User login
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the extremely common presence of dangerous fentanyl in opioids and other drugs, a new study finds. More than 95% said they’d use the inexpensive strips again.
said study lead author addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
Researchers in Vancouver distributed take-home fentanyl test strip kits at 10 sites that allow users to test their illicit drugs. The 218 participants performed 1,680 tests, mainly (73%) for opioids, over 3 months in 2019. Of the participants, 61% were male, and the average age was 36 (interquartile range, 29-47). About 30% described themselves as indigenous Canadians (First Nations).
About 90% of the opioid samples tested at home were positive for fentanyl, about the same level as samples tested at clinics. Fentanyl is very potent and linked to the huge rise in overdose deaths in the United States.
Fentanyl test strips aren’t new. According to the Harm Reduction Coalition, they originally were developed to detect fentanyl in urine samples but were jury-rigged in Vancouver to work on samples of illicit drugs. “We literally just repurposed it,” Dr. Klaire said. “It’s the same strip.”
Users test their drugs by dissolving a small sample in water. Then then dip the test strip, which provides readings similar to those in a pregnancy test. If a sample turns up positive for fentanyl, Dr. Klaire said, users may discard the drug or “be more careful with it.”
When asked what they would do if a sample turned up positive, 27% said they’d make a “positive change,” such as using less or using more slowly (n = 45) or making sure that someone else is present in case of an overdose (n = 26). But most, 71%, reported no change in behavior.
Previously, researchers in Rhode Island and North Carolina also found that some users adopted safer behaviors – such as throwing out their drugs or using less often – after testing their drugs with the strips.
The strips cost about 75 cents, Dr. Klaire said.
Harm-reduction strategies are controversial, and fentanyl test strips aren’t any exemption. “The entire approach is based on the premise that a drug user poised to use a drug is making rational choices, is weighing pros and cons, and is thinking completely logically about his or her drug use. Based on my clinical experience, I know this could not be further from the truth,” wrote Elinore F. McCance-Katz, MD, PhD, assistant secretary for Mental Health and Substance Use with the Department of Health & Human Services, in a 2018 blog post.
But Dr. Klaire said the patients in the new study are highly dependent on opioids. “The drug supply is heavily contaminated [with fentanyl],” he said, “but even when people know it’s contaminated, they still need to go ahead and use it.”
In an interview, epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., who has conducted fentanyl test strip research, called the study results “compelling.”
“The researchers found that the fentanyl test strips had a very high level of acceptability – over 95% said they would use the strips again – which is remarkably similar to what we found in our work here in Rhode Island,” he said. “Taken together, these studies show that take-home test strips are a feasible, acceptable, and effective strategy for people who use drugs to reduce their risk of fentanyl overdose.”
He added that “fentanyl test strips help people make more informed decisions about their drug use and reducing their risk of overdose.”
However, he said, “one of important limitations of the strips is that they do not detect all contaminants that put persons at risk of overdose. Just because a test result is negative does not mean that the drug is 100% safe.”
Kimberly Sue, MD, PhD, medical director of the National Harm Reduction Coalition, said in an interview that the research is “important,” but noted that many drug users already have been using fentanyl test strips on their own. “We should be focusing on investing in variety of other interventions that could keep more people safe against nonfatal and fatal opioid overdoses, including structural interventions such as safe supply, housing and community with appropriate supports, low barrier access to medication for opioid use disorder, and safe consumption spaces,” she said.
No study funding was reported. Dr. Klaire disclosed participating in a research fellowship and a research in addiction medical scholars program, both funded by the National Institute of Drug Abuse. Dr. Sue reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with one of the coauthors of the Vancouver study.
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the extremely common presence of dangerous fentanyl in opioids and other drugs, a new study finds. More than 95% said they’d use the inexpensive strips again.
said study lead author addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
Researchers in Vancouver distributed take-home fentanyl test strip kits at 10 sites that allow users to test their illicit drugs. The 218 participants performed 1,680 tests, mainly (73%) for opioids, over 3 months in 2019. Of the participants, 61% were male, and the average age was 36 (interquartile range, 29-47). About 30% described themselves as indigenous Canadians (First Nations).
About 90% of the opioid samples tested at home were positive for fentanyl, about the same level as samples tested at clinics. Fentanyl is very potent and linked to the huge rise in overdose deaths in the United States.
Fentanyl test strips aren’t new. According to the Harm Reduction Coalition, they originally were developed to detect fentanyl in urine samples but were jury-rigged in Vancouver to work on samples of illicit drugs. “We literally just repurposed it,” Dr. Klaire said. “It’s the same strip.”
Users test their drugs by dissolving a small sample in water. Then then dip the test strip, which provides readings similar to those in a pregnancy test. If a sample turns up positive for fentanyl, Dr. Klaire said, users may discard the drug or “be more careful with it.”
When asked what they would do if a sample turned up positive, 27% said they’d make a “positive change,” such as using less or using more slowly (n = 45) or making sure that someone else is present in case of an overdose (n = 26). But most, 71%, reported no change in behavior.
Previously, researchers in Rhode Island and North Carolina also found that some users adopted safer behaviors – such as throwing out their drugs or using less often – after testing their drugs with the strips.
The strips cost about 75 cents, Dr. Klaire said.
Harm-reduction strategies are controversial, and fentanyl test strips aren’t any exemption. “The entire approach is based on the premise that a drug user poised to use a drug is making rational choices, is weighing pros and cons, and is thinking completely logically about his or her drug use. Based on my clinical experience, I know this could not be further from the truth,” wrote Elinore F. McCance-Katz, MD, PhD, assistant secretary for Mental Health and Substance Use with the Department of Health & Human Services, in a 2018 blog post.
But Dr. Klaire said the patients in the new study are highly dependent on opioids. “The drug supply is heavily contaminated [with fentanyl],” he said, “but even when people know it’s contaminated, they still need to go ahead and use it.”
In an interview, epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., who has conducted fentanyl test strip research, called the study results “compelling.”
“The researchers found that the fentanyl test strips had a very high level of acceptability – over 95% said they would use the strips again – which is remarkably similar to what we found in our work here in Rhode Island,” he said. “Taken together, these studies show that take-home test strips are a feasible, acceptable, and effective strategy for people who use drugs to reduce their risk of fentanyl overdose.”
He added that “fentanyl test strips help people make more informed decisions about their drug use and reducing their risk of overdose.”
However, he said, “one of important limitations of the strips is that they do not detect all contaminants that put persons at risk of overdose. Just because a test result is negative does not mean that the drug is 100% safe.”
Kimberly Sue, MD, PhD, medical director of the National Harm Reduction Coalition, said in an interview that the research is “important,” but noted that many drug users already have been using fentanyl test strips on their own. “We should be focusing on investing in variety of other interventions that could keep more people safe against nonfatal and fatal opioid overdoses, including structural interventions such as safe supply, housing and community with appropriate supports, low barrier access to medication for opioid use disorder, and safe consumption spaces,” she said.
No study funding was reported. Dr. Klaire disclosed participating in a research fellowship and a research in addiction medical scholars program, both funded by the National Institute of Drug Abuse. Dr. Sue reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with one of the coauthors of the Vancouver study.
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the extremely common presence of dangerous fentanyl in opioids and other drugs, a new study finds. More than 95% said they’d use the inexpensive strips again.
said study lead author addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
Researchers in Vancouver distributed take-home fentanyl test strip kits at 10 sites that allow users to test their illicit drugs. The 218 participants performed 1,680 tests, mainly (73%) for opioids, over 3 months in 2019. Of the participants, 61% were male, and the average age was 36 (interquartile range, 29-47). About 30% described themselves as indigenous Canadians (First Nations).
About 90% of the opioid samples tested at home were positive for fentanyl, about the same level as samples tested at clinics. Fentanyl is very potent and linked to the huge rise in overdose deaths in the United States.
Fentanyl test strips aren’t new. According to the Harm Reduction Coalition, they originally were developed to detect fentanyl in urine samples but were jury-rigged in Vancouver to work on samples of illicit drugs. “We literally just repurposed it,” Dr. Klaire said. “It’s the same strip.”
Users test their drugs by dissolving a small sample in water. Then then dip the test strip, which provides readings similar to those in a pregnancy test. If a sample turns up positive for fentanyl, Dr. Klaire said, users may discard the drug or “be more careful with it.”
When asked what they would do if a sample turned up positive, 27% said they’d make a “positive change,” such as using less or using more slowly (n = 45) or making sure that someone else is present in case of an overdose (n = 26). But most, 71%, reported no change in behavior.
Previously, researchers in Rhode Island and North Carolina also found that some users adopted safer behaviors – such as throwing out their drugs or using less often – after testing their drugs with the strips.
The strips cost about 75 cents, Dr. Klaire said.
Harm-reduction strategies are controversial, and fentanyl test strips aren’t any exemption. “The entire approach is based on the premise that a drug user poised to use a drug is making rational choices, is weighing pros and cons, and is thinking completely logically about his or her drug use. Based on my clinical experience, I know this could not be further from the truth,” wrote Elinore F. McCance-Katz, MD, PhD, assistant secretary for Mental Health and Substance Use with the Department of Health & Human Services, in a 2018 blog post.
But Dr. Klaire said the patients in the new study are highly dependent on opioids. “The drug supply is heavily contaminated [with fentanyl],” he said, “but even when people know it’s contaminated, they still need to go ahead and use it.”
In an interview, epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., who has conducted fentanyl test strip research, called the study results “compelling.”
“The researchers found that the fentanyl test strips had a very high level of acceptability – over 95% said they would use the strips again – which is remarkably similar to what we found in our work here in Rhode Island,” he said. “Taken together, these studies show that take-home test strips are a feasible, acceptable, and effective strategy for people who use drugs to reduce their risk of fentanyl overdose.”
He added that “fentanyl test strips help people make more informed decisions about their drug use and reducing their risk of overdose.”
However, he said, “one of important limitations of the strips is that they do not detect all contaminants that put persons at risk of overdose. Just because a test result is negative does not mean that the drug is 100% safe.”
Kimberly Sue, MD, PhD, medical director of the National Harm Reduction Coalition, said in an interview that the research is “important,” but noted that many drug users already have been using fentanyl test strips on their own. “We should be focusing on investing in variety of other interventions that could keep more people safe against nonfatal and fatal opioid overdoses, including structural interventions such as safe supply, housing and community with appropriate supports, low barrier access to medication for opioid use disorder, and safe consumption spaces,” she said.
No study funding was reported. Dr. Klaire disclosed participating in a research fellowship and a research in addiction medical scholars program, both funded by the National Institute of Drug Abuse. Dr. Sue reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with one of the coauthors of the Vancouver study.
FROM CPDD 2020
Cushing’s and COVID-19: Nontraditional symptoms keys to assessment, treatments
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
Community programs improve psychosis outcomes
Community-based services that tap into local environments not only reduce the duration of untreated psychosis (DUP) but also provide improved long-term outcomes for patients with first-episode psychosis (FEP), results of two new studies show.
In the first study, investigators led by Vinod Srihari, MD, director of specialized treatment early in psychosis at Yale University, New Haven, Conn., developed a program to reduce DUP to complement their first-episode service (FES).
Through a combination of mass media and social media campaigns, outreach events with local professionals, and rapid triage, the team was able to nearly halve the time from diagnosis to initiation of antipsychotic treatment.
In the second study, a team led by Delbert G. Robinson, MD, of Hofstra University, Hempstead, N.Y., conducted a 5-year follow-up of RAISE-ETP, the first U.S. randomized trial to compare a 2-year comprehensive early intervention service (EIS) with usual care.
These trial results showed that, among more than 400 FEP patients, the EIS significantly improved both symptoms and quality of life and reduced inpatient days in comparison with standard care.
The research was scheduled to be presented at the Congress of the Schizophrenia International Research Society (SIRS) 2020, but the meeting was canceled because of the coronavirus pandemic.
Norwegian model
Dr. Srihari and colleagues note that a specialized treatment early in psychosis (STEP), which delivers a specialty team–based FES, was established at their institution in 2006.
However, in a bid to reduce DUP, in 2015 they launched MindMap, a 4-year early-detection campaign based on the Scandinavian TIPS Early Detection in Psychosis Study.
Dr. Srihari said in an interview that they visited the team that developed the TIPS program in Norway “to try to understand what elements of their approach had resulted in a successful reduction of DUP.”
He pointed out that the health care system in Norway has “more reliable pathways to care, with an ability to route people in more predictable ways from primary care to secondary care, and so on.”
In the United States, “there’s no expectation that people will go through a primary care provider,” he said. He noted that patients “make their way to specialty care in many different ways.”
Dr. Srihari said, “The other change we realized we’d have to make was that, since TIPS had been completed many years back, the media environment had changed substantially.
“At the time that TIPS was done, there was no such thing as social media, whereas when we began to think about designing our campaign, we thought social media would be a very efficient and also cost-effective way to target young people.”
MindMap, which covered a 10-town catchment area that has a population of 400,000, targeted both demand- and supply-side aspects of DUP. The former focused on delays in identifying illness and help-seeking, and the latter concentrated on referral and treatment access delays.
The researchers used a combination of mass media and social media messaging, professional detailing, and the rapid triage of referrals.
The media campaign included Facebook, Twitter, and other social media platforms that allowed the team to “target individuals in a somewhat more fine-grained way than mass media allows,” Dr. Srihari said. They focused on individuals in a particular age range and geographic location.
The professional detailing encompassed mental health agencies, emergency departments, and inpatient units, as well as colleges, college counseling centers, high schools, and police departments. The team hosted meetings, “often at local restaurants, where we provided a meal and provided some general education about what our mission was,” said Dr. Srihari. The researchers followed up these meetings with more in-person visits.
“The third arm was finding ways to basically eliminate any kind of a waiting time at our front door, such as ensuring that transportation issues were circumvented, in addition to cutting through any ambivalence they might have about finally making the leap to come to the center.”
More rapid treatment
Over the course of the baseline year and the 4 years of the MindMap program, almost 1,500 individuals were assessed. Of these, approximately 200 were eligible, and almost all were enrolled.
The researchers measured DUP at two time points from the onset of psychosis – the initiation of antipsychotic treatment (DUP1) and the initiation of FES care. Across the study period, they found that DUP1 fell significantly between the pre- and postprogram assessments, from 329 days to 185 days (P = .03). By contrast, there was no change over the same period for the Prevention and Recovery in Early Psychosis FES program in Boston, which served as a comparator.
There was also a cumulative effect on DUP, with each year of the 4-year program associated with a 46-day reduction in DUP1. However, the significant reduction was restricted to the third quintile of DUP1 and was not found in the other quintiles of DUP1 or for DUP2, despite all measures showing a consistent trend for reduction over time.
Dr. Srihari acknowledges that the team was “disappointed” that DUP2 did not fall significantly in their study. He suggested, “It might take longer for agencies to change their workloads and refer patients to STEP, which is what ended up resulting in the DUP2 not dropping as quickly.”
To see whether there was indeed a time lag in changes to practice, the team conducted an analysis in which they cut out the first year from the results and analyzed only the last 3 years. Then “we do see a decline in DUP2,” he said.
The study’s full results are currently being prepared for publication, and the investigators are considering relaunching the initiative.
“The question we are having now is how to resource the campaign without the research funds and which parts of it we think we can launch sustainably so we can continue the reduction of DUP,” Dr. Srihari said.
Plans may include developing partnerships with local businesses to help fund the media costs and working with the state government to build a learning health care network, which would make it easier for mental health agencies to consult with the team on problematic cases.
“We’re trying to reduce DUP referrals on the supply side by providing this kind of learning health collaborative ... that we also think might be fiscally a more sustainable way to do this vs. what we did in MindMap,” which would be “very expensive” to implement on a statewide basis, Dr. Srihari added.
Long-term benefit
In the second study, Dr. Robinson and colleagues highlight that EISs have been implemented worldwide for FEP patients and have been associated with improved outcomes.
However, these services typically provide care for a limited period, and cross-sectional follow-up studies have identified few advantages in comparison with standard care.
To provide a more robust longitudinal assessment of the ongoing effects of an EIS, the team conducted a 5-year follow-up of the first U.S.-based, multicenter, randomized clinical trial comparing an EIS, NAVIGATE, with usual clinical care in FEP.
RAISE-ETP was conducted at 34 sites across the United States. Seventeen sites provided NAVIGATE to 223 individuals with FEP, and the remaining 17 sites provided usual care to 181 patients.
NAVIGATE, which continued for 2 years, consisted of treatments and services delivered by a coordinated team of providers. Those services included the following:
- Education on schizophrenia and its treatment for patients and their families.
- Symptom and relapse prevention medication, using a computerized decision support system.
- Strategies for illness management building personal resilience.
- A supported employment/education model.
Patients were assessed every 6 months for up to 60 months via a video link using the Heinrichs-Carpenter Quality of Life Scale (QLS) and the Positive and Negative Syndrome Scale (PANSS).
The average age of the participants was 23 years; 78% of those who received NAVIGATE and 66% of those who received usual care were male. The opportunity for each participant to engage in NAVIGATE treatment lasted an average of 33.8 months. The longest was 44.4 months.
Over 5 years, NAVIGATE was associated with a significant improvement over usual care in QLS scores by an average of 13.14 units (P < .001). PANSS scores improved by an average of 7.73 units (P < .002). QLS scores were not affected either by the length of opportunity to participate in NAVIGATE or by DUP, the team reports. Patients who received NAVIGATE also had an average of 2.5 fewer inpatient days, compared with those on usual care (P = .02).
The investigators note that the study “provides compelling evidence of a substantial long-term benefit for FEP treatment with the NAVIGATE EIS, compared with standard care.”
A ‘great message’
Commenting on the findings in an interview, Ragy R. Girgis, MD, associate professor of clinical psychiatry at Columbia University, New York, and the New York State Psychiatric Institute, said
Dr. Girgis, who was not involved in either study, said that the research is “a great message.” He noted that it is “really important for people to know and it’s really important that we’re still doing research in those areas.”
However, he noted that psychosocial interventions such as these “sometimes take a lot of work.” Dr. Girgis said that it is “so easy to just give people a medication” but that approach has its own disadvantages, including adverse effects and sometimes a lack of efficacy.
“Psychosocial interventions, on the other hand, are very well tolerated by people. They are very effective, but they may require a lot more manpower, and in some ways they can also be more expensive.
“So this is a dialectic that we oftentimes have to deal with when we figure out the right balance between psychosocial vs. medication types of treatments,” he said.
STEP has received research funding from the National Institutes of Health and the Patrick and Catherine Weldon Donaghue Medical Research Foundation. RAISE-ETP was funded by the National Institute of Mental Health as part of the Recovery After an Initial Schizophrenia Episode (RAISE) Project. Dr. Gopal is an employee of Janssen Research & Development, and owns stock/equity in Johnson & Johnson. Dr. Girgis has received research support from Genentech, BioAdvantex, Allegran/Forest, and Otsuka, and royalties from Wipf and Stock and Routledge/Taylor and Francis.
A version of this article originally appeared on Medscape.com.
Community-based services that tap into local environments not only reduce the duration of untreated psychosis (DUP) but also provide improved long-term outcomes for patients with first-episode psychosis (FEP), results of two new studies show.
In the first study, investigators led by Vinod Srihari, MD, director of specialized treatment early in psychosis at Yale University, New Haven, Conn., developed a program to reduce DUP to complement their first-episode service (FES).
Through a combination of mass media and social media campaigns, outreach events with local professionals, and rapid triage, the team was able to nearly halve the time from diagnosis to initiation of antipsychotic treatment.
In the second study, a team led by Delbert G. Robinson, MD, of Hofstra University, Hempstead, N.Y., conducted a 5-year follow-up of RAISE-ETP, the first U.S. randomized trial to compare a 2-year comprehensive early intervention service (EIS) with usual care.
These trial results showed that, among more than 400 FEP patients, the EIS significantly improved both symptoms and quality of life and reduced inpatient days in comparison with standard care.
The research was scheduled to be presented at the Congress of the Schizophrenia International Research Society (SIRS) 2020, but the meeting was canceled because of the coronavirus pandemic.
Norwegian model
Dr. Srihari and colleagues note that a specialized treatment early in psychosis (STEP), which delivers a specialty team–based FES, was established at their institution in 2006.
However, in a bid to reduce DUP, in 2015 they launched MindMap, a 4-year early-detection campaign based on the Scandinavian TIPS Early Detection in Psychosis Study.
Dr. Srihari said in an interview that they visited the team that developed the TIPS program in Norway “to try to understand what elements of their approach had resulted in a successful reduction of DUP.”
He pointed out that the health care system in Norway has “more reliable pathways to care, with an ability to route people in more predictable ways from primary care to secondary care, and so on.”
In the United States, “there’s no expectation that people will go through a primary care provider,” he said. He noted that patients “make their way to specialty care in many different ways.”
Dr. Srihari said, “The other change we realized we’d have to make was that, since TIPS had been completed many years back, the media environment had changed substantially.
“At the time that TIPS was done, there was no such thing as social media, whereas when we began to think about designing our campaign, we thought social media would be a very efficient and also cost-effective way to target young people.”
MindMap, which covered a 10-town catchment area that has a population of 400,000, targeted both demand- and supply-side aspects of DUP. The former focused on delays in identifying illness and help-seeking, and the latter concentrated on referral and treatment access delays.
The researchers used a combination of mass media and social media messaging, professional detailing, and the rapid triage of referrals.
The media campaign included Facebook, Twitter, and other social media platforms that allowed the team to “target individuals in a somewhat more fine-grained way than mass media allows,” Dr. Srihari said. They focused on individuals in a particular age range and geographic location.
The professional detailing encompassed mental health agencies, emergency departments, and inpatient units, as well as colleges, college counseling centers, high schools, and police departments. The team hosted meetings, “often at local restaurants, where we provided a meal and provided some general education about what our mission was,” said Dr. Srihari. The researchers followed up these meetings with more in-person visits.
“The third arm was finding ways to basically eliminate any kind of a waiting time at our front door, such as ensuring that transportation issues were circumvented, in addition to cutting through any ambivalence they might have about finally making the leap to come to the center.”
More rapid treatment
Over the course of the baseline year and the 4 years of the MindMap program, almost 1,500 individuals were assessed. Of these, approximately 200 were eligible, and almost all were enrolled.
The researchers measured DUP at two time points from the onset of psychosis – the initiation of antipsychotic treatment (DUP1) and the initiation of FES care. Across the study period, they found that DUP1 fell significantly between the pre- and postprogram assessments, from 329 days to 185 days (P = .03). By contrast, there was no change over the same period for the Prevention and Recovery in Early Psychosis FES program in Boston, which served as a comparator.
There was also a cumulative effect on DUP, with each year of the 4-year program associated with a 46-day reduction in DUP1. However, the significant reduction was restricted to the third quintile of DUP1 and was not found in the other quintiles of DUP1 or for DUP2, despite all measures showing a consistent trend for reduction over time.
Dr. Srihari acknowledges that the team was “disappointed” that DUP2 did not fall significantly in their study. He suggested, “It might take longer for agencies to change their workloads and refer patients to STEP, which is what ended up resulting in the DUP2 not dropping as quickly.”
To see whether there was indeed a time lag in changes to practice, the team conducted an analysis in which they cut out the first year from the results and analyzed only the last 3 years. Then “we do see a decline in DUP2,” he said.
The study’s full results are currently being prepared for publication, and the investigators are considering relaunching the initiative.
“The question we are having now is how to resource the campaign without the research funds and which parts of it we think we can launch sustainably so we can continue the reduction of DUP,” Dr. Srihari said.
Plans may include developing partnerships with local businesses to help fund the media costs and working with the state government to build a learning health care network, which would make it easier for mental health agencies to consult with the team on problematic cases.
“We’re trying to reduce DUP referrals on the supply side by providing this kind of learning health collaborative ... that we also think might be fiscally a more sustainable way to do this vs. what we did in MindMap,” which would be “very expensive” to implement on a statewide basis, Dr. Srihari added.
Long-term benefit
In the second study, Dr. Robinson and colleagues highlight that EISs have been implemented worldwide for FEP patients and have been associated with improved outcomes.
However, these services typically provide care for a limited period, and cross-sectional follow-up studies have identified few advantages in comparison with standard care.
To provide a more robust longitudinal assessment of the ongoing effects of an EIS, the team conducted a 5-year follow-up of the first U.S.-based, multicenter, randomized clinical trial comparing an EIS, NAVIGATE, with usual clinical care in FEP.
RAISE-ETP was conducted at 34 sites across the United States. Seventeen sites provided NAVIGATE to 223 individuals with FEP, and the remaining 17 sites provided usual care to 181 patients.
NAVIGATE, which continued for 2 years, consisted of treatments and services delivered by a coordinated team of providers. Those services included the following:
- Education on schizophrenia and its treatment for patients and their families.
- Symptom and relapse prevention medication, using a computerized decision support system.
- Strategies for illness management building personal resilience.
- A supported employment/education model.
Patients were assessed every 6 months for up to 60 months via a video link using the Heinrichs-Carpenter Quality of Life Scale (QLS) and the Positive and Negative Syndrome Scale (PANSS).
The average age of the participants was 23 years; 78% of those who received NAVIGATE and 66% of those who received usual care were male. The opportunity for each participant to engage in NAVIGATE treatment lasted an average of 33.8 months. The longest was 44.4 months.
Over 5 years, NAVIGATE was associated with a significant improvement over usual care in QLS scores by an average of 13.14 units (P < .001). PANSS scores improved by an average of 7.73 units (P < .002). QLS scores were not affected either by the length of opportunity to participate in NAVIGATE or by DUP, the team reports. Patients who received NAVIGATE also had an average of 2.5 fewer inpatient days, compared with those on usual care (P = .02).
The investigators note that the study “provides compelling evidence of a substantial long-term benefit for FEP treatment with the NAVIGATE EIS, compared with standard care.”
A ‘great message’
Commenting on the findings in an interview, Ragy R. Girgis, MD, associate professor of clinical psychiatry at Columbia University, New York, and the New York State Psychiatric Institute, said
Dr. Girgis, who was not involved in either study, said that the research is “a great message.” He noted that it is “really important for people to know and it’s really important that we’re still doing research in those areas.”
However, he noted that psychosocial interventions such as these “sometimes take a lot of work.” Dr. Girgis said that it is “so easy to just give people a medication” but that approach has its own disadvantages, including adverse effects and sometimes a lack of efficacy.
“Psychosocial interventions, on the other hand, are very well tolerated by people. They are very effective, but they may require a lot more manpower, and in some ways they can also be more expensive.
“So this is a dialectic that we oftentimes have to deal with when we figure out the right balance between psychosocial vs. medication types of treatments,” he said.
STEP has received research funding from the National Institutes of Health and the Patrick and Catherine Weldon Donaghue Medical Research Foundation. RAISE-ETP was funded by the National Institute of Mental Health as part of the Recovery After an Initial Schizophrenia Episode (RAISE) Project. Dr. Gopal is an employee of Janssen Research & Development, and owns stock/equity in Johnson & Johnson. Dr. Girgis has received research support from Genentech, BioAdvantex, Allegran/Forest, and Otsuka, and royalties from Wipf and Stock and Routledge/Taylor and Francis.
A version of this article originally appeared on Medscape.com.
Community-based services that tap into local environments not only reduce the duration of untreated psychosis (DUP) but also provide improved long-term outcomes for patients with first-episode psychosis (FEP), results of two new studies show.
In the first study, investigators led by Vinod Srihari, MD, director of specialized treatment early in psychosis at Yale University, New Haven, Conn., developed a program to reduce DUP to complement their first-episode service (FES).
Through a combination of mass media and social media campaigns, outreach events with local professionals, and rapid triage, the team was able to nearly halve the time from diagnosis to initiation of antipsychotic treatment.
In the second study, a team led by Delbert G. Robinson, MD, of Hofstra University, Hempstead, N.Y., conducted a 5-year follow-up of RAISE-ETP, the first U.S. randomized trial to compare a 2-year comprehensive early intervention service (EIS) with usual care.
These trial results showed that, among more than 400 FEP patients, the EIS significantly improved both symptoms and quality of life and reduced inpatient days in comparison with standard care.
The research was scheduled to be presented at the Congress of the Schizophrenia International Research Society (SIRS) 2020, but the meeting was canceled because of the coronavirus pandemic.
Norwegian model
Dr. Srihari and colleagues note that a specialized treatment early in psychosis (STEP), which delivers a specialty team–based FES, was established at their institution in 2006.
However, in a bid to reduce DUP, in 2015 they launched MindMap, a 4-year early-detection campaign based on the Scandinavian TIPS Early Detection in Psychosis Study.
Dr. Srihari said in an interview that they visited the team that developed the TIPS program in Norway “to try to understand what elements of their approach had resulted in a successful reduction of DUP.”
He pointed out that the health care system in Norway has “more reliable pathways to care, with an ability to route people in more predictable ways from primary care to secondary care, and so on.”
In the United States, “there’s no expectation that people will go through a primary care provider,” he said. He noted that patients “make their way to specialty care in many different ways.”
Dr. Srihari said, “The other change we realized we’d have to make was that, since TIPS had been completed many years back, the media environment had changed substantially.
“At the time that TIPS was done, there was no such thing as social media, whereas when we began to think about designing our campaign, we thought social media would be a very efficient and also cost-effective way to target young people.”
MindMap, which covered a 10-town catchment area that has a population of 400,000, targeted both demand- and supply-side aspects of DUP. The former focused on delays in identifying illness and help-seeking, and the latter concentrated on referral and treatment access delays.
The researchers used a combination of mass media and social media messaging, professional detailing, and the rapid triage of referrals.
The media campaign included Facebook, Twitter, and other social media platforms that allowed the team to “target individuals in a somewhat more fine-grained way than mass media allows,” Dr. Srihari said. They focused on individuals in a particular age range and geographic location.
The professional detailing encompassed mental health agencies, emergency departments, and inpatient units, as well as colleges, college counseling centers, high schools, and police departments. The team hosted meetings, “often at local restaurants, where we provided a meal and provided some general education about what our mission was,” said Dr. Srihari. The researchers followed up these meetings with more in-person visits.
“The third arm was finding ways to basically eliminate any kind of a waiting time at our front door, such as ensuring that transportation issues were circumvented, in addition to cutting through any ambivalence they might have about finally making the leap to come to the center.”
More rapid treatment
Over the course of the baseline year and the 4 years of the MindMap program, almost 1,500 individuals were assessed. Of these, approximately 200 were eligible, and almost all were enrolled.
The researchers measured DUP at two time points from the onset of psychosis – the initiation of antipsychotic treatment (DUP1) and the initiation of FES care. Across the study period, they found that DUP1 fell significantly between the pre- and postprogram assessments, from 329 days to 185 days (P = .03). By contrast, there was no change over the same period for the Prevention and Recovery in Early Psychosis FES program in Boston, which served as a comparator.
There was also a cumulative effect on DUP, with each year of the 4-year program associated with a 46-day reduction in DUP1. However, the significant reduction was restricted to the third quintile of DUP1 and was not found in the other quintiles of DUP1 or for DUP2, despite all measures showing a consistent trend for reduction over time.
Dr. Srihari acknowledges that the team was “disappointed” that DUP2 did not fall significantly in their study. He suggested, “It might take longer for agencies to change their workloads and refer patients to STEP, which is what ended up resulting in the DUP2 not dropping as quickly.”
To see whether there was indeed a time lag in changes to practice, the team conducted an analysis in which they cut out the first year from the results and analyzed only the last 3 years. Then “we do see a decline in DUP2,” he said.
The study’s full results are currently being prepared for publication, and the investigators are considering relaunching the initiative.
“The question we are having now is how to resource the campaign without the research funds and which parts of it we think we can launch sustainably so we can continue the reduction of DUP,” Dr. Srihari said.
Plans may include developing partnerships with local businesses to help fund the media costs and working with the state government to build a learning health care network, which would make it easier for mental health agencies to consult with the team on problematic cases.
“We’re trying to reduce DUP referrals on the supply side by providing this kind of learning health collaborative ... that we also think might be fiscally a more sustainable way to do this vs. what we did in MindMap,” which would be “very expensive” to implement on a statewide basis, Dr. Srihari added.
Long-term benefit
In the second study, Dr. Robinson and colleagues highlight that EISs have been implemented worldwide for FEP patients and have been associated with improved outcomes.
However, these services typically provide care for a limited period, and cross-sectional follow-up studies have identified few advantages in comparison with standard care.
To provide a more robust longitudinal assessment of the ongoing effects of an EIS, the team conducted a 5-year follow-up of the first U.S.-based, multicenter, randomized clinical trial comparing an EIS, NAVIGATE, with usual clinical care in FEP.
RAISE-ETP was conducted at 34 sites across the United States. Seventeen sites provided NAVIGATE to 223 individuals with FEP, and the remaining 17 sites provided usual care to 181 patients.
NAVIGATE, which continued for 2 years, consisted of treatments and services delivered by a coordinated team of providers. Those services included the following:
- Education on schizophrenia and its treatment for patients and their families.
- Symptom and relapse prevention medication, using a computerized decision support system.
- Strategies for illness management building personal resilience.
- A supported employment/education model.
Patients were assessed every 6 months for up to 60 months via a video link using the Heinrichs-Carpenter Quality of Life Scale (QLS) and the Positive and Negative Syndrome Scale (PANSS).
The average age of the participants was 23 years; 78% of those who received NAVIGATE and 66% of those who received usual care were male. The opportunity for each participant to engage in NAVIGATE treatment lasted an average of 33.8 months. The longest was 44.4 months.
Over 5 years, NAVIGATE was associated with a significant improvement over usual care in QLS scores by an average of 13.14 units (P < .001). PANSS scores improved by an average of 7.73 units (P < .002). QLS scores were not affected either by the length of opportunity to participate in NAVIGATE or by DUP, the team reports. Patients who received NAVIGATE also had an average of 2.5 fewer inpatient days, compared with those on usual care (P = .02).
The investigators note that the study “provides compelling evidence of a substantial long-term benefit for FEP treatment with the NAVIGATE EIS, compared with standard care.”
A ‘great message’
Commenting on the findings in an interview, Ragy R. Girgis, MD, associate professor of clinical psychiatry at Columbia University, New York, and the New York State Psychiatric Institute, said
Dr. Girgis, who was not involved in either study, said that the research is “a great message.” He noted that it is “really important for people to know and it’s really important that we’re still doing research in those areas.”
However, he noted that psychosocial interventions such as these “sometimes take a lot of work.” Dr. Girgis said that it is “so easy to just give people a medication” but that approach has its own disadvantages, including adverse effects and sometimes a lack of efficacy.
“Psychosocial interventions, on the other hand, are very well tolerated by people. They are very effective, but they may require a lot more manpower, and in some ways they can also be more expensive.
“So this is a dialectic that we oftentimes have to deal with when we figure out the right balance between psychosocial vs. medication types of treatments,” he said.
STEP has received research funding from the National Institutes of Health and the Patrick and Catherine Weldon Donaghue Medical Research Foundation. RAISE-ETP was funded by the National Institute of Mental Health as part of the Recovery After an Initial Schizophrenia Episode (RAISE) Project. Dr. Gopal is an employee of Janssen Research & Development, and owns stock/equity in Johnson & Johnson. Dr. Girgis has received research support from Genentech, BioAdvantex, Allegran/Forest, and Otsuka, and royalties from Wipf and Stock and Routledge/Taylor and Francis.
A version of this article originally appeared on Medscape.com.
Lifestyle changes may explain skin lesions in pandemic-era patients
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
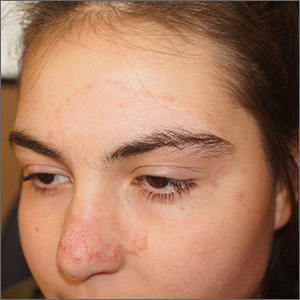
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
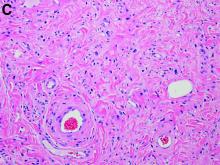
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
Once again, no survival benefit with PCI, surgery in stable CAD
Coronary revascularization does not confer a survival advantage over initial medical therapy in patients with stable ischemic heart disease (SIHD) but reduces unstable angina, according to a new study-level meta-analysis.
Routine upfront revascularization is also associated with less spontaneous myocardial infarction but this is at the cost of increased procedural infarctions, reported lead investigator Sripal Bangalore, MD, of New York University.
“These relationships should be taken into consideration for shared decision-making for the management of patients with stable ischemic heart disease,” he said in a late-breaking trial session at PCR e-Course 2020, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR).
The results, simultaneously published in Circulation, are consistent with last year’s ISCHEMIA trial and other contemporary trials, such as COURAGE, FAME 2, and BARI 2D, that have failed to show a reduction in mortality with revascularization alone in SIHD. Guidelines continue, however, to recommend revascularization to improve survival in SIHD based on trials performed in the 1980s when medical therapy was limited, Dr. Bangalore observed.
The updated meta-analysis included 14 randomized controlled trials, including the aforementioned, and 14,877 patients followed for a weighted mean of 4.5 years. Most trials enrolled patients who had preserved left ventricular function and low symptom burden (Canadian Cardiovascular Society Class I/II).
In the revascularization group, 87.5% of patients underwent any revascularization. Percutaneous coronary intervention (PCI) was the first procedure in 71.3% and bypass surgery the first choice in 16.2%. In eight trials, stents were used in at least 50% of PCI patients; drug-eluting stents were mainly used in FAME 2, ISCHEMIA, and ISCHEMIA-CKD.
In eight trials, statins were used in at least 50% of patients. Nearly 1 in 3 patients (31.9%) treated initially with medical therapy underwent revascularization during follow-up.
Results show no reduction in mortality risk with routine revascularization in the overall analysis (relative risk, 0.99; 95% confidence interval, 0.90-1.09) or when analyzed by whether studies did or did not use stents (P for interaction = .85).
Trial sequential analysis also showed that the cumulative z-curve crossed the futility boundary, “suggesting we have great data to show that there is lack of even a 10% reduction in death with revascularization,” Dr. Bangalore said.
Results were very similar for cardiovascular death (RR, 0.92; 95% CI, 0.80-1.06), including when analyzed by study stent status (P for interaction = .60).
There was no significant reduction in overall MI risk with revascularization, although a borderline significant 11% decrease in MIs was found in the contemporary stent era trials (RR, 0.89; 95% CI, 0.80-0.998).
Revascularization was associated with a 148% increase in the risk of procedural MI (RR, 2.48; 95% CI, 1.86-3.31) but reduced risk of spontaneous MI (RR, 0.76; 95% CI, 0.67-0.85).
Unstable angina was reduced in patients undergoing revascularization (RR, 0.64; 95% CI, 0.45-0.92), driven by a 55% reduction in the contemporary stent era trials. Freedom from angina was also greater with routine revascularization but the difference was modest, Dr. Bangalore said. There was no difference between the two strategies in heart failure or stroke.
“This meta-analysis is well done but really doesn’t change what we already know,” Rasha Al-Lamee, MBBS, of Imperial College, London, said in an interview. “The most important message is that intervention in stable CAD does not change survival. We don’t need to rush to intervene: We have time to plan the best strategy for each patient and to modify our plans based on their response.”
The analysis addresses some of the issues with previous meta-analyses that have included trials that were not strictly stable CAD trials such as SWISSI-2, COMPARE-ACUTE, and DANAMI-3-PRIMULTI, she noted. “However a study like this is only as good as the trials that are included. We must remember that unblinded trials really cannot be used to accurately assess endpoints that are prone to bias such as unstable angina and freedom from angina.”
Following the presentation, dedicated discussant Davide Capodanno, MD, PhD, of the University of Catania (Italy) said, “We have seen beyond any doubt that there is no difference in mortality. For cardiovascular death, it’s pretty much the same. It’s a little bit more mixed and nuanced, the story of myocardial infarction.”
“Additional science is needed to understand the prognostic implications,” he said. “Of course we know that spontaneous myocardial infarction is bad, but I’m not so sure about periprocedural MI. Is this something that is as important as spontaneous myocardial infarction?”
The meta-analysis is the largest ever performed, but there was clinical heterogeneity in the individual studies, especially in the definition of MI, Dr. Capodanno observed. Because of the use of trial-level data rather than patient-level data, the analysis also could not account for adherence to treatment or the effect of stent type or medication dosage.
The MI issue really depends on the trial definition of MI, Dr. Al-Lamee said. “We need long-term follow-up from ISCHEMIA to understand what it means for our patients. While revascularization clearly increases procedural MI rates, it also results in lower spontaneous MI rates with no impact on overall MI or death,” she said. “We will only know if these MIs are important if we see what impact they have in the long term.”
Although the meta-analysis combined data from several decades, it’s likely that the outdated revascularization techniques in the older trials are balanced out by the outdated medical therapy in the same trials, Dr. Al-Lamee observed.
The new findings can certainly be used in patient-physician discussions, with more follow-up from ISCHEMIA to provide additional insights, she said.
“We will of course hear more about the placebo-controlled efficacy of PCI in the blinded ORBITA-2 trial. And I would really like to see some of the older studies of patients and perceptions of the effect of PCI repeated,” Dr. Al-Lamee said. “Now we have more data, are we informing our patients and referrers correctly of the impact of our procedures, and do they truly choose revascularization with a true awareness of what it does and does not do?”
Dr. Bangalore reported grants from the National Heart, Lung, and Blood Institute and Abbott Vascular; and serving on the advisory boards of Abbott Vascular, Biotronik, Meril, SMT, Pfizer, Amgen, and Reata. Dr. Al-Lamee reported speaker’s honorarium from Philips Volcano and Menarini Pharmaceuticals. Dr. Capodanno has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Coronary revascularization does not confer a survival advantage over initial medical therapy in patients with stable ischemic heart disease (SIHD) but reduces unstable angina, according to a new study-level meta-analysis.
Routine upfront revascularization is also associated with less spontaneous myocardial infarction but this is at the cost of increased procedural infarctions, reported lead investigator Sripal Bangalore, MD, of New York University.
“These relationships should be taken into consideration for shared decision-making for the management of patients with stable ischemic heart disease,” he said in a late-breaking trial session at PCR e-Course 2020, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR).
The results, simultaneously published in Circulation, are consistent with last year’s ISCHEMIA trial and other contemporary trials, such as COURAGE, FAME 2, and BARI 2D, that have failed to show a reduction in mortality with revascularization alone in SIHD. Guidelines continue, however, to recommend revascularization to improve survival in SIHD based on trials performed in the 1980s when medical therapy was limited, Dr. Bangalore observed.
The updated meta-analysis included 14 randomized controlled trials, including the aforementioned, and 14,877 patients followed for a weighted mean of 4.5 years. Most trials enrolled patients who had preserved left ventricular function and low symptom burden (Canadian Cardiovascular Society Class I/II).
In the revascularization group, 87.5% of patients underwent any revascularization. Percutaneous coronary intervention (PCI) was the first procedure in 71.3% and bypass surgery the first choice in 16.2%. In eight trials, stents were used in at least 50% of PCI patients; drug-eluting stents were mainly used in FAME 2, ISCHEMIA, and ISCHEMIA-CKD.
In eight trials, statins were used in at least 50% of patients. Nearly 1 in 3 patients (31.9%) treated initially with medical therapy underwent revascularization during follow-up.
Results show no reduction in mortality risk with routine revascularization in the overall analysis (relative risk, 0.99; 95% confidence interval, 0.90-1.09) or when analyzed by whether studies did or did not use stents (P for interaction = .85).
Trial sequential analysis also showed that the cumulative z-curve crossed the futility boundary, “suggesting we have great data to show that there is lack of even a 10% reduction in death with revascularization,” Dr. Bangalore said.
Results were very similar for cardiovascular death (RR, 0.92; 95% CI, 0.80-1.06), including when analyzed by study stent status (P for interaction = .60).
There was no significant reduction in overall MI risk with revascularization, although a borderline significant 11% decrease in MIs was found in the contemporary stent era trials (RR, 0.89; 95% CI, 0.80-0.998).
Revascularization was associated with a 148% increase in the risk of procedural MI (RR, 2.48; 95% CI, 1.86-3.31) but reduced risk of spontaneous MI (RR, 0.76; 95% CI, 0.67-0.85).
Unstable angina was reduced in patients undergoing revascularization (RR, 0.64; 95% CI, 0.45-0.92), driven by a 55% reduction in the contemporary stent era trials. Freedom from angina was also greater with routine revascularization but the difference was modest, Dr. Bangalore said. There was no difference between the two strategies in heart failure or stroke.
“This meta-analysis is well done but really doesn’t change what we already know,” Rasha Al-Lamee, MBBS, of Imperial College, London, said in an interview. “The most important message is that intervention in stable CAD does not change survival. We don’t need to rush to intervene: We have time to plan the best strategy for each patient and to modify our plans based on their response.”
The analysis addresses some of the issues with previous meta-analyses that have included trials that were not strictly stable CAD trials such as SWISSI-2, COMPARE-ACUTE, and DANAMI-3-PRIMULTI, she noted. “However a study like this is only as good as the trials that are included. We must remember that unblinded trials really cannot be used to accurately assess endpoints that are prone to bias such as unstable angina and freedom from angina.”
Following the presentation, dedicated discussant Davide Capodanno, MD, PhD, of the University of Catania (Italy) said, “We have seen beyond any doubt that there is no difference in mortality. For cardiovascular death, it’s pretty much the same. It’s a little bit more mixed and nuanced, the story of myocardial infarction.”
“Additional science is needed to understand the prognostic implications,” he said. “Of course we know that spontaneous myocardial infarction is bad, but I’m not so sure about periprocedural MI. Is this something that is as important as spontaneous myocardial infarction?”
The meta-analysis is the largest ever performed, but there was clinical heterogeneity in the individual studies, especially in the definition of MI, Dr. Capodanno observed. Because of the use of trial-level data rather than patient-level data, the analysis also could not account for adherence to treatment or the effect of stent type or medication dosage.
The MI issue really depends on the trial definition of MI, Dr. Al-Lamee said. “We need long-term follow-up from ISCHEMIA to understand what it means for our patients. While revascularization clearly increases procedural MI rates, it also results in lower spontaneous MI rates with no impact on overall MI or death,” she said. “We will only know if these MIs are important if we see what impact they have in the long term.”
Although the meta-analysis combined data from several decades, it’s likely that the outdated revascularization techniques in the older trials are balanced out by the outdated medical therapy in the same trials, Dr. Al-Lamee observed.
The new findings can certainly be used in patient-physician discussions, with more follow-up from ISCHEMIA to provide additional insights, she said.
“We will of course hear more about the placebo-controlled efficacy of PCI in the blinded ORBITA-2 trial. And I would really like to see some of the older studies of patients and perceptions of the effect of PCI repeated,” Dr. Al-Lamee said. “Now we have more data, are we informing our patients and referrers correctly of the impact of our procedures, and do they truly choose revascularization with a true awareness of what it does and does not do?”
Dr. Bangalore reported grants from the National Heart, Lung, and Blood Institute and Abbott Vascular; and serving on the advisory boards of Abbott Vascular, Biotronik, Meril, SMT, Pfizer, Amgen, and Reata. Dr. Al-Lamee reported speaker’s honorarium from Philips Volcano and Menarini Pharmaceuticals. Dr. Capodanno has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Coronary revascularization does not confer a survival advantage over initial medical therapy in patients with stable ischemic heart disease (SIHD) but reduces unstable angina, according to a new study-level meta-analysis.
Routine upfront revascularization is also associated with less spontaneous myocardial infarction but this is at the cost of increased procedural infarctions, reported lead investigator Sripal Bangalore, MD, of New York University.
“These relationships should be taken into consideration for shared decision-making for the management of patients with stable ischemic heart disease,” he said in a late-breaking trial session at PCR e-Course 2020, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR).
The results, simultaneously published in Circulation, are consistent with last year’s ISCHEMIA trial and other contemporary trials, such as COURAGE, FAME 2, and BARI 2D, that have failed to show a reduction in mortality with revascularization alone in SIHD. Guidelines continue, however, to recommend revascularization to improve survival in SIHD based on trials performed in the 1980s when medical therapy was limited, Dr. Bangalore observed.
The updated meta-analysis included 14 randomized controlled trials, including the aforementioned, and 14,877 patients followed for a weighted mean of 4.5 years. Most trials enrolled patients who had preserved left ventricular function and low symptom burden (Canadian Cardiovascular Society Class I/II).
In the revascularization group, 87.5% of patients underwent any revascularization. Percutaneous coronary intervention (PCI) was the first procedure in 71.3% and bypass surgery the first choice in 16.2%. In eight trials, stents were used in at least 50% of PCI patients; drug-eluting stents were mainly used in FAME 2, ISCHEMIA, and ISCHEMIA-CKD.
In eight trials, statins were used in at least 50% of patients. Nearly 1 in 3 patients (31.9%) treated initially with medical therapy underwent revascularization during follow-up.
Results show no reduction in mortality risk with routine revascularization in the overall analysis (relative risk, 0.99; 95% confidence interval, 0.90-1.09) or when analyzed by whether studies did or did not use stents (P for interaction = .85).
Trial sequential analysis also showed that the cumulative z-curve crossed the futility boundary, “suggesting we have great data to show that there is lack of even a 10% reduction in death with revascularization,” Dr. Bangalore said.
Results were very similar for cardiovascular death (RR, 0.92; 95% CI, 0.80-1.06), including when analyzed by study stent status (P for interaction = .60).
There was no significant reduction in overall MI risk with revascularization, although a borderline significant 11% decrease in MIs was found in the contemporary stent era trials (RR, 0.89; 95% CI, 0.80-0.998).
Revascularization was associated with a 148% increase in the risk of procedural MI (RR, 2.48; 95% CI, 1.86-3.31) but reduced risk of spontaneous MI (RR, 0.76; 95% CI, 0.67-0.85).
Unstable angina was reduced in patients undergoing revascularization (RR, 0.64; 95% CI, 0.45-0.92), driven by a 55% reduction in the contemporary stent era trials. Freedom from angina was also greater with routine revascularization but the difference was modest, Dr. Bangalore said. There was no difference between the two strategies in heart failure or stroke.
“This meta-analysis is well done but really doesn’t change what we already know,” Rasha Al-Lamee, MBBS, of Imperial College, London, said in an interview. “The most important message is that intervention in stable CAD does not change survival. We don’t need to rush to intervene: We have time to plan the best strategy for each patient and to modify our plans based on their response.”
The analysis addresses some of the issues with previous meta-analyses that have included trials that were not strictly stable CAD trials such as SWISSI-2, COMPARE-ACUTE, and DANAMI-3-PRIMULTI, she noted. “However a study like this is only as good as the trials that are included. We must remember that unblinded trials really cannot be used to accurately assess endpoints that are prone to bias such as unstable angina and freedom from angina.”
Following the presentation, dedicated discussant Davide Capodanno, MD, PhD, of the University of Catania (Italy) said, “We have seen beyond any doubt that there is no difference in mortality. For cardiovascular death, it’s pretty much the same. It’s a little bit more mixed and nuanced, the story of myocardial infarction.”
“Additional science is needed to understand the prognostic implications,” he said. “Of course we know that spontaneous myocardial infarction is bad, but I’m not so sure about periprocedural MI. Is this something that is as important as spontaneous myocardial infarction?”
The meta-analysis is the largest ever performed, but there was clinical heterogeneity in the individual studies, especially in the definition of MI, Dr. Capodanno observed. Because of the use of trial-level data rather than patient-level data, the analysis also could not account for adherence to treatment or the effect of stent type or medication dosage.
The MI issue really depends on the trial definition of MI, Dr. Al-Lamee said. “We need long-term follow-up from ISCHEMIA to understand what it means for our patients. While revascularization clearly increases procedural MI rates, it also results in lower spontaneous MI rates with no impact on overall MI or death,” she said. “We will only know if these MIs are important if we see what impact they have in the long term.”
Although the meta-analysis combined data from several decades, it’s likely that the outdated revascularization techniques in the older trials are balanced out by the outdated medical therapy in the same trials, Dr. Al-Lamee observed.
The new findings can certainly be used in patient-physician discussions, with more follow-up from ISCHEMIA to provide additional insights, she said.
“We will of course hear more about the placebo-controlled efficacy of PCI in the blinded ORBITA-2 trial. And I would really like to see some of the older studies of patients and perceptions of the effect of PCI repeated,” Dr. Al-Lamee said. “Now we have more data, are we informing our patients and referrers correctly of the impact of our procedures, and do they truly choose revascularization with a true awareness of what it does and does not do?”
Dr. Bangalore reported grants from the National Heart, Lung, and Blood Institute and Abbott Vascular; and serving on the advisory boards of Abbott Vascular, Biotronik, Meril, SMT, Pfizer, Amgen, and Reata. Dr. Al-Lamee reported speaker’s honorarium from Philips Volcano and Menarini Pharmaceuticals. Dr. Capodanno has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Worrisome health disparities among transgender adults
Background: The transgender population historically has not been identified in population research. Little is known about their health care needs.
Study design: Survey review.
Setting: Large, continuously operative health survey.
Synopsis: The Centers for Disease Control and Prevention added an optional Sexual Orientation and Gender Identity module to the Behavioral Risk Factor Surveillance System in 2014. Compared with non–transgender responders, transgender adults (0.55% of responders) were more likely to report “fair” or “poor” health status (24.5% vs. 18.2%), were more likely to have experienced severe mental distress in the last 30 days (20.3% vs. 11.6), and were more likely to be physically inactive (35% vs. 25.6%), smoke cigarettes (19.2% vs. 16.3%), and lack health care coverage (20.1% vs. 14.6%).
Bottom line: Transgender adults report worse physical and mental health status. Physicians should consider these disparities during screening and treatment.
Citation: Baker K. Findings from the Behavioral Risk Factor Surveillance System on health-related quality of life among U.S. transgender adults, 2014-2017. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2018.7931.
Dr. Hoegh is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The transgender population historically has not been identified in population research. Little is known about their health care needs.
Study design: Survey review.
Setting: Large, continuously operative health survey.
Synopsis: The Centers for Disease Control and Prevention added an optional Sexual Orientation and Gender Identity module to the Behavioral Risk Factor Surveillance System in 2014. Compared with non–transgender responders, transgender adults (0.55% of responders) were more likely to report “fair” or “poor” health status (24.5% vs. 18.2%), were more likely to have experienced severe mental distress in the last 30 days (20.3% vs. 11.6), and were more likely to be physically inactive (35% vs. 25.6%), smoke cigarettes (19.2% vs. 16.3%), and lack health care coverage (20.1% vs. 14.6%).
Bottom line: Transgender adults report worse physical and mental health status. Physicians should consider these disparities during screening and treatment.
Citation: Baker K. Findings from the Behavioral Risk Factor Surveillance System on health-related quality of life among U.S. transgender adults, 2014-2017. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2018.7931.
Dr. Hoegh is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The transgender population historically has not been identified in population research. Little is known about their health care needs.
Study design: Survey review.
Setting: Large, continuously operative health survey.
Synopsis: The Centers for Disease Control and Prevention added an optional Sexual Orientation and Gender Identity module to the Behavioral Risk Factor Surveillance System in 2014. Compared with non–transgender responders, transgender adults (0.55% of responders) were more likely to report “fair” or “poor” health status (24.5% vs. 18.2%), were more likely to have experienced severe mental distress in the last 30 days (20.3% vs. 11.6), and were more likely to be physically inactive (35% vs. 25.6%), smoke cigarettes (19.2% vs. 16.3%), and lack health care coverage (20.1% vs. 14.6%).
Bottom line: Transgender adults report worse physical and mental health status. Physicians should consider these disparities during screening and treatment.
Citation: Baker K. Findings from the Behavioral Risk Factor Surveillance System on health-related quality of life among U.S. transgender adults, 2014-2017. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2018.7931.
Dr. Hoegh is a hospitalist at the University of Colorado at Denver, Aurora.
What is your diagnosis? - July 2020
Fibroepithelial polyp of the hypopharynx
Our patient underwent an upper endoscopy to evaluate symptoms of refractory gastroesophageal reflux disease and was found to have a large hiatal hernia. Upon careful endoscopic withdrawal, the polyp was briefly visualized as it was pulled back into the oropharynx. The patient was referred for flexible laryngoscopy that confirmed a polypoid mass involving the right lateral piriform wall. She subsequently underwent direct laryngoscopy with harmonic scalpel-assisted excision of the lesion leading to resolution of her symptom of oropharyngeal dysphagia. The surgical specimen measured 3 × 1.4 × 0.4 cm. Pathology demonstrated benign overlying squamous mucosa with submucosa composed of bland spindle cells and fat, consistent with a benign fibroepithelial polyp (Figure C, original magnification × 100; stain: hematoxylin and eosin).
Fibroepithelial polyps are rare benign lesions of the hypopharynx and proximal esophagus that can lead to oropharyngeal dysphagia.1 Larger hypopharyngeal polyps have been associated with aspiration and airway compromise.1 Owing to their proximal location, these lesions are more readily identified under flexible laryngoscopy, but can also be observed with esophagogastroduodenoscopy. Cross-sectional imaging of the neck can be considered for patients with oropharyngeal dysphagia and a normal video-swallow study. Although the underlying pathogenesis remains unclear, inflammation or infection may play a role, especially in smokers.2 The rate of recurrence after resection is low.1
Further evaluation for her symptomatic hiatal hernia was performed and the patient ultimately underwent a laparoscopic Nissen fundoplication with wedge gastroplasty, leading to improvement in her symptoms of gastroesophageal reflux disease. This case illustrates that, although esophagogastroduodenoscopy is not considered the first step in the evaluation of patients with oropharyngeal dysphagia, a careful examination can sometimes reveal the diagnosis.
References
1. Caceres M, et al. Large pedunculated polyps originating in the esophagus and hypopharynx. Ann Thorac Surg. 2006;81:393-6.
2. Maskey AP, et al. Endobronchial fibroepithelial polyp. J Bronchology Interv Pulmonol. 2012;19:313-4.
Fibroepithelial polyp of the hypopharynx
Our patient underwent an upper endoscopy to evaluate symptoms of refractory gastroesophageal reflux disease and was found to have a large hiatal hernia. Upon careful endoscopic withdrawal, the polyp was briefly visualized as it was pulled back into the oropharynx. The patient was referred for flexible laryngoscopy that confirmed a polypoid mass involving the right lateral piriform wall. She subsequently underwent direct laryngoscopy with harmonic scalpel-assisted excision of the lesion leading to resolution of her symptom of oropharyngeal dysphagia. The surgical specimen measured 3 × 1.4 × 0.4 cm. Pathology demonstrated benign overlying squamous mucosa with submucosa composed of bland spindle cells and fat, consistent with a benign fibroepithelial polyp (Figure C, original magnification × 100; stain: hematoxylin and eosin).
Fibroepithelial polyps are rare benign lesions of the hypopharynx and proximal esophagus that can lead to oropharyngeal dysphagia.1 Larger hypopharyngeal polyps have been associated with aspiration and airway compromise.1 Owing to their proximal location, these lesions are more readily identified under flexible laryngoscopy, but can also be observed with esophagogastroduodenoscopy. Cross-sectional imaging of the neck can be considered for patients with oropharyngeal dysphagia and a normal video-swallow study. Although the underlying pathogenesis remains unclear, inflammation or infection may play a role, especially in smokers.2 The rate of recurrence after resection is low.1
Further evaluation for her symptomatic hiatal hernia was performed and the patient ultimately underwent a laparoscopic Nissen fundoplication with wedge gastroplasty, leading to improvement in her symptoms of gastroesophageal reflux disease. This case illustrates that, although esophagogastroduodenoscopy is not considered the first step in the evaluation of patients with oropharyngeal dysphagia, a careful examination can sometimes reveal the diagnosis.
References
1. Caceres M, et al. Large pedunculated polyps originating in the esophagus and hypopharynx. Ann Thorac Surg. 2006;81:393-6.
2. Maskey AP, et al. Endobronchial fibroepithelial polyp. J Bronchology Interv Pulmonol. 2012;19:313-4.
Fibroepithelial polyp of the hypopharynx
Our patient underwent an upper endoscopy to evaluate symptoms of refractory gastroesophageal reflux disease and was found to have a large hiatal hernia. Upon careful endoscopic withdrawal, the polyp was briefly visualized as it was pulled back into the oropharynx. The patient was referred for flexible laryngoscopy that confirmed a polypoid mass involving the right lateral piriform wall. She subsequently underwent direct laryngoscopy with harmonic scalpel-assisted excision of the lesion leading to resolution of her symptom of oropharyngeal dysphagia. The surgical specimen measured 3 × 1.4 × 0.4 cm. Pathology demonstrated benign overlying squamous mucosa with submucosa composed of bland spindle cells and fat, consistent with a benign fibroepithelial polyp (Figure C, original magnification × 100; stain: hematoxylin and eosin).
Fibroepithelial polyps are rare benign lesions of the hypopharynx and proximal esophagus that can lead to oropharyngeal dysphagia.1 Larger hypopharyngeal polyps have been associated with aspiration and airway compromise.1 Owing to their proximal location, these lesions are more readily identified under flexible laryngoscopy, but can also be observed with esophagogastroduodenoscopy. Cross-sectional imaging of the neck can be considered for patients with oropharyngeal dysphagia and a normal video-swallow study. Although the underlying pathogenesis remains unclear, inflammation or infection may play a role, especially in smokers.2 The rate of recurrence after resection is low.1
Further evaluation for her symptomatic hiatal hernia was performed and the patient ultimately underwent a laparoscopic Nissen fundoplication with wedge gastroplasty, leading to improvement in her symptoms of gastroesophageal reflux disease. This case illustrates that, although esophagogastroduodenoscopy is not considered the first step in the evaluation of patients with oropharyngeal dysphagia, a careful examination can sometimes reveal the diagnosis.
References
1. Caceres M, et al. Large pedunculated polyps originating in the esophagus and hypopharynx. Ann Thorac Surg. 2006;81:393-6.
2. Maskey AP, et al. Endobronchial fibroepithelial polyp. J Bronchology Interv Pulmonol. 2012;19:313-4.
Scaly nose plaque
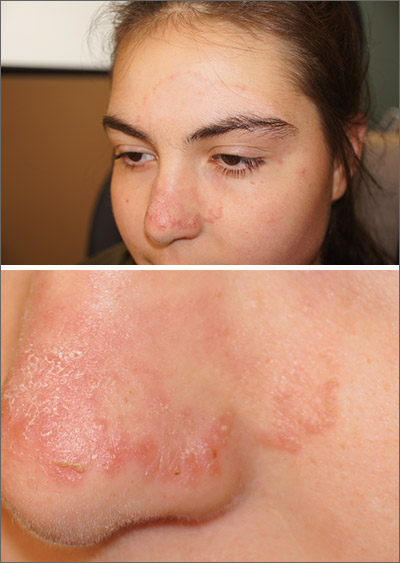
An annular morphology was appreciated on close inspection and small pustules were seen at the edges—features consistent with tinea faciei, a fungal infection of facial skin. A skin exam did not reveal any scaling or erythema on the scalp, hands, feet, trunk, or nails. The diagnosis was confirmed during the visit with a skin scraping and examination in potassium hydroxide with parker pen blue ink (Swartz-Lamkins stain) which revealed hyphae. The diagnosis was made with the knowledge that a history of eczema increases the risk of fungal, viral, and bacterial infections due to an impaired skin barrier.
Tinea faciei is an uncommon diagnosis that often is misdiagnosed as facial dermatitis, rosacea, or acne. The differential diagnosis also includes discoid lupus and psoriasis. Rarely is the annular presentation as obvious as it was here. Diagnosing tinea faciei in a patient can be made more challenging if the patient is already being treated with steroids. That’s because the steroids may decrease the clinical signs of tinea and allow subtle, slow progression of disease.
The location of fungal disease has implications for treatment. While some cases of tinea faciei may respond to topical antifungals, involvement of the eyebrows and glandular structures of the mid-face are beyond the depth of penetration of topical formulations. In these cases, systemic antifungals such as terbinafine, griseofulvin, or itraconazole are more effective.
Because of eyebrow and glandular involvement, this patient was given oral terbinafine 250 mg/d for 3 weeks and the lesion cleared completely in that time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular tinea faciei. J Pediatr. 2020;221:255-256.

An annular morphology was appreciated on close inspection and small pustules were seen at the edges—features consistent with tinea faciei, a fungal infection of facial skin. A skin exam did not reveal any scaling or erythema on the scalp, hands, feet, trunk, or nails. The diagnosis was confirmed during the visit with a skin scraping and examination in potassium hydroxide with parker pen blue ink (Swartz-Lamkins stain) which revealed hyphae. The diagnosis was made with the knowledge that a history of eczema increases the risk of fungal, viral, and bacterial infections due to an impaired skin barrier.
Tinea faciei is an uncommon diagnosis that often is misdiagnosed as facial dermatitis, rosacea, or acne. The differential diagnosis also includes discoid lupus and psoriasis. Rarely is the annular presentation as obvious as it was here. Diagnosing tinea faciei in a patient can be made more challenging if the patient is already being treated with steroids. That’s because the steroids may decrease the clinical signs of tinea and allow subtle, slow progression of disease.
The location of fungal disease has implications for treatment. While some cases of tinea faciei may respond to topical antifungals, involvement of the eyebrows and glandular structures of the mid-face are beyond the depth of penetration of topical formulations. In these cases, systemic antifungals such as terbinafine, griseofulvin, or itraconazole are more effective.
Because of eyebrow and glandular involvement, this patient was given oral terbinafine 250 mg/d for 3 weeks and the lesion cleared completely in that time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

An annular morphology was appreciated on close inspection and small pustules were seen at the edges—features consistent with tinea faciei, a fungal infection of facial skin. A skin exam did not reveal any scaling or erythema on the scalp, hands, feet, trunk, or nails. The diagnosis was confirmed during the visit with a skin scraping and examination in potassium hydroxide with parker pen blue ink (Swartz-Lamkins stain) which revealed hyphae. The diagnosis was made with the knowledge that a history of eczema increases the risk of fungal, viral, and bacterial infections due to an impaired skin barrier.
Tinea faciei is an uncommon diagnosis that often is misdiagnosed as facial dermatitis, rosacea, or acne. The differential diagnosis also includes discoid lupus and psoriasis. Rarely is the annular presentation as obvious as it was here. Diagnosing tinea faciei in a patient can be made more challenging if the patient is already being treated with steroids. That’s because the steroids may decrease the clinical signs of tinea and allow subtle, slow progression of disease.
The location of fungal disease has implications for treatment. While some cases of tinea faciei may respond to topical antifungals, involvement of the eyebrows and glandular structures of the mid-face are beyond the depth of penetration of topical formulations. In these cases, systemic antifungals such as terbinafine, griseofulvin, or itraconazole are more effective.
Because of eyebrow and glandular involvement, this patient was given oral terbinafine 250 mg/d for 3 weeks and the lesion cleared completely in that time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular tinea faciei. J Pediatr. 2020;221:255-256.
Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular tinea faciei. J Pediatr. 2020;221:255-256.
Part 2: Controlling BP in Diabetes Patients
Previously, I introduced the topic of self-care for patients with diabetes to prevent complications. Now let’s explore how to help reduce risk for cardiovascular conditions in these patients, starting with blood pressure control.
CASE CONTINUED
Mr. W’s vitals include a heart rate of 82; BP, 150/86 mm Hg; and O2 saturation, 98%. He is afebrile. You consider how to best manage glucose control and reduce the risk for cardiovascular conditions.
Reducing the Risk for Cardiovascular Conditions
The ADA recommends at least annual systematic assessment of cardiovascular risk factors, including weight, hypertension, dyslipidemia, chronic kidney disease (CKD), and presence of albuminuria.2 Managing these conditions to the standards supported by currently available evidence should reduce the risk for ASCVD in patients such as Mr. W. Two newer medication classes—glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors—offer potential benefit in reducing cardiovascular risk.15,16 Consider these medications for patients with diabetes or known ASCVD or for those who are at high risk for ASCVD and/or CKD.2,7
Furthermore, the ADA recommends using a risk calculator, such as the ASCVD Risk Estimator Plus created by the American College of Cardiology/American Heart Association (see http://tools.acc.org/ASCVD-Risk-Estimator-Plus), to stratify the 10-year risk for a first ASCVD event.2 This calculator can produce results that can help guide an individualized risk-reduction treatment plan for each patient. Also, consider low-dose aspirin for primary prevention in those at high risk for ASCVD (10-year risk > 10%) and for secondary prevention of ASCVD in those who have already had a cardiovascular event.2,7
Setting and Meeting BP Goals
Hypertension is common in patients with diabetes, with a recent study suggesting that ≥ 67% of these patients have elevated BP.17 Significant evidence demonstrates that BP control reduces morbidity and mortality in diabetes.18 Although the importance of BP control in this setting is widely known, recent studies have demonstrated that only 30% to 42% of affected patients meet their BP goals.19,20
How to make a BP goal. Guideline recommendations for setting specific BP goals have varied slightly over the past several years and are influenced by known comorbidities such as ASCVD and CKD. Patients should be part of the decision-making process to individualize goals based on their circumstances and safety. A BP goal of < 130/80 mm Hg is generally acceptable for patients who are known to have ASCVD or who are at high risk (≥ 15% risk) for ASCVD in the next 10 years.7 A goal of < 140/90 mm Hg is considered appropriate in those with a lower risk for ASCVD.7,8,21,22
Medications. Selecting an appropriate antihypertensive medication relies on multiple factors. Evidence supports the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for diabetes, and both the AACE and ADA recommend these medications as an initial treatment option.2,7 They help reduce the progression of kidney disease in patients with albuminuria and may improve cardiovascular outcomes.23-27 When additional agents are needed to meet BP goals, the ADA recommends thiazide-like diuretics (chlorthalidone and indapamide) or calcium channel blockers (dihydropyridine).2 Although some hyperglycemic adverse effects have been observed with use of thiazide-like diuretics, these might be outweighed by the benefit of BP control.24
Continue to: Monitor the patient's BP
Monitor the patient’s BP at every visit, and advise the patient to regularly measure his or her BP at home with a BP cuff. Patients who may need assistance with at-home monitoring can be directed to an online guide on how to accurately measure their BP (see www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home). For those who report consistently above-goal measurements at home, advise them to check their BP cuff, because an ill-fitting cuff is a well-known cause of inaccurate measurement. Patients also should be assessed for medication nonadherence, white coat hypertension, and secondary hypertension.7,8 If a patient’s BP is truly above goal, a step-up in therapy may be appropriate because without adequate BP control, the benefit in mortality and morbidity may not be fully realized.28
In Part 3, we’ll check in with Mr. W and discuss which patients require assessment for dyslipidemia. We’ll also explore the treatments, such as statin therapy, for this condition.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
Previously, I introduced the topic of self-care for patients with diabetes to prevent complications. Now let’s explore how to help reduce risk for cardiovascular conditions in these patients, starting with blood pressure control.
CASE CONTINUED
Mr. W’s vitals include a heart rate of 82; BP, 150/86 mm Hg; and O2 saturation, 98%. He is afebrile. You consider how to best manage glucose control and reduce the risk for cardiovascular conditions.
Reducing the Risk for Cardiovascular Conditions
The ADA recommends at least annual systematic assessment of cardiovascular risk factors, including weight, hypertension, dyslipidemia, chronic kidney disease (CKD), and presence of albuminuria.2 Managing these conditions to the standards supported by currently available evidence should reduce the risk for ASCVD in patients such as Mr. W. Two newer medication classes—glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors—offer potential benefit in reducing cardiovascular risk.15,16 Consider these medications for patients with diabetes or known ASCVD or for those who are at high risk for ASCVD and/or CKD.2,7
Furthermore, the ADA recommends using a risk calculator, such as the ASCVD Risk Estimator Plus created by the American College of Cardiology/American Heart Association (see http://tools.acc.org/ASCVD-Risk-Estimator-Plus), to stratify the 10-year risk for a first ASCVD event.2 This calculator can produce results that can help guide an individualized risk-reduction treatment plan for each patient. Also, consider low-dose aspirin for primary prevention in those at high risk for ASCVD (10-year risk > 10%) and for secondary prevention of ASCVD in those who have already had a cardiovascular event.2,7
Setting and Meeting BP Goals
Hypertension is common in patients with diabetes, with a recent study suggesting that ≥ 67% of these patients have elevated BP.17 Significant evidence demonstrates that BP control reduces morbidity and mortality in diabetes.18 Although the importance of BP control in this setting is widely known, recent studies have demonstrated that only 30% to 42% of affected patients meet their BP goals.19,20
How to make a BP goal. Guideline recommendations for setting specific BP goals have varied slightly over the past several years and are influenced by known comorbidities such as ASCVD and CKD. Patients should be part of the decision-making process to individualize goals based on their circumstances and safety. A BP goal of < 130/80 mm Hg is generally acceptable for patients who are known to have ASCVD or who are at high risk (≥ 15% risk) for ASCVD in the next 10 years.7 A goal of < 140/90 mm Hg is considered appropriate in those with a lower risk for ASCVD.7,8,21,22
Medications. Selecting an appropriate antihypertensive medication relies on multiple factors. Evidence supports the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for diabetes, and both the AACE and ADA recommend these medications as an initial treatment option.2,7 They help reduce the progression of kidney disease in patients with albuminuria and may improve cardiovascular outcomes.23-27 When additional agents are needed to meet BP goals, the ADA recommends thiazide-like diuretics (chlorthalidone and indapamide) or calcium channel blockers (dihydropyridine).2 Although some hyperglycemic adverse effects have been observed with use of thiazide-like diuretics, these might be outweighed by the benefit of BP control.24
Continue to: Monitor the patient's BP
Monitor the patient’s BP at every visit, and advise the patient to regularly measure his or her BP at home with a BP cuff. Patients who may need assistance with at-home monitoring can be directed to an online guide on how to accurately measure their BP (see www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home). For those who report consistently above-goal measurements at home, advise them to check their BP cuff, because an ill-fitting cuff is a well-known cause of inaccurate measurement. Patients also should be assessed for medication nonadherence, white coat hypertension, and secondary hypertension.7,8 If a patient’s BP is truly above goal, a step-up in therapy may be appropriate because without adequate BP control, the benefit in mortality and morbidity may not be fully realized.28
In Part 3, we’ll check in with Mr. W and discuss which patients require assessment for dyslipidemia. We’ll also explore the treatments, such as statin therapy, for this condition.
Previously, I introduced the topic of self-care for patients with diabetes to prevent complications. Now let’s explore how to help reduce risk for cardiovascular conditions in these patients, starting with blood pressure control.
CASE CONTINUED
Mr. W’s vitals include a heart rate of 82; BP, 150/86 mm Hg; and O2 saturation, 98%. He is afebrile. You consider how to best manage glucose control and reduce the risk for cardiovascular conditions.
Reducing the Risk for Cardiovascular Conditions
The ADA recommends at least annual systematic assessment of cardiovascular risk factors, including weight, hypertension, dyslipidemia, chronic kidney disease (CKD), and presence of albuminuria.2 Managing these conditions to the standards supported by currently available evidence should reduce the risk for ASCVD in patients such as Mr. W. Two newer medication classes—glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors—offer potential benefit in reducing cardiovascular risk.15,16 Consider these medications for patients with diabetes or known ASCVD or for those who are at high risk for ASCVD and/or CKD.2,7
Furthermore, the ADA recommends using a risk calculator, such as the ASCVD Risk Estimator Plus created by the American College of Cardiology/American Heart Association (see http://tools.acc.org/ASCVD-Risk-Estimator-Plus), to stratify the 10-year risk for a first ASCVD event.2 This calculator can produce results that can help guide an individualized risk-reduction treatment plan for each patient. Also, consider low-dose aspirin for primary prevention in those at high risk for ASCVD (10-year risk > 10%) and for secondary prevention of ASCVD in those who have already had a cardiovascular event.2,7
Setting and Meeting BP Goals
Hypertension is common in patients with diabetes, with a recent study suggesting that ≥ 67% of these patients have elevated BP.17 Significant evidence demonstrates that BP control reduces morbidity and mortality in diabetes.18 Although the importance of BP control in this setting is widely known, recent studies have demonstrated that only 30% to 42% of affected patients meet their BP goals.19,20
How to make a BP goal. Guideline recommendations for setting specific BP goals have varied slightly over the past several years and are influenced by known comorbidities such as ASCVD and CKD. Patients should be part of the decision-making process to individualize goals based on their circumstances and safety. A BP goal of < 130/80 mm Hg is generally acceptable for patients who are known to have ASCVD or who are at high risk (≥ 15% risk) for ASCVD in the next 10 years.7 A goal of < 140/90 mm Hg is considered appropriate in those with a lower risk for ASCVD.7,8,21,22
Medications. Selecting an appropriate antihypertensive medication relies on multiple factors. Evidence supports the use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for diabetes, and both the AACE and ADA recommend these medications as an initial treatment option.2,7 They help reduce the progression of kidney disease in patients with albuminuria and may improve cardiovascular outcomes.23-27 When additional agents are needed to meet BP goals, the ADA recommends thiazide-like diuretics (chlorthalidone and indapamide) or calcium channel blockers (dihydropyridine).2 Although some hyperglycemic adverse effects have been observed with use of thiazide-like diuretics, these might be outweighed by the benefit of BP control.24
Continue to: Monitor the patient's BP
Monitor the patient’s BP at every visit, and advise the patient to regularly measure his or her BP at home with a BP cuff. Patients who may need assistance with at-home monitoring can be directed to an online guide on how to accurately measure their BP (see www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings/monitoring-your-blood-pressure-at-home). For those who report consistently above-goal measurements at home, advise them to check their BP cuff, because an ill-fitting cuff is a well-known cause of inaccurate measurement. Patients also should be assessed for medication nonadherence, white coat hypertension, and secondary hypertension.7,8 If a patient’s BP is truly above goal, a step-up in therapy may be appropriate because without adequate BP control, the benefit in mortality and morbidity may not be fully realized.28
In Part 3, we’ll check in with Mr. W and discuss which patients require assessment for dyslipidemia. We’ll also explore the treatments, such as statin therapy, for this condition.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
1. Centers for Disease Control and Prevention. Diabetes incidence and prevalence. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/incidence-2017.html. Published 2018. Accessed June 18, 2020.
2. Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. American Diabetes Association Clinical Diabetes. 2020;38(1):10-38.
3. Chen Y, Sloan FA, Yashkin AP. Adherence to diabetes guidelines for screening, physical activity and medication and onset of complications and death. J Diabetes Complications. 2015;29(8):1228-1233.
4. Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care. 2017;5(1):e000406.
5. Center for Disease Control and Prevention. Preventive care practices. Diabetes Report Card 2017. www.cdc.gov/diabetes/library/reports/reportcard/preventive-care.html. Published 2018. Accessed June 18, 2020.
6. Arnold SV, de Lemos JA, Rosenson RS, et al; GOULD Investigators. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620.
7. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract Endocr Pract. 2020;26(1):107-139.
8. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S37-S47.
9. Beck J, Greenwood DA, Blanton L, et al; 2017 Standards Revision Task Force. 2017 National Standards for diabetes self-management education and support. Diabetes Educ. 2017;43(5): 449-464.
10. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(6):926-943.
11. Association of Diabetes Care & Education Specialists. Find a diabetes education program in your area. www.diabeteseducator.org/living-with-diabetes/find-an-education-program. Accessed June 15, 2020.
12. Estruch R, Ros E, Salas-Salvadó J, et al; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. NEJM. 2018;378(25):e34.
13. Centers for Disease Control and Prevention. Tips for better sleep. Sleep and sleep disorders. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html. Reviewed July 15, 2016. Accessed June 18, 2020.
14. Doumit J, Prasad B. Sleep Apnea in Type 2 Diabetes. Diabetes Spectrum. 2016; 29(1): 14-19.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
17. Trends in Blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens. 2009;27(9):1908-1916.
18. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603-615.
19. Vouri SM, Shaw RF, Waterbury NV, et al. Prevalence of achievement of A1c, blood pressure, and cholesterol (ABC) goal in veterans with diabetes. J Manag Care Pharm. 2011;17(4):304-312.
20. Kudo N, Yokokawa H, Fukuda H, et al. Achievement of target blood pressure levels among Japanese workers with hypertension and healthy lifestyle characteristics associated with therapeutic failure. Plos One. 2015;10(7):e0133641.
21. Carey RM, Whelton PK; 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension guideline. Ann Intern Med. 2018;168(5):351-358.
22. Deedwania PC. Blood pressure control in diabetes mellitus. Circulation. 2011;123:2776–2778.
23. Catalá-López F, Saint-Gerons DM, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med. 2016;13(3):e1001971.
24. Furberg CD, Wright JT Jr, Davis BR, et al; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997.
25. Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation). J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):18-20.
26. Tatti P, Pahor M, Byington RP, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21(4):597-603.
27. Schrier RW, Estacio RO, Jeffers B. Appropriate Blood Pressure Control in NIDDM (ABCD) Trial. Diabetologia. 1996;39(12):1646-1654.
28. Hansson L, Zanchetti A, Carruthers SG, et al; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet. 1998;351(9118):1755-1762.
29. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681.
30. Fu AZ, Zhang Q, Davies MJ, et al. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-1040.
31. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387-2397
32. Sabatine MS, Giugliano RP, Keech AC, et al; the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
33. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome | NEJM. N Engl J Med. 2018;379:2097-2107.
34. Icosapent ethyl [package insert]. Bridgewater, NJ: Amarin Pharma, Inc.; 2019.
35. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22
36. Bolton WK. Renal Physicians Association Clinical practice guideline: appropriate patient preparation for renal replacement therapy: guideline number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
37. American Diabetes Association. Pharmacologic Approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110.
38. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279-290.
39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1-59.
40. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154.
41. Gupta V, Bansal R, Gupta A, Bhansali A. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Indian J Ophthalmol. 2014;62(8):851-856.
42. Pérez MA, Bruce BB, Newman NJ, Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. The Neurologist. 2012;18(6):350-355.
A message from new president, Bishr Omary
Dear colleagues,
I have the privilege and honor to serve as AGA president as of June 1, 2020. When we look back at the first half of 2020, we will remember the COVID-19 pandemic and the unimaginable loss of life, morbidity, and economic impact it had. We will also remember the grief and anger that have characterized the recent weeks. I hope that the second half of 2020 will be a time that reshapes us for the better and allows us to seize the opportunity to make meaningful changes, in addition to recovering from the impact of the pandemic. The ongoing protests for the past 16 days against police brutality finally have our country recognizing front-and-center injustices facing African Americans.
While recognition of an injustice is a start, it is essentially meaningless unless action is taken to ensure equity in all facets of society. Of particular interest to AGA is access to health care without bias, addressing racial disparities in health care, diversity within the practice of GI, and supporting the careers of diverse researchers. AGA has a diversity policy and a solid history of programs supporting minority physicians and researchers. We know that’s not enough and AGA, with our dedicated committees, staff, and leadership, will continue to implement and assess plans for meaningful improvements. Watch for more on this topic in the future.
In addition, AGA took a pledge with our GI sister organizations to “continue to advocate for diversity in our staff and governance, grant awards to research health care disparities, ensure quality care for all, and work tirelessly to reduce inequalities in health care delivery and access.” We plan to honor this pledge with our own efforts and by making a concerted effort to work with AASLD, ACG, ASGE, DHPA, and other societies, colleagues, and friends.
The COVID-19 pandemic has been a major challenge for our practices and to our research community. To all AGA members, please know that we have your back with a stream of practice guidance, business support, advocacy, and funding. You can find these resources collected at www.gastro.org/COVID.
My special thanks to the following AGA members, among several AGA staff and expert participants, for making these resources possible and highly engaging:
- Maria Abreu, who oversees our weekly COVID Connection webinar.
- Shahnaz Sultan and Joseph Lim whose Guidelines and Clinical Practice Update committees have generated evidence-based practice guidance at an incredible pace.
- Vivek Kaul and Vijay Shah who lead regular townhall webinars with division chiefs to share how GI divisions are pivoting to address the numerous current challenges.
- Rhonda Souza, chair of AGA Council, which is already thinking about how to make DDW 2021 a success.
Throughout my time as AGA president, I plan to communicate with you on a regular basis and welcome your input and suggestions. Watch the AGA Community for updates and announcements. Every other month, I plan to host a Townhall with the AGA President webinar on Zoom, where we can gather to hear from AGA leaders and staff on their work. My first webinar is planned for July 10, 2020, at 11 a.m. United States Eastern time. Watch for more info to come.
My goals are to build on what past president Hashem El-Serag has initiated and to work closely with John Inadomi (president-elect), John Carethers (vice president), the AGA Governing Board, committees, and staff. Along these lines, we will work tirelessly to support AGA domestic and international members and the gastroenterology community needs, be it patient care and those who provide the care, basic and clinical scientific discovery, education and training, advocacy, and ABIM recertification. I look forward to working with you and for you throughout the year.
Sincerely,
Bishr Omary, MD, PhD, AGAF
AGA Institute President
Dear colleagues,
I have the privilege and honor to serve as AGA president as of June 1, 2020. When we look back at the first half of 2020, we will remember the COVID-19 pandemic and the unimaginable loss of life, morbidity, and economic impact it had. We will also remember the grief and anger that have characterized the recent weeks. I hope that the second half of 2020 will be a time that reshapes us for the better and allows us to seize the opportunity to make meaningful changes, in addition to recovering from the impact of the pandemic. The ongoing protests for the past 16 days against police brutality finally have our country recognizing front-and-center injustices facing African Americans.
While recognition of an injustice is a start, it is essentially meaningless unless action is taken to ensure equity in all facets of society. Of particular interest to AGA is access to health care without bias, addressing racial disparities in health care, diversity within the practice of GI, and supporting the careers of diverse researchers. AGA has a diversity policy and a solid history of programs supporting minority physicians and researchers. We know that’s not enough and AGA, with our dedicated committees, staff, and leadership, will continue to implement and assess plans for meaningful improvements. Watch for more on this topic in the future.
In addition, AGA took a pledge with our GI sister organizations to “continue to advocate for diversity in our staff and governance, grant awards to research health care disparities, ensure quality care for all, and work tirelessly to reduce inequalities in health care delivery and access.” We plan to honor this pledge with our own efforts and by making a concerted effort to work with AASLD, ACG, ASGE, DHPA, and other societies, colleagues, and friends.
The COVID-19 pandemic has been a major challenge for our practices and to our research community. To all AGA members, please know that we have your back with a stream of practice guidance, business support, advocacy, and funding. You can find these resources collected at www.gastro.org/COVID.
My special thanks to the following AGA members, among several AGA staff and expert participants, for making these resources possible and highly engaging:
- Maria Abreu, who oversees our weekly COVID Connection webinar.
- Shahnaz Sultan and Joseph Lim whose Guidelines and Clinical Practice Update committees have generated evidence-based practice guidance at an incredible pace.
- Vivek Kaul and Vijay Shah who lead regular townhall webinars with division chiefs to share how GI divisions are pivoting to address the numerous current challenges.
- Rhonda Souza, chair of AGA Council, which is already thinking about how to make DDW 2021 a success.
Throughout my time as AGA president, I plan to communicate with you on a regular basis and welcome your input and suggestions. Watch the AGA Community for updates and announcements. Every other month, I plan to host a Townhall with the AGA President webinar on Zoom, where we can gather to hear from AGA leaders and staff on their work. My first webinar is planned for July 10, 2020, at 11 a.m. United States Eastern time. Watch for more info to come.
My goals are to build on what past president Hashem El-Serag has initiated and to work closely with John Inadomi (president-elect), John Carethers (vice president), the AGA Governing Board, committees, and staff. Along these lines, we will work tirelessly to support AGA domestic and international members and the gastroenterology community needs, be it patient care and those who provide the care, basic and clinical scientific discovery, education and training, advocacy, and ABIM recertification. I look forward to working with you and for you throughout the year.
Sincerely,
Bishr Omary, MD, PhD, AGAF
AGA Institute President
Dear colleagues,
I have the privilege and honor to serve as AGA president as of June 1, 2020. When we look back at the first half of 2020, we will remember the COVID-19 pandemic and the unimaginable loss of life, morbidity, and economic impact it had. We will also remember the grief and anger that have characterized the recent weeks. I hope that the second half of 2020 will be a time that reshapes us for the better and allows us to seize the opportunity to make meaningful changes, in addition to recovering from the impact of the pandemic. The ongoing protests for the past 16 days against police brutality finally have our country recognizing front-and-center injustices facing African Americans.
While recognition of an injustice is a start, it is essentially meaningless unless action is taken to ensure equity in all facets of society. Of particular interest to AGA is access to health care without bias, addressing racial disparities in health care, diversity within the practice of GI, and supporting the careers of diverse researchers. AGA has a diversity policy and a solid history of programs supporting minority physicians and researchers. We know that’s not enough and AGA, with our dedicated committees, staff, and leadership, will continue to implement and assess plans for meaningful improvements. Watch for more on this topic in the future.
In addition, AGA took a pledge with our GI sister organizations to “continue to advocate for diversity in our staff and governance, grant awards to research health care disparities, ensure quality care for all, and work tirelessly to reduce inequalities in health care delivery and access.” We plan to honor this pledge with our own efforts and by making a concerted effort to work with AASLD, ACG, ASGE, DHPA, and other societies, colleagues, and friends.
The COVID-19 pandemic has been a major challenge for our practices and to our research community. To all AGA members, please know that we have your back with a stream of practice guidance, business support, advocacy, and funding. You can find these resources collected at www.gastro.org/COVID.
My special thanks to the following AGA members, among several AGA staff and expert participants, for making these resources possible and highly engaging:
- Maria Abreu, who oversees our weekly COVID Connection webinar.
- Shahnaz Sultan and Joseph Lim whose Guidelines and Clinical Practice Update committees have generated evidence-based practice guidance at an incredible pace.
- Vivek Kaul and Vijay Shah who lead regular townhall webinars with division chiefs to share how GI divisions are pivoting to address the numerous current challenges.
- Rhonda Souza, chair of AGA Council, which is already thinking about how to make DDW 2021 a success.
Throughout my time as AGA president, I plan to communicate with you on a regular basis and welcome your input and suggestions. Watch the AGA Community for updates and announcements. Every other month, I plan to host a Townhall with the AGA President webinar on Zoom, where we can gather to hear from AGA leaders and staff on their work. My first webinar is planned for July 10, 2020, at 11 a.m. United States Eastern time. Watch for more info to come.
My goals are to build on what past president Hashem El-Serag has initiated and to work closely with John Inadomi (president-elect), John Carethers (vice president), the AGA Governing Board, committees, and staff. Along these lines, we will work tirelessly to support AGA domestic and international members and the gastroenterology community needs, be it patient care and those who provide the care, basic and clinical scientific discovery, education and training, advocacy, and ABIM recertification. I look forward to working with you and for you throughout the year.
Sincerely,
Bishr Omary, MD, PhD, AGAF
AGA Institute President