User login
Study IDs microbial signature of celiac disease in children
Eleven operational taxonomic units (OTUs) of fecal bacteria were less abundant in children with celiac disease than in healthy children, according to the findings of a study published in Gastroenterology.
This microbial signature correctly identified approximately four out of five cases of celiac disease, regardless of whether children were newly diagnosed or had already modified their diet, reported Konstantina Zafeiropoulou and Ben Nichols, PhD, of the Glasgow Royal Infirmary. “It is not clear whether the microbes identified [in this study] contribute to the pathogenesis of celiac disease or are the result of it. Future research should explore the role of the disease-specific species identified here,” the researchers wrote in Gastroenterology.
Celiac disease is multifactorial. While up to 40% of people are genetically predisposed, only a small proportion develop it, suggesting that environmental factors are key to pathogenesis. Recent studies have linked celiac disease with alterations in the gut microbiome, but it is unclear whether dysbiosis is pathogenic or a secondary effect of disease processes such as nutrient malabsorption, or whether dysbiosis is present at disease onset or results from a gluten-free diet.
For the study, the researchers performed gas chromatography and 16S ribosomal RNA sequencing of fecal samples from 141 children, including 20 with newly biopsy-confirmed, previously untreated celiac disease, 45 who were previously diagnosed and on a gluten-free diet, 19 unaffected siblings, and 57 healthy children who were not on regular medications and had no history of chronic gastrointestinal symptoms. A single fecal sample was tested for all but the previously untreated children, who were tested at baseline and then after 6 and 12 months on a gluten-free diet.
Children with new-onset celiac disease showed no evidence of dysbiosis, while a gluten-free diet explained up to 2.8% of variation in microbiota between patients and controls. Microbial alpha diversity, a measure of species-level diversity, was generally similar among groups, but between 3% and 5% of all taxa differed. Irrespective of treatment, the decreased abundance of the 11 OTUs was diagnostic for celiac disease with an error rate of 21.5% (P < .001 vs. random classification). Notably, most of these 11 discrepant OTUs were associated with nutrient or food group intake and with biomarkers of gluten ingestion, the researchers said. Gas chromatography showed that, after patients started a gluten-free diet, fecal levels of butyrate and ammonia decreased.
“Even though we identified differences in the abundance of a few species between patients with untreated celiac disease and healthy controls, the profound microbial dysbiosis noted in Crohn’s disease was not observed, at least using crude diversity indices,” the investigators commented. “Although several alterations in the intestinal microbiota of children with established celiac disease appear to be effects of a gluten-free diet, there are specific bacteria that are distinct biomarkers of celiac disease.”
Future research might involve performing in vitro tests of “candidate” bacteria, coculturing these bacteria with human immune cells, and studying whether dietary interventions alter the relative abundance of these bacteria in the gut microbiome, the researchers said.
Nutricia Research Foundation, the Biotechnology and Biological Sciences Research Council, and The Catherine McEwan Foundation provided funding. Three coinvestigators disclosed ties to Nutricia, 4D Pharma, AbbVie, Celltrion, Janssen, Takeda, and several other pharmaceutical companies. One coinvestigator reported chairing the working group for ISLI Europe. The remaining investigators reported having no conflicts of interest.
SOURCE: Zafeiropoulou K et al. Gastroenterology. 2020 Aug 10;S0016-5085(20)35023-X. doi: 10.1053/j.gastro.2020.08.007.
It is well known that gluten ingestion in genetically susceptible individuals does not guarantee celiac disease, and research over the past decade has searched for environmental triggers. Gut microbiota play a role in activation of innate immunity, which leads to the adaptive immune response and the small bowel damage that is characteristic of celiac disease. The authors of this study sought to identify whether there is a distinct microbial pattern among celiac disease patients, both those with treated and untreated disease, in comparison with healthy controls and healthy siblings.
A significantly different microbial profile and metabolites were identified in subjects on gluten-free diets. The consequences of the gluten-free diet are an important consideration when committing a patient to this life-long therapy. The microbiome changes may play a role in persistent symptoms and the increased health conditions we see in treated celiac disease. Those on a gluten-free diet have other micronutrient deficiencies in addition to microbiome changes and the health sequelae of this are not fully understood. A gluten-free diet focused on restoring the normal gut flora through probiotic or gluten-free prebiotic or fiber supplementation in celiac disease patients could prove beneficial.
Dawn Wiese Adams, MD, MS, is assistant professor and medical director, Center for Human Nutrition, department of gastroenterology, hepatology, and nutrition, Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
It is well known that gluten ingestion in genetically susceptible individuals does not guarantee celiac disease, and research over the past decade has searched for environmental triggers. Gut microbiota play a role in activation of innate immunity, which leads to the adaptive immune response and the small bowel damage that is characteristic of celiac disease. The authors of this study sought to identify whether there is a distinct microbial pattern among celiac disease patients, both those with treated and untreated disease, in comparison with healthy controls and healthy siblings.
A significantly different microbial profile and metabolites were identified in subjects on gluten-free diets. The consequences of the gluten-free diet are an important consideration when committing a patient to this life-long therapy. The microbiome changes may play a role in persistent symptoms and the increased health conditions we see in treated celiac disease. Those on a gluten-free diet have other micronutrient deficiencies in addition to microbiome changes and the health sequelae of this are not fully understood. A gluten-free diet focused on restoring the normal gut flora through probiotic or gluten-free prebiotic or fiber supplementation in celiac disease patients could prove beneficial.
Dawn Wiese Adams, MD, MS, is assistant professor and medical director, Center for Human Nutrition, department of gastroenterology, hepatology, and nutrition, Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
It is well known that gluten ingestion in genetically susceptible individuals does not guarantee celiac disease, and research over the past decade has searched for environmental triggers. Gut microbiota play a role in activation of innate immunity, which leads to the adaptive immune response and the small bowel damage that is characteristic of celiac disease. The authors of this study sought to identify whether there is a distinct microbial pattern among celiac disease patients, both those with treated and untreated disease, in comparison with healthy controls and healthy siblings.
A significantly different microbial profile and metabolites were identified in subjects on gluten-free diets. The consequences of the gluten-free diet are an important consideration when committing a patient to this life-long therapy. The microbiome changes may play a role in persistent symptoms and the increased health conditions we see in treated celiac disease. Those on a gluten-free diet have other micronutrient deficiencies in addition to microbiome changes and the health sequelae of this are not fully understood. A gluten-free diet focused on restoring the normal gut flora through probiotic or gluten-free prebiotic or fiber supplementation in celiac disease patients could prove beneficial.
Dawn Wiese Adams, MD, MS, is assistant professor and medical director, Center for Human Nutrition, department of gastroenterology, hepatology, and nutrition, Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
Eleven operational taxonomic units (OTUs) of fecal bacteria were less abundant in children with celiac disease than in healthy children, according to the findings of a study published in Gastroenterology.
This microbial signature correctly identified approximately four out of five cases of celiac disease, regardless of whether children were newly diagnosed or had already modified their diet, reported Konstantina Zafeiropoulou and Ben Nichols, PhD, of the Glasgow Royal Infirmary. “It is not clear whether the microbes identified [in this study] contribute to the pathogenesis of celiac disease or are the result of it. Future research should explore the role of the disease-specific species identified here,” the researchers wrote in Gastroenterology.
Celiac disease is multifactorial. While up to 40% of people are genetically predisposed, only a small proportion develop it, suggesting that environmental factors are key to pathogenesis. Recent studies have linked celiac disease with alterations in the gut microbiome, but it is unclear whether dysbiosis is pathogenic or a secondary effect of disease processes such as nutrient malabsorption, or whether dysbiosis is present at disease onset or results from a gluten-free diet.
For the study, the researchers performed gas chromatography and 16S ribosomal RNA sequencing of fecal samples from 141 children, including 20 with newly biopsy-confirmed, previously untreated celiac disease, 45 who were previously diagnosed and on a gluten-free diet, 19 unaffected siblings, and 57 healthy children who were not on regular medications and had no history of chronic gastrointestinal symptoms. A single fecal sample was tested for all but the previously untreated children, who were tested at baseline and then after 6 and 12 months on a gluten-free diet.
Children with new-onset celiac disease showed no evidence of dysbiosis, while a gluten-free diet explained up to 2.8% of variation in microbiota between patients and controls. Microbial alpha diversity, a measure of species-level diversity, was generally similar among groups, but between 3% and 5% of all taxa differed. Irrespective of treatment, the decreased abundance of the 11 OTUs was diagnostic for celiac disease with an error rate of 21.5% (P < .001 vs. random classification). Notably, most of these 11 discrepant OTUs were associated with nutrient or food group intake and with biomarkers of gluten ingestion, the researchers said. Gas chromatography showed that, after patients started a gluten-free diet, fecal levels of butyrate and ammonia decreased.
“Even though we identified differences in the abundance of a few species between patients with untreated celiac disease and healthy controls, the profound microbial dysbiosis noted in Crohn’s disease was not observed, at least using crude diversity indices,” the investigators commented. “Although several alterations in the intestinal microbiota of children with established celiac disease appear to be effects of a gluten-free diet, there are specific bacteria that are distinct biomarkers of celiac disease.”
Future research might involve performing in vitro tests of “candidate” bacteria, coculturing these bacteria with human immune cells, and studying whether dietary interventions alter the relative abundance of these bacteria in the gut microbiome, the researchers said.
Nutricia Research Foundation, the Biotechnology and Biological Sciences Research Council, and The Catherine McEwan Foundation provided funding. Three coinvestigators disclosed ties to Nutricia, 4D Pharma, AbbVie, Celltrion, Janssen, Takeda, and several other pharmaceutical companies. One coinvestigator reported chairing the working group for ISLI Europe. The remaining investigators reported having no conflicts of interest.
SOURCE: Zafeiropoulou K et al. Gastroenterology. 2020 Aug 10;S0016-5085(20)35023-X. doi: 10.1053/j.gastro.2020.08.007.
Eleven operational taxonomic units (OTUs) of fecal bacteria were less abundant in children with celiac disease than in healthy children, according to the findings of a study published in Gastroenterology.
This microbial signature correctly identified approximately four out of five cases of celiac disease, regardless of whether children were newly diagnosed or had already modified their diet, reported Konstantina Zafeiropoulou and Ben Nichols, PhD, of the Glasgow Royal Infirmary. “It is not clear whether the microbes identified [in this study] contribute to the pathogenesis of celiac disease or are the result of it. Future research should explore the role of the disease-specific species identified here,” the researchers wrote in Gastroenterology.
Celiac disease is multifactorial. While up to 40% of people are genetically predisposed, only a small proportion develop it, suggesting that environmental factors are key to pathogenesis. Recent studies have linked celiac disease with alterations in the gut microbiome, but it is unclear whether dysbiosis is pathogenic or a secondary effect of disease processes such as nutrient malabsorption, or whether dysbiosis is present at disease onset or results from a gluten-free diet.
For the study, the researchers performed gas chromatography and 16S ribosomal RNA sequencing of fecal samples from 141 children, including 20 with newly biopsy-confirmed, previously untreated celiac disease, 45 who were previously diagnosed and on a gluten-free diet, 19 unaffected siblings, and 57 healthy children who were not on regular medications and had no history of chronic gastrointestinal symptoms. A single fecal sample was tested for all but the previously untreated children, who were tested at baseline and then after 6 and 12 months on a gluten-free diet.
Children with new-onset celiac disease showed no evidence of dysbiosis, while a gluten-free diet explained up to 2.8% of variation in microbiota between patients and controls. Microbial alpha diversity, a measure of species-level diversity, was generally similar among groups, but between 3% and 5% of all taxa differed. Irrespective of treatment, the decreased abundance of the 11 OTUs was diagnostic for celiac disease with an error rate of 21.5% (P < .001 vs. random classification). Notably, most of these 11 discrepant OTUs were associated with nutrient or food group intake and with biomarkers of gluten ingestion, the researchers said. Gas chromatography showed that, after patients started a gluten-free diet, fecal levels of butyrate and ammonia decreased.
“Even though we identified differences in the abundance of a few species between patients with untreated celiac disease and healthy controls, the profound microbial dysbiosis noted in Crohn’s disease was not observed, at least using crude diversity indices,” the investigators commented. “Although several alterations in the intestinal microbiota of children with established celiac disease appear to be effects of a gluten-free diet, there are specific bacteria that are distinct biomarkers of celiac disease.”
Future research might involve performing in vitro tests of “candidate” bacteria, coculturing these bacteria with human immune cells, and studying whether dietary interventions alter the relative abundance of these bacteria in the gut microbiome, the researchers said.
Nutricia Research Foundation, the Biotechnology and Biological Sciences Research Council, and The Catherine McEwan Foundation provided funding. Three coinvestigators disclosed ties to Nutricia, 4D Pharma, AbbVie, Celltrion, Janssen, Takeda, and several other pharmaceutical companies. One coinvestigator reported chairing the working group for ISLI Europe. The remaining investigators reported having no conflicts of interest.
SOURCE: Zafeiropoulou K et al. Gastroenterology. 2020 Aug 10;S0016-5085(20)35023-X. doi: 10.1053/j.gastro.2020.08.007.
FROM GASTROENTEROLOGY
Key clinical point: A novel microbial signature distinguished children with celiac disease from healthy controls.
Major finding: Eleven operational taxonomic units (OTUs) were less abundant in fecal samples from children with treated and untreated celiac disease than in healthy controls. The microbial signature was diagnostic for celiac disease with an error rate of 21.5% (P < .001 compared with random classification).
Study details: Gas chromatography and 16S ribosomal RNA sequencing of fecal samples from 141 children: 20 with new-onset celiac disease, 45 with an established diagnosis who were on a gluten-free diet, 19 unaffected siblings, and 57 healthy children. Also, a prospective study of fecal samples from 13 newly diagnosed children after 6 and 12 months on a gluten-free diet.
Disclosures: Nutricia Research Foundation, the Biotechnology and Biological Sciences Research Council, and The Catherine McEwan Foundation provided funding. Three coinvestigators disclosed ties to Nutricia, 4D Pharma, Abbvie, Janssen, Takeda, Celltrion, and several other pharmaceutical companies. One coinvestigator reported chairing the working group for ISLI Europe. The remaining investigators reported having no conflicts of interest.
Source: Zafeiropoulou K et al. Gastroenterology. 2020 Aug 10;S0016-5085(20)35023-X. doi: 10.1053/j.gastro.2020.08.007.
Great Barrington coauthor backs off strict reliance on herd immunity
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
What imaging can disclose about suspected stroke and its Tx
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
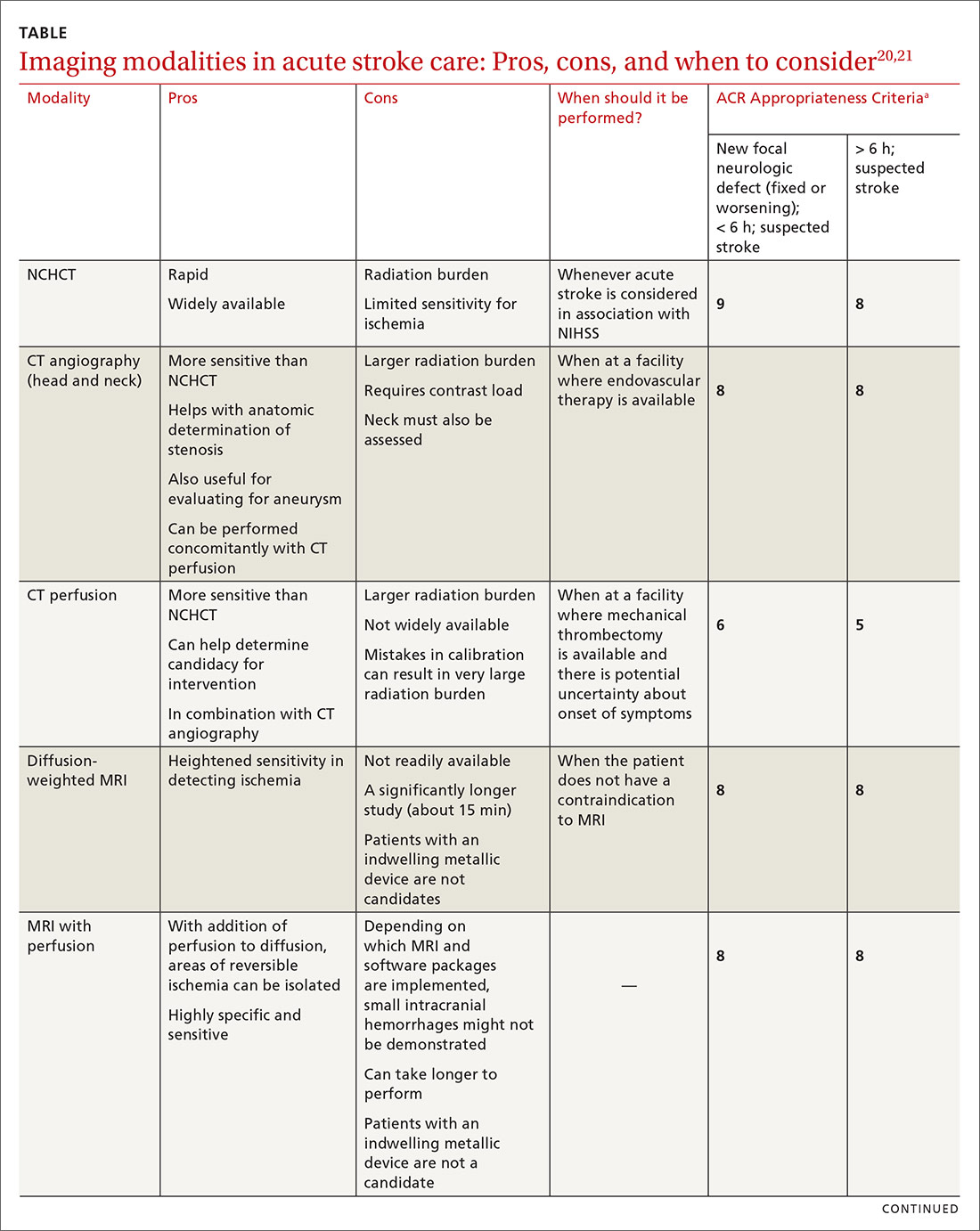
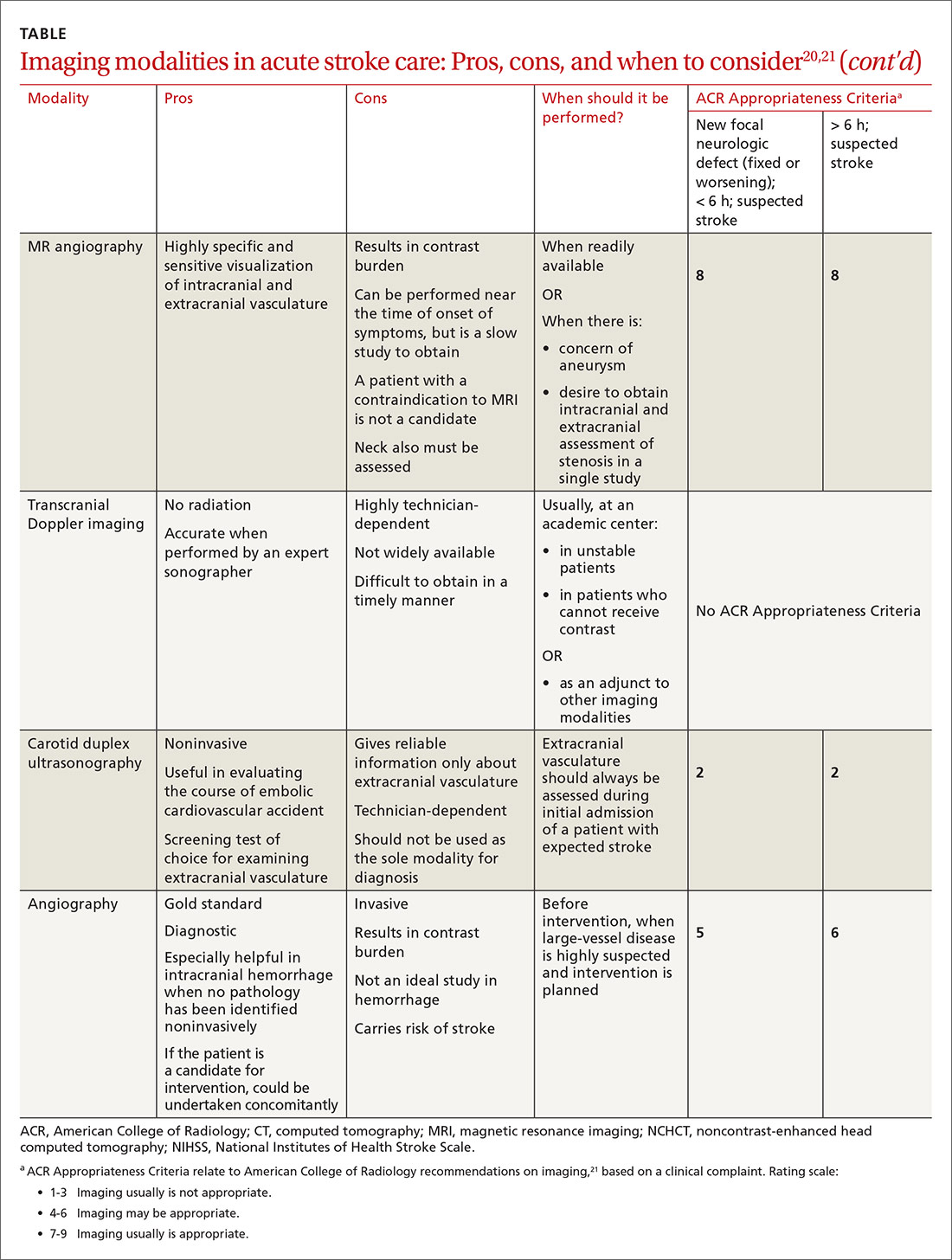
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3
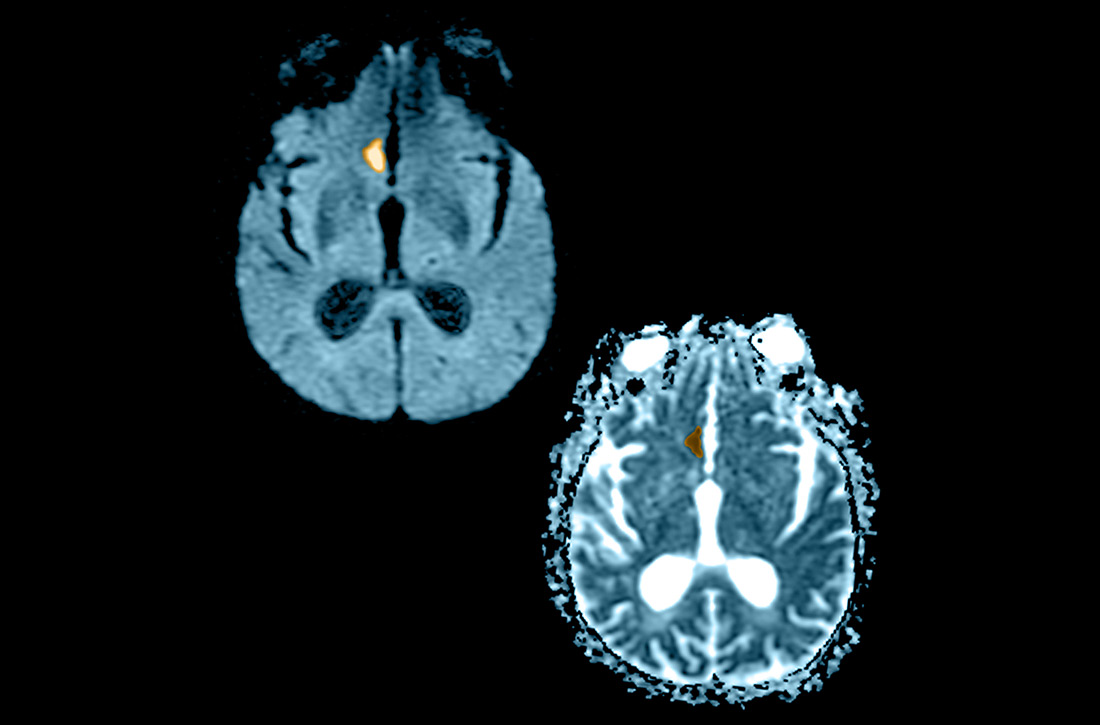
NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25
Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3

NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25

Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
Stroke ranks second behind heart disease as the leading cause of mortality worldwide, accounting for 1 of every 19 deaths,1 and remains a serious cause of morbidity. Best practices in stroke diagnosis and management can seem elusive to front-line clinicians, for 2 reasons: the rate of proliferation and nuance in stroke medicine and the fact that the typical scope of primary care practice exists apart from much of the diagnostic tools and management schema provided in stroke centers.2 In this article, we describe and update the diagnosis of stroke and review imaging modalities, their nuances, and their application in practice.
Diagnosis of acute stroke
Acute stroke is diagnosed upon observation of new neurologic deficits and congruent neuroimaging. Some updated definitions favor a silent form of cerebral ischemia manifested by imaging pathology only; this form is not discussed in this article. Although there are several characteristically distinct stroke syndromes, there is no way to clinically distinguish ischemic pathology from hemorrhagic pathology.
Some common symptoms that should prompt evaluation for stroke are part of the American Stroke Association FAST mnemonic designed to promote public health awareness3-5:
f ace droopinga rm weaknesss peech difficultyt ime to call 911.
Other commonly reported stroke symptoms include unilateral weakness or numbness, confusion, word-finding difficulty, visual problems, difficulty ambulating, dizziness, loss of balance or coordination, and thunderclap headache. A stroke should also be considered in the presence of any new focal neurologic deficit.3,4
Stroke patients should be triaged by emergency medical services using a stroke screening scale, such as BE-FAST5 (a modification of FAST that adds balance and eye assessments); the Los Angeles Prehospital Stroke Screen (LAPSS)6,7; the Rapid Arterial oCclusion Evaluation (RACE)8; and the Cincinnati Prehospital Stroke Severity Scale (CP-SSS)9,10 (see “Stroke screening scales for early identification and triage"). Studies have not found that any single prehospital stroke scale is superior to the others for reliably predicting large-vessel occlusion; therefore, prehospital assessment is typically based on practice patterns in a given locale.11 A patient (or family member or caregiver) who seeks your care for stroke symptoms should be told to call 911 and get emergency transport to a health care facility that can capably administer intravenous (IV) thrombolysis.a
SIDEBAR
Stroke screening scales for early identification and triage
National Institutes of Health Stroke Scale
www.stroke.nih.gov/resources/scale.htm
FAST
www.stroke.org/en/help-and-support/resource-library/fast-materials
BE-FAST
www.ahajournals.org/doi/10.1161/STROKEAHA.116.015169
Los Angeles Prehospital Stroke Screen (LAPSS)
http://stroke.ucla.edu/workfiles/prehospital-screen.pdf
Rapid Artery Occlusion Evaluation (RACE)
www.mdcalc.com/rapid-arterial-occlusion-evaluation-race-scale-stroke
Cincinnati Prehospital Stroke Severity Scale (CP-SSS)
https://www.mdcalc.com/cincinnati-prehospital-stroke-severity-scale-cp-sss
First responders should elicit “last-known-normal” time; this critical information can aid in diagnosis and drive therapeutic options, especially if patients are unaccompanied at time of transport to a higher echelon of care. A point-of-care blood glucose test should be performed by emergency medical staff, with dextrose administered for a level < 45 mg/dL. Establishing IV access for fluids, medications, and contrast can be considered if it does not delay transport. A 12-lead electrocardiogram can also be considered, again, as long as it does not delay transport to a facility capable of providing definitive therapy. Notification by emergency services staff before arrival and transport of the patient to such a facility is the essential element of prehospital care, and should be prioritized above ancillary testing beyond the stroke assessment.14
Guidelines recommend use of the National Institutes of Health Stroke Scale (NIHSS; www.stroke.nih.gov/resources/scale.htm) for clinical evaluation upon arrival at the ED.15 Although no scale has been identified that can reliably predict large-vessel occlusion amenable to endovascular therapy (EVT), no other score has been found to outperform the NIHSS in achieving meaningful patient outcomes.16 Furthermore, NIHSS has been validated to track clinical changes in response to therapy, is widely utilized, and is free.
Continue to: A criticism of the NIHSS...
A criticism of the NIHSS is its bias toward left-hemispheric ischemic pathology.17 NIHSS includes 11 questions on a scale of 0 to 42; typically, a score < 4 is associated with a higher chance of a positive clinical outcome.18 There is no minimum or maximum NIHSS score that precludes treatment with thrombolysis or EVT.
Other commonly used scores in acute stroke include disability assessments. The modified Rankin scale, which is used most often, features a score of 0 (symptom-free) to 6 (death). A modified Rankin scale score of 0 or 1 is considered an indication of a favorable outcome after stroke.19 Note that these functional scores are not always part of an acute assessment but can be done early in the clinical course to gauge the response to treatment, and are collected for stroke-center certification.
Imaging modalities
Imaging is recommended within 20 minutes of arrival in the ED in a stroke patient who might be a candidate for thrombolysis or thrombectomy.3 There, imaging modalities commonly performed are noncontrast-enhanced head computed tomography (NCHCT); computed tomography (CT) angiography, with or without perfusion; and diffusion-weighted magnetic resonance imaging (MRI).20,21 In addition, more highly specialized imaging modalities are available for the evaluation of the stroke patient in specific, often limited, circumstances. All these modalities are described below and compared in the TABLE,20,21 using the ACR Appropriateness Criteria (of the American College of Radiology),21 which are guidelines for appropriate imaging of stroke, based on a clinical complaint. Separate recommendations and appraisals are offered by the most recent American Heart Association/American Stroke Association (AHA/ASA) guideline.3

NCHCT. This study should be performed within 20 minutes after arrival at the ED because it provides rapid assessment of intracerebral hemorrhage, can effectively corroborate the diagnosis of some stroke mimickers, and identifies some candidates for EVT or thrombolysis3,21,22 (typically, the decision to proceed with EVT is based on adjunct imaging studies discussed in a bit). Evaluation for intracerebral hemorrhage is required prior to administering thrombolysis. Ischemic changes can be seen with variable specificity and sensitivity on NCHCT, depending on how much time has passed since the original insult. In all historical trials, CT was the only imaging modality used in the diagnosis of acute ischemic stroke (AIS) that suggested benefit from IV thrombolysis.23-25

Acute, subacute, and chronic changes can be seen on NCHCT, although the modality has limited sensitivity for identifying AIS (ie, approximately 75% within 6 hours after the original insult):
- Acute findings on NCHCT include intracellular edema, which causes loss of the gray matter–white matter interface and effacement of the cortical sulci. This occurs as a result of increased cellular uptake of water in response to ischemia and cell death, resulting in a decreased density of tissue (hypoattenuation) in affected areas.
- Subacute changes appear in the 2- to 5-day window, including vasogenic edema with greater mass effect, hypoattenuation, and well-defined margins.3,20,21
- Chronic vascular findings on NCHCT include loss of brain tissue and hypoattenuation.
Continue to: NCHCT is typically performed...
NCHCT is typically performed in advance of other adjunct imaging modalities.3,20,21 Baseline NCHCT can be performed on patients with advanced kidney disease and those who have an indwelling metallic device.
CT angiography is performed with timed contrast, providing a 3-dimensional representation of the cerebral vasculature; the entire intracranial and extracranial vasculature, including the aortic arch, can be mapped in approximately 60 seconds. CT angiography is sensitive in identifying areas of stenosis > 50% and identifies clinically significant areas of stenosis up to approximately 90% of the time.26 For this reason, it is particularly helpful in identifying candidates for an interventional strategy beyond pharmacotherapeutic thrombolysis. In addition, CT angiography can visualize aneurysmal dilation and dissection, and help with the planning of interventions—specifically, the confident administration of thrombolysis or more specific planning for target lesions and EVT.
It also can help identify a host of vascular phenomena, such as arteriovenous malformations, Moyamoya disease (progressive arterial blockage within the basal ganglia and compensatory microvascularization), and some vasculopathies.20,27 In intracranial hemorrhage, CT with angiography can help evaluate for structural malformations and identify patients at risk of hematoma expansion.22
CT perfusion. Many stroke centers will perform a CT perfusion study,28 which encompasses as many as 3 different CT sequences:
- NCHCT
- vertex-to-arch angiography with contrast bolus
- administration of contrast and capture of a dynamic sequence through 1 or 2 slabs of tissue, allowing for the generation of maps of cerebral blood flow (CBF), mean transit time (MTT), and cerebral blood volume (CBV) of the entire cerebral vasculature.
The interplay of these 3 sequences drives characterization of lesions (ie, CBF = CBV/MTT). An infarct is characterized by low CBF, low CBV, and elevated MTT. In penumbral tissue, MTT is elevated but CBF is slightly decreased and CBV is normal or increased. Using CT perfusion, areas throughout the ischemic penumbra can be surveyed for favorable interventional characteristics.20,29
Continue to: A CT perfusion study adds...
A CT perfusion study adds at least 60 seconds to NCHCT. This modality can be useful in planning interventions and for stratifying appropriateness of reperfusion strategies in strokes of unknown duration.3,30 CT perfusion can be performed on any multidetector CT scan but (1) requires specialized software and expertise to interpret and (2) subjects the patient to a significant radiation dose, which, if incorrectly administered, can be considerably higher than intended.20,26,27
Diffusion-weighted MRI. This is the most sensitive study for demonstrating early ischemic changes; however, limitations include lack of availability, contraindication in patients with metallic indwelling implants, and duration of the study—although, at some stroke centers, diffusion-weighted MRI can be performed in ≤ 10 minutes.
MRI and NCHCT have comparable sensitivity in detecting intracranial hemorrhage. MRI is likely more sensitive in identifying areas of microhemorrhage: In diffusion-weighted MRI, the sensitivity of stroke detection increases to > 95%.31 The modality relies on the comparable movement of water through damaged vs normal neuronal tissue. Diffusion-weighted MRI does not require administration of concomitant contrast, which can be a benefit in patients who are allergic to gadolinium-based contrast agents or have advanced kidney disease that precludes the use of contrast. It typically does not result in adequate characterization of extracranial vasculature.
Other MRI modalities. These MRI extensions include magnetic resonance (MR) perfusion and MR angiography. Whereas diffusion-weighted MRI (discussed above) offers the most rapid and sensitive evaluation for ischemia, fluid-attenuated inversion recovery (FLAIR) imaging has been utilized as a comparator to isolated diffusion-weighted MRI to help determine stroke duration. FLAIR signal positivity typically occurs 6 to 24 hours after the initial insult but is negative in stroke that occurred < 3 hours earlier.32
MRI is limited, in terms of availability and increased study duration, especially when it comes to timely administration of thrombolysis. A benefit of this modality is less radiation and, as noted, superior sensitivity for ischemia. Diffusion-weighted MRI combined with MR perfusion analysis can help isolate areas of the ischemic penumbra. MR perfusion is performed for a similar reason as CT perfusion, although logistical execution across those modalities is significantly different. Considerations for choosing MR perfusion or CT perfusion should be made on an individual basis and based on available local resources and accepted local practice patterns.26
Continue to: In the subacute setting...
In the subacute setting, MR perfusion and MR angiography of the head and the neck are often performed to identify stenosis, dissection, and more subtle mimickers of cerebrovascular accident not ascertained on initial CT evaluation. These studies are typically performed well outside the window for thrombolysis or intervention.26 No guidelines specifically direct or recommend this practice pattern. The superior sensitivity and cerebral blood flow mapping of MR perfusion and MR angiography might be useful for validating a suspected diagnosis of ischemic stroke and providing phenotypic information about AIS events.
Transcranial Doppler imaging relies on bony windows to assess intracranial vascular flow, velocity, direction, and reactivity. This information can be utilized to diagnose stenosis or occlusion. This modality is principally used to evaluate for stenosis in the anterior circulation (sensitivity, 70%-90%; specificity, 90%-95%).20 Evaluation of the basilar, vertebral, and internal carotid arteries is less accurate (sensitivity, 55%-80%).20 Transcranial Doppler imaging is also used to assess for cerebral vasospasm after subarachnoid hemorrhage, monitor sickle cell disease patients’ overall risk for ischemic stroke, and augment thrombolysis. It is limited by the availability of an expert technician, and therefore is typically reserved for unstable patients or those who cannot receive contrast.20
Carotid duplex ultrasonography. A dynamic study such as duplex ultrasonography can be strongly considered for flow imaging of the extracranial carotids to evaluate for stenosis. Indications for carotid stenting or endarterectomy include 50% to 79% occlusion of the carotid artery on the same side as a recent transient ischemic attack or AIS. Carotid stenosis > 80% warrants consideration for intervention independent of a recent cerebrovascular accident. Interventions are typically performed 2 to 14 days after stroke.33 Although this study is of limited utility in the hyperacute setting, it is recommended within 24 hours after nondisabling stroke in the carotid territory, when (1) the patient is otherwise a candidate for a surgical or procedural intervention to address the stenosis and (2) none of the aforementioned studies that focus on neck vasculature have been performed.
Conventional (digital subtraction) angiography is the gold standard for mapping cerebrovascular disease because it is dynamic and highly accurate. It is, however, typically limited by the number of required personnel, its invasive nature, and the requirement for IV contrast. This study is performed during intra-arterial intervention techniques, including stent retrieval and intra-arterial thrombolysis.26
Impact of imaging on treatment
Imaging helps determine the cause and some characteristics of stroke, both of which can help determine therapy. Strokes can be broadly subcategorized as hemorrhagic or ischemic; recent studies suggest that 87% are ischemic.34 Knowledge of the historic details of the event, the patient (eg, known atrial fibrillation, anticoagulant use, history of falls), and findings on imaging can contribute to determine the cause of AIS, and can facilitate communication and consultation between the primary care physician and inpatient teams.35
Continue to: Best practices for stroke treatment...
Best practices for stroke treatment are based on the cause of the event.3 To identify the likely cause, the aforementioned characteristics are incorporated into one of the scoring systems, which seek to clarify either the cause or the phenotypic appearance of the AIS, which helps direct further testing and treatment. (The ASCOD36 and TOAST37 classification schemes are commonly used phenotypic and causative classifications, respectively.) Several (not all) of the broad phenotypic imaging patterns, with myriad clinical manifestations, are reviewed below. They include:
- Embolic stroke, which, classically, involves end circulation and therefore has cortical involvement. Typically, these originate from the heart or large extracranial arteries, and higher rates of atrial fibrillation and hypercoagulable states are implicated.
- Thrombotic stroke, which, typically, is from large vessels or small vessels, and occurs as a result of atherosclerosis. These strokes are more common at the origins or bifurcations of vessels. Symptoms of thrombotic stroke classically wax and wane slightly more frequently. Lacunar strokes are typically from thrombotic causes, although there are rare episodes of an embolic source contributing to a lacunar stroke syndrome.38
There is evidence for using MRI discrepancies between diffusion-weighted and FLAIR imaging to time AIS findings in so-called wake-up strokes.39 The rationale is that strokes < 4.5 hours old can be identified because they would have abnormal diffusion imaging components but normal findings with FLAIR. When these criteria were utilized in considering whether to treat with thrombolysis, there was a statistically significant improvement in 90-day modified Rankin scale (odds ratio = 1.61; 95% confidence interval, 1.09-2.36), but also an increased probability of death and intracerebral hemorrhage.39
A recent multicenter, randomized, open-label trial, with blinded outcomes assessment, showcased the efficacy of thrombectomy as an adjunct when ischemic brain territory was identified without frank infarction, as ascertained by CT perfusion within the anterior circulation. This trial showed that thrombectomy could be performed as long as 16 hours after the patient was last well-appearing and still result in an improved outcome with favorable imaging characteristics (on the modified Rankin scale, an ordinal score of 4 with medical therapy and an ordinal score of 3 with EVT [odds ratio = 2.77; 95% confidence interval, 1.63-4.70]).29 A 2018 multicenter, prospective, randomized trial with blinded assessment of endpoints extended this idea, demonstrating that, when there was mismatch of the clinical deficit (ie, high NIHSS score) and infarct volume (measured on diffusion-weighted MRI or CT perfusion), thrombectomy as late as 24 hours after the patient was last known to be well was beneficial for lesions in the anterior circulation—specifically, the intracranial internal carotid artery or the proximal middle cerebral artery.40
a Whether local emergency departments (EDs) should be bypassed in favor of a specialized stroke center is the subject of debate. The 2019 American Heart Association/American Stroke Association guidelines note the AHA’s Mission: Lifeline Stroke EMS algorithm, which bypasses the nearest ED in feared cases of large-vessel occlusion if travel to a comprehensive stroke center can be accomplished within 30 minutes of arrival at the scene. This is based on expert consensus.3,12,13
CORRESPONDENCE
Brian Ford, MD, 4301 Jones Bridge Road, Bethesda, MD; [email protected].
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67-e492.
2. Darves B. Collaboration key to post-stroke follow-up. ACP Internist. October 2009. https://acpinternist.org/archives/2009/10/stroke.htm. Accessed September 22, 2020.
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50e344-e418.
4. Sacco RL, Kasner SE, Broderick JP, et al; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089.
5. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance, Eyes, Face, Arm, Speech, Time): Reducing the proportion of strokes missed using the FAST mnemonic. 2017;48:479-481.
6. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke. 2000;31:71-76.
7. Llanes JN, Kidwell CS, Starkman S, et al. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46-50.
8. Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45:87-91.
9. Katz BS, McMullan JT, Sucharew H, et al. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;466:1508-1512.
10. Kummer BR, et al. External validation of the Cincinnati Prehospital Stroke Severity Scale. J Stroke Cerebrovasc Dis. 2016;25:1270-1274.
11. Beume L-A, Hieber M, Kaller CP, et al. Large vessel occlusion in acute stroke. Stroke. 2018;49:2323-2329.
12. Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004512.
13. American Heart Association (Mission: Lifeline—Stroke). Emergency medical services acute stroke routing. 2020. www.heart.org/-/media/files/professional/quality-improvement/mission-lifeline/2_25_2020/ds15698-qi-ems-algorithm_update-2142020.pdf?la=en. Accessed October 8, 2020.
14. Glober NK, Sporer KA, Guluma KZ, et al. Acute stroke: current evidence-based recommendations for prehospital care. West J Emerg Med. 2016;17:104-128.
15. NIH stroke scale. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health. www.stroke.nih.gov/resources/scale.htm. Accessed October 10, 2020.
16. Smith EE, Kent DM, Bulsara KR, et al; . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e111-e122.
17. Woo D, Broderick JP, Kothari RU, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA Stroke Study Group. Stroke. 1999;30:2355-2359.
18. Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126-131.
19. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091-1096.
20. Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67-76.
21. Salmela MB, Mortazavi S, Jagadeesan BD, et al. ACR Appropriateness Criteria® Cerebrovascular Disease. J Am Coll Radiol. 2017;14:S34-S61.
22. Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-60.
23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
24. The Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 1995;333:1581-1587.
25. Albers GW, Clark WM, Madden KP, et al. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493-495.
26. Khan R, Nael K, Erly W. Acute stroke imaging: what clinicians need to know. Am J Med. 2013;126:379-386.
27. Latchaw RE, Alberts MJ, Lev MH, et al; . Recommendations for managing of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646-3678.
28. Vagal A, Meganathan K, Kleindorfer DO, et al. Increasing use of computed tomographic perfusion and computed tomographic angiograms in acute ischemic stroke from 2006 to 2010. Stroke. 2014;45:1029-1034.
29. Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
30. Demeestere J, Wouters A, Christensen S, et al. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51:1017-1024.
31. Chalela JA, Kidwell CS, Nentwich LM, et al, Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293-298.
32. Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39-44.
33. Wabnitz AM, Turan TN. Symptomatic carotid artery stenosis: surgery, stenting, or medical therapy? Curr Treat Options Cardiovasc Med. 2017;19:62.
34. Muir KW, Santosh C. Imaging of acute stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii19-iii28.
35. Cameron JI, Tsoi C, Marsella A.Optimizing stroke systems of care by enhancing transitions across care environments. Stroke. 2008;39:2637-2643.
36. Amarenco P, Bogousslavsky J, Caplan LR, et al. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1-5.
37. Adams HP Jr, Bendixen BH, Kappelle LJ. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41.
38. Cacciatore A, Russo LS Jr. Lacunar infarction as an embolic complication of cardiac and arch angiography. Stroke. 1991;22:1603-1605.
39. Thomalla G, Simonsen CZ, Boutitie F, et al; WAKE-UP Investigators. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611-622.
40. Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11-21.
Hospitals poised to launch first COVID-19 vaccines in clinicians
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
United States adds nearly 74,000 more children with COVID-19
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.
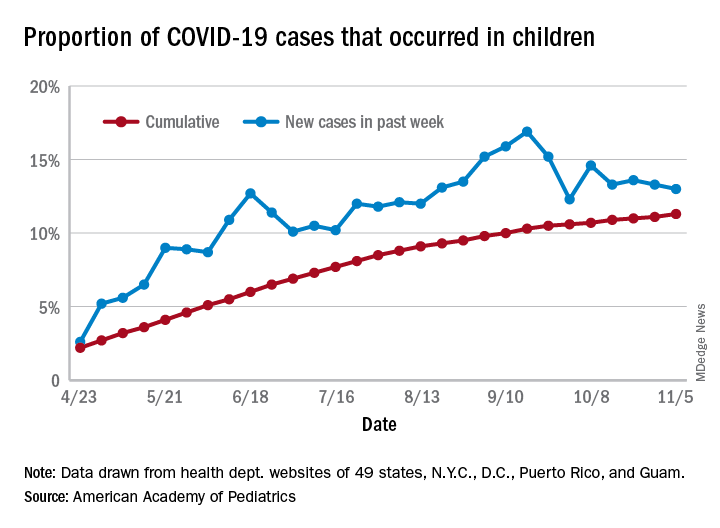
The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
Food insecurity called urgent issue you must address
and advocate on behalf of those experiencing or at risk of food insecurity, according to Kofi Essel, MD, MPH, a pediatrician at Children’s National Hospital in Washington.
More than one in four adults are dealing with food access hardships during the pandemic, Dr. Essel said at the virtual annual meeting of the American Academy of Pediatrics. Food insecurity is often interchangeable with hunger and refers to limited or uncertain availability of foods that are nutritious and safe.
“Food insecurity is as much about the threat of deprivation as it is about deprivation itself: A food-insecure life means a life lived in fear of hunger, and the psychological toll that takes,” according to a 2020 New York Times photo feature on food insecurity by Brenda Ann Kenneally that Dr. Essel quoted.
The lived experience of food insecure households includes food anxiety, a preoccupation with being able to get enough food that takes up cognitive bandwidth and prevents people from being able to focus on other important things. Another feature of food-insecure homes is a monotony of diet, which often involves an increase in caloric density and decrease in nutritional quality. As food insecurity grows more dire, adults’ food intake decreases, and then children’s intake decreases as adults seek out any way to get food, including “socially unacceptable” ways, which can include food pantries and bartering for food.
Food insecurity is associated with a wide range of negative outcomes even after accounting for other confounders, including decreased overall health, mental health, and educational outcomes. It’s also associated with an increase in developmental delays, hospitalizations, iron deficiency, asthma, and birth defects, among other problems. Somewhat paradoxically, it’s associated with both an increase and a decrease in obesity in the research.
Megan J. Gray, MD, MPH, assistant professor of pediatrics and population health at Dell Medical School at the The University of Texas at Austin, attended Dr. Essel’s session because food insecurity during COVID-19 now affects about half her patients, according to screening research she’s conducted.
“I wanted to learn more about the nuances of screening and using language and talking points that are helpful with families and with staff in building a culture of discussing food insecurity in our clinics,” Dr. Gray said in an interview. “What I’ve learned in my clinic is that if we don’t ask about it, families aren’t telling us – food insecurity is hiding in plain sight.”
She particularly appreciated Dr. Essel’s slides on the progression of food insecurity and how they acknowledged the mental health burden of food insecurity among parents.
“Right now during COVID-19, I see more patients I would call ‘socially complex’ rather than ‘medically complex,’ ” she said. “We all need to get a crash course in social work and Dr. Essel’s presentation is a great starting place.”
Screening for food insecurity
Beginning in 2015, an AAP policy statement charged pediatricians to “screen and intervene” with regard to food insecurity and their patients, Dr. Essel said. The statement also called for pediatricians to advocate for programs and policies that end childhood food insecurity.
The policy statement recommended a validated two-question screening tool called the Hunger Vital Sign:
1. “Within the past 12 months, we worried whether our food would run out before we got money to buy more.”
2. “Within the past 12 months, the food that we bought just didn’t last and we didn’t have money to get more.”
But in screening, you need to be conscious of how dignity intersects with food insecurity concerns, Dr. Essel said.
“We need to create dignity for our families,” he said. “We need to create a safe environment for our families and use appropriate tools when necessary to be able to identify families that are struggling with food insecurity.”
That need is seen in research on food screening. The Hunger Vital Signs questions can be asked with a dichotomous variable, as a yes/no question, or on a Likert scale, though the latter is a more complex way to ask.
A 2017 study found, however, that asking with “yes/no” answers missed more than a quarter of at-risk families. In the AAP survey using “yes/no” answers, 31% of families screened positive for being at risk of food insecurity, compared with 46% when the same question was asked on a Likert scale. It seems the ability to answer with “sometimes” feels “safer” than answering “yes,” Dr. Essel said.
Another factor that potentially affects answers is how doctors ask. In a March 2020 study at a single primary care practice, 16% of families screened positive with yes/no responses to a food insecurity screen when the questions were written, compared with 10% of positive screens with verbal responses (P < .001).
Epidemiology of food insecurity
The most updated United States Department of Agriculture report on food insecurity released in September shows the United States finally reached prerecession levels in 2019, with 11% of families designated as “food insecure.” But 2019 data cannot show what has occurred since the pandemic.
Further, the numbers are higher in households with children: Fourteen percent, or one in seven households with children, are experiencing food insecurity. Racial and ethnic disparities in food insecurity have remained consistent over the past 2 decades, with about twice as many Black and Hispanic homes experiencing food insecurity as White homes.
More recent research using Census Household Pulse Surveys has found a tremendous increase in food insecurity for children in 2020. One in three Black children and one in four Hispanic children are food insecure, according to these surveys. The rates are one in six for Asian households and one in ten for White households.
“The disparity is consistent,” Dr. Essel said. “We see what COVID has done. We once may have described it as a great equalizer – everyone is touched in the same way – but the reality is, this is actually a great magnifier. It’s revealing to us and magnifying disparities that have existed for far too long and has really allowed us to see it in a new way.”
A big part of disparities in food insecurity is disparities in wealth, “the safety net or cushion for families when things go wrong,” Dr. Essel said. The median wealth of White Americans in 2016 was $171,000, compared to $20,700 among Latinx Americans and $17,600 among Black Americans, according to the Federal Reserve Board Survey of Consumer Finances.
Food insecurity interventions
Federal nutrition programs – such as Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and school meal programs – are key to addressing food insecurity, Dr. Essel said.
“They have a long track record of rescuing families out of poverty, of rescuing families from food security and improving overall health of families,” he said.
But emergency food relief programs are important as well. Four in 10 families currently coming into food pantries are new recipients, and these resources have seen a 60% increase in clients, he said.
“This is utterly unreasonable for them to be able to manage,” he said. “Food pantries are essential but inadequate to compensate for large numbers of families,” even while they also may be the only option for families unable or unwilling to access federal programs. For example, for every one meal that food banks can provide, SNAP can provide nine meals, Dr. Essel said. Further, during times of economic downtown, every SNAP $1 spent generates $1.50 to $2 in economic activity.
Currently, the Pandemic Electronic Benefit Transfer (P-EBT) program provides benefits to families for school breakfast and lunch and has been extended through December 2021. Another federal pandemic response was to increase SNAP to the maximum household benefit for families, about $646 for a family of four, although 40% of households were already receiving the maximum benefit.
Food insecurity advocacy
You can advocate for any one of multiple pillars when it comes to food insecurity, Dr. Essel said. “Food cannot solve food insecurity by itself,” he said. “We have to think about root causes – systemic causes – and think about unemployment, livable wage, systemic racism, oppression, an inequitable food system. All of these things are pillars that any of you can advocate for when recognizing a family that is struggling with food insecurity.”
He offered several suggestions for advocacy:
- Join your local AAP chapter and prioritize food insecurity.
- Join a local antihunger task force.
- Make your clinical environment as safe as possible for families to respond to questions about food insecurity.
- Know what’s happening in your community immigrant populations.
- Provide up-to-date information to families about eligibility for federal programs.
- Share stories through op-eds and letters to the editor, and by contacting congressional representatives and providing expert testimony to school boards and city councils.
- Educate others about food insecurity through the above channels and on social media.
Jessica Lazerov, MD, a general pediatrician at Children’s National Anacostia and assistant professor of pediatrics at George Washington University, Washington, said the session was fantastic.
“Dr. Essel went beyond the basics of food insecurity, delving into the root causes, potential solutions, and important considerations when screening for food insecurity in practice,” Dr. Lazerov said in an interview. “I enjoyed his focus on advocacy, as well as the fact that he spent a bit of time reviewing how the COVID pandemic has affected food insecurity. I truly felt empowered to take my advocacy efforts a step further as Dr. Essel laid out concrete, actionable next steps, as well as a review of the most relevant and current information about food insecurity.”
Dr. Essel, Dr. Lazerov, and Dr. Gray have no relevant financial disclosures.
and advocate on behalf of those experiencing or at risk of food insecurity, according to Kofi Essel, MD, MPH, a pediatrician at Children’s National Hospital in Washington.
More than one in four adults are dealing with food access hardships during the pandemic, Dr. Essel said at the virtual annual meeting of the American Academy of Pediatrics. Food insecurity is often interchangeable with hunger and refers to limited or uncertain availability of foods that are nutritious and safe.
“Food insecurity is as much about the threat of deprivation as it is about deprivation itself: A food-insecure life means a life lived in fear of hunger, and the psychological toll that takes,” according to a 2020 New York Times photo feature on food insecurity by Brenda Ann Kenneally that Dr. Essel quoted.
The lived experience of food insecure households includes food anxiety, a preoccupation with being able to get enough food that takes up cognitive bandwidth and prevents people from being able to focus on other important things. Another feature of food-insecure homes is a monotony of diet, which often involves an increase in caloric density and decrease in nutritional quality. As food insecurity grows more dire, adults’ food intake decreases, and then children’s intake decreases as adults seek out any way to get food, including “socially unacceptable” ways, which can include food pantries and bartering for food.
Food insecurity is associated with a wide range of negative outcomes even after accounting for other confounders, including decreased overall health, mental health, and educational outcomes. It’s also associated with an increase in developmental delays, hospitalizations, iron deficiency, asthma, and birth defects, among other problems. Somewhat paradoxically, it’s associated with both an increase and a decrease in obesity in the research.
Megan J. Gray, MD, MPH, assistant professor of pediatrics and population health at Dell Medical School at the The University of Texas at Austin, attended Dr. Essel’s session because food insecurity during COVID-19 now affects about half her patients, according to screening research she’s conducted.
“I wanted to learn more about the nuances of screening and using language and talking points that are helpful with families and with staff in building a culture of discussing food insecurity in our clinics,” Dr. Gray said in an interview. “What I’ve learned in my clinic is that if we don’t ask about it, families aren’t telling us – food insecurity is hiding in plain sight.”
She particularly appreciated Dr. Essel’s slides on the progression of food insecurity and how they acknowledged the mental health burden of food insecurity among parents.
“Right now during COVID-19, I see more patients I would call ‘socially complex’ rather than ‘medically complex,’ ” she said. “We all need to get a crash course in social work and Dr. Essel’s presentation is a great starting place.”
Screening for food insecurity
Beginning in 2015, an AAP policy statement charged pediatricians to “screen and intervene” with regard to food insecurity and their patients, Dr. Essel said. The statement also called for pediatricians to advocate for programs and policies that end childhood food insecurity.
The policy statement recommended a validated two-question screening tool called the Hunger Vital Sign:
1. “Within the past 12 months, we worried whether our food would run out before we got money to buy more.”
2. “Within the past 12 months, the food that we bought just didn’t last and we didn’t have money to get more.”
But in screening, you need to be conscious of how dignity intersects with food insecurity concerns, Dr. Essel said.
“We need to create dignity for our families,” he said. “We need to create a safe environment for our families and use appropriate tools when necessary to be able to identify families that are struggling with food insecurity.”
That need is seen in research on food screening. The Hunger Vital Signs questions can be asked with a dichotomous variable, as a yes/no question, or on a Likert scale, though the latter is a more complex way to ask.
A 2017 study found, however, that asking with “yes/no” answers missed more than a quarter of at-risk families. In the AAP survey using “yes/no” answers, 31% of families screened positive for being at risk of food insecurity, compared with 46% when the same question was asked on a Likert scale. It seems the ability to answer with “sometimes” feels “safer” than answering “yes,” Dr. Essel said.
Another factor that potentially affects answers is how doctors ask. In a March 2020 study at a single primary care practice, 16% of families screened positive with yes/no responses to a food insecurity screen when the questions were written, compared with 10% of positive screens with verbal responses (P < .001).
Epidemiology of food insecurity
The most updated United States Department of Agriculture report on food insecurity released in September shows the United States finally reached prerecession levels in 2019, with 11% of families designated as “food insecure.” But 2019 data cannot show what has occurred since the pandemic.
Further, the numbers are higher in households with children: Fourteen percent, or one in seven households with children, are experiencing food insecurity. Racial and ethnic disparities in food insecurity have remained consistent over the past 2 decades, with about twice as many Black and Hispanic homes experiencing food insecurity as White homes.
More recent research using Census Household Pulse Surveys has found a tremendous increase in food insecurity for children in 2020. One in three Black children and one in four Hispanic children are food insecure, according to these surveys. The rates are one in six for Asian households and one in ten for White households.
“The disparity is consistent,” Dr. Essel said. “We see what COVID has done. We once may have described it as a great equalizer – everyone is touched in the same way – but the reality is, this is actually a great magnifier. It’s revealing to us and magnifying disparities that have existed for far too long and has really allowed us to see it in a new way.”
A big part of disparities in food insecurity is disparities in wealth, “the safety net or cushion for families when things go wrong,” Dr. Essel said. The median wealth of White Americans in 2016 was $171,000, compared to $20,700 among Latinx Americans and $17,600 among Black Americans, according to the Federal Reserve Board Survey of Consumer Finances.
Food insecurity interventions
Federal nutrition programs – such as Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and school meal programs – are key to addressing food insecurity, Dr. Essel said.
“They have a long track record of rescuing families out of poverty, of rescuing families from food security and improving overall health of families,” he said.
But emergency food relief programs are important as well. Four in 10 families currently coming into food pantries are new recipients, and these resources have seen a 60% increase in clients, he said.
“This is utterly unreasonable for them to be able to manage,” he said. “Food pantries are essential but inadequate to compensate for large numbers of families,” even while they also may be the only option for families unable or unwilling to access federal programs. For example, for every one meal that food banks can provide, SNAP can provide nine meals, Dr. Essel said. Further, during times of economic downtown, every SNAP $1 spent generates $1.50 to $2 in economic activity.
Currently, the Pandemic Electronic Benefit Transfer (P-EBT) program provides benefits to families for school breakfast and lunch and has been extended through December 2021. Another federal pandemic response was to increase SNAP to the maximum household benefit for families, about $646 for a family of four, although 40% of households were already receiving the maximum benefit.
Food insecurity advocacy
You can advocate for any one of multiple pillars when it comes to food insecurity, Dr. Essel said. “Food cannot solve food insecurity by itself,” he said. “We have to think about root causes – systemic causes – and think about unemployment, livable wage, systemic racism, oppression, an inequitable food system. All of these things are pillars that any of you can advocate for when recognizing a family that is struggling with food insecurity.”
He offered several suggestions for advocacy:
- Join your local AAP chapter and prioritize food insecurity.
- Join a local antihunger task force.
- Make your clinical environment as safe as possible for families to respond to questions about food insecurity.
- Know what’s happening in your community immigrant populations.
- Provide up-to-date information to families about eligibility for federal programs.
- Share stories through op-eds and letters to the editor, and by contacting congressional representatives and providing expert testimony to school boards and city councils.
- Educate others about food insecurity through the above channels and on social media.
Jessica Lazerov, MD, a general pediatrician at Children’s National Anacostia and assistant professor of pediatrics at George Washington University, Washington, said the session was fantastic.
“Dr. Essel went beyond the basics of food insecurity, delving into the root causes, potential solutions, and important considerations when screening for food insecurity in practice,” Dr. Lazerov said in an interview. “I enjoyed his focus on advocacy, as well as the fact that he spent a bit of time reviewing how the COVID pandemic has affected food insecurity. I truly felt empowered to take my advocacy efforts a step further as Dr. Essel laid out concrete, actionable next steps, as well as a review of the most relevant and current information about food insecurity.”
Dr. Essel, Dr. Lazerov, and Dr. Gray have no relevant financial disclosures.
and advocate on behalf of those experiencing or at risk of food insecurity, according to Kofi Essel, MD, MPH, a pediatrician at Children’s National Hospital in Washington.
More than one in four adults are dealing with food access hardships during the pandemic, Dr. Essel said at the virtual annual meeting of the American Academy of Pediatrics. Food insecurity is often interchangeable with hunger and refers to limited or uncertain availability of foods that are nutritious and safe.
“Food insecurity is as much about the threat of deprivation as it is about deprivation itself: A food-insecure life means a life lived in fear of hunger, and the psychological toll that takes,” according to a 2020 New York Times photo feature on food insecurity by Brenda Ann Kenneally that Dr. Essel quoted.
The lived experience of food insecure households includes food anxiety, a preoccupation with being able to get enough food that takes up cognitive bandwidth and prevents people from being able to focus on other important things. Another feature of food-insecure homes is a monotony of diet, which often involves an increase in caloric density and decrease in nutritional quality. As food insecurity grows more dire, adults’ food intake decreases, and then children’s intake decreases as adults seek out any way to get food, including “socially unacceptable” ways, which can include food pantries and bartering for food.
Food insecurity is associated with a wide range of negative outcomes even after accounting for other confounders, including decreased overall health, mental health, and educational outcomes. It’s also associated with an increase in developmental delays, hospitalizations, iron deficiency, asthma, and birth defects, among other problems. Somewhat paradoxically, it’s associated with both an increase and a decrease in obesity in the research.
Megan J. Gray, MD, MPH, assistant professor of pediatrics and population health at Dell Medical School at the The University of Texas at Austin, attended Dr. Essel’s session because food insecurity during COVID-19 now affects about half her patients, according to screening research she’s conducted.
“I wanted to learn more about the nuances of screening and using language and talking points that are helpful with families and with staff in building a culture of discussing food insecurity in our clinics,” Dr. Gray said in an interview. “What I’ve learned in my clinic is that if we don’t ask about it, families aren’t telling us – food insecurity is hiding in plain sight.”
She particularly appreciated Dr. Essel’s slides on the progression of food insecurity and how they acknowledged the mental health burden of food insecurity among parents.
“Right now during COVID-19, I see more patients I would call ‘socially complex’ rather than ‘medically complex,’ ” she said. “We all need to get a crash course in social work and Dr. Essel’s presentation is a great starting place.”
Screening for food insecurity
Beginning in 2015, an AAP policy statement charged pediatricians to “screen and intervene” with regard to food insecurity and their patients, Dr. Essel said. The statement also called for pediatricians to advocate for programs and policies that end childhood food insecurity.
The policy statement recommended a validated two-question screening tool called the Hunger Vital Sign:
1. “Within the past 12 months, we worried whether our food would run out before we got money to buy more.”
2. “Within the past 12 months, the food that we bought just didn’t last and we didn’t have money to get more.”
But in screening, you need to be conscious of how dignity intersects with food insecurity concerns, Dr. Essel said.
“We need to create dignity for our families,” he said. “We need to create a safe environment for our families and use appropriate tools when necessary to be able to identify families that are struggling with food insecurity.”
That need is seen in research on food screening. The Hunger Vital Signs questions can be asked with a dichotomous variable, as a yes/no question, or on a Likert scale, though the latter is a more complex way to ask.
A 2017 study found, however, that asking with “yes/no” answers missed more than a quarter of at-risk families. In the AAP survey using “yes/no” answers, 31% of families screened positive for being at risk of food insecurity, compared with 46% when the same question was asked on a Likert scale. It seems the ability to answer with “sometimes” feels “safer” than answering “yes,” Dr. Essel said.
Another factor that potentially affects answers is how doctors ask. In a March 2020 study at a single primary care practice, 16% of families screened positive with yes/no responses to a food insecurity screen when the questions were written, compared with 10% of positive screens with verbal responses (P < .001).
Epidemiology of food insecurity
The most updated United States Department of Agriculture report on food insecurity released in September shows the United States finally reached prerecession levels in 2019, with 11% of families designated as “food insecure.” But 2019 data cannot show what has occurred since the pandemic.
Further, the numbers are higher in households with children: Fourteen percent, or one in seven households with children, are experiencing food insecurity. Racial and ethnic disparities in food insecurity have remained consistent over the past 2 decades, with about twice as many Black and Hispanic homes experiencing food insecurity as White homes.
More recent research using Census Household Pulse Surveys has found a tremendous increase in food insecurity for children in 2020. One in three Black children and one in four Hispanic children are food insecure, according to these surveys. The rates are one in six for Asian households and one in ten for White households.
“The disparity is consistent,” Dr. Essel said. “We see what COVID has done. We once may have described it as a great equalizer – everyone is touched in the same way – but the reality is, this is actually a great magnifier. It’s revealing to us and magnifying disparities that have existed for far too long and has really allowed us to see it in a new way.”
A big part of disparities in food insecurity is disparities in wealth, “the safety net or cushion for families when things go wrong,” Dr. Essel said. The median wealth of White Americans in 2016 was $171,000, compared to $20,700 among Latinx Americans and $17,600 among Black Americans, according to the Federal Reserve Board Survey of Consumer Finances.
Food insecurity interventions
Federal nutrition programs – such as Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and school meal programs – are key to addressing food insecurity, Dr. Essel said.
“They have a long track record of rescuing families out of poverty, of rescuing families from food security and improving overall health of families,” he said.
But emergency food relief programs are important as well. Four in 10 families currently coming into food pantries are new recipients, and these resources have seen a 60% increase in clients, he said.
“This is utterly unreasonable for them to be able to manage,” he said. “Food pantries are essential but inadequate to compensate for large numbers of families,” even while they also may be the only option for families unable or unwilling to access federal programs. For example, for every one meal that food banks can provide, SNAP can provide nine meals, Dr. Essel said. Further, during times of economic downtown, every SNAP $1 spent generates $1.50 to $2 in economic activity.
Currently, the Pandemic Electronic Benefit Transfer (P-EBT) program provides benefits to families for school breakfast and lunch and has been extended through December 2021. Another federal pandemic response was to increase SNAP to the maximum household benefit for families, about $646 for a family of four, although 40% of households were already receiving the maximum benefit.
Food insecurity advocacy
You can advocate for any one of multiple pillars when it comes to food insecurity, Dr. Essel said. “Food cannot solve food insecurity by itself,” he said. “We have to think about root causes – systemic causes – and think about unemployment, livable wage, systemic racism, oppression, an inequitable food system. All of these things are pillars that any of you can advocate for when recognizing a family that is struggling with food insecurity.”
He offered several suggestions for advocacy:
- Join your local AAP chapter and prioritize food insecurity.
- Join a local antihunger task force.
- Make your clinical environment as safe as possible for families to respond to questions about food insecurity.
- Know what’s happening in your community immigrant populations.
- Provide up-to-date information to families about eligibility for federal programs.
- Share stories through op-eds and letters to the editor, and by contacting congressional representatives and providing expert testimony to school boards and city councils.
- Educate others about food insecurity through the above channels and on social media.
Jessica Lazerov, MD, a general pediatrician at Children’s National Anacostia and assistant professor of pediatrics at George Washington University, Washington, said the session was fantastic.
“Dr. Essel went beyond the basics of food insecurity, delving into the root causes, potential solutions, and important considerations when screening for food insecurity in practice,” Dr. Lazerov said in an interview. “I enjoyed his focus on advocacy, as well as the fact that he spent a bit of time reviewing how the COVID pandemic has affected food insecurity. I truly felt empowered to take my advocacy efforts a step further as Dr. Essel laid out concrete, actionable next steps, as well as a review of the most relevant and current information about food insecurity.”
Dr. Essel, Dr. Lazerov, and Dr. Gray have no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 2020
Proinflammatory dietary pattern linked to higher CV risk
Dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease (CVD) and stroke in a new pooled analysis of three prospective cohort studies.
The analysis included 210,145 U.S. women and men followed for up to 32 years in the Nurses’ Health Studies I and II and the Health Professionals Follow-up Study.
After adjustment for use of anti-inflammatory medications and CVD risk factors, those whose dietary pattern ranked in the highest quintile of inflammatory potential had a 38% higher risk of CVD (hazard ratio comparing highest with lowest quintiles, 1.38), a 46% higher risk of coronary heart disease (HR, 1.46), and a 28% higher risk of stroke (HR, 1.28) (all P for trend < .001).
Jun Li, MD, PhD, and colleagues at Harvard School of Public Health and Harvard Medical School, Boston, published the findings of their study in the Nov. 10 issue of the Journal of the American College of Cardiology.
The inflammatory potential of a diet was assessed using a food-based, dietary index called the “empirical dietary inflammatory pattern” or EDIP.
In an interview, Dr. Li explained that the EDIP was developed 4 years ago by many of the same authors involved with this study, including nutrition heavyweights Walter C. Willett, MD, DrPH, and Frank B. Hu, MD, PhD, both from Harvard.
“We summarized all the foods people eat into 39 defined food groups and did a reduced-rank regression analysis that looked at these 39 food groups and three inflammatory markers – interleukin-6, C-reactive protein, and tumor necrosis factor–alpha receptor 2. We found 18 food groups that are most predictive of these biomarkers, and the EDIP was calculated as the weighted sum of these 18 food groups.”
Individuals who had higher intakes of green-leafy vegetables (kale, spinach, arugula), dark-yellow vegetables (pumpkin, yellow peppers, carrots), whole grains, fruits, tea, coffee and wine had lower long-term CVD risk than those with higher intakes of red meat, processed meat, organ meat, refined carbohydrates, and sweetened beverages.
The associations were consistent across cohorts and between sexes and remained significant in multiple sensitivity analysis that adjusted for alcohol consumption, smoking pack-years, use of lipid-lowering and antihypertensive medications, sodium intake, and blood pressure.
In a secondary analysis, diets with higher inflammatory potential were also associated with significantly higher biomarker levels indicative of more systemic, vascular, and metabolic inflammation, as well as less favorable lipid profiles.
“We wanted to be able to provide guidance on dietary patterns and food combinations,” said Dr. Li. “If you tell people to eat more polyunsaturated fats instead of saturated fat or trans fat, most people don’t know what foods are higher and lower in those nutrients. Also, many foods have different nutrients – some of which are good and some of which are bad – so we wanted to help people find the foods with the higher proportion of healthy nutrients rather than point out specific nutrients to avoid.”
Researchers used prospectively gathered data from the Nurses’ Health Studies I and II starting from 1984 and from the Health Professionals Follow-up Study. After excluding participants with missing diet information or previously diagnosed heart disease, stroke or cancer, over 210,000 participants were included in the analysis. Participants completed a survey every 4 years to ascertain dietary intake.
Prevention, not treatment
In an editorial comment, Ramon Estruch, MD, PhD, from the Hospital Clinic in Barcelona, and colleagues suggested that it might be time for better dietary guidelines.
“A better knowledge of health protection provided by different foods and dietary patterns, mainly their anti-inflammatory properties, should provide the basis for designing even healthier dietary patterns to protect against heart disease,” the editorialists wrote.
They added extra-virgin olive oil, fatty fish, and tomatoes to the list of foods with “established anti-inflammatory activity.”
In a comment, Dr. Estruch said the findings of this new study are confirmatory of the PREDIMED trial, which showed a reduction in risk of major CV events in individuals at high cardiovascular risk assigned to an anti-inflammatory Mediterranean diet pattern supplemented with extra-virgin olive oil or nuts as compared with those assigned to a reduced-fat diet.
“The study of Jun Li et al. confirms that an anti-inflammatory diet is useful to prevent cardiovascular events and, more important, that healthy dietary patterns may be even healthier if subjects increase consumption of foods with the highest anti-inflammatory potential,” he said, adding that “mechanistic explanations add plausibility to the results of observational studies.”
Dr. Estruch was the principal investigator of PREDIMED. This trial was originally published in 2013 and then retracted and republished in 2018, with some required corrections, but the results had not materially changed.
Dr. Li is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Boston Nutrition Obesity Research Center. Dr. Estruch disclosed no financial relationships relevant to the contents of this article.
A version of this article originally appeared on Medscape.com.
Dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease (CVD) and stroke in a new pooled analysis of three prospective cohort studies.
The analysis included 210,145 U.S. women and men followed for up to 32 years in the Nurses’ Health Studies I and II and the Health Professionals Follow-up Study.
After adjustment for use of anti-inflammatory medications and CVD risk factors, those whose dietary pattern ranked in the highest quintile of inflammatory potential had a 38% higher risk of CVD (hazard ratio comparing highest with lowest quintiles, 1.38), a 46% higher risk of coronary heart disease (HR, 1.46), and a 28% higher risk of stroke (HR, 1.28) (all P for trend < .001).
Jun Li, MD, PhD, and colleagues at Harvard School of Public Health and Harvard Medical School, Boston, published the findings of their study in the Nov. 10 issue of the Journal of the American College of Cardiology.
The inflammatory potential of a diet was assessed using a food-based, dietary index called the “empirical dietary inflammatory pattern” or EDIP.
In an interview, Dr. Li explained that the EDIP was developed 4 years ago by many of the same authors involved with this study, including nutrition heavyweights Walter C. Willett, MD, DrPH, and Frank B. Hu, MD, PhD, both from Harvard.
“We summarized all the foods people eat into 39 defined food groups and did a reduced-rank regression analysis that looked at these 39 food groups and three inflammatory markers – interleukin-6, C-reactive protein, and tumor necrosis factor–alpha receptor 2. We found 18 food groups that are most predictive of these biomarkers, and the EDIP was calculated as the weighted sum of these 18 food groups.”
Individuals who had higher intakes of green-leafy vegetables (kale, spinach, arugula), dark-yellow vegetables (pumpkin, yellow peppers, carrots), whole grains, fruits, tea, coffee and wine had lower long-term CVD risk than those with higher intakes of red meat, processed meat, organ meat, refined carbohydrates, and sweetened beverages.
The associations were consistent across cohorts and between sexes and remained significant in multiple sensitivity analysis that adjusted for alcohol consumption, smoking pack-years, use of lipid-lowering and antihypertensive medications, sodium intake, and blood pressure.
In a secondary analysis, diets with higher inflammatory potential were also associated with significantly higher biomarker levels indicative of more systemic, vascular, and metabolic inflammation, as well as less favorable lipid profiles.
“We wanted to be able to provide guidance on dietary patterns and food combinations,” said Dr. Li. “If you tell people to eat more polyunsaturated fats instead of saturated fat or trans fat, most people don’t know what foods are higher and lower in those nutrients. Also, many foods have different nutrients – some of which are good and some of which are bad – so we wanted to help people find the foods with the higher proportion of healthy nutrients rather than point out specific nutrients to avoid.”
Researchers used prospectively gathered data from the Nurses’ Health Studies I and II starting from 1984 and from the Health Professionals Follow-up Study. After excluding participants with missing diet information or previously diagnosed heart disease, stroke or cancer, over 210,000 participants were included in the analysis. Participants completed a survey every 4 years to ascertain dietary intake.
Prevention, not treatment
In an editorial comment, Ramon Estruch, MD, PhD, from the Hospital Clinic in Barcelona, and colleagues suggested that it might be time for better dietary guidelines.
“A better knowledge of health protection provided by different foods and dietary patterns, mainly their anti-inflammatory properties, should provide the basis for designing even healthier dietary patterns to protect against heart disease,” the editorialists wrote.
They added extra-virgin olive oil, fatty fish, and tomatoes to the list of foods with “established anti-inflammatory activity.”
In a comment, Dr. Estruch said the findings of this new study are confirmatory of the PREDIMED trial, which showed a reduction in risk of major CV events in individuals at high cardiovascular risk assigned to an anti-inflammatory Mediterranean diet pattern supplemented with extra-virgin olive oil or nuts as compared with those assigned to a reduced-fat diet.
“The study of Jun Li et al. confirms that an anti-inflammatory diet is useful to prevent cardiovascular events and, more important, that healthy dietary patterns may be even healthier if subjects increase consumption of foods with the highest anti-inflammatory potential,” he said, adding that “mechanistic explanations add plausibility to the results of observational studies.”
Dr. Estruch was the principal investigator of PREDIMED. This trial was originally published in 2013 and then retracted and republished in 2018, with some required corrections, but the results had not materially changed.
Dr. Li is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Boston Nutrition Obesity Research Center. Dr. Estruch disclosed no financial relationships relevant to the contents of this article.
A version of this article originally appeared on Medscape.com.
Dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease (CVD) and stroke in a new pooled analysis of three prospective cohort studies.
The analysis included 210,145 U.S. women and men followed for up to 32 years in the Nurses’ Health Studies I and II and the Health Professionals Follow-up Study.
After adjustment for use of anti-inflammatory medications and CVD risk factors, those whose dietary pattern ranked in the highest quintile of inflammatory potential had a 38% higher risk of CVD (hazard ratio comparing highest with lowest quintiles, 1.38), a 46% higher risk of coronary heart disease (HR, 1.46), and a 28% higher risk of stroke (HR, 1.28) (all P for trend < .001).
Jun Li, MD, PhD, and colleagues at Harvard School of Public Health and Harvard Medical School, Boston, published the findings of their study in the Nov. 10 issue of the Journal of the American College of Cardiology.
The inflammatory potential of a diet was assessed using a food-based, dietary index called the “empirical dietary inflammatory pattern” or EDIP.
In an interview, Dr. Li explained that the EDIP was developed 4 years ago by many of the same authors involved with this study, including nutrition heavyweights Walter C. Willett, MD, DrPH, and Frank B. Hu, MD, PhD, both from Harvard.
“We summarized all the foods people eat into 39 defined food groups and did a reduced-rank regression analysis that looked at these 39 food groups and three inflammatory markers – interleukin-6, C-reactive protein, and tumor necrosis factor–alpha receptor 2. We found 18 food groups that are most predictive of these biomarkers, and the EDIP was calculated as the weighted sum of these 18 food groups.”
Individuals who had higher intakes of green-leafy vegetables (kale, spinach, arugula), dark-yellow vegetables (pumpkin, yellow peppers, carrots), whole grains, fruits, tea, coffee and wine had lower long-term CVD risk than those with higher intakes of red meat, processed meat, organ meat, refined carbohydrates, and sweetened beverages.
The associations were consistent across cohorts and between sexes and remained significant in multiple sensitivity analysis that adjusted for alcohol consumption, smoking pack-years, use of lipid-lowering and antihypertensive medications, sodium intake, and blood pressure.
In a secondary analysis, diets with higher inflammatory potential were also associated with significantly higher biomarker levels indicative of more systemic, vascular, and metabolic inflammation, as well as less favorable lipid profiles.
“We wanted to be able to provide guidance on dietary patterns and food combinations,” said Dr. Li. “If you tell people to eat more polyunsaturated fats instead of saturated fat or trans fat, most people don’t know what foods are higher and lower in those nutrients. Also, many foods have different nutrients – some of which are good and some of which are bad – so we wanted to help people find the foods with the higher proportion of healthy nutrients rather than point out specific nutrients to avoid.”
Researchers used prospectively gathered data from the Nurses’ Health Studies I and II starting from 1984 and from the Health Professionals Follow-up Study. After excluding participants with missing diet information or previously diagnosed heart disease, stroke or cancer, over 210,000 participants were included in the analysis. Participants completed a survey every 4 years to ascertain dietary intake.
Prevention, not treatment
In an editorial comment, Ramon Estruch, MD, PhD, from the Hospital Clinic in Barcelona, and colleagues suggested that it might be time for better dietary guidelines.
“A better knowledge of health protection provided by different foods and dietary patterns, mainly their anti-inflammatory properties, should provide the basis for designing even healthier dietary patterns to protect against heart disease,” the editorialists wrote.
They added extra-virgin olive oil, fatty fish, and tomatoes to the list of foods with “established anti-inflammatory activity.”
In a comment, Dr. Estruch said the findings of this new study are confirmatory of the PREDIMED trial, which showed a reduction in risk of major CV events in individuals at high cardiovascular risk assigned to an anti-inflammatory Mediterranean diet pattern supplemented with extra-virgin olive oil or nuts as compared with those assigned to a reduced-fat diet.
“The study of Jun Li et al. confirms that an anti-inflammatory diet is useful to prevent cardiovascular events and, more important, that healthy dietary patterns may be even healthier if subjects increase consumption of foods with the highest anti-inflammatory potential,” he said, adding that “mechanistic explanations add plausibility to the results of observational studies.”
Dr. Estruch was the principal investigator of PREDIMED. This trial was originally published in 2013 and then retracted and republished in 2018, with some required corrections, but the results had not materially changed.
Dr. Li is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Boston Nutrition Obesity Research Center. Dr. Estruch disclosed no financial relationships relevant to the contents of this article.
A version of this article originally appeared on Medscape.com.
67-year-old man • upper extremity pain & edema • recent diagnosis of heart failure • Dx?
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.
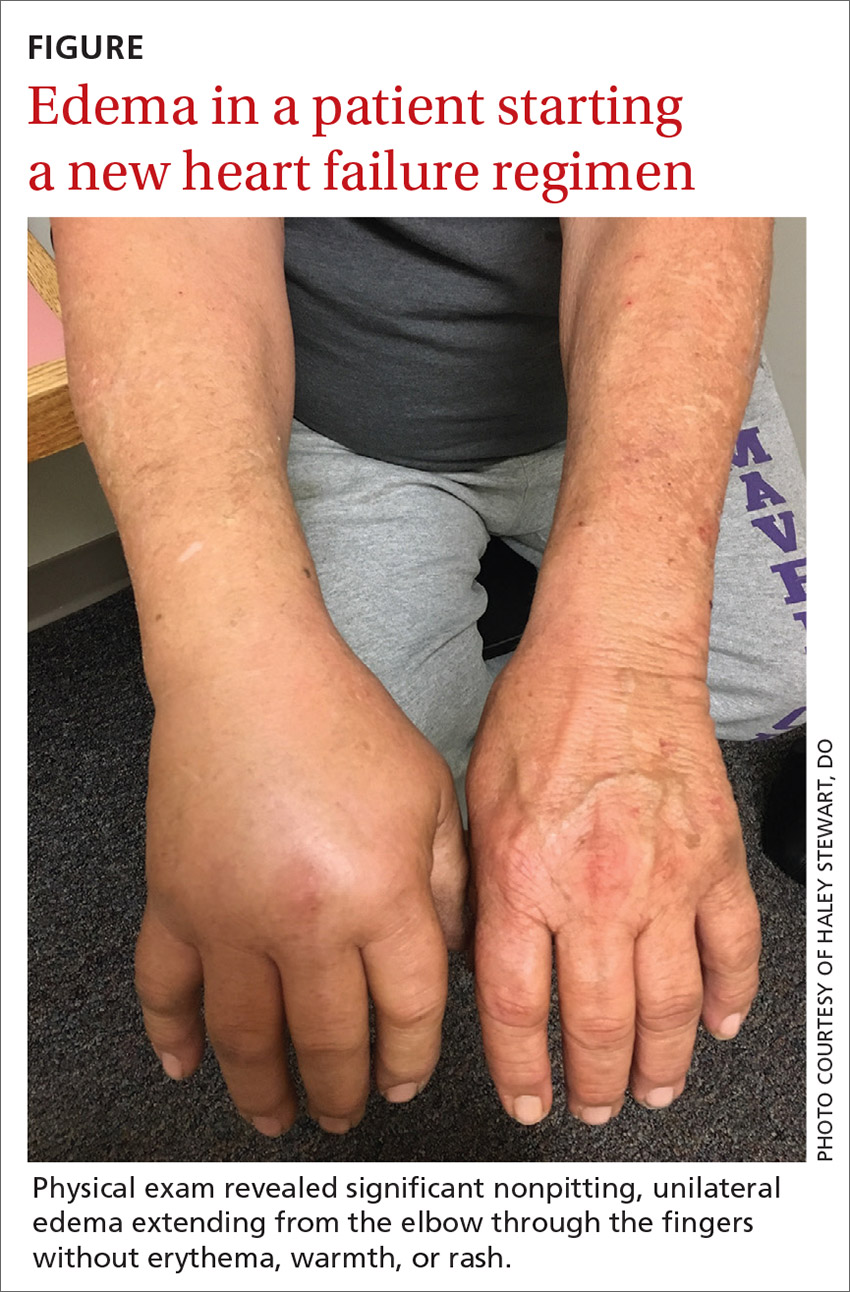
Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.

Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
THE CASE
A 67-year-old man with a history of gout, tobacco use, hypertension, hyperlipidemia, prediabetes, and newly diagnosed heart failure with reduced ejection fraction presented with a new concern for sudden-onset, atraumatic right upper extremity pain and swelling. The patient had awakened with these symptoms and on the following day went to the emergency department (ED) for evaluation. Review of the ED documentation highlighted that the patient was afebrile and was found to have a slight leukocytosis (11.7 x 103/µL) and an elevated C-reactive protein level (4 mg/dL; normal range, 0.3 to 1 mg/dL). A right upper extremity x-ray was unremarkable. The patient was treated with cephalexin and colchicine for cellulitis and possible acute gout.
Three days after the ED visit, the patient presented to his primary care clinic, reporting adherence to the prescribed therapies (cephalexin and colchicine) but no improvement in symptoms. He was again afebrile, and his blood pressure was controlled to goal (118/80 mm Hg). On exam, he had significant nonpitting, unilateral edema extending from the elbow through the fingers without erythema, warmth, or rash (FIGURE). A right upper extremity ultrasound was obtained; results were negative for deep vein thrombosis.

Medication reconciliation completed during the clinic visit revealed that the patient had started and continued to take newly prescribed medications for the treatment of heart failure, including metoprolol succinate, lisinopril, and furosemide. The patient confirmed that these were started 7 days prior to symptom onset.
THE DIAGNOSIS
Given the clinical resemblance to angioedema and the recent initiation of lisinopril, the patient was asked to hold this medication. He was also advised to discontinue the cephalexin and colchicine, given low suspicion for cellulitis and gout. Six days later, he returned to clinic and reported significantly improved pain and swelling.
DISCUSSION
Angioedema is a common condition in the United States, affecting approximately 15% of the general population.1 When associated with hypotension, respiratory compromise, and other end-organ dysfunction, it is treated as anaphylaxis. Angioedema without anaphylaxis can be categorized as either histaminergic or nonhistaminergic; the former is more common.2
Certain patient and disease characteristics are more prevalent in select subsets of angioedema, although there are no features that automatically identify an etiology. Here are some factors to consider:
Recent exposures. Within the histaminergic category, allergic angioedema has the longest list of potential causes, including medications (notably, antibiotics, nonsteroidal anti-inflammatory drugs, opiates, and perioperative medications), foods, latex, and insect stings and/or bites.2 Nonhistaminergic subtypes, which include hereditary and acquired angioedema, are caused by deficiencies or mutations in complement or coagulation pathways, which can be more challenging to diagnose.
Continue to: Acquired angioedema may also...
Acquired angioedema may also be associated with the use of angiotensin-converting enzyme (ACE) inhibitors. Risk factors for ACE inhibitor–induced angioedema include history of smoking, increasing age, and female gender.3 African-American race has been correlated with increased incidence of angioedema, with rates 4 to 5 times that of Whites,1 but race is now identified as a social and not a biological construct and should not be relied on to make medical decisions about prescribing.
The rate of occurrence for ACE inhibitor–induced angioedema is highest within the first 30 days of medication use2; however, it can occur anytime. The absolute risk has been estimated as 0.3% per year.4
Patient age. Histaminergic angioedema can occur at any age. The hereditary subtype of nonhistaminergic angioedema is more common in younger individuals, typically occurring in infancy to the second decade of life, and tends to run in families, while the acquired subtype often manifests in adults older than 40.2
Physical exam findings. The typical manifestation of nonhistaminergic angioedema is firm, nonpitting, nonpruritic swelling resulting from fluid shifts to the reticular dermis and subcutaneous or submucosal tissue. In comparison, histaminergic reactions commonly involve deeper dermal tissue.
Commonly affected anatomic sites also vary by angioedema type but do not directly distinguish a cause. Allergic and ACE inhibitor–induced subtypes more commonly involve the lips, tongue, larynx, and face, whereas hereditary and other acquired etiologies are more likely to affect the periphery, abdomen, face, larynx, and genitourinary systems.2 So the way that this patient presented was a bit unusual.
Continue to: Symptom history
Symptom history. Allergic angioedema often has a rapid onset and resolution, whereas hereditary and acquired subtypes appear more gradually.2 While the presence of urticaria distinguishes a histaminergic reaction, both histaminergic and nonhistaminergic angioedema may manifest without this symptom.
In our patient, the timeline of gradual symptom manifestation and the physical exam findings, as well as the patient’s age, tobacco history, and recent initiation of an ACE inhibitor, made acquired angioedema a more likely etiology.
Treatment for ACE inhibitor–induced angioedema, in addition to airway support, entails drug discontinuation. This typically leads to symptom resolution within 24 to 48 hours.2 Treatment with corticosteroids, antihistamines, and epinephrine is usually ineffective. Switching to an alternative ACE inhibitor is not recommended, as other members of the class carry the same risk. Instead, angiotensin receptor blockers (ARBs) are an appropriate substitute, as the incidence of cross-reactivity in ACE inhibitor–intolerant patients is estimated to be 10% or less,5 and the risk for recurrence has been shown to be no different than with placebo.3,4
Our patient was transitioned to losartan 25 mg/d without recurrence of his symptoms and with continued blood pressure control (125/60 mm Hg).
THE TAKEAWAY
Angioedema is a common condition. While many medications are associated with histaminergic angioedema, ACE inhibitors are a common cause of the acquired subtype of nonhistaminergic angioedema. Commonly affected sites include the lips, tongue, and face; however, this diagnosis is not dependent on location and may manifest at other sites, as seen in this case. Treatment involves medication discontinuation. When switching the patient’s medication, other members of the ACE inhibitor class should be avoided. ARBs are an appropriate alternative without increased risk for recurrence.
CORRESPONDENCE
Katherine Montag Schafer, University of Minnesota— Department of Family Medicine and Community Health, 1414 Maryland Avenue E, St Paul, MN 55106; [email protected]
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
1. Temiño VM, Peebles RS Jr. The spectrum and treatment of angioedema. Am J Med. 2008;121:282-286.
2. Moellman JJ, Bernstein JA, Lindsell CA, et al; American College of Allergy, Asthma & Immunology (ACAAI), Society for Academic Emergency Medicine (SAEM). A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21:469-484.
3. Zuraw BL, Bernstein JA, Lang DM, et al; American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013;131:1491-1493.
4. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012;110:383-391.
5. Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2011;45:520-524.
Comment & Controversy
OBG Manag. 2020 November; 32(11).
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Robert L. Barbieri, MD
(Editorial; July 2020)
Alternative option to the Fetal Pillow
I enjoyed Dr. Barbieri’s editorial on the Fetal Pillow. I worry, however, that applying high air pressure to the upper vagina could result in an air embolism.
I have experienced good results using a vacuum cup. Like the pillow, it distributes the force more evenly than a hand. Also, the handle makes elevation of the vertex much less awkward and allows elevation to a higher station. Whatever approach is employed, using an open internal monitor catheter allows for a gentler procedure than when brute force alone is used to “break the seal” to allow ingress of air into the uterine cavity (at just 1 atmosphere of pressure).
John H. Sand, MD
Ellensburg, Washington
Cost of device must be considered
The information on the Fetal Pillow in Dr. Barbieri’s timely editorial, while limited in scope, does make the device look like a promising option.
One of my institution’s biggest issues relates to cost. We have had some interest in incorporating the Fetal Pillow into our practice, and we have been quoted a rate of about $600.00 per device. I had our Fetal Pillow representative look into reimbursement and have been informed that, at least in our region, there has been no reimbursement for the cost.
When I look at the cost of a hospital stay for a normal spontaneous vaginal delivery (NSVD), the cost of the Fetal Pillow would actually add 15% to 20% to that stay. Now, one must consider also the cost of extension of the uterine incision versus the cost of the Fetal Pillow. When we did a superficial look at when the Fetal Pillow might be used versus how many uterine extensions we experienced, the cost of the Fetal Pillow over a year far exceeded the cost of the uterine extensions. Without reimbursement, this appeared unsustainable. It has been interesting as some sites had no awareness of cost and the fact that essentially “the system” was absorbing those costs.
This issue is worthy of thought but likely one that most obstetricians will not consider.
Casey Morris, MD
Downers Grove, Illinois
Tip for dislodging the fetal head
I read Dr. Barbieri’s editorial regarding the Fetal Pillow and would like to share my experience. Over the last 30 years, I have used a simple trick. After entering the pelvic cavity, we push on the lower uterine segment toward the fundus prior to uterine incision. This helps dislodge the fetal head. Occasionally, you can feel the “pop” when the suction is broken, which sets the head free. We then proceed with the uterine incision and delivery of the head. We have had great success over the years, and the poor nurse does not have to go under the drapes.
Walter Kobasa Jr, MD
Wilmington, Delaware
Dr. Barbieri responds
I appreciate the recommendations and insights of Drs. Sand, Morris, and Kobasa. As I mentioned in the editorial on the Fetal Pillow, there are many clinical pearls about management of a second stage, deep-transverse cephalic arrest at the time of cesarean delivery, including to extend or T the uterine incision, push with a hand from below, reverse breech extraction, use a Coyne spoon, administer nitroglycerine or terbutaline, break the vaginal suction before attempting delivery, and incise a Bandl ring. Dr. Sand adds vaginal placement of a vacuum cup to our armamentarium, and Dr. Kobasa recommends dislodging the fetal head with a push on the lower uterine segment before making the hysterotomy incision. I thank Dr. Morris for correcting my failure to report the cost of the Fetal Pillow, reporting a quoted price of $600 for each Fetal Pillow. I agree with Dr. Morris that physicians have an important responsibility to be good stewards of health care resources and weigh the benefits and costs of our decisions.
Continue to: In your practice, are you planning to have a chaperone present for all intimate examinations?
In your practice, are you planning to have a chaperone present for all intimate examinations?
Robert L. Barbieri, MD
(Editorial; June 2020)
Enough is enough
I have always thought that many doctors who write opinions and pontificate about what should be done in practice live in la-la land. This editorial, for me, confirms it.
I personally am becoming tired of all this: dividing the specialty into obstetricians and gynecologists; pelvic exams are not necessary during annual visits; HPV testing by patients at home; doing away with Pap smears; Pap smears are not necessary for patients after a certain age; scribes in your footsteps to document all findings in the EMR; heaven forbid you do not ask the patient if she has a fire extinguisher in her house or some other stupid information; interpreters for people who speak Mongolian because their partner should not be used to interpret for them; and so on.
Now you want us to have a chaperone for every pelvic exam! Not any chaperone, but a specialized one! You worry about the sanctity and privacy of the patient but now have 2 additional people in the room for the patient’s exam. First, most patients prefer to have the least number of people looking at their bodies during an exam, especially a pelvic exam. Second, where do we get the money to support all of this? Does this type of policy make any sense? Are lawyers now controlling what medical care is all about? Is that what is now considered quality medical care?
By the way, I am not a burned out physician. I use common sense and consider what is best for my patients in everything that I do. If a patient requests a chaperone, my medical assistant will come to the room and provide that service. You do not need to be specialized to provide this service! Ivory tower people have lost all common sense. You consider yourselves the authorities in whatever medical field you specialize in, but let me tell you something: You really are not.
I know I will be criticized and demonized publicly by many; however, I have the courage to say what, in my opinion, I feel is right and what is wrong. Many physicians are afraid to do so, and, like sheep, will comply with your misguided opinions. I truly do not mean any disrespect to your knowledge and good intentions. I just think that enough is enough!
Gabriel G. Hakim, MD
Waterbury, Connecticut
Dr. Barbieri responds
In response to my editorial on the American College of Obstetricians and Gynecologists (ACOG) recommendation that chaperones be present for intimate examinations (ACOG Committee Opinion No. 796), Dr. Hakim outlines many concerns with the rapidly evolving practice of medicine.1 I am confident that the ACOG Committee on Ethics wisely considered the benefits, costs, and unintended consequences of the recommendation. The United States Veterans Administration, the Royal College of Obstetricians and Gynaecologists, and the American College Health Association endorse a similar recommendation. I do not think the distinguished members of the committees who issued the recommendation “live in la-la land.”
Reference
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50.
OBG Manag. 2020 November; 32(11).
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Robert L. Barbieri, MD
(Editorial; July 2020)
Alternative option to the Fetal Pillow
I enjoyed Dr. Barbieri’s editorial on the Fetal Pillow. I worry, however, that applying high air pressure to the upper vagina could result in an air embolism.
I have experienced good results using a vacuum cup. Like the pillow, it distributes the force more evenly than a hand. Also, the handle makes elevation of the vertex much less awkward and allows elevation to a higher station. Whatever approach is employed, using an open internal monitor catheter allows for a gentler procedure than when brute force alone is used to “break the seal” to allow ingress of air into the uterine cavity (at just 1 atmosphere of pressure).
John H. Sand, MD
Ellensburg, Washington
Cost of device must be considered
The information on the Fetal Pillow in Dr. Barbieri’s timely editorial, while limited in scope, does make the device look like a promising option.
One of my institution’s biggest issues relates to cost. We have had some interest in incorporating the Fetal Pillow into our practice, and we have been quoted a rate of about $600.00 per device. I had our Fetal Pillow representative look into reimbursement and have been informed that, at least in our region, there has been no reimbursement for the cost.
When I look at the cost of a hospital stay for a normal spontaneous vaginal delivery (NSVD), the cost of the Fetal Pillow would actually add 15% to 20% to that stay. Now, one must consider also the cost of extension of the uterine incision versus the cost of the Fetal Pillow. When we did a superficial look at when the Fetal Pillow might be used versus how many uterine extensions we experienced, the cost of the Fetal Pillow over a year far exceeded the cost of the uterine extensions. Without reimbursement, this appeared unsustainable. It has been interesting as some sites had no awareness of cost and the fact that essentially “the system” was absorbing those costs.
This issue is worthy of thought but likely one that most obstetricians will not consider.
Casey Morris, MD
Downers Grove, Illinois
Tip for dislodging the fetal head
I read Dr. Barbieri’s editorial regarding the Fetal Pillow and would like to share my experience. Over the last 30 years, I have used a simple trick. After entering the pelvic cavity, we push on the lower uterine segment toward the fundus prior to uterine incision. This helps dislodge the fetal head. Occasionally, you can feel the “pop” when the suction is broken, which sets the head free. We then proceed with the uterine incision and delivery of the head. We have had great success over the years, and the poor nurse does not have to go under the drapes.
Walter Kobasa Jr, MD
Wilmington, Delaware
Dr. Barbieri responds
I appreciate the recommendations and insights of Drs. Sand, Morris, and Kobasa. As I mentioned in the editorial on the Fetal Pillow, there are many clinical pearls about management of a second stage, deep-transverse cephalic arrest at the time of cesarean delivery, including to extend or T the uterine incision, push with a hand from below, reverse breech extraction, use a Coyne spoon, administer nitroglycerine or terbutaline, break the vaginal suction before attempting delivery, and incise a Bandl ring. Dr. Sand adds vaginal placement of a vacuum cup to our armamentarium, and Dr. Kobasa recommends dislodging the fetal head with a push on the lower uterine segment before making the hysterotomy incision. I thank Dr. Morris for correcting my failure to report the cost of the Fetal Pillow, reporting a quoted price of $600 for each Fetal Pillow. I agree with Dr. Morris that physicians have an important responsibility to be good stewards of health care resources and weigh the benefits and costs of our decisions.
Continue to: In your practice, are you planning to have a chaperone present for all intimate examinations?
In your practice, are you planning to have a chaperone present for all intimate examinations?
Robert L. Barbieri, MD
(Editorial; June 2020)
Enough is enough
I have always thought that many doctors who write opinions and pontificate about what should be done in practice live in la-la land. This editorial, for me, confirms it.
I personally am becoming tired of all this: dividing the specialty into obstetricians and gynecologists; pelvic exams are not necessary during annual visits; HPV testing by patients at home; doing away with Pap smears; Pap smears are not necessary for patients after a certain age; scribes in your footsteps to document all findings in the EMR; heaven forbid you do not ask the patient if she has a fire extinguisher in her house or some other stupid information; interpreters for people who speak Mongolian because their partner should not be used to interpret for them; and so on.
Now you want us to have a chaperone for every pelvic exam! Not any chaperone, but a specialized one! You worry about the sanctity and privacy of the patient but now have 2 additional people in the room for the patient’s exam. First, most patients prefer to have the least number of people looking at their bodies during an exam, especially a pelvic exam. Second, where do we get the money to support all of this? Does this type of policy make any sense? Are lawyers now controlling what medical care is all about? Is that what is now considered quality medical care?
By the way, I am not a burned out physician. I use common sense and consider what is best for my patients in everything that I do. If a patient requests a chaperone, my medical assistant will come to the room and provide that service. You do not need to be specialized to provide this service! Ivory tower people have lost all common sense. You consider yourselves the authorities in whatever medical field you specialize in, but let me tell you something: You really are not.
I know I will be criticized and demonized publicly by many; however, I have the courage to say what, in my opinion, I feel is right and what is wrong. Many physicians are afraid to do so, and, like sheep, will comply with your misguided opinions. I truly do not mean any disrespect to your knowledge and good intentions. I just think that enough is enough!
Gabriel G. Hakim, MD
Waterbury, Connecticut
Dr. Barbieri responds
In response to my editorial on the American College of Obstetricians and Gynecologists (ACOG) recommendation that chaperones be present for intimate examinations (ACOG Committee Opinion No. 796), Dr. Hakim outlines many concerns with the rapidly evolving practice of medicine.1 I am confident that the ACOG Committee on Ethics wisely considered the benefits, costs, and unintended consequences of the recommendation. The United States Veterans Administration, the Royal College of Obstetricians and Gynaecologists, and the American College Health Association endorse a similar recommendation. I do not think the distinguished members of the committees who issued the recommendation “live in la-la land.”
Reference
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50.
OBG Manag. 2020 November; 32(11).
The Fetal Pillow: A new option for delivering the deeply impacted fetal head
Robert L. Barbieri, MD
(Editorial; July 2020)
Alternative option to the Fetal Pillow
I enjoyed Dr. Barbieri’s editorial on the Fetal Pillow. I worry, however, that applying high air pressure to the upper vagina could result in an air embolism.
I have experienced good results using a vacuum cup. Like the pillow, it distributes the force more evenly than a hand. Also, the handle makes elevation of the vertex much less awkward and allows elevation to a higher station. Whatever approach is employed, using an open internal monitor catheter allows for a gentler procedure than when brute force alone is used to “break the seal” to allow ingress of air into the uterine cavity (at just 1 atmosphere of pressure).
John H. Sand, MD
Ellensburg, Washington
Cost of device must be considered
The information on the Fetal Pillow in Dr. Barbieri’s timely editorial, while limited in scope, does make the device look like a promising option.
One of my institution’s biggest issues relates to cost. We have had some interest in incorporating the Fetal Pillow into our practice, and we have been quoted a rate of about $600.00 per device. I had our Fetal Pillow representative look into reimbursement and have been informed that, at least in our region, there has been no reimbursement for the cost.
When I look at the cost of a hospital stay for a normal spontaneous vaginal delivery (NSVD), the cost of the Fetal Pillow would actually add 15% to 20% to that stay. Now, one must consider also the cost of extension of the uterine incision versus the cost of the Fetal Pillow. When we did a superficial look at when the Fetal Pillow might be used versus how many uterine extensions we experienced, the cost of the Fetal Pillow over a year far exceeded the cost of the uterine extensions. Without reimbursement, this appeared unsustainable. It has been interesting as some sites had no awareness of cost and the fact that essentially “the system” was absorbing those costs.
This issue is worthy of thought but likely one that most obstetricians will not consider.
Casey Morris, MD
Downers Grove, Illinois
Tip for dislodging the fetal head
I read Dr. Barbieri’s editorial regarding the Fetal Pillow and would like to share my experience. Over the last 30 years, I have used a simple trick. After entering the pelvic cavity, we push on the lower uterine segment toward the fundus prior to uterine incision. This helps dislodge the fetal head. Occasionally, you can feel the “pop” when the suction is broken, which sets the head free. We then proceed with the uterine incision and delivery of the head. We have had great success over the years, and the poor nurse does not have to go under the drapes.
Walter Kobasa Jr, MD
Wilmington, Delaware
Dr. Barbieri responds
I appreciate the recommendations and insights of Drs. Sand, Morris, and Kobasa. As I mentioned in the editorial on the Fetal Pillow, there are many clinical pearls about management of a second stage, deep-transverse cephalic arrest at the time of cesarean delivery, including to extend or T the uterine incision, push with a hand from below, reverse breech extraction, use a Coyne spoon, administer nitroglycerine or terbutaline, break the vaginal suction before attempting delivery, and incise a Bandl ring. Dr. Sand adds vaginal placement of a vacuum cup to our armamentarium, and Dr. Kobasa recommends dislodging the fetal head with a push on the lower uterine segment before making the hysterotomy incision. I thank Dr. Morris for correcting my failure to report the cost of the Fetal Pillow, reporting a quoted price of $600 for each Fetal Pillow. I agree with Dr. Morris that physicians have an important responsibility to be good stewards of health care resources and weigh the benefits and costs of our decisions.
Continue to: In your practice, are you planning to have a chaperone present for all intimate examinations?
In your practice, are you planning to have a chaperone present for all intimate examinations?
Robert L. Barbieri, MD
(Editorial; June 2020)
Enough is enough
I have always thought that many doctors who write opinions and pontificate about what should be done in practice live in la-la land. This editorial, for me, confirms it.
I personally am becoming tired of all this: dividing the specialty into obstetricians and gynecologists; pelvic exams are not necessary during annual visits; HPV testing by patients at home; doing away with Pap smears; Pap smears are not necessary for patients after a certain age; scribes in your footsteps to document all findings in the EMR; heaven forbid you do not ask the patient if she has a fire extinguisher in her house or some other stupid information; interpreters for people who speak Mongolian because their partner should not be used to interpret for them; and so on.
Now you want us to have a chaperone for every pelvic exam! Not any chaperone, but a specialized one! You worry about the sanctity and privacy of the patient but now have 2 additional people in the room for the patient’s exam. First, most patients prefer to have the least number of people looking at their bodies during an exam, especially a pelvic exam. Second, where do we get the money to support all of this? Does this type of policy make any sense? Are lawyers now controlling what medical care is all about? Is that what is now considered quality medical care?
By the way, I am not a burned out physician. I use common sense and consider what is best for my patients in everything that I do. If a patient requests a chaperone, my medical assistant will come to the room and provide that service. You do not need to be specialized to provide this service! Ivory tower people have lost all common sense. You consider yourselves the authorities in whatever medical field you specialize in, but let me tell you something: You really are not.
I know I will be criticized and demonized publicly by many; however, I have the courage to say what, in my opinion, I feel is right and what is wrong. Many physicians are afraid to do so, and, like sheep, will comply with your misguided opinions. I truly do not mean any disrespect to your knowledge and good intentions. I just think that enough is enough!
Gabriel G. Hakim, MD
Waterbury, Connecticut
Dr. Barbieri responds
In response to my editorial on the American College of Obstetricians and Gynecologists (ACOG) recommendation that chaperones be present for intimate examinations (ACOG Committee Opinion No. 796), Dr. Hakim outlines many concerns with the rapidly evolving practice of medicine.1 I am confident that the ACOG Committee on Ethics wisely considered the benefits, costs, and unintended consequences of the recommendation. The United States Veterans Administration, the Royal College of Obstetricians and Gynaecologists, and the American College Health Association endorse a similar recommendation. I do not think the distinguished members of the committees who issued the recommendation “live in la-la land.”
Reference
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50.
No link shown between thyroid dysfunction and heart failure
Thyroid dysfunction had virtually no independent impact on survival in a retrospective study of nearly 5,000 English patients with chronic heart failure, adding to evidence that subclinical thyroid disorders in these patients requires no special management beyond ongoing monitoring.
“Although thyroid dysfunction is related to outcome in patients with chronic heart failure, the association disappears when adjustment is made for established prognostic variables, such as age, NT-proBNP [N-terminal of the prohormone brain natriuretic peptide], and [New York Heart Association] class,” wrote Nathan A. Samuel, MBChB, and coauthors in the American Journal of Cardiology.
Results from several earlier studies had shown evidence for reduced survival in heart failure patients with thyroid dysfunction, but in analyses that did not adjust for heart failure severity, such as a 2013 report that used data from the Sudden Cardiac Death in Heart Failure Trial SCD-HeFT. Other studies that adjusted for heart failure severity based on serum level of natriuretic peptides did not show significant associations between thyroid function and mortality, and when those results couple with the new report they together minimize the immediate risk from subclinical thyroid dysfunction faced by heart failure patients, wrote the authors of the new report.
Don’t treat subclinical thyroid dysfunction
“Our results suggest that subclinical thyroid disease has little impact on outcomes, and that we should not treat subclinical hypothyroidism in the expectation of improving outlook,” said Andrew L. Clark, MD, senior author on the new report and professor and head of the department of academic cardiology at Hull (England) York Medical School.
“Both hyper-and hypothyroidism can cause heart failure, so thyroid function should always be checked in patients when they present with heart failure. A small proportion of patients have heart failure that is potentially reversible” with thyroid-directed treatment, Dr. Clark said in an interview.
But “subclinical disease should probably not be treated, although we have not conducted a clinical trial that proves this assertion. We speculate, based on our findings, that such a trial is unlikely to be positive.”
Patients with subclinical thyroid disorders, particularly subclinical hypothyroidism, “need to be followed and treated should they develop clinical disease,” he maintained. “Except in extreme circumstances, such as the handful of patients who might have gross myxedema and may be near coma, thyroid replacement therapy for those [with heart failure] who have clinical hypothyroidism should follow standard lines.”
It is important to monitor thyroid function,” agreed Dr. Samuel, a researcher in the department of academic cardiology at Hull York Medical School. “We found that thyroxine use was most common among patients with hyperthyroidism, suggesting that they were previously hypothyroid and had received inappropriate treatment.”
Confounder adjustment mitigates the thyroid link
The new analysis used data collected from 6,782 consecutive heart failure patients enrolled during 2000-2018 at a community heart failure clinic that serves patients in the region of Hull, England. The researchers identified 4,992 of these patients with confirmed heart failure and adequate data for their analyses, including 2,997 (60%) with heart failure with reduced ejection fraction (HFrEF) and 1,995 (40%) with heart failure with normal ejection fraction (HFnEF, the term used by the authors but often called heart failure with preserved ejection fraction).
Thyroid hormone levels showed that 90% of these patients were euthyroid, 6% were hyperthyroid, and 4% were hypothyroid, rates consistent with prior reports for both the general population and heart failure patients. Only 12 patients (0.2%) had overt hypothyroidism, and fewer that 1% (about 45 patients) had overt hyperthyroidism. Patients averaged about 73 years of age, and during a median 4.6 years of follow-up 58% died.
Both the hypo- and hyperthyroid patients showed significantly higher mortality rates than euthyroid patients in a univariate analysis. But the patients with thyroid dysfunction also had more comorbidities, more severe heart failure symptoms measured by NYHA functional class, and more severe heart failure measured as higher serum levels of NT-proBNP.
In a multivariate analysis that adjusted for these factors, the significant differences disappeared among the entire group of heart failure patients for the outcomes of all-cause mortality, and mortality or hospitalization with heart failure. The multivariate analysis also showed no significant association between higher levels of thyroid-stimulating hormone (TSH) and all-cause death or death plus heart failure hospitalization among the patients with HFrEF.
Among patients with HFnEF, the multivariate adjusted analysis showed a small increase in both mortality and mortality plus hospitalization for heart failure, a 2% rise for each of these two endpoints for each 1 mIU/L increase in TSH, the authors reported. Although the P value for each of these two significant differences among patients with HFnEF was .02, the 95% confidence interval included 1.00 and ranged from 1.00 to 1.04.
The multivariate analysis identified three variables with the strongest associations with all-cause mortality: older age, higher levels of NT-proBNP, and higher NYHA class indicating greater functional impairment.
The results support the hypothesis that “worsening heart failure can lead to down-regulation of thyroid hormone signaling,” the authors suggested. Their study is also “the first to examine the prognostic significance of thyroid dysfunction in a large population of patients with HFnEF.” This analysis showed a “weak but significant association between increasing TSH and both mortality and the composite endpoint in patients with HFnEF.”
“HFnEF is a heterogeneous group of conditions that are difficult to diagnose in many cases. Therefore, future studies are needed to provide further clarity on the effect of thyroid dysfunction in these patients,” Dr. Samuel said.
The study received no commercial funding. Dr. Samuel and Dr. Clark had no disclosures.
SOURCE: Samuel NA et al. Am J Cardiol. 2020 Oct 24. doi: 10.1016/j.amjcard.2020.10.034.
Thyroid dysfunction had virtually no independent impact on survival in a retrospective study of nearly 5,000 English patients with chronic heart failure, adding to evidence that subclinical thyroid disorders in these patients requires no special management beyond ongoing monitoring.
“Although thyroid dysfunction is related to outcome in patients with chronic heart failure, the association disappears when adjustment is made for established prognostic variables, such as age, NT-proBNP [N-terminal of the prohormone brain natriuretic peptide], and [New York Heart Association] class,” wrote Nathan A. Samuel, MBChB, and coauthors in the American Journal of Cardiology.
Results from several earlier studies had shown evidence for reduced survival in heart failure patients with thyroid dysfunction, but in analyses that did not adjust for heart failure severity, such as a 2013 report that used data from the Sudden Cardiac Death in Heart Failure Trial SCD-HeFT. Other studies that adjusted for heart failure severity based on serum level of natriuretic peptides did not show significant associations between thyroid function and mortality, and when those results couple with the new report they together minimize the immediate risk from subclinical thyroid dysfunction faced by heart failure patients, wrote the authors of the new report.
Don’t treat subclinical thyroid dysfunction
“Our results suggest that subclinical thyroid disease has little impact on outcomes, and that we should not treat subclinical hypothyroidism in the expectation of improving outlook,” said Andrew L. Clark, MD, senior author on the new report and professor and head of the department of academic cardiology at Hull (England) York Medical School.
“Both hyper-and hypothyroidism can cause heart failure, so thyroid function should always be checked in patients when they present with heart failure. A small proportion of patients have heart failure that is potentially reversible” with thyroid-directed treatment, Dr. Clark said in an interview.
But “subclinical disease should probably not be treated, although we have not conducted a clinical trial that proves this assertion. We speculate, based on our findings, that such a trial is unlikely to be positive.”
Patients with subclinical thyroid disorders, particularly subclinical hypothyroidism, “need to be followed and treated should they develop clinical disease,” he maintained. “Except in extreme circumstances, such as the handful of patients who might have gross myxedema and may be near coma, thyroid replacement therapy for those [with heart failure] who have clinical hypothyroidism should follow standard lines.”
It is important to monitor thyroid function,” agreed Dr. Samuel, a researcher in the department of academic cardiology at Hull York Medical School. “We found that thyroxine use was most common among patients with hyperthyroidism, suggesting that they were previously hypothyroid and had received inappropriate treatment.”
Confounder adjustment mitigates the thyroid link
The new analysis used data collected from 6,782 consecutive heart failure patients enrolled during 2000-2018 at a community heart failure clinic that serves patients in the region of Hull, England. The researchers identified 4,992 of these patients with confirmed heart failure and adequate data for their analyses, including 2,997 (60%) with heart failure with reduced ejection fraction (HFrEF) and 1,995 (40%) with heart failure with normal ejection fraction (HFnEF, the term used by the authors but often called heart failure with preserved ejection fraction).
Thyroid hormone levels showed that 90% of these patients were euthyroid, 6% were hyperthyroid, and 4% were hypothyroid, rates consistent with prior reports for both the general population and heart failure patients. Only 12 patients (0.2%) had overt hypothyroidism, and fewer that 1% (about 45 patients) had overt hyperthyroidism. Patients averaged about 73 years of age, and during a median 4.6 years of follow-up 58% died.
Both the hypo- and hyperthyroid patients showed significantly higher mortality rates than euthyroid patients in a univariate analysis. But the patients with thyroid dysfunction also had more comorbidities, more severe heart failure symptoms measured by NYHA functional class, and more severe heart failure measured as higher serum levels of NT-proBNP.
In a multivariate analysis that adjusted for these factors, the significant differences disappeared among the entire group of heart failure patients for the outcomes of all-cause mortality, and mortality or hospitalization with heart failure. The multivariate analysis also showed no significant association between higher levels of thyroid-stimulating hormone (TSH) and all-cause death or death plus heart failure hospitalization among the patients with HFrEF.
Among patients with HFnEF, the multivariate adjusted analysis showed a small increase in both mortality and mortality plus hospitalization for heart failure, a 2% rise for each of these two endpoints for each 1 mIU/L increase in TSH, the authors reported. Although the P value for each of these two significant differences among patients with HFnEF was .02, the 95% confidence interval included 1.00 and ranged from 1.00 to 1.04.
The multivariate analysis identified three variables with the strongest associations with all-cause mortality: older age, higher levels of NT-proBNP, and higher NYHA class indicating greater functional impairment.
The results support the hypothesis that “worsening heart failure can lead to down-regulation of thyroid hormone signaling,” the authors suggested. Their study is also “the first to examine the prognostic significance of thyroid dysfunction in a large population of patients with HFnEF.” This analysis showed a “weak but significant association between increasing TSH and both mortality and the composite endpoint in patients with HFnEF.”
“HFnEF is a heterogeneous group of conditions that are difficult to diagnose in many cases. Therefore, future studies are needed to provide further clarity on the effect of thyroid dysfunction in these patients,” Dr. Samuel said.
The study received no commercial funding. Dr. Samuel and Dr. Clark had no disclosures.
SOURCE: Samuel NA et al. Am J Cardiol. 2020 Oct 24. doi: 10.1016/j.amjcard.2020.10.034.
Thyroid dysfunction had virtually no independent impact on survival in a retrospective study of nearly 5,000 English patients with chronic heart failure, adding to evidence that subclinical thyroid disorders in these patients requires no special management beyond ongoing monitoring.
“Although thyroid dysfunction is related to outcome in patients with chronic heart failure, the association disappears when adjustment is made for established prognostic variables, such as age, NT-proBNP [N-terminal of the prohormone brain natriuretic peptide], and [New York Heart Association] class,” wrote Nathan A. Samuel, MBChB, and coauthors in the American Journal of Cardiology.
Results from several earlier studies had shown evidence for reduced survival in heart failure patients with thyroid dysfunction, but in analyses that did not adjust for heart failure severity, such as a 2013 report that used data from the Sudden Cardiac Death in Heart Failure Trial SCD-HeFT. Other studies that adjusted for heart failure severity based on serum level of natriuretic peptides did not show significant associations between thyroid function and mortality, and when those results couple with the new report they together minimize the immediate risk from subclinical thyroid dysfunction faced by heart failure patients, wrote the authors of the new report.
Don’t treat subclinical thyroid dysfunction
“Our results suggest that subclinical thyroid disease has little impact on outcomes, and that we should not treat subclinical hypothyroidism in the expectation of improving outlook,” said Andrew L. Clark, MD, senior author on the new report and professor and head of the department of academic cardiology at Hull (England) York Medical School.
“Both hyper-and hypothyroidism can cause heart failure, so thyroid function should always be checked in patients when they present with heart failure. A small proportion of patients have heart failure that is potentially reversible” with thyroid-directed treatment, Dr. Clark said in an interview.
But “subclinical disease should probably not be treated, although we have not conducted a clinical trial that proves this assertion. We speculate, based on our findings, that such a trial is unlikely to be positive.”
Patients with subclinical thyroid disorders, particularly subclinical hypothyroidism, “need to be followed and treated should they develop clinical disease,” he maintained. “Except in extreme circumstances, such as the handful of patients who might have gross myxedema and may be near coma, thyroid replacement therapy for those [with heart failure] who have clinical hypothyroidism should follow standard lines.”
It is important to monitor thyroid function,” agreed Dr. Samuel, a researcher in the department of academic cardiology at Hull York Medical School. “We found that thyroxine use was most common among patients with hyperthyroidism, suggesting that they were previously hypothyroid and had received inappropriate treatment.”
Confounder adjustment mitigates the thyroid link
The new analysis used data collected from 6,782 consecutive heart failure patients enrolled during 2000-2018 at a community heart failure clinic that serves patients in the region of Hull, England. The researchers identified 4,992 of these patients with confirmed heart failure and adequate data for their analyses, including 2,997 (60%) with heart failure with reduced ejection fraction (HFrEF) and 1,995 (40%) with heart failure with normal ejection fraction (HFnEF, the term used by the authors but often called heart failure with preserved ejection fraction).
Thyroid hormone levels showed that 90% of these patients were euthyroid, 6% were hyperthyroid, and 4% were hypothyroid, rates consistent with prior reports for both the general population and heart failure patients. Only 12 patients (0.2%) had overt hypothyroidism, and fewer that 1% (about 45 patients) had overt hyperthyroidism. Patients averaged about 73 years of age, and during a median 4.6 years of follow-up 58% died.
Both the hypo- and hyperthyroid patients showed significantly higher mortality rates than euthyroid patients in a univariate analysis. But the patients with thyroid dysfunction also had more comorbidities, more severe heart failure symptoms measured by NYHA functional class, and more severe heart failure measured as higher serum levels of NT-proBNP.
In a multivariate analysis that adjusted for these factors, the significant differences disappeared among the entire group of heart failure patients for the outcomes of all-cause mortality, and mortality or hospitalization with heart failure. The multivariate analysis also showed no significant association between higher levels of thyroid-stimulating hormone (TSH) and all-cause death or death plus heart failure hospitalization among the patients with HFrEF.
Among patients with HFnEF, the multivariate adjusted analysis showed a small increase in both mortality and mortality plus hospitalization for heart failure, a 2% rise for each of these two endpoints for each 1 mIU/L increase in TSH, the authors reported. Although the P value for each of these two significant differences among patients with HFnEF was .02, the 95% confidence interval included 1.00 and ranged from 1.00 to 1.04.
The multivariate analysis identified three variables with the strongest associations with all-cause mortality: older age, higher levels of NT-proBNP, and higher NYHA class indicating greater functional impairment.
The results support the hypothesis that “worsening heart failure can lead to down-regulation of thyroid hormone signaling,” the authors suggested. Their study is also “the first to examine the prognostic significance of thyroid dysfunction in a large population of patients with HFnEF.” This analysis showed a “weak but significant association between increasing TSH and both mortality and the composite endpoint in patients with HFnEF.”
“HFnEF is a heterogeneous group of conditions that are difficult to diagnose in many cases. Therefore, future studies are needed to provide further clarity on the effect of thyroid dysfunction in these patients,” Dr. Samuel said.
The study received no commercial funding. Dr. Samuel and Dr. Clark had no disclosures.
SOURCE: Samuel NA et al. Am J Cardiol. 2020 Oct 24. doi: 10.1016/j.amjcard.2020.10.034.
FROM THE AMERICAN JOURNAL OF CARDIOLOGY