User login
SGLT2 inhibitors, developed for T2D, now ‘belong to cardiologists and nephrologists’
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
It’s passé to think of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drugs as agents that primarily treat hyperglycemia because their major clinical role has rapidly morphed into treating or preventing heart failure and chronic kidney disease.
This change suddenly thrust primary responsibility for prescribing these drug into the hands of cardiologists and nephrologists, though endocrinologists, diabetologists, and primary care physicians remain in the prescribing mix, experts agreed at the virtual annual scientific sessions of the American Diabetes Association.
“Glucose lowering plays little or no role in the cardiorenal protection from drugs in the sodium-glucose cotransporter 2 inhibitor class,” said David Z. Cherney, MD, a nephrologist and professor of medicine at the University of Toronto.
The SGLT2 inhibitor drugs “belong to cardiologists and nephrologists,” declared endocrinologist Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif.
But therein lies a problem. “Cardiologists and nephrologists often say that they don’t want to start SGLT2 inhibitors because they do not want to interfere with the glucose reducing medications a patient takes,” Dr. Cherney added.
“Cardiologists are absolutely afraid to prescribe SGLT2 inhibitors,” claimed John J.V. McMurray MD, a professor of medical cardiology at the University of Glasgow. “Cardiologists need to talk with diabetologists about the importance of treating heart failure” in patients with type 2 diabetes (T2D), and diabetologists “need to help cardiologists understand how to use these and other effective glucose-lowering drugs that reduce cardiovascular disease risk,” said Dr. McMurray during the ADA sessions.
“I don’t think any medical specialty owns this drug class,” said Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center. “No permission is needed” from an endocrinologist for another specialist to prescribe an SGLT2 inhibitor to patients with T2D or to appropriate patients without diabetes, he maintained.
The need for greater involvement by cardiologists in prescribing SGLT2 inhibitors to patients with T2D was underscored in findings recently reported by Dr. Inzucchi and associates. They analyzed the physician encounters that patients with T2D had with cardiologists and endocrinologists during 2017 at two U.S. health systems: one centered around clinicians affiliated with Yale Medicine and Yale University, and a second with clinicians drawn from the staffs of the Saint Luke’s Health System, including Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
During 2017, the two systems has outpatient encounters with 109,747 patients with T2D, who averaged 67 years of age and were roughly evenly split between women and men: 43% had prevalent cardiovascular disease, including 30% with coronary artery disease and 15% with heart failure. These patients had more than 110,000 physician visits, and the number of these consultations with a cardiologist was double the number with an endocrinologist, Dr. Inzucchi and associates recently reported (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
Among the 30% of T2D patients with prevalent cardiovascular disease, the consultation rate with a cardiologist was four times greater than with an endocrinologist; among the 15% with heart failure, a visit with a cardiologist was nearly seven times more common that with an endocrinologist.
“Based on these data, cardiovascular specialists encouraging the use of these medications, or, if comfortable, actually prescribing these medications, would likely significantly hasten the adoption of evidence-based glucose-lowering therapies in those patients most apt to benefit from them,” concluded the study’s authors.
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Handelsman has been a consultant to or speaker on behalf of Amarin, Amgen, Applied Therapeutic, AstraZeneca, Boehringer Ingelheim, Esperion, Gilead, Janssen, Merck, Merck-Pfizer, Novo Nordisk, Regeneron, and Sanofi. Dr. McMurray’s employer, the University of Glasgow, received payments from AstraZeneca for his involvement in trials involving dapagliflozin. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics.
FROM ADA 2020
Provide support in uncertain times
A sense of safety and stability, both emotional and physical, is crucial in promoting the healthy development of youth. Between the global pandemic, need for social distancing, economic downturn, and increased awareness of racial disparities, for many this sense of stability has been rattled.
School closures have led to a loss of social interaction, challenges to continued academic growth, and, for some students, lack of access to nutrition and increased food insecurity. For students with learning or mental health challenges, closures may have eliminated or significantly reduced desperately needed supports received in school.1 While these trying circumstances have been difficult for many, the transition back to school in the fall also may be challenging because of the uncertainty about what this will look like and possible change in routine. Some students or their families may have anxiety about returning, either because of a history of adverse experiences at school such as bullying, or because of fears about exposure for themselves or others to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The past several months also brought about greater awareness of systemic racial disparities, whether as reflected in health care, education, or the criminal justice system. According to the Centers for Disease Control and Prevention data, Latinx and African-American individuals in the United States have had a threefold greater chance of contracting SARS-CoV-2 and have a twofold greater risk of death, compared with white people in the same communities.2 Other social determinants of health – economic stability, education, social factors such as incarceration and discrimination, and neighborhood factors including access to healthy food – play a role in this vulnerability.
The pandemic has resulted in a need for social distancing, and as a result, isolation. Children and teens exposed to the news may have anxiety about what they see or hear. Additional pressures in the family can include economic uncertainty, loss of employment for the primary wage earner of the household, or stress related to family members being first responders.
Any one of these factors is a potentially significant stressor, so how do we best support youth to help them survive and hopefully thrive during this time?
- It is important to establish a sense of routine; this can help create a sense of stability and safety. Recognizing that circumstances are not the same as they were 5 or 6 months ago, encouraging structure should not come at the cost of preserving connection.
- Note positive behavior and choices made by children and make sure they know it was observed.
- Many children have experienced increased screen time with the lack of structure of the traditional school day or summer camp and extracurricular activities. Limiting screen time and being mindful of its potential impact on mood is prudent.
- Self-care for parents and guardians is important. This time is stressful for the adults of the household, let alone children who are learning self-regulation skills.
- Listen to children’s or teens’ concerns and share information in developmentally appropriate ways. It is okay to not have all of the answers.
- Balance fostering a sense of gratitude with not invalidating a child’s or teen’s experience. Showing empathy during this time is vital. While there may be other soccer seasons, it is normal to experience grief about the loss of experiences during this time.
- Parents and guardians know their children best, so it is prudent for them to be mindful of concerning changes such as an increase in sadness, anxiety, or irritability that negatively impacts daily functioning such as sleeping, eating, or relationships with family and friends.
- Promote social interactions with appropriate safeguards in place. Unfortunately, the number of SARS-CoV-2 infections is increasing in multiple states, and there is the potential to return to some of the previous restrictions. However, encouraging social interaction while following local guidelines and with cautions such as limiting the number of people present, meeting outside, or considering interacting with others who are similarly social distancing can help foster social connection and development.
- Maintain connection digitally when in-person contact is not an option.3 Social groups, places of worship, and other activities have been agile in developing virtual communities. Communication by voice and/or video is thought to be more powerful than by written communication (text, email) alone.4 However, it is important to consider those who may have limited to no access to electronic methods.
- Encourage open communication with children about diversity and bias, and consider how our interactions with others may affect our children’s perspectives.5
- As providers, it is crucial that we address structural and institutional systems that negatively impact the health, safety, and access to care including our Black, indigenous, and people of color (BIPOC) and lesbian, gay, bisexual, transgender/transsexual, queer/questioning, intersex, and allied/asexual/aromantic/agender (LGBTQIA) patients.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. Dr. Strange has no relevant financial disclosures. Email her at [email protected].
Online resources for parents and families
- Child Mind Institute: Coping With the Coronavirus Crisis: Supporting Your Kids.
- American Psychological Association: Talking with children about discrimination.
- Common Sense Media: Help with determining appropriateness of media for children.
Hotlines
- National Suicide Prevention Hotline: 1-800-273-8255
- GLBT National Hotline: 888-843-4564
- The California Peer-Run Warm Line: 1-855-845-7415
- Trevor Project: 866-488-7386 or text TREVOR to 1-202-304-1200
- Trans Lifeline: 877-565-8860
- Crisis Text Line: Text HOME to 741741
References
1. JAMA Pediatr. 2020 Apr 14. doi: 10.1001/jamapediatrics.2020.1456.
2. CDC: COVID-19 in Racial and Ethnic Minority Groups.
3. JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4469.
4. JAMA Intern Med. 2020 Apr 10. doi: 10.1001/jamainternmed.2020.1562.
5. American Psychological Association: Talking with children about discrimination.
A sense of safety and stability, both emotional and physical, is crucial in promoting the healthy development of youth. Between the global pandemic, need for social distancing, economic downturn, and increased awareness of racial disparities, for many this sense of stability has been rattled.
School closures have led to a loss of social interaction, challenges to continued academic growth, and, for some students, lack of access to nutrition and increased food insecurity. For students with learning or mental health challenges, closures may have eliminated or significantly reduced desperately needed supports received in school.1 While these trying circumstances have been difficult for many, the transition back to school in the fall also may be challenging because of the uncertainty about what this will look like and possible change in routine. Some students or their families may have anxiety about returning, either because of a history of adverse experiences at school such as bullying, or because of fears about exposure for themselves or others to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The past several months also brought about greater awareness of systemic racial disparities, whether as reflected in health care, education, or the criminal justice system. According to the Centers for Disease Control and Prevention data, Latinx and African-American individuals in the United States have had a threefold greater chance of contracting SARS-CoV-2 and have a twofold greater risk of death, compared with white people in the same communities.2 Other social determinants of health – economic stability, education, social factors such as incarceration and discrimination, and neighborhood factors including access to healthy food – play a role in this vulnerability.
The pandemic has resulted in a need for social distancing, and as a result, isolation. Children and teens exposed to the news may have anxiety about what they see or hear. Additional pressures in the family can include economic uncertainty, loss of employment for the primary wage earner of the household, or stress related to family members being first responders.
Any one of these factors is a potentially significant stressor, so how do we best support youth to help them survive and hopefully thrive during this time?
- It is important to establish a sense of routine; this can help create a sense of stability and safety. Recognizing that circumstances are not the same as they were 5 or 6 months ago, encouraging structure should not come at the cost of preserving connection.
- Note positive behavior and choices made by children and make sure they know it was observed.
- Many children have experienced increased screen time with the lack of structure of the traditional school day or summer camp and extracurricular activities. Limiting screen time and being mindful of its potential impact on mood is prudent.
- Self-care for parents and guardians is important. This time is stressful for the adults of the household, let alone children who are learning self-regulation skills.
- Listen to children’s or teens’ concerns and share information in developmentally appropriate ways. It is okay to not have all of the answers.
- Balance fostering a sense of gratitude with not invalidating a child’s or teen’s experience. Showing empathy during this time is vital. While there may be other soccer seasons, it is normal to experience grief about the loss of experiences during this time.
- Parents and guardians know their children best, so it is prudent for them to be mindful of concerning changes such as an increase in sadness, anxiety, or irritability that negatively impacts daily functioning such as sleeping, eating, or relationships with family and friends.
- Promote social interactions with appropriate safeguards in place. Unfortunately, the number of SARS-CoV-2 infections is increasing in multiple states, and there is the potential to return to some of the previous restrictions. However, encouraging social interaction while following local guidelines and with cautions such as limiting the number of people present, meeting outside, or considering interacting with others who are similarly social distancing can help foster social connection and development.
- Maintain connection digitally when in-person contact is not an option.3 Social groups, places of worship, and other activities have been agile in developing virtual communities. Communication by voice and/or video is thought to be more powerful than by written communication (text, email) alone.4 However, it is important to consider those who may have limited to no access to electronic methods.
- Encourage open communication with children about diversity and bias, and consider how our interactions with others may affect our children’s perspectives.5
- As providers, it is crucial that we address structural and institutional systems that negatively impact the health, safety, and access to care including our Black, indigenous, and people of color (BIPOC) and lesbian, gay, bisexual, transgender/transsexual, queer/questioning, intersex, and allied/asexual/aromantic/agender (LGBTQIA) patients.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. Dr. Strange has no relevant financial disclosures. Email her at [email protected].
Online resources for parents and families
- Child Mind Institute: Coping With the Coronavirus Crisis: Supporting Your Kids.
- American Psychological Association: Talking with children about discrimination.
- Common Sense Media: Help with determining appropriateness of media for children.
Hotlines
- National Suicide Prevention Hotline: 1-800-273-8255
- GLBT National Hotline: 888-843-4564
- The California Peer-Run Warm Line: 1-855-845-7415
- Trevor Project: 866-488-7386 or text TREVOR to 1-202-304-1200
- Trans Lifeline: 877-565-8860
- Crisis Text Line: Text HOME to 741741
References
1. JAMA Pediatr. 2020 Apr 14. doi: 10.1001/jamapediatrics.2020.1456.
2. CDC: COVID-19 in Racial and Ethnic Minority Groups.
3. JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4469.
4. JAMA Intern Med. 2020 Apr 10. doi: 10.1001/jamainternmed.2020.1562.
5. American Psychological Association: Talking with children about discrimination.
A sense of safety and stability, both emotional and physical, is crucial in promoting the healthy development of youth. Between the global pandemic, need for social distancing, economic downturn, and increased awareness of racial disparities, for many this sense of stability has been rattled.
School closures have led to a loss of social interaction, challenges to continued academic growth, and, for some students, lack of access to nutrition and increased food insecurity. For students with learning or mental health challenges, closures may have eliminated or significantly reduced desperately needed supports received in school.1 While these trying circumstances have been difficult for many, the transition back to school in the fall also may be challenging because of the uncertainty about what this will look like and possible change in routine. Some students or their families may have anxiety about returning, either because of a history of adverse experiences at school such as bullying, or because of fears about exposure for themselves or others to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The past several months also brought about greater awareness of systemic racial disparities, whether as reflected in health care, education, or the criminal justice system. According to the Centers for Disease Control and Prevention data, Latinx and African-American individuals in the United States have had a threefold greater chance of contracting SARS-CoV-2 and have a twofold greater risk of death, compared with white people in the same communities.2 Other social determinants of health – economic stability, education, social factors such as incarceration and discrimination, and neighborhood factors including access to healthy food – play a role in this vulnerability.
The pandemic has resulted in a need for social distancing, and as a result, isolation. Children and teens exposed to the news may have anxiety about what they see or hear. Additional pressures in the family can include economic uncertainty, loss of employment for the primary wage earner of the household, or stress related to family members being first responders.
Any one of these factors is a potentially significant stressor, so how do we best support youth to help them survive and hopefully thrive during this time?
- It is important to establish a sense of routine; this can help create a sense of stability and safety. Recognizing that circumstances are not the same as they were 5 or 6 months ago, encouraging structure should not come at the cost of preserving connection.
- Note positive behavior and choices made by children and make sure they know it was observed.
- Many children have experienced increased screen time with the lack of structure of the traditional school day or summer camp and extracurricular activities. Limiting screen time and being mindful of its potential impact on mood is prudent.
- Self-care for parents and guardians is important. This time is stressful for the adults of the household, let alone children who are learning self-regulation skills.
- Listen to children’s or teens’ concerns and share information in developmentally appropriate ways. It is okay to not have all of the answers.
- Balance fostering a sense of gratitude with not invalidating a child’s or teen’s experience. Showing empathy during this time is vital. While there may be other soccer seasons, it is normal to experience grief about the loss of experiences during this time.
- Parents and guardians know their children best, so it is prudent for them to be mindful of concerning changes such as an increase in sadness, anxiety, or irritability that negatively impacts daily functioning such as sleeping, eating, or relationships with family and friends.
- Promote social interactions with appropriate safeguards in place. Unfortunately, the number of SARS-CoV-2 infections is increasing in multiple states, and there is the potential to return to some of the previous restrictions. However, encouraging social interaction while following local guidelines and with cautions such as limiting the number of people present, meeting outside, or considering interacting with others who are similarly social distancing can help foster social connection and development.
- Maintain connection digitally when in-person contact is not an option.3 Social groups, places of worship, and other activities have been agile in developing virtual communities. Communication by voice and/or video is thought to be more powerful than by written communication (text, email) alone.4 However, it is important to consider those who may have limited to no access to electronic methods.
- Encourage open communication with children about diversity and bias, and consider how our interactions with others may affect our children’s perspectives.5
- As providers, it is crucial that we address structural and institutional systems that negatively impact the health, safety, and access to care including our Black, indigenous, and people of color (BIPOC) and lesbian, gay, bisexual, transgender/transsexual, queer/questioning, intersex, and allied/asexual/aromantic/agender (LGBTQIA) patients.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. Dr. Strange has no relevant financial disclosures. Email her at [email protected].
Online resources for parents and families
- Child Mind Institute: Coping With the Coronavirus Crisis: Supporting Your Kids.
- American Psychological Association: Talking with children about discrimination.
- Common Sense Media: Help with determining appropriateness of media for children.
Hotlines
- National Suicide Prevention Hotline: 1-800-273-8255
- GLBT National Hotline: 888-843-4564
- The California Peer-Run Warm Line: 1-855-845-7415
- Trevor Project: 866-488-7386 or text TREVOR to 1-202-304-1200
- Trans Lifeline: 877-565-8860
- Crisis Text Line: Text HOME to 741741
References
1. JAMA Pediatr. 2020 Apr 14. doi: 10.1001/jamapediatrics.2020.1456.
2. CDC: COVID-19 in Racial and Ethnic Minority Groups.
3. JAMA. 2020 Mar 23. doi: 10.1001/jama.2020.4469.
4. JAMA Intern Med. 2020 Apr 10. doi: 10.1001/jamainternmed.2020.1562.
5. American Psychological Association: Talking with children about discrimination.
Residents, fellows will get minimum 6 weeks leave for caregiving
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
Guidance addresses elders with diabetes during COVID-19
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Repetitive hits to the head tied to depression, poor cognition in later life
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
Jaw pigmentation
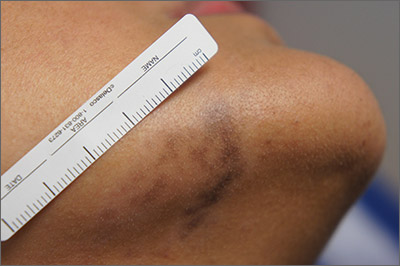
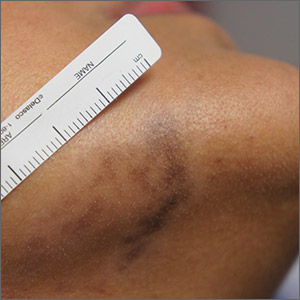
Exam and biopsy led to a diagnosis of erythema dyschromicum perstans. Notably, the punch biopsy was performed as superficially as possible to avoid injuring the marginal mandibular branch of the facial nerve. Histology showed focal vacuolar interface changes, pigmentary incontinence, and prominent dermal melanophages—consistent with erythema dyschromicum perstans.
Erythema dyschromicum perstans is an uncommon dermal macular hyperpigmentation that can affect patients of all ages. It is more often seen in patients with Fitzpatrick skin types III and IV. Some patients have a preceding inflammatory phase with associated erythema.
It is believed that erythema dyschromicum perstans may be a form of pigmented lichen planus with similar histologic changes. Genetic predisposition and medications, such as penicillamine and omeprazole, have been suggested as predisposing risk factors, although these risk factors are not always present. Similarly, cases of erythema dyschromicum perstans associated with human immunodeficiency virus and hepatitis C virus have been reported, but a causal link has not been established. Commonly affected sites include the face and neck, although patches on the trunk and extremities occur, as well.
The differential diagnosis includes lichen planus, discoid lupus, drug-induced hyperpigmentation, Hansen disease, and fixed drug eruption.
Erythema dyschromicum perstans is resistant to most therapies, yet it may clear spontaneously over the years. Therapies that have been successful in case reports include Q-switched ruby laser, topical steroids, UV therapy, isotretinoin, and clofazimine. This patient used desonide cream 0.05% bid for 3 weeks without improvement. She then used camouflage concealer for a year, at which point the area had faded almost completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Gutierrez D, Krueger LD, Tan A, et al. Proton pump inhibitor-induced erythema dyschromicum perstans-like pigmentation. JAAD Case Rep. 2019;5:701-703.

Exam and biopsy led to a diagnosis of erythema dyschromicum perstans. Notably, the punch biopsy was performed as superficially as possible to avoid injuring the marginal mandibular branch of the facial nerve. Histology showed focal vacuolar interface changes, pigmentary incontinence, and prominent dermal melanophages—consistent with erythema dyschromicum perstans.
Erythema dyschromicum perstans is an uncommon dermal macular hyperpigmentation that can affect patients of all ages. It is more often seen in patients with Fitzpatrick skin types III and IV. Some patients have a preceding inflammatory phase with associated erythema.
It is believed that erythema dyschromicum perstans may be a form of pigmented lichen planus with similar histologic changes. Genetic predisposition and medications, such as penicillamine and omeprazole, have been suggested as predisposing risk factors, although these risk factors are not always present. Similarly, cases of erythema dyschromicum perstans associated with human immunodeficiency virus and hepatitis C virus have been reported, but a causal link has not been established. Commonly affected sites include the face and neck, although patches on the trunk and extremities occur, as well.
The differential diagnosis includes lichen planus, discoid lupus, drug-induced hyperpigmentation, Hansen disease, and fixed drug eruption.
Erythema dyschromicum perstans is resistant to most therapies, yet it may clear spontaneously over the years. Therapies that have been successful in case reports include Q-switched ruby laser, topical steroids, UV therapy, isotretinoin, and clofazimine. This patient used desonide cream 0.05% bid for 3 weeks without improvement. She then used camouflage concealer for a year, at which point the area had faded almost completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Exam and biopsy led to a diagnosis of erythema dyschromicum perstans. Notably, the punch biopsy was performed as superficially as possible to avoid injuring the marginal mandibular branch of the facial nerve. Histology showed focal vacuolar interface changes, pigmentary incontinence, and prominent dermal melanophages—consistent with erythema dyschromicum perstans.
Erythema dyschromicum perstans is an uncommon dermal macular hyperpigmentation that can affect patients of all ages. It is more often seen in patients with Fitzpatrick skin types III and IV. Some patients have a preceding inflammatory phase with associated erythema.
It is believed that erythema dyschromicum perstans may be a form of pigmented lichen planus with similar histologic changes. Genetic predisposition and medications, such as penicillamine and omeprazole, have been suggested as predisposing risk factors, although these risk factors are not always present. Similarly, cases of erythema dyschromicum perstans associated with human immunodeficiency virus and hepatitis C virus have been reported, but a causal link has not been established. Commonly affected sites include the face and neck, although patches on the trunk and extremities occur, as well.
The differential diagnosis includes lichen planus, discoid lupus, drug-induced hyperpigmentation, Hansen disease, and fixed drug eruption.
Erythema dyschromicum perstans is resistant to most therapies, yet it may clear spontaneously over the years. Therapies that have been successful in case reports include Q-switched ruby laser, topical steroids, UV therapy, isotretinoin, and clofazimine. This patient used desonide cream 0.05% bid for 3 weeks without improvement. She then used camouflage concealer for a year, at which point the area had faded almost completely.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Gutierrez D, Krueger LD, Tan A, et al. Proton pump inhibitor-induced erythema dyschromicum perstans-like pigmentation. JAAD Case Rep. 2019;5:701-703.
Gutierrez D, Krueger LD, Tan A, et al. Proton pump inhibitor-induced erythema dyschromicum perstans-like pigmentation. JAAD Case Rep. 2019;5:701-703.
Ready for PRIME time? Newly ID’d cells predict RA flares
A newly identified circulating cell type may be a reliable marker for impending RA flares. The discovery and description of the cells, which bear a “striking” similarity to synovial fibroblasts, provide important clues to the origins of RA and progressive joint inflammation, investigators say.
By studying longitudinally collected blood samples from four patients with RA over 4 years, Dana E. Orange, MD and colleagues at Rockefeller University, New York, identified a pattern of B-cell activation and expansion of circulating cells that are negative for CD45 and CD31 expression, and positive for PDPN, dubbed preinflammatory mesenchymal or “PRIME” cells.
Expansion of PRIME cells in circulation increased dramatically in the weeks leading up to a flare and decreased during a flare, suggesting the possibility of a serum assay for predicting flares and allowing for early intervention to ameliorate or prevent disabling consequences, the investigators wrote in a study published in the New England Journal of Medicine.
“Our hope is that this will be a diagnostic in the future, but we need to study it in more patients to see how it will perform,” Dr. Orange said in an interview, adding that the cells, if shown to be pathogenic, could also be targets for new therapeutic strategies.
RNA sequencing
Dr. Orange and colleagues discovered the PRIME cells through a novel clinical and technical protocol involving home collection of blood by patients and longitudinal RNA sequencing to study gene expression profiles during times of both disease quiescence and flares, and noticed a distinct pattern of PRIME cell expansion, depletion, and gene expression.
“Looking at their gene expression profiles, they overlapped with fibroblasts that reside in inflamed rheumatoid arthritis synovium, and in an animal model those types of fibroblasts were important for allowing entry of inflammatory infiltrates around the joint,” she said.
PRIME cells may be a precursor of synovial fibroblasts, which have been implicated by some researchers in the spread of RA between joints, Dr. Orange added.
Patients do homework
The investigators began by enrolling four patients, followed for 1-4 years, who met 2010 American College of Rheumatology–European League Against Rheumatism criteria and who were seropositive for anti–cyclic citrullinated peptide antibodies.
They assessed disease activity from patient homes weekly or during escalation of flares up to four times daily, with the Routine Assessment of Patient Index Data 3 (RAPID3) questionnaire, as well as monthly clinic visits. At clinic visits during flares, disease activity was assessed using both the RAPID3 and 28-joint Disease Activity Score.
The patients performed fingerstick blood collection and mailed the samples overnight each week to Rockefeller University, where RNA was extracted and sequenced. The investigators identified gene transcripts that were differentially expressed in blood prior to flares, and compared them with data profiles derived from synovial single-cell RNA sequencing.
To validate the findings, the researchers used flow cytometry and sorted blood-cell RNA sequencing of samples from an additional 19 patients with RA.
They found that a total of 2,613 genes were differentially expressed during a flare, compared with baseline, and that expression of 1,437 of these genes was increased during a flare, with the remaining 1,176 decreased during flares.
Before the storm
Focusing on two flare-antecedent clusters of genes, they identified one cluster of transcripts that increased 2 weeks before a flare, enriched with genes coding for developmental pathways for naive B cells (that is, not yet exposed to antigens) and leukocytes.
The second cluster included gene transcripts that increased during the week before a flare, then decreased over the duration of the flare. Genes in this cluster were enriched for pathways that were unexpected in typical blood specimens, including genes involved in cartilage morphogenesis, endochondral bone growth, and extracellular matrix organization. The gene activity suggested the presence of a mesenchymal cell, they wrote.
The RNA expression profiles of these newly identified PRIME cells were very similar to those of synovial fibroblasts, and the investigators speculated that PRIME cells may be synovial fibroblast precursors.
They proposed a model of RA exacerbation in which PRIME cells become activated by B cells in the weeks immediately preceding a flare, and then migrate out of blood into the synovium.
The investigators are currently investigating “how reproducible this signature is in different flares in patients on different types of background therapy, and then we’re very interested in looking at the upstream triggers of the B cell and the PRIME cell,” Dr. Orange said.
“One of the reasons this is very exciting is that there are these signatures that can be found when patients are clearly asymptomatic but about to flare, and if we can intervene at that time, then the patients won’t have to live through a flare, they won’t have to have that experience,” she said.
Pros and cons
In an editorial accompanying the study, Ellen M. Gravallese, MD, from Brigham and Women’s Hospital in Boston and William H. Robinson, MD, PhD, from Stanford (Calif.) University and the Veterans Affairs Palo Alto (Calif.) Health Care System wrote that the study demonstrates an important method for identifying genetic contributions to many different types of disease.
“Orange and colleagues show that intensively collected longitudinal data from a small sample of patients can be used to identify dysregulated transcriptional signatures that are not recognized by classical cross-sectional studies. This study illustrates the exciting potential of longitudinal genomics to identify key antecedents of disease flares in an approach that may be applicable to the investigation of pathogenic and protective immune responses in a wide range of human diseases,” they wrote.
Rheumatology researcher Christopher D. Buckley, MBBS, DPhil, from the University of Birmingham (England), who was not involved in the study, said that the use of blood samples is both a strength and a weakness of the study.
“Blood is much easier to get than synovial tissue, but synovial tissue is important. If I’m trying to look at the blood and trying to make an inference about what’s going on in the synovium, if I don’t look at the synovium I don’t know what the link between the blood and synovium is,” he said in an interview.
On the plus side, “the big advantage about looking at blood is that you do multiple time points, which is really cool,” he said.
Dr. Buckley is a coauthor of a recent paper in Nature Medicine – published after the study by Dr. Orange and colleagues was accepted by the New England Journal of Medicine – showing that a population of macrophages in synovium was highly predictive of remission in patients with RA, and that therapeutic modulation of these macrophages has the potential as a treatment strategy for RA.
“We are very keen to understand the cellular basis of disease. We’re very good at understanding genes, but genes have to work in cells, and cells make organs, so the cells are critical,” he said.
The paper adds fuel to a controversy that has been raging among rheumatology researchers for more than a decade: the “flying fibroblast” hypothesis, which suggests that fibroblasts can migrate from one joint to another, hence spreading the disease in a manner akin to cancer metastases.
“It’s been quite controversial whether these cells like fibroblasts can exist in the blood, or whether they’re found in sufficient number in the blood,” Dr. Buckley said. “The fact that they have identified these PRIME cells is fascinating, because that’s going to cause us to go back and reinvestigate the whole flying fibroblast story.”
His colleague John Isaacs, MBBS, PhD, from Newcastle (England) University, is principal investigator for the BIO-FLARE study, in which participants with RA stop taking their disease-modifying antirheumatic drugs under close supervision of researchers. The investigators then study the patients looking for flare signals as well as the biology of flares themselves.
“As it happens, our protocols would not pick up this particular cell, because we have not been focusing on the stroma, at least not in peripheral blood. We’ve all been looking at synovium as part of BIO-FLARE,” he said in an interview. “We will be looking for this cell now that we have seen this research, and certainly we would want to replicate.”
He agreed with Dr. Buckley’s observation that the PRIME cell data may revive the flying fibroblast hypothesis. “This is a great paper in a top clinical journal. What isn’t there is mechanism. That’s the thing we’re all going to want to understand now: Where do the cells come from, how do they actually trigger flares, and how do they go down as flare starts?”
Both Dr. Buckley and Dr. Isaacs agreed that the study findings point to important new avenues of research, but also noted that the study was small, involving a total of only 23 patients, and that replication of the findings and elucidation of the mechanism of PRIME cell generation and disposition will be required.
The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the PRIME cells. Dr. Buckley and Dr. Isaacs reported no relevant conflicts of interest.
SOURCE: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
A newly identified circulating cell type may be a reliable marker for impending RA flares. The discovery and description of the cells, which bear a “striking” similarity to synovial fibroblasts, provide important clues to the origins of RA and progressive joint inflammation, investigators say.
By studying longitudinally collected blood samples from four patients with RA over 4 years, Dana E. Orange, MD and colleagues at Rockefeller University, New York, identified a pattern of B-cell activation and expansion of circulating cells that are negative for CD45 and CD31 expression, and positive for PDPN, dubbed preinflammatory mesenchymal or “PRIME” cells.
Expansion of PRIME cells in circulation increased dramatically in the weeks leading up to a flare and decreased during a flare, suggesting the possibility of a serum assay for predicting flares and allowing for early intervention to ameliorate or prevent disabling consequences, the investigators wrote in a study published in the New England Journal of Medicine.
“Our hope is that this will be a diagnostic in the future, but we need to study it in more patients to see how it will perform,” Dr. Orange said in an interview, adding that the cells, if shown to be pathogenic, could also be targets for new therapeutic strategies.
RNA sequencing
Dr. Orange and colleagues discovered the PRIME cells through a novel clinical and technical protocol involving home collection of blood by patients and longitudinal RNA sequencing to study gene expression profiles during times of both disease quiescence and flares, and noticed a distinct pattern of PRIME cell expansion, depletion, and gene expression.
“Looking at their gene expression profiles, they overlapped with fibroblasts that reside in inflamed rheumatoid arthritis synovium, and in an animal model those types of fibroblasts were important for allowing entry of inflammatory infiltrates around the joint,” she said.
PRIME cells may be a precursor of synovial fibroblasts, which have been implicated by some researchers in the spread of RA between joints, Dr. Orange added.
Patients do homework
The investigators began by enrolling four patients, followed for 1-4 years, who met 2010 American College of Rheumatology–European League Against Rheumatism criteria and who were seropositive for anti–cyclic citrullinated peptide antibodies.
They assessed disease activity from patient homes weekly or during escalation of flares up to four times daily, with the Routine Assessment of Patient Index Data 3 (RAPID3) questionnaire, as well as monthly clinic visits. At clinic visits during flares, disease activity was assessed using both the RAPID3 and 28-joint Disease Activity Score.
The patients performed fingerstick blood collection and mailed the samples overnight each week to Rockefeller University, where RNA was extracted and sequenced. The investigators identified gene transcripts that were differentially expressed in blood prior to flares, and compared them with data profiles derived from synovial single-cell RNA sequencing.
To validate the findings, the researchers used flow cytometry and sorted blood-cell RNA sequencing of samples from an additional 19 patients with RA.
They found that a total of 2,613 genes were differentially expressed during a flare, compared with baseline, and that expression of 1,437 of these genes was increased during a flare, with the remaining 1,176 decreased during flares.
Before the storm
Focusing on two flare-antecedent clusters of genes, they identified one cluster of transcripts that increased 2 weeks before a flare, enriched with genes coding for developmental pathways for naive B cells (that is, not yet exposed to antigens) and leukocytes.
The second cluster included gene transcripts that increased during the week before a flare, then decreased over the duration of the flare. Genes in this cluster were enriched for pathways that were unexpected in typical blood specimens, including genes involved in cartilage morphogenesis, endochondral bone growth, and extracellular matrix organization. The gene activity suggested the presence of a mesenchymal cell, they wrote.
The RNA expression profiles of these newly identified PRIME cells were very similar to those of synovial fibroblasts, and the investigators speculated that PRIME cells may be synovial fibroblast precursors.
They proposed a model of RA exacerbation in which PRIME cells become activated by B cells in the weeks immediately preceding a flare, and then migrate out of blood into the synovium.
The investigators are currently investigating “how reproducible this signature is in different flares in patients on different types of background therapy, and then we’re very interested in looking at the upstream triggers of the B cell and the PRIME cell,” Dr. Orange said.
“One of the reasons this is very exciting is that there are these signatures that can be found when patients are clearly asymptomatic but about to flare, and if we can intervene at that time, then the patients won’t have to live through a flare, they won’t have to have that experience,” she said.
Pros and cons
In an editorial accompanying the study, Ellen M. Gravallese, MD, from Brigham and Women’s Hospital in Boston and William H. Robinson, MD, PhD, from Stanford (Calif.) University and the Veterans Affairs Palo Alto (Calif.) Health Care System wrote that the study demonstrates an important method for identifying genetic contributions to many different types of disease.
“Orange and colleagues show that intensively collected longitudinal data from a small sample of patients can be used to identify dysregulated transcriptional signatures that are not recognized by classical cross-sectional studies. This study illustrates the exciting potential of longitudinal genomics to identify key antecedents of disease flares in an approach that may be applicable to the investigation of pathogenic and protective immune responses in a wide range of human diseases,” they wrote.
Rheumatology researcher Christopher D. Buckley, MBBS, DPhil, from the University of Birmingham (England), who was not involved in the study, said that the use of blood samples is both a strength and a weakness of the study.
“Blood is much easier to get than synovial tissue, but synovial tissue is important. If I’m trying to look at the blood and trying to make an inference about what’s going on in the synovium, if I don’t look at the synovium I don’t know what the link between the blood and synovium is,” he said in an interview.
On the plus side, “the big advantage about looking at blood is that you do multiple time points, which is really cool,” he said.
Dr. Buckley is a coauthor of a recent paper in Nature Medicine – published after the study by Dr. Orange and colleagues was accepted by the New England Journal of Medicine – showing that a population of macrophages in synovium was highly predictive of remission in patients with RA, and that therapeutic modulation of these macrophages has the potential as a treatment strategy for RA.
“We are very keen to understand the cellular basis of disease. We’re very good at understanding genes, but genes have to work in cells, and cells make organs, so the cells are critical,” he said.
The paper adds fuel to a controversy that has been raging among rheumatology researchers for more than a decade: the “flying fibroblast” hypothesis, which suggests that fibroblasts can migrate from one joint to another, hence spreading the disease in a manner akin to cancer metastases.
“It’s been quite controversial whether these cells like fibroblasts can exist in the blood, or whether they’re found in sufficient number in the blood,” Dr. Buckley said. “The fact that they have identified these PRIME cells is fascinating, because that’s going to cause us to go back and reinvestigate the whole flying fibroblast story.”
His colleague John Isaacs, MBBS, PhD, from Newcastle (England) University, is principal investigator for the BIO-FLARE study, in which participants with RA stop taking their disease-modifying antirheumatic drugs under close supervision of researchers. The investigators then study the patients looking for flare signals as well as the biology of flares themselves.
“As it happens, our protocols would not pick up this particular cell, because we have not been focusing on the stroma, at least not in peripheral blood. We’ve all been looking at synovium as part of BIO-FLARE,” he said in an interview. “We will be looking for this cell now that we have seen this research, and certainly we would want to replicate.”
He agreed with Dr. Buckley’s observation that the PRIME cell data may revive the flying fibroblast hypothesis. “This is a great paper in a top clinical journal. What isn’t there is mechanism. That’s the thing we’re all going to want to understand now: Where do the cells come from, how do they actually trigger flares, and how do they go down as flare starts?”
Both Dr. Buckley and Dr. Isaacs agreed that the study findings point to important new avenues of research, but also noted that the study was small, involving a total of only 23 patients, and that replication of the findings and elucidation of the mechanism of PRIME cell generation and disposition will be required.
The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the PRIME cells. Dr. Buckley and Dr. Isaacs reported no relevant conflicts of interest.
SOURCE: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
A newly identified circulating cell type may be a reliable marker for impending RA flares. The discovery and description of the cells, which bear a “striking” similarity to synovial fibroblasts, provide important clues to the origins of RA and progressive joint inflammation, investigators say.
By studying longitudinally collected blood samples from four patients with RA over 4 years, Dana E. Orange, MD and colleagues at Rockefeller University, New York, identified a pattern of B-cell activation and expansion of circulating cells that are negative for CD45 and CD31 expression, and positive for PDPN, dubbed preinflammatory mesenchymal or “PRIME” cells.
Expansion of PRIME cells in circulation increased dramatically in the weeks leading up to a flare and decreased during a flare, suggesting the possibility of a serum assay for predicting flares and allowing for early intervention to ameliorate or prevent disabling consequences, the investigators wrote in a study published in the New England Journal of Medicine.
“Our hope is that this will be a diagnostic in the future, but we need to study it in more patients to see how it will perform,” Dr. Orange said in an interview, adding that the cells, if shown to be pathogenic, could also be targets for new therapeutic strategies.
RNA sequencing
Dr. Orange and colleagues discovered the PRIME cells through a novel clinical and technical protocol involving home collection of blood by patients and longitudinal RNA sequencing to study gene expression profiles during times of both disease quiescence and flares, and noticed a distinct pattern of PRIME cell expansion, depletion, and gene expression.
“Looking at their gene expression profiles, they overlapped with fibroblasts that reside in inflamed rheumatoid arthritis synovium, and in an animal model those types of fibroblasts were important for allowing entry of inflammatory infiltrates around the joint,” she said.
PRIME cells may be a precursor of synovial fibroblasts, which have been implicated by some researchers in the spread of RA between joints, Dr. Orange added.
Patients do homework
The investigators began by enrolling four patients, followed for 1-4 years, who met 2010 American College of Rheumatology–European League Against Rheumatism criteria and who were seropositive for anti–cyclic citrullinated peptide antibodies.
They assessed disease activity from patient homes weekly or during escalation of flares up to four times daily, with the Routine Assessment of Patient Index Data 3 (RAPID3) questionnaire, as well as monthly clinic visits. At clinic visits during flares, disease activity was assessed using both the RAPID3 and 28-joint Disease Activity Score.
The patients performed fingerstick blood collection and mailed the samples overnight each week to Rockefeller University, where RNA was extracted and sequenced. The investigators identified gene transcripts that were differentially expressed in blood prior to flares, and compared them with data profiles derived from synovial single-cell RNA sequencing.
To validate the findings, the researchers used flow cytometry and sorted blood-cell RNA sequencing of samples from an additional 19 patients with RA.
They found that a total of 2,613 genes were differentially expressed during a flare, compared with baseline, and that expression of 1,437 of these genes was increased during a flare, with the remaining 1,176 decreased during flares.
Before the storm
Focusing on two flare-antecedent clusters of genes, they identified one cluster of transcripts that increased 2 weeks before a flare, enriched with genes coding for developmental pathways for naive B cells (that is, not yet exposed to antigens) and leukocytes.
The second cluster included gene transcripts that increased during the week before a flare, then decreased over the duration of the flare. Genes in this cluster were enriched for pathways that were unexpected in typical blood specimens, including genes involved in cartilage morphogenesis, endochondral bone growth, and extracellular matrix organization. The gene activity suggested the presence of a mesenchymal cell, they wrote.
The RNA expression profiles of these newly identified PRIME cells were very similar to those of synovial fibroblasts, and the investigators speculated that PRIME cells may be synovial fibroblast precursors.
They proposed a model of RA exacerbation in which PRIME cells become activated by B cells in the weeks immediately preceding a flare, and then migrate out of blood into the synovium.
The investigators are currently investigating “how reproducible this signature is in different flares in patients on different types of background therapy, and then we’re very interested in looking at the upstream triggers of the B cell and the PRIME cell,” Dr. Orange said.
“One of the reasons this is very exciting is that there are these signatures that can be found when patients are clearly asymptomatic but about to flare, and if we can intervene at that time, then the patients won’t have to live through a flare, they won’t have to have that experience,” she said.
Pros and cons
In an editorial accompanying the study, Ellen M. Gravallese, MD, from Brigham and Women’s Hospital in Boston and William H. Robinson, MD, PhD, from Stanford (Calif.) University and the Veterans Affairs Palo Alto (Calif.) Health Care System wrote that the study demonstrates an important method for identifying genetic contributions to many different types of disease.
“Orange and colleagues show that intensively collected longitudinal data from a small sample of patients can be used to identify dysregulated transcriptional signatures that are not recognized by classical cross-sectional studies. This study illustrates the exciting potential of longitudinal genomics to identify key antecedents of disease flares in an approach that may be applicable to the investigation of pathogenic and protective immune responses in a wide range of human diseases,” they wrote.
Rheumatology researcher Christopher D. Buckley, MBBS, DPhil, from the University of Birmingham (England), who was not involved in the study, said that the use of blood samples is both a strength and a weakness of the study.
“Blood is much easier to get than synovial tissue, but synovial tissue is important. If I’m trying to look at the blood and trying to make an inference about what’s going on in the synovium, if I don’t look at the synovium I don’t know what the link between the blood and synovium is,” he said in an interview.
On the plus side, “the big advantage about looking at blood is that you do multiple time points, which is really cool,” he said.
Dr. Buckley is a coauthor of a recent paper in Nature Medicine – published after the study by Dr. Orange and colleagues was accepted by the New England Journal of Medicine – showing that a population of macrophages in synovium was highly predictive of remission in patients with RA, and that therapeutic modulation of these macrophages has the potential as a treatment strategy for RA.
“We are very keen to understand the cellular basis of disease. We’re very good at understanding genes, but genes have to work in cells, and cells make organs, so the cells are critical,” he said.
The paper adds fuel to a controversy that has been raging among rheumatology researchers for more than a decade: the “flying fibroblast” hypothesis, which suggests that fibroblasts can migrate from one joint to another, hence spreading the disease in a manner akin to cancer metastases.
“It’s been quite controversial whether these cells like fibroblasts can exist in the blood, or whether they’re found in sufficient number in the blood,” Dr. Buckley said. “The fact that they have identified these PRIME cells is fascinating, because that’s going to cause us to go back and reinvestigate the whole flying fibroblast story.”
His colleague John Isaacs, MBBS, PhD, from Newcastle (England) University, is principal investigator for the BIO-FLARE study, in which participants with RA stop taking their disease-modifying antirheumatic drugs under close supervision of researchers. The investigators then study the patients looking for flare signals as well as the biology of flares themselves.
“As it happens, our protocols would not pick up this particular cell, because we have not been focusing on the stroma, at least not in peripheral blood. We’ve all been looking at synovium as part of BIO-FLARE,” he said in an interview. “We will be looking for this cell now that we have seen this research, and certainly we would want to replicate.”
He agreed with Dr. Buckley’s observation that the PRIME cell data may revive the flying fibroblast hypothesis. “This is a great paper in a top clinical journal. What isn’t there is mechanism. That’s the thing we’re all going to want to understand now: Where do the cells come from, how do they actually trigger flares, and how do they go down as flare starts?”
Both Dr. Buckley and Dr. Isaacs agreed that the study findings point to important new avenues of research, but also noted that the study was small, involving a total of only 23 patients, and that replication of the findings and elucidation of the mechanism of PRIME cell generation and disposition will be required.
The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the PRIME cells. Dr. Buckley and Dr. Isaacs reported no relevant conflicts of interest.
SOURCE: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A newly identified cell type may be predictive of RA flares.
Major finding: Preinflammatory mesenchymal cells were expanded in the week preceding a flare and decreased during the flare.
Study details: A longitudinal observational study of 23 patients with RA.
Disclosures: The study was supported by grants from the National Institutes of Health, Simons Foundation, Robertson Foundation, Rheumatology Research Foundation, Bernard and Irene Schwartz Foundation, the Iris and Junming Le Foundation, and Rockefeller University. Dr. Orange disclosed a provisional patent for the discovery of the preinflammatory mesenchymal cells.
Source: Orange DE et al. N Engl J Med. 2020 Jul 15. doi: 10.1056/NEJMoa2004114.
Novel program cuts weight retention after gestational diabetes
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
An online, lifestyle-based weight loss initiative known as the Balance After Baby (BAB) program is effective at reducing weight retention a year after birth among women with recent gestational diabetes.
Specifically, results of the study were positive in women of most ethnicities, bar those of a small group of Hispanic origin.
Jacinda Nicklas, MD, from the University of Colorado at Denver, Aurora, presented findings of the BAB trial during the virtual annual scientific sessions of the American Diabetes Association. She was coprincipal investigator alongside Ellen Seely, MD, from Brigham and Women’s Hospital, Boston.
“Looking at the entire population of women on the BAB program, there was a trend in weight loss from 6 weeks postpartum to 12 months (P = .09), and significantly less postpartum weight retention at 12 months (P = .04),” Dr. Nicklas said.
“Through this effect on postpartum weight retention, the BAB program has potential to delay or prevent development of type 2 diabetes in women with recent gestational diabetes, while the web-based, remote nature of the program is scalable and very relevant in current times,” she added. “However, the lack of efficacy in Hispanic women means it needs to be modified to be successful in this ethnic group.”
Frank Qian, MD, who also presented during the same session, said the BAB program has potential as a viable way of preventing both future pregnancy complications and the progression to overt type 2 diabetes in this high-risk population.
“Large-scale epidemiologic studies show us that weight gain from pregnancy is a major risk factor for long-term cardiometabolic risk, particularly for women with a history of gestational diabetes,” he observed. “In turn, it is critical to implement lifestyle interventions that can help women get as close to the weight they were before pregnancy as possible and keep that weight off.”
Postpartum weight retention a modifiable risk factor for type 2 diabetes
Current evidence shows that a large proportion of women who develop gestational diabetes go on to develop type 2 diabetes within 10 years and that women with a history of gestational diabetes are more likely to retain or gain weight postpartum.
Dr. Nicklas also pointed out that obesity and weight gain are the strongest modifiable risk factors for type 2 diabetes.
“We know from the Diabetes Prevention Program [DPP] that an intensive lifestyle program in women who had had gestational diabetes led to a 53% reduction in type 2 diabetes,” Dr. Nicklas noted.
However, she added there were barriers to adhering to the intensive DPP program – which required 16 one-on-one meetings in the first 24 weeks – including travel, as some participants lived quite remotely, or family responsibilities. Consequently, Dr. Nicklas and colleagues developed the BAB pilot trial, which involved web-based delivery with remote coaching.
The trial involved women with a history of gestational diabetes who were, on average, 7 weeks postpartum. The key outcome was weight at 12 months, compared with both 6-week postpartum weight and prepregnancy weight.
Based on encouraging results in the pilot trial – in which the intervention group showed significant weight loss from 6-week postpartum weight and in 12-month weight retention – a larger, two-site trial was initiated, the BAB Intervention randomized, controlled trial.
Outcome measures were the same as for the pilot study. The 181 participants were aged 18-45 years, had recent gestational diabetes, and had a mean prepregnancy body mass index of approximately 29 kg/m2. Around half were college educated, and 28% were from lower income households. Overall, 48% were white, 22% Asian, 17% African American, and 13% were of other ethnicities, with just over a third being Hispanic.
The initial study visit was at 6 weeks postpartum. Women were randomized to the behavioral intervention website plus a lifestyle coach group or to a control group that consisted of a website plus knowledge links.
The intervention website required women to complete some DPP-derived and bonus modules, and also featured action plans, tracked weight and steps, and had a direct link to contact their lifestyle coach. Follow-up visits were held at 6 and 12 months and A1c, waist circumference, and height/weight were measured. A total of 86% eligible women completed the 6- and 12-month visits.
Why didn’t the BAB program work in Hispanic women?
“The overall result showed that weight change from 6 weeks postpartum to 12 months revealed a slight gain in the control group of 1.3 pounds and a loss in the intervention group of 1.8 pounds, resulting in a between-group difference of 3.1 pounds [P = .09],” reported Dr. Nicklas. Adjustment for gestational weight gain and breastfeeding had no substantial effect.
When 12-month weight retention versus prepregnancy weight was assessed, the former was halved in participants in the BAB program.
The control group gained a mean of 10.1 pounds, and those in the intervention group gained a mean of 5.3 pounds, equivalent to a difference of 4.8 pounds (P = .04).
A prespecified analysis was conducted of 120 non-Hispanic women. At 12 months, weight retention, compared with prepregnancy weight showed an increase of 9 pounds in the control group versus 1.8 pounds in the intervention group (P = .01).
By comparison, in the small group of Hispanic women only, weight retention at 12 months compared to prepregnancy weight showed a 12.7-pound increase and a 13.3-pound increase in the control and intervention groups respectively, reported Dr. Nicklas.
Addressing the key question of why the BAB program was ineffective in Hispanic women, Dr. Nicklas said, “The literature tells us that low income Hispanic women are twice as likely to experience postpartum weight retention compared to white non-Hispanic women. But we also know that low-income Hispanic women generally engage less with interventions, and there is a higher acceptance of overweight among this ethnic group.”
The researchers hope to follow the women from their trial to determine who progresses to type 2 diabetes.
“Hispanic women are a high-risk population for gestational diabetes and type 2 diabetes, and we plan to identify the best options to help Hispanic women with a history of gestational diabetes prevent type 2 diabetes,” Dr. Nicklas said in an interview.
Dr. Qian also remarked on the differences observed in the weight loss outcomes for non-Hispanic versus Hispanic women, noting that it highlights the importance of studying lifestyle interventions in diverse populations. “Environmental and cultural factors that may differ across different racial or ethnic groups could impact the effectiveness of such interventions.
Dr. Nicklas and Dr. Qian have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ADA 2020
COVID-19: A primary care perspective
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at [email protected].
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at [email protected].
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at [email protected].
This article was updated 7/16/2020.
PD-1 Signaling in Extramammary Paget Disease
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Resident Pearls
- Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts, while secondary EMPD is an extension of malignant cells from an underlying internal neoplasm.
- Surgical margin clearance in EMPD often is problematic, with high recurrence rates indicating the need for additional treatment modalities.
- Programmed cell death receptor 1 (PD-1) signaling can serve as a potential target in EMPD. Further studies and clinical trials are needed to test the efficacy of PD-1 inhibitors in unresectable or invasive/metastatic EMPD.