User login
Transabdominal cerclage for managing recurrent pregnancy loss
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
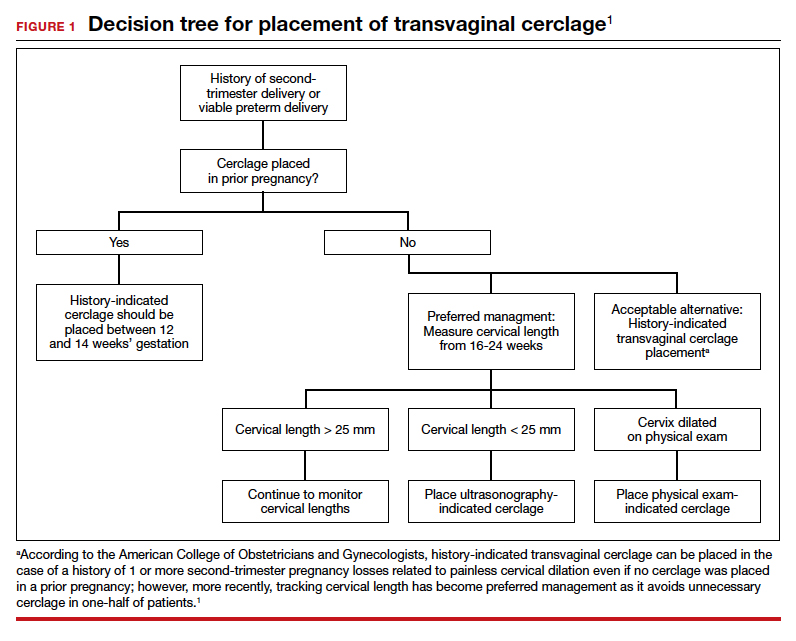
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7
Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
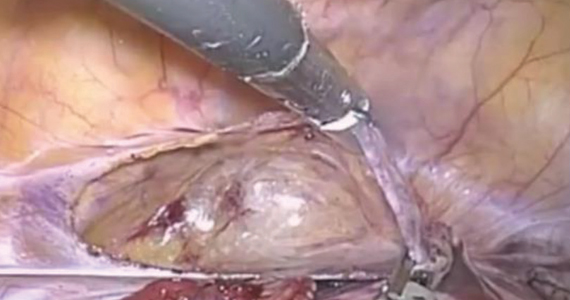
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.
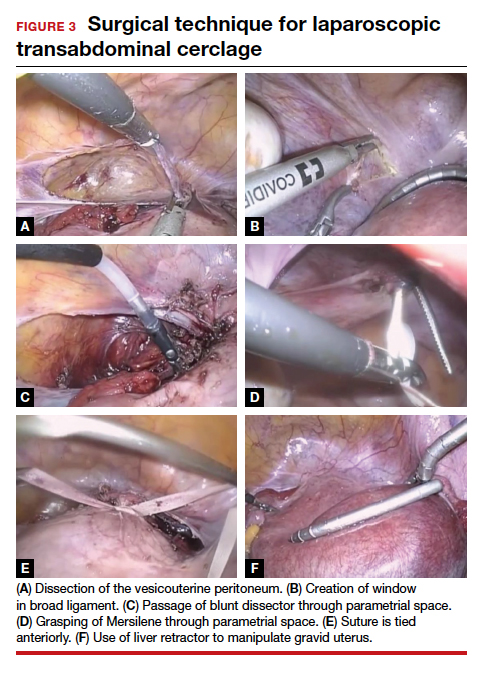
Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7

Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.

Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
CASE A woman with recurrent pregnancy loss
A 38-year-old woman (G4P0221) presents to your office for preconception counseling. Her history is significant for the following: a spontaneous pregnancy loss at 15 weeks’ gestation; a pregnancy loss at 17 weeks secondary to preterm premature rupture of membranes (PPROM); a cesarean delivery at 30 weeks and 6 days’ gestation after placement of a transvaginal cerclage at 20 weeks for cervical dilation noted on physical exam (the child now has developmental delays); and most recently a delivery at 24 weeks and 4 days due to preterm labor with subsequent neonatal demise (this followed a transvaginal cerclage placed at 13 weeks and 6 days).
How would you counsel this patient?
Cervical insufficiency describes the inability of the cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, labor, or both in the second trimester.1 This condition affects an estimated 1% of obstetric patients and 8% of women with recurrent losses who have experienced a second-trimester loss.2
Diagnosis of cervical insufficiency is based on a history of painless cervical dilation after the first trimester with expulsion of the pregnancy in the second trimester before 24 weeks of gestation without contractions and in the absence of other pathology, such as bleeding, infection, or ruptured membranes.1 Diagnosis also can be made by noting cervical dilation on physical exam during the second trimester; more recently, short cervical length on transvaginal ultrasonography in the second trimester has been used to try to predict when a cervical cerclage may be indicated, although sonographic cervical length is more a marker for risk of preterm birth than for cervical insufficiency specifically.1,3
Given the considerable emotional and physical distress that patients experience with recurrent second-trimester losses and the significant neonatal morbidity and mortality that can occur with preterm delivery, substantial efforts are made to prevent these outcomes by treating patients with cervical insufficiency and those at risk for preterm delivery.
Transvaginal cerclage: A treatment mainstay
Standard treatment options for cervical insufficiency depend on the patient’s history. One of the treatment mainstays for women with prior second-trimester losses or preterm deliveries is transvaginal cervical cerclage. A transvaginal cerclage can be placed using either a Shirodkar technique, in which the vesicocervical mucosa is dissected and a suture is placed as close to the internal cervical os as possible, or a McDonald technique, in which a purse-string suture is placed around the cervicovaginal junction. No randomized trials have compared the effectiveness of these 2 methods, but most observational studies show no difference, and one suggests that the Shirodkar technique may be more effective in obese women specifically.4-6
Indications for transvaginal cerclage. The indication for transvaginal cerclage is based on history, physical exam, or ultrasonography.
A physical-exam indication is the most straightforward of the 3. Transvaginal cerclage placement is indicated if on physical exam in the second trimester a patient has cervical dilation without contractions or infection.1,7
A history-indicated cerclage (typically placed between 12 and 14 weeks’ gestation) is based on a cerclage having been placed in a prior pregnancy due to painless cervical dilation in the second trimester (either ultrasonography- or physical-exam indicated), and it also can be considered in the case of a history of 1 or more second-trimester pregnancy losses related to painless cervical dilation.1
More recent evidence suggests that in patients with 1 prior second-trimester loss or preterm delivery, serial sonographic cervical length can be measured safely from 16 to 24 weeks, with a cerclage being placed only if cervical length decreases to less than 25 mm. By using the ultrasonography-based indication, unnecessary history-indicated cerclages for 1 prior second-trimester or preterm birth can be avoided in more than one-half of patients (FIGURE 1).1,7

Efficacy. The effectiveness of transvaginal cerclage varies by the indication. Authors of a 2017 Cochrane review found an overall reduced risk of giving birth before 34 weeks’ gestation for any indication, with an average relative risk of 0.77.2 Other recent studies showed the following8-10:
- a 63% delivery rate after 28 weeks’ gestation for physical-exam indicated cerclages in the presence of bulging amniotic membranes
- an 86.2% delivery rate after 32 weeks’ gestation for ultrasonography-indicated cerclages
- an 86% delivery rate after 32 weeks’ gestation for a history-indicated cerclage in patients with 2 or more prior second-trimester losses.
Success rates, especially for ultrasonography- and history-indicated cerclage, are thus high. For the 14% who still fail these methods, however, a different management strategy is needed, which is where transabdominal cerclage comes into play.
Continue to: Transabdominal cerclage is an option for certain patients...
Transabdominal cerclage is an option for certain patients
In transabdominal cerclage, an abdominal approach is used to place a stitch at the cervicouterine junction. With this approach, the cerclage can reach a closer proximity to the internal os compared with the vaginal approach, providing better support of the cervical tissue (FIGURE 2).11 Whether performed via laparotomy or laparoscopy, the transabdominal cerclage procedure likely carries higher morbidity than a transvaginal approach, and cesarean delivery is required after placement.

Since transvaginal cerclage often is successful, in most cases the transabdominal approach should not be viewed as the first-line treatment for cervical insufficiency if a history-indicated transvaginal cerclage has not been attempted. For women who fail a history-indicated transvaginal cerclage, however, a transabdominal cerclage has been proven to decrease the rate of preterm delivery and PPROM compared with attempting another history-indicated transvaginal cerclage.11,12
A recent systematic review of pregnancy outcomes after transabdominal cerclage placement reported neonatal survival of 96.5% and an 83% delivery rate after 34 weeks’ gestation.13 Thus, even among a population that failed transvaginal cerclage, a transabdominal cerclage has a high success rate in providing a good pregnancy outcome (TABLE). Transabdominal cerclage also can be considered as first-line treatment in patients who had prior cervical surgery or cervical deformities that might preclude the ability to place a cerclage transvaginally.

CASE Continued: A candidate for transabdominal cerclage
Given the patient’s poor obstetric history, which includes a preterm delivery and neonatal loss despite a history-indicated cerclage, you recommend that the patient have a transabdominal cerclage placed as the procedure has been proven to increase the chances of neonatal survival and delivery after 34 weeks in women with a similar obstetric history. The patient is interested in this option and asks about how this cerclage is placed and when it would need to be placed during her next pregnancy.
Surgical technique for transabdominal cerclage placement
A transabdominal cerclage can be placed via laparotomy, laparoscopy, or robot-assisted laparoscopy. No differences in obstetric outcomes have been shown between the laparotomy and laparoscopic approaches.14,15 Given the benefits of minimally invasive surgery, a laparoscopic or robot-assisted approach is preferred when feasible.
Additionally, for ease of placement, transabdominal cerclage can be placed prior to conception—known as interval placement—or during pregnancy between 10 and 14 weeks (preferably closer to 10 weeks). Because of the increased difficulty in placing a cerclage in the gravid uterus, interval transabdominal cerclage placement is recommended when possible.13,16 Authors of one observational study noted that improved obstetric outcomes occurred with interval placement compared with cerclage placement between 9 and 10 weeks’ gestation, with a delivery rate at more than 34 weeks’ gestation in 90% versus 74% of patients, respectively.16
Continue to: Steps for interval cerclage and during pregnancy...
Steps for interval cerclage and during pregnancy
Our practice is to place transabdominal cerclage via conventional laparoscopy as an interval procedure when possible. We find no benefit in using robotic assistance.
For an interval procedure, the patient is placed in a dorsal lithotomy position, and we place a 10-mm umbilical port, 2 lateral 5-mm ports, 1 suprapubic 5-mm port, and a uterine manipulator. We use a flexible laparoscope to provide optimal visualization of the pelvis from any angle.
The first step of the surgery involves dissecting the vesicouterine peritoneum in order to move the bladder inferiorly (FIGURE 3A). Uterine arteries are then identified lateral to the cervix as part of this dissection, and a window is created in the inferior aspect of the broad ligament just anterior and lateral to the insertion of the uterosacral ligaments onto the uterus, with care taken to avoid the uterine vessels superiorly (FIGURE 3B). Two 5-mm Mersilene tape sutures are then tied together to create 1 suture with a needle at each end. This is then passed into the abdomen, and 1 needle is passed through the parametrial space at the level of the internal os inferior to the uterine vessels on 1 side of the uterus while the other needle is passed through the parametrial space on the opposite side.

Alternatively, rather than using the suture needles, a blunt dissector can be passed through this same space bilaterally (FIGURE 3C) via the suprapubic port and can pull the Mersilene tape through the parametrial space (FIGURE 3D). The suture is then tied anterior at the level of the internal os intracorporally (FIGURE 3E), and the needles are cut off the suture and removed from the abdomen.
To perform transabdominal cerclage when the patient is pregnant, a few modifications are needed to help with placement. First, the patient may be placed in supine position since a uterine manipulator cannot be used. Second, use of a flexible laparoscope becomes even more imperative in order to properly see around the gravid uterus. Lastly, a 5-mm laparoscopic liver retractor can be used to aid in blunt manipulation of the gravid uterus (FIGURE 3F). (The surgical video below highlights the steps to transabdominal cerclage placement in a pregnant patient.) All other port placements and steps to dissection and suture placement are the same as in interval placement.

CASE Continued: Patient pursues transabdominal cerclage
You explain to your patient that ideally the cerclage should be placed now in a laparoscopic fashion before she becomes pregnant. You then refer her to a local gynecologic surgeon who places many laparoscopic transabdominal cerclages. She undergoes the procedure, becomes pregnant, and after presenting in labor at 35 weeks’ gestation has a cesarean delivery. Her baby is born without any neonatal complications, and the patient is overjoyed with the outcome.
Management during and after pregnancy
Pregnant patients with a transabdominal cerclage are precluded from having a vaginal delivery and must deliver via cesarean. During the antepartum period, patients are managed in the same manner as those who have a transvaginal cerclage. Delivery via cesarean at the onset of regular contractions is recommended to reduce the risk of uterine rupture. In the absence of labor, scheduled cesarean is performed at term.
Our practice is to schedule cesarean delivery at 38 weeks’ gestation, although there are no data or consensus to support a specific gestational age between 37 and 39 weeks. Unlike a transvaginal cerclage, a transabdominal cerclage can be left in place for use in subsequent pregnancies. Data are limited on whether the transabdominal cerclage should be removed in women who no longer desire childbearing and whether there are long-term sequelae if the suture is left in situ.17
Continue to: Complications and risks of abdominal cerclage...
Complications and risks of abdominal cerclage
As the data suggest and our experience confirms, transabdominal cerclage is highly successful in patients who have failed a history-indicated transvaginal cerclage; however, the transabdominal approach carries a higher surgical risk. Risks include intraoperative hemorrhage, conversion to laparotomy, and a range of rare surgical and obstetric complications, such as bladder injury and PPROM.13,18
If a patient experiences a fetal loss in the first trimester, a dilation and curettage (D&C) can be performed, with good obstetric outcomes in subsequent pregnancies.19 If the patient experiences an early-to-mid second-trimester loss, some studies suggest that a dilation and evacuation (D&E) of the uterus can be done with sufficient dilation of the cervix to accommodate up to a 15-mm cannula and Sopher forceps.19 Laminaria also may be used in this process. However, no data exist regarding success of future pregnancies and transabdominal cerclage integrity after a D&E.20 If the cerclage prevents successful dilation of the cervix, the cerclage must be removed laparoscopically prior to performing the D&E.
In late second-trimester and third-trimester loss, the cerclage must be removed to allow passage of the fetus and placenta prior to a D&E or an induction of labor.20
For patients with PPROM or preterm labor, data are limited regarding management recommendations. However, in these complex cases, we strongly recommend an individualized approach and co-management with maternal-fetal medicine specialists.
CASE Resolved
The cerclage is left in place during the patient’s cesarean delivery, and her postpartum course is uneventful. She continued without complications for the next year, at which time she sees you in the office with plans to have another pregnancy later in the year. You counsel her that her abdominal cerclage will still be effective and that she can get pregnant with expectations of similar outcomes as her previous pregnancy. She thanks you for everything and reports that she hopes to return later in the year for her first prenatal visit. ●
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 pt 1): 372-379.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6(6):CD008991.
- Brown R, Gagnon R, Delisle M-F. No. 373—cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41:233-247.
- Odibo AO, Berghella V, To MS, et al. Shirodkar versus McDonald cerclage for the prevention of preterm birth in women with short cervical length. Am J Perinatol. 2007;24: 55-60.
- Basbug A, Bayrak M, Dogan O, et al. McDonald versus modified Shirodkar rescue cerclage in women with prolapsed fetal membranes. J Matern Fetal Neonatal Med. 2020;33: 1075-1097.
- Figueroa R, Crowell R, Martinez A, et al. McDonald versus Shirodkar cervical cerclage for the prevention of preterm birth: impact of body mass index. J Matern Fetal Neonatal Med. 2019;32:3408-3414.
- Suhag A, Berghella V. Cervical cerclage. Clin Obstet Gynecol. 2014;57:557-567.
- Bayrak M, Gul A, Goynumer G. Rescue cerclage when foetal membranes prolapse into the vagina. J Obstet Gynaecol. 2017;37:471-475.
- Drassinower D, Coviello E, Landy HJ, et al. Outcomes after periviable ultrasound-indicated cerclage. J Matern Fetal Neonatal Med. 2019;32:932-938.
- Lee KN, Whang EJ, Chang KH, et al. History-indicated cerclage: the association between previous preterm history and cerclage outcome. Obstet Gynecol Sci. 2018;61:23-29. doi:10.5468/ogs.2018.61.1.23.
- Sneider K, Christiansen OB, Sundtoft IB, et al. Recurrence rates after abdominal and vaginal cerclages in women with cervical insufficiency: a validated cohort study. Arch Gynecol Obstet. 2017;295:859-866.
- Davis G, Berghella V, Talucci M, et al. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836-839.
- Moawad GN, Tyan P, Bracke T, et al. Systematic review of transabdominal cerclage placed via laparoscopy for the prevention of preterm birth. J Mimim Invasive Gynecol. 2018;25:277-286.
- Burger NB, Brölmann HAM, Einarsson JI, et al. Effectiveness of abdominal cerclage placed via laparotomy or laparoscopy: systematic review. J Minim Invasive Gynecol. 2011;18:696-704.
- Kim S, Hill A, Menderes G, et al. Minimally invasive abdominal cerclage compared to laparotomy: a comparison of surgical and obstetric outcomes. J Robot Surg. 2018;12:295-301.
- Dawood F, Farquharson RG. Transabdominal cerclage: preconceptual versus first trimester insertion. Eur J Obstet Gynecol Reprod Biol. 2016;199:27-31.
- Hawkins E, Nimaroff M. Vaginal erosion of an abdominal cerclage 7 years after laparoscopic placement. Obstet Gynecol. 2014;123(2 pt 2 suppl 2):420-423.
- Foster TL, Moore ES, Sumners JE. Operative complications and fetal morbidity encountered in 300 prophylactic transabdominal cervical cerclage procedures by one obstetric surgeon. J Obstet Gynaecol. 2011;31:713-717.
- Dethier D, Lassey SC, Pilliod R, et al. Uterine evacuation in the setting of transabdominal cerclage. Contraception. 2020;101:174-177.
- Martin A, Lathrop E. Controversies in family planning: management of second-trimester losses in the setting of an abdominal cerclage. Contraception. 2013;87:728-731.
Statins and ICH: new meta-analysis
The meta-analysis was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Coauthor Abhi Pandhi, MD, the University of Tennessee Health Science Center, Memphis, explained that some previous studies have suggested that statin therapy may be associated with an increased risk for ICH, especially at higher doses. Other studies, however, have failed to confirm this and have shown an increase in cardiovascular events if statins are stopped.
To look further into this issue, Dr. Pandhi and colleagues conducted a meta-analysis of 19 clinical studies involving patients who had a history of cardiovascular or cerebrovascular events and who had been treated with statins. A total of 35,842 patients were included.
Results showed that statin use was not significantly associated with the risk for combined primary and secondary ICH (relative risk, 1.03; 95% confidence interval, 0.85–1.08). But the risk for cerebral ischemia (stroke and transient ischemic attack) was significantly lower in those who received statins (RR, 0.79; 95% CI, 0.61–0.87).
“Overall, we found no effect of statins on the risk of ICH, and benefits on reducing ischemic events are clear,” Dr. Pandhi said.
Increased secondary ICH?
However, a sensitivity analysis showed a trend toward a higher risk for secondary ICH among those who were assigned to statin treatment (odds ratio, 1.87; 95% CI, 0.91–3.86).
“While this may suggest an increased risk of secondary ICH, when we look at the big picture, putting all the data together, and given that ischemic events are far more common than ICH, the risk of stopping statins and losing the protection against ischemic events is probably greater than any harm even in patients with underlying risk factors for ICH,” Dr. Pandhi concluded.
Commenting on the study, Michael Szarek, PhD, who has also conducted research in this field, said: “The results of this meta-analysis appear to be consistent with individual randomized trials of statins in patients with cerebrovascular disease that have shown clear benefit in terms of ischemic stroke or TIA and potential harm in terms of hemorrhagic stroke.”
Dr. Szarek is chair and professor in the Department of Epidemiology and Biostatistics at the SUNY Downstate Health Sciences University, New York City.
“However, the much greater frequency of ischemic events, coupled with benefits in coronary and peripheral vascular territories, suggest the risk/benefit of statin treatment remains favorable in this patient population, with the possible exception of patients with a history of hemorrhagic stroke,” he added.
Also commenting on this latest meta-analysis, Pamela Rist, ScD, associate epidemiologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston, who has also authored studies in this area, said the results of this study seem similar to prior results from meta-analyses of clinical trials.
“It will be interesting to see the full manuscript to learn more about the sensitivity analyses they conducted and why they may have observed a nonsignificant increased risk of secondary ICH among some individuals using statins,” Dr. Rist added.
“Based on prior published meta-analyses of statin use and ICH, any potential increase in risk of hemorrhagic stroke is probably outweighed by the reduction in ischemic stroke and other cardiovascular events,” she concluded.
Dr. Pandhi has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Ishfaq A et al. AAN 2020. Abstract S9.010.
The meta-analysis was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Coauthor Abhi Pandhi, MD, the University of Tennessee Health Science Center, Memphis, explained that some previous studies have suggested that statin therapy may be associated with an increased risk for ICH, especially at higher doses. Other studies, however, have failed to confirm this and have shown an increase in cardiovascular events if statins are stopped.
To look further into this issue, Dr. Pandhi and colleagues conducted a meta-analysis of 19 clinical studies involving patients who had a history of cardiovascular or cerebrovascular events and who had been treated with statins. A total of 35,842 patients were included.
Results showed that statin use was not significantly associated with the risk for combined primary and secondary ICH (relative risk, 1.03; 95% confidence interval, 0.85–1.08). But the risk for cerebral ischemia (stroke and transient ischemic attack) was significantly lower in those who received statins (RR, 0.79; 95% CI, 0.61–0.87).
“Overall, we found no effect of statins on the risk of ICH, and benefits on reducing ischemic events are clear,” Dr. Pandhi said.
Increased secondary ICH?
However, a sensitivity analysis showed a trend toward a higher risk for secondary ICH among those who were assigned to statin treatment (odds ratio, 1.87; 95% CI, 0.91–3.86).
“While this may suggest an increased risk of secondary ICH, when we look at the big picture, putting all the data together, and given that ischemic events are far more common than ICH, the risk of stopping statins and losing the protection against ischemic events is probably greater than any harm even in patients with underlying risk factors for ICH,” Dr. Pandhi concluded.
Commenting on the study, Michael Szarek, PhD, who has also conducted research in this field, said: “The results of this meta-analysis appear to be consistent with individual randomized trials of statins in patients with cerebrovascular disease that have shown clear benefit in terms of ischemic stroke or TIA and potential harm in terms of hemorrhagic stroke.”
Dr. Szarek is chair and professor in the Department of Epidemiology and Biostatistics at the SUNY Downstate Health Sciences University, New York City.
“However, the much greater frequency of ischemic events, coupled with benefits in coronary and peripheral vascular territories, suggest the risk/benefit of statin treatment remains favorable in this patient population, with the possible exception of patients with a history of hemorrhagic stroke,” he added.
Also commenting on this latest meta-analysis, Pamela Rist, ScD, associate epidemiologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston, who has also authored studies in this area, said the results of this study seem similar to prior results from meta-analyses of clinical trials.
“It will be interesting to see the full manuscript to learn more about the sensitivity analyses they conducted and why they may have observed a nonsignificant increased risk of secondary ICH among some individuals using statins,” Dr. Rist added.
“Based on prior published meta-analyses of statin use and ICH, any potential increase in risk of hemorrhagic stroke is probably outweighed by the reduction in ischemic stroke and other cardiovascular events,” she concluded.
Dr. Pandhi has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Ishfaq A et al. AAN 2020. Abstract S9.010.
The meta-analysis was presented online as part of the 2020 American Academy of Neurology Science Highlights.
Coauthor Abhi Pandhi, MD, the University of Tennessee Health Science Center, Memphis, explained that some previous studies have suggested that statin therapy may be associated with an increased risk for ICH, especially at higher doses. Other studies, however, have failed to confirm this and have shown an increase in cardiovascular events if statins are stopped.
To look further into this issue, Dr. Pandhi and colleagues conducted a meta-analysis of 19 clinical studies involving patients who had a history of cardiovascular or cerebrovascular events and who had been treated with statins. A total of 35,842 patients were included.
Results showed that statin use was not significantly associated with the risk for combined primary and secondary ICH (relative risk, 1.03; 95% confidence interval, 0.85–1.08). But the risk for cerebral ischemia (stroke and transient ischemic attack) was significantly lower in those who received statins (RR, 0.79; 95% CI, 0.61–0.87).
“Overall, we found no effect of statins on the risk of ICH, and benefits on reducing ischemic events are clear,” Dr. Pandhi said.
Increased secondary ICH?
However, a sensitivity analysis showed a trend toward a higher risk for secondary ICH among those who were assigned to statin treatment (odds ratio, 1.87; 95% CI, 0.91–3.86).
“While this may suggest an increased risk of secondary ICH, when we look at the big picture, putting all the data together, and given that ischemic events are far more common than ICH, the risk of stopping statins and losing the protection against ischemic events is probably greater than any harm even in patients with underlying risk factors for ICH,” Dr. Pandhi concluded.
Commenting on the study, Michael Szarek, PhD, who has also conducted research in this field, said: “The results of this meta-analysis appear to be consistent with individual randomized trials of statins in patients with cerebrovascular disease that have shown clear benefit in terms of ischemic stroke or TIA and potential harm in terms of hemorrhagic stroke.”
Dr. Szarek is chair and professor in the Department of Epidemiology and Biostatistics at the SUNY Downstate Health Sciences University, New York City.
“However, the much greater frequency of ischemic events, coupled with benefits in coronary and peripheral vascular territories, suggest the risk/benefit of statin treatment remains favorable in this patient population, with the possible exception of patients with a history of hemorrhagic stroke,” he added.
Also commenting on this latest meta-analysis, Pamela Rist, ScD, associate epidemiologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston, who has also authored studies in this area, said the results of this study seem similar to prior results from meta-analyses of clinical trials.
“It will be interesting to see the full manuscript to learn more about the sensitivity analyses they conducted and why they may have observed a nonsignificant increased risk of secondary ICH among some individuals using statins,” Dr. Rist added.
“Based on prior published meta-analyses of statin use and ICH, any potential increase in risk of hemorrhagic stroke is probably outweighed by the reduction in ischemic stroke and other cardiovascular events,” she concluded.
Dr. Pandhi has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Ishfaq A et al. AAN 2020. Abstract S9.010.
AAN 2020
Vaccine maintenance improves relapse-free survival in BRCA wild-type ovarian cancer
The autologous tumor cell vaccine gemogenovatucel-T (Vigil Ovarian) is well tolerated as maintenance therapy in stage III-IV ovarian cancer patients and may improve relapse-free survival, particularly in BRCA wild-type disease, according to findings from the ongoing VITAL study.
In patients with and without BRCA1/2 mutations, the median relapse-free survival was longer with gemogenovatucel-T maintenance than with placebo, but the difference did not reach statistical significance (P = .065).
However, among patients with wild-type BRCA, the median relapse-free survival was significantly longer with gemogenovatucel-T (P = .0007).
Rodney P. Rocconi, MD, of the Mitchell Cancer Institute at University of South Alabama, Mobile, reported these results in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancers. The meeting was canceled because of the COVID-19 pandemic. Some data have been updated from the abstract.
Study rationale
Gemogenovatucel-T (formerly called FANG) is an autologous tumor cell vaccine transfected with a plasmid encoding granulocyte-macrophage colony–stimulating factor and a novel bifunctional short hairpin interfering RNA targeting furin convertase.
“In the era of personalized, targeted medicine, I think this is about as personalized as you can get, this type of vaccine,” Dr. Rocconi said. “Essentially, we harvest patients’ own cancer cells and create a vaccine that is targeted to the antigens on their cells so that it recognizes only that patient’s cancer.”
The vaccine also helps recruit immune cells to the area and has a very limited off-target effect, Dr. Rocconi added.
He noted that gemogenovatucel-T previously demonstrated promising efficacy and limited side effects in a phase 1 study that included patients with advanced ovarian cancer (Mol Ther. 2012 Mar;20[3]:679-86).
“So we thought that, in ovarian cancer, as a maintenance therapy, it made a lot of sense,” Dr. Rocconi said, noting that the overall prognosis for advanced epithelial ovarian cancer remains limited.
Treatment and toxicity
Dr. Rocconi and colleagues reported data on 91 patients in the VITAL study. The patients had achieved a complete response after frontline surgery and chemotherapy, and they were randomized to maintenance with gemogenovatucel-T or placebo.
Patients had a median time from surgery to randomization of 208.5 days in the gemogenovatucel-T group and 200 days in the control group. The patients were treated with 1 x 107 cells/mL of gemogenovatucel-T or placebo intradermally once a month for up to 12 doses.
Gemogenovatucel-T was well tolerated. No added overall toxicity was noted in the gemogenovatucel-T group versus the control group, and no grade 4/5 toxicities were observed, Dr. Rocconi said. Grade 2/3 toxic events were observed in 8% of patients in the gemogenovatucel-T group, compared with 18% in the control group. The most common events were nausea and musculoskeletal pain in the gemogenovatucel-T group, and were bone pain and fatigue in the control group.
Relapse-free and overall survival
In the entire cohort, the median relapse-free survival was longer with gemogenovatucel-T maintenance – 12.6 months versus 8.4 months with placebo (hazard ratio, 0.69) – but the difference did not reach statistical significance (P = .065).
However, in the 67 patients with wild-type BRCA, the median relapse-free survival was 19.4 months with gemogenovatucel-T and 14.8 months with placebo, a statistically significant difference (HR, 0.459; P = .0007).
The median overall survival was not reached in the BRCA wild-type patients treated with gemogenovatucel-T, and it was 41.4 months from the time of randomization in those who received placebo (HR, 0.417; P = .02).
No benefit was seen with gemogenovatucel-T in patients with known BRCA1/2 mutations, Dr. Rocconi said.
‘Encouraging’ results
The overall improvement in the gemogenovatucel-T group was encouraging, particularly in a maintenance-type trial, Dr. Rocconi said. He noted that prior treatments for maintenance have received approval based on shorter survival gains, and the finding of particular benefit in BRCA wild-type disease could have important implications for a population that usually has lesser benefit from treatments, compared with patients who have BRCA mutations.
“So this result is very unique,” Dr. Rocconi said, explaining that about 85% of ovarian cancer patients have BRCA wild-type disease; with this treatment, patients with wild-type BRCA may achieve similar survival rates as those seen in BRCA-mutant disease.
“I think, in general, immunotherapy has been somewhat disappointing in ovarian cancer, so to have a targeted vaccine work in ovarian cancer, just broadly ... is pretty noteworthy,” he said. “We’re really excited, obviously, about the overall success we’ve seen for all patients, but most importantly in those with BRCA wild type. This is a pretty marked significance in recurrence-free intervals and overall survival, and we’re definitely pleased with that.”
Next steps
The findings from this trial have been submitted for publication, and efforts are underway to determine next steps through communication with the Food and Drug Administration, Dr. Rocconi said.
Additionally, other studies are underway to assess gemogenovatucel-T in patients who fall in “the middle ground” – that is, patients who have BRCA wild-type disease but have “some homologous recombination deficiency where the tumor itself might be BRCA deficient or have some other type of deficiency,” Dr. Rocconi explained.
“So we’re trying to tease out specifically what is going on across all the different variations of ovarian cancer patients, and also looking for potential biomarkers for predicting response,” he said. “What we would like to see is a companion test where we’re able to predict which patients can really respond and do best with this technology, and, that way, we know how to stratify patients most appropriately.”
The current trial was sponsored by Gradalis. Dr. Rocconi disclosed relationships with Gradalis, Genentech, Clovis, and Johnson & Johnson.
SOURCE: Rocconi RP et al. SGO 2020, Abstract LBA7.
The autologous tumor cell vaccine gemogenovatucel-T (Vigil Ovarian) is well tolerated as maintenance therapy in stage III-IV ovarian cancer patients and may improve relapse-free survival, particularly in BRCA wild-type disease, according to findings from the ongoing VITAL study.
In patients with and without BRCA1/2 mutations, the median relapse-free survival was longer with gemogenovatucel-T maintenance than with placebo, but the difference did not reach statistical significance (P = .065).
However, among patients with wild-type BRCA, the median relapse-free survival was significantly longer with gemogenovatucel-T (P = .0007).
Rodney P. Rocconi, MD, of the Mitchell Cancer Institute at University of South Alabama, Mobile, reported these results in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancers. The meeting was canceled because of the COVID-19 pandemic. Some data have been updated from the abstract.
Study rationale
Gemogenovatucel-T (formerly called FANG) is an autologous tumor cell vaccine transfected with a plasmid encoding granulocyte-macrophage colony–stimulating factor and a novel bifunctional short hairpin interfering RNA targeting furin convertase.
“In the era of personalized, targeted medicine, I think this is about as personalized as you can get, this type of vaccine,” Dr. Rocconi said. “Essentially, we harvest patients’ own cancer cells and create a vaccine that is targeted to the antigens on their cells so that it recognizes only that patient’s cancer.”
The vaccine also helps recruit immune cells to the area and has a very limited off-target effect, Dr. Rocconi added.
He noted that gemogenovatucel-T previously demonstrated promising efficacy and limited side effects in a phase 1 study that included patients with advanced ovarian cancer (Mol Ther. 2012 Mar;20[3]:679-86).
“So we thought that, in ovarian cancer, as a maintenance therapy, it made a lot of sense,” Dr. Rocconi said, noting that the overall prognosis for advanced epithelial ovarian cancer remains limited.
Treatment and toxicity
Dr. Rocconi and colleagues reported data on 91 patients in the VITAL study. The patients had achieved a complete response after frontline surgery and chemotherapy, and they were randomized to maintenance with gemogenovatucel-T or placebo.
Patients had a median time from surgery to randomization of 208.5 days in the gemogenovatucel-T group and 200 days in the control group. The patients were treated with 1 x 107 cells/mL of gemogenovatucel-T or placebo intradermally once a month for up to 12 doses.
Gemogenovatucel-T was well tolerated. No added overall toxicity was noted in the gemogenovatucel-T group versus the control group, and no grade 4/5 toxicities were observed, Dr. Rocconi said. Grade 2/3 toxic events were observed in 8% of patients in the gemogenovatucel-T group, compared with 18% in the control group. The most common events were nausea and musculoskeletal pain in the gemogenovatucel-T group, and were bone pain and fatigue in the control group.
Relapse-free and overall survival
In the entire cohort, the median relapse-free survival was longer with gemogenovatucel-T maintenance – 12.6 months versus 8.4 months with placebo (hazard ratio, 0.69) – but the difference did not reach statistical significance (P = .065).
However, in the 67 patients with wild-type BRCA, the median relapse-free survival was 19.4 months with gemogenovatucel-T and 14.8 months with placebo, a statistically significant difference (HR, 0.459; P = .0007).
The median overall survival was not reached in the BRCA wild-type patients treated with gemogenovatucel-T, and it was 41.4 months from the time of randomization in those who received placebo (HR, 0.417; P = .02).
No benefit was seen with gemogenovatucel-T in patients with known BRCA1/2 mutations, Dr. Rocconi said.
‘Encouraging’ results
The overall improvement in the gemogenovatucel-T group was encouraging, particularly in a maintenance-type trial, Dr. Rocconi said. He noted that prior treatments for maintenance have received approval based on shorter survival gains, and the finding of particular benefit in BRCA wild-type disease could have important implications for a population that usually has lesser benefit from treatments, compared with patients who have BRCA mutations.
“So this result is very unique,” Dr. Rocconi said, explaining that about 85% of ovarian cancer patients have BRCA wild-type disease; with this treatment, patients with wild-type BRCA may achieve similar survival rates as those seen in BRCA-mutant disease.
“I think, in general, immunotherapy has been somewhat disappointing in ovarian cancer, so to have a targeted vaccine work in ovarian cancer, just broadly ... is pretty noteworthy,” he said. “We’re really excited, obviously, about the overall success we’ve seen for all patients, but most importantly in those with BRCA wild type. This is a pretty marked significance in recurrence-free intervals and overall survival, and we’re definitely pleased with that.”
Next steps
The findings from this trial have been submitted for publication, and efforts are underway to determine next steps through communication with the Food and Drug Administration, Dr. Rocconi said.
Additionally, other studies are underway to assess gemogenovatucel-T in patients who fall in “the middle ground” – that is, patients who have BRCA wild-type disease but have “some homologous recombination deficiency where the tumor itself might be BRCA deficient or have some other type of deficiency,” Dr. Rocconi explained.
“So we’re trying to tease out specifically what is going on across all the different variations of ovarian cancer patients, and also looking for potential biomarkers for predicting response,” he said. “What we would like to see is a companion test where we’re able to predict which patients can really respond and do best with this technology, and, that way, we know how to stratify patients most appropriately.”
The current trial was sponsored by Gradalis. Dr. Rocconi disclosed relationships with Gradalis, Genentech, Clovis, and Johnson & Johnson.
SOURCE: Rocconi RP et al. SGO 2020, Abstract LBA7.
The autologous tumor cell vaccine gemogenovatucel-T (Vigil Ovarian) is well tolerated as maintenance therapy in stage III-IV ovarian cancer patients and may improve relapse-free survival, particularly in BRCA wild-type disease, according to findings from the ongoing VITAL study.
In patients with and without BRCA1/2 mutations, the median relapse-free survival was longer with gemogenovatucel-T maintenance than with placebo, but the difference did not reach statistical significance (P = .065).
However, among patients with wild-type BRCA, the median relapse-free survival was significantly longer with gemogenovatucel-T (P = .0007).
Rodney P. Rocconi, MD, of the Mitchell Cancer Institute at University of South Alabama, Mobile, reported these results in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancers. The meeting was canceled because of the COVID-19 pandemic. Some data have been updated from the abstract.
Study rationale
Gemogenovatucel-T (formerly called FANG) is an autologous tumor cell vaccine transfected with a plasmid encoding granulocyte-macrophage colony–stimulating factor and a novel bifunctional short hairpin interfering RNA targeting furin convertase.
“In the era of personalized, targeted medicine, I think this is about as personalized as you can get, this type of vaccine,” Dr. Rocconi said. “Essentially, we harvest patients’ own cancer cells and create a vaccine that is targeted to the antigens on their cells so that it recognizes only that patient’s cancer.”
The vaccine also helps recruit immune cells to the area and has a very limited off-target effect, Dr. Rocconi added.
He noted that gemogenovatucel-T previously demonstrated promising efficacy and limited side effects in a phase 1 study that included patients with advanced ovarian cancer (Mol Ther. 2012 Mar;20[3]:679-86).
“So we thought that, in ovarian cancer, as a maintenance therapy, it made a lot of sense,” Dr. Rocconi said, noting that the overall prognosis for advanced epithelial ovarian cancer remains limited.
Treatment and toxicity
Dr. Rocconi and colleagues reported data on 91 patients in the VITAL study. The patients had achieved a complete response after frontline surgery and chemotherapy, and they were randomized to maintenance with gemogenovatucel-T or placebo.
Patients had a median time from surgery to randomization of 208.5 days in the gemogenovatucel-T group and 200 days in the control group. The patients were treated with 1 x 107 cells/mL of gemogenovatucel-T or placebo intradermally once a month for up to 12 doses.
Gemogenovatucel-T was well tolerated. No added overall toxicity was noted in the gemogenovatucel-T group versus the control group, and no grade 4/5 toxicities were observed, Dr. Rocconi said. Grade 2/3 toxic events were observed in 8% of patients in the gemogenovatucel-T group, compared with 18% in the control group. The most common events were nausea and musculoskeletal pain in the gemogenovatucel-T group, and were bone pain and fatigue in the control group.
Relapse-free and overall survival
In the entire cohort, the median relapse-free survival was longer with gemogenovatucel-T maintenance – 12.6 months versus 8.4 months with placebo (hazard ratio, 0.69) – but the difference did not reach statistical significance (P = .065).
However, in the 67 patients with wild-type BRCA, the median relapse-free survival was 19.4 months with gemogenovatucel-T and 14.8 months with placebo, a statistically significant difference (HR, 0.459; P = .0007).
The median overall survival was not reached in the BRCA wild-type patients treated with gemogenovatucel-T, and it was 41.4 months from the time of randomization in those who received placebo (HR, 0.417; P = .02).
No benefit was seen with gemogenovatucel-T in patients with known BRCA1/2 mutations, Dr. Rocconi said.
‘Encouraging’ results
The overall improvement in the gemogenovatucel-T group was encouraging, particularly in a maintenance-type trial, Dr. Rocconi said. He noted that prior treatments for maintenance have received approval based on shorter survival gains, and the finding of particular benefit in BRCA wild-type disease could have important implications for a population that usually has lesser benefit from treatments, compared with patients who have BRCA mutations.
“So this result is very unique,” Dr. Rocconi said, explaining that about 85% of ovarian cancer patients have BRCA wild-type disease; with this treatment, patients with wild-type BRCA may achieve similar survival rates as those seen in BRCA-mutant disease.
“I think, in general, immunotherapy has been somewhat disappointing in ovarian cancer, so to have a targeted vaccine work in ovarian cancer, just broadly ... is pretty noteworthy,” he said. “We’re really excited, obviously, about the overall success we’ve seen for all patients, but most importantly in those with BRCA wild type. This is a pretty marked significance in recurrence-free intervals and overall survival, and we’re definitely pleased with that.”
Next steps
The findings from this trial have been submitted for publication, and efforts are underway to determine next steps through communication with the Food and Drug Administration, Dr. Rocconi said.
Additionally, other studies are underway to assess gemogenovatucel-T in patients who fall in “the middle ground” – that is, patients who have BRCA wild-type disease but have “some homologous recombination deficiency where the tumor itself might be BRCA deficient or have some other type of deficiency,” Dr. Rocconi explained.
“So we’re trying to tease out specifically what is going on across all the different variations of ovarian cancer patients, and also looking for potential biomarkers for predicting response,” he said. “What we would like to see is a companion test where we’re able to predict which patients can really respond and do best with this technology, and, that way, we know how to stratify patients most appropriately.”
The current trial was sponsored by Gradalis. Dr. Rocconi disclosed relationships with Gradalis, Genentech, Clovis, and Johnson & Johnson.
SOURCE: Rocconi RP et al. SGO 2020, Abstract LBA7.
FROM SGO 2020
Petechiae and Ecchymoses on the Arm
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
A 70-year-old man who had been admitted to the hospital a week prior for a right groin abscess overlying the site of a femoral graft developed a purpuric rash isolated to the left arm of 1 day's duration. The dermatology service was consulted by vascular surgery. The patient denied prior episodes of a similar rash, and there was no associated pruritus or pain. There was no history of trauma to the area. His medical history was remarkable for type 2 diabetes mellitus, hypertension, and peripheral vascular disease. His surgical history was notable for bilateral popliteal aneurysm repair and right femoral aneurysm repair. No pertinent family history was elicited. Cefepime, metronidazole, and vancomycin were administered for the groin abscess. Additional medications included amlodipine, atorvastatin, diltiazem, gabapentin, heparin, insulin, oxycodone, and acetaminophen.
Physical examination revealed broad ecchymoses on the left forearm with scattered petechiae as well as linear ecchymoses on the left upper arm distributed in the area where a sphygmomanometer cuff was applied. Full-body skin examination confirmed that the distribution of the petechiae and ecchymoses was limited to the left arm. The patient was normotensive at the time of examination. Laboratory evaluation revealed a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL), a platelet count of 279,000/µL (reference range, 150,000-350,000/µL), and a glucose level of 121 mg/dL (reference range, 70-110 mg/dL).
COVID-19 pulmonary severity ascribed to coagulation differences
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
FROM THE BRITISH JOURNAL OF HEMATOLOGY
New angiotensin studies in COVID-19 give more reassurance
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Don’t delay antibiotic treatment in elderly patients with UTI
Background: If left untreated, UTIs may lead to severe complications. Although campaigns aimed at decreasing unnecessary prescriptions have reduced the number of antibiotic prescriptions for UTI, a concurrent rise in the rates of gram-negative bloodstream infections (BSIs) has also been observed.
Study design: Retrospective, population-based cohort study with data compiled from primary care records from 2007 to 2015 linked to hospital episode statistics and death records.
Setting: General practices in England.
Synopsis: The investigators analyzed 312,896 UTI episodes among 157,264 unique patients (65 years of age or older) during the study period. Exclusion criteria included asymptomatic bacteriuria and complicated UTI. Of 271,070 patients who received antibiotics on the day of presentation with symptoms, 0.2% developed BSI within 60 days versus 2.2% of patients in whom antibiotics were delayed and 2.9% among patients not prescribed antibiotics. After adjustment for comorbidities, sex, and socioeconomic status, patients in whom antibiotics were deferred had a 7.12-fold greater odds of BSI, compared with the immediate-antibiotic group. BSIs were more common among men and older patients. All-cause mortality, a secondary outcome, was 1.16-fold higher with deferred antibiotics and 2.18 times higher with no antibiotics.
While the cohort studied was very large, a causal relationship cannot be firmly established in this observational study. Also, researchers were unable to include laboratory data, such as urinalysis and culture, in their analysis.
Bottom line: Delayed prescription of antibiotics for elderly patients presenting with UTI in primary care settings was associated with higher rates of BSI and death.
Citation: Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ. 2019 Feb;364:1525.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Background: If left untreated, UTIs may lead to severe complications. Although campaigns aimed at decreasing unnecessary prescriptions have reduced the number of antibiotic prescriptions for UTI, a concurrent rise in the rates of gram-negative bloodstream infections (BSIs) has also been observed.
Study design: Retrospective, population-based cohort study with data compiled from primary care records from 2007 to 2015 linked to hospital episode statistics and death records.
Setting: General practices in England.
Synopsis: The investigators analyzed 312,896 UTI episodes among 157,264 unique patients (65 years of age or older) during the study period. Exclusion criteria included asymptomatic bacteriuria and complicated UTI. Of 271,070 patients who received antibiotics on the day of presentation with symptoms, 0.2% developed BSI within 60 days versus 2.2% of patients in whom antibiotics were delayed and 2.9% among patients not prescribed antibiotics. After adjustment for comorbidities, sex, and socioeconomic status, patients in whom antibiotics were deferred had a 7.12-fold greater odds of BSI, compared with the immediate-antibiotic group. BSIs were more common among men and older patients. All-cause mortality, a secondary outcome, was 1.16-fold higher with deferred antibiotics and 2.18 times higher with no antibiotics.
While the cohort studied was very large, a causal relationship cannot be firmly established in this observational study. Also, researchers were unable to include laboratory data, such as urinalysis and culture, in their analysis.
Bottom line: Delayed prescription of antibiotics for elderly patients presenting with UTI in primary care settings was associated with higher rates of BSI and death.
Citation: Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ. 2019 Feb;364:1525.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Background: If left untreated, UTIs may lead to severe complications. Although campaigns aimed at decreasing unnecessary prescriptions have reduced the number of antibiotic prescriptions for UTI, a concurrent rise in the rates of gram-negative bloodstream infections (BSIs) has also been observed.
Study design: Retrospective, population-based cohort study with data compiled from primary care records from 2007 to 2015 linked to hospital episode statistics and death records.
Setting: General practices in England.
Synopsis: The investigators analyzed 312,896 UTI episodes among 157,264 unique patients (65 years of age or older) during the study period. Exclusion criteria included asymptomatic bacteriuria and complicated UTI. Of 271,070 patients who received antibiotics on the day of presentation with symptoms, 0.2% developed BSI within 60 days versus 2.2% of patients in whom antibiotics were delayed and 2.9% among patients not prescribed antibiotics. After adjustment for comorbidities, sex, and socioeconomic status, patients in whom antibiotics were deferred had a 7.12-fold greater odds of BSI, compared with the immediate-antibiotic group. BSIs were more common among men and older patients. All-cause mortality, a secondary outcome, was 1.16-fold higher with deferred antibiotics and 2.18 times higher with no antibiotics.
While the cohort studied was very large, a causal relationship cannot be firmly established in this observational study. Also, researchers were unable to include laboratory data, such as urinalysis and culture, in their analysis.
Bottom line: Delayed prescription of antibiotics for elderly patients presenting with UTI in primary care settings was associated with higher rates of BSI and death.
Citation: Gharbi M et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ. 2019 Feb;364:1525.
Dr. Torres is a hospitalist at Massachusetts General Hospital.
Renal function data improve risk stratification in patients with PAH
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
FROM THE JOURNAL OF HEART AND LUNG TRANSPLANTATION
FDA approves hyaluronic acid filler for lip augmentation, perioral rhytids
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
, the manufacturer has announced.
Approval was supported by results of a phase 3 clinical trial in which a lower amount of Restylane Kysse was needed to see an improvement in lip fullness (1.82 mL) vs. a comparator (2.24 mL), according to the press release issued by Galderma. After 1 year, 78% of those who received the Restylane product were satisfied, and it was also shown to be safe and well tolerated, the release said.
In the statement, the company said that it is “working to determine the appropriate launch timing and availability” of this new product.
[email protected]
Moving beyond the hospital ward
SHM is entering an exciting new chapter in its history because we will soon see Dr. Eric Howell take the reins from Dr. Larry Wellikson as CEO, as we watch Dr. Danielle Scheurer assume the role of president from Dr. Chris Frost, and as a side note, I will try to fill Dr. Scheurer’s shoes as physician editor of The Hospitalist.
This changing of the guard of SHM’s leadership will take place amid the backdrop of an acrimonious presidential election and the emergence of a novel coronavirus that threatens to upend the typical routines of our social and professional lives.
Without a doubt, our leaders, whether national, regional, or local, will be at the helm during one of the most uncertain times in the history of modern health care. Will we see a U.S. President who is a proponent of supporting the Affordable Care Act? Will we see further erosion of Obamacare under a second term of President Trump? Will we see rural hospitals continue to close or shrink1 as their margins get squeezed by skyrocketing denials for inpatient status in favor of observation or outpatient status?2
Forces that seem beyond our control threaten to drastically alter our professions and even our livelihoods. In the space of the few weeks during which I began and finished this piece, every day brought a whole new world of changes in my hospital, town, state, and country. No leader can predict the future with any semblance of certitude.
In the face of these swirling winds of uncertainty, what is clear is that maintaining our commitment as hospitalists to providing evidence-based, high-quality care to our patients while providing support to our colleagues in the health care industry will greatly benefit from collaborating effectively under the “big tent” philosophy of SHM. Over my career, I have benefited from great role models and colleagues as my career took me from primary care med-peds to the “new” field of hospital medicine as a med-peds hospitalist, to a leadership role in pediatric hospital medicine. I have also benefited from “learning opportunities,” as I have made my fair share of mistakes in efforts to improve systems of care. Nearly all of these mistakes share a common thread – not collaborating effectively with critical stakeholders, both within and outside of my institution.3 As this pandemic progresses, I am (and likely you are) witnessing your leaders succeed or fail based on their ability to collaborate across the institution.
As a field, we risk making similar errors by being too narrowly focused as we strive to improve the care of our patients. Recently, Dr. Russell Buhr and his colleagues at the University of California, Los Angeles, demonstrated that a majority of 30-day readmissions for chronic obstructive pulmonary disease (COPD) are due to non-COPD diagnoses.4 As we discharge our COPD patients, we may be satisfied that we’ve “tuned up” our patient’s COPD, but have we adequately arranged for appropriate ongoing care of their other medical problems? This requires an activity undertaken less and less these days in medicine – a conversation between hospitalists and outpatient medical providers. The coronavirus disease 2019 (COVID-19) pandemic has made this more challenging, but I can assure you that you can neither transmit nor catch the coronavirus from a phone call.
Perhaps we can learn from our hospitalist colleagues trained in family medicine. A recent study found that hospitalists in a team made up of family medicine–trained physicians in an academic health center achieved a 33% shorter length of stay for patients from the family medicine clinic, after adjustment for disease, demographics, and disease severity.5 The conclusion of the authors was that this was likely caused by greater familiarity with outpatient resources. I would conjecture that family medicine hospitalists were also more likely to have a conversation with a patient’s outpatient primary care provider (PCP).
Of course, I am the first to admit that chatting with a PCP is not as easy as it used to be – when we could bump into each other in the doctor’s lounge drinking coffee or in radiology while pulling x-ray films (remember those?) – and in the age of COVID-19, these interactions are even less likely. It can take considerable time and effort to get PCP colleagues on the phone unless you’re chummy enough to have their cell phone numbers. And time is a resource in short supply because most hospital medicine groups are understaffed – in the 2018 SHM State of Hospital Medicine (SoHM) Report, 66.4% of responding groups had open positions, with a median of 12% understaffing reported. The 2020 SoHM report is being compiled as we speak, but I suspect this situation will not have improved, and as the pandemic strikes, staffing models have been completely blown up.
To dig ourselves out of this staffing hole and still stay under (or not too over) budget, bringing more advanced practice providers (APP) into our groups/divisions will be needed. We must recognize, however, that APPs can’t just be hired rapidly and thrown into the schedule. As Tracy Cardin, ACNP-BC, SFHM, stated in her December 2019 blog post on the Hospital Leader website, leaders need to implement consistent onboarding, training, and support of APPs, just as they would for any other hospitalist in their group.6 Physician hospitalists need to develop and maintain proven competency in effectively interacting with APPs practicing at the top of their skills and productivity. No time has ever proven the need to allow APPs to practice at the top of their skills than the age of COVID-19.7
But if your “field” doesn’t even recognize you at all? That is the fate of many providers left behind by the field of pediatric hospital medicine. Over the past year, we have seen PHM attain a great achievement in its recognition as a board-certified subspecialty established by the American Board of Pediatrics (ABP), only to have the process beset by allegations of gender and maternal bias. While a groundswell of opposition from pediatric hospitalists triggered by the exclusion of applicants to the Practice Pathway to board certification led the ABP to remove the practice interruption criteria, other potential sources of gender and maternal bias remain.8
This does not even address pediatric hospitalists trained in family medicine who cannot be eligible for PHM board certification through experience or fellowship, med-peds trained pediatric hospitalists who cannot quality because of insufficient time spent on pediatric inpatient care, newborn hospitalists (who do not qualify), and APPs specialized in pediatric inpatient care. While it is completely understandable that the ABP cannot provide a certification pathway for all of these groups, this still leaves a gap for these providers when it comes to being in a professional community that supports their professional development, ongoing education, and training. Fortunately, leaders of the three societies that have significant numbers of pediatric hospitalists – SHM, American Academy of Pediatrics, and Academic Pediatric Association – are working to develop a PHM designation outside of the ABP board certification pathway that will extend the professional community to those left out of board certification.
As we move bravely into this new era of SHM, our clarion call is to collaborate whenever and wherever we can, with our practice administrators, APPs, outpatient providers, subspecialist providers, and patient/family advocates – pandemic or no pandemic. In fact, what this pandemic has shown us is that rapid cycle, fully 360-degree collaboration is the only way hospitalists and hospital leaders will weather the storms of changing reimbursement, pandemics, or politics. This will be our challenge for the next decade, to ensure that SHM collaboratively moves beyond the confines of the hospital ward.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. Frakt A. A Sense of Alarm as Rural Hospitals Keep Closing. The New York Times. 2018. https://www.nytimes.com/2018/10/29/upshot/a-sense-of-alarm-as-rural-hospitals-keep-closing.html. Accessed February 28, 2020.
2. Poonacha TK, Chamoun F. The burden of prior authorizations and denials in health care. Medpage Today’s KevinMD. 2019. https://www.kevinmd.com/blog/2019/12/the-burden-of-prior-authorizations-and-denials-in-health-care.html. Accessed February 28, 2020.
3. 10 reasons healthcare leaders fail and how to prevent them. Becker’s Hospital Review. 2015. https://www.beckershospitalreview.com/hospital-management-administration/10-reasons-healthcare-leaders-fail-and-how-to-prevent-them.html. Accessed March 15, 2020
4. Buhr RG et al. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC Health Serv Res. 2019;19:701.
5. Garrison GM et al. Family medicine patients have shorter length of stay when cared for on a family medicine inpatient service. J Prim Care Community Health. 2019. doi: 10.1177/2150132719840517.
6. Cardin T. Work the Program for NP/PAs, and the Program Will Work. The Hospital Leader: Official Blog of SHM. 2019. https://thehospitalleader.org/work-the-program-for-np-pas-and-the-program-will-work/
7. Mittman DE. More physician assistants are ready to help with COVID-19 – now governors must empower them. The Hill. 2020. https://thehill.com/opinion/healthcare/489985-more-physician-assistants-are-ready-to-help-with-covid-19-now-governors. Accessed March 31, 2020.
8. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019;14:630-2.
SHM is entering an exciting new chapter in its history because we will soon see Dr. Eric Howell take the reins from Dr. Larry Wellikson as CEO, as we watch Dr. Danielle Scheurer assume the role of president from Dr. Chris Frost, and as a side note, I will try to fill Dr. Scheurer’s shoes as physician editor of The Hospitalist.
This changing of the guard of SHM’s leadership will take place amid the backdrop of an acrimonious presidential election and the emergence of a novel coronavirus that threatens to upend the typical routines of our social and professional lives.
Without a doubt, our leaders, whether national, regional, or local, will be at the helm during one of the most uncertain times in the history of modern health care. Will we see a U.S. President who is a proponent of supporting the Affordable Care Act? Will we see further erosion of Obamacare under a second term of President Trump? Will we see rural hospitals continue to close or shrink1 as their margins get squeezed by skyrocketing denials for inpatient status in favor of observation or outpatient status?2
Forces that seem beyond our control threaten to drastically alter our professions and even our livelihoods. In the space of the few weeks during which I began and finished this piece, every day brought a whole new world of changes in my hospital, town, state, and country. No leader can predict the future with any semblance of certitude.
In the face of these swirling winds of uncertainty, what is clear is that maintaining our commitment as hospitalists to providing evidence-based, high-quality care to our patients while providing support to our colleagues in the health care industry will greatly benefit from collaborating effectively under the “big tent” philosophy of SHM. Over my career, I have benefited from great role models and colleagues as my career took me from primary care med-peds to the “new” field of hospital medicine as a med-peds hospitalist, to a leadership role in pediatric hospital medicine. I have also benefited from “learning opportunities,” as I have made my fair share of mistakes in efforts to improve systems of care. Nearly all of these mistakes share a common thread – not collaborating effectively with critical stakeholders, both within and outside of my institution.3 As this pandemic progresses, I am (and likely you are) witnessing your leaders succeed or fail based on their ability to collaborate across the institution.
As a field, we risk making similar errors by being too narrowly focused as we strive to improve the care of our patients. Recently, Dr. Russell Buhr and his colleagues at the University of California, Los Angeles, demonstrated that a majority of 30-day readmissions for chronic obstructive pulmonary disease (COPD) are due to non-COPD diagnoses.4 As we discharge our COPD patients, we may be satisfied that we’ve “tuned up” our patient’s COPD, but have we adequately arranged for appropriate ongoing care of their other medical problems? This requires an activity undertaken less and less these days in medicine – a conversation between hospitalists and outpatient medical providers. The coronavirus disease 2019 (COVID-19) pandemic has made this more challenging, but I can assure you that you can neither transmit nor catch the coronavirus from a phone call.
Perhaps we can learn from our hospitalist colleagues trained in family medicine. A recent study found that hospitalists in a team made up of family medicine–trained physicians in an academic health center achieved a 33% shorter length of stay for patients from the family medicine clinic, after adjustment for disease, demographics, and disease severity.5 The conclusion of the authors was that this was likely caused by greater familiarity with outpatient resources. I would conjecture that family medicine hospitalists were also more likely to have a conversation with a patient’s outpatient primary care provider (PCP).
Of course, I am the first to admit that chatting with a PCP is not as easy as it used to be – when we could bump into each other in the doctor’s lounge drinking coffee or in radiology while pulling x-ray films (remember those?) – and in the age of COVID-19, these interactions are even less likely. It can take considerable time and effort to get PCP colleagues on the phone unless you’re chummy enough to have their cell phone numbers. And time is a resource in short supply because most hospital medicine groups are understaffed – in the 2018 SHM State of Hospital Medicine (SoHM) Report, 66.4% of responding groups had open positions, with a median of 12% understaffing reported. The 2020 SoHM report is being compiled as we speak, but I suspect this situation will not have improved, and as the pandemic strikes, staffing models have been completely blown up.
To dig ourselves out of this staffing hole and still stay under (or not too over) budget, bringing more advanced practice providers (APP) into our groups/divisions will be needed. We must recognize, however, that APPs can’t just be hired rapidly and thrown into the schedule. As Tracy Cardin, ACNP-BC, SFHM, stated in her December 2019 blog post on the Hospital Leader website, leaders need to implement consistent onboarding, training, and support of APPs, just as they would for any other hospitalist in their group.6 Physician hospitalists need to develop and maintain proven competency in effectively interacting with APPs practicing at the top of their skills and productivity. No time has ever proven the need to allow APPs to practice at the top of their skills than the age of COVID-19.7
But if your “field” doesn’t even recognize you at all? That is the fate of many providers left behind by the field of pediatric hospital medicine. Over the past year, we have seen PHM attain a great achievement in its recognition as a board-certified subspecialty established by the American Board of Pediatrics (ABP), only to have the process beset by allegations of gender and maternal bias. While a groundswell of opposition from pediatric hospitalists triggered by the exclusion of applicants to the Practice Pathway to board certification led the ABP to remove the practice interruption criteria, other potential sources of gender and maternal bias remain.8
This does not even address pediatric hospitalists trained in family medicine who cannot be eligible for PHM board certification through experience or fellowship, med-peds trained pediatric hospitalists who cannot quality because of insufficient time spent on pediatric inpatient care, newborn hospitalists (who do not qualify), and APPs specialized in pediatric inpatient care. While it is completely understandable that the ABP cannot provide a certification pathway for all of these groups, this still leaves a gap for these providers when it comes to being in a professional community that supports their professional development, ongoing education, and training. Fortunately, leaders of the three societies that have significant numbers of pediatric hospitalists – SHM, American Academy of Pediatrics, and Academic Pediatric Association – are working to develop a PHM designation outside of the ABP board certification pathway that will extend the professional community to those left out of board certification.
As we move bravely into this new era of SHM, our clarion call is to collaborate whenever and wherever we can, with our practice administrators, APPs, outpatient providers, subspecialist providers, and patient/family advocates – pandemic or no pandemic. In fact, what this pandemic has shown us is that rapid cycle, fully 360-degree collaboration is the only way hospitalists and hospital leaders will weather the storms of changing reimbursement, pandemics, or politics. This will be our challenge for the next decade, to ensure that SHM collaboratively moves beyond the confines of the hospital ward.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. Frakt A. A Sense of Alarm as Rural Hospitals Keep Closing. The New York Times. 2018. https://www.nytimes.com/2018/10/29/upshot/a-sense-of-alarm-as-rural-hospitals-keep-closing.html. Accessed February 28, 2020.
2. Poonacha TK, Chamoun F. The burden of prior authorizations and denials in health care. Medpage Today’s KevinMD. 2019. https://www.kevinmd.com/blog/2019/12/the-burden-of-prior-authorizations-and-denials-in-health-care.html. Accessed February 28, 2020.
3. 10 reasons healthcare leaders fail and how to prevent them. Becker’s Hospital Review. 2015. https://www.beckershospitalreview.com/hospital-management-administration/10-reasons-healthcare-leaders-fail-and-how-to-prevent-them.html. Accessed March 15, 2020
4. Buhr RG et al. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC Health Serv Res. 2019;19:701.
5. Garrison GM et al. Family medicine patients have shorter length of stay when cared for on a family medicine inpatient service. J Prim Care Community Health. 2019. doi: 10.1177/2150132719840517.
6. Cardin T. Work the Program for NP/PAs, and the Program Will Work. The Hospital Leader: Official Blog of SHM. 2019. https://thehospitalleader.org/work-the-program-for-np-pas-and-the-program-will-work/
7. Mittman DE. More physician assistants are ready to help with COVID-19 – now governors must empower them. The Hill. 2020. https://thehill.com/opinion/healthcare/489985-more-physician-assistants-are-ready-to-help-with-covid-19-now-governors. Accessed March 31, 2020.
8. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019;14:630-2.
SHM is entering an exciting new chapter in its history because we will soon see Dr. Eric Howell take the reins from Dr. Larry Wellikson as CEO, as we watch Dr. Danielle Scheurer assume the role of president from Dr. Chris Frost, and as a side note, I will try to fill Dr. Scheurer’s shoes as physician editor of The Hospitalist.
This changing of the guard of SHM’s leadership will take place amid the backdrop of an acrimonious presidential election and the emergence of a novel coronavirus that threatens to upend the typical routines of our social and professional lives.
Without a doubt, our leaders, whether national, regional, or local, will be at the helm during one of the most uncertain times in the history of modern health care. Will we see a U.S. President who is a proponent of supporting the Affordable Care Act? Will we see further erosion of Obamacare under a second term of President Trump? Will we see rural hospitals continue to close or shrink1 as their margins get squeezed by skyrocketing denials for inpatient status in favor of observation or outpatient status?2
Forces that seem beyond our control threaten to drastically alter our professions and even our livelihoods. In the space of the few weeks during which I began and finished this piece, every day brought a whole new world of changes in my hospital, town, state, and country. No leader can predict the future with any semblance of certitude.
In the face of these swirling winds of uncertainty, what is clear is that maintaining our commitment as hospitalists to providing evidence-based, high-quality care to our patients while providing support to our colleagues in the health care industry will greatly benefit from collaborating effectively under the “big tent” philosophy of SHM. Over my career, I have benefited from great role models and colleagues as my career took me from primary care med-peds to the “new” field of hospital medicine as a med-peds hospitalist, to a leadership role in pediatric hospital medicine. I have also benefited from “learning opportunities,” as I have made my fair share of mistakes in efforts to improve systems of care. Nearly all of these mistakes share a common thread – not collaborating effectively with critical stakeholders, both within and outside of my institution.3 As this pandemic progresses, I am (and likely you are) witnessing your leaders succeed or fail based on their ability to collaborate across the institution.
As a field, we risk making similar errors by being too narrowly focused as we strive to improve the care of our patients. Recently, Dr. Russell Buhr and his colleagues at the University of California, Los Angeles, demonstrated that a majority of 30-day readmissions for chronic obstructive pulmonary disease (COPD) are due to non-COPD diagnoses.4 As we discharge our COPD patients, we may be satisfied that we’ve “tuned up” our patient’s COPD, but have we adequately arranged for appropriate ongoing care of their other medical problems? This requires an activity undertaken less and less these days in medicine – a conversation between hospitalists and outpatient medical providers. The coronavirus disease 2019 (COVID-19) pandemic has made this more challenging, but I can assure you that you can neither transmit nor catch the coronavirus from a phone call.
Perhaps we can learn from our hospitalist colleagues trained in family medicine. A recent study found that hospitalists in a team made up of family medicine–trained physicians in an academic health center achieved a 33% shorter length of stay for patients from the family medicine clinic, after adjustment for disease, demographics, and disease severity.5 The conclusion of the authors was that this was likely caused by greater familiarity with outpatient resources. I would conjecture that family medicine hospitalists were also more likely to have a conversation with a patient’s outpatient primary care provider (PCP).
Of course, I am the first to admit that chatting with a PCP is not as easy as it used to be – when we could bump into each other in the doctor’s lounge drinking coffee or in radiology while pulling x-ray films (remember those?) – and in the age of COVID-19, these interactions are even less likely. It can take considerable time and effort to get PCP colleagues on the phone unless you’re chummy enough to have their cell phone numbers. And time is a resource in short supply because most hospital medicine groups are understaffed – in the 2018 SHM State of Hospital Medicine (SoHM) Report, 66.4% of responding groups had open positions, with a median of 12% understaffing reported. The 2020 SoHM report is being compiled as we speak, but I suspect this situation will not have improved, and as the pandemic strikes, staffing models have been completely blown up.
To dig ourselves out of this staffing hole and still stay under (or not too over) budget, bringing more advanced practice providers (APP) into our groups/divisions will be needed. We must recognize, however, that APPs can’t just be hired rapidly and thrown into the schedule. As Tracy Cardin, ACNP-BC, SFHM, stated in her December 2019 blog post on the Hospital Leader website, leaders need to implement consistent onboarding, training, and support of APPs, just as they would for any other hospitalist in their group.6 Physician hospitalists need to develop and maintain proven competency in effectively interacting with APPs practicing at the top of their skills and productivity. No time has ever proven the need to allow APPs to practice at the top of their skills than the age of COVID-19.7
But if your “field” doesn’t even recognize you at all? That is the fate of many providers left behind by the field of pediatric hospital medicine. Over the past year, we have seen PHM attain a great achievement in its recognition as a board-certified subspecialty established by the American Board of Pediatrics (ABP), only to have the process beset by allegations of gender and maternal bias. While a groundswell of opposition from pediatric hospitalists triggered by the exclusion of applicants to the Practice Pathway to board certification led the ABP to remove the practice interruption criteria, other potential sources of gender and maternal bias remain.8
This does not even address pediatric hospitalists trained in family medicine who cannot be eligible for PHM board certification through experience or fellowship, med-peds trained pediatric hospitalists who cannot quality because of insufficient time spent on pediatric inpatient care, newborn hospitalists (who do not qualify), and APPs specialized in pediatric inpatient care. While it is completely understandable that the ABP cannot provide a certification pathway for all of these groups, this still leaves a gap for these providers when it comes to being in a professional community that supports their professional development, ongoing education, and training. Fortunately, leaders of the three societies that have significant numbers of pediatric hospitalists – SHM, American Academy of Pediatrics, and Academic Pediatric Association – are working to develop a PHM designation outside of the ABP board certification pathway that will extend the professional community to those left out of board certification.
As we move bravely into this new era of SHM, our clarion call is to collaborate whenever and wherever we can, with our practice administrators, APPs, outpatient providers, subspecialist providers, and patient/family advocates – pandemic or no pandemic. In fact, what this pandemic has shown us is that rapid cycle, fully 360-degree collaboration is the only way hospitalists and hospital leaders will weather the storms of changing reimbursement, pandemics, or politics. This will be our challenge for the next decade, to ensure that SHM collaboratively moves beyond the confines of the hospital ward.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. Frakt A. A Sense of Alarm as Rural Hospitals Keep Closing. The New York Times. 2018. https://www.nytimes.com/2018/10/29/upshot/a-sense-of-alarm-as-rural-hospitals-keep-closing.html. Accessed February 28, 2020.
2. Poonacha TK, Chamoun F. The burden of prior authorizations and denials in health care. Medpage Today’s KevinMD. 2019. https://www.kevinmd.com/blog/2019/12/the-burden-of-prior-authorizations-and-denials-in-health-care.html. Accessed February 28, 2020.
3. 10 reasons healthcare leaders fail and how to prevent them. Becker’s Hospital Review. 2015. https://www.beckershospitalreview.com/hospital-management-administration/10-reasons-healthcare-leaders-fail-and-how-to-prevent-them.html. Accessed March 15, 2020
4. Buhr RG et al. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC Health Serv Res. 2019;19:701.
5. Garrison GM et al. Family medicine patients have shorter length of stay when cared for on a family medicine inpatient service. J Prim Care Community Health. 2019. doi: 10.1177/2150132719840517.
6. Cardin T. Work the Program for NP/PAs, and the Program Will Work. The Hospital Leader: Official Blog of SHM. 2019. https://thehospitalleader.org/work-the-program-for-np-pas-and-the-program-will-work/
7. Mittman DE. More physician assistants are ready to help with COVID-19 – now governors must empower them. The Hill. 2020. https://thehill.com/opinion/healthcare/489985-more-physician-assistants-are-ready-to-help-with-covid-19-now-governors. Accessed March 31, 2020.
8. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019;14:630-2.