User login
Intraoperative Tissue Expansion to Allow Primary Linear Closure of 2 Large Adjacent Surgical Defects
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
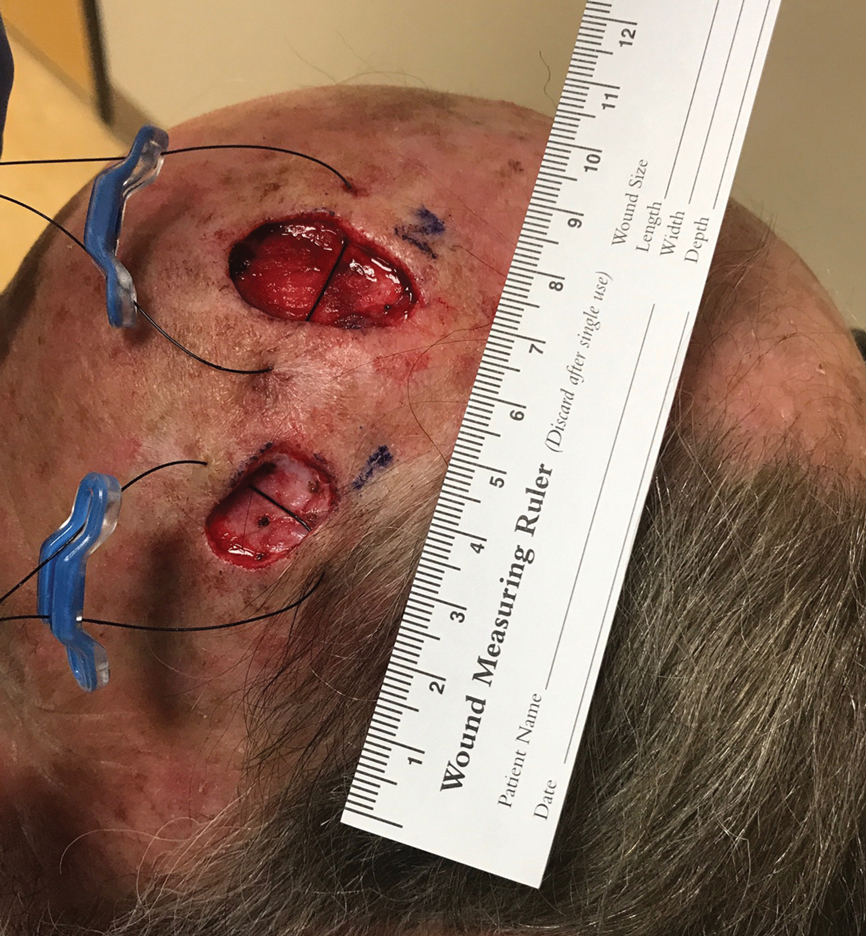
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

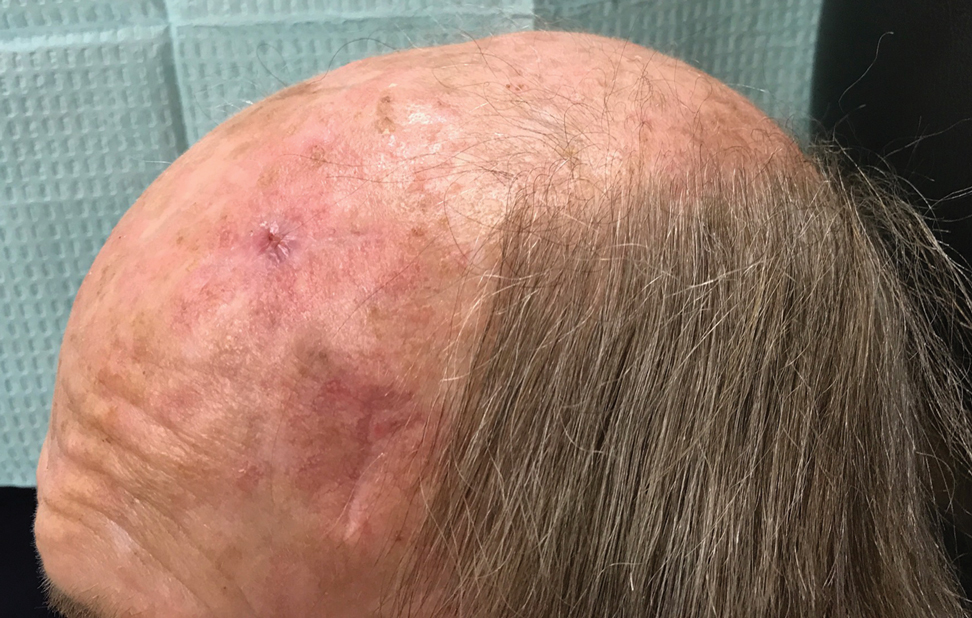
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3

After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).

Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3

After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.

The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).

Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
The Gips Procedure for Pilonidal Disease: A Retrospective Review of Adolescent Patients
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
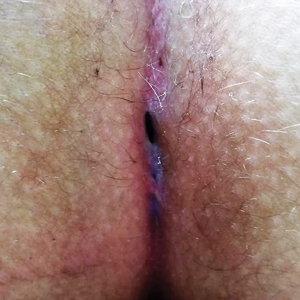
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
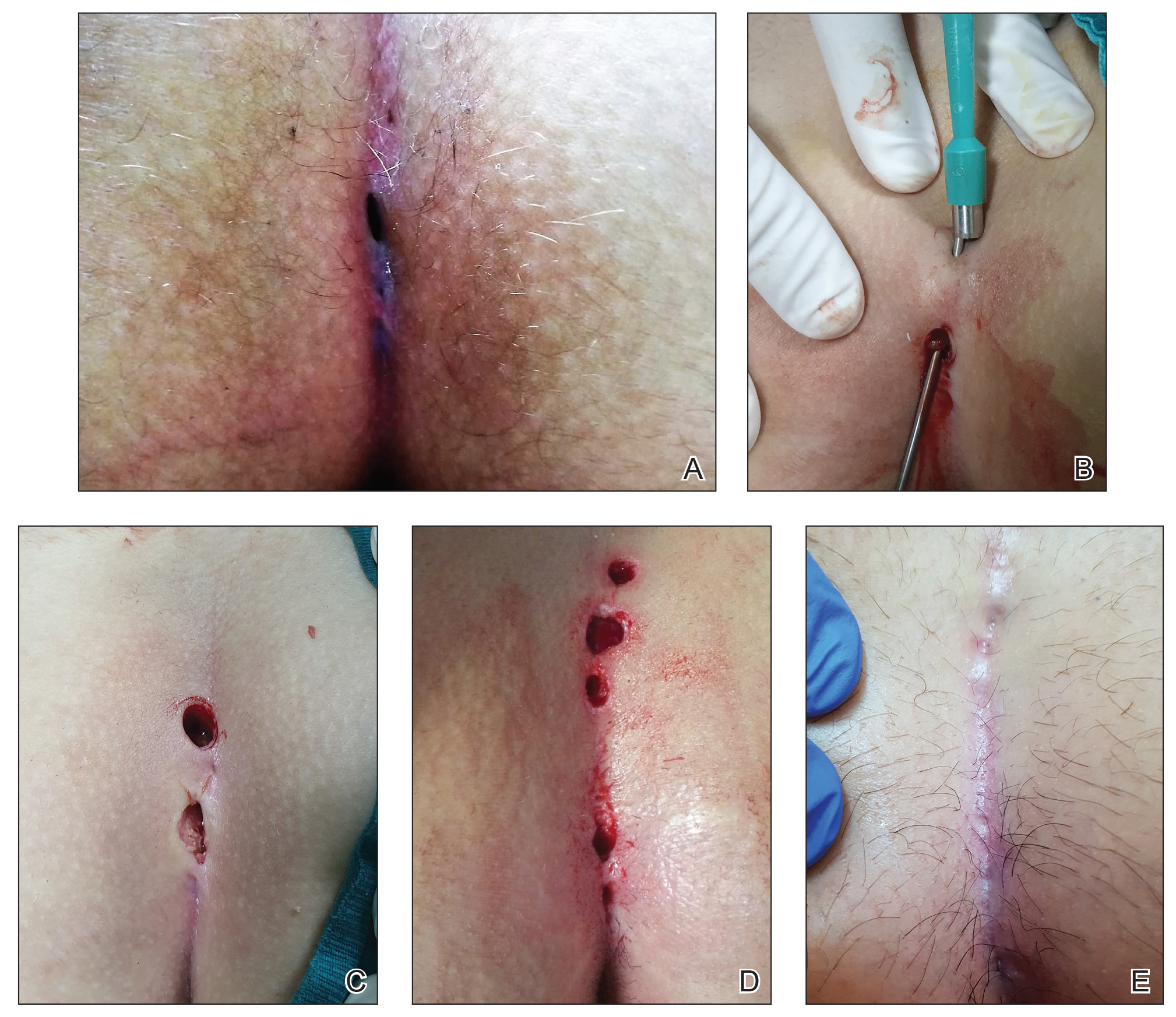
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.

Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.

Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Practice Points
- The Gips procedure is an easy-to-use outpatient procedure for adolescents with pilonidal disease.
- This procedure has a high success rate and does not restrict school or social activities.
FIT unfit for inpatient, emergency settings
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Biologics in Pediatric Psoriasis and Atopic Dermatitis: Revolutionizing the Treatment Landscape
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.
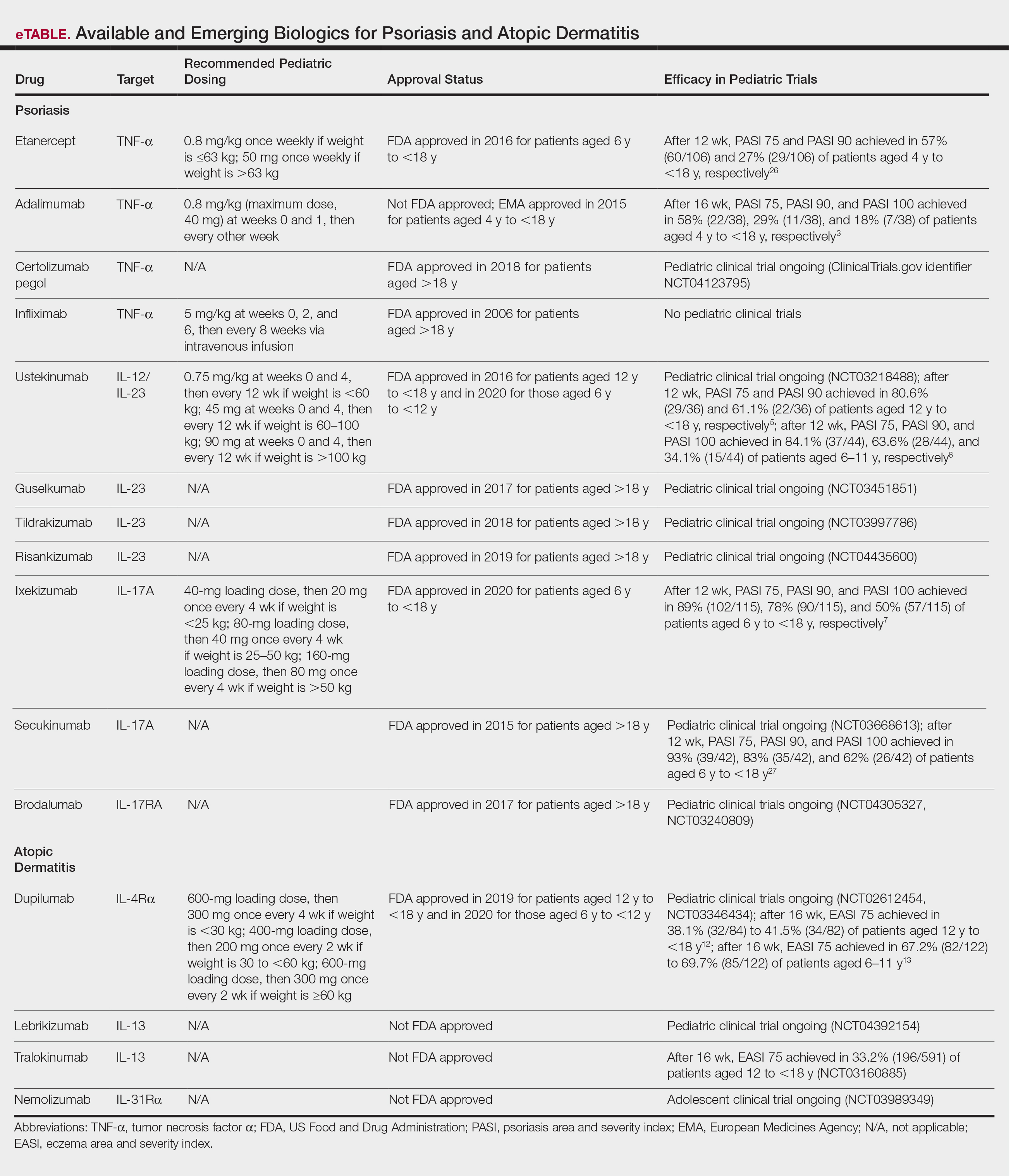
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.

Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.

- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Aging with HIV adds to comorbidity burden
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
FROM IDWEEK 2020
Biometric changes on fitness trackers, smartwatches detect COVID-19
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
Adding cetuximab to afatinib provides no benefit in EGFR-mutant NSCLC
In addition, toxicity was greater with the afatinib/cetuximab combination, according to study author Sarah S. Goldberg, MD, of Yale University, New Haven, Conn.
Dr. Goldberg and colleagues reported these results, from the SWOG S1403 trial, in the Journal of Clinical Oncology.
The authors noted that activating EGFR mutations are present in about 15% of patients with lung adenocarcinomas in Western populations and the mutations confer heightened sensitivity to EGFR tyrosine kinase inhibitors (TKIs). EGFR-TKIs have been shown to improve clinical outcomes, quality of life, and toxicity when compared with chemotherapy.
Based on better outcomes over chemotherapy, the third-generation EGFR-TKI osimertinib is now the standard treatment for patients with T790M-mediated resistance, but osimertinib is not effective in TKI-resistant T790M-negative disease, the authors pointed out.
In a phase 1b trial of patients with EGFR-mutant NSCLC with acquired resistance to first-generation agents, afatinib/cetuximab produced a response rate of 29% and comparable activity regardless of T790M status.
The aim of the SWOG S1403 study was to test whether adding cetuximab to afatinib would improve PFS over afatinib alone in patients with treatment-naive, EGFR-mutant NSCLC by preventing or delaying resistance.
Trial details
The phase 2, multicenter trial included 168 eligible patients with EGFR-mutant NSCLC without prior treatment of advanced disease. The patients’ median age was 66 years (range, 27-93 years), and 66% were women.
The most common histology was adenocarcinoma (96%). EGFR exon 19 deletions were detected in 64% of patients and L858R point mutations in 36%.
Patients were randomly assigned 2:1 to receive afatinib at 40 mg orally daily plus cetuximab at 500 mg/m2 intravenously every 2 weeks or afatinib at 40 mg alone. Patients received diphenhydramine at 50 mg intravenously before the first dose of cetuximab to prevent hypersensitivity reaction, and it was recommended before subsequent doses.
Patients continued on treatment until disease progression, symptomatic deterioration, unacceptable toxicity, pregnancy, treatment delay greater than 28 days, or patient decision. The study’s primary endpoint was PFS.
Further accrual not supported
At the interim analysis, the SWOG data safety and monitoring committee decided there was insufficient evidence to support further accrual, and the trial was closed.
The primary endpoint analysis revealed a median PFS of 11.9 months in the afatinib/cetuximab group and 13.4 months in the afatinib-alone group (hazard ratio, 1.01; 95% confidence interval, 0.72-1.43; P = .94). A subset analysis showed no PFS differences based on clinical or tumor characteristics.
Overall survival, time to response, and overall response rate were not improved in the afatinib/cetuximab arm.
PFS and overall survival were longer in patients with tumors harboring exon 19 deletions than in patients with L858R mutations. However, there were no mutation subtype–based PFS and overall survival differences between the treatment arms.
Grade 3 or higher treatment-related adverse events were more common in the combination arm than in the monotherapy arm (72% and 40%, respectively; P < .0001).
The most common grade 3 or higher treatment-related adverse events (in the combination and monotherapy arms, respectively) were acneiform rash (27% and 2%), maculopapular rash (13% and 0%), and diarrhea (15% and 20%).
Patients receiving afatinib plus cetuximab required dose reductions more often (56.7% vs. 26.2%), and treatment discontinuation because of an adverse event was more frequent in the combination arm (14% vs. 11%).
Turn toward third-generation drug
“Treatment with a single-agent EGFR-TKI remains the standard of care for patients with EGFR-mutant NSCLC,” Dr. Goldberg and colleagues wrote.
Why the combination of afatinib and cetuximab, which has demonstrated activity in the resistance setting, failed in the first-line setting remains unclear, Dr. Goldberg observed in an interview.
She noted that about one-quarter of patients receiving the afatinib/cetuximab combination discontinued cetuximab because of toxicity.
“That’s a good amount. It could be part of the explanation,” Dr. Goldberg said.
Investigators are currently analyzing collected tissue and blood samples in an effort to identify biomarkers that could potentially predict subgroups receiving benefit.
“That’s our next step: looking at specific EGFR mutation types, comutation types, and amplification of other genes and of EGFR,” Dr. Goldberg said.
She noted that, because of a superior side-effect profile and possibly greater efficacy, osimertinib is being used in the first-line setting.
“So now the idea of combining an EGFR-TKI with an EGFR antibody is still an active area of research, but with osimertinib rather than a first- or second-generation drug,” she said.
This research was supported by Boehringer Ingelheim, Eli Lilly, grants from the National Institutes of Health/National Cancer Institute, The Hope Foundation Career Development Award, and the SWOG and NIH Yale SPORE in Lung Cancer Grant. Dr. Goldberg and colleagues disclosed relationships with many pharmaceutical companies.
SOURCE: Goldberg SB et al. J Clin Oncol. 2020 Oct 6. doi: 10.1200/JCO.20.01149.
In addition, toxicity was greater with the afatinib/cetuximab combination, according to study author Sarah S. Goldberg, MD, of Yale University, New Haven, Conn.
Dr. Goldberg and colleagues reported these results, from the SWOG S1403 trial, in the Journal of Clinical Oncology.
The authors noted that activating EGFR mutations are present in about 15% of patients with lung adenocarcinomas in Western populations and the mutations confer heightened sensitivity to EGFR tyrosine kinase inhibitors (TKIs). EGFR-TKIs have been shown to improve clinical outcomes, quality of life, and toxicity when compared with chemotherapy.
Based on better outcomes over chemotherapy, the third-generation EGFR-TKI osimertinib is now the standard treatment for patients with T790M-mediated resistance, but osimertinib is not effective in TKI-resistant T790M-negative disease, the authors pointed out.
In a phase 1b trial of patients with EGFR-mutant NSCLC with acquired resistance to first-generation agents, afatinib/cetuximab produced a response rate of 29% and comparable activity regardless of T790M status.
The aim of the SWOG S1403 study was to test whether adding cetuximab to afatinib would improve PFS over afatinib alone in patients with treatment-naive, EGFR-mutant NSCLC by preventing or delaying resistance.
Trial details
The phase 2, multicenter trial included 168 eligible patients with EGFR-mutant NSCLC without prior treatment of advanced disease. The patients’ median age was 66 years (range, 27-93 years), and 66% were women.
The most common histology was adenocarcinoma (96%). EGFR exon 19 deletions were detected in 64% of patients and L858R point mutations in 36%.
Patients were randomly assigned 2:1 to receive afatinib at 40 mg orally daily plus cetuximab at 500 mg/m2 intravenously every 2 weeks or afatinib at 40 mg alone. Patients received diphenhydramine at 50 mg intravenously before the first dose of cetuximab to prevent hypersensitivity reaction, and it was recommended before subsequent doses.
Patients continued on treatment until disease progression, symptomatic deterioration, unacceptable toxicity, pregnancy, treatment delay greater than 28 days, or patient decision. The study’s primary endpoint was PFS.
Further accrual not supported
At the interim analysis, the SWOG data safety and monitoring committee decided there was insufficient evidence to support further accrual, and the trial was closed.
The primary endpoint analysis revealed a median PFS of 11.9 months in the afatinib/cetuximab group and 13.4 months in the afatinib-alone group (hazard ratio, 1.01; 95% confidence interval, 0.72-1.43; P = .94). A subset analysis showed no PFS differences based on clinical or tumor characteristics.
Overall survival, time to response, and overall response rate were not improved in the afatinib/cetuximab arm.
PFS and overall survival were longer in patients with tumors harboring exon 19 deletions than in patients with L858R mutations. However, there were no mutation subtype–based PFS and overall survival differences between the treatment arms.
Grade 3 or higher treatment-related adverse events were more common in the combination arm than in the monotherapy arm (72% and 40%, respectively; P < .0001).
The most common grade 3 or higher treatment-related adverse events (in the combination and monotherapy arms, respectively) were acneiform rash (27% and 2%), maculopapular rash (13% and 0%), and diarrhea (15% and 20%).
Patients receiving afatinib plus cetuximab required dose reductions more often (56.7% vs. 26.2%), and treatment discontinuation because of an adverse event was more frequent in the combination arm (14% vs. 11%).
Turn toward third-generation drug
“Treatment with a single-agent EGFR-TKI remains the standard of care for patients with EGFR-mutant NSCLC,” Dr. Goldberg and colleagues wrote.
Why the combination of afatinib and cetuximab, which has demonstrated activity in the resistance setting, failed in the first-line setting remains unclear, Dr. Goldberg observed in an interview.
She noted that about one-quarter of patients receiving the afatinib/cetuximab combination discontinued cetuximab because of toxicity.
“That’s a good amount. It could be part of the explanation,” Dr. Goldberg said.
Investigators are currently analyzing collected tissue and blood samples in an effort to identify biomarkers that could potentially predict subgroups receiving benefit.
“That’s our next step: looking at specific EGFR mutation types, comutation types, and amplification of other genes and of EGFR,” Dr. Goldberg said.
She noted that, because of a superior side-effect profile and possibly greater efficacy, osimertinib is being used in the first-line setting.
“So now the idea of combining an EGFR-TKI with an EGFR antibody is still an active area of research, but with osimertinib rather than a first- or second-generation drug,” she said.
This research was supported by Boehringer Ingelheim, Eli Lilly, grants from the National Institutes of Health/National Cancer Institute, The Hope Foundation Career Development Award, and the SWOG and NIH Yale SPORE in Lung Cancer Grant. Dr. Goldberg and colleagues disclosed relationships with many pharmaceutical companies.
SOURCE: Goldberg SB et al. J Clin Oncol. 2020 Oct 6. doi: 10.1200/JCO.20.01149.
In addition, toxicity was greater with the afatinib/cetuximab combination, according to study author Sarah S. Goldberg, MD, of Yale University, New Haven, Conn.
Dr. Goldberg and colleagues reported these results, from the SWOG S1403 trial, in the Journal of Clinical Oncology.
The authors noted that activating EGFR mutations are present in about 15% of patients with lung adenocarcinomas in Western populations and the mutations confer heightened sensitivity to EGFR tyrosine kinase inhibitors (TKIs). EGFR-TKIs have been shown to improve clinical outcomes, quality of life, and toxicity when compared with chemotherapy.
Based on better outcomes over chemotherapy, the third-generation EGFR-TKI osimertinib is now the standard treatment for patients with T790M-mediated resistance, but osimertinib is not effective in TKI-resistant T790M-negative disease, the authors pointed out.
In a phase 1b trial of patients with EGFR-mutant NSCLC with acquired resistance to first-generation agents, afatinib/cetuximab produced a response rate of 29% and comparable activity regardless of T790M status.
The aim of the SWOG S1403 study was to test whether adding cetuximab to afatinib would improve PFS over afatinib alone in patients with treatment-naive, EGFR-mutant NSCLC by preventing or delaying resistance.
Trial details
The phase 2, multicenter trial included 168 eligible patients with EGFR-mutant NSCLC without prior treatment of advanced disease. The patients’ median age was 66 years (range, 27-93 years), and 66% were women.
The most common histology was adenocarcinoma (96%). EGFR exon 19 deletions were detected in 64% of patients and L858R point mutations in 36%.
Patients were randomly assigned 2:1 to receive afatinib at 40 mg orally daily plus cetuximab at 500 mg/m2 intravenously every 2 weeks or afatinib at 40 mg alone. Patients received diphenhydramine at 50 mg intravenously before the first dose of cetuximab to prevent hypersensitivity reaction, and it was recommended before subsequent doses.
Patients continued on treatment until disease progression, symptomatic deterioration, unacceptable toxicity, pregnancy, treatment delay greater than 28 days, or patient decision. The study’s primary endpoint was PFS.
Further accrual not supported
At the interim analysis, the SWOG data safety and monitoring committee decided there was insufficient evidence to support further accrual, and the trial was closed.
The primary endpoint analysis revealed a median PFS of 11.9 months in the afatinib/cetuximab group and 13.4 months in the afatinib-alone group (hazard ratio, 1.01; 95% confidence interval, 0.72-1.43; P = .94). A subset analysis showed no PFS differences based on clinical or tumor characteristics.
Overall survival, time to response, and overall response rate were not improved in the afatinib/cetuximab arm.
PFS and overall survival were longer in patients with tumors harboring exon 19 deletions than in patients with L858R mutations. However, there were no mutation subtype–based PFS and overall survival differences between the treatment arms.
Grade 3 or higher treatment-related adverse events were more common in the combination arm than in the monotherapy arm (72% and 40%, respectively; P < .0001).
The most common grade 3 or higher treatment-related adverse events (in the combination and monotherapy arms, respectively) were acneiform rash (27% and 2%), maculopapular rash (13% and 0%), and diarrhea (15% and 20%).
Patients receiving afatinib plus cetuximab required dose reductions more often (56.7% vs. 26.2%), and treatment discontinuation because of an adverse event was more frequent in the combination arm (14% vs. 11%).
Turn toward third-generation drug
“Treatment with a single-agent EGFR-TKI remains the standard of care for patients with EGFR-mutant NSCLC,” Dr. Goldberg and colleagues wrote.
Why the combination of afatinib and cetuximab, which has demonstrated activity in the resistance setting, failed in the first-line setting remains unclear, Dr. Goldberg observed in an interview.
She noted that about one-quarter of patients receiving the afatinib/cetuximab combination discontinued cetuximab because of toxicity.
“That’s a good amount. It could be part of the explanation,” Dr. Goldberg said.
Investigators are currently analyzing collected tissue and blood samples in an effort to identify biomarkers that could potentially predict subgroups receiving benefit.
“That’s our next step: looking at specific EGFR mutation types, comutation types, and amplification of other genes and of EGFR,” Dr. Goldberg said.
She noted that, because of a superior side-effect profile and possibly greater efficacy, osimertinib is being used in the first-line setting.
“So now the idea of combining an EGFR-TKI with an EGFR antibody is still an active area of research, but with osimertinib rather than a first- or second-generation drug,” she said.
This research was supported by Boehringer Ingelheim, Eli Lilly, grants from the National Institutes of Health/National Cancer Institute, The Hope Foundation Career Development Award, and the SWOG and NIH Yale SPORE in Lung Cancer Grant. Dr. Goldberg and colleagues disclosed relationships with many pharmaceutical companies.
SOURCE: Goldberg SB et al. J Clin Oncol. 2020 Oct 6. doi: 10.1200/JCO.20.01149.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Primary care workforce expanding, but mostly in cities
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Arcuate eruption on the back
A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.

A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
Black patients less likely to receive H. pylori eradication testing
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
FROM ACG 2020