User login
Apps for applying to ObGyn residency programs in the era of virtual interviews
The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
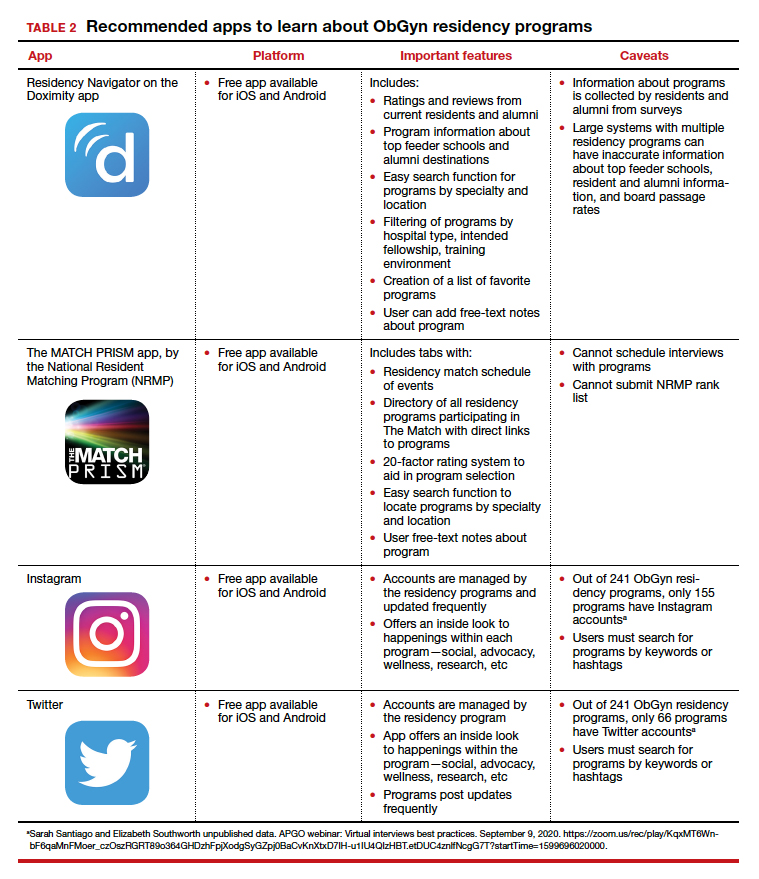
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.
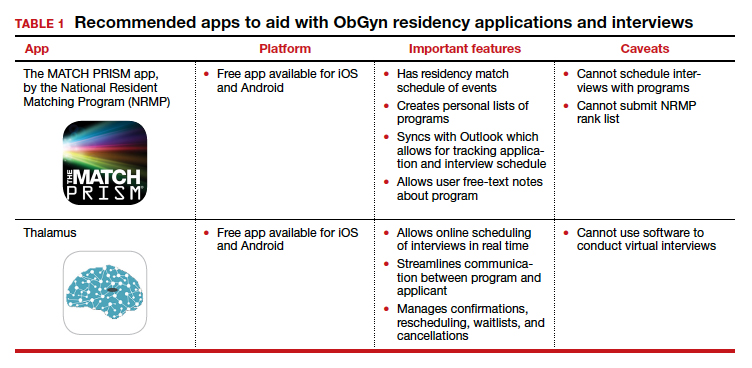
We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●
- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.

We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●

The coronavirus disease 2019 (COVID-19) pandemic has upended the traditional 2020–2021 application season for ObGyn residency programs. In May 2020, the 2 national ObGyn education organizations, the Association of Professors of Gynecology and Obstetrics (APGO) and Council on Resident Education in ObGyn (CREOG), issued guidelines to ensure a fair and equitable application process.1 These guidelines are consistent with recommendations from the Association of American Medical Colleges (AAMC) and the Coalition for Physician Accountability. Important recommendations include:
- limiting away rotations
- being flexible in the number of specialty-specific letters of recommendation required
- encouraging residency programs to develop alternate means of conveying information about their curriculum.
In addition, these statements provide timing on when programs should release interview offers and when to begin interviews. Finally, programs are required to commit to online interviews and virtual visits for all applicants, including local students, rather than in-person interviews.
Here, we focus on identifying apps that students can use to help them with the application process—apps for the nuts and bolts of applying and interviewing and apps to learn more about individual programs.
Students must use the Electronic Residency Application Service (ERAS) platform from AAMC to enter their information and register with the National Resident Matching Program (NRMP). Students also must use the ERAS to submit their applications to their selected residency programs. The ERAS platform does not include an app to aid in the completion or submission of an application. The NRMP has developed the MATCH PRISM app, but this does not allow students to register for the match or submit their rank list. To learn about how to schedule interviews, residency programs may use one of the following sources: ERAS, Interview Broker, or Thalamus. Moreover, APGO/CREOG has partnered with Thalamus for the upcoming application cycle, which provides residency programs and applicants tools for application management, interview scheduling, and itinerary building. Thalamus offers a free app.
This year offers some unique challenges. The application process for ObGyn residencies is likely to be more competitive, and students face the added stress of having to navigate the interview season:
- without away rotations (audition interviews)
- without in-person visits of the city/hospital/program or social events before or after interview day
- with an all-virtual interview day.
Continue to: To find information on individual residency programs...
To find information on individual residency programs, the APGO website lists the FREIDA and APGO Residency Directories, which are not apps. Students are also aware of the Doximity Residency Navigator, which does include an app. The NRMP MATCH PRISM app is another resource, as it provides students with a directory of residency programs and information about each program.
The American College of Obstetricians and Gynecologists (ACOG) recognizes that residency program websites and social media will be crucial in helping applicants learn about individual programs, faculty, and residents. As such, ACOG hosted a Virtual Residency Showcase in September 2020 in which programs posted content on Instagram and Twitter using the hashtag #ACOG-ResWeek20.2 Similarly, APGO and CREOG produced a report containing a social media directory, which lists individual residency programs and whether or not they have a social media handle/account.3 In a recent webinar,4 Drs. Sarah Santiago and Elizabeth Southworth noted that the number of residency programs that have an Instagram account more than doubled (from 60 to 128) between May and September 2020.

We present 2 tables describing the important features and caveats of apps available to students to assist them with residency applications this year—TABLE 1 summarizes apps to aid with applications and interviews; TABLE 2 lists apps designed for students to learn more about individual residency programs. We wish all of this year’s students every success in their search for the right program. ●

- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
- Association of Professors of Gynecology and Obstetrics, Council on Resident Education in ObGyn. Updated APGO and CREOG Residency Application Response to COVID-19. https://www.apgo.org/wp-content/uploads/2020/05 /Updated-APGO-CREOG-Residency-Response-to -COVID-19-.pdf. Accessed October 27, 2020.
- https://www.acog.org/education-and-events/webinars /virtual-residency-showcase. Accessed October 4, 2020.
- Social media directory-ObGyn. https://docs.google.com /spreadsheets/d/e/2PACX-1vQ6boyn7FWV9tEhfQp1o3 XJgNIPNBQ3qCYf4IpV-rOPcd212J-HNR84p0r85nXrAz MvOmcNlgjywDP/pubhtml?gid=1472916499&single =true. Accessed October 27, 2020.
- APGO webinar: Virtual interviews best practices. September 9, 2020. https://zoom.us/rec/play/KqxMT6Wnb F6qaMnFMoer_czOszRGRT89o364GHDzhFpjXodgSyGZpj 0BaCvKnXtxD7IH-u1IU4QIzHBT.etDUC4znlfNcgG7T?start Time=1599696020000. Accessed October 4, 2020.
When Female Patients with MS Ask About Breastfeeding, Here’s What to Tell Them
Chances are your female patients of childbearing age with multiple sclerosis—particularly if they become pregnant—will ask about breastfeeding. What are they likely to ask, and how should you answer? Here’s a quick rundown.
What kind of impact will breastfeeding have on my child?
We know that MS is not a genetic disease per se-it is neither autosomal recessive nor dominant. But there is an increased risk among family members, particularly first-degree relatives. If a patient asks, you can tell them it appears that infants who are breastfed are less likely to develop pediatric-onset MS.
In 2017, Brenton and colleagues asked individuals who experienced pediatric-onset MS (n=36) and those in a control group (n=72) to complete a questionnaire that covered breastfeeding history and other birth and demographic features. While most demographic and birth features were similar, 36% of those in the pediatric-onset MS group reported being breastfed, compared with 71% of controls. Individuals who were not breastfed were nearly 4.5 times more likely to be diagnosed with pediatric-onset MS.
How will breastfeeding impact my risk of MS relapse after giving birth?
The issue of breastfeeding and MS relapses is somewhat controversial. In 1988, Nelson and colleagues found that among 191 women with MS who became pregnant, 10% relapsed during pregnancy, but relapse rate rose to 34% during the 9 months after birth. Moreover, nearly 4 in 10 of those who breastfed experienced exacerbations, versus 3 in 10 among those who did not.
However, more recent studies demonstrate no association with breastfeeding and relapse. Just this year, Gould and colleagues published a study showing that among 466 pregnancies, annualized relapse rates declined during pregnancy, and there was no increase seen in the postpartum period. Moreover, women who exclusively breastfed saw their risk of an early postpartum relapse lowered by 63%.
In late 2019, Krysko and colleagues published a meta-analysis of 24 studies involving nearly 3,000 women with MS which showed that breastfeeds were 43% less likely to experience postpartum relapse compared with their non-breastfeeding counterparts. The link was stronger in studies where women breastfed exclusively.
The bottom line: There is a plurality of physicians who believe that breastfeeding has a protective effect – and most will tell you that you should recommend exclusive breastfeeding.
What medicines can I take that will not adversely affect me and my baby?
Once a woman knows that breastfeeding could help her offspring avoid developing MS, and minimize her chance of a postpartum relapse, she will likely ask what to do about medications. You answer will depends on what she’s taking.
- Drugs she can take with relative peace of mind. Most experts believe it is safe to take corticosteroids and breastfeed. In fact, women who relapse while breastfeeding will in all likelihood be given intravenous corticosteroids, such as methylprednisolone. These medications are present in the blood at very low levels, peak an hour after infusion, and quickly dissipate. So, it’s important to tell your patients to delay breastfeeding by 2 to 4 hours after they receive the steroid.
- Drugs that are potentially concerning and require close monitoring. For the so-called platform therapies—such as interferon beta/glatiramer acetate, natalizumab, and their generic equivalents—there are no large studies that clearly demonstrate safety. Still, they are generally thought to be safe. Be sure to heed FDA labeling: weigh breastfeeding benefit against the potential risk
- Drug to avoid entirely. Under no circumstances should breastfeeding women receive teriflunomide, cladribine, alemtuzumab, or mitoxantrone. The jury is still out on rituximab—which is not yet approved for MS in the United States—and ocrelizumab. For now, err on the safe side and switch to another therapy.
Chances are your female patients of childbearing age with multiple sclerosis—particularly if they become pregnant—will ask about breastfeeding. What are they likely to ask, and how should you answer? Here’s a quick rundown.
What kind of impact will breastfeeding have on my child?
We know that MS is not a genetic disease per se-it is neither autosomal recessive nor dominant. But there is an increased risk among family members, particularly first-degree relatives. If a patient asks, you can tell them it appears that infants who are breastfed are less likely to develop pediatric-onset MS.
In 2017, Brenton and colleagues asked individuals who experienced pediatric-onset MS (n=36) and those in a control group (n=72) to complete a questionnaire that covered breastfeeding history and other birth and demographic features. While most demographic and birth features were similar, 36% of those in the pediatric-onset MS group reported being breastfed, compared with 71% of controls. Individuals who were not breastfed were nearly 4.5 times more likely to be diagnosed with pediatric-onset MS.
How will breastfeeding impact my risk of MS relapse after giving birth?
The issue of breastfeeding and MS relapses is somewhat controversial. In 1988, Nelson and colleagues found that among 191 women with MS who became pregnant, 10% relapsed during pregnancy, but relapse rate rose to 34% during the 9 months after birth. Moreover, nearly 4 in 10 of those who breastfed experienced exacerbations, versus 3 in 10 among those who did not.
However, more recent studies demonstrate no association with breastfeeding and relapse. Just this year, Gould and colleagues published a study showing that among 466 pregnancies, annualized relapse rates declined during pregnancy, and there was no increase seen in the postpartum period. Moreover, women who exclusively breastfed saw their risk of an early postpartum relapse lowered by 63%.
In late 2019, Krysko and colleagues published a meta-analysis of 24 studies involving nearly 3,000 women with MS which showed that breastfeeds were 43% less likely to experience postpartum relapse compared with their non-breastfeeding counterparts. The link was stronger in studies where women breastfed exclusively.
The bottom line: There is a plurality of physicians who believe that breastfeeding has a protective effect – and most will tell you that you should recommend exclusive breastfeeding.
What medicines can I take that will not adversely affect me and my baby?
Once a woman knows that breastfeeding could help her offspring avoid developing MS, and minimize her chance of a postpartum relapse, she will likely ask what to do about medications. You answer will depends on what she’s taking.
- Drugs she can take with relative peace of mind. Most experts believe it is safe to take corticosteroids and breastfeed. In fact, women who relapse while breastfeeding will in all likelihood be given intravenous corticosteroids, such as methylprednisolone. These medications are present in the blood at very low levels, peak an hour after infusion, and quickly dissipate. So, it’s important to tell your patients to delay breastfeeding by 2 to 4 hours after they receive the steroid.
- Drugs that are potentially concerning and require close monitoring. For the so-called platform therapies—such as interferon beta/glatiramer acetate, natalizumab, and their generic equivalents—there are no large studies that clearly demonstrate safety. Still, they are generally thought to be safe. Be sure to heed FDA labeling: weigh breastfeeding benefit against the potential risk
- Drug to avoid entirely. Under no circumstances should breastfeeding women receive teriflunomide, cladribine, alemtuzumab, or mitoxantrone. The jury is still out on rituximab—which is not yet approved for MS in the United States—and ocrelizumab. For now, err on the safe side and switch to another therapy.
Chances are your female patients of childbearing age with multiple sclerosis—particularly if they become pregnant—will ask about breastfeeding. What are they likely to ask, and how should you answer? Here’s a quick rundown.
What kind of impact will breastfeeding have on my child?
We know that MS is not a genetic disease per se-it is neither autosomal recessive nor dominant. But there is an increased risk among family members, particularly first-degree relatives. If a patient asks, you can tell them it appears that infants who are breastfed are less likely to develop pediatric-onset MS.
In 2017, Brenton and colleagues asked individuals who experienced pediatric-onset MS (n=36) and those in a control group (n=72) to complete a questionnaire that covered breastfeeding history and other birth and demographic features. While most demographic and birth features were similar, 36% of those in the pediatric-onset MS group reported being breastfed, compared with 71% of controls. Individuals who were not breastfed were nearly 4.5 times more likely to be diagnosed with pediatric-onset MS.
How will breastfeeding impact my risk of MS relapse after giving birth?
The issue of breastfeeding and MS relapses is somewhat controversial. In 1988, Nelson and colleagues found that among 191 women with MS who became pregnant, 10% relapsed during pregnancy, but relapse rate rose to 34% during the 9 months after birth. Moreover, nearly 4 in 10 of those who breastfed experienced exacerbations, versus 3 in 10 among those who did not.
However, more recent studies demonstrate no association with breastfeeding and relapse. Just this year, Gould and colleagues published a study showing that among 466 pregnancies, annualized relapse rates declined during pregnancy, and there was no increase seen in the postpartum period. Moreover, women who exclusively breastfed saw their risk of an early postpartum relapse lowered by 63%.
In late 2019, Krysko and colleagues published a meta-analysis of 24 studies involving nearly 3,000 women with MS which showed that breastfeeds were 43% less likely to experience postpartum relapse compared with their non-breastfeeding counterparts. The link was stronger in studies where women breastfed exclusively.
The bottom line: There is a plurality of physicians who believe that breastfeeding has a protective effect – and most will tell you that you should recommend exclusive breastfeeding.
What medicines can I take that will not adversely affect me and my baby?
Once a woman knows that breastfeeding could help her offspring avoid developing MS, and minimize her chance of a postpartum relapse, she will likely ask what to do about medications. You answer will depends on what she’s taking.
- Drugs she can take with relative peace of mind. Most experts believe it is safe to take corticosteroids and breastfeed. In fact, women who relapse while breastfeeding will in all likelihood be given intravenous corticosteroids, such as methylprednisolone. These medications are present in the blood at very low levels, peak an hour after infusion, and quickly dissipate. So, it’s important to tell your patients to delay breastfeeding by 2 to 4 hours after they receive the steroid.
- Drugs that are potentially concerning and require close monitoring. For the so-called platform therapies—such as interferon beta/glatiramer acetate, natalizumab, and their generic equivalents—there are no large studies that clearly demonstrate safety. Still, they are generally thought to be safe. Be sure to heed FDA labeling: weigh breastfeeding benefit against the potential risk
- Drug to avoid entirely. Under no circumstances should breastfeeding women receive teriflunomide, cladribine, alemtuzumab, or mitoxantrone. The jury is still out on rituximab—which is not yet approved for MS in the United States—and ocrelizumab. For now, err on the safe side and switch to another therapy.
How mental health care would look under a Trump vs. Biden administration
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Updated heart failure measures add newer meds
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
Safety measures for lab monitoring of mineralocorticoid receptor agonist therapy, performance measures for sacubitril/valsartan, cardiac resynchronization therapy and titration of medications, and quality measures based on patient-reported outcomes are among the updates the joint task force of the American College of Cardiology and the American Heart Association have made to performance and quality measures for managing adults with heart failure.
The revisions, published online Nov. 2 in the Journal of the American College of Cardiology, update the 2011 ACC/AHA heart failure measure set, writing committee vice chair Gregg C. Fonarow, MD, said in an interview. The 2011 measure set predates the 2015 approval of the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan for heart failure in adults.
Measures stress dosages, strength of evidence
“For the first time the heart failure performance measure sets also focus on not just the use of guideline-recommended medication at any dose, but on utilizing the doses that are evidence-based and guideline recommended so long as they are well tolerated,” said Dr. Fonarow, interim chief of cardiology at the University of California, Los Angeles. “The measure set now includes assessment of patients being treated with doses of medications at 50% or greater of target dose in the absence of contraindications or documented intolerance.”
The update includes seven new performance measures, two quality measures, and one structural measure. The performance measures come from the strongest recommendations – that is, a class of recommendation of 1 (strong) or 3 (no benefit or harmful, process to be avoided) – in the 2017 ACC/AHA/Heart Failure Society of American heart failure guideline update published in Circulation.
In addition to the 2017 update, the writing committee also reviewed existing performance measures. “Those management strategies, diagnostic testing, medications, and devices with the strongest evidence and highest level of guideline recommendations were further considered for inclusion in the performance measure set,” Dr. Fonarow said. “The measures went through extensive review by peer reviewers and approval from the organizations represented.”
Specifically, the update includes measures for monitoring serum potassium after starting mineralocorticoid receptor antagonists therapy, and cardiac resynchronization therapy for patients with heart failure with reduced ejection fraction already on guideline-directed therapy. “This therapy can significantly improve functional capacity and outcomes in appropriately selected patients,” Dr. Fonarow said.
New and retired measures
The update adds two performance measures for titration of medications based on dose, either reaching 50% of the recommended dose for a variety of medications, including ARNI, or documenting that the dose wasn’t tolerated for other reason for not using the dose.
The new structural measure calls for facility participation in a heart failure registry. The revised measure set now consists of 18 measures in all.
The update retired one measure from the 2011 set: left ventricular ejection fraction assessment for inpatients. The committee cited its use above 97% as the reason, but LVEF in outpatients remains a measure.
The following tree measures have been revised:
- Patient self-care education has moved from performance measure to quality measure because of concerns about the accuracy of self-care education documentation and limited evidence of improved outcomes with better documentation.
- ACE inhibitor or angiotensin receptor blocker therapy for left ventricular systolic dysfunction adds ARNI therapy to align with the 2017 ACC/AHA/HFSA update.
- Postdischarge appointments shifts from performance to quality measure and include a 7-day limit.
Measures future research should focus on, noted Dr. Fonarow, include the use of sodium glucose cotransporter 2 (SGLT2) inhibitors for heart failure, including in patients without diabetes. “Since the ACC/AHA heart failure guidelines had not yet been updated to recommend these therapies they could not be included in this performance measure set,” he said.
He also said “an urgent need” exists for further research into treatments for heart failure with preserved ejection fraction along with optimal implementation strategies.
“If these ACC/AHA heart failure performance measures were applied in all settings in which patients with heart failure in the United States are being cared for, and optimal and equitable conformity with each of these measures were achieved, over 100,000 lives a year of patients with heart failure could be saved,” he said. “There’s in an urgent need to measure and improve heart failure care quality.”
Dr. Fonarow reported financial relationships with Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis.
SOURCE: American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2020 Nov 2;76:2527-64.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Mirikizumab beats placebo, secukinumab for psoriasis
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
FROM THE EADV CONGRESS
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
New technologies aim to improve ovarian cancer detection
Encouraging trends abound in the management of ovarian cancer. As rates of ovarian disease continue to decline, there has also been a notable increase in tools for detecting it earlier in its course.
To better understand these developments, this news organization reached out to Rebecca Stone, MD, an ovarian cancer expert and associate professor of gynecologic oncology at Johns Hopkins University, in Baltimore, Maryland. This interview has been edited for length and clarity.
There has been a decline in the rates of ovarian cancer in recent years. What are the possible causes of this?Dr. Stone: The number of new cases in the United States has actually been declining over the past 2 decades. This is thought to be attributable to the increased prescribing of oral contraceptive pills in the late 1990s and the uptake of preventive measures, such as risk-reducing gynecologic surgery for women with genetic predisposition to ovarian cancer, as well as opportunistic salpingectomy in the general population. Opportunistic salpingectomy was introduced about 10 years ago. It is a surgical means for primary prevention of tubo-ovarian cancer by removing both fallopian tubes at the time of elective surgery for women who have completed childbearing or in lieu of “tying the tubes” for women who desire permanent surgical sterility.
What can you tell us about a recent study suggesting that high-grade serous epithelial ovarian cancer may be detected earlier in the course of the disease by testing for TP53 clonal variants in DNA from Papanicolaou (Pap) tests performed during cervical cancer screening?
The idea here is that early mutational events that ultimately result in the development of epithelial ovarian cancer can be detected by performing gene sequencing on genetic material collected at the time of routine Pap smear screening done for cervical cancer. Pap tests are known to contain cells and genetic material shed from the fallopian tubes, where the precancerous lesions thought to give rise to epithelial ovarian cancer, predominantly serous epithelial ovarian cancers, start.
p53 gene mutations are thought to occur early in the evolution of ovarian cancer. There are data indicating that these mutations actually occur in cells lining the fallopian tubes. Polymerase chain reaction–based DNA/gene sequencing performed on cervical fluid collected by Pap smears could detect these p53-mutated cells shed from the fallopian tubes.
A strength of this study is that it included healthy controls. None of their Pap smears screened positive for the p53 mutations, unlike the Pap smears of women predating their diagnosis of ovarian cancer. Limitations of the study include the fact that it had a small sample size. Findings will need to be confirmed in a larger patient population.
Also, the study only looked for p53 gene mutations. Ovarian cancers, like other cancers, are largely thought to occur when there is a buildup of mutations in critical genes that result in uncontrolled cell growth and division. These genetic changes/mutations are acquired during a person’s lifetime. Thus, there are likely early genetic changes/mutations that occur in addition to p53 mutations that ultimately lead to the development of ovarian cancer. Detecting these along with p53 mutations could improve the sensitivity/detection rate of the screening strategy that the authors are investigating.
Finally, this screening strategy may not prove effective for the early detection of all histologic subtypes of epithelial ovarian cancer or for nonepithelial ovarian cancers.
What other recent developments in the diagnosis of ovarian cancer should clinicians be aware of?
Liquid biopsies using circulating tumor DNA (ctDNA) have shown promising results for cancer detection and management, including ovarian cancer. However, further clarification is needed to define the minimum tumor size/burden detectable using ctDNA-based approaches. Moreover, large prospective studies are needed to determine the clinical utility of ctDNA detection for early diagnosis of ovarian cancer and its impact on patient outcomes.
DNA methylation is an early event in carcinogenesis and can be detected in blood plasma samples from cancer patients. Data related to the discovery and validation of discriminated methylated DNA marker candidates extracted from ovarian cancer tissues were presented at the American Society of Clinical Oncology meeting this year. Findings were subsequently evaluated in plasma from women with and without ovarian cancer.
In addition to blood, peritoneal fluid and uterine lavage have been used to obtain cell pellets that are used for the identification of common mutant genes – TP53, BRCA1, and BRCA2. These body fluids have also been shown as the source of tumor-derived material that can be used to differentiate between ovarian cancer patients and healthy individuals.
Further studies are needed to determine the sensitivity and specificity of other noninvasive tests for the diagnosis of ovarian cancer.
The American Cancer Society issued a statement that the human papillomavirus (HPV) test is the preferred cervical cancer screening tool. Why do they prefer the HPV test over the Pap test?
The American Cancer Society recommends that cervical cancer testing (screening) begin at age 25 years. Women aged 25-65 years should have a primary HPV test every 5 years. If primary HPV testing is not available, screening may be done with either a co-test that combines an HPV test with a Pap test every 5 years or a Pap test alone every 3 years.
The HPV test is widely available. The cost of an HPV test is approximately $44 (unit cost, 2014 USD). The cost of a Pap test is approximately $30 (unit cost, 2014 USD).
The HPV test is preferred over cytologic testing (Pap) for several reasons.
Firstly, in well-designed studies, the sensitivity of a single Pap smear for detecting high-grade precancer of the cervix is around 50%, which is less than optimal for a cancer screening test. Sensitivity means the chance that, if you have the disease (in this case, high-grade precancer of the cervix), the test will detect it. In particular, cytology is known to have an even more limited ability to detect glandular precancers, which arise in the endocervical canal rather than on or in close proximity to the exterior surface of the cervix (ectocervix). Thus, HPV-based screening programs hold the promise of improving detection of cervical adenocarcinoma.
Secondly, to function reliably, cytology programs require substantial infrastructure, highly qualified human resources, and a well‐defined quality-control system, which have proved to be costly and difficult to implement. This results in global disparities in cytology-based cervical cancer screening programs.
Thirdly, although co‐testing with both cytology and HPV tests is an option for screening programs, studies have confirmed that there is limited benefit from adding cytology to HPV screening. Long‐term studies from Kaiser Permanente that included over 1 million women found that HPV testing has a very high negative predictive value for precancerous lesions. Women with negative HPV tests were very unlikely to develop precancerous lesions in the following 5 years. The 5‐year risk of high-grade precancer or cancer of the cervix following a negative HPV test was 0.14%, whereas for women with a negative cytology, it was 0.31%. The screening benefit of co‐testing is largely driven by HPV testing and not cytology.
So, in summary, HPV testing is preferred over cytologic screening for cervical cancer, given its improved sensitivity and quality assurance, the opportunity to automate testing, and ultimately, its prospect of reducing the overall number of lifetime screenings for women.
Dr. Stone has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Encouraging trends abound in the management of ovarian cancer. As rates of ovarian disease continue to decline, there has also been a notable increase in tools for detecting it earlier in its course.
To better understand these developments, this news organization reached out to Rebecca Stone, MD, an ovarian cancer expert and associate professor of gynecologic oncology at Johns Hopkins University, in Baltimore, Maryland. This interview has been edited for length and clarity.
There has been a decline in the rates of ovarian cancer in recent years. What are the possible causes of this?Dr. Stone: The number of new cases in the United States has actually been declining over the past 2 decades. This is thought to be attributable to the increased prescribing of oral contraceptive pills in the late 1990s and the uptake of preventive measures, such as risk-reducing gynecologic surgery for women with genetic predisposition to ovarian cancer, as well as opportunistic salpingectomy in the general population. Opportunistic salpingectomy was introduced about 10 years ago. It is a surgical means for primary prevention of tubo-ovarian cancer by removing both fallopian tubes at the time of elective surgery for women who have completed childbearing or in lieu of “tying the tubes” for women who desire permanent surgical sterility.
What can you tell us about a recent study suggesting that high-grade serous epithelial ovarian cancer may be detected earlier in the course of the disease by testing for TP53 clonal variants in DNA from Papanicolaou (Pap) tests performed during cervical cancer screening?
The idea here is that early mutational events that ultimately result in the development of epithelial ovarian cancer can be detected by performing gene sequencing on genetic material collected at the time of routine Pap smear screening done for cervical cancer. Pap tests are known to contain cells and genetic material shed from the fallopian tubes, where the precancerous lesions thought to give rise to epithelial ovarian cancer, predominantly serous epithelial ovarian cancers, start.
p53 gene mutations are thought to occur early in the evolution of ovarian cancer. There are data indicating that these mutations actually occur in cells lining the fallopian tubes. Polymerase chain reaction–based DNA/gene sequencing performed on cervical fluid collected by Pap smears could detect these p53-mutated cells shed from the fallopian tubes.
A strength of this study is that it included healthy controls. None of their Pap smears screened positive for the p53 mutations, unlike the Pap smears of women predating their diagnosis of ovarian cancer. Limitations of the study include the fact that it had a small sample size. Findings will need to be confirmed in a larger patient population.
Also, the study only looked for p53 gene mutations. Ovarian cancers, like other cancers, are largely thought to occur when there is a buildup of mutations in critical genes that result in uncontrolled cell growth and division. These genetic changes/mutations are acquired during a person’s lifetime. Thus, there are likely early genetic changes/mutations that occur in addition to p53 mutations that ultimately lead to the development of ovarian cancer. Detecting these along with p53 mutations could improve the sensitivity/detection rate of the screening strategy that the authors are investigating.
Finally, this screening strategy may not prove effective for the early detection of all histologic subtypes of epithelial ovarian cancer or for nonepithelial ovarian cancers.
What other recent developments in the diagnosis of ovarian cancer should clinicians be aware of?
Liquid biopsies using circulating tumor DNA (ctDNA) have shown promising results for cancer detection and management, including ovarian cancer. However, further clarification is needed to define the minimum tumor size/burden detectable using ctDNA-based approaches. Moreover, large prospective studies are needed to determine the clinical utility of ctDNA detection for early diagnosis of ovarian cancer and its impact on patient outcomes.
DNA methylation is an early event in carcinogenesis and can be detected in blood plasma samples from cancer patients. Data related to the discovery and validation of discriminated methylated DNA marker candidates extracted from ovarian cancer tissues were presented at the American Society of Clinical Oncology meeting this year. Findings were subsequently evaluated in plasma from women with and without ovarian cancer.
In addition to blood, peritoneal fluid and uterine lavage have been used to obtain cell pellets that are used for the identification of common mutant genes – TP53, BRCA1, and BRCA2. These body fluids have also been shown as the source of tumor-derived material that can be used to differentiate between ovarian cancer patients and healthy individuals.
Further studies are needed to determine the sensitivity and specificity of other noninvasive tests for the diagnosis of ovarian cancer.
The American Cancer Society issued a statement that the human papillomavirus (HPV) test is the preferred cervical cancer screening tool. Why do they prefer the HPV test over the Pap test?
The American Cancer Society recommends that cervical cancer testing (screening) begin at age 25 years. Women aged 25-65 years should have a primary HPV test every 5 years. If primary HPV testing is not available, screening may be done with either a co-test that combines an HPV test with a Pap test every 5 years or a Pap test alone every 3 years.
The HPV test is widely available. The cost of an HPV test is approximately $44 (unit cost, 2014 USD). The cost of a Pap test is approximately $30 (unit cost, 2014 USD).
The HPV test is preferred over cytologic testing (Pap) for several reasons.
Firstly, in well-designed studies, the sensitivity of a single Pap smear for detecting high-grade precancer of the cervix is around 50%, which is less than optimal for a cancer screening test. Sensitivity means the chance that, if you have the disease (in this case, high-grade precancer of the cervix), the test will detect it. In particular, cytology is known to have an even more limited ability to detect glandular precancers, which arise in the endocervical canal rather than on or in close proximity to the exterior surface of the cervix (ectocervix). Thus, HPV-based screening programs hold the promise of improving detection of cervical adenocarcinoma.
Secondly, to function reliably, cytology programs require substantial infrastructure, highly qualified human resources, and a well‐defined quality-control system, which have proved to be costly and difficult to implement. This results in global disparities in cytology-based cervical cancer screening programs.
Thirdly, although co‐testing with both cytology and HPV tests is an option for screening programs, studies have confirmed that there is limited benefit from adding cytology to HPV screening. Long‐term studies from Kaiser Permanente that included over 1 million women found that HPV testing has a very high negative predictive value for precancerous lesions. Women with negative HPV tests were very unlikely to develop precancerous lesions in the following 5 years. The 5‐year risk of high-grade precancer or cancer of the cervix following a negative HPV test was 0.14%, whereas for women with a negative cytology, it was 0.31%. The screening benefit of co‐testing is largely driven by HPV testing and not cytology.
So, in summary, HPV testing is preferred over cytologic screening for cervical cancer, given its improved sensitivity and quality assurance, the opportunity to automate testing, and ultimately, its prospect of reducing the overall number of lifetime screenings for women.
Dr. Stone has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Encouraging trends abound in the management of ovarian cancer. As rates of ovarian disease continue to decline, there has also been a notable increase in tools for detecting it earlier in its course.
To better understand these developments, this news organization reached out to Rebecca Stone, MD, an ovarian cancer expert and associate professor of gynecologic oncology at Johns Hopkins University, in Baltimore, Maryland. This interview has been edited for length and clarity.
There has been a decline in the rates of ovarian cancer in recent years. What are the possible causes of this?Dr. Stone: The number of new cases in the United States has actually been declining over the past 2 decades. This is thought to be attributable to the increased prescribing of oral contraceptive pills in the late 1990s and the uptake of preventive measures, such as risk-reducing gynecologic surgery for women with genetic predisposition to ovarian cancer, as well as opportunistic salpingectomy in the general population. Opportunistic salpingectomy was introduced about 10 years ago. It is a surgical means for primary prevention of tubo-ovarian cancer by removing both fallopian tubes at the time of elective surgery for women who have completed childbearing or in lieu of “tying the tubes” for women who desire permanent surgical sterility.
What can you tell us about a recent study suggesting that high-grade serous epithelial ovarian cancer may be detected earlier in the course of the disease by testing for TP53 clonal variants in DNA from Papanicolaou (Pap) tests performed during cervical cancer screening?
The idea here is that early mutational events that ultimately result in the development of epithelial ovarian cancer can be detected by performing gene sequencing on genetic material collected at the time of routine Pap smear screening done for cervical cancer. Pap tests are known to contain cells and genetic material shed from the fallopian tubes, where the precancerous lesions thought to give rise to epithelial ovarian cancer, predominantly serous epithelial ovarian cancers, start.
p53 gene mutations are thought to occur early in the evolution of ovarian cancer. There are data indicating that these mutations actually occur in cells lining the fallopian tubes. Polymerase chain reaction–based DNA/gene sequencing performed on cervical fluid collected by Pap smears could detect these p53-mutated cells shed from the fallopian tubes.
A strength of this study is that it included healthy controls. None of their Pap smears screened positive for the p53 mutations, unlike the Pap smears of women predating their diagnosis of ovarian cancer. Limitations of the study include the fact that it had a small sample size. Findings will need to be confirmed in a larger patient population.
Also, the study only looked for p53 gene mutations. Ovarian cancers, like other cancers, are largely thought to occur when there is a buildup of mutations in critical genes that result in uncontrolled cell growth and division. These genetic changes/mutations are acquired during a person’s lifetime. Thus, there are likely early genetic changes/mutations that occur in addition to p53 mutations that ultimately lead to the development of ovarian cancer. Detecting these along with p53 mutations could improve the sensitivity/detection rate of the screening strategy that the authors are investigating.
Finally, this screening strategy may not prove effective for the early detection of all histologic subtypes of epithelial ovarian cancer or for nonepithelial ovarian cancers.
What other recent developments in the diagnosis of ovarian cancer should clinicians be aware of?
Liquid biopsies using circulating tumor DNA (ctDNA) have shown promising results for cancer detection and management, including ovarian cancer. However, further clarification is needed to define the minimum tumor size/burden detectable using ctDNA-based approaches. Moreover, large prospective studies are needed to determine the clinical utility of ctDNA detection for early diagnosis of ovarian cancer and its impact on patient outcomes.
DNA methylation is an early event in carcinogenesis and can be detected in blood plasma samples from cancer patients. Data related to the discovery and validation of discriminated methylated DNA marker candidates extracted from ovarian cancer tissues were presented at the American Society of Clinical Oncology meeting this year. Findings were subsequently evaluated in plasma from women with and without ovarian cancer.
In addition to blood, peritoneal fluid and uterine lavage have been used to obtain cell pellets that are used for the identification of common mutant genes – TP53, BRCA1, and BRCA2. These body fluids have also been shown as the source of tumor-derived material that can be used to differentiate between ovarian cancer patients and healthy individuals.
Further studies are needed to determine the sensitivity and specificity of other noninvasive tests for the diagnosis of ovarian cancer.
The American Cancer Society issued a statement that the human papillomavirus (HPV) test is the preferred cervical cancer screening tool. Why do they prefer the HPV test over the Pap test?
The American Cancer Society recommends that cervical cancer testing (screening) begin at age 25 years. Women aged 25-65 years should have a primary HPV test every 5 years. If primary HPV testing is not available, screening may be done with either a co-test that combines an HPV test with a Pap test every 5 years or a Pap test alone every 3 years.
The HPV test is widely available. The cost of an HPV test is approximately $44 (unit cost, 2014 USD). The cost of a Pap test is approximately $30 (unit cost, 2014 USD).
The HPV test is preferred over cytologic testing (Pap) for several reasons.
Firstly, in well-designed studies, the sensitivity of a single Pap smear for detecting high-grade precancer of the cervix is around 50%, which is less than optimal for a cancer screening test. Sensitivity means the chance that, if you have the disease (in this case, high-grade precancer of the cervix), the test will detect it. In particular, cytology is known to have an even more limited ability to detect glandular precancers, which arise in the endocervical canal rather than on or in close proximity to the exterior surface of the cervix (ectocervix). Thus, HPV-based screening programs hold the promise of improving detection of cervical adenocarcinoma.
Secondly, to function reliably, cytology programs require substantial infrastructure, highly qualified human resources, and a well‐defined quality-control system, which have proved to be costly and difficult to implement. This results in global disparities in cytology-based cervical cancer screening programs.
Thirdly, although co‐testing with both cytology and HPV tests is an option for screening programs, studies have confirmed that there is limited benefit from adding cytology to HPV screening. Long‐term studies from Kaiser Permanente that included over 1 million women found that HPV testing has a very high negative predictive value for precancerous lesions. Women with negative HPV tests were very unlikely to develop precancerous lesions in the following 5 years. The 5‐year risk of high-grade precancer or cancer of the cervix following a negative HPV test was 0.14%, whereas for women with a negative cytology, it was 0.31%. The screening benefit of co‐testing is largely driven by HPV testing and not cytology.
So, in summary, HPV testing is preferred over cytologic screening for cervical cancer, given its improved sensitivity and quality assurance, the opportunity to automate testing, and ultimately, its prospect of reducing the overall number of lifetime screenings for women.
Dr. Stone has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Molecular features of medulloblastoma may help personalize radiotherapy
Results from this analysis were reported at the American Society for Radiation Oncology Annual Meeting 2020.
“Today, molecular diagnostics play a critical role in the classification of tumors, particularly medulloblastoma,” noted lead investigator Jeff M. Michalski, MD, of Washington University St. Louis, Mo.
“It is now recognized that medulloblastoma can be subgrouped into four distinct entities with unique demographics and tumor behaviors,” he said.
Those four groups are the SHH subgroup, the WNT subgroup, group 3, and group 4.
Study rationale and details
The benefits of current multimodality therapy in controlling and curing medulloblastoma come at the cost of toxicity, especially for younger patients, in terms of neurocognitive deficits, secondary cancers, and growth and neuro-endocrine abnormalities.
With this in mind, Dr. Michalski and colleagues conducted a phase 3 trial to test two strategies for reducing radiation in average-risk medulloblastoma without compromising outcomes.
After craniospinal irradiation (CSI), all patients were randomized to a radiation boost to the whole posterior fossa (PFRT) or an involved field volume (IFRT). Patients aged 8-21 years received CSI at the standard dose (23.4 Gy). Patients aged 3-7 years were randomized to standard-dose CSI or low-dose CSI (18 Gy).
There were 464 patients in whom PFRT to IFRT could be compared and 226 patients in whom standard and low-dose CSI could be compared.
Only 362 patients had sufficient tumor tissue to allow for classification into molecular subgroups. Among these patients, 43.1% fell into the group 4 subgroup, 21.0% into the group 3 subgroup, 18.2% into the SHH subgroup, and 17.7% into the WNT subgroup.
Survival results
The trial’s primary outcomes were event-free and overall survival. Events were defined as progression, recurrence, death, or second malignancy.
For the whole cohort, boost volume did not significantly affect outcomes. IFRT and PFRT yielded similar 5-year event-free survival (82.5% vs. 80.5%; P = .44) and overall survival (84.6% vs. 85.2%; P = .44). However, CSI dose did affect outcomes, with the low dose inferior to the standard dose on both 5-year event-free survival (71.4% vs. 82.9%; P = .028) and overall survival (77.5% vs. 85.6%; P = .049).
In analyses stratified by molecular subgroup, event-free survival did not differ significantly by boost volume within subgroups, except for the SHH subgroup, within which PFRT yielded worse outcomes (P = .018). Similarly, event-free survival did not differ significantly by CSI dose within subgroups, except for group 4, within which the low dose yielded a worse outcome (P = .047).
When specific genomic alterations were also considered, patients in the SHH group had worse outcomes if they had chromosome 14q loss, chromosome 10q loss, or p53 mutation. Patients in group 3 had worse outcomes if they had MYC amplification, iso-chromosome 17q, or both of these abnormalities.
No significant correlations were seen for group 4 patients, and there were too few patients in the WNT subgroup to assess correlations.
“Survival rates following reduced radiation boost volumes were comparable to standard treatment volumes for the primary tumor site. Interestingly, the SHH subgroup had worse event-free survival with whole posterior fossa radiation therapy,” Dr. Michalski commented. “Reduced dose of craniospinal axis irradiation was associated with higher event rates and worse survival, and group 4 was the primary subgroup that drove these inferior outcomes.”
“Specific genomic abnormalities are associated with worse outcomes, and future trials should consider subgroup and these genomic abnormalities in their study design,” he recommended.
‘A new era’ of risk stratification
Current evidence “leads us to conclude that certain molecular subgroups and specific genetic abnormalities within the subgroups are primary drivers of outcome and can be associated with far worse outcomes than what the conventional risk definition might suggest,” said invited discussant Stephanie Terezakis, MD, of the University of Minnesota in Minneapolis.
“In fact, differences in outcomes are greater between molecular subgroups and genomic abnormalities in large trial cohorts than between clinical trials when we use conventional risk definitions,” Dr. Terezakis said.
The ACNS0331 trial’s subgroup findings demonstrate that one size of therapy may not fit all, she elaborated. For example, some patients with favorable tumor biology may still be able to receive deintensified therapy and maintain excellent outcomes, whereas other patients with unfavorable tumor biology could potentially be newly classified as high risk and eligible for intensified therapy.
“This is the subject of ongoing discussions today to try to inform this next generation of trials, to see how we risk-stratify patients,” Dr. Terezakis concluded. “This model of conducting a national clinical trial with biologic endpoints has allowed us to usher in a new era where tumor biology may potentially guide our treatment approaches and lead to more personalized cancer care.”
The trial was funded by the National Cancer Institute, The Brain Tumor Charity, and St. Jude Children’s Research Hospital. Dr. Michalski disclosed relationships with ViewRay, Boston Scientific, Merck, and Blue Earth Diagnostics. Dr. Terezakis disclosed scientific grants from the Radiation Oncology Institute and the Sarcoma Foundation of America.
SOURCE: Michalski JM et al. ASTRO 2020, Abstract 1.
Results from this analysis were reported at the American Society for Radiation Oncology Annual Meeting 2020.
“Today, molecular diagnostics play a critical role in the classification of tumors, particularly medulloblastoma,” noted lead investigator Jeff M. Michalski, MD, of Washington University St. Louis, Mo.
“It is now recognized that medulloblastoma can be subgrouped into four distinct entities with unique demographics and tumor behaviors,” he said.
Those four groups are the SHH subgroup, the WNT subgroup, group 3, and group 4.
Study rationale and details
The benefits of current multimodality therapy in controlling and curing medulloblastoma come at the cost of toxicity, especially for younger patients, in terms of neurocognitive deficits, secondary cancers, and growth and neuro-endocrine abnormalities.
With this in mind, Dr. Michalski and colleagues conducted a phase 3 trial to test two strategies for reducing radiation in average-risk medulloblastoma without compromising outcomes.
After craniospinal irradiation (CSI), all patients were randomized to a radiation boost to the whole posterior fossa (PFRT) or an involved field volume (IFRT). Patients aged 8-21 years received CSI at the standard dose (23.4 Gy). Patients aged 3-7 years were randomized to standard-dose CSI or low-dose CSI (18 Gy).
There were 464 patients in whom PFRT to IFRT could be compared and 226 patients in whom standard and low-dose CSI could be compared.
Only 362 patients had sufficient tumor tissue to allow for classification into molecular subgroups. Among these patients, 43.1% fell into the group 4 subgroup, 21.0% into the group 3 subgroup, 18.2% into the SHH subgroup, and 17.7% into the WNT subgroup.
Survival results
The trial’s primary outcomes were event-free and overall survival. Events were defined as progression, recurrence, death, or second malignancy.
For the whole cohort, boost volume did not significantly affect outcomes. IFRT and PFRT yielded similar 5-year event-free survival (82.5% vs. 80.5%; P = .44) and overall survival (84.6% vs. 85.2%; P = .44). However, CSI dose did affect outcomes, with the low dose inferior to the standard dose on both 5-year event-free survival (71.4% vs. 82.9%; P = .028) and overall survival (77.5% vs. 85.6%; P = .049).
In analyses stratified by molecular subgroup, event-free survival did not differ significantly by boost volume within subgroups, except for the SHH subgroup, within which PFRT yielded worse outcomes (P = .018). Similarly, event-free survival did not differ significantly by CSI dose within subgroups, except for group 4, within which the low dose yielded a worse outcome (P = .047).
When specific genomic alterations were also considered, patients in the SHH group had worse outcomes if they had chromosome 14q loss, chromosome 10q loss, or p53 mutation. Patients in group 3 had worse outcomes if they had MYC amplification, iso-chromosome 17q, or both of these abnormalities.
No significant correlations were seen for group 4 patients, and there were too few patients in the WNT subgroup to assess correlations.
“Survival rates following reduced radiation boost volumes were comparable to standard treatment volumes for the primary tumor site. Interestingly, the SHH subgroup had worse event-free survival with whole posterior fossa radiation therapy,” Dr. Michalski commented. “Reduced dose of craniospinal axis irradiation was associated with higher event rates and worse survival, and group 4 was the primary subgroup that drove these inferior outcomes.”
“Specific genomic abnormalities are associated with worse outcomes, and future trials should consider subgroup and these genomic abnormalities in their study design,” he recommended.
‘A new era’ of risk stratification
Current evidence “leads us to conclude that certain molecular subgroups and specific genetic abnormalities within the subgroups are primary drivers of outcome and can be associated with far worse outcomes than what the conventional risk definition might suggest,” said invited discussant Stephanie Terezakis, MD, of the University of Minnesota in Minneapolis.
“In fact, differences in outcomes are greater between molecular subgroups and genomic abnormalities in large trial cohorts than between clinical trials when we use conventional risk definitions,” Dr. Terezakis said.
The ACNS0331 trial’s subgroup findings demonstrate that one size of therapy may not fit all, she elaborated. For example, some patients with favorable tumor biology may still be able to receive deintensified therapy and maintain excellent outcomes, whereas other patients with unfavorable tumor biology could potentially be newly classified as high risk and eligible for intensified therapy.
“This is the subject of ongoing discussions today to try to inform this next generation of trials, to see how we risk-stratify patients,” Dr. Terezakis concluded. “This model of conducting a national clinical trial with biologic endpoints has allowed us to usher in a new era where tumor biology may potentially guide our treatment approaches and lead to more personalized cancer care.”
The trial was funded by the National Cancer Institute, The Brain Tumor Charity, and St. Jude Children’s Research Hospital. Dr. Michalski disclosed relationships with ViewRay, Boston Scientific, Merck, and Blue Earth Diagnostics. Dr. Terezakis disclosed scientific grants from the Radiation Oncology Institute and the Sarcoma Foundation of America.
SOURCE: Michalski JM et al. ASTRO 2020, Abstract 1.
Results from this analysis were reported at the American Society for Radiation Oncology Annual Meeting 2020.
“Today, molecular diagnostics play a critical role in the classification of tumors, particularly medulloblastoma,” noted lead investigator Jeff M. Michalski, MD, of Washington University St. Louis, Mo.
“It is now recognized that medulloblastoma can be subgrouped into four distinct entities with unique demographics and tumor behaviors,” he said.
Those four groups are the SHH subgroup, the WNT subgroup, group 3, and group 4.
Study rationale and details
The benefits of current multimodality therapy in controlling and curing medulloblastoma come at the cost of toxicity, especially for younger patients, in terms of neurocognitive deficits, secondary cancers, and growth and neuro-endocrine abnormalities.
With this in mind, Dr. Michalski and colleagues conducted a phase 3 trial to test two strategies for reducing radiation in average-risk medulloblastoma without compromising outcomes.
After craniospinal irradiation (CSI), all patients were randomized to a radiation boost to the whole posterior fossa (PFRT) or an involved field volume (IFRT). Patients aged 8-21 years received CSI at the standard dose (23.4 Gy). Patients aged 3-7 years were randomized to standard-dose CSI or low-dose CSI (18 Gy).
There were 464 patients in whom PFRT to IFRT could be compared and 226 patients in whom standard and low-dose CSI could be compared.
Only 362 patients had sufficient tumor tissue to allow for classification into molecular subgroups. Among these patients, 43.1% fell into the group 4 subgroup, 21.0% into the group 3 subgroup, 18.2% into the SHH subgroup, and 17.7% into the WNT subgroup.
Survival results
The trial’s primary outcomes were event-free and overall survival. Events were defined as progression, recurrence, death, or second malignancy.
For the whole cohort, boost volume did not significantly affect outcomes. IFRT and PFRT yielded similar 5-year event-free survival (82.5% vs. 80.5%; P = .44) and overall survival (84.6% vs. 85.2%; P = .44). However, CSI dose did affect outcomes, with the low dose inferior to the standard dose on both 5-year event-free survival (71.4% vs. 82.9%; P = .028) and overall survival (77.5% vs. 85.6%; P = .049).
In analyses stratified by molecular subgroup, event-free survival did not differ significantly by boost volume within subgroups, except for the SHH subgroup, within which PFRT yielded worse outcomes (P = .018). Similarly, event-free survival did not differ significantly by CSI dose within subgroups, except for group 4, within which the low dose yielded a worse outcome (P = .047).
When specific genomic alterations were also considered, patients in the SHH group had worse outcomes if they had chromosome 14q loss, chromosome 10q loss, or p53 mutation. Patients in group 3 had worse outcomes if they had MYC amplification, iso-chromosome 17q, or both of these abnormalities.
No significant correlations were seen for group 4 patients, and there were too few patients in the WNT subgroup to assess correlations.
“Survival rates following reduced radiation boost volumes were comparable to standard treatment volumes for the primary tumor site. Interestingly, the SHH subgroup had worse event-free survival with whole posterior fossa radiation therapy,” Dr. Michalski commented. “Reduced dose of craniospinal axis irradiation was associated with higher event rates and worse survival, and group 4 was the primary subgroup that drove these inferior outcomes.”
“Specific genomic abnormalities are associated with worse outcomes, and future trials should consider subgroup and these genomic abnormalities in their study design,” he recommended.
‘A new era’ of risk stratification
Current evidence “leads us to conclude that certain molecular subgroups and specific genetic abnormalities within the subgroups are primary drivers of outcome and can be associated with far worse outcomes than what the conventional risk definition might suggest,” said invited discussant Stephanie Terezakis, MD, of the University of Minnesota in Minneapolis.
“In fact, differences in outcomes are greater between molecular subgroups and genomic abnormalities in large trial cohorts than between clinical trials when we use conventional risk definitions,” Dr. Terezakis said.
The ACNS0331 trial’s subgroup findings demonstrate that one size of therapy may not fit all, she elaborated. For example, some patients with favorable tumor biology may still be able to receive deintensified therapy and maintain excellent outcomes, whereas other patients with unfavorable tumor biology could potentially be newly classified as high risk and eligible for intensified therapy.
“This is the subject of ongoing discussions today to try to inform this next generation of trials, to see how we risk-stratify patients,” Dr. Terezakis concluded. “This model of conducting a national clinical trial with biologic endpoints has allowed us to usher in a new era where tumor biology may potentially guide our treatment approaches and lead to more personalized cancer care.”
The trial was funded by the National Cancer Institute, The Brain Tumor Charity, and St. Jude Children’s Research Hospital. Dr. Michalski disclosed relationships with ViewRay, Boston Scientific, Merck, and Blue Earth Diagnostics. Dr. Terezakis disclosed scientific grants from the Radiation Oncology Institute and the Sarcoma Foundation of America.
SOURCE: Michalski JM et al. ASTRO 2020, Abstract 1.
FROM ASTRO 2020
A guide to the new agents reshaping ovarian cancer treatment
The treatment of ovarian cancer has evolved considerably in the last few years, with the approval of several PARP inhibitors, antiangiogenic agents, and other therapies for a multitude of indications. Additional treatments are likely to soon join this already diverse spectrum of available options, if their promising efficacy and safety continues to be borne out in ongoing research.
To better understand the individual merits and potential drawbacks of these treatments, Medscape recently spoke with Rebecca Stone, MD, an ovarian cancer expert and associate professor of gynecologic oncology at Johns Hopkins University, Baltimore. This interview has been edited for length and clarity.
Medscape: We’re starting to see preliminary data on pamiparib , an investigational inhibitor of PARP1 and PARP2, for the treatment of ovarian cancer. What is the evidence supporting this drug?
Dr. Stone: Currently, six different PARP inhibitors – olaparib, rucaparib, veliparib, niraparib, talazoparib, and pamiparib – have been in clinical development at different stages. In clinical applications, PARP inhibitors, including olaparib, rucaparib, niraparib, and talazoparib, have demonstrated sustained antitumor responses as single agents in patients with BRCA1 or BRCA2 mutations. Those with Food and Drug Administration indications in ovarian cancer include olaparib, rucaparib, and niraparib. The preclinical and clinical data with pamiparib is limited as of now. But, in a xenograft breast cancer model, it was found to be over 10 times more potent than olaparib.
If approved, where would pamiparib fit in the treatment paradigm for ovarian cancer?
It would potentially fit as monotherapy as well as in combination with agents other than standard chemotherapy for the treatment of BRCA mutated ovarian cancer. It could also be considered for maintenance therapy at the conclusion of chemotherapy treatment of newly diagnosed or recurrent BRCA-mutated ovarian cancer.
What adverse events are associated with pamiparib? How does the toxicity profile compare with other drugs for ovarian cancer?
With respect to PARP inhibitors, the differences in potency (PARP trapping) correlate with their toxicity profiles. The most common adverse events are gastrointestinal, hematologic, and constitutional (fatigue). Even though it is difficult to compare toxicities across different trials with heterogeneous patient populations, there are a few points worth noting.
Rucaparib leads to inhibition of renal transporter proteins involved in secretion of creatinine and can lead to increased creatinine (any grade: 15%; grade 3: ≤1%). Transaminitis is generally self-limiting and highest with rucaparib (any grade: 34%; grade 3: 10%). Hematologic toxicities are the highest with niraparib (any grade: thrombocytopenia 61%, anemia 50%, neutropenia 30%; grade ≥3: thrombocytopenia 34%, anemia 25%, neutropenia 20%).
Toxicities are more common in the first few cycles of treatment, warranting closer early monitoring. This differs somewhat from the gastrointestinal, hematological, and constitutional (fatigue) adverse events that we see with common chemotherapeutic agents used to treat ovarian cancer, which are generally cumulative.
PARP inhibitor treatment is also associated with an increased risk of developing myelodysplastic syndrome/acute myeloid leukemia (MDS/AML). That being said, therapy-related MDS/AML is a well-recognized complication of conventional chemotherapy used to treat a variety of primary malignancies, including ovarian cancer.
The expected toxicity profile for pamiparib is based on what we have seen with the other PARP inhibitors. This includes any grade nausea (50%), fatigue (33%), anemia (20%), vomiting (15%), and neutropenia (13%). Toxicity of grade 3 or higher includes anemia (13%), neutropenia (8%), and fatigue (5%).
Where do the newest drugs to be approved for ovarian cancer in recent years fit within the treatment paradigm? What do the research findings show about their efficacy and safety?
Data from phase 2/3 trials support the use of PARP inhibitors as monotherapy as well as in combination with other agents (most commonly agents other than standard chemotherapy) for the treatment of BRCA mutated or otherwise homologous recombination-deficient (HRD) ovarian cancer. They can also be considered for maintenance therapy at the conclusion of treatment of newly diagnosed or recurrent BRCA-mutated/HRD ovarian cancer.
Large phase 3 studies have resulted in the approval of the antiangiogenic agent bevacizumab in combination with chemotherapy for the treatment of newly diagnosed and recurrent ovarian cancer, as well as for maintenance therapy at the conclusion of combination chemotherapy plus bevacizumab treatment of newly diagnosed (GOG 218 and ICON 7 trials) or recurrent ovarian cancer (GOG 218, OCEANS, and AURELIA trials). The most common toxicity with antiangiogenic agents is hypertension. Women also commonly experience arthralgia/myalgia. There is an increased risk of proteinuria, blood clots, bleeding, and serious gastrointestinal events such as fistula and bowel perforation.
Data from the phase 2 KEYNOTE 158 trial support pembrolizumab for microsatellite high or mismatch repair-deficient ovarian cancers. Common side effects associated with the use of pembrolizumab include fatigue, itchy skin, diarrhea, nausea, decreased appetite, rash, fever, cough, difficulty breathing, musculoskeletal pain, constipation, and joint pain. Pembrolizumab can cause the immune system to attack normal organs and tissues in the body resulting in serious side effects, including inflammation of such organs as the lungs, colon, liver, endocrine glands, and kidneys.
Evidence for hormonal therapy (i.e., aromatase inhibitors like letrozole) for the treatment of newly diagnosed and recurrent low-grade serous/endometrioid epithelial ovarian cancer comes from largely retrospective cohort studies. A large phase 3 study, now enrolling, will examine if letrozole monotherapy/maintenance is non-inferior to intravenous paclitaxel/carboplatin and maintenance letrozole with respect to progression-free survival in women with stage II-IV primary low-grade serous carcinoma of the ovary or peritoneum after primary surgical cytoreduction.
Hormonal therapies are generally very well tolerated. Common side effects may include hot flashes, warmth or redness in the face or chest, headache, dizziness, weakness, bone pain, muscle or joint pain, swelling, weight gain, increased sweating, or increased cholesterol in the blood.
What other drugs are in development for ovarian cancer?
VEGF receptor tyrosine kinase inhibitors, such as cediranib, are in development. Anlotinib is another drug being investigated. It is a new multi-target tyrosine kinase inhibitor that targets VEGFR, PDGFR, and FGFR. Drugs targeting folate-alpha receptor, such as mirvetuximab, are under investigation, particularly for patients with high folate-alpha receptor membrane staining by immunohistochemistry. Drugs targeting cell cycle arrest, such as CDK4/6 inhibitors, are also being considered.
Can you provide some of the highlights of ovarian cancer research presented at this year’s American Society of Clinical Oncology meeting ?
My take is that we have gone from a monotonous landscape of platinum doublet chemotherapy to an exciting, diversified landscape over the past several years. All of this activity has driven median overall survival up from 3 years to 5 years and progression-free survival following first platinum sensitive recurrence to well beyond 6 months.
Since last year’s meeting, we have seen several new approvals, including niraparib for the treatment of BRCA mutated and HRD disease, as well as for first-line maintenance in all comers. In May, the FDA expanded the indication for olaparib to include its combination with bevacizumab as first-line maintenance for BRCA-mutated and HRD disease based on the results of PAOLA-1. With certainty, our treatment paradigms will continue to evolve in response to these and other new data.
At this year’s meeting, the SOLO-2 investigators revealed the first overall survival data for second-line PARP inhibitor maintenance, which is the first suggestion that PARP inhibitor maintenance improves overall survival.
We have a new understanding about the genetics of long-term responders to rucaparib on ARIEL-2.
We also understand how the role of secondary cytoreductive surgery and how nonchemotherapy options for the treatment of platinum sensitive relapse compare in terms of efficacy and toxicity (i.e., AVANOVA-2 and GY004 trials). We see again the importance of R0 cytoreduction when surgery is pursued. Achieving anything less than R0 cytoreduction for the treatment of first platinum sensitive recurrence may translate into shorter survival, compared with chemotherapy alone.
We are also becoming increasingly familiar with the limited therapeutic benefit of single-agent anti-PD-1/PD-L1, which is so different from our experience in mismatch repair-deficient endometrial cancer. In the small percentage of responders, there are some durable responses and a suggestion of particular efficacy among women with clear cell ovarian cancer.
What other recent findings in ovarian cancer research should oncologists be aware of?
Data supporting improved efficacy of a gastrointestinal-type chemotherapy regimen for mucinous epithelial ovarian cancers come from a retrospective cohort study of patients with ovarian mucinous carcinoma who received postoperative adjuvant chemotherapy at two academic centers.
Identification of inactivating SMARCA4 mutations as the driver of small cell carcinoma of the ovary, hypercalcemic type, and the idea that CDK4/6 inhibitors could be effectively repurposed to treat this rare but highly aggressive type of ovarian cancer is also new and exciting.
Dr. Stone has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The treatment of ovarian cancer has evolved considerably in the last few years, with the approval of several PARP inhibitors, antiangiogenic agents, and other therapies for a multitude of indications. Additional treatments are likely to soon join this already diverse spectrum of available options, if their promising efficacy and safety continues to be borne out in ongoing research.
To better understand the individual merits and potential drawbacks of these treatments, Medscape recently spoke with Rebecca Stone, MD, an ovarian cancer expert and associate professor of gynecologic oncology at Johns Hopkins University, Baltimore. This interview has been edited for length and clarity.
Medscape: We’re starting to see preliminary data on pamiparib , an investigational inhibitor of PARP1 and PARP2, for the treatment of ovarian cancer. What is the evidence supporting this drug?
Dr. Stone: Currently, six different PARP inhibitors – olaparib, rucaparib, veliparib, niraparib, talazoparib, and pamiparib – have been in clinical development at different stages. In clinical applications, PARP inhibitors, including olaparib, rucaparib, niraparib, and talazoparib, have demonstrated sustained antitumor responses as single agents in patients with BRCA1 or BRCA2 mutations. Those with Food and Drug Administration indications in ovarian cancer include olaparib, rucaparib, and niraparib. The preclinical and clinical data with pamiparib is limited as of now. But, in a xenograft breast cancer model, it was found to be over 10 times more potent than olaparib.
If approved, where would pamiparib fit in the treatment paradigm for ovarian cancer?
It would potentially fit as monotherapy as well as in combination with agents other than standard chemotherapy for the treatment of BRCA mutated ovarian cancer. It could also be considered for maintenance therapy at the conclusion of chemotherapy treatment of newly diagnosed or recurrent BRCA-mutated ovarian cancer.
What adverse events are associated with pamiparib? How does the toxicity profile compare with other drugs for ovarian cancer?
With respect to PARP inhibitors, the differences in potency (PARP trapping) correlate with their toxicity profiles. The most common adverse events are gastrointestinal, hematologic, and constitutional (fatigue). Even though it is difficult to compare toxicities across different trials with heterogeneous patient populations, there are a few points worth noting.
Rucaparib leads to inhibition of renal transporter proteins involved in secretion of creatinine and can lead to increased creatinine (any grade: 15%; grade 3: ≤1%). Transaminitis is generally self-limiting and highest with rucaparib (any grade: 34%; grade 3: 10%). Hematologic toxicities are the highest with niraparib (any grade: thrombocytopenia 61%, anemia 50%, neutropenia 30%; grade ≥3: thrombocytopenia 34%, anemia 25%, neutropenia 20%).
Toxicities are more common in the first few cycles of treatment, warranting closer early monitoring. This differs somewhat from the gastrointestinal, hematological, and constitutional (fatigue) adverse events that we see with common chemotherapeutic agents used to treat ovarian cancer, which are generally cumulative.
PARP inhibitor treatment is also associated with an increased risk of developing myelodysplastic syndrome/acute myeloid leukemia (MDS/AML). That being said, therapy-related MDS/AML is a well-recognized complication of conventional chemotherapy used to treat a variety of primary malignancies, including ovarian cancer.
The expected toxicity profile for pamiparib is based on what we have seen with the other PARP inhibitors. This includes any grade nausea (50%), fatigue (33%), anemia (20%), vomiting (15%), and neutropenia (13%). Toxicity of grade 3 or higher includes anemia (13%), neutropenia (8%), and fatigue (5%).
Where do the newest drugs to be approved for ovarian cancer in recent years fit within the treatment paradigm? What do the research findings show about their efficacy and safety?
Data from phase 2/3 trials support the use of PARP inhibitors as monotherapy as well as in combination with other agents (most commonly agents other than standard chemotherapy) for the treatment of BRCA mutated or otherwise homologous recombination-deficient (HRD) ovarian cancer. They can also be considered for maintenance therapy at the conclusion of treatment of newly diagnosed or recurrent BRCA-mutated/HRD ovarian cancer.
Large phase 3 studies have resulted in the approval of the antiangiogenic agent bevacizumab in combination with chemotherapy for the treatment of newly diagnosed and recurrent ovarian cancer, as well as for maintenance therapy at the conclusion of combination chemotherapy plus bevacizumab treatment of newly diagnosed (GOG 218 and ICON 7 trials) or recurrent ovarian cancer (GOG 218, OCEANS, and AURELIA trials). The most common toxicity with antiangiogenic agents is hypertension. Women also commonly experience arthralgia/myalgia. There is an increased risk of proteinuria, blood clots, bleeding, and serious gastrointestinal events such as fistula and bowel perforation.
Data from the phase 2 KEYNOTE 158 trial support pembrolizumab for microsatellite high or mismatch repair-deficient ovarian cancers. Common side effects associated with the use of pembrolizumab include fatigue, itchy skin, diarrhea, nausea, decreased appetite, rash, fever, cough, difficulty breathing, musculoskeletal pain, constipation, and joint pain. Pembrolizumab can cause the immune system to attack normal organs and tissues in the body resulting in serious side effects, including inflammation of such organs as the lungs, colon, liver, endocrine glands, and kidneys.
Evidence for hormonal therapy (i.e., aromatase inhibitors like letrozole) for the treatment of newly diagnosed and recurrent low-grade serous/endometrioid epithelial ovarian cancer comes from largely retrospective cohort studies. A large phase 3 study, now enrolling, will examine if letrozole monotherapy/maintenance is non-inferior to intravenous paclitaxel/carboplatin and maintenance letrozole with respect to progression-free survival in women with stage II-IV primary low-grade serous carcinoma of the ovary or peritoneum after primary surgical cytoreduction.
Hormonal therapies are generally very well tolerated. Common side effects may include hot flashes, warmth or redness in the face or chest, headache, dizziness, weakness, bone pain, muscle or joint pain, swelling, weight gain, increased sweating, or increased cholesterol in the blood.
What other drugs are in development for ovarian cancer?
VEGF receptor tyrosine kinase inhibitors, such as cediranib, are in development. Anlotinib is another drug being investigated. It is a new multi-target tyrosine kinase inhibitor that targets VEGFR, PDGFR, and FGFR. Drugs targeting folate-alpha receptor, such as mirvetuximab, are under investigation, particularly for patients with high folate-alpha receptor membrane staining by immunohistochemistry. Drugs targeting cell cycle arrest, such as CDK4/6 inhibitors, are also being considered.
Can you provide some of the highlights of ovarian cancer research presented at this year’s American Society of Clinical Oncology meeting ?
My take is that we have gone from a monotonous landscape of platinum doublet chemotherapy to an exciting, diversified landscape over the past several years. All of this activity has driven median overall survival up from 3 years to 5 years and progression-free survival following first platinum sensitive recurrence to well beyond 6 months.
Since last year’s meeting, we have seen several new approvals, including niraparib for the treatment of BRCA mutated and HRD disease, as well as for first-line maintenance in all comers. In May, the FDA expanded the indication for olaparib to include its combination with bevacizumab as first-line maintenance for BRCA-mutated and HRD disease based on the results of PAOLA-1. With certainty, our treatment paradigms will continue to evolve in response to these and other new data.
At this year’s meeting, the SOLO-2 investigators revealed the first overall survival data for second-line PARP inhibitor maintenance, which is the first suggestion that PARP inhibitor maintenance improves overall survival.
We have a new understanding about the genetics of long-term responders to rucaparib on ARIEL-2.
We also understand how the role of secondary cytoreductive surgery and how nonchemotherapy options for the treatment of platinum sensitive relapse compare in terms of efficacy and toxicity (i.e., AVANOVA-2 and GY004 trials). We see again the importance of R0 cytoreduction when surgery is pursued. Achieving anything less than R0 cytoreduction for the treatment of first platinum sensitive recurrence may translate into shorter survival, compared with chemotherapy alone.
We are also becoming increasingly familiar with the limited therapeutic benefit of single-agent anti-PD-1/PD-L1, which is so different from our experience in mismatch repair-deficient endometrial cancer. In the small percentage of responders, there are some durable responses and a suggestion of particular efficacy among women with clear cell ovarian cancer.
What other recent findings in ovarian cancer research should oncologists be aware of?
Data supporting improved efficacy of a gastrointestinal-type chemotherapy regimen for mucinous epithelial ovarian cancers come from a retrospective cohort study of patients with ovarian mucinous carcinoma who received postoperative adjuvant chemotherapy at two academic centers.
Identification of inactivating SMARCA4 mutations as the driver of small cell carcinoma of the ovary, hypercalcemic type, and the idea that CDK4/6 inhibitors could be effectively repurposed to treat this rare but highly aggressive type of ovarian cancer is also new and exciting.
Dr. Stone has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The treatment of ovarian cancer has evolved considerably in the last few years, with the approval of several PARP inhibitors, antiangiogenic agents, and other therapies for a multitude of indications. Additional treatments are likely to soon join this already diverse spectrum of available options, if their promising efficacy and safety continues to be borne out in ongoing research.
To better understand the individual merits and potential drawbacks of these treatments, Medscape recently spoke with Rebecca Stone, MD, an ovarian cancer expert and associate professor of gynecologic oncology at Johns Hopkins University, Baltimore. This interview has been edited for length and clarity.
Medscape: We’re starting to see preliminary data on pamiparib , an investigational inhibitor of PARP1 and PARP2, for the treatment of ovarian cancer. What is the evidence supporting this drug?
Dr. Stone: Currently, six different PARP inhibitors – olaparib, rucaparib, veliparib, niraparib, talazoparib, and pamiparib – have been in clinical development at different stages. In clinical applications, PARP inhibitors, including olaparib, rucaparib, niraparib, and talazoparib, have demonstrated sustained antitumor responses as single agents in patients with BRCA1 or BRCA2 mutations. Those with Food and Drug Administration indications in ovarian cancer include olaparib, rucaparib, and niraparib. The preclinical and clinical data with pamiparib is limited as of now. But, in a xenograft breast cancer model, it was found to be over 10 times more potent than olaparib.
If approved, where would pamiparib fit in the treatment paradigm for ovarian cancer?
It would potentially fit as monotherapy as well as in combination with agents other than standard chemotherapy for the treatment of BRCA mutated ovarian cancer. It could also be considered for maintenance therapy at the conclusion of chemotherapy treatment of newly diagnosed or recurrent BRCA-mutated ovarian cancer.
What adverse events are associated with pamiparib? How does the toxicity profile compare with other drugs for ovarian cancer?
With respect to PARP inhibitors, the differences in potency (PARP trapping) correlate with their toxicity profiles. The most common adverse events are gastrointestinal, hematologic, and constitutional (fatigue). Even though it is difficult to compare toxicities across different trials with heterogeneous patient populations, there are a few points worth noting.
Rucaparib leads to inhibition of renal transporter proteins involved in secretion of creatinine and can lead to increased creatinine (any grade: 15%; grade 3: ≤1%). Transaminitis is generally self-limiting and highest with rucaparib (any grade: 34%; grade 3: 10%). Hematologic toxicities are the highest with niraparib (any grade: thrombocytopenia 61%, anemia 50%, neutropenia 30%; grade ≥3: thrombocytopenia 34%, anemia 25%, neutropenia 20%).
Toxicities are more common in the first few cycles of treatment, warranting closer early monitoring. This differs somewhat from the gastrointestinal, hematological, and constitutional (fatigue) adverse events that we see with common chemotherapeutic agents used to treat ovarian cancer, which are generally cumulative.
PARP inhibitor treatment is also associated with an increased risk of developing myelodysplastic syndrome/acute myeloid leukemia (MDS/AML). That being said, therapy-related MDS/AML is a well-recognized complication of conventional chemotherapy used to treat a variety of primary malignancies, including ovarian cancer.
The expected toxicity profile for pamiparib is based on what we have seen with the other PARP inhibitors. This includes any grade nausea (50%), fatigue (33%), anemia (20%), vomiting (15%), and neutropenia (13%). Toxicity of grade 3 or higher includes anemia (13%), neutropenia (8%), and fatigue (5%).
Where do the newest drugs to be approved for ovarian cancer in recent years fit within the treatment paradigm? What do the research findings show about their efficacy and safety?
Data from phase 2/3 trials support the use of PARP inhibitors as monotherapy as well as in combination with other agents (most commonly agents other than standard chemotherapy) for the treatment of BRCA mutated or otherwise homologous recombination-deficient (HRD) ovarian cancer. They can also be considered for maintenance therapy at the conclusion of treatment of newly diagnosed or recurrent BRCA-mutated/HRD ovarian cancer.
Large phase 3 studies have resulted in the approval of the antiangiogenic agent bevacizumab in combination with chemotherapy for the treatment of newly diagnosed and recurrent ovarian cancer, as well as for maintenance therapy at the conclusion of combination chemotherapy plus bevacizumab treatment of newly diagnosed (GOG 218 and ICON 7 trials) or recurrent ovarian cancer (GOG 218, OCEANS, and AURELIA trials). The most common toxicity with antiangiogenic agents is hypertension. Women also commonly experience arthralgia/myalgia. There is an increased risk of proteinuria, blood clots, bleeding, and serious gastrointestinal events such as fistula and bowel perforation.
Data from the phase 2 KEYNOTE 158 trial support pembrolizumab for microsatellite high or mismatch repair-deficient ovarian cancers. Common side effects associated with the use of pembrolizumab include fatigue, itchy skin, diarrhea, nausea, decreased appetite, rash, fever, cough, difficulty breathing, musculoskeletal pain, constipation, and joint pain. Pembrolizumab can cause the immune system to attack normal organs and tissues in the body resulting in serious side effects, including inflammation of such organs as the lungs, colon, liver, endocrine glands, and kidneys.
Evidence for hormonal therapy (i.e., aromatase inhibitors like letrozole) for the treatment of newly diagnosed and recurrent low-grade serous/endometrioid epithelial ovarian cancer comes from largely retrospective cohort studies. A large phase 3 study, now enrolling, will examine if letrozole monotherapy/maintenance is non-inferior to intravenous paclitaxel/carboplatin and maintenance letrozole with respect to progression-free survival in women with stage II-IV primary low-grade serous carcinoma of the ovary or peritoneum after primary surgical cytoreduction.
Hormonal therapies are generally very well tolerated. Common side effects may include hot flashes, warmth or redness in the face or chest, headache, dizziness, weakness, bone pain, muscle or joint pain, swelling, weight gain, increased sweating, or increased cholesterol in the blood.
What other drugs are in development for ovarian cancer?
VEGF receptor tyrosine kinase inhibitors, such as cediranib, are in development. Anlotinib is another drug being investigated. It is a new multi-target tyrosine kinase inhibitor that targets VEGFR, PDGFR, and FGFR. Drugs targeting folate-alpha receptor, such as mirvetuximab, are under investigation, particularly for patients with high folate-alpha receptor membrane staining by immunohistochemistry. Drugs targeting cell cycle arrest, such as CDK4/6 inhibitors, are also being considered.
Can you provide some of the highlights of ovarian cancer research presented at this year’s American Society of Clinical Oncology meeting ?
My take is that we have gone from a monotonous landscape of platinum doublet chemotherapy to an exciting, diversified landscape over the past several years. All of this activity has driven median overall survival up from 3 years to 5 years and progression-free survival following first platinum sensitive recurrence to well beyond 6 months.
Since last year’s meeting, we have seen several new approvals, including niraparib for the treatment of BRCA mutated and HRD disease, as well as for first-line maintenance in all comers. In May, the FDA expanded the indication for olaparib to include its combination with bevacizumab as first-line maintenance for BRCA-mutated and HRD disease based on the results of PAOLA-1. With certainty, our treatment paradigms will continue to evolve in response to these and other new data.
At this year’s meeting, the SOLO-2 investigators revealed the first overall survival data for second-line PARP inhibitor maintenance, which is the first suggestion that PARP inhibitor maintenance improves overall survival.
We have a new understanding about the genetics of long-term responders to rucaparib on ARIEL-2.
We also understand how the role of secondary cytoreductive surgery and how nonchemotherapy options for the treatment of platinum sensitive relapse compare in terms of efficacy and toxicity (i.e., AVANOVA-2 and GY004 trials). We see again the importance of R0 cytoreduction when surgery is pursued. Achieving anything less than R0 cytoreduction for the treatment of first platinum sensitive recurrence may translate into shorter survival, compared with chemotherapy alone.
We are also becoming increasingly familiar with the limited therapeutic benefit of single-agent anti-PD-1/PD-L1, which is so different from our experience in mismatch repair-deficient endometrial cancer. In the small percentage of responders, there are some durable responses and a suggestion of particular efficacy among women with clear cell ovarian cancer.
What other recent findings in ovarian cancer research should oncologists be aware of?
Data supporting improved efficacy of a gastrointestinal-type chemotherapy regimen for mucinous epithelial ovarian cancers come from a retrospective cohort study of patients with ovarian mucinous carcinoma who received postoperative adjuvant chemotherapy at two academic centers.
Identification of inactivating SMARCA4 mutations as the driver of small cell carcinoma of the ovary, hypercalcemic type, and the idea that CDK4/6 inhibitors could be effectively repurposed to treat this rare but highly aggressive type of ovarian cancer is also new and exciting.
Dr. Stone has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.














