User login
How effective is that face mask?
More and more, the streets of America are looking like those of Eastern countries (such as China) during previous public health crises. Americans are wearing face masks.
In addition to social distancing and hand washing, face masks are a primary defense against COVID-19. N95 face masks protect against 95% of the particles that are likely to transmit respiratory infection microbes. Last month, the Centers for Disease Control and Prevention (CDC) recommended that we all use masks, in addition to social distancing, in public settings. Since there will not be a sufficient supply of N95 masks for the general public (and they are difficult to fit and wear properly), we are left with surgical masks and so-called DIY (do-it-yourself) masks. But do DIY face masks protect against COVID-19?
The National Academies of Sciences, Engineering, and Medicine published a scientific review of fabric face masks last month.1 They found 7 studies that evaluated either the ability of the mask to protect the wearer or to prevent the spread of infectious particles from a wearer. Performance ranged from very poor to 50% filtration depending on the material used. Jayaraman2 found a filtration rate of 50% for 4 layers of polyester knitted cut-pile fabric, the best material he tested. Davies3 compared a 2-layer cotton DIY mask with a surgical face mask and found that the cotton mask was 3 times less effective. And in the only randomized trial of cotton masks, the cotton 2-layer masks performed much worse than medical masks in protecting from respiratory infection (relative risk [RR] = 13).4 A study of COVID-19-infected patients found that neither surgical nor cotton masks were effective at blocking the virus from disseminating during coughing.5
The most recent lab testing of DIY masks was done at Wake Forest Institute for Regenerative Medicine, where they tested a variety of materials; the results were somewhat encouraging.6 The best homemade masks were those with “2 layers of high-quality, heavyweight ‘quilter’s cotton’ with a thread count of 180 or more, and those with especially tight weave and thicker thread such as batiks.”6 The best homemade masks achieved 79% filtration. But single-layer masks or double-layer designs of lower quality, lightweight cotton achieved as little as 1% filtration.
The bottom line: Mass production and use of N95-type masks would be most effective in preventing transmission in general public settings, but this seems unlikely. Surgical masks are next best. Well-constructed DIY masks are the last resort but can provide some protection against infection.
1. Besser R, Fischhoff B; National Academy of Sciences, Engineering, and Medicine. Rapid Expert Consultation on the Effectiveness of Fabric Masks for the COVID-19 Pandemic (April 8, 2020). www.nap.edu/read/25776/chapter/1. Published April 8, 2020. Accessed April 28, 2020.
2. Jayaraman S. Pandemic flu—textile solutions pilot: design and development of innovative medical masks [final technical report]. Atlanta, GA: Georgia Institute of Technology; 2012.
3. Davies A, Thompson K, Giri K, et al. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413-418.
4. MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5:e006577.
5. Bae S, Kim MC, Kim JY, et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: a controlled comparison in 4 patients [published online ahead of print April 6, 2020]. Ann Intern Med. 2020.
6. Wake Forest Baptist Medical Center. Testing shows type of cloth used in homemade masks makes a difference, doctors say. https://newsroom.wakehealth.edu/News-Releases/2020/04/Testing-Shows-Type-of-Cloth-Used-in-Homemade-Masks-Makes-a-Difference. Published April 2, 2020. Accessed April 28, 2020.
More and more, the streets of America are looking like those of Eastern countries (such as China) during previous public health crises. Americans are wearing face masks.
In addition to social distancing and hand washing, face masks are a primary defense against COVID-19. N95 face masks protect against 95% of the particles that are likely to transmit respiratory infection microbes. Last month, the Centers for Disease Control and Prevention (CDC) recommended that we all use masks, in addition to social distancing, in public settings. Since there will not be a sufficient supply of N95 masks for the general public (and they are difficult to fit and wear properly), we are left with surgical masks and so-called DIY (do-it-yourself) masks. But do DIY face masks protect against COVID-19?
The National Academies of Sciences, Engineering, and Medicine published a scientific review of fabric face masks last month.1 They found 7 studies that evaluated either the ability of the mask to protect the wearer or to prevent the spread of infectious particles from a wearer. Performance ranged from very poor to 50% filtration depending on the material used. Jayaraman2 found a filtration rate of 50% for 4 layers of polyester knitted cut-pile fabric, the best material he tested. Davies3 compared a 2-layer cotton DIY mask with a surgical face mask and found that the cotton mask was 3 times less effective. And in the only randomized trial of cotton masks, the cotton 2-layer masks performed much worse than medical masks in protecting from respiratory infection (relative risk [RR] = 13).4 A study of COVID-19-infected patients found that neither surgical nor cotton masks were effective at blocking the virus from disseminating during coughing.5
The most recent lab testing of DIY masks was done at Wake Forest Institute for Regenerative Medicine, where they tested a variety of materials; the results were somewhat encouraging.6 The best homemade masks were those with “2 layers of high-quality, heavyweight ‘quilter’s cotton’ with a thread count of 180 or more, and those with especially tight weave and thicker thread such as batiks.”6 The best homemade masks achieved 79% filtration. But single-layer masks or double-layer designs of lower quality, lightweight cotton achieved as little as 1% filtration.
The bottom line: Mass production and use of N95-type masks would be most effective in preventing transmission in general public settings, but this seems unlikely. Surgical masks are next best. Well-constructed DIY masks are the last resort but can provide some protection against infection.
More and more, the streets of America are looking like those of Eastern countries (such as China) during previous public health crises. Americans are wearing face masks.
In addition to social distancing and hand washing, face masks are a primary defense against COVID-19. N95 face masks protect against 95% of the particles that are likely to transmit respiratory infection microbes. Last month, the Centers for Disease Control and Prevention (CDC) recommended that we all use masks, in addition to social distancing, in public settings. Since there will not be a sufficient supply of N95 masks for the general public (and they are difficult to fit and wear properly), we are left with surgical masks and so-called DIY (do-it-yourself) masks. But do DIY face masks protect against COVID-19?
The National Academies of Sciences, Engineering, and Medicine published a scientific review of fabric face masks last month.1 They found 7 studies that evaluated either the ability of the mask to protect the wearer or to prevent the spread of infectious particles from a wearer. Performance ranged from very poor to 50% filtration depending on the material used. Jayaraman2 found a filtration rate of 50% for 4 layers of polyester knitted cut-pile fabric, the best material he tested. Davies3 compared a 2-layer cotton DIY mask with a surgical face mask and found that the cotton mask was 3 times less effective. And in the only randomized trial of cotton masks, the cotton 2-layer masks performed much worse than medical masks in protecting from respiratory infection (relative risk [RR] = 13).4 A study of COVID-19-infected patients found that neither surgical nor cotton masks were effective at blocking the virus from disseminating during coughing.5
The most recent lab testing of DIY masks was done at Wake Forest Institute for Regenerative Medicine, where they tested a variety of materials; the results were somewhat encouraging.6 The best homemade masks were those with “2 layers of high-quality, heavyweight ‘quilter’s cotton’ with a thread count of 180 or more, and those with especially tight weave and thicker thread such as batiks.”6 The best homemade masks achieved 79% filtration. But single-layer masks or double-layer designs of lower quality, lightweight cotton achieved as little as 1% filtration.
The bottom line: Mass production and use of N95-type masks would be most effective in preventing transmission in general public settings, but this seems unlikely. Surgical masks are next best. Well-constructed DIY masks are the last resort but can provide some protection against infection.
1. Besser R, Fischhoff B; National Academy of Sciences, Engineering, and Medicine. Rapid Expert Consultation on the Effectiveness of Fabric Masks for the COVID-19 Pandemic (April 8, 2020). www.nap.edu/read/25776/chapter/1. Published April 8, 2020. Accessed April 28, 2020.
2. Jayaraman S. Pandemic flu—textile solutions pilot: design and development of innovative medical masks [final technical report]. Atlanta, GA: Georgia Institute of Technology; 2012.
3. Davies A, Thompson K, Giri K, et al. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413-418.
4. MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5:e006577.
5. Bae S, Kim MC, Kim JY, et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: a controlled comparison in 4 patients [published online ahead of print April 6, 2020]. Ann Intern Med. 2020.
6. Wake Forest Baptist Medical Center. Testing shows type of cloth used in homemade masks makes a difference, doctors say. https://newsroom.wakehealth.edu/News-Releases/2020/04/Testing-Shows-Type-of-Cloth-Used-in-Homemade-Masks-Makes-a-Difference. Published April 2, 2020. Accessed April 28, 2020.
1. Besser R, Fischhoff B; National Academy of Sciences, Engineering, and Medicine. Rapid Expert Consultation on the Effectiveness of Fabric Masks for the COVID-19 Pandemic (April 8, 2020). www.nap.edu/read/25776/chapter/1. Published April 8, 2020. Accessed April 28, 2020.
2. Jayaraman S. Pandemic flu—textile solutions pilot: design and development of innovative medical masks [final technical report]. Atlanta, GA: Georgia Institute of Technology; 2012.
3. Davies A, Thompson K, Giri K, et al. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413-418.
4. MacIntyre CR, Seale H, Dung TC, et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5:e006577.
5. Bae S, Kim MC, Kim JY, et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: a controlled comparison in 4 patients [published online ahead of print April 6, 2020]. Ann Intern Med. 2020.
6. Wake Forest Baptist Medical Center. Testing shows type of cloth used in homemade masks makes a difference, doctors say. https://newsroom.wakehealth.edu/News-Releases/2020/04/Testing-Shows-Type-of-Cloth-Used-in-Homemade-Masks-Makes-a-Difference. Published April 2, 2020. Accessed April 28, 2020.
Silent brain infarcts found in 3% of AFib patients, tied to cognitive decline
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
FROM HEART RHYTHM 2020
Hypertriglyceridemia: A strategic approach
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15

The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
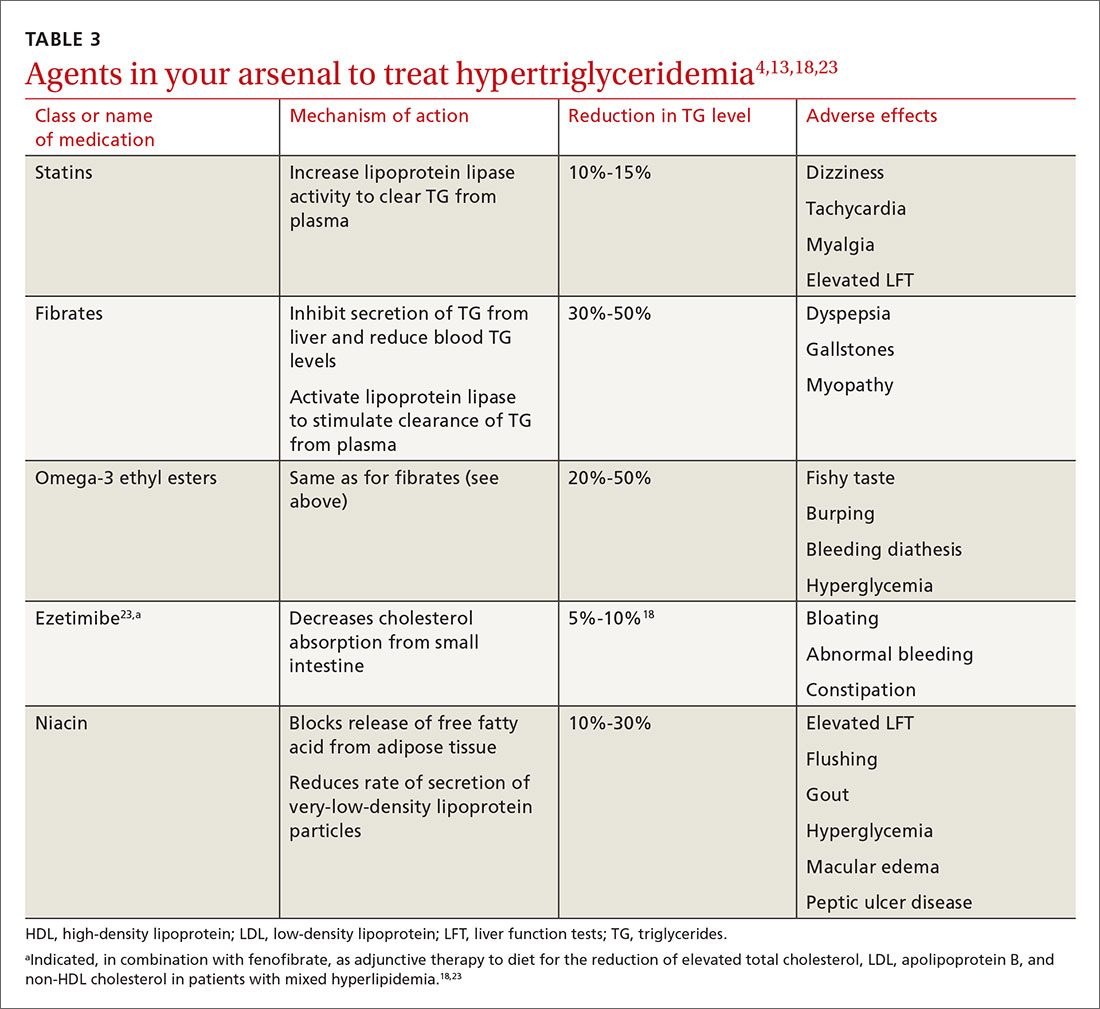
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
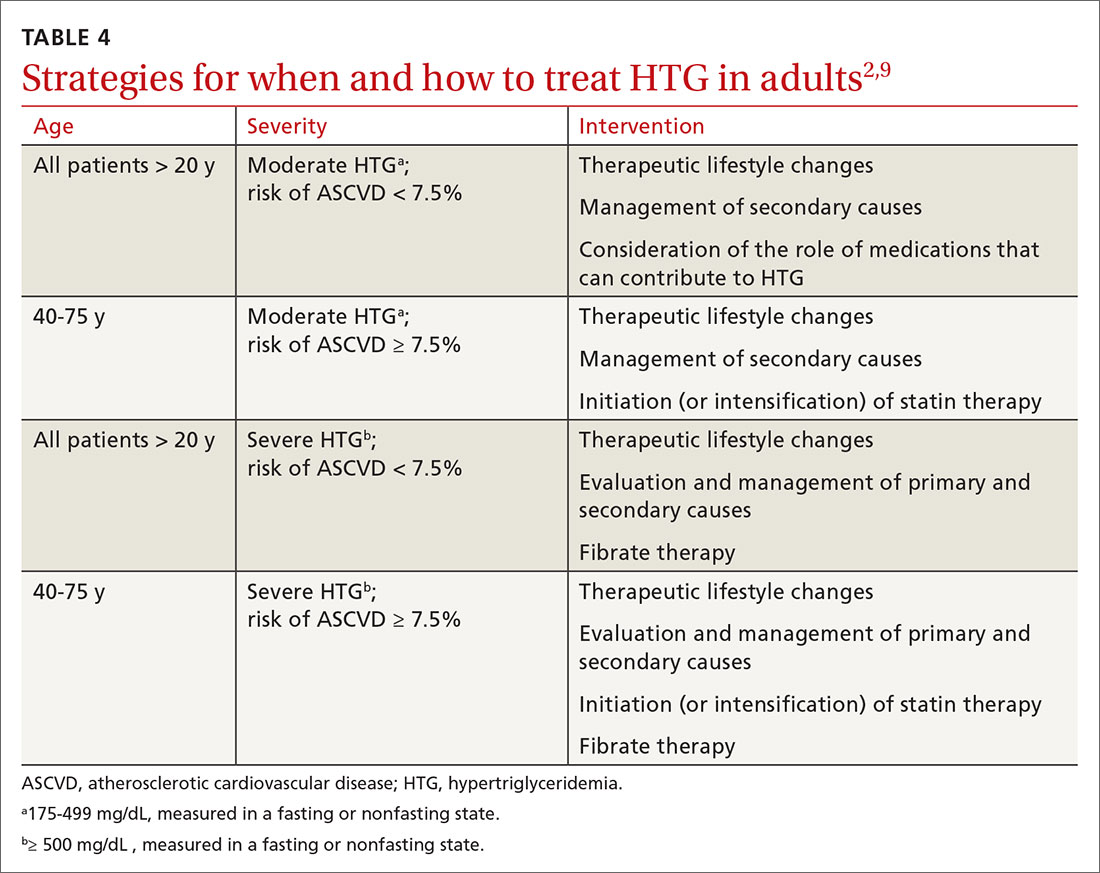
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; [email protected].
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?

CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.

Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15

The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2

Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).

Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; [email protected].
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?

CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.

Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15

The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2

Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).

Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; [email protected].
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
PRACTICE RECOMMENDATIONS
› Evaluate patients for hypertriglyceridemia when they have a comorbid condition (eg, type 2 diabetes, obesity, hypothyroidism, metabolic syndrome, alcoholism). B
› Do not require fasting status when evaluating triglycerides in a lipid panel. B
› Make therapeutic lifestyle changes first-line treatment for hypertriglyceridemia. C
› Prescribe fibrates for severe hypertriglyceridemia to reduce the risk and recurrence of pancreatitis. A
› Prescribe a statin and an omega-3 fatty acid (fish oil) to lower the triglyceride level and thus reduce resulting atherogenicity when the risk of atherosclerotic cardiovascular disease is > 7.5%. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
E-cigarette users topped 8 million in 2018
according to a report from the National Center for Health Statistics.
Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
according to a report from the National Center for Health Statistics.

Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
according to a report from the National Center for Health Statistics.

Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
COVID-19 in pregnancy: Supplement oxygen if saturation dips below 94%
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
OBSTETRICS & GYNECOLOGY
Too much or too little sleep spikes constipation
Individuals who sleep more or less than average report significantly more constipation, compared with normal sleepers, based on data from 14,590 adults.
“Normal sleep duration is thought to be essential for healthy bowel function; however, the effect of either limited or excessive sleep duration on bowel patterns is poorly understood,” Adeyinka Adejumo, MD, of North Shore Medical Center, Salem, Mass., and colleagues wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
To examine the association between sleep duration and bowel function, the researchers identified 14,590 adults aged 20 years and older who completed questionnaires on sleep and bowel health as part of the National Health and Nutrition Examination Survey (NHANES) during 2005-2010.
Sleep was divided into three categories based on standards from the National Sleep Foundation: short (less than 7 hours), normal (7-8 hours) and long (more than 8 hours).
Overall, constipation rates were significantly lower among normal sleepers (8.3%) compared with both short and long sleepers (11.0% and 12.5%, respectively; P < .0001 for both).
Bowel function was defined as normal, constipation, or diarrhea based on stool form and bowel movements per week. After controlling for demographic, lifestyle, and dietary factors, long sleepers and short sleepers were 61% and 38% more likely, respectively, to report constipation, compared with normal sleepers.
However, sleep duration was not related to diarrhea, the researchers noted. In addition, “A sensitivity analysis revealed that sleep duration did not mediate the relationship between comorbid factors (such as overall health, poverty index, obesity, and body mass index) and constipation,” they wrote.
The results suggest that decreased sleep is associated with constipation among adults in the United States, the researchers said. However, “further studies are needed to evaluate the physiologic mechanisms driving the impact of sleep duration on bowel function to determine whether sleep disorders or their underlying causes affect constipation,” they concluded.
“This study was necessary because up to 50% of Americans suffer from sleep disorders, out of which abnormal sleep duration is one of the most common and underdiagnosed, and associated with other diseases such as hypertension and diabetes,” Dr. Adejumo said in an interview. “However, disorders of bowel function (constipation and diarrhea), which affect almost 10%-15% of the population and result in significant health care burden, such as higher cost, hospital visits, abdominal discomfort, have not been studied among individuals with suboptimal sleep duration.”
Dr. Adejumo said he and his colleagues were surprised by their findings. “Although, based on our hypothesis, we thought that sleeping too long may be associated with constipation, we were shocked to note similar results among people who also sleep for short durations,” he noted.
“Previous studies had suggested that bowel contraction slows down considerably during sleep. It, therefore, will make sense that sleeping for too long may result in suppressed bowel motility and decreased bowel movement,” he said. “However, our results showed similar findings among short sleepers. We do not know the exact mechanism of these results. It may be that short sleep resulted in inadequate bowel rest, bowel muscle fatigue, and, subsequently, decreased bowel movement,” said Dr. Adejumo. “Or it may also be that brain-gut signaling pathways are disrupted among short sleepers, as is seen among IBS patients after a poor night sleep, resulting in higher constipation,” he added.
Clinicians should be aware of the impact of both short and long sleep on constipation, said Dr. Adejumo. “Individuals who are unable to have adequate periods of sleep due to other diseases, including insomnia, disrupted job schedules, or conditions with too long sleep, such as narcolepsy, may all additionally suffer from constipation. Such patients may need regular evaluation and treatment for constipation to improve their discomfort,” he said.
“To confirm our findings, other clinical studies with more granular data on sleep and constipation, are needed, as well as translational research to uncover the potential mechanisms of these findings,” he emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Adejumo A et al. DDW 2020. Abstract Sa1711.
Individuals who sleep more or less than average report significantly more constipation, compared with normal sleepers, based on data from 14,590 adults.
“Normal sleep duration is thought to be essential for healthy bowel function; however, the effect of either limited or excessive sleep duration on bowel patterns is poorly understood,” Adeyinka Adejumo, MD, of North Shore Medical Center, Salem, Mass., and colleagues wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
To examine the association between sleep duration and bowel function, the researchers identified 14,590 adults aged 20 years and older who completed questionnaires on sleep and bowel health as part of the National Health and Nutrition Examination Survey (NHANES) during 2005-2010.
Sleep was divided into three categories based on standards from the National Sleep Foundation: short (less than 7 hours), normal (7-8 hours) and long (more than 8 hours).
Overall, constipation rates were significantly lower among normal sleepers (8.3%) compared with both short and long sleepers (11.0% and 12.5%, respectively; P < .0001 for both).
Bowel function was defined as normal, constipation, or diarrhea based on stool form and bowel movements per week. After controlling for demographic, lifestyle, and dietary factors, long sleepers and short sleepers were 61% and 38% more likely, respectively, to report constipation, compared with normal sleepers.
However, sleep duration was not related to diarrhea, the researchers noted. In addition, “A sensitivity analysis revealed that sleep duration did not mediate the relationship between comorbid factors (such as overall health, poverty index, obesity, and body mass index) and constipation,” they wrote.
The results suggest that decreased sleep is associated with constipation among adults in the United States, the researchers said. However, “further studies are needed to evaluate the physiologic mechanisms driving the impact of sleep duration on bowel function to determine whether sleep disorders or their underlying causes affect constipation,” they concluded.
“This study was necessary because up to 50% of Americans suffer from sleep disorders, out of which abnormal sleep duration is one of the most common and underdiagnosed, and associated with other diseases such as hypertension and diabetes,” Dr. Adejumo said in an interview. “However, disorders of bowel function (constipation and diarrhea), which affect almost 10%-15% of the population and result in significant health care burden, such as higher cost, hospital visits, abdominal discomfort, have not been studied among individuals with suboptimal sleep duration.”
Dr. Adejumo said he and his colleagues were surprised by their findings. “Although, based on our hypothesis, we thought that sleeping too long may be associated with constipation, we were shocked to note similar results among people who also sleep for short durations,” he noted.
“Previous studies had suggested that bowel contraction slows down considerably during sleep. It, therefore, will make sense that sleeping for too long may result in suppressed bowel motility and decreased bowel movement,” he said. “However, our results showed similar findings among short sleepers. We do not know the exact mechanism of these results. It may be that short sleep resulted in inadequate bowel rest, bowel muscle fatigue, and, subsequently, decreased bowel movement,” said Dr. Adejumo. “Or it may also be that brain-gut signaling pathways are disrupted among short sleepers, as is seen among IBS patients after a poor night sleep, resulting in higher constipation,” he added.
Clinicians should be aware of the impact of both short and long sleep on constipation, said Dr. Adejumo. “Individuals who are unable to have adequate periods of sleep due to other diseases, including insomnia, disrupted job schedules, or conditions with too long sleep, such as narcolepsy, may all additionally suffer from constipation. Such patients may need regular evaluation and treatment for constipation to improve their discomfort,” he said.
“To confirm our findings, other clinical studies with more granular data on sleep and constipation, are needed, as well as translational research to uncover the potential mechanisms of these findings,” he emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Adejumo A et al. DDW 2020. Abstract Sa1711.
Individuals who sleep more or less than average report significantly more constipation, compared with normal sleepers, based on data from 14,590 adults.
“Normal sleep duration is thought to be essential for healthy bowel function; however, the effect of either limited or excessive sleep duration on bowel patterns is poorly understood,” Adeyinka Adejumo, MD, of North Shore Medical Center, Salem, Mass., and colleagues wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
To examine the association between sleep duration and bowel function, the researchers identified 14,590 adults aged 20 years and older who completed questionnaires on sleep and bowel health as part of the National Health and Nutrition Examination Survey (NHANES) during 2005-2010.
Sleep was divided into three categories based on standards from the National Sleep Foundation: short (less than 7 hours), normal (7-8 hours) and long (more than 8 hours).
Overall, constipation rates were significantly lower among normal sleepers (8.3%) compared with both short and long sleepers (11.0% and 12.5%, respectively; P < .0001 for both).
Bowel function was defined as normal, constipation, or diarrhea based on stool form and bowel movements per week. After controlling for demographic, lifestyle, and dietary factors, long sleepers and short sleepers were 61% and 38% more likely, respectively, to report constipation, compared with normal sleepers.
However, sleep duration was not related to diarrhea, the researchers noted. In addition, “A sensitivity analysis revealed that sleep duration did not mediate the relationship between comorbid factors (such as overall health, poverty index, obesity, and body mass index) and constipation,” they wrote.
The results suggest that decreased sleep is associated with constipation among adults in the United States, the researchers said. However, “further studies are needed to evaluate the physiologic mechanisms driving the impact of sleep duration on bowel function to determine whether sleep disorders or their underlying causes affect constipation,” they concluded.
“This study was necessary because up to 50% of Americans suffer from sleep disorders, out of which abnormal sleep duration is one of the most common and underdiagnosed, and associated with other diseases such as hypertension and diabetes,” Dr. Adejumo said in an interview. “However, disorders of bowel function (constipation and diarrhea), which affect almost 10%-15% of the population and result in significant health care burden, such as higher cost, hospital visits, abdominal discomfort, have not been studied among individuals with suboptimal sleep duration.”
Dr. Adejumo said he and his colleagues were surprised by their findings. “Although, based on our hypothesis, we thought that sleeping too long may be associated with constipation, we were shocked to note similar results among people who also sleep for short durations,” he noted.
“Previous studies had suggested that bowel contraction slows down considerably during sleep. It, therefore, will make sense that sleeping for too long may result in suppressed bowel motility and decreased bowel movement,” he said. “However, our results showed similar findings among short sleepers. We do not know the exact mechanism of these results. It may be that short sleep resulted in inadequate bowel rest, bowel muscle fatigue, and, subsequently, decreased bowel movement,” said Dr. Adejumo. “Or it may also be that brain-gut signaling pathways are disrupted among short sleepers, as is seen among IBS patients after a poor night sleep, resulting in higher constipation,” he added.
Clinicians should be aware of the impact of both short and long sleep on constipation, said Dr. Adejumo. “Individuals who are unable to have adequate periods of sleep due to other diseases, including insomnia, disrupted job schedules, or conditions with too long sleep, such as narcolepsy, may all additionally suffer from constipation. Such patients may need regular evaluation and treatment for constipation to improve their discomfort,” he said.
“To confirm our findings, other clinical studies with more granular data on sleep and constipation, are needed, as well as translational research to uncover the potential mechanisms of these findings,” he emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Adejumo A et al. DDW 2020. Abstract Sa1711.
FROM DDW 2020
Key clinical point: Both short sleepers and long sleepers reported significantly more constipation compared with normal sleepers.
Major finding: Overall constipation rates were 8.3% in normal sleepers, compared with 11.0% for short sleepers and 12.5% for long sleepers.
Study details: The data come from 14,590 adults who participated in NHANES between 2005 and 2010.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Adejumo A et al. DDW 2020. Abstract Sa1711.
With life in the balance, a pediatric palliative care program expands its work to adults
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
L-thyroxine no help for older patients with symptomatic SCH
A new analysis of the large, randomized TRUST trial shows that L-thyroxine does not improve pronounced symptoms in older people with subclinical hypothyroidism.
The original trial established that the synthetic hormone did not improve symptoms in the overall trial population, a finding that called into question the routine prescribing of thyroid medication for this patient group.
But questions lingered as to whether patients with a higher burden of symptoms might still benefit from treatment with L-thyroxine.
For their research, published in Annals of Internal Medicine, Maria de Montmollin, MD, of the University of Bern (Switzerland), looked at results for 638 subjects randomized to L-thyroxine treatment (50 mcg daily for most patients) or placebo and followed for at least 1 year in the Thyroid Hormone Therapy for Older Adults With Subclinical Hypothyroidism (TRUST) trial (N Engl J Med. 2017;376:2534-2544). All were 65 years or older and met the criteria for subclinical hypothyroidism, defined as persistent elevated TSH levels (4.60-19.99 mIU/L) in combination with a normal free-thyroxine level.
Dr. de Montmollin and her colleagues identified 132 participants with high hypothyroid symptom burden at baseline and 133 patients with high scores for tiredness, using the Thyroid-Related Quality-of-Life Patient-Reported Outcome Questionnaire. Cutoffs were a baseline symptoms score of higher than 30 (on a 1-100 scale), or a tiredness score of over 40.
At 1 year, researchers saw no statistically significant improvements in either measure for the L-thyroxine treated patients, compared with placebo.
Among the patients with high symptom burden, those on L-thyroxine saw a score improvement of –12.3 points, compared with –10.4 for those on placebo, for an adjusted between-group difference of –2.0 (95% confidence interval, –5.5 to 1.5; P = 0.27). Tiredness scores also improved similarly, dropping 8.9 points for L-thyroxine–treated patients, compared with –10.9 for those receiving placebo, for an adjusted between-group difference of 0.0 (95% CI, –4.1 to 4.0; P = 0.99).
Dr. de Montmollin and colleagues also noted no significant between-group differences in two secondary measures they looked at in the study: patient self-reported quality of life and handgrip strength, an objective measure of weakness.
The results “do not support the hypothesis that the subgroup of adults with SCH [subclinical hypothyroidism] and high symptom burden before treatment benefit from L-thyroxine therapy,” the investigators wrote in their analysis. “This may be because of regression to the mean, the natural history of SCH, or the placebo effect and may explain why many persons with symptomatic SCH and their treating physicians are convinced that L-thyroxine is beneficial,” they added.
In an interview, Dr. de Montmollin commented that treating physicians “should reconsider prescribing or offering L-thyroxine to older adults with SCH, even those with consistent symptoms, because there is no clear evidence for its benefit in treating SCH to date and a risk of harm related to overtreatment is still possible. In addition, it is associated with unnecessary costs for the patient and for the health system.”
The investigators mentioned several limitations to their study, including its post hoc design and a small sample size. Additionally, they wrote, the findings “cannot exclude the possibility that a rare subgroup with greater symptom burden would benefit from L-thyroxine therapy” or that more aggressive treatment leading to lower TSH levels would confer benefit.
The study was sponsored by the National Health Service Greater Glasgow and Clyde Health Board, while the TRUST trial was sponsored by the European Union and with medication donated by Merck. Dr. de Montmollin and her coauthors disclosed no financial ties to industry.
SOURCE: De Montmollin et al. Ann Intern Med 2020 May 5. doi: 10.7326/M19-3193.
A new analysis of the large, randomized TRUST trial shows that L-thyroxine does not improve pronounced symptoms in older people with subclinical hypothyroidism.
The original trial established that the synthetic hormone did not improve symptoms in the overall trial population, a finding that called into question the routine prescribing of thyroid medication for this patient group.
But questions lingered as to whether patients with a higher burden of symptoms might still benefit from treatment with L-thyroxine.
For their research, published in Annals of Internal Medicine, Maria de Montmollin, MD, of the University of Bern (Switzerland), looked at results for 638 subjects randomized to L-thyroxine treatment (50 mcg daily for most patients) or placebo and followed for at least 1 year in the Thyroid Hormone Therapy for Older Adults With Subclinical Hypothyroidism (TRUST) trial (N Engl J Med. 2017;376:2534-2544). All were 65 years or older and met the criteria for subclinical hypothyroidism, defined as persistent elevated TSH levels (4.60-19.99 mIU/L) in combination with a normal free-thyroxine level.
Dr. de Montmollin and her colleagues identified 132 participants with high hypothyroid symptom burden at baseline and 133 patients with high scores for tiredness, using the Thyroid-Related Quality-of-Life Patient-Reported Outcome Questionnaire. Cutoffs were a baseline symptoms score of higher than 30 (on a 1-100 scale), or a tiredness score of over 40.
At 1 year, researchers saw no statistically significant improvements in either measure for the L-thyroxine treated patients, compared with placebo.
Among the patients with high symptom burden, those on L-thyroxine saw a score improvement of –12.3 points, compared with –10.4 for those on placebo, for an adjusted between-group difference of –2.0 (95% confidence interval, –5.5 to 1.5; P = 0.27). Tiredness scores also improved similarly, dropping 8.9 points for L-thyroxine–treated patients, compared with –10.9 for those receiving placebo, for an adjusted between-group difference of 0.0 (95% CI, –4.1 to 4.0; P = 0.99).
Dr. de Montmollin and colleagues also noted no significant between-group differences in two secondary measures they looked at in the study: patient self-reported quality of life and handgrip strength, an objective measure of weakness.
The results “do not support the hypothesis that the subgroup of adults with SCH [subclinical hypothyroidism] and high symptom burden before treatment benefit from L-thyroxine therapy,” the investigators wrote in their analysis. “This may be because of regression to the mean, the natural history of SCH, or the placebo effect and may explain why many persons with symptomatic SCH and their treating physicians are convinced that L-thyroxine is beneficial,” they added.
In an interview, Dr. de Montmollin commented that treating physicians “should reconsider prescribing or offering L-thyroxine to older adults with SCH, even those with consistent symptoms, because there is no clear evidence for its benefit in treating SCH to date and a risk of harm related to overtreatment is still possible. In addition, it is associated with unnecessary costs for the patient and for the health system.”
The investigators mentioned several limitations to their study, including its post hoc design and a small sample size. Additionally, they wrote, the findings “cannot exclude the possibility that a rare subgroup with greater symptom burden would benefit from L-thyroxine therapy” or that more aggressive treatment leading to lower TSH levels would confer benefit.
The study was sponsored by the National Health Service Greater Glasgow and Clyde Health Board, while the TRUST trial was sponsored by the European Union and with medication donated by Merck. Dr. de Montmollin and her coauthors disclosed no financial ties to industry.
SOURCE: De Montmollin et al. Ann Intern Med 2020 May 5. doi: 10.7326/M19-3193.
A new analysis of the large, randomized TRUST trial shows that L-thyroxine does not improve pronounced symptoms in older people with subclinical hypothyroidism.
The original trial established that the synthetic hormone did not improve symptoms in the overall trial population, a finding that called into question the routine prescribing of thyroid medication for this patient group.
But questions lingered as to whether patients with a higher burden of symptoms might still benefit from treatment with L-thyroxine.
For their research, published in Annals of Internal Medicine, Maria de Montmollin, MD, of the University of Bern (Switzerland), looked at results for 638 subjects randomized to L-thyroxine treatment (50 mcg daily for most patients) or placebo and followed for at least 1 year in the Thyroid Hormone Therapy for Older Adults With Subclinical Hypothyroidism (TRUST) trial (N Engl J Med. 2017;376:2534-2544). All were 65 years or older and met the criteria for subclinical hypothyroidism, defined as persistent elevated TSH levels (4.60-19.99 mIU/L) in combination with a normal free-thyroxine level.
Dr. de Montmollin and her colleagues identified 132 participants with high hypothyroid symptom burden at baseline and 133 patients with high scores for tiredness, using the Thyroid-Related Quality-of-Life Patient-Reported Outcome Questionnaire. Cutoffs were a baseline symptoms score of higher than 30 (on a 1-100 scale), or a tiredness score of over 40.
At 1 year, researchers saw no statistically significant improvements in either measure for the L-thyroxine treated patients, compared with placebo.
Among the patients with high symptom burden, those on L-thyroxine saw a score improvement of –12.3 points, compared with –10.4 for those on placebo, for an adjusted between-group difference of –2.0 (95% confidence interval, –5.5 to 1.5; P = 0.27). Tiredness scores also improved similarly, dropping 8.9 points for L-thyroxine–treated patients, compared with –10.9 for those receiving placebo, for an adjusted between-group difference of 0.0 (95% CI, –4.1 to 4.0; P = 0.99).
Dr. de Montmollin and colleagues also noted no significant between-group differences in two secondary measures they looked at in the study: patient self-reported quality of life and handgrip strength, an objective measure of weakness.
The results “do not support the hypothesis that the subgroup of adults with SCH [subclinical hypothyroidism] and high symptom burden before treatment benefit from L-thyroxine therapy,” the investigators wrote in their analysis. “This may be because of regression to the mean, the natural history of SCH, or the placebo effect and may explain why many persons with symptomatic SCH and their treating physicians are convinced that L-thyroxine is beneficial,” they added.
In an interview, Dr. de Montmollin commented that treating physicians “should reconsider prescribing or offering L-thyroxine to older adults with SCH, even those with consistent symptoms, because there is no clear evidence for its benefit in treating SCH to date and a risk of harm related to overtreatment is still possible. In addition, it is associated with unnecessary costs for the patient and for the health system.”
The investigators mentioned several limitations to their study, including its post hoc design and a small sample size. Additionally, they wrote, the findings “cannot exclude the possibility that a rare subgroup with greater symptom burden would benefit from L-thyroxine therapy” or that more aggressive treatment leading to lower TSH levels would confer benefit.
The study was sponsored by the National Health Service Greater Glasgow and Clyde Health Board, while the TRUST trial was sponsored by the European Union and with medication donated by Merck. Dr. de Montmollin and her coauthors disclosed no financial ties to industry.
SOURCE: De Montmollin et al. Ann Intern Med 2020 May 5. doi: 10.7326/M19-3193.
FROM ANNALS OF INTERNAL MEDICINE
Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Operation Quack Hack: FDA moves to stop fraudulent COVID-19 products
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.
As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.