User login
FDA approves new indications for pembrolizumab
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD, of Harvard Medical School, Boston.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders, Dr. Jones said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
However, postprocedure problems don’t always equal regrets, he said. “Although many women [in the 2014 study] reported negative thoughts and health issues after weight loss surgery, none of them said they regret undergoing the procedure,” he noted.
To further examine decision regret in patients who underwent gastric bypass and gastric banding, Dr. Jones participated in a study of patients’ attitudes 4 years after gastric bypass and gastric banding (Obes Surg. 2019;29:1624-31).
“Weight loss surgery is neither risk free nor universally effective, yet few studies have examined what proportion of patients regret having undergone weight loss surgery,” he noted.
Dr. Jones and colleagues interviewed patients at two weight loss surgery centers and used specific metrics and a multivariate analysis to examine associations among weight loss, quality of life, and decision regret.
A total of 205 Roux-en-Y gastric bypass (RYGB) patients responded at 1 year after surgery: 181, 156, and 134 patients responded at 2, 3, and 4 years, respectively.
At 1 year, 2% reported regret and that they would not choose the surgery again, and by 4 years, 5% reported regret, based on overall regret scores greater than 50. In addition, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects.
The researchers also interviewed gastric band patients: 170, 157, 146, and 123 responded at years 1,2,3, and 4.
Overall, 8% of these patients expressed regret at 1 year, and 20% expressed regret at 4 years, said Dr. Jones.
“Almost 20% did not think they made the right decision,” he said.
Dr. Jones noted. An average weight loss of 7.4% of excess body weight was associated with regret scores greater than 50, while an average weight loss of 21.1% was associated with regret scores less than 50, he said.
In addition, poor sexual function, but not weight loss or other quality-of-life factors was significantly associated with regret among RYGB patients.
Many surgeons are performing sleeve gastrectomies, which appear to yield greater weight loss than gastric banding and fewer complications than gastric bypass, said Dr. Jones. His study did not include sleeve gastrectomies, but “I expect a sleeve gastrectomy to do pretty well in this analysis,” and to be associated with less patient regret, he said.
Overall, better patient education is key to improving patients’ experiences and reducing feelings of regret, said Dr. Jones.
“The better patients understand the difference between band, bypass, and sleeve preoperatively, the better we can set expectations,” he said. Dr. Jones’ institution has developed an app for laparoscopic sleeve that guides patients through the process from preop through postoperative stay, he noted.
Given the association between amount of weight lost and regret, “setting expectations is very important,” and could include not only written consent but also webinars, information sessions, and apps for patients in advance to help mitigate regrets after the procedure, Dr. Jones concluded.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Jones disclosed serving on the medical advisory board for Allurion.
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD, of Harvard Medical School, Boston.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders, Dr. Jones said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
However, postprocedure problems don’t always equal regrets, he said. “Although many women [in the 2014 study] reported negative thoughts and health issues after weight loss surgery, none of them said they regret undergoing the procedure,” he noted.
To further examine decision regret in patients who underwent gastric bypass and gastric banding, Dr. Jones participated in a study of patients’ attitudes 4 years after gastric bypass and gastric banding (Obes Surg. 2019;29:1624-31).
“Weight loss surgery is neither risk free nor universally effective, yet few studies have examined what proportion of patients regret having undergone weight loss surgery,” he noted.
Dr. Jones and colleagues interviewed patients at two weight loss surgery centers and used specific metrics and a multivariate analysis to examine associations among weight loss, quality of life, and decision regret.
A total of 205 Roux-en-Y gastric bypass (RYGB) patients responded at 1 year after surgery: 181, 156, and 134 patients responded at 2, 3, and 4 years, respectively.
At 1 year, 2% reported regret and that they would not choose the surgery again, and by 4 years, 5% reported regret, based on overall regret scores greater than 50. In addition, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects.
The researchers also interviewed gastric band patients: 170, 157, 146, and 123 responded at years 1,2,3, and 4.
Overall, 8% of these patients expressed regret at 1 year, and 20% expressed regret at 4 years, said Dr. Jones.
“Almost 20% did not think they made the right decision,” he said.
Dr. Jones noted. An average weight loss of 7.4% of excess body weight was associated with regret scores greater than 50, while an average weight loss of 21.1% was associated with regret scores less than 50, he said.
In addition, poor sexual function, but not weight loss or other quality-of-life factors was significantly associated with regret among RYGB patients.
Many surgeons are performing sleeve gastrectomies, which appear to yield greater weight loss than gastric banding and fewer complications than gastric bypass, said Dr. Jones. His study did not include sleeve gastrectomies, but “I expect a sleeve gastrectomy to do pretty well in this analysis,” and to be associated with less patient regret, he said.
Overall, better patient education is key to improving patients’ experiences and reducing feelings of regret, said Dr. Jones.
“The better patients understand the difference between band, bypass, and sleeve preoperatively, the better we can set expectations,” he said. Dr. Jones’ institution has developed an app for laparoscopic sleeve that guides patients through the process from preop through postoperative stay, he noted.
Given the association between amount of weight lost and regret, “setting expectations is very important,” and could include not only written consent but also webinars, information sessions, and apps for patients in advance to help mitigate regrets after the procedure, Dr. Jones concluded.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Jones disclosed serving on the medical advisory board for Allurion.
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD, of Harvard Medical School, Boston.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders, Dr. Jones said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
However, postprocedure problems don’t always equal regrets, he said. “Although many women [in the 2014 study] reported negative thoughts and health issues after weight loss surgery, none of them said they regret undergoing the procedure,” he noted.
To further examine decision regret in patients who underwent gastric bypass and gastric banding, Dr. Jones participated in a study of patients’ attitudes 4 years after gastric bypass and gastric banding (Obes Surg. 2019;29:1624-31).
“Weight loss surgery is neither risk free nor universally effective, yet few studies have examined what proportion of patients regret having undergone weight loss surgery,” he noted.
Dr. Jones and colleagues interviewed patients at two weight loss surgery centers and used specific metrics and a multivariate analysis to examine associations among weight loss, quality of life, and decision regret.
A total of 205 Roux-en-Y gastric bypass (RYGB) patients responded at 1 year after surgery: 181, 156, and 134 patients responded at 2, 3, and 4 years, respectively.
At 1 year, 2% reported regret and that they would not choose the surgery again, and by 4 years, 5% reported regret, based on overall regret scores greater than 50. In addition, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects.
The researchers also interviewed gastric band patients: 170, 157, 146, and 123 responded at years 1,2,3, and 4.
Overall, 8% of these patients expressed regret at 1 year, and 20% expressed regret at 4 years, said Dr. Jones.
“Almost 20% did not think they made the right decision,” he said.
Dr. Jones noted. An average weight loss of 7.4% of excess body weight was associated with regret scores greater than 50, while an average weight loss of 21.1% was associated with regret scores less than 50, he said.
In addition, poor sexual function, but not weight loss or other quality-of-life factors was significantly associated with regret among RYGB patients.
Many surgeons are performing sleeve gastrectomies, which appear to yield greater weight loss than gastric banding and fewer complications than gastric bypass, said Dr. Jones. His study did not include sleeve gastrectomies, but “I expect a sleeve gastrectomy to do pretty well in this analysis,” and to be associated with less patient regret, he said.
Overall, better patient education is key to improving patients’ experiences and reducing feelings of regret, said Dr. Jones.
“The better patients understand the difference between band, bypass, and sleeve preoperatively, the better we can set expectations,” he said. Dr. Jones’ institution has developed an app for laparoscopic sleeve that guides patients through the process from preop through postoperative stay, he noted.
Given the association between amount of weight lost and regret, “setting expectations is very important,” and could include not only written consent but also webinars, information sessions, and apps for patients in advance to help mitigate regrets after the procedure, Dr. Jones concluded.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Jones disclosed serving on the medical advisory board for Allurion.
FROM MISS
Leadership & Professional Development: Engaging Patients as Stakeholders
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
“Nothing about us without us” (Latin: ”Nihil de nobis, sine nobis”)
Hospitalists are at the forefront of decisions, innovations, and system-improvement projects that impact hospitalized patients. However, many of our decisions—while centered on patient care—fail to include their perspectives or views.
In his book Total Leadership, Stewart Friedman describes the importance of identifying and engaging key stakeholders.1 Friedman exhorts leaders to engage stakeholders in conversations to “confirm or correct your current understanding of stakeholder expectations.” In other words, instead of assuming what stakeholders want, ask and verify before proceeding.
Although hospitalists frequently include stakeholders such as nurses, pharmacists, and therapists in system-improvement initiatives, engaging patients is less common.
Why do we omit patients as stakeholders? There are considerable barriers to seeking patient input. The busy hospital environment or the acuity of a patient’s illness may, for instance, limit engagement between hospital caregivers and patients. Further, the power imbalance between physicians and patients may make it uncomfortable for the patient to offer direct feedback.
However, the importance of patient input is increasingly recognized by researchers. For example, community-based participatory research “involves community members or recipients of interventions in all phases of the research process.”2 Similarly, we believe hospitalists should engage patients when designing new clinical initiatives.
Examples from some institutions provide further support of this concept. The Dana Farber Cancer Institute created a patient and family advisory council in response to the loss of trust over errors and in the face of community outrage over an impending joint venture. While the scope was initially limited to the collection of feedback regarding patient satisfaction and preferences, the council evolved to become an integral part of organizational decision making. Patient contributions were subsequently assimilated into policies, continuous improvement teams, and even search committees. Additional benefits included patient-generated initiatives such as “patient rounds.”3 Specifically soliciting input from hospitalized patients to inform hospital-based interventions may be uncommon, but this practice holds the potential to yield vital insights.4
We have experienced this benefit at our institution. For example, before implementing an inpatient addiction medicine consult service, we asked hospitalized patients struggling with addiction about their needs. The patient voice highlighted a lack of trust for hospital providers and led directly to the inclusion of peer-recovery mentors as part of the consulting team.5
Many organizations, including our own, have instituted a patient/family advisory committee comprising former patients and family members who participate voluntarily in projects and provide input. This resource can serve as an excellent platform for patient involvement. At the University of Michigan, the patient and family advisory council provides input on every major institutional decision, from the construction of a new building to the introduction of a new clinical service. This “hardwired” practice ensures that patients’ voices and views are incorporated into major health system decisions.
In order to engage patients as stakeholders, we recommend: (1) Be sensitive to the power imbalance between clinicians and patients and recognize that hospitalized patients may not feel comfortable providing direct feedback. (2) Familiarize yourself with your institution’s patient/family advisory committee. If one does not exist, consider soliciting responses from patients via interviews and/or postdischarge surveys. (3) Deliberately seek the opinions, experience, and values of patients or their representatives. (4) For projects aimed at improving patient experience, include patients among your key stakeholders.
Involving patients as stakeholders requires effort; however, it has potential to reap valuable rewards, making healthcare improvements more effective, inclusive, and healing.
Acknowledgments
The authors wish to thank Jeffrey S. Stewart for his contributions and feedback on this topic and manuscript.
Disclosures
The authors have nothing to disclose.
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
1. Friedman S. Total Leadership: Be a Better Leader, Have a Richer Life (With New Preface). Boston, Massachusetts: Harvard Business Review Press; 2014.
2. Minkler M. Community-based research partnerships: challenges and opportunities. J Urban Health. 2005;82(2 Suppl 2):ii3-12. https://doi.org/10.1093/jurban/jti034
3. Ponte PR, Conlin G, Conway JB, et al. Making patient-centered care come alive: achieving full integration of the patient’s perspective. J Nurs Adm. 2003;33(2):82-90. https://doi.org/10.1097/00005110-200302000-00004
4. O’Leary KJ, Chapman MM, Foster S, O’Hara L, Henschen BL, Cameron KA. Frequently hospitalized patients’ perceptions of factors contributing to high hospital use. J Hosp Med. 2019;14(9):521-526. https://doi.org/10.12788/jhm.3175
5. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. https://doi.org/10.1007/s11606-016-3919-4
© 2020 Society of Hospital Medicine
Psychiatric manifestations of sport-related concussion
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
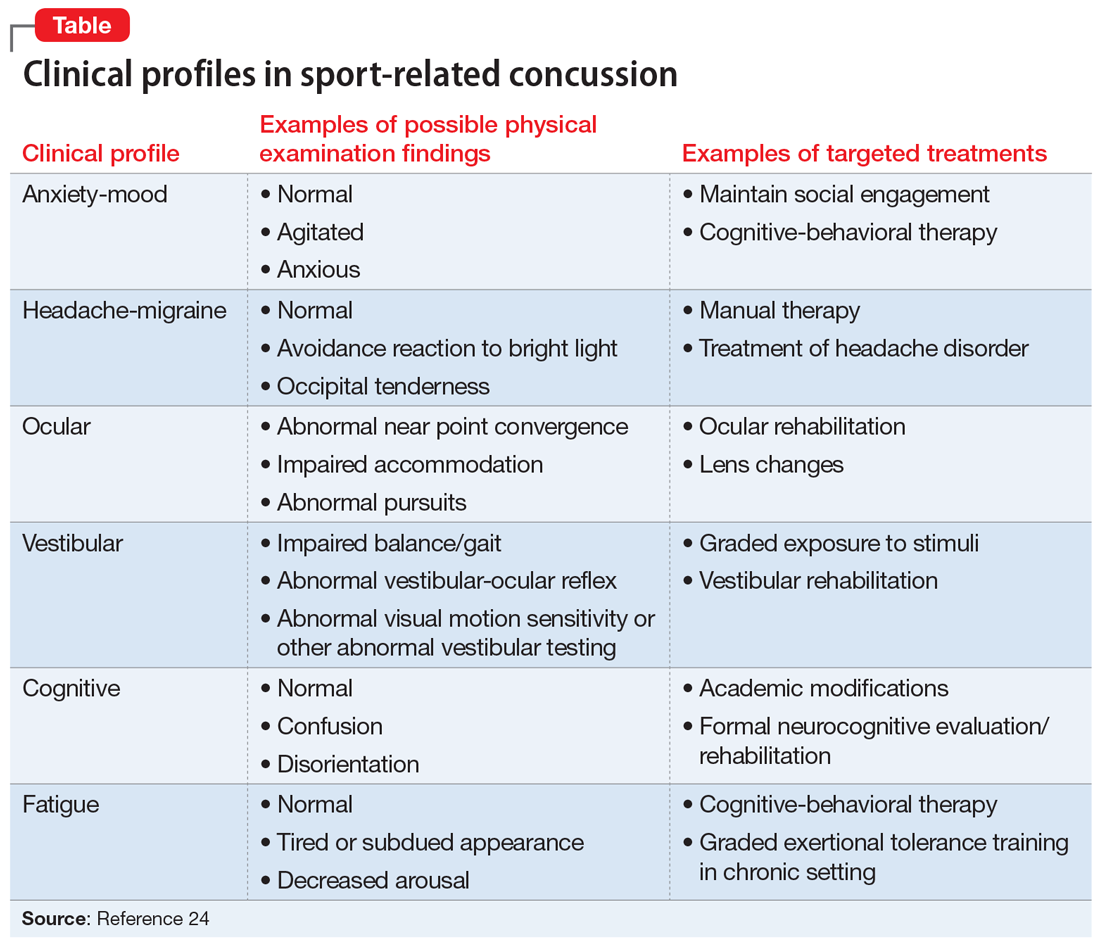
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.
Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
Ms. J, age 19, is a Division I collegiate volleyball player who recently sustained her third sport-related concussion (SRC). She has no psychiatric history but does have a history of migraine, and her headaches have worsened since the most recent SRC. She has a family history of depression (mother and her sole sibling). Ms. J recently experienced the loss of her coach, someone she greatly admired, in a motor vehicle accident. She is referred to outpatient psychiatry for assessment of mood symptoms that are persisting 1 month after the SRC. Upon assessment, she is found to meet 8 of the 9 criteria for a major depressive episode, including suicidality with vague plans but no intent to end her life.
Although Ms. J does not have a history of psychiatric illness, her psychiatrist recognizes that she has factors that increase her risk of developing depression post-SRC, and of poor recovery from SRC. These include pre-existing symptoms, such as her history of migraine, which is common in patients after SRC. Additionally, a family history of psychiatric disorders and high life stressors (eg, recent loss of her coach) are risk factors for a poor SRC recovery.1 Due to these risk factors and the severity of Ms. J’s symptoms—which include suicidal ideation—the psychiatrist believes that her depressive symptoms might be unlikely to improve in the coming weeks, so he establishes a diagnosis of “depressive disorder due to another medical condition (concussion)” because the development of her depressive symptoms coincided with the SRC. If Ms. J had a pre-existing mood disorder, or if her depression had not developed until later in the post-injury period, it would have been more difficult to establish confidently that the depressive episode was a direct physiologic consequence of the SRC; if that had been the case, the diagnosis probably would have been unspecified or other specified depressive disorder.2
SRC is a traumatic brain injury (TBI) induced by biomechanical forces, typically resulting in short-lived impairment of neurologic function, although signs and symptoms may evolve over minutes to hours.3 It largely reflects functional, rather than structural, brain disturbances.3 SRC has been deemed a “neuropsychiatric syndrome” because psychiatric manifestations are common.4 There may be a myriad of biopsychosocial factors involved in the etiology of psychiatric symptoms in an individual who sustains an SRC. For example, SRC may have a direct physiologic cause of psychiatric symptoms based on the location and degree of injury to the brain. Additionally, pre-existing psychiatric symptoms might increase the likelihood of sustaining an SRC. Finally, as with any major injury, illness, or event, stressors associated with SRC may cause psychiatric symptoms.
Regardless of causal factors, psychiatrists should be comfortable with managing psychiatric symptoms that commonly accompany this condition. This article highlights possible psychiatric manifestations of SRC and delineates high-yield management considerations. Although it focuses on concussions that occur in the context of sport, much of the information applies to patients who experience concussions from other causes.
SRC and depression
Changes in mood, emotion, and behavior are common following SRC. On the Sport Concussion Assessment Tool 5 (SCAT5),5 which is a standardized tool used to evaluate athletes suspected of having sustained a concussion, most symptoms overlap with those attributable to anxiety and depression.4,6 These include5:
- feeling slowed down
- “not feeling right”
- difficulty concentrating
- fatigue or loss of energy
- feeling more emotional
- irritability
- sadness
- feeling nervous or anxious
- difficulty falling asleep.
A recent systematic review of mental health outcomes of SRC in athletes found that the most commonly described and studied psychiatric symptoms following SRC were depression, anxiety, and impulsivity.7 The most rigorous study included in this review found depressive symptoms in 20% of collegiate athletes following SRC (all tested within 41 days of the SRC) vs 5% in the control group.8 These researchers delineated factors that predicted depressive symptoms after SRC (Box 18). Data were insufficient to draw conclusions about the association between SRC and other psychiatric symptoms, such as anxiety.8
Box 1
- Baseline depressive symptoms
- Baseline “post-concussion” symptoms
- Lower estimated premorbid intelligence
- Nonwhite ethnicity
- Increased number of games missed following injury
- Age of first participation in organized sport (more depression in athletes with fewer years of experience)
Source: Reference 8
Psychiatric manifestations of concussion in retired athletes may shed light on the long-term impact of SRC on psychiatric disorders, particularly depression. Hutchison et al9 conducted a systematic review of mental health outcomes of SRC in retired athletes.Two of the included studies that measured clinically diagnosed disorders found positive associations between self-reported concussion and clinically diagnosed depression.10,11 Hutchison et al9 found insufficient data to draw conclusions about depression and a lifetime history of subconcussive impacts—a topic that is receiving growing attention.
Continue to: Regarding a dose-response relationship...
Regarding a dose-response relationship in retired athletes, Guskiewicz et al11 reported a 3-fold increased risk of depression among retired professional football players who had experienced ≥3 SRCs. Five years later, the same research group reported a 5.8-fold increased risk of depression in retired professional football players after 5 to 9 concussions.10 In sum, there is evidence to suggest that the more SRCs an athlete sustains, the more likely they are to develop depression. Moreover, depression may persist or develop long after an SRC occurs.
Suicide risk
While suicide among athletes, especially football players, who have experienced concussion has received relatively widespread media attention, the risk of suicide in former professional football players appears to be significantly lower than in the general population.12 A recent large systematic review and meta-analysis reported on 713,706 individuals diagnosed with concussion and/or mild TBI and 6,236,010 individuals with no such diagnoses.13 It found a 2-fold higher risk of suicide in individuals who experienced concussion and/or mild TBI, but because participants were not necessarily athletes, it is difficult to extrapolate these findings to the athlete population.
Other psychiatric symptoms associated with SRC
Posttraumatic stress disorder (PTSD). Some athletes experience PTSD symptoms shortly after SRC, and these can be missed if clinicians do not specifically ask about them.14 For example, substantial proportions of athletes who have had an SRC report making efforts to avoid sport situations that are similar to how and where their SRC occurred (19%), having trouble keeping thoughts about sustaining the SRC out of their heads (18%), experiencing flashbacks of sustaining the SRC (13%), and having nightmares about sustaining the SRC (8%).14 Posttraumatic stress disorder may have a negative impact on an athlete’s performance because a fear of re-injury might lead them to avoid rehabilitation exercises and inhibit their effort.15-18
Attention-deficit/hyperactivity disorder (ADHD) is commonly comorbid with SRC.19,20 It is not known if pre-existing ADHD makes sustaining a concussion more likely (eg, because the athlete is distractible and thus does not notice when an opponent is about to hit them hard) and/or if a history of concussion makes ADHD more likely to develop (eg, because something about the concussed brain is changed in a way that leads to ADHD). Additionally, in some cases, ADHD has been associated with prolonged recovery from SRC.3,21
Immediate medical evaluation and cognitive assessment
Any patient in whom an SRC is suspected should undergo a medical evaluation immediately, whether in a physician’s office, emergency department, or on the sideline of a sports event. This medical evaluation should incorporate a clinical neurologic assessment, including evaluation of mental status/cognition, oculomotor function, gross sensorimotor, coordination, gait, vestibular function, and balance.3
Continue to: There is no single guideline...
There is no single guideline on how and when a neuropsychology referral is warranted.22 Insurance coverage for neurocognitive testing varies. Regardless of formal referral to neuropsychology, assessment of cognitive function is an important aspect of SRC management and is a factor in return-to-school and return-to-play decisions.3,22 Screening tools, such as the SCAT5, are useful in acute and subacute settings (ie, up to 3 to 5 days after injury); clinicians often use serial monitoring to track the resolution of symptoms.3 If pre-season baseline cognitive test results are available, clinicians may compare them to post-SRC results, but this should not be the sole basis of management decisions.3,22
Diagnosing psychiatric disorders in patients with SRC
Diagnosis of psychiatric symptoms and disorders associated with SRC can be challenging.7 There are no concussion-specific rating scales or diagnostic criteria for psychiatric disorders unique to patients who have sustained SRC. As a result, clinicians are left to use standard DSM-5 criteria for the diagnosis of psychiatric disorders in patients with SRC. Importantly, psychiatric symptoms must be distinguished from disorders. For example, Kontos et al23 reported significantly worse depressive symptoms following SRC, but not at the level to meet the criteria for major depressive disorder. This is an important distinction, because a psychiatrist might be less likely to initiate pharmacotherapy for a patient with SRC who has only a few depressive symptoms and is only 1 week post-SRC, vs for one who has had most symptoms of a major depressive episode for several weeks.
The American Medical Society for Sports Medicine has proposed 6 overlapping clinical profiles in patients with SRC (see the Table).24 Most patients with SRC have features of multiple clinical profiles.24 Anxiety/mood is one of these profiles. The impetus for developing these profiles was the recognition of heterogeneity among concussion presentations. Identification of the clinical profile(s) into which a patient’s symptoms fall might allow for more specific prognostication and targeted treatment.24 For example, referral to a psychiatrist obviously would be appropriate for a patient for whom anxiety/mood symptoms are prominent.

Treatment options for psychiatric sequelae of SRC
Both psychosocial and medical principles of management of psychiatric manifestations of SRC are important. Psychosocially, clinicians should address factors that may contribute to delayed SRC recovery (Box 225-30).
Box 2
- Recommend a progressive increase in exercise after a brief period of rest (often ameliorates psychiatric symptoms, as opposed to the historical approach of “cocoon therapy” in which the patient was to rest for prolonged periods of time in a darkened room so as to minimize brain stimulation)25
- Allow social activities, including team meetings (restriction of such activities has been associated with increased post-SRC depression)26
- Encourage members of the athlete’s “entourage” (team physicians, athletic trainers, coaches, teammates, and parents) to provide support27
- Educate coaches and teammates about how to make supportive statements because they often have trouble knowing how to do so27
- Recommend psychotherapy for mental and other physical symptoms of SRC that are moderate to severe or that persist longer than 4 weeks after the SRC28
- Recommend minimization of use of alcohol and other substances29,30
SRC: sport-related concussion
No medications are FDA-approved for SRC or associated psychiatric symptoms, and there is minimal evidence to support the use of specific medications.31 Most athletes with SRC recover quickly—typically within 2 weeks—and do not need medication.4,32 When medications are needed, start with low dosing and titrate slowly.33,34
Continue to: For patients with SRC who experience insomnia...
For patients with SRC who experience insomnia, clinicians should focus on sleep hygiene and, if needed, cognitive-behavioral therapy for insomnia (CBT-I).31 If medication is needed, melatonin may be a first-line agent.31,35,36 Trazodone may be a second option.32 Benzodiazepines typically are avoided because of their negative impact on cognition.31
For patients with SRC who have depression, selective serotonin reuptake inhibitors (SSRIs) may simultaneously improve depressed mood31 and cognition.37 Tricyclic antidepressants (TCAs) are sometimes used to treat headaches, depression, anxiety, and/or insomnia after SRC,32 but adverse effects such as sedation and weight gain may limit their use in athletes. Theoretically, serotonin-norepinephrine reuptake inhibitors might have some of the same benefits as TCAs with fewer adverse effects, but they have not been well studied in patients with SRC.
For patients with SRC who have cognitive dysfunction (eg, deficits in attention and processing speed), there is some evidence for treatment with stimulants.31,37 However, these medications are prohibited by many athletic governing organizations, including professional sports leagues, the National Collegiate Athletic Association (NCAA), and the World Anti-Doping Agency.4 If an athlete was receiving stimulants for ADHD before sustaining an SRC, there is no evidence that these medications should be stopped.
Consider interdisciplinary collaboration
Throughout the course of management, psychiatrists should consider if and when it is necessary to consult with other specialties such as primary care, sports medicine, neurology, and neuropsychology. As with many psychiatric symptoms and disorders, collaboration with an interdisciplinary team is recommended. Primary care, sports medicine, or neurology should be involved in the management of patients with SRC. Choice of which of those 3 specialties in particular will depend on comfort level and experience with managing SRC of the individual providers in question as well as availability of each provider type in a given community.
Additionally, psychiatrists may wonder if and when they should refer patients with SRC for neuroimaging. Because SRC is a functional, rather than structural, brain disturbance, neuroimaging is not typically pursued because results would be expected to be normal.3 However, when in doubt, consultation with the interdisciplinary team can guide this decision. Factors that may lead to a decision to obtain neuroimaging include:
- an abnormal neurologic examination
- prolonged loss of consciousness
- unexpected persistence of symptoms (eg, 6 to 12 weeks)
- worsening symptoms.22
Continue to: If imaging is deemed necessary...
If imaging is deemed necessary for a patient with an acute SRC, brain CT is typically the imaging modality of choice; however, if imaging is deemed necessary due to the persistence of symptoms, then MRI is often the preferred test because it provides more detailed information and does not expose the patient to ionizing radiation.22 While results are often normal, the ordering clinician should be prepared for the possibility of incidental findings, such as cysts or aneurysms, and the need for further consultation with other clinicians to weigh in on such findings.22
CASE CONTINUED
Ms. J is prescribed extended-release venlafaxine, 37.5 mg every morning for 5 days, and then is switched to 75 mg every morning. The psychiatrist hopes that venlafaxine might simultaneously offer benefit for Ms. J’s depression and migraine headaches. Venlafaxine is not FDA-approved for migraine, and there is more evidence supporting TCAs for preventing migraine. However, Ms. J is adamant that she does not want to take a medication, such as a TCA, that could cause weight gain or sedation, which could be problematic in her sport. The psychiatrist also tells Ms. J to avoid substances of abuse, and emphasizes the importance of good sleep hygiene. Finally, the psychiatrist communicates with the interdisciplinary medical team, which is helping Ms. J with gradual return-to-school and return-to-sport strategies and ensuring continued social involvement with the team even as she is held out from sport.
Ultimately, Ms. J’s extended-release venlafaxine is titrated to 150 mg every morning. After 2 months on this dose, her depressive symptoms remit. After her other symptoms remit, Ms. J has difficulty returning to certain practice drills that remind her of what she was doing when she sustained the SRC. She says that while participating in these drills, she has intrusive thoughts and images of the experience of her most recent concussion. She works with her psychiatrist on a gradual program of exposure therapy so she can return to all types of practice. Ms. J says she wishes to continue playing volleyball; however, together with her parents and treatment team, she decides that any additional SRCs might lead her to retire from the sport.
Bottom Line
Psychiatric symptoms are common after sport-related concussion (SRC). The nature of the relationship between concussion and mental health is not firmly established. Post-SRC psychiatric symptoms need to be carefully managed to avoid unnecessary treatment or restrictions.
Related Resources
- National Collegiate Athletic Association. Concussion. www.ncaa.org/sport-science-institute/concussion.
- American Academy of Neurology. Sports concussion resources. www.aan.com/tools-and-resources/practicing-neurologists-administrators/patient-resources/sports-concussion-resources. Published 2020.
Drug Brand Names
Trazodone • Desyrel
Venlafaxine • Effexor
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
1. Morgan CD, Zuckerman SL, Lee YM, et al. Predictors of postconcussion syndrome after sports-related concussion in young athletes: a matched case-control study. J Neurosurg Pediatr. 2015;15(6):589-598.
2. Jorge RE, Arciniegas DB. Mood disorders after TBI. Psychiatr Clin North Am. 2014;37(1):13-29.
3. McCrory P, Meeuwisse W, Dvor˘ák J, et al. Consensus statement on concussion in sport—the 5th International Conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847.
4. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667-699.
5. Echemendia RJ, Meeuwisse W, McCrory P, et al. The sport concussion assessment tool 5th edition (SCAT5): background and rationale. Br J Sports Med. 2017;51:848-850.
6. Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65(7):601.
7. Rice SM, Parker AG, Rosenbaum S, et al. Sport-related concussion outcomes in elite athletes: a systematic review. Sports Med. 2018;48(2):447-465.
8. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-255.
9. Hutchison MG, Di Battista AP, McCoskey J, et al. Systematic review of mental health measures associated with concussive and subconcussive head trauma in former athletes. Int J Psychophysiol. 2018;132(Pt A):55-61.
10. Kerr GA, Stirling AE. Parents’ reflections on their child’s experiences of emotionally abusive coaching practices. J Appl Sport Psychol. 2012;24(2):191-206.
11. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909.
12. Lehman EJ, Hein MJ, Gersic CM. Suicide mortality among retired National Football League players who played 5 or more seasons. Am J Sports Med. 2016;44(10):2486-2491.
13. Fralick M, Sy E, Hassan A, et al. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2018;76(2):144-151.
14. Brassil HE, Salvatore AP. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin Transl Med. 2018;7:25.
15. Bateman A, Morgan KAD. The postinjury psychological sequelae of high-level Jamaican athletes: exploration of a posttraumatic stress disorder-self-efficacy conceptualization. J Sport Rehabil. 2019;28(2):144-152.
16. Brewer BW, Van Raalte JL, Cornelius AE, et al. Psychological factors, rehabilitation adherence, and rehabilitation outcome after anterior cruciate ligament reconstruction. Rehabil Psychol. 2000;45(1):20-37.
17. Putukian M, Echemendia RJ. Psychological aspects of serious head injury in the competitive athlete. Clin Sports Med. 2003;22(33):617-630.
18. James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among Veterans with and without PTSD and mild TBI. Mil Med. 2014;179(4):357-363.
19. Harmon KG, Drezner J, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;47(1):15-26.
20. Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med. 2016;26(2):120-127.
21. Esfandiari A, Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2011;30(3):611-627.
22. Mahooti N. Sport-related concussion: acute management and chronic postconcussive issues. Chld Adolesc Psychiatric Clin N Am. 2018;27(1):93-108.
23. Kontos AP, Covassin T, Elbin RJ, et al. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch Phys Med Rehabil. 2012;93(10):1751-1756.
24. Harmon KG, Clugston JR, Dec K, et al. American Medical Society for Sports Medicine position statement on concussion in sport. Clin J Sport Med. 2019;29(2):87-100.
25. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current Sports Medicine Reports. 2013;12(6):370-376.
26. Schneider KJ, Iverson GL, Emery CA, et al. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304-307.
27. Wayment HA, Huffman AH. Psychosocial experiences of concussed collegiate athletes: the role of emotional support in the recovery process. J Am Coll Health. 2020;68(4):438-443.
28. Todd R, Bhalerao S, Vu MT, et al. Understanding the psychiatric effects of concussion on constructed identity in hockey players: implications for health professionals. PLoS ONE. 2018;13(2):e0192125.
29. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140.
30. Gaetz M. The multi-factorial origins of chronic traumatic encephalopathy (CTE) symptomatology in post-career athletes: the athlete post-career adjustment (AP-CA) model. Med Hypotheses. 2017;102:130-143.
31. Meehan WP. Medical therapies for concussion. Clin Sports Med. 2011;30(1):115-124.
32. Broglio SP, Collins MW, Williams RM, et al. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213-231.
33. Arciniegas DB, Silver JM, McAllister TW. Stimulants and acetylcholinesterase inhibitors for the treatment of cognitive impairment after traumatic brain injury. Psychopharm Review. 2008;43(12):91-97.
34. Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
35. Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity/mortality after craniocerebral trauma. J Pineal Res. 2007;42(1):1-11.
36. Samantaray S, Das A, Thakore NP, et al. Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res. 2009;47(2):134-142.
37. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
COVID-19’s effects on emergency psychiatry
Coronavirus disease 2019 (COVID-19) is affecting every aspect of medical care. Much has been written about overwhelmed hospital settings, the financial devastation to outpatient treatment centers, and an impending pandemic of mental illness that the existing underfunded and fragmented mental health system would not be prepared to weather. Although COVID-19 has undeniably affected the practice of emergency psychiatry, its impact has been surprising and complex. In this article, I describe the effects COVID-19 has had on our psychiatric emergency service, and how the pandemic has affected me personally.
How the pandemic affected our psychiatric ED
The Comprehensive Psychiatric Emergency Program (CPEP) in Buffalo, New York, is part of the emergency department (ED) in the local county hospital and is staffed by faculty from the Department of Psychiatry at the University at Buffalo. It was developed to provide evaluations of acutely psychiatrically ill individuals, to determine their treatment needs and facilitate access to the appropriate level of care.
Before COVID-19, as the only fully staffed psychiatric emergency service in the region, CPEP would routinely be called upon to serve many functions for which it was not designed. For example, people who had difficulty accessing psychiatric care in the community might come to CPEP expecting treatment for chronic conditions. Additionally, due to systemic deficiencies and limited resources, police and other community agencies refer individuals to CPEP who either have illnesses unrelated to current circumstances or who are not psychiatrically ill but unmanageable because of aggression or otherwise unresolvable social challenges such as homelessness, criminal behavior, poor parenting and other family strains, or general dissatisfaction with life. Parents unable to set limits with bored or defiant children might leave them in CPEP, hoping to transfer the parenting role, just as law enforcement officers who feel impotent to apply meaningful sanctions to non-felonious offenders might bring them to CPEP seeking containment. Labeling these problems as psychiatric emergencies has made it more palatable to leave these individuals in our care. These types of visits have contributed to the substantial growth of CPEP in recent years, in terms of annual patient visits, number of children abandoned and their lengths of stay in the CPEP, among other metrics.
The impact of the COVID-19 pandemic on an emergency psychiatry service that is expected to be all things to all people has been interesting. For the first few weeks of the societal shutdown, the patient flow was unchanged. However, during this time, the usual overcrowding created a feeling of vulnerability to contagion that sparked an urgency to minimize the census. Superhuman efforts were fueled by an unspoken sense of impending doom, and wait times dropped from approximately 17 hours to 3 or 4 hours. This state of hypervigilance was impossible to sustain indefinitely, and inevitably those efforts were exhausted. As adrenaline waned, the focus turned toward family and self-preservation. Nursing and social work staff began cancelling shifts, as did part-time physicians who contracted services with our department. Others, however, were drawn to join the front-line fight.
Trends in psychiatric ED usage during the pandemic
As COVID-19 spread, local media reported the paucity of personal protective equipment (PPE) and created the sense that no one would receive hospital treatment unless they were on the brink of death. Consequently, total visits to the ED began to slow. During April, CPEP saw 25% fewer visits than average. This reduction was partly attributable to cohorting patients with any suspicion of infection in a designated area within the medical ED, with access to remote evaluation by CPEP psychiatrists via telemedicine. In addition, the characteristics and circumstances of patients presenting to CPEP began to change (Table).
Children/adolescents. In the months before COVID-19’s spread to the United States, there had been an exponential surge in child visits to CPEP, with >200 such visits in January 2020. When schools closed on March 13, school-related stress abruptly abated, and during April, child visits dropped to 89. This reduction might have been due in part to increased access to outpatient treatment via telemedicine or telephone appointments. In our affiliated clinics, both new patient visits and remote attendance to appointments by established patients increased substantially, likely contributing to a decreased reliance on the CPEP for treatment. Limited Family Court operations, though, left already-frustrated police without much recourse when called to intervene with adolescent offenders. CPEP once again served an untraditional role, facilitating the removal of these disruptive individuals from potentially dangerous circumstances, under the guise of behavioral emergencies.
Suicidality. While nonemergent visits declined, presentations related to suicidality persisted. In the United States, suicide rates have increased annually for decades. This trend has also been observed locally, with early evidence suggesting that the changes inflicted by COVID-19 perpetuated the surge in suicidal thinking and behavior, but with a change in character. Some of this is likely related to financial stress and social disruption, though job loss seems more likely to result in increased substance use than suicidality. Even more distressing to those coming to CPEP was anxiety about the illness itself, social isolation, and loss. The death of a loved one is painful enough, but disrupting the grief process by preventing people from visiting family members dying in hospitals or gathering for funerals has been devastating. Reports of increased gun sales undoubtedly associated with fears of social decay caused by the pandemic are concerning with regard to patients with suicidality, because shooting has emerged as the means most likely to result in completed suicide.1 The imposition of social distancing directly isolated some individuals, increasing suicidality. Limitations on gathering in groups disrupted other sources of social support as well, such as religious services, clubhouses, and meetings of 12-step programs such as Alcoholics Anonymous. This could increase suicidality, either directly for more vulnerable patients or indirectly by compromising sobriety and thereby adding to the risk for suicide.
Continue to: Substance use disorders (SUDs)
Substance use disorders (SUDs). Presentations to CPEP by patients with SUDs surged, but the patient profile changed, undoubtedly influenced by the pandemic. Requests for detoxification became less frequent because people who were not in severe distress avoided the hospital. At the same time, alcohol-dependent individuals who might typically avoid clinical attention were requiring emergent medical attention for delirium. This is attributable to a combination of factors, including nutritional depletion, and a lack of access to alcohol leading to abrupt withdrawal or consumption of unconventional sources of alcohol, such as hand sanitizer, or hard liquor (over beer). Amphetamine use appears to have increased, although the observed surge may simply be related to the conspicuousness of stimulant intoxication for someone who is sheltering in place. There was a noticeable uptick in overdoses (primarily with opioids) requiring CPEP evaluation, which was possibly related to a reduction of available beds in inpatient rehabilitation facilities as a result of social distancing rules.
Patients with chronic mental illness. Many experts anticipated an increase in hospital visits by individuals with chronic mental illness expected to decompensate as a result of reduced access to community treatment resources.2 Closing courts did not prevent remote sessions for inpatient retention and treatment over objection, but did result in the expiration of many Assisted Outpatient Treatment orders by restricting renewal hearings, which is circuitously beginning to fulfill this prediction. On the other hand, an impressive community response has managed to continue meeting the needs of most of these patients. Dedicated mental health clinics have recruited mobile teams or developed carefully scheduled, nursing-run “shot clinics” to ensure that patients who require long-acting injectable medications or medication-assisted treatment for SUDs continue to receive treatment.
New-onset psychosis. A new population of patients with acute mania and psychosis also seems to have surfaced during this pandemic. Previously high-functioning individuals in their 30s, 40s, and 50s without a history of mental illness were presenting with new-onset psychotic symptoms. These are individuals who may have been characteristically anxious, or had a “Type A personality,” but were social and employed. The cause is unclear, but given the extreme uncertainty and the political climate COVID-19 brings, it is possible that the pandemic may have triggered these episodes. These individuals and their families now have the stress of learning to navigate the mental health system added to the anxiety COVID-19 brings to most households.
Homelessness. Limitations on occupancy have reduced the availability of beds in shelters and residences, resulting in increased homelessness. Locally, authorities estimated that the homeless population has grown nearly threefold as a result of bussing in from neighboring counties with fewer resources, flight from New York City, and the urgent release from jail of nonviolent offenders, many of whom had no place to go for shelter. New emergency shelter beds have not fully compensated for the relative shortage, leading individuals who had been avoiding the hospital due to fear of infection to CPEP looking for a place to stay.
Home stressors. Whereas CPEP visits by children initially decreased, after 6 weeks, the relief from school pressures appears to have been replaced by weariness from stresses at home, and the number of children presenting with depression, SUDs, and behavioral disruptions has increased. Domestic violence involving children and adults increased. Factors that might be contributing to this include the forced proximity of family members who would typically need intermittent interpersonal distance, and an obligation to care for children who would normally be in school or for disabled loved ones now unable to attend day programs or respite services. After months of enduring the pressure of these conflicts and the resulting emotional strain, patient volumes in CPEP have begun slowly returning toward the expected average, particularly since the perceived threat of coming to the hospital has attenuated.
Continue to: Personal challenges
Personal challenges
For me, COVID-19 has brought the chance to grow and learn, fumbling at times to provide the best care when crisis abounds and when not much can be said to ease the appropriate emotional distress our patients experience. The lines between what is pathological anxiety, what level of anxiety causes functional impairment, and what can realistically be expected to respond to psychiatric treatment have become blurred. At the same time, I have come across some of the sickest patients I have ever encountered.
In some ways, my passion for psychiatry has been rekindled by COVID-19, sparking an enthusiasm to teach and inspire students to pursue careers in this wonderful field of medicine. Helping to care for patients in the absence of a cure can necessitate the application of creativity and thoughtfulness to relieve suffering, thereby teaching the art of healing above offering treatment alone. Unfortunately, replacing actual patient contact with remote learning deprives students of this unique educational opportunity. Residents who attempt to continue training while limiting exposure to patients may mitigate their own risk but could also be missing an opportunity to learn how to balance their needs with making their patients’ well-being a priority. This raises the question of how the next generation of medical students and residents will learn to navigate future crises. Gruesome media depictions of haunting experiences witnessed by medical professionals exposed to an enormity of loss and death, magnified by the suicide deaths of 2 front-line workers in New York City, undoubtedly contribute to the instinct driving the protection of students and residents in this way.
The gratitude the public expresses toward me for simply continuing to do my job brings an expectation of heroism I did not seek, and with which I am uncomfortable. For me, exceptionally poised to analyze and over-analyze myriad aspects of an internal conflict that is exhausting to balance, it all generates frustration and guilt more than anything.
I am theoretically at lower risk than intubating anesthesiologists, emergency medicine physicians, and emergency medical technicians who face patients with active COVID-19. Nevertheless, daily proximity to so many patients naturally generates fear. I convince myself that performing video consultations to the medical ED is an adaptation necessary to preserve PPE, to keep me healthy through reduced exposure, to be available to patients longer, and to support the emotional health of the medical staff who are handing over that headset to patients “under investigation.” At the same time, I am secretly relieved to avoid entering those rooms and taunting death, or even worse, risking exposing my family to the virus. The threat of COVID-19 can be so consuming that it becomes easy to forget that most individuals infected are asymptomatic and therefore difficult to quickly identify.
So I continue to sit with patients face-to-face all day. Many of them are not capable of following masking and distancing recommendations, and are more prone to spitting and biting than their counterparts in the medical ED. I must ignore this threat and convince myself I am safe to be able to place my responsibility to patient care above my own needs and do my job.
Continue to: Most of my colleagues exhibit...
Most of my colleagues exhibit an effortless bravery, even if we all naturally waver briefly at times. I am proud to stand shoulder-to-shoulder every day with these clinicians, and other staff, from police to custodians, as we continue to care for the people of this community. Despite the lower clinical burden, each day we expend significant emotional energy struggling with unexpected and unique challenges, including the burden of facing the unknown. Everyone is under stress right now. For most, the effects will be transient. For some, the damage might be permanent. For others, this stress has brought out the best in us. But knowing that physicians are particularly prone to burnout, how long can the current state of hypervigilance be maintained?
What will the future hold?
The COVID-19 era has brought fewer patients through the door of my psychiatric ED; however, just like everywhere else in the world, everything has changed. The only thing that is certain is that further change is inevitable, and we must adapt to the challenge and learn from it. As unsettling as disruptions to the status quo can be, human behavior dictates that we have the option to seize opportunities created by instability to produce superior outcomes, which can be accomplished only by looking at things anew. The question is whether we will revert to the pre-COVID-19 dysfunctional use of psychiatric emergency services, or can we use what we have learned—particularly about the value of telepsychiatry—to pursue a more effective system based on an improved understanding of the mental health treatment needs of our community. While technology is proving that social distancing requires only space between people, and not necessarily social separation, there is a risk that excessive use of remote treatment could compromise the therapeutic relationship with our patients. Despite emerging opportunities, it is difficult to direct change in a productive way when the future is uncertain.
The continuous outpouring of respect for clinicians is morale-boosting. Behind closed doors, however, news that this county hospital failed to qualify for any of the second round of federal support funding because the management of COVID-19 patients has been too effective brought a new layer of unanticipated stress. This is the only hospital in 7 counties operating a psychiatric emergency service. The mandatory, “voluntary” furloughs expected of nursing and social work staff are only now being scheduled to occur over the next couple of months. And just in time for patient volumes to return to normal. How can we continue to provide quality care, let alone build changes into practice, with reduced nursing and support staff?
It is promising, however, that in the midst of social distancing, the shared experience of endeavoring to overcome COVID-19 has promoted a connectedness among individuals who might otherwise never cross paths. This observation has bolstered my confidence in the capacity for resilience of the mental health system and the individuals within it. The reality is that we are all in this together. Differences should matter less in the face of altered perceptions of mortality. Despite the stress, suicide becomes a less reasonable choice when the value of life is magnified by pandemic circumstances. Maybe there will be even less of a need for psychiatric emergency services in the wake of COVID-19, rather than the anticipated wave of mental health crises. Until we know for sure, it is only through fellowship and continued dedication to healing that the ED experience will continue to be a positive one.
Bottom Line
Coronavirus disease 2019 (COVID-19) led to changes in the characteristics and circumstances of patients presenting to our psychiatric emergency service. Despite a lower clinical burden, each day we expended significant emotional energy struggling with unexpected and unique challenges. We can use what we have learned from COVID-19 to pursue a more effective system based on an improved understanding of the mental health treatment needs of our community.
Related Resource
- American Association for Emergency Psychiatry, American College of Emergency Physicians, American Psychiatric Association, Coalition on Psychiatric Emergencies, Crisis Residential Association, and the Emergency Nurses Association. Joint statement for care of patients with behavioral health emergencies and suspected or confirmed COVID-19. https://aaep.memberclicks.net/assets/joint-statement-covid-behavioral-health.pdf.
1. Wang J, Sumner SA, Simon TR, et al. Trends in the incidence and lethality of suicidal acts in the United States, 2006-2015 [published online April 22, 2020]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.0596.
2. Reger MA, Stanley IH, Joiner TE. Suicide mortality and coronavirus disease 2019--a perfect storm? [published online April 10, 2020]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.1060.
Coronavirus disease 2019 (COVID-19) is affecting every aspect of medical care. Much has been written about overwhelmed hospital settings, the financial devastation to outpatient treatment centers, and an impending pandemic of mental illness that the existing underfunded and fragmented mental health system would not be prepared to weather. Although COVID-19 has undeniably affected the practice of emergency psychiatry, its impact has been surprising and complex. In this article, I describe the effects COVID-19 has had on our psychiatric emergency service, and how the pandemic has affected me personally.
How the pandemic affected our psychiatric ED
The Comprehensive Psychiatric Emergency Program (CPEP) in Buffalo, New York, is part of the emergency department (ED) in the local county hospital and is staffed by faculty from the Department of Psychiatry at the University at Buffalo. It was developed to provide evaluations of acutely psychiatrically ill individuals, to determine their treatment needs and facilitate access to the appropriate level of care.
Before COVID-19, as the only fully staffed psychiatric emergency service in the region, CPEP would routinely be called upon to serve many functions for which it was not designed. For example, people who had difficulty accessing psychiatric care in the community might come to CPEP expecting treatment for chronic conditions. Additionally, due to systemic deficiencies and limited resources, police and other community agencies refer individuals to CPEP who either have illnesses unrelated to current circumstances or who are not psychiatrically ill but unmanageable because of aggression or otherwise unresolvable social challenges such as homelessness, criminal behavior, poor parenting and other family strains, or general dissatisfaction with life. Parents unable to set limits with bored or defiant children might leave them in CPEP, hoping to transfer the parenting role, just as law enforcement officers who feel impotent to apply meaningful sanctions to non-felonious offenders might bring them to CPEP seeking containment. Labeling these problems as psychiatric emergencies has made it more palatable to leave these individuals in our care. These types of visits have contributed to the substantial growth of CPEP in recent years, in terms of annual patient visits, number of children abandoned and their lengths of stay in the CPEP, among other metrics.
The impact of the COVID-19 pandemic on an emergency psychiatry service that is expected to be all things to all people has been interesting. For the first few weeks of the societal shutdown, the patient flow was unchanged. However, during this time, the usual overcrowding created a feeling of vulnerability to contagion that sparked an urgency to minimize the census. Superhuman efforts were fueled by an unspoken sense of impending doom, and wait times dropped from approximately 17 hours to 3 or 4 hours. This state of hypervigilance was impossible to sustain indefinitely, and inevitably those efforts were exhausted. As adrenaline waned, the focus turned toward family and self-preservation. Nursing and social work staff began cancelling shifts, as did part-time physicians who contracted services with our department. Others, however, were drawn to join the front-line fight.
Trends in psychiatric ED usage during the pandemic
As COVID-19 spread, local media reported the paucity of personal protective equipment (PPE) and created the sense that no one would receive hospital treatment unless they were on the brink of death. Consequently, total visits to the ED began to slow. During April, CPEP saw 25% fewer visits than average. This reduction was partly attributable to cohorting patients with any suspicion of infection in a designated area within the medical ED, with access to remote evaluation by CPEP psychiatrists via telemedicine. In addition, the characteristics and circumstances of patients presenting to CPEP began to change (Table).

Children/adolescents. In the months before COVID-19’s spread to the United States, there had been an exponential surge in child visits to CPEP, with >200 such visits in January 2020. When schools closed on March 13, school-related stress abruptly abated, and during April, child visits dropped to 89. This reduction might have been due in part to increased access to outpatient treatment via telemedicine or telephone appointments. In our affiliated clinics, both new patient visits and remote attendance to appointments by established patients increased substantially, likely contributing to a decreased reliance on the CPEP for treatment. Limited Family Court operations, though, left already-frustrated police without much recourse when called to intervene with adolescent offenders. CPEP once again served an untraditional role, facilitating the removal of these disruptive individuals from potentially dangerous circumstances, under the guise of behavioral emergencies.
Suicidality. While nonemergent visits declined, presentations related to suicidality persisted. In the United States, suicide rates have increased annually for decades. This trend has also been observed locally, with early evidence suggesting that the changes inflicted by COVID-19 perpetuated the surge in suicidal thinking and behavior, but with a change in character. Some of this is likely related to financial stress and social disruption, though job loss seems more likely to result in increased substance use than suicidality. Even more distressing to those coming to CPEP was anxiety about the illness itself, social isolation, and loss. The death of a loved one is painful enough, but disrupting the grief process by preventing people from visiting family members dying in hospitals or gathering for funerals has been devastating. Reports of increased gun sales undoubtedly associated with fears of social decay caused by the pandemic are concerning with regard to patients with suicidality, because shooting has emerged as the means most likely to result in completed suicide.1 The imposition of social distancing directly isolated some individuals, increasing suicidality. Limitations on gathering in groups disrupted other sources of social support as well, such as religious services, clubhouses, and meetings of 12-step programs such as Alcoholics Anonymous. This could increase suicidality, either directly for more vulnerable patients or indirectly by compromising sobriety and thereby adding to the risk for suicide.
Continue to: Substance use disorders (SUDs)
Substance use disorders (SUDs). Presentations to CPEP by patients with SUDs surged, but the patient profile changed, undoubtedly influenced by the pandemic. Requests for detoxification became less frequent because people who were not in severe distress avoided the hospital. At the same time, alcohol-dependent individuals who might typically avoid clinical attention were requiring emergent medical attention for delirium. This is attributable to a combination of factors, including nutritional depletion, and a lack of access to alcohol leading to abrupt withdrawal or consumption of unconventional sources of alcohol, such as hand sanitizer, or hard liquor (over beer). Amphetamine use appears to have increased, although the observed surge may simply be related to the conspicuousness of stimulant intoxication for someone who is sheltering in place. There was a noticeable uptick in overdoses (primarily with opioids) requiring CPEP evaluation, which was possibly related to a reduction of available beds in inpatient rehabilitation facilities as a result of social distancing rules.
Patients with chronic mental illness. Many experts anticipated an increase in hospital visits by individuals with chronic mental illness expected to decompensate as a result of reduced access to community treatment resources.2 Closing courts did not prevent remote sessions for inpatient retention and treatment over objection, but did result in the expiration of many Assisted Outpatient Treatment orders by restricting renewal hearings, which is circuitously beginning to fulfill this prediction. On the other hand, an impressive community response has managed to continue meeting the needs of most of these patients. Dedicated mental health clinics have recruited mobile teams or developed carefully scheduled, nursing-run “shot clinics” to ensure that patients who require long-acting injectable medications or medication-assisted treatment for SUDs continue to receive treatment.
New-onset psychosis. A new population of patients with acute mania and psychosis also seems to have surfaced during this pandemic. Previously high-functioning individuals in their 30s, 40s, and 50s without a history of mental illness were presenting with new-onset psychotic symptoms. These are individuals who may have been characteristically anxious, or had a “Type A personality,” but were social and employed. The cause is unclear, but given the extreme uncertainty and the political climate COVID-19 brings, it is possible that the pandemic may have triggered these episodes. These individuals and their families now have the stress of learning to navigate the mental health system added to the anxiety COVID-19 brings to most households.
Homelessness. Limitations on occupancy have reduced the availability of beds in shelters and residences, resulting in increased homelessness. Locally, authorities estimated that the homeless population has grown nearly threefold as a result of bussing in from neighboring counties with fewer resources, flight from New York City, and the urgent release from jail of nonviolent offenders, many of whom had no place to go for shelter. New emergency shelter beds have not fully compensated for the relative shortage, leading individuals who had been avoiding the hospital due to fear of infection to CPEP looking for a place to stay.
Home stressors. Whereas CPEP visits by children initially decreased, after 6 weeks, the relief from school pressures appears to have been replaced by weariness from stresses at home, and the number of children presenting with depression, SUDs, and behavioral disruptions has increased. Domestic violence involving children and adults increased. Factors that might be contributing to this include the forced proximity of family members who would typically need intermittent interpersonal distance, and an obligation to care for children who would normally be in school or for disabled loved ones now unable to attend day programs or respite services. After months of enduring the pressure of these conflicts and the resulting emotional strain, patient volumes in CPEP have begun slowly returning toward the expected average, particularly since the perceived threat of coming to the hospital has attenuated.
Continue to: Personal challenges
Personal challenges
For me, COVID-19 has brought the chance to grow and learn, fumbling at times to provide the best care when crisis abounds and when not much can be said to ease the appropriate emotional distress our patients experience. The lines between what is pathological anxiety, what level of anxiety causes functional impairment, and what can realistically be expected to respond to psychiatric treatment have become blurred. At the same time, I have come across some of the sickest patients I have ever encountered.
In some ways, my passion for psychiatry has been rekindled by COVID-19, sparking an enthusiasm to teach and inspire students to pursue careers in this wonderful field of medicine. Helping to care for patients in the absence of a cure can necessitate the application of creativity and thoughtfulness to relieve suffering, thereby teaching the art of healing above offering treatment alone. Unfortunately, replacing actual patient contact with remote learning deprives students of this unique educational opportunity. Residents who attempt to continue training while limiting exposure to patients may mitigate their own risk but could also be missing an opportunity to learn how to balance their needs with making their patients’ well-being a priority. This raises the question of how the next generation of medical students and residents will learn to navigate future crises. Gruesome media depictions of haunting experiences witnessed by medical professionals exposed to an enormity of loss and death, magnified by the suicide deaths of 2 front-line workers in New York City, undoubtedly contribute to the instinct driving the protection of students and residents in this way.
The gratitude the public expresses toward me for simply continuing to do my job brings an expectation of heroism I did not seek, and with which I am uncomfortable. For me, exceptionally poised to analyze and over-analyze myriad aspects of an internal conflict that is exhausting to balance, it all generates frustration and guilt more than anything.
I am theoretically at lower risk than intubating anesthesiologists, emergency medicine physicians, and emergency medical technicians who face patients with active COVID-19. Nevertheless, daily proximity to so many patients naturally generates fear. I convince myself that performing video consultations to the medical ED is an adaptation necessary to preserve PPE, to keep me healthy through reduced exposure, to be available to patients longer, and to support the emotional health of the medical staff who are handing over that headset to patients “under investigation.” At the same time, I am secretly relieved to avoid entering those rooms and taunting death, or even worse, risking exposing my family to the virus. The threat of COVID-19 can be so consuming that it becomes easy to forget that most individuals infected are asymptomatic and therefore difficult to quickly identify.
So I continue to sit with patients face-to-face all day. Many of them are not capable of following masking and distancing recommendations, and are more prone to spitting and biting than their counterparts in the medical ED. I must ignore this threat and convince myself I am safe to be able to place my responsibility to patient care above my own needs and do my job.
Continue to: Most of my colleagues exhibit...
Most of my colleagues exhibit an effortless bravery, even if we all naturally waver briefly at times. I am proud to stand shoulder-to-shoulder every day with these clinicians, and other staff, from police to custodians, as we continue to care for the people of this community. Despite the lower clinical burden, each day we expend significant emotional energy struggling with unexpected and unique challenges, including the burden of facing the unknown. Everyone is under stress right now. For most, the effects will be transient. For some, the damage might be permanent. For others, this stress has brought out the best in us. But knowing that physicians are particularly prone to burnout, how long can the current state of hypervigilance be maintained?
What will the future hold?
The COVID-19 era has brought fewer patients through the door of my psychiatric ED; however, just like everywhere else in the world, everything has changed. The only thing that is certain is that further change is inevitable, and we must adapt to the challenge and learn from it. As unsettling as disruptions to the status quo can be, human behavior dictates that we have the option to seize opportunities created by instability to produce superior outcomes, which can be accomplished only by looking at things anew. The question is whether we will revert to the pre-COVID-19 dysfunctional use of psychiatric emergency services, or can we use what we have learned—particularly about the value of telepsychiatry—to pursue a more effective system based on an improved understanding of the mental health treatment needs of our community. While technology is proving that social distancing requires only space between people, and not necessarily social separation, there is a risk that excessive use of remote treatment could compromise the therapeutic relationship with our patients. Despite emerging opportunities, it is difficult to direct change in a productive way when the future is uncertain.
The continuous outpouring of respect for clinicians is morale-boosting. Behind closed doors, however, news that this county hospital failed to qualify for any of the second round of federal support funding because the management of COVID-19 patients has been too effective brought a new layer of unanticipated stress. This is the only hospital in 7 counties operating a psychiatric emergency service. The mandatory, “voluntary” furloughs expected of nursing and social work staff are only now being scheduled to occur over the next couple of months. And just in time for patient volumes to return to normal. How can we continue to provide quality care, let alone build changes into practice, with reduced nursing and support staff?
It is promising, however, that in the midst of social distancing, the shared experience of endeavoring to overcome COVID-19 has promoted a connectedness among individuals who might otherwise never cross paths. This observation has bolstered my confidence in the capacity for resilience of the mental health system and the individuals within it. The reality is that we are all in this together. Differences should matter less in the face of altered perceptions of mortality. Despite the stress, suicide becomes a less reasonable choice when the value of life is magnified by pandemic circumstances. Maybe there will be even less of a need for psychiatric emergency services in the wake of COVID-19, rather than the anticipated wave of mental health crises. Until we know for sure, it is only through fellowship and continued dedication to healing that the ED experience will continue to be a positive one.
Bottom Line
Coronavirus disease 2019 (COVID-19) led to changes in the characteristics and circumstances of patients presenting to our psychiatric emergency service. Despite a lower clinical burden, each day we expended significant emotional energy struggling with unexpected and unique challenges. We can use what we have learned from COVID-19 to pursue a more effective system based on an improved understanding of the mental health treatment needs of our community.
Related Resource
- American Association for Emergency Psychiatry, American College of Emergency Physicians, American Psychiatric Association, Coalition on Psychiatric Emergencies, Crisis Residential Association, and the Emergency Nurses Association. Joint statement for care of patients with behavioral health emergencies and suspected or confirmed COVID-19. https://aaep.memberclicks.net/assets/joint-statement-covid-behavioral-health.pdf.
Coronavirus disease 2019 (COVID-19) is affecting every aspect of medical care. Much has been written about overwhelmed hospital settings, the financial devastation to outpatient treatment centers, and an impending pandemic of mental illness that the existing underfunded and fragmented mental health system would not be prepared to weather. Although COVID-19 has undeniably affected the practice of emergency psychiatry, its impact has been surprising and complex. In this article, I describe the effects COVID-19 has had on our psychiatric emergency service, and how the pandemic has affected me personally.
How the pandemic affected our psychiatric ED
The Comprehensive Psychiatric Emergency Program (CPEP) in Buffalo, New York, is part of the emergency department (ED) in the local county hospital and is staffed by faculty from the Department of Psychiatry at the University at Buffalo. It was developed to provide evaluations of acutely psychiatrically ill individuals, to determine their treatment needs and facilitate access to the appropriate level of care.
Before COVID-19, as the only fully staffed psychiatric emergency service in the region, CPEP would routinely be called upon to serve many functions for which it was not designed. For example, people who had difficulty accessing psychiatric care in the community might come to CPEP expecting treatment for chronic conditions. Additionally, due to systemic deficiencies and limited resources, police and other community agencies refer individuals to CPEP who either have illnesses unrelated to current circumstances or who are not psychiatrically ill but unmanageable because of aggression or otherwise unresolvable social challenges such as homelessness, criminal behavior, poor parenting and other family strains, or general dissatisfaction with life. Parents unable to set limits with bored or defiant children might leave them in CPEP, hoping to transfer the parenting role, just as law enforcement officers who feel impotent to apply meaningful sanctions to non-felonious offenders might bring them to CPEP seeking containment. Labeling these problems as psychiatric emergencies has made it more palatable to leave these individuals in our care. These types of visits have contributed to the substantial growth of CPEP in recent years, in terms of annual patient visits, number of children abandoned and their lengths of stay in the CPEP, among other metrics.
The impact of the COVID-19 pandemic on an emergency psychiatry service that is expected to be all things to all people has been interesting. For the first few weeks of the societal shutdown, the patient flow was unchanged. However, during this time, the usual overcrowding created a feeling of vulnerability to contagion that sparked an urgency to minimize the census. Superhuman efforts were fueled by an unspoken sense of impending doom, and wait times dropped from approximately 17 hours to 3 or 4 hours. This state of hypervigilance was impossible to sustain indefinitely, and inevitably those efforts were exhausted. As adrenaline waned, the focus turned toward family and self-preservation. Nursing and social work staff began cancelling shifts, as did part-time physicians who contracted services with our department. Others, however, were drawn to join the front-line fight.
Trends in psychiatric ED usage during the pandemic
As COVID-19 spread, local media reported the paucity of personal protective equipment (PPE) and created the sense that no one would receive hospital treatment unless they were on the brink of death. Consequently, total visits to the ED began to slow. During April, CPEP saw 25% fewer visits than average. This reduction was partly attributable to cohorting patients with any suspicion of infection in a designated area within the medical ED, with access to remote evaluation by CPEP psychiatrists via telemedicine. In addition, the characteristics and circumstances of patients presenting to CPEP began to change (Table).

Children/adolescents. In the months before COVID-19’s spread to the United States, there had been an exponential surge in child visits to CPEP, with >200 such visits in January 2020. When schools closed on March 13, school-related stress abruptly abated, and during April, child visits dropped to 89. This reduction might have been due in part to increased access to outpatient treatment via telemedicine or telephone appointments. In our affiliated clinics, both new patient visits and remote attendance to appointments by established patients increased substantially, likely contributing to a decreased reliance on the CPEP for treatment. Limited Family Court operations, though, left already-frustrated police without much recourse when called to intervene with adolescent offenders. CPEP once again served an untraditional role, facilitating the removal of these disruptive individuals from potentially dangerous circumstances, under the guise of behavioral emergencies.
Suicidality. While nonemergent visits declined, presentations related to suicidality persisted. In the United States, suicide rates have increased annually for decades. This trend has also been observed locally, with early evidence suggesting that the changes inflicted by COVID-19 perpetuated the surge in suicidal thinking and behavior, but with a change in character. Some of this is likely related to financial stress and social disruption, though job loss seems more likely to result in increased substance use than suicidality. Even more distressing to those coming to CPEP was anxiety about the illness itself, social isolation, and loss. The death of a loved one is painful enough, but disrupting the grief process by preventing people from visiting family members dying in hospitals or gathering for funerals has been devastating. Reports of increased gun sales undoubtedly associated with fears of social decay caused by the pandemic are concerning with regard to patients with suicidality, because shooting has emerged as the means most likely to result in completed suicide.1 The imposition of social distancing directly isolated some individuals, increasing suicidality. Limitations on gathering in groups disrupted other sources of social support as well, such as religious services, clubhouses, and meetings of 12-step programs such as Alcoholics Anonymous. This could increase suicidality, either directly for more vulnerable patients or indirectly by compromising sobriety and thereby adding to the risk for suicide.
Continue to: Substance use disorders (SUDs)
Substance use disorders (SUDs). Presentations to CPEP by patients with SUDs surged, but the patient profile changed, undoubtedly influenced by the pandemic. Requests for detoxification became less frequent because people who were not in severe distress avoided the hospital. At the same time, alcohol-dependent individuals who might typically avoid clinical attention were requiring emergent medical attention for delirium. This is attributable to a combination of factors, including nutritional depletion, and a lack of access to alcohol leading to abrupt withdrawal or consumption of unconventional sources of alcohol, such as hand sanitizer, or hard liquor (over beer). Amphetamine use appears to have increased, although the observed surge may simply be related to the conspicuousness of stimulant intoxication for someone who is sheltering in place. There was a noticeable uptick in overdoses (primarily with opioids) requiring CPEP evaluation, which was possibly related to a reduction of available beds in inpatient rehabilitation facilities as a result of social distancing rules.
Patients with chronic mental illness. Many experts anticipated an increase in hospital visits by individuals with chronic mental illness expected to decompensate as a result of reduced access to community treatment resources.2 Closing courts did not prevent remote sessions for inpatient retention and treatment over objection, but did result in the expiration of many Assisted Outpatient Treatment orders by restricting renewal hearings, which is circuitously beginning to fulfill this prediction. On the other hand, an impressive community response has managed to continue meeting the needs of most of these patients. Dedicated mental health clinics have recruited mobile teams or developed carefully scheduled, nursing-run “shot clinics” to ensure that patients who require long-acting injectable medications or medication-assisted treatment for SUDs continue to receive treatment.
New-onset psychosis. A new population of patients with acute mania and psychosis also seems to have surfaced during this pandemic. Previously high-functioning individuals in their 30s, 40s, and 50s without a history of mental illness were presenting with new-onset psychotic symptoms. These are individuals who may have been characteristically anxious, or had a “Type A personality,” but were social and employed. The cause is unclear, but given the extreme uncertainty and the political climate COVID-19 brings, it is possible that the pandemic may have triggered these episodes. These individuals and their families now have the stress of learning to navigate the mental health system added to the anxiety COVID-19 brings to most households.
Homelessness. Limitations on occupancy have reduced the availability of beds in shelters and residences, resulting in increased homelessness. Locally, authorities estimated that the homeless population has grown nearly threefold as a result of bussing in from neighboring counties with fewer resources, flight from New York City, and the urgent release from jail of nonviolent offenders, many of whom had no place to go for shelter. New emergency shelter beds have not fully compensated for the relative shortage, leading individuals who had been avoiding the hospital due to fear of infection to CPEP looking for a place to stay.
Home stressors. Whereas CPEP visits by children initially decreased, after 6 weeks, the relief from school pressures appears to have been replaced by weariness from stresses at home, and the number of children presenting with depression, SUDs, and behavioral disruptions has increased. Domestic violence involving children and adults increased. Factors that might be contributing to this include the forced proximity of family members who would typically need intermittent interpersonal distance, and an obligation to care for children who would normally be in school or for disabled loved ones now unable to attend day programs or respite services. After months of enduring the pressure of these conflicts and the resulting emotional strain, patient volumes in CPEP have begun slowly returning toward the expected average, particularly since the perceived threat of coming to the hospital has attenuated.
Continue to: Personal challenges
Personal challenges
For me, COVID-19 has brought the chance to grow and learn, fumbling at times to provide the best care when crisis abounds and when not much can be said to ease the appropriate emotional distress our patients experience. The lines between what is pathological anxiety, what level of anxiety causes functional impairment, and what can realistically be expected to respond to psychiatric treatment have become blurred. At the same time, I have come across some of the sickest patients I have ever encountered.
In some ways, my passion for psychiatry has been rekindled by COVID-19, sparking an enthusiasm to teach and inspire students to pursue careers in this wonderful field of medicine. Helping to care for patients in the absence of a cure can necessitate the application of creativity and thoughtfulness to relieve suffering, thereby teaching the art of healing above offering treatment alone. Unfortunately, replacing actual patient contact with remote learning deprives students of this unique educational opportunity. Residents who attempt to continue training while limiting exposure to patients may mitigate their own risk but could also be missing an opportunity to learn how to balance their needs with making their patients’ well-being a priority. This raises the question of how the next generation of medical students and residents will learn to navigate future crises. Gruesome media depictions of haunting experiences witnessed by medical professionals exposed to an enormity of loss and death, magnified by the suicide deaths of 2 front-line workers in New York City, undoubtedly contribute to the instinct driving the protection of students and residents in this way.
The gratitude the public expresses toward me for simply continuing to do my job brings an expectation of heroism I did not seek, and with which I am uncomfortable. For me, exceptionally poised to analyze and over-analyze myriad aspects of an internal conflict that is exhausting to balance, it all generates frustration and guilt more than anything.
I am theoretically at lower risk than intubating anesthesiologists, emergency medicine physicians, and emergency medical technicians who face patients with active COVID-19. Nevertheless, daily proximity to so many patients naturally generates fear. I convince myself that performing video consultations to the medical ED is an adaptation necessary to preserve PPE, to keep me healthy through reduced exposure, to be available to patients longer, and to support the emotional health of the medical staff who are handing over that headset to patients “under investigation.” At the same time, I am secretly relieved to avoid entering those rooms and taunting death, or even worse, risking exposing my family to the virus. The threat of COVID-19 can be so consuming that it becomes easy to forget that most individuals infected are asymptomatic and therefore difficult to quickly identify.
So I continue to sit with patients face-to-face all day. Many of them are not capable of following masking and distancing recommendations, and are more prone to spitting and biting than their counterparts in the medical ED. I must ignore this threat and convince myself I am safe to be able to place my responsibility to patient care above my own needs and do my job.
Continue to: Most of my colleagues exhibit...
Most of my colleagues exhibit an effortless bravery, even if we all naturally waver briefly at times. I am proud to stand shoulder-to-shoulder every day with these clinicians, and other staff, from police to custodians, as we continue to care for the people of this community. Despite the lower clinical burden, each day we expend significant emotional energy struggling with unexpected and unique challenges, including the burden of facing the unknown. Everyone is under stress right now. For most, the effects will be transient. For some, the damage might be permanent. For others, this stress has brought out the best in us. But knowing that physicians are particularly prone to burnout, how long can the current state of hypervigilance be maintained?
What will the future hold?
The COVID-19 era has brought fewer patients through the door of my psychiatric ED; however, just like everywhere else in the world, everything has changed. The only thing that is certain is that further change is inevitable, and we must adapt to the challenge and learn from it. As unsettling as disruptions to the status quo can be, human behavior dictates that we have the option to seize opportunities created by instability to produce superior outcomes, which can be accomplished only by looking at things anew. The question is whether we will revert to the pre-COVID-19 dysfunctional use of psychiatric emergency services, or can we use what we have learned—particularly about the value of telepsychiatry—to pursue a more effective system based on an improved understanding of the mental health treatment needs of our community. While technology is proving that social distancing requires only space between people, and not necessarily social separation, there is a risk that excessive use of remote treatment could compromise the therapeutic relationship with our patients. Despite emerging opportunities, it is difficult to direct change in a productive way when the future is uncertain.
The continuous outpouring of respect for clinicians is morale-boosting. Behind closed doors, however, news that this county hospital failed to qualify for any of the second round of federal support funding because the management of COVID-19 patients has been too effective brought a new layer of unanticipated stress. This is the only hospital in 7 counties operating a psychiatric emergency service. The mandatory, “voluntary” furloughs expected of nursing and social work staff are only now being scheduled to occur over the next couple of months. And just in time for patient volumes to return to normal. How can we continue to provide quality care, let alone build changes into practice, with reduced nursing and support staff?
It is promising, however, that in the midst of social distancing, the shared experience of endeavoring to overcome COVID-19 has promoted a connectedness among individuals who might otherwise never cross paths. This observation has bolstered my confidence in the capacity for resilience of the mental health system and the individuals within it. The reality is that we are all in this together. Differences should matter less in the face of altered perceptions of mortality. Despite the stress, suicide becomes a less reasonable choice when the value of life is magnified by pandemic circumstances. Maybe there will be even less of a need for psychiatric emergency services in the wake of COVID-19, rather than the anticipated wave of mental health crises. Until we know for sure, it is only through fellowship and continued dedication to healing that the ED experience will continue to be a positive one.
Bottom Line
Coronavirus disease 2019 (COVID-19) led to changes in the characteristics and circumstances of patients presenting to our psychiatric emergency service. Despite a lower clinical burden, each day we expended significant emotional energy struggling with unexpected and unique challenges. We can use what we have learned from COVID-19 to pursue a more effective system based on an improved understanding of the mental health treatment needs of our community.
Related Resource
- American Association for Emergency Psychiatry, American College of Emergency Physicians, American Psychiatric Association, Coalition on Psychiatric Emergencies, Crisis Residential Association, and the Emergency Nurses Association. Joint statement for care of patients with behavioral health emergencies and suspected or confirmed COVID-19. https://aaep.memberclicks.net/assets/joint-statement-covid-behavioral-health.pdf.
1. Wang J, Sumner SA, Simon TR, et al. Trends in the incidence and lethality of suicidal acts in the United States, 2006-2015 [published online April 22, 2020]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.0596.
2. Reger MA, Stanley IH, Joiner TE. Suicide mortality and coronavirus disease 2019--a perfect storm? [published online April 10, 2020]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.1060.
1. Wang J, Sumner SA, Simon TR, et al. Trends in the incidence and lethality of suicidal acts in the United States, 2006-2015 [published online April 22, 2020]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.0596.
2. Reger MA, Stanley IH, Joiner TE. Suicide mortality and coronavirus disease 2019--a perfect storm? [published online April 10, 2020]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.1060.
The hidden dangers of supplements: A case of substance-induced psychosis
“You are what you eat,” my mother always said, and structured our dinner plates according to the USDA food pyramid. We dutifully consumed leafy greens, and prior to medical school I invested time and money into healthy diet choices. I drank green smoothies, pureed baby food for my children, read up on the pH balancing diet, grew sprouts on windowsills, bought organic.
With the stressors and time constraints of managing medical school and a family, nutrition tumbled down the ladder of priorities until eventually my family was subsisting on chicken nuggets, pizza, and peanut butter. Intern year has only added the occasional candy bar from the doctors’ lounge. I experienced a vague sense of loss for something I had once valued, but simultaneously felt dismissive of trendy topics such as omega-3 fatty acids and antioxidants in the face of myocardial infarctions and liver failure. A biochemistry professor once scoffed at “the laypeople’s obsession with toxins,” and nutrition received zero attention in our medical school curriculum or board exams.
However, a clinical experience on the inpatient psychiatric unit made me reevaluate the importance nutrition should have in both our personal lives and the practice of medicine. This is the case of an otherwise healthy young man with no psychiatric history who suffered a psychotic break after ingesting an excess of a supplement he purchased online with the purpose of improving his performance at a high-stress job.
CASE REPORT
Mr. K, a 28-year-old computer programmer, was voluntarily admitted to the inpatient psychiatry unit for paranoia and persecutory delusions along with auditory hallucinations. His father reported that Mr. K had been behaving erratically for several days prior to admission and was subsequently found wandering in the street.
On admission, Mr. K was not oriented to place or situation. He was unkempt and guarded, and claimed people were following him. His urine toxicology screen and blood alcohol levels were negative.
While hospitalized, Mr. K was hyperverbal and delusional. He related that at work he had been developing programs to make slaves in the computer, “algorithms for orchestration,” and that he was uncomfortable with the ethical implications. He eventually endorsed having purchased the supplement phenylethylamine (PEA) to improve his focus, and ingesting “two substantial scoops of the crystalline substance.”
We did not initiate any psychiatric medications. On the third day of his hospitalization, Mr. K was alert, oriented, euthymic, relaxed, and had a full range of affect; upon discharge we advised him to discard the PEA and avoid stimulants. He complied, quit his high-stress job, and had no subsequent psychotic symptoms in the 7 months since discharge.
Continue to: Dietary supplements carry risks
Dietary supplements carry risks
According to the FDA, dietary supplements are regulated as food, but many have strong biologic effects or may even contain drugs.1 More than 18% of Americans use herbal or nutritional therapies as part of their health regimen.2 However, many over-the-counter remedies have been found to exhibit psychotropic effects,3 and many more are purported to impact mental and physical health with little to no scientific research into these claims or potential adverse effects.
Phenylethylamine is sold as a nutritional supplement and marketed for its purported beneficial effects on weight loss, mood, and focus.4 However, PEA is known to act as a natural amphetamine and to play a role in the development of neuropsychiatric disorders.5 It is an endogenous psychotogenic molecule that has been previously theorized as a cause for primary psychosis.6 Phenylethylamine interacts with the same receptor ligand that responds to amphetamine and related compounds (such as methamphetamine and 3,4-methylenedioxy-methamphetamine [MDMA]), the genetic coding for which is located in an area of DNA associated with schizophrenia: chromosome 6q23.2.7 While the mechanisms and details of these interactions remain poorly understood, this case of PEA-induced psychosis represents a glimpse into the potential psychoactive properties of this readily available nutritional supplement.
This patient’s cautionary tale has given me pause regarding both my family’s nutrition and the oft-neglected dietary portion of the social history. Also, several subsequent patient experiences hearken back to my mother’s words regarding the importance of healthy eating. A patient with phenylketonuria presented with psychosis after running out of her formula and consuming junk food. Another patient with severely elevated blood glucose levels presented with confusion. I have come to realize that ingestion impacts presentation, or, in other words, you are what you eat.
1. US Food and Drug Administration. Dietary supplements. https://www.fda.gov/consumers/consumer-updates/dietary-supplements. Accessed December 11, 2019.
2. Tindle H, Davis R, Philips R, et al. Trends in use of complementary and alternative medicine by US adults: 1997-2002. Altern Ther Health Med. 2005;11(1):42-49.
3. Sarris J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytotherapy Research. 2018;32(7):1147-1162.
4. Irsfeld M, Spadafore M, Prüß BM. β-phenylethylamine, a small molecule with a large impact. WebmedCentral. 2013;4(9):4409.
5. Wolf M, Mosnaim A. Phenylethylamine in neuropsychiatric disorders. Gen Pharmacol. 1983;14(4):385-390.
6. Janssen P, Leysen J, Megens A, et al. Does phenylethylamine act as an endogenous amphetamine in some patients? In J Neuropsychopharmacol. 1999;2(3):229-240.
7. Zucchi R, Chiellini G, Scanlan TS, et al. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149(8):967-978.
“You are what you eat,” my mother always said, and structured our dinner plates according to the USDA food pyramid. We dutifully consumed leafy greens, and prior to medical school I invested time and money into healthy diet choices. I drank green smoothies, pureed baby food for my children, read up on the pH balancing diet, grew sprouts on windowsills, bought organic.
With the stressors and time constraints of managing medical school and a family, nutrition tumbled down the ladder of priorities until eventually my family was subsisting on chicken nuggets, pizza, and peanut butter. Intern year has only added the occasional candy bar from the doctors’ lounge. I experienced a vague sense of loss for something I had once valued, but simultaneously felt dismissive of trendy topics such as omega-3 fatty acids and antioxidants in the face of myocardial infarctions and liver failure. A biochemistry professor once scoffed at “the laypeople’s obsession with toxins,” and nutrition received zero attention in our medical school curriculum or board exams.
However, a clinical experience on the inpatient psychiatric unit made me reevaluate the importance nutrition should have in both our personal lives and the practice of medicine. This is the case of an otherwise healthy young man with no psychiatric history who suffered a psychotic break after ingesting an excess of a supplement he purchased online with the purpose of improving his performance at a high-stress job.
CASE REPORT
Mr. K, a 28-year-old computer programmer, was voluntarily admitted to the inpatient psychiatry unit for paranoia and persecutory delusions along with auditory hallucinations. His father reported that Mr. K had been behaving erratically for several days prior to admission and was subsequently found wandering in the street.
On admission, Mr. K was not oriented to place or situation. He was unkempt and guarded, and claimed people were following him. His urine toxicology screen and blood alcohol levels were negative.
While hospitalized, Mr. K was hyperverbal and delusional. He related that at work he had been developing programs to make slaves in the computer, “algorithms for orchestration,” and that he was uncomfortable with the ethical implications. He eventually endorsed having purchased the supplement phenylethylamine (PEA) to improve his focus, and ingesting “two substantial scoops of the crystalline substance.”
We did not initiate any psychiatric medications. On the third day of his hospitalization, Mr. K was alert, oriented, euthymic, relaxed, and had a full range of affect; upon discharge we advised him to discard the PEA and avoid stimulants. He complied, quit his high-stress job, and had no subsequent psychotic symptoms in the 7 months since discharge.
Continue to: Dietary supplements carry risks
Dietary supplements carry risks
According to the FDA, dietary supplements are regulated as food, but many have strong biologic effects or may even contain drugs.1 More than 18% of Americans use herbal or nutritional therapies as part of their health regimen.2 However, many over-the-counter remedies have been found to exhibit psychotropic effects,3 and many more are purported to impact mental and physical health with little to no scientific research into these claims or potential adverse effects.
Phenylethylamine is sold as a nutritional supplement and marketed for its purported beneficial effects on weight loss, mood, and focus.4 However, PEA is known to act as a natural amphetamine and to play a role in the development of neuropsychiatric disorders.5 It is an endogenous psychotogenic molecule that has been previously theorized as a cause for primary psychosis.6 Phenylethylamine interacts with the same receptor ligand that responds to amphetamine and related compounds (such as methamphetamine and 3,4-methylenedioxy-methamphetamine [MDMA]), the genetic coding for which is located in an area of DNA associated with schizophrenia: chromosome 6q23.2.7 While the mechanisms and details of these interactions remain poorly understood, this case of PEA-induced psychosis represents a glimpse into the potential psychoactive properties of this readily available nutritional supplement.
This patient’s cautionary tale has given me pause regarding both my family’s nutrition and the oft-neglected dietary portion of the social history. Also, several subsequent patient experiences hearken back to my mother’s words regarding the importance of healthy eating. A patient with phenylketonuria presented with psychosis after running out of her formula and consuming junk food. Another patient with severely elevated blood glucose levels presented with confusion. I have come to realize that ingestion impacts presentation, or, in other words, you are what you eat.
“You are what you eat,” my mother always said, and structured our dinner plates according to the USDA food pyramid. We dutifully consumed leafy greens, and prior to medical school I invested time and money into healthy diet choices. I drank green smoothies, pureed baby food for my children, read up on the pH balancing diet, grew sprouts on windowsills, bought organic.
With the stressors and time constraints of managing medical school and a family, nutrition tumbled down the ladder of priorities until eventually my family was subsisting on chicken nuggets, pizza, and peanut butter. Intern year has only added the occasional candy bar from the doctors’ lounge. I experienced a vague sense of loss for something I had once valued, but simultaneously felt dismissive of trendy topics such as omega-3 fatty acids and antioxidants in the face of myocardial infarctions and liver failure. A biochemistry professor once scoffed at “the laypeople’s obsession with toxins,” and nutrition received zero attention in our medical school curriculum or board exams.
However, a clinical experience on the inpatient psychiatric unit made me reevaluate the importance nutrition should have in both our personal lives and the practice of medicine. This is the case of an otherwise healthy young man with no psychiatric history who suffered a psychotic break after ingesting an excess of a supplement he purchased online with the purpose of improving his performance at a high-stress job.
CASE REPORT
Mr. K, a 28-year-old computer programmer, was voluntarily admitted to the inpatient psychiatry unit for paranoia and persecutory delusions along with auditory hallucinations. His father reported that Mr. K had been behaving erratically for several days prior to admission and was subsequently found wandering in the street.
On admission, Mr. K was not oriented to place or situation. He was unkempt and guarded, and claimed people were following him. His urine toxicology screen and blood alcohol levels were negative.
While hospitalized, Mr. K was hyperverbal and delusional. He related that at work he had been developing programs to make slaves in the computer, “algorithms for orchestration,” and that he was uncomfortable with the ethical implications. He eventually endorsed having purchased the supplement phenylethylamine (PEA) to improve his focus, and ingesting “two substantial scoops of the crystalline substance.”
We did not initiate any psychiatric medications. On the third day of his hospitalization, Mr. K was alert, oriented, euthymic, relaxed, and had a full range of affect; upon discharge we advised him to discard the PEA and avoid stimulants. He complied, quit his high-stress job, and had no subsequent psychotic symptoms in the 7 months since discharge.
Continue to: Dietary supplements carry risks
Dietary supplements carry risks
According to the FDA, dietary supplements are regulated as food, but many have strong biologic effects or may even contain drugs.1 More than 18% of Americans use herbal or nutritional therapies as part of their health regimen.2 However, many over-the-counter remedies have been found to exhibit psychotropic effects,3 and many more are purported to impact mental and physical health with little to no scientific research into these claims or potential adverse effects.
Phenylethylamine is sold as a nutritional supplement and marketed for its purported beneficial effects on weight loss, mood, and focus.4 However, PEA is known to act as a natural amphetamine and to play a role in the development of neuropsychiatric disorders.5 It is an endogenous psychotogenic molecule that has been previously theorized as a cause for primary psychosis.6 Phenylethylamine interacts with the same receptor ligand that responds to amphetamine and related compounds (such as methamphetamine and 3,4-methylenedioxy-methamphetamine [MDMA]), the genetic coding for which is located in an area of DNA associated with schizophrenia: chromosome 6q23.2.7 While the mechanisms and details of these interactions remain poorly understood, this case of PEA-induced psychosis represents a glimpse into the potential psychoactive properties of this readily available nutritional supplement.
This patient’s cautionary tale has given me pause regarding both my family’s nutrition and the oft-neglected dietary portion of the social history. Also, several subsequent patient experiences hearken back to my mother’s words regarding the importance of healthy eating. A patient with phenylketonuria presented with psychosis after running out of her formula and consuming junk food. Another patient with severely elevated blood glucose levels presented with confusion. I have come to realize that ingestion impacts presentation, or, in other words, you are what you eat.
1. US Food and Drug Administration. Dietary supplements. https://www.fda.gov/consumers/consumer-updates/dietary-supplements. Accessed December 11, 2019.
2. Tindle H, Davis R, Philips R, et al. Trends in use of complementary and alternative medicine by US adults: 1997-2002. Altern Ther Health Med. 2005;11(1):42-49.
3. Sarris J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytotherapy Research. 2018;32(7):1147-1162.
4. Irsfeld M, Spadafore M, Prüß BM. β-phenylethylamine, a small molecule with a large impact. WebmedCentral. 2013;4(9):4409.
5. Wolf M, Mosnaim A. Phenylethylamine in neuropsychiatric disorders. Gen Pharmacol. 1983;14(4):385-390.
6. Janssen P, Leysen J, Megens A, et al. Does phenylethylamine act as an endogenous amphetamine in some patients? In J Neuropsychopharmacol. 1999;2(3):229-240.
7. Zucchi R, Chiellini G, Scanlan TS, et al. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149(8):967-978.
1. US Food and Drug Administration. Dietary supplements. https://www.fda.gov/consumers/consumer-updates/dietary-supplements. Accessed December 11, 2019.
2. Tindle H, Davis R, Philips R, et al. Trends in use of complementary and alternative medicine by US adults: 1997-2002. Altern Ther Health Med. 2005;11(1):42-49.
3. Sarris J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytotherapy Research. 2018;32(7):1147-1162.
4. Irsfeld M, Spadafore M, Prüß BM. β-phenylethylamine, a small molecule with a large impact. WebmedCentral. 2013;4(9):4409.
5. Wolf M, Mosnaim A. Phenylethylamine in neuropsychiatric disorders. Gen Pharmacol. 1983;14(4):385-390.
6. Janssen P, Leysen J, Megens A, et al. Does phenylethylamine act as an endogenous amphetamine in some patients? In J Neuropsychopharmacol. 1999;2(3):229-240.
7. Zucchi R, Chiellini G, Scanlan TS, et al. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149(8):967-978.
Rethinking the language of substance abuse
In December 2019, Seattle Seahawks wide receiver Josh Gordon was suspended indefinitely from the NFL for violation of the league’s substance abuse policy. Gordon, once known as one of the most promising wide receivers of the last few decades, had a tumultuous relationship with the NFL as a result of his struggles with substance use. However, the headlines from major sports and news outlets often describe Gordon and other professional and collegiate athletes who struggle with substance use as “violating policies of abuse.” Media coverage of such athletes frequently imposes labels such as “violation” and “abuse,” implying a greater level of personal responsibility and willful misconduct than the biological process of addiction would typically allow. Gordon’s story brought attention not only to the adversity and impairments of substance use, but also the stigmatizing language that often accompanies it.
Shifting to less stigmatizing terminology
In DMS-5, use of the terminology substance use disorder fosters a more biologically-based model of behavior, and encourages recovery-oriented terminology.1 However, for most collegiate and professional sports leagues, the policies regarding substance use often use the term substance abuse, which can perpetuate stigma and a misunderstanding of the underpinnings of substance use, insinuating a sense of personal responsibility, deliberate misconduct, and criminality. When an individual is referred to as an “abuser” of substances, this might suggest that they are willful perpetrators of the disease on themselves, and thus may be undeserving of care.2 Individuals referred to as “substance abusers” rather than having a substance use disorder are more likely to be subjected to negative perceptions and evaluations of their behaviors, particularly by clinicians.
Individuals with substance use disorders are often viewed more negatively than individuals with physical or other psychiatric disorders, and are among the most stigmatized and marginalized groups in health care.4,5 Today, lawmakers, advocates, and health care professionals across the country are working to integrate destigmatizing language into media, policy, and educational settings in order to characterize substance use as a neurobiological process rather than a moral fault.6 For example, legislation in Maine passed in 2018 removed references to stigmatizing terms in policies related to substance use, replacing substance abuse and drug addict with recovery-oriented terminology such as substance use disorder and person with a substance use disorder.7
Individuals with substance use disorders often fear judgment and stigma during clinical encounters, and commonly cite this as a reason to avoid seeking care.8 Words matter, and if we are not careful, the language we use can convey meaning and attitudes that perpetuate the stigma that prevents so many from accessing treatment.9,10 Individuals with a substance use disorder should feel institutionally supported, and the language of policies and the clinicians who treat these patients should reflect this as well.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Wakeman SE. Language and addiction: choosing words wisely. Am J Public Health. 2013;103(4):e1‐e2.
3. Kelly JF, Westerhoff CM. Does it matter how we refer to individuals with substance-related conditions? A randomized study of two commonly used terms. Int J Drug Policy. 2010;21(3):202‐207.
4. Corrigan PW, Kuwabara SA, O’Shaughnessy J. The public stigma of mental illness and drug addiction: findings from a stratified random sample. Journal of Social Work. 2009;9(2):139-147.
5. Barry CL, McGinty EE, Pescosolido BA, et al. Stigma, discrimination, treatment effectiveness, and policy: public views about drug addiction and mental illness. Psychiatr Serv. 2014;65(10):1269‐1272.
6. Office of National Drug Control Policy. Changing the language of addiction. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Published January 9, 2017. Accessed June 8, 2020.
7. Flaherty N. Why language matters when describing substance use disorder in Maine. http://www.mainepublic.org/post/why-language-matters-when-describing-substance-use-disorder-maine. Published May 16, 2018. Accessed June 8, 2020.
8. Merrill JO, Rhodes LA, Deyo RA, et al. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327‐333.
9. Yang LH, Wong LY, Grivel MM, et al. Stigma and substance use disorders: an international phenomenon. Curr Opin Psychiatry. 2017;30(5):378‐388.
10. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217‐221.
In December 2019, Seattle Seahawks wide receiver Josh Gordon was suspended indefinitely from the NFL for violation of the league’s substance abuse policy. Gordon, once known as one of the most promising wide receivers of the last few decades, had a tumultuous relationship with the NFL as a result of his struggles with substance use. However, the headlines from major sports and news outlets often describe Gordon and other professional and collegiate athletes who struggle with substance use as “violating policies of abuse.” Media coverage of such athletes frequently imposes labels such as “violation” and “abuse,” implying a greater level of personal responsibility and willful misconduct than the biological process of addiction would typically allow. Gordon’s story brought attention not only to the adversity and impairments of substance use, but also the stigmatizing language that often accompanies it.
Shifting to less stigmatizing terminology
In DMS-5, use of the terminology substance use disorder fosters a more biologically-based model of behavior, and encourages recovery-oriented terminology.1 However, for most collegiate and professional sports leagues, the policies regarding substance use often use the term substance abuse, which can perpetuate stigma and a misunderstanding of the underpinnings of substance use, insinuating a sense of personal responsibility, deliberate misconduct, and criminality. When an individual is referred to as an “abuser” of substances, this might suggest that they are willful perpetrators of the disease on themselves, and thus may be undeserving of care.2 Individuals referred to as “substance abusers” rather than having a substance use disorder are more likely to be subjected to negative perceptions and evaluations of their behaviors, particularly by clinicians.
Individuals with substance use disorders are often viewed more negatively than individuals with physical or other psychiatric disorders, and are among the most stigmatized and marginalized groups in health care.4,5 Today, lawmakers, advocates, and health care professionals across the country are working to integrate destigmatizing language into media, policy, and educational settings in order to characterize substance use as a neurobiological process rather than a moral fault.6 For example, legislation in Maine passed in 2018 removed references to stigmatizing terms in policies related to substance use, replacing substance abuse and drug addict with recovery-oriented terminology such as substance use disorder and person with a substance use disorder.7
Individuals with substance use disorders often fear judgment and stigma during clinical encounters, and commonly cite this as a reason to avoid seeking care.8 Words matter, and if we are not careful, the language we use can convey meaning and attitudes that perpetuate the stigma that prevents so many from accessing treatment.9,10 Individuals with a substance use disorder should feel institutionally supported, and the language of policies and the clinicians who treat these patients should reflect this as well.
In December 2019, Seattle Seahawks wide receiver Josh Gordon was suspended indefinitely from the NFL for violation of the league’s substance abuse policy. Gordon, once known as one of the most promising wide receivers of the last few decades, had a tumultuous relationship with the NFL as a result of his struggles with substance use. However, the headlines from major sports and news outlets often describe Gordon and other professional and collegiate athletes who struggle with substance use as “violating policies of abuse.” Media coverage of such athletes frequently imposes labels such as “violation” and “abuse,” implying a greater level of personal responsibility and willful misconduct than the biological process of addiction would typically allow. Gordon’s story brought attention not only to the adversity and impairments of substance use, but also the stigmatizing language that often accompanies it.
Shifting to less stigmatizing terminology
In DMS-5, use of the terminology substance use disorder fosters a more biologically-based model of behavior, and encourages recovery-oriented terminology.1 However, for most collegiate and professional sports leagues, the policies regarding substance use often use the term substance abuse, which can perpetuate stigma and a misunderstanding of the underpinnings of substance use, insinuating a sense of personal responsibility, deliberate misconduct, and criminality. When an individual is referred to as an “abuser” of substances, this might suggest that they are willful perpetrators of the disease on themselves, and thus may be undeserving of care.2 Individuals referred to as “substance abusers” rather than having a substance use disorder are more likely to be subjected to negative perceptions and evaluations of their behaviors, particularly by clinicians.
Individuals with substance use disorders are often viewed more negatively than individuals with physical or other psychiatric disorders, and are among the most stigmatized and marginalized groups in health care.4,5 Today, lawmakers, advocates, and health care professionals across the country are working to integrate destigmatizing language into media, policy, and educational settings in order to characterize substance use as a neurobiological process rather than a moral fault.6 For example, legislation in Maine passed in 2018 removed references to stigmatizing terms in policies related to substance use, replacing substance abuse and drug addict with recovery-oriented terminology such as substance use disorder and person with a substance use disorder.7
Individuals with substance use disorders often fear judgment and stigma during clinical encounters, and commonly cite this as a reason to avoid seeking care.8 Words matter, and if we are not careful, the language we use can convey meaning and attitudes that perpetuate the stigma that prevents so many from accessing treatment.9,10 Individuals with a substance use disorder should feel institutionally supported, and the language of policies and the clinicians who treat these patients should reflect this as well.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Wakeman SE. Language and addiction: choosing words wisely. Am J Public Health. 2013;103(4):e1‐e2.
3. Kelly JF, Westerhoff CM. Does it matter how we refer to individuals with substance-related conditions? A randomized study of two commonly used terms. Int J Drug Policy. 2010;21(3):202‐207.
4. Corrigan PW, Kuwabara SA, O’Shaughnessy J. The public stigma of mental illness and drug addiction: findings from a stratified random sample. Journal of Social Work. 2009;9(2):139-147.
5. Barry CL, McGinty EE, Pescosolido BA, et al. Stigma, discrimination, treatment effectiveness, and policy: public views about drug addiction and mental illness. Psychiatr Serv. 2014;65(10):1269‐1272.
6. Office of National Drug Control Policy. Changing the language of addiction. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Published January 9, 2017. Accessed June 8, 2020.
7. Flaherty N. Why language matters when describing substance use disorder in Maine. http://www.mainepublic.org/post/why-language-matters-when-describing-substance-use-disorder-maine. Published May 16, 2018. Accessed June 8, 2020.
8. Merrill JO, Rhodes LA, Deyo RA, et al. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327‐333.
9. Yang LH, Wong LY, Grivel MM, et al. Stigma and substance use disorders: an international phenomenon. Curr Opin Psychiatry. 2017;30(5):378‐388.
10. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217‐221.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Wakeman SE. Language and addiction: choosing words wisely. Am J Public Health. 2013;103(4):e1‐e2.
3. Kelly JF, Westerhoff CM. Does it matter how we refer to individuals with substance-related conditions? A randomized study of two commonly used terms. Int J Drug Policy. 2010;21(3):202‐207.
4. Corrigan PW, Kuwabara SA, O’Shaughnessy J. The public stigma of mental illness and drug addiction: findings from a stratified random sample. Journal of Social Work. 2009;9(2):139-147.
5. Barry CL, McGinty EE, Pescosolido BA, et al. Stigma, discrimination, treatment effectiveness, and policy: public views about drug addiction and mental illness. Psychiatr Serv. 2014;65(10):1269‐1272.
6. Office of National Drug Control Policy. Changing the language of addiction. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Published January 9, 2017. Accessed June 8, 2020.
7. Flaherty N. Why language matters when describing substance use disorder in Maine. http://www.mainepublic.org/post/why-language-matters-when-describing-substance-use-disorder-maine. Published May 16, 2018. Accessed June 8, 2020.
8. Merrill JO, Rhodes LA, Deyo RA, et al. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327‐333.
9. Yang LH, Wong LY, Grivel MM, et al. Stigma and substance use disorders: an international phenomenon. Curr Opin Psychiatry. 2017;30(5):378‐388.
10. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217‐221.
More on the travesty of pre-authorization
We were delighted to read Dr. Nasrallah’s coruscating editorial about the deceptive, unethical, and clinically harmful practice of insurance companies requiring pre-authorization before granting coverage of psychotropic medications that are not on their short list of inexpensive alternatives (“Pre-authorization is illegal, unethical, and adversely disrupts patient care.” From the Editor,
Brian S. Barnett, MD
Staff Psychiatrist
Cleveland Clinic
Lutheran Hospital
Cleveland, Ohio
J. Alexander Bodkin, MD
Chief
Clinical Psychopharmacology
Research Program
McLean Hospital
Harvard Medical School
Belmont, Massachusetts
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I thank Dr. Nasrallah for bringing up the issue of pre-authorization in his editorial and could not agree with him more. As a practicing geriatric psychiatrist—for several decades—I experienced all of what he so nicely summarized, and more. The amount and degree of humiliation, frustration, and (mainly) waste of time have been painful and unacceptable. As he said: It must be stopped! The question is “How?” Hopefully this editorial triggers some activity against pre-authorization. It was time somebody addressed this problem.
Istvan Boksay, MD, PhD
Private psychiatric practice
New York, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I thank Dr. Nasrallah...
I thank Dr. Nasrallah for his editorial about pre-authorization, which was well organized and had a perfect headline. In succinct paragraphs, it says what we practitioners have wanted to say for years. If only the American Psychiatric Association and American Medical Association would take up the cause, perhaps some limitations might be put on this corporate intrusion into our practice. Pre-authorization may save insurance companies money, but its cost in time, frustration, and clinical outcomes adds a considerable burden to the financial problems of health care in the United States.
John Buckley, MD
Private psychiatric practice
Glen Arm, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I thank Dr. Nasrallah so much for his editorial. These types of clinically useless administrative tasks are invisible barriers to mental health care access, because the time utilized to complete these tasks can easily be used to see one more patient who needs to be treated. However, I also wonder how we as psychiatrists can move forward so that our psychiatric organizations and legislative bodies can take further action to the real barriers to health care and effective interventions.
Ranvinder Kaur Rai, MD
Private psychiatric practice
Fremont, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I read with interest...
I read with interest Dr. Nasrallah’s editorials “We are physicians, not providers, and we treat patients, not clients!” (From the Editor,
Dr. Nasrallah’s strong advocacy against the use of the term “provider” is long overdue. I distinctly remember the insidious onset of the use of the terms provider and “consumer” during my years as a medical director of a mental health center. The inception of the provider/consumer terminology can be construed as striving for cultural correctness when psychiatry was going through its own identity crisis in response to deinstitutionalization and the destruction of the so-called myth of psychiatrists as paternalistic and all-powerful. Managed care as the business model of medicine further destroyed the perception of the psychiatric physician as noble and caring, and demythologized the physician–patient relationship. It is amazing how the term provider has persisted and become part of the language of medicine. During the last 20 years or so, psychiatric and medical professional organizations have done little to squash the usage of the term.
Furthermore, the concept of pre-authorization is not new to medicine, but has insidiously become part of the tasks of the psychiatric physician. It has morphed into more than having to obtain approval for using a branded medication over a cheaper generic alternative to having to obtain approval for the use of any medication that does not fall under the approved tier. Even antipsychotics (generally a protected class) have not been immune.
Both the use of the term provider and the concept of pre-authorization require more than the frustration and indignation of a clinical psychiatrist. It requires the determination of professional psychiatric organizations and those with power to fight the gradual but ever-deteriorating authority of medical practice and the role of the psychiatric physician.
Elizabeth A. Varas, MD
Private psychiatric practice
Westwood, New Jersey
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
We were delighted to read Dr. Nasrallah’s coruscating editorial about the deceptive, unethical, and clinically harmful practice of insurance companies requiring pre-authorization before granting coverage of psychotropic medications that are not on their short list of inexpensive alternatives (“Pre-authorization is illegal, unethical, and adversely disrupts patient care.” From the Editor,
Brian S. Barnett, MD
Staff Psychiatrist
Cleveland Clinic
Lutheran Hospital
Cleveland, Ohio
J. Alexander Bodkin, MD
Chief
Clinical Psychopharmacology
Research Program
McLean Hospital
Harvard Medical School
Belmont, Massachusetts
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I thank Dr. Nasrallah for bringing up the issue of pre-authorization in his editorial and could not agree with him more. As a practicing geriatric psychiatrist—for several decades—I experienced all of what he so nicely summarized, and more. The amount and degree of humiliation, frustration, and (mainly) waste of time have been painful and unacceptable. As he said: It must be stopped! The question is “How?” Hopefully this editorial triggers some activity against pre-authorization. It was time somebody addressed this problem.
Istvan Boksay, MD, PhD
Private psychiatric practice
New York, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I thank Dr. Nasrallah...
I thank Dr. Nasrallah for his editorial about pre-authorization, which was well organized and had a perfect headline. In succinct paragraphs, it says what we practitioners have wanted to say for years. If only the American Psychiatric Association and American Medical Association would take up the cause, perhaps some limitations might be put on this corporate intrusion into our practice. Pre-authorization may save insurance companies money, but its cost in time, frustration, and clinical outcomes adds a considerable burden to the financial problems of health care in the United States.
John Buckley, MD
Private psychiatric practice
Glen Arm, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I thank Dr. Nasrallah so much for his editorial. These types of clinically useless administrative tasks are invisible barriers to mental health care access, because the time utilized to complete these tasks can easily be used to see one more patient who needs to be treated. However, I also wonder how we as psychiatrists can move forward so that our psychiatric organizations and legislative bodies can take further action to the real barriers to health care and effective interventions.
Ranvinder Kaur Rai, MD
Private psychiatric practice
Fremont, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I read with interest...
I read with interest Dr. Nasrallah’s editorials “We are physicians, not providers, and we treat patients, not clients!” (From the Editor,
Dr. Nasrallah’s strong advocacy against the use of the term “provider” is long overdue. I distinctly remember the insidious onset of the use of the terms provider and “consumer” during my years as a medical director of a mental health center. The inception of the provider/consumer terminology can be construed as striving for cultural correctness when psychiatry was going through its own identity crisis in response to deinstitutionalization and the destruction of the so-called myth of psychiatrists as paternalistic and all-powerful. Managed care as the business model of medicine further destroyed the perception of the psychiatric physician as noble and caring, and demythologized the physician–patient relationship. It is amazing how the term provider has persisted and become part of the language of medicine. During the last 20 years or so, psychiatric and medical professional organizations have done little to squash the usage of the term.
Furthermore, the concept of pre-authorization is not new to medicine, but has insidiously become part of the tasks of the psychiatric physician. It has morphed into more than having to obtain approval for using a branded medication over a cheaper generic alternative to having to obtain approval for the use of any medication that does not fall under the approved tier. Even antipsychotics (generally a protected class) have not been immune.
Both the use of the term provider and the concept of pre-authorization require more than the frustration and indignation of a clinical psychiatrist. It requires the determination of professional psychiatric organizations and those with power to fight the gradual but ever-deteriorating authority of medical practice and the role of the psychiatric physician.
Elizabeth A. Varas, MD
Private psychiatric practice
Westwood, New Jersey
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
We were delighted to read Dr. Nasrallah’s coruscating editorial about the deceptive, unethical, and clinically harmful practice of insurance companies requiring pre-authorization before granting coverage of psychotropic medications that are not on their short list of inexpensive alternatives (“Pre-authorization is illegal, unethical, and adversely disrupts patient care.” From the Editor,
Brian S. Barnett, MD
Staff Psychiatrist
Cleveland Clinic
Lutheran Hospital
Cleveland, Ohio
J. Alexander Bodkin, MD
Chief
Clinical Psychopharmacology
Research Program
McLean Hospital
Harvard Medical School
Belmont, Massachusetts
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I thank Dr. Nasrallah for bringing up the issue of pre-authorization in his editorial and could not agree with him more. As a practicing geriatric psychiatrist—for several decades—I experienced all of what he so nicely summarized, and more. The amount and degree of humiliation, frustration, and (mainly) waste of time have been painful and unacceptable. As he said: It must be stopped! The question is “How?” Hopefully this editorial triggers some activity against pre-authorization. It was time somebody addressed this problem.
Istvan Boksay, MD, PhD
Private psychiatric practice
New York, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I thank Dr. Nasrallah...
I thank Dr. Nasrallah for his editorial about pre-authorization, which was well organized and had a perfect headline. In succinct paragraphs, it says what we practitioners have wanted to say for years. If only the American Psychiatric Association and American Medical Association would take up the cause, perhaps some limitations might be put on this corporate intrusion into our practice. Pre-authorization may save insurance companies money, but its cost in time, frustration, and clinical outcomes adds a considerable burden to the financial problems of health care in the United States.
John Buckley, MD
Private psychiatric practice
Glen Arm, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I thank Dr. Nasrallah so much for his editorial. These types of clinically useless administrative tasks are invisible barriers to mental health care access, because the time utilized to complete these tasks can easily be used to see one more patient who needs to be treated. However, I also wonder how we as psychiatrists can move forward so that our psychiatric organizations and legislative bodies can take further action to the real barriers to health care and effective interventions.
Ranvinder Kaur Rai, MD
Private psychiatric practice
Fremont, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I read with interest...
I read with interest Dr. Nasrallah’s editorials “We are physicians, not providers, and we treat patients, not clients!” (From the Editor,
Dr. Nasrallah’s strong advocacy against the use of the term “provider” is long overdue. I distinctly remember the insidious onset of the use of the terms provider and “consumer” during my years as a medical director of a mental health center. The inception of the provider/consumer terminology can be construed as striving for cultural correctness when psychiatry was going through its own identity crisis in response to deinstitutionalization and the destruction of the so-called myth of psychiatrists as paternalistic and all-powerful. Managed care as the business model of medicine further destroyed the perception of the psychiatric physician as noble and caring, and demythologized the physician–patient relationship. It is amazing how the term provider has persisted and become part of the language of medicine. During the last 20 years or so, psychiatric and medical professional organizations have done little to squash the usage of the term.
Furthermore, the concept of pre-authorization is not new to medicine, but has insidiously become part of the tasks of the psychiatric physician. It has morphed into more than having to obtain approval for using a branded medication over a cheaper generic alternative to having to obtain approval for the use of any medication that does not fall under the approved tier. Even antipsychotics (generally a protected class) have not been immune.
Both the use of the term provider and the concept of pre-authorization require more than the frustration and indignation of a clinical psychiatrist. It requires the determination of professional psychiatric organizations and those with power to fight the gradual but ever-deteriorating authority of medical practice and the role of the psychiatric physician.
Elizabeth A. Varas, MD
Private psychiatric practice
Westwood, New Jersey
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
COVID-19 and the precipitous dismantlement of societal norms
As the life-altering coronavirus disease 2019 (COVID-19) pandemic gradually ebbs, we are all its survivors. Now, we are experiencing COVID-19 fatigue, trying to emerge from its dense fog that pervaded every facet of our lives. We are fully cognizant that there will not be a return to the previous “normal.” The pernicious virus had a transformative effect that did not spare any component of our society. Full recovery will not be easy.
As the uncertainty lingers about another devastating return of the pandemic later this year, we can see the reverberation of this invisible assault on human existence. Although a relatively small fraction of the population lost their lives, the rest of us are valiantly trying to readjust to the multiple ways our world has changed. Consider the following abrupt and sweeping burdens inflicted by the pandemic within a few short weeks:
Mental health. The acute stress of thanatophobia generated a triad of anxiety, depression, and nosophobia on a large scale. The demand for psychiatric care rapidly escalated. Suicide rate increased not only because of the stress of being locked down at home (alien to most people’s lifestyle) but because of the coincidental timing of the pandemic during April and May, the peak time of year for suicide. Animal researchers use immobilization as a paradigm to stress a rat or mouse. Many humans immobilized during the pandemic have developed exquisite empathy towards those rodents! The impact on children may also have long-term effects because playing and socializing with friends is a vital part of their lives. Parents have noticed dysphoria and acting out among their children, and an intense compensatory preoccupation with video games and electronic communications with friends.
Physical health. Medical care focused heavily on COVID-19 victims, to the detriment of all other medical conditions. Non-COVID-19 hospital admissions plummeted, and all elective surgeries and procedures were put on hold, depriving many people of medical care they badly needed. Emergency department (ED) visits also declined dramatically, including the usual flow of heart attacks, stroke, pulmonary embolus, asthma attacks, etc. The minimization of driving greatly reduced the admission of accident victims to EDs. Colonoscopies, cardiac stents, hip replacements, MRIs, mammography, and other procedures that are vital to maintain health and quality of life were halted. Dentists shuttered their practices due to the high risk of infection from exposure to oral secretions and breathing. One can only imagine the suffering of having a toothache with no dental help available, and how that might lead to narcotic abuse.
Social health. The imperative of social distancing disrupted most ordinary human activities, such as dining out, sitting in an auditorium for Grand Rounds or a lecture, visiting friends at their homes, the cherished interactions between grandparents and grandchildren (the lack of which I painfully experienced), and even seeing each other’s smiles behind the ubiquitous masks. And forget about hugging or kissing. The aversion to being near anyone who is coughing or sneezing led to an adaptive social paranoia and the social shunning of anyone who appeared to have an upper respiratory infection, even if it was unrelated to COVID-19.
Redemption for the pharmaceutical industry. The deadly pandemic intensified the public’s awareness of the importance of developing treatments and vaccines for COVID-19. The often-demonized pharmaceutical companies, with their extensive R&D infrastructure, emerged as a major source of hope for discovering an effective treatment for the coronavirus infection, or—better still—one or more vaccines that will enable society to return to its normal functions. It was quite impressive how many pharmaceutical companies “came to the rescue” with clinical trials to repurpose existing medications or to develop new ones. It was very encouraging to see multiple vaccine candidates being developed and expedited for testing around the world. A process that usually takes years was reduced to a few months, thanks to the existing technical infrastructure and thousands of scientists who enable rapid drug development. It is possible that the public may gradually modify its perception of the pharmaceutical industry from a “corporate villain” to an “indispensable health industry” for urgent medical crises such as a pandemic, and also for hundreds of medical diseases that are still in need of safe, effective therapies.
Economic burden. The unimaginable nightmare scenario of a total shutdown of all businesses led to the unprecedented loss of millions of jobs and livelihoods, reflected in miles-long lines of families at food banks. Overnight, the government switched from worrying about its $20-trillion deficit to printing several more trillion dollars to rescue the economy from collapse. The huge magnitude of a trillion can be appreciated if one is aware that it takes roughly 32 years to count to 1 billion, and 32,000 years to count to 1 trillion. Stimulating the economy while the gross domestic product threatens to sink by terrifying percentages (20% to 30%) was urgently needed, even though it meant mortgaging the future, especially when interest rates, and servicing the debt, will inevitably rise from the current zero to much higher levels in the future. The collapse of the once-thriving airline industry (bookings were down an estimated 98%) is an example of why desperate measures were needed to salvage an economy paralyzed by a viral pandemic.
Continue to: Political repercussions
Political repercussions. In our already hyperpartisan country, the COVID-19 crisis created more fissures across party lines. The blame game escalated as each side tried to exploit the crisis for political gain during a presidential election year. None of the leaders, from mayors to governors to the president, had any notion of how to wisely manage an unforeseen catastrophic pandemic. Thus, a political cacophony has developed, further exacerbating the public’s anxiety and uncertainty, especially about how and when the pandemic will end.
Education disruption. Never before have all schools and colleges around the country abruptly closed and sent students of all ages to shelter at home. Massive havoc ensued, with a wholesale switch to solitary online learning, the loss of the unique school and college social experience in the classroom and on campus, and the loss of experiencing commencement to receive a diploma (an important milestone for every graduate). Even medical students were not allowed to complete their clinical rotations and were sent home to attend online classes. A complete paradigm shift emerged about entrance exams: the SAT and ACT were eliminated for college applicants, and the MCAT for medical school applicants. This was unthinkable before the pandemic descended upon us, but benchmarks suddenly evaporated to adjust to the new reality. Then there followed disastrous financial losses by institutions of higher learning as well as academic medical centers and teaching hospitals, all slashing their budgets, furloughing employees, cutting salaries, and eliminating programs. Even the “sacred” tenure of senior faculty became a casualty of the financial “exigency.” Children’s nutrition suffered, especially among those in lower socioeconomic groups for whom the main meal of the day was the school lunch, and was made worse by their parents’ loss of income. For millions of people, the emotional toll was inevitable following the draconian measure of closing all educational institutions to contain the spread of the pandemic.
Family burden. Sheltering at home might have been fun for a few days, but after many weeks, it festered into a major stress, especially for those living in a small house, condominium, or apartment. The resilience of many families was tested as the exercise of freedoms collided with the fear of getting infected. Families were deprived of celebrating birthdays, weddings, funerals, graduation parties, retirement parties, Mother’s Day, Father’s Day, and various religious holidays, including Easter, Passover, and Eid al-Fitr.
Sexual burden. Intimacy and sexual contact between consenting adults living apart were sacrificed on the altar of the pernicious viral pandemic. Mandatory social distancing of 6 feet or more to avoid each other’s droplets emanating from simple speech, not just sneezing or coughing, makes intimacy practically impossible. Thus, physical closeness became taboo, and avoiding another person’s saliva or body secretions became a must to avoid contracting the virus. Being single was quite a lonely experience during this pandemic!
Entertainment deprivation. Americans are known to thrive on an extensive diet of spectator sports. Going to football, basketball, baseball, or hockey games to root for one’s team is intrinsically American. The pursuit of happiness extends to attending concerts, movies, Broadway shows, theme parks, and cruises with thousands of others. The pandemic ripped all those pleasurable leisure activities from our daily lives, leaving a big hole in people’s lives at the precise time fun activities were needed as a useful diversion from the dismal stress of a pandemic. To make things worse, it is uncertain when (if ever) such group activities will be restored, especially if the pandemic returns with another wave. But optimists would hurry to remind us that the “Roaring 20s” blossomed in the decade following the 1918 Spanish Flu pandemic.
Continue to: Legal system
Legal system. Astounding changes were instigated by the pandemic, such as the release of thousands of inmates, including felons, to avoid the spread of the virus in crowded prisons. For us psychiatrists, the silver lining in that unexpected action is that many of those released were patients with mental illness who were incarcerated because of the lack of hospitals that would take them. The police started issuing citations instead of arresting and jailing violators. Enforcement of the law was welcome when it targeted those who gouged the public for personal profit during the scarcity of masks, sanitizers, or even toilet paper and soap.
Medical practice. In addition to delaying medical care for patients, the freeze on so-called elective surgeries or procedures (many of which were actually necessary) was financially ruinous for physicians. Another regrettable consequence of the pandemic is a drop in pediatric vaccinations because parents were reluctant to take their children to the pediatrician. On a more positive note, the massive switch to telehealth was advantageous for both patients and psychiatrists because this technology is well-suited for psychiatric care. Fortunately, regulations that hampered telepsychiatry practice were substantially loosened or eliminated, and even the usually sacrosanct HIPAA regulations were temporarily sidelined.
Medical research. Both human and animal research came to a screeching halt, and many research assistants were furloughed. Data collection was disrupted, and a generation of scientific and medical discoveries became a casualty of the pandemic.
Medical literature. It was stunning to see how quickly COVID-19 occupied most of the pages of prominent journals. The scholarly articles were frankly quite useful, covering topics ranging from risk factors to early symptoms to treatment and pathophysiology across multiple organs. As with other paradigm shifts, there was an accelerated publication push, sometimes with expedited peer reviews to inform health care workers and the public while the pandemic was still raging. However, a couple of very prominent journals had to retract flawed articles that were hastily published without the usual due diligence and rigorous peer review. The pandemic clearly disrupted the science publishing process.
Travel effects. The steep reduction of flights (by 98%) was financially catastrophic, not only for airline companies but to business travel across the country. However, fewer cars on the road resulted in fewer accidents and deaths, and also reduced pollution. Paradoxically, to prevent crowding in subways, trains, and buses, officials reversed their traditional instructions and advised the public to drive their own cars instead of using public transportation!
Continue to: Heroism of front-line medical personnel
Heroism of front-line medical personnel. Everyone saluted and prayed for the health care professionals working at the bedside of highly infectious patients who needed 24/7 intensive care. Many have died while carrying out the noble but hazardous medical duties. Those heroes deserve our lasting respect and admiration.
The COVID-19 pandemic insidiously permeated and altered every aspect of our complex society and revealed how fragile our “normal lifestyle” really is. It is possible that nothing will ever be the same again, and an uneasy sense of vulnerability will engulf us as we cautiously return to a “new normal.” Even our language has expanded with the lexicon of pandemic terminology (Table). We all pray and hope that this plague never returns. And let’s hope one or more vaccines are developed soon so we can manage future recurrences like the annual flu season. In the meantime, keep your masks and sanitizers close by…
Postscript: Shortly after I completed this editorial, the ongoing COVID-19 plague was overshadowed by the scourge of racism, with massive protests, at times laced by violence, triggered by the death of a black man in custody of the police, under condemnable circumstances. The COVID-19 pandemic and the necessary social distancing it requires were temporarily ignored during the ensuing protests. The combined effect of those overlapping scourges are jarring to the country’s psyche, complicating and perhaps sabotaging the social recovery from the pandemic.
As the life-altering coronavirus disease 2019 (COVID-19) pandemic gradually ebbs, we are all its survivors. Now, we are experiencing COVID-19 fatigue, trying to emerge from its dense fog that pervaded every facet of our lives. We are fully cognizant that there will not be a return to the previous “normal.” The pernicious virus had a transformative effect that did not spare any component of our society. Full recovery will not be easy.
As the uncertainty lingers about another devastating return of the pandemic later this year, we can see the reverberation of this invisible assault on human existence. Although a relatively small fraction of the population lost their lives, the rest of us are valiantly trying to readjust to the multiple ways our world has changed. Consider the following abrupt and sweeping burdens inflicted by the pandemic within a few short weeks:
Mental health. The acute stress of thanatophobia generated a triad of anxiety, depression, and nosophobia on a large scale. The demand for psychiatric care rapidly escalated. Suicide rate increased not only because of the stress of being locked down at home (alien to most people’s lifestyle) but because of the coincidental timing of the pandemic during April and May, the peak time of year for suicide. Animal researchers use immobilization as a paradigm to stress a rat or mouse. Many humans immobilized during the pandemic have developed exquisite empathy towards those rodents! The impact on children may also have long-term effects because playing and socializing with friends is a vital part of their lives. Parents have noticed dysphoria and acting out among their children, and an intense compensatory preoccupation with video games and electronic communications with friends.
Physical health. Medical care focused heavily on COVID-19 victims, to the detriment of all other medical conditions. Non-COVID-19 hospital admissions plummeted, and all elective surgeries and procedures were put on hold, depriving many people of medical care they badly needed. Emergency department (ED) visits also declined dramatically, including the usual flow of heart attacks, stroke, pulmonary embolus, asthma attacks, etc. The minimization of driving greatly reduced the admission of accident victims to EDs. Colonoscopies, cardiac stents, hip replacements, MRIs, mammography, and other procedures that are vital to maintain health and quality of life were halted. Dentists shuttered their practices due to the high risk of infection from exposure to oral secretions and breathing. One can only imagine the suffering of having a toothache with no dental help available, and how that might lead to narcotic abuse.
Social health. The imperative of social distancing disrupted most ordinary human activities, such as dining out, sitting in an auditorium for Grand Rounds or a lecture, visiting friends at their homes, the cherished interactions between grandparents and grandchildren (the lack of which I painfully experienced), and even seeing each other’s smiles behind the ubiquitous masks. And forget about hugging or kissing. The aversion to being near anyone who is coughing or sneezing led to an adaptive social paranoia and the social shunning of anyone who appeared to have an upper respiratory infection, even if it was unrelated to COVID-19.
Redemption for the pharmaceutical industry. The deadly pandemic intensified the public’s awareness of the importance of developing treatments and vaccines for COVID-19. The often-demonized pharmaceutical companies, with their extensive R&D infrastructure, emerged as a major source of hope for discovering an effective treatment for the coronavirus infection, or—better still—one or more vaccines that will enable society to return to its normal functions. It was quite impressive how many pharmaceutical companies “came to the rescue” with clinical trials to repurpose existing medications or to develop new ones. It was very encouraging to see multiple vaccine candidates being developed and expedited for testing around the world. A process that usually takes years was reduced to a few months, thanks to the existing technical infrastructure and thousands of scientists who enable rapid drug development. It is possible that the public may gradually modify its perception of the pharmaceutical industry from a “corporate villain” to an “indispensable health industry” for urgent medical crises such as a pandemic, and also for hundreds of medical diseases that are still in need of safe, effective therapies.
Economic burden. The unimaginable nightmare scenario of a total shutdown of all businesses led to the unprecedented loss of millions of jobs and livelihoods, reflected in miles-long lines of families at food banks. Overnight, the government switched from worrying about its $20-trillion deficit to printing several more trillion dollars to rescue the economy from collapse. The huge magnitude of a trillion can be appreciated if one is aware that it takes roughly 32 years to count to 1 billion, and 32,000 years to count to 1 trillion. Stimulating the economy while the gross domestic product threatens to sink by terrifying percentages (20% to 30%) was urgently needed, even though it meant mortgaging the future, especially when interest rates, and servicing the debt, will inevitably rise from the current zero to much higher levels in the future. The collapse of the once-thriving airline industry (bookings were down an estimated 98%) is an example of why desperate measures were needed to salvage an economy paralyzed by a viral pandemic.
Continue to: Political repercussions
Political repercussions. In our already hyperpartisan country, the COVID-19 crisis created more fissures across party lines. The blame game escalated as each side tried to exploit the crisis for political gain during a presidential election year. None of the leaders, from mayors to governors to the president, had any notion of how to wisely manage an unforeseen catastrophic pandemic. Thus, a political cacophony has developed, further exacerbating the public’s anxiety and uncertainty, especially about how and when the pandemic will end.
Education disruption. Never before have all schools and colleges around the country abruptly closed and sent students of all ages to shelter at home. Massive havoc ensued, with a wholesale switch to solitary online learning, the loss of the unique school and college social experience in the classroom and on campus, and the loss of experiencing commencement to receive a diploma (an important milestone for every graduate). Even medical students were not allowed to complete their clinical rotations and were sent home to attend online classes. A complete paradigm shift emerged about entrance exams: the SAT and ACT were eliminated for college applicants, and the MCAT for medical school applicants. This was unthinkable before the pandemic descended upon us, but benchmarks suddenly evaporated to adjust to the new reality. Then there followed disastrous financial losses by institutions of higher learning as well as academic medical centers and teaching hospitals, all slashing their budgets, furloughing employees, cutting salaries, and eliminating programs. Even the “sacred” tenure of senior faculty became a casualty of the financial “exigency.” Children’s nutrition suffered, especially among those in lower socioeconomic groups for whom the main meal of the day was the school lunch, and was made worse by their parents’ loss of income. For millions of people, the emotional toll was inevitable following the draconian measure of closing all educational institutions to contain the spread of the pandemic.
Family burden. Sheltering at home might have been fun for a few days, but after many weeks, it festered into a major stress, especially for those living in a small house, condominium, or apartment. The resilience of many families was tested as the exercise of freedoms collided with the fear of getting infected. Families were deprived of celebrating birthdays, weddings, funerals, graduation parties, retirement parties, Mother’s Day, Father’s Day, and various religious holidays, including Easter, Passover, and Eid al-Fitr.
Sexual burden. Intimacy and sexual contact between consenting adults living apart were sacrificed on the altar of the pernicious viral pandemic. Mandatory social distancing of 6 feet or more to avoid each other’s droplets emanating from simple speech, not just sneezing or coughing, makes intimacy practically impossible. Thus, physical closeness became taboo, and avoiding another person’s saliva or body secretions became a must to avoid contracting the virus. Being single was quite a lonely experience during this pandemic!
Entertainment deprivation. Americans are known to thrive on an extensive diet of spectator sports. Going to football, basketball, baseball, or hockey games to root for one’s team is intrinsically American. The pursuit of happiness extends to attending concerts, movies, Broadway shows, theme parks, and cruises with thousands of others. The pandemic ripped all those pleasurable leisure activities from our daily lives, leaving a big hole in people’s lives at the precise time fun activities were needed as a useful diversion from the dismal stress of a pandemic. To make things worse, it is uncertain when (if ever) such group activities will be restored, especially if the pandemic returns with another wave. But optimists would hurry to remind us that the “Roaring 20s” blossomed in the decade following the 1918 Spanish Flu pandemic.
Continue to: Legal system
Legal system. Astounding changes were instigated by the pandemic, such as the release of thousands of inmates, including felons, to avoid the spread of the virus in crowded prisons. For us psychiatrists, the silver lining in that unexpected action is that many of those released were patients with mental illness who were incarcerated because of the lack of hospitals that would take them. The police started issuing citations instead of arresting and jailing violators. Enforcement of the law was welcome when it targeted those who gouged the public for personal profit during the scarcity of masks, sanitizers, or even toilet paper and soap.
Medical practice. In addition to delaying medical care for patients, the freeze on so-called elective surgeries or procedures (many of which were actually necessary) was financially ruinous for physicians. Another regrettable consequence of the pandemic is a drop in pediatric vaccinations because parents were reluctant to take their children to the pediatrician. On a more positive note, the massive switch to telehealth was advantageous for both patients and psychiatrists because this technology is well-suited for psychiatric care. Fortunately, regulations that hampered telepsychiatry practice were substantially loosened or eliminated, and even the usually sacrosanct HIPAA regulations were temporarily sidelined.
Medical research. Both human and animal research came to a screeching halt, and many research assistants were furloughed. Data collection was disrupted, and a generation of scientific and medical discoveries became a casualty of the pandemic.
Medical literature. It was stunning to see how quickly COVID-19 occupied most of the pages of prominent journals. The scholarly articles were frankly quite useful, covering topics ranging from risk factors to early symptoms to treatment and pathophysiology across multiple organs. As with other paradigm shifts, there was an accelerated publication push, sometimes with expedited peer reviews to inform health care workers and the public while the pandemic was still raging. However, a couple of very prominent journals had to retract flawed articles that were hastily published without the usual due diligence and rigorous peer review. The pandemic clearly disrupted the science publishing process.
Travel effects. The steep reduction of flights (by 98%) was financially catastrophic, not only for airline companies but to business travel across the country. However, fewer cars on the road resulted in fewer accidents and deaths, and also reduced pollution. Paradoxically, to prevent crowding in subways, trains, and buses, officials reversed their traditional instructions and advised the public to drive their own cars instead of using public transportation!
Continue to: Heroism of front-line medical personnel
Heroism of front-line medical personnel. Everyone saluted and prayed for the health care professionals working at the bedside of highly infectious patients who needed 24/7 intensive care. Many have died while carrying out the noble but hazardous medical duties. Those heroes deserve our lasting respect and admiration.
The COVID-19 pandemic insidiously permeated and altered every aspect of our complex society and revealed how fragile our “normal lifestyle” really is. It is possible that nothing will ever be the same again, and an uneasy sense of vulnerability will engulf us as we cautiously return to a “new normal.” Even our language has expanded with the lexicon of pandemic terminology (Table). We all pray and hope that this plague never returns. And let’s hope one or more vaccines are developed soon so we can manage future recurrences like the annual flu season. In the meantime, keep your masks and sanitizers close by…

Postscript: Shortly after I completed this editorial, the ongoing COVID-19 plague was overshadowed by the scourge of racism, with massive protests, at times laced by violence, triggered by the death of a black man in custody of the police, under condemnable circumstances. The COVID-19 pandemic and the necessary social distancing it requires were temporarily ignored during the ensuing protests. The combined effect of those overlapping scourges are jarring to the country’s psyche, complicating and perhaps sabotaging the social recovery from the pandemic.
As the life-altering coronavirus disease 2019 (COVID-19) pandemic gradually ebbs, we are all its survivors. Now, we are experiencing COVID-19 fatigue, trying to emerge from its dense fog that pervaded every facet of our lives. We are fully cognizant that there will not be a return to the previous “normal.” The pernicious virus had a transformative effect that did not spare any component of our society. Full recovery will not be easy.
As the uncertainty lingers about another devastating return of the pandemic later this year, we can see the reverberation of this invisible assault on human existence. Although a relatively small fraction of the population lost their lives, the rest of us are valiantly trying to readjust to the multiple ways our world has changed. Consider the following abrupt and sweeping burdens inflicted by the pandemic within a few short weeks:
Mental health. The acute stress of thanatophobia generated a triad of anxiety, depression, and nosophobia on a large scale. The demand for psychiatric care rapidly escalated. Suicide rate increased not only because of the stress of being locked down at home (alien to most people’s lifestyle) but because of the coincidental timing of the pandemic during April and May, the peak time of year for suicide. Animal researchers use immobilization as a paradigm to stress a rat or mouse. Many humans immobilized during the pandemic have developed exquisite empathy towards those rodents! The impact on children may also have long-term effects because playing and socializing with friends is a vital part of their lives. Parents have noticed dysphoria and acting out among their children, and an intense compensatory preoccupation with video games and electronic communications with friends.
Physical health. Medical care focused heavily on COVID-19 victims, to the detriment of all other medical conditions. Non-COVID-19 hospital admissions plummeted, and all elective surgeries and procedures were put on hold, depriving many people of medical care they badly needed. Emergency department (ED) visits also declined dramatically, including the usual flow of heart attacks, stroke, pulmonary embolus, asthma attacks, etc. The minimization of driving greatly reduced the admission of accident victims to EDs. Colonoscopies, cardiac stents, hip replacements, MRIs, mammography, and other procedures that are vital to maintain health and quality of life were halted. Dentists shuttered their practices due to the high risk of infection from exposure to oral secretions and breathing. One can only imagine the suffering of having a toothache with no dental help available, and how that might lead to narcotic abuse.
Social health. The imperative of social distancing disrupted most ordinary human activities, such as dining out, sitting in an auditorium for Grand Rounds or a lecture, visiting friends at their homes, the cherished interactions between grandparents and grandchildren (the lack of which I painfully experienced), and even seeing each other’s smiles behind the ubiquitous masks. And forget about hugging or kissing. The aversion to being near anyone who is coughing or sneezing led to an adaptive social paranoia and the social shunning of anyone who appeared to have an upper respiratory infection, even if it was unrelated to COVID-19.
Redemption for the pharmaceutical industry. The deadly pandemic intensified the public’s awareness of the importance of developing treatments and vaccines for COVID-19. The often-demonized pharmaceutical companies, with their extensive R&D infrastructure, emerged as a major source of hope for discovering an effective treatment for the coronavirus infection, or—better still—one or more vaccines that will enable society to return to its normal functions. It was quite impressive how many pharmaceutical companies “came to the rescue” with clinical trials to repurpose existing medications or to develop new ones. It was very encouraging to see multiple vaccine candidates being developed and expedited for testing around the world. A process that usually takes years was reduced to a few months, thanks to the existing technical infrastructure and thousands of scientists who enable rapid drug development. It is possible that the public may gradually modify its perception of the pharmaceutical industry from a “corporate villain” to an “indispensable health industry” for urgent medical crises such as a pandemic, and also for hundreds of medical diseases that are still in need of safe, effective therapies.
Economic burden. The unimaginable nightmare scenario of a total shutdown of all businesses led to the unprecedented loss of millions of jobs and livelihoods, reflected in miles-long lines of families at food banks. Overnight, the government switched from worrying about its $20-trillion deficit to printing several more trillion dollars to rescue the economy from collapse. The huge magnitude of a trillion can be appreciated if one is aware that it takes roughly 32 years to count to 1 billion, and 32,000 years to count to 1 trillion. Stimulating the economy while the gross domestic product threatens to sink by terrifying percentages (20% to 30%) was urgently needed, even though it meant mortgaging the future, especially when interest rates, and servicing the debt, will inevitably rise from the current zero to much higher levels in the future. The collapse of the once-thriving airline industry (bookings were down an estimated 98%) is an example of why desperate measures were needed to salvage an economy paralyzed by a viral pandemic.
Continue to: Political repercussions
Political repercussions. In our already hyperpartisan country, the COVID-19 crisis created more fissures across party lines. The blame game escalated as each side tried to exploit the crisis for political gain during a presidential election year. None of the leaders, from mayors to governors to the president, had any notion of how to wisely manage an unforeseen catastrophic pandemic. Thus, a political cacophony has developed, further exacerbating the public’s anxiety and uncertainty, especially about how and when the pandemic will end.
Education disruption. Never before have all schools and colleges around the country abruptly closed and sent students of all ages to shelter at home. Massive havoc ensued, with a wholesale switch to solitary online learning, the loss of the unique school and college social experience in the classroom and on campus, and the loss of experiencing commencement to receive a diploma (an important milestone for every graduate). Even medical students were not allowed to complete their clinical rotations and were sent home to attend online classes. A complete paradigm shift emerged about entrance exams: the SAT and ACT were eliminated for college applicants, and the MCAT for medical school applicants. This was unthinkable before the pandemic descended upon us, but benchmarks suddenly evaporated to adjust to the new reality. Then there followed disastrous financial losses by institutions of higher learning as well as academic medical centers and teaching hospitals, all slashing their budgets, furloughing employees, cutting salaries, and eliminating programs. Even the “sacred” tenure of senior faculty became a casualty of the financial “exigency.” Children’s nutrition suffered, especially among those in lower socioeconomic groups for whom the main meal of the day was the school lunch, and was made worse by their parents’ loss of income. For millions of people, the emotional toll was inevitable following the draconian measure of closing all educational institutions to contain the spread of the pandemic.
Family burden. Sheltering at home might have been fun for a few days, but after many weeks, it festered into a major stress, especially for those living in a small house, condominium, or apartment. The resilience of many families was tested as the exercise of freedoms collided with the fear of getting infected. Families were deprived of celebrating birthdays, weddings, funerals, graduation parties, retirement parties, Mother’s Day, Father’s Day, and various religious holidays, including Easter, Passover, and Eid al-Fitr.
Sexual burden. Intimacy and sexual contact between consenting adults living apart were sacrificed on the altar of the pernicious viral pandemic. Mandatory social distancing of 6 feet or more to avoid each other’s droplets emanating from simple speech, not just sneezing or coughing, makes intimacy practically impossible. Thus, physical closeness became taboo, and avoiding another person’s saliva or body secretions became a must to avoid contracting the virus. Being single was quite a lonely experience during this pandemic!
Entertainment deprivation. Americans are known to thrive on an extensive diet of spectator sports. Going to football, basketball, baseball, or hockey games to root for one’s team is intrinsically American. The pursuit of happiness extends to attending concerts, movies, Broadway shows, theme parks, and cruises with thousands of others. The pandemic ripped all those pleasurable leisure activities from our daily lives, leaving a big hole in people’s lives at the precise time fun activities were needed as a useful diversion from the dismal stress of a pandemic. To make things worse, it is uncertain when (if ever) such group activities will be restored, especially if the pandemic returns with another wave. But optimists would hurry to remind us that the “Roaring 20s” blossomed in the decade following the 1918 Spanish Flu pandemic.
Continue to: Legal system
Legal system. Astounding changes were instigated by the pandemic, such as the release of thousands of inmates, including felons, to avoid the spread of the virus in crowded prisons. For us psychiatrists, the silver lining in that unexpected action is that many of those released were patients with mental illness who were incarcerated because of the lack of hospitals that would take them. The police started issuing citations instead of arresting and jailing violators. Enforcement of the law was welcome when it targeted those who gouged the public for personal profit during the scarcity of masks, sanitizers, or even toilet paper and soap.
Medical practice. In addition to delaying medical care for patients, the freeze on so-called elective surgeries or procedures (many of which were actually necessary) was financially ruinous for physicians. Another regrettable consequence of the pandemic is a drop in pediatric vaccinations because parents were reluctant to take their children to the pediatrician. On a more positive note, the massive switch to telehealth was advantageous for both patients and psychiatrists because this technology is well-suited for psychiatric care. Fortunately, regulations that hampered telepsychiatry practice were substantially loosened or eliminated, and even the usually sacrosanct HIPAA regulations were temporarily sidelined.
Medical research. Both human and animal research came to a screeching halt, and many research assistants were furloughed. Data collection was disrupted, and a generation of scientific and medical discoveries became a casualty of the pandemic.
Medical literature. It was stunning to see how quickly COVID-19 occupied most of the pages of prominent journals. The scholarly articles were frankly quite useful, covering topics ranging from risk factors to early symptoms to treatment and pathophysiology across multiple organs. As with other paradigm shifts, there was an accelerated publication push, sometimes with expedited peer reviews to inform health care workers and the public while the pandemic was still raging. However, a couple of very prominent journals had to retract flawed articles that were hastily published without the usual due diligence and rigorous peer review. The pandemic clearly disrupted the science publishing process.
Travel effects. The steep reduction of flights (by 98%) was financially catastrophic, not only for airline companies but to business travel across the country. However, fewer cars on the road resulted in fewer accidents and deaths, and also reduced pollution. Paradoxically, to prevent crowding in subways, trains, and buses, officials reversed their traditional instructions and advised the public to drive their own cars instead of using public transportation!
Continue to: Heroism of front-line medical personnel
Heroism of front-line medical personnel. Everyone saluted and prayed for the health care professionals working at the bedside of highly infectious patients who needed 24/7 intensive care. Many have died while carrying out the noble but hazardous medical duties. Those heroes deserve our lasting respect and admiration.
The COVID-19 pandemic insidiously permeated and altered every aspect of our complex society and revealed how fragile our “normal lifestyle” really is. It is possible that nothing will ever be the same again, and an uneasy sense of vulnerability will engulf us as we cautiously return to a “new normal.” Even our language has expanded with the lexicon of pandemic terminology (Table). We all pray and hope that this plague never returns. And let’s hope one or more vaccines are developed soon so we can manage future recurrences like the annual flu season. In the meantime, keep your masks and sanitizers close by…

Postscript: Shortly after I completed this editorial, the ongoing COVID-19 plague was overshadowed by the scourge of racism, with massive protests, at times laced by violence, triggered by the death of a black man in custody of the police, under condemnable circumstances. The COVID-19 pandemic and the necessary social distancing it requires were temporarily ignored during the ensuing protests. The combined effect of those overlapping scourges are jarring to the country’s psyche, complicating and perhaps sabotaging the social recovery from the pandemic.
New-onset psychosis while being treated for coronavirus
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.
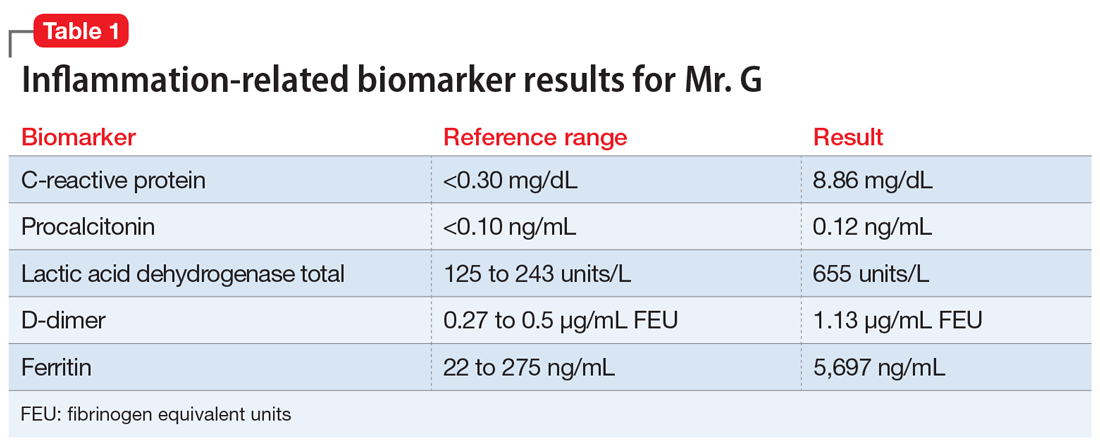
Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.
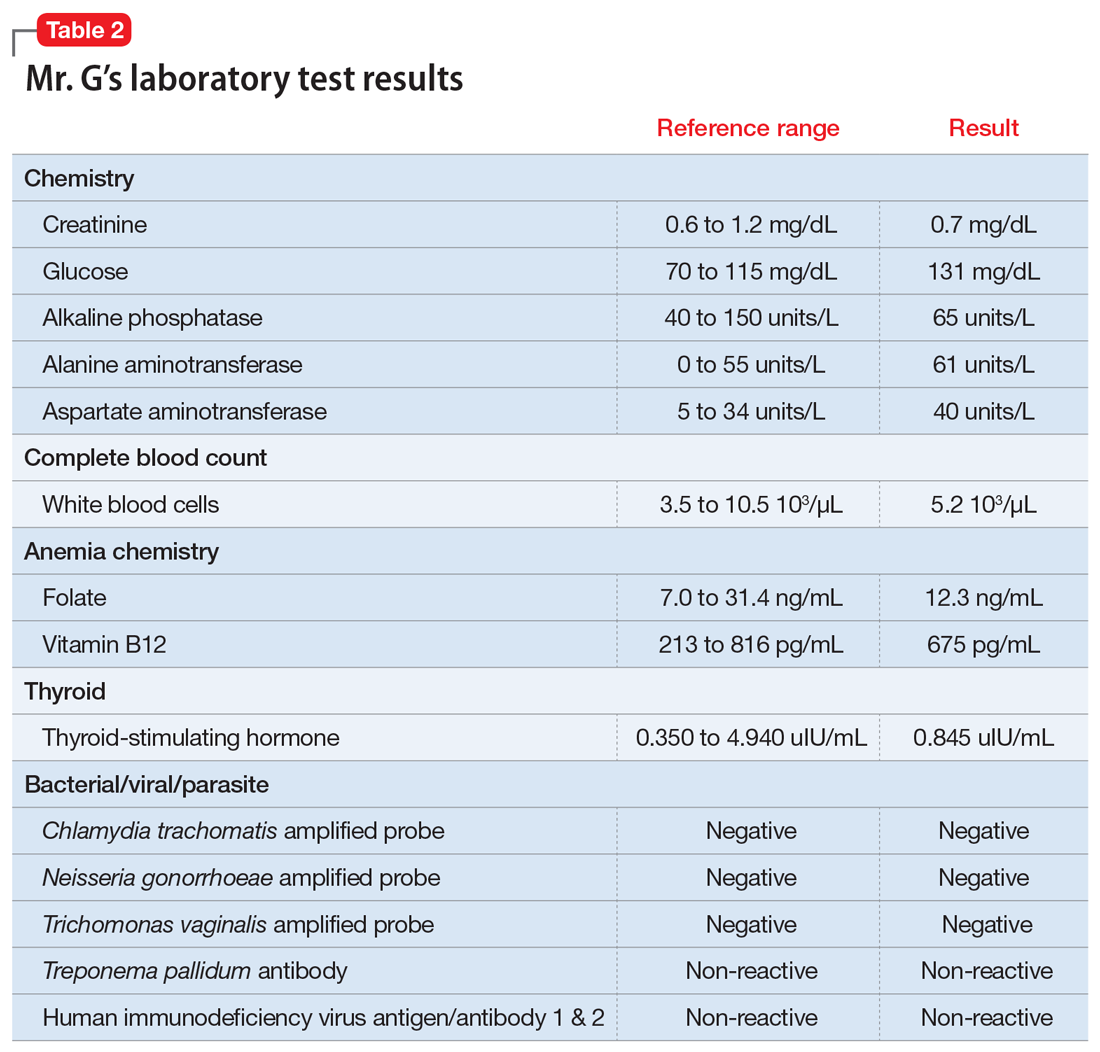
Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
CASE Agitated, psychotic, and COVID-19–positive
Mr. G, age 56, is brought to the emergency department (ED) by emergency medical services (EMS) after his girlfriend reports that he was trying to climb into the “fiery furnace” to “burn the devil within him.” Mr. G had recently tested positive for coronavirus disease 2019 (COVID-19) via polymerase chain reaction and had been receiving treatment for it. In the ED, he is distressed and repeatedly exclaims, “The devil is alive!” He insists on covering himself with blankets, despite diaphoresis and soaking through his clothing within minutes. Because he does not respond to attempted redirection, the ED clinicians administer a single dose of IM haloperidol, 2 mg, for agitation.
HISTORY Multiple ED visits and hospitalizations
Mr. G, who has no known psychiatric history, lives with his girlfriend of 10 years. His medical history includes chronic obstructive pulmonary disease and prostate cancer. In 2015, he had a radical prostatectomy, without chemotherapy. His social history includes childhood neglect, which prompted him to leave home when he was a teenager. Mr. G had earned his general education development certificate and worked at a small retail store.
Mr. G had no previous history of mental health treatment per self-report, collateral information from his girlfriend, and chart review. He reported no known family psychiatric history. He did not endorse past psychiatric admissions or suicide attempts, nor previous periods of mania, depression, or psychosis. He said he used illicit substances as a teen, but denied using alcohol, tobacco products, or illicit substances in the past 20 years.
Mr. G recently had multiple ED visits and hospitalizations due to ongoing signs and symptoms associated with his COVID-19 diagnosis, primarily worsening shortness of breath and cough. Eleven days before EMS brought him to the ED at his girlfriend’s request, Mr. G had presented to the ED with chief complaints of shortness of breath and dry cough (Day 0). He reported that he had been “running a fever” for 2 days. In the ED, his initial vital signs were notable only for a temperature of 100.9°F (38.28°C). He was diagnosed with “acute viral syndrome” and received 1 dose of IV ceftriaxone, 2 g, and IV azithromycin, 500 mg. On Day 2, the ED clinicians prescribed a 4-day course of oral azithromycin, 250 mg/d, and discharged him home.
On Day 3, Mr. G returned to the ED with similar complaints—congestion and productive cough. He tested positive for COVID-19, and the ED discharged him home with quarantine instructions. Hours later, he returned to the ED via EMS with chief complaints of chest pain, diarrhea, and myalgias. He was prescribed a 5-day course ofoseltamivir, 75 mg twice daily, and azithromycin, 250 mg/d. The ED again discharged him home.
On Day 4, Mr. G returned to the ED for a fourth time. His chief complaint was worsening shortness of breath. His oxygen saturation was 94% on room air; it improved to 96% on 2 L of oxygen. His chest X-ray showed diffuse reticulonodular opacities throughout his bilateral lung fields and increased airspace opacification in the bilateral lower lobes. The ED admitted Mr. G to an internal medicine unit, where the primary treatment team enrolled him in a clinical trial. As part of the trial, Mr. G received hydroxychloroquine, 400 mg, on Day 4 and Day 5. The placebo-controlled component of the trial involved Mr. G receiving daily infusions of either remdesivir or placebo on Day 6 through Day 8. On Day 8, Mr. G was discharged home.
On Day 9, Mr. G returned to the ED with a chief complaint that his “thermometer wasn’t working” at home. The ED readmitted him to the internal medicine unit. On Day 9 through Day 11, Mr. G received daily doses of
Continue to: During the second hospitalization...
During the second hospitalization, nursing staff reported that Mr. G seemed religiously preoccupied and once reported seeing angels and demons. He was observed sitting in a chair praying to Allah that he would “come in on a horse to chop all the workers’ heads off.”
On Day 11, Mr. G was discharged home. Later that evening, the EMS brought him back in the ED due to his girlfriend’s concerns about his mental state.
EVALUATION Talks to God
On Day 12, psychiatry is consulted to evaluate Mr. G’s new-onset psychosis. Mr. G is alert and oriented to person, place, and time. His speech is loud, though the amount and rate are unremarkable. He displays no psychomotor agitation. His thought process is tangential and focuses on religious themes, specifically referring to Islam. He reports auditory hallucinations of God speaking directly to him. Mr. G states, “I am here because of a miraculous transformation from death back to life. Do you believe in God? Which God do you believe in? There are 2 Gods and only one of them is the true God. He is the God of all the 7 heavens and His true name is Allah, only one God, one faith. Allah is a ball of energy.”
Mr. G’s girlfriend provides collateral information that Mr. G had been raised Christian but was not religious as an adult. She says that he had never spoken about being Muslim. She adds that she had never known him to speak much about religion.
[polldaddy:10572249]
The authors’ observations
The etiology of new-onset psychosis can be related to several factors, including primary psychiatric illnesses, use of illicit substances, sequelae of general medical conditions, or adverse effects of prescribed medications. We considered each of these in the differential diagnosis for Mr. G.
Continue to: Psychiatric illness or illicit substance use
Psychiatric illness or illicit substance use. Because Mr. G was 56 years old and had no known psychiatric history or family psychiatric history, a primary psychiatric illness seemed less likely. Substance-induced psychosis related to illicit substance use also seemed unlikely because he denied using illicit substances, and an expanded urine drug screen was negative.

Psychosis due to a general medical condition. Results from Mr. G’s laboratory workup show marked elevation in multiple inflammation-related biomarkers (Table 1), consistent with the inflammatory profile seen with COVID-19 infection. However, results from several laboratory tests for potential etiologies of new-onset psychosis due to a general medical condition were negative (Table 2). Based on Mr. G’s history of prostate cancer, we considered the possibility of metastatic space-occupying lesions of the brain; however, Mr. G’s head CT showed no acute intracranial abnormalities. Another possible etiology we considered was COVID-19–induced encephalitis; however, Mr. G’s brain MRI with and without contrast showed no evidence of acute or chronic intracranial changes.

Medication-induced psychosis. After largely ruling out primary psychiatric illnesses, illicit substances, and sequelae of general medical conditions, we turned our attention to prescribed medications as a potential etiology of Mr. G’s new-onset psychosis. During his initial hospitalization, Mr. G had been prescribed 2 doses of hydroxychloroquine, 400 mg, to treat his diagnosis of COVID-19. Because none of the other medications he received were reported to have neuropsychiatric adverse effects, including psychosis, hydroxychloroquine-induced psychosis was therefore the primary team’s working diagnosis.
EVALUATION Request to leave AMA
On Day 13, Mr. G requests to leave the hospital against medical advice (AMA). Until this point, he had voluntarily remained in the hospital, which he repeatedly referred to as “Heaven.” When asked to describe his medical condition, Mr. G replies, “God told me my condition is far beyond man’s understanding.” He denies that he is positive for COVID-19. He states, “I am cured, and the real fight has just begun.”
At the recommendation of the psychiatry consultation-liaison (C-L) service, the primary treatment team determines that Mr. G does not have capacity to leave AMA. The team is concerned that because of his psychotic symptoms, Mr. G would be unable to understand and follow his quarantine instructions. He remains hospitalized on a medical hold.
Continue to: The authors' observations
The authors’ observations
One important consideration this case highlighted was potential third-party responsibility clinicians and hospital systems may face if they discharge a patient with a communicable illness who is unable to follow precautions based on a psychiatric condition.1 That concern was based on Mr. G’s reported desire to pursue missions “beyond man’s understanding,” which he felt compelled to complete, and which could unnecessarily place the public at risk. The psychiatry C-L service consulted the local health department and conferred with the hospital’s legal representatives, who agreed with the plan to keep Mr. G in the hospital for his safety as well as for the public’s safety.
TREATMENT Oral haloperidol
The psychiatry C-L service recommends initiating an antipsychotic. On Day 13, Mr. G starts oral haloperidol, 2.5 mg twice a day, to address his ongoing psychotic symptoms. On Day 14, the treatment team increases the dosage to 5 mg twice a day. Mr. G tolerates the haloperidol and gradually begins to improve. He demonstrates improved sleep, normal speech volume, less religious preoccupation, and a considerably improved understanding of his medical condition.
The authors’ observations
Mr. G’s initial psychiatric evaluation demonstrated an acute onset of psychotic symptoms, without evidence of delirium. Psychosis secondary to a general medical condition (such as COVID-19) and hydroxychloroquine-induced psychotic disorder topped our initial considerations in the differential diagnosis of this case. While the exact neuropsychiatric sequelae of COVID-19 are not yet clear, previous experiences with viral pandemics and case studies from the current pandemic demonstrate a wide variety of possible neuropsychiatric manifestations. Mood symptoms, psychosis, and encephalopathy represent some of the neuropsychiatric complications observed with past viral pandemics.2 Neuropsychiatric symptoms may be triggered by the virus itself, or from the host’s immune response to the infection.3 To further complicate matters, neuropsychiatric symptoms may manifest during the acute viral infection, or may surface later, as subacute or chronic neuropsychiatric illness.
Neuropsychiatric adverse events
Mr. G developed psychotic symptoms within the first few days of receiving hydroxychloroquine, which is consistent with the scant literature on this topic.8 Based on the available information, hydroxychloroquine remains the most likely etiology of his new-onset psychotic symptoms. Mr. G’s case is one example of the possible neuropsychiatric presentations clinicians may face while treating a novel viral illness.
Continue to: OUTCOME Homeward-bound
OUTCOME Homeward-bound
By Day 18, Mr. G’s psychotic symptoms have significantly improved. He is able to rationally process information about his COVID-19 diagnosis and the recommended quarantine instructions he needs to follow after discharge. He is cleared by infection control and discharged home to return to living with his girlfriend.
Mr. G attends his follow-up psychiatric appointment remotely 2 weeks after discharge. He reports that since discharge, he has continued taking his prescribed haloperidol, 5 mg twice a day. He demonstrates improved insight into his medical condition, acknowledging his COVID-19–positive status, and confirms that he has been following quarantine instructions. He does not report ongoing auditory or visual hallucinations, and is no longer religiously preoccupied. He says he is looking forward to being medically cleared to return to work.
The authors’ observations
This case highlights the need for prospective, longitudinal screening and monitoring of neuropsychiatric symptoms as part of the public health response to COVID-19. The case also highlights the importance of careful monitoring for adverse events, including neuropsychiatric symptoms, during clinical trials that involve experimental treatments. The long-term prognosis for individuals such as Mr. G who develop neuropsychiatric symptoms during acute COVID-19 infection remains unknown. Similarly, subacute and chronic neuropsychiatric manifestations that may develop after resolution of acute COVID-19 infection are unknown at this time. However, we can learn from past viral pandemics and anticipate that neuropsychiatric sequelae are likely to occur and should be part of the public health response to the pandemic.
Bottom Line
The coronavirus disease 2019 pandemic provides multiple clinical challenges pertinent to psychiatry. Neuropsychiatric symptoms may manifest from delirium, viral infection, host immune response, or adverse reactions to experimental treatments. These potential neuropsychiatric symptoms may complicate medical treatment. They can also raise important ethical and legal considerations, such as weighing patient autonomy vs third-party responsibility to the public at large.
Related Resources
- Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. 2020. doi: 10.1016/j.psym.2020.05.012.
- Vlessides M. COVID-19 and psychosis: is there a link? Medscape Medical News. https://www.medscape.com/viewarticle/930224. Published May 8, 2020.
Drug Brand Names
Azithromycin • Zithromax
Ceftriaxone • Rocephin
Chloroquine • Aralen
Haloperidol • Haldol
Hydroxychloroquine • Plaquenil
Levofloxacin • Levaquin
Oseltamivir • Tamiflu
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.
1. Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. [published online ahead of print, May 8, 2020]. J Am Acad Psychiatry Law.
2. Menninger Ka. Psychoses associated with influenza: I. general data: statistical analysis. JAMA. 1919;72(4):235-241.
3. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity. 2020. doi:10.1016/j.bbi.2020.04.027.
4. Alkadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385-391.
5. Bogaczewicz A, Sobów T. Psychiatric adverse effects of chloroquine. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-114.
6. Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends. 2020;14(2):139-143.
7. Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283.
8. Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics. 2014;55(4):409-413.