User login
Nebulized surfactant shows promise in large cohort
Nebulized delivery of surfactant reduced the need for intubation and liquid surfactant administration by half among newborns with signs of respiratory distress syndrome, according to results from a large randomized, multicenter trial.
Neonatologists have long sought alternatives to intubation for administering surfactant to newborns with respiratory distress syndrome (RDS). An effective noninvasive aerosolized treatment has remained elusive, with small clinical trials that have produced mixed results.
In research published in Pediatrics, James J. Cummings, MD, of Albany (N.Y.) Medical Center, and colleagues, randomized 457 infants (mean 33 weeks’ gestational age) with signs of RDS to either usual care or a nebulized bovine surfactant. Infants were recruited at 22 neonatal ICUs in the United States. Investigators were not blinded to treatment allocation and the decision to intubate was left up to the individual treating physician, because to do so, the authors wrote, would add “pragmatic strength” to the study, and “be ethically compliant with the infant’s best interest.”
Infants in the study received usual care or up to three treatments 4 or more hours apart of 35 mg/mL calfactant suspension, 210 mg phospholipid/kg body weight delivered into the mouth through a nebulizer modified with a pacifier. Dr. Cummings and colleagues found that intubation and liquid surfactant administration within the first 4 days after birth was 26% in the intervention group and 50% in the usual care group (P < .001).
The results remained significant after investigators adjusted for gestational age, birth weight, age when randomized, sex, delivery mode, and antenatal steroids. Rates of intubation for surfactant administration were lower for infants in the intervention group in all gestational age brackets except the youngest (23-24 weeks); all of these infants needed intubation. Respiratory support at days 3, 7, and 28 did not differ between study groups.
“Our study is the first to reveal the efficacy of an aerosolized surfactant delivery system that does not require a respiratory circuit interface,” the investigators wrote.
In previous trials of aerosolized surfactants, they noted, treatment was delivered with nasal continuous positive airway pressure. “By using a separate, pacifier interface, both the aerosol delivery and [nasal continuous positive airway pressure] flow can be managed independently, which should allow for safer patient care.”
Dr. Cummings and colleagues also acknowledged several important limitations of their study, including its nonmasked, nonblinded design, and that it enrolled few infants with less than 28 weeks’ gestation. It takes 1-2 hours to deliver aerosolized calfactant, and “we did not want to delay definitive treatment.”
In an editorial comment accompanying the study, Kirsten Glaser, MD, of the University of Leipzig (Germany), and Clyde Wright, MD of the University of Colorado at Denver, Aurora, called the results promising. “Importantly, application of surfactant aerosols was well tolerated by using a modified nebulizer with a pacifier interface.”
“Clinicians were aware that every infant randomly assigned to the nebulized surfactant arm received the intervention,” they wrote. “It is possible that clinicians delayed intubation and endotracheal surfactant instillation in this group, being biased by aerosolization and the hypothesis of lower risk of air leak and lung injury.”
Dr. Glaser and Dr. Wright further lamented that there were no formal criteria for administering surfactant therapy, and that the infants in the study might not be representative of those most in need of treatment. Today, bronchopulmonary dysplasia “primarily affects infants born at less than 28 weeks’ gestational age,” a minority of the infants recruited for this study, they wrote, urging further investigation in this patient group.
In an interview, neonatologist Roger F. Soll, MD, the H. Wallace Professor of Neonatology at the Larner College of Medicine at University of Vermont in Burlington, echoed the editorialists’ concerns that the study’s pragmatic design left a number of key questions unanswered. “It’s a promising study that laudably recruited on an order of magnitude more infants than any like it in the past,” Dr. Soll said. “And the kids who got the therapy seemed to do better, which is exciting. But with the broad entry criteria, the lack of formal diagnosis of RDS, and the outcome measures ultimately potentially biased by lack of blinding, it doesn’t give us the answers we need yet to consider aerosolized treatment.”
ONY Biotech, manufacturer of the study drug Infasurf, sponsored the trial. Dr. Cummings and one coauthor disclosed consulting arrangements with the sponsor, and another coauthor is an employee of the sponsor. The remaining investigators had no relevant financial disclosures. Dr. Glaser and Dr. Wright disclosed no conflicts of interest related to their editorial; Dr. Wright’s work was supported by the National Institutes of Health. Dr. Soll is president of the Vermont Oxford Network and coordinating editor of Cochrane Neonatal.
SOURCE: Cummings JJ et al. Pediatrics. 2020;146(5):e2020021576.
Nebulized delivery of surfactant reduced the need for intubation and liquid surfactant administration by half among newborns with signs of respiratory distress syndrome, according to results from a large randomized, multicenter trial.
Neonatologists have long sought alternatives to intubation for administering surfactant to newborns with respiratory distress syndrome (RDS). An effective noninvasive aerosolized treatment has remained elusive, with small clinical trials that have produced mixed results.
In research published in Pediatrics, James J. Cummings, MD, of Albany (N.Y.) Medical Center, and colleagues, randomized 457 infants (mean 33 weeks’ gestational age) with signs of RDS to either usual care or a nebulized bovine surfactant. Infants were recruited at 22 neonatal ICUs in the United States. Investigators were not blinded to treatment allocation and the decision to intubate was left up to the individual treating physician, because to do so, the authors wrote, would add “pragmatic strength” to the study, and “be ethically compliant with the infant’s best interest.”
Infants in the study received usual care or up to three treatments 4 or more hours apart of 35 mg/mL calfactant suspension, 210 mg phospholipid/kg body weight delivered into the mouth through a nebulizer modified with a pacifier. Dr. Cummings and colleagues found that intubation and liquid surfactant administration within the first 4 days after birth was 26% in the intervention group and 50% in the usual care group (P < .001).
The results remained significant after investigators adjusted for gestational age, birth weight, age when randomized, sex, delivery mode, and antenatal steroids. Rates of intubation for surfactant administration were lower for infants in the intervention group in all gestational age brackets except the youngest (23-24 weeks); all of these infants needed intubation. Respiratory support at days 3, 7, and 28 did not differ between study groups.
“Our study is the first to reveal the efficacy of an aerosolized surfactant delivery system that does not require a respiratory circuit interface,” the investigators wrote.
In previous trials of aerosolized surfactants, they noted, treatment was delivered with nasal continuous positive airway pressure. “By using a separate, pacifier interface, both the aerosol delivery and [nasal continuous positive airway pressure] flow can be managed independently, which should allow for safer patient care.”
Dr. Cummings and colleagues also acknowledged several important limitations of their study, including its nonmasked, nonblinded design, and that it enrolled few infants with less than 28 weeks’ gestation. It takes 1-2 hours to deliver aerosolized calfactant, and “we did not want to delay definitive treatment.”
In an editorial comment accompanying the study, Kirsten Glaser, MD, of the University of Leipzig (Germany), and Clyde Wright, MD of the University of Colorado at Denver, Aurora, called the results promising. “Importantly, application of surfactant aerosols was well tolerated by using a modified nebulizer with a pacifier interface.”
“Clinicians were aware that every infant randomly assigned to the nebulized surfactant arm received the intervention,” they wrote. “It is possible that clinicians delayed intubation and endotracheal surfactant instillation in this group, being biased by aerosolization and the hypothesis of lower risk of air leak and lung injury.”
Dr. Glaser and Dr. Wright further lamented that there were no formal criteria for administering surfactant therapy, and that the infants in the study might not be representative of those most in need of treatment. Today, bronchopulmonary dysplasia “primarily affects infants born at less than 28 weeks’ gestational age,” a minority of the infants recruited for this study, they wrote, urging further investigation in this patient group.
In an interview, neonatologist Roger F. Soll, MD, the H. Wallace Professor of Neonatology at the Larner College of Medicine at University of Vermont in Burlington, echoed the editorialists’ concerns that the study’s pragmatic design left a number of key questions unanswered. “It’s a promising study that laudably recruited on an order of magnitude more infants than any like it in the past,” Dr. Soll said. “And the kids who got the therapy seemed to do better, which is exciting. But with the broad entry criteria, the lack of formal diagnosis of RDS, and the outcome measures ultimately potentially biased by lack of blinding, it doesn’t give us the answers we need yet to consider aerosolized treatment.”
ONY Biotech, manufacturer of the study drug Infasurf, sponsored the trial. Dr. Cummings and one coauthor disclosed consulting arrangements with the sponsor, and another coauthor is an employee of the sponsor. The remaining investigators had no relevant financial disclosures. Dr. Glaser and Dr. Wright disclosed no conflicts of interest related to their editorial; Dr. Wright’s work was supported by the National Institutes of Health. Dr. Soll is president of the Vermont Oxford Network and coordinating editor of Cochrane Neonatal.
SOURCE: Cummings JJ et al. Pediatrics. 2020;146(5):e2020021576.
Nebulized delivery of surfactant reduced the need for intubation and liquid surfactant administration by half among newborns with signs of respiratory distress syndrome, according to results from a large randomized, multicenter trial.
Neonatologists have long sought alternatives to intubation for administering surfactant to newborns with respiratory distress syndrome (RDS). An effective noninvasive aerosolized treatment has remained elusive, with small clinical trials that have produced mixed results.
In research published in Pediatrics, James J. Cummings, MD, of Albany (N.Y.) Medical Center, and colleagues, randomized 457 infants (mean 33 weeks’ gestational age) with signs of RDS to either usual care or a nebulized bovine surfactant. Infants were recruited at 22 neonatal ICUs in the United States. Investigators were not blinded to treatment allocation and the decision to intubate was left up to the individual treating physician, because to do so, the authors wrote, would add “pragmatic strength” to the study, and “be ethically compliant with the infant’s best interest.”
Infants in the study received usual care or up to three treatments 4 or more hours apart of 35 mg/mL calfactant suspension, 210 mg phospholipid/kg body weight delivered into the mouth through a nebulizer modified with a pacifier. Dr. Cummings and colleagues found that intubation and liquid surfactant administration within the first 4 days after birth was 26% in the intervention group and 50% in the usual care group (P < .001).
The results remained significant after investigators adjusted for gestational age, birth weight, age when randomized, sex, delivery mode, and antenatal steroids. Rates of intubation for surfactant administration were lower for infants in the intervention group in all gestational age brackets except the youngest (23-24 weeks); all of these infants needed intubation. Respiratory support at days 3, 7, and 28 did not differ between study groups.
“Our study is the first to reveal the efficacy of an aerosolized surfactant delivery system that does not require a respiratory circuit interface,” the investigators wrote.
In previous trials of aerosolized surfactants, they noted, treatment was delivered with nasal continuous positive airway pressure. “By using a separate, pacifier interface, both the aerosol delivery and [nasal continuous positive airway pressure] flow can be managed independently, which should allow for safer patient care.”
Dr. Cummings and colleagues also acknowledged several important limitations of their study, including its nonmasked, nonblinded design, and that it enrolled few infants with less than 28 weeks’ gestation. It takes 1-2 hours to deliver aerosolized calfactant, and “we did not want to delay definitive treatment.”
In an editorial comment accompanying the study, Kirsten Glaser, MD, of the University of Leipzig (Germany), and Clyde Wright, MD of the University of Colorado at Denver, Aurora, called the results promising. “Importantly, application of surfactant aerosols was well tolerated by using a modified nebulizer with a pacifier interface.”
“Clinicians were aware that every infant randomly assigned to the nebulized surfactant arm received the intervention,” they wrote. “It is possible that clinicians delayed intubation and endotracheal surfactant instillation in this group, being biased by aerosolization and the hypothesis of lower risk of air leak and lung injury.”
Dr. Glaser and Dr. Wright further lamented that there were no formal criteria for administering surfactant therapy, and that the infants in the study might not be representative of those most in need of treatment. Today, bronchopulmonary dysplasia “primarily affects infants born at less than 28 weeks’ gestational age,” a minority of the infants recruited for this study, they wrote, urging further investigation in this patient group.
In an interview, neonatologist Roger F. Soll, MD, the H. Wallace Professor of Neonatology at the Larner College of Medicine at University of Vermont in Burlington, echoed the editorialists’ concerns that the study’s pragmatic design left a number of key questions unanswered. “It’s a promising study that laudably recruited on an order of magnitude more infants than any like it in the past,” Dr. Soll said. “And the kids who got the therapy seemed to do better, which is exciting. But with the broad entry criteria, the lack of formal diagnosis of RDS, and the outcome measures ultimately potentially biased by lack of blinding, it doesn’t give us the answers we need yet to consider aerosolized treatment.”
ONY Biotech, manufacturer of the study drug Infasurf, sponsored the trial. Dr. Cummings and one coauthor disclosed consulting arrangements with the sponsor, and another coauthor is an employee of the sponsor. The remaining investigators had no relevant financial disclosures. Dr. Glaser and Dr. Wright disclosed no conflicts of interest related to their editorial; Dr. Wright’s work was supported by the National Institutes of Health. Dr. Soll is president of the Vermont Oxford Network and coordinating editor of Cochrane Neonatal.
SOURCE: Cummings JJ et al. Pediatrics. 2020;146(5):e2020021576.
FROM PEDIATRICS
The future of pediatrics
Things will change. That is a constant. The practice of pediatrics will be different in the future. The pandemic has changed some things; mostly it has accelerated changes, advancements, improvements, and losses that were already occurring. Telemedicine will play a more prominent role in the future. The finances of solo and small-group practice have become more difficult.
As I wrote my prior column on the character traits/virtues of an admirable physician, I also began brainstorming this column on the traits of an admirable profession. Then the American Academy of Pediatrics’ virtual National Conference & Exhibition had many presentations encouraging pediatricians to adopt a conglomeration of activities in their offices. I became skeptical. Which should be selected? To make a wise choice, I review the major goals of medicine, which I have evolved to embrace as the quadruple aims.
First and hopefully always foremost, the health professions are dedicated to the health of their patients and, hopefully, the population at large. This trait dates to the Hippocratic Oath.
Second, physicians have a stewardship over a vast collection of knowledge, skills, resources, and funds. When I started my career, U.S. health care had increased from 6% of the gross domestic product to 9%, nearly twice that of other developed nations, and was expanding rapidly, contributing to widespread economic problems including the national debt. The health economists of the 1980’s made dire predictions that the nation was headed up to 12% of the GDP, which would cause the sky to start falling. Last I checked U.S. health care is approaching 18% of the GDP. The sky seems intact, although the oceans are rising and the hillsides are burning.
Managed care of the 1990s became focused on the consumer experience. Evaluations of physicians and nurses became dependent on consumer surveys. I recall one survey about the care I personally had received as day surgery. It was mostly about scheduling, being greeted on arrival, the waiting room, and other fluff. Only 1 of the over 20 questions had any bearing on whether I thought the diagnosis was correct, the treatment was effective, or my physician was competent. As a cancer patient, my priorities were not aligned with that survey’s concept of quality.
From 2008, I recall the Triple Aim: “Improving the U.S. health care system requires simultaneous pursuit of three aims: improving the experience of care, improving the health of populations, and reducing per capita costs of health care.”
Over the ensuing decade, physician wellness has been added to make a quadruple aim. If the system isn’t professionally rewarding, burnout occurs. Skills and experience are lost. The best and brightest are not attracted to the specialty. Quality goes down. So physicians must factor this into decisions about the future of pediatrics.
There are many social determinants of health that have large impacts on the population health of children, and it does not necessarily follow that I should spend my patient care time on those determinants. As a professional, I have a responsibility to ensure that I am treating important problems that match my extensive (and expensive) training, knowledge, skills, and experience.
I recently read a persuasive argument that caring for ADHD is an important and doable part of modern general pediatrics. I agree, but I agreed with the proponent’s idea 25 years ago when I joined a large group and saw my own ADHD patients. Change can be slow.
Pharmacology options for anxiety have become safer, more effective, and better understood in children. General pediatricians may now be able to provide important, earlier, and accessible intervention for pediatric anxiety and other mental health issues.
Food insecurity is a worsening issue during the pandemic, but not one which I have specialized abilities to address. A brochure listing available local resources could be posted in waiting rooms and exam rooms. Is spending time asking about it during a visit the best use of a pediatrician’s time? That is a choice a professional needs to make. It may depend on your patient panel and community resources. In the past, I was more inclined to focus on medical care and donate the extra income to my church’s food bank. But the world has changed. Perhaps the pediatrician’s office of the 2020s is a department store, with social workers, psychologists, and therapists located under the same roof. It reminds me of the Mayo model. Wealthy people would travel to Rochester for an executive physical. That physical would frequently recommend seeing a couple specialists before leaving town. It is an effective model but also luxurious.
Racism causes major harms, both to physical health and mental health. Is asking about it a wise use of limited time for well-child visits? What resources will you offer?
Climate change, hurricanes, and wildfires are harming children. Is debating the issue with your patient’s parents productive? I am zealous about the topic. I spend considerable time and money promoting the credibility of science within various religious organizations, but I try to avoid bringing politics into my interactions with patients.
As a professional, your choices may be different. Many people are telling you what you should care about. The executive well-child visit would be beneficial, but it would also take 2 hours. Don’t be misled into spending too much effort on issues not in your expertise. Choose wisely.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at [email protected].
Things will change. That is a constant. The practice of pediatrics will be different in the future. The pandemic has changed some things; mostly it has accelerated changes, advancements, improvements, and losses that were already occurring. Telemedicine will play a more prominent role in the future. The finances of solo and small-group practice have become more difficult.
As I wrote my prior column on the character traits/virtues of an admirable physician, I also began brainstorming this column on the traits of an admirable profession. Then the American Academy of Pediatrics’ virtual National Conference & Exhibition had many presentations encouraging pediatricians to adopt a conglomeration of activities in their offices. I became skeptical. Which should be selected? To make a wise choice, I review the major goals of medicine, which I have evolved to embrace as the quadruple aims.
First and hopefully always foremost, the health professions are dedicated to the health of their patients and, hopefully, the population at large. This trait dates to the Hippocratic Oath.
Second, physicians have a stewardship over a vast collection of knowledge, skills, resources, and funds. When I started my career, U.S. health care had increased from 6% of the gross domestic product to 9%, nearly twice that of other developed nations, and was expanding rapidly, contributing to widespread economic problems including the national debt. The health economists of the 1980’s made dire predictions that the nation was headed up to 12% of the GDP, which would cause the sky to start falling. Last I checked U.S. health care is approaching 18% of the GDP. The sky seems intact, although the oceans are rising and the hillsides are burning.
Managed care of the 1990s became focused on the consumer experience. Evaluations of physicians and nurses became dependent on consumer surveys. I recall one survey about the care I personally had received as day surgery. It was mostly about scheduling, being greeted on arrival, the waiting room, and other fluff. Only 1 of the over 20 questions had any bearing on whether I thought the diagnosis was correct, the treatment was effective, or my physician was competent. As a cancer patient, my priorities were not aligned with that survey’s concept of quality.
From 2008, I recall the Triple Aim: “Improving the U.S. health care system requires simultaneous pursuit of three aims: improving the experience of care, improving the health of populations, and reducing per capita costs of health care.”
Over the ensuing decade, physician wellness has been added to make a quadruple aim. If the system isn’t professionally rewarding, burnout occurs. Skills and experience are lost. The best and brightest are not attracted to the specialty. Quality goes down. So physicians must factor this into decisions about the future of pediatrics.
There are many social determinants of health that have large impacts on the population health of children, and it does not necessarily follow that I should spend my patient care time on those determinants. As a professional, I have a responsibility to ensure that I am treating important problems that match my extensive (and expensive) training, knowledge, skills, and experience.
I recently read a persuasive argument that caring for ADHD is an important and doable part of modern general pediatrics. I agree, but I agreed with the proponent’s idea 25 years ago when I joined a large group and saw my own ADHD patients. Change can be slow.
Pharmacology options for anxiety have become safer, more effective, and better understood in children. General pediatricians may now be able to provide important, earlier, and accessible intervention for pediatric anxiety and other mental health issues.
Food insecurity is a worsening issue during the pandemic, but not one which I have specialized abilities to address. A brochure listing available local resources could be posted in waiting rooms and exam rooms. Is spending time asking about it during a visit the best use of a pediatrician’s time? That is a choice a professional needs to make. It may depend on your patient panel and community resources. In the past, I was more inclined to focus on medical care and donate the extra income to my church’s food bank. But the world has changed. Perhaps the pediatrician’s office of the 2020s is a department store, with social workers, psychologists, and therapists located under the same roof. It reminds me of the Mayo model. Wealthy people would travel to Rochester for an executive physical. That physical would frequently recommend seeing a couple specialists before leaving town. It is an effective model but also luxurious.
Racism causes major harms, both to physical health and mental health. Is asking about it a wise use of limited time for well-child visits? What resources will you offer?
Climate change, hurricanes, and wildfires are harming children. Is debating the issue with your patient’s parents productive? I am zealous about the topic. I spend considerable time and money promoting the credibility of science within various religious organizations, but I try to avoid bringing politics into my interactions with patients.
As a professional, your choices may be different. Many people are telling you what you should care about. The executive well-child visit would be beneficial, but it would also take 2 hours. Don’t be misled into spending too much effort on issues not in your expertise. Choose wisely.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at [email protected].
Things will change. That is a constant. The practice of pediatrics will be different in the future. The pandemic has changed some things; mostly it has accelerated changes, advancements, improvements, and losses that were already occurring. Telemedicine will play a more prominent role in the future. The finances of solo and small-group practice have become more difficult.
As I wrote my prior column on the character traits/virtues of an admirable physician, I also began brainstorming this column on the traits of an admirable profession. Then the American Academy of Pediatrics’ virtual National Conference & Exhibition had many presentations encouraging pediatricians to adopt a conglomeration of activities in their offices. I became skeptical. Which should be selected? To make a wise choice, I review the major goals of medicine, which I have evolved to embrace as the quadruple aims.
First and hopefully always foremost, the health professions are dedicated to the health of their patients and, hopefully, the population at large. This trait dates to the Hippocratic Oath.
Second, physicians have a stewardship over a vast collection of knowledge, skills, resources, and funds. When I started my career, U.S. health care had increased from 6% of the gross domestic product to 9%, nearly twice that of other developed nations, and was expanding rapidly, contributing to widespread economic problems including the national debt. The health economists of the 1980’s made dire predictions that the nation was headed up to 12% of the GDP, which would cause the sky to start falling. Last I checked U.S. health care is approaching 18% of the GDP. The sky seems intact, although the oceans are rising and the hillsides are burning.
Managed care of the 1990s became focused on the consumer experience. Evaluations of physicians and nurses became dependent on consumer surveys. I recall one survey about the care I personally had received as day surgery. It was mostly about scheduling, being greeted on arrival, the waiting room, and other fluff. Only 1 of the over 20 questions had any bearing on whether I thought the diagnosis was correct, the treatment was effective, or my physician was competent. As a cancer patient, my priorities were not aligned with that survey’s concept of quality.
From 2008, I recall the Triple Aim: “Improving the U.S. health care system requires simultaneous pursuit of three aims: improving the experience of care, improving the health of populations, and reducing per capita costs of health care.”
Over the ensuing decade, physician wellness has been added to make a quadruple aim. If the system isn’t professionally rewarding, burnout occurs. Skills and experience are lost. The best and brightest are not attracted to the specialty. Quality goes down. So physicians must factor this into decisions about the future of pediatrics.
There are many social determinants of health that have large impacts on the population health of children, and it does not necessarily follow that I should spend my patient care time on those determinants. As a professional, I have a responsibility to ensure that I am treating important problems that match my extensive (and expensive) training, knowledge, skills, and experience.
I recently read a persuasive argument that caring for ADHD is an important and doable part of modern general pediatrics. I agree, but I agreed with the proponent’s idea 25 years ago when I joined a large group and saw my own ADHD patients. Change can be slow.
Pharmacology options for anxiety have become safer, more effective, and better understood in children. General pediatricians may now be able to provide important, earlier, and accessible intervention for pediatric anxiety and other mental health issues.
Food insecurity is a worsening issue during the pandemic, but not one which I have specialized abilities to address. A brochure listing available local resources could be posted in waiting rooms and exam rooms. Is spending time asking about it during a visit the best use of a pediatrician’s time? That is a choice a professional needs to make. It may depend on your patient panel and community resources. In the past, I was more inclined to focus on medical care and donate the extra income to my church’s food bank. But the world has changed. Perhaps the pediatrician’s office of the 2020s is a department store, with social workers, psychologists, and therapists located under the same roof. It reminds me of the Mayo model. Wealthy people would travel to Rochester for an executive physical. That physical would frequently recommend seeing a couple specialists before leaving town. It is an effective model but also luxurious.
Racism causes major harms, both to physical health and mental health. Is asking about it a wise use of limited time for well-child visits? What resources will you offer?
Climate change, hurricanes, and wildfires are harming children. Is debating the issue with your patient’s parents productive? I am zealous about the topic. I spend considerable time and money promoting the credibility of science within various religious organizations, but I try to avoid bringing politics into my interactions with patients.
As a professional, your choices may be different. Many people are telling you what you should care about. The executive well-child visit would be beneficial, but it would also take 2 hours. Don’t be misled into spending too much effort on issues not in your expertise. Choose wisely.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at [email protected].
Treatments for COVID-19: Update for hospitalists
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Most patients with COVID-19 will have a mild presentation and not require hospitalization or any treatment. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19, which includes pneumonia, hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and septic shock.
Currently, there is no clinically proven specific antiviral treatment for COVID-19. A few antivirals and treatment modalities have been studied and used, with the hope of decreasing mortality and improving recovery time for those with moderate to severe cases of COVID-19.
Remdesivir
The antiviral remdesivir was the second drug to receive emergency use authorization by the Food and Drug Administration for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with an oxygen saturation less than 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO).
Remdesivir is a nucleotide analogue that has shown in vitro antiviral activity against a range of RNA viruses. It acts by causing premature termination of viral RNA transcription. Remdesivir is administered intravenously and the recommended dose is 200 mg on day 1, followed by 100 mg daily for various time courses.
A few clinical studies have reported benefits of remdesivir rather than no remdesivir for treatment of severe COVID-19 in hospitalized patients. The Infectious Diseases Society of America (IDSA) recommends 5 days of remdesivir in patients with severe COVID-19 on noninvasive supplemental oxygen and 10 days treatment for those on mechanical ventilation and ECMO. In a randomized, uncontrolled, phase 3 trial, investigators compared 5-day (n = 200) versus 10-day (n = 197) courses of remdesivir in patients with severe COVID-19. Clinical data revealed no differences in outcomes in the two groups.
Common reported adverse effects of the drug include elevated alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) and gastrointestinal symptoms including nausea, vomiting, and hematochezia. There is insufficient data on using remdesivir in patients requiring dialysis.
Corticosteroids
Is dexamethasone effective for treating COVID-19? In the early days of the COVID-19 pandemic, corticosteroids were not recommended with the fear that, if started too soon, you could blunt the body’s natural defense system and that could allow the virus to thrive. Recent clinical data has shown clinical benefits and decreased mortality with the use of dexamethasone in patients with severe COVID-19 infection because glucocorticoids may modulate inflammation-mediated lung injury and reduce progression to respiratory failure and death.
The Recovery Trial was an open label study which used 6-mg once-daily doses of dexamethasone for up to 10 days or until hospital discharge if sooner. The study concluded that the use of dexamethasone for up to 10 days in hospitalized patients with severe COVID-19 resulted in lower 28-day mortality than usual care.
Dexamethasone is recommended in COVID-19 patients who require supplemental oxygen. If dexamethasone is not available, alternative forms of steroids – prednisone, methylprednisolone, or hydrocortisone – can be used. However, there is no clear evidence that the use of other steroids provides the same benefit as dexamethasone.
Both the IDSA and National Institutes of Health guidelines have recommended the use of steroids. However, clinicians should closely monitor the adverse effects like hyperglycemia, secondary infections, psychiatric effects, and avascular necrosis.
Convalescent plasma
Convalescent plasma is a blood product believed to provide passive antibody therapy through the transmission of neutralizing viral antibodies. Convalescent plasma has been used for decades for different viral infections including the treatment of H1N1 influenza virus, polio, chicken pox, measles, SARS-CoV-1, and MERS-CoV.
On Aug. 23, 2020, the FDA issued an emergency use authorization for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients. The FDA recommends neutralizing antibodies of at least 1:160. However, such assays have not been widely available and titers in plasma have often not been assessed prior to infusion.
There is no current standard recommended dosing. Most study protocols infuse 1-2 units of convalescent plasma for persons with COVID-19.
There is insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Existing data suggest that, if a benefit exists, convalescent plasma is most useful when given early and with a high titer of neutralizing antibodies.
The adverse effects of convalescent plasma is very similar to the receipt of other blood products, including allergic reactions to the plasma, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and acquisition of infections, though the latter is rare because of the rigorous screening process.
Tocilizumab
Tocilizumab is a recombinant humanized monoclonal antibody that binds to interleukin (IL)-6 receptors. Tocilizumab is currently FDA approved for the treatment of severe or life-threatening cytokine release syndrome that is associated with chimeric antigen–receptor (CAR) T-cell therapy and for the treatment of rheumatologic disorders.
The interest in using tocilizumab to treat persons with COVID-19 is based on the observations that a subset of patients with COVID-19 develop a severe inflammatory response that can result in cytokine storm resulting in ARDS, multiorgan failure, and potentially death. Very high levels of IL-6 have been observed in these individuals, thereby suggesting IL-6 may play a central role in the acute clinical decompensation seen with severe COVID-19.
The optimal dosing of tocilizumab in patients with COVID-19 is not known. The FDA recommends dosing of tocilizumab for cytokine release syndrome should not exceed 800 mg. There is limited data about the potential benefit of tocilizumab in patients with COVID-19. The COVACTA trial showed no difference between tocilizumab and placebo in regard to mortality. The time to hospital discharge was shorter in patients treated with tocilizumab; however, the difference was not statistically significant.
Reported adverse effects of tocilizumab include increase in ALT and AST, increased risk of serious infections (especially tuberculosis and invasive fungal infections), reactivation of hepatitis B virus, and rare reports of gastrointestinal perforation.
Hydroxychloroquine
Hydroxycholoroquine (HCQ) and its sister drug chloroquine, have been used for many decades as treatment for malaria and autoimmune diseases. HCQ gained widespread popularity in the early days of the COVID-19 pandemic when clinical studies showed that it had significant in vitro activity against SARS-CoV-2, which provided the rationale for its use in the treatment and prevention of COVID-19 infection.
It was the first drug that was authorized for emergency use by the FDA during the COVID-19 pandemic. However, On June 15, 2020, because of accumulating harmful data, the FDA revoked the emergency authorization use of HCQ as a COVID-19 treatment.
Randomized controlled trials showed that patients treated with HCQ experienced a longer hospital stay with increase in mortality rates and increased likelihood of being placed on mechanical ventilation. In addition, studies revealed an increase in QT prolongation in patients treated with HCQ, especially when coadministered with azithromycin, which can lead to torsades de pointes, ventricular tachycardia, and sudden cardiac death.
The IDSA and National Institutes of Health, both recommend against the use of hydroxychloroquine with or without azithromycin to treat COVID-19 because the harms outweigh the benefits, even if high quality RCTs were to become available in the future.
Other drugs
There have been experimental studies on other medications for the treatment of COVID-19, including losartan, amlodipine, ivermectin, famotidine, Anakinra, Bruton’s tyrosine kinase inhibitors such as ibrutinib, and Janus kinase inhibitors, such as tofacitinib. Additionally, a few supplements such as vitamin C, vitamin D, and zinc have been used in both inpatient and outpatient settings for COVID-19 treatment. Polyclonal antibodies are being investigated in phase 3 trials. However, the data is insufficient, and the effectiveness of these drugs is unknown. The COVID-19 treatment guidelines panel recommends against the use of these treatment modalities.
Dr Tiyouh is an infectious diseases physician at Keystone Health in Chambersburg, Pa. Dr. Tenneti completed medical school at Vydehi Institute of Medical Sciences and Research Centre in Karnataka, India, and is interested in pursuing internal medicine residency. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg Hospital and Waynesboro (Pa.) Hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson, and a member of the editorial advisory board for The Hospitalist.
Sources
Goldman JD et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMoa2015301.
Beigel JH et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569-78.
National Institutes of Health. COVID-19 Treatment Guidelines.
Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19.
Joyner et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791-7.
Luo P et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020 Jul;92(7):814-8.
Centers for Disease Control and Prevention. Healthcare Workers: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
University of Washington. COVID-19 Treatments: Prescribing Information, Clinical Studies, and Slide Decks.
Purple toe lesion
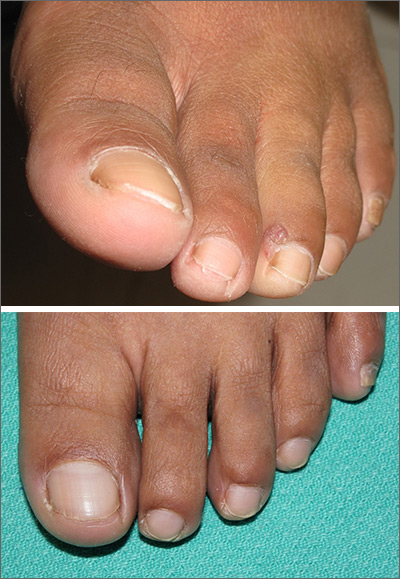
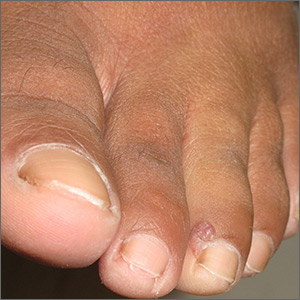
An excisional biopsy revealed that this was an eccrine poroma, a benign neoplasm of sweat gland tissue in the epidermis.
This lesion was clearly not a wart, as it lacked the common verrucous and keratotic features one would expect, and it did not respond to wart treatments. Other diagnoses that might be considered with a lesion like this include pyogenic granuloma, periungual fibroma, and squamous cell carcinoma.
Poromas are well demarcated papules that grow slowly or are stable in size. They are most commonly flesh colored and smooth, but poromas may also appear verrucous, pigmented, or ulcerated. They are usually found on acral skin—particularly the palms or soles. Due to their location, poromas bleed easily, which is what usually prompts patients to seek care. Friable lesions can mimic acral melanoma.
Poromas occur most often in patients over 40 years of age and are evenly distributed among sexes and skin types. Cases have been associated with trauma, radiation, and chemotherapy. Although exceedingly rare, malignant transformation can occur in the form of eccrine porocarcinoma—a larger tumor that can grow rapidly and that has metastatic potential.
Treatment is optional—but desirable—when lesions are painful or bleed easily. Surgical excision is curative. Shave biopsy or curettage coupled with electrocautery of the base is also curative. Recurrence rates are very low with either method of treatment.
In this case, an excisional biopsy of the lateral nail fold was both diagnostic and curative (second image). The patient remained clear 9 months after treatment.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.

An excisional biopsy revealed that this was an eccrine poroma, a benign neoplasm of sweat gland tissue in the epidermis.
This lesion was clearly not a wart, as it lacked the common verrucous and keratotic features one would expect, and it did not respond to wart treatments. Other diagnoses that might be considered with a lesion like this include pyogenic granuloma, periungual fibroma, and squamous cell carcinoma.
Poromas are well demarcated papules that grow slowly or are stable in size. They are most commonly flesh colored and smooth, but poromas may also appear verrucous, pigmented, or ulcerated. They are usually found on acral skin—particularly the palms or soles. Due to their location, poromas bleed easily, which is what usually prompts patients to seek care. Friable lesions can mimic acral melanoma.
Poromas occur most often in patients over 40 years of age and are evenly distributed among sexes and skin types. Cases have been associated with trauma, radiation, and chemotherapy. Although exceedingly rare, malignant transformation can occur in the form of eccrine porocarcinoma—a larger tumor that can grow rapidly and that has metastatic potential.
Treatment is optional—but desirable—when lesions are painful or bleed easily. Surgical excision is curative. Shave biopsy or curettage coupled with electrocautery of the base is also curative. Recurrence rates are very low with either method of treatment.
In this case, an excisional biopsy of the lateral nail fold was both diagnostic and curative (second image). The patient remained clear 9 months after treatment.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

An excisional biopsy revealed that this was an eccrine poroma, a benign neoplasm of sweat gland tissue in the epidermis.
This lesion was clearly not a wart, as it lacked the common verrucous and keratotic features one would expect, and it did not respond to wart treatments. Other diagnoses that might be considered with a lesion like this include pyogenic granuloma, periungual fibroma, and squamous cell carcinoma.
Poromas are well demarcated papules that grow slowly or are stable in size. They are most commonly flesh colored and smooth, but poromas may also appear verrucous, pigmented, or ulcerated. They are usually found on acral skin—particularly the palms or soles. Due to their location, poromas bleed easily, which is what usually prompts patients to seek care. Friable lesions can mimic acral melanoma.
Poromas occur most often in patients over 40 years of age and are evenly distributed among sexes and skin types. Cases have been associated with trauma, radiation, and chemotherapy. Although exceedingly rare, malignant transformation can occur in the form of eccrine porocarcinoma—a larger tumor that can grow rapidly and that has metastatic potential.
Treatment is optional—but desirable—when lesions are painful or bleed easily. Surgical excision is curative. Shave biopsy or curettage coupled with electrocautery of the base is also curative. Recurrence rates are very low with either method of treatment.
In this case, an excisional biopsy of the lateral nail fold was both diagnostic and curative (second image). The patient remained clear 9 months after treatment.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
Virtual AHA 2020 may influence template for postpandemic scientific sessions
Cardiologists are already old hands at virtual meetings this year and are fast becoming experts on Zoom and other teleconferencing platforms, if not on how to unmute their microphones.
With expectations perhaps elevated and the new communications genre’s novelty on the wane, the American Heart Association (AHA) Scientific Sessions 2020 has a chance to both innovate with familiar formats and captivate with the field’s latest research findings.
Although the virtual AHA 2020 might not satisfy longings for face-to-face networking, shop talk, or kidding around over coffee, it will feature many traditional elements of the live conferences adapted for ear buds and small screens. They include late-breaking science (LBS) presentations and panel discussions, poster and live oral abstract presentations, meet-the-trialist talks, fireside-chat discussion forums, early career events, and satellite symposia.
The event may well hold lessons for future iterations of AHA Scientific Sessions in the postpandemic world, which some foresee as, potentially, an amalgam of the time-honored live format and a robust, complementary online presence.
“I can’t commit to exactly what AHA sessions will look like next November; I think that’s still being looked at,” the organization’s president-elect Donald M. Lloyd-Jones, MD, ScM, chair of the AHA Committee on Scientific Sessions Programming, told theheart.org | Medscape Cardiology.
There’s no debating that a live conference is valuable “for career networking and other opportunities, so I don’t think we can do without it. That has to be an important part of it,” he said. “When we can safely, of course.”
Still, “the virtual platform democratizes, right? I mean, it just allows greater access for a broader audience, and I think that’s important, too,” said Lloyd-Jones, MD, Northwestern University Feinberg School of Medicine, Chicago.
“I don’t think we’ll ever go completely back to it being all in-person,” he said. “I think the world has changed, and we’ll have to adapt our platforms to recognize that.”
Online, at least, meeting registrants will get a better look at Anthony Fauci, MD, than one might from the middle rows of a vast ballroom-turned-auditorium. Fauci is scheduled to speak on “Public Health and Scientific Challenges” during the Main Event Session “Latest Insights on COVID 19 and Cardiovascular Disease,” slated for the meeting’s final day.
Fauci has directed the National Institute of Allergy and Infectious Diseases (NIAID) since 1984, and has been celebrated for his leadership roles in the battles against AIDS and Ebola virus. Today, his name is close to a household word for his service as a prominent though embattled member of the White House Coronavirus Task Force.
The virtual AHA sessions will feature a core collection of LBS presentations from often high-profile clinical trials and other studies the organization deems worthy of special attention. There are nine such presentations arrayed across the meeting’s five days — from Friday, November 13 to Tuesday, November 17 — at times listed in this story and throughout the AHA Scientific Session program synched with the Central Standard Time (CST) zone of the AHA’s home office in Dallas.
Late-Breaking Science 1. Friday, November 13, 10:30 AM - 11:30 AM CST
The LBS sessions launch with the GALACTIC-HF trial, which — the world recently learned — may expand the burgeoning list of meds shown to improve clinical outcomes in chronic heart failure (HF) with reduced ejection fraction (HFrEF).
In cursory top-line results announced last month, those in the trial of more than 8000 patients who were randomly assigned to receive omecamtiv mecarbil (Amgen/Cytokinetics/Servier) showed a slight but significant benefit for the primary end point of cardiovascular (CV) death or HF events. The hazard ratio (HR), compared with standard care, was 0.92 (95% CI, 0.86 - 0.99; P = .025), noted a press release from Amgen.
Among the announcement’s few other details was a short take on safety outcomes: no difference in risk for “adverse events, including major ischemic cardiac events,” between the active and control groups. The presentation is sure to provide further insights and caveats, if any, along with other information crucial to the study’s interpretation.
Next on the schedule is the closely watched AFFIRM-AHF, billed as the first major outcomes trial of iron administration to iron-deficient patients with acute HF. It randomly assigned more than 1000 such patients to receive IV ferric carboxymaltose or a placebo. The first dose was given in-hospital and subsequent doses at home for 24 weeks or until patients were no longer iron deficient. They were followed to 1 year for the primary end point of recurrent HF hospitalizations or CV death.
The session wraps with the VITAL Rhythm trial, a substudy of the doubly randomized VITAL trial that explored the effects of vitamin D and omega-3 fatty acid supplementation on CV and cancer risk in more than 25,000 patients in the community. The substudy explored the effects of two active therapies, a preparation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Omacor, Reliant Pharmaceuticals) or vitamin D3 supplements, on new-onset atrial fibrillation (AF) as the primary end point; it also looked at risk for sudden death.
Late-Breaking Science 2. Friday, November 13, 12:00 PM - 1:00 PM CST
Dominating the session in two presentations, the (TIPS)-3 trial explored a polypill primary-prevention strategy and daily aspirin with vitamin D supplementation in three separate placebo-controlled comparisons in more than 5700 “intermediate risk” participants 55 years and older, mostly in developing countries.
The daily polypill in this trial is a combination of hydrochlorothiazide 25 mg, atenolol 100 mg, ramipril 10 mg, and simvastatin 40 mg; aspirin was given at 75 mg daily and vitamin D at 60,000 IU monthly.
The participants are followed for a primary end point composed of major CV disease, HF, resuscitated cardiac arrest, or ischemia-driven revascularization for the polypill comparison; CV events or cancer for the aspirin comparison; and fracture risk for the vitamin D component of the trial.
In the Swedish Cardiopulmonary Bioimage Study (SCAPIS), presented third in the session, a random sample of adults from throughout Sweden, projected at about 30,000, underwent a 2-day evaluation for metabolic risk factors plus ultrasound and coronary and lung CT scans. The group has been followed for risks for myocardial infarction (MI), sudden death, and other cardiac diseases; and chronic obstructive pulmonary disease (COPD) and other lung disorders.
Late-Breaking Science 3. Saturday, November 14, 12:00 PM - 1:00 PM CST
The field may learn more mechanistically about MI associated with nonobstructed coronary arteries (MINOCA) than ever before from the Heart Attack Research Program-Imaging Study (HARP). The observational study is enrolling a projected 450 patients with suspected MI and ischemic symptoms who were referred for cardiac catheterization.
Their evaluation includes coronary optical coherence tomographic (OCT) scanning and cardiac magnetic resonance (CMR) imaging for evidence of coronary plaque disruption as the primary end point. The patients are to be followed for 10 years for a composite of death, unstable angina, stroke, recurrent MI, diagnostic or interventional catheterization, and cardiac hospitalization.
The major direct oral anticoagulant (DOAC) comparisons with warfarin in atrial fibrillation (AF) didn’t include many patients with prosthetic valve implants. In contrast, the RIVER trial enrolled 1005 adults with either persistent or paroxysmal AF and bioprosthetic mitral valves and assigned them to rivaroxaban 20 mg or the vitamin K antagonist.
The presentation will include the noninferiority primary outcome of major clinical events, which is stroke, transient ischemic attack (TIA), major bleeding, death from any cause, valve thrombosis, other systemic embolism, or HF hospitalization over 12 months.
This session also includes ALPHEUS, a trial pitting ticagrelor (Brilinta/Brilique, AstraZeneca) against mainstay clopidogrel in a setting that is mostly uncharted for such comparisons, elective percutaneous coronary intervention (PCI).
About 1900 patients with stable coronary disease were randomly assigned to a month of treatment with either agent on top of continuous aspirin. The primary end point is PCI-related MI or myocardial injury within 48 hours of the procedure.
Late-Breaking Science 4. Sunday, November 15, 9:00 AM - 10:00 AM CST
The Self-Assessment Method for Statin Side-effects Or Nocebo (SAMSON) trial may be one of the AHA 2020 frontrunners for early buzz and anticipation. So it’s with some irony that it’s also among the smallest of the LBS studies, at 60 patients, which was nonetheless considered sufficient due to its unusual design.
SAMSON is the latest and perhaps most rigorous attempt to clarify whether symptoms, especially muscle pain or discomfort, attributed to statins by many patients are pharmacologic in origin or, rather, a nocebo effect from negative expectations about statin side effects.
The study patients, all of whom had previously halted statins because of side effects, were assigned to follow three separate regimens, each for month, in a randomized order; they did that four times, for a total of 12 months. The regimens consisted of atorvastatin 20 mg daily, a placebo, or neither.
Patients kept daily logs of any perceived side effects. Parity between side effects experienced on the statin and the placebo would point to a nocebo effect, whereas a significant excess on atorvastatin would suggest they are direct drug effects.
The session also features two randomized trials each on a unique omega-3 fatty acid preparation for either secondary prevention or high-risk primary prevention, in both cases compared with a corn-oil placebo.
The Omega-3 Fatty Acids in Elderly Patients with Myocardial Infarction (OMEMI) trial randomly assigned more than 1000 elderly post-MI patients to take Pikasol (Orkla Care) at 1.8 g EPA and DHA per day or the placebo. It looked for all-cause mortality, nonfatal MI, stroke, revascularization, or hospitalization for new or worsened HF over 24 months.
The STRENGTH trial, with a planned enrollment of about 13,000 high-vascular-risk patients, looked primarily at the effect of daily treatment with Epanova (AstraZeneca), which also contains DHA and EPA, on the composite of CV death, nonfatal MI or stroke, coronary revascularization, and hospitalization for unstable angina. The trial was halted early for low likelihood of benefit, AstraZeneca announced in January of this year.
Late-Breaking Science 5. Sunday, November 15, 7:15 PM - 8:30 PM CST
Slated for the session is the primary analysis of the PIONEER 3 trial, conducted in the United States, Europe, and Japan. It compared the BuMA Supreme biodegradable drug-coated stent (SinoMed) with the durable Xience (Abbott Vascular) and Promus (Boston Scientific) drug-eluting stents. The trial followed more than 1600 patients treated for chronic stable angina or acute coronary syndrome (ACS) for the 1-year composite of cardiac death, target-vessel-related MI, and clinically driven target-lesion revascularization.
Late-Breaking Science 6. Monday, November 16, 9:00 AM - 10:00 AM CST
The EARLY-AF trial enrolled 303 patients with symptomatic paroxysmal or persistent AF suitable for catheter ablation, assigning them to pulmonary vein isolation (PVI) by cryoablation using the Arctic Front (Medtronic) system or antiarrhythmic drug therapy for rhythm control. The primary end point is time to recurrence of AF, atrial flutter, or atrial tachycardia, whether symptomatic or asymptomatic, as determined by implantable loop recorder. Patients will also be followed for symptoms and arrhythmia burden.
Also in the session, the SEARCH-AF study randomized almost 400 patients undergoing cardiac surgery who were engaged subacutely with one of two commercial portable cardiac rhythm monitoring devices (CardioSTAT, Icentia; or SEEQ, Medtronic) or, alternatively, to receive usual postoperative care
The patients, considered to be at high risk for stroke with no history of AF, were followed for the primary end point of cumulative burden of AF or atrial flutter exceeding 6 minutes or documentation of either arrhythmia by 12-lead ECG within 30 days.
Two other studies in the session look at different approaches to AF screening, one using a handheld ECG monitor in the primary care setting and the other wearable monitors in the form of a patch or wristband. The VITAL-AF presentation is titled “Screening for Atrial Fibrillation in Older Adults at Primary Care Visits Using Single Lead Electrocardiograms.” The other presentation, on the study mSToPS, is called “Three-Year Clinical Outcomes in a Nationwide, Randomized, Pragmatic Clinical Trial of Atrial Fibrillation Screening — Mhealth Screening to Prevent Strokes.”
Late-Breaking Science 7. Monday, November 16, 7:00 PM - 8:30 PM CST
In the randomized FIDELIO-DKD trial with more than 5700 patients with type 2 diabetes and associated kidney disease, those assigned to the novel mineralocorticoid receptor antagonist (MRA) finerenone (Bayer) showed an 18% drop in risk for adverse renal events, including death from renal causes (P = .001), over a median of 2.6 years. That primary outcome was previously presented in detail at a nephrology meeting and published in the New England Journal of Medicine in October.
Patients on the MRA showed a similar reduction in a composite CV-event end point, it was also reported at that time. A follow-up presentation at the AHA sessions promises to dive deeper into the trial’s CV outcomes.
In the RAPID-CTCA study, slated next for the session, 1749 patients with suspected or confirmed intermediate-risk ACS were randomly assigned to undergo computed tomographic coronary angiography (CTCA) for guiding treatment decisions or a standard-of-care strategy. It followed patients for the primary end point of death or nonfatal MI over 1 year.
Rilonacept (Arcalyst, Kiniksa/Regeneron) is an interleukin-1α and -1β inhibitor used in several autoinflammatory diseases that went unsuccessfully before regulators for the treatment of gout. The RHAPSODY trial has now explored its use against recurrent pericarditis in a randomized trial that entered 86 patients 12 years and older who had previously experienced at least three episodes.
In top-line results reported to investors in June, patients assigned to receive the drug instead of placebo in weekly injections showed a 96% drop in risk for pericarditis recurrence and “no or minimal pain” on more than 90% of days in the trial. A full presentation is expected during this LBS session.
Also on the schedule is the THALES study, which led the US Food and Drug Administration (FDA) to expand indications for ticagrelor to include stroke prevention in patients with a history of acute ischemic stroke or high-risk TIA based on the trial’s primary results published in July.
In THALES, more than 11,000 patients with mild to moderate acute noncardiogenic ischemic stroke or TIA were randomly assigned within 24 hours to start on daily aspirin with or without ticagrelor given as a 180 mg loading dose followed by 90 mg twice daily for 30 days.
At the end of a month, it was reported, those on dual antiplatelet therapy showed a 17% risk reduction (P = .02) for the primary end point of stroke or death, at the cost of a slight but significant increase in “severe” bleeding (0.5% vs 0.1%; P = .001).
The session is to conclude with two related studies that fell victim in part to the COVID-19 pandemic, both of which explored sotagliflozin (Zynquista, Sanofi/Lexicon), an inhibitor of both sodium-glucose cotransporters 1 and 2 (SGLT1 and SGLT2, respectively) in patients with type 2 diabetes.
SOLOIST-WHF had entered 1222 such patients hospitalized with urgent or worsening HF at 466 centers and randomly assigned them to receive sotagliflozin or placebo; they were followed for the composite of CV death or HF events. SCORED reached an enrollment of 10,584 patients with diabetes and chronic kidney disease at 754 hospitals, following them for the same primary end point.
Lexicon announced in March that the trials would be “closed out early” because of the unavailability of funding “together with uncertainties relating to the COVID-19 pandemic on the trials.” The LBS presentation is expected to include analyses of available data; SOLOIST-WHF launched in summer 2018 and SCORED began in November 2017.
Late-Breaking Science 8. Tuesday, November 17, 9:00 AM - 10:00 AM CST
Most of this LBS session is devoted to the AHA COVID-19 Cardiovascular Disease registry, which is looking at the hospital journey, clinical course, and outcomes of patients hospitalized with SARS-CoV-2 infections at centers participating in the organization’s Get With The Guidelines (GWTG) quality-improvement program. As of September, the registry included data from more than 15,000 patients.
Scheduled presentations include a summary of the registry’s design and initial results; an analysis of racial and ethnic variation in therapy and clinical outcomes; an exploration of how body mass index influenced outcomes, including death, use of mechanical ventilation, and cardiovascular end points, in patients with COVID-19; and a deep dive into the relation between CV disease and clinical outcomes in the cohort.
The last of this LBS block’s five talks will cover the randomized Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) trial, which compared vaccination with high-dose trivalent influenza vaccine or a standard-dose quadrivalent vaccine in 5388 adults with a history of hospitalization for either MI or HF. Patients were required to have at least one other CV risk factor, such as older age, reduced left ventricular ejection fraction, or diabetes.
INVESTED tracked the patients at 190 centers across an initial pilot flu season and three subsequent flu seasons for the primary end point of death from any cause or cardiopulmonary hospitalization.
The trial is one of at least three that have been looking at the effect of flu vaccination on cardiovascular outcomes; results from the other two — IAMI, with more than 2500 participants, and RCT-IVVE, with an enrollment of 4871 — are planned for presentation in 2021, theheart.org | Medscape Cardiology recently reported.
Late-Breaking Science 9. Tuesday, November 17, 12:00 PM - 1:00 PM CST
The conference’s concluding LBS session features three studies that relied on technologic strategies for modifying patient compliance and other care behaviors and one that used human-centered design principles to develop a group-care model aimed improving the management of diabetes, hypertension, and other noncommunicable diseases in economically disadvantaged regions of Kenya.
The EPIC-HF trial tested a strategy for improving HFrEF medication-plan engagement by use of a video and documents delivered to patients several times by email or text prior to their follow-up clinic appointments. The strategy was compared with usual care for its effect on HF-medication optimization over 1 month and 1 year in a total of 306 patients.
Following EPIC-HF on the schedule is the MYROAD trial, looking at the efficacy of discharge instructions provided to patients with acute HF as an audio recording that they and their physicians could replay on demand, the idea being to increase adherence to the instructions. The trial’s 1073 patients were assigned to the novel strategy or usual care and followed for HF rehospitalization within 30 days.
MYROAD is to be followed by a presentation entitled “Digital Care Transformation: One-Year Report of >5,000 Patients Enrolled in a Remote Algorithm-Based CV Risk Management Program to Achieve Optimal Lipid and Hypertension Control.”
Rounding out the LBS session: the Bridging Income Generation With Group Integrated Care (BIGPIC) program, a pilot study that developed and executed “a healthcare delivery model targeting health behaviors, medication adherence, and financial barriers to accessing healthcare” in four rural counties in Kenya.
The model features locally developed plans, tailored for regional needs, that are said to “combine the benefits of microfinance with the peer support available through group medical care to enhance management of hypertension and diabetes.” The microfinance component is aimed at improving household economies to alleviate the financial burden of care and clinic attendance, and for the health effects of improved quality of life.
The study randomized 2890 adults with diabetes or prediabetes to one of four groups: usual care plus microfinance group support, group medical visits only or combined with microfinance group support, or usual care only. They were followed for changes in systolic blood pressure and CV-risk score over 12 months.
Lloyd-Jones and Fauci declared no conflicts.
This article first appeared on Medscape.com.
Cardiologists are already old hands at virtual meetings this year and are fast becoming experts on Zoom and other teleconferencing platforms, if not on how to unmute their microphones.
With expectations perhaps elevated and the new communications genre’s novelty on the wane, the American Heart Association (AHA) Scientific Sessions 2020 has a chance to both innovate with familiar formats and captivate with the field’s latest research findings.
Although the virtual AHA 2020 might not satisfy longings for face-to-face networking, shop talk, or kidding around over coffee, it will feature many traditional elements of the live conferences adapted for ear buds and small screens. They include late-breaking science (LBS) presentations and panel discussions, poster and live oral abstract presentations, meet-the-trialist talks, fireside-chat discussion forums, early career events, and satellite symposia.
The event may well hold lessons for future iterations of AHA Scientific Sessions in the postpandemic world, which some foresee as, potentially, an amalgam of the time-honored live format and a robust, complementary online presence.
“I can’t commit to exactly what AHA sessions will look like next November; I think that’s still being looked at,” the organization’s president-elect Donald M. Lloyd-Jones, MD, ScM, chair of the AHA Committee on Scientific Sessions Programming, told theheart.org | Medscape Cardiology.
There’s no debating that a live conference is valuable “for career networking and other opportunities, so I don’t think we can do without it. That has to be an important part of it,” he said. “When we can safely, of course.”
Still, “the virtual platform democratizes, right? I mean, it just allows greater access for a broader audience, and I think that’s important, too,” said Lloyd-Jones, MD, Northwestern University Feinberg School of Medicine, Chicago.
“I don’t think we’ll ever go completely back to it being all in-person,” he said. “I think the world has changed, and we’ll have to adapt our platforms to recognize that.”
Online, at least, meeting registrants will get a better look at Anthony Fauci, MD, than one might from the middle rows of a vast ballroom-turned-auditorium. Fauci is scheduled to speak on “Public Health and Scientific Challenges” during the Main Event Session “Latest Insights on COVID 19 and Cardiovascular Disease,” slated for the meeting’s final day.
Fauci has directed the National Institute of Allergy and Infectious Diseases (NIAID) since 1984, and has been celebrated for his leadership roles in the battles against AIDS and Ebola virus. Today, his name is close to a household word for his service as a prominent though embattled member of the White House Coronavirus Task Force.
The virtual AHA sessions will feature a core collection of LBS presentations from often high-profile clinical trials and other studies the organization deems worthy of special attention. There are nine such presentations arrayed across the meeting’s five days — from Friday, November 13 to Tuesday, November 17 — at times listed in this story and throughout the AHA Scientific Session program synched with the Central Standard Time (CST) zone of the AHA’s home office in Dallas.
Late-Breaking Science 1. Friday, November 13, 10:30 AM - 11:30 AM CST
The LBS sessions launch with the GALACTIC-HF trial, which — the world recently learned — may expand the burgeoning list of meds shown to improve clinical outcomes in chronic heart failure (HF) with reduced ejection fraction (HFrEF).
In cursory top-line results announced last month, those in the trial of more than 8000 patients who were randomly assigned to receive omecamtiv mecarbil (Amgen/Cytokinetics/Servier) showed a slight but significant benefit for the primary end point of cardiovascular (CV) death or HF events. The hazard ratio (HR), compared with standard care, was 0.92 (95% CI, 0.86 - 0.99; P = .025), noted a press release from Amgen.
Among the announcement’s few other details was a short take on safety outcomes: no difference in risk for “adverse events, including major ischemic cardiac events,” between the active and control groups. The presentation is sure to provide further insights and caveats, if any, along with other information crucial to the study’s interpretation.
Next on the schedule is the closely watched AFFIRM-AHF, billed as the first major outcomes trial of iron administration to iron-deficient patients with acute HF. It randomly assigned more than 1000 such patients to receive IV ferric carboxymaltose or a placebo. The first dose was given in-hospital and subsequent doses at home for 24 weeks or until patients were no longer iron deficient. They were followed to 1 year for the primary end point of recurrent HF hospitalizations or CV death.
The session wraps with the VITAL Rhythm trial, a substudy of the doubly randomized VITAL trial that explored the effects of vitamin D and omega-3 fatty acid supplementation on CV and cancer risk in more than 25,000 patients in the community. The substudy explored the effects of two active therapies, a preparation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Omacor, Reliant Pharmaceuticals) or vitamin D3 supplements, on new-onset atrial fibrillation (AF) as the primary end point; it also looked at risk for sudden death.
Late-Breaking Science 2. Friday, November 13, 12:00 PM - 1:00 PM CST
Dominating the session in two presentations, the (TIPS)-3 trial explored a polypill primary-prevention strategy and daily aspirin with vitamin D supplementation in three separate placebo-controlled comparisons in more than 5700 “intermediate risk” participants 55 years and older, mostly in developing countries.
The daily polypill in this trial is a combination of hydrochlorothiazide 25 mg, atenolol 100 mg, ramipril 10 mg, and simvastatin 40 mg; aspirin was given at 75 mg daily and vitamin D at 60,000 IU monthly.
The participants are followed for a primary end point composed of major CV disease, HF, resuscitated cardiac arrest, or ischemia-driven revascularization for the polypill comparison; CV events or cancer for the aspirin comparison; and fracture risk for the vitamin D component of the trial.
In the Swedish Cardiopulmonary Bioimage Study (SCAPIS), presented third in the session, a random sample of adults from throughout Sweden, projected at about 30,000, underwent a 2-day evaluation for metabolic risk factors plus ultrasound and coronary and lung CT scans. The group has been followed for risks for myocardial infarction (MI), sudden death, and other cardiac diseases; and chronic obstructive pulmonary disease (COPD) and other lung disorders.
Late-Breaking Science 3. Saturday, November 14, 12:00 PM - 1:00 PM CST
The field may learn more mechanistically about MI associated with nonobstructed coronary arteries (MINOCA) than ever before from the Heart Attack Research Program-Imaging Study (HARP). The observational study is enrolling a projected 450 patients with suspected MI and ischemic symptoms who were referred for cardiac catheterization.
Their evaluation includes coronary optical coherence tomographic (OCT) scanning and cardiac magnetic resonance (CMR) imaging for evidence of coronary plaque disruption as the primary end point. The patients are to be followed for 10 years for a composite of death, unstable angina, stroke, recurrent MI, diagnostic or interventional catheterization, and cardiac hospitalization.
The major direct oral anticoagulant (DOAC) comparisons with warfarin in atrial fibrillation (AF) didn’t include many patients with prosthetic valve implants. In contrast, the RIVER trial enrolled 1005 adults with either persistent or paroxysmal AF and bioprosthetic mitral valves and assigned them to rivaroxaban 20 mg or the vitamin K antagonist.
The presentation will include the noninferiority primary outcome of major clinical events, which is stroke, transient ischemic attack (TIA), major bleeding, death from any cause, valve thrombosis, other systemic embolism, or HF hospitalization over 12 months.
This session also includes ALPHEUS, a trial pitting ticagrelor (Brilinta/Brilique, AstraZeneca) against mainstay clopidogrel in a setting that is mostly uncharted for such comparisons, elective percutaneous coronary intervention (PCI).
About 1900 patients with stable coronary disease were randomly assigned to a month of treatment with either agent on top of continuous aspirin. The primary end point is PCI-related MI or myocardial injury within 48 hours of the procedure.
Late-Breaking Science 4. Sunday, November 15, 9:00 AM - 10:00 AM CST
The Self-Assessment Method for Statin Side-effects Or Nocebo (SAMSON) trial may be one of the AHA 2020 frontrunners for early buzz and anticipation. So it’s with some irony that it’s also among the smallest of the LBS studies, at 60 patients, which was nonetheless considered sufficient due to its unusual design.
SAMSON is the latest and perhaps most rigorous attempt to clarify whether symptoms, especially muscle pain or discomfort, attributed to statins by many patients are pharmacologic in origin or, rather, a nocebo effect from negative expectations about statin side effects.
The study patients, all of whom had previously halted statins because of side effects, were assigned to follow three separate regimens, each for month, in a randomized order; they did that four times, for a total of 12 months. The regimens consisted of atorvastatin 20 mg daily, a placebo, or neither.
Patients kept daily logs of any perceived side effects. Parity between side effects experienced on the statin and the placebo would point to a nocebo effect, whereas a significant excess on atorvastatin would suggest they are direct drug effects.
The session also features two randomized trials each on a unique omega-3 fatty acid preparation for either secondary prevention or high-risk primary prevention, in both cases compared with a corn-oil placebo.
The Omega-3 Fatty Acids in Elderly Patients with Myocardial Infarction (OMEMI) trial randomly assigned more than 1000 elderly post-MI patients to take Pikasol (Orkla Care) at 1.8 g EPA and DHA per day or the placebo. It looked for all-cause mortality, nonfatal MI, stroke, revascularization, or hospitalization for new or worsened HF over 24 months.
The STRENGTH trial, with a planned enrollment of about 13,000 high-vascular-risk patients, looked primarily at the effect of daily treatment with Epanova (AstraZeneca), which also contains DHA and EPA, on the composite of CV death, nonfatal MI or stroke, coronary revascularization, and hospitalization for unstable angina. The trial was halted early for low likelihood of benefit, AstraZeneca announced in January of this year.
Late-Breaking Science 5. Sunday, November 15, 7:15 PM - 8:30 PM CST
Slated for the session is the primary analysis of the PIONEER 3 trial, conducted in the United States, Europe, and Japan. It compared the BuMA Supreme biodegradable drug-coated stent (SinoMed) with the durable Xience (Abbott Vascular) and Promus (Boston Scientific) drug-eluting stents. The trial followed more than 1600 patients treated for chronic stable angina or acute coronary syndrome (ACS) for the 1-year composite of cardiac death, target-vessel-related MI, and clinically driven target-lesion revascularization.
Late-Breaking Science 6. Monday, November 16, 9:00 AM - 10:00 AM CST
The EARLY-AF trial enrolled 303 patients with symptomatic paroxysmal or persistent AF suitable for catheter ablation, assigning them to pulmonary vein isolation (PVI) by cryoablation using the Arctic Front (Medtronic) system or antiarrhythmic drug therapy for rhythm control. The primary end point is time to recurrence of AF, atrial flutter, or atrial tachycardia, whether symptomatic or asymptomatic, as determined by implantable loop recorder. Patients will also be followed for symptoms and arrhythmia burden.
Also in the session, the SEARCH-AF study randomized almost 400 patients undergoing cardiac surgery who were engaged subacutely with one of two commercial portable cardiac rhythm monitoring devices (CardioSTAT, Icentia; or SEEQ, Medtronic) or, alternatively, to receive usual postoperative care
The patients, considered to be at high risk for stroke with no history of AF, were followed for the primary end point of cumulative burden of AF or atrial flutter exceeding 6 minutes or documentation of either arrhythmia by 12-lead ECG within 30 days.
Two other studies in the session look at different approaches to AF screening, one using a handheld ECG monitor in the primary care setting and the other wearable monitors in the form of a patch or wristband. The VITAL-AF presentation is titled “Screening for Atrial Fibrillation in Older Adults at Primary Care Visits Using Single Lead Electrocardiograms.” The other presentation, on the study mSToPS, is called “Three-Year Clinical Outcomes in a Nationwide, Randomized, Pragmatic Clinical Trial of Atrial Fibrillation Screening — Mhealth Screening to Prevent Strokes.”
Late-Breaking Science 7. Monday, November 16, 7:00 PM - 8:30 PM CST
In the randomized FIDELIO-DKD trial with more than 5700 patients with type 2 diabetes and associated kidney disease, those assigned to the novel mineralocorticoid receptor antagonist (MRA) finerenone (Bayer) showed an 18% drop in risk for adverse renal events, including death from renal causes (P = .001), over a median of 2.6 years. That primary outcome was previously presented in detail at a nephrology meeting and published in the New England Journal of Medicine in October.
Patients on the MRA showed a similar reduction in a composite CV-event end point, it was also reported at that time. A follow-up presentation at the AHA sessions promises to dive deeper into the trial’s CV outcomes.
In the RAPID-CTCA study, slated next for the session, 1749 patients with suspected or confirmed intermediate-risk ACS were randomly assigned to undergo computed tomographic coronary angiography (CTCA) for guiding treatment decisions or a standard-of-care strategy. It followed patients for the primary end point of death or nonfatal MI over 1 year.
Rilonacept (Arcalyst, Kiniksa/Regeneron) is an interleukin-1α and -1β inhibitor used in several autoinflammatory diseases that went unsuccessfully before regulators for the treatment of gout. The RHAPSODY trial has now explored its use against recurrent pericarditis in a randomized trial that entered 86 patients 12 years and older who had previously experienced at least three episodes.
In top-line results reported to investors in June, patients assigned to receive the drug instead of placebo in weekly injections showed a 96% drop in risk for pericarditis recurrence and “no or minimal pain” on more than 90% of days in the trial. A full presentation is expected during this LBS session.
Also on the schedule is the THALES study, which led the US Food and Drug Administration (FDA) to expand indications for ticagrelor to include stroke prevention in patients with a history of acute ischemic stroke or high-risk TIA based on the trial’s primary results published in July.
In THALES, more than 11,000 patients with mild to moderate acute noncardiogenic ischemic stroke or TIA were randomly assigned within 24 hours to start on daily aspirin with or without ticagrelor given as a 180 mg loading dose followed by 90 mg twice daily for 30 days.
At the end of a month, it was reported, those on dual antiplatelet therapy showed a 17% risk reduction (P = .02) for the primary end point of stroke or death, at the cost of a slight but significant increase in “severe” bleeding (0.5% vs 0.1%; P = .001).
The session is to conclude with two related studies that fell victim in part to the COVID-19 pandemic, both of which explored sotagliflozin (Zynquista, Sanofi/Lexicon), an inhibitor of both sodium-glucose cotransporters 1 and 2 (SGLT1 and SGLT2, respectively) in patients with type 2 diabetes.
SOLOIST-WHF had entered 1222 such patients hospitalized with urgent or worsening HF at 466 centers and randomly assigned them to receive sotagliflozin or placebo; they were followed for the composite of CV death or HF events. SCORED reached an enrollment of 10,584 patients with diabetes and chronic kidney disease at 754 hospitals, following them for the same primary end point.
Lexicon announced in March that the trials would be “closed out early” because of the unavailability of funding “together with uncertainties relating to the COVID-19 pandemic on the trials.” The LBS presentation is expected to include analyses of available data; SOLOIST-WHF launched in summer 2018 and SCORED began in November 2017.
Late-Breaking Science 8. Tuesday, November 17, 9:00 AM - 10:00 AM CST
Most of this LBS session is devoted to the AHA COVID-19 Cardiovascular Disease registry, which is looking at the hospital journey, clinical course, and outcomes of patients hospitalized with SARS-CoV-2 infections at centers participating in the organization’s Get With The Guidelines (GWTG) quality-improvement program. As of September, the registry included data from more than 15,000 patients.
Scheduled presentations include a summary of the registry’s design and initial results; an analysis of racial and ethnic variation in therapy and clinical outcomes; an exploration of how body mass index influenced outcomes, including death, use of mechanical ventilation, and cardiovascular end points, in patients with COVID-19; and a deep dive into the relation between CV disease and clinical outcomes in the cohort.
The last of this LBS block’s five talks will cover the randomized Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) trial, which compared vaccination with high-dose trivalent influenza vaccine or a standard-dose quadrivalent vaccine in 5388 adults with a history of hospitalization for either MI or HF. Patients were required to have at least one other CV risk factor, such as older age, reduced left ventricular ejection fraction, or diabetes.
INVESTED tracked the patients at 190 centers across an initial pilot flu season and three subsequent flu seasons for the primary end point of death from any cause or cardiopulmonary hospitalization.
The trial is one of at least three that have been looking at the effect of flu vaccination on cardiovascular outcomes; results from the other two — IAMI, with more than 2500 participants, and RCT-IVVE, with an enrollment of 4871 — are planned for presentation in 2021, theheart.org | Medscape Cardiology recently reported.
Late-Breaking Science 9. Tuesday, November 17, 12:00 PM - 1:00 PM CST
The conference’s concluding LBS session features three studies that relied on technologic strategies for modifying patient compliance and other care behaviors and one that used human-centered design principles to develop a group-care model aimed improving the management of diabetes, hypertension, and other noncommunicable diseases in economically disadvantaged regions of Kenya.
The EPIC-HF trial tested a strategy for improving HFrEF medication-plan engagement by use of a video and documents delivered to patients several times by email or text prior to their follow-up clinic appointments. The strategy was compared with usual care for its effect on HF-medication optimization over 1 month and 1 year in a total of 306 patients.
Following EPIC-HF on the schedule is the MYROAD trial, looking at the efficacy of discharge instructions provided to patients with acute HF as an audio recording that they and their physicians could replay on demand, the idea being to increase adherence to the instructions. The trial’s 1073 patients were assigned to the novel strategy or usual care and followed for HF rehospitalization within 30 days.
MYROAD is to be followed by a presentation entitled “Digital Care Transformation: One-Year Report of >5,000 Patients Enrolled in a Remote Algorithm-Based CV Risk Management Program to Achieve Optimal Lipid and Hypertension Control.”
Rounding out the LBS session: the Bridging Income Generation With Group Integrated Care (BIGPIC) program, a pilot study that developed and executed “a healthcare delivery model targeting health behaviors, medication adherence, and financial barriers to accessing healthcare” in four rural counties in Kenya.
The model features locally developed plans, tailored for regional needs, that are said to “combine the benefits of microfinance with the peer support available through group medical care to enhance management of hypertension and diabetes.” The microfinance component is aimed at improving household economies to alleviate the financial burden of care and clinic attendance, and for the health effects of improved quality of life.
The study randomized 2890 adults with diabetes or prediabetes to one of four groups: usual care plus microfinance group support, group medical visits only or combined with microfinance group support, or usual care only. They were followed for changes in systolic blood pressure and CV-risk score over 12 months.
Lloyd-Jones and Fauci declared no conflicts.
This article first appeared on Medscape.com.
Cardiologists are already old hands at virtual meetings this year and are fast becoming experts on Zoom and other teleconferencing platforms, if not on how to unmute their microphones.
With expectations perhaps elevated and the new communications genre’s novelty on the wane, the American Heart Association (AHA) Scientific Sessions 2020 has a chance to both innovate with familiar formats and captivate with the field’s latest research findings.
Although the virtual AHA 2020 might not satisfy longings for face-to-face networking, shop talk, or kidding around over coffee, it will feature many traditional elements of the live conferences adapted for ear buds and small screens. They include late-breaking science (LBS) presentations and panel discussions, poster and live oral abstract presentations, meet-the-trialist talks, fireside-chat discussion forums, early career events, and satellite symposia.
The event may well hold lessons for future iterations of AHA Scientific Sessions in the postpandemic world, which some foresee as, potentially, an amalgam of the time-honored live format and a robust, complementary online presence.
“I can’t commit to exactly what AHA sessions will look like next November; I think that’s still being looked at,” the organization’s president-elect Donald M. Lloyd-Jones, MD, ScM, chair of the AHA Committee on Scientific Sessions Programming, told theheart.org | Medscape Cardiology.
There’s no debating that a live conference is valuable “for career networking and other opportunities, so I don’t think we can do without it. That has to be an important part of it,” he said. “When we can safely, of course.”
Still, “the virtual platform democratizes, right? I mean, it just allows greater access for a broader audience, and I think that’s important, too,” said Lloyd-Jones, MD, Northwestern University Feinberg School of Medicine, Chicago.
“I don’t think we’ll ever go completely back to it being all in-person,” he said. “I think the world has changed, and we’ll have to adapt our platforms to recognize that.”
Online, at least, meeting registrants will get a better look at Anthony Fauci, MD, than one might from the middle rows of a vast ballroom-turned-auditorium. Fauci is scheduled to speak on “Public Health and Scientific Challenges” during the Main Event Session “Latest Insights on COVID 19 and Cardiovascular Disease,” slated for the meeting’s final day.
Fauci has directed the National Institute of Allergy and Infectious Diseases (NIAID) since 1984, and has been celebrated for his leadership roles in the battles against AIDS and Ebola virus. Today, his name is close to a household word for his service as a prominent though embattled member of the White House Coronavirus Task Force.
The virtual AHA sessions will feature a core collection of LBS presentations from often high-profile clinical trials and other studies the organization deems worthy of special attention. There are nine such presentations arrayed across the meeting’s five days — from Friday, November 13 to Tuesday, November 17 — at times listed in this story and throughout the AHA Scientific Session program synched with the Central Standard Time (CST) zone of the AHA’s home office in Dallas.
Late-Breaking Science 1. Friday, November 13, 10:30 AM - 11:30 AM CST
The LBS sessions launch with the GALACTIC-HF trial, which — the world recently learned — may expand the burgeoning list of meds shown to improve clinical outcomes in chronic heart failure (HF) with reduced ejection fraction (HFrEF).
In cursory top-line results announced last month, those in the trial of more than 8000 patients who were randomly assigned to receive omecamtiv mecarbil (Amgen/Cytokinetics/Servier) showed a slight but significant benefit for the primary end point of cardiovascular (CV) death or HF events. The hazard ratio (HR), compared with standard care, was 0.92 (95% CI, 0.86 - 0.99; P = .025), noted a press release from Amgen.
Among the announcement’s few other details was a short take on safety outcomes: no difference in risk for “adverse events, including major ischemic cardiac events,” between the active and control groups. The presentation is sure to provide further insights and caveats, if any, along with other information crucial to the study’s interpretation.
Next on the schedule is the closely watched AFFIRM-AHF, billed as the first major outcomes trial of iron administration to iron-deficient patients with acute HF. It randomly assigned more than 1000 such patients to receive IV ferric carboxymaltose or a placebo. The first dose was given in-hospital and subsequent doses at home for 24 weeks or until patients were no longer iron deficient. They were followed to 1 year for the primary end point of recurrent HF hospitalizations or CV death.
The session wraps with the VITAL Rhythm trial, a substudy of the doubly randomized VITAL trial that explored the effects of vitamin D and omega-3 fatty acid supplementation on CV and cancer risk in more than 25,000 patients in the community. The substudy explored the effects of two active therapies, a preparation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Omacor, Reliant Pharmaceuticals) or vitamin D3 supplements, on new-onset atrial fibrillation (AF) as the primary end point; it also looked at risk for sudden death.
Late-Breaking Science 2. Friday, November 13, 12:00 PM - 1:00 PM CST
Dominating the session in two presentations, the (TIPS)-3 trial explored a polypill primary-prevention strategy and daily aspirin with vitamin D supplementation in three separate placebo-controlled comparisons in more than 5700 “intermediate risk” participants 55 years and older, mostly in developing countries.
The daily polypill in this trial is a combination of hydrochlorothiazide 25 mg, atenolol 100 mg, ramipril 10 mg, and simvastatin 40 mg; aspirin was given at 75 mg daily and vitamin D at 60,000 IU monthly.
The participants are followed for a primary end point composed of major CV disease, HF, resuscitated cardiac arrest, or ischemia-driven revascularization for the polypill comparison; CV events or cancer for the aspirin comparison; and fracture risk for the vitamin D component of the trial.
In the Swedish Cardiopulmonary Bioimage Study (SCAPIS), presented third in the session, a random sample of adults from throughout Sweden, projected at about 30,000, underwent a 2-day evaluation for metabolic risk factors plus ultrasound and coronary and lung CT scans. The group has been followed for risks for myocardial infarction (MI), sudden death, and other cardiac diseases; and chronic obstructive pulmonary disease (COPD) and other lung disorders.
Late-Breaking Science 3. Saturday, November 14, 12:00 PM - 1:00 PM CST
The field may learn more mechanistically about MI associated with nonobstructed coronary arteries (MINOCA) than ever before from the Heart Attack Research Program-Imaging Study (HARP). The observational study is enrolling a projected 450 patients with suspected MI and ischemic symptoms who were referred for cardiac catheterization.
Their evaluation includes coronary optical coherence tomographic (OCT) scanning and cardiac magnetic resonance (CMR) imaging for evidence of coronary plaque disruption as the primary end point. The patients are to be followed for 10 years for a composite of death, unstable angina, stroke, recurrent MI, diagnostic or interventional catheterization, and cardiac hospitalization.
The major direct oral anticoagulant (DOAC) comparisons with warfarin in atrial fibrillation (AF) didn’t include many patients with prosthetic valve implants. In contrast, the RIVER trial enrolled 1005 adults with either persistent or paroxysmal AF and bioprosthetic mitral valves and assigned them to rivaroxaban 20 mg or the vitamin K antagonist.
The presentation will include the noninferiority primary outcome of major clinical events, which is stroke, transient ischemic attack (TIA), major bleeding, death from any cause, valve thrombosis, other systemic embolism, or HF hospitalization over 12 months.
This session also includes ALPHEUS, a trial pitting ticagrelor (Brilinta/Brilique, AstraZeneca) against mainstay clopidogrel in a setting that is mostly uncharted for such comparisons, elective percutaneous coronary intervention (PCI).
About 1900 patients with stable coronary disease were randomly assigned to a month of treatment with either agent on top of continuous aspirin. The primary end point is PCI-related MI or myocardial injury within 48 hours of the procedure.
Late-Breaking Science 4. Sunday, November 15, 9:00 AM - 10:00 AM CST
The Self-Assessment Method for Statin Side-effects Or Nocebo (SAMSON) trial may be one of the AHA 2020 frontrunners for early buzz and anticipation. So it’s with some irony that it’s also among the smallest of the LBS studies, at 60 patients, which was nonetheless considered sufficient due to its unusual design.
SAMSON is the latest and perhaps most rigorous attempt to clarify whether symptoms, especially muscle pain or discomfort, attributed to statins by many patients are pharmacologic in origin or, rather, a nocebo effect from negative expectations about statin side effects.
The study patients, all of whom had previously halted statins because of side effects, were assigned to follow three separate regimens, each for month, in a randomized order; they did that four times, for a total of 12 months. The regimens consisted of atorvastatin 20 mg daily, a placebo, or neither.
Patients kept daily logs of any perceived side effects. Parity between side effects experienced on the statin and the placebo would point to a nocebo effect, whereas a significant excess on atorvastatin would suggest they are direct drug effects.
The session also features two randomized trials each on a unique omega-3 fatty acid preparation for either secondary prevention or high-risk primary prevention, in both cases compared with a corn-oil placebo.
The Omega-3 Fatty Acids in Elderly Patients with Myocardial Infarction (OMEMI) trial randomly assigned more than 1000 elderly post-MI patients to take Pikasol (Orkla Care) at 1.8 g EPA and DHA per day or the placebo. It looked for all-cause mortality, nonfatal MI, stroke, revascularization, or hospitalization for new or worsened HF over 24 months.
The STRENGTH trial, with a planned enrollment of about 13,000 high-vascular-risk patients, looked primarily at the effect of daily treatment with Epanova (AstraZeneca), which also contains DHA and EPA, on the composite of CV death, nonfatal MI or stroke, coronary revascularization, and hospitalization for unstable angina. The trial was halted early for low likelihood of benefit, AstraZeneca announced in January of this year.
Late-Breaking Science 5. Sunday, November 15, 7:15 PM - 8:30 PM CST
Slated for the session is the primary analysis of the PIONEER 3 trial, conducted in the United States, Europe, and Japan. It compared the BuMA Supreme biodegradable drug-coated stent (SinoMed) with the durable Xience (Abbott Vascular) and Promus (Boston Scientific) drug-eluting stents. The trial followed more than 1600 patients treated for chronic stable angina or acute coronary syndrome (ACS) for the 1-year composite of cardiac death, target-vessel-related MI, and clinically driven target-lesion revascularization.
Late-Breaking Science 6. Monday, November 16, 9:00 AM - 10:00 AM CST
The EARLY-AF trial enrolled 303 patients with symptomatic paroxysmal or persistent AF suitable for catheter ablation, assigning them to pulmonary vein isolation (PVI) by cryoablation using the Arctic Front (Medtronic) system or antiarrhythmic drug therapy for rhythm control. The primary end point is time to recurrence of AF, atrial flutter, or atrial tachycardia, whether symptomatic or asymptomatic, as determined by implantable loop recorder. Patients will also be followed for symptoms and arrhythmia burden.
Also in the session, the SEARCH-AF study randomized almost 400 patients undergoing cardiac surgery who were engaged subacutely with one of two commercial portable cardiac rhythm monitoring devices (CardioSTAT, Icentia; or SEEQ, Medtronic) or, alternatively, to receive usual postoperative care
The patients, considered to be at high risk for stroke with no history of AF, were followed for the primary end point of cumulative burden of AF or atrial flutter exceeding 6 minutes or documentation of either arrhythmia by 12-lead ECG within 30 days.
Two other studies in the session look at different approaches to AF screening, one using a handheld ECG monitor in the primary care setting and the other wearable monitors in the form of a patch or wristband. The VITAL-AF presentation is titled “Screening for Atrial Fibrillation in Older Adults at Primary Care Visits Using Single Lead Electrocardiograms.” The other presentation, on the study mSToPS, is called “Three-Year Clinical Outcomes in a Nationwide, Randomized, Pragmatic Clinical Trial of Atrial Fibrillation Screening — Mhealth Screening to Prevent Strokes.”
Late-Breaking Science 7. Monday, November 16, 7:00 PM - 8:30 PM CST
In the randomized FIDELIO-DKD trial with more than 5700 patients with type 2 diabetes and associated kidney disease, those assigned to the novel mineralocorticoid receptor antagonist (MRA) finerenone (Bayer) showed an 18% drop in risk for adverse renal events, including death from renal causes (P = .001), over a median of 2.6 years. That primary outcome was previously presented in detail at a nephrology meeting and published in the New England Journal of Medicine in October.
Patients on the MRA showed a similar reduction in a composite CV-event end point, it was also reported at that time. A follow-up presentation at the AHA sessions promises to dive deeper into the trial’s CV outcomes.
In the RAPID-CTCA study, slated next for the session, 1749 patients with suspected or confirmed intermediate-risk ACS were randomly assigned to undergo computed tomographic coronary angiography (CTCA) for guiding treatment decisions or a standard-of-care strategy. It followed patients for the primary end point of death or nonfatal MI over 1 year.
Rilonacept (Arcalyst, Kiniksa/Regeneron) is an interleukin-1α and -1β inhibitor used in several autoinflammatory diseases that went unsuccessfully before regulators for the treatment of gout. The RHAPSODY trial has now explored its use against recurrent pericarditis in a randomized trial that entered 86 patients 12 years and older who had previously experienced at least three episodes.
In top-line results reported to investors in June, patients assigned to receive the drug instead of placebo in weekly injections showed a 96% drop in risk for pericarditis recurrence and “no or minimal pain” on more than 90% of days in the trial. A full presentation is expected during this LBS session.
Also on the schedule is the THALES study, which led the US Food and Drug Administration (FDA) to expand indications for ticagrelor to include stroke prevention in patients with a history of acute ischemic stroke or high-risk TIA based on the trial’s primary results published in July.
In THALES, more than 11,000 patients with mild to moderate acute noncardiogenic ischemic stroke or TIA were randomly assigned within 24 hours to start on daily aspirin with or without ticagrelor given as a 180 mg loading dose followed by 90 mg twice daily for 30 days.
At the end of a month, it was reported, those on dual antiplatelet therapy showed a 17% risk reduction (P = .02) for the primary end point of stroke or death, at the cost of a slight but significant increase in “severe” bleeding (0.5% vs 0.1%; P = .001).
The session is to conclude with two related studies that fell victim in part to the COVID-19 pandemic, both of which explored sotagliflozin (Zynquista, Sanofi/Lexicon), an inhibitor of both sodium-glucose cotransporters 1 and 2 (SGLT1 and SGLT2, respectively) in patients with type 2 diabetes.
SOLOIST-WHF had entered 1222 such patients hospitalized with urgent or worsening HF at 466 centers and randomly assigned them to receive sotagliflozin or placebo; they were followed for the composite of CV death or HF events. SCORED reached an enrollment of 10,584 patients with diabetes and chronic kidney disease at 754 hospitals, following them for the same primary end point.
Lexicon announced in March that the trials would be “closed out early” because of the unavailability of funding “together with uncertainties relating to the COVID-19 pandemic on the trials.” The LBS presentation is expected to include analyses of available data; SOLOIST-WHF launched in summer 2018 and SCORED began in November 2017.
Late-Breaking Science 8. Tuesday, November 17, 9:00 AM - 10:00 AM CST
Most of this LBS session is devoted to the AHA COVID-19 Cardiovascular Disease registry, which is looking at the hospital journey, clinical course, and outcomes of patients hospitalized with SARS-CoV-2 infections at centers participating in the organization’s Get With The Guidelines (GWTG) quality-improvement program. As of September, the registry included data from more than 15,000 patients.
Scheduled presentations include a summary of the registry’s design and initial results; an analysis of racial and ethnic variation in therapy and clinical outcomes; an exploration of how body mass index influenced outcomes, including death, use of mechanical ventilation, and cardiovascular end points, in patients with COVID-19; and a deep dive into the relation between CV disease and clinical outcomes in the cohort.
The last of this LBS block’s five talks will cover the randomized Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) trial, which compared vaccination with high-dose trivalent influenza vaccine or a standard-dose quadrivalent vaccine in 5388 adults with a history of hospitalization for either MI or HF. Patients were required to have at least one other CV risk factor, such as older age, reduced left ventricular ejection fraction, or diabetes.
INVESTED tracked the patients at 190 centers across an initial pilot flu season and three subsequent flu seasons for the primary end point of death from any cause or cardiopulmonary hospitalization.
The trial is one of at least three that have been looking at the effect of flu vaccination on cardiovascular outcomes; results from the other two — IAMI, with more than 2500 participants, and RCT-IVVE, with an enrollment of 4871 — are planned for presentation in 2021, theheart.org | Medscape Cardiology recently reported.
Late-Breaking Science 9. Tuesday, November 17, 12:00 PM - 1:00 PM CST
The conference’s concluding LBS session features three studies that relied on technologic strategies for modifying patient compliance and other care behaviors and one that used human-centered design principles to develop a group-care model aimed improving the management of diabetes, hypertension, and other noncommunicable diseases in economically disadvantaged regions of Kenya.
The EPIC-HF trial tested a strategy for improving HFrEF medication-plan engagement by use of a video and documents delivered to patients several times by email or text prior to their follow-up clinic appointments. The strategy was compared with usual care for its effect on HF-medication optimization over 1 month and 1 year in a total of 306 patients.
Following EPIC-HF on the schedule is the MYROAD trial, looking at the efficacy of discharge instructions provided to patients with acute HF as an audio recording that they and their physicians could replay on demand, the idea being to increase adherence to the instructions. The trial’s 1073 patients were assigned to the novel strategy or usual care and followed for HF rehospitalization within 30 days.
MYROAD is to be followed by a presentation entitled “Digital Care Transformation: One-Year Report of >5,000 Patients Enrolled in a Remote Algorithm-Based CV Risk Management Program to Achieve Optimal Lipid and Hypertension Control.”
Rounding out the LBS session: the Bridging Income Generation With Group Integrated Care (BIGPIC) program, a pilot study that developed and executed “a healthcare delivery model targeting health behaviors, medication adherence, and financial barriers to accessing healthcare” in four rural counties in Kenya.
The model features locally developed plans, tailored for regional needs, that are said to “combine the benefits of microfinance with the peer support available through group medical care to enhance management of hypertension and diabetes.” The microfinance component is aimed at improving household economies to alleviate the financial burden of care and clinic attendance, and for the health effects of improved quality of life.
The study randomized 2890 adults with diabetes or prediabetes to one of four groups: usual care plus microfinance group support, group medical visits only or combined with microfinance group support, or usual care only. They were followed for changes in systolic blood pressure and CV-risk score over 12 months.
Lloyd-Jones and Fauci declared no conflicts.
This article first appeared on Medscape.com.
Poverty raises depression risk in patients with cystic fibrosis
Poor people with chronic illness have greater difficulty managing their disease than do their better-off counterparts, and a new study confirms this reality for patients with cystic fibrosis.
and anxiety symptoms, according to a new cross-sectional study. The data were drawn from the Cystic Fibrosis Foundation’s Success with Therapies Research Consortium.
“Assessing the special challenges that individuals with lower SES face, including financial barriers, is essential to understand how we can address the unique combinations of adherence barriers. In other chronic disorders, financial barriers or lower socioeconomic status is associated with nonadherence, but this relationship has not been well established in cystic fibrosis,” said Kimberly Dickinson, MD, MPH, of Johns Hopkins University, Baltimore, during her presentation of the results at the virtual North American Cystic Fibrosis Conference.
“I’ve always thought that my patients in the poorer population were doing worse, and I think this demonstrates that that’s true,” said Robert Giusti, MD, in an interview. Dr. Giusti is a clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center in New York. He was not involved in the study.
“These are very pertinent issues, especially if you think about the pandemic, and some of the issues related to mental health. It just highlights the importance of socioeconomic status and screening for some of the known risk factors so that we can develop interventions or programs to provide equitable care to all of our cystic fibrosis patients,” said Ryan Perkins, MD, who moderated the session where the study was presented. He is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, also in Boston.
The researchers looked retrospectively at 1 year’s worth of pharmacy refill receipts and number of times prescriptions were refilled versus the number of times prescribed, then calculated medicinal possession ratios. This was cross-referenced with annual household income and insurance status of patients with CF at 12 pediatric and 9 adult CF care centers, for a total of 376 patients (128 pediatric and 248 adult).
In this population, 32% of participants had public or no insurance, 68% had private or military insurance. The public/no insurance group was more likely than the private/military insurance group to report having trouble paying for treatments, food, or critical expenses related to CF care (23.3% vs. 12.1%, respectively); feeling symptoms on most days of depression (42.5% vs. 31.3%) or anxiety (40.0% vs. 28.5%); and experiencing conflict or stress with loved ones over treatments (30.0% vs. 20.3%) (P < .05 for all).
In all, 35% had a household income less than $40,000 per year, 33% between $44,000 and $100,000, and 32% higher than $100,000. The low-income group had a lower composite medication possession ratio (0.41) than the middle- (0.44) or high-income (0.52) groups, were more likely to have trouble paying for treatments, food, or treatment-related expenses (25%, 18%, 4%, respectively); were more likely most days to report symptoms of depression (43%, 34%, 26%) or anxiety (40%, 32%, 24%), and to have concerns about whether treatments were effective (42%, 27%, 29%). They were more likely to not be able to maintain a daily schedule or routine for treatments (28%, 22%, 14%).
The study showed that adherence barriers and suboptimal adherence are issues that cross all socioeconomic categories, though they were more problematic in the lowest bracket. Greater anxiety and depression among lower income individuals and those with private or no insurance was a key finding, according to Dr. Dickinson. “It highlights the importance of screening for mental health comorbidities that may impact non-adherence,” she said.
The study received funding from the Cystic Fibrosis Foundation. Dr. Dickinson, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
Poor people with chronic illness have greater difficulty managing their disease than do their better-off counterparts, and a new study confirms this reality for patients with cystic fibrosis.
and anxiety symptoms, according to a new cross-sectional study. The data were drawn from the Cystic Fibrosis Foundation’s Success with Therapies Research Consortium.
“Assessing the special challenges that individuals with lower SES face, including financial barriers, is essential to understand how we can address the unique combinations of adherence barriers. In other chronic disorders, financial barriers or lower socioeconomic status is associated with nonadherence, but this relationship has not been well established in cystic fibrosis,” said Kimberly Dickinson, MD, MPH, of Johns Hopkins University, Baltimore, during her presentation of the results at the virtual North American Cystic Fibrosis Conference.
“I’ve always thought that my patients in the poorer population were doing worse, and I think this demonstrates that that’s true,” said Robert Giusti, MD, in an interview. Dr. Giusti is a clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center in New York. He was not involved in the study.
“These are very pertinent issues, especially if you think about the pandemic, and some of the issues related to mental health. It just highlights the importance of socioeconomic status and screening for some of the known risk factors so that we can develop interventions or programs to provide equitable care to all of our cystic fibrosis patients,” said Ryan Perkins, MD, who moderated the session where the study was presented. He is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, also in Boston.
The researchers looked retrospectively at 1 year’s worth of pharmacy refill receipts and number of times prescriptions were refilled versus the number of times prescribed, then calculated medicinal possession ratios. This was cross-referenced with annual household income and insurance status of patients with CF at 12 pediatric and 9 adult CF care centers, for a total of 376 patients (128 pediatric and 248 adult).

In this population, 32% of participants had public or no insurance, 68% had private or military insurance. The public/no insurance group was more likely than the private/military insurance group to report having trouble paying for treatments, food, or critical expenses related to CF care (23.3% vs. 12.1%, respectively); feeling symptoms on most days of depression (42.5% vs. 31.3%) or anxiety (40.0% vs. 28.5%); and experiencing conflict or stress with loved ones over treatments (30.0% vs. 20.3%) (P < .05 for all).
In all, 35% had a household income less than $40,000 per year, 33% between $44,000 and $100,000, and 32% higher than $100,000. The low-income group had a lower composite medication possession ratio (0.41) than the middle- (0.44) or high-income (0.52) groups, were more likely to have trouble paying for treatments, food, or treatment-related expenses (25%, 18%, 4%, respectively); were more likely most days to report symptoms of depression (43%, 34%, 26%) or anxiety (40%, 32%, 24%), and to have concerns about whether treatments were effective (42%, 27%, 29%). They were more likely to not be able to maintain a daily schedule or routine for treatments (28%, 22%, 14%).
The study showed that adherence barriers and suboptimal adherence are issues that cross all socioeconomic categories, though they were more problematic in the lowest bracket. Greater anxiety and depression among lower income individuals and those with private or no insurance was a key finding, according to Dr. Dickinson. “It highlights the importance of screening for mental health comorbidities that may impact non-adherence,” she said.
The study received funding from the Cystic Fibrosis Foundation. Dr. Dickinson, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
Poor people with chronic illness have greater difficulty managing their disease than do their better-off counterparts, and a new study confirms this reality for patients with cystic fibrosis.
and anxiety symptoms, according to a new cross-sectional study. The data were drawn from the Cystic Fibrosis Foundation’s Success with Therapies Research Consortium.
“Assessing the special challenges that individuals with lower SES face, including financial barriers, is essential to understand how we can address the unique combinations of adherence barriers. In other chronic disorders, financial barriers or lower socioeconomic status is associated with nonadherence, but this relationship has not been well established in cystic fibrosis,” said Kimberly Dickinson, MD, MPH, of Johns Hopkins University, Baltimore, during her presentation of the results at the virtual North American Cystic Fibrosis Conference.
“I’ve always thought that my patients in the poorer population were doing worse, and I think this demonstrates that that’s true,” said Robert Giusti, MD, in an interview. Dr. Giusti is a clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center in New York. He was not involved in the study.
“These are very pertinent issues, especially if you think about the pandemic, and some of the issues related to mental health. It just highlights the importance of socioeconomic status and screening for some of the known risk factors so that we can develop interventions or programs to provide equitable care to all of our cystic fibrosis patients,” said Ryan Perkins, MD, who moderated the session where the study was presented. He is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, also in Boston.
The researchers looked retrospectively at 1 year’s worth of pharmacy refill receipts and number of times prescriptions were refilled versus the number of times prescribed, then calculated medicinal possession ratios. This was cross-referenced with annual household income and insurance status of patients with CF at 12 pediatric and 9 adult CF care centers, for a total of 376 patients (128 pediatric and 248 adult).

In this population, 32% of participants had public or no insurance, 68% had private or military insurance. The public/no insurance group was more likely than the private/military insurance group to report having trouble paying for treatments, food, or critical expenses related to CF care (23.3% vs. 12.1%, respectively); feeling symptoms on most days of depression (42.5% vs. 31.3%) or anxiety (40.0% vs. 28.5%); and experiencing conflict or stress with loved ones over treatments (30.0% vs. 20.3%) (P < .05 for all).
In all, 35% had a household income less than $40,000 per year, 33% between $44,000 and $100,000, and 32% higher than $100,000. The low-income group had a lower composite medication possession ratio (0.41) than the middle- (0.44) or high-income (0.52) groups, were more likely to have trouble paying for treatments, food, or treatment-related expenses (25%, 18%, 4%, respectively); were more likely most days to report symptoms of depression (43%, 34%, 26%) or anxiety (40%, 32%, 24%), and to have concerns about whether treatments were effective (42%, 27%, 29%). They were more likely to not be able to maintain a daily schedule or routine for treatments (28%, 22%, 14%).
The study showed that adherence barriers and suboptimal adherence are issues that cross all socioeconomic categories, though they were more problematic in the lowest bracket. Greater anxiety and depression among lower income individuals and those with private or no insurance was a key finding, according to Dr. Dickinson. “It highlights the importance of screening for mental health comorbidities that may impact non-adherence,” she said.
The study received funding from the Cystic Fibrosis Foundation. Dr. Dickinson, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
FROM NACFC 2020
.
Biden plan to lower Medicare eligibility age to 60 faces hostility from hospitals
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Dripping, dabbing, and bongs: Can’t tell the players without a scorecard
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Fenway data, the final frontier
Data, as we all know, have taken over the world. ”
Statistical objectivity is in, individuality is out. You may have taught for 30 years and gained a sense for which child has a problem that needs intervention and which one just needs patience and time to develop. You may have managed patients for decades and have a hunch about who needs immediate help and who can be watched. But “senses” and “hunches” can’t be measured and therefore do not exist, or better, don’t count. Numbers count!
Data-obsession reflects what Germans call the Zeitgeist, the spirit of the age. But the Germans will have to come up with a different word for our age, won’t they? Nobody can measure a “spirit.”
Still, you know the spirit’s there, when it knocks you over and stomps on you.
The one sphere of life that has resisted being reduced to numbers is sports. In sports, you don’t need complex analysis to know who’s No. 1 and who’s number everything else. No. 1 crosses the finish line first, wins the most games, knocks out the opponent. The one lying on the mat is No. 2.
Of course, sports always had lots of numbers. Baseball fans have always known about batting averages, runs batted in, earned run averages. But there were always those individual intangibles that goggle the eyes of small boys and keep sportswriters in business: this athlete’s “ferocious drive,” that one’s “will to win,” the way a third “always comes through in the clutch.” Pitchers who couldn’t throw fast anymore were “crafty.” Grizzled, tobacco-chewing scouts could sense which youngster “looked like a ballplayer.”
As if you didn’t already know, you can tell how old I am to talk this way. Bill James and his statistical acolytes put paid to that old kind of thinking a long time ago. Read Moneyball or see the movie. In sports too, it’s now all about the stats.
To generate flagging interest among the young for America’s now-stodgy pastime, Major League Baseball has brought out Statcast 2.0., which adds, according to a recent news story, “Doppler-based tracking of pitch velocity, exit velocity, launch angles, and spin rates, and defensive tracking of players.” Multicamera arrays produce “biomechanical imaging and skeletal models that can help pitchers with delivery issues or batters with swing path quandaries.”
And so we have lots of new data to ponder: exit velocity – how fast a hit ball leaves the bat; launch angle – what angle it leaves at; spin rate – how fast a thrown curveball spins; and defensive tracking – how many feet this shortstop can move left to snag a ground ball, or a right-fielder to catch a fly. And there are new, composite stats, like OPS (on-base plus slugging). I will not try to explain OPS, because it is a mathematical abstraction that I cannot grasp. It signifies a blend of on-base percentage and slugging percentage, which to me is like what you get when you blend a tomato with a broccoli. Or something.
And, stats aside, you do still have to win. Not long ago the Boston Red Sox had a relief pitcher whose spin rate was splendid, but he couldn’t get anybody out.
The real aim of the new broadcast innovations noted above comes at the end of the report:
In an effort to at least reach, if not grow, a younger fan base, MLB from now on will focus on video engagement, gaming, and augmented reality on Snapchat.
You got it: the goal is to reduce baseball to a video game, and its players to gaming characters, perhaps with big contracts and marketing deals. Hey, check out that dude’s OPS!
You can’t measure a Zeitgeist, but you certainly know when it’s sitting on your chest. Your respirations get depressed. Measurably.
Yeah, I sound like every cranky old man in history. But hey – I’m Emeritus! See this column’s title!
In addition, the article has one more detail:
Curiosity about whether a fly ball to deep right field at Fenway Park would be a home run at Yankee Stadium can be satisfied by overlaying the Yankee Stadium footprint on top of Fenway.
Maybe it would satisfy you, buddy, but anything that superimposes Yankee Stadium on top of Fenway Park dissatisfies me by a factor of 6.7!
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available online. Write to him at [email protected].
Data, as we all know, have taken over the world. ”
Statistical objectivity is in, individuality is out. You may have taught for 30 years and gained a sense for which child has a problem that needs intervention and which one just needs patience and time to develop. You may have managed patients for decades and have a hunch about who needs immediate help and who can be watched. But “senses” and “hunches” can’t be measured and therefore do not exist, or better, don’t count. Numbers count!
Data-obsession reflects what Germans call the Zeitgeist, the spirit of the age. But the Germans will have to come up with a different word for our age, won’t they? Nobody can measure a “spirit.”
Still, you know the spirit’s there, when it knocks you over and stomps on you.
The one sphere of life that has resisted being reduced to numbers is sports. In sports, you don’t need complex analysis to know who’s No. 1 and who’s number everything else. No. 1 crosses the finish line first, wins the most games, knocks out the opponent. The one lying on the mat is No. 2.
Of course, sports always had lots of numbers. Baseball fans have always known about batting averages, runs batted in, earned run averages. But there were always those individual intangibles that goggle the eyes of small boys and keep sportswriters in business: this athlete’s “ferocious drive,” that one’s “will to win,” the way a third “always comes through in the clutch.” Pitchers who couldn’t throw fast anymore were “crafty.” Grizzled, tobacco-chewing scouts could sense which youngster “looked like a ballplayer.”
As if you didn’t already know, you can tell how old I am to talk this way. Bill James and his statistical acolytes put paid to that old kind of thinking a long time ago. Read Moneyball or see the movie. In sports too, it’s now all about the stats.
To generate flagging interest among the young for America’s now-stodgy pastime, Major League Baseball has brought out Statcast 2.0., which adds, according to a recent news story, “Doppler-based tracking of pitch velocity, exit velocity, launch angles, and spin rates, and defensive tracking of players.” Multicamera arrays produce “biomechanical imaging and skeletal models that can help pitchers with delivery issues or batters with swing path quandaries.”
And so we have lots of new data to ponder: exit velocity – how fast a hit ball leaves the bat; launch angle – what angle it leaves at; spin rate – how fast a thrown curveball spins; and defensive tracking – how many feet this shortstop can move left to snag a ground ball, or a right-fielder to catch a fly. And there are new, composite stats, like OPS (on-base plus slugging). I will not try to explain OPS, because it is a mathematical abstraction that I cannot grasp. It signifies a blend of on-base percentage and slugging percentage, which to me is like what you get when you blend a tomato with a broccoli. Or something.
And, stats aside, you do still have to win. Not long ago the Boston Red Sox had a relief pitcher whose spin rate was splendid, but he couldn’t get anybody out.
The real aim of the new broadcast innovations noted above comes at the end of the report:
In an effort to at least reach, if not grow, a younger fan base, MLB from now on will focus on video engagement, gaming, and augmented reality on Snapchat.
You got it: the goal is to reduce baseball to a video game, and its players to gaming characters, perhaps with big contracts and marketing deals. Hey, check out that dude’s OPS!
You can’t measure a Zeitgeist, but you certainly know when it’s sitting on your chest. Your respirations get depressed. Measurably.
Yeah, I sound like every cranky old man in history. But hey – I’m Emeritus! See this column’s title!
In addition, the article has one more detail:
Curiosity about whether a fly ball to deep right field at Fenway Park would be a home run at Yankee Stadium can be satisfied by overlaying the Yankee Stadium footprint on top of Fenway.
Maybe it would satisfy you, buddy, but anything that superimposes Yankee Stadium on top of Fenway Park dissatisfies me by a factor of 6.7!
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available online. Write to him at [email protected].
Data, as we all know, have taken over the world. ”
Statistical objectivity is in, individuality is out. You may have taught for 30 years and gained a sense for which child has a problem that needs intervention and which one just needs patience and time to develop. You may have managed patients for decades and have a hunch about who needs immediate help and who can be watched. But “senses” and “hunches” can’t be measured and therefore do not exist, or better, don’t count. Numbers count!
Data-obsession reflects what Germans call the Zeitgeist, the spirit of the age. But the Germans will have to come up with a different word for our age, won’t they? Nobody can measure a “spirit.”
Still, you know the spirit’s there, when it knocks you over and stomps on you.
The one sphere of life that has resisted being reduced to numbers is sports. In sports, you don’t need complex analysis to know who’s No. 1 and who’s number everything else. No. 1 crosses the finish line first, wins the most games, knocks out the opponent. The one lying on the mat is No. 2.
Of course, sports always had lots of numbers. Baseball fans have always known about batting averages, runs batted in, earned run averages. But there were always those individual intangibles that goggle the eyes of small boys and keep sportswriters in business: this athlete’s “ferocious drive,” that one’s “will to win,” the way a third “always comes through in the clutch.” Pitchers who couldn’t throw fast anymore were “crafty.” Grizzled, tobacco-chewing scouts could sense which youngster “looked like a ballplayer.”
As if you didn’t already know, you can tell how old I am to talk this way. Bill James and his statistical acolytes put paid to that old kind of thinking a long time ago. Read Moneyball or see the movie. In sports too, it’s now all about the stats.
To generate flagging interest among the young for America’s now-stodgy pastime, Major League Baseball has brought out Statcast 2.0., which adds, according to a recent news story, “Doppler-based tracking of pitch velocity, exit velocity, launch angles, and spin rates, and defensive tracking of players.” Multicamera arrays produce “biomechanical imaging and skeletal models that can help pitchers with delivery issues or batters with swing path quandaries.”
And so we have lots of new data to ponder: exit velocity – how fast a hit ball leaves the bat; launch angle – what angle it leaves at; spin rate – how fast a thrown curveball spins; and defensive tracking – how many feet this shortstop can move left to snag a ground ball, or a right-fielder to catch a fly. And there are new, composite stats, like OPS (on-base plus slugging). I will not try to explain OPS, because it is a mathematical abstraction that I cannot grasp. It signifies a blend of on-base percentage and slugging percentage, which to me is like what you get when you blend a tomato with a broccoli. Or something.
And, stats aside, you do still have to win. Not long ago the Boston Red Sox had a relief pitcher whose spin rate was splendid, but he couldn’t get anybody out.
The real aim of the new broadcast innovations noted above comes at the end of the report:
In an effort to at least reach, if not grow, a younger fan base, MLB from now on will focus on video engagement, gaming, and augmented reality on Snapchat.
You got it: the goal is to reduce baseball to a video game, and its players to gaming characters, perhaps with big contracts and marketing deals. Hey, check out that dude’s OPS!
You can’t measure a Zeitgeist, but you certainly know when it’s sitting on your chest. Your respirations get depressed. Measurably.
Yeah, I sound like every cranky old man in history. But hey – I’m Emeritus! See this column’s title!
In addition, the article has one more detail:
Curiosity about whether a fly ball to deep right field at Fenway Park would be a home run at Yankee Stadium can be satisfied by overlaying the Yankee Stadium footprint on top of Fenway.
Maybe it would satisfy you, buddy, but anything that superimposes Yankee Stadium on top of Fenway Park dissatisfies me by a factor of 6.7!
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available online. Write to him at [email protected].
90-year-old man • dyspnea • lower extremity edema • limitations in daily activities • Dx?
THE CASE
An obese 90-year-old White man presented for a 1-month follow-up with his family physician after being hospitalized for an acute exacerbation of heart failure (HF). In addition to New York Heart Association (NYHA) Class III heart failure with reduced ejection fraction (HFrEF), he had a history of tobacco abuse, hyperlipidemia, atrial fibrillation, coronary artery disease, stage 3 chronic kidney disease, and benign prostatic hyperplasia. The patient’s family accompanied him during the visit to discuss hospice care.
The patient complained of persistent shortness of breath that limited his activities of daily living (ADLs) and lower extremity and scrotal edema. He denied chest pain, orthopnea, paroxysmal nocturnal dyspnea, ascites, nocturia, and nocturnal cough.
The patient had undergone a coronary artery bypass graft 23 years earlier. His HF was being managed with metoprolol tartrate 25 mg bid, spironolactone 25 mg/d, and furosemide 80 mg/d.
Examination revealed bilateral 3+ pitting edema in the lower extremities midway up the shin, crackles to the inferior scapula bilaterally, and a 3/6 systolic murmur with regular rate and rhythm. The remainder of the physical exam was normal. The patient’s vitals were within normal limits, with an oxygen saturation of 90%.
The patient’s most recent chest x-ray demonstrated mild cardiomegaly. An echocardiogram showed an ejection fraction of 44% with severe bi-atrial enlargement, moderate-to-severe mitral regurgitation, and mild-to-moderate aortic insufficiency. His brain natriuretic peptide (BNP) was 915 pg/mL (normal range for patients ages 75-99 years, < 450 pg/mL).
THE DIAGNOSIS
The differential diagnosis for the patient’s shortness of breath included chronic obstructive pulmonary disease secondary to his smoking history, pulmonary embolus, respiratory infection, anemia, and medication-related adverse effects. The patient’s history of renal disease merited consideration of a nephrotic syndrome causing low albumin, which could explain his edema. Another possible cause of the edema was venous insufficiency. However, given the patient’s extensive cardiac history, the most likely explanation for his shortness of breath and edema was congestive HF that was unresponsive to the current diuretic regimen.
Several changes to the patient’s medications were made. Lisinopril 2.5 mg/d was started due to the mortality benefit of angiotensin-converting enzyme inhibitors in the treatment of HFrEF.1 Metoprolol tartrate 25 mg/d was transitioned to metoprolol succinate 50 mg/d, as only the longer-acting succinate version has shown mortality benefit in HFrEF.1 (Other beta-blockers with mortality benefit include carvedilol and bisoprolol.1) The furosemide 80 mg/d was replaced with torsemide 100 mg/d to provide an enhanced diuretic effect for symptomatic relief. The spironolactone dose was not increased due to concerns about the patient’s renal function. Of note, spironolactone was included in the patient’s regimen based on his NYHA classification, as well as the potential mortality benefits and improvement in edema seen in HFrEF patients.1 Spironolactone can be used with loop and/or thiazide diuretics in the treatment of HF.
Continue to: Within 5 days...
Within 5 days, the patient had lost 6 lb and his oxygen saturation had improved from 90% to 95%. He reported improvements in his breathing and was able to move around more easily.
DISCUSSION
There are several possible explanations for torsemide’s superior diuretic effect in this patient. Unlike furosemide, torsemide absorption is not influenced by intestinal edema, which is commonly seen in patients with HF. It has a longer half-life and improved bioavailability that is not altered by food intake. Torsemide also inhibits the actions of aldosterone through its interaction with the renin-angiotensin-aldosterone system and aldosterone receptor, leading to further diuresis and reduced cardiac remodeling.2
What the evidence shows. The TORIC trial was an open-label, nonrandomized, post-marketing surveillance study of 1377 patients with NYHA Class II–III HF who received diuretic therapy with torsemide 10 mg/d, furosemide 40 mg/d, or another diuretic for 12 months.3 Significantly lower total mortality and cardiac mortality was found in the torsemide group; in addition, a significantly greater proportion of patients in the torsemide group showed improvement in NYHA classification.3 Murray et al reported a reduction in hospitalization rates with torsemide therapy vs furosemide therapy in a randomized trial of 234 HF patients (32% vs 17%, P = 0.01).4 The ASCEND-HF trial, a large international acute HF trial comparing torsemide with furosemide, demonstrated a nonsignificant reduction in 30-day and 180-day events (all-cause mortality or HF hospitalization) in those receiving torsemide, after risk adjustment.5 Torsemide has also been shown to improve quality of life compared to furosemide.6
Preliminary results from the TORNADO trial,7 a multicenter randomized controlled trial, demonstrated superior symptom improvement in HF patients taking torsemide compared to those taking furosemide.8 The preliminary endpoint—a composite of improvement in NYHA class, improvement in distance of at least 50 m during a 6-minute walk test, and a decrease in fluid retention of at least 0.5 ohms at 3-month follow-up—was achieved by 94% and 58% of patients on torsemide and furosemide, respectively (P = 0.03).8 A total of 7 patients (3 in the torsemide and 4 in the furosemide group) were hospitalized for worsening HF during the follow-up period.8
A 2020 meta-analysis of more than 19,000 patients compared furosemide to torsemide and found a number needed to treat (NNT) of 23 to prevent a hospitalization due to HF; an NNT of 5 for improvement in NYHA functional status; and an NNT of 40 for reduction in cardiac mortality.9
Continue to: Our patient
Our patient reported feeling “great” at the 6-week follow-up appointment, with significant improvement in breathing and ability to perform his ADLs. His NYHA classification improved to Class II. He had lost 26 pounds (back to his weight 9 months prior), and his oxygen saturation was 97%.
On exam, the bilateral peripheral edema in his lower extremities had improved from 3+ to 1+, with the edema extending just distal to the mid-shin. Only mild crackles were present at the lung bases. The remainder of his physical examination was unchanged. His vital signs were within normal limits with no signs of hypotension. A basic metabolic panel was obtained to confirm his electrolytes were still within normal limits. His BNP had decreased to 230 pg/mL.
The patient declined the referral for hospice evaluation due to the significant improvement in his symptoms.
THE TAKEAWAY
A significant clinical improvement and improved quality of life were achieved with the transition from furosemide to torsemide. It is apparent that the patient’s furosemide had an inferior diuretic effect compared to torsemide, whether that be secondary to his dose or due to the unpredictable nature of furosemide’s bioavailability, especially in the setting of intestinal edema.
A growing body of literature9-11 suggests torsemide’s superiority over furosemide with no signs of increased adverse effects. Although additional prospective, head-to-head trials are needed, at this point in time it is appropriate to consider the use of torsemide in a patient with HF who does not seem to be fully responding to furosemide.
CORRESPONDENCE
Ryan Paulus, DO, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
2. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-333.
3. Cosín J, Díez J; TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507-513.
4. Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513-520.
5. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:404-411.
6. Müller K, Gamba G, Jaquet F, et al. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793-801.
7. Balsam P, Ozierański K, Tymińska A, et al. The impact of torasemide on haemodynamic and neurohormonal stress, and cardiac remodelling in heart failure—TORNADO: a study protocol for a randomized controlled trial. Trials. 2017;18:36.
8. Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J. 2019;26:661-668.
9. Abraham B, Megaly M, Sous M, et al. Meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol. 2020;125:92-99.
10. Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Comparative analysis of long-term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77-86.
11. Täger T, Fröhlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83-90.
THE CASE
An obese 90-year-old White man presented for a 1-month follow-up with his family physician after being hospitalized for an acute exacerbation of heart failure (HF). In addition to New York Heart Association (NYHA) Class III heart failure with reduced ejection fraction (HFrEF), he had a history of tobacco abuse, hyperlipidemia, atrial fibrillation, coronary artery disease, stage 3 chronic kidney disease, and benign prostatic hyperplasia. The patient’s family accompanied him during the visit to discuss hospice care.
The patient complained of persistent shortness of breath that limited his activities of daily living (ADLs) and lower extremity and scrotal edema. He denied chest pain, orthopnea, paroxysmal nocturnal dyspnea, ascites, nocturia, and nocturnal cough.
The patient had undergone a coronary artery bypass graft 23 years earlier. His HF was being managed with metoprolol tartrate 25 mg bid, spironolactone 25 mg/d, and furosemide 80 mg/d.
Examination revealed bilateral 3+ pitting edema in the lower extremities midway up the shin, crackles to the inferior scapula bilaterally, and a 3/6 systolic murmur with regular rate and rhythm. The remainder of the physical exam was normal. The patient’s vitals were within normal limits, with an oxygen saturation of 90%.
The patient’s most recent chest x-ray demonstrated mild cardiomegaly. An echocardiogram showed an ejection fraction of 44% with severe bi-atrial enlargement, moderate-to-severe mitral regurgitation, and mild-to-moderate aortic insufficiency. His brain natriuretic peptide (BNP) was 915 pg/mL (normal range for patients ages 75-99 years, < 450 pg/mL).
THE DIAGNOSIS
The differential diagnosis for the patient’s shortness of breath included chronic obstructive pulmonary disease secondary to his smoking history, pulmonary embolus, respiratory infection, anemia, and medication-related adverse effects. The patient’s history of renal disease merited consideration of a nephrotic syndrome causing low albumin, which could explain his edema. Another possible cause of the edema was venous insufficiency. However, given the patient’s extensive cardiac history, the most likely explanation for his shortness of breath and edema was congestive HF that was unresponsive to the current diuretic regimen.
Several changes to the patient’s medications were made. Lisinopril 2.5 mg/d was started due to the mortality benefit of angiotensin-converting enzyme inhibitors in the treatment of HFrEF.1 Metoprolol tartrate 25 mg/d was transitioned to metoprolol succinate 50 mg/d, as only the longer-acting succinate version has shown mortality benefit in HFrEF.1 (Other beta-blockers with mortality benefit include carvedilol and bisoprolol.1) The furosemide 80 mg/d was replaced with torsemide 100 mg/d to provide an enhanced diuretic effect for symptomatic relief. The spironolactone dose was not increased due to concerns about the patient’s renal function. Of note, spironolactone was included in the patient’s regimen based on his NYHA classification, as well as the potential mortality benefits and improvement in edema seen in HFrEF patients.1 Spironolactone can be used with loop and/or thiazide diuretics in the treatment of HF.
Continue to: Within 5 days...
Within 5 days, the patient had lost 6 lb and his oxygen saturation had improved from 90% to 95%. He reported improvements in his breathing and was able to move around more easily.
DISCUSSION
There are several possible explanations for torsemide’s superior diuretic effect in this patient. Unlike furosemide, torsemide absorption is not influenced by intestinal edema, which is commonly seen in patients with HF. It has a longer half-life and improved bioavailability that is not altered by food intake. Torsemide also inhibits the actions of aldosterone through its interaction with the renin-angiotensin-aldosterone system and aldosterone receptor, leading to further diuresis and reduced cardiac remodeling.2
What the evidence shows. The TORIC trial was an open-label, nonrandomized, post-marketing surveillance study of 1377 patients with NYHA Class II–III HF who received diuretic therapy with torsemide 10 mg/d, furosemide 40 mg/d, or another diuretic for 12 months.3 Significantly lower total mortality and cardiac mortality was found in the torsemide group; in addition, a significantly greater proportion of patients in the torsemide group showed improvement in NYHA classification.3 Murray et al reported a reduction in hospitalization rates with torsemide therapy vs furosemide therapy in a randomized trial of 234 HF patients (32% vs 17%, P = 0.01).4 The ASCEND-HF trial, a large international acute HF trial comparing torsemide with furosemide, demonstrated a nonsignificant reduction in 30-day and 180-day events (all-cause mortality or HF hospitalization) in those receiving torsemide, after risk adjustment.5 Torsemide has also been shown to improve quality of life compared to furosemide.6
Preliminary results from the TORNADO trial,7 a multicenter randomized controlled trial, demonstrated superior symptom improvement in HF patients taking torsemide compared to those taking furosemide.8 The preliminary endpoint—a composite of improvement in NYHA class, improvement in distance of at least 50 m during a 6-minute walk test, and a decrease in fluid retention of at least 0.5 ohms at 3-month follow-up—was achieved by 94% and 58% of patients on torsemide and furosemide, respectively (P = 0.03).8 A total of 7 patients (3 in the torsemide and 4 in the furosemide group) were hospitalized for worsening HF during the follow-up period.8
A 2020 meta-analysis of more than 19,000 patients compared furosemide to torsemide and found a number needed to treat (NNT) of 23 to prevent a hospitalization due to HF; an NNT of 5 for improvement in NYHA functional status; and an NNT of 40 for reduction in cardiac mortality.9
Continue to: Our patient
Our patient reported feeling “great” at the 6-week follow-up appointment, with significant improvement in breathing and ability to perform his ADLs. His NYHA classification improved to Class II. He had lost 26 pounds (back to his weight 9 months prior), and his oxygen saturation was 97%.
On exam, the bilateral peripheral edema in his lower extremities had improved from 3+ to 1+, with the edema extending just distal to the mid-shin. Only mild crackles were present at the lung bases. The remainder of his physical examination was unchanged. His vital signs were within normal limits with no signs of hypotension. A basic metabolic panel was obtained to confirm his electrolytes were still within normal limits. His BNP had decreased to 230 pg/mL.
The patient declined the referral for hospice evaluation due to the significant improvement in his symptoms.
THE TAKEAWAY
A significant clinical improvement and improved quality of life were achieved with the transition from furosemide to torsemide. It is apparent that the patient’s furosemide had an inferior diuretic effect compared to torsemide, whether that be secondary to his dose or due to the unpredictable nature of furosemide’s bioavailability, especially in the setting of intestinal edema.
A growing body of literature9-11 suggests torsemide’s superiority over furosemide with no signs of increased adverse effects. Although additional prospective, head-to-head trials are needed, at this point in time it is appropriate to consider the use of torsemide in a patient with HF who does not seem to be fully responding to furosemide.
CORRESPONDENCE
Ryan Paulus, DO, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
THE CASE
An obese 90-year-old White man presented for a 1-month follow-up with his family physician after being hospitalized for an acute exacerbation of heart failure (HF). In addition to New York Heart Association (NYHA) Class III heart failure with reduced ejection fraction (HFrEF), he had a history of tobacco abuse, hyperlipidemia, atrial fibrillation, coronary artery disease, stage 3 chronic kidney disease, and benign prostatic hyperplasia. The patient’s family accompanied him during the visit to discuss hospice care.
The patient complained of persistent shortness of breath that limited his activities of daily living (ADLs) and lower extremity and scrotal edema. He denied chest pain, orthopnea, paroxysmal nocturnal dyspnea, ascites, nocturia, and nocturnal cough.
The patient had undergone a coronary artery bypass graft 23 years earlier. His HF was being managed with metoprolol tartrate 25 mg bid, spironolactone 25 mg/d, and furosemide 80 mg/d.
Examination revealed bilateral 3+ pitting edema in the lower extremities midway up the shin, crackles to the inferior scapula bilaterally, and a 3/6 systolic murmur with regular rate and rhythm. The remainder of the physical exam was normal. The patient’s vitals were within normal limits, with an oxygen saturation of 90%.
The patient’s most recent chest x-ray demonstrated mild cardiomegaly. An echocardiogram showed an ejection fraction of 44% with severe bi-atrial enlargement, moderate-to-severe mitral regurgitation, and mild-to-moderate aortic insufficiency. His brain natriuretic peptide (BNP) was 915 pg/mL (normal range for patients ages 75-99 years, < 450 pg/mL).
THE DIAGNOSIS
The differential diagnosis for the patient’s shortness of breath included chronic obstructive pulmonary disease secondary to his smoking history, pulmonary embolus, respiratory infection, anemia, and medication-related adverse effects. The patient’s history of renal disease merited consideration of a nephrotic syndrome causing low albumin, which could explain his edema. Another possible cause of the edema was venous insufficiency. However, given the patient’s extensive cardiac history, the most likely explanation for his shortness of breath and edema was congestive HF that was unresponsive to the current diuretic regimen.
Several changes to the patient’s medications were made. Lisinopril 2.5 mg/d was started due to the mortality benefit of angiotensin-converting enzyme inhibitors in the treatment of HFrEF.1 Metoprolol tartrate 25 mg/d was transitioned to metoprolol succinate 50 mg/d, as only the longer-acting succinate version has shown mortality benefit in HFrEF.1 (Other beta-blockers with mortality benefit include carvedilol and bisoprolol.1) The furosemide 80 mg/d was replaced with torsemide 100 mg/d to provide an enhanced diuretic effect for symptomatic relief. The spironolactone dose was not increased due to concerns about the patient’s renal function. Of note, spironolactone was included in the patient’s regimen based on his NYHA classification, as well as the potential mortality benefits and improvement in edema seen in HFrEF patients.1 Spironolactone can be used with loop and/or thiazide diuretics in the treatment of HF.
Continue to: Within 5 days...
Within 5 days, the patient had lost 6 lb and his oxygen saturation had improved from 90% to 95%. He reported improvements in his breathing and was able to move around more easily.
DISCUSSION
There are several possible explanations for torsemide’s superior diuretic effect in this patient. Unlike furosemide, torsemide absorption is not influenced by intestinal edema, which is commonly seen in patients with HF. It has a longer half-life and improved bioavailability that is not altered by food intake. Torsemide also inhibits the actions of aldosterone through its interaction with the renin-angiotensin-aldosterone system and aldosterone receptor, leading to further diuresis and reduced cardiac remodeling.2
What the evidence shows. The TORIC trial was an open-label, nonrandomized, post-marketing surveillance study of 1377 patients with NYHA Class II–III HF who received diuretic therapy with torsemide 10 mg/d, furosemide 40 mg/d, or another diuretic for 12 months.3 Significantly lower total mortality and cardiac mortality was found in the torsemide group; in addition, a significantly greater proportion of patients in the torsemide group showed improvement in NYHA classification.3 Murray et al reported a reduction in hospitalization rates with torsemide therapy vs furosemide therapy in a randomized trial of 234 HF patients (32% vs 17%, P = 0.01).4 The ASCEND-HF trial, a large international acute HF trial comparing torsemide with furosemide, demonstrated a nonsignificant reduction in 30-day and 180-day events (all-cause mortality or HF hospitalization) in those receiving torsemide, after risk adjustment.5 Torsemide has also been shown to improve quality of life compared to furosemide.6
Preliminary results from the TORNADO trial,7 a multicenter randomized controlled trial, demonstrated superior symptom improvement in HF patients taking torsemide compared to those taking furosemide.8 The preliminary endpoint—a composite of improvement in NYHA class, improvement in distance of at least 50 m during a 6-minute walk test, and a decrease in fluid retention of at least 0.5 ohms at 3-month follow-up—was achieved by 94% and 58% of patients on torsemide and furosemide, respectively (P = 0.03).8 A total of 7 patients (3 in the torsemide and 4 in the furosemide group) were hospitalized for worsening HF during the follow-up period.8
A 2020 meta-analysis of more than 19,000 patients compared furosemide to torsemide and found a number needed to treat (NNT) of 23 to prevent a hospitalization due to HF; an NNT of 5 for improvement in NYHA functional status; and an NNT of 40 for reduction in cardiac mortality.9
Continue to: Our patient
Our patient reported feeling “great” at the 6-week follow-up appointment, with significant improvement in breathing and ability to perform his ADLs. His NYHA classification improved to Class II. He had lost 26 pounds (back to his weight 9 months prior), and his oxygen saturation was 97%.
On exam, the bilateral peripheral edema in his lower extremities had improved from 3+ to 1+, with the edema extending just distal to the mid-shin. Only mild crackles were present at the lung bases. The remainder of his physical examination was unchanged. His vital signs were within normal limits with no signs of hypotension. A basic metabolic panel was obtained to confirm his electrolytes were still within normal limits. His BNP had decreased to 230 pg/mL.
The patient declined the referral for hospice evaluation due to the significant improvement in his symptoms.
THE TAKEAWAY
A significant clinical improvement and improved quality of life were achieved with the transition from furosemide to torsemide. It is apparent that the patient’s furosemide had an inferior diuretic effect compared to torsemide, whether that be secondary to his dose or due to the unpredictable nature of furosemide’s bioavailability, especially in the setting of intestinal edema.
A growing body of literature9-11 suggests torsemide’s superiority over furosemide with no signs of increased adverse effects. Although additional prospective, head-to-head trials are needed, at this point in time it is appropriate to consider the use of torsemide in a patient with HF who does not seem to be fully responding to furosemide.
CORRESPONDENCE
Ryan Paulus, DO, 590 Manning Drive, Chapel Hill, NC 27599; [email protected]
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
2. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-333.
3. Cosín J, Díez J; TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507-513.
4. Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513-520.
5. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:404-411.
6. Müller K, Gamba G, Jaquet F, et al. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793-801.
7. Balsam P, Ozierański K, Tymińska A, et al. The impact of torasemide on haemodynamic and neurohormonal stress, and cardiac remodelling in heart failure—TORNADO: a study protocol for a randomized controlled trial. Trials. 2017;18:36.
8. Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J. 2019;26:661-668.
9. Abraham B, Megaly M, Sous M, et al. Meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol. 2020;125:92-99.
10. Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Comparative analysis of long-term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77-86.
11. Täger T, Fröhlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83-90.
1. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239.
2. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323-333.
3. Cosín J, Díez J; TORIC investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507-513.
4. Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513-520.
5. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF Trial). Am J Cardiol. 2016;117:404-411.
6. Müller K, Gamba G, Jaquet F, et al. Torasemide vs furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793-801.
7. Balsam P, Ozierański K, Tymińska A, et al. The impact of torasemide on haemodynamic and neurohormonal stress, and cardiac remodelling in heart failure—TORNADO: a study protocol for a randomized controlled trial. Trials. 2017;18:36.
8. Balsam P, Ozierański K, Marchel M, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized TORNADO trial. Cardiol J. 2019;26:661-668.
9. Abraham B, Megaly M, Sous M, et al. Meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol. 2020;125:92-99.
10. Balsam P, Ozierański K, Kapłon-Cieślicka A, et al. Comparative analysis of long-term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77-86.
11. Täger T, Fröhlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure—a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83-90.