User login
The SHM Fellow designation: Class of 2021
Spotlight on Tanisha Hamilton, MD, FHM
As we navigate a time unlike any other, it is clear that the value hospitalists provide is growing stronger as the hospital medicine field expands. Many Society of Hospital Medicine members look to its Fellows program as a worthwhile opportunity to distinguish themselves as leaders in the field and accelerate their careers in the specialty.
An active member of SHM since 2012 and member of its 2020 class of Fellows, Tanisha Hamilton, MD, FHM, is one of these ambitious individuals.
Dr. Hamilton is based at Baylor University Medical Center in Dallas, an affiliate of Baylor Scott & White Health. Known for personalized health and wellness care, Dr. Hamilton has more than 14 years of experience in the medical field.
Her love for the hospital medicine specialty is rooted in its diversity and complexity of patient cases – something that she knew would innately complement her personality. She says that an invaluable aspect of working in the field is the ability to interact and connect with people from all walks of life.
“My patients keep me motivated in this space. Learning from my patients and having the responsibility of serving as their advocate is incredibly rewarding,” Dr. Hamilton said. “I hope my patients feel like I’ve helped to make a difference in their lives, if only for just a moment.”
When reflecting on why she joined SHM 8 years ago, Dr. Hamilton said she was encouraged to do so because of its like-minded membership community and professional development opportunities, including the Fellows program.
“I applied to SHM’s Fellows program because I’m committed to the specialty. Hospital medicine is an ever-changing field loaded with opportunities to enhance personal and professional career growth,” said Dr. Hamilton. “To me, SHM’s Fellow in Hospital Medicine [FHM] designation demonstrates the ability to make a contribution to the field and to be an instrument for change.”
She credits receiving her designation as a distinction that has opened doors to other career-enhancing opportunities and networking resources, including an expansive global community, program development at her institution, and positions within SHM. Since earning her FHM designation, Dr. Hamilton has become an engaged member of the annual meeting committee and the North Central Texas Chapter.
“Since we are taking our annual conference virtual for SHM Converge in 2021, I’m excited to see how we can transform a meeting of more than 5,000 attendees into a full digital experience with interactive workshops, exhibits, research competitions, and more,” Dr. Hamilton said. “It’s certainly going to be a challenge, but I know that our meetings department and annual conference committee will make it a success!”
As Dr. Hamilton looks forward in her hospital medicine career, she is committed to making a positive impact on the field and for her patients.
In the future, Dr. Hamilton hopes to share curriculum she recently developed and sponsored around diversity, equity, and inclusion with her team at Baylor University Medical Center.
“Following the tragic deaths of numerous individuals, including Breonna Taylor, Ahmaud Abery, and George Floyd, and other people of color who have died because of COVID-19, I have felt compelled to educate my colleagues on how to curtail systemic racism, sexism, religious discrimination, and xenophobia in health care,” Dr. Hamilton said. “This curriculum includes courses on health disparities and cultural competencies, launching a lecture series, and other educational components.”
While 2020 has been a trying year, Dr. Hamilton remains hopeful for a prosperous future.
“When I think of the future of hospital medicine, I am hopeful that hospitalists will have a more prominent role in changing the direction of our health care system,” she said. “The pandemic has made the world realize the importance of hospital medicine. We, as hospitalists, are a critical part of its infrastructure and its success.”
If you would like to join Dr. Hamilton and other like-minded hospital medicine leaders in accelerating your career, SHM is currently recruiting for the Fellows and Senior Fellows class of 2021. Applications are open until Nov. 20, 2020. These designations are available across a variety of membership categories, including physicians, nurse practitioners, physician assistants, and qualified practice administrators. Dedicated to promoting excellence, innovation, and quality improvement in patient care, Fellows designations provide members with a distinguishing credential as established pioneers in the industry.
For more information and to review your eligibility, visit hospitalmedicine.org/fellows.
Ms. Cowan is a communications specialist at the Society of Hospital Medicine.
Spotlight on Tanisha Hamilton, MD, FHM
Spotlight on Tanisha Hamilton, MD, FHM
As we navigate a time unlike any other, it is clear that the value hospitalists provide is growing stronger as the hospital medicine field expands. Many Society of Hospital Medicine members look to its Fellows program as a worthwhile opportunity to distinguish themselves as leaders in the field and accelerate their careers in the specialty.
An active member of SHM since 2012 and member of its 2020 class of Fellows, Tanisha Hamilton, MD, FHM, is one of these ambitious individuals.
Dr. Hamilton is based at Baylor University Medical Center in Dallas, an affiliate of Baylor Scott & White Health. Known for personalized health and wellness care, Dr. Hamilton has more than 14 years of experience in the medical field.
Her love for the hospital medicine specialty is rooted in its diversity and complexity of patient cases – something that she knew would innately complement her personality. She says that an invaluable aspect of working in the field is the ability to interact and connect with people from all walks of life.
“My patients keep me motivated in this space. Learning from my patients and having the responsibility of serving as their advocate is incredibly rewarding,” Dr. Hamilton said. “I hope my patients feel like I’ve helped to make a difference in their lives, if only for just a moment.”
When reflecting on why she joined SHM 8 years ago, Dr. Hamilton said she was encouraged to do so because of its like-minded membership community and professional development opportunities, including the Fellows program.
“I applied to SHM’s Fellows program because I’m committed to the specialty. Hospital medicine is an ever-changing field loaded with opportunities to enhance personal and professional career growth,” said Dr. Hamilton. “To me, SHM’s Fellow in Hospital Medicine [FHM] designation demonstrates the ability to make a contribution to the field and to be an instrument for change.”
She credits receiving her designation as a distinction that has opened doors to other career-enhancing opportunities and networking resources, including an expansive global community, program development at her institution, and positions within SHM. Since earning her FHM designation, Dr. Hamilton has become an engaged member of the annual meeting committee and the North Central Texas Chapter.
“Since we are taking our annual conference virtual for SHM Converge in 2021, I’m excited to see how we can transform a meeting of more than 5,000 attendees into a full digital experience with interactive workshops, exhibits, research competitions, and more,” Dr. Hamilton said. “It’s certainly going to be a challenge, but I know that our meetings department and annual conference committee will make it a success!”
As Dr. Hamilton looks forward in her hospital medicine career, she is committed to making a positive impact on the field and for her patients.
In the future, Dr. Hamilton hopes to share curriculum she recently developed and sponsored around diversity, equity, and inclusion with her team at Baylor University Medical Center.
“Following the tragic deaths of numerous individuals, including Breonna Taylor, Ahmaud Abery, and George Floyd, and other people of color who have died because of COVID-19, I have felt compelled to educate my colleagues on how to curtail systemic racism, sexism, religious discrimination, and xenophobia in health care,” Dr. Hamilton said. “This curriculum includes courses on health disparities and cultural competencies, launching a lecture series, and other educational components.”
While 2020 has been a trying year, Dr. Hamilton remains hopeful for a prosperous future.
“When I think of the future of hospital medicine, I am hopeful that hospitalists will have a more prominent role in changing the direction of our health care system,” she said. “The pandemic has made the world realize the importance of hospital medicine. We, as hospitalists, are a critical part of its infrastructure and its success.”
If you would like to join Dr. Hamilton and other like-minded hospital medicine leaders in accelerating your career, SHM is currently recruiting for the Fellows and Senior Fellows class of 2021. Applications are open until Nov. 20, 2020. These designations are available across a variety of membership categories, including physicians, nurse practitioners, physician assistants, and qualified practice administrators. Dedicated to promoting excellence, innovation, and quality improvement in patient care, Fellows designations provide members with a distinguishing credential as established pioneers in the industry.
For more information and to review your eligibility, visit hospitalmedicine.org/fellows.
Ms. Cowan is a communications specialist at the Society of Hospital Medicine.
As we navigate a time unlike any other, it is clear that the value hospitalists provide is growing stronger as the hospital medicine field expands. Many Society of Hospital Medicine members look to its Fellows program as a worthwhile opportunity to distinguish themselves as leaders in the field and accelerate their careers in the specialty.
An active member of SHM since 2012 and member of its 2020 class of Fellows, Tanisha Hamilton, MD, FHM, is one of these ambitious individuals.
Dr. Hamilton is based at Baylor University Medical Center in Dallas, an affiliate of Baylor Scott & White Health. Known for personalized health and wellness care, Dr. Hamilton has more than 14 years of experience in the medical field.
Her love for the hospital medicine specialty is rooted in its diversity and complexity of patient cases – something that she knew would innately complement her personality. She says that an invaluable aspect of working in the field is the ability to interact and connect with people from all walks of life.
“My patients keep me motivated in this space. Learning from my patients and having the responsibility of serving as their advocate is incredibly rewarding,” Dr. Hamilton said. “I hope my patients feel like I’ve helped to make a difference in their lives, if only for just a moment.”
When reflecting on why she joined SHM 8 years ago, Dr. Hamilton said she was encouraged to do so because of its like-minded membership community and professional development opportunities, including the Fellows program.
“I applied to SHM’s Fellows program because I’m committed to the specialty. Hospital medicine is an ever-changing field loaded with opportunities to enhance personal and professional career growth,” said Dr. Hamilton. “To me, SHM’s Fellow in Hospital Medicine [FHM] designation demonstrates the ability to make a contribution to the field and to be an instrument for change.”
She credits receiving her designation as a distinction that has opened doors to other career-enhancing opportunities and networking resources, including an expansive global community, program development at her institution, and positions within SHM. Since earning her FHM designation, Dr. Hamilton has become an engaged member of the annual meeting committee and the North Central Texas Chapter.
“Since we are taking our annual conference virtual for SHM Converge in 2021, I’m excited to see how we can transform a meeting of more than 5,000 attendees into a full digital experience with interactive workshops, exhibits, research competitions, and more,” Dr. Hamilton said. “It’s certainly going to be a challenge, but I know that our meetings department and annual conference committee will make it a success!”
As Dr. Hamilton looks forward in her hospital medicine career, she is committed to making a positive impact on the field and for her patients.
In the future, Dr. Hamilton hopes to share curriculum she recently developed and sponsored around diversity, equity, and inclusion with her team at Baylor University Medical Center.
“Following the tragic deaths of numerous individuals, including Breonna Taylor, Ahmaud Abery, and George Floyd, and other people of color who have died because of COVID-19, I have felt compelled to educate my colleagues on how to curtail systemic racism, sexism, religious discrimination, and xenophobia in health care,” Dr. Hamilton said. “This curriculum includes courses on health disparities and cultural competencies, launching a lecture series, and other educational components.”
While 2020 has been a trying year, Dr. Hamilton remains hopeful for a prosperous future.
“When I think of the future of hospital medicine, I am hopeful that hospitalists will have a more prominent role in changing the direction of our health care system,” she said. “The pandemic has made the world realize the importance of hospital medicine. We, as hospitalists, are a critical part of its infrastructure and its success.”
If you would like to join Dr. Hamilton and other like-minded hospital medicine leaders in accelerating your career, SHM is currently recruiting for the Fellows and Senior Fellows class of 2021. Applications are open until Nov. 20, 2020. These designations are available across a variety of membership categories, including physicians, nurse practitioners, physician assistants, and qualified practice administrators. Dedicated to promoting excellence, innovation, and quality improvement in patient care, Fellows designations provide members with a distinguishing credential as established pioneers in the industry.
For more information and to review your eligibility, visit hospitalmedicine.org/fellows.
Ms. Cowan is a communications specialist at the Society of Hospital Medicine.
Low-dose radiotherapy for lung inflammation in severe COVID-19
The first study to suggest benefit from low-dose radiotherapy for severe COVID-19–induced pneumonia involved only 20 patients, but the results were so promising that two larger randomized trials are now underway.
“RESCUE-119 was a trial based on the hypothesis that low-dose radiation therapy may help eliminate the stormy cytokine release and unchecked edema in hospitalized COVID-19 patients,” said Mohammed Khan, MD, PhD, Winship Cancer Institute of Emory University, Atlanta.
“We found patients had a quicker improvement in their time to clinical recovery with low-dose radiation therapy, compared to controls, and this was significant even in this small cohort of patients,” he said.
Dr. Khan was speaking at a special press briefing held during the virtual American Society for Radiation Oncology Annual Meeting 2020.
A total of 20 patients were involved in the trial. Ten patients were treated with low-dose radiotherapy; 10 others, who served as control patients, were treated with the best supportive care and COVID-directed therapies. The control patients were matched for age and comorbidities. All these patients were hospitalized and were oxygen dependent, Dr. Khan noted. In addition, for all patients, serial x-rays demonstrated consolidation and damage in the lung.
The intervention consisted of whole-lung low-dose radiotherapy delivered at a dose of 1.5 Gy.
The first five patients were assessed at an interim endpoint of 7 days to confirm the safety of the procedure. Subsequently, a total of 10 patients were treated with radiotherapy and were followed to day 28.
The main study endpoints were time to clinical recovery, determined on the basis of the patient’s being taken off oxygen, and improvement, evidenced on either serial x-rays or by inflammatory biomarkers.
The median time to clinical recovery was almost three times faster for the patients who received low-dose radiotherapy, at a median of 3 days; for control patients, the median was 12 days (P = .048).
“We also saw a trend toward getting patients out of hospital sooner,” Dr. Khan added. The mean time to hospital discharge was 12 days for the patients who received low-dose radiotherapy, compared with 20 days for control patients (P = .19).
Only one patient required intubation after receiving low-dose radiotherapy, whereas 4 of 10 control patients required some sort of intubation (P = .12), he noted.
Investigators also saw improvements on serial x-rays in 9 of 10 patients treated with low-dose radiotherapy, compared with only 4 patients in the control group. There was also a significant improvement in delirium among the low-dose radiotherapy group compared with control patients (P < .01). Before receiving low-dose radiotherapy, C-reactive protein levels increased by 22% per day. After receiving the 1.5-Gy radiation treatment, there was a sharp reduction in C-reactive protein levels (P < .01) as well as in lactate dehydrogenase levels (P = .03).
Overall survival, however, did not differ between the two treatment groups; 90% of both groups were alive at day 28.
“By focally dampening cytokine hyperactivation, [low-dose radiotherapy] may improve COVID-19 outcomes through immunomodulation,” Dr. Khan explained.
VENTED and PRE-VENT trials
These results from the small RESCUE-119 trial led to the launch of two larger phase 2 trials, the VENTED and the PRE-VENT trials, noted Arnab Chakravarti, MD, professor and chair of radiation oncology, the Ohio State University Comprehensive Cancer Center, Columbus.
To be enrolled in the VENTED trial, patients must have received mechanical ventilation. They will receive at least one dose of ultra-low-dose bilateral whole-lung radiotherapy, with the option of receiving a second dose. The primary objective is 30-day mortality rate.
“The hypothesis is that low-dose thoracic radiation will decrease inflammation and improve outcomes for these intubated COVID-19 patients,” Dr. Chakravarti explained.
The PRE-VENT trial will explore low-dose thoracic radiotherapy for hospitalized patients with severe respiratory compromise who have not yet been intubated. Two doses of low-dose radiotherapy will be tested and compared. The primary study objective is to determine which of the two doses appears to be the most efficacious, Dr. Chakravarti noted.
“The ultimate question to which we remain agnostic is whether the potential benefits of low-dose radiation therapy outweigh the risks,” he said.
Low-dose radiotherapy is readily available in most countries, unlike the newly developed COVID-19 drugs, which are only available in the developed world, he noted. “This creates a bit more economic equity in terms of COVID-19 treatment.”
In addition, it may offer a therapeutic option that could be useful in the future, “as low-dose radiation therapy does not discriminate against various viruses that may cause another pandemic,” he commented. It could offer “a stopgap measure where we don’t have to shut down society completely, which, as we have all witnessed, can cause tremendous financial and social unrest.”
Reasonable question
Whether or not radiotherapy has value for the short-term management of severe pulmonary inflammation caused by COVID-19 is a reasonable question to evaluate in clinical trials, commented discussant Ramesh Rengan, MD, PhD, professor and chair, department of radiation oncology, University of Washington, Seattle.
He noted that inflammatory cells are highly sensitive to radiation, and low-dose radiotherapy has been used effectively in other inflammatory conditions, such as arthritis. Indeed, before the discovery of antibiotics, low-dose radiation was used with reasonable efficacy to treat pneumonia.
“The pneumonia associated with this viral infection is a bit unique in that what happens is the infection triggers an inflammatory cascade – the so-called cytokine storm – that essentially overwhelms the lungs, thereby leading, unfortunately, to mortality,” Dr. Rengan noted. “So a big focus of our energy is how to stop this inflammatory cascade from occurring.”
Corticosteroids are currently the only therapeutic intervention that has shown any mortality benefit in COVID-19, he pointed out.
The question now being asked is: “Can we suppress inflammation specifically within the lung?” Dr. Rengan continued. The main problem with radiotherapy is that it has different effects on various tissues, both immediately and over the long term.
“The immediate benefit that we will likely see from these studies is the immediate sterilization of inflammatory cells,” he said. However, injury to normal lung tissue from low-dose radiotherapy could lead to inflammation weeks or months later, and this could contribute to the disease burden and increase the risk of dying.
Dr. Rengan also noted that there are some very real practical concerns about offering radiotherapy to COVID-19 patients, including potential COVID-19 transmission to vulnerable cancer patients.
Nevertheless, Dr. Rengan said the results to date are very important and that ongoing trials will provide important new information about the long-term impact of this particular treatment in high-risk patients.
“This is a race to the bottom – we are trying to find the lowest possible dose of radiation therapy that we can deliver to sterilize these inflammatory cells without creating any harm to the surrounding tissue,” he said.
“It also brings radiation oncologists into the fight against this deadly disease,” he added.
Dr. Rengan has received honoraria from Novocur and has served as a consultant to AstraZeneca.
A version of this article originally appeared on Medscape.com.
The first study to suggest benefit from low-dose radiotherapy for severe COVID-19–induced pneumonia involved only 20 patients, but the results were so promising that two larger randomized trials are now underway.
“RESCUE-119 was a trial based on the hypothesis that low-dose radiation therapy may help eliminate the stormy cytokine release and unchecked edema in hospitalized COVID-19 patients,” said Mohammed Khan, MD, PhD, Winship Cancer Institute of Emory University, Atlanta.
“We found patients had a quicker improvement in their time to clinical recovery with low-dose radiation therapy, compared to controls, and this was significant even in this small cohort of patients,” he said.
Dr. Khan was speaking at a special press briefing held during the virtual American Society for Radiation Oncology Annual Meeting 2020.
A total of 20 patients were involved in the trial. Ten patients were treated with low-dose radiotherapy; 10 others, who served as control patients, were treated with the best supportive care and COVID-directed therapies. The control patients were matched for age and comorbidities. All these patients were hospitalized and were oxygen dependent, Dr. Khan noted. In addition, for all patients, serial x-rays demonstrated consolidation and damage in the lung.
The intervention consisted of whole-lung low-dose radiotherapy delivered at a dose of 1.5 Gy.
The first five patients were assessed at an interim endpoint of 7 days to confirm the safety of the procedure. Subsequently, a total of 10 patients were treated with radiotherapy and were followed to day 28.
The main study endpoints were time to clinical recovery, determined on the basis of the patient’s being taken off oxygen, and improvement, evidenced on either serial x-rays or by inflammatory biomarkers.
The median time to clinical recovery was almost three times faster for the patients who received low-dose radiotherapy, at a median of 3 days; for control patients, the median was 12 days (P = .048).
“We also saw a trend toward getting patients out of hospital sooner,” Dr. Khan added. The mean time to hospital discharge was 12 days for the patients who received low-dose radiotherapy, compared with 20 days for control patients (P = .19).
Only one patient required intubation after receiving low-dose radiotherapy, whereas 4 of 10 control patients required some sort of intubation (P = .12), he noted.
Investigators also saw improvements on serial x-rays in 9 of 10 patients treated with low-dose radiotherapy, compared with only 4 patients in the control group. There was also a significant improvement in delirium among the low-dose radiotherapy group compared with control patients (P < .01). Before receiving low-dose radiotherapy, C-reactive protein levels increased by 22% per day. After receiving the 1.5-Gy radiation treatment, there was a sharp reduction in C-reactive protein levels (P < .01) as well as in lactate dehydrogenase levels (P = .03).
Overall survival, however, did not differ between the two treatment groups; 90% of both groups were alive at day 28.
“By focally dampening cytokine hyperactivation, [low-dose radiotherapy] may improve COVID-19 outcomes through immunomodulation,” Dr. Khan explained.
VENTED and PRE-VENT trials
These results from the small RESCUE-119 trial led to the launch of two larger phase 2 trials, the VENTED and the PRE-VENT trials, noted Arnab Chakravarti, MD, professor and chair of radiation oncology, the Ohio State University Comprehensive Cancer Center, Columbus.
To be enrolled in the VENTED trial, patients must have received mechanical ventilation. They will receive at least one dose of ultra-low-dose bilateral whole-lung radiotherapy, with the option of receiving a second dose. The primary objective is 30-day mortality rate.
“The hypothesis is that low-dose thoracic radiation will decrease inflammation and improve outcomes for these intubated COVID-19 patients,” Dr. Chakravarti explained.
The PRE-VENT trial will explore low-dose thoracic radiotherapy for hospitalized patients with severe respiratory compromise who have not yet been intubated. Two doses of low-dose radiotherapy will be tested and compared. The primary study objective is to determine which of the two doses appears to be the most efficacious, Dr. Chakravarti noted.
“The ultimate question to which we remain agnostic is whether the potential benefits of low-dose radiation therapy outweigh the risks,” he said.
Low-dose radiotherapy is readily available in most countries, unlike the newly developed COVID-19 drugs, which are only available in the developed world, he noted. “This creates a bit more economic equity in terms of COVID-19 treatment.”
In addition, it may offer a therapeutic option that could be useful in the future, “as low-dose radiation therapy does not discriminate against various viruses that may cause another pandemic,” he commented. It could offer “a stopgap measure where we don’t have to shut down society completely, which, as we have all witnessed, can cause tremendous financial and social unrest.”
Reasonable question
Whether or not radiotherapy has value for the short-term management of severe pulmonary inflammation caused by COVID-19 is a reasonable question to evaluate in clinical trials, commented discussant Ramesh Rengan, MD, PhD, professor and chair, department of radiation oncology, University of Washington, Seattle.
He noted that inflammatory cells are highly sensitive to radiation, and low-dose radiotherapy has been used effectively in other inflammatory conditions, such as arthritis. Indeed, before the discovery of antibiotics, low-dose radiation was used with reasonable efficacy to treat pneumonia.
“The pneumonia associated with this viral infection is a bit unique in that what happens is the infection triggers an inflammatory cascade – the so-called cytokine storm – that essentially overwhelms the lungs, thereby leading, unfortunately, to mortality,” Dr. Rengan noted. “So a big focus of our energy is how to stop this inflammatory cascade from occurring.”
Corticosteroids are currently the only therapeutic intervention that has shown any mortality benefit in COVID-19, he pointed out.
The question now being asked is: “Can we suppress inflammation specifically within the lung?” Dr. Rengan continued. The main problem with radiotherapy is that it has different effects on various tissues, both immediately and over the long term.
“The immediate benefit that we will likely see from these studies is the immediate sterilization of inflammatory cells,” he said. However, injury to normal lung tissue from low-dose radiotherapy could lead to inflammation weeks or months later, and this could contribute to the disease burden and increase the risk of dying.
Dr. Rengan also noted that there are some very real practical concerns about offering radiotherapy to COVID-19 patients, including potential COVID-19 transmission to vulnerable cancer patients.
Nevertheless, Dr. Rengan said the results to date are very important and that ongoing trials will provide important new information about the long-term impact of this particular treatment in high-risk patients.
“This is a race to the bottom – we are trying to find the lowest possible dose of radiation therapy that we can deliver to sterilize these inflammatory cells without creating any harm to the surrounding tissue,” he said.
“It also brings radiation oncologists into the fight against this deadly disease,” he added.
Dr. Rengan has received honoraria from Novocur and has served as a consultant to AstraZeneca.
A version of this article originally appeared on Medscape.com.
The first study to suggest benefit from low-dose radiotherapy for severe COVID-19–induced pneumonia involved only 20 patients, but the results were so promising that two larger randomized trials are now underway.
“RESCUE-119 was a trial based on the hypothesis that low-dose radiation therapy may help eliminate the stormy cytokine release and unchecked edema in hospitalized COVID-19 patients,” said Mohammed Khan, MD, PhD, Winship Cancer Institute of Emory University, Atlanta.
“We found patients had a quicker improvement in their time to clinical recovery with low-dose radiation therapy, compared to controls, and this was significant even in this small cohort of patients,” he said.
Dr. Khan was speaking at a special press briefing held during the virtual American Society for Radiation Oncology Annual Meeting 2020.
A total of 20 patients were involved in the trial. Ten patients were treated with low-dose radiotherapy; 10 others, who served as control patients, were treated with the best supportive care and COVID-directed therapies. The control patients were matched for age and comorbidities. All these patients were hospitalized and were oxygen dependent, Dr. Khan noted. In addition, for all patients, serial x-rays demonstrated consolidation and damage in the lung.
The intervention consisted of whole-lung low-dose radiotherapy delivered at a dose of 1.5 Gy.
The first five patients were assessed at an interim endpoint of 7 days to confirm the safety of the procedure. Subsequently, a total of 10 patients were treated with radiotherapy and were followed to day 28.
The main study endpoints were time to clinical recovery, determined on the basis of the patient’s being taken off oxygen, and improvement, evidenced on either serial x-rays or by inflammatory biomarkers.
The median time to clinical recovery was almost three times faster for the patients who received low-dose radiotherapy, at a median of 3 days; for control patients, the median was 12 days (P = .048).
“We also saw a trend toward getting patients out of hospital sooner,” Dr. Khan added. The mean time to hospital discharge was 12 days for the patients who received low-dose radiotherapy, compared with 20 days for control patients (P = .19).
Only one patient required intubation after receiving low-dose radiotherapy, whereas 4 of 10 control patients required some sort of intubation (P = .12), he noted.
Investigators also saw improvements on serial x-rays in 9 of 10 patients treated with low-dose radiotherapy, compared with only 4 patients in the control group. There was also a significant improvement in delirium among the low-dose radiotherapy group compared with control patients (P < .01). Before receiving low-dose radiotherapy, C-reactive protein levels increased by 22% per day. After receiving the 1.5-Gy radiation treatment, there was a sharp reduction in C-reactive protein levels (P < .01) as well as in lactate dehydrogenase levels (P = .03).
Overall survival, however, did not differ between the two treatment groups; 90% of both groups were alive at day 28.
“By focally dampening cytokine hyperactivation, [low-dose radiotherapy] may improve COVID-19 outcomes through immunomodulation,” Dr. Khan explained.
VENTED and PRE-VENT trials
These results from the small RESCUE-119 trial led to the launch of two larger phase 2 trials, the VENTED and the PRE-VENT trials, noted Arnab Chakravarti, MD, professor and chair of radiation oncology, the Ohio State University Comprehensive Cancer Center, Columbus.
To be enrolled in the VENTED trial, patients must have received mechanical ventilation. They will receive at least one dose of ultra-low-dose bilateral whole-lung radiotherapy, with the option of receiving a second dose. The primary objective is 30-day mortality rate.
“The hypothesis is that low-dose thoracic radiation will decrease inflammation and improve outcomes for these intubated COVID-19 patients,” Dr. Chakravarti explained.
The PRE-VENT trial will explore low-dose thoracic radiotherapy for hospitalized patients with severe respiratory compromise who have not yet been intubated. Two doses of low-dose radiotherapy will be tested and compared. The primary study objective is to determine which of the two doses appears to be the most efficacious, Dr. Chakravarti noted.
“The ultimate question to which we remain agnostic is whether the potential benefits of low-dose radiation therapy outweigh the risks,” he said.
Low-dose radiotherapy is readily available in most countries, unlike the newly developed COVID-19 drugs, which are only available in the developed world, he noted. “This creates a bit more economic equity in terms of COVID-19 treatment.”
In addition, it may offer a therapeutic option that could be useful in the future, “as low-dose radiation therapy does not discriminate against various viruses that may cause another pandemic,” he commented. It could offer “a stopgap measure where we don’t have to shut down society completely, which, as we have all witnessed, can cause tremendous financial and social unrest.”
Reasonable question
Whether or not radiotherapy has value for the short-term management of severe pulmonary inflammation caused by COVID-19 is a reasonable question to evaluate in clinical trials, commented discussant Ramesh Rengan, MD, PhD, professor and chair, department of radiation oncology, University of Washington, Seattle.
He noted that inflammatory cells are highly sensitive to radiation, and low-dose radiotherapy has been used effectively in other inflammatory conditions, such as arthritis. Indeed, before the discovery of antibiotics, low-dose radiation was used with reasonable efficacy to treat pneumonia.
“The pneumonia associated with this viral infection is a bit unique in that what happens is the infection triggers an inflammatory cascade – the so-called cytokine storm – that essentially overwhelms the lungs, thereby leading, unfortunately, to mortality,” Dr. Rengan noted. “So a big focus of our energy is how to stop this inflammatory cascade from occurring.”
Corticosteroids are currently the only therapeutic intervention that has shown any mortality benefit in COVID-19, he pointed out.
The question now being asked is: “Can we suppress inflammation specifically within the lung?” Dr. Rengan continued. The main problem with radiotherapy is that it has different effects on various tissues, both immediately and over the long term.
“The immediate benefit that we will likely see from these studies is the immediate sterilization of inflammatory cells,” he said. However, injury to normal lung tissue from low-dose radiotherapy could lead to inflammation weeks or months later, and this could contribute to the disease burden and increase the risk of dying.
Dr. Rengan also noted that there are some very real practical concerns about offering radiotherapy to COVID-19 patients, including potential COVID-19 transmission to vulnerable cancer patients.
Nevertheless, Dr. Rengan said the results to date are very important and that ongoing trials will provide important new information about the long-term impact of this particular treatment in high-risk patients.
“This is a race to the bottom – we are trying to find the lowest possible dose of radiation therapy that we can deliver to sterilize these inflammatory cells without creating any harm to the surrounding tissue,” he said.
“It also brings radiation oncologists into the fight against this deadly disease,” he added.
Dr. Rengan has received honoraria from Novocur and has served as a consultant to AstraZeneca.
A version of this article originally appeared on Medscape.com.
NfL blood biomarker captures suboptimal treatment response in MS
a new study has shown.
The study found that current serum NfL levels predicted relapses, disability worsening, and MRI activity in the following year independent of standard metrics for treatment monitoring, such as relapse rate, disability worsening, and MRI findings. The biomarker also detected subclinical disease activity in patients with no evidence of disease activity (NEDA3), as measured by absence of previous relapses, worsening score on the Expanded Disability Status Scale (EDSS), or brain lesion formation on MRI.
“Our data in this well-characterized large real-world cohort supports the value of serum NfL levels for treatment monitoring in MS clinical practice,” lead author Özgür Yaldizli, MD, concluded.
Dr. Yaldizli, who is a consultant neurologist at University Hospital Basel (Switzerland), presented the findings at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“This is the first study to compare NfL simultaneously with other markers of disease progression, such as MRI lesions and relapse rate in treated patients. We show that NfL gives a unique signal that is not captured by other markers,” Dr. Yaldizli said.
“This is likely the largest study of NfL in MS to date, with more than 7,000 samples from well-characterized MS patients followed longitudinally for more than 5 years of sampling and including high quality data on MRI and clinical examinations. It is the first time all these factors have been combined so that we can see how NfL compares with other markers of disease progression in predicting clinical events and monitoring treatment efficacy,” said senior author Jens Kuhle, MD, PhD, also from University Hospital Basel.
Large normative database for reference
The researchers also reported a large normative database of NfL values with data from more than 8,000 healthy controls. “This is the largest normative database to date, that gives us reliable reference values for NfL across a range of ages and comorbidities,” Dr. Kuhle noted.
In his presentation, Dr. Yaldizli explained that NfL is a neuronal cytoskeletal protein released into the cerebrospinal fluid and blood following neuroaxonal injury. Although numerous studies have shown that serum NfL is associated with clinical and MRI disease activity and treatment response, it is not clear whether serum NfL under established disease-modifying therapy (DMT) can identify patients with suboptimal treatment response, compared with standard clinical and MRI activity measures.
This study addressed that question in the large real-world Swiss MS cohort.
The study involved 1,366 patients (88.8% with relapsing remitting MS [RRMS], 5.4% with secondary progressive MS, and 5.8% with primary progressive MS) receiving DMT for at least 3 months from seven MS centers. The median disease duration was 7.2 years. Serum NfL was measured every 6 or 12 months with NF-Light assay on the latest-generation HDX platform (blinded for clinical and MRI data). The median follow-up was 4.9 years. There was an average of five samples per patient, with a total of 7462 samples.
Results showed that NfL levels were higher in older patients (14.5% per 10 years), those with secondary progressive MS (12.4% vs. RRMS), those with primary progressive MS (14.4% vs. RRMS), and in those who had a relapse in the last 4 months (53.4%).
NfL levels were 13.4% lower in patients receiving oral DMT (vs. untreated patients) and 17.7% in patients receiving monoclonal antibodies (vs. untreated patients).
In the large cohort of healthy controls, NfL levels also increased with age, but levels in patients with MS were higher than in controls across the whole age spectrum.
To obtain a measure of deviation from normal, the authors converted NfL levels to z score, which express how much (in terms of number of standard deviations) a measurement differs from mean values found in healthy controls of the same age. Effects were more pronounced with use of z score derived from the normative database than with use of absolute NfL levels even after adjustment for age.
In the univariate analysis, serum NfL z score predicted relapse or EDSS worsening in the following year: The higher the z score, the higher the risk for relapse or EDSS worsening. Patients with an NfL z score greater than 1 had a 41% higher risk for relapse or EDSS worsening in the following year, compared with those whose z score was less than 1 (odds ratio, 1.41).
Patients with an NfL z score exceeding 1.5 had an 80% higher risk for relapse or EDSS worsening in the following year than did those whose score was below 1.5 (OR, 1.8).
Patients with an NfL z score greater than 2 had a 2.3 times higher risk for relapse or EDSS worsening in the following year versus those with a score below 2. (P < 0.001 for all comparisons.)
A screen for nervous system conditions?
Dr, Kuhle reported that NfL is being used on an individual basis in clinical practice at present – at certain MS centers. “One of the problems is not having reliable reference values, so this database of normative values will be very helpful in developing those,” he said. “We see an increase in NfL with age in healthy controls. In order to know what pathological levels are, we need to know what normal levels are in controls throughout the spectrum of ages and other comorbidities, which also play a role. If we normalize these, then we can work out the MS signal in a more efficient way.”
Dr. Kuhle believes that, in the future, NfL may be used to screen for nervous system disease. “NfL is a measure of neuronal health independent of MS. If we have increased levels, we should be worried.”
There is a “high level of energy in this field,” he added. “In future, it could be like having a cholesterol test at present – picking up that something is not right and indicating the need for more tests.”
Dr. Yaldizli suggested that NfL monitoring could also help to individualize and optimize use of MS treatments. “There is a huge unmet need in MS. While we have a plethora of treatment options, we are struggling to individualize and monitor treatments. If NfL levels increase, this is likely a strong indication to change treatment even if there are no other overt symptoms.”
Commenting on the current study, ACTRIMS president, Jeffrey Cohen, MD, Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, called it “an important study.”
“NfL clearly can detect disease activity and distinguish efficacy of DMT in groups of patients,” Dr. Cohen said.
“This study shows that NfL can be used to monitor DMT efficacy in individual patients and can detect suboptimal treatment response in patients with NEDA (i.e., who appear stable by the measures we typically employ in practice),” he added.
Dr. Yaldizli sits on advisory boards for Sanofi Genzyme, Novartis, Biogen, and Novartis. Dr. Kuhle reported no relevant disclosures.
This article first appeared on Medscape.com.
a new study has shown.
The study found that current serum NfL levels predicted relapses, disability worsening, and MRI activity in the following year independent of standard metrics for treatment monitoring, such as relapse rate, disability worsening, and MRI findings. The biomarker also detected subclinical disease activity in patients with no evidence of disease activity (NEDA3), as measured by absence of previous relapses, worsening score on the Expanded Disability Status Scale (EDSS), or brain lesion formation on MRI.
“Our data in this well-characterized large real-world cohort supports the value of serum NfL levels for treatment monitoring in MS clinical practice,” lead author Özgür Yaldizli, MD, concluded.
Dr. Yaldizli, who is a consultant neurologist at University Hospital Basel (Switzerland), presented the findings at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“This is the first study to compare NfL simultaneously with other markers of disease progression, such as MRI lesions and relapse rate in treated patients. We show that NfL gives a unique signal that is not captured by other markers,” Dr. Yaldizli said.
“This is likely the largest study of NfL in MS to date, with more than 7,000 samples from well-characterized MS patients followed longitudinally for more than 5 years of sampling and including high quality data on MRI and clinical examinations. It is the first time all these factors have been combined so that we can see how NfL compares with other markers of disease progression in predicting clinical events and monitoring treatment efficacy,” said senior author Jens Kuhle, MD, PhD, also from University Hospital Basel.
Large normative database for reference
The researchers also reported a large normative database of NfL values with data from more than 8,000 healthy controls. “This is the largest normative database to date, that gives us reliable reference values for NfL across a range of ages and comorbidities,” Dr. Kuhle noted.
In his presentation, Dr. Yaldizli explained that NfL is a neuronal cytoskeletal protein released into the cerebrospinal fluid and blood following neuroaxonal injury. Although numerous studies have shown that serum NfL is associated with clinical and MRI disease activity and treatment response, it is not clear whether serum NfL under established disease-modifying therapy (DMT) can identify patients with suboptimal treatment response, compared with standard clinical and MRI activity measures.
This study addressed that question in the large real-world Swiss MS cohort.
The study involved 1,366 patients (88.8% with relapsing remitting MS [RRMS], 5.4% with secondary progressive MS, and 5.8% with primary progressive MS) receiving DMT for at least 3 months from seven MS centers. The median disease duration was 7.2 years. Serum NfL was measured every 6 or 12 months with NF-Light assay on the latest-generation HDX platform (blinded for clinical and MRI data). The median follow-up was 4.9 years. There was an average of five samples per patient, with a total of 7462 samples.
Results showed that NfL levels were higher in older patients (14.5% per 10 years), those with secondary progressive MS (12.4% vs. RRMS), those with primary progressive MS (14.4% vs. RRMS), and in those who had a relapse in the last 4 months (53.4%).
NfL levels were 13.4% lower in patients receiving oral DMT (vs. untreated patients) and 17.7% in patients receiving monoclonal antibodies (vs. untreated patients).
In the large cohort of healthy controls, NfL levels also increased with age, but levels in patients with MS were higher than in controls across the whole age spectrum.
To obtain a measure of deviation from normal, the authors converted NfL levels to z score, which express how much (in terms of number of standard deviations) a measurement differs from mean values found in healthy controls of the same age. Effects were more pronounced with use of z score derived from the normative database than with use of absolute NfL levels even after adjustment for age.
In the univariate analysis, serum NfL z score predicted relapse or EDSS worsening in the following year: The higher the z score, the higher the risk for relapse or EDSS worsening. Patients with an NfL z score greater than 1 had a 41% higher risk for relapse or EDSS worsening in the following year, compared with those whose z score was less than 1 (odds ratio, 1.41).
Patients with an NfL z score exceeding 1.5 had an 80% higher risk for relapse or EDSS worsening in the following year than did those whose score was below 1.5 (OR, 1.8).
Patients with an NfL z score greater than 2 had a 2.3 times higher risk for relapse or EDSS worsening in the following year versus those with a score below 2. (P < 0.001 for all comparisons.)
A screen for nervous system conditions?
Dr, Kuhle reported that NfL is being used on an individual basis in clinical practice at present – at certain MS centers. “One of the problems is not having reliable reference values, so this database of normative values will be very helpful in developing those,” he said. “We see an increase in NfL with age in healthy controls. In order to know what pathological levels are, we need to know what normal levels are in controls throughout the spectrum of ages and other comorbidities, which also play a role. If we normalize these, then we can work out the MS signal in a more efficient way.”
Dr. Kuhle believes that, in the future, NfL may be used to screen for nervous system disease. “NfL is a measure of neuronal health independent of MS. If we have increased levels, we should be worried.”
There is a “high level of energy in this field,” he added. “In future, it could be like having a cholesterol test at present – picking up that something is not right and indicating the need for more tests.”
Dr. Yaldizli suggested that NfL monitoring could also help to individualize and optimize use of MS treatments. “There is a huge unmet need in MS. While we have a plethora of treatment options, we are struggling to individualize and monitor treatments. If NfL levels increase, this is likely a strong indication to change treatment even if there are no other overt symptoms.”
Commenting on the current study, ACTRIMS president, Jeffrey Cohen, MD, Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, called it “an important study.”
“NfL clearly can detect disease activity and distinguish efficacy of DMT in groups of patients,” Dr. Cohen said.
“This study shows that NfL can be used to monitor DMT efficacy in individual patients and can detect suboptimal treatment response in patients with NEDA (i.e., who appear stable by the measures we typically employ in practice),” he added.
Dr. Yaldizli sits on advisory boards for Sanofi Genzyme, Novartis, Biogen, and Novartis. Dr. Kuhle reported no relevant disclosures.
This article first appeared on Medscape.com.
a new study has shown.
The study found that current serum NfL levels predicted relapses, disability worsening, and MRI activity in the following year independent of standard metrics for treatment monitoring, such as relapse rate, disability worsening, and MRI findings. The biomarker also detected subclinical disease activity in patients with no evidence of disease activity (NEDA3), as measured by absence of previous relapses, worsening score on the Expanded Disability Status Scale (EDSS), or brain lesion formation on MRI.
“Our data in this well-characterized large real-world cohort supports the value of serum NfL levels for treatment monitoring in MS clinical practice,” lead author Özgür Yaldizli, MD, concluded.
Dr. Yaldizli, who is a consultant neurologist at University Hospital Basel (Switzerland), presented the findings at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“This is the first study to compare NfL simultaneously with other markers of disease progression, such as MRI lesions and relapse rate in treated patients. We show that NfL gives a unique signal that is not captured by other markers,” Dr. Yaldizli said.
“This is likely the largest study of NfL in MS to date, with more than 7,000 samples from well-characterized MS patients followed longitudinally for more than 5 years of sampling and including high quality data on MRI and clinical examinations. It is the first time all these factors have been combined so that we can see how NfL compares with other markers of disease progression in predicting clinical events and monitoring treatment efficacy,” said senior author Jens Kuhle, MD, PhD, also from University Hospital Basel.
Large normative database for reference
The researchers also reported a large normative database of NfL values with data from more than 8,000 healthy controls. “This is the largest normative database to date, that gives us reliable reference values for NfL across a range of ages and comorbidities,” Dr. Kuhle noted.
In his presentation, Dr. Yaldizli explained that NfL is a neuronal cytoskeletal protein released into the cerebrospinal fluid and blood following neuroaxonal injury. Although numerous studies have shown that serum NfL is associated with clinical and MRI disease activity and treatment response, it is not clear whether serum NfL under established disease-modifying therapy (DMT) can identify patients with suboptimal treatment response, compared with standard clinical and MRI activity measures.
This study addressed that question in the large real-world Swiss MS cohort.
The study involved 1,366 patients (88.8% with relapsing remitting MS [RRMS], 5.4% with secondary progressive MS, and 5.8% with primary progressive MS) receiving DMT for at least 3 months from seven MS centers. The median disease duration was 7.2 years. Serum NfL was measured every 6 or 12 months with NF-Light assay on the latest-generation HDX platform (blinded for clinical and MRI data). The median follow-up was 4.9 years. There was an average of five samples per patient, with a total of 7462 samples.
Results showed that NfL levels were higher in older patients (14.5% per 10 years), those with secondary progressive MS (12.4% vs. RRMS), those with primary progressive MS (14.4% vs. RRMS), and in those who had a relapse in the last 4 months (53.4%).
NfL levels were 13.4% lower in patients receiving oral DMT (vs. untreated patients) and 17.7% in patients receiving monoclonal antibodies (vs. untreated patients).
In the large cohort of healthy controls, NfL levels also increased with age, but levels in patients with MS were higher than in controls across the whole age spectrum.
To obtain a measure of deviation from normal, the authors converted NfL levels to z score, which express how much (in terms of number of standard deviations) a measurement differs from mean values found in healthy controls of the same age. Effects were more pronounced with use of z score derived from the normative database than with use of absolute NfL levels even after adjustment for age.
In the univariate analysis, serum NfL z score predicted relapse or EDSS worsening in the following year: The higher the z score, the higher the risk for relapse or EDSS worsening. Patients with an NfL z score greater than 1 had a 41% higher risk for relapse or EDSS worsening in the following year, compared with those whose z score was less than 1 (odds ratio, 1.41).
Patients with an NfL z score exceeding 1.5 had an 80% higher risk for relapse or EDSS worsening in the following year than did those whose score was below 1.5 (OR, 1.8).
Patients with an NfL z score greater than 2 had a 2.3 times higher risk for relapse or EDSS worsening in the following year versus those with a score below 2. (P < 0.001 for all comparisons.)
A screen for nervous system conditions?
Dr, Kuhle reported that NfL is being used on an individual basis in clinical practice at present – at certain MS centers. “One of the problems is not having reliable reference values, so this database of normative values will be very helpful in developing those,” he said. “We see an increase in NfL with age in healthy controls. In order to know what pathological levels are, we need to know what normal levels are in controls throughout the spectrum of ages and other comorbidities, which also play a role. If we normalize these, then we can work out the MS signal in a more efficient way.”
Dr. Kuhle believes that, in the future, NfL may be used to screen for nervous system disease. “NfL is a measure of neuronal health independent of MS. If we have increased levels, we should be worried.”
There is a “high level of energy in this field,” he added. “In future, it could be like having a cholesterol test at present – picking up that something is not right and indicating the need for more tests.”
Dr. Yaldizli suggested that NfL monitoring could also help to individualize and optimize use of MS treatments. “There is a huge unmet need in MS. While we have a plethora of treatment options, we are struggling to individualize and monitor treatments. If NfL levels increase, this is likely a strong indication to change treatment even if there are no other overt symptoms.”
Commenting on the current study, ACTRIMS president, Jeffrey Cohen, MD, Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, called it “an important study.”
“NfL clearly can detect disease activity and distinguish efficacy of DMT in groups of patients,” Dr. Cohen said.
“This study shows that NfL can be used to monitor DMT efficacy in individual patients and can detect suboptimal treatment response in patients with NEDA (i.e., who appear stable by the measures we typically employ in practice),” he added.
Dr. Yaldizli sits on advisory boards for Sanofi Genzyme, Novartis, Biogen, and Novartis. Dr. Kuhle reported no relevant disclosures.
This article first appeared on Medscape.com.
FROM MSVIRTUAL 2020
Pediatric Procedural Dermatology
Performing dermatologic procedures in infants, children, and teenagers presents many unique challenges. There may be unique diagnoses, different instruments, differences in skin biology, or different approaches to pain management and anesthesia; the inclusion of a third party (caregivers) in decision processes; or a need to assess maturity level or to optimize outcomes over the patient’s lifetime. The field of pediatric procedural dermatology is broad. This article reviews some of the more common procedures performed by pediatric dermatologists and some of the more common ethical and quality-of-life (QOL) considerations one might face in procedural pediatric dermatology. (The textbook Procedural Pediatric Dermatology1 offers a thorough discussion of this topic.)
Quality of Life
More often than not, procedures are performed in pediatric dermatology to improve QOL rather than to prevent morbidity or mortality. In the case of many self-limited conditions, such as ingrown nails or pyogenic granulomas, it is clear that intervention will improve the patient’s QOL. In the case of warts and molluscum contagiosum, emotional, social, and cultural considerations play a large role in determining whether an intervention will improve QOL. Finally, some conditions, such as genodermatoses, giant congenital melanocytic nevi, and large vascular malformations, may be associated with additional systemic symptoms and may not have good treatment options for cure. In these cases, procedural interventions will result in a mixture of positive and negative QOL outcomes that can occur at the same time.
Bemmels et al2 published a qualitative study that provides a good foundation for understanding the positive and negative effects of procedural interventions on children and teenagers. In their study, children and teenagers who underwent reconstructive surgery for craniofacial differences noted improved self-esteem and reduced stigmatization. However, they also experienced negative outcomes, including an addiction to attaining a perfect surgical face, missing school for treatments, difficulty adjusting to an evolving appearance, anxiety related to not knowing when treatments will end, and experiencing stigma related to undergoing surgery.2 Thus, a comprehensive plan for the management of children who need ongoing procedures should include some level of psychosocial support. Two good references on supporting young patients with visible differences include CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference3 and Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development.4
Ethics
Ethical decisions in pediatric procedural dermatology differ from adult dermatology in 3 major ways: (1) the involvement of a third party (ie, parents or legal guardians), (2) the need to assess the maturity of the patient, and (3) the need to know local laws in the jurisdiction in which care is being provided. Ethical dilemmas occur when the desires of the child, parents/guardians, and dermatologist are not in alignment. In these cases, it is important to be prepared with a moral or ethical framework to guide decision-making when conflicts occur. Two great resources are the best interest standard5 and the publication entitled, “Informed Consent in Decision-making in Pediatric Practice,” from the American Academy of Pediatrics.6
In pediatrics, it often is better to conceptualize medical decision-making as a combination of informed permission and assent of the patient rather than informed consent. Informed permission describes how a parent or surrogate makes decisions for the child or adolescent and is similar to informed consent. A parent’s informed permission may be in conflict with a child’s wishes, but it is assumed that the parent is acting in the best interest of the child. Assent of the patient is the process of obtaining a minor’s agreement to undergo an intervention even though he/she may lack legal authority or decision-making capacity to provide standard informed consent. It is important to respect the child’s right to assent to interventions to the extent that their maturity level permits to develop trust with the dermatologist and medical encounters in general.
These differences emphasize an active process in which the patient, caregiver, and physician are all involved in the health care process and allow for increasing inclusion of the child as is developmentally appropriate. In the end, however, parents have the legal authority to give or withhold permission for a procedure.7 When this conflicts with a child’s dissent, the dermatologist will need to objectively explore the reasons for the conflict and decide if a procedure is not in the child’s best interests. If a mutual understanding cannot be reached between the dermatologist and parents, obtaining a second opinion is a good option.8
Common Diagnoses
The most common diagnoses unique to procedural pediatric dermatology include congenital melanocytic nevi, vascular anomalies, midline lesions, epidermal nevi, and pilomatricomas. Prior to intervening on these lesions, it is important to consider evaluating for associated diseases.
Congenital Melanocytic Nevi
Nevus Outreach has published best practices for the management of congenital melanocytic nevi.9 In newborns with a congenital melanocytic nevus greater than 3 cm in diameter or more than 20 satellite lesions, it is recommended that magnetic resonance imaging (MRI) of the brain and spine with and without gadolinium contrast be obtained before 6 months of age. Within the first 6 months of life, these children also should see ophthalmologists, neurologists, pediatric dermatologists, and plastic surgeons. These early referrals will help to establish a baseline for the patient and plan for possible interventions, if needed. Additionally, before 3 years of age, every child should be referred to psychology, even if he/she is asymptomatic.10
Vascular Anomalies
Prior to intervening on a vascular anomaly, it is important to accurately classify the lesion. Once the lesion is classified, an evaluation and treatment plan can be developed. The International Society for the Study of Vascular Anomalies has published a detailed classification guide that is a useful starting point in the management of vascular anomalies.11 Once a diagnosis is confirmed, further evaluation may include imaging, specialty referrals, genetic testing, biopsy, or blood tests, and a pediatric dermatologist usually helps to coordinate the care of patients with complex vascular anomalies.
Midline Lesions
Certain lesions in the midline may have a higher risk for neural tube dysraphism, and imaging should be performed prior to any procedural intervention.12 Midline cutaneous findings that are highly likely to be associated with dysraphism are lipomas, acrochordons, pseudotails, true tails, aplasia cutis congenita, congenital scars, dermoid cysts, dermoid sinuses, and infantile hemangiomas that are greater than 2.5 cm in diameter. An MRI should be performed for all high-risk lesions. Intermediate-risk lesions are atypical dimples (>5 mm in diameter or >2.5 cm from the anal verge), hemangiomas less than 2.5 cm in diameter, and hypertrichosis. An ultrasound can screen for spinal dysraphism in these cases as long as imaging is performed prior to 6 months of age. If the child is older than 6 months, an MRI should be performed. Low-risk lesions that do not require imaging are simple dimples, hyperpigmentation, hypopigmentation, melanocytic nevi, port-wine stains, and telangiectases.
Epidermal Nevi
Children with epidermal nevi should have a complete physical examination, focusing on the skeletal system, central nervous system, and eyes. There are no specifically recommended imaging studies or referrals; however, several diagnostic clues can aid in the diagnosis of an epidermal nevus syndrome13:
• Schimmelpenning syndrome: extensive nevus sebaceous and bowing or pain in the legs after 2 years of age
• Phacomatosis pigmentokeratotica: nevus sebaceous and nevus spilus
• Nevus comedonicus syndrome: ipsilateral cataract
• Angora hair nevus syndrome: soft white hair within the nevus
• Becker nevus syndrome: breast hypoplasia
• Proteus syndrome: cerebriform plantar changes
• PIK3CA-related overgrowth spectrum: lipomas, macrodactyly, and/or vascular malformations
• Congenital hemidysplasia with ichthyosiform erythroderma and limb defects: inflammatory epidermal nevi, lateralization, ptychotropism, and ipsilateral limb defects
• Conradi-Hünermann-Happle syndrome: scaly red epidermal nevi without hair follicles and asymmetric limb shortening
Pilomatricomas
In addition to the tent sign—an angulated shape can be appreciated by stretching the skin overlying pilomatricomas—diagnosis of pilomatricoma can be confirmed by transillumination with an otoscope. In this case, a dark shadow typically is cast distal to where the otoscope touches the skin.14 In the case of multiple lesions, the patient should be evaluated for signs of myotonic dystrophy, Turner syndrome, and Gardner syndrome.15
Common Procedures
Pulsed Dye Laser
The pulsed dye laser is the most common laser used for red-colored lesions such as port-wine stains, facial telangiectases, and superficial hemangiomas. It also can be used to treat erythematous scars, verrucae, and psoriasis. In large vascular lesions, it typically is employed at 0.45 to 10 milliseconds every 4 to 6 weeks for 10 or more treatments. Port-wine stains preferably are treated within the first few months of life to provide the most fading without the need for general anesthesia.16 On the other hand, systemic therapy with propranolol is preferred over lasers for infantile hemangiomas.17
Long-Pulsed Alexandrite Laser (755 nm)
The alexandrite laser often is used to treat deeper vascular lesions such as venous lakes and hypertrophic port-wine stains. The operator needs to be cautious, as this laser has a higher incidence of scarring at the settings used to treat vascular lesions (typically fluences around 60–85 J/cm2).18 It also may be used for hair reduction in disorders with hypertrichosis or hidradenitis suppurativa.19
Long-Pulsed Nd:YAG Laser
The long-pulsed Nd:YAG laser also can be used to treat deep vascular lesions and remove unwanted hair. Because of its low window of safety in the treatment of vascular lesions, the alexandrite laser usually is preferred. However, it is the preferred laser for treatment of unwanted hair and hidradenitis suppurativa in darker skin types. It often provides a 50% reduction in hair density after 9 treatments.20
Quality-Switched Lasers
Pigment granules in melanosomes and tattoo particles are targeted with quality-switched (QS) lasers. Typically, a device will contain a combination of QS 532-nm potassium-titanyl-phosphate (KTP) lasers, QS 1064-nm Nd:YAG lasers, and QS 755-nm alexandrite lasers in 1 machine. In general, shorter wavelengths are used to treat epidermal lesions such as ephelides, lentigines, and café-au-lait macules. Longer wavelengths are used to treat deeper lesions such as nevus of Ota. A 2017 review suggested that café-au-lait macules with ragged borders (so-called coast of Maine borders) may respond well to QS lasers.21
Ablative Lasers
The 10,600-nm
Fractionated Lasers
Fractionated lasers can be nonablative (several devices are available in the 1410- to 1927-nm range) or ablative (CO2 or erbium:YAG). In pediatrics, they are usually used to treat burn scars, traumatic scars, and mild to moderate acne scarring.22 The most common side effects from fractionated lasers are prolonged erythema or hyperpigmentation. In addition, it typically takes at least 3 treatments to notice improvements.
Excisions
Pediatric procedural dermatologists remove a variety of unique lesions through excision. A few tips are provided for some of the more common lesions that may be excised in children.
Accessory Tragi
Prior to excising an accessory tragus, the surgeon should consider documenting a facial nerve examination, as accessory tragi can be associated with complete or partial facial nerve dysfunction. Additionally, there usually is an underlying cartilage structure present within the tragus. The cartilage stalk also should be addressed during the excision to avoid a continued palpable deformity after excision.
Dermoid Cysts
Dermoid cysts are the most commonly diagnosed benign orbital lesion in children.23 Exophytic periorbital lesions, which extend outside the orbital rim, can be removed through an infrabrow incision. Endophytic periorbital lesions, which are inside the orbital rim, should be removed through a crease incision. Midline lesions may have an intracranial extension and should be imaged through MRI and/or a computed tomography.24 Because dermoid cysts usually are located below the orbicularis oculi muscle, the muscle should be fixed with a suture prior to closing with skin sutures.
Pilomatricomas
Typically, a linear incision is made overlying the lesion, and then the underlying tumor is removed with sharp or blunt dissection. However, if the overlying skin has been stretched thin, a lenticular excision that includes the thinned skin may improve cosmesis.
Congenital Nevi
Large congenital nevi typically are removed through staged excisions. Lower extremity lesions are best removed before 10 months of age or before walking begins to minimize wound tension. However, if the procedure is not performed in infancy, it is best to wait until walking becomes stable.25 In older children, it is advisable to splint the affected lower extremity for 2 weeks to prevent dehiscence. The interval between excisions typically is 4 to 6 weeks for small lesions and 3 months for larger nevi.
Conclusion
Procedural pediatric dermatology is a broad and emerging field. As this article highlights, children are not small versions of adults and have unique biology, diseases, therapies, social situations, and ethical challenges from adults. This article provides a superficial overview of some of the more common issues faced by pediatric dermatologists and providers who perform procedures on infants, children, and teenagers. Readers who are interested in obtaining a more in-depth understanding of procedural pediatric dermatology should look at Procedural Pediatric Dermatology,1 the first textbook to provide expert opinion and evidence-based information on procedural management of pediatric skin conditions.
- Krakowski AC. Procedural Pediatric Dermatology. Phialdelphia, PA: Wolters Kluwer; 2011.
- Bemmels H, Biesecker B, Schmidt J, et al. Psychological and social factors in undergoing reconstructive surgery among individuals with craniofacial conditions: an exploratory study. Cleft Palate Craniofac J. 2013;50:158-167.
- Clarke A, Thompson AR, Jenkinson E, et al. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference. Chichester, West Sussex: Wiley-Blackwell; 2013.
- Ginsburg KR, Ramirez McClain ZB, eds. Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development. 2nd ed. Itasca, IL: American Academy of Pediatrics; 2020.
- Kopelman LM. The best interests standard for incompetent or incapacitated patients of all ages. J Law Med Ethics. 2007;35:187-196.
- Katz AL, Webb SA; Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485.
- Michon K. Emancipation of minors. NOLO website. https://www.nolo.com/legal-encyclopedia/emancipation-of-minors-32237.html. Accessed October 14, 2020.
- Cobb C, Bercovitch L. Ethical dilemmas. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:7-10.
- Nevus Outreach, Inc., releases best practice guidelines [news release]. Bartlesville, OK: Nevus Outreach Inc; July 7, 2018. https://www.nevus.org/matrices/page_file_download.php?id=239. Accessed October 14, 2020.
- 10. Masnari O, Neuhaus K, Aegerter T, et al. Predictors of health-related quality of life and psychological adjustment in children and adolescents with congenital melanocytic nevi: analysis of parent reports. J Pediatr Psychol. 2019;44:714-725.
- ISSVA classification for vascular anomalies. International Society for the Study of Vascular Anomalies website. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Approved April 2014. Revised May 2018. Accessed October 14, 2020.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Berreto-Chang OL, Gorell ES, Yamaguma MA, et al. Diagnosis of pilomatricoma using an otoscope. Pediatr Dermatol. 2010;27:554-557.
- Danielson-Cohen A, Lin SJ, Hughes CA, et al. Head and neck pilomatrixoma in children. Arch Otolaryngol Head Neck Surg. 2001;127:1481-1483.
- Jeon H, Bernstein LJ, Belkin DA, et al. Pulsed dye laser treatment of port-wine stains in infancy without the need for general anesthesia. JAMA Dermatol. 2019;155:435-441.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37:1268-1278.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:76-90.
- Rao K, Sankar TK. Long-pulsed Nd:YAG laser-assisted hair removal in Fitzpatrick skin types IV-VI. Lasers Med Sci. 2011;26:623-626.
- Belkin DA, Neckman JP, Jeon H, et al. Response to laser treatment of café au lait macules based on morphologic features. JAMA Dermatol. 2017;153:1158-1161.
- Kelly K, Lehmer L. Laser surgery. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:92-106.
- Eldesouky MA, Elbakary MA. Orbital dermoid cyst: classification and its impact on surgical management. Semin Ophthalmol. 2018;33:170-174.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Metz BJ. Procedural pediatric dermatology. Dermatol Clin. 2013;31:337-346.
Performing dermatologic procedures in infants, children, and teenagers presents many unique challenges. There may be unique diagnoses, different instruments, differences in skin biology, or different approaches to pain management and anesthesia; the inclusion of a third party (caregivers) in decision processes; or a need to assess maturity level or to optimize outcomes over the patient’s lifetime. The field of pediatric procedural dermatology is broad. This article reviews some of the more common procedures performed by pediatric dermatologists and some of the more common ethical and quality-of-life (QOL) considerations one might face in procedural pediatric dermatology. (The textbook Procedural Pediatric Dermatology1 offers a thorough discussion of this topic.)
Quality of Life
More often than not, procedures are performed in pediatric dermatology to improve QOL rather than to prevent morbidity or mortality. In the case of many self-limited conditions, such as ingrown nails or pyogenic granulomas, it is clear that intervention will improve the patient’s QOL. In the case of warts and molluscum contagiosum, emotional, social, and cultural considerations play a large role in determining whether an intervention will improve QOL. Finally, some conditions, such as genodermatoses, giant congenital melanocytic nevi, and large vascular malformations, may be associated with additional systemic symptoms and may not have good treatment options for cure. In these cases, procedural interventions will result in a mixture of positive and negative QOL outcomes that can occur at the same time.
Bemmels et al2 published a qualitative study that provides a good foundation for understanding the positive and negative effects of procedural interventions on children and teenagers. In their study, children and teenagers who underwent reconstructive surgery for craniofacial differences noted improved self-esteem and reduced stigmatization. However, they also experienced negative outcomes, including an addiction to attaining a perfect surgical face, missing school for treatments, difficulty adjusting to an evolving appearance, anxiety related to not knowing when treatments will end, and experiencing stigma related to undergoing surgery.2 Thus, a comprehensive plan for the management of children who need ongoing procedures should include some level of psychosocial support. Two good references on supporting young patients with visible differences include CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference3 and Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development.4
Ethics
Ethical decisions in pediatric procedural dermatology differ from adult dermatology in 3 major ways: (1) the involvement of a third party (ie, parents or legal guardians), (2) the need to assess the maturity of the patient, and (3) the need to know local laws in the jurisdiction in which care is being provided. Ethical dilemmas occur when the desires of the child, parents/guardians, and dermatologist are not in alignment. In these cases, it is important to be prepared with a moral or ethical framework to guide decision-making when conflicts occur. Two great resources are the best interest standard5 and the publication entitled, “Informed Consent in Decision-making in Pediatric Practice,” from the American Academy of Pediatrics.6
In pediatrics, it often is better to conceptualize medical decision-making as a combination of informed permission and assent of the patient rather than informed consent. Informed permission describes how a parent or surrogate makes decisions for the child or adolescent and is similar to informed consent. A parent’s informed permission may be in conflict with a child’s wishes, but it is assumed that the parent is acting in the best interest of the child. Assent of the patient is the process of obtaining a minor’s agreement to undergo an intervention even though he/she may lack legal authority or decision-making capacity to provide standard informed consent. It is important to respect the child’s right to assent to interventions to the extent that their maturity level permits to develop trust with the dermatologist and medical encounters in general.
These differences emphasize an active process in which the patient, caregiver, and physician are all involved in the health care process and allow for increasing inclusion of the child as is developmentally appropriate. In the end, however, parents have the legal authority to give or withhold permission for a procedure.7 When this conflicts with a child’s dissent, the dermatologist will need to objectively explore the reasons for the conflict and decide if a procedure is not in the child’s best interests. If a mutual understanding cannot be reached between the dermatologist and parents, obtaining a second opinion is a good option.8
Common Diagnoses
The most common diagnoses unique to procedural pediatric dermatology include congenital melanocytic nevi, vascular anomalies, midline lesions, epidermal nevi, and pilomatricomas. Prior to intervening on these lesions, it is important to consider evaluating for associated diseases.
Congenital Melanocytic Nevi
Nevus Outreach has published best practices for the management of congenital melanocytic nevi.9 In newborns with a congenital melanocytic nevus greater than 3 cm in diameter or more than 20 satellite lesions, it is recommended that magnetic resonance imaging (MRI) of the brain and spine with and without gadolinium contrast be obtained before 6 months of age. Within the first 6 months of life, these children also should see ophthalmologists, neurologists, pediatric dermatologists, and plastic surgeons. These early referrals will help to establish a baseline for the patient and plan for possible interventions, if needed. Additionally, before 3 years of age, every child should be referred to psychology, even if he/she is asymptomatic.10
Vascular Anomalies
Prior to intervening on a vascular anomaly, it is important to accurately classify the lesion. Once the lesion is classified, an evaluation and treatment plan can be developed. The International Society for the Study of Vascular Anomalies has published a detailed classification guide that is a useful starting point in the management of vascular anomalies.11 Once a diagnosis is confirmed, further evaluation may include imaging, specialty referrals, genetic testing, biopsy, or blood tests, and a pediatric dermatologist usually helps to coordinate the care of patients with complex vascular anomalies.
Midline Lesions
Certain lesions in the midline may have a higher risk for neural tube dysraphism, and imaging should be performed prior to any procedural intervention.12 Midline cutaneous findings that are highly likely to be associated with dysraphism are lipomas, acrochordons, pseudotails, true tails, aplasia cutis congenita, congenital scars, dermoid cysts, dermoid sinuses, and infantile hemangiomas that are greater than 2.5 cm in diameter. An MRI should be performed for all high-risk lesions. Intermediate-risk lesions are atypical dimples (>5 mm in diameter or >2.5 cm from the anal verge), hemangiomas less than 2.5 cm in diameter, and hypertrichosis. An ultrasound can screen for spinal dysraphism in these cases as long as imaging is performed prior to 6 months of age. If the child is older than 6 months, an MRI should be performed. Low-risk lesions that do not require imaging are simple dimples, hyperpigmentation, hypopigmentation, melanocytic nevi, port-wine stains, and telangiectases.
Epidermal Nevi
Children with epidermal nevi should have a complete physical examination, focusing on the skeletal system, central nervous system, and eyes. There are no specifically recommended imaging studies or referrals; however, several diagnostic clues can aid in the diagnosis of an epidermal nevus syndrome13:
• Schimmelpenning syndrome: extensive nevus sebaceous and bowing or pain in the legs after 2 years of age
• Phacomatosis pigmentokeratotica: nevus sebaceous and nevus spilus
• Nevus comedonicus syndrome: ipsilateral cataract
• Angora hair nevus syndrome: soft white hair within the nevus
• Becker nevus syndrome: breast hypoplasia
• Proteus syndrome: cerebriform plantar changes
• PIK3CA-related overgrowth spectrum: lipomas, macrodactyly, and/or vascular malformations
• Congenital hemidysplasia with ichthyosiform erythroderma and limb defects: inflammatory epidermal nevi, lateralization, ptychotropism, and ipsilateral limb defects
• Conradi-Hünermann-Happle syndrome: scaly red epidermal nevi without hair follicles and asymmetric limb shortening
Pilomatricomas
In addition to the tent sign—an angulated shape can be appreciated by stretching the skin overlying pilomatricomas—diagnosis of pilomatricoma can be confirmed by transillumination with an otoscope. In this case, a dark shadow typically is cast distal to where the otoscope touches the skin.14 In the case of multiple lesions, the patient should be evaluated for signs of myotonic dystrophy, Turner syndrome, and Gardner syndrome.15
Common Procedures
Pulsed Dye Laser
The pulsed dye laser is the most common laser used for red-colored lesions such as port-wine stains, facial telangiectases, and superficial hemangiomas. It also can be used to treat erythematous scars, verrucae, and psoriasis. In large vascular lesions, it typically is employed at 0.45 to 10 milliseconds every 4 to 6 weeks for 10 or more treatments. Port-wine stains preferably are treated within the first few months of life to provide the most fading without the need for general anesthesia.16 On the other hand, systemic therapy with propranolol is preferred over lasers for infantile hemangiomas.17
Long-Pulsed Alexandrite Laser (755 nm)
The alexandrite laser often is used to treat deeper vascular lesions such as venous lakes and hypertrophic port-wine stains. The operator needs to be cautious, as this laser has a higher incidence of scarring at the settings used to treat vascular lesions (typically fluences around 60–85 J/cm2).18 It also may be used for hair reduction in disorders with hypertrichosis or hidradenitis suppurativa.19
Long-Pulsed Nd:YAG Laser
The long-pulsed Nd:YAG laser also can be used to treat deep vascular lesions and remove unwanted hair. Because of its low window of safety in the treatment of vascular lesions, the alexandrite laser usually is preferred. However, it is the preferred laser for treatment of unwanted hair and hidradenitis suppurativa in darker skin types. It often provides a 50% reduction in hair density after 9 treatments.20
Quality-Switched Lasers
Pigment granules in melanosomes and tattoo particles are targeted with quality-switched (QS) lasers. Typically, a device will contain a combination of QS 532-nm potassium-titanyl-phosphate (KTP) lasers, QS 1064-nm Nd:YAG lasers, and QS 755-nm alexandrite lasers in 1 machine. In general, shorter wavelengths are used to treat epidermal lesions such as ephelides, lentigines, and café-au-lait macules. Longer wavelengths are used to treat deeper lesions such as nevus of Ota. A 2017 review suggested that café-au-lait macules with ragged borders (so-called coast of Maine borders) may respond well to QS lasers.21
Ablative Lasers
The 10,600-nm
Fractionated Lasers
Fractionated lasers can be nonablative (several devices are available in the 1410- to 1927-nm range) or ablative (CO2 or erbium:YAG). In pediatrics, they are usually used to treat burn scars, traumatic scars, and mild to moderate acne scarring.22 The most common side effects from fractionated lasers are prolonged erythema or hyperpigmentation. In addition, it typically takes at least 3 treatments to notice improvements.
Excisions
Pediatric procedural dermatologists remove a variety of unique lesions through excision. A few tips are provided for some of the more common lesions that may be excised in children.
Accessory Tragi
Prior to excising an accessory tragus, the surgeon should consider documenting a facial nerve examination, as accessory tragi can be associated with complete or partial facial nerve dysfunction. Additionally, there usually is an underlying cartilage structure present within the tragus. The cartilage stalk also should be addressed during the excision to avoid a continued palpable deformity after excision.
Dermoid Cysts
Dermoid cysts are the most commonly diagnosed benign orbital lesion in children.23 Exophytic periorbital lesions, which extend outside the orbital rim, can be removed through an infrabrow incision. Endophytic periorbital lesions, which are inside the orbital rim, should be removed through a crease incision. Midline lesions may have an intracranial extension and should be imaged through MRI and/or a computed tomography.24 Because dermoid cysts usually are located below the orbicularis oculi muscle, the muscle should be fixed with a suture prior to closing with skin sutures.
Pilomatricomas
Typically, a linear incision is made overlying the lesion, and then the underlying tumor is removed with sharp or blunt dissection. However, if the overlying skin has been stretched thin, a lenticular excision that includes the thinned skin may improve cosmesis.
Congenital Nevi
Large congenital nevi typically are removed through staged excisions. Lower extremity lesions are best removed before 10 months of age or before walking begins to minimize wound tension. However, if the procedure is not performed in infancy, it is best to wait until walking becomes stable.25 In older children, it is advisable to splint the affected lower extremity for 2 weeks to prevent dehiscence. The interval between excisions typically is 4 to 6 weeks for small lesions and 3 months for larger nevi.
Conclusion
Procedural pediatric dermatology is a broad and emerging field. As this article highlights, children are not small versions of adults and have unique biology, diseases, therapies, social situations, and ethical challenges from adults. This article provides a superficial overview of some of the more common issues faced by pediatric dermatologists and providers who perform procedures on infants, children, and teenagers. Readers who are interested in obtaining a more in-depth understanding of procedural pediatric dermatology should look at Procedural Pediatric Dermatology,1 the first textbook to provide expert opinion and evidence-based information on procedural management of pediatric skin conditions.
Performing dermatologic procedures in infants, children, and teenagers presents many unique challenges. There may be unique diagnoses, different instruments, differences in skin biology, or different approaches to pain management and anesthesia; the inclusion of a third party (caregivers) in decision processes; or a need to assess maturity level or to optimize outcomes over the patient’s lifetime. The field of pediatric procedural dermatology is broad. This article reviews some of the more common procedures performed by pediatric dermatologists and some of the more common ethical and quality-of-life (QOL) considerations one might face in procedural pediatric dermatology. (The textbook Procedural Pediatric Dermatology1 offers a thorough discussion of this topic.)
Quality of Life
More often than not, procedures are performed in pediatric dermatology to improve QOL rather than to prevent morbidity or mortality. In the case of many self-limited conditions, such as ingrown nails or pyogenic granulomas, it is clear that intervention will improve the patient’s QOL. In the case of warts and molluscum contagiosum, emotional, social, and cultural considerations play a large role in determining whether an intervention will improve QOL. Finally, some conditions, such as genodermatoses, giant congenital melanocytic nevi, and large vascular malformations, may be associated with additional systemic symptoms and may not have good treatment options for cure. In these cases, procedural interventions will result in a mixture of positive and negative QOL outcomes that can occur at the same time.
Bemmels et al2 published a qualitative study that provides a good foundation for understanding the positive and negative effects of procedural interventions on children and teenagers. In their study, children and teenagers who underwent reconstructive surgery for craniofacial differences noted improved self-esteem and reduced stigmatization. However, they also experienced negative outcomes, including an addiction to attaining a perfect surgical face, missing school for treatments, difficulty adjusting to an evolving appearance, anxiety related to not knowing when treatments will end, and experiencing stigma related to undergoing surgery.2 Thus, a comprehensive plan for the management of children who need ongoing procedures should include some level of psychosocial support. Two good references on supporting young patients with visible differences include CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference3 and Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development.4
Ethics
Ethical decisions in pediatric procedural dermatology differ from adult dermatology in 3 major ways: (1) the involvement of a third party (ie, parents or legal guardians), (2) the need to assess the maturity of the patient, and (3) the need to know local laws in the jurisdiction in which care is being provided. Ethical dilemmas occur when the desires of the child, parents/guardians, and dermatologist are not in alignment. In these cases, it is important to be prepared with a moral or ethical framework to guide decision-making when conflicts occur. Two great resources are the best interest standard5 and the publication entitled, “Informed Consent in Decision-making in Pediatric Practice,” from the American Academy of Pediatrics.6
In pediatrics, it often is better to conceptualize medical decision-making as a combination of informed permission and assent of the patient rather than informed consent. Informed permission describes how a parent or surrogate makes decisions for the child or adolescent and is similar to informed consent. A parent’s informed permission may be in conflict with a child’s wishes, but it is assumed that the parent is acting in the best interest of the child. Assent of the patient is the process of obtaining a minor’s agreement to undergo an intervention even though he/she may lack legal authority or decision-making capacity to provide standard informed consent. It is important to respect the child’s right to assent to interventions to the extent that their maturity level permits to develop trust with the dermatologist and medical encounters in general.
These differences emphasize an active process in which the patient, caregiver, and physician are all involved in the health care process and allow for increasing inclusion of the child as is developmentally appropriate. In the end, however, parents have the legal authority to give or withhold permission for a procedure.7 When this conflicts with a child’s dissent, the dermatologist will need to objectively explore the reasons for the conflict and decide if a procedure is not in the child’s best interests. If a mutual understanding cannot be reached between the dermatologist and parents, obtaining a second opinion is a good option.8
Common Diagnoses
The most common diagnoses unique to procedural pediatric dermatology include congenital melanocytic nevi, vascular anomalies, midline lesions, epidermal nevi, and pilomatricomas. Prior to intervening on these lesions, it is important to consider evaluating for associated diseases.
Congenital Melanocytic Nevi
Nevus Outreach has published best practices for the management of congenital melanocytic nevi.9 In newborns with a congenital melanocytic nevus greater than 3 cm in diameter or more than 20 satellite lesions, it is recommended that magnetic resonance imaging (MRI) of the brain and spine with and without gadolinium contrast be obtained before 6 months of age. Within the first 6 months of life, these children also should see ophthalmologists, neurologists, pediatric dermatologists, and plastic surgeons. These early referrals will help to establish a baseline for the patient and plan for possible interventions, if needed. Additionally, before 3 years of age, every child should be referred to psychology, even if he/she is asymptomatic.10
Vascular Anomalies
Prior to intervening on a vascular anomaly, it is important to accurately classify the lesion. Once the lesion is classified, an evaluation and treatment plan can be developed. The International Society for the Study of Vascular Anomalies has published a detailed classification guide that is a useful starting point in the management of vascular anomalies.11 Once a diagnosis is confirmed, further evaluation may include imaging, specialty referrals, genetic testing, biopsy, or blood tests, and a pediatric dermatologist usually helps to coordinate the care of patients with complex vascular anomalies.
Midline Lesions
Certain lesions in the midline may have a higher risk for neural tube dysraphism, and imaging should be performed prior to any procedural intervention.12 Midline cutaneous findings that are highly likely to be associated with dysraphism are lipomas, acrochordons, pseudotails, true tails, aplasia cutis congenita, congenital scars, dermoid cysts, dermoid sinuses, and infantile hemangiomas that are greater than 2.5 cm in diameter. An MRI should be performed for all high-risk lesions. Intermediate-risk lesions are atypical dimples (>5 mm in diameter or >2.5 cm from the anal verge), hemangiomas less than 2.5 cm in diameter, and hypertrichosis. An ultrasound can screen for spinal dysraphism in these cases as long as imaging is performed prior to 6 months of age. If the child is older than 6 months, an MRI should be performed. Low-risk lesions that do not require imaging are simple dimples, hyperpigmentation, hypopigmentation, melanocytic nevi, port-wine stains, and telangiectases.
Epidermal Nevi
Children with epidermal nevi should have a complete physical examination, focusing on the skeletal system, central nervous system, and eyes. There are no specifically recommended imaging studies or referrals; however, several diagnostic clues can aid in the diagnosis of an epidermal nevus syndrome13:
• Schimmelpenning syndrome: extensive nevus sebaceous and bowing or pain in the legs after 2 years of age
• Phacomatosis pigmentokeratotica: nevus sebaceous and nevus spilus
• Nevus comedonicus syndrome: ipsilateral cataract
• Angora hair nevus syndrome: soft white hair within the nevus
• Becker nevus syndrome: breast hypoplasia
• Proteus syndrome: cerebriform plantar changes
• PIK3CA-related overgrowth spectrum: lipomas, macrodactyly, and/or vascular malformations
• Congenital hemidysplasia with ichthyosiform erythroderma and limb defects: inflammatory epidermal nevi, lateralization, ptychotropism, and ipsilateral limb defects
• Conradi-Hünermann-Happle syndrome: scaly red epidermal nevi without hair follicles and asymmetric limb shortening
Pilomatricomas
In addition to the tent sign—an angulated shape can be appreciated by stretching the skin overlying pilomatricomas—diagnosis of pilomatricoma can be confirmed by transillumination with an otoscope. In this case, a dark shadow typically is cast distal to where the otoscope touches the skin.14 In the case of multiple lesions, the patient should be evaluated for signs of myotonic dystrophy, Turner syndrome, and Gardner syndrome.15
Common Procedures
Pulsed Dye Laser
The pulsed dye laser is the most common laser used for red-colored lesions such as port-wine stains, facial telangiectases, and superficial hemangiomas. It also can be used to treat erythematous scars, verrucae, and psoriasis. In large vascular lesions, it typically is employed at 0.45 to 10 milliseconds every 4 to 6 weeks for 10 or more treatments. Port-wine stains preferably are treated within the first few months of life to provide the most fading without the need for general anesthesia.16 On the other hand, systemic therapy with propranolol is preferred over lasers for infantile hemangiomas.17
Long-Pulsed Alexandrite Laser (755 nm)
The alexandrite laser often is used to treat deeper vascular lesions such as venous lakes and hypertrophic port-wine stains. The operator needs to be cautious, as this laser has a higher incidence of scarring at the settings used to treat vascular lesions (typically fluences around 60–85 J/cm2).18 It also may be used for hair reduction in disorders with hypertrichosis or hidradenitis suppurativa.19
Long-Pulsed Nd:YAG Laser
The long-pulsed Nd:YAG laser also can be used to treat deep vascular lesions and remove unwanted hair. Because of its low window of safety in the treatment of vascular lesions, the alexandrite laser usually is preferred. However, it is the preferred laser for treatment of unwanted hair and hidradenitis suppurativa in darker skin types. It often provides a 50% reduction in hair density after 9 treatments.20
Quality-Switched Lasers
Pigment granules in melanosomes and tattoo particles are targeted with quality-switched (QS) lasers. Typically, a device will contain a combination of QS 532-nm potassium-titanyl-phosphate (KTP) lasers, QS 1064-nm Nd:YAG lasers, and QS 755-nm alexandrite lasers in 1 machine. In general, shorter wavelengths are used to treat epidermal lesions such as ephelides, lentigines, and café-au-lait macules. Longer wavelengths are used to treat deeper lesions such as nevus of Ota. A 2017 review suggested that café-au-lait macules with ragged borders (so-called coast of Maine borders) may respond well to QS lasers.21
Ablative Lasers
The 10,600-nm
Fractionated Lasers
Fractionated lasers can be nonablative (several devices are available in the 1410- to 1927-nm range) or ablative (CO2 or erbium:YAG). In pediatrics, they are usually used to treat burn scars, traumatic scars, and mild to moderate acne scarring.22 The most common side effects from fractionated lasers are prolonged erythema or hyperpigmentation. In addition, it typically takes at least 3 treatments to notice improvements.
Excisions
Pediatric procedural dermatologists remove a variety of unique lesions through excision. A few tips are provided for some of the more common lesions that may be excised in children.
Accessory Tragi
Prior to excising an accessory tragus, the surgeon should consider documenting a facial nerve examination, as accessory tragi can be associated with complete or partial facial nerve dysfunction. Additionally, there usually is an underlying cartilage structure present within the tragus. The cartilage stalk also should be addressed during the excision to avoid a continued palpable deformity after excision.
Dermoid Cysts
Dermoid cysts are the most commonly diagnosed benign orbital lesion in children.23 Exophytic periorbital lesions, which extend outside the orbital rim, can be removed through an infrabrow incision. Endophytic periorbital lesions, which are inside the orbital rim, should be removed through a crease incision. Midline lesions may have an intracranial extension and should be imaged through MRI and/or a computed tomography.24 Because dermoid cysts usually are located below the orbicularis oculi muscle, the muscle should be fixed with a suture prior to closing with skin sutures.
Pilomatricomas
Typically, a linear incision is made overlying the lesion, and then the underlying tumor is removed with sharp or blunt dissection. However, if the overlying skin has been stretched thin, a lenticular excision that includes the thinned skin may improve cosmesis.
Congenital Nevi
Large congenital nevi typically are removed through staged excisions. Lower extremity lesions are best removed before 10 months of age or before walking begins to minimize wound tension. However, if the procedure is not performed in infancy, it is best to wait until walking becomes stable.25 In older children, it is advisable to splint the affected lower extremity for 2 weeks to prevent dehiscence. The interval between excisions typically is 4 to 6 weeks for small lesions and 3 months for larger nevi.
Conclusion
Procedural pediatric dermatology is a broad and emerging field. As this article highlights, children are not small versions of adults and have unique biology, diseases, therapies, social situations, and ethical challenges from adults. This article provides a superficial overview of some of the more common issues faced by pediatric dermatologists and providers who perform procedures on infants, children, and teenagers. Readers who are interested in obtaining a more in-depth understanding of procedural pediatric dermatology should look at Procedural Pediatric Dermatology,1 the first textbook to provide expert opinion and evidence-based information on procedural management of pediatric skin conditions.
- Krakowski AC. Procedural Pediatric Dermatology. Phialdelphia, PA: Wolters Kluwer; 2011.
- Bemmels H, Biesecker B, Schmidt J, et al. Psychological and social factors in undergoing reconstructive surgery among individuals with craniofacial conditions: an exploratory study. Cleft Palate Craniofac J. 2013;50:158-167.
- Clarke A, Thompson AR, Jenkinson E, et al. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference. Chichester, West Sussex: Wiley-Blackwell; 2013.
- Ginsburg KR, Ramirez McClain ZB, eds. Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development. 2nd ed. Itasca, IL: American Academy of Pediatrics; 2020.
- Kopelman LM. The best interests standard for incompetent or incapacitated patients of all ages. J Law Med Ethics. 2007;35:187-196.
- Katz AL, Webb SA; Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485.
- Michon K. Emancipation of minors. NOLO website. https://www.nolo.com/legal-encyclopedia/emancipation-of-minors-32237.html. Accessed October 14, 2020.
- Cobb C, Bercovitch L. Ethical dilemmas. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:7-10.
- Nevus Outreach, Inc., releases best practice guidelines [news release]. Bartlesville, OK: Nevus Outreach Inc; July 7, 2018. https://www.nevus.org/matrices/page_file_download.php?id=239. Accessed October 14, 2020.
- 10. Masnari O, Neuhaus K, Aegerter T, et al. Predictors of health-related quality of life and psychological adjustment in children and adolescents with congenital melanocytic nevi: analysis of parent reports. J Pediatr Psychol. 2019;44:714-725.
- ISSVA classification for vascular anomalies. International Society for the Study of Vascular Anomalies website. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Approved April 2014. Revised May 2018. Accessed October 14, 2020.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Berreto-Chang OL, Gorell ES, Yamaguma MA, et al. Diagnosis of pilomatricoma using an otoscope. Pediatr Dermatol. 2010;27:554-557.
- Danielson-Cohen A, Lin SJ, Hughes CA, et al. Head and neck pilomatrixoma in children. Arch Otolaryngol Head Neck Surg. 2001;127:1481-1483.
- Jeon H, Bernstein LJ, Belkin DA, et al. Pulsed dye laser treatment of port-wine stains in infancy without the need for general anesthesia. JAMA Dermatol. 2019;155:435-441.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37:1268-1278.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:76-90.
- Rao K, Sankar TK. Long-pulsed Nd:YAG laser-assisted hair removal in Fitzpatrick skin types IV-VI. Lasers Med Sci. 2011;26:623-626.
- Belkin DA, Neckman JP, Jeon H, et al. Response to laser treatment of café au lait macules based on morphologic features. JAMA Dermatol. 2017;153:1158-1161.
- Kelly K, Lehmer L. Laser surgery. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:92-106.
- Eldesouky MA, Elbakary MA. Orbital dermoid cyst: classification and its impact on surgical management. Semin Ophthalmol. 2018;33:170-174.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Metz BJ. Procedural pediatric dermatology. Dermatol Clin. 2013;31:337-346.
- Krakowski AC. Procedural Pediatric Dermatology. Phialdelphia, PA: Wolters Kluwer; 2011.
- Bemmels H, Biesecker B, Schmidt J, et al. Psychological and social factors in undergoing reconstructive surgery among individuals with craniofacial conditions: an exploratory study. Cleft Palate Craniofac J. 2013;50:158-167.
- Clarke A, Thompson AR, Jenkinson E, et al. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference. Chichester, West Sussex: Wiley-Blackwell; 2013.
- Ginsburg KR, Ramirez McClain ZB, eds. Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development. 2nd ed. Itasca, IL: American Academy of Pediatrics; 2020.
- Kopelman LM. The best interests standard for incompetent or incapacitated patients of all ages. J Law Med Ethics. 2007;35:187-196.
- Katz AL, Webb SA; Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485.
- Michon K. Emancipation of minors. NOLO website. https://www.nolo.com/legal-encyclopedia/emancipation-of-minors-32237.html. Accessed October 14, 2020.
- Cobb C, Bercovitch L. Ethical dilemmas. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:7-10.
- Nevus Outreach, Inc., releases best practice guidelines [news release]. Bartlesville, OK: Nevus Outreach Inc; July 7, 2018. https://www.nevus.org/matrices/page_file_download.php?id=239. Accessed October 14, 2020.
- 10. Masnari O, Neuhaus K, Aegerter T, et al. Predictors of health-related quality of life and psychological adjustment in children and adolescents with congenital melanocytic nevi: analysis of parent reports. J Pediatr Psychol. 2019;44:714-725.
- ISSVA classification for vascular anomalies. International Society for the Study of Vascular Anomalies website. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Approved April 2014. Revised May 2018. Accessed October 14, 2020.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Berreto-Chang OL, Gorell ES, Yamaguma MA, et al. Diagnosis of pilomatricoma using an otoscope. Pediatr Dermatol. 2010;27:554-557.
- Danielson-Cohen A, Lin SJ, Hughes CA, et al. Head and neck pilomatrixoma in children. Arch Otolaryngol Head Neck Surg. 2001;127:1481-1483.
- Jeon H, Bernstein LJ, Belkin DA, et al. Pulsed dye laser treatment of port-wine stains in infancy without the need for general anesthesia. JAMA Dermatol. 2019;155:435-441.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37:1268-1278.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:76-90.
- Rao K, Sankar TK. Long-pulsed Nd:YAG laser-assisted hair removal in Fitzpatrick skin types IV-VI. Lasers Med Sci. 2011;26:623-626.
- Belkin DA, Neckman JP, Jeon H, et al. Response to laser treatment of café au lait macules based on morphologic features. JAMA Dermatol. 2017;153:1158-1161.
- Kelly K, Lehmer L. Laser surgery. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:92-106.
- Eldesouky MA, Elbakary MA. Orbital dermoid cyst: classification and its impact on surgical management. Semin Ophthalmol. 2018;33:170-174.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Metz BJ. Procedural pediatric dermatology. Dermatol Clin. 2013;31:337-346.
Practice Points
- Children who require repetitive laser or surgical procedures over time benefit from regular monitoring of psychosocial needs.
- The informed consent process for children differs from adult procedural dermatology and should be adjusted to the maturity level of the patient.
- Common diagnoses unique to procedural pediatric dermatology that may require additional investigation include congenital melanocytic nevi, vascular anomalies, epidermal nevi, and midline lesions.
- Specific measures can be performed to improve outcomes when removing accessory tragi, dermoid cysts, pilomatricomas, and congenital nevi.
Are HMAS appropriate for posttransplant maintenance in acute leukemias?
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
FROM ALF 2020
Bonds and Bridges: The Role of Social Capital in Building a More Diverse Dermatology Workforce
As our specialty seeks to address its lack of racial diversity, many dermatologists have answered recent calls to action.1,2 As we work toward dismantling systemic issues that have created pervasive inequality in our residency application review and interview processes, consideration also should be given to psychosocial issues that underrepresented-in-medicine (UIM) students face before their applications come to our attention. In this article, we explore how potential differences in the social capital of UIM and other disadvantaged dermatology residency applicants contribute to persistent homogeneity among dermatology training programs and the workforce.
The Theory of Capital
The concepts of economic, social, and cultural capital originate from the writings of social theorist Pierre Bourdieu.3 All 3 forms of capital are interconnected, and they relate to each other in ways that often facilitate social division and inequality. Economic capital denotes an individual’s economic resources or wealth, while cultural capital refers to the knowledge, behaviors, and skills that demonstrate his/her economic class (eg, communication style, table manners).3 Social capital refers to an individual’s interpersonal connections in personal and professional settings and can be subdivided into 3 categories: bonds, bridges, and linkages.4,5 Herein, we will focus on bonds and bridges.
It has been suggested that bonds are important for “getting by,” while bridges are critical for “getting ahead.”5 Bonds refer to close relationships within a community of people with shared characteristics, such as racial/ethnic identity and culture, access to information, and resources (eg, family, friends). These bonds provide trust, safety, and financial and emotional support; however, they are considered to be inward-looking and can promote exclusion and homogeneity.5
On the other hand, bridges refer to social relationships that extend outward beyond one’s close circle of family and friends to other people with shared interests and goals who may have different social or cultural identities (eg, professional colleagues). These bridges are considered to be outward-looking and provide many benefits to individuals and society. They link diverse individuals, which tends to increase tolerance and disrupt stereotypes, and they facilitate the sharing of ideas, information, and innovation. Additionally, bridges between individuals from different networks facilitate access to increased resources and opportunities for all parties.5
The 3 forms of capital are inextricably linked. For example, with economic capital, a child’s family can purchase access to a prestigious private high school, where he/she will gain valuable social capital through bridges with other students and their families. At this school, the child also will accumulate cultural capital that increases his/her sense of belonging in these circles. Subsequently, both the social and cultural capital accumulated at this private high school can be exchanged for economic capital via social networks, skills, values, and behaviors that facilitate entry into higher education and professional training. As such, these 3 forms of capital work together to continue social/class divisions, hierarchies, and ultimately inequality.
Impact of Social Capital in Pursuing a Medical Career
For medical students whose bonds (ie, close family, friends) include physicians or other health care professionals, the journey to studying medicine and entering their chosen specialty will be facilitated by financial security, valuable “inside information” about the application process, study skills, and even clinical guidance. Additionally, these students will have access to professional networks for mentorship, shadowing experiences, and other potential advantages. Furthermore, social capital is associated with higher self-esteem,6 which likely improves academic performance and wards off imposter syndrome in these students.
For medical students from lower socioeconomic status backgrounds or those whose inner circles do not include physicians or other health care professionals, accumulating the social and cultural capital needed to successfully navigate a medical career is more difficult. Although they may receive support and encouragement from family and friends, they will not have access to the same valuable information and connections that facilitate success; rather, they will have a further distance to travel, and this distance should be acknowledged in the residency application review process.
Acquiring Social Capital as a UIM Student
Despite the benefits of social and cultural capital, acquiring them takes a toll. For those UIM students who start life from a disadvantaged place, the accumulation of social capital does not come easily; rather, it demands effort and time that has the potential to detract from a student’s focus on the academic demands of medical education.7 Programs that attempt to improve disadvantaged students’ access to credible information, role models, and mentors can help lift some of the burden from the individual student’s shoulders. For example, studies have demonstrated the benefits of harnessing technology to enhance mentorship programs that increase social capital of disadvantaged populations.8-11 This approach already is in progress, bolstered by advances made in digital communications during the coronavirus disease 2019 pandemic.12 Student-led networking groups that connect remotely have been shown to build social capital bonds and bridges that facilitate collaborative learning, relationship building, and information sharing.8-11 There are existing online UIM student networks that individual dermatologists, institutions, and national organizations can partner with to facilitate the construction of bridges between these UIM student groups and dermatologists who can provide accurate, high-yield information and professional networking; however, one limitation of this suggestion is the disparate access to technology in the UIM community.
Final Thoughts
It is important to note that assumptions should not be made about the level of economic, social, or cultural capital an individual possesses based on his/her race or ethnicity. Instead, mentors should attempt to be available to a diverse pool of students; take the time to get to know these students; and then provide the types of mentorship, information, exposure, and networking that each individual student needs. Another approach is to make a concerted effort to ensure that all students receive the same amount and quality of information about medical education and our specialty regardless of their level of economic, cultural, or social capital. Moreover, beyond the promotion of diversity through increasing numbers of UIM applicants, we should seek to reshape our specialty into a space that does not require students to subdue their existing diverse forms of capital but rather to bring these different perspectives and lived experiences to the table.13
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:7.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Bourdieu P. The forms of capital. In: Richardson J, ed. Handbook of Theory and Research for the Sociology of Education. Westport, CT: Greenwood; 1986:241-258.
- Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360-1380.
- Putnam RD. Bowling alone: America’s declining social capital. J Democracy. 1995;6:65-78.
- Han S. Longitudinal association between social capital and self-esteem: a matter of context. Psychiatry Research. 2015;226:340-346.
- Kirschling JM. Building social capital: leading and leveraging constituencies outside the college. J Nurs Educ. 2004;43:517-519.
- Radlick RL, Svedberg P, Nygren JM, et al. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial [published online March 17, 2020]. JMIR Research Protocols. doi:10.2196/16472.
- Koh LC, Walker R, Wollersheim D, et al. I think someone is walking with me: the use of mobile phone for social capital development among women in four refugee communities. Int J Migration Health Social Care. 2018;14:411-424.
- Hartley A, Kassam AA. Social networking for learning in higher education: capitalising on social capital. ResearchGate website.https://www.researchgate.net/publication/311097860_Social_Networking_for_Learning_in_Higher_Education_Capitalising_on_Social_Capital. Published November 2016. Accessed October 19, 2020.
- Zalon ML. Using technology to build community in professional associations. J Contin Educ Nurs. 2008;39:235-240.
- Stewart CR, Chernoff KA, Wildman HF, et al. Recommendations for medical student preparedness and equity for dermatology residency applications during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E225-E226.
- Brosnan C, Southgate E, Outram S, et al. Experiences of medical students who are first in family to attend university. Med Educ. 2016;50:842-851.
As our specialty seeks to address its lack of racial diversity, many dermatologists have answered recent calls to action.1,2 As we work toward dismantling systemic issues that have created pervasive inequality in our residency application review and interview processes, consideration also should be given to psychosocial issues that underrepresented-in-medicine (UIM) students face before their applications come to our attention. In this article, we explore how potential differences in the social capital of UIM and other disadvantaged dermatology residency applicants contribute to persistent homogeneity among dermatology training programs and the workforce.
The Theory of Capital
The concepts of economic, social, and cultural capital originate from the writings of social theorist Pierre Bourdieu.3 All 3 forms of capital are interconnected, and they relate to each other in ways that often facilitate social division and inequality. Economic capital denotes an individual’s economic resources or wealth, while cultural capital refers to the knowledge, behaviors, and skills that demonstrate his/her economic class (eg, communication style, table manners).3 Social capital refers to an individual’s interpersonal connections in personal and professional settings and can be subdivided into 3 categories: bonds, bridges, and linkages.4,5 Herein, we will focus on bonds and bridges.
It has been suggested that bonds are important for “getting by,” while bridges are critical for “getting ahead.”5 Bonds refer to close relationships within a community of people with shared characteristics, such as racial/ethnic identity and culture, access to information, and resources (eg, family, friends). These bonds provide trust, safety, and financial and emotional support; however, they are considered to be inward-looking and can promote exclusion and homogeneity.5
On the other hand, bridges refer to social relationships that extend outward beyond one’s close circle of family and friends to other people with shared interests and goals who may have different social or cultural identities (eg, professional colleagues). These bridges are considered to be outward-looking and provide many benefits to individuals and society. They link diverse individuals, which tends to increase tolerance and disrupt stereotypes, and they facilitate the sharing of ideas, information, and innovation. Additionally, bridges between individuals from different networks facilitate access to increased resources and opportunities for all parties.5
The 3 forms of capital are inextricably linked. For example, with economic capital, a child’s family can purchase access to a prestigious private high school, where he/she will gain valuable social capital through bridges with other students and their families. At this school, the child also will accumulate cultural capital that increases his/her sense of belonging in these circles. Subsequently, both the social and cultural capital accumulated at this private high school can be exchanged for economic capital via social networks, skills, values, and behaviors that facilitate entry into higher education and professional training. As such, these 3 forms of capital work together to continue social/class divisions, hierarchies, and ultimately inequality.
Impact of Social Capital in Pursuing a Medical Career
For medical students whose bonds (ie, close family, friends) include physicians or other health care professionals, the journey to studying medicine and entering their chosen specialty will be facilitated by financial security, valuable “inside information” about the application process, study skills, and even clinical guidance. Additionally, these students will have access to professional networks for mentorship, shadowing experiences, and other potential advantages. Furthermore, social capital is associated with higher self-esteem,6 which likely improves academic performance and wards off imposter syndrome in these students.
For medical students from lower socioeconomic status backgrounds or those whose inner circles do not include physicians or other health care professionals, accumulating the social and cultural capital needed to successfully navigate a medical career is more difficult. Although they may receive support and encouragement from family and friends, they will not have access to the same valuable information and connections that facilitate success; rather, they will have a further distance to travel, and this distance should be acknowledged in the residency application review process.
Acquiring Social Capital as a UIM Student
Despite the benefits of social and cultural capital, acquiring them takes a toll. For those UIM students who start life from a disadvantaged place, the accumulation of social capital does not come easily; rather, it demands effort and time that has the potential to detract from a student’s focus on the academic demands of medical education.7 Programs that attempt to improve disadvantaged students’ access to credible information, role models, and mentors can help lift some of the burden from the individual student’s shoulders. For example, studies have demonstrated the benefits of harnessing technology to enhance mentorship programs that increase social capital of disadvantaged populations.8-11 This approach already is in progress, bolstered by advances made in digital communications during the coronavirus disease 2019 pandemic.12 Student-led networking groups that connect remotely have been shown to build social capital bonds and bridges that facilitate collaborative learning, relationship building, and information sharing.8-11 There are existing online UIM student networks that individual dermatologists, institutions, and national organizations can partner with to facilitate the construction of bridges between these UIM student groups and dermatologists who can provide accurate, high-yield information and professional networking; however, one limitation of this suggestion is the disparate access to technology in the UIM community.
Final Thoughts
It is important to note that assumptions should not be made about the level of economic, social, or cultural capital an individual possesses based on his/her race or ethnicity. Instead, mentors should attempt to be available to a diverse pool of students; take the time to get to know these students; and then provide the types of mentorship, information, exposure, and networking that each individual student needs. Another approach is to make a concerted effort to ensure that all students receive the same amount and quality of information about medical education and our specialty regardless of their level of economic, cultural, or social capital. Moreover, beyond the promotion of diversity through increasing numbers of UIM applicants, we should seek to reshape our specialty into a space that does not require students to subdue their existing diverse forms of capital but rather to bring these different perspectives and lived experiences to the table.13
As our specialty seeks to address its lack of racial diversity, many dermatologists have answered recent calls to action.1,2 As we work toward dismantling systemic issues that have created pervasive inequality in our residency application review and interview processes, consideration also should be given to psychosocial issues that underrepresented-in-medicine (UIM) students face before their applications come to our attention. In this article, we explore how potential differences in the social capital of UIM and other disadvantaged dermatology residency applicants contribute to persistent homogeneity among dermatology training programs and the workforce.
The Theory of Capital
The concepts of economic, social, and cultural capital originate from the writings of social theorist Pierre Bourdieu.3 All 3 forms of capital are interconnected, and they relate to each other in ways that often facilitate social division and inequality. Economic capital denotes an individual’s economic resources or wealth, while cultural capital refers to the knowledge, behaviors, and skills that demonstrate his/her economic class (eg, communication style, table manners).3 Social capital refers to an individual’s interpersonal connections in personal and professional settings and can be subdivided into 3 categories: bonds, bridges, and linkages.4,5 Herein, we will focus on bonds and bridges.
It has been suggested that bonds are important for “getting by,” while bridges are critical for “getting ahead.”5 Bonds refer to close relationships within a community of people with shared characteristics, such as racial/ethnic identity and culture, access to information, and resources (eg, family, friends). These bonds provide trust, safety, and financial and emotional support; however, they are considered to be inward-looking and can promote exclusion and homogeneity.5
On the other hand, bridges refer to social relationships that extend outward beyond one’s close circle of family and friends to other people with shared interests and goals who may have different social or cultural identities (eg, professional colleagues). These bridges are considered to be outward-looking and provide many benefits to individuals and society. They link diverse individuals, which tends to increase tolerance and disrupt stereotypes, and they facilitate the sharing of ideas, information, and innovation. Additionally, bridges between individuals from different networks facilitate access to increased resources and opportunities for all parties.5
The 3 forms of capital are inextricably linked. For example, with economic capital, a child’s family can purchase access to a prestigious private high school, where he/she will gain valuable social capital through bridges with other students and their families. At this school, the child also will accumulate cultural capital that increases his/her sense of belonging in these circles. Subsequently, both the social and cultural capital accumulated at this private high school can be exchanged for economic capital via social networks, skills, values, and behaviors that facilitate entry into higher education and professional training. As such, these 3 forms of capital work together to continue social/class divisions, hierarchies, and ultimately inequality.
Impact of Social Capital in Pursuing a Medical Career
For medical students whose bonds (ie, close family, friends) include physicians or other health care professionals, the journey to studying medicine and entering their chosen specialty will be facilitated by financial security, valuable “inside information” about the application process, study skills, and even clinical guidance. Additionally, these students will have access to professional networks for mentorship, shadowing experiences, and other potential advantages. Furthermore, social capital is associated with higher self-esteem,6 which likely improves academic performance and wards off imposter syndrome in these students.
For medical students from lower socioeconomic status backgrounds or those whose inner circles do not include physicians or other health care professionals, accumulating the social and cultural capital needed to successfully navigate a medical career is more difficult. Although they may receive support and encouragement from family and friends, they will not have access to the same valuable information and connections that facilitate success; rather, they will have a further distance to travel, and this distance should be acknowledged in the residency application review process.
Acquiring Social Capital as a UIM Student
Despite the benefits of social and cultural capital, acquiring them takes a toll. For those UIM students who start life from a disadvantaged place, the accumulation of social capital does not come easily; rather, it demands effort and time that has the potential to detract from a student’s focus on the academic demands of medical education.7 Programs that attempt to improve disadvantaged students’ access to credible information, role models, and mentors can help lift some of the burden from the individual student’s shoulders. For example, studies have demonstrated the benefits of harnessing technology to enhance mentorship programs that increase social capital of disadvantaged populations.8-11 This approach already is in progress, bolstered by advances made in digital communications during the coronavirus disease 2019 pandemic.12 Student-led networking groups that connect remotely have been shown to build social capital bonds and bridges that facilitate collaborative learning, relationship building, and information sharing.8-11 There are existing online UIM student networks that individual dermatologists, institutions, and national organizations can partner with to facilitate the construction of bridges between these UIM student groups and dermatologists who can provide accurate, high-yield information and professional networking; however, one limitation of this suggestion is the disparate access to technology in the UIM community.
Final Thoughts
It is important to note that assumptions should not be made about the level of economic, social, or cultural capital an individual possesses based on his/her race or ethnicity. Instead, mentors should attempt to be available to a diverse pool of students; take the time to get to know these students; and then provide the types of mentorship, information, exposure, and networking that each individual student needs. Another approach is to make a concerted effort to ensure that all students receive the same amount and quality of information about medical education and our specialty regardless of their level of economic, cultural, or social capital. Moreover, beyond the promotion of diversity through increasing numbers of UIM applicants, we should seek to reshape our specialty into a space that does not require students to subdue their existing diverse forms of capital but rather to bring these different perspectives and lived experiences to the table.13
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:7.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Bourdieu P. The forms of capital. In: Richardson J, ed. Handbook of Theory and Research for the Sociology of Education. Westport, CT: Greenwood; 1986:241-258.
- Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360-1380.
- Putnam RD. Bowling alone: America’s declining social capital. J Democracy. 1995;6:65-78.
- Han S. Longitudinal association between social capital and self-esteem: a matter of context. Psychiatry Research. 2015;226:340-346.
- Kirschling JM. Building social capital: leading and leveraging constituencies outside the college. J Nurs Educ. 2004;43:517-519.
- Radlick RL, Svedberg P, Nygren JM, et al. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial [published online March 17, 2020]. JMIR Research Protocols. doi:10.2196/16472.
- Koh LC, Walker R, Wollersheim D, et al. I think someone is walking with me: the use of mobile phone for social capital development among women in four refugee communities. Int J Migration Health Social Care. 2018;14:411-424.
- Hartley A, Kassam AA. Social networking for learning in higher education: capitalising on social capital. ResearchGate website.https://www.researchgate.net/publication/311097860_Social_Networking_for_Learning_in_Higher_Education_Capitalising_on_Social_Capital. Published November 2016. Accessed October 19, 2020.
- Zalon ML. Using technology to build community in professional associations. J Contin Educ Nurs. 2008;39:235-240.
- Stewart CR, Chernoff KA, Wildman HF, et al. Recommendations for medical student preparedness and equity for dermatology residency applications during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E225-E226.
- Brosnan C, Southgate E, Outram S, et al. Experiences of medical students who are first in family to attend university. Med Educ. 2016;50:842-851.
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:7.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Bourdieu P. The forms of capital. In: Richardson J, ed. Handbook of Theory and Research for the Sociology of Education. Westport, CT: Greenwood; 1986:241-258.
- Granovetter MS. The strength of weak ties. Am J Sociol. 1973;78:1360-1380.
- Putnam RD. Bowling alone: America’s declining social capital. J Democracy. 1995;6:65-78.
- Han S. Longitudinal association between social capital and self-esteem: a matter of context. Psychiatry Research. 2015;226:340-346.
- Kirschling JM. Building social capital: leading and leveraging constituencies outside the college. J Nurs Educ. 2004;43:517-519.
- Radlick RL, Svedberg P, Nygren JM, et al. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial [published online March 17, 2020]. JMIR Research Protocols. doi:10.2196/16472.
- Koh LC, Walker R, Wollersheim D, et al. I think someone is walking with me: the use of mobile phone for social capital development among women in four refugee communities. Int J Migration Health Social Care. 2018;14:411-424.
- Hartley A, Kassam AA. Social networking for learning in higher education: capitalising on social capital. ResearchGate website.https://www.researchgate.net/publication/311097860_Social_Networking_for_Learning_in_Higher_Education_Capitalising_on_Social_Capital. Published November 2016. Accessed October 19, 2020.
- Zalon ML. Using technology to build community in professional associations. J Contin Educ Nurs. 2008;39:235-240.
- Stewart CR, Chernoff KA, Wildman HF, et al. Recommendations for medical student preparedness and equity for dermatology residency applications during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E225-E226.
- Brosnan C, Southgate E, Outram S, et al. Experiences of medical students who are first in family to attend university. Med Educ. 2016;50:842-851.
Practice Points
- Achieving diversity in the field of dermatology will require a concerted effort to equalize access to mentorship, information, exposure, and networking for students of all backgrounds.
- Valuing diverse forms of capital in applicants ultimately will strengthen the dermatology workforce through inclusion of various lived experiences and perspectives.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis in Pediatric Patients
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
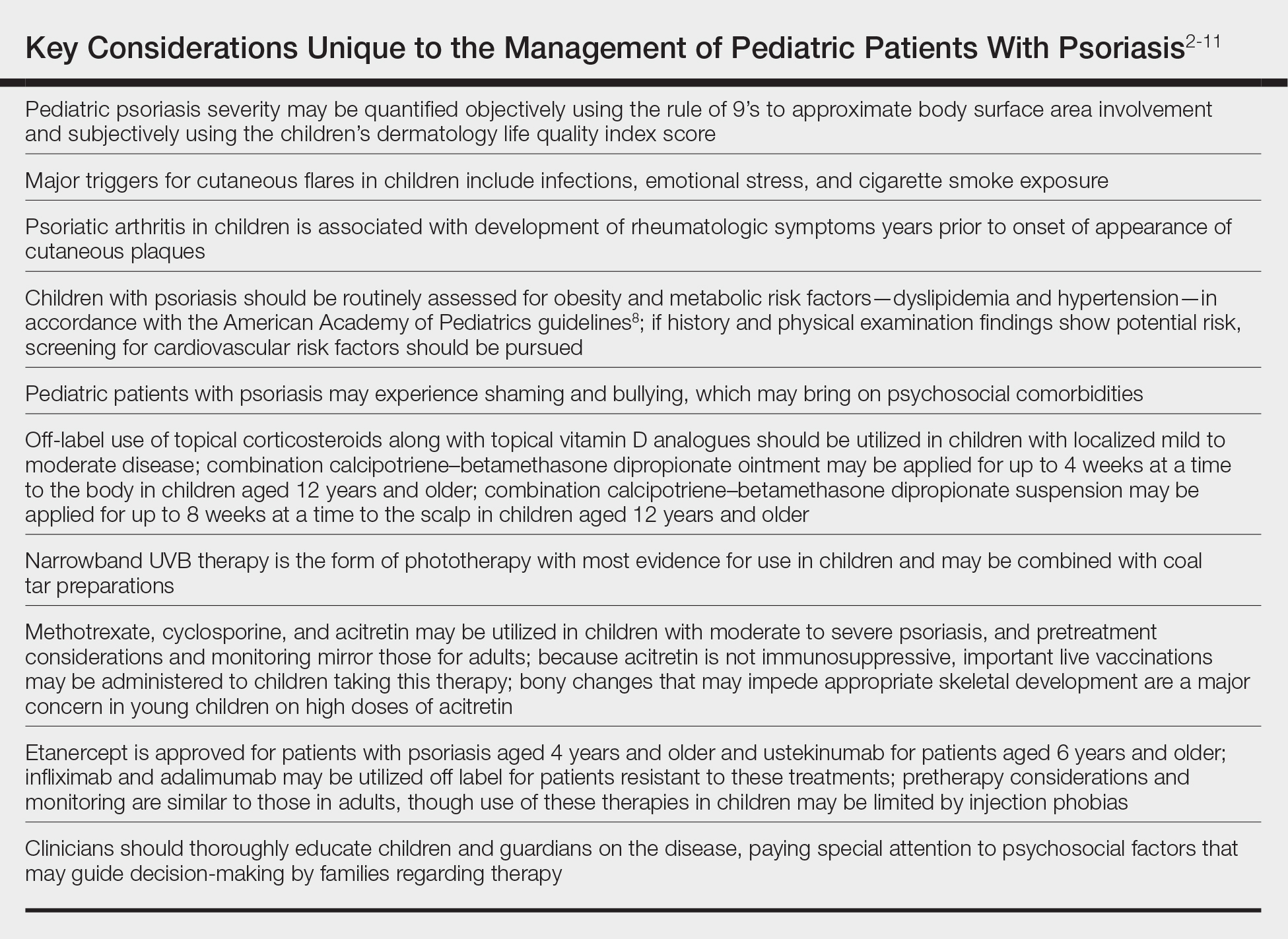
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
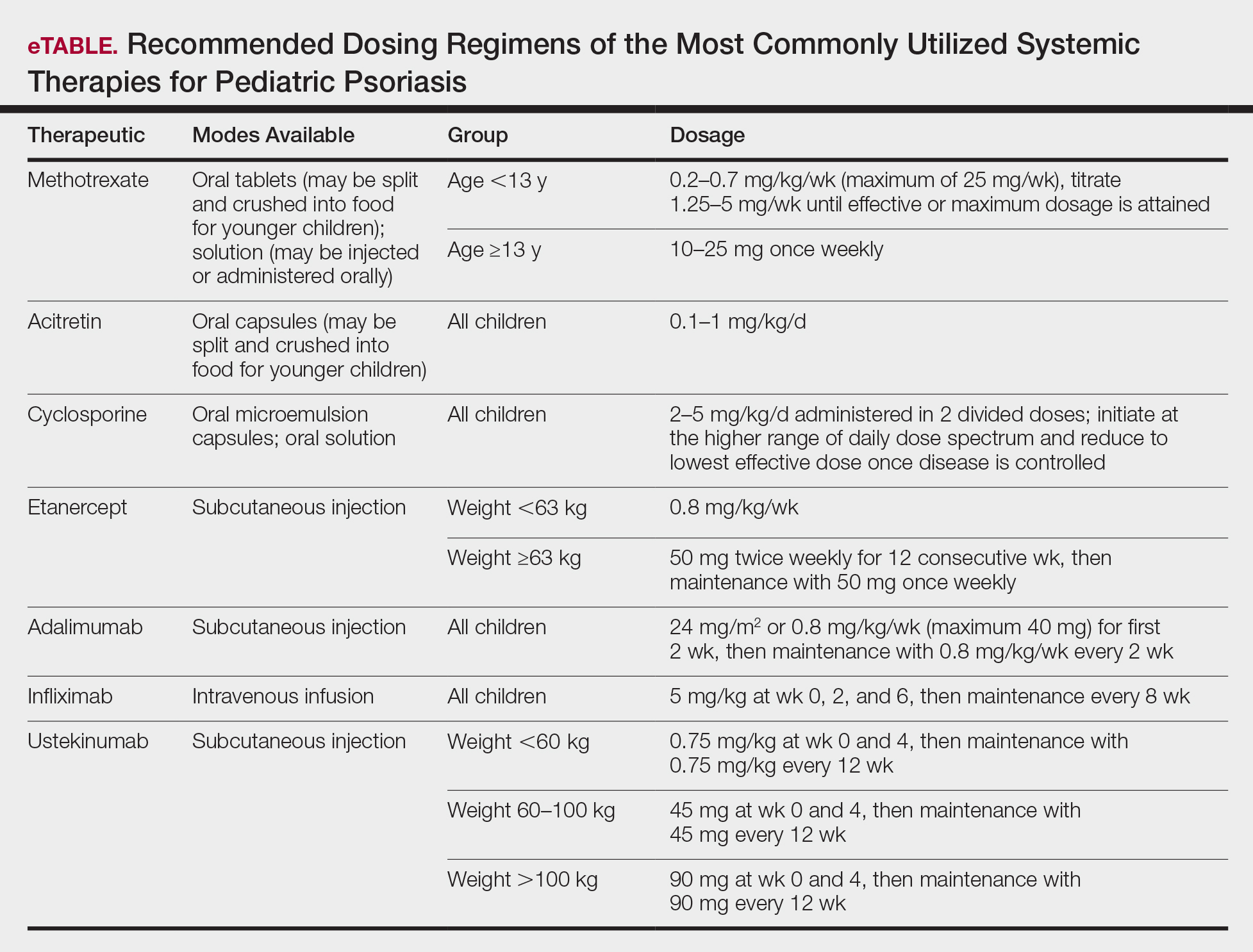
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
Practice Points
- For children, several environmental factors may prompt psoriasis flares, and it is critical to identify and eliminate these triggers.
- Although the use of biologics may be limited by cost and injection phobias in children, they may be an appropriate option for children with moderate to severe psoriasis when other therapies have failed. A growing body of literature is establishing the safety and effectiveness of biologics in children.
- Clinicians should thoroughly educate parents/ guardians on the course of psoriasis and treatment options as well as pay special attention to treatment goals and psychosocial factors that may guide decision-making regarding therapy.
Palmoplantar Eruption in a Patient With Mercury Poisoning
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.
An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.
Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.

An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.

Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.

An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.

Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Practice Points
- The dermatologic and histologic presentation of mercury exposure may be nonspecific, requiring a high degree of clinical suspicion to make a diagnosis.
- Mercury exposure should be included in the differential diagnosis in patients presenting with a rash of the palms and soles, especially in young patients with systemic symptoms.
Cystic fibrosis treatment: Triple combination benefits patients with advanced disease
New CFTR [cystic fibrosis transmembrane conductance regulator] modulator therapies can offer life-altering benefits to some patients with cystic fibrosis, even those with advanced disease.
, according to a multicenter analysis of patients taking elexacaftor, tezacaftor, and ivacaftor.
The study participants had a percent predicted forced expiratory volume in 1 second (ppFEV1) of 40% or below, or other high-risk factors. Researchers compared them to control patients who were genetically ineligible for triple combination therapy.
Previous studies of such patients on individual drugs or previous combinations showed increases in lung function in patients with advanced disease, though the magnitude of improvement varied across regimens. “With this improvement, it’s unclear how CFTR modulators should affect lung transplant referral timing,” Brent Bermingham, MD, said during a presentation of the study at the virtual North American Cystic Fibrosis Conference.
“The rationale for our study was that despite patients with advanced lung disease being excluded from phase III trials (of elexacaftor, tezacaftor, and ivacaftor), they are receiving a therapy with an unknown clinical efficacy and safety profile,” said Dr. Bermingham, a pulmonary and critical care fellow at the Medical University of South Carolina, Charleston.
Lung transplant referral guidelines recommend that physicians initiate discussions about the potential benefit of lung transplant when FEV1 drops below 50% of the predicted value. Patients should be referred for a transplant when the value is below 50% and rapidly declining (>20% decline in the past 12 months), when it drops below 40% with accompanying predictors of shortened survival, or when it drops below 30%. The guidelines were published before approval of triple combination therapy.
The researchers conducted an open-label retrospective analysis of 60 patients started on triple combination therapy between September 2019 and February 2020 at three centers in the Southeast. They compared percent predicted ppFEV1 values prior to initiation of therapy to ppFEV1 values obtained 2-12 weeks after the start of therapy. Patients on therapy were compared with 10 genetically ineligible controls. The two groups were generally similar aside from genetic status, though 100% of the therapy group had pancreatic insufficiency, compared with 90% of controls (P = .013).
The therapeutic group experienced a 7.8% increase in ppFEV1 after starting therapy (P < .001), compared with a 0.5% decrease in controls (P = .65). Before initiation of therapy, 33% of the therapy group met the criteria for initiating a transplant discussion, while 67% had been recommended for transplant. After therapy, 55% met the criteria for discussion, 33% were recommended for transplant, and 12% no longer met the criteria for discussion of transplantation. Fifty percent of controls were in discussion, and this dropped to 40%, while 50% were referred for transplantation, and this increased to 60%. On therapy, transplant referral candidates had an increase of forced vital capacity from 48.9 to 59.16 (P < .001).
Adverse events were rare, with only one discontinuation that occurred following a lung transplant and was not believed to be treatment related.
“Our study had a large number of patients taken from multiple centers, which suggests generalizabilty and real-world experience,” said Dr. Bermingham.
The results are encouraging, said Robert J. Giusti, MD, clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center.
“We’re all remarking how wonderful patients feel these days. It’s really a disease-altering treatment. But for the high-risk group, whose FEV1 is less than 40%, those are the patients we’re more concerned about because we thought maybe they had too much lung disease, and that they wouldn’t benefit from triple combination. But they seem to be improving, so that’s very reassuring,” said Dr. Giusti, who was not involved in the study.
The study received funding from the Cystic Fibrosis Foundation and Dartmouth College. Dr. Bermingham and Dr. Giusti have no relevant financial disclosures.
SOURCE: Bermingham B et al. NACFC 2020, Abstract 645.
New CFTR [cystic fibrosis transmembrane conductance regulator] modulator therapies can offer life-altering benefits to some patients with cystic fibrosis, even those with advanced disease.
, according to a multicenter analysis of patients taking elexacaftor, tezacaftor, and ivacaftor.
The study participants had a percent predicted forced expiratory volume in 1 second (ppFEV1) of 40% or below, or other high-risk factors. Researchers compared them to control patients who were genetically ineligible for triple combination therapy.
Previous studies of such patients on individual drugs or previous combinations showed increases in lung function in patients with advanced disease, though the magnitude of improvement varied across regimens. “With this improvement, it’s unclear how CFTR modulators should affect lung transplant referral timing,” Brent Bermingham, MD, said during a presentation of the study at the virtual North American Cystic Fibrosis Conference.
“The rationale for our study was that despite patients with advanced lung disease being excluded from phase III trials (of elexacaftor, tezacaftor, and ivacaftor), they are receiving a therapy with an unknown clinical efficacy and safety profile,” said Dr. Bermingham, a pulmonary and critical care fellow at the Medical University of South Carolina, Charleston.
Lung transplant referral guidelines recommend that physicians initiate discussions about the potential benefit of lung transplant when FEV1 drops below 50% of the predicted value. Patients should be referred for a transplant when the value is below 50% and rapidly declining (>20% decline in the past 12 months), when it drops below 40% with accompanying predictors of shortened survival, or when it drops below 30%. The guidelines were published before approval of triple combination therapy.
The researchers conducted an open-label retrospective analysis of 60 patients started on triple combination therapy between September 2019 and February 2020 at three centers in the Southeast. They compared percent predicted ppFEV1 values prior to initiation of therapy to ppFEV1 values obtained 2-12 weeks after the start of therapy. Patients on therapy were compared with 10 genetically ineligible controls. The two groups were generally similar aside from genetic status, though 100% of the therapy group had pancreatic insufficiency, compared with 90% of controls (P = .013).
The therapeutic group experienced a 7.8% increase in ppFEV1 after starting therapy (P < .001), compared with a 0.5% decrease in controls (P = .65). Before initiation of therapy, 33% of the therapy group met the criteria for initiating a transplant discussion, while 67% had been recommended for transplant. After therapy, 55% met the criteria for discussion, 33% were recommended for transplant, and 12% no longer met the criteria for discussion of transplantation. Fifty percent of controls were in discussion, and this dropped to 40%, while 50% were referred for transplantation, and this increased to 60%. On therapy, transplant referral candidates had an increase of forced vital capacity from 48.9 to 59.16 (P < .001).
Adverse events were rare, with only one discontinuation that occurred following a lung transplant and was not believed to be treatment related.
“Our study had a large number of patients taken from multiple centers, which suggests generalizabilty and real-world experience,” said Dr. Bermingham.
The results are encouraging, said Robert J. Giusti, MD, clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center.
“We’re all remarking how wonderful patients feel these days. It’s really a disease-altering treatment. But for the high-risk group, whose FEV1 is less than 40%, those are the patients we’re more concerned about because we thought maybe they had too much lung disease, and that they wouldn’t benefit from triple combination. But they seem to be improving, so that’s very reassuring,” said Dr. Giusti, who was not involved in the study.
The study received funding from the Cystic Fibrosis Foundation and Dartmouth College. Dr. Bermingham and Dr. Giusti have no relevant financial disclosures.
SOURCE: Bermingham B et al. NACFC 2020, Abstract 645.
New CFTR [cystic fibrosis transmembrane conductance regulator] modulator therapies can offer life-altering benefits to some patients with cystic fibrosis, even those with advanced disease.
, according to a multicenter analysis of patients taking elexacaftor, tezacaftor, and ivacaftor.
The study participants had a percent predicted forced expiratory volume in 1 second (ppFEV1) of 40% or below, or other high-risk factors. Researchers compared them to control patients who were genetically ineligible for triple combination therapy.
Previous studies of such patients on individual drugs or previous combinations showed increases in lung function in patients with advanced disease, though the magnitude of improvement varied across regimens. “With this improvement, it’s unclear how CFTR modulators should affect lung transplant referral timing,” Brent Bermingham, MD, said during a presentation of the study at the virtual North American Cystic Fibrosis Conference.
“The rationale for our study was that despite patients with advanced lung disease being excluded from phase III trials (of elexacaftor, tezacaftor, and ivacaftor), they are receiving a therapy with an unknown clinical efficacy and safety profile,” said Dr. Bermingham, a pulmonary and critical care fellow at the Medical University of South Carolina, Charleston.
Lung transplant referral guidelines recommend that physicians initiate discussions about the potential benefit of lung transplant when FEV1 drops below 50% of the predicted value. Patients should be referred for a transplant when the value is below 50% and rapidly declining (>20% decline in the past 12 months), when it drops below 40% with accompanying predictors of shortened survival, or when it drops below 30%. The guidelines were published before approval of triple combination therapy.
The researchers conducted an open-label retrospective analysis of 60 patients started on triple combination therapy between September 2019 and February 2020 at three centers in the Southeast. They compared percent predicted ppFEV1 values prior to initiation of therapy to ppFEV1 values obtained 2-12 weeks after the start of therapy. Patients on therapy were compared with 10 genetically ineligible controls. The two groups were generally similar aside from genetic status, though 100% of the therapy group had pancreatic insufficiency, compared with 90% of controls (P = .013).
The therapeutic group experienced a 7.8% increase in ppFEV1 after starting therapy (P < .001), compared with a 0.5% decrease in controls (P = .65). Before initiation of therapy, 33% of the therapy group met the criteria for initiating a transplant discussion, while 67% had been recommended for transplant. After therapy, 55% met the criteria for discussion, 33% were recommended for transplant, and 12% no longer met the criteria for discussion of transplantation. Fifty percent of controls were in discussion, and this dropped to 40%, while 50% were referred for transplantation, and this increased to 60%. On therapy, transplant referral candidates had an increase of forced vital capacity from 48.9 to 59.16 (P < .001).
Adverse events were rare, with only one discontinuation that occurred following a lung transplant and was not believed to be treatment related.
“Our study had a large number of patients taken from multiple centers, which suggests generalizabilty and real-world experience,” said Dr. Bermingham.
The results are encouraging, said Robert J. Giusti, MD, clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center.
“We’re all remarking how wonderful patients feel these days. It’s really a disease-altering treatment. But for the high-risk group, whose FEV1 is less than 40%, those are the patients we’re more concerned about because we thought maybe they had too much lung disease, and that they wouldn’t benefit from triple combination. But they seem to be improving, so that’s very reassuring,” said Dr. Giusti, who was not involved in the study.
The study received funding from the Cystic Fibrosis Foundation and Dartmouth College. Dr. Bermingham and Dr. Giusti have no relevant financial disclosures.
SOURCE: Bermingham B et al. NACFC 2020, Abstract 645.
FROM NACFC 2020
What’s Eating You? Human Flea (Pulex irritans)
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.

Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.

Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Practice Points
- The human flea, Pulex irritans, is a vector for various human diseases including the bubonic plague, bartonellosis, and rickettsioses.
- Presenting symptoms of flea bites include intensely pruritic, urticarial to vesicular papules on exposed areas of skin.
- The primary method of flea control includes a combination of insecticidal products and insect growth regulators.