the investigators, who said that finding supports the value of HSCT “even in the era of highly active novel agents.”
Turning to results of the second randomization, Dr. Cavo and colleagues said the VRD consolidation strategy resulted in median progression-free survival that was significantly improved versus no consolidation, at 58.9 months and 45.5 months, respectively (P = .014).
While this is an important study, the added benefit of HSCT intensification therapy is in question given the high rates of MRD being reported for potent, daratumumab-based four-drug combination regimens, according to a related commentary by Omar Nadeem, MD, and Irene M. Ghobrial, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston.
“We are entering an era in which novel combinations have shown unprecedented efficacy and future studies with or without HSCT will be needed to answer these key questions,” wrote Dr. Nadeem and Dr. Ghobrial.
Dr. Cavo and colleagues said the estimated 5-year rate of overall survival in EMN02/HO95 was “comparable” between arms, at 75% for the HSCT strategy and 72% for VMP.
“Although this suggests that delaying HSCT to a later time is not harmful, a substantial proportion of patients may become ineligible for high-dose melphalan at first relapse,” they said, noting that 63% of VMP-treated patients went on to receive salvage HSCT.
Further follow-up could demonstrate a survival advantage, as seen in other studies, they added.
Dr. Cavo reported that he has received honoraria from multiple pharmaceutical companies, and is a member of speakers bureaus for Janssen and Celgene. Dr. Nadeem and Dr. Ghobrial reported serving on the advisory boards of multiple pharmaceutical companies.
SOURCE: Cavo M et al. Lancet Haematol. 2020 Apr 30. doi: 10.1016/S2352-3026(20)30099-5.
While upfront autologous transplantation has bested bortezomib-based intensification therapy in a large, randomized multiple myeloma (MM) trial, investigators and observers say more research will be needed to determine the optimal treatment strategy in the era of novel agents.
Autologous hematopoietic stem cell transplantation (HSCT) extended progression-free survival by almost 15 months compared with bortezomib, melphalan, and prednisone (VMP) intensification therapy for the treatment of newly diagnosed multiple myeloma, according to results of the randomized, phase 3 trial of 1,503 patients enrolled at 172 centers in the European Myeloma Network.
That finding could provide more fodder for the ongoing debate over the role of upfront autologous HSCT as a gold-standard intensification treatment for patients who can tolerate myeloablative doses of chemotherapy in light of the proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies that are now available.
However, the study is not without limitations, including the use of bortezomib, cyclophosphamide, and dexamethasone (VCD) as induction therapy, according to the investigators, led by Michele Cavo, MD, with the Seràgnoli Institute of Hematology at Bologna (Italy) University.
The VCD regimen was one of the most frequently used induction regimens back when the trial was designed, but now it’s considered “less optimal” versus regimens such as bortezomib, lenalidomide, and dexamethasone (VRD), according to Dr. Cavo and coinvestigators.
Besides, the field is moving forward based on clinical trials of “highly active” daratumumab-based four-drug regimens, which appear to enhance rates of response and minimal residual disease (MRD) negativity when given as induction before autologous HSCT and as consolidation afterward, they said.
“Final results from these studies should be awaited before a shift from routine use of upfront autologous HSCT to delayed HSCT or alternative treatment strategies driven by MRD status can be offered to patients with newly diagnosed multiple myeloma who are fit for high-dose chemotherapy,” Dr. Cavo and colleagues noted in their report, available in The Lancet Hematology.
The multicenter, randomized, open-label, phase 3 study by Dr. Cavo and coinvestigators, known as EMN02/HO95, included patients up to 65 years of age with symptomatic multiple myeloma, measurable disease, and WHO performance of 0 to 2. Patients were treated with VCD induction therapy, followed by randomization to either VMP or autologous HSCT after high-dose melphalan. In a second randomization, patients were assigned to either VRD consolidation therapy or no consolidation. All patients then received lenalidomide maintenance therapy until progression.
Median progression-free survival was 56.7 months in patients initially randomized to HSCT, compared with 41.9 months for MP (hazard ratio, 0.73; 95% confidence interval, 0.62-0.85; P = .0001), according to the investigators, who said that finding supports the value of HSCT “even in the era of highly active novel agents.”
Turning to results of the second randomization, Dr. Cavo and colleagues said the VRD consolidation strategy resulted in median progression-free survival that was significantly improved versus no consolidation, at 58.9 months and 45.5 months, respectively (P = .014).
While this is an important study, the added benefit of HSCT intensification therapy is in question given the high rates of MRD being reported for potent, daratumumab-based four-drug combination regimens, according to a related commentary by Omar Nadeem, MD, and Irene M. Ghobrial, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston.
“We are entering an era in which novel combinations have shown unprecedented efficacy and future studies with or without HSCT will be needed to answer these key questions,” wrote Dr. Nadeem and Dr. Ghobrial.
Dr. Cavo and colleagues said the estimated 5-year rate of overall survival in EMN02/HO95 was “comparable” between arms, at 75% for the HSCT strategy and 72% for VMP.
“Although this suggests that delaying HSCT to a later time is not harmful, a substantial proportion of patients may become ineligible for high-dose melphalan at first relapse,” they said, noting that 63% of VMP-treated patients went on to receive salvage HSCT.
Further follow-up could demonstrate a survival advantage, as seen in other studies, they added.
Dr. Cavo reported that he has received honoraria from multiple pharmaceutical companies, and is a member of speakers bureaus for Janssen and Celgene. Dr. Nadeem and Dr. Ghobrial reported serving on the advisory boards of multiple pharmaceutical companies.
SOURCE: Cavo M et al. Lancet Haematol. 2020 Apr 30. doi: 10.1016/S2352-3026(20)30099-5.
While upfront autologous transplantation has bested bortezomib-based intensification therapy in a large, randomized multiple myeloma (MM) trial, investigators and observers say more research will be needed to determine the optimal treatment strategy in the era of novel agents.
Autologous hematopoietic stem cell transplantation (HSCT) extended progression-free survival by almost 15 months compared with bortezomib, melphalan, and prednisone (VMP) intensification therapy for the treatment of newly diagnosed multiple myeloma, according to results of the randomized, phase 3 trial of 1,503 patients enrolled at 172 centers in the European Myeloma Network.
That finding could provide more fodder for the ongoing debate over the role of upfront autologous HSCT as a gold-standard intensification treatment for patients who can tolerate myeloablative doses of chemotherapy in light of the proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies that are now available.
However, the study is not without limitations, including the use of bortezomib, cyclophosphamide, and dexamethasone (VCD) as induction therapy, according to the investigators, led by Michele Cavo, MD, with the Seràgnoli Institute of Hematology at Bologna (Italy) University.
The VCD regimen was one of the most frequently used induction regimens back when the trial was designed, but now it’s considered “less optimal” versus regimens such as bortezomib, lenalidomide, and dexamethasone (VRD), according to Dr. Cavo and coinvestigators.
Besides, the field is moving forward based on clinical trials of “highly active” daratumumab-based four-drug regimens, which appear to enhance rates of response and minimal residual disease (MRD) negativity when given as induction before autologous HSCT and as consolidation afterward, they said.
“Final results from these studies should be awaited before a shift from routine use of upfront autologous HSCT to delayed HSCT or alternative treatment strategies driven by MRD status can be offered to patients with newly diagnosed multiple myeloma who are fit for high-dose chemotherapy,” Dr. Cavo and colleagues noted in their report, available in The Lancet Hematology.
The multicenter, randomized, open-label, phase 3 study by Dr. Cavo and coinvestigators, known as EMN02/HO95, included patients up to 65 years of age with symptomatic multiple myeloma, measurable disease, and WHO performance of 0 to 2. Patients were treated with VCD induction therapy, followed by randomization to either VMP or autologous HSCT after high-dose melphalan. In a second randomization, patients were assigned to either VRD consolidation therapy or no consolidation. All patients then received lenalidomide maintenance therapy until progression.
Median progression-free survival was 56.7 months in patients initially randomized to HSCT, compared with 41.9 months for MP (hazard ratio, 0.73; 95% confidence interval, 0.62-0.85; P = .0001), according to the investigators, who said that finding supports the value of HSCT “even in the era of highly active novel agents.”
Turning to results of the second randomization, Dr. Cavo and colleagues said the VRD consolidation strategy resulted in median progression-free survival that was significantly improved versus no consolidation, at 58.9 months and 45.5 months, respectively (P = .014).
While this is an important study, the added benefit of HSCT intensification therapy is in question given the high rates of MRD being reported for potent, daratumumab-based four-drug combination regimens, according to a related commentary by Omar Nadeem, MD, and Irene M. Ghobrial, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston.
“We are entering an era in which novel combinations have shown unprecedented efficacy and future studies with or without HSCT will be needed to answer these key questions,” wrote Dr. Nadeem and Dr. Ghobrial.
Dr. Cavo and colleagues said the estimated 5-year rate of overall survival in EMN02/HO95 was “comparable” between arms, at 75% for the HSCT strategy and 72% for VMP.
“Although this suggests that delaying HSCT to a later time is not harmful, a substantial proportion of patients may become ineligible for high-dose melphalan at first relapse,” they said, noting that 63% of VMP-treated patients went on to receive salvage HSCT.
Further follow-up could demonstrate a survival advantage, as seen in other studies, they added.
Dr. Cavo reported that he has received honoraria from multiple pharmaceutical companies, and is a member of speakers bureaus for Janssen and Celgene. Dr. Nadeem and Dr. Ghobrial reported serving on the advisory boards of multiple pharmaceutical companies.
SOURCE: Cavo M et al. Lancet Haematol. 2020 Apr 30. doi: 10.1016/S2352-3026(20)30099-5.
FROM THE LANCET HEMATOLOGY
Starting school later in the morning improves adolescents’ sleep
according to results from a cohort study.
For their research published in JAMA Pediatrics, Rachel Widome, PhD, of the University of Minnesota, Minneapolis, and colleagues followed a cohort of students at five public high schools in suburban and rural Minneapolis, randomly selecting 455 (225 girls; mean age, 15 years) for wrist actigraphy to track sleep and activity.
The students were followed up over 2 years, from 2016 to 2018. Sleep and activity were monitored at baseline, at year 1, and at year 2. Baseline monitoring lasted a month, and each follow-up monitoring period lasted over 2 months.
Although all the high schools in the study had early start times when the study began, two moved within the first year to delay their starting times to after 8:30 a.m., after a decision by the local school district. The other three schools retained start times of 7:30 a.m. This allowed investigators to compare students’ sleep patterns between start times for an extended period.
Dr. Widome and colleagues found significant improvements in sleep at 1 year that did not attenuate in the second year. At the end of year 2, students in the delayed-start schools slept 43 minutes more on weeknights than their early-starting peers (95% confidence interval, 25-61, P < .001.) The investigators did not see significant between-group differences in weeknight bedtimes. On weekends, students in the delayed-start group slept a mean 34 minutes less at year 2 (95% CI, –65 to –3, P = .03) than their peers in the early groups.
The researchers described the study’s design, a natural experiment with long follow-up and objectively measured sleep data, as its key strength. “No previous studies have been performed of sufficient quality to conclude that later start times cause students to get more sleep and that this effect can be sustained,” they concluded.
In an editorial comment, Erika R. Cheng, PhD, and Aaron E. Carroll, MD, of Indiana University, Indianapolis, wrote that the study provides strong evidence that delaying early school start times “would help adolescents get the sleep they need to thrive,” and belies the commonly held argument that delayed school times would merely lead to them staying awake later on school nights.
Adolescents “experience natural circadian and physiological brain changes that shift their sleep preference to go to bed and wake up later than adults or younger children,” Dr. Cheng and Dr. Carroll noted, with 12th graders’ bedtimes typically after 11 p.m. on weekdays. Regardless, “more than 40% of high schools in the United States start before 8 a.m., and more than 20% of middle schools start at 7:45 a.m. or earlier.”
Dr. Cheng and Dr. Carroll cautioned that the population in this study comprised “relatively affluent students and schools,” and that there were “socioeconomic and racial differences in student characteristics between schools that did and did not adopt the later start times.” For instance, they noted, nearly 90% of students in the delayed-start schools reported having at least one college-educated parent, while in the comparison schools fewer than 75% did. Unmeasured characteristics associated with parent education may have “influenced the school district’s decision to delay schools’ start times and had an effect on student sleep duration.”
Dr. Widome and colleagues’ study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the authors received support from a grant from the Minnesota Population Center. One coauthor acknowledged receiving a consulting fee from Jazz Pharmaceuticals. Dr. Cheng and Dr. Carroll disclosed no relevant conflicts of interest.
SOURCES: Widome R et al. JAMA Pedatr. 2020 Apr 27. doi: 10.1001/jamapediatrics.2020.0344; Cheng ER, Carroll AE. JAMA Pediatr. 2020 Apr 27. oi: 10.1001/jamapediatrics.2020.0351.
according to results from a cohort study.
For their research published in JAMA Pediatrics, Rachel Widome, PhD, of the University of Minnesota, Minneapolis, and colleagues followed a cohort of students at five public high schools in suburban and rural Minneapolis, randomly selecting 455 (225 girls; mean age, 15 years) for wrist actigraphy to track sleep and activity.
The students were followed up over 2 years, from 2016 to 2018. Sleep and activity were monitored at baseline, at year 1, and at year 2. Baseline monitoring lasted a month, and each follow-up monitoring period lasted over 2 months.
Although all the high schools in the study had early start times when the study began, two moved within the first year to delay their starting times to after 8:30 a.m., after a decision by the local school district. The other three schools retained start times of 7:30 a.m. This allowed investigators to compare students’ sleep patterns between start times for an extended period.
Dr. Widome and colleagues found significant improvements in sleep at 1 year that did not attenuate in the second year. At the end of year 2, students in the delayed-start schools slept 43 minutes more on weeknights than their early-starting peers (95% confidence interval, 25-61, P < .001.) The investigators did not see significant between-group differences in weeknight bedtimes. On weekends, students in the delayed-start group slept a mean 34 minutes less at year 2 (95% CI, –65 to –3, P = .03) than their peers in the early groups.
The researchers described the study’s design, a natural experiment with long follow-up and objectively measured sleep data, as its key strength. “No previous studies have been performed of sufficient quality to conclude that later start times cause students to get more sleep and that this effect can be sustained,” they concluded.
In an editorial comment, Erika R. Cheng, PhD, and Aaron E. Carroll, MD, of Indiana University, Indianapolis, wrote that the study provides strong evidence that delaying early school start times “would help adolescents get the sleep they need to thrive,” and belies the commonly held argument that delayed school times would merely lead to them staying awake later on school nights.
Adolescents “experience natural circadian and physiological brain changes that shift their sleep preference to go to bed and wake up later than adults or younger children,” Dr. Cheng and Dr. Carroll noted, with 12th graders’ bedtimes typically after 11 p.m. on weekdays. Regardless, “more than 40% of high schools in the United States start before 8 a.m., and more than 20% of middle schools start at 7:45 a.m. or earlier.”
Dr. Cheng and Dr. Carroll cautioned that the population in this study comprised “relatively affluent students and schools,” and that there were “socioeconomic and racial differences in student characteristics between schools that did and did not adopt the later start times.” For instance, they noted, nearly 90% of students in the delayed-start schools reported having at least one college-educated parent, while in the comparison schools fewer than 75% did. Unmeasured characteristics associated with parent education may have “influenced the school district’s decision to delay schools’ start times and had an effect on student sleep duration.”
Dr. Widome and colleagues’ study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the authors received support from a grant from the Minnesota Population Center. One coauthor acknowledged receiving a consulting fee from Jazz Pharmaceuticals. Dr. Cheng and Dr. Carroll disclosed no relevant conflicts of interest.
SOURCES: Widome R et al. JAMA Pedatr. 2020 Apr 27. doi: 10.1001/jamapediatrics.2020.0344; Cheng ER, Carroll AE. JAMA Pediatr. 2020 Apr 27. oi: 10.1001/jamapediatrics.2020.0351.
according to results from a cohort study.
For their research published in JAMA Pediatrics, Rachel Widome, PhD, of the University of Minnesota, Minneapolis, and colleagues followed a cohort of students at five public high schools in suburban and rural Minneapolis, randomly selecting 455 (225 girls; mean age, 15 years) for wrist actigraphy to track sleep and activity.
The students were followed up over 2 years, from 2016 to 2018. Sleep and activity were monitored at baseline, at year 1, and at year 2. Baseline monitoring lasted a month, and each follow-up monitoring period lasted over 2 months.
Although all the high schools in the study had early start times when the study began, two moved within the first year to delay their starting times to after 8:30 a.m., after a decision by the local school district. The other three schools retained start times of 7:30 a.m. This allowed investigators to compare students’ sleep patterns between start times for an extended period.
Dr. Widome and colleagues found significant improvements in sleep at 1 year that did not attenuate in the second year. At the end of year 2, students in the delayed-start schools slept 43 minutes more on weeknights than their early-starting peers (95% confidence interval, 25-61, P < .001.) The investigators did not see significant between-group differences in weeknight bedtimes. On weekends, students in the delayed-start group slept a mean 34 minutes less at year 2 (95% CI, –65 to –3, P = .03) than their peers in the early groups.
The researchers described the study’s design, a natural experiment with long follow-up and objectively measured sleep data, as its key strength. “No previous studies have been performed of sufficient quality to conclude that later start times cause students to get more sleep and that this effect can be sustained,” they concluded.
In an editorial comment, Erika R. Cheng, PhD, and Aaron E. Carroll, MD, of Indiana University, Indianapolis, wrote that the study provides strong evidence that delaying early school start times “would help adolescents get the sleep they need to thrive,” and belies the commonly held argument that delayed school times would merely lead to them staying awake later on school nights.
Adolescents “experience natural circadian and physiological brain changes that shift their sleep preference to go to bed and wake up later than adults or younger children,” Dr. Cheng and Dr. Carroll noted, with 12th graders’ bedtimes typically after 11 p.m. on weekdays. Regardless, “more than 40% of high schools in the United States start before 8 a.m., and more than 20% of middle schools start at 7:45 a.m. or earlier.”
Dr. Cheng and Dr. Carroll cautioned that the population in this study comprised “relatively affluent students and schools,” and that there were “socioeconomic and racial differences in student characteristics between schools that did and did not adopt the later start times.” For instance, they noted, nearly 90% of students in the delayed-start schools reported having at least one college-educated parent, while in the comparison schools fewer than 75% did. Unmeasured characteristics associated with parent education may have “influenced the school district’s decision to delay schools’ start times and had an effect on student sleep duration.”
Dr. Widome and colleagues’ study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the authors received support from a grant from the Minnesota Population Center. One coauthor acknowledged receiving a consulting fee from Jazz Pharmaceuticals. Dr. Cheng and Dr. Carroll disclosed no relevant conflicts of interest.
SOURCES: Widome R et al. JAMA Pedatr. 2020 Apr 27. doi: 10.1001/jamapediatrics.2020.0344; Cheng ER, Carroll AE. JAMA Pediatr. 2020 Apr 27. oi: 10.1001/jamapediatrics.2020.0351.
FROM JAMA PEDIATRICS
Patients with epilepsy may underreport seizures, survey finds
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
according to survey results presented online as part of the 2020 American Academy of Neurology Science Highlights.
Clinicians, for their part, may underestimate the number of seizures that go unreported. This disconnect may contribute to complacency about epilepsy treatment regimens among patients, caregivers, and health care professionals (HCPs), despite continuations in seizures. “Improved reporting of all seizure occurrences and more frequent discussion of potential treatment changes, initiated by all groups, may be needed to optimize treatment outcomes,” said Patricia E. Penovich, MD, a neurologist at Minnesota Epilepsy Group in St. Paul, and colleagues.
To evaluate treatment complacency among adult patients with epilepsy, caregivers, and HCPs, Dr. Penovich and collaborators analyzed data from the STEP survey (Seize the Truth about Epilepsy Perceptions), which was conducted between February and March 2019. In all, 400 adults with epilepsy, 201 caregivers, and 258 HCPs completed the survey. The HCPs included 96 epileptologists, 112 general neurologists, and 50 nurse practitioners or physician assistants.
Patients had an average epilepsy duration of 16 years, and 58% were on at least their third antiepileptic drug (AED). In the past year, 52% of patients had 1-9 seizures, and 31% had 10 or more seizures. “Patients estimated reporting 45% of their seizures to their HCPs, and for the seizures not reported, 57% provided reasoning that they were not serious enough to mention,” reported Dr. Penovich and colleagues. “Alternatively, HCPs estimated that patients report 73% of seizures.”
Survey participants most frequently selected HCPs as the ones to initiate conversations about changing AEDs or increasing dosage. “Patient-initiated discussions were reported by 39% of patients for changing AEDs and 27% of patients for increasing AED dosage; 25% of patients reported they were likely to ask their HCP about changing treatments in the next 12 months,” the authors said. Discussion of vagus nerve stimulation was reported by 21% of HCPs, and 10% reported discussion of responsive neurostimulation. HCPs also discussed surgical options such as hemispherectomy (3%), corpus callosotomy and multiple subpial transection (4%), lobe resection (8%), and lesionectomy (11%).
Among patients with 13 or more seizures per year, 27% reported referral to an epilepsy center. Most survey participants – 61% of patients and HCPs and 68% of caregivers – “reported a desire for a treatment map that tells patients to see an epileptologist/specialist as soon as they have symptoms,” the researchers said.
“What we would like to think is that we are getting the whole scoop and the honest scoop” about seizure activity, Dr. Penovich said in an interview. “What this shows us is that that’s probably not always true. Some health care providers understand that the patients do not tell them everything,” but the degree of seizure underreporting may be surprising.
Dr. Penovich has seen this phenomenon in practice. In some cases, caregivers return to the office to explain that a patient did not report all of their seizures. Other patients may omit entire days of seizures in their diaries as an oversight. In addition, patients may not report seizures to avoid having a driver’s license revoked. In some instances, clinicians may not take the time to discuss seizure activity in detail.
Having an accurate picture of seizure activity is an “important part of working with our patients, particularly when we are trying to get them to the point of being seizure free,” said Dr. Penovich.
Failing a first or second AED indicates a greater likelihood that medication will not stop a patient’s seizures, “but it does not mean that you will not be controlled,” Dr. Penovich said. More medications, surgical options, and investigative treatments have become available. Still, AED trials should not prevent a timely referral to an epilepsy center. “You don’t need to go through 10 or 15 before you get referred” to an epilepsy center, she said.
The STEP survey was conducted by Kantar Health on behalf of SK Life Science. Dr. Penovich has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from SK Life Science, Neurelis, GW Pharmaceuticals, Engage Therapeutics, and UCB Pharma. A coauthor was employed by Kantar Health. Other coauthors disclosed compensation from SK Life Science and various pharmaceutical companies.
SOURCE: Penovich PE et al. AAN 2020, Abstract S59.007.
FROM AAN 2020
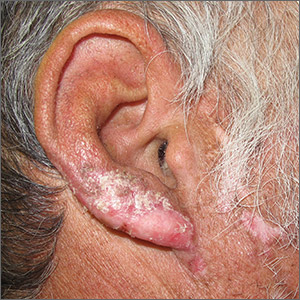
Ear and nose plaques
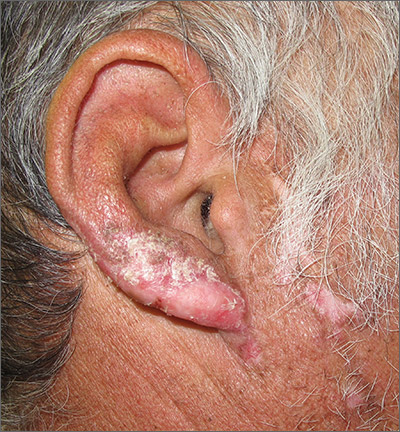
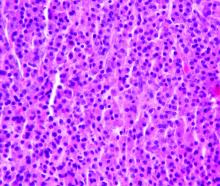
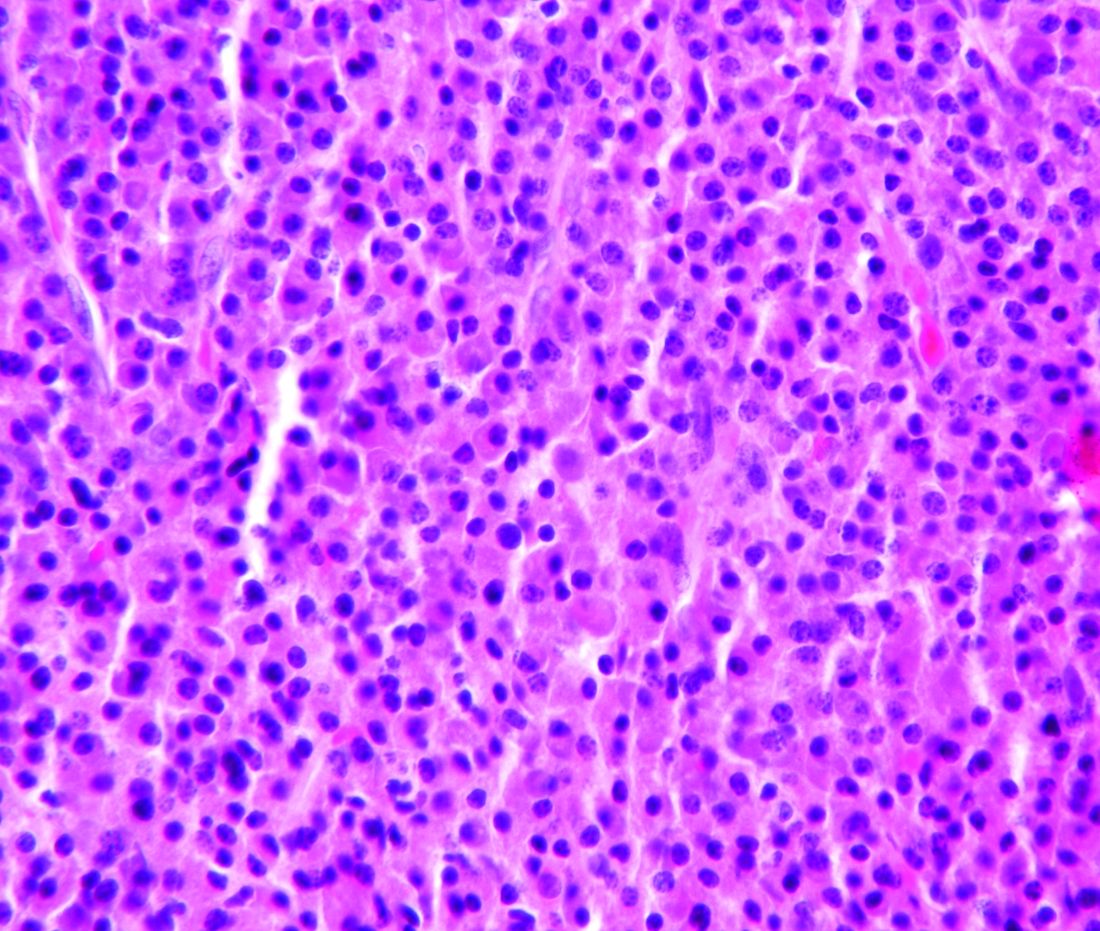
Based on the clinical appearance of hyperpigmented and hypopigmented scaly plaques in sun-exposed facial areas (with associated scarring and follicular plugging in affected lesions), the patient was given a diagnosis of discoid lupus erythematosus. The diagnosis was confirmed with a punch biopsy that showed characteristic interface dermatitis with superficial and deep perivascular and peri-adnexal lymphocytes. Direct immunofluorescence showed a band of C3 at the dermo-epidermal junction, consistent with various forms of cutaneous lupus.
A review of systems was negative for signs of systemic disease, including a lack of weakness, joint pain/swelling, fever, or known blood clots. An antinuclear antibody panel was also negative, largely excluding systemic disease.
Discoid lupus erythematosus is a form of cutaneous lupus. Patients often are genetically predisposed to autoimmune disease, and disease may be triggered by sun exposure. Up to 80% of patients develop lesions that are limited to the head and neck. Up to 25% of patients develop systemic lupus erythematosus that may occur months—or even decades—after the initial diagnosis. Lesions may lead to longstanding scars; this cannot always be prevented.
Two- to 3-week trials of topical potent or superpotent steroids applied to affected plaques may lead to clearance. Additionally, lesions may be treated with intralesional triamcinolone 3 mg/mL to 10 mg/mL and repeated every 3 to 4 weeks. Systemic therapies include hydroxychloroquine, chloroquine, retinoids, steroid sparing immunomodulators, and rarely prednisone.
This patient tolerated topical therapy well and though some scars remained, he was able to control flares with 2- to 3-week courses of clobetasol 0.05% twice daily. He is followed 2 to 3 times annually and has not developed signs or serologic findings of systemic disease over several years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Jessop S, Whitelaw DA, Grainge MJ, et al. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954.

Based on the clinical appearance of hyperpigmented and hypopigmented scaly plaques in sun-exposed facial areas (with associated scarring and follicular plugging in affected lesions), the patient was given a diagnosis of discoid lupus erythematosus. The diagnosis was confirmed with a punch biopsy that showed characteristic interface dermatitis with superficial and deep perivascular and peri-adnexal lymphocytes. Direct immunofluorescence showed a band of C3 at the dermo-epidermal junction, consistent with various forms of cutaneous lupus.
A review of systems was negative for signs of systemic disease, including a lack of weakness, joint pain/swelling, fever, or known blood clots. An antinuclear antibody panel was also negative, largely excluding systemic disease.
Discoid lupus erythematosus is a form of cutaneous lupus. Patients often are genetically predisposed to autoimmune disease, and disease may be triggered by sun exposure. Up to 80% of patients develop lesions that are limited to the head and neck. Up to 25% of patients develop systemic lupus erythematosus that may occur months—or even decades—after the initial diagnosis. Lesions may lead to longstanding scars; this cannot always be prevented.
Two- to 3-week trials of topical potent or superpotent steroids applied to affected plaques may lead to clearance. Additionally, lesions may be treated with intralesional triamcinolone 3 mg/mL to 10 mg/mL and repeated every 3 to 4 weeks. Systemic therapies include hydroxychloroquine, chloroquine, retinoids, steroid sparing immunomodulators, and rarely prednisone.
This patient tolerated topical therapy well and though some scars remained, he was able to control flares with 2- to 3-week courses of clobetasol 0.05% twice daily. He is followed 2 to 3 times annually and has not developed signs or serologic findings of systemic disease over several years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Based on the clinical appearance of hyperpigmented and hypopigmented scaly plaques in sun-exposed facial areas (with associated scarring and follicular plugging in affected lesions), the patient was given a diagnosis of discoid lupus erythematosus. The diagnosis was confirmed with a punch biopsy that showed characteristic interface dermatitis with superficial and deep perivascular and peri-adnexal lymphocytes. Direct immunofluorescence showed a band of C3 at the dermo-epidermal junction, consistent with various forms of cutaneous lupus.
A review of systems was negative for signs of systemic disease, including a lack of weakness, joint pain/swelling, fever, or known blood clots. An antinuclear antibody panel was also negative, largely excluding systemic disease.
Discoid lupus erythematosus is a form of cutaneous lupus. Patients often are genetically predisposed to autoimmune disease, and disease may be triggered by sun exposure. Up to 80% of patients develop lesions that are limited to the head and neck. Up to 25% of patients develop systemic lupus erythematosus that may occur months—or even decades—after the initial diagnosis. Lesions may lead to longstanding scars; this cannot always be prevented.
Two- to 3-week trials of topical potent or superpotent steroids applied to affected plaques may lead to clearance. Additionally, lesions may be treated with intralesional triamcinolone 3 mg/mL to 10 mg/mL and repeated every 3 to 4 weeks. Systemic therapies include hydroxychloroquine, chloroquine, retinoids, steroid sparing immunomodulators, and rarely prednisone.
This patient tolerated topical therapy well and though some scars remained, he was able to control flares with 2- to 3-week courses of clobetasol 0.05% twice daily. He is followed 2 to 3 times annually and has not developed signs or serologic findings of systemic disease over several years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Jessop S, Whitelaw DA, Grainge MJ, et al. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954.
Jessop S, Whitelaw DA, Grainge MJ, et al. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954.
NSCLC: FDA approves capmatinib and companion assay
as detected by an FDA-approved test.
The FDA also approved the FoundationOne CDx assay (F1CDx) as a companion diagnostic for capmatinib. F1CDx is a next-generation sequencing-based, in vitro diagnostic device that detects several mutations, including MET exon 14 skipping mutations.
Capmatinib is a selective, reversible inhibitor of MET tyrosine kinase and the first treatment FDA-approved for NSCLC with MET exon 14 skipping mutations.
Capmatinib was granted accelerated approval based on overall response rate and response duration in the GEOMETRY mono-1 trial, the FDA said. Results from this trial were recently presented at the AACR Virtual Annual Meeting I.
The phase 2 trial enrolled 97 patients with metastatic NSCLC and confirmed MET exon 14 skipping mutations, 69 of whom were previously treated and 28 of whom were treatment naive. The patients received capmatinib at 400 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 68% in the treatment-naive patients and 41% in the previously treated patients. The median duration of response was 12.6 months and 9.7 months, respectively, according to the FDA.
The most common adverse events (occurring in at least 20% of patients) were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite.
The full prescribing information for capmatinib is available for download from the FDA website.
The FDA granted the approval of capmatinib to Novartis Pharmaceuticals Corporation and the approval of the F1CDx companion diagnostic to Foundation Medicine.
as detected by an FDA-approved test.
The FDA also approved the FoundationOne CDx assay (F1CDx) as a companion diagnostic for capmatinib. F1CDx is a next-generation sequencing-based, in vitro diagnostic device that detects several mutations, including MET exon 14 skipping mutations.
Capmatinib is a selective, reversible inhibitor of MET tyrosine kinase and the first treatment FDA-approved for NSCLC with MET exon 14 skipping mutations.
Capmatinib was granted accelerated approval based on overall response rate and response duration in the GEOMETRY mono-1 trial, the FDA said. Results from this trial were recently presented at the AACR Virtual Annual Meeting I.
The phase 2 trial enrolled 97 patients with metastatic NSCLC and confirmed MET exon 14 skipping mutations, 69 of whom were previously treated and 28 of whom were treatment naive. The patients received capmatinib at 400 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 68% in the treatment-naive patients and 41% in the previously treated patients. The median duration of response was 12.6 months and 9.7 months, respectively, according to the FDA.
The most common adverse events (occurring in at least 20% of patients) were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite.
The full prescribing information for capmatinib is available for download from the FDA website.
The FDA granted the approval of capmatinib to Novartis Pharmaceuticals Corporation and the approval of the F1CDx companion diagnostic to Foundation Medicine.
as detected by an FDA-approved test.
The FDA also approved the FoundationOne CDx assay (F1CDx) as a companion diagnostic for capmatinib. F1CDx is a next-generation sequencing-based, in vitro diagnostic device that detects several mutations, including MET exon 14 skipping mutations.
Capmatinib is a selective, reversible inhibitor of MET tyrosine kinase and the first treatment FDA-approved for NSCLC with MET exon 14 skipping mutations.
Capmatinib was granted accelerated approval based on overall response rate and response duration in the GEOMETRY mono-1 trial, the FDA said. Results from this trial were recently presented at the AACR Virtual Annual Meeting I.
The phase 2 trial enrolled 97 patients with metastatic NSCLC and confirmed MET exon 14 skipping mutations, 69 of whom were previously treated and 28 of whom were treatment naive. The patients received capmatinib at 400 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate was 68% in the treatment-naive patients and 41% in the previously treated patients. The median duration of response was 12.6 months and 9.7 months, respectively, according to the FDA.
The most common adverse events (occurring in at least 20% of patients) were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite.
The full prescribing information for capmatinib is available for download from the FDA website.
The FDA granted the approval of capmatinib to Novartis Pharmaceuticals Corporation and the approval of the F1CDx companion diagnostic to Foundation Medicine.
Capmatinib shows impressive results in METex14-mutated NSCLC
according to a presentation at the AACR virtual meeting I.
The duration of response was impressive in both treatment-naive and previously treated patients, according to presenter Edward B. Garon, MD, of the University of California, Los Angeles.
In view of these responses, Dr. Garon urged early molecular testing in NSCLC.
He also noted that capmatinib produced responses in patients with brain metastases. However, because of small patient numbers, additional study is needed to validate the intracranial efficacy of capmatinib and ascertain mechanisms of resistance.
Study rationale and details
METex14 mutations are reported in up to 4% of patients with NSCLC and portend poor outcomes with chemotherapy and immune checkpoint inhibitors (PLoS One 2014; 9:e107677; Ann Oncol 2018;29:2085-91).
Capmatinib is a highly selective, reversible, and potent inhibitor of MET tyrosine kinase that crosses the blood-brain barrier.
In the phase 2 GEOMETRY mono-1 study, Dr. Garon and colleagues tested capmatinib, given at 400 mg orally twice a day, in patients with METex14-mutated, ALK and EGFR wild-type, stage IIIB/IV NSCLC. Patients with neurologically stable or asymptomatic brain metastases were eligible.
Dr. Garon presented safety data for all patients enrolled in this study and efficacy data for patients in cohorts 4 and 5b. Cohort 4 enrolled patients who received prior systemic therapy for advanced disease, and cohort 5b enrolled treatment-naive patients. Both cohorts had METex14 gene mutations but not amplification.
Efficacy
There were 97 patients evaluable for efficacy – 69 previously treated and 28 treatment naive. The median age in both cohorts was 71 years, most patients were female (58% of previously treated and 64.3% of treatment-naive patients), and most were never-smokers (58% and 64.3%, respectively). Adenocarcinoma was the predominant histology.
The overall response rate, per an independent review committee, was 40.6% in previously treated patients and 67.9% in treatment-naive patients.
Waterfall plots showed deep responses, with only four cases of disease progression in the previously treated cohort and none in the treatment-naive cohort.
Responses occurred rapidly. Many responses exceeded 1 year and were ongoing at the data cut-off. The median response duration was 9.72 months in previously treated patients and 11.14 months in treatment-naive patients.
There were 13 patients with evaluable baseline brain metastases (3.3 brain lesions per patient [range, 1-8]). Twelve patients had intracranial disease control, and seven patients (54%) had intracranial response. Four patients had complete resolution of all brain lesions.
Intracranial responses were generally seen by the first radiologic evaluation and occurred as rapidly as systemic responses.
Safety
With safety data on all 334 patients in the trial, the GEOMETRY mono-1 study is the largest reported experience with capmatinib in NSCLC patients. The median treatment exposure time was 14.9 weeks.
Overall, 35.6% of patients experienced a grade 3/4 adverse event (AE). Grade 4 AEs were observed in 4.5% of patients, and there were no treatment-related deaths.
Peripheral edema (41.6%), nausea (33.2%), increased blood creatinine (19.5%), and vomiting (18.9%) were the most frequent AEs of any grade.
In all, 21.9% of patients required dose adjustments due to treatment-related AEs, and 11.1% of patients stopped treatment because of an AE.
This study was sponsored by Novartis. Dr. Garon disclosed relationships with Novartis, AstraZeneca, Bristol-Myers Squibb, Dracen, Dynavax, Eli Lilly, EMD Serono, Genentech, GSK, Iovance, Merck, Mirati, and Neon.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Garon EB et al. AACR 2020, Abstract CT082.
according to a presentation at the AACR virtual meeting I.
The duration of response was impressive in both treatment-naive and previously treated patients, according to presenter Edward B. Garon, MD, of the University of California, Los Angeles.
In view of these responses, Dr. Garon urged early molecular testing in NSCLC.
He also noted that capmatinib produced responses in patients with brain metastases. However, because of small patient numbers, additional study is needed to validate the intracranial efficacy of capmatinib and ascertain mechanisms of resistance.
Study rationale and details
METex14 mutations are reported in up to 4% of patients with NSCLC and portend poor outcomes with chemotherapy and immune checkpoint inhibitors (PLoS One 2014; 9:e107677; Ann Oncol 2018;29:2085-91).
Capmatinib is a highly selective, reversible, and potent inhibitor of MET tyrosine kinase that crosses the blood-brain barrier.
In the phase 2 GEOMETRY mono-1 study, Dr. Garon and colleagues tested capmatinib, given at 400 mg orally twice a day, in patients with METex14-mutated, ALK and EGFR wild-type, stage IIIB/IV NSCLC. Patients with neurologically stable or asymptomatic brain metastases were eligible.
Dr. Garon presented safety data for all patients enrolled in this study and efficacy data for patients in cohorts 4 and 5b. Cohort 4 enrolled patients who received prior systemic therapy for advanced disease, and cohort 5b enrolled treatment-naive patients. Both cohorts had METex14 gene mutations but not amplification.
Efficacy
There were 97 patients evaluable for efficacy – 69 previously treated and 28 treatment naive. The median age in both cohorts was 71 years, most patients were female (58% of previously treated and 64.3% of treatment-naive patients), and most were never-smokers (58% and 64.3%, respectively). Adenocarcinoma was the predominant histology.
The overall response rate, per an independent review committee, was 40.6% in previously treated patients and 67.9% in treatment-naive patients.
Waterfall plots showed deep responses, with only four cases of disease progression in the previously treated cohort and none in the treatment-naive cohort.
Responses occurred rapidly. Many responses exceeded 1 year and were ongoing at the data cut-off. The median response duration was 9.72 months in previously treated patients and 11.14 months in treatment-naive patients.
There were 13 patients with evaluable baseline brain metastases (3.3 brain lesions per patient [range, 1-8]). Twelve patients had intracranial disease control, and seven patients (54%) had intracranial response. Four patients had complete resolution of all brain lesions.
Intracranial responses were generally seen by the first radiologic evaluation and occurred as rapidly as systemic responses.
Safety
With safety data on all 334 patients in the trial, the GEOMETRY mono-1 study is the largest reported experience with capmatinib in NSCLC patients. The median treatment exposure time was 14.9 weeks.
Overall, 35.6% of patients experienced a grade 3/4 adverse event (AE). Grade 4 AEs were observed in 4.5% of patients, and there were no treatment-related deaths.
Peripheral edema (41.6%), nausea (33.2%), increased blood creatinine (19.5%), and vomiting (18.9%) were the most frequent AEs of any grade.
In all, 21.9% of patients required dose adjustments due to treatment-related AEs, and 11.1% of patients stopped treatment because of an AE.
This study was sponsored by Novartis. Dr. Garon disclosed relationships with Novartis, AstraZeneca, Bristol-Myers Squibb, Dracen, Dynavax, Eli Lilly, EMD Serono, Genentech, GSK, Iovance, Merck, Mirati, and Neon.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Garon EB et al. AACR 2020, Abstract CT082.
according to a presentation at the AACR virtual meeting I.
The duration of response was impressive in both treatment-naive and previously treated patients, according to presenter Edward B. Garon, MD, of the University of California, Los Angeles.
In view of these responses, Dr. Garon urged early molecular testing in NSCLC.
He also noted that capmatinib produced responses in patients with brain metastases. However, because of small patient numbers, additional study is needed to validate the intracranial efficacy of capmatinib and ascertain mechanisms of resistance.
Study rationale and details
METex14 mutations are reported in up to 4% of patients with NSCLC and portend poor outcomes with chemotherapy and immune checkpoint inhibitors (PLoS One 2014; 9:e107677; Ann Oncol 2018;29:2085-91).
Capmatinib is a highly selective, reversible, and potent inhibitor of MET tyrosine kinase that crosses the blood-brain barrier.
In the phase 2 GEOMETRY mono-1 study, Dr. Garon and colleagues tested capmatinib, given at 400 mg orally twice a day, in patients with METex14-mutated, ALK and EGFR wild-type, stage IIIB/IV NSCLC. Patients with neurologically stable or asymptomatic brain metastases were eligible.
Dr. Garon presented safety data for all patients enrolled in this study and efficacy data for patients in cohorts 4 and 5b. Cohort 4 enrolled patients who received prior systemic therapy for advanced disease, and cohort 5b enrolled treatment-naive patients. Both cohorts had METex14 gene mutations but not amplification.
Efficacy
There were 97 patients evaluable for efficacy – 69 previously treated and 28 treatment naive. The median age in both cohorts was 71 years, most patients were female (58% of previously treated and 64.3% of treatment-naive patients), and most were never-smokers (58% and 64.3%, respectively). Adenocarcinoma was the predominant histology.
The overall response rate, per an independent review committee, was 40.6% in previously treated patients and 67.9% in treatment-naive patients.
Waterfall plots showed deep responses, with only four cases of disease progression in the previously treated cohort and none in the treatment-naive cohort.
Responses occurred rapidly. Many responses exceeded 1 year and were ongoing at the data cut-off. The median response duration was 9.72 months in previously treated patients and 11.14 months in treatment-naive patients.
There were 13 patients with evaluable baseline brain metastases (3.3 brain lesions per patient [range, 1-8]). Twelve patients had intracranial disease control, and seven patients (54%) had intracranial response. Four patients had complete resolution of all brain lesions.
Intracranial responses were generally seen by the first radiologic evaluation and occurred as rapidly as systemic responses.
Safety
With safety data on all 334 patients in the trial, the GEOMETRY mono-1 study is the largest reported experience with capmatinib in NSCLC patients. The median treatment exposure time was 14.9 weeks.
Overall, 35.6% of patients experienced a grade 3/4 adverse event (AE). Grade 4 AEs were observed in 4.5% of patients, and there were no treatment-related deaths.
Peripheral edema (41.6%), nausea (33.2%), increased blood creatinine (19.5%), and vomiting (18.9%) were the most frequent AEs of any grade.
In all, 21.9% of patients required dose adjustments due to treatment-related AEs, and 11.1% of patients stopped treatment because of an AE.
This study was sponsored by Novartis. Dr. Garon disclosed relationships with Novartis, AstraZeneca, Bristol-Myers Squibb, Dracen, Dynavax, Eli Lilly, EMD Serono, Genentech, GSK, Iovance, Merck, Mirati, and Neon.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Garon EB et al. AACR 2020, Abstract CT082.
FROM AACR 2020
A multicenter RCT makes a case for transabdominal cerclage
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
Since the 1950s, when Shirodkar (1955) and McDonald (1957) published their seminal works detailing a transvaginal method to suture a “weak” cervix, clinicians and researchers have debated the indications for and utility of cerclage for preventing pregnancy loss and preterm birth.1,2
Originally based on a history of recurrent mid-trimester loss (that is, a clinical diagnosis of cervical insufficiency), cerclage has been expanded to capture both ultrasonography and physical-exam indications. While cerclage has proven useful in select patient populations, an infrequent but vexing problem is what to do when a woman has experienced 1 or more (transvaginal) cerclage “failures.”
With a dearth of well-controlled, randomized data to support the use of cerclage for either history- or physical-exam indications, it is not surprising that we still debate whether the Shirodkar method is superior to the McDonald technique as well as how to best manage a patient when either or both methods previously resulted in an unsatisfactory outcome.
First randomized study to directly compare cerclage techniques
Fortunately, Shennan and colleagues in the United Kingdom have greatly enlarged our knowledge in this area by performing the first well-powered, 3-arm, randomized trial of transabdominal cerclage (TAC) compared with both high and low vaginal cerclage (HVC, LVC).3 They analyzed data for 111 women who were randomly assigned to TAC
(n = 39), HVC (n = 39), or LVC (n = 33).
Interestingly, the investigators chose to not attach conventional eponymous labels to their transvaginal methods, and they do not even provide a reference or detailed description of the surgical methods, telling us instead that, “Techniques used were left to the local clinician’s discretion.” Writing also that HVC cases, like the transabdominal surgeries, were carried out in specialty centers, they implied that additional training was required for the HVC. I inferred that indeed they actually were performing the McDonald and Shirodkar transvaginal methods and with possible by-physician, local modifications.
I am certain that the authors’ results did not surprise proponents of transabdominal cerclage for transvaginal cerclage failures, defined in this trial as prior birth from 14 to 28 weeks’ gestation. Since some clinicians use a more generous definition of cerclage failure (such as birth at less than 34 weeks), this study population was clearly at high risk for poor outcomes; in fact, more than 90% of each group had experienced at least 2 prior mid-trimester losses. As anticipated with randomization, other characteristics were well distributed across the 3 groups.
Continue to: Transabdominal cerclage significantly reduced preterm birth rates...
Transabdominal cerclage significantly reduced preterm birth rates
Using a primary outcome of preterm birth less than 32 weeks, which concentrates neonatal morbidities, the investigators observed an overall 4.5-fold higher rate of preterm birth in the transvaginal cohorts compared with the transabdominal patients (33% and 38% versus 8%, respectively). Comparing the TAC group individually with both LVC and HVC groups, the relative risk of preterm birth was 0.20 compared with the HVC group and 0.23 compared with the LVC group, reflecting an approximate 80% reduction.
Not surprising to me, the investigators observed nearly identical outcomes between the HVC and LVC cohorts, substantiating my bias that the 2 transvaginal methods are similarly effective. Opponents will quickly remind me that the study was not well-powered to detect a clinically significant difference between these 2 groups; touché!
Risks of TAC. We all know that, despite its now-proven benefits, the transabdominal approach is associated with a risk of special complications, including the surgical risks of placement (and removal) of the cerclage, the management of fetal death beyond approximately 14 weeks, and the absolute requisite for hysterotomy/cesarean birth. While serious complications are rare, in the trial by Shennan and colleagues none were recorded in the 39 TAC cases. Nevertheless, for women with no children or only prior early births, the risks seem to be justified; the number needed to treat was less than 4 to prevent 1 birth at less than 32 weeks and was 5.3 to prevent a fetal loss.
TAC is an option for select patients
Given that TAC now can be successfully placed using minimally invasive surgery, either prior to or following conception, this study provides unique level I evidence that should not be discounted and should further be considered in the context of confirming prior cohort studies that suggested a significant benefit. Although specialized training is required and the procedure may involve travel to a specialty center, the weight of clinical data clearly supports the use of TAC.
In summary, based largely on the trial by Shennan and colleagues, women with prior failed vaginal cerclage can and should be counseled regarding the availability of TAC and given the opportunity to weigh the reported risks and benefits. ●
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
1. Shirodkar VN. A new method of operative treatment for habitual abortion in the second trimester of pregnancy. Antiseptic. 1955;52:299-303.
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynecol Br Emp. 1957;64:346-350.
3. Shennan A, Chandiramani M, Bennett P, et al. MAVRIC: a multicenter randomized trial of transabdominal vs transvaginal cervical cerclage. Am J Obstet Gynecol. 2020;222:261.e1-261.e9.
Do women treated with ceftriaxone and doxycycline for PID benefit from added metronidazole to broaden anaerobic coverage?
Wiesenfeld HC, Meyn LA, Darville T, et al. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. February 13, 2020. doi:10.1093/cid/ciaa101.
EXPERT COMMENTARY
Pelvic inflammatory disease remains prevalent among young women and is commonly diagnosed in emergency departments and sexually transmitted disease (STD) clinics. This tubal infection is associated with significant reproductive sequelae, including tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. In addition, these women remain at risk for recurrent PID.
Bacterial vaginosis is present in more than half of women with PID. Not surprisingly, anaerobic microorganisms are more commonly isolated from the upper genital tract of patients with acute PID than either Neisseria gonorrhoeae or Chlamydia trachomatis, yet recommended antimicrobial regimens do not necessarily include antibiotics with an excellent antianaerobic spectrum.
Details of the study
In a randomized, double-blind, placebo-controlled trial, Wiesenfeld and colleagues enrolled women from hospital emergency departments or an STD clinic with symptoms of lower abdominal or pelvic pain associated with pelvic organ tenderness. The 233 study participants were randomly assigned to 2 treatment arms: ceftriaxone, doxycycline, and placebo (n = 117) or ceftriaxone, doxycycline, and metronidazole (n = 116).
Findings. Women treated with metronidazole were less likely to have pelvic organ tenderness a month after enrollment compared with the placebo group (9% vs 20%, respectively). Although the clinical cure rates at 30 days were statistically similar in both arms of the study, those receiving metronidazole had a 97% clinical cure rate while those not treated with metronidazole had a 90% clinical cure rate
(P = .38).
Moreover, the concurrent disorders of bacterial vaginosis and trichomonas vaginitis were more effectively treated in the metronidazole group, and fewer women had positive follow-up endometrial cultures for anaerobic bacteria compared with the placebo group (8% vs 21%, respectively).
The anticipated gastrointestinal adverse effects of a combination doxycycline-and-metronidazole regimen was a significant concern; however, combination therapy was no more likely to cause gastrointestinal adverse effects than doxycycline alone.
Continue to: Study strengths and limitations...
Study strengths and limitations
This well-designed randomized, double-blinded clinical trial was performed by clinical investigators experienced in the clinical diagnosis of PID. The demography of the population and their history of C trachomatis, N gonorrhoeae, plus the concurrent diagnosis of bacterial vaginosis make the diagnosis believable and real world, and these factors contribute to the generalizability of the study results.
However, PID is an imprecise clinical diagnosis (specificity averages 65%) when held to the gold standard of diagnostic laparoscopy to confirm the presence of acute salpingitis. Given the reticence of investigators and clinicians to embark on such an invasive procedure to confirm this diagnosis, endometrial biopsy showing evidence of histologic acute endometritis has been offered as an alternative gold standard. Confirmation of acute endometritis in the trial participants would have enhanced the validity of this study.
This study challenges a long held, but never proven, belief that the combination of doxycycline and metronidazole would be poorly tolerated as a combination antimicrobial regimen. It also further solidifies the role of anaerobic bacteria as major players in the microbial etiology of acute PID. In addition, it appears that treating bacterial vaginosis concurrently may lessen the likelihood of endometrial recolonization with anaerobic bacteria. ●
Metronidazole should be added routinely to the standard antibiotic regimen of ceftriaxone and doxycycline for the treatment of women with PID.
DAVID E. SOPER, MD
Wiesenfeld HC, Meyn LA, Darville T, et al. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. February 13, 2020. doi:10.1093/cid/ciaa101.
EXPERT COMMENTARY
Pelvic inflammatory disease remains prevalent among young women and is commonly diagnosed in emergency departments and sexually transmitted disease (STD) clinics. This tubal infection is associated with significant reproductive sequelae, including tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. In addition, these women remain at risk for recurrent PID.
Bacterial vaginosis is present in more than half of women with PID. Not surprisingly, anaerobic microorganisms are more commonly isolated from the upper genital tract of patients with acute PID than either Neisseria gonorrhoeae or Chlamydia trachomatis, yet recommended antimicrobial regimens do not necessarily include antibiotics with an excellent antianaerobic spectrum.
Details of the study
In a randomized, double-blind, placebo-controlled trial, Wiesenfeld and colleagues enrolled women from hospital emergency departments or an STD clinic with symptoms of lower abdominal or pelvic pain associated with pelvic organ tenderness. The 233 study participants were randomly assigned to 2 treatment arms: ceftriaxone, doxycycline, and placebo (n = 117) or ceftriaxone, doxycycline, and metronidazole (n = 116).
Findings. Women treated with metronidazole were less likely to have pelvic organ tenderness a month after enrollment compared with the placebo group (9% vs 20%, respectively). Although the clinical cure rates at 30 days were statistically similar in both arms of the study, those receiving metronidazole had a 97% clinical cure rate while those not treated with metronidazole had a 90% clinical cure rate
(P = .38).
Moreover, the concurrent disorders of bacterial vaginosis and trichomonas vaginitis were more effectively treated in the metronidazole group, and fewer women had positive follow-up endometrial cultures for anaerobic bacteria compared with the placebo group (8% vs 21%, respectively).
The anticipated gastrointestinal adverse effects of a combination doxycycline-and-metronidazole regimen was a significant concern; however, combination therapy was no more likely to cause gastrointestinal adverse effects than doxycycline alone.
Continue to: Study strengths and limitations...
Study strengths and limitations
This well-designed randomized, double-blinded clinical trial was performed by clinical investigators experienced in the clinical diagnosis of PID. The demography of the population and their history of C trachomatis, N gonorrhoeae, plus the concurrent diagnosis of bacterial vaginosis make the diagnosis believable and real world, and these factors contribute to the generalizability of the study results.
However, PID is an imprecise clinical diagnosis (specificity averages 65%) when held to the gold standard of diagnostic laparoscopy to confirm the presence of acute salpingitis. Given the reticence of investigators and clinicians to embark on such an invasive procedure to confirm this diagnosis, endometrial biopsy showing evidence of histologic acute endometritis has been offered as an alternative gold standard. Confirmation of acute endometritis in the trial participants would have enhanced the validity of this study.
This study challenges a long held, but never proven, belief that the combination of doxycycline and metronidazole would be poorly tolerated as a combination antimicrobial regimen. It also further solidifies the role of anaerobic bacteria as major players in the microbial etiology of acute PID. In addition, it appears that treating bacterial vaginosis concurrently may lessen the likelihood of endometrial recolonization with anaerobic bacteria. ●
Metronidazole should be added routinely to the standard antibiotic regimen of ceftriaxone and doxycycline for the treatment of women with PID.
DAVID E. SOPER, MD
Wiesenfeld HC, Meyn LA, Darville T, et al. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. February 13, 2020. doi:10.1093/cid/ciaa101.
EXPERT COMMENTARY
Pelvic inflammatory disease remains prevalent among young women and is commonly diagnosed in emergency departments and sexually transmitted disease (STD) clinics. This tubal infection is associated with significant reproductive sequelae, including tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. In addition, these women remain at risk for recurrent PID.
Bacterial vaginosis is present in more than half of women with PID. Not surprisingly, anaerobic microorganisms are more commonly isolated from the upper genital tract of patients with acute PID than either Neisseria gonorrhoeae or Chlamydia trachomatis, yet recommended antimicrobial regimens do not necessarily include antibiotics with an excellent antianaerobic spectrum.
Details of the study
In a randomized, double-blind, placebo-controlled trial, Wiesenfeld and colleagues enrolled women from hospital emergency departments or an STD clinic with symptoms of lower abdominal or pelvic pain associated with pelvic organ tenderness. The 233 study participants were randomly assigned to 2 treatment arms: ceftriaxone, doxycycline, and placebo (n = 117) or ceftriaxone, doxycycline, and metronidazole (n = 116).
Findings. Women treated with metronidazole were less likely to have pelvic organ tenderness a month after enrollment compared with the placebo group (9% vs 20%, respectively). Although the clinical cure rates at 30 days were statistically similar in both arms of the study, those receiving metronidazole had a 97% clinical cure rate while those not treated with metronidazole had a 90% clinical cure rate
(P = .38).
Moreover, the concurrent disorders of bacterial vaginosis and trichomonas vaginitis were more effectively treated in the metronidazole group, and fewer women had positive follow-up endometrial cultures for anaerobic bacteria compared with the placebo group (8% vs 21%, respectively).
The anticipated gastrointestinal adverse effects of a combination doxycycline-and-metronidazole regimen was a significant concern; however, combination therapy was no more likely to cause gastrointestinal adverse effects than doxycycline alone.
Continue to: Study strengths and limitations...
Study strengths and limitations
This well-designed randomized, double-blinded clinical trial was performed by clinical investigators experienced in the clinical diagnosis of PID. The demography of the population and their history of C trachomatis, N gonorrhoeae, plus the concurrent diagnosis of bacterial vaginosis make the diagnosis believable and real world, and these factors contribute to the generalizability of the study results.
However, PID is an imprecise clinical diagnosis (specificity averages 65%) when held to the gold standard of diagnostic laparoscopy to confirm the presence of acute salpingitis. Given the reticence of investigators and clinicians to embark on such an invasive procedure to confirm this diagnosis, endometrial biopsy showing evidence of histologic acute endometritis has been offered as an alternative gold standard. Confirmation of acute endometritis in the trial participants would have enhanced the validity of this study.
This study challenges a long held, but never proven, belief that the combination of doxycycline and metronidazole would be poorly tolerated as a combination antimicrobial regimen. It also further solidifies the role of anaerobic bacteria as major players in the microbial etiology of acute PID. In addition, it appears that treating bacterial vaginosis concurrently may lessen the likelihood of endometrial recolonization with anaerobic bacteria. ●
Metronidazole should be added routinely to the standard antibiotic regimen of ceftriaxone and doxycycline for the treatment of women with PID.
DAVID E. SOPER, MD