User login
Increasing ear pain and headache
A previously healthy 12-year-old boy with normal development presented to his primary care physician (PCP) with a 1-week history of moderate ear pain. He was given a diagnosis of acute otitis media (AOM) and prescribed a 7-day course of amoxicillin. Although the child’s history was otherwise unremarkable, the mother reported that she’d had a deep venous thrombosis and pulmonary embolism a year earlier.
The boy continued to experience intermittent left ear pain 2 weeks after completing his antibiotic treatment, leading the PCP to refer him to an otolaryngologist. An examination by the otolaryngologist revealed a cloudy, bulging tympanic membrane. The patient was prescribed amoxicillin/clavulanate and ofloxacin ear drops.
Two days later, he was admitted to the emergency department (ED) due to worsening left ear pain and a new-onset left-sided headache. His left tympanic membrane was normal, with no tenderness or erythema of the mastoid. His vital signs were normal. He was afebrile and discharged home.
A week later, he returned to the ED with worsening ear pain and severe persistent headache, which was localized in the left occipital, left frontal, and retro-orbital regions. He denied light or sound sensitivity, nausea, vomiting, or increased lacrimation. He was tearful on examination due to the pain. He had no meningismus and normal fundi. A neurologic examination was nonlateralizing. Laboratory tests showed a normal complete blood count but increased C-reactive protein at 113 mg/dL (normal, < 0.3 mg/dL) and an erythrocyte sedimentation rate of 88 mm/hr (normal, 0-20 mm/hr).
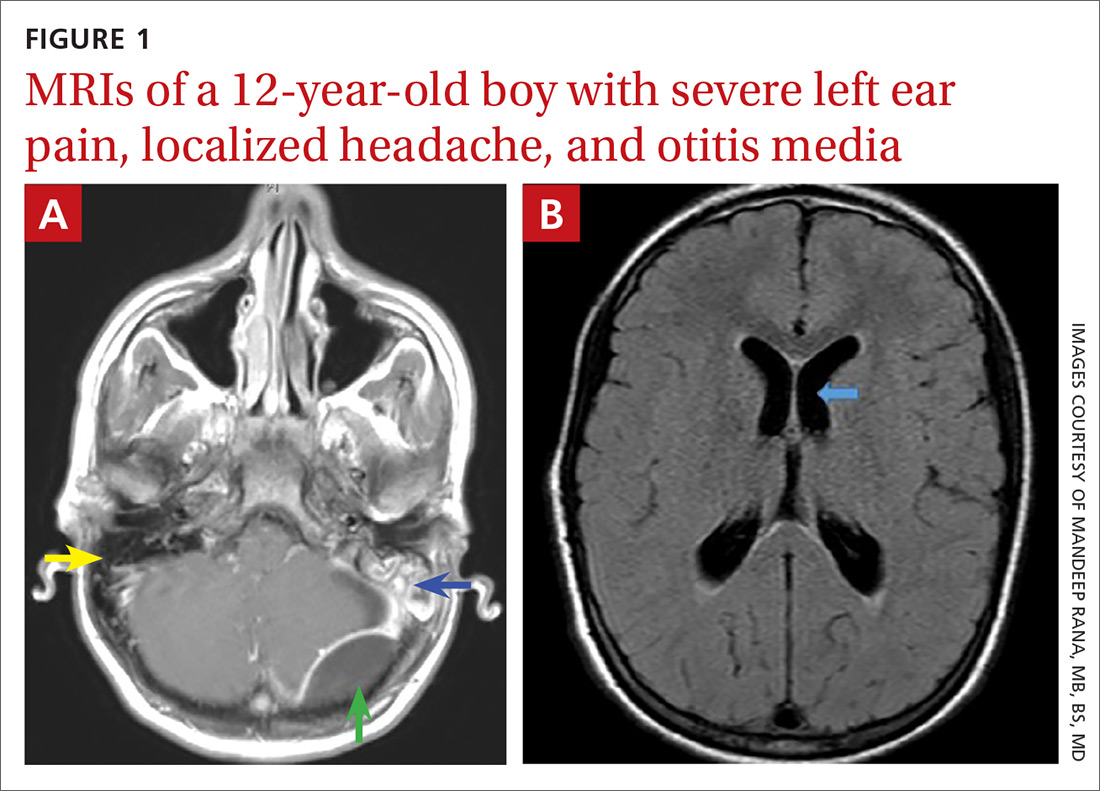
Magnetic resonance imaging was ordered (FIGURES 1A and 1B), and Neurosurgery and Otolaryngology were consulted.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Acute mastoiditis with epidural abscess
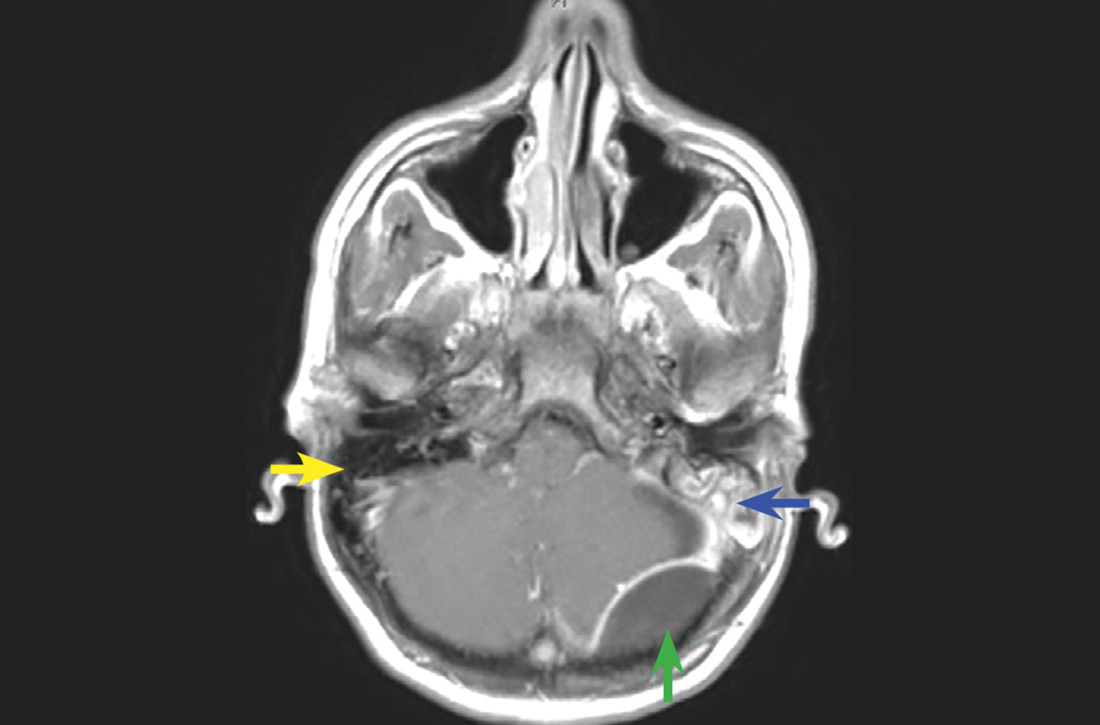
The contrast-enhanced cranial MRI scan (FIGURE 1A) revealed a case of acute mastoiditis with fluid in the left mastoid (blue arrow) and a large epidural abscess in the left posterior fossa (green arrow). The normal right mastoid was air-filled (yellow arrow). The T2-weighted MRI scan (FIGURE 1B) showed mild dilatation of the lateral ventricles (blue arrow) secondary to compression on the fourth ventricle by mass effect from the epidural abscess.
Acute mastoiditis—a complication of AOM—is an inflammatory process of mastoid air cells, which are contiguous to the middle ear cleft. In one large study of 61,783 inpatient children admitted with AOM, acute mastoiditis was reported as the most common complication in 1505 (2.4%) of the cases.1 The 2000-2012 national estimated incidence rate of pediatric mastoiditis has ranged from a high of 2.7 per 100,000 population in 2006 to a low of 1.8 per 100,000 in 2012.2 Clinical features of mastoiditis include localized mastoid tenderness, swelling, erythema, fluctuance, protrusion of the auricle, and ear pain.3
The clinical presentation of epidural abscess can be subtle with fever, headache, neck pain, and changes in mental status developing over several days.1 Focal deficits and seizures are relatively uncommon. In a review of 308 children with acute mastoiditis (3 with an epidural abscess), high-grade fever and high absolute neutrophil count and C-reactive protein levels were associated with the development of complications of mastoiditis, including hearing loss, sinus venous thrombosis, intracranial abscess, and cranial nerve palsies.4
Venous sinus thrombosis was part of the differential
When we were caring for this patient, the differential diagnosis included a cranial extension of AOM. Venous sinus thrombosis was also considered, given the family history of a hypercoagulable state. The patient did not have any features suggesting primary headache syndromes, such as migraine, tension type, or cluster headache.
The differential for a patient complaining of ear pain also includes postauricular lymphadenopathy, mumps, periauricular cellulitis (with and without otitis externa), perichondritis of the auricle, and tumors involving the mastoid bone.4
Continue to: Imaging and treatment
Imaging and treatment
Imaging of temporal bone is not recommended to make a diagnosis of mastoiditis in children with characteristic clinical findings. When imaging is needed, contrast-enhanced computed tomography (CT) is best to help visualize changes in temporal bone. If intracranial complications are suspected, cranial MRI with contrast or cranial CT with contrast can be ordered (depending on availability).5
Conservative management with intravenous antimicrobial therapy and middle ear drainage with myringotomy is indicated for a child with uncomplicated acute or subacute mastoiditis. For patients with suppurative extracranial or intracranial complications, aggressive surgical management is needed.5
Treatment for this patient included craniotomy, evacuation of the epidural abscess, and mastoidectomy. A culture obtained from the abscess showed Streptococcus intermedius. He was treated with broad-spectrum antibiotics, including ceftriaxone, vancomycin, and metronidazole. Within a week of surgery, he was discharged from the hospital and continued antibiotic treatment for 6 weeks via a peripherally inserted central catheter line.
1. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg. 2016;154:366-370.
2. King LM, Bartoces M, Hersh AL, et al. National incidence of pediatric mastoiditis in the United States, 2000-2012: creating a baseline for public health surveillance. Pediatr Infect Dis J. 2019;38:e14-e16.
3. Pang LH, Barakate MS, Havas TE. Mastoiditis in a paediatric population: a review of 11 years’ experience in management. Int J Pediatr Otorhinolaryngol. 2009;73:1520.
4. Bilavsky E, Yarden-Bilavsky H, Samra Z, et al. Clinical, laboratory, and microbiological differences between children with simple or complicated mastoiditis. Int J Pediatr Otorhinolaryngol. 2009;73:1270-1273.
5. Chesney J, Black A, Choo D. What is the best practice for acute mastoiditis in children? Laryngoscope. 2014;124:1057-1059.
A previously healthy 12-year-old boy with normal development presented to his primary care physician (PCP) with a 1-week history of moderate ear pain. He was given a diagnosis of acute otitis media (AOM) and prescribed a 7-day course of amoxicillin. Although the child’s history was otherwise unremarkable, the mother reported that she’d had a deep venous thrombosis and pulmonary embolism a year earlier.
The boy continued to experience intermittent left ear pain 2 weeks after completing his antibiotic treatment, leading the PCP to refer him to an otolaryngologist. An examination by the otolaryngologist revealed a cloudy, bulging tympanic membrane. The patient was prescribed amoxicillin/clavulanate and ofloxacin ear drops.
Two days later, he was admitted to the emergency department (ED) due to worsening left ear pain and a new-onset left-sided headache. His left tympanic membrane was normal, with no tenderness or erythema of the mastoid. His vital signs were normal. He was afebrile and discharged home.
A week later, he returned to the ED with worsening ear pain and severe persistent headache, which was localized in the left occipital, left frontal, and retro-orbital regions. He denied light or sound sensitivity, nausea, vomiting, or increased lacrimation. He was tearful on examination due to the pain. He had no meningismus and normal fundi. A neurologic examination was nonlateralizing. Laboratory tests showed a normal complete blood count but increased C-reactive protein at 113 mg/dL (normal, < 0.3 mg/dL) and an erythrocyte sedimentation rate of 88 mm/hr (normal, 0-20 mm/hr).
Magnetic resonance imaging was ordered (FIGURES 1A and 1B), and Neurosurgery and Otolaryngology were consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Acute mastoiditis with epidural abscess
The contrast-enhanced cranial MRI scan (FIGURE 1A) revealed a case of acute mastoiditis with fluid in the left mastoid (blue arrow) and a large epidural abscess in the left posterior fossa (green arrow). The normal right mastoid was air-filled (yellow arrow). The T2-weighted MRI scan (FIGURE 1B) showed mild dilatation of the lateral ventricles (blue arrow) secondary to compression on the fourth ventricle by mass effect from the epidural abscess.
Acute mastoiditis—a complication of AOM—is an inflammatory process of mastoid air cells, which are contiguous to the middle ear cleft. In one large study of 61,783 inpatient children admitted with AOM, acute mastoiditis was reported as the most common complication in 1505 (2.4%) of the cases.1 The 2000-2012 national estimated incidence rate of pediatric mastoiditis has ranged from a high of 2.7 per 100,000 population in 2006 to a low of 1.8 per 100,000 in 2012.2 Clinical features of mastoiditis include localized mastoid tenderness, swelling, erythema, fluctuance, protrusion of the auricle, and ear pain.3
The clinical presentation of epidural abscess can be subtle with fever, headache, neck pain, and changes in mental status developing over several days.1 Focal deficits and seizures are relatively uncommon. In a review of 308 children with acute mastoiditis (3 with an epidural abscess), high-grade fever and high absolute neutrophil count and C-reactive protein levels were associated with the development of complications of mastoiditis, including hearing loss, sinus venous thrombosis, intracranial abscess, and cranial nerve palsies.4
Venous sinus thrombosis was part of the differential
When we were caring for this patient, the differential diagnosis included a cranial extension of AOM. Venous sinus thrombosis was also considered, given the family history of a hypercoagulable state. The patient did not have any features suggesting primary headache syndromes, such as migraine, tension type, or cluster headache.
The differential for a patient complaining of ear pain also includes postauricular lymphadenopathy, mumps, periauricular cellulitis (with and without otitis externa), perichondritis of the auricle, and tumors involving the mastoid bone.4
Continue to: Imaging and treatment
Imaging and treatment
Imaging of temporal bone is not recommended to make a diagnosis of mastoiditis in children with characteristic clinical findings. When imaging is needed, contrast-enhanced computed tomography (CT) is best to help visualize changes in temporal bone. If intracranial complications are suspected, cranial MRI with contrast or cranial CT with contrast can be ordered (depending on availability).5
Conservative management with intravenous antimicrobial therapy and middle ear drainage with myringotomy is indicated for a child with uncomplicated acute or subacute mastoiditis. For patients with suppurative extracranial or intracranial complications, aggressive surgical management is needed.5
Treatment for this patient included craniotomy, evacuation of the epidural abscess, and mastoidectomy. A culture obtained from the abscess showed Streptococcus intermedius. He was treated with broad-spectrum antibiotics, including ceftriaxone, vancomycin, and metronidazole. Within a week of surgery, he was discharged from the hospital and continued antibiotic treatment for 6 weeks via a peripherally inserted central catheter line.
A previously healthy 12-year-old boy with normal development presented to his primary care physician (PCP) with a 1-week history of moderate ear pain. He was given a diagnosis of acute otitis media (AOM) and prescribed a 7-day course of amoxicillin. Although the child’s history was otherwise unremarkable, the mother reported that she’d had a deep venous thrombosis and pulmonary embolism a year earlier.
The boy continued to experience intermittent left ear pain 2 weeks after completing his antibiotic treatment, leading the PCP to refer him to an otolaryngologist. An examination by the otolaryngologist revealed a cloudy, bulging tympanic membrane. The patient was prescribed amoxicillin/clavulanate and ofloxacin ear drops.
Two days later, he was admitted to the emergency department (ED) due to worsening left ear pain and a new-onset left-sided headache. His left tympanic membrane was normal, with no tenderness or erythema of the mastoid. His vital signs were normal. He was afebrile and discharged home.
A week later, he returned to the ED with worsening ear pain and severe persistent headache, which was localized in the left occipital, left frontal, and retro-orbital regions. He denied light or sound sensitivity, nausea, vomiting, or increased lacrimation. He was tearful on examination due to the pain. He had no meningismus and normal fundi. A neurologic examination was nonlateralizing. Laboratory tests showed a normal complete blood count but increased C-reactive protein at 113 mg/dL (normal, < 0.3 mg/dL) and an erythrocyte sedimentation rate of 88 mm/hr (normal, 0-20 mm/hr).
Magnetic resonance imaging was ordered (FIGURES 1A and 1B), and Neurosurgery and Otolaryngology were consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Acute mastoiditis with epidural abscess
The contrast-enhanced cranial MRI scan (FIGURE 1A) revealed a case of acute mastoiditis with fluid in the left mastoid (blue arrow) and a large epidural abscess in the left posterior fossa (green arrow). The normal right mastoid was air-filled (yellow arrow). The T2-weighted MRI scan (FIGURE 1B) showed mild dilatation of the lateral ventricles (blue arrow) secondary to compression on the fourth ventricle by mass effect from the epidural abscess.
Acute mastoiditis—a complication of AOM—is an inflammatory process of mastoid air cells, which are contiguous to the middle ear cleft. In one large study of 61,783 inpatient children admitted with AOM, acute mastoiditis was reported as the most common complication in 1505 (2.4%) of the cases.1 The 2000-2012 national estimated incidence rate of pediatric mastoiditis has ranged from a high of 2.7 per 100,000 population in 2006 to a low of 1.8 per 100,000 in 2012.2 Clinical features of mastoiditis include localized mastoid tenderness, swelling, erythema, fluctuance, protrusion of the auricle, and ear pain.3
The clinical presentation of epidural abscess can be subtle with fever, headache, neck pain, and changes in mental status developing over several days.1 Focal deficits and seizures are relatively uncommon. In a review of 308 children with acute mastoiditis (3 with an epidural abscess), high-grade fever and high absolute neutrophil count and C-reactive protein levels were associated with the development of complications of mastoiditis, including hearing loss, sinus venous thrombosis, intracranial abscess, and cranial nerve palsies.4
Venous sinus thrombosis was part of the differential
When we were caring for this patient, the differential diagnosis included a cranial extension of AOM. Venous sinus thrombosis was also considered, given the family history of a hypercoagulable state. The patient did not have any features suggesting primary headache syndromes, such as migraine, tension type, or cluster headache.
The differential for a patient complaining of ear pain also includes postauricular lymphadenopathy, mumps, periauricular cellulitis (with and without otitis externa), perichondritis of the auricle, and tumors involving the mastoid bone.4
Continue to: Imaging and treatment
Imaging and treatment
Imaging of temporal bone is not recommended to make a diagnosis of mastoiditis in children with characteristic clinical findings. When imaging is needed, contrast-enhanced computed tomography (CT) is best to help visualize changes in temporal bone. If intracranial complications are suspected, cranial MRI with contrast or cranial CT with contrast can be ordered (depending on availability).5
Conservative management with intravenous antimicrobial therapy and middle ear drainage with myringotomy is indicated for a child with uncomplicated acute or subacute mastoiditis. For patients with suppurative extracranial or intracranial complications, aggressive surgical management is needed.5
Treatment for this patient included craniotomy, evacuation of the epidural abscess, and mastoidectomy. A culture obtained from the abscess showed Streptococcus intermedius. He was treated with broad-spectrum antibiotics, including ceftriaxone, vancomycin, and metronidazole. Within a week of surgery, he was discharged from the hospital and continued antibiotic treatment for 6 weeks via a peripherally inserted central catheter line.
1. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg. 2016;154:366-370.
2. King LM, Bartoces M, Hersh AL, et al. National incidence of pediatric mastoiditis in the United States, 2000-2012: creating a baseline for public health surveillance. Pediatr Infect Dis J. 2019;38:e14-e16.
3. Pang LH, Barakate MS, Havas TE. Mastoiditis in a paediatric population: a review of 11 years’ experience in management. Int J Pediatr Otorhinolaryngol. 2009;73:1520.
4. Bilavsky E, Yarden-Bilavsky H, Samra Z, et al. Clinical, laboratory, and microbiological differences between children with simple or complicated mastoiditis. Int J Pediatr Otorhinolaryngol. 2009;73:1270-1273.
5. Chesney J, Black A, Choo D. What is the best practice for acute mastoiditis in children? Laryngoscope. 2014;124:1057-1059.
1. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg. 2016;154:366-370.
2. King LM, Bartoces M, Hersh AL, et al. National incidence of pediatric mastoiditis in the United States, 2000-2012: creating a baseline for public health surveillance. Pediatr Infect Dis J. 2019;38:e14-e16.
3. Pang LH, Barakate MS, Havas TE. Mastoiditis in a paediatric population: a review of 11 years’ experience in management. Int J Pediatr Otorhinolaryngol. 2009;73:1520.
4. Bilavsky E, Yarden-Bilavsky H, Samra Z, et al. Clinical, laboratory, and microbiological differences between children with simple or complicated mastoiditis. Int J Pediatr Otorhinolaryngol. 2009;73:1270-1273.
5. Chesney J, Black A, Choo D. What is the best practice for acute mastoiditis in children? Laryngoscope. 2014;124:1057-1059.
Five pediatric heart health practices that may be unnecessary
the American Academy of Pediatrics explained in guidance released Nov. 2.
The AAP Section on Cardiology and Cardiac Surgery developed the recommendations as part of the Choosing Wisely campaign after reviewing evidence pertaining to practices common during pediatric visits, such as routinely ordering an electrocardiogram (ECG) as part of a sports exam.
The guidance lets physicians know what is not necessary or not indicated, with noted exceptions, Christopher S. Snyder, MD, chair of the section, said in an interview.
In all cases, family history is key, said Dr. Snyder, who is also chief of the division of pediatric cardiology at University Hospitals Cleveland Medical Center. That means taking the time necessary to ask about aunts, uncles, and all first-degree relatives, not just asking the single question of whether a patient has a family history of cardiac problems.
The following are the targeted practices and the AAP’s guidance on each.
ECG for sports participation
A screening ECG should not be ordered as part of a routine sports entry examination in otherwise healthy patients who have no symptoms and no personal or family history of cardiac disease, the committee says.
Some medical societies argue that all children who participate in sports should have an ECG, but, Dr. Snyder said, “Currently there are no data that support that, especially in the United States.”
ECGs often yield false positive findings, he noted: “About 10% of them will say the child is a little abnormal.”
That can be a particular problem in places with few or no pediatric cardiologists because kids can become sidelined from sports without access to experts who could clear them.
“In the U.S.,” he said, “we believe that the preparticipation physical exam and screening, which is routine for all high school athletes for sure and most athletes who compete in sports, is currently good enough.”
However, he warned, patients with a family history of heart disease need to see a pediatric cardiologist and “those patients need an ECG.”
The test is not perfect, though, he noted: “You could get your screening, go home, get a fever, COVID, something like that, and come back and have myocarditis and drop dead.”
ECG before ADHD therapy
Similarly, a screening ECG is not routinely needed before initiating therapy for ADHD in asymptomatic, otherwise healthy children who have no personal or family history of cardiac disease, according to the new guidance.
Dr. Snyder said that it has become routine for children to undergo an ECG before ADHD therapy, but evidence doesn’t support the practice, and with the rise in the number of ADHD diagnoses, the tests have increasingly become a burden.
Twenty years ago, the prevalence of ADHD was 3%-4%, Dr. Snyder said. It is now almost threefold higher.
The AAP committee points out that, when ECG abnormalities are identified, they rarely lead to a change in ADHD therapy. Additionally, the typical stimulants used to treat ADHD “have never shown any major effect on the heart,” Dr. Snyder said.
“Black box warnings have been put on these medications, but nothing has been found in the very routine stimulants in normal, routine doses to warrant an ECG,” he said.
Echocardiogram for syncope
The committee says routine use of echocardiograms for children with syncope is unnecessary unless a child has a concerning history or ECG abnormalities.
Most patient who have true syncope or are passing out or fainting are diagnosed through thorough family history, Dr. Snyder said.
“The vast majority of those need an ECG to rule out one other cause that can do this and a physical exam. If those things are normal, there really is no indication to do an echocardiogram,” he said.
“If the patient passes out while they’re running, they pass out doing strenuous exercise, or they pass out for 10-15 minutes as opposed to 20 seconds – those are the ones that need a thorough cardiac workup. But routine passing out, waking up in seconds, those do not.”
Echocardiogram for chest pain
Children with chest pain do not need an echocardiogram unless an ECG is abnormal or the patient has a concerning history, according to the new recommendations.
Too often, Dr. Snyder said, providers treat kids as they would adults.
“Often it comes down to what you learn in medical school,” Dr. Snyder said. “In medical school, we have 6 weeks of cardiology and we had 1 hour of pediatric cardiology.”
That younger patients will clog their arteries with fatty foods and high lipids “is really exceptionally rare,” Dr. Snyder said.
Chest pain “rarely, if ever” means heart attack in younger children, he added.
A thorough history and complete physical exam are critical, “without jumping immediately to an echocardiogram, which 99.9% of the time is going to be normal,” he said.
Troponins for chest pain
In addition, a typical workup for pediatric chest pain need not include evaluating troponins unless there is a concerning history or ECG abnormalities.
Snyder notes that kids with chest pain are often brought to emergency departments that are not pediatric specific, and thus clinicians turn to the standard treatment for adults with chest pain: ECG and troponin.
“The reason we in pediatric cardiology don’t love this is that troponins tend not to be specific just for heart in kids,” Dr. Snyder said. “If someone has anginal chest pain – shortness of breath, chest pain doing anything and everything, [chest pain that] occurs when they’re exercising, feels like an elephant standing on their chest – then we do encourage troponins on those patients.”
The guidance discourages ordering troponins without careful consideration of the patient’s age and condition, he said.
This list was developed by faculty in Pediatric Cardiology at University Hospitals in Cleveland. It was revised and approved by the AAP Section on Cardiology and Cardiac Surgery and the AAP Executive Committee.
A version of this article originally appeared on Medscape.com.
the American Academy of Pediatrics explained in guidance released Nov. 2.
The AAP Section on Cardiology and Cardiac Surgery developed the recommendations as part of the Choosing Wisely campaign after reviewing evidence pertaining to practices common during pediatric visits, such as routinely ordering an electrocardiogram (ECG) as part of a sports exam.
The guidance lets physicians know what is not necessary or not indicated, with noted exceptions, Christopher S. Snyder, MD, chair of the section, said in an interview.
In all cases, family history is key, said Dr. Snyder, who is also chief of the division of pediatric cardiology at University Hospitals Cleveland Medical Center. That means taking the time necessary to ask about aunts, uncles, and all first-degree relatives, not just asking the single question of whether a patient has a family history of cardiac problems.
The following are the targeted practices and the AAP’s guidance on each.
ECG for sports participation
A screening ECG should not be ordered as part of a routine sports entry examination in otherwise healthy patients who have no symptoms and no personal or family history of cardiac disease, the committee says.
Some medical societies argue that all children who participate in sports should have an ECG, but, Dr. Snyder said, “Currently there are no data that support that, especially in the United States.”
ECGs often yield false positive findings, he noted: “About 10% of them will say the child is a little abnormal.”
That can be a particular problem in places with few or no pediatric cardiologists because kids can become sidelined from sports without access to experts who could clear them.
“In the U.S.,” he said, “we believe that the preparticipation physical exam and screening, which is routine for all high school athletes for sure and most athletes who compete in sports, is currently good enough.”
However, he warned, patients with a family history of heart disease need to see a pediatric cardiologist and “those patients need an ECG.”
The test is not perfect, though, he noted: “You could get your screening, go home, get a fever, COVID, something like that, and come back and have myocarditis and drop dead.”
ECG before ADHD therapy
Similarly, a screening ECG is not routinely needed before initiating therapy for ADHD in asymptomatic, otherwise healthy children who have no personal or family history of cardiac disease, according to the new guidance.
Dr. Snyder said that it has become routine for children to undergo an ECG before ADHD therapy, but evidence doesn’t support the practice, and with the rise in the number of ADHD diagnoses, the tests have increasingly become a burden.
Twenty years ago, the prevalence of ADHD was 3%-4%, Dr. Snyder said. It is now almost threefold higher.
The AAP committee points out that, when ECG abnormalities are identified, they rarely lead to a change in ADHD therapy. Additionally, the typical stimulants used to treat ADHD “have never shown any major effect on the heart,” Dr. Snyder said.
“Black box warnings have been put on these medications, but nothing has been found in the very routine stimulants in normal, routine doses to warrant an ECG,” he said.
Echocardiogram for syncope
The committee says routine use of echocardiograms for children with syncope is unnecessary unless a child has a concerning history or ECG abnormalities.
Most patient who have true syncope or are passing out or fainting are diagnosed through thorough family history, Dr. Snyder said.
“The vast majority of those need an ECG to rule out one other cause that can do this and a physical exam. If those things are normal, there really is no indication to do an echocardiogram,” he said.
“If the patient passes out while they’re running, they pass out doing strenuous exercise, or they pass out for 10-15 minutes as opposed to 20 seconds – those are the ones that need a thorough cardiac workup. But routine passing out, waking up in seconds, those do not.”
Echocardiogram for chest pain
Children with chest pain do not need an echocardiogram unless an ECG is abnormal or the patient has a concerning history, according to the new recommendations.
Too often, Dr. Snyder said, providers treat kids as they would adults.
“Often it comes down to what you learn in medical school,” Dr. Snyder said. “In medical school, we have 6 weeks of cardiology and we had 1 hour of pediatric cardiology.”
That younger patients will clog their arteries with fatty foods and high lipids “is really exceptionally rare,” Dr. Snyder said.
Chest pain “rarely, if ever” means heart attack in younger children, he added.
A thorough history and complete physical exam are critical, “without jumping immediately to an echocardiogram, which 99.9% of the time is going to be normal,” he said.
Troponins for chest pain
In addition, a typical workup for pediatric chest pain need not include evaluating troponins unless there is a concerning history or ECG abnormalities.
Snyder notes that kids with chest pain are often brought to emergency departments that are not pediatric specific, and thus clinicians turn to the standard treatment for adults with chest pain: ECG and troponin.
“The reason we in pediatric cardiology don’t love this is that troponins tend not to be specific just for heart in kids,” Dr. Snyder said. “If someone has anginal chest pain – shortness of breath, chest pain doing anything and everything, [chest pain that] occurs when they’re exercising, feels like an elephant standing on their chest – then we do encourage troponins on those patients.”
The guidance discourages ordering troponins without careful consideration of the patient’s age and condition, he said.
This list was developed by faculty in Pediatric Cardiology at University Hospitals in Cleveland. It was revised and approved by the AAP Section on Cardiology and Cardiac Surgery and the AAP Executive Committee.
A version of this article originally appeared on Medscape.com.
the American Academy of Pediatrics explained in guidance released Nov. 2.
The AAP Section on Cardiology and Cardiac Surgery developed the recommendations as part of the Choosing Wisely campaign after reviewing evidence pertaining to practices common during pediatric visits, such as routinely ordering an electrocardiogram (ECG) as part of a sports exam.
The guidance lets physicians know what is not necessary or not indicated, with noted exceptions, Christopher S. Snyder, MD, chair of the section, said in an interview.
In all cases, family history is key, said Dr. Snyder, who is also chief of the division of pediatric cardiology at University Hospitals Cleveland Medical Center. That means taking the time necessary to ask about aunts, uncles, and all first-degree relatives, not just asking the single question of whether a patient has a family history of cardiac problems.
The following are the targeted practices and the AAP’s guidance on each.
ECG for sports participation
A screening ECG should not be ordered as part of a routine sports entry examination in otherwise healthy patients who have no symptoms and no personal or family history of cardiac disease, the committee says.
Some medical societies argue that all children who participate in sports should have an ECG, but, Dr. Snyder said, “Currently there are no data that support that, especially in the United States.”
ECGs often yield false positive findings, he noted: “About 10% of them will say the child is a little abnormal.”
That can be a particular problem in places with few or no pediatric cardiologists because kids can become sidelined from sports without access to experts who could clear them.
“In the U.S.,” he said, “we believe that the preparticipation physical exam and screening, which is routine for all high school athletes for sure and most athletes who compete in sports, is currently good enough.”
However, he warned, patients with a family history of heart disease need to see a pediatric cardiologist and “those patients need an ECG.”
The test is not perfect, though, he noted: “You could get your screening, go home, get a fever, COVID, something like that, and come back and have myocarditis and drop dead.”
ECG before ADHD therapy
Similarly, a screening ECG is not routinely needed before initiating therapy for ADHD in asymptomatic, otherwise healthy children who have no personal or family history of cardiac disease, according to the new guidance.
Dr. Snyder said that it has become routine for children to undergo an ECG before ADHD therapy, but evidence doesn’t support the practice, and with the rise in the number of ADHD diagnoses, the tests have increasingly become a burden.
Twenty years ago, the prevalence of ADHD was 3%-4%, Dr. Snyder said. It is now almost threefold higher.
The AAP committee points out that, when ECG abnormalities are identified, they rarely lead to a change in ADHD therapy. Additionally, the typical stimulants used to treat ADHD “have never shown any major effect on the heart,” Dr. Snyder said.
“Black box warnings have been put on these medications, but nothing has been found in the very routine stimulants in normal, routine doses to warrant an ECG,” he said.
Echocardiogram for syncope
The committee says routine use of echocardiograms for children with syncope is unnecessary unless a child has a concerning history or ECG abnormalities.
Most patient who have true syncope or are passing out or fainting are diagnosed through thorough family history, Dr. Snyder said.
“The vast majority of those need an ECG to rule out one other cause that can do this and a physical exam. If those things are normal, there really is no indication to do an echocardiogram,” he said.
“If the patient passes out while they’re running, they pass out doing strenuous exercise, or they pass out for 10-15 minutes as opposed to 20 seconds – those are the ones that need a thorough cardiac workup. But routine passing out, waking up in seconds, those do not.”
Echocardiogram for chest pain
Children with chest pain do not need an echocardiogram unless an ECG is abnormal or the patient has a concerning history, according to the new recommendations.
Too often, Dr. Snyder said, providers treat kids as they would adults.
“Often it comes down to what you learn in medical school,” Dr. Snyder said. “In medical school, we have 6 weeks of cardiology and we had 1 hour of pediatric cardiology.”
That younger patients will clog their arteries with fatty foods and high lipids “is really exceptionally rare,” Dr. Snyder said.
Chest pain “rarely, if ever” means heart attack in younger children, he added.
A thorough history and complete physical exam are critical, “without jumping immediately to an echocardiogram, which 99.9% of the time is going to be normal,” he said.
Troponins for chest pain
In addition, a typical workup for pediatric chest pain need not include evaluating troponins unless there is a concerning history or ECG abnormalities.
Snyder notes that kids with chest pain are often brought to emergency departments that are not pediatric specific, and thus clinicians turn to the standard treatment for adults with chest pain: ECG and troponin.
“The reason we in pediatric cardiology don’t love this is that troponins tend not to be specific just for heart in kids,” Dr. Snyder said. “If someone has anginal chest pain – shortness of breath, chest pain doing anything and everything, [chest pain that] occurs when they’re exercising, feels like an elephant standing on their chest – then we do encourage troponins on those patients.”
The guidance discourages ordering troponins without careful consideration of the patient’s age and condition, he said.
This list was developed by faculty in Pediatric Cardiology at University Hospitals in Cleveland. It was revised and approved by the AAP Section on Cardiology and Cardiac Surgery and the AAP Executive Committee.
A version of this article originally appeared on Medscape.com.
Which behavioral health screening tool should you use—and when?
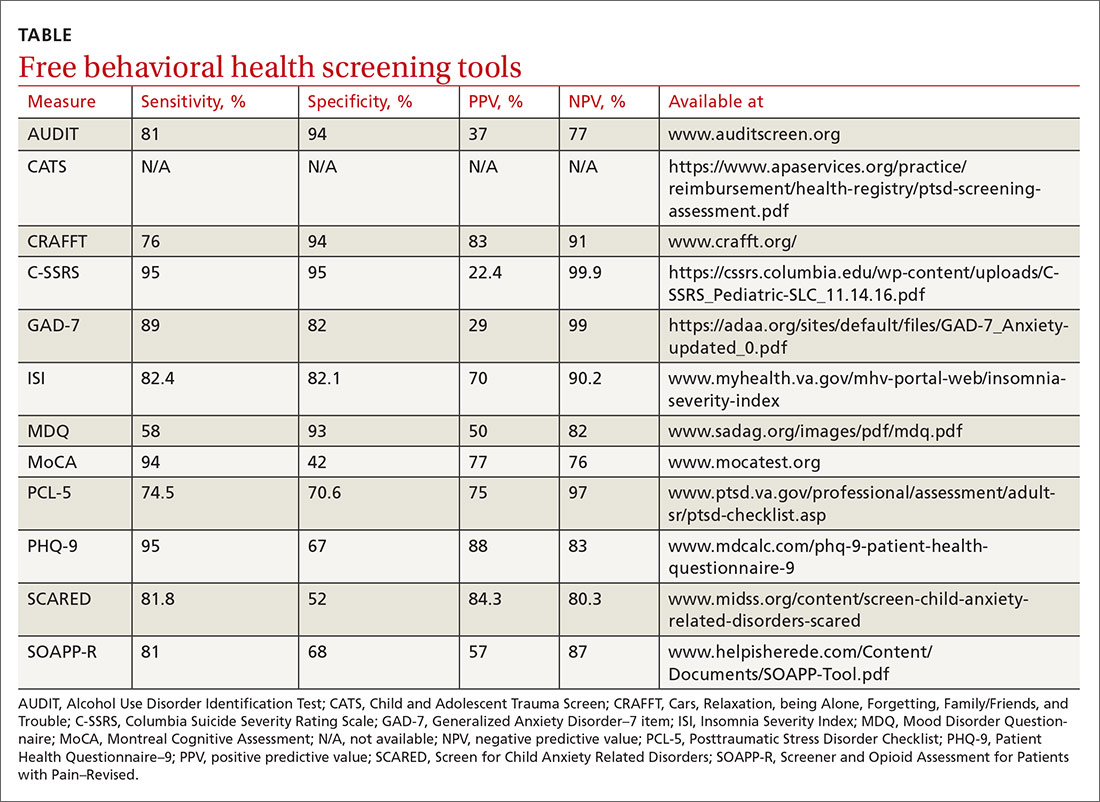
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3
Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
Continue to: The C-SSRS covers...
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
Continue to: The Insomnia Severity Index...
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Continue to: Opioid medications...
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Posttraumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher,
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
Continue to: The MoCA is a simple...
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues.
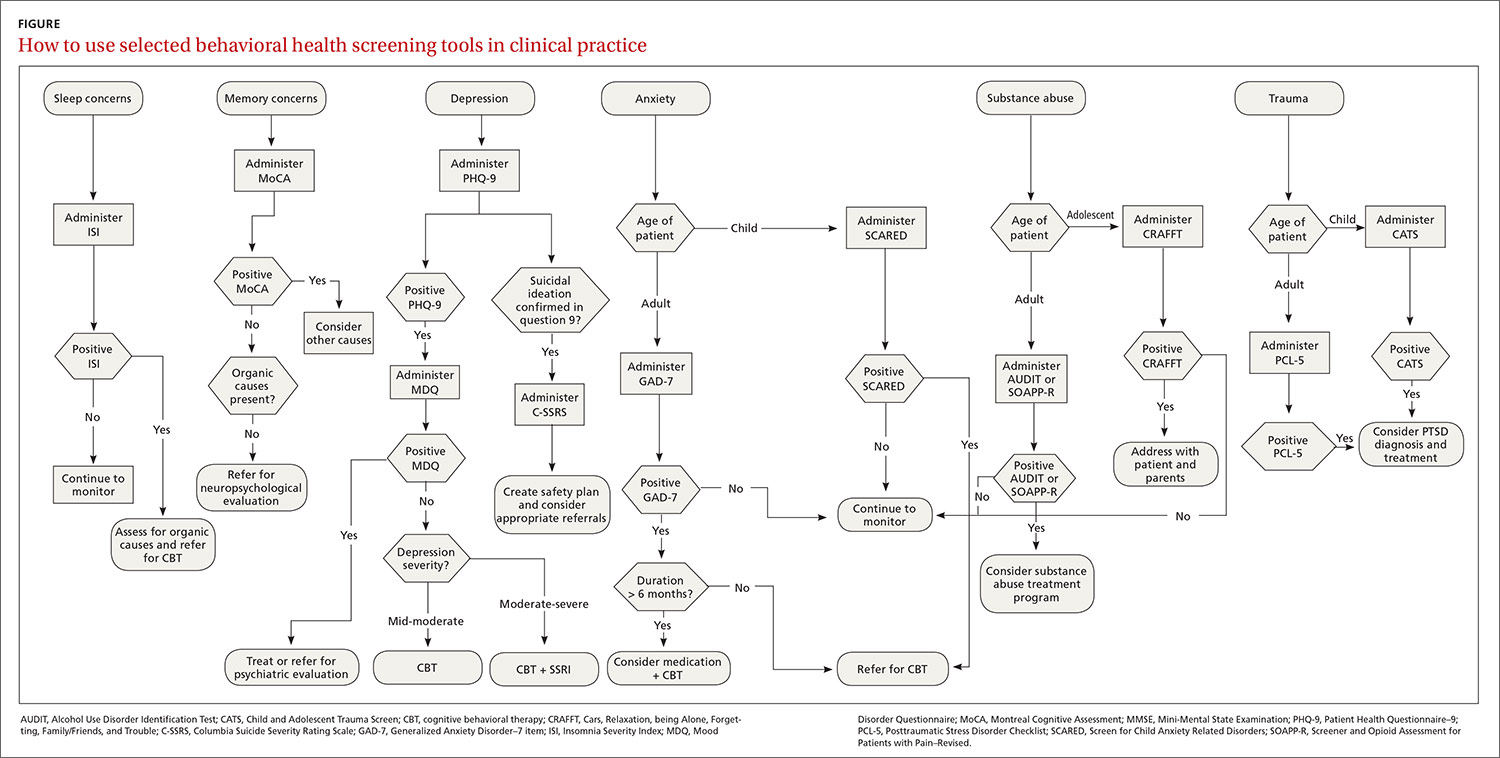
The FIGURE can be used to aid in the clinical decision-making process.
CORRESPONDENCE
Rebecca Sewell, PsyD, Bon Secours Mercy Health, 2213 Cherry Street, Toledo, OH 4360; [email protected].
1. Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
2. Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
3. Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
4. Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
5. Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016,2:127-138.
6. Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
7. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
9. Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
10. CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
11. Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
12. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
13. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
14. DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
15. Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
16. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
17. Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
18. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
19. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
21. Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
22. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
24. Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
26. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
27. Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
28. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360-372.
29. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
30. DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23, 2020.
31. Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
32. Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
34. Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
35. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
Continue to: The C-SSRS covers...
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
Continue to: The Insomnia Severity Index...
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Continue to: Opioid medications...
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Posttraumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher,
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
Continue to: The MoCA is a simple...
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues.
The FIGURE can be used to aid in the clinical decision-making process.

CORRESPONDENCE
Rebecca Sewell, PsyD, Bon Secours Mercy Health, 2213 Cherry Street, Toledo, OH 4360; [email protected].
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
Continue to: The C-SSRS covers...
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
Continue to: The Insomnia Severity Index...
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Continue to: Opioid medications...
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Posttraumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher,
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
Continue to: The MoCA is a simple...
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues.
The FIGURE can be used to aid in the clinical decision-making process.

CORRESPONDENCE
Rebecca Sewell, PsyD, Bon Secours Mercy Health, 2213 Cherry Street, Toledo, OH 4360; [email protected].
1. Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
2. Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
3. Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
4. Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
5. Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016,2:127-138.
6. Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
7. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
9. Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
10. CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
11. Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
12. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
13. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
14. DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
15. Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
16. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
17. Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
18. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
19. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
21. Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
22. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
24. Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
26. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
27. Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
28. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360-372.
29. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
30. DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23, 2020.
31. Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
32. Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
34. Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
35. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
1. Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
2. Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
3. Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
4. Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
5. Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016,2:127-138.
6. Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
7. Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
9. Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
10. CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
11. Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
12. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
13. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
14. DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
15. Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
16. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
17. Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
18. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
19. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
20. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
21. Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
22. Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
24. Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
26. Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
27. Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
28. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R). J Pain. 2008;9:360-372.
29. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
30. DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23, 2020.
31. Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
32. Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
33. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
34. Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
35. Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
Pfizer vaccine data show 90% efficacy in early results
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
Proposed Medicare rule would expand CGM coverage
A new proposed rule from the Centers for Medicare & Medicaid Services (CMS) would expand coverage for continuous glucose monitors (CGMs) under Medicare to include devices that aren’t approved for making treatment decisions.
If accepted, the proposed rule would classify all approved CGMs as durable medical equipment under Medicare Part B and establish payment amounts for all related supplies. The move primarily affects Medtronic’s Guardian Connect System, which has not been approved by the U.S. Food and Drug Administration to replace the need for fingersticks in determining insulin or other glucose-lowering medication dosing.
Two other CGM systems, the Dexcom G6 and Abbott Libre, have “therapeutic” indications and are, therefore, already covered under Medicare, as is the combined insulin pump–CGM Tandem Diabetes Care Control-IQ Technology system.
According to a CMS statement, “CGMs that are not approved for use in making diabetes treatment decisions can be used to alert beneficiaries about potentially dangerous glucose levels while they sleep and that they should further test their glucose levels using a blood glucose monitor. ... This proposal would give Medicare beneficiaries and their physicians a wider range of technology and devices to choose from in managing diabetes.”
Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic said in an interview that the company is “very encouraged” by the proposal. “Importantly, the proposed rule would enable continuity of therapy for people on Medtronic insulin pumps aging into Medicare – including Medtronic hybrid closed loop systems, which automatically adjust insulin delivery based on readings from the integrated CGM.”
The type 1 diabetes research and advocacy organization JDRF also applauded the proposed rule, noting in a statement, “CGM technology can be an integral component of artificial pancreas systems and important on its own to significantly improve diabetes management and enable users to avoid potential crises and risks for long-term complications. JDRF is heartened by this proposed change as it has long advocated for coverage, affordability and choice of all therapies to help ensure people with T1D have what they need to survive.”
The proposal is part of a broader set of proposed changes to Medicare Durable Medical Equipment, Prosthetics, Orthotic Devices and Supplies (DMEPOS) coverage and payment policies. Comments on the entire document can be submitted through Jan. 4, 2021 to the Federal Register.
A new proposed rule from the Centers for Medicare & Medicaid Services (CMS) would expand coverage for continuous glucose monitors (CGMs) under Medicare to include devices that aren’t approved for making treatment decisions.
If accepted, the proposed rule would classify all approved CGMs as durable medical equipment under Medicare Part B and establish payment amounts for all related supplies. The move primarily affects Medtronic’s Guardian Connect System, which has not been approved by the U.S. Food and Drug Administration to replace the need for fingersticks in determining insulin or other glucose-lowering medication dosing.
Two other CGM systems, the Dexcom G6 and Abbott Libre, have “therapeutic” indications and are, therefore, already covered under Medicare, as is the combined insulin pump–CGM Tandem Diabetes Care Control-IQ Technology system.
According to a CMS statement, “CGMs that are not approved for use in making diabetes treatment decisions can be used to alert beneficiaries about potentially dangerous glucose levels while they sleep and that they should further test their glucose levels using a blood glucose monitor. ... This proposal would give Medicare beneficiaries and their physicians a wider range of technology and devices to choose from in managing diabetes.”
Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic said in an interview that the company is “very encouraged” by the proposal. “Importantly, the proposed rule would enable continuity of therapy for people on Medtronic insulin pumps aging into Medicare – including Medtronic hybrid closed loop systems, which automatically adjust insulin delivery based on readings from the integrated CGM.”
The type 1 diabetes research and advocacy organization JDRF also applauded the proposed rule, noting in a statement, “CGM technology can be an integral component of artificial pancreas systems and important on its own to significantly improve diabetes management and enable users to avoid potential crises and risks for long-term complications. JDRF is heartened by this proposed change as it has long advocated for coverage, affordability and choice of all therapies to help ensure people with T1D have what they need to survive.”
The proposal is part of a broader set of proposed changes to Medicare Durable Medical Equipment, Prosthetics, Orthotic Devices and Supplies (DMEPOS) coverage and payment policies. Comments on the entire document can be submitted through Jan. 4, 2021 to the Federal Register.
A new proposed rule from the Centers for Medicare & Medicaid Services (CMS) would expand coverage for continuous glucose monitors (CGMs) under Medicare to include devices that aren’t approved for making treatment decisions.
If accepted, the proposed rule would classify all approved CGMs as durable medical equipment under Medicare Part B and establish payment amounts for all related supplies. The move primarily affects Medtronic’s Guardian Connect System, which has not been approved by the U.S. Food and Drug Administration to replace the need for fingersticks in determining insulin or other glucose-lowering medication dosing.
Two other CGM systems, the Dexcom G6 and Abbott Libre, have “therapeutic” indications and are, therefore, already covered under Medicare, as is the combined insulin pump–CGM Tandem Diabetes Care Control-IQ Technology system.
According to a CMS statement, “CGMs that are not approved for use in making diabetes treatment decisions can be used to alert beneficiaries about potentially dangerous glucose levels while they sleep and that they should further test their glucose levels using a blood glucose monitor. ... This proposal would give Medicare beneficiaries and their physicians a wider range of technology and devices to choose from in managing diabetes.”
Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic said in an interview that the company is “very encouraged” by the proposal. “Importantly, the proposed rule would enable continuity of therapy for people on Medtronic insulin pumps aging into Medicare – including Medtronic hybrid closed loop systems, which automatically adjust insulin delivery based on readings from the integrated CGM.”
The type 1 diabetes research and advocacy organization JDRF also applauded the proposed rule, noting in a statement, “CGM technology can be an integral component of artificial pancreas systems and important on its own to significantly improve diabetes management and enable users to avoid potential crises and risks for long-term complications. JDRF is heartened by this proposed change as it has long advocated for coverage, affordability and choice of all therapies to help ensure people with T1D have what they need to survive.”
The proposal is part of a broader set of proposed changes to Medicare Durable Medical Equipment, Prosthetics, Orthotic Devices and Supplies (DMEPOS) coverage and payment policies. Comments on the entire document can be submitted through Jan. 4, 2021 to the Federal Register.
Cystic fibrosis patients’ vulnerability to COVID-19 infection: Preliminary data ease fears
But early results suggest that social distance measures and perhaps the younger average age of individuals with CF have prevented a severe impact on this patient population.
Not all of the news is good. Some research suggests that posttransplant individuals may be at greater risk of severe outcomes. However, researchers warned that the data are too sparse to draw firm conclusions, and ongoing analyses of patient registries and other sources should lend greater insight into the burden of COVID-19 among individuals with CF. Those were some of the conclusions presented at a session of the virtual North American Cystic Fibrosis Conference.
D.B. Sanders, MD, who is a pediatric pulmonologist at Riley Hospital for Children and the Indiana University, both in Indianapolis, presented data from the Cystic Fibrosis Foundation’s Patient Registry, which includes patients in the United States. As in other populations, he showed that health care use has gone down among individuals with CF. From April to September 2019, 81% of clinical encounters were in the clinic and 12% in the hospital. Over the same period in 2020, those numbers dropped to 35% and 4%, respectively, with 30% by phone or computer. In-person health care use rebounded somewhat between July 1 and Sept. 16, with 53% of encounters at the clinic, 5% at the hospital, and 28% conducted virtually. There were also dips in forced expiratory volume in one second (FEV1) and microbiology testing, from about 90% occurring during health encounters at the end of 2019 to fewer than 10% of encounters by April.
As of Aug. 17, Dr. Sanders reported that 3,048 individuals with CF had been tested for COVID-19, with 174 positive results.
Racial and ethnic disparities in positive test results seen in other populations were also observable among individuals with CF. Several groups made up a higher proportion of COVID-19–positive CF patients than the general CF population, including Hispanics (18% vs. 9%), Blacks (7% vs. 5%), and individuals with FEV1 value less than 40% predicted (14% vs. 8%).
As of Sept. 17, there had been 51 hospitalizations and two deaths in the United States among 212 individuals with CF who tested positive for COVID-19, with increasing numbers that mirror trends in the U.S. population. One death occurred in a patient with advanced lung disease, the other in a post–lung transplant patient. “Thankfully [the numbers are] not higher, but this is being followed very closely,” said Dr. Sanders during his presentation.
One encouraging bit of news was that hospitalizations among individuals with CF have dropped since the start of the pandemic. “I think this shows how good our families are at socially distancing, wearing masks, and now that they not being exposed to viruses, I think we’re seeing the fruits of this with fewer hospitalizations,” said Dr. Sanders. He noted that it’s possible some of the decline could have been to reluctance to go to the hospital, and the introduction of triple combination cystic fibrosis transmembrane conductance regulator modulator therapy has also likely contributed. “We were already seeing fewer hospitalizations even before the pandemic hit,” he said.
At the session, Rebecca Cosgriff, director of data and quality improvement at the Cystic Fibrosis Trust in the United Kingdom, presented an international perspective on COVID-19 cases among individuals with CF. At the beginning of the pandemic, the Cystic Fibrosis Global Registry Harmonization Group recruited country coordinators to collect anonymized data on infections, hospitalizations, and other outcomes. In April, the group published its initial findings from 40 cases in eight countries, which concluded that these cases generally resembled the broader population in clinical course, which assuaged initial fears.
Ms. Cosgriff reported on results from a second round of data collection with a cutoff date of June 19, which expanded to 19 countries and included many from South America and more in Europe. The network encompassed about 85,000 individuals with CF, and tallied 181 cases of COVID-19. A total of 149 cases were nontransplant, and 32 were posttransplant (28 lung only). Fully 15% of the nontransplant group were over age 40 years, compared with 41% in the transplant group. Homozygous F508del mutations were more common in the posttransplant group (59% vs. 36%). However, lung function, as estimated by the best FEV1 measured in the previous year prior to infection, differed between the nontransplant (73%) and posttransplant (80%) COVID-19 patients.
Across all age groups, hospitalizations were more common in patients with best FEV1 percentage predicted values less than 70% (P = .001). Ms. Cosgriff also expressed concern about the posttransplant group. “Across all outcomes that might be indicative of infection severity – hospitalization, ICU admission, new supplementary oxygen, and non-invasive ventilation – the proportion of the posttransplant group was higher across the board,” she said during her presentation.
There were seven deaths. Ms. Cosgriff noted that there were too few deaths to analyze trends, but she presented a slide showing characteristics of deceased patients. “Factors like being post–lung transplant, being male, having less FEV1 than predicted, being over 40, or having CF-related diabetes, all appear pretty frequently amongst the cohort of people who died,” she said.
Overall, the results of these surveys are encouraging, suggesting that early fears that COVID-19 cases could be more severe among individuals with CF may not have been borne out so far. Dr. Sanders noted in his talk that there aren’t enough cases in the U.S. cohort to show links to risk factors with statistical significance. “But thankfully we’re not seeing a host of negative outcomes,” he said.
Dr. Sanders and Ms Cosgriff have no relevant financial disclosures.
But early results suggest that social distance measures and perhaps the younger average age of individuals with CF have prevented a severe impact on this patient population.
Not all of the news is good. Some research suggests that posttransplant individuals may be at greater risk of severe outcomes. However, researchers warned that the data are too sparse to draw firm conclusions, and ongoing analyses of patient registries and other sources should lend greater insight into the burden of COVID-19 among individuals with CF. Those were some of the conclusions presented at a session of the virtual North American Cystic Fibrosis Conference.
D.B. Sanders, MD, who is a pediatric pulmonologist at Riley Hospital for Children and the Indiana University, both in Indianapolis, presented data from the Cystic Fibrosis Foundation’s Patient Registry, which includes patients in the United States. As in other populations, he showed that health care use has gone down among individuals with CF. From April to September 2019, 81% of clinical encounters were in the clinic and 12% in the hospital. Over the same period in 2020, those numbers dropped to 35% and 4%, respectively, with 30% by phone or computer. In-person health care use rebounded somewhat between July 1 and Sept. 16, with 53% of encounters at the clinic, 5% at the hospital, and 28% conducted virtually. There were also dips in forced expiratory volume in one second (FEV1) and microbiology testing, from about 90% occurring during health encounters at the end of 2019 to fewer than 10% of encounters by April.
As of Aug. 17, Dr. Sanders reported that 3,048 individuals with CF had been tested for COVID-19, with 174 positive results.
Racial and ethnic disparities in positive test results seen in other populations were also observable among individuals with CF. Several groups made up a higher proportion of COVID-19–positive CF patients than the general CF population, including Hispanics (18% vs. 9%), Blacks (7% vs. 5%), and individuals with FEV1 value less than 40% predicted (14% vs. 8%).
As of Sept. 17, there had been 51 hospitalizations and two deaths in the United States among 212 individuals with CF who tested positive for COVID-19, with increasing numbers that mirror trends in the U.S. population. One death occurred in a patient with advanced lung disease, the other in a post–lung transplant patient. “Thankfully [the numbers are] not higher, but this is being followed very closely,” said Dr. Sanders during his presentation.
One encouraging bit of news was that hospitalizations among individuals with CF have dropped since the start of the pandemic. “I think this shows how good our families are at socially distancing, wearing masks, and now that they not being exposed to viruses, I think we’re seeing the fruits of this with fewer hospitalizations,” said Dr. Sanders. He noted that it’s possible some of the decline could have been to reluctance to go to the hospital, and the introduction of triple combination cystic fibrosis transmembrane conductance regulator modulator therapy has also likely contributed. “We were already seeing fewer hospitalizations even before the pandemic hit,” he said.
At the session, Rebecca Cosgriff, director of data and quality improvement at the Cystic Fibrosis Trust in the United Kingdom, presented an international perspective on COVID-19 cases among individuals with CF. At the beginning of the pandemic, the Cystic Fibrosis Global Registry Harmonization Group recruited country coordinators to collect anonymized data on infections, hospitalizations, and other outcomes. In April, the group published its initial findings from 40 cases in eight countries, which concluded that these cases generally resembled the broader population in clinical course, which assuaged initial fears.
Ms. Cosgriff reported on results from a second round of data collection with a cutoff date of June 19, which expanded to 19 countries and included many from South America and more in Europe. The network encompassed about 85,000 individuals with CF, and tallied 181 cases of COVID-19. A total of 149 cases were nontransplant, and 32 were posttransplant (28 lung only). Fully 15% of the nontransplant group were over age 40 years, compared with 41% in the transplant group. Homozygous F508del mutations were more common in the posttransplant group (59% vs. 36%). However, lung function, as estimated by the best FEV1 measured in the previous year prior to infection, differed between the nontransplant (73%) and posttransplant (80%) COVID-19 patients.
Across all age groups, hospitalizations were more common in patients with best FEV1 percentage predicted values less than 70% (P = .001). Ms. Cosgriff also expressed concern about the posttransplant group. “Across all outcomes that might be indicative of infection severity – hospitalization, ICU admission, new supplementary oxygen, and non-invasive ventilation – the proportion of the posttransplant group was higher across the board,” she said during her presentation.
There were seven deaths. Ms. Cosgriff noted that there were too few deaths to analyze trends, but she presented a slide showing characteristics of deceased patients. “Factors like being post–lung transplant, being male, having less FEV1 than predicted, being over 40, or having CF-related diabetes, all appear pretty frequently amongst the cohort of people who died,” she said.
Overall, the results of these surveys are encouraging, suggesting that early fears that COVID-19 cases could be more severe among individuals with CF may not have been borne out so far. Dr. Sanders noted in his talk that there aren’t enough cases in the U.S. cohort to show links to risk factors with statistical significance. “But thankfully we’re not seeing a host of negative outcomes,” he said.
Dr. Sanders and Ms Cosgriff have no relevant financial disclosures.
But early results suggest that social distance measures and perhaps the younger average age of individuals with CF have prevented a severe impact on this patient population.
Not all of the news is good. Some research suggests that posttransplant individuals may be at greater risk of severe outcomes. However, researchers warned that the data are too sparse to draw firm conclusions, and ongoing analyses of patient registries and other sources should lend greater insight into the burden of COVID-19 among individuals with CF. Those were some of the conclusions presented at a session of the virtual North American Cystic Fibrosis Conference.
D.B. Sanders, MD, who is a pediatric pulmonologist at Riley Hospital for Children and the Indiana University, both in Indianapolis, presented data from the Cystic Fibrosis Foundation’s Patient Registry, which includes patients in the United States. As in other populations, he showed that health care use has gone down among individuals with CF. From April to September 2019, 81% of clinical encounters were in the clinic and 12% in the hospital. Over the same period in 2020, those numbers dropped to 35% and 4%, respectively, with 30% by phone or computer. In-person health care use rebounded somewhat between July 1 and Sept. 16, with 53% of encounters at the clinic, 5% at the hospital, and 28% conducted virtually. There were also dips in forced expiratory volume in one second (FEV1) and microbiology testing, from about 90% occurring during health encounters at the end of 2019 to fewer than 10% of encounters by April.
As of Aug. 17, Dr. Sanders reported that 3,048 individuals with CF had been tested for COVID-19, with 174 positive results.
Racial and ethnic disparities in positive test results seen in other populations were also observable among individuals with CF. Several groups made up a higher proportion of COVID-19–positive CF patients than the general CF population, including Hispanics (18% vs. 9%), Blacks (7% vs. 5%), and individuals with FEV1 value less than 40% predicted (14% vs. 8%).
As of Sept. 17, there had been 51 hospitalizations and two deaths in the United States among 212 individuals with CF who tested positive for COVID-19, with increasing numbers that mirror trends in the U.S. population. One death occurred in a patient with advanced lung disease, the other in a post–lung transplant patient. “Thankfully [the numbers are] not higher, but this is being followed very closely,” said Dr. Sanders during his presentation.
One encouraging bit of news was that hospitalizations among individuals with CF have dropped since the start of the pandemic. “I think this shows how good our families are at socially distancing, wearing masks, and now that they not being exposed to viruses, I think we’re seeing the fruits of this with fewer hospitalizations,” said Dr. Sanders. He noted that it’s possible some of the decline could have been to reluctance to go to the hospital, and the introduction of triple combination cystic fibrosis transmembrane conductance regulator modulator therapy has also likely contributed. “We were already seeing fewer hospitalizations even before the pandemic hit,” he said.
At the session, Rebecca Cosgriff, director of data and quality improvement at the Cystic Fibrosis Trust in the United Kingdom, presented an international perspective on COVID-19 cases among individuals with CF. At the beginning of the pandemic, the Cystic Fibrosis Global Registry Harmonization Group recruited country coordinators to collect anonymized data on infections, hospitalizations, and other outcomes. In April, the group published its initial findings from 40 cases in eight countries, which concluded that these cases generally resembled the broader population in clinical course, which assuaged initial fears.
Ms. Cosgriff reported on results from a second round of data collection with a cutoff date of June 19, which expanded to 19 countries and included many from South America and more in Europe. The network encompassed about 85,000 individuals with CF, and tallied 181 cases of COVID-19. A total of 149 cases were nontransplant, and 32 were posttransplant (28 lung only). Fully 15% of the nontransplant group were over age 40 years, compared with 41% in the transplant group. Homozygous F508del mutations were more common in the posttransplant group (59% vs. 36%). However, lung function, as estimated by the best FEV1 measured in the previous year prior to infection, differed between the nontransplant (73%) and posttransplant (80%) COVID-19 patients.
Across all age groups, hospitalizations were more common in patients with best FEV1 percentage predicted values less than 70% (P = .001). Ms. Cosgriff also expressed concern about the posttransplant group. “Across all outcomes that might be indicative of infection severity – hospitalization, ICU admission, new supplementary oxygen, and non-invasive ventilation – the proportion of the posttransplant group was higher across the board,” she said during her presentation.
There were seven deaths. Ms. Cosgriff noted that there were too few deaths to analyze trends, but she presented a slide showing characteristics of deceased patients. “Factors like being post–lung transplant, being male, having less FEV1 than predicted, being over 40, or having CF-related diabetes, all appear pretty frequently amongst the cohort of people who died,” she said.
Overall, the results of these surveys are encouraging, suggesting that early fears that COVID-19 cases could be more severe among individuals with CF may not have been borne out so far. Dr. Sanders noted in his talk that there aren’t enough cases in the U.S. cohort to show links to risk factors with statistical significance. “But thankfully we’re not seeing a host of negative outcomes,” he said.
Dr. Sanders and Ms Cosgriff have no relevant financial disclosures.
FROM NACFC 2020
Denosumab favored over alendronate for BMD protection in glucocorticoid-induced osteoporosis
Denosumab boosted bone mineral density (BMD) over 12 months to a greater extent than did alendronate in a randomized, 12-month study. The investigator-initiated research compared BMD at the lumbar spine and elsewhere among people with systemic lupus erythematosus (SLE) and other autoimmune conditions. Long-term glucocorticoid therapy places some people in this group at higher risk for adverse effects of bone density loss.
“Glucocorticoids remain the mainstay of treatment of rheumatic diseases, but [they are] a major risk factor for osteoporosis and fracture,” study author Chi Chiu Mok, MD, said in an interview.
Compared with baseline, adults randomly assigned to denosumab had a 3.5% increase in lumbar spine BMD at 12 months, compared with 2.5% among those taking alendronate, a significant difference. Dr. Mok, a consultant and honorary associate professor in the department of medicine and nuclear medicine at Tuen Mun Hospital in Hong Kong, presented the study results at the virtual annual meeting of the American College of Rheumatology.
“Given the knowledge that denosumab is more effective than alendronate in raising spinal BMD in chronic users of GCs without increasing adverse events, this drug may be considered as an alternative first-line therapy in higher-risk patients and in those who are contraindicated for the oral bisphosphonates,” he said.
Cost considerations
Denosumab is a human monoclonal antibody administered as a subcutaneous injection, available under the brand names Prolia and Xgeva. Alendronate is an oral agent available as both generic and brand name formulations.
“Yes, denosumab is more expensive, more costly than oral alendronate, but our study shows efficacy is better for steroid users,” Dr. Mok said in answer to a question about cost disparity between the two agents during his presentation at the meeting. “For patients who are contraindicated or have low compliance for bisphosphonate, or are high-risk patients, I recommend first-line use of denosumab.”
Researchers previously studied these agents, including a smaller study by Dr. Mok and colleagues that showed a BMD benefit after switching people on an oral bisphosphonate to denosumab. However, he said, “There is a paucity of data regarding comparative efficacy of denosumab and the bisphosphonates in long-term steroid users.”
To explore any differences in a larger patient population, the investigators randomly assigned adults with SLE and other autoimmune conditions to the two treatments: denosumab 60 mg subcutaneoulsy every 6 months or oral alendronate 70 mg/week. All patients also received 3,000 mg calcium and 1,000 IU vitamin D3 (cholecalciferol) each day.
After three discontinuations in denosumab cohort and four in the alendronate group, the researchers evaluated 69 people taking denosumab and 70 others taking alendronate. The discontinuations were caused by noncompliance, Dr. Mok said, not by adverse events.
Adverse events were reported, but the rate did not differ significantly between groups. Dr. Mok highlighted some notable differences, including more minor infections and arthralgias reported in the denosumab cohort. Chest discomfort was reported in one denosumab recipient versus no patients in the alendronate group. Dyspepsia/upper GI symptoms and dizziness/vertigo occurred more often in the alendronate group.
Women were 96% of the study population, and mean age was 50 years. A majority, 81%, had underlying SLE. Other diagnoses included rheumatoid arthritis, myositis, antineutrophil cytoplasmic antibody–associated vasculitis, and polymyalgia rheumatica. The mean dose of prednisolone at study entry was 5.1 mg/day.
Key BMD and biomarker findings
BMD increased significantly in the spine, hip, and femoral neck in both treatment groups by 12 months. However, after adjustment for baseline BMD and covariates including age, menopause, and history of fracture, the gains in the denosumab group were significantly higher.
The increase in lumbar spine BMD at 12 months of 3.5% in the denosumab group versus 2.5% in the alendronate group was statistically significant (P = .045). Less significant was a 0.9% increase at the hip in the denosumab patients versus 1.6% in the alendronate group (P = .10), as well as femoral neck BMD gains of 1% in the denosumab group versus 1.5% in the alendronate group (P = .86).
Furthermore, “denosumab was more potent in suppressing the bone markers at 12 months,” Dr. Mok said.
Specifically, the percentage decrease in serum PINP (procollagen type I N-terminal propeptide) levels in the denosumab group was significantly greater than in the alendronate group (P = .001). Likewise, the decrease in CTX (C-terminal telopeptide of type I collagen) was significantly greater in the denosumab cohort versus the alendronate cohort (P < .001).
“Dr. Mok’s study was a well-controlled investigation. The superiority of denosumab was impressive, especially given the small group sizes of 69 and 70,” session comoderator Gregg Silverman, MD, professor in the department of internal medicine and the department of pathology at New York University, said when asked for comment.
“However, bone density measurements may not tell the whole story. These results support a bigger and much larger-scale study to confirm that rates of fracture on denosumab are also reduced.”
No new symptomatic fractures occurred in either group during the study. The investigators are evaluating for any new radiologic fractures, with results pending.
Dr. Mok said “results of our study in Asian patients are largely confirmatory” of a previous 2018 comparison study and a 2019 comparison study, each sponsored by Amgen.
A small sample size, short duration of treatment, and the open-label design were limitations of the study.
The trial was an investigator-initiated study. Dr. Mok and colleagues had no relevant financial disclosures. Dr. Silverman had no relevant financial disclosures.
SOURCE: Mok CC et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract 1442.
Denosumab boosted bone mineral density (BMD) over 12 months to a greater extent than did alendronate in a randomized, 12-month study. The investigator-initiated research compared BMD at the lumbar spine and elsewhere among people with systemic lupus erythematosus (SLE) and other autoimmune conditions. Long-term glucocorticoid therapy places some people in this group at higher risk for adverse effects of bone density loss.
“Glucocorticoids remain the mainstay of treatment of rheumatic diseases, but [they are] a major risk factor for osteoporosis and fracture,” study author Chi Chiu Mok, MD, said in an interview.
Compared with baseline, adults randomly assigned to denosumab had a 3.5% increase in lumbar spine BMD at 12 months, compared with 2.5% among those taking alendronate, a significant difference. Dr. Mok, a consultant and honorary associate professor in the department of medicine and nuclear medicine at Tuen Mun Hospital in Hong Kong, presented the study results at the virtual annual meeting of the American College of Rheumatology.
“Given the knowledge that denosumab is more effective than alendronate in raising spinal BMD in chronic users of GCs without increasing adverse events, this drug may be considered as an alternative first-line therapy in higher-risk patients and in those who are contraindicated for the oral bisphosphonates,” he said.
Cost considerations
Denosumab is a human monoclonal antibody administered as a subcutaneous injection, available under the brand names Prolia and Xgeva. Alendronate is an oral agent available as both generic and brand name formulations.
“Yes, denosumab is more expensive, more costly than oral alendronate, but our study shows efficacy is better for steroid users,” Dr. Mok said in answer to a question about cost disparity between the two agents during his presentation at the meeting. “For patients who are contraindicated or have low compliance for bisphosphonate, or are high-risk patients, I recommend first-line use of denosumab.”
Researchers previously studied these agents, including a smaller study by Dr. Mok and colleagues that showed a BMD benefit after switching people on an oral bisphosphonate to denosumab. However, he said, “There is a paucity of data regarding comparative efficacy of denosumab and the bisphosphonates in long-term steroid users.”
To explore any differences in a larger patient population, the investigators randomly assigned adults with SLE and other autoimmune conditions to the two treatments: denosumab 60 mg subcutaneoulsy every 6 months or oral alendronate 70 mg/week. All patients also received 3,000 mg calcium and 1,000 IU vitamin D3 (cholecalciferol) each day.
After three discontinuations in denosumab cohort and four in the alendronate group, the researchers evaluated 69 people taking denosumab and 70 others taking alendronate. The discontinuations were caused by noncompliance, Dr. Mok said, not by adverse events.
Adverse events were reported, but the rate did not differ significantly between groups. Dr. Mok highlighted some notable differences, including more minor infections and arthralgias reported in the denosumab cohort. Chest discomfort was reported in one denosumab recipient versus no patients in the alendronate group. Dyspepsia/upper GI symptoms and dizziness/vertigo occurred more often in the alendronate group.
Women were 96% of the study population, and mean age was 50 years. A majority, 81%, had underlying SLE. Other diagnoses included rheumatoid arthritis, myositis, antineutrophil cytoplasmic antibody–associated vasculitis, and polymyalgia rheumatica. The mean dose of prednisolone at study entry was 5.1 mg/day.
Key BMD and biomarker findings
BMD increased significantly in the spine, hip, and femoral neck in both treatment groups by 12 months. However, after adjustment for baseline BMD and covariates including age, menopause, and history of fracture, the gains in the denosumab group were significantly higher.
The increase in lumbar spine BMD at 12 months of 3.5% in the denosumab group versus 2.5% in the alendronate group was statistically significant (P = .045). Less significant was a 0.9% increase at the hip in the denosumab patients versus 1.6% in the alendronate group (P = .10), as well as femoral neck BMD gains of 1% in the denosumab group versus 1.5% in the alendronate group (P = .86).
Furthermore, “denosumab was more potent in suppressing the bone markers at 12 months,” Dr. Mok said.
Specifically, the percentage decrease in serum PINP (procollagen type I N-terminal propeptide) levels in the denosumab group was significantly greater than in the alendronate group (P = .001). Likewise, the decrease in CTX (C-terminal telopeptide of type I collagen) was significantly greater in the denosumab cohort versus the alendronate cohort (P < .001).
“Dr. Mok’s study was a well-controlled investigation. The superiority of denosumab was impressive, especially given the small group sizes of 69 and 70,” session comoderator Gregg Silverman, MD, professor in the department of internal medicine and the department of pathology at New York University, said when asked for comment.
“However, bone density measurements may not tell the whole story. These results support a bigger and much larger-scale study to confirm that rates of fracture on denosumab are also reduced.”
No new symptomatic fractures occurred in either group during the study. The investigators are evaluating for any new radiologic fractures, with results pending.
Dr. Mok said “results of our study in Asian patients are largely confirmatory” of a previous 2018 comparison study and a 2019 comparison study, each sponsored by Amgen.
A small sample size, short duration of treatment, and the open-label design were limitations of the study.
The trial was an investigator-initiated study. Dr. Mok and colleagues had no relevant financial disclosures. Dr. Silverman had no relevant financial disclosures.
SOURCE: Mok CC et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract 1442.
Denosumab boosted bone mineral density (BMD) over 12 months to a greater extent than did alendronate in a randomized, 12-month study. The investigator-initiated research compared BMD at the lumbar spine and elsewhere among people with systemic lupus erythematosus (SLE) and other autoimmune conditions. Long-term glucocorticoid therapy places some people in this group at higher risk for adverse effects of bone density loss.
“Glucocorticoids remain the mainstay of treatment of rheumatic diseases, but [they are] a major risk factor for osteoporosis and fracture,” study author Chi Chiu Mok, MD, said in an interview.
Compared with baseline, adults randomly assigned to denosumab had a 3.5% increase in lumbar spine BMD at 12 months, compared with 2.5% among those taking alendronate, a significant difference. Dr. Mok, a consultant and honorary associate professor in the department of medicine and nuclear medicine at Tuen Mun Hospital in Hong Kong, presented the study results at the virtual annual meeting of the American College of Rheumatology.
“Given the knowledge that denosumab is more effective than alendronate in raising spinal BMD in chronic users of GCs without increasing adverse events, this drug may be considered as an alternative first-line therapy in higher-risk patients and in those who are contraindicated for the oral bisphosphonates,” he said.
Cost considerations
Denosumab is a human monoclonal antibody administered as a subcutaneous injection, available under the brand names Prolia and Xgeva. Alendronate is an oral agent available as both generic and brand name formulations.
“Yes, denosumab is more expensive, more costly than oral alendronate, but our study shows efficacy is better for steroid users,” Dr. Mok said in answer to a question about cost disparity between the two agents during his presentation at the meeting. “For patients who are contraindicated or have low compliance for bisphosphonate, or are high-risk patients, I recommend first-line use of denosumab.”
Researchers previously studied these agents, including a smaller study by Dr. Mok and colleagues that showed a BMD benefit after switching people on an oral bisphosphonate to denosumab. However, he said, “There is a paucity of data regarding comparative efficacy of denosumab and the bisphosphonates in long-term steroid users.”
To explore any differences in a larger patient population, the investigators randomly assigned adults with SLE and other autoimmune conditions to the two treatments: denosumab 60 mg subcutaneoulsy every 6 months or oral alendronate 70 mg/week. All patients also received 3,000 mg calcium and 1,000 IU vitamin D3 (cholecalciferol) each day.
After three discontinuations in denosumab cohort and four in the alendronate group, the researchers evaluated 69 people taking denosumab and 70 others taking alendronate. The discontinuations were caused by noncompliance, Dr. Mok said, not by adverse events.
Adverse events were reported, but the rate did not differ significantly between groups. Dr. Mok highlighted some notable differences, including more minor infections and arthralgias reported in the denosumab cohort. Chest discomfort was reported in one denosumab recipient versus no patients in the alendronate group. Dyspepsia/upper GI symptoms and dizziness/vertigo occurred more often in the alendronate group.
Women were 96% of the study population, and mean age was 50 years. A majority, 81%, had underlying SLE. Other diagnoses included rheumatoid arthritis, myositis, antineutrophil cytoplasmic antibody–associated vasculitis, and polymyalgia rheumatica. The mean dose of prednisolone at study entry was 5.1 mg/day.
Key BMD and biomarker findings
BMD increased significantly in the spine, hip, and femoral neck in both treatment groups by 12 months. However, after adjustment for baseline BMD and covariates including age, menopause, and history of fracture, the gains in the denosumab group were significantly higher.
The increase in lumbar spine BMD at 12 months of 3.5% in the denosumab group versus 2.5% in the alendronate group was statistically significant (P = .045). Less significant was a 0.9% increase at the hip in the denosumab patients versus 1.6% in the alendronate group (P = .10), as well as femoral neck BMD gains of 1% in the denosumab group versus 1.5% in the alendronate group (P = .86).
Furthermore, “denosumab was more potent in suppressing the bone markers at 12 months,” Dr. Mok said.
Specifically, the percentage decrease in serum PINP (procollagen type I N-terminal propeptide) levels in the denosumab group was significantly greater than in the alendronate group (P = .001). Likewise, the decrease in CTX (C-terminal telopeptide of type I collagen) was significantly greater in the denosumab cohort versus the alendronate cohort (P < .001).
“Dr. Mok’s study was a well-controlled investigation. The superiority of denosumab was impressive, especially given the small group sizes of 69 and 70,” session comoderator Gregg Silverman, MD, professor in the department of internal medicine and the department of pathology at New York University, said when asked for comment.
“However, bone density measurements may not tell the whole story. These results support a bigger and much larger-scale study to confirm that rates of fracture on denosumab are also reduced.”
No new symptomatic fractures occurred in either group during the study. The investigators are evaluating for any new radiologic fractures, with results pending.
Dr. Mok said “results of our study in Asian patients are largely confirmatory” of a previous 2018 comparison study and a 2019 comparison study, each sponsored by Amgen.
A small sample size, short duration of treatment, and the open-label design were limitations of the study.
The trial was an investigator-initiated study. Dr. Mok and colleagues had no relevant financial disclosures. Dr. Silverman had no relevant financial disclosures.
SOURCE: Mok CC et al. Arthritis Rheumatol. 2020;72(suppl 10). ACR 2020, Abstract 1442.
FROM ACR 2020
Abrocitinib highly effective as long-term monotherapy in AD
through 48 weeks of follow-up in the JADE EXTEND study, Kristian Reich, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The head-turning outcomes achieved at the higher studied dose of 200 mg once daily as monotherapy – namely, 87% of patients had an EASI-75 response, defined as at least a 75% reduction from baseline in Eczema Area and Severity Index score, and 62% had an EASI-90 response – herald a new era in the management of atopic dermatitis, predicted Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany).
“I think we will see an evolution in the treatment goals in atopic dermatitis. It’s really good to see nearly 90% of the patients achieved EASI-75 over time. I am completely convinced that if you ultimately want to have a happy patient, you will see treatment goals moving up. We have already seen this in psoriasis. I want to see drugs that give the majority of my patients an EASI-75. And ultimately I want to see EASI-90 for my patients,” he said.
Concurrent with his presentation at the EADV congress, Pfizer announced it has filed for marketing approval of abrocitinib at 100 mg and 200 mg once daily for the treatment of moderate to severe AD. The Food and Drug Administration has granted the application priority review status, with a decision due next April. The company has also filed for marketing approval with the European Medicines Agency.
The JADE EXTEND study is an ongoing extension of the previously reported phase 3, randomized, double-blind, placebo-controlled, 12-week JADE MONO-1 and JADE MONO-2 trials. The two trials included a total of 309 patients on abrocitinib at 200 mg/day and 314 on the selective Janus kinase (JAK) 1 inhibitor at 100 mg/day, 519 of whom subsequently entered the long-term extension study on their same dose. The 70% who required no supplemental topical therapy through 48 weeks were the focus of the analysis presented by Dr. Reich.
The proportion of strong responders increased up until the week 24 or 36 assessments, then remained steady until week 48. For example, the EASI-75 rate in patients on abrocitinib at 200 mg/day rose from 82.5% at week 16, to 86.2% at week 24, 90.1% at week 36, and reached 87.2% at week 48. The EASI-90 rates at the same time points were 56.7%, 64.5%, 65.5%, and 61.6%, respectively. And the EASI-100 rates were 24%, 31.6%, 29.6%, and 24%, respectively.
Not surprisingly, the EASI-75 rates in patients on abrocitinib at 100 mg/day were less robust: 64.4% at week 16, 75.5% at week 24, 74.5% at week 36, and 68% at week 48.
An Investigator’s Global Assessment score of 0 or 1 – that is, clear or almost clear – was achieved at week 16 in 55% of patients on 200 mg/day, 64.5% at week 24, 66% at week 36, and 60.5% at week 48. In patients on the 100-mg dose, the corresponding figures were 36.5%, 46.6%, 53.3%, and 45.2%.
A hallmark of all of the JAK inhibitors under study for AD is what Dr. Reich characterized as “an amazingly fast reduction of itch,” the dominant symptom of the disease. A clinically meaningful reduction of at least 4 points in the Peak Pruritus Numerical Rating Scale – a response of 4 or greater is considered clinically important – from the mean baseline score of 7.1 was present at week 12 in 56.3% of patients on abrocitinib at 200 mg, in 74.3% at week 16, and in 72.5% at week 48. The proportion of patients achieving this endpoint on 100 mg was 41.6% at week 12, 49.4% at week 16, and 52% at week 48.
Serious treatment-emergent adverse events occurred in 6.1% of JADE EXTEND participants on abrocitinib at 100 mg and 12.8% of those on 200 mg. These events included oral herpes and elevated creatine phosphokinase levels. The sole case of pulmonary embolism that occurred during the study was deemed unrelated to treatment.
“What this is telling me here is there are no signals that we haven’t seen earlier with this drug and with other JAK inhibitors before,” the dermatologist observed. “But I want to see more data. I want to see the overall safety, not just for a year, but for 2, 3, 4, and 5 years.”
Asked by an audience member if nonresponsiveness to one JAK inhibitor predicts nonresponse to others, Dr. Reich speculated that it’s likely to be so. He noted that all three of the JAK inhibitors furthest along in the developmental pipeline for atopic dermatitis – abrocitinib, baricitinib, and upadacitinib – are inhibitors of JAK 1, although baricitinib also targets JAK 2.
“I would think that if you really are a nonresponder to any of these that it will be hard to get a good response with the others. We’re not talking about antibodies here, where there may be different epitopes. The affinity is different, and we have seen that if you have no response to a weak TNF [tumor necrosis factor] inhibitor, you can still have a response to a strong TNF inhibitor. I don’t expect the same here,” according to Dr. Reich.
He reported serving as an adviser to and paid clinical research for Pfizer, which sponsored JADE EXTEND, as well as more than two dozen other pharmaceutical companies.
through 48 weeks of follow-up in the JADE EXTEND study, Kristian Reich, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The head-turning outcomes achieved at the higher studied dose of 200 mg once daily as monotherapy – namely, 87% of patients had an EASI-75 response, defined as at least a 75% reduction from baseline in Eczema Area and Severity Index score, and 62% had an EASI-90 response – herald a new era in the management of atopic dermatitis, predicted Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany).
“I think we will see an evolution in the treatment goals in atopic dermatitis. It’s really good to see nearly 90% of the patients achieved EASI-75 over time. I am completely convinced that if you ultimately want to have a happy patient, you will see treatment goals moving up. We have already seen this in psoriasis. I want to see drugs that give the majority of my patients an EASI-75. And ultimately I want to see EASI-90 for my patients,” he said.
Concurrent with his presentation at the EADV congress, Pfizer announced it has filed for marketing approval of abrocitinib at 100 mg and 200 mg once daily for the treatment of moderate to severe AD. The Food and Drug Administration has granted the application priority review status, with a decision due next April. The company has also filed for marketing approval with the European Medicines Agency.
The JADE EXTEND study is an ongoing extension of the previously reported phase 3, randomized, double-blind, placebo-controlled, 12-week JADE MONO-1 and JADE MONO-2 trials. The two trials included a total of 309 patients on abrocitinib at 200 mg/day and 314 on the selective Janus kinase (JAK) 1 inhibitor at 100 mg/day, 519 of whom subsequently entered the long-term extension study on their same dose. The 70% who required no supplemental topical therapy through 48 weeks were the focus of the analysis presented by Dr. Reich.
The proportion of strong responders increased up until the week 24 or 36 assessments, then remained steady until week 48. For example, the EASI-75 rate in patients on abrocitinib at 200 mg/day rose from 82.5% at week 16, to 86.2% at week 24, 90.1% at week 36, and reached 87.2% at week 48. The EASI-90 rates at the same time points were 56.7%, 64.5%, 65.5%, and 61.6%, respectively. And the EASI-100 rates were 24%, 31.6%, 29.6%, and 24%, respectively.
Not surprisingly, the EASI-75 rates in patients on abrocitinib at 100 mg/day were less robust: 64.4% at week 16, 75.5% at week 24, 74.5% at week 36, and 68% at week 48.
An Investigator’s Global Assessment score of 0 or 1 – that is, clear or almost clear – was achieved at week 16 in 55% of patients on 200 mg/day, 64.5% at week 24, 66% at week 36, and 60.5% at week 48. In patients on the 100-mg dose, the corresponding figures were 36.5%, 46.6%, 53.3%, and 45.2%.
A hallmark of all of the JAK inhibitors under study for AD is what Dr. Reich characterized as “an amazingly fast reduction of itch,” the dominant symptom of the disease. A clinically meaningful reduction of at least 4 points in the Peak Pruritus Numerical Rating Scale – a response of 4 or greater is considered clinically important – from the mean baseline score of 7.1 was present at week 12 in 56.3% of patients on abrocitinib at 200 mg, in 74.3% at week 16, and in 72.5% at week 48. The proportion of patients achieving this endpoint on 100 mg was 41.6% at week 12, 49.4% at week 16, and 52% at week 48.
Serious treatment-emergent adverse events occurred in 6.1% of JADE EXTEND participants on abrocitinib at 100 mg and 12.8% of those on 200 mg. These events included oral herpes and elevated creatine phosphokinase levels. The sole case of pulmonary embolism that occurred during the study was deemed unrelated to treatment.
“What this is telling me here is there are no signals that we haven’t seen earlier with this drug and with other JAK inhibitors before,” the dermatologist observed. “But I want to see more data. I want to see the overall safety, not just for a year, but for 2, 3, 4, and 5 years.”
Asked by an audience member if nonresponsiveness to one JAK inhibitor predicts nonresponse to others, Dr. Reich speculated that it’s likely to be so. He noted that all three of the JAK inhibitors furthest along in the developmental pipeline for atopic dermatitis – abrocitinib, baricitinib, and upadacitinib – are inhibitors of JAK 1, although baricitinib also targets JAK 2.
“I would think that if you really are a nonresponder to any of these that it will be hard to get a good response with the others. We’re not talking about antibodies here, where there may be different epitopes. The affinity is different, and we have seen that if you have no response to a weak TNF [tumor necrosis factor] inhibitor, you can still have a response to a strong TNF inhibitor. I don’t expect the same here,” according to Dr. Reich.
He reported serving as an adviser to and paid clinical research for Pfizer, which sponsored JADE EXTEND, as well as more than two dozen other pharmaceutical companies.
through 48 weeks of follow-up in the JADE EXTEND study, Kristian Reich, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The head-turning outcomes achieved at the higher studied dose of 200 mg once daily as monotherapy – namely, 87% of patients had an EASI-75 response, defined as at least a 75% reduction from baseline in Eczema Area and Severity Index score, and 62% had an EASI-90 response – herald a new era in the management of atopic dermatitis, predicted Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany).
“I think we will see an evolution in the treatment goals in atopic dermatitis. It’s really good to see nearly 90% of the patients achieved EASI-75 over time. I am completely convinced that if you ultimately want to have a happy patient, you will see treatment goals moving up. We have already seen this in psoriasis. I want to see drugs that give the majority of my patients an EASI-75. And ultimately I want to see EASI-90 for my patients,” he said.
Concurrent with his presentation at the EADV congress, Pfizer announced it has filed for marketing approval of abrocitinib at 100 mg and 200 mg once daily for the treatment of moderate to severe AD. The Food and Drug Administration has granted the application priority review status, with a decision due next April. The company has also filed for marketing approval with the European Medicines Agency.
The JADE EXTEND study is an ongoing extension of the previously reported phase 3, randomized, double-blind, placebo-controlled, 12-week JADE MONO-1 and JADE MONO-2 trials. The two trials included a total of 309 patients on abrocitinib at 200 mg/day and 314 on the selective Janus kinase (JAK) 1 inhibitor at 100 mg/day, 519 of whom subsequently entered the long-term extension study on their same dose. The 70% who required no supplemental topical therapy through 48 weeks were the focus of the analysis presented by Dr. Reich.
The proportion of strong responders increased up until the week 24 or 36 assessments, then remained steady until week 48. For example, the EASI-75 rate in patients on abrocitinib at 200 mg/day rose from 82.5% at week 16, to 86.2% at week 24, 90.1% at week 36, and reached 87.2% at week 48. The EASI-90 rates at the same time points were 56.7%, 64.5%, 65.5%, and 61.6%, respectively. And the EASI-100 rates were 24%, 31.6%, 29.6%, and 24%, respectively.
Not surprisingly, the EASI-75 rates in patients on abrocitinib at 100 mg/day were less robust: 64.4% at week 16, 75.5% at week 24, 74.5% at week 36, and 68% at week 48.
An Investigator’s Global Assessment score of 0 or 1 – that is, clear or almost clear – was achieved at week 16 in 55% of patients on 200 mg/day, 64.5% at week 24, 66% at week 36, and 60.5% at week 48. In patients on the 100-mg dose, the corresponding figures were 36.5%, 46.6%, 53.3%, and 45.2%.
A hallmark of all of the JAK inhibitors under study for AD is what Dr. Reich characterized as “an amazingly fast reduction of itch,” the dominant symptom of the disease. A clinically meaningful reduction of at least 4 points in the Peak Pruritus Numerical Rating Scale – a response of 4 or greater is considered clinically important – from the mean baseline score of 7.1 was present at week 12 in 56.3% of patients on abrocitinib at 200 mg, in 74.3% at week 16, and in 72.5% at week 48. The proportion of patients achieving this endpoint on 100 mg was 41.6% at week 12, 49.4% at week 16, and 52% at week 48.
Serious treatment-emergent adverse events occurred in 6.1% of JADE EXTEND participants on abrocitinib at 100 mg and 12.8% of those on 200 mg. These events included oral herpes and elevated creatine phosphokinase levels. The sole case of pulmonary embolism that occurred during the study was deemed unrelated to treatment.
“What this is telling me here is there are no signals that we haven’t seen earlier with this drug and with other JAK inhibitors before,” the dermatologist observed. “But I want to see more data. I want to see the overall safety, not just for a year, but for 2, 3, 4, and 5 years.”
Asked by an audience member if nonresponsiveness to one JAK inhibitor predicts nonresponse to others, Dr. Reich speculated that it’s likely to be so. He noted that all three of the JAK inhibitors furthest along in the developmental pipeline for atopic dermatitis – abrocitinib, baricitinib, and upadacitinib – are inhibitors of JAK 1, although baricitinib also targets JAK 2.
“I would think that if you really are a nonresponder to any of these that it will be hard to get a good response with the others. We’re not talking about antibodies here, where there may be different epitopes. The affinity is different, and we have seen that if you have no response to a weak TNF [tumor necrosis factor] inhibitor, you can still have a response to a strong TNF inhibitor. I don’t expect the same here,” according to Dr. Reich.
He reported serving as an adviser to and paid clinical research for Pfizer, which sponsored JADE EXTEND, as well as more than two dozen other pharmaceutical companies.
FROM THE EADV CONGRESS
VA joins Pentagon in recruiting volunteers for COVID vaccine trials
according to officials with the VA and Operation Warp Speed, the Trump administration’s initiative to fast-track a coronavirus vaccine.
The largely unpublicized effort follows a Department of Defense announcement in September that it has partnered with AstraZeneca to recruit volunteers at five of its medical facilities, which are separate from the VA system. DOD is also is in talks with developers of other vaccine candidates, although officials won’t say which ones.
Both federal departments have long experience in medical research and diverse populations – a crucial component of effective clinical trials, said J. Stephen Morrison, senior vice president and director of global health policy at the Center for Strategic and International Studies, a bipartisan think tank in Washington.
Since active troops are essential to national security, and veterans are extremely vulnerable to COVID-19, both departments have a vested interest in supporting the development of safe, effective vaccines, Mr. Morrison said.
“On the DOD active servicemen and -women side, it’s a question of making sure they’re ready, they are protected,” Mr. Morrison said. “With VA, their population, all elderly and infirm with underlying conditions, they could really be suffering if we don’t get a vaccine.”
According to a VA website, of its 20 medical centers involved, 17 would be part of the Johnson & Johnson vaccine trial, while the three others are recruiting – or have completed recruitment – for advanced-stage trials for Moderna, AstraZeneca, and Pfizer vaccines.
Matthew Hepburn, MD, head of vaccine development at Operation Warp Speed, said the VA effort lets veterans contribute to the overall well-being of the country.
“This is another way they can continue to serve in this way, fighting the pandemic as a volunteer,” Dr. Hepburn said during a discussion of vaccine and therapeutics development hosted by the Heritage Foundation on Oct. 27.
It’s not unusual for the military to participate in multicenter trials for treatments of ailments as diverse as cancer and trauma. Historically, many vaccines have been tested first by the military.
In the general population, clinicians often have difficulty recruiting African Americans and other minorities for medical research, and “the military provides a rich opportunity to find volunteers for those groups,” said retired Rear Adm. Thomas Cullison, MD, a doctor and former deputy surgeon general for the Navy.
Military health facilities are held to the same standards as private research facilities, he said.
No service members will be required to participate in the COVID vaccine trials. All volunteers will be paid by the developer.
Support for routine vaccinations runs high in the military, but some have expressed concerns about new vaccines and mandatory inoculations, especially for anthrax. In a 2002 federal study, 85% of those who received that vaccine reported an adverse reaction, with just under half noticing minor redness at the injection site. But nearly a quarter of the side effects reported were more systemic, including fevers, chills, fatigue and joint pain.
That survey of a small group of National Guard and Reserve members found that, while 73% said they believe immunizations are effective, two-thirds said they did not support the mandatory anthrax program, and 6 in 10 said they were not satisfied with the information they were given on the vaccines.
To quell concerns over the military’s role in supporting COVID vaccine development, the Pentagon has reiterated that troops or their dependents interested in participating in the research must provide voluntary written consent, and they will be allowed to take part only if they will be in the same location for the length of the research, expected to last at least 2 years.
In addition, active-duty members such as new recruits and boot camp participants will not be allowed to volunteer because they are “considered vulnerable from an ethical and regulatory standpoint,” an official said.
At the VA, officials are seeking to recruit healthy veterans aged 18-65 years old who are not pregnant and may be at risk for exposure. As with trials conducted in civilian facilities, participants will be paid by the developer, VA spokesperson Christina Noel said.
Also, VA nurses and caseworkers also are being asked to identify their sickest, highest-risk patients to determine who should be at the top of the list once a vaccine is approved, according to a VA nurse and other health officials who asked not to be identified because they were not authorized to speak with the press.
The U.S. military has a long history of contributing to research on vaccines, including a key role in developing inoculations against yellow fever and adenovirus, and the Walter Reed Army Institute of Research is developing its own vaccine against the coronavirus.
Some segments of the population remain skeptical of federal medical experiments. A survey by AP-NORC in May found that Black people are particularly reluctant to get the coronavirus vaccine. Many have concerns about federal research in part because of associations with the infamous Tuskegee Institute syphilis experiments, in which U.S. Public Health Service officials intentionally withheld a cure from Black men infected with the disease.
But Mr. Morrison, of the Center for Strategic and International Studies, said the Defense Department and VA are a “natural fit” for the COVID vaccine trials.
“DOD has lots of expertise. They know how to vaccinate; they know how to reach communities. They have a whole science infrastructure and research-and-development infrastructure. And when you are thinking what the mission of VA is, [VA] sees this is part of their mission,” Mr. Morrison said.
The Defense Department announced its agreement with AstraZeneca in September, shortly before the drugmaker’s vaccine trial was put on hold to study a serious medical condition that one participant reported. That research was approved by the Food and Drug Administration to begin again Oct. 23. The military plans to restart its efforts to recruit 3,000 volunteers.
The Pentagon has also signed an agreement with another vaccine developer, the head of the Defense Health Agency, Army Lt. Gen. Ronald Place, told reporters Oct. 8. He wouldn’t provide the company’s name.
Senator Elizabeth Warren (D-Mass.) and Senator Mazie Hirono (D-Hawaii) have called, unsuccessfully, for the Senate Armed Services Committee to investigate what they say is a lack of Pentagon transparency on its role in vaccine development and distribution. The Defense Department has awarded more than $6 billion in Operation Warp Speed contracts through an intermediary, Advanced Technology International, and the two senators want more information about those contracts.
“There may well be a valuable role for DoD officials in [Operation Warp Speed] – particularly given the department’s logistical capacity,” they wrote to the committee chair and ranking member. “But it is important that Congress conduct appropriate oversight of, and understand, DoD’s activities in this area.”
Neither department has disclosed the financial arrangements they have made with developers to support the vaccine research.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
according to officials with the VA and Operation Warp Speed, the Trump administration’s initiative to fast-track a coronavirus vaccine.
The largely unpublicized effort follows a Department of Defense announcement in September that it has partnered with AstraZeneca to recruit volunteers at five of its medical facilities, which are separate from the VA system. DOD is also is in talks with developers of other vaccine candidates, although officials won’t say which ones.
Both federal departments have long experience in medical research and diverse populations – a crucial component of effective clinical trials, said J. Stephen Morrison, senior vice president and director of global health policy at the Center for Strategic and International Studies, a bipartisan think tank in Washington.
Since active troops are essential to national security, and veterans are extremely vulnerable to COVID-19, both departments have a vested interest in supporting the development of safe, effective vaccines, Mr. Morrison said.
“On the DOD active servicemen and -women side, it’s a question of making sure they’re ready, they are protected,” Mr. Morrison said. “With VA, their population, all elderly and infirm with underlying conditions, they could really be suffering if we don’t get a vaccine.”
According to a VA website, of its 20 medical centers involved, 17 would be part of the Johnson & Johnson vaccine trial, while the three others are recruiting – or have completed recruitment – for advanced-stage trials for Moderna, AstraZeneca, and Pfizer vaccines.
Matthew Hepburn, MD, head of vaccine development at Operation Warp Speed, said the VA effort lets veterans contribute to the overall well-being of the country.
“This is another way they can continue to serve in this way, fighting the pandemic as a volunteer,” Dr. Hepburn said during a discussion of vaccine and therapeutics development hosted by the Heritage Foundation on Oct. 27.
It’s not unusual for the military to participate in multicenter trials for treatments of ailments as diverse as cancer and trauma. Historically, many vaccines have been tested first by the military.
In the general population, clinicians often have difficulty recruiting African Americans and other minorities for medical research, and “the military provides a rich opportunity to find volunteers for those groups,” said retired Rear Adm. Thomas Cullison, MD, a doctor and former deputy surgeon general for the Navy.
Military health facilities are held to the same standards as private research facilities, he said.
No service members will be required to participate in the COVID vaccine trials. All volunteers will be paid by the developer.
Support for routine vaccinations runs high in the military, but some have expressed concerns about new vaccines and mandatory inoculations, especially for anthrax. In a 2002 federal study, 85% of those who received that vaccine reported an adverse reaction, with just under half noticing minor redness at the injection site. But nearly a quarter of the side effects reported were more systemic, including fevers, chills, fatigue and joint pain.
That survey of a small group of National Guard and Reserve members found that, while 73% said they believe immunizations are effective, two-thirds said they did not support the mandatory anthrax program, and 6 in 10 said they were not satisfied with the information they were given on the vaccines.
To quell concerns over the military’s role in supporting COVID vaccine development, the Pentagon has reiterated that troops or their dependents interested in participating in the research must provide voluntary written consent, and they will be allowed to take part only if they will be in the same location for the length of the research, expected to last at least 2 years.
In addition, active-duty members such as new recruits and boot camp participants will not be allowed to volunteer because they are “considered vulnerable from an ethical and regulatory standpoint,” an official said.
At the VA, officials are seeking to recruit healthy veterans aged 18-65 years old who are not pregnant and may be at risk for exposure. As with trials conducted in civilian facilities, participants will be paid by the developer, VA spokesperson Christina Noel said.
Also, VA nurses and caseworkers also are being asked to identify their sickest, highest-risk patients to determine who should be at the top of the list once a vaccine is approved, according to a VA nurse and other health officials who asked not to be identified because they were not authorized to speak with the press.
The U.S. military has a long history of contributing to research on vaccines, including a key role in developing inoculations against yellow fever and adenovirus, and the Walter Reed Army Institute of Research is developing its own vaccine against the coronavirus.
Some segments of the population remain skeptical of federal medical experiments. A survey by AP-NORC in May found that Black people are particularly reluctant to get the coronavirus vaccine. Many have concerns about federal research in part because of associations with the infamous Tuskegee Institute syphilis experiments, in which U.S. Public Health Service officials intentionally withheld a cure from Black men infected with the disease.
But Mr. Morrison, of the Center for Strategic and International Studies, said the Defense Department and VA are a “natural fit” for the COVID vaccine trials.
“DOD has lots of expertise. They know how to vaccinate; they know how to reach communities. They have a whole science infrastructure and research-and-development infrastructure. And when you are thinking what the mission of VA is, [VA] sees this is part of their mission,” Mr. Morrison said.
The Defense Department announced its agreement with AstraZeneca in September, shortly before the drugmaker’s vaccine trial was put on hold to study a serious medical condition that one participant reported. That research was approved by the Food and Drug Administration to begin again Oct. 23. The military plans to restart its efforts to recruit 3,000 volunteers.
The Pentagon has also signed an agreement with another vaccine developer, the head of the Defense Health Agency, Army Lt. Gen. Ronald Place, told reporters Oct. 8. He wouldn’t provide the company’s name.
Senator Elizabeth Warren (D-Mass.) and Senator Mazie Hirono (D-Hawaii) have called, unsuccessfully, for the Senate Armed Services Committee to investigate what they say is a lack of Pentagon transparency on its role in vaccine development and distribution. The Defense Department has awarded more than $6 billion in Operation Warp Speed contracts through an intermediary, Advanced Technology International, and the two senators want more information about those contracts.
“There may well be a valuable role for DoD officials in [Operation Warp Speed] – particularly given the department’s logistical capacity,” they wrote to the committee chair and ranking member. “But it is important that Congress conduct appropriate oversight of, and understand, DoD’s activities in this area.”
Neither department has disclosed the financial arrangements they have made with developers to support the vaccine research.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
according to officials with the VA and Operation Warp Speed, the Trump administration’s initiative to fast-track a coronavirus vaccine.
The largely unpublicized effort follows a Department of Defense announcement in September that it has partnered with AstraZeneca to recruit volunteers at five of its medical facilities, which are separate from the VA system. DOD is also is in talks with developers of other vaccine candidates, although officials won’t say which ones.
Both federal departments have long experience in medical research and diverse populations – a crucial component of effective clinical trials, said J. Stephen Morrison, senior vice president and director of global health policy at the Center for Strategic and International Studies, a bipartisan think tank in Washington.
Since active troops are essential to national security, and veterans are extremely vulnerable to COVID-19, both departments have a vested interest in supporting the development of safe, effective vaccines, Mr. Morrison said.
“On the DOD active servicemen and -women side, it’s a question of making sure they’re ready, they are protected,” Mr. Morrison said. “With VA, their population, all elderly and infirm with underlying conditions, they could really be suffering if we don’t get a vaccine.”
According to a VA website, of its 20 medical centers involved, 17 would be part of the Johnson & Johnson vaccine trial, while the three others are recruiting – or have completed recruitment – for advanced-stage trials for Moderna, AstraZeneca, and Pfizer vaccines.
Matthew Hepburn, MD, head of vaccine development at Operation Warp Speed, said the VA effort lets veterans contribute to the overall well-being of the country.
“This is another way they can continue to serve in this way, fighting the pandemic as a volunteer,” Dr. Hepburn said during a discussion of vaccine and therapeutics development hosted by the Heritage Foundation on Oct. 27.
It’s not unusual for the military to participate in multicenter trials for treatments of ailments as diverse as cancer and trauma. Historically, many vaccines have been tested first by the military.
In the general population, clinicians often have difficulty recruiting African Americans and other minorities for medical research, and “the military provides a rich opportunity to find volunteers for those groups,” said retired Rear Adm. Thomas Cullison, MD, a doctor and former deputy surgeon general for the Navy.
Military health facilities are held to the same standards as private research facilities, he said.
No service members will be required to participate in the COVID vaccine trials. All volunteers will be paid by the developer.
Support for routine vaccinations runs high in the military, but some have expressed concerns about new vaccines and mandatory inoculations, especially for anthrax. In a 2002 federal study, 85% of those who received that vaccine reported an adverse reaction, with just under half noticing minor redness at the injection site. But nearly a quarter of the side effects reported were more systemic, including fevers, chills, fatigue and joint pain.
That survey of a small group of National Guard and Reserve members found that, while 73% said they believe immunizations are effective, two-thirds said they did not support the mandatory anthrax program, and 6 in 10 said they were not satisfied with the information they were given on the vaccines.
To quell concerns over the military’s role in supporting COVID vaccine development, the Pentagon has reiterated that troops or their dependents interested in participating in the research must provide voluntary written consent, and they will be allowed to take part only if they will be in the same location for the length of the research, expected to last at least 2 years.
In addition, active-duty members such as new recruits and boot camp participants will not be allowed to volunteer because they are “considered vulnerable from an ethical and regulatory standpoint,” an official said.
At the VA, officials are seeking to recruit healthy veterans aged 18-65 years old who are not pregnant and may be at risk for exposure. As with trials conducted in civilian facilities, participants will be paid by the developer, VA spokesperson Christina Noel said.
Also, VA nurses and caseworkers also are being asked to identify their sickest, highest-risk patients to determine who should be at the top of the list once a vaccine is approved, according to a VA nurse and other health officials who asked not to be identified because they were not authorized to speak with the press.
The U.S. military has a long history of contributing to research on vaccines, including a key role in developing inoculations against yellow fever and adenovirus, and the Walter Reed Army Institute of Research is developing its own vaccine against the coronavirus.
Some segments of the population remain skeptical of federal medical experiments. A survey by AP-NORC in May found that Black people are particularly reluctant to get the coronavirus vaccine. Many have concerns about federal research in part because of associations with the infamous Tuskegee Institute syphilis experiments, in which U.S. Public Health Service officials intentionally withheld a cure from Black men infected with the disease.
But Mr. Morrison, of the Center for Strategic and International Studies, said the Defense Department and VA are a “natural fit” for the COVID vaccine trials.
“DOD has lots of expertise. They know how to vaccinate; they know how to reach communities. They have a whole science infrastructure and research-and-development infrastructure. And when you are thinking what the mission of VA is, [VA] sees this is part of their mission,” Mr. Morrison said.
The Defense Department announced its agreement with AstraZeneca in September, shortly before the drugmaker’s vaccine trial was put on hold to study a serious medical condition that one participant reported. That research was approved by the Food and Drug Administration to begin again Oct. 23. The military plans to restart its efforts to recruit 3,000 volunteers.
The Pentagon has also signed an agreement with another vaccine developer, the head of the Defense Health Agency, Army Lt. Gen. Ronald Place, told reporters Oct. 8. He wouldn’t provide the company’s name.
Senator Elizabeth Warren (D-Mass.) and Senator Mazie Hirono (D-Hawaii) have called, unsuccessfully, for the Senate Armed Services Committee to investigate what they say is a lack of Pentagon transparency on its role in vaccine development and distribution. The Defense Department has awarded more than $6 billion in Operation Warp Speed contracts through an intermediary, Advanced Technology International, and the two senators want more information about those contracts.
“There may well be a valuable role for DoD officials in [Operation Warp Speed] – particularly given the department’s logistical capacity,” they wrote to the committee chair and ranking member. “But it is important that Congress conduct appropriate oversight of, and understand, DoD’s activities in this area.”
Neither department has disclosed the financial arrangements they have made with developers to support the vaccine research.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
What to know as ACA heads to Supreme Court – again
The case, California v. Texas, is the result of a change to the health law made by Congress in 2017. As part of a major tax bill, Congress reduced to zero the penalty for not having health insurance. But it was that penalty – a tax – that the high court ruled made the law constitutional in a 2012 decision, argues a group of Republican state attorneys general. Without the tax, they say in their suit, the rest of the law must fall, too.
After originally contending that the entire law should not be struck down when the suit was filed in 2018, the Trump administration changed course in 2019 and joined the GOP officials who brought the case.
Here are some key questions and answers about the case.
What are the possibilities for how the court could rule?
There is a long list of ways this could play out.
The justices could declare the entire law unconstitutional – which is what a federal district judge in Texas ruled in December 2018. But legal experts say that’s not the most likely outcome of this case.
First, the court may avoid deciding the case on its merits entirely by ruling that the plaintiffs do not have “standing” to sue. The central issue in the case is whether the requirement in the law to have insurance – which remains even though Congress eliminated the penalty or tax – is constitutional. But states are not subject to the so-called individual mandate, so some analysts suggest the Republican officials have no standing. In addition, questions have been raised about the individual plaintiffs in the case, two consultants from Texas who argue that they felt compelled to buy insurance even without a possible penalty.
The court could also rule that, by eliminating the penalty but not the rest of the mandate (which Congress could not do in that 2017 tax bill for procedural reasons), lawmakers “didn’t mean to coerce anyone to do anything, and so there’s no constitutional problem,” University of Michigan law professor Nicholas Bagley said in a recent webinar for the NIHCM Foundation, the Commonwealth Fund, and the University of Southern California’s Center for Health Journalism.
Or, said Bagley, the court could rule that, without the tax, the requirement to have health insurance is unconstitutional, but the rest of the law is not. In that case, the justices might strike the mandate only, which would have basically no impact.
It gets more complicated if the court decides that, as the plaintiffs argue, the individual mandate language without the penalty is unconstitutional and so closely tied to other parts of the law that some of them must fall as well.
Even there the court has choices. One option would be, as the Trump administration originally argued, to strike down the mandate and just the pieces of the law most closely related to it – which happen to include the insurance protections for people with preexisting conditions, an extremely popular provision of the law. The two parts are connected because the original purpose of the mandate was to make sure enough healthy people sign up for insurance to offset the added costs to insurers of sicker people.
Another option, of course, would be for the court to follow the lead of the Texas judge and strike down the entire law.
While that’s not the most likely outcome, said Bagley, if it happens it could be “a hot mess” for the nation’s entire health care system. As just one example, he said, “every hospital is getting paid pursuant to changes made by the ACA. How do you even go about making payments if the thing that you are looking to guide what those payments ought to be is itself invalid?”
What impact will new Justice Amy Coney Barrett have?
Perhaps a lot. Before the death of Justice Ruth Bader Ginsburg, most court observers thought the case was highly unlikely to result in the entire law being struck down. That’s because Chief Justice John Roberts voted to uphold the law in 2012, and again when it was challenged in a less sweeping way in 2015.
But with Barrett replacing Ginsburg, even if Roberts joined the court’s remaining three liberals they could still be outvoted by the other five conservatives. Barrett was coy about her views on the Affordable Care Act during her confirmation hearings in October, but she has written that she thinks Roberts was wrong to uphold the law in 2012.
Could a new president and Congress make the case go away?
Many have suggested that, if Joe Biden assumes the presidency, his Justice Department could simply drop the case. But the administration did not bring the case; the GOP state officials did. And while normally the Justice Department’s job is to defend existing laws in court, in this case the ACA is being defended by a group of Democratic state attorneys general. A new administration could change that position, but that’s not the same as dropping the case.
Congress, on the other hand, could easily make the case moot. It could add back even a nominal financial penalty for not having insurance. It could eliminate the mandate altogether, although that would require 60 votes in the Senate under current rules. Congress could also pass a “severability” provision saying that, if any portion of the law is struck down, the rest should remain.
“The problem is not technical,” said Bagley. “It’s political.”
What is the timeline for a decision? Could the court delay implementation of its ruling?
The court usually hears oral arguments in a case months before it issues a decision. Unless the decision is unanimous or turns out to be very simple, Bagley said, he would expect to see an opinion “sometime in the spring.”
As to whether the court could find some or all of the law unconstitutional but delay when its decision takes effect, Bagley said that happened from time to time as recently as the 1970s. “That practice has been more or less abandoned,” he said, but in the case of a law so large, “you could imagine the Supreme Court using its discretion to say the decision wouldn’t take effect immediately.”
If the court does invalidate the entire ACA, Congress could act to fix things, but it’s unclear if it will be able to, especially if Republicans still control the Senate. If the justices strike the law, Bagley said, “I honestly think the likeliest outcome is that Congress runs around like a chicken with its head cut off, doesn’t come to a deal, and we’re back to where we were before 2010” when the ACA passed.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The case, California v. Texas, is the result of a change to the health law made by Congress in 2017. As part of a major tax bill, Congress reduced to zero the penalty for not having health insurance. But it was that penalty – a tax – that the high court ruled made the law constitutional in a 2012 decision, argues a group of Republican state attorneys general. Without the tax, they say in their suit, the rest of the law must fall, too.
After originally contending that the entire law should not be struck down when the suit was filed in 2018, the Trump administration changed course in 2019 and joined the GOP officials who brought the case.
Here are some key questions and answers about the case.
What are the possibilities for how the court could rule?
There is a long list of ways this could play out.
The justices could declare the entire law unconstitutional – which is what a federal district judge in Texas ruled in December 2018. But legal experts say that’s not the most likely outcome of this case.
First, the court may avoid deciding the case on its merits entirely by ruling that the plaintiffs do not have “standing” to sue. The central issue in the case is whether the requirement in the law to have insurance – which remains even though Congress eliminated the penalty or tax – is constitutional. But states are not subject to the so-called individual mandate, so some analysts suggest the Republican officials have no standing. In addition, questions have been raised about the individual plaintiffs in the case, two consultants from Texas who argue that they felt compelled to buy insurance even without a possible penalty.
The court could also rule that, by eliminating the penalty but not the rest of the mandate (which Congress could not do in that 2017 tax bill for procedural reasons), lawmakers “didn’t mean to coerce anyone to do anything, and so there’s no constitutional problem,” University of Michigan law professor Nicholas Bagley said in a recent webinar for the NIHCM Foundation, the Commonwealth Fund, and the University of Southern California’s Center for Health Journalism.
Or, said Bagley, the court could rule that, without the tax, the requirement to have health insurance is unconstitutional, but the rest of the law is not. In that case, the justices might strike the mandate only, which would have basically no impact.
It gets more complicated if the court decides that, as the plaintiffs argue, the individual mandate language without the penalty is unconstitutional and so closely tied to other parts of the law that some of them must fall as well.
Even there the court has choices. One option would be, as the Trump administration originally argued, to strike down the mandate and just the pieces of the law most closely related to it – which happen to include the insurance protections for people with preexisting conditions, an extremely popular provision of the law. The two parts are connected because the original purpose of the mandate was to make sure enough healthy people sign up for insurance to offset the added costs to insurers of sicker people.
Another option, of course, would be for the court to follow the lead of the Texas judge and strike down the entire law.
While that’s not the most likely outcome, said Bagley, if it happens it could be “a hot mess” for the nation’s entire health care system. As just one example, he said, “every hospital is getting paid pursuant to changes made by the ACA. How do you even go about making payments if the thing that you are looking to guide what those payments ought to be is itself invalid?”
What impact will new Justice Amy Coney Barrett have?
Perhaps a lot. Before the death of Justice Ruth Bader Ginsburg, most court observers thought the case was highly unlikely to result in the entire law being struck down. That’s because Chief Justice John Roberts voted to uphold the law in 2012, and again when it was challenged in a less sweeping way in 2015.
But with Barrett replacing Ginsburg, even if Roberts joined the court’s remaining three liberals they could still be outvoted by the other five conservatives. Barrett was coy about her views on the Affordable Care Act during her confirmation hearings in October, but she has written that she thinks Roberts was wrong to uphold the law in 2012.
Could a new president and Congress make the case go away?
Many have suggested that, if Joe Biden assumes the presidency, his Justice Department could simply drop the case. But the administration did not bring the case; the GOP state officials did. And while normally the Justice Department’s job is to defend existing laws in court, in this case the ACA is being defended by a group of Democratic state attorneys general. A new administration could change that position, but that’s not the same as dropping the case.
Congress, on the other hand, could easily make the case moot. It could add back even a nominal financial penalty for not having insurance. It could eliminate the mandate altogether, although that would require 60 votes in the Senate under current rules. Congress could also pass a “severability” provision saying that, if any portion of the law is struck down, the rest should remain.
“The problem is not technical,” said Bagley. “It’s political.”
What is the timeline for a decision? Could the court delay implementation of its ruling?
The court usually hears oral arguments in a case months before it issues a decision. Unless the decision is unanimous or turns out to be very simple, Bagley said, he would expect to see an opinion “sometime in the spring.”
As to whether the court could find some or all of the law unconstitutional but delay when its decision takes effect, Bagley said that happened from time to time as recently as the 1970s. “That practice has been more or less abandoned,” he said, but in the case of a law so large, “you could imagine the Supreme Court using its discretion to say the decision wouldn’t take effect immediately.”
If the court does invalidate the entire ACA, Congress could act to fix things, but it’s unclear if it will be able to, especially if Republicans still control the Senate. If the justices strike the law, Bagley said, “I honestly think the likeliest outcome is that Congress runs around like a chicken with its head cut off, doesn’t come to a deal, and we’re back to where we were before 2010” when the ACA passed.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The case, California v. Texas, is the result of a change to the health law made by Congress in 2017. As part of a major tax bill, Congress reduced to zero the penalty for not having health insurance. But it was that penalty – a tax – that the high court ruled made the law constitutional in a 2012 decision, argues a group of Republican state attorneys general. Without the tax, they say in their suit, the rest of the law must fall, too.
After originally contending that the entire law should not be struck down when the suit was filed in 2018, the Trump administration changed course in 2019 and joined the GOP officials who brought the case.
Here are some key questions and answers about the case.
What are the possibilities for how the court could rule?
There is a long list of ways this could play out.
The justices could declare the entire law unconstitutional – which is what a federal district judge in Texas ruled in December 2018. But legal experts say that’s not the most likely outcome of this case.
First, the court may avoid deciding the case on its merits entirely by ruling that the plaintiffs do not have “standing” to sue. The central issue in the case is whether the requirement in the law to have insurance – which remains even though Congress eliminated the penalty or tax – is constitutional. But states are not subject to the so-called individual mandate, so some analysts suggest the Republican officials have no standing. In addition, questions have been raised about the individual plaintiffs in the case, two consultants from Texas who argue that they felt compelled to buy insurance even without a possible penalty.
The court could also rule that, by eliminating the penalty but not the rest of the mandate (which Congress could not do in that 2017 tax bill for procedural reasons), lawmakers “didn’t mean to coerce anyone to do anything, and so there’s no constitutional problem,” University of Michigan law professor Nicholas Bagley said in a recent webinar for the NIHCM Foundation, the Commonwealth Fund, and the University of Southern California’s Center for Health Journalism.
Or, said Bagley, the court could rule that, without the tax, the requirement to have health insurance is unconstitutional, but the rest of the law is not. In that case, the justices might strike the mandate only, which would have basically no impact.
It gets more complicated if the court decides that, as the plaintiffs argue, the individual mandate language without the penalty is unconstitutional and so closely tied to other parts of the law that some of them must fall as well.
Even there the court has choices. One option would be, as the Trump administration originally argued, to strike down the mandate and just the pieces of the law most closely related to it – which happen to include the insurance protections for people with preexisting conditions, an extremely popular provision of the law. The two parts are connected because the original purpose of the mandate was to make sure enough healthy people sign up for insurance to offset the added costs to insurers of sicker people.
Another option, of course, would be for the court to follow the lead of the Texas judge and strike down the entire law.
While that’s not the most likely outcome, said Bagley, if it happens it could be “a hot mess” for the nation’s entire health care system. As just one example, he said, “every hospital is getting paid pursuant to changes made by the ACA. How do you even go about making payments if the thing that you are looking to guide what those payments ought to be is itself invalid?”
What impact will new Justice Amy Coney Barrett have?
Perhaps a lot. Before the death of Justice Ruth Bader Ginsburg, most court observers thought the case was highly unlikely to result in the entire law being struck down. That’s because Chief Justice John Roberts voted to uphold the law in 2012, and again when it was challenged in a less sweeping way in 2015.
But with Barrett replacing Ginsburg, even if Roberts joined the court’s remaining three liberals they could still be outvoted by the other five conservatives. Barrett was coy about her views on the Affordable Care Act during her confirmation hearings in October, but she has written that she thinks Roberts was wrong to uphold the law in 2012.
Could a new president and Congress make the case go away?
Many have suggested that, if Joe Biden assumes the presidency, his Justice Department could simply drop the case. But the administration did not bring the case; the GOP state officials did. And while normally the Justice Department’s job is to defend existing laws in court, in this case the ACA is being defended by a group of Democratic state attorneys general. A new administration could change that position, but that’s not the same as dropping the case.
Congress, on the other hand, could easily make the case moot. It could add back even a nominal financial penalty for not having insurance. It could eliminate the mandate altogether, although that would require 60 votes in the Senate under current rules. Congress could also pass a “severability” provision saying that, if any portion of the law is struck down, the rest should remain.
“The problem is not technical,” said Bagley. “It’s political.”
What is the timeline for a decision? Could the court delay implementation of its ruling?
The court usually hears oral arguments in a case months before it issues a decision. Unless the decision is unanimous or turns out to be very simple, Bagley said, he would expect to see an opinion “sometime in the spring.”
As to whether the court could find some or all of the law unconstitutional but delay when its decision takes effect, Bagley said that happened from time to time as recently as the 1970s. “That practice has been more or less abandoned,” he said, but in the case of a law so large, “you could imagine the Supreme Court using its discretion to say the decision wouldn’t take effect immediately.”
If the court does invalidate the entire ACA, Congress could act to fix things, but it’s unclear if it will be able to, especially if Republicans still control the Senate. If the justices strike the law, Bagley said, “I honestly think the likeliest outcome is that Congress runs around like a chicken with its head cut off, doesn’t come to a deal, and we’re back to where we were before 2010” when the ACA passed.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.