User login
Proposed withdrawal of approval of preterm drug: Two opposing views
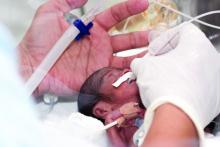
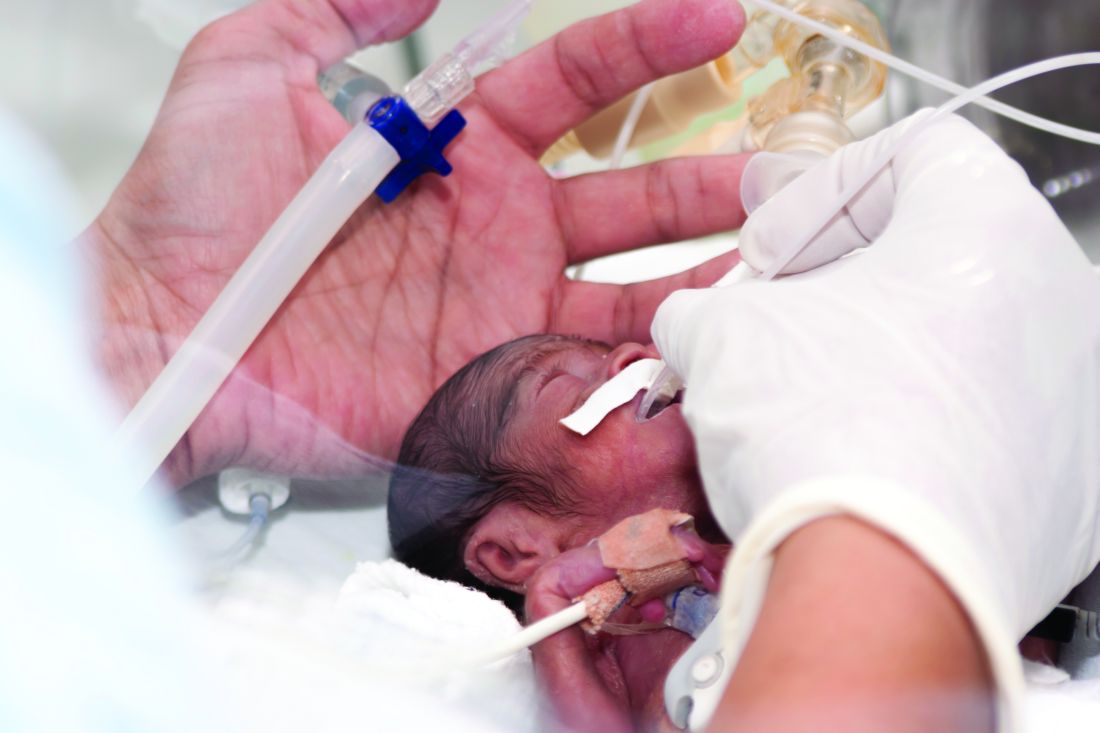
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Primary care journals address systemic racism in medicine
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
How to help families get through climate-related disasters
Wildfires burned millions of acres in California, Oregon, and Washington this year. Record numbers of tropical storms and hurricanes formed in the Atlantic. “Climate change is here. Disasters are here. They are going to be increasing, which is why we want to talk about this and talk about how pediatricians can help and respond to these events,” Scott Needle, MD, said at the annual meeting American Academy of Pediatrics, held virtually this year.

said Dr. Needle, chief medical officer of Elica Health Centers in Sacramento, California. “We can be a positive influence in terms of getting out proactive messaging and keeping people informed.”
The Federal Emergency Management Agency (FEMA) 2019 National Household Survey found that about half of households had an emergency plan. A theme across surveys is that, although households take some steps to get ready for disasters, the public generally “is not as prepared for these events as they really need to be,” Dr. Needle said.
The AAP, the Red Cross, and FEMA are among the organizations that offer planning guides, most of which emphasize three simple things: have a kit, have a plan, and be informed, he said.
To prepare for a disaster, parents might refill a child’s medications ahead of time if possible, Dr. Needle suggested. And during the COVID-19 pandemic, families should add masks, sanitizers, and wipes to their go-bags.
Physicians also can help families by asking how they are coping.
Wildfire smoke
“Smoke from wildfires can blanket large, large areas,” Mark Miller, MD, MPH, said during the presentation at the AAP meeting. “This year, we have seen wildfire smoke from the western states reach all the way to the East Coast. So this impacts your patients and your own families sometimes, regardless of wherever you live.”
Children may be more vulnerable to wildfire smoke because they often spend more time outdoors and tend to be more active. In addition, their ongoing development means exposure to air pollutants could have lifelong consequences, said Dr. Miller, who recently reviewed the effects of wildfire smoke on children.
“Children with asthma should have some information about wildfires built into their asthma management plan,” said Dr. Miller, who is affiliated with Western States Pediatric Environmental Health Specialty Unit (PEHSU) and University of California, San Francisco. Pollutants are associated with respiratory visits and admissions, asthma exacerbations, decreased lung function, and neurocognitive effects. They also may be carcinogenic.
A study in monkeys found that smoke exposure during California wildfires in 2008 was associated with immune dysregulation and compromised lung function in adolescence.
Another study of three cohorts of children in southern California found that air pollutant levels were associated with children’s lung function.
Organizations have provided resources on creating cleaner air spaces during wildfires, including guides to build DIY air filter fans. AirNow.gov provides air quality and fire maps that can inform decisions about school closures and outdoor activities. Communities should prioritize establishing schools as clean air shelters, Dr. Miller suggested.
Studies have found that respirators and medical masks may decrease children’s exposure to smoke. Children should not use face coverings, however, if they are younger than 2 years, if they are not able to remove the face covering on their own or tell an adult that they need help, or if they have difficulty breathing with a face covering. Younger children should be observed by an adult.
During the pandemic, families should be aware that some types of masks are sold only for health care use, many foreign respirators are counterfeit, and cloth masks used for COVID-19 are not suitable for reducing wildfire smoke exposure, Dr. Miller said.
Hazards may linger
Long-term mental health issues may be the disaster consequence that pediatricians encounter most often, Dr. Needle said.
Eighteen months after a major wildfire in Canada, more than one-third of middle and high school students in one community had probable posttraumatic stress disorder (that is, intrusive thoughts, avoidance, and increased arousal). In addition, 31% of students had probable depression. Rates were elevated relative to a control group of students in another community that was not affected by the fire.
Findings indicate that a patient’s degree of exposure to a disaster affects the likelihood of adverse outcomes. On the other hand, resiliency may help mitigate adverse effects. “The hope is that if we can find ways to encourage resiliency before or in the aftermath of an event, we may be able to, in a sense, reduce some of these mental health sequelae,” Dr. Needle said.
Posttraumatic reactions in kids are likely after a disaster. “They may not rise to the level of a diagnosable condition, but they are very common in kids,” he said. “It is important to at least be able to counsel parents to recognize some of the common reactions,” such as acting withdrawn or aggressive, somatic complaints, and having trouble sleeping.
The AAP has a policy statement that encourages talking to children about their concerns with honest and age-appropriate responses, he noted.
When returning to an area after a disaster, many hazards may remain, such as floodwaters, ash pits, mold, and carbon monoxide from generators. “Generally speaking, you don’t want to have kids return to these areas until it is safe,” Dr. Needle said.
Exacerbation of existing conditions – perhaps because of lost medications, smoke exposure, or stress – may be another common problem. Other problems after a disaster could include domestic violence (direct or witnessed) and substance abuse.
“We have a responsibility to take care of our own health as well,” Dr. Needle added. “You can’t take care of others if you’re not taking care of yourself. It’s not being selfish. As a matter of fact, it’s being prudent. It’s survival.”
Dr. Needle and Dr. Miller had no relevant financial disclosures. Dr. Miller’s presentation was supported by the AAP and funded in part by the Agency for Toxic Substances and Disease Registry. The U.S. Environmental Protection Agency (EPA) provides funding support for the Pediatric Environmental Health Specialty Unit.
Wildfires burned millions of acres in California, Oregon, and Washington this year. Record numbers of tropical storms and hurricanes formed in the Atlantic. “Climate change is here. Disasters are here. They are going to be increasing, which is why we want to talk about this and talk about how pediatricians can help and respond to these events,” Scott Needle, MD, said at the annual meeting American Academy of Pediatrics, held virtually this year.

said Dr. Needle, chief medical officer of Elica Health Centers in Sacramento, California. “We can be a positive influence in terms of getting out proactive messaging and keeping people informed.”
The Federal Emergency Management Agency (FEMA) 2019 National Household Survey found that about half of households had an emergency plan. A theme across surveys is that, although households take some steps to get ready for disasters, the public generally “is not as prepared for these events as they really need to be,” Dr. Needle said.
The AAP, the Red Cross, and FEMA are among the organizations that offer planning guides, most of which emphasize three simple things: have a kit, have a plan, and be informed, he said.
To prepare for a disaster, parents might refill a child’s medications ahead of time if possible, Dr. Needle suggested. And during the COVID-19 pandemic, families should add masks, sanitizers, and wipes to their go-bags.
Physicians also can help families by asking how they are coping.
Wildfire smoke
“Smoke from wildfires can blanket large, large areas,” Mark Miller, MD, MPH, said during the presentation at the AAP meeting. “This year, we have seen wildfire smoke from the western states reach all the way to the East Coast. So this impacts your patients and your own families sometimes, regardless of wherever you live.”
Children may be more vulnerable to wildfire smoke because they often spend more time outdoors and tend to be more active. In addition, their ongoing development means exposure to air pollutants could have lifelong consequences, said Dr. Miller, who recently reviewed the effects of wildfire smoke on children.
“Children with asthma should have some information about wildfires built into their asthma management plan,” said Dr. Miller, who is affiliated with Western States Pediatric Environmental Health Specialty Unit (PEHSU) and University of California, San Francisco. Pollutants are associated with respiratory visits and admissions, asthma exacerbations, decreased lung function, and neurocognitive effects. They also may be carcinogenic.
A study in monkeys found that smoke exposure during California wildfires in 2008 was associated with immune dysregulation and compromised lung function in adolescence.
Another study of three cohorts of children in southern California found that air pollutant levels were associated with children’s lung function.
Organizations have provided resources on creating cleaner air spaces during wildfires, including guides to build DIY air filter fans. AirNow.gov provides air quality and fire maps that can inform decisions about school closures and outdoor activities. Communities should prioritize establishing schools as clean air shelters, Dr. Miller suggested.
Studies have found that respirators and medical masks may decrease children’s exposure to smoke. Children should not use face coverings, however, if they are younger than 2 years, if they are not able to remove the face covering on their own or tell an adult that they need help, or if they have difficulty breathing with a face covering. Younger children should be observed by an adult.
During the pandemic, families should be aware that some types of masks are sold only for health care use, many foreign respirators are counterfeit, and cloth masks used for COVID-19 are not suitable for reducing wildfire smoke exposure, Dr. Miller said.
Hazards may linger
Long-term mental health issues may be the disaster consequence that pediatricians encounter most often, Dr. Needle said.
Eighteen months after a major wildfire in Canada, more than one-third of middle and high school students in one community had probable posttraumatic stress disorder (that is, intrusive thoughts, avoidance, and increased arousal). In addition, 31% of students had probable depression. Rates were elevated relative to a control group of students in another community that was not affected by the fire.
Findings indicate that a patient’s degree of exposure to a disaster affects the likelihood of adverse outcomes. On the other hand, resiliency may help mitigate adverse effects. “The hope is that if we can find ways to encourage resiliency before or in the aftermath of an event, we may be able to, in a sense, reduce some of these mental health sequelae,” Dr. Needle said.
Posttraumatic reactions in kids are likely after a disaster. “They may not rise to the level of a diagnosable condition, but they are very common in kids,” he said. “It is important to at least be able to counsel parents to recognize some of the common reactions,” such as acting withdrawn or aggressive, somatic complaints, and having trouble sleeping.
The AAP has a policy statement that encourages talking to children about their concerns with honest and age-appropriate responses, he noted.
When returning to an area after a disaster, many hazards may remain, such as floodwaters, ash pits, mold, and carbon monoxide from generators. “Generally speaking, you don’t want to have kids return to these areas until it is safe,” Dr. Needle said.
Exacerbation of existing conditions – perhaps because of lost medications, smoke exposure, or stress – may be another common problem. Other problems after a disaster could include domestic violence (direct or witnessed) and substance abuse.
“We have a responsibility to take care of our own health as well,” Dr. Needle added. “You can’t take care of others if you’re not taking care of yourself. It’s not being selfish. As a matter of fact, it’s being prudent. It’s survival.”
Dr. Needle and Dr. Miller had no relevant financial disclosures. Dr. Miller’s presentation was supported by the AAP and funded in part by the Agency for Toxic Substances and Disease Registry. The U.S. Environmental Protection Agency (EPA) provides funding support for the Pediatric Environmental Health Specialty Unit.
Wildfires burned millions of acres in California, Oregon, and Washington this year. Record numbers of tropical storms and hurricanes formed in the Atlantic. “Climate change is here. Disasters are here. They are going to be increasing, which is why we want to talk about this and talk about how pediatricians can help and respond to these events,” Scott Needle, MD, said at the annual meeting American Academy of Pediatrics, held virtually this year.

said Dr. Needle, chief medical officer of Elica Health Centers in Sacramento, California. “We can be a positive influence in terms of getting out proactive messaging and keeping people informed.”
The Federal Emergency Management Agency (FEMA) 2019 National Household Survey found that about half of households had an emergency plan. A theme across surveys is that, although households take some steps to get ready for disasters, the public generally “is not as prepared for these events as they really need to be,” Dr. Needle said.
The AAP, the Red Cross, and FEMA are among the organizations that offer planning guides, most of which emphasize three simple things: have a kit, have a plan, and be informed, he said.
To prepare for a disaster, parents might refill a child’s medications ahead of time if possible, Dr. Needle suggested. And during the COVID-19 pandemic, families should add masks, sanitizers, and wipes to their go-bags.
Physicians also can help families by asking how they are coping.
Wildfire smoke
“Smoke from wildfires can blanket large, large areas,” Mark Miller, MD, MPH, said during the presentation at the AAP meeting. “This year, we have seen wildfire smoke from the western states reach all the way to the East Coast. So this impacts your patients and your own families sometimes, regardless of wherever you live.”
Children may be more vulnerable to wildfire smoke because they often spend more time outdoors and tend to be more active. In addition, their ongoing development means exposure to air pollutants could have lifelong consequences, said Dr. Miller, who recently reviewed the effects of wildfire smoke on children.
“Children with asthma should have some information about wildfires built into their asthma management plan,” said Dr. Miller, who is affiliated with Western States Pediatric Environmental Health Specialty Unit (PEHSU) and University of California, San Francisco. Pollutants are associated with respiratory visits and admissions, asthma exacerbations, decreased lung function, and neurocognitive effects. They also may be carcinogenic.
A study in monkeys found that smoke exposure during California wildfires in 2008 was associated with immune dysregulation and compromised lung function in adolescence.
Another study of three cohorts of children in southern California found that air pollutant levels were associated with children’s lung function.
Organizations have provided resources on creating cleaner air spaces during wildfires, including guides to build DIY air filter fans. AirNow.gov provides air quality and fire maps that can inform decisions about school closures and outdoor activities. Communities should prioritize establishing schools as clean air shelters, Dr. Miller suggested.
Studies have found that respirators and medical masks may decrease children’s exposure to smoke. Children should not use face coverings, however, if they are younger than 2 years, if they are not able to remove the face covering on their own or tell an adult that they need help, or if they have difficulty breathing with a face covering. Younger children should be observed by an adult.
During the pandemic, families should be aware that some types of masks are sold only for health care use, many foreign respirators are counterfeit, and cloth masks used for COVID-19 are not suitable for reducing wildfire smoke exposure, Dr. Miller said.
Hazards may linger
Long-term mental health issues may be the disaster consequence that pediatricians encounter most often, Dr. Needle said.
Eighteen months after a major wildfire in Canada, more than one-third of middle and high school students in one community had probable posttraumatic stress disorder (that is, intrusive thoughts, avoidance, and increased arousal). In addition, 31% of students had probable depression. Rates were elevated relative to a control group of students in another community that was not affected by the fire.
Findings indicate that a patient’s degree of exposure to a disaster affects the likelihood of adverse outcomes. On the other hand, resiliency may help mitigate adverse effects. “The hope is that if we can find ways to encourage resiliency before or in the aftermath of an event, we may be able to, in a sense, reduce some of these mental health sequelae,” Dr. Needle said.
Posttraumatic reactions in kids are likely after a disaster. “They may not rise to the level of a diagnosable condition, but they are very common in kids,” he said. “It is important to at least be able to counsel parents to recognize some of the common reactions,” such as acting withdrawn or aggressive, somatic complaints, and having trouble sleeping.
The AAP has a policy statement that encourages talking to children about their concerns with honest and age-appropriate responses, he noted.
When returning to an area after a disaster, many hazards may remain, such as floodwaters, ash pits, mold, and carbon monoxide from generators. “Generally speaking, you don’t want to have kids return to these areas until it is safe,” Dr. Needle said.
Exacerbation of existing conditions – perhaps because of lost medications, smoke exposure, or stress – may be another common problem. Other problems after a disaster could include domestic violence (direct or witnessed) and substance abuse.
“We have a responsibility to take care of our own health as well,” Dr. Needle added. “You can’t take care of others if you’re not taking care of yourself. It’s not being selfish. As a matter of fact, it’s being prudent. It’s survival.”
Dr. Needle and Dr. Miller had no relevant financial disclosures. Dr. Miller’s presentation was supported by the AAP and funded in part by the Agency for Toxic Substances and Disease Registry. The U.S. Environmental Protection Agency (EPA) provides funding support for the Pediatric Environmental Health Specialty Unit.
FROM AAP 2020
Choosing pharmacotherapy for bipolar disorder requires a risk-benefit analysis
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Dermatologists as Social Media Contributors During the COVID-19 Pandemic
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
Practice Points
- With the coronavirus disease 2019 (COVID-19) pandemic, strict physical distancing measures have made patients and providers alike reliant on global digital social networks such as Instagram, Twitter, and Facebook to facilitate information sharing about COVID-19.
- Dermatologists should utilize social media as a platform to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during the global pandemic.
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Overlap Syndrome in a Patient With Relapsing Polychondritis
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.

Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.

Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.

Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
Practice Points
- The clinical presentation of relapsing polychondritis (RP) may demonstrate cutaneous manifestations other than the typical inflammation of cartilage-rich structures.
- Approximately 30% of patients with RP will have another autoimmune disease.
New return-to-play recommendations for athletes with COVID-19
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Cirrhosis, Child-Pugh score predict ERCP complications
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
Cirrhosis may increase the risk of complications from endoscopic retrograde cholangiopancreatography (ERCP), according to a retrospective study involving almost 700 patients.
The study also showed that Child-Pugh class was a better predictor of risk than Model for End-Stage Liver Disease (MELD) score, reported lead author Michelle Bernshteyn, MD, a third-year internal medicine resident at State University of New York, Syracuse , and colleagues.
“There remains a scarcity in the literature regarding complications and adverse effects after ERCP in cirrhotic patients, particularly those incorporating Child-Pugh class and MELD score or type of intervention as predictors,” Dr. Bernshteyn said during a virtual presentation at the American College of Gastroenterology annual meeting. “Furthermore, literature review demonstrates inconsistency among results.”
To gain clarity, Dr. Bernshteyn and colleagues reviewed electronic medical records from 692 patients who underwent ERCP, of whom 174 had cirrhosis and 518 did not. For all patients, the investigators analyzed demographics, comorbidities, indications for ERCP, type of sedation, type of intervention, and complications within a 30-day period. Complications included bleeding, pancreatitis, cholangitis, perforation, mortality caused by ERCP, and mortality from other causes. Patients with cirrhosis were further analyzed based on etiology of cirrhosis, Child-Pugh class, and MELD score.
The analysis revealed that complications were significantly more common in patients with cirrhosis than in those without cirrhosis (21.30% vs. 13.51%; P = .015). No specific complications were significantly more common in patients with cirrhosis than in those without cirrhosis.
In patients with cirrhosis, 41.18% of Child-Pugh class C patients had complications, compared with 15.15% of class B patients and 19.30% of class A patients (P = .010). In contrast, MELD scores were not significantly associated with adverse events.
Further analysis showed that, in patients without cirrhosis, diagnostic-only ERCP and underlying chronic obstructive pulmonary disease were associated with high rates of complications (P = .039 and P = .003, respectively). In patients with cirrhosis, underlying chronic obstructive pulmonary disease and hypertension predicted adverse events (P = .009 and P = .003, respectively).
“The results of our study reaffirm that liver cirrhosis has an impact on the occurrence of complications during ERCP,” Dr. Bernshteyn said. “Child-Pugh class seems to be more reliable as compared to MELD score in predicting complications of ERCP in cirrhosis patients,” she added. “However, we are also aware that Child-Pugh and MELD scores are complementary to each other while evaluating outcomes of any surgery in patients with cirrhosis.”
In 2017, Udayakumar Navaneethan, MD, a gastroenterologist at AdventHealth Orlando’s Center for Interventional Endoscopy, and an assistant professor at the University of Central Florida, Orlando, and colleagues published a national database study concerning the safety of ERCP in patients with liver cirrhosis.
“[The present] study is important as it highlights the fact that ERCP is associated with significant complications in cirrhotic patients compared to those without cirrhosis,” Dr. Navaneethan said when asked to comment. “Also, Child-Pugh score appeared to be more reliable than MELD score in predicting complications of ERCP in cirrhotic patients.”
He went on to explain relevance for practicing clinicians. “The clinical implications of the study are that a detailed risk-benefit discussion needs to be done with patients with liver cirrhosis, particularly with advanced liver disease Child-Pugh class C, irrespective of the etiology,” Dr. Navaneethan said. “ERCP should be performed when there is clear evidence that the benefits outweigh the risks.” The investigators and Dr. Navaneethan reported no conflicts of interest.
SOURCE: Bernshteyn M et al. ACG 2020, Abstract S0982.
FROM ACG 2020
Surgery may not be needed with locally advanced rectal cancer
A short course of radiation therapy followed by neoadjuvant chemotherapy resulted in a clinical complete response (CR) in almost half of 90 patients with locally advanced rectal cancer, allowing the majority of responders to skip surgical resection, a retrospective study indicates.
Specifically, at a median follow-up of 16.6 months for living patients, the initial clinical CR rate was 48% overall.
“While we do not have enough follow-up yet to know the late side-effect profile of this regimen, our preliminary results are promising,” Re-I Chin, MD, of Washington University School of Medicine, St. Louis, Missouri, told Medscape Medical News in an email.
The study was presented at the virtual 2020 meeting of the American Society of Radiation Oncology (ASTRO).
“Certainly, longer follow-up will be needed in this study, but none of the observed patients to date has experienced an unsalvageable failure,” said meeting discussant Amol Narang, MD, of Johns Hopkins University, Baltimore, Maryland.
He reminded conference attendees that, despite good evidence supporting equivalency in oncologic outcomes between short-course radiation and long-course chemoradiation, the former is “highly underutilized in the US” with a mere 1% usage rate between 2004 and 2014.
The current study’s short-course treatment approach was compared in this setting to long-course chemoradiation and adjuvant chemotherapy in the RAPIDO trial reported at the 2020 annual meeting of the American Society of Clinical Oncology (ASCO), Narang pointed out.
Short-course patients had a higher rate of pathological complete response (pCR) and a lower rate of treatment failure compared with patients who received long-course chemoradiation and adjuvant chemotherapy; both patient groups underwent total mesorectal excision — which is different from the current analysis. The RAPIDO investigators concluded that the approach featuring the short course “can be considered as a new standard of care.”
Narang said the data collectively “begs the question as to whether the superiority of long-course chemoradiation should really have to be demonstrated to justify its use.”
But Chin highlighted toxicity issues. “Historically, there have been concerns regarding toxicity with short-course radiation therapy since it requires larger doses of radiation given over a shorter period of time,” Chin explained. “But [the short course] is particularly convenient for patients since it saves them more than a month of daily trips to the radiation oncology department.”
Seven local failures
The single-center study involved patients with newly diagnosed, nonmetastatic rectal adenocarcinoma treated with short-course radiation therapy followed by chemotherapy in 2018 and 2019. Nearly all (96%) had locally advanced disease, with either a T3/T4 tumor or node-positive disease. Median tumor size was 4.6 cm.
“Many of the patients in the study had low lying tumors,” Chin reported, with a median distance from the anal verge of 7 cm.
Radiation therapy was delivered in 25 Gy given in five fractions over 5 consecutive days, with the option to boost the dose to 30 Gy or 35 Gy in five fractions if extra-mesorectal lymph nodes were involved. Conventional 3D or intensity-modulated radiation was used and all patients completed treatment.
The median interval between diagnosis of rectal cancer and initiation of radiation therapy was 1.4 months, while the median interval between completion of radiation to initiation of chemotherapy was 2.7 weeks.
The most common chemotherapy regimen was FOLFOX – consisting of leucovorin, fluorouracil (5-FU), and oxaliplatin – or modified FOLFOX. For patients who received six or more cycles of chemotherapy, the median time spent on treatment was 3.9 months.
For those who completed at least six cycles of chemotherapy, the overall clinical CR was 51%, and, for patients with locally advanced disease, the clinical CR rate was 49%. Five of the 43 patients who achieved an initial clinical CR still underwent surgical resection because of patient or physician preference. Among this small group of patients, 4 of the 5 achieved a pCR, and the remaining patient achieved a pathological partial response (pPR).
A total of 42 patients (47% of the group) achieved a partial response following the radiation plus chemotherapy paradigm, and one patient had progressive disease. All underwent surgical resection. One patient did not complete chemotherapy and did not get surgery and subsequently died.
This left 38 patients to be managed nonoperatively. In this nonoperative cohort, 79% of patients continued to have a clinical CR at the end of follow-up. Of the 7 patients with local failure, 6 were salvaged by surgery, one was salvaged by chemotherapy, and all 7 treatment failures had no evidence of disease at last follow-up.
Of the small group of 5 patients who achieved an initial clinical CR following short-course radiation therapy and neoadjuvant chemotherapy, there were no further events in this group, whereas, for patients who achieved only an initial partial response or who had progressive disease, 72% remained event-free.
Approximately half of those who achieved a clinical CR to the treatment regimen had no late gastrointestinal toxicities, and no grade 3 or 4 toxicities were observed. “Surgical resection of tumors — even without a permanent stoma — can result in significantly decreased bowel function, so our goal is to treat patients without surgery and maintain good bowel function,” Chin noted.
“For rectal cancer, both short-course radiation therapy and nonoperative management are emerging treatment paradigms that may be more cost-effective and convenient compared to long-course chemoradiation followed by surgery, [especially since] the COVID-19 pandemic...has spurred changes in clinical practices in radiation oncology,” she said.
Chin has disclosed no relevant financial relationships. Narang reports receiving research support from Boston Scientific.
This article first appeared on Medscape.com.
A short course of radiation therapy followed by neoadjuvant chemotherapy resulted in a clinical complete response (CR) in almost half of 90 patients with locally advanced rectal cancer, allowing the majority of responders to skip surgical resection, a retrospective study indicates.
Specifically, at a median follow-up of 16.6 months for living patients, the initial clinical CR rate was 48% overall.
“While we do not have enough follow-up yet to know the late side-effect profile of this regimen, our preliminary results are promising,” Re-I Chin, MD, of Washington University School of Medicine, St. Louis, Missouri, told Medscape Medical News in an email.
The study was presented at the virtual 2020 meeting of the American Society of Radiation Oncology (ASTRO).
“Certainly, longer follow-up will be needed in this study, but none of the observed patients to date has experienced an unsalvageable failure,” said meeting discussant Amol Narang, MD, of Johns Hopkins University, Baltimore, Maryland.
He reminded conference attendees that, despite good evidence supporting equivalency in oncologic outcomes between short-course radiation and long-course chemoradiation, the former is “highly underutilized in the US” with a mere 1% usage rate between 2004 and 2014.
The current study’s short-course treatment approach was compared in this setting to long-course chemoradiation and adjuvant chemotherapy in the RAPIDO trial reported at the 2020 annual meeting of the American Society of Clinical Oncology (ASCO), Narang pointed out.
Short-course patients had a higher rate of pathological complete response (pCR) and a lower rate of treatment failure compared with patients who received long-course chemoradiation and adjuvant chemotherapy; both patient groups underwent total mesorectal excision — which is different from the current analysis. The RAPIDO investigators concluded that the approach featuring the short course “can be considered as a new standard of care.”
Narang said the data collectively “begs the question as to whether the superiority of long-course chemoradiation should really have to be demonstrated to justify its use.”
But Chin highlighted toxicity issues. “Historically, there have been concerns regarding toxicity with short-course radiation therapy since it requires larger doses of radiation given over a shorter period of time,” Chin explained. “But [the short course] is particularly convenient for patients since it saves them more than a month of daily trips to the radiation oncology department.”
Seven local failures
The single-center study involved patients with newly diagnosed, nonmetastatic rectal adenocarcinoma treated with short-course radiation therapy followed by chemotherapy in 2018 and 2019. Nearly all (96%) had locally advanced disease, with either a T3/T4 tumor or node-positive disease. Median tumor size was 4.6 cm.
“Many of the patients in the study had low lying tumors,” Chin reported, with a median distance from the anal verge of 7 cm.
Radiation therapy was delivered in 25 Gy given in five fractions over 5 consecutive days, with the option to boost the dose to 30 Gy or 35 Gy in five fractions if extra-mesorectal lymph nodes were involved. Conventional 3D or intensity-modulated radiation was used and all patients completed treatment.
The median interval between diagnosis of rectal cancer and initiation of radiation therapy was 1.4 months, while the median interval between completion of radiation to initiation of chemotherapy was 2.7 weeks.
The most common chemotherapy regimen was FOLFOX – consisting of leucovorin, fluorouracil (5-FU), and oxaliplatin – or modified FOLFOX. For patients who received six or more cycles of chemotherapy, the median time spent on treatment was 3.9 months.
For those who completed at least six cycles of chemotherapy, the overall clinical CR was 51%, and, for patients with locally advanced disease, the clinical CR rate was 49%. Five of the 43 patients who achieved an initial clinical CR still underwent surgical resection because of patient or physician preference. Among this small group of patients, 4 of the 5 achieved a pCR, and the remaining patient achieved a pathological partial response (pPR).
A total of 42 patients (47% of the group) achieved a partial response following the radiation plus chemotherapy paradigm, and one patient had progressive disease. All underwent surgical resection. One patient did not complete chemotherapy and did not get surgery and subsequently died.
This left 38 patients to be managed nonoperatively. In this nonoperative cohort, 79% of patients continued to have a clinical CR at the end of follow-up. Of the 7 patients with local failure, 6 were salvaged by surgery, one was salvaged by chemotherapy, and all 7 treatment failures had no evidence of disease at last follow-up.
Of the small group of 5 patients who achieved an initial clinical CR following short-course radiation therapy and neoadjuvant chemotherapy, there were no further events in this group, whereas, for patients who achieved only an initial partial response or who had progressive disease, 72% remained event-free.
Approximately half of those who achieved a clinical CR to the treatment regimen had no late gastrointestinal toxicities, and no grade 3 or 4 toxicities were observed. “Surgical resection of tumors — even without a permanent stoma — can result in significantly decreased bowel function, so our goal is to treat patients without surgery and maintain good bowel function,” Chin noted.
“For rectal cancer, both short-course radiation therapy and nonoperative management are emerging treatment paradigms that may be more cost-effective and convenient compared to long-course chemoradiation followed by surgery, [especially since] the COVID-19 pandemic...has spurred changes in clinical practices in radiation oncology,” she said.
Chin has disclosed no relevant financial relationships. Narang reports receiving research support from Boston Scientific.
This article first appeared on Medscape.com.
A short course of radiation therapy followed by neoadjuvant chemotherapy resulted in a clinical complete response (CR) in almost half of 90 patients with locally advanced rectal cancer, allowing the majority of responders to skip surgical resection, a retrospective study indicates.
Specifically, at a median follow-up of 16.6 months for living patients, the initial clinical CR rate was 48% overall.
“While we do not have enough follow-up yet to know the late side-effect profile of this regimen, our preliminary results are promising,” Re-I Chin, MD, of Washington University School of Medicine, St. Louis, Missouri, told Medscape Medical News in an email.
The study was presented at the virtual 2020 meeting of the American Society of Radiation Oncology (ASTRO).
“Certainly, longer follow-up will be needed in this study, but none of the observed patients to date has experienced an unsalvageable failure,” said meeting discussant Amol Narang, MD, of Johns Hopkins University, Baltimore, Maryland.
He reminded conference attendees that, despite good evidence supporting equivalency in oncologic outcomes between short-course radiation and long-course chemoradiation, the former is “highly underutilized in the US” with a mere 1% usage rate between 2004 and 2014.
The current study’s short-course treatment approach was compared in this setting to long-course chemoradiation and adjuvant chemotherapy in the RAPIDO trial reported at the 2020 annual meeting of the American Society of Clinical Oncology (ASCO), Narang pointed out.
Short-course patients had a higher rate of pathological complete response (pCR) and a lower rate of treatment failure compared with patients who received long-course chemoradiation and adjuvant chemotherapy; both patient groups underwent total mesorectal excision — which is different from the current analysis. The RAPIDO investigators concluded that the approach featuring the short course “can be considered as a new standard of care.”
Narang said the data collectively “begs the question as to whether the superiority of long-course chemoradiation should really have to be demonstrated to justify its use.”
But Chin highlighted toxicity issues. “Historically, there have been concerns regarding toxicity with short-course radiation therapy since it requires larger doses of radiation given over a shorter period of time,” Chin explained. “But [the short course] is particularly convenient for patients since it saves them more than a month of daily trips to the radiation oncology department.”
Seven local failures
The single-center study involved patients with newly diagnosed, nonmetastatic rectal adenocarcinoma treated with short-course radiation therapy followed by chemotherapy in 2018 and 2019. Nearly all (96%) had locally advanced disease, with either a T3/T4 tumor or node-positive disease. Median tumor size was 4.6 cm.
“Many of the patients in the study had low lying tumors,” Chin reported, with a median distance from the anal verge of 7 cm.
Radiation therapy was delivered in 25 Gy given in five fractions over 5 consecutive days, with the option to boost the dose to 30 Gy or 35 Gy in five fractions if extra-mesorectal lymph nodes were involved. Conventional 3D or intensity-modulated radiation was used and all patients completed treatment.
The median interval between diagnosis of rectal cancer and initiation of radiation therapy was 1.4 months, while the median interval between completion of radiation to initiation of chemotherapy was 2.7 weeks.
The most common chemotherapy regimen was FOLFOX – consisting of leucovorin, fluorouracil (5-FU), and oxaliplatin – or modified FOLFOX. For patients who received six or more cycles of chemotherapy, the median time spent on treatment was 3.9 months.
For those who completed at least six cycles of chemotherapy, the overall clinical CR was 51%, and, for patients with locally advanced disease, the clinical CR rate was 49%. Five of the 43 patients who achieved an initial clinical CR still underwent surgical resection because of patient or physician preference. Among this small group of patients, 4 of the 5 achieved a pCR, and the remaining patient achieved a pathological partial response (pPR).
A total of 42 patients (47% of the group) achieved a partial response following the radiation plus chemotherapy paradigm, and one patient had progressive disease. All underwent surgical resection. One patient did not complete chemotherapy and did not get surgery and subsequently died.
This left 38 patients to be managed nonoperatively. In this nonoperative cohort, 79% of patients continued to have a clinical CR at the end of follow-up. Of the 7 patients with local failure, 6 were salvaged by surgery, one was salvaged by chemotherapy, and all 7 treatment failures had no evidence of disease at last follow-up.
Of the small group of 5 patients who achieved an initial clinical CR following short-course radiation therapy and neoadjuvant chemotherapy, there were no further events in this group, whereas, for patients who achieved only an initial partial response or who had progressive disease, 72% remained event-free.
Approximately half of those who achieved a clinical CR to the treatment regimen had no late gastrointestinal toxicities, and no grade 3 or 4 toxicities were observed. “Surgical resection of tumors — even without a permanent stoma — can result in significantly decreased bowel function, so our goal is to treat patients without surgery and maintain good bowel function,” Chin noted.
“For rectal cancer, both short-course radiation therapy and nonoperative management are emerging treatment paradigms that may be more cost-effective and convenient compared to long-course chemoradiation followed by surgery, [especially since] the COVID-19 pandemic...has spurred changes in clinical practices in radiation oncology,” she said.
Chin has disclosed no relevant financial relationships. Narang reports receiving research support from Boston Scientific.
This article first appeared on Medscape.com.
Burnout risk may be exacerbated by COVID crisis
New kinds of job stress multiply in unusual times
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
New kinds of job stress multiply in unusual times
New kinds of job stress multiply in unusual times
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.
Clarissa Barnes, MD, a hospitalist at Avera McKennan Hospital in Sioux Falls, S.D., and until recently medical director of Avera’s LIGHT Program, a wellness-oriented service for doctors, nurse practitioners, and physician assistants, watched the COVID-19 crisis unfold up close in her community and her hospital. Sioux Falls traced its surge of COVID patients to an outbreak at a local meatpacking plant.
“In the beginning, we didn’t know much about the virus and its communicability, although we have since gotten a better handle on that,” she said. “We had questions: Should we give patients more fluids – or less? Steroids or not? In my experience as a hospitalist I never had patients die every day on my shift, but that was happening with COVID.” The crisis imposed serious stresses on frontline providers, and hospitalists were concerned about personal safety and exposure risk – not just for themselves but for their families.
“The first time I worked on the COVID unit, I moved into the guest room in our home, apart from my husband and our young children,” Dr. Barnes said. “Ultimately I caught the virus, although I have since recovered.” Her experience has highlighted how existing issues of job stress and burnout in hospital medicine have been exacerbated by COVID-19. Even physicians who consider themselves healthy may have little emotional reserve to draw upon in a crisis of this magnitude.
“We are social distancing at work, wearing masks, not eating together with our colleagues – with less camaraderie and social support than we used to have,” she said. “I feel exhausted and there’s no question that my colleagues and I have sacrificed a lot to deal with the pandemic.” Add to that the second front of the COVID-19 crisis, Dr. Barnes said, which is “fighting the medical information wars, trying to correct misinformation put out there by people. Physicians who have been on the front lines of the pandemic know how demoralizing it can be to have people negate your first-hand experience.”
The situation has gotten better in Sioux Falls, Dr. Barnes said, although cases have started rising in the state again. The stress, while not gone, is reduced. For some doctors, “COVID reminded us of why we do what we do. Some of the usual bureaucratic requirements were set aside and we could focus on what our patients needed and how to take care of them.”
Taking job stress seriously
Tiffani Panek, MA, SFHM, CLHM, administrator of the division of hospital medicine at Johns Hopkins Bayview Medical Center in Baltimore, said job stress is a major issue for hospitalist groups.
“We take it seriously here, and use a survey tool to measure morale in our group annually,” she said. “So far, knock on wood, Baltimore has not been one of the big hot spots, but we’ve definitely had waves of COVID patients.”
The Bayview hospitalist group has a diversified set of leaders, including a wellness director. “They’re always checking up on our people, keeping an eye on those who are most vulnerable. One of the stressors we hadn’t thought about before was for our people who live alone. With the isolation and lockdown, they haven’t been able to socialize, so we’ve made direct outreach, asking people how they were doing,” Ms. Panek said. “People know we’ve got their back – professionally and personally. They know, if there’s something we can do to help, we will do it.”
Bayview Medical Center has COVID-specific units and non-COVID units, and has tried to rotate hospitalist assignments because more than a couple days in a row spent wearing full personal protective equipment (PPE) is exhausting, Ms. Panek said. The group also allocated a respite room just outside the biocontainment unit, with a computer and opportunities for providers to just sit and take a breather – with appropriate social distancing. “It’s not fancy, but you just have to wear a mask, not full PPE.”
The Hopkins hospitalist group’s wellness director, Catherine Washburn, MD, also a working hospitalist, said providers are exhausted, and trying to transition to the new normal is a moving target.
“It’s hard for anyone to say what our lives will look like in 6 months,” she said. “People in our group have lost family members to COVID, or postponed major life events, like weddings. We acknowledge losses together as a group, and celebrate things worth celebrating, like babies or birthdays.”
Greatest COVID caseload
Joshua Case, MD, hospitalist medical director for 16 acute care hospitals of Northwell Health serving metropolitan New York City and Long Island, said his group’s hospitalists and other staff worked incredibly hard during the surge of COVID-19 patients in New York. “Northwell likely cared for more COVID patients than any other health care system in the U.S., if not the world.
“It’s vastly different now. We went from a peak of thousands of cases per day down to about 70-90 new cases a day across our system. We’re lucky our system recognized that COVID could be an issue early on, with all of the multifaceted stressors on patient care,” Dr. Case said. “We’ve done whatever we could to give people time off, especially as the census started to come down. We freed up as many supportive mental health services as we could, working with the health system’s employee assistance program.”
Northwell gave out numbers for the psychiatry department, with clinicians available 24/7 for a confidential call, along with outside volunteers and a network of trauma psychologists. “Our system also provided emergency child care for staff, including hospitalists, wherever we could, drawing upon community resources,” Dr. Case added.
“We recognize that we’re all in the same foxhole. That’s been a helpful attitude – recognizing that it’s okay to be upset in a crisis and to have trouble dealing with what’s going on,” he said. “We need to acknowledge that some of us are suffering and try to encourage people to face it head on. For a lot of physicians, especially those who were redeployed here from other departments, it was important just to have us ask if they were doing okay.”
Brian Schroeder, MHA, FACHE, FHM, assistant vice president for hospital and emergency medicine for Atrium Health, based in Charlotte, N.C., said one of the biggest sources of stress on his staff has been the constant pace of change – whether local hospital protocols, state policies, or guidelines from the Centers for Disease Control and Prevention. “The updating is difficult to keep up with. A lot of our physicians get worried and anxious that they’re not following the latest guidelines or correctly doing what they should be doing to care for COVID patients. One thing we’ve done to alleviate some of that fear and anxiety is through weekly huddles with our hospital teams, focusing on changes relevant to their work. We also have weekly ‘all-hands’ meetings for our 250 providers across 13 acute and four postacute facilities.”
Before COVID, it was difficult to get everyone together as one big group from hospitals up to 5 hours apart, but with the Microsoft Teams platform, they can all meet together.
“At the height of the pandemic, we’d convene weekly and share national statistics, organizational statistics, testing updates, changes to protocols,” Mr. Schroeder said. As the pace of change has slowed, these meetings were cut back to monthly. “Our physicians feel we are passing on information as soon as we get it. They know we’ll always tell them what we know.”
Sarah Richards, MD, assistant professor of internal medicine at the University of Nebraska, Omaha, who heads the Society of Hospital Medicine’s Well-Being Task Force, formed to address staff stress in the COVID environment, said there are things that health care systems can do to help mitigate job stress and burnout. But broader issues may need to be addressed at a national level. “SHM is trying to understand work-related stress – and to identify resources that could support doctors, so they can spend more of their time doing what they enjoy most, which is taking care of patients,” she said.
“We also recognize that people have had very different experiences, depending on geography, and at the individual level stressors are experienced very differently,” Dr. Richard noted. “One of the most common stressors we’ve heard from doctors is the challenge of caring for patients who are lonely and isolated in their hospital rooms, suffering and dying in new ways. In low-incidence areas, doctors are expressing guilt because they aren’t under as much stress as their colleagues. In high-incidence areas, doctors are already experiencing posttraumatic stress disorder.”
SHM’s Well-Being Task Force is working on a tool to help normalize these stressors and encourage open conversations about mental health issues. A guide called “HM COVID Check-in Guide for Self & Peers” is designed to help hospitalists break the culture of silence around well-being and burnout during COVID-19 and how people are handling and processing the pandemic experience. It is expected to be completed later this year, Dr. Richards said. Other SHM projects and resources for staff support are also in the works.
The impact on women doctors
In a recent Journal of Hospital Medicine article entitled “Collateral Damage: How COVID-19 is Adversely Impacting Women Physicians,” hospitalist Yemisi Jones, MD, medical director of continuing medical education at Cincinnati Children’s Hospital Medical Center, and colleagues argue that preexisting gender inequities in compensation, academic rank and leadership positions for physicians have made the COVID-19 crisis even more burdensome on female hospitalists.1
“Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up,” they write. COVID may intensify workplace inequalities, with a lack of recognition of the undue strain that group policies place on women.
“Often women suffer in silence,” said coauthor Jennifer O’Toole, MD, MEd, director of education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center and program director of the internal medicine–pediatrics residency. “We are not always the best self-advocates, although many of us are working on that.”
When women in hospital medicine take leadership roles, these often tend to involve mutual support activities, taking care of colleagues, and promoting collaborative work environments, Dr. Jones added. The stereotypical example is the committee that organizes celebrations when group members get married or have babies.
These activities can take a lot of time, she said. “We need to pay attention to that kind of role in our groups, because it’s important to the cohesiveness of the group. But it often goes unrecognized and doesn’t translate into the currency of promotion and leadership in medicine. When women go for promotions in the future, how will what happened during the COVID crisis impact their opportunities?”
What is the answer to overcoming these systemic inequities? Start with making sure women are part of the leadership team, with responsibilities for group policies, schedules, and other important decisions. “Look at your group’s leadership – particularly the higher positions. If it’s not diverse, ask why. ‘What is it about the structure of our group?’ Make a more concerted effort in your recruitment and retention,” Dr. Jones said.
The JHM article also recommends closely monitoring the direct and indirect effects of COVID-19 on female hospitalists, inquiring specifically about the needs of women in the organization, and ensuring that diversity, inclusion, and equity efforts are not suspended during the pandemic. Gender-based disparities in pay also need a closer look, and not just one time but reviewed periodically and adjusted accordingly.
Mentoring for early career women is important, but more so is sponsorship – someone in a high-level leadership role in the group sponsoring women who are rising up the career ladder, Dr. O’Toole said. “Professional women tend to be overmentored and undersponsored.”
What are the answers?
Ultimately, listening is key to try to help people get through the pandemic, Dr. Washburn said. “People become burned out when they feel leadership doesn’t understand their needs or doesn’t hear their concerns. Our group leaders all do clinical work, so they are seen as one of us. They try very hard; they have listening ears. But listening is just the first step. Next step is to work creatively to get the identified needs met.”
A few years ago, Johns Hopkins developed training in enhanced communication in health care for all hospital providers, including nurses and doctors, encouraging them to get trained in how to actively listen and address their patients’ emotional and social experiences as well as disease, Dr. Washburn explained. Learning how to listen better to patients can enhance skills at listening to colleagues, and vice versa. “We recognize the importance of better communication – for reducing sentinel events in the hospital and also for preventing staff burnout.”
Dr. Barnes also does physician coaching, and says a lot of that work is helping people achieve clarity on their core values. “Healing patients is a core identify for physicians; we want to take care of people. But other things can get in the way of that, and hospitalist groups can work at minimizing those barriers. We also need to learn, as hospitalists, that we work in a group. You need to be creative in how you do your team building, especially now, when you can no longer get together for dinner. Whatever it is, how do we bring our team back together? The biggest source of support for many hospitalists, beyond their family, is the group.”
Dr. Case said there is a longer-term need to study the root causes of burnout in hospitalists and to identify the issues that cause job stress. “What is modifiable? How can we tackle it? I see that as big part of my job every day. Being a physician is hard enough as it is. Let’s work to resolve those issues that add needlessly to the stress.”
“I think the pandemic brought a magnifying glass to how important a concern staff stress is,” Ms. Panek said. Resilience is important.
“We were working in our group on creating a culture that values trust and transparency, and then the COVID crisis hit,” she said. “But you can still keep working on those things. We would not have been as good or as positive as we were in managing this crisis without that preexisting culture to draw upon. We always said it was important. Now we know that’s true.”
Reference
1. Jones Y et al. Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians. J Hosp Med. 2020 August;15(8):507-9.