User login
Continued Dosing of Oritavancin for Complicated Gram-Positive Infections
Oritavancin is a lipoglycopeptide antibiotic. The US Food and Drug Administration (FDA) approved oritavancin in 2014 for adults with acute bacterial skin and skin structure infections (ABSSSI).1 The antibiotic is currently FDA approved for infections caused by Gram-positive organisms, including methicillin-resistant and methicillinsusceptible Staphylococcus aureus (MRSA, MSSA), a variety of Streptococcus species, and vancomycin-susceptible Enterococcus faecalis (VSE). Oritavancin demonstrates concentrationdependent bactericidal activity and has a half-life of 245 hours. This half-life allows for treatment of ABSSSI with a single 1,200 mg IV dose, which has been shown to be noninferior to vancomycin dosed twice daily for 7 to 10 days.1-3
Proposal for Expanded Uses
Although the approved indication for oritavancin is narrow, in vitro studies have shown that oritavancin also has activity against vancomycin-resistant enterococci (VRE), and rabbit studies have demonstrated its excellent bone penetration.4,5 These findings have raised the question of whether oritavancin can be safely and effectively used for infections such as endocarditis, osteomyelitis, and bacteremia, which are often caused by invasive Grampositive organisms. These types of invasive infections, particularly when MRSA is implicated, generally require IV antibiotic therapy for several weeks, often with vancomycin.6
To avoid long hospital stays solely for antibiotic administration, health care practitioners will often use outpatient parenteral antimicrobial therapy (OPAT). However, using OPAT presents many challenges due to the need for frequent dosing, the risk of peripheral or central-line infections, and therapeutic drug monitoring when using vancomycin; additionally, administration and line care oftentimes require caregiver support, which may not be present for all patients.7 Concerns also have been raised regarding the use of OPAT in patients with a history of IV drug use due to the potential increased risk of line infections or line abuse. Few studies have explored OPAT in this population, and the Infectious Diseases Society of America OPAT guidelines recommend that the decision to use OPAT should be made on a case-by-case basis.7 Thus, patients who are deemed inappropriate for OPAT oftentimes remain hospitalized or reside briefly in nursing facilities solely for antibiotic administration
Oritavancin’s long half-life and potent activity against Gram-positive organisms has led to increased interest in off-label use of infrequent dosing intervals, such as weekly, to treat complicated and invasive infections. Weekly rather than daily dosing would allow for less burdensome antibiotic administration regimens and shorter hospital stays especially for patients who are not candidates for OPAT.
Efficacy of Continued Dosing
This proposed weekly dosing pattern, referred to as continued dosing or a multiple-dose regimen, has gained traction in the literature. To date, no randomized controlled trials have been conducted to assess oritavancin’s efficacy in off-label indications or continued dosing, but several case reports and retrospective cohort analyses show promising outcomes.8-16 In an analysis of data from the Clinical and Historic Registry and Orbactiv Medical Evaluation (CHROME) patient registry, 32 patients received multiple doses of oritavancin for complicated Gram-positive infections with a 93.8% overall clinical success rate, including success rates of 90.9% (10/11) for general bone and joint infections and 87.5% (7/8) for patients diagnosed specifically with osteomyelitis.8
Patients received between 2 and 10 doses of 1,200 mg IV given every 6 to 14 days. Johnson and colleagues report using oritavancin 1,200 mg IV every other day for 3 doses followed by 1,200 mg IV once weekly for a patient with daptomycin- and vancomycin-resistant Enterococcus endocarditis, resulting in negative blood cultures while on therapy.9 However, source control via valve replacement and postoperative oritavancin 1,200 mg IV twice weekly for 10 weeks was required to fully clear the infection.
Schulz and colleagues published a retrospective cohort analysis of 17 patients who received multiple doses of oritavancin for complicated bacterial infections, including osteomyelitis, pneumonia, and bacteremia.10 They reported 100% of patients were either successfully cured or had demonstrable improvements in their infections by using a 1,200 mg IV loading dose followed by 800 mg IV if the second dose was given within 7 days or 1,200 mg IV if the second dose was given more than 10 days later. Patients received between 2 and 18 total doses, with 6 out of 17 (35%) receiving only 2 doses. One patient who received 18 doses was an outlier, as her treatment goal was palliative suppression due to an infected endovascular graft that could not be removed.
In a published case series, 1 of 10 patients receiving oritavancin for invasive Grampositive infections received multiple doses of oritavancin for an MSSA deep tissue infection.11 The 3 total doses (strength not reported) were separated by 19 days and 14 days and resulted in cure. Several case reports and a retrospective chart review study specifically show the effectiveness of oritavancin for osteomyelitis caused by MSSA, MRSA, and VRE.12-16 However, dosing strategies varied widely after the initial 1,200 mg IV loading dose.
Drug Interactions, Safety, and Tolerability
Oritavancin has minimal drug-drug interactions, the most notable being with anticoagulants. 1 Use of IV heparin within 120 hours of oritavancin administration can falsely elevate activated partial thromboplastin time (aPTT) levels; therefore, heparin should not be monitored with aPTT during this period. Oritavancin also can artificially prolong international normalized ratio (INR) values for up to 12 hours, and dose adjustments based on INRs during this window are not recommended. Of note, factor Xa laboratory monitoring is unaffected by oritavancin, as it does not depend on phospholipid reagents as do aPTT and INR measurements.
Oritavancin has been shown to be well tolerated when dosed according to both the package insert and continued dosing strategies. The most common adverse effects (AEs) (≥ 3%), occurring at similar rates to vancomycin, are nausea, vomiting, diarrhea, headache, and limb and subcutaneous abscesses.1 Infusion reactions also have been reported, although they are usually reversible on slowing or stopping the infusion. It is worth noting that the use of oritavancin for osteomyelitis is not recommended in the product labeling, as an increased rate of osteomyelitis was observed in the oritavancin vs IV vancomycin groups for the treatment of patients with acute bacterial skin and skin structure infection (SOLO) trials (0.6% in oritavancin group vs 0.1% in vancomycin group, statistical significance not reported).17 However, it was postulated that these osteomyelitis cases were likely present, yet not recognized, at baseline and were not the result of administering oritavancin. This conclusion is further corroborated by previously presented research demonstrating successful cure of osteomyelitis with continued dosing strategies.12-16
Many patients receiving multiple doses of oritavancin did not experience AEs or laboratory abnormalities.13,15 Four of 17 patients (24%) in one retrospective review experienced AEs, including infusion reactions, anemia, and leukopenia; all were reversible on discontinuation of oritavancin, and contributions of other antibiotics in some cases could not be ruled out.10 One patient experienced taste disturbance for several hours after each infusion, and a second had documented hearing loss after 3 doses of oritavancin in a 33-day period, though she had received 6 weeks of IV vancomycin prior to oritavancin.11,12 A patient treated for daptomycin- and vancomycinresistant Enterococcus faecium prosthetic valve endocarditis experienced nausea, anorexia, and minor liver function test (LFT) abnormalities after cumulative oritavancin exposure over 18 weeks.9 On discontinuation of the drug, nausea and anorexia improved, and LFTs normalized 11 months later. Overall, AEs reported with continued dosing of oritavancin have been minimal and largely reversible, mimicking the AEs in the product labeling for traditional dosing. This suggests that using a continued dosing strategy may not result in worse or more frequent AEs, though randomized controlled trials are needed to fully ascertain these preliminary findings.
Conclusions
The literature supporting the use of oritavancin beyond single-dose administration for ABSSSI is growing. Continued dosing regimens have been well tolerated and have resulted in clinical cure for many patients with barriers to first-line treatment and complicated or invasive infections. While randomized controlled trials are needed to concretely demonstrate the efficacy and safety of continued dosing of oritavancin, it may fill an important treatment niche in this era of growing antibiotic resistance and increasing complexity of patient cases.
1. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2019.
2. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422
3. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778
4. Sweeney D, Stoneburner A, Shinabarger DL, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72(2):622-624. doi.10.1093/jac/dkw451
5. Lehoux D, Ostiguy V, Vadieux C, et al. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501-6505. doi:10.1128/AAC.00981-15
6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi:10.1093/cid/ciq146
7. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi:10.1093/cid/ciy745
8. Redell M, Seirra-Hoffman M, Assi Maha, et al. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019;6(11):ofz479. doi:10.1093/ofid/ofz479
9. Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi:10.1093/ofid/ofv156
10. Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multipledose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38(1):152-159. doi:10.1002/phar.2057
11. Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6(2):277-289. doi:10.1007/s40121-017-0156-z
12. Delaportas DJ, Estrada SJ, Darmelio M. Successful treatment of methicillin susceptible Staphylococcus aureus osteomyelitis with oritavancin. Pharmacotherapy. 2017;37(8):e90-e92. doi:10.1002/phar.1957
13. Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents. 2019;53(4):429-434. doi:10.1016/j.ijantimicag.2018.11.023
14. Dahesh S, Wong B, Nizet V, Sakoulas G, Tran TT, Aitken SL. Treatment of multidrug-resistant vancomycinresistant Enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019;63(7):e02622-18. doi:10.1128/AAC.02622-18
15. Foster RA, Philavong KP, Weissman S, Tang X, Bookstaver PB. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant Enterococci osteomyelitis. Infect Dis Clin Pract. 2018;26(2):97-99. doi:10.1097/IPC.0000000000000517
16. Ruggero M, Ziegler M, Tebas P, Binkley A, Kelly B. Successful treatment of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis with outpatient oritavancin therapy. Infect Dis Clin Pract. 2018;26(3):141-144. doi:10.1097/IPC.0000000000000599
17. Corey GR, Loutit J, Moeck G, et al. Single intravenous dose of oritavancin for treatment of acute skin and skin structure infections caused by gram-positive bacteria: summary of safety analysis from the phase 3 SOLO studies. Antimicrob Agents Chemother. 2018;62(4):e01919- 17. doi:10.1128/AAC.01919-17
Oritavancin is a lipoglycopeptide antibiotic. The US Food and Drug Administration (FDA) approved oritavancin in 2014 for adults with acute bacterial skin and skin structure infections (ABSSSI).1 The antibiotic is currently FDA approved for infections caused by Gram-positive organisms, including methicillin-resistant and methicillinsusceptible Staphylococcus aureus (MRSA, MSSA), a variety of Streptococcus species, and vancomycin-susceptible Enterococcus faecalis (VSE). Oritavancin demonstrates concentrationdependent bactericidal activity and has a half-life of 245 hours. This half-life allows for treatment of ABSSSI with a single 1,200 mg IV dose, which has been shown to be noninferior to vancomycin dosed twice daily for 7 to 10 days.1-3
Proposal for Expanded Uses
Although the approved indication for oritavancin is narrow, in vitro studies have shown that oritavancin also has activity against vancomycin-resistant enterococci (VRE), and rabbit studies have demonstrated its excellent bone penetration.4,5 These findings have raised the question of whether oritavancin can be safely and effectively used for infections such as endocarditis, osteomyelitis, and bacteremia, which are often caused by invasive Grampositive organisms. These types of invasive infections, particularly when MRSA is implicated, generally require IV antibiotic therapy for several weeks, often with vancomycin.6
To avoid long hospital stays solely for antibiotic administration, health care practitioners will often use outpatient parenteral antimicrobial therapy (OPAT). However, using OPAT presents many challenges due to the need for frequent dosing, the risk of peripheral or central-line infections, and therapeutic drug monitoring when using vancomycin; additionally, administration and line care oftentimes require caregiver support, which may not be present for all patients.7 Concerns also have been raised regarding the use of OPAT in patients with a history of IV drug use due to the potential increased risk of line infections or line abuse. Few studies have explored OPAT in this population, and the Infectious Diseases Society of America OPAT guidelines recommend that the decision to use OPAT should be made on a case-by-case basis.7 Thus, patients who are deemed inappropriate for OPAT oftentimes remain hospitalized or reside briefly in nursing facilities solely for antibiotic administration
Oritavancin’s long half-life and potent activity against Gram-positive organisms has led to increased interest in off-label use of infrequent dosing intervals, such as weekly, to treat complicated and invasive infections. Weekly rather than daily dosing would allow for less burdensome antibiotic administration regimens and shorter hospital stays especially for patients who are not candidates for OPAT.
Efficacy of Continued Dosing
This proposed weekly dosing pattern, referred to as continued dosing or a multiple-dose regimen, has gained traction in the literature. To date, no randomized controlled trials have been conducted to assess oritavancin’s efficacy in off-label indications or continued dosing, but several case reports and retrospective cohort analyses show promising outcomes.8-16 In an analysis of data from the Clinical and Historic Registry and Orbactiv Medical Evaluation (CHROME) patient registry, 32 patients received multiple doses of oritavancin for complicated Gram-positive infections with a 93.8% overall clinical success rate, including success rates of 90.9% (10/11) for general bone and joint infections and 87.5% (7/8) for patients diagnosed specifically with osteomyelitis.8
Patients received between 2 and 10 doses of 1,200 mg IV given every 6 to 14 days. Johnson and colleagues report using oritavancin 1,200 mg IV every other day for 3 doses followed by 1,200 mg IV once weekly for a patient with daptomycin- and vancomycin-resistant Enterococcus endocarditis, resulting in negative blood cultures while on therapy.9 However, source control via valve replacement and postoperative oritavancin 1,200 mg IV twice weekly for 10 weeks was required to fully clear the infection.
Schulz and colleagues published a retrospective cohort analysis of 17 patients who received multiple doses of oritavancin for complicated bacterial infections, including osteomyelitis, pneumonia, and bacteremia.10 They reported 100% of patients were either successfully cured or had demonstrable improvements in their infections by using a 1,200 mg IV loading dose followed by 800 mg IV if the second dose was given within 7 days or 1,200 mg IV if the second dose was given more than 10 days later. Patients received between 2 and 18 total doses, with 6 out of 17 (35%) receiving only 2 doses. One patient who received 18 doses was an outlier, as her treatment goal was palliative suppression due to an infected endovascular graft that could not be removed.
In a published case series, 1 of 10 patients receiving oritavancin for invasive Grampositive infections received multiple doses of oritavancin for an MSSA deep tissue infection.11 The 3 total doses (strength not reported) were separated by 19 days and 14 days and resulted in cure. Several case reports and a retrospective chart review study specifically show the effectiveness of oritavancin for osteomyelitis caused by MSSA, MRSA, and VRE.12-16 However, dosing strategies varied widely after the initial 1,200 mg IV loading dose.
Drug Interactions, Safety, and Tolerability
Oritavancin has minimal drug-drug interactions, the most notable being with anticoagulants. 1 Use of IV heparin within 120 hours of oritavancin administration can falsely elevate activated partial thromboplastin time (aPTT) levels; therefore, heparin should not be monitored with aPTT during this period. Oritavancin also can artificially prolong international normalized ratio (INR) values for up to 12 hours, and dose adjustments based on INRs during this window are not recommended. Of note, factor Xa laboratory monitoring is unaffected by oritavancin, as it does not depend on phospholipid reagents as do aPTT and INR measurements.
Oritavancin has been shown to be well tolerated when dosed according to both the package insert and continued dosing strategies. The most common adverse effects (AEs) (≥ 3%), occurring at similar rates to vancomycin, are nausea, vomiting, diarrhea, headache, and limb and subcutaneous abscesses.1 Infusion reactions also have been reported, although they are usually reversible on slowing or stopping the infusion. It is worth noting that the use of oritavancin for osteomyelitis is not recommended in the product labeling, as an increased rate of osteomyelitis was observed in the oritavancin vs IV vancomycin groups for the treatment of patients with acute bacterial skin and skin structure infection (SOLO) trials (0.6% in oritavancin group vs 0.1% in vancomycin group, statistical significance not reported).17 However, it was postulated that these osteomyelitis cases were likely present, yet not recognized, at baseline and were not the result of administering oritavancin. This conclusion is further corroborated by previously presented research demonstrating successful cure of osteomyelitis with continued dosing strategies.12-16
Many patients receiving multiple doses of oritavancin did not experience AEs or laboratory abnormalities.13,15 Four of 17 patients (24%) in one retrospective review experienced AEs, including infusion reactions, anemia, and leukopenia; all were reversible on discontinuation of oritavancin, and contributions of other antibiotics in some cases could not be ruled out.10 One patient experienced taste disturbance for several hours after each infusion, and a second had documented hearing loss after 3 doses of oritavancin in a 33-day period, though she had received 6 weeks of IV vancomycin prior to oritavancin.11,12 A patient treated for daptomycin- and vancomycinresistant Enterococcus faecium prosthetic valve endocarditis experienced nausea, anorexia, and minor liver function test (LFT) abnormalities after cumulative oritavancin exposure over 18 weeks.9 On discontinuation of the drug, nausea and anorexia improved, and LFTs normalized 11 months later. Overall, AEs reported with continued dosing of oritavancin have been minimal and largely reversible, mimicking the AEs in the product labeling for traditional dosing. This suggests that using a continued dosing strategy may not result in worse or more frequent AEs, though randomized controlled trials are needed to fully ascertain these preliminary findings.
Conclusions
The literature supporting the use of oritavancin beyond single-dose administration for ABSSSI is growing. Continued dosing regimens have been well tolerated and have resulted in clinical cure for many patients with barriers to first-line treatment and complicated or invasive infections. While randomized controlled trials are needed to concretely demonstrate the efficacy and safety of continued dosing of oritavancin, it may fill an important treatment niche in this era of growing antibiotic resistance and increasing complexity of patient cases.
Oritavancin is a lipoglycopeptide antibiotic. The US Food and Drug Administration (FDA) approved oritavancin in 2014 for adults with acute bacterial skin and skin structure infections (ABSSSI).1 The antibiotic is currently FDA approved for infections caused by Gram-positive organisms, including methicillin-resistant and methicillinsusceptible Staphylococcus aureus (MRSA, MSSA), a variety of Streptococcus species, and vancomycin-susceptible Enterococcus faecalis (VSE). Oritavancin demonstrates concentrationdependent bactericidal activity and has a half-life of 245 hours. This half-life allows for treatment of ABSSSI with a single 1,200 mg IV dose, which has been shown to be noninferior to vancomycin dosed twice daily for 7 to 10 days.1-3
Proposal for Expanded Uses
Although the approved indication for oritavancin is narrow, in vitro studies have shown that oritavancin also has activity against vancomycin-resistant enterococci (VRE), and rabbit studies have demonstrated its excellent bone penetration.4,5 These findings have raised the question of whether oritavancin can be safely and effectively used for infections such as endocarditis, osteomyelitis, and bacteremia, which are often caused by invasive Grampositive organisms. These types of invasive infections, particularly when MRSA is implicated, generally require IV antibiotic therapy for several weeks, often with vancomycin.6
To avoid long hospital stays solely for antibiotic administration, health care practitioners will often use outpatient parenteral antimicrobial therapy (OPAT). However, using OPAT presents many challenges due to the need for frequent dosing, the risk of peripheral or central-line infections, and therapeutic drug monitoring when using vancomycin; additionally, administration and line care oftentimes require caregiver support, which may not be present for all patients.7 Concerns also have been raised regarding the use of OPAT in patients with a history of IV drug use due to the potential increased risk of line infections or line abuse. Few studies have explored OPAT in this population, and the Infectious Diseases Society of America OPAT guidelines recommend that the decision to use OPAT should be made on a case-by-case basis.7 Thus, patients who are deemed inappropriate for OPAT oftentimes remain hospitalized or reside briefly in nursing facilities solely for antibiotic administration
Oritavancin’s long half-life and potent activity against Gram-positive organisms has led to increased interest in off-label use of infrequent dosing intervals, such as weekly, to treat complicated and invasive infections. Weekly rather than daily dosing would allow for less burdensome antibiotic administration regimens and shorter hospital stays especially for patients who are not candidates for OPAT.
Efficacy of Continued Dosing
This proposed weekly dosing pattern, referred to as continued dosing or a multiple-dose regimen, has gained traction in the literature. To date, no randomized controlled trials have been conducted to assess oritavancin’s efficacy in off-label indications or continued dosing, but several case reports and retrospective cohort analyses show promising outcomes.8-16 In an analysis of data from the Clinical and Historic Registry and Orbactiv Medical Evaluation (CHROME) patient registry, 32 patients received multiple doses of oritavancin for complicated Gram-positive infections with a 93.8% overall clinical success rate, including success rates of 90.9% (10/11) for general bone and joint infections and 87.5% (7/8) for patients diagnosed specifically with osteomyelitis.8
Patients received between 2 and 10 doses of 1,200 mg IV given every 6 to 14 days. Johnson and colleagues report using oritavancin 1,200 mg IV every other day for 3 doses followed by 1,200 mg IV once weekly for a patient with daptomycin- and vancomycin-resistant Enterococcus endocarditis, resulting in negative blood cultures while on therapy.9 However, source control via valve replacement and postoperative oritavancin 1,200 mg IV twice weekly for 10 weeks was required to fully clear the infection.
Schulz and colleagues published a retrospective cohort analysis of 17 patients who received multiple doses of oritavancin for complicated bacterial infections, including osteomyelitis, pneumonia, and bacteremia.10 They reported 100% of patients were either successfully cured or had demonstrable improvements in their infections by using a 1,200 mg IV loading dose followed by 800 mg IV if the second dose was given within 7 days or 1,200 mg IV if the second dose was given more than 10 days later. Patients received between 2 and 18 total doses, with 6 out of 17 (35%) receiving only 2 doses. One patient who received 18 doses was an outlier, as her treatment goal was palliative suppression due to an infected endovascular graft that could not be removed.
In a published case series, 1 of 10 patients receiving oritavancin for invasive Grampositive infections received multiple doses of oritavancin for an MSSA deep tissue infection.11 The 3 total doses (strength not reported) were separated by 19 days and 14 days and resulted in cure. Several case reports and a retrospective chart review study specifically show the effectiveness of oritavancin for osteomyelitis caused by MSSA, MRSA, and VRE.12-16 However, dosing strategies varied widely after the initial 1,200 mg IV loading dose.
Drug Interactions, Safety, and Tolerability
Oritavancin has minimal drug-drug interactions, the most notable being with anticoagulants. 1 Use of IV heparin within 120 hours of oritavancin administration can falsely elevate activated partial thromboplastin time (aPTT) levels; therefore, heparin should not be monitored with aPTT during this period. Oritavancin also can artificially prolong international normalized ratio (INR) values for up to 12 hours, and dose adjustments based on INRs during this window are not recommended. Of note, factor Xa laboratory monitoring is unaffected by oritavancin, as it does not depend on phospholipid reagents as do aPTT and INR measurements.
Oritavancin has been shown to be well tolerated when dosed according to both the package insert and continued dosing strategies. The most common adverse effects (AEs) (≥ 3%), occurring at similar rates to vancomycin, are nausea, vomiting, diarrhea, headache, and limb and subcutaneous abscesses.1 Infusion reactions also have been reported, although they are usually reversible on slowing or stopping the infusion. It is worth noting that the use of oritavancin for osteomyelitis is not recommended in the product labeling, as an increased rate of osteomyelitis was observed in the oritavancin vs IV vancomycin groups for the treatment of patients with acute bacterial skin and skin structure infection (SOLO) trials (0.6% in oritavancin group vs 0.1% in vancomycin group, statistical significance not reported).17 However, it was postulated that these osteomyelitis cases were likely present, yet not recognized, at baseline and were not the result of administering oritavancin. This conclusion is further corroborated by previously presented research demonstrating successful cure of osteomyelitis with continued dosing strategies.12-16
Many patients receiving multiple doses of oritavancin did not experience AEs or laboratory abnormalities.13,15 Four of 17 patients (24%) in one retrospective review experienced AEs, including infusion reactions, anemia, and leukopenia; all were reversible on discontinuation of oritavancin, and contributions of other antibiotics in some cases could not be ruled out.10 One patient experienced taste disturbance for several hours after each infusion, and a second had documented hearing loss after 3 doses of oritavancin in a 33-day period, though she had received 6 weeks of IV vancomycin prior to oritavancin.11,12 A patient treated for daptomycin- and vancomycinresistant Enterococcus faecium prosthetic valve endocarditis experienced nausea, anorexia, and minor liver function test (LFT) abnormalities after cumulative oritavancin exposure over 18 weeks.9 On discontinuation of the drug, nausea and anorexia improved, and LFTs normalized 11 months later. Overall, AEs reported with continued dosing of oritavancin have been minimal and largely reversible, mimicking the AEs in the product labeling for traditional dosing. This suggests that using a continued dosing strategy may not result in worse or more frequent AEs, though randomized controlled trials are needed to fully ascertain these preliminary findings.
Conclusions
The literature supporting the use of oritavancin beyond single-dose administration for ABSSSI is growing. Continued dosing regimens have been well tolerated and have resulted in clinical cure for many patients with barriers to first-line treatment and complicated or invasive infections. While randomized controlled trials are needed to concretely demonstrate the efficacy and safety of continued dosing of oritavancin, it may fill an important treatment niche in this era of growing antibiotic resistance and increasing complexity of patient cases.
1. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2019.
2. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422
3. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778
4. Sweeney D, Stoneburner A, Shinabarger DL, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72(2):622-624. doi.10.1093/jac/dkw451
5. Lehoux D, Ostiguy V, Vadieux C, et al. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501-6505. doi:10.1128/AAC.00981-15
6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi:10.1093/cid/ciq146
7. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi:10.1093/cid/ciy745
8. Redell M, Seirra-Hoffman M, Assi Maha, et al. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019;6(11):ofz479. doi:10.1093/ofid/ofz479
9. Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi:10.1093/ofid/ofv156
10. Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multipledose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38(1):152-159. doi:10.1002/phar.2057
11. Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6(2):277-289. doi:10.1007/s40121-017-0156-z
12. Delaportas DJ, Estrada SJ, Darmelio M. Successful treatment of methicillin susceptible Staphylococcus aureus osteomyelitis with oritavancin. Pharmacotherapy. 2017;37(8):e90-e92. doi:10.1002/phar.1957
13. Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents. 2019;53(4):429-434. doi:10.1016/j.ijantimicag.2018.11.023
14. Dahesh S, Wong B, Nizet V, Sakoulas G, Tran TT, Aitken SL. Treatment of multidrug-resistant vancomycinresistant Enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019;63(7):e02622-18. doi:10.1128/AAC.02622-18
15. Foster RA, Philavong KP, Weissman S, Tang X, Bookstaver PB. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant Enterococci osteomyelitis. Infect Dis Clin Pract. 2018;26(2):97-99. doi:10.1097/IPC.0000000000000517
16. Ruggero M, Ziegler M, Tebas P, Binkley A, Kelly B. Successful treatment of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis with outpatient oritavancin therapy. Infect Dis Clin Pract. 2018;26(3):141-144. doi:10.1097/IPC.0000000000000599
17. Corey GR, Loutit J, Moeck G, et al. Single intravenous dose of oritavancin for treatment of acute skin and skin structure infections caused by gram-positive bacteria: summary of safety analysis from the phase 3 SOLO studies. Antimicrob Agents Chemother. 2018;62(4):e01919- 17. doi:10.1128/AAC.01919-17
1. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2019.
2. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422
3. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778
4. Sweeney D, Stoneburner A, Shinabarger DL, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72(2):622-624. doi.10.1093/jac/dkw451
5. Lehoux D, Ostiguy V, Vadieux C, et al. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501-6505. doi:10.1128/AAC.00981-15
6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi:10.1093/cid/ciq146
7. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi:10.1093/cid/ciy745
8. Redell M, Seirra-Hoffman M, Assi Maha, et al. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019;6(11):ofz479. doi:10.1093/ofid/ofz479
9. Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi:10.1093/ofid/ofv156
10. Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multipledose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38(1):152-159. doi:10.1002/phar.2057
11. Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6(2):277-289. doi:10.1007/s40121-017-0156-z
12. Delaportas DJ, Estrada SJ, Darmelio M. Successful treatment of methicillin susceptible Staphylococcus aureus osteomyelitis with oritavancin. Pharmacotherapy. 2017;37(8):e90-e92. doi:10.1002/phar.1957
13. Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents. 2019;53(4):429-434. doi:10.1016/j.ijantimicag.2018.11.023
14. Dahesh S, Wong B, Nizet V, Sakoulas G, Tran TT, Aitken SL. Treatment of multidrug-resistant vancomycinresistant Enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019;63(7):e02622-18. doi:10.1128/AAC.02622-18
15. Foster RA, Philavong KP, Weissman S, Tang X, Bookstaver PB. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant Enterococci osteomyelitis. Infect Dis Clin Pract. 2018;26(2):97-99. doi:10.1097/IPC.0000000000000517
16. Ruggero M, Ziegler M, Tebas P, Binkley A, Kelly B. Successful treatment of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis with outpatient oritavancin therapy. Infect Dis Clin Pract. 2018;26(3):141-144. doi:10.1097/IPC.0000000000000599
17. Corey GR, Loutit J, Moeck G, et al. Single intravenous dose of oritavancin for treatment of acute skin and skin structure infections caused by gram-positive bacteria: summary of safety analysis from the phase 3 SOLO studies. Antimicrob Agents Chemother. 2018;62(4):e01919- 17. doi:10.1128/AAC.01919-17
Proposed RA guidelines: Maximize methotrexate before switching
New proposed guidelines for managing rheumatoid arthritis (RA) recommend that methotrexate (MTX) be used aggressively before other treatment options.
Previous guidelines, last updated in 2015, had not ranked the order of the treatments, said Liana Fraenkel, MD, MPH, principal investigator for the American College of Rheumatology’s treatment guidelines.
“There’s a strong emphasis on maximizing methotrexate using various means before switching to a biologic or JAK [Janus kinase] inhibitor,” she said in a press conference at the virtual annual meeting of the American College of Rheumatology. The guidelines draft was developed collaboratively with clinicians, researchers, and patients. In addition, the authors conducted a comprehensive review of the literature.
Dr. Fraenkel, of Yale University in New Haven, Conn., said the exception for maximizing MTX would be for patients with low disease activity for whom treatments with other medications, such as hydroxychloroquine (HCQ) and sulfasalazine, are feasible, she said.
Stop defaulting to prednisone
Another recommendation urges against the use of prednisone as a default treatment.
“We should really be trying to maximize disease-modifying antirheumatic drugs [DMARDs] and try to push the needle away from using prednisone as frequently as we do,” she said.
Dr. Fraenkel said the panel wanted to emphasize that “even lower doses of prednisone can be harmful.”
She noted that patients on the guidelines panel said it’s hard to taper off prednisone.
Don Thomas, MD, who is in private rheumatology practice in Greenbelt, Md., said in an interview he loves the guidelines.
“Most of my patients are not on steroids,” he said, “which is a godsend because of the great therapies we have.”
He said he was glad to see support for exhausting methotrexate options first before trying new treatments.
“Too many of us are not as aggressive as we should be with using methotrexate initially,” he said.
Specific recommendations
In the proposed guidelines, MTX alone is strongly recommended over HCQ or sulfasalazine and is conditionally recommended over a conventional synthetic DMARD dual or triple combination. MTX alone is also conditionally recommended over MTX in combination with a tumor necrosis factor (TNF) inhibitor and is strongly recommended over MTX in combination with a non-TNF inhibitor, a biologic, or a targeted synthetic DMARD.
For patients with low disease activity who have not taken DMARDs, HCQ is recommended over other conventional synthetic DMARDs. Sulfasalazine is recommended over MTX, and MTX is recommended over leflunomide.
For initial treatment, oral MTX is conditionally recommended over subcutaneous administration. For patients who are not tolerating the oral version, “recommend split-dose or subcutaneous or increasing folic acid over switching to a new DMARD,” she said.
Dr. Fraenkel said the oral recommendation was based largely on patient preference.
Use of glucocorticoids
For patients who need glucocorticoids to remain at target, adding or switching DMARDs is recommended over continuing glucocorticoids, the guidelines indicate.
“For patients on DMARDs and not at target, adding or switching DMARDs with or without the use of intraarticular glucocorticoids is conditionally recommended over the use of intraarticular glucocorticoids alone,” the proposed guidelines advise.
Tapering
Tapering should only be considered for patients “who have been at target for at least 6 months,” she said. “In these patients, continuation of all DMARDs at their current dose is conditionally recommended over any dose reduction.”
Dose reduction is recommended over gradual discontinuation, and gradual discontinuation is recommended over abruptly stopping.
Dr. Fraenkel acknowledged that the level of evidence is low to very low for many of the recommendations (only 7 of 44 recommendations were classified as strong), which, she said, underscores the importance of shared decision making for RA.
She added, “We really need trials to address clinically important questions driven by patients and not simply driven by [having] a new molecule to test.”
ACR says the final version of the proposed guidelines is expected to be simultaneously published in Arthritis Care and Research and Arthritis and Rheumatology by the end of the year.
These guidelines are focused on pharmacologic agents. Separate ACR guidelines will address nonpharmacologic management of RA and vaccine recommendations for inflammatory disease.
Dr. Fraenkel and Dr. Thomas have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New proposed guidelines for managing rheumatoid arthritis (RA) recommend that methotrexate (MTX) be used aggressively before other treatment options.
Previous guidelines, last updated in 2015, had not ranked the order of the treatments, said Liana Fraenkel, MD, MPH, principal investigator for the American College of Rheumatology’s treatment guidelines.
“There’s a strong emphasis on maximizing methotrexate using various means before switching to a biologic or JAK [Janus kinase] inhibitor,” she said in a press conference at the virtual annual meeting of the American College of Rheumatology. The guidelines draft was developed collaboratively with clinicians, researchers, and patients. In addition, the authors conducted a comprehensive review of the literature.
Dr. Fraenkel, of Yale University in New Haven, Conn., said the exception for maximizing MTX would be for patients with low disease activity for whom treatments with other medications, such as hydroxychloroquine (HCQ) and sulfasalazine, are feasible, she said.
Stop defaulting to prednisone
Another recommendation urges against the use of prednisone as a default treatment.
“We should really be trying to maximize disease-modifying antirheumatic drugs [DMARDs] and try to push the needle away from using prednisone as frequently as we do,” she said.
Dr. Fraenkel said the panel wanted to emphasize that “even lower doses of prednisone can be harmful.”
She noted that patients on the guidelines panel said it’s hard to taper off prednisone.
Don Thomas, MD, who is in private rheumatology practice in Greenbelt, Md., said in an interview he loves the guidelines.
“Most of my patients are not on steroids,” he said, “which is a godsend because of the great therapies we have.”
He said he was glad to see support for exhausting methotrexate options first before trying new treatments.
“Too many of us are not as aggressive as we should be with using methotrexate initially,” he said.
Specific recommendations
In the proposed guidelines, MTX alone is strongly recommended over HCQ or sulfasalazine and is conditionally recommended over a conventional synthetic DMARD dual or triple combination. MTX alone is also conditionally recommended over MTX in combination with a tumor necrosis factor (TNF) inhibitor and is strongly recommended over MTX in combination with a non-TNF inhibitor, a biologic, or a targeted synthetic DMARD.
For patients with low disease activity who have not taken DMARDs, HCQ is recommended over other conventional synthetic DMARDs. Sulfasalazine is recommended over MTX, and MTX is recommended over leflunomide.
For initial treatment, oral MTX is conditionally recommended over subcutaneous administration. For patients who are not tolerating the oral version, “recommend split-dose or subcutaneous or increasing folic acid over switching to a new DMARD,” she said.
Dr. Fraenkel said the oral recommendation was based largely on patient preference.
Use of glucocorticoids
For patients who need glucocorticoids to remain at target, adding or switching DMARDs is recommended over continuing glucocorticoids, the guidelines indicate.
“For patients on DMARDs and not at target, adding or switching DMARDs with or without the use of intraarticular glucocorticoids is conditionally recommended over the use of intraarticular glucocorticoids alone,” the proposed guidelines advise.
Tapering
Tapering should only be considered for patients “who have been at target for at least 6 months,” she said. “In these patients, continuation of all DMARDs at their current dose is conditionally recommended over any dose reduction.”
Dose reduction is recommended over gradual discontinuation, and gradual discontinuation is recommended over abruptly stopping.
Dr. Fraenkel acknowledged that the level of evidence is low to very low for many of the recommendations (only 7 of 44 recommendations were classified as strong), which, she said, underscores the importance of shared decision making for RA.
She added, “We really need trials to address clinically important questions driven by patients and not simply driven by [having] a new molecule to test.”
ACR says the final version of the proposed guidelines is expected to be simultaneously published in Arthritis Care and Research and Arthritis and Rheumatology by the end of the year.
These guidelines are focused on pharmacologic agents. Separate ACR guidelines will address nonpharmacologic management of RA and vaccine recommendations for inflammatory disease.
Dr. Fraenkel and Dr. Thomas have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New proposed guidelines for managing rheumatoid arthritis (RA) recommend that methotrexate (MTX) be used aggressively before other treatment options.
Previous guidelines, last updated in 2015, had not ranked the order of the treatments, said Liana Fraenkel, MD, MPH, principal investigator for the American College of Rheumatology’s treatment guidelines.
“There’s a strong emphasis on maximizing methotrexate using various means before switching to a biologic or JAK [Janus kinase] inhibitor,” she said in a press conference at the virtual annual meeting of the American College of Rheumatology. The guidelines draft was developed collaboratively with clinicians, researchers, and patients. In addition, the authors conducted a comprehensive review of the literature.
Dr. Fraenkel, of Yale University in New Haven, Conn., said the exception for maximizing MTX would be for patients with low disease activity for whom treatments with other medications, such as hydroxychloroquine (HCQ) and sulfasalazine, are feasible, she said.
Stop defaulting to prednisone
Another recommendation urges against the use of prednisone as a default treatment.
“We should really be trying to maximize disease-modifying antirheumatic drugs [DMARDs] and try to push the needle away from using prednisone as frequently as we do,” she said.
Dr. Fraenkel said the panel wanted to emphasize that “even lower doses of prednisone can be harmful.”
She noted that patients on the guidelines panel said it’s hard to taper off prednisone.
Don Thomas, MD, who is in private rheumatology practice in Greenbelt, Md., said in an interview he loves the guidelines.
“Most of my patients are not on steroids,” he said, “which is a godsend because of the great therapies we have.”
He said he was glad to see support for exhausting methotrexate options first before trying new treatments.
“Too many of us are not as aggressive as we should be with using methotrexate initially,” he said.
Specific recommendations
In the proposed guidelines, MTX alone is strongly recommended over HCQ or sulfasalazine and is conditionally recommended over a conventional synthetic DMARD dual or triple combination. MTX alone is also conditionally recommended over MTX in combination with a tumor necrosis factor (TNF) inhibitor and is strongly recommended over MTX in combination with a non-TNF inhibitor, a biologic, or a targeted synthetic DMARD.
For patients with low disease activity who have not taken DMARDs, HCQ is recommended over other conventional synthetic DMARDs. Sulfasalazine is recommended over MTX, and MTX is recommended over leflunomide.
For initial treatment, oral MTX is conditionally recommended over subcutaneous administration. For patients who are not tolerating the oral version, “recommend split-dose or subcutaneous or increasing folic acid over switching to a new DMARD,” she said.
Dr. Fraenkel said the oral recommendation was based largely on patient preference.
Use of glucocorticoids
For patients who need glucocorticoids to remain at target, adding or switching DMARDs is recommended over continuing glucocorticoids, the guidelines indicate.
“For patients on DMARDs and not at target, adding or switching DMARDs with or without the use of intraarticular glucocorticoids is conditionally recommended over the use of intraarticular glucocorticoids alone,” the proposed guidelines advise.
Tapering
Tapering should only be considered for patients “who have been at target for at least 6 months,” she said. “In these patients, continuation of all DMARDs at their current dose is conditionally recommended over any dose reduction.”
Dose reduction is recommended over gradual discontinuation, and gradual discontinuation is recommended over abruptly stopping.
Dr. Fraenkel acknowledged that the level of evidence is low to very low for many of the recommendations (only 7 of 44 recommendations were classified as strong), which, she said, underscores the importance of shared decision making for RA.
She added, “We really need trials to address clinically important questions driven by patients and not simply driven by [having] a new molecule to test.”
ACR says the final version of the proposed guidelines is expected to be simultaneously published in Arthritis Care and Research and Arthritis and Rheumatology by the end of the year.
These guidelines are focused on pharmacologic agents. Separate ACR guidelines will address nonpharmacologic management of RA and vaccine recommendations for inflammatory disease.
Dr. Fraenkel and Dr. Thomas have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ACR 2020
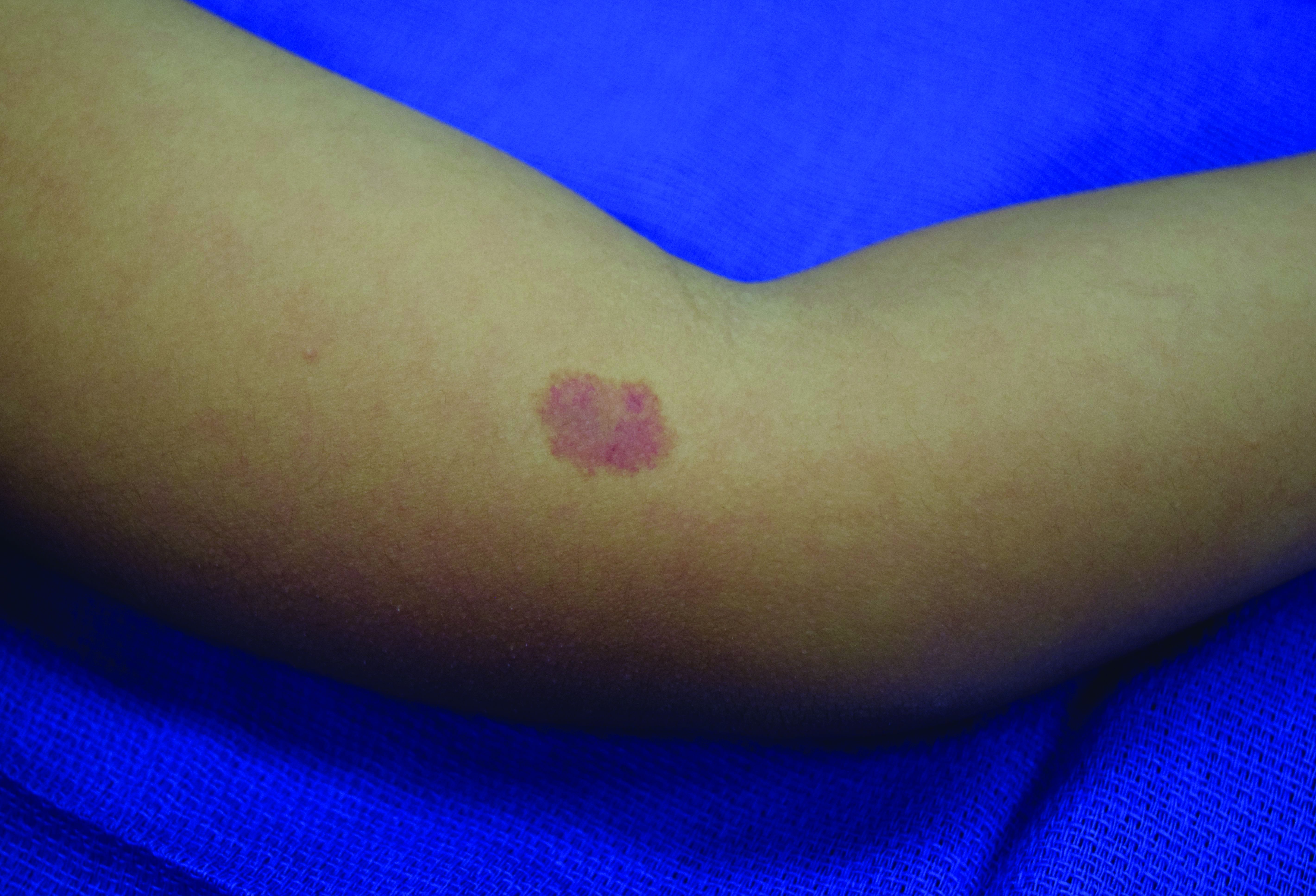
Painful Papules on the Arms
The Diagnosis: Piloleiomyoma
Leiomyoma cutis, also known as cutaneous leiomyoma, is a benign smooth muscle tumor first described in 1854.1 Cutaneous leiomyoma is comprised of 3 distinct types that depend on the origin of smooth muscle tumor: piloleiomyoma (arrector pili muscle), angioleiomyoma (tunica media of arteries/veins), and genital leiomyoma (dartos muscle of the scrotum and labia majora, erectile muscle of nipple).2 It affects both sexes equally, though some reports have noted an increased prevalence in females. Piloleiomyomas commonly present on the extensor surfaces of the extremities (solitary) and trunk (multiple).1 Tumors most often present as firm flesh-colored or pink-brown papulonodules. They can be linear, dermatomal, segmental, or diffuse, and often are painful. Clinical differential diagnosis for painful skin tumors is aided by the acronym "BLEND AN EGG": blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor.3 For isolated lesions, surgical excision is the treatment of choice. For numerous lesions in which excision would not be feasible, intralesional corticosteroids, medications (eg, calcium channel blockers, alpha blockers, nitroglycerin), and botulinum toxin have been used for pain relief.4
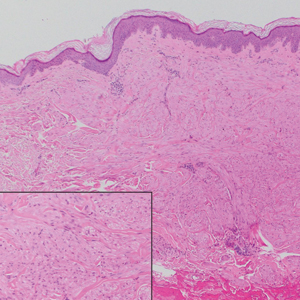
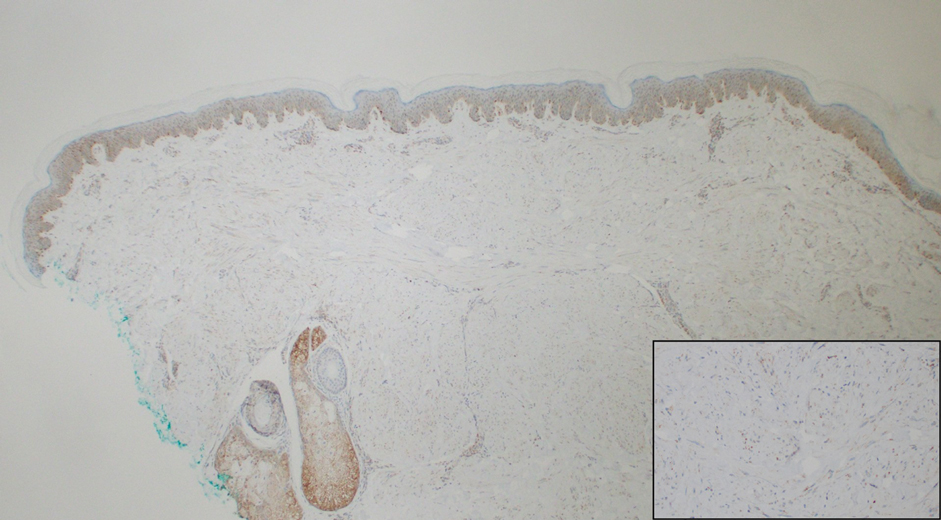
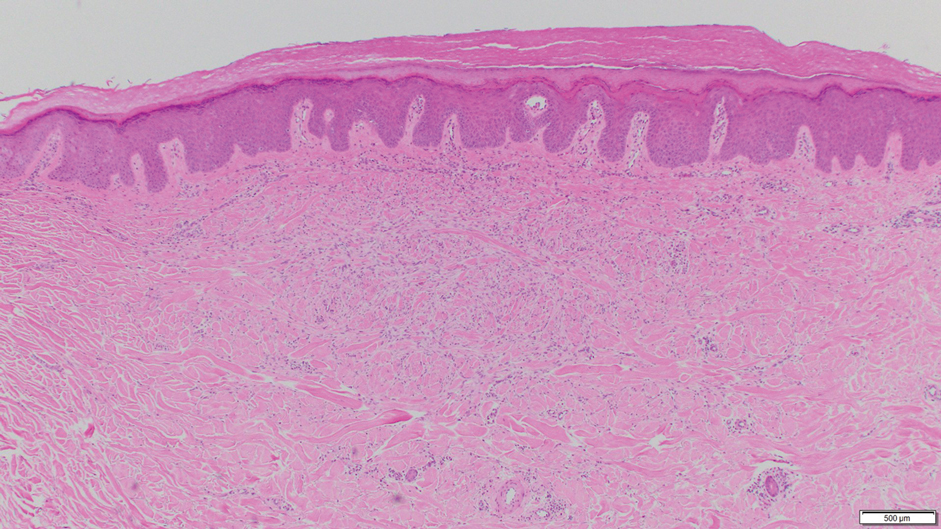
Notably, multiple cutaneous leiomyomas can be seen in association with uterine leiomyomas in Reed syndrome due to an autosomal-dominant or de novo mutation in the fumarate hydratase gene, FH. Reed syndrome is associated with a lifetime risk for renal cell carcinoma (hereditary leiomyomatosis and renal cell cancer) in 15% of cases with FH mutations.5 In our patient, both immunohistochemical staining and blood testing for FH were performed. Immunohistochemistry revealed notably diminished staining with only weak patchy granular cytoplasmic staining present (Figure 1). Genetic testing revealed heterozygosity for a pathogenic variant of the FH gene, consistent with a diagnosis of Reed syndrome.
Histologically, the differential diagnosis includes other spindle cell tumors, such as dermatofibroma, neurofibroma, and dermatomyofibroma. The histologic appearance varies depending on the type, with piloleiomyoma typically located within the reticular dermis with possible subcutaneous extension. Fascicles of eosinophilic smooth muscle cells in an interlacing arrangement often ramify between neighboring dermal collagen; these smooth muscle cells contain cigar-shaped, blunt-ended nuclei with a perinuclear clear vacuole. Marked epidermal hyperplasia is possible.6 A close association with a nearby hair follicle frequently is noted. Although differentiated smooth muscle cells usually are evident on hematoxylin and eosin, positive staining for smooth muscle actin (SMA) and desmin can aid in diagnosis.7 Immunohistochemical staining for FH has proven to be highly specific (97.6%) with moderate sensitivity (70.0%).8 Angioleiomyomas appear as well-demarcated dermal to subcutaneous tumors composed of smooth muscle cells surrounding thick-walled vaculature.9 Scrotal and vulvar leiomyomas are composed of eosinophilic spindle cells, though vulvar leiomyomas have shown epithelioid differentiation.10 Nipple leiomyomas appear similar to piloleiomyomas on histology with interlacing smooth muscle fiber bundles.
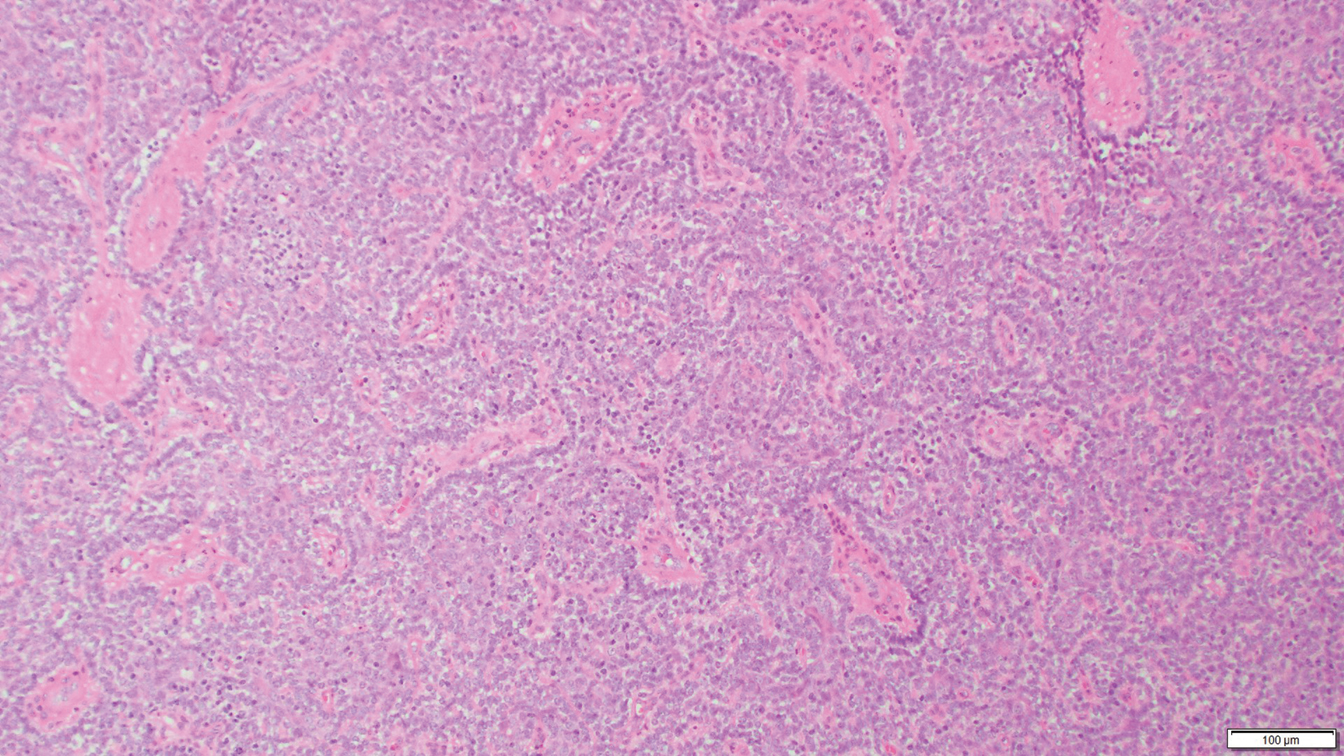
Eccrine spiradenoma is a relatively uncommon adnexal tumor derived from eccrine sweat glands. It most often presents as a small, painful or tender, intradermal nodule (or rarely as nodules) on the head or ventral trunk.11 There is no sexual predilection. It affects adults at any age but most often from 15 to 35 years. Although rare, malignant transformation is possible. Histologically, eccrine spiradenomas appear as a well-demarcated dermal tumor composed of bland basaloid cells with minimal cytoplasm, often with numerous admixed lymphocytes and variably prominent vasculature (Figure 2). Eosinophilic basement membrane material can be seen within or surrounding the nodules of tumor cells. Multiple spiradenomas can occur in the setting of Brooke-Spiegler syndrome, which is an autosomal-dominant disorder due to an inherited mutation in the CYLD gene. Spiradenomas are benign neoplasms, and surgical excision with clear margins is the treatment of choice.12
Dermatofibroma, also known as cutaneous benign fibrous histiocytoma, is a firm, flesh-colored papule or nodule that most often presents on the lower extremities. It typically is seen in women aged 20 to 40 years.13 The etiology is uncertain, and dermatofibromas often spontaneously develop, though there are inconsistent reports of development with local trauma including insect bites and puncture wounds. The dimple sign refers to skin dimpling with lateral pressure.13 Most commonly, dermatofibromas consist of a dermal proliferation of bland fibroblastic cells with entrapment of dermal collagen bundles at the periphery of the tumors (Figure 3). The fibroblastic cells often are paler and less eosinophilic than smooth muscle cells seen in cutaneous leiomyomas, with tapered nuclei that lack a perinuclear vacuole. Admixed histocytes and other inflammatory cells often are present. Overlying epidermal hyperplasia and/or hyperpigmentation also may be present. Numerous histologic variants have been described, including cellular, epithelioid, aneurysmal, atypical, and hemosiderotic types.14 Immunohistochemical stains may show patchy positive staining for SMA, but h-caldesmon and desmin typically are negative.
Neurofibroma is a tumor derived from neuromesenchymal tissue with nerve axons. They form through neuromesenchyme (eg, Schwann cells, mast cells, perineural cells, endoneural fibroblast) proliferation. Solitary neurofibromas occur most commonly in adults and have no gender predilection. The most common presentation is an asymptomatic, solitary, soft, flesh-colored papulonodule.15 Clinical variants include pigmented, diffuse, and plexiform, with plexiform neurofibromas almost always being consistent with a diagnosis of neurofibromatosis type 1. Histologically, neurofibromas present as dermal or subcutaneous nodules composed of randomly arranged spindle cells with wavy tapered nuclei within a loose collagenous stroma (Figure 4).16 The spindle cells in neurofibromas will stain positively for S-100 protein and SOX-10 and negatively for SMA and desmin.

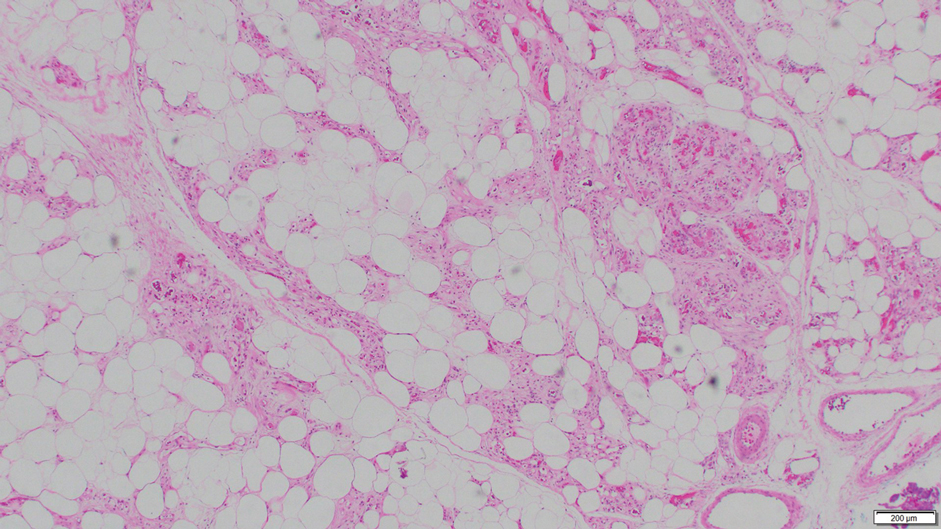
Angiolipoma is a benign tumor composed of adipocytes that also contains vasculature.17 The majority of cases are of unknown etiology, though familial cases have been described. They typically present as multiple painful or tender (differentiating from lipomas) subcutaneous swellings over the forearms in individuals aged 20 to 30 years.18 On histopathology, angiolipomas appear as well-circumscribed subcutaneous tumors containing mature adipocytes intermixed with small capillary vessels, some of which contain luminal fibrin thrombi (Figure 5).
- Malik K, Patel P, Chen J, et al. Leiomyoma cutis: a focused review on presentation, management, and association with malignancy. Am J Clin Dermatol. 2015;16:35-46.
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Delfino S, Toto V, Brunetti B, et al. Recurrent atypical eccrine spiradenoma of the forehead. In Vivo. 2008;22:821-823.
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60:325-328.
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644.
- Raj S, Calonje E, Kraus M, et al. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19:2-9.
- Choi JH, Ro JY. Cutaneous spindle cell neoplasms: pattern-based diagnostic approach. Arch Pathol Lab Med. 2018;142:958-972.
- Carter CS, Skala SL, Chinnaiyan AM, et al. Immunohistochemical characterization of fumarate hydratase (FH) and succinate dehydrogenase (SDH) in cutaneous leiomyomas for detection of familial cancer syndromes. Am J Surg Pathol. 2017;41:801-809.
- Kanitakis J. Angioleiomyoma of the auricle: an unusual tumor on a rare location. Case Rep Otolaryngol. 2017;2017:1-3.
- Tavassoli FA, Norris HJ. Smooth muscle tumors of the vulva. Obstet Gynecol. 1979;53:213-217.
- Phukan J, Sinha A, Pal S. Fine needle aspiration cytology of eccrine spiradenoma of back: report of a rare case. J Lab Physicians. 2014;6:130.
- Zheng Y, Tian Q, Wang J, et al. Differential diagnosis of eccrine spiradenoma: a case report. Exp Ther Med. 2014;8:1097-1101.
- Bandyopadhyay MR, Besra M, Dutta S, et al. Dermatofibroma: atypical presentations. Indian J Dermatol. 2016;61:121.
- Commons JD, Parish L, Yazdanian S, et al. Dermatofibroma: a curious tumor. Skinmed. 2012;10:268-270.
- Lee YB, Lee JI, Park HJ, et al. Solitary neurofibromas: does an uncommon site exist? Ann Dermatol. 2012;24:101-102.
- Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology. 2018;91:S5-S13.
- Howard WR. Angiolipoma. Arch Dermatol. 1960;82:924.
- Ghosh S, Haldar BA. Multiple angiolipomas. Indian J Dermatol Venereol Leprol. 1990;56:143-144.
The Diagnosis: Piloleiomyoma
Leiomyoma cutis, also known as cutaneous leiomyoma, is a benign smooth muscle tumor first described in 1854.1 Cutaneous leiomyoma is comprised of 3 distinct types that depend on the origin of smooth muscle tumor: piloleiomyoma (arrector pili muscle), angioleiomyoma (tunica media of arteries/veins), and genital leiomyoma (dartos muscle of the scrotum and labia majora, erectile muscle of nipple).2 It affects both sexes equally, though some reports have noted an increased prevalence in females. Piloleiomyomas commonly present on the extensor surfaces of the extremities (solitary) and trunk (multiple).1 Tumors most often present as firm flesh-colored or pink-brown papulonodules. They can be linear, dermatomal, segmental, or diffuse, and often are painful. Clinical differential diagnosis for painful skin tumors is aided by the acronym "BLEND AN EGG": blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor.3 For isolated lesions, surgical excision is the treatment of choice. For numerous lesions in which excision would not be feasible, intralesional corticosteroids, medications (eg, calcium channel blockers, alpha blockers, nitroglycerin), and botulinum toxin have been used for pain relief.4
Notably, multiple cutaneous leiomyomas can be seen in association with uterine leiomyomas in Reed syndrome due to an autosomal-dominant or de novo mutation in the fumarate hydratase gene, FH. Reed syndrome is associated with a lifetime risk for renal cell carcinoma (hereditary leiomyomatosis and renal cell cancer) in 15% of cases with FH mutations.5 In our patient, both immunohistochemical staining and blood testing for FH were performed. Immunohistochemistry revealed notably diminished staining with only weak patchy granular cytoplasmic staining present (Figure 1). Genetic testing revealed heterozygosity for a pathogenic variant of the FH gene, consistent with a diagnosis of Reed syndrome.

Histologically, the differential diagnosis includes other spindle cell tumors, such as dermatofibroma, neurofibroma, and dermatomyofibroma. The histologic appearance varies depending on the type, with piloleiomyoma typically located within the reticular dermis with possible subcutaneous extension. Fascicles of eosinophilic smooth muscle cells in an interlacing arrangement often ramify between neighboring dermal collagen; these smooth muscle cells contain cigar-shaped, blunt-ended nuclei with a perinuclear clear vacuole. Marked epidermal hyperplasia is possible.6 A close association with a nearby hair follicle frequently is noted. Although differentiated smooth muscle cells usually are evident on hematoxylin and eosin, positive staining for smooth muscle actin (SMA) and desmin can aid in diagnosis.7 Immunohistochemical staining for FH has proven to be highly specific (97.6%) with moderate sensitivity (70.0%).8 Angioleiomyomas appear as well-demarcated dermal to subcutaneous tumors composed of smooth muscle cells surrounding thick-walled vaculature.9 Scrotal and vulvar leiomyomas are composed of eosinophilic spindle cells, though vulvar leiomyomas have shown epithelioid differentiation.10 Nipple leiomyomas appear similar to piloleiomyomas on histology with interlacing smooth muscle fiber bundles.
Eccrine spiradenoma is a relatively uncommon adnexal tumor derived from eccrine sweat glands. It most often presents as a small, painful or tender, intradermal nodule (or rarely as nodules) on the head or ventral trunk.11 There is no sexual predilection. It affects adults at any age but most often from 15 to 35 years. Although rare, malignant transformation is possible. Histologically, eccrine spiradenomas appear as a well-demarcated dermal tumor composed of bland basaloid cells with minimal cytoplasm, often with numerous admixed lymphocytes and variably prominent vasculature (Figure 2). Eosinophilic basement membrane material can be seen within or surrounding the nodules of tumor cells. Multiple spiradenomas can occur in the setting of Brooke-Spiegler syndrome, which is an autosomal-dominant disorder due to an inherited mutation in the CYLD gene. Spiradenomas are benign neoplasms, and surgical excision with clear margins is the treatment of choice.12

Dermatofibroma, also known as cutaneous benign fibrous histiocytoma, is a firm, flesh-colored papule or nodule that most often presents on the lower extremities. It typically is seen in women aged 20 to 40 years.13 The etiology is uncertain, and dermatofibromas often spontaneously develop, though there are inconsistent reports of development with local trauma including insect bites and puncture wounds. The dimple sign refers to skin dimpling with lateral pressure.13 Most commonly, dermatofibromas consist of a dermal proliferation of bland fibroblastic cells with entrapment of dermal collagen bundles at the periphery of the tumors (Figure 3). The fibroblastic cells often are paler and less eosinophilic than smooth muscle cells seen in cutaneous leiomyomas, with tapered nuclei that lack a perinuclear vacuole. Admixed histocytes and other inflammatory cells often are present. Overlying epidermal hyperplasia and/or hyperpigmentation also may be present. Numerous histologic variants have been described, including cellular, epithelioid, aneurysmal, atypical, and hemosiderotic types.14 Immunohistochemical stains may show patchy positive staining for SMA, but h-caldesmon and desmin typically are negative.

Neurofibroma is a tumor derived from neuromesenchymal tissue with nerve axons. They form through neuromesenchyme (eg, Schwann cells, mast cells, perineural cells, endoneural fibroblast) proliferation. Solitary neurofibromas occur most commonly in adults and have no gender predilection. The most common presentation is an asymptomatic, solitary, soft, flesh-colored papulonodule.15 Clinical variants include pigmented, diffuse, and plexiform, with plexiform neurofibromas almost always being consistent with a diagnosis of neurofibromatosis type 1. Histologically, neurofibromas present as dermal or subcutaneous nodules composed of randomly arranged spindle cells with wavy tapered nuclei within a loose collagenous stroma (Figure 4).16 The spindle cells in neurofibromas will stain positively for S-100 protein and SOX-10 and negatively for SMA and desmin.

Angiolipoma is a benign tumor composed of adipocytes that also contains vasculature.17 The majority of cases are of unknown etiology, though familial cases have been described. They typically present as multiple painful or tender (differentiating from lipomas) subcutaneous swellings over the forearms in individuals aged 20 to 30 years.18 On histopathology, angiolipomas appear as well-circumscribed subcutaneous tumors containing mature adipocytes intermixed with small capillary vessels, some of which contain luminal fibrin thrombi (Figure 5).

The Diagnosis: Piloleiomyoma
Leiomyoma cutis, also known as cutaneous leiomyoma, is a benign smooth muscle tumor first described in 1854.1 Cutaneous leiomyoma is comprised of 3 distinct types that depend on the origin of smooth muscle tumor: piloleiomyoma (arrector pili muscle), angioleiomyoma (tunica media of arteries/veins), and genital leiomyoma (dartos muscle of the scrotum and labia majora, erectile muscle of nipple).2 It affects both sexes equally, though some reports have noted an increased prevalence in females. Piloleiomyomas commonly present on the extensor surfaces of the extremities (solitary) and trunk (multiple).1 Tumors most often present as firm flesh-colored or pink-brown papulonodules. They can be linear, dermatomal, segmental, or diffuse, and often are painful. Clinical differential diagnosis for painful skin tumors is aided by the acronym "BLEND AN EGG": blue rubber bleb nevus, leiomyoma, eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomangioma, and granular cell tumor.3 For isolated lesions, surgical excision is the treatment of choice. For numerous lesions in which excision would not be feasible, intralesional corticosteroids, medications (eg, calcium channel blockers, alpha blockers, nitroglycerin), and botulinum toxin have been used for pain relief.4
Notably, multiple cutaneous leiomyomas can be seen in association with uterine leiomyomas in Reed syndrome due to an autosomal-dominant or de novo mutation in the fumarate hydratase gene, FH. Reed syndrome is associated with a lifetime risk for renal cell carcinoma (hereditary leiomyomatosis and renal cell cancer) in 15% of cases with FH mutations.5 In our patient, both immunohistochemical staining and blood testing for FH were performed. Immunohistochemistry revealed notably diminished staining with only weak patchy granular cytoplasmic staining present (Figure 1). Genetic testing revealed heterozygosity for a pathogenic variant of the FH gene, consistent with a diagnosis of Reed syndrome.

Histologically, the differential diagnosis includes other spindle cell tumors, such as dermatofibroma, neurofibroma, and dermatomyofibroma. The histologic appearance varies depending on the type, with piloleiomyoma typically located within the reticular dermis with possible subcutaneous extension. Fascicles of eosinophilic smooth muscle cells in an interlacing arrangement often ramify between neighboring dermal collagen; these smooth muscle cells contain cigar-shaped, blunt-ended nuclei with a perinuclear clear vacuole. Marked epidermal hyperplasia is possible.6 A close association with a nearby hair follicle frequently is noted. Although differentiated smooth muscle cells usually are evident on hematoxylin and eosin, positive staining for smooth muscle actin (SMA) and desmin can aid in diagnosis.7 Immunohistochemical staining for FH has proven to be highly specific (97.6%) with moderate sensitivity (70.0%).8 Angioleiomyomas appear as well-demarcated dermal to subcutaneous tumors composed of smooth muscle cells surrounding thick-walled vaculature.9 Scrotal and vulvar leiomyomas are composed of eosinophilic spindle cells, though vulvar leiomyomas have shown epithelioid differentiation.10 Nipple leiomyomas appear similar to piloleiomyomas on histology with interlacing smooth muscle fiber bundles.
Eccrine spiradenoma is a relatively uncommon adnexal tumor derived from eccrine sweat glands. It most often presents as a small, painful or tender, intradermal nodule (or rarely as nodules) on the head or ventral trunk.11 There is no sexual predilection. It affects adults at any age but most often from 15 to 35 years. Although rare, malignant transformation is possible. Histologically, eccrine spiradenomas appear as a well-demarcated dermal tumor composed of bland basaloid cells with minimal cytoplasm, often with numerous admixed lymphocytes and variably prominent vasculature (Figure 2). Eosinophilic basement membrane material can be seen within or surrounding the nodules of tumor cells. Multiple spiradenomas can occur in the setting of Brooke-Spiegler syndrome, which is an autosomal-dominant disorder due to an inherited mutation in the CYLD gene. Spiradenomas are benign neoplasms, and surgical excision with clear margins is the treatment of choice.12

Dermatofibroma, also known as cutaneous benign fibrous histiocytoma, is a firm, flesh-colored papule or nodule that most often presents on the lower extremities. It typically is seen in women aged 20 to 40 years.13 The etiology is uncertain, and dermatofibromas often spontaneously develop, though there are inconsistent reports of development with local trauma including insect bites and puncture wounds. The dimple sign refers to skin dimpling with lateral pressure.13 Most commonly, dermatofibromas consist of a dermal proliferation of bland fibroblastic cells with entrapment of dermal collagen bundles at the periphery of the tumors (Figure 3). The fibroblastic cells often are paler and less eosinophilic than smooth muscle cells seen in cutaneous leiomyomas, with tapered nuclei that lack a perinuclear vacuole. Admixed histocytes and other inflammatory cells often are present. Overlying epidermal hyperplasia and/or hyperpigmentation also may be present. Numerous histologic variants have been described, including cellular, epithelioid, aneurysmal, atypical, and hemosiderotic types.14 Immunohistochemical stains may show patchy positive staining for SMA, but h-caldesmon and desmin typically are negative.

Neurofibroma is a tumor derived from neuromesenchymal tissue with nerve axons. They form through neuromesenchyme (eg, Schwann cells, mast cells, perineural cells, endoneural fibroblast) proliferation. Solitary neurofibromas occur most commonly in adults and have no gender predilection. The most common presentation is an asymptomatic, solitary, soft, flesh-colored papulonodule.15 Clinical variants include pigmented, diffuse, and plexiform, with plexiform neurofibromas almost always being consistent with a diagnosis of neurofibromatosis type 1. Histologically, neurofibromas present as dermal or subcutaneous nodules composed of randomly arranged spindle cells with wavy tapered nuclei within a loose collagenous stroma (Figure 4).16 The spindle cells in neurofibromas will stain positively for S-100 protein and SOX-10 and negatively for SMA and desmin.

Angiolipoma is a benign tumor composed of adipocytes that also contains vasculature.17 The majority of cases are of unknown etiology, though familial cases have been described. They typically present as multiple painful or tender (differentiating from lipomas) subcutaneous swellings over the forearms in individuals aged 20 to 30 years.18 On histopathology, angiolipomas appear as well-circumscribed subcutaneous tumors containing mature adipocytes intermixed with small capillary vessels, some of which contain luminal fibrin thrombi (Figure 5).

- Malik K, Patel P, Chen J, et al. Leiomyoma cutis: a focused review on presentation, management, and association with malignancy. Am J Clin Dermatol. 2015;16:35-46.
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Delfino S, Toto V, Brunetti B, et al. Recurrent atypical eccrine spiradenoma of the forehead. In Vivo. 2008;22:821-823.
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60:325-328.
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644.
- Raj S, Calonje E, Kraus M, et al. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19:2-9.
- Choi JH, Ro JY. Cutaneous spindle cell neoplasms: pattern-based diagnostic approach. Arch Pathol Lab Med. 2018;142:958-972.
- Carter CS, Skala SL, Chinnaiyan AM, et al. Immunohistochemical characterization of fumarate hydratase (FH) and succinate dehydrogenase (SDH) in cutaneous leiomyomas for detection of familial cancer syndromes. Am J Surg Pathol. 2017;41:801-809.
- Kanitakis J. Angioleiomyoma of the auricle: an unusual tumor on a rare location. Case Rep Otolaryngol. 2017;2017:1-3.
- Tavassoli FA, Norris HJ. Smooth muscle tumors of the vulva. Obstet Gynecol. 1979;53:213-217.
- Phukan J, Sinha A, Pal S. Fine needle aspiration cytology of eccrine spiradenoma of back: report of a rare case. J Lab Physicians. 2014;6:130.
- Zheng Y, Tian Q, Wang J, et al. Differential diagnosis of eccrine spiradenoma: a case report. Exp Ther Med. 2014;8:1097-1101.
- Bandyopadhyay MR, Besra M, Dutta S, et al. Dermatofibroma: atypical presentations. Indian J Dermatol. 2016;61:121.
- Commons JD, Parish L, Yazdanian S, et al. Dermatofibroma: a curious tumor. Skinmed. 2012;10:268-270.
- Lee YB, Lee JI, Park HJ, et al. Solitary neurofibromas: does an uncommon site exist? Ann Dermatol. 2012;24:101-102.
- Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology. 2018;91:S5-S13.
- Howard WR. Angiolipoma. Arch Dermatol. 1960;82:924.
- Ghosh S, Haldar BA. Multiple angiolipomas. Indian J Dermatol Venereol Leprol. 1990;56:143-144.
- Malik K, Patel P, Chen J, et al. Leiomyoma cutis: a focused review on presentation, management, and association with malignancy. Am J Clin Dermatol. 2015;16:35-46.
- Malhotra P, Walia H, Singh A, et al. Leiomyoma cutis: a clinicopathological series of 37 cases. Indian J Dermatol. 2010;55:337-341.
- Delfino S, Toto V, Brunetti B, et al. Recurrent atypical eccrine spiradenoma of the forehead. In Vivo. 2008;22:821-823.
- Onder M, Adis¸en E. A new indication of botulinum toxin: leiomyoma-related pain. J Am Acad Dermatol. 2009;60:325-328.
- Menko FH, Maher ER, Schmidt LS, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637-644.
- Raj S, Calonje E, Kraus M, et al. Cutaneous pilar leiomyoma: clinicopathologic analysis of 53 lesions in 45 patients. Am J Dermatopathol. 1997;19:2-9.
- Choi JH, Ro JY. Cutaneous spindle cell neoplasms: pattern-based diagnostic approach. Arch Pathol Lab Med. 2018;142:958-972.
- Carter CS, Skala SL, Chinnaiyan AM, et al. Immunohistochemical characterization of fumarate hydratase (FH) and succinate dehydrogenase (SDH) in cutaneous leiomyomas for detection of familial cancer syndromes. Am J Surg Pathol. 2017;41:801-809.
- Kanitakis J. Angioleiomyoma of the auricle: an unusual tumor on a rare location. Case Rep Otolaryngol. 2017;2017:1-3.
- Tavassoli FA, Norris HJ. Smooth muscle tumors of the vulva. Obstet Gynecol. 1979;53:213-217.
- Phukan J, Sinha A, Pal S. Fine needle aspiration cytology of eccrine spiradenoma of back: report of a rare case. J Lab Physicians. 2014;6:130.
- Zheng Y, Tian Q, Wang J, et al. Differential diagnosis of eccrine spiradenoma: a case report. Exp Ther Med. 2014;8:1097-1101.
- Bandyopadhyay MR, Besra M, Dutta S, et al. Dermatofibroma: atypical presentations. Indian J Dermatol. 2016;61:121.
- Commons JD, Parish L, Yazdanian S, et al. Dermatofibroma: a curious tumor. Skinmed. 2012;10:268-270.
- Lee YB, Lee JI, Park HJ, et al. Solitary neurofibromas: does an uncommon site exist? Ann Dermatol. 2012;24:101-102.
- Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology. 2018;91:S5-S13.
- Howard WR. Angiolipoma. Arch Dermatol. 1960;82:924.
- Ghosh S, Haldar BA. Multiple angiolipomas. Indian J Dermatol Venereol Leprol. 1990;56:143-144.
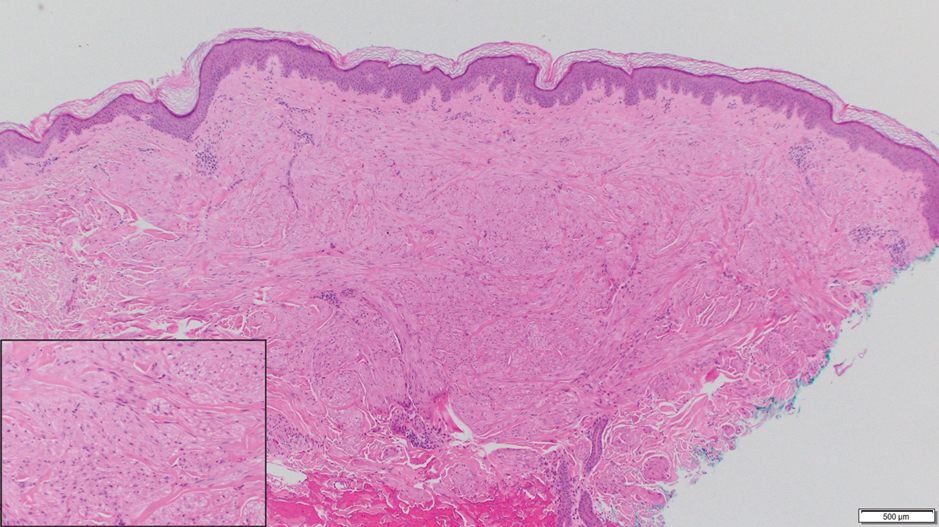
A 36-year-old woman presented with multiple new-onset, firm, tender, subcutaneous papules and nodules involving the upper arms and shoulders.
PCI success vs. meds only in diabetes may depend on LDL-C control
In order for percutaneous coronary intervention (PCI) to shine, compared with meds alone in patients with type-2 diabetes and stable coronary disease (CAD), it needs help from aggressive control of LDL cholesterol (LDL-C) levels, suggests a patient-level meta-analysis of three major randomized trials.
Performing PCI in such patients with diabetes conferred further benefit over optimal medical therapy (OMT) for major adverse cardiac or cerebrovascular events (MACCE) only among those whose LDL-C levels had been pushed below the guidelines-specified threshold of 70 mg/dL within 1 year.
At that level of LDL-C control, PCI, compared with the meds-alone strategy, was followed by a nearly 40% drop in 4-year risk for the composite endpoint, which consisted of death from any cause or nonfatal myocardial infarction (MI) or stroke.
Also for patients reaching a 1-year LDL-C of <70 mg/dL, the risk of MACCE was similar for those who had been assigned to coronary bypass surgery (CABG), compared with PCI. But that risk was significantly lower for the CABG group among those reaching LDL-C levels above that threshold.
“The strategy of revascularization with the LDL lowering, that’s the combination that seems to be a winner” in such patients with diabetes and stable CAD, lead author Michael E. Farkouh, MD, MSc, said in an interview.
If their LDL-C “stays above 70 mg/dL, they don’t really enjoy any benefit of PCI. It’s a message to our interventional community to really drive that LDL down,” said Dr. Farkouh, of the University of Toronto. “Not only with statins, but perhaps with PCSK9 inhibitors, ezetimibe, and other therapies to lower that LDL-C.”
The analysis, published Nov. 2 in the Journal of the American College of Cardiology, pooled more than 4,000 patients with diabetes and stable CAD randomized in the BARI 2D, FREEDOM, and COURAGE trials.
The new study adds a twist to an ongoing theme throughout some meta-analyses and clinical trials like ISCHEMIA since the results of COURAGE were unveiled 13 years ago. The latter trial famously saw no significant difference in death, MI, or stroke in patients with stable CAD assigned to OMT with or without PCI. That set off years of controversy about the relative merits of the revascularization and meds-only strategies in stable CAD that persists today.
But, Dr. Farkouh proposed, whether PCI improves clinical outcomes, compared with meds alone, at least in patients with diabetes, may be tied to the success of LDL-C-lowering therapies in reaching that goal, which in the current study was below 70 mg/dL.
“In this analysis of pooled data from the three major trials, we demonstrate that attaining that level of LDL-C at 1 year portends a better outcome for PCI” in patients with diabetes and stable CAD, he said.
The findings “probably need to be studied further, but it is compelling to think that if we can drive the LDL-C down by one year after the procedure, we have better outcomes with PCI,” compared with a meds-only strategy in patients with diabetes and stable CAD. “That really vindicates a lot of those who believe in PCI,” Dr. Farkouh said.
“What’s surprising to me is, if the patient has an LDL less than 70, why is it that there is a benefit of PCI, compared to medical therapy alone? Because they’re already so aggressively managed, you would think there shouldn’t be a benefit,” Sripal Bangalore, MD, MHA, New York University, said in an interview. “For me, that part is difficult to understand.”
The finding somewhat contradicts the results of ISCHEMIA, in which OMT – including LDL-C-lowering therapy – was considered more aggressive than usually managed in practice, Bangalore said. Yet the trial saw no outcomes difference between PCI and the more conservative approach, leading some to speculate that PCI may be a better choice when, for whatever reason, medical therapy isn’t optimal.
The observed superiority of PCI over meds-only at the lowest LDL-C levels is, according to Dr. Banagalore, “more likely because of residual confounding, given the fact that they’re combining three different trials, which are aimed to address different sets of questions.” He was an investigator with the FREEDOM and ISCHEMIA trials but isn’t associated with the current report.
The main message from this observational analysis is that “of course, we want to get the LDL as low as possible in these patients with demonstrated cardiovascular disease and diabetes,” Donald M. Lloyd-Jones, MD, ScM, Northwestern University, Chicago, said in an interview. “Every one of these patients should be shooting for as low an LDL as possible.”
Regardless of revascularization strategy, he said, “we have to get people on a high-intensity statin, or at least their maximally targeted dose, and have a careful and thoughtful conversation about whether they need additional lowering with, perhaps, ezetimibe, if they’re not below the thresholds we’d like to see them at, in this case, 70 mg/dL.”
Still, the current findings that the relative effects of PCI and CABG in these patients may vary by degree of LDL-C reduction “are interesting, but would have to be tested a little bit more directly,” said Dr. Lloyd-Jones, who is not affiliated with the analysis.
An accompanying editorial, which also acknowledges the study’s limitations, says its results “are relevant for clinical practice and may pave the way toward the generation of novel personalized medicine models that can optimize care of patients with type-2 diabetes.”
They “support the concept of an individualized treatment strategy that accounts for a patient’s LDL-C level to estimate clinical outcomes and expected treatment effects after therapeutic interventions,” say the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
“For daily practice, these results also underscore the importance of follow-up LDL-C measurements, both as a risk stratifier and as an indicator for therapy adjustments,” they write, noting that “current guidelines provide no formal recommendation on when to check LDL-C after PCI.”
The meta-analysis followed a total of 4050 patients with diabetes and stable CAD from the three randomized trials, those with evaluable baseline and follow-up LDL-C measurements, for a median of 4 years after the 1-year LDL-C assessment. At that time, at least 90% of patients in each of the trials had statin prescriptions, the group reported.
At one year, 34.5% of the total cohort had an LDL-C <70 mg/dL; their mean was 55.8 mg/dL.
And 42.2% had an LDL-C from 70 mg/dL to <100 mg/dL; their mean was 83.4 mg/dL. Compared with patients with an LDL-C <70 mg/dL, their adjusted hazard ratio for the composite endpoint was not elevated at 1.07 (95% CI, 0.86-1.32, P = .54).
Finally, 23.2% had an LDL-C ≥100 mg/dL; the mean was 123.0 mg/dL. Compared with the group with the lowest 1-year LDL-C, their adjusted HR for MACCE was increased at 1.46 (95% CI, 1.15 - 1.85, P = .002).
That HR among the 42.3% of patients in the PCI cohort, compared with the 33.3% assigned to meds only, climbed significantly only among those in the lowest 1-year LDL-C stratum: HR, 0.61 (95% CI, 0.40-0.91, P = .016). Corresponding HRs in the mid-range and highest 1-year LDL strata were close to unity and nonsignificant at P = .71 and P = .98, respectively.
On the other hand, the 24.4% of patients assigned to CABG showed better MACCE outcomes than those in the meds-only group across all three 1-year LDL-C strata.
The risk of MACCE wasn’t significantly altered by CABG, compared with PCI among patients achieving a 1-year LDL-C less than 70 mg/dL. However, it fell by about one-half for CABG vs. PCI in both the mid-range and highest 1-year LDL-C strata, P = .003 and P = .022, respectively.
Dr. Bangalore said he’s entirely behind the results of the study’s comparison of PCI and CABG. “It’s exactly the hypothesis that I’ve been putting forward, that if you want to achieve results as good as CABG, do PCI with aggressive medical therapy.” That means second-generation drug-eluting stents for the target lesions, “and aggressive medical therapy to address all of the nontarget lesions, specifically in diabetics.”
It’s possible, Dr. Lloyd-Jones said, that there is “no longer a dichotomy between revascularization strategies,” with respect to clinical outcomes, in such patients who maintain an LDL less than 70 mg/dL, as the study suggests.
“But I wonder, if it had continued for another 4 years of follow-up, whether we would see the CABG patients start to have more events,” such that the CABG advantage goes away at higher LDL-C levels, he proposed.
Or, Dr. Lloyd-Jones speculated, if all patients had achieved LDL-C below 70 mg/dL, “would there be such a difference between the PCI and CABG groups? My bet would be that it would be small or abolished.”
Dr. Farkouh discloses receiving research grants from Amgen, Novo Nordisk, and Novartis. Disclosures for the other study authors can be found with the original article. Editorialist Dr. Navarese discloses receiving consulting fees or honoraria from Abbott, AstraZeneca, Amgen, Bayer, Sanofi, and Pfizer; and grants from Abbott and Amgen. Dr. Lloyd-Jones has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
In order for percutaneous coronary intervention (PCI) to shine, compared with meds alone in patients with type-2 diabetes and stable coronary disease (CAD), it needs help from aggressive control of LDL cholesterol (LDL-C) levels, suggests a patient-level meta-analysis of three major randomized trials.
Performing PCI in such patients with diabetes conferred further benefit over optimal medical therapy (OMT) for major adverse cardiac or cerebrovascular events (MACCE) only among those whose LDL-C levels had been pushed below the guidelines-specified threshold of 70 mg/dL within 1 year.
At that level of LDL-C control, PCI, compared with the meds-alone strategy, was followed by a nearly 40% drop in 4-year risk for the composite endpoint, which consisted of death from any cause or nonfatal myocardial infarction (MI) or stroke.
Also for patients reaching a 1-year LDL-C of <70 mg/dL, the risk of MACCE was similar for those who had been assigned to coronary bypass surgery (CABG), compared with PCI. But that risk was significantly lower for the CABG group among those reaching LDL-C levels above that threshold.
“The strategy of revascularization with the LDL lowering, that’s the combination that seems to be a winner” in such patients with diabetes and stable CAD, lead author Michael E. Farkouh, MD, MSc, said in an interview.
If their LDL-C “stays above 70 mg/dL, they don’t really enjoy any benefit of PCI. It’s a message to our interventional community to really drive that LDL down,” said Dr. Farkouh, of the University of Toronto. “Not only with statins, but perhaps with PCSK9 inhibitors, ezetimibe, and other therapies to lower that LDL-C.”
The analysis, published Nov. 2 in the Journal of the American College of Cardiology, pooled more than 4,000 patients with diabetes and stable CAD randomized in the BARI 2D, FREEDOM, and COURAGE trials.
The new study adds a twist to an ongoing theme throughout some meta-analyses and clinical trials like ISCHEMIA since the results of COURAGE were unveiled 13 years ago. The latter trial famously saw no significant difference in death, MI, or stroke in patients with stable CAD assigned to OMT with or without PCI. That set off years of controversy about the relative merits of the revascularization and meds-only strategies in stable CAD that persists today.
But, Dr. Farkouh proposed, whether PCI improves clinical outcomes, compared with meds alone, at least in patients with diabetes, may be tied to the success of LDL-C-lowering therapies in reaching that goal, which in the current study was below 70 mg/dL.
“In this analysis of pooled data from the three major trials, we demonstrate that attaining that level of LDL-C at 1 year portends a better outcome for PCI” in patients with diabetes and stable CAD, he said.
The findings “probably need to be studied further, but it is compelling to think that if we can drive the LDL-C down by one year after the procedure, we have better outcomes with PCI,” compared with a meds-only strategy in patients with diabetes and stable CAD. “That really vindicates a lot of those who believe in PCI,” Dr. Farkouh said.
“What’s surprising to me is, if the patient has an LDL less than 70, why is it that there is a benefit of PCI, compared to medical therapy alone? Because they’re already so aggressively managed, you would think there shouldn’t be a benefit,” Sripal Bangalore, MD, MHA, New York University, said in an interview. “For me, that part is difficult to understand.”
The finding somewhat contradicts the results of ISCHEMIA, in which OMT – including LDL-C-lowering therapy – was considered more aggressive than usually managed in practice, Bangalore said. Yet the trial saw no outcomes difference between PCI and the more conservative approach, leading some to speculate that PCI may be a better choice when, for whatever reason, medical therapy isn’t optimal.
The observed superiority of PCI over meds-only at the lowest LDL-C levels is, according to Dr. Banagalore, “more likely because of residual confounding, given the fact that they’re combining three different trials, which are aimed to address different sets of questions.” He was an investigator with the FREEDOM and ISCHEMIA trials but isn’t associated with the current report.
The main message from this observational analysis is that “of course, we want to get the LDL as low as possible in these patients with demonstrated cardiovascular disease and diabetes,” Donald M. Lloyd-Jones, MD, ScM, Northwestern University, Chicago, said in an interview. “Every one of these patients should be shooting for as low an LDL as possible.”
Regardless of revascularization strategy, he said, “we have to get people on a high-intensity statin, or at least their maximally targeted dose, and have a careful and thoughtful conversation about whether they need additional lowering with, perhaps, ezetimibe, if they’re not below the thresholds we’d like to see them at, in this case, 70 mg/dL.”
Still, the current findings that the relative effects of PCI and CABG in these patients may vary by degree of LDL-C reduction “are interesting, but would have to be tested a little bit more directly,” said Dr. Lloyd-Jones, who is not affiliated with the analysis.
An accompanying editorial, which also acknowledges the study’s limitations, says its results “are relevant for clinical practice and may pave the way toward the generation of novel personalized medicine models that can optimize care of patients with type-2 diabetes.”
They “support the concept of an individualized treatment strategy that accounts for a patient’s LDL-C level to estimate clinical outcomes and expected treatment effects after therapeutic interventions,” say the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
“For daily practice, these results also underscore the importance of follow-up LDL-C measurements, both as a risk stratifier and as an indicator for therapy adjustments,” they write, noting that “current guidelines provide no formal recommendation on when to check LDL-C after PCI.”
The meta-analysis followed a total of 4050 patients with diabetes and stable CAD from the three randomized trials, those with evaluable baseline and follow-up LDL-C measurements, for a median of 4 years after the 1-year LDL-C assessment. At that time, at least 90% of patients in each of the trials had statin prescriptions, the group reported.
At one year, 34.5% of the total cohort had an LDL-C <70 mg/dL; their mean was 55.8 mg/dL.
And 42.2% had an LDL-C from 70 mg/dL to <100 mg/dL; their mean was 83.4 mg/dL. Compared with patients with an LDL-C <70 mg/dL, their adjusted hazard ratio for the composite endpoint was not elevated at 1.07 (95% CI, 0.86-1.32, P = .54).
Finally, 23.2% had an LDL-C ≥100 mg/dL; the mean was 123.0 mg/dL. Compared with the group with the lowest 1-year LDL-C, their adjusted HR for MACCE was increased at 1.46 (95% CI, 1.15 - 1.85, P = .002).
That HR among the 42.3% of patients in the PCI cohort, compared with the 33.3% assigned to meds only, climbed significantly only among those in the lowest 1-year LDL-C stratum: HR, 0.61 (95% CI, 0.40-0.91, P = .016). Corresponding HRs in the mid-range and highest 1-year LDL strata were close to unity and nonsignificant at P = .71 and P = .98, respectively.
On the other hand, the 24.4% of patients assigned to CABG showed better MACCE outcomes than those in the meds-only group across all three 1-year LDL-C strata.
The risk of MACCE wasn’t significantly altered by CABG, compared with PCI among patients achieving a 1-year LDL-C less than 70 mg/dL. However, it fell by about one-half for CABG vs. PCI in both the mid-range and highest 1-year LDL-C strata, P = .003 and P = .022, respectively.
Dr. Bangalore said he’s entirely behind the results of the study’s comparison of PCI and CABG. “It’s exactly the hypothesis that I’ve been putting forward, that if you want to achieve results as good as CABG, do PCI with aggressive medical therapy.” That means second-generation drug-eluting stents for the target lesions, “and aggressive medical therapy to address all of the nontarget lesions, specifically in diabetics.”
It’s possible, Dr. Lloyd-Jones said, that there is “no longer a dichotomy between revascularization strategies,” with respect to clinical outcomes, in such patients who maintain an LDL less than 70 mg/dL, as the study suggests.
“But I wonder, if it had continued for another 4 years of follow-up, whether we would see the CABG patients start to have more events,” such that the CABG advantage goes away at higher LDL-C levels, he proposed.
Or, Dr. Lloyd-Jones speculated, if all patients had achieved LDL-C below 70 mg/dL, “would there be such a difference between the PCI and CABG groups? My bet would be that it would be small or abolished.”
Dr. Farkouh discloses receiving research grants from Amgen, Novo Nordisk, and Novartis. Disclosures for the other study authors can be found with the original article. Editorialist Dr. Navarese discloses receiving consulting fees or honoraria from Abbott, AstraZeneca, Amgen, Bayer, Sanofi, and Pfizer; and grants from Abbott and Amgen. Dr. Lloyd-Jones has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
In order for percutaneous coronary intervention (PCI) to shine, compared with meds alone in patients with type-2 diabetes and stable coronary disease (CAD), it needs help from aggressive control of LDL cholesterol (LDL-C) levels, suggests a patient-level meta-analysis of three major randomized trials.
Performing PCI in such patients with diabetes conferred further benefit over optimal medical therapy (OMT) for major adverse cardiac or cerebrovascular events (MACCE) only among those whose LDL-C levels had been pushed below the guidelines-specified threshold of 70 mg/dL within 1 year.
At that level of LDL-C control, PCI, compared with the meds-alone strategy, was followed by a nearly 40% drop in 4-year risk for the composite endpoint, which consisted of death from any cause or nonfatal myocardial infarction (MI) or stroke.
Also for patients reaching a 1-year LDL-C of <70 mg/dL, the risk of MACCE was similar for those who had been assigned to coronary bypass surgery (CABG), compared with PCI. But that risk was significantly lower for the CABG group among those reaching LDL-C levels above that threshold.
“The strategy of revascularization with the LDL lowering, that’s the combination that seems to be a winner” in such patients with diabetes and stable CAD, lead author Michael E. Farkouh, MD, MSc, said in an interview.
If their LDL-C “stays above 70 mg/dL, they don’t really enjoy any benefit of PCI. It’s a message to our interventional community to really drive that LDL down,” said Dr. Farkouh, of the University of Toronto. “Not only with statins, but perhaps with PCSK9 inhibitors, ezetimibe, and other therapies to lower that LDL-C.”
The analysis, published Nov. 2 in the Journal of the American College of Cardiology, pooled more than 4,000 patients with diabetes and stable CAD randomized in the BARI 2D, FREEDOM, and COURAGE trials.
The new study adds a twist to an ongoing theme throughout some meta-analyses and clinical trials like ISCHEMIA since the results of COURAGE were unveiled 13 years ago. The latter trial famously saw no significant difference in death, MI, or stroke in patients with stable CAD assigned to OMT with or without PCI. That set off years of controversy about the relative merits of the revascularization and meds-only strategies in stable CAD that persists today.
But, Dr. Farkouh proposed, whether PCI improves clinical outcomes, compared with meds alone, at least in patients with diabetes, may be tied to the success of LDL-C-lowering therapies in reaching that goal, which in the current study was below 70 mg/dL.
“In this analysis of pooled data from the three major trials, we demonstrate that attaining that level of LDL-C at 1 year portends a better outcome for PCI” in patients with diabetes and stable CAD, he said.
The findings “probably need to be studied further, but it is compelling to think that if we can drive the LDL-C down by one year after the procedure, we have better outcomes with PCI,” compared with a meds-only strategy in patients with diabetes and stable CAD. “That really vindicates a lot of those who believe in PCI,” Dr. Farkouh said.
“What’s surprising to me is, if the patient has an LDL less than 70, why is it that there is a benefit of PCI, compared to medical therapy alone? Because they’re already so aggressively managed, you would think there shouldn’t be a benefit,” Sripal Bangalore, MD, MHA, New York University, said in an interview. “For me, that part is difficult to understand.”
The finding somewhat contradicts the results of ISCHEMIA, in which OMT – including LDL-C-lowering therapy – was considered more aggressive than usually managed in practice, Bangalore said. Yet the trial saw no outcomes difference between PCI and the more conservative approach, leading some to speculate that PCI may be a better choice when, for whatever reason, medical therapy isn’t optimal.
The observed superiority of PCI over meds-only at the lowest LDL-C levels is, according to Dr. Banagalore, “more likely because of residual confounding, given the fact that they’re combining three different trials, which are aimed to address different sets of questions.” He was an investigator with the FREEDOM and ISCHEMIA trials but isn’t associated with the current report.
The main message from this observational analysis is that “of course, we want to get the LDL as low as possible in these patients with demonstrated cardiovascular disease and diabetes,” Donald M. Lloyd-Jones, MD, ScM, Northwestern University, Chicago, said in an interview. “Every one of these patients should be shooting for as low an LDL as possible.”
Regardless of revascularization strategy, he said, “we have to get people on a high-intensity statin, or at least their maximally targeted dose, and have a careful and thoughtful conversation about whether they need additional lowering with, perhaps, ezetimibe, if they’re not below the thresholds we’d like to see them at, in this case, 70 mg/dL.”
Still, the current findings that the relative effects of PCI and CABG in these patients may vary by degree of LDL-C reduction “are interesting, but would have to be tested a little bit more directly,” said Dr. Lloyd-Jones, who is not affiliated with the analysis.
An accompanying editorial, which also acknowledges the study’s limitations, says its results “are relevant for clinical practice and may pave the way toward the generation of novel personalized medicine models that can optimize care of patients with type-2 diabetes.”
They “support the concept of an individualized treatment strategy that accounts for a patient’s LDL-C level to estimate clinical outcomes and expected treatment effects after therapeutic interventions,” say the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
“For daily practice, these results also underscore the importance of follow-up LDL-C measurements, both as a risk stratifier and as an indicator for therapy adjustments,” they write, noting that “current guidelines provide no formal recommendation on when to check LDL-C after PCI.”
The meta-analysis followed a total of 4050 patients with diabetes and stable CAD from the three randomized trials, those with evaluable baseline and follow-up LDL-C measurements, for a median of 4 years after the 1-year LDL-C assessment. At that time, at least 90% of patients in each of the trials had statin prescriptions, the group reported.
At one year, 34.5% of the total cohort had an LDL-C <70 mg/dL; their mean was 55.8 mg/dL.
And 42.2% had an LDL-C from 70 mg/dL to <100 mg/dL; their mean was 83.4 mg/dL. Compared with patients with an LDL-C <70 mg/dL, their adjusted hazard ratio for the composite endpoint was not elevated at 1.07 (95% CI, 0.86-1.32, P = .54).
Finally, 23.2% had an LDL-C ≥100 mg/dL; the mean was 123.0 mg/dL. Compared with the group with the lowest 1-year LDL-C, their adjusted HR for MACCE was increased at 1.46 (95% CI, 1.15 - 1.85, P = .002).
That HR among the 42.3% of patients in the PCI cohort, compared with the 33.3% assigned to meds only, climbed significantly only among those in the lowest 1-year LDL-C stratum: HR, 0.61 (95% CI, 0.40-0.91, P = .016). Corresponding HRs in the mid-range and highest 1-year LDL strata were close to unity and nonsignificant at P = .71 and P = .98, respectively.
On the other hand, the 24.4% of patients assigned to CABG showed better MACCE outcomes than those in the meds-only group across all three 1-year LDL-C strata.
The risk of MACCE wasn’t significantly altered by CABG, compared with PCI among patients achieving a 1-year LDL-C less than 70 mg/dL. However, it fell by about one-half for CABG vs. PCI in both the mid-range and highest 1-year LDL-C strata, P = .003 and P = .022, respectively.
Dr. Bangalore said he’s entirely behind the results of the study’s comparison of PCI and CABG. “It’s exactly the hypothesis that I’ve been putting forward, that if you want to achieve results as good as CABG, do PCI with aggressive medical therapy.” That means second-generation drug-eluting stents for the target lesions, “and aggressive medical therapy to address all of the nontarget lesions, specifically in diabetics.”
It’s possible, Dr. Lloyd-Jones said, that there is “no longer a dichotomy between revascularization strategies,” with respect to clinical outcomes, in such patients who maintain an LDL less than 70 mg/dL, as the study suggests.
“But I wonder, if it had continued for another 4 years of follow-up, whether we would see the CABG patients start to have more events,” such that the CABG advantage goes away at higher LDL-C levels, he proposed.
Or, Dr. Lloyd-Jones speculated, if all patients had achieved LDL-C below 70 mg/dL, “would there be such a difference between the PCI and CABG groups? My bet would be that it would be small or abolished.”
Dr. Farkouh discloses receiving research grants from Amgen, Novo Nordisk, and Novartis. Disclosures for the other study authors can be found with the original article. Editorialist Dr. Navarese discloses receiving consulting fees or honoraria from Abbott, AstraZeneca, Amgen, Bayer, Sanofi, and Pfizer; and grants from Abbott and Amgen. Dr. Lloyd-Jones has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Infectious disease is an increasing threat from climate change
“I would argue that the most important reason to care about climate change is because of our children,” Saul Hymes, MD, said at the annual meeting of the American Academy of Pediatrics, held virtually this year.
“Being able to point out to people how climate change harms the health of their children and affects their children’s risk of infections is a particularly effective argument to make,” said Dr. Hymes, a pediatric infectious diseases specialist at Stony Brook (N.Y.) University.
Rachel Boykan, MD, a pediatrician at the university, found Dr. Hymes’ presentation excellent and highly relevant to issues all health care workers treating children face, even beyond infectious disease.
“It was data focused but also understandable for a broad audience,” Dr. Boykan, who was not involved in the presentation, said in an interview. “He explained the science of climate change in a way that all physicians, but especially pediatricians, would find relevant. I suspect if people who were listening didn’t already prioritize the issues of climate change, they certainly did after hearing the talk.”
She also appreciated that Dr. Hymes addressed how climate change affects everyone in both their professional and personal lives.
“We need to be prepared to address the clinical issues that ensue after a natural disaster, and we need to be advocates for change so that we can slow down the climate changes we are all dealing with,” said Dr. Boykan, adding that the presentation was also inspiring. “He presented many different viewpoints and many ways to be involved and to be an advocate. I would think that a good number of people who were there would be energized to do something differently to combat climate change.”
The multitudinous impacts of climate change
The impact of climate change on human health is broad and far-reaching, Dr. Hymes said. It doesn’t require much imagination to recognize that rising global temperatures can lead to prolonged extreme heat waves that can cause heat-related deaths and illnesses. But other effects can be more gradual or subtle. Changes in outdoor air quality can affect weather patterns, pollen counts, and air pollution that can increase risk of asthma, allergies, as well as acute and chronic respiratory and cardiovascular disease.
Sea level rise, more frequent and severe hurricanes, storm surges, and extreme precipitation all can lead to contaminated water and destruction of essential infrastructure. In addition to drowning and injuries from the storms themselves, these changes have mental health consequences, and can lead to gastrointestinal and other illnesses, including water-borne infectious disease. The distribution and prevalence of vector-borne diseases also will shift with changes in temperature, precipitation, and other weather patterns.
Distribution, prevalence of vector-borne diseases shift with climate change
One of the most common bacteria transmitted by vectors in the United States is Borrelia burgdorferi, the cause of Lyme disease. Transmitted by deer ticks, Lyme disease is listed by the Environmental Protection Agency as an indicator of climate change’s impact on human health and is becoming more common every year. Cases doubled from 1990 to 2014, from 4 to 8 cases per 100,000 people.
Increases were most dramatic in the Northeast, where Lyme disease is endemic. States such as Maine, Vermont, and New Hampshire all saw increases of 80-100 more cases per 100,000 people. Evidence now shows that Lyme disease is moving north as the climate warms. Toronto, for example, has seen more than a 400% increase in cases in less than a decade, from 128 cases per 100,000 people in 2009 to 700 cases per 100,000 in 2015.
“It’s a known phenomenon that climate change affects more northerly latitudes disproportionately to more than southerly latitudes,” Dr. Hymes said. He shared a 2013 study providing evidence that climate change is expanding the range of Lyme disease. Even when controlling for other confounding factors, the research found that areas being warmed proportionately more by climate change also are experiencing greater Lyme incidence. While Lyme cases declined in several Western and Deep South states, it significantly increased in nearly every Northeast state as well as Idaho, Arizona, and states in the northern Midwest near the Great Lakes.
“We find that this impact of climate change on the movement of vectors like ticks affects more than just Lyme disease,” Dr. Hymes said. Amblyomma americanum, the Lone Star tick, has historically been restricted to the southern United States but is now found further north, even up to New England. It carries bacteria that can cause multiple illnesses, including ehrlichiosis, heartland virus, and tularemia.
An alpha-gal meat allergy associated with this tick can lead to anaphylaxis about 6 hours after a person eats red meat or pork. Prevalence of this allergy, first reported in Georgia in 1989-1991, has been increasing and moving further north, and the Lone Star tick is a particularly heat-tolerant and heat-loving tick.
Climate change also affects how long during the year people are at risk. Lyme disease, for example, typically lasted from April/May to October, when ticks then hibernated during the cold weather. But the warming climate has expanded Lyme season: Local Lyme cases have begun occurring into November through January on Long Island over the past 5 years.
The impact of seasonal changes on infectious diseases overall is difficult to predict. The seasons for cold weather diseases such as influenza and respiratory syncytial virus, for example, may become shorter or milder while viruses more common in the summer, such as enteroviruses, may become a risk year-round.
Natural disasters pose multiple risks
Natural disasters can pose immediate dangers to families and have a significant impact on mental health, but that’s not their only potential impact.
“Severe weather events such as hurricanes, floods, and tornadoes are well established in the climate change literature as an effect of increased temperatures and more volatile weather systems, but they also have a significant effect on infectious diseases and on children in particular,” Dr. Hymes said. “Hurricanes and flash floods can cause increases in infectious disease outbreaks through a variety of different ways.”
They can bring saltwater, freshwater, and sometimes soil organisms into the food and water supplies, and lead to sewage contamination from overloaded sewers, overflowing storm drains, and loss of power or pumps. Displaced animal vectors, such as rats, can lead to spread of other diseases, such as plague, hantavirus, typhus, and rabies.
Examples of saltwater organisms include Vibrio, Aeromonas, and Mycobacterium marinum, all of which can cause infections in wounds and/or diarrheal illness or bacteremia. Similarly, organisms from freshwater and soil that can cause serious illness or death include Aeromonas, Pseudomonas, Amebiasis, Giardia, and Legionella. Without access to clean water, or with contamination from overflowing sewage, cryptosporidium, Escherichia coli, salmonella, typhoid, norovirus, hepatitis A and E, and even cholera can also become problems as well.
In Houston following Hurricane Harvey, for example, cellulitis cases doubled and included infections from organisms different from the usual suspects. Scrapes and cuts that occurred during the storm also festered sooner.
Cases of disease linked to Hurricane Katrina in a Centers for Disease Control and Prevention report included 6 cases of cholera, 17 cases of other vibrio – including five that resulted in death – and reported cases of norovirus, Escherichia coli, salmonella, and influenza and pneumonia from overcrowding of evacuees.
You can help in a variety of ways
You can play several key roles as the world’s climate changes, starting with preparing for the changes. You should familiarize themselves with new and emerging infections, or those that have been around a while but not seen in your areas, such as Lyme, Zika, and Dengue.
“If you haven’t seen them already, you likely will due to movements of vector-borne infections that can occur due to climate change,” Dr. Hymes said. “You also want to expect the usual common diseases, but maybe at unsuspected times,” he added. “If you have a pediatric patient who looks like they have Coxsackie virus but it’s February, if it’s been a warm February, it may very well be Coxsackie virus.”
Following natural disasters such as floods, hurricanes and tornadoes, consider who your patients are. If they’re evacuees, are they living in overcrowded conditions? Do they have access to clean water? If not, explain the need to boil water if they can, or to use iodine tablets or a portable pump filter. Consider that some infections may involve unexpected or odd organisms, such as legionella pneumonia or vibrio cellulitis, and contact your local infectious disease doctor as needed.
You also can make personal lifestyle changes that, while small, can add up in the aggregate in reducing carbon footprints, such as purchasing an electric or hybrid car and converting their homes to solar power.
“For very little money, you can purchase carbon offsets,” Dr. Hymes said, such as $10-$15 a month for wind power offsets with home electricity or $5-$10 a month for car or plane travel.
“But really, the most important thing we can do as pediatricians is educate,” Dr. Hymes said. “Taking opportunities every day in your office to educate your patients and educate your colleagues about the importance of climate change in our patients’ health and our own children’s health is super, super important.”
Dr. Hymes and Dr. Boykan had no relevant financial disclosures.
“I would argue that the most important reason to care about climate change is because of our children,” Saul Hymes, MD, said at the annual meeting of the American Academy of Pediatrics, held virtually this year.
“Being able to point out to people how climate change harms the health of their children and affects their children’s risk of infections is a particularly effective argument to make,” said Dr. Hymes, a pediatric infectious diseases specialist at Stony Brook (N.Y.) University.
Rachel Boykan, MD, a pediatrician at the university, found Dr. Hymes’ presentation excellent and highly relevant to issues all health care workers treating children face, even beyond infectious disease.
“It was data focused but also understandable for a broad audience,” Dr. Boykan, who was not involved in the presentation, said in an interview. “He explained the science of climate change in a way that all physicians, but especially pediatricians, would find relevant. I suspect if people who were listening didn’t already prioritize the issues of climate change, they certainly did after hearing the talk.”
She also appreciated that Dr. Hymes addressed how climate change affects everyone in both their professional and personal lives.
“We need to be prepared to address the clinical issues that ensue after a natural disaster, and we need to be advocates for change so that we can slow down the climate changes we are all dealing with,” said Dr. Boykan, adding that the presentation was also inspiring. “He presented many different viewpoints and many ways to be involved and to be an advocate. I would think that a good number of people who were there would be energized to do something differently to combat climate change.”
The multitudinous impacts of climate change
The impact of climate change on human health is broad and far-reaching, Dr. Hymes said. It doesn’t require much imagination to recognize that rising global temperatures can lead to prolonged extreme heat waves that can cause heat-related deaths and illnesses. But other effects can be more gradual or subtle. Changes in outdoor air quality can affect weather patterns, pollen counts, and air pollution that can increase risk of asthma, allergies, as well as acute and chronic respiratory and cardiovascular disease.
Sea level rise, more frequent and severe hurricanes, storm surges, and extreme precipitation all can lead to contaminated water and destruction of essential infrastructure. In addition to drowning and injuries from the storms themselves, these changes have mental health consequences, and can lead to gastrointestinal and other illnesses, including water-borne infectious disease. The distribution and prevalence of vector-borne diseases also will shift with changes in temperature, precipitation, and other weather patterns.
Distribution, prevalence of vector-borne diseases shift with climate change
One of the most common bacteria transmitted by vectors in the United States is Borrelia burgdorferi, the cause of Lyme disease. Transmitted by deer ticks, Lyme disease is listed by the Environmental Protection Agency as an indicator of climate change’s impact on human health and is becoming more common every year. Cases doubled from 1990 to 2014, from 4 to 8 cases per 100,000 people.
Increases were most dramatic in the Northeast, where Lyme disease is endemic. States such as Maine, Vermont, and New Hampshire all saw increases of 80-100 more cases per 100,000 people. Evidence now shows that Lyme disease is moving north as the climate warms. Toronto, for example, has seen more than a 400% increase in cases in less than a decade, from 128 cases per 100,000 people in 2009 to 700 cases per 100,000 in 2015.
“It’s a known phenomenon that climate change affects more northerly latitudes disproportionately to more than southerly latitudes,” Dr. Hymes said. He shared a 2013 study providing evidence that climate change is expanding the range of Lyme disease. Even when controlling for other confounding factors, the research found that areas being warmed proportionately more by climate change also are experiencing greater Lyme incidence. While Lyme cases declined in several Western and Deep South states, it significantly increased in nearly every Northeast state as well as Idaho, Arizona, and states in the northern Midwest near the Great Lakes.
“We find that this impact of climate change on the movement of vectors like ticks affects more than just Lyme disease,” Dr. Hymes said. Amblyomma americanum, the Lone Star tick, has historically been restricted to the southern United States but is now found further north, even up to New England. It carries bacteria that can cause multiple illnesses, including ehrlichiosis, heartland virus, and tularemia.
An alpha-gal meat allergy associated with this tick can lead to anaphylaxis about 6 hours after a person eats red meat or pork. Prevalence of this allergy, first reported in Georgia in 1989-1991, has been increasing and moving further north, and the Lone Star tick is a particularly heat-tolerant and heat-loving tick.
Climate change also affects how long during the year people are at risk. Lyme disease, for example, typically lasted from April/May to October, when ticks then hibernated during the cold weather. But the warming climate has expanded Lyme season: Local Lyme cases have begun occurring into November through January on Long Island over the past 5 years.
The impact of seasonal changes on infectious diseases overall is difficult to predict. The seasons for cold weather diseases such as influenza and respiratory syncytial virus, for example, may become shorter or milder while viruses more common in the summer, such as enteroviruses, may become a risk year-round.
Natural disasters pose multiple risks
Natural disasters can pose immediate dangers to families and have a significant impact on mental health, but that’s not their only potential impact.
“Severe weather events such as hurricanes, floods, and tornadoes are well established in the climate change literature as an effect of increased temperatures and more volatile weather systems, but they also have a significant effect on infectious diseases and on children in particular,” Dr. Hymes said. “Hurricanes and flash floods can cause increases in infectious disease outbreaks through a variety of different ways.”
They can bring saltwater, freshwater, and sometimes soil organisms into the food and water supplies, and lead to sewage contamination from overloaded sewers, overflowing storm drains, and loss of power or pumps. Displaced animal vectors, such as rats, can lead to spread of other diseases, such as plague, hantavirus, typhus, and rabies.
Examples of saltwater organisms include Vibrio, Aeromonas, and Mycobacterium marinum, all of which can cause infections in wounds and/or diarrheal illness or bacteremia. Similarly, organisms from freshwater and soil that can cause serious illness or death include Aeromonas, Pseudomonas, Amebiasis, Giardia, and Legionella. Without access to clean water, or with contamination from overflowing sewage, cryptosporidium, Escherichia coli, salmonella, typhoid, norovirus, hepatitis A and E, and even cholera can also become problems as well.
In Houston following Hurricane Harvey, for example, cellulitis cases doubled and included infections from organisms different from the usual suspects. Scrapes and cuts that occurred during the storm also festered sooner.
Cases of disease linked to Hurricane Katrina in a Centers for Disease Control and Prevention report included 6 cases of cholera, 17 cases of other vibrio – including five that resulted in death – and reported cases of norovirus, Escherichia coli, salmonella, and influenza and pneumonia from overcrowding of evacuees.
You can help in a variety of ways
You can play several key roles as the world’s climate changes, starting with preparing for the changes. You should familiarize themselves with new and emerging infections, or those that have been around a while but not seen in your areas, such as Lyme, Zika, and Dengue.
“If you haven’t seen them already, you likely will due to movements of vector-borne infections that can occur due to climate change,” Dr. Hymes said. “You also want to expect the usual common diseases, but maybe at unsuspected times,” he added. “If you have a pediatric patient who looks like they have Coxsackie virus but it’s February, if it’s been a warm February, it may very well be Coxsackie virus.”
Following natural disasters such as floods, hurricanes and tornadoes, consider who your patients are. If they’re evacuees, are they living in overcrowded conditions? Do they have access to clean water? If not, explain the need to boil water if they can, or to use iodine tablets or a portable pump filter. Consider that some infections may involve unexpected or odd organisms, such as legionella pneumonia or vibrio cellulitis, and contact your local infectious disease doctor as needed.
You also can make personal lifestyle changes that, while small, can add up in the aggregate in reducing carbon footprints, such as purchasing an electric or hybrid car and converting their homes to solar power.
“For very little money, you can purchase carbon offsets,” Dr. Hymes said, such as $10-$15 a month for wind power offsets with home electricity or $5-$10 a month for car or plane travel.
“But really, the most important thing we can do as pediatricians is educate,” Dr. Hymes said. “Taking opportunities every day in your office to educate your patients and educate your colleagues about the importance of climate change in our patients’ health and our own children’s health is super, super important.”
Dr. Hymes and Dr. Boykan had no relevant financial disclosures.
“I would argue that the most important reason to care about climate change is because of our children,” Saul Hymes, MD, said at the annual meeting of the American Academy of Pediatrics, held virtually this year.
“Being able to point out to people how climate change harms the health of their children and affects their children’s risk of infections is a particularly effective argument to make,” said Dr. Hymes, a pediatric infectious diseases specialist at Stony Brook (N.Y.) University.
Rachel Boykan, MD, a pediatrician at the university, found Dr. Hymes’ presentation excellent and highly relevant to issues all health care workers treating children face, even beyond infectious disease.
“It was data focused but also understandable for a broad audience,” Dr. Boykan, who was not involved in the presentation, said in an interview. “He explained the science of climate change in a way that all physicians, but especially pediatricians, would find relevant. I suspect if people who were listening didn’t already prioritize the issues of climate change, they certainly did after hearing the talk.”
She also appreciated that Dr. Hymes addressed how climate change affects everyone in both their professional and personal lives.
“We need to be prepared to address the clinical issues that ensue after a natural disaster, and we need to be advocates for change so that we can slow down the climate changes we are all dealing with,” said Dr. Boykan, adding that the presentation was also inspiring. “He presented many different viewpoints and many ways to be involved and to be an advocate. I would think that a good number of people who were there would be energized to do something differently to combat climate change.”
The multitudinous impacts of climate change
The impact of climate change on human health is broad and far-reaching, Dr. Hymes said. It doesn’t require much imagination to recognize that rising global temperatures can lead to prolonged extreme heat waves that can cause heat-related deaths and illnesses. But other effects can be more gradual or subtle. Changes in outdoor air quality can affect weather patterns, pollen counts, and air pollution that can increase risk of asthma, allergies, as well as acute and chronic respiratory and cardiovascular disease.
Sea level rise, more frequent and severe hurricanes, storm surges, and extreme precipitation all can lead to contaminated water and destruction of essential infrastructure. In addition to drowning and injuries from the storms themselves, these changes have mental health consequences, and can lead to gastrointestinal and other illnesses, including water-borne infectious disease. The distribution and prevalence of vector-borne diseases also will shift with changes in temperature, precipitation, and other weather patterns.
Distribution, prevalence of vector-borne diseases shift with climate change
One of the most common bacteria transmitted by vectors in the United States is Borrelia burgdorferi, the cause of Lyme disease. Transmitted by deer ticks, Lyme disease is listed by the Environmental Protection Agency as an indicator of climate change’s impact on human health and is becoming more common every year. Cases doubled from 1990 to 2014, from 4 to 8 cases per 100,000 people.
Increases were most dramatic in the Northeast, where Lyme disease is endemic. States such as Maine, Vermont, and New Hampshire all saw increases of 80-100 more cases per 100,000 people. Evidence now shows that Lyme disease is moving north as the climate warms. Toronto, for example, has seen more than a 400% increase in cases in less than a decade, from 128 cases per 100,000 people in 2009 to 700 cases per 100,000 in 2015.
“It’s a known phenomenon that climate change affects more northerly latitudes disproportionately to more than southerly latitudes,” Dr. Hymes said. He shared a 2013 study providing evidence that climate change is expanding the range of Lyme disease. Even when controlling for other confounding factors, the research found that areas being warmed proportionately more by climate change also are experiencing greater Lyme incidence. While Lyme cases declined in several Western and Deep South states, it significantly increased in nearly every Northeast state as well as Idaho, Arizona, and states in the northern Midwest near the Great Lakes.
“We find that this impact of climate change on the movement of vectors like ticks affects more than just Lyme disease,” Dr. Hymes said. Amblyomma americanum, the Lone Star tick, has historically been restricted to the southern United States but is now found further north, even up to New England. It carries bacteria that can cause multiple illnesses, including ehrlichiosis, heartland virus, and tularemia.
An alpha-gal meat allergy associated with this tick can lead to anaphylaxis about 6 hours after a person eats red meat or pork. Prevalence of this allergy, first reported in Georgia in 1989-1991, has been increasing and moving further north, and the Lone Star tick is a particularly heat-tolerant and heat-loving tick.
Climate change also affects how long during the year people are at risk. Lyme disease, for example, typically lasted from April/May to October, when ticks then hibernated during the cold weather. But the warming climate has expanded Lyme season: Local Lyme cases have begun occurring into November through January on Long Island over the past 5 years.
The impact of seasonal changes on infectious diseases overall is difficult to predict. The seasons for cold weather diseases such as influenza and respiratory syncytial virus, for example, may become shorter or milder while viruses more common in the summer, such as enteroviruses, may become a risk year-round.
Natural disasters pose multiple risks
Natural disasters can pose immediate dangers to families and have a significant impact on mental health, but that’s not their only potential impact.
“Severe weather events such as hurricanes, floods, and tornadoes are well established in the climate change literature as an effect of increased temperatures and more volatile weather systems, but they also have a significant effect on infectious diseases and on children in particular,” Dr. Hymes said. “Hurricanes and flash floods can cause increases in infectious disease outbreaks through a variety of different ways.”
They can bring saltwater, freshwater, and sometimes soil organisms into the food and water supplies, and lead to sewage contamination from overloaded sewers, overflowing storm drains, and loss of power or pumps. Displaced animal vectors, such as rats, can lead to spread of other diseases, such as plague, hantavirus, typhus, and rabies.
Examples of saltwater organisms include Vibrio, Aeromonas, and Mycobacterium marinum, all of which can cause infections in wounds and/or diarrheal illness or bacteremia. Similarly, organisms from freshwater and soil that can cause serious illness or death include Aeromonas, Pseudomonas, Amebiasis, Giardia, and Legionella. Without access to clean water, or with contamination from overflowing sewage, cryptosporidium, Escherichia coli, salmonella, typhoid, norovirus, hepatitis A and E, and even cholera can also become problems as well.
In Houston following Hurricane Harvey, for example, cellulitis cases doubled and included infections from organisms different from the usual suspects. Scrapes and cuts that occurred during the storm also festered sooner.
Cases of disease linked to Hurricane Katrina in a Centers for Disease Control and Prevention report included 6 cases of cholera, 17 cases of other vibrio – including five that resulted in death – and reported cases of norovirus, Escherichia coli, salmonella, and influenza and pneumonia from overcrowding of evacuees.
You can help in a variety of ways
You can play several key roles as the world’s climate changes, starting with preparing for the changes. You should familiarize themselves with new and emerging infections, or those that have been around a while but not seen in your areas, such as Lyme, Zika, and Dengue.
“If you haven’t seen them already, you likely will due to movements of vector-borne infections that can occur due to climate change,” Dr. Hymes said. “You also want to expect the usual common diseases, but maybe at unsuspected times,” he added. “If you have a pediatric patient who looks like they have Coxsackie virus but it’s February, if it’s been a warm February, it may very well be Coxsackie virus.”
Following natural disasters such as floods, hurricanes and tornadoes, consider who your patients are. If they’re evacuees, are they living in overcrowded conditions? Do they have access to clean water? If not, explain the need to boil water if they can, or to use iodine tablets or a portable pump filter. Consider that some infections may involve unexpected or odd organisms, such as legionella pneumonia or vibrio cellulitis, and contact your local infectious disease doctor as needed.
You also can make personal lifestyle changes that, while small, can add up in the aggregate in reducing carbon footprints, such as purchasing an electric or hybrid car and converting their homes to solar power.
“For very little money, you can purchase carbon offsets,” Dr. Hymes said, such as $10-$15 a month for wind power offsets with home electricity or $5-$10 a month for car or plane travel.
“But really, the most important thing we can do as pediatricians is educate,” Dr. Hymes said. “Taking opportunities every day in your office to educate your patients and educate your colleagues about the importance of climate change in our patients’ health and our own children’s health is super, super important.”
Dr. Hymes and Dr. Boykan had no relevant financial disclosures.
FROM AAP 2020
How to assess and relieve that perplexing rashless itch
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.
An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2
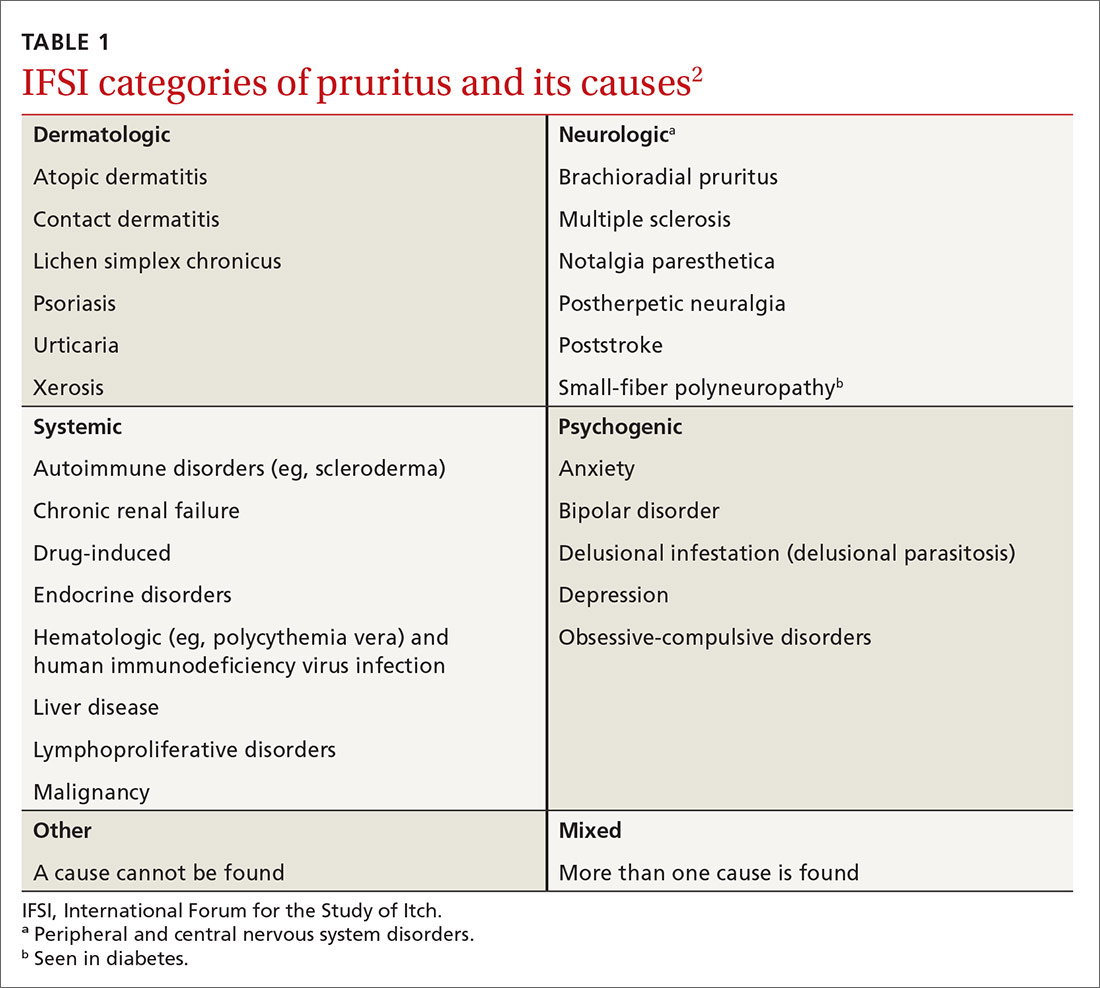
A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2
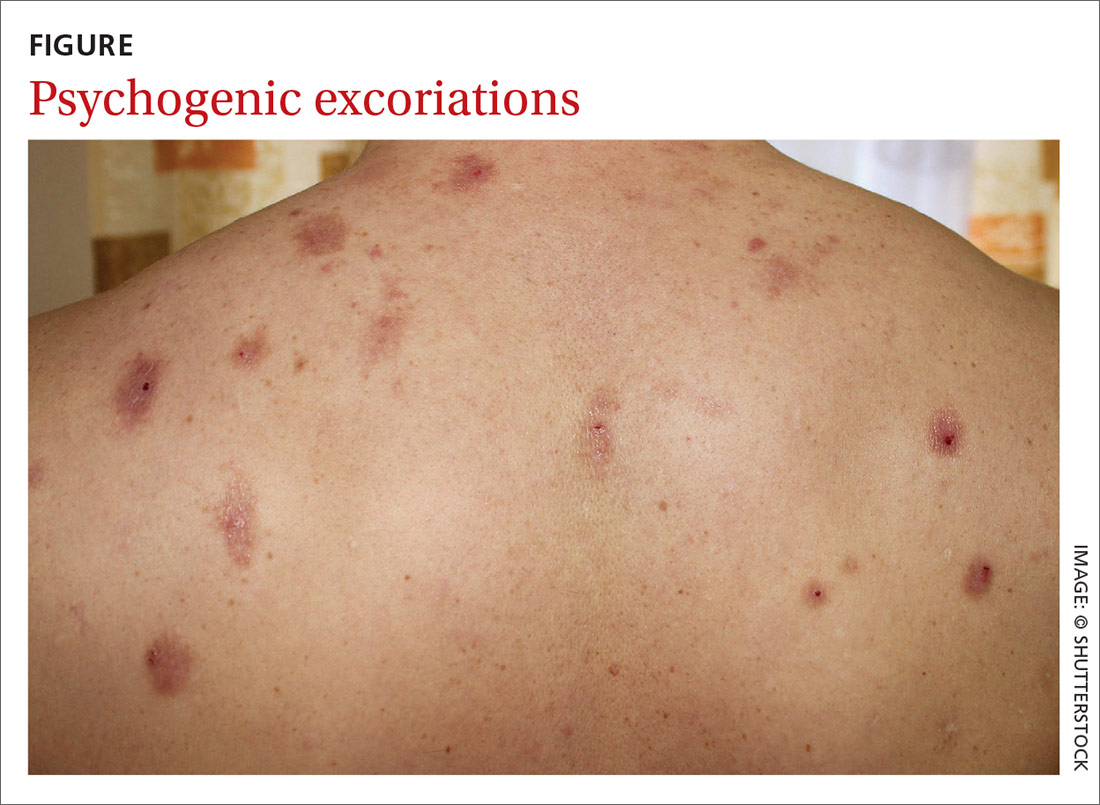
Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17
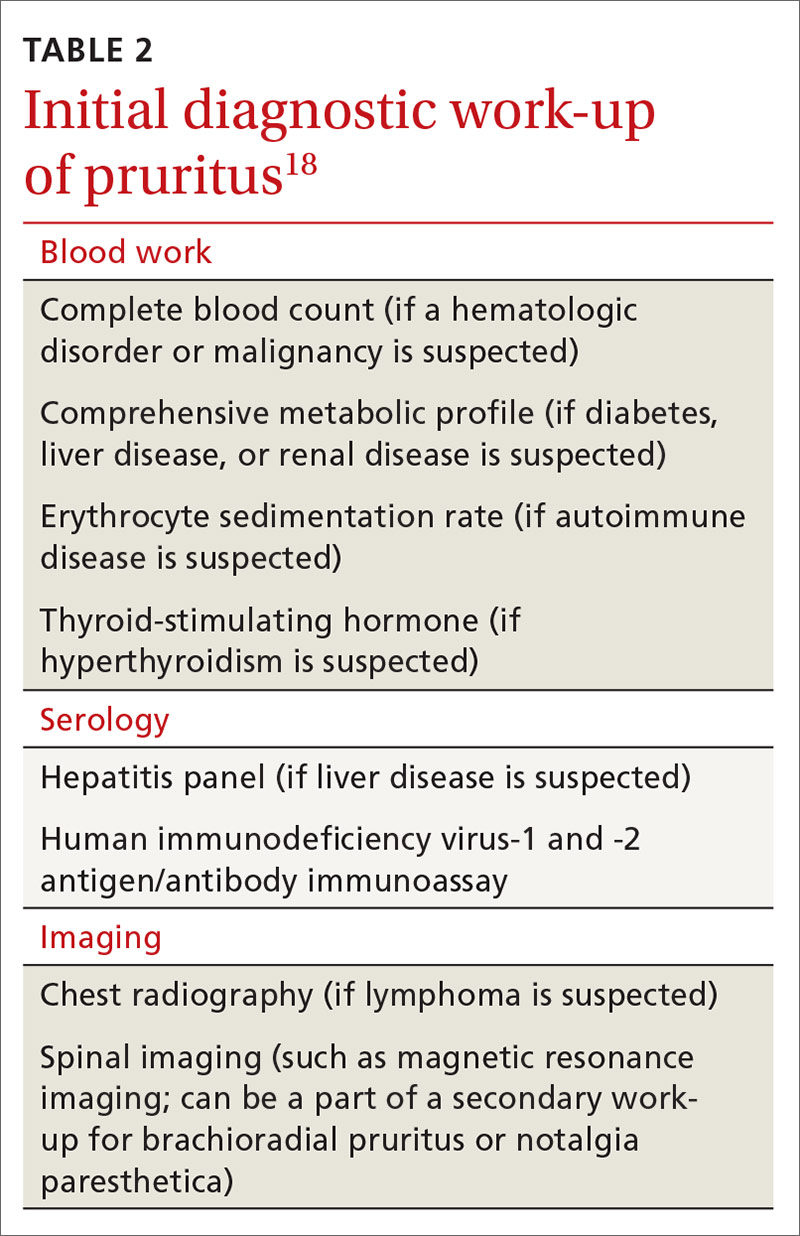
Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7
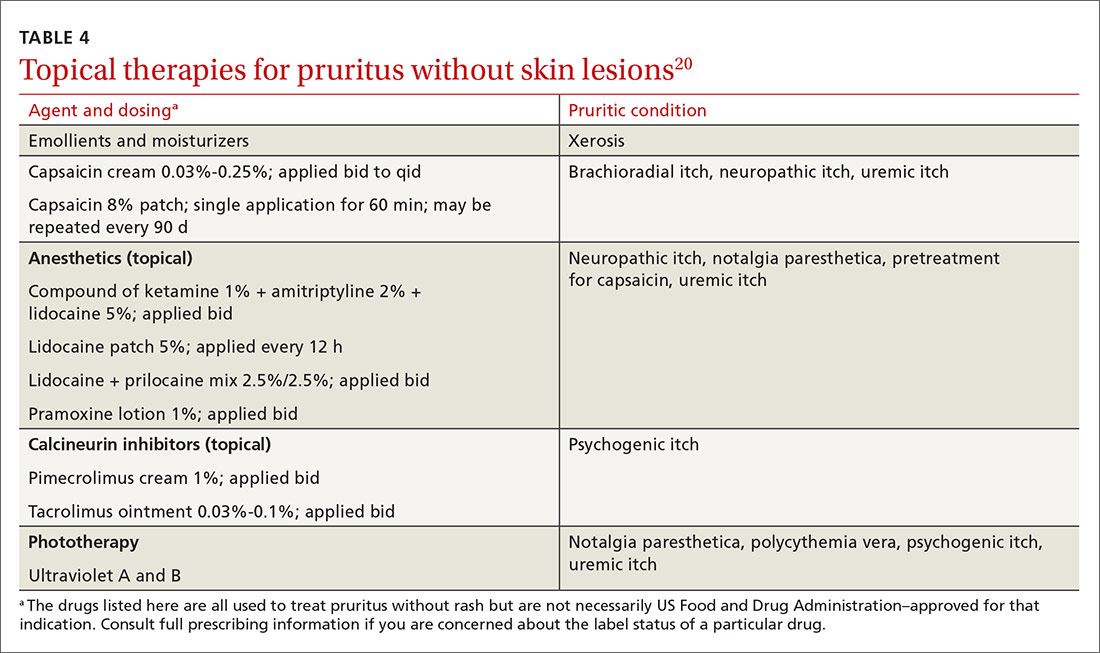
Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
Pruritus, defined as a sensation that induces a desire to scratch1 and classified as acute or chronic (lasting > 6 weeks),2 is one of the most common complaints among primary care patients: Approximately 1% of ambulatory visits in the United States are linked to pruritus.3
Chronic pruritus impairs quality of life; its impact has been compared to that of chronic pain.4 Treatment should therefore be instituted promptly. Although this condition might appear benign, chronic pruritus can be a symptom of a serious condition, as we describe here. When persistent pruritus is refractory to treatment, systemic causes should be fully explored.
In this article, we discuss the pathogenesis and management of pruritus without skin eruption in the adult nonpregnant patient. We also present practice recommendations to help you determine whether your patient’s pruritus is indicative of a serious systemic condition.

An incomplete understanding of the pathophysiology of pruritus
The pathophysiology of pruritus is not fully understood. It is generally recognized, however, that pruritus starts in the peripheral nerves located in the dermal–epidermal junction of the skin.5 The sensation is then transmitted along unmyelinated slow-conducting C fibers to the dorsal horn of the spinal cord.5,6 There are 2 types of C fibers that transmit the itch impulse6: A histamine-dependent type and a non-histamine-dependent type, which might explain why pruritus can be refractory to antihistamine treatment.6
Once the itch impulse has moved from the spinal cord, it travels along the spinothalamic tract up to the contralateral thalamus.1 From there, the impulse ascends to the cerebral cortex.1 In the cortex, the impulse triggers multiple areas of the brain, such as those responsible for sensation, motor function, reward, memory, and emotion.7
Several chemical mediators have been found to be peripheral and central inducers of pruritus: histamine, endogenous opioids, substance P, and serotonin.2 There are indications that certain receptors, such as mu-opioid receptors and kappa-opioid receptors, are key contributors to itch as well.2

A diverse etiology
The International Forum for the Study of Itch (IFSI) has established 6 main categories of causes of pruritus(TABLE 1)2:
- dermatologic
- systemic
- neurologic
- psychogenic
- mixed
- other.
Continue to: In this review...
In this review, we focus on the work-up and management of 3 of those categories: systemic, neurologic, and psychogenic causes of pruritus.
Systemic causes
Research has shown that 14% to 24% of patients who seek the care of a dermatologist for chronic itch without skin lesions have a systemic illness.8
Renal disease. Approximately 40% of patients with end-stage renal disease who are on hemodialysis or peritoneal dialysis have uremic pruritus.2 The itch is mostly generalized but can be pronounced on the back. For most patients, the itch is worse at night, causing a major impact on quality of life.6
Liver disease. In hepatic disease, there is often impairment in the secretion of bile, which can lead to cholestatic pruritus.2 This condition commonly affects the hands and feet first; later, it becomes generalized.2 Cholestatic pruritus can be elicited by tight-fitting clothing. Relief is not achieved by scratching.9 This type of itch effects 70% of patients with primary biliary cirrhosis and 15% of patients with hepatitis C infection.9
Hematologic disorders. Pruritus is a hallmark symptom of polycythemia rubra vera. Almost 50% of patients with this disorder report pruritus that occurs after exposure to water9; aquagenic pruritus can precede the formal diagnosis of polycythemia rubra vera by years.2 It has been speculated that platelet aggregation in this disorder leads to release of serotonin and histamine, which, in turn, causes itch.9
Continue to: Endocrine disorders
Endocrine disorders. Approximately 4% to 11% of patients with thyrotoxicosis have pruritus.1 It has been suggested that vasodilation, increased skin temperature, and a decreased itch threshold from untreated Graves disease might be inciting factors.
Malignancy. In generalized chronic pruritus without a known cause, strongly consider the likelihood of underlying malignancy8,10; for 10% of these patients, their chronic pruritus is a paraneoplastic sign. Paraneoplastic pruritus is characterized as an itch that predates clinical onset, or occurs early in the course, of a malignancy.9 The condition is most strongly linked to cancers of the liver, gallbladder, biliary tract, hematologic system, and skin.11
Chronic pruritus affects 30% of patients with Hodgkin lymphoma.9 General pruritus can precede this diagnosis by months, even years.1 In Hodgkin lymphoma patients who are in remission, a return of pruritic symptoms can be a harbinger of recurrence.9
Neurologic causes
A recent study found that 8% to 15% of patients referred to a dermatology clinic for chronic pruritus without skin eruption had underlying neurologic pathology.12 Although the specific mechanisms of neuropathic itch are still poorly understood, it has been theorized that the itch emanates from neuronal damage, which can come from peripheral or central nervous system lesions.9
Brachioradial pruritus. There are divergent theories about the etiology of brachioradial pruritus. One hypothesis is that the condition is caused by cervical nerve-root impingement at the level of C5-C8 that leads to nerve damage2; another is that chronic exposure to sunlight causes injury to peripheral cutaneous nerves.2 Brachioradial pruritus is localized to the dorsolateral forearm; it can also involve the neck, back, shoulder, upper arm, and chest, unilaterally and bilaterally. This pruritus can be intermittent and become worse upon exposure to sunlight.2
Continue to: Notalgia paresthetica
Notalgia paresthetica. This condition might also cause neuropathic pruritus as a consequence of nerve impingement. The itch of notalgia paresthesia is located on the skin, medial to the scapular border on the upper or mid-back.2 It has been postulated that the itch is caused by nerve entrapment of the posterior rami of spinal nerves arising from T2-T6.9 However, another theory suggests that the itch is caused by damage to peripheral nerves.9 The itch of notalgia paresthetica can wax and wane.2
Poststroke pruritus. Brain lesions, most often caused by stroke, can cause neuropathic itch. One of the best-known syndromes related to poststroke itch is Wallenberg syndrome (ischemia from a lateral medullary infarction), which typically presents with itch, thermalgic hypoesthesia of the face, cerebellar dysfunction, nausea, and vomiting.7
Shingles. More than one-half of patients who develop postherpetic neuralgia as a consequence of a herpes zoster infection also develop neuropathic pruritus.9 It is thought that postherpetic pruritus shares a comparable pathophysiology with postherpetic neuralgia, in which neurons involved in itch stimuli become damaged.7
Diabetes mellitus. Pruritus from diabetes can be classified as systemic or neuropathic. Diabetes is one of the most common causes of small-fiber polyneuropathy, which can cause neuropathic pruritus.13
Multiple sclerosis. Central nervous system lesions that affect sensory pathways can lead to neuropathic itch in multiple sclerosis. Patients can have severe episodes of generalized pruritus. It has been hypothesized that the neuropathic itch in multiple sclerosis is induced by activation of artificial synapses in demyelinated areas.2
Continue to: Psychogenic pruritus
Psychogenic pruritus
Chronic pruritus can be a comorbidity of psychiatric illness. A retrospective study found that pruritus occurs in 32% to 42% of psychiatric inpatients.14 Depression, anxiety, bipolar disorders, obsessive–compulsive disorders, somatoform disorders, psychosis, and substance abuse all have a strong link to psychogenic excoriation.15 Psychogenic excoriation, which can cause secondary skin lesions, occurs in psychiatric patients who excessively pick and scratch normal skin because they perceive an itch sensation or have a delusion of infestation.2 Affected skin can be marked by scattered crusted lesions (FIGURE) anywhere on the body that the patient can reach—most commonly, the extremities.2

Delusion of infestation. Patients with a delusion of infestation have a strong belief that their body is infected by some kind of insect or microorganism.16 Before a diagnosis of delusion of infestation can be made, other organic causes must be excluded, including withdrawal from such substances as cocaine, amphetamines, and alcohol.16 Patients with a delusion of infestation can have, and maintain, a symptomatic response with continuing use of an atypical antipsychotic agent, including risperidone and olanzapine.17
Evaluation and diagnostic work-up
A thorough medical history, review of systems, medication review, social history, and family history are important when evaluating a patient with chronic pruritus.18 These items can be valuable in formulating a differential diagnosis, even before a physical examination.
Physical examination. The physical exam should include detailed inspection of the entire skin and hair18; such a comprehensive physical exam can determine whether the source of the itch is cutaneous.7 This, in turn, can help further narrow the differential diagnosis. It is crucial that the physical exam include palpation of the liver, spleen, lymph nodes, and thyroid for organomegaly,8 which could indicate a serious systemic condition, such as lymphoma.
The ice-pack sign—in which an ice pack is applied to the pruritic area, the patient experiences immediate relief of pruritus, and the itch returns soon after the ice pack is removed—is considered pathognomonic for brachioradial pruritus.19
Continue to: Chronic pruritus with abnormal findings...
Chronic pruritus with abnormal findings on the physical exam should prompt an initial work-up.18 Also consider an initial work-up for a patient with chronic pruritus whose symptom has not been relieved with conservative treatment.18
Laboratory testing. The initial laboratory work-up could include any of the following evaluations: complete blood count, measurement of thyroid-stimulating hormone, comprehensive metabolic panel (liver function, renal function, and the serum glucose level) and the erythrocyte sedimentation rate (TABLE 2).18 If warranted by the evaluation and physical exam, blood work can also include serologic studies for human immunodeficiency virus infection and hepatitis.17

Imaging. Chest radiography should be performed if there is suspicion of malignancy, such as lymphoma.7 Although brachioradial pruritus and notalgia paresthetica have been postulated to be caused by impingement of spinal nerves, obtaining spinal imaging, such as magnetic resonance imaging, as part of the initial work-up is not recommended; because spinal images might not show evidence of spinal disease, obtaining spinal imaging is not a requirement before treating brachioradial pruritus and notalgia paresthetica. Do consider spinal imaging, however, for patients in whom brachioradial pruritus or notalgia paresthetica is suspected and conservative treatment has not produced a response.
Treatment: Nondrug approaches, topicals, systemic agents
Start conservatively. Treatment of pruritus should begin with behavior modification and nonpharmacotherapeutic options (TABLE 38). Educate the patient that scratching might cause secondary skin lesions; empowering them with that knowledge is sometimes enough to help break the scratching cycling—especially if the patient combines behavior modification with proper skin hydration with an emollient. To prevent secondary skin lesions through involuntary scratching, consider recommending that lesions be covered with an occlusive dressing or protective clothing.13

Stress has been shown to make chronic itch worse; therefore, stress-reduction activities, such as exercise, meditation, and yoga, might be helpful.20 For patients in whom pruritus has a psychological component, referral to a psychiatrist or psychologist might be therapeutic.
Continue to: When a patient complains...
When a patient complains of severe pruritus at first presentation, consider pharmacotherapy in conjunction with nonpharmacotherapeutic options. Several of the more effective topical therapies for pruritusa are listed in TABLE 4.20 Well-known systemic agents for this purpose are reviewed below and listed in TABLE 5.7

Systemic treatment
Antihistamines. A staple in the treatment of pruritus for many years, antihistamines are not effective for all causes; however, they are effective in treating paraneoplastic pruritus.20 First-generation antihistamines, with their sedating effect, can be useful for patients who experience generalized pruritus at night.20

Anticonvulsants. Gabapentin and pregabalin are analogs of the neurotransmitter gamma-aminobutyric acid.20 This drug class is helpful in neuropathic pruritus specifically caused by impingements, such as brachioradial pruritus and notalgia paresthetica.20 In addition, of all systemic therapies used to treat uremic pruritus, gabapentin has, in clinical trials, most consistently been found effective for uremic pruritus.6 (Note: Use renal dosing of gabapentin in patients with renal failure.)
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs; eg, fluvoxamine, paroxetine, and sertraline) might cause itch to subside by increasing the serotonin level, which, in turn, works to decrease inflammatory substances that cause itch.7 SSRIs have been used to treat patients with psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.7
Tricyclic antidepressants (eg, amitriptyline and doxepin) lessen the itch by antagonizing histamine receptors and through anticholinergic mechanisms. Tricyclics are best used in the treatment of psychogenic and nocturnal itch.7
Continue to: Mirtazapine...
Mirtazapine, a tetracyclic antidepressant, works in patients with uremic pruritus, psychogenic pruritus, cholestatic pruritus, and paraneoplastic pruritus.1
Substance P antagonist. Aprepitant, a neurokinin receptor I antagonist, is a newer agent that inhibits binding of the itch mediator substance P to the neurokinin receptor. The drug has been found helpful in patients with drug-induced, paraneoplastic, and brachioradial pruritus.7
Opioid-receptor agents. Naltrexone, as a mu opioid-receptor antagonist, has shown promise as a treatment for uremic pruritus and cholestatic pruritus. Nalfurafine, a kappa opioid-receptor agonist, is emerging as a possible therapy for uremic pruritus.7
Bile-acid sequestrants. A few small studies have shown that treatment with a bile-acid sequestrant, such as cholestyramine and ursodiol, induces moderate improvement in symptoms in patients with cholestatic pruritus.21
CORRESPONDENCE
Matasha Russell, MD, Department of Family and Community Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin Street, JJL 324, Houston, TX 77030; [email protected].
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
1. Tarikci N, E, S, et al. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015:803752.
2. Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625-1634.
3. Silverberg JI, Kantor RW, Dalal P. A comprehensive conceptual model of the experience of chronic itch in adults. Am J Clin Dermatol. 2018;19:759-769.
4. Matterne U, Apfelbacher CJ, Vogelgsang L, et al. Incidence and determinants of chronic pruritus: a population based cohort study. Acta Derm Venereol. 2013;93:532-537.
5. Moses S. Pruritus. Am Fam Physician. 2003;68:1135-1142.
6. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35:383-391.
7. Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
8. Reamy BV, Bunt C. A diagnostic approach to pruritus. Am Fam Physician. 2011;84:195-202.
9. M. Current concepts of pathophysiology, epidemiology and classification of pruritus. Srp Arh Celok Lek. 2014;142:106-112.
10. Fett N, Haynes K, Propert KJ, et al. Five-year malignancy incidence in patients with chronic pruritus: a population-based cohort study aimed at limiting unnecessary screening practices. J Am Acad Dermatol. 2014;70:651-658.
11. Larson VA, Tang O, S, et al. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80:931-937.
12. Rosen JD, Fostini AC, Chan YH, et al. Cross-sectional study of clinical distinctions between neuropathic and inflammatory pruritus. J Am Acad Dermatol. 2018;79:1143-1144.
13. Oaklander AL. Neuropathic itch. Semin Cutan Med Surg. 2011;30:87-92.
14. Ferm I, Sterner M, Wallengren J. Somatic and psychiatric comorbidity in patients with chronic pruritus. Acta Derm Venereol. 2010;90:395-400.
15. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. 2019;58:3-23.
16. Koo J, Lebwohl A. Psychodermatology: the mind and skin connection. Am Fam Physician. 2001;64:1873-1878.
17. Bewley AP, Lepping P, Freudenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol.2010;163:1-2.
18. Clerc C-J, Misery L. A literature review of senile pruritus: from diagnosis to treatment. Acta Derm Venereol. 2017;97:433-440.
19. Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
20. Sanders KM, Nattkemper LA, Yosipovitch G. Advances in understanding itching and scratching: a new era of targeted treatments [version 1]. F1000Res. 2016;5 F1000 Faculty Rev–2042.
21. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123.
PRACTICE RECOMMENDATIONS
› Undertake a diagnostic work-up for systemic causes of pruritus in patients who have a chronic, generalized itch and abnormal findings on physical examination. C
› Prescribe gabapentin for its effectiveness in treating pruritus caused by uremic and neurologic itch. B
› Consider prescribing one of the bile-acid sequestrants in patients with cholestatic pruritus because these agents can provide moderate relief of the symptom. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 4-year-old presented to our pediatric dermatology clinic for evaluation of asymptomatic "brown spots."
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
Capillary malformation-arteriovenous malformation syndrome
with or without arteriovenous malformations, as well as arteriovenous fistulas (AVFs). CM-AVM is an autosomal dominant disorder.1 CM-AVM type 1 is caused by mutations in the RASA1 gene, and CM-AVM type 2 is caused by mutations in the EPHB4 gene.2 Approximately 70% of patients with RASA1-associated CM-AVM syndrome and 80% of patients with EPHB4-associated CM-AVM syndrome have an affected parent, while the remainder have de novo variants.1
In patients with CM-AVM syndrome, CMs are often present at birth and more are typically acquired over time. CMs are characteristically 1-3 cm in diameter, round or oval, dull red or red-brown macules and patches with a blanched halo.3 Some CMs may be warm to touch indicating a possible underlying AVM or AVF.4 This can be confirmed by Doppler ultrasound, which would demonstrate increased arterial flow.4 CMs are most commonly located on the face and limbs and may present in isolation, but approximately one-third of patients have associated AVMs and AVFs.1,5 These high-flow vascular malformations may be present in skin, muscle, bone, brain, and/or spine and may be asymptomatic or lead to serious sequelae, including bleeding, congestive heart failure, and neurologic complications, such as migraine headaches, seizures, or even stroke.5 Symptoms from intracranial and spinal high-flow lesions usually present in early childhood and affect approximately 7% of patients.3
The diagnosis of CM-AVM should be suspected in an individual with numerous characteristic CMs and may be supported by the presence of AVMs and AVFs, family history of CM-AVM, and/or identification of RASA1 or EPHB4 mutation by molecular genetic testing.1,3 Although there are no consensus protocols for imaging CM-AVM patients, MRI of the brain and spine is recommended at diagnosis to identify underlying high-flow lesions.1 This may allow for early treatment before the development of symptoms.1 Any lesions identified on screening imaging may require regular surveillance, which is best determined by discussion with the radiologist.1 Although there are no reports of patients with negative results on screening imaging who later develop AVMs or AVFs, there should be a low threshold for repeat imaging in patients who develop new symptoms or physical exam findings.3,4
It has previously been suggested that the CMs in CM-AVM may actually represent early or small AVMs and pulsed-dye laser (PDL) treatment was not recommended because of concern for potential progression of lesions.4 However, a recent study demonstrated good response to PDL in patients with CM-AVM with no evidence of worsening or recurrence of lesions with long-term follow-up.6 Treatment of CMs that cause cosmetic concerns may be considered following discussion of risks and benefits with a dermatologist. Management of AVMs and AVFs requires a multidisciplinary team that, depending on location and symptoms of these features, may require the expertise of specialists such as neurosurgery, surgery, orthopedics, cardiology, and/or interventional radiology.1
Given the suspicion for CM-AVM in our patient, further workup was completed. A skin biopsy was consistent with CM. Genetic testing with the Vascular Malformations Panel, Sequencing and Deletion/Duplication revealed a pathogenic variant in the RASA1 gene and a variant of unknown clinical significance in the TEK gene. Parental genetic testing for the RASA1 mutation was negative, supporting a de novo mutation in the patient. CNS imaging showed a small developmental venous malformation in the brain that neurosurgery did not think was clinically significant. At the most recent follow-up at age 8 years, our patient had developed a few new small CMs but was otherwise well.
Dr. Leszczynska is trained in pediatrics and is the current dermatology research fellow at the University of Texas at Austin. Ms. Croce is a dermatology-trained pediatric nurse practitioner and PhD student at the University of Texas at Austin School of Nursing. Dr. Diaz is chief of pediatric dermatology at Dell Children’s Medical Center, Austin, assistant professor of pediatrics and medicine (dermatology), and dermatology residency associate program director at University of Texas at Austin . The authors have no relevant conflicts of interest to disclose. Donna Bilu Martin, MD, is the editor of this column.
References
1. Bayrak-Toydemir P, Stevenson D. Capillary Malformation-Arteriovenous Malformation Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; February 22, 2011.
2.Yu J et al. Pediatr Dermatol. 2017 Sep;34(5):e227-30.
3. Orme CM et al. Pediatr Dermatol. 2013 Jul-Aug;30(4):409-15.
4. Weitz NA et al. Pediatr Dermatol. 2015 Jan-Feb;32(1):76-84.
5. Revencu N et al. Hum Mutat. 2013 Dec;34(12):1632-41.
6. Iznardo H et al. Pediatr Dermatol. 2020 Mar;37(2):342-44.
RECIPE trial cooks up gout therapy improvement
Adding the immunomodulator mycophenolate mofetil (MMF) to therapy with pegloticase (Krystexxa) may improve outcomes in patients with refractory gout, results of the proof-of-concept RECIPE trial suggest.
In the phase 2 trial, 19 of 22 patients randomized to received pegloticase and MMF achieved the primary outcome of serum uric acid levels below 6 mg/dL at week 12, compared with 4 of 10 patients assigned to pegloticase and placebo, reported Puja Khanna MD, MPH, of the University of Michigan, Ann Arbor, and colleagues.
“The use of MMF was associated with statistically significant and clinically meaningful impact on the proportion of subjects who achieved and maintained a serum urate of less than 6 mg/dL. Short-term concomitant use of MMF with pegloticase was generally well tolerated, and the estimated rates of adverse events were comparable between the groups,” she said during the virtual annual meeting of the American College of Rheumatology.
Pegloticase is a pegylated recombinant form of porcine uricase that has been shown to be effective in the treatment of gout in patients for whom other therapies have failed.
The drug’s use is limited, however, by immunogenicity, with high antipegloticase antibody titers associated with a loss of response.
“The PEG portion of the molecule, the polyethylene glycol component, can initiate an immune response that would cause significant infusion reactions and preclude further use of the medication for our patients,” explained Suleman Bhana, MD, a rheumatologist with Crystal Run Healthcare in New York’s Hudson Valley, who was not involved in the study.
“By trying to attenuate that immune response by whatever means one can, that could reduce the risk of these infusion reactions and lead to longevity and continuing efficacy of the medication,” he said.
Study details
The RECIPE trial was designed to test whether concomitant immunomodulation could prolong the efficacy of pegloticase therapy by dampening immune reactions.
Investigators enrolled patients 18 years and older who met 2015 ACR/European League Against Rheumatism gout classification criteria and had chronic refractory disease, defined as having symptoms inadequately controlled with oral urate-lowering therapy or a contraindication to ULT.
A total of 42 patients from five rheumatology practices were screened, and 35 were randomized on a 3:1 basis. In the intention-to-treat analysis of the results, the investigators included 32 patients: 22 in the MMF/pegloticase group and 10 placebo-treated controls who had received at least one dose of pegloticase.
Men comprised approximately 90% of the patients in each study arm, with the mean patient age around 55 years. In both groups, patients had a median of one gout flare in the prior year, and a mean duration of gout of 13 years plus a few months.
The patients’ prior urate-lowering agents included allopurinol and febuxostat, and patients had received colchicine, NSAIDs, and corticosteroids for acute gout.
The mean serum urate levels at baseline were 8.9 mg/dL in the MMF group, and 9.8 mg/dL in the placebo group.
Patients were given either MMF 1 g twice daily or a placebo during a 2-week run-in, with the assigned medications continuing for the first 12 weeks concomitantly with pegloticase. The uricase was given intravenously at a dose of 8 mg every 2 weeks for a total of 12 infusions.
As noted before, 86% of patients in the MMF arm (19 of 22) reached the primary outcome of serum uric acid levels below 6 mg/dL by week 12, compared with 40% (4 of 10) in the placebo arm (P = .01).
Week 24 serum uric acid response, a secondary endpoint, was sustained in 68% of patients in the MMF arm, compared with 30% in the placebo arm (P = .03).
“We found no significant differences between the groups in the absolute change in serum urate from baseline to week 24, or from week 12 to 24. We also did not find any differences between the treatment arms for the PROMIS [Patient Reported Outcomes Measurement Information System] or for the Gout Impact Scale,” Dr. Khanna said.
The most commonly reported adverse events included gout flares in 13% of patients in the MMF group and 3% in the placebo group, cardiac disorders in 3% versus 2%, respectively, and gastrointestinal disorders in 9% versus 2%.
Adverse events that occurred only in the MMF group included infections (3%), musculoskeletal and connective tissue disorders (18%), and respiratory events.
Three patients in the placebo arm had infusion reactions, two of which occurred during the first infusion, and one during the second. One of the reactions was considered serious and required hospitalization, but all infusion reactions resolved and none were fatal. There were no infusion reactions in the MMF arm.
Maybe methotrexate instead?
“The efficacy data for mycophenolate in the RECIPE study are convincing, and suggest that this combination substantially increases the proportion of people who respond to pegloticase,” commented Nicola Dalbeth, MD, professor of medicine at the University of Auckland (New Zealand), who moderated the session where the RECIPE data were reported.
“Previous open-label studies of methotrexate with pegloticase [e.g., the MIRROR study] suggest that methotrexate is another effective option to increase the response to pegloticase. However, at this stage, placebo-controlled trials of methotrexate have not been reported. I think a key consideration will be safety, and which option [methotrexate vs. mycophenolate] is safer, noting that many patients with severe gout have important comorbidities, including chronic kidney disease, diabetes, and liver disease,” she said.
Dr. Bhana also noted that there are multiple factors that might determine the choice of MMF or methotrexate as an immunomodulatory partner for pegloticase.
“Some gout patients have chronic kidney disease or a variety of comorbidities – high uric acid can also cause kidney damage – and if they have a kidney illness, methotrexate may not be a safe medicine because there’s a risk of further toxicity that can lead to bone marrow suppression, which I have seen personally in patients, and in this case mycophenolate would be the preferred option,” he said.
The study was sponsored by the University of Alabama at Birmingham, with collaboration from the University of Michigan, as well as the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Horizon, which makes pegloticase. Dr. Khanna disclosed grant and research support from Dyve, Selecta, and Sobi, and consulting for Sobi and Horizon. Dr. Dalbeth disclosed relationships with AstraZeneca, AbbVie, Arthrosi, Dyve, Selecta, and Janssen. Dr. Bhana disclosed nonbranded consulting work for Horizon.
SOURCE: Khanna P et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 0952.
Adding the immunomodulator mycophenolate mofetil (MMF) to therapy with pegloticase (Krystexxa) may improve outcomes in patients with refractory gout, results of the proof-of-concept RECIPE trial suggest.
In the phase 2 trial, 19 of 22 patients randomized to received pegloticase and MMF achieved the primary outcome of serum uric acid levels below 6 mg/dL at week 12, compared with 4 of 10 patients assigned to pegloticase and placebo, reported Puja Khanna MD, MPH, of the University of Michigan, Ann Arbor, and colleagues.
“The use of MMF was associated with statistically significant and clinically meaningful impact on the proportion of subjects who achieved and maintained a serum urate of less than 6 mg/dL. Short-term concomitant use of MMF with pegloticase was generally well tolerated, and the estimated rates of adverse events were comparable between the groups,” she said during the virtual annual meeting of the American College of Rheumatology.
Pegloticase is a pegylated recombinant form of porcine uricase that has been shown to be effective in the treatment of gout in patients for whom other therapies have failed.
The drug’s use is limited, however, by immunogenicity, with high antipegloticase antibody titers associated with a loss of response.
“The PEG portion of the molecule, the polyethylene glycol component, can initiate an immune response that would cause significant infusion reactions and preclude further use of the medication for our patients,” explained Suleman Bhana, MD, a rheumatologist with Crystal Run Healthcare in New York’s Hudson Valley, who was not involved in the study.
“By trying to attenuate that immune response by whatever means one can, that could reduce the risk of these infusion reactions and lead to longevity and continuing efficacy of the medication,” he said.
Study details
The RECIPE trial was designed to test whether concomitant immunomodulation could prolong the efficacy of pegloticase therapy by dampening immune reactions.
Investigators enrolled patients 18 years and older who met 2015 ACR/European League Against Rheumatism gout classification criteria and had chronic refractory disease, defined as having symptoms inadequately controlled with oral urate-lowering therapy or a contraindication to ULT.
A total of 42 patients from five rheumatology practices were screened, and 35 were randomized on a 3:1 basis. In the intention-to-treat analysis of the results, the investigators included 32 patients: 22 in the MMF/pegloticase group and 10 placebo-treated controls who had received at least one dose of pegloticase.
Men comprised approximately 90% of the patients in each study arm, with the mean patient age around 55 years. In both groups, patients had a median of one gout flare in the prior year, and a mean duration of gout of 13 years plus a few months.
The patients’ prior urate-lowering agents included allopurinol and febuxostat, and patients had received colchicine, NSAIDs, and corticosteroids for acute gout.
The mean serum urate levels at baseline were 8.9 mg/dL in the MMF group, and 9.8 mg/dL in the placebo group.
Patients were given either MMF 1 g twice daily or a placebo during a 2-week run-in, with the assigned medications continuing for the first 12 weeks concomitantly with pegloticase. The uricase was given intravenously at a dose of 8 mg every 2 weeks for a total of 12 infusions.
As noted before, 86% of patients in the MMF arm (19 of 22) reached the primary outcome of serum uric acid levels below 6 mg/dL by week 12, compared with 40% (4 of 10) in the placebo arm (P = .01).
Week 24 serum uric acid response, a secondary endpoint, was sustained in 68% of patients in the MMF arm, compared with 30% in the placebo arm (P = .03).
“We found no significant differences between the groups in the absolute change in serum urate from baseline to week 24, or from week 12 to 24. We also did not find any differences between the treatment arms for the PROMIS [Patient Reported Outcomes Measurement Information System] or for the Gout Impact Scale,” Dr. Khanna said.
The most commonly reported adverse events included gout flares in 13% of patients in the MMF group and 3% in the placebo group, cardiac disorders in 3% versus 2%, respectively, and gastrointestinal disorders in 9% versus 2%.
Adverse events that occurred only in the MMF group included infections (3%), musculoskeletal and connective tissue disorders (18%), and respiratory events.
Three patients in the placebo arm had infusion reactions, two of which occurred during the first infusion, and one during the second. One of the reactions was considered serious and required hospitalization, but all infusion reactions resolved and none were fatal. There were no infusion reactions in the MMF arm.
Maybe methotrexate instead?
“The efficacy data for mycophenolate in the RECIPE study are convincing, and suggest that this combination substantially increases the proportion of people who respond to pegloticase,” commented Nicola Dalbeth, MD, professor of medicine at the University of Auckland (New Zealand), who moderated the session where the RECIPE data were reported.
“Previous open-label studies of methotrexate with pegloticase [e.g., the MIRROR study] suggest that methotrexate is another effective option to increase the response to pegloticase. However, at this stage, placebo-controlled trials of methotrexate have not been reported. I think a key consideration will be safety, and which option [methotrexate vs. mycophenolate] is safer, noting that many patients with severe gout have important comorbidities, including chronic kidney disease, diabetes, and liver disease,” she said.
Dr. Bhana also noted that there are multiple factors that might determine the choice of MMF or methotrexate as an immunomodulatory partner for pegloticase.
“Some gout patients have chronic kidney disease or a variety of comorbidities – high uric acid can also cause kidney damage – and if they have a kidney illness, methotrexate may not be a safe medicine because there’s a risk of further toxicity that can lead to bone marrow suppression, which I have seen personally in patients, and in this case mycophenolate would be the preferred option,” he said.
The study was sponsored by the University of Alabama at Birmingham, with collaboration from the University of Michigan, as well as the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Horizon, which makes pegloticase. Dr. Khanna disclosed grant and research support from Dyve, Selecta, and Sobi, and consulting for Sobi and Horizon. Dr. Dalbeth disclosed relationships with AstraZeneca, AbbVie, Arthrosi, Dyve, Selecta, and Janssen. Dr. Bhana disclosed nonbranded consulting work for Horizon.
SOURCE: Khanna P et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 0952.
Adding the immunomodulator mycophenolate mofetil (MMF) to therapy with pegloticase (Krystexxa) may improve outcomes in patients with refractory gout, results of the proof-of-concept RECIPE trial suggest.
In the phase 2 trial, 19 of 22 patients randomized to received pegloticase and MMF achieved the primary outcome of serum uric acid levels below 6 mg/dL at week 12, compared with 4 of 10 patients assigned to pegloticase and placebo, reported Puja Khanna MD, MPH, of the University of Michigan, Ann Arbor, and colleagues.
“The use of MMF was associated with statistically significant and clinically meaningful impact on the proportion of subjects who achieved and maintained a serum urate of less than 6 mg/dL. Short-term concomitant use of MMF with pegloticase was generally well tolerated, and the estimated rates of adverse events were comparable between the groups,” she said during the virtual annual meeting of the American College of Rheumatology.
Pegloticase is a pegylated recombinant form of porcine uricase that has been shown to be effective in the treatment of gout in patients for whom other therapies have failed.
The drug’s use is limited, however, by immunogenicity, with high antipegloticase antibody titers associated with a loss of response.
“The PEG portion of the molecule, the polyethylene glycol component, can initiate an immune response that would cause significant infusion reactions and preclude further use of the medication for our patients,” explained Suleman Bhana, MD, a rheumatologist with Crystal Run Healthcare in New York’s Hudson Valley, who was not involved in the study.
“By trying to attenuate that immune response by whatever means one can, that could reduce the risk of these infusion reactions and lead to longevity and continuing efficacy of the medication,” he said.
Study details
The RECIPE trial was designed to test whether concomitant immunomodulation could prolong the efficacy of pegloticase therapy by dampening immune reactions.
Investigators enrolled patients 18 years and older who met 2015 ACR/European League Against Rheumatism gout classification criteria and had chronic refractory disease, defined as having symptoms inadequately controlled with oral urate-lowering therapy or a contraindication to ULT.
A total of 42 patients from five rheumatology practices were screened, and 35 were randomized on a 3:1 basis. In the intention-to-treat analysis of the results, the investigators included 32 patients: 22 in the MMF/pegloticase group and 10 placebo-treated controls who had received at least one dose of pegloticase.
Men comprised approximately 90% of the patients in each study arm, with the mean patient age around 55 years. In both groups, patients had a median of one gout flare in the prior year, and a mean duration of gout of 13 years plus a few months.
The patients’ prior urate-lowering agents included allopurinol and febuxostat, and patients had received colchicine, NSAIDs, and corticosteroids for acute gout.
The mean serum urate levels at baseline were 8.9 mg/dL in the MMF group, and 9.8 mg/dL in the placebo group.
Patients were given either MMF 1 g twice daily or a placebo during a 2-week run-in, with the assigned medications continuing for the first 12 weeks concomitantly with pegloticase. The uricase was given intravenously at a dose of 8 mg every 2 weeks for a total of 12 infusions.
As noted before, 86% of patients in the MMF arm (19 of 22) reached the primary outcome of serum uric acid levels below 6 mg/dL by week 12, compared with 40% (4 of 10) in the placebo arm (P = .01).
Week 24 serum uric acid response, a secondary endpoint, was sustained in 68% of patients in the MMF arm, compared with 30% in the placebo arm (P = .03).
“We found no significant differences between the groups in the absolute change in serum urate from baseline to week 24, or from week 12 to 24. We also did not find any differences between the treatment arms for the PROMIS [Patient Reported Outcomes Measurement Information System] or for the Gout Impact Scale,” Dr. Khanna said.
The most commonly reported adverse events included gout flares in 13% of patients in the MMF group and 3% in the placebo group, cardiac disorders in 3% versus 2%, respectively, and gastrointestinal disorders in 9% versus 2%.
Adverse events that occurred only in the MMF group included infections (3%), musculoskeletal and connective tissue disorders (18%), and respiratory events.
Three patients in the placebo arm had infusion reactions, two of which occurred during the first infusion, and one during the second. One of the reactions was considered serious and required hospitalization, but all infusion reactions resolved and none were fatal. There were no infusion reactions in the MMF arm.
Maybe methotrexate instead?
“The efficacy data for mycophenolate in the RECIPE study are convincing, and suggest that this combination substantially increases the proportion of people who respond to pegloticase,” commented Nicola Dalbeth, MD, professor of medicine at the University of Auckland (New Zealand), who moderated the session where the RECIPE data were reported.
“Previous open-label studies of methotrexate with pegloticase [e.g., the MIRROR study] suggest that methotrexate is another effective option to increase the response to pegloticase. However, at this stage, placebo-controlled trials of methotrexate have not been reported. I think a key consideration will be safety, and which option [methotrexate vs. mycophenolate] is safer, noting that many patients with severe gout have important comorbidities, including chronic kidney disease, diabetes, and liver disease,” she said.
Dr. Bhana also noted that there are multiple factors that might determine the choice of MMF or methotrexate as an immunomodulatory partner for pegloticase.
“Some gout patients have chronic kidney disease or a variety of comorbidities – high uric acid can also cause kidney damage – and if they have a kidney illness, methotrexate may not be a safe medicine because there’s a risk of further toxicity that can lead to bone marrow suppression, which I have seen personally in patients, and in this case mycophenolate would be the preferred option,” he said.
The study was sponsored by the University of Alabama at Birmingham, with collaboration from the University of Michigan, as well as the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Horizon, which makes pegloticase. Dr. Khanna disclosed grant and research support from Dyve, Selecta, and Sobi, and consulting for Sobi and Horizon. Dr. Dalbeth disclosed relationships with AstraZeneca, AbbVie, Arthrosi, Dyve, Selecta, and Janssen. Dr. Bhana disclosed nonbranded consulting work for Horizon.
SOURCE: Khanna P et al. Arthritis Rheumatol. 2020;72(suppl 10), Abstract 0952.
FROM ACR 2020
A high proportion of SARS-CoV-2–infected university students are asymptomatic
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.
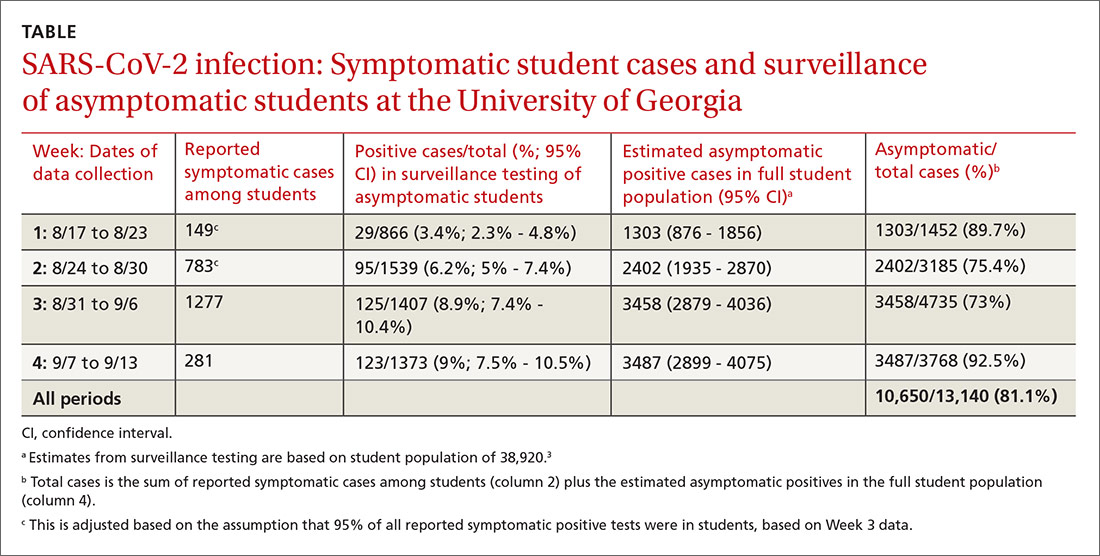
Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
[email protected]
The authors reported no potential conflict of interest relevant to this article.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.

Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
[email protected]
The authors reported no potential conflict of interest relevant to this article.
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.

Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
[email protected]
The authors reported no potential conflict of interest relevant to this article.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
Whales, seals, and dolphins: Will SARS-CoV-2–contaminated wastewater prove a killer?
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
FROM SCIENCE OF THE TOTAL ENVIRONMENT