User login
Evidence-based management of early pregnancy loss
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


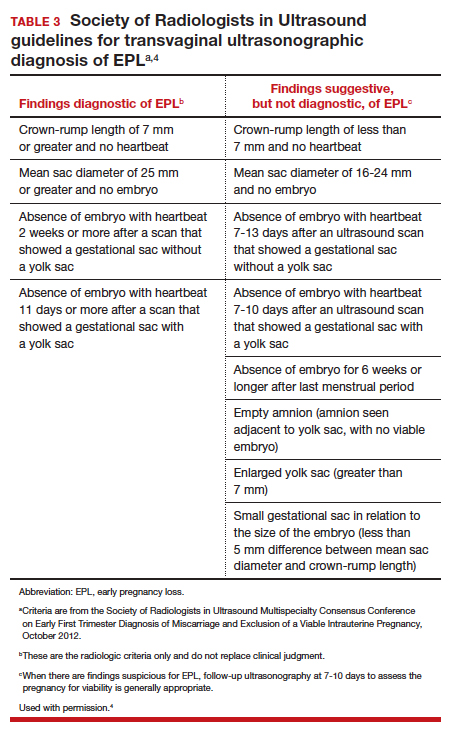
Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.
Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
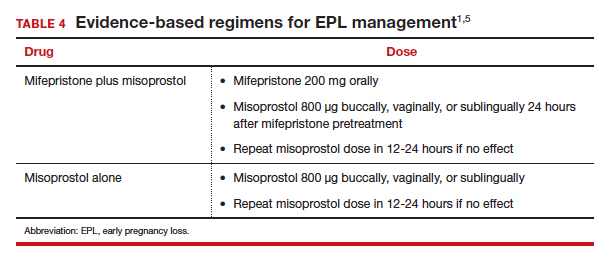
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.
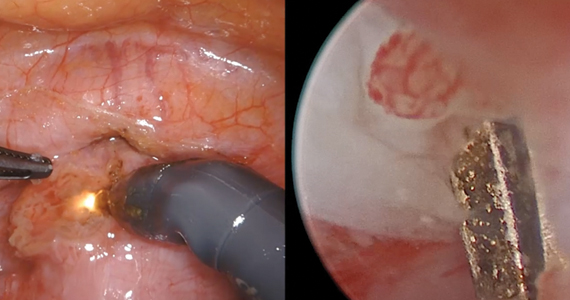
Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
Telemedicine: Navigating legal issues
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
Free videoconferencing apps for the ObGyn

The COVID-19 pandemic has created a metamorphosis in human interactions. One way we have adapted is our increased use of virtual platforms for tasks such as lectures, meetings, interviews, conferences, and patient care via telemedicine.1 Virtual platforms have allowed for the continuation of existing programs and facilitated new collaborations ranging from international webinars on patient care to national lectures for residents and fellows in ObGyn. New virtual platforms continue to emerge. We present here a review of free virtual communication apps available to the ObGyn care provider.
We used the term “videoconference” to search the Apple and Google Play app stores between May 29, 2020, and June 1, 2020. A total of 25 apps that offered both audio and videoconferencing were identified. All were free for download, but the majority required an ongoing paid subscription fee for the service. Thirteen programs were either completely free or offered a free version of their services. Based on our review and a systematic analysis, we selected 5 apps to feature here: Cisco Webex Meetings, Free Conference Call, Jitsi Meet, Microsoft Teams, and Zoom.
Featured videoconferencing apps
Cisco Webex Meetings and Free Conference Call offer an easy video meeting setup from both a smartphone and a desktop app. They provide seamless access to functions on the virtual main page, including chat with other participants in the meeting and screen sharing. These apps both require screen recording in order to share screens.
Jitsi Meet is a web app usable on an iPhone or Android as well as on a desktop through the meet.jit.si website. No account is required. On the app or website, the user creates a meeting name and shares the unique URL or meeting name with invitees to join the videoconference. The mobile app and website both offer a “raise your hand” feature, full screen and/or gallery (tile) view, group chat, and live streaming. In both settings, users may lock the meeting and require a password. Additional features through the website include screen sharing, recording the meeting, blurred background, muting all participants, and sharing YouTube videos.
The Microsoft Teams app asks you the purpose of signing up on the website—“use for school,” “with friends and family,” or “for work.” If you choose “with friends and family,” the app directs you to Skype. Choosing the “for work” function directs you to complete your free registration. Microsoft Teams requires participants to create teams; thus, others participating in the videoconference need to have their own account. However, “guest access” also is available.
On the Zoom platform, immediate and scheduled meetings can be set up on the app as well as on the website, or directly on Microsoft Outlook and Google Calendar if the plug-in has been established. The desktop and smartphone apps are similar in function and provide access to personalized settings.
For patient care, since HIPAA (Health Insurance Portability and Accountability Act) protection is a concern, we recommend following guidelines at the user’s institution regarding use of apps such as Epic Haiku for telehealth visits. For teaching and interacting with colleagues, we recommend Cisco Webex, Free Conference Call, Microsoft Teams, and Zoom, keeping in mind the time limitations of each app for the free account.
Overall, these 5 apps are easy to set up and user-friendly. Deciding which program to choose will depend on the number of participants allowed for a meeting and the duration of the meeting, as these two factors seem to be the most constraining among the free videoconferencing apps. ●
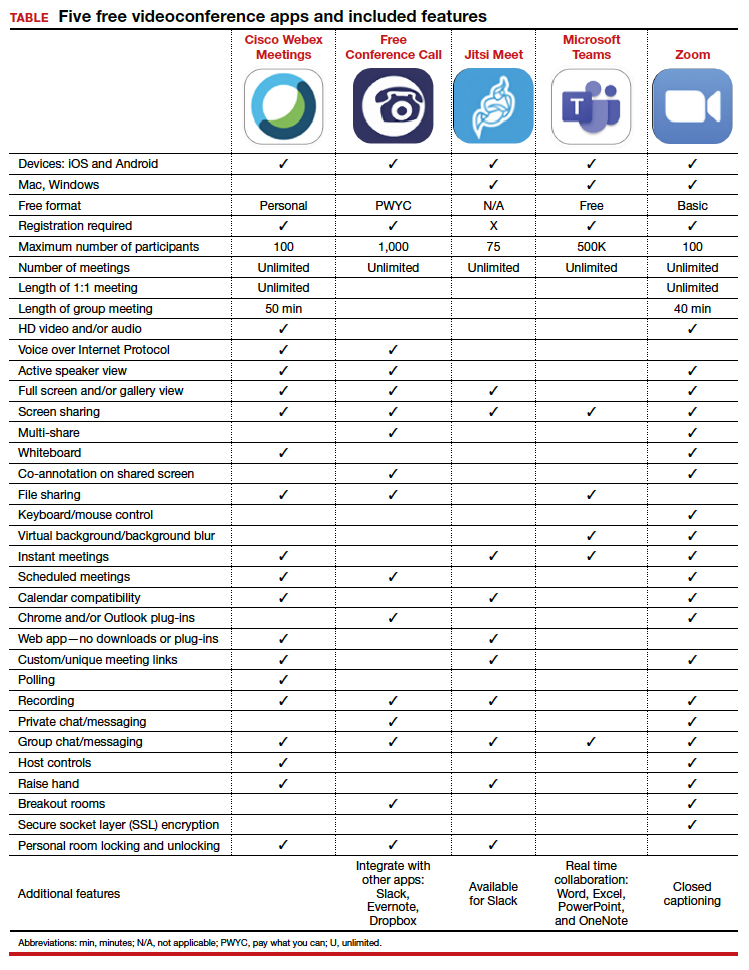
- Karram M, Baum N. Telemedicine: a primer for today’s ObGyn. OBG Manag. 2020;32(5):28-32.

The COVID-19 pandemic has created a metamorphosis in human interactions. One way we have adapted is our increased use of virtual platforms for tasks such as lectures, meetings, interviews, conferences, and patient care via telemedicine.1 Virtual platforms have allowed for the continuation of existing programs and facilitated new collaborations ranging from international webinars on patient care to national lectures for residents and fellows in ObGyn. New virtual platforms continue to emerge. We present here a review of free virtual communication apps available to the ObGyn care provider.
We used the term “videoconference” to search the Apple and Google Play app stores between May 29, 2020, and June 1, 2020. A total of 25 apps that offered both audio and videoconferencing were identified. All were free for download, but the majority required an ongoing paid subscription fee for the service. Thirteen programs were either completely free or offered a free version of their services. Based on our review and a systematic analysis, we selected 5 apps to feature here: Cisco Webex Meetings, Free Conference Call, Jitsi Meet, Microsoft Teams, and Zoom.
Featured videoconferencing apps
Cisco Webex Meetings and Free Conference Call offer an easy video meeting setup from both a smartphone and a desktop app. They provide seamless access to functions on the virtual main page, including chat with other participants in the meeting and screen sharing. These apps both require screen recording in order to share screens.
Jitsi Meet is a web app usable on an iPhone or Android as well as on a desktop through the meet.jit.si website. No account is required. On the app or website, the user creates a meeting name and shares the unique URL or meeting name with invitees to join the videoconference. The mobile app and website both offer a “raise your hand” feature, full screen and/or gallery (tile) view, group chat, and live streaming. In both settings, users may lock the meeting and require a password. Additional features through the website include screen sharing, recording the meeting, blurred background, muting all participants, and sharing YouTube videos.
The Microsoft Teams app asks you the purpose of signing up on the website—“use for school,” “with friends and family,” or “for work.” If you choose “with friends and family,” the app directs you to Skype. Choosing the “for work” function directs you to complete your free registration. Microsoft Teams requires participants to create teams; thus, others participating in the videoconference need to have their own account. However, “guest access” also is available.
On the Zoom platform, immediate and scheduled meetings can be set up on the app as well as on the website, or directly on Microsoft Outlook and Google Calendar if the plug-in has been established. The desktop and smartphone apps are similar in function and provide access to personalized settings.
For patient care, since HIPAA (Health Insurance Portability and Accountability Act) protection is a concern, we recommend following guidelines at the user’s institution regarding use of apps such as Epic Haiku for telehealth visits. For teaching and interacting with colleagues, we recommend Cisco Webex, Free Conference Call, Microsoft Teams, and Zoom, keeping in mind the time limitations of each app for the free account.
Overall, these 5 apps are easy to set up and user-friendly. Deciding which program to choose will depend on the number of participants allowed for a meeting and the duration of the meeting, as these two factors seem to be the most constraining among the free videoconferencing apps. ●


The COVID-19 pandemic has created a metamorphosis in human interactions. One way we have adapted is our increased use of virtual platforms for tasks such as lectures, meetings, interviews, conferences, and patient care via telemedicine.1 Virtual platforms have allowed for the continuation of existing programs and facilitated new collaborations ranging from international webinars on patient care to national lectures for residents and fellows in ObGyn. New virtual platforms continue to emerge. We present here a review of free virtual communication apps available to the ObGyn care provider.
We used the term “videoconference” to search the Apple and Google Play app stores between May 29, 2020, and June 1, 2020. A total of 25 apps that offered both audio and videoconferencing were identified. All were free for download, but the majority required an ongoing paid subscription fee for the service. Thirteen programs were either completely free or offered a free version of their services. Based on our review and a systematic analysis, we selected 5 apps to feature here: Cisco Webex Meetings, Free Conference Call, Jitsi Meet, Microsoft Teams, and Zoom.
Featured videoconferencing apps
Cisco Webex Meetings and Free Conference Call offer an easy video meeting setup from both a smartphone and a desktop app. They provide seamless access to functions on the virtual main page, including chat with other participants in the meeting and screen sharing. These apps both require screen recording in order to share screens.
Jitsi Meet is a web app usable on an iPhone or Android as well as on a desktop through the meet.jit.si website. No account is required. On the app or website, the user creates a meeting name and shares the unique URL or meeting name with invitees to join the videoconference. The mobile app and website both offer a “raise your hand” feature, full screen and/or gallery (tile) view, group chat, and live streaming. In both settings, users may lock the meeting and require a password. Additional features through the website include screen sharing, recording the meeting, blurred background, muting all participants, and sharing YouTube videos.
The Microsoft Teams app asks you the purpose of signing up on the website—“use for school,” “with friends and family,” or “for work.” If you choose “with friends and family,” the app directs you to Skype. Choosing the “for work” function directs you to complete your free registration. Microsoft Teams requires participants to create teams; thus, others participating in the videoconference need to have their own account. However, “guest access” also is available.
On the Zoom platform, immediate and scheduled meetings can be set up on the app as well as on the website, or directly on Microsoft Outlook and Google Calendar if the plug-in has been established. The desktop and smartphone apps are similar in function and provide access to personalized settings.
For patient care, since HIPAA (Health Insurance Portability and Accountability Act) protection is a concern, we recommend following guidelines at the user’s institution regarding use of apps such as Epic Haiku for telehealth visits. For teaching and interacting with colleagues, we recommend Cisco Webex, Free Conference Call, Microsoft Teams, and Zoom, keeping in mind the time limitations of each app for the free account.
Overall, these 5 apps are easy to set up and user-friendly. Deciding which program to choose will depend on the number of participants allowed for a meeting and the duration of the meeting, as these two factors seem to be the most constraining among the free videoconferencing apps. ●

- Karram M, Baum N. Telemedicine: a primer for today’s ObGyn. OBG Manag. 2020;32(5):28-32.
- Karram M, Baum N. Telemedicine: a primer for today’s ObGyn. OBG Manag. 2020;32(5):28-32.
Pulmonary function tests can’t substitute for high-resolution CT in early systemic sclerosis ILD screening
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
FROM ARTHRITIS & RHEUMATOLOGY
Isthmocele repair: Simultaneous hysteroscopy and robotic-assisted laparoscopy

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues
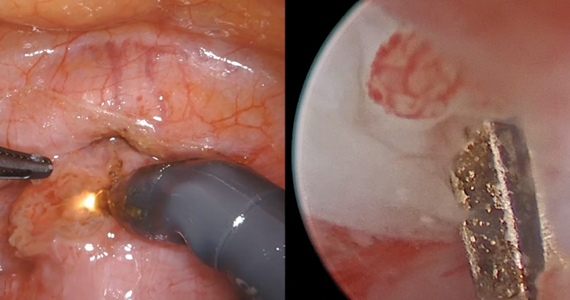
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues


An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
Lipophilic statins linked to lower mortality in ovarian cancer
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
FROM AACR 2020
Tendyne device shows promise for mitral annular calcification
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
REPORTING FROM EUROPCR 2020
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
Kawthar Al Ajmi, MSc, of the University of Manchester (England), and colleagues published these findings in JAMA Network Open.
With almost a quarter of breast cancers thought to be preventable in the United Kingdom, “it is important to understand the contribution of modifiable risk factors ... and how they affect or add to the inherited genetic factors,” the researchers wrote.
To that end, the team reviewed 91,217 white, postmenopausal women in the United Kingdom Biobank, an ongoing longitudinal study of the contribution of genetic, environmental, and lifestyle risk factors in disease. There were 2,728 women who developed breast cancer at a median follow-up of 10 years.
The investigators used a polygenic risk score to categorize subjects as low, intermediate, or high genetic risk. The score was constructed using 305 single-nucleotide variants.
Within each risk group, the researchers divided women by the presence or absence of five lifestyle factors previously associated with a lower risk of breast cancer: healthy weight, regular exercise, no use of hormone replacement therapy beyond 5 years, no oral contraceptive use, and alcohol intake no more than twice a week.
Women with four or more of these factors were deemed to have a favorable lifestyle. Women with two or three factors had an intermediate lifestyle, and women with fewer factors had an unfavorable lifestyle.
Results
The data showed an association between breast cancer and a body mass index of 25 or higher (relative risk, 1.14), no regular physical activity (RR, 1.12), alcohol intake at least three times per week (RR, 1.11), and use of hormone replacement therapy for 5 or more years (RR, 1.23). History of oral contraceptive use was not associated with breast cancer risk (RR, 1.02), but this factor remained a part of the lifestyle classification.
In the low genetic risk group, an intermediate lifestyle (hazard ratio, 1.40; 95% CI, 1.09-1.80) and an unfavorable lifestyle (HR, 1.63; 95% CI, 1.14-2.34) were both associated with a higher risk of breast cancer, compared with a favorable lifestyle.
In the intermediate genetic risk group, intermediate (HR, 1.37; 95% CI, 1.12-1.68) and unfavorable lifestyles (HR 1.94; 95% CI, 1.46-2.58) were again associated with higher breast cancer risk, compared with a favorable lifestyle .
Even in the high genetic risk group, intermediate (HR, 1.13; 95% CI, 0.98-1.31) and unfavorable lifestyles (HR, 1.39; 95% CI, 1.11-1.74) were associated with increased breast cancer risk. Results were adjusted for both age and family history.
In the end, “a healthier lifestyle ... appeared to be associated with a reduced level of risk for [breast cancer], even if the women were at higher genetic risk,” the researchers wrote. “Our findings suggest that women may be able to alter or reduce their risk of developing [breast cancer] by following healthier lifestyles,” regardless of genetic predisposition.
‘Surprising’ findings
It’s “surprising that these lifestyle changes lowered the risk of breast cancer,” said Charles Shapiro, MD, of the Icahn School of Medicine at Mount Sinai in New York, who was not involved in this study.
The study “requires replication,” he said. “On the other hand, these lifestyle changes promote overall health and certainly are associated with decreased risks of cardiovascular disease, the number one killer of women.”
“Patients always want to know what they can do above and beyond screening mammograms to reduce their risk of developing breast cancer,” said William Gradishar, MD, of Northwestern University in Chicago, who was not involved in the study.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” he said.
Among the study’s limitations, it’s unclear how the findings apply to nonwhite, nonpostmenopausal women, and the analysis did not differentiate between breast cancer subtypes.
In addition, although oral contraceptives have been linked to breast cancer in the past, there was no association in this study. Possible explanations could be that the investigators did not take into account duration of use, age of last use, and type or oral contraceptive used, they noted.
This research was funded by the National Institute for Health Research Manchester Biomedical Research Centre, the Alan Turing Institute, and a Cancer Research UK Integrated Cancer Epidemiology Programme grant. The investigators, Dr. Gradishar, and Dr. Shapiro have no relevant disclosures.
SOURCE: Al Ajmi K et al. JAMA Netw Open. 2020;3(4):e203760.
FROM JAMA NETWORK OPEN
Study supports changing classification of renal cell carcinoma
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
FROM CANCER
Despite guidelines, children receive opioids and steroids for pneumonia and sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
FROM PEDIATRICS