User login
Recent-onset bloody nodule
A 45-year-old man presented to the Dermatology Clinic with a 4-month history of a bump on his left upper back. The lesion was tender and had been draining clear fluid and intermittent blood; he denied any preceding trauma. He had been seen both by his primary care physician and by a physician at an urgent care clinic, where he was told to use an antibiotic ointment and benzoyl peroxide daily on the area and advised to seek a dermatology consult should it not resolve. He did not see any improvement from these measures.
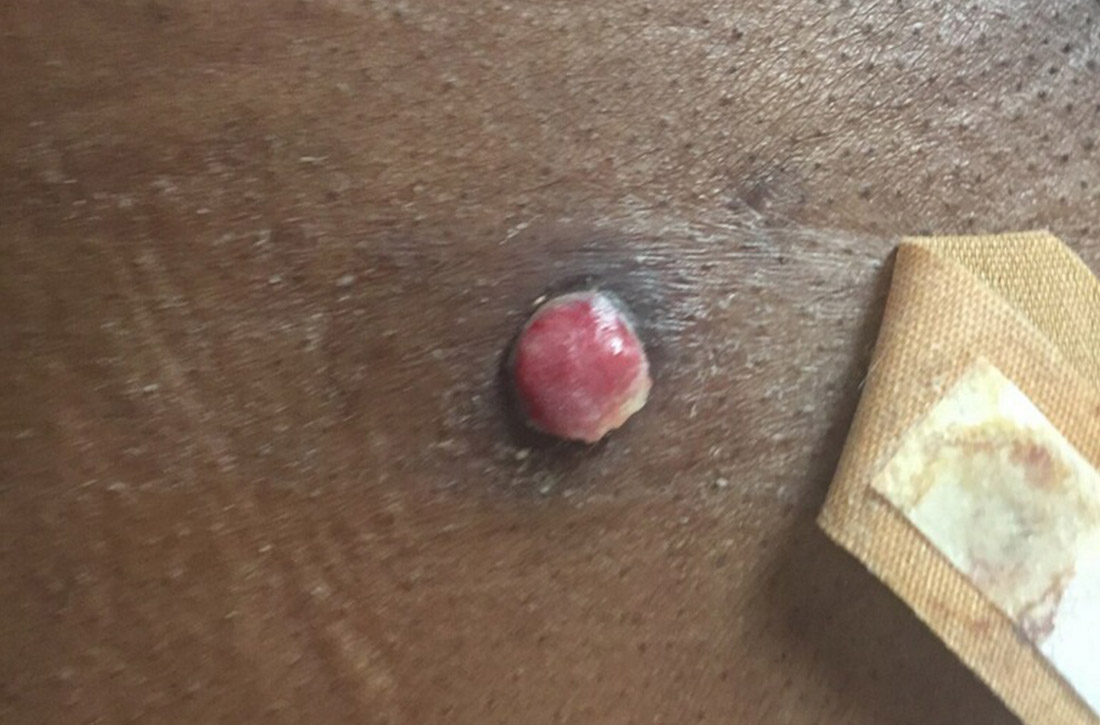
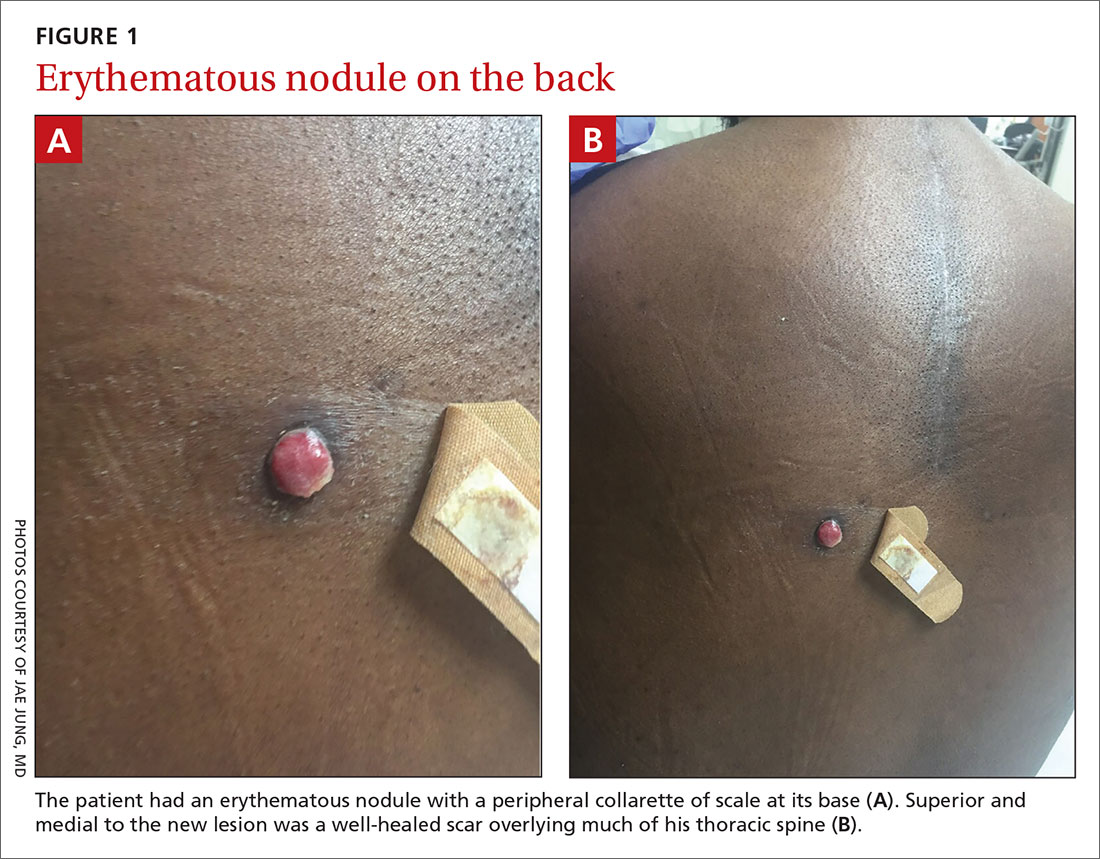
Physical exam revealed a 0.8-cm erythematous nodule with a peripheral collarette of scale at its base. The bandage used to cover the nodule was stained with hemorrhagic crust (FIGURE 1A). Superior and medial to the new lesion was a well-healed scar overlying much of the patient’s thoracic spine (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic renal cell carcinoma
The nodule initially appeared to be a benign pyogenic granuloma. In fact, a biopsy of the nodule showed a profile similar to that of a pyogenic granuloma and it exhibited granulation tissue. However, further questioning revealed that the patient had a history of metastatic clear cell renal carcinoma. (The scar was from a prior unrelated orthopedic surgery.) Immunohistochemical stains showed positive staining in the cells of interest for PAX8 and CK8, 2 markers for renal cell lineage.
Cutaneous metastasis to the skin is a rare event, representing roughly 2% of all skin tumors.1 Anatomically, lesions tend to appear on the head and neck in men and anterior chest and abdomen in women.2 Eruptions on the back, as seen in our patient, are relatively rare. The primary source of the metastasis also is gender dependent. Melanoma is the most common source overall; but in women, breast cancer represents the large majority of cutaneous metastases3 while in men lung, large intestine, and oral cavity tumors are the most common origin.3 Renal metastases are the fourth most common cause in men.3
The clinical morphology of cutaneous metastases is protean; the most common manifestations are nodules, papules, plaques, tumors, and ulcers.2 Rare manifestations include alopecia plaques, erysipelas, herpes zoster–like eruptions,4 and pyogenic granuloma–like manifestations, as in our case. Pyogenic granuloma–like manifestations have been described in renal cell carcinoma, breast carcinoma, acute myelogenous leukemia,5 and hepatocellular carcinoma.6
Differential includes an array of erythematous nodules
The differential diagnosis of a lesion with the appearance of a pyogenic granuloma is variable.
Pyogenic granulomas tend to arise over a short period of time. They are more common in children and pregnant women. Pyogenic granulomas can manifest anywhere but often are reported on the digits and extremities. Clinical history is important to ensure no history of internal malignancy.
Continue to: Bartonella henselae
Bartonella henselae, known as “cat scratch disease,” also can present as a friable, erythematous nodule reminiscent of a pyogenic granuloma. Patients with bartonella henselae usually are immunocompromised and/or have had close contact with a cat.7
Kaposi sarcoma is a vascular tumor that may manifest as erythematous papules or nodules. Erythematous or violaceous patches or plaques may be present before a nodule arises. Kaposi sarcoma may manifest on the legs of elderly patients or anywhere on immunocompromised patients. Immunohistochemical stains for human herpesvirus-8 can clinch the diagnosis.8
Amelanotic melanoma may be impossible to discern clinically from a pyogenic granuloma. It appears as erythematous, violaceous, or flesh-colored nodules. Histologic evaluation is paramount in the diagnosis.9
Clinical suspicion should prompt a biopsy
The diagnosis of metastatic renal cell carcinoma is made on clinical suspicion and skin biopsy. Dermoscopy is an important tool in the evaluation of primary cutaneous tumors. Due to the rarity of cutaneous metastases, studies on dermoscopic findings in cutaneous metastases are limited to case series. One series showed a vascular dermoscopy pattern in 15 of 17 cases (88%).10
In light of this nonspecific pattern, it’s wise to consider biopsy of a pyogenic granuloma–like lesion or one with a vascular pattern on dermoscopy in any patient with a history of malignancy. Any lesion suspected of being a pyogenic granuloma that does not respond to conservative measures also would warrant a biopsy. Definitive diagnosis is made based upon histologic evaluation.
Continue to: Surgery is the cornerstone of treatment
Surgery is the cornerstone of treatment
Upon diagnosis, immediate referral for further local and systemic control is recommended. Treatment may consist of any combination of surgery, chemotherapy, immunotherapy, or radiation.11
In this case, our patient was referred to Oncology for further treatment. Unfortunately, cutaneous metastases portend a very poor prognosis, with approximate survival times of 7.5 months.12
CORRESPONDENCE
M. Tye Haeberle, MD, 3810 Springhurst Boulevard, Ste 200, Louisville, KY 40241; [email protected]
1. Nashan D, Meiss F, Braun-Flaco M, et al. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23:567-580.
2. Alcaraz IM, Cerroni LM, Rütten AM, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
3. Lookingbill D, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
4. Hussein MR. Skin metastases: a pathologist’s perspective. J Cutan Pathol. 2010;37:E1-E20.
5. Hager C, Cohen P. Cutaneous lesions of metastatic visceral malignancy mimicking pyogenic granuloma. Cancer Invest. 1999;17:385-390.
6. Kubota Y, Koga T, Nakayama J. Cutaneous metastasis from hepatocellular carcinoma resembling pyogenic granuloma. Clin Exp Dermatol. 1999;24:78-80.
7. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203-219.
8. Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460.
9. Wee E, Wolfe R, Mclean C, et al. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79:645-651.
10. Chernoff K, Marghoob A, Lacouture M, et al. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;4:429-433.
11. Adibi M, Thomas AZ, Borregales LD, et al. Surgical considerations for patients with metastatic renal cell carcinoma. Urol Oncol. 2015;33:528-537.
12. Saeed S, Keehm C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 1994;31:419-430.
A 45-year-old man presented to the Dermatology Clinic with a 4-month history of a bump on his left upper back. The lesion was tender and had been draining clear fluid and intermittent blood; he denied any preceding trauma. He had been seen both by his primary care physician and by a physician at an urgent care clinic, where he was told to use an antibiotic ointment and benzoyl peroxide daily on the area and advised to seek a dermatology consult should it not resolve. He did not see any improvement from these measures.
Physical exam revealed a 0.8-cm erythematous nodule with a peripheral collarette of scale at its base. The bandage used to cover the nodule was stained with hemorrhagic crust (FIGURE 1A). Superior and medial to the new lesion was a well-healed scar overlying much of the patient’s thoracic spine (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic renal cell carcinoma
The nodule initially appeared to be a benign pyogenic granuloma. In fact, a biopsy of the nodule showed a profile similar to that of a pyogenic granuloma and it exhibited granulation tissue. However, further questioning revealed that the patient had a history of metastatic clear cell renal carcinoma. (The scar was from a prior unrelated orthopedic surgery.) Immunohistochemical stains showed positive staining in the cells of interest for PAX8 and CK8, 2 markers for renal cell lineage.
Cutaneous metastasis to the skin is a rare event, representing roughly 2% of all skin tumors.1 Anatomically, lesions tend to appear on the head and neck in men and anterior chest and abdomen in women.2 Eruptions on the back, as seen in our patient, are relatively rare. The primary source of the metastasis also is gender dependent. Melanoma is the most common source overall; but in women, breast cancer represents the large majority of cutaneous metastases3 while in men lung, large intestine, and oral cavity tumors are the most common origin.3 Renal metastases are the fourth most common cause in men.3
The clinical morphology of cutaneous metastases is protean; the most common manifestations are nodules, papules, plaques, tumors, and ulcers.2 Rare manifestations include alopecia plaques, erysipelas, herpes zoster–like eruptions,4 and pyogenic granuloma–like manifestations, as in our case. Pyogenic granuloma–like manifestations have been described in renal cell carcinoma, breast carcinoma, acute myelogenous leukemia,5 and hepatocellular carcinoma.6
Differential includes an array of erythematous nodules
The differential diagnosis of a lesion with the appearance of a pyogenic granuloma is variable.
Pyogenic granulomas tend to arise over a short period of time. They are more common in children and pregnant women. Pyogenic granulomas can manifest anywhere but often are reported on the digits and extremities. Clinical history is important to ensure no history of internal malignancy.
Continue to: Bartonella henselae
Bartonella henselae, known as “cat scratch disease,” also can present as a friable, erythematous nodule reminiscent of a pyogenic granuloma. Patients with bartonella henselae usually are immunocompromised and/or have had close contact with a cat.7
Kaposi sarcoma is a vascular tumor that may manifest as erythematous papules or nodules. Erythematous or violaceous patches or plaques may be present before a nodule arises. Kaposi sarcoma may manifest on the legs of elderly patients or anywhere on immunocompromised patients. Immunohistochemical stains for human herpesvirus-8 can clinch the diagnosis.8
Amelanotic melanoma may be impossible to discern clinically from a pyogenic granuloma. It appears as erythematous, violaceous, or flesh-colored nodules. Histologic evaluation is paramount in the diagnosis.9
Clinical suspicion should prompt a biopsy
The diagnosis of metastatic renal cell carcinoma is made on clinical suspicion and skin biopsy. Dermoscopy is an important tool in the evaluation of primary cutaneous tumors. Due to the rarity of cutaneous metastases, studies on dermoscopic findings in cutaneous metastases are limited to case series. One series showed a vascular dermoscopy pattern in 15 of 17 cases (88%).10
In light of this nonspecific pattern, it’s wise to consider biopsy of a pyogenic granuloma–like lesion or one with a vascular pattern on dermoscopy in any patient with a history of malignancy. Any lesion suspected of being a pyogenic granuloma that does not respond to conservative measures also would warrant a biopsy. Definitive diagnosis is made based upon histologic evaluation.
Continue to: Surgery is the cornerstone of treatment
Surgery is the cornerstone of treatment
Upon diagnosis, immediate referral for further local and systemic control is recommended. Treatment may consist of any combination of surgery, chemotherapy, immunotherapy, or radiation.11
In this case, our patient was referred to Oncology for further treatment. Unfortunately, cutaneous metastases portend a very poor prognosis, with approximate survival times of 7.5 months.12
CORRESPONDENCE
M. Tye Haeberle, MD, 3810 Springhurst Boulevard, Ste 200, Louisville, KY 40241; [email protected]
A 45-year-old man presented to the Dermatology Clinic with a 4-month history of a bump on his left upper back. The lesion was tender and had been draining clear fluid and intermittent blood; he denied any preceding trauma. He had been seen both by his primary care physician and by a physician at an urgent care clinic, where he was told to use an antibiotic ointment and benzoyl peroxide daily on the area and advised to seek a dermatology consult should it not resolve. He did not see any improvement from these measures.
Physical exam revealed a 0.8-cm erythematous nodule with a peripheral collarette of scale at its base. The bandage used to cover the nodule was stained with hemorrhagic crust (FIGURE 1A). Superior and medial to the new lesion was a well-healed scar overlying much of the patient’s thoracic spine (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic renal cell carcinoma
The nodule initially appeared to be a benign pyogenic granuloma. In fact, a biopsy of the nodule showed a profile similar to that of a pyogenic granuloma and it exhibited granulation tissue. However, further questioning revealed that the patient had a history of metastatic clear cell renal carcinoma. (The scar was from a prior unrelated orthopedic surgery.) Immunohistochemical stains showed positive staining in the cells of interest for PAX8 and CK8, 2 markers for renal cell lineage.
Cutaneous metastasis to the skin is a rare event, representing roughly 2% of all skin tumors.1 Anatomically, lesions tend to appear on the head and neck in men and anterior chest and abdomen in women.2 Eruptions on the back, as seen in our patient, are relatively rare. The primary source of the metastasis also is gender dependent. Melanoma is the most common source overall; but in women, breast cancer represents the large majority of cutaneous metastases3 while in men lung, large intestine, and oral cavity tumors are the most common origin.3 Renal metastases are the fourth most common cause in men.3
The clinical morphology of cutaneous metastases is protean; the most common manifestations are nodules, papules, plaques, tumors, and ulcers.2 Rare manifestations include alopecia plaques, erysipelas, herpes zoster–like eruptions,4 and pyogenic granuloma–like manifestations, as in our case. Pyogenic granuloma–like manifestations have been described in renal cell carcinoma, breast carcinoma, acute myelogenous leukemia,5 and hepatocellular carcinoma.6
Differential includes an array of erythematous nodules
The differential diagnosis of a lesion with the appearance of a pyogenic granuloma is variable.
Pyogenic granulomas tend to arise over a short period of time. They are more common in children and pregnant women. Pyogenic granulomas can manifest anywhere but often are reported on the digits and extremities. Clinical history is important to ensure no history of internal malignancy.
Continue to: Bartonella henselae
Bartonella henselae, known as “cat scratch disease,” also can present as a friable, erythematous nodule reminiscent of a pyogenic granuloma. Patients with bartonella henselae usually are immunocompromised and/or have had close contact with a cat.7
Kaposi sarcoma is a vascular tumor that may manifest as erythematous papules or nodules. Erythematous or violaceous patches or plaques may be present before a nodule arises. Kaposi sarcoma may manifest on the legs of elderly patients or anywhere on immunocompromised patients. Immunohistochemical stains for human herpesvirus-8 can clinch the diagnosis.8
Amelanotic melanoma may be impossible to discern clinically from a pyogenic granuloma. It appears as erythematous, violaceous, or flesh-colored nodules. Histologic evaluation is paramount in the diagnosis.9
Clinical suspicion should prompt a biopsy
The diagnosis of metastatic renal cell carcinoma is made on clinical suspicion and skin biopsy. Dermoscopy is an important tool in the evaluation of primary cutaneous tumors. Due to the rarity of cutaneous metastases, studies on dermoscopic findings in cutaneous metastases are limited to case series. One series showed a vascular dermoscopy pattern in 15 of 17 cases (88%).10
In light of this nonspecific pattern, it’s wise to consider biopsy of a pyogenic granuloma–like lesion or one with a vascular pattern on dermoscopy in any patient with a history of malignancy. Any lesion suspected of being a pyogenic granuloma that does not respond to conservative measures also would warrant a biopsy. Definitive diagnosis is made based upon histologic evaluation.
Continue to: Surgery is the cornerstone of treatment
Surgery is the cornerstone of treatment
Upon diagnosis, immediate referral for further local and systemic control is recommended. Treatment may consist of any combination of surgery, chemotherapy, immunotherapy, or radiation.11
In this case, our patient was referred to Oncology for further treatment. Unfortunately, cutaneous metastases portend a very poor prognosis, with approximate survival times of 7.5 months.12
CORRESPONDENCE
M. Tye Haeberle, MD, 3810 Springhurst Boulevard, Ste 200, Louisville, KY 40241; [email protected]
1. Nashan D, Meiss F, Braun-Flaco M, et al. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23:567-580.
2. Alcaraz IM, Cerroni LM, Rütten AM, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
3. Lookingbill D, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
4. Hussein MR. Skin metastases: a pathologist’s perspective. J Cutan Pathol. 2010;37:E1-E20.
5. Hager C, Cohen P. Cutaneous lesions of metastatic visceral malignancy mimicking pyogenic granuloma. Cancer Invest. 1999;17:385-390.
6. Kubota Y, Koga T, Nakayama J. Cutaneous metastasis from hepatocellular carcinoma resembling pyogenic granuloma. Clin Exp Dermatol. 1999;24:78-80.
7. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203-219.
8. Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460.
9. Wee E, Wolfe R, Mclean C, et al. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79:645-651.
10. Chernoff K, Marghoob A, Lacouture M, et al. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;4:429-433.
11. Adibi M, Thomas AZ, Borregales LD, et al. Surgical considerations for patients with metastatic renal cell carcinoma. Urol Oncol. 2015;33:528-537.
12. Saeed S, Keehm C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 1994;31:419-430.
1. Nashan D, Meiss F, Braun-Flaco M, et al. Cutaneous metastases from internal malignancies. Dermatol Ther. 2010;23:567-580.
2. Alcaraz IM, Cerroni LM, Rütten AM, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
3. Lookingbill D, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
4. Hussein MR. Skin metastases: a pathologist’s perspective. J Cutan Pathol. 2010;37:E1-E20.
5. Hager C, Cohen P. Cutaneous lesions of metastatic visceral malignancy mimicking pyogenic granuloma. Cancer Invest. 1999;17:385-390.
6. Kubota Y, Koga T, Nakayama J. Cutaneous metastasis from hepatocellular carcinoma resembling pyogenic granuloma. Clin Exp Dermatol. 1999;24:78-80.
7. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203-219.
8. Patel RM, Goldblum JR, Hsi ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456-460.
9. Wee E, Wolfe R, Mclean C, et al. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79:645-651.
10. Chernoff K, Marghoob A, Lacouture M, et al. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;4:429-433.
11. Adibi M, Thomas AZ, Borregales LD, et al. Surgical considerations for patients with metastatic renal cell carcinoma. Urol Oncol. 2015;33:528-537.
12. Saeed S, Keehm C, Morgan M. Cutaneous metastases: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol. 1994;31:419-430.
How to responsibly engage with social media during disasters
A few months into the COVID-19 pandemic, social media’s role in the rapid spread of information is undeniable. From the beginning, Chinese ophthalmologist Li Wenliang, MD, first raised the alarm to his classmates through WeChat, a messaging and social media app. Since that time, individuals, groups, organizations, government agencies, and mass media outlets have used social media to share ideas and disseminate information. Individuals check in on loved ones and update others on their own safety. Networks of clinicians discuss patient presentations, new therapeutics, management strategies, and institutional protocols. Multiple organizations including the Federal Emergency Management Agency, the Centers for Disease Control and Prevention, and the World Health Organization use Facebook, Instagram, or Twitter accounts to provide updates on ongoing efforts and spread public health messaging.
Unfortunately, not all information is trustworthy. Social media outlets have been used to spread misinformation and conspiracy theories, and to promote false treatments. Google, YouTube, and Facebook are now actively trying to reduce the viral spread of misleading information and to block hoaxes. With the increasing amount of news and information consumed and disseminated via social media, clinicians need to critically appraise information presented on those platforms, and to be familiar with how to use them to disseminate informed, effective, and responsible information.
Appraisal of social media content
Traditional scholarly communication exists in many forms and includes observations, anecdotes, perspectives, case reports, and research. Each form involves differing levels of academic rigor and standards of evaluation. Electronic content and online resources pose a unique challenge because there is no standardized method for assessing impact and quality. Proposed scales for evaluation of online resources such as Medical Education Translational Resources: Impact and Quality (METRIQ),1 Academic Life in Emergency Medicine Approved Instructional Resources (AliEM AIR) scoring system,2 and the Social Media Index3 are promising and can be used to guide critical appraisal of social media content.
The same skepticism and critical thinking applied to traditional resources should be applied when evaluating online resources. The scales listed above include questions such as:
- How accurate is the data presented and conclusions drawn?
- Does the content reflect evidence-based medicine?
- Has the content undergone an editorial process?
- Who are the authors and what are their credentials?
- Are there potential biases or conflicts of interest present?
- Have references been cited?
- How does this content affect/change clinical practice?
While these proposed review metrics may not apply to all forms of social media content, clinicians should be discerning when consuming or disseminating online content.
Strategies for effective communication on social media
In addition to appraising social media content, clinicians also should be able to craft effective messages on social media to spread trustworthy content. The CDC offers guidelines and best practices for social media communication4,5 and the WHO has created a framework for effective communications.6 Both organizations recognize social media as a powerful communication tool that has the potential to greatly impact public health efforts.
Some key principles highlighted from these sources include the following:
- Identify an audience and make messages relevant. Taking time to listen to key stakeholders within the target audience (individuals, health care providers, communities, policy-makers, organizations) allows for better understanding of baseline knowledge, attitudes, and beliefs that may drive concerns and ultimately helps to tailor the messaging.
- Make messages accessible. Certain social media platforms are more often utilized for specific target audiences. Verbiage used should take into account the health literacy of the audience. A friendly, professional, conversational tone encourages interaction and dialogue.
- Engage the audience by offering something actionable. Changing behavior is a daunting task that involves multiple steps. Encouraging behavioral changes initially at an individual level has the potential to influence community practices and policies.
- Communication should be timely. It should address current and urgent topics. Keep abreast of the situation as it evolves to ensure messaging stays relevant. Deliver consistent messaging and updates.
- Sources must be credible. It is important to be transparent about expertise and honest about what is known and unknown about the topic.
- Content should be understandable. In addition to using plain language, visual aids and real stories can be used to reinforce messages.
Use social media responsibly
Clinicians have a responsibility to use social media to disseminate credible content, refute misleading content, and create accurate content. When clinicians share health-related information via social media, it should be appraised skeptically and crafted responsibly because that message can have profound implications on public health. Mixed messaging that is contradictory, inconsistent, or unclear can lead to panic and confusion. By recognizing the important role of social media in access to information and as a tool for public health messaging and crisis communication, clinicians have an obligation to consider both the positive and negative impacts as messengers in that space.
Dr. Ren is a pediatric emergency medicine fellow at Children’s National Hospital, Washington. Dr. Simpson is a pediatric emergency medicine attending and medical director of emergency preparedness of Children’s National Hospital. They do not have any disclosures or conflicts of interest. Email Dr. Ren and Dr. Simpson at [email protected].
References
1. AEM Educ Train. 2019;3(4):387-92.
2. Ann Emerg Med. 2016;68(6):729-35.
3. Ann Emerg Med. 2018;72(6):696-702.
4. CDC Guide to Writing for Social Media.
5. The Health Communicator’s Social Media Toolkit.
6. WHO Strategic Communications Framework for effective communications.
A few months into the COVID-19 pandemic, social media’s role in the rapid spread of information is undeniable. From the beginning, Chinese ophthalmologist Li Wenliang, MD, first raised the alarm to his classmates through WeChat, a messaging and social media app. Since that time, individuals, groups, organizations, government agencies, and mass media outlets have used social media to share ideas and disseminate information. Individuals check in on loved ones and update others on their own safety. Networks of clinicians discuss patient presentations, new therapeutics, management strategies, and institutional protocols. Multiple organizations including the Federal Emergency Management Agency, the Centers for Disease Control and Prevention, and the World Health Organization use Facebook, Instagram, or Twitter accounts to provide updates on ongoing efforts and spread public health messaging.
Unfortunately, not all information is trustworthy. Social media outlets have been used to spread misinformation and conspiracy theories, and to promote false treatments. Google, YouTube, and Facebook are now actively trying to reduce the viral spread of misleading information and to block hoaxes. With the increasing amount of news and information consumed and disseminated via social media, clinicians need to critically appraise information presented on those platforms, and to be familiar with how to use them to disseminate informed, effective, and responsible information.
Appraisal of social media content
Traditional scholarly communication exists in many forms and includes observations, anecdotes, perspectives, case reports, and research. Each form involves differing levels of academic rigor and standards of evaluation. Electronic content and online resources pose a unique challenge because there is no standardized method for assessing impact and quality. Proposed scales for evaluation of online resources such as Medical Education Translational Resources: Impact and Quality (METRIQ),1 Academic Life in Emergency Medicine Approved Instructional Resources (AliEM AIR) scoring system,2 and the Social Media Index3 are promising and can be used to guide critical appraisal of social media content.
The same skepticism and critical thinking applied to traditional resources should be applied when evaluating online resources. The scales listed above include questions such as:
- How accurate is the data presented and conclusions drawn?
- Does the content reflect evidence-based medicine?
- Has the content undergone an editorial process?
- Who are the authors and what are their credentials?
- Are there potential biases or conflicts of interest present?
- Have references been cited?
- How does this content affect/change clinical practice?
While these proposed review metrics may not apply to all forms of social media content, clinicians should be discerning when consuming or disseminating online content.
Strategies for effective communication on social media
In addition to appraising social media content, clinicians also should be able to craft effective messages on social media to spread trustworthy content. The CDC offers guidelines and best practices for social media communication4,5 and the WHO has created a framework for effective communications.6 Both organizations recognize social media as a powerful communication tool that has the potential to greatly impact public health efforts.
Some key principles highlighted from these sources include the following:
- Identify an audience and make messages relevant. Taking time to listen to key stakeholders within the target audience (individuals, health care providers, communities, policy-makers, organizations) allows for better understanding of baseline knowledge, attitudes, and beliefs that may drive concerns and ultimately helps to tailor the messaging.
- Make messages accessible. Certain social media platforms are more often utilized for specific target audiences. Verbiage used should take into account the health literacy of the audience. A friendly, professional, conversational tone encourages interaction and dialogue.
- Engage the audience by offering something actionable. Changing behavior is a daunting task that involves multiple steps. Encouraging behavioral changes initially at an individual level has the potential to influence community practices and policies.
- Communication should be timely. It should address current and urgent topics. Keep abreast of the situation as it evolves to ensure messaging stays relevant. Deliver consistent messaging and updates.
- Sources must be credible. It is important to be transparent about expertise and honest about what is known and unknown about the topic.
- Content should be understandable. In addition to using plain language, visual aids and real stories can be used to reinforce messages.
Use social media responsibly
Clinicians have a responsibility to use social media to disseminate credible content, refute misleading content, and create accurate content. When clinicians share health-related information via social media, it should be appraised skeptically and crafted responsibly because that message can have profound implications on public health. Mixed messaging that is contradictory, inconsistent, or unclear can lead to panic and confusion. By recognizing the important role of social media in access to information and as a tool for public health messaging and crisis communication, clinicians have an obligation to consider both the positive and negative impacts as messengers in that space.
Dr. Ren is a pediatric emergency medicine fellow at Children’s National Hospital, Washington. Dr. Simpson is a pediatric emergency medicine attending and medical director of emergency preparedness of Children’s National Hospital. They do not have any disclosures or conflicts of interest. Email Dr. Ren and Dr. Simpson at [email protected].
References
1. AEM Educ Train. 2019;3(4):387-92.
2. Ann Emerg Med. 2016;68(6):729-35.
3. Ann Emerg Med. 2018;72(6):696-702.
4. CDC Guide to Writing for Social Media.
5. The Health Communicator’s Social Media Toolkit.
6. WHO Strategic Communications Framework for effective communications.
A few months into the COVID-19 pandemic, social media’s role in the rapid spread of information is undeniable. From the beginning, Chinese ophthalmologist Li Wenliang, MD, first raised the alarm to his classmates through WeChat, a messaging and social media app. Since that time, individuals, groups, organizations, government agencies, and mass media outlets have used social media to share ideas and disseminate information. Individuals check in on loved ones and update others on their own safety. Networks of clinicians discuss patient presentations, new therapeutics, management strategies, and institutional protocols. Multiple organizations including the Federal Emergency Management Agency, the Centers for Disease Control and Prevention, and the World Health Organization use Facebook, Instagram, or Twitter accounts to provide updates on ongoing efforts and spread public health messaging.
Unfortunately, not all information is trustworthy. Social media outlets have been used to spread misinformation and conspiracy theories, and to promote false treatments. Google, YouTube, and Facebook are now actively trying to reduce the viral spread of misleading information and to block hoaxes. With the increasing amount of news and information consumed and disseminated via social media, clinicians need to critically appraise information presented on those platforms, and to be familiar with how to use them to disseminate informed, effective, and responsible information.
Appraisal of social media content
Traditional scholarly communication exists in many forms and includes observations, anecdotes, perspectives, case reports, and research. Each form involves differing levels of academic rigor and standards of evaluation. Electronic content and online resources pose a unique challenge because there is no standardized method for assessing impact and quality. Proposed scales for evaluation of online resources such as Medical Education Translational Resources: Impact and Quality (METRIQ),1 Academic Life in Emergency Medicine Approved Instructional Resources (AliEM AIR) scoring system,2 and the Social Media Index3 are promising and can be used to guide critical appraisal of social media content.
The same skepticism and critical thinking applied to traditional resources should be applied when evaluating online resources. The scales listed above include questions such as:
- How accurate is the data presented and conclusions drawn?
- Does the content reflect evidence-based medicine?
- Has the content undergone an editorial process?
- Who are the authors and what are their credentials?
- Are there potential biases or conflicts of interest present?
- Have references been cited?
- How does this content affect/change clinical practice?
While these proposed review metrics may not apply to all forms of social media content, clinicians should be discerning when consuming or disseminating online content.
Strategies for effective communication on social media
In addition to appraising social media content, clinicians also should be able to craft effective messages on social media to spread trustworthy content. The CDC offers guidelines and best practices for social media communication4,5 and the WHO has created a framework for effective communications.6 Both organizations recognize social media as a powerful communication tool that has the potential to greatly impact public health efforts.
Some key principles highlighted from these sources include the following:
- Identify an audience and make messages relevant. Taking time to listen to key stakeholders within the target audience (individuals, health care providers, communities, policy-makers, organizations) allows for better understanding of baseline knowledge, attitudes, and beliefs that may drive concerns and ultimately helps to tailor the messaging.
- Make messages accessible. Certain social media platforms are more often utilized for specific target audiences. Verbiage used should take into account the health literacy of the audience. A friendly, professional, conversational tone encourages interaction and dialogue.
- Engage the audience by offering something actionable. Changing behavior is a daunting task that involves multiple steps. Encouraging behavioral changes initially at an individual level has the potential to influence community practices and policies.
- Communication should be timely. It should address current and urgent topics. Keep abreast of the situation as it evolves to ensure messaging stays relevant. Deliver consistent messaging and updates.
- Sources must be credible. It is important to be transparent about expertise and honest about what is known and unknown about the topic.
- Content should be understandable. In addition to using plain language, visual aids and real stories can be used to reinforce messages.
Use social media responsibly
Clinicians have a responsibility to use social media to disseminate credible content, refute misleading content, and create accurate content. When clinicians share health-related information via social media, it should be appraised skeptically and crafted responsibly because that message can have profound implications on public health. Mixed messaging that is contradictory, inconsistent, or unclear can lead to panic and confusion. By recognizing the important role of social media in access to information and as a tool for public health messaging and crisis communication, clinicians have an obligation to consider both the positive and negative impacts as messengers in that space.
Dr. Ren is a pediatric emergency medicine fellow at Children’s National Hospital, Washington. Dr. Simpson is a pediatric emergency medicine attending and medical director of emergency preparedness of Children’s National Hospital. They do not have any disclosures or conflicts of interest. Email Dr. Ren and Dr. Simpson at [email protected].
References
1. AEM Educ Train. 2019;3(4):387-92.
2. Ann Emerg Med. 2016;68(6):729-35.
3. Ann Emerg Med. 2018;72(6):696-702.
4. CDC Guide to Writing for Social Media.
5. The Health Communicator’s Social Media Toolkit.
6. WHO Strategic Communications Framework for effective communications.
Performance of the Veterans Choice Program for Improving Access to Colonoscopy at a Tertiary VA Facility
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
Urgent and Emergent Eye Care Strategies to Protect Against COVID-19
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
The Duty to Care and Its Exceptions in a Pandemic
As of April 9, 2020, the Centers for Disease Control and Prevention (CDC) reported that 9,282 health care providers in the US had contracted COVID-19, and 27 had died of the virus.2 Medscape reports the toll as much higher. Thousands more nurses, doctors, epidemiologists, social workers, physician assistants, dentists, pharmacists, and other health care workers from Italy, China, and dozens of other countries have died fighting this plague.3
The truth is no one knows how many health care workers are actually sick or even have died. State and federal governments have not been routinely and specifically tracking that data, making these already grim statistics likely a gross underestimation.4 While not all of these health care providers were exposed to COVID-19 in the line of duty, many were, and many more will be as the pandemic subsides in one epicenter only to erupt in another, and smolders for months until a vaccine quenches it.
Each of those lost lives of promise had a story of hard work and sacrifice to become a health care professional, of friends and family who loved and cared for them when ill, who need and grieve for them, now gone far too soon. Nor should we forget to mourn all of the administrative professionals, the line and support staff of health care facilities, who also perished fighting the pestilence. It is fitting then, that this second editorial in my pledge to write each month about COVID-19 until the pandemic ends, be about the duty to care and its limits.
The duty to care is among the most fundamental and ancient ethical obligations of health care providers. It is included even in modern codes of ethics like that of the American Medical Association and American Nurses Association. The obligation to not abandon patients is even more compelling for the Military Health System, Veterans Health Administration (VHA), and the US Public Health Service whose health care mission also is a public trust. The duty is rooted in the fiduciary nature of the health professions in which the interests of the patient should take priority over other considerations, including a risk to their own health and life. Prioritization though has exceptions. Physician and attorney David Orentlicher points out the unconditional obligation that bound physicians in the 14th century Black Death, or the 1918 Spanish influenza, now admits exceptions and qualifications.5
The exception that has become the object of greatest concern to health care workers is personal protective equipment (PPE). In modern public health ethics, health care systems and state and federal governments have a corresponding ethical obligation of reciprocity toward their employees whose work places them at elevated risk of harm—in this case, COVID-19 exposure. The principle of reciprocity encompasses the measures and materials that health care institutions need to provide to health care workers to reasonably minimize the risk of viral transmission. The reasonableness standard does not demand that there be zero risk. It does require that health care workers have adequate and appropriate PPE so that in fulfilling their duty to care they are not exposed to a disproportionate risk.
This last assertion has been the subject of controversy in the media and consternation on the part of health care professionals for several disconcerting reasons. First and foremost, a cascade failure on the part of government and industry has resulted in PPE being the scarcest health care resource in this pandemic.6 The shortage is as serious as that of the life-saving ventilators that are rightly at the center of most crisis standards resource allocation plans.7 Second, the guidance from the CDC and other authoritative sources continues to change. This is, in part, to adjust to the even more rapid pace of knowledge about the virus and its behavior and to adapt to the reality of insufficient PPE.8
Understandably, health care providers, especially those on the frontlines, may lose trust in the scientific experts and the leadership of their institutions, compounding the climate of moral distress in a public health crisis. Health care workers in the community, and even in federal service, have launched socially distanced protests and taken to social media to voice their concern and rally assistance.9,10 In response, VHA Executive-in-Charge Richard Stone, MD, admitted that VHA does have a shortage of PPE in a Washington Post interview.11 He outlined how the organization plans to address staff concerns. The article also reported only a 4% absentee rate of VHA staff as opposed to the 40% that plans predicted was possible. This demonstrates once more the dedication of VHA health care professionals and workers to fulfill their duty to care for veterans even amid fears about inadequate PPE.
In the epigraph, Albert Camus captures the uncertainty and fear that as humans all health care providers experience as they face the unpredictable but very real threat of COVID-19.1 Camus expresses even more strongly the devotion to duty of health care providers to care for vulnerable ill patients in need despite the inherent threat in a highly transmissible and potentially deadly infection that is inextricably linked to that caring. Orentlicher wisely opines that the integrity of the health professions and their respected role in society benefit from a strong duty to care.5 The best way to promote that duty is to do all in our power to protect those who willingly brave the pestilence to treat, and hope and pray someday to cure COVID-19.
1. Camus A. The Plague. Vintage Books: New York; 1948:120.
2. CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19— United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
3. In memoriam: healthcare workers who have died of COVID-19. https://www.medscape.com/viewarticle/927976. Updated April 21, 2020. Accessed April 22, 2020.
4. Galvin G. The great unknown: how many health care workers have coronavirus? https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Published April 3, 2020. Accessed April 22, 2020.
5. Orentlicher D. The physician’s duty to treat during pandemics. Am J Public Health. 2018;108(11):1459-1461.
6. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. [Published online ahead of print, 2020 Mar 25.] N Engl J Med. 2020;10.1056/NEJMp2006141.
7. New York State Task Force on Life and the Law, New York State Department of Health. Ventilator allocation guidelines. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published November 2015. Accessed April 22, 2020.
8. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): Strategies to optimize PPE and equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Updated April 3, 2020. Accessed April 22, 2020.
9. Wentling N. ‘It’s out of control’: VA nurses demand more protection against coronavirus. https://www.stripes.com/news/veterans/va-nurses-demand-more-protection-against-coronavirus-1.626910. Updated April 21, 2020. Accessed April 22, 2020.
10. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html. Updated March 22, 2020. Accessed April 22, 2020.
11. Rein L. VA health chief acknowledges a shortage of protective gear for its hospital workers. https://www.washingtonpost.com/politics/va-health-chief-acknowledges-a-shortage-of-protective-gear-for-its-hospital-workers/2020/04/24/4c1bcd5e-84bf-11ea-ae26-989cfce1c7c7_story.html. Published April 25, 2020. Accessed April 27, 2020.
As of April 9, 2020, the Centers for Disease Control and Prevention (CDC) reported that 9,282 health care providers in the US had contracted COVID-19, and 27 had died of the virus.2 Medscape reports the toll as much higher. Thousands more nurses, doctors, epidemiologists, social workers, physician assistants, dentists, pharmacists, and other health care workers from Italy, China, and dozens of other countries have died fighting this plague.3
The truth is no one knows how many health care workers are actually sick or even have died. State and federal governments have not been routinely and specifically tracking that data, making these already grim statistics likely a gross underestimation.4 While not all of these health care providers were exposed to COVID-19 in the line of duty, many were, and many more will be as the pandemic subsides in one epicenter only to erupt in another, and smolders for months until a vaccine quenches it.
Each of those lost lives of promise had a story of hard work and sacrifice to become a health care professional, of friends and family who loved and cared for them when ill, who need and grieve for them, now gone far too soon. Nor should we forget to mourn all of the administrative professionals, the line and support staff of health care facilities, who also perished fighting the pestilence. It is fitting then, that this second editorial in my pledge to write each month about COVID-19 until the pandemic ends, be about the duty to care and its limits.
The duty to care is among the most fundamental and ancient ethical obligations of health care providers. It is included even in modern codes of ethics like that of the American Medical Association and American Nurses Association. The obligation to not abandon patients is even more compelling for the Military Health System, Veterans Health Administration (VHA), and the US Public Health Service whose health care mission also is a public trust. The duty is rooted in the fiduciary nature of the health professions in which the interests of the patient should take priority over other considerations, including a risk to their own health and life. Prioritization though has exceptions. Physician and attorney David Orentlicher points out the unconditional obligation that bound physicians in the 14th century Black Death, or the 1918 Spanish influenza, now admits exceptions and qualifications.5
The exception that has become the object of greatest concern to health care workers is personal protective equipment (PPE). In modern public health ethics, health care systems and state and federal governments have a corresponding ethical obligation of reciprocity toward their employees whose work places them at elevated risk of harm—in this case, COVID-19 exposure. The principle of reciprocity encompasses the measures and materials that health care institutions need to provide to health care workers to reasonably minimize the risk of viral transmission. The reasonableness standard does not demand that there be zero risk. It does require that health care workers have adequate and appropriate PPE so that in fulfilling their duty to care they are not exposed to a disproportionate risk.
This last assertion has been the subject of controversy in the media and consternation on the part of health care professionals for several disconcerting reasons. First and foremost, a cascade failure on the part of government and industry has resulted in PPE being the scarcest health care resource in this pandemic.6 The shortage is as serious as that of the life-saving ventilators that are rightly at the center of most crisis standards resource allocation plans.7 Second, the guidance from the CDC and other authoritative sources continues to change. This is, in part, to adjust to the even more rapid pace of knowledge about the virus and its behavior and to adapt to the reality of insufficient PPE.8
Understandably, health care providers, especially those on the frontlines, may lose trust in the scientific experts and the leadership of their institutions, compounding the climate of moral distress in a public health crisis. Health care workers in the community, and even in federal service, have launched socially distanced protests and taken to social media to voice their concern and rally assistance.9,10 In response, VHA Executive-in-Charge Richard Stone, MD, admitted that VHA does have a shortage of PPE in a Washington Post interview.11 He outlined how the organization plans to address staff concerns. The article also reported only a 4% absentee rate of VHA staff as opposed to the 40% that plans predicted was possible. This demonstrates once more the dedication of VHA health care professionals and workers to fulfill their duty to care for veterans even amid fears about inadequate PPE.
In the epigraph, Albert Camus captures the uncertainty and fear that as humans all health care providers experience as they face the unpredictable but very real threat of COVID-19.1 Camus expresses even more strongly the devotion to duty of health care providers to care for vulnerable ill patients in need despite the inherent threat in a highly transmissible and potentially deadly infection that is inextricably linked to that caring. Orentlicher wisely opines that the integrity of the health professions and their respected role in society benefit from a strong duty to care.5 The best way to promote that duty is to do all in our power to protect those who willingly brave the pestilence to treat, and hope and pray someday to cure COVID-19.
As of April 9, 2020, the Centers for Disease Control and Prevention (CDC) reported that 9,282 health care providers in the US had contracted COVID-19, and 27 had died of the virus.2 Medscape reports the toll as much higher. Thousands more nurses, doctors, epidemiologists, social workers, physician assistants, dentists, pharmacists, and other health care workers from Italy, China, and dozens of other countries have died fighting this plague.3
The truth is no one knows how many health care workers are actually sick or even have died. State and federal governments have not been routinely and specifically tracking that data, making these already grim statistics likely a gross underestimation.4 While not all of these health care providers were exposed to COVID-19 in the line of duty, many were, and many more will be as the pandemic subsides in one epicenter only to erupt in another, and smolders for months until a vaccine quenches it.
Each of those lost lives of promise had a story of hard work and sacrifice to become a health care professional, of friends and family who loved and cared for them when ill, who need and grieve for them, now gone far too soon. Nor should we forget to mourn all of the administrative professionals, the line and support staff of health care facilities, who also perished fighting the pestilence. It is fitting then, that this second editorial in my pledge to write each month about COVID-19 until the pandemic ends, be about the duty to care and its limits.
The duty to care is among the most fundamental and ancient ethical obligations of health care providers. It is included even in modern codes of ethics like that of the American Medical Association and American Nurses Association. The obligation to not abandon patients is even more compelling for the Military Health System, Veterans Health Administration (VHA), and the US Public Health Service whose health care mission also is a public trust. The duty is rooted in the fiduciary nature of the health professions in which the interests of the patient should take priority over other considerations, including a risk to their own health and life. Prioritization though has exceptions. Physician and attorney David Orentlicher points out the unconditional obligation that bound physicians in the 14th century Black Death, or the 1918 Spanish influenza, now admits exceptions and qualifications.5
The exception that has become the object of greatest concern to health care workers is personal protective equipment (PPE). In modern public health ethics, health care systems and state and federal governments have a corresponding ethical obligation of reciprocity toward their employees whose work places them at elevated risk of harm—in this case, COVID-19 exposure. The principle of reciprocity encompasses the measures and materials that health care institutions need to provide to health care workers to reasonably minimize the risk of viral transmission. The reasonableness standard does not demand that there be zero risk. It does require that health care workers have adequate and appropriate PPE so that in fulfilling their duty to care they are not exposed to a disproportionate risk.
This last assertion has been the subject of controversy in the media and consternation on the part of health care professionals for several disconcerting reasons. First and foremost, a cascade failure on the part of government and industry has resulted in PPE being the scarcest health care resource in this pandemic.6 The shortage is as serious as that of the life-saving ventilators that are rightly at the center of most crisis standards resource allocation plans.7 Second, the guidance from the CDC and other authoritative sources continues to change. This is, in part, to adjust to the even more rapid pace of knowledge about the virus and its behavior and to adapt to the reality of insufficient PPE.8
Understandably, health care providers, especially those on the frontlines, may lose trust in the scientific experts and the leadership of their institutions, compounding the climate of moral distress in a public health crisis. Health care workers in the community, and even in federal service, have launched socially distanced protests and taken to social media to voice their concern and rally assistance.9,10 In response, VHA Executive-in-Charge Richard Stone, MD, admitted that VHA does have a shortage of PPE in a Washington Post interview.11 He outlined how the organization plans to address staff concerns. The article also reported only a 4% absentee rate of VHA staff as opposed to the 40% that plans predicted was possible. This demonstrates once more the dedication of VHA health care professionals and workers to fulfill their duty to care for veterans even amid fears about inadequate PPE.
In the epigraph, Albert Camus captures the uncertainty and fear that as humans all health care providers experience as they face the unpredictable but very real threat of COVID-19.1 Camus expresses even more strongly the devotion to duty of health care providers to care for vulnerable ill patients in need despite the inherent threat in a highly transmissible and potentially deadly infection that is inextricably linked to that caring. Orentlicher wisely opines that the integrity of the health professions and their respected role in society benefit from a strong duty to care.5 The best way to promote that duty is to do all in our power to protect those who willingly brave the pestilence to treat, and hope and pray someday to cure COVID-19.
1. Camus A. The Plague. Vintage Books: New York; 1948:120.
2. CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19— United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
3. In memoriam: healthcare workers who have died of COVID-19. https://www.medscape.com/viewarticle/927976. Updated April 21, 2020. Accessed April 22, 2020.
4. Galvin G. The great unknown: how many health care workers have coronavirus? https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Published April 3, 2020. Accessed April 22, 2020.
5. Orentlicher D. The physician’s duty to treat during pandemics. Am J Public Health. 2018;108(11):1459-1461.
6. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. [Published online ahead of print, 2020 Mar 25.] N Engl J Med. 2020;10.1056/NEJMp2006141.
7. New York State Task Force on Life and the Law, New York State Department of Health. Ventilator allocation guidelines. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published November 2015. Accessed April 22, 2020.
8. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): Strategies to optimize PPE and equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Updated April 3, 2020. Accessed April 22, 2020.
9. Wentling N. ‘It’s out of control’: VA nurses demand more protection against coronavirus. https://www.stripes.com/news/veterans/va-nurses-demand-more-protection-against-coronavirus-1.626910. Updated April 21, 2020. Accessed April 22, 2020.
10. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html. Updated March 22, 2020. Accessed April 22, 2020.
11. Rein L. VA health chief acknowledges a shortage of protective gear for its hospital workers. https://www.washingtonpost.com/politics/va-health-chief-acknowledges-a-shortage-of-protective-gear-for-its-hospital-workers/2020/04/24/4c1bcd5e-84bf-11ea-ae26-989cfce1c7c7_story.html. Published April 25, 2020. Accessed April 27, 2020.
1. Camus A. The Plague. Vintage Books: New York; 1948:120.
2. CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19— United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
3. In memoriam: healthcare workers who have died of COVID-19. https://www.medscape.com/viewarticle/927976. Updated April 21, 2020. Accessed April 22, 2020.
4. Galvin G. The great unknown: how many health care workers have coronavirus? https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Published April 3, 2020. Accessed April 22, 2020.
5. Orentlicher D. The physician’s duty to treat during pandemics. Am J Public Health. 2018;108(11):1459-1461.
6. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. [Published online ahead of print, 2020 Mar 25.] N Engl J Med. 2020;10.1056/NEJMp2006141.
7. New York State Task Force on Life and the Law, New York State Department of Health. Ventilator allocation guidelines. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published November 2015. Accessed April 22, 2020.
8. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): Strategies to optimize PPE and equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Updated April 3, 2020. Accessed April 22, 2020.
9. Wentling N. ‘It’s out of control’: VA nurses demand more protection against coronavirus. https://www.stripes.com/news/veterans/va-nurses-demand-more-protection-against-coronavirus-1.626910. Updated April 21, 2020. Accessed April 22, 2020.
10. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html. Updated March 22, 2020. Accessed April 22, 2020.
11. Rein L. VA health chief acknowledges a shortage of protective gear for its hospital workers. https://www.washingtonpost.com/politics/va-health-chief-acknowledges-a-shortage-of-protective-gear-for-its-hospital-workers/2020/04/24/4c1bcd5e-84bf-11ea-ae26-989cfce1c7c7_story.html. Published April 25, 2020. Accessed April 27, 2020.
What does COVID-19 mean for child safety?
In my home county of San Diego, school closure has meant some 800,000 children staying home.1 Parents love and are committed to care for their children, but as these parents struggle with food insecurity and mass unemployment, local pediatricians are joining their national colleagues in worrying about rising rates of child abuse.
Dr. Gwendolyn Wright, a local pediatrician at Scripps Coastal Medical Center, San Diego, explains. “Obviously, it’s easy for tempers to flare,” during this stressful time, “so there is increased risk for child abuse. And there’s no one else with eyes on the kids. Usually, there would be teachers at schools and other childcare workers who would have eyes on the kid. And now there is none of that extra protection.”
2018 data from the National Child Abuse and Neglect Data System showed that in 91.7% of child abuse cases, one or more parent perpetrated the abuse.2 Prior reporting in our county showed that calls to the child abuse hotline went down nearly 60% a week after school closure.3 However, this is not necessarily good news. NCANDS data show that educational personnel report 20% of child abuse cases – far more than the number of cases reported by social services, medical professionals, or family members.2
Teachers, childcare workers, law enforcement, and medical professionals all are mandated reporters, meaning that they are legally obligated to report any suspected cases of child abuse to Child Welfare Services. Accordingly, they receive training on how to spot signs of child abuse.
Sometimes, the signs are obvious, sometimes subtle. Subtle injuries are called “sentinel” injuries. In a landmark study published in Pediatrics in 2013, a “sentinel” injury was defined as “a previous injury reported in the medical history that was suspicious for abuse because the infant could not cruise, or the explanation was implausible.” Sentinel injuries can be mild bruising or oral injuries in a young infant. These injuries suggest “there may be escalating and repeated violence toward the infant” that can culminate in death.4,5
In this study, severely abused infants were 4.4 times more likely to initially have come to the doctor with a sentinel injury. Of concern, 42% of parents of definitely abused children reported that a medical provider was aware of the sentinel injury. Of these cases, 56% did not show evidence that a professional was worried about abuse. These data show that medical professionals do miss cases of child abuse.
The cost of child abuse is real and lifelong. According to a policy statement from the American Academy of Pediatrics Council on Child Abuse and Neglect, a quarter of kids who suffer abusive head trauma die. Of the survivors, nearly 70% “have some degree of lasting neurological impairment.”5
Given the potentially disastrous consequences of child abuse, we must stay vigilant about child abuse. In our own profession, we must educate trainees and update experienced pediatricians about suspecting child abuse and reporting. For example, child abuse can be suspected and reported based on telemedicine interactions. The burden of proof for reporting child abuse is only “reasonable suspicion,” not “beyond a reasonable doubt.” In our communities, we must engage with local Child Welfare Services workers and educate them about sentinel injuries. And finally, in our practices, we must build families up with awareness, resources, and coping mechanisms to prevent abuse from happening in the first place.
Dr. Helen C. Wang, associate professor of pediatrics at the University of California, San Diego, talks to parents about managing stress early and often. She says, “I start counseling families at the prenatal visit. I do talk to families about what they liked to do before children. What brought you joy? What communities do you spend time with? And what have you been doing now?”
It can be hard to reconcile prior hobbies with the current recommendations of social distancing. “Now it’s more ‘Do FaceTime’ and ‘Do Zoom’ and spend more time with your extended family,” says Dr. Wang.
By caring for themselves, parents can better protect their children from mistreatment and injury. Healthychildren.org, the parent-facing website of the AAP, offers several tips for parenting in times of stress.
In this unusual time of COVID-19, it is more important than ever to provide parents with suggestions and strategies that will help them – and their children – survive this health crisis. By educating ourselves and our communities about child abuse, we as pediatricians can fulfill our mandate in keeping kids healthy and thriving.
Dr. Parekh is a pediatric resident at University of California, San Diego. She has no financial disclosures. Email Dr. Parekh at [email protected].
References
1. Early childhood age group in California. kidsdata.org.
2. U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2020). Child Maltreatment 2018.
3. Hong Joe. “School closures lead to troubling drop in child abuse reports.” KPBS. 2020 Mar 27.
4. Pediatrics. 2013 Apr;131(4):701-7.
5. Pediatrics. 2020;145(4):e20200203.
In my home county of San Diego, school closure has meant some 800,000 children staying home.1 Parents love and are committed to care for their children, but as these parents struggle with food insecurity and mass unemployment, local pediatricians are joining their national colleagues in worrying about rising rates of child abuse.
Dr. Gwendolyn Wright, a local pediatrician at Scripps Coastal Medical Center, San Diego, explains. “Obviously, it’s easy for tempers to flare,” during this stressful time, “so there is increased risk for child abuse. And there’s no one else with eyes on the kids. Usually, there would be teachers at schools and other childcare workers who would have eyes on the kid. And now there is none of that extra protection.”
2018 data from the National Child Abuse and Neglect Data System showed that in 91.7% of child abuse cases, one or more parent perpetrated the abuse.2 Prior reporting in our county showed that calls to the child abuse hotline went down nearly 60% a week after school closure.3 However, this is not necessarily good news. NCANDS data show that educational personnel report 20% of child abuse cases – far more than the number of cases reported by social services, medical professionals, or family members.2
Teachers, childcare workers, law enforcement, and medical professionals all are mandated reporters, meaning that they are legally obligated to report any suspected cases of child abuse to Child Welfare Services. Accordingly, they receive training on how to spot signs of child abuse.
Sometimes, the signs are obvious, sometimes subtle. Subtle injuries are called “sentinel” injuries. In a landmark study published in Pediatrics in 2013, a “sentinel” injury was defined as “a previous injury reported in the medical history that was suspicious for abuse because the infant could not cruise, or the explanation was implausible.” Sentinel injuries can be mild bruising or oral injuries in a young infant. These injuries suggest “there may be escalating and repeated violence toward the infant” that can culminate in death.4,5
In this study, severely abused infants were 4.4 times more likely to initially have come to the doctor with a sentinel injury. Of concern, 42% of parents of definitely abused children reported that a medical provider was aware of the sentinel injury. Of these cases, 56% did not show evidence that a professional was worried about abuse. These data show that medical professionals do miss cases of child abuse.
The cost of child abuse is real and lifelong. According to a policy statement from the American Academy of Pediatrics Council on Child Abuse and Neglect, a quarter of kids who suffer abusive head trauma die. Of the survivors, nearly 70% “have some degree of lasting neurological impairment.”5
Given the potentially disastrous consequences of child abuse, we must stay vigilant about child abuse. In our own profession, we must educate trainees and update experienced pediatricians about suspecting child abuse and reporting. For example, child abuse can be suspected and reported based on telemedicine interactions. The burden of proof for reporting child abuse is only “reasonable suspicion,” not “beyond a reasonable doubt.” In our communities, we must engage with local Child Welfare Services workers and educate them about sentinel injuries. And finally, in our practices, we must build families up with awareness, resources, and coping mechanisms to prevent abuse from happening in the first place.
Dr. Helen C. Wang, associate professor of pediatrics at the University of California, San Diego, talks to parents about managing stress early and often. She says, “I start counseling families at the prenatal visit. I do talk to families about what they liked to do before children. What brought you joy? What communities do you spend time with? And what have you been doing now?”
It can be hard to reconcile prior hobbies with the current recommendations of social distancing. “Now it’s more ‘Do FaceTime’ and ‘Do Zoom’ and spend more time with your extended family,” says Dr. Wang.
By caring for themselves, parents can better protect their children from mistreatment and injury. Healthychildren.org, the parent-facing website of the AAP, offers several tips for parenting in times of stress.
In this unusual time of COVID-19, it is more important than ever to provide parents with suggestions and strategies that will help them – and their children – survive this health crisis. By educating ourselves and our communities about child abuse, we as pediatricians can fulfill our mandate in keeping kids healthy and thriving.
Dr. Parekh is a pediatric resident at University of California, San Diego. She has no financial disclosures. Email Dr. Parekh at [email protected].
References
1. Early childhood age group in California. kidsdata.org.
2. U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2020). Child Maltreatment 2018.
3. Hong Joe. “School closures lead to troubling drop in child abuse reports.” KPBS. 2020 Mar 27.
4. Pediatrics. 2013 Apr;131(4):701-7.
5. Pediatrics. 2020;145(4):e20200203.
In my home county of San Diego, school closure has meant some 800,000 children staying home.1 Parents love and are committed to care for their children, but as these parents struggle with food insecurity and mass unemployment, local pediatricians are joining their national colleagues in worrying about rising rates of child abuse.
Dr. Gwendolyn Wright, a local pediatrician at Scripps Coastal Medical Center, San Diego, explains. “Obviously, it’s easy for tempers to flare,” during this stressful time, “so there is increased risk for child abuse. And there’s no one else with eyes on the kids. Usually, there would be teachers at schools and other childcare workers who would have eyes on the kid. And now there is none of that extra protection.”
2018 data from the National Child Abuse and Neglect Data System showed that in 91.7% of child abuse cases, one or more parent perpetrated the abuse.2 Prior reporting in our county showed that calls to the child abuse hotline went down nearly 60% a week after school closure.3 However, this is not necessarily good news. NCANDS data show that educational personnel report 20% of child abuse cases – far more than the number of cases reported by social services, medical professionals, or family members.2
Teachers, childcare workers, law enforcement, and medical professionals all are mandated reporters, meaning that they are legally obligated to report any suspected cases of child abuse to Child Welfare Services. Accordingly, they receive training on how to spot signs of child abuse.
Sometimes, the signs are obvious, sometimes subtle. Subtle injuries are called “sentinel” injuries. In a landmark study published in Pediatrics in 2013, a “sentinel” injury was defined as “a previous injury reported in the medical history that was suspicious for abuse because the infant could not cruise, or the explanation was implausible.” Sentinel injuries can be mild bruising or oral injuries in a young infant. These injuries suggest “there may be escalating and repeated violence toward the infant” that can culminate in death.4,5
In this study, severely abused infants were 4.4 times more likely to initially have come to the doctor with a sentinel injury. Of concern, 42% of parents of definitely abused children reported that a medical provider was aware of the sentinel injury. Of these cases, 56% did not show evidence that a professional was worried about abuse. These data show that medical professionals do miss cases of child abuse.
The cost of child abuse is real and lifelong. According to a policy statement from the American Academy of Pediatrics Council on Child Abuse and Neglect, a quarter of kids who suffer abusive head trauma die. Of the survivors, nearly 70% “have some degree of lasting neurological impairment.”5
Given the potentially disastrous consequences of child abuse, we must stay vigilant about child abuse. In our own profession, we must educate trainees and update experienced pediatricians about suspecting child abuse and reporting. For example, child abuse can be suspected and reported based on telemedicine interactions. The burden of proof for reporting child abuse is only “reasonable suspicion,” not “beyond a reasonable doubt.” In our communities, we must engage with local Child Welfare Services workers and educate them about sentinel injuries. And finally, in our practices, we must build families up with awareness, resources, and coping mechanisms to prevent abuse from happening in the first place.
Dr. Helen C. Wang, associate professor of pediatrics at the University of California, San Diego, talks to parents about managing stress early and often. She says, “I start counseling families at the prenatal visit. I do talk to families about what they liked to do before children. What brought you joy? What communities do you spend time with? And what have you been doing now?”
It can be hard to reconcile prior hobbies with the current recommendations of social distancing. “Now it’s more ‘Do FaceTime’ and ‘Do Zoom’ and spend more time with your extended family,” says Dr. Wang.
By caring for themselves, parents can better protect their children from mistreatment and injury. Healthychildren.org, the parent-facing website of the AAP, offers several tips for parenting in times of stress.
In this unusual time of COVID-19, it is more important than ever to provide parents with suggestions and strategies that will help them – and their children – survive this health crisis. By educating ourselves and our communities about child abuse, we as pediatricians can fulfill our mandate in keeping kids healthy and thriving.
Dr. Parekh is a pediatric resident at University of California, San Diego. She has no financial disclosures. Email Dr. Parekh at [email protected].
References
1. Early childhood age group in California. kidsdata.org.
2. U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2020). Child Maltreatment 2018.
3. Hong Joe. “School closures lead to troubling drop in child abuse reports.” KPBS. 2020 Mar 27.
4. Pediatrics. 2013 Apr;131(4):701-7.
5. Pediatrics. 2020;145(4):e20200203.
Onyx stent meets DAPT performance goal in bleeding-risk patients
Results from a prospective, multicenter, uncontrolled series of just over 1,500 patients with high bleeding risk who underwent coronary revascularization with a polymer-based, zotarolimus-eluting stent showed that these patients could safely receive dual-antiplatelet therapy (DAPT)for just 1 month.
This finding sets the stage for a new labeled indication for this device and management strategy in this patient population.
Results from the Onyx ONE Clear study “met its primary endpoint, with favorable rates of ischemic outcomes from 1-12 months after DAPT discontinuation within a high risk population of HBR [high-bleeding-risk] patients,” Ajay J. Kirtane, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. The rate of cardiac death or MI during months 1-12 of follow-up while patients were on single-antiplatelet therapy (SAPT) with either aspirin or a P2Y12 inhibitor, usually clopidogrel, was 7.0%, compared with a prespecified performance goal of 9.7% or less, a goal set in consultation with and approval from the Food and Drug Administration based on the results from earlier, short DAPT studies in HBR patients.
“We hope these data will support our submission to the FDA for a 1-month DAPT indication for high-bleeding-risk patients treated with Resolute Onyx,” the polymer-based, zotarolimus-eluting stent tested in the study, said an officer with Medtronic, the company that sponsored this study and markets this stent, in a written statement. Currently, no stent has received a U.S. indication for just 1 month of DAPT treatment.
“The Onyx ONE Clear study represents the largest analysis of 1-month DAPT among commercially available DES [drug-eluting stents], and extends findings from the Onyx ONE [randomized, controlled trial] assuring the safety of a 1-month DAPT strategy among selected patients with high bleeding risk,” said David E. Kandzari, MD, director of interventional cardiology at Piedmont Healthcare in Atlanta and coprincipal investigator for the study along with Dr. Kirtane.
“Despite the patient complexity included in the study, the observation of a favorably low rate of ischemic events despite abbreviated DAPT is consistent with a theme from other contemporary studies that, among HBR patients, bleeding risk rather than ischemic risk should guide clinical decision making regarding DAPT duration,” Dr. Kandzari said in an interview.
Two similar trials
The Onyx ONE Clear results were consistent with findings from a study with a somewhat similar design, LEADERS FREE II, a single-arm study that assessed the safety and efficacy of BioFreedom, a polymer-free umirolimus-coated coronary stent, in HBR patients treated with DAPT for 1 month followed by SAPT.
LEADERS FREE II showed a 12-month cardiac death or MI rate of 8.6% that compared favorably with the 12.3% 1-year rate among similar patients who received bare-metal stents and a similar timing of DAPT and SAPT in a historical control group (Circ Cardiovasc Interv. 2020 Apr 13. doi: 10.1161/CIRCINTERVENTIONS.119.008603). The primary goal of LEADERS FREE II was to serve as the pivotal trial for FDA approval of the BioFreedom stent, but as of May 2020 the FDA had not approved this stent for U.S. use.
Results of another recent study, Onyx ONE, that supplied more than half the patients included in the Onyx ONE Clear analysis, showed that, in a head-to-head comparison of the Onxy and BioFreedom stents in 1,996 HBR patients treated with DAPT for 1 month followed by 11 months of SAPT, the Onyx stent was noninferior for both a primary safety outcome and a secondary efficacy outcome (N Engl J Med. 2020 Mar 26;382[13]:1208-18).
“The major differences” between the Onyx and BioFreedom stents in the patients studied in Onxy ONE Clear and in LEADERS FREE II “lie in the fact that BioFreedom is not approved in the U.S., and that Onyx is a current generation, preferred DES platform for both conventional and HBR patients,” Dr. Kirtane said in an interview.
“Because of the performance characteristics of Onyx, as well as the fact that ONYX ONE studied a far more complex group of patients than other shorter DAPT studies with conventional DES, I personally feel that there will be a preference to use this stent as a result of these data,” added Dr. Kirtane, professor of medicine at Columbia University and director of the coronary catheterization laboratory at New York–Presbyterian Hospital in New York.
The results from Onyx ONE “are critical for changing practice” among U.S. interventionalists, commented Sunil V. Rao, MD, an interventional cardiologist and professor of medicine at Duke University, Durham, N.C. Based on the new findings, U.S. operators performing percutaneous coronary interventions “will feel comfortable stopping DAPT in patients who are at high bleeding risk,” he said in an interview.
Although the results from LEADERS FREE II showed that the BioFreedom stent was superior to a bare-metal stent with 1 month of DAPT in HBR patients, and the results from Onyx ONE showed that the Onyx stent was noninferior to BioFreedom in this setting, “it’s important not to assume that there is a class effect across DES platforms. Each platform has a different drug and different stent design, so the interventional community needs to see these data for each DES,” Dr. Rao maintained.
Onyx ONE Clear design
Onyx ONE Clear enrolled a total of 1,506 patients, including more than 1,000 patients who received the Onyx stent in the Onyx ONE trial and an additional 752 patients enrolled in the United States and Japan, but 263 of these patients had an adverse event during their first 30 days or follow-up leaving 1,506 patients eligible to continue into the Onyx ONE Clear analysis, and with 1,491 patients followed through 12 months. Patients were an average age of 74 years, a little over two-thirds were men, 49% had a recent acute coronary syndrome event and 41% had chronic coronary syndrome. The choice of which antiplatelet agent to continue when patients transitioned to SAPT after 30 days on DAPT was left to the discretion of the physicians for each enrolled patient.
One issue these studies did not address was whether 1 month is the ideal duration for DAPT before switching to SAPT in HBR patients following coronary stenting, or whether longer DAPT durations produce even better outcomes. “It was important to establish what happens if we need to stop DAPT early.” The Onyx ONE and Onyx ONE Clear studies “provide much-needed data informing clinicians of the risks and safety of SAPT after 1 month in appropriately selected patients,” Dr. Kirtane said.
“The results do not indicate that all HBR patients should be treated with 1 month [of] DAPT, but instead demonstrate the safety and effectiveness of this strategy when clinically appropriate.” This scenario “is quite common, given that HBR patients represent up to a third” of patients undergoing percutaneous coronary intervention, Dr. Kandzari said.
Onyx ONE and Onyx ONE Clear were sponsored by Medtronic, the company that markets the Onyx coronary stent. Dr. Kirtane’s institution has received research support from Medtronic, and from Abbott Vascular, Abiomed, Boston Scientific, Cathworks, CSI, Philips, ReCor Medical, and Siemens. Dr. Kandzari has received personal fees and research grants from medtronic, personal fees from Biotronik and Cardiovascular Systems, and research grants from Biotronik, Boston Scientific, and Cardiovascular Systems. Dr. Rao has received personal fees from Medtronic, as well as from CSI and Philips.
SOURCE: Kirtane AJ et al. ACC 2020, Abstract 903-06.
Results from a prospective, multicenter, uncontrolled series of just over 1,500 patients with high bleeding risk who underwent coronary revascularization with a polymer-based, zotarolimus-eluting stent showed that these patients could safely receive dual-antiplatelet therapy (DAPT)for just 1 month.
This finding sets the stage for a new labeled indication for this device and management strategy in this patient population.
Results from the Onyx ONE Clear study “met its primary endpoint, with favorable rates of ischemic outcomes from 1-12 months after DAPT discontinuation within a high risk population of HBR [high-bleeding-risk] patients,” Ajay J. Kirtane, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. The rate of cardiac death or MI during months 1-12 of follow-up while patients were on single-antiplatelet therapy (SAPT) with either aspirin or a P2Y12 inhibitor, usually clopidogrel, was 7.0%, compared with a prespecified performance goal of 9.7% or less, a goal set in consultation with and approval from the Food and Drug Administration based on the results from earlier, short DAPT studies in HBR patients.
“We hope these data will support our submission to the FDA for a 1-month DAPT indication for high-bleeding-risk patients treated with Resolute Onyx,” the polymer-based, zotarolimus-eluting stent tested in the study, said an officer with Medtronic, the company that sponsored this study and markets this stent, in a written statement. Currently, no stent has received a U.S. indication for just 1 month of DAPT treatment.
“The Onyx ONE Clear study represents the largest analysis of 1-month DAPT among commercially available DES [drug-eluting stents], and extends findings from the Onyx ONE [randomized, controlled trial] assuring the safety of a 1-month DAPT strategy among selected patients with high bleeding risk,” said David E. Kandzari, MD, director of interventional cardiology at Piedmont Healthcare in Atlanta and coprincipal investigator for the study along with Dr. Kirtane.
“Despite the patient complexity included in the study, the observation of a favorably low rate of ischemic events despite abbreviated DAPT is consistent with a theme from other contemporary studies that, among HBR patients, bleeding risk rather than ischemic risk should guide clinical decision making regarding DAPT duration,” Dr. Kandzari said in an interview.
Two similar trials
The Onyx ONE Clear results were consistent with findings from a study with a somewhat similar design, LEADERS FREE II, a single-arm study that assessed the safety and efficacy of BioFreedom, a polymer-free umirolimus-coated coronary stent, in HBR patients treated with DAPT for 1 month followed by SAPT.
LEADERS FREE II showed a 12-month cardiac death or MI rate of 8.6% that compared favorably with the 12.3% 1-year rate among similar patients who received bare-metal stents and a similar timing of DAPT and SAPT in a historical control group (Circ Cardiovasc Interv. 2020 Apr 13. doi: 10.1161/CIRCINTERVENTIONS.119.008603). The primary goal of LEADERS FREE II was to serve as the pivotal trial for FDA approval of the BioFreedom stent, but as of May 2020 the FDA had not approved this stent for U.S. use.
Results of another recent study, Onyx ONE, that supplied more than half the patients included in the Onyx ONE Clear analysis, showed that, in a head-to-head comparison of the Onxy and BioFreedom stents in 1,996 HBR patients treated with DAPT for 1 month followed by 11 months of SAPT, the Onyx stent was noninferior for both a primary safety outcome and a secondary efficacy outcome (N Engl J Med. 2020 Mar 26;382[13]:1208-18).
“The major differences” between the Onyx and BioFreedom stents in the patients studied in Onxy ONE Clear and in LEADERS FREE II “lie in the fact that BioFreedom is not approved in the U.S., and that Onyx is a current generation, preferred DES platform for both conventional and HBR patients,” Dr. Kirtane said in an interview.
“Because of the performance characteristics of Onyx, as well as the fact that ONYX ONE studied a far more complex group of patients than other shorter DAPT studies with conventional DES, I personally feel that there will be a preference to use this stent as a result of these data,” added Dr. Kirtane, professor of medicine at Columbia University and director of the coronary catheterization laboratory at New York–Presbyterian Hospital in New York.
The results from Onyx ONE “are critical for changing practice” among U.S. interventionalists, commented Sunil V. Rao, MD, an interventional cardiologist and professor of medicine at Duke University, Durham, N.C. Based on the new findings, U.S. operators performing percutaneous coronary interventions “will feel comfortable stopping DAPT in patients who are at high bleeding risk,” he said in an interview.
Although the results from LEADERS FREE II showed that the BioFreedom stent was superior to a bare-metal stent with 1 month of DAPT in HBR patients, and the results from Onyx ONE showed that the Onyx stent was noninferior to BioFreedom in this setting, “it’s important not to assume that there is a class effect across DES platforms. Each platform has a different drug and different stent design, so the interventional community needs to see these data for each DES,” Dr. Rao maintained.
Onyx ONE Clear design
Onyx ONE Clear enrolled a total of 1,506 patients, including more than 1,000 patients who received the Onyx stent in the Onyx ONE trial and an additional 752 patients enrolled in the United States and Japan, but 263 of these patients had an adverse event during their first 30 days or follow-up leaving 1,506 patients eligible to continue into the Onyx ONE Clear analysis, and with 1,491 patients followed through 12 months. Patients were an average age of 74 years, a little over two-thirds were men, 49% had a recent acute coronary syndrome event and 41% had chronic coronary syndrome. The choice of which antiplatelet agent to continue when patients transitioned to SAPT after 30 days on DAPT was left to the discretion of the physicians for each enrolled patient.
One issue these studies did not address was whether 1 month is the ideal duration for DAPT before switching to SAPT in HBR patients following coronary stenting, or whether longer DAPT durations produce even better outcomes. “It was important to establish what happens if we need to stop DAPT early.” The Onyx ONE and Onyx ONE Clear studies “provide much-needed data informing clinicians of the risks and safety of SAPT after 1 month in appropriately selected patients,” Dr. Kirtane said.
“The results do not indicate that all HBR patients should be treated with 1 month [of] DAPT, but instead demonstrate the safety and effectiveness of this strategy when clinically appropriate.” This scenario “is quite common, given that HBR patients represent up to a third” of patients undergoing percutaneous coronary intervention, Dr. Kandzari said.
Onyx ONE and Onyx ONE Clear were sponsored by Medtronic, the company that markets the Onyx coronary stent. Dr. Kirtane’s institution has received research support from Medtronic, and from Abbott Vascular, Abiomed, Boston Scientific, Cathworks, CSI, Philips, ReCor Medical, and Siemens. Dr. Kandzari has received personal fees and research grants from medtronic, personal fees from Biotronik and Cardiovascular Systems, and research grants from Biotronik, Boston Scientific, and Cardiovascular Systems. Dr. Rao has received personal fees from Medtronic, as well as from CSI and Philips.
SOURCE: Kirtane AJ et al. ACC 2020, Abstract 903-06.
Results from a prospective, multicenter, uncontrolled series of just over 1,500 patients with high bleeding risk who underwent coronary revascularization with a polymer-based, zotarolimus-eluting stent showed that these patients could safely receive dual-antiplatelet therapy (DAPT)for just 1 month.
This finding sets the stage for a new labeled indication for this device and management strategy in this patient population.
Results from the Onyx ONE Clear study “met its primary endpoint, with favorable rates of ischemic outcomes from 1-12 months after DAPT discontinuation within a high risk population of HBR [high-bleeding-risk] patients,” Ajay J. Kirtane, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic. The rate of cardiac death or MI during months 1-12 of follow-up while patients were on single-antiplatelet therapy (SAPT) with either aspirin or a P2Y12 inhibitor, usually clopidogrel, was 7.0%, compared with a prespecified performance goal of 9.7% or less, a goal set in consultation with and approval from the Food and Drug Administration based on the results from earlier, short DAPT studies in HBR patients.
“We hope these data will support our submission to the FDA for a 1-month DAPT indication for high-bleeding-risk patients treated with Resolute Onyx,” the polymer-based, zotarolimus-eluting stent tested in the study, said an officer with Medtronic, the company that sponsored this study and markets this stent, in a written statement. Currently, no stent has received a U.S. indication for just 1 month of DAPT treatment.
“The Onyx ONE Clear study represents the largest analysis of 1-month DAPT among commercially available DES [drug-eluting stents], and extends findings from the Onyx ONE [randomized, controlled trial] assuring the safety of a 1-month DAPT strategy among selected patients with high bleeding risk,” said David E. Kandzari, MD, director of interventional cardiology at Piedmont Healthcare in Atlanta and coprincipal investigator for the study along with Dr. Kirtane.
“Despite the patient complexity included in the study, the observation of a favorably low rate of ischemic events despite abbreviated DAPT is consistent with a theme from other contemporary studies that, among HBR patients, bleeding risk rather than ischemic risk should guide clinical decision making regarding DAPT duration,” Dr. Kandzari said in an interview.
Two similar trials
The Onyx ONE Clear results were consistent with findings from a study with a somewhat similar design, LEADERS FREE II, a single-arm study that assessed the safety and efficacy of BioFreedom, a polymer-free umirolimus-coated coronary stent, in HBR patients treated with DAPT for 1 month followed by SAPT.
LEADERS FREE II showed a 12-month cardiac death or MI rate of 8.6% that compared favorably with the 12.3% 1-year rate among similar patients who received bare-metal stents and a similar timing of DAPT and SAPT in a historical control group (Circ Cardiovasc Interv. 2020 Apr 13. doi: 10.1161/CIRCINTERVENTIONS.119.008603). The primary goal of LEADERS FREE II was to serve as the pivotal trial for FDA approval of the BioFreedom stent, but as of May 2020 the FDA had not approved this stent for U.S. use.
Results of another recent study, Onyx ONE, that supplied more than half the patients included in the Onyx ONE Clear analysis, showed that, in a head-to-head comparison of the Onxy and BioFreedom stents in 1,996 HBR patients treated with DAPT for 1 month followed by 11 months of SAPT, the Onyx stent was noninferior for both a primary safety outcome and a secondary efficacy outcome (N Engl J Med. 2020 Mar 26;382[13]:1208-18).
“The major differences” between the Onyx and BioFreedom stents in the patients studied in Onxy ONE Clear and in LEADERS FREE II “lie in the fact that BioFreedom is not approved in the U.S., and that Onyx is a current generation, preferred DES platform for both conventional and HBR patients,” Dr. Kirtane said in an interview.
“Because of the performance characteristics of Onyx, as well as the fact that ONYX ONE studied a far more complex group of patients than other shorter DAPT studies with conventional DES, I personally feel that there will be a preference to use this stent as a result of these data,” added Dr. Kirtane, professor of medicine at Columbia University and director of the coronary catheterization laboratory at New York–Presbyterian Hospital in New York.
The results from Onyx ONE “are critical for changing practice” among U.S. interventionalists, commented Sunil V. Rao, MD, an interventional cardiologist and professor of medicine at Duke University, Durham, N.C. Based on the new findings, U.S. operators performing percutaneous coronary interventions “will feel comfortable stopping DAPT in patients who are at high bleeding risk,” he said in an interview.
Although the results from LEADERS FREE II showed that the BioFreedom stent was superior to a bare-metal stent with 1 month of DAPT in HBR patients, and the results from Onyx ONE showed that the Onyx stent was noninferior to BioFreedom in this setting, “it’s important not to assume that there is a class effect across DES platforms. Each platform has a different drug and different stent design, so the interventional community needs to see these data for each DES,” Dr. Rao maintained.
Onyx ONE Clear design
Onyx ONE Clear enrolled a total of 1,506 patients, including more than 1,000 patients who received the Onyx stent in the Onyx ONE trial and an additional 752 patients enrolled in the United States and Japan, but 263 of these patients had an adverse event during their first 30 days or follow-up leaving 1,506 patients eligible to continue into the Onyx ONE Clear analysis, and with 1,491 patients followed through 12 months. Patients were an average age of 74 years, a little over two-thirds were men, 49% had a recent acute coronary syndrome event and 41% had chronic coronary syndrome. The choice of which antiplatelet agent to continue when patients transitioned to SAPT after 30 days on DAPT was left to the discretion of the physicians for each enrolled patient.
One issue these studies did not address was whether 1 month is the ideal duration for DAPT before switching to SAPT in HBR patients following coronary stenting, or whether longer DAPT durations produce even better outcomes. “It was important to establish what happens if we need to stop DAPT early.” The Onyx ONE and Onyx ONE Clear studies “provide much-needed data informing clinicians of the risks and safety of SAPT after 1 month in appropriately selected patients,” Dr. Kirtane said.
“The results do not indicate that all HBR patients should be treated with 1 month [of] DAPT, but instead demonstrate the safety and effectiveness of this strategy when clinically appropriate.” This scenario “is quite common, given that HBR patients represent up to a third” of patients undergoing percutaneous coronary intervention, Dr. Kandzari said.
Onyx ONE and Onyx ONE Clear were sponsored by Medtronic, the company that markets the Onyx coronary stent. Dr. Kirtane’s institution has received research support from Medtronic, and from Abbott Vascular, Abiomed, Boston Scientific, Cathworks, CSI, Philips, ReCor Medical, and Siemens. Dr. Kandzari has received personal fees and research grants from medtronic, personal fees from Biotronik and Cardiovascular Systems, and research grants from Biotronik, Boston Scientific, and Cardiovascular Systems. Dr. Rao has received personal fees from Medtronic, as well as from CSI and Philips.
SOURCE: Kirtane AJ et al. ACC 2020, Abstract 903-06.
FROM ACC 2020
Postcolonoscopy antibiotics linked with IBS
Antibiotic exposure within 14 days after screening colonoscopy may increase risk of irritable bowel syndrome (IBS), based on a retrospective analysis of more than 400,000 individuals.
Antibiotic use in the 2 weeks leading up to colonoscopy also trended toward an association with IBS, reported lead author Ravy Vajravelu, MD, of University of Pennsylvania, Philadelphia, and colleagues.
“Laboratory studies in mice have demonstrated that colon cleansing in conjunction with systemic antibiotic use can cause persistent intestinal dysbiosis,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19. “Because perturbation of the gut microbiome is thought to be a trigger for the development of IBS, we sought to assess whether humans who undergo bowel cleanse for colonoscopy in conjunction with antibiotic exposure develop new-onset IBS or IBS-related symptoms.”
According to Dr. Vajravelu, previous human studies have shown that bowel cleansing or antibiotics can alter the baseline gut microbiome, but no previous human research explored the impact of both triggers at once.
The present study involved individuals 50 years or older from the OptumInsight Clinformatics database who underwent screening colonoscopy between 2000 and 2016. Those with preexisting gastrointestinal conditions or symptoms within 180 days leading up to colonoscopy were excluded, leaving 402,259 individuals in the final cohort. From this group, individuals were identified who had exposure to antibiotics within 14 days before and/or after colonoscopy.
The primary outcome was a diagnosis of IBS in the 180 days following the antibiotic exposure window. Secondary outcomes included newly diagnosed diarrhea, change in bowel habits, and abdominal pain. A variety of covariates were tested through multivariable logistical regression, including gastrointestinal infections, medical comorbidities, and demographic factors, with only sex and age remaining in the final model.
Across the cohort, 2% of patients received antibiotics either before or after colonoscopy, while 1% had exposure both before and after. A total of 1,002 individuals (0.2%) were diagnosed with IBS within a median time frame of 112 days.
Multivariate analysis revealed that individuals exposed to antibiotics in the 14 days following colonoscopy had a 77% increased risk of developing IBS (adjusted odds ratio, 1.77; 95% confidence interval, 1.31-2.39). To a lesser degree, and not quite achieving statistical significance, trends toward an association were found for antibiotic exposure before colonoscopy (aOR, 1.38; 95% CI, 0.99-1.92), and for antibiotic exposure both before and after colonoscopy (aOR, 1.41; 95% CI, 0.97-2.04).
Dr. Vajravelu said that these preliminary findings are currently undergoing further analysis.
“In particular, we are interested in determining whether antibiotics that target gram-negative bacteria, which are abundant in the gut, have a greater association with subsequent IBS,” Dr. Vajravelu said.
In addition, they are taking steps to eliminate other confounding factors.
“The main objective of these new analyses is to ensure that the association between bowel cleanse and antibiotics with subsequent IBS is not related to the reasons antibiotics were prescribed initially,” Dr. Vajravelu said. “For example, someone experiencing diarrhea could receive a trial of empiric antibiotics and then receive a colonoscopy when the diarrhea does not resolve. In [the present analysis], we avoided including individuals like this by including only those who underwent screening colonoscopy, and therefore did not have any prior documented GI symptoms. In our [ongoing] analyses, we are including additional restrictions to strengthen the findings.”
If the findings do hold, Dr. Vajravelu suggested that they may have clinical implications.
“[I]t may be important to review whether patients scheduled for colonoscopy have received recent antibiotics and warn them to avoid antibiotics after colonoscopy, if possible,” Dr. Vajravelu said. “Additionally, for gastroenterologists, these data may underscore the importance of adhering to preprocedural antibiotic prophylaxis guidelines put forth by GI societies.”The investigators disclosed relationships with Merck, Pfizer, Gilead, and others.
SOURCE: Vajravelu R et al. DDW 2020. Abstract 404.
Antibiotic exposure within 14 days after screening colonoscopy may increase risk of irritable bowel syndrome (IBS), based on a retrospective analysis of more than 400,000 individuals.
Antibiotic use in the 2 weeks leading up to colonoscopy also trended toward an association with IBS, reported lead author Ravy Vajravelu, MD, of University of Pennsylvania, Philadelphia, and colleagues.
“Laboratory studies in mice have demonstrated that colon cleansing in conjunction with systemic antibiotic use can cause persistent intestinal dysbiosis,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19. “Because perturbation of the gut microbiome is thought to be a trigger for the development of IBS, we sought to assess whether humans who undergo bowel cleanse for colonoscopy in conjunction with antibiotic exposure develop new-onset IBS or IBS-related symptoms.”
According to Dr. Vajravelu, previous human studies have shown that bowel cleansing or antibiotics can alter the baseline gut microbiome, but no previous human research explored the impact of both triggers at once.
The present study involved individuals 50 years or older from the OptumInsight Clinformatics database who underwent screening colonoscopy between 2000 and 2016. Those with preexisting gastrointestinal conditions or symptoms within 180 days leading up to colonoscopy were excluded, leaving 402,259 individuals in the final cohort. From this group, individuals were identified who had exposure to antibiotics within 14 days before and/or after colonoscopy.
The primary outcome was a diagnosis of IBS in the 180 days following the antibiotic exposure window. Secondary outcomes included newly diagnosed diarrhea, change in bowel habits, and abdominal pain. A variety of covariates were tested through multivariable logistical regression, including gastrointestinal infections, medical comorbidities, and demographic factors, with only sex and age remaining in the final model.
Across the cohort, 2% of patients received antibiotics either before or after colonoscopy, while 1% had exposure both before and after. A total of 1,002 individuals (0.2%) were diagnosed with IBS within a median time frame of 112 days.
Multivariate analysis revealed that individuals exposed to antibiotics in the 14 days following colonoscopy had a 77% increased risk of developing IBS (adjusted odds ratio, 1.77; 95% confidence interval, 1.31-2.39). To a lesser degree, and not quite achieving statistical significance, trends toward an association were found for antibiotic exposure before colonoscopy (aOR, 1.38; 95% CI, 0.99-1.92), and for antibiotic exposure both before and after colonoscopy (aOR, 1.41; 95% CI, 0.97-2.04).
Dr. Vajravelu said that these preliminary findings are currently undergoing further analysis.
“In particular, we are interested in determining whether antibiotics that target gram-negative bacteria, which are abundant in the gut, have a greater association with subsequent IBS,” Dr. Vajravelu said.
In addition, they are taking steps to eliminate other confounding factors.
“The main objective of these new analyses is to ensure that the association between bowel cleanse and antibiotics with subsequent IBS is not related to the reasons antibiotics were prescribed initially,” Dr. Vajravelu said. “For example, someone experiencing diarrhea could receive a trial of empiric antibiotics and then receive a colonoscopy when the diarrhea does not resolve. In [the present analysis], we avoided including individuals like this by including only those who underwent screening colonoscopy, and therefore did not have any prior documented GI symptoms. In our [ongoing] analyses, we are including additional restrictions to strengthen the findings.”
If the findings do hold, Dr. Vajravelu suggested that they may have clinical implications.
“[I]t may be important to review whether patients scheduled for colonoscopy have received recent antibiotics and warn them to avoid antibiotics after colonoscopy, if possible,” Dr. Vajravelu said. “Additionally, for gastroenterologists, these data may underscore the importance of adhering to preprocedural antibiotic prophylaxis guidelines put forth by GI societies.”The investigators disclosed relationships with Merck, Pfizer, Gilead, and others.
SOURCE: Vajravelu R et al. DDW 2020. Abstract 404.
Antibiotic exposure within 14 days after screening colonoscopy may increase risk of irritable bowel syndrome (IBS), based on a retrospective analysis of more than 400,000 individuals.
Antibiotic use in the 2 weeks leading up to colonoscopy also trended toward an association with IBS, reported lead author Ravy Vajravelu, MD, of University of Pennsylvania, Philadelphia, and colleagues.
“Laboratory studies in mice have demonstrated that colon cleansing in conjunction with systemic antibiotic use can cause persistent intestinal dysbiosis,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19. “Because perturbation of the gut microbiome is thought to be a trigger for the development of IBS, we sought to assess whether humans who undergo bowel cleanse for colonoscopy in conjunction with antibiotic exposure develop new-onset IBS or IBS-related symptoms.”
According to Dr. Vajravelu, previous human studies have shown that bowel cleansing or antibiotics can alter the baseline gut microbiome, but no previous human research explored the impact of both triggers at once.
The present study involved individuals 50 years or older from the OptumInsight Clinformatics database who underwent screening colonoscopy between 2000 and 2016. Those with preexisting gastrointestinal conditions or symptoms within 180 days leading up to colonoscopy were excluded, leaving 402,259 individuals in the final cohort. From this group, individuals were identified who had exposure to antibiotics within 14 days before and/or after colonoscopy.
The primary outcome was a diagnosis of IBS in the 180 days following the antibiotic exposure window. Secondary outcomes included newly diagnosed diarrhea, change in bowel habits, and abdominal pain. A variety of covariates were tested through multivariable logistical regression, including gastrointestinal infections, medical comorbidities, and demographic factors, with only sex and age remaining in the final model.
Across the cohort, 2% of patients received antibiotics either before or after colonoscopy, while 1% had exposure both before and after. A total of 1,002 individuals (0.2%) were diagnosed with IBS within a median time frame of 112 days.
Multivariate analysis revealed that individuals exposed to antibiotics in the 14 days following colonoscopy had a 77% increased risk of developing IBS (adjusted odds ratio, 1.77; 95% confidence interval, 1.31-2.39). To a lesser degree, and not quite achieving statistical significance, trends toward an association were found for antibiotic exposure before colonoscopy (aOR, 1.38; 95% CI, 0.99-1.92), and for antibiotic exposure both before and after colonoscopy (aOR, 1.41; 95% CI, 0.97-2.04).
Dr. Vajravelu said that these preliminary findings are currently undergoing further analysis.
“In particular, we are interested in determining whether antibiotics that target gram-negative bacteria, which are abundant in the gut, have a greater association with subsequent IBS,” Dr. Vajravelu said.
In addition, they are taking steps to eliminate other confounding factors.
“The main objective of these new analyses is to ensure that the association between bowel cleanse and antibiotics with subsequent IBS is not related to the reasons antibiotics were prescribed initially,” Dr. Vajravelu said. “For example, someone experiencing diarrhea could receive a trial of empiric antibiotics and then receive a colonoscopy when the diarrhea does not resolve. In [the present analysis], we avoided including individuals like this by including only those who underwent screening colonoscopy, and therefore did not have any prior documented GI symptoms. In our [ongoing] analyses, we are including additional restrictions to strengthen the findings.”
If the findings do hold, Dr. Vajravelu suggested that they may have clinical implications.
“[I]t may be important to review whether patients scheduled for colonoscopy have received recent antibiotics and warn them to avoid antibiotics after colonoscopy, if possible,” Dr. Vajravelu said. “Additionally, for gastroenterologists, these data may underscore the importance of adhering to preprocedural antibiotic prophylaxis guidelines put forth by GI societies.”The investigators disclosed relationships with Merck, Pfizer, Gilead, and others.
SOURCE: Vajravelu R et al. DDW 2020. Abstract 404.
FROM DDW 2020
Hospitalist movers and shakers – May 2020
Pediatric hospitalists Linda Bloom, MD, Corina Sandru, MD, and Ilana Price MD, all from Reading Hospital – Tower Health (West Reading, Pa.) recently earned board certification in pediatric hospital medicine from the American Board of Pediatrics. This was the first certification of its kind given by the ABP.
Sitting for the board certification exam required ABP certification and meeting the training requirements set for pediatric hospital medicine, which was recognized as a subspecialty in 2016.
Felipe Castorena, MD, recently received the Humanitarian Award at the Northwell Health Hospital Medicine Academic Summit. Dr. Castorena was honored for the volunteer work he did with underserved communities in the Dominican Republic in October 2018.
Dr. Castorena worked with the Dr. Almanzar Foundation, providing medical care that included vaccine administration, surgery, and general checkups. A native of Mexico, Dr. Castorena is a hospitalist at Phelps Hospital in Sleepy Hollow, N.Y.
Alteon Health has named Frank Kelley, MD, as one of three 2019 Facility Medical Directors of the Year. Dr. Kelley serves as director of hospital medicine at University Hospitals Portage Medical Center (Ravenna, Ohio). Alteon began managing the Portage hospitalist program in 2006.
Dr. Kelley was recognized for exhibiting “exemplary leadership and professionalism … mentoring their physicians and advance practice providers while improving department performance.” He is one of three winners among Alteon’s 125 clinical sites.
Amina Ahmed, MD, recently was named chief medical officer for CareOne, New Jersey’s largest family-owned-and-operated senior-living/post–acute care operator.
A board-certified internist, Dr. Ahmed most recently was chief of hospitalist medicine and post–acute care at Summit Medical Group (Berkeley Heights, N.J.).
Ikenna Ibe, MD, has been promoted to vice president of medical affairs and chief medical officer at Virginia Commonwealth University Health Community Memorial Hospital (Richmond, Va.). Dr. Ibe will be charged with creating a stronger connect between staff at VCU Health CMH and the clinical programs at VCU Medical Center’s main campus.
Dr. Ibe has been medical director of the hospitalist group since starting at VCU Health CMH in 2018. He will continue to care for patients and guide the hospitalist program while in his new role until his replacement is found. He previously directed the hospitalist program at Richmond’s St. Mary’s Hospital.
The medical staff at Saint Thomas Rutherford Hospital (Murfreesboro, Tenn.) has voted David Sellers, MD, to be chief of staff for a 2-year term that began in January 2020.
Dr. Sellers is the lead hospitalist at Ascension Saint Thomas Rutherford. Dr. Sellers, as chief of staff, will chair the hospital’s Medical Executive Committee, as well as serving as the staff’s advocate at overall board meetings. In addition, he will seek continuing education opportunities for staff, and safeguard that the staff aligns along board policies.
Angela Shippy, MD, FHM, has been promoted to senior vice president and chief medical officer at Memorial Hermann Health System (Houston). In addition, Dr. Shippy will continue to execute her duties as the system’s chief quality officer, a position she has held for the past 5 years.
Dr. Shippy has worked in management throughout her career, serving as chief medical officer at HCA Healthcare’s Gulf Coast Division and as vice president of medical affairs at St. Luke’s Episcopal Hospital, where she also was a hospitalist.
Munir Ahmed, MD, an internist with a quarter century’s worth of experience in Cape Cod, Mass., has been named chief transformation officer with Community Health Center of Cape Cod. Dr. Ahmed will be tasked with creating improvements in clinical outcomes and expanding the facility’s use of emerging technology.
Dr. Ahmed previously worked as a hospitalist and internist at Cape Cod Hospital (East Sandwich, Mass.), where he specialized in hypertension, diabetes, geriatrics, hospice, and palliative care.
A new obstetrics hospitalist program is coming to Bayhealth Kent Campus (Dover, Del.), which has partnered with the national OB Hospitalist Group (Greenville, S.C.). The OB hospitalists will cover labor and delivery, as well as emergency and trauma, 24 hours a day, 7 days a week.
The program will be advantageous for Bayhealth patients who may not have a primary doctor, as the hospitalists will work to ensure safe deliveries and perform C-sections as needed.
The OB Hospitalist Group is the nation’s largest and only dedicated ob.gyn. hospitalist provider with more than 1,000 clinicians in close to 200 facilities across 33 states.
Pediatric hospitalists Linda Bloom, MD, Corina Sandru, MD, and Ilana Price MD, all from Reading Hospital – Tower Health (West Reading, Pa.) recently earned board certification in pediatric hospital medicine from the American Board of Pediatrics. This was the first certification of its kind given by the ABP.
Sitting for the board certification exam required ABP certification and meeting the training requirements set for pediatric hospital medicine, which was recognized as a subspecialty in 2016.
Felipe Castorena, MD, recently received the Humanitarian Award at the Northwell Health Hospital Medicine Academic Summit. Dr. Castorena was honored for the volunteer work he did with underserved communities in the Dominican Republic in October 2018.
Dr. Castorena worked with the Dr. Almanzar Foundation, providing medical care that included vaccine administration, surgery, and general checkups. A native of Mexico, Dr. Castorena is a hospitalist at Phelps Hospital in Sleepy Hollow, N.Y.
Alteon Health has named Frank Kelley, MD, as one of three 2019 Facility Medical Directors of the Year. Dr. Kelley serves as director of hospital medicine at University Hospitals Portage Medical Center (Ravenna, Ohio). Alteon began managing the Portage hospitalist program in 2006.
Dr. Kelley was recognized for exhibiting “exemplary leadership and professionalism … mentoring their physicians and advance practice providers while improving department performance.” He is one of three winners among Alteon’s 125 clinical sites.
Amina Ahmed, MD, recently was named chief medical officer for CareOne, New Jersey’s largest family-owned-and-operated senior-living/post–acute care operator.
A board-certified internist, Dr. Ahmed most recently was chief of hospitalist medicine and post–acute care at Summit Medical Group (Berkeley Heights, N.J.).
Ikenna Ibe, MD, has been promoted to vice president of medical affairs and chief medical officer at Virginia Commonwealth University Health Community Memorial Hospital (Richmond, Va.). Dr. Ibe will be charged with creating a stronger connect between staff at VCU Health CMH and the clinical programs at VCU Medical Center’s main campus.
Dr. Ibe has been medical director of the hospitalist group since starting at VCU Health CMH in 2018. He will continue to care for patients and guide the hospitalist program while in his new role until his replacement is found. He previously directed the hospitalist program at Richmond’s St. Mary’s Hospital.
The medical staff at Saint Thomas Rutherford Hospital (Murfreesboro, Tenn.) has voted David Sellers, MD, to be chief of staff for a 2-year term that began in January 2020.
Dr. Sellers is the lead hospitalist at Ascension Saint Thomas Rutherford. Dr. Sellers, as chief of staff, will chair the hospital’s Medical Executive Committee, as well as serving as the staff’s advocate at overall board meetings. In addition, he will seek continuing education opportunities for staff, and safeguard that the staff aligns along board policies.
Angela Shippy, MD, FHM, has been promoted to senior vice president and chief medical officer at Memorial Hermann Health System (Houston). In addition, Dr. Shippy will continue to execute her duties as the system’s chief quality officer, a position she has held for the past 5 years.
Dr. Shippy has worked in management throughout her career, serving as chief medical officer at HCA Healthcare’s Gulf Coast Division and as vice president of medical affairs at St. Luke’s Episcopal Hospital, where she also was a hospitalist.
Munir Ahmed, MD, an internist with a quarter century’s worth of experience in Cape Cod, Mass., has been named chief transformation officer with Community Health Center of Cape Cod. Dr. Ahmed will be tasked with creating improvements in clinical outcomes and expanding the facility’s use of emerging technology.
Dr. Ahmed previously worked as a hospitalist and internist at Cape Cod Hospital (East Sandwich, Mass.), where he specialized in hypertension, diabetes, geriatrics, hospice, and palliative care.
A new obstetrics hospitalist program is coming to Bayhealth Kent Campus (Dover, Del.), which has partnered with the national OB Hospitalist Group (Greenville, S.C.). The OB hospitalists will cover labor and delivery, as well as emergency and trauma, 24 hours a day, 7 days a week.
The program will be advantageous for Bayhealth patients who may not have a primary doctor, as the hospitalists will work to ensure safe deliveries and perform C-sections as needed.
The OB Hospitalist Group is the nation’s largest and only dedicated ob.gyn. hospitalist provider with more than 1,000 clinicians in close to 200 facilities across 33 states.
Pediatric hospitalists Linda Bloom, MD, Corina Sandru, MD, and Ilana Price MD, all from Reading Hospital – Tower Health (West Reading, Pa.) recently earned board certification in pediatric hospital medicine from the American Board of Pediatrics. This was the first certification of its kind given by the ABP.
Sitting for the board certification exam required ABP certification and meeting the training requirements set for pediatric hospital medicine, which was recognized as a subspecialty in 2016.
Felipe Castorena, MD, recently received the Humanitarian Award at the Northwell Health Hospital Medicine Academic Summit. Dr. Castorena was honored for the volunteer work he did with underserved communities in the Dominican Republic in October 2018.
Dr. Castorena worked with the Dr. Almanzar Foundation, providing medical care that included vaccine administration, surgery, and general checkups. A native of Mexico, Dr. Castorena is a hospitalist at Phelps Hospital in Sleepy Hollow, N.Y.
Alteon Health has named Frank Kelley, MD, as one of three 2019 Facility Medical Directors of the Year. Dr. Kelley serves as director of hospital medicine at University Hospitals Portage Medical Center (Ravenna, Ohio). Alteon began managing the Portage hospitalist program in 2006.
Dr. Kelley was recognized for exhibiting “exemplary leadership and professionalism … mentoring their physicians and advance practice providers while improving department performance.” He is one of three winners among Alteon’s 125 clinical sites.
Amina Ahmed, MD, recently was named chief medical officer for CareOne, New Jersey’s largest family-owned-and-operated senior-living/post–acute care operator.
A board-certified internist, Dr. Ahmed most recently was chief of hospitalist medicine and post–acute care at Summit Medical Group (Berkeley Heights, N.J.).
Ikenna Ibe, MD, has been promoted to vice president of medical affairs and chief medical officer at Virginia Commonwealth University Health Community Memorial Hospital (Richmond, Va.). Dr. Ibe will be charged with creating a stronger connect between staff at VCU Health CMH and the clinical programs at VCU Medical Center’s main campus.
Dr. Ibe has been medical director of the hospitalist group since starting at VCU Health CMH in 2018. He will continue to care for patients and guide the hospitalist program while in his new role until his replacement is found. He previously directed the hospitalist program at Richmond’s St. Mary’s Hospital.
The medical staff at Saint Thomas Rutherford Hospital (Murfreesboro, Tenn.) has voted David Sellers, MD, to be chief of staff for a 2-year term that began in January 2020.
Dr. Sellers is the lead hospitalist at Ascension Saint Thomas Rutherford. Dr. Sellers, as chief of staff, will chair the hospital’s Medical Executive Committee, as well as serving as the staff’s advocate at overall board meetings. In addition, he will seek continuing education opportunities for staff, and safeguard that the staff aligns along board policies.
Angela Shippy, MD, FHM, has been promoted to senior vice president and chief medical officer at Memorial Hermann Health System (Houston). In addition, Dr. Shippy will continue to execute her duties as the system’s chief quality officer, a position she has held for the past 5 years.
Dr. Shippy has worked in management throughout her career, serving as chief medical officer at HCA Healthcare’s Gulf Coast Division and as vice president of medical affairs at St. Luke’s Episcopal Hospital, where she also was a hospitalist.
Munir Ahmed, MD, an internist with a quarter century’s worth of experience in Cape Cod, Mass., has been named chief transformation officer with Community Health Center of Cape Cod. Dr. Ahmed will be tasked with creating improvements in clinical outcomes and expanding the facility’s use of emerging technology.
Dr. Ahmed previously worked as a hospitalist and internist at Cape Cod Hospital (East Sandwich, Mass.), where he specialized in hypertension, diabetes, geriatrics, hospice, and palliative care.
A new obstetrics hospitalist program is coming to Bayhealth Kent Campus (Dover, Del.), which has partnered with the national OB Hospitalist Group (Greenville, S.C.). The OB hospitalists will cover labor and delivery, as well as emergency and trauma, 24 hours a day, 7 days a week.
The program will be advantageous for Bayhealth patients who may not have a primary doctor, as the hospitalists will work to ensure safe deliveries and perform C-sections as needed.
The OB Hospitalist Group is the nation’s largest and only dedicated ob.gyn. hospitalist provider with more than 1,000 clinicians in close to 200 facilities across 33 states.
Postpartum IUD placement • breastfeeding • difficulty maintaining milk supply • Dx?
THE CASE
A 28-year-old G1P1 initially presented to the family medicine clinic 4 weeks postpartum to discuss possibilities for contraception. She had received her prenatal care through a midwife and had had a successful home delivery. She was exclusively breastfeeding her infant daughter but wanted to ensure adequate spacing between her pregnancies.
During the discussion of possible options, the patient revealed that she had previously had an intrauterine device (IUD) placed and expressed interest in using this method again. A levonorgestrel-releasing IUD (Mirena) was placed at 6 weeks postpartum, after a negative pregnancy test was obtained.
The patient returned to the clinic about 6 months later with complaints of increased difficulty maintaining her milk supply.
THE DIAGNOSIS
The patient had taken a home pregnancy test, which was positive—a finding confirmed in clinic via a urine pregnancy test.
Gestational age. Since the patient had an IUD in place and had been exclusively breastfeeding, gestational age was difficult to determine. A quantitative human chorionic gonadotropin (hCG) test showed an hCG level of 12,469 U/L, consistent with a 4-to-8-week pregnancy. An ultrasound performed the next day showed a single intrauterine pregnancy at 21 weeks.
IUD location. There was also the question of the location of the IUD and whether it would interfere with the patient’s ability to maintain the pregnancy. On ultrasound, the IUD was noted within the cervix and myometrium. After discussion of the risks, the patient chose to leave it in place.
DISCUSSION
IUDs are among the most effective forms of contraception; levonorgestrel-releasing IUDs are more effective than copper IUDs.1 The rates of failure in the first year of use are 0.8% and 0.2% for copper and levonorgestrel-releasing IUDs, respectively.1
Continue to: The Lactational Amenorrhea Method
The Lactational Amenorrhea Method (LAM), which is defined as providing infant nutrition exclusively through breastmilk during the first 6 months postpartum, also provides protection against pregnancy. LAM has a failure rate of 0% to 1.5%.2
It is not surprising that this patient thought she was adequately protected against pregnancy. That said, no contraceptive method is foolproof (as this case demonstrates).
Risks to the pregnancy. When pregnancy does occur with an IUD in place, the patient should be informed of the possible risks to the pregnancy. These include complications such as spontaneous abortion, chorioamnionitis, and preterm delivery.3 Risk is further increased if the IUD is malpositioned (as this one was), meaning that any part of the IUD is located in the lower uterine segment, myometrium, or endocervical canal.4,5
Removal of the IUD is generally recommended if the device and its strings can be located, although removal does not completely mitigate risk. In a study done in Egypt, 46 of 52 IUDs were removed successfully, with 2 spontaneous abortions as a result.6 Of note, the IUDs extracted in this study were Lippes loop and copper models, not levonorgestrel-releasing IUDs such as our patient had. There is a single case report7 of a patient who had a Mirena inserted very early in a pregnancy; the IUD had to be left in place due to the risk for miscarriage, but she was able to carry the infant to term and did not experience any adverse effects.
Our patient
The patient delivered a male infant vaginally at term without issue. However, the IUD was not expelled during this process. Ultrasound showed that it was embedded in the posterior myometrium with a hypoechoic tract. The patient was referred to Gynecology, and the IUD was successfully removed.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Even the most reliable method of contraception can fail—so pregnancy should always be in the differential diagnosis for a sexually active woman. Location of IUD placement is important; it must be in the right place to be effective. The tenets of LAM must be followed precisely in order for breastfeeding to provide protection against pregnancy. Patients can successfully carry a pregnancy to term with an IUD, as this patient did, but it places them at higher risk for ectopic pregnancy, premature rupture of membranes, and infection.
The author thanks Jenny Walters, lactation consultant, for her assistance in the preparation of the manuscript.
CORRESPONDENCE
Hannah Maxfield, MD, 3901 Rainbow Boulevard, MS 4010, Kansas City, KS 66160; [email protected]
1. Heinemann K, Reed S, Moehner S, et al. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception. 2015;91:280-283.
2. Labbok MH. Postpartum sexuality and the Lactational Amenorrhea Method for contraception. Clin Obstet Gynecol. 2015;58:915-927.
3. Ganer H, Levy A, Ohel I, et al. Pregnancy outcome in women with an intrauterine contraceptive device. Am J Obstet Gynecol. 2009;201:381.e1-e5.
4. Moschos E, Twickler D. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-e6.
5. Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430.
6. Assaf A, Gohar M, Saad S, et al. Removal of intrauterine devices with missing tails during early pregnancy. Contraception. 1992;45:541-546.
7. Gardyszewska A, Czajkowski K. Application of levonorgestrel-releasing intrauterine system in early pregnancy: a case report [article in Polish]. Ginekol Pol. 2012;83:950-952.
THE CASE
A 28-year-old G1P1 initially presented to the family medicine clinic 4 weeks postpartum to discuss possibilities for contraception. She had received her prenatal care through a midwife and had had a successful home delivery. She was exclusively breastfeeding her infant daughter but wanted to ensure adequate spacing between her pregnancies.
During the discussion of possible options, the patient revealed that she had previously had an intrauterine device (IUD) placed and expressed interest in using this method again. A levonorgestrel-releasing IUD (Mirena) was placed at 6 weeks postpartum, after a negative pregnancy test was obtained.
The patient returned to the clinic about 6 months later with complaints of increased difficulty maintaining her milk supply.
THE DIAGNOSIS
The patient had taken a home pregnancy test, which was positive—a finding confirmed in clinic via a urine pregnancy test.
Gestational age. Since the patient had an IUD in place and had been exclusively breastfeeding, gestational age was difficult to determine. A quantitative human chorionic gonadotropin (hCG) test showed an hCG level of 12,469 U/L, consistent with a 4-to-8-week pregnancy. An ultrasound performed the next day showed a single intrauterine pregnancy at 21 weeks.
IUD location. There was also the question of the location of the IUD and whether it would interfere with the patient’s ability to maintain the pregnancy. On ultrasound, the IUD was noted within the cervix and myometrium. After discussion of the risks, the patient chose to leave it in place.
DISCUSSION
IUDs are among the most effective forms of contraception; levonorgestrel-releasing IUDs are more effective than copper IUDs.1 The rates of failure in the first year of use are 0.8% and 0.2% for copper and levonorgestrel-releasing IUDs, respectively.1
Continue to: The Lactational Amenorrhea Method
The Lactational Amenorrhea Method (LAM), which is defined as providing infant nutrition exclusively through breastmilk during the first 6 months postpartum, also provides protection against pregnancy. LAM has a failure rate of 0% to 1.5%.2
It is not surprising that this patient thought she was adequately protected against pregnancy. That said, no contraceptive method is foolproof (as this case demonstrates).
Risks to the pregnancy. When pregnancy does occur with an IUD in place, the patient should be informed of the possible risks to the pregnancy. These include complications such as spontaneous abortion, chorioamnionitis, and preterm delivery.3 Risk is further increased if the IUD is malpositioned (as this one was), meaning that any part of the IUD is located in the lower uterine segment, myometrium, or endocervical canal.4,5
Removal of the IUD is generally recommended if the device and its strings can be located, although removal does not completely mitigate risk. In a study done in Egypt, 46 of 52 IUDs were removed successfully, with 2 spontaneous abortions as a result.6 Of note, the IUDs extracted in this study were Lippes loop and copper models, not levonorgestrel-releasing IUDs such as our patient had. There is a single case report7 of a patient who had a Mirena inserted very early in a pregnancy; the IUD had to be left in place due to the risk for miscarriage, but she was able to carry the infant to term and did not experience any adverse effects.
Our patient
The patient delivered a male infant vaginally at term without issue. However, the IUD was not expelled during this process. Ultrasound showed that it was embedded in the posterior myometrium with a hypoechoic tract. The patient was referred to Gynecology, and the IUD was successfully removed.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Even the most reliable method of contraception can fail—so pregnancy should always be in the differential diagnosis for a sexually active woman. Location of IUD placement is important; it must be in the right place to be effective. The tenets of LAM must be followed precisely in order for breastfeeding to provide protection against pregnancy. Patients can successfully carry a pregnancy to term with an IUD, as this patient did, but it places them at higher risk for ectopic pregnancy, premature rupture of membranes, and infection.
The author thanks Jenny Walters, lactation consultant, for her assistance in the preparation of the manuscript.
CORRESPONDENCE
Hannah Maxfield, MD, 3901 Rainbow Boulevard, MS 4010, Kansas City, KS 66160; [email protected]
THE CASE
A 28-year-old G1P1 initially presented to the family medicine clinic 4 weeks postpartum to discuss possibilities for contraception. She had received her prenatal care through a midwife and had had a successful home delivery. She was exclusively breastfeeding her infant daughter but wanted to ensure adequate spacing between her pregnancies.
During the discussion of possible options, the patient revealed that she had previously had an intrauterine device (IUD) placed and expressed interest in using this method again. A levonorgestrel-releasing IUD (Mirena) was placed at 6 weeks postpartum, after a negative pregnancy test was obtained.
The patient returned to the clinic about 6 months later with complaints of increased difficulty maintaining her milk supply.
THE DIAGNOSIS
The patient had taken a home pregnancy test, which was positive—a finding confirmed in clinic via a urine pregnancy test.
Gestational age. Since the patient had an IUD in place and had been exclusively breastfeeding, gestational age was difficult to determine. A quantitative human chorionic gonadotropin (hCG) test showed an hCG level of 12,469 U/L, consistent with a 4-to-8-week pregnancy. An ultrasound performed the next day showed a single intrauterine pregnancy at 21 weeks.
IUD location. There was also the question of the location of the IUD and whether it would interfere with the patient’s ability to maintain the pregnancy. On ultrasound, the IUD was noted within the cervix and myometrium. After discussion of the risks, the patient chose to leave it in place.
DISCUSSION
IUDs are among the most effective forms of contraception; levonorgestrel-releasing IUDs are more effective than copper IUDs.1 The rates of failure in the first year of use are 0.8% and 0.2% for copper and levonorgestrel-releasing IUDs, respectively.1
Continue to: The Lactational Amenorrhea Method
The Lactational Amenorrhea Method (LAM), which is defined as providing infant nutrition exclusively through breastmilk during the first 6 months postpartum, also provides protection against pregnancy. LAM has a failure rate of 0% to 1.5%.2
It is not surprising that this patient thought she was adequately protected against pregnancy. That said, no contraceptive method is foolproof (as this case demonstrates).
Risks to the pregnancy. When pregnancy does occur with an IUD in place, the patient should be informed of the possible risks to the pregnancy. These include complications such as spontaneous abortion, chorioamnionitis, and preterm delivery.3 Risk is further increased if the IUD is malpositioned (as this one was), meaning that any part of the IUD is located in the lower uterine segment, myometrium, or endocervical canal.4,5
Removal of the IUD is generally recommended if the device and its strings can be located, although removal does not completely mitigate risk. In a study done in Egypt, 46 of 52 IUDs were removed successfully, with 2 spontaneous abortions as a result.6 Of note, the IUDs extracted in this study were Lippes loop and copper models, not levonorgestrel-releasing IUDs such as our patient had. There is a single case report7 of a patient who had a Mirena inserted very early in a pregnancy; the IUD had to be left in place due to the risk for miscarriage, but she was able to carry the infant to term and did not experience any adverse effects.
Our patient
The patient delivered a male infant vaginally at term without issue. However, the IUD was not expelled during this process. Ultrasound showed that it was embedded in the posterior myometrium with a hypoechoic tract. The patient was referred to Gynecology, and the IUD was successfully removed.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Even the most reliable method of contraception can fail—so pregnancy should always be in the differential diagnosis for a sexually active woman. Location of IUD placement is important; it must be in the right place to be effective. The tenets of LAM must be followed precisely in order for breastfeeding to provide protection against pregnancy. Patients can successfully carry a pregnancy to term with an IUD, as this patient did, but it places them at higher risk for ectopic pregnancy, premature rupture of membranes, and infection.
The author thanks Jenny Walters, lactation consultant, for her assistance in the preparation of the manuscript.
CORRESPONDENCE
Hannah Maxfield, MD, 3901 Rainbow Boulevard, MS 4010, Kansas City, KS 66160; [email protected]
1. Heinemann K, Reed S, Moehner S, et al. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception. 2015;91:280-283.
2. Labbok MH. Postpartum sexuality and the Lactational Amenorrhea Method for contraception. Clin Obstet Gynecol. 2015;58:915-927.
3. Ganer H, Levy A, Ohel I, et al. Pregnancy outcome in women with an intrauterine contraceptive device. Am J Obstet Gynecol. 2009;201:381.e1-e5.
4. Moschos E, Twickler D. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-e6.
5. Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430.
6. Assaf A, Gohar M, Saad S, et al. Removal of intrauterine devices with missing tails during early pregnancy. Contraception. 1992;45:541-546.
7. Gardyszewska A, Czajkowski K. Application of levonorgestrel-releasing intrauterine system in early pregnancy: a case report [article in Polish]. Ginekol Pol. 2012;83:950-952.
1. Heinemann K, Reed S, Moehner S, et al. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception. 2015;91:280-283.
2. Labbok MH. Postpartum sexuality and the Lactational Amenorrhea Method for contraception. Clin Obstet Gynecol. 2015;58:915-927.
3. Ganer H, Levy A, Ohel I, et al. Pregnancy outcome in women with an intrauterine contraceptive device. Am J Obstet Gynecol. 2009;201:381.e1-e5.
4. Moschos E, Twickler D. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-e6.
5. Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430.
6. Assaf A, Gohar M, Saad S, et al. Removal of intrauterine devices with missing tails during early pregnancy. Contraception. 1992;45:541-546.
7. Gardyszewska A, Czajkowski K. Application of levonorgestrel-releasing intrauterine system in early pregnancy: a case report [article in Polish]. Ginekol Pol. 2012;83:950-952.